Reactions at α-position
|
|
|
- Alexander Strickland
- 6 years ago
- Views:
Transcription
1 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that can occur with carbonyl compounds, however, is to react an electrophile with the carbonyl E C 2 E The electrophile adds to the α-position and allows the synthesis of a variety of substituted carbonyl compounds by reacting different electrophiles
2 Reactions at α-position In order to react with electrophiles at the α-position, the carbonyl compound needs to be nucleophilic at the α-position There are two general methods to become nucleophilic at α-position: 1) React through the enol form K 5 x 10-9 keto C 2 enol Br Br C 2 Br A carbonyl compound is in equilibrium with an enol Typically the equilibrium for a ketone though lies heavily in the keto form The enol form, however, is more reactive than an alkene and can undergo similar reactions as observed with reactions with π bonds
3 Reactions at α-position 2) To make a carbonyl compound even more nucleophilic at the α-position, a base can be added to form an enolate base C 2 C 2 E C 2 E The α-position of a ketone is relatively acidic (pka ~19) because the anion is stabilized by resonance with the carbonyl oxygen The negatively charged enolate anion can react with an electrophile to form a new bond between the α-carbon and the electrophilic atom
4 Reactions at α-position Since the enolate anion resonates between two atoms, it is important to recognize which atom will react preferentially with an electrophile E E E C 2 E Reaction at carbon C 2 C 2 C 2 Reaction at oxygen In order to make this prediction, it is important to recognize which orbital is reacting As in all nucleophilic reactions, the M of the nucleophile is reacting with the LUM of the electrophile Consider the M for the enolate nucleophile: The charge in the M for the unsymmetrical enolate is far greater on the carbon than the oxygen (this is offset by a greater electron density in the lowest occupied orbital) Enolate structure M of enolate Therefore the enolate reacts preferentially at the carbon site
5 Reactions at α-position To form an enolate therefore a base can be reacted with a carbonyl compound to deprotonate the hydrogen on the α-carbon Realize, however, that most strong bases are also strong nucleophiles (remember factors in S N 2 versus E2 reactions) A base/nucleophile used could react either by reaction at carbonyl carbon or by abstracting the hydrogen on the α-carbon base/nucleophile C 2 NUC Formation of enolate Reaction at carbonyl Which pathway is preferred depends on the choice of base/nucleophile used
6 Reactions at α-position To generate enolate need to use a base that will not act as a nucleophile Common choice is to use lithium diisopropylamide (LDA) N BuLi N Li LDA LDA is a strong base (pka of conjugate is in high 30 s), while it is very bulky so it will not react as nucleophile on carbonyl LDA will therefore quantitatively deprotonate α-carbon without reacting at carbonyl carbon LDA C 2
7 Reactions at α-position The type of carbonyl compound will also affect the enolate formation Due to the resonance stabilization of some of the carboxylic acid derivatives, the pka values vary amongst different carbonyl compounds pka of conjugate C 2 C 2 C 2 () 2 N C 2 N RC C N Aldehydes are typically lower pka than ketones Esters and amides are less acidic Amidate is more acidic than α-carbon Therefore while LDA will quantitatively deprotonate the α-carbon, hydroxide or alkoxide bases (pka ~ 16) will only deprotonate a small fraction of molecules Na LDA C 2 C 2
8 Reactions at α-position The keto/enol equilibrium is also affected by the structure of the carbonyl compound K C 2 C 2 Both ketones and aldehydes highly favor keto form, but aldehyde have relatively more enol form present 3 β-dicarbonyl compounds have a much higher concentration of enol form due to intramolecular hydrogen bond Enol form is highly favored with phenol due to aromatic stabilization
9 Reactions at α-position The amount of enol present is increased in either acidic or basic conditions + 2 C 2 Na 2 C 2 C 2 Formation of enol allows hydrogens on α-carbon to be exchanged NaD, D 2 D+, D 2 D 3 C CD 3 D 3 C CD 3
10 Racemization of Enols and Enolates A consequence of the formation of enols or enolates is the α-carbon goes from sp 3 (and potentially chiral) to sp 2 (and therefore planar and achiral) hybridization + + or α-carbon is chiral α-carbon is planar racemic When the keto form is regenerated, the chirality at the α-carbon is lost The α-position therefore becomes racemic if there is an α-hydrogen present
11 alogenation When enols are generated in the presence of dihalogen compounds, an electrophilic reaction occurs which places a halogen on the α-carbon + 2 C 2 Br Br Br C 2 In acidic conditions the halogenation is stopped at one addition because the protonated carbonyl compound is less stable after a halogen has been added Br C 2 Br C 2 Positive charge is less stable with adjacent C-Br bond
12 alogenation In basic conditions, however, an enolate is generated instead of an enol Na C 2 Br Br Br C 2 The enolate is more stable with an attached halogen and therefore under basic conditions the α-position is polyhalogenated Br C 2 Na C Br Br Br CBr 2 More stable anion Reaction will continue until all α-hydrogens are replaced with halogen Br 2 R Na R CBr 3
13 aloform Reaction When the α-carbon is a methyl group, the basic halogenation places three halogens on carbon Br 2 R Na R CBr 3 Under the basic conditions of the reaction, however, the three halogens convert the methyl group into a good leaving group and thus the hydroxide can react at carbonyl carbon R CBr 3 Na R CBr 3 R CBr 3 bromoform The reaction thus will convert a methyl ketone into a carboxylic acid Called a haloform reaction because the common name for a trihalogen substituted carbon is a haloform (chloroform, bromoform or iodoform)
14 alogenation of Carboxylic Acids Carboxylic acids can also be halogenated in the α-position, but the acid halide needs to be formed first PBr 3 Br 2 Br Br Br 2 Br Br The acid halide can easily be converted back into the acid with water work-up Br Br 2 Br N 3! N 2 alanine These α-bromo acids are very convenient compounds to prepare α-amino acids with reaction with ammonia
15 Alkylation of Enolates Enolates are very useful to form new C-C bonds by reacting the enolate with alkyl halides LDA Br C 2 C 2 Allows formation of new C-C bond at the α-position, works best with methyl or 1 halides as more sterically hindered alkyl halides react through E2 mechanism When using symmetrical ketones, alkylation at either α-position generates the same product, but when using unsymmetrical ketones two different products can be obtained C 2 LDA 2 C C 2 or C Br Br The conditions used to form the enolate determines which is favored 2 C C 2 C
16 Alkylation of Enolates Differences in enolate formation control preferential pathway LDA C 2 2 C C 2 2 C C 2 C C ydrogen is easier to abstract, therefore this is the kinetic enolate Double bond of enolate is more stable, therefore this is the thermodynamic enolate When trying to control kinetic versus thermodynamic, typically the temperature can be used as the lower temperature favors kinetic and the higher temperature favors thermodynamic 1) LDA, -78 C 2) Br C 2 2 C C 2 1) LDA, 40 C 2) Br C 2 C
17 Alkylation of Enolates Alkylation of ketones is therefore relatively straightforward, add one equivalent of LDA at either low temperature for kinetic enolate and high temperature for thermodynamic enolate and then add the required alkyl halide ther types of carbonyl compounds can also be alkylated using these conditions Esters: 1) LDA 2) Br C 2 C Acids: With esters there is only one α-position and therefore alkylation occurs at this site Na LDA Br C 2 C 2 C C With carboxylic acids, first need to deprotonate the acidic hydrogen before deprotonating at α-position, alkylation will then occur at the α-position
18 Alkylation of Enolates Aldehydes: LDA C 2 C 2 C Alkylation of aldehydes can sometimes be problematic because the aldehyde carbonyl is more reactive than a ketone, therefore the enolate formed can react with the carbonyl (called an aldol reaction to be seen shortly) A way to circumvent this potential problem, the aldehyde can be converted to an imine RN 2 N R LDA N R 1) Br 2) 2 C 2 C 2 C C The imine anion can react with the alkyl halide and then the α-alkylated imine can be hydrolyzed back to the aldehyde with water
19 Alkylation of Enolates β-dicarbonyl: Na Br A distinct advantage with β-dicarbonyl compounds is the α-hydrogen is more acidic and can be quantitatively deprotonated with alkoxide base When discussing carboxylic acid derivatives, also observed that when a β-keto ester is hydrolyzed to the acid form a decarboxylation readily occurs Na! 2 C C 2 Thus this allows a much easier method to alkylate a ketone without needing to use LDA nor controlling kinetic versus thermodynamic (only obtain anion α to both carbonyls)
20 Alkylation of Enolates Another option to alkylate a ketone instead of needing to form an enolate is to react the ketone with a secondary amine to form an enamine N N C 2 The enamine can then react with an alkyl halide to alkylate the compound N Br N 2 C 2 C 2 C 2 The imminium ion that forms after alkylation is easily hydrolyzed with water to the ketone The enamine is less reactive than an enolate, but more reactive than an enol
21 Aldol Reaction As mentioned when forming enolates with aldehydes a potential problem is an aldol reaction Na C 2 C 2 C Aldol product Instead of merely being a potential side product, the aldol reaction can be favored by forming the enolate with alkoxide bases While the enolate is only formed in small concentration due to the differences in pka, each enolate that is generated is in the presence of an excess of aldehyde After work-up the product will contain an aldehyde (ald) and a β- hydroxy (ol) functionality, a characteristic of an aldol reaction is the formation of a β-hydroxy carbonyl Alexander Borodin ( ) Borodin is more famous today as a composer, but coinvented the aldol reaction and this could just as easily been called the Borodin reaction
22 Aldol Reaction The β-hydroxy ketone compounds obtained after an aldol reaction can also be dehydrated + The dehydration can occur under either acidic or basic conditions, although the dehydration is typically much easier under acidic conditions The dehydration is favored compared to other alcohols dehydrating to alkenes due to the conjugation of the obtained α,β-unsaturated alkene with the carbonyl As the conjugation increases, sometimes it is difficult to isolate the β-hydroxy carbonyl and only the α,β-unsaturated carbonyl is obtained Aldol reactions can occur with either aldehydes or ketones 1) Na 2) + C
23 Aldol Reaction If a compound contains both an enolizable position and a different carbonyl, then an intramolecular aldol reaction can occur to form a new ring Na 2 nce formed the β-hydroxy ketone can also dehydrate to form the α,β-unsaturated ketone When there are multiple enolizable positions, must consider the different types of possible products Na 2 2 C 5-membered rings are more stable than 7-membered, typically intramolecular aldol reactions are favored in forming either 5- or 6-membered rings
24 Crossed Aldol Reaction In addition to considering different enolizable positions in an intramolecular aldol reaction, when two different carbonyls are reacted in an aldol a variety of products are obtained Na C 2 C 2 C C C 2 2 C C 2 C C 3 3 C 2 C C 2 C 2 If the two carbonyls are both present, then the enolate could form on either nce formed, each enolate could react with either carbonyl that is present to yield 4 different products (assuming the compounds don t dehydrate to yield potentially more products) All four products will be obtained in similar amounts as the reactivity difference between different ketones is minimal This is called a crossed aldol or mixed aldol
25 Crossed Aldol Reaction While reacting two different ketones with alkoxide base is impractical due to the variety of products obtained, the desired product would only be obtained in low yield after a difficult separation, there are methods to react two different carbonyls in an aldol reaction efficiently A simple solution is if one of the two carbonyls does not have an enolizable position Na 2 C 2 nly enolate possible The enolate formed could still react with either carbonyl to generate two different products, but since an aldehyde is more reactive than a ketone benzaldehyde will react preferentially Due to the extra conjugation, more than likely only the dehydrated product will be obtained
26 Crossed Aldol Reaction The vast majority of time, however, there will be two carbonyls that either both have enolizable positions or the reactivity of the two carbonyls are similar, in these cases more than one product will be obtained if using alkoxide bases A solution for these cases is to quantitatively form the enolate rather than having an equilibrium between the enolate and keto forms with weak base LDA 3C2C 2 C C 2 2 C C First, quantitatively form the enolate from the desired ketone Then in a second step add the appropriate electrophilic carbonyl to react and only one product will be obtained By controlling the order of steps, any of the desired aldol products can be obtained LDA C 2 2 C C 2 C 2 C 2
27 Crossed Aldol Reaction The main difference is that the weak base only forms a small amount of enolate and thus once this enolate is generated it is in the presence of the ketone form to react Therefore both carbonyls would need to be present at the same time and thus a variety of products are obtained Na C 2 C 2 C C C 2 2 C C 2 C C 3 3 C 2 C C 2 C 2 All obtained in ~equal yield To synthesize only one, which enolate is required can be determined from the structure 1) LDA 2) 2 C C 2 nly product C 2 C 2
28 Claisen Condensation An aldol reaction refers to any reaction between an enolate nucleophile and a carbonyl electrophile When using ketone or aldehyde carbonyls, the reaction is equilibrium controlled When the electrophilic carbonyl is an ester, however, an irreversible last step occurs to drive the reaction to completion These aldol reactions with an ester are called Claisen condensations Na Difference in ketone and ester pka allows ketone enolate to be formed C 2 Na β-diketone formed has an acidic methylene (pka ~10) that is deprotonated in these basic conditions
29 Claisen Condensation Claisen condensation can also occur with only an ester present Na 2 C The enolate is harder to form due to the less acidic ester, but if it is the only carbonyl present it can still form Want to use same alkoxide as ester used, otherwise a transesterification will occur Na Rainer Ludwig Claisen ( ) Will generate a β-keto ester after acidifying the solution
30 Dieckmann Condensation An intramolecular Claisen condensation is called a Dieckmann condensation Na 2 C Ketone is more acidic than ester (6-membered ring more stable than 4) Walter Dieckmann ( ) In presence of alkoxide base, diketone will be deprotonated to drive reaction Dieckmann condensation can also occur with diester compounds to generate β-keto ester Na +, 2! The β-keto ester can then be hydrolyzed to acid and decarboxylated
31 Knoevenagel Reaction Another variant of the aldol condensation involves the formation of an enolate from an acidic position, usually a β-dicarbonyl, using an amine base N Due to the more acidic β-dicarbonyl compound, the enolate can be formed with amine base If generated in presence of ketone or aldehyde, an aldol reaction occurs which typically readily dehydrates A key factor in a Knoevenagel reaction is the extra stability of the formed enolate, allows formation exlusively at more acidic position even in presence of the less acidic ketone or aldehyde and thus can be formed even with weaker bases (typically amines) Emil Knoevenagel ( )
32 Michael Reaction Michael reactions, or sometimes called Michael additions, can occur when the electrophile has an α,β unsaturation NUC NUC NUC NUC 1,2 addition 1,4 addition (Michael) E NUC E When reacting with a nucleophile, the nucleophile can react in two different ways: 1) React directly on the carbonyl carbon (called a 1,2 addition) 2) React instead at the β-position (called a 1,4 addition) In a 1,4 addition, initially an enolate is formed which can be neutralized in work-up to reobtain the carbonyl Arthur Michael ( ) r the enolate can be reacted with a different electrophile in a second step to create a product that has substitution at both the α and β positions
33 Michael Reaction Whether a reaction proceeds with 1,2 addition or 1,4 addition (Michael) is often dependent upon the type of nucleophile being used Strong nucleophiles often favor 1,2 addition MgBr Grignard reagents and hydride delivery agents (LA) favor 1,2 addition Stabilized nucleophiles, however, favor 1,4 addition ( ) 2 CuLi Cuprates favor 1,4 addition ther stabilized nucleophiles favoring Michael addition are β-dicarbonyl enolates and enamines
34 Michael Reaction Michael addition using β-dicarbonyl enolates Na If a β-diester is used, then the ester can be hydrolyzed and decarboxylated Michael addition using an enamine 1) +, 2 2)! N N +, 2 The imminium salts generated initially can be hydrolyzed to the ketone
35 Michael Reaction When an enamine is used as the Michael donor with an α,β unsaturated carbonyl as the Michael acceptor, the reaction is called a Stork reaction after its inventor The Stork reaction allows the formation of a 1,5 dicarbonyl compound 1) N 2) +, 2 An advantage for the Stork reaction is that an enolate of a ketone generally reacts in a 1,2 addition Gilbert Stork (b 1921) By forming the enamine first, a Michael addition can occur instead
36 Michael Reaction We observed an example of a Michael reaction when discussing radical reactions in an earlier chapter Calicheamicin γ 1 S NC 2 Michael addition S NC 2 binding group binding group DNA Bergman cyclization S binding group C 2 N DNA diradical DNA cleavage 2 S binding group C 2 N
37 Robinson Annulation Many of these reactions can be used in combination to create interesting structures, one combination is to do a Michael reaction followed by an intramolecular aldol reaction (called a Robinson annulation) Na A small amount of enolate is formed by reacting a ketone with an alkoxide base Eventually the Michael addition will occur The Michael product under these conditions can equilibrate to place enolate at other α-carbon By placing enolate at this position, an intramolecular aldol reaction can occur that generates a 6-membered ring Na Robert Robinson ( ) Upon work-up this aldol dehydrates to form π bond C 2
38 Robinson Annulation Robinson annulation is a convenient method to synthesize polycyclic ring junctions The two α-carbons have different acidities and thus reaction occurs selectively at more acidic position Na Allows synthesis of fused polycyclic structures in high yield For example, this fused ring system is similar to steroid ring structures
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
What is in Common for the Following Reactions, and How Do They Work?
 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Another Equilibrium: Reaction At The α-position
 Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3 alogenation At The α-position Br 2, 2 C 2 Br
Another Equilibrium: Reaction At The α-position D 3 + D 3 C CD 3 2 C 2 the keto form the enol form chanism: + C 2 C 2 C 2 D D 2 D 2 repeat 5 times D 3 C CD 3 alogenation At The α-position Br 2, 2 C 2 Br
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Summary of π Bond Chemistry
 Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
ORGANIC - CLUTCH CH CONDENSATION CHEMISTRY.
 !! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
!! www.clutchprep.com CNCEPT: CNDENSATIN REACTINS A condensation reaction spontaneously combines two or more molecules with the loss of a smaller molecule. Instead of just reacting with electophiles, enolates
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Conjugate Addition Reactions 2:02 PM
 1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
1 Conjugate Addition vs Direct Addition What is Conjugate Addition? Conjugate addition refers to nucleophilic addition directed to the electrophilic carbon of the C=C (double bond) in a,bunsaturated systems.
Chapter 23. Alpha Substitution of Carbonyl Compounds.
 1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
18: Reactions of Enolate Ions and Enols
 18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
Module No and Title. PAPER No: 5 ; TITLE : Organic Chemistry-II MODULE No: 25 ; TITLE: S E 1 reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction
 P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
Lecture 23. The Aldol Condensation. an Aldol! April 12, 2018 O H O H - Chemistry 328N
 Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
ENOLATES IN ORGANIC SYNTHESIS
 ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
CHAPTER 24 HW: CARBONYL CONDENSATIONS
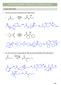 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Carbonyl Chemistry IV + C O C. Lecture 10. Chemistry /30/02
 arbonyl hemistry IV Ō - + Lecture 10 Addition of Nitrogen Nucleophiles Primary Amines RN 2 Imines Secondary Amines R 2 N Enamines ydrazine derivatives RNN 2 ydrazones ydroxyl Amine N 2 ximes Imine Formation
arbonyl hemistry IV Ō - + Lecture 10 Addition of Nitrogen Nucleophiles Primary Amines RN 2 Imines Secondary Amines R 2 N Enamines ydrazine derivatives RNN 2 ydrazones ydroxyl Amine N 2 ximes Imine Formation
20.3 Alkylation of Enolate Anions
 864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Amines. Amines are organic compounds containing a nitrogen functionality. primary secondary tertiary quaternary
 Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
CHAPTER 23 HW: ENOLS + ENOLATES
 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
20.10 Conjugate Additions
 894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
Amines Reading Study Problems Key Concepts and Skills Lecture Topics: Amines: structure and nomenclature
 Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
How to make pyridines: the Hantzsch pyridine synthesis
 ow to make pyridines: the antzsch pyridine synthesis 1191 Zinc in acetic acid (Chapter 24) reduces the oxime to the amine and we can start the synthesis by doing the conjugate addition and then reducing
ow to make pyridines: the antzsch pyridine synthesis 1191 Zinc in acetic acid (Chapter 24) reduces the oxime to the amine and we can start the synthesis by doing the conjugate addition and then reducing
Chapter 11 Reaction of Alcohols
 Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
The problem is that your product still has a-protons, and can keep on forming enolates to get more methyl groups added:
 Lecture 13 Notes November 7, 2012 We are going to start with some examples of proline-catalyzed reactions. In each case, you can draw a mechanism that would involve proline as a chiral catalyst, and one
Lecture 13 Notes November 7, 2012 We are going to start with some examples of proline-catalyzed reactions. In each case, you can draw a mechanism that would involve proline as a chiral catalyst, and one
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
O Enolate. = Electrophile/ Lewis acid. = Electrophile/ Lewis acid O B. Enolate. = Electrophile/ Lewis acid. Enolate. O O Enolate
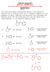 CM 234, Spring 2017 QUIZ #12 ANSWER KEY (hit the RETURN Button to return to the Main Quiz Page) QUESTIN 1 MC34r The following Aldol condensation product was formed by reaction of an enolate anion and a
CM 234, Spring 2017 QUIZ #12 ANSWER KEY (hit the RETURN Button to return to the Main Quiz Page) QUESTIN 1 MC34r The following Aldol condensation product was formed by reaction of an enolate anion and a
Reversible Additions to carbonyls: Weak Nucleophiles Relative Reactivity of carbonyls: Hydration of Ketones and Aldehydes
 Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Reversible Additions to carbonyls: Weak Nucleophiles Weak nucleophiles, such as water, alcohols, and amines, require acid or base catalysis to undergo addition to carbonyl compounds Relative Reactivity
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
MITOCW watch?v=gboyppj9ok4
 MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
Topic 9. Aldehydes & Ketones
 Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Practice Synthetic Problems: CHEM 235 Page 2
 Practice Synthetic Problems: CM 235 Page 2 Syntheses based on diethyl malonate, ethyl acetoacetate, etc. Using diethyl malonate and any other necessary organic reagents, show a synthesis of: a) 2,2-dimethyl-1,3-propanediamine
Practice Synthetic Problems: CM 235 Page 2 Syntheses based on diethyl malonate, ethyl acetoacetate, etc. Using diethyl malonate and any other necessary organic reagents, show a synthesis of: a) 2,2-dimethyl-1,3-propanediamine
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
When H and OH add to the alkyne, an enol is formed, which rearranges to form a carbonyl (C=O) group:
 Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Next Up: Addition of, : The next two reactions are the Markovnikov and non-markovnikov additions of and to an alkyne But you will not see alcohols form in this reaction! When and add to the alkyne, an
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
CHEM 345 Problem Set 07 Key
 CHEM 345 Problem Set 07 Key 1.) Fill in the appropriate reaction arrow. The starting material is on the left, the product is on the right. Use. Simple Ring Size. 5 and 6 are favored. 3 is not. That s it.
CHEM 345 Problem Set 07 Key 1.) Fill in the appropriate reaction arrow. The starting material is on the left, the product is on the right. Use. Simple Ring Size. 5 and 6 are favored. 3 is not. That s it.
Chem 263 Nov 3, 2016
 hem 263 Nov 3, 2016 Preparation of Aldehydes from Acid alides? + l l acid chloride aka acyl chloride aldehyde Needed: 2 Actual eagents: 2 /Pd Al This is lithium tri-t-butoxy aluminum hydride, a very sterically
hem 263 Nov 3, 2016 Preparation of Aldehydes from Acid alides? + l l acid chloride aka acyl chloride aldehyde Needed: 2 Actual eagents: 2 /Pd Al This is lithium tri-t-butoxy aluminum hydride, a very sterically
Aldehydes, Ketones and Carboxylic acids
 Teacher Orientation Aldehydes, Ketones and Carboxylic Acids contains following topics: Nomenclature Preparation Properties Student Orientation Preparation and Properties Of Aldehydes, Ketones and Carboxylic
Teacher Orientation Aldehydes, Ketones and Carboxylic Acids contains following topics: Nomenclature Preparation Properties Student Orientation Preparation and Properties Of Aldehydes, Ketones and Carboxylic
به نام خدا روشهای سنتز مواد آلی
 به نام خدا روشهای سنتز مواد آلی 1 References: 1. Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry: Reactions and Synthesis (Part B), 5th ed., Springer, 2007. 2. Carey, F. A.; Sundberg, R. J. Advanced
به نام خدا روشهای سنتز مواد آلی 1 References: 1. Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry: Reactions and Synthesis (Part B), 5th ed., Springer, 2007. 2. Carey, F. A.; Sundberg, R. J. Advanced
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
ANSWER GUIDE APRIL/MAY 2006 EXAMINATIONS CHEMISTRY 249H
 AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
Lecture 13A 05/11/12. Amines. [Sn2; Hofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis]
![Lecture 13A 05/11/12. Amines. [Sn2; Hofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis] Lecture 13A 05/11/12. Amines. [Sn2; Hofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis]](/thumbs/86/93838793.jpg) Lecture 13A 05/11/12 Amines [Sn2; ofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis] Curtius and ofmann rearrangements Both of these, in principle,
Lecture 13A 05/11/12 Amines [Sn2; ofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis] Curtius and ofmann rearrangements Both of these, in principle,
Aldehydes & Ketones LEVEL I. Phenol (enol form) Phenol is aromatic, so equiulibrium is shifted to the right hand side. b) O
 Subjective Problems Aldehydes & Ketones LEVEL I 1. a) 2,4cyclohexadiene-1-one (keto form) Phenol (enol form) Phenol is aromatic, so equiulibrium is shifted to the right hand side. b) Base This ketone is
Subjective Problems Aldehydes & Ketones LEVEL I 1. a) 2,4cyclohexadiene-1-one (keto form) Phenol (enol form) Phenol is aromatic, so equiulibrium is shifted to the right hand side. b) Base This ketone is
THE CHEMISTRY OF THE CARBONYL GROUP
 TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
Chem 263 March 7, 2006
 Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Lecture 24 Two Germans and an Englishman
 Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Lecture 23. Amines. Chemistry 328N. April 12, 2016
 Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Chapter 22 The Chemistry of Enolate Ions, Enols, and
 Organic Chemistry, 5th ed. Marc Loudon Chapter 22 The Chemistry of Enolate Ions, Enols, and α,β Unsaturated Carbonyl Compounds Eric J. Kantorowski California Polytechnic State University San Luis Obispo,
Organic Chemistry, 5th ed. Marc Loudon Chapter 22 The Chemistry of Enolate Ions, Enols, and α,β Unsaturated Carbonyl Compounds Eric J. Kantorowski California Polytechnic State University San Luis Obispo,
Lecture 19. Carboxylic Acids O C OH O R C O - + H + O - Chemistry 328N
 Lecture 19 arboxylic Acids R R - + + R - March 29, 2018 Review: Selectivity in Reduction LiAl 4 reduces any and all carbonyl compounds to the corresponding alcohols NaB 4 only reduces aldehydes and ketone
Lecture 19 arboxylic Acids R R - + + R - March 29, 2018 Review: Selectivity in Reduction LiAl 4 reduces any and all carbonyl compounds to the corresponding alcohols NaB 4 only reduces aldehydes and ketone
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
c. Oxidizing agent shown here oxidizes 2º alcohols to ketones and 1º alcohols to carboxylic acids. 3º alcohols DO NOT REACT.
 Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Tautomerism and Keto Enol Equilibrium
 Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
Ethers can be symmetrical or not:
 Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
Acid/Base stuff Beauchamp 1
 cid/base stuff Beauchamp 1 Problems You should be able to match a pk a value with its acid in each group below and explain the differences. You should be able to draw an arrow-pushing mechanism with general
cid/base stuff Beauchamp 1 Problems You should be able to match a pk a value with its acid in each group below and explain the differences. You should be able to draw an arrow-pushing mechanism with general
Organic Chemistry Review: Topic 10 & Topic 20
 Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
CH 3 CHCH 3 CH 3 CHCH 3 Isopropyl cation. Oxomium ion intermediate. intermediate (an electrophile)
 Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
