Objective 14. Develop synthesis strategies for organic synthesis.
|
|
|
- Trevor Ferguson
- 6 years ago
- Views:
Transcription
1 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show bonds breaking and forming show delocalized electrons with resonance structures. Key ideas: Starting material --> target compound. Count # of C s bigger or smaller compound. ID functional groups in starting material and target compound. What part(s) of the starting material and target compound are the same? ID bonds that break/form. Brainstorm possible reactions.
2 Reaction Roadmap: Which groups has an α-c? (2 types of α-c) What are β-keto acids/esters used for? What group makes α,β-unsaturated carbonyls? alkyne alkane alkene alkyl halide amide acid ester alcohol R-M aldehyde ketone nitriles Diols, cyanohydrins, imines epoxide Ether acetal w/ ROH
3 The Journey!
4 ORGANIC SYNTHESIS STRATEGIES Convert one functional group to another Move a Functional Group from 1 C to adjacent C Make a C-C bond to make a bigger molecule How? See Structural Features è Tells you Reaction Type
5 Structural Features tell us: Which atom or bond reacts Reaction Type How to make a Compound C=C pi bond (including conjugated diene and aromatic) C-O, C-X, C-N compounds: carbon, leaving group (HOH, ROH, NH 3, X - ), H bonded to carbon, epoxide C=O compounds: carbonyl carbon, carbonyl oxygen, carbon (C next to carbonyl C), carbon TWO structural features: -hydroxy aldehyde/ketone (see enolates), -unsaturated aldehyde/ketone (see enolates) -keto ester (see ester enolates)
6 Reaction Type Acid-Base Nucleophilic Substitution Nucleophilic Acyl Substitution EAS Elimination Electrophilic Addition Nucleophilic Addition Structural Features Acid H (see pk table) Base (see pk table) carbon, LG Reacts with Nu: - carbon, H bonded to carbon, LG Reacts with Nu: - C=C pi bond Reacts with E +
7 Fill in the Table. Classify carbon, H bonded to or carbon, enolate. What is used to make a C-C bond? Atom Type Nucleophiles Electrophiles hydrogen carbon nitrogen oxygen X Bond
8 Many Organic Reactions are Reversible Example: OR How does ethanol react? How can you make ethanol? Ethanol Structural Features: carbon and LG ==> Substitution H bonded to carbon and LG ==> Elimination Basic O and Acidic H ==> Acid-Base C-O bond ==> Oxidation Several Ways Ethanol Reacts è Several Ways to Make Ethanol
9 The following reaction has been reported in the chemical literature. Write the structure of the product(s). Structural Features: Carbonyl C Carbonyl C base H on carbon no H on carbon H on carbon H on carbon
10 Identify the reagents appropriate for each step in the following synthesis: Structural Features: Carbonyl C Carbonyl C C=C H on C Br on C, unsat d H on C H on C H on C
11 Aldehydes/Ketones and Acids Can be Halogenated at the α-c via an Enol Mechanism involves an enol. Compare the reaction of an enol with X 2 to an alkene with X 2. Does NOT work with acids, esters, or amides (not converted to enols) under these conditions. Different conditions (Hell-Volhard-Zelinsky)
12 Outline a reasonable mechanism for the following reaction: Structural Features: Carbonyl C What else? Carbonyl C What else?
13 Suggest reagents appropriate for each step in the synthesis. Structural Features: Carbonyl C Carbonyl C What else? Br on which C?
14 ORGANIC SYNTHESIS STRATEGIES How? See Structural Features è Tells you Reaction Type Convert One Functional Group To Another Structural Features: carbon and LG ==> Substitution Structural Features: C-O bond to C-O, C=O ==> Oxidation
15 ORGANIC SYNTHESIS STRATEGIES How? See Structural Features è Tells you Reaction Type Move a Functional Group from 1 C to adjacent C Structural Features: C and LG è Sub H on C and LG è Elim LG on different C H on C
16 ORGANIC SYNTHESIS STRATEGIES How? See Structural Features è Tells you Reaction Type Make a C-C bond to make a bigger molecule Use a carbon nucleophile and a carbon electrophile ID structural features: addition ID structural features: substitution Which C is electrophilic? Which carbon nucleophile to use? RMgX, CN -, acetylide ion, enolate or ester enolate
17 C&EN, 6/2/14, p. 24 ( ) Eastman Chemical Invents a New Cleaning Solvent for household and industrial cleaning products that meets the EPA standards for toxicity and VOC content. Trade Name: Omnia Propose an efficient synthesis of Omnia from. Replaces: ethylene glycol monobutyl ether (CA hazardous substance) dipropylene glycol monomethyl ether (VOC)
18 Propose an efficient synthesis of Omnia from. Trade Name: Omnia Structural Features: carbonyl C and carbonyl O (ester) H on C OH on C ( -hydroxy ester, not a -keto ester) O-H group: O is basic, H is acidic
19 3/25/13, CEN, p. 15 Limits Of Lithium It helps millions with bipolar disorder, but toxicity problems and side effects have scientists looking for alternatives by analyzing the drug s mysterious mechanism. Subanesthetic infusion of Ketamine (anesthetic agent) can relieve the symptoms of depression and suicidal urges in a matter of hours. The effect lasts about a week, whereas commonly prescribed antidepressants usually take weeks to work. (C&EN, Aug. 23, 2010, page 8). Design a synthesis of ketamine from: Ketamine Structural Features: How to connect the 2 molecules? Structural Features:
20 Note: Aldol and Claisen reactions form a C-C bond What else can an enolate or ester enolate ion react with? +?? > +?? >
21 Use Enolate in Nu: - substitution reaction Problems with this method: 1. Elimination competes with substitution 2. Poor yield Is there a Better way? (Klein, Organic Chemistry, p. 1063)
22 Objective: use malonic ester synthesis and acetoacetic ester synthesis to make a C-C bond α to carbonyl C Malonic ester synthesis: Lengthen chain by 2 carbons Use diethyl malonate Remove α-h to form enolate Enolate = Nu: - Acetoacetic ester synthesis: Lengthen chain by 3 carbons Use ethyl acetoacetate Remove α-h to form enolate
23 Alkylate the α carbon in an aldehyde/ketone: Use Acetoacetic Ester. Alkylate α carbon in an organic acid: Use Malonic Ester. Decarboxylation Reactions Are Important in Biology
24 Sirrus Company makes 1,1-disubstituted alkene monomers for use in adhesives, sealants, coatings, and inks. What type of reaction occurs?
25 Other ORGANIC SYNTHESIS STRATEGIES Shorten (Break C-C bond) a carbon chain: 1. ozonolysis 2. Decarboxylation Reaction Dicarboxylic acid (with 1 C between -COOH) -- heat --> acid + CO 2 E.g., Malonic acid (propanedioic acid) decarboxylation: Which bond breaks?
26 Other ORGANIC SYNTHESIS STRATEGIES Shorten (Break C-C bond) a carbon chain: 2. Break a C-C bond by decarboxylation of a. dicarboxylic acid b. β-keto acid -- heat --> CO 2 + enol ----> ketone 1 C from -COOH is α-c Draw structure of product 2 C from -COOH is β-c
27 Other ORGANIC SYNTHESIS STRATEGIES Shorten (Break C-C bond) a carbon chain: 3. Biology: Glucose (C 6 ) undergoes glycolysis to form pyruvate (C 3 ). (reverse aldol condensation) Which bond breaks?
28 Identify Compounds A, B, and C in the following synthetic sequence. NaOCH 2 CH 3 Compound A (C 6 H 10 O 2 ) > + H 2 C(CO 2 CH 2 CH 3 ) 2 ethanol Compound B (C 13 H 22 O 6 ) heat CH 3 CH(CH 2 CO 2 H) 2 < Compound C (C 7 H 10 O 6 ) H 2 C(CO 2 CH 2 CH 3 ) 2 = malonic ester
29 Objective: describe the mechanism of this reaction Compare C-C bond making methods: 1. Grignard (to make ROH) 2. Acetylide ion (alkyne ---> other functional groups) 3. CN substitution (to make nitrile ---> convert to acid) 4. Enolate ion (Aldol, Claisen, RX, malonic ester, acetoacetic ester)
30 Conjugated dienes undergo 1,2 and 1,4 addition (Klein, Ch. 17) α,β-unsaturated ketones undergo 1,2 and 1,4 addition (Michael Reaction) How do you make an α,β -unsaturated ketone?
31 Common Michael Donors and Acceptors (see Klein, Table 22.2, p. 1067) Michael Donors Michael Acceptors R 2 CuLi (RMgX too strong)
32 CEN, 4/2/12, p. 8 New Class of Superbases Cyclopropenimine is a very strong base (3.5 orders of magnitude higher than that of comparable guanidines) and catalyzes reactions up to 800 times faster. a. Explain why Cyclopropenimine is a strong base. (Hint: draw the structure of the conjugate acid of Cyclopropenimine.) b. With which reactant does this base react? c. Draw the structure of the product. (Hint: Michael addition)
33 CEN, 4/2/12, p. 8 New Class of Superbases Cyclopropenimine is a very strong base (3.5 orders of magnitude higher than that of comparable guanidines) and catalyzes reactions up to 800 times faster.
34 It is pretty easy to form a C-C bond at an - C. But it is NOT easy to form a C-C bond at the - C. -substituted carbonyls are common in insecticides and drug candidates. A new -arylation makes an intermediate used to synthesize serotonin reuptake inhibitors. (
35 3/18/13, CEN, p. 11 Synthetic Shortcut To Erythromycin Precursor Where does the carbonyl C come from? 1978: 33 steps 1979: 39 steps 2009: 23 steps 2013: 14 steps
36
37 Making Drugs: Multistep synthesis often involves undesirable materials, e.g., genotoxic impurities that damage DNA and may cause cancer.
38 CEN, 9/27/10, Genotoxic Impurities
39 CEN, 9/27/10, Genotoxic Impurities
40 5/13/13 Clicking Pyrroles Into Place Map the atoms in reactants and products.
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Key ideas: In EAS, pi bond is Nu and undergoes addition.
 Objective 7. Apply addition and elimination concepts to predict electrophilic aromatic substitution reactions (EAS) of benzene and monosubstituted benzenes. Skills: Draw structure ID structural features
Objective 7. Apply addition and elimination concepts to predict electrophilic aromatic substitution reactions (EAS) of benzene and monosubstituted benzenes. Skills: Draw structure ID structural features
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Alkynes Are Found in Natural Products
 Objective 13 Apply Reactivity Principles to Electrophilic Addition Reactions 2: Alkynes Identify structural features (pi bond) and electrophiles Use curved arrows to predict product Alkynes Are Found in
Objective 13 Apply Reactivity Principles to Electrophilic Addition Reactions 2: Alkynes Identify structural features (pi bond) and electrophiles Use curved arrows to predict product Alkynes Are Found in
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Objective 2. Identify and understand an organic oxidation and reduction reactions.
 Objective 2. Identify and understand an organic oxidation and reduction reactions. Skills: Draw structure ID structural features and reactive sites (atom that is being oxidized or reduced) ID Nu - and
Objective 2. Identify and understand an organic oxidation and reduction reactions. Skills: Draw structure ID structural features and reactive sites (atom that is being oxidized or reduced) ID Nu - and
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline
 Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Many Organic compounds are acids or bases (or both) Many Organic compounds undergo acid-base reactions
 Objective 4 Intro to Reactivity 1: identify acids and bases using Lewis definition. Use curved arrows to show how base reacts with acid. Relate strength to pk a. Determine direction of equilibrium. Use
Objective 4 Intro to Reactivity 1: identify acids and bases using Lewis definition. Use curved arrows to show how base reacts with acid. Relate strength to pk a. Determine direction of equilibrium. Use
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Chapter 23. Alpha Substitution of Carbonyl Compounds.
 1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
What is in Common for the Following Reactions, and How Do They Work?
 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
Chapter 20: Carboxylic Acids
 1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Summary of π Bond Chemistry
 Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Lecture 23. Amines. Chemistry 328N. April 12, 2016
 Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Spring Term 2012 Dr. Williams (309 Zurn, ex 2386)
 Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
COURSE UNIT DESCRIPTION. Dept. Organic Chemistry, Vilnius University. Type of the course unit
 Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Aldehydes and Ketones: Nucleophilic Addition Reactions
 Aldehydes and Ketones: Nucleophilic Addition Reactions Why this Chapter? Much of organic chemistry involves the chemistry of carbonyl compounds Aldehydes/ketones are intermediates in synthesis of pharmaceutical
Aldehydes and Ketones: Nucleophilic Addition Reactions Why this Chapter? Much of organic chemistry involves the chemistry of carbonyl compounds Aldehydes/ketones are intermediates in synthesis of pharmaceutical
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
18.8 Oxidation. Oxidation by silver ion requires an alkaline medium
 18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Lecture 23. The Aldol Condensation. an Aldol! April 12, 2018 O H O H - Chemistry 328N
 Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
Objective 11. Apply Reactivity Principles to Substitution and Elimination Reactions: compare size and strength of nucleophile to predict major product
 Objective 11 Apply Reactivity Principles to Substitution and Elimination Reactions: compare size and strength of nucleophile to predict major product Given Reactants ----> Predict Products How to figure
Objective 11 Apply Reactivity Principles to Substitution and Elimination Reactions: compare size and strength of nucleophile to predict major product Given Reactants ----> Predict Products How to figure
Chapter 18: Ketones and Aldehydes. I. Introduction
 1 Chapter 18: Ketones and Aldehydes I. Introduction We have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The three-dimensional
1 Chapter 18: Ketones and Aldehydes I. Introduction We have already encountered numerous examples of this functional group (ketones, aldehydes, carboxylic acids, acid chlorides, etc). The three-dimensional
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
Naming Organic Halides. Properties of Organic Halides
 Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
Carbonyl Chemistry IV: Enolate Alkylations and Aldols. Aldol Madness O O O N M + substrate. aldehyde. (Z)-enolate H
 Carbonyl Chemistry IV: nolate Alkylations and Aldols Paul Bracher Chem 30 Section 9 Section Agenda 1) o office hours Thursday 2) The Great Joe Young is covering section next onday 3) andout: Carbonyl Chemistry
Carbonyl Chemistry IV: nolate Alkylations and Aldols Paul Bracher Chem 30 Section 9 Section Agenda 1) o office hours Thursday 2) The Great Joe Young is covering section next onday 3) andout: Carbonyl Chemistry
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Chapter 20: Aldehydes and Ketones
 hem A225 Notes Page 67 I. Introduction hapter 20: Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group (=) with no other heteroatoms attached. An aldehyde has at least one hydrogen attached;
hem A225 Notes Page 67 I. Introduction hapter 20: Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group (=) with no other heteroatoms attached. An aldehyde has at least one hydrogen attached;
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Alkyl phenyl ketones are usually named by adding the acyl group as prefix to phenone.
 Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water.
 Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 12: Carbonyl Compounds II
 Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Alcohol Synthesis. Dr. Sapna Gupta
 Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
Chapter 9. Organic Chemistry: The Infinite Variety of Carbon Compounds. Organic Chemistry
 Chapter 9 Organic Chemistry: The Infinite Variety of Carbon Compounds Organic Chemistry Organic chemistry is defined as the chemistry of carbon compounds. Of tens of millions of known chemical compounds,
Chapter 9 Organic Chemistry: The Infinite Variety of Carbon Compounds Organic Chemistry Organic chemistry is defined as the chemistry of carbon compounds. Of tens of millions of known chemical compounds,
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
1. What is the major organic product obtained from the following sequence of reactions?
 CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
OCR (A) Chemistry A-level. Module 6: Organic Chemistry and Analysis
 OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
ROADMAP FOR REACTIONS Chapter 6
 RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
10. Alkyl Halides. What Is an Alkyl Halide. An organic compound containing at least one carbonhalogen
 10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
Physical Properties. Alcohols can be: CH CH 2 OH CH 2 CH 3 C OH CH 3. Secondary alcohol. Primary alcohol. Tertiary alcohol
 Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Lecture 24 Two Germans and an Englishman
 Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
2. Hydrohalogenation: Propylene reacts with HBr to form 2-bromopropane.
 Objective 12. Apply reactivity principles to Electrophilic Addition reactions 1: alkenes identify structural features (pi bond) and electrophiles, use curved arrows to predict product. Structural features:
Objective 12. Apply reactivity principles to Electrophilic Addition reactions 1: alkenes identify structural features (pi bond) and electrophiles, use curved arrows to predict product. Structural features:
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
ORGANIC - CLUTCH CH ALCOHOLS AND CARBONYL COMPOUNDS.
 !! www.clutchprep.com CONCEPT: INTRO TO REDOX Oxidation reactions involve an increase in the content of a molecule Reduction reactions involve an increase in the content of a molecule EXAMPLE: Label the
!! www.clutchprep.com CONCEPT: INTRO TO REDOX Oxidation reactions involve an increase in the content of a molecule Reduction reactions involve an increase in the content of a molecule EXAMPLE: Label the
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers and Epoxides. Chapter Organic Chemistry, 8th Edition John McMurry
 Alcohols, Ethers and Epoxides Chapter 17-18 Organic Chemistry, 8th Edition John McMurry 1 Introduction Structure and Bonding Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 2
Alcohols, Ethers and Epoxides Chapter 17-18 Organic Chemistry, 8th Edition John McMurry 1 Introduction Structure and Bonding Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 2
20.10 Conjugate Additions
 894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds.
 This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
Cape Cod Community College
 Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs:
Cape Cod Community College Departmental Syllabus Prepared by the Department of Natural Sciences & Applied Technology Date of Departmental Approval: February 3, 2014 Date Approved by Curriculum and Programs:
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Luckily this intermediate has three saturated carbons between the carbonyls, which again points to a Michael reaction:
 Retrosynthesis Practice Problems Answer Key October 1, 2013 1. Draw a retrosynthesis for how to make the compound shown below from starting materials with eight or fewer carbon atoms. The first step is
Retrosynthesis Practice Problems Answer Key October 1, 2013 1. Draw a retrosynthesis for how to make the compound shown below from starting materials with eight or fewer carbon atoms. The first step is
CHAPTER 23 HW: ENOLS + ENOLATES
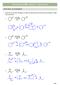 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
18: Reactions of Enolate Ions and Enols
 18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES
 ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name when given the structure, and draw the structure given the name of open-chain and monocyclic
ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name when given the structure, and draw the structure given the name of open-chain and monocyclic
Chapter 19 Carboxylic Acids
 Carboxylic acids have the formula RCO2H. Nomenclature Chapter 19 Carboxylic Acids For the parent alkane, drop the terminal e and add the suffix oic acid. The parent alkane is the longest continuous chain
Carboxylic acids have the formula RCO2H. Nomenclature Chapter 19 Carboxylic Acids For the parent alkane, drop the terminal e and add the suffix oic acid. The parent alkane is the longest continuous chain
Aldehydes, Ketones and Carboxylic acids
 Teacher Orientation Aldehydes, Ketones and Carboxylic Acids contains following topics: Nomenclature Preparation Properties Student Orientation Preparation and Properties Of Aldehydes, Ketones and Carboxylic
Teacher Orientation Aldehydes, Ketones and Carboxylic Acids contains following topics: Nomenclature Preparation Properties Student Orientation Preparation and Properties Of Aldehydes, Ketones and Carboxylic
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
CHAPTER 20: MORE ABOUT OXIDATION REDUCTION REACTIONS Oxidation Reduction Reactions of Organic Compounds: An Overview
 CHAPTER 20: MORE ABOUT OXIDATION REDUCTION REACTIONS In an oxidation-reduction reaction (redox reaction), one species loses electrons and one gains electrons. The species that loses electrons is oxidized,
CHAPTER 20: MORE ABOUT OXIDATION REDUCTION REACTIONS In an oxidation-reduction reaction (redox reaction), one species loses electrons and one gains electrons. The species that loses electrons is oxidized,
REALLY, REALLY STRONG BASES. DO NOT FORGET THIS!!!!!
 CHEM 345 Problem Set 4 Key Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. To use them effectively, it will require some knowledge from
CHEM 345 Problem Set 4 Key Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. To use them effectively, it will require some knowledge from
Learning Guide for Chapter 11 - Alkenes I
 Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
