Examples of spontaneity in terms of increased spatial arrangements Notes on General Chemistry
|
|
|
- Eustace Harris
- 5 years ago
- Views:
Transcription
1 Examples of spontaneity in terms of increased spatial arrangements Notes on General Chemistry Last updated Thursday, March 8, :40:15-05:00 Copyright 2007 Dan Dill Department of Chemistry, Boston University, Boston MA Here are some examples showing how spontaneous changes we are familiar with trace to an increase in the number of arrangements of particles. To analyze the examples, we use the generalized counting expression, Hi + j + kl! W = ÅÅÅÅÅÅÅÅ i! j! k! to get the number of unique ways i objects of one kind, j objects of another kind, and k objects of a third kind can be arranged. If there are only two different kinds of objects, this expression reduces to Hi + jl! W = ÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅ. i! j! If there are more than three different kinds of objects, the expression can be extended by adding the additional terms in the numerator and additional factorials in the denominator. à A gas evenly fills its container Here is a model of j = 3 particles confined in successively more possible positions. The maximum number of arrangements corresponds the particles spread out over the greatest number of possible positions. W = ÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅ H j + L ÅÅÅÅÅÅ! H3 + 0L! = = 1. j!! 3! 0! W = ÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅ H j + L ÅÅÅÅÅÅ! H3 + 2L! = = 10. j!! 3! 2! W = ÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅ H j + L ÅÅÅÅÅÅ! H3 + 6L! = = 84. j!! 3! 6!
2 2 Counting distinguishable arrangements à Two gases evenly mix Here is a model of 4 particles of one kind (light circles) and 4 particles of another kind (dark circles) confined in a lattice of 16 possible positions. The lattice is separated into halves by a fixed membrane that is permeable to both kinds of particles. The maximum number of arrangements corresponds to each kind of particle evenly spread out over the two halves of the whole lattice. In this arrangement, the particles are segregated in the different sides of the container. H4 + 4L! W = W left W right = 4! 4! H4 + 4L! = 70 μ 70 = ! 4! In this arrangement, the one particle of each type has moved to the other side of the container, and the result is a large increase in the number of possible arrangements, H L! W = W left W right = ÅÅÅÅÅÅÅÅÅÅ 1! 3! 4! H L! ÅÅÅÅÅÅÅÅÅÅ = 280 μ 280 = ! 1! 4! In this arrangement, the particles of each type are evenly distributed on both sides if the container, and the result is the maximum number of possible arrangements, H L! W = W left W right = ÅÅÅÅÅÅÅÅÅÅ 2! 2! 4! H L! ÅÅÅÅÅÅÅÅÅÅ = 420 μ 420 = ! 2! 4!
3 Counting distinguishable arrangements 3 à Two regions of a gas come to a common pressure Here is a model of 2 particles confined in one region and 6 particles of confined in another region. The two regions are separated by an impermeable barrier that is free to move, thereby changing the volume (the number possible positions) of the two regions. The maximum number of arrangements corresponds to the same pressure (number of particles per possible positions) in each region. In this initial configuration the number of arrangements is H2 + 6L! W = W left W right = 2! 6! H6 + 2L! = 28 μ 28 = 784, 6! 2! and the pressure on the left is too low, Dp = p left - p right = 2 ê 8 RT - 6 ê 8 RT=-1 ê 2 RT =-0.50 RT. Moving the barrier left decreases the volume on the left and increases the volume on the right. This results in an increase in the number of arrangements, H2 + 4L! W = W left W right = 2! 4! H6 + 4L! = 15 μ 210 = 3150, 6! 4! and a reduction in the pressure difference, Dp = p left - p right = 2 ê 6 RT - 6 ê 10 RT =-4 ê 15 RT =-0.27 RT. Further decreasing the volume on the left side and increasing the volume on the right side, results in an additional increase in the number of arrangements, H2 + 2L! W = W left W right = 2! 2! H6 + 6L! = 6 μ 924 = 5544, 6! 6! and now the pressure is the same on both sides, Dp = p left - p right = 2 ê 4 RT - 6 ê 12 R T = 0.
4 4 Counting distinguishable arrangements Further decreasing the volume of the left side and increasing the volume of the right side, results in a decrease in the number of arrangements, H2 + 0L! W = W left W right = 2! 0! H6 + 8L! = 1 μ 3003 = 3003, 6! 8! and reappearance of a pressure difference, Dp = p left - p right = 2 ê 2 RT - 6 ê 14 RT =+4 ê 7 RT =+0.57 RT.
5 Counting distinguishable arrangements 5 à A pressure difference develops across a semipermeable membrane Here is a model of 2 particles of one kind (light squares) and 6 particles of another kind (dark squares). The membrane is impermeable to the light colored particles but permeable to the dark colored particles. Here is an initial arrangement. H L! W = W left W right = ÅÅÅÅÅÅÅÅÅÅ 2! 2! 8! H4 + 8L! = 2970 μ 495 = ! 8! If one solvent particle moves to the right, there is a decrease in the number of arrangements. H L! W = W left W right = ÅÅÅÅÅÅÅÅÅÅ 2! 1! 9! H5 + 7L! = 660 μ 792 = ! 7! If instead one solvent particle moves to the left, there is an increase in the number of arrangements. H L! W = W left W right = ÅÅÅÅÅÅÅÅÅÅ 2! 3! 7! H3 + 9L! = 7920 μ 220 = ! 9! If a second solvent particle moves to the left, there is a decrease in the number of arrangements. H L! W = W left W right = ÅÅÅÅÅÅÅÅÅÅ 2! 4! 6! H2 + 10L! ÅÅÅÅ = μ 66 = ! 10! If all of the solvent particle move to the left, there is a large decrease in the number of arrangements. H L! W = W left W right = ÅÅÅÅÅÅÅÅÅÅ 2! 6! 4! H0 + 12L! ÅÅÅÅ = μ 1 = ! 12!
6 6 Counting distinguishable arrangements The maximum number of arrangements corresponds to equal numbers of the dark particles on either side of the membrane, n dark,left = n dark,right. Since the light particles cannot pass through the membrane, there is a pressure difference when the number of arrangements is a maximum, P=p left - p right = n light + n dark,left ÅÅÅÅÅÅÅÅÅÅÅÅ ÅÅÅÅÅÅÅÅÅÅÅ RT - n dark,right Å V V RT = n light ÅÅÅÅÅÅÅÅÅÅÅÅÅ V RT. This pressure difference the osmotic pressure P depends only on the concentration of the particles that cannot pass through the membrane.
Workshop: Average values and uncertainties Quantum aspects of physical chemistry
 Workshop: Average values and uncertainties Quantum aspects of physical chemistry http://quantum.bu.edu/pltl/2/2.pdf Last updated Tuesday, November 15, 2005 12:56:11-05:00 Copyright 2005 Dan Dill (dan@bu.edu)
Workshop: Average values and uncertainties Quantum aspects of physical chemistry http://quantum.bu.edu/pltl/2/2.pdf Last updated Tuesday, November 15, 2005 12:56:11-05:00 Copyright 2005 Dan Dill (dan@bu.edu)
Spontaneity: Second law of thermodynamics CH102 General Chemistry, Spring 2012, Boston University
 Spontaneity: Second law of thermodynamics CH102 General Chemistry, Spring 2012, Boston University three or four forces and, as capstone, a minimalist cosmic constitution to legislate their use: Article
Spontaneity: Second law of thermodynamics CH102 General Chemistry, Spring 2012, Boston University three or four forces and, as capstone, a minimalist cosmic constitution to legislate their use: Article
Workshop: One-electron-atom orbitals Quantum aspects of physical chemistry
 Workshop: One-electron-atom orbitals Quantum aspects of physical chemistry http://quantum.bu.edu/pltl/7.pdf Last updated Tuesday, November 15, 25 12:58:14-5: Copyright 25 Dan Dill (dan@bu.edu) Department
Workshop: One-electron-atom orbitals Quantum aspects of physical chemistry http://quantum.bu.edu/pltl/7.pdf Last updated Tuesday, November 15, 25 12:58:14-5: Copyright 25 Dan Dill (dan@bu.edu) Department
SOE: Symmetry Overlap Energy Notes on General Chemistry
 SOE: Symmetry Overlap Energy Notes on General Chemistry http://quantum.bu.edu/notes/generalchemistry/soesymmetryoverlapenergy.pdf Last updated Thursday, December 13, 2007 15:31:32-05:00 Copyright 2007
SOE: Symmetry Overlap Energy Notes on General Chemistry http://quantum.bu.edu/notes/generalchemistry/soesymmetryoverlapenergy.pdf Last updated Thursday, December 13, 2007 15:31:32-05:00 Copyright 2007
Example one-dimensional quantum systems Notes on Quantum Mechanics
 Eample one-dimensional quantum sstems Notes on Quantum Mechanics http://quantum.bu.edu/notes/quantummechanics/eampledquantumsstems.pdf Last updated Wednesda, October 0, 004 6:03:47-05:00 Copright 004 Dan
Eample one-dimensional quantum sstems Notes on Quantum Mechanics http://quantum.bu.edu/notes/quantummechanics/eampledquantumsstems.pdf Last updated Wednesda, October 0, 004 6:03:47-05:00 Copright 004 Dan
Workshop 5: Rovibrational and rovibronic spectra of gaseous HCl CH351 Physical Chemistry, Fall 2004
 Workshop 5: Rovibrational and rovibronic spectra of gaseous HCl CH351 Physical Chemistry, Fall 2004 http://quantum.bu.edu/courses/ch351/pltl/5.pdf Last updated Thursday, December 16, 2004 10:47:55-05:00
Workshop 5: Rovibrational and rovibronic spectra of gaseous HCl CH351 Physical Chemistry, Fall 2004 http://quantum.bu.edu/courses/ch351/pltl/5.pdf Last updated Thursday, December 16, 2004 10:47:55-05:00
1 Integration of Rational Functions Using Partial Fractions
 MTH Fall 008 Essex County College Division of Mathematics Handout Version 4 September 8, 008 Integration of Rational Functions Using Partial Fractions In the past it was far more usual to simplify or combine
MTH Fall 008 Essex County College Division of Mathematics Handout Version 4 September 8, 008 Integration of Rational Functions Using Partial Fractions In the past it was far more usual to simplify or combine
Wave functions and quantization workshop CH112 Workshop 9, Spring 2003
 Wave functions and quantization workshop CH112 Workshop 9, Spring 2003 http://quantum.bu.edu/notes/quantummechanics/wavefunctionsandquantizationworkshop.pdf Last updated Friday, October 31, 2003 16:33:01
Wave functions and quantization workshop CH112 Workshop 9, Spring 2003 http://quantum.bu.edu/notes/quantummechanics/wavefunctionsandquantizationworkshop.pdf Last updated Friday, October 31, 2003 16:33:01
in Trigonometry Name Section 6.1 Law of Sines Important Vocabulary
 Name Chapter 6 Additional Topics in Trigonometry Section 6.1 Law of Sines Objective: In this lesson you learned how to use the Law of Sines to solve oblique triangles and how to find the areas of oblique
Name Chapter 6 Additional Topics in Trigonometry Section 6.1 Law of Sines Objective: In this lesson you learned how to use the Law of Sines to solve oblique triangles and how to find the areas of oblique
Workshop 4: Diatomic molecule vibrational and rotational spectra CH351 Physical Chemistry, Fall 2004
 Workshop 4: Diatomic molecule vibrational and rotational spectra CH35 Physical Chemistry, Fall 004 http://quantum.bu.edu/courses/ch35/pltl/4.pdf Last updated Monday, November 9, 004 6:59:3-05:00 Copyright
Workshop 4: Diatomic molecule vibrational and rotational spectra CH35 Physical Chemistry, Fall 004 http://quantum.bu.edu/courses/ch35/pltl/4.pdf Last updated Monday, November 9, 004 6:59:3-05:00 Copyright
ÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅ
 HW.nb HW #. D scattering (a) The delta function potential requires a discontinuity of the wave function, y' H+0L - y' H-0L = ÅÅÅÅÅÅÅÅÅÅÅÅ m m yh0l, while the wavefunction itself is continuous. SolveA9
HW.nb HW #. D scattering (a) The delta function potential requires a discontinuity of the wave function, y' H+0L - y' H-0L = ÅÅÅÅÅÅÅÅÅÅÅÅ m m yh0l, while the wavefunction itself is continuous. SolveA9
Colligative properties CH102 General Chemistry, Spring 2011, Boston University
 Colligative properties CH12 General Chemistry, Spring 211, Boston University here are four colligative properties. vapor-pressure lowering boiling-point elevation freezing-point depression osmotic pressure
Colligative properties CH12 General Chemistry, Spring 211, Boston University here are four colligative properties. vapor-pressure lowering boiling-point elevation freezing-point depression osmotic pressure
Atomic wavefunction family album Notes on General Chemistry
 Atomic wavefunction family album Notes on General Chemistry http://quantum.bu.edu/notes/generalchemistry/atomicwavefunctionfamilyalbum.pdf Last updated Monday, November 7, 2005 14:31:48-05:00 Copyright
Atomic wavefunction family album Notes on General Chemistry http://quantum.bu.edu/notes/generalchemistry/atomicwavefunctionfamilyalbum.pdf Last updated Monday, November 7, 2005 14:31:48-05:00 Copyright
Solutions to the 2005 AP Calculus AB Exam Free Response Questions
 Solutions to the 00 AP Calculus AB Exam Free Response Questions Louis A. Talman Department of Mathematical & Computer Sciences Metropolitan State College of Denver Problem. FindRootA ÅÅÅÅ 4 + Sin@p xd
Solutions to the 00 AP Calculus AB Exam Free Response Questions Louis A. Talman Department of Mathematical & Computer Sciences Metropolitan State College of Denver Problem. FindRootA ÅÅÅÅ 4 + Sin@p xd
Recipe for p bonds in polyatomic molecules Notes on General Chemistry
 Recipe for p bonds in polyatomic molecules Notes on General Chemistry http://quantum.bu.edu/notes/generalchemistry/recipeforpibondsinpolyatomicmolecules.pdf Last updated Thursday, December 20, 2007 11:08:18-05:00
Recipe for p bonds in polyatomic molecules Notes on General Chemistry http://quantum.bu.edu/notes/generalchemistry/recipeforpibondsinpolyatomicmolecules.pdf Last updated Thursday, December 20, 2007 11:08:18-05:00
Time Independent Perturbation Theory
 apr_0-may_5.nb: 5/4/04::9:56:8 Time Independent Perturbation Theory Note: In producing a "final" vrsion of these notes I decided to change my notation from that used in class and by Sakurai. In class,
apr_0-may_5.nb: 5/4/04::9:56:8 Time Independent Perturbation Theory Note: In producing a "final" vrsion of these notes I decided to change my notation from that used in class and by Sakurai. In class,
CHAPTER 6 : LITERATURE REVIEW
 CHAPTER 6 : LITERATURE REVIEW Chapter : LITERATURE REVIEW 77 M E A S U R I N G T H E E F F I C I E N C Y O F D E C I S I O N M A K I N G U N I T S A B S T R A C T A n o n l i n e a r ( n o n c o n v e
CHAPTER 6 : LITERATURE REVIEW Chapter : LITERATURE REVIEW 77 M E A S U R I N G T H E E F F I C I E N C Y O F D E C I S I O N M A K I N G U N I T S A B S T R A C T A n o n l i n e a r ( n o n c o n v e
P E R E N C O - C H R I S T M A S P A R T Y
 L E T T I C E L E T T I C E I S A F A M I L Y R U N C O M P A N Y S P A N N I N G T W O G E N E R A T I O N S A N D T H R E E D E C A D E S. B A S E D I N L O N D O N, W E H A V E T H E P E R F E C T R
L E T T I C E L E T T I C E I S A F A M I L Y R U N C O M P A N Y S P A N N I N G T W O G E N E R A T I O N S A N D T H R E E D E C A D E S. B A S E D I N L O N D O N, W E H A V E T H E P E R F E C T R
à FIELD EFFECT TRANSISTORS
 Prof.M.G.Guvench à FIELD EFFECT TRANSISTORS ü FET: CONTENTS Principles of Operation Models: DC, S.S.A.C. and SPICE Applications: AC coupled S.S. Amplifiers ü FET: NAMES JFET Junction Field Effect Transistor
Prof.M.G.Guvench à FIELD EFFECT TRANSISTORS ü FET: CONTENTS Principles of Operation Models: DC, S.S.A.C. and SPICE Applications: AC coupled S.S. Amplifiers ü FET: NAMES JFET Junction Field Effect Transistor
Molecular structure: Diatomic molecules in the rigid rotor and harmonic oscillator approximations Notes on Quantum Mechanics
 Molecular structure: Datomc molecules n the rgd rotor and harmonc oscllator approxmatons Notes on Quantum Mechancs http://quantum.bu.edu/notes/quantummechancs/molecularstructuredatomc.pdf Last updated
Molecular structure: Datomc molecules n the rgd rotor and harmonc oscllator approxmatons Notes on Quantum Mechancs http://quantum.bu.edu/notes/quantummechancs/molecularstructuredatomc.pdf Last updated
Copyright 2017 Dan Dill 1
 TP An acid has K a 1.0 10 7 at 25 o C. In a c a 0.40 M solution of this acid solution, H 3 O 1. 0.40 2. 0.040 3. 0.0010 4. 0.0020 5. 0.00020 6. 0.00040 7. Something else Lecture 19 CH102 A1 (MWF 9:05 am)
TP An acid has K a 1.0 10 7 at 25 o C. In a c a 0.40 M solution of this acid solution, H 3 O 1. 0.40 2. 0.040 3. 0.0010 4. 0.0020 5. 0.00020 6. 0.00040 7. Something else Lecture 19 CH102 A1 (MWF 9:05 am)
The coalescent process
 The coalescent process Introduction Random drift can be seen in several ways Forwards in time: variation in allele frequency Backwards in time: a process of inbreeding//coalescence Allele frequencies Random
The coalescent process Introduction Random drift can be seen in several ways Forwards in time: variation in allele frequency Backwards in time: a process of inbreeding//coalescence Allele frequencies Random
Depression of the Freezing Point
 Depression of the Freezing Point If a solute is dissolved in the liquid at the triple point, the escaping tendency or vapor pressure of the liquid solvent is lowered below that of the pure solid solvent.
Depression of the Freezing Point If a solute is dissolved in the liquid at the triple point, the escaping tendency or vapor pressure of the liquid solvent is lowered below that of the pure solid solvent.
T i t l e o f t h e w o r k : L a M a r e a Y o k o h a m a. A r t i s t : M a r i a n o P e n s o t t i ( P l a y w r i g h t, D i r e c t o r )
 v e r. E N G O u t l i n e T i t l e o f t h e w o r k : L a M a r e a Y o k o h a m a A r t i s t : M a r i a n o P e n s o t t i ( P l a y w r i g h t, D i r e c t o r ) C o n t e n t s : T h i s w o
v e r. E N G O u t l i n e T i t l e o f t h e w o r k : L a M a r e a Y o k o h a m a A r t i s t : M a r i a n o P e n s o t t i ( P l a y w r i g h t, D i r e c t o r ) C o n t e n t s : T h i s w o
The Pythagorean Means
 Pythagorean-Mean-M6.nb The Pythagorean Mean Prof. Dr. J. Ziegenbalg Intitut für Mathematik und Informatik Univerity of Education, Karlruhe electronic mail: homepage: ziegenbalg@ph-karlruhe.de http://www.ph-karlruhe.de/wp/ziegenbalg/
Pythagorean-Mean-M6.nb The Pythagorean Mean Prof. Dr. J. Ziegenbalg Intitut für Mathematik und Informatik Univerity of Education, Karlruhe electronic mail: homepage: ziegenbalg@ph-karlruhe.de http://www.ph-karlruhe.de/wp/ziegenbalg/
Copyright 2017 Dan Dill 1
 TP Identify the correct statement about the redox reaction Zn 2 Cu Cu 2 Zn. 1. Zn 2 is the reducing agent because it gains electrons 2. Cu is the reducing agent because it gains electrons 3. Zn 2 is the
TP Identify the correct statement about the redox reaction Zn 2 Cu Cu 2 Zn. 1. Zn 2 is the reducing agent because it gains electrons 2. Cu is the reducing agent because it gains electrons 3. Zn 2 is the
à 10. DC (DIRECT-COUPLED) AMPLIFIERS
 0.DC-Amps-X.nb à 0. DC (DIRECT-COUPLED) AMPLIFIERS ü AC COUPLED SMALL SIGNAL AMPLIFIERS ADVANTAGES:. Signal, load and the amplifier bias are separate. One can work on the bias calculations stage by stage
0.DC-Amps-X.nb à 0. DC (DIRECT-COUPLED) AMPLIFIERS ü AC COUPLED SMALL SIGNAL AMPLIFIERS ADVANTAGES:. Signal, load and the amplifier bias are separate. One can work on the bias calculations stage by stage
Q 1 = 23.8 M = Q 3 = 29.8 IQR = 6 The numbers are in order and there are 18 pieces of data so the median is the average of the 9th and 10th
 Sample Exam #1, Math 01 1. Use the data set given below to answer all of the following questions. 14.0, 18.4, 1.6,.1, 3.8, 4.3, 5.9, 6.5, 7.5, 9., 9.3, 9.4, 9.7, 9.8, 30., 30.8, 31.9, 33.5 HaL Use the
Sample Exam #1, Math 01 1. Use the data set given below to answer all of the following questions. 14.0, 18.4, 1.6,.1, 3.8, 4.3, 5.9, 6.5, 7.5, 9., 9.3, 9.4, 9.7, 9.8, 30., 30.8, 31.9, 33.5 HaL Use the
Tricks of the Trade. Edited by Paul Abbott. Interpolation with Noise Vittorio G. Caffa. The Mathematica Journal
 The Mathematica Journal Tricks of the Trade Edited by Paul Abbott This is a column of programming tricks and techniques, most of which, we hope, will be contributed by our readers, either directly as submissions
The Mathematica Journal Tricks of the Trade Edited by Paul Abbott This is a column of programming tricks and techniques, most of which, we hope, will be contributed by our readers, either directly as submissions
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 9 8//4 University of Washington Department of Chemistry Chemistry 45/456 Summer Quarter 04. Solutions that re Very, Very on-ideal In the prior lectures on non-ideal solution behavior, we have considered
Lecture 9 8//4 University of Washington Department of Chemistry Chemistry 45/456 Summer Quarter 04. Solutions that re Very, Very on-ideal In the prior lectures on non-ideal solution behavior, we have considered
Exploring Large-scale Gravitational Quantization without in Planetary Systems, Galaxies, and the Universe
 Exploring Large-scale Gravitational Quantization without in Planetary Systems, Galaxies, and the Universe Howard G. Preston 1 & Franklin Potter 2,3 Abstract We explore a theory of large-scale gravitational
Exploring Large-scale Gravitational Quantization without in Planetary Systems, Galaxies, and the Universe Howard G. Preston 1 & Franklin Potter 2,3 Abstract We explore a theory of large-scale gravitational
Simple Mixtures. Chapter 7 of Atkins: Section
 Simple Mixtures Chapter 7 of Atkins: Section 7.5-7.8 Colligative Properties Boiling point elevation Freezing point depression Solubility Osmotic Pressure Activities Solvent Activity Solute Activity Regular
Simple Mixtures Chapter 7 of Atkins: Section 7.5-7.8 Colligative Properties Boiling point elevation Freezing point depression Solubility Osmotic Pressure Activities Solvent Activity Solute Activity Regular
Chem 260 Quiz - Chapter 4 (11/19/99)
 Chem 260 Quiz - Chapter 4 (11/19/99) Name (print) Signature Terms in bold: phase transitions transition temperature phase diagram phase boundaries vapor pressure thermal analysis dynamic equilibrium boiling
Chem 260 Quiz - Chapter 4 (11/19/99) Name (print) Signature Terms in bold: phase transitions transition temperature phase diagram phase boundaries vapor pressure thermal analysis dynamic equilibrium boiling
7. DESIGN OF AC-COUPLED BJT AMPLIFIERS FOR MAXIMUM UNDISTORTED VOLTAGE SWING
 à 7. DESIGN OF AC-COUPLED BJT AMPLIFIERS FOR MAXIMUM UNDISTORTED VOLTAGE SWING Figure. AC coupled common emitter amplifier circuit ü The DC Load Line V CC = I CQ + V CEQ + R E I EQ I EQ = I CQ + I BQ I
à 7. DESIGN OF AC-COUPLED BJT AMPLIFIERS FOR MAXIMUM UNDISTORTED VOLTAGE SWING Figure. AC coupled common emitter amplifier circuit ü The DC Load Line V CC = I CQ + V CEQ + R E I EQ I EQ = I CQ + I BQ I
Chapter 12.4 Colligative Properties of Solutions Objectives List and define the colligative properties of solutions. Relate the values of colligative
 Chapter 12.4 Colligative Properties of Solutions Objectives List and define the colligative properties of solutions. Relate the values of colligative properties to the concentrations of solutions. Calculate
Chapter 12.4 Colligative Properties of Solutions Objectives List and define the colligative properties of solutions. Relate the values of colligative properties to the concentrations of solutions. Calculate
Vapor Pressure Lowering explains the value of putting antifreeze in your radiator to keep a car from overheating
 Colligative Properties- Page 1 Colligative Properties (Sect. 8.14-8.17) By definition a colligative property is a solution property (a property of mixtures) for which it is the amount of solute dissolved
Colligative Properties- Page 1 Colligative Properties (Sect. 8.14-8.17) By definition a colligative property is a solution property (a property of mixtures) for which it is the amount of solute dissolved
Lecture 14. Entropy relationship to heat
 Lecture 14 Entropy relationship to heat Reading: Lecture 14, today: Chapter 7: 7.20 end Lecture 15, Wednesday: Ref. (2) 2/29/16 1 Hemoglobin and probability Oxygen binding molecule. Its quaternary structure
Lecture 14 Entropy relationship to heat Reading: Lecture 14, today: Chapter 7: 7.20 end Lecture 15, Wednesday: Ref. (2) 2/29/16 1 Hemoglobin and probability Oxygen binding molecule. Its quaternary structure
Student Exploration: Colligative Properties
 Name: Date: Student Exploration: Colligative Properties Vocabulary: boiling point, colligative property, concentration, dissociate, freezing point, manometer, osmosis, osmotic pressure, solute, solution,
Name: Date: Student Exploration: Colligative Properties Vocabulary: boiling point, colligative property, concentration, dissociate, freezing point, manometer, osmosis, osmotic pressure, solute, solution,
Applications of integrals
 ApplicationsofIntegrals.nb Applications of integrals There are many applications of definite integrals and we cannot include all of them in a document intended to be a review. However there are some very
ApplicationsofIntegrals.nb Applications of integrals There are many applications of definite integrals and we cannot include all of them in a document intended to be a review. However there are some very
5.4 Liquid Mixtures. G i. + n B. = n A. )+ n B. + RT ln x A. + RT ln x B. G = nrt ( x A. ln x A. Δ mix. + x B S = nr( x A
 5.4 Liquid Mixtures Key points 1. The Gibbs energy of mixing of two liquids to form an ideal solution is calculated in the same way as for two perfect gases 2. A regular solution is one in which the entropy
5.4 Liquid Mixtures Key points 1. The Gibbs energy of mixing of two liquids to form an ideal solution is calculated in the same way as for two perfect gases 2. A regular solution is one in which the entropy
Approximate formulas for the distance term in far and near field interferometry Tim Cornwell, NRAO
 EVLA Memo 75 Approximate formulas for the distance term in far and near field interferometry Tim Cornwell, NRAO tcornwel@nrao.edu Introduction In many applications in wide field and near field interferometry,
EVLA Memo 75 Approximate formulas for the distance term in far and near field interferometry Tim Cornwell, NRAO tcornwel@nrao.edu Introduction In many applications in wide field and near field interferometry,
Homework Key #7 - Phy 375R
 HMWK7-75R.nb Homewor Ke #7 - Ph 75R Problem #: See Ke for Homewor # Problem #: Transform the lne element... The nverse transformaton s t' = ÅÅÅÅ t tanh- ÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅ + ÅÅÅÅÅ ' = $%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%
HMWK7-75R.nb Homewor Ke #7 - Ph 75R Problem #: See Ke for Homewor # Problem #: Transform the lne element... The nverse transformaton s t' = ÅÅÅÅ t tanh- ÅÅÅÅÅÅÅÅÅÅÅÅÅÅÅ + ÅÅÅÅÅ ' = $%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%
A generalization of Amdahl's law and relative conditions of parallelism
 A generalization of Amdahl's law and relative conditions of arallelism Author: Gianluca Argentini, New Technologies and Models, Riello Grou, Legnago (VR), Italy. E-mail: gianluca.argentini@riellogrou.com
A generalization of Amdahl's law and relative conditions of arallelism Author: Gianluca Argentini, New Technologies and Models, Riello Grou, Legnago (VR), Italy. E-mail: gianluca.argentini@riellogrou.com
1. Calculating arrangements due to distribution of molecules: Wpos(a,b,c,.) =
 Chemistry 102 Spring 2018 Discussion #11, Chapter 17 Student name A name Section Review and hings you should know when you leave Discussion today: http://quantum.bu.edu/courses/ch102-spring-2016/notes/colligativeproperties-2014.pdf
Chemistry 102 Spring 2018 Discussion #11, Chapter 17 Student name A name Section Review and hings you should know when you leave Discussion today: http://quantum.bu.edu/courses/ch102-spring-2016/notes/colligativeproperties-2014.pdf
Difference Equations. Representation in "closed form" Formula solutions
 Difference-Equations-3-ClosedForm.nb Difference Equations - Part 3 - Representation in "closed form" Formula solutions Prof. Dr. J. Ziegenbalg Institut für Mathemati und Informati Pädagogische Hochschule
Difference-Equations-3-ClosedForm.nb Difference Equations - Part 3 - Representation in "closed form" Formula solutions Prof. Dr. J. Ziegenbalg Institut für Mathemati und Informati Pädagogische Hochschule
Damped Rolling Sphere II
 DapedRollingSphere(II).nb Daped Rolling Sphere II 008 October 30 printed 008 October 30 This adds daping to the case of a rolling sphere solved using the constraint transforation. Alost all of this notebook
DapedRollingSphere(II).nb Daped Rolling Sphere II 008 October 30 printed 008 October 30 This adds daping to the case of a rolling sphere solved using the constraint transforation. Alost all of this notebook
The Mathematica Journal Tricks of the Trade
 The Mathematica Joural Tricks of the Trade Edited by Paul Abbott This is a colum of programmig tricks ad techiques, most of which, we hope, will be cotributed by our readers, either directly as submissios
The Mathematica Joural Tricks of the Trade Edited by Paul Abbott This is a colum of programmig tricks ad techiques, most of which, we hope, will be cotributed by our readers, either directly as submissios
19. Steerable kernels
 329 9. Introduction 9. Steerable kernels 9. Introduction Clearly, orientation of structure is a multi-scale concept. On a small scale, the local orientation of structural elements, such as edges and ridges,
329 9. Introduction 9. Steerable kernels 9. Introduction Clearly, orientation of structure is a multi-scale concept. On a small scale, the local orientation of structural elements, such as edges and ridges,
Solutions. Part I. The Mathematica Notebook. Section 1. Cell structure: Input/Output. Section 2. Mixing Text and Mathematics
 Solutions To derive the maximum benefit from these exercises, make an honest effort to create the Mathematica code before consulting the solution offered here. Part I. The Mathematica Notebook Section.
Solutions To derive the maximum benefit from these exercises, make an honest effort to create the Mathematica code before consulting the solution offered here. Part I. The Mathematica Notebook Section.
ALE 24. Colligative Properties (Part 2)
 Name Chem 162, Section: Group Number: ALE 24. Colligative Properties (Part 2) (Reference: 13.6 Silberberg 5 th edition) Why is calcium chloride spread on highways in the North during the Winter? The Model:
Name Chem 162, Section: Group Number: ALE 24. Colligative Properties (Part 2) (Reference: 13.6 Silberberg 5 th edition) Why is calcium chloride spread on highways in the North during the Winter? The Model:
A Quick Introduction to Vectors, Vector Calculus, and Coordinate Systems
 Copyright 2006, David A. Randall Revised Wed, Feb 06, :36:23 A Quick Introduction to Vectors, Vector Calculus, and Coordinate Systems David A. Randall Department of Atmospheric Science Colorado State University,
Copyright 2006, David A. Randall Revised Wed, Feb 06, :36:23 A Quick Introduction to Vectors, Vector Calculus, and Coordinate Systems David A. Randall Department of Atmospheric Science Colorado State University,
A Numerical Study on Natural Convection in a Square Inclined Porous Enclosure Filled with an Ag Nanoliquids
 Applied Mathematical Sciences, Vol. 11, 2017, no. 34, 1695-1703 HIKARI Ltd, www.m-hikari.com https://doi.org/10.12988/ams.2017.74143 A Numerical Study on Natural Convection in a Square Inclined Porous
Applied Mathematical Sciences, Vol. 11, 2017, no. 34, 1695-1703 HIKARI Ltd, www.m-hikari.com https://doi.org/10.12988/ams.2017.74143 A Numerical Study on Natural Convection in a Square Inclined Porous
Daily Skill Builders:
 Daily Skill Builders: Pre-Algebra By WENDI SILVANO COPYRIGHT 2008 Mark Twain Media, Inc. ISBN 978-1-58037-445-3 Printing No. CD-404086 Mark Twain Media, Inc., Publishers Distributed by Carson-Dellosa Publishing
Daily Skill Builders: Pre-Algebra By WENDI SILVANO COPYRIGHT 2008 Mark Twain Media, Inc. ISBN 978-1-58037-445-3 Printing No. CD-404086 Mark Twain Media, Inc., Publishers Distributed by Carson-Dellosa Publishing
Name Class Date. Read the words in the box. Read the sentences. Fill in each blank with the word or phrase that best completes the sentence.
 Skills Worksheet Directed Reading B Section: Mixtures PROPERTIES OF MIXTURES mixture compound physical identity 1. A combination of substances that are not chemically combined is called a(n). 2. Two or
Skills Worksheet Directed Reading B Section: Mixtures PROPERTIES OF MIXTURES mixture compound physical identity 1. A combination of substances that are not chemically combined is called a(n). 2. Two or
Overview. Types of Solutions. Intermolecular forces in solution. Concentration terms. Colligative properties. Osmotic Pressure 2 / 46
 1 / 46 2 / 46 Overview Types of Solutions. Intermolecular forces in solution Concentration terms Colligative properties Osmotic Pressure 3 / 46 Solutions and Colloids A solution is a homogeneous mixture
1 / 46 2 / 46 Overview Types of Solutions. Intermolecular forces in solution Concentration terms Colligative properties Osmotic Pressure 3 / 46 Solutions and Colloids A solution is a homogeneous mixture
Announcements. It is critical that you are keeping up. Ask or see me if you need help. Lecture slides updated and homework solutions posted.
 Announcements Dec. 18 Hour Exam 1 C-109 Start time 6PM Coverage is Chapter 12 and 13. 10-multiple choice 3-fairly short problems 3-longer problem solving 100 point Exam Lecture slides updated and homework
Announcements Dec. 18 Hour Exam 1 C-109 Start time 6PM Coverage is Chapter 12 and 13. 10-multiple choice 3-fairly short problems 3-longer problem solving 100 point Exam Lecture slides updated and homework
Thermodynamics IV - Free Energy and Chemical Equilibria Chemical Potential (Partial Molar Gibbs Free Energy)
 Thermodynamics IV - Free Energy and Chemical Equilibria Chemical Potential (Partial Molar Gibbs Free Energy) increase in the Gibbs free energy of the system when 1 mole of i is added to a large amount
Thermodynamics IV - Free Energy and Chemical Equilibria Chemical Potential (Partial Molar Gibbs Free Energy) increase in the Gibbs free energy of the system when 1 mole of i is added to a large amount
STAT 501 Assignment 1 Name Spring Written Assignment: Due Monday, January 22, in class. Please write your answers on this assignment
 STAT 5 Assignment Name Spring Reading Assignment: Johnson and Wichern, Chapter, Sections.5 and.6, Chapter, and Chapter. Review matrix operations in Chapter and Supplement A. Examine the matrix properties
STAT 5 Assignment Name Spring Reading Assignment: Johnson and Wichern, Chapter, Sections.5 and.6, Chapter, and Chapter. Review matrix operations in Chapter and Supplement A. Examine the matrix properties
Copyright 2017 Dan Dill 1
 TP The order of normal boiling points is Acetone, CH 3 C O CH 3 30.8 Diethyl ether, CH 3 CH 2 2 O 71.7 Ethanol, CH 3 CH 2 OH 7.87 Water, H 2 O 3.17 0 Lecture 13 CH1 A1 (MWF 9:05 am) Friday, October 6,
TP The order of normal boiling points is Acetone, CH 3 C O CH 3 30.8 Diethyl ether, CH 3 CH 2 2 O 71.7 Ethanol, CH 3 CH 2 OH 7.87 Water, H 2 O 3.17 0 Lecture 13 CH1 A1 (MWF 9:05 am) Friday, October 6,
Linear system of the Schrödinger equation Notes on Quantum Mechanics
 Lnear sstem of the Schrödnger equaton Notes on Quantum Mechancs htt://quantum.bu.edu/notes/quantummechancs/lnearsstems.df Last udated Wednesda, October 9, 003 :0:08 Corght 003 Dan Dll (dan@bu.edu) Deartment
Lnear sstem of the Schrödnger equaton Notes on Quantum Mechancs htt://quantum.bu.edu/notes/quantummechancs/lnearsstems.df Last udated Wednesda, October 9, 003 :0:08 Corght 003 Dan Dll (dan@bu.edu) Deartment
Section 8.4: Ordinary Differential equations
 Section8_4.nb Section 8.4: Ordinary Differential equations Created by Tomas de-camino-beck tomasd@math.ualberta.ca SIR model Let ihtl be the infected at time t and shtl suceptibles. Note that we are not
Section8_4.nb Section 8.4: Ordinary Differential equations Created by Tomas de-camino-beck tomasd@math.ualberta.ca SIR model Let ihtl be the infected at time t and shtl suceptibles. Note that we are not
Chapter 11 section 6 and Chapter 8 Sections 1-4 from Atkins
 Lecture Announce: Chapter 11 section 6 and Chapter 8 Sections 1-4 from Atkins Outline: osmotic pressure electrolyte solutions phase diagrams of mixtures Gibbs phase rule liquid-vapor distillation azeotropes
Lecture Announce: Chapter 11 section 6 and Chapter 8 Sections 1-4 from Atkins Outline: osmotic pressure electrolyte solutions phase diagrams of mixtures Gibbs phase rule liquid-vapor distillation azeotropes
Lecture Presentation. Chapter 12. Solutions. Sherril Soman, Grand Valley State University Pearson Education, Inc.
 Lecture Presentation Chapter 12 Solutions Sherril Soman, Grand Valley State University Thirsty Seawater Drinking seawater can cause dehydration. Seawater Is a homogeneous mixture of salts with water Contains
Lecture Presentation Chapter 12 Solutions Sherril Soman, Grand Valley State University Thirsty Seawater Drinking seawater can cause dehydration. Seawater Is a homogeneous mixture of salts with water Contains
REMOVE THIS PAGE PRIOR TO STARTING EXAM.
 REMOVE THIS PAGE PRIOR TO STARTING EXAM. 1 2 ANSWER KEY CHEMISTRY F14O3 SECOND EXAM 11/10/99 PROFESSOR J. MORROW PRINT NAME, LAST: FIRST: I.D.# : MAXIMUM POINT VALUE IS IN PARENTHESES 1. (15) 9. (15) 17.
REMOVE THIS PAGE PRIOR TO STARTING EXAM. 1 2 ANSWER KEY CHEMISTRY F14O3 SECOND EXAM 11/10/99 PROFESSOR J. MORROW PRINT NAME, LAST: FIRST: I.D.# : MAXIMUM POINT VALUE IS IN PARENTHESES 1. (15) 9. (15) 17.
Slide 1 / 69. Slide 2 / 69. Slide 3 / 69. Whole Numbers. Table of Contents. Prime and Composite Numbers
 Slide 1 / 69 Whole Numbers Table of Contents Slide 2 / 69 Prime and Composite Numbers Prime Factorization Common Factors Greatest Common Factor Relatively Prime Least Common Multiple Slide 3 / 69 Prime
Slide 1 / 69 Whole Numbers Table of Contents Slide 2 / 69 Prime and Composite Numbers Prime Factorization Common Factors Greatest Common Factor Relatively Prime Least Common Multiple Slide 3 / 69 Prime
Mathematica for Theoretical Physics
 Mathematica for Theoretical Physics Mathematica for Theoretical Physics Electrodynamics, Quantum Mechanics, General Relativity, and Fractals Second Edition Gerd Baumann CD-ROM Included Gerd Baumann Department
Mathematica for Theoretical Physics Mathematica for Theoretical Physics Electrodynamics, Quantum Mechanics, General Relativity, and Fractals Second Edition Gerd Baumann CD-ROM Included Gerd Baumann Department
Enduring Understandings & Essential Knowledge for AP Chemistry
 Enduring Understandings & Essential Knowledge for AP Chemistry Big Idea 1: The chemical elements are fundamental building materials of matter, and all matter can be understood in terms of arrangements
Enduring Understandings & Essential Knowledge for AP Chemistry Big Idea 1: The chemical elements are fundamental building materials of matter, and all matter can be understood in terms of arrangements
Lecture 03/30 Monday, March 30, :46 AM
 Chem 110A Page 1 Lecture 03/30 Monday, March 30, 2009 9:46 AM Notes 0330 Audio recording started: 10:02 AM Monday, March 30, 2009 Chapter 1: Ideal Gases Macroscopic properties of gases Equation of state
Chem 110A Page 1 Lecture 03/30 Monday, March 30, 2009 9:46 AM Notes 0330 Audio recording started: 10:02 AM Monday, March 30, 2009 Chapter 1: Ideal Gases Macroscopic properties of gases Equation of state
Physical Properties of Solutions
 Physical Properties of Solutions Chapter 12 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 12.1- Types of solutions A solution is a homogenous mixture of 2 or
Physical Properties of Solutions Chapter 12 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 12.1- Types of solutions A solution is a homogenous mixture of 2 or
Life, Order, Thermodynamics
 Life, Order, Thermodynamics 1 A key question about order How order is created and maintained in biological systems? Through energy fluxes Biological Physics, updated 1st ed. ( Philip C. Nelson) 2 Energy:
Life, Order, Thermodynamics 1 A key question about order How order is created and maintained in biological systems? Through energy fluxes Biological Physics, updated 1st ed. ( Philip C. Nelson) 2 Energy:
JFET CAPACITANCE CALCULATIONS
 JFET CAPACITANCE CALCULATIONS JFET CAPACITANCE CALCULATIONS In order to simplify the design procedure for the frequency response of the JFET amplifier we will consider effect of each capacitance separately.
JFET CAPACITANCE CALCULATIONS JFET CAPACITANCE CALCULATIONS In order to simplify the design procedure for the frequency response of the JFET amplifier we will consider effect of each capacitance separately.
Copyright 2017 Dan Dill 1
 TP In pure water at 25, there is only a tiny, tiny amount of self ionization, ~ 7 mol/l. In pure water, how many water molecules are there for every OH ion? 1. 1 2. 3. 0 4.,000 5. 1,000,000 6.,000,000
TP In pure water at 25, there is only a tiny, tiny amount of self ionization, ~ 7 mol/l. In pure water, how many water molecules are there for every OH ion? 1. 1 2. 3. 0 4.,000 5. 1,000,000 6.,000,000
Design of Experiments. Factorial experiments require a lot of resources
 Design of Experiments Factorial experiments require a lot of resources Sometimes real-world practical considerations require us to design experiments in specialized ways. The design of an experiment is
Design of Experiments Factorial experiments require a lot of resources Sometimes real-world practical considerations require us to design experiments in specialized ways. The design of an experiment is
KEMS448 Physical Chemistry Advanced Laboratory Work. Freezing Point Depression
 KEMS448 Physical Chemistry Advanced Laboratory Work Freezing Point Depression 1 Introduction Colligative properties are properties of liquids that depend only on the amount of dissolved matter (concentration),
KEMS448 Physical Chemistry Advanced Laboratory Work Freezing Point Depression 1 Introduction Colligative properties are properties of liquids that depend only on the amount of dissolved matter (concentration),
Chapter 11 Review Packet
 Chapter 11 Review Packet Name Multiple Choice Portion: 1. Which of the following terms is not a quantitative description of a solution? a. molarity b. molality c. mole fraction d. supersaturation 2. Which
Chapter 11 Review Packet Name Multiple Choice Portion: 1. Which of the following terms is not a quantitative description of a solution? a. molarity b. molality c. mole fraction d. supersaturation 2. Which
Diffusion By Cindy Grigg
 Diffusion By Cindy Grigg 1 Why do we smell bread baking throughout the house? The answer is diffusion. A good way to describe diffusion is the moving of molecules from a place where they are concentrated
Diffusion By Cindy Grigg 1 Why do we smell bread baking throughout the house? The answer is diffusion. A good way to describe diffusion is the moving of molecules from a place where they are concentrated
School of Chemical & Biological Engineering, Konkuk University
 School of Chemical & iological Engineering, Konkuk University Lecture 7 Ch. 5 Simple Mixtures Colligative properties Prof. Yo-Sep Min Physical Chemistry I, Spring 2009 Ch. 5-2 he presence of a solute in
School of Chemical & iological Engineering, Konkuk University Lecture 7 Ch. 5 Simple Mixtures Colligative properties Prof. Yo-Sep Min Physical Chemistry I, Spring 2009 Ch. 5-2 he presence of a solute in
Cell Membranes and Permeability Laboratory
 Cell Membranes and Permeability Laboratory Do all chemical substances pass in and out of a cell membrane with equal ease? Do chemical substances move from areas of high concentration to areas of low concentration
Cell Membranes and Permeability Laboratory Do all chemical substances pass in and out of a cell membrane with equal ease? Do chemical substances move from areas of high concentration to areas of low concentration
- Let's look at how things dissolve into water, since aqueous solutions are quite common. sucrose (table sugar)
 68 HOW THINGS DISSOLVE - Let's look at how things dissolve into water, since aqueous solutions are quite common. sucrose (table sugar)... what happens? - Water molecules pull the sugar molecules out of
68 HOW THINGS DISSOLVE - Let's look at how things dissolve into water, since aqueous solutions are quite common. sucrose (table sugar)... what happens? - Water molecules pull the sugar molecules out of
Chemistry 163B Colligative Properties Challenged Penpersonship Notes
 Chemistry 163 Colligative Properties Challenged Penpersonship Notes 1 colligative properties o solutions colligative One entry ound. Main Entry: col li ga tive Pronunciation: kä-lə- gā-tiv, kə- li-gə-tiv
Chemistry 163 Colligative Properties Challenged Penpersonship Notes 1 colligative properties o solutions colligative One entry ound. Main Entry: col li ga tive Pronunciation: kä-lə- gā-tiv, kə- li-gə-tiv
Soln Notes February 17, 2017
 Chapter 15 Solutions You are responsible for reading/notes on Section 15.4 Heterogeneous Mixtures p.476-479 What is a SOLUTION? SOLUTE vs SOLVENT Characteristics of Solutions: Soluble/ Insoluble Solvation
Chapter 15 Solutions You are responsible for reading/notes on Section 15.4 Heterogeneous Mixtures p.476-479 What is a SOLUTION? SOLUTE vs SOLVENT Characteristics of Solutions: Soluble/ Insoluble Solvation
6-4 Atomic structure Trilogy
 6-4 Atomic structure Trilogy.0 Figure shows a helium atom. Figure. Use the words in the box to label the diagram. electron neutron proton [2 marks].2 An alpha particle is the same as the nucleus of a helium
6-4 Atomic structure Trilogy.0 Figure shows a helium atom. Figure. Use the words in the box to label the diagram. electron neutron proton [2 marks].2 An alpha particle is the same as the nucleus of a helium
Unit B: Cells and Systems
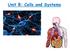 Unit B: Cells and Systems Topic 4: Fluid Movement in Cells The Cell Membrane A cell membrane allows some to enter or leave the cell, while stopping other substances. It is a selectively membrane. (A permeable
Unit B: Cells and Systems Topic 4: Fluid Movement in Cells The Cell Membrane A cell membrane allows some to enter or leave the cell, while stopping other substances. It is a selectively membrane. (A permeable
2. Foundations of scale-space
 2. Foundations of scale-space 13 2. Foundations of scale-space "There are many paths to the top of the mountain, but the view is always the same" -Chinese proverb. 2.1 Constraints for an uncommitted front-end
2. Foundations of scale-space 13 2. Foundations of scale-space "There are many paths to the top of the mountain, but the view is always the same" -Chinese proverb. 2.1 Constraints for an uncommitted front-end
Department of Mathematical and Statistical Sciences University of Alberta
 MATH 214 (R1) Winter 2008 Intermediate Calculus I Solutions to Problem Set #8 Completion Date: Friday March 14, 2008 Department of Mathematical and Statistical Sciences University of Alberta Question 1.
MATH 214 (R1) Winter 2008 Intermediate Calculus I Solutions to Problem Set #8 Completion Date: Friday March 14, 2008 Department of Mathematical and Statistical Sciences University of Alberta Question 1.
Intermolecular Forces
 Intermolecular Forces! When two molecules approach one another, they are attracted to some extent! Polar molecules are attracted through the electrostatic interaction of their dipole moments! Non-polar
Intermolecular Forces! When two molecules approach one another, they are attracted to some extent! Polar molecules are attracted through the electrostatic interaction of their dipole moments! Non-polar
Disorder and Entropy. Disorder and Entropy
 Disorder and Entropy Suppose I have 10 particles that can be in one of two states either the blue state or the red state. How many different ways can we arrange those particles among the states? All particles
Disorder and Entropy Suppose I have 10 particles that can be in one of two states either the blue state or the red state. How many different ways can we arrange those particles among the states? All particles
Ways of Expressing Concentrations of Solutions. Solutions
 Ways of Expressing Concentrations of Mole Fraction (X) X A = moles of A total moles in solution In some applications, one needs the mole fraction of solvent, not solute make sure you find the quantity
Ways of Expressing Concentrations of Mole Fraction (X) X A = moles of A total moles in solution In some applications, one needs the mole fraction of solvent, not solute make sure you find the quantity
Student Name: Module 1: Elements of Art and Principles of Design Study Guide
 Before attempting the Module Exam, you should be familiar with the concepts below. Use your notes from each lesson to help you complete this self check worksheet. Review any lessons where you feel a lack
Before attempting the Module Exam, you should be familiar with the concepts below. Use your notes from each lesson to help you complete this self check worksheet. Review any lessons where you feel a lack
Mixtures and Solutions
 Mixtures and Solutions Section 14.1 Heterogeneous and Homogeneous Mixtures In your textbook, read about suspensions and colloids. For each statement below, write true or false. 1. A solution is a mixture
Mixtures and Solutions Section 14.1 Heterogeneous and Homogeneous Mixtures In your textbook, read about suspensions and colloids. For each statement below, write true or false. 1. A solution is a mixture
Chapter 9 Lesson 1: Substances and Mixtures
 Chapter 9 Lesson 1: Substances and Mixtures Vocabulary -Substance -Heterogeneous mixture -Mixture -Homogeneous mixture -Solution Matter: Substances and Mixtures How do compounds and mixtures differ? Because
Chapter 9 Lesson 1: Substances and Mixtures Vocabulary -Substance -Heterogeneous mixture -Mixture -Homogeneous mixture -Solution Matter: Substances and Mixtures How do compounds and mixtures differ? Because
- Applications: In chemistry, this effect is often used to determine the molecular weight of an unknown molecule.
 73 FREEZING POINT DEPRESSION concentration of solute (molality) Freezing point depression constant (for SOLVENT) Freezing point depression: The amount the freezing temperature is LOWERED by the solute.
73 FREEZING POINT DEPRESSION concentration of solute (molality) Freezing point depression constant (for SOLVENT) Freezing point depression: The amount the freezing temperature is LOWERED by the solute.
Worksheet for Exploration 21.1: Engine Efficiency W Q H U
 Worksheet for Exploration 21.1: Engine Efficiency In this animation, N = nr (i.e., k B = 1). This, then, gives the ideal gas law as PV = NT. Assume an ideal monatomic gas. The efficiency of an engine is
Worksheet for Exploration 21.1: Engine Efficiency In this animation, N = nr (i.e., k B = 1). This, then, gives the ideal gas law as PV = NT. Assume an ideal monatomic gas. The efficiency of an engine is
AP Review Unit 2. Solutions and Solution Equilibrium. Copyright John Sayles 1
 AP Review Unit 2 Solutions and Solution Equilibrium Copyright 2004 - John Sayles 1 The Solution Process Break solute-solute bonds IMF s if covalent Ionic bonds if ionic Break solvent-solvent bonds Likely
AP Review Unit 2 Solutions and Solution Equilibrium Copyright 2004 - John Sayles 1 The Solution Process Break solute-solute bonds IMF s if covalent Ionic bonds if ionic Break solvent-solvent bonds Likely
Chapter 13. Properties of Solutions
 Chapter 13 Properties of Solutions Warm - Up Why doesn t salt dissolve in nonpolar solvents such as hexane? How does the orientation of water around Na + differ from the orientation of water around Cl
Chapter 13 Properties of Solutions Warm - Up Why doesn t salt dissolve in nonpolar solvents such as hexane? How does the orientation of water around Na + differ from the orientation of water around Cl
CHEM1101 Example Multiple Choice Questions
 HM0 xample Multiple hoice Questions The following multiple choice questions are provided to illustrate the type of questions used in this section of the paper and to provide you with extra practice. It
HM0 xample Multiple hoice Questions The following multiple choice questions are provided to illustrate the type of questions used in this section of the paper and to provide you with extra practice. It
Solution Formation. Copyright Houghton Mifflin Company.All rights reserved. Presentation of Lecture Outlines, 12 2
 Solutions Solution Formation A solution is a homogeneous mixture of two or more substances, consisting of ions or molecules. (See Animation: Solution Equilibrium). A colloid, although it also appears to
Solutions Solution Formation A solution is a homogeneous mixture of two or more substances, consisting of ions or molecules. (See Animation: Solution Equilibrium). A colloid, although it also appears to
Virtual Solution Lab::
 Name A Solution is a type of homogeneous mixture formed when one substance dissolves in another. The particles of the mixing substances are evenly spread throughout. The substance that is dissolved is
Name A Solution is a type of homogeneous mixture formed when one substance dissolves in another. The particles of the mixing substances are evenly spread throughout. The substance that is dissolved is
a) Write the reaction that occurs (pay attention to and label ends correctly) 5 AGCTG CAGCT > 5 AGCTG 3 3 TCGAC 5
 Chem 315 Applications Practice Problem Set 1.As you learned in Chem 315, DNA higher order structure formation is a two step process. The first step, nucleation, is entropically the least favorable. The
Chem 315 Applications Practice Problem Set 1.As you learned in Chem 315, DNA higher order structure formation is a two step process. The first step, nucleation, is entropically the least favorable. The
Topic 6 Gases and Colligative Properties
 Topic 6 Gases and Colligative Properties Boyle noticed an inverse relationship between volume and pressure. Pressure x volume = constant PV = a V V P 1/P Charles found the volume of a gas, at constant
Topic 6 Gases and Colligative Properties Boyle noticed an inverse relationship between volume and pressure. Pressure x volume = constant PV = a V V P 1/P Charles found the volume of a gas, at constant
