Recipe for p bonds in polyatomic molecules Notes on General Chemistry
|
|
|
- Ralf Booth
- 5 years ago
- Views:
Transcription
1 Recipe for p bonds in polyatomic molecules Notes on General Chemistry Last updated Thursday, December 20, :08:18-05:00 Copyright 2007 Dan Dill (dan@bu.edu) Department of Chemistry, Boston University, Boston MA Bonding in polyatomic molecules (molecules with more than two atoms) is treated in two parts. The first part accounts for the connectivity of the atoms, that is the bonds between the atoms. This is treated as a framework of (localized) s bonds built from overlapping hybridized AO's. The second part accounts for multiple bonds. This is treated as a framework of p MO's built from overlapping unhybridized AO's. Here are illustrations of these two aspects, for formaldehyde, H 2 C=O. Framework of s bonds (left panel) and p bonds (right panel) in formaldehyde. The p antibonding orbital is not shown. The hybridization on the O is determine by the C. Adapted from Clayden et al., Organic Chemistry (Oxford University Press, 2000), pages 108 (bottom figure) and 136 (top figure). Copyright Oxford University Press, We have learned how to connect atoms with a s framework built from sp, sp 2, sp 3 hybrid orbitals. Here we describe how to treat multiple bonds, using a recipe for building p frameworks. In the following, the distinction is made between a central atom and a terminal atom. A central atom has two or more atoms attached to it. A terminal atom is an atom (other than H) that is connected to a central atom. Here are two examples of this distinction. In formaldehyde, H 2 C=O, the C the only central atom, and the double bonded O is the only terminal atom. In formic acid, HC(=O)OH, the C and single bonded O are central atoms, and the double bonded O is a terminal atom. à Recipe overview The recipe is based on the Lewis structure of the molecule. If there are equivalent resonance Lewis structures, just one of these is needed.
2 2 Recipe for p bonds in polyatomic molecules Step 1: Determine the number of p atomic orbitals (AO's) not used in the s framework. This is the number of p AO's that are available to form the same number of p molecular orbitals (MO's). Step 2: Sketch the corresponding number of p MO's, and determine their bond character (bonding, nonbonding, or antibonding) and their relative energies. Step 3: Determine the number of electrons that there will be in the p MO's. Step 4: Assign all of the p electrons using aufbau and Hund rules, and calculate the p bond order. à Recipe details Here is a description of how to carry out each step, using the examples of CO 2 and formate, HCOO -, the conjugate base of formic acid, HCOOH. Format (left) and CO 2 (right) Lewis structures. Step 1: Number of unhybridized p AO's available to form p MO's The key to this step is the following. The hybridization on each terminal atom is determined by the central atom to which it is attached. In formate, the C is sp 2 hybridized and in CO 2 the C is sp hybridized. The result is that then there will be one or more unhybridized AO's on each terminal atom that are perpendicular to the s framework. The double bonded O's in formate and CO 2 illustrate that terminal atoms that look identical in Lewis structures do not have the same hybridization and do not form identical orbital interactions. The s framework consists of the s bonds and nonbonded hybrid AO's on terminal atoms. Here are the s frameworks of CO 2 and HCOO -. Format (left) and CO 2 (right) s frameworks. Since the unhybridized p AO's on adjacent central atoms are perpendicular to the s framework, all of these p AO's are available to form p MO's. In CO 2, since C is sp, both O's are sp. This means that there are two unhybridized, mutually perpendicular p AO's on each of the three atoms, for a total of six p AO's, which form six p MO's. In HCOO -, since C is sp 2, both O's are sp 2. This means that there is one unhybridized p AO on each of the three atoms, for a total of three p AO's, which form three p MO's. Unhybridized p AO's on a terminal atom are closer to the other AO's on the same atom than they would be if all p AO's were hybridized. This means that there is an energy cost due to increased
3 Recipe for p bonds in polyatomic molecules 3 electron cloud repulsion on the terminal atom. This energy cost is overcome by the lowering of energy made possible by the formation of p MO's. There are two sources of the energy lowering due to p MO formation. First, bonding p MO's result in increased electrical attraction by shifting electron clouds toward pairs of nuclei. Second, the electron clouds of bonding p MO's will have larger wavelength than the individual p AO's, and so smaller kinetic energy. That is, the reason some lone pairs on terminal atoms are in unhybridized p AO's is the energy cost is paid for by the energy return made possible by p MO formation. Step 2: Sketch the p MO's, and determine their bond character and relative energies The general procedure is to use the number of loops that there must be in the MO electron wave to guide the relative phasing of the p AO's, and to use electronegativities to determine relative amounts of AO's. The lowest-energy MO must have one loop, the second-lowest must have two loops, etc. The one-loop (lowest-energy) MO will have greatest contribution from AO's on most electronegative atoms, and The maximum-loop (highest-energy) MO will have greatest contribution from AO's on least electronegative atoms. The lowest-energy (one-loop) MO has all of the p AO's in phase. Degenerate pair of lowest energy (one-loop) p MO's in CO 2. Formate has just a single one-loop p MO. The O AO's predominate, since O is more electronegative than C and these are bonding MO's (denoted by their blue color). Since O is more electronegative than C, the O AO's predominate. For CO 2, there are two degenerate (equal-energy), perpendicular one-loop MO's. This is because the unhybridized p AO's consist of two perpendicular sets of three AO's each. AO's from different sets have zero net overlap (that is incorrect relative symmetry) and so do not interact with each other. These are bonding MO's. The two degenerate second lowest-energy (two-loop) MO's consist of a group of adjacent p AO's with one relative phase, and a group of adjacent AO's with the other relative phase. Degenerate pair of second lowest energy (two-loop) p MO's in CO 2. Formate has just a single two-loop p MO. There is no C contribution to these MO's. Because the AO's overlap hardly at all, these are nonbonding MO's (denoted by their black color). The reason there is no C AO in these MO's is that such an MO electron cloud would be lopsided, which is inconsistent with the equal C O bond lengths in these molecules. Because the O AO's are not adjacent, they overlap hardly at all and so these are nonbonding MO's. The third lowest-energy (three-loop) MO's consist of AO's alternating in relative phase. Degenerate pair of third lowest energy (three-loop) p MO's in CO 2. Formate has just a single three-loop p MO. The C AO's predominate, since O is more electronegative than C and these are antibonding MO's (denoted by their red color). These are antibonding MO's. Since O is more electronegative than C, the C AO's predominate. Step 3: Number of electrons in the p MO's: visual method A visual way to determine the number of p electrons is to compare the full Lewis structure with just its s framework part. For CO 2 the comparison is
4 4 Recipe for p bonds in polyatomic molecules CO 2 Lewis structure (left) and s framework (right). One lone pair (shown in red) on each O and one pair from each double bond are the eight electrons in the p MO's There are two lone pairs (shown in red) on the O's and two bond pairs that are not in the s framework. These are the eight electrons that will be in the CO 2 p MO's. The visual comparison for formate is Format Lewis structure (left) and s framework (right). One lone pair (shown in red) and one pair from the double bond are the four electrons in the p MO's There is one lone pair (shown in red) on the single bonded O and one bond pair that are not in the s framework. These are the four electrons that will be in the formate p MO's. Step 3: Number of electrons in the p MO's: calculational method A calculational method to determine the number of p electrons begins by writing that these electrons come from two places: number of p electrons = pelectrons from central atom multiple bonds + p electrons from terminal atom lone pairs The number of p electrons from each central atom multiple bond is bond electrons - 2, that is, the number of electrons in the s part of the multiple bond. To compute the p electrons from terminal (non-h) atom lone pairs, subtract from the terminal atom lone pairs the number of pairs in the s framework. The number of lone pairs in the s framework is steric number - 1, since the terminal atom has one connection and so the remainder of the steric number (SN) must be made up of lone pairs. This means that the lone pairs left over, and so available as p electrons, is lone pairs - HSN - 1L. Putting everything together, we get number of p electrons = sum of central atom Hshared elecrons - 2L + sum of terminal atom Hlone pairs - HSN - 1LL μ 2 For CO 2, there are two double bonds, the steric number is SN = 2 (since each atom is sp), and there are two terminal atoms (ignoring the H), each with two lone pairs. Hence number of p electrons = H4-2L + H4-2L + H2 - H2-1LL μ 2 + H2 - H2-1LL μ 2 = = 8 For HCOO -, there is one double bond, the steric number is SN = 3 (since each atom is sp 2 ), and there are two terminal atoms, one with two lone pairs and one with three lone pairs. Hence number of p electrons = H2 - H3-1LL μ 2 + H3 - H3-1LL μ 2 = = 4
5 Recipe for p bonds in polyatomic molecules 5 Another method is to subtract all of the electrons in the s framework from the total number of electrons. See if you can express this as a formula. Step 4: Assign p electrons to MO's and calculate p bond order In CO 2 we need to assign eight electrons to the p MO's. Four electrons are paired in the two one-loop MO's, contributing 2 to the p bond order. The remaining four electrons are paired in the two twoloop MO's; since these MO's are nonbonding, the contribution to the p bond order is 0. The net result is a p bond order = = 2, delocalized over C and the two O's. CO 2 p MO's. The degenerate pair of bonding orbitals (in blue) corresponds to one loop in the p MO electron wave. The degenerate pair of nonbonding orbitals (in black) corresponds to two loops in the p MO electron wave. The degenerate pair of antibonding orbitals (in red) corresponds to three loops in the p MO electron wave. In HCOO - we need to assign four electrons to the p MO's. Two electrons are paired in the one-loop MO, contributing 1 to the p bond order. The remaining two electrons are paired in the two-loop MO; since this MO is nonbonding, the contribution to the p bond order is 0. The net result is p bond order = = 1, delocalized over C and the two O's. Formate p MO's. The bonding orbital (in blue) corresponds to one loop in the p MO electron wave. The nonbonding orbital (in black) corresponds to two loops in the p MO electron wave. The antibonding orbital (in red) corresponds to three loops in the p MO electron wave. à Practice examples For each of the following molecules, è give the number of unhybridized p AO's, è sketch that number of MO's, è give the number of electrons in the p MO's, and è compute the bond order. SO 2 H 2 CO
Hybrid AO's and polyatomic MO's. CH101 Fall 2012 Boston University
 CH101 Fall 2012 Boston University Figures on slides 4 9 are used with permission from Clayden et al., Organic Chemistry (Oxford University Press, 2000), 2007 Oxford University Press. Figures on slides
CH101 Fall 2012 Boston University Figures on slides 4 9 are used with permission from Clayden et al., Organic Chemistry (Oxford University Press, 2000), 2007 Oxford University Press. Figures on slides
SOE: Symmetry Overlap Energy Notes on General Chemistry
 SOE: Symmetry Overlap Energy Notes on General Chemistry http://quantum.bu.edu/notes/generalchemistry/soesymmetryoverlapenergy.pdf Last updated Thursday, December 13, 2007 15:31:32-05:00 Copyright 2007
SOE: Symmetry Overlap Energy Notes on General Chemistry http://quantum.bu.edu/notes/generalchemistry/soesymmetryoverlapenergy.pdf Last updated Thursday, December 13, 2007 15:31:32-05:00 Copyright 2007
MOLECULAR ORBITAL THEORY Chapter 10.8, Morrison and Boyd
 MOLECULAR ORBITAL THEORY Chapter 10.8, Morrison and Boyd more understanding: why oxygen is paramagnetic, why H2 + exists; explanation of excited electronic states (e.g., visible spectra) eliminates need
MOLECULAR ORBITAL THEORY Chapter 10.8, Morrison and Boyd more understanding: why oxygen is paramagnetic, why H2 + exists; explanation of excited electronic states (e.g., visible spectra) eliminates need
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
2. Constructive and destructive interference (in phase and out-of-phase interaction) a. Sigma bond is achieved by head on overlap
 Discussion #1 Chapter 10 CH102 2018 MOs TF s name: Your name: Discussion Section: 1. Atomic Orbital (s, p, d, f) vs. Molecular Orbital (σ, σ *, NB, π, π *, π nb ) a. Total Number of MO =Total Number of
Discussion #1 Chapter 10 CH102 2018 MOs TF s name: Your name: Discussion Section: 1. Atomic Orbital (s, p, d, f) vs. Molecular Orbital (σ, σ *, NB, π, π *, π nb ) a. Total Number of MO =Total Number of
Chapter 9 Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity (which atoms are physically connected). By noting the number of bonding and nonbonding electron
Chapter 9 Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity (which atoms are physically connected). By noting the number of bonding and nonbonding electron
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model.
 Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Chemistry: The Central Science. Chapter 9: Molecular Geometry and Bonding Theory
 Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Andrew Rosen *Note: If you can rotate a molecule to have one isomer equal to another, they are both the same
 *Note: If you can rotate a molecule to have one isomer equal to another, they are both the same *Note: For hybridization, if an SP 2 is made, there is one unhybridized p orbital (because p usually has
*Note: If you can rotate a molecule to have one isomer equal to another, they are both the same *Note: For hybridization, if an SP 2 is made, there is one unhybridized p orbital (because p usually has
Lecture 16 C1403 October 31, Molecular orbital theory: molecular orbitals and diatomic molecules
 Lecture 16 C1403 October 31, 2005 18.1 Molecular orbital theory: molecular orbitals and diatomic molecules 18.2 Valence bond theory: hybridized orbitals and polyatomic molecules. From steric number to
Lecture 16 C1403 October 31, 2005 18.1 Molecular orbital theory: molecular orbitals and diatomic molecules 18.2 Valence bond theory: hybridized orbitals and polyatomic molecules. From steric number to
Molecular shape is determined by the number of bonds that form around individual atoms.
 Chapter 9 CH 180 Major Concepts: Molecular shape is determined by the number of bonds that form around individual atoms. Sublevels (s, p, d, & f) of separate atoms may overlap and result in hybrid orbitals
Chapter 9 CH 180 Major Concepts: Molecular shape is determined by the number of bonds that form around individual atoms. Sublevels (s, p, d, & f) of separate atoms may overlap and result in hybrid orbitals
Molecular Geometry and Bonding Theories. Chapter 9
 Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories MOLECULAR SHAPES 2 Molecular Shapes Lewis Structures show bonding and lone pairs do not denote shape Use Lewis Structures to determine shapes Molecular
Chapter 9 Molecular Geometry and Bonding Theories MOLECULAR SHAPES 2 Molecular Shapes Lewis Structures show bonding and lone pairs do not denote shape Use Lewis Structures to determine shapes Molecular
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Constructing a MO of NH 3. Nitrogen AO symmetries are
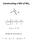 Constructing a MO of NH 3 Nitrogen AO symmetries are To develop a MO scheme for NH 3 assume that only the 2s and2p orbitals of nitrogen interact with the hydrogen 1s orbitals (i.e., the nitrogen 1s orbital
Constructing a MO of NH 3 Nitrogen AO symmetries are To develop a MO scheme for NH 3 assume that only the 2s and2p orbitals of nitrogen interact with the hydrogen 1s orbitals (i.e., the nitrogen 1s orbital
Chapter 9. Molecular Geometries and Bonding Theories. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
General Chemistry I (2012) Lecture by B. H. Hong
 3.8 The Limitations of Lewis's Theory 3.9 Molecular Orbitals The valence-bond (VB) and molecular orbital (MO) theories are both procedures for constructing approximate wavefunctions of electrons. The MO
3.8 The Limitations of Lewis's Theory 3.9 Molecular Orbitals The valence-bond (VB) and molecular orbital (MO) theories are both procedures for constructing approximate wavefunctions of electrons. The MO
Chapter 9. Molecular Geometries and Bonding Theories. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Chapter 9 Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories molecular shapes the VSEPR model molecular shape and molecular polarity covalent bonding and orbital overlap hybrid orbitals multiple bonds 9.1 Molecular
Chapter 9 Molecular Geometry and Bonding Theories molecular shapes the VSEPR model molecular shape and molecular polarity covalent bonding and orbital overlap hybrid orbitals multiple bonds 9.1 Molecular
Structure and Bonding of Organic Molecules
 Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Chapter 9. Covalent Bonding: Orbitals. Copyright 2017 Cengage Learning. All Rights Reserved.
 Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents (9.1) (9.2) (9.3) (9.4) (9.5) (9.6) Hybridization and the localized electron model The molecular orbital model Bonding in homonuclear diatomic
Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents (9.1) (9.2) (9.3) (9.4) (9.5) (9.6) Hybridization and the localized electron model The molecular orbital model Bonding in homonuclear diatomic
1s atomic orbital 2s atomic orbital 2s atomic orbital (with node) 2px orbital 2py orbital 2pz orbital
 Atomic Orbitals 1s atomic orbital 2s atomic orbital 2s atomic orbital (with node) 2px orbital 2py orbital 2pz orbital Valence Bond Theory and ybridized Atomic Orbitals Bonding in 2 1s 1s Atomic Orbital
Atomic Orbitals 1s atomic orbital 2s atomic orbital 2s atomic orbital (with node) 2px orbital 2py orbital 2pz orbital Valence Bond Theory and ybridized Atomic Orbitals Bonding in 2 1s 1s Atomic Orbital
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. Dr. V.M. Williamson Texas A & M University Student Version
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. VSEPR: Electronic Geometries VSEPR
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Chapter 3. Orbitals and Bonding
 Chapter 3. Orbitals and Bonding What to master Assigning Electrons to Atomic Orbitals Constructing Bonding and Antibonding Molecular Orbitals with Simple MO Theory Understanding Sigma and Pi Bonds Identifying
Chapter 3. Orbitals and Bonding What to master Assigning Electrons to Atomic Orbitals Constructing Bonding and Antibonding Molecular Orbitals with Simple MO Theory Understanding Sigma and Pi Bonds Identifying
Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories
 C h e m i s t r y 1 A : C h a p t e r 1 0 P a g e 1 Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories Homework: Read Chapter 10: Work out sample/practice
C h e m i s t r y 1 A : C h a p t e r 1 0 P a g e 1 Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories Homework: Read Chapter 10: Work out sample/practice
Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
Chemistry 2000 Lecture 2: LCAO-MO theory for homonuclear diatomic molecules
 Chemistry 2000 Lecture 2: LCAO-MO theory for homonuclear diatomic molecules Marc R. Roussel January 5, 2018 Marc R. Roussel Homonuclear diatomics January 5, 2018 1 / 17 MO theory for homonuclear diatomic
Chemistry 2000 Lecture 2: LCAO-MO theory for homonuclear diatomic molecules Marc R. Roussel January 5, 2018 Marc R. Roussel Homonuclear diatomics January 5, 2018 1 / 17 MO theory for homonuclear diatomic
BONDING THEORIES Chapter , Carey
 BONDING THEORIES Chapter 10.6-10.7, Carey The Covalent Chemical Bond (9.2) FIG I Potential Energy Change to Form H2 What is a chemical bond? Why do chemical bonds occur? Descriptions of bonding: Valence
BONDING THEORIES Chapter 10.6-10.7, Carey The Covalent Chemical Bond (9.2) FIG I Potential Energy Change to Form H2 What is a chemical bond? Why do chemical bonds occur? Descriptions of bonding: Valence
Symmetry and Molecular Orbitals (I)
 Symmetry and Molecular Orbitals (I) Simple Bonding Model http://chiuserv.ac.nctu.edu.tw/~htchiu/chemistry/fall-2005/chemical-bonds.htm Lewis Structures Octet Rule Resonance Formal Charge Oxidation Number
Symmetry and Molecular Orbitals (I) Simple Bonding Model http://chiuserv.ac.nctu.edu.tw/~htchiu/chemistry/fall-2005/chemical-bonds.htm Lewis Structures Octet Rule Resonance Formal Charge Oxidation Number
Chapter 9: Molecular Geometry and Bonding Theories
 Chapter 9: Molecular Geometry and Bonding Theories 9.1 Molecular Geometries -Bond angles: angles made by the lines joining the nuclei of the atoms in a molecule -Bond angles determine overall shape of
Chapter 9: Molecular Geometry and Bonding Theories 9.1 Molecular Geometries -Bond angles: angles made by the lines joining the nuclei of the atoms in a molecule -Bond angles determine overall shape of
Chapter 10 Theories of Covalent Bonding
 Chapter 10 Theories of Covalent Bonding 1 Atomic Orbitals Molecules Bonding and 2 Molecular Structure Questions How are molecules held together? Why is O 2 paramagnetic? And how is this property connected
Chapter 10 Theories of Covalent Bonding 1 Atomic Orbitals Molecules Bonding and 2 Molecular Structure Questions How are molecules held together? Why is O 2 paramagnetic? And how is this property connected
Chapter 9 Molecular Geometry and Bonding Theories
 Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Chapter 1 Carbon Compounds and Chemical Bonds
 Chapter 1 Carbon Compounds and Chemical Bonds Introduction Organic Chemistry The chemistry of the compounds of carbon The human body is largely composed of organic compounds Organic chemistry plays a central
Chapter 1 Carbon Compounds and Chemical Bonds Introduction Organic Chemistry The chemistry of the compounds of carbon The human body is largely composed of organic compounds Organic chemistry plays a central
17/11/2010. Lewis structures
 Reading assignment: 8.5-8.8 As you read ask yourself: How can I use Lewis structures to account for bonding in covalent molecules? What are the differences between single, double and triple bonds in terms
Reading assignment: 8.5-8.8 As you read ask yourself: How can I use Lewis structures to account for bonding in covalent molecules? What are the differences between single, double and triple bonds in terms
Molecular Orbital Theory This means that the coefficients in the MO will not be the same!
 Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of atomic orbitals to each MO is not equal. Some MO s will have more contribution from AO s on
Diatomic molecules: Heteronuclear molecules In heteronuclear diatomic molecules, the relative contribution of atomic orbitals to each MO is not equal. Some MO s will have more contribution from AO s on
Molecular shape is only discussed when there are three or more atoms connected (diatomic shape is obvious).
 Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is
Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is
CHAPTER 5: Bonding Theories - Explaining Molecular Geometry. Chapter Outline
 CHAPTER 5: Bonding Theories - Explaining Molecular Geometry Chapter Outline 5.1 Molecular Shape 5.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) 5.3 Polar Bonds and Polar Molecules» What Makes
CHAPTER 5: Bonding Theories - Explaining Molecular Geometry Chapter Outline 5.1 Molecular Shape 5.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) 5.3 Polar Bonds and Polar Molecules» What Makes
Chapter 4 Symmetry and Chemical Bonding
 Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4. Molecular Structure and Orbitals
 Chapter 4 Molecular Structure and Orbitals Chapter 4 Table of Contents (4.1) (4.2) (4.3) (4.4) (4.5) (4.6) (4.7) Molecular structure: The VSEPR model Bond polarity and dipole moments Hybridization and
Chapter 4 Molecular Structure and Orbitals Chapter 4 Table of Contents (4.1) (4.2) (4.3) (4.4) (4.5) (4.6) (4.7) Molecular structure: The VSEPR model Bond polarity and dipole moments Hybridization and
Molecular Shape and Molecular Polarity. Molecular Shape and Molecular Polarity. Molecular Shape and Molecular Polarity
 Molecular Shape and Molecular Polarity When there is a difference in electronegativity between two atoms, then the bond between them is polar. It is possible for a molecule to contain polar bonds, but
Molecular Shape and Molecular Polarity When there is a difference in electronegativity between two atoms, then the bond between them is polar. It is possible for a molecule to contain polar bonds, but
Chapter 9. and Bonding Theories
 Chemistry, The Central Science, 11th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The
Chemistry, The Central Science, 11th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The
Chemistry 2000 Lecture 8: Valence bond theory
 Chemistry 000 Lecture 8: Valence bond theory Marc R. Roussel January 9, 08 Marc R. Roussel Valence bond theory January 9, 08 / 5 MO theory: a recap A molecular orbital is a one-electron wavefunction which,
Chemistry 000 Lecture 8: Valence bond theory Marc R. Roussel January 9, 08 Marc R. Roussel Valence bond theory January 9, 08 / 5 MO theory: a recap A molecular orbital is a one-electron wavefunction which,
Chapter 12: Chemical Bonding II: Additional Aspects
 General Chemistry Principles and Modern Applications Petrucci Harwood Herring 8 th Edition Chapter 12: Chemical Bonding II: Additional Aspects Philip Dutton University of Windsor, Canada N9B 3P4 Prentice-Hall
General Chemistry Principles and Modern Applications Petrucci Harwood Herring 8 th Edition Chapter 12: Chemical Bonding II: Additional Aspects Philip Dutton University of Windsor, Canada N9B 3P4 Prentice-Hall
Chapter 5. Molecular Orbitals
 Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Chapter 4 Symmetry and Chemical Bonding
 Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
General Chemistry. Contents. Chapter 12: Chemical Bonding II: Additional Aspects What a Bonding Theory Should Do. Potential Energy Diagram
 General Chemistry Principles and Modern Applications Petrucci Harwood Herring 8 th Edition Chapter 12: Chemical Bonding II: Additional Aspects Philip Dutton University of Windsor, Canada N9B 3P4 Contents
General Chemistry Principles and Modern Applications Petrucci Harwood Herring 8 th Edition Chapter 12: Chemical Bonding II: Additional Aspects Philip Dutton University of Windsor, Canada N9B 3P4 Contents
Wave functions and quantization workshop CH112 Workshop 9, Spring 2003
 Wave functions and quantization workshop CH112 Workshop 9, Spring 2003 http://quantum.bu.edu/notes/quantummechanics/wavefunctionsandquantizationworkshop.pdf Last updated Friday, October 31, 2003 16:33:01
Wave functions and quantization workshop CH112 Workshop 9, Spring 2003 http://quantum.bu.edu/notes/quantummechanics/wavefunctionsandquantizationworkshop.pdf Last updated Friday, October 31, 2003 16:33:01
Chapter 9. and Bonding Theories. Molecular Shapes. What Determines the Shape of a Molecule? 3/8/2013
 Chemistry, The Central Science, 10th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 9 Theories John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice-Hall,
Chemistry, The Central Science, 10th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten Chapter 9 Theories John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice-Hall,
Molecular Bond Theory
 Molecular Bond Theory Short comings of the localized electron model: electrons are not really localized so the concept of resonance was added no direct information about bond energies Molecular Orbital
Molecular Bond Theory Short comings of the localized electron model: electrons are not really localized so the concept of resonance was added no direct information about bond energies Molecular Orbital
Chapter 10. Structure Determines Properties! Molecular Geometry. Chemical Bonding II
 Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
Hückel Molecular Orbital (HMO) Theory
 Hückel Molecular Orbital (HMO) Theory A simple quantum mechanical concept that gives important insight into the properties of large molecules Why HMO theory The first MO theory that could be applied to
Hückel Molecular Orbital (HMO) Theory A simple quantum mechanical concept that gives important insight into the properties of large molecules Why HMO theory The first MO theory that could be applied to
Atomic wavefunction family album Notes on General Chemistry
 Atomic wavefunction family album Notes on General Chemistry http://quantum.bu.edu/notes/generalchemistry/atomicwavefunctionfamilyalbum.pdf Last updated Monday, November 7, 2005 14:31:48-05:00 Copyright
Atomic wavefunction family album Notes on General Chemistry http://quantum.bu.edu/notes/generalchemistry/atomicwavefunctionfamilyalbum.pdf Last updated Monday, November 7, 2005 14:31:48-05:00 Copyright
1.14 the orbital view of bonding:
 1.14 the orbital view of bonding: The sigma bond Dr. Abdullah Saleh/236-3 1 A limitation of Lewis Theory of Bonding It does not explain the three dimensional geometries of molecules! Dr. Abdullah Saleh/236-3
1.14 the orbital view of bonding: The sigma bond Dr. Abdullah Saleh/236-3 1 A limitation of Lewis Theory of Bonding It does not explain the three dimensional geometries of molecules! Dr. Abdullah Saleh/236-3
Chapter 10: Chemical Bonding II. Bonding Theories
 Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then
 1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
Ch. 9- Molecular Geometry and Bonding Theories
 Ch. 9- Molecular Geometry and Bonding Theories 9.0 Introduction A. Lewis structures do not show one of the most important aspects of molecules- their overall shapes B. The shape and size of molecules-
Ch. 9- Molecular Geometry and Bonding Theories 9.0 Introduction A. Lewis structures do not show one of the most important aspects of molecules- their overall shapes B. The shape and size of molecules-
General Physical Chemistry II
 General Physical Chemistry II Lecture 13 Aleksey Kocherzhenko October 16, 2014" Last time " The Hückel method" Ø Used to study π systems of conjugated molecules" Ø π orbitals are treated separately from
General Physical Chemistry II Lecture 13 Aleksey Kocherzhenko October 16, 2014" Last time " The Hückel method" Ø Used to study π systems of conjugated molecules" Ø π orbitals are treated separately from
Copyright 2015 Dan Dill 1
 1. The kinetic energy of the antibonding of a pair of AO's is... Questions on Symmetry, Overlap, Energy CH1 Fall 2015 Boston University 1. greater than that for 2. about the same that for 3. less than
1. The kinetic energy of the antibonding of a pair of AO's is... Questions on Symmetry, Overlap, Energy CH1 Fall 2015 Boston University 1. greater than that for 2. about the same that for 3. less than
Valence Bond Theory. Localized Electron Model. Hybridize the Orbitals! Overlap and Bonding. Atomic Orbitals are. mmmkay. Overlap and Bonding
 Valence Bond Theory Atomic Orbitals are bad mmmkay Overlap and Bonding Lewis taught us to think of covalent bonds forming through the sharing of electrons by adjacent atoms. In such an approach this can
Valence Bond Theory Atomic Orbitals are bad mmmkay Overlap and Bonding Lewis taught us to think of covalent bonds forming through the sharing of electrons by adjacent atoms. In such an approach this can
Experiment 15: Atomic Orbitals, Bond Length, and Molecular Orbitals
 Experiment 15: Atomic Orbitals, Bond Length, and Molecular Orbitals Introduction Molecular orbitals result from the mixing of atomic orbitals that overlap during the bonding process allowing the delocalization
Experiment 15: Atomic Orbitals, Bond Length, and Molecular Orbitals Introduction Molecular orbitals result from the mixing of atomic orbitals that overlap during the bonding process allowing the delocalization
Chapter 9. Molecular Geometry and Bonding Theories
 9.1 Molecular Shapes Read Sec. 9.1 and 9.2, then complete the Sample and Practice Exercises in these sections. Sample Exercise 9.1 (p. 347) Use the VSEPR model to predict the molecular geometries of a)
9.1 Molecular Shapes Read Sec. 9.1 and 9.2, then complete the Sample and Practice Exercises in these sections. Sample Exercise 9.1 (p. 347) Use the VSEPR model to predict the molecular geometries of a)
Lecture 16 C1403 October 31, Molecular orbital theory: molecular orbitals and diatomic molecules
 Lecture 16 C1403 October 31, 2005 18.1 Molecular orbital theory: molecular orbitals and diatomic molecules 18.2 Valence bond theory: hybridized orbitals and polyatomic molecules Bond order, bond lengths,
Lecture 16 C1403 October 31, 2005 18.1 Molecular orbital theory: molecular orbitals and diatomic molecules 18.2 Valence bond theory: hybridized orbitals and polyatomic molecules Bond order, bond lengths,
Molecular Orbital Theory
 Molecular Orbital Theory Paramagnetic properties of O 2 pranjoto utomo Covalent Bonding Theory Valence Bond Theory useful for deriving shapes/polarity simple but inaccurate/deficient Molecular Orbital
Molecular Orbital Theory Paramagnetic properties of O 2 pranjoto utomo Covalent Bonding Theory Valence Bond Theory useful for deriving shapes/polarity simple but inaccurate/deficient Molecular Orbital
Topic 2. Structure and Bonding Models of Covalent Compounds of p-block Elements
 Topic 2 2-1 Structure and Bonding Models of Covalent Compounds of p-block Elements Bonding 2-2 Many different approaches to describe bonding: Ionic Bonding: Elements with large electronegativity differences;
Topic 2 2-1 Structure and Bonding Models of Covalent Compounds of p-block Elements Bonding 2-2 Many different approaches to describe bonding: Ionic Bonding: Elements with large electronegativity differences;
Carbon Compounds. Chemical Bonding Part 1b
 Carbon Compounds Chemical Bonding Part 1b Board Notes Introduction to VSEPR Organic Formulas Various Representations " dimethyl ether C 2 H 6 O " propyl alcohol C 3 H 8 O 3D representations " Wedges and
Carbon Compounds Chemical Bonding Part 1b Board Notes Introduction to VSEPR Organic Formulas Various Representations " dimethyl ether C 2 H 6 O " propyl alcohol C 3 H 8 O 3D representations " Wedges and
Carbon Compounds and Chemical Bonds
 Carbon Compounds and Chemical Bonds Introduction Organic Chemistry The chemistry of the compounds of carbon The human body is largely composed of organic compounds Organic chemistry plays a central role
Carbon Compounds and Chemical Bonds Introduction Organic Chemistry The chemistry of the compounds of carbon The human body is largely composed of organic compounds Organic chemistry plays a central role
Molecular Orbitals. Chapter 9. Sigma bonding orbitals. Sigma bonding orbitals. Pi bonding orbitals. Sigma and pi bonds
 Molecular Orbitals Chapter 9 Orbitals and Covalent Bond The overlap of atomic orbitals from separate atoms makes molecular orbitals Each molecular orbital has room for two electrons Two types of MO Sigma
Molecular Orbitals Chapter 9 Orbitals and Covalent Bond The overlap of atomic orbitals from separate atoms makes molecular orbitals Each molecular orbital has room for two electrons Two types of MO Sigma
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
Chapter 9. Covalent Bonding: Orbitals
 Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents 9.1 Hybridization and the Localized Electron Model 9.2 The Molecular Orbital Model 9.3 Bonding in Homonuclear Diatomic Molecules 9.4 Bonding
Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents 9.1 Hybridization and the Localized Electron Model 9.2 The Molecular Orbital Model 9.3 Bonding in Homonuclear Diatomic Molecules 9.4 Bonding
Lecture Presentation. Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Electronic Bonding of Atoms Joel M Williams 2012
 Electronic Bonding of Atoms Joel M Williams 2012 Bonded atoms make up our physical world. The principal parts that provide this composition are positive nuclei and negative electrons. While these may be
Electronic Bonding of Atoms Joel M Williams 2012 Bonded atoms make up our physical world. The principal parts that provide this composition are positive nuclei and negative electrons. While these may be
Wave Equations of Polyatomic Molecules
 Wave Equations of Polyatomic Molecules! Approximate wave functions are sought by combining atomic wave functions for the bonded atoms.! Several different approaches have been taken to constructing trial
Wave Equations of Polyatomic Molecules! Approximate wave functions are sought by combining atomic wave functions for the bonded atoms.! Several different approaches have been taken to constructing trial
Lecture 14 Chemistry 362 M. Darensbourg 2017 Spring term. Molecular orbitals for diatomics
 Lecture 14 Chemistry 362 M. Darensbourg 2017 Spring term Molecular orbitals for diatomics Molecular Orbital Theory of the Chemical Bond Simplest example - H 2 : two H atoms H A and H B Only two a.o.'s
Lecture 14 Chemistry 362 M. Darensbourg 2017 Spring term Molecular orbitals for diatomics Molecular Orbital Theory of the Chemical Bond Simplest example - H 2 : two H atoms H A and H B Only two a.o.'s
Bonding and Physical Properties The Molecular Orbital Theory
 Bonding and Physical Properties The Molecular Orbital Theory Ø Developed by F. Hund and R. S. Mulliken in 1932 Ø Diagram of molecular energy levels Ø Magnetic and spectral properties Paramagnetic vs. Diamagnetic
Bonding and Physical Properties The Molecular Orbital Theory Ø Developed by F. Hund and R. S. Mulliken in 1932 Ø Diagram of molecular energy levels Ø Magnetic and spectral properties Paramagnetic vs. Diamagnetic
Review Outline Chemistry 1B, Fall 2012
 Review Outline Chemistry 1B, Fall 2012 -------------------------------------- Chapter 12 -------------------------------------- I. Experiments and findings related to origin of quantum mechanics A. Planck:
Review Outline Chemistry 1B, Fall 2012 -------------------------------------- Chapter 12 -------------------------------------- I. Experiments and findings related to origin of quantum mechanics A. Planck:
Chapter 9 Molecular Geometry Valence Bond and Molecular Orbital Theory
 Chapter 9 Molecular Geometry Valence Bond and Molecular Orbital Theory Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and how they are used to model covalent bonding.
Chapter 9 Molecular Geometry Valence Bond and Molecular Orbital Theory Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and how they are used to model covalent bonding.
Activity Hybrid Atomic Orbitals
 Activity 201 8 Hybrid Atomic Orbitals Directions: This Guided Learning Activity (GLA) discusses Hybrid Atomic Orbitals, which are the basis for Valence Bond Theory. Part A introduces σ- and π-bonds. Part
Activity 201 8 Hybrid Atomic Orbitals Directions: This Guided Learning Activity (GLA) discusses Hybrid Atomic Orbitals, which are the basis for Valence Bond Theory. Part A introduces σ- and π-bonds. Part
We can model covalent bonding in molecules in essentially two ways:
 CHEM 2060 Lecture 22: VB Theory L22-1 PART FIVE: The Covalent Bond We can model covalent bonding in molecules in essentially two ways: 1. Localized Bonds (retains electron pair concept of Lewis Structures)
CHEM 2060 Lecture 22: VB Theory L22-1 PART FIVE: The Covalent Bond We can model covalent bonding in molecules in essentially two ways: 1. Localized Bonds (retains electron pair concept of Lewis Structures)
Carbon-based molecules are held together by covalent bonds between atoms
 hapter 1: hemical bonding and structure in organic compounds arbon-based molecules are held together by covalent bonds between atoms omposition: Mainly nonmetals; especially,, O, N, S, P and the halogens
hapter 1: hemical bonding and structure in organic compounds arbon-based molecules are held together by covalent bonds between atoms omposition: Mainly nonmetals; especially,, O, N, S, P and the halogens
Valence Bond Theory Considers the interaction of separate atoms brought together as they form a molecule. Lewis structures Resonance considerations
 CHEM 511 chapter 2 page 1 of 11 Chapter 2 Molecular Structure and Bonding Read the section on Lewis dot structures, we will not cover this in class. If you have problems, seek out a general chemistry text.
CHEM 511 chapter 2 page 1 of 11 Chapter 2 Molecular Structure and Bonding Read the section on Lewis dot structures, we will not cover this in class. If you have problems, seek out a general chemistry text.
7. Arrange the molecular orbitals in order of increasing energy and add the electrons.
 Molecular Orbital Theory I. Introduction. A. Ideas. 1. Start with nuclei at their equilibrium positions. 2. onstruct a set of orbitals that cover the complete nuclear framework, called molecular orbitals
Molecular Orbital Theory I. Introduction. A. Ideas. 1. Start with nuclei at their equilibrium positions. 2. onstruct a set of orbitals that cover the complete nuclear framework, called molecular orbitals
Exam 2 Review Problems
 09-107 onors Chemistry Carnegie Mellon University Exam 2 Review Problems Exam 2 will be held on Tuesday, ctober 30, in Porter all A18C. We will be there from 2:45 to 4:45 pm. Disclaimer: This review sheet
09-107 onors Chemistry Carnegie Mellon University Exam 2 Review Problems Exam 2 will be held on Tuesday, ctober 30, in Porter all A18C. We will be there from 2:45 to 4:45 pm. Disclaimer: This review sheet
Valence Bond Theory - Description
 Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
CHEMISTRY - ZUMDAHL 2E CH.4 - MOLECULAR STRUCTURE AND ORBITALS.
 !! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
!! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
Instructor Supplemental Solutions to Problems. Organic Chemistry 5th Edition
 Instructor Supplemental Solutions to Problems Marc Loudon Joseph G. Stowell to accompany Organic Chemistry 5th Edition This manual provides the solutions to the problems that are not provided in the Study
Instructor Supplemental Solutions to Problems Marc Loudon Joseph G. Stowell to accompany Organic Chemistry 5th Edition This manual provides the solutions to the problems that are not provided in the Study
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron
Chemistry 2000 Lecture 1: Introduction to the molecular orbital theory
 Chemistry 2000 Lecture 1: Introduction to the molecular orbital theory Marc R. Roussel January 5, 2018 Marc R. Roussel Introduction to molecular orbitals January 5, 2018 1 / 24 Review: quantum mechanics
Chemistry 2000 Lecture 1: Introduction to the molecular orbital theory Marc R. Roussel January 5, 2018 Marc R. Roussel Introduction to molecular orbitals January 5, 2018 1 / 24 Review: quantum mechanics
Experiment 21 Lewis structures and VSEPR Theory
 Experiment 21 Lewis structures and VSEPR Theory Introduction 1. Lewis Structures and Formal Charge LG.N. Lewis, at the University of California at Berkeley devised a simple way to understand the nature
Experiment 21 Lewis structures and VSEPR Theory Introduction 1. Lewis Structures and Formal Charge LG.N. Lewis, at the University of California at Berkeley devised a simple way to understand the nature
Chapter 18 Molecular orbitals and spectroscopy Conjugation of bonds and resonance structures
 Chapter 18 Molecular orbitals and spectroscopy 18.1 Diatomic molecules 18.2 Polyatomic molecules 18.3 Conjugation of bonds and resonance structures 18.4 The interaction of light and matter (spectroscopy)
Chapter 18 Molecular orbitals and spectroscopy 18.1 Diatomic molecules 18.2 Polyatomic molecules 18.3 Conjugation of bonds and resonance structures 18.4 The interaction of light and matter (spectroscopy)
CHAPTER 9 COVALENT BONDING: ORBITALS 323
 APTER 9 OVALET BODIG: ORBITALS 323 2 3 2 2 2 3 3 2 2 3 2 3 O * * 2 o; most of the carbons are not in the same plane since a majority of carbon atoms exhibit a tetrahedral structure (19.5 bond angles).
APTER 9 OVALET BODIG: ORBITALS 323 2 3 2 2 2 3 3 2 2 3 2 3 O * * 2 o; most of the carbons are not in the same plane since a majority of carbon atoms exhibit a tetrahedral structure (19.5 bond angles).
Polarity in Molecules!
 Chapter 9 part 2: Polarity in Molecules, Valence Bond Theory! Read:!!BLB 9.3 5! W:!!BLB 9.33, 35, 38!!!Packet 9:8-11! Know:! bond angles and geometry! polarity of molecules! Polarity in Molecules! " Just
Chapter 9 part 2: Polarity in Molecules, Valence Bond Theory! Read:!!BLB 9.3 5! W:!!BLB 9.33, 35, 38!!!Packet 9:8-11! Know:! bond angles and geometry! polarity of molecules! Polarity in Molecules! " Just
QUANTUM MECHANICS AND MOLECULAR STRUCTURE
 6 QUANTUM MECHANICS AND MOLECULAR STRUCTURE 6.1 Quantum Picture of the Chemical Bond 6.2 Exact Molecular Orbital for the Simplest Molecule: H + 2 6.3 Molecular Orbital Theory and the Linear Combination
6 QUANTUM MECHANICS AND MOLECULAR STRUCTURE 6.1 Quantum Picture of the Chemical Bond 6.2 Exact Molecular Orbital for the Simplest Molecule: H + 2 6.3 Molecular Orbital Theory and the Linear Combination
Chapter 9. Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory
 Chapter 9 Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Lewis Theory Lewis theory generally predicts trends in properties, but does not give good numerical predictions.
Chapter 9 Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Lewis Theory Lewis theory generally predicts trends in properties, but does not give good numerical predictions.
EXAM II Material. Part I Chemical Bonding I Lewis Theory Chapter 9 pages A. Drawing electron dot structures HOW TO:
 CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
Covalent Bonding: Orbitals
 Hybridization and the Localized Electron Model Covalent Bonding: Orbitals A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new orbitals of equal
Hybridization and the Localized Electron Model Covalent Bonding: Orbitals A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new orbitals of equal
Chapter 9. Chemical Bonding II: Molecular Geometry and Bonding Theories
 Chapter 9 Chemical Bonding II: Molecular Geometry and Bonding Theories Topics Molecular Geometry Molecular Geometry and Polarity Valence Bond Theory Hybridization of Atomic Orbitals Hybridization in Molecules
Chapter 9 Chemical Bonding II: Molecular Geometry and Bonding Theories Topics Molecular Geometry Molecular Geometry and Polarity Valence Bond Theory Hybridization of Atomic Orbitals Hybridization in Molecules
Organic Chemistry Lecture I. Dr. John D. Spence
 HEMISTRY 3 Organic hemistry Lecture I Dr. John D. Spence jdspence@scu.edu jspence@csus.eduedu http://www.csus.edu/indiv/s/spencej What is Organic hemistry? 780 s hemistry of compounds from living organisms
HEMISTRY 3 Organic hemistry Lecture I Dr. John D. Spence jdspence@scu.edu jspence@csus.eduedu http://www.csus.edu/indiv/s/spencej What is Organic hemistry? 780 s hemistry of compounds from living organisms
