Zinc Chloride-activated Waste Carbon Powder for Decolourization of Methylene Blue
|
|
|
- Flora Berry
- 5 years ago
- Views:
Transcription
1 Jurnal Tknologi Full papr Zinc Chlorid-acivad Was Carbon Powdr for Dcolourizaion of Mhyln Blu Muhammad Abbas Ahmad Zaini a*, Ting Chiw Ngiik a, Mohd. Johari Kamaruddin a, Sii Hamidah Mohd. Sapar a, Mohd. Azizi Ch Yunus a a Cnr of Lipids Enginring and Applid Rsarch (CLEAR), Univrsii Tknologi Malaysia, UTM Johor Bahru, Johor, Malaysia *Corrsponding auhor: abbas@chm.um.my Aricl hisory Rcivd :1 Ocobr 2013 Rcivd in rvisd form : 17 Novmbr 2013 Accpd :27 January 2014 Graphical absrac Absrac Was carbon powdr from pyrolysis of discardd yr was convrd ino acivad carbon for dcolourizaion of myln blu (MB). Th prcursor was acivad using ZnCl 2 (ZC), rcycld ZnCl 2 soluion from h arlir acivaion (RC), and irradiad war (MC). Acivad carbons wr characrizd according o surfac ara and morphology. Th valus of surfac ara wr rcordd as 288, 104 and 113 m 2 /g for ZC, RC and MC, rspcivly. Th dcolourizaion of MB was obsrvd o hav h following ordr: ZC>MC>RC. Th maximum adsorpion capaciy of MB by ZC was found o b 154 mg/g. Adsorpion daa for all acivad carbons sudid obyd Rdlich-Prson isohrm for which h procss could b dscribd as mixd faurs of Langmuir and Frundlich modls. Th kinics daa wr wllfid o psudo-scond-ordr modl, suggsing h chmisorpion procss. Kywords: Acivad carbon; irradiad war acivaion; mhyln blu; was carbon powdr; zinc chlorid acivaion 2014 Pnrbi UTM Prss. All righs rsrvd. 1.0 INTRODUCTION Th rlas of dys from xil indusris ino rciving war affcs h auaic craurs bcaus som dys ar highly oxic and carcinognic [1-3]. Dys block h passag of sunligh ino h sram, hus dsroy h lif cycl and food chain in war, and disrup h biodivrsiy wihin. Drioraing ualiy of war will also bring bad implicaions o public halh and daily lif. Mhyln blu (MB) is commonly usd in dying xil and mdicinal ramns [2-5]. I is no srongly hazardous bu can caus som harmful ffcs such as y injury, difficul brahing, nausa, vomiing, profus swaing, Hinz body formaion, diarrha, gasriis, mnal confusion, cyanosis, jaundic, issu ncrosis in humans and mhmoglobinmia [1-5]. Ths halh ffcs hav riggrd srong masur for MB rmoval as on of h major nvironmnal concrns. Mhods for dy rmoval includ adsorpion, chmical coagulaion, ion xchang, biological ramn and lcrolysis [2,3,6]. Of hs, adsorpion by acivad carbon is highly ffciv o rmov dy bcaus of h larg surfac ara and uniu xural propris of acivad carbon [7-8]. Howvr, acivad carbon prcursors lik coal and prolum pich ar no rnwabl, whil rgnraion of h spn acivad carbon is rlaivly xpnsiv. This scnario has rsuld in h sarchs for alrnaiv carbonacous prcursors ha ar abundanly availabl and low cos. On of h promising candidas undr his cagory is was carbon powdr, a pyrolysis by-produc of bulk was yr. Acivad carbon can b prpard hrough wo common rous, namly physical acivaion and chmical acivaion. Acivaing agns for physical acivaion ar CO2 and sam, whil chmical agns such as KOH, ZnCl2, H3PO4, K2CO3, c. ar commonly usd in chmical acivaion. Chmical acivaion is usually bing carrid ou a a lowr mpraur wihou carbonizaion, and usually rsuls in a highr yild compard o physical acivaion [9-12]. Th aim of his work was o invsiga h ponial of ZnCl2- acivad was carbon powdr for MB rmoval from auous soluion. Amps wr also mad o aciva h carbon by using rcovrd zinc chlorid soluion and irradiad war nvironmn. Th ffcs of iniial dy concnraion and agiaion im wr xamind and discussd. 2.0 EXPERIMENTAL 2.1 Marials All chmicals wr of analyical-ragn grad. Was carbon powdr was obaind from Buki Bau Brickmills (M) Sdn. Bhd. Mhyln blu (MB) was usd as h dy o b rad. Figur 1 67:2 (2014) ISSN
2 38 Muhammad Abbas Ahmad Zaini al. / Jurnal Tknologi (Scincs & Enginring) 67:2 (2014), shows h molcular srucur of MB, and is gnral propris ar lisd in Tabl 1. MB is a basic dy which carris a posiiv charg (caionic dy). Figur 1 Molcular srucur of mhyln blu Tabl 1 Characrisics of mhyln blu Chmical formula C 16H 18N 3SCl IUPAC nam 3,7-bis(Dimhylamino)- phnohiazin-5-ium chlorid Molar mass g/mol Colour indx numbr Wavlngh, λ 663 nm Solubiliy in war 1 g/25 ml 2.2 Prparaion of Acivad Carbon Th was carbon powdr was pr-rad a 250 C in furnac for abou 1.5h o rmov oil covring is surfac. Chmical acivaion: h prcursor was imprgnad wih ZnCl2 a imprgnaion raio (chmical agn ovr prcursor) of 1. Acivaion was prformd a 550 C for 1.5h in furnac. Th acivad carbon was washd using disilld war in a soxhl uni o rcovr ZnCl2. Th acivaion procdurs wr rpad using h rcycld ZnCl2 soluion o produc anohr acivad carbon. Acivad carbons wr dsignad as ZC and RC for acivaion using frsh and rcycld ZnCl2 soluion, rspcivly. Irradiad war acivaion: h prcursor was soakd in disilld war and acivad undr microwav a half magniud of powr innsiy for 20 minus. Th rsulan acivad carbon was dsignad as MC. Th acivad carbons wr drid in an ovn prior o characrizaion and adsorpion sudis. C 0 C m V whr C0 and C (mg/l) ar iniial and uilibrium concnraions, rspcivly, V (ml) is h volum of h soluion and m (g) is h mass of dry adsorbn. Euilibrium daa wr analyzd using h commonly usd isohrm modls, namly Langmuir, Frundlich and Rdlich-Prson modls. Adsorpion kinics: Acivad carbon ha dmonsrad h highs MB rmoval in uilibrium sudis was slcd for kinics valuaion. A fixd amoun of 0.1 g of acivad carbon was addd o diffrn bachs of 50 ml MB soluion having concnraions of 50 and 300 mg/l. Th rsidual concnraion was masurd a prs im inrvals for 72 hours. Kinics daa wr fid o psudo-firs ordr, psudo-scond ordr and inraparicl diffusion modls, and h rspciv consans wr drmind. 3.0 RESULTS AND DISCUSSION 3.1 Characrisics of Acivad Carbon Figur 2 rprsns h hrmogravimric profil of was carbon powdr bfor acivaion. (1) 2.3 Characrizaion of Acivad Carbon Thrmal profil of h prcursor was drmind undr N2 flow a a haing ra of 10 C/min by hrmogravimric analysis (TGA7, PrkinElmr). Th surfac ara of acivad carbon was masurd using surfac ara analyzr (Micromriics PulsChmiSorb 2705, USA), whil h surfac morphology was drmind using SEM insrumn (Philips XL 40, Nhrlands). 2.4 Adsorpion Sudis Euilibrium isohrms: Mhyln blu (MB) was mployd as h polluan prob in adsorpion. In bach adsorpion, 0.1g of acivad carbon was addd ino conical flasks conaining 50mL of MB of varying concnraions (50 o 300ppm). Th soluion ph was no adjusd, and masurd as 5.5±0.3 for all iniial concnraions sudid. Th flasks wr sald, and h mixurs wr allowd o uilibra on orbial shakr a 28±1 C and 90rpm for 72 hours. Thrafr, h soluions wr filrd and h rsidual concnraions wr drmind using Visibl Spcrophoomr (Biochrom Libra S6, UK) a a wavlngh of 690 nm. Th amoun of mhyln blu adsorbd a uilibrium, (mg/g), was calculad by simpl mass balanc, Figur 2 Thrmogravimric analysis of was carbon powdr bfor acivaion Th profil shows hr main rgions of considrabl wigh loss, i.., a mpraurs 300, 500 and 800 C. Th firs rgion indicas h rmoval of oil ha covrs h surfac of carbon powdr. I should b nod ha h raw marial conains almos ngligibl amoun of moisur, and i dos no mix wih war in is as-rcivd condiion. For h carbon powdr o b homognously imprgnad in ZnCl2 soluion, h layr of oil mus firs b rmovd. Th scond and hird rgions signify h rlas of volail and furhr dcomposiion of marial. Th prcursor originally conains high carbon conn; 90.9% combusibl marial and 9.1% ash. Du o is fin powdr form ha prsumably consiss of dns aromaic or graphiic srucur, longr duraion was ruird o burn h prcursor ino ash. This xplains why h wigh loss was only 25% alhough h mpraur was clos o 1000 C. Tabl 2 shows h yild and surfac ara of acivad carbons.
3 39 Muhammad Abbas Ahmad Zaini al. / Jurnal Tknologi (Scincs & Enginring) 67:2 (2014), Tabl 2 Yild and surfac ara of acivad carbons Acivad carbon Yild (%) Surfac ara (m 2 /g) ZC MC RC (a) Th yild of acivad carbon was drmind from h wigh of rsulan produc ovr h wigh of was carbon powdr afr h pr-ramn. In gnral, h acivaion of was yr powdr givs a br yild compard o ohr carbonacous marial bcaus h prcursor was radily rich in carbon conn [11]. Th valus rpord in his work wr somwha grar compard o prvious rlad works [13]. A dcras in yild indicas ha hr was burn-off of carbon marial during h acivaion. Rsuls show ha h yild of ZC is slighly highr compard o ha of RC and MC. A highr ZnCl2 raio is xpcd o inhibi h rlas of volail mar from h prcursor [14]. Th raio of rcovrd ZnCl2 usd o prpar RC is prsumably lssr han 1 du o som amoun ha has alrady bn usd up and/or vaporad from h firs acivaion, hus rsuling in a lowr yild of RZ. From Tabl 2, h valus of surfac ara wr rcordd as 288 and 104 m 2 /g for ZC and RC, rspcivly. A highr surfac ara of ZC ovr RC provd ha ZnCl2 imprgnaion raio plays an imporan rol during h acivaion, whrin is amoun drmins h surfac ara of acivad carbon. Nowihsanding ha, h uilizaion of rcovrd ZnCl2 for subsun acivaion was sill fasibl o produc acivad carbon wih high surfac ara. MC showd a lowr yild bcaus microwav haing could rach high mpraur in a shorr priod, hus spding up h rlas of ar or volail mar [15]. Th surfac ara of MC was as good as ha of RC, suggsing h ponial of using irradiad war in acivad carbon prparaion. Figur 3 shows h SEM imags of acivad carbons. Th formaion of ml, vsicls, prcipias of inorganic sals and surfac ching wr obsrvd on h surfac of acivad carbons. Undr h sam magnificaion, ZC showd a largr paricl siz compard o MC and RC du o h dominanc of acivaing agn. Comparabl morphology bwn MC and RC signifis a much lowr amoun of ZnCl2 usd o aciva RC. All acivad carbons displayd irrgular srucurs wih cracks and crvics which confirmd h amorphous and hrognous srucurs. Howvr, h por xurs ar no clarly obsrvd from hs SEM imags probably du o h fin powdr form. (b) (c) 3.2 Euilibrium Isohrms In his work, h ph of h soluion was no adjusd prior o adsorpion. A h nd of h procss, h valus of uilibrium ph wr found o b consisn for all acivad carbons sudid, and wr masurd as 6.1±0.2. Th consisn valus of uilibrium ph suggsd ha h amoun of proons adsorbd for ach acivad carbon is uniform alhough h surfac ara of acivad carbon is diffrn; ca. abou mmol/g proon was adsorbd. Thrfor, i is adua o uiliz singl componn isohrm modls o rprsn h adsorpion daa. Figur 4 displays h rmoval of MB by acivad carbons a diffrn iniial concnraions. Figur 3 SEM imags of acivad carbons (a) ZC, (b) MC and (c) RC
4 40 Muhammad Abbas Ahmad Zaini al. / Jurnal Tknologi (Scincs & Enginring) 67:2 (2014), Figur 4 Upak of mhyln blu a diffrn iniial concnraions afr 72 hours Th rmoval of MB by all acivad carbons was found o incras wih incrasing iniial concnraion. A iniial concnraion of 50 mg/l, all acivad carbons dmonsrad an idnical rmoval of MB a 25 mg/g which was uivaln o 100% rmoval. I implis h fasibiliy of using irradiad war, and rcovrd ZnCl2 for subsun acivad carbon prparaion, ha suis for adsorpion a lowr concnraion. As iniial concnraion incrass o 300 mg/l, h dcolourizaion of MB was found o follow h ordr: ZC>MC>RC, which corrsponds dircly o hir valus of surfac ara. ZC was abl o rmov narly 100% MB a concnraion approaching 200 mg/l, whil MC and RC displayd a dcrasing rnd of prcn rmoval a concnraions abov 50 mg/l. In gnral, h incras of iniial concnraion incrass h driving forc for adsorpion. A highr concnraions, howvr, h prsnc of adsorba molculs ar highr han h surfac sis can accommoda hus dcrasing h prcn rmoval. Adsorpion is a procss ha is dpndn on h iniial concnraion. Thus, if h surfac of acivad carbon is complly saurad, no chang in upak capaciy could b obsrvd vn as h concnraion incrass. This can b visualizd by h upak of RC a concnraions of 200 and 300 mg/l. Thr commonly usd isohrm modls for singl solu adsorpion, namly Langmuir [16-17], Frundlich [18] and Rdlich-Prson [19] modls wr usd o dscrib h adsorpion daa. Th Langmuir isohrm indicas monolayr adsorpion ono a complly homognous surfac, and is givn as, Q0bC 1 bc whr Q0 (mg/g) is h maximum upak pr uni mass of adsorbn o form a compl monolayr on h surfac of adsorbn, and b (L/mg) is a consan rlad o h affiniy of h binding sis. Th mpirical Frundlich isohrm basd on sorpion on a hrognous surfac is givn by, F 1 n (2) K C (3) Prson isohrm which combins h faurs of h Langmuir and Frundlich uaions can b dscribd as, AC (4) 1 BC g whr A, B and g ar all h Rdlich-Prson consans, and 0<g<1. Th Rdlich-Prson uaion bcoms Hnry s law whn g uals 0, whil i bcoms Langmuir modl whn g is uniy. Th non-linar modls wr solvd using Solvr add-in of Microsof Excl, and hir applicabiliy was dducd basd on cofficin of drminaion (R 2 ). Figur 5 shows h uilibrium adsorpion of MB ono acivad carbons, and h rspciv consans of h isohrm modls ar abulad in Tabl 3. All acivad carbons sudid displayd a concav shap upak capaciy agains uilibrium concnraion which indicas favourabl and rapid adsorpion in dilu soluion. Th valus of maximum rmoval wr rcordd as 154, 83 and 59 mg/g for ZC, MC and RC rspcivly. All hr modls wr found o corrla h adsorpion daa wll. Howvr, h Frundlich and Rdlich- Prson modls wr mor fid o linar approximaion; h R 2 valus ar clos o uniy. Th Frundlich modl suggss ha h upak of MB by acivad carbons is a normal adsorpion ono hrognous surfac. This could b suppord by h surfac morphology of acivad carbons (Figur 3). Th applicabiliy of Rdlich-Prson modl indicas h adsorpion procss as join faurs bwn Langmuir (monolayr adsorpion) and Frundlich (adsorpion on hrognous surfac) modls. As can b sn from Figur 5(a), h rmoval of MB obyd Frundlich isohrm a lowr uilibrium concnraion, and swichd o Langmuir isohrm as h upak bgan o lvl-off. Among h acivad carbons sudid, ZC dmonsrad a grar adsorpion affiniy for MB. I is likly du o is high surfac ara, which allows for grar inracion probabiliis bwn h surfac sis and adsorba molculs spcially a lowr concnraion. Tabl 3 Consans of isohrm modls Isohrm modl Acivad carbon ZC MC RC Langmuir Q 0 (mg/g) b (L/mg) R² Frundlich K F (mg/g)(l/mg) 1/n /n R² Rdlich-Prson A (L/g) B g R whr KF and 1/n, ar h Frundlich consans which corrspond o h maximum adsorpion capaciy and innsiy, rspcivly. Th 1/n valu ranging from 0 o 1 is considrd o rprsn surfac hrogniy. I is also suggsd ha h n valus ranging bwn 2 and 10 rprsn favourabl adsorpion procss. Th Rdlich-
5 41 Muhammad Abbas Ahmad Zaini al. / Jurnal Tknologi (Scincs & Enginring) 67:2 (2014), (a) whr k2 (g/mg.h) is h ra consan of psudo-scond-ordr adsorpion. Th iniial adsorpion ra, h, of psudo-scond-ordr as approaching zro is dfind as, h k (7) 2 2 Half-adsorpion im, 1/2, is dfind as h im ruird for h adsorpion o ak up half as much MB as is uilibrium valu. I is ofn usd as a masur of h adsorpion ra, and is xprssd as, k (8) 2 Figur 5 Euilibrium adsorpion of mhyln blu. (a) Lins wr prdicd from Langmuir (solid) and Frundlich (dashd) modls; (b) Lins wr prdicd from Rdlich-Prson modl 3.3 Adsorpion Kinics A br undrsanding of h mchanism and ra of adsorpion is imporan o dsign h adsorpion procss. This par dscribs h kinics valuaion of ZC a wo concnraions, i.., 50 and 300 mg/l. ZC was slcd bcaus i prforms br han h ohr wo acivad carbons, whil h wo concnraions wr chosn o valua h kinics in dilud soluion and a h poin approaching surfac sauraion. Th kinics daa was valuad by using hr kinics modls, namly psudo-firs ordr [20], Ho s psudo-scond ordr [21] and inraparicl diffusion modls [22]. Psudo-firs ordr modl is givn by, k 1 1 (5) whr (mg/g) is h amoun of MB adsorbd a im (h), and k1 (h 1 ) is h ra consan of firs ordr adsorpion. Ho s psudoscond-ordr uaion basd on chmical rlad adsorpion is xprssd as, (b) 2 k2 1 k (6) 2 Th diffusion mchanism and ra-conrolling sps in h adsorpion procss could b xplaind by h Wbr and Morris inraparicl diffusion modl [22], k 0.5 P C whr kp (mg/g.h 0.5 ) is h inraparicl diffusion ra consan, and C is h inrcp ha givs an ida abou h boundary layr hicknss, i.., h largr h inrcp, h grar h boundary layr ffc. All kinics consans wr solvd using Solvr add-in, givn h condiion whr h sum of suard rror is h las hus yild h opimum valu of corrlaion of drminaion (R 2 ). Figur 6 illusras h kinics profils of ZC a wo diffrn concnraions, and h rspciv consans ar abulad in Tabl 4. Th convx upward kinics curvs gnrally indica srong and favourabl MB adsorpion, and h uilibrium upak ovr h sudid priod was rasonably allid wih Figur 4. From Figur 6, h upak of MB was found o incras wih incrasing im upon raching a plaau a abou 30 min and 48 h for iniial concnraions of 50 and 300 mg/l, rspcivly. ZC displayd a rapid incras in adsorpion in dilud soluion during h firs fw minus, afr which h upak sars o incras gradually. Narly 100% MB rmoval (24.8 mg/g) was aaind afr 30 min h soluion was in conac wih h adsorbn. Howvr, a much slowr rmoval was obsrvd for MB concnraion of 300 mg/l. Th kinics daa wr fid o h commonly usd kinics modls o lucida h mchanisms of MB rmoval ono acivad carbon. Tabl 4 Consans of kinics modl (9) Iniial mhyln blu concnraion (mg/l) , Exp (mg/g) Psudo-firs-ordr k min h -1, Modl (mg/g) R Psudo-scond-ordr k g/mg.min g/mg.h h 42.4 g/mg.min 124 g/mg.h 1/ min h, Modl (mg/g) R Inraparicl diffusion modl k P min h -0.5 C R
6 42 Muhammad Abbas Ahmad Zaini al. / Jurnal Tknologi (Scincs & Enginring) 67:2 (2014), (a) (b) (b) Figur 7 Inraparicl diffusion modl for mhyln blu rmoval a concnraions of (a) 50 and (b) 300 mg/l Figur 6 Kinics of mhyln blu rmoval a iniial concnraions of (a) 50 and (b) 300 mg/l. Lins wr prdicd from psudo-firs-ordr (dashd) and psudo-scond-ordr (solid) modls (a) From Tabl 4, Ho s psudo-scond ordr kinics modl rprsns a rasonably br R 2 valus compard o h ohr wo kinics modls. Th calculad valus of MB upak (, Modl) wr also in agrmn wih h xprimnal valus (, Exp). MB rmoval ono ZC could hrfor b sufficinly dscribd as chmisopion or chmically drivn adsorpion [21]. Th ra consan for adsorpion in dilu soluion (50 mg/l) is mor han 500 ims grar han ha in 300 mg/l MB soluion. Also, h iniial adsorpion ra, h for dilu soluion is narly 20 ims highr. This phnomnon could b xplaind by h fac ha hr ar plny availabl sis o accommoda MB molculs a lowr concnraion which offrs fas adsorpion ra. As h concnraion incrass, mor adsorba molculs ar comping for limid numbr of availabl sis hus dcrasing h ra consan. Nowihsanding ha, a grar concnraion gradin in auous soluion aracs mor adsorba molculs o lodg on h acivad carbon surfac hus incrasing h upak capaciy; which is ru unil h surfac sauraion is rachd. Adsorpion usually follows hr conscuiv sps, i.., (i) film diffusion which ranspors h adsorba from h bulk soluion o h xrnal surfac of h adsorbn, (ii) paricl diffusion which ranspors adsorba from h liuid film o h solid surfac, and (iii) Adsorba-adsorbn inracion lading o h adsorpion. Th mchanism of adsorpion could b dscribd by h inraparicl diffusion modl [22]. Figur 7 shows h inraparicl diffusion modl for mhyln blu rmoval ono ZC, and h rspciv consans ar abulad in Tabl 4. From h plos, hr ar wo conncing lins of diffrn slops; h iniial par (sp slop) indicas h iniial sag of adsorpion ha rflcs h hicknss of boundary layr, and h scond par rprsns h ffc of inraparicl diffusion. Inraparicl diffusion is h ra-limiing sp (h slows sp in MB adsorpion ha drmins h ra consan) whn h wo lins yild a sraigh lin hrough origin whn combind. I happns whn h rsisanc in h film is almos ngligibl bcaus of hin boundary layr. Howvr, i was no h cas for ZC bcaus h adsorpion daa did no fi o h modl. Thus, h inraparicl diffusion is no h only ra-limiing sp ha is conrolling h adsorpion of MB; i is xpcd ha ohr coopraiv sps ar also involvd including film diffusion and/or chmisorpion.
7 43 Muhammad Abbas Ahmad Zaini al. / Jurnal Tknologi (Scincs & Enginring) 67:2 (2014), Tabl 5 Rmoval of mhyln blu by various adsorbns Adsorbn Maximu m upak (mg/g) ph Surfac ara (m 2 /g) Rfrnc ZnCl 2-acivad a was carbon [23] Acivad carbon fibr [24] Muli-walld carbon nanoubs [25] ZnCl 2-acivad corn husk carbon [26] rcovrd ZnCl2 and irradiad war dmonsrad fasibl mhyln blu adsorpion in dilu soluion. Dcolourizaion of mhyln blu ono acivad carbons could b dscribd by Rdlich-Prson modl, whil h kinics daa wr br fid o Ho s psudo-scond ordr modl. Acivad carbons prpard in his work could bcom a ponial candida for mhyln blu rmoval. Acknowldgmn Th auhors grafully acknowldg h suppor of Minisry of Highr Educaion Malaysia and Univrsii Tknologi Malaysia hrough UTM-Rsarch Univrsiy Gran (No. 07J58). Gypsum [27] Rfrncs Hyacinh roo powdr [28] Naural Jordanian Tripoli [29] MgO nanoparicl [30] ZnCl 2-acivad pa shlls carbon [31] Poplar lavs [32] Sugar xracd spn ric biomass [33] ZnCl 2-acivad ric husk carbon [34] ZnCl 2-acivad carbon powdr Irradiad waracivad carbon powdr Prsn sudy Prsn sudy Tabl 5 summarizs som sudis on h rmoval of MB by various adsorbns. A numbr of auhors wr siln on wo imporan paramrs ha ar usually rpord o hav influnc on h rmoval of MB, i.., surfac ara of adsorbn/acivad carbon, and h soluion ph a which h adsorpion was carrid ou. MB is a caionic dy, and hrfor a basic nvironmn is favourabl for adsorpion bcaus surfac dproonaion would arac mor MB molculs o h adsorbn. Howvr, som sudis [25-26] rpord ha soluion ph grar han 4 would no giv much diffrnc in h upak of MB. Compard o ohr adsorbns rpord in liraur, ZC and MC dmonsrad comparabl prformanc for MB rmoval a ph 5.5 (non-adjusd); which will b a mri for indusrial applicaions. 4.0 CONCLUSION Acivad carbons wr prpard using ZnCl2, rcovrd ZnCl2 and irradiad-war nvironmn for mhyln blu rmoval from auous soluion. ZnCl2-acivad carbon powdr showd a grar rmoval of mhyln blu bcaus of is highr surfac ara han h ohr wo acivad carbons. Acivad carbons prpard using [1] Environmnal Procion Agncy Profil of h Txil Indusry. Washingon: U.S. EPA. [2] Robinson, T., G. McMullan, R. Marchan, and P. Nigam Rmdiaion of Dys in Txil Efflun: A Criical Rviw on Currn Tramn Tchnologis wih a Proposd Alrnaiv. Biorsourc Tchnology. 77: [3] Forgacs, E., T. Csrhai, and G. Oros Rmoval of Synhic Dys from Waswars: A Rviw. Environmnal Inrnaional. 30: [4] Schirmr, R. H., B. Coulibaly, A. Sich, M. Schiwin, H. Mrkl, J. Eubl, K. Bckr, H. Bchr, O. Mullr, T. Zich, W. Schik, and B. Kouya Mhyln Blu as an Animalarial Agn. Rdox Rpor. 8: [5] Kupfr, A., C. Aschlimann, B. Wrmuh, and T. Crny T Prophylaxis and Rvrsal of Ifosfamid Encphalopahy wih Mhyln Blu. Lanc. 343: [6] Anjanyulu, Y., N. S. Chary, and D. S. S. Raj Dcolorisaion of Indusrial Effluns- Availabl Mhods and Emrging Tchnologis- A Rviw. Rviws Environmnal Scinc Biochnology. 4: [7] Bansal, R.C., and M. Goyal Acivad Carbon Adsorpion. Nw York: Taylor & Francis. [8] Rafaullaha, M., O. Sulaiman, R. Hashim, and A. Ahmad Adsorpion of Mhyln Blu on Low-Cos Adsorbns: A rviw. Journal of Hazardous Marials. 177: [9] Dng, H., L. Yang, G. Tao, and J. Dai Prparaion and Characrizaion of Acivad Carbon from Coon Salk by Microwav Assisd Chmical Acivaion: Applicaion in Mhyln Blu Adsorpion from Auous Soluion. Journal of Hazardous Marials. 166: [10] Marsh, H., and F. Rodriguz-Rinoso Acivad Carbon. Amsrdam: Elsvir. [11] Zaini, M. A. A., R. Okayama, and M. Machida Adsorpion of Auous Mal Ions on Cal-Manur-Compos basd Acivad Carbons. Journal of Hazardous Marials. 170: [12] Zaini, M. A. A., Y. Amano, and M. Machida Adsorpion of Havy Mals ono Acivad Carbons drivd from Polyacryloniril Fibr. Journal of Hazardous Marials. 180: [13] Mui, E. L. K., D. C. K. Ko, and G. McKay Producion of Aciv Carbons from Was Tyrs- A Rviw. Carbon. 42: [14] Qian, Q., M. Machida, and H. Tasumoo Prparaion of Acivad Carbons from Cal-Manur-Compos by Zinc Chlorid Acivaion. Biorsourc Tchnology. 98: [15] Huang, L., Y. Sun, W. Wang, Q. Yu, and T. Yang Comparaiv Sudy on Characrizaion of Acivad Carbons Prpard by Microwav and Convnional Haing Mhods and Applicaion in Rmoval of Oxyracyclin (OTC). Chmical Enginring Journal. 171: [16] Langmuir, I Th Consiuion and Fundamnal Propris of Solids and Liuids. Par I. Solid. Journal of h Amrican Chmical Sociy. 38: [17] Langmuir, I Th Adsorpion of Gass on Plan Surfacs of Glass, Mica and Plainum. Journal of h Amrican Chmical Sociy. 40: [18] Frundlich, H. M. F Abou h Adsorpion in Soluion (Ubr di Adsorpion in Losungn). Zischrif fur Physikalisch Chmi. 57A: [19] Rdlich, O., and D.vL. Prson A Usful Adsorpion Isohrm. Th Journal of Physical Chmisry. 63: 1024.
8 44 Muhammad Abbas Ahmad Zaini al. / Jurnal Tknologi (Scincs & Enginring) 67:2 (2014), [20] Lagrgrn, S Abou h Thory of So-calld Adsorpion of Solubl Subsancs (Kungliga Svnska Vnskapsakadmins). Handlingar. 24: [21] Ho, Y.S., and G. McKay A Comparison of Chmisorpion Kinics Modls Applid o Polluan Rmoval on Various Sorbns. Trans IChmE. 76B: [22] Wbr, W.J., and J.C. Morris Kinics of Adsorpion on Carbon from Soluion. Journal of h Saniary Enginring Division Amrican Sociy of Civil Enginrs. 89: [23] Sarici-Ozdmir, C Adsorpion and Dsorpion Kinics Bhaviour of Mhyln Blu ono Acivad Carbon. Physicochmical Problms of Minral Procssing. 48: [24] Ivans, M.G., T.A. Saviskaya, T.N. Nvar, and D.D. Grinshpan Adsorpiv and Srucural Characrisics of Carbon Sorbns. Inorganic Marials. 47: [25] Shahryari, Z., A.S. Goharrizi, and M. Azadi Exprimnal Sudy of Mhyln Blu Adsorpion from Auous Soluions ono Carbon Nano Tubs. Inrnaional Journal of War Rsourcs and Environmnal Enginring. 2: [26] Khodai, M., N. Ghasmi, B. Moradi, and M. Rahimi Rmoval of Mhyln Blu from Waswar by Adsorpion ono ZnCl 2 Acivad Corn Husk Carbon Euilibrium Sudis. Journal of Chmisry. In prss. [27] Rauf, M.A., I. Shhadh, A. Ahmd, and A. Al-Zamly Rmoval of Mhyln Blu from Auous Soluion by Using Gypsum as a Low Cos Adsorbn. World Acadmy of Scinc, Enginring and Tchnology. 31: [28] Soni, M., A.K.Sharma, J.K.Srivasava, and J.S. Yadav Adsorpiv Rmoval of Mhyln Blu Dy from an Auous Soluion using War Hyacinh Roo Powdr as a Low Cos Adsorbn. Inrnaional Journal of Chmical Scincs and Applicaions. 3: [29] ALzaydin, A.S Adsorpion of Mhyln Blu from Auous Soluion ono a Low-Cos Naural Jordanian Tripoli. Amrican Journal of Environmnal Scincs. 5: [30] Wanchanhuk, R., and A. Thapol Th Kinic Sudy of Mhyln Blu Adsorpion ovr MgO from PVA Tmpla Prparaion. Journal of Environmnal Scinc and Tchnology. 4: [31] Gcgl, U., G. Ozcan, and G.C. Gurpinar Rmoval of Mhyln Blu from Auous Soluion by Acivad Carbon Prpard from Pa Shlls (Pisum Saivum). Journal of Chmisry. In prss. [32] Han, X., X. Niu, and X. Ma Adsorpion Characrisics of Mhyln Blu on Poplar Laf in Bach Mod: Euilibrium, Kinics and Thrmodynamics. Koran Journal of Chmical Enginring. 29: [33] Ur-Rhman, M.S., I. Kim, and J-I. Han Adsorpion of Mhyln Blu Dy from Auous Soluion by Sugar Exracd Spn Ric Biomass. Carbohydra Polymrs. 90: [34] Sharma, Y. C., Uma, and S. N. Upadhyay An Economically Viabl Rmoval of Mhyln Blu by Adsorpion on Acivad Carbon Prpard from Ric Husk. Th Canadian Journal of Chmical Enginring. 82:
Pelagia Research Library
 Availabl onlin a www.plagiarsarchlibrary.com Plagia Rsarch Library Dr Chmica Sinica, 04, 5(): 8-3 ISSN: 0976-8505 CODEN (USA) CSHIA5 Sorpion dynamics of acid and basic dys ono acivad carbon drivd from
Availabl onlin a www.plagiarsarchlibrary.com Plagia Rsarch Library Dr Chmica Sinica, 04, 5(): 8-3 ISSN: 0976-8505 CODEN (USA) CSHIA5 Sorpion dynamics of acid and basic dys ono acivad carbon drivd from
Removal of Atrazine from Aqueous Solutions Using HNO 3 Treated Oil Palm Shell-Based Adsorbent
 Rmoval of Arazin from Aquous Soluions Using HNO 3 Trad Oil Palm Shll-Basd Adsorbn I.A.W. Tan, L.L.P. Lim, and K.T. Lim Absrac Acivad carbon is a prominn marial for adsorpion of arazin, howvr is usag is
Rmoval of Arazin from Aquous Soluions Using HNO 3 Trad Oil Palm Shll-Basd Adsorbn I.A.W. Tan, L.L.P. Lim, and K.T. Lim Absrac Acivad carbon is a prominn marial for adsorpion of arazin, howvr is usag is
COD removal from industrial wastewater using activated carbon prepared from animal horns
 African Journal of Biochnology Vol. 7 (2), pp. 37-3, 5 Novmbr, 2008 Availabl onlin a hp://www.acadmicjournals.org/ajb ISSN 684 535 2008 Acadmic Journals Full Lngh Rsarch Papr COD rmoval from indusrial
African Journal of Biochnology Vol. 7 (2), pp. 37-3, 5 Novmbr, 2008 Availabl onlin a hp://www.acadmicjournals.org/ajb ISSN 684 535 2008 Acadmic Journals Full Lngh Rsarch Papr COD rmoval from indusrial
Department of Chemistry, Thiru Vi Ka Govt. Arts College, Thiruvarur , India. 3
 Scholars Journal of Enginring and Tchnology (SJET) Sch. J. Eng. Tch., 24; 2(2B):258-263 Scholars Acadmic and Scinific Publishr (An Inrnaional Publishr for Acadmic and Scinific Rsourcs) www.saspublishr.com
Scholars Journal of Enginring and Tchnology (SJET) Sch. J. Eng. Tch., 24; 2(2B):258-263 Scholars Acadmic and Scinific Publishr (An Inrnaional Publishr for Acadmic and Scinific Rsourcs) www.saspublishr.com
Keywords: Adsorption modeling, Crystal violet, Isotherms, Kinetics, Natural adsorbent. 1. INTRODUCTION 2. MATERIALS AND METHODS
 Inrnaional Journal of Enhancd Rsarch in Scinc Tchnology & Enginring, ISSN: 239-7463 Vol. 4 Issu 6, Jun-25, pp: (55-66), Impac Facor:.252, Availabl onlin a: www.rpublicaions.com Bach Adsorpion Sysm of Hazardous
Inrnaional Journal of Enhancd Rsarch in Scinc Tchnology & Enginring, ISSN: 239-7463 Vol. 4 Issu 6, Jun-25, pp: (55-66), Impac Facor:.252, Availabl onlin a: www.rpublicaions.com Bach Adsorpion Sysm of Hazardous
Journal of Chemical and Pharmaceutical Research, 2014, 6(6): Research Article
 Availabl onlin www.jocpr.com Journal of hmical and Pharmacuical Rsarch, 214, 6(6):154-159 Rsarch Aricl ISSN : 975-7384 ODEN(USA) : JPR5 Th adsorpion of nickl ions in auous soluion by chiosan gl bads Muing
Availabl onlin www.jocpr.com Journal of hmical and Pharmacuical Rsarch, 214, 6(6):154-159 Rsarch Aricl ISSN : 975-7384 ODEN(USA) : JPR5 Th adsorpion of nickl ions in auous soluion by chiosan gl bads Muing
Copper (II) Uptake by Adsorption Using Palmyra Palm Nut
 Availabl onlin a www.plagiarsarchlibrary.com Plagia Rsarch Library Advancs in Applid Scinc Rsarch, 0, (6):66-75 ISSN: 0976-860 CODEN (USA): AASRFC Coppr (II) Upak by Adsorpion Using Palmyra Palm Nu Josph
Availabl onlin a www.plagiarsarchlibrary.com Plagia Rsarch Library Advancs in Applid Scinc Rsarch, 0, (6):66-75 ISSN: 0976-860 CODEN (USA): AASRFC Coppr (II) Upak by Adsorpion Using Palmyra Palm Nu Josph
Biosorption of acid dyes using spent brewery grains: Characterization and modeling
 Inrnaional Journal of Applid Scinc and Enginring 2009. 7, 2: 115-125 Biosorpion of acid dys using spn brwry grains: Characrizaion and modling Dr.V.Jaikumar a, *, K.Sahish Kumar b, D.Gnana Prakash b a Dparmn
Inrnaional Journal of Applid Scinc and Enginring 2009. 7, 2: 115-125 Biosorpion of acid dys using spn brwry grains: Characrizaion and modling Dr.V.Jaikumar a, *, K.Sahish Kumar b, D.Gnana Prakash b a Dparmn
UPTAKE OF METHYLENE BLUE DYE FROM AQUEOUS SOLUTIONS ONTO CHEMICAL ACTIVATED CARDOON (CYNARA CARDUNCULUS)
 Procdings of h 14 h Inrnaional Confrnc on Environmnal Scinc and Tchnology Rhods, Grc, 3-5 Spmbr 2015 UPTAKE OF METHYLENE BLUE DYE FROM AQUEOUS SOLUTIONS ONTO CHEMICAL ACTIVATED CARDOON (CYNARA CARDUNCULUS)
Procdings of h 14 h Inrnaional Confrnc on Environmnal Scinc and Tchnology Rhods, Grc, 3-5 Spmbr 2015 UPTAKE OF METHYLENE BLUE DYE FROM AQUEOUS SOLUTIONS ONTO CHEMICAL ACTIVATED CARDOON (CYNARA CARDUNCULUS)
Cd(II) ADSORPTION FROM AQUEOUS SOLUTION BY ACTIVATED CARBON FROM SUGAR BEET PULP IMPREGNATED WITH PHOSPHORIC ACID
 Volum No. 9 REPRINT pp. 5-58 Cd(II) ADSORPTION FROM AQUEOUS SOLUTION BY ACTIVATED CARBON FROM SUGAR BEET PULP IMPREGNATED WITH PHOSPHORIC ACID Ahm Özr - Fikr Tümn Angrsr. 855 Frising - Grmany Phon: ++9
Volum No. 9 REPRINT pp. 5-58 Cd(II) ADSORPTION FROM AQUEOUS SOLUTION BY ACTIVATED CARBON FROM SUGAR BEET PULP IMPREGNATED WITH PHOSPHORIC ACID Ahm Özr - Fikr Tümn Angrsr. 855 Frising - Grmany Phon: ++9
Equilibrium Kinetics and Isotherm Studies of Cu (II) Adsorption from Waste Water onto Alkali Activated Oil Palm Ash
 Amrican Journal of Applid Scincs 8 (3): 230-237, 20 ISSN 546-9239 200 Scinc Publicaions Euilibrium Kinics and Isohrm Sudis of Cu (II) Adsorpion from Was War ono Alkali Acivad Oil Palm Ash Zaira Zaman Chowdhury,
Amrican Journal of Applid Scincs 8 (3): 230-237, 20 ISSN 546-9239 200 Scinc Publicaions Euilibrium Kinics and Isohrm Sudis of Cu (II) Adsorpion from Was War ono Alkali Acivad Oil Palm Ash Zaira Zaman Chowdhury,
Chemically Modified Cornulaca monacantha Biomass for Bioadsorption of Hg (II) from Contaminated Water: Adsorption Mechanism
 Journal of Environmnal Procion, 3, 4, 8-89 hp://dx.doi.org/.436/jp.3.4333 Publishd Onlin March 3 (hp://www.scirp.org/journal/jp) Chmically Modifid Cornulaca monacanha Biomass for Bioadsorpion of Hg (II)
Journal of Environmnal Procion, 3, 4, 8-89 hp://dx.doi.org/.436/jp.3.4333 Publishd Onlin March 3 (hp://www.scirp.org/journal/jp) Chmically Modifid Cornulaca monacanha Biomass for Bioadsorpion of Hg (II)
A MATHEMATICAL MODEL FOR NATURAL COOLING OF A CUP OF TEA
 MTHEMTICL MODEL FOR NTURL COOLING OF CUP OF TE 1 Mrs.D.Kalpana, 2 Mr.S.Dhvarajan 1 Snior Lcurr, Dparmn of Chmisry, PSB Polychnic Collg, Chnnai, India. 2 ssisan Profssor, Dparmn of Mahmaics, Dr.M.G.R Educaional
MTHEMTICL MODEL FOR NTURL COOLING OF CUP OF TE 1 Mrs.D.Kalpana, 2 Mr.S.Dhvarajan 1 Snior Lcurr, Dparmn of Chmisry, PSB Polychnic Collg, Chnnai, India. 2 ssisan Profssor, Dparmn of Mahmaics, Dr.M.G.R Educaional
2.1. Differential Equations and Solutions #3, 4, 17, 20, 24, 35
 MATH 5 PS # Summr 00.. Diffrnial Equaions and Soluions PS.# Show ha ()C #, 4, 7, 0, 4, 5 ( / ) is a gnral soluion of h diffrnial quaion. Us a compur or calculaor o skch h soluions for h givn valus of h
MATH 5 PS # Summr 00.. Diffrnial Equaions and Soluions PS.# Show ha ()C #, 4, 7, 0, 4, 5 ( / ) is a gnral soluion of h diffrnial quaion. Us a compur or calculaor o skch h soluions for h givn valus of h
UNIT #5 EXPONENTIAL AND LOGARITHMIC FUNCTIONS
 Answr Ky Nam: Da: UNIT # EXPONENTIAL AND LOGARITHMIC FUNCTIONS Par I Qusions. Th prssion is quivaln o () () 6 6 6. Th ponnial funcion y 6 could rwrin as y () y y 6 () y y (). Th prssion a is quivaln o
Answr Ky Nam: Da: UNIT # EXPONENTIAL AND LOGARITHMIC FUNCTIONS Par I Qusions. Th prssion is quivaln o () () 6 6 6. Th ponnial funcion y 6 could rwrin as y () y y 6 () y y (). Th prssion a is quivaln o
Spring 2006 Process Dynamics, Operations, and Control Lesson 2: Mathematics Review
 Spring 6 Procss Dynamics, Opraions, and Conrol.45 Lsson : Mahmaics Rviw. conx and dircion Imagin a sysm ha varis in im; w migh plo is oupu vs. im. A plo migh imply an quaion, and h quaion is usually an
Spring 6 Procss Dynamics, Opraions, and Conrol.45 Lsson : Mahmaics Rviw. conx and dircion Imagin a sysm ha varis in im; w migh plo is oupu vs. im. A plo migh imply an quaion, and h quaion is usually an
Lecture 2: Current in RC circuit D.K.Pandey
 Lcur 2: urrn in circui harging of apacior hrough Rsisr L us considr a capacior of capacianc is conncd o a D sourc of.m.f. E hrough a rsisr of rsisanc R and a ky K in sris. Whn h ky K is swichd on, h charging
Lcur 2: urrn in circui harging of apacior hrough Rsisr L us considr a capacior of capacianc is conncd o a D sourc of.m.f. E hrough a rsisr of rsisanc R and a ky K in sris. Whn h ky K is swichd on, h charging
JMES, 2017 Volume 8, Issue 3, Page ICMES2016, 1-3 Dec. 2016, Oujda
 Journal of Marials and Environmnal Scincs ISSN: 08-508 Copyrigh 07, Univrsiy of Mohammd Prmir Oujda Morocco JMES, 07 Volum 8, Issu 3, Pag 784-800 hp://www.jmarnvironsci.com/ ICMES06, -3 Dc. 06, Oujda Dcolourizaion
Journal of Marials and Environmnal Scincs ISSN: 08-508 Copyrigh 07, Univrsiy of Mohammd Prmir Oujda Morocco JMES, 07 Volum 8, Issu 3, Pag 784-800 hp://www.jmarnvironsci.com/ ICMES06, -3 Dc. 06, Oujda Dcolourizaion
The influences on the Phosphate Removal from Aqueous Solution by using red mud
 Inrnaional Journal of Innovaiv Sudis in Scincs and Enginring Tchnology ISSN 2455-4863 (Onlin) www.ijiss.org Volum: 3 Issu: 6 Jun 2017 Th influncs on h Phospha Rmoval from Aquous Soluion by using rd mud
Inrnaional Journal of Innovaiv Sudis in Scincs and Enginring Tchnology ISSN 2455-4863 (Onlin) www.ijiss.org Volum: 3 Issu: 6 Jun 2017 Th influncs on h Phospha Rmoval from Aquous Soluion by using rd mud
N.A. Adesola Babarinde 1* ; Jonathan O. Babalola 2 ; John Adegoke 1 ; Esther O. George 1 ; Henrietta O. Okeke 1 ; and Ayoola T.
 Kinic, Euilibrium, and Thrmodynamic Sudis of h Biosorpion of Cd(II),, and from Auous Soluions using Coconu (Cocos nucifra) Laf. N.A. Adsola Babarind * ; Jonahan O. Babalola 2 ; John Adgok ; Eshr O. Gorg
Kinic, Euilibrium, and Thrmodynamic Sudis of h Biosorpion of Cd(II),, and from Auous Soluions using Coconu (Cocos nucifra) Laf. N.A. Adsola Babarind * ; Jonahan O. Babalola 2 ; John Adgok ; Eshr O. Gorg
4.1 The Uniform Distribution Def n: A c.r.v. X has a continuous uniform distribution on [a, b] when its pdf is = 1 a x b
![4.1 The Uniform Distribution Def n: A c.r.v. X has a continuous uniform distribution on [a, b] when its pdf is = 1 a x b 4.1 The Uniform Distribution Def n: A c.r.v. X has a continuous uniform distribution on [a, b] when its pdf is = 1 a x b](/thumbs/71/65779443.jpg) 4. Th Uniform Disribuion Df n: A c.r.v. has a coninuous uniform disribuion on [a, b] whn is pdf is f x a x b b a Also, b + a b a µ E and V Ex4. Suppos, h lvl of unblivabiliy a any poin in a Transformrs
4. Th Uniform Disribuion Df n: A c.r.v. has a coninuous uniform disribuion on [a, b] whn is pdf is f x a x b b a Also, b + a b a µ E and V Ex4. Suppos, h lvl of unblivabiliy a any poin in a Transformrs
Institute of Actuaries of India
 Insiu of Acuaris of India ubjc CT3 Probabiliy and Mahmaical aisics Novmbr Examinaions INDICATIVE OLUTION Pag of IAI CT3 Novmbr ol. a sampl man = 35 sampl sandard dviaion = 36.6 b for = uppr bound = 35+*36.6
Insiu of Acuaris of India ubjc CT3 Probabiliy and Mahmaical aisics Novmbr Examinaions INDICATIVE OLUTION Pag of IAI CT3 Novmbr ol. a sampl man = 35 sampl sandard dviaion = 36.6 b for = uppr bound = 35+*36.6
Charging of capacitor through inductor and resistor
 cur 4&: R circui harging of capacior hrough inducor and rsisor us considr a capacior of capacianc is conncd o a D sourc of.m.f. E hrough a rsisr of rsisanc R, an inducor of inducanc and a y K in sris.
cur 4&: R circui harging of capacior hrough inducor and rsisor us considr a capacior of capacianc is conncd o a D sourc of.m.f. E hrough a rsisr of rsisanc R, an inducor of inducanc and a y K in sris.
The Comparison of Red Mud Modification by Acid and Heat for Phosphate Removal from Aqueous Solution
 Inrnaional Journal of Innovaiv Sudis in Scincs and Enginring Tchnology ISSN 2455-4863 (Onlin) www.ijiss.org Volum: 2 Issu: 12 Dcmbr 2016 Th Comparison of Rd Mud Modificaion by Acid and Ha for Phospha Rmoval
Inrnaional Journal of Innovaiv Sudis in Scincs and Enginring Tchnology ISSN 2455-4863 (Onlin) www.ijiss.org Volum: 2 Issu: 12 Dcmbr 2016 Th Comparison of Rd Mud Modificaion by Acid and Ha for Phospha Rmoval
Investigation on Biosorption of Reactive Blue 140 by Dead Biomass of Aspergillus niger HM11: Kinetics and Isotherm Studies
 Global Journal of Biochnology & Biochmisry 4 (): 169-178, 9 ISSN 78-466X IDOSI Publicaions, 9 Invsigaion on Biosorpion of Raciv Blu 14 by Dad Biomass of Asprgillus nigr HM11: Kinics and Isohrm Sudis 1
Global Journal of Biochnology & Biochmisry 4 (): 169-178, 9 ISSN 78-466X IDOSI Publicaions, 9 Invsigaion on Biosorpion of Raciv Blu 14 by Dad Biomass of Asprgillus nigr HM11: Kinics and Isohrm Sudis 1
The removal of chloramphenicol from water through adsorption on activated carbon
 E3S Wb of Confrncs 9, 0008 (07) EEMS 07 DOI: 0.05/3sconf/0790008 Th rmoval of chloramphnicol from war hrough adsorpion on acivad carbon Joanna Lach,*, Agniszka Ocipa-Kubicka Czsochowa Univrsiy of Tchnology,
E3S Wb of Confrncs 9, 0008 (07) EEMS 07 DOI: 0.05/3sconf/0790008 Th rmoval of chloramphnicol from war hrough adsorpion on acivad carbon Joanna Lach,*, Agniszka Ocipa-Kubicka Czsochowa Univrsiy of Tchnology,
REMOVAL OF BASIC BLUE 3 AND REACTIVE ORANGE 16 BY ADSORPTION ONTO QUARTENIZED SUGAR CANE BAGASSE
 Th Malaysian Journal of Analyical Scincs, Vol 13 No 2 (29): 185-193 REMOVAL OF BASIC BLUE 3 AND REACTIVE ORANGE 16 BY ADSORPTION ONTO QUARTENIZED SUGAR CANE BAGASSE (Pnyingkiran Basik Biru 3 Dan Rakif
Th Malaysian Journal of Analyical Scincs, Vol 13 No 2 (29): 185-193 REMOVAL OF BASIC BLUE 3 AND REACTIVE ORANGE 16 BY ADSORPTION ONTO QUARTENIZED SUGAR CANE BAGASSE (Pnyingkiran Basik Biru 3 Dan Rakif
Comparative analysis of the efficiencies of two low cost adsorbents in the removal of Cr(VI) and Ni(II) from aqueous solution
 African Journal of Environmnal Scinc and Tchnology Vol. 3 (11), pp. 360-369, Novmbr 2009 Availabl onlin a hp://www.acadmicjournals.org/ajs ISSN 1991-637X 2009 Acadmic Journals Full Lngh Rsarch Papr Comparaiv
African Journal of Environmnal Scinc and Tchnology Vol. 3 (11), pp. 360-369, Novmbr 2009 Availabl onlin a hp://www.acadmicjournals.org/ajs ISSN 1991-637X 2009 Acadmic Journals Full Lngh Rsarch Papr Comparaiv
Biosorption of Azure Dye with Sunflower Seed Hull: Estimation of Equilibrium, Thermodynamic and Kinetic Parameters
 Inrnaional Journal of Enginring Rsarch and Dvlopmn -ISSN: 2278-67X, p-issn: 2278-8X, www.ijrd.com Volum, Issu 6 (Jun 24), PP.26-4 Biosorpion of Azur Dy wih Sunflowr Sd Hull: Esimaion of Equilibrium, Thrmodynamic
Inrnaional Journal of Enginring Rsarch and Dvlopmn -ISSN: 2278-67X, p-issn: 2278-8X, www.ijrd.com Volum, Issu 6 (Jun 24), PP.26-4 Biosorpion of Azur Dy wih Sunflowr Sd Hull: Esimaion of Equilibrium, Thrmodynamic
Received 28 May, 2018; Accepted 6 June, 2018.
 Vol. (8), pp. xxx-xxx, Augus 8 DOI:.5897/AJEST8.xxxx Aricl Numbr: xxxxx ISSN: 996-786 Copyrigh 8 Auhor(s) rain h copyrigh of his aricl hp://www.acadmicjournals.org/ajest African Journal of Environmnal
Vol. (8), pp. xxx-xxx, Augus 8 DOI:.5897/AJEST8.xxxx Aricl Numbr: xxxxx ISSN: 996-786 Copyrigh 8 Auhor(s) rain h copyrigh of his aricl hp://www.acadmicjournals.org/ajest African Journal of Environmnal
General Article Application of differential equation in L-R and C-R circuit analysis by classical method. Abstract
 Applicaion of Diffrnial... Gnral Aricl Applicaion of diffrnial uaion in - and C- circui analysis by classical mhod. ajndra Prasad gmi curr, Dparmn of Mahmaics, P.N. Campus, Pokhara Email: rajndraprasadrgmi@yahoo.com
Applicaion of Diffrnial... Gnral Aricl Applicaion of diffrnial uaion in - and C- circui analysis by classical mhod. ajndra Prasad gmi curr, Dparmn of Mahmaics, P.N. Campus, Pokhara Email: rajndraprasadrgmi@yahoo.com
Microscopic Flow Characteristics Time Headway - Distribution
 CE57: Traffic Flow Thory Spring 20 Wk 2 Modling Hadway Disribuion Microscopic Flow Characrisics Tim Hadway - Disribuion Tim Hadway Dfiniion Tim Hadway vrsus Gap Ahmd Abdl-Rahim Civil Enginring Dparmn,
CE57: Traffic Flow Thory Spring 20 Wk 2 Modling Hadway Disribuion Microscopic Flow Characrisics Tim Hadway - Disribuion Tim Hadway Dfiniion Tim Hadway vrsus Gap Ahmd Abdl-Rahim Civil Enginring Dparmn,
UNSTEADY FLOW OF A FLUID PARTICLE SUSPENSION BETWEEN TWO PARALLEL PLATES SUDDENLY SET IN MOTION WITH SAME SPEED
 006-0 Asian Rsarch Publishing work (ARP). All righs rsrvd. USTEADY FLOW OF A FLUID PARTICLE SUSPESIO BETWEE TWO PARALLEL PLATES SUDDELY SET I MOTIO WITH SAME SPEED M. suniha, B. Shankr and G. Shanha 3
006-0 Asian Rsarch Publishing work (ARP). All righs rsrvd. USTEADY FLOW OF A FLUID PARTICLE SUSPESIO BETWEE TWO PARALLEL PLATES SUDDELY SET I MOTIO WITH SAME SPEED M. suniha, B. Shankr and G. Shanha 3
Use of Surface Modified Silica gel factory Waste for Removal of 2,4-D Pesticide from Agricultural Wastewater: A case study
 In. J. Environ. Rs., 6(4):995-006, Auumn 202 ISSN: 735-6865 Us of Surfac Modifid Silica gl facory Was for Rmoval of 2,4-D Psicid from Agriculural Waswar: A cas sudy Konr, S., Pal, A. 2 and Adak, A. 3*
In. J. Environ. Rs., 6(4):995-006, Auumn 202 ISSN: 735-6865 Us of Surfac Modifid Silica gl facory Was for Rmoval of 2,4-D Psicid from Agriculural Waswar: A cas sudy Konr, S., Pal, A. 2 and Adak, A. 3*
The transition:transversion rate ratio vs. the T-ratio.
 PhyloMah Lcur 8 by Dan Vandrpool March, 00 opics of Discussion ransiion:ransvrsion ra raio Kappa vs. ransiion:ransvrsion raio raio alculaing h xpcd numbr of subsiuions using marix algbra Why h nral im
PhyloMah Lcur 8 by Dan Vandrpool March, 00 opics of Discussion ransiion:ransvrsion ra raio Kappa vs. ransiion:ransvrsion raio raio alculaing h xpcd numbr of subsiuions using marix algbra Why h nral im
Applied Statistics and Probability for Engineers, 6 th edition October 17, 2016
 Applid Saisics and robabiliy for Enginrs, 6 h diion Ocobr 7, 6 CHATER Scion - -. a d. 679.. b. d. 88 c d d d. 987 d. 98 f d.. Thn, = ln. =. g d.. Thn, = ln.9 =.. -7. a., by symmry. b.. d...6. 7.. c...
Applid Saisics and robabiliy for Enginrs, 6 h diion Ocobr 7, 6 CHATER Scion - -. a d. 679.. b. d. 88 c d d d. 987 d. 98 f d.. Thn, = ln. =. g d.. Thn, = ln.9 =.. -7. a., by symmry. b.. d...6. 7.. c...
EXERCISE - 01 CHECK YOUR GRASP
 DIFFERENTIAL EQUATION EXERCISE - CHECK YOUR GRASP 7. m hn D() m m, D () m m. hn givn D () m m D D D + m m m m m m + m m m m + ( m ) (m ) (m ) (m + ) m,, Hnc numbr of valus of mn will b. n ( ) + c sinc
DIFFERENTIAL EQUATION EXERCISE - CHECK YOUR GRASP 7. m hn D() m m, D () m m. hn givn D () m m D D D + m m m m m m + m m m m + ( m ) (m ) (m ) (m + ) m,, Hnc numbr of valus of mn will b. n ( ) + c sinc
+Modelling the Sorption of Zn 2+ Ions onto Luffa cylindrica
 Amrican Journal of Scinc and Tchnology 218; 5(1): 1-13 hp://www.aasci.org/journal/ajs ISSN: 2375-3846 +Modlling h Sorpion of Zn 2+ Ions ono Luffa cylindrica Oboh Innocn O. 1, Aluyor Emmanul O. 2 1 Dparmn
Amrican Journal of Scinc and Tchnology 218; 5(1): 1-13 hp://www.aasci.org/journal/ajs ISSN: 2375-3846 +Modlling h Sorpion of Zn 2+ Ions ono Luffa cylindrica Oboh Innocn O. 1, Aluyor Emmanul O. 2 1 Dparmn
5. An object moving along an x-coordinate axis with its scale measured in meters has a velocity of 6t
 AP CALCULUS FINAL UNIT WORKSHEETS ACCELERATION, VELOCTIY AND POSITION In problms -, drmin h posiion funcion, (), from h givn informaion.. v (), () = 5. v ()5, () = b g. a (), v() =, () = -. a (), v() =
AP CALCULUS FINAL UNIT WORKSHEETS ACCELERATION, VELOCTIY AND POSITION In problms -, drmin h posiion funcion, (), from h givn informaion.. v (), () = 5. v ()5, () = b g. a (), v() =, () = -. a (), v() =
On the Derivatives of Bessel and Modified Bessel Functions with Respect to the Order and the Argument
 Inrnaional Rsarch Journal of Applid Basic Scincs 03 Aailabl onlin a wwwirjabscom ISSN 5-838X / Vol 4 (): 47-433 Scinc Eplorr Publicaions On h Driais of Bssl Modifid Bssl Funcions wih Rspc o h Ordr h Argumn
Inrnaional Rsarch Journal of Applid Basic Scincs 03 Aailabl onlin a wwwirjabscom ISSN 5-838X / Vol 4 (): 47-433 Scinc Eplorr Publicaions On h Driais of Bssl Modifid Bssl Funcions wih Rspc o h Ordr h Argumn
Comparison the New Kinetics Equation of Noncompetitive Sorption Cd(II) and Zn(II) onto Green Sorbent Horse Dung Humic Acid (HD-HA)
 Availabl onlin a BCREC Wbsi: hps://bcrc.undip.ac.id Bullin of Chmical Racion Enginring & Caalysis, 13 (3) 2018, 475-488 Rsarch Aricl Comparison h Nw Kinics Equaion of Noncompiiv Sorpion Cd(II) and Zn(II)
Availabl onlin a BCREC Wbsi: hps://bcrc.undip.ac.id Bullin of Chmical Racion Enginring & Caalysis, 13 (3) 2018, 475-488 Rsarch Aricl Comparison h Nw Kinics Equaion of Noncompiiv Sorpion Cd(II) and Zn(II)
Modelling the Effect of Dosage on the Biosorption of Ni 2+ Ions onto Luffa Cylindrica
 Journal of Marials Scincs and Applicaions 2018; 4(1): 1-9 hp://www.aasci.org/journal/jmsa ISSN: 2381-0998 (Prin); ISSN: 2381-1005 (Onlin) Modlling h Effc of Dosag on h Biosorpion of Ni 2+ Ions ono Luffa
Journal of Marials Scincs and Applicaions 2018; 4(1): 1-9 hp://www.aasci.org/journal/jmsa ISSN: 2381-0998 (Prin); ISSN: 2381-1005 (Onlin) Modlling h Effc of Dosag on h Biosorpion of Ni 2+ Ions ono Luffa
EE 434 Lecture 22. Bipolar Device Models
 EE 434 Lcur 22 Bipolar Dvic Modls Quiz 14 Th collcor currn of a BJT was masurd o b 20mA and h bas currn masurd o b 0.1mA. Wha is h fficincy of injcion of lcrons coming from h mir o h collcor? 1 And h numbr
EE 434 Lcur 22 Bipolar Dvic Modls Quiz 14 Th collcor currn of a BJT was masurd o b 20mA and h bas currn masurd o b 0.1mA. Wha is h fficincy of injcion of lcrons coming from h mir o h collcor? 1 And h numbr
AMMONIUM ADSORPTION ON NATURAL ZEOLITE (CLINOPTILOLITE): ADSORPTION ISOTHERMS AND KINETICS MODELING
 ISSN 1848-0071 UDC 549.67+544.723=111 Rcivd: 2012-06-15 Accpd: 2012-07-12 Original scinific papr AMMONIUM ADSORPTION ON NATURAL ZEOLITE (CLINOPTILOLITE): ADSORPTION ISOTHERMS AND KINETICS MODELING DAJANA
ISSN 1848-0071 UDC 549.67+544.723=111 Rcivd: 2012-06-15 Accpd: 2012-07-12 Original scinific papr AMMONIUM ADSORPTION ON NATURAL ZEOLITE (CLINOPTILOLITE): ADSORPTION ISOTHERMS AND KINETICS MODELING DAJANA
AR(1) Process. The first-order autoregressive process, AR(1) is. where e t is WN(0, σ 2 )
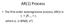 AR() Procss Th firs-ordr auorgrssiv procss, AR() is whr is WN(0, σ ) Condiional Man and Varianc of AR() Condiional man: Condiional varianc: ) ( ) ( Ω Ω E E ) var( ) ) ( var( ) var( σ Ω Ω Ω Ω E Auocovarianc
AR() Procss Th firs-ordr auorgrssiv procss, AR() is whr is WN(0, σ ) Condiional Man and Varianc of AR() Condiional man: Condiional varianc: ) ( ) ( Ω Ω E E ) var( ) ) ( var( ) var( σ Ω Ω Ω Ω E Auocovarianc
A THREE COMPARTMENT MATHEMATICAL MODEL OF LIVER
 A THREE COPARTENT ATHEATICAL ODEL OF LIVER V. An N. Ch. Paabhi Ramacharyulu Faculy of ahmaics, R D collgs, Hanamonda, Warangal, India Dparmn of ahmaics, Naional Insiu of Tchnology, Warangal, India E-ail:
A THREE COPARTENT ATHEATICAL ODEL OF LIVER V. An N. Ch. Paabhi Ramacharyulu Faculy of ahmaics, R D collgs, Hanamonda, Warangal, India Dparmn of ahmaics, Naional Insiu of Tchnology, Warangal, India E-ail:
Batch Biosorption of Metanil Yellow from Aqueous Solution on Egg Membrane: Kinetics and Mechanism
 Inrnaional Journal of Chmical, Marial and Environmnal Rsarch 018, 5 (): 155-164 Rsarch aricl INTERNATIONAL JOURNAL OF CHEMICAL, MATERIAL AND ENVIRONMENTAL RESEARCH An Inrnaional Opn Fr Accss, Pr Rviwd
Inrnaional Journal of Chmical, Marial and Environmnal Rsarch 018, 5 (): 155-164 Rsarch aricl INTERNATIONAL JOURNAL OF CHEMICAL, MATERIAL AND ENVIRONMENTAL RESEARCH An Inrnaional Opn Fr Accss, Pr Rviwd
7.4 QUANTUM MECHANICAL TREATMENT OF FLUCTUATIONS *
 Andri Tokmakoff, MIT Dparmn of Chmisry, 5/19/5 7-11 7.4 QUANTUM MECANICAL TREATMENT OF FLUCTUATIONS * Inroducion and Prviw Now h origin of frquncy flucuaions is inracions of our molcul (or mor approprialy
Andri Tokmakoff, MIT Dparmn of Chmisry, 5/19/5 7-11 7.4 QUANTUM MECANICAL TREATMENT OF FLUCTUATIONS * Inroducion and Prviw Now h origin of frquncy flucuaions is inracions of our molcul (or mor approprialy
4.3 Design of Sections for Flexure (Part II)
 Prsrssd Concr Srucurs Dr. Amlan K Sngupa and Prof. Dvdas Mnon 4. Dsign of Scions for Flxur (Par II) This scion covrs h following opics Final Dsign for Typ Mmrs Th sps for Typ 1 mmrs ar xplaind in Scion
Prsrssd Concr Srucurs Dr. Amlan K Sngupa and Prof. Dvdas Mnon 4. Dsign of Scions for Flxur (Par II) This scion covrs h following opics Final Dsign for Typ Mmrs Th sps for Typ 1 mmrs ar xplaind in Scion
Decline Curves. Exponential decline (constant fractional decline) Harmonic decline, and Hyperbolic decline.
 Dlin Curvs Dlin Curvs ha lo flow ra vs. im ar h mos ommon ools for forasing roduion and monioring wll rforman in h fild. Ths urvs uikly show by grahi mans whih wlls or filds ar roduing as xd or undr roduing.
Dlin Curvs Dlin Curvs ha lo flow ra vs. im ar h mos ommon ools for forasing roduion and monioring wll rforman in h fild. Ths urvs uikly show by grahi mans whih wlls or filds ar roduing as xd or undr roduing.
whereby we can express the phase by any one of the formulas cos ( 3 whereby we can express the phase by any one of the formulas
 Third In-Class Exam Soluions Mah 6, Profssor David Lvrmor Tusday, 5 April 07 [0] Th vrical displacmn of an unforcd mass on a spring is givn by h 5 3 cos 3 sin a [] Is his sysm undampd, undr dampd, criically
Third In-Class Exam Soluions Mah 6, Profssor David Lvrmor Tusday, 5 April 07 [0] Th vrical displacmn of an unforcd mass on a spring is givn by h 5 3 cos 3 sin a [] Is his sysm undampd, undr dampd, criically
CHAPTER CHAPTER14. Expectations: The Basic Tools. Prepared by: Fernando Quijano and Yvonn Quijano
 Expcaions: Th Basic Prpard by: Frnando Quijano and Yvonn Quijano CHAPTER CHAPTER14 2006 Prnic Hall Businss Publishing Macroconomics, 4/ Olivir Blanchard 14-1 Today s Lcur Chapr 14:Expcaions: Th Basic Th
Expcaions: Th Basic Prpard by: Frnando Quijano and Yvonn Quijano CHAPTER CHAPTER14 2006 Prnic Hall Businss Publishing Macroconomics, 4/ Olivir Blanchard 14-1 Today s Lcur Chapr 14:Expcaions: Th Basic Th
An Indian Journal FULL PAPER. Trade Science Inc. A stage-structured model of a single-species with density-dependent and birth pulses ABSTRACT
 [Typ x] [Typ x] [Typ x] ISSN : 974-7435 Volum 1 Issu 24 BioTchnology 214 An Indian Journal FULL PAPE BTAIJ, 1(24), 214 [15197-1521] A sag-srucurd modl of a singl-spcis wih dnsiy-dpndn and birh pulss LI
[Typ x] [Typ x] [Typ x] ISSN : 974-7435 Volum 1 Issu 24 BioTchnology 214 An Indian Journal FULL PAPE BTAIJ, 1(24), 214 [15197-1521] A sag-srucurd modl of a singl-spcis wih dnsiy-dpndn and birh pulss LI
CPSC 211 Data Structures & Implementations (c) Texas A&M University [ 259] B-Trees
![CPSC 211 Data Structures & Implementations (c) Texas A&M University [ 259] B-Trees CPSC 211 Data Structures & Implementations (c) Texas A&M University [ 259] B-Trees](/thumbs/74/70718364.jpg) CPSC 211 Daa Srucurs & Implmnaions (c) Txas A&M Univrsiy [ 259] B-Trs Th AVL r and rd-black r allowd som variaion in h lnghs of h diffrn roo-o-laf pahs. An alrnaiv ida is o mak sur ha all roo-o-laf pahs
CPSC 211 Daa Srucurs & Implmnaions (c) Txas A&M Univrsiy [ 259] B-Trs Th AVL r and rd-black r allowd som variaion in h lnghs of h diffrn roo-o-laf pahs. An alrnaiv ida is o mak sur ha all roo-o-laf pahs
Bioreduction of Thallium and Cadmium Toxicity from Industrial Wastewater using Microalgae
 A publicaion of CHEMICAL ENGINEERING TRANSACTIONS VOL. 57, 7 Gus Ediors: Sauro Pirucci, Jiří Jaromír Klmš, Laura Piazza, Srafim Bakalis Copyrigh 7, AIDIC Srvizi S.r.l. ISBN 978-88-9568-48-8; ISSN 83-96
A publicaion of CHEMICAL ENGINEERING TRANSACTIONS VOL. 57, 7 Gus Ediors: Sauro Pirucci, Jiří Jaromír Klmš, Laura Piazza, Srafim Bakalis Copyrigh 7, AIDIC Srvizi S.r.l. ISBN 978-88-9568-48-8; ISSN 83-96
Lecture 1: Growth and decay of current in RL circuit. Growth of current in LR Circuit. D.K.Pandey
 cur : Growh and dcay of currn in circui Growh of currn in Circui us considr an inducor of slf inducanc is conncd o a DC sourc of.m.f. E hrough a rsisr of rsisanc and a ky K in sris. Whn h ky K is swichd
cur : Growh and dcay of currn in circui Growh of currn in Circui us considr an inducor of slf inducanc is conncd o a DC sourc of.m.f. E hrough a rsisr of rsisanc and a ky K in sris. Whn h ky K is swichd
Chemistry and Materials Research ISSN (Print) ISSN (Online) Vol.7 No.5, 2015
 Chmisry and Marials Rsarch ISSN 4-34 (Prin) ISSN 5-956 (Onlin) Vol.7 No.5, 5 www.iis.org Rmoval o Phnol rom Pain Waswar by Adsorpion ono Phosphoric Acid Acivad Carbon Producd rom Coconu Shll: Isohrmal
Chmisry and Marials Rsarch ISSN 4-34 (Prin) ISSN 5-956 (Onlin) Vol.7 No.5, 5 www.iis.org Rmoval o Phnol rom Pain Waswar by Adsorpion ono Phosphoric Acid Acivad Carbon Producd rom Coconu Shll: Isohrmal
Equilibrium sorption of divalent metal ions onto groundnut (Arachis hypogaea) shell: kinetics, isotherm and thermodynamics
 ISSN: 2410-9649 Babarind Chmisry and Onyiaocha Inrnaional / Chmisry 2(1) (2016) Inrnaional 37-46 2(1) (2016) 37-46 iscinic.org. Equilibrium sorpion of divaln mal ions ono groundnu (Arachis hypogaa) shll:
ISSN: 2410-9649 Babarind Chmisry and Onyiaocha Inrnaional / Chmisry 2(1) (2016) Inrnaional 37-46 2(1) (2016) 37-46 iscinic.org. Equilibrium sorpion of divaln mal ions ono groundnu (Arachis hypogaa) shll:
On the Speed of Heat Wave. Mihály Makai
 On h Spd of Ha Wa Mihály Maai maai@ra.bm.hu Conns Formulaion of h problm: infini spd? Local hrmal qulibrium (LTE hypohsis Balanc quaion Phnomnological balanc Spd of ha wa Applicaion in plasma ranspor 1.
On h Spd of Ha Wa Mihály Maai maai@ra.bm.hu Conns Formulaion of h problm: infini spd? Local hrmal qulibrium (LTE hypohsis Balanc quaion Phnomnological balanc Spd of ha wa Applicaion in plasma ranspor 1.
Accepted 31 January, 2012
 Inrnaional Journal of Physical Scincs Vol. 7(9), pp. 376-385, 23 Fbruary, 202 Availabl onlin a hp://www.acadmicjournals.org/ijps DOI: 0.5897/IJPS.597 ISSN 992-950 202 Acadmic Journals Full Lngh Rsarch
Inrnaional Journal of Physical Scincs Vol. 7(9), pp. 376-385, 23 Fbruary, 202 Availabl onlin a hp://www.acadmicjournals.org/ijps DOI: 0.5897/IJPS.597 ISSN 992-950 202 Acadmic Journals Full Lngh Rsarch
ERROR ANALYSIS A.J. Pintar and D. Caspary Department of Chemical Engineering Michigan Technological University Houghton, MI September, 2012
 ERROR AALYSIS AJ Pinar and D Caspary Dparmn of Chmical Enginring Michigan Tchnological Univrsiy Houghon, MI 4993 Spmbr, 0 OVERVIEW Exprimnaion involvs h masurmn of raw daa in h laboraory or fild I is assumd
ERROR AALYSIS AJ Pinar and D Caspary Dparmn of Chmical Enginring Michigan Tchnological Univrsiy Houghon, MI 4993 Spmbr, 0 OVERVIEW Exprimnaion involvs h masurmn of raw daa in h laboraory or fild I is assumd
DEPARTMENT OF ELECTRICAL &ELECTRONICS ENGINEERING SIGNALS AND SYSTEMS. Assoc. Prof. Dr. Burak Kelleci. Spring 2018
 DEPARTMENT OF ELECTRICAL &ELECTRONICS ENGINEERING SIGNALS AND SYSTEMS Aoc. Prof. Dr. Burak Kllci Spring 08 OUTLINE Th Laplac Tranform Rgion of convrgnc for Laplac ranform Invr Laplac ranform Gomric valuaion
DEPARTMENT OF ELECTRICAL &ELECTRONICS ENGINEERING SIGNALS AND SYSTEMS Aoc. Prof. Dr. Burak Kllci Spring 08 OUTLINE Th Laplac Tranform Rgion of convrgnc for Laplac ranform Invr Laplac ranform Gomric valuaion
Experimental and Computer Aided Study of Anisotropic Behavior of Material to Reduce the Metal Forming Defects
 ISSN 2395-1621 Exprimnal and Compur Aidd Sudy of Anisoropic Bhavior of Marial o Rduc h Mal Forming Dfcs #1 Tausif N. Momin, #2 Vishal B.Bhagwa 1 ausifnmomin@gmail.com 2 bhagwavb@gmail.com #12 Mchanical
ISSN 2395-1621 Exprimnal and Compur Aidd Sudy of Anisoropic Bhavior of Marial o Rduc h Mal Forming Dfcs #1 Tausif N. Momin, #2 Vishal B.Bhagwa 1 ausifnmomin@gmail.com 2 bhagwavb@gmail.com #12 Mchanical
Lecture 1: Numerical Integration The Trapezoidal and Simpson s Rule
 Lcur : Numrical ngraion Th Trapzoidal and Simpson s Rul A problm Th probabiliy of a normally disribud (man µ and sandard dviaion σ ) vn occurring bwn h valus a and b is B A P( a x b) d () π whr a µ b -
Lcur : Numrical ngraion Th Trapzoidal and Simpson s Rul A problm Th probabiliy of a normally disribud (man µ and sandard dviaion σ ) vn occurring bwn h valus a and b is B A P( a x b) d () π whr a µ b -
3.9 Carbon Contamination & Fractionation
 3.9 arbon onaminaion & Fracionaion Bcaus h raio / in a sampl dcrass wih incrasing ag - du o h coninuous dcay of - a small addd impuriy of modrn naural carbon causs a disproporionaly larg shif in ag. (
3.9 arbon onaminaion & Fracionaion Bcaus h raio / in a sampl dcrass wih incrasing ag - du o h coninuous dcay of - a small addd impuriy of modrn naural carbon causs a disproporionaly larg shif in ag. (
Economics 302 (Sec. 001) Intermediate Macroeconomic Theory and Policy (Spring 2011) 3/28/2012. UW Madison
 Economics 302 (Sc. 001) Inrmdia Macroconomic Thory and Policy (Spring 2011) 3/28/2012 Insrucor: Prof. Mnzi Chinn Insrucor: Prof. Mnzi Chinn UW Madison 16 1 Consumpion Th Vry Forsighd dconsumr A vry forsighd
Economics 302 (Sc. 001) Inrmdia Macroconomic Thory and Policy (Spring 2011) 3/28/2012 Insrucor: Prof. Mnzi Chinn Insrucor: Prof. Mnzi Chinn UW Madison 16 1 Consumpion Th Vry Forsighd dconsumr A vry forsighd
Modelling of three dimensional liquid steel flow in continuous casting process
 AMME 2003 12h Modlling of hr dimnsional liquid sl flow in coninuous casing procss M. Jani, H. Dyja, G. Banasz, S. Brsi Insiu of Modlling and Auomaion of Plasic Woring Procsss, Faculy of Marial procssing
AMME 2003 12h Modlling of hr dimnsional liquid sl flow in coninuous casing procss M. Jani, H. Dyja, G. Banasz, S. Brsi Insiu of Modlling and Auomaion of Plasic Woring Procsss, Faculy of Marial procssing
The Optimal Timing of Transition to New Environmental Technology in Economic Growth
 h Opimal iming of ransiion o Nw Environmnal chnology in Economic Growh h IAEE Europan Confrnc 7- Spmbr, 29 Vinna, Ausria Akira AEDA and akiko NAGAYA yoo Univrsiy Background: Growh and h Environmn Naural
h Opimal iming of ransiion o Nw Environmnal chnology in Economic Growh h IAEE Europan Confrnc 7- Spmbr, 29 Vinna, Ausria Akira AEDA and akiko NAGAYA yoo Univrsiy Background: Growh and h Environmn Naural
Phosphonate-Functionalized Polystyrene Microspheres with Controlled Zeta Potential for Efficient Uranium Sorption
 Elctronic Supplmntary Matrial (ESI) for RSC Advancs. This journal is Th Royal Socity of Chmistry 2016 Supporting Information Phosphonat-Functionalizd Polystyrn Microsphrs with Controlld Zta Potntial for
Elctronic Supplmntary Matrial (ESI) for RSC Advancs. This journal is Th Royal Socity of Chmistry 2016 Supporting Information Phosphonat-Functionalizd Polystyrn Microsphrs with Controlld Zta Potntial for
ROLE OF SAWDUST IN THE REMOVAL OF IRON FROM AQUEOUS SOLUTION
 AJSTD Vol. 23 Issu 3 pp. 223-229 (2006) ROLE OF SAWDUST IN THE REMOVAL OF IRON FROM AQUEOUS SOLUTION H.B. Snin *, O. Subhi, R. Rosliza, N. Kancono, M.S. Azhar, S. Hasiah, and W.B. Wan Nik Faculty of Scinc
AJSTD Vol. 23 Issu 3 pp. 223-229 (2006) ROLE OF SAWDUST IN THE REMOVAL OF IRON FROM AQUEOUS SOLUTION H.B. Snin *, O. Subhi, R. Rosliza, N. Kancono, M.S. Azhar, S. Hasiah, and W.B. Wan Nik Faculty of Scinc
Lagrangian for RLC circuits using analogy with the classical mechanics concepts
 Lagrangian for RLC circuis using analogy wih h classical mchanics concps Albrus Hariwangsa Panuluh and Asan Damanik Dparmn of Physics Educaion, Sanaa Dharma Univrsiy Kampus III USD Paingan, Maguwoharjo,
Lagrangian for RLC circuis using analogy wih h classical mchanics concps Albrus Hariwangsa Panuluh and Asan Damanik Dparmn of Physics Educaion, Sanaa Dharma Univrsiy Kampus III USD Paingan, Maguwoharjo,
Logistic equation of Human population growth (generalization to the case of reactive environment).
 Logisic quaion of Human populaion growh gnralizaion o h cas of raciv nvironmn. Srg V. Ershkov Insiu for Tim aur Exploraions M.V. Lomonosov's Moscow Sa Univrsi Lninski gor - Moscow 999 ussia -mail: srgj-rshkov@andx.ru
Logisic quaion of Human populaion growh gnralizaion o h cas of raciv nvironmn. Srg V. Ershkov Insiu for Tim aur Exploraions M.V. Lomonosov's Moscow Sa Univrsi Lninski gor - Moscow 999 ussia -mail: srgj-rshkov@andx.ru
Wave Equation (2 Week)
 Rfrnc Wav quaion ( Wk 6.5 Tim-armonic filds 7. Ovrviw 7. Plan Wavs in Losslss Mdia 7.3 Plan Wavs in Loss Mdia 7.5 Flow of lcromagnic Powr and h Poning Vcor 7.6 Normal Incidnc of Plan Wavs a Plan Boundaris
Rfrnc Wav quaion ( Wk 6.5 Tim-armonic filds 7. Ovrviw 7. Plan Wavs in Losslss Mdia 7.3 Plan Wavs in Loss Mdia 7.5 Flow of lcromagnic Powr and h Poning Vcor 7.6 Normal Incidnc of Plan Wavs a Plan Boundaris
Impulsive Differential Equations. by using the Euler Method
 Applid Mahmaical Scincs Vol. 4 1 no. 65 19 - Impulsiv Diffrnial Equaions by using h Eulr Mhod Nor Shamsidah B Amir Hamzah 1 Musafa bin Mama J. Kaviumar L Siaw Chong 4 and Noor ani B Ahmad 5 1 5 Dparmn
Applid Mahmaical Scincs Vol. 4 1 no. 65 19 - Impulsiv Diffrnial Equaions by using h Eulr Mhod Nor Shamsidah B Amir Hamzah 1 Musafa bin Mama J. Kaviumar L Siaw Chong 4 and Noor ani B Ahmad 5 1 5 Dparmn
Mundell-Fleming I: Setup
 Mundll-Flming I: Sup In ISLM, w had: E ( ) T I( i π G T C Y ) To his, w now add n xpors, which is a funcion of h xchang ra: ε E P* P ( T ) I( i π ) G T NX ( ) C Y Whr NX is assumd (Marshall Lrnr condiion)
Mundll-Flming I: Sup In ISLM, w had: E ( ) T I( i π G T C Y ) To his, w now add n xpors, which is a funcion of h xchang ra: ε E P* P ( T ) I( i π ) G T NX ( ) C Y Whr NX is assumd (Marshall Lrnr condiion)
Adsorción de fenobarbital en carbones activados comerciales. Equilibrio cinético e isotermo de adsorción
 Rvisa CENIC Cincias Químicas, Vol. 45, pp. 17-183, 014. Adsorción d fnobarbial n carbons acivados comrcials. Euilibrio cinéico isormo d adsorción Carlos Albro Ry-Mafull, Julio Csar-Llopiz, Albro Iglsias-Crvo,
Rvisa CENIC Cincias Químicas, Vol. 45, pp. 17-183, 014. Adsorción d fnobarbial n carbons acivados comrcials. Euilibrio cinéico isormo d adsorción Carlos Albro Ry-Mafull, Julio Csar-Llopiz, Albro Iglsias-Crvo,
Elementary Differential Equations and Boundary Value Problems
 Elmnar Diffrnial Equaions and Boundar Valu Problms Boc. & DiPrima 9 h Ediion Chapr : Firs Ordr Diffrnial Equaions 00600 คณ ตศาสตร ว ศวกรรม สาขาว ชาว ศวกรรมคอมพ วเตอร ป การศ กษา /55 ผศ.ดร.อร ญญา ผศ.ดร.สมศ
Elmnar Diffrnial Equaions and Boundar Valu Problms Boc. & DiPrima 9 h Ediion Chapr : Firs Ordr Diffrnial Equaions 00600 คณ ตศาสตร ว ศวกรรม สาขาว ชาว ศวกรรมคอมพ วเตอร ป การศ กษา /55 ผศ.ดร.อร ญญา ผศ.ดร.สมศ
Let s look again at the first order linear differential equation we are attempting to solve, in its standard form:
 Th Ingraing Facor Mhod In h prvious xampls of simpl firs ordr ODEs, w found h soluions by algbraically spara h dpndn variabl- and h indpndn variabl- rms, and wri h wo sids of a givn quaion as drivaivs,
Th Ingraing Facor Mhod In h prvious xampls of simpl firs ordr ODEs, w found h soluions by algbraically spara h dpndn variabl- and h indpndn variabl- rms, and wri h wo sids of a givn quaion as drivaivs,
I) Title: Rational Expectations and Adaptive Learning. II) Contents: Introduction to Adaptive Learning
 I) Til: Raional Expcaions and Adapiv Larning II) Conns: Inroducion o Adapiv Larning III) Documnaion: - Basdvan, Olivir. (2003). Larning procss and raional xpcaions: an analysis using a small macroconomic
I) Til: Raional Expcaions and Adapiv Larning II) Conns: Inroducion o Adapiv Larning III) Documnaion: - Basdvan, Olivir. (2003). Larning procss and raional xpcaions: an analysis using a small macroconomic
Principles of Humidity Dalton s law
 Principls of Humidity Dalton s law Air is a mixtur of diffrnt gass. Th main gas componnts ar: Gas componnt volum [%] wight [%] Nitrogn N 2 78,03 75,47 Oxygn O 2 20,99 23,20 Argon Ar 0,93 1,28 Carbon dioxid
Principls of Humidity Dalton s law Air is a mixtur of diffrnt gass. Th main gas componnts ar: Gas componnt volum [%] wight [%] Nitrogn N 2 78,03 75,47 Oxygn O 2 20,99 23,20 Argon Ar 0,93 1,28 Carbon dioxid
A batch study of adsorption equilibrium and kinetic for methylene blue onto synthesized zeolite
 Journal of marials and Environmnal Scincs ISSN : 8-58 Copyrigh 7, Univrsiy of Mohammd Prmir Oujda Morocco JMES, 7 Volum 8, Issu, Pag 539-55 hp://www.jmarnvironsci.com/ A bach sudy of adsorpion uilibrium
Journal of marials and Environmnal Scincs ISSN : 8-58 Copyrigh 7, Univrsiy of Mohammd Prmir Oujda Morocco JMES, 7 Volum 8, Issu, Pag 539-55 hp://www.jmarnvironsci.com/ A bach sudy of adsorpion uilibrium
Modeling and Experimental Investigation on the Internal Leakage in a CO2 Rotary Vane Expander
 urdu Univrsiy urdu -ubs Inrnaional Comprssor Enginring Confrnc School of chanical Enginring 2008 odling and Exprimnal Invsigaion on h Inrnal Lakag in a CO2 Roary Van Expandr Bingchun Yang Xi an Jiaoong
urdu Univrsiy urdu -ubs Inrnaional Comprssor Enginring Confrnc School of chanical Enginring 2008 odling and Exprimnal Invsigaion on h Inrnal Lakag in a CO2 Roary Van Expandr Bingchun Yang Xi an Jiaoong
H is equal to the surface current J S
 Chapr 6 Rflcion and Transmission of Wavs 6.1 Boundary Condiions A h boundary of wo diffrn mdium, lcromagnic fild hav o saisfy physical condiion, which is drmind by Maxwll s quaion. This is h boundary condiion
Chapr 6 Rflcion and Transmission of Wavs 6.1 Boundary Condiions A h boundary of wo diffrn mdium, lcromagnic fild hav o saisfy physical condiion, which is drmind by Maxwll s quaion. This is h boundary condiion
Transfer function and the Laplace transformation
 Lab No PH-35 Porland Sa Univriy A. La Roa Tranfr funcion and h Laplac ranformaion. INTRODUTION. THE LAPLAE TRANSFORMATION L 3. TRANSFER FUNTIONS 4. ELETRIAL SYSTEMS Analyi of h hr baic paiv lmn R, and
Lab No PH-35 Porland Sa Univriy A. La Roa Tranfr funcion and h Laplac ranformaion. INTRODUTION. THE LAPLAE TRANSFORMATION L 3. TRANSFER FUNTIONS 4. ELETRIAL SYSTEMS Analyi of h hr baic paiv lmn R, and
Midterm Examination (100 pts)
 Econ 509 Spring 2012 S.L. Parn Midrm Examinaion (100 ps) Par I. 30 poins 1. Dfin h Law of Diminishing Rurns (5 ps.) Incrasing on inpu, call i inpu x, holding all ohr inpus fixd, on vnuall runs ino h siuaion
Econ 509 Spring 2012 S.L. Parn Midrm Examinaion (100 ps) Par I. 30 poins 1. Dfin h Law of Diminishing Rurns (5 ps.) Incrasing on inpu, call i inpu x, holding all ohr inpus fixd, on vnuall runs ino h siuaion
Routing in Delay Tolerant Networks
 Rouing in Dlay Tolran Nworks Primary Rfrnc: S. Jain K. Fall and R. Para Rouing in a Dlay Tolran Nwork SIGCOMM 04 Aug. 30-Sp. 3 2004 Porland Orgon USA Sudn lcur by: Soshan Bali (748214) mail : sbali@ic.ku.du
Rouing in Dlay Tolran Nworks Primary Rfrnc: S. Jain K. Fall and R. Para Rouing in a Dlay Tolran Nwork SIGCOMM 04 Aug. 30-Sp. 3 2004 Porland Orgon USA Sudn lcur by: Soshan Bali (748214) mail : sbali@ic.ku.du
Asymptotic Solutions of Fifth Order Critically Damped Nonlinear Systems with Pair Wise Equal Eigenvalues and another is Distinct
 Qus Journals Journal of Rsarch in Applid Mahmaics Volum ~ Issu (5 pp: -5 ISSN(Onlin : 94-74 ISSN (Prin:94-75 www.usjournals.org Rsarch Papr Asympoic Soluions of Fifh Ordr Criically Dampd Nonlinar Sysms
Qus Journals Journal of Rsarch in Applid Mahmaics Volum ~ Issu (5 pp: -5 ISSN(Onlin : 94-74 ISSN (Prin:94-75 www.usjournals.org Rsarch Papr Asympoic Soluions of Fifh Ordr Criically Dampd Nonlinar Sysms
3(8 ) (8 x x ) 3x x (8 )
 Scion - CHATER -. a d.. b. d.86 c d 8 d d.9997 f g 6. d. d. Thn, = ln. =. =.. d Thn, = ln.9 =.7 8 -. a d.6 6 6 6 6 8 8 8 b 9 d 6 6 6 8 c d.8 6 6 6 6 8 8 7 7 d 6 d.6 6 6 6 6 6 6 8 u u u u du.9 6 6 6 6 6
Scion - CHATER -. a d.. b. d.86 c d 8 d d.9997 f g 6. d. d. Thn, = ln. =. =.. d Thn, = ln.9 =.7 8 -. a d.6 6 6 6 6 8 8 8 b 9 d 6 6 6 8 c d.8 6 6 6 6 8 8 7 7 d 6 d.6 6 6 6 6 6 6 8 u u u u du.9 6 6 6 6 6
14.02 Principles of Macroeconomics Problem Set 5 Fall 2005
 40 Principls of Macroconomics Problm S 5 Fall 005 Posd: Wdnsday, Novmbr 6, 005 Du: Wdnsday, Novmbr 3, 005 Plas wri your nam AND your TA s nam on your problm s Thanks! Exrcis I Tru/Fals? Explain Dpnding
40 Principls of Macroconomics Problm S 5 Fall 005 Posd: Wdnsday, Novmbr 6, 005 Du: Wdnsday, Novmbr 3, 005 Plas wri your nam AND your TA s nam on your problm s Thanks! Exrcis I Tru/Fals? Explain Dpnding
Azimuthal angular correlations between heavy flavour decay electrons and charged hadrons in pp collisions at s = 2.76 TeV in ALICE
 Azimuhal angular corrlaions bwn havy flavour dcay lcrons and chargd hadrons in pp collisions a s = 2.76 TV in ALICE DEEPA THOMAS FOR THE ALICE COLLABORATION INTERNATIONAL SCHOOL OF SUBNUCLEAR PHYSICS ERICE,
Azimuhal angular corrlaions bwn havy flavour dcay lcrons and chargd hadrons in pp collisions a s = 2.76 TV in ALICE DEEPA THOMAS FOR THE ALICE COLLABORATION INTERNATIONAL SCHOOL OF SUBNUCLEAR PHYSICS ERICE,
Boyce/DiPrima 9 th ed, Ch 2.1: Linear Equations; Method of Integrating Factors
 Boc/DiPrima 9 h d, Ch.: Linar Equaions; Mhod of Ingraing Facors Elmnar Diffrnial Equaions and Boundar Valu Problms, 9 h diion, b William E. Boc and Richard C. DiPrima, 009 b John Wil & Sons, Inc. A linar
Boc/DiPrima 9 h d, Ch.: Linar Equaions; Mhod of Ingraing Facors Elmnar Diffrnial Equaions and Boundar Valu Problms, 9 h diion, b William E. Boc and Richard C. DiPrima, 009 b John Wil & Sons, Inc. A linar
Chapter 12 Introduction To The Laplace Transform
 Chapr Inroducion To Th aplac Tranorm Diniion o h aplac Tranorm - Th Sp & Impul uncion aplac Tranorm o pciic uncion 5 Opraional Tranorm Applying h aplac Tranorm 7 Invr Tranorm o Raional uncion 8 Pol and
Chapr Inroducion To Th aplac Tranorm Diniion o h aplac Tranorm - Th Sp & Impul uncion aplac Tranorm o pciic uncion 5 Opraional Tranorm Applying h aplac Tranorm 7 Invr Tranorm o Raional uncion 8 Pol and
CSE 245: Computer Aided Circuit Simulation and Verification
 CSE 45: Compur Aidd Circui Simulaion and Vrificaion Fall 4, Sp 8 Lcur : Dynamic Linar Sysm Oulin Tim Domain Analysis Sa Equaions RLC Nwork Analysis by Taylor Expansion Impuls Rspons in im domain Frquncy
CSE 45: Compur Aidd Circui Simulaion and Vrificaion Fall 4, Sp 8 Lcur : Dynamic Linar Sysm Oulin Tim Domain Analysis Sa Equaions RLC Nwork Analysis by Taylor Expansion Impuls Rspons in im domain Frquncy
1. Inverse Matrix 4[(3 7) (02)] 1[(0 7) (3 2)] Recall that the inverse of A is equal to:
![1. Inverse Matrix 4[(3 7) (02)] 1[(0 7) (3 2)] Recall that the inverse of A is equal to: 1. Inverse Matrix 4[(3 7) (02)] 1[(0 7) (3 2)] Recall that the inverse of A is equal to:](/thumbs/84/89365453.jpg) Rfrncs Brnank, B. and I. Mihov (1998). Masuring monary policy, Quarrly Journal of Economics CXIII, 315-34. Blanchard, O. R. Proi (00). An mpirical characrizaion of h dynamic ffcs of changs in govrnmn spnding
Rfrncs Brnank, B. and I. Mihov (1998). Masuring monary policy, Quarrly Journal of Economics CXIII, 315-34. Blanchard, O. R. Proi (00). An mpirical characrizaion of h dynamic ffcs of changs in govrnmn spnding
Final Exam : Solutions
 Comp : Algorihm and Daa Srucur Final Exam : Soluion. Rcuriv Algorihm. (a) To bgin ind h mdian o {x, x,... x n }. Sinc vry numbr xcp on in h inrval [0, n] appar xacly onc in h li, w hav ha h mdian mu b
Comp : Algorihm and Daa Srucur Final Exam : Soluion. Rcuriv Algorihm. (a) To bgin ind h mdian o {x, x,... x n }. Sinc vry numbr xcp on in h inrval [0, n] appar xacly onc in h li, w hav ha h mdian mu b
Homotopy perturbation technique
 Comput. Mthods Appl. Mch. Engrg. 178 (1999) 257±262 www.lsvir.com/locat/cma Homotopy prturbation tchniqu Ji-Huan H 1 Shanghai Univrsity, Shanghai Institut of Applid Mathmatics and Mchanics, Shanghai 272,
Comput. Mthods Appl. Mch. Engrg. 178 (1999) 257±262 www.lsvir.com/locat/cma Homotopy prturbation tchniqu Ji-Huan H 1 Shanghai Univrsity, Shanghai Institut of Applid Mathmatics and Mchanics, Shanghai 272,
British Journal of Economics, Finance and Management Sciences 64 October 2011, Vol. 2 (1)
 riish Journal of conomics, Financ and Managmn Scincs 64 Ocobr 2011, ol. 2 (1 An mpirical valuaion of Using h Rsidual Incom Modl for rdicion of Sock ric Mhdi Sarikhani Dparmn of Accouning, Safashahr ranch,
riish Journal of conomics, Financ and Managmn Scincs 64 Ocobr 2011, ol. 2 (1 An mpirical valuaion of Using h Rsidual Incom Modl for rdicion of Sock ric Mhdi Sarikhani Dparmn of Accouning, Safashahr ranch,
Double Slits in Space and Time
 Doubl Slis in Sac an Tim Gorg Jons As has bn ror rcnly in h mia, a am l by Grhar Paulus has monsra an inrsing chniqu for ionizing argon aoms by using ulra-shor lasr ulss. Each lasr uls is ffcivly on an
Doubl Slis in Sac an Tim Gorg Jons As has bn ror rcnly in h mia, a am l by Grhar Paulus has monsra an inrsing chniqu for ionizing argon aoms by using ulra-shor lasr ulss. Each lasr uls is ffcivly on an
A HAMILTON-JACOBI TREATMENT OF DISSIPATIVE SYSTEMS
 Europan Scinific Journal Ocobr 13 diion vol9, No3 ISSN: 1857 7881 (Prin) - ISSN 1857-7431 A AMILTON-JACOBI TREATMENT OF DISSIPATIVE SYSTEMS Ola A Jarab'ah Tafila Tchnical Univrsiy, Tafila, Jordan Khald
Europan Scinific Journal Ocobr 13 diion vol9, No3 ISSN: 1857 7881 (Prin) - ISSN 1857-7431 A AMILTON-JACOBI TREATMENT OF DISSIPATIVE SYSTEMS Ola A Jarab'ah Tafila Tchnical Univrsiy, Tafila, Jordan Khald
Computational prediction of high ZT of n-type Mg 3 Sb 2 - based compounds with isotropic thermoelectric conduction performance
 Elcronic Supplnary Marial (ES for Physical Chisry Chical Physics. This journal is h Ownr Sociis 08 Supporing nforaion Copuaional prdicion of high ZT of n-yp Mg 3 Sb - basd copounds wih isoropic hrolcric
Elcronic Supplnary Marial (ES for Physical Chisry Chical Physics. This journal is h Ownr Sociis 08 Supporing nforaion Copuaional prdicion of high ZT of n-yp Mg 3 Sb - basd copounds wih isoropic hrolcric
