Yves J. M. Bollen, Sanne M. Nabuurs, Willem J. H. van Berkel, and Carlo P. M. van Mierlo
|
|
|
- Joanna Hill
- 5 years ago
- Views:
Transcription
1 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 280, No. 9, Issue of March 4, pp , by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Last In, First Out THE ROLE OF COFACTOR BINDING IN FLAVODOXIN FOLDING* Received for publication, November 15, 2004, and in revised form, December 22, 2004 Published, JBC Papers in Press, January 4, 2005, DOI /jbc.M Yves J. M. Bollen, Sanne M. Nabuurs, Willem J. H. van Berkel, and Carlo P. M. van Mierlo From the Department of Agrotechnology and Food Sciences, Laboratory of Biochemistry, Wageningen University, Dreijenlaan 3, NL-6703 HA Wageningen, The Netherlands Although many proteins require the binding of a ligand to be functional, the role of ligand binding during folding is scarcely investigated. Here, we have reported the influence of the flavin mononucleotide (FMN) cofactor on the global stability and folding kinetics of Azotobacter vinelandii holoflavodoxin. Earlier studies have revealed that A. vinelandii apoflavodoxin kinetically folds according to the four-state mechanism: I 1 N unfolded apoflavodoxin N I 2 N native apoflavodoxin. I 1 is an off-pathway molten globule-like intermediate that populates during denaturant-induced equilibrium unfolding; I 2 is a high energy on-pathway folding intermediate that never populates to a significant extent. Here, we have presented extensive denaturant-induced equilibrium unfolding data of holoflavodoxin, holoflavodoxin with excess FMN, and apoflavodoxin as well as kinetic folding and unfolding data of holoflavodoxin. All folding data are excellently described by a five-state mechanism: I 1 FMN N unfolded apoflavodoxin FMN N I 2 FMN N native apoflavodoxin FMN N holoflavodoxin. The last step in flavodoxin folding is thus the binding of FMN to native apoflavodoxin. I 1,I 2, and unfolded apoflavodoxin do not interact to a significant extent with FMN. The autonomous formation of native apoflavodoxin is essential during holoflavodoxin folding. Excess FMN does not accelerate holoflavodoxin folding, and FMN does not act as a nucleation site for folding. The stability of holoflavodoxin is so high that even under strongly denaturing conditions FMN needs to be released first before global unfolding of the protein can occur. Both in vivo and in vitro the folding of a protein is a complex process. Current knowledge about this process largely stems from in vitro studies of small proteins ( 20 kda). The general picture that has emerged is that the topology of the native state is a key factor in determining the rate with which a protein folds (1). In addition, many proteins form one or more metastable intermediates while folding. Some of these intermediates are interpreted to be productive species that accelerate the folding process, whereas others appear to be misfolded conformations (2). The role an intermediate has in kinetic folding also depends to some extent on the experimental conditions used (3). * The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. Present address: Dept. of Structural Biology, Faculty of Earth and Life Sciences, Vrije Universiteit, 1081 HV Amsterdam, The Netherlands. To whom correspondence should be addressed. Tel.: ; Fax: ; carlo.vanmierlo@wur.nl Many proteins require the binding of a non-covalently bound ligand to be functional. The ligand, or cofactor, can vary from a simple metal ion to a large organic molecule. The influences of cofactor binding on the structure of the protein are manifold. For example, apocytochrome c is largely unstructured; it only folds upon incorporation of its heme cofactor (4). In contrast, apoflavodoxin is structurally identical to holoflavodoxin, except for increased dynamics in the region where the flavin cofactor binds (5, 6). The quaternary structure of a protein also can depend on the binding of a cofactor. For example, inactive monomeric apolipoamide dehydrogenase forms active dimers after incorporation of FAD (7). Although many proteins require the binding of a non-covalently bound ligand to be functional, the role of ligand binding during folding is scarcely investigated. In principle, a ligand could bind to an unfolded protein and reduce the conformational freedom of the polypeptide, thereby reducing the conformational space sampled during protein folding. Such ligands might potentially speed up the folding process by acting as a nucleation site. In some cases, ligands indeed remain bound to the unfolded protein (8, 9). However, in other cases, ligands may not interact with non-native protein states and only become incorporated in the protein during the final stages of protein folding. In vivo, chaperones may also play a role in the incorporation of a ligand. As kinetic folding studies of proteins in the presence of their ligands are sparse, the kinetic role of ligand binding during protein folding remains unclear. Flavoproteins offer a good opportunity to study the role of ligand binding during protein folding. The flavin cofactor is generally non-covalently bound and can be reversibly removed (10). Reconstitution of the holoprotein from its constituents can be studied because of changes in spectroscopic properties that accompany flavin binding. Here, the influence of the flavin mononucleotide (FMN) 1 cofactor on the folding of the 179-residue Azotobacter vinelandii flavodoxin was investigated. Flavodoxins are monomeric proteins (Fig. 1) that function as low potential one-electron carriers in many microorganisms. Both the denaturant-induced equilibrium unfolding and the kinetic folding of A. vinelandii apoflavodoxin, i.e. flavodoxin in the absence of its cofactor, have recently been studied in great detail (11 14). Apoflavodoxin is structurally identical to holoflavodoxin except for increased dynamics in the flavin binding region (5, 6). Apoflavodoxin kinetic folding can be described by the model shown in Equation 1 (13) I 1 ^ U ^ I 2 ^ N (Eq. 1) where U and N are unfolded and native apoflavodoxin, respec- 1 The abbreviations used are: FMN, flavin mononucleotide; GuHCl, guanidinium chloride. This paper is available on line at
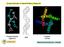
2 The Role of Cofactor Binding in Flavodoxin Folding 7837 prep grade column (Amersham Biosciences) to remove apoflavodoxin molecules in an oligomeric state (13). The apoflavodoxin concentration was determined spectrophotometrically using an extinction coefficient at 280 nm of 29 mm 1 cm 1 (17). Dissociation Constant of the Apoflavodoxin-FMN complex The dissociation constant of the apoflavodoxin-fmn complex was determined using the quenching of FMN fluorescence upon binding to the apoprotein (18). A solution of 1.5 ml containing 210 nm FMN (based on the extinction coefficient at 445 nm of 12.2 mm 1 cm 1 (19)) in 100 mm potassium pyrophosphate, ph 6.0, was titrated with aliquots of 4.1 M apoflavodoxin in the same buffer. After each addition of protein, the system was allowed to equilibrate for 5 min in the dark. Subsequently, the FMN fluorescence intensity was determined using a Cary eclipse fluorimeter equipped with a peltier accessory operating at 25 C (Varian, Palo Alto, CA). Excitation was at 445 nm with a slit of 5 nm; emission was recorded at 525 nm with a slit of 10 nm. The dissociation constant of the apoflavodoxin-fmn complex was determined by fitting the fluorescence emission to Equation 2, a slightly modified equation compared with the one described in Ref. 19, F df end F dc F FIG. 1.Molscript schematic drawing (30) of the x-ray structure of Azotobacter chroococcum flavodoxin (31), the sequence of which is 95% identical to A. vinelandii flavodoxin. The secondary structure depicted is based on the NMR spectroscopic data of A. vinelandii holoflavodoxin (5). The FMN cofactor is shown in dark gray in ball-and-stick representation. tively, and I 1 and I 2 are folding intermediates. During denaturant-induced equilibrium unfolding apoflavodoxin populates the relatively stable folding intermediate I 1, which has molten globule-like characteristics. During kinetic A. vinelandii apoflavodoxin folding intermediate I 1 acts as a trap. Intermediate I 1 is off the direct folding route between unfolded and native apoflavodoxin and has to unfold before native apoflavodoxin can be formed. Some folding apoflavodoxin molecules manage to circumvent this trap and fold via a direct and rapid route to the native state, on which the second folding intermediate I 2 is located. This second intermediate is highly unstable and never populates to a significant extent and is thus not observed during denaturant-induced equilibrium unfolding of apoflavodoxin. All folding apoflavodoxin molecules pass through this high energy intermediate before reaching the native state. In this study we reported on (i) the kinetics of FMN binding to native A. vinelandii apoflavodoxin, (ii) the kinetics of FMN binding during A. vinelandii holoflavodoxin folding, (iii) the release of FMN during A. vinelandii holoflavodoxin unfolding, and (iv) the influence of FMN binding on the global stability of A. vinelandii holoflavodoxin. By doing so, the role of FMN during A. vinelandii flavodoxin folding was deciphered. EXPERIMENTAL PROCEDURES Materials Guanidinium chloride (GuHCl, ultrapure) and potassium pyrophosphate were from Sigma. In holoflavodoxin equilibrium unfolding experiments, FMN purchased from Sigma was used without further purification. In all other experiments FMN obtained during the preparation of apoflavodoxin (see below) and purified by reverse-phase HPLC was used. Protein Expression and Purification Recombinant A. vinelandii C69A holoflavodoxin was obtained and purified as described previously (5, 11). The holoflavodoxin concentration was determined spectrophotometrically using an extinction coefficient of 11.3 mm 1 cm 1 at 452 nm (15). Apoflavodoxin was subsequently prepared by trichloroacetic acid precipitation (11, 16) followed by gel filtration on a Superdex 200 C A K D dc F C A K D dc F 2 4C A dc F 2 (Eq. 2) where F is the observed fluorescence intensity after each addition, d the dilution factor (initial volume/total volume), F end the remaining fluorescence intensity after the titration (resulting from both fluorescence of holoflavodoxin and traces of flavin impurities that are unable to bind to apoflavodoxin), F the difference in molar emission intensity between holoflavodoxin and free FMN, C F the initial concentration of FMN, C A the total protein concentration (i.e. apo holo), and K D the dissociation constant of the apoflavodoxin-fmn complex. The fit resulted in values for C A that indicate an apoflavodoxin stock solution of 4.4 M instead of 4.1 M. As a consequence, an extinction coefficient for A. vinelandii apoflavodoxin at 280 nm of 27 mm 1 cm 1 was used in the rest of this study instead of the value of 29 mm 1 cm 1 reported elsewhere (17). FMN Binding Kinetics Kinetic FMN binding experiments were performed on a BioLogic (Claix, France) SFM-4 stopped-flow apparatus. Solutions were thermostated at 25 C using a circulating water bath. FMN and apoflavodoxin, both in 100 mm potassium pyrophosphate, ph 6.0, were mixed in different ratios. Pseudo-first-order kinetics were obtained by assuring at least a 10-fold excess of apoflavodoxin relative to FMN. The final FMN concentration was 0.10 M in all cases, except for the two highest protein concentrations (i.e. 10 and 12.5 M apoflavodoxin) where an FMN concentration of 1.0 M was used. Binding of FMN to apoflavodoxin was monitored via the accompanying quenching of the FMN fluorescence. The excitation wavelength was 446 nm using an 8-nm slit, and emission was recorded above 475 nm using a cutoff filter. Exponential equations were fitted to the kinetic traces using ProFit (Quantumsoft, Zürich, Switzerland). Denaturant-induced Equilibrium Unfolding of Holoflavodoxin Three GuHCl-induced equilibrium unfolding experiments were done: one with apoflavodoxin, one with holoflavodoxin, and one with holoflavodoxin in the presence of 100 M excess FMN. Unfolding was monitored by circular dichroism (CD) and fluorescence spectroscopy. Before measurements, all samples were equilibrated for at least 12 h at 25 C in the dark. The protein concentration was 4.0 M in all cases. Steady-state far-uv CD measurements were performed on a Jasco J715 spectropolarimeter (Tokyo, Japan) equipped with a PTC-348WI peltier temperature control system. The cell chamber was purged with dry nitrogen gas at a flow rate of 5 liters/min. Calibration was performed with a solution of ammonium d10-camphorsulfonate in nanopure water, the concentration (0.06% w/v) of which was checked spectrophotometrically. GuHCl unfolding samples were measured in a 1-mm quartz cuvette (Starna, Hainault, England) at 222 and 255 nm, and the corresponding signal was averaged over 3 min/wavelength at a temperature of 25 C. The ellipticity at 255 nm was subtracted from the 222 nm ellipticity as a baseline value. Before and after each measurement series, 222 and 255 nm ellipticity values ( ) of a buffer solution (i.e. 100 mm potassium pyrophosphate, ph 6.0) were recorded. The average net buffer ellipticity (i.e ) was subtracted from all sample ellipticities to allow quantitative comparison of ellipticity values that result from different experiments. Tryptophan fluorescence intensity was determined in a 1-ml quartz cuvette (excitation path length 1 cm) using a Cary eclipse fluorimeter equipped with a peltier accessory operating at 25 C (Varian). Excita-
3 7838 The Role of Cofactor Binding in Flavodoxin Folding tion was at 280 nm with a slit of 5 nm; emission was measured for 5 s at 350 nm with a slit of 2.5 nm. In the presence of 100 M excess FMN, fluorescence emission was recorded using a 0.5-ml quartz cuvette that was positioned with its long side (1-cm length) perpendicular to the excitation beam, resulting in an excitation path length of 2 mm. Unfolding of holoflavodoxin in the absence of excess FMN was also followed by recording the FMN fluorescence at 525 nm with a slit of 2.5 nm for a period of 5 s. A four-state model for equilibrium unfolding (holoflavodoxin N native apoflavodoxin FMN N I 1 FMN N unfolded apoflavodoxin FMN; see Results and Discussion ) was fitted globally to the unfolding data of apoflavodoxin, holoflavodoxin, and holoflavodoxin with 100 M excess FMN using ProFit. Each GuHCl-induced equilibrium unfolding curve as observed by fluorescence or CD spectroscopy is described by Equation 3 Y obs j j j D j (Eq. 3) where j is the molar spectroscopic property of species j in a specific unfolding curve in the absence of denaturant, j describes the dependence of the molar spectroscopic property on the concentration denaturant, [D]. The observed spectroscopic signal Y (i.e. fluorescence or CD intensity) is the summation of contributions of all components, j (i.e. holoflavodoxin, native apoflavodoxin, I 1, and unfolded flavodoxin). The fractional population [j] of species j in a specific unfolding experiment and at a specific denaturant concentration is determined by the global fit of the four-state model described above. The spectroscopic properties of a particular protein species in different experiments are identical. However, the spectroscopic properties detected of a particular species differ slightly between different experiments. This effect is due to the concentration of free FMN that differs between the experiments. For example, FMN absorbs light, and as a result the fluorescence emission of unfolded apoflavodoxin in the presence of FMN in an unfolding experiment is slightly less compared with that of an unfolding experiment in which no FMN is present. As a consequence, the spectroscopic parameters were treated as local parameters in the fitting routine (see below for exceptions). To properly weigh each data point in the global fitting procedure, all unfolding curves were recorded with the same number of data points and each data point was weighted according to the corresponding standard error. Native apoflavodoxin hardly populates in holoflavodoxin unfolding experiments. Consequently, in these holoflavodoxin unfolding experiments the spectroscopic properties of native apoflavodoxin (i.e. the and -values in Equation 3) could not be determined. However, the latter spectroscopic parameters could be accurately determined in the apoflavodoxin unfolding experiment. We assumed that these parameters do not depend on the presence of holoflavodoxin or of free FMN. In the global fit of all unfolding data the -values, as well as the -values, of native apoflavodoxin were set to be equal. Their fitted values were mainly determined by the apoflavodoxin unfolding experiment. The denaturant concentration dependence of the spectroscopic properties of a specific species (i.e. the -values in Equation 3) can be determined accurately only when the species populates for 100% over a significant range of denaturant concentrations. However, the equilibrium folding intermediate I 1 does not populate for 100%, and thus the denaturant dependence of its CD and fluorescence intensity (i.e. -values) cannot be determined. Therefore, the -values of I 1 are fixed to 0 (13). Denaturant Dependence of Holoflavodoxin Folding Kinetics Folding kinetics of holoflavodoxin were measured by monitoring the FMN binding to folding apoflavodoxin molecules at 25 C. Unfolded apoflavodoxin in 3.0 M GuHCl and a solution of FMN, both in 100 mm potassium pyrophosphate, ph 6.0, were mixed in a BioLogic SFM-4 stopped-flow machine to a final FMN concentration of 10 M, a final protein concentration of 1.0 M, and GuHCl concentrations ranging between 0.3 and 1.2 M. To determine the fluorescence intensity of unbound FMN in this denaturant range, blank experiments were performed without apoflavodoxin. Binding of FMN to apoflavodoxin was monitored via the accompanying quenching of FMN fluorescence. The excitation wavelength was 446 nm using an 8-nm slit and recording emission above 475 nm using a cutoff filter. Kinetic traces were fitted to exponential equations using ProFit. The influence of Xaa-Pro peptide bond isomerization on the observed holoflavodoxin folding kinetics was examined by repeating the experiment described above with freshly unfolded apoflavodoxin, which has all Xaa-Pro peptide bonds in the native trans conformation. This was achieved by using a double-jump stopped-flow experiment in which native apoflavodoxin was first unfolded for a period of 600 ms in 3.0 M FIG. 2. Determination of the dissociation constant of the A. vinelandii apoflavodoxin-fmn complex using the quenching of FMN fluorescence upon its binding to apoflavodoxin. A 210-nM FMN solution was titrated with aliquots of a 4.1- M apoflavodoxin solution. Equation 2 is fitted to the resulting fluorescence intensity data as described under Experimental Procedures. The dissociation constant is determined to be ( ) M in 100 mm potassium pyrophosphate, ph 6.0, at 25 C. GuHCl. The resulting freshly unfolded apoflavodoxin solution was subsequently immediately mixed with 100 mm potassium pyrophosphate, ph 6.0, containing FMN to final concentrations of 10 M FMN, 1.0 M apoflavodoxin, and 0.5 M GuHCl. Denaturant Dependence of Holoflavodoxin Unfolding Kinetics The denaturant dependence of the unfolding kinetics of holoflavodoxin was measured by mixing holoflavodoxin and GuHCl solutions, both in 100 mm potassium pyrophosphate, ph 6.0, in a BioLogic SFM-4 stoppedflow machine to a final protein concentration of 1.0 M and final GuHCl concentrations that range between 3.5 and 5.7 M. Unfolding of holoflavodoxin was monitored by the release of FMN, which results in a strong increase of the FMN fluorescence intensity. The samples were excited at 446 nm using an 8-nm slit, and emission was recorded above 475 nm using a cutoff filter. RESULTS AND DISCUSSION Dissociation Constant of the A. vinelandii Apoflavodoxin- FMN Complex The FMN dissociation constant of A. vinelandii holoflavodoxin in 100 mm potassium pyrophosphate at ph 6.0 is determined by a procedure in which aliquots of apoflavodoxin are titrated to an FMN solution (see Experimental Procedures ). In this procedure, use is made of the quenching of FMN fluorescence upon its binding to apoflavodoxin (19, 20). The corresponding binding curve is shown in Fig. 2, and the fitted dissociation constant turns out to be ( ) M. This value is identical within error to the reported value of ( ) M for A. vinelandii apoflavodoxin in 50 mm sodium phosphate at ph 7.0 (21). Model Used to Describe Denaturant-induced A. vinelandii Holoflavodoxin Unfolding The GuHCl-induced equilibrium unfolding of A. vinelandii apoflavodoxin is a three-state process and involves the population of an intermediate as shown in Equation 4 Apo ^ I 1 ^ U (Eq. 4) with Apo being native apoflavodoxin, I 1 the folding intermediate with molten globule-like characteristics (11, 13), and U the unfolded protein. The kinetic apoflavodoxin folding intermediate I 2 (Equation 1) is not incorporated in Equation 4 as it is shown not to populate to a detectable level during denaturantinduced equilibrium unfolding of apoflavodoxin (13). Consequently, if FMN would only bind to native apoflavodoxin, holoflavodoxin denaturant-induced equilibrium unfolding would be expected to be a four-state process as shown in Equation 5
4 The Role of Cofactor Binding in Flavodoxin Folding 7839 Holo ^ Apo FMN ^ I 1 FMN ^ U FMN (Eq. 5) with Holo being holoflavodoxin. Equation 5 involves three equilibrium constants as shown in Equation 6 K 1 Holo / Apo FMN K 2 Apo / I 1 K 3 I 1 / U (Eq. 6) in which K 1 is the inverse of the dissociation constant K D of the apoflavodoxin-fmn complex determined above. The denaturant concentration dependence of these equilibrium constants is given by Equation 7 K i D K i 0 exp m i D /RT (Eq. 7) in which [D] represents the denaturant concentration, K i (0) is the equilibrium constant (i.e. K 1, K 2,orK 3 of Equation 6) in the absence of denaturant, m i is a constant of proportionality that describes the denaturant dependence of the equilibrium constant K i, R is the gas constant, and T is the absolute temperature. Each equilibrium constant in Equation 6 is related to a free energy difference G as shown in Equation 8. G i RTln K i RTln K i 0 m i D (Eq. 8) The concentration of free FMN in solution affects the ratio between apo- and holoflavodoxin concentrations according to Equation 6. Consequently, the apparent stability of holoflavodoxin against unfolding, G app unf, depends on the concentration of free FMN, as is expressed in Equation 9 (22) G app unf G apo RTln 1 FMN K D (Eq. 9) in which G apo is the global stability of native apoflavodoxin. Upon denaturant-induced unfolding of a fraction of the holoflavodoxin molecules, FMN is released and this affects the apparent stability of the remaining holoflavodoxin molecules according to Equation 9. Ignoring the latter phenomenon in the analysis of equilibrium unfolding data of a ligand-binding protein will result in wrong stability values. Denaturant-induced Unfolding Shows That A. vinelandii Holoflavodoxin Unfolds according to a Four-state Process Because holoflavodoxin equilibrium unfolding is expected to be a four-state process (Equation 5), it is not likely that the stabilities and corresponding m-values of all the species involved can be extracted from a single equilibrium unfolding experiment. To determine these parameters, unfolding curves of apoflavodoxin, holoflavodoxin, and holoflavodoxin with 100 M FMN (i.e. a 25-fold excess of FMN relative to holoflavodoxin) were determined. This excess of FMN ensures a virtually constant FMN concentration during denaturant-induced holoflavodoxin unfolding and contributes 7.43 kcal/mol to the holoflavodoxin stability in the absence of denaturant (Equation 9). The GuHCl-induced equilibrium unfolding data were obtained by using tryptophan fluorescence (Fig. 3, A and B), FMN fluorescence (Fig. 3C), and CD spectroscopy (Fig. 3D). All unfolding curves were analyzed globally employing the four-state unfolding model discussed (Equation 5; also see Experimental Procedures ). The dissociation constant (K 1 ) 1 (Equation 6) is fixed at zero molar denaturant to M as determined above. In the case of holoflavodoxin unfolding without excess FMN, the free FMN concentration is set to equal the total protein concentration (i.e. 4 M) minus the actual concentration of holoflavodoxin. In case of holoflavodoxin unfolding with excess FMN, the free FMN concentration is fixed to 100 M. In our analysis both unfolded flavodoxin and the flavodoxin folding intermediate I 1 did not interact to a significant extent with FMN as is experimentally supported below. Thus, the stabilities of native apoflavodoxin and of its folding intermediate I 1, both relative to unfolded apoflavodoxin, do not change between the three flavodoxin equilibrium unfolding experiments. Identical fluorimeter settings are used to record the unfolding curves of apoflavodoxin and of holoflavodoxin without excess FMN. These settings need to be altered in case of holoflavodoxin unfolding with excess FMN because of the high optical density of FMN at 280 nm. Therefore, the fluorescence intensity values of holoflavodoxin with excess FMN cannot be directly compared with those of apoflavodoxin and holoflavodoxin in Fig. 3A. All four flavodoxin unfolding curves determined by fluorescence spectroscopy are well described by the four-state unfolding model (Equation 5) as the global fit results show (Fig. 3, A C). Native holoflavodoxin without excess FMN is less fluorescent than native apoflavodoxin (Fig. 3A) as the tryptophan fluorescence in holoflavodoxin is quenched due to the bound FMN molecule. Upon unfolding of apo- and holoflavodoxin, virtually identical fluorescence intensity values at 350 nm were obtained (Fig. 3, A and B). The small differences observed were most likely caused by the absorption of free FMN at 280 nm. This showed that FMN does thus not interact to a significant extent with unfolded flavodoxin. The excellent fit of the four-state unfolding model to the 350-nm as well as the 525-nm fluorescence data showed that, indeed, FMN does not significantly interact with I 1 or with unfolded flavodoxin. In the case of holoflavodoxin without excess FMN a hump is observed at 2.6 M GuHCl in its unfolding curve measured by tryptophan fluorescence (Fig. 3B). This hump is caused by the population of I 1 and its fluorescence properties. FMN does not interact to a significant extent with I 1 as supported by the monitoring of holoflavodoxin unfolding without excess FMN via the fluorescence intensity of FMN at 525 nm (Fig. 3C). At this wavelength FMN gives a high fluorescence intensity when free in solution, whereas the fluorescence of FMN bound to holoflavodoxin is quenched. Note that the hump in the 350-nm tryptophan fluorescence data (Fig. 3B) is not observed in the 525-nm flavin fluorescence data, which shows that I 1, just as U, does not interact with FMN. All three flavodoxin unfolding curves determined by CD at 222 nm (Fig. 3D) are also well described by the four-state unfolding model (Equation 5). Native holoflavodoxin without excess FMN gives a stronger CD signal at 222 nm than native apoflavodoxin (Fig. 3D). This is attributed to the formation of some secondary structure in the FMN binding site of flavodoxin upon binding of FMN (5, 6). Unfolded apo- and holoflavodoxin have virtually identical ellipticity values (Fig. 3D). This supports that the presence of FMN does not affect the conformation of unfolded flavodoxin. In the case of holoflavodoxin with 100 M excess FMN, the ellipticity at 222 nm is 1 millidegree less negative compared with the ellipticity of holoflavodoxin without excess FMN (Fig. 3D). This difference is attributed to the ellipticity of 100 M free FMN, which gives a positive CD signal at 222 nm of 1 millidegree (data not shown). The results of the global fit of Equation 5 to the flavodoxin unfolding data are summarized in Table I. The equilibrium populations of holoflavodoxin, native apoflavodoxin, I 1, and of unfolded flavodoxin, with and without 100 M excess FMN, are shown in Fig. 4 as a function of denaturant concentration. In summary, the denaturant-induced unfolding experiments show that equilibrium unfolding of A. vinelandii holoflavodoxin is well described by the four-state unfolding model holoflavodoxin N native apoflavodoxin FMN N I 1 FMN N unfolded apoflavodoxin FMN. Global Stability of Holoflavodoxin The stabilities of native apoflavodoxin and of the folding intermediate I 1 and corre-
5 7840 The Role of Cofactor Binding in Flavodoxin Folding FIG.3.GuHCl-induced equilibrium unfolding data of A. vinelandii flavodoxin. The solid lines are the results of a global fit of a four-state unfolding model (holoflavodoxin N native apoflavodoxin FMN N I 1 FMN N unfolded apoflavodoxin FMN, Equation 5) to all data presented, as described in the main text. In this model, FMN is either bound to the protein to form holoflavodoxin or it is free in solution. A, the unfolding of apoflavodoxin (O), holoflavodoxin ( ), and of holoflavodoxin with 100 M excess FMN (Œ) as monitored by tryptophan fluorescence at 350 nm. The fluorescence intensity values of the samples of holoflavodoxin with 100 M FMN cannot be directly compared with those of the apoflavodoxin and holoflavodoxin samples as the excess of FMN leads to a high optical density of the corresponding samples at the excitation wavelength used and thus requires modified spectrometer settings. B, zoom-in graph of the apoflavodoxin (O) and holoflavodoxin ( ) data presented in panel A that shows the hump that resides at the high denaturant concentration part of the transition zone of the unfolding curve of holoflavodoxin. This hump is caused by the population of I 1 and its fluorescence properties. C, unfolding of holoflavodoxin without excess FMN as monitored by FMN fluorescence at 525 nm. Note that the hump seen in panel B is not present here as I 1, just as U, does not interact with FMN. D, the monitoring by CD at 222 nm of the unfolding of apoflavodoxin (O), holoflavodoxin ( ), and of holoflavodoxin with 100 M excess FMN (Œ). The unfolding experiments are done at 25 C in 100 mm potassium pyrophosphate, ph 6.0, and the protein concentration is 4.0 M. sponding denaturant concentration dependences (i.e. m-values) presented in Table I are within error identical to values reported previously for denaturant-induced apoflavodoxin (un)- folding (13). However, the seven unfolding curves presented here result in much lower fitting errors. The recording of unfolding curves of flavodoxin at different FMN concentrations (i.e. no FMN present, with equimolar amounts of FMN, and with excess FMN) together with the determination of the holoflavodoxin dissociation constant is a powerful approach to determine the stability of apo- and holoflavodoxin with high accuracy. A similar approach, in which the ligand concentration is varied, can be used to accurately determine the stability of other ligand-binding proteins. The stability of a cofactor-binding protein is expected to equal the sum of the stability of the apoprotein and the free energy that is gained upon binding of the cofactor (22). Wittung-Stafshede and co-workers (23) propose that FMN binding has no effect on the denaturant-induced equilibrium unfolding of Desulfovibrio desulfuricans flavodoxin, whereas the presence of FMN speeds up the folding of this protein (24). The resulting thermodynamic conflict is handled by Wittung- Stafshede and co-workers by assuming that unfolded D. desulfuricans flavodoxin binds to FMN with nanomolar affinity, which is a rather remarkable assumption. In contrast, the results presented here show that the presence of FMN has a clear effect on the denaturant-induced equilibrium unfolding of A. vinelandii flavodoxin (Fig. 3, A and D). Apo- and holoflavodoxin start to unfold at largely different concentrations of denaturant, and no appreciable interaction between FMN and unfolded apoflavodoxin molecules is observed. In addition, kinetic holoflavodoxin folding experiments showed that FMN does not accelerate A. vinelandii flavodoxin folding (see The Kinetics of A. vinelandii Holoflavodoxin Folding ). The equilibrium unfolding data of A. vinelandii flavodoxin are described by a four-state folding model (Equation 5) in which only native apoflavodoxin interacts with FMN. Consequently, we concluded that in accordance with thermodynamic theory the global stability of A. vinelandii holoflavodoxin equals the sum of the global stability of apoflavodoxin and the free energy associated with FMN binding to native apoflavodoxin. The global stability of A. vinelandii holoflavodoxin in the presence of 100 M FMN is kcal/mol (Table I, Equation 9). The Kinetics of FMN Binding to A. vinelandii Apoflavodoxin Involve Two Rate Constants The kinetics of FMN binding to apoflavodoxin were determined at different protein concentrations. Pseudo-first-order kinetics were obtained by using at least a 10-fold excess of apoflavodoxin relative to FMN. In Fig. 5A a fluorescence intensity trace of FMN mixed with native apoflavodoxin is shown. A sum of two exponentials is required to properly describe the data (Fig. 5, B and C). Two rate constants for FMN binding to apoflavodoxin were observed at all protein concentrations used, with both rate constants linearly depending on the protein concentration (Fig. 6A). In addition, two distinct rate constants for FMN binding were also observed at all GuHCl concentrations at which apoflavodoxin is still native (i.e. up to 1.0 M GuHCl) (data not shown). The larger binding rate constant is associated with a large amplitude (93 2% of the total signal on average), whereas the slower binding process contributes for 7 2% to the observed FMN binding kinetics (Fig. 6B). The small amplitude associated with the slower binding process causes the observed scatter in the corresponding binding rate constants (Fig. 6A). Second-order rate constants for FMN binding to excess A. vinelandii apoflavodoxin of and M 1 s 1, respectively, were derived from the protein concentration dependence of
6 The Role of Cofactor Binding in Flavodoxin Folding 7841 TABLE I Thermodynamic parameters obtained from the GuHCl-induced equilibrium unfolding of A. vinelandii holoflavodoxin A four-state model for equilibrium unfolding (holoflavodoxin N native apoflavodoxin FMN N I 1 FMN N unfolded apoflavodoxin FMN, Equations 3 7) is fitted to the unfolding data of apoflavodoxin, holoflavodoxin, and of holoflavodoxin with 100 M excess FMN (see Results and Discussion ). The free energy differences in the absence of denaturant ( G) and corresponding denaturant concentration dependencies (m) are given for the equilibrium between unfolded flavodoxin U and the apoflavodoxin folding intermediate I 1 ( G UI and m UI ), for the equilibrium between intermediate I 1 and native apoflavodoxin ( G IA and m IA ), for the equilibrium between unfolded and native apoflavodoxin ( G UA and m UA ), and for the equilibrium between native apo- and holoflavodoxin in the theoretical presence of 1 M FMN ( G b and m b ), respectively. The errors given are standard fitting errors. G UI (kcal/mol) m UI (kcal/mol M 1 ) G IA (kcal/mol) m IA (kcal/mol M 1 ) G UA (kcal/mol) m UA (kcal/mol M 1 ) G b (kcal/mol) a m b (kcal/mol M 1 ) a RTln(K D ), with K D M, the value determined for the FMN dissociation constant of A. vinelandii holoflavodoxin based on the data presented in Figure 2. FIG. 4.Normalized equilibrium population of holoflavodoxin (solid line), native apoflavodoxin (dotted line), folding intermediate I 1 (dashed line), and unfolded apoflavodoxin (dash-dotted line) during the equilibrium unfolding of holoflavodoxin without excess FMN (A) and with 100 M excess FMN (B), respectively. The populations are derived from the results of the four-state global fit to the experimental flavodoxin unfolding data shown in Fig. 3 and summarized in Table I. both FMN binding rate constants (Fig. 6A). The errors on the second-order rate constants were estimated using a Chisquared test (25). What causes the observed bi-exponential FMN binding kinetics of A. vinelandii apoflavodoxin? In the case of Desulfovibrio vulgaris apoflavodoxin monophasic FMN binding kinetics are observed in the absence of inorganic phosphate in the buffer, whereas in the presence of phosphate FMN binding is biphasic (26). Based on these observations and additional studies (27) it was proposed that in the absence of free phosphate the 5 -phosphate of FMN binds first to apoflavodoxin followed by binding of the isoalloxazine ring. Inorganic phosphate was proposed to bind to the phosphate binding site of a fraction of the D. vulgaris apoflavodoxin molecules, leading to a preformed isoalloxazine binding site (26). The latter allows the isoalloxazine ring of FMN to bind first to apoflavodoxin in these molecules. Consequently, biphasic FMN binding kinetics were observed for D. vulgaris apoflavodoxin in the presence of phosphate. The slower kinetic phase was assigned to the phosphate-first binding mode and the faster phase to the ring-first binding mode (26). The studies of Murray and Swenson (26) prompted us to determine the amount of free inorganic phosphate in the buffer we used (i.e. 100 mm potassium pyrophosphate). 31 P NMR spectroscopy showed that 2% of the 31 P nuclei present in this buffer is present as phosphate (data not shown). Thus 4 mm inorganic phosphate is present in the FMN binding studies of A. vinelandii apoflavodoxin. Most likely, the bi-exponential FMN binding kinetics of A. vinelandii apoflavodoxin are caused by the binding of inorganic phosphate to the phosphate binding site of a fraction of the A. vinelandii apoflavodoxin molecules. The slower kinetic phase observed would be due to the phosphate-first binding mode of FMN to A. vinelandii apoflavodoxin molecules that contain no inorganic phosphate. A. vinelandii apoflavodoxin molecules that have an inorganic phosphate ion bound would cause the faster kinetic phase observed due to the ring-first binding mode of FMN to these molecules, just as is observed for D. vulgaris apoflavodoxin (26). The Rate of FMN Release Determines the A. vinelandii Holoflavodoxin Unfolding Rate Holoflavodoxin unfolding is measured via the corresponding release of FMN, which results in a strong increase of its fluorescence intensity (Fig. 7A). At each GuHCl concentration studied (i.e M GuHCl), only one unfolding rate constant was observed (Fig. 7B). Clearly, the natural logarithm of the holoflavodoxin unfolding rate constant depends linearly on the GuHCl concentration. The linear extrapolation of the holoflavodoxin unfolding rate constants presented in Fig. 7B to 0 molar GuHCl leads to a holoflavodoxin unfolding rate constant in water of ( ) 10 5 s 1, and the corresponding m-value is kcal/mol M 1. The errors on the rate constant and m-value were estimated using a Chisquared test (25). It was previously suggested based on hydrogen/deuterium exchange results that FMN release is probably the ratelimiting step in A. vinelandii holoflavodoxin unfolding (5, 28), as is also observed for D. vulgaris holoflavodoxin unfolding (29). If true, the A. vinelandii holoflavodoxin unfolding rate constant should equal the rate constant for FMN release from holoflavodoxin. The rate constant for FMN release can be deduced using the rate constant for FMN binding to A. vinelandii apoflavodoxin and the dissociation constant of the apoflavodoxin-fmn complex, both of which have been experimentally determined in this study. The slowest FMN binding rate constant observed (i.e M 1 s 1 ) most likely results from binding of FMN to apoflavodoxin molecules that contain no inorganic phosphate ion. The dissociation constant K D of the apoflavodoxin-fmn complex is determined to be ( ) M. This dissociation constant describes the relative amounts of holoflavodoxin and apoflavodoxin at equilibrium. The rate constant for release of FMN from holoflavodoxin equals the rate constant for FMN binding to apoflavodoxin the dissociation constant of the apoflavodoxin-fmn complex and is (9 2) 10 5 s 1. This value matches with the holoflavodoxin unfolding rate constant in water of ( ) 10 5 s 1. Thus, FMN release is indeed the rate-limiting step in A. vinelandii holoflavodoxin global unfolding. The data shown in Fig. 7B represent the GuHCl concentration dependence of the rate constant for FMN release from A. vinelandii holoflavodoxin. Extrapolation of these data shows
7 7842 The Role of Cofactor Binding in Flavodoxin Folding FIG. 5. Two rate constants describe FMN binding to native A. vinelandii apoflavodoxin. A, kinetics of FMN binding to native apoflavodoxin as monitored by the quenching of the FMN fluorescence emission above 475 nm in a stopped-flow instrument. B, residuals of a fit of a single exponential equation to the data. C, residuals of a fit of a sum of two exponential equations to the data with the fitted rate constants being and s 1, respectively. The final flavodoxin concentration is 3 M, and the final FMN concentration is 0.10 M, both in 100 mm potassium pyrophosphate. ph 6.0, at 25 C. FIG. 6.Observed rate constants for FMN binding to A. vinelandii apoflavodoxin as a function of the final protein concentration in 100 mm potassium pyrophosphate, ph 6.0, at 25 C. The final FMN concentration is 0.10 M in all cases, except for the two highest protein concentrations used (i.e. 10 and 12.5 M), where it is 1.0 M. A, the protein concentration dependence of both observed rate constants is fitted to a straight line that crosses the origin; the corresponding slopes are the pseudo-first-order rate constants. The faster of the two observed FMN binding rate constants ( ) fits to a pseudo-first-order rate constant of M 1 s 1, whereas the slower one (O) fits to a pseudo-first-order rate constant of M 1 s 1. B, relative amplitudes associated with the faster ( ) and the slower (O) observed rate constants for FMN binding to apoflavodoxin as a function of the protein concentration. The error bars give the standard fitting errors of the rate constants and of the relative amplitudes, respectively. FIG. 7. Unfolding kinetics of A. vinelandii holoflavodoxin monitored by FMN fluorescence emission. A, fluorescence emission spectrum of free FMN (gray lines) and of the same concentration of FMN in the presence of an equimolar amount of A. vinelandii apoflavodoxin (black lines), showing the effect of the absence (dashed lines) and the presence (solid lines) of4m GuHCl. In the absence of denaturant, FMN binds to apoflavodoxin; as a result, its fluorescence intensity is strongly quenched. Apoflavodoxin is unfolded in 4 M GuHCl; as a result, the FMN fluorescence emission spectrum in the presence of an equimolar amount of apoflavodoxin and 4 M GuHCl is indistinguishable from the one of free FMN in 4 M GuHCl. Apoflavodoxin (when present) and FMN concentrations are 1 M in 100 mm potassium pyrophosphate, ph 6.0, at 25 C. Excitation is at 446 nm, and a 5-nm slit is used. B, natural logarithm of the single observed rate constant for A. vinelandii holoflavodoxin unfolding (O) as a function of the GuHCl concentration. At the X sign two data points overlap. The solid line is the result of a linear fit to all data shown. The unfolding rate constant extrapolated to water is ( ) 10 5 s 1, and the corresponding m-value is kcal/mol M 1. The error bars show the standard errors of the rate constants. The experiments are done at 25 C, and final conditions are 1.0 M flavodoxin in 100 mm potassium pyrophosphate, ph 6.0. that in the absence of denaturant A. vinelandii holoflavodoxin unfolds approximately once every 3 h. The Kinetics of A. vinelandii Holoflavodoxin Folding The folding kinetics of apoflavodoxin from A. vinelandii in the absence of FMN have been studied in great detail (13). Singlejump and interrupted refolding experiments showed that the refolding kinetics of A. vinelandii apoflavodoxin are complex and involve four processes, with rate constants 1 to 4 in order of decreasing value. All four processes yield native apoflavodoxin molecules. The fastest process with rate constant 1 corresponds to the rapid formation of native apoflavodoxin along the most direct folding route from unfolded to native
8 The Role of Cofactor Binding in Flavodoxin Folding 7843 FIG. 8. Folding kinetics of A. vinelandii holoflavodoxin. A, time-dependent fluorescence intensity resulting from FMN binding to folding A. vinelandii apoflavodoxin molecules (black line) detected via the quenching of the FMN fluorescence above 475 nm. The trace is obtained by mixing unfolded apoflavodoxin (in 3.0 M GuHCl) with buffer containing FMN to final conditions of 10 M FMN, 1.0 M flavodoxin, 0.50 M GuHCl, 100 mm potassium pyrophosphate, ph 6.0, at 25 C. Under these final conditions apoflavodoxin is in its native state. The gray line shows the trace obtained when repeating the same experiment in the absence of protein. The steady decrease in fluorescence emission observed in the latter trace is caused by the time-dependent photobleaching of FMN. B, chevron plot of the two largest observed rate constants for A. vinelandii holoflavodoxin folding as observed by changes in FMN fluorescence above 475 nm (, 1 ; f, 2 ). For comparison, the corresponding A. vinelandii apoflavodoxin folding rate constants as observed by changes in tryptophan fluorescence intensity are shown as well (open symbols). The two smallest folding rate constants for both apo- and holoflavodoxin folding (i.e. 3 and 4 ) are not shown as they do not inform about the folding mechanism of flavodoxin, because they originate from Xaa-Pro peptide bond isomerizations. Up to 0.7 M GuHCl, the folding rate constants observed for both holo- and apoflavodoxin are identical within error. The 1 and 2 rate constants for refolding of freshly unfolded apoflavodoxin (made by unfolding apoflavodoxin for a period of 600 ms in 3.0 M GuHCl) in the presence of FMN at 0.5 M GuHCl are shown in gray. The rate constants are plotted on a logarithmic scale. Final conditions are 1.0 M flavodoxin in 100 mm potassium pyrophosphate, ph 6.0, at 25 C. In the case of holoflavodoxin, 10 M FMN is present as well. Apoflavodoxin folding data are taken from Ref. 13. protein. Only a small fraction (11%) (13) of the folding apoflavodoxin molecules follows the direct folding route. On this route (unfolded apoflavodoxin N I 2 N native apoflavodoxin), a high energy intermediate I 2 is transiently formed. The process with rate constant 2 corresponds to the formation of native apoflavodoxin via a relatively stable intermediate I 1 (I 1 N unfolded apoflavodoxin N I 2 N native apoflavodoxin, Equation 1). The unfolding of the latter intermediate is the rate-limiting step in the formation of native apoflavodoxin with rate constant 2. The two slowest folding processes (with rate constants 3 and 4 ) are because of Xaa-Pro peptide bond isomerizations in the unfolded state (13). In this study the influence of FMN on the folding kinetics of A. vinelandii flavodoxin was investigated. Unfolded apoflavodoxin in 3.0 M GuHCl was mixed into buffer containing FMN, and the subsequent binding of FMN to the folding apoflavodoxin molecules was monitored, as shown in Fig. 8A. A sum of three exponential equations was needed to properly fit the time-dependent fluorescence signal obtained, which led to the identification of three observable folding rate constants. The slowest folding rate constant observed in the holoflavodoxin folding experiment is equal to 3 observed during apoflavodoxin kinetic folding, which originates from Xaa-Pro peptide bond isomerizations (a rate constant of 0.3 s 1, data not shown). The slowest apoflavodoxin folding rate constant 4 of 0.03 s 1 in the absence of denaturant, which also originates from Xaa-Pro peptide bond isomerizations, cannot be extracted from the data. Rate constant 4 was not sufficiently sampled in the holoflavodoxin kinetic folding traces, as these traces were recorded for a period of only 10 s. As both 3 and 4 originate from Xaa-Pro peptide bond isomerizations, they do not inform about the folding mechanism of both apo- and holoflavodoxin. The dependence of the two fastest rate constants for both apoflavodoxin folding and holoflavodoxin folding (i.e. 1 and 2 ) on the GuHCl concentration is shown in Fig. 8B. Upto0.7 M GuHCl, the rate constants for holoflavodoxin folding as observed via FMN binding are identical within error to those observed for apoflavodoxin folding. Clearly, excess FMN does not accelerate flavodoxin folding, and FMN thus does not act as a nucleation site for flavodoxin folding. Instead, in the presence of excess FMN, formation of native apoflavodoxin is the ratelimiting process in the folding of A. vinelandii holoflavodoxin. Above 0.7 M GuHCl, the FMN binding rate constants deviated from the apoflavodoxin folding rate constants. At these concentrations of denaturant both folding and unfolding processes contributed to the observed kinetics, and as holoflavodoxin unfolds slower than apoflavodoxin, the resulting observed rate constants for holoflavodoxin folding were lower than those of apoflavodoxin folding. The largest folding rate observed during apoflavodoxin folding (i.e. 1 ) contains contributions from the formation of folding intermediates with non-native Xaa-Pro peptide bond isomers (13). In case these intermediates are able to bind FMN, this could influence the observed holoflavodoxin folding kinetics as monitored by FMN binding (Fig. 8B). However, freshly unfolded apoflavodoxin gave upon folding in the presence of FMN at 0.5 M GuHCl rate constants for FMN binding that were identical within error to the rate constants for folding of equilibrium unfolded apo- and holoflavodoxin (Fig. 8B). In freshly unfolded apoflavodoxin (made by unfolding apoflavodoxin for 600 ms in 3.0 M GuHCl) the vast majority of the Xaa-Pro peptide bond isomers was native. Hence, apoflavodoxin folding intermediates with non-native Xaa-Pro peptide bonds did not bind FMN. In Fig. 8A the time-dependent fluorescence intensity of free FMN in a kinetic folding experiment is shown, recorded under identical circumstances as for holoflavodoxin folding, except that now no protein was present. The initial fluorescence intensity of FMN during kinetic holoflavodoxin folding was somewhat (2%) higher than observed in the blank experiment in which flavodoxin was absent. This higher intensity might suggest a weak association of FMN with the folding apoflavodoxin molecules, which would result in FMN being less accessible to GuHCl compared with unbound FMN. We observed that the fluorescence intensity of free FMN was quenched by the presence of GuHCl (24% quenching at 0.5 M GuHCl, data not shown). No indications for the association of FMN with apoflavodoxin unfolded in 4 M GuHCl were observed. First, at this denaturant concentration the fluorescence emission spectra of FMN in the presence and in the absence of apoflavodoxin were identical (Fig. 7A). Second, 1 H NMR spectra of holoflavodoxin unfolded in 4 M GuHCl showed sharp resonances of FMN protons at frequencies that coincided within the spectral resolution of 0.01
ph-jump-induced Folding and Unfolding Studies of Barstar: Evidence for Multiple Folding and Unfolding Pathways
 Biochemistry 2001, 40, 15267-15279 15267 ph-jump-induced Folding and Unfolding Studies of Barstar: Evidence for Multiple Folding and Unfolding Pathways Bhadresh R. Rami and Jayant B. Udgaonkar* National
Biochemistry 2001, 40, 15267-15279 15267 ph-jump-induced Folding and Unfolding Studies of Barstar: Evidence for Multiple Folding and Unfolding Pathways Bhadresh R. Rami and Jayant B. Udgaonkar* National
Protein folding can be described by using a free energy
 The folding energy landscape of apoflavodoxin is rugged: Hydrogen exchange reveals nonproductive misfolded intermediates Yves J. M. Bollen*, Monique B. Kamphuis, and Carlo P. M. van Mierlo *Department
The folding energy landscape of apoflavodoxin is rugged: Hydrogen exchange reveals nonproductive misfolded intermediates Yves J. M. Bollen*, Monique B. Kamphuis, and Carlo P. M. van Mierlo *Department
Lecture 11: Protein Folding & Stability
 Structure - Function Protein Folding: What we know Lecture 11: Protein Folding & Stability 1). Amino acid sequence dictates structure. 2). The native structure represents the lowest energy state for a
Structure - Function Protein Folding: What we know Lecture 11: Protein Folding & Stability 1). Amino acid sequence dictates structure. 2). The native structure represents the lowest energy state for a
Protein Folding & Stability. Lecture 11: Margaret A. Daugherty. Fall Protein Folding: What we know. Protein Folding
 Lecture 11: Protein Folding & Stability Margaret A. Daugherty Fall 2003 Structure - Function Protein Folding: What we know 1). Amino acid sequence dictates structure. 2). The native structure represents
Lecture 11: Protein Folding & Stability Margaret A. Daugherty Fall 2003 Structure - Function Protein Folding: What we know 1). Amino acid sequence dictates structure. 2). The native structure represents
Isothermal experiments characterize time-dependent aggregation and unfolding
 1 Energy Isothermal experiments characterize time-dependent aggregation and unfolding Technical ote Introduction Kinetic measurements have, for decades, given protein scientists insight into the mechanisms
1 Energy Isothermal experiments characterize time-dependent aggregation and unfolding Technical ote Introduction Kinetic measurements have, for decades, given protein scientists insight into the mechanisms
Supporting Information
 Supporting Information Materials and Methods: Tris-hydroxymethylaminomethane (Tris) was purchased from USB. All the other reagents used in the experiments were purchased from Sigma. All the DNA oligonucleotides
Supporting Information Materials and Methods: Tris-hydroxymethylaminomethane (Tris) was purchased from USB. All the other reagents used in the experiments were purchased from Sigma. All the DNA oligonucleotides
SUPPORTING INFORMATION. Photoswitchable catalysis by a nanozyme mediated by a light-sensitive cofactor
 SUPPORTING INFORMATION Photoswitchable catalysis by a nanozyme mediated by a light-sensitive cofactor Simona Neri, Sergio García Martín, Cristian Pezzato, Leonard J. Prins* Department of Chemical Sciences,
SUPPORTING INFORMATION Photoswitchable catalysis by a nanozyme mediated by a light-sensitive cofactor Simona Neri, Sergio García Martín, Cristian Pezzato, Leonard J. Prins* Department of Chemical Sciences,
ion mobility spectrometry IR spectroscopy
 Debasmita Gho 29.10.2016 Introducti on Owing to its accuracy, sensitivity, and speed, mass spectrometry (MS) coupled to fragmentation techniques is the method of choice for determining the primary structure
Debasmita Gho 29.10.2016 Introducti on Owing to its accuracy, sensitivity, and speed, mass spectrometry (MS) coupled to fragmentation techniques is the method of choice for determining the primary structure
CD Basis Set of Spectra that is used is that derived from comparing the spectra of globular proteins whose secondary structures are known from X-ray
 CD Basis Set of Spectra that is used is that derived from comparing the spectra of globular proteins whose secondary structures are known from X-ray crystallography An example of the use of CD Modeling
CD Basis Set of Spectra that is used is that derived from comparing the spectra of globular proteins whose secondary structures are known from X-ray crystallography An example of the use of CD Modeling
Thermodynamic Stability of Hoogsteen and Watson-Crick. Base Pairs in the Presence of Histone H3-Mimicking Peptide
 Supporting information Thermodynamic Stability of Hoogsteen and Watson-Crick Base Pairs in the Presence of Histone H3-Mimicking Peptide Smritimoy Pramanik a, Kaori Nakamura a, Kenji Usui a,b, Shu-ichi
Supporting information Thermodynamic Stability of Hoogsteen and Watson-Crick Base Pairs in the Presence of Histone H3-Mimicking Peptide Smritimoy Pramanik a, Kaori Nakamura a, Kenji Usui a,b, Shu-ichi
Methods for the study of the conformation of folding intermediates
 7.88 Lecture Notes - 9 7.24/7.88J/5.48J The Protein Folding and Human Disease Fluorescence spectroscopy Denaturation and Denaturing agents Denatured State as a random coil (First Approx.) Renaturation/Refolding
7.88 Lecture Notes - 9 7.24/7.88J/5.48J The Protein Folding and Human Disease Fluorescence spectroscopy Denaturation and Denaturing agents Denatured State as a random coil (First Approx.) Renaturation/Refolding
Electronic Supplementary Information
 Electronic Supplementary Information Influence of DNA-binding on the photochromic equilibrium of a chromene derivative Sergey V. Paramonov, Vladimir Lokshin, Heiko Ihmels, Olga A. Fedorova Contents: Synthesis
Electronic Supplementary Information Influence of DNA-binding on the photochromic equilibrium of a chromene derivative Sergey V. Paramonov, Vladimir Lokshin, Heiko Ihmels, Olga A. Fedorova Contents: Synthesis
I. Proteomics by Mass Spectrometry 1. What is an internal standard and what does it accomplish analytically?
 Name I. Proteomics by Mass Spectrometry 1. What is an internal standard and what does it accomplish analytically? Internal standards are standards added intentionally to all samples, standards and blanks.
Name I. Proteomics by Mass Spectrometry 1. What is an internal standard and what does it accomplish analytically? Internal standards are standards added intentionally to all samples, standards and blanks.
4. Circular Dichroism - Spectroscopy
 4. Circular Dichroism - Spectroscopy The optical rotatory dispersion (ORD) and the circular dichroism (CD) are special variations of absorption spectroscopy in the UV and VIS region of the spectrum. The
4. Circular Dichroism - Spectroscopy The optical rotatory dispersion (ORD) and the circular dichroism (CD) are special variations of absorption spectroscopy in the UV and VIS region of the spectrum. The
Biology Chemistry & Physics of Biomolecules. Examination #1. Proteins Module. September 29, Answer Key
 Biology 5357 Chemistry & Physics of Biomolecules Examination #1 Proteins Module September 29, 2017 Answer Key Question 1 (A) (5 points) Structure (b) is more common, as it contains the shorter connection
Biology 5357 Chemistry & Physics of Biomolecules Examination #1 Proteins Module September 29, 2017 Answer Key Question 1 (A) (5 points) Structure (b) is more common, as it contains the shorter connection
for Single mixing applications
 RAPID KINETICS AND SPECTROSCOPY SFM-20 2 SYRINGE STOPPED-FLOW SYSTEM for Single mixing applications The SFM-20 is a high performance, modular stopped-flow system, designed for single mixing rapid kinetics
RAPID KINETICS AND SPECTROSCOPY SFM-20 2 SYRINGE STOPPED-FLOW SYSTEM for Single mixing applications The SFM-20 is a high performance, modular stopped-flow system, designed for single mixing rapid kinetics
Characterization of the Unfolding of Ribonuclease A by a Pulsed Hydrogen Exchange Study: Evidence for Competing Pathways for Unfolding
 Characterization of the Unfolding of Ribonuclease A by a Pulsed Hydrogen Exchange Study: Evidence for Competing Pathways for Unfolding Juhi Juneja and Jayant B. Udgaonkar* National Centre for Biological
Characterization of the Unfolding of Ribonuclease A by a Pulsed Hydrogen Exchange Study: Evidence for Competing Pathways for Unfolding Juhi Juneja and Jayant B. Udgaonkar* National Centre for Biological
Supplementary Information
 Supplementary Information Adenosyltransferase Tailors and Delivers Coenzyme B 12 Dominique Padovani 1,2, Tetyana Labunska 2, Bruce A. Palfey 1, David P. Ballou 1 and Ruma Banerjee 1,2 * 1 Biological Chemistry
Supplementary Information Adenosyltransferase Tailors and Delivers Coenzyme B 12 Dominique Padovani 1,2, Tetyana Labunska 2, Bruce A. Palfey 1, David P. Ballou 1 and Ruma Banerjee 1,2 * 1 Biological Chemistry
Awanish Kumar, Anjeeta Rani and Pannuru Venkatesu* Department of Chemistry, University of Delhi, Delhi
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 Supplimentary Informations
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2014 Supplimentary Informations
type GroEL-GroES complex. Crystals were grown in buffer D (100 mm HEPES, ph 7.5,
 Supplementary Material Supplementary Materials and Methods Structure Determination of SR1-GroES-ADP AlF x SR1-GroES-ADP AlF x was purified as described in Materials and Methods for the wild type GroEL-GroES
Supplementary Material Supplementary Materials and Methods Structure Determination of SR1-GroES-ADP AlF x SR1-GroES-ADP AlF x was purified as described in Materials and Methods for the wild type GroEL-GroES
A Single Outer Sphere Mutation Stabilizes apo- Mn Superoxide Dismutase by 35 C and. Disfavors Mn Binding.
 Supporting information for A Single Outer Sphere Mutation Stabilizes apo- Mn Superoxide Dismutase by 35 C and Disfavors Mn Binding. Anne-Frances Miller* and Ting Wang Department of Chemistry, University
Supporting information for A Single Outer Sphere Mutation Stabilizes apo- Mn Superoxide Dismutase by 35 C and Disfavors Mn Binding. Anne-Frances Miller* and Ting Wang Department of Chemistry, University
Protein Folding & Stability. Lecture 11: Margaret A. Daugherty. Fall How do we go from an unfolded polypeptide chain to a
 Lecture 11: Protein Folding & Stability Margaret A. Daugherty Fall 2004 How do we go from an unfolded polypeptide chain to a compact folded protein? (Folding of thioredoxin, F. Richards) Structure - Function
Lecture 11: Protein Folding & Stability Margaret A. Daugherty Fall 2004 How do we go from an unfolded polypeptide chain to a compact folded protein? (Folding of thioredoxin, F. Richards) Structure - Function
two slits and 5 slits
 Electronic Spectroscopy 2015January19 1 1. UV-vis spectrometer 1.1. Grating spectrometer 1.2. Single slit: 1.2.1. I diffracted intensity at relative to un-diffracted beam 1.2.2. I - intensity of light
Electronic Spectroscopy 2015January19 1 1. UV-vis spectrometer 1.1. Grating spectrometer 1.2. Single slit: 1.2.1. I diffracted intensity at relative to un-diffracted beam 1.2.2. I - intensity of light
Transformations of Oxidized Multiwalled Carbon Nanotubes Exposed to UVC (254nm) Irradiation. Supporting Information
 Electronic Supplementary Material (ESI) for Environmental Science: Nano. This journal is The Royal Society of Chemistry 2014 Transformations of Oxidized Multiwalled Carbon Nanotubes Exposed to UVC (254nm)
Electronic Supplementary Material (ESI) for Environmental Science: Nano. This journal is The Royal Society of Chemistry 2014 Transformations of Oxidized Multiwalled Carbon Nanotubes Exposed to UVC (254nm)
Lecture 21 (11/3/17) Protein Stability, Folding, and Dynamics Hydrophobic effect drives protein folding
 Reading: Ch4; 142-151 Problems: Ch4 (text); 14, 16 Ch6 (text); 1, 4 NEXT (after exam) Reading: Ch8; 310-312, 279-285, 285-289 Ch24; 957-961 Problems: Ch8 (text); 1,2,22 Ch8 (study-guide:facts); 1,2,3,4,5,9,10
Reading: Ch4; 142-151 Problems: Ch4 (text); 14, 16 Ch6 (text); 1, 4 NEXT (after exam) Reading: Ch8; 310-312, 279-285, 285-289 Ch24; 957-961 Problems: Ch8 (text); 1,2,22 Ch8 (study-guide:facts); 1,2,3,4,5,9,10
MOLEBIO LAB #4: Using a Spectrophotometer
 Introduction: Spectrophotometry MOLEBIO LAB #4: Using a Spectrophotometer Many kinds of molecules interact with or absorb specific types of radiant energy in a predictable fashion. For example, when while
Introduction: Spectrophotometry MOLEBIO LAB #4: Using a Spectrophotometer Many kinds of molecules interact with or absorb specific types of radiant energy in a predictable fashion. For example, when while
Presenter: She Zhang
 Presenter: She Zhang Introduction Dr. David Baker Introduction Why design proteins de novo? It is not clear how non-covalent interactions favor one specific native structure over many other non-native
Presenter: She Zhang Introduction Dr. David Baker Introduction Why design proteins de novo? It is not clear how non-covalent interactions favor one specific native structure over many other non-native
Visible and IR Absorption Spectroscopy. Andrew Rouff and Kyle Chau
 Visible and IR Absorption Spectroscopy Andrew Rouff and Kyle Chau The Basics wavelength= (λ) original intensity= Ι o sample slab thickness= dl Final intensity= I f ε = molar extinction coefficient -di=
Visible and IR Absorption Spectroscopy Andrew Rouff and Kyle Chau The Basics wavelength= (λ) original intensity= Ι o sample slab thickness= dl Final intensity= I f ε = molar extinction coefficient -di=
Supporting Information for:
 Supporting Information for: A Simple Molecular Model for Thermophilic Adaptation of Functional Nucleic Acids Joshua M. Blose *, Scott K. Silverman, and Philip C. Bevilacqua * * Department of Chemistry,
Supporting Information for: A Simple Molecular Model for Thermophilic Adaptation of Functional Nucleic Acids Joshua M. Blose *, Scott K. Silverman, and Philip C. Bevilacqua * * Department of Chemistry,
UNIT 3 CHEMISTRY. Fundamental Principles in Chemistry
 UNIT 3 CHEMISTRY NOTE: This list has been compiled based on the topics covered in the 2016 Master Class program. Once all of the 2017 Chemistry program materials have been finalised, this summary will
UNIT 3 CHEMISTRY NOTE: This list has been compiled based on the topics covered in the 2016 Master Class program. Once all of the 2017 Chemistry program materials have been finalised, this summary will
Substrate-dependent switching of the allosteric binding mechanism of a dimeric enzyme
 Supplementary Information: Substrate-dependent switching of the allosteric binding mechanism of a dimeric enzyme Lee Freiburger, 1 Teresa Miletti, 1 Siqi Zhu, 1 Oliver Baettig, Albert Berghuis, Karine
Supplementary Information: Substrate-dependent switching of the allosteric binding mechanism of a dimeric enzyme Lee Freiburger, 1 Teresa Miletti, 1 Siqi Zhu, 1 Oliver Baettig, Albert Berghuis, Karine
Experimental Methods in Kinetics (2014)
 IIIa- 35 Experimental Methods in Kinetics (2014) Initial examples assume start R = A 0 + B 0 etc., P = 0 follow reaction forward initial rate, etc. measure [A] vs. t Chemical methods: Could mix and take
IIIa- 35 Experimental Methods in Kinetics (2014) Initial examples assume start R = A 0 + B 0 etc., P = 0 follow reaction forward initial rate, etc. measure [A] vs. t Chemical methods: Could mix and take
Chirascan 6-Cell Peltier Cell Holder: Rapid Optimisation of Buffer Conditions for Stabilising Protein Therapeutics
 CHIRASCAN SERIES APPLICATION NOTE Chirascan 6-Cell Peltier Cell Holder: Rapid Optimisation of Buffer Conditions for Stabilising Protein Therapeutics Abstract: The selection of buffer conditions that maximise
CHIRASCAN SERIES APPLICATION NOTE Chirascan 6-Cell Peltier Cell Holder: Rapid Optimisation of Buffer Conditions for Stabilising Protein Therapeutics Abstract: The selection of buffer conditions that maximise
BIOPHYSICAL CHARACTERIZATION OF PROTEIN FOLDING AND MISFOLDING
 BIOPHYSICAL CHARACTERIZATION OF PROTEIN FOLDING AND MISFOLDING A Dissertation by JASON PETER SCHMITTSCHMITT Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of
BIOPHYSICAL CHARACTERIZATION OF PROTEIN FOLDING AND MISFOLDING A Dissertation by JASON PETER SCHMITTSCHMITT Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of
Supporting Information
 Supporting Information Wiley-VCH 27 6941 Weinheim, Germany Homogeneous Biocatalysis in both Fluorous Biphasic and Supercritical Carbon Dioxide Systems H. R. Hobbs, H. M. Kirke, M. Poliakoff, N.R. T ho
Supporting Information Wiley-VCH 27 6941 Weinheim, Germany Homogeneous Biocatalysis in both Fluorous Biphasic and Supercritical Carbon Dioxide Systems H. R. Hobbs, H. M. Kirke, M. Poliakoff, N.R. T ho
A Determination of DNA-DAPI Binding using Fluorescence Spectroscopy
 CHEM 311L Revision 1.2 A Determination of DNA-DAPI Binding using Fluorescence Spectroscopy In this Laboratory Exercise, we will determine the binding constant K f for complex formation between 4'-6-diamidino-2-phenylindole
CHEM 311L Revision 1.2 A Determination of DNA-DAPI Binding using Fluorescence Spectroscopy In this Laboratory Exercise, we will determine the binding constant K f for complex formation between 4'-6-diamidino-2-phenylindole
Chapter 6. The interaction of Src SH2 with the focal adhesion kinase catalytic domain studied by NMR
 The interaction of Src SH2 with the focal adhesion kinase catalytic domain studied by NMR 103 Abstract The interaction of the Src SH2 domain with the catalytic domain of FAK, including the Y397 SH2 domain
The interaction of Src SH2 with the focal adhesion kinase catalytic domain studied by NMR 103 Abstract The interaction of the Src SH2 domain with the catalytic domain of FAK, including the Y397 SH2 domain
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi: 1.138/nchem.892 Mutual modulation Modulation between membrane-embedded Membrane Embedded receptor Receptors clustering lustering and ligand binding in lipid membranes Ligand
SUPPLEMENTARY INFORMATION doi: 1.138/nchem.892 Mutual modulation Modulation between membrane-embedded Membrane Embedded receptor Receptors clustering lustering and ligand binding in lipid membranes Ligand
Principles of Physical Biochemistry
 Principles of Physical Biochemistry Kensal E. van Hold e W. Curtis Johnso n P. Shing Ho Preface x i PART 1 MACROMOLECULAR STRUCTURE AND DYNAMICS 1 1 Biological Macromolecules 2 1.1 General Principles
Principles of Physical Biochemistry Kensal E. van Hold e W. Curtis Johnso n P. Shing Ho Preface x i PART 1 MACROMOLECULAR STRUCTURE AND DYNAMICS 1 1 Biological Macromolecules 2 1.1 General Principles
Effect of alkaline ph on sunflower 11S protein
 J Biosci, Vol. 11, Numbers 1 4, March 1987, pp. 351 360. Printed in India Effect of alkaline ph on sunflower 11S protein G. SRIPAD* and M. S. NARASINGA RAO Protein Technology Discipline, Central Food Technological
J Biosci, Vol. 11, Numbers 1 4, March 1987, pp. 351 360. Printed in India Effect of alkaline ph on sunflower 11S protein G. SRIPAD* and M. S. NARASINGA RAO Protein Technology Discipline, Central Food Technological
EXPERIMENT 14. ACID DISSOCIATION CONSTANT OF METHYL RED 1
 EXPERIMET 14. ACID DISSOCIATIO COSTAT OF METHYL RED 1 The acid dissociation constant, Ka, of a dye is determined using spectrophotometry. Introduction In aqueous solution, methyl red is a zwitterion and
EXPERIMET 14. ACID DISSOCIATIO COSTAT OF METHYL RED 1 The acid dissociation constant, Ka, of a dye is determined using spectrophotometry. Introduction In aqueous solution, methyl red is a zwitterion and
Spectrometric Titrations
 Jürgen Polster Heinrich Lachmann Spectrometric Titrations Analysis of Chemical Equilibria VCH Contents Part I The Theoretical and Methodological Basis of Spectrometric Titration 1 Basic Principles and
Jürgen Polster Heinrich Lachmann Spectrometric Titrations Analysis of Chemical Equilibria VCH Contents Part I The Theoretical and Methodological Basis of Spectrometric Titration 1 Basic Principles and
The investigation of the photokinetics of a platinum organoamine complex using the Cary 50/60
 The investigation of the photokinetics of a platinum organoamine complex using the Cary 50/60 Application Note Chemical Author Jeffrey J. Comerford, PhD. Agilent Technologies, Inc. Mulgrave, Victoria 3170,
The investigation of the photokinetics of a platinum organoamine complex using the Cary 50/60 Application Note Chemical Author Jeffrey J. Comerford, PhD. Agilent Technologies, Inc. Mulgrave, Victoria 3170,
A. K. R. Junker et al 1 Investigating subtle 4f vs. 5f...
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 A. K. R. Junker et al 1 Investigating subtle 4f vs. 5f... Electronic Supplementary Information
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 A. K. R. Junker et al 1 Investigating subtle 4f vs. 5f... Electronic Supplementary Information
1. Use the Data for RNAse to estimate:
 Chem 78 - - Spr 1 03/14/01 Assignment 4 - Answers Thermodynamic Analysis of RNAseA Denaturation by UV- Vis Difference Absorption Spectroscopy (and Differential Scanning Calorimetry). The accompanying excel
Chem 78 - - Spr 1 03/14/01 Assignment 4 - Answers Thermodynamic Analysis of RNAseA Denaturation by UV- Vis Difference Absorption Spectroscopy (and Differential Scanning Calorimetry). The accompanying excel
Circular Dichroism & Optical Rotatory Dispersion. Proteins (KCsa) Polysaccharides (agarose) DNA CHEM 305. Many biomolecules are α-helical!
 Circular Dichroism & Optical Rotatory Dispersion Polysaccharides (agarose) DNA Proteins (KCsa) Many biomolecules are α-helical! How can we measure the amount and changes in amount of helical structure
Circular Dichroism & Optical Rotatory Dispersion Polysaccharides (agarose) DNA Proteins (KCsa) Many biomolecules are α-helical! How can we measure the amount and changes in amount of helical structure
Analytical Biochemistry
 Analytical Biochemistry 389 (2009) 165 170 Contents lists available at ScienceDirect Analytical Biochemistry journal homepage: www.elsevier.com/locate/yabio Revisiting absorbance at 230 nm as a protein
Analytical Biochemistry 389 (2009) 165 170 Contents lists available at ScienceDirect Analytical Biochemistry journal homepage: www.elsevier.com/locate/yabio Revisiting absorbance at 230 nm as a protein
A General Approach for Detecting Folding Intermediates from Steady-State and Time-Resolved Fluorescence of Single-Tryptophan-Containing Proteins
 pubs.acs.org/biochemistry A General Approach for Detecting Folding Intermediates from Steady-State and Time-Resolved Fluorescence of Single-Tryptophan-Containing Proteins Sergey P. Laptenok,,, Nina V.
pubs.acs.org/biochemistry A General Approach for Detecting Folding Intermediates from Steady-State and Time-Resolved Fluorescence of Single-Tryptophan-Containing Proteins Sergey P. Laptenok,,, Nina V.
Chem 310 rd. 3 Homework Set Answers
 -1- Chem 310 rd 3 Homework Set Answers 1. A double line labeled S 0 represents the _ground electronic_ state and the _ground vibrational_ state of a molecule in an excitation state diagram. Light absorption
-1- Chem 310 rd 3 Homework Set Answers 1. A double line labeled S 0 represents the _ground electronic_ state and the _ground vibrational_ state of a molecule in an excitation state diagram. Light absorption
Free Energy. because H is negative doesn't mean that G will be negative and just because S is positive doesn't mean that G will be negative.
 Biochemistry 462a Bioenergetics Reading - Lehninger Principles, Chapter 14, pp. 485-512 Practice problems - Chapter 14: 2-8, 10, 12, 13; Physical Chemistry extra problems, free energy problems Free Energy
Biochemistry 462a Bioenergetics Reading - Lehninger Principles, Chapter 14, pp. 485-512 Practice problems - Chapter 14: 2-8, 10, 12, 13; Physical Chemistry extra problems, free energy problems Free Energy
J- vs. H-type assembly: pentamethine cyanine (Cy5) as near IR chiroptical. reporter
 S1 Supporting Information: J- vs. H-type assembly: pentamethine cyanine (Cy5) as near IR chiroptical reporter Larysa I. Markova, a,b Vladimir L. Malinovskii, a Leonid D. Patsenker b and Robert Häner* a
S1 Supporting Information: J- vs. H-type assembly: pentamethine cyanine (Cy5) as near IR chiroptical reporter Larysa I. Markova, a,b Vladimir L. Malinovskii, a Leonid D. Patsenker b and Robert Häner* a
Protein folding. Today s Outline
 Protein folding Today s Outline Review of previous sessions Thermodynamics of folding and unfolding Determinants of folding Techniques for measuring folding The folding process The folding problem: Prediction
Protein folding Today s Outline Review of previous sessions Thermodynamics of folding and unfolding Determinants of folding Techniques for measuring folding The folding process The folding problem: Prediction
Pig Muscle Lactate Dehydrogenase with Oxidized Nicotinamide-Adenine
 Biochem. J. (1973) 135, 81-85 Printed in Great Britain 81 The Kinetics of the Interconversion of Intermediates of the Reaction of Pig Muscle Dehydrogenase with Oxidized Nicotinamide-Adenine Dinucleotide
Biochem. J. (1973) 135, 81-85 Printed in Great Britain 81 The Kinetics of the Interconversion of Intermediates of the Reaction of Pig Muscle Dehydrogenase with Oxidized Nicotinamide-Adenine Dinucleotide
Supporting Information. for. Formation of 1,10-phenanthroline-N,N -dioxide under mild conditions: the kinetics and
 Supporting Information for Formation of 1,10-phenanthroline-N,N -dioxide under mild conditions: the kinetics and mechanism of the oxidation of 1,10-phenanthroline by peroxomonosulfate ion (Oxone) Gábor
Supporting Information for Formation of 1,10-phenanthroline-N,N -dioxide under mild conditions: the kinetics and mechanism of the oxidation of 1,10-phenanthroline by peroxomonosulfate ion (Oxone) Gábor
Supplementary Information. Overlap between folding and functional energy landscapes for. adenylate kinase conformational change
 Supplementary Information Overlap between folding and functional energy landscapes for adenylate kinase conformational change by Ulrika Olsson & Magnus Wolf-Watz Contents: 1. Supplementary Note 2. Supplementary
Supplementary Information Overlap between folding and functional energy landscapes for adenylate kinase conformational change by Ulrika Olsson & Magnus Wolf-Watz Contents: 1. Supplementary Note 2. Supplementary
Experimental Design and Data Collection
 A. Checks to run to make sure the instrument is in good working condition B. Decide which experiment type is most appropriate, equilibrium or velocity C. Buffer considerations D. Speed selection and length
A. Checks to run to make sure the instrument is in good working condition B. Decide which experiment type is most appropriate, equilibrium or velocity C. Buffer considerations D. Speed selection and length
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1.
 Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Table S1. Overview of used PDZK1 constructs and their binding affinities to peptides. Related to figure 1. PDZK1 constru cts Amino acids MW [kda] KD [μm] PEPT2-CT- FITC KD [μm] NHE3-CT- FITC KD [μm] PDZK1-CT-
Supplementary Information for. Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase
 Supplementary Information for Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase Megan L Matthews, Wei-chen Chang, Andrew P Layne, Linde A Miles, Carsten Krebs, J Martin
Supplementary Information for Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase Megan L Matthews, Wei-chen Chang, Andrew P Layne, Linde A Miles, Carsten Krebs, J Martin
1901 Application of Spectrophotometry
 1901 Application of Spectrophotometry Chemical Analysis Problem: 1 Application of Spectroscopy Organic Compounds Organic compounds with single bonds absorb in the UV region because electrons from single
1901 Application of Spectrophotometry Chemical Analysis Problem: 1 Application of Spectroscopy Organic Compounds Organic compounds with single bonds absorb in the UV region because electrons from single
1 st European Union Science Olympiad in Dublin, Ireland. task B
 1 st European Union Science Olympiad in Dublin, Ireland task B Task B The Properties of Proteins Introduction In this task you will investigate some of the properties of proteins. Proteins consist of a
1 st European Union Science Olympiad in Dublin, Ireland task B Task B The Properties of Proteins Introduction In this task you will investigate some of the properties of proteins. Proteins consist of a
Supporting Information
 Supporting Information Figure S1. 2D ( 1 H- 1 H) COSY90 NMR (300 MHz, 3:1 TFA:TFA-d) spectrum of oligo(l-glu-co- 25%L-Cys) synthesized using from 7:3 L-Glu-(Et) 2 :L-Cys-Et, 0.5 M total substrate concentration,
Supporting Information Figure S1. 2D ( 1 H- 1 H) COSY90 NMR (300 MHz, 3:1 TFA:TFA-d) spectrum of oligo(l-glu-co- 25%L-Cys) synthesized using from 7:3 L-Glu-(Et) 2 :L-Cys-Et, 0.5 M total substrate concentration,
Complex Promoted by Electron-Deficient Alkenes. Brian V. Popp and Shannon S. Stahl*
 Oxidatively-Induced Reductive Elimination of Dioxygen from an η 2 -Peroxopalladium(II) Complex Promoted by Electron-Deficient Alkenes Brian V. Popp and Shannon S. Stahl* Department of Chemistry, University
Oxidatively-Induced Reductive Elimination of Dioxygen from an η 2 -Peroxopalladium(II) Complex Promoted by Electron-Deficient Alkenes Brian V. Popp and Shannon S. Stahl* Department of Chemistry, University
often display a deep green color due to where the SPR occurs (i.e., the wavelength of light that interacts with this specific morphology).
 Synthesis-Dependent Catalytic Properties of Gold Nanoparticles Nanoscience is the study of materials that have dimensions, intuitively, on the nanoscale, typically between 1 100 nm. This field has received
Synthesis-Dependent Catalytic Properties of Gold Nanoparticles Nanoscience is the study of materials that have dimensions, intuitively, on the nanoscale, typically between 1 100 nm. This field has received
Inter-flavin electron transfer in cytochrome P450 reductase effects of solvent and ph identify hidden complexity in mechanism
 Inter-flavin electron transfer in cytochrome P450 reductase effects of solvent and ph identify hidden complexity in mechanism Sibylle Brenner, Sam Hay, Andrew W. Munro and Nigel S. Scrutton Manchester
Inter-flavin electron transfer in cytochrome P450 reductase effects of solvent and ph identify hidden complexity in mechanism Sibylle Brenner, Sam Hay, Andrew W. Munro and Nigel S. Scrutton Manchester
LAB #3: FLUROESCENCE SPECTROSCOPY AND ELECTRON TRANSFER (This lab is adapted from the U of MN Phsyical Chemistry lab manual)
 Chemistry 372 Gustavus Adolphus College A. Purpose LAB #3: FLUROESCENCE SPECTROSCOPY AND ELECTRON TRANSFER (This lab is adapted from the U of MN Phsyical Chemistry lab manual) In this experiment, you will
Chemistry 372 Gustavus Adolphus College A. Purpose LAB #3: FLUROESCENCE SPECTROSCOPY AND ELECTRON TRANSFER (This lab is adapted from the U of MN Phsyical Chemistry lab manual) In this experiment, you will
Protein Folding experiments and theory
 Protein Folding experiments and theory 1, 2,and 3 Protein Structure Fig. 3-16 from Lehninger Biochemistry, 4 th ed. The 3D structure is not encoded at the single aa level Hydrogen Bonding Shared H atom
Protein Folding experiments and theory 1, 2,and 3 Protein Structure Fig. 3-16 from Lehninger Biochemistry, 4 th ed. The 3D structure is not encoded at the single aa level Hydrogen Bonding Shared H atom
Nature Structural and Molecular Biology: doi: /nsmb Supplementary Figure 1. Definition and assessment of ciap1 constructs.
 Supplementary Figure 1 Definition and assessment of ciap1 constructs. (a) ciap1 constructs used in this study are shown as primary structure schematics with domains colored as in the main text. Mutations
Supplementary Figure 1 Definition and assessment of ciap1 constructs. (a) ciap1 constructs used in this study are shown as primary structure schematics with domains colored as in the main text. Mutations
Supporting Information for. Near infrared-to-blue photon upconversion by exploiting direct. S-T absorption of a molecular sensitizer
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2017 Supporting Information for Near infrared-to-blue photon upconversion by
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2017 Supporting Information for Near infrared-to-blue photon upconversion by
Calculate a rate given a species concentration change.
 Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
( ) x10 8 m. The energy in a mole of 400 nm photons is calculated by: ' & sec( ) ( & % ) 6.022x10 23 photons' E = h! = hc & 6.
 Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
Chemistry 524--Final Exam--Keiderling May 4, :30 -?? pm SES
 Chemistry 524--Final Exam--Keiderling May 4, 2011 3:30 -?? pm -- 4286 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted. No open books or
Chemistry 524--Final Exam--Keiderling May 4, 2011 3:30 -?? pm -- 4286 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted. No open books or
Lecture 27. Transition States and Enzyme Catalysis
 Lecture 27 Transition States and Enzyme Catalysis Reading for Today: Chapter 15 sections B and C Chapter 16 next two lectures 4/8/16 1 Pop Question 9 Binding data for your thesis protein (YTP), binding
Lecture 27 Transition States and Enzyme Catalysis Reading for Today: Chapter 15 sections B and C Chapter 16 next two lectures 4/8/16 1 Pop Question 9 Binding data for your thesis protein (YTP), binding
Supporting Information
 Supporting Information Verification of specific G-quadruplex structure by using a novel cyanine dye supramolecular assembly: I. Recognizing mixed G-quadruplex in human telomeres Qianfan Yang, Junfeng Xiang,
Supporting Information Verification of specific G-quadruplex structure by using a novel cyanine dye supramolecular assembly: I. Recognizing mixed G-quadruplex in human telomeres Qianfan Yang, Junfeng Xiang,
Osmolyte-Induced Folding of an Intrinsically Disordered Protein: Folding Mechanism in the Absence of Ligand
 586 Biochemistry 21, 49, 586 596 DOI: 1.121/bi1222h Osmolyte-Induced Folding of an Intrinsically Disordered Protein: Folding Mechanism in the Absence of Ligand Yu-Chu Chang and Terrence G. Oas* Department
586 Biochemistry 21, 49, 586 596 DOI: 1.121/bi1222h Osmolyte-Induced Folding of an Intrinsically Disordered Protein: Folding Mechanism in the Absence of Ligand Yu-Chu Chang and Terrence G. Oas* Department
Circular Dichroism. For students of HI Computational Structural Biology
 T H E U N I V E R S I T Y of T E X A S S C H O O L O F H E A L T H I N F O R M A T I O N S C I E N C E S A T H O U S T O N Circular Dichroism For students of HI 6001-125 Computational Structural Biology
T H E U N I V E R S I T Y of T E X A S S C H O O L O F H E A L T H I N F O R M A T I O N S C I E N C E S A T H O U S T O N Circular Dichroism For students of HI 6001-125 Computational Structural Biology
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing
 Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Supplementary Figure 1. SDS-PAGE analysis of GFP oligomer variants with different linkers. Oligomer mixtures were applied to a PAGE gel containing 0.1% SDS without boiling. The gel was analyzed by a fluorescent
Outline. The ensemble folding kinetics of protein G from an all-atom Monte Carlo simulation. Unfolded Folded. What is protein folding?
 The ensemble folding kinetics of protein G from an all-atom Monte Carlo simulation By Jun Shimada and Eugine Shaknovich Bill Hawse Dr. Bahar Elisa Sandvik and Mehrdad Safavian Outline Background on protein
The ensemble folding kinetics of protein G from an all-atom Monte Carlo simulation By Jun Shimada and Eugine Shaknovich Bill Hawse Dr. Bahar Elisa Sandvik and Mehrdad Safavian Outline Background on protein
The ROXI Colorimeter & Fluorimeter. Laboratory Application I. Colorimetric measurements via Beer s Law.
 The ROXI Colorimeter & Fluorimeter. Laboratory Application I. Colorimetric measurements via Beer s Law. Required Supplies & Costs: RGB LED; $1.95 Light Sensors; $3.95 ea 3-way switch; $6.54 3 ohm resistor;
The ROXI Colorimeter & Fluorimeter. Laboratory Application I. Colorimetric measurements via Beer s Law. Required Supplies & Costs: RGB LED; $1.95 Light Sensors; $3.95 ea 3-way switch; $6.54 3 ohm resistor;
Corn Syrup Analysis E-51-1 PHOSPHORUS
 Corn Syrup Analysis E-51-1 PRINCIPLE SCOPE The sample is ignited in the presence of a fixative to destroy organic matter and convert phosphorus to inorganic phosphates which are not volatilized during
Corn Syrup Analysis E-51-1 PRINCIPLE SCOPE The sample is ignited in the presence of a fixative to destroy organic matter and convert phosphorus to inorganic phosphates which are not volatilized during
Degree of labeling (DOL)
 Degree of labeling (DOL) A written explanation for the determination of the degree of labeling when using NuLink reagents. The DOL is the average number of reagent molecules that have been covalently attached
Degree of labeling (DOL) A written explanation for the determination of the degree of labeling when using NuLink reagents. The DOL is the average number of reagent molecules that have been covalently attached
Title Quantitative Analysis of a Solution Containing Cobalt and Copper
 Title Quantitative Analysis of a Solution Containing Cobalt and Copper Name Manraj Gill (Lab partner: Tanner Adams) Abstract In this series of experiments, we determine the concentrations of two metal
Title Quantitative Analysis of a Solution Containing Cobalt and Copper Name Manraj Gill (Lab partner: Tanner Adams) Abstract In this series of experiments, we determine the concentrations of two metal
Binding Theory Equations for Affinity and Kinetics Analysis
 Technology Note #101 Binding Theory Equations for Affinity and Kinetics Analysis This technology note summarizes important equations underlying the theory of binding of solute analytes to surface-tethered
Technology Note #101 Binding Theory Equations for Affinity and Kinetics Analysis This technology note summarizes important equations underlying the theory of binding of solute analytes to surface-tethered
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/1/9/e1500511/dc1 Supplementary Materials for Contractility parameters of human -cardiac myosin with the hypertrophic cardiomyopathy mutation R403Q show loss of
advances.sciencemag.org/cgi/content/full/1/9/e1500511/dc1 Supplementary Materials for Contractility parameters of human -cardiac myosin with the hypertrophic cardiomyopathy mutation R403Q show loss of
Circular dichroism spectroscopic studies on structures formed by telomeric DNA sequences in vitro
 Circular dichroism spectroscopic studies on structures formed by telomeric DNA sequences in vitro ZHANG Xiaoyan 1, CAO Enhua 1, SUN Xueguang 1 & BAI Chunli 2 1. Institute of Biophysics, Chinese Academy
Circular dichroism spectroscopic studies on structures formed by telomeric DNA sequences in vitro ZHANG Xiaoyan 1, CAO Enhua 1, SUN Xueguang 1 & BAI Chunli 2 1. Institute of Biophysics, Chinese Academy
Kang, Lin-Woo, Ph.D. Professor Department of Biological Sciences Konkuk University Seoul, Korea nd Semester
 Kang, Lin-Woo, Ph.D. Professor Department of Biological Sciences Konkuk University Seoul, Korea 2018. 2 nd Semester Absorbance Assay (280 nm) Considerations for use Absorbance assays are fast and
Kang, Lin-Woo, Ph.D. Professor Department of Biological Sciences Konkuk University Seoul, Korea 2018. 2 nd Semester Absorbance Assay (280 nm) Considerations for use Absorbance assays are fast and
Hydrogen Bond Switching among Flavin and. Amino Acids Determines the Nature of Proton- Coupled Electron Transfer in BLUF.
 Hydrogen Bond Switching among Flavin and Amino Acids Determines the Nature of Proton- Coupled Electron Transfer in BLUF Photoreceptors Tilo Mathes 1,2, Jingyi Zhu 1, Ivo H.M. van Stokkum 1, M.L. Groot
Hydrogen Bond Switching among Flavin and Amino Acids Determines the Nature of Proton- Coupled Electron Transfer in BLUF Photoreceptors Tilo Mathes 1,2, Jingyi Zhu 1, Ivo H.M. van Stokkum 1, M.L. Groot
Novel fluorescent cationic benzothiazole dye response to G-quadruplex aptamer as a novel K + sensor
 Electronic Supplementary Material (ESI) for Analyst. This journal is The Royal Society of Chemistry 2017 Novel fluorescent cationic benzothiazole dye response to G-quadruplex aptamer as a novel K + sensor
Electronic Supplementary Material (ESI) for Analyst. This journal is The Royal Society of Chemistry 2017 Novel fluorescent cationic benzothiazole dye response to G-quadruplex aptamer as a novel K + sensor
BCHS 6229 Protein Structure and Function. Lecture 3 (October 18, 2011) Protein Folding: Forces, Mechanisms & Characterization
 BCHS 6229 Protein Structure and Function Lecture 3 (October 18, 2011) Protein Folding: Forces, Mechanisms & Characterization 1 The folding problem One of the greatest unsolved problems of Science The folding
BCHS 6229 Protein Structure and Function Lecture 3 (October 18, 2011) Protein Folding: Forces, Mechanisms & Characterization 1 The folding problem One of the greatest unsolved problems of Science The folding
Contents. xiii. Preface v
 Contents Preface Chapter 1 Biological Macromolecules 1.1 General PrincipIes 1.1.1 Macrornolecules 1.2 1.1.2 Configuration and Conformation Molecular lnteractions in Macromolecular Structures 1.2.1 Weak
Contents Preface Chapter 1 Biological Macromolecules 1.1 General PrincipIes 1.1.1 Macrornolecules 1.2 1.1.2 Configuration and Conformation Molecular lnteractions in Macromolecular Structures 1.2.1 Weak
LAB 05 Enzyme Action
 LAB 05 Enzyme Action Objectives: Name the substrate and products of the peroxidase-catalyzed reaction. To understand the terms: enzyme, activation energy, active site, ph, and denaturation. Distinguish
LAB 05 Enzyme Action Objectives: Name the substrate and products of the peroxidase-catalyzed reaction. To understand the terms: enzyme, activation energy, active site, ph, and denaturation. Distinguish
The Aβ40 and Aβ42 peptides self-assemble into separate homomolecular fibrils in binary mixtures but cross-react during primary nucleation
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2015 The Aβ40 and Aβ42 peptides self-assemble into separate homomolecular fibrils in binary
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2015 The Aβ40 and Aβ42 peptides self-assemble into separate homomolecular fibrils in binary
CONFOCHECK. Innovation with Integrity. Infrared Protein Analysis FT-IR
 CONFOCHECK Infrared Protein Analysis Innovation with Integrity FT-IR CONFOCHECK: FT-IR System for Protein Analytics FT-IR Protein Analysis Infrared spectroscopy measures molecular vibrations due to the
CONFOCHECK Infrared Protein Analysis Innovation with Integrity FT-IR CONFOCHECK: FT-IR System for Protein Analytics FT-IR Protein Analysis Infrared spectroscopy measures molecular vibrations due to the
it is assumed that only EH and ESH are catalytically active Michaelis-Menten equation for this model is:
 initial rates for many enzymatic reactions exhibit bell-shaped curves as a function of ph curves reflect the ionizations of certain amino acid residues that must be in a specific ionization state for enzyme
initial rates for many enzymatic reactions exhibit bell-shaped curves as a function of ph curves reflect the ionizations of certain amino acid residues that must be in a specific ionization state for enzyme
Optical Sensing for a Changing Planet. Smart Cuvettes!
 Optical Sensing for a Changing Planet Smart Cuvettes! phuvettes for optical ph measurements foxyvette for optical po 2 measurements phoxyvettes for both ph and po 2 727.230.1697 727.230.6845 fax info@spectrecology.com
Optical Sensing for a Changing Planet Smart Cuvettes! phuvettes for optical ph measurements foxyvette for optical po 2 measurements phoxyvettes for both ph and po 2 727.230.1697 727.230.6845 fax info@spectrecology.com
Reaction Thermodynamics
 Reaction Thermodynamics Thermodynamics reflects the degree to which a reaction is favored or disfavored Recall: G = Gibbs free energy = the energy available to do work ΔG = change in G of the system as
Reaction Thermodynamics Thermodynamics reflects the degree to which a reaction is favored or disfavored Recall: G = Gibbs free energy = the energy available to do work ΔG = change in G of the system as
Lecture 26: More on Gel Filtration Chromatography and the Trypsin Resurrection Experiment
 Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2018 Lecture 26: More on Gel Filtration Chromatography and the Trypsin Resurrection Experiment 12 April 2018 c David P. Goldenberg University
Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2018 Lecture 26: More on Gel Filtration Chromatography and the Trypsin Resurrection Experiment 12 April 2018 c David P. Goldenberg University
Super-Resolution Monitoring of Mitochondrial Dynamics upon. Time-Gated Photo-Triggered Release of Nitric Oxide
 Supporting Information for Super-Resolution Monitoring of Mitochondrial Dynamics upon Time-Gated Photo-Triggered Release of Nitric Oxide Haihong He a, Zhiwei Ye b, Yi Xiao b, *, Wei Yang b, *, Xuhong Qian
Supporting Information for Super-Resolution Monitoring of Mitochondrial Dynamics upon Time-Gated Photo-Triggered Release of Nitric Oxide Haihong He a, Zhiwei Ye b, Yi Xiao b, *, Wei Yang b, *, Xuhong Qian
Kinetic Isotope Effects
 1 Experiment 31 Kinetic Isotope Effects Isotopic substitution is a useful technique for the probing of reaction mechanisms. The change of an isotope may affect the reaction rate in a number of ways, providing
1 Experiment 31 Kinetic Isotope Effects Isotopic substitution is a useful technique for the probing of reaction mechanisms. The change of an isotope may affect the reaction rate in a number of ways, providing
Department of Chemistry and Biochemistry University of Lethbridge. Biochemistry II. Bioenergetics
 Department of Chemistry and Biochemistry University of Lethbridge II. Bioenergetics Slide 1 Bioenergetics Bioenergetics is the quantitative study of energy relationships and energy conversion in biological
Department of Chemistry and Biochemistry University of Lethbridge II. Bioenergetics Slide 1 Bioenergetics Bioenergetics is the quantitative study of energy relationships and energy conversion in biological
the erythrocyte membrane (fluorescent probes)
 Proc. Natl. Acad. Sci. USA Vol. 77, No. 12, pp. 7147-7151, December 1980 Biochemistry Kinetic study of the interaction of oxy- and deoxyhemoglobins with the erythrocyte membrane (fluorescent probes) N.
Proc. Natl. Acad. Sci. USA Vol. 77, No. 12, pp. 7147-7151, December 1980 Biochemistry Kinetic study of the interaction of oxy- and deoxyhemoglobins with the erythrocyte membrane (fluorescent probes) N.
