COMPARISON OF PROCESS OF DIFFUSION OF INTERSTITIAL OXYGEN ATOMS AND INTERSTITIAL HYDROGEN MOLECULES IN SILICON AND
|
|
|
- Shanon Chapman
- 5 years ago
- Views:
Transcription
1 COMPARISON OF PROCESS OF DIFFUSION OF INTERSTITIAL OXYGEN ATOMS AND INTERSTITIAL HYDROGEN MOLECULES IN SILICON AND GERMANIUM CRYSTALS: QUANTUMCHEMICAL SIMULATION Vasilii Gusakov Joint Institute of Solid State and Semiconductor Physics, P. Brovka str. 17, Minsk, Belarus The theoretical analysis of the process of diffusion of interstitial oxygen atoms and hydrogen molecules in silicon and germanium crystals has been performed. The calculated values of the activation energy and pre-exponential factor for an interstitial oxygen atom E(Si) = 2.59 ev, E(Ge) = 2.05 ev, D (Si)= 0.28 cm 2 s -1, D (Ge)= 0.39 cm 2 s -1 and interstitial hydrogen molecule E(Si) = ev, E(Ge) = ev D (Si)= cm 2 s -1, D (Ge)= cm 2 s -1 are in an excellent agreement with experimental ones and for the first time describe perfectly an experimental temperature dependence of an interstitial oxygen atom and hydrogen molecules diffusion constant in Si and Ge crystals. It is shown, that for a case of impurity atom with a strong interaction with a lattice (interstitial oxygen atom) process of diffusion has a cooperative nature - the activation energy and pre-exponential are controlled by the optimum position of three nearest lattice atoms. For a case of extended defect with a weak interaction (an interstitial hydrogen molecule) process of diffusion is determined by the activation barrier subjected to fluctuations connected with rotation of a hydrogen molecule. The effect of hydrostatic pressure on the process of diffusion is discussed also. INTRODUCTION Development of theoretical methods of determining the diffusivity of atoms in crystals is of great interest not only from a fundamental understanding microscopic process of diffusion, but also from a practical point of view. The reasoning is that practically all technological processes of microelectronics in some way or another are connected to diffusion of atoms
2 (defects) in crystals. Moreover, the diffusion in crystals (nanostructures) occurs very often under extreme conditions (very high temperatures, fields of stress, interfacial regions, nanomaterials etc) and that essentially impedes, makes expensive or even impossible an experimental research. However until now there have been many obscure questions related to the microscopic mechanism of diffusion in crystals, whenever migration of an impurity atom involves the breaking and forming of covalent bonds. Recently I advanced the general method of calculation of coefficient of diffusion of interstitial atom of oxygen in silicon and germanium crystals [1, 2]. Theoretical analysis performed in [1, 2] enables to calculate the diffusion coefficient-the preexponential factor and activation energy both at normal pressure and at a hydrostatic compression of a crystal lattice. The calculated values of coefficient of diffusion are in very good agreement with experimental results [1, 2]. An interstitial oxygen atom is strongly interacting atom with a crystal lattice. In this connection it is obviously important to consider other limiting case - diffusion of a particle weakly interacting with a crystal lattice and comparison of features of diffusion of strongly and weakly interacting particles with a crystal lattice. As a characteristic example in this article the comparative analysis of process of diffusion of interstitial oxygen atom (particle strongly interacting with a crystal lattice) and the interstitial hydrogen molecule(particle weakly interacting with a crystal lattice) in silicon (germanium) crystals under normal and hydrostatic pressure (HP) is carried out. To the best of my knowledge, no effects of HP on the H 2 diffusivity have been considered yet. THEORETICAL APPROACH Let us consider the physical parameters determining the diffusion process of an atom in a crystal. Modeling by the method of random walk results in the following general expression for the diffusion constant:
3 2 d N D = 2d s et Γ (1) where d is diffusion jump distance, N et is the number of the equivalent trajectories leaving the starting point, d s is the dimension of space (in our case d s =3), Γ is the average frequency of jumps on the distance d. In the case of a system consisting of N atoms, using the reaction-rate theory [3], the value of Г may be written in the following form N i= 1 ( o) i λ 1 i 0 E Γ = = exp a, 2π N (2) kbt λ ( b) i where Ea is the adiabatic potential energy difference between the saddle point and the stable one, 2( ob, ) λ i are the eigenvalues of the matrix (with respect to mass-weighted internal 2 ij eff i j coordinates) K U / f, = f ( ) Ueff f1,..., f m denotes the potential as a function of the internal degrees of freedom. The indices (b) and (o) indicate that the corresponding quantities are evaluated at the saddle point and local minimum, respectively. Thus, the diffusion constant D is determined by the following diffusion parameters: the length of diffusion jumps (d), the diffusion barrier ( Ea jumps (N et ) and the eigenvalues matrix ( ), the number of equivalent ways leaving the starting point of diffusion 2( ob, ) λ i ). The calculation of diffusion parameters was performed in a cluster approximation. For comparison with the previous calculations different methods such as empirical potential (MM2), semiempirical (AM1, PM3, PM5) and ab-initio (RHF, LDA) have been used for the calculation of the cluster total energy. Depending on the method of total energy calculation the cluster size was varied from 17 Si atoms (ab-initio methods) up to 10 3 Si atoms (semiempirical and empirical potential methods).
4 RESULTS AND DISCUSSION The starting and the final points of the diffusion jump correspond to the equilibrium configuration of an interstitial oxygen and interstitial hydrogen molecule (H 2 ) in a crystal. Individual oxygen atom occupies interstitial bond-center (BC) position in silicon and to diffuse by jumping between the neighboring BC sites. Hence the starting and the final points of the diffusion jump correspond to the equilibrium BC configuration of an interstitial oxygen atom (O i ) in silicon. The calculated equilibrium configuration of O i and the local vibration frequency of the asymmetric stretching mode (B 1 ) takes the following values: d =1.63 Å (RHF, 6- Si O 31G ** ), Å (MM2), 1.61 Å (PM5), Si O Si =161.6 o (RHF, 6-31G ** ), o (MM2), 171 o (PM5), ν O = 1214 cm -1 (RHF, 6-31G ** ), 1091 cm -1 (AM1), 1078 cm -1 (LDA) and are in a good agreement with experimental [4, 5] and recently calculated ones [6, 7]. The calculated value of the potential barrier for the rotation of O i around Si-Si axis equals E ϕ 20 mev (PM5). As E ϕ is much less than k B T (at diffusion temperatures) an interstitial oxygen atom jumps on any of six nearest Si-Si bonds (Fig. 1.) and, hence, in Formula (1) the parameters N et = 6 and d=0.19 nm. The calculated equilibrium configuration of H 2 corresponds to position of the centre of mass of a molecule in the tetrahedral interstitial (T) site of crystal. The calculated equilibrium configuration of H 2 and the stretch vibrational frequencies takes the following values: = nm, (Si) =0.074 nm, n(si) = 3413 cm -1, n(ge) = 3586 cm -1 (PM3, PM5) and are d H H d H H in a good agreement with recently calculated ones [8]. The Mulliken population analysis provides a qualitative value for the effective charge associated with each atom. Both H atoms in H 2 carry a small positive charge (Q(H 2 ) ~+0.06e). Some electron density is transferred from the H 2 molecule to its Si neighbors. The calculated value of the potential barrier for the rotation of (Ge) H 2 equals E φ 6 mev (PM5). The barrier E for rotation of the H H dumbbell around its φ CM is much less than k B T, implying a nearly-free rotator at the T site. Owing to thermal fluctuations the CM moves randomly in the volume limited by isoenergetic surface with the
5 maximal displacement r max nm. The value r is in a good agreement with the max results of modeling by a molecular dynamic method [8]. Since the isoenergetic surface is anisotropic (calculated normalized diagonal matrix of the second derivatives in T configurations has the following value 2 xxe = 1, 0.79, 0.7 ), the diffusion transition occurs in a direction of maximal displacement H 2 between the nearest T configurations along the trigonal axis from T to hexagonal to T sites (T-H-T path). In this case the number of equivalent trajectories N et = 1 and the calculated distance of diffusion transition has the following value d = nm. To determine the activation barrier of diffusion the simulation of probable diffusion trajectories was performed. The oxygen atom (hydrogen molecule) was displaced from the equilibrium configuration along a trajectory in the direction to a new equilibrium position (nearest Si-Si bonds for the case of O i or T site for the case of H 2 molecule). Owing to thermal fluctuations crystal lattice atoms may occupy any permissible positions at the given position of O i or H 2 but all of these configurations differ in the total energy of a crystal. Since the diffusion constant exponentially depends on the diffusion barrier (2) we should pick the minimal value of Ea E = min[ E ( S ) E ( O)], (3) a cl G cl where Ecl ( S G ) and Ecl ( O) are the total cluster energy on the surface S G (on this surface energy has a local maximum) and in local minimum O (equilibrium configuration), respectively. It means that for determining of the minimal value of Ea we should minimize total energy of cluster as a function of atoms coordinates of crystal lattice. Along the given trajectory the minimal total cluster energy has been calculated and among the set of calculated trajectories the extreme trajectory satisfying condition (3) was selected. The simulation has revealed an important fact for understanding of the diffusion process. Ecl ( S G ) and hence Ea depends on the number of the crystal lattice atoms (n) nearest to diffused particle (O i or H 2 ) involved in the
6 minimization of the cluster total energy. The activation barrier Ea (n) decreases with increase of the number of crystal lattice atoms participating in minimization (Fig 1.). Therefore, first of all, we should determine how many of the nearest crystal lattice atoms are involved in the diffusion process. A diffused particle can overcome a barrier at any optimum configuration of crystal lattice atoms. However the relative number of particles diffused at the given optimum configuration of crystal lattice atoms is proportional to the product of probabilities of occurrence of the optimum configuration P occ and probability of diffusion jump P dj (P dj exp(- DE a (n)/k B T)). The probability of occurrence of an optimum configuration out of n atoms have been calculated on the basis of geometrical definition of probability (the problem of a random collisions) and in this case the product P occ P dj may be written as n 1 τ ( n) E ( ) exp a n PoccPdj n, τ ( n) kbt (4) where n is the number of atoms in the optimum configuration, τ ( n) and τ ( n) are the period of formation and lifetime of the given optimum configuration, respectively, E a (n) is the diffusion barrier. Usually τ ( n)/ τ ( n) is much less than one [3]. The dependence P occ P dj as function of n Si atoms involved in the minimization is depicted in the insets of Fig. 1. Calculations have shown, that P occ P dj for interstitial oxygen atom has a sharp maximum at n=3 which more than an order of magnitude exceeds P (n) for n=2, 4, 5 Hence, essentially all O i atoms overcome the diffusion barrier when three nearest Si atoms (Si atoms connected to O i atom before and after diffusion jump Si(1) - O initial Si(2) O final Si(3)) are in the optimum configuration. The result obtained suggests that for a case of impurity atom with a strong interaction with a lattice (interstitial oxygen atom) process of diffusion has a cooperative nature - the activation energy and pre-exponential are controlled by the optimum position of three nearest lattice atoms and the diffusion parameters should be calculated for the given configuration. For interstitial hydrogen
7 molecules P occ P dj (n) is a rapidly decreasing function. It means that practically all H 2 molecules diffuse without correlation with the position of the nearest atoms of crystal lattice and in this case the diffusion transition is not a cooperative transition. Let s consider variation of total cluster energy lengthways of diffusion trajectories. A number of the dependences calculated near to a maximum of a potential energy are presented in Fig. 2. For interstitial hydrogen molecule the maximum of a potential barrier is located symmetrically relative initial and final points of diffusion transition of H 2 and potential function of energy is a saddle surfaces. As orientation of H 2 molecule in a saddle point is arbitrary, the activation barrier is a stochastic function of orientation of H 2 molecule and the process of diffusion of a rotating molecule should be considered as process of diffusion in a fluctuating potential [9]. The activation barrier was evaluated for three orientations of H 2 molecule in a saddle point (the axis of a H 2 molecule is directed lengthways x. y, z coordinate axes where the axis x was perpendicular planes H). For a molecule of hydrogen the diffusion transition is not cooperative, and as consequence, the maximum of a potential barrier is located symmetrically concerning initial and final points of direct and reverse diffusion transition. In the case of interstitial oxygen atom the variation of total cluster energy lengthways of diffusion trajectories has essentially other nature (Fig. 2.) For the extreme trajectory (3) the maximum of the potential energy (a saddle point of Oi migration) is displaced from the midpoint of the path in a direction to a final point of diffusion transition and a direct trajectory do not coincide with a return trajectory. The displacement of a maximum of potential energy is caused by cooperative nature of diffusion transition - correlated displacement of three atoms - Si(1) - O initial - Si(2) - O final - Si(3) occurs, and the displacement is far more for Ge crystals. In a vicinity of a maximum the abrupt variation of potential energy is observed. Similar dependence of potential energy lengthways of diffusion trajectories is characteristic for atoms strongly interacting with a crystal lattice. Really, abrupt variation of potential energy in vicinity of a maximum is caused by reconfiguration of an electronic subsystem of a crystal. In the course of transition of O i atom
8 from one equilibrium configuration in another the breaking of old and formation of new covalent Si-O bonds takes place. The process of reconfiguration of an electronic subsystem will occur in the case when oxygen and neighboring silicon atoms owing to thermal fluctuations get in the region of configuration space G (bounded by the critical surface S G ) where the electronic reconfiguration leads to lowering of the crystal total energy. Matrix 2( ob, ) λ i necessary for the calculation of the pre-exponential factor D 0 was evaluated as follows. At the equilibrium configuration of interstitial oxygen O i or interstitial hydrogen molecule H 2 and at the intersection point of the extreme trajectory of O i or H 2 with surface S G the square-law interpolation of the potential energy U ( f 1,..., f ) eff m (f 1 f m are coordinates of O i or H 2 and nearest Si atoms) has been constructed and 2( ob, ) λ i has been obtained at once by diagonalization of K ij. Calculated values of pre-exponential factor and activation barrier for diffusion of interstitial oxygen atom and interstitial hydrogen molecules are presented in Table I and Fig. 3. On Figure 3 one can see the excellent agreement between the calculated and experimental temperature dependences of the diffusion coefficient in all temperature range T= o C for O i and T= o C for H 2. For better understanding of O i and H 2 diffusion the influence of compressive hydrostatic pressure (HP) on the diffusion coefficient has been evaluated. This is particularly interesting as high HP has been found to enhance strongly the oxygen agglomeration at elevated temperatures [10 13]. Recently [1, 2, 14] I have shown that under HP the activation barrier of diffusion of interstitial oxygen in Si crystals is decreased and that is the reason to enhance strongly the oxygen agglomeration at elevated temperatures. Modelling of influence of HP upon the diffusion of interstitial hydrogen molecule was carried out as in [14]. The cluster has been conventionally divided into internal (R < R 0 ) and external parts (R>R 0 ). The internal part includes diffused particle and is selected in such a manner that the increase in R 0 does not result in essential change of the equilibrium structure of O i or H 2 defect at pressure P=0 (usually R o equals nm). The pressure has been modeled by replacement of the equilibrium length of bonds in the external part of the cluster with the length of Si-Si bonds that are characteristic (calculated
9 from experimental value of compressibility modulus) for the given pressure. Upon minimization of the cluster total energy, the lengths of bonds at R>R 0 did not vary, and the minimization was carried out on the coordinates of O i or H 2 and crystal lattice atoms being in the internal part of the cluster. The further evaluation of E a (P) was carried out similarly to the case P=0. Calculations have revealed that HP leads to variation of the activation barrier. Linear interpolation of E a (P) dependence E ( P) / E (0) = 1+ γ P (5) a a result in the following value of γ (O i ) = -( ) Pa -1 and γ (H 2 ) = ( ) Pa -1, P is the HP. The calculated pressure dependence of the O i diffusivity (without any adjustable parameters) corresponds well to an enhanced growth of the oxygen-related thermal donors (TDs) observed experimentally [1, 2, 14]. It is interesting to note that HP decreases the activation barrier for diffusion of interstitial oxygen (γ (O i ) < 0) also increases activation barrier for diffusion of an interstitial hydrogen molecule (γ (H 2 ) > 0). Qualitatively it is possible to explain the result obtained as follows. In a case of interstitial oxygen atom the value of activation barrier is determined by deformation of covalent Si-O bond in a saddle point. HP results in compression of a crystal and the distance between initial and final points of diffusion transition is decreased. Hence, in a saddle point the value of deformation of covalent Si-O bonds will be less and the activation barrier will decrease with increase of pressure. In the case of interstitial hydrogen molecule the activation barrier is determined by interaction of H 2 molecule and the nearest Si atoms in the saddle point. Under HP the distance between Si atoms in a saddle point is decreased, overlapping of molecular orbitals of hydrogen molecules and Si atoms are increased and, hence, the growth of activation barrier is observed. The calculated value g(h 2 ) has a sufficiently big average mistake that points to a possible nonlinear dependence of E a (P). Moreover, as preliminary calculations have shown in case of diffusion of interstitial H 2 in Ge and Si crystals the pre-exponential factor is a function of HP and that demands more detailed calculations of coefficient of diffusion of H 2 under hydrostatic pressure. These calculations are in progress.
10 SUMMARY In summary, a theoretical modeling and comparative analysis of the interstitial oxygen and interstitial hydrogen molecule diffusivity in silicon and germanium crystals at normal and hydrostatic pressure has been presented. On the basis of the results obtained it is possible to draw the following conclusions. The process of diffusion of strongly interacting particle (interstitial oxygen atom) with crystal lattice is a cooperative process. Three nearest crystal lattice Si (Ge) atoms are involved in an elementary oxygen jump from a bond-center site to another bond-center site along a path in the (110) plane. It is precisely their optimum position (corresponding to a local minimum of the crystal total energy) which determines the value of the diffusion parameters of an interstitial oxygen atom in silicon and germanium. For the case of weakly interacting particle with crystal lattice (interstitial hydrogen molecules) the process of diffusion is not correlated with the position of crystal lattice atoms. But in the case of H 2 the diffusion parameters is subjected to fluctuations connected with rotation of a hydrogen molecule. The theoretically determined values of the diffusion potential barrier and pre-exponential factor are in excellent agreement with experimental ones and describe very well the experimental temperature dependence of diffusion constant in Si crystals. Hydrostatic pressure gives rise to the decrease of the diffusion potential barrier for interstitial oxygen and increases the diffusion potential barrier for interstitial hydrogen molecule in Si crystals Such a pressure dependence of O i diffusivity appears most likely to be responsible for the HP enhancement in generation of the oxygenrelated thermal donors. ACKNOWLEDGMENT The author is grateful to the CADRES, INTAS and the State Committee for Science and Technology and Fund for Fundamental Research of the Republic of Belarus (grants , ) for financial supports.
11 REFERENCES 1. Vasilii Gusakov, J. Phys.: Condens. Matter 17 (2005) S Vasilii Gusakov, Solid State Phenomena (2005) P. Hänggi, P. Talkner, and M. Borcovec, Rev. Mod. Phys. 62 (1990) R. C. Newman and B. Jones in: Oxygen in Silicon, Semiconductors and Semimetals,, edited by F Shimura (Academic Press, London) 42(1994) R. C. Newman, J. Phys.: Condens. Matter 12 (2000) R M. Pesola, J. von Boehm, T. Mattila et al. Phys. Rev. B 60 (1999) J. Coutinho, R. Jones, P. R. Briddon et al. Phys. Rev. B 62 (2000) S. K. Estreicher, K. Wells, P. A. Fedders and Pablo Ordejòn, J. Phys.: Condens. Matter 13 (2001) P. Reimann and P. Hanggi, Lect. Notes in Physics 484 (1998) A. Misiuk, Mater. Phys. Mech. 1 (2000) V. V. Emtsev, B. A. Andreev, A. Misiuk et al. Appl. Phys. Lett. 71 (1997) V. V. Emtsev Jr, C. A. J. Ammerlaan, V. V. Emtsev et al. Phys. Stat. Sol. (b) 235 (200) I. V. Antonova, A. Misiuk, V. P. Popov et al. Physica B 225 (996) Vasilii Gusakov, Leonid Murin, J. Phys. B (2003) J. C. Mikkelsen, Matter. Res. Soc. Symp. Proc. 59 (1986) V. P. Markevich, M. Suezava, L. I. Murin, Materials Science and Engineering B 58 (1999) N. M. Johnson and C. Herring, Phys. Rev B 43 (1991) J.W. Corbett, R. S. McDonald and G. D. Watkins, J. Phys. Chem. Solids 25 (1964) 873.
12 Table I. Calculated values of diffusion parameter for interstitial oxygen and interstitial hydrogen molecules in silicon and germanium crystals Si Ge Theory Experiment Theory Experiment [18] [15, 16, 17] O i H 2 O i H 2 O i H 2 O i H 2 DE a, ev D 0, cm 2 s ÿ ( )ÿ ÿ
13 CAPTURES ON FIGURES Figure 1. Diffusion barrier DE a (n) as a function of the number of Si atoms involved in minimization of the total cluster energy. In the inset the probability P occ P dj of occurrence of an optimum configuration out of n atoms is presented (Dt(n)/t(n)=0.01). Figure 2. Variation of total cluster energy lengthways of diffusion trajectories. Figure 3. Temperature dependence of diffusion constant of interstitial oxygen atom and interstitial hydrogen molecules in silicon. Points experiment [15, 17], line theory.
14 P(N at )/P(3) P(Nat)/P(1) E a (ev) Si(8) Si(2) Si(9) N Si(7) at Si(4) O i Si(3) Si(5) Si(6) Ε a (ev) H 2 Si(2) Si(5) Si(3) Si(4) 2 4 N at N at N at 1
15 H 2 E, ab. u O i O i E, ab. u H d, nm -3.6 H d, nm 2
16 10-8 O i H D, cm 2 s D, cm 2 s /T, K /T, K -1 3
Lee, Young Joo; von Boehm, J.; Nieminen, Risto Simulation of the kinetics of oxygen complexes in crystalline silicon
 Powered by TCPDF (www.tcpdf.org) This is an electronic reprint of the original article. This reprint may differ from the original in pagination and typographic detail. Lee, Young Joo; von Boehm, J.; Nieminen,
Powered by TCPDF (www.tcpdf.org) This is an electronic reprint of the original article. This reprint may differ from the original in pagination and typographic detail. Lee, Young Joo; von Boehm, J.; Nieminen,
3.091 Introduction to Solid State Chemistry. Lecture Notes No. 5a ELASTIC BEHAVIOR OF SOLIDS
 3.091 Introduction to Solid State Chemistry Lecture Notes No. 5a ELASTIC BEHAVIOR OF SOLIDS 1. INTRODUCTION Crystals are held together by interatomic or intermolecular bonds. The bonds can be covalent,
3.091 Introduction to Solid State Chemistry Lecture Notes No. 5a ELASTIC BEHAVIOR OF SOLIDS 1. INTRODUCTION Crystals are held together by interatomic or intermolecular bonds. The bonds can be covalent,
Kinetic lattice Monte Carlo simulations of diffusion processes in Si and SiGe alloys
 Kinetic lattice Monte Carlo simulations of diffusion processes in Si and SiGe alloys, Scott Dunham Department of Electrical Engineering Multiscale Modeling Hierarchy Configuration energies and transition
Kinetic lattice Monte Carlo simulations of diffusion processes in Si and SiGe alloys, Scott Dunham Department of Electrical Engineering Multiscale Modeling Hierarchy Configuration energies and transition
Asymmetry of Endofullerenes with Silver Atoms
 Asymmetry of Endofullerenes with Silver Atoms V.S. Gurin Physico-Chemical Research Institute, Belarusian State University Leningradskaja str., 14, Minsk, 220080, BELARUS; E-mail: gurin@bsu.by; gurinvs@lycos.com
Asymmetry of Endofullerenes with Silver Atoms V.S. Gurin Physico-Chemical Research Institute, Belarusian State University Leningradskaja str., 14, Minsk, 220080, BELARUS; E-mail: gurin@bsu.by; gurinvs@lycos.com
Structural and Electronic Properties of Small Silicon Nanoclusters
 Structural and Electronic Properties of Small Silicon Nanoclusters Prabodh Sahai Saxena a* and Amerendra Singh Sanger b a Physics Department, Lakshmi Narain College of Technology Excellence, Bhopal, India.
Structural and Electronic Properties of Small Silicon Nanoclusters Prabodh Sahai Saxena a* and Amerendra Singh Sanger b a Physics Department, Lakshmi Narain College of Technology Excellence, Bhopal, India.
Lecture 1. OUTLINE Basic Semiconductor Physics. Reading: Chapter 2.1. Semiconductors Intrinsic (undoped) silicon Doping Carrier concentrations
 Lecture 1 OUTLINE Basic Semiconductor Physics Semiconductors Intrinsic (undoped) silicon Doping Carrier concentrations Reading: Chapter 2.1 EE105 Fall 2007 Lecture 1, Slide 1 What is a Semiconductor? Low
Lecture 1 OUTLINE Basic Semiconductor Physics Semiconductors Intrinsic (undoped) silicon Doping Carrier concentrations Reading: Chapter 2.1 EE105 Fall 2007 Lecture 1, Slide 1 What is a Semiconductor? Low
Joint ICTP-IAEA Workshop on Physics of Radiation Effect and its Simulation for Non-Metallic Condensed Matter.
 2359-3 Joint ICTP-IAEA Workshop on Physics of Radiation Effect and its Simulation for Non-Metallic Condensed Matter 13-24 August 2012 Electrically active defects in semiconductors induced by radiation
2359-3 Joint ICTP-IAEA Workshop on Physics of Radiation Effect and its Simulation for Non-Metallic Condensed Matter 13-24 August 2012 Electrically active defects in semiconductors induced by radiation
Lecture 2. Semiconductor Physics. Sunday 4/10/2015 Semiconductor Physics 1-1
 Lecture 2 Semiconductor Physics Sunday 4/10/2015 Semiconductor Physics 1-1 Outline Intrinsic bond model: electrons and holes Charge carrier generation and recombination Intrinsic semiconductor Doping:
Lecture 2 Semiconductor Physics Sunday 4/10/2015 Semiconductor Physics 1-1 Outline Intrinsic bond model: electrons and holes Charge carrier generation and recombination Intrinsic semiconductor Doping:
Density of states for electrons and holes. Distribution function. Conduction and valence bands
 Intrinsic Semiconductors In the field of semiconductors electrons and holes are usually referred to as free carriers, or simply carriers, because it is these particles which are responsible for carrying
Intrinsic Semiconductors In the field of semiconductors electrons and holes are usually referred to as free carriers, or simply carriers, because it is these particles which are responsible for carrying
Structure and dynamics of the diarsenic complex in crystalline silicon
 Structure and dynamics of the diarsenic complex in crystalline silicon Scott A. Harrison, Thomas F. Edgar, and Gyeong S. Hwang* Department of Chemical Engineering, University of Texas, Austin, Texas 78713,
Structure and dynamics of the diarsenic complex in crystalline silicon Scott A. Harrison, Thomas F. Edgar, and Gyeong S. Hwang* Department of Chemical Engineering, University of Texas, Austin, Texas 78713,
Russia. St. Petersburg, , Russia
 PACS 81.07.Bc, 61.72.Cc An asymptotic formula for displacement field in triangular lattice with vacancy V.A. Tsaplin 1, V.A. Kuzkin 1,2 vtsaplin@yandex.ru, kuzkinva@gmail.com 1 Institute for Problems in
PACS 81.07.Bc, 61.72.Cc An asymptotic formula for displacement field in triangular lattice with vacancy V.A. Tsaplin 1, V.A. Kuzkin 1,2 vtsaplin@yandex.ru, kuzkinva@gmail.com 1 Institute for Problems in
Electro - Principles I
 Electro - Principles I Page 10-1 Atomic Theory It is necessary to know what goes on at the atomic level of a semiconductor so the characteristics of the semiconductor can be understood. In many cases a
Electro - Principles I Page 10-1 Atomic Theory It is necessary to know what goes on at the atomic level of a semiconductor so the characteristics of the semiconductor can be understood. In many cases a
Semiconductor physics I. The Crystal Structure of Solids
 Lecture 3 Semiconductor physics I The Crystal Structure of Solids 1 Semiconductor materials Types of solids Space lattices Atomic Bonding Imperfection and doping in SOLIDS 2 Semiconductor Semiconductors
Lecture 3 Semiconductor physics I The Crystal Structure of Solids 1 Semiconductor materials Types of solids Space lattices Atomic Bonding Imperfection and doping in SOLIDS 2 Semiconductor Semiconductors
VIRTUAL LATTICE TECHNIQUE AND THE INTERATOMIC POTENTIALS OF ZINC-BLEND-TYPE BINARY COMPOUNDS
 Modern Physics Letters B, Vol. 16, Nos. 5 & 6 (2002) 187 194 c World Scientific Publishing Company VIRTUAL LATTICE TECHNIQUE AND THE INTERATOMIC POTENTIALS OF ZINC-BLEND-TYPE BINARY COMPOUNDS LIU YING,
Modern Physics Letters B, Vol. 16, Nos. 5 & 6 (2002) 187 194 c World Scientific Publishing Company VIRTUAL LATTICE TECHNIQUE AND THE INTERATOMIC POTENTIALS OF ZINC-BLEND-TYPE BINARY COMPOUNDS LIU YING,
collisions of electrons. In semiconductor, in certain temperature ranges the conductivity increases rapidly by increasing temperature
 1.9. Temperature Dependence of Semiconductor Conductivity Such dependence is one most important in semiconductor. In metals, Conductivity decreases by increasing temperature due to greater frequency of
1.9. Temperature Dependence of Semiconductor Conductivity Such dependence is one most important in semiconductor. In metals, Conductivity decreases by increasing temperature due to greater frequency of
Introduction to DFTB. Marcus Elstner. July 28, 2006
 Introduction to DFTB Marcus Elstner July 28, 2006 I. Non-selfconsistent solution of the KS equations DFT can treat up to 100 atoms in routine applications, sometimes even more and about several ps in MD
Introduction to DFTB Marcus Elstner July 28, 2006 I. Non-selfconsistent solution of the KS equations DFT can treat up to 100 atoms in routine applications, sometimes even more and about several ps in MD
Supporting information for Polymer interactions with Reduced Graphene Oxide: Van der Waals binding energies of Benzene on defected Graphene
 Supporting information for Polymer interactions with Reduced Graphene Oxide: Van der Waals binding energies of Benzene on defected Graphene Mohamed Hassan, Michael Walter *,,, and Michael Moseler, Freiburg
Supporting information for Polymer interactions with Reduced Graphene Oxide: Van der Waals binding energies of Benzene on defected Graphene Mohamed Hassan, Michael Walter *,,, and Michael Moseler, Freiburg
Kinetic Monte Carlo: from transition probabilities to transition rates
 Kinetic Monte Carlo: from transition probabilities to transition rates With MD we can only reproduce the dynamics of the system for 100 ns. Slow thermallyactivated processes, such as diffusion, cannot
Kinetic Monte Carlo: from transition probabilities to transition rates With MD we can only reproduce the dynamics of the system for 100 ns. Slow thermallyactivated processes, such as diffusion, cannot
Diffusion. Diffusion = the spontaneous intermingling of the particles of two or more substances as a result of random thermal motion
 Diffusion Diffusion = the spontaneous intermingling of the particles of two or more substances as a result of random thermal motion Fick s First Law Γ ΔN AΔt Γ = flux ΔN = number of particles crossing
Diffusion Diffusion = the spontaneous intermingling of the particles of two or more substances as a result of random thermal motion Fick s First Law Γ ΔN AΔt Γ = flux ΔN = number of particles crossing
Unmanageable Defects in Proton- Irradiated Silicon: a Factual Outlook for Positron Probing N. Yu. Arutyunov 1,2, M. Elsayed 1, R.
 Unmanageable Defects in Proton- Irradiated Silicon: a Factual Outlook for Positron Probing N. Yu. Arutyunov 1,2, M. Elsayed 1, R. Krause-Rehberg 1 1 Department of Physics, Martin Luther University, 06120
Unmanageable Defects in Proton- Irradiated Silicon: a Factual Outlook for Positron Probing N. Yu. Arutyunov 1,2, M. Elsayed 1, R. Krause-Rehberg 1 1 Department of Physics, Martin Luther University, 06120
Mat E 272 Lecture 25: Electrical properties of materials
 Mat E 272 Lecture 25: Electrical properties of materials December 6, 2001 Introduction: Calcium and copper are both metals; Ca has a valence of +2 (2 electrons per atom) while Cu has a valence of +1 (1
Mat E 272 Lecture 25: Electrical properties of materials December 6, 2001 Introduction: Calcium and copper are both metals; Ca has a valence of +2 (2 electrons per atom) while Cu has a valence of +1 (1
Stress in Flip-Chip Solder Bumps due to Package Warpage -- Matt Pharr
 Stress in Flip-Chip Bumps due to Package Warpage -- Matt Pharr Introduction As the size of microelectronic devices continues to decrease, interconnects in the devices are scaling down correspondingly.
Stress in Flip-Chip Bumps due to Package Warpage -- Matt Pharr Introduction As the size of microelectronic devices continues to decrease, interconnects in the devices are scaling down correspondingly.
ATOMISTIC MODELING OF BORON ACTIVATION AND DIFFUSION IN STRAINED SIGE
 ATOMISTIC MODELING OF BORON ACTIVATION AND DIFFUSION IN STRAINED SIGE Scott T. Dunham,, Jakyoung Song, and Chihak Ahn Dept. of Electrical Engineering, Dept. of Physics University of Washington, Box 35500,
ATOMISTIC MODELING OF BORON ACTIVATION AND DIFFUSION IN STRAINED SIGE Scott T. Dunham,, Jakyoung Song, and Chihak Ahn Dept. of Electrical Engineering, Dept. of Physics University of Washington, Box 35500,
Introduction to Engineering Materials ENGR2000. Dr.Coates
 Introduction to Engineering Materials ENGR2000 Chapter 18: Electrical Properties Dr.Coates 18.2 Ohm s Law V = IR where R is the resistance of the material, V is the voltage and I is the current. l R A
Introduction to Engineering Materials ENGR2000 Chapter 18: Electrical Properties Dr.Coates 18.2 Ohm s Law V = IR where R is the resistance of the material, V is the voltage and I is the current. l R A
Computer simulation of the Poisson's ratio of soft polydisperse discs at zero temperature
 Computer simulation of the Poisson's ratio of soft polydisperse discs at zero temperature JAKUB NAROJCZYK KRZYSZTOF W. WOJCIECHOWSKI Institute of Molecular Physics Polish Academy of Sciences ul. M. Smoluchowskiego
Computer simulation of the Poisson's ratio of soft polydisperse discs at zero temperature JAKUB NAROJCZYK KRZYSZTOF W. WOJCIECHOWSKI Institute of Molecular Physics Polish Academy of Sciences ul. M. Smoluchowskiego
dynamics computer code, whiccan simulate the motion of atoms at a surface and We coembininewton's equation for the nuclei,
 DUI FILE COpy T DTIC ANNUAL LETTER REPORT BY 0. F. ELEC33 20KE AD-A224 069 P.1. FOR N00014-85-K-0442 U Theory of electronic states and formations energies of.defect comp exes, interstitial defects, and
DUI FILE COpy T DTIC ANNUAL LETTER REPORT BY 0. F. ELEC33 20KE AD-A224 069 P.1. FOR N00014-85-K-0442 U Theory of electronic states and formations energies of.defect comp exes, interstitial defects, and
Molecular Dynamics Simulations of Fusion Materials: Challenges and Opportunities (Recent Developments)
 Molecular Dynamics Simulations of Fusion Materials: Challenges and Opportunities (Recent Developments) Fei Gao gaofeium@umich.edu Limitations of MD Time scales Length scales (PBC help a lot) Accuracy of
Molecular Dynamics Simulations of Fusion Materials: Challenges and Opportunities (Recent Developments) Fei Gao gaofeium@umich.edu Limitations of MD Time scales Length scales (PBC help a lot) Accuracy of
The samples used in these calculations were arranged as perfect diamond crystal of
 Chapter 5 Results 5.1 Hydrogen Diffusion 5.1.1 Computational Details The samples used in these calculations were arranged as perfect diamond crystal of a2 2 2 unit cells, i.e. 64 carbon atoms. The effect
Chapter 5 Results 5.1 Hydrogen Diffusion 5.1.1 Computational Details The samples used in these calculations were arranged as perfect diamond crystal of a2 2 2 unit cells, i.e. 64 carbon atoms. The effect
Physics 211B : Problem Set #0
 Physics 211B : Problem Set #0 These problems provide a cross section of the sort of exercises I would have assigned had I taught 211A. Please take a look at all the problems, and turn in problems 1, 4,
Physics 211B : Problem Set #0 These problems provide a cross section of the sort of exercises I would have assigned had I taught 211A. Please take a look at all the problems, and turn in problems 1, 4,
Basic Semiconductor Physics
 6 Basic Semiconductor Physics 6.1 Introduction With this chapter we start with the discussion of some important concepts from semiconductor physics, which are required to understand the operation of solar
6 Basic Semiconductor Physics 6.1 Introduction With this chapter we start with the discussion of some important concepts from semiconductor physics, which are required to understand the operation of solar
Lecture 2 Electrons and Holes in Semiconductors
 EE 471: Transport Phenomena in Solid State Devices Spring 2018 Lecture 2 Electrons and Holes in Semiconductors Bryan Ackland Department of Electrical and Computer Engineering Stevens Institute of Technology
EE 471: Transport Phenomena in Solid State Devices Spring 2018 Lecture 2 Electrons and Holes in Semiconductors Bryan Ackland Department of Electrical and Computer Engineering Stevens Institute of Technology
Supplementary Materials
 Supplementary Materials Sample characterization The presence of Si-QDs is established by Transmission Electron Microscopy (TEM), by which the average QD diameter of d QD 2.2 ± 0.5 nm has been determined
Supplementary Materials Sample characterization The presence of Si-QDs is established by Transmission Electron Microscopy (TEM), by which the average QD diameter of d QD 2.2 ± 0.5 nm has been determined
Comparisons of DFT-MD, TB- MD and classical MD calculations of radiation damage and plasmawallinteractions
 CMS Comparisons of DFT-MD, TB- MD and classical MD calculations of radiation damage and plasmawallinteractions Kai Nordlund Department of Physics and Helsinki Institute of Physics University of Helsinki,
CMS Comparisons of DFT-MD, TB- MD and classical MD calculations of radiation damage and plasmawallinteractions Kai Nordlund Department of Physics and Helsinki Institute of Physics University of Helsinki,
Structure, energetics, and vibrational properties of Si-H bond dissociation in silicon
 PHYSICAL REVIEW B VOLUME 59, NUMBER 20 15 MAY 1999-II Structure, energetics, and vibrational properties of Si-H bond dissociation in silicon Blair Tuttle Department of Physics, University of Illinois,
PHYSICAL REVIEW B VOLUME 59, NUMBER 20 15 MAY 1999-II Structure, energetics, and vibrational properties of Si-H bond dissociation in silicon Blair Tuttle Department of Physics, University of Illinois,
EECS143 Microfabrication Technology
 EECS143 Microfabrication Technology Professor Ali Javey Introduction to Materials Lecture 1 Evolution of Devices Yesterday s Transistor (1947) Today s Transistor (2006) Why Semiconductors? Conductors e.g
EECS143 Microfabrication Technology Professor Ali Javey Introduction to Materials Lecture 1 Evolution of Devices Yesterday s Transistor (1947) Today s Transistor (2006) Why Semiconductors? Conductors e.g
arxiv: v1 [cond-mat.stat-mech] 6 Mar 2008
![arxiv: v1 [cond-mat.stat-mech] 6 Mar 2008 arxiv: v1 [cond-mat.stat-mech] 6 Mar 2008](/thumbs/86/93817801.jpg) CD2dBS-v2 Convergence dynamics of 2-dimensional isotropic and anisotropic Bak-Sneppen models Burhan Bakar and Ugur Tirnakli Department of Physics, Faculty of Science, Ege University, 35100 Izmir, Turkey
CD2dBS-v2 Convergence dynamics of 2-dimensional isotropic and anisotropic Bak-Sneppen models Burhan Bakar and Ugur Tirnakli Department of Physics, Faculty of Science, Ege University, 35100 Izmir, Turkey
Energetics in Ice VI
 Energetics in Ice VI Nishanth Sasankan 2011 NSF / REU PROJECT Physics Department University of Notre Dame Advisor: Dr. Kathie E. Newman Abstract There are many different phases of Ice, which exist in different
Energetics in Ice VI Nishanth Sasankan 2011 NSF / REU PROJECT Physics Department University of Notre Dame Advisor: Dr. Kathie E. Newman Abstract There are many different phases of Ice, which exist in different
EFFECTS OF STOICHIOMETRY ON POINT DEFECTS AND IMPURITIES IN GALLIUM NITRIDE
 EFFECTS OF STOICHIOMETRY ON POINT DEFECTS AND IMPURITIES IN GALLIUM NITRIDE C. G. VAN DE WALLE AND J. E. NORTHRUP Palo Alto Research Center, 3333 Coyote Hill Road, Palo Alto, CA 930, USA E-mail: vandewalle@parc.com
EFFECTS OF STOICHIOMETRY ON POINT DEFECTS AND IMPURITIES IN GALLIUM NITRIDE C. G. VAN DE WALLE AND J. E. NORTHRUP Palo Alto Research Center, 3333 Coyote Hill Road, Palo Alto, CA 930, USA E-mail: vandewalle@parc.com
6.5 mm. ε = 1%, r = 9.4 mm. ε = 3%, r = 3.1 mm
 Supplementary Information Supplementary Figures Gold wires Substrate Compression holder 6.5 mm Supplementary Figure 1 Picture of the compression holder. 6.5 mm ε = 0% ε = 1%, r = 9.4 mm ε = 2%, r = 4.7
Supplementary Information Supplementary Figures Gold wires Substrate Compression holder 6.5 mm Supplementary Figure 1 Picture of the compression holder. 6.5 mm ε = 0% ε = 1%, r = 9.4 mm ε = 2%, r = 4.7
3.1 Introduction to Semiconductors. Y. Baghzouz ECE Department UNLV
 3.1 Introduction to Semiconductors Y. Baghzouz ECE Department UNLV Introduction In this lecture, we will cover the basic aspects of semiconductor materials, and the physical mechanisms which are at the
3.1 Introduction to Semiconductors Y. Baghzouz ECE Department UNLV Introduction In this lecture, we will cover the basic aspects of semiconductor materials, and the physical mechanisms which are at the
Clustering of Defects and Impurities in Hydrogenated Single-Crystal Silicon
 Semiconductors, Vol. 36, No. 3, 00, pp. 39 49. Translated from Fizika i Tekhnika Poluprovodnikov, Vol. 36, No. 3, 00, pp. 57 68. Original Russian Text Copyright 00 by Abdulin, Gorelkinskiœ, Mukashev, Tokmoldin.
Semiconductors, Vol. 36, No. 3, 00, pp. 39 49. Translated from Fizika i Tekhnika Poluprovodnikov, Vol. 36, No. 3, 00, pp. 57 68. Original Russian Text Copyright 00 by Abdulin, Gorelkinskiœ, Mukashev, Tokmoldin.
ADVANCED UNDERGRADUATE LABORATORY EXPERIMENT 20. Semiconductor Resistance, Band Gap, and Hall Effect
 ADVANCED UNDERGRADUATE LABORATORY EXPERIMENT 20 Semiconductor Resistance, Band Gap, and Hall Effect Revised: November 1996 by David Bailey March 1990 by John Pitre & Taek-Soon Yoon Introduction Solid materials
ADVANCED UNDERGRADUATE LABORATORY EXPERIMENT 20 Semiconductor Resistance, Band Gap, and Hall Effect Revised: November 1996 by David Bailey March 1990 by John Pitre & Taek-Soon Yoon Introduction Solid materials
Electronic Structure and van der Waals Interactions in the Stability and Mobility of Point Defects in Semiconductors
 Electronic Structure and van der Waals Interactions in the Stability and Mobility of Point Defects in Semiconductors Wang Gao and Alexandre Tkatchenko Fritz-Haber-Institut der Max-Planck-Gesellschaft,
Electronic Structure and van der Waals Interactions in the Stability and Mobility of Point Defects in Semiconductors Wang Gao and Alexandre Tkatchenko Fritz-Haber-Institut der Max-Planck-Gesellschaft,
Basic cell design. Si cell
 Basic cell design Si cell 1 Concepts needed to describe photovoltaic device 1. energy bands in semiconductors: from bonds to bands 2. free carriers: holes and electrons, doping 3. electron and hole current:
Basic cell design Si cell 1 Concepts needed to describe photovoltaic device 1. energy bands in semiconductors: from bonds to bands 2. free carriers: holes and electrons, doping 3. electron and hole current:
The Semiconductor in Equilibrium
 Lecture 6 Semiconductor physics IV The Semiconductor in Equilibrium Equilibrium, or thermal equilibrium No external forces such as voltages, electric fields. Magnetic fields, or temperature gradients are
Lecture 6 Semiconductor physics IV The Semiconductor in Equilibrium Equilibrium, or thermal equilibrium No external forces such as voltages, electric fields. Magnetic fields, or temperature gradients are
Introductory Nanotechnology ~ Basic Condensed Matter Physics ~
 Introductory Nanotechnology ~ Basic Condensed Matter Physics ~ Atsufumi Hirohata Department of Electronics Go into Nano-Scale Lateral Size [m] 10-3 10-6 Micron-scale Sub-Micron-scale Nano-scale Human hair
Introductory Nanotechnology ~ Basic Condensed Matter Physics ~ Atsufumi Hirohata Department of Electronics Go into Nano-Scale Lateral Size [m] 10-3 10-6 Micron-scale Sub-Micron-scale Nano-scale Human hair
VERY SHORT ANSWER TYPE QUESTIONS (1 Mark)
 UNIT I 10 Chemistry-XII THE SOLID STATE VERY SHORT ANSWER TYPE QUESTIONS (1 Mark) Q. 1. What do you mean by paramagnetic substance? Ans. Weakly attracted by magnetic eld and these substances are made of
UNIT I 10 Chemistry-XII THE SOLID STATE VERY SHORT ANSWER TYPE QUESTIONS (1 Mark) Q. 1. What do you mean by paramagnetic substance? Ans. Weakly attracted by magnetic eld and these substances are made of
Supporting Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2015 Supporting Information Single Layer Lead Iodide: Computational Exploration of Structural, Electronic
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2015 Supporting Information Single Layer Lead Iodide: Computational Exploration of Structural, Electronic
Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together.
 Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together. For example Nacl In the Nacl lattice, each Na atom is
Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together. For example Nacl In the Nacl lattice, each Na atom is
Evidence of a second acceptor state of the E center in Si1-x Gex
 Powered by TCPDF (www.tcpdf.org) This is an electronic reprint of the original article. This reprint may differ from the original in pagination and typographic detail. Author(s): Kuitunen, K. & Tuomisto,
Powered by TCPDF (www.tcpdf.org) This is an electronic reprint of the original article. This reprint may differ from the original in pagination and typographic detail. Author(s): Kuitunen, K. & Tuomisto,
Nonlinear Elasticity in Wurtzite GaN/AlN Planar Superlattices and Quantum Dots
 Vol. 108 (2005) ACTA PHYSICA POLONICA A No. 5 Proceedings of the XXXIV International School of Semiconducting Compounds, Jaszowiec 2005 Nonlinear Elasticity in Wurtzite GaN/AlN Planar Superlattices and
Vol. 108 (2005) ACTA PHYSICA POLONICA A No. 5 Proceedings of the XXXIV International School of Semiconducting Compounds, Jaszowiec 2005 Nonlinear Elasticity in Wurtzite GaN/AlN Planar Superlattices and
Lecture 3b. Bonding Model and Dopants. Reading: (Cont d) Notes and Anderson 2 sections
 Lecture 3b Bonding Model and Dopants Reading: (Cont d) Notes and Anderson 2 sections 2.3-2.7 The need for more control over carrier concentration Without help the total number of carriers (electrons and
Lecture 3b Bonding Model and Dopants Reading: (Cont d) Notes and Anderson 2 sections 2.3-2.7 The need for more control over carrier concentration Without help the total number of carriers (electrons and
PN Junction
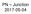 P Junction 2017-05-04 Definition Power Electronics = semiconductor switches are used Analogue amplifier = high power loss 250 200 u x 150 100 u Udc i 50 0 0 50 100 150 200 250 300 350 400 i,u dc i,u u
P Junction 2017-05-04 Definition Power Electronics = semiconductor switches are used Analogue amplifier = high power loss 250 200 u x 150 100 u Udc i 50 0 0 50 100 150 200 250 300 350 400 i,u dc i,u u
Supporting Online Material (1)
 Supporting Online Material The density functional theory (DFT) calculations were carried out using the dacapo code (http://www.fysik.dtu.dk/campos), and the RPBE (1) generalized gradient correction (GGA)
Supporting Online Material The density functional theory (DFT) calculations were carried out using the dacapo code (http://www.fysik.dtu.dk/campos), and the RPBE (1) generalized gradient correction (GGA)
Edinburgh Research Explorer
 Edinburgh Research Explorer Ab initio calculations of the self-interstitial in silicon Citation for published version: Clark, SJ & Ackland, GJ 1997, 'Ab initio calculations of the self-interstitial in
Edinburgh Research Explorer Ab initio calculations of the self-interstitial in silicon Citation for published version: Clark, SJ & Ackland, GJ 1997, 'Ab initio calculations of the self-interstitial in
On the Possibility of Metastable Metallic Hydrogen
 On the Possibility of Metastable Metallic Hydrogen Jeffrey M. McMahon Department of Physics & Astronomy March 14, 2017 Jeffrey M. McMahon (WSU) March 14, 2017 1 / 19 The Problem of Metallic Hydrogen In
On the Possibility of Metastable Metallic Hydrogen Jeffrey M. McMahon Department of Physics & Astronomy March 14, 2017 Jeffrey M. McMahon (WSU) March 14, 2017 1 / 19 The Problem of Metallic Hydrogen In
Kinetics. Rate of change in response to thermodynamic forces
 Kinetics Rate of change in response to thermodynamic forces Deviation from local equilibrium continuous change T heat flow temperature changes µ atom flow composition changes Deviation from global equilibrium
Kinetics Rate of change in response to thermodynamic forces Deviation from local equilibrium continuous change T heat flow temperature changes µ atom flow composition changes Deviation from global equilibrium
Charge Carriers in Semiconductor
 Charge Carriers in Semiconductor To understand PN junction s IV characteristics, it is important to understand charge carriers behavior in solids, how to modify carrier densities, and different mechanisms
Charge Carriers in Semiconductor To understand PN junction s IV characteristics, it is important to understand charge carriers behavior in solids, how to modify carrier densities, and different mechanisms
EE301 Electronics I , Fall
 EE301 Electronics I 2018-2019, Fall 1. Introduction to Microelectronics (1 Week/3 Hrs.) Introduction, Historical Background, Basic Consepts 2. Rewiev of Semiconductors (1 Week/3 Hrs.) Semiconductor materials
EE301 Electronics I 2018-2019, Fall 1. Introduction to Microelectronics (1 Week/3 Hrs.) Introduction, Historical Background, Basic Consepts 2. Rewiev of Semiconductors (1 Week/3 Hrs.) Semiconductor materials
3 - Ice and crystalline water
 3 - Ice and crystalline water All ice is not crystalline not all crystalline water is ice H 2 O solid phases (From Savage) Some general comments on the phase diagram All crystalline ice is tetrahedrically
3 - Ice and crystalline water All ice is not crystalline not all crystalline water is ice H 2 O solid phases (From Savage) Some general comments on the phase diagram All crystalline ice is tetrahedrically
Electroluminescence from Silicon and Germanium Nanostructures
 Electroluminescence from silicon Silicon Getnet M. and Ghoshal S.K 35 ORIGINAL ARTICLE Electroluminescence from Silicon and Germanium Nanostructures Getnet Melese* and Ghoshal S. K.** Abstract Silicon
Electroluminescence from silicon Silicon Getnet M. and Ghoshal S.K 35 ORIGINAL ARTICLE Electroluminescence from Silicon and Germanium Nanostructures Getnet Melese* and Ghoshal S. K.** Abstract Silicon
Introduction to Semiconductor Physics. Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India
 Introduction to Semiconductor Physics 1 Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India http://folk.uio.no/ravi/cmp2013 Review of Semiconductor Physics Semiconductor fundamentals
Introduction to Semiconductor Physics 1 Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India http://folk.uio.no/ravi/cmp2013 Review of Semiconductor Physics Semiconductor fundamentals
The Morse Potential. ChE Reactive Process Engineering. Let s have a look at a very simple model reaction :
 The Morse Potential L7-1 Let s have a look at a very simple model reaction : We can describe the F + H-H energy of this system based on the relative position of the atoms. This energy is (in the most
The Morse Potential L7-1 Let s have a look at a very simple model reaction : We can describe the F + H-H energy of this system based on the relative position of the atoms. This energy is (in the most
Au-C Au-Au. g(r) r/a. Supplementary Figures
 g(r) Supplementary Figures 60 50 40 30 20 10 0 Au-C Au-Au 2 4 r/a 6 8 Supplementary Figure 1 Radial bond distributions for Au-C and Au-Au bond. The zero density regime between the first two peaks in g
g(r) Supplementary Figures 60 50 40 30 20 10 0 Au-C Au-Au 2 4 r/a 6 8 Supplementary Figure 1 Radial bond distributions for Au-C and Au-Au bond. The zero density regime between the first two peaks in g
DFT EXERCISES. FELIPE CERVANTES SODI January 2006
 DFT EXERCISES FELIPE CERVANTES SODI January 2006 http://www.csanyi.net/wiki/space/dftexercises Dr. Gábor Csányi 1 Hydrogen atom Place a single H atom in the middle of a largish unit cell (start with a
DFT EXERCISES FELIPE CERVANTES SODI January 2006 http://www.csanyi.net/wiki/space/dftexercises Dr. Gábor Csányi 1 Hydrogen atom Place a single H atom in the middle of a largish unit cell (start with a
4.2 Molecular orbitals and atomic orbitals Consider a linear chain of four identical atoms representing a hypothetical molecule.
 4. Molecular orbitals and atomic orbitals Consider a linear chain of four identical atoms representing a hypothetical molecule. Suppose that each atomic wavefunction is 1s wavefunction. This system of
4. Molecular orbitals and atomic orbitals Consider a linear chain of four identical atoms representing a hypothetical molecule. Suppose that each atomic wavefunction is 1s wavefunction. This system of
EE495/695 Introduction to Semiconductors I. Y. Baghzouz ECE Department UNLV
 EE495/695 Introduction to Semiconductors I Y. Baghzouz ECE Department UNLV Introduction Solar cells have always been aligned closely with other electronic devices. We will cover the basic aspects of semiconductor
EE495/695 Introduction to Semiconductors I Y. Baghzouz ECE Department UNLV Introduction Solar cells have always been aligned closely with other electronic devices. We will cover the basic aspects of semiconductor
Bonding and Elastic Properties in Ti 2 AC (A = Ga or Tl)
 Printed in the Republic of Korea http://dx.doi.org/10.5012/jkcs.2013.57.1.35 Bonding and Elastic Properties in Ti 2 AC (A = Ga or Tl) Dae-Bok Kang* Department of Chemistry, Kyungsung University, Busan
Printed in the Republic of Korea http://dx.doi.org/10.5012/jkcs.2013.57.1.35 Bonding and Elastic Properties in Ti 2 AC (A = Ga or Tl) Dae-Bok Kang* Department of Chemistry, Kyungsung University, Busan
Self-compensating incorporation of Mn in Ga 1 x Mn x As
 Self-compensating incorporation of Mn in Ga 1 x Mn x As arxiv:cond-mat/0201131v1 [cond-mat.mtrl-sci] 9 Jan 2002 J. Mašek and F. Máca Institute of Physics, Academy of Sciences of the CR CZ-182 21 Praha
Self-compensating incorporation of Mn in Ga 1 x Mn x As arxiv:cond-mat/0201131v1 [cond-mat.mtrl-sci] 9 Jan 2002 J. Mašek and F. Máca Institute of Physics, Academy of Sciences of the CR CZ-182 21 Praha
The photovoltaic effect occurs in semiconductors where there are distinct valence and
 How a Photovoltaic Cell Works The photovoltaic effect occurs in semiconductors where there are distinct valence and conduction bands. (There are energies at which electrons can not exist within the solid)
How a Photovoltaic Cell Works The photovoltaic effect occurs in semiconductors where there are distinct valence and conduction bands. (There are energies at which electrons can not exist within the solid)
Supporting Information
 Supporting Information Fluorination of Metal Phthalocyanines: Single-Crystal Growth, Efficient N-Channel Organic Field-Effect Transistors, and Structure- Property Relationships Hui Jiang 1*, Jun Ye 2,
Supporting Information Fluorination of Metal Phthalocyanines: Single-Crystal Growth, Efficient N-Channel Organic Field-Effect Transistors, and Structure- Property Relationships Hui Jiang 1*, Jun Ye 2,
Atomistic simulations of point defect diffusion in Si and SiGe
 Atomistic simulations of point defect diffusion in Si and SiGe P. Pochet, D. Caliste, K. Rushchanskii, F. Lançon & T. Deutsch CEA UJF INAC Institute for Nanoscience and Cryogenics Partially founded by
Atomistic simulations of point defect diffusion in Si and SiGe P. Pochet, D. Caliste, K. Rushchanskii, F. Lançon & T. Deutsch CEA UJF INAC Institute for Nanoscience and Cryogenics Partially founded by
Dynamical Monte-Carlo Simulation of Surface Kinetics
 Dynamical Monte-Carlo Simulation of Surface Kinetics V. Guerra and J. Loureiro Centro de Física dos Plasmas, Instituto Superior Técnico, 1049-001 Lisboa, Portugal Abstract. This wor presents a theoretical
Dynamical Monte-Carlo Simulation of Surface Kinetics V. Guerra and J. Loureiro Centro de Física dos Plasmas, Instituto Superior Técnico, 1049-001 Lisboa, Portugal Abstract. This wor presents a theoretical
So why is sodium a metal? Tungsten Half-filled 5d band & half-filled 6s band. Insulators. Interaction of metals with light?
 Bonding in Solids: Metals, Insulators, & CHEM 107 T. Hughbanks Delocalized bonding in Solids Think of a pure solid as a single, very large molecule. Use our bonding pictures to try to understand properties.
Bonding in Solids: Metals, Insulators, & CHEM 107 T. Hughbanks Delocalized bonding in Solids Think of a pure solid as a single, very large molecule. Use our bonding pictures to try to understand properties.
Atoms? All matters on earth made of atoms (made up of elements or combination of elements).
 Chapter 1 Atoms? All matters on earth made of atoms (made up of elements or combination of elements). Atomic Structure Atom is the smallest particle of an element that can exist in a stable or independent
Chapter 1 Atoms? All matters on earth made of atoms (made up of elements or combination of elements). Atomic Structure Atom is the smallest particle of an element that can exist in a stable or independent
Atomic configuration of boron pile-up at the Si/SiO 2 interface
 Atomic configuration of boron pile-up at the Si/SiO 2 interface Masayuki Furuhashi, a) Tetsuya Hirose, Hiroshi Tsuji, Masayuki Tachi, and Kenji Taniguchi Department of Electronics and Information Systems,
Atomic configuration of boron pile-up at the Si/SiO 2 interface Masayuki Furuhashi, a) Tetsuya Hirose, Hiroshi Tsuji, Masayuki Tachi, and Kenji Taniguchi Department of Electronics and Information Systems,
The pathway to reorientation in ammonium fluoride
 14 April 2000 Ž. Chemical Physics Letters 320 2000 487 491 www.elsevier.nlrlocatercplett The pathway to reorientation in ammonium fluoride A. Alavi a, R.M. Lynden-Bell a,), R.J.C. Brown b a Atomistic Simulation
14 April 2000 Ž. Chemical Physics Letters 320 2000 487 491 www.elsevier.nlrlocatercplett The pathway to reorientation in ammonium fluoride A. Alavi a, R.M. Lynden-Bell a,), R.J.C. Brown b a Atomistic Simulation
Ion Implantation ECE723
 Ion Implantation Topic covered: Process and Advantages of Ion Implantation Ion Distribution and Removal of Lattice Damage Simulation of Ion Implantation Range of Implanted Ions Ion Implantation is the
Ion Implantation Topic covered: Process and Advantages of Ion Implantation Ion Distribution and Removal of Lattice Damage Simulation of Ion Implantation Range of Implanted Ions Ion Implantation is the
DIFFUSION IN SOLIDS. IE-114 Materials Science and General Chemistry Lecture-5
 DIFFUSION IN SOLIDS IE-114 Materials Science and General Chemistry Lecture-5 Diffusion The mechanism by which matter is transported through matter. It is related to internal atomic movement. Atomic movement;
DIFFUSION IN SOLIDS IE-114 Materials Science and General Chemistry Lecture-5 Diffusion The mechanism by which matter is transported through matter. It is related to internal atomic movement. Atomic movement;
Diamond. Covalent Insulators and Semiconductors. Silicon, Germanium, Gray Tin. Chem 462 September 24, 2004
 Covalent Insulators and Chem 462 September 24, 2004 Diamond Pure sp 3 carbon All bonds staggered- ideal d(c-c) - 1.54 Å, like ethane Silicon, Germanium, Gray Tin Diamond structure Si and Ge: semiconductors
Covalent Insulators and Chem 462 September 24, 2004 Diamond Pure sp 3 carbon All bonds staggered- ideal d(c-c) - 1.54 Å, like ethane Silicon, Germanium, Gray Tin Diamond structure Si and Ge: semiconductors
Electrically active defects in semiconductors induced by radiation
 Electrically active defects in semiconductors induced by radiation Ivana Capan Rudjer Boskovic Institute, Croatia http://www.irb.hr/users/capan Outline Radiation damage Capacitance transient techniques
Electrically active defects in semiconductors induced by radiation Ivana Capan Rudjer Boskovic Institute, Croatia http://www.irb.hr/users/capan Outline Radiation damage Capacitance transient techniques
Modified Maxwell-Garnett equation for the effective transport coefficients in inhomogeneous media
 J. Phys. A: Math. Gen. 3 (998) 7227 7234. Printed in the UK PII: S0305-4470(98)93976-2 Modified Maxwell-Garnett equation for the effective transport coefficients in inhomogeneous media Juris Robert Kalnin
J. Phys. A: Math. Gen. 3 (998) 7227 7234. Printed in the UK PII: S0305-4470(98)93976-2 Modified Maxwell-Garnett equation for the effective transport coefficients in inhomogeneous media Juris Robert Kalnin
KATIHAL FİZİĞİ MNT-510
 KATIHAL FİZİĞİ MNT-510 YARIİLETKENLER Kaynaklar: Katıhal Fiziği, Prof. Dr. Mustafa Dikici, Seçkin Yayıncılık Katıhal Fiziği, Şakir Aydoğan, Nobel Yayıncılık, Physics for Computer Science Students: With
KATIHAL FİZİĞİ MNT-510 YARIİLETKENLER Kaynaklar: Katıhal Fiziği, Prof. Dr. Mustafa Dikici, Seçkin Yayıncılık Katıhal Fiziği, Şakir Aydoğan, Nobel Yayıncılık, Physics for Computer Science Students: With
Determination of properties in semiconductor materials by applying Matlab
 Determination of properties in semiconductor materials by applying Matlab Carlos Figueroa. 1, Raúl Riera A. 2 1 Departamento de Ingeniería Industrial. Universidad de Sonora A.P. 5-088, Hermosillo, Sonora.
Determination of properties in semiconductor materials by applying Matlab Carlos Figueroa. 1, Raúl Riera A. 2 1 Departamento de Ingeniería Industrial. Universidad de Sonora A.P. 5-088, Hermosillo, Sonora.
Ab initio phonon calculations in mixed systems
 Ab initio phonon calculations in mixed systems Andrei Postnikov apostnik@uos.de Outline: Experiment vs. ab initio theory Ways of theory: linear response and frozen phonon approaches Applications: Be x
Ab initio phonon calculations in mixed systems Andrei Postnikov apostnik@uos.de Outline: Experiment vs. ab initio theory Ways of theory: linear response and frozen phonon approaches Applications: Be x
Ionic Bonding. Example: Atomic Radius: Na (r = 0.192nm) Cl (r = 0.099nm) Ionic Radius : Na (r = 0.095nm) Cl (r = 0.181nm)
 Ionic Bonding Ion: an atom or molecule that gains or loses electrons (acquires an electrical charge). Atoms form cations (+charge), when they lose electrons, or anions (- charge), when they gain electrons.
Ionic Bonding Ion: an atom or molecule that gains or loses electrons (acquires an electrical charge). Atoms form cations (+charge), when they lose electrons, or anions (- charge), when they gain electrons.
Vacancy generation during Cu diffusion in GaAs M. Elsayed PhD. Student
 Vacancy generation during Cu diffusion in GaAs M. Elsayed PhD. Student Martin Luther University-FB Physik IV Halle-Wittenberg Outlines Principles of PAS vacancy in Semiconductors and shallow positron traps
Vacancy generation during Cu diffusion in GaAs M. Elsayed PhD. Student Martin Luther University-FB Physik IV Halle-Wittenberg Outlines Principles of PAS vacancy in Semiconductors and shallow positron traps
Outline. Introduction: graphene. Adsorption on graphene: - Chemisorption - Physisorption. Summary
 Outline Introduction: graphene Adsorption on graphene: - Chemisorption - Physisorption Summary 1 Electronic band structure: Electronic properties K Γ M v F = 10 6 ms -1 = c/300 massless Dirac particles!
Outline Introduction: graphene Adsorption on graphene: - Chemisorption - Physisorption Summary 1 Electronic band structure: Electronic properties K Γ M v F = 10 6 ms -1 = c/300 massless Dirac particles!
ICCP Project 2 - Advanced Monte Carlo Methods Choose one of the three options below
 ICCP Project 2 - Advanced Monte Carlo Methods Choose one of the three options below Introduction In statistical physics Monte Carlo methods are considered to have started in the Manhattan project (1940
ICCP Project 2 - Advanced Monte Carlo Methods Choose one of the three options below Introduction In statistical physics Monte Carlo methods are considered to have started in the Manhattan project (1940
Theoretical Studies of Self-Diffusion and Dopant Clustering in Semiconductors
 phys. stat. sol. (b) 233, No., 24 30 (2002) Theoretical Studies of Self-Diffusion and Dopant Clustering in Semiconductors B. P. Uberuaga )(a), G. Henkelman (b), H. Jónsson (b, c), S. T. Dunham (d), W.
phys. stat. sol. (b) 233, No., 24 30 (2002) Theoretical Studies of Self-Diffusion and Dopant Clustering in Semiconductors B. P. Uberuaga )(a), G. Henkelman (b), H. Jónsson (b, c), S. T. Dunham (d), W.
MTLE-6120: Advanced Electronic Properties of Materials. Intrinsic and extrinsic semiconductors. Reading: Kasap:
 MTLE-6120: Advanced Electronic Properties of Materials 1 Intrinsic and extrinsic semiconductors Reading: Kasap: 5.1-5.6 Band structure and conduction 2 Metals: partially filled band(s) i.e. bands cross
MTLE-6120: Advanced Electronic Properties of Materials 1 Intrinsic and extrinsic semiconductors Reading: Kasap: 5.1-5.6 Band structure and conduction 2 Metals: partially filled band(s) i.e. bands cross
Polarons in linear chains of fullerenes
 Polarons in linear chains of fullerenes V. A. Levashov, A. A. Remova, and V. R. Belosludov a) Institute of Inorganic Chemistry, 630090 Novosibirsk, Russia Submitted 6 September 1996 Pis ma Zh. Éksp. Teor.
Polarons in linear chains of fullerenes V. A. Levashov, A. A. Remova, and V. R. Belosludov a) Institute of Inorganic Chemistry, 630090 Novosibirsk, Russia Submitted 6 September 1996 Pis ma Zh. Éksp. Teor.
From Last Time Important new Quantum Mechanical Concepts. Atoms and Molecules. Today. Symmetry. Simple molecules.
 Today From Last Time Important new Quantum Mechanical Concepts Indistinguishability: Symmetries of the wavefunction: Symmetric and Antisymmetric Pauli exclusion principle: only one fermion per state Spin
Today From Last Time Important new Quantum Mechanical Concepts Indistinguishability: Symmetries of the wavefunction: Symmetric and Antisymmetric Pauli exclusion principle: only one fermion per state Spin
EE143 Fall 2016 Microfabrication Technologies. Evolution of Devices
 EE143 Fall 2016 Microfabrication Technologies Prof. Ming C. Wu wu@eecs.berkeley.edu 511 Sutardja Dai Hall (SDH) 1-1 Evolution of Devices Yesterday s Transistor (1947) Today s Transistor (2006) 1-2 1 Why
EE143 Fall 2016 Microfabrication Technologies Prof. Ming C. Wu wu@eecs.berkeley.edu 511 Sutardja Dai Hall (SDH) 1-1 Evolution of Devices Yesterday s Transistor (1947) Today s Transistor (2006) 1-2 1 Why
ATOMISTIC MODELING OF DIFFUSION IN ALUMINUM
 ATOMISTIC MODELING OF DIFFUSION IN ALUMINUM S. GRABOWSKI, K. KADAU and P. ENTEL Theoretische Physik, Gerhard-Mercator-Universität Duisburg, 47048 Duisburg, Germany (Received...) Abstract We present molecular-dynamics
ATOMISTIC MODELING OF DIFFUSION IN ALUMINUM S. GRABOWSKI, K. KADAU and P. ENTEL Theoretische Physik, Gerhard-Mercator-Universität Duisburg, 47048 Duisburg, Germany (Received...) Abstract We present molecular-dynamics
Micron School of Materials Science and Engineering. Problem Set 9 Solutions
 Problem Set 9 Solutions 1. Mobility in extrinsic semiconductors is affected by phonon scattering and impurity scattering. Thoroughly explain the mobility plots for the following figures from your textbook
Problem Set 9 Solutions 1. Mobility in extrinsic semiconductors is affected by phonon scattering and impurity scattering. Thoroughly explain the mobility plots for the following figures from your textbook
Module 6 : PHYSICS OF SEMICONDUCTOR DEVICES Lecture 32 : Bonding in Solids
 Module 6 : PHYSICS OF SEMICONDUCTOR DEVICES Lecture 32 : Bonding in Solids Objectives In this course you will learn the following Bonding in solids. Ionic and covalent bond. Structure of Silicon Concept
Module 6 : PHYSICS OF SEMICONDUCTOR DEVICES Lecture 32 : Bonding in Solids Objectives In this course you will learn the following Bonding in solids. Ionic and covalent bond. Structure of Silicon Concept
Chapter 10. Liquids and Solids
 Chapter 10 Liquids and Solids Chapter 10 Table of Contents 10.1 Intermolecular Forces 10.2 The Liquid State 10.3 An Introduction to Structures and Types of Solids 10.4 Structure and Bonding in Metals 10.5
Chapter 10 Liquids and Solids Chapter 10 Table of Contents 10.1 Intermolecular Forces 10.2 The Liquid State 10.3 An Introduction to Structures and Types of Solids 10.4 Structure and Bonding in Metals 10.5
22 Path Optimisation Methods
 22 Path Optimisation Methods 204 22 Path Optimisation Methods Many interesting chemical and physical processes involve transitions from one state to another. Typical examples are migration paths for defects
22 Path Optimisation Methods 204 22 Path Optimisation Methods Many interesting chemical and physical processes involve transitions from one state to another. Typical examples are migration paths for defects
Electronic Structure Theory for Periodic Systems: The Concepts. Christian Ratsch
 Electronic Structure Theory for Periodic Systems: The Concepts Christian Ratsch Institute for Pure and Applied Mathematics and Department of Mathematics, UCLA Motivation There are 10 20 atoms in 1 mm 3
Electronic Structure Theory for Periodic Systems: The Concepts Christian Ratsch Institute for Pure and Applied Mathematics and Department of Mathematics, UCLA Motivation There are 10 20 atoms in 1 mm 3
