TEACHER NOTES MIDDLE GRADES SCIENCE NSPIRED
|
|
|
- Merryl Miller
- 5 years ago
- Views:
Transcription
1 Science Objectives Students will describe the three states of matter. Students will analyze changes of state graphically. Students will ascertain the melting and boiling points of different substances. Vocabulary absolute zero boiling point change of state freezing point gas liquid melting point solid About the Lesson This lesson visually shows the behavior of particles in a substance as the temperature changes. As a result, students will: Understand the behavior of particles as substances change state. Identify the melting and boiling points of 3 substances by reading a graph. TI-Nspire Navigator Send out the Changing_States.tns file. Monitor student progress using Class Capture. Use Live Presenter to spotlight student answers. Activity Materials Compatible TI Technologies: TI-Nspire CX Handhelds, TI-Nspire Apps for ipad, TI-Nspire Software Tech Tips: This activity includes class Nspire CX handheld. It is also appropriate for use with the TI-Nspire family of products including TI-Nspire software and TI-Nspire App. Slight variations to these directions may be required if using other technologies besides the handheld. Watch for additional Tech Tips throughout the activity for the specific technology you are using. Access free tutorials at captures taken from the TI- calculators/pd/us/online- Learning/Tutorials Lesson Materials: Student Activity Changing_States _Student.doc Changing_States _Student.pdf TI-Nspire document Changing_States.tns 2014 Texas Instruments Incorporated 1 education.ti.com
2 Discussion Points and Possible Answers Have students read the background information on matter and changes of state on the student activity sheet. Students should already have some background on the states of matter. If you place the water in a pan and heat it at a high temperature, the water begins to boil and create steam. Steam is a gas. So, for matter to change from liquid to gas, energy (in the form of heat) needs to be added. Matter is generally considered to exist in three states: solid, liquid, and gas. The particles that make up matter are in continual motion. This motion varies from vibrations in a more or less fixed position (solid), to sliding over one another (liquid), to freely moving in all directions (gas). At absolute zero ( 273 o C or 0 K), matter has its lowest kinetic energy. Move to pages Have students answer questions 1 3 on either the handheld, on the activity sheet, or both. Q1. Matter is usually considered to exist in one of state(s). Answer: C. three 2014 Texas Instruments Incorporated 2 education.ti.com
3 Q2. All molecular motion is believed to stop at. Answer: C. 0 K Q3. The atoms of which state of matter rest in relatively fixed positions? Answer: D. solid Move to pages 2.1 and 2.2. Students will look at three Experiments and see the temperature at which the substances changes state. The three substances are (1) water (HOH), (2) ethyl alcohol (C 2 H 5 OH), and (3) iron (Fe). 1. Students will choose Experiment 1 by using the lower up/down arrows until experiment 1 appears (if it is not already chosen). 2. They can raise the temperature by using the upper time slider and then observe the effect on the behavior of the particles. Have students answer questions 4 6 on their activity sheet only. Q4. What happens to the particles as more energy is added? Answer: The particles begin to move farther apart. Q5. What happens to the temperature of the substance as more heat is added? Answer: It increases Texas Instruments Incorporated 3 education.ti.com
4 Q6. Look at the portions of the graph where the temperature remains constant (where the line is flat), even though heat is still being added. Describe what is happening here. Answer: The substance is absorbing heat but not increasing in temperature, while the material changes state. 3. Students record the melting point in the data table below. 4. Students record the boiling point in the data table below. 5. Students complete the Data Table below for Experiment 2 and Experiment 3 following the same steps. Data Table Data Experiment 1 Experiment 2 Experiment 3 (HOH) (C 2 H 5 OH) (Fe) Melting point 0 C 114 C 1535 C Boiling point 100 C 78 C 2750 C Tech Tip: You may want students to reset the Time variable to 0 before moving between Experiments. If the time is not set back to 0, the next Experiment starts at the current Time. Move to pages Have students answer questions 7 12 on either the handheld, on the activity sheet, or both. Q7. The melting point for the substance in Experiment 2 is. Answer: A. 114 C 2014 Texas Instruments Incorporated 4 education.ti.com
5 Q8. The boiling point for this substance in Experiment 2 is. Answer: D. 78 C Q9. When the temperature is 273 C, the particles of a substance. Answer: theoretically have no movement (absolute zero) Q10. As the temperature increases, the amount of movement of the particles increases. Answer: A. always Q11. In the liquid state, most of the movement of particles is. Answer: A. horizontal Q12. How does the temperature change during any change of state? Explain. Answer: Temperature remains constant during any change of state. Energy is used to break or form bonds between molecules, so changes of state are constant temperature processes. TI-Nspire Navigator Opportunities Use TI-Nspire Navigator to capture screen shots of student progress and to retrieve the file from each student at the end of the class period. The student questions can be electronically graded and added to the student portfolio. Wrap Up When students are finished with the activity, retrieve the.tns file using TI-Nspire Navigator. Save grades to Portfolio. Discuss activity questions using Slide Show. Assessment Formative assessment will consist of questions embedded in the.tns file. The questions will be graded when the.tns file is retrieved. The Slide Show will be utilized to give students immediate feedback on their assessment. Summative assessment will consist of questions/problems on the chapter test Texas Instruments Incorporated 5 education.ti.com
It s Just a Lunar Phase
 Science Objectives Students will identify patterns in data associated with the lunar phases. Students will describe how the relative positions of the Earth, the Moon, and the Sun cause lunar phases. Students
Science Objectives Students will identify patterns in data associated with the lunar phases. Students will describe how the relative positions of the Earth, the Moon, and the Sun cause lunar phases. Students
TEACHER NOTES MIDDLE GRADES SCIENCE NSPIRED
 Science Objectives Students will investigate and differentiate between planets, moons, and asteroids. Students will understand how planets are classified. Vocabulary asteroid planet moon gravity orbital
Science Objectives Students will investigate and differentiate between planets, moons, and asteroids. Students will understand how planets are classified. Vocabulary asteroid planet moon gravity orbital
TEACHER NOTES SCIENCE NSPIRED
 Science Objectives Students will transfer electrons from a metal to a nonmetal. Students will relate this transfer to the formation of positive and negative ions. Student will learn how to write formulas
Science Objectives Students will transfer electrons from a metal to a nonmetal. Students will relate this transfer to the formation of positive and negative ions. Student will learn how to write formulas
TEACHER NOTES SCIENCE NSPIRED
 Science Objectives Students will investigate the effects of mass, spring constant, and initial displacement on the motion of an object oscillating on a spring. Students will observe the relationships between
Science Objectives Students will investigate the effects of mass, spring constant, and initial displacement on the motion of an object oscillating on a spring. Students will observe the relationships between
Science Friction! TEACHER NOTES. Science Objectives. Vocabulary. About the Lesson. TI-Nspire Navigator. Activity Materials
 Science Objectives Students will investigate the role of friction in the motion of a hero running across a concrete surface. Students will learn about Newton s second and third laws of motion and how they
Science Objectives Students will investigate the role of friction in the motion of a hero running across a concrete surface. Students will learn about Newton s second and third laws of motion and how they
First Derivative Test TEACHER NOTES
 Math Objectives Students will relate the first derivative of a function to its critical s and identify which of these critical s are local extrema. Students will visualize why the first derivative test
Math Objectives Students will relate the first derivative of a function to its critical s and identify which of these critical s are local extrema. Students will visualize why the first derivative test
TEACHER NOTES SCIENCE NSPIRED
 Science Objectives Students will explore the force two charges exert on each other. Students will observe how the force depends upon the magnitude of the two charges and the distance separating them. Students
Science Objectives Students will explore the force two charges exert on each other. Students will observe how the force depends upon the magnitude of the two charges and the distance separating them. Students
Electric Field Hockey
 Science Objectives Students will describe an electric field and electric field lines. Students will describe what happens when two like charges interact and when two unlike charges interact. Students will
Science Objectives Students will describe an electric field and electric field lines. Students will describe what happens when two like charges interact and when two unlike charges interact. Students will
Sum of an Infinite Geometric Series
 Math Objectives Students will understand how a unit square can be divided into an infinite number of pieces. Students will understand a justification for the following theorem: The sum of the infinite
Math Objectives Students will understand how a unit square can be divided into an infinite number of pieces. Students will understand a justification for the following theorem: The sum of the infinite
TEACHER NOTES MATH NSPIRED
 Math Objectives Students will learn that for a continuous non-negative function f, one interpretation of the definite integral f ( x ) dx is the area of a the region bounded above by the graph of y = f(x),
Math Objectives Students will learn that for a continuous non-negative function f, one interpretation of the definite integral f ( x ) dx is the area of a the region bounded above by the graph of y = f(x),
Crossing the Asymptote
 Math Objectives Students will test whether the graph of a given rational function crosses its horizontal asymptote. Students will examine the relationship among the coefficients of the polynomials in the
Math Objectives Students will test whether the graph of a given rational function crosses its horizontal asymptote. Students will examine the relationship among the coefficients of the polynomials in the
Function Notation TEACHER NOTES MATH NSPIRED. Math Objectives. Vocabulary. About the Lesson. TI-Nspire Navigator System. Activity Materials
 Math Objectives Students will understand function notation and the distinction between an input value x, a function f, and a function output f(x) Students will determine the rule for a function machine
Math Objectives Students will understand function notation and the distinction between an input value x, a function f, and a function output f(x) Students will determine the rule for a function machine
TEACHER NOTES MATH NSPIRED
 Math Objectives Students will solve linear equations in one variable graphically and algebraically. Students will explore what it means for an equation to be balanced both graphically and algebraically.
Math Objectives Students will solve linear equations in one variable graphically and algebraically. Students will explore what it means for an equation to be balanced both graphically and algebraically.
A Magnetic Attraction
 Science Objectives Students will map and describe the magnetic field around a permanent magnet or electromagnet. Students will determine the direction and strength of the force a magnet exerts on a current-carrying
Science Objectives Students will map and describe the magnetic field around a permanent magnet or electromagnet. Students will determine the direction and strength of the force a magnet exerts on a current-carrying
Influencing Regression
 Math Objectives Students will recognize that one point can influence the correlation coefficient and the least-squares regression line. Students will differentiate between an outlier and an influential
Math Objectives Students will recognize that one point can influence the correlation coefficient and the least-squares regression line. Students will differentiate between an outlier and an influential
Ice Cream, Cool Science
 Objectives Ice Cream, Cool Science Students will identify the differences between the three main states of matter. Students will understand that temperature and pressure can cause one state of matter to
Objectives Ice Cream, Cool Science Students will identify the differences between the three main states of matter. Students will understand that temperature and pressure can cause one state of matter to
Friction: Your Friend or Your Enemy?
 Science Objectives Students will determine what factors affect the friction between two surfaces. Students will relate the forces needed to drag different shoes across a table at a constant speed. Students
Science Objectives Students will determine what factors affect the friction between two surfaces. Students will relate the forces needed to drag different shoes across a table at a constant speed. Students
Special Right Triangles
 Math Objectives Students will identify the relationship between the lengths of the shorter and longer legs in a 30-60 -90 right triangle. Students will determine the relationship between the shorter leg
Math Objectives Students will identify the relationship between the lengths of the shorter and longer legs in a 30-60 -90 right triangle. Students will determine the relationship between the shorter leg
TEACHER NOTES MIDDLE GRADES SCIENCE NSPIRED
 Science Objectives Students will plot recent earthquakes and volcanoes in the Pacific Ocean on a map to discover the relationship between these events and the plate boundaries. Students will use their
Science Objectives Students will plot recent earthquakes and volcanoes in the Pacific Ocean on a map to discover the relationship between these events and the plate boundaries. Students will use their
Solving Stoichiometry Problems
 Science Objectives Students will write ratios from balanced chemical equations. Students will use -to-mass conversion to determine mass of reactants and products iven the number of s. Students will calculate
Science Objectives Students will write ratios from balanced chemical equations. Students will use -to-mass conversion to determine mass of reactants and products iven the number of s. Students will calculate
What Is a Solution to a System?
 Math Objectives Students will understand what it means for an ordered pair to be a solution to a linear equation. Students will understand what it means for an ordered pair to be a solution to a system
Math Objectives Students will understand what it means for an ordered pair to be a solution to a linear equation. Students will understand what it means for an ordered pair to be a solution to a system
Polynomials Factors, Roots, and Zeros TEACHER NOTES MATH NSPIRED
 Math Objectives Students will discover that the zeros of the linear factors are the zeros of the polynomial function. Students will discover that the real zeros of a polynomial function are the zeros of
Math Objectives Students will discover that the zeros of the linear factors are the zeros of the polynomial function. Students will discover that the real zeros of a polynomial function are the zeros of
Multiplying Inequalities by Negative Numbers
 Math Objectives Students will relate the inequality symbol to the location of points on a number line. Students will recognize, moreover, that the value of a number depends on its position on the number
Math Objectives Students will relate the inequality symbol to the location of points on a number line. Students will recognize, moreover, that the value of a number depends on its position on the number
End Behavior of Polynomial Functions TEACHER NOTES MATH NSPIRED
 Math Objectives Students will recognize the similarities and differences among power and polynomial functions of various degrees. Students will describe the effects of changes in the leading coefficient
Math Objectives Students will recognize the similarities and differences among power and polynomial functions of various degrees. Students will describe the effects of changes in the leading coefficient
Standard Error & Sample Means
 Math Objectives Students will recognize that samples have smaller variability than the population. Students will recognize that the variability in samples is a function of sample size, n, and that the
Math Objectives Students will recognize that samples have smaller variability than the population. Students will recognize that the variability in samples is a function of sample size, n, and that the
TEACHER NOTES MATH NSPIRED
 Math Objectives Students will produce various graphs of Taylor polynomials. Students will discover how the accuracy of a Taylor polynomial is associated with the degree of the Taylor polynomial. Students
Math Objectives Students will produce various graphs of Taylor polynomials. Students will discover how the accuracy of a Taylor polynomial is associated with the degree of the Taylor polynomial. Students
Sweating Alcohol TEACHER NOTES SCIENCE NSPIRED. Science Objectives. Math Objectives. Materials Needed. Vocabulary.
 Science Objectives Students will collect data for the cooling rates of water and isopropyl alcohol. Students will compare and contrast the cooling rate data, both graphically and numerically. Students
Science Objectives Students will collect data for the cooling rates of water and isopropyl alcohol. Students will compare and contrast the cooling rate data, both graphically and numerically. Students
Ice Cream, Cool Science
 Objectives Students will identify the differences between the three main states of matter. Students will understand that temperature and pressure can cause one state of matter to turn into another state
Objectives Students will identify the differences between the three main states of matter. Students will understand that temperature and pressure can cause one state of matter to turn into another state
TEACHER NOTES SCIENCE NSPIRED
 Science Objectives Students will determine the relationship between mass and volume. Students will mathematically describe the relationship between mass and volume. Students will relate the slope of a
Science Objectives Students will determine the relationship between mass and volume. Students will mathematically describe the relationship between mass and volume. Students will relate the slope of a
Rose Curve / G. TEACHER NOTES MATH NSPIRED: PRECALCULUS. Math Objectives. Vocabulary. About the Lesson. TI-Nspire Navigator TM
 Math Objectives Students will understand the role of the values of a and n in the equation r = asin(nθ). Students will be able to predict the number of petals and their length by examining the polar equation.
Math Objectives Students will understand the role of the values of a and n in the equation r = asin(nθ). Students will be able to predict the number of petals and their length by examining the polar equation.
Epsilon-Delta Window Challenge TEACHER NOTES MATH NSPIRED
 Math Objectives Students will interpret the formal definition (epsilon-delta) of limit in terms of graphing window dimensions. Students will use this interpretation to make judgments of whether a limit
Math Objectives Students will interpret the formal definition (epsilon-delta) of limit in terms of graphing window dimensions. Students will use this interpretation to make judgments of whether a limit
Building Concepts: Solving Systems of Equations Algebraically
 Lesson Overview In this TI-Nspire lesson, students will investigate pathways for solving systems of linear equations algebraically. There are many effective solution pathways for a system of two linear
Lesson Overview In this TI-Nspire lesson, students will investigate pathways for solving systems of linear equations algebraically. There are many effective solution pathways for a system of two linear
Kinetics Activity 1 Objective
 Grade level: 9-12 Subject: Chemistry Time required: 45 to 90 minutes Kinetic by Todd Morstein Study of Chemical Rates of Reaction An introductory lesson on determining the differential rate law and rate
Grade level: 9-12 Subject: Chemistry Time required: 45 to 90 minutes Kinetic by Todd Morstein Study of Chemical Rates of Reaction An introductory lesson on determining the differential rate law and rate
Regressions of Olympic Proportions
 About the Lesson In this activity, students use the Manual-Fit and Linear Regression commands to find lines of best fit to model data from the Olympic Games. As a result, students will: Develop and evaluate
About the Lesson In this activity, students use the Manual-Fit and Linear Regression commands to find lines of best fit to model data from the Olympic Games. As a result, students will: Develop and evaluate
Vectors in Component Form
 Vectors in Component Form Student Activity 7 8 9 10 11 12 Introduction TI-Nspire & TI-Nspire CAS Investigation Student 30 min Vectors are used to represent quantities that have both magnitude and direction.
Vectors in Component Form Student Activity 7 8 9 10 11 12 Introduction TI-Nspire & TI-Nspire CAS Investigation Student 30 min Vectors are used to represent quantities that have both magnitude and direction.
TEACHER NOTES MATH NSPIRED
 Math Objectives Students will understand how various data transformations can be used to achieve a linear relationship and, consequently, how to use the methods of linear regression to obtain a line of
Math Objectives Students will understand how various data transformations can be used to achieve a linear relationship and, consequently, how to use the methods of linear regression to obtain a line of
Section 16.3 Phase Changes
 Section 16.3 Phase Changes Solid Liquid Gas 3 Phases of Matter Density of Matter How packed matter is (The amount of matter in a given space) Solid: Liquid: Gas: High Density Medium Density Low Density
Section 16.3 Phase Changes Solid Liquid Gas 3 Phases of Matter Density of Matter How packed matter is (The amount of matter in a given space) Solid: Liquid: Gas: High Density Medium Density Low Density
Solving Stoichiometry Problems
 Open the TI-Nspire document Solving_Stoichiometry_Problems.tns In this lesson you will use simulations of three combustion reactions to develop skills necessary to solve stoichiometric problems. Move to
Open the TI-Nspire document Solving_Stoichiometry_Problems.tns In this lesson you will use simulations of three combustion reactions to develop skills necessary to solve stoichiometric problems. Move to
Matter. Gas. Solid Liquid. Both shape and volume are not fixed. It has a fixed shape and a fixed volume.
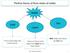 Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
Chemistry A: States of Matter Packet Name: Hour: Page!1. Chemistry A States of Matter Packet
 Chemistry A: States of Matter Packet Name: Hour: Page!1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page!2 Worksheet #1: States of Matter In this packet we will
Chemistry A: States of Matter Packet Name: Hour: Page!1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page!2 Worksheet #1: States of Matter In this packet we will
States of Matter. What physical changes and energy changes occur as matter goes from one state to another?
 Name States of Matter Date What physical changes and energy changes occur as matter goes from one state to another? Before You Read Before you read the chapter, think about what you know about states of
Name States of Matter Date What physical changes and energy changes occur as matter goes from one state to another? Before You Read Before you read the chapter, think about what you know about states of
Building Concepts: What is an Equation?
 Lesson Overview Algebraic Focus: How do equations differ from expressions? What does it mean to have a solution to an equation? In this lesson students begin by establishing the difference between an expression
Lesson Overview Algebraic Focus: How do equations differ from expressions? What does it mean to have a solution to an equation? In this lesson students begin by establishing the difference between an expression
Finite Differences TEACHER NOTES MATH NSPIRED
 Math Objectives Students will be able to recognize that the first set of finite differences for a linear function will be constant. Students will be able to recognize that the second set of finite differences
Math Objectives Students will be able to recognize that the first set of finite differences for a linear function will be constant. Students will be able to recognize that the second set of finite differences
SD: How Far is Typical? TEACHER NOTES
 Math Objectives Students will identify the standard deviation as a measure of spread about the mean of a distribution and loosely interpret it as an "average" or typical distance from the mean. Students
Math Objectives Students will identify the standard deviation as a measure of spread about the mean of a distribution and loosely interpret it as an "average" or typical distance from the mean. Students
Area Measures and Right Triangles
 Math Objectives Students will open or create a TI-Nspire document with a right triangle that has equilateral triangles on its sides. The areas of the equilateral triangles are measured and displayed on
Math Objectives Students will open or create a TI-Nspire document with a right triangle that has equilateral triangles on its sides. The areas of the equilateral triangles are measured and displayed on
THE PHASES OF MATTER. Solid: holds its shape and does not flow. The molecules in a solid vibrate in place, but on average, don t move very far.
 THE QUESTIONS What are the phases of matter? What makes these phases different from each other? What is the difference between melting, freezing, boiling and condensation? How do you interpret a Temperature
THE QUESTIONS What are the phases of matter? What makes these phases different from each other? What is the difference between melting, freezing, boiling and condensation? How do you interpret a Temperature
Chapter 21: Temperature, Heat and Expansion
 Chapter 21: Temperature, Heat and Expansion All matter solid, liquid and gas is made of atoms or molecules, which are continually jiggling. As this jiggling is a movement, all these particles must have
Chapter 21: Temperature, Heat and Expansion All matter solid, liquid and gas is made of atoms or molecules, which are continually jiggling. As this jiggling is a movement, all these particles must have
Chemistry A: States of Matter Packet Name: Hour: Page 1. Chemistry A States of Matter Packet
 Chemistry A: States of Matter Packet Name: Hour: Page 1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page 2 Worksheet #1: States of Matter In this packet we will
Chemistry A: States of Matter Packet Name: Hour: Page 1 Chemistry A States of Matter Packet Chemistry A: States of Matter Packet Name: Hour: Page 2 Worksheet #1: States of Matter In this packet we will
TEACHER NOTES: ICE CUBE POSTER
 TEACHER NOTES: NATIONAL CURRICULUM LINKS THE PARTICULATE NATURE OF MATTER the properties of the different states of matter (solid, liquid and gas) in terms of the particle model, including gas pressure
TEACHER NOTES: NATIONAL CURRICULUM LINKS THE PARTICULATE NATURE OF MATTER the properties of the different states of matter (solid, liquid and gas) in terms of the particle model, including gas pressure
Vectors in Component Form
 Vectors in Component Form Student Activity - Answers 7 8 9 10 11 12 Introduction TI-Nspire & TI-Nspire CAS Investigation Student 30 min Vectors are used to represent quantities that have both magnitude
Vectors in Component Form Student Activity - Answers 7 8 9 10 11 12 Introduction TI-Nspire & TI-Nspire CAS Investigation Student 30 min Vectors are used to represent quantities that have both magnitude
Chapter 8. Chapter 8. Preview. Bellringer. Chapter 8. Particles of Matter. Objectives. Chapter 8. Particles of Matter, continued
 States of Matter Preview Bellringer Section 2 Behavior of Gases In the kitchen, you might find three different forms of water. What are these three forms of water, and where exactly in the kitchen would
States of Matter Preview Bellringer Section 2 Behavior of Gases In the kitchen, you might find three different forms of water. What are these three forms of water, and where exactly in the kitchen would
Chapter 22 States of matter. Section 1 matter Section 2 Changes of State
 Chapter 22 States of matter Section 1 matter Section 2 Changes of State States of Matter is a physical property ***Matter is made of atoms Atoms form chemical bonds to make matter **** Atoms vibrate constantly
Chapter 22 States of matter Section 1 matter Section 2 Changes of State States of Matter is a physical property ***Matter is made of atoms Atoms form chemical bonds to make matter **** Atoms vibrate constantly
Chapter 3. Preview. Section 1 Three States of Matter. Section 2 Behavior of Gases. Section 3 Changes of State. States of Matter.
 States of Matter Preview Section 1 Three States of Matter Section 2 Behavior of Gases Section 3 Changes of State Concept Mapping Section 1 Three States of Matter Bellringer In the kitchen, you might find
States of Matter Preview Section 1 Three States of Matter Section 2 Behavior of Gases Section 3 Changes of State Concept Mapping Section 1 Three States of Matter Bellringer In the kitchen, you might find
INPUT~ Explore It! Station Directions: This is one of the four INPUT stations. They may be completed in any order.
 INPUT~ Explore It! Station Directions: This is one of the four INPUT stations. They may be completed in any order. One member of the group will read the task cards in order. The group will be responsible
INPUT~ Explore It! Station Directions: This is one of the four INPUT stations. They may be completed in any order. One member of the group will read the task cards in order. The group will be responsible
Epsilon-Delta Window Challenge Name Student Activity
 Open the TI-Nspire document Epsilon-Delta.tns. In this activity, you will get to visualize what the formal definition of limit means in terms of a graphing window challenge. Informally, lim f( x) Lmeans
Open the TI-Nspire document Epsilon-Delta.tns. In this activity, you will get to visualize what the formal definition of limit means in terms of a graphing window challenge. Informally, lim f( x) Lmeans
From Rumor to Chaos TEACHER NOTES MATH NSPIRED. Math Objectives. Vocabulary. About the Lesson. TI-Nspire Navigator System
 Math Objectives Students will model the spread of a rumor using a recursive sequence. Students will understand that variations in the recursive sequence modeling the spread of a rumor or a disease can
Math Objectives Students will model the spread of a rumor using a recursive sequence. Students will understand that variations in the recursive sequence modeling the spread of a rumor or a disease can
Chemistry Heat Review. Heat: Temperature: Enthalpy: Calorimetry: Activation energy:
 Chemistry Heat Review Name Date Vocabulary Heat: Temperature: Enthalpy: Calorimetry: Activation energy: Formulas Heat of phase change Heat for temperature increase Heat of reaction Endothermic/Exothermic
Chemistry Heat Review Name Date Vocabulary Heat: Temperature: Enthalpy: Calorimetry: Activation energy: Formulas Heat of phase change Heat for temperature increase Heat of reaction Endothermic/Exothermic
SPH3U1 Lesson 03 Energy
 THERMAL ENERGY AND LATENT HEAT LEARNING GOALS Students will learn: Heat changes the amount of thermal energy in an object Temperature is a measure of the average thermal energy in an object Heat capacity
THERMAL ENERGY AND LATENT HEAT LEARNING GOALS Students will learn: Heat changes the amount of thermal energy in an object Temperature is a measure of the average thermal energy in an object Heat capacity
Name: Block: Date: Student Notes. OBJECTIVE Students will investigate the relationship between temperature and the change of the state of matter.
 Name: Block: Date: LCPS Core Experience Heat Transfer Student Notes OBJECTIVE Students will investigate the relationship between temperature and the change of the state of matter. LINK 1. Particles in
Name: Block: Date: LCPS Core Experience Heat Transfer Student Notes OBJECTIVE Students will investigate the relationship between temperature and the change of the state of matter. LINK 1. Particles in
Term Info Picture. Anything that has mass and takes up space; everything is made of matter.
 Characteristics, Changes, and States of Matter S8P1. Obtain, evaluate, and communicate information about the structure and properties of matter. B. Develop and use models to describe the movement of particles
Characteristics, Changes, and States of Matter S8P1. Obtain, evaluate, and communicate information about the structure and properties of matter. B. Develop and use models to describe the movement of particles
States of Matter 1 of 21 Boardworks Ltd 2016
 States of Matter 1 of 21 Boardworks Ltd 2016 States of Matter 2 of 21 Boardworks Ltd 2016 What are the three states of matter? 3 of 21 Boardworks Ltd 2016 At any given temperature, all substances exist
States of Matter 1 of 21 Boardworks Ltd 2016 States of Matter 2 of 21 Boardworks Ltd 2016 What are the three states of matter? 3 of 21 Boardworks Ltd 2016 At any given temperature, all substances exist
1 Three States of Matter
 CHAPTER 3 1 Three States of Matter SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is matter made of? What are the three most common
CHAPTER 3 1 Three States of Matter SECTION States of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is matter made of? What are the three most common
Lesson 02: Physical Properties of Matter. 01 Matter
 Chemistry 11, Physical Properties, Unit 02 1 Lesson 02: Physical Properties of Matter 01 Matter Almost everything in the universe is made of matter matter has volume matter has mass matter is made up of
Chemistry 11, Physical Properties, Unit 02 1 Lesson 02: Physical Properties of Matter 01 Matter Almost everything in the universe is made of matter matter has volume matter has mass matter is made up of
States of Matter. Changes in State
 CHAPTER 8 States of Matter LESSON 2 Changes in State What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with
CHAPTER 8 States of Matter LESSON 2 Changes in State What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with
Solids (cont.) Describe the movement of particles in a solid and the forces between them.
 Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together. Solids (cont.) Describe the movement
Solids A solid is matter that has a definite shape and a definite volume. The attractive forces between the particles in a solid are strong and pull them close together. Solids (cont.) Describe the movement
4. Every CHANGE in matter includes a change in, which is conserved in a chemical reaction and. TRANSFORMED from one form to another.
 Part C: Lesson 1.4 - Read pages 20-29 to answer these questions. Add to notes - 1. A physical change alters the form or appearance of matter, such as Change in size, a change in SIZE or. SHAPE shape, or
Part C: Lesson 1.4 - Read pages 20-29 to answer these questions. Add to notes - 1. A physical change alters the form or appearance of matter, such as Change in size, a change in SIZE or. SHAPE shape, or
Worksheet: Coulomb Force Simulation
 Screen shot of simulation showing force between two charged particles. Worksheet: Coulomb Force Simulation Key Topic/Concept: Coulomb s Law and Newton s 3 rd Law Materials: One guide sheet for each student
Screen shot of simulation showing force between two charged particles. Worksheet: Coulomb Force Simulation Key Topic/Concept: Coulomb s Law and Newton s 3 rd Law Materials: One guide sheet for each student
Foundations of Chemistry
 Name Foundations of Chemistry What is matter, and how does it change? Date Before You Read Before you read the chapter, think about what you know about matter and how it changes Record three things that
Name Foundations of Chemistry What is matter, and how does it change? Date Before You Read Before you read the chapter, think about what you know about matter and how it changes Record three things that
Chapter Review USING VOCABULARY UNDERSTANDING CONCEPTS. Skills Worksheet
 Skills Worksheet Chapter Review USING VOCABULARY 1. Academic Vocabulary Which of the following words means a set of steps or events? a. reaction b. process c. principle d. role For each pair of terms,
Skills Worksheet Chapter Review USING VOCABULARY 1. Academic Vocabulary Which of the following words means a set of steps or events? a. reaction b. process c. principle d. role For each pair of terms,
Calorimetry. A calorimeter is a device in which this energy transfer takes place
 Calorimetry One technique for measuring specific heat involves heating a material, adding it to a sample of water, and recording the final temperature This technique is known as calorimetry A calorimeter
Calorimetry One technique for measuring specific heat involves heating a material, adding it to a sample of water, and recording the final temperature This technique is known as calorimetry A calorimeter
Name Chemistry / / SOL Questions Chapter 9 For each of the following, fill in the correct answer on the BLUE side of the scantron.
 Name Chemistry / / SOL Questions Chapter 9 For each of the following, fill in the correct answer on the BLUE side of the scantron. 1. Which number on the graph to the right represents the effect of the
Name Chemistry / / SOL Questions Chapter 9 For each of the following, fill in the correct answer on the BLUE side of the scantron. 1. Which number on the graph to the right represents the effect of the
Exercises Evaporation (page 451) 23.2 Condensation (pages )
 Exercises 23.1 Evaporation (page 451) 1. The four forms in which matter exists solid, liquid, gas, and plasma are called. 2. Water that is left out in an open container will eventually. 3. Is the following
Exercises 23.1 Evaporation (page 451) 1. The four forms in which matter exists solid, liquid, gas, and plasma are called. 2. Water that is left out in an open container will eventually. 3. Is the following
Lesson 9: States of Matter
 Lesson 9: States of Matter Do Now 6O, 6S 11.8.18 Take out HW 6.14 to be checked. Copy info into CJ keep CJ out and open on desk throughout class. On Do Now Page #5, copy and answer: 1. If you use a magnet
Lesson 9: States of Matter Do Now 6O, 6S 11.8.18 Take out HW 6.14 to be checked. Copy info into CJ keep CJ out and open on desk throughout class. On Do Now Page #5, copy and answer: 1. If you use a magnet
TIphysics.com. Physics. Resolving Vectors ID: 9899
 Resolving Vectors ID: 9899 By Charles W. Eaker Time required 30 minutes Topic: Kinematics Graphically resolve a vector into perpendicular components. Resolve a vector into perpendicular components using
Resolving Vectors ID: 9899 By Charles W. Eaker Time required 30 minutes Topic: Kinematics Graphically resolve a vector into perpendicular components. Resolve a vector into perpendicular components using
Chapter 9. Preview. Objectives Defining Temperature. Thermal Equilibrium. Thermal Expansion Measuring Temperature. Section 1 Temperature and
 Section 1 Temperature and Thermal Equilibrium Preview Objectives Defining Temperature Thermal Equilibrium Thermal Expansion Measuring Temperature Section 1 Temperature and Thermal Equilibrium Objectives
Section 1 Temperature and Thermal Equilibrium Preview Objectives Defining Temperature Thermal Equilibrium Thermal Expansion Measuring Temperature Section 1 Temperature and Thermal Equilibrium Objectives
Thermal energy 7 TH GRADE SCIENCE
 Thermal energy 7 TH GRADE SCIENCE Temperature There s more to temperature than the idea of hot and cold. Remember that all matter is made up of tiny particles that are constantly moving even in solid objects.
Thermal energy 7 TH GRADE SCIENCE Temperature There s more to temperature than the idea of hot and cold. Remember that all matter is made up of tiny particles that are constantly moving even in solid objects.
* Defining Temperature * Temperature is proportional to the kinetic energy of atoms and molecules. * Temperature * Internal energy
 * Defining Temperature * We associate temperature with how hot or cold an object feels. * Our sense of touch serves as a qualitative indicator of temperature. * Energy must be either added or removed from
* Defining Temperature * We associate temperature with how hot or cold an object feels. * Our sense of touch serves as a qualitative indicator of temperature. * Energy must be either added or removed from
EVAPORATION AND INTERMOLECULAR ATTRACTIONS From Chemistry with Vernier, Vernier Software and Technology LABQUEST 9
 EVAPORATION AND INTERMOLECULAR ATTRACTIONS From Chemistry with Vernier, Vernier Software and Technology LABQUEST 9 Westminster College In this experiment, Temperature Probes are placed in various liquids.
EVAPORATION AND INTERMOLECULAR ATTRACTIONS From Chemistry with Vernier, Vernier Software and Technology LABQUEST 9 Westminster College In this experiment, Temperature Probes are placed in various liquids.
SAM Teachers Guide Phase Change Overview Learning Objectives Possible Student Pre/Misconceptions
 SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion. Students observe and manipulate
SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion. Students observe and manipulate
SAM Teachers Guide Phase Change Overview Learning Objectives Possible Student Pre/Misconceptions
 SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion and observing and manipulating
SAM Teachers Guide Phase Change Overview Students review the atomic arrangements for each state of matter, following trajectories of individual atoms to observe their motion and observing and manipulating
Heating Curve Worksheet If this curve is read from right to left, it is a Cooling Curve.
 Heating Curve Worksheet If this curve is read from right to left, it is a Cooling Curve. The diagram below illustrates the steps involved to convert 10g of solid ice at -20 C to 10g of gaseous steam at
Heating Curve Worksheet If this curve is read from right to left, it is a Cooling Curve. The diagram below illustrates the steps involved to convert 10g of solid ice at -20 C to 10g of gaseous steam at
Matter & Energy. Objectives: properties and structures of the different states of matter.
 Matter & Energy Objectives: 1. Use the kinetic theory to describe the properties and structures of the different states of matter. 2. Describe energy transfers involved in changes of state. 3. Describe
Matter & Energy Objectives: 1. Use the kinetic theory to describe the properties and structures of the different states of matter. 2. Describe energy transfers involved in changes of state. 3. Describe
Liquids and Intermolecular Forces. Course Learning Outcomes for Unit I. Reading Assignment. Unit Lesson UNIT I STUDY GUIDE
 UNIT I STUDY GUIDE Liquids and Intermolecular Forces Course Learning Outcomes for Unit I Upon completion of this unit, students should be able to: 1. Identify the intermolecular attractive interactions
UNIT I STUDY GUIDE Liquids and Intermolecular Forces Course Learning Outcomes for Unit I Upon completion of this unit, students should be able to: 1. Identify the intermolecular attractive interactions
How Does the Sun s Energy Cause Rain?
 1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
1.2 Investigate 3.3 Read How Does the Sun s Energy Cause Rain? In the water-cycle simulation, you observed water change from a liquid to a gas, and then back to a liquid falling to the bottom of the container.
P6 Molecules and matter. Student Book answers. P6.1 Density. Question Answer Marks Guidance. 1 a m 3 (= 0.80 m 0.60 m 0.
 P6. Density a 0.024 m 3 (= 0.80 m 0.60 m 0.05 m) b = 2500 kg/m 3 2 a 36 g 48 g = 88 g 2 b =. g/cm 3 3 a i 0.000 40 m 3 (= 0.0 m 0.080 m 0.05 m) 3 a ii = 9 000 kg/m 3 3 b v = = 7.9 0 8 m 3 thickness t =
P6. Density a 0.024 m 3 (= 0.80 m 0.60 m 0.05 m) b = 2500 kg/m 3 2 a 36 g 48 g = 88 g 2 b =. g/cm 3 3 a i 0.000 40 m 3 (= 0.0 m 0.080 m 0.05 m) 3 a ii = 9 000 kg/m 3 3 b v = = 7.9 0 8 m 3 thickness t =
Physical Science Exam 3 Study Guide. Dr. Karoline Rostamiani. Chapter 3
 Chapter 3 Section 1 States of Matter What is matter made of? What are the three most common states of matter? How do particles behave in each state of matter? Solids, Liquids, and Gases Materials can be
Chapter 3 Section 1 States of Matter What is matter made of? What are the three most common states of matter? How do particles behave in each state of matter? Solids, Liquids, and Gases Materials can be
4 Discuss and evaluate the 5th state of matter. 3 - Differentiate among the four states of matter in terms of energy,
 Goal: Differentiate among the four states of matter in terms of energy, particle motion, and phase transitions. 4 States of Mater Sections 3.1, 3.2 4 Discuss and evaluate the 5 th state of matter. 3 -
Goal: Differentiate among the four states of matter in terms of energy, particle motion, and phase transitions. 4 States of Mater Sections 3.1, 3.2 4 Discuss and evaluate the 5 th state of matter. 3 -
Unit 13 Lesson 1 What Are Solids, Liquids, and Gases? Copyright Houghton Mifflin Harcourt Publishing Company
 Unit 13 Lesson 1 What Are Solids, Liquids, and Gases? Copyright Houghton Mifflin Harcourt Publishing Company What s the Matter? Matter has mass and volume. It cannot be created or destroyed. Mass is the
Unit 13 Lesson 1 What Are Solids, Liquids, and Gases? Copyright Houghton Mifflin Harcourt Publishing Company What s the Matter? Matter has mass and volume. It cannot be created or destroyed. Mass is the
TIphysics.com. Physics. Collisions in One Dimension ID: 8879
 Collisions in One Dimension ID: 8879 By Charles W. Eaker Time required 45 minutes Topic: Momentum and Collisions Compare kinetic energy and momentum. Classify collisions as elastic or inelastic. Compare
Collisions in One Dimension ID: 8879 By Charles W. Eaker Time required 45 minutes Topic: Momentum and Collisions Compare kinetic energy and momentum. Classify collisions as elastic or inelastic. Compare
THERMODYNAMICS. Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer.
 THERMODYNAMICS Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer. Central Heating Objectives: After finishing this unit,
THERMODYNAMICS Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer. Central Heating Objectives: After finishing this unit,
Chapter 14 9/21/15. Solids, Liquids & Gasses. Essential Questions! Kinetic Theory! Gas State! Gas State!
 Chapter 14 Solids, Liquids & Gasses Essential Questions What is the kinetic theory of matter? How do particles move in the different states of matter? How do particles behave at the boiling and melting
Chapter 14 Solids, Liquids & Gasses Essential Questions What is the kinetic theory of matter? How do particles move in the different states of matter? How do particles behave at the boiling and melting
Preview. Heat Section 1. Section 1 Temperature and Thermal Equilibrium. Section 2 Defining Heat. Section 3 Changes in Temperature and Phase
 Heat Section 1 Preview Section 1 Temperature and Thermal Equilibrium Section 2 Defining Heat Section 3 Changes in Temperature and Phase Heat Section 1 TEKS The student is expected to: 6E describe how the
Heat Section 1 Preview Section 1 Temperature and Thermal Equilibrium Section 2 Defining Heat Section 3 Changes in Temperature and Phase Heat Section 1 TEKS The student is expected to: 6E describe how the
solid IMF>liquid IMF>gas IMF Draw a diagram to represent the 3 common states of matter of a given substance: solid liquid gas
 Thermochemistry Part 1 Notes States of Matter and Intermolecular Forces (IMF) Chemistry HP At the end of this unit, students should be able to: Describe the various states of matter in terms of kinetic
Thermochemistry Part 1 Notes States of Matter and Intermolecular Forces (IMF) Chemistry HP At the end of this unit, students should be able to: Describe the various states of matter in terms of kinetic
! Name: STUDENT JOURNAL Week 13 Radioactivity & Physical Changes
 ! Name: Period: STUDENT JOURNAL Week 13 Radioactivity & Physical Changes Overarching Goal for the Week: Distinguish between the types of physical changes and the change in particle motion Learning Objectives:
! Name: Period: STUDENT JOURNAL Week 13 Radioactivity & Physical Changes Overarching Goal for the Week: Distinguish between the types of physical changes and the change in particle motion Learning Objectives:
Forensics with TI-Nspire Technology
 Forensics with TI-Nspire Technology 2013 Texas Instruments Incorporated 1 education.ti.com About the Lesson This lab uses the identification of an unknown drug to demonstrate the differences between chemical
Forensics with TI-Nspire Technology 2013 Texas Instruments Incorporated 1 education.ti.com About the Lesson This lab uses the identification of an unknown drug to demonstrate the differences between chemical
2 Changes of State KEY IDEAS READING TOOLBOX ADDING AND REMOVING ENERGY. States of Matter. As you read this section, keep these questions in mind:
 CHAPTER 3 States of Matter 2 Changes of State SECTION KEY IDEAS As you read this section, keep these questions in mind: What happens when a substance changes from one state of matter to another? What happens
CHAPTER 3 States of Matter 2 Changes of State SECTION KEY IDEAS As you read this section, keep these questions in mind: What happens when a substance changes from one state of matter to another? What happens
CHAPTER 4 - STATES OF MATTER. Mr. Polard Physical Science Ingomar Middle School
 CHAPTER 4 - STATES OF MATTER Mr. Polard Physical Science Ingomar Middle School SECTION 1 MATTER VOCABULARY SECTION 1 Matter : anything that takes up space and has mass (pg 72, 102) Solid : Matter with
CHAPTER 4 - STATES OF MATTER Mr. Polard Physical Science Ingomar Middle School SECTION 1 MATTER VOCABULARY SECTION 1 Matter : anything that takes up space and has mass (pg 72, 102) Solid : Matter with
Before Statement After
 Thermal Energy Thermal Energy, Temperature, and Heat What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with
Thermal Energy Thermal Energy, Temperature, and Heat What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with
AQA (Trilogy) Combined Science GCSE Unit 6.3 Particle Model of Matter
 AQA (Trilogy) Combined Science GCSE Unit 6.3 Particle Model of Matter Test (Levels 4 9) Time allowed: 50 minutes Question Links to Student Progress Sheet Score Total Marks Available Score Estimated Grade
AQA (Trilogy) Combined Science GCSE Unit 6.3 Particle Model of Matter Test (Levels 4 9) Time allowed: 50 minutes Question Links to Student Progress Sheet Score Total Marks Available Score Estimated Grade
Phase Changes. Measuring temperature during phase changes
 Objective The purpose of this activity is to analyze temperature changes in water as a result of a physical state transition, formulating an hypothesis about that phenomenon and testing it, using the Labdisc
Objective The purpose of this activity is to analyze temperature changes in water as a result of a physical state transition, formulating an hypothesis about that phenomenon and testing it, using the Labdisc
