Two-body polyelectrolyte-mediated bridging interactions
|
|
|
- Octavia Long
- 6 years ago
- Views:
Transcription
1 JOURNAL OF CHEMICAL PHYSICS VOLUME 118, NUMBER 4 JUNE 3 Two-body polyelectrolyte-mediated bridging interactions Rudi Podgornik Laboratoire de Physique des Solides, Université Paris Sud, Bat 51, 9145 Orsay Cedex, France and Department of Physics, University of Ljubljana, Jadranska 19, 1 Ljubljana, Slovenia Received January 3; accepted 5 March 3 We investigate theoretically polyelectrolyte bridging interactions on the two-body level. The model system is composed of two macroions with two oppositely charged flexible chains. The electrostatic interactions are treated on the Debye Hückel level. The formal level of the theory is provided by the Feynman Kleinert variational method generalized to include also self-interactions between polyelectrolyte segments. The variational equations are shown to exhibit two solution branches corresponding to strong and weak coupling, whereas conformations of the chain can be described as weakly or strongly paired. We investigate the effective pair interaction between the macroions in the parameter space and comment on the relevance of the calculation for bridging interactions in experimental context. 3 American Institute of Physics. DOI: 1.163/ I. INTRODUCTION Polyelectrolytes are ubiquitous in colloidal systems and play a fundamental role in determining the interactions between as well as stability and structure of various molecular assemblies. 1 Their effect on colloidal interactions has been studied and exploited in various technological contexts ranging from the paper industry to the pharmaceutical industry. It seems, however, that their most basic role is played in the biological context where their importance can be hardly overestimated. They are an essential and fundamental component of the cellular environment and make their mark in its every structural and functional aspect. 3 The behavior of polyelectrolytes in biological context has without any doubt been one of the focuses of soft matter research for quite a few years now. 4 The intense work on the interactions and mesophase behavior of the most studied polyelectrolyte in the biological context if not in general i.e., DNA has elucidated many fascinating physical aspects of this molecule and the repercussions that they have on the structure and function of biological matter. 5 The mesoscopic interactions between many DNA molecules 6 and elastic properties of single DNA molecules in different solution conditions 7 have been measured directly and are understood on a fundamental physical level. Not all biological polyelectrolytes or all solution conditions have been or indeed can be studied at quite the same level of detail. Sometimes a lot less information than direct measurement of molecular interactions at all macromolecular densities is experimentally available. Studies at low macromolecular densities in systems where polyelectrolyte behavior is expected to show its mark usually only lead to second virial coefficients and not complete interaction curves. 8 1 This is not due to poorly designed experimental setup, but shows a rather fundamental limit in the amount of information that can be provided by experiments at these conditions. In this situation one has to rely heavily on different models of the mesoscopic interaction potential and the way they transpire through the measured second virial coefficient. This is the first point of departure of this work. The second one is a very peculiar interaction in polyelectrolyte systems, where long charged polymers can mediate interactions between macroions of opposite charge Ref. 11 and references therein. The term bridging interactions is usually applied to this situation where a single chain can adsorb to different macroions and via its connectivity and elasticity mediate attractive interactions between them. These interactions have been studied intensively both experimentally 1 as well as theoretically Surface force apparatus and atomic force microscopy have provided direct data on the separation dependence of the bridging interaction between macroscopic surfaces with polyelectrolyte chains either grafted or in chemical equilibrium with a bulk solution. 1 Theoretical work has added a clear mesoscopic picture for the bridging interaction between macroscopic surfaces and elucidated the effects of salt and nonelectrostatic excluded volume effects on the strength and range of this interaction Since it is based on sometimes severe model or formal restrictions, there is no single theoretical approach that is able to account for all experimentally observed details or is able to explore in comparable details all the regions of the parameter phase space. 11 The fact that the effects of the bridging interaction between small macroions 15 as opposed to macroscopic surfaces 11 have been studied to a much smaller extent thus makes a strong point for its re-evaluation. The main motivation for this task is recent experiments on the second virial coefficient of the nucleosomal core particles NCP in the low-density regime at various solution conditions. 1,17 NCPs represent the lowest level of the chromatin organization in eucaryotes and have recently been resolved at an atomic resolution. 18 They consist of a histone protein octamer core with 146 bp of DNA tightly wrapped around it, giving it an approximate cylindrical shape of a radius 55 Å, a height of 57 Å, and a structural charge of 5. This complex is stable in an aqueous solution from 1 to 75 mm monovalent salt ionic strength. 17 The charged histone N-termini or N-tails can desorb from this complex 1-966/3/118(4)/1186/11/$ American Institute of Physics Downloaded 15 Jun 3 to Redistribution subject to AIP license or copyright, see
2 J. Chem. Phys., Vol. 118, No. 4, June 3 Two-body polyelectrolyte mediated bridging interactions 1187 and basically play the role of grafted flexible polyelectrolyte chains of an approximate total charge of 9. They remain essentially adsorbed to the DNA segment of the NCP at low ionic strength, but tend to assume a more extended conformation as the ionic strength is increased. 1 The application of classical 19 and manometric osmometry provided data on the osmotic pressure of the NCP in NaCl solutions of variable ionic strength from which the second virial coefficient was deduced quite accurately. 1 It was demonstrated that the second virial coefficient is a nonmonotonic function of the ionic strength, and a plausible though tentative hypothesis was given that the nonmonotonicity of the second virial coefficient might be due to the bridging interaction mediated by the extended N-tails of the NCPs. 1 A very similar nonmonotonic dependence of the second virial coefficient was seen also in apoferritin solutions where a bridging interpretation is hard to envision. 9 Motivated by this important experimental result and its tentative interpretation, we embarked on a detailed study of the interaction between charged macroions with grafted oppositely charged polyelectrolyte tails as a function of the ionic strength of a monovalent bathing salt solution. The level of the theoretical calculation had to be considered that would allow for a straightforward evaluation of the second virial coefficient, being an integral of the underlying interaction potential, and its dependence on the ionic strength. Also since the N-tails in the motivating experiment cannot be considered as infinitely long i.e., there are finite-size effects that need to be taken properly into account it seems that a mean-field MF theory of bridging interactions as formulated for the case of interacting charged planes and based on the ground-state dominance ansatz 13,16 cannot be simply implemented to the present case. Finite-size effects are quite difficult to deal with on the MF level, especially if one needs to evaluate the interaction potential between macroions in a very large ideally infinite range of separations. In view of all this we formulated a variational 1 two-particle twomacroion theory of the bridging interaction that starts from an explicit mesoscopic polyelectrolyte model and includes the interactions of the polyelectrolyte chain with the macroions, the interactions of the chain with itself and, connected with it, the effect of the electrostatic stiffening of the chain, as well as the configurational entropy of the chain. In some respects the theory proposed here could be viewed upon as a variational version of the Asakura Oosawa theory. The finite size of the chain can be relatively straightforwardly dealt with on this level of the theory and gives rise to important features of the two-particle bridging interaction that are lost in the simplest, ground-state dominance formulation of the MF theories. The organization of the paper is as follows: We will first describe the model and give an introduction to a modified variational Feynman Kleinert approach to the polyelectrolyte chains. We will derive the main equations and solve them numerically for different conditions. We will show that in general the bridging interactions for this model system comes in two varieties that we dub the strong- and weakcoupling limits. The form of the total interaction between the macroions will be obtained numerically for all values of the intermacroion separation as a function of system parameters such as the amount of fixed charge on the macroions, the length of the polyelectrolyte chain, and the screening length of the intervening bathing solution. We will discuss the ramifications of these results for the salt dependence of the second virial coefficient and point to the possible shortcomings of the calculation and guess about a way to possibly circumvent them. We will finally comment on the significance of the present calculation for the understanding of the bridging interaction in the NCP solution system. II. MODEL The model system that we take as the starting point of our theoretical investigation of the bridging interaction is quite simple: it is composed of two spherical point macroions with M negative fixed charges plus two oppositely charged chains, each with N monomers, one per each monomer. The pair interaction potential u(r,r) between all the charges in the system will be taken of the screened Coulomb Debye Hückel form 1 ur,r e e rr 4 rr ee or else uk k, 1 in real or Fourier space, the form we will need later on. is the inverse Debye length, e is the elementary charge, one per each Kuhn s length, and the rest of the notation is standard. Obviously, counterions are not explicitly included in this model. The interaction potential between the polyelectrolyte and macroion charges is assumed of a similar form: viz., ext r e 1e e rr1 e e e rr, 4 rr 1 4 rr where r 1, r, etc., are the positions of macroions and their charges are e 1 e Me, etc. Our model is thus a very straightforward generalization of many macroions of the model used in polyelectrolyte adsorption studies. 3 We will use a standard Edwards model 4 for the polyelectrolyte chain where the mesoscopic Hamiltonian has contributions from chain connectivity, interactions between the segments of the chain and the interaction with an external field due to the presence of two macroions. It is written as Hr i n 3 N ṙi ndn i1 N N u ri n,r j n dn dn 1 i, j1 N ext r i n dn, i1 where is the Kuhn s length and u(r,r) is the pair interaction potential, while ext (r) is the external interaction potential. The indices i, j stand for the two polyelectrolyte chains. 3 Downloaded 15 Jun 3 to Redistribution subject to AIP license or copyright, see
3 1188 J. Chem. Phys., Vol. 118, No. 4, June 3 Rudi Podgornik FIG. 1. Schematic representation of the model and the nature of the variational solutions. LHS: the strong-coupling solution branch. The conformation of the chain is determined mostly by the interaction with the two macroions. It can be either in the weakly upper or strongly lower drawing paired configuration, depending on the separations between the macroions. The attractive bridging interaction is strongest in the strongly paired configuration. RHS: the weak-coupling solution branch. The conformation of the chain is determined mostly by the self-interactions of the chains. They are in the weakly paired configuration for any separation between the macroions. On approach the interpenetration of the monomer clouds the coronas leads to prevalently repulsive interactions. Note that the macroions are treated as point particles in the calculation and that their size is thus exaggerated in this drawing. Figure 1 schematically presents the mesoscopic model on which the present evaluation of the bridging interactions is based. Clearly, the nonpairwise additive effects, such as bridging between multiple macroions mediated by a single chain, have been completely disregarded in this model. The finite macroion size effects have also been disregarded. Also, the model is based on a linear theory Debye Hückel of Coulomb interactions and thus cannot capture nonlinearities such as charge renormalization or counterion condensation. It can, however, take into account the electrostatic stiffening of the chain as well as the finite chain size effects. The grafting of the chains to their respective macroions is not taken into account explicitly on the Hamiltonian level. First of all, in this model system the effects of grafting are small or indeed negligible 15 to the extent that they are always overpowered by the much stronger electrostatic interactions. This would of course not be the case for, e.g., electrostatic brushes where grafting has to enter the description of the chain already at the Hamiltonian level. The grafting of the chains is only taken into account via their center-of-mass coordinates in the way explained later. III. VARIATIONAL ANSATZ AND FORMALISM Since the interactions with external fields as well as the self-interactions along the polymer chains are highly nonlinear in terms of their spatial dependence, the statistical integral corresponding to Eq. 3 is in general impossible to evaluate analytically and is difficult to evaluate even approximately. Instead of taking recourse to a numerical approximation, we will rather introduce a harmonic variational ansatz 5,6 that will make the evaluation of the statistical integral straightforward. The parameters of the variational ansatz will be chosen so as to minimize the upper bound of the exact free energy. This procedure is generally known as the Feynman Kleinert variational method, 6 and its application to the case of self-interacting polymer chains in the bulk has been discussed in detail elsewhere. 1 It was shown that it captures all the salient features of polyelectrolyte behavior in the bulk. The details of the variational approach are as follows: 1 the two polyelectrolyte chains are treated as Gaussian blobs positioned at their centers-of-mass, r 1 and r, respectively. The width of the Gaussian blobs is determined variationally from the strength of the external fields as well as selfinteractions along the chain. 6 The Gaussian blobs thus behave as effective particles with finite extensions. In this respect we can claim that the present theoretical framework represents a kind of Asakura Oosawa theory where the effective size of the macroions is determined variationally. The final statistical integral is then obtained by integrating over the two centers-of-mass, r 1 and r, i.e., the coordinates of the effective Asakura Oosawa particles, or indeed by finding the configuration of the centers-of-mass that gives the largest contribution to this integral. 7 For the variational ansatz corresponding to two effective Gaussian chains we will chose a general harmonic Hamiltonian of the form 1 H r i n 3 i1 N ṙi ndn 3 i r i Nr i nr i dn i1 NLr 1,r, with periodic boundary conditions for r i (n). This ansatz is obviously still dependent on r i for i1,, which stand for the centers-of-mass of the two chains, i.e., r i (1/N) N r i (n)dn, as well as the functions i (r i ) and L(r 1,r ), which will be determined variationally. The term with i (r i ) obviously represents an external harmonic potential, centered on r i, which acts either to confine or expand the chain, depending on its sign. A simple limiting form of can be derived only for the case of a single self-interacting chain and is given in Ref. 1. In Eq. 4 this term was taken with a positive sign, but we will argue later that it can as well be negative. The term NL(r 1,r ) simply represents the value of this harmonic external potential at the centers-of-mass of both chains. Again, a simple limiting form of NL can be derived only for the case of a single self-interacting chain. 1 As will become clear when we proceed, both quantities depend in a complicated way on the interactions between the monomers as well as on the interactions between the monomers and external macroions. The statistical integral for the variational ansatz can be obtained in the following form: 1 N Dr 1 n Dr ne H r i n d 3 r 1 d 3 r e F r 1,r. 4 5 Downloaded 15 Jun 3 to Redistribution subject to AIP license or copyright, see
4 J. Chem. Phys., Vol. 118, No. 4, June 3 Two-body polyelectrolyte mediated bridging interactions 1189 The two polymer chains are thus represented as two effective Gaussian particles with an effective Hamiltonian given by F (r 1,r ). The details of the implementation of the Feynman Kleinert ansatz 6 for selfinteracting polymer chains have been given before, 1 and we will rely on the formal developments described in that work. First of all, let us introduce the radius of gyration defined as a i r i 1 N ri nr 3N i dn 1 3 i L i N, where L(x)coth x1/x is the standard Langevin function. One can then derive 1 that minimization of the upper bound of the variational free energy with respect to the function L(r 1,r ) leads to the following equation: with NLr 1,r 3 i r i Na i r i i1 Wr 1,r, Wr 1,r d 3 r ext rr,r 1,r 1 d 3 r d 3 r r,r 1,r ur,rr,r 1,r. W(r 1,r ) obviously represents the total interaction free energy, due to self-interactions as well as interactions with external fields, of a smeared monomer cloud with a Gaussian density distribution. (r,r 1,r ) stands for the combined monomer density function of the two chains and has the form r,r 1,r a1 r,r 1 a r,r, where for each of the chains the monomer density distribution function is given by N ai r,r i a i 3/ exp rr i a i or else ai kn exp k a i in real and Fourier space, the form we will need later on. Taking now the form of the self-interaction and the external fields as in Eqs. 1 and, we obtain the following result: Wr 1,r F a1 r 1 r k k1 k1 k1 W i W a1,a r 1,r. F a r r k 11 Here r i stand for the position of the two macroins to be distinguished from the position of the two centers-of-mass of the polymer chains, r i ). F s are due to the interaction of the chains with external fields and can be written in the form F ai rr d3 k 3 ai kuke ik rr. 1 The self-interactions of the chains correspond to the terms W i and are given by W i d3 k 3 ai k uk, 13 and finally the interactions between the two chains that can be derived in the form W a1,a r,r d3 k 3 a1 kuk a ke ik rr. 14 This is all straightforward generalization of the variational theory set up previously for a single chain. 1 The functions i (r i ) are next obtained by minimizing the upper bound to the exact free energy with respect to a i, leading to 3 i r i N a i Wr 1,r. 15 The effective center-of-mass free energy of the two polymer chains is finally given by the expression F r 1,r 3 i1 log sinh in i N Wr 1,r 3 i Na i i1 F r 1,r Wr 1,r, 16 where we separated out the harmonic part of the free energy F (r 1,r ). The first two terms of this variational free energy represent the entropy of the Gaussian chain, and the last one is due to the interactions with the external fields and self-interactions. These are the basic equations of the Feynman Kleinert variational theory as applied to the selfinteracting polyelectrolyte chains. They are still quite complicated because of the dependence on the center-of-mass coordinates r i and the final integration over these variables in Eq. 5. If there are no external fields that break the translational symmetry of the problem, it can be easily seen 1 that the dependence on r i vanishes and the solution of the variational equations is straightforward. With external fields the final quite complicated r i integration can be obtained only numerically. In the case that F (r 1,r ) scales with a positive power of N and N is large enough; there is, however, an additional quite accurate approximation to circumvent this final integration. 7 It consists of the saddle point evaluation of the final integration with respect to r i that is, of an additional minimization of F (r 1,r ) with respect to r 1 as well as r. This means that Eq. 5 can be written in an approximate form Downloaded 15 Jun 3 to Redistribution subject to AIP license or copyright, see
5 119 J. Chem. Phys., Vol. 118, No. 4, June 3 Rudi Podgornik Nd 3 r 1 d 3 r e F r 1,r e F r * 1r *, 17 where r 1 *, r * are given as solutions of the saddle point condition F r 1 *,r * r 1 * F r 1 *,r * * r. 18 Thus we obtain a simple explicit and accurate estimate for the free energy of two self-interacting polyelectrolyte chains in an external field: viz., FkT (N)F (r 1 *,r * ). In what follows we will always assume that all the solutions of the variational equations have to be symmetric with respect to the two chains. There is no reason on the pair-potential level to assume otherwise. Since the solution of variational equations is in general quite complicated, we give here a little preview of what exactly we will be calculating in what follows. We will show that grosso modo the solution of the variational problem has two branches depending on the relative magnitudes of the interaction with external fields and the self-interaction and mutual interaction of the chains. The two branches of the solution are the following. i What we call a strong-coupling branch, which corresponds to i (r i ) in the variational equation 5 and thus to the dominance of the interactions of the chains with the external macroion fields, the self-interaction and mutual interaction of the chains being a small perturbation. The strong-coupling branch furthermore bifurcates into two different subbranches depending on the solution of the additional minimization implied by Eq. 18: intheweakly paired subbranch the chains are associated each with its own grafting macroion and in the strongly paired subbranch both chains share the two macroions on the average. ii And what we term a weak-coupling branch where i (r i ) and thus corresponds to the case where the selfinteraction and mutual interaction of the chain are dominant and the interactions with external macroion fields are perturbative. Coupling in both cases thus refers to coupling with the external macroion field. In both cases we can in general observe some bridging effects, but they are several orders of magnitude stronger in the first case. Nevertheless, they are always present to some extent. After this introductory survey of the nature of the solutions of the variational problem, we are ready to find these solutions explicitly. A note on the grafting of the chain is in order at this point. Both in the weakly paired as well as the strongly paired states the electrostatic adsorption energy more than the grafting itself determines the statistics of the chain. Grafting the chains, by fixing, e.g., r i () to be at the surface of the macroion, would change none of the conclusions reached below, provided of course that the size of the macroions is small compared to the size of the chains and that we have only one chain associated with each of the macroions. It would, however, introduce some serious complications into our formalism, thus obscuring its straightforward interpretation. IV. SOLUTION OF THE VARIATIONAL EQUATIONS We are now ready to solve the general variational equations for the case of two polyelectrolyte chains with two external point macroions. As we already announced, we consider only symmetric solutions for which a 1 a a, but in general with r 1 r. This symmetrization will be applied to the results derived below in their final form. A little straightforward algebra then leads to the following form of the total variational free energy, Eq. 16: F ai rr BMN 3/ W i BN a f 1,a, a f 1 & a rr, a &, W a1,a r,r 4 BN f a 1 arr,a B e /4 kt was introduced above as the Bjerrum length. Also, we defined the following function: u sin uye f y,t u yu t du 4y ey e yt e yt Erfc y t y e Erfc yt t, where Erfc(x) is the standard complementary error function. On the other hand, the variational equation 15 can be obtained just as straightforwardly as Wr a 1,r 1 BN a 3 3/ M k1 Ng r 1r a g & a r 1 r a k, &,a Ng,a. 1 A similar equation could be obtained also for (/a )W(r 1,r ) except that r 1 on the right-hand side RHS would be turned into r. The following new function was defined above gy,t f y,t 1. What the variational equation 1 really asserts is which terms are important in determining the statistical conformation of the chain: i.e., a i in our case. The first term on the RHS of Eq. 1 is due to the interactions with the macroions, the second one is due to the interactions between the two chains, and the last one is the self-interaction of the chains. The conformation of the chain as described by a i is thus determined by the relative magnitudes of these three terms. Downloaded 15 Jun 3 to Redistribution subject to AIP license or copyright, see
6 J. Chem. Phys., Vol. 118, No. 4, June 3 Two-body polyelectrolyte mediated bridging interactions 1191 The properties of the solution of the variational equation 1 first of all depend crucially on the sign of the RHS of Eq. 1, thus on the fact whether is positive or negative. The sign of this term tells for each value of the separation between the macroions whether it is the interactions with the external fields or the self-interactions of the chain that determine the statistical conformation of the chain. In view of the form of the variational ansatz, Eq. 4, the positive sign corresponds to a general confinement of the chain if compared to the case with no interactions. We will refer to the ensuing interactions between the two macroions mediated by the polyelectrolyte chain as the strong-coupling limit. In the opposite case the chain is expanded if compared to the case with no interactions, and we will refer to the ensuing polyelectrolyte mediated interactions as the weakcoupling limit. Both terms will be explained further below. The final closure for this system of variational equations is provided by the relation between and a, Eq. 6. Since in this model the external macroions break the translational symmetry of the system, we apply also the minimization condition, Eq. 18, with respect to r 1 as well as r, in order to avoid the final complicated integral over the centers-of-mass of the two polyelectrolyte chains. This minimization introduces additional features of the solutions of the variational equations. Taking into account the Gaussian-like form of the function f 1 (y,t), we realize that there are in fact two different symmetric solutions to Eq. 18: i r 1 r 1 and r r, i.e., each of the chain remains associated with its grafting macroion, and ii r 1 r 1 (r 1 r ), i.e., each chain is shared by the two macroions symmetrically. Here we assumed that the first chain is grafted to the first macroion while the second one is grafted to the second macroion. We refer to the configuration of the polyelectrolye chains in the first case as weakly paired and in the second case as strongly paired. The terms are self-explanatory: in the first two cases the chain is confined to one of the macroions, whereas in the second case it is confined or bound by both of them. A schematic representation of the solutions of the variational equations is presented in Fig. 1. V. STRONG-COUPLING LIMIT Once again strong coupling means that external fields dominate the statistical configuration of the chain and thus. In this domain of the parameter space the effect of the interactions of the polyelectrolyte chain with the macroions, the term proportional to M in Eq. 1, determines the overall configuration of the chain. The strong-coupling limit entails, however, two different polyelectrolyte equilibrium states as discussed above, depending on the minimization with respect to r 1, r : the first state, stable for small values of the separation between the macroions, is due to the strong-pairing configuration of the chain with r 1 r (r 1 1 r ). The variational equation for in this case reads 3 BN a 3 Mg & 5/ ar 1 r a, &3Ng,a, 3 while the corresponding free energy has the form f 1 ar & 1 F F r 1 r BN a 7/ M r, a &6Nf 1,a. 4 The form of the dependence F (r 1 r ) is of course given implicitely via the dependence of and a. Once again, the chain here is bound to both macroions and its statistical properties are dominated by the interaction with the charges on the macroions. One would expect that the polyelectrolyte mediated interactions between the macroions would be strongest in this case. Obviously, for large enough r 1 r the RHS of Eq. 3 can become negative, going first through zero. This is due to the fact that g(y,t) is a decaying function of y. At this point the above solution ceases to be stable and we have a transition from the strongly paired to weakly paired branch of the strong-coupling limit. The transition depends on the macroion parameters such as the magnitude of their charges as well as the length of the chains. In this sense it represents a finite-size of the chains effect. The weakly paired configuration is characterized by r 1 r 1 and r r and is the stable branch at larger separations between the macroions. Here the variational equation for becomes 3 BN a 3 M g 3/, a Ng r 1r a & &g & a r 1 r a,,a Ng,a. 5 The corresponding free energy in this case can be obtained as f 1, a F F r 1 r BN a 5/ M f 1 & a r 1 r, a Nf 1,. & &4Nf 1 r 1r a,a 6 The most important term to determine the conformation of the chain is the interaction with the single macroion and is thus only weakly dependent on the separation between them. These are the first and last terms on the RHS of Eq. 5. The separation-dependent terms act only as a perturbation to these terms. It is thus to be expected that the polyelectrolyte mediated interactions will be much weaker in this case. Also in order to get the interaction free energy in the weakly paired configuration one needs in addition to subtract the terms that do not depend on the separation between the macroions from the total free energy. This is the standard way to get the interaction free energy. Again, the form of the dependence F (r 1 r ) is given implicitely via the dependence of and a. Downloaded 15 Jun 3 to Redistribution subject to AIP license or copyright, see
7 119 J. Chem. Phys., Vol. 118, No. 4, June 3 Rudi Podgornik The form of the solution of Eqs. 17 and 5 as well as the corresponding polyelectrolyte-mediated interaction free energy is presented in Fig.. We see that at small enough separations the chain is in the strongly paired configuration, being confined symmetrically by both macroions. In this regime the external field trying to confine the chain to both macroions wins over the chain entropy that is expanding the chain. The entropy of the parts of the chain spanning the region between the macroions is quite low. Its size, as described by a, in this case depends monotonically on the separation between the macroions that are effectively stretching it. At the instability point, reached at a well-defined value of the separation between the macroions, the chain entropy scores a partial victory over the interactions with the macroions forcing the chain to remain close to the macroion to which it is grafted. At this transition the chain basically relaxes the low-entropy configurations of its parts confined between both macroions by snapping back to the macroion to which it is grafted. After that the size of the chain is basically determined solely through the interactions of the chain with its grafting macroion and remains constant with separation between the macroions. These conclusions reached on the basis of the two chain variational approach are very similar to the existing simulation data 15 for one chain in the field of two macroions. This scenario of chain conformations is clearly illustrated in Figs. and 3 where one can follow the transition of the chain from the strongly paired to the weakly paired configuration via the changes of a as a function of the separation between the macroions for two different values of the ionic strength of the univalent salt solution: viz., 1 and 6 mm. Clearly, the overall effect of the salt is to quench the magnitude of the bridging interaction. We note about one order of magnitude difference in the strength of the bridging attraction at both salt activities. The increase in salt activity also quenches the difference between the weakly paired and strongly paired configurations. If we compare the radius of gyration of the chain lower graphs in Figs. and 3, wesee that at higher salt the separation between the macroions has a smaller effect on the size of the chain. The ensuing chain-mediated interaction free energy follows closely the equilibrium configuration of the chain between the macroions. For a strongly paired chain the interaction free energy shows a pronounced attractive contribution stemming from the coupling MN term in Eq. 4. At the point where the chain snaps from the strongly paired to the weakly paired configuration there is also a corresponding jump in the free energy due to much-lesspronounced chain-mediated interactions. It is interesting to analyze the asymptotic behavior of the interaction free energy, Eq. 6, in the weakly paired branch of the solution. Expanding f 1 (y,t) for large values of y, we see that all the chain-dependent parts of the free energy finally just renormalize the direct Debye Hückel interactions between the macroions. Thus asymptotically instead of a Debye Hückel interaction of strength proportional to M, we simply end up with its strength being proportional to (MN). The chain FIG.. F and a in the strong-coupling limit. The upper graph shows the dependence of F for M1 for N1 losenge, 3square, and 1 circle at 1 mm on the separation between the macroions r 1 r. The lower graph shows the dependence of the size of the chain a on the separation between the macroions for the same values of parameters. The length of the chain N obviously determines the separation between macroions where the snapping of the chain between the strongly paired and weakly paired states occurs. In the strongly paired configuration we have a well-developed regime of attractive bridging interactions, leading to a an effective screened Coulomb repulsion in the weakly paired regime. In all cases 1. The bold line represents the pure Debye Hückel interactions between the macroions. snapped back to the grafting macroion and simply renormalized its charge. There is one interesting remark that we can make here. Gurovitch and Sans 8 studied polyelectrolyte adsorption of a single chain to a point charged macroion. Their case thus correspond to a weakly paired branch at infinite separation between the macroions in our terminology, which would correspond to Eq. 5 with r 1 r, leading to 3 BN a 3 Mg 3/, a 7 &Ng,a. Clearly, in the vanishing salt limit a, which is in fact the case treated in Ref. 8, the polyelectrolyte chain can adsorb until its charge or the number of monomers becomes equal to N 3/ M; there is thus maximal overcharging in the amount of 3/.83, which is indeed very close to the value derived by them by a completely different method viz., 15/6.5. Though the approach of these authors has been criticized, 8 our results are more then consistent with theirs. More could be said on polyelectrolyte ad- Downloaded 15 Jun 3 to Redistribution subject to AIP license or copyright, see
8 J. Chem. Phys., Vol. 118, No. 4, June 3 Two-body polyelectrolyte mediated bridging interactions 1193 In this case the effect of electrostatic self-interaction of the chain, the term proportional to N in Eq. 18, determines the overall configuration of the chain. If the effect of the external fields would be indeed negligible, we have shown in a previous publication 1 that the electrostatic interactions would stiffen up the chain and give it a rodlike appearance quantified by the scaling an. We expect that even with external fields originating at the macroions the chain will essentially assume this type of extended configurations in this limit, modified by the perturbative effect of both macroions. Simulations of single-chain adsorption 3 are completely consistent with this picture since for large N protruding rodlike tails are observed that correspond to electrostatically stiffened portions of the chain. Calculations of Nguyen and Shklovskii 9 also lead to the same qualitative picture of chain adsorption in this limit. Again, here we are not interested in adsorption per se, but rather in the bridging effects in interaction between the macroions, so we will skip the detailed comparison of our work with polyelectrolyte adsorption studies. With the external fields present the stiffening of the chain depends on the details i.e., the strength of the term proportional to M in the variational equation 18 and is difficult to quantify in scaling terms, unless the effect of the macroions is thoroughly negligible, which is not the situation we are trying to investigate. Since here the effect of the macroions is small, the polyelectrolyte chains can never be strongly paired by both macroions. We thus remain solely with the weakly paired configuration of the chains due to the grafting to the macroions. The solution of the variational equation 18 thus only has one branch in this case and is given by 3 BN a Ng r 1r 3 a,a Ng,a FIG. 3. F and a in the strong-coupling limit. The upper graph shows the dependence of F for M1 for N1, 3, 1 at 6 mm on the separation between the macroions r 1 r. The lower graph shows the dependence of the size of the chain a on the separation between the macroions for the same values of parameters. The bold line represents the pure Debye Hückel interactions between the macroions. sorption and overcharging, 3 but we will focus here strictly on the interaction: i.e., bridging aspects of the problem. VI. WEAK-COUPLING LIMIT M g 3/, &g a & a r 1 r a,. & 8 Clearly, this equation is obtained by the substitution i from Eq. 18. This transformation should be taken into account also in Eq. 6, leading to a 1 3 L N, 9 ) where now L(x)1/xcot x. The interaction with the macroion, the M term in Eq. 8, can modify the value of the size of the chain, but it has no effect anymore on the stability of the solution. The numerical solutions to Eq. 8 are presented in Figs. 4 and 5. Clearly, the size of the polyelectrolyte chain in this case shows no discontinuities, though it is still, to a lesser extent than before, effected by the positions of the two macroions. The weak-coupling term thus seems appropriate for the behavior of the chain in this region of the parameter space. The free energy is now given by an equation similar to Eq. 6, but with the change i well taken into account in F (r 1 r ). It leads to the following result: F 6 log sin N N BN a 5/ M r, a 3 Na f 1, a f & 1 & a r 1 &4Nf 1 r 1r a,a Nf 1,a, 3 again because the solution of the variational equations here remains on a single branch all the time, showing no jump from one stable branch to another one snapping of the chain. The free energy shows no discontinuities either, though it still depends on the separation between macroions. This state of affairs introduces new features in the polyelectrolyte-mediated interactions. First of all, there is a Downloaded 15 Jun 3 to Redistribution subject to AIP license or copyright, see
9 1194 J. Chem. Phys., Vol. 118, No. 4, June 3 Rudi Podgornik FIG. 4. F and a in the weak-coupling limit for M4 and N5,, 1 at 1 mm. There exists only a weakly paired state in this case and the effect of the external fields of the macroions is much less pronounced then in the strong coupling limit. The length of the chain N determines primarily the steric repulsion effect due to the interpenetration of stiffened grafted chains, the coronas of both macroions, on close approach. The inset shows the residual very-weak-coupling interaction at large separations between macroions with N1. The size of the chain tends to grow slightly on approach of the macroions because of the interpenetration of the coronas of both macroions. The bold line represents the pure Debye Hückel interactions between the macroions. clearly discernible see Fig. 4 repulsion at smaller separations. It is due to the interpenetrating coronas, i.e., extended configurations of the grafted chains, on approach of the macroions. If there would be many chains grafted to both macroions, this incipient repulsion would develop into a fullblown brush repulsion regime. Since we have only one chain per macroion, the effect of coronal interpenetration is rather weak, but nevertheless clearly discernible. Its range depends on the size of the chain N as well as the amount of salt which regulates the overall extension of the chain. Figure 5 clearly shows that salt quenches the coronal repulsion. It is only at larger separations see the inset of Fig. 4 that residual, indeed very weak coupling, is finally discerned. The electrostatically extended chains can still make weak bridges to the other macroions, but since this can happen only at sufficiently large separations, the ensuing bridging is much attenuated. On adding the salt this effect is displaced towards smaller separations because the extent of the chain is diminished by the salt as well. For larger ionic strengths we are thus left with weak coupling at smaller separations see Fig. 5, where it is nevertheless stronger than in small salt compare again inset to Fig. 4. Bridging, no matter what its FIG. 5. F and a in the weak-coupling limit for M4 and N5,, 1 at 6 mm. Clearly, again, the overall effect of the salt is to quench the effects of the macroion fields. There is weak residual bridging at very small separation now: note the scale of the interactions energy, which goes smoothly into a weak corona repulsion at larger separations. The details of both the residual bridging as well as corona repulsions depend on the values of M and N. The bold line represents the pure Debye Hückel interactions between the macroions. source, is of course strongly dependent on the separations between the macroions and is in general stronger for smaller separations. Clearly, the weak-coupling interaction in the case of large salt resembles much more sticky macroions than relatively long-range bridging interaction. In the weak-coupling limit there is thus an additional feature stemming from the polyelectrolyte mediated interactions which is due to coronal interpenetration and marks the incipient brush repulsion that would be developed fully if more chains were grafted to each macroions. The weak bridging attraction in this case is overall small and is in constant competition with coronal interpenetration interactions. VII. DISCUSSION The polyelectrolyte bridging interaction analyzed here is obviously very rich in its features and depends crucially on the region of the parameter space under investigation. We showed that attractive bridging interactions effectively come in two varieties: the strong coupling, where interactions between the chains and the macroions are dominant, and the weak coupling, where self-interactions of the chains are dominant. Bridging interaction can be obtained in both cases but is a couple of orders of magnitude larger in the strongcoupling limit. It the weak-coupling limit the attractive Downloaded 15 Jun 3 to Redistribution subject to AIP license or copyright, see
10 J. Chem. Phys., Vol. 118, No. 4, June 3 Two-body polyelectrolyte mediated bridging interactions 1195 bridging interactions are overwhelmed by the incipient electrostatic repulsions between the overlapping polyelectrolyte coronas as well. In both cases, however, the effective polyelectrolyte-mediated interaction potential is strongly nonmonotonic and shows pronounced variation with respect to the length of the polyelectrolyte chains and the screening length of the underlying electrostatic interactions. The variational formulation of the bridging interaction problem, which lies at the basis of our approach, has several advantages as well as drawbacks. The main feature of the formalism is that it allows for the transition between the strong pairing and weak pairing states of the polyelectrolyte chains which is clearly a finite-size effect and would thus be missed on the ground-state dominance level. The latter has been used successfully for the polyelectrolyte-mediated interactions between macroscopic surfaces with intervening long polyelectrolyte chains. 13,16 Another feature of the variational formulation is that it can describe the snapping of the chain at large enough macroion separations which is the most important feature of a finite chain size effect. We need to reiterate again that this phenomenon is absent in the ground-state dominance ansatz. The snapping of the chain from the configuration where it is partitioned between both macroions to one where it is adsorbed to a single one is of course due to an interplay and balance between chain adsorption energy, chain self-interaction energy, and configurational entropy of the chain. The balance depends on the size of the chain and the separation between the macroions and leads to an abrupt transition between the two configurations that has a well-discerned imprint also on the polyelectrolyte-mediated interaction between the macroions. In general, one sees bridging only for chain cofigurations where it is partitioned by the two macroions symmetricaly, i.e., in what we dubbed the strong-pairing configuration. The attractive bridging interaction is typicaly about 1 times stronger in the strong-coupling limit if compared to its weak-coupling counterpart whence the designation of the two limiting cases. This is intuitively quite easy to grasp, since one can expect strong coupling to emerge only when the interactions between the chain and the macroions dominate the statistical properties of the system. If however the dominant interactions in the system are self-interactions of the chains, the polyelectrolytes clearly mediate only insignificantly the interactions between the macroions. In this case a much more important feature of the interaction is the interpenetration of the polyelectrolyte coronas, which can in some cases lead to pronounced repulsions between dressed macroions. These repusions would clearly stabilize the macroion interactions. The main drawback of the present analysis of the bridging interaction problem, apart from it being a purely pairwise additive formulation, is the linearized Debye Hückel form of electrostatics. All nonlinear effects are thus a priori excluded. On this level the main effect of the salt is to attenuate the bridging interaction as well as the repulsive interaction between the polyelectrolyte coronas. In this respect the variational approach is inferior to the ground-state dominance ansatz. One possible way out would be to formulate also the electrostatic part of the problem on a variational level where all the Debye Hückel parameters would be determined self-consistently. We leave this exercise for future work. Another important omission of our method is the size of the macroions that does not feature explicitly in our formulation. The finite size of the chain in weakly paired or strongly paired configuration clearly showed by our numerical results see the lower graphs in Figs. and 3 is thus not due to the finite size of the adsorbing macroions as in more realistic simulations, 15,3 but is an entropy energy competition effect: high adsorption energy versus low configurational entropy in the weakly paired state, leading to the finite size of the weakly paired state even with a point adsorbing macroion. The omission of the finite size of the macroion tends to overestimate the polyelectrolyte-mediated interactions and underestimate the size of the chain in the weakly paired as well as strongly paired configurations. This type of finite-size effects can be straightforwardly incorporated into the statistics of free, noninteracting chains, 3 but would be unfortunately difficult to incorporate into the Feynman Kleinert variational method and were thus ignored in our formulation. Alternative approaches would thus have to be considered. 31 At this stage it does not seem reasonable to compare the second virial coefficient derived from our calculation of the effective pair interactions with the experiment on NCPs. 1 There would be just too many adjustments that one would have to put in by hand, but that would have a crucial effect on the ensuing numerical results: the effective charge of the macroion due to nonlinear Poisson Boltzmann effect, the effective charge of the chains which depends strongly on the local ionic equilibrium of the dissociable amino acid groups, the effective length of the chains that are free enough to behave as flexible polyelectrolytes, etc. Nevertheless, if one chooses to ignore all these additional complications, the virial coefficient in the strong-coupling limit comes out always monotonically decreasing dependent on the screening length: i.e., ionic strength. No nonmonotonic effects, of the type that feature so prominently in experimental results, 1 are ever seen for any reasonable values of parameters i.e., the charges on the macroions, the length of the chain, the charges on the chain. Basing our conclusion on the analysis presented above, we are inclined to believe that the polyelectrolyte bridging itself never leads to a nonmonotonic second virial coefficient. One nevertheless has to keep in mind that our model calculation is based on many constraints that are not entirely realistic. Our work represents an alternative formulation of the polyelectrolyte bridging interaction between two small macroions on the two-particle level. The finite-size effects of the chain length make obviously a strong imprint on the bridging interaction. These effects have not been studied previously analytically and are missed by the more popular ground-state dominance mean-field approach. In this respect we believe our work adds an important feature to our understanding of the phenomenon of polymer-mediated interactions. Downloaded 15 Jun 3 to Redistribution subject to AIP license or copyright, see
Molecular attractions:
 Molecular attractions: a.) van der Waals interactions b.) electrostatic correlation interactions c.) polyelectrolyte bridging interactions Rudi Podgornik Laboratory of Physical and Structural Biology National
Molecular attractions: a.) van der Waals interactions b.) electrostatic correlation interactions c.) polyelectrolyte bridging interactions Rudi Podgornik Laboratory of Physical and Structural Biology National
Long-range many-body polyelectrolyte bridging interactions
 THE JOURNAL OF CHEMICAL PHYSICS 122, 204902 2005 Long-range many-body polyelectrolyte bridging interactions Rudi Podgornik a Department of Physics, University of Ljubljana, Jadranska 19, 1000 Ljubljana,
THE JOURNAL OF CHEMICAL PHYSICS 122, 204902 2005 Long-range many-body polyelectrolyte bridging interactions Rudi Podgornik a Department of Physics, University of Ljubljana, Jadranska 19, 1000 Ljubljana,
2 Structure. 2.1 Coulomb interactions
 2 Structure 2.1 Coulomb interactions While the information needed for reproduction of living systems is chiefly maintained in the sequence of macromolecules, any practical use of this information must
2 Structure 2.1 Coulomb interactions While the information needed for reproduction of living systems is chiefly maintained in the sequence of macromolecules, any practical use of this information must
Effect of Polyelectrolyte Adsorption on Intercolloidal Forces
 5042 J. Phys. Chem. B 1999, 103, 5042-5057 Effect of Polyelectrolyte Adsorption on Intercolloidal Forces Itamar Borukhov, David Andelman,*, and Henri Orland School of Physics and Astronomy, Raymond and
5042 J. Phys. Chem. B 1999, 103, 5042-5057 Effect of Polyelectrolyte Adsorption on Intercolloidal Forces Itamar Borukhov, David Andelman,*, and Henri Orland School of Physics and Astronomy, Raymond and
Proteins polymer molecules, folded in complex structures. Konstantin Popov Department of Biochemistry and Biophysics
 Proteins polymer molecules, folded in complex structures Konstantin Popov Department of Biochemistry and Biophysics Outline General aspects of polymer theory Size and persistent length of ideal linear
Proteins polymer molecules, folded in complex structures Konstantin Popov Department of Biochemistry and Biophysics Outline General aspects of polymer theory Size and persistent length of ideal linear
EXAM I COURSE TFY4310 MOLECULAR BIOPHYSICS December Suggested resolution
 page 1 of 7 EXAM I COURSE TFY4310 MOLECULAR BIOPHYSICS December 2013 Suggested resolution Exercise 1. [total: 25 p] a) [t: 5 p] Describe the bonding [1.5 p] and the molecular orbitals [1.5 p] of the ethylene
page 1 of 7 EXAM I COURSE TFY4310 MOLECULAR BIOPHYSICS December 2013 Suggested resolution Exercise 1. [total: 25 p] a) [t: 5 p] Describe the bonding [1.5 p] and the molecular orbitals [1.5 p] of the ethylene
Mechanics, Heat, Oscillations and Waves Prof. V. Balakrishnan Department of Physics Indian Institute of Technology, Madras
 Mechanics, Heat, Oscillations and Waves Prof. V. Balakrishnan Department of Physics Indian Institute of Technology, Madras Lecture - 21 Central Potential and Central Force Ready now to take up the idea
Mechanics, Heat, Oscillations and Waves Prof. V. Balakrishnan Department of Physics Indian Institute of Technology, Madras Lecture - 21 Central Potential and Central Force Ready now to take up the idea
3 Biopolymers Uncorrelated chains - Freely jointed chain model
 3.4 Entropy When we talk about biopolymers, it is important to realize that the free energy of a biopolymer in thermal equilibrium is not constant. Unlike solids, biopolymers are characterized through
3.4 Entropy When we talk about biopolymers, it is important to realize that the free energy of a biopolymer in thermal equilibrium is not constant. Unlike solids, biopolymers are characterized through
Computer simulation methods (2) Dr. Vania Calandrini
 Computer simulation methods (2) Dr. Vania Calandrini in the previous lecture: time average versus ensemble average MC versus MD simulations equipartition theorem (=> computing T) virial theorem (=> computing
Computer simulation methods (2) Dr. Vania Calandrini in the previous lecture: time average versus ensemble average MC versus MD simulations equipartition theorem (=> computing T) virial theorem (=> computing
Interfacial forces and friction on the nanometer scale: A tutorial
 Interfacial forces and friction on the nanometer scale: A tutorial M. Ruths Department of Chemistry University of Massachusetts Lowell Presented at the Nanotribology Tutorial/Panel Session, STLE/ASME International
Interfacial forces and friction on the nanometer scale: A tutorial M. Ruths Department of Chemistry University of Massachusetts Lowell Presented at the Nanotribology Tutorial/Panel Session, STLE/ASME International
EFFECTS OF ADDED ELECTROLYTES ON THE STRUCTURE OF CHARGED POLYMERIC MICELLES
 Soft Materials, 3(2&3): 89 120, (2006) Copyright # Taylor & Francis Group, LLC ISSN 1539-445X print/1539-4468 online DOI: 10.1080/15394450600801228 EFFECTS OF DDED ELECTROLYTES ON THE STRUCTURE OF CHRGED
Soft Materials, 3(2&3): 89 120, (2006) Copyright # Taylor & Francis Group, LLC ISSN 1539-445X print/1539-4468 online DOI: 10.1080/15394450600801228 EFFECTS OF DDED ELECTROLYTES ON THE STRUCTURE OF CHRGED
Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia
 University of Groningen Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia IMPORTANT NOTE: You are advised to consult the publisher's version
University of Groningen Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia IMPORTANT NOTE: You are advised to consult the publisher's version
Aqueous solutions. Solubility of different compounds in water
 Aqueous solutions Solubility of different compounds in water The dissolution of molecules into water (in any solvent actually) causes a volume change of the solution; the size of this volume change is
Aqueous solutions Solubility of different compounds in water The dissolution of molecules into water (in any solvent actually) causes a volume change of the solution; the size of this volume change is
Chemistry C : Polymers Section. Dr. Edie Sevick, Research School of Chemistry, ANU. 4.0 Polymers at interfaces
 Chemistry C302-2006: Polymers Section Dr. Edie Sevick, Research School of Chemistry, ANU 4.0 Polymers at interfaces In this section, we begin to investigate the conformation at interfaces, including multiplechains
Chemistry C302-2006: Polymers Section Dr. Edie Sevick, Research School of Chemistry, ANU 4.0 Polymers at interfaces In this section, we begin to investigate the conformation at interfaces, including multiplechains
Supplementary Information for: Controlling Cellular Uptake of Nanoparticles with ph-sensitive Polymers
 Supplementary Information for: Controlling Cellular Uptake of Nanoparticles with ph-sensitive Polymers Hong-ming Ding 1 & Yu-qiang Ma 1,2, 1 National Laboratory of Solid State Microstructures and Department
Supplementary Information for: Controlling Cellular Uptake of Nanoparticles with ph-sensitive Polymers Hong-ming Ding 1 & Yu-qiang Ma 1,2, 1 National Laboratory of Solid State Microstructures and Department
V = 2ze 2 n. . a. i=1
 IITS: Statistical Physics in Biology Assignment # 3 KU Leuven 5/29/2013 Coulomb Interactions & Polymers 1. Flory Theory: The Coulomb energy of a ball of charge Q and dimension R in d spacial dimensions
IITS: Statistical Physics in Biology Assignment # 3 KU Leuven 5/29/2013 Coulomb Interactions & Polymers 1. Flory Theory: The Coulomb energy of a ball of charge Q and dimension R in d spacial dimensions
8.592J HST.452J: Statistical Physics in Biology
 Assignment # 4 8.592J HST.452J: Statistical Physics in Biology Coulomb Interactions 1. Flory Theory: The Coulomb energy of a ball of charge Q and dimension R in d spacial dimensions scales as Q 2 E c.
Assignment # 4 8.592J HST.452J: Statistical Physics in Biology Coulomb Interactions 1. Flory Theory: The Coulomb energy of a ball of charge Q and dimension R in d spacial dimensions scales as Q 2 E c.
Final Morphology of Complex Materials
 120314 Final Morphology of Complex Materials 1) Proteins are the prototypical model for hierarchy. a) Give the generic chemical structure for an amino acid and a protein molecule (a tripeptide). b) Label
120314 Final Morphology of Complex Materials 1) Proteins are the prototypical model for hierarchy. a) Give the generic chemical structure for an amino acid and a protein molecule (a tripeptide). b) Label
Theoretische Physik 2: Elektrodynamik (Prof. A-S. Smith) Home assignment 11
 WiSe 22..23 Prof. Dr. A-S. Smith Dipl.-Phys. Matthias Saba am Lehrstuhl für Theoretische Physik I Department für Physik Friedrich-Alexander-Universität Erlangen-Nürnberg Problem. Theoretische Physik 2:
WiSe 22..23 Prof. Dr. A-S. Smith Dipl.-Phys. Matthias Saba am Lehrstuhl für Theoretische Physik I Department für Physik Friedrich-Alexander-Universität Erlangen-Nürnberg Problem. Theoretische Physik 2:
Hydrogels in charged solvents
 Hydrogels in charged solvents Peter Košovan, Christian Holm, Tobias Richter 27. May 2013 Institute for Computational Physics Pfaffenwaldring 27 D-70569 Stuttgart Germany Tobias Richter (ICP, Stuttgart)
Hydrogels in charged solvents Peter Košovan, Christian Holm, Tobias Richter 27. May 2013 Institute for Computational Physics Pfaffenwaldring 27 D-70569 Stuttgart Germany Tobias Richter (ICP, Stuttgart)
Colloidal Suspension Rheology Chapter 1 Study Questions
 Colloidal Suspension Rheology Chapter 1 Study Questions 1. What forces act on a single colloidal particle suspended in a flowing fluid? Discuss the dependence of these forces on particle radius. 2. What
Colloidal Suspension Rheology Chapter 1 Study Questions 1. What forces act on a single colloidal particle suspended in a flowing fluid? Discuss the dependence of these forces on particle radius. 2. What
Chiral selection in wrapping, crossover, and braiding of DNA mediated by asymmetric bend-writhe elasticity
 http://www.aimspress.com/ AIMS Biophysics, 2(4): 666-694. DOI: 10.3934/biophy.2015.4.666 Received date 28 August 2015, Accepted date 29 October 2015, Published date 06 November 2015 Research article Chiral
http://www.aimspress.com/ AIMS Biophysics, 2(4): 666-694. DOI: 10.3934/biophy.2015.4.666 Received date 28 August 2015, Accepted date 29 October 2015, Published date 06 November 2015 Research article Chiral
Confinement of polymer chains and gels
 Confinement of polymer chains and gels Nefeli Georgoulia - Student number: 70732831 1 Introduction Confinement of polymer chains is significant in industrial as well as biological applications. For this
Confinement of polymer chains and gels Nefeli Georgoulia - Student number: 70732831 1 Introduction Confinement of polymer chains is significant in industrial as well as biological applications. For this
Colloids as nucleons
 Colloids as nucleons Willem Kegel & Jan Groenewold Van t Hoff Laboratory Utrecht University The Netherlands Finite-size equilibrium structures macroscopic phase separation Equilibrium clusters & periodic
Colloids as nucleons Willem Kegel & Jan Groenewold Van t Hoff Laboratory Utrecht University The Netherlands Finite-size equilibrium structures macroscopic phase separation Equilibrium clusters & periodic
Swelling and Collapse of Single Polymer Molecules and Gels.
 Swelling and Collapse of Single Polymer Molecules and Gels. Coil-Globule Transition in Single Polymer Molecules. the coil-globule transition If polymer chains are not ideal, interactions of non-neighboring
Swelling and Collapse of Single Polymer Molecules and Gels. Coil-Globule Transition in Single Polymer Molecules. the coil-globule transition If polymer chains are not ideal, interactions of non-neighboring
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian. which is given in spherical coordinates by (2)
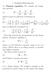 1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
The effect of surface dipoles and of the field generated by a polarization gradient on the repulsive force
 Journal of Colloid and Interface Science 263 (2003) 156 161 www.elsevier.com/locate/jcis The effect of surface dipoles and of the field generated by a polarization gradient on the repulsive force Haohao
Journal of Colloid and Interface Science 263 (2003) 156 161 www.elsevier.com/locate/jcis The effect of surface dipoles and of the field generated by a polarization gradient on the repulsive force Haohao
8.333: Statistical Mechanics I Problem Set # 5 Due: 11/22/13 Interacting particles & Quantum ensembles
 8.333: Statistical Mechanics I Problem Set # 5 Due: 11/22/13 Interacting particles & Quantum ensembles 1. Surfactant condensation: N surfactant molecules are added to the surface of water over an area
8.333: Statistical Mechanics I Problem Set # 5 Due: 11/22/13 Interacting particles & Quantum ensembles 1. Surfactant condensation: N surfactant molecules are added to the surface of water over an area
S(l) bl + c log l + d, with c 1.8k B. (2.71)
 2.4 DNA structure DNA molecules come in a wide range of length scales, from roughly 50,000 monomers in a λ-phage, 6 0 9 for human, to 9 0 0 nucleotides in the lily. The latter would be around thirty meters
2.4 DNA structure DNA molecules come in a wide range of length scales, from roughly 50,000 monomers in a λ-phage, 6 0 9 for human, to 9 0 0 nucleotides in the lily. The latter would be around thirty meters
Surface Forces & Liquid Films (Answers to Exercise Problems)
 //5 Surface Forces & Liquid Films (nswers to Exercise Problems) Wuge H. Briscoe wuge.briscoe@bris.ac.uk URL: wugebrisco7.wix.com/briscoegroup Exercise : van der Waals forces & liquid films When octane
//5 Surface Forces & Liquid Films (nswers to Exercise Problems) Wuge H. Briscoe wuge.briscoe@bris.ac.uk URL: wugebrisco7.wix.com/briscoegroup Exercise : van der Waals forces & liquid films When octane
Math Methods for Polymer Physics Lecture 1: Series Representations of Functions
 Math Methods for Polymer Physics ecture 1: Series Representations of Functions Series analysis is an essential tool in polymer physics and physical sciences, in general. Though other broadly speaking,
Math Methods for Polymer Physics ecture 1: Series Representations of Functions Series analysis is an essential tool in polymer physics and physical sciences, in general. Though other broadly speaking,
PHASE TRANSITIONS IN SOFT MATTER SYSTEMS
 OUTLINE: Topic D. PHASE TRANSITIONS IN SOFT MATTER SYSTEMS Definition of a phase Classification of phase transitions Thermodynamics of mixing (gases, polymers, etc.) Mean-field approaches in the spirit
OUTLINE: Topic D. PHASE TRANSITIONS IN SOFT MATTER SYSTEMS Definition of a phase Classification of phase transitions Thermodynamics of mixing (gases, polymers, etc.) Mean-field approaches in the spirit
Kinetics of surfactant adsorption: the free energy approach
 Colloids and Surfaces A: Physicochemical and Engineering Aspects 183 185 (2001) 259 276 www.elsevier.nl/locate/colsurfa Kinetics of surfactant adsorption: the free energy approach Haim Diamant 1, Gil Ariel,
Colloids and Surfaces A: Physicochemical and Engineering Aspects 183 185 (2001) 259 276 www.elsevier.nl/locate/colsurfa Kinetics of surfactant adsorption: the free energy approach Haim Diamant 1, Gil Ariel,
Equations of State. Equations of State (EoS)
 Equations of State (EoS) Equations of State From molecular considerations, identify which intermolecular interactions are significant (including estimating relative strengths of dipole moments, polarizability,
Equations of State (EoS) Equations of State From molecular considerations, identify which intermolecular interactions are significant (including estimating relative strengths of dipole moments, polarizability,
Appendix C - Persistence length 183. Consider an ideal chain with N segments each of length a, such that the contour length L c is
 Appendix C - Persistence length 183 APPENDIX C - PERSISTENCE LENGTH Consider an ideal chain with N segments each of length a, such that the contour length L c is L c = Na. (C.1) If the orientation of each
Appendix C - Persistence length 183 APPENDIX C - PERSISTENCE LENGTH Consider an ideal chain with N segments each of length a, such that the contour length L c is L c = Na. (C.1) If the orientation of each
of Nebraska - Lincoln
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Xiao Cheng Zeng Publications Published Research - Department of Chemistry 10-1-2006 Homogeneous nucleation at high supersaturation
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Xiao Cheng Zeng Publications Published Research - Department of Chemistry 10-1-2006 Homogeneous nucleation at high supersaturation
Khinchin s approach to statistical mechanics
 Chapter 7 Khinchin s approach to statistical mechanics 7.1 Introduction In his Mathematical Foundations of Statistical Mechanics Khinchin presents an ergodic theorem which is valid also for systems that
Chapter 7 Khinchin s approach to statistical mechanics 7.1 Introduction In his Mathematical Foundations of Statistical Mechanics Khinchin presents an ergodic theorem which is valid also for systems that
Multimedia : Boundary Lubrication Podcast, Briscoe, et al. Nature , ( )
 3.05 Nanomechanics of Materials and Biomaterials Thursday 04/05/07 Prof. C. Ortiz, MITDMSE I LECTURE 14: TE ELECTRICAL DOUBLE LAYER (EDL) Outline : REVIEW LECTURE #11 : INTRODUCTION TO TE ELECTRICAL DOUBLE
3.05 Nanomechanics of Materials and Biomaterials Thursday 04/05/07 Prof. C. Ortiz, MITDMSE I LECTURE 14: TE ELECTRICAL DOUBLE LAYER (EDL) Outline : REVIEW LECTURE #11 : INTRODUCTION TO TE ELECTRICAL DOUBLE
CE 530 Molecular Simulation
 1 CE 530 Molecular Simulation Lecture 14 Molecular Models David A. Kofke Department of Chemical Engineering SUNY Buffalo kofke@eng.buffalo.edu 2 Review Monte Carlo ensemble averaging, no dynamics easy
1 CE 530 Molecular Simulation Lecture 14 Molecular Models David A. Kofke Department of Chemical Engineering SUNY Buffalo kofke@eng.buffalo.edu 2 Review Monte Carlo ensemble averaging, no dynamics easy
Non-bonded interactions
 speeding up the number-crunching Marcus Elstner and Tomáš Kubař May 8, 2015 why care? key to understand biomolecular structure and function binding of a ligand efficiency of a reaction color of a chromophore
speeding up the number-crunching Marcus Elstner and Tomáš Kubař May 8, 2015 why care? key to understand biomolecular structure and function binding of a ligand efficiency of a reaction color of a chromophore
Effective charge saturation in colloidal suspensions
 JOURNAL OF CHEMICAL PHYSICS VOLUME 117, NUMBER 17 1 NOVEMBER 2002 Effective charge saturation in colloidal suspensions Lydéric Bocquet Laboratoire de Physique de l E.N.S. de Lyon, UMR CNRS 5672, 46 Allée
JOURNAL OF CHEMICAL PHYSICS VOLUME 117, NUMBER 17 1 NOVEMBER 2002 Effective charge saturation in colloidal suspensions Lydéric Bocquet Laboratoire de Physique de l E.N.S. de Lyon, UMR CNRS 5672, 46 Allée
2.4 DNA structure. S(l) bl + c log l + d, with c 1.8k B. (2.72)
 2.4 DNA structure DNA molecules come in a wide range of length scales, from roughly 50,000 monomers in a λ-phage, 6 0 9 for human, to 9 0 0 nucleotides in the lily. The latter would be around thirty meters
2.4 DNA structure DNA molecules come in a wide range of length scales, from roughly 50,000 monomers in a λ-phage, 6 0 9 for human, to 9 0 0 nucleotides in the lily. The latter would be around thirty meters
 Title Theory of solutions in the energy r of the molecular flexibility Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2003), 9702 Issue Date 2003-11-08 URL http://hdl.handle.net/2433/50354
Title Theory of solutions in the energy r of the molecular flexibility Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2003), 9702 Issue Date 2003-11-08 URL http://hdl.handle.net/2433/50354
Collective behavior, from particles to fields
 978-0-51-87341-3 - Statistical Physics of Fields 1 Collective behavior, from particles to fields 1.1 Introduction One of the most successful aspects of physics in the twentieth century was revealing the
978-0-51-87341-3 - Statistical Physics of Fields 1 Collective behavior, from particles to fields 1.1 Introduction One of the most successful aspects of physics in the twentieth century was revealing the
On the Chemical Free Energy of the Electrical Double Layer
 1114 Langmuir 23, 19, 1114-112 On the Chemical Free Energy of the Electrical Double Layer Marian Manciu and Eli Ruckenstein* Department of Chemical Engineering, State University of New York at Buffalo,
1114 Langmuir 23, 19, 1114-112 On the Chemical Free Energy of the Electrical Double Layer Marian Manciu and Eli Ruckenstein* Department of Chemical Engineering, State University of New York at Buffalo,
The Potential Energy Surface (PES) And the Basic Force Field Chem 4021/8021 Video II.iii
 The Potential Energy Surface (PES) And the Basic Force Field Chem 4021/8021 Video II.iii Fundamental Points About Which to Be Thinking It s clear the PES is useful, so how can I construct it for an arbitrary
The Potential Energy Surface (PES) And the Basic Force Field Chem 4021/8021 Video II.iii Fundamental Points About Which to Be Thinking It s clear the PES is useful, so how can I construct it for an arbitrary
Electrochemical Properties of Materials for Electrical Energy Storage Applications
 Electrochemical Properties of Materials for Electrical Energy Storage Applications Lecture Note 3 October 11, 2013 Kwang Kim Yonsei Univ., KOREA kbkim@yonsei.ac.kr 39 Y 88.91 8 O 16.00 7 N 14.01 34 Se
Electrochemical Properties of Materials for Electrical Energy Storage Applications Lecture Note 3 October 11, 2013 Kwang Kim Yonsei Univ., KOREA kbkim@yonsei.ac.kr 39 Y 88.91 8 O 16.00 7 N 14.01 34 Se
Module 8: "Stability of Colloids" Lecture 38: "" The Lecture Contains: Calculation for CCC (n c )
 The Lecture Contains: Calculation for CCC (n c ) Relation between surface charge and electrostatic potential Extensions to DLVO theory file:///e /courses/colloid_interface_science/lecture38/38_1.htm[6/16/2012
The Lecture Contains: Calculation for CCC (n c ) Relation between surface charge and electrostatic potential Extensions to DLVO theory file:///e /courses/colloid_interface_science/lecture38/38_1.htm[6/16/2012
UNDERSTANDING BOLTZMANN S ANALYSIS VIA. Contents SOLVABLE MODELS
 UNDERSTANDING BOLTZMANN S ANALYSIS VIA Contents SOLVABLE MODELS 1 Kac ring model 2 1.1 Microstates............................ 3 1.2 Macrostates............................ 6 1.3 Boltzmann s entropy.......................
UNDERSTANDING BOLTZMANN S ANALYSIS VIA Contents SOLVABLE MODELS 1 Kac ring model 2 1.1 Microstates............................ 3 1.2 Macrostates............................ 6 1.3 Boltzmann s entropy.......................
Citation PHYSICAL REVIEW E (2002), 65(6) RightCopyright 2002 American Physical So
 TitleStatistical mechanics of two hard d Author(s) Munakata, T; Hu, G Citation PHYSICAL REVIEW E (2002), 65(6) Issue Date 2002-06 URL http://hdl.handle.net/2433/50310 RightCopyright 2002 American Physical
TitleStatistical mechanics of two hard d Author(s) Munakata, T; Hu, G Citation PHYSICAL REVIEW E (2002), 65(6) Issue Date 2002-06 URL http://hdl.handle.net/2433/50310 RightCopyright 2002 American Physical
Electrostatic Interactions in Mixtures of Cationic and Anionic Biomolecules: Bulk Structures and Induced Surface Pattern Formation
 Electrostatic Interactions in Mixtures of Cationic and Anionic Biomolecules: Bulk Structures and Induced Surface Pattern Formation Monica Olvera de la Cruz F. J. Solis, P. Gonzalez- Mozuleos (theory) E.
Electrostatic Interactions in Mixtures of Cationic and Anionic Biomolecules: Bulk Structures and Induced Surface Pattern Formation Monica Olvera de la Cruz F. J. Solis, P. Gonzalez- Mozuleos (theory) E.
Phys 450 Spring 2011 Solution set 6. A bimolecular reaction in which A and B combine to form the product P may be written as:
 Problem Phys 45 Spring Solution set 6 A bimolecular reaction in which A and combine to form the product P may be written as: k d A + A P k d k a where k d is a diffusion-limited, bimolecular rate constant
Problem Phys 45 Spring Solution set 6 A bimolecular reaction in which A and combine to form the product P may be written as: k d A + A P k d k a where k d is a diffusion-limited, bimolecular rate constant
Correlated and decorrelated positional and orientational order in the nucleosomal core particle mesophases
 EUROPHYSICS LETTERS 5 March 2005 Europhys. Lett., 69 (6), pp. 07 023 (2005) DOI: 0.209/epl/i2004-0437-5 Correlated and decorrelated positional and orientational order in the nucleosomal core particle mesophases
EUROPHYSICS LETTERS 5 March 2005 Europhys. Lett., 69 (6), pp. 07 023 (2005) DOI: 0.209/epl/i2004-0437-5 Correlated and decorrelated positional and orientational order in the nucleosomal core particle mesophases
Roland R. Netz Max-Planck Institute for Colloids and Interfaces, Potsdam, Germany
 282 2 Electrochemical Double Layers 2.7 Polyelectrolytes in Solution and at Surfaces Roland R. Netz Max-Planck Institute for Colloids and Interfaces, Potsdam, Germany David Andelman School of Physics and
282 2 Electrochemical Double Layers 2.7 Polyelectrolytes in Solution and at Surfaces Roland R. Netz Max-Planck Institute for Colloids and Interfaces, Potsdam, Germany David Andelman School of Physics and
Chapter 6. Phase transitions. 6.1 Concept of phase
 Chapter 6 hase transitions 6.1 Concept of phase hases are states of matter characterized by distinct macroscopic properties. ypical phases we will discuss in this chapter are liquid, solid and gas. Other
Chapter 6 hase transitions 6.1 Concept of phase hases are states of matter characterized by distinct macroscopic properties. ypical phases we will discuss in this chapter are liquid, solid and gas. Other
Introduction. Chapter The Purpose of Statistical Mechanics
 Chapter 1 Introduction 1.1 The Purpose of Statistical Mechanics Statistical Mechanics is the mechanics developed to treat a collection of a large number of atoms or particles. Such a collection is, for
Chapter 1 Introduction 1.1 The Purpose of Statistical Mechanics Statistical Mechanics is the mechanics developed to treat a collection of a large number of atoms or particles. Such a collection is, for
INTERMOLECULAR AND SURFACE FORCES
 INTERMOLECULAR AND SURFACE FORCES SECOND EDITION JACOB N. ISRAELACHVILI Department of Chemical & Nuclear Engineering and Materials Department University of California, Santa Barbara California, USA ACADEMIC
INTERMOLECULAR AND SURFACE FORCES SECOND EDITION JACOB N. ISRAELACHVILI Department of Chemical & Nuclear Engineering and Materials Department University of California, Santa Barbara California, USA ACADEMIC
Supporting Information for Lysozyme Adsorption in ph-responsive Hydrogel Thin-Films: The non-trivial Role of Acid-Base Equilibrium
 Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 215 Supporting Information for Lysozyme Adsorption in ph-responsive Hydrogel Thin-Films: The non-trivial
Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 215 Supporting Information for Lysozyme Adsorption in ph-responsive Hydrogel Thin-Films: The non-trivial
Proteins in solution: charge-tuning, cluster formation, liquid-liquid phase separation, and crystallization
 HERCULES Specialized Course: Non-atomic resolution scattering in biology and soft matter Grenoble, September 14-19, 2014 Proteins in solution: charge-tuning, cluster formation, liquid-liquid phase separation,
HERCULES Specialized Course: Non-atomic resolution scattering in biology and soft matter Grenoble, September 14-19, 2014 Proteins in solution: charge-tuning, cluster formation, liquid-liquid phase separation,
Polymer Dynamics and Rheology
 Polymer Dynamics and Rheology 1 Polymer Dynamics and Rheology Brownian motion Harmonic Oscillator Damped harmonic oscillator Elastic dumbbell model Boltzmann superposition principle Rubber elasticity and
Polymer Dynamics and Rheology 1 Polymer Dynamics and Rheology Brownian motion Harmonic Oscillator Damped harmonic oscillator Elastic dumbbell model Boltzmann superposition principle Rubber elasticity and
Current Opinion in Colloid & Interface Science
 Current Opinion in Colloid & Interface Science 13 (2008) 376 388 Contents lists available at ScienceDirect Current Opinion in Colloid & Interface Science journal homepage: www.elsevier.com/locate/cocis
Current Opinion in Colloid & Interface Science 13 (2008) 376 388 Contents lists available at ScienceDirect Current Opinion in Colloid & Interface Science journal homepage: www.elsevier.com/locate/cocis
Effect of protein shape on multibody interactions between membrane inclusions
 PHYSICAL REVIEW E VOLUME 61, NUMBER 4 APRIL 000 Effect of protein shape on multibody interactions between membrane inclusions K. S. Kim, 1, * John Neu, and George Oster 3, 1 Department of Physics, Graduate
PHYSICAL REVIEW E VOLUME 61, NUMBER 4 APRIL 000 Effect of protein shape on multibody interactions between membrane inclusions K. S. Kim, 1, * John Neu, and George Oster 3, 1 Department of Physics, Graduate
Computer Simulations of Soft Nanoparticles and Their Interactions with DNA-Like Polyelectrolytes. STOLL, Serge. Abstract
 Book Chapter Computer Simulations of Soft Nanoparticles and Their Interactions with DNA-Like Polyelectrolytes STOLL, Serge Abstract The design of functional molecular architectures and formation of electrostatic
Book Chapter Computer Simulations of Soft Nanoparticles and Their Interactions with DNA-Like Polyelectrolytes STOLL, Serge Abstract The design of functional molecular architectures and formation of electrostatic
Big Idea #5: The laws of thermodynamics describe the essential role of energy and explain and predict the direction of changes in matter.
 KUDs for Unit 6: Chemical Bonding Textbook Reading: Chapters 8 & 9 Big Idea #2: Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ion, or molecules
KUDs for Unit 6: Chemical Bonding Textbook Reading: Chapters 8 & 9 Big Idea #2: Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ion, or molecules
arxiv:cond-mat/ v1 [cond-mat.soft] 9 Aug 1997
![arxiv:cond-mat/ v1 [cond-mat.soft] 9 Aug 1997 arxiv:cond-mat/ v1 [cond-mat.soft] 9 Aug 1997](/thumbs/89/99391124.jpg) Depletion forces between two spheres in a rod solution. K. Yaman, C. Jeppesen, C. M. Marques arxiv:cond-mat/9708069v1 [cond-mat.soft] 9 Aug 1997 Department of Physics, U.C.S.B., CA 93106 9530, U.S.A. Materials
Depletion forces between two spheres in a rod solution. K. Yaman, C. Jeppesen, C. M. Marques arxiv:cond-mat/9708069v1 [cond-mat.soft] 9 Aug 1997 Department of Physics, U.C.S.B., CA 93106 9530, U.S.A. Materials
Lecture 6 - Introduction to Electricity
 Lecture 6 - Introduction to Electricity A Puzzle... We are all familiar with visualizing an integral as the area under a curve. For example, a b f[x] dx equals the sum of the areas of the rectangles of
Lecture 6 - Introduction to Electricity A Puzzle... We are all familiar with visualizing an integral as the area under a curve. For example, a b f[x] dx equals the sum of the areas of the rectangles of
Introduction The gramicidin A (ga) channel forms by head-to-head association of two monomers at their amino termini, one from each bilayer leaflet. Th
 Abstract When conductive, gramicidin monomers are linked by six hydrogen bonds. To understand the details of dissociation and how the channel transits from a state with 6H bonds to ones with 4H bonds or
Abstract When conductive, gramicidin monomers are linked by six hydrogen bonds. To understand the details of dissociation and how the channel transits from a state with 6H bonds to ones with 4H bonds or
An electrokinetic LB based model for ion transport and macromolecular electrophoresis
 An electrokinetic LB based model for ion transport and macromolecular electrophoresis Raffael Pecoroni Supervisor: Michael Kuron July 8, 2016 1 Introduction & Motivation So far an mesoscopic coarse-grained
An electrokinetic LB based model for ion transport and macromolecular electrophoresis Raffael Pecoroni Supervisor: Michael Kuron July 8, 2016 1 Introduction & Motivation So far an mesoscopic coarse-grained
Chapter 18. Remarks on partial differential equations
 Chapter 8. Remarks on partial differential equations If we try to analyze heat flow or vibration in a continuous system such as a building or an airplane, we arrive at a kind of infinite system of ordinary
Chapter 8. Remarks on partial differential equations If we try to analyze heat flow or vibration in a continuous system such as a building or an airplane, we arrive at a kind of infinite system of ordinary
Step 1. Step 2. g l = g v. dg = 0 We have shown that over a plane surface of water. g v g l = ρ v R v T ln e/e sat. this can be rewritten
 The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
Experiment 2 Electric Field Mapping
 Experiment 2 Electric Field Mapping I hear and I forget. I see and I remember. I do and I understand Anonymous OBJECTIVE To visualize some electrostatic potentials and fields. THEORY Our goal is to explore
Experiment 2 Electric Field Mapping I hear and I forget. I see and I remember. I do and I understand Anonymous OBJECTIVE To visualize some electrostatic potentials and fields. THEORY Our goal is to explore
The Wave Function. Chapter The Harmonic Wave Function
 Chapter 3 The Wave Function On the basis of the assumption that the de Broglie relations give the frequency and wavelength of some kind of wave to be associated with a particle, plus the assumption that
Chapter 3 The Wave Function On the basis of the assumption that the de Broglie relations give the frequency and wavelength of some kind of wave to be associated with a particle, plus the assumption that
Nuclear Science Research Group
 UR-NCUR 05-12 THE UNIVERSITY OF ROCHESTER Nuclear Science Research Group ROCHESTER, NEW YORK 14267-0216, USA Compound Nucleus with Surface Entropy; A Unified Phenomenology of Particle Evaporation, Fission,
UR-NCUR 05-12 THE UNIVERSITY OF ROCHESTER Nuclear Science Research Group ROCHESTER, NEW YORK 14267-0216, USA Compound Nucleus with Surface Entropy; A Unified Phenomenology of Particle Evaporation, Fission,
1 Fundamentals. 1.1 Overview. 1.2 Units: Physics 704 Spring 2018
 Physics 704 Spring 2018 1 Fundamentals 1.1 Overview The objective of this course is: to determine and fields in various physical systems and the forces and/or torques resulting from them. The domain of
Physics 704 Spring 2018 1 Fundamentals 1.1 Overview The objective of this course is: to determine and fields in various physical systems and the forces and/or torques resulting from them. The domain of
CHARGED POLYMERS THE STORY SO FAR
 CHARGED POLYMERS THE STORY SO FAR Andrey V Dobrynin Institute of Materials Science &Department of Physics University of Connecticut What are polyelectrolytes? Poly(styrene sulfonate) CH-CH 2 SO Na Poly(methacrylic
CHARGED POLYMERS THE STORY SO FAR Andrey V Dobrynin Institute of Materials Science &Department of Physics University of Connecticut What are polyelectrolytes? Poly(styrene sulfonate) CH-CH 2 SO Na Poly(methacrylic
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 22, March 20, 2006
 Chem 350/450 Physical Chemistry II Quantum Mechanics 3 Credits Spring Semester 006 Christopher J. Cramer Lecture, March 0, 006 Some material in this lecture has been adapted from Cramer, C. J. Essentials
Chem 350/450 Physical Chemistry II Quantum Mechanics 3 Credits Spring Semester 006 Christopher J. Cramer Lecture, March 0, 006 Some material in this lecture has been adapted from Cramer, C. J. Essentials
A mathematical model for a copolymer in an emulsion
 J Math Chem (2010) 48:83 94 DOI 10.1007/s10910-009-9564-y ORIGINAL PAPER A mathematical model for a copolymer in an emulsion F. den Hollander N. Pétrélis Received: 3 June 2007 / Accepted: 22 April 2009
J Math Chem (2010) 48:83 94 DOI 10.1007/s10910-009-9564-y ORIGINAL PAPER A mathematical model for a copolymer in an emulsion F. den Hollander N. Pétrélis Received: 3 June 2007 / Accepted: 22 April 2009
Electrostatic correlations and fluctuations for ion binding to a finite length polyelectrolyte
 THE JOURNAL OF CHEMICAL PHYSICS 122, 044903 2005 Electrostatic correlations and fluctuations for ion binding to a finite length polyelectrolyte Zhi-Jie Tan and Shi-Jie Chen a) Department of Physics and
THE JOURNAL OF CHEMICAL PHYSICS 122, 044903 2005 Electrostatic correlations and fluctuations for ion binding to a finite length polyelectrolyte Zhi-Jie Tan and Shi-Jie Chen a) Department of Physics and
with a proper choice of the potential U(r). Clearly, we should include the potential of the ions, U ion (r):.
 The Hartree Equations So far we have ignored the effects of electron-electron (e-e) interactions by working in the independent electron approximation. In this lecture, we shall discuss how this effect
The Hartree Equations So far we have ignored the effects of electron-electron (e-e) interactions by working in the independent electron approximation. In this lecture, we shall discuss how this effect
Theory of Interfacial Tension of Partially Miscible Liquids
 Theory of Interfacial Tension of Partially Miscible Liquids M.-E. BOUDH-HIR and G.A. MANSOORI * University of Illinois at Chicago (M/C 063) Chicago, Illinois USA 60607-7052 Abstract The aim of this work
Theory of Interfacial Tension of Partially Miscible Liquids M.-E. BOUDH-HIR and G.A. MANSOORI * University of Illinois at Chicago (M/C 063) Chicago, Illinois USA 60607-7052 Abstract The aim of this work
THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION
 THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION AND CALIBRATION Calculation of turn and beta intrinsic propensities. A statistical analysis of a protein structure
THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION AND CALIBRATION Calculation of turn and beta intrinsic propensities. A statistical analysis of a protein structure
Problem Set Number 01, MIT (Winter-Spring 2018)
 Problem Set Number 01, 18.377 MIT (Winter-Spring 2018) Rodolfo R. Rosales (MIT, Math. Dept., room 2-337, Cambridge, MA 02139) February 28, 2018 Due Thursday, March 8, 2018. Turn it in (by 3PM) at the Math.
Problem Set Number 01, 18.377 MIT (Winter-Spring 2018) Rodolfo R. Rosales (MIT, Math. Dept., room 2-337, Cambridge, MA 02139) February 28, 2018 Due Thursday, March 8, 2018. Turn it in (by 3PM) at the Math.
Multimedia : Fibronectin and Titin unfolding simulation movies.
 I LECTURE 21: SINGLE CHAIN ELASTICITY OF BIOMACROMOLECULES: THE GIANT PROTEIN TITIN AND DNA Outline : REVIEW LECTURE #2 : EXTENSIBLE FJC AND WLC... 2 STRUCTURE OF MUSCLE AND TITIN... 3 SINGLE MOLECULE
I LECTURE 21: SINGLE CHAIN ELASTICITY OF BIOMACROMOLECULES: THE GIANT PROTEIN TITIN AND DNA Outline : REVIEW LECTURE #2 : EXTENSIBLE FJC AND WLC... 2 STRUCTURE OF MUSCLE AND TITIN... 3 SINGLE MOLECULE
Chapter 2. Dielectric Theories
 Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
A Summary of Economic Methodology
 A Summary of Economic Methodology I. The Methodology of Theoretical Economics All economic analysis begins with theory, based in part on intuitive insights that naturally spring from certain stylized facts,
A Summary of Economic Methodology I. The Methodology of Theoretical Economics All economic analysis begins with theory, based in part on intuitive insights that naturally spring from certain stylized facts,
Chain Stiffness and Attachment-Dependent Attraction between Polyelectrolyte-Grafted Colloids
 15886 J. Phys. Chem. B 2010, 114, 15886 15896 Chain Stiffness and Attachment-Dependent Attraction between Polyelectrolyte-Grafted Colloids Gaurav Arya* Department of NanoEngineering, 9500 Gilman DriVe,
15886 J. Phys. Chem. B 2010, 114, 15886 15896 Chain Stiffness and Attachment-Dependent Attraction between Polyelectrolyte-Grafted Colloids Gaurav Arya* Department of NanoEngineering, 9500 Gilman DriVe,
An Introduction to Lattice Vibrations
 An Introduction to Lattice Vibrations Andreas Wacker 1 Mathematical Physics, Lund University November 3, 2015 1 Introduction Ideally, the atoms in a crystal are positioned in a regular manner following
An Introduction to Lattice Vibrations Andreas Wacker 1 Mathematical Physics, Lund University November 3, 2015 1 Introduction Ideally, the atoms in a crystal are positioned in a regular manner following
Lectures 11-13: Electrostatics of Salty Solutions
 Lectures 11-13: Electrostatics of Salty Solutions Lecturer: Brigita Urbanc Office: 1-909 (E-mail: brigita@drexel.edu) Course website: www.physics.drexel.edu/~brigita/courses/biophys_011-01/ 1 Water as
Lectures 11-13: Electrostatics of Salty Solutions Lecturer: Brigita Urbanc Office: 1-909 (E-mail: brigita@drexel.edu) Course website: www.physics.drexel.edu/~brigita/courses/biophys_011-01/ 1 Water as
7.5 Partial Fractions and Integration
 650 CHPTER 7. DVNCED INTEGRTION TECHNIQUES 7.5 Partial Fractions and Integration In this section we are interested in techniques for computing integrals of the form P(x) dx, (7.49) Q(x) where P(x) and
650 CHPTER 7. DVNCED INTEGRTION TECHNIQUES 7.5 Partial Fractions and Integration In this section we are interested in techniques for computing integrals of the form P(x) dx, (7.49) Q(x) where P(x) and
2 One-dimensional models in discrete time
 2 One-dimensional models in discrete time So far, we have assumed that demographic events happen continuously over time and can thus be written as rates. For many biological species with overlapping generations
2 One-dimensional models in discrete time So far, we have assumed that demographic events happen continuously over time and can thus be written as rates. For many biological species with overlapping generations
An Introduction to namic Light Scattering by Macromole cules
 An Introduction to namic Light Scattering by Macromole cules Kenneth S. Schmitz Department of Chemistry University of Missouri-Kansas Kansas City, Missouri City ACADEMIC PRESS, INC. Harcourt Brace Jovanovich,
An Introduction to namic Light Scattering by Macromole cules Kenneth S. Schmitz Department of Chemistry University of Missouri-Kansas Kansas City, Missouri City ACADEMIC PRESS, INC. Harcourt Brace Jovanovich,
Statistical Thermodynamics Exercise 11 HS Exercise 11
 Exercise 11 Release: 412215 on-line Return: 1112215 your assistant Discussion: 1512215 your tutorial room Macromolecules (or polymers) are large molecules consisting of smaller subunits (referred to as
Exercise 11 Release: 412215 on-line Return: 1112215 your assistant Discussion: 1512215 your tutorial room Macromolecules (or polymers) are large molecules consisting of smaller subunits (referred to as
Lecture 2 and 3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability
 Lecture 2 and 3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions: Van der Waals Interactions
Lecture 2 and 3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions: Van der Waals Interactions
Polymers in a slabs and slits with attracting walls
 Polymers in a slabs and slits with attracting walls Aleks Richard Martin Enzo Orlandini Thomas Prellberg Andrew Rechnitzer Buks van Rensburg Stu Whittington The Universities of Melbourne, Toronto and Padua
Polymers in a slabs and slits with attracting walls Aleks Richard Martin Enzo Orlandini Thomas Prellberg Andrew Rechnitzer Buks van Rensburg Stu Whittington The Universities of Melbourne, Toronto and Padua
Write your name here:
 MSE 102, Fall 2013 Final Exam Write your name here: Instructions: Answer all questions to the best of your abilities. Be sure to write legibly and state your answers clearly. The point values for each
MSE 102, Fall 2013 Final Exam Write your name here: Instructions: Answer all questions to the best of your abilities. Be sure to write legibly and state your answers clearly. The point values for each
Molecular Modeling -- Lecture 15 Surfaces and electrostatics
 Molecular Modeling -- Lecture 15 Surfaces and electrostatics Molecular surfaces The Hydrophobic Effect Electrostatics Poisson-Boltzmann Equation Electrostatic maps Electrostatic surfaces in MOE 15.1 The
Molecular Modeling -- Lecture 15 Surfaces and electrostatics Molecular surfaces The Hydrophobic Effect Electrostatics Poisson-Boltzmann Equation Electrostatic maps Electrostatic surfaces in MOE 15.1 The
Chapter 2 Direct Current Circuits
 Chapter 2 Direct Current Circuits 2.1 Introduction Nowadays, our lives are increasingly dependent upon the availability of devices that make extensive use of electric circuits. The knowledge of the electrical
Chapter 2 Direct Current Circuits 2.1 Introduction Nowadays, our lives are increasingly dependent upon the availability of devices that make extensive use of electric circuits. The knowledge of the electrical
Amorphous Polymers: Polymer Conformation Laboratory 1: Module 1
 D E P A R T M E N T O F M A T E R I A L S S C I E N C E A N D E N G I N E E R I N G M A S S A C H U S E T T S I N S T I T U T E O F T E C H N O L O G Y 3.014 Materials Laboratory Fall 2008 Amorphous Polymers:
D E P A R T M E N T O F M A T E R I A L S S C I E N C E A N D E N G I N E E R I N G M A S S A C H U S E T T S I N S T I T U T E O F T E C H N O L O G Y 3.014 Materials Laboratory Fall 2008 Amorphous Polymers:
Potts And XY, Together At Last
 Potts And XY, Together At Last Daniel Kolodrubetz Massachusetts Institute of Technology, Center for Theoretical Physics (Dated: May 16, 212) We investigate the behavior of an XY model coupled multiplicatively
Potts And XY, Together At Last Daniel Kolodrubetz Massachusetts Institute of Technology, Center for Theoretical Physics (Dated: May 16, 212) We investigate the behavior of an XY model coupled multiplicatively
Polymerization and force generation
 Polymerization and force generation by Eric Cytrynbaum April 8, 2008 Equilibrium polymer in a box An equilibrium polymer is a polymer has no source of extraneous energy available to it. This does not mean
Polymerization and force generation by Eric Cytrynbaum April 8, 2008 Equilibrium polymer in a box An equilibrium polymer is a polymer has no source of extraneous energy available to it. This does not mean
