Molecular Modeling -- Lecture 15 Surfaces and electrostatics
|
|
|
- Leon Davidson
- 6 years ago
- Views:
Transcription
1 Molecular Modeling -- Lecture 15 Surfaces and electrostatics Molecular surfaces The Hydrophobic Effect Electrostatics Poisson-Boltzmann Equation Electrostatic maps Electrostatic surfaces in MOE
2 15.1 The hydrophobic effect 2
3 Molecular surface Water that sits on the surface behaves differently than water that is surrounded by water. So, surfaces can be used to model solvation. water protein
4 How does water interact with a polar versus a non-polar surface? + Water has a partial charge separation along the O-H bonds -- a dipole low energy waters On a non-polar surface it loses entropy and does not gain enthalpy. - high energy waters On a polar surface it loses entropy and gains enthalpy through Coulombic intereactions (charge-charge) - - +
5 Shape dependence. Convex, contact surface Toroidal surface Concave, reentrant surface Water loses entropy in proportion to degrees of freedom lost. A water on a convex surface loses less entropy than a water in a concave surface.
6 Hydrophobic collapse releases high energy waters in proportion to the buried surface area. Purple waters have reduced entropy. Only the waters associated with the blue group are shown. The green group also has its low-entropy waters.
7 Nature abhors a vacuum no, not this kind... There is only one way to make space empty, but many ways to fill it. The entropy is larger if number of different states available to the system is larger. Lower entropy implies higher free energy (unfavorable).
8 Better fit, less wasted space, lower energy. If we compare two surfacesurface interactions, all else being equal, the one with more void spaces has a higher free energy, and is therefore less favorable. The one that fills space has a lower energy. higher E lower E
9 The Hydrophobic Effect What happens to the surface as two hydrophobic spheres come together?? closest positions of probe centers
10 The Hydrophobic Effect White area is excluded volume. Water can't go closer than one radius (1.4 Å). SES
11 The Hydrophobic Effect First, SAS (green) is increased by new "toroidal" surface (brown). SES
12 The Hydrophobic Effect Toroidal surface (brown) grows faster than atom surface is lost. SAS goes up. SES
13 The Hydrophobic Effect At short distance, toroidal surface stops growing, atom surface shrinks faster. SES
14 Is there an energy barrier to hydrophobic collapse? (yes) VDW ΔG ** SES Volume, SAS 0 5.8Å Distance between pairs of hydrophobic spheres of radius 1.5Å Solvation energy ΔG parallels SES, not SAS or excluded volume. Thus hydrophobic surface area is related to solvation energy. ** (Rank & Baker, Czaplewski & Sheraga)
15 Summary The hydrophobic effect is related to water -- specifically to the solvation of the hydrophobic surface. The solvation energy is composed of enthalpy (Coulombs law) and entropy. Loss of entropy depends on the surface shape. There is a barrier to hydrophobic collapse, caused by transient shape changes. 15
16 15.2 Electrostatics 16
17 Partial charges Quantum mechanical calculations can tell us the electron distributions around atom nuclei. If there are more or less electrons that belong to a given atom nucleus, it has a non-zero charge. Formal charge is the net charge of an ion or ionized group. Partial charge is the charge introduced by polarization of electron clouds. E.g. the backbone amide group N-H. Which atoms has partial positive charge and which has partial negative charge? What about the carbonyl group C=O? Critical for calculating the electrostatic potential map and surface!
18 Coulomb's Law: Energy of a charge-charge interaction E = q iq j εr ij where q i is the charge on particle i, q j is the charge on particle j, r ij is the distance between them, and ε is the dielectric constant. This is the energy required to bring two charges from infinite distance to the distance r ij in a medium with dielectric constant ε.
19 Dielectric (die electric?) The dielectric ε is the degree to which a medium disperses charge, or the ability to reduce electrostatic interaction. Charge dispersion can happen by rotating dipoles (like water) or by induced polarization (aromatics). In a higher dielectric, the charges feel less force. The energy to pull two like charges together is less, and the energy to push two opposite charges apart is less. Water has ε of ~ 80, while inside the hydrophobic core of a protein ε is 2-4. E = q iq j εr ij + + ε (must be (vacuum))
20 Electrostatic potential map. - - E(x) = q q i εr ix + φ(x) = εr ix Assuming a dielectric constant ε, the energy for putting a charge q at any position r is summed using Coulomb's Law. Then, we define the electrostatic potential as Φ=E/q. In this space we can draw contours where Φ is constant (isopotential surfaces). Isopotential surfaces are useful for considering the docking of charged or polar ligands to a protein. q i
21 Electrostatic potential map of a non-homogeneous medium An electrostatic potential map Φ(x) is the energy landscape for a point charge brought from infinite distance to point x. (a) the electrostatic potential map for a single charge in low dielectric (membrane). (b) High dielectric (water). (c) point charge on the surface of a membrane. isopotential lines water (a) (b) membrane (c)
22 Charge density Like mass density, charge density is the sum over all point sources of charge, divided by the volume. In this case, however, the value summed is signed. The charge density at r, ρ(r), can be expressed as, Where qi is the charge (which may be a partial charge). δ(r-r i ) is the delta function, which is 1 if r == r i and zero otherwise.
23 Electric field An electric field E(r) is the amplitude and direction of force on a particle of unit (+) charge, sitting at location r, which depends on the charge density distribution ρ(r). The electric field is the gradient of the electrostatic potential 23 map.
24 Poisson's equation If charges are movable in the space r, then charge density ρ(r) can move to find the lowest energy. The condition for this equilibrium is that the charge density ρ(r) is everywhere inversely proportional to the curvature of the electrostatic potential. 2 φ( r) = ρ( r) The Laplacian of φ. the dielectric "del" means the gradient, or 3-dimensional 1st derivative (d/dx)i+(d/dy)j+(d/dz)k 2 means, the divergence of the gradient. 2 φ is the 3-dimensional 2nd derivative. It expresses the degree to which a point in space is a sink or a source. Think mountain or valley. ε phi is the electrostatic potential. So... if we know ρ, we can get φ, by solving Poisson s equation. rho is the charge density. DelPhi is a program that calculates the electrostatic potential map, developed by Barry Honig at Columbia University
25 Two problems when calculating electrostatics... (1) Mobile charges redistribute themselves in the electric field. When an ion moves to a position of opposite electrostatic potential, it changes the charge distribution ρ(r), which in turn changes the electrostatic potential. E.g. Na + and Cl - in a protein solution or in our body. (2) We can't assume that the dielectric is a constant throughout our system. The dielectric is the ability of the medium to disperse the charge. Solid or semisolid objects have a much lower dielectric than liquids, especially polar liquids like water.
26 Boltzmann distribution of charges Boltzmann states that the number density of ions in an electric field or map is related to the electrostatic potential as follows: number density of ions (charges) ( ) = Ie ρ r φ r ( ) k b T energy to bring a charge to point r from infinity Boltzmann constant bulk number density ionic strength So... if we know φ, we can get ρ by solving the Boltzmann equation. NOTE: take the log of both sides, change variable names and you get Gibbs Free Energy: ΔG = -RTlog(K eq )
27 Poisson-Boltzmann equation ε( r) φ( r) κφ( r) = 4πρ( r) Features of the P-B equation: It expresses the relationship between the electrostatic potential and the charge distribution. It has a position specific dielectric ε(r). It has a position specific charge density ρ(r). It has a position specific electrostatic potential φ(r). It has a Boltzmann correction κ to account for redistributed charges. The P-B equation must hold for any two functions ρ and φ. ρ and φ are solved numerically, over the space r.
28 Variable dielectric 80 4 Generally, we assume there is a ε for inside the protein (green, ε=2 or 4), and a ε for outside in the solvent (ε=80).
29 P-B algorithm Until converged { For each grid point, Calculate the 2nd derivative of the electrostatic potential φ given the charge density ρ, in each direction. } For each grid point, Calculate the new charge density ρ given the 2nd derivative of the electrostatic potential φ. 29
30 Projecting the electrostatic potential onto the surface For each point on the molecular surface { Find the 8 bordering gridpoints. Find the electrostatic potential φ by 3-way linear interpolation. Convert to a color. } -X 0 +X 30
31 Projecting the electrostatic potential onto the surface Active sites and binding surfaces can be found by coloring the surface by the electrostatic potential. Here we ignore all grid points except the ones closest to the surface. A ligand should have a complementary electrostatic surface AND a complementary shape!
32 Exercise/Demo: Use MOE to view the electrostatics on the molecular surface Display the Connolly surface of the protein. Calculate the electrostatic potential. Limit the surface by selected atoms. Show complementary surfaces. (1bgs chains A and E) Slice through the surface.
Molecular Modeling Lecture 11 side chain modeling rotamers rotamer explorer buried cavities.
 Molecular Modeling 218 Lecture 11 side chain modeling rotamers rotamer explorer buried cavities. Sidechain Rotamers Discrete approximation of the continuous space of backbone angles. Sidechain conformations
Molecular Modeling 218 Lecture 11 side chain modeling rotamers rotamer explorer buried cavities. Sidechain Rotamers Discrete approximation of the continuous space of backbone angles. Sidechain conformations
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015,
 Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
Mirror Charges, Hydrogen Bonds, And the Water-Dielectric Interface
 Mirror Charges, ydrogen Bonds, And the Water-Dielectric Interface Kevin Cahill cahill@unm.edu http://dna.phys.unm.edu/ 1 Field Energy The energy E of an electric field E( x, t) is the space integral of
Mirror Charges, ydrogen Bonds, And the Water-Dielectric Interface Kevin Cahill cahill@unm.edu http://dna.phys.unm.edu/ 1 Field Energy The energy E of an electric field E( x, t) is the space integral of
Chapter 4. Electric Fields in Matter
 Chapter 4. Electric Fields in Matter 4.1.2 Induced Dipoles What happens to a neutral atom when it is placed in an electric field E? The atom now has a tiny dipole moment p, in the same direction as E.
Chapter 4. Electric Fields in Matter 4.1.2 Induced Dipoles What happens to a neutral atom when it is placed in an electric field E? The atom now has a tiny dipole moment p, in the same direction as E.
Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse
 March 4, 2016 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse 1 Foothold principles: Newton s Laws Newton 0: An object responds only to the
March 4, 2016 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse 1 Foothold principles: Newton s Laws Newton 0: An object responds only to the
Biophysics II. Hydrophobic Bio-molecules. Key points to be covered. Molecular Interactions in Bio-molecular Structures - van der Waals Interaction
 Biophysics II Key points to be covered By A/Prof. Xiang Yang Liu Biophysics & Micro/nanostructures Lab Department of Physics, NUS 1. van der Waals Interaction 2. Hydrogen bond 3. Hydrophilic vs hydrophobic
Biophysics II Key points to be covered By A/Prof. Xiang Yang Liu Biophysics & Micro/nanostructures Lab Department of Physics, NUS 1. van der Waals Interaction 2. Hydrogen bond 3. Hydrophilic vs hydrophobic
BIOC : Homework 1 Due 10/10
 Contact information: Name: Student # BIOC530 2012: Homework 1 Due 10/10 Department Email address The following problems are based on David Baker s lectures of forces and protein folding. When numerical
Contact information: Name: Student # BIOC530 2012: Homework 1 Due 10/10 Department Email address The following problems are based on David Baker s lectures of forces and protein folding. When numerical
2 Structure. 2.1 Coulomb interactions
 2 Structure 2.1 Coulomb interactions While the information needed for reproduction of living systems is chiefly maintained in the sequence of macromolecules, any practical use of this information must
2 Structure 2.1 Coulomb interactions While the information needed for reproduction of living systems is chiefly maintained in the sequence of macromolecules, any practical use of this information must
I: Life and Energy. Lecture 2: Solutions and chemical potential; Osmotic pressure (B Lentz).
 I: Life and Energy Lecture 1: What is life? An attempt at definition. Energy, heat, and work: Temperature and thermal equilibrium. The First Law. Thermodynamic states and state functions. Reversible and
I: Life and Energy Lecture 1: What is life? An attempt at definition. Energy, heat, and work: Temperature and thermal equilibrium. The First Law. Thermodynamic states and state functions. Reversible and
Enduring Understandings & Essential Knowledge for AP Chemistry
 Enduring Understandings & Essential Knowledge for AP Chemistry Big Idea 1: The chemical elements are fundamental building materials of matter, and all matter can be understood in terms of arrangements
Enduring Understandings & Essential Knowledge for AP Chemistry Big Idea 1: The chemical elements are fundamental building materials of matter, and all matter can be understood in terms of arrangements
Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse
 March 1, 2013 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse 1 Foothold principles: Newton s Laws Newton 0: An object responds only to the
March 1, 2013 Prof. E. F. Redish Theme Music: Duke Ellington Take the A Train Cartoon: Lynn Johnson For Better or for Worse 1 Foothold principles: Newton s Laws Newton 0: An object responds only to the
Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together.
 Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together. For example Nacl In the Nacl lattice, each Na atom is
Bonding in solids The interaction of electrons in neighboring atoms of a solid serves the very important function of holding the crystal together. For example Nacl In the Nacl lattice, each Na atom is
Atoms & Their Interactions
 Lecture 2 Atoms & Their Interactions Si: the heart of electronic materials Intel, 300mm Si wafer, 200 μm thick and 48-core CPU ( cloud computing on a chip ) Twin Creeks Technologies, San Jose, Si wafer,
Lecture 2 Atoms & Their Interactions Si: the heart of electronic materials Intel, 300mm Si wafer, 200 μm thick and 48-core CPU ( cloud computing on a chip ) Twin Creeks Technologies, San Jose, Si wafer,
Electrolytes. Chapter Basics = = 131 2[ ]. (c) From both of the above = = 120 8[
![Electrolytes. Chapter Basics = = 131 2[ ]. (c) From both of the above = = 120 8[ Electrolytes. Chapter Basics = = 131 2[ ]. (c) From both of the above = = 120 8[](/thumbs/83/88791608.jpg) Chapter 1 Electrolytes 1.1 Basics Here we consider species that dissociate into positively and negatively charged species in solution. 1. Consider: 1 H (g) + 1 Cl (g) + ()+ () = { } = (+ )+ ( ) = 167[
Chapter 1 Electrolytes 1.1 Basics Here we consider species that dissociate into positively and negatively charged species in solution. 1. Consider: 1 H (g) + 1 Cl (g) + ()+ () = { } = (+ )+ ( ) = 167[
Free energy, electrostatics, and the hydrophobic effect
 Protein Physics 2016 Lecture 3, January 26 Free energy, electrostatics, and the hydrophobic effect Magnus Andersson magnus.andersson@scilifelab.se Theoretical & Computational Biophysics Recap Protein structure
Protein Physics 2016 Lecture 3, January 26 Free energy, electrostatics, and the hydrophobic effect Magnus Andersson magnus.andersson@scilifelab.se Theoretical & Computational Biophysics Recap Protein structure
Solutions and Non-Covalent Binding Forces
 Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
BIOC 530 Fall, 2011 BIOC 530
 Fall, 2011 Course Information Course Description Graduate-level discussion of the structure, function, and chemistry of proteins and nucleic acids, control of enzymatic reactions. Please see the course
Fall, 2011 Course Information Course Description Graduate-level discussion of the structure, function, and chemistry of proteins and nucleic acids, control of enzymatic reactions. Please see the course
Physics 272: Electricity and Magnetism. Mark Palenik Thursday June 14 th
 Physics 272: Electricity and Magnetism Mark Palenik Thursday June 14 th Topics Finish up dipoles Polarization of materials Conductors Charging/discharging iclicker (not credit) An atom is placed in a UNIFORM
Physics 272: Electricity and Magnetism Mark Palenik Thursday June 14 th Topics Finish up dipoles Polarization of materials Conductors Charging/discharging iclicker (not credit) An atom is placed in a UNIFORM
Aqueous solutions. Solubility of different compounds in water
 Aqueous solutions Solubility of different compounds in water The dissolution of molecules into water (in any solvent actually) causes a volume change of the solution; the size of this volume change is
Aqueous solutions Solubility of different compounds in water The dissolution of molecules into water (in any solvent actually) causes a volume change of the solution; the size of this volume change is
Biochemistry 675, Lecture 8 Electrostatic Interactions
 Biochemistry 675, Lecture 8 Electrostatic Interactions Previous Classes Hydrophobic Effect Hydrogen Bonds Today: Electrostatic Interactions Reading: Handout:P.R. Bergethon & E.R. Simons, Biophysical Chemistry,
Biochemistry 675, Lecture 8 Electrostatic Interactions Previous Classes Hydrophobic Effect Hydrogen Bonds Today: Electrostatic Interactions Reading: Handout:P.R. Bergethon & E.R. Simons, Biophysical Chemistry,
The change in free energy on transferring an ion from a medium of low dielectric constantε1 to one of high dielectric constant ε2:
 The Born Energy of an Ion The free energy density of an electric field E arising from a charge is ½(ε 0 ε E 2 ) per unit volume Integrating the energy density of an ion over all of space = Born energy:
The Born Energy of an Ion The free energy density of an electric field E arising from a charge is ½(ε 0 ε E 2 ) per unit volume Integrating the energy density of an ion over all of space = Born energy:
Multimedia : Boundary Lubrication Podcast, Briscoe, et al. Nature , ( )
 3.05 Nanomechanics of Materials and Biomaterials Thursday 04/05/07 Prof. C. Ortiz, MITDMSE I LECTURE 14: TE ELECTRICAL DOUBLE LAYER (EDL) Outline : REVIEW LECTURE #11 : INTRODUCTION TO TE ELECTRICAL DOUBLE
3.05 Nanomechanics of Materials and Biomaterials Thursday 04/05/07 Prof. C. Ortiz, MITDMSE I LECTURE 14: TE ELECTRICAL DOUBLE LAYER (EDL) Outline : REVIEW LECTURE #11 : INTRODUCTION TO TE ELECTRICAL DOUBLE
Biochemistry 530: Introduction to Structural Biology. Autumn Quarter 2014 BIOC 530
 Biochemistry 530: Introduction to Structural Biology Autumn Quarter 2014 Course Information Course Description Graduate-level discussion of the structure, function, and chemistry of proteins and nucleic
Biochemistry 530: Introduction to Structural Biology Autumn Quarter 2014 Course Information Course Description Graduate-level discussion of the structure, function, and chemistry of proteins and nucleic
Chapter 2. Dielectric Theories
 Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
Non-bonded interactions
 speeding up the number-crunching Marcus Elstner and Tomáš Kubař May 8, 2015 why care? key to understand biomolecular structure and function binding of a ligand efficiency of a reaction color of a chromophore
speeding up the number-crunching Marcus Elstner and Tomáš Kubař May 8, 2015 why care? key to understand biomolecular structure and function binding of a ligand efficiency of a reaction color of a chromophore
Chapter 4. Electrostatic Fields in Matter
 Chapter 4. Electrostatic Fields in Matter 4.1. Polarization 4.2. The Field of a Polarized Object 4.3. The Electric Displacement 4.4. Linear Dielectrics 4.5. Energy in dielectric systems 4.6. Forces on
Chapter 4. Electrostatic Fields in Matter 4.1. Polarization 4.2. The Field of a Polarized Object 4.3. The Electric Displacement 4.4. Linear Dielectrics 4.5. Energy in dielectric systems 4.6. Forces on
Other Cells. Hormones. Viruses. Toxins. Cell. Bacteria
 Other Cells Hormones Viruses Toxins Cell Bacteria ΔH < 0 reaction is exothermic, tells us nothing about the spontaneity of the reaction Δ H > 0 reaction is endothermic, tells us nothing about the spontaneity
Other Cells Hormones Viruses Toxins Cell Bacteria ΔH < 0 reaction is exothermic, tells us nothing about the spontaneity of the reaction Δ H > 0 reaction is endothermic, tells us nothing about the spontaneity
Electron Configuration and Periodic Trends - Chapter 5 section 3 Guided Notes
 Electron Configuration and Periodic Trends - Chapter 5 section 3 Guided Notes There are several important atomic characteristics that show predictable that you should know. Atomic Radius The first and
Electron Configuration and Periodic Trends - Chapter 5 section 3 Guided Notes There are several important atomic characteristics that show predictable that you should know. Atomic Radius The first and
Step 1: Solute particles must separate from each other. Since energy must be absorbed to overcome the forces of attraction between solute particles,
 Step 1: Solute particles must separate from each other. Since energy must be absorbed to overcome the forces of attraction between solute particles, this process is endothermic. Step 2: Solvent particles
Step 1: Solute particles must separate from each other. Since energy must be absorbed to overcome the forces of attraction between solute particles, this process is endothermic. Step 2: Solvent particles
Chapter 17 & 18. Electric Field and Electric Potential
 Chapter 17 & 18 Electric Field and Electric Potential Electric Field Maxwell developed an approach to discussing fields An electric field is said to exist in the region of space around a charged object
Chapter 17 & 18 Electric Field and Electric Potential Electric Field Maxwell developed an approach to discussing fields An electric field is said to exist in the region of space around a charged object
Phase Equilibria and Molecular Solutions Jan G. Korvink and Evgenii Rudnyi IMTEK Albert Ludwig University Freiburg, Germany
 Phase Equilibria and Molecular Solutions Jan G. Korvink and Evgenii Rudnyi IMTEK Albert Ludwig University Freiburg, Germany Preliminaries Learning Goals Phase Equilibria Phase diagrams and classical thermodynamics
Phase Equilibria and Molecular Solutions Jan G. Korvink and Evgenii Rudnyi IMTEK Albert Ludwig University Freiburg, Germany Preliminaries Learning Goals Phase Equilibria Phase diagrams and classical thermodynamics
Chemical symbols. Know names and symbols of elements #1 30, plus. Rb, Cs, Sr, Ba, Ag, Au, Cd, Hg, Pt, Ga, Ge, As, Sn, Pb, Se, Br, I, and U
 Chemical symbols Know names and symbols of elements #1 30, plus Rb, Cs, Sr, Ba, Ag, Au, Cd, Hg, Pt, Ga, Ge, As, Sn, Pb, Se, Br, I, and U Coulomb s Law F = attractive/repulsive force Q 1, Q 2 = charges
Chemical symbols Know names and symbols of elements #1 30, plus Rb, Cs, Sr, Ba, Ag, Au, Cd, Hg, Pt, Ga, Ge, As, Sn, Pb, Se, Br, I, and U Coulomb s Law F = attractive/repulsive force Q 1, Q 2 = charges
Basics of Thermodynamics: Easy learning by Dr. Anjana Sen
 Basics of Thermodynamics: Easy learning by Dr. Anjana Sen Part 1: Theory and concept Part 2: Definitions and equations Part 3: Laws of Thermodynamics Part 1: theory and concept Thermodynamics means conversion
Basics of Thermodynamics: Easy learning by Dr. Anjana Sen Part 1: Theory and concept Part 2: Definitions and equations Part 3: Laws of Thermodynamics Part 1: theory and concept Thermodynamics means conversion
Why Proteins Fold. How Proteins Fold? e - ΔG/kT. Protein Folding, Nonbonding Forces, and Free Energy
 Why Proteins Fold Proteins are the action superheroes of the body. As enzymes, they make reactions go a million times faster. As versatile transport vehicles, they carry oxygen and antibodies to fight
Why Proteins Fold Proteins are the action superheroes of the body. As enzymes, they make reactions go a million times faster. As versatile transport vehicles, they carry oxygen and antibodies to fight
Lecture 15: Enzymes & Kinetics. Mechanisms ROLE OF THE TRANSITION STATE. H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl. Margaret A. Daugherty.
 Lecture 15: Enzymes & Kinetics Mechanisms Margaret A. Daugherty Fall 2004 ROLE OF THE TRANSITION STATE Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products
Lecture 15: Enzymes & Kinetics Mechanisms Margaret A. Daugherty Fall 2004 ROLE OF THE TRANSITION STATE Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products
Electric Force and Charges. Conceptual Physics 11 th Edition. Electric Force and Charges
 Conceptual Physics 11 th Edition Central rule of electricity Opposite charges attract one another; like charges repel. Chapter 22: ELECTROSTATICS This lecture will help you understand: Electrical Forces
Conceptual Physics 11 th Edition Central rule of electricity Opposite charges attract one another; like charges repel. Chapter 22: ELECTROSTATICS This lecture will help you understand: Electrical Forces
2.2.2 Bonding and Structure
 2.2.2 Bonding and Structure Ionic Bonding Definition: Ionic bonding is the electrostatic force of attraction between oppositely charged ions formed by electron transfer. Metal atoms lose electrons to form
2.2.2 Bonding and Structure Ionic Bonding Definition: Ionic bonding is the electrostatic force of attraction between oppositely charged ions formed by electron transfer. Metal atoms lose electrons to form
This semester. Books
 Models mostly proteins from detailed to more abstract models Some simulation methods This semester Books None necessary for my group and Prof Rarey Molecular Modelling: Principles and Applications Leach,
Models mostly proteins from detailed to more abstract models Some simulation methods This semester Books None necessary for my group and Prof Rarey Molecular Modelling: Principles and Applications Leach,
Ch. 5 - The Periodic Table
 Ch. 5 - The Periodic Table 250 Atomic Radius (pm) 200 150 100 50 0 0 5 10 15 20 Atomic Number III. Periodic Trends (p. 140-154) I II III A. Periodic Law When elements are arranged in order of increasing
Ch. 5 - The Periodic Table 250 Atomic Radius (pm) 200 150 100 50 0 0 5 10 15 20 Atomic Number III. Periodic Trends (p. 140-154) I II III A. Periodic Law When elements are arranged in order of increasing
Chapter 1. Topic: Overview of basic principles
 Chapter 1 Topic: Overview of basic principles Four major themes of biochemistry I. What are living organism made from? II. How do organism acquire and use energy? III. How does an organism maintain its
Chapter 1 Topic: Overview of basic principles Four major themes of biochemistry I. What are living organism made from? II. How do organism acquire and use energy? III. How does an organism maintain its
ONETEP PB/SA: Application to G-Quadruplex DNA Stability. Danny Cole
 ONETEP PB/SA: Application to G-Quadruplex DNA Stability Danny Cole Introduction Historical overview of structure and free energy calculation of complex molecules using molecular mechanics and continuum
ONETEP PB/SA: Application to G-Quadruplex DNA Stability Danny Cole Introduction Historical overview of structure and free energy calculation of complex molecules using molecular mechanics and continuum
Molecular Forces in Biological Systems - Electrostatic Interactions; - Shielding of charged objects in solution
 Molecular Forces in Biological Systems - Electrostatic Interactions; - Shielding of charged objects in solution Electrostatic self-energy, effects of size and dielectric constant q q r r ε ε 1 ε 2? δq
Molecular Forces in Biological Systems - Electrostatic Interactions; - Shielding of charged objects in solution Electrostatic self-energy, effects of size and dielectric constant q q r r ε ε 1 ε 2? δq
MCB100A/Chem130 MidTerm Exam 2 April 4, 2013
 MCBA/Chem Miderm Exam 2 April 4, 2 Name Student ID rue/false (2 points each).. he Boltzmann constant, k b sets the energy scale for observing energy microstates 2. Atoms with favorable electronic configurations
MCBA/Chem Miderm Exam 2 April 4, 2 Name Student ID rue/false (2 points each).. he Boltzmann constant, k b sets the energy scale for observing energy microstates 2. Atoms with favorable electronic configurations
Bchem 675 Lecture 9 Electrostatics-Lecture 2 Debye-Hückel: Continued Counter ion condensation
 Bchem 675 Lecture 9 Electrostatics-Lecture 2 Debye-Hückel: Continued Counter ion condensation Ion:ion interactions What is the free energy of ion:ion interactions ΔG i-i? Consider an ion in a solution
Bchem 675 Lecture 9 Electrostatics-Lecture 2 Debye-Hückel: Continued Counter ion condensation Ion:ion interactions What is the free energy of ion:ion interactions ΔG i-i? Consider an ion in a solution
We have considered how Coulombic attractions and repulsions help to organize electrons in atoms and ions.
 CHEM 2060 Lecture 10: Electrostatics L10-1 Electrostatics of Atoms & Molecules We have considered how Coulombic attractions and repulsions help to organize electrons in atoms and ions. We now look at Coulombic
CHEM 2060 Lecture 10: Electrostatics L10-1 Electrostatics of Atoms & Molecules We have considered how Coulombic attractions and repulsions help to organize electrons in atoms and ions. We now look at Coulombic
Physics 272: Electricity and Magnetism. Mark Palenik Wednesday June 13 th
 Physics 272: Electricity and Magnetism Mark Palenik Wednesday June 13 th Todays topics Polarization and electric field Polarization of atoms Transfer of charge Charged Particles Net charge: sum of all
Physics 272: Electricity and Magnetism Mark Palenik Wednesday June 13 th Todays topics Polarization and electric field Polarization of atoms Transfer of charge Charged Particles Net charge: sum of all
Lecture 2-3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability
 Lecture 2-3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions Van der Waals Interactions
Lecture 2-3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions Van der Waals Interactions
One Q partial negative, the other partial negative Ø H- bonding particularly strong. Abby Carroll 2
 Chemistry Notes v Polarity Experiment Ø Things involved Polarity Solubility Dispersion Ø Polarity Shaving cream has soap steric acid Water is polar Food coloring is polar/ionic because dissolved Like dissolves
Chemistry Notes v Polarity Experiment Ø Things involved Polarity Solubility Dispersion Ø Polarity Shaving cream has soap steric acid Water is polar Food coloring is polar/ionic because dissolved Like dissolves
Mechanics, Heat, Oscillations and Waves Prof. V. Balakrishnan Department of Physics Indian Institute of Technology, Madras
 Mechanics, Heat, Oscillations and Waves Prof. V. Balakrishnan Department of Physics Indian Institute of Technology, Madras Lecture - 21 Central Potential and Central Force Ready now to take up the idea
Mechanics, Heat, Oscillations and Waves Prof. V. Balakrishnan Department of Physics Indian Institute of Technology, Madras Lecture - 21 Central Potential and Central Force Ready now to take up the idea
Lecture: P1_Wk1_L1 IntraMolecular Interactions. Ron Reifenberger Birck Nanotechnology Center Purdue University 2012
 Lecture: IntraMolecular Interactions Distinguish between IntraMolecular (within a molecule) and InterMolecular (between molecules) Ron Reifenberger Birck Nanotechnology Center Purdue University 2012 1
Lecture: IntraMolecular Interactions Distinguish between IntraMolecular (within a molecule) and InterMolecular (between molecules) Ron Reifenberger Birck Nanotechnology Center Purdue University 2012 1
Protein Folding & Stability. Lecture 11: Margaret A. Daugherty. Fall How do we go from an unfolded polypeptide chain to a
 Lecture 11: Protein Folding & Stability Margaret A. Daugherty Fall 2004 How do we go from an unfolded polypeptide chain to a compact folded protein? (Folding of thioredoxin, F. Richards) Structure - Function
Lecture 11: Protein Folding & Stability Margaret A. Daugherty Fall 2004 How do we go from an unfolded polypeptide chain to a compact folded protein? (Folding of thioredoxin, F. Richards) Structure - Function
Phys 102 Lecture 2 Coulomb s Law & Electric Dipoles
 Phys 102 Lecture 2 Coulomb s Law & Electric Dipoles 1 Today we will... Get practice using Coulomb s law & vector addition Learn about electric dipoles Apply these concepts! Molecular interactions Polar
Phys 102 Lecture 2 Coulomb s Law & Electric Dipoles 1 Today we will... Get practice using Coulomb s law & vector addition Learn about electric dipoles Apply these concepts! Molecular interactions Polar
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit. Lecture 2 (9/11/17)
 16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
Molecular Simulation III
 Molecular Simulation III Quantum Chemistry Classical Mechanics E = Ψ H Ψ ΨΨ U = E bond +E angle +E torsion +E non-bond Molecular Dynamics Jeffry D. Madura Department of Chemistry & Biochemistry Center
Molecular Simulation III Quantum Chemistry Classical Mechanics E = Ψ H Ψ ΨΨ U = E bond +E angle +E torsion +E non-bond Molecular Dynamics Jeffry D. Madura Department of Chemistry & Biochemistry Center
Step 1. Step 2. g l = g v. dg = 0 We have shown that over a plane surface of water. g v g l = ρ v R v T ln e/e sat. this can be rewritten
 The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
SAM Teachers Guide Chemical Bonds
 SAM Teachers Guide Chemical Bonds Overview Students discover that the type of bond formed ionic, non polar covalent, or polar covalent depends on the electronegativity of the two atoms that are bonded
SAM Teachers Guide Chemical Bonds Overview Students discover that the type of bond formed ionic, non polar covalent, or polar covalent depends on the electronegativity of the two atoms that are bonded
Chapter 9. Chemical Bonding I: The Lewis Model. HIV-Protease. Lecture Presentation
 Lecture Presentation Chapter 9 Chemical Bonding I: The Lewis Model HIV-Protease HIV-protease is a protein synthesized by the human immunodeficiency virus (HIV). This particular protein is crucial to the
Lecture Presentation Chapter 9 Chemical Bonding I: The Lewis Model HIV-Protease HIV-protease is a protein synthesized by the human immunodeficiency virus (HIV). This particular protein is crucial to the
WS 1: Ionic Bonds 1. Charge on particle 1= q1 Charge on particle 2 = q2
 Part I: The Ionic Bonding Model: i WS 1: Ionic Bonds 1 Trends in ionization energies and electron affinities indicate that some elements for ions more readily than others. We know that ions with opposite
Part I: The Ionic Bonding Model: i WS 1: Ionic Bonds 1 Trends in ionization energies and electron affinities indicate that some elements for ions more readily than others. We know that ions with opposite
Chapter 8: Bonding. Section 8.1: Lewis Dot Symbols
 Chapter 8: Bonding Section 8.1: Lewis Dot Symbols The Lewis electron dot symbol is named after Gilbert Lewis. In the Lewis dot symbol, the element symbol represents the nucleus and the inner electrons.
Chapter 8: Bonding Section 8.1: Lewis Dot Symbols The Lewis electron dot symbol is named after Gilbert Lewis. In the Lewis dot symbol, the element symbol represents the nucleus and the inner electrons.
End-of-Chapter Exercises
 End-of-Chapter Exercises Exercises 1 12 are primarily conceptual questions designed to see whether you understand the main concepts of the chapter. 1. (a) If the electric field at a particular point is
End-of-Chapter Exercises Exercises 1 12 are primarily conceptual questions designed to see whether you understand the main concepts of the chapter. 1. (a) If the electric field at a particular point is
4/4/2013. Covalent Bonds a bond that results in the sharing of electron pairs between two atoms.
 A chemical bond is a mutual electrical attraction between the nucleus and valence electrons of different atoms that binds the atoms together. Why bond? As independent particles, atoms have a high potential
A chemical bond is a mutual electrical attraction between the nucleus and valence electrons of different atoms that binds the atoms together. Why bond? As independent particles, atoms have a high potential
Docking. GBCB 5874: Problem Solving in GBCB
 Docking Benzamidine Docking to Trypsin Relationship to Drug Design Ligand-based design QSAR Pharmacophore modeling Can be done without 3-D structure of protein Receptor/Structure-based design Molecular
Docking Benzamidine Docking to Trypsin Relationship to Drug Design Ligand-based design QSAR Pharmacophore modeling Can be done without 3-D structure of protein Receptor/Structure-based design Molecular
Life is a chemical process
 CHEMISTRY FOR LIFE Life is a chemical process Relies on and is subject to chemistry Must obey the laws of physics Biologists study Chemistry because all living things are made of matter. Matter undergoes
CHEMISTRY FOR LIFE Life is a chemical process Relies on and is subject to chemistry Must obey the laws of physics Biologists study Chemistry because all living things are made of matter. Matter undergoes
Solvation and Macromolecular Structure. The structure and dynamics of biological macromolecules are strongly influenced by water:
 Overview Solvation and Macromolecular Structure The structure and dynamics of biological macromolecules are strongly influenced by water: Electrostatic effects: charges are screened by water molecules
Overview Solvation and Macromolecular Structure The structure and dynamics of biological macromolecules are strongly influenced by water: Electrostatic effects: charges are screened by water molecules
Chapter-2 (Page 22-37) Physical and Chemical Properties of Water
 Chapter-2 (Page 22-37) Physical and Chemical Properties of Water Introduction About 70% of the mass of the human body is water. Water is central to biochemistry for the following reasons: 1- Biological
Chapter-2 (Page 22-37) Physical and Chemical Properties of Water Introduction About 70% of the mass of the human body is water. Water is central to biochemistry for the following reasons: 1- Biological
Definition: Electricity at rest (stationary)
 Electrostatics Definition: Electricity at rest (stationary) Static means to stand and is used in Mechanical Engineering to study forces on bridges and other structures. Statue, stasis, stationary, ecstatic,
Electrostatics Definition: Electricity at rest (stationary) Static means to stand and is used in Mechanical Engineering to study forces on bridges and other structures. Statue, stasis, stationary, ecstatic,
Water, water everywhere,; not a drop to drink. Consumption resulting from how environment inhabited Deforestation disrupts water cycle
 Chapter 3 Water: The Matrix of Life Overview n n n Water, water everywhere,; not a drop to drink Only 3% of world s water is fresh How has this happened Consumption resulting from how environment inhabited
Chapter 3 Water: The Matrix of Life Overview n n n Water, water everywhere,; not a drop to drink Only 3% of world s water is fresh How has this happened Consumption resulting from how environment inhabited
Periodic Trends. Elemental Properties and Patterns
 Periodic Trends Elemental Properties and Patterns The Periodic Law Dimitri Mendeleev was the first scientist to publish an organized periodic table of the known elements. He was perpetually in trouble
Periodic Trends Elemental Properties and Patterns The Periodic Law Dimitri Mendeleev was the first scientist to publish an organized periodic table of the known elements. He was perpetually in trouble
SAM Teacher s Guide Protein Partnering and Function
 SAM Teacher s Guide Protein Partnering and Function Overview Students explore protein molecules physical and chemical characteristics and learn that these unique characteristics enable other molecules
SAM Teacher s Guide Protein Partnering and Function Overview Students explore protein molecules physical and chemical characteristics and learn that these unique characteristics enable other molecules
Lectures 11-13: Electrostatics of Salty Solutions
 Lectures 11-13: Electrostatics of Salty Solutions Lecturer: Brigita Urbanc Office: 1-909 (E-mail: brigita@drexel.edu) Course website: www.physics.drexel.edu/~brigita/courses/biophys_011-01/ 1 Water as
Lectures 11-13: Electrostatics of Salty Solutions Lecturer: Brigita Urbanc Office: 1-909 (E-mail: brigita@drexel.edu) Course website: www.physics.drexel.edu/~brigita/courses/biophys_011-01/ 1 Water as
What is this? Electrons: charge, mass? Atom. Negative charge(-), mass = 0. The basic unit of matter. Made of subatomic particles:
 Chemical Bonds What is this? Atom The basic unit of matter. Electrons: charge, mass? Negative charge(-), mass = 0 Made of subatomic particles: Protons: charge, mass? Positive charge (+), mass = 1 Neutrons:
Chemical Bonds What is this? Atom The basic unit of matter. Electrons: charge, mass? Negative charge(-), mass = 0 Made of subatomic particles: Protons: charge, mass? Positive charge (+), mass = 1 Neutrons:
Big Idea: Ionic Bonds: Ionic Bonds: Metals: Nonmetals: Covalent Bonds: Ionic Solids: What ions do atoms form? Electron Electron
 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
CHAPTER 2 INTERATOMIC FORCES. atoms together in a solid?
 CHAPTER 2 INTERATOMIC FORCES What kind of force holds the atoms together in a solid? Interatomic Binding All of the mechanisms which cause bonding between the atoms derive from electrostatic interaction
CHAPTER 2 INTERATOMIC FORCES What kind of force holds the atoms together in a solid? Interatomic Binding All of the mechanisms which cause bonding between the atoms derive from electrostatic interaction
Köhler Curve. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
= (-22) = +2kJ /mol
 Lecture 8: Thermodynamics & Protein Stability Assigned reading in Campbell: Chapter 4.4-4.6 Key Terms: DG = -RT lnk eq = DH - TDS Transition Curve, Melting Curve, Tm DH calculation DS calculation van der
Lecture 8: Thermodynamics & Protein Stability Assigned reading in Campbell: Chapter 4.4-4.6 Key Terms: DG = -RT lnk eq = DH - TDS Transition Curve, Melting Curve, Tm DH calculation DS calculation van der
Non-bonded interactions
 speeding up the number-crunching continued Marcus Elstner and Tomáš Kubař December 3, 2013 why care? number of individual pair-wise interactions bonded interactions proportional to N: O(N) non-bonded interactions
speeding up the number-crunching continued Marcus Elstner and Tomáš Kubař December 3, 2013 why care? number of individual pair-wise interactions bonded interactions proportional to N: O(N) non-bonded interactions
File ISM04. Properties of Colloids I
 File ISM04 Properties of Colloids I 1 Colloids Small things, but much bigger than a molecule Background: Foundations of Colloid Science Vol I & II, R.J. Hunter Physical Chemistry, P.W. Atkins, Chapter
File ISM04 Properties of Colloids I 1 Colloids Small things, but much bigger than a molecule Background: Foundations of Colloid Science Vol I & II, R.J. Hunter Physical Chemistry, P.W. Atkins, Chapter
Chapter (2) Gauss s Law
 Chapter (2) Gauss s Law How you can determine the amount of charge within a closed surface by examining the electric field on the surface! What is meant by electric flux and how you can calculate it. How
Chapter (2) Gauss s Law How you can determine the amount of charge within a closed surface by examining the electric field on the surface! What is meant by electric flux and how you can calculate it. How
Alek Marenich Cramer -Truhlar Group. University of Minnesota
 and Computational Electrochemistry Alek Marenich Cramer -Truhlar Group University of Minnesota Outline Solvation and charge models Energetics and dynamics in solution Computational electrochemistry Future
and Computational Electrochemistry Alek Marenich Cramer -Truhlar Group University of Minnesota Outline Solvation and charge models Energetics and dynamics in solution Computational electrochemistry Future
AP Physics C. Electric Potential and Capacitance. Free Response Problems
 AP Physics C Electric Potential and Capacitance Free Response Problems 1. Two stationary point charges + are located on the y-axis at a distance L from the origin, as shown above. A third charge +q is
AP Physics C Electric Potential and Capacitance Free Response Problems 1. Two stationary point charges + are located on the y-axis at a distance L from the origin, as shown above. A third charge +q is
Notes: Most of the material presented in this chapter is taken from Jackson, Chap. 2, 3, and 4, and Di Bartolo, Chap. 2. 2π nx i a. ( ) = G n.
 Chapter. Electrostatic II Notes: Most of the material presented in this chapter is taken from Jackson, Chap.,, and 4, and Di Bartolo, Chap... Mathematical Considerations.. The Fourier series and the Fourier
Chapter. Electrostatic II Notes: Most of the material presented in this chapter is taken from Jackson, Chap.,, and 4, and Di Bartolo, Chap... Mathematical Considerations.. The Fourier series and the Fourier
Chapter 2 - Water 9/8/2014. Water exists as a H-bonded network with an average of 4 H-bonds per molecule in ice and 3.4 in liquid. 104.
 Chapter 2 - Water Water exists as a -bonded network with an average of 4 -bonds per molecule in ice and 3.4 in liquid. 104.5 o -bond: An electrostatic attraction between polarized molecules containing
Chapter 2 - Water Water exists as a -bonded network with an average of 4 -bonds per molecule in ice and 3.4 in liquid. 104.5 o -bond: An electrostatic attraction between polarized molecules containing
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian. which is given in spherical coordinates by (2)
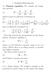 1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
3. Solutions W = N!/(N A!N B!) (3.1) Using Stirling s approximation ln(n!) = NlnN N: ΔS mix = k (N A lnn + N B lnn N A lnn A N B lnn B ) (3.
 3. Solutions Many biological processes occur between molecules in aqueous solution. In addition, many protein and nucleic acid molecules adopt three-dimensional structure ( fold ) in aqueous solution.
3. Solutions Many biological processes occur between molecules in aqueous solution. In addition, many protein and nucleic acid molecules adopt three-dimensional structure ( fold ) in aqueous solution.
Mechanics, Heat, Oscillations and Waves Prof. V. Balakrishnan Department of Physics Indian Institute of Technology, Madras
 Mechanics, Heat, Oscillations and Waves Prof. V. Balakrishnan Department of Physics Indian Institute of Technology, Madras Lecture 05 The Fundamental Forces of Nature In this lecture, we will discuss the
Mechanics, Heat, Oscillations and Waves Prof. V. Balakrishnan Department of Physics Indian Institute of Technology, Madras Lecture 05 The Fundamental Forces of Nature In this lecture, we will discuss the
Electric Charge and the Electrostatic Force
 Electric Charge and the Electrostatic Force Goals and Introduction When two electrically-charged objects are brought near each other, they can either attract or repel, depending on the sign of each of
Electric Charge and the Electrostatic Force Goals and Introduction When two electrically-charged objects are brought near each other, they can either attract or repel, depending on the sign of each of
Lecture 11: Protein Folding & Stability
 Structure - Function Protein Folding: What we know Lecture 11: Protein Folding & Stability 1). Amino acid sequence dictates structure. 2). The native structure represents the lowest energy state for a
Structure - Function Protein Folding: What we know Lecture 11: Protein Folding & Stability 1). Amino acid sequence dictates structure. 2). The native structure represents the lowest energy state for a
Protein Folding & Stability. Lecture 11: Margaret A. Daugherty. Fall Protein Folding: What we know. Protein Folding
 Lecture 11: Protein Folding & Stability Margaret A. Daugherty Fall 2003 Structure - Function Protein Folding: What we know 1). Amino acid sequence dictates structure. 2). The native structure represents
Lecture 11: Protein Folding & Stability Margaret A. Daugherty Fall 2003 Structure - Function Protein Folding: What we know 1). Amino acid sequence dictates structure. 2). The native structure represents
Sunyia Hussain 06/15/2012 ChE210D final project. Hydration Dynamics at a Hydrophobic Surface. Abstract:
 Hydration Dynamics at a Hydrophobic Surface Sunyia Hussain 6/15/212 ChE21D final project Abstract: Water is the universal solvent of life, crucial to the function of all biomolecules. Proteins, membranes,
Hydration Dynamics at a Hydrophobic Surface Sunyia Hussain 6/15/212 ChE21D final project Abstract: Water is the universal solvent of life, crucial to the function of all biomolecules. Proteins, membranes,
12/15/2015. Newton per Coulomb N/C. vector. A model of the mechanism for electrostatic interactions. The Electric Field
 Chapter 15 Lecture The Electric Field A model of the mechanism for electrostatic interactions A model for electric interactions, suggested by Michael Faraday, involves some sort of electric disturbance
Chapter 15 Lecture The Electric Field A model of the mechanism for electrostatic interactions A model for electric interactions, suggested by Michael Faraday, involves some sort of electric disturbance
14 September 2013 On the paper by Loss et al. about stabilizing the toric code by coupling to a scalar field. (arxiv: )
 14 September 2013 On the paper by Loss et al. about stabilizing the toric code by coupling to a scalar field. (arxiv:1309.0621) Consider the Hamiltonian (where w(x) is a classical source for a massless
14 September 2013 On the paper by Loss et al. about stabilizing the toric code by coupling to a scalar field. (arxiv:1309.0621) Consider the Hamiltonian (where w(x) is a classical source for a massless
schematic diagram; EGF binding, dimerization, phosphorylation, Grb2 binding, etc.
 Lecture 1: Noncovalent Biomolecular Interactions Bioengineering and Modeling of biological processes -e.g. tissue engineering, cancer, autoimmune disease Example: RTK signaling, e.g. EGFR Growth responses
Lecture 1: Noncovalent Biomolecular Interactions Bioengineering and Modeling of biological processes -e.g. tissue engineering, cancer, autoimmune disease Example: RTK signaling, e.g. EGFR Growth responses
Saba Al Fayoumi. Tamer Barakat. Dr. Mamoun Ahram + Dr. Diala Abu-Hassan
 1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
Chapter 13: Phenomena
 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 8: E & M (Electricity & Magnetism or Electromagnetism)
 Chapter 8: E & M (Electricity & Magnetism or Electromagnetism) Electric charge & electric force Coulomb s Law Electrons & basic facts about atoms (mainly review) Charge conservation Electric current &
Chapter 8: E & M (Electricity & Magnetism or Electromagnetism) Electric charge & electric force Coulomb s Law Electrons & basic facts about atoms (mainly review) Charge conservation Electric current &
Why is it called a periodic table?
 The Periodic Table Why is it called a periodic table? The properties of the elements in the table repeat in a "periodic" way (specific pattern). Periodic law: There is a periodic repetition of chemical
The Periodic Table Why is it called a periodic table? The properties of the elements in the table repeat in a "periodic" way (specific pattern). Periodic law: There is a periodic repetition of chemical
Phys498BIO; Prof. Paul Selvin Hw #9 Assigned Wed. 4/18/12: Due 4/25/08
 1. Ionic Movements Across a Permeable Membrane: The Nernst Potential. In class we showed that if a non-permeable membrane separates a solution with high [KCl] from a solution with low [KCl], the net charge
1. Ionic Movements Across a Permeable Membrane: The Nernst Potential. In class we showed that if a non-permeable membrane separates a solution with high [KCl] from a solution with low [KCl], the net charge
Physics 240 Fall 2003: Exam #1. Please print your name: Please list your discussion section number: Please list your discussion instructor:
 Physics 4 Fall 3: Exam #1 Please print your name: Please list your discussion section number: Please list your discussion instructor: Form #1 Instructions 1. Fill in your name above. This will be a 1.5
Physics 4 Fall 3: Exam #1 Please print your name: Please list your discussion section number: Please list your discussion instructor: Form #1 Instructions 1. Fill in your name above. This will be a 1.5
32 Electrostatics. Electrostatics involves electric charges, the forces between them, and their behavior in materials.
 Electrostatics involves electric charges, the forces between them, and their behavior in materials. Electrostatics, or electricity at rest, involves electric charges, the forces between them, and their
Electrostatics involves electric charges, the forces between them, and their behavior in materials. Electrostatics, or electricity at rest, involves electric charges, the forces between them, and their
40 46, 51, ,
 cha02680_fm.indd Page xxvi 12/27/12 4:05 PM GG-009 /Volumes/107/GO01228/CHANG_11E/ANCILLARY/CHANG/007_665610_1_P1 BIG IDEA 1: The chemical elements are fundamental building materials of matter, and all
cha02680_fm.indd Page xxvi 12/27/12 4:05 PM GG-009 /Volumes/107/GO01228/CHANG_11E/ANCILLARY/CHANG/007_665610_1_P1 BIG IDEA 1: The chemical elements are fundamental building materials of matter, and all
