5.1 Alkene Nomenclature
|
|
|
- Damian Bennett
- 6 years ago
- Views:
Transcription
1 5.1 Alkene Nomenclature
2 Alkenes Alkenes are hydrocarbons that contain a carbon-carbon double bond also called "olefins" characterized by molecular formula n 2n said to be "unsaturated"
3 Alkene Nomenclature Ethene or Ethylene (both are acceptable IUPA names) Propene (Propylene is sometimes used but is not an acceptable IUPA name)
4 Alkene Nomenclature ) Find the longest continuous chain that includes the double bond. 2) Replace the -ane ending of the unbranched alkane having the same number of carbons by -ene. 3) Number the chain in the direction that gives the lowest number to the doubly bonded carbon.
5 Alkene Nomenclature Butene 1) Find the longest continuous chain that includes the double bond. 2) Replace the -ane ending of the unbranched alkane having the same number of carbons by -ene. 3) Number the chain in the direction that gives the lowest number to the doubly bonded carbon.
6 Alkene Nomenclature 2 2 Br 3 4) If a substituent is present, identify its position by number. The double bond takes precedence over alkyl groups and halogens when the chain is numbered. The compound shown above is 4-bromo-3-methyl-1-butene.
7 Alkene Nomenclature 2 2 O 3 4) If a substituent is present, identify its position by number. ydroxyl groups take precedence over the double bond when the chain is numbered. The compound shown above is 2-methyl-3-buten-1-ol.
8 Alkenyl Groups methylene 2 vinyl 2 allyl 2 2 isopropenyl 2 3
9 ycloalkene Nomenclature yclohexene 1) Replace the -ane ending of the cycloalkane having the same number of carbons by -ene.
10 ycloalkene Nomenclature ) Replace the -ane ending of the cycloalkane having the same number of carbons by -ene. 2) Number through the double bond in the direction that gives the lower number to the first-appearing substituent.
11 ycloalkene Nomenclature 3 6-Ethyl-1-methylcyclohexene 2 3 1) Replace the -ane ending of the cycloalkane having the same number of carbons by -ene. 2) Number through the double bond in the direction that gives the lower number to the first-appearing substituent.
12 5.12 Structure and Bonding in Alkenes
13 Structure of Ethylene bond angles: -- = 117 bond distances: -- = 121 = 110 pm = = 134 pm planar
14 Bonding in Ethylene σ σ σ σ σ Framework of σ bonds Each carbon is sp 2 hybridized
15 Bonding in Ethylene Each carbon has a half-filled filled p orbital
16 Bonding in Ethylene Side-by by-side overlap of half- filled p orbitals gives a π bond
17 5.3 Isomerism in Alkenes
18 Isomers Isomers are different compounds that have the same molecular formula.
19 Isomers onstitutional isomers Stereoisomers
20 Isomers onstitutional isomers different connectivity Stereoisomers same connectivity; different arrangement of atoms in space
21 Isomers onstitutional isomers Stereoisomers consider the isomeric alkenes of molecular formula 4 8
22 Butene 2-Methylpropene cis-2-butene trans-2-butene
23 Butene 2-Methylpropene 3 3 onstitutional isomers cis-2-butene
24 Butene 2-Methylpropene 3 onstitutional isomers 3 trans-2-butene
25 Stereoisomers cis-2-butene trans-2-butene
26 Stereochemical Notation cis (identical or analogous substitutents on same side) trans (identical or analogous substituents on opposite sides)
27 Figure 5.2 Interconversion of stereoisomeric alkenes does not normally occur. Requires that π component of double bond be broken. cis trans
28 Figure 5.2 cis trans
29 5.4 Naming Steroisomeric Alkenes by the E-Z E Z Notational System
30 Stereochemical Notation 3 ( 2 ) ( 2 ) 6 O 2 Oleic acid cis and trans are useful when substituents are identical or analogous (oleic acid has a cis double bond) cis and trans are ambiguous when analogies are not obvious
31 Example l Br F What is needed: 1) systematic body of rules for ranking substituents 2) new set of stereochemical symbols other than cis and trans
32 The E-Z E Z Notational System E : Z : higher ranked substituents on opposite sides higher ranked substituents on same side higher lower
33 The E-Z E Z Notational System E : Z : higher ranked substituents on opposite sides higher ranked substituents on same side lower higher
34 The E-Z E Z Notational System E : Z : higher ranked substituents on opposite sides higher ranked substituents on same side higher lower lower higher Entgegen
35 The E-Z E Z Notational System E : Z : higher ranked substituents on opposite sides higher ranked substituents on same side higher lower higher higher lower higher lower lower Entgegen Zusammen
36 Answer: They are ranked in order of decreasing atomic number. higher The E-Z E Z Notational System Question: ow are substituents ranked? lower higher higher lower higher lower lower Entgegen Zusammen
37 The ahn-ingold Ingold-Prelog (IP) System The system that we use was devised by R. S. ahn Sir hristopher Ingold Vladimir Prelog Their rules for ranking groups were devised in connection with a different kind of stereochemistry one one that we will discuss in hapter 7 but 7 have been adapted to alkene stereochemistry.
38 Table 5.1 IP Rules (1) igher atomic number outranks lower atomic number Br > F l > higher Br l higher lower F lower
39 Table 5.1 IP Rules (1) igher atomic number outranks lower atomic number Br > F l > higher Br l higher lower F lower (Z )-1-Bromo-2-chloro-1-fluoroethene
40 Table 5.1 IP Rules (2) When two atoms are identical, compare the atoms attached to them on the basis of their atomic numbers. Precedence is established at the first point of difference. 2 3 outranks 3 (,,) (,,)
41 Table 5.1 IP Rules (3) Work outward from the point of attachment, comparing all the atoms attached to a particular atom before proceeding further along the chain. ( ( 3 ) 2 outranks 2 2 O (,,),) (,,)
42 Table 5.1 IP Rules (4) Evaluate substituents one by one. Don't add atomic numbers within groups. 2 O outranks ( 3 ) 3 (O,,) (,,)
43 Table 5.1 IP Rules (5) An atom that is multiply bonded to another atom is considered to be replicated as a substituent on that atom. = =O outranks 2 O (O,O,),) (O,,)
44 Table 5.1 IP Rules A table of commonly encountered substituents ranked according to precedence is given on the inside back cover of the text.
Question. Chapter 5 Structure and Preparation of Alkenes (C n H 2n ): Elimination Reactions
 hapter 5 Structure and Preparation of Alkenes ( n 2n ): Elimination Reactions The molecular formula of β-arotene is 40 On catalytic hydrogenation, β-carotene is converted to a saturated hydrocarbon of
hapter 5 Structure and Preparation of Alkenes ( n 2n ): Elimination Reactions The molecular formula of β-arotene is 40 On catalytic hydrogenation, β-carotene is converted to a saturated hydrocarbon of
1. Root of name depends on longest chain of C containing the double bond; ends in "ene"
 Alkenes (β-carotene, an antioxidant pigment) n 2n (acyclic) n 2n-2 (cyclic) R R R R Key features sp 2 -hybridized carbons, 120 o bond angles σ + π bonding between = planar geometry around = "unsaturated"
Alkenes (β-carotene, an antioxidant pigment) n 2n (acyclic) n 2n-2 (cyclic) R R R R Key features sp 2 -hybridized carbons, 120 o bond angles σ + π bonding between = planar geometry around = "unsaturated"
Alkenes and Alkynes 10/27/2010. Chapter 7. Alkenes and Alkynes. Alkenes and Alkynes
 Chapter 7 Alkenes and Alkynes CHP6 Problems: 6.1-13, 16-34, 36. CHP7 Problems: 7.1-23, 25-28, 31-34, 37-39, 41-47, 49-56. Alkenes and Alkynes Alkene (or olefin ) Hydrocarbon that contains a carbon-carbon
Chapter 7 Alkenes and Alkynes CHP6 Problems: 6.1-13, 16-34, 36. CHP7 Problems: 7.1-23, 25-28, 31-34, 37-39, 41-47, 49-56. Alkenes and Alkynes Alkene (or olefin ) Hydrocarbon that contains a carbon-carbon
Chapter 7 Alkenes; Elimination Reactions
 hapter 7 Alkenes; Elimination Reactions Alkenes Alkenes contain a carbon-carbon double bond. They are named as derivatives of alkanes with the suffix -ane changed to -ene. The parent alkane is the longest
hapter 7 Alkenes; Elimination Reactions Alkenes Alkenes contain a carbon-carbon double bond. They are named as derivatives of alkanes with the suffix -ane changed to -ene. The parent alkane is the longest
eg ethylene (IUPAC: ethene), C 2
 Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Alkenes. Alkenes are unsaturated aliphatic hydrocarbons.
 Alkenes Alkenes Each member contains one double covalent bond between two C atoms. Draw condensed structural formulas of first three members of alkenes family. Alkenes are unsaturated aliphatic hydrocarbons.
Alkenes Alkenes Each member contains one double covalent bond between two C atoms. Draw condensed structural formulas of first three members of alkenes family. Alkenes are unsaturated aliphatic hydrocarbons.
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Chapter 24 From Petroleum to Pharmaceuticals
 hapter 24 From Petroleum to Pharmaceuticals 24.1 Petroleum Refining and the ydrocarbons 24.2 Functional Groups and Organic Synthesis 24.3 Pesticides and Pharmaceuticals IR Tutor and Infrared Spectroscopy
hapter 24 From Petroleum to Pharmaceuticals 24.1 Petroleum Refining and the ydrocarbons 24.2 Functional Groups and Organic Synthesis 24.3 Pesticides and Pharmaceuticals IR Tutor and Infrared Spectroscopy
Lecture 9 Organic Chemistry 1
 EM 232 Organic hemistry I at hicago Lecture 9 Organic hemistry 1 Prof. Duncan J. Wardrop 02/09/2010 1 Functional Group larification Although they can be viewed as ethers, epoxides are classified as distinct,
EM 232 Organic hemistry I at hicago Lecture 9 Organic hemistry 1 Prof. Duncan J. Wardrop 02/09/2010 1 Functional Group larification Although they can be viewed as ethers, epoxides are classified as distinct,
Introduction to Alkenes and Alkynes
 Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Unsaturated hydrocarbons. Chapter 13
 Unsaturated hydrocarbons Chapter 13 Unsaturated hydrocarbons Hydrocarbons which contain at least one C-C multiple (double or triple) bond. The multiple bond is a site for chemical reactions in these molecules.
Unsaturated hydrocarbons Chapter 13 Unsaturated hydrocarbons Hydrocarbons which contain at least one C-C multiple (double or triple) bond. The multiple bond is a site for chemical reactions in these molecules.
H H C C. Alkenes C n H 2n unsaturated hydrocarbons. C 2 H 4 ethylene. Functional group = carbon-carbon double bond
 Alkenes C n H 2n unsaturated hydrocarbons C 2 H 4 ethylene H H C C H H Functional group = carbon-carbon double bond sp 2 hybridization => flat, 120 o bond angles σ bond & π bond => H 2 C=CH 2 No rotation
Alkenes C n H 2n unsaturated hydrocarbons C 2 H 4 ethylene H H C C H H Functional group = carbon-carbon double bond sp 2 hybridization => flat, 120 o bond angles σ bond & π bond => H 2 C=CH 2 No rotation
Chapter 12 Alkenes and Alkynes
 BR M 102 lass Notes hapter 12 Page 1 of 8 hapter 12 Alkenes and Alkynes * alkenes = double bonds * alkynes triple bonds * aromatics or arenes alternating double and single bonds such as in benzene * saturated
BR M 102 lass Notes hapter 12 Page 1 of 8 hapter 12 Alkenes and Alkynes * alkenes = double bonds * alkynes triple bonds * aromatics or arenes alternating double and single bonds such as in benzene * saturated
Unsaturated Hydrocarbons
 Unsaturated ydrocarbons hemical Formulas and Unsaturation n n 2n n 2n+2 n 2n+2 hemical Formulas and Unsaturation n n n n 2n n 2n hemical Formulas and Unsaturation ydrocarbons Saturated ydrocarbons Unsaturated
Unsaturated ydrocarbons hemical Formulas and Unsaturation n n 2n n 2n+2 n 2n+2 hemical Formulas and Unsaturation n n n n 2n n 2n hemical Formulas and Unsaturation ydrocarbons Saturated ydrocarbons Unsaturated
Introduction to Alkenes. Structure and Reactivity
 4 4 Introduction to Alkenes. Structure and Reactivity Alkenes are hydrocarbons that contain one or more carbon carbon double bonds. Alkenes are sometimes called olefins, particularly in the chemical industry.
4 4 Introduction to Alkenes. Structure and Reactivity Alkenes are hydrocarbons that contain one or more carbon carbon double bonds. Alkenes are sometimes called olefins, particularly in the chemical industry.
Classes of Alkenes. Alkenes and Alkynes. Saturated compounds (alkanes): Have the maximum number of hydrogen atoms attached to each carbon atom.
 Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
STRUCTURE, ISOMERISM AND NOMENCLATURE OF ORGANIC COMPOUNDS
 II STRUCTURE, ISOMERISM AND NOMENCLATURE OF ORGANIC COMPOUNDS I. OBJECTIVES AND BACKGROUND This exercise will give you an opportunity to experience the three-dimensional nature of molecules and to visualize
II STRUCTURE, ISOMERISM AND NOMENCLATURE OF ORGANIC COMPOUNDS I. OBJECTIVES AND BACKGROUND This exercise will give you an opportunity to experience the three-dimensional nature of molecules and to visualize
Unit 7 Part 1 Introduction to Organic Chemistry Nomenclature and Isomerism in Simple Organic Compounds UNIT 7 INTRODUCTION TO ORGANIC CHEMISTRY
 Unit 7 Part 1 Introduction to Organic hemistry Nomenclature and Isomerism in Simple Organic ompounds UNIT 7 INTRODUTION TO ORGANI EMISTRY PART 1 NOMENLATURE AND ISOMERISM IN SIMPLE ORGANI MOLEULES ontents
Unit 7 Part 1 Introduction to Organic hemistry Nomenclature and Isomerism in Simple Organic ompounds UNIT 7 INTRODUTION TO ORGANI EMISTRY PART 1 NOMENLATURE AND ISOMERISM IN SIMPLE ORGANI MOLEULES ontents
Chapter 6: Alkenes: Structure and Reactivity
 hapter 6: Alkenes: Structure and eactivity overage: 1. Degrees of Unsaturation 2. Nomenclature cis/trans E/Z 3. Alkene Stability 4. Electrophilic Addition eactions/markovinikov s ule 5. The ammond Postulate
hapter 6: Alkenes: Structure and eactivity overage: 1. Degrees of Unsaturation 2. Nomenclature cis/trans E/Z 3. Alkene Stability 4. Electrophilic Addition eactions/markovinikov s ule 5. The ammond Postulate
Reading: Chapter 4 Practice Problems: in text problems and 19 22, 24, 26, 27, 29, 30, 33 35, 39, 40.
 Reading: Chapter Practice Problems: in text problems and 19 22, 24, 26, 27, 29, 30, 33 35, 39, 40. Alkenes: Structure, Nomenclature, Stability, and an Introduction to Reactivity Alkenes are unsaturated
Reading: Chapter Practice Problems: in text problems and 19 22, 24, 26, 27, 29, 30, 33 35, 39, 40. Alkenes: Structure, Nomenclature, Stability, and an Introduction to Reactivity Alkenes are unsaturated
Ch.6 Alkenes: Structure and Reactivity
 alkene = olefin 2 2 Ethylene α-pinene 3 β-arotene (orange pigment and vitamin A precursor) 6.1 Industrial Preparation and Use of Alkenes ompounds derived industrially from ethylene 2 2 Ethylene (26 million
alkene = olefin 2 2 Ethylene α-pinene 3 β-arotene (orange pigment and vitamin A precursor) 6.1 Industrial Preparation and Use of Alkenes ompounds derived industrially from ethylene 2 2 Ethylene (26 million
Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) Can act as weak nucleophiles
 Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Lab Workshop 1: Nomenclature of alkane and cycloalkanes
 Lab Workshop 1: Nomenclature of alkane and cycloalkanes Each student work group choose a Leader (reads activity out loud, poses questions to group), Facilitator (makes sure everyone is participating equally,
Lab Workshop 1: Nomenclature of alkane and cycloalkanes Each student work group choose a Leader (reads activity out loud, poses questions to group), Facilitator (makes sure everyone is participating equally,
Isomerism in Alkanes, Haloalkanes, and Alkenes using Molecular Models
 EXPERIMENT 1 Isomerism in Alkanes, aloalkanes, and Alkenes using Molecular Models Materials Needed - Molecular model kit Relevant Textbook Reading Denniston, chap 11.2-11.4, 12.1-12.3 Background In uncharged,
EXPERIMENT 1 Isomerism in Alkanes, aloalkanes, and Alkenes using Molecular Models Materials Needed - Molecular model kit Relevant Textbook Reading Denniston, chap 11.2-11.4, 12.1-12.3 Background In uncharged,
Chem 121 Winter 2016: Section 03, Sample Problems. Alkenes and Alkynes
 Chem 121 Winter 2016: Section 03, Sample Problems Alkenes and Alkynes Problems adapted from Chemistry - The Central Science, 3 rd edition. 24.2 Consider the hydrocarbon drawn below. (a) What is the hybridisation
Chem 121 Winter 2016: Section 03, Sample Problems Alkenes and Alkynes Problems adapted from Chemistry - The Central Science, 3 rd edition. 24.2 Consider the hydrocarbon drawn below. (a) What is the hybridisation
Organic Chemistry The Functional Group Approach. Organic Chemistry The Functional Group Approach
 Organic Chemistry The Functional Group Approach OH Br alkane (no F.G.) alcohol halide alkene non-polar (grease, fats) O NH alkyne aromatic aldehyde/ketone imine linear flat Organic Chemistry The Functional
Organic Chemistry The Functional Group Approach OH Br alkane (no F.G.) alcohol halide alkene non-polar (grease, fats) O NH alkyne aromatic aldehyde/ketone imine linear flat Organic Chemistry The Functional
Chapter 2. Alkanes and Cycloalkanes; Conformational and Geometrical Isomerism
 Chapter 2 Alkanes and Cycloalkanes; Conformational and Geometrical Isomerism Hydrocarbons are compounds that contain only carbon and hydrogen. There are three main classes of hydrocarbons, based on the
Chapter 2 Alkanes and Cycloalkanes; Conformational and Geometrical Isomerism Hydrocarbons are compounds that contain only carbon and hydrogen. There are three main classes of hydrocarbons, based on the
3.1 Organic: Basic Concepts
 . rganic: Basic oncepts ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds only Unsaturated : ontains a = double bond
. rganic: Basic oncepts ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds only Unsaturated : ontains a = double bond
Chapter 27: Structure and Bonding
 Chapter 27: Structure and Bonding 1 Atomic Orbitals: Wave functions that represent the probability of finding electrons in a specific region of space s, p, d, f orbitals In organic chemistry, need to concentrate
Chapter 27: Structure and Bonding 1 Atomic Orbitals: Wave functions that represent the probability of finding electrons in a specific region of space s, p, d, f orbitals In organic chemistry, need to concentrate
C C. sp 2. π M.O. atomic. orbitals. carbon 1. σ M.O. molecular. orbitals. H C C rotate D. D H zero overlap of p orbitals: π bond broken!
 Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BDE ~ 80 kcal/mol π = BDE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in
Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BDE ~ 80 kcal/mol π = BDE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in
Organic Chemistry Unit #2: Structure of Alkanes, Cycloalkanes, and Alkenes
 Organic hemistry Unit #2: Structure of Alkanes, ycloalkanes, and Alkenes Bring your model kits to class we will to learn to use them! Objectives: by the end of this unit, you should be able to... Interconvert
Organic hemistry Unit #2: Structure of Alkanes, ycloalkanes, and Alkenes Bring your model kits to class we will to learn to use them! Objectives: by the end of this unit, you should be able to... Interconvert
Chem 341 Organic Chemistry I Lecture Summary 16 October 01, 2007
 hem 341 Organic hemistry I Lecture Summary 16 October 01, 2007 hapter 6 - Alkenes: Structure and eactivity Nomenclature Double bond geometry is important in biology. For example, the trans to cis isomerization
hem 341 Organic hemistry I Lecture Summary 16 October 01, 2007 hapter 6 - Alkenes: Structure and eactivity Nomenclature Double bond geometry is important in biology. For example, the trans to cis isomerization
Unsaturated Hydrocarbons
 Interchapter G Unsaturated ydrocarbons The flame from an acetylene torch. Acetylene, which is used extensively in welding, is an example of an unsaturated hydrocarbon. University Science Books, 2011. All
Interchapter G Unsaturated ydrocarbons The flame from an acetylene torch. Acetylene, which is used extensively in welding, is an example of an unsaturated hydrocarbon. University Science Books, 2011. All
Chapter 13 Alkenes and Alkynes & Aromatic Compounds
 Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
A. Structure and Nomenclature. Introduction of Organic Chemistry. Unit 2: Structure of Alkanes, Cycloalkanes, and Alkenes
 Organic hemistry #2 1 Introduction of Organic hemistry. Unit 2: Structure of Alkanes, ycloalkanes, and Alkenes Bring your model kits to class we will to learn to use them! Objectives: by the end of this
Organic hemistry #2 1 Introduction of Organic hemistry. Unit 2: Structure of Alkanes, ycloalkanes, and Alkenes Bring your model kits to class we will to learn to use them! Objectives: by the end of this
Alkenes. Bonding and Structure: Carbons in the double bond of butene are sp 2 hybridized. Side on p-p orbital overlap creates a π-bond.
 Alkenes Bonding and Structure: Carbons in the double bond of butene are sp 2 hybridized. Side on p-p orbital overlap creates a π-bond. Angles around the carbons in the double bond are ~ 120º. Thus, all
Alkenes Bonding and Structure: Carbons in the double bond of butene are sp 2 hybridized. Side on p-p orbital overlap creates a π-bond. Angles around the carbons in the double bond are ~ 120º. Thus, all
Assigning Stereochemistry I What is stereochemistry?
 S. Lievens, March 0 University of alifornia, Davis For use in UDavis hemistry 8/8 Series Assigning Stereochemistry I What is stereochemistry? Types of isomers As organic molecules get larger (more than
S. Lievens, March 0 University of alifornia, Davis For use in UDavis hemistry 8/8 Series Assigning Stereochemistry I What is stereochemistry? Types of isomers As organic molecules get larger (more than
It is possible for organic molecules with the same molecular formula to have different structures
 Isomerism It is possible for organic molecules with the same molecular formula to have different structures Definition- Structural isomers: same molecular formula different structures (or structural formulae)
Isomerism It is possible for organic molecules with the same molecular formula to have different structures Definition- Structural isomers: same molecular formula different structures (or structural formulae)
MOLECULER MODELS/ISOMERS ORGANIC STRUCTURES AND NAMING
 REVISED 10/14 EMISTRY 1101L MOLEULER MODELS/ISOMERS ORGANI STRUTURES AND NAMING NOTE: This lab does not require safety glasses or lab coats. INTRODUTION Electron Dot Structures: Electron dot structures,
REVISED 10/14 EMISTRY 1101L MOLEULER MODELS/ISOMERS ORGANI STRUTURES AND NAMING NOTE: This lab does not require safety glasses or lab coats. INTRODUTION Electron Dot Structures: Electron dot structures,
Alkanes and Cycloalkanes
 Chapter 3 Alkanes and Cycloalkanes Two types Saturated hydrocarbons Unsaturated hydrocarbons 3.1 Alkanes Also referred as aliphatic hydrocarbons General formula: CnH2n+2 (straight chain) and CnH2n (cyclic)
Chapter 3 Alkanes and Cycloalkanes Two types Saturated hydrocarbons Unsaturated hydrocarbons 3.1 Alkanes Also referred as aliphatic hydrocarbons General formula: CnH2n+2 (straight chain) and CnH2n (cyclic)
Alkenes, Alkynes and Aromatic
 BIO-ORGANI EMISTRY (Organic hemistry for Biology Students) (SQBS 1603) Alkenes, Alkynes and Aromatic Dr Nik Ahmad Nizam Bin Nik Malek, BSc (Ind. hem.)(utm), MSc (hem)(utm), PhD (hem)(utm), A.M.I. Senior
BIO-ORGANI EMISTRY (Organic hemistry for Biology Students) (SQBS 1603) Alkenes, Alkynes and Aromatic Dr Nik Ahmad Nizam Bin Nik Malek, BSc (Ind. hem.)(utm), MSc (hem)(utm), PhD (hem)(utm), A.M.I. Senior
Alkanes and Cycloalkanes
 Alkanes and Cycloalkanes Alkanes molecules consisting of carbons and hydrogens in the following ratio: C n H 2n+2 Therefore, an alkane having 4 carbons would have 2(4) + 2 hydrogens, which equals 10 hydrogens.
Alkanes and Cycloalkanes Alkanes molecules consisting of carbons and hydrogens in the following ratio: C n H 2n+2 Therefore, an alkane having 4 carbons would have 2(4) + 2 hydrogens, which equals 10 hydrogens.
BRCC CHM 102 Class Notes Chapter 11 Page 1 of 9
 BRCC CHM 102 Class Notes Chapter 11 Page 1 of 9 Chapter 11 Alkanes and Cycloalkanes hydrocarbons compounds that contain only carbon and hydrogen * 4 families: 1) alkanes only single bonds (includes cycloalkanes)
BRCC CHM 102 Class Notes Chapter 11 Page 1 of 9 Chapter 11 Alkanes and Cycloalkanes hydrocarbons compounds that contain only carbon and hydrogen * 4 families: 1) alkanes only single bonds (includes cycloalkanes)
OCR AS Chemistry A H032 for first assessment in Complete Tutor Notes. Section: Alkenes Boomer Publications
 OR AS hemistry A 032 for first assessment in 206 omplete Tutor Notes www.boomerchemistry.com Section: 4..3 Alkenes E/Z Isomerism Alkenes Addition polymers 205 Boomer Publications page 43 page 45 page 5
OR AS hemistry A 032 for first assessment in 206 omplete Tutor Notes www.boomerchemistry.com Section: 4..3 Alkenes E/Z Isomerism Alkenes Addition polymers 205 Boomer Publications page 43 page 45 page 5
Chapter 3. Alkenes And Alkynes
 Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
AP Chemistry Chapter 22 - Organic and Biological Molecules
 AP Chemistry Chapter - Organic and Biological Molecules.1 Alkanes: Saturated Hydrocarbons A. Straight-chain Hydrocarbons 1. Straight-chain alkanes have the formula C n H n+. Carbons are sp hybridized The
AP Chemistry Chapter - Organic and Biological Molecules.1 Alkanes: Saturated Hydrocarbons A. Straight-chain Hydrocarbons 1. Straight-chain alkanes have the formula C n H n+. Carbons are sp hybridized The
Alkenes. Structure, Nomenclature, and an introduc1on to Reac1vity Thermodynamics and Kine1cs
 Alkenes Structure, Nomenclature, and an introduc1on to Reac1vity Thermodynamics and Kine1cs 1 Alkene - Hydrocarbon With Carbon- Carbon Double Bond Also called an olefin but alkene is be>er Hydrocarbon
Alkenes Structure, Nomenclature, and an introduc1on to Reac1vity Thermodynamics and Kine1cs 1 Alkene - Hydrocarbon With Carbon- Carbon Double Bond Also called an olefin but alkene is be>er Hydrocarbon
3.1 Organic: Basic Concepts
 3.1 rganic: Basic oncepts ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds only Unsaturated : ontains a = double bond
3.1 rganic: Basic oncepts ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds only Unsaturated : ontains a = double bond
Review: Atoms and Orbitals. Electrons = and charged; held
 hapter 1 Review: Atoms and Orbitals 1.1 Molecules are composed of Atoms be broken into smaller, stable units (except by physicists Elements are : Pb Au Atom = Nucleus + Electrons: Nucleus =,, charged core
hapter 1 Review: Atoms and Orbitals 1.1 Molecules are composed of Atoms be broken into smaller, stable units (except by physicists Elements are : Pb Au Atom = Nucleus + Electrons: Nucleus =,, charged core
General formula is CnH2n. Propene. But-1-ene. C-C pi bond. Formation of π bond in alkenes p orbitals Rotation can occur around a sigma bond
 4.1.3 Alkenes Alkenes are unsaturated hydrocarbons General formula is n2n Alkenes contain a carboncarbon double bond somewhere in their structure Ethene Propene Numbers need to be added to the name when
4.1.3 Alkenes Alkenes are unsaturated hydrocarbons General formula is n2n Alkenes contain a carboncarbon double bond somewhere in their structure Ethene Propene Numbers need to be added to the name when
Topic 1.5 INTRODUCTION TO ORGANIC CHEMISTRY. Introduction to Organic Chemistry Nomenclature Isomerism
 Topic 1.5 INTRODUTION TO ORGANI EMISTRY Introduction to Organic hemistry Nomenclature Isomerism 1. arbon compounds INTRODUTION TO ORGANI EMISTRY Organic chemistry is the chemistry of carbon compounds.
Topic 1.5 INTRODUTION TO ORGANI EMISTRY Introduction to Organic hemistry Nomenclature Isomerism 1. arbon compounds INTRODUTION TO ORGANI EMISTRY Organic chemistry is the chemistry of carbon compounds.
Alkenes. Dr. Munther A. M-Ali For 1 st Stage Setudents
 Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
ORGANIC CHEMISTRY- 1
 ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
4.1.3 Alkenes. N Goalby chemrevise.org. Formation of π bond p orbitals C C C C. Alkenes contain a carboncarbon. General formula is CnH2n
 4.1.3 Alkenes Alkenes are unsaturated hydrocarbons General formula is n2n Alkenes contain a carboncarbon double bond somewhere in their structure Ethene Propene Numbers need to be added to the name when
4.1.3 Alkenes Alkenes are unsaturated hydrocarbons General formula is n2n Alkenes contain a carboncarbon double bond somewhere in their structure Ethene Propene Numbers need to be added to the name when
5.5 Physical Properties of Alkenes
 5.5 Physical Properties of Alkenes Dipole moments What is direction of dipole moment? Does a methyl group donate electrons to the double bond, or does it withdraw them? µ = 0 D 3 µ = 0.3 D µ = 1.4 D Dipole
5.5 Physical Properties of Alkenes Dipole moments What is direction of dipole moment? Does a methyl group donate electrons to the double bond, or does it withdraw them? µ = 0 D 3 µ = 0.3 D µ = 1.4 D Dipole
Chem101 General Chemistry. Lecture 11 Unsaturated Hydrocarbons
 hem101 General hemistry Lecture 11 Unsaturated ydrocarbons Unsaturated ydrocarbons ontain one or more double or triple carbon-carbon bond. University of Wisconsin-Eau laire hem101 - Lecture 11 2 Unsaturated
hem101 General hemistry Lecture 11 Unsaturated ydrocarbons Unsaturated ydrocarbons ontain one or more double or triple carbon-carbon bond. University of Wisconsin-Eau laire hem101 - Lecture 11 2 Unsaturated
3.1 Introduction to Organic Chemistry
 3.1 Introduction to Organic hemistry Organic hemistry is the study of carbon chemistry as carbon has the ability to join together in chains, rings, balls etc. arbon also joins with other elements easily
3.1 Introduction to Organic hemistry Organic hemistry is the study of carbon chemistry as carbon has the ability to join together in chains, rings, balls etc. arbon also joins with other elements easily
Alkenes. Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula C n H 2n. Nomenclature
 Alkenes Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula n 2n. Nomenclature Alkenes are named in the same manner as alkanes with the following adjustments. 1. Find the longest
Alkenes Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula n 2n. Nomenclature Alkenes are named in the same manner as alkanes with the following adjustments. 1. Find the longest
CHAPTER 3 ALKENES, ALKYNES & CONJUGATE DIENES
 CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
Chapter 25 Organic and Biological Chemistry
 Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Chemistry 20 Chapters 2 Alkanes
 Chemistry 20 Chapters 2 Alkanes ydrocarbons: a large family of organic compounds and they contain only carbon and hydrogen. ydrocarbons are divided into two groups: 1. Saturated hydrocarbon: a hydrocarbon
Chemistry 20 Chapters 2 Alkanes ydrocarbons: a large family of organic compounds and they contain only carbon and hydrogen. ydrocarbons are divided into two groups: 1. Saturated hydrocarbon: a hydrocarbon
Organic Nomenclature
 University of Puget Sound Department of Chemistry Chem 111 Spring, 2010 Organic Nomenclature LEARNING GOALS AND ASSESSMENTS 1. Be familiar with the structure and nomenclature of organic compounds. a. Identify
University of Puget Sound Department of Chemistry Chem 111 Spring, 2010 Organic Nomenclature LEARNING GOALS AND ASSESSMENTS 1. Be familiar with the structure and nomenclature of organic compounds. a. Identify
Chapter 3 AN INTRODUCTION TO ORGANIC COMPOUNDS NOMENCLATURE, PHYSICAL PROPERTIES, REPRESENTATION OF STRUCTURE AND
 ORGANIC CHEMISTRY, 2 ND EDITION PAULA YURKANIS BRUICE Chapter 3 AN INTRODUCTION TO ORGANIC COMPOUNDS NOMENCLATURE, PHYSICAL PROPERTIES, AND REPRESENTATION OF STRUCTURE RAED M. AL-ZOUBI, ASSISTANT PROFESSOR
ORGANIC CHEMISTRY, 2 ND EDITION PAULA YURKANIS BRUICE Chapter 3 AN INTRODUCTION TO ORGANIC COMPOUNDS NOMENCLATURE, PHYSICAL PROPERTIES, AND REPRESENTATION OF STRUCTURE RAED M. AL-ZOUBI, ASSISTANT PROFESSOR
Organic Chemistry is the chemistry of compounds containing.
 Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
nsaturated Hydrocarbons: Alkenes, Cycloalkenes and Dienes
 240 Chem nsaturated Hydrocarbons: Alkenes, Cycloalkenes and Dienes 1 Chapter 3 Alkenes or Olefines Crabon-Carbon double bond C n H 2n Hybridization in Alkenes: 1.34 A 2 Nomenclature of Alkenes and Cycloalkenes
240 Chem nsaturated Hydrocarbons: Alkenes, Cycloalkenes and Dienes 1 Chapter 3 Alkenes or Olefines Crabon-Carbon double bond C n H 2n Hybridization in Alkenes: 1.34 A 2 Nomenclature of Alkenes and Cycloalkenes
ALKENES. select the longest chain of C atoms containing the double bond; end in ENE
 Alkenes 1 ALKENES Structure form a homologous series of general formula n 2n - non cyclic alkenes only contain a carbon-carbon double bond somewhere in their structure unsaturated hydrocarbons - can still
Alkenes 1 ALKENES Structure form a homologous series of general formula n 2n - non cyclic alkenes only contain a carbon-carbon double bond somewhere in their structure unsaturated hydrocarbons - can still
MOLECULAR MODELS : STEREOISOMERS
 MM.1 MOLEULAR MODELS : STEREOISOMERS Note: No pre-laboratory summary is required for this experiment, but there are some topics you most probably need to review from 351 and you may want to start work
MM.1 MOLEULAR MODELS : STEREOISOMERS Note: No pre-laboratory summary is required for this experiment, but there are some topics you most probably need to review from 351 and you may want to start work
Chapter 20 (part 2) Organic Chemistry
 Chapter 20 (part 2) Organic Chemistry Section 20.7 Alkenes and Alkynes Alkenes: hydrocarbons that contain a carbon carbon double bond. [C n H 2n ] CH 3 CH=CH 2 propene Alkynes: hydrocarbons containing
Chapter 20 (part 2) Organic Chemistry Section 20.7 Alkenes and Alkynes Alkenes: hydrocarbons that contain a carbon carbon double bond. [C n H 2n ] CH 3 CH=CH 2 propene Alkynes: hydrocarbons containing
Chemistry 110. Bettelheim, Brown, Campbell & Farrell. Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes.
 Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes
 Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Organic Chemistry. A. Introduction
 Organic Chemistry A. Introduction 1. Organic chemistry is defined as the chemistry of CARBON compounds. There are a huge number of organic compounds. This results from the fact that carbon forms chains
Organic Chemistry A. Introduction 1. Organic chemistry is defined as the chemistry of CARBON compounds. There are a huge number of organic compounds. This results from the fact that carbon forms chains
Isomerism CH 4 C 2 H 6 C 3 H 8 C 4 H 10 C 5 H 12. Constitutional isomers...
 Isomerism 4 2 6 3 8 4 10 5 12 onstitutional isomers... 3 8 Positional isomers... Functional isomers... ow many constitutional isomers are there for the formula 4 8? arbon atoms are often classified as
Isomerism 4 2 6 3 8 4 10 5 12 onstitutional isomers... 3 8 Positional isomers... Functional isomers... ow many constitutional isomers are there for the formula 4 8? arbon atoms are often classified as
2. Hydrocarbons. 2.1 Composition of Petroleum
 2. Hydrocarbons 2.1 Composition of Petroleum Naturally occurring petroleum is composed of organic chemicals: approximately 11 to 13% hydrogen and 84 to 87% carbon. Traces of oxygen, sulfur, nitrogen and
2. Hydrocarbons 2.1 Composition of Petroleum Naturally occurring petroleum is composed of organic chemicals: approximately 11 to 13% hydrogen and 84 to 87% carbon. Traces of oxygen, sulfur, nitrogen and
Chapter 6 H 2 H 3 C C H CH 3 C H H 2 C C CH 3. (b) =2 H 2 C C C H H C H CH 2 C CH 3 H 3 C C C CH 3. (c) =2
 hapter 6 6.1 alculate the degree of the unsaturation in the following hydrocarbons: 8 14 ; 5 6 (c) 12 20 (d) 20 32 (e) 40 56 =2 =3 (c) =3 (d) =5 (e) =13 6.2 alculate the degree of the unsaturation in the
hapter 6 6.1 alculate the degree of the unsaturation in the following hydrocarbons: 8 14 ; 5 6 (c) 12 20 (d) 20 32 (e) 40 56 =2 =3 (c) =3 (d) =5 (e) =13 6.2 alculate the degree of the unsaturation in the
University of Sydney Chemistry 1B (CHEM1102) Organic Chemistry Lecture Notes
 University of Sydney hemistry 1B (EM1102) rganic hemistry Lecture Notes Topic 1 Introduction & isomers 2 Topic 2 Alkenes, alkynes, arenes 20 Topic 3 Structure determination 27 Topic 4 Alcohols and amines
University of Sydney hemistry 1B (EM1102) rganic hemistry Lecture Notes Topic 1 Introduction & isomers 2 Topic 2 Alkenes, alkynes, arenes 20 Topic 3 Structure determination 27 Topic 4 Alcohols and amines
unsaturated (one or more pi bonds) alkanes alkenes alkynes benzene naming alkanes C 4 H 10 C 5 H 12 C 6 H 14 C 7 H 16 C 8 H 18 C 9 H 20 C 10 H 22
 hapter 4: Alkanes and ycloalkanes [Sections: 4.1-4.14] Basic Organic ompound Nomenclature hydrocarbons: comprised of just carbon and hydrogen saturated (no pi bonds) unsaturated (one or more pi bonds)
hapter 4: Alkanes and ycloalkanes [Sections: 4.1-4.14] Basic Organic ompound Nomenclature hydrocarbons: comprised of just carbon and hydrogen saturated (no pi bonds) unsaturated (one or more pi bonds)
Alkanes and Cycloalkanes
 Alkanes and Cycloalkanes Families of Organic Compounds Organic compounds can be grouped into families by their common structural features We shall survey the nature of the compounds in a tour of the families
Alkanes and Cycloalkanes Families of Organic Compounds Organic compounds can be grouped into families by their common structural features We shall survey the nature of the compounds in a tour of the families
Vision. Cis-trans isomerism is key to vision. How rods work H 3 C CH 3. Protein opsin. 11-cis-retinal. Opsin. Rhodopsin.
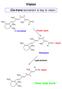 Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
STEREOISOMERISM - GEOMETRICAL ISOMERISM
 STEREOISOMERISM - GEOMETRICAL ISOMERISM Geometric isomerism (also known as cis-trans isomerism or E-Z isomerism) is a form of stereoisomerism. This page explains what stereoisomers are and how you recognise
STEREOISOMERISM - GEOMETRICAL ISOMERISM Geometric isomerism (also known as cis-trans isomerism or E-Z isomerism) is a form of stereoisomerism. This page explains what stereoisomers are and how you recognise
UNIT (7) ORGANIC COMPOUNDS: HYDROCARBONS
 UNIT (7) RGANI MPUNDS: YDRARBNS rganic chemistry is the study carbon containing compounds. 7.1 Bonding in rganic ompounds rganic compounds are made up of only a few elements and the bonding is almost entirely
UNIT (7) RGANI MPUNDS: YDRARBNS rganic chemistry is the study carbon containing compounds. 7.1 Bonding in rganic ompounds rganic compounds are made up of only a few elements and the bonding is almost entirely
Common Elements in Organic Compounds
 Organic hemistry ommon Elements in Organic ompounds lassification of ydrocarbons Alkanes Alkanes have the general formula n 2n+2 where n = 1,2,3, only single covalent bonds saturated hydrocarbons because
Organic hemistry ommon Elements in Organic ompounds lassification of ydrocarbons Alkanes Alkanes have the general formula n 2n+2 where n = 1,2,3, only single covalent bonds saturated hydrocarbons because
Chapters 2 & 25: Covalent bonds & Organic Chemistry
 hapters 2 & 25: ovalent bonds & Organic hemistry Read: BLB 2.6, 2.9; 25.1-25.4 (only nomenclature in Table 25.1, NOT reactions) W: BLB 2:43, 45, 69, 76, 77 BLB 25:11, 12, 25, 40a, c-f Packet Organic:1
hapters 2 & 25: ovalent bonds & Organic hemistry Read: BLB 2.6, 2.9; 25.1-25.4 (only nomenclature in Table 25.1, NOT reactions) W: BLB 2:43, 45, 69, 76, 77 BLB 25:11, 12, 25, 40a, c-f Packet Organic:1
ISOMERISM - A general survey
 Isomerism 1 ISOMERISM - A general survey STRUTURAL ISOMERS have the same molecular formula but different structural formulae They occur due to variations in... the carbon skeleton AIN ISOMERISM 2 2 positions
Isomerism 1 ISOMERISM - A general survey STRUTURAL ISOMERS have the same molecular formula but different structural formulae They occur due to variations in... the carbon skeleton AIN ISOMERISM 2 2 positions
Hydrogen iodide is a strong acid and will drive the reverse reaction, meaning the forward reaction will not occur.
 EM 261 Oct 18, 2018 Photosynthesis and Related Reactions O 2 2 O 6 12 O 6 2 O N 3, S, Fe, u, o, other Natural Products D-Glucose R O R OR Ionic substitution S N 1 & R X X 2 hv Petroleum/ Alkanes R N 2
EM 261 Oct 18, 2018 Photosynthesis and Related Reactions O 2 2 O 6 12 O 6 2 O N 3, S, Fe, u, o, other Natural Products D-Glucose R O R OR Ionic substitution S N 1 & R X X 2 hv Petroleum/ Alkanes R N 2
Chapter 2 Alkanes and Cycloalkanes: Introduction to Hydrocarbons
 Chapter 2 Alkanes and Cycloalkanes: Introduction to Hydrocarbons 2.1 Classes of Hydrocarbons Classes of Hydrocarbons Hydrocarbons only contain carbon and hydrogen atoms. Hydrocarbons are either classed
Chapter 2 Alkanes and Cycloalkanes: Introduction to Hydrocarbons 2.1 Classes of Hydrocarbons Classes of Hydrocarbons Hydrocarbons only contain carbon and hydrogen atoms. Hydrocarbons are either classed
ORGANIC CHEMISTRY. Classification of organic compounds
 ORGANIC CHEMISTRY Organic chemistry is very important branch of chemistry and it study the compounds which contain carbon (C) and hydrogen (H), in general, and may contains other atoms such as oxygen (O),
ORGANIC CHEMISTRY Organic chemistry is very important branch of chemistry and it study the compounds which contain carbon (C) and hydrogen (H), in general, and may contains other atoms such as oxygen (O),
Chapter 4 - Nomenclature and Conformations of Alkanes and Cycloalkanes 1
 Andrew Rosen Chapter 4 - Nomenclature and Conformations of Alkanes and Cycloalkanes 1 4.1 - Introduction to Alkanes and Cycloalkanes - Alkanes are hydrocarbons with all carbon-carbon single bonds - Alkenes
Andrew Rosen Chapter 4 - Nomenclature and Conformations of Alkanes and Cycloalkanes 1 4.1 - Introduction to Alkanes and Cycloalkanes - Alkanes are hydrocarbons with all carbon-carbon single bonds - Alkenes
Chemistry 123: Physical and Organic Chemistry Topic 1: Organic Chemistry
 Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
The carbon-carbon double bond is the distinguishing feature of alkenes.
 Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Chapter 13 Alkenes and Alkynes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 13 Alkenes and Alkynes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 13 Alkenes and Alkynes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
Chapter 3: Structure and Nomenclature of Organic Compounds Focus on Alkanes
 hapter 3: Structure and Nomenclature of rganic ompounds Focus on Alkanes rganic molecules are composed of one or more functional groups attached to one or more hydrocarbon groups (alkyl or groups) I. Functional
hapter 3: Structure and Nomenclature of rganic ompounds Focus on Alkanes rganic molecules are composed of one or more functional groups attached to one or more hydrocarbon groups (alkyl or groups) I. Functional
Chapter 12: Unsaturated Hydrocarbons
 Chapter 12: Unsaturated Hydrocarbons UNSATURATED HYDROCARBONS contain carbon-carbon multiple bonds. Alkenes C=C double bonds Alkynes triple bonds Aromatics benzene rings 1 2 NAMING ALKENES Step 1: Name
Chapter 12: Unsaturated Hydrocarbons UNSATURATED HYDROCARBONS contain carbon-carbon multiple bonds. Alkenes C=C double bonds Alkynes triple bonds Aromatics benzene rings 1 2 NAMING ALKENES Step 1: Name
Chapter 11. Introduction to Organic Chemistry
 hapter 11 Introduction to rganic hemistry Properties of arbon and its compounds 2 Properties of arbon and its compounds 3 Properties of arbon and its compounds 4 Properties of arbon and its compounds 5
hapter 11 Introduction to rganic hemistry Properties of arbon and its compounds 2 Properties of arbon and its compounds 3 Properties of arbon and its compounds 4 Properties of arbon and its compounds 5
CHEM 203 Exam 1. Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 CHEM 203 Exam 1 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following elements is a large percentage of both the earth's
CHEM 203 Exam 1 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following elements is a large percentage of both the earth's
Organic Chemistry, Second Edition. Janice Gorzynski Smith University of Hawai i. Chapter 10 Alkenes
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
3. Organic Compounds: Alkanes and Cycloalkanes
 3. Organic Compounds: Alkanes and Cycloalkanes Based on McMurry s Organic Chemistry, 6 th edition, Chapter 3 2003 Ronald Kluger Department of Chemistry University of Toronto 1 Families of Organic Compounds!
3. Organic Compounds: Alkanes and Cycloalkanes Based on McMurry s Organic Chemistry, 6 th edition, Chapter 3 2003 Ronald Kluger Department of Chemistry University of Toronto 1 Families of Organic Compounds!
Chemistry 11. Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons
 Chemistry 11 Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons 2 1. Unsaturated hydrocarbons So far, we have studied the hydrocarbons in which atoms are connected exclusively by
Chemistry 11 Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons 2 1. Unsaturated hydrocarbons So far, we have studied the hydrocarbons in which atoms are connected exclusively by
Chapter 3 Alkenes and Alkynes. Excluded sections 3.15&3.16
 Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
There are several ways to draw an organic compound, mainly being display formulae, 3D structure and skeletal structure.
 Introduction The basis of Organic Chemistry is the fact that Carbon molecules can produce long chains which can produce a number of different molecules. The reason why Carbon is such an ideal building
Introduction The basis of Organic Chemistry is the fact that Carbon molecules can produce long chains which can produce a number of different molecules. The reason why Carbon is such an ideal building
