ISOMERISM - A general survey
|
|
|
- Beatrice Freeman
- 5 years ago
- Views:
Transcription
1 Isomerism 1 ISOMERISM - A general survey STRUTURAL ISOMERS have the same molecular formula but different structural formulae They occur due to variations in... the carbon skeleton AIN ISOMERISM 2 2 positions of a functional group on a chain POSITION ISOMERISM 2 2 l l 2 l l l relative positions on a benzene ring functional group FUNTIONAL GROUP ISOMERISM l O l l O Differences between isomers Boiling Point straight chain isomers have higher boiling points than branched chain isomers the greater the degree of branching the lower the boiling point branching decreases the effectiveness of intermolecular attractive forces less energy has to be put in to separate the molecules boiling points also vary between isomers containing different functional groups e.g alcohols and ethers - due to permanent dipole-dipole interactions or hydrogen bonding. hemical properties Most isomers show similar chemical properties if the same functional group is present. owever, it is best to have a look at each structure and apply any knowledge of the chemical reactions of the compounds in question.
2 2 Isomerism E/Z ISOMERISM Occurrence a form of stereoisomerism. found in alkenes, it occurs due to the restricted rotation of = double bonds certain forms are known as IS and TRANS to occur, there must be two different groups/atoms on one end of the = and two different groups/atoms on the other end 2 different groups 2 different groups on this end on this end is-trans if there are two s and two non-hydrogen groups attached to each carbon IS groups on the same side of the double bond TRANS groups are across the double bond Both molecules have the double bond in the same position but the atoms occupy different positions within space. Quick check are two similar atoms, or groups of atoms attached to the same end of the =? if so you will not get E/Z isomers A B A and B are E/Z isomers, isn t. It is a structural isomer of the other two. E/Z a newer system based on the masses of groups/atoms attached to the = IP rules are used to allocate priority the heavier the atom / group attached to the = bond, the higher its priority... higher mass I > > l > F > lower mass = = higher priority 7 > 2 5 > > lower priority Z ZUSAMMEN igher priority groups are on the same side of = E ENTGEGEN igher priority groups are on opposite sides of =
3 Isomerism IP RULES they work out if a geometrical isomer is E or Z named after the people who developed the system... ahn-ingold-prelog groups/atoms either side of the = are prioritised according to their mass higher mass number = higher priority 4-methylheptane higher priorities are on the same side of the = (Z) isomer (15) (1) 7 (4) 2 5 (29) 7 (4) (29) 2 5 higher priorities are on the same side of the = (E) isomer Properties E/Z isomers have different physical properties (e.g. boiling point) and sometimes react differently in certain chemical reactions. Q.1 Work out all the possible structural isomers of pentene 5 10 and hexene ow many exhibit E/Z isomerism? OPTIAL ISOMERISM Occurrence another form of stereoisomerism occurs when compounds have... non-superimposable mirror images B D A A D B Existence the two different forms are known as OPTIAL ISOMERS or ENANTIOMERS and occur when molecules have a IRAL centre. to find such a centre, look for an ASYMMETRI ARBON ATOM... one with four different atoms, or arranged tetrahedrally around it. example 5 2 O two forms exist which are non-superimposable mirror images of each other; i.e. you can t stack one form exactly on top of the other. Difference isomers differ in their reaction to plane-polarised light one isomer rotates light to the right, the other to the left rotation of light is measured using a polarimeter
4 4 Isomerism A POLARIMETER Light source Polarising filter produces plane polarised light Filter is rotated until the light appears brightest Sample under examination rotation is measured by observing the polarised light as it emerges towards the observer. If the light appears to have... turned to the right turned to the left DEXTROROTATORY LAEVOROTATORY d or + form l or - form Racemate a mixture of the two enantiomers (dl) or (±) is a racemic mixture the opposite optical effects of each isomer cancel each other out Examples Optical activity is widespread in nature, biochemistry and pharmaceuticals. e.g. OO N 2 OO O 2-aminopropanoic acid 2-hydroxypropanoic acid (alanine) (lactic acid) The drug thalidomide is optically active but only one of the optical isomers is effective. Many years ago women gave birth to babies with abnormalities caused by taking thalidomide tablets which contained some of the wrong enantiomer. Practical problems laboratory reactions are more likely to make mixtures than those in the body a larger dose will be needed if a drug contains a mixture of enantiomers the non-reactive isomer may be dangerous (as in thalidomide)
5 Isomerism 5 Q.2 ow many structural isomers of 6 14 are optically active? ow many structural isomers of butanol, 4 9 O, are optically active? Q. Which of the following can exist as enantiomers? a) 2-bromopropane b) 2-bromobutane c) 2-bromopentane d) -bromopentane e) (O) 2 5 f) (O) Q.4 Why is there the possibility of enantiomers being formed when butanone undergoes nucleophilic addition with N? Do all carbonyl compounds produce a mixture of products with N? If not, why not? ISOMERISM STRUTURAL ISOMERS Same molecular formula - different structural formula STEREOISOMERS Same structural formula, different arrangement of groups in space. E/Z (cis-trans) ISOMERS Groups fixed in different space as a result of the restricted rotation of double bonds etc. OPTIAL ISOMERS Groups fixed in different space as a result of the asymmetry of the structure. Non-superimposable mirror images of each other.
It is possible for organic molecules with the same molecular formula to have different structures
 Isomerism It is possible for organic molecules with the same molecular formula to have different structures Definition- Structural isomers: same molecular formula different structures (or structural formulae)
Isomerism It is possible for organic molecules with the same molecular formula to have different structures Definition- Structural isomers: same molecular formula different structures (or structural formulae)
OPTICAL ISOMERISM UNIT-1
 OPTICAL ISOMERISM UNIT-1 K.Anita priyadharshini, Lecturer, Dept.of Pharmaceutical Chemistry, SRM College of Pharmacy TYPES OF ISOMERISM CHAIN ISOMERISM STRUCTURAL ISOMERISM Same molecular formula but different
OPTICAL ISOMERISM UNIT-1 K.Anita priyadharshini, Lecturer, Dept.of Pharmaceutical Chemistry, SRM College of Pharmacy TYPES OF ISOMERISM CHAIN ISOMERISM STRUCTURAL ISOMERISM Same molecular formula but different
Optical Isomerism. Types of isomerism. chemrevise.org 20/08/2013. N Goalby Chemrevise.org. Isomerism. Structural isomerism.
 ptical Isomerism N Goalby hemrevise.org Types of isomerism Isomerism Structural isomerism Stereoisomerism Geometric isomerism ptical isomerism 1 ptical Isomerism ptical isomerism occurs in carbon compounds
ptical Isomerism N Goalby hemrevise.org Types of isomerism Isomerism Structural isomerism Stereoisomerism Geometric isomerism ptical isomerism 1 ptical Isomerism ptical isomerism occurs in carbon compounds
Organic: module 4 revision guide. Basic definitions to know. Drawing Displayed formulae
 opyright Goalby Bancroft's School rganic: module 4 revision guide ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds
opyright Goalby Bancroft's School rganic: module 4 revision guide ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds
CH 3 C 2 H 5. Tetrahedral Stereochemistry
 Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
PRESENTATION ISOMERISM. Dr. Susmita Bajpai
 PRESENTATION OF ISOMERISM Dr. Susmita Bajpai Department Chemistry B.N.D. College, Kanpur ISOMERISM What is isomerism:- The compounds which have the some molecular formula but differ from each other in
PRESENTATION OF ISOMERISM Dr. Susmita Bajpai Department Chemistry B.N.D. College, Kanpur ISOMERISM What is isomerism:- The compounds which have the some molecular formula but differ from each other in
02/07/2017. Isomerism. Structural isomerism. 1. Structural isomerism different linkages of atoms. Same molecular formula Different structural formulae
 hain isomerism Position isomerism Metamerism Tautomerism Functional group isomerism Geometrical isomerism Optical isomerism 02/07/2017 Isomerism The presence of two or more compounds which has the same
hain isomerism Position isomerism Metamerism Tautomerism Functional group isomerism Geometrical isomerism Optical isomerism 02/07/2017 Isomerism The presence of two or more compounds which has the same
Lecture 4: 12.4 Isomerism
 Lecture 4: 12.4 Isomerism Learning Outcomes: At the end of the lesson the students should be able to : Define isomerism. Explain constitutional isomerism. chain isomers positional isomers functional group
Lecture 4: 12.4 Isomerism Learning Outcomes: At the end of the lesson the students should be able to : Define isomerism. Explain constitutional isomerism. chain isomers positional isomers functional group
Stereochemistry. Based on McMurry s Organic Chemistry, 6 th edition
 Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry! Some objects are not the same as their mirror images (technically, they have no plane of symmetry)! A right-hand glove
geometric isomers (diastereomers)
 Symmetry Monarch butterfly: bilateral symmetry= mirror symmetry Whenever winds blow butterflies find a new place on the willow tree -Basho (~6-69) 5 hapter 7: Stereochemistry - three-dimensional arrangement
Symmetry Monarch butterfly: bilateral symmetry= mirror symmetry Whenever winds blow butterflies find a new place on the willow tree -Basho (~6-69) 5 hapter 7: Stereochemistry - three-dimensional arrangement
3.7 Organic naming and Isomerism continued
 3.7 rganic naming and Isomerism continued See chapter 3.1 for basic naming of organic molecules. This chapter extends the naming for functional groups met in next few chapters homologous series functional
3.7 rganic naming and Isomerism continued See chapter 3.1 for basic naming of organic molecules. This chapter extends the naming for functional groups met in next few chapters homologous series functional
CHAPTER 26 STEREOISOMERISM SOLUTIONS TO REVIEW QUESTIONS. ƒ C Cl ƒ
 EINS26-400-417.v1.qxd 11/9/07 1:13 PM Page 400 APTER 26 STEREOISOMERISM SOLUTIONS TO REVIEW QUESTIONS 1. A chiral carbon atom is one to which four different atoms or groups are attached and is a center
EINS26-400-417.v1.qxd 11/9/07 1:13 PM Page 400 APTER 26 STEREOISOMERISM SOLUTIONS TO REVIEW QUESTIONS 1. A chiral carbon atom is one to which four different atoms or groups are attached and is a center
Lecture Topics: I. Stereochemistry Stereochemistry is the study of the three dimensional structure of molecules
 Stereochemistry eading: Wade chapter 5, sections 5-- 5-7 Study Problems: 5-26, 5-3, 5-32, 5-33, 5-34 Key oncepts and Skills: assify molecules as chiral or achiral, and identify planes of symmetry. Identify
Stereochemistry eading: Wade chapter 5, sections 5-- 5-7 Study Problems: 5-26, 5-3, 5-32, 5-33, 5-34 Key oncepts and Skills: assify molecules as chiral or achiral, and identify planes of symmetry. Identify
Organic Chemistry HL IB CHEMISTRY HL
 Organic Chemistry HL IB CHEMISTRY HL Understandings: Nucleophilic Substitution Reactions: SN1 represents a nucleophilic unimolecular substitution reaction and SN2 represents a nucleophilic bimolecular
Organic Chemistry HL IB CHEMISTRY HL Understandings: Nucleophilic Substitution Reactions: SN1 represents a nucleophilic unimolecular substitution reaction and SN2 represents a nucleophilic bimolecular
Assigning Stereochemistry I What is stereochemistry?
 S. Lievens, March 0 University of alifornia, Davis For use in UDavis hemistry 8/8 Series Assigning Stereochemistry I What is stereochemistry? Types of isomers As organic molecules get larger (more than
S. Lievens, March 0 University of alifornia, Davis For use in UDavis hemistry 8/8 Series Assigning Stereochemistry I What is stereochemistry? Types of isomers As organic molecules get larger (more than
Empirical formula: shows the simplest whole number ratio of atoms of each element in the compound
 3.7 rganic naming and Isomerism ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds only Unsaturated : ontains a = double
3.7 rganic naming and Isomerism ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds only Unsaturated : ontains a = double
3.1 Organic: Basic Concepts
 3.1 rganic: Basic oncepts ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds only Unsaturated : ontains a = double bond
3.1 rganic: Basic oncepts ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds only Unsaturated : ontains a = double bond
ALKENES. select the longest chain of C atoms containing the double bond; end in ENE
 Alkenes 1 ALKENES Structure form a homologous series of general formula n 2n - non cyclic alkenes only contain a carbon-carbon double bond somewhere in their structure unsaturated hydrocarbons - can still
Alkenes 1 ALKENES Structure form a homologous series of general formula n 2n - non cyclic alkenes only contain a carbon-carbon double bond somewhere in their structure unsaturated hydrocarbons - can still
MOLECULAR MODELS : STEREOISOMERS
 MM.1 MOLEULAR MODELS : STEREOISOMERS Note: No pre-laboratory summary is required for this experiment, but there are some topics you most probably need to review from 351 and you may want to start work
MM.1 MOLEULAR MODELS : STEREOISOMERS Note: No pre-laboratory summary is required for this experiment, but there are some topics you most probably need to review from 351 and you may want to start work
C 4 H 10 O. butanol. diethyl ether. different carbon skeleton different functional group different position of FG
 hapter 5: Stereoisomerism- three-dimensional arrangement of atoms (groups) in space 5. verview of Isomerism Isomers: different chemical compounds with the same formula onstitutional isomers: same formula,
hapter 5: Stereoisomerism- three-dimensional arrangement of atoms (groups) in space 5. verview of Isomerism Isomers: different chemical compounds with the same formula onstitutional isomers: same formula,
240 Chem. Stereochemistry. Chapter 5
 240 Chem Stereochemistry Chapter 5 1 Isomerism Isomers are different compounds that have the same molecular formula. Constitutional isomers are isomers that differ because their atoms are connected in
240 Chem Stereochemistry Chapter 5 1 Isomerism Isomers are different compounds that have the same molecular formula. Constitutional isomers are isomers that differ because their atoms are connected in
Chem!stry. Optical Isomerism
 hem!stry ame: ( ) lass: Date: / / ptical Isomerism ptical isomers are compounds that share the same molecular formula, but which rotate plane polarised light in opposite directions, either clockwise or
hem!stry ame: ( ) lass: Date: / / ptical Isomerism ptical isomers are compounds that share the same molecular formula, but which rotate plane polarised light in opposite directions, either clockwise or
E30 ENANTIOMERS Chirality in organic chemistry
 E30 ENANTIMERS hirality in organic chemistry TE TASK To investigate the nature of chirality in organic chemistry. TE SKILLS By the end of the experiment you should be able to: use molecular modelling kits
E30 ENANTIMERS hirality in organic chemistry TE TASK To investigate the nature of chirality in organic chemistry. TE SKILLS By the end of the experiment you should be able to: use molecular modelling kits
Chapter 5 Stereochemistry. Stereoisomers
 Chapter 5 Stereochemistry Stereoisomers Same bonding sequence Different arrangement in space Example: OOC-C=C-COO has two geometric (cis-trans) isomers: COO COO COO COO Stereochemistry Slide 5-2 1 Chirality
Chapter 5 Stereochemistry Stereoisomers Same bonding sequence Different arrangement in space Example: OOC-C=C-COO has two geometric (cis-trans) isomers: COO COO COO COO Stereochemistry Slide 5-2 1 Chirality
10/4/2010. Sequence Rules for Specifying Configuration. Sequence Rules for Specifying Configuration. 5.5 Sequence Rules for Specifying.
 5.5 Sequence Rules for Specifying Configuration Configuration The three-dimensional arrangement of substituents at a chirality center Sequence rules to specify the configuration of a chirality center:
5.5 Sequence Rules for Specifying Configuration Configuration The three-dimensional arrangement of substituents at a chirality center Sequence rules to specify the configuration of a chirality center:
Stereochemistry Structural or constitutional isomers... have the same molecular formula but different connectivity (skeletal, positional, functional)
 Stereochemistry Structural or constitutional isomers... have the same molecular formula but different connectivity (skeletal, positional, functional) Stereoisomers... have the same connectivity but a different
Stereochemistry Structural or constitutional isomers... have the same molecular formula but different connectivity (skeletal, positional, functional) Stereoisomers... have the same connectivity but a different
Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop
 Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop What are isomers? Isomers are molecules with the same molecular formula, but different arrangements of atoms. There are different types
Chemistry 102 Organic Chemistry: Introduction to Isomers Workshop What are isomers? Isomers are molecules with the same molecular formula, but different arrangements of atoms. There are different types
Stereochemistry CHAPTER SUMMARY
 2 7 2 7. Introduction APTER SUMMARY Isomers are compounds with identical molecular formulas but different structural formulas. Structural or constitutional isomers differ in the bonding arrangement of
2 7 2 7. Introduction APTER SUMMARY Isomers are compounds with identical molecular formulas but different structural formulas. Structural or constitutional isomers differ in the bonding arrangement of
For more info visit
 Bond Fission: a) Homolytic fission: Each atom separates with one electron, leading to the formation of highly reactive entities called radicals, owing their reactivity to their unpaired electron. b) Heterolytic
Bond Fission: a) Homolytic fission: Each atom separates with one electron, leading to the formation of highly reactive entities called radicals, owing their reactivity to their unpaired electron. b) Heterolytic
Chemistry M11 Spring 2010 Examination #3 ANSWER KEY pp. 1
 Chemistry M11 Spring 2010 Examination #3 ASWER KEY pp. 1 or Questions 1 2, consider the hydrogenation of trans-3-methyl-2-pentene in the presence of a transition metal catalyst. 1. C Determine the major
Chemistry M11 Spring 2010 Examination #3 ASWER KEY pp. 1 or Questions 1 2, consider the hydrogenation of trans-3-methyl-2-pentene in the presence of a transition metal catalyst. 1. C Determine the major
Stereochemistry. Conformers: Compounds that differ by orientation of atoms in space. They are interconvertible via rotation about single bonds.
 Stereochemistry Terms onformers: ompounds that differ by orientation of atoms in space. They are interconvertible via rotation about single bonds. onstitutional isomers (also called structural isomers):
Stereochemistry Terms onformers: ompounds that differ by orientation of atoms in space. They are interconvertible via rotation about single bonds. onstitutional isomers (also called structural isomers):
Question. Chapter 5 Structure and Preparation of Alkenes (C n H 2n ): Elimination Reactions
 hapter 5 Structure and Preparation of Alkenes ( n 2n ): Elimination Reactions The molecular formula of β-arotene is 40 On catalytic hydrogenation, β-carotene is converted to a saturated hydrocarbon of
hapter 5 Structure and Preparation of Alkenes ( n 2n ): Elimination Reactions The molecular formula of β-arotene is 40 On catalytic hydrogenation, β-carotene is converted to a saturated hydrocarbon of
Topic 5 Stereochemistry and optical isomers Isomerism
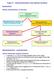 Topic 5 Stereochemistry and optical isomers Isomerism Recap lassification of isomers same molecular formula onstitutional Different nature/sequence of bonds Stereoisomers Different arrangement of groups
Topic 5 Stereochemistry and optical isomers Isomerism Recap lassification of isomers same molecular formula onstitutional Different nature/sequence of bonds Stereoisomers Different arrangement of groups
Amides, Amino acids and Chirality
 R hemistry A 432 Amides, Amino Acids & hirality Amides, Amino acids and hirality aming of Amides The amide functional group consists of a carbonyl group bonded to the nitrogen of an amine. Like amines,
R hemistry A 432 Amides, Amino Acids & hirality Amides, Amino acids and hirality aming of Amides The amide functional group consists of a carbonyl group bonded to the nitrogen of an amine. Like amines,
Lesson 4. Molecular Geometry and Isomers II. Lesson 4 CH 3 HO H OH
 Lesson 4 Molecular Geometry and Isomers II 4 Lesson 4 3 O O 3 Organic Edge A. Structural Isomers (onstitutional Isomers) 1. Structural isomers are molecules that share the same molecular formula but differ
Lesson 4 Molecular Geometry and Isomers II 4 Lesson 4 3 O O 3 Organic Edge A. Structural Isomers (onstitutional Isomers) 1. Structural isomers are molecules that share the same molecular formula but differ
Chapter 6. Isomers and Stereochemistry
 Chapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
Chapter 6. Isomers and Stereochemistry Learning objectives: 1. Differentiate chiral and achiral molecules. 2. Recognize and draw structural isomers (constitutional isomers), stereoisomers including enantiomers
Stereochemistry. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects.
 Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers
Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 6. Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry Some objects
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 6. Stereochemistry Based on McMurry s Organic Chemistry, 6 th edition Stereochemistry Some objects
Isomerism. Introduction
 Isomerism Introduction The existence of two or more compounds with same molecular formula but different properties (physical, chemical or both) is known as isomerism; and the compounds themselves are called
Isomerism Introduction The existence of two or more compounds with same molecular formula but different properties (physical, chemical or both) is known as isomerism; and the compounds themselves are called
OCR AS Chemistry A H032 for first assessment in Complete Tutor Notes. Section: Alkenes Boomer Publications
 OR AS hemistry A 032 for first assessment in 206 omplete Tutor Notes www.boomerchemistry.com Section: 4..3 Alkenes E/Z Isomerism Alkenes Addition polymers 205 Boomer Publications page 43 page 45 page 5
OR AS hemistry A 032 for first assessment in 206 omplete Tutor Notes www.boomerchemistry.com Section: 4..3 Alkenes E/Z Isomerism Alkenes Addition polymers 205 Boomer Publications page 43 page 45 page 5
Name. Optical Isomers
 Name KEY Lab Day Optical Isomers Introduction: Stereoisomers are compounds that have the same structural formulas, but differ in their spatial arrangements. Two major types of stereoisomers are geometric
Name KEY Lab Day Optical Isomers Introduction: Stereoisomers are compounds that have the same structural formulas, but differ in their spatial arrangements. Two major types of stereoisomers are geometric
Worksheet Chapter 10: Organic chemistry glossary
 Worksheet 10.1 Chapter 10: Organic chemistry glossary Addition elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is
Worksheet 10.1 Chapter 10: Organic chemistry glossary Addition elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is
Stereochemistry. 3-dimensional Aspects of Tetrahedral Atoms
 Stereochemistry 3-dimensional Aspects of Tetrahedral Atoms Chiral Entire molecules or simply atoms that do not possess a plane of symmetry are called chiral. Conversely, the term achiral is applied to
Stereochemistry 3-dimensional Aspects of Tetrahedral Atoms Chiral Entire molecules or simply atoms that do not possess a plane of symmetry are called chiral. Conversely, the term achiral is applied to
CHEM 263 Oct 18, Do they have the same molecular formula?
 EM 263 ct 8, 206 To compare the relationship of 2 structures: Do they have the same molecular formula? o ot isomers Do they have the same sequence of atoms (i.e. connectivity)? o onstitutional or tructural
EM 263 ct 8, 206 To compare the relationship of 2 structures: Do they have the same molecular formula? o ot isomers Do they have the same sequence of atoms (i.e. connectivity)? o onstitutional or tructural
3.1 Organic: Basic Concepts
 . rganic: Basic oncepts ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds only Unsaturated : ontains a = double bond
. rganic: Basic oncepts ydrocarbon is a compound consisting of hydrogen and carbon only Basic definitions to know Saturated: ontain single carbon-carbon bonds only Unsaturated : ontains a = double bond
Isomerism in Alkanes, Haloalkanes, and Alkenes using Molecular Models
 EXPERIMENT 1 Isomerism in Alkanes, aloalkanes, and Alkenes using Molecular Models Materials Needed - Molecular model kit Relevant Textbook Reading Denniston, chap 11.2-11.4, 12.1-12.3 Background In uncharged,
EXPERIMENT 1 Isomerism in Alkanes, aloalkanes, and Alkenes using Molecular Models Materials Needed - Molecular model kit Relevant Textbook Reading Denniston, chap 11.2-11.4, 12.1-12.3 Background In uncharged,
Suggested answers to in-text activities and unit-end exercises Topic 8 Unit 30
 Suggested answers to in-text activities and unit-end exercises In-text activities Checkpoint (page 60) 1 Any two of the following: 2 a) A 2-bromo-2-methylpropane B 1-bromobutane b) 3 4 a) position isomers
Suggested answers to in-text activities and unit-end exercises In-text activities Checkpoint (page 60) 1 Any two of the following: 2 a) A 2-bromo-2-methylpropane B 1-bromobutane b) 3 4 a) position isomers
9. Stereochemistry. Stereochemistry
 9. Stereochemistry Stereochemistry Some objects are not the same as their mirror images (technically, they have no plane of symmetry) A right-hand glove is different than a left-hand glove (See Figure
9. Stereochemistry Stereochemistry Some objects are not the same as their mirror images (technically, they have no plane of symmetry) A right-hand glove is different than a left-hand glove (See Figure
1. Root of name depends on longest chain of C containing the double bond; ends in "ene"
 Alkenes (β-carotene, an antioxidant pigment) n 2n (acyclic) n 2n-2 (cyclic) R R R R Key features sp 2 -hybridized carbons, 120 o bond angles σ + π bonding between = planar geometry around = "unsaturated"
Alkenes (β-carotene, an antioxidant pigment) n 2n (acyclic) n 2n-2 (cyclic) R R R R Key features sp 2 -hybridized carbons, 120 o bond angles σ + π bonding between = planar geometry around = "unsaturated"
5.1 Alkene Nomenclature
 5.1 Alkene Nomenclature Alkenes Alkenes are hydrocarbons that contain a carbon-carbon double bond also called "olefins" characterized by molecular formula n 2n said to be "unsaturated" Alkene Nomenclature
5.1 Alkene Nomenclature Alkenes Alkenes are hydrocarbons that contain a carbon-carbon double bond also called "olefins" characterized by molecular formula n 2n said to be "unsaturated" Alkene Nomenclature
Introduction to Organic Chemistry, Unit 3: Chirality at Carbon Centers Bring your model kits to class!
 Introduction to rganic hemistry, Unit 3: hirality at arbon enters Bring your model kits to class! rganic hemistry #3 1 bjectives: by the end of this unit, you should be able to... Identify the chiral centres
Introduction to rganic hemistry, Unit 3: hirality at arbon enters Bring your model kits to class! rganic hemistry #3 1 bjectives: by the end of this unit, you should be able to... Identify the chiral centres
Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a
 Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a Hard coal, which is high in carbon content any straight-chain or branched-chain
Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a Hard coal, which is high in carbon content any straight-chain or branched-chain
Mechanism: We therefore describe the mechanism as a S N 2 mechanism.
 OH Mechanism: This is known as a bimolecular reaction as there are 2 molecules involved in the slow step. We therefore describe the mechanism as a S N 2 mechanism. We see an inversion in this process around
OH Mechanism: This is known as a bimolecular reaction as there are 2 molecules involved in the slow step. We therefore describe the mechanism as a S N 2 mechanism. We see an inversion in this process around
4.1.3 Alkenes. N Goalby chemrevise.org. Formation of π bond p orbitals C C C C. Alkenes contain a carboncarbon. General formula is CnH2n
 4.1.3 Alkenes Alkenes are unsaturated hydrocarbons General formula is n2n Alkenes contain a carboncarbon double bond somewhere in their structure Ethene Propene Numbers need to be added to the name when
4.1.3 Alkenes Alkenes are unsaturated hydrocarbons General formula is n2n Alkenes contain a carboncarbon double bond somewhere in their structure Ethene Propene Numbers need to be added to the name when
Atomic Properties of Carbon
 Organic Compounds Atomic Properties of Carbon Organic molecules have structural complexity and chemical diversity. Carbon can lose 4 electrons and have the same electronic configuration as He. OR Carbon
Organic Compounds Atomic Properties of Carbon Organic molecules have structural complexity and chemical diversity. Carbon can lose 4 electrons and have the same electronic configuration as He. OR Carbon
Alkenes CnH2n Ethene Propene But-2-ene But-1-ene exposed high electron density one sigma (σ) bond and one pi (π) bond.
 Alkenes Alkenes are unsaturated hydrocarbons General formula is n2n Alkenes contain a carboncarbon double bond somewhere in their structure Ethene Propene Numbers need to be added to the name when positional
Alkenes Alkenes are unsaturated hydrocarbons General formula is n2n Alkenes contain a carboncarbon double bond somewhere in their structure Ethene Propene Numbers need to be added to the name when positional
Basic Stereochemical Considerations
 Basic Stereochemical Considerations Key words: chirality, chiral carbon, enantiomers, diastereomers, absolute configuration, relative configuration, optical activity 1 Key Concepts Basics of projection
Basic Stereochemical Considerations Key words: chirality, chiral carbon, enantiomers, diastereomers, absolute configuration, relative configuration, optical activity 1 Key Concepts Basics of projection
General formula is CnH2n. Propene. But-1-ene. C-C pi bond. Formation of π bond in alkenes p orbitals Rotation can occur around a sigma bond
 4.1.3 Alkenes Alkenes are unsaturated hydrocarbons General formula is n2n Alkenes contain a carboncarbon double bond somewhere in their structure Ethene Propene Numbers need to be added to the name when
4.1.3 Alkenes Alkenes are unsaturated hydrocarbons General formula is n2n Alkenes contain a carboncarbon double bond somewhere in their structure Ethene Propene Numbers need to be added to the name when
Chapter 5: Stereoisomerism
 hapter 5: Stereoisomerism [Sections: 5.1-5.9] 1. dentifying Types of somers Same MolecularFormula? A B compounds are not isomers Same onnectivity? D E constitutional isomers have different names (parent
hapter 5: Stereoisomerism [Sections: 5.1-5.9] 1. dentifying Types of somers Same MolecularFormula? A B compounds are not isomers Same onnectivity? D E constitutional isomers have different names (parent
Chapter 5 Stereochemistry
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 5 Stereochemistry Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 5 Stereochemistry Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
4Types of Isomers. 1. Structural Isomers/(Constitutional) 2. Geometric Isomers/(Cis/Trans) 3. Optical Isomers A. Enantiomers B.
 4Types of Isomers 1. Structural Isomers/(Constitutional) 2. Geometric Isomers/(Cis/Trans) 3. Optical Isomers A. Enantiomers B. Diastereomers 4Types of Isomers C 4 10 C 4 10 O O O O O O O O O O O O C 3
4Types of Isomers 1. Structural Isomers/(Constitutional) 2. Geometric Isomers/(Cis/Trans) 3. Optical Isomers A. Enantiomers B. Diastereomers 4Types of Isomers C 4 10 C 4 10 O O O O O O O O O O O O C 3
CHAPTER 5. Stereoisomers
 CHAPTER 5 Stereoisomers We have already covered two kinds of isomerism: Constitutional Isomers (structural isomers) Stereoisomers Examples of Constitutional Isomers: Examples of Stereoisomers: Another
CHAPTER 5 Stereoisomers We have already covered two kinds of isomerism: Constitutional Isomers (structural isomers) Stereoisomers Examples of Constitutional Isomers: Examples of Stereoisomers: Another
Enantiomers. nonsuperimposable mirror image Both Configuration will be opposite. Both Configuration will be opposite
 Optical Isomerism Isomerism of Organic Molecules: Two chiral centers Many organic compounds have more than one asymmetric carbon. The more asymmetric carbons a compound has, the more number of stereoisomers
Optical Isomerism Isomerism of Organic Molecules: Two chiral centers Many organic compounds have more than one asymmetric carbon. The more asymmetric carbons a compound has, the more number of stereoisomers
Chapter 5 Stereochemistry
 Chapter 5 Stereochemistry References: 1. Title: Organic Chemistry (fifth edition) Author: Paula Yurkanis Bruice Publisher: Pearson International Edition 2. Title: Stereokimia Author: Poh Bo Long Publisher:
Chapter 5 Stereochemistry References: 1. Title: Organic Chemistry (fifth edition) Author: Paula Yurkanis Bruice Publisher: Pearson International Edition 2. Title: Stereokimia Author: Poh Bo Long Publisher:
Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
 Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
CHEMISTRY FOR THE IB DIPLOMA CAMBRIDGE UNIVERSITY PRESS
 10 rganic chemistry Revision checklist I am able to: explain, using an example, what is meant by the term homologous series sketch a graph of boiling point against number of carbons for the straight-chain
10 rganic chemistry Revision checklist I am able to: explain, using an example, what is meant by the term homologous series sketch a graph of boiling point against number of carbons for the straight-chain
2/25/2015. Chapter 4. Introduction to Organic Compounds. Outline. Lecture Presentation. 4.1 Alkanes: The Simplest Organic Compounds
 Lecture Presentation Outline Chapter 4 Introduction to Organic Compounds 4.2 Representing Structures of Organic Compounds Julie Klare Fortis College Smyrna, GA Alkanes are structurally simple organic compounds
Lecture Presentation Outline Chapter 4 Introduction to Organic Compounds 4.2 Representing Structures of Organic Compounds Julie Klare Fortis College Smyrna, GA Alkanes are structurally simple organic compounds
Chapter 6 Principles of Stereochemistry
 6.1 (a) This compound is chiral. Methane is achiral. Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 6 Principles of Stereochemistry Solutions to In-Text Problems
6.1 (a) This compound is chiral. Methane is achiral. Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 6 Principles of Stereochemistry Solutions to In-Text Problems
Organic Chemistry. Chemical Bonding and Structure (2)
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Chemical Bonding and Structure (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty of Industrial Science & Technology seema@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Chemical Bonding and Structure (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty of Industrial Science & Technology seema@ump.edu.my
Pentane (C5H12) exists in three form
 ISOMERISM The phenomenon of existence of two or more compounds with same molecular formula but different properties ( physical, chemical or both) is known as isomerism and the compound exhibiting this
ISOMERISM The phenomenon of existence of two or more compounds with same molecular formula but different properties ( physical, chemical or both) is known as isomerism and the compound exhibiting this
Due Date: 2) What is the relationship between the following compounds?
 Assignment #5 Name CHEM201 Student #: Due Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What type of isomers are CH3CH2OCH3 and CH3CH2CH2OH?
Assignment #5 Name CHEM201 Student #: Due Date: MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What type of isomers are CH3CH2OCH3 and CH3CH2CH2OH?
STEREOGENIC CENTER (Chiral Center,Asymmetric Center) Atom (usually carbon) to which 4 different groups are attached: W Z C X Y
 STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
Experiment 6. Stereochemistry
 Experiment 6. Stereochemistry Introduction Organic molecules with the same molecular formula but different arrangements of atoms are called isomers. Structural isomers are different because the location
Experiment 6. Stereochemistry Introduction Organic molecules with the same molecular formula but different arrangements of atoms are called isomers. Structural isomers are different because the location
H 3 C. staggered H 2 C
 EMISTRY 104 elp Sheet #3 Organic-Part II: ISOMERS (Text: h 2: 2.9, h 6: 6.11, 6.5, h 7: 7.2f) Do topics appropriate for your lecture Prepared by Dr. Tony Jacob http://www.chem.wisc.edu/areas/clc (Resource
EMISTRY 104 elp Sheet #3 Organic-Part II: ISOMERS (Text: h 2: 2.9, h 6: 6.11, 6.5, h 7: 7.2f) Do topics appropriate for your lecture Prepared by Dr. Tony Jacob http://www.chem.wisc.edu/areas/clc (Resource
Chapter 24 From Petroleum to Pharmaceuticals
 hapter 24 From Petroleum to Pharmaceuticals 24.1 Petroleum Refining and the ydrocarbons 24.2 Functional Groups and Organic Synthesis 24.3 Pesticides and Pharmaceuticals IR Tutor and Infrared Spectroscopy
hapter 24 From Petroleum to Pharmaceuticals 24.1 Petroleum Refining and the ydrocarbons 24.2 Functional Groups and Organic Synthesis 24.3 Pesticides and Pharmaceuticals IR Tutor and Infrared Spectroscopy
(1) Check to see if the two compounds are identical. (2) Recall the definitions of stereoisomers, conformational isomers, and constitutional isomers.
 MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
Chemistry 123: Physical and Organic Chemistry Topic 1: Organic Chemistry
 Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Text Sections (N0 1.9, 9-11) Homework: Chapter 1:
 CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
Organic Chemistry. Unit 10
 Organic Chemistry Unit 10 Halides Primary Carbons Secondary Carbons Tertiary Carbons IMPORTANCE?? REACTIONS!! Benzene C6H6 Aromatic functional group - C6H5 (IUPAC name - phenyl) Substitution Reactions
Organic Chemistry Unit 10 Halides Primary Carbons Secondary Carbons Tertiary Carbons IMPORTANCE?? REACTIONS!! Benzene C6H6 Aromatic functional group - C6H5 (IUPAC name - phenyl) Substitution Reactions
CH 3. Section Resources
 22.3 1 FOCUS Objectives 22.3.1 Explain why structural isomers have different properties. 22.3.2 Describe the conditions under which geometric isomers are possible. 22.3.3 Identify optical isomers. Guide
22.3 1 FOCUS Objectives 22.3.1 Explain why structural isomers have different properties. 22.3.2 Describe the conditions under which geometric isomers are possible. 22.3.3 Identify optical isomers. Guide
Counterclockwise (Leftward turning) S. 3 2 Clockwise (Rightward turning) R
 Stereochemistry I and S in Alkanes A chiral carbon has four different groups attached to it, the differences may be several bonds away, but so long as they are different in any way the groups are considered
Stereochemistry I and S in Alkanes A chiral carbon has four different groups attached to it, the differences may be several bonds away, but so long as they are different in any way the groups are considered
CHEMISTRY - TRO 4E CH.21 - ORGANIC CHEMISTRY.
 !! www.clutchprep.com TOPI: ORGANI EMISTRY Organic hemistry is the study of carbon and the other common nonmetals it is connected to:,, &. Some organic molecules are made of just carbons and hydrogens
!! www.clutchprep.com TOPI: ORGANI EMISTRY Organic hemistry is the study of carbon and the other common nonmetals it is connected to:,, &. Some organic molecules are made of just carbons and hydrogens
Chem 341 Jasperse Ch. 9 Handouts 1
 Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
Chem 341 Jasperse Ch. 9 andouts 1 Ch. 9 Stereochemistry Stereoisomers have the same condensed formulas and basic bonding sequence, but have different 3-dimensional shape and cannot be interconverted 9.1,2
STRUCTURE, ISOMERISM AND NOMENCLATURE OF ORGANIC COMPOUNDS
 II STRUCTURE, ISOMERISM AND NOMENCLATURE OF ORGANIC COMPOUNDS I. OBJECTIVES AND BACKGROUND This exercise will give you an opportunity to experience the three-dimensional nature of molecules and to visualize
II STRUCTURE, ISOMERISM AND NOMENCLATURE OF ORGANIC COMPOUNDS I. OBJECTIVES AND BACKGROUND This exercise will give you an opportunity to experience the three-dimensional nature of molecules and to visualize
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CHEMISTRY PAPER No. : 7 MODULE No. : 23 (Optical Isomerism)
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 7 : Inorganic Chemistry-II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) 23
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 7 : Inorganic Chemistry-II (Metal-Ligand Bonding, Electronic Spectra and Magnetic Properties of Transition Metal Complexes) 23
Calculate a rate given a species concentration change.
 Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
(a) (i) Give the equation representing the overall reaction. (1) Give the equation representing the formation of the electrophile.
 1. Benzene reacts with concentrated nitric acid in the presence of concentrated sulphuric acid at about 50 º in an electrophilic substitution reaction to give nitrobenzene. (a) Give the equation representing
1. Benzene reacts with concentrated nitric acid in the presence of concentrated sulphuric acid at about 50 º in an electrophilic substitution reaction to give nitrobenzene. (a) Give the equation representing
University of Sydney Chemistry 1B (CHEM1102) Organic Chemistry Lecture Notes
 University of Sydney hemistry 1B (EM1102) rganic hemistry Lecture Notes Topic 1 Introduction & isomers 2 Topic 2 Alkenes, alkynes, arenes 20 Topic 3 Structure determination 27 Topic 4 Alcohols and amines
University of Sydney hemistry 1B (EM1102) rganic hemistry Lecture Notes Topic 1 Introduction & isomers 2 Topic 2 Alkenes, alkynes, arenes 20 Topic 3 Structure determination 27 Topic 4 Alcohols and amines
Chemistry: The Central Science. Chapter 24: Chemistry of Coordination Compounds
 Chemistry: The Central Science Chapter 24: Chemistry of Coordination Compounds Metal compounds with complex assemblies of metals surrounded by molecules and ions are called coordination compounds 24.3:
Chemistry: The Central Science Chapter 24: Chemistry of Coordination Compounds Metal compounds with complex assemblies of metals surrounded by molecules and ions are called coordination compounds 24.3:
Stereochemistry Terminology for two pure isomeric compounds, both of which are chiral? A pair of stereoisomers
 Name Last, irst STEECEMISTY This handout will help you understand stereoisomerism, naming conventions and relationships between stereoisomers. I hope that you will use this to help you study for exam 1.
Name Last, irst STEECEMISTY This handout will help you understand stereoisomerism, naming conventions and relationships between stereoisomers. I hope that you will use this to help you study for exam 1.
HISTORY OF ORGANIC CHEMISTRY
 ISTORY OF ORGANI EMISTRY In the early days of chemistry, scientists classified chemical substances into 2 groups: 1. Inorganic: those that were composed of minerals, such as rocks and nonliving matter.
ISTORY OF ORGANI EMISTRY In the early days of chemistry, scientists classified chemical substances into 2 groups: 1. Inorganic: those that were composed of minerals, such as rocks and nonliving matter.
STEREOGENIC CENTER (Chiral Center,Asymmetric Center)
 STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
STEREOGENI ENTER (hiral enter,asymmetric enter) Atom (usually carbon) to which 4 different groups are attached: W Z X Y Many, but not all, molecules which contain a stereogenic center are chiral. (A molecule
Chapters 2 & 25: Covalent bonds & Organic Chemistry
 hapters 2 & 25: ovalent bonds & Organic hemistry Read: BLB 2.6, 2.9; 25.1-25.4 (only nomenclature in Table 25.1, NOT reactions) W: BLB 2:43, 45, 69, 76, 77 BLB 25:11, 12, 25, 40a, c-f Packet Organic:1
hapters 2 & 25: ovalent bonds & Organic hemistry Read: BLB 2.6, 2.9; 25.1-25.4 (only nomenclature in Table 25.1, NOT reactions) W: BLB 2:43, 45, 69, 76, 77 BLB 25:11, 12, 25, 40a, c-f Packet Organic:1
Chapter 3: Stereochemistry & Chirality
 Chapter 3: Stereochemistry & Chirality 1. Chiral & Achiral Compounds - Identifying Stereocenters 2. Assigning R & S configurations 3. Diastereomers - Molecules with two or more stereocenters 4. Properties
Chapter 3: Stereochemistry & Chirality 1. Chiral & Achiral Compounds - Identifying Stereocenters 2. Assigning R & S configurations 3. Diastereomers - Molecules with two or more stereocenters 4. Properties
Organic Chemistry is the chemistry of compounds containing.
 Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Chapter 3 The Chemistry of Carbon
 Complex molecules assembled like TinkerToys Chapter 3 The Chemistry of Carbon Why study Carbon? All living things are made of cells Cells ~72% H 2 O ~3% salts (Na, Cl, K ) ~25% carbon compounds carbohydrates
Complex molecules assembled like TinkerToys Chapter 3 The Chemistry of Carbon Why study Carbon? All living things are made of cells Cells ~72% H 2 O ~3% salts (Na, Cl, K ) ~25% carbon compounds carbohydrates
Alkenes and Alkynes 10/27/2010. Chapter 7. Alkenes and Alkynes. Alkenes and Alkynes
 Chapter 7 Alkenes and Alkynes CHP6 Problems: 6.1-13, 16-34, 36. CHP7 Problems: 7.1-23, 25-28, 31-34, 37-39, 41-47, 49-56. Alkenes and Alkynes Alkene (or olefin ) Hydrocarbon that contains a carbon-carbon
Chapter 7 Alkenes and Alkynes CHP6 Problems: 6.1-13, 16-34, 36. CHP7 Problems: 7.1-23, 25-28, 31-34, 37-39, 41-47, 49-56. Alkenes and Alkynes Alkene (or olefin ) Hydrocarbon that contains a carbon-carbon
HYDROCARBON COMPOUNDS
 YDROARBON OMPOUNDS hapter Quiz lassify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. ydrocarbons are unsaturated. 22.2 2. The IUPA name for 3(2)33 is butane. 22.1
YDROARBON OMPOUNDS hapter Quiz lassify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 1. ydrocarbons are unsaturated. 22.2 2. The IUPA name for 3(2)33 is butane. 22.1
Structure of Coordination Compounds
 Chapter 22 COORDINATION CHEMISTRY (Part II) Dr. Al Saadi 1 Structure of Coordination Compounds The geometry of coordination compounds plays a significant role in determining their properties. The structure
Chapter 22 COORDINATION CHEMISTRY (Part II) Dr. Al Saadi 1 Structure of Coordination Compounds The geometry of coordination compounds plays a significant role in determining their properties. The structure
