[H O ][CH COO ] [CH COOH] 0.2 x
|
|
|
- Evan Fox
- 6 years ago
- Views:
Transcription
1 CEM1612 Answers to Problem Sheet 6 1. (a) 0.2 M acetic acid As acetic acid is a weak acid, [ 3 O + ] must be calculated: C 3 2 O 3 O + C 3 initial 0.2 large 0 0 change - negligible + + final 0.2 large The equilibrium constant K a is given by: K a [ O ][C ] = = [C ] As pk a = 4.76 = log 10 K a so K a = As K a is very small, 0.2 ~ 0.2 and hence: 2 = or = M = [ 3 O + ] ence, the p is given by: p = log 10 [ 3 O + ] = log 10 [0.0019] = 2.7 (b) 0.2 M sodium acetate As C 3 is a weak base, [O ] must be calculated in a similar way: C 3 2 O O C 3 initial 0.2 large 0 0 change - negligible + + final 0.2 large The equilibrium constant K b is given by: K - [O ][C 2 3] b = = - [C ] For an acid and its conjugate base: so pk a + pk b = pk b = = 9.24
2 As pk b = 9.24, K b = Again, K b is very small, 0.2 ~ 0.2 and hence: 2 = or = M = [O ] ence, the po is given by: po = log 10 [O ] = log 10 [ ] = 5.0 Finally, p + po = 14 so p = = 9.0 (c) A buffer that is 0.2 M in acetic acid and 0.2 M in sodium acetate This solution contains an acid and its conjugate base so the enderson- asselbalch equation can be used: p = pk a + log10 As [acetic acid] = [sodium acetate], log10 = log 10(1) = 0 and so p = pk a = (a) As the α- group has the lowest pk a value, it is the most acidic. At p = 1.81, the α- group is in equilibrium with its conjugate base. The pk a values of the imidazole - and the α- + 3 groups are higher than the p value, both eist predominately in the protonated form at p = acid conjugate base (b) As p = 6.05 is higher than its pk a value, the α- group will eist predominately in its conjugate base form. The pk a value for the α- 3 + group is higher than the p value so it will eist predominately in its protonated form. The ring - group will be in equilibrium with its conjugate base form.
3 3 3 acid conjugate base (c) As p = 9.15 is higher than their pk a values, the α- and imidazole - groups will eist predominately in their conjugate base forms. The α- 3 group is in equilibrium with its conjugate base form The equilibrium of interest is: acid conjugate base 2 PO 4 - (aq) + 2 O(l) PO 4 2- (aq) + 3 O + (l) where 2 PO 4 _ is the acid and PO 4 2- is the base. The enderson-asselbalch equation can be used to calculate the required concentrations of each: p = pk a + log10 or log10 = p pk a log 10 = ( ) = 0.20 ence, = Both the acid and base have initial concentrations of 0.10 M so the ratio of the volume of each required is: V base = V acid = 1.58
4 When the two solutions are added together, their total volume = V base + V acid = 1.0 L. ence, V acid = V base V base = 1.0 V base 1.58 so V base = 0.61 L and V acid = 1.0 V base = 0.39 L 4. The concentration of the acid that has dissociated at equilibrium can be calculated from the p: A 2 O 3 O + A initial 0.6 large 0 0 change - negligible + + final 0.6 large At equilibrium, [ 3 O + ] = [ 3 O + ] from A + [ 3 O + ] from water. As [ 3 O + ] from water ~ 10-7, it can be assumed that the p is due to the [ 3 O + ] from A. Therefore: so p = log 10 [ 3 O + ] ~ log[ 3 O + ] from A = log = 4.0 = 10 4 M The initial concentration of A is 0.6 M. The percentage dissociation is: + from A 4 4 [ O 3 ] = 100% = 100% = 0.017% [ A] + [ A ] (a) The titration is a 1:1 reaction between a weak acid and a strong base. (i) Before addition of any base, the p of the M acetic solution follows the same method used in Q1(a). Following the same steps (with a M solution in place of a 0.2 M solution) gives the equilibrium constant K a is given by: K a [ O ][C ] = = [C ] With the approimation that ~ 0.100, 2 = or = M = [ 3 O + ] ence, the p is given by: p = log 10 [ 3 O + ] = log 10 [ ] = 2.88 (ii) Addition of the strong base O - (aq) leads to the neutralization reaction:
5 C 3 (aq) + O - (aq) à C 3 - (aq) + 2 O(l) The number of moles of C 3 - is equal to the number of moles of O - (aq) which have been added. The number of moles of C 3 remaining is equal to the initial number of moles of C 3 minus the number of moles of O - (aq) which have been added. Initially, there is 50.0 ml of M C 3 so n(c 3 ) initial = concentration volume = M L = mol 25.0 ml of M ao contains n(o - ) added = concentration volume = M L = mol Thus, after addition and the neutralization reaction, there are: n(c 3 ) = n(c 3 ) initial - n(o - ) added = ( ) mol = mol n(c 3 - ) = n(o - ) added = mol These quantities are now in a solution with total volume ( ) ml = 75.0 ml, so their concentrations are: c(c 3 ) = n / V = mol / L = M c(c 3 - ) = n / V = mol / L = M Using the enderson-asselbalch equation gives p = pk a + log M = log10 = M At the ½ equivalence point, when the number of moles of base added is equal to ½ the number of moles of acid originally present, the p is equal to the pk a. (iii) 45.0 ml of M ao corresponds to n(o - ) added = concentration volume = M L = mol Thus, after addition and the neutralization reaction, there are: n(c 3 ) = n(c 3 ) initial - n(o - ) added = ( ) mol = mol
6 n(c 3 - ) = n(o - ) added = mol These quantities are now in a solution with total volume ( ) ml = 95.0 ml, so their concentrations are: c(c 3 ) = n / V = mol / L = M c(c 3 - ) = n / V = mol / L = M Using the enderson-asselbalch equation gives M p = log10 = M 5.71 (ote: In fact one could have skipped the conversion of moles into concentration step, because the volume of solution is the same for C 3 and C3 - and it would just cancel out) (iv) The addition of 50.0 ml of M ao corresponds to the equivalence point as the number of moles of added base is equal to the number of moles of acid initially present. owever, the p is not equal to 7 for this point of the titration of a weak acid with a strong base ml of M ao corresponds mol so whilst this leads to n(c 3 ) = 0.00 mol, it also leads to n(c 3 - ) = mol. A solution of a weak base has p > 7. As the total volume is ( ) ml = ml, the concentration of C 3 - is: c(c 3 - ) = n / V = mol / L = M The calculation of the p now follows that in Q1(b) with the concentration of M: K - [O ][C 2 3] b - [C3 ] = = = As pk b = 9.24, K b = Again, K b is very small, ~ and hence: 2 = or = M = [O ] ence, the po is given by: po = log 10 [O ] = log 10 [ ] = 5.27 Finally, p + po = 14 so
7 p = = 8.73 (v) Beyond the equivalence point, ecess base is being added to a solution of a weak base and the p is controlled by the ecess amount of O - (aq). The calculation of the p is then eactly the same as in Q7(a)(v) from Problem Sheet ml of M ao corresponds to mol. Of this, mol is used to react with the acid. The total volume is ( ) L = L. ence ( mol) ( mol) [O (aq)] = = M (0.105 L) ence, po = -log 10 ( ) = 2,32 and p = = (vi) 75.0 ml of M ao corresponds to mol Of this, mol is used to react with the acid and the total volume is ( ) L = L. ence ( mol) ( mol) [O (aq)] = = M (0.125 L) ence, po = -log 10 (0.0200) = 1.70 and p = = 12.3 (b) Using these values, the p curve for the titration can be constructed and is shown below (in pink) p volume of ao added / ml (c) The figure also includes the data from Q7 in Problem Sheet 5 for a strong acid / strong base titration (in blue). For a weak acid / strong base titration: (i) The initial p is higher. For a strong acid and a weak acid with the same concentration, the p of the strong acid is lower.
8 (ii) (iii) (iv) The p at the ½ equivalence point is equal to the pk a of the weak acid. At the equivalence point, the solution contains weak base and so the p > 7. After the equivalence point, the p is determined only the concentration of ecess strong base and is thus the same for the two titrations.
Amino acids have the general structure: All amino acids have at least two acidic protons. One is the proton
 Worksheet rganic Acids and Bases II Amino Acids Acids are proton donors in aqueous solutions: A + 2 3 + + A - acid base conjugate conjugate base acid The strength of an acid is measured by the [ 3 + ]
Worksheet rganic Acids and Bases II Amino Acids Acids are proton donors in aqueous solutions: A + 2 3 + + A - acid base conjugate conjugate base acid The strength of an acid is measured by the [ 3 + ]
5 Acid Base Reactions
 Aubrey High School AP Chemistry 5 Acid Base Reactions 1. Consider the formic acid, HCOOH. K a of formic acid = 1.8 10 4 a. Calculate the ph of a 0.20 M solution of formic acid. Name Period Date / / 5.2
Aubrey High School AP Chemistry 5 Acid Base Reactions 1. Consider the formic acid, HCOOH. K a of formic acid = 1.8 10 4 a. Calculate the ph of a 0.20 M solution of formic acid. Name Period Date / / 5.2
Buffer Calculations. The Standard Equilibrium Approach to Calculating a Buffer s ph
 Buffer Calculations A buffer is a solution that has the ability to resist a change in ph upon the addition of a strong acid or a strong base. For a buffer to exist it must satisfy two conditions: (1) the
Buffer Calculations A buffer is a solution that has the ability to resist a change in ph upon the addition of a strong acid or a strong base. For a buffer to exist it must satisfy two conditions: (1) the
CHEM J-3 June 2012
 CEM1611 2012-J-3 June 2012 In a standard acid-base titration, 25.00 ml of 0.1043 M a solution was found to react exactly with 28.45 ml of an Cl solution of unknown concentration. What is the p of the unknown
CEM1611 2012-J-3 June 2012 In a standard acid-base titration, 25.00 ml of 0.1043 M a solution was found to react exactly with 28.45 ml of an Cl solution of unknown concentration. What is the p of the unknown
Lecture 12. Acid/base reactions. Equilibria in aqueous solutions.
 Lecture 12 Acid/base reactions. Equilibria in aqueous solutions. Titrations Kotz 7 th ed. Section 18.3, pp.821-832. In a titration a solution of accurately known concentration is added gradually added
Lecture 12 Acid/base reactions. Equilibria in aqueous solutions. Titrations Kotz 7 th ed. Section 18.3, pp.821-832. In a titration a solution of accurately known concentration is added gradually added
Titration of a Weak Acid with a Strong Base
 Titration of a Weak Acid with a Strong Base Weak Acid w/ Strong Base Overall: INITIAL ph: Weak acids do not fully dissociate we need to do an ICE table to determine initial ph. We expect it to be weakly
Titration of a Weak Acid with a Strong Base Weak Acid w/ Strong Base Overall: INITIAL ph: Weak acids do not fully dissociate we need to do an ICE table to determine initial ph. We expect it to be weakly
Kotz 7 th ed. Section 18.3, pp
 Lecture 15 Acid/base reactions. Equilibria in aqueous solutions. Titrations Kotz 7 th ed. Section 18.3, pp.821-832. In a titration a solution of accurately known concentration is added gradually added
Lecture 15 Acid/base reactions. Equilibria in aqueous solutions. Titrations Kotz 7 th ed. Section 18.3, pp.821-832. In a titration a solution of accurately known concentration is added gradually added
School of Chemistry, University of KwaZulu-Natal, Howard College Campus, Durban. CHEM191 Tutorial 1: Buffers
 School of Chemistry, University of KwaZulu-Natal, Howard College Campus, Durban CHEM191 Tutorial 1: Buffers Preparing a Buffer 1. How many moles of NH 4 Cl must be added to 1.0 L of 0.05 M NH 3 to form
School of Chemistry, University of KwaZulu-Natal, Howard College Campus, Durban CHEM191 Tutorial 1: Buffers Preparing a Buffer 1. How many moles of NH 4 Cl must be added to 1.0 L of 0.05 M NH 3 to form
Buffer Effectiveness, Titrations & ph curves. Section
 Buffer Effectiveness, Titrations & ph curves Section 16.3-16.4 Buffer effectiveness Buffer effectiveness refers to the ability of a buffer to resist ph change Effective buffers only neutralize small to
Buffer Effectiveness, Titrations & ph curves Section 16.3-16.4 Buffer effectiveness Buffer effectiveness refers to the ability of a buffer to resist ph change Effective buffers only neutralize small to
Chapter 17 Answers. Practice Examples [H3O ] 0.018M, 1a. HF = M. 1b. 30 drops. 2a.
![Chapter 17 Answers. Practice Examples [H3O ] 0.018M, 1a. HF = M. 1b. 30 drops. 2a. Chapter 17 Answers. Practice Examples [H3O ] 0.018M, 1a. HF = M. 1b. 30 drops. 2a.](/thumbs/95/123080455.jpg) Chapter 17 Answers Practice Examples 1a. + [HO ] 0.018M, 1b. 0 drops [HF] = 0.8 M. [H O + ] = 0.10 M, HF = 0.97 M. a. + HO 1.10 M, CHO = 0.150 M. b. 15g NaCHO a. The hydronium ion and the acetate ion react
Chapter 17 Answers Practice Examples 1a. + [HO ] 0.018M, 1b. 0 drops [HF] = 0.8 M. [H O + ] = 0.10 M, HF = 0.97 M. a. + HO 1.10 M, CHO = 0.150 M. b. 15g NaCHO a. The hydronium ion and the acetate ion react
Chapter 17. Additional Aspects of Aqueous Equilibria. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 17 Additional Aspects of John D. Bookstaver St. Charles Community College Cottleville, MO The Common-Ion Effect Consider a solution of acetic acid: CH 3 COOH(aq) + H 2 O(l)
Lecture Presentation Chapter 17 Additional Aspects of John D. Bookstaver St. Charles Community College Cottleville, MO The Common-Ion Effect Consider a solution of acetic acid: CH 3 COOH(aq) + H 2 O(l)
Chapter 15. Acid-Base Equilibria
 Chapter 15 Acid-Base Equilibria Section 15.1 Solutions of Acids or Bases Containing a Common Ion Common Ion Effect Shift in equilibrium position that occurs because of the addition of an ion already involved
Chapter 15 Acid-Base Equilibria Section 15.1 Solutions of Acids or Bases Containing a Common Ion Common Ion Effect Shift in equilibrium position that occurs because of the addition of an ion already involved
x x10. Hydromiun ion already in solution before acid added. NH 3 /NH4+ buffer solution
 10/15/01 Commonion effect In the last chapter, we calculated the [H 3 O ] of a M O as 6.010 5 M. The percent dissociation for this solution would be: More Acid and Base Chemistry 6.010 5 100 0.089% [H
10/15/01 Commonion effect In the last chapter, we calculated the [H 3 O ] of a M O as 6.010 5 M. The percent dissociation for this solution would be: More Acid and Base Chemistry 6.010 5 100 0.089% [H
Understanding the shapes of acid-base titration curves AP Chemistry
 Understanding the shapes of acidbase titration curves AP Chemistry Neutralization Reactions go to Completion Every acidbase reaction produces another acid and another base. A neutralization reaction is
Understanding the shapes of acidbase titration curves AP Chemistry Neutralization Reactions go to Completion Every acidbase reaction produces another acid and another base. A neutralization reaction is
Acid Base Titrations
 ChemActivity CA47b Acid Base Titrations Model 1 Titration of a strong acid with a strong base. 20.00 ml of HNO 3 is titrated with 0.10 M NaOH. The acid-base reaction is The net ionic reaction is HNO 3
ChemActivity CA47b Acid Base Titrations Model 1 Titration of a strong acid with a strong base. 20.00 ml of HNO 3 is titrated with 0.10 M NaOH. The acid-base reaction is The net ionic reaction is HNO 3
Chapter 17 Additional Aspects of
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 17 Additional Aspects of AP Chemistry 2014-15 North Nova Education Centre Mr. Gauthier
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 17 Additional Aspects of AP Chemistry 2014-15 North Nova Education Centre Mr. Gauthier
Chapter 17 Additional Aspects of
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 17 Additional Aspects of John D. Bookstaver St. Charles Community College Cottleville,
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 17 Additional Aspects of John D. Bookstaver St. Charles Community College Cottleville,
Questions #4-5 The following two questions refer to the following system: A 1.0L solution contains 0.25M HF and 0.60M NaF (Ka for HF = 7.2 x 10-4 ).
 Multiple Choice 1) A solution contains 0.250 M HA (K a = 1.0 x 10-6 ) and 0.45 M NaA. What is the ph after 0.10 mole of HCl is added to 1.00L of this solution? a. 3.17 b. 3.23 c. 6.00 d. 10.77 e. 10.83
Multiple Choice 1) A solution contains 0.250 M HA (K a = 1.0 x 10-6 ) and 0.45 M NaA. What is the ph after 0.10 mole of HCl is added to 1.00L of this solution? a. 3.17 b. 3.23 c. 6.00 d. 10.77 e. 10.83
Lecture #11-Buffers and Titrations The Common Ion Effect
 Lecture #11-Buffers and Titrations The Common Ion Effect The Common Ion Effect Shift in position of an equilibrium caused by the addition of an ion taking part in the reaction HA(aq) + H2O(l) A - (aq)
Lecture #11-Buffers and Titrations The Common Ion Effect The Common Ion Effect Shift in position of an equilibrium caused by the addition of an ion taking part in the reaction HA(aq) + H2O(l) A - (aq)
A 95 g/mol B 102 /mol C 117 g/mol D 126 g/mol E 152 g/mol
 Titrations In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete.
Titrations In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete.
Note that side chains serve as a) stabilizers of protein structure, b) reactive centers, and c) micro-environments. * + H 3 N-C-COOH H 2 N-C-COO -
 BIOCEMISTRY I AMINO ACIDS I. Amino Acid Structure One of the most important macromolecules (chains of distinct molecular units) in the biosphere is protein. Proteins are needed for catalysis, reaction,
BIOCEMISTRY I AMINO ACIDS I. Amino Acid Structure One of the most important macromolecules (chains of distinct molecular units) in the biosphere is protein. Proteins are needed for catalysis, reaction,
Chapter 16. The Danger of Antifreeze. Buffers. Aqueous Equilibrium
 hapter 16 Aqueous Equilibrium The Danger of Antifreeze Each year, thousands of pets and wildlife die from consuming antifreeze Most brands of antifreeze contain ethylene glycol sweet taste and initial
hapter 16 Aqueous Equilibrium The Danger of Antifreeze Each year, thousands of pets and wildlife die from consuming antifreeze Most brands of antifreeze contain ethylene glycol sweet taste and initial
= (CO 2. H) pk a2 = (NH 2. The stronger base is associated with K b1
 Solutions - Chapter 0 3. Proline: pk a =.95 (C H) pk a = 0.640 (NH ) NH H The stronger base is associated with K b ; thus NH stronger base H = - NH - NH H = - NH H weaker base pk a.95 pk a 0.640 pk w 4
Solutions - Chapter 0 3. Proline: pk a =.95 (C H) pk a = 0.640 (NH ) NH H The stronger base is associated with K b ; thus NH stronger base H = - NH - NH H = - NH H weaker base pk a.95 pk a 0.640 pk w 4
ACIDS, BASES & ACID/BASE EQUILIBRIA
 Chapter 5 ACIDS, BASES & ACID/BASE EQUILIBRIA ill, Petrucci, McCreary & Perry 4 th Ed. ACIDS & BASES The Arrhenius Definition: In Water: Acid Substance which increases the concentration of ydrogen Ion,
Chapter 5 ACIDS, BASES & ACID/BASE EQUILIBRIA ill, Petrucci, McCreary & Perry 4 th Ed. ACIDS & BASES The Arrhenius Definition: In Water: Acid Substance which increases the concentration of ydrogen Ion,
CHEMISTRY 102 Fall 2010 Hour Exam III. 1. My answers for this Chemistry 102 exam should be graded with the answer sheet associated with:
 1. My answers for this Chemistry 10 exam should be graded with the answer sheet associated with: a) Form A b) Form B c) Form C d) Form D e) Form E Consider the titration of 30.0 ml of 0.30 M HCN by 0.10
1. My answers for this Chemistry 10 exam should be graded with the answer sheet associated with: a) Form A b) Form B c) Form C d) Form D e) Form E Consider the titration of 30.0 ml of 0.30 M HCN by 0.10
Acids and Bases. CHEM 102 T. Hughbanks. In following equilibrium, will reactants or products be favored? Strong acid (HCl) + Strong base (NaOH)
 Acids and Bases According to the Brønsted Lowry theory, all acid base reactions can be written as equilibria involving the acid and base and their conjugates. CEM 102 T. ughbanks All proton transfer reactions
Acids and Bases According to the Brønsted Lowry theory, all acid base reactions can be written as equilibria involving the acid and base and their conjugates. CEM 102 T. ughbanks All proton transfer reactions
6. ACIDS AND BASES PREVIOUS EAMCET BITS
 1 6. ACIDS AND BASES PREVIOUS EAMCET BITS 1. p of a buffer solution decreases by 0.02 untis when 0.12g of acetic acid is added to 250ml of a buffer solution of acetic acid and Potassium acetate at 27 0
1 6. ACIDS AND BASES PREVIOUS EAMCET BITS 1. p of a buffer solution decreases by 0.02 untis when 0.12g of acetic acid is added to 250ml of a buffer solution of acetic acid and Potassium acetate at 27 0
1. What colour would 1.0 M HCl be in an indicator mixture consisting of phenol red and
 cids Practice Test 2 1. What colour would 1.0 M Hl be in an indicator mixture consisting of phenol red and thymolphthalein? red blue yellow colourless 2. uring a titration, what volume of 0.500 M KOH is
cids Practice Test 2 1. What colour would 1.0 M Hl be in an indicator mixture consisting of phenol red and thymolphthalein? red blue yellow colourless 2. uring a titration, what volume of 0.500 M KOH is
Acid-Base Titration Solution Key
 Key CH3NH2(aq) H2O(l) CH3NH3 (aq) OH - (aq) Kb = 4.38 x 10-4 In aqueous solution of methylamine at 25 C, the hydroxide ion concentration is 1.50 x 10-3 M. In answering the following, assume that temperature
Key CH3NH2(aq) H2O(l) CH3NH3 (aq) OH - (aq) Kb = 4.38 x 10-4 In aqueous solution of methylamine at 25 C, the hydroxide ion concentration is 1.50 x 10-3 M. In answering the following, assume that temperature
Buffer Solutions. Buffer Solutions
 Buffer Solutions A buffer solution is comprised of a mixture of an acid (base) with its conjugate base (acid) that resists changes in ph when additional acid or base is added The Henderson-Hasselbalch
Buffer Solutions A buffer solution is comprised of a mixture of an acid (base) with its conjugate base (acid) that resists changes in ph when additional acid or base is added The Henderson-Hasselbalch
SCH4U Chapter 8 review
 Name: Class: Date: SCH4U Chapter 8 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which statement does not describe a characteristic of acidic
Name: Class: Date: SCH4U Chapter 8 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which statement does not describe a characteristic of acidic
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which one of the following is the weakest acid? 1) A) HF (Ka = 6.8 10-4) B) HNO2 (Ka
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which one of the following is the weakest acid? 1) A) HF (Ka = 6.8 10-4) B) HNO2 (Ka
Acids and Bases. Reviewing Vocabulary CHAPTER ASSESSMENT CHAPTER 19. Compare and contrast each of the following terms.
 Acids and Bases Reviewing Vocabulary Compare and contrast each of the following terms. 1. Arrhenius model, Brønsted-Lowry model 2. acid ionization constant, base ionization constant 3. conjugate acid,
Acids and Bases Reviewing Vocabulary Compare and contrast each of the following terms. 1. Arrhenius model, Brønsted-Lowry model 2. acid ionization constant, base ionization constant 3. conjugate acid,
Acid-Base Solutions - Applications
 Acid-Base Solutions - Applications 1 The Common Ion Effect Consider the equilibrium established when acetic acid, HC 2 H 3 O 2, is added to water. CH 3 COOH(aq) + H 2 O(l) CH 3 COO - (aq) + H 3 O + (aq)
Acid-Base Solutions - Applications 1 The Common Ion Effect Consider the equilibrium established when acetic acid, HC 2 H 3 O 2, is added to water. CH 3 COOH(aq) + H 2 O(l) CH 3 COO - (aq) + H 3 O + (aq)
CHEM1109 Answers to Problem Sheet Isotonic solutions have the same osmotic pressure. The osmotic pressure, Π, is given by:
 CHEM1109 Answers to Problem Sheet 5 1. Isotonic solutions have the same osmotic pressure. The osmotic pressure, Π, is given by: Π = MRT where M is the molarity of the solution. Hence, M = Π 5 (8.3 10 atm)
CHEM1109 Answers to Problem Sheet 5 1. Isotonic solutions have the same osmotic pressure. The osmotic pressure, Π, is given by: Π = MRT where M is the molarity of the solution. Hence, M = Π 5 (8.3 10 atm)
Chapter 15. Acid-Base Equilibria
 Chapter 15 Acid-Base Equilibria The Common Ion Effect The common-ion effect is the shift in an ionic equilibrium caused by the addition of a solute that provides an ion already involved in the equilibrium
Chapter 15 Acid-Base Equilibria The Common Ion Effect The common-ion effect is the shift in an ionic equilibrium caused by the addition of a solute that provides an ion already involved in the equilibrium
Midterm Exam III. CHEM 181: Introduction to Chemical Principles November 25, 2014 Answer Key
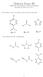 Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
CHAPTER FIFTEEN APPLICATIONS OF AQUEOUS EQUILIBRIA. For Review
 CHAPTER FIFTEEN APPLICATIONS OF AQUEOUS EQUILIBRIA For Review 1. A common ion is an ion that appears in an equilibrium reaction but came from a source other than that reaction. Addition of a common ion
CHAPTER FIFTEEN APPLICATIONS OF AQUEOUS EQUILIBRIA For Review 1. A common ion is an ion that appears in an equilibrium reaction but came from a source other than that reaction. Addition of a common ion
Chapter 2 - Water 9/8/2014. Water exists as a H-bonded network with an average of 4 H-bonds per molecule in ice and 3.4 in liquid. 104.
 Chapter 2 - Water Water exists as a -bonded network with an average of 4 -bonds per molecule in ice and 3.4 in liquid. 104.5 o -bond: An electrostatic attraction between polarized molecules containing
Chapter 2 - Water Water exists as a -bonded network with an average of 4 -bonds per molecule in ice and 3.4 in liquid. 104.5 o -bond: An electrostatic attraction between polarized molecules containing
Strong Acids and Bases C020
 Strong Acids and Bases C020 Strong Acids and Bases 1 Before discussing acids and bases examine the concept of chemical equilibrium At reaction is at equilibrium when it is proceeding forward and backwards
Strong Acids and Bases C020 Strong Acids and Bases 1 Before discussing acids and bases examine the concept of chemical equilibrium At reaction is at equilibrium when it is proceeding forward and backwards
SUPPLEMENTAL HOMEWORK SOLUTIONS WEEK 8
 SUPPLEMETAL MEWRK SLUTIS WEEK 8 Assignment for Tuesday, March 7 th 7.36 a) + + b) + + c) 6 14 4 + 6 14 4 + + d) 6 5 3 7 + 6 5 7 + Be sure to write the correct charges for the products. 7.4 a) l + l + b)
SUPPLEMETAL MEWRK SLUTIS WEEK 8 Assignment for Tuesday, March 7 th 7.36 a) + + b) + + c) 6 14 4 + 6 14 4 + + d) 6 5 3 7 + 6 5 7 + Be sure to write the correct charges for the products. 7.4 a) l + l + b)
Titration a solution of known concentration, called a standard solution
 Acid-Base Titrations Titration is a form of analysis in which we measure the volume of material of known concentration sufficient to react with the substance being analyzed. Titration a solution of known
Acid-Base Titrations Titration is a form of analysis in which we measure the volume of material of known concentration sufficient to react with the substance being analyzed. Titration a solution of known
Responses of Chemical Equilibria
 Responses of Chemical Equilibria Chapter 9 of Atkins: Section 9.5 The Response of Chemical Equilibria to Conditions Acid-base equilibria in water The ph of acids and bases Acid-base titrations The ph curve
Responses of Chemical Equilibria Chapter 9 of Atkins: Section 9.5 The Response of Chemical Equilibria to Conditions Acid-base equilibria in water The ph of acids and bases Acid-base titrations The ph curve
The ph of aqueous salt solutions
 The ph of aqueous salt solutions Sometimes (most times), the salt of an acid-base neutralization reaction can influence the acid/base properties of water. NaCl dissolved in water: ph = 7 NaC 2 H 3 O 2
The ph of aqueous salt solutions Sometimes (most times), the salt of an acid-base neutralization reaction can influence the acid/base properties of water. NaCl dissolved in water: ph = 7 NaC 2 H 3 O 2
+ H 2 O HPO 4. (a) In this system, there are two acid-base conjugate pairs. These are (1) HPO4
 1 The dihydrogenphosphate-hydrogenphosphate ion system is an important buffer in the human body. H 2 PO 4 H 2 O HPO 4 2 H 3 O (a) In this system, there are two acid-base conjugate pairs. These are acid
1 The dihydrogenphosphate-hydrogenphosphate ion system is an important buffer in the human body. H 2 PO 4 H 2 O HPO 4 2 H 3 O (a) In this system, there are two acid-base conjugate pairs. These are acid
CHEMISTRY 102 Fall 2010 Hour Exam III Page My answers for this Chemistry 102 exam should be graded with the answer sheet associated with:
 Hour Exam III Page 1 1. My answers for this Chemistry 102 exam should be graded with the answer sheet associated with: a) Form A b) Form B c) Form C d) Form D e) Form E Consider the titration of 30.0 ml
Hour Exam III Page 1 1. My answers for this Chemistry 102 exam should be graded with the answer sheet associated with: a) Form A b) Form B c) Form C d) Form D e) Form E Consider the titration of 30.0 ml
Chemistry 222. Start mol End mol
 Chemistry Spring 019 Exam 3: Chapters 8-10 Name 80 Points Complete problem 1 and four of problems 6. CLEARLY mark the problem you do not want graded. You must show your work to receive credit for problems
Chemistry Spring 019 Exam 3: Chapters 8-10 Name 80 Points Complete problem 1 and four of problems 6. CLEARLY mark the problem you do not want graded. You must show your work to receive credit for problems
Now, the excess strong base will react: HA + OH - A - + H 2 O Start mol End mol
 Chemistry Spring 016 Exam 3: Chapters 8-10 Name 80 Points Complete problem 1 and four of problems -6. CLEARLY mark the problem you do not want graded. You must show your work to receive credit for problems
Chemistry Spring 016 Exam 3: Chapters 8-10 Name 80 Points Complete problem 1 and four of problems -6. CLEARLY mark the problem you do not want graded. You must show your work to receive credit for problems
Chapter 15 Acid-Base Equilibria
 Chapter 15 Acid-Base Equilibria Acid-Base Equilibria 15.1 Solutions of Acids or Bases Containing a Common Ion A. Common Ion 1. Ion provided in solution by an aqueous acid (or base) as well as a salt a.
Chapter 15 Acid-Base Equilibria Acid-Base Equilibria 15.1 Solutions of Acids or Bases Containing a Common Ion A. Common Ion 1. Ion provided in solution by an aqueous acid (or base) as well as a salt a.
Chemistry 102 Discussion #8, Chapter 14_key Student name TA name Section
 Chemistry 102 Discussion #8, Chapter 14_key Student name TA name Section 1. If 1.0 liter solution has 5.6mol HCl, 5.mol NaOH and 0.0mol NaA is added together what is the ph when the resulting solution
Chemistry 102 Discussion #8, Chapter 14_key Student name TA name Section 1. If 1.0 liter solution has 5.6mol HCl, 5.mol NaOH and 0.0mol NaA is added together what is the ph when the resulting solution
Chem 106 Thursday, March 10, Chapter 17 Acids and Bases
 Chem 106 Thursday, March 10, 2011 Chapter 17 Acids and Bases K a and acid strength Acid + base reactions: Four types (s +s, s + w, w + s, and w + w) Determining K from concentrations and ph ph of aqueous
Chem 106 Thursday, March 10, 2011 Chapter 17 Acids and Bases K a and acid strength Acid + base reactions: Four types (s +s, s + w, w + s, and w + w) Determining K from concentrations and ph ph of aqueous
K a =
 Q1.The acid dissociation constant, K a, for ethanoic acid is given by the expression K a = The value of K a for ethanoic acid is 1.74 10 5 mol dm 3 at 25 C. A buffer solution is prepared using ethanoic
Q1.The acid dissociation constant, K a, for ethanoic acid is given by the expression K a = The value of K a for ethanoic acid is 1.74 10 5 mol dm 3 at 25 C. A buffer solution is prepared using ethanoic
K w. Acids and bases 8/24/2009. Acids and Bases 9 / 03 / Ionization of water. Proton Jumping Large proton and hydroxide mobility
 Chapter 2 Water Acids and Bases 9 / 03 / 2009 1. How is the molecular structure of water related to physical and chemical behavior? 2. What is a Hydrogen Bond? 3Wh 3. What are Acids Aid and db Bases? 4.
Chapter 2 Water Acids and Bases 9 / 03 / 2009 1. How is the molecular structure of water related to physical and chemical behavior? 2. What is a Hydrogen Bond? 3Wh 3. What are Acids Aid and db Bases? 4.
Chemistry 102 Summary July 24 th. Question: Sketch a generic curve for a diprotic acid titration with a strong base. Answer:
 Polyprotic Acid Titrations * Chemistry 102 Summary July 24 th Question: Sketch a generic curve for a diprotic acid titration with a strong base. Answer: Question: Consider the titration curve of 50.0 ml
Polyprotic Acid Titrations * Chemistry 102 Summary July 24 th Question: Sketch a generic curve for a diprotic acid titration with a strong base. Answer: Question: Consider the titration curve of 50.0 ml
Chapter 5. Acid/Base Reactions
 hapter 5. cid/base Reactions 5. Log p Diagrams (Graphical Solutions) 6. itration 7. Buffers and Buffer Intensity 8. arbonate System 7 Water hemistry 5. Log p Diagrams p as an independent variable Develop
hapter 5. cid/base Reactions 5. Log p Diagrams (Graphical Solutions) 6. itration 7. Buffers and Buffer Intensity 8. arbonate System 7 Water hemistry 5. Log p Diagrams p as an independent variable Develop
Chapter 15. Titration Curves for Complex Acid/Base Systems
 Chapter 15 Titration Curves for Complex Acid/Base Systems Polyfunctional acids and bases Carbonic acid/bicarbonate buffer system Buffers for human blood ph = 7.35-7.45 CO 2(g) + H 2 O H 2 CO 3(aq) H 2
Chapter 15 Titration Curves for Complex Acid/Base Systems Polyfunctional acids and bases Carbonic acid/bicarbonate buffer system Buffers for human blood ph = 7.35-7.45 CO 2(g) + H 2 O H 2 CO 3(aq) H 2
What is an acid? What is a base?
 What is an acid? What is a base? Properties of an acid Sour taste Turns litmus paper red Conducts electric current Some acids are strong and some are weak Properties of a base Bitter taste Slippery to
What is an acid? What is a base? Properties of an acid Sour taste Turns litmus paper red Conducts electric current Some acids are strong and some are weak Properties of a base Bitter taste Slippery to
Part One: Pure Solutions of Weak Acids, Bases (water plus a single electrolyte solute)
 CHAPTER 16: ACID-BASE EQUILIBRIA Part One: Pure Solutions of Weak Acids, Bases (water plus a single electrolyte solute) A. Weak Monoprotic Acids. (Section 16.1) 1. Solution of Acetic Acid: 2. See Table
CHAPTER 16: ACID-BASE EQUILIBRIA Part One: Pure Solutions of Weak Acids, Bases (water plus a single electrolyte solute) A. Weak Monoprotic Acids. (Section 16.1) 1. Solution of Acetic Acid: 2. See Table
Chem 1102 Semester 1, 2011 ACIDS AND BASES
 Chem 1102 Semester 1, 2011 ACIDS AND BASES Acids and Bases Lecture 23: Weak Acids and Bases Calculations involving pk a and pk b Strong Acids and Bases Lecture 24: Polyprotic Acids Salts of Acids and Bases
Chem 1102 Semester 1, 2011 ACIDS AND BASES Acids and Bases Lecture 23: Weak Acids and Bases Calculations involving pk a and pk b Strong Acids and Bases Lecture 24: Polyprotic Acids Salts of Acids and Bases
Solution equilibria Effect of common ion Ex. HF K a = For a 1.0 M solution of HF: about ~2.7% dissociation
 15,16 Solution equilibria Effect of common ion Ex. F K a = 7. 10 4 For a 1.0 M solution of F: about ~.7% dissociation With the addition of 1.0 M NaF F (aq) + (aq) + F (aq) final 1.0-x x 1.0+x (M) K a x
15,16 Solution equilibria Effect of common ion Ex. F K a = 7. 10 4 For a 1.0 M solution of F: about ~.7% dissociation With the addition of 1.0 M NaF F (aq) + (aq) + F (aq) final 1.0-x x 1.0+x (M) K a x
Unit 9: Acid and Base Multiple Choice Practice
 Unit 9: Acid and Base Multiple Choice Practice Name June 14, 2017 1. Consider the following acidbase equilibrium: HCO3 H2O H2CO3 OH In the reaction above, the BrönstedLowry acids are: A. H2O and OH B.
Unit 9: Acid and Base Multiple Choice Practice Name June 14, 2017 1. Consider the following acidbase equilibrium: HCO3 H2O H2CO3 OH In the reaction above, the BrönstedLowry acids are: A. H2O and OH B.
Lecture 8. Making a Buffer. Buffers. Professor Hicks Inorganic Chemistry (CHE152)
 Lecture 8 Professor Hicks Inorganic Chemistry (CHE152) Making a Buffer Buffers buffers = solutions that resist ph changes act by neutralizing added acid or base made by preparing a solution of a weak acid/base
Lecture 8 Professor Hicks Inorganic Chemistry (CHE152) Making a Buffer Buffers buffers = solutions that resist ph changes act by neutralizing added acid or base made by preparing a solution of a weak acid/base
ph calculations MUDr. Jan Pláteník, PhD Brønsted-Lowry concept of acids and bases Acid is a proton donor Base is a proton acceptor
 ph calculations MUDr. Jan Pláteník, PhD Brønsted-Lowry concept of acids and bases Acid is a proton donor Base is a proton acceptor HCl(aq) + H 2 O(l) H 3 O + (aq) + Cl - (aq) Acid Base Conjugate acid Conjugate
ph calculations MUDr. Jan Pláteník, PhD Brønsted-Lowry concept of acids and bases Acid is a proton donor Base is a proton acceptor HCl(aq) + H 2 O(l) H 3 O + (aq) + Cl - (aq) Acid Base Conjugate acid Conjugate
Chapter 17 Additional Aspects of Aqueous Equilibria (Part A)
 Chapter 17 Additional Aspects of Aqueous Equilibria (Part A) Often, there are many equilibria going on in an aqueous solution. So, we must determine the dominant equilibrium (i.e. the equilibrium reaction
Chapter 17 Additional Aspects of Aqueous Equilibria (Part A) Often, there are many equilibria going on in an aqueous solution. So, we must determine the dominant equilibrium (i.e. the equilibrium reaction
Titration region & buffer calculations. An acid base region is a circumstance
 CH102 Summer 1 2012 Boston University An acid base region is a circumstance not a sequence of operations Region 1: Only weak acid (base) Region 2: Both weak acid (base) and its conjugate base (acid) Region
CH102 Summer 1 2012 Boston University An acid base region is a circumstance not a sequence of operations Region 1: Only weak acid (base) Region 2: Both weak acid (base) and its conjugate base (acid) Region
Acid Base Equilibria
 Acid Base Equilibria Acid Ionization, also known as acid dissociation, is the process in where an acid reacts with water to produce a hydrogen ion and the conjugate base ion. HC 2 H 3 O 2(aq) H + (aq)
Acid Base Equilibria Acid Ionization, also known as acid dissociation, is the process in where an acid reacts with water to produce a hydrogen ion and the conjugate base ion. HC 2 H 3 O 2(aq) H + (aq)
Acids and Bases Written Response
 Acids and Bases Written Response January 1999 4. Consider the salt sodium oxalate, Na2C2O4. a) Write the dissociation equation for sodium oxalate. (1 mark) b) A 1.0M solution of sodium oxalate turns pink
Acids and Bases Written Response January 1999 4. Consider the salt sodium oxalate, Na2C2O4. a) Write the dissociation equation for sodium oxalate. (1 mark) b) A 1.0M solution of sodium oxalate turns pink
Titration Curves equivalence point
 1 Here is an example of a titration curve, produced when a strong base is added to a strong acid. This curve shows how ph varies as 0.100 M NaOH is added to 50.0 ml of 0.100 M HCl. The equivalence point
1 Here is an example of a titration curve, produced when a strong base is added to a strong acid. This curve shows how ph varies as 0.100 M NaOH is added to 50.0 ml of 0.100 M HCl. The equivalence point
Strong & Weak Acid (ph, pka, Kw) Question Paper
 For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ Strong & Weak cid (ph, pka, Kw) Question Paper Level Subject Exam oard Topic Sub Topic ooklet Paper Type Level hemistry Edexcel
For more awesome GSE and level resources, visit us at www.savemyexams.co.uk/ Strong & Weak cid (ph, pka, Kw) Question Paper Level Subject Exam oard Topic Sub Topic ooklet Paper Type Level hemistry Edexcel
Analytical Chemistry Lecture III by/ Dr. Ekhlas Q. J. BUFFER SOLUTIONS
 Analytical Chemistry Lecture III by/ Dr. Ekhlas Q. J. BUFFER SOLUTIONS Buffer solutions Definition Solutions which resist changes in ph when small quantities of acid or alkali are added. a solution that
Analytical Chemistry Lecture III by/ Dr. Ekhlas Q. J. BUFFER SOLUTIONS Buffer solutions Definition Solutions which resist changes in ph when small quantities of acid or alkali are added. a solution that
5/10/2017. Chapter 10. Acids, Bases, and Salts
 Chapter 10. Acids, Bases, and Salts Introduction to Inorganic Chemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chapter 10. Acids, Bases, and Salts Introduction to Inorganic Chemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
ACID-BASE EQUILIBRIA. Chapter 14 Big Idea Six
 ACID-BASE EQUILIBRIA Chapter 14 Big Idea Six Acid-Base Equilibria Common Ion Effect in Acids and Bases Buffer SoluDons for Controlling ph Buffer Capacity ph-titradon Curves Acid-Base TitraDon Indicators
ACID-BASE EQUILIBRIA Chapter 14 Big Idea Six Acid-Base Equilibria Common Ion Effect in Acids and Bases Buffer SoluDons for Controlling ph Buffer Capacity ph-titradon Curves Acid-Base TitraDon Indicators
Ch. 17 Applications of Aqueous Equilibria: Buffers and Titrations
 Ch. 17 Applications of Aqueous Equilibria: Buffers and Titrations Sec 1 The Common-Ion Effect: The dissociation of a weak electrolyte decreases when a strong electrolyte that has an ion in common with
Ch. 17 Applications of Aqueous Equilibria: Buffers and Titrations Sec 1 The Common-Ion Effect: The dissociation of a weak electrolyte decreases when a strong electrolyte that has an ion in common with
Consider a 1.0 L solution of 0.10 M acetic acid. Acetic acid is a weak acid only a small percent of the weak acid is ionized
 Chemistry 12 Acid- Base Equilibrium V Name: Date: Block: 1. Buffers 2. Hydrolysis Buffers An acid- base buffer is a solution that resists changes in ph following the addition of relatively small amounts
Chemistry 12 Acid- Base Equilibrium V Name: Date: Block: 1. Buffers 2. Hydrolysis Buffers An acid- base buffer is a solution that resists changes in ph following the addition of relatively small amounts
AP Chemistry: Acid-Base Chemistry Practice Problems
 Name AP Chemistry: Acid-Base Chemistry Practice Problems Date Due Directions: Write your answers to the following questions in the space provided. For problem solving, show all of your work. Make sure
Name AP Chemistry: Acid-Base Chemistry Practice Problems Date Due Directions: Write your answers to the following questions in the space provided. For problem solving, show all of your work. Make sure
Chemistry 2000 Lecture 19: Organic acids
 Chemistry 2000 Lecture 19: Organic acids Marc R. Roussel March 8, 2018 Marc R. Roussel Chemistry 2000 Lecture 19: Organic acids March 8, 2018 1 / 22 Organic acids The acid dissociation constant, K a The
Chemistry 2000 Lecture 19: Organic acids Marc R. Roussel March 8, 2018 Marc R. Roussel Chemistry 2000 Lecture 19: Organic acids March 8, 2018 1 / 22 Organic acids The acid dissociation constant, K a The
Dilutions 4/8/2013. Steps involved in preparing solutions from pure solids. Steps involved in preparing solutions from pure solids
 Steps involved in preparing solutions from pure solids Steps involved in preparing solutions from pure solids Calculate the amount of solid required Weigh out the solid Place in an appropriate volumetric
Steps involved in preparing solutions from pure solids Steps involved in preparing solutions from pure solids Calculate the amount of solid required Weigh out the solid Place in an appropriate volumetric
Grace King High School Chemistry Test Review
 CHAPTER 19 Acids, Bases & Salts 1. ACIDS Grace King High School Chemistry Test Review UNITS 7 SOLUTIONS &ACIDS & BASES Arrhenius definition of Acid: Contain Hydrogen and produce Hydrogen ion (aka proton),
CHAPTER 19 Acids, Bases & Salts 1. ACIDS Grace King High School Chemistry Test Review UNITS 7 SOLUTIONS &ACIDS & BASES Arrhenius definition of Acid: Contain Hydrogen and produce Hydrogen ion (aka proton),
Last Time Strong Acid/Strong Base Titration. Today. Titration determining something about an unknown by reacting it with a known solution
 Today Titration determining something about an unknown by reacting it with a known solution Neutralization (again) we'll need this to figure out titration Solubility The easiest of all the equilibria we'll
Today Titration determining something about an unknown by reacting it with a known solution Neutralization (again) we'll need this to figure out titration Solubility The easiest of all the equilibria we'll
Chem Chapter 18: Sect 1-3 Common Ion Effect; Buffers ; Acid-Base Titrations Sect 4-5 Ionic solubility Sect 6-7 Complex Formation
 Chem 106 3--011 Chapter 18: Sect 1-3 Common Ion Effect; Buffers ; Acid-Base Titrations Sect 4-5 Ionic solubility Sect 6-7 Complex Formation 3//011 1 The net ionic equation for the reaction of KOH(aq) and
Chem 106 3--011 Chapter 18: Sect 1-3 Common Ion Effect; Buffers ; Acid-Base Titrations Sect 4-5 Ionic solubility Sect 6-7 Complex Formation 3//011 1 The net ionic equation for the reaction of KOH(aq) and
Chapter 17 Additional Aspects of Aqueous Equilibria (Part A)
 Chapter 17 Additional Aspects of Aqueous Equilibria (Part A) What is a dominant equilibrium? How do we define major species? Reactions between acids and bases 1. Strong Acids + Strong Base The reaction
Chapter 17 Additional Aspects of Aqueous Equilibria (Part A) What is a dominant equilibrium? How do we define major species? Reactions between acids and bases 1. Strong Acids + Strong Base The reaction
Gateway 125,126,130 Fall 2006 Exam 3 p1. Section (circle one): 601 (Colin) 602 (Brannon) 603 (Mali) 604 (Xiaomu)
 Gateway 125,126,130 Fall 2006 Exam 3 p1 Gateway General Chemistry 125/126/130 Exam 3 December 5, 2006 (8:00-10:00pm) Name Section (circle one): 601 (Colin) 602 (Brannon) 603 (Mali) 604 (Xiaomu) The exam
Gateway 125,126,130 Fall 2006 Exam 3 p1 Gateway General Chemistry 125/126/130 Exam 3 December 5, 2006 (8:00-10:00pm) Name Section (circle one): 601 (Colin) 602 (Brannon) 603 (Mali) 604 (Xiaomu) The exam
Monoprotic Acid/Base Equilibria. Monoprotic Acid/Base Equilibria
 Monoprotic Acid/Base Equilibria Strong acids and bases: What is the ph of 0.10 M HCl? How do you calculate it? Why? Concentration (F) 0.10 (10-1 ) 0.01 (10-2 ) 0.001 (10-3 ) 0.0001 (10-4 ) 0.00001 (10-5
Monoprotic Acid/Base Equilibria Strong acids and bases: What is the ph of 0.10 M HCl? How do you calculate it? Why? Concentration (F) 0.10 (10-1 ) 0.01 (10-2 ) 0.001 (10-3 ) 0.0001 (10-4 ) 0.00001 (10-5
Chapter 15 Acid Base Equilibria
 Buffer Solutions The ph changes by a large amount even when a small amount of acid or base is added to pure water: Chapter 15 Acid Base Equilibria A buffer solution is a solution which resists a change
Buffer Solutions The ph changes by a large amount even when a small amount of acid or base is added to pure water: Chapter 15 Acid Base Equilibria A buffer solution is a solution which resists a change
Unless otherwise stated, all images in this file have been reproduced from:
 Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, Chemistry, 3 rd Edition 2016 (John Wiley & Sons) The University of Sydney Page
Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, Chemistry, 3 rd Edition 2016 (John Wiley & Sons) The University of Sydney Page
Chapter 17 Homework Problem Solutions
 Chapter 17 Homework Problem Solutions 17.40 D 2 O D + + OD, K w = [D + ] [OD ] = 8.9 10 16 Since [D + ] = [OD ], we can rewrite the above expression to give: 8.9 10 16 = ([D + ]) 2, [D + ] = 3.0 10 8 M
Chapter 17 Homework Problem Solutions 17.40 D 2 O D + + OD, K w = [D + ] [OD ] = 8.9 10 16 Since [D + ] = [OD ], we can rewrite the above expression to give: 8.9 10 16 = ([D + ]) 2, [D + ] = 3.0 10 8 M
Acid-Base Equilibria. 1.NH 4 Cl 2.NaCl 3.KC 2 H 3 O 2 4.NaNO 2. Acid-Ionization Equilibria. Acid-Ionization Equilibria
 Acid-Ionization Equilibria Acid-Base Equilibria Acid ionization (or acid dissociation) is the reaction of an acid with water to produce hydronium ion (hydrogen ion) and the conjugate base anion. (See Animation:
Acid-Ionization Equilibria Acid-Base Equilibria Acid ionization (or acid dissociation) is the reaction of an acid with water to produce hydronium ion (hydrogen ion) and the conjugate base anion. (See Animation:
Buffer Effectiveness 19
 Buffer Effectiveness 19 Buffer Effectiveness What makes a buffer effective? A buffer should be able to neutralize small to moderate amounts of added acid or base Too much added acid or base will destroy
Buffer Effectiveness 19 Buffer Effectiveness What makes a buffer effective? A buffer should be able to neutralize small to moderate amounts of added acid or base Too much added acid or base will destroy
Acid-Base Equilibria (Chapter 10.) Problems: 2,3,6,13,16,18,21,30,31,33
 Acid-Base Equilibria (Chapter 10.) Problems: 2,3,6,13,16,18,21,30,31,33 Review acid-base theory and titrations. For all titrations, at the equivalence point, the two reactants have completely reacted with
Acid-Base Equilibria (Chapter 10.) Problems: 2,3,6,13,16,18,21,30,31,33 Review acid-base theory and titrations. For all titrations, at the equivalence point, the two reactants have completely reacted with
Grade A buffer: is a solution that resists changes in its ph upon small additions of acid or base.sq1
 Chapter 15 Lesson Plan Grade 12 402. The presence of a common ion decreases the dissociation. BQ1 Calculate the ph of 0.10M CH 3 COOH. Ka = 1.8 10-5. [H + ] = = ( )( ) = 1.34 10-3 M ph = 2.87 Calculate
Chapter 15 Lesson Plan Grade 12 402. The presence of a common ion decreases the dissociation. BQ1 Calculate the ph of 0.10M CH 3 COOH. Ka = 1.8 10-5. [H + ] = = ( )( ) = 1.34 10-3 M ph = 2.87 Calculate
Bellwork: Answer these in your notes. What is the [H + ] of a solution with a ph of 4.90? Name this acid: H 3 PO 4. Name this base: KOH
![Bellwork: Answer these in your notes. What is the [H + ] of a solution with a ph of 4.90? Name this acid: H 3 PO 4. Name this base: KOH Bellwork: Answer these in your notes. What is the [H + ] of a solution with a ph of 4.90? Name this acid: H 3 PO 4. Name this base: KOH](/thumbs/83/87658619.jpg) Bellwork: Answer these in your notes. What is the [H + ] of a solution with a ph of 4.90? Name this acid: H 3 PO 4 Name this base: KOH Stoichiometry The stoichiometry of an acid-base neutralization reaction
Bellwork: Answer these in your notes. What is the [H + ] of a solution with a ph of 4.90? Name this acid: H 3 PO 4 Name this base: KOH Stoichiometry The stoichiometry of an acid-base neutralization reaction
Objectives To prepare a dilute solution of a weak acid. To prepare a buffer of a specific ph value.
 E x p e r i m e n t Chemistry Is phun! Objectives To prepare a dilute solution of a weak acid. To prepare a buffer of a specific ph value. To observe the effects of adding acid and base to a buffer solution.
E x p e r i m e n t Chemistry Is phun! Objectives To prepare a dilute solution of a weak acid. To prepare a buffer of a specific ph value. To observe the effects of adding acid and base to a buffer solution.
Chapter 17. Additional Aspects of Aqueous Equilibria. Lecture Presentation. James F. Kirby Quinnipiac University Hamden, CT
 Lecture Presentation Chapter 17 Additional Aspects of James F. Kirby Quinnipiac University Hamden, CT Effect of Acetate on the Acetic Acid Equilibrium Acetic acid is a weak acid: CH 3 COOH(aq) H + (aq)
Lecture Presentation Chapter 17 Additional Aspects of James F. Kirby Quinnipiac University Hamden, CT Effect of Acetate on the Acetic Acid Equilibrium Acetic acid is a weak acid: CH 3 COOH(aq) H + (aq)
Completion of acid/base/buffer chemistry. Hanson Activity Clicker quiz 3/11/2013. Chs 7 8 of Zumdahl
 Completion of acid/base/buffer chemistry Chs 7 8 of Zumdahl Hanson Activity 16 3 Discuss Key Questions 1 of Activity 16 3, page 301, with your partner for three minutes. The clicker quiz will commence
Completion of acid/base/buffer chemistry Chs 7 8 of Zumdahl Hanson Activity 16 3 Discuss Key Questions 1 of Activity 16 3, page 301, with your partner for three minutes. The clicker quiz will commence
CHEM1902/ N-4 November 2004
 CHEM190/4 004-N-4 November 004 Teeth are made from hydroxyapatite, Ca 5 (PO 4 ) 3 OH. Why does an acidic medium promote tooth decay and how can the decay be stopped using fluoridation of drinking water?
CHEM190/4 004-N-4 November 004 Teeth are made from hydroxyapatite, Ca 5 (PO 4 ) 3 OH. Why does an acidic medium promote tooth decay and how can the decay be stopped using fluoridation of drinking water?
Solutions are aqueous and the temperature is 25 C unless stated otherwise.
 Solutions are aqueous and the temperature is 25 C unless stated otherwise. 1. According to the Arrhenius definition, an acid is a substance that produces ions in aqueous solution. A. H C. OH B. H + D.
Solutions are aqueous and the temperature is 25 C unless stated otherwise. 1. According to the Arrhenius definition, an acid is a substance that produces ions in aqueous solution. A. H C. OH B. H + D.
Ch. 14/15: Acid-Base Equilibria Sections 14.6, 14.7, 15.1, 15.2
 Ch. 14/15: Acid-Base Equilibria Sections 14.6, 14.7, 15.1, 15.2 Creative Commons License Images and tables in this file have been used from the following sources: OpenStax: Creative Commons Attribution
Ch. 14/15: Acid-Base Equilibria Sections 14.6, 14.7, 15.1, 15.2 Creative Commons License Images and tables in this file have been used from the following sources: OpenStax: Creative Commons Attribution
Department of Chemistry University of Texas at Austin
 Polyprotic and Special Cases Calculations Supplemental Worksheet KEY For the following polyprotic acid questions: Citric acid (H3C6H5O6) Ka1 = 8.4 x 10 4 Ka2 = 1.8 x 10 5 Ka3 = 4.0 x 10 6 Oxalic acid (H2C2O4)
Polyprotic and Special Cases Calculations Supplemental Worksheet KEY For the following polyprotic acid questions: Citric acid (H3C6H5O6) Ka1 = 8.4 x 10 4 Ka2 = 1.8 x 10 5 Ka3 = 4.0 x 10 6 Oxalic acid (H2C2O4)
Acid-Base Equilibria. 1.NH 4 Cl 2.NaCl 3.KC 2 H 3 O 2 4.NaNO 2. Solutions of a Weak Acid or Base
 Acid-Base Equilibria 1 Will the following salts be acidic, basic or neutral in aqueous solution? 1.NH 4 Cl.NaCl.KC H O 4.NaNO A = acidic B = basic C = neutral Solutions of a Weak Acid or Base The simplest
Acid-Base Equilibria 1 Will the following salts be acidic, basic or neutral in aqueous solution? 1.NH 4 Cl.NaCl.KC H O 4.NaNO A = acidic B = basic C = neutral Solutions of a Weak Acid or Base The simplest
The Common Ion Effect
 Chapter 17 ACID BASE EQUILIBRIA (Part I) Dr. Al Saadi 1 17.1 The Common Ion Effect A phenomenon known as the common ion effect states that: When a compound containing an ion in common with an already dissolved
Chapter 17 ACID BASE EQUILIBRIA (Part I) Dr. Al Saadi 1 17.1 The Common Ion Effect A phenomenon known as the common ion effect states that: When a compound containing an ion in common with an already dissolved
