Note that side chains serve as a) stabilizers of protein structure, b) reactive centers, and c) micro-environments. * + H 3 N-C-COOH H 2 N-C-COO -
|
|
|
- Bruno McKenzie
- 5 years ago
- Views:
Transcription
1 BIOCEMISTRY I AMINO ACIDS I. Amino Acid Structure One of the most important macromolecules (chains of distinct molecular units) in the biosphere is protein. Proteins are needed for catalysis, reaction, support, stability, binding and thousands of other functions. The distinct molecular units, or building blocks, that make up proteins are called amino acids. amino >> 3 N -C"-COO << carboxyl terminal terminal (N-term) R (C-term) An amino acid is an organic molecule with a central carbon, called the "-carbon, connected to 1) a hydrogen, 2) an amino group (called the "-amino group), 3) a carboxylic acid group (called the "-carboxyl group), and 4) a variable group (or side chain) designated -R. The "-amino and "-carboxyl (also referred to as "-carbonyl) groups are the terminals of the amino acid. The "-amino, called the N-terminal, is considered the beginning, or front, of the amino acid. The "-carbonyl, called the C-terminal, is the end, or back, of the amino acid. L-amino acid D-amino acid R 3 N -C-COO 3 N -C-COO R There are 20 common amino acids and all have R-groups distinct from the other "-carbon attachments except one (whose R-group is hydrogen). This means that the "-carbon of nineteen of the common amino acids is chiral (or asymmetric and optically active). The one common amino acid that is not chiral, glycine, is achiral. For any asymmetric center that is tetrahedral, there are two possible arrangements of atoms on the chiral atom. The For many possible structures are not the same, but are mirror images of each other. biochemical building blocks, these are designated D- and L-configurations. Note that side chains serve as a) stabilizers of protein structure, b) reactive centers, and c) micro-environments. II. Amino Acids Ionization The terminal functional groups of amino acids are weak acids. The "-carboxyl group is a carboxylic acid. Oxygen is highly electronegative, and its attraction for the electrons that hold the hydrogen is great. ence, the bond between the oxygen and the hydrogen has fair ionic character. The hydrogen is easily stripped from the oxygen (dissociable) as hydrogen ion ( ) at higher p levels. Low p Form igh p Form 3 N-C-COO 2 N-C-COO - R R At low p everything is protonated. high p everything is deprotonated. At In a solution with a low p, there are so many hydrogen ions that the probability that the "-carboxyl group will permanently lose its hydrogen (be deprotonated) is almost zero. But at p levels above 2 or so, is lower and the carbonyl group can be deprotonated, leaving a negative charge on the oxygen. A carboxyl group will have a low pk a. The "-amino group is an amine; an even weaker acid than the carboxylic acid type. Nitrogen is only fairly electronegative, and its attraction for the electrons that hold the hydrogen is just a bit stronger than hydrogen s attraction for them. ence, the bond has poor ionic character. ydrogen ion is not easily stripped from the nitrogen and tt p levels below 9 or so, an amino group will be protonated, and have a positive charge. An amino group will have a high pk a. The p of the solution determines the overall charge of an amino acid. At median p levels (between about 6 to 8), the "-carboxyl group of most amino acid molecules in a solution will be deprotonated, and have a negative charge). Under the same conditions, the "-amino group of most amino acids in solution will be protonated (and have a positive charge). This form of an amino acid with no dissociable hydrogen on the R-group is called a zwitterion (a neutral molecule that has cationic and anionic functional groups). Zwitterion 3 N-C-COO - R
2 The R-group of several amino acids is polar and contains dissociable hydrogens. Like the terminal groups, the charge on these groups is a function of p. Acidic functional groups will deprotonate (lose hydrogen ion) in solutions with a lower p than basic or other nonacidic functional groups. The ionization (protonation or deprotonation) of various amino acid functional groups is important when considering biological reactions. We can examine what happens during protonation/deportonation by analyzing the titration of an amino acids. When an amino acid is titrated, we typically start with an amino acid solution at one extreme of the p scale or the other, then raise or lower the p by the addition of strong base or acid. Lets look at the titration curve of a simple amino acid like glycine. Glycine 3 N-C-COO Glycine has dissociable hydrogens on the N and C-terminals. The "-carbonyl group has a pk of about 2.4 and the "-amino group has a pk of about 9.8. In a solution with a p lower than 2.4, most of the molecules will be protonated, at all functional groups; hence most will have a 1 charge: MOSTLY 3 N -C 2 -COO below this pk As the p is raised (by the addition of base), the in solution is lowered because it reacts with O - to make 2 O; glycine starts to deprotonate. By the time the solution reaches a p of 2.4, half the glycine molecules will have a protonated carbonyl group while the other half will be deprotonated. alf will have a 1 charge, the other half will have no net charge: [ 3 N -C 2 -COO - ]/[ 3 N -C 2 -COO] = 1.0 As the p is raised more, the concentration eventually falls so low that by a p of 6.1, every molecule s carbonyl group will be deprotonated, and all molecules will have no net charge; there will only be zwitterions in solution. This p is the isoelectric point of the amino acid glycine. The isoelectric point is the p at which all molecules have no net charge. The symbol for isoelectric point is pi: ONLY 3 N -C 2 -COO - exists at this p Above the isoelectric point, most of the molecules have no charge, but we start to see deprotonation of the "-amino group. The N-term group starts to deprotonate and some molecules acquire an overall negative charge: MOSTLY 3 N -C 2 -COO - exists above the pi By the time the p of the solution is the same as the pk of the "-amino group, 9.8, there are equal amounts of molecules with no net charge and molecules with a -1 charge: [ 2 N-C 2 -COO - ]/[ 3 N -C 2 -COO - ] = 1.0 At a p above the pk of the "-amino group (9.8 for glycine), most of the molecules will have a negative charge: MOSTLY 2 N-C 2 -COO - exists above this pk In solutions with a p much above (~3 units) the pk of the "-amino group, there will only be molecules with an overall negative charge. For glycine, this is around 12 or more: ONLY 2 N-C 2 -COO - exists above this p Be any of this as it may, when a molecule deprotonates, it is acting as a weak acid, and the deprotonation of a weak acid produces its conjugate base. The relation between the p of a solution and relative amounts of conjugates in solution may be discerned using the enderson- asselbalch equation: p = pk log([conjugate Base]/[Weak Acid]) = pk log([a - ]/[A]) The relative amounts of the conjugates is therefore given by: [A - ]/[A] = 10 p-pk
3 A certain amino acid has a functional group with a pk of If 60% of amino acids in solution must be protonated at that group for some reaction to work properly, determine the p required to ensure the reaction. The protonated form is the acid, A, while the deprotonated form is the conjugate base, A -. If 60% must be protonated, [A - ] = 60, and [A] = 40, p = pk log([a - ]/[A]) = 4.50 log(60/40) ~ 4.68 The solution p should be 4.68 for the reaction to work properly. III. The Relation Between p and Charge The strength of the bond between a dissociable proton and the atom it is attached to is a function of the attraction of the atom to the electrons in the bond with the proton (electronegativity). The greater the atoms attraction for the electrons (its electronegativity), the weaker its bond to the proton, the easier it is to lose the proton! Some environmental factors interact with the functional group, knocking off or pulling away the proton. The lower the electronegativity of the atom holding the proton, the harder the environment must act to remove it. A. At low p, there is an abundance of hydrogen ions in solution. Groups with dissociable hydrogens are acted upon by the environment and hydrogens are stripped off. owever, there are so many hydrogen ions in solution that as soon as a proton is removed or pulled away, its replaced by another from solution. The functional group, for all practical purposes, is never without the hydrogen; everything is always protonated! Therefore, amino acids have a positive charge at low p (in acidic solution). B. At high p there is almost no hydrogen ions in solution. Groups with dissociable hydrogens are acted upon by the environment and protons are stripped off. Since there are no hydrogen ions in solution, the protons are not replaced. The functional group, for all practical purposes, never has the hydrogen; everything is always deprotonated! Therefore, amino acids have a negative charge at high p (in basic solution). A. The Quantitative Relation Between p and Charge For any weak acid, A, the dissociable proton is removed according to the equation: A(aq) 2 O(l) º 3 O (aq) A - (aq) T h e e q u i l i b r i u m c o n s t a n t, K a ( t h e a c i d dissociation constant), for the dissociation of the proton from the acid is equal to [ ][A - ]/[A]. Note that is simply short for 3 O. This equation may be rearranged to produce: p = pk a log([a - ]/[A]), the so-called enderson-asselbalch equation. This expresses the relation between the ratio of the conjugates and the p of the system. Each conjugate represents a form of the substance that has a particular charge. The ratio of those forms is given by the equation: [A - ]/[A] = 10 p-pk where the pk is the -log of the dissociation constant for the acid (A). For simple amino acids that don t have dissociable hydrogens on the side chains, the acid is the form of the molecule with a net charge of 1, 3 N -CR-COO. The conjugate base of the acid is the zwitterion, 3 N -CR-COO -, a molecule with no net charge. So [A - ]/[A] is the ratio of the 0 charge form to the 1 charge form: [ 3 N -CR-COO - ]/[ 3 N -CR-COO]. Note, if p = pk, [ 3 N -CR-COO - ]=[ 3 N -CR-COO]. If the zwitterion form loses a hydrogen ion, it becomes, 2 N-CR-COO - ; a molecule with a -1 charge. The ratio of acid, 0 charge form, to conjugate base, -1 charge form, is still given by 10 p-pk. But now we d use the pk for the zwitterion, the pk for the dissociation of the hydrogen ion from the "-amino group.
4 The ratio of the conjugates is only determined for a valid p range of the particular conjugates in question. For example, for the 1 and 0 forms, 3 N -CR-COO and 3 N -CR-COO -, this is for any p up to the average of the pk s for the 1 and the 0 forms: (pk COO pk N3 )/2. Below that p there are only the 1 charge and 0 charge forms. At that p there is only the 0 charge form; there is no 1 charge form or -1 charge form. Above that p up to maybe 3-5 p units above the pk N3, there is only the 0 charge form and the -1 charge form (there is no 1 charge form). So you d only be concerned with ratios for those forms. At any p above 3-5 p units of that pk, there is only the -1 form. 1) For example, glycine has a pk COO ~ 2.4 and a pk N3 ~ 9.8. What fraction of glycine molecules will have a -1 charge in a solution with a p of 5? 1 form 0 form -1 form 3 N -CR-COO º 3 N -CR-COO - º 2 N-CR-COO - The average of the two pk s is (9.82.4)/2 ~ 6.1. Below a p of 6.1, the population consists only of the 1 form and the 0 form. At a p of 6.1 the population is all molecules with no charge (so 6.1 is the isoelectric point). And above a p of 6.1, the population consists only of molecules with 0 charge and molecules with a -1 charge. So there are no molecules with a -1 charge at a p of 5. 2) What fraction of glycine molecules will have a 1 charge at a p of 8.0? Above a p of 6.1, the population consists only of molecules with 0 charge and molecules with a -1 charge. So there are no molecules with a 1 charge at a p of 8. 3) What fraction of glycine molecules will have a 1 charge at a p of 3.0? The 1 form only occurs below a p of 6.1, and for p values less than 6.1, we only use the pk for the "-carboxyl group. So the form with the protonated "-carboxyl group is acid ( 3 N -CR-COO; the 1 form), and the form with the deprotonated "-carboxyl group is its conjugate base ( 3 N -CR-COO - ; the 0 form). [ 3 N -CR-COO - ]/[ 3 N -CR-COO] = = 3.98/1 The 1 form = 1 and the 0 form = Thus the fraction that is 1 is 1/(3.981) = 0.2 or about 20%. 4) istidine s "-carboxyl and "-amino groups have pks of 1.80 and 9.33 respectively. It has a side chain with a dissociable amino group (-N ) whose pk is If this amino acid is required in a reaction where the hydrogen of the side chain is transferred to some acceptor, what fraction of histidine molecules may participate in the reaction in a solution that has a p of 7.2? N -CN -COO º 3 N -CN -COO - º 3 N -CN-COO - º 2 N-CN-COO No more 2 No more 1 Up to a p of 3.92, ( )/2, only 2 and 1 molecules exists. Up to a p of 7.69, ( )/2, only the 1 and 0 molecules exists. Above this p, there are no molecules with hydrogens to donate on the side chain, so we work with the previous pk, 6.04 (the pk for the 1 and 0 forms). [ 3 N -CN-COO - ]/[ 3 N -CN -COO - ] = = 14.5/1 The concentration of the deprotonated form is 14.5 times greater than that of the protonated form. The fraction protonated is 1/(14.51) ~ or about 6.5% of the histidine molecules in solution may be used in the reaction.
5 B. The p of Amino Acid Solutions (this section is in development) When amino acids are dissolved in water, they act as weak acids and weak bases. The p of pure water is 7, so the predominant form of amino acid in pure water is: 3 N -CR-COO - Some fraction of these molecules will take a proton from water to reprotonate the carboxyl terminal while another fraction will donate hydrogen to water, deprotonating the amino terminal. 3 N -CR-COO - (aq) 2 O(l) º 3 N -CR-COO(aq) O - (aq) 3 N -CR-COO - (aq) 2 O(l) º 2 N-CR-COO - (aq) 3 O (aq) K b K a the sum of these reactions yields: 2 3 N -CR-COO - (aq)2 2 O(l) º 2 N-CR-COO - (aq) 3 N -CR-COO(aq)O - (aq) 3 O (aq) K a K b K a K b = [ 3 O ][O - ][ 2 N-CR-COO - ][ 3 N -CR-COO]/[ 3 N -CR-COO - ] 2 = K w [ 2 N-CR-COO - ][ 3 N -CR-COO]/[ 3 N -CR-COO - ] 2 so that K a K b /K w = [ 2 N-CR-COO - ][ 3 N -CR-COO]/[ 3 N -CR-COO - ] 2 So for a solution of amino acid with two dissociable functional groups, the equilibrium amounts will be: 2 3 N -CR-COO - (aq)2 2 O(l) º 2 N-CR-COO - (aq) 3 N -CR-COO(aq)O - (aq) 3 O (aq) n-2x x x K a K b = [3O ][O - ]x 2 /(n-2x) 2 = x 2 /(n 2-4nx 4x 2 ) where [3O ][O - ] = K w K a K b /K w = x 2 /(n-2x) 2 = x 2 /(n 2-4nx 4x 2 ) (K a K b /K w )n 2 - (K a K b /K w )4nx (K a K b /K w )4x 2 = x 2 [4(K a K b /K w )-1]x 2 - (K a K b /K w )4nx (K a K b /K w )n 2 = 0 x = (K a K b /K w )4n ± SQRT([(K a K b /K w )4n] 2 - [16(K a K b /K w )-4](K a K b /K w )n 2 ])/[8(K a K b /K w )-2] So for a 0.1M solution of glycine (K COO = 0.005, K N3 = 2.5x10-10 ) 3 N -C 2 -COO - (aq) 2 O(l) º 3 N -C 2 -COO(aq) O - (aq) K b = 2.0x N -C 2 -COO - (aq) 2 O(l) º 2 N-C 2 -COO - (aq) 3 O (aq) K a = 2.5x10-10 K a K b /K w = 5.0x10-8 = [ 2 N-C 2 -COO - ][ 3 N -C 2 -COO]/[ 3 N -CR-COO - ] 2 = x 2 /(0.1-2x) 2 = x 2 / x4x 2 x 2 = 5x x10-8 x 2x10-7 x 2 0 ~ 1x 2 2x10-8 x - 5x10-10 x ~ 2.24x10-5 = [ ]??? so p ~ 4.65 IV. Isoelectric Points The isoelectric point, pi, is the p where 100% of the molecules have a net charge of zero. Operationally, this is the average of the pk values for the acid of the zwitterion form and the pk of the zwitterion form. Consider glycine below: 1-form 0-form -1-form pk COO pk N3 3 N -C-COO º 3 N -C-COO - º 2 N-C-COO - The pk of the "-carboxyl group (pk COO ) is 2.34 while that of the "-amino group (pk N3 ) is The acid of the zwitterion is the 1 form, its pk is pk COO. The zwitterion s pk is pk N3. The isoelectric point is the average of these pks: pi = (pk COO pk N3 )/2 = ( )/2 = 5.97 In a solution whose p is 5.97, all glycine molecules will have a net charge of zero. Many amino acids have more than 2 pks because the side chain contains functional groups that have dissociable hydrogens (like amines or carboxylic acids).
6 Lysine 3 N-C-COO (C 2 ) 4 )N 3 This is the low p form of the amino acid lysine; it has a 2 net charge. Lysine has an amine side chain with a pk a of 10.53; it has three dissociable hydrogens. The zwitterion form has deprotonated "-carbonyl and "-amino groups. The acid of that form simply has a deprotonated "-carbonyl group. Its pi is: (pk "-N3 pk R-N3 )/2 = ( )/2 = 9.74 Aspartic Acid 3 N-C-COO C 2 )COO On the left is the low p form of the amino acid aspartic acid; it has a 1 net charge. Aspartic acid has a carboxylic side chain with a pk a of 3.65; it also has three dissociable hydrogens. The zwitterion form has a deprotonated "-carbonyl group. The acid of that form has no deprotonated groups. Its pi is: (pk "-COO pk R-COO )/2 = ( )/2 = 2.77
Chapter In each case the conjugate base is obtained by removing a proton from the acid: (a) OH (b) I (c)
 Practice Exercises 16.1 Conjugate acid base pairs (a), (c), and (f) (b) The conjugate base of I is I (d) The conjugate base of N 2 is N 2 and the conjugate base of N 4 is N 3 (e) The conjugate acid of
Practice Exercises 16.1 Conjugate acid base pairs (a), (c), and (f) (b) The conjugate base of I is I (d) The conjugate base of N 2 is N 2 and the conjugate base of N 4 is N 3 (e) The conjugate acid of
Water. Water participates in H-bonding with biomolecules.
 Water Most biochemical reactions occur in an aqueous environment. Water is highly polar because of its bent geometry. Water is highly cohesive because of intermolecular hydrogen bonding. Water participates
Water Most biochemical reactions occur in an aqueous environment. Water is highly polar because of its bent geometry. Water is highly cohesive because of intermolecular hydrogen bonding. Water participates
Amino Acids and Peptides
 Amino Acids Amino Acids and Peptides Amino acid a compound that contains both an amino group and a carboxyl group α-amino acid an amino acid in which the amino group is on the carbon adjacent to the carboxyl
Amino Acids Amino Acids and Peptides Amino acid a compound that contains both an amino group and a carboxyl group α-amino acid an amino acid in which the amino group is on the carbon adjacent to the carboxyl
2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS
 2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS 2.1 Water and Polarity Both geometry and properties of molecule determine polarity Electronegativity - The tendency of an atom to attract electrons to itself
2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS 2.1 Water and Polarity Both geometry and properties of molecule determine polarity Electronegativity - The tendency of an atom to attract electrons to itself
ACIDS AND BASES. Note: For most of the acid-base reactions, we will be using the Bronsted-Lowry definitions.
 DEFINITIONS: ACIDS AND BASES Arrhenius Definition An acid in aqueous solution produces H + ions. A base in aqueous solution produces OH - ions. Bronsted Lowry Theory An acid is a proton donor A base is
DEFINITIONS: ACIDS AND BASES Arrhenius Definition An acid in aqueous solution produces H + ions. A base in aqueous solution produces OH - ions. Bronsted Lowry Theory An acid is a proton donor A base is
HA(aq) H + (aq) + A (aq) We can write an equilibrium constant expression for this dissociation: [ ][ ]
![HA(aq) H + (aq) + A (aq) We can write an equilibrium constant expression for this dissociation: [ ][ ] HA(aq) H + (aq) + A (aq) We can write an equilibrium constant expression for this dissociation: [ ][ ]](/thumbs/87/96059823.jpg) 16.6 Weak Acids Weak acids are only partially ionized in aqueous solution. There is a mixture of ions and un-ionized acid in solution. Therefore, weak acids are in equilibrium: Or: HA(aq) + H 2 O(l) H
16.6 Weak Acids Weak acids are only partially ionized in aqueous solution. There is a mixture of ions and un-ionized acid in solution. Therefore, weak acids are in equilibrium: Or: HA(aq) + H 2 O(l) H
Weak acids are only partially ionized in aqueous solution: mixture of ions and un-ionized acid in solution.
 16.6 Weak Acids Weak acids are only partially ionized in aqueous solution: mixture of ions and un-ionized acid in solution. Therefore, weak acids are in equilibrium: HA(aq) + H 2 O(l) H 3 O + (aq) + A
16.6 Weak Acids Weak acids are only partially ionized in aqueous solution: mixture of ions and un-ionized acid in solution. Therefore, weak acids are in equilibrium: HA(aq) + H 2 O(l) H 3 O + (aq) + A
Amino acids have the general structure: All amino acids have at least two acidic protons. One is the proton
 Worksheet rganic Acids and Bases II Amino Acids Acids are proton donors in aqueous solutions: A + 2 3 + + A - acid base conjugate conjugate base acid The strength of an acid is measured by the [ 3 + ]
Worksheet rganic Acids and Bases II Amino Acids Acids are proton donors in aqueous solutions: A + 2 3 + + A - acid base conjugate conjugate base acid The strength of an acid is measured by the [ 3 + ]
Chemistry 2000 Lecture 19: Organic acids
 Chemistry 2000 Lecture 19: Organic acids Marc R. Roussel March 8, 2018 Marc R. Roussel Chemistry 2000 Lecture 19: Organic acids March 8, 2018 1 / 22 Organic acids The acid dissociation constant, K a The
Chemistry 2000 Lecture 19: Organic acids Marc R. Roussel March 8, 2018 Marc R. Roussel Chemistry 2000 Lecture 19: Organic acids March 8, 2018 1 / 22 Organic acids The acid dissociation constant, K a The
C H C H 3. aspirin CHEMISTRY Topic #4: Organic Chemistry Fall 2018 Dr. Susan Findlay See Exercises in Topic 12
 = = 3 EMISTY 2000 aspirin Topic #4: rganic hemistry Fall 2018 Dr. Susan Findlay See Exercises in Topic 12 rganic Acids (arboxylic Acids) When you hear the term organic acid, it s generally referring to
= = 3 EMISTY 2000 aspirin Topic #4: rganic hemistry Fall 2018 Dr. Susan Findlay See Exercises in Topic 12 rganic Acids (arboxylic Acids) When you hear the term organic acid, it s generally referring to
Content : Properties of amino acids.. Separation and Analysis of Amino Acids
 قسم الكيمياء الحيوية.دولت على سالمه د. استاذ الكيمياء الحيوية ٢٠١٥-٢٠١٤ المحاضرة الثانية 1 Content : Properties of amino acids.. Separation and Analysis of Amino Acids 2 3 Physical Properties of Amino
قسم الكيمياء الحيوية.دولت على سالمه د. استاذ الكيمياء الحيوية ٢٠١٥-٢٠١٤ المحاضرة الثانية 1 Content : Properties of amino acids.. Separation and Analysis of Amino Acids 2 3 Physical Properties of Amino
Properties of Amino Acids
 Biochemistry Department Date:19/9/ 2017 Properties of Amino Acids Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology 1 Intended Learning Outcomes (ILOs) By the end of this lecture,
Biochemistry Department Date:19/9/ 2017 Properties of Amino Acids Prof.Dr./ FAYDA Elazazy Professor of Biochemistry and Molecular Biology 1 Intended Learning Outcomes (ILOs) By the end of this lecture,
A. Two of the common amino acids are analyzed. Amino acid X and amino acid Y both have an isoionic point in the range of
 Questions with Answers- Amino Acids & Peptides A. Two of the common amino acids are analyzed. Amino acid X and amino acid Y both have an isoionic point in the range of 5.0-6.5 (Questions 1-4) 1. Which
Questions with Answers- Amino Acids & Peptides A. Two of the common amino acids are analyzed. Amino acid X and amino acid Y both have an isoionic point in the range of 5.0-6.5 (Questions 1-4) 1. Which
CHEM J-3 June 2012
 CEM1611 2012-J-3 June 2012 In a standard acid-base titration, 25.00 ml of 0.1043 M a solution was found to react exactly with 28.45 ml of an Cl solution of unknown concentration. What is the p of the unknown
CEM1611 2012-J-3 June 2012 In a standard acid-base titration, 25.00 ml of 0.1043 M a solution was found to react exactly with 28.45 ml of an Cl solution of unknown concentration. What is the p of the unknown
Topic 4.8 AMINO ACIDS. Structure Acid-Base Properties Condensation Reactions Proteins
 Topic 4.8 AMI AIDS Structure Acid-Base Properties ondensation eactions Proteins STUTUE F AMI AIDS Amino acids are molecules containing an amine group and a carboxylic acid group. aturally occurring amino
Topic 4.8 AMI AIDS Structure Acid-Base Properties ondensation eactions Proteins STUTUE F AMI AIDS Amino acids are molecules containing an amine group and a carboxylic acid group. aturally occurring amino
Buffer solutions المحاليل المنظمة
 Buffer solutions المحاليل المنظمة Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Understanding ph balance The human
Buffer solutions المحاليل المنظمة Presented by Dr. Mohammad Saadeh The requirements for the Pharmaceutical Biochemistry I Philadelphia University Faculty of pharmacy Understanding ph balance The human
CHEM 109A Organic Chemistry
 CHEM 109A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter 2 Acids and Bases Central to Understanding Organic Chemistry Draw the conjugate acid of each of the following:
CHEM 109A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter 2 Acids and Bases Central to Understanding Organic Chemistry Draw the conjugate acid of each of the following:
3/30/2015. Third energy level. Second energy level. Energy absorbed. First energy level. Atomic nucleus. Energy released (as light)
 Chapter 2 An Introduction Chemistry Lecture 2: Energy Levels and Chemical Bonding Electrons are always moving Outside the nucleus in atomic orbitals Maybe usually Average distance from nucleus (size of
Chapter 2 An Introduction Chemistry Lecture 2: Energy Levels and Chemical Bonding Electrons are always moving Outside the nucleus in atomic orbitals Maybe usually Average distance from nucleus (size of
2. Acids and Bases. Grossman, CHE Definitions.
 Grossman, CE 230 2. Acids and Bases. 2.1 Definitions. Brønsted acids are proton donors, and Brønsted bases are proton acceptors. Examples of Brønsted acids: Cl, Br, 2 S 4,, + 2, + 4, 3, C 3 C 2, C 2 CC
Grossman, CE 230 2. Acids and Bases. 2.1 Definitions. Brønsted acids are proton donors, and Brønsted bases are proton acceptors. Examples of Brønsted acids: Cl, Br, 2 S 4,, + 2, + 4, 3, C 3 C 2, C 2 CC
Chemistry: The Central Science. Chapter 16: Acid-Base Equilibria. 16.1: Acids and Bases: A Brief Review
 Chemistry: The Central Science Chapter 16: Acid-Base Equilibria 16.1: Acids and Bases: A Brief Review Acids have a sour taste and cause certain dyes to change color Base have a bitter taste and feel slippery
Chemistry: The Central Science Chapter 16: Acid-Base Equilibria 16.1: Acids and Bases: A Brief Review Acids have a sour taste and cause certain dyes to change color Base have a bitter taste and feel slippery
Water, water everywhere,; not a drop to drink. Consumption resulting from how environment inhabited Deforestation disrupts water cycle
 Chapter 3 Water: The Matrix of Life Overview n n n Water, water everywhere,; not a drop to drink Only 3% of world s water is fresh How has this happened Consumption resulting from how environment inhabited
Chapter 3 Water: The Matrix of Life Overview n n n Water, water everywhere,; not a drop to drink Only 3% of world s water is fresh How has this happened Consumption resulting from how environment inhabited
Full file at Chapter 2 Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
1) Which of the following represents the breaking of a noncovalent interaction? Topic: The Nature of Noncovalent Interactions
 Multiple Choice Questions 1) Which of the following represents the breaking of a noncovalent interaction? A) hydrolysis of an ester B) dissolving of salt crystals C) ionization of water D) decomposition
Multiple Choice Questions 1) Which of the following represents the breaking of a noncovalent interaction? A) hydrolysis of an ester B) dissolving of salt crystals C) ionization of water D) decomposition
Chemistry. for the life and medical sciences. Mitch Fry and Elizabeth Page. second edition
 hemistry for the life and medical sciences Mitch Fry and Elizabeth Page second edition ontents Preface to the second edition Preface to the first edition about the authors ix x xi 1 elements, atoms and
hemistry for the life and medical sciences Mitch Fry and Elizabeth Page second edition ontents Preface to the second edition Preface to the first edition about the authors ix x xi 1 elements, atoms and
Acid-Base Chemistry. Chapter Brønsted Acid-Base Chemistry R P
 Chapter 5 Acid-Base Chemistry 5.1 Brønsted Acid-Base Chemistry R P The equilibrium constant (K eq ) gives quick insight into whether the reactant or product is more stable, and the extent to which the
Chapter 5 Acid-Base Chemistry 5.1 Brønsted Acid-Base Chemistry R P The equilibrium constant (K eq ) gives quick insight into whether the reactant or product is more stable, and the extent to which the
Acids and Bases. CHEM 102 T. Hughbanks. In following equilibrium, will reactants or products be favored? Strong acid (HCl) + Strong base (NaOH)
 Acids and Bases According to the Brønsted Lowry theory, all acid base reactions can be written as equilibria involving the acid and base and their conjugates. CEM 102 T. ughbanks All proton transfer reactions
Acids and Bases According to the Brønsted Lowry theory, all acid base reactions can be written as equilibria involving the acid and base and their conjugates. CEM 102 T. ughbanks All proton transfer reactions
Chapter 2: Acids and Bases
 hapter 2: Acids and Bases 32 hapter 2: Acids and Bases Problems 2.1 Write each acid- reaction as a proton-transfer reaction. Label which reactant is the acid and which the, as well as which product is
hapter 2: Acids and Bases 32 hapter 2: Acids and Bases Problems 2.1 Write each acid- reaction as a proton-transfer reaction. Label which reactant is the acid and which the, as well as which product is
Acids and Bases. A strong base is a substance that completely ionizes in aqueous solutions to give a cation and a hydroxide ion.
 Acid-Base Theories Arrhenius Acids and Bases (1884) Acids and Bases An acid is a substance that, when dissolved in water, increases the concentration of hydrogen ions. A base is a substance that, when
Acid-Base Theories Arrhenius Acids and Bases (1884) Acids and Bases An acid is a substance that, when dissolved in water, increases the concentration of hydrogen ions. A base is a substance that, when
Chapter 16. Acid-Base Equilibria
 Chapter 16. Acid-Base Equilibria 16.1 Acids and Bases: A Brief Review Acids taste sour and cause certain dyes to change color. Bases taste bitter and feel soapy. Arrhenius concept of acids and bases: An
Chapter 16. Acid-Base Equilibria 16.1 Acids and Bases: A Brief Review Acids taste sour and cause certain dyes to change color. Bases taste bitter and feel soapy. Arrhenius concept of acids and bases: An
IB Chemistry ABS Introduction An acid was initially considered a substance that would produce H + ions in water.
 IB Chemistry ABS Introduction An acid was initially considered a substance that would produce H + ions in water. The Brønsted-Lowry definition of an acid is a species that can donate an H + ion to any
IB Chemistry ABS Introduction An acid was initially considered a substance that would produce H + ions in water. The Brønsted-Lowry definition of an acid is a species that can donate an H + ion to any
[H O ][CH COO ] [CH COOH] 0.2 x
![[H O ][CH COO ] [CH COOH] 0.2 x [H O ][CH COO ] [CH COOH] 0.2 x](/thumbs/73/68762023.jpg) CEM1612 Answers to Problem Sheet 6 1. (a) 0.2 M acetic acid As acetic acid is a weak acid, [ 3 O + ] must be calculated: C 3 2 O 3 O + C 3 initial 0.2 large 0 0 change - negligible + + final 0.2 large
CEM1612 Answers to Problem Sheet 6 1. (a) 0.2 M acetic acid As acetic acid is a weak acid, [ 3 O + ] must be calculated: C 3 2 O 3 O + C 3 initial 0.2 large 0 0 change - negligible + + final 0.2 large
Acid-Base Chemistry & Organic Compounds. Chapter 2
 Acid-Base Chemistry & Organic Compounds Chapter 2 Brønsted Lowry Acids & Bases! Brønsted-Lowry Acid: Proton (H + ) Donor! Brønsted-Lowry Base: Proton (H + ) Acceptor! General reaction: HA + B: A - + BH
Acid-Base Chemistry & Organic Compounds Chapter 2 Brønsted Lowry Acids & Bases! Brønsted-Lowry Acid: Proton (H + ) Donor! Brønsted-Lowry Base: Proton (H + ) Acceptor! General reaction: HA + B: A - + BH
Chapter 16: Acid Base Equilibria Chapter 16 Acid-Base Equilibria Learning Standards & Objectives;
 Chapter 16: Acid Base Equilibria Chapter 16 Acid-Base Equilibria Learning Standards & Objectives; Chapter 16 AP16-1,2-01 AP16-1,2-02 AP16-1,2-03 AP16-3,4-01 AP16-3,4-02 AP16-5-01 AP16-6,7-01 AP16-6,7-02
Chapter 16: Acid Base Equilibria Chapter 16 Acid-Base Equilibria Learning Standards & Objectives; Chapter 16 AP16-1,2-01 AP16-1,2-02 AP16-1,2-03 AP16-3,4-01 AP16-3,4-02 AP16-5-01 AP16-6,7-01 AP16-6,7-02
SUPPLEMENTAL HOMEWORK SOLUTIONS WEEK 8
 SUPPLEMETAL MEWRK SLUTIS WEEK 8 Assignment for Tuesday, March 7 th 7.36 a) + + b) + + c) 6 14 4 + 6 14 4 + + d) 6 5 3 7 + 6 5 7 + Be sure to write the correct charges for the products. 7.4 a) l + l + b)
SUPPLEMETAL MEWRK SLUTIS WEEK 8 Assignment for Tuesday, March 7 th 7.36 a) + + b) + + c) 6 14 4 + 6 14 4 + + d) 6 5 3 7 + 6 5 7 + Be sure to write the correct charges for the products. 7.4 a) l + l + b)
INTRODUCTION. Amino acids occurring in nature have the general structure shown below:
 Biochemistry I Laboratory Amino Acid Thin Layer Chromatography INTRODUCTION The primary importance of amino acids in cell structure and metabolism lies in the fact that they serve as building blocks for
Biochemistry I Laboratory Amino Acid Thin Layer Chromatography INTRODUCTION The primary importance of amino acids in cell structure and metabolism lies in the fact that they serve as building blocks for
Amino Acids, Polypeptides and Proteins. An -amino acid is a carboxylic acid which has an amino group attached to the carbon to the COOH.
 Amino Acids, Polypeptides and Proteins An -amino acid is a carboxylic acid which has an amino group attached to the carbon to the OO. Polypeptides and proteins are composed, either completely or partially,
Amino Acids, Polypeptides and Proteins An -amino acid is a carboxylic acid which has an amino group attached to the carbon to the OO. Polypeptides and proteins are composed, either completely or partially,
Aqueous Equilibria, Part 1 AP Chemistry Lecture Outline
 Aqueous Equilibria, Part 1 AP Chemistry Lecture Outline Name: Acids and Bases Arrhenius...acids increase the when dissolved in H 2 O....bases increase the when dissolved in H 2 O. e.g., HCl and NaOH Bronsted-Lowry
Aqueous Equilibria, Part 1 AP Chemistry Lecture Outline Name: Acids and Bases Arrhenius...acids increase the when dissolved in H 2 O....bases increase the when dissolved in H 2 O. e.g., HCl and NaOH Bronsted-Lowry
Chapter 17 Acids and Bases
 Chapter 17 Acids and Bases - we are all familiar with 'acids' - depicted on television as burning liquids - from foods (i.e. vinegar) - taste "sour" or "tart' - less familiar with 'bases' - taste "bitter"
Chapter 17 Acids and Bases - we are all familiar with 'acids' - depicted on television as burning liquids - from foods (i.e. vinegar) - taste "sour" or "tart' - less familiar with 'bases' - taste "bitter"
10/16/17 ACIDS AND BASES, DEFINED WATER IS AMPHOTERIC OUTLINE. 9.1 Properties of Acids and Bases. 9.2 ph. 9.3 Buffers
 ACIDS AND BASES, DEFINED A hydrogen atom contains a proton and an electron, thus a hydrogen ion (H + ) is a proton: Acids: Proton (H + ) transfer between molecules is the basis of acid/base chemistry Ø
ACIDS AND BASES, DEFINED A hydrogen atom contains a proton and an electron, thus a hydrogen ion (H + ) is a proton: Acids: Proton (H + ) transfer between molecules is the basis of acid/base chemistry Ø
2.2.2 Bonding and Structure
 2.2.2 Bonding and Structure Ionic Bonding Definition: Ionic bonding is the electrostatic force of attraction between oppositely charged ions formed by electron transfer. Metal atoms lose electrons to form
2.2.2 Bonding and Structure Ionic Bonding Definition: Ionic bonding is the electrostatic force of attraction between oppositely charged ions formed by electron transfer. Metal atoms lose electrons to form
Chapter 16 Acids and Bases. Chapter 16 Acids and Bases
 . Chapter 16 Acids and Bases 1 Some Definitions Arrhenius Acid: Substance that, when dissolved in water, increases the concentration of hydrogen ions. Base: Substance that, when dissolved in water, increases
. Chapter 16 Acids and Bases 1 Some Definitions Arrhenius Acid: Substance that, when dissolved in water, increases the concentration of hydrogen ions. Base: Substance that, when dissolved in water, increases
Chapter 2 Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens. There are
Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens. There are
Chemical and Physical Properties of Organic Molecules
 Chemical and Physical Properties of Organic Molecules I.Elements A. Chemical symbols: C H O P S N C=carbon, H=hydrogen, O=oxygen, P=phosphorus, S=sulfur, N=nitrogen B. Top 3 Earth s surface = O, Si, Al
Chemical and Physical Properties of Organic Molecules I.Elements A. Chemical symbols: C H O P S N C=carbon, H=hydrogen, O=oxygen, P=phosphorus, S=sulfur, N=nitrogen B. Top 3 Earth s surface = O, Si, Al
11/14/10. Properties of Acids! CHAPTER 15 Acids and Bases. Table 18.1
 11/14/10 CHAPTER 15 Acids and Bases 15-1 Properties of Acids! Sour taste React with active metals i.e., Al, Zn, Fe, but not Cu, Ag, or Au 2 Al + 6 HCl 2 AlCl3 + 3 H2 corrosive React with carbonates, producing
11/14/10 CHAPTER 15 Acids and Bases 15-1 Properties of Acids! Sour taste React with active metals i.e., Al, Zn, Fe, but not Cu, Ag, or Au 2 Al + 6 HCl 2 AlCl3 + 3 H2 corrosive React with carbonates, producing
Chapter 8. Acidity, Basicity and pk a
 Chapter 8 Acidity, Basicity and pk a p182 In this reaction water is acting as a base, according to our definition above, by accepting a proton from HCl which in turn is acting as an acid by donating a
Chapter 8 Acidity, Basicity and pk a p182 In this reaction water is acting as a base, according to our definition above, by accepting a proton from HCl which in turn is acting as an acid by donating a
Acid Base Equilibria
 Acid Base Equilibria Acid Ionization, also known as acid dissociation, is the process in where an acid reacts with water to produce a hydrogen ion and the conjugate base ion. HC 2 H 3 O 2(aq) H + (aq)
Acid Base Equilibria Acid Ionization, also known as acid dissociation, is the process in where an acid reacts with water to produce a hydrogen ion and the conjugate base ion. HC 2 H 3 O 2(aq) H + (aq)
Chapter 4: Carbon and the Molecular Diversity of Life. 1. Organic Molecules 2. Chemical Groups
 Chapter 4: Carbon and the Molecular Diversity of Life 1. Organic Molecules 2. Chemical Groups 1. Organic Molecules Chapter Reading pp. 57-62 Elements in Biological Molecules Biological macromolecules are
Chapter 4: Carbon and the Molecular Diversity of Life 1. Organic Molecules 2. Chemical Groups 1. Organic Molecules Chapter Reading pp. 57-62 Elements in Biological Molecules Biological macromolecules are
1. Organic Molecules. Elements in Biological Molecules 2/13/2016. Chapter 4: Carbon and the Molecular Diversity of Life
 Chapter 4: Carbon and the Molecular Diversity of Life 1. Organic Molecules 2. Chemical Groups 1. Organic Molecules Chapter Reading pp. 57-62 Elements in Biological Molecules Biological macromolecules are
Chapter 4: Carbon and the Molecular Diversity of Life 1. Organic Molecules 2. Chemical Groups 1. Organic Molecules Chapter Reading pp. 57-62 Elements in Biological Molecules Biological macromolecules are
1. Amino Acids and Peptides Structures and Properties
 1. Amino Acids and Peptides Structures and Properties Chemical nature of amino acids The!-amino acids in peptides and proteins (excluding proline) consist of a carboxylic acid ( COOH) and an amino ( NH
1. Amino Acids and Peptides Structures and Properties Chemical nature of amino acids The!-amino acids in peptides and proteins (excluding proline) consist of a carboxylic acid ( COOH) and an amino ( NH
Chemical Properties of Amino Acids
 hemical Properties of Amino Acids Protein Function Make up about 15% of the cell and have many functions in the cell 1. atalysis: enzymes 2. Structure: muscle proteins 3. Movement: myosin, actin 4. Defense:
hemical Properties of Amino Acids Protein Function Make up about 15% of the cell and have many functions in the cell 1. atalysis: enzymes 2. Structure: muscle proteins 3. Movement: myosin, actin 4. Defense:
Chem(Bio) Week 6 Expt F: Amino Acid pka from potentiometry. Identification of an Amino Acid through Potentiometric Titration
 Objectives: Identification of an Amino Acid through Potentiometric Titration Keywords: Amino acid, zwitterions, enderson asselbach, pka, p arry out rough and then an accurate potentiometric titration of
Objectives: Identification of an Amino Acid through Potentiometric Titration Keywords: Amino acid, zwitterions, enderson asselbach, pka, p arry out rough and then an accurate potentiometric titration of
Equilibrium constant
 Equilibrium constant Equilibrium constant Many reactions that occur in nature are reversible and do not proceed to completion. They come to an equilibrium where the net velocity = 0 The velocity of forward
Equilibrium constant Equilibrium constant Many reactions that occur in nature are reversible and do not proceed to completion. They come to an equilibrium where the net velocity = 0 The velocity of forward
Chapter 14. Acids and Bases
 Chapter 14 Acids and Bases Section 14.1 The Nature of Acids and Bases Models of Acids and Bases Arrhenius: Acids produce H + ions in solution, bases produce OH - ions. Brønsted Lowry: Acids are proton
Chapter 14 Acids and Bases Section 14.1 The Nature of Acids and Bases Models of Acids and Bases Arrhenius: Acids produce H + ions in solution, bases produce OH - ions. Brønsted Lowry: Acids are proton
NAME. EXAM I I. / 36 September 25, 2000 Biochemistry I II. / 26 BICH421/621 III. / 38 TOTAL /100
 EXAM I I. / 6 September 25, 2000 Biochemistry I II. / 26 BIH421/621 III. / 8 TOTAL /100 I. MULTIPLE HOIE (6 points) hoose the BEST answer to the question by circling the appropriate letter. 1. An amino
EXAM I I. / 6 September 25, 2000 Biochemistry I II. / 26 BIH421/621 III. / 8 TOTAL /100 I. MULTIPLE HOIE (6 points) hoose the BEST answer to the question by circling the appropriate letter. 1. An amino
Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions 11 SUMMARY Section 2.1 Section 2.2 Section 2.3 Section 2.4 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive
Chapter 2 Water: The Solvent for Biochemical Reactions 11 SUMMARY Section 2.1 Section 2.2 Section 2.3 Section 2.4 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive
CHAPTER 18 ACID-BASE EQUILIBRIA
 CAPTER 18 ACID-BASE EQUILIBRIA FOLLOW UP PROBLEMS 18.1A Plan: Examine the formulas and classify each as an acid or base. Strong acids are the hydrohalic acids Cl, Br, and I, and oxoacids in which the number
CAPTER 18 ACID-BASE EQUILIBRIA FOLLOW UP PROBLEMS 18.1A Plan: Examine the formulas and classify each as an acid or base. Strong acids are the hydrohalic acids Cl, Br, and I, and oxoacids in which the number
Chapter 14 Acids and Bases
 Properties of Acids and Bases Chapter 14 Acids and Bases Svante Arrhenius (1859-1927) First to develop a theory for acids and bases in aqueous solution Arrhenius Acids Compounds which dissolve (dissociate)
Properties of Acids and Bases Chapter 14 Acids and Bases Svante Arrhenius (1859-1927) First to develop a theory for acids and bases in aqueous solution Arrhenius Acids Compounds which dissolve (dissociate)
1.1 The Fundamental Chemistry of life
 1.1 The Fundamental Chemistry of life Matter makes up everything in the universe, including all living organisms. Matter is composed of elements, a pure substance that cannot be broken down into simpler
1.1 The Fundamental Chemistry of life Matter makes up everything in the universe, including all living organisms. Matter is composed of elements, a pure substance that cannot be broken down into simpler
Chapter 2 Chemistry. The World of Elements. Why are we studying chemistry? Models of atoms. The Basics. Atomic structure determines behavior
 Chapter 2 Chemistry The World of Elements What? You thought you were all done with the Periodic Table? NEVER! Why are we studying chemistry? Biology has chemistry at its foundation Models of atoms Yeah,
Chapter 2 Chemistry The World of Elements What? You thought you were all done with the Periodic Table? NEVER! Why are we studying chemistry? Biology has chemistry at its foundation Models of atoms Yeah,
SBI4U BIOCHEMISTRY. Atoms, Bonding & Molecular Polarity
 SBI4U BIOCHEMISTRY Atoms, Bonding & Molecular Polarity 6 types of atoms make up 99% of all living organisms Naturally Occurring Elements in the Human Body Element Symbol Atomic # % of human body weight
SBI4U BIOCHEMISTRY Atoms, Bonding & Molecular Polarity 6 types of atoms make up 99% of all living organisms Naturally Occurring Elements in the Human Body Element Symbol Atomic # % of human body weight
Aqueous Equilibria: Acids and Bases
 /3/014 Aqueous Equilibria: Acids and Bases Ch. 16 What is an? What is a? There are actually multiple definitions Arrhenius: Dealt with species in aqueous solutions. Most basic definition of acis. Acid:
/3/014 Aqueous Equilibria: Acids and Bases Ch. 16 What is an? What is a? There are actually multiple definitions Arrhenius: Dealt with species in aqueous solutions. Most basic definition of acis. Acid:
CHAPTER 29 HW: AMINO ACIDS + PROTEINS
 CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
may contain one or more neutrons
 Biology 115 Fall 2001 Campos/Saupe Atoms and Molecules I. Introduction - living things are composed of the same chemical elements as the nonliving world and obey the same physical and chemical laws - living
Biology 115 Fall 2001 Campos/Saupe Atoms and Molecules I. Introduction - living things are composed of the same chemical elements as the nonliving world and obey the same physical and chemical laws - living
Acid-Base Chemistry. Introduction to Reaction Mechanism
 Acid-Base Chemistry Introduction to Reaction Mechanism What is an acid and what is a base? Bronsted-Lowry definition Acids are proton donors (A-H) e.g. HCl, H 2 SO 4, HBr, HNO 3, HI etc. Bronsted-Lowry
Acid-Base Chemistry Introduction to Reaction Mechanism What is an acid and what is a base? Bronsted-Lowry definition Acids are proton donors (A-H) e.g. HCl, H 2 SO 4, HBr, HNO 3, HI etc. Bronsted-Lowry
Midterm Exam III. CHEM 181: Introduction to Chemical Principles November 25, 2014 Answer Key
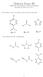 Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Assessment Schedule 2017 Scholarship Chemistry (93102)
 Scholarship Chemistry (93102) 2017 page 1 of 9 Assessment Schedule 2017 Scholarship Chemistry (93102) Evidence Statement Q Evidence Level 3 Scholarship Outstanding ONE (a)(i) Lewis structure: [Total electron
Scholarship Chemistry (93102) 2017 page 1 of 9 Assessment Schedule 2017 Scholarship Chemistry (93102) Evidence Statement Q Evidence Level 3 Scholarship Outstanding ONE (a)(i) Lewis structure: [Total electron
Wednesday, February 25, Acid and Base Reactions
 Acid and Base Reactions Dilute aqueous solution of acetic acid, C3COO Aqueous solution of sodium hydroxide, NaO The role of the ydrogen Ion Cl (aq) Æ + (aq) + Cl - (aq) What does the neutral atom consist
Acid and Base Reactions Dilute aqueous solution of acetic acid, C3COO Aqueous solution of sodium hydroxide, NaO The role of the ydrogen Ion Cl (aq) Æ + (aq) + Cl - (aq) What does the neutral atom consist
Chapter 2 Polar Covalent Bonds; Acids and Bases SAMPLE. Chapter Outline
 Chapter 2 Polar Covalent Bonds; Acids and Bases Chapter utline I. Polar covalent bonds (Sections 2.1 2.3). A. Electronegativity (Section 2.1). 1. Although some bonds are totally ionic and some are totally
Chapter 2 Polar Covalent Bonds; Acids and Bases Chapter utline I. Polar covalent bonds (Sections 2.1 2.3). A. Electronegativity (Section 2.1). 1. Although some bonds are totally ionic and some are totally
The Chemical Basis of Life
 The Chemical Basis of Life Chapter 2 Objectives Identify the four elements that make up 96% of living matter. Distinguish between the following pairs of terms: neutron and proton, atomic number and mass
The Chemical Basis of Life Chapter 2 Objectives Identify the four elements that make up 96% of living matter. Distinguish between the following pairs of terms: neutron and proton, atomic number and mass
2. Polar Covalent Bonds: Acids and Bases
 2. Polar Covalent Bonds: Acids and Bases Based on McMurry s Organic Chemistry, 6 th edition, Chapter 2 2003 Ronald Kluger Department of Chemistry University of Toronto 2.1 Polar Covalent Bonds: Electronegativity!
2. Polar Covalent Bonds: Acids and Bases Based on McMurry s Organic Chemistry, 6 th edition, Chapter 2 2003 Ronald Kluger Department of Chemistry University of Toronto 2.1 Polar Covalent Bonds: Electronegativity!
Ionic Equilibria. In the Brönsted Lowry classification, acids and bases may be anions such as HSO 4
 Ionic Equilibria Brönsted Lowry Theory According to the Brönsted Lowry theory, an acid is a substance, charged or uncharged, that is capable of donating a proton, and a base is a substance, charged or
Ionic Equilibria Brönsted Lowry Theory According to the Brönsted Lowry theory, an acid is a substance, charged or uncharged, that is capable of donating a proton, and a base is a substance, charged or
CHAPTER 6--- CHEMISTRY IN BIOLOGY. Miss Queen
 CHAPTER 6--- CHEMISTRY IN BIOLOGY Miss Queen SECTION 6.1 Atoms, Elements, Compounds COMPOSITION OF MATTER Matter - Everything in universe is composed of matter Matter is anything that occupies space or
CHAPTER 6--- CHEMISTRY IN BIOLOGY Miss Queen SECTION 6.1 Atoms, Elements, Compounds COMPOSITION OF MATTER Matter - Everything in universe is composed of matter Matter is anything that occupies space or
Cu 2+ (aq) + 4NH 3(aq) = Cu(NH 3) 4 2+ (aq) I (aq) + I 2(aq) = I 3 (aq) Fe 3+ (aq) + 6H 2O(l) = Fe(H 2O) 6 3+ (aq) Strong acids
 There are three definitions for acids and bases we will need to understand. Arrhenius Concept: an acid supplies H + to an aqueous solution. A base supplies OH to an aqueous solution. This is the oldest
There are three definitions for acids and bases we will need to understand. Arrhenius Concept: an acid supplies H + to an aqueous solution. A base supplies OH to an aqueous solution. This is the oldest
AP Biology. Why are we studying chemistry? Chapter 2. The Chemical Context of Life. The Basics. The World of Elements.
 Chapter 2. The Chemical Context of Life Why are we studying chemistry? Biology has chemistry at its foundation The Basics The World of Elements Everything is made of matter Matter is made of atoms Atoms
Chapter 2. The Chemical Context of Life Why are we studying chemistry? Biology has chemistry at its foundation The Basics The World of Elements Everything is made of matter Matter is made of atoms Atoms
Introductory Biochemistry
 Introductory Biochemistry Instructors Dr. Nafez Abu Tarboush Dr. Mamoun Ahram Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 6 th edition Recommended electronic
Introductory Biochemistry Instructors Dr. Nafez Abu Tarboush Dr. Mamoun Ahram Recommended textbooks Biochemistry; Mary K. Campbell and Shawn O. Farrell, Brooks Cole; 6 th edition Recommended electronic
CHAPTER 8: ACID/BASE EQUILIBRIUM
 CHAPTER 8: ACID/BASE EQUILIBRIUM Already mentioned acid-base reactions in Chapter 6 when discussing reaction types. One way to define acids and bases is using the Brønsted-Lowry definitions. A Brønsted-Lowry
CHAPTER 8: ACID/BASE EQUILIBRIUM Already mentioned acid-base reactions in Chapter 6 when discussing reaction types. One way to define acids and bases is using the Brønsted-Lowry definitions. A Brønsted-Lowry
Periodic Table. 8/3/2006 MEDC 501 Fall
 Periodic Table 8/3/2006 MEDC 501 Fall 2006 1 rbitals Shapes of rbitals s - orbital p -orbital 8/3/2006 MEDC 501 Fall 2006 2 Ionic Bond - acl Electronic Structure 11 a :: 1s 2 2s 2 2p x2 2p y2 2p z2 3s
Periodic Table 8/3/2006 MEDC 501 Fall 2006 1 rbitals Shapes of rbitals s - orbital p -orbital 8/3/2006 MEDC 501 Fall 2006 2 Ionic Bond - acl Electronic Structure 11 a :: 1s 2 2s 2 2p x2 2p y2 2p z2 3s
1014NSC Fundamentals of Biochemistry Semester Summary
 1014NSC Fundamentals of Biochemistry Semester Summary Griffith University, Nathan Campus Semester 1, 2014 Topics include: - Water & ph - Protein Diversity - Nucleic Acids - DNA Replication - Transcription
1014NSC Fundamentals of Biochemistry Semester Summary Griffith University, Nathan Campus Semester 1, 2014 Topics include: - Water & ph - Protein Diversity - Nucleic Acids - DNA Replication - Transcription
Covalent bonds can have ionic character These are polar covalent bonds
 Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character These are polar covalent bonds Bonding electrons attracted more strongly by one atom than by the other Electron distribution
Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character These are polar covalent bonds Bonding electrons attracted more strongly by one atom than by the other Electron distribution
Can you see atoms? M
 Can you see atoms? http://www.youtube.com/watch?v=s_okfvbzd9 M 2. Life requires about 25 chemical elements About 25 of the 92 natural elements are known to be essential for life. Four elements - carbon
Can you see atoms? http://www.youtube.com/watch?v=s_okfvbzd9 M 2. Life requires about 25 chemical elements About 25 of the 92 natural elements are known to be essential for life. Four elements - carbon
Lecture Presentation. Chapter 16. Acid Base Equilibria. John D. Bookstaver St. Charles Community College Cottleville, MO Pearson Education, Inc.
 Lecture Presentation Chapter 16 Acid Base Equilibria John D. Bookstaver St. Charles Community College Cottleville, MO Some Definitions Arrhenius An acid is a substance that, when dissolved in water, increases
Lecture Presentation Chapter 16 Acid Base Equilibria John D. Bookstaver St. Charles Community College Cottleville, MO Some Definitions Arrhenius An acid is a substance that, when dissolved in water, increases
Acid Dissociation Constant
 CE 131 Lecture 37 Lewis Acids and Bases Chapter 16: pp. 800-802. Acid Dissociation Constant C 2 3 2 + 2 3 + + C 2 3-2 [ 3 + ][C 2 3-2 ] K = [ 2 ][C 2 3 2 ] [ 3 + ][C 2 3-2 ] K a = K [ 2 ] = [C 2 3 2 ]
CE 131 Lecture 37 Lewis Acids and Bases Chapter 16: pp. 800-802. Acid Dissociation Constant C 2 3 2 + 2 3 + + C 2 3-2 [ 3 + ][C 2 3-2 ] K = [ 2 ][C 2 3 2 ] [ 3 + ][C 2 3-2 ] K a = K [ 2 ] = [C 2 3 2 ]
Life is a chemical process
 CHEMISTRY FOR LIFE WHY STUDY CHEMISTRY? Chemistry is the ultimate (basic) cause of all physiological processes Interactions of atoms produce chemical changes Chemical reactions involve a transfer of energy
CHEMISTRY FOR LIFE WHY STUDY CHEMISTRY? Chemistry is the ultimate (basic) cause of all physiological processes Interactions of atoms produce chemical changes Chemical reactions involve a transfer of energy
Content : Properties of amino acids.. Separation and Analysis of Amino Acids
 قسم الكيمياء الحيوية.دولت على سالمه د استاذ الكيمياء الحيوية ٢٠١٥-٢٠١٤ المحاضرة الثانية Content : Properties of amino acids.. Separation and Analysis of Amino Acids 2 -3 A. Physical properties 1. Solubility:
قسم الكيمياء الحيوية.دولت على سالمه د استاذ الكيمياء الحيوية ٢٠١٥-٢٠١٤ المحاضرة الثانية Content : Properties of amino acids.. Separation and Analysis of Amino Acids 2 -3 A. Physical properties 1. Solubility:
Chapter 16. Chemistry, The Central Science, 11th edition Theodore L. Brown, H. Eugene LeMay, Jr., and Bruce E. Bursten
 Chemistry, The Central Science, 11th edition Theodore L. Brown, H. Eugene LeMay, Jr., Bruce E. Bursten Chapter 16 John D. Bookstaver St. Charles Community College Cottleville, MO Some Definitions Arrhenius
Chemistry, The Central Science, 11th edition Theodore L. Brown, H. Eugene LeMay, Jr., Bruce E. Bursten Chapter 16 John D. Bookstaver St. Charles Community College Cottleville, MO Some Definitions Arrhenius
Chemical Bonding. Chemical Bonding 20/03/2015. The atomic radius increases from right to left. The atomic radius increases from top to bottom
 Chemical Bonding Atomic Radius: This distance from the nucleus to the outermost electron. Chemical Bonding Chemistry 11 Two factors must be taken into consideration in explaining this periodic trend: Increasing
Chemical Bonding Atomic Radius: This distance from the nucleus to the outermost electron. Chemical Bonding Chemistry 11 Two factors must be taken into consideration in explaining this periodic trend: Increasing
Carbon and the Molecular Diversity of Life
 Chapter 4 Carbon and the Molecular Diversity of Life PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Overview: Carbon The Backbone of Biological Molecules
Chapter 4 Carbon and the Molecular Diversity of Life PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Overview: Carbon The Backbone of Biological Molecules
Talk n Acids & Bases... Lady Dog! Definitions
 Talk n Acids & Bases... Lady Dog! Definitions So far in this course, we have looked at processes in chemistry that deal with, or are best explained by, ionic salts or molecules. Now we will turn our attention
Talk n Acids & Bases... Lady Dog! Definitions So far in this course, we have looked at processes in chemistry that deal with, or are best explained by, ionic salts or molecules. Now we will turn our attention
Titration curve of amino acids. BCH 312 [Practical]
![Titration curve of amino acids. BCH 312 [Practical] Titration curve of amino acids. BCH 312 [Practical]](/thumbs/95/122777605.jpg) Titration curve of amino acids BCH 312 [Practical] Titration curve Titration Curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of acid or alkali.
Titration curve of amino acids BCH 312 [Practical] Titration curve Titration Curves are produced by monitoring the ph of a given volume of a sample solution after successive addition of acid or alkali.
Basic Chemistry for Biology. Honors Biology
 Basic Chemistry for Biology Honors Biology 2013-2014 Composition of Matter Matter - Everything in universe is composed of matter Matter is anything that occupies space or has mass Mass quantity of matter
Basic Chemistry for Biology Honors Biology 2013-2014 Composition of Matter Matter - Everything in universe is composed of matter Matter is anything that occupies space or has mass Mass quantity of matter
Lecture 7. Acids. non-metals form anions. metals form cations H+ - Professor Hicks Inorganic Chemistry (CHE152) + anion. molecular compounds
 Lecture 7 Professor icks Inorganic Chemistry (CE152) Acids + + anion + - anion substances that release + ions when dissolved Strong acids Cl NO 3 2 SO 4 + Cl - + NO - 3 2 + SO 2-4 hydrochloric acid nitric
Lecture 7 Professor icks Inorganic Chemistry (CE152) Acids + + anion + - anion substances that release + ions when dissolved Strong acids Cl NO 3 2 SO 4 + Cl - + NO - 3 2 + SO 2-4 hydrochloric acid nitric
Acids Bases and Salts Acid
 Acids Bases and Salts Acid ph less than 7.0 Sour taste Electrolyte Names of Acids Binary acids Contain only 2 elements Begin with hydro; end with ic Ternary acids Ex: H 2 S = hydrosulfuric Contain a polyatomic
Acids Bases and Salts Acid ph less than 7.0 Sour taste Electrolyte Names of Acids Binary acids Contain only 2 elements Begin with hydro; end with ic Ternary acids Ex: H 2 S = hydrosulfuric Contain a polyatomic
Acids, Bases and Salts. Chapters 19
 Acids, Bases and Salts Chapters 19 Acid - Base Theories Section 19.1 What are common examples of acids and bases? What properties do you know about acids and bases? Arrhenius acids In 1887 A swedish Chemist,
Acids, Bases and Salts Chapters 19 Acid - Base Theories Section 19.1 What are common examples of acids and bases? What properties do you know about acids and bases? Arrhenius acids In 1887 A swedish Chemist,
CHAPTER 14 ACIDS AND BASES
 CHAPTER 14 ACIDS AND BASES Topics Definition of acids and bases Bronsted-Lowry Concept Dissociation constant of weak acids Acid strength Calculating ph for strong and weak acids and bases Polyprotic acids
CHAPTER 14 ACIDS AND BASES Topics Definition of acids and bases Bronsted-Lowry Concept Dissociation constant of weak acids Acid strength Calculating ph for strong and weak acids and bases Polyprotic acids
Chemistry Review: Atoms
 Chemistry Review: Atoms Atoms are made up : nucleus containing protons and neutrons orbitals containing electrons (2, 8, 8,...). Valence electrons outermost electrons Chemistry Review: Atoms All atoms
Chemistry Review: Atoms Atoms are made up : nucleus containing protons and neutrons orbitals containing electrons (2, 8, 8,...). Valence electrons outermost electrons Chemistry Review: Atoms All atoms
g. Looking at the equation, one can conclude that H 2 O has accepted a proton from HONH 3 HONH 3
 Chapter 14 Acids and Bases I. Bronsted Lowry Acids and Bases a. According to Brønsted- Lowry, an acid is a proton donor and a base is a proton acceptor. Therefore, in an acid- base reaction, a proton (H
Chapter 14 Acids and Bases I. Bronsted Lowry Acids and Bases a. According to Brønsted- Lowry, an acid is a proton donor and a base is a proton acceptor. Therefore, in an acid- base reaction, a proton (H
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility
 (P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
(P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
ADVANCED PLACEMENT CHEMISTRY ACIDS, BASES, AND AQUEOUS EQUILIBRIA
 ADVANCED PLACEMENT CHEMISTRY ACIDS, BASES, AND AQUEOUS EQUILIBRIA Acids- taste sour Bases(alkali)- taste bitter and feel slippery Arrhenius concept- acids produce hydrogen ions in aqueous solution while
ADVANCED PLACEMENT CHEMISTRY ACIDS, BASES, AND AQUEOUS EQUILIBRIA Acids- taste sour Bases(alkali)- taste bitter and feel slippery Arrhenius concept- acids produce hydrogen ions in aqueous solution while
BIOLOGY 101. CHAPTER 4: Carbon and the Molecular Diversity of Life: Carbon: the Backbone of Life
 BIOLOGY 101 CHAPTER 4: Carbon and the Molecular Diversity of Life: CONCEPTS: 4.1 Organic chemistry is the study of carbon compounds 4.2 Carbon atoms can form diverse molecules by bonding to four other
BIOLOGY 101 CHAPTER 4: Carbon and the Molecular Diversity of Life: CONCEPTS: 4.1 Organic chemistry is the study of carbon compounds 4.2 Carbon atoms can form diverse molecules by bonding to four other
Acids and Bases: Molecular Structure and Acidity
 Tutorial Contents A. Introduction B. Resonance C. Atomic Radius D. Electronegativity E. Inductive Effect F. Exercises G. Exercise Solutions Acids and Bases: Molecular Structure and Acidity Review the Acids
Tutorial Contents A. Introduction B. Resonance C. Atomic Radius D. Electronegativity E. Inductive Effect F. Exercises G. Exercise Solutions Acids and Bases: Molecular Structure and Acidity Review the Acids
