Plant Plumes Volume 2: Some Coal-Fired Power Plants in the
|
|
|
- Derrick Stone
- 5 years ago
- Views:
Transcription
1 Particle Formation and Growth in Power Plant Plumes Volume : Some Coal-Fired Power Plants in the Western United States EA-315, Volume Research Project 33-1 Final Report, May 1983 Prepared by UNIVERSITY OF WASHINGTON Cloud and Aerosol Research Group Atmospheric Sciences Department Seattle, Washington Principal Investigators P. V. Hobbs D. A. Hegg M. W. EItgroth L. F. Radke Prepared for Electric Power Research Institute 341 Hillview Avenue Palo Alto, California 9434 EPRI Project Manager C. Hakkarinen Environmental Physics and Chemistry Program Energy Analysis and Environment Division
2 ORDERING INFORMATION Requests for copies of this report should be directed to Research Reports Center (RRC), Box 549, Palo Alto, CA 9433, (415) There is no charge for reports requested by EPRI member utilities and affiliates, U.S. utility associations, U.S. government agencies (federal, state, and local), media, and foreign organizations with which EPRI has an information exchange agreement. On request, RRC will send a catalog of EPRI reports. Copyright (C) 1983 Electric Power Research Institute. Inc. All rights reserved. NOTICE This report was prepared by the organizaiion(s) named below as an account of work sponsored by Ihe Electric Power Research Institute. Inc. (EPRI). Neither EPRI. members of EPRI, Ihe organization(s) named below, any person acting on behalf of any of them- (a) makes any warranty, express or implied, with respect to the use of any information, apparatus, method, or process disclosed in this reporl or that such use may nol infringe privately owned rights: or (b) assumes any liabilities with respect to Ihe use of. or for damages resulting from the use of. any information, apparatus, method, process disclosed in this report. Prepared by University of Washington Seattle, Washington
3 ABSTRACT Airborne measurements have been made of the concentrations of particulate sulfur, sulfate, nitrate, total particle volume, Aitken nuclei and various trace gases in the plumes of six coal-fired power plants situated in the West and Midwest of the United States. Simultaneous measurements of relevant meteorological parameters (e.g. relative humidity, temperature, ultraviolet light) were also taken. Gas-toparticle conversion rates were calculated from these data by four different techniques. The SO^-to-SCL conversion rates ranged from to 5.7%/hr. The rate correlated well (r.9) with a parameter indicative of the reaction of plume SO^ with ambient OH radicals. Further support for the importance of this type of reaction was provided by an observed inverse relationship between the ratio of Aitken nuclei to SOn concentrations and a conservative plume tracer. A sophisticated plume model predicts that this type of relationship wil be most prevalent for plume SO^ reacting with radicals, or radical-precursors, from the ambient air. The plume nitrate data showed that significant nitrate formation occurred in the plumes on only one of the five flights on which sufficient nitrate data were collected to permit a decision on this point. The NO -to-nitrate conversion rate on this flight was ^.4%/hr for distances of 4.8 to 43. km from the stack. The SOp-to-sulfate conversion rates, measured simultaneously, ranged from.7 to.8%/hr. The rates at which new particles were nucleated in the plumes were evaluated and the ratio of this nucleation rate to the rate of formation of new particle volume? 5-3 was calculated. The ratio ranged from-.7 x 1 to 3.9 x 1 particles pm with a mean of 7.4 x 14 +/-. x 15 particles v-m 3. The data suggest that this ratio varies with plant locale. Results from a model of particle interactions in power plant plumes indicate that this ratio is important in determining the lightscattering coefficient of the particles in a plume and visibility degradation. The relationship between rates of formation of new particle surface area and new particle volume in the plumes was also explored. It was found that the particle surface area varied as the three-fifths power of the rate of formation of new particle volume. iii
4 EPRI PERSPECTIVE PROJECT DESCRIPTION Sulfur oxides and nitrogen oxides have been implicated as major contributors to visibility impairment and acidic precipitation in the United States. Formation of small particles that scatter light, producing "haze" and act as condensation nuclei for raindrops has been reported to occur in the plumes of fossil-fueled power plants as well as other locations. The studies described in this two-volume report involved theoretical and field investigations of the rates of formation and size distributions of particles in six coal-fired power plants in the midwestern and western United States. PROJECT OBJECTIVES The objectives of the project, which spanned some five years, were to measure particle size distributions in coal-fired power plant plumes at various locations and under a variety of meteorological conditions. In addition, a three-dimensional numerical model was developed and refined for simulating particle formation and growth in plumes. PROJECT RESULTS The measured rates of conversion of sulfur dioxide to particulate sulfate ranged from to 5.7% per hour. However, most observations fell within a narrower range of.1 to 1.% per hour. Reaction rates were all found to depend on travel time from the stack and ultraviolet light intensity. The PHOENIX model predictions of maximum gas-to-particle conversion rates that occur near the edges of the plume are in agreement with the measurements. Elsewhere in the plume, the model predictions were about a factor of 1 lower than the measurements. The PHOENIX model has been applied in recent years by other sponsors to the specific question of haze generation in western plumes. Charles Hakkarinen, Project Manager Energy Analysis and Environment Division
5 ACKNOWLEDGMENTS This work was supported by the Energy Analysis and Environment Division of the Electric Power Research Institute under Contract RP33-1 to the University of Washington. We wish to express our thanks to Dr. C. Hakkarinen of EPRI for his help and interest, and to the Pacific Power and Light Company, the Arizona Public Service Company, Basin Electric Power Cooperative, the Minnkota Power Cooperative, Northern States Power, and the Texas Utilities Service Company for their cooperation in this study. vii
6 CONTENTS Section SUMMARY REVIEW OF PREVIOUS WORK AND SCOPE OF STUDY STATEMENT OF PROBLEM PREVIOUS STUDIES Laboratory Studies of Gas-to-Particle Conversion Field Studies of Gas-to-Particle Conversion Field Studies of Particles in Plumes SCOPE OF THE PRESENT STUDY INSTRUMENTATION AND DESIGN OF THE FIELD STUDY THE UNIVERSITY OF WASHINGTON AIRCRAFT FACILITY Aitken Nucleus Counter Electrical Aerosol Analyzer Royco Counter Ozone Analyzer Oxides of Nitrogen Analyzer Sulfur Analyzers Hydrocarbon Analysis Pallflex Filter Samples Teflon Filters LOCATIONS AND CHARACTERISTICS OF THE POWER PLANTS Centra1ia Four Corners LeIand-Olds Minnkota Sherburne County Big Brown DESIGN OF THE FIELD STUDY Plume Dynamics Meteorological and Chemical Parameters STUDIED ix
7 CONTENTS Section 3 RESULTS OF THE FIELD STUDY THE DATA BASE PARTICLE DATA FILTER DATA PARTICLE SIZE DISTRIBUTIONS HYDROCARBON DATA METEOROLOGICAL DATA 4 ANALYSIS OF DATA METHODOLOGIES FOR DETERMINING GAS-TO-PARTICLE CONVERSION THE Total Particle Volume Method Aitken Nucleus Production Method Particulate Filter Method PHOENIX PLUME MODEL Meteorological Gas Reactions Particle Dynamics PHYSICAL ASPECTS OF GAS-TO-PARTICLE Nucleation and Condensation Particle Volume Formation and SO;? CONVERSION Concentrations Asymptotic Behavior of the Particles in the Plumes Light Scattering CHEMICAL ASPECTS OF GAS-TO-PARTICLE CONVERSION Chemical Nature of the Conversion Products Possible Factors Influencing the SO^ Conversion Rate RATES
8 CONTENTS Section 5 REFERENCES APPENDIX A: ANALYSIS OF VARIANCES APPENDIX B: DERIVATION OF THE [OH] PARAMETER APPENDIX C: GAS-TO-PARTICLE CONVERSION RATES PREDICTED BY THE PHOENIX MODEL xi
9 ILLUSTRATIONS -1 Particle- and gas-measuring instruments aboard the University of Washington s B-3 aircraft. - Cal ibration curve for the flash vaporization apparatus. 3-1 Scattergram of [SO^] derived from two different techniques. 3- Particle number distribution in the Centralia plume at a range of 3. km on August, Particle number distribution in the Four Corners plume at a range of 6.4 km on 3, Particle number distribution in the LeIand-Otds plume at a range of 3. km on 16,. 3-5 Particle number distribution in the Minnkota plume at a range of 3. km on 16,. 3-5 Particle number distribution in the Minnkota plume at a range of 3. km on 16,. 3-6 Particle number distribution in the Sherburne County plume at a range of 3. km on,. 3-7 Particle number distribution in the Big Brown plume at a range of 4.8 km on 6,. 3-8 Particle volume distribution in the Big Brown plume at a range of 4.8 km on 6,. 3-9 Particle volume distribution in the Sherburne County plume at a range of 3. km on,. 4-1 P (r) as a function of particle radius r. 4- Schematic of the processes considered in the University of Washington s PHOENIX plume model 4-3 Percentage of gas-to-particle conversion products forming new particles in the plume of the Sherburne County power plant at a range of 4.3 km on ;, as predicted by the PHOENIX plume model 4-4 Percentage of gas-to-particle conversion products forming new particles in the plume of the LeIand-Olds power plant at a range of 9.1 km on 16,, as predicted by the PHOENIX plume model 4-5 Percentage of gas-to-particle conversion products forming new particles in the plume of the LeIand-Olds power plant at a range of 17.8 km on 16,, as predicted by PHOENIX plume model xiii
10 Figure ILLUSTRATIONS 4-6 Excess concentrations (dn/d log D) in units of cm"3 of particles 4- of D UL55 pm measured in the plume of the LeIand-Olds power plant on 16,. (a) measured, (b) predicted by the PHOENIX plume model 4-7 Excess concentration (dn/d log D) in units of 14 cm" of particles 4-1 D ^.4 ym in the plume of the LeIand-Olds power plant on 16,. (a) measured, predicted by the PHOENIX plume model 4-8 Comparisons of the relationship of particle surface area to the rate 4-3 of formation of particle volume measured in the power plant discussed in this study. 4-9 Comparisons of measurements of the light-scattering coefficient bg 4-5 with values predicted by the PHOENIX plume model across the plumes of (a) the Big Brown plant at ranges of 31.5 and 5 km on 6,, (b) the Sherburne County plant at ranges of 3.7 and km on,, and (c) the LeIand-Olds plant at ranges of 9.3 and 19 km on 16,. 4-1 Plot of SO^-to-SO^ conversion rate (derived from ion-exchange 4-3 chromatography) against the right-hand side of Eq C-1 Homogeneous gas-to-particle conversion rates (in units of % SO^/hr) C- across the plume of the Big Brown power plant at a range of 49 km on 6, as predicted by the PHOENIX plume model C- Homogeneous gas-to-particle conversion rates (in units of % SO^/hr) C-3 across the plume of the LeIand-Olds Power plant at a range of 9.1 km on 16, as predicted by the PHOENIX plume model C-3 Homogeneous gas-to-particle conversion rates (in units of % S/hr) C-4 across the plume of the LeIand-Olds power plant at a range of 9.1 km on 16, as predicted by the PHOENIX plume model C-4 Homogeneous gas-to-particle conversion rates (in units of % SOo/hr) C-5 across the plume of the LeIand-Olds power plant at a range of T7.8 km on 16, as predicted by the PHOENIX plume model page xiv
11 TABLES Table 1-1 Summary of SO^ Oxidation Processes Relevant to Gas-to-Particle Conversion in Power Plant Plumes 1- Summary of SO^ 1-3 Summary of N^ Reactions on Various Particulate Species Oxidation Processes Relevant to Gas-to-Particle Conversion in Power Plant Plumes 1-4 Results of Previous Field Measurements on the Rates of SO? Oxidation in Power Plant Plumes -1 Particle- and gas-measuring instruments aboard the University of Washington s B-3 aircraft - Characteristics of Power Plants Studied 3-1 Data Flights of B-3 Used in This Study 3- Particle Volume and Aitken Nucleus Measurements 3-3 Particulate Sulfate Concentrations Derived from Flash Vaporization Analysis of Pallflex Filters 3-4 Particulate Sulfate Measurements Derived from the X-Ray Fluorescence Analysis of Teflon Filters 3-5 Particulate Sulfate and Nitrate Concentrations Derived from Ion-Exchange Chromatography Analysis of Teflon Filters 3-6 Comparison of Particulate Sulfate Concentrations Obtained by Three Different Techniques 3-7 Hydrocarbon Concentrations Measured in Ambient Air in the Vicinity of Five of the Power Plants Discussed in This Study 4-1 Gas-to-Particle Conversion Rates Calculated by Three Different Techniques. The Rates are Given in Terms of SO^ Conversion Rates Assuming the Conversion Product is Sulfunc Acid 4- Rates of Nucleation of Aitken Nuclei and Values of the Coagulation Coefficient KQ Derived from Measurements of Particle Size Distributions in Power Plant Plumes 4-3 Comparison of Gas-to-Particle Conversion Rates Deduced from the Total Particle Volume and the Particulate Filter Methods 4-4 The Variance (o) in the Fraction (C/A) of the Volume of New Particles to the Total Volume of New Material Formed by Gasto-Particle Conversion in the Power Plant Plumes. 4-5 Some Parameters Relevant to the Physics of Particle Formation in the Plumes 4-6 Parameters Employed in the Factor Analysis 4-7 Correlation Between SO^ Concentrations and the Ratio of Aitken Nucleus to SO^ Concentrations xv
12 SUMMARY RESULTS OF THIS STUDY Gas-to-particle (g-to-p) conversion rates have been measured in the plumes of six coal-fired power plants situated in the West and Midwest of the United States. The conversion rate was estimated from measurements of the changes in the total volume of particles in the plume, the production rate of Aitken nuclei and three different particulate filter analysis techniques (flash vaporization. X-ray fluorescence and ion-exchange chromatography). Comparison of sulfate concentrations derived from X-ray fluorescence and ion-exchange chromatography showed fair agreement-indicating that most of the particulate sulfur is sulfate. Comparison of g-to-p conversion rates estimated from the changes in total particle volume with those derived from particulate filter data showed, in general that the major portion of the g-to-p conversion product was sulfate. However, in some instances, the g-to-p conversion rate based on total particle volume was higher than the rate based on particulate filter analysis; this suggests that conversion products other than sulfate may sometimes be formed in power plant plumes. One possible conversion product, other than sulfate, which has been postulated to be produced in power plant plumes, is nitrate. To evaluate this hypothesis some nitrate measurements were made in the plumes. The data suggest that nitrate formation, in general was of little importance during the flights in which measurements were obtained. An estimate of the NO -to-nitrate conversion rate was made for one of the cases studied at the Big Brown (Texas) power plant and was found to be ^.4%/hr for distances of 4.8 to 43. km. The SO^-to-sulfate conversion rates, measured simultaneously, ranged from.7 to.8%/hr and increased with travel time from the stack. The rates at which new particles were nucleated in the plumes were evaluated and the ratio of this nucleation rate to the rate of formation of new particle volume 5 was calculated. The ratio was found to range from.7 x 1 to 3.9 x 1 particles pm 3 with a mean value of 7.4 x 14 +/-. x 15 particles pm~ This ratio is S-1
13 indicative of the fraction of g-to-p conversion product which forms new particles --the remainder of the conversion product condensing directly onto already existing particles. The large variation in this ratio, some of which is attributable to differing plant locale, suggests regional differences in the g-to-p conversion process. Results from the PHOENIX pl ume model suggest that it should be possible to predict this ratio on the basis of a modified version of the theory of McMurry (7). The PHOENIX model outputs suggest the importance of the above ratio in dfelermining the light-scattering coefficient and visibility degradation. Further analysis was made of the relationship between the rates of formation of new particle surface area and new particle volume. The data were found to be in fair agreement with the theoretical relationship of McMurry and Friedlander (64) in which the particle surface area varies as the rate of formation of new particle volume raised to the 3/5 power. This suggests that the size distribution of the particles in the plume becomes self-preserving. The SOp-to-particulate sulfate conversion rates were found to range from to 5.7%/hr--the higher rates general ly occurring in the Southwest. This range is in agreement with previous studies conducted both in the Eastern United States and at the Four Corners power plant in New Mexico. The SO., g-to-p conversion rate was also found to depend on travel time from the stack and UV ight intensity. Both the physics and the chemistry of the g-to-p conversion process(es) suggest that the dominant (though not necessarily the sole) conversion mechanism in the plumes studied is the oxidation of SOn by OH radicals. A significant correlation (r.9) was found between the conversion rates and a parameter indicative of this reaction. The results of this study have suggested several areas in which further efforts might yi,e1d valuable information. While the data col lected in this study suggest that the SOp-to-sulfate conversion process is predominantly SO? oxidation by OH radicals this has not been conclusively demonstrated. An extension of the present work would be to correlate measured g-to-p conversion rates with actual measurements of OH concentrations. Furthermore, this study was conducted preferentially in fair weather when free S-
14 radical reactions would be at their maximum importance. More data should be gathered under cloudy weather conditions when aqueous processes might dominate. The question of the source of the variation in the fraction of new particle volume that appears as new particles has not been fully answered by this study. More precise data on particle surface area, volume formation rates, coll ision frequencies, and particle nucleation rates are necessary to determine possible relationships between these parameters and the fraction of new particle volume that forms new particles. The use of a refined version of the theory of McMurry (7) in the PHOENIX plume model appears to be a potential ly useful tool in attacking this question. Further data are needed on nitrate concentrations in power plant plumes in order to evaluate more fully the importance of nitrate formation to the overall g-to-p conversion process. In view of the low nitrate concentrations that we measured, much larger sample volumes will be needed than those obtained in this study; volumes as high as liters may be necessary. Analysis for particulate organics should also be carried out together with a more detailed analysis of background hydrocarbons to identify possible particulate organic precursors. The results of this study suggest that regional differences may exist in the g-to-p conversion process. Sufficient data should be gathered at each of several different locales to allow parameters such as the ratio of particle nucleation rate to particle volume formation rate to be determined with great statistical precision at each site. Statistically meaningful intersite comparisons could then be made. S-3
15 Section REVIEW OF PREVIOUS WORK AND SCOPE OF STUDY STATEMENT OF PROBLEM In view of the projected increase in reliance on coal as a major source of energy in the United States, studies of the effects on air quality of the emissions from coal-fired electric power plants have taken on increasing importance. In addition to the particles directly emitted into the atmosphere from such plants, certain trace gas emissions are converted in the atmosphere into liquid drops or solid particles by gas-to-particle (g-to-p) conversion. The products of g-to-p conversion are thought to play an important role in such diverse areas as visibility degradation [Waggoner et a1. (1 )], climatology [Bolin and Charlson ()], and the production of acid rain [Marsh (3) Scott (4)]. The gas that has been most intensely studied in this regard is sulfur dioxide a likely precursor for the ubiquitous sulfate particles in the atmosphere, and a gas for which coalfired power plants are the largest anthropogenic point sources. However, there is increasing evidence that atmospheric nitrates produced by the oxidation of anthropogenic NO also play a role in the formation of acid rain [Likens et a1 (5) Marsh (3)]; coal-fired electric power plants are major sources of N^. While the nature and magnitude of the g-to-p conversion process are of primary interest, considerable attention has been paid recently to the physical aspects of the conversion process. For example, the nucleation rate of secondary particles, the fraction of the g-to-p conversion products that form new particles (as opposed to that which condenses onto existing particles) and the relationship between particle surface area and volume formation rate of particles, are all topics of importance [Whitby et a1 (6); Friedlander (7)]. Unfortunately, despite the fact that emissions from coal-fired power plants have received considerable attention over the last two decades, there is still no consensus regarding either the rates or nature of the g-to-p conversion processes [Leyy et a1 (8) Forrest and Newman (9); Husar et a1 (1)]. Furthermore, 1-1
16 most previous field studies have been conducted on power plants situated in the eastern part of the United States. Since air qual ity in the eastern states is unique, the results of the earlier studies may not be applicable to the western states, where many coal-fired power plants are projected to be built. In Volume of this Report [Hobbs et a1 (11 )], we described studies of the plumes from two coal-fired power plants situated in the western United States. Emphasis was placed on a delineation of the overall evolution of particles in the plumes and on a numerical model of power plant plumes. In this volume an account is given of investigations carried out on six coal-fired power plants situated in the West and Midwest of the United States. Emphasis is placed on elucidation of the g-to-p conversion processes in these plumes and on physical aspects related to the growth of particles. PREVIOUS STUDIES Laboratory Studies of Gas-to-Partide Conversion Most laboratory studies of g-to-p conversion have dealt with SO? oxidation and sulfate formation. Relatively little work has yet been done on nitrate and organic particulate formation, both of which may be of importance in coal-fired power plant plumes. Studies concerned with the homogeneous gas phase oxidation of SO? liquid phase oxidation of SO? have been reviewed recently by Calvert et a1 (1), those dealing with the by Hegg and Hobbs (13), and those deal ing with heterogeneous reactions on particles by Urone et a1 (14). Table 1-1 and 1- summarize the oxidation processes discussed in these reviews which are relevant to particulate production in power plant plumes. Probably the single most important reaction is the homogeneous gas phase oxidation of SO? by OH radicals [Calvert (1)]. However, under various conditions, any of the reactions shown in Table 1-1 could make a substantial contribution to the g-to-p conversion rate. Indeed, it is likely that many of these processes occur simultaneously in plumes under most atmospheric conditions. Possible particulate nitrogen formation mechanisms of relevance to coal-fired power plant plumes are summarized in Table 1-3. Considerably less is known about particulate nitrogen formation than is the case with sulfate formation. 1-
17 SUMMARY OF SO^ Table 1-1 OXIDATION PROCESSES RELEVANT TO GAS-TO-PARTICLE CONVERSION IN POWER PLANT PLUMES Process (a) SO^ + OH + (M) HSOg (+M) SO^ + HO? OH + SOg SO? + CH^ CHgO + SO^ SO? + CH^CHOO SO^ + CHgCHO Rate in the Atmosphere (%/h) Reference Homogeneous Gas Phase.4.7 Davis et a1 (15) Calvert et a1 (1) Calvert et a1 (1) Catvert et a1 (1) Cox & Penkett (16) Calvert et a1 (1) SO 3 SO 3 SO 3 SO +? Oy " (b) Homogeneous Liquid Phase Fe +3 so] + ^.1: ^ SO +.4 Penkett 18) Larson et a1 19) Mn^ & Fe"3. Barrie & Georgii () + Soot -. SO^ SO^ +.13 Brimblecombe & Spedding 17) Fast Chang et a1 1) (c) Heterogeneous SO^ + Soot + ^ SO^? SOr, + aerosol particles with * various trace metals SO,? Novakov et a1 ) Urone et a1 (14) *See Table 1-1-3
18 Table 1- SUMMARY OF SO? REACTIONS ON VARIOUS PARTICIPATE SPECIES FROM URONE ET AL. (14) Particulate Species CaCOg Cr^3 ^5 NaCI A^ CaO AI^Og/CaO PbO PbO^ pe^ Fe^3 SO^ Reacted (%/min)
19 Table 1-3 SUMMARY OF N^ OXIDATION PROCESSES RELEVANT TO GAS-TO-PARTICLE CONVERSION IN POWER PLANT PLUMES Process (a) NO? + OH(+M) HNOg (+M) N^ + Og N^ + Og NO + RO NORO N^ + RO NO?RO NOn + RCOOg RCOOgNO^ Rate in the Atmosphere (%/h) Homogeneous Gas ^13 ^1? Fast Phase Reference Cox (3) Leighton (4) Calvert et a1 (1) N^ + Og NOg + Og NO (g) + H^SO^ (&) NOg (b) Homogeneous Liquid Phase? Penkett (18) +? Cox (3) (c) Heterogeneous Processes NO + Soot NH, + amines + amides? NH-, + Soot W + a"n"es + amides? Chang & Novakov (5) Chang & Novakov (5) 1-5
20 Once again, the reaction of most importance is clearly the hydroxyl oxidation reaction. One interesting consequence of this is that the SO., and NO., in coalfired power plant plumes must compete for the available OH radicals. Possible mechanisms for organic particle formation under conditions resembling those in coal-fired power plant plumes are as yet merely speculative. Reactions such as the methyl peroxy (CH^) oxidation of SO^, peroxyacyi (RCOO^) oxidation of NO?, shown in Table 1-3, will produce organic shown in Table 1-1 or the sulfur and organic nitrogen compounds, respectively. It is unl ikely, however, that these reactions are major sources of particulate orgam cs. That particulate organics can be formed under conditions similar to those in power plant plumes has been suggested by numerous smog chamber studies [e.g. Leighton (4); Kocmond and Yang (6)]. There is one question of considerable importance in g-to-p conversions in plumes on which some laboratory data are available, namely, whether the new particulate mass is created by the formation of new particles or by the condensation of the gases onto existing particles. Cox 3) has stated, on the basis of theoretical calculations and smog chamber experiments, that while oxidation of NO will not provide sufficient vapor pressure of HNO-, to produce heteromolecular nucleation, sufficient HpSO^, can be produced, at least under urban conditions, to produce new particles. He therefore predicts that HNOo produced by g-to-p conversion should condense onto existing particles but that H?SO^ may produce new mass either by heteromolecular nucleation or by condensation. McMurry (7) has investigated, theoretically, the conditions under which either nucleation or condensation would be the dominant fate of g-to-p conversion products and found them dependent on the available surface area of particles and the g-to-p conversion rate. Field Studies of Gas-to-Particle Conversion Previous field studies of g-to-p conversion in power plant plumes have concentrated on determining the SO? oxidation-sulfate formation rate and the influence of various meteorological parameters on this rate. A summary of the SO,, oxidation rates that have been derived is given in Table 1-4. It can be seen that most recent studies place the oxidation rate between and 1%/h. While some of the variations shown, both inter- and infra-study, could plausibly be attributed to 1-6
21 Table 1-4 RESULTS OF PREVIOUS FIELD MEASUREMENTS ON THE RATES OF SO,, OXIDATION IN POWER PLANT PLUMES Power Plant Location Colbert Plant (Alabama) 33 MW Plant (Eastern U.S. Frankfurt am Main (West Germany) Crystal River (Florida) Keystone (Pennsylvania) Labadie (Missouri Four Corners (New Mexico) Labadie and Portage des Sioi (Missouri Muscle Shoals (Alabama) Kyger Creek (Ohio) Labadie (Missouri Four Corners (new Mexico) Labadie (Missouri Cumberland (Tennessee) JX Great Canadian Oil Sands (Alberta, Canada) Central ia (Washington Four Corners (New Mexico) SOo Oxidation rate (%/h) Method SOn & SO., measurements C- SO/, decay relative to SFg SOn decay relative to CO^ SOo decay relative to sub-micron particles ^S/^S ratio, change with oxidation Total change in particle volume Sub-micron sulfate particles & SO^ change of ratio with time Particulate sulfur to total sulfur ratio Particulate sulfur to total sulfur ratio CCN production (CCN to SO^ ratios) Particulate sulfur to total sulfur ratio Particulate sulfur to total sulfur ratio Particulate sulfur to total sulfur ratio Total change in particle volume Reference Gartrell et at (8) Dennis et a1 (9) Weber (3) Stephens & (31 McCaldin Newman et a1 (3) Cantrell & Whitby (33) Ursenbach et a1 (34) Forrest & Newman (9) Giliani et a1 (35) Pueschel & Van Valin (36) Husar et a1 (1) Meagher et a1 (37) Lusis et a1 (38) Hobbs et a1 (39) 1-7
22 differing environmental conditions, there is as yet little agreement as to the relevant independent variables and the dependence of the conversion rate on them. For example, while the studies of Gartrell et a1 (8) Stephens and McCaldin (31 and Weber (3) showed the SO^ oxidation rate to be positively correlated with relative humidity, no such dependence was found in the larger studies by Forrest and Newman (9) or Hobbs et a1 (39 ). A correlation between the SO? g-to-p conversion rate and relative humidity would be in accord with a large number of smog chamber studies [e.g. Cox (3) Wood et a1 (4) Shen and Springer (41)]. Furthermore, while Cantrell and Whitby (33) found an increase in the g-to-p conversion rate with travel time, and Gillani et a1 (35) found an increase with increasing ultraviolet (UV) insolation (travel time x UV intensity) Forrest and Newman (9) found, if anything, a decrease of SOy conversion rate with travel time. Field Studies of Particles in Plumes Whitby et a1 (6) Cantrell and Whitby (33) and Husar et a1 (1) were the first to report on detailed measurements of particles in plumes. All three studies were on the plume from the Labadie coal power plant in Missouri Particle nucleation rates ranging from to 179 cm 3 s were found, with the highest rates occurring in the early morning. The average percentage of the mass produced by g-to-p conversion that formed particles was about 5%, indicating that condensation of the g-to-p conversion products onto existing particles was dominant. Good agreement was found between field data and smog chamber studies with respect to the number of new particles formed per unit volume of material formed by g-to-p conversion, and between the volume of the g-to-p conversion products and the SO,, concentration. Finally, a suggestively high correlation was found between the excess of the light-scattering coefficient in the plume over that in the ambient air and the excess of particulate sulfur concentration in the plume. However, Husar et a1 (1) caution that this correlation was based on a very limited data set. If significant, this correlation would be strong evidence for a relationship between g-to-p conversion and visibility degradation which has been widely proposed. Further work involving detailed measurements of particle distributions in plumes has been reported by Hobbs et a1 (39) and Eitgroth and Hobbs (4). These workers report on measurements taken in the plumes of two coal-fired power plant situated in Washington State and New Mexico. Particle nucleation rates were found to range from.9 to 593 cm" s~ The particle size distributions found in these two plumes differed considerably in shape from those reported for the Labadie plume. 1-8
23 Specifically, the position and number of the particle modes in the plumes of the two western power plants showed considerably more variation than was the case in the Labadie plume. Furthermore, the measurements in the two western plumes showed that, in several cases, the bulk of the new particle volume being created appeared at particle sizes greater than the submicron region where light-scattering is important. This suggests that the correlation between light-scattering and new sulfate particle volume found in the Labadie plume may not always hold. SCOPE OF THE PRESENT STUDY In the present report we describe the results of measurements and analysis of data gathered in the plumes of six coal-fired power plants situated in various parts of the Western and Midwestern United States. The measurements include particle size spectra, trace gas concentrations (SO^, N^, ^, hydrocarbons) particulate sulfate, sulfur, and nitrate concentrations, as well as relevant meteorological parameters. From this data base, g-to-p conversion rates are estimated using several different techniques and comparisons are made with previous field and laboratory results and the the University of Washington s PHOENIX plume model [Eitgroth and Hobbs (4 )]. Particle nucleation rates, volume formation rates, and the surface areas, as well as other parameters indicative of the physics of the g-to-p conversion process, are also derived. These field measurements are also compared with previous laboratory and field studies and with the PHOENIX plume model Furthermore, an attempt is made to determine the effects of various chemical meteorological and particle parameters upon the SO^-to-SO^ conversion process. Finally, particulate nitrate concentrations in power plant plumes are discussed and an estimate is made of the NOp-to-NOo conversion rate for one case where adequate data were collected. 1-9
24 Section INSTRUMENTATION AND DESIGN OF THE FIELD STUDY THE UNVERSITY OF WASHINGTON AIRCRAFT FACILITY All of the data to be described in this study were collected aboard the University of Washington s B-3 research aircraft. Most of the instrumentation available on the aircraft is listed in Table -1 The instrumentation used to measure particles and trace gases in this study are described briefly below. A schematic drawing of the instrumentation system is shown in Fig. -1 Aitken Nucleus Counter The concentrations of Aitken nuclei were measured with two different automatic counters. During fl ights at the Central ia and Four Corners power plants, an inhouse Aitken nucleus counter, which has been described by Hegg et a1 (43) was employed. For the remainder of the flights, a General Electric CN counter (Model CNC-) was employed. This instrument is a rapid-expansion type cloud chamber which has been calibrated (indirectly) against a Pollak counter. Based on the study of Liu and Kirn (44) the GE counter is assumed to detect all particles with a radius greater than ^.7 ym. It measured concentrations from 1-1 cm" Aboard the B-3 aircraft, air samples were passed into both the GE and the inhousebuilt Aitken nucleus counters from a 8-liter bag sampler made of neoprene. This bag is filled in ^3 s by ram air from the ram-air line shown in Fig. -1 As an air sample leaves the bag to go to the Aitken nucleus counter (or any other instrument sampling from the 8-liter bag sampler), it passes through a diffusion drier filled with desiccant which reduces the relative humidity to 5%. Measurements show that particle losses through this system are less than 1% for min holding time in the bag. Electrical Aerosol Analyzer An electrical aerosol analyzer (EAA) was used to measure the size distribution of particles from.3 to. pm diameter. The principle of operation is based on the relationship between charge, particle size and particle mobility described by Mhitby and dark (45) The instrument has been described in detail by Liu et a1 (46) The size range measured by the EAA is divided into ten discrete -1
25 PARTICLE- AND GAS-MEASURING Table -1 INSTRUMENTS ABOARD THE UNIVERSITY OF WASHINGTON S B-3 AIRCRAFT temserdiur^ re s^ance " Type bridge Range (O.l C) Lrror) Angle PhotogrdDhs1^ attack lype Rdnge (and r) Cambridge Htjt^ casac^ance Meteorology (O.?l) s-1 W 3 S-1 Dzo^et (C;hfl) (3) Syslemi Rotary m-^ m-3 ra 1 panicles ignt-scattcring (9 1) Royco fioyco ^oudpart icles nucleus panicle"5 Meteorology Bollay (modified in-house) (detects (-11) (-51) t Lun) cm-3 1 t 1 polarization Lignl- Polarizing iarcicles-1- Concentrations^ a\y,cu "rtmis Measuring (pdi-licles ^ 1-1 (pjrl-icles ID 1, O c. 3 in cn-i TiTe+ Systron km) (1 tu^ "11 in-1" Lightscdtteri-ig Hedding Integrating Gyrocompass Doppler Meteorology Sperry (11) (:?!) 1"^ m"1 m"1 tinen displayed analysis) magnetic m-3 photoelectric Fppley Laboratory m s
26 AUTOMATIC VALVE SEQUENTIAL BAG SAMPLER (FOR OPC 8 EAA)-t ELECTRICAL AEROSOL ANALYZER (EAA) 8 MASS MONITOR ^INTEGRATING / NEPHELOMETER ISOKINETIC PROBE STATIC PRESSURE TRANSDUCER ^31 HEATED CHAMBER ISOKINETIC PUMP PROBE FOR MANUAL BAG SAMPLE (UP TO 3 M3 CAPACITY) GAS ANALYSIS SYSTEM (NO, NOa.SOz, AND 3) OPTICAL PARTICLE COUNTERS (OPC I & II) FRONT AXIALLY SCATTERING SPECTROMETER PROBE INLET FOR ISOKINETIC PROBE open SENSOR ISOKINETIC PUMP Figure -1 Particle- and gas-measuring instruments aboard the University of Washington s B-3 aircraft. Meteorological instrumentation is not shown. -3
27 ranges or channels bounded by.3 pm at the lower end and increasing by onequarter of an order of magnitude per channel up to. um. It takes about min for the instrument to process a sample. Air samples for the EAA are taken from the 8-l iter bag sampler. Flowmeters on the EAA were adjusted at each altitude to ensure proper instrument response. Royco Counter The Royco particle counter measured the size distribution of particles from.3 to 1 pm in diameter by measuring the amount of energy from an incandescent light beam which is scattered at 9 by particles passing through the beam. The instrument has been modified from the commercially available model by using a low-noise, photo-multipl ier tube for detecting the scattering light and replacing the original pulse-height analyzer with a high-speed, 16-channet pulse-height analyzer. The size ranges in which the concentrations of particles are measured are (in pm, diameter).3-.4,.4-.5,.5-.6,.6-.7,.7-.8,.8-1.,.-1.,.-1.5,.5-.,.-3., 3.-4., 4.-5., 5.-6., 6.-8., 8.-1., The instrument was calibrated using polystyrene-latex spheres, as described by Eitgroth (47) The air sample for the Royco is taken from the 8-liter bag sampler mentioned above. Ozone Analyzer Ozone concentrations were measured with a Monitor Labs 841OA ozone analyzer. This instrument measured ozone by monitoring the output of light energy from the chemiluminescent ozone-ethylene reaction. It has a response time of less than 5 s for the scale used in this study (-. ppm) and a detection limit of about 5 ppb. It was periodically cal ibrated against the Monitor Labs Model 85 Permacal Cal ibration Source. This source is essentially an ultraviolet (UV) lamp which irradiates air flowing by it to produce ozone in that air flow. It, in turn, was calibrated by a chemituminescent ozone analyzer standardized by comparison to neutral buffered potassium iodide analysis. The calibration accuracy is given at +/-5% relative to the titration. Oxides of Nitrogen Analyzer Nitric oxide and nitrogen dioxide (NO and NO-,, respectively) were measured with a Monitor Labs Model 844. It has a response time of 5 s on the range (-. ppm) generally used in this study. A higher range (-.5 ppm) was occasionally used -4
28 which has a 9% response time of 11.5 s and a 63% response time of 5 s. While the manufacturer quotes a minimum detection imit of ppb, laboratory tests at the University of Washington indicate that the lower limit for the atmosphere is 7-1 ppb NO and 1- ppb for NOo. The NO (NO.) + NO) analyzer was periodically calibrated against a Monitor Labs x ^ Model 85 Permacal Calibration Source. This source was calibrated by the manufacturer by means of a chemiluminescent analyzer standardized by comparison with gas-phase titration analysis. The calibration accuracy is given as +/-5% relative to the titration. Sulfur Analyzers During flights at the Centralia and Four Corners power plants, the sulfur analyzer employed was the Meloy SA 16- flame photometric detector. This instrument detects photons given off by excited sutfur in a strongly reducing hydrogen-air flame. Because of the very low atmospheric concentrations of sulfur-containing gases other than SOo, the only gaseous sulfur-containing species the analyzer detects is SOo The Meloy SA 16- has a 9% response time of less than 1 s and a detection limit of about 1 ppb SOo. During the flights at the LeIand-Olds, Big Brown and Sherburne County power plants, a Meloy SA 85 flame photometric detector was employed. While operating on the same principle as the Meloy SA 16-, this instrument has a detection limit of.5 ppb SOo and measures concentrations of SOo from.5 ppb to ppb on four linear scales. The model employed was a rapid time response model with a 9% response time of less than s. It should be noted that, while both sulfur analyzers detect particulate sulfur, particulate sulfur species are quite low in atmospheric concentrations compared to SOo. The Meloy analyzers, furthermore, are relatively inefficient in detecting particulate sulfur when compared to gaseous sulfur detection [Hegg et a1 (43)]. The SA 16- and the SA 85 are therefore considered to be SOo detectors. Both sulfur analyzers were periodically calibrated against a Meloy Model CS-1 SOo cal ibration source. This cal ibration source consists of a permeation tube in a temperature-controlled air bath and was factory-calibrated with the calibration warranted correct to within +/-% of the NBS Certified Standard. -5
29 Both sulfur analyzers sampled directly from the ram air line shown in Fig -1 (as did the ozone and NO analyzers) A Hydrocarbon Analysis The ambient concentrations of C^ to C. hydrocarbons, methane, and CO were determined by taking "grab samples" of air in 6-liter, electro-polished, stainlesssteel canisters. These canisters were then sent to Washington State University where analysis was performed by means of gas chromatography-mass spectroscopy following procedures described by Rasmussen et a1 (48) All canisters were analyzed within 6 days of sample collection. Studies conducted at Washington State have shown these canisters to quantitatively retain ight hydrocarbons for periods exceeding 4 weeks. The detection limit for the Co through Cr hydrocarbons -3 was.1 pg m with a precision of %. The detection imit for the methane and CO analysis was.3 ppm with a precision of 1%. Pali flex Filter Samples Particulate sulfur samples were collected on Pallflex glass-fiber filters by vacuum sampl ing from a 5-liter polyethylene bag sampler (Fig. -1 The filters were analyzed for sulfur by the flash vaporization technique described by Roberts (49) as modified by Husar et a1 (5) The apparatus was cal ibrated by the vaporization of gravimetricaliy determined calibration standards. The calibration curve for this apparatus is shown in Fig. - and is similar to that shown by Husar et a1 (5). Further details on this technique are given in Table -1 It should be emphasized that this technique measures particulate sulfur, not sulfate. Teflon Filters Particles were also col lected on Teflon membrane filters manufactured by Ghia Corporation ( ym pore size). Once again, sampling was done from the 5-liter bag sampler. These filters were sent to the EPA laboratories at Research Triangle Park for analysis by X-ray fluorescence and ion-exchange chromatography following procedures described by Stevens et a1 (51 The X-ray fluorescence analysis yielded the concentration of particulate sulfur and of some trace metals. The ion-exchange chromatography analysis yielded the concentrations of particulate sulfate and nitrate. Comparison of the X-ray fluorescence values of particulate sutfur concentration with the ion-exchange chromatography values of particulate sulfate concentration will therefore involve the multiplication of the particulate -6
30 NANOGRAMS SULFUR Figure -. Calibration curve for the flash vaporization apparatus. The regression lines shown have values of R.>..95.
31 sulfur concentration by the mass ratio of sulfate to sulfur to yield equivalent sulfate concentrations. The detection limits for both X-ray fluorescence and ion-exchange chromatography analysis are species-dependent and are given by Stevens et a1 (51 LOCATIONS AND CHARACTERISTICS OF THE Centralia POWER PLANTS STUDIED The Centralia coal-fired electric power plant is situated in an east-westoriented river valley and is subject to the prevailing rainy weather characteristic of western Washington. The surrounding vegetation is mostly evergreen trees, primarily fir. Plant operation data are shown in Table -. Particle emissions from the stack are relatively low due to the installation, in series, of two electrostatic precipitators with an overall mass removal efficiency of 99.9% (the efficiency decreases with decreasing particle size). Four Corners The Four Corners coal-fired electric power plant is located near Farmington, New Mexico. The terrain around the plant is the flat-topped mesa-river valley topography typical of northern New Mexico. The vegetation consists of sparse sage and some pine trees on the mesa tops. Plant operating data are shown in Table -. While the particulate mass loading of the flue gas is rather high, this is due primarily to the large size (5- \im diameter) of a relatively small number of fly-ash particles. The number concentrations of particles in the plume are less than or equal to a few hundred per cm3 and are comparable to background concentrations at a range of a few kilometers. The plant is equipped with electrostatic precipitators of about 97% mass removal efficiency. Letand-Olds The LeIand-Olds coal-fired electric power plant is situated near Bismarck, North Dakota. The terrain around the plant is the roll ing hill topography of the northern Great Plains. The vegetation is sparse and varied. -8
32 Table - CHARACTERISTICS OF POWER PLANTS STUDIED ^ Plant Centralia, Four Corners, WA WA " Le1and-1ds, ND Minnkota, ND Characteristics of Surrounding Area Semi-Rural, Wet Rural, Dry Rural Dry Rural Dry Power Sulfur Capacity Content of (MW) Coal (%) "u 7.7 Ash Content of Coal (%) Flue Concentration of SO^ (ppm) Flue Concentration of NO. (ppm) Particulate Loading of Flue Gas (kg/hr) 6 x x 13.8 x 13.5 x 1 Volume Flow (m- /hr at STP) 6.7 x x 16.7 x x 16 Sherburne County, MN Big Brown, TX Urban, High relative humidity Rural, Low relative humidity but high water vapor pressure ^ ^ x 1. x 16
33 The plant is equipped with electrostatic precipitators of 99% mass removal efficiency. Other plant operating data are listed in Table -. Minnkota The Minnkota coal-fired electric power plant is located ^15 miles from the Leiand- Olds plant. Furthermore, the plant is virtually a duplicate of the LeIand-Olds plant with the exception of the technique employed for air pollution abatement. The Minnkota plant uses wet scrubbers rather than the electrostatic precipitators used at LeIand-Olds. While the data collected at Minnkota are very sparse, a comparison of these data with the LeIand-Olds data is of considerable interest with respect to the relative efficiencies of the two pollution control techniques, and these are are therefore included in this study. Plant operating data are shown in Table -. Sherburne County The Sherburne County coal-fired electric power plant is located northwest of the Minneapol is-st. Paul urban complex. The terrain is quite flat and the vegetation fairly extensive consisting of a mixture of evergreen and deciduous trees. The plant employs wet scrubbers for both gaseous and particulate mass emission control The SO^ flue gas concentration (and presumably the NO flue gas concentration as well although this was not measured) is consequently lower than that of the other plants included in this study. Data on particulate removal efficiency are not available. Plant operating data are shown in Table -. Big Brown The Big Brown coal-fired electric power plant is located near waco, Texas in the relatively flat terrain characteristic of East-Central Texas. The vegetation consists of scattered pines and some deciduous groves. The plant is equipped with electrostatic precipitators of 97.3% mass removal efficiency. Other plant operating data are shown in Table -. -1
34 DESIGN OF THE FIELD STUDY Pl ume Dynamics The primary question addressed in this study, gas-to-particle conversion in coalfired electric power plant plumes, is intimately connected with overall plume dynamics. For example, possible production of Aitken nuclei by g-to-p conversion can only be determined after taking into account the effects of plume dilution and particle coagulation. Furthermore, some of the rapid reactions postulated to produce particle-forming vapors may well be imited by the mixing of the plume with ambient air. It is thus necessary to consider the g-to-p conversion problem within the framework of a dynamic plume model The simple Lagrangian box-model described by Hegg et a1 (43) will be used. The specific equations employed in the analysis are described in Section 4. However, a brief discussion of the data necessary for the analyses performed in Section 4 is given below. A conservative tracer of the plume is necessary in order to estimate the rates at which the plume mixes with the ambient air. It was assumed that sulfur dioxide was conserved over the time intervals between successive sampling ranges (usually hr). While this assumption contradicts, in principle, any hypothesis of oxidation of SO? to particulate sulfate in the plume, in practice the assumption is reasonable since any conversion process should result, at most, in only a few percent loss of SO? per hr. This loss is small conpared to the uncertainty in the SO? concentrations simply due to the lack of precision in the measurements (roughly +/-5%). The decrease in plume SO? concentrations due to mixing, on the other hand, can be orders of magnitude. It is therefore reasonable to neglect the effect of g-to-p conversion on plume SO? concentrations when employing such concentrations to evaluate plume mixing. Another process which can, in principle, have a substantial effect on plume SO? concentration is dry deposition. Once again, however, the practical effect of this process is small Care was taken to ensure that all plumes analyzed had little, if any, contact with the ground. Furthermore, for the distances over which the plumes in this study were measured, previous work has shown that SO? deposition will almost always be considerably less than the uncertainty of measurement in the SO? concentration measurements [Gillani et a1 (35) Husar et a1 (1)]. Certainly the effect of deposition on the concentration of SO? in the plume will always be far less than that of mixing over the distances analyzed in this study. -11
Plant Plumes Volume 1: Field Observations and Theoretical
 Particle Formation and Growth in Power Plant Plumes Volume 1: Field Observations and Theoretical Studies of the Evolution of Particles in the Plumes From Coal-Fired Electric Power Plants EA-3105, Volume
Particle Formation and Growth in Power Plant Plumes Volume 1: Field Observations and Theoretical Studies of the Evolution of Particles in the Plumes From Coal-Fired Electric Power Plants EA-3105, Volume
Lab 4 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
CHAPTER 8. AEROSOLS 8.1 SOURCES AND SINKS OF AEROSOLS
 1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
ATOC 3500/CHEM 3152 Week 9, March 8, 2016
 ATOC 3500/CHEM 3152 Week 9, March 8, 2016 Hand back Midterm Exams (average = 84) Interaction of atmospheric constituents with light Haze and Visibility Aerosol formation processes (more detail) Haze and
ATOC 3500/CHEM 3152 Week 9, March 8, 2016 Hand back Midterm Exams (average = 84) Interaction of atmospheric constituents with light Haze and Visibility Aerosol formation processes (more detail) Haze and
Who is polluting the Columbia River Gorge?
 Who is polluting the Columbia River Gorge? Final report to the Yakima Nation Prepared by: Dan Jaffe, Ph.D Northwest Air Quality, Inc. 7746 Ravenna Avenue NE Seattle WA 98115 NW_airquality@hotmail.com December
Who is polluting the Columbia River Gorge? Final report to the Yakima Nation Prepared by: Dan Jaffe, Ph.D Northwest Air Quality, Inc. 7746 Ravenna Avenue NE Seattle WA 98115 NW_airquality@hotmail.com December
Big Bend Regional Aerosol & Visibility Observational Study
 Big Bend Regional Aerosol & Visibility Observational Study BRAVO - Results Bret Schichtel National Park Service, Schichtel@cira.colostate.edu Presented at the BRAVO Public Meeting Alpine, Texas September
Big Bend Regional Aerosol & Visibility Observational Study BRAVO - Results Bret Schichtel National Park Service, Schichtel@cira.colostate.edu Presented at the BRAVO Public Meeting Alpine, Texas September
Lab 6 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2007 Lab 6 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 6: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2007 Lab 6 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 6: This experiment will involve determining
NOTES AND CORRESPONDENCE. High Aitken Nucleus Concentrations above Cloud Tops in the Arctic
 779 NOTES AND CORRESPONDENCE High Aitken Nucleus Concentrations above Cloud Tops in the Arctic TIMOTHY J. GARRETT* AND PETER V. HOBBS Atmospheric Sciences Department, University of Washington, Seattle,
779 NOTES AND CORRESPONDENCE High Aitken Nucleus Concentrations above Cloud Tops in the Arctic TIMOTHY J. GARRETT* AND PETER V. HOBBS Atmospheric Sciences Department, University of Washington, Seattle,
STP-TS THERMOPHYSICAL PROPERTIES OF WORKING GASES USED IN WORKING GAS TURBINE APPLICATIONS
 THERMOPHYSICAL PROPERTIES OF WORKING GASES USED IN WORKING GAS TURBINE APPLICATIONS THERMOPHYSICAL PROPERTIES OF WORKING GASES USED IN GAS TURBINE APPLICATIONS Prepared by: ASME Standards Technology, LLC
THERMOPHYSICAL PROPERTIES OF WORKING GASES USED IN WORKING GAS TURBINE APPLICATIONS THERMOPHYSICAL PROPERTIES OF WORKING GASES USED IN GAS TURBINE APPLICATIONS Prepared by: ASME Standards Technology, LLC
ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1
 ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1 Note Page numbers refer to Daniel Jacob s online textbook: http://acmg.seas.harvard.edu/publications/ jacobbook/index.html Atmos = vapor + sphaira
ATOC 3500/CHEM 3151 Air Pollution Chemistry Lecture 1 Note Page numbers refer to Daniel Jacob s online textbook: http://acmg.seas.harvard.edu/publications/ jacobbook/index.html Atmos = vapor + sphaira
The Atmospheric Chemistry and Physics of Ammonia
 The Atmospheric Chemistry and Physics of Ammonia Russell Dickerson Dept. Meteorology, The University of Maryland Presented at the National Atmospheric Deposition Program Ammonia Workshop October 23, 2003
The Atmospheric Chemistry and Physics of Ammonia Russell Dickerson Dept. Meteorology, The University of Maryland Presented at the National Atmospheric Deposition Program Ammonia Workshop October 23, 2003
Fine Particles: Why We Care
 Fine Particles: Why We Care Visibility/Radiative Forcing Health Effects A function of chemical composition PM2.5 Mostly 1) Sulfate 2) Carbonaceous - Organic - Elemental (Soot) 3) Metals, minerals, Metals,
Fine Particles: Why We Care Visibility/Radiative Forcing Health Effects A function of chemical composition PM2.5 Mostly 1) Sulfate 2) Carbonaceous - Organic - Elemental (Soot) 3) Metals, minerals, Metals,
C (s) + O 2 (g) CO 2 (g) S (s) + O 2 (g) SO 2 (g)
 Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Gaseous CEMS technology
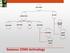 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle
 Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
Visibility and Air Quality Measurement Methods Used by the National Park Service
 Visibility and Air Quality Measurement Methods Used by the National Park Service U.S. Department of the Interior National Park Service Air Quality Division February 1990 Visibility and Air Quality Measurement
Visibility and Air Quality Measurement Methods Used by the National Park Service U.S. Department of the Interior National Park Service Air Quality Division February 1990 Visibility and Air Quality Measurement
MODULE 4.3 Atmospheric analysis of particulates
 MODULE 4.3 Atmospheric analysis of particulates Measurement And Characterisation Of The Particulate Content 1 Total particulate concentration 1 Composition of the particulate 1 Determination of particle
MODULE 4.3 Atmospheric analysis of particulates Measurement And Characterisation Of The Particulate Content 1 Total particulate concentration 1 Composition of the particulate 1 Determination of particle
Aerosols AP sizes AP types Sources Sinks Amount and lifetime Aerosol radiative effects. Aerosols. Trude Storelvmo Aerosols 1 / 21
 Aerosols Trude Storelvmo Aerosols 1 / 21 Aerosols: Definition Definition of an aerosol: disperse system with air as carrier gas and a solid or liquid or a mixture of both as disperse phases. Aerosol particles
Aerosols Trude Storelvmo Aerosols 1 / 21 Aerosols: Definition Definition of an aerosol: disperse system with air as carrier gas and a solid or liquid or a mixture of both as disperse phases. Aerosol particles
Experimental Techniques for Studying Surface Chemistry in Smog Chambers
 Experimental Techniques for Studying Surface Chemistry in Smog Chambers Laura T. Iraci, Jeffrey C. Johnston and David M. Golden SRI International, Menlo Park, CA Chemical reactions occurring on the walls
Experimental Techniques for Studying Surface Chemistry in Smog Chambers Laura T. Iraci, Jeffrey C. Johnston and David M. Golden SRI International, Menlo Park, CA Chemical reactions occurring on the walls
Continuous measurement of airborne particles and gases
 Continuous measurement of airborne particles and gases Jeff Collett and Taehyoung Lee Atmospheric Science Department Colorado State University Funding: USDA/AES and NPS Outline Why measure particles and
Continuous measurement of airborne particles and gases Jeff Collett and Taehyoung Lee Atmospheric Science Department Colorado State University Funding: USDA/AES and NPS Outline Why measure particles and
Effect of aging on cloud nucleating properties of atmospheric aerosols
 Effect of aging on cloud nucleating properties of atmospheric aerosols Yan Ma Nanjing University of Information Science & Technology The 1st Regional GEOS-Chem Asia Meeting 2015 年 5 月 8 日 Indirect effect
Effect of aging on cloud nucleating properties of atmospheric aerosols Yan Ma Nanjing University of Information Science & Technology The 1st Regional GEOS-Chem Asia Meeting 2015 年 5 月 8 日 Indirect effect
Atmospheric New Particle Formation and Climate Sensitivity: the CLOUD experiment.
 Atmospheric New Particle Formation and Climate Sensitivity: the CLOUD experiment 4th April 2017 Molteni Ugo ugo.molteni@psi.ch About me Master degree in Applied and Environmental Chemistry at the University
Atmospheric New Particle Formation and Climate Sensitivity: the CLOUD experiment 4th April 2017 Molteni Ugo ugo.molteni@psi.ch About me Master degree in Applied and Environmental Chemistry at the University
Chapter Eight: Conclusions and Future Work
 2004 PhD Thesis 202 Chapter Eight: Conclusions and Future Work 8.1 Conclusions The Aerodyne aerosol mass spectrometer is capable of providing quantitative information on the chemical composition of the
2004 PhD Thesis 202 Chapter Eight: Conclusions and Future Work 8.1 Conclusions The Aerodyne aerosol mass spectrometer is capable of providing quantitative information on the chemical composition of the
CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION
 i CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION 1 1.1 MIXING RATIO 1 1.2 NUMBER DENSITY 2 1.3 PARTIAL PRESSURE 6 PROBLEMS 10 1.1 Fog formation 10 1.2 Phase partitioning of water in cloud 10 1.3 The ozone
i CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION 1 1.1 MIXING RATIO 1 1.2 NUMBER DENSITY 2 1.3 PARTIAL PRESSURE 6 PROBLEMS 10 1.1 Fog formation 10 1.2 Phase partitioning of water in cloud 10 1.3 The ozone
Combustion Generated Pollutants
 Combustion Generated Pollutants New Delhi Peking Climate change Combustion Generated Pollutants Greenhouse gases: CO 2, methane, N 2 O, CFCs, particulates, etc. Hydrocarbons: Toxins and a major contributor
Combustion Generated Pollutants New Delhi Peking Climate change Combustion Generated Pollutants Greenhouse gases: CO 2, methane, N 2 O, CFCs, particulates, etc. Hydrocarbons: Toxins and a major contributor
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR
 AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
Sampling. Information is helpful in implementing control measures for reducing pollutant concentration to acceptable levels
 Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
The Atmosphere and Atmospheric Energy Chapter 3 and 4
 The Atmosphere and Atmospheric Energy Chapter 3 and 4 Size of the Earth s Atmosphere Atmosphere produced over 4.6 billion years of development Protects us from radiation Completely surrounds the earth
The Atmosphere and Atmospheric Energy Chapter 3 and 4 Size of the Earth s Atmosphere Atmosphere produced over 4.6 billion years of development Protects us from radiation Completely surrounds the earth
Air quality impacts of oil and gas development in the Bakken formation region
 Air quality impacts of oil and gas development in the Bakken formation region J. L. Collett, Jr. 1, A. Evanoski Cole 1, A. Prenni 2, D. Day 2, A. Sullivan 1, Y. Li 1, B. Sive 2, Y. Zhou 1, A. Hecobian
Air quality impacts of oil and gas development in the Bakken formation region J. L. Collett, Jr. 1, A. Evanoski Cole 1, A. Prenni 2, D. Day 2, A. Sullivan 1, Y. Li 1, B. Sive 2, Y. Zhou 1, A. Hecobian
Reference pg and in Textbook
 Reference pg. 154-164 and 188-202 in Textbook Combustion Reactions During combustion (burning) of fossil fuels, collisions between the molecules of the fuel and oxygen result in the formation of new molecules.
Reference pg. 154-164 and 188-202 in Textbook Combustion Reactions During combustion (burning) of fossil fuels, collisions between the molecules of the fuel and oxygen result in the formation of new molecules.
Validation of a new flow-reactor for the study of secondary organic aerosol (SOA) formation
 Validation of a new flow-reactor for the study of secondary organic aerosol (SOA) formation M. Duncianu*(1,2), V. Riffault (1,2), A. Tomas (1,2), P. Coddeville (1,2) (1) Université Lille Nord de France,
Validation of a new flow-reactor for the study of secondary organic aerosol (SOA) formation M. Duncianu*(1,2), V. Riffault (1,2), A. Tomas (1,2), P. Coddeville (1,2) (1) Université Lille Nord de France,
UV3000. Accurate, precise, and portable ambient gas point analyzer
 UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
RS DYNAMICS ECOPROBE 5. Portable IR/PID Gas Analyzer PID. PID and IR Analyzers
 RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
Gas, Cloudwater, and Rain Hydrogen Peroxide and Methylhydroperoxide Measurements in RICO
 Gas, Cloudwater, and Rain Hydrogen Peroxide and Methylhydroperoxide Measurements in RICO Brian G. Heikes, Center for Atmospheric Chemical Studies, Graduate School of Oceanography, University of Rhode Island
Gas, Cloudwater, and Rain Hydrogen Peroxide and Methylhydroperoxide Measurements in RICO Brian G. Heikes, Center for Atmospheric Chemical Studies, Graduate School of Oceanography, University of Rhode Island
Project-Based Inquiry Science: Air Quality Storyline. Air Quality: What s the Big Question? How Can You Improve Air Quality in Your Community?
 Project-Based Inquiry Science: Air Quality Storyline Air Quality: What s the Big Question? How Can You Improve Air Quality in Your Community? In the Introduction to Air Quality, students read a parable
Project-Based Inquiry Science: Air Quality Storyline Air Quality: What s the Big Question? How Can You Improve Air Quality in Your Community? In the Introduction to Air Quality, students read a parable
Insights Into Atmospheric Organic Aerosols Using An Aerosol Mass Spectrometer
 Insights Into Atmospheric Organic Aerosols Using An Aerosol Mass Spectrometer A thesis submitted to the University of Manchester Institute of Science and Technology for the degree of Doctor of Philosophy
Insights Into Atmospheric Organic Aerosols Using An Aerosol Mass Spectrometer A thesis submitted to the University of Manchester Institute of Science and Technology for the degree of Doctor of Philosophy
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth
 FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
End of Ozone Season Report
 End of Ozone Season Report Central Ohio: April 1 through October 31, 2016 The Mid-Ohio Regional Planning Commission (MORPC) is part of a network of agencies across the country that issues daily air quality
End of Ozone Season Report Central Ohio: April 1 through October 31, 2016 The Mid-Ohio Regional Planning Commission (MORPC) is part of a network of agencies across the country that issues daily air quality
Tananyag fejlesztés idegen nyelven
 Tananyag fejlesztés idegen nyelven Prevention of the atmosphere KÖRNYEZETGAZDÁLKODÁSI AGRÁRMÉRNÖKI MSC (MSc IN AGRO-ENVIRONMENTAL STUDIES) Fundamentals to atmospheric chemical reactions. The stratospheric
Tananyag fejlesztés idegen nyelven Prevention of the atmosphere KÖRNYEZETGAZDÁLKODÁSI AGRÁRMÉRNÖKI MSC (MSc IN AGRO-ENVIRONMENTAL STUDIES) Fundamentals to atmospheric chemical reactions. The stratospheric
1.1 Introduction to the Particulate Nature of Matter and Chemical Change MATTER. Homogeneous (SOLUTIONS)
 TOPIC 1: STOICHIOMETRIC RELATIONS 1.1 Introduction to the Particulate Nature of Matter and Chemical Change MATTER Mass Volume Particles Particles in constant motion MATTER Pure Matters Mixtures ELEMENTS
TOPIC 1: STOICHIOMETRIC RELATIONS 1.1 Introduction to the Particulate Nature of Matter and Chemical Change MATTER Mass Volume Particles Particles in constant motion MATTER Pure Matters Mixtures ELEMENTS
Over the course of this unit, you have learned about different
 70 People and Weather TA L K I N G I T O V E R Over the course of this unit, you have learned about different aspects of earth s weather and atmosphere. Atmospheric scientists, climatologists, hydrologists,
70 People and Weather TA L K I N G I T O V E R Over the course of this unit, you have learned about different aspects of earth s weather and atmosphere. Atmospheric scientists, climatologists, hydrologists,
Luminescence transitions. Fluorescence spectroscopy
 Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
The Application of Method TO-15 to Naphthalene Measurements in Indoor Air
 The Application of Method TO-15 to Naphthalene Measurements in Indoor Air Extended Abstract #13 Heidi C. Hayes and Diane J. Benton Air Toxics Ltd. 180 Blue Ravine Rd. Ste. B Folsom, CA 95630 INTRODUCTION
The Application of Method TO-15 to Naphthalene Measurements in Indoor Air Extended Abstract #13 Heidi C. Hayes and Diane J. Benton Air Toxics Ltd. 180 Blue Ravine Rd. Ste. B Folsom, CA 95630 INTRODUCTION
The Atmosphere. All of it. In one hour. Mikael Witte 10/27/2010
 The Atmosphere All of it. In one hour. Mikael Witte 10/27/2010 Outline Structure Dynamics - heat transport Composition Trace constituent compounds Some Atmospheric Processes Ozone destruction in stratosphere
The Atmosphere All of it. In one hour. Mikael Witte 10/27/2010 Outline Structure Dynamics - heat transport Composition Trace constituent compounds Some Atmospheric Processes Ozone destruction in stratosphere
The Atmosphere. Characteristics of the Atmosphere. Section 23.1 Objectives. Chapter 23. Chapter 23 Modern Earth Science. Section 1
 The Atmosphere Chapter 23 Modern Earth Science Characteristics of the Atmosphere Chapter 23 Section 1 Section 23.1 Objectives Describe the composition of Earth s atmosphere. Explain how two types of barometers
The Atmosphere Chapter 23 Modern Earth Science Characteristics of the Atmosphere Chapter 23 Section 1 Section 23.1 Objectives Describe the composition of Earth s atmosphere. Explain how two types of barometers
Regional methane emissions estimates in northern Pennsylvania gas fields using a mesoscale atmospheric inversion system
 Regional methane emissions estimates in northern Pennsylvania gas fields using a mesoscale atmospheric inversion system Thomas Lauvaux1, A. Deng1, B. Gaudet1, S. J. Richardson1, N. L. Miles1, J. N. Ciccarelli1,2,
Regional methane emissions estimates in northern Pennsylvania gas fields using a mesoscale atmospheric inversion system Thomas Lauvaux1, A. Deng1, B. Gaudet1, S. J. Richardson1, N. L. Miles1, J. N. Ciccarelli1,2,
(for tutoring, homework help, or help with online classes)
 www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. chem10b 18.2-30 What is the final stage in municipal water treatment? A. aeration B. settling C. removal of added fluoride
www.tutor-homework.com (for tutoring, homework help, or help with online classes) 1. chem10b 18.2-30 What is the final stage in municipal water treatment? A. aeration B. settling C. removal of added fluoride
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
Abstract. 1 Introduction
 Measuring and modelling of aerosol chemical composition for the SANA intensive field campaigns W. Seidl, G. Brunnemann, L. Kins, D. Kohler, E. Kohler, K. ReiBwig, K. RouB, Th. Seller, R. Dugli Meteorologisches
Measuring and modelling of aerosol chemical composition for the SANA intensive field campaigns W. Seidl, G. Brunnemann, L. Kins, D. Kohler, E. Kohler, K. ReiBwig, K. RouB, Th. Seller, R. Dugli Meteorologisches
Clever Catch Weather Ball Question and Answer Sheets
 Clever Catch Weather Ball Question and Answer Sheets 1. Too much exposure to can cause skin cancer. B. Ultraviolet radiation 2. The layer of the atmosphere closest to the Earth s surface is the 3. Some
Clever Catch Weather Ball Question and Answer Sheets 1. Too much exposure to can cause skin cancer. B. Ultraviolet radiation 2. The layer of the atmosphere closest to the Earth s surface is the 3. Some
Stoichiometric relationships 1
 Stoichiometric relationships 1 Chapter outline Describe the three states of matter. Recall that atoms of diff erent elements combine in fi xed ratios to form compounds which have diff erent properties
Stoichiometric relationships 1 Chapter outline Describe the three states of matter. Recall that atoms of diff erent elements combine in fi xed ratios to form compounds which have diff erent properties
NATS 101 Section 13: Lecture 31. Air Pollution Part II
 NATS 101 Section 13: Lecture 31 Air Pollution Part II Last time we talked mainly about two types of smog:. 1. London-type smog 2. L.A.-type smog or photochemical smog What are the necessary ingredients
NATS 101 Section 13: Lecture 31 Air Pollution Part II Last time we talked mainly about two types of smog:. 1. London-type smog 2. L.A.-type smog or photochemical smog What are the necessary ingredients
Worldwide Pollution Control Association
 Worldwide Pollution Control Association WPCA- Southern Company Mercury Seminar October 30-31, 2012 All presentations posted on this website are copyrighted by the Worldwide Pollution Control Association
Worldwide Pollution Control Association WPCA- Southern Company Mercury Seminar October 30-31, 2012 All presentations posted on this website are copyrighted by the Worldwide Pollution Control Association
Progress on Application of Modal Aerosol Dynamics to CAM
 Progress on Application of Modal Aerosol Dynamics to CAM Xiaohong Liu, Steve Ghan, Richard Easter, Rahul Zaveri, Yun Qian (Pacific Northwest National Laboratory) Jean-Francois Lamarque, Peter Hess, Natalie
Progress on Application of Modal Aerosol Dynamics to CAM Xiaohong Liu, Steve Ghan, Richard Easter, Rahul Zaveri, Yun Qian (Pacific Northwest National Laboratory) Jean-Francois Lamarque, Peter Hess, Natalie
 Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. All are 3 points each. 1. First set. How can you tell these apart?
Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. All are 3 points each. 1. First set. How can you tell these apart?
Chemistry of SO 2 in tropospheric volcanic plumes
 Chemistry of SO 2 in tropospheric volcanic plumes by Dr. Lizzette A. Rodríguez Iglesias Department of Geology University of Puerto Rico Mayagüez Campus Photo: L. Rodriguez http://volcano-pictures.info/glossary/volcanic_gas.html
Chemistry of SO 2 in tropospheric volcanic plumes by Dr. Lizzette A. Rodríguez Iglesias Department of Geology University of Puerto Rico Mayagüez Campus Photo: L. Rodriguez http://volcano-pictures.info/glossary/volcanic_gas.html
Phase State and Physical Properties of Ambient and Laboratory. Generated Secondary Organic Aerosol
 1 2 Phase State and Physical Properties of Ambient and Laboratory Generated Secondary Organic Aerosol 3 4 5 6 7 8 9 10 11 Rachel E. O Brien, 1, 2* Alexander Neu, 1 Scott A. Epstein, 3 Amanda C. MacMillan,
1 2 Phase State and Physical Properties of Ambient and Laboratory Generated Secondary Organic Aerosol 3 4 5 6 7 8 9 10 11 Rachel E. O Brien, 1, 2* Alexander Neu, 1 Scott A. Epstein, 3 Amanda C. MacMillan,
 Contributors: Arlene Fiore 1, Lee Murray 1, Luke Valin 1, Olivia Clifton 1, Jean Guo 1 Author: Melissa Seto 1 Analysis of Wisconsin 2007 High-Ozone event Section 1A: Can satellite NO 2 columns inform us
Contributors: Arlene Fiore 1, Lee Murray 1, Luke Valin 1, Olivia Clifton 1, Jean Guo 1 Author: Melissa Seto 1 Analysis of Wisconsin 2007 High-Ozone event Section 1A: Can satellite NO 2 columns inform us
Differential Mobility Particle Sizer (Aerosol measurements)
 Institute for Atmospheric and Climate Science - IACETH Atmospheric Physics Lab Work Differential Mobility Particle Sizer (Aerosol measurements) Abstract A differential mobility particle sizer (DMPS) is
Institute for Atmospheric and Climate Science - IACETH Atmospheric Physics Lab Work Differential Mobility Particle Sizer (Aerosol measurements) Abstract A differential mobility particle sizer (DMPS) is
Reactive Nitrogen Monitoring
 Reactive Nitrogen Monitoring Some definitions NOy NO + NO 2 + NO 3 + 2xN2 2 O 5 + HNO 3 + HONO + HO 2 NO 2 + RONO 2 (organic nitrates such as PAN and alkyl nitrates) + RONO (organic nitrites) + NO 3 -
Reactive Nitrogen Monitoring Some definitions NOy NO + NO 2 + NO 3 + 2xN2 2 O 5 + HNO 3 + HONO + HO 2 NO 2 + RONO 2 (organic nitrates such as PAN and alkyl nitrates) + RONO (organic nitrites) + NO 3 -
Studies of the uptake of gaseous ethyl-3-ethoxy propionate onto ammonium sulfate and ammonium nitrate aerosol particles
 Air Pollution XIII 519 Studies of the uptake of gaseous ethyl-3-ethoxy propionate onto ammonium sulfate and ammonium nitrate aerosol particles K. D. Stewart & J. M. Andino Department of Environmental Engineering
Air Pollution XIII 519 Studies of the uptake of gaseous ethyl-3-ethoxy propionate onto ammonium sulfate and ammonium nitrate aerosol particles K. D. Stewart & J. M. Andino Department of Environmental Engineering
Measuring Total Reactive N and its Composition
 Measuring Total Reactive N and its Composition Bret A. Schichtel 1, Katie Benedict 2, Christian M. Carrico 2, Anthony Prenni 2, Jr. 2, Ezra Levin 2, Derek Day 3, Doris Chen 2, John Ray 1, William C. Malm
Measuring Total Reactive N and its Composition Bret A. Schichtel 1, Katie Benedict 2, Christian M. Carrico 2, Anthony Prenni 2, Jr. 2, Ezra Levin 2, Derek Day 3, Doris Chen 2, John Ray 1, William C. Malm
CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION
 1 CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION The objective of atmospheric chemistry is to understand the factors that control the concentrations of chemical species in the atmosphere. In this book
1 CHAPTER 1. MEASURES OF ATMOSPHERIC COMPOSITION The objective of atmospheric chemistry is to understand the factors that control the concentrations of chemical species in the atmosphere. In this book
A) usually less B) dark colored and rough D) light colored with a smooth surface A) transparency of the atmosphere D) rough, black surface
 1. Base your answer to the following question on the diagram below which shows two identical houses, A and B, in a city in North Carolina. One house was built on the east side of a factory, and the other
1. Base your answer to the following question on the diagram below which shows two identical houses, A and B, in a city in North Carolina. One house was built on the east side of a factory, and the other
Atmospheric Oxidation Mechanisms of Unsaturated Oxygenated VOCs
 Atmospheric Oxidation Mechanisms of Unsaturated Oxygenated VOCs R. Thévenet, G. Thiault, E. Vésine, G. Laverdet, A. Mellouki, G. Le Bras LCSR-CNRS-1C, Avenue de la recherche scientifique 4571, Orléans,
Atmospheric Oxidation Mechanisms of Unsaturated Oxygenated VOCs R. Thévenet, G. Thiault, E. Vésine, G. Laverdet, A. Mellouki, G. Le Bras LCSR-CNRS-1C, Avenue de la recherche scientifique 4571, Orléans,
Preliminary Conceptual Model Development
 Preliminary Conceptual Model Development Develop preliminary conceptual models regarding the sources of haze at every Class I area in the WRAP region Site-specific summaries of the descriptive material
Preliminary Conceptual Model Development Develop preliminary conceptual models regarding the sources of haze at every Class I area in the WRAP region Site-specific summaries of the descriptive material
Performance Characterization of A New Cam System M.J. Koskelo 1, J.C. Rodgers 2, D.C. Nelson 2, A.R. McFarland 3 and C.A. Ortiz 3
 Performance Characterization of A New Cam System M.J. Koskelo 1, J.C. Rodgers 2, D.C. Nelson 2, A.R. McFarland 3 and C.A. Ortiz 3 1 CANBERRA Industries, Meriden, CT 06450 2 Los Alamos National Laboratory,
Performance Characterization of A New Cam System M.J. Koskelo 1, J.C. Rodgers 2, D.C. Nelson 2, A.R. McFarland 3 and C.A. Ortiz 3 1 CANBERRA Industries, Meriden, CT 06450 2 Los Alamos National Laboratory,
Atmospheric chemistry Acidification
 Atmospheric chemistry Acidification Presented by Pontus Roldin Most material from Erik Swietlicki Avd. för Kärnfysik Fysiska institutionen Lunds universitet Acidification 1 Acidification Sulphur- and nitrogen-containing
Atmospheric chemistry Acidification Presented by Pontus Roldin Most material from Erik Swietlicki Avd. för Kärnfysik Fysiska institutionen Lunds universitet Acidification 1 Acidification Sulphur- and nitrogen-containing
UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j
 UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j 1 Environmental Air Pollution The addition of any harmful material (having harmful effects on our lives) to our atmosphere is called
UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j 1 Environmental Air Pollution The addition of any harmful material (having harmful effects on our lives) to our atmosphere is called
 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
ATMOSPHERE: ORIGIN, COMPOSITION, AND STRUCTURE
 CHAPTER 2 ATMOSPHERE: ORIGIN, COMPOSITION, AND STRUCTURE MULTIPLE CHOICE QUESTIONS 1. A relatively thin envelope of gases and particles that encircles the planet is known as a. the jet stream. *b. the
CHAPTER 2 ATMOSPHERE: ORIGIN, COMPOSITION, AND STRUCTURE MULTIPLE CHOICE QUESTIONS 1. A relatively thin envelope of gases and particles that encircles the planet is known as a. the jet stream. *b. the
Stoichiometry SUPPLEMENTAL PROBLEMS CHAPTER 12. 3Si(s) 2N 2 N 4. (g) 0 Si 3. (s) PO 4. the reaction. Cr(s) H 3. (aq) 0.
 CHAPTER 12 Stoichiometry 1. Silicon nitride is used in the manufacturing of high-temperature thermal insulation for heat engines and turbines. It is produced by the following 3Si(s) 2N 2 (g) 0 Si 3 N 4
CHAPTER 12 Stoichiometry 1. Silicon nitride is used in the manufacturing of high-temperature thermal insulation for heat engines and turbines. It is produced by the following 3Si(s) 2N 2 (g) 0 Si 3 N 4
Chasing Aerosol Particles Down to Nano Sizes
 Chasing Aerosol Particles Down to Nano Sizes ERC Seminar 13 June 2013 Armin Sorooshian Chemical & Environmental Engineering Atmospheric Sciences University of Arizona Outline of Talk 1. What are aerosol
Chasing Aerosol Particles Down to Nano Sizes ERC Seminar 13 June 2013 Armin Sorooshian Chemical & Environmental Engineering Atmospheric Sciences University of Arizona Outline of Talk 1. What are aerosol
The Atmosphere - Chapter Characteristics of the Atmosphere
 Section Objectives Describe the composition of Earth s atmosphere. Explain how two types of barometers work. Identify the layers of the atmosphere. Identify two effects of air pollution. The Atmosphere
Section Objectives Describe the composition of Earth s atmosphere. Explain how two types of barometers work. Identify the layers of the atmosphere. Identify two effects of air pollution. The Atmosphere
Jianfei Peng et al. Correspondence to: Jianfei Peng Min Hu and Renyi Zhang
 Supplement of Atmos. Chem. Phys., 17, 10333 10348, 2017 https://doi.org/10.5194/acp-17-10333-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License.
Supplement of Atmos. Chem. Phys., 17, 10333 10348, 2017 https://doi.org/10.5194/acp-17-10333-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License.
Fundamentals of Particle Counting
 Fundamentals of Particle Counting 1 Particle Counting: Remains the most significant technique for determining the cleanliness level of a fluid Useful as a tool for qualification and monitoring cleanroom
Fundamentals of Particle Counting 1 Particle Counting: Remains the most significant technique for determining the cleanliness level of a fluid Useful as a tool for qualification and monitoring cleanroom
Ambient Ammonium Contribution to total Nitrogen Deposition
 Ambient Ammonium Contribution to total Nitrogen Deposition 2016 NADP Annual Meeting Santa Fe, New Mexico November 3, 2016 Rich Scheffe, Jason Lynch, Donna Schwede, James Kelly, Halil Cakir, Adam Reff U.S.
Ambient Ammonium Contribution to total Nitrogen Deposition 2016 NADP Annual Meeting Santa Fe, New Mexico November 3, 2016 Rich Scheffe, Jason Lynch, Donna Schwede, James Kelly, Halil Cakir, Adam Reff U.S.
Warning!! Chapter 5 Gases. Chapter Objectives. Chapter Objectives. Chapter Objectives. Air Pollution
 Warning!! Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 5 Gases These slides contains visual aids for learning BUT they are NOT the actual lecture notes! Failure to attend to lectures most
Warning!! Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 5 Gases These slides contains visual aids for learning BUT they are NOT the actual lecture notes! Failure to attend to lectures most
correspondence Science Center, SRI International, Menlo Park, Calif FIG. 1. Los Angeles area flight paths, 16 December 1979.
 correspondence Airborne Lidar Plume and Haze Analyzer (ALPHA-1) Edward E. Uthe, Norman B. Nielsen, and Walter L. Jimison, Atmospheric Science Center, SRI International, Menlo Park, Calif. 94025 Abstract
correspondence Airborne Lidar Plume and Haze Analyzer (ALPHA-1) Edward E. Uthe, Norman B. Nielsen, and Walter L. Jimison, Atmospheric Science Center, SRI International, Menlo Park, Calif. 94025 Abstract
Quantitative chemistry Atomic structure Periodicity
 IB chemistry Units 1-3 review Quantitative chemistry Significant figures The mole- be able to convert to number of particles and mass Finding empirical and molecular formulas from mass percentage States
IB chemistry Units 1-3 review Quantitative chemistry Significant figures The mole- be able to convert to number of particles and mass Finding empirical and molecular formulas from mass percentage States
Chapter 4 Lesson 1: Describing Earth s Atmosphere
 Chapter 4 Lesson 1: Describing Earth s Atmosphere Vocabulary Importance of Earth s Atmosphere The atmosphere is a thin layer of gases surrounding Earth. o Contains the oxygen and water needed for life.
Chapter 4 Lesson 1: Describing Earth s Atmosphere Vocabulary Importance of Earth s Atmosphere The atmosphere is a thin layer of gases surrounding Earth. o Contains the oxygen and water needed for life.
CHEMISTRY HIGHER LEVEL
 *P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2009 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
*P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2009 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
Final Exam: Monday March 17 3:00-6:00 pm (here in Center 113) Slides from Review Sessions are posted on course website:
 Final Exam: Monday March 17 3:00-6:00 pm (here in Center 113) 35% of total grade Format will be all multiple choice (~70 questions) Final exam will cover entire course - material since 2 nd midterm weighted
Final Exam: Monday March 17 3:00-6:00 pm (here in Center 113) 35% of total grade Format will be all multiple choice (~70 questions) Final exam will cover entire course - material since 2 nd midterm weighted
Lecture- 08 Emission and absorption spectra
 Atomic and Molecular Absorption Spectrometry for Pollution Monitoring Dr. J R Mudakavi Department of Chemical Engineering Indian Institute of Science, Bangalore Lecture- 08 Emission and absorption spectra
Atomic and Molecular Absorption Spectrometry for Pollution Monitoring Dr. J R Mudakavi Department of Chemical Engineering Indian Institute of Science, Bangalore Lecture- 08 Emission and absorption spectra
UNIVERSITY OF VICTORIA CHEMISTRY 102 Midterm Test 1 January 31, pm (60 minutes) DISPLAY YOUR STUDENT ID CARD ON THE TOP OF YOUR DESK NOW
 Version A UNIVERSITY OF VICTORIA CHEMISTRY 102 Midterm Test 1 January 31, 2014 5-6 pm (60 minutes) Version A DISPLAY YOUR STUDENT ID CARD ON THE TOP OF YOUR DESK NOW Answer all multiple choice questions
Version A UNIVERSITY OF VICTORIA CHEMISTRY 102 Midterm Test 1 January 31, 2014 5-6 pm (60 minutes) Version A DISPLAY YOUR STUDENT ID CARD ON THE TOP OF YOUR DESK NOW Answer all multiple choice questions
In the space provided, write the letter of the description that best matches the term or phrase. as waves. thermosphere
 Skills Worksheet Concept Review In the space provided, write the letter of the description that best matches the term or phrase. 1. layers of the atmosphere 2. radiation 3. conduction 4. convection 5.
Skills Worksheet Concept Review In the space provided, write the letter of the description that best matches the term or phrase. 1. layers of the atmosphere 2. radiation 3. conduction 4. convection 5.
Multicusp Sources for Ion Beam Lithography Applications
 LBL-3 6645 UC-406 Multicusp Sources for Ion Beam Lithography Applications K.N. Leung, P. H e n, W.B. Kunkel, Y. Lee, L. Perkins, D. Pickard, M. Sarstedt, M. Weber, and M.D. Williams Accelerator and Fusion
LBL-3 6645 UC-406 Multicusp Sources for Ion Beam Lithography Applications K.N. Leung, P. H e n, W.B. Kunkel, Y. Lee, L. Perkins, D. Pickard, M. Sarstedt, M. Weber, and M.D. Williams Accelerator and Fusion
ENHANCED MERCURY CONTROL BY MANAGING SCR SYSTEMS FOR MERCURY AND NOx
 ENHANCED MERCURY CONTROL BY MANAGING SCR SYSTEMS FOR MERCURY AND NOx Paper # 38 Power Plant Air Pollutant Control MEGA Symposium, 2012 Scott Hinton W.S. Hinton & Associates 1612 Smugglers Cove Circle,
ENHANCED MERCURY CONTROL BY MANAGING SCR SYSTEMS FOR MERCURY AND NOx Paper # 38 Power Plant Air Pollutant Control MEGA Symposium, 2012 Scott Hinton W.S. Hinton & Associates 1612 Smugglers Cove Circle,
Name: Regents Review Quiz #1 2016
 Name: Regents Review Quiz #1 2016 1. Which two particle diagrams represent mixtures of diatomic elements? A) A and B B) A and C C) B and C D) B and D 2. At STP, which physical property of aluminum always
Name: Regents Review Quiz #1 2016 1. Which two particle diagrams represent mixtures of diatomic elements? A) A and B B) A and C C) B and C D) B and D 2. At STP, which physical property of aluminum always
AN INTRODUCTION TO ATOMIC SPECTROSCOPY
 AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
AN INTRODUCTION TO ATOMIC SPECTROSCOPY Atomic spectroscopy deals with the absorption, emission, or fluorescence by atom or elementary ions. Two regions of the spectrum yield atomic information- the UV-visible
White Paper. Overview: NDIR Definition:
 Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
ABSTRACT INTRODUCTION
 The role of clouds in transformation and removal of air pollutants D. Moller, G. Mauersberger Department for Air Chemistry, Fraunhofer Institut for Atmospheric Environmental Research, D-1199 Berlin, Germany
The role of clouds in transformation and removal of air pollutants D. Moller, G. Mauersberger Department for Air Chemistry, Fraunhofer Institut for Atmospheric Environmental Research, D-1199 Berlin, Germany
CHEMISTRY - HIGHER LEVEL
 M34 AN ROINN OIDEACHAIS AGUS EOLAÍOCHTA LEAVING CERTIFICATE EXAMINATION, 2002 CHEMISTRY - HIGHER LEVEL TUESDAY, 18 JUNE - AFTERNOON 2.00 to 5.00 400 MARKS Answer eight questions in all These must include
M34 AN ROINN OIDEACHAIS AGUS EOLAÍOCHTA LEAVING CERTIFICATE EXAMINATION, 2002 CHEMISTRY - HIGHER LEVEL TUESDAY, 18 JUNE - AFTERNOON 2.00 to 5.00 400 MARKS Answer eight questions in all These must include
PROBLEMS Sources of CO Sources of tropospheric ozone
 220 PROBLEMS 11. 1 Sources of CO The two principal sources of CO to the atmosphere are oxidation of CH 4 and combustion. Mean rate constants for oxidation of CH 4 and CO by OH in the troposphere are k
220 PROBLEMS 11. 1 Sources of CO The two principal sources of CO to the atmosphere are oxidation of CH 4 and combustion. Mean rate constants for oxidation of CH 4 and CO by OH in the troposphere are k
Glossary of Common Laboratory Terms
 Accuracy A measure of how close a measured value is to the true value. Assessed by means of percent recovery of spikes and standards. Aerobic Atmospheric or dissolved oxygen is available. Aliquot A measured
Accuracy A measure of how close a measured value is to the true value. Assessed by means of percent recovery of spikes and standards. Aerobic Atmospheric or dissolved oxygen is available. Aliquot A measured
UV Hound Series. Dependable High Quality Air Monitoring. Accurate Readings Within Seconds. Portable UVDOAS Multi-gas Analyzers. Multi-Gas Capability
 UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor
 13th North American Waste to Energy Conference May 23-25, 2005, Orlando, Florida USA NAWTEC13-3158 Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor G. J. Aldina Jeffrey
13th North American Waste to Energy Conference May 23-25, 2005, Orlando, Florida USA NAWTEC13-3158 Validation Study of FTIR-Based Emissions Measurements at a Municipal Waste Combustor G. J. Aldina Jeffrey
Acid rain long recognized as a problem; the air pollution problem of the 80s, but it is still with us
 Aqueous Atmospheric Chemistry: Acid Rain Review Henry s Law: scavenging of water-soluble gases into clouds, fogs, and rain Review normal ph of rainwater ~ 5.6 due to dissolved CO 2 Acid precipitation a
Aqueous Atmospheric Chemistry: Acid Rain Review Henry s Law: scavenging of water-soluble gases into clouds, fogs, and rain Review normal ph of rainwater ~ 5.6 due to dissolved CO 2 Acid precipitation a
