Sampling. Information is helpful in implementing control measures for reducing pollutant concentration to acceptable levels
|
|
|
- Hilary Ray
- 6 years ago
- Views:
Transcription
1 Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution control programme. Monitoring is important: Air quality can be evaluated Information is helpful in implementing control measures for reducing pollutant concentration to acceptable levels Assessing the effect of air pollution control strategies
2 Classification of sampling methods: 1. Sampling of impurities of every nature (Ranging from particulate matter to gases) 2. Sampling under various environmental conditions (ranging from samples taken from chimneys to samples taken in the open air) 3. Sampling methods varying according to the time factor (Ranging from intermittent to continuous sampling)
3 Air Quality measurement is undertaken in two situations: 1. Ambient air quality measurement 2. Stack monitoring Ambient air quality measurement: Where the pollutant levels in the ambient atmosphere are measured. Stack sampling: It deals with the pollutants emitted from a source such as smoke stack and is known as stack sampling. It provides information on the nature and quantities of various pollutants that are emitted into the atmosphere.
4 Difficulties encountered in sampling: Collecting samples of true representative character Errors arising from methods used for the collection and separation of the various components of pollution. Difficulty in preventing any change in the concentration of particulate matter in suspension, as a result of sampling operation.
5 Preliminary considerations and stages of sampling: Following principles should be followed to ensure correct sampling 1. Statistical studies Important to establish the basic data e.g. size and frequency of sampling It use the basic principles of the probability 2. Size of samples The sample should be large enough to make analysis possible. 3. Change in the sample during and after sampling
6 4. Continuous and intermittent sampling An automatic continuous recording apparatus is preferable. 5. Sampling of volatile constituents Carry out sampling with large volumes of air (To avoid error) 6. Sampling of particulates Errors may be introduced due to agglomeration or breaking up of particulate matter.
7 In order to eliminate the sources of error Sampling should be carried out under conditions that are as isokinetics as possible A gas stream should be sampled as far as possible in the same direction and at the same speed itself but never counter-current The collection surface should be as close as possible to the source of the gas stream. To avoid reducing the efficiency of sampling, deposits and condensation should not be allowed to form on the walls of sampling vessels.
8 of waste gas Sampling Difficulties are encountered due to high temperature, lack of uniformity in the composition of gas flow and difference in speed due to disturbances. Hence to avoid error, the gas stream should be sampled at several points and maximum number of samples should be taken to get the average value. Sampling in the open air Difficulties arise due to High dilution of the pollutants dispersed in air Consequent need to collect large number of samples Difficulty of sampling under iso-kinetic conditions.
9 To minimize the error of sampling continuous recording instruments should be used and samples should be collected at various places. Also meteorological data like wind speed and direction, should be collected to know their effects on dilution or concentration of pollutant in the air. Basic consideration of air sampling 1. The sample collected must be representative in terms of time and location 2. The sample volume should be large enough to permit accurate analysis 3. The sampling rate must be such as to provide maximum efficiency of collection 4. The duration of sampling and frequency of sampling should reflect accurately the occurrence of fluctuations in pollution level 5. The contaminants must not be modified or altered in the process of collection
10 Air sampling set-up Sampling (a) Sample collector Flow meter P low Pump (b) Flow meter Sample collector P high Pump
11 Sample collector Nature of pollutants Method of analysis to be used Sample collector To collect gaseous pollutants To collect Particulates matter Grab sampling Absorption in liquid Adsorption on solids Freeze out sampling Sedimentation (Dust fall Jar) High volume filtration (Hi volume sampler) Tape sampler Impingement Electrostatic Precipitation Thermal Precipitation
12 Instruments for sampling waste gases and for atmospheric sampling 1. Devices for general use 2. Devices for sampling gases and vapors 1. Devices for general use Meters They are used to determine accurately the volume of the gas collected. They are fitted with manometers and thermometers to indicate the pressure and temperature of the gas stream sampled.
13 Probes Sampling They are tubes suitable for penetrating into the gas stream and should be constructed of material which are non-corrosive and which can withstand special temperature conditions. They should be constructed of materials (S. S, Glass or Quartz) that do not react with the substances to be sampled. A probe should have suitable length and diameter. To ensure iso-kinetic sampling condition the opening of he probe should face the gas stream to be sampled.
14 probe Sampling
15 Suction Devices Sampling Any suction device that has the required volumetric capacity can be used. Vacuum pumps driven by electric motors are very commonly used.
16 2. Devices for sampling gases and vapors Absorbers: Effluent gases are passed through absorber (scrubbers) which contains liquid absorbents that remove one or more of the pollutants in the gas stream. The efficiency of this process depends on 1. Amount of surface contact between gas and liquid 2. Contact time 3. Concentration of absorbing medium 4. Speed of reaction between the absorbent and gas Absorbent are being used to remove sulphur dioxide, hydrogen sulphide, shulphur trioxide and fluorides and oxides of nitrogen
17 Equipments using the principle of absorption for the removal of gaseous pollutants includes (1) Packed tower (2) Plate tower (3) bubble cap plate tower (4) Spray tower (5) Liquid jet scrubber absorber. A gas can be sampled by means of a suitable absorption reagent. For this purpose, U-shaped absorbers are used. This absorber is filled with a certain amount of reagent and fitted with a porous glass partition. So that the air or gas led into them passes through the reagent solution in the form of fine bubbles thus ensuring intimate contact Sampling is carried out at an average rate of about liters per hour of gas stream Oxides of sulphur, Oxides of nitrogen, Ammonia, Hydrogen sulphide, Hydrochloric acid, Hydrofluoric acid, Hydrocynic acid, ozone, H/C, organic solvent are measured (0.1 ppm by volume)
18 Sampling train
19 Various gas absorbing devices. (A-B) Simple bubblers (C) Midget impinger (c) Greenburg Smith standard impinger (E-F) Fritted absorbers (G-H) Spiral type absorbers
20 Utility and specification of various absorbers for air sampling
21 Gas absorbing devices
22 Adsorbers Sampling Adsorption is brought about by aspiring the air or gas to be sampled through adsorption column containing silica gel, activated charcoal or another suitable agents. After adsorption, the different pollutants can be extracted from the column in various ways. e.g. by rising the temperature Difficulties: Selecting a suitable adsorbing medium Application: used for ozone and light H/C
23 Condensers Sampling The gas stream sampled is cooled in suitable containers, thus bringing about the condensation of the Volatile substances. Condensation trap can be arranged either series or parallel at decreasing temperature. Using various coolants e.g. ice, liquid air or liquid nitrogen can separate the components separated by fractional condensation. Used for the sampling of odoriferous substances.
24 Condensers Sampling The gas stream sampled is cooled in suitable containers, thus bringing about the condensation of the Volatile substances. Condensation trap can be arranged either series or parallel at decreasing temperature. Using various coolants e.g. ice, liquid air or liquid nitrogen can separate the components separated by fractional condensation. Used for the sampling of odoriferous substances.
25 Collector under reduced pressure Sampling For some substances like nitric acid and aldehydes having high molecular weight, absorption in aqueous solution is sometimes incomplete. In such cases, it is to use bottles of known volume of colleting under a pressure reduced to 200 mm Hg or even less. The absorbent solution chosen is first introduced into the bottle and the pressure is thus reduced. Then the sample is admitted until the internal and external pressures are equal and the container is shaken continuously so as to ensure maximum absorption. Use for sampling the oxides on N 2
26 Plastics containers Sampling Special polyethylene bags are used for collecting and transporting large volume of air. Advantages: They can be used for successive analysis of small fractions of sample taken. Polyethylene is inert with respect to many substances including SO 2, and formaldehyde Plastics bags are not suitable for collecting and storing aerosol suspensions because of the possible generation of electrostatic charges, as a result of which the aerosol tend to move towards the walls and condense on them. Used for grab sampling and sample storage before analysis
27 Samples for Mass Spectrometric analysis Sampling for mass spectrometric analysis can be carried out by compressing the gas sample in a pressure flask so as to concentrate a large quantity of gas in a small volume or by fling evacuated containers.
28 Duration of Sampling Period Sampling Two types of sampling are used (1) Short period or Spot sampling and (2) Continuous sampling It is used to evaluate peak and average concentration over definite time intervals. Spot sampling Samples are collected over periods varying from less than 30 minutes to several hours for specific proposes. The choice of sampling period depends upon Nature of the component under study Its stability to oxidation, light or other factors such as sensitivity, accuracy and precision of the analytical method to be used.
29 Short sampling is useful for the random checking of pollution at many points. Such samples have limited value because pollution levels fluctuate widely depending on meteorological conditions, topographical features and various factors associated with sources of pollution (e.g. Mass rates of emission of pollutants form smoke stacks, the temperature, velocity and density of stack gases the height of smoke stacks, the distribution of sources and downwind distance form the sources to the points where the measurement are made).
30 Continuous sampling: Sampling Techniques are useful in systematic studies of the nature and extent of air pollution if the data are to be value for epidemiological surveys, for evaluating the potential hazards to man, animal or vegetation and for control programmes. It can be carried out by the subsequent analysis of pollutant by appropriate analytical techniques.
31 Location of sampling sites Sampling Sampling sites must be carefully selected so as o be representatives of the area under study. A representative number of sampling stations for a given area may be established by means of a preliminary survey whose objectives should be 1. To gather information on the nature and magnitude of the emission from principal sources of pollution. 2. To review the available climatologically and meteorological data 3. To gather data o the conc. Of pollutant in areas of severe and slight air pollution
32 Collection of Gaseous air Pollutants: 1. Grab sampling 2. Absorption in liquid 3. Adsorption on solids 4. Freeze-out Sampling 1. Grab sampling In grab sampling the sample is collected by filling an evacuated flask or an inflatable bag. Plastics bag are widely used. Bag sampling: Disadvantages: Losses caused by moisture condensation or diffusion through the walls of the bag. The losses can be minimized by performing the analysis immediately following collection. Grab sampling may be taken using rigid wall containers made from glass or stainless steel.
33 Various devices for collection of gaseous air samples
34 Absorption in liquid Sampling Absorption separates the desire pollutant form air either through direct solubility in the absorbing medium or by chemical reaction. 1. Fitted glass absorber 2. Impingers Fitted glass absorber The gas stream is broken up into extremely small bubbles, thus promoting an intimate contact between the gas and the liquid. Frits designated coarse (50 µm pore size) are used for air sampling. The glass frit can become blocked and is difficult to clean after use. So pre filter the air prior to sampling.
35 Impingers Sampling In the Impingers the gas stream is impinged at high velocity onto a flat surface thus providing good contact between the gas and liquid. The flat surface can be the bottom of the collector or a specially designed pate. Two types of Impingers: (1) Wet Impingers (2) Dry Impingers Wet Impingers: Collect a particle by causing them to impinge a surface submerged in a liquid. Dry Impingers: Referred to as impactors collect particles by impaction on a dry surface.
36 In both the apparatus, collection results as result of inertial force as the particles tend to resist a change in the direction when the air stream is deflected by a surface or other obstacle. The efficient is very high whose diameter is 1 µ or grater. There are two types if Impingers (wet collector) Greenberg- Smith Midget type Devices can handled sample flow rate of about 30 and 3 liter per minute respectively Easy to clean and maintain.
37 Impingers
38 Sampling Midget fitted impingers Standard midget impingers
39 Adsorption: Sampling This method is based on the tendency of gases to be adsorbed on the surface of solid materials. The sample air is passed through a packed column containing a finely divided solid adsorbent on whose surface the pollutants are retained and concentrated. Solid adsorbent: Granular porous solids: Activated Charcoal, Silica gel After adsorption, the sample gases are desorbed for analysis. This may be accomplished by heating the adsorbent to volatilize the trapped materials or by washing it with a liquid solvent. Most organic vapors are analyses by gas chromatographic techniques that directly use the adsorption of the gases. Disadvantages: Desorption of gases are complicated
40 Freeze out Sampling: Sampling In freeze out sampling a series of cold traps, which are maintained at progressively lower temperature, are used to draw the air sample, whereby the pollutants are condensed. The traps are brought to the laboratory, the samples are removed and analyses by means of gas chromatographic, infrared or ultraviolet, spectrophotometer, and mass spectrometry or by wet chemical means. Disadvantages: Plugging of the system because of Ice formation
41 Freeze out Sampling: Sampling Coolants used for freeze-out traps Coolant Temp. Attained ( 0 C) Ice-water 0 Ice-salt -21 Dry ice and -79 acetone Liquid air -147 Liquid oxygen -183 Liquid nitrogen -196
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Sampling for Gases and Vapors
 Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Methods of pollution control and waste management - laboratory. Adsorptive removal of volatile organic compounds from gases streams
 Methods of pollution control and waste management - laboratory Adsorptive removal of volatile organic compounds from gases streams Manual for experiment 17 dr Hanna Wilczura-Wachnik and dr inż. Jadwiga
Methods of pollution control and waste management - laboratory Adsorptive removal of volatile organic compounds from gases streams Manual for experiment 17 dr Hanna Wilczura-Wachnik and dr inż. Jadwiga
Raw Material Finished product By product Exposure standards Length of shift Physical environment
 Measurement &Sampling Part 1 Introduction to Sampling What to sample? - Hazard Raw Material Finished product By product Exposure standards Length of shift Recognition Physical environment To Determine
Measurement &Sampling Part 1 Introduction to Sampling What to sample? - Hazard Raw Material Finished product By product Exposure standards Length of shift Recognition Physical environment To Determine
MODULE 4.3 Atmospheric analysis of particulates
 MODULE 4.3 Atmospheric analysis of particulates Measurement And Characterisation Of The Particulate Content 1 Total particulate concentration 1 Composition of the particulate 1 Determination of particle
MODULE 4.3 Atmospheric analysis of particulates Measurement And Characterisation Of The Particulate Content 1 Total particulate concentration 1 Composition of the particulate 1 Determination of particle
Chapter 7 Separation of Particles from a Gas
 Chapter 7 Separation of Particles from a Gas For either gas cleaning (removal of dusts) or recovery of particulate products Separation Mechanisms Sedimentation : Settling chamber, centrifuge Migration
Chapter 7 Separation of Particles from a Gas For either gas cleaning (removal of dusts) or recovery of particulate products Separation Mechanisms Sedimentation : Settling chamber, centrifuge Migration
NITROGEN AND ITS COMPOUNDS Q30 (i) Explain how the following would affect the yield of ammonia. An increase in (i). Pressure.
 NAME SCHOOL INDEX NUMBER DATE NITROGEN AND ITS COMPOUNDS 1. 1990 Q30 (i) Explain how the following would affect the yield of ammonia. An increase in (i). Pressure. (2 marks) marks)... (ii) Temperature
NAME SCHOOL INDEX NUMBER DATE NITROGEN AND ITS COMPOUNDS 1. 1990 Q30 (i) Explain how the following would affect the yield of ammonia. An increase in (i). Pressure. (2 marks) marks)... (ii) Temperature
4023 Synthesis of cyclopentanone-2-carboxylic acid ethyl ester from adipic acid diethyl ester
 NP 4023 Synthesis of cyclopentanone-2-carboxylic acid ethyl ester from adipic acid diethyl ester NaEt C 10 H 18 4 Na C 2 H 6 C 8 H 12 3 (202.2) (23.0) (46.1) (156.2) Classification Reaction types and substance
NP 4023 Synthesis of cyclopentanone-2-carboxylic acid ethyl ester from adipic acid diethyl ester NaEt C 10 H 18 4 Na C 2 H 6 C 8 H 12 3 (202.2) (23.0) (46.1) (156.2) Classification Reaction types and substance
Sodium Chloride - Analytical Standard
 Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
Sodium Chloride - Analytical Standard Determination of Total Mercury Former numbering: ECSS/CN 312-1982 & ESPA/CN-E-106-1994 1. SCOPE AND FIELD OF APPLICATION The present EuSalt Analytical Standard describes
ANALYTICAL METHOD DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Air sampling and analysis
 DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Replaces: Dated: Author: Date: AM-No.: New New Nils Arne Jentoft 18.06.2014 0 CHANGES This procedure is new. 1 SCOPE This document describes
DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Replaces: Dated: Author: Date: AM-No.: New New Nils Arne Jentoft 18.06.2014 0 CHANGES This procedure is new. 1 SCOPE This document describes
Chapter 3: Source Measurement Techniques
 Chapter 3 Source Measurement Techniques Measurement Methods Method 18, Measurement of Gaseous Organic Compound Emissions by Gas Chromatography Method 25, Determination of Total Gaseous Non-Methane Organic
Chapter 3 Source Measurement Techniques Measurement Methods Method 18, Measurement of Gaseous Organic Compound Emissions by Gas Chromatography Method 25, Determination of Total Gaseous Non-Methane Organic
Lab 4 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 4 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 4: This experiment will involve determining
Recap: Introduction 12/1/2015. EVE 402 Air Pollution Generation and Control. Adsorption
 EVE 402 Air Pollution Generation and Control Chapter #6 Lectures Adsorption Recap: Solubility: the extent of absorption into the bulk liquid after the gas has diffused through the interface An internal
EVE 402 Air Pollution Generation and Control Chapter #6 Lectures Adsorption Recap: Solubility: the extent of absorption into the bulk liquid after the gas has diffused through the interface An internal
Basic Digestion Principles
 Basic Digestion Principles 1 From Samples to Solutions Direct Analytical Method Solid Sample Problems: Mech. Sample Preparation (Grinding, Sieving, Weighing, Pressing, Polishing,...) Solid Sample Autosampler
Basic Digestion Principles 1 From Samples to Solutions Direct Analytical Method Solid Sample Problems: Mech. Sample Preparation (Grinding, Sieving, Weighing, Pressing, Polishing,...) Solid Sample Autosampler
UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j
 UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j 1 Environmental Air Pollution The addition of any harmful material (having harmful effects on our lives) to our atmosphere is called
UNIT 4 Part 2 Measurements of Environmental Air Pollution Parameters j 1 Environmental Air Pollution The addition of any harmful material (having harmful effects on our lives) to our atmosphere is called
--> Buy True-PDF --> Auto-delivered in 0~10 minutes. GB Translated English of Chinese Standard: GB5009.
 Translated English of Chinese Standard: GB5009.17-2014 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF GB THE PEOPLE S REPUBLIC OF CHINA National Food Safety Standard-Determination
Translated English of Chinese Standard: GB5009.17-2014 www.chinesestandard.net Sales@ChineseStandard.net NATIONAL STANDARD OF GB THE PEOPLE S REPUBLIC OF CHINA National Food Safety Standard-Determination
High-Pressure Volumetric Analyzer
 High-Pressure Volumetric Analyzer High-Pressure Volumetric Analysis HPVA II Benefits Dual free-space measurement for accurate isotherm data Free space can be measured or entered Correction for non-ideality
High-Pressure Volumetric Analyzer High-Pressure Volumetric Analysis HPVA II Benefits Dual free-space measurement for accurate isotherm data Free space can be measured or entered Correction for non-ideality
A P T I : A T M O S P H E R I C S A M P L I N G C O U R S E
 Chapter 6 This chapter will take approximately 1 hour and 45 minutes to complete. Gaseous Sampling O B J E C T I V E S Terminal Learning Objective At the end of this chapter, the student will be able to
Chapter 6 This chapter will take approximately 1 hour and 45 minutes to complete. Gaseous Sampling O B J E C T I V E S Terminal Learning Objective At the end of this chapter, the student will be able to
Wet FGD Chemistry and Performance Factors
 Wet FGD Chemistry and Performance Factors Gordon Maller URS Corporation Presented at: 2008 Power Gen Conference December 1, 2008 Presentation Outline FGD Chemistry Overview Effect of Key Process Variables
Wet FGD Chemistry and Performance Factors Gordon Maller URS Corporation Presented at: 2008 Power Gen Conference December 1, 2008 Presentation Outline FGD Chemistry Overview Effect of Key Process Variables
BUSIA SUB-COUNTY JET 2016
 Name Index No.. School... 233/1 CHEMISTRY THEORY Paper 1 Time: 2 Hours BUSIA SUB-COUNTY JET 2016 Kenya Certificate of Secondary Education (K.C.S.E) INSTRUCTIONS TO CANDIDATES. Answer all the questions
Name Index No.. School... 233/1 CHEMISTRY THEORY Paper 1 Time: 2 Hours BUSIA SUB-COUNTY JET 2016 Kenya Certificate of Secondary Education (K.C.S.E) INSTRUCTIONS TO CANDIDATES. Answer all the questions
EXECUTIVE SUMMARY. especially in last 50 years. Industries, especially power industry, are the large anthropogenic
 EXECUTIVE SUMMARY Introduction The concentration of CO 2 in atmosphere has increased considerably in last 100 years, especially in last 50 years. Industries, especially power industry, are the large anthropogenic
EXECUTIVE SUMMARY Introduction The concentration of CO 2 in atmosphere has increased considerably in last 100 years, especially in last 50 years. Industries, especially power industry, are the large anthropogenic
GRAVIMETRIC ANALYSIS
 GRAVIMETRIC ANALYSIS Gravimetric methods are quantitative methods in which the mass of the analyte or some compound that is chemically related to the analyte is determined. What are the steps in a gravimetric
GRAVIMETRIC ANALYSIS Gravimetric methods are quantitative methods in which the mass of the analyte or some compound that is chemically related to the analyte is determined. What are the steps in a gravimetric
Volumetric Analysis. Quantitative analysis answers the second question
 Volumetric Analysis Volumetric analysis is a form of quantitative analysis involving the measuring of volumes of reacting solutions, it involves the use of titrations. When buying food we often have two
Volumetric Analysis Volumetric analysis is a form of quantitative analysis involving the measuring of volumes of reacting solutions, it involves the use of titrations. When buying food we often have two
ph AND WATER Comparable substance
 BACKGROUND ph AND WATER ph 15 The ph of a solution is a measure of its hydrogen ion (H + ) concentration. A solution with the same amount of H + as pure water has a ph value of 7 and is said to be neutral.
BACKGROUND ph AND WATER ph 15 The ph of a solution is a measure of its hydrogen ion (H + ) concentration. A solution with the same amount of H + as pure water has a ph value of 7 and is said to be neutral.
4. a) Complete the nuclear equation below. (1mk) b) 37 37
 KCSE 2006 CHEMISTRY PAPER 1 QUESTIONS 1. (a) What is meant by isomerism? (1mark) (b) Draw and name two isomers of butane. (2 marks) 2. The diagram below represent a set-up that was used to show that part
KCSE 2006 CHEMISTRY PAPER 1 QUESTIONS 1. (a) What is meant by isomerism? (1mark) (b) Draw and name two isomers of butane. (2 marks) 2. The diagram below represent a set-up that was used to show that part
PRINCIPLES AND APPLICATION OF CHROMATOGRAPHY. Dr. P. Jayachandra Reddy Mpharm PhD Principal & professor KTPC
 PRINCIPLES AND APPLICATION OF CHROMATOGRAPHY Dr. P. Jayachandra Reddy Mpharm PhD Principal & professor KTPC CHROMATOGRAPHY Laboratory technique for the Separation of mixtures Chroma -"color" and graphein
PRINCIPLES AND APPLICATION OF CHROMATOGRAPHY Dr. P. Jayachandra Reddy Mpharm PhD Principal & professor KTPC CHROMATOGRAPHY Laboratory technique for the Separation of mixtures Chroma -"color" and graphein
Gravimetric Methods of Analysis
 Gravimetric Methods of Analysis Chapter 8 Gravimetric Analysis Skoog Book Page 179-198 Do Problems: 1,2,4,9,10,11,14,16,21,27,30,33 Chapter 9 Electrolyte Effects Activities effective concentration and
Gravimetric Methods of Analysis Chapter 8 Gravimetric Analysis Skoog Book Page 179-198 Do Problems: 1,2,4,9,10,11,14,16,21,27,30,33 Chapter 9 Electrolyte Effects Activities effective concentration and
Gaseous CEMS technology
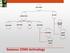 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
Gas Chromatography. Presented By Mr. Venkateswarlu Mpharm KTPC
 Gas Chromatography Gas Chromatography Presented By Mr. Venkateswarlu Mpharm KTPC What is Gas Chromatography? It is also known as Gas-Liquid Chromatography (GLC) GAS CHROMATOGRAPHY Separation of gaseous
Gas Chromatography Gas Chromatography Presented By Mr. Venkateswarlu Mpharm KTPC What is Gas Chromatography? It is also known as Gas-Liquid Chromatography (GLC) GAS CHROMATOGRAPHY Separation of gaseous
Matter Properties and Changes
 Matter Properties and Changes What is matter? anything that takes up space (volume) and has mass everything around you is made up of matter matter has 3 main states: solid, liquid, and gas Physical Property
Matter Properties and Changes What is matter? anything that takes up space (volume) and has mass everything around you is made up of matter matter has 3 main states: solid, liquid, and gas Physical Property
 Chapter No. 2 EXPERIMENTAL TECHNIQUES IN CHEMISTRY SHORT QUESTIONS WITH ANSWERS Q.1 Define analytical chemistry? The branch of chemistry which deals with the qualitative and quantitative analyses of sample
Chapter No. 2 EXPERIMENTAL TECHNIQUES IN CHEMISTRY SHORT QUESTIONS WITH ANSWERS Q.1 Define analytical chemistry? The branch of chemistry which deals with the qualitative and quantitative analyses of sample
Thermochemistry/Calorimetry. Determination of the enthalpy of vaporization of liquids LEC 02. What you need: What you can learn about
 LEC 02 Thermochemistry/Calorimetry Determination of the enthalpy of vaporization of liquids What you can learn about Enthalpy of vaporisation Entropy of vaporisation Trouton s rule Calorimetry Heat capacity
LEC 02 Thermochemistry/Calorimetry Determination of the enthalpy of vaporization of liquids What you can learn about Enthalpy of vaporisation Entropy of vaporisation Trouton s rule Calorimetry Heat capacity
Attention is drawn to the following places, which may be of interest for search:
 B03C MAGNETIC OR ELECTROSTATIC SEPARATION OF SOLID MATERIALS FROM SOLID MATERIALS OR FLUIDS; SEPARATION BY HIGH-VOLTAGE ELECTRIC FIELDS (filters making use of electricity or magnetism B01D 35/06; separating
B03C MAGNETIC OR ELECTROSTATIC SEPARATION OF SOLID MATERIALS FROM SOLID MATERIALS OR FLUIDS; SEPARATION BY HIGH-VOLTAGE ELECTRIC FIELDS (filters making use of electricity or magnetism B01D 35/06; separating
Aerosol Dynamics. Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008
 Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Chemistry Instrumental Analysis Lecture 34. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
On the Emissivity of Silver Coated Panels, Effect of Long Term Stability and Effect of Coating Thickness
 JET P(98)57 P A eladarakis W Obert On the Emissivity of Silver Coated Panels, Effect of Long Term Stability and Effect of Coating Thickness This document is intended for publication in the open literature.
JET P(98)57 P A eladarakis W Obert On the Emissivity of Silver Coated Panels, Effect of Long Term Stability and Effect of Coating Thickness This document is intended for publication in the open literature.
Thin Layer Chromatography
 Thin Layer Chromatography Thin-layer chromatography involves the same principles as column chromatography, it also is a form of solid-liquid adsorption chromatography. In this case, however, the solid
Thin Layer Chromatography Thin-layer chromatography involves the same principles as column chromatography, it also is a form of solid-liquid adsorption chromatography. In this case, however, the solid
CHEMISTRY HIGHER LEVEL
 *P15* Pre-Leaving Certificate Examination, 2012 Triailscrúdú na hardteistiméireachta, 2012 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
*P15* Pre-Leaving Certificate Examination, 2012 Triailscrúdú na hardteistiméireachta, 2012 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
Real-Time Detection: From Gisclard et al.: A Simple Device for Air Analysis. AIHA Quarterly, 14(1):23-25 (1953)
 Real-Time Detection: 1953 From Gisclard et al.: A Simple Device for Air Analysis. AIHA Quarterly, 14(1):23-25 (1953) Sampling Gases and Vapors Gas: A state of matter characterized by very low density and
Real-Time Detection: 1953 From Gisclard et al.: A Simple Device for Air Analysis. AIHA Quarterly, 14(1):23-25 (1953) Sampling Gases and Vapors Gas: A state of matter characterized by very low density and
METHOD 3520C CONTINUOUS LIQUID-LIQUID EXTRACTION
 METHOD 3520C CONTINUOUS LIQUID-LIQUID EXTRACTION 1.0 SCOPE AND APPLICATION 1.1 This method describes a procedure for isolating organic compounds from aqueous samples. The method also describes concentration
METHOD 3520C CONTINUOUS LIQUID-LIQUID EXTRACTION 1.0 SCOPE AND APPLICATION 1.1 This method describes a procedure for isolating organic compounds from aqueous samples. The method also describes concentration
 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
Chemistry Instrumental Analysis Lecture 31. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 31 High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) Solvent Delivery
Chemistry 4631 Instrumental Analysis Lecture 31 High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) High Performance Liquid Chromatography (HPLC) Solvent Delivery
MIXTURES, COMPOUNDS, & SOLUTIONS
 MIXTURES, COMPOUNDS, & SOLUTIONS As with elements, few compounds are found pure in nature and usually found as mixtures with other compounds. A mixture is a combination of two or more substances that are
MIXTURES, COMPOUNDS, & SOLUTIONS As with elements, few compounds are found pure in nature and usually found as mixtures with other compounds. A mixture is a combination of two or more substances that are
Post-Show HOT AND COLD. Gases. Liquids. Solids. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show HOT AND COLD After the Show We recently presented a Hot and Cold show at your school, and thought you and your students might like to continue investigating this topic.
Traveling Science Shows Post-Show HOT AND COLD After the Show We recently presented a Hot and Cold show at your school, and thought you and your students might like to continue investigating this topic.
Name AP Chemistry / / Chapter 5 Collected AP Exam Free Response Questions Answers
 Name AP Chemistry / / Chapter 5 Collected AP Exam Free Response Questions 1980 2010 - Answers 1982 - #5 (a) From the standpoint of the kinetic-molecular theory, discuss briefly the properties of gas molecules
Name AP Chemistry / / Chapter 5 Collected AP Exam Free Response Questions 1980 2010 - Answers 1982 - #5 (a) From the standpoint of the kinetic-molecular theory, discuss briefly the properties of gas molecules
THEORETICAL DETERMINATION OF THE SAMPLING RATES OF DIFFUSION SAMPLERS FOR VOCS AND ALDEHYDES
 THEORETICAL DETERMINATION OF THE SAMPLING RATES OF DIFFUSION SAMPLERS FOR VOCS AND ALDEHYDES J Kouzaki 1*, S Sato 1, S Nakai 1, Y Shirasuna 2, K Hirano 2 1 Graduate School of Environmental and Information
THEORETICAL DETERMINATION OF THE SAMPLING RATES OF DIFFUSION SAMPLERS FOR VOCS AND ALDEHYDES J Kouzaki 1*, S Sato 1, S Nakai 1, Y Shirasuna 2, K Hirano 2 1 Graduate School of Environmental and Information
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY
 5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY SUB-TOPIC 1.2 METHODS OF PURIFICATION AND ANALYSIS LEARNING
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY SUB-TOPIC 1.2 METHODS OF PURIFICATION AND ANALYSIS LEARNING
MAHESH TUTORIALS I.C.S.E.
 MAHESH TUTORIALS I.C.S.E. GRADE - X (2017-2018) Exam No. : MT/ICSE/SEMI PRELIM - I-SET -A 008 Sulphuric acid, Ammonia, Analytical Chemistry, Organic Chemistry HCl, Nitric acid, Metallurgy Chemistry SCIENCE
MAHESH TUTORIALS I.C.S.E. GRADE - X (2017-2018) Exam No. : MT/ICSE/SEMI PRELIM - I-SET -A 008 Sulphuric acid, Ammonia, Analytical Chemistry, Organic Chemistry HCl, Nitric acid, Metallurgy Chemistry SCIENCE
Glossary of Common Laboratory Terms
 Accuracy A measure of how close a measured value is to the true value. Assessed by means of percent recovery of spikes and standards. Aerobic Atmospheric or dissolved oxygen is available. Aliquot A measured
Accuracy A measure of how close a measured value is to the true value. Assessed by means of percent recovery of spikes and standards. Aerobic Atmospheric or dissolved oxygen is available. Aliquot A measured
Air Monitoring. Semi-continuous determination of ambient air quality
 Air Monitoring Semi-continuous determination of ambient air quality The Particle Into Liquid Sampler a simple solution for the determination of ions in aerosol particles 02 Combustion of fossil fuels for
Air Monitoring Semi-continuous determination of ambient air quality The Particle Into Liquid Sampler a simple solution for the determination of ions in aerosol particles 02 Combustion of fossil fuels for
ICSE Board Class IX Chemistry Paper 3 Solution
 ICSE Board Class IX Chemistry Paper 3 Solution SECTION I Answer 1 i. The number of electrons, that atom can lose, gain or share during a chemical reaction is called its valency. ii. Solute: A solute is
ICSE Board Class IX Chemistry Paper 3 Solution SECTION I Answer 1 i. The number of electrons, that atom can lose, gain or share during a chemical reaction is called its valency. ii. Solute: A solute is
Test Booklet. Subject: SC, Grade: HS CST High School Chemistry Part 2. Student name:
 Test Booklet Subject: SC, Grade: HS Student name: Author: California District: California Released Tests Printed: Thursday January 16, 2014 1 Theoretically, when an ideal gas in a closed container cools,
Test Booklet Subject: SC, Grade: HS Student name: Author: California District: California Released Tests Printed: Thursday January 16, 2014 1 Theoretically, when an ideal gas in a closed container cools,
METHOD 7060A ARSENIC (ATOMIC ABSORPTION, FURNACE TECHNIQUE)
 METHOD 7060A ARSENIC (ATOMIC ABSORPTION, FURNACE TECHNIQUE) 1.0 SCOPE AND APPLICATION 1.1 Method 7060 is an atomic absorption procedure approved for determining the concentration of arsenic in wastes,
METHOD 7060A ARSENIC (ATOMIC ABSORPTION, FURNACE TECHNIQUE) 1.0 SCOPE AND APPLICATION 1.1 Method 7060 is an atomic absorption procedure approved for determining the concentration of arsenic in wastes,
Developing an Improved Impinger-Based Method for Measuring Gaseous Hydrogen Chloride in Cement and Lime Kiln Emissions
 Developing an Improved Impinger-Based Method for Measuring Gaseous Hydrogen Chloride in Cement and Lime Kiln Emissions Laura L. Kinner and James W. Peeler Emission Monitoring Inc. Raleigh, NC 27612 Daniel
Developing an Improved Impinger-Based Method for Measuring Gaseous Hydrogen Chloride in Cement and Lime Kiln Emissions Laura L. Kinner and James W. Peeler Emission Monitoring Inc. Raleigh, NC 27612 Daniel
Carbonate content. SCAN-N 32:98 Revised White, green and black liquors and burnt lime sludge
 Revised 1998 White, green and black liquors and burnt lime sludge Carbonate content 0 Introduction This SCAN-test Method replaces SCAN-N 32:88 from which it differs in that it, in addition to white and
Revised 1998 White, green and black liquors and burnt lime sludge Carbonate content 0 Introduction This SCAN-test Method replaces SCAN-N 32:88 from which it differs in that it, in addition to white and
Basic Equipments and Instruments used in Chemistry laboratory: Balance: It is an instrument for measuring mass.
 Basic Equipments and Instruments used in Chemistry laboratory: Balance: It is an instrument for measuring mass. Pipettes: They are used to transfer of known volumes of liquids from one container to another.
Basic Equipments and Instruments used in Chemistry laboratory: Balance: It is an instrument for measuring mass. Pipettes: They are used to transfer of known volumes of liquids from one container to another.
78% : component of atmosphere! 21% : 1% : Changes depending on origin of air: - originated over - originated over Ozone = O 3 Definition:
 Unit 6 Part 1 Meteorology Name: Composition and Structure of the Atmosphere SWBAT: Describe the composition of the atmosphere. Diagram/describe the layers of the earth s atmosphere. Weather Climate Atmospheric
Unit 6 Part 1 Meteorology Name: Composition and Structure of the Atmosphere SWBAT: Describe the composition of the atmosphere. Diagram/describe the layers of the earth s atmosphere. Weather Climate Atmospheric
not to be republished NCERT THE technique of chromatography is vastly used for the separation, Chromatography UNIT-5 EXPERIMENT 5.
 UNIT-5 Chromatography THE technique of chromatography is vastly used for the separation, purification and identification of compounds. According to IUPAC, chromatography is a physical method of separation
UNIT-5 Chromatography THE technique of chromatography is vastly used for the separation, purification and identification of compounds. According to IUPAC, chromatography is a physical method of separation
Vacuum Pumps. Two general classes exist: Gas transfer physical removal of matter. Mechanical, diffusion, turbomolecular
 Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Method for the determination of 1,3-butadiene
 Federation of the Employment Accidents Insurance Institutions of Germany (Hauptverband der Berufsgenossenschaften) Centre for Accident Prevention and Occupational Medicine Alte Heerstraße 111, 53757 Sankt
Federation of the Employment Accidents Insurance Institutions of Germany (Hauptverband der Berufsgenossenschaften) Centre for Accident Prevention and Occupational Medicine Alte Heerstraße 111, 53757 Sankt
models (three-dimensional representation containing essential structure of
 Unit 2 Matter The universe consists of matter and energy. Chemistry is the branch of science the studies matter as well as the changes it undergoes and the energy changes that accompany such transformations.
Unit 2 Matter The universe consists of matter and energy. Chemistry is the branch of science the studies matter as well as the changes it undergoes and the energy changes that accompany such transformations.
Unit 11: Chapters 15 and 16
 Unit 11: Chapters 15 and 16 Water and Solution Chemistry What makes Water Special? Extensive Hydrogen Bonding!! Unusually... high surface tension low vapor pressure high specific heat capacity high molar
Unit 11: Chapters 15 and 16 Water and Solution Chemistry What makes Water Special? Extensive Hydrogen Bonding!! Unusually... high surface tension low vapor pressure high specific heat capacity high molar
Physicochemical Processes
 Lecture 3 Physicochemical Processes Physicochemical Processes Air stripping Carbon adsorption Steam stripping Chemical oxidation Supercritical fluids Membrane processes 1 1. Air Stripping A mass transfer
Lecture 3 Physicochemical Processes Physicochemical Processes Air stripping Carbon adsorption Steam stripping Chemical oxidation Supercritical fluids Membrane processes 1 1. Air Stripping A mass transfer
Angel International School - Manipay 1 st Term Examination November, 2016 Chemistry
 c Grade 9 Angel International School - Manipay 1 st Term Examination November, 2016 Chemistry Duration: 01:30 Hours Index No:- PART I Underline the correct answers 1) Identify the correct statement about
c Grade 9 Angel International School - Manipay 1 st Term Examination November, 2016 Chemistry Duration: 01:30 Hours Index No:- PART I Underline the correct answers 1) Identify the correct statement about
Surface Chemistry & States of Matter
 Surface Chemistry & States of Matter S. Sunil Kumar Lecturer in Chemistry 1. Adsorption is a. Colligative property b. Oxidation process c. Reduction process d. Surface phenomenon Ans. d 2. When adsorption
Surface Chemistry & States of Matter S. Sunil Kumar Lecturer in Chemistry 1. Adsorption is a. Colligative property b. Oxidation process c. Reduction process d. Surface phenomenon Ans. d 2. When adsorption
What type of samples are common? Time spent on different operations during LC analyses. Number of samples? Aims. Sources of error. Sample preparation
 What type of samples are common? Sample preparation 1 2 Number of samples? Time spent on different operations during LC analyses 3 4 Sources of error Aims Sample has to be representative Sample has to
What type of samples are common? Sample preparation 1 2 Number of samples? Time spent on different operations during LC analyses 3 4 Sources of error Aims Sample has to be representative Sample has to
Continuous measurement of airborne particles and gases
 Continuous measurement of airborne particles and gases Jeff Collett and Taehyoung Lee Atmospheric Science Department Colorado State University Funding: USDA/AES and NPS Outline Why measure particles and
Continuous measurement of airborne particles and gases Jeff Collett and Taehyoung Lee Atmospheric Science Department Colorado State University Funding: USDA/AES and NPS Outline Why measure particles and
Generation and absorption of CO2 gas
 Generation and absorption of CO2 gas CO2 is generated by dissolving carbonates in in hydrochloric acid according to the following equation: CaCO3(s) + 2 HCl(l) = CaCl2(aq) + CO2(g) + H2O(l) One convenient
Generation and absorption of CO2 gas CO2 is generated by dissolving carbonates in in hydrochloric acid according to the following equation: CaCO3(s) + 2 HCl(l) = CaCl2(aq) + CO2(g) + H2O(l) One convenient
BRIEFING. Pharmacopeial Discussion Group Sign Off Document Attributes EP JP USP Definition Loss on drying Readily carbonizable substances
 BRIEFING Saccharin, NF 22 page 2825 and page 1711 of PF 29(5) [Sept. Oct. 2003]. The United States Pharmacopeia is the coordinating pharmacopeia for the international harmonization of the compendial standards
BRIEFING Saccharin, NF 22 page 2825 and page 1711 of PF 29(5) [Sept. Oct. 2003]. The United States Pharmacopeia is the coordinating pharmacopeia for the international harmonization of the compendial standards
Physical Separations and Chromatography
 Lab #5A & B: Physical Separations and Chromatography Individual Objectives: At the end of these experiments you should be able to: Ø Distinguish between Rf and tr; chromatograph and chromatogram; adsorption
Lab #5A & B: Physical Separations and Chromatography Individual Objectives: At the end of these experiments you should be able to: Ø Distinguish between Rf and tr; chromatograph and chromatogram; adsorption
SEPARATION BY BARRIER
 SEPARATION BY BARRIER SEPARATION BY BARRIER Phase 1 Feed Barrier Phase 2 Separation by barrier uses a barrier which restricts and/or enhances the movement of certain chemical species with respect to other
SEPARATION BY BARRIER SEPARATION BY BARRIER Phase 1 Feed Barrier Phase 2 Separation by barrier uses a barrier which restricts and/or enhances the movement of certain chemical species with respect to other
Lab 6 Major Anions In Atmospheric Aerosol Particles
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2007 Lab 6 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 6: This experiment will involve determining
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2007 Lab 6 Major Anions In Atmospheric Aerosol Particles Purpose of Lab 6: This experiment will involve determining
Downloaded from
 Matter in Our Surroundings 1. Which state of matter is characterized by the following properties : (0 A substance with a fixed arrangement of particles. (I'O A substance that has large distances between
Matter in Our Surroundings 1. Which state of matter is characterized by the following properties : (0 A substance with a fixed arrangement of particles. (I'O A substance that has large distances between
PRACTICAL QUESTIONS TEST TUBE REACTIONS 4&11 Questions. Dr Chris Clay
 PRACTICAL QUESTIONS TEST TUBE REACTIONS 4&11 Questions Dr Chris Clay http://drclays-alevelchemistry.com/ Q1.(a) A sample of solid chromium(iii) hydroxide displays amphoteric character when treated separately
PRACTICAL QUESTIONS TEST TUBE REACTIONS 4&11 Questions Dr Chris Clay http://drclays-alevelchemistry.com/ Q1.(a) A sample of solid chromium(iii) hydroxide displays amphoteric character when treated separately
Rule 2. Rule 1. Rule 4. Rule 3. Rule 5. Rule 6. Rule 7. Rule 8
 Rule 1 Follow the directions in your course reader, of your teaching assistant and of your instructor. They are usually much more experienced doing chemistry. Rule 3 When in doubt, ask. This will make
Rule 1 Follow the directions in your course reader, of your teaching assistant and of your instructor. They are usually much more experienced doing chemistry. Rule 3 When in doubt, ask. This will make
Which particle diagram represents molecules of only one compound in the gaseous phase?
 Name: 1) Which species represents a chemical compound? 9114-1 - Page 1 NaHCO3 NH4 + Na N2 2) 3) 4) Which substance represents a compound? Co(s) O2(g) CO(g) C(s) Which terms are used to identify pure substances?
Name: 1) Which species represents a chemical compound? 9114-1 - Page 1 NaHCO3 NH4 + Na N2 2) 3) 4) Which substance represents a compound? Co(s) O2(g) CO(g) C(s) Which terms are used to identify pure substances?
Generation of monodisperse aerosols through condensation nuclei control
 Air Pollution XV 505 Generation of monodisperse aerosols through condensation nuclei control H. M. Kadlimatti 1, S. Gangamma 2 & S. K. Varghese 3 1 Department of Mechanical Engineering, Basaveshwar Engineering
Air Pollution XV 505 Generation of monodisperse aerosols through condensation nuclei control H. M. Kadlimatti 1, S. Gangamma 2 & S. K. Varghese 3 1 Department of Mechanical Engineering, Basaveshwar Engineering
SOIL ORGANIC CONTENT USING UV-VIS METHOD
 Test Procedure for SOIL ORGANIC CONTENT USING UV-VIS METHOD TxDOT Designation: Tex-148-E Effective Date: March 2016 1. SCOPE 1.1 This method determines the soil organic content based on the amount of humic
Test Procedure for SOIL ORGANIC CONTENT USING UV-VIS METHOD TxDOT Designation: Tex-148-E Effective Date: March 2016 1. SCOPE 1.1 This method determines the soil organic content based on the amount of humic
WATER IN THE ATMOSPHERE
 WATER IN THE ATMOSPHERE During a rainstorm, the air feels moist On a clear, cloudless day, the air may feel dry As the sun heats the land and oceans, the amount of water in the atmosphere changes Water
WATER IN THE ATMOSPHERE During a rainstorm, the air feels moist On a clear, cloudless day, the air may feel dry As the sun heats the land and oceans, the amount of water in the atmosphere changes Water
ESTIMATION OF UNCERTAINTY IN THE DETERMINATION OF NITROGEN OXIDES EMISSIONS
 Chemical Eng. Dept., ISEL From the SelectedWorks of João F Gomes 2006 ESTIMATION OF UNCERTAINTY IN THE DETERMINATION OF NITROGEN OXIDES EMISSIONS João F Gomes Available at: https://works.bepress.com/joao_gomes/9/
Chemical Eng. Dept., ISEL From the SelectedWorks of João F Gomes 2006 ESTIMATION OF UNCERTAINTY IN THE DETERMINATION OF NITROGEN OXIDES EMISSIONS João F Gomes Available at: https://works.bepress.com/joao_gomes/9/
C (s) + O 2 (g) CO 2 (g) S (s) + O 2 (g) SO 2 (g)
 Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Vapor Intrusion Sampling Options: Performance Data for Canisters, Badges, and Sorbent Tubes for VOCs
 Vapor Intrusion Sampling Options: Performance Data for s, Badges, and Sorbent Tubes for VOCs Linda S. Coyne SKC Inc., 863 Valley View Road, Eighty Four, PA 1533 George Havalias, Maria C. Echarte American
Vapor Intrusion Sampling Options: Performance Data for s, Badges, and Sorbent Tubes for VOCs Linda S. Coyne SKC Inc., 863 Valley View Road, Eighty Four, PA 1533 George Havalias, Maria C. Echarte American
In terms of production, nitric acid is the third most widely produced acid across the world.
 In terms of production, nitric acid is the third most widely produced acid across the world. It has a wide range of uses in agriculture, industry and medicine where it is used as a fertiliser and in the
In terms of production, nitric acid is the third most widely produced acid across the world. It has a wide range of uses in agriculture, industry and medicine where it is used as a fertiliser and in the
Acyl chloride/ acid anhydride
 3.14 Synthetic routes poly(alkene) dihalogenoalkane KH aqueous under reflux Nu Sub diol high pressure catalyst Step 1 H 2 S 4 EAdd Step 2 H 2 warm hydrolysis alcohol alkene conc. H 2 S 4 or conc. H 3 P
3.14 Synthetic routes poly(alkene) dihalogenoalkane KH aqueous under reflux Nu Sub diol high pressure catalyst Step 1 H 2 S 4 EAdd Step 2 H 2 warm hydrolysis alcohol alkene conc. H 2 S 4 or conc. H 3 P
TECHNICAL BRODIFACOUM
 BRODIFACOUM Full specification: Approved 10 December 1999 1. Specification 1.1 Description The material shall consist of brodifacoum together with related manufacturing impurities and shall be in the form
BRODIFACOUM Full specification: Approved 10 December 1999 1. Specification 1.1 Description The material shall consist of brodifacoum together with related manufacturing impurities and shall be in the form
for free revision past papers visit:
 NAME ADM NO:. STUNDENT S SIGNATURE DATE.. SCHOOL 233/2 FORM THREE CHEMISTRY THEORY Paper 2 END YEAR 2017 EXAMS. Time: 2 Hrs FORM THREE CHEMISTRY 233/2 INSTRUCTIONS TO CANDIDATES Write your Name and Index
NAME ADM NO:. STUNDENT S SIGNATURE DATE.. SCHOOL 233/2 FORM THREE CHEMISTRY THEORY Paper 2 END YEAR 2017 EXAMS. Time: 2 Hrs FORM THREE CHEMISTRY 233/2 INSTRUCTIONS TO CANDIDATES Write your Name and Index
Chromatography & instrumentation in Organic Chemistry
 Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
METHOD 7B - DETERMINATION OF NITROGEN OXIDE EMISSIONS FROM STATIONARY SOURCES (ULTRAVIOLET SPECTROPHOTOMETRIC METHOD)
 683 METHOD 7B - DETERMINATION OF NITROGEN OXIDE EMISSIONS FROM STATIONARY SOURCES (ULTRAVIOLET SPECTROPHOTOMETRIC METHOD) NOTE: This method does not include all of the specifications (e.g., equipment and
683 METHOD 7B - DETERMINATION OF NITROGEN OXIDE EMISSIONS FROM STATIONARY SOURCES (ULTRAVIOLET SPECTROPHOTOMETRIC METHOD) NOTE: This method does not include all of the specifications (e.g., equipment and
NaOH (aq) + HCl (aq) NaCl (aq) + H 2 O (l)
 EXPERIMENT 21 Molarity of a Hydrochloric Acid Solution by Titration INTRODUCTION Volumetric analysis is a general term meaning any method in which a volume measurement is the critical operation; however,
EXPERIMENT 21 Molarity of a Hydrochloric Acid Solution by Titration INTRODUCTION Volumetric analysis is a general term meaning any method in which a volume measurement is the critical operation; however,
for free past papers visit
 NAME SCHOOL ------------------------------- INDEX NO. ----------------------------- SIGNATURE -------------------------- DATE ---------------------------------- 233/2 CHEMISTRYPAPER 2 (Theory) TIME: 2
NAME SCHOOL ------------------------------- INDEX NO. ----------------------------- SIGNATURE -------------------------- DATE ---------------------------------- 233/2 CHEMISTRYPAPER 2 (Theory) TIME: 2
Chapter content. Reference
 Chapter 7 HPLC Instrumental Analysis Rezaul Karim Environmental Science and Technology Jessore University of Science and Technology Chapter content Liquid Chromatography (LC); Scope; Principles Instrumentation;
Chapter 7 HPLC Instrumental Analysis Rezaul Karim Environmental Science and Technology Jessore University of Science and Technology Chapter content Liquid Chromatography (LC); Scope; Principles Instrumentation;
Water and Aqueous Systems
 Water and Aqueous Systems The Water Molecule: a Review Water is a simple tri-atomic molecule, H 2 O Each O-H bond is highly polar, because of the high electronegativity of the oxygen (N, O, F, and Cl have
Water and Aqueous Systems The Water Molecule: a Review Water is a simple tri-atomic molecule, H 2 O Each O-H bond is highly polar, because of the high electronegativity of the oxygen (N, O, F, and Cl have
1. Preliminary qualitative analysis of unknown substances (liquid or solid).
 Name of Procedure: Ultraviolet Spectroscopy Suggested Uses: 1. Preliminary qualitative analysis of unknown substances (liquid or solid). 2. Quantitative analysis of known compounds. Apparatus Used to Perform
Name of Procedure: Ultraviolet Spectroscopy Suggested Uses: 1. Preliminary qualitative analysis of unknown substances (liquid or solid). 2. Quantitative analysis of known compounds. Apparatus Used to Perform
CHAPTER 1: Chemistry, An Introduction
 CHAPTER 1: Chemistry, An Introduction science: the study of nature to explain what one observes 1.4 THE SCIENTIFIC METHOD: How Chemists Think Applying the Scientific Method 1. Make an observation, and
CHAPTER 1: Chemistry, An Introduction science: the study of nature to explain what one observes 1.4 THE SCIENTIFIC METHOD: How Chemists Think Applying the Scientific Method 1. Make an observation, and
Diquat 1,1 -ethylene-2,2 -bipyridium dibromide salt Paraquat 1,1 -dimethyl-4,4 -bipyridium dichloride salt Initial Preparation
 EPA Method 549.2 Revision 1.0 Determination of Diquat and Paraquat in Drinking Water by Liquid-Solid Extraction and High Performance Liquid Chromatography with Ultraviolet Detection* UCT Products: ENVIRO-CLEAN
EPA Method 549.2 Revision 1.0 Determination of Diquat and Paraquat in Drinking Water by Liquid-Solid Extraction and High Performance Liquid Chromatography with Ultraviolet Detection* UCT Products: ENVIRO-CLEAN
The Particulate Nature of Matter
 The Particulate Nature of Matter Matter: Matter is defined as anything that has mass and takes space. There are 3 states of matter: Solids Liquids Gases Scientists have developed a model called the kinetic
The Particulate Nature of Matter Matter: Matter is defined as anything that has mass and takes space. There are 3 states of matter: Solids Liquids Gases Scientists have developed a model called the kinetic
Structural characterization begins with a purity check!
 Structural characterization begins with a purity check! Refractive Index Melting Point Elemental Analysis (EA) Thin Layer Chromatography (TLC) High Performance Liquid Chromatography (HPLC) Course: 59-320
Structural characterization begins with a purity check! Refractive Index Melting Point Elemental Analysis (EA) Thin Layer Chromatography (TLC) High Performance Liquid Chromatography (HPLC) Course: 59-320
Chapter 7 Solid Surface
 Chapter 7 Solid Surface Definition of solid : A matter that is rigid and resists stress. Difference between solid and liquid surface : Liquid : always in equilibrium and equipotential. (Fig 7.1a,b) Solid
Chapter 7 Solid Surface Definition of solid : A matter that is rigid and resists stress. Difference between solid and liquid surface : Liquid : always in equilibrium and equipotential. (Fig 7.1a,b) Solid
2B Air, Oxygen, Carbon Dioxide and Water
 Air, Oxygen, Carbon Dioxide and Water Air, oxygen and carbon dioxide are important chemicals in our everyday lives. Knowledge of their properties helps us to develop an understanding of the role they play.
Air, Oxygen, Carbon Dioxide and Water Air, oxygen and carbon dioxide are important chemicals in our everyday lives. Knowledge of their properties helps us to develop an understanding of the role they play.
DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD
 Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
