Zumdahl, Hfst. 9. Intermoleculaire Binding. Intermoleculaire Binding (overzicht modellen 2) Intermoleculaire Binding (overzicht modellen)
|
|
|
- Kenneth Walters
- 5 years ago
- Views:
Transcription
1 Zumdahl, Hfst. 9 Hybridisatie (9.1) orbital theorie ( ) bindingsvolgorde binding diatomic molecules binding heteronucleaire moleculen Combinatie LE en M theorie (9.5) p. 351: A bond will form if the energy of the aggregate is lower than that of the separate atoms NB: voor het verklaren van het bestaan van moleculen, die dus een lage toestand vertegenwoordigen, zijn steeds uitgebreidere modellen nodig. (overzicht modellen) (overzicht modellen 2) Quantumchemie is overkoepelend model plossing vaak onmogelijk (complexe moleculen) Benadering nu mogelijk met computermodellen Echter, onderstaande modellen gebaseerde op quantumchemie stellen ons in staat >90 of zelfs 99% van alle moleculen te begrijpen. Gelokaliseerde electronmodellen (Hoofdstuk 8) - Van Ionbinding tot Covalente Binding - Valence Bond model: valentie-electronen spelen hoofdrol bij chemische binding Covalent: Lewis model voor Electronenverdeling in moleculen i Resonantie VSEPR Niet alleen electronen-verdeling, maar ook verklaring waarom chemische binding in organische moleculen stabiel zijn waarom ze een bepaalde ruimtelijke structuur hebben Hybridisatie (Hfk. 9.1) Verklaring van het bestaan van een aantal bijzonder moleculen en eigenschappen die niet te verklaren zijn met de voorgaande modellen Gedelokaliseerde electronmodellen (hfk ) - M theorie Localized Electron Model (8-9.1) of atomic Underlying assumption: A molecule is composed of atoms that are bound together by sharing pairs of electrons using the atomic of the bound atoms. Hfst. 8: ionbinding covalent; Lewis Hfst 9: gehybridiseerde atomic Definition Model Most important types; examples sp 3 methaan sp 2 etheen sp ethyn Additional types that explain exceptions to ctet-rule sp 2 d / sp 3 d 2 / sp 3 d 2 1
2 - Definition -Model The mixing of atomic to form special for bonding Quantumchemie: het is mogelijk oplossingen te vinden van de Schrodinger vergelijking die aangeven dat in plaats van s en p atomic er gemixte bestaan rond een atoom die onderling gelijkwaardig zijn Voorbeelden: sp 3, sp 2, sp 1. Per atoom kunnen atomic s, p (en evt. d) samen verschillende gehybridiseerde orbitalen vormen 2. Deze kunnen vervolgens een binding aangaan met een ander atoom 1. Met een s of p orbitaal 2. Met eveneens een gehybridiseerd orbitaal 3. Dit leidt tot eenσ- of π-binding 3. Elk individueel atoom reageert zo, dat de laagste toestand voor het kan worden bereikt Hybridized - uitwerking sp 3 hybridisatie whenever a set of 4 equivalent tetrahedral atomic is required by an atom in a molecule, this model assumes that the atom adopts a set of sp 3 sp 2 analogous: whenever 3 equivalent, triagonale bindingshoeken 120 o sp analogous: whenever 2 equivalent,.. Straight, bindingshoeken 180 o NTABENE: als de Lewisstructuur een vrij electronenpaar aangeeft bij een atoom, dan telt dat als een binding. Hybridized uitwerking 2 sp 2 : in dit geval onstaan er 3 gelijkwaardige orbitalen voor bindingen of vrije electronenparen in die richtingen 1 vrij p-orbital, (oorspronkelijk atomic orbital) in vlak loodrecht op de driehoek sp: - 2 gelijkwaardige orbitalen 2 vrije p-orbitalen loodrecht op de as van de sp orbitalen en loodrecht op elkaar (p y en p z Hybridisation vorming van binding Hybridized - Voorbeelden Twee typen bindings-orbitaal: er worden σ-bond orbital en/of π-bond orbitalen gevormd Altijd één sigma (σ) binding welke langs de internucleaire as ligt. Combinatie van (, sp, sp2, sp3) met (, sp, sp2, sp3); Combinatie van ( met ) 0, 1 of 2 pi (π ) bond orbital Deze ligt in de ruimte boven en onder de internucleaire as Bestaat altijd uit een combinatie van twee niet-gehybridiseerde atomic p orbitalen (p y en p z ) Bonding around C-atom: all four sp 3 maybe used for σ-bonding (CH 4 ) or combination of sp 2 and π-bonding of (double bond) (C 2 H 4 ) or combination of sp and 2 π-bonds of (triple bond) (C 2 H 2,C 2 ) 2
3 Hybridized - voorbeelden The Localized Electron Model Bonding around -atom: sp 3 : two for σ-bonding, 2 free electron pairs example: CH 3 H one for σ-bonding, 3 free electron pairs, example: H 3 C - ; sp 2 : one double bond (σ- ) and π-bonding of orbital; 2 free electron pairs; example: C 2 (double bond), formaldehyde H 2 C sp: one for σ-bonding and 2 π-bonds of (triple bond); example: C Procedure to draw the Loc. Electron Model 1. Draw the Lewis structure(s) ( ) 2. Determine the arrangement of electron pairs (VSEPR model;) VSEPR: valence shell electron-pair repulsion model, Specify the necessary hybrid (9.1) Name Example rbitals for σ-bonding Free p- π-bonding sp 3 methaan 4 0 sp 2 etheen 3 1 sp ethyn 2 2 sp 2 d / sp 3 d / sp 3 d 2?? hybr. type hybr. type C for C: for : BF - 4 for B: for F: XeF 2 for Xe: for F: S 2 for S: for : 1. Draw Lewis-structure 2. Determine electron pair arrangement (VSEPR) 3. Determine hybridisation required per atom - procedure 1. Draw Lewis-structure: (p. 377 Zumdahl) sum all valence electrons; divide them so that all atoms to achieve NGEC (Noble Gas Electronic Configuration; I.e. 8 electron pairs (ctet rule) all atoms net zero formal charge or charge as low as possible; net sum of electrons (lone pairs + half bonding pairs) = valence of atom 2. Determine electron pair arrangement (VSEPR) spatial arrangement to minimize electron pair repulsions (8.13; p. 390 Zumdahl) - procedure (2) 3. Determine hybridisation required per atom From step 1: locations of double/triple bonds From step 2: spatial arrangement (NB step can be skipped first, and checked later on) Per atom: derive from step 1 the number N of free p- to accommodate all double/triple bonds; sp x ; and x = 3-N; N>2: d orbital involved! Per atom: check whether spatial arrangement of hybrid fits with result step 2. 3
4 - result C for C: sp for : sp BF - 4 for B: sp 3 for F: sp 3 XeF 2 for Xe: dsp 3 for F: sp 3 S 2 for S: sp 2 for : sp 2 Prediction of shape of molecule Consider the allene molecule H 2 C=C=CH 2 A) Are all four hydrogen atoms in the same plane? B) If not, what is their spatial relationship; Explain orbital theorie Gelokaliseerde electronmodellen Ionbinding Lewisstructuren VSEPR Valence Bond model Hybridisatie Gedelokaliseerde electronmodellen M theorie orbital theorie ( ) bindingsvolgorde binding diatomic molecules binding heteronucleaire moleculen Combinatie LE en M theorie (9.5) M theory: rbitals M theory Yet another model? Basic assumptions M energy diagram - H 2 - He 2 - Li 2 - B 2 - verview Combination M-VB Model: Electrons shared between two atoms can be thought to be in a rbital that is predicted by the Schrodinger Equation. Assumption: Analogous to atomic, molecules will strive to reach minimum energy configuration Consequence: existing M s will be filled from lowest energy onwards. 4
5 M theory - assumptions (cont d) In order to participate in M s, atomic must overlap in space. Therefore, only valence of atoms contribute significantly to Ms. M - Another model M offers quantum-chemically correct explanation of structure of molecules that were previously described as exhibiting resonance structures M is new theory that explains and supports the model simplification of the resonance structure theory In addition, offers explanation why complex reaction mechanisms occur Example: atmospheric ZNE chemistry! M - Again, natural systems evolve to state of minimum energy M - (1) H 2 M theory predicts that atomic (A) recombine to yield: M s with lower energy than A s: Bonding rbitals M s with higher energy than A s: Anti-Bonding rbitals σ * σ H H 2 H M - (2) He 2 Bond rder (B) He σ * σ He 2 He M helps to predict which molecules are stable, and which are not; indicator: B Difference between the number of electrons in bonding M s and number of electrons in anti-bonding M s divided by two. B is indication of relative stability of a molecular bond, and hence of a molecule 5
6 M - (3) Li 2 Be 2 M energy levels: B 2 σ * σ * s-p orbital mixing verzicht: p.436 σ x * π y * π z * σ x π y π z σ σ Li Li 2 Li What is B of both these molecules Which one is likely to be stable? Be Be 2 Be B σ * σ B 2 B M - energy levels Shift for B 2 C 2 N 2 N 2 σ x * π y * π z * σ x π y π z M - energy levels No shift for 2 F 2 2 σ x * π y * π z * π y π z σ x σ * σ * N σ N 2 N σ 2 M - energy levels N σ x * Magnetism Use for diatomic molecules adjacent in Per. Syst. N CN etc. π y * π z * σ x π y π z σ * Paramagnetism unpaired electrons attracted to induced magnetic field Diamagnetism paired electrons repelled from induced magnetic field σ 2 6
7 utcomes of M Model M theorie 1 As bond order increases, bond energy increases and bond length decreases. 2 N 2 has a triple bond, and a correspondingly high bond energy. 3 2 is paramagnetic. This is predicted by the M model, not by the Lewis model, which predicts diamagnetism. M diagram H 2 - He 2 Li 2 B 2 verzicht Combinatie M-VB Combining LE and M Models σ bonds can be described as being localized. π bonding must be treated as being delocalized. 7
Chapter 9. Covalent Bonding: Orbitals
 Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents 9.1 Hybridization and the Localized Electron Model 9.2 The Molecular Orbital Model 9.3 Bonding in Homonuclear Diatomic Molecules 9.4 Bonding
Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents 9.1 Hybridization and the Localized Electron Model 9.2 The Molecular Orbital Model 9.3 Bonding in Homonuclear Diatomic Molecules 9.4 Bonding
Molecular Geometry and Bonding Theories. Chapter 9
 Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Chapter 9. Covalent Bonding: Orbitals. Copyright 2017 Cengage Learning. All Rights Reserved.
 Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents (9.1) (9.2) (9.3) (9.4) (9.5) (9.6) Hybridization and the localized electron model The molecular orbital model Bonding in homonuclear diatomic
Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents (9.1) (9.2) (9.3) (9.4) (9.5) (9.6) Hybridization and the localized electron model The molecular orbital model Bonding in homonuclear diatomic
Chapter 9. Covalent Bonding: Orbitals
 Chapter 9 Covalent Bonding: Orbitals EXERCISE! Draw the Lewis structure for methane, CH 4. What is the shape of a methane molecule? tetrahedral What are the bond angles? 109.5 o H H C H H Copyright Cengage
Chapter 9 Covalent Bonding: Orbitals EXERCISE! Draw the Lewis structure for methane, CH 4. What is the shape of a methane molecule? tetrahedral What are the bond angles? 109.5 o H H C H H Copyright Cengage
Chapter 4. Molecular Structure and Orbitals
 Chapter 4 Molecular Structure and Orbitals Chapter 4 Table of Contents (4.1) (4.2) (4.3) (4.4) (4.5) (4.6) (4.7) Molecular structure: The VSEPR model Bond polarity and dipole moments Hybridization and
Chapter 4 Molecular Structure and Orbitals Chapter 4 Table of Contents (4.1) (4.2) (4.3) (4.4) (4.5) (4.6) (4.7) Molecular structure: The VSEPR model Bond polarity and dipole moments Hybridization and
Chapter 9. Covalent Bonding: Orbitals
 Chapter 9. Covalent onding: Orbitals Models to explain the structures and/or energies of the covalent molecules Localized Electron (LE) onding Model Lewis Structure Valence Shell Electron Pair Repulsion
Chapter 9. Covalent onding: Orbitals Models to explain the structures and/or energies of the covalent molecules Localized Electron (LE) onding Model Lewis Structure Valence Shell Electron Pair Repulsion
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Farthest apart
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Chapter 10. VSEPR Model: Geometries
 Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Chapter 10 Molecular Geometry VSEPR Model: Geometries Valence Shell Electron Pair Repulsion Theory Electron pairs repel and get as far apart as possible Example: Water Four electron pairs Two bonds Two
Covalent Compounds: Bonding Theories and Molecular Structure
 CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
CHM 123 Chapter 8 Covalent Compounds: Bonding Theories and Molecular Structure 8.1 Molecular shapes and VSEPR theory VSEPR theory proposes that the geometric arrangement of terminal atoms, or groups of
Ch. 9- Molecular Geometry and Bonding Theories
 Ch. 9- Molecular Geometry and Bonding Theories 9.0 Introduction A. Lewis structures do not show one of the most important aspects of molecules- their overall shapes B. The shape and size of molecules-
Ch. 9- Molecular Geometry and Bonding Theories 9.0 Introduction A. Lewis structures do not show one of the most important aspects of molecules- their overall shapes B. The shape and size of molecules-
Chapter 9 Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories molecular shapes the VSEPR model molecular shape and molecular polarity covalent bonding and orbital overlap hybrid orbitals multiple bonds 9.1 Molecular
Chapter 9 Molecular Geometry and Bonding Theories molecular shapes the VSEPR model molecular shape and molecular polarity covalent bonding and orbital overlap hybrid orbitals multiple bonds 9.1 Molecular
General and Inorganic Chemistry I.
 General and Inorganic Chemistry I. Lecture 1 István Szalai Eötvös University István Szalai (Eötvös University) Lecture 1 1 / 29 Outline István Szalai (Eötvös University) Lecture 1 2 / 29 Lewis Formulas
General and Inorganic Chemistry I. Lecture 1 István Szalai Eötvös University István Szalai (Eötvös University) Lecture 1 1 / 29 Outline István Szalai (Eötvös University) Lecture 1 2 / 29 Lewis Formulas
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Hybridisation of Atomic Orbitals
 Lecture 7 CHEM101 Hybridisation of Atomic Orbitals Dr. Noha Osman Learning Outcomes Understand the valence bond theory Understand the concept of hybridization. Understand the different types of orbital
Lecture 7 CHEM101 Hybridisation of Atomic Orbitals Dr. Noha Osman Learning Outcomes Understand the valence bond theory Understand the concept of hybridization. Understand the different types of orbital
Chapter 9 Molecular Geometry and Bonding Theories
 Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Chemistry: The Central Science. Chapter 9: Molecular Geometry and Bonding Theory
 Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
VSEPR Model. Valence-Shell Electron-Pair Repulsion Bonds (single or multiple) and lone pairs are thought of as charge clouds
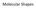 Molecular Shapes VSEPR Model Valence-Shell Electron-Pair Repulsion Bonds (single or multiple) and lone pairs are thought of as charge clouds They repel each other and stay as far away from each other as
Molecular Shapes VSEPR Model Valence-Shell Electron-Pair Repulsion Bonds (single or multiple) and lone pairs are thought of as charge clouds They repel each other and stay as far away from each other as
For more info visit Chemical bond is the attractive force which holds various constituents together in a molecule.
 Chemical bond:- Chemical bond is the attractive force which holds various constituents together in a molecule. There are three types of chemical bonds: Ionic Bond, Covalent Bond, Coordinate Bond. Octet
Chemical bond:- Chemical bond is the attractive force which holds various constituents together in a molecule. There are three types of chemical bonds: Ionic Bond, Covalent Bond, Coordinate Bond. Octet
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model.
 Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Molecular Geometry and Chemical Bonding Theory
 Molecular Geometry and Chemical Bonding Theory The Valence -Shell Electron -Pair Repulsion (VSEPR) Model predicts the shapes of the molecules and ions by assuming that the valence shell electron pairs
Molecular Geometry and Chemical Bonding Theory The Valence -Shell Electron -Pair Repulsion (VSEPR) Model predicts the shapes of the molecules and ions by assuming that the valence shell electron pairs
Chapter 8. Molecular Shapes. Valence Shell Electron Pair Repulsion Theory (VSEPR) What Determines the Shape of a Molecule?
 PowerPoint to accompany Molecular Shapes Chapter 8 Molecular Geometry and Bonding Theories Figure 8.2 The shape of a molecule plays an important role in its reactivity. By noting the number of bonding
PowerPoint to accompany Molecular Shapes Chapter 8 Molecular Geometry and Bonding Theories Figure 8.2 The shape of a molecule plays an important role in its reactivity. By noting the number of bonding
COVALENT BONDING: ORBITALS
 COVALENT BONDING: ORBITALS The localized electron model views a molecule as a collection of atoms bound together by sharing electrons between their atomic orbitals. The arrangement of valence electrons
COVALENT BONDING: ORBITALS The localized electron model views a molecule as a collection of atoms bound together by sharing electrons between their atomic orbitals. The arrangement of valence electrons
CHAPTER TEN MOLECULAR GEOMETRY MOLECULAR GEOMETRY V S E P R CHEMICAL BONDING II: MOLECULAR GEOMETRY AND HYBRIDIZATION OF ATOMIC ORBITALS
 CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
Molecular Orbitals. Chapter 9. Sigma bonding orbitals. Sigma bonding orbitals. Pi bonding orbitals. Sigma and pi bonds
 Molecular Orbitals Chapter 9 Orbitals and Covalent Bond The overlap of atomic orbitals from separate atoms makes molecular orbitals Each molecular orbital has room for two electrons Two types of MO Sigma
Molecular Orbitals Chapter 9 Orbitals and Covalent Bond The overlap of atomic orbitals from separate atoms makes molecular orbitals Each molecular orbital has room for two electrons Two types of MO Sigma
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9 - Covalent Bonding: Orbitals
 Chapter 9 - Covalent Bonding: Orbitals 9.1 Hybridization and the Localized Electron Model A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new
Chapter 9 - Covalent Bonding: Orbitals 9.1 Hybridization and the Localized Electron Model A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new
Chapter 9: Molecular Geometry and Bonding Theories
 Chapter 9: Molecular Geometry and Bonding Theories 9.1 Molecular Geometries -Bond angles: angles made by the lines joining the nuclei of the atoms in a molecule -Bond angles determine overall shape of
Chapter 9: Molecular Geometry and Bonding Theories 9.1 Molecular Geometries -Bond angles: angles made by the lines joining the nuclei of the atoms in a molecule -Bond angles determine overall shape of
Chapters 9&10 Structure and Bonding Theories
 Chapters 9&10 Structure and Bonding Theories Ionic Radii Ions, just like atoms, follow a periodic trend in their radii. The metal ions in a given period are smaller than the non-metal ions in the same
Chapters 9&10 Structure and Bonding Theories Ionic Radii Ions, just like atoms, follow a periodic trend in their radii. The metal ions in a given period are smaller than the non-metal ions in the same
Molecular shape is determined by the number of bonds that form around individual atoms.
 Chapter 9 CH 180 Major Concepts: Molecular shape is determined by the number of bonds that form around individual atoms. Sublevels (s, p, d, & f) of separate atoms may overlap and result in hybrid orbitals
Chapter 9 CH 180 Major Concepts: Molecular shape is determined by the number of bonds that form around individual atoms. Sublevels (s, p, d, & f) of separate atoms may overlap and result in hybrid orbitals
Organic Chemistry. Review Information for Unit 1. VSEPR Hybrid Orbitals Polar Molecules
 rganic hemistry Review Information for Unit 1 VSEPR ybrid rbitals Polar Molecules VSEPR The valence shell electron pair repulsion model (VSEPR) can be used to predict the geometry around a particular atom
rganic hemistry Review Information for Unit 1 VSEPR ybrid rbitals Polar Molecules VSEPR The valence shell electron pair repulsion model (VSEPR) can be used to predict the geometry around a particular atom
Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
Molecular Structure and Orbitals
 CHEM 1411 General Chemistry Chemistry: An Atoms First Approach by Zumdahl 2 5 Molecular Structure and Orbitals Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and
CHEM 1411 General Chemistry Chemistry: An Atoms First Approach by Zumdahl 2 5 Molecular Structure and Orbitals Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and
Lewis Structure and Electron Dot Models
 Lewis Structure and Electron Dot Models The Lewis Structure is a method of displaying the electrons present in any given atom or compound. Steps: 1. Make a skeleton structure 2. Count all e- available
Lewis Structure and Electron Dot Models The Lewis Structure is a method of displaying the electrons present in any given atom or compound. Steps: 1. Make a skeleton structure 2. Count all e- available
1s atomic orbital 2s atomic orbital 2s atomic orbital (with node) 2px orbital 2py orbital 2pz orbital
 Atomic Orbitals 1s atomic orbital 2s atomic orbital 2s atomic orbital (with node) 2px orbital 2py orbital 2pz orbital Valence Bond Theory and ybridized Atomic Orbitals Bonding in 2 1s 1s Atomic Orbital
Atomic Orbitals 1s atomic orbital 2s atomic orbital 2s atomic orbital (with node) 2px orbital 2py orbital 2pz orbital Valence Bond Theory and ybridized Atomic Orbitals Bonding in 2 1s 1s Atomic Orbital
Chapter 9. Chemical Bonding II: Molecular Geometry and Bonding Theories
 Chapter 9 Chemical Bonding II: Molecular Geometry and Bonding Theories Topics Molecular Geometry Molecular Geometry and Polarity Valence Bond Theory Hybridization of Atomic Orbitals Hybridization in Molecules
Chapter 9 Chemical Bonding II: Molecular Geometry and Bonding Theories Topics Molecular Geometry Molecular Geometry and Polarity Valence Bond Theory Hybridization of Atomic Orbitals Hybridization in Molecules
Chapter 10 Chemical Bonding II
 Chapter 10 Chemical Bonding II Valence Bond Theory Valence Bond Theory: A quantum mechanical model which shows how electron pairs are shared in a covalent bond. Bond forms between two atoms when the following
Chapter 10 Chemical Bonding II Valence Bond Theory Valence Bond Theory: A quantum mechanical model which shows how electron pairs are shared in a covalent bond. Bond forms between two atoms when the following
SHAPES OF MOLECULES (VSEPR MODEL)
 1 SAPES MLEULES (VSEPR MDEL) Valence Shell Electron-Pair Repulsion model - Electron pairs surrounding atom spread out as to minimize repulsion. - Electron pairs can be bonding pairs (including multiple
1 SAPES MLEULES (VSEPR MDEL) Valence Shell Electron-Pair Repulsion model - Electron pairs surrounding atom spread out as to minimize repulsion. - Electron pairs can be bonding pairs (including multiple
Molecular Shape and Molecular Polarity. Molecular Shape and Molecular Polarity. Molecular Shape and Molecular Polarity
 Molecular Shape and Molecular Polarity When there is a difference in electronegativity between two atoms, then the bond between them is polar. It is possible for a molecule to contain polar bonds, but
Molecular Shape and Molecular Polarity When there is a difference in electronegativity between two atoms, then the bond between them is polar. It is possible for a molecule to contain polar bonds, but
Chapters 8 and 9. Octet Rule Breakers Shapes
 Chapters 8 and 9 Octet Rule Breakers Shapes Bond Energies Bond Energy (review): The energy needed to break one mole of covalent bonds in the gas phase Breaking bonds consumes energy; forming bonds releases
Chapters 8 and 9 Octet Rule Breakers Shapes Bond Energies Bond Energy (review): The energy needed to break one mole of covalent bonds in the gas phase Breaking bonds consumes energy; forming bonds releases
Chapter 9 Molecular Geometry Valence Bond and Molecular Orbital Theory
 Chapter 9 Molecular Geometry Valence Bond and Molecular Orbital Theory Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and how they are used to model covalent bonding.
Chapter 9 Molecular Geometry Valence Bond and Molecular Orbital Theory Chapter Objectives: Learn the basics of Valence Bond Theory and Molecular Orbital Theory and how they are used to model covalent bonding.
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
Lecture Presentation. Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Chapter 13: Phenomena
 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Molecular shape is only discussed when there are three or more atoms connected (diatomic shape is obvious).
 Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is
Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is
Chapter 10. Structure Determines Properties! Molecular Geometry. Chemical Bonding II
 Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
CHAPTER 5: Bonding Theories - Explaining Molecular Geometry. Chapter Outline
 CHAPTER 5: Bonding Theories - Explaining Molecular Geometry Chapter Outline 5.1 Molecular Shape 5.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) 5.3 Polar Bonds and Polar Molecules» What Makes
CHAPTER 5: Bonding Theories - Explaining Molecular Geometry Chapter Outline 5.1 Molecular Shape 5.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) 5.3 Polar Bonds and Polar Molecules» What Makes
PART 3 Chemical Bonds, Valence Bond Method, and Molecular Shapes. Reference: Chapter 9 10 in textbook
 PART 3 Chemical Bonds, Valence Bond Method, and Molecular Shapes Reference: Chapter 9 10 in textbook 1 Valence Electrons Valence ae Electron Define: the outer shell electrons Important for determination
PART 3 Chemical Bonds, Valence Bond Method, and Molecular Shapes Reference: Chapter 9 10 in textbook 1 Valence Electrons Valence ae Electron Define: the outer shell electrons Important for determination
Chapter 10 Theories of Covalent Bonding
 Chapter 10 Theories of Covalent Bonding 1 Atomic Orbitals Molecules Bonding and 2 Molecular Structure Questions How are molecules held together? Why is O 2 paramagnetic? And how is this property connected
Chapter 10 Theories of Covalent Bonding 1 Atomic Orbitals Molecules Bonding and 2 Molecular Structure Questions How are molecules held together? Why is O 2 paramagnetic? And how is this property connected
At the end of this lesson, students should be able to :
 At the end of this lesson, students should be able to : (a) Explain Valence Shell Electron Pair Repulsion theory (VSEPR) (b) Draw the basic molecular shapes: linear, planar, tetrahedral, and octahedral.
At the end of this lesson, students should be able to : (a) Explain Valence Shell Electron Pair Repulsion theory (VSEPR) (b) Draw the basic molecular shapes: linear, planar, tetrahedral, and octahedral.
CHEMISTRY. Chapter 10 Theories of Bonding and Structure. The Molecular Nature of Matter. Jespersen Brady Hyslop SIXTH EDITION
 CHEMISTRY The Molecular Nature of Matter SIXTH EDITION Jespersen Brady Hyslop Chapter 10 Theories of Bonding and Structure Copyright 2012 by John Wiley & Sons, Inc. Molecular Structures Molecules containing
CHEMISTRY The Molecular Nature of Matter SIXTH EDITION Jespersen Brady Hyslop Chapter 10 Theories of Bonding and Structure Copyright 2012 by John Wiley & Sons, Inc. Molecular Structures Molecules containing
Chapter 10. Geometry
 Chapter 10 Molec cular Geometry 1 CHAPTER OUTLINE Molecular Geometry Molecular Polarity VSEPR Model Summary of Molecular Shapes Hybridization Molecular Orbital Theory Bond Angles 2 MOLECULAR GEOMETRY Molecular
Chapter 10 Molec cular Geometry 1 CHAPTER OUTLINE Molecular Geometry Molecular Polarity VSEPR Model Summary of Molecular Shapes Hybridization Molecular Orbital Theory Bond Angles 2 MOLECULAR GEOMETRY Molecular
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron
Shapes of Molecules and Hybridization
 Shapes of Molecules and Hybridization A. Molecular Geometry Lewis structures provide us with the number and types of bonds around a central atom, as well as any NB electron pairs. They do not tell us the
Shapes of Molecules and Hybridization A. Molecular Geometry Lewis structures provide us with the number and types of bonds around a central atom, as well as any NB electron pairs. They do not tell us the
I. Multiple Choice Questions (Type-I)
 I. Multiple Choice Questions (Type-I) 1. Isostructural species are those which have the same shape and hybridisation. Among the given species identify the isostructural pairs. (i) [NF 3 and BF 3 ] [BF
I. Multiple Choice Questions (Type-I) 1. Isostructural species are those which have the same shape and hybridisation. Among the given species identify the isostructural pairs. (i) [NF 3 and BF 3 ] [BF
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
What Do Molecules Look Like?
 What Do Molecules Look Like? The Lewis Dot Structure approach provides some insight into molecular structure in terms of bonding, but what about 3D geometry? Recall that we have two types of electron pairs:
What Do Molecules Look Like? The Lewis Dot Structure approach provides some insight into molecular structure in terms of bonding, but what about 3D geometry? Recall that we have two types of electron pairs:
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories MOLECULAR SHAPES 2 Molecular Shapes Lewis Structures show bonding and lone pairs do not denote shape Use Lewis Structures to determine shapes Molecular
Chapter 9 Molecular Geometry and Bonding Theories MOLECULAR SHAPES 2 Molecular Shapes Lewis Structures show bonding and lone pairs do not denote shape Use Lewis Structures to determine shapes Molecular
Carbon and Its Compounds
 Chapter 1 Carbon and Its Compounds Copyright 2018 by Nelson Education Limited 1 1.2 Organic Molecules from the Inside Out I: The Modelling of Atoms Copyright 2018 by Nelson Education Limited 2 s orbitals:
Chapter 1 Carbon and Its Compounds Copyright 2018 by Nelson Education Limited 1 1.2 Organic Molecules from the Inside Out I: The Modelling of Atoms Copyright 2018 by Nelson Education Limited 2 s orbitals:
Section 8.1 The Covalent Bond
 Section 8.1 The Covalent Bond Apply the octet rule to atoms that form covalent bonds. Describe the formation of single, double, and triple covalent bonds. Contrast sigma and pi bonds. Relate the strength
Section 8.1 The Covalent Bond Apply the octet rule to atoms that form covalent bonds. Describe the formation of single, double, and triple covalent bonds. Contrast sigma and pi bonds. Relate the strength
Downloaded from
 Remedial action plan :- Chemical bonding # focus on important topic 1. Octet rule 2. Lewis structure Q.7. The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly.
Remedial action plan :- Chemical bonding # focus on important topic 1. Octet rule 2. Lewis structure Q.7. The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly.
Lewis Dot Structures for Methane, CH 4 The central C atom is bonded by single bonds (-) to 4 individual H atoms
 Chapter 10 (Hill/Petrucci/McCreary/Perry Bonding Theory and Molecular Structure This chapter deals with two additional approaches chemists use to describe chemical bonding: valence-shell electron pair
Chapter 10 (Hill/Petrucci/McCreary/Perry Bonding Theory and Molecular Structure This chapter deals with two additional approaches chemists use to describe chemical bonding: valence-shell electron pair
SMK SULTAN ISMAIL JB, NUR FATHIN SUHANA BT AYOB
 SMK SULTAN ISMAIL JB, NUR FATHIN SUHANA BT AYOB POLAR AND NON POLAR BONDS BOND POLARITY 1. Atoms with different electronegative from polar bonds (difference in EN) 2. Depicted as polar arrow : 3. Example
SMK SULTAN ISMAIL JB, NUR FATHIN SUHANA BT AYOB POLAR AND NON POLAR BONDS BOND POLARITY 1. Atoms with different electronegative from polar bonds (difference in EN) 2. Depicted as polar arrow : 3. Example
Valence Bond Theory - Description
 Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Covalent Bonding: Orbitals
 Hybridization and the Localized Electron Model Covalent Bonding: Orbitals A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new orbitals of equal
Hybridization and the Localized Electron Model Covalent Bonding: Orbitals A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new orbitals of equal
Chapter Molecules are 3D. Shapes and Bonds. Chapter 9 1. Chemical Bonding and Molecular Structure
 Chapter 9 Chemical Bonding and Molecular Structure 1 Shape 9.1 Molecules are 3D Angle Linear 180 Planar triangular (trigonal planar) 120 Tetrahedral 109.5 2 Shapes and Bonds Imagine a molecule where the
Chapter 9 Chemical Bonding and Molecular Structure 1 Shape 9.1 Molecules are 3D Angle Linear 180 Planar triangular (trigonal planar) 120 Tetrahedral 109.5 2 Shapes and Bonds Imagine a molecule where the
Hybridization and Molecular Orbital (MO) Theory
 ybridization and Molecular Orbital (MO) Theory Chapter 10 istorical Models G.N.Lewis and I. Langmuir (~1920) laid out foundations Ionic species were formed by electron transfer Covalent molecules arise
ybridization and Molecular Orbital (MO) Theory Chapter 10 istorical Models G.N.Lewis and I. Langmuir (~1920) laid out foundations Ionic species were formed by electron transfer Covalent molecules arise
EXAM II Material. Part I Chemical Bonding I Lewis Theory Chapter 9 pages A. Drawing electron dot structures HOW TO:
 CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
Example: Write the Lewis structure of XeF 4. Example: Write the Lewis structure of I 3-. Example: Select the favored resonance structure of the PO 4
 Expanded valence shells (extended octets) more than 8e - around a central atom Extended octets are formed only by atoms with vacant d-orbitals in the valence shell (p-elements from the third or later periods)
Expanded valence shells (extended octets) more than 8e - around a central atom Extended octets are formed only by atoms with vacant d-orbitals in the valence shell (p-elements from the third or later periods)
CHEMISTRY 112 LECTURE EXAM II Material
 CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
CHEMISTRY 112 LECTURE EXAM II Material Part I Chemical Bonding I Lewis Theory Chapter 9 pages 376-386 A. Drawing electron dot structures HOW TO: 1. Write e- dot structure for the individual atoms. 2. a)
Subtopic 4.2 MOLECULAR SHAPE AND POLARITY
 Subtopic 4.2 MOLECULAR SHAPE AND POLARITY 1 LEARNING OUTCOMES (covalent bonding) 1. Draw the Lewis structure of covalent molecules (octet rule such as NH 3, CCl 4, H 2 O, CO 2, N 2 O 4, and exception to
Subtopic 4.2 MOLECULAR SHAPE AND POLARITY 1 LEARNING OUTCOMES (covalent bonding) 1. Draw the Lewis structure of covalent molecules (octet rule such as NH 3, CCl 4, H 2 O, CO 2, N 2 O 4, and exception to
Chapter 9 Molecular Geometries. and Bonding Theories
 Chapter 9 Molecular Geometries and Bonding Theories Coverage of Chapter 9 9.1 All 9.2 All 9.3 All 9.4 All 9.5 Omit Hybridization Involving d Orbitals 9.6 All 9.7 and 9.8 Omit ALL MOLECULAR SHAPES The shape
Chapter 9 Molecular Geometries and Bonding Theories Coverage of Chapter 9 9.1 All 9.2 All 9.3 All 9.4 All 9.5 Omit Hybridization Involving d Orbitals 9.6 All 9.7 and 9.8 Omit ALL MOLECULAR SHAPES The shape
Shapes of Molecules. Lewis structures are useful but don t allow prediction of the shape of a molecule.
 Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
Shapes of Molecules Lewis structures are useful but don t allow prediction of the shape of a molecule. H O H H O H Can use a simple theory based on electron repulsion to predict structure (for non-transition
Covalent Bonding - Orbitals
 Covalent Bonding - Orbitals ybridization - The Blending of Orbitals + = Poodle + Cocker Spaniel = Cockapoo + = s orbital + p orbital = sp orbital What Proof Exists for ybridization? We have studied electron
Covalent Bonding - Orbitals ybridization - The Blending of Orbitals + = Poodle + Cocker Spaniel = Cockapoo + = s orbital + p orbital = sp orbital What Proof Exists for ybridization? We have studied electron
Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories
 C h e m i s t r y 1 A : C h a p t e r 1 0 P a g e 1 Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories Homework: Read Chapter 10: Work out sample/practice
C h e m i s t r y 1 A : C h a p t e r 1 0 P a g e 1 Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories Homework: Read Chapter 10: Work out sample/practice
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. Dr. V.M. Williamson Texas A & M University Student Version
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. VSEPR: Electronic Geometries VSEPR
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Symmetry and Molecular Orbitals (I)
 Symmetry and Molecular Orbitals (I) Simple Bonding Model http://chiuserv.ac.nctu.edu.tw/~htchiu/chemistry/fall-2005/chemical-bonds.htm Lewis Structures Octet Rule Resonance Formal Charge Oxidation Number
Symmetry and Molecular Orbitals (I) Simple Bonding Model http://chiuserv.ac.nctu.edu.tw/~htchiu/chemistry/fall-2005/chemical-bonds.htm Lewis Structures Octet Rule Resonance Formal Charge Oxidation Number
Chemical Bonds. Chapter 6
 Chemical Bonds Chapter 6 1 Ch. 6 Chemical Bonding I. How and Why Atoms Bond A. Vocabulary B. Chemical Bonds - Basics C. Chemical Bonds Types D. Chemical Bonds Covalent E. Drawing Lewis Diagrams F. Bond
Chemical Bonds Chapter 6 1 Ch. 6 Chemical Bonding I. How and Why Atoms Bond A. Vocabulary B. Chemical Bonds - Basics C. Chemical Bonds Types D. Chemical Bonds Covalent E. Drawing Lewis Diagrams F. Bond
Chapter 10: Chemical Bonding II. Bonding Theories
 Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
Activity Hybrid Atomic Orbitals
 Activity 201 8 Hybrid Atomic Orbitals Directions: This Guided Learning Activity (GLA) discusses Hybrid Atomic Orbitals, which are the basis for Valence Bond Theory. Part A introduces σ- and π-bonds. Part
Activity 201 8 Hybrid Atomic Orbitals Directions: This Guided Learning Activity (GLA) discusses Hybrid Atomic Orbitals, which are the basis for Valence Bond Theory. Part A introduces σ- and π-bonds. Part
Molecular Shapes and VSEPR (Valence Shell Electron Pair Repulsion Theory)
 AP Chemistry Ms. Ye Name Date Block Molecular Shapes and VSEPR (Valence Shell Electron Pair Repulsion Theory) Go to bit.ly/vseprshapes Introduction Atoms bond to satisfy their need for more electrons.
AP Chemistry Ms. Ye Name Date Block Molecular Shapes and VSEPR (Valence Shell Electron Pair Repulsion Theory) Go to bit.ly/vseprshapes Introduction Atoms bond to satisfy their need for more electrons.
Unit IV. Covalent Bonding
 Unit IV. Covalent Bonding READING ASSIGNMENT 1: Read 16.1 pp. 437-451. Complete section review questions 1-12. Lewis Theory of Covalent Bonding- The driving force of bond formation is the desire of each
Unit IV. Covalent Bonding READING ASSIGNMENT 1: Read 16.1 pp. 437-451. Complete section review questions 1-12. Lewis Theory of Covalent Bonding- The driving force of bond formation is the desire of each
Valence Bond Theory. Localized Electron Model. Hybridize the Orbitals! Overlap and Bonding. Atomic Orbitals are. mmmkay. Overlap and Bonding
 Valence Bond Theory Atomic Orbitals are bad mmmkay Overlap and Bonding Lewis taught us to think of covalent bonds forming through the sharing of electrons by adjacent atoms. In such an approach this can
Valence Bond Theory Atomic Orbitals are bad mmmkay Overlap and Bonding Lewis taught us to think of covalent bonds forming through the sharing of electrons by adjacent atoms. In such an approach this can
Chemistry 2000 Lecture 8: Valence bond theory
 Chemistry 000 Lecture 8: Valence bond theory Marc R. Roussel January 9, 08 Marc R. Roussel Valence bond theory January 9, 08 / 5 MO theory: a recap A molecular orbital is a one-electron wavefunction which,
Chemistry 000 Lecture 8: Valence bond theory Marc R. Roussel January 9, 08 Marc R. Roussel Valence bond theory January 9, 08 / 5 MO theory: a recap A molecular orbital is a one-electron wavefunction which,
Valence Bond Model and Hybridization
 Valence Bond Model and ybridization APPENDIX 4 1 Concepts The key ideas required to understand this section are: Concept Book page reference VSEPR theory 65 More advanced ideas about electronic structure
Valence Bond Model and ybridization APPENDIX 4 1 Concepts The key ideas required to understand this section are: Concept Book page reference VSEPR theory 65 More advanced ideas about electronic structure
Andrew Rosen *Note: If you can rotate a molecule to have one isomer equal to another, they are both the same
 *Note: If you can rotate a molecule to have one isomer equal to another, they are both the same *Note: For hybridization, if an SP 2 is made, there is one unhybridized p orbital (because p usually has
*Note: If you can rotate a molecule to have one isomer equal to another, they are both the same *Note: For hybridization, if an SP 2 is made, there is one unhybridized p orbital (because p usually has
Chapter 9 Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity (which atoms are physically connected). By noting the number of bonding and nonbonding electron
Chapter 9 Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity (which atoms are physically connected). By noting the number of bonding and nonbonding electron
14.1 Shapes of molecules and ions (HL)
 14.1 Shapes of molecules and ions (HL) The octet is the most common electron arrangement because of its stability. Exceptions: a) Fewer electrons (incomplete octet) if the central atom is a small atoms,
14.1 Shapes of molecules and ions (HL) The octet is the most common electron arrangement because of its stability. Exceptions: a) Fewer electrons (incomplete octet) if the central atom is a small atoms,
CHEMISTRY. Chapter 8 ADVANCED THEORIES OF COVALENT BONDING Kevin Kolack, Ph.D. The Cooper Union HW problems: 6, 7, 12, 21, 27, 29, 41, 47, 49
 CHEMISTRY Chapter 8 ADVANCED THEORIES OF COVALENT BONDING Kevin Kolack, Ph.D. The Cooper Union HW problems: 6, 7, 12, 21, 27, 29, 41, 47, 49 2 CH. 8 OUTLINE 8.1 Valence Bond Theory 8.2 Hybrid Atomic Orbitals
CHEMISTRY Chapter 8 ADVANCED THEORIES OF COVALENT BONDING Kevin Kolack, Ph.D. The Cooper Union HW problems: 6, 7, 12, 21, 27, 29, 41, 47, 49 2 CH. 8 OUTLINE 8.1 Valence Bond Theory 8.2 Hybrid Atomic Orbitals
Lecture outline: Section 9. theory 2. Valence bond theory 3. Molecular orbital theory. S. Ensign, Chem. 1210
 Lecture outline: Section 9 Molecular l geometry and bonding theories 1. Valence shell electron pair repulsion theory 2. Valence bond theory 3. Molecular orbital theory 1 Ionic bonding Covalent bonding
Lecture outline: Section 9 Molecular l geometry and bonding theories 1. Valence shell electron pair repulsion theory 2. Valence bond theory 3. Molecular orbital theory 1 Ionic bonding Covalent bonding
Covalent Bonding Introduction, 2. Chapter 7 Covalent Bonding. Figure 7.1 The Hydrogen Molecule. Outline. Covalent Bonding Introduction, 1. Figure 7.
 Covalent Bonding Introduction, 2 William L. Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 7 Covalent Bonding Electron density Electrons are located between nuclei Electrostatic
Covalent Bonding Introduction, 2 William L. Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 7 Covalent Bonding Electron density Electrons are located between nuclei Electrostatic
Ex. 1) F F bond in F = 0 < % covalent, no transfer of electrons
 #60 Notes Unit 8: Bonding Ch. Bonding I. Bond Character Bonds are usually combinations of ionic and covalent character. The electronegativity difference is used to determine a bond s character. Electronegativity
#60 Notes Unit 8: Bonding Ch. Bonding I. Bond Character Bonds are usually combinations of ionic and covalent character. The electronegativity difference is used to determine a bond s character. Electronegativity
Chapter 9. Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory
 Chapter 9 Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Lewis Theory Lewis theory generally predicts trends in properties, but does not give good numerical predictions.
Chapter 9 Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Lewis Theory Lewis theory generally predicts trends in properties, but does not give good numerical predictions.
Periodic Trends. Homework: Lewis Theory. Elements of his theory:
 Periodic Trends There are various trends on the periodic table that need to be understood to explain chemical bonding. These include: Atomic/Ionic Radius Ionization Energy Electronegativity Electron Affinity
Periodic Trends There are various trends on the periodic table that need to be understood to explain chemical bonding. These include: Atomic/Ionic Radius Ionization Energy Electronegativity Electron Affinity
Ch. 9 Practice Questions
 Ch. 9 Practice Questions 1. The hybridization of the carbon atom in the cation CH + 3 is: A) sp 2 B) sp 3 C) dsp D) sp E) none of these 2. In the molecule C 2 H 4 the valence orbitals of the carbon atoms
Ch. 9 Practice Questions 1. The hybridization of the carbon atom in the cation CH + 3 is: A) sp 2 B) sp 3 C) dsp D) sp E) none of these 2. In the molecule C 2 H 4 the valence orbitals of the carbon atoms
AP CHEMISTRY: BONDING THEORIES REVIEW KEY p. 1
 AP CHEMISTRY: BONDING THEORIES REVIEW KEY p. 1 1) a) O-H PC b) Cs-Cl I c) H-Cl PC d) Br-Br NPC 2) differences in electronegativity determines amount of ity O3 0, P8 0, NO.5, CO2 1.0, CH4.4, H2S.4 answer
AP CHEMISTRY: BONDING THEORIES REVIEW KEY p. 1 1) a) O-H PC b) Cs-Cl I c) H-Cl PC d) Br-Br NPC 2) differences in electronegativity determines amount of ity O3 0, P8 0, NO.5, CO2 1.0, CH4.4, H2S.4 answer
Covalent Bonds: overlap of orbitals σ-bond π-bond Molecular Orbitals
 Covalent Bonding What is covalent bonding? Covalent Bonds: overlap of orbitals σ-bond π-bond Molecular Orbitals Hybrid Orbital Formation Shapes of Hybrid Orbitals Hybrid orbitals and Multiple Bonds resonance
Covalent Bonding What is covalent bonding? Covalent Bonds: overlap of orbitals σ-bond π-bond Molecular Orbitals Hybrid Orbital Formation Shapes of Hybrid Orbitals Hybrid orbitals and Multiple Bonds resonance
Class XI: Chemistry Chapter 4: Chemical Bonding and Molecular Structure Top Concepts
 1 Class XI: Chemistry Chapter 4: Chemical Bonding and Molecular Structure Top Concepts 1. The attractive force which holds together the constituent particles (atoms, ions or molecules) in chemical species
1 Class XI: Chemistry Chapter 4: Chemical Bonding and Molecular Structure Top Concepts 1. The attractive force which holds together the constituent particles (atoms, ions or molecules) in chemical species
Chapter 12: Chemical Bonding II: Additional Aspects
 General Chemistry Principles and Modern Applications Petrucci Harwood Herring 8 th Edition Chapter 12: Chemical Bonding II: Additional Aspects Philip Dutton University of Windsor, Canada N9B 3P4 Prentice-Hall
General Chemistry Principles and Modern Applications Petrucci Harwood Herring 8 th Edition Chapter 12: Chemical Bonding II: Additional Aspects Philip Dutton University of Windsor, Canada N9B 3P4 Prentice-Hall
