Nicholas V. Sushkin ISBN: DISSERTATION.COM
|
|
|
- Emil Logan
- 5 years ago
- Views:
Transcription
1 An Improved Form for the Electrostatic Interactions of Polyelectrolytes in Solution and its Implications for the Analysis of QELSS Experiments on Sodium Dodecyl Sulfate and Cetyl Trimethyl Ammonium Bromide Solutions by Nicholas V. Sushkin ISBN: DISSERTATION.COM 1997
2
3 ABSTRACT The electrostatic interaction between two charged spheres in the presence of a screening electrolyte is calculated at the level of the linearized Debye{Huckel theory. The calculation is performed analytically as a multipole expansion by applying two-center spherical harmonic expansions and symbolic manipulation methods. I focus on charge{ charge and charge{induced dipole interactions, calculated for two spheres of possibly unequal size. The former interaction is given to good approximation by the familiar Debye{Huckel form q 1 q 2 exp[,(r, 2a)]=[r(1 + a) 2 ]. The new results are the charge{induced dipole interactions. Physically, these terms arise from two sources: (i) surface polarization charge at the surface of each sphere, and (ii) exclusion of the counterion cloud of each sphere from the volume occupied by the other sphere. With parameters appropriate for micelles, the charge{induced dipole interactions dominate the charge{charge interaction at small separations. Quasi-elastic light scattering measurements of the diusion of sodium dodecyl sulfate (SDS) and cetyl trimethyl ammonium bromide (CTAB) micelles in aqueous solutions, and the diusion of mesoscopic optical probes through the same solutions, were carried out at 35 C and multiple solvent ionic strengths. Assuming a spherical micelle, I deduced the micelle radius, aggregation number, charge, and hydration from nonlinear least-squares ts to both probe and mutual diusion data. For SDS micelles the charge that I nd is lower than reported in the literature [Hayter, J. B.;
4 ii Penfold, J. Colloid & Polymer Science 1983, 261, 1022; Triolo, R.; Caponetti, E.; Graziano, V. J. Phys. Chem. 1985, 89, ] because I used an improved functional form of the micellar electrostatic interaction. I nd a smaller aggregation number and a larger micellar hydration than literature values. My analysis of CTAB data implies extensive micellar growth, and failure of the spherical micelle assumption.
5 ACKNOWLEDGMENTS I would like to thank my advisor, Professor George D. J. Phillies, for his patient guidance and constant encouragement through all my years of study and research at WPI. From him I learned a lot of experimental and theoretical physics, technical writing, and the meaning of hard work. The support of a part of this work by the NSF under grant CHE is gratefully acknowledged. I would like to thank the Department of Physics, and particularly its Department Heads, Professors Stephen Jasperson and Thomas Keil, for providing me with nancial assistantship. The department's support allowed me to conduct research and enjoy decent living conditions in the United States. I am grateful to Professors L. R. Ram-Mohan and Harvey Gould for serving on my thesis committee. Roger Steele and Severin Ritchie provided valuable technical assistance. I thank them for contributing their time, skill, and smile to my experiments. Department secretaries Jean Flynn, Earline Rich, and Sue Milkman made sure that everything ran smoothly.
6 CONTENTS Contents : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : v List of Figures : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : ix List of Tables : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : xi 1. General Introduction : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : 1 2. Interaction of Polyelectrolytes : : : : : : : : : : : : : : : : : : : : : : : : : Introduction Historical Electrostatic Interactions General Results Electrostatic Interactions { Closed Forms via Truncation Numerical Results for Micelles Discussion Conclusions Microscopic parameters of CTAB and SDS Micelles ::: : : : : : : : : : : Introduction Historical Technique
7 Contents v 3.4 Experiment Experimental Results Theory Analysis Discussion Conclusions General Conclusions : : : : : : : : : : : : : : : : : : : : : : : : : : : : : : 111 Appendix 113
8 LIST OF FIGURES 1.1 Bond structure of CTAB (top) and a schematic representation of a micelle (bottom). CTA + has a non-polar carbon alkyl tail chain and a polar quaternary ammonium head group. Filled circles in the micelle represent head groups Regions and variables used in the solution of Poisson's equation. I and III are the interiors of the two polyelectrolytes, with dielectric constants i, and xed charges e j located at r j. region II is the solvent with dielectric constant! and charge density =, 2 2 = Coordinates for the calculation. j and j are measured with respect to the line of centers Cross term P 0000 of the electrostatic interaction energy of two spheres, normalized relative to Phillies' analytic approximant, 10 Eq. (2.78). Here w = i = 20 and r 1 = r 3 = a. At large R and small a, the analytic approximant becomes virtually exact Self term P B 0000 of the electrostatic interaction energy of two spheres, normalized relative to Phillies' analytic approximant, 10 Eq. (2.78). Here w = i = 20 and r 1 = r 3 = a
9 List of Figures vii 2.5 Cross term P 0000 and self term P0000, B in units of k B T for T = 298, for identical spherical polyelectrolytes with a = 25 A, Q 1 = Q 3 = 1, and w = i = 20 at various ionic strengths. At small R and large a, the self-term dominates the better-known cross term; at large R and small a the cross term is dominant Cross term P 0000 and self term P0000, B in units of k B T for T = 298, for polyelectrolytes having radii r 1 = 25 A, r 3 = 350 A, charges Q 1 = Q 3 = 1, and w = i = 20 at various ionic strengths. At small R and large a, the self-term dominates the better-known cross term; at large R and small a the cross term is dominant The interaction energy at several ionic strengths for spherical polyelectrolytes of equal radius 25 A and opposite charge 10e, in units of k B T at 298 K Induced dipole (top) and counterion-exclusion eects (bottom). The dipole induced in each sphere by the charge of the other sphere causes the spheres to repel or attract each other depending on the ratio between dielectric constants of spheres and the solution. Exclusion of part of the counterion cloud of one sphere by the excluded volume of the other sphere is always unfavorable, contributing to sphere repulsion Self term P B 0000 of the interaction energy (in units of k B T at 298K) for spherical polyelectrolytes of radius 25 A and unit charge, at several ionic strengths. Here w = i = 0:2; for w = i < 1, P B 0000 may be either attractive or repulsive
10 List of Figures viii 2.10 Cross (P 0000 ) and self (P0000) B terms in the interaction energy at several ionic strengths for identical spherical polyelectrolytes of radius 25 A and unit charge, in units of k B T at 298 K. Here w = i = 1:0; the dielectric constant discontinuity at the polyelectrolyte surfaces has been removed. In this system, P B 0000 is due entirely to the exclusion of the counterion cloud by the other sphere Electrostatic interaction energies in units of kt for spheres of unequal size, showing (a) cross and (b) self terms as functions of intersphere distance in reduced units. Spheres bear one electron charge and have radii r 1 and r 3, with = 20, I =0:1M, and T = 298K Electrostatic interaction energies in units of kt for touching spheres of unequal size, showing (a) cross and (b) self terms as functions of sphere radii r 1 and r 3. Spheres bear one electron charge; system has with = 20, I =0:1M, and T = 298K Experimental setup for measuring mutual and probe diusion coecient with QELSS Interference of light scattered by two particles in a QELSS experiment. k i is the wavevector of the incident light, k s is the wavevector of the scattered light, and Q = k s, k i is the scattering vector Mutual diusion coecient D m of SDS micelles at temperature 35 C against surfactant concentration c at NaCl concentrations 0.1 M (), 0.2 M (4), 0.3 M (2), 0.4 M () and 0.5 M (5). Lines represent linear least{squares ts to D m and D p to obtain microscopic properties of micelles (see text). Data was taken by John Ren
11 List of Figures ix 3.4 Probe diusion coecient D p of 67 nm polystyrene spheres in solutions of SDS:NaCl:H 2 O micelles at temperature 35 C against SDS concentration c at NaCl concentrations 0.1 M (), 0.2 M (4), 0.3 M (2), 0.4 M () and 0.5 M (5). Lines represent linear least{squares ts to D m and D p to obtain microscopic properties of micelles (see text). Data was taken by Delphine Clomenil Mutual diusion coecient D m of CTAB micelles at temperature 35 C against surfactant concentration c at NaBr concentrations 0.01 M (), 0.05 M (4), 0.1 M (2), 0.2 M () and 0.3 M (5). Lines represent linear least{squares ts to D m and D p to obtain microscopic properties of micelles. Dashed parabola represents a quadratic t to 0.01 M NaBr data to obtain the initial slope and intercept, as used in the ts (see text). Data was taken by the author Probe diusion coecient D p of 240 nm polystyrene spheres in solutions of CTAB micelles in NaBr:H 2 O at temperature 35 C against CTAB concentration c at NaBr concentrations 0.01 M (), 0.05 M (4), 0.1 M (2), 0.2 M () and 0.3 M (5). Lines represent linear least{ squares ts to D m and D p to obtain microscopic properties of micelles (see text). Data was taken by the author
12 LIST OF TABLES 3.1 Analysis of SDS D m and D p data using my sphere-sphere electrostatic potential and eqs. (3.1) { (3.5) and (3.26) { (3.35). Asterisk indicates an inferred parameter. I is the salt concentration, a m and a p are the micelle and probe radii, q m and q p are the corresponding charges (for all ionic strengths, q p =10000), a c is the micellar radius of closest approach, N a is the micellar aggregation number, h is the micellar hydration number inwater molecules per surfactant molecule, is the micellar hydration in grams of water per gram of surfactant. R w is the equivalent water shell thickness, and v is the micellar partial volume Analysis of SDS D m and D p data using the Debye-Huckel potential and eqs. (3.1) { (3.5) and (3.26) { (3.35). Symbols are as in Table Analysis of CTAB D m and D p data, using my sphere-sphere electrostatic potential and eqs. (3.1) { (3.5) and (3.26) { (3.35). All symbols are as in Table
13 List of Tables xi 3.4 Analysis of SDS D m and D p data using my sphere{sphere electrostatic potential and micellar growth model described by Eq. (3.41). N 0 and k 1 are dened in Eq. (3.41); m is the micellar fractional charge, a 0 m is the micellar radius at zero surfactant concentration. Columns 2{4 of the table summarize results of ts with k 1 xed at 8.76 (g/mol), 1 4. In ts described by the last three columns k 1 is a free parameter
14 1. GENERAL INTRODUCTION This thesis uses experimental and theoretical approaches to treat a problem of micelle diusion in solutions. In the experimental part I measure the mutual diusion coecient of micelles and the diusion coecient of optical probes in a solution containing micelles using Quasi-Elastic Light Scattering Spectroscopy (QELSS). In the theoretical part I enhance and develop further the existing theory and use it to interpret the results of my experiment in terms of micellar microscopic parameters. This chapter briey familiarizes the reader with terms used and problems treated in this thesis. Micelles are aggregates of dissolved amphiphilic molecules. In an amphiphilic molecule one can point out spatially separated parts that exhibit substantially different solvation properties. Thus, an amphiphilic molecule in aqueous solution has a hydrophilic and a hydrophobic part. Thermal equilibration creates an eective force that aggregates amphiphilic molecules and orients them so that all hydrophobic molecular parts are inside the aggregate, whereas all hydrophilic molecular parts are facing outwards into the solvent (Figure 1.1). A micelle is thought to have a dened closed shell shape, where the shell is formed by amphiphilic molecules aligned parallel to each other and perpendicular to the surface of the shell. Micelles are usually spherical or slightly elongated, but under certain conditions micelles can grow into very long worm-like entangled structures. The materials whose molecules form shells and layers in the same way molecules are
15 1. General Introduction 2 arranged in the micellar shell are called surfactants. A micelle may be charged if the constituent amphiphilic molecules carry a charge or a dipolar moment. Micelles of cationic surfactants are positively charged and micelles of anionic surfactants are negatively charged. Charged micelles in solutions of electrolytes interact via a screened Coulomb potential, which is one of the foci of this thesis. In a water{oil emulsion containing dissolved amphiphilic molecules, the micelles are formed around oil micro-droplets, coating the oil micro-droplets with a shell of surfactant. The amphiphilic molecules enhance wetting of the oil by essentially binding the oil droplets to the surrounding water. The property of binding oil to water makes amphiphilic molecules wonderful candidates for soap making. In fact, a molecule of the most popular commercial detergent, sodium lauryl sulfate, is the best example of an amphiphilic molecule. Sodium lauryl sulfate is an inexpensive detergent commonly used in cosmetic cleansers, hair shampoos, bath and shower gels, bubble baths, etc. (the ingredients of your favorite product in your bathroom). (Sodium lauryl sulfate is also probably the most potentially dangerous ingredient used in skin and hair-care products. Sodium lauryl sulfate is used in testing-labs as the standard skin irritant to compare the healing properties of other ingredients.) In this thesis I study micelles of sodium dodecyl sulfate and cetyl trimethyl ammonium bromide surfactants. Sodium dodecyl sulfate (SDS, NaC 12 H 25 SO 4 ) is a man-made form of the naturally occuring sodium lauryl sulfate. In aqueous solution an SDS molecule dissociates into a sodium and a dodecyl sulfate (DS, ) ions. A DS, ion consists of a non-polar alkyl chain of 12 carbon atoms, the \tail", and a polar sulfate group, the \head". SDS is amphiphilic because its polar head is hy-
16 1. General Introduction 3 drophilic and the non-polar tail is hydrophobic. SDS is an anionic surfactant, so SDS micelles are negatively charged. A molecule of cetyl trimethyl ammonium bromide (CTAB, C 16 H 33 (CH 3 ) 3 NBr) dissociates into a bromide and a cetyl trimethyl ammonium (CTA + ) ion. ACTA + ion consists of a non-polar sixteen carbon alkyl tail chain and a polar quaternary ammonium head group (Figure 1.1). CTA + is a cation, so CTAB is a cationic surfactant, and CTAB micelles are positively charged. From the measured micellar diusion constant, I deduce the micellar radius, the micellar charge, and other micellar microscopic parameters. In addition to the dependence of the micellar diusion coecient D m on the micellar radius and hydrodynamic drag coecient, D m depends on the micellar charge and the micellar concentration. D m depends on the micellar charge because a QELLS experiment measures D m as the average relaxation rate of micellar concentration gradients induced by thermal uctuations. An increase in the micellar charge increases micellar repulsion, and therefore increases the relaxation rate of micellar concentration gradients. Also, in general, D m is larger in more concentrated micellar systems, where micelles are in average closer to each other and repel each other stronger than in dilute micellar systems. However, if micelles attract each other, D m may decrease with the micellar concentration. I measured the dependence of D m and D p of CTAB in aqueous solutions with added NaBr on the CTAB concentration for several solution ionic strengths. John Ren and Delphine Clomenil measured D m and D p of SDS in NaCl:water as a function of SDS concentration. Analysis of the data using the old form for the electrostatic potential of two charged spheres q 1 q 2 exp[,(r, 2a)]=[r(1 + a) 2 ] failed to produce consistent results. Using a novel mathematical treatment and a computer technique, I found a corrected form for the electrostatic interaction potential and an analytical
17 1. General Introduction 4 CH 3... CH 2 CH 2 N + CH 3 Br - CH 3 CH 3 Fig. 1.1: Bond structure of CTAB (top) and a schematic representation of a micelle (bottom). CTA + has a non-polar carbon alkyl tail chain and a polar quaternary ammonium head group. Filled circles in the micelle represent head groups.
18 1. General Introduction 5 form for a good approximation to the potential. Analysis of experimental data on CTAB and SDS using the correct form for the electrostatic interaction potential between micelles and between micelles and optical probes produced consistent micellar microscopic parameters. The systems studied here, besides being interesting in their own right, are a model for a variety of fundamental and practical phenomena, such as self-organization of complex structures, intracellular diusion in living cells, and enzymatic postprocessing of polymers in biochemical engineering. Industrial applications of micelleforming surfactants include detergents, cosmetics, drug delivery, environmental remeditation, and enhanced oil recovery. Developing an improved form for the sphere-sphere electrostatic interaction potential is very important for understanding of vesicle and cell-cell adhesion. Vesicles are closed-lm structures which are being employed as simple containers to transport drugs within the blood system. Improvements of the electrostatic interaction potential may help better understand the protein folding problem. All proteins have a certain energy associated with them, and it is the natural tendency to minimize this energy which causes a protein to fold as it does. Proteins are surronded by an aqueous solution in the body, and this interaction with solvent aswell as with itself helps to dictate how a protein folds. My work may be applied to correct the electrostatic interaction between two parts of a protein or between two proteins in the presence of screening solvent. Simultaneous analysis of probe and mutual diusion of charged micelles implemented here for the rst time may be used to measure the anionic surfactant adsorption on environmental contaminants. The surfactant adsorption measurements are
19 1. General Introduction 6 necessary to identify and characterize surfactants suitable for environmental remeditation applications. Surfactant ooding is technically one of the most promising enhanced oil recovery processes. Surfactants make it easier for water to move oil droplets through a reservoir to producing wells. The surfactants act like detergents, breaking down the oil's resistance to the water and allowing the oil to be carried through the reservoir's tiny rock pores. The surfactant adsorption measurements are necessary to identify and characterize surfactants suitable for oil recovery applications. Chapter 2 of this thesis derives the correct form for the electrostatic interaction potential of two charged spheres in a electrolyte solution. Chapter 3 studies diusion of sodium dodecyl sulfate and cetyl trimethyl ammonium bromide micelles using QELLS and deduces microscopic parameters of micelles using results of Chapter 2. Chapter 4 gives concluding remarks and proposes future work. The Appendix gives the Mathematica code used to obtain the results of Chapter 2.
20 2. INTERACTION OF POLYELECTROLYTES 2.1 Introduction The objective of this chapter is to treat the electrostatic interaction energy of a pair of charged dielectric spheres immersed in a screening medium. Physical systems realizing this model include charged micelles, proteins, and spherical colloids in aqueous electrolyte solutions. Electrostatic interactions of polyelectrolytes play an important role in a wide variety of physical problems, including solubility and salting-out eects, 2,3 protein titration, 4 the eect of substituents on organic acids' pk, 5 the stability of lyophobic colloids, 6,7 the excess chemical potential of polyelectrolyte solutions, 8,10 and the concentration dependence of the diusion coecient. 11 My own interest in this problem arose from my studies of light scattering by micellar solutions of sodium dodecyl sulphate or cetyl trimethyl ammonium bromide. In these experiments, which are described in more detail in Chapter 3 of this thesis, I used quasi-elastic light scattering spectroscopy (QELSS) to determine the concentration dependence of the mutual diusion coecient D m of the micelles; I also used QELSS to measure the single-particle diusion coecient D p of polystyrene latex probes suspended in the micellar solutions. Eorts to interpret my measurements in terms of fundamental hydrodynamic theory 12 and a simple hard-sphere plus Debye{ Huckel potential led to unsatisfactory values for micelle charge and aggregation num-
21 2. Interaction of Polyelectrolytes 8 bers. A thorough examination of alternative explanations for the diculty led me to improvements in the theory based the following calculations. Section 2.2 of this chapter describes previous work on the subject of electrostatic interaction of ions in solution. Section 2.3 obtains the electrostatic interaction energy in the form of a spherical harmonic expansion with open-form expressions for the expansion coecients. In Sec. 2.4, computer symbolic manipulation methods are used to nd closed form approximations for the expansion coecients. Finding the closed form requires truncation and inversion of an initially innite matrix; in Sec. 2.4 the process is shown to be numerically convergent. Section 2.5 presents numerical evaluations of the closed-form terms using parameters appropriate to micelles in salt solution. Closing sections compare my ndings with the literature and give conclusions. The work described here has been reported in the literature Historical In 1923 Debye and Huckel presented a linear theory 13 for the electrostatic potential created by a single macroion in an electrolyte. Their macroion charge distribution was spherically symmetric; all higher moments of the charge distribution vanished. For simple electrolyte producing ions of the valence types q s, present at concentrations c s, the potential in the region outside the macroion (r) is described by Poisson- Boltzmann equation r 2 (r) =, 4 X c s q s e,qs(r) ; (2.1) w s
22 2. Interaction of Polyelectrolytes 9 where w is the dielectric constant of the solution, =1=k B T, k B is the Boltzmann's constant, and T is the absolute temperature. With the electroneutrality condition X s c s q s =0 (2.2) applied, linearization of the exponential gives the linear Poisson-Boltzmann equation: r 2 (r) = 2 (r); (2.3) where 2 = 4 w X s q 2 sc s : (2.4) In the region inside the macroion the appropriate equation for (r) is the Laplace equation r 2 (r) =0: (2.5) Since the macroion is xed at the origin and (r) is spherically symmetric, it is convenient to use sphericall coordinates. Equations 2.3 and 2.5 become 1 = 2 (r); (2.6) and 1 =0: (2.7)
23 2. Interaction of Polyelectrolytes 10 The spherically symmetric general solutions to these equations are (r) = A 1e,r r + B 1e r ; r>a and r (r) = A 2 r + B 2 0 <r<a; (2.8) where A 1 ;A 2 ;B 1, and B 2 are constants of integration. The boundary conditions used to solve for unknown constants of integration in Eq. (2.8) are that (r) must vanish as r!1and that (r) and d=dr must be continuous at the spherical surface of the macroion. The main result of the Debye{Huckel theory is the screened coulombic potential (r) = qe,(r,a) r(1 + a) ; (2.9) where q is the charge and a is the radius of the spherical macroion. Kirkwood 2 extended the linear Debye{Huckel theory to account for the nonspherical charge distribution in the macroion. The macroion was allowed to have nonvanishing dipole, quadrupole, and higher multipole momenta of the charge distribution. The general solutions of eqs. 2.3 and 2.5 contained spherical harmonics of all orders. Using the same boundary conditions, Kirkwood obtained an expression for the electrostatic potential of the macroion at all points in space as a function of position and all multipole momenta of the macroion charge distribution. Kirkwood 3 calculated the potential energy of dipolar ions of spherical and prolate ellipsoidal shapes. Kirkwood used the Laplace and linearized Poisson-Boltzmann equations (eqs. 2.3 and 2.5) to describe the electrostatic potential, but Kirkwood conned his treatment to limiting laws approached at high dilution and idealized
24 2. Interaction of Polyelectrolytes 11 the solvent as a structureless dielectric continuum that does not cause electrostatic screening. Kirkwood neglected the terms small in the ratio of dielectric constants of the dipolar ion and the solution, i = w. In his treatment of non-spherical dipolar ions Kirkwood included the \salting-out" forces between an ion in solution and a dipolar ion. The \salting-out" forces represent a repulsion between a real ion and its image charge distribution in the cavity occupied by the dipolar ion in the solvent. Kirkwood claimed that a similar eect arising from the interaction of the dipolar ion and an image distribution in the ionic cavity was usually of much smaller magnitude and could be neglected. Kirkwood and Shumaker 8 extended the linear Debye{Huckel theory to include interactions between pairs of macroions. The macroions were allowed to have nonvanishing charge multipole moments. Kirkwood and Shumaker computed terms in the excess chemical potential of the polyelectrolytes arising from uctuations in the net polyelectrolyte charge. The potential of average force between two ions W (r) was calculated by averaging the potential V (R) of the force in xed orientation and charge conguration of the ions as exp(,w(r)) = hexp(,v )i Av W (R) = hv (R)i Av, 2 hv 2 i Av,hVi 2 Av + O( 2 ); V (R) = X i;k q i q k w R ik (2.10) where R ik is the scalar distance between charge group i of macroion 1 and charge group k of the macroion 2, q i and q k are the charges of the respective groups, and the average was taken over all orientations and over all charge congurations of the two
25 2. Interaction of Polyelectrolytes 12 macroions, with uncorrelated distribution functions. The potential W (R) leads Kirkwood and Shumaker to a long-range attractive force diminishing as the inverse cube of the distance between the two macroions in the absence of screening. A force of this range fails to yield convergent expressions for the thermodynamic functions. For spherical macroions Kirkwood and Shumaker included a Debye{Huckel factor for screening of this electrostatic interaction of the form W 0 (R) =W (R) e,2(r,a 12) (1 + a 12 ) 2 ; (2.11) where a 12 is the sum of the radii of the two macroions. Bahe 9 suggested that the eect of a point charge on water is to polarize the water locally, reducing the local dielectric constant from 80 to approximately 4. Bahe calculated the interaction of the gradient of the dielectric constant near the surface of an ion immersed in water, with the electric eld generated by another ion. In Bahe's model the ion is an incompressible sphere with a charge located at the center; and water is a continuous dielectric medium with the properties of liquid water and the dielectric constant described by a piecewise linear function of distance from the center of the ion. For an ion with radius 1.5 A Bahe assumed that the dielectric constant of water increases linearly from 3 to 81 as the distance from the center increases from 1.5 to 4.5 A. The dielectric constant of water is 81 for distances larger than 4.5 A. Bahe calculated the volume force vector ~F acting on a region of space in a dielectric
26 2. Interaction of Polyelectrolytes 13 as ~F = ~E, 0 ~rk 2 E2 + 0 r 2 ~ E dk 2 dg g ; (2.12) where is the charge density, ~E is the electric eld, k is the dielectric constant, and g is the mass density. Bahe obtained the energy of two ions by integrating the combination of the Coulomb force and the volume force given by Eq. (2.12) from 1 (zero point of electric energy) to R. The resultant electrostatic energy of two ions is E = q +q, k 1 R + 0 q k 2 0 dk dr V 1 sea R 3 ; (2.13) where q + and q, are the ion charges, k 0 is the dielectric constant at the surface of the ion, and V sea is the volume of gradient sea around the ion. Phillies 10 has extended the results of Kirkwood 8 by allowing the macroions to have dipole and quadrupole moments. His approach 10 was to solve the Debye{ Huckel model numerically, obtaining the interaction energy of charges, dipoles, and quadrupoles, and then to nd simple analytic approximations for these interactions. The resultant simplied electrostatic interaction potential was of the form V (R) = Q 1Q 2 w e,r R C Q 1D 2 cos 2 w e,r (1 + R) R 2 C 0 C 1 + D 1 D 2 e,r [2+2R +(R) 2 ] cos 1 cos 2 C 2 w R D 1 D 2 sin 1 sin 2 cos( 2, 1 ) e,r (1 + R) C w R 1; 2 (2.14) 3 where Q is the net charge of an ion, D is the magnitude of its dipole moment (whichis taken to lie along the orientation vector), the subscripts 1 and 2 distinguish between
27 2. Interaction of Polyelectrolytes 14 variables associated with dierent ions, w is the dielectric constant of the solvent, i is the dielectric constant inside the ions, = w = i, a is the radius of both ions, and where C 0 = C 1 = e a 1+a 3e a [2+2a +(a) 2 +(1+a)=] : (2.15) Phillies 10 also calculated the excess chemical potential arising from uctuation of charges Q 1 and Q 2 and dipole moments D 1 and D 2. Here I will show that recent developments in spherical harmonic expansions 14 and computer symbolic manipulation techniques permit a purely analytic determination of the expressions that Ref. 10 obtained numerically. I will present open-form expressions and closed-form approximations for the electrostatic interactions between two spherical polyelectrolytes of unequal radii. In addition to the long range charge{ charge term V (R) = Q2 exp[,(r, 2a)] (2.16) w R (1 + a) 2 of Verwey and Overbeek, 7 I obtain shorter-range charge-induced dipole terms often overlooked. (Here Q and a are the colloid charge and radius, and w and are the solvent dielectric constant and inverse Debye length, respectively, while R is the center-to-center distance.) To anticipate my most important result, at short but physically interesting distances, with parameters appropriate to ionic micelles, charge-induced dipole terms dominate the charge{charge term of eq Fisher et al. 24{26 studied approximately the same problem. Fisher et al. preceded
28 2. Interaction of Polyelectrolytes 15 me in identifying the importance of the charge-induced dipole (q1, 2 q2) 2 terms for the electrostatic interaction of two non-pointlike charged spheres. My results dier from those of Fisher et al. in that they obtained at intermediate stages series expansions in a and exp(,r)=r which they truncated at low order in each variable. In contrast, I retained all terms of higher order in both exp(,r)=r and a. 2.3 Electrostatic Interactions General Results In this section I calculate the electrostatic interaction energy U(R) of a pair of polyelectrolytes in a solution containing a simple electrolyte. The complete model (Fig. 2.1) contains two dielectric spheres 1 and 2 having radii r 1 and r 3, center-to-center distance R, and dielectric constant i. Each sphere contains xed charges e j at locations r j, with j 2 (1;N) for the rst sphere and j 2 (1;M) for the second sphere. The spheres divide space into three regions, namely region I within the rst sphere, region II in the intervening space, and region III within the second sphere. The solvent, which contains a simple electrolyte, has dielectric constant w. It is convenient to dene = w = i. The local charge density in the solvent (region II) is induced by the xed charges on the two polyelectrolytes, and is assumed to be given by the linearized Poisson{Boltzmann equation. From this equation, the charge density (r) in region II is (r) =, 2 2 w 4 (2.17)
29 2. Interaction of Polyelectrolytes 16 where in cgs units 2 = 8Ne2 I 1000 w k B T : (2.18) Here is the Debye{Huckel inverse screening length, e is the electron charge, N is Avogadro's number, I is the molar ionic strength of the solvent, T is the absolute temperature, k B is Boltzmann's constant, 1000 converts moles/liter to moles cm,3, and 2 (r) istheelectrostatic potential at r. I II e j III r 1 R r 3 Fig. 2.1: Regions and variables used in the solution of Poisson's equation. I and III are the interiors of the two polyelectrolytes, with dielectric constants i, and xed charges e j located at r j. region II is the solvent with dielectric constant! and charge density =, 2 2 =4. Figure 2.2 shows the (spherical polar) coordinates used in these calculations. Coordinates are r = (r;;) centered on sphere 1 and r 0 = (r 0 ; 0 ; 0 ) centered on sphere 2. If r and r 0 refer to the same point, = 0. The conventions are the same as in Phillies, 10 except that and 0 are measured from the line of centers of the spheres in the same direction, rather than in opposite directions as in Ref. 10. Inow solve Poisson's equation (in regions I and III) and the linearized Poisson{ Boltzmann equation (in region II) to compute the electrostatic potential in each region. A charging process gives the interaction energy of the two spheres. The
30 2. Interaction of Polyelectrolytes 17 φ ^x r r Θ φ ^x Θ ^z ^y ^y Fig. 2.2: Coordinates for the calculation. j and j are measured with respect to the line of centers.
31 2. Interaction of Polyelectrolytes 18 underlying dierential equations for are linear, so the electrostatic potential arising from the xed charges in each sphere can legitimately be calculated while neglecting the contributions to the potential created by the xed charges in the other sphere. If the linearized Poisson{Boltzmann equation were replaced with its correct nonlinear generalization, this neglect would no longer be justiable. and the specied xed charges in the two spheres are nally used to calculate U(R). U(R) contains four terms. Two terms refer to the interaction of the xed charges within each sphere with the electrostatic potential created within that sphere by the xed charges in the other sphere. I call these terms the cross terms of U(R). These terms vanish as R! 1. The other two terms refer to the interaction of the xed charges in each sphere with the electrostatic potential those charges induce in their own sphere. I call these terms the self terms of U(R). The self terms include interactions between charges within each sphere, ions in the surrounding solvent, and the induced polarization and excluded volume of the other sphere. The self terms depend on R, but do not vanish as R!1. The model is symmetric under exchange of labels between spheres 1 and 2, and regions I and III. It is therefore necessary to calculate explicitly only the self and cross terms created by the xed charges in one of the two spheres. The self and cross terms arising from the charges in the other sphere may be obtained by symmetry on the labels. I now obtain the terms arising from the xed charges in region I. The potential j induced in region j by these xed charges follows from r 2 1 (r) =, 4 i NX j=1 e j (r, r j ); (2.19)
32 2. Interaction of Polyelectrolytes 19 r 2 2 = 2 2 ; (2.20) and r 2 3 =0: (2.21) The Poisson and Poisson{Boltzmann equations are solved by expanding the charge distribution and potential in each region in terms of spherical harmonics and appropriate radial functions. The expansion coecients are determined by the boundary conditions and the xed charges. In regions I and III, can be written in terms of spherical harmonics Y lm (; ) or Y lm ( 0 ; 0 ), and powers of r or r 0, respectively, namely 1 = 1X lx l=0 m=,l (A lm r,(l+1) + B lm r l )Y lm (; ); (2.22) and 3 = 1X lx l=0 m=,l (J lm (r 0 ) l + K lm (r 0 ),(l+1) )Y lm ( 0 ; 0 ): (2.23) In region II, the potential is determined by Eq. (2.20) and the requirement that the potential should go to zero at large r. Kirkwood 2 has shown that a complete set
33 2. Interaction of Polyelectrolytes 20 of solutions for Eq that satisfy the condition! 0asr!1is = 1X lx l=0 m=,l C lm e,r r l+1 K l (r)y (; ); (2.24) where K l (r) isthelth-order polynomial part of the solution to the radial part of Eq. (2.20). The K l (r) are explicitly given by K l (r) = lx s=0 2 s l!(2l, s)! (2l)!(l, s)! (r)s : (2.25) Kirkwood's solution [Eq. 2.24] is equally valid for coordinates centered on either sphere. For my problem, a complete solution to eq is usefully written as the sum of two complete sets of these solutions, one centered on each sphere, namely 2 = 1X lx l=0 m=,l Elm e,r K l (r) r l+1 Y lm (; )+ F lme,r0 K l (r 0 ) (r 0 Y ) l+1 lm ( 0 ; 0 ) : (2.26) The potential and the perpendicular component of the electric eld satisfy boundary conditions that determine relations between the expansion coecients and the xed charges. At the j { k boundary, these conditions are j = k (2.27) and = ; (2.28)
34 2. Interaction of Polyelectrolytes 21 where ^r is the normal to the boundary, j is the dielectric constant in region j and j is the electrostatic potential in region j. In order to use the boundary conditions to solve for the B lm, E lm, F lm, and J lm, it is highly convenient to be able to write 2 on the surface of either sphere entirely in terms of spherical harmonics centered on a single sphere. To do this, functions f(r)y lm (; ) centered on sphere 1must be expanded in terms of functions f 0 (r 0 )Y l0 m 0(0 ; 0 ) centered on sphere 2, and vice versa. A general procedure for obtaining the needed expansion was obtained by Silverstone and Moats, 14 who showed how to express a function F (r) =f(r)y LM (; ) in terms of spherical harmonics and radial functions centered on a new origin displaced from r = 0 by R = (R; R ; R ). From Ref. 14, F (r) maybeexpanded as F (r, R) = 1X l =0 l + L + even Xl+L =jl,lj ll (r;r) lx m=,l C LMlm Y ;M,m ( R ; R )Y lm (; ) (2.29) where C LMlm = Z d Y ;M,m(; )Y lm(; )Y LM (; ); (2.30) ll (r;r)= 2(,1)l R (L+l+)=2 X a=0 (L+l+,2a)=2 X b=0 D llab ( r R )2b,l,1 Z r+r jr,rj ( r0 R )2a,L+1 f(r 0 ) dr 0 ; (2.31)
35 2. Interaction of Polyelectrolytes 22 D llab = 1= [(2a)!!(2a, 2L, 1)!!(2b, 2l, 1)!!(L + l +, 2a, 2b)!! (2b)!!(L + l,, 2a, 2b, 1)!!] ; (2.32) (2N)!!=2 N N!; (2.33) (2N, 1)!! = (2N)!=(2N)!!; (2.34) and (,2N, 1)!! = (,1) N =(2N, 1)!! (2.35) Here d =d d sin(), while R and R are the angular components of the vector R. The Y lm (; ) follow the normalization of Edmonds, 15 so Y lm (; ) =(,1)m Y l;,m (; ). I now solve general forms of the electrostatic potential in Regions I, II, and III (eqs. 2.22, 2.26, and 2.23) and boundary conditions at boundaries of the Regions I { II and II { III (eqs and 2.28) for integration constants A lm, B lm, and J lm. The constants A lm are determined by the xed charges within region I. Solution of Eq. (2.19) combined with Eq. (2.22) gives A lm = nx e j (r j ) l j=1 i 4 lm( j ; j ) 2l +1 : (2.36) Y Since I am computing the part of due to the charges in region I, the potential is nonsingular in region III; K lm = 0 for all l and m. Using the Silverstone{Moats expansion, the term of Eq. (2.26) containing F lm
We have considered how Coulombic attractions and repulsions help to organize electrons in atoms and ions.
 CHEM 2060 Lecture 10: Electrostatics L10-1 Electrostatics of Atoms & Molecules We have considered how Coulombic attractions and repulsions help to organize electrons in atoms and ions. We now look at Coulombic
CHEM 2060 Lecture 10: Electrostatics L10-1 Electrostatics of Atoms & Molecules We have considered how Coulombic attractions and repulsions help to organize electrons in atoms and ions. We now look at Coulombic
Module 4: "Surface Thermodynamics" Lecture 21: "" The Lecture Contains: Effect of surfactant on interfacial tension. Objectives_template
 The Lecture Contains: Effect of surfactant on interfacial tension file:///e /courses/colloid_interface_science/lecture21/21_1.htm[6/16/2012 1:10:36 PM] Surface Thermodynamics: Roles of Surfactants and
The Lecture Contains: Effect of surfactant on interfacial tension file:///e /courses/colloid_interface_science/lecture21/21_1.htm[6/16/2012 1:10:36 PM] Surface Thermodynamics: Roles of Surfactants and
The change in free energy on transferring an ion from a medium of low dielectric constantε1 to one of high dielectric constant ε2:
 The Born Energy of an Ion The free energy density of an electric field E arising from a charge is ½(ε 0 ε E 2 ) per unit volume Integrating the energy density of an ion over all of space = Born energy:
The Born Energy of an Ion The free energy density of an electric field E arising from a charge is ½(ε 0 ε E 2 ) per unit volume Integrating the energy density of an ion over all of space = Born energy:
Critical Micellization Concentration Determination using Surface Tension Phenomenon
 Critical Micellization Concentration Determination using Phenomenon 1. Introduction Surface-active agents (surfactants) were already known in ancient times, when their properties were used in everyday
Critical Micellization Concentration Determination using Phenomenon 1. Introduction Surface-active agents (surfactants) were already known in ancient times, when their properties were used in everyday
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit. Lecture 2 (9/11/17)
 16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
The effect of surface dipoles and of the field generated by a polarization gradient on the repulsive force
 Journal of Colloid and Interface Science 263 (2003) 156 161 www.elsevier.com/locate/jcis The effect of surface dipoles and of the field generated by a polarization gradient on the repulsive force Haohao
Journal of Colloid and Interface Science 263 (2003) 156 161 www.elsevier.com/locate/jcis The effect of surface dipoles and of the field generated by a polarization gradient on the repulsive force Haohao
Colloidal dispersion
 Dispersed Systems Dispersed systems consist of particulate matter, known as the dispersed phase, distributed throughout a continuous or dispersion medium. The dispersed material may range in size from
Dispersed Systems Dispersed systems consist of particulate matter, known as the dispersed phase, distributed throughout a continuous or dispersion medium. The dispersed material may range in size from
*blood and bones contain colloids. *milk is a good example of a colloidal dispersion.
 Chap. 3. Colloids 3.1. Introduction - Simple definition of a colloid: a macroscopically heterogeneous system where one component has dimensions in between molecules and macroscopic particles like sand
Chap. 3. Colloids 3.1. Introduction - Simple definition of a colloid: a macroscopically heterogeneous system where one component has dimensions in between molecules and macroscopic particles like sand
INTERMOLECULAR AND SURFACE FORCES
 INTERMOLECULAR AND SURFACE FORCES SECOND EDITION JACOB N. ISRAELACHVILI Department of Chemical & Nuclear Engineering and Materials Department University of California, Santa Barbara California, USA ACADEMIC
INTERMOLECULAR AND SURFACE FORCES SECOND EDITION JACOB N. ISRAELACHVILI Department of Chemical & Nuclear Engineering and Materials Department University of California, Santa Barbara California, USA ACADEMIC
Electrostatic Double Layer Force: Part III
 NPTEL Chemical Engineering Interfacial Engineering Module 3: Lecture 4 Electrostatic Double Layer Force: Part III Dr. Pallab Ghosh Associate Professor Department of Chemical Engineering IIT Guwahati, Guwahati
NPTEL Chemical Engineering Interfacial Engineering Module 3: Lecture 4 Electrostatic Double Layer Force: Part III Dr. Pallab Ghosh Associate Professor Department of Chemical Engineering IIT Guwahati, Guwahati
Electrolytes. Chapter Basics = = 131 2[ ]. (c) From both of the above = = 120 8[
![Electrolytes. Chapter Basics = = 131 2[ ]. (c) From both of the above = = 120 8[ Electrolytes. Chapter Basics = = 131 2[ ]. (c) From both of the above = = 120 8[](/thumbs/83/88791608.jpg) Chapter 1 Electrolytes 1.1 Basics Here we consider species that dissociate into positively and negatively charged species in solution. 1. Consider: 1 H (g) + 1 Cl (g) + ()+ () = { } = (+ )+ ( ) = 167[
Chapter 1 Electrolytes 1.1 Basics Here we consider species that dissociate into positively and negatively charged species in solution. 1. Consider: 1 H (g) + 1 Cl (g) + ()+ () = { } = (+ )+ ( ) = 167[
Full file at Chapter 2 Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
Solutions and Non-Covalent Binding Forces
 Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
Particle Characterization Laboratories, Inc.
 Analytical services Particle size analysis Dynamic Light Scattering Static Light Scattering Sedimentation Diffraction Zeta Potential Analysis Single Point Titration Isoelectric point determination Aqueous
Analytical services Particle size analysis Dynamic Light Scattering Static Light Scattering Sedimentation Diffraction Zeta Potential Analysis Single Point Titration Isoelectric point determination Aqueous
Heat Capacity of Water A) heat capacity amount of heat required to change a substance s temperature by exactly 1 C
 CHEMISTRY Ch. 13 Notes: Water and Its Solutions NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 13.1 Notes I. Water Molecule Characteristics POLAR molecule (a
CHEMISTRY Ch. 13 Notes: Water and Its Solutions NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 13.1 Notes I. Water Molecule Characteristics POLAR molecule (a
Chapter-2 (Page 22-37) Physical and Chemical Properties of Water
 Chapter-2 (Page 22-37) Physical and Chemical Properties of Water Introduction About 70% of the mass of the human body is water. Water is central to biochemistry for the following reasons: 1- Biological
Chapter-2 (Page 22-37) Physical and Chemical Properties of Water Introduction About 70% of the mass of the human body is water. Water is central to biochemistry for the following reasons: 1- Biological
we1 = j+dq + = &/ET, (2)
 EUROPHYSICS LETTERS Europhys. Lett., 24 (S), pp. 693-698 (1993) 10 December 1993 On Debye-Huckel s Theory. I. M. MLADENOV Central Laboratory of Biophysics, Bulgarian Academy of Sciences Acad. G. Bonchev
EUROPHYSICS LETTERS Europhys. Lett., 24 (S), pp. 693-698 (1993) 10 December 1993 On Debye-Huckel s Theory. I. M. MLADENOV Central Laboratory of Biophysics, Bulgarian Academy of Sciences Acad. G. Bonchev
Multimedia : Boundary Lubrication Podcast, Briscoe, et al. Nature , ( )
 3.05 Nanomechanics of Materials and Biomaterials Thursday 04/05/07 Prof. C. Ortiz, MITDMSE I LECTURE 14: TE ELECTRICAL DOUBLE LAYER (EDL) Outline : REVIEW LECTURE #11 : INTRODUCTION TO TE ELECTRICAL DOUBLE
3.05 Nanomechanics of Materials and Biomaterials Thursday 04/05/07 Prof. C. Ortiz, MITDMSE I LECTURE 14: TE ELECTRICAL DOUBLE LAYER (EDL) Outline : REVIEW LECTURE #11 : INTRODUCTION TO TE ELECTRICAL DOUBLE
Aqueous solutions. Solubility of different compounds in water
 Aqueous solutions Solubility of different compounds in water The dissolution of molecules into water (in any solvent actually) causes a volume change of the solution; the size of this volume change is
Aqueous solutions Solubility of different compounds in water The dissolution of molecules into water (in any solvent actually) causes a volume change of the solution; the size of this volume change is
Water and solutions. Prof. Ramune Morkuniene, Biochemistry Dept., LUHS
 Water and solutions Prof. Ramune Morkuniene, Biochemistry Dept., LUHS Characteristics of water molecule Hydrophylic, hydrophobic and amphipatic compounds Types of real solutions Electrolytes and non- electrolytes
Water and solutions Prof. Ramune Morkuniene, Biochemistry Dept., LUHS Characteristics of water molecule Hydrophylic, hydrophobic and amphipatic compounds Types of real solutions Electrolytes and non- electrolytes
Atoms & Their Interactions
 Lecture 2 Atoms & Their Interactions Si: the heart of electronic materials Intel, 300mm Si wafer, 200 μm thick and 48-core CPU ( cloud computing on a chip ) Twin Creeks Technologies, San Jose, Si wafer,
Lecture 2 Atoms & Their Interactions Si: the heart of electronic materials Intel, 300mm Si wafer, 200 μm thick and 48-core CPU ( cloud computing on a chip ) Twin Creeks Technologies, San Jose, Si wafer,
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian. which is given in spherical coordinates by (2)
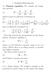 1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
Molecular Driving Forces
 Molecular Driving Forces Statistical Thermodynamics in Chemistry and Biology SUBGfittingen 7 At 216 513 073 / / Ken A. Dill Sarina Bromberg With the assistance of Dirk Stigter on the Electrostatics chapters
Molecular Driving Forces Statistical Thermodynamics in Chemistry and Biology SUBGfittingen 7 At 216 513 073 / / Ken A. Dill Sarina Bromberg With the assistance of Dirk Stigter on the Electrostatics chapters
Protein separation and characterization
 Address:800 S Wineville Avenue, Ontario, CA 91761,USA Website:www.aladdin-e.com Email USA: tech@aladdin-e.com Email EU: eutech@aladdin-e.com Email Asia Pacific: cntech@aladdin-e.com Protein separation
Address:800 S Wineville Avenue, Ontario, CA 91761,USA Website:www.aladdin-e.com Email USA: tech@aladdin-e.com Email EU: eutech@aladdin-e.com Email Asia Pacific: cntech@aladdin-e.com Protein separation
Electrolyte Solutions
 Chapter 8 Electrolyte Solutions In the last few chapters of this book, we will deal with several specific types of chemical systems. The first one is solutions and equilibria involving electrolytes, which
Chapter 8 Electrolyte Solutions In the last few chapters of this book, we will deal with several specific types of chemical systems. The first one is solutions and equilibria involving electrolytes, which
H O H. Chapter 3: Outline-2. Chapter 3: Outline-1
 Chapter 3: utline-1 Molecular Nature of Water Noncovalent Bonding Ionic interactions van der Waals Forces Thermal Properties of Water Solvent Properties of Water ydrogen Bonds ydrophilic, hydrophobic,
Chapter 3: utline-1 Molecular Nature of Water Noncovalent Bonding Ionic interactions van der Waals Forces Thermal Properties of Water Solvent Properties of Water ydrogen Bonds ydrophilic, hydrophobic,
On the Chemical Free Energy of the Electrical Double Layer
 1114 Langmuir 23, 19, 1114-112 On the Chemical Free Energy of the Electrical Double Layer Marian Manciu and Eli Ruckenstein* Department of Chemical Engineering, State University of New York at Buffalo,
1114 Langmuir 23, 19, 1114-112 On the Chemical Free Energy of the Electrical Double Layer Marian Manciu and Eli Ruckenstein* Department of Chemical Engineering, State University of New York at Buffalo,
Big Idea #5: The laws of thermodynamics describe the essential role of energy and explain and predict the direction of changes in matter.
 KUDs for Unit 6: Chemical Bonding Textbook Reading: Chapters 8 & 9 Big Idea #2: Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ion, or molecules
KUDs for Unit 6: Chemical Bonding Textbook Reading: Chapters 8 & 9 Big Idea #2: Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ion, or molecules
States of matter Part 1
 Physical pharmacy I 1. States of matter (2 Lectures) 2. Thermodynamics (2 Lectures) 3. Solution of non-electrolyte 4. Solution of electrolyte 5. Ionic equilibria 6. Buffered and isotonic solution Physical
Physical pharmacy I 1. States of matter (2 Lectures) 2. Thermodynamics (2 Lectures) 3. Solution of non-electrolyte 4. Solution of electrolyte 5. Ionic equilibria 6. Buffered and isotonic solution Physical
Intermolecular and Intramolecular Forces. Introduction
 Intermolecular and Intramolecular Forces Introduction Atoms can form stable units called molecules by sharing electrons. The formation of molecules is the result of intramolecular bonding (within the molecule)
Intermolecular and Intramolecular Forces Introduction Atoms can form stable units called molecules by sharing electrons. The formation of molecules is the result of intramolecular bonding (within the molecule)
States of matter Part 1. Lecture 1. University of Kerbala. Hamid Alghurabi Assistant Lecturer in Pharmaceutics. Physical Pharmacy
 Physical pharmacy I 1. States of matter (2 Lectures) 2. Thermodynamics (2 Lectures) 3. Solution of non-electrolyte 4. Solution of electrolyte 5. Ionic equilibria 6. Buffered and isotonic solution Physical
Physical pharmacy I 1. States of matter (2 Lectures) 2. Thermodynamics (2 Lectures) 3. Solution of non-electrolyte 4. Solution of electrolyte 5. Ionic equilibria 6. Buffered and isotonic solution Physical
Dielectrics. Lecture 20: Electromagnetic Theory. Professor D. K. Ghosh, Physics Department, I.I.T., Bombay
 What are dielectrics? Dielectrics Lecture 20: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay So far we have been discussing electrostatics in either vacuum or in a conductor.
What are dielectrics? Dielectrics Lecture 20: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay So far we have been discussing electrostatics in either vacuum or in a conductor.
Module 8: "Stability of Colloids" Lecture 37: "" The Lecture Contains: DLVO Theory. Effect of Concentration. Objectives_template
 The Lecture Contains: DLVO Theory Effect of Concentration file:///e /courses/colloid_interface_science/lecture37/37_1.htm[6/16/2012 1:02:12 PM] Studying the stability of colloids is an important topic
The Lecture Contains: DLVO Theory Effect of Concentration file:///e /courses/colloid_interface_science/lecture37/37_1.htm[6/16/2012 1:02:12 PM] Studying the stability of colloids is an important topic
Interfacial Phenomena
 Physical Pharmacy Lecture 4 Interfacial Phenomena Assistant Lecturer in Pharmaceutics Overview Liquid interfaces Surface tension Interfacial tension Surface free energy Measurement of tensions Spreading
Physical Pharmacy Lecture 4 Interfacial Phenomena Assistant Lecturer in Pharmaceutics Overview Liquid interfaces Surface tension Interfacial tension Surface free energy Measurement of tensions Spreading
Complete and precise descriptions based on quantum mechanics exist for the Coulombic/Electrostatic force. These are used to describe materials.
 The forces of nature: 1. Strong forces hold protons and neutrons together (exchange of mesons) 2. Weak interactions are involved in some kinds of radioactive decay (β-decay) 3. Coulombic or electrostatic
The forces of nature: 1. Strong forces hold protons and neutrons together (exchange of mesons) 2. Weak interactions are involved in some kinds of radioactive decay (β-decay) 3. Coulombic or electrostatic
Chapter 2 Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens. There are
Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens. There are
 Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
CHEM J-9 June 2012
 CEM1901 2012-J-9 June 2012 Explain, with the aid of a diagram labelling all the key components, how sodium stearate (C 17 35 CNa) can stabilise long-chain non-polar hydrocarbons ( grease ) in water. Marks
CEM1901 2012-J-9 June 2012 Explain, with the aid of a diagram labelling all the key components, how sodium stearate (C 17 35 CNa) can stabilise long-chain non-polar hydrocarbons ( grease ) in water. Marks
Theoretische Physik 2: Elektrodynamik (Prof. A-S. Smith) Home assignment 11
 WiSe 22..23 Prof. Dr. A-S. Smith Dipl.-Phys. Matthias Saba am Lehrstuhl für Theoretische Physik I Department für Physik Friedrich-Alexander-Universität Erlangen-Nürnberg Problem. Theoretische Physik 2:
WiSe 22..23 Prof. Dr. A-S. Smith Dipl.-Phys. Matthias Saba am Lehrstuhl für Theoretische Physik I Department für Physik Friedrich-Alexander-Universität Erlangen-Nürnberg Problem. Theoretische Physik 2:
Atoms can form stable units called molecules by sharing electrons.
 Atoms can form stable units called molecules by sharing electrons. The formation of molecules is the result of intramolecular bonding (within the molecule) e.g. ionic, covalent. Forces that cause the aggregation
Atoms can form stable units called molecules by sharing electrons. The formation of molecules is the result of intramolecular bonding (within the molecule) e.g. ionic, covalent. Forces that cause the aggregation
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015,
 Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
Chapter 11 Properties of Solutions
 Chapter 11 Properties of Solutions Solutions Homogeneous mixtures of two or more substances Composition is uniform throughout the sample No chemical reaction between the components of the mixture Solvents
Chapter 11 Properties of Solutions Solutions Homogeneous mixtures of two or more substances Composition is uniform throughout the sample No chemical reaction between the components of the mixture Solvents
2 Structure. 2.1 Coulomb interactions
 2 Structure 2.1 Coulomb interactions While the information needed for reproduction of living systems is chiefly maintained in the sequence of macromolecules, any practical use of this information must
2 Structure 2.1 Coulomb interactions While the information needed for reproduction of living systems is chiefly maintained in the sequence of macromolecules, any practical use of this information must
Colloidal Suspension Rheology Chapter 1 Study Questions
 Colloidal Suspension Rheology Chapter 1 Study Questions 1. What forces act on a single colloidal particle suspended in a flowing fluid? Discuss the dependence of these forces on particle radius. 2. What
Colloidal Suspension Rheology Chapter 1 Study Questions 1. What forces act on a single colloidal particle suspended in a flowing fluid? Discuss the dependence of these forces on particle radius. 2. What
DLVO Theory and Non-DLVO Forces
 NPTEL Chemical Engineering Interfacial Engineering Module 3: Lecture 5 DLVO Theory and Non-DLVO Forces Dr. Pallab Ghosh Associate Professor Department of Chemical Engineering IIT Guwahati, Guwahati 781039
NPTEL Chemical Engineering Interfacial Engineering Module 3: Lecture 5 DLVO Theory and Non-DLVO Forces Dr. Pallab Ghosh Associate Professor Department of Chemical Engineering IIT Guwahati, Guwahati 781039
V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes
 V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes MIT Student 1. Poisson-Nernst-Planck Equations The Nernst-Planck Equation is a conservation of mass equation that describes the influence of
V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes MIT Student 1. Poisson-Nernst-Planck Equations The Nernst-Planck Equation is a conservation of mass equation that describes the influence of
CHEMISTRY Ch. 14 Notes: Mixtures and Solutions NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 CHEMISTRY Ch. 14 Notes: Mixtures and Solutions NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 14.1 notes I. Types of mixtures (mixture a physical blend of substances)
CHEMISTRY Ch. 14 Notes: Mixtures and Solutions NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 14.1 notes I. Types of mixtures (mixture a physical blend of substances)
Scientists learned that elements in same group on PT react in a similar way. Why?
 Unit 5: Bonding Scientists learned that elements in same group on PT react in a similar way Why? They all have the same number of valence electrons.which are electrons in the highest occupied energy level
Unit 5: Bonding Scientists learned that elements in same group on PT react in a similar way Why? They all have the same number of valence electrons.which are electrons in the highest occupied energy level
Chapter 13 States of Matter Forces of Attraction 13.3 Liquids and Solids 13.4 Phase Changes
 Chapter 13 States of Matter 13.2 Forces of Attraction 13.3 Liquids and Solids 13.4 Phase Changes I. Forces of Attraction (13.2) Intramolecular forces? (forces within) Covalent Bonds, Ionic Bonds, and metallic
Chapter 13 States of Matter 13.2 Forces of Attraction 13.3 Liquids and Solids 13.4 Phase Changes I. Forces of Attraction (13.2) Intramolecular forces? (forces within) Covalent Bonds, Ionic Bonds, and metallic
Apparent molar volume of sodium chloride in mixed solvent at different temperatures
 Ultra Chemistry Vol. 8(2), 205-210 (2012). Apparent molar volume of sodium chloride in mixed solvent at different temperatures C.K. RATH 1, N.C. ROUT 2, S.P. DAS 3 and P.K. MISHRA 4 1 Department of Chemistry,
Ultra Chemistry Vol. 8(2), 205-210 (2012). Apparent molar volume of sodium chloride in mixed solvent at different temperatures C.K. RATH 1, N.C. ROUT 2, S.P. DAS 3 and P.K. MISHRA 4 1 Department of Chemistry,
Peter Debye and Electrochemistry
 Peter Debye and Electrochemistry A K Shukla and T Prem Kumar A K Shukla is Professor and Chairman, Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore. His research interests
Peter Debye and Electrochemistry A K Shukla and T Prem Kumar A K Shukla is Professor and Chairman, Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore. His research interests
Precipitation Reactions of Protein. By Sandip Kanazariya
 Precipitation Reactions of Protein By Sandip Kanazariya PRECIPITATION REACTIONS OF ALBUMIN Solubility of protein depends on proportion & distribution of polar hydrophilic end & non - polar hydrophobic
Precipitation Reactions of Protein By Sandip Kanazariya PRECIPITATION REACTIONS OF ALBUMIN Solubility of protein depends on proportion & distribution of polar hydrophilic end & non - polar hydrophobic
Chapter 6 Chemical Bonding
 Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
CS 2, HCN, BeF 2 Trigonal planar. Cl 120 BF 3, AlCl 3, SO 3, NO 3-, CO NCl 3,PF 3,ClO 3,H 3 O + ...
 Shape of molecules Name No bonding pairs No lone pairs Diagram Bond angle Examples linear 2 0 l Be l 180 2, S 2, N, Be 2 Trigonal planar 3 0 l l 120 B 3, All 3, S 3, N 3-, 2-3 B Tetrahedral 4 0 109.5 Sil
Shape of molecules Name No bonding pairs No lone pairs Diagram Bond angle Examples linear 2 0 l Be l 180 2, S 2, N, Be 2 Trigonal planar 3 0 l l 120 B 3, All 3, S 3, N 3-, 2-3 B Tetrahedral 4 0 109.5 Sil
Solvation Studies on Sodium Dodecyl Sulphate in aqueous solutions at different temperatures
 IOSR Journal of Applied Physics (IOSR-JAP) e-issn: 2278-4861.Volume 6, Issue 5 Ver. I (Sep.-Oct. 2014), PP 63-67 Solvation Studies on Sodium Dodecyl Sulphate in aqueous solutions at different temperatures
IOSR Journal of Applied Physics (IOSR-JAP) e-issn: 2278-4861.Volume 6, Issue 5 Ver. I (Sep.-Oct. 2014), PP 63-67 Solvation Studies on Sodium Dodecyl Sulphate in aqueous solutions at different temperatures
Contents. Preface XI Symbols and Abbreviations XIII. 1 Introduction 1
 V Contents Preface XI Symbols and Abbreviations XIII 1 Introduction 1 2 Van der Waals Forces 5 2.1 Van der Waals Forces Between Molecules 5 2.1.1 Coulomb Interaction 5 2.1.2 Monopole Dipole Interaction
V Contents Preface XI Symbols and Abbreviations XIII 1 Introduction 1 2 Van der Waals Forces 5 2.1 Van der Waals Forces Between Molecules 5 2.1.1 Coulomb Interaction 5 2.1.2 Monopole Dipole Interaction
Chapter 12 Intermolecular Forces and Liquids
 Chapter 12 Intermolecular Forces and Liquids Jeffrey Mack California State University, Sacramento Why? Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature
Chapter 12 Intermolecular Forces and Liquids Jeffrey Mack California State University, Sacramento Why? Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature
Water, water everywhere,; not a drop to drink. Consumption resulting from how environment inhabited Deforestation disrupts water cycle
 Chapter 3 Water: The Matrix of Life Overview n n n Water, water everywhere,; not a drop to drink Only 3% of world s water is fresh How has this happened Consumption resulting from how environment inhabited
Chapter 3 Water: The Matrix of Life Overview n n n Water, water everywhere,; not a drop to drink Only 3% of world s water is fresh How has this happened Consumption resulting from how environment inhabited
Chem 406 Biophysical Chemistry Lecture 1 Transport Processes, Sedimentation & Diffusion
 Chem 406 Biophysical Chemistry Lecture 1 Transport Processes, Sedimentation & Diusion I. Introduction A. There are a group o biophysical techniques that are based on transport processes. 1. Transport processes
Chem 406 Biophysical Chemistry Lecture 1 Transport Processes, Sedimentation & Diusion I. Introduction A. There are a group o biophysical techniques that are based on transport processes. 1. Transport processes
Personalised Learning Checklists Edexcel Combined: Chemistry Paper 1
 Edexcel (combined) Chemistry Topics (1SC0) from 2016 - Paper 1 (Topic 1 parts a&b) Topic Student Checklist R A G Describe how the Dalton model of an atom has changed over time because of the discovery
Edexcel (combined) Chemistry Topics (1SC0) from 2016 - Paper 1 (Topic 1 parts a&b) Topic Student Checklist R A G Describe how the Dalton model of an atom has changed over time because of the discovery
Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed.
 Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed. by Steven S. Zumdahl & Donald J. DeCoste University of Illinois Chapter 12 Chemical Bonding Structure
Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed. by Steven S. Zumdahl & Donald J. DeCoste University of Illinois Chapter 12 Chemical Bonding Structure
Water, ph and pka. Lecture 2: Margaret A. Daugherty. Fall Water: What makes it so good for life? Solvent properties.
 Lecture 2: Water, ph and pka Margaret A. Daugherty Fall 2004 Water: What makes it so good for life? Structure ice vs. water or more technically solid vs. liquid Solvent properties High heat capacity High
Lecture 2: Water, ph and pka Margaret A. Daugherty Fall 2004 Water: What makes it so good for life? Structure ice vs. water or more technically solid vs. liquid Solvent properties High heat capacity High
Lecture 15. Polar vs Non-Polar Substances. Professor Hicks Inorganic Chemistry II (CHE151)
 Lecture 15 Professor icks Inorganic hemistry II (E151) Polar vs on-polar Substances Ionic compounds Molecules with significant dipole moments (from polar bonds) Molecules with little or no dipole moment,
Lecture 15 Professor icks Inorganic hemistry II (E151) Polar vs on-polar Substances Ionic compounds Molecules with significant dipole moments (from polar bonds) Molecules with little or no dipole moment,
Ionic polysaccharides, solubility, interactions with surfactants, particle formation, and deposition
 Ionic polysaccharides, solubility, interactions with surfactants, particle formation, and deposition Björn Lindman, Tommy Nylander, Maria Miguel and Lennart Piculell Physical Chemistry 1, Lund University,
Ionic polysaccharides, solubility, interactions with surfactants, particle formation, and deposition Björn Lindman, Tommy Nylander, Maria Miguel and Lennart Piculell Physical Chemistry 1, Lund University,
Supporting information. The role of halide ions in the anisotropic growth. of gold nanoparticles: a microscopic, atomistic.
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 26 Supporting information The role of halide ions in the anisotropic growth of gold
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 26 Supporting information The role of halide ions in the anisotropic growth of gold
Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions 11 SUMMARY Section 2.1 Section 2.2 Section 2.3 Section 2.4 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive
Chapter 2 Water: The Solvent for Biochemical Reactions 11 SUMMARY Section 2.1 Section 2.2 Section 2.3 Section 2.4 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive
READING. Review of Intermolecular Forces & Liquids (Chapter 12) Ion-Ion Forces. Ion-Dipole Energies
 Review of Intermolecular Forces & Liquids (Chapter 12) CEM 102 T. ughbanks READIG We will very briefly review the underlying concepts from Chapters 12 on intermolecular forces since it is relevant to Chapter
Review of Intermolecular Forces & Liquids (Chapter 12) CEM 102 T. ughbanks READIG We will very briefly review the underlying concepts from Chapters 12 on intermolecular forces since it is relevant to Chapter
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 25: CHROMATOGRAPHIC METHODS AND CAPILLARY ELECTROPHORESIS
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 25: CHROMATOGRAPHIC METHODS AND CAPILLARY ELECTROPHORESIS CHAPTER 25: Opener Aa CHAPTER 25: Opener Ab CHAPTER 25: Opener B 25-1 Ion-Exchange
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 25: CHROMATOGRAPHIC METHODS AND CAPILLARY ELECTROPHORESIS CHAPTER 25: Opener Aa CHAPTER 25: Opener Ab CHAPTER 25: Opener B 25-1 Ion-Exchange
Surface interactions part 1: Van der Waals Forces
 CHEM-E150 Interfacial Phenomena in Biobased Systems Surface interactions part 1: Van der Waals Forces Monika Österberg Spring 018 Content Colloidal stability van der Waals Forces Surface Forces and their
CHEM-E150 Interfacial Phenomena in Biobased Systems Surface interactions part 1: Van der Waals Forces Monika Österberg Spring 018 Content Colloidal stability van der Waals Forces Surface Forces and their
Lecture 5: Macromolecules, polymers and DNA
 1, polymers and DNA Introduction In this lecture, we focus on a subfield of soft matter: macromolecules and more particularly on polymers. As for the previous chapter about surfactants and electro kinetics,
1, polymers and DNA Introduction In this lecture, we focus on a subfield of soft matter: macromolecules and more particularly on polymers. As for the previous chapter about surfactants and electro kinetics,
Chapter 2 - Water 9/8/2014. Water exists as a H-bonded network with an average of 4 H-bonds per molecule in ice and 3.4 in liquid. 104.
 Chapter 2 - Water Water exists as a -bonded network with an average of 4 -bonds per molecule in ice and 3.4 in liquid. 104.5 o -bond: An electrostatic attraction between polarized molecules containing
Chapter 2 - Water Water exists as a -bonded network with an average of 4 -bonds per molecule in ice and 3.4 in liquid. 104.5 o -bond: An electrostatic attraction between polarized molecules containing
Chapter 3. Crystal Binding
 Chapter 3. Crystal Binding Energy of a crystal and crystal binding Cohesive energy of Molecular crystals Ionic crystals Metallic crystals Elasticity What causes matter to exist in three different forms?
Chapter 3. Crystal Binding Energy of a crystal and crystal binding Cohesive energy of Molecular crystals Ionic crystals Metallic crystals Elasticity What causes matter to exist in three different forms?
Elements react to attain stable (doublet or octet) electronic configurations of the noble gases.
 digitalteachers.co.ug Chemical bonding This chapter teaches the different types and names of bonds that exist in substances that keep their constituent particles together. We will understand how these
digitalteachers.co.ug Chemical bonding This chapter teaches the different types and names of bonds that exist in substances that keep their constituent particles together. We will understand how these
Micellar Effect on The Reaction of Acetophenone with Phenylhydrazine
 Asian Journal of Chemistry Vol. 22, No. 2 (1), 829-833 Micellar Effect on The Reaction of Acetophenone with Phenylhydrazine K. NAGAJYOTHI*, P.S. RAGHAVAN and R. GOPALAN Department of Chemistry, Aarupadai
Asian Journal of Chemistry Vol. 22, No. 2 (1), 829-833 Micellar Effect on The Reaction of Acetophenone with Phenylhydrazine K. NAGAJYOTHI*, P.S. RAGHAVAN and R. GOPALAN Department of Chemistry, Aarupadai
Physics and Chemistry of Interfaces
 Hans Jürgen Butt, Karlheinz Graf, and Michael Kappl Physics and Chemistry of Interfaces Second, Revised and Enlarged Edition WILEY- VCH WILEY-VCH Verlag GmbH & Co. KGaA Contents Preface XI 1 Introduction
Hans Jürgen Butt, Karlheinz Graf, and Michael Kappl Physics and Chemistry of Interfaces Second, Revised and Enlarged Edition WILEY- VCH WILEY-VCH Verlag GmbH & Co. KGaA Contents Preface XI 1 Introduction
NANO 243/CENG 207 Course Use Only
 L8. Drug Dispersion and Diffusion in Biological Systems (2) April 26, 2018 2. Diffusion in Water (3) Diffusion of small molecule in water drug molecules/solvents are identical to solvent Assume molecules
L8. Drug Dispersion and Diffusion in Biological Systems (2) April 26, 2018 2. Diffusion in Water (3) Diffusion of small molecule in water drug molecules/solvents are identical to solvent Assume molecules
Applied Surfactants: Principles and Applications
 Applied Surfactants: Principles and Applications Tadros, Tharwat F. ISBN-13: 9783527306299 Table of Contents Preface. 1 Introduction. 1.1 General Classification of Surface Active Agents. 1.2 Anionic Surfactants.
Applied Surfactants: Principles and Applications Tadros, Tharwat F. ISBN-13: 9783527306299 Table of Contents Preface. 1 Introduction. 1.1 General Classification of Surface Active Agents. 1.2 Anionic Surfactants.
Surface chemistry. Liquid-gas, solid-gas and solid-liquid surfaces. Levente Novák István Bányai Zoltán Nagy Department of Physical Chemistry
 Surface chemistry. Liquid-gas, solid-gas and solid-liquid surfaces. Levente Novák István Bányai Zoltán Nagy Department of Physical Chemistry Surfaces and Interfaces Defining of interfacial region Types
Surface chemistry. Liquid-gas, solid-gas and solid-liquid surfaces. Levente Novák István Bányai Zoltán Nagy Department of Physical Chemistry Surfaces and Interfaces Defining of interfacial region Types
Intermolecular Forces & Condensed Phases
 Intermolecular Forces & Condensed Phases CHEM 107 T. Hughbanks READING We will discuss some of Chapter 5 that we skipped earlier (Van der Waals equation, pp. 145-8), but this is just a segue into intermolecular
Intermolecular Forces & Condensed Phases CHEM 107 T. Hughbanks READING We will discuss some of Chapter 5 that we skipped earlier (Van der Waals equation, pp. 145-8), but this is just a segue into intermolecular
IB Chemistry Solutions Gasses and Energy
 Solutions A solution is a homogeneous mixture it looks like one substance. An aqueous solution will be a clear mixture with only one visible phase. Be careful with the definitions of clear and colourless.
Solutions A solution is a homogeneous mixture it looks like one substance. An aqueous solution will be a clear mixture with only one visible phase. Be careful with the definitions of clear and colourless.
Definition of Matter. Subatomic particles 8/20/2012
 Interplay of Biology and Chemistry Here is a link to the video these beetles are fairly common locally an amazing adaptation, and a good example of chemistry and physics in biology. Also look for creationist-evolutionist
Interplay of Biology and Chemistry Here is a link to the video these beetles are fairly common locally an amazing adaptation, and a good example of chemistry and physics in biology. Also look for creationist-evolutionist
Solids, Liquids and Gases We have already covered these phases of matter. See online section 5.2
 Chapter 10 This chapter begins to answer the questions: So now that I now what atoms and molecules look like, how do these structures translate into what I see in the world around me. Reading Assignment:
Chapter 10 This chapter begins to answer the questions: So now that I now what atoms and molecules look like, how do these structures translate into what I see in the world around me. Reading Assignment:
Chapter 8. Basic Concepts of Chemical Bonding
 Chapter 8. Basic Concepts of Chemical Bonding 8.1 Lewis Symbols and the Octet Rule When atoms or ions are strongly attracted to one another, we say that there is a chemical bond between them. In chemical
Chapter 8. Basic Concepts of Chemical Bonding 8.1 Lewis Symbols and the Octet Rule When atoms or ions are strongly attracted to one another, we say that there is a chemical bond between them. In chemical
Liquid Chromatography
 Liquid Chromatography 1. Introduction and Column Packing Material 2. Retention Mechanisms in Liquid Chromatography 3. Method Development 4. Column Preparation 5. General Instrumental aspects 6. Detectors
Liquid Chromatography 1. Introduction and Column Packing Material 2. Retention Mechanisms in Liquid Chromatography 3. Method Development 4. Column Preparation 5. General Instrumental aspects 6. Detectors
Life is a chemical process
 CHEMISTRY FOR LIFE Life is a chemical process Relies on and is subject to chemistry Must obey the laws of physics Biologists study Chemistry because all living things are made of matter. Matter undergoes
CHEMISTRY FOR LIFE Life is a chemical process Relies on and is subject to chemistry Must obey the laws of physics Biologists study Chemistry because all living things are made of matter. Matter undergoes
Chapter 11. Intermolecular Forces and Liquids & Solids
 Chapter 11 Intermolecular Forces and Liquids & Solids The Kinetic Molecular Theory of Liquids & Solids Gases vs. Liquids & Solids difference is distance between molecules Liquids Molecules close together;
Chapter 11 Intermolecular Forces and Liquids & Solids The Kinetic Molecular Theory of Liquids & Solids Gases vs. Liquids & Solids difference is distance between molecules Liquids Molecules close together;
Chemical Bonding Basic Concepts
 Chemical Bonding Basic Concepts Valence electrons are the outer shell electrons of an atom. The valence electrons are the electrons that particpate in chemical bonding. Group e - configuration # of valence
Chemical Bonding Basic Concepts Valence electrons are the outer shell electrons of an atom. The valence electrons are the electrons that particpate in chemical bonding. Group e - configuration # of valence
Sodium, Na. Gallium, Ga CHEMISTRY Topic #2: The Chemical Alphabet Fall 2017 Dr. Susan Findlay See Exercises 9.2 to 9.7.
 Sodium, Na Gallium, Ga CHEMISTRY 1000 Topic #2: The Chemical Alphabet Fall 2017 Dr. Susan Findlay See Exercises 9.2 to 9.7 Forms of Carbon Kinetic Molecular Theory of Matter The kinetic-molecular theory
Sodium, Na Gallium, Ga CHEMISTRY 1000 Topic #2: The Chemical Alphabet Fall 2017 Dr. Susan Findlay See Exercises 9.2 to 9.7 Forms of Carbon Kinetic Molecular Theory of Matter The kinetic-molecular theory
Test bank for Chemistry An Introduction to General Organic and Biological Chemistry 12th Edition by Timberlake
 Test bank for Chemistry An Introduction to General Organic and Biological Chemistry 12th Edition by Timberlake Link download full: http://testbankair.com/download/test-bank-for-chemistry-an-introduction-to-general-organic-and-biological-chemistry-12th-edition-by-timberlak
Test bank for Chemistry An Introduction to General Organic and Biological Chemistry 12th Edition by Timberlake Link download full: http://testbankair.com/download/test-bank-for-chemistry-an-introduction-to-general-organic-and-biological-chemistry-12th-edition-by-timberlak
Learning Objectives. Learning Objectives (cont.) Chapter 2: Basic Chemistry 1. Lectures by Tariq Alalwan, Ph.D.
 Biology, 10e Mader Lectures by Tariq Alalwan, Ph.D. Learning Objectives Name the principal chemical elements in living things. Compare the physical properties (mass and charge) and locations of electrons,
Biology, 10e Mader Lectures by Tariq Alalwan, Ph.D. Learning Objectives Name the principal chemical elements in living things. Compare the physical properties (mass and charge) and locations of electrons,
Atomic structure & interatomic bonding. Chapter two
 Atomic structure & interatomic bonding Chapter two 1 Atomic Structure Mass Charge Proton 1.67 х 10-27 kg + 1.60 х 10-19 C Neutron 1.67 х 10-27 kg Neutral Electron 9.11 х 10-31 kg - 1.60 х 10-19 C Electron
Atomic structure & interatomic bonding Chapter two 1 Atomic Structure Mass Charge Proton 1.67 х 10-27 kg + 1.60 х 10-19 C Neutron 1.67 х 10-27 kg Neutral Electron 9.11 х 10-31 kg - 1.60 х 10-19 C Electron
Monolayers. Factors affecting the adsorption from solution. Adsorption of amphiphilic molecules on solid support
 Monolayers Adsorption as process Adsorption of gases on solids Adsorption of solutions on solids Factors affecting the adsorption from solution Adsorption of amphiphilic molecules on solid support Adsorption
Monolayers Adsorption as process Adsorption of gases on solids Adsorption of solutions on solids Factors affecting the adsorption from solution Adsorption of amphiphilic molecules on solid support Adsorption
Self-assembly Structures of Block Copolymers in Selective Solvents and of Polysaccharide- Surfactant Mixtures
 Self-assembly Structures of Block Copolymers in Selective Solvents and of Polysaccharide- Surfactant Mixtures Björn Lindman, Physical Chemistry, Lund University, Sweden Center of Excellence Contributions
Self-assembly Structures of Block Copolymers in Selective Solvents and of Polysaccharide- Surfactant Mixtures Björn Lindman, Physical Chemistry, Lund University, Sweden Center of Excellence Contributions
Chapter 23 Introduction to Analytical Separations
 Chapter 23 Introduction to Analytical Separations Homework Due Monday April 24 Problems 23-1, 23-2, 23-7, 23-15, 23-27, 23-29, 23-32 Analytical Separations: Universal approach to analyzing complex mixtures
Chapter 23 Introduction to Analytical Separations Homework Due Monday April 24 Problems 23-1, 23-2, 23-7, 23-15, 23-27, 23-29, 23-32 Analytical Separations: Universal approach to analyzing complex mixtures
Water and Aqueous Systems
 Water and Aqueous Systems The Water Molecule: a Review Water is a simple tri-atomic molecule, H 2 O Each O-H bond is highly polar, because of the high electronegativity of the oxygen (N, O, F, and Cl have
Water and Aqueous Systems The Water Molecule: a Review Water is a simple tri-atomic molecule, H 2 O Each O-H bond is highly polar, because of the high electronegativity of the oxygen (N, O, F, and Cl have
Potentials, periodicity
 Potentials, periodicity Lecture 2 1/23/18 1 Survey responses 2 Topic requests DFT (10), Molecular dynamics (7), Monte Carlo (5) Machine Learning (4), High-throughput, Databases (4) NEB, phonons, Non-equilibrium
Potentials, periodicity Lecture 2 1/23/18 1 Survey responses 2 Topic requests DFT (10), Molecular dynamics (7), Monte Carlo (5) Machine Learning (4), High-throughput, Databases (4) NEB, phonons, Non-equilibrium
Lecture 5: Electrostatic Interactions & Screening
 Lecture 5: Electrostatic Interactions & Screening Lecturer: Prof. Brigita Urbanc (brigita@drexel.edu) PHYS 461 & 561, Fall 2009-2010 1 A charged particle (q=+1) in water, at the interface between water
Lecture 5: Electrostatic Interactions & Screening Lecturer: Prof. Brigita Urbanc (brigita@drexel.edu) PHYS 461 & 561, Fall 2009-2010 1 A charged particle (q=+1) in water, at the interface between water
Lec.1 Chemistry Of Water
 Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Liquids and Solutions
 Liquids and Solutions Introduction This course examines the properties of liquids and solutions at both the thermodynamic and the molecular level. The main topics are: Liquids, Ideal and Regular Solutions,
Liquids and Solutions Introduction This course examines the properties of liquids and solutions at both the thermodynamic and the molecular level. The main topics are: Liquids, Ideal and Regular Solutions,
Chapter 9. Chemical Bonding I: The Lewis Model. HIV-Protease. Lecture Presentation
 Lecture Presentation Chapter 9 Chemical Bonding I: The Lewis Model HIV-Protease HIV-protease is a protein synthesized by the human immunodeficiency virus (HIV). This particular protein is crucial to the
Lecture Presentation Chapter 9 Chemical Bonding I: The Lewis Model HIV-Protease HIV-protease is a protein synthesized by the human immunodeficiency virus (HIV). This particular protein is crucial to the
