Molecular asphaltene models based on Clar sextet theory
|
|
|
- Mildred Warren
- 5 years ago
- Views:
Transcription
1 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Molecular asphaltene models based on Clar sextet theory Francisco J. Martín-Martínez 1, Elham H. Fini 2 and Markus J. Buehler 1 1 Laboratory for Atomistic and Molecular Mechanics (LAMM), Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, 77 Massachusetts Ave., Cambridge, MA, USA. 2 Department of Civil, Architectural and Environmental Engineering, North Carolina A&T State University, 434 McNair Hall, 1601 E. Market St., Greensboro, NC Corresponding author, mbuehler@mit.edu SUPPLEMENTARY INFORMATION Clar s theory of the aromatic sextet Initially developed in 1972, Clar s theory of the aromatic sextet 1,2,3 represents the delocalization of six π-electrons in a single benzene-like ring with a circle inscribed in the hexagon, which denotes a Clar sextet (See Figure s1a). Consequently, two adjacent rings cannot host a circle at the same time, since it would mean 12 π-electrons for just 10 carbon atoms. Furthermore, according to this theory the structure with largest number of π-sextets is the most stable among all possible configurations. Based on these simple statements, Clar s theory of the aromatic sextet predicts the aromaticity distribution of PAHs and explains the stability and many of their chemical properties. 4,5,6,7,8 For instance, in the case of phenanthrene (Figure s1b) the resonance structure with two Clar sextets is more representative than the one with a single inscribed circle, and therefore outer rings have larger local aromaticity than the central one. 9,10,11 On the other hand, anthracene shows three equivalent Clar configurations and therefore the resulting structure is a superposition of the three, with similar aromaticity for the three rings. 9,10,11 As a general idea, a PAH with a given number of aromatic π-sextets is kinetically more stable than its isomers with less aromatic π- sextets. 1,2,3,12 Therefore, the higher the numbers of circles we draw in the structure, the more stable the molecule would be (see isomers in Figure s1d). 13 Further information can be found in a recent review by Solà. 3 This theory has been even proved useful in the study of many novel nanostructures such as carbon nanotubes, 14,15,16,17 graphene nanoribbons, 18,19,20,21 carbon nanocones, 22 and carbon nanotori. 19
2 Figure s1. (a) Clar sextet representation of the delocalization of six π-electrons resulting from the resonance of two Kekulé bond configurations with alternating single and double bonds. (b) Phenanthrene Clar structures with two and one Clar sextets respectively. (c) Anthracene equivalent Clar configurations that result in a final structure superposition of the three. (d) Clar structure of benzo [qr] naphto [2,1,8,7-fghi] pentacene (left) and tribenzo [fg, ij, rst] pentaphene (right).
3 PAH representative of the FAR region of asphaltenes Aiming to reduce the size of the model systems for DFT calculations, we consider smaller PAHs that contain the same FAR region. These PAHs retain the same electronic distribution and geometrical effects in the aromatic system, but with considerably smaller number of atoms. Three different molecular structures for representing A1 are investigated. The planarity of the aromatic structure is studied using the Cremer-Pople pucker amplitude. 23 This method provides a general quantification of the ring deformation by introducing ring-puckering coordinates that can be applied without approximation to any cyclic molecule, given only the coordinates of the nuclear positions of the atoms in the ring. It is also independent on the number and type of atoms and the size of the ring. The coloring is done with the color scale provided by the PaperChain representation in VMD, 24 where Cremer-Pople pucker amplitude is calculated and color-coded, 25,26 using Hill-Reilly method. 27 In this coordinate system the radius Q means the magnitude of puckering, measuring the deviation from the perfectly flat six-membered ring (Q = 0). In this scale, red rings are planar, green rings are highly deformed and the orange-colored ones are partially deformed. This representation allows us to study the deformation in the polyaromatic core of the molecules under study. Figure s2 depicts the fully optimized geometries of these molecules, where the rings are colorcoded following the Cremer-Pople pucker amplitude, as implemented in VMD molecular visualization. The values of Q are shown inside the hexagons. These color-coding allow us to quickly visualize qualitatively the deformation of the rings with respect to planarity. Red rings are those completely planar while green ones are the most deformed and the values closer to zero. The first model is the most simplified one, where hydrogen atoms saturate all terminal carbons in the structure (Figure s2a). In the second model, methane groups saturate those positions where aliphatic chains should be in the complete molecule (Figure s2b). The third model shows the complete structure where all aliphatic chains are included (Figure s2c). Remarkably, pentane effect does not depend on the aliphatic chains, but the strain that undergoes the polycyclic aromatic core remains in the three molecules independently on the edge configuration, as it is clearly noticed by the same colors for the Cremer-Pople pucker amplitude. Obviously, the higher deformation takes place in the cyclohexane ring, which is not aromatic at all, while the polycyclic aromatic system goes from orange (partially deformed) to red (planar).
4 Figure s2. Different models for A1 molecule color-coded with pucker Cremer and Pople amplitude. The values of Q are shown inside the hexagons. (a) Simplified model with hydrogen atoms saturating all carbon atoms. (b) Model with methane groups saturating positions of aliphatic chains. (c) Complete molecular structure.
5 Supplemental references (1) Clar, E. The Aromatic Sextet; Wiley: New York, (2) Randić, M. Chemical Reviews 2003, 103, (3) Solà, M. Frontiers in Chemistry 2013, 1. (4) Biermann, D.; Schmidt, W. Journal of the American Chemical Society 1980, 102, (5) Schleyer, P. v. R.; Manoharan, M.; Jiao, H.; Stahl, F. Organic Letters 2001, 3, (6) Cheng, M.-F.; Li, W.-K. Chemical Physics Letters 2003, 368, 630. (7) Ciesielski, A.; Krygowski, T. M.; Cyrański, M. K. Journal of Chemical Information and Modeling 2008, 48, (8) Poater, J.; Visser, R.; Solà, M.; Bickelhaupt, F. M. The Journal of Organic Chemistry 2007, 72, (9) Schulman, J. M.; Disch, R. L. The Journal of Physical Chemistry A 1999, 103, (10) Cyrański, M. K.; Stępień, B. T.; Krygowski, T. M. Tetrahedron 2000, 56, (11) Portella, G.; Poater, J.; Bofill, J. M.; Alemany, P.; Solà, M. The Journal of Organic Chemistry 2005, 70, (12) Ruiz-Morales, Y. The Journal of Physical Chemistry A 2004, 108, (13) Clar, E.; Zander, M. Journal of the Chemical Society (Resumed) 1958, (14) Martín-Martínez, F. J.; Melchor, S.; Dobado, J. A. Organic Letters 2008, 10, (15) Martín-Martínez, F. J.; Fias, S.; Van Lier, G.; De Proft, F.; Geerlings, P. Chemistry A European Journal 2012, 18, (16) Baldoni, M.; Selli, D.; Sgamellotti, A.; Mercuri, F. The Journal of Physical Chemistry C 2008, 113, 862. (17) Baldoni, M.; Sgamellotti, A.; Mercuri, F. Organic Letters 2007, 9, (18) Martin-Martinez, F. J.; Fias, S.; Van Lier, G.; De Proft, F.; Geerlings, P. Physical Chemistry Chemical Physics 2013, 15, (19) Baldoni, M.; Sgamellotti, A.; Mercuri, F. Chemical Physics Letters 2008, 464, 202. (20) Wassmann, T.; Seitsonen, A. P.; Saitta, A. M.; Lazzeri, M.; Mauri, F. Physical Review Letters 2008, 101, (21) Wassmann, T.; Seitsonen, A. P.; Saitta, A. M.; Lazzeri, M.; Mauri, F. Journal of the American Chemical Society 2010, 132, (22) Balaban, A. T.; Klein, D. J. The Journal of Physical Chemistry C 2009, 113, (23) Cremer, D.; Pople, J. A. Journal of the American Chemical Society 1975, 97, (24) Humphrey;, W.; Dalke;, A.; Schulten, K. Journal of Molecular Graphics 1996, 14, 33. (25) Cross, S.; Kuttel, M. M.; Stone, J. E.; Gain, J. E. Journal of Molecular Graphics and Modelling 2009, 28, 131. (26) Kuttel, M.; Gain, J.; Burger, A.; Eborn, I. Journal of Molecular Graphics and Modelling 2006, 25, 380. (27) Hill, A. D.; Reilly, P. J. Journal of Chemical Information and Modeling 2007, 47, 1031.
6 XYZ Coordinates A1-M1 C C C C C C C C C C C C C C C C C C C C C C C C C C S C C C C H H H H H H H H H H H H H H C H H
7 C H H C H H C H H A1-M2 C C C C C C C C C C C H H C C C H C H S C H C H C C H C H C C C H H H C C H C C
8 C C C H H C H H C H H C H H C H H A1-M3 C C C C C C C C C C C H H C C C H C H C H C H C H H C C H C C C C
9 C H C H H C H H C H H C H H S C C H C H C H C H A2-M1 C C C C C C C C C C C C H H H C C C C C C C C C C
10 C C C C H H H C H H C H H C H H C H H N H C H C H C C C H C H H H A2-M2 C C C C C C C C C C C C H H H C
11 C C C C C C C C H C C C C C H H H H C H H C H H C C C C H C H C H H C H H C H H N H A2-M3 C C C C C C C
12 C C C C C H H H C C C C C C C C C H C C C C C H H H H C H H C H H C C H H C H H C C C H C H C H H N H
13 A3-M1 C C C C C C C C C C C C C C H C C C H H C C H C H C H H C H H C H H C H H H H H H O H A3-M2 C C C C
14 C C C C C C C C C C H C C C H C H C H H C H H C H H C H H H H O H C C H H H H Frequencies (cm -1 ) A1-M
15 A1-M
16
17 A1-M
18 A2-M
19 A2-M
20 A2-M
21 A3-M
22 A3-M
23
Clar Sextet Theory for low-dimensional carbon nanostructures: an efficient approach based on chemical criteria
 Clar Sextet Theory for low-dimensional carbon nanostructures: an efficient approach based on chemical criteria Matteo Baldoni Fachbereich Chemie, Technische Universität Dresden, Germany Department of Chemistry
Clar Sextet Theory for low-dimensional carbon nanostructures: an efficient approach based on chemical criteria Matteo Baldoni Fachbereich Chemie, Technische Universität Dresden, Germany Department of Chemistry
How to Find the Fries Structures for Benzenoid Hydrocarbons
 Symmetry 00,, 0-00; doi:0.0/sym00 Article OEN ACCESS symmetry ISSN 0- www.mdpi.com/journal/symmetry How to Find the Fries Structures for Benzenoid Hydrocarbons Arkadiusz Ciesielski,, *, Tadeusz M. Krygowski
Symmetry 00,, 0-00; doi:0.0/sym00 Article OEN ACCESS symmetry ISSN 0- www.mdpi.com/journal/symmetry How to Find the Fries Structures for Benzenoid Hydrocarbons Arkadiusz Ciesielski,, *, Tadeusz M. Krygowski
Chemistry 11. Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons
 Chemistry 11 Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons 2 1. Unsaturated hydrocarbons So far, we have studied the hydrocarbons in which atoms are connected exclusively by
Chemistry 11 Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons 2 1. Unsaturated hydrocarbons So far, we have studied the hydrocarbons in which atoms are connected exclusively by
Local Aromaticity of [n]acenes, [n]phenacenes, and [n]helicenes (n ) 1-9)
![Local Aromaticity of [n]acenes, [n]phenacenes, and [n]helicenes (n ) 1-9) Local Aromaticity of [n]acenes, [n]phenacenes, and [n]helicenes (n ) 1-9)](/thumbs/93/113685377.jpg) Local Aromaticity of [n]acenes, [n]phenacenes, and [n]helicenes (n ) 1-9) Guillem Portella, Jordi Poater,*, Josep M. Bofill, Pere Alemany, and Miquel Solà*, Institut de Química Computacional and Departament
Local Aromaticity of [n]acenes, [n]phenacenes, and [n]helicenes (n ) 1-9) Guillem Portella, Jordi Poater,*, Josep M. Bofill, Pere Alemany, and Miquel Solà*, Institut de Química Computacional and Departament
Chapter 2. Alkanes and Cycloalkanes; Conformational and Geometrical Isomerism
 Chapter 2 Alkanes and Cycloalkanes; Conformational and Geometrical Isomerism Hydrocarbons are compounds that contain only carbon and hydrogen. There are three main classes of hydrocarbons, based on the
Chapter 2 Alkanes and Cycloalkanes; Conformational and Geometrical Isomerism Hydrocarbons are compounds that contain only carbon and hydrogen. There are three main classes of hydrocarbons, based on the
Electronic and Aromatic properties of Graphene and Nanographenes of various kinds: New Insights and Results
 Electronic and Aromatic properties of Graphene and Nanographenes of various kinds: New Insights and Results Aristides D. Zdetsis 1,2*, Eleftherios N. Economou 2 1 Molecular Engineering Laboratory, Department
Electronic and Aromatic properties of Graphene and Nanographenes of various kinds: New Insights and Results Aristides D. Zdetsis 1,2*, Eleftherios N. Economou 2 1 Molecular Engineering Laboratory, Department
Alicyclic Hydrocarbons can be classified into: Cycloalkanes Cycloalkenes Cycloalkynes
 Cycloalkanes Open-chain The carbon atoms are attached to one another to form chains Ex: CH 3 -CH 2 -CH 2 -CH 3 n-butane Cyclic compounds the carbon atoms are arranged to form rings called: cyclic compounds,
Cycloalkanes Open-chain The carbon atoms are attached to one another to form chains Ex: CH 3 -CH 2 -CH 2 -CH 3 n-butane Cyclic compounds the carbon atoms are arranged to form rings called: cyclic compounds,
Organic Chemistry. Second Edition. Chapter 18 Aromatic Compounds. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 18 Aromatic Compounds Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 18.1 Introduction to Aromatic Compounds
Organic Chemistry Second Edition David Klein Chapter 18 Aromatic Compounds Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 18.1 Introduction to Aromatic Compounds
Atomic Structure of Benzene Which Accounts for Resonance Energy
 1 Atomic Structure of Benzene Which Accounts for Resonance Energy (This work is dedicated to Kathleen Lonsdale) 1 Raji Heyrovska 1 Institute of Biophysics, Academy of Sciences of the Czech Republic, Královopolská
1 Atomic Structure of Benzene Which Accounts for Resonance Energy (This work is dedicated to Kathleen Lonsdale) 1 Raji Heyrovska 1 Institute of Biophysics, Academy of Sciences of the Czech Republic, Královopolská
Clar number of catacondensed benzenoid hydrocarbons
 Clar number of catacondensed benzenoid hydrocarbons Sandi Klavžar a,, Petra Žigert a, Ivan Gutman b, a Department of Mathematics, PEF, University of Maribor, Koroška 160, 2000 Maribor, Slovenia b Faculty
Clar number of catacondensed benzenoid hydrocarbons Sandi Klavžar a,, Petra Žigert a, Ivan Gutman b, a Department of Mathematics, PEF, University of Maribor, Koroška 160, 2000 Maribor, Slovenia b Faculty
Self assembly of Carbon Atoms in Interstellar Space and Formation Mechanism of Naphthalene from Small Precursors: A Molecular Dynamics Study
 Self assembly of Carbon Atoms in Interstellar Space and Formation Mechanism of Naphthalene from Small Precursors: A Molecular Dynamics Study Niladri Patra UIC Advisor: Dr. H. R. Sadeghpour ITAMP Large
Self assembly of Carbon Atoms in Interstellar Space and Formation Mechanism of Naphthalene from Small Precursors: A Molecular Dynamics Study Niladri Patra UIC Advisor: Dr. H. R. Sadeghpour ITAMP Large
All organic compounds contain carbon, however, not all carbon containing compounds are classified as organic. Organic compounds covalently bonded
 Chapter 20 All organic compounds contain carbon, however, not all carbon containing compounds are classified as organic. Organic compounds covalently bonded compounds containing carbon, excluding carbonates
Chapter 20 All organic compounds contain carbon, however, not all carbon containing compounds are classified as organic. Organic compounds covalently bonded compounds containing carbon, excluding carbonates
Benzene a remarkable compound. Chapter 14 Aromatic Compounds. Some proposed structures for C 6 H 6. Dimethyl substituted benzenes are called xylenes
 Benzene a remarkable compound Chapter 14 Aromatic Compounds Discovered by Faraday 1825 Formula C 6 H 6 Highly unsaturated, but remarkably stable Whole new class of benzene derivatives called aromatic compounds
Benzene a remarkable compound Chapter 14 Aromatic Compounds Discovered by Faraday 1825 Formula C 6 H 6 Highly unsaturated, but remarkably stable Whole new class of benzene derivatives called aromatic compounds
Valence electrons octet rule. Lewis structure Lewis structures
 Lewis Dot Diagrams Valence electrons are the electrons in the outermost energy level of an atom. An element with a full octet of valence electrons has a stable configuration. The tendency of bonded atoms
Lewis Dot Diagrams Valence electrons are the electrons in the outermost energy level of an atom. An element with a full octet of valence electrons has a stable configuration. The tendency of bonded atoms
Alkanes 3/27/17. Hydrocarbons: Compounds made of hydrogen and carbon only. Aliphatic (means fat ) - Open chain Aromatic - ring. Alkane Alkene Alkyne
 Alkanes EQ 1. How will I define Hydrocarbons? 2. Compare and contrast the 3 types of hydrocarbons (Alkanes, alkenes, alkynes). Hydrocarbons: Compounds made of hydrogen and carbon only. Aliphatic (means
Alkanes EQ 1. How will I define Hydrocarbons? 2. Compare and contrast the 3 types of hydrocarbons (Alkanes, alkenes, alkynes). Hydrocarbons: Compounds made of hydrogen and carbon only. Aliphatic (means
STEREOCHEMISTRY OF ALKANES AND CYCLOALKANES CONFORMATIONAL ISOMERS
 STEREOCHEMISTRY OF ALKANES AND CYCLOALKANES CONFORMATIONAL ISOMERS 1 CONFORMATIONAL ISOMERS Stereochemistry concerned with the 3-D aspects of molecules Rotation is possible around C-C bonds in openchain
STEREOCHEMISTRY OF ALKANES AND CYCLOALKANES CONFORMATIONAL ISOMERS 1 CONFORMATIONAL ISOMERS Stereochemistry concerned with the 3-D aspects of molecules Rotation is possible around C-C bonds in openchain
Objective 3. Draw resonance structures, use curved arrows, determine extent of delocalization. Identify major/minor contributor.
 Objective 3 Draw resonance structures, use curved arrows, determine extent of delocalization. Identify major/minor contributor. Structure Should Fit Experimental Data The chemical formula of benzene is
Objective 3 Draw resonance structures, use curved arrows, determine extent of delocalization. Identify major/minor contributor. Structure Should Fit Experimental Data The chemical formula of benzene is
A critical approach toward resonance-assistance in the intramolecular hydrogen bond
 Electronic Supplementary Material (ESI) for Photochemical & Photobiological Sciences. This journal is The Royal Society of Chemistry and Owner Societies 2015 Supplementary Information for A critical approach
Electronic Supplementary Material (ESI) for Photochemical & Photobiological Sciences. This journal is The Royal Society of Chemistry and Owner Societies 2015 Supplementary Information for A critical approach
Alkanes. Introduction
 Introduction Alkanes Recall that alkanes are aliphatic hydrocarbons having C C and C H bonds. They can be categorized as acyclic or cyclic. Acyclic alkanes have the molecular formula C n H 2n+2 (where
Introduction Alkanes Recall that alkanes are aliphatic hydrocarbons having C C and C H bonds. They can be categorized as acyclic or cyclic. Acyclic alkanes have the molecular formula C n H 2n+2 (where
Supplementary Figure 1 Irregular arrangement of E,E-8-mer on TMA. STM height images formed when
 Supplementary Figure 1 Irregular arrangement of E,E-8-mer on TMA. STM height images formed when a 5 µl heptanoic acid solution of E,E-8-mer is applied on: (a) a TMA templated HOPG substrate, U s = +1.00
Supplementary Figure 1 Irregular arrangement of E,E-8-mer on TMA. STM height images formed when a 5 µl heptanoic acid solution of E,E-8-mer is applied on: (a) a TMA templated HOPG substrate, U s = +1.00
Class XI Chapter 13 Hydrocarbons Chemistry
 Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Interplay between Intramolecular Resonance-Assisted Hydrogen Bonding and Local Aromaticity. II. 1,3-Dihydroxyaryl-2-aldehydes
 Article Subscriber access provided by American Chemical Society Interplay between Intramolecular Resonance-Assisted Hydrogen Bonding and Local Aromaticity. II. 1,3-Dihydroxyaryl-2-aldehydes Marcin Palusiak,
Article Subscriber access provided by American Chemical Society Interplay between Intramolecular Resonance-Assisted Hydrogen Bonding and Local Aromaticity. II. 1,3-Dihydroxyaryl-2-aldehydes Marcin Palusiak,
MULTIPLE EQUIVALENT STRUCTURES: RESONANCE
 MULTIPLE EQUIVALENT STRUCTURES: RESONANCE There is a rather large class of molecules for which one has no difficulty writing Lewis structures; in fact, we can write more than one valid structure for a
MULTIPLE EQUIVALENT STRUCTURES: RESONANCE There is a rather large class of molecules for which one has no difficulty writing Lewis structures; in fact, we can write more than one valid structure for a
Naming Aromatic Compounds (Benzene as the Parent)
 aming Aromatic Compounds (Benzene as the Parent) If the the alkyl chain is smaller than the ring use the ring as the parent Monosubstituted benzenes are named the same way as other hydrocarbons using benzene
aming Aromatic Compounds (Benzene as the Parent) If the the alkyl chain is smaller than the ring use the ring as the parent Monosubstituted benzenes are named the same way as other hydrocarbons using benzene
Aromatic Compounds I
 2302272 Org Chem II Part I Lecture 1 Aromatic Compounds I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 16 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 1 Aromatic Compounds I Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 16 in Organic Chemistry, 8 th Edition, L.
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURES Dr Ali El-Agamey. Organic Chemistry, 7 th Edition L. G. Wade, Jr.
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURES 11-12 Dr Ali El-Agamey Organic Chemistry, 7 th Edition L. G. Wade, Jr. Amines 2010, Prentice Hall Reactions N,N-Disubstituted amides 2 o amine
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURES 11-12 Dr Ali El-Agamey Organic Chemistry, 7 th Edition L. G. Wade, Jr. Amines 2010, Prentice Hall Reactions N,N-Disubstituted amides 2 o amine
Name Date Class HYDROCARBONS
 22.1 HYDROCARBONS Section Review Objectives Describe the relationship between number of valence electrons and bonding in carbon Define and describe alkanes Relate the polarity of hydrocarbons to their
22.1 HYDROCARBONS Section Review Objectives Describe the relationship between number of valence electrons and bonding in carbon Define and describe alkanes Relate the polarity of hydrocarbons to their
BRCC CHM 102 Class Notes Chapter 13 Page 1 of 6
 BRCC CHM 102 ass Notes Chapter 13 Page 1 of 6 Chapter 13 Benzene and Its Derivatives aliphatic hydrocarbons include alkanes, alkenes, and alkynes aromatic hydrocarbons compounds that contain one or more
BRCC CHM 102 ass Notes Chapter 13 Page 1 of 6 Chapter 13 Benzene and Its Derivatives aliphatic hydrocarbons include alkanes, alkenes, and alkynes aromatic hydrocarbons compounds that contain one or more
Introduction to Aromaticity
 Introduction to Aromaticity Historical Timeline: 1 Spotlight on Benzene: 2 Early 19 th century chemists derive benzene formula (C 6 H 6 ) and molecular mass (78). Carbon to hydrogen ratio of 1:1 suggests
Introduction to Aromaticity Historical Timeline: 1 Spotlight on Benzene: 2 Early 19 th century chemists derive benzene formula (C 6 H 6 ) and molecular mass (78). Carbon to hydrogen ratio of 1:1 suggests
12/27/2010. Chapter 14 Aromatic Compounds
 Nomenclature of Benzene Derivatives Benzene is the parent name for some monosubstituted benzenes; the substituent name is added as a prefix Chapter 14 Aromatic Compounds For other monosubstituted benzenes,
Nomenclature of Benzene Derivatives Benzene is the parent name for some monosubstituted benzenes; the substituent name is added as a prefix Chapter 14 Aromatic Compounds For other monosubstituted benzenes,
A test of Clar aromatic sextet theory
 J. Serb. Chem. Soc. 78 (10) 1539 1546 (2013) UDC 541:547.52+547.53+549.746 JSCS 4516 Original scientific paper A test of Clar aromatic sextet theory IVAN GUTMAN* #, SLAVKO RADENKOVIĆ #, MARIJA ANTIĆ and
J. Serb. Chem. Soc. 78 (10) 1539 1546 (2013) UDC 541:547.52+547.53+549.746 JSCS 4516 Original scientific paper A test of Clar aromatic sextet theory IVAN GUTMAN* #, SLAVKO RADENKOVIĆ #, MARIJA ANTIĆ and
17.24 To name the compounds use the directions from Answer 17.3.
 Benzene and Aromatic Compounds 7 7 7.2 If benzene could be described by a single Kekulé structure, only one product would form in Reaction [], but there would be four (not three) dibromobenzenes (A ),
Benzene and Aromatic Compounds 7 7 7.2 If benzene could be described by a single Kekulé structure, only one product would form in Reaction [], but there would be four (not three) dibromobenzenes (A ),
Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
 Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a
 Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a Hard coal, which is high in carbon content any straight-chain or branched-chain
Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a Hard coal, which is high in carbon content any straight-chain or branched-chain
Hydrocarbons. Table Alkanes
 Hydrocarbons Hydrocarbons may be divided into two groups, aliphatic and aromatic. The aliphatics can be subdivided into i) alkanes, ii) alkenes, and iii) alkynes. The alkanes contain single covalent bonds
Hydrocarbons Hydrocarbons may be divided into two groups, aliphatic and aromatic. The aliphatics can be subdivided into i) alkanes, ii) alkenes, and iii) alkynes. The alkanes contain single covalent bonds
State Key Laboratory of Molecular Reaction Dynamics, Dalian Institute of Chemical Physics, Chinese Academic of Sciences, Dalian , P. R. China.
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supplementary Information for: Theoretical exploration of MgH 2 and graphene nano-flake in cyclohexane:
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supplementary Information for: Theoretical exploration of MgH 2 and graphene nano-flake in cyclohexane:
12.1 AROMATIC COMPOUNDS
 12 Arenes and Aromaticity estradiol 12.1 AROMATIC COMPOUNDS O O O O C 3 C 3 O O safrole (oil of sassafras) O methyl salicylate (oil of wintergreen) O vanillin (vanilla) C 3 N C 3 C 3 CC 2 ibuprofen C C
12 Arenes and Aromaticity estradiol 12.1 AROMATIC COMPOUNDS O O O O C 3 C 3 O O safrole (oil of sassafras) O methyl salicylate (oil of wintergreen) O vanillin (vanilla) C 3 N C 3 C 3 CC 2 ibuprofen C C
Chapter 13 Alkenes and Alkynes & Aromatic Compounds
 Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
7. Arrange the molecular orbitals in order of increasing energy and add the electrons.
 Molecular Orbital Theory I. Introduction. A. Ideas. 1. Start with nuclei at their equilibrium positions. 2. onstruct a set of orbitals that cover the complete nuclear framework, called molecular orbitals
Molecular Orbital Theory I. Introduction. A. Ideas. 1. Start with nuclei at their equilibrium positions. 2. onstruct a set of orbitals that cover the complete nuclear framework, called molecular orbitals
Reading Skill Practice
 system. This process is explained on page 698. create a flowchart that describes the steps for naming branched-chain alkanes using the IUPAC A flowchart can help you to remember the order in which events
system. This process is explained on page 698. create a flowchart that describes the steps for naming branched-chain alkanes using the IUPAC A flowchart can help you to remember the order in which events
Lecture 2 Nomenclature of hydrocarbons 1
 Lecture 2 Nomenclature of hydrocarbons 1 Simplest Organic Molecules: The carbon atoms in organic molecules covalently bind to each other as well as to atoms of other elements. The most common of these
Lecture 2 Nomenclature of hydrocarbons 1 Simplest Organic Molecules: The carbon atoms in organic molecules covalently bind to each other as well as to atoms of other elements. The most common of these
Application of graph theory and topological models for the determination of fundamentals of the aromatic character of pi-conjugated hydrocarbons*
 Pure Appl. Chem., Vol. 84, No. 4, pp. 1069 1088, 2012. http://dx.doi.org/10.1351/pac-con-11-11-20 2012 IUPAC, Publication date (Web): 19 March 2012 Application of graph theory and topological models for
Pure Appl. Chem., Vol. 84, No. 4, pp. 1069 1088, 2012. http://dx.doi.org/10.1351/pac-con-11-11-20 2012 IUPAC, Publication date (Web): 19 March 2012 Application of graph theory and topological models for
The maximum forcing number of a polyomino
 AUSTRALASIAN JOURNAL OF COMBINATORICS Volume 69(3) (2017), Pages 306 314 The maximum forcing number of a polyomino Yuqing Lin Mujiangshan Wang School of Electrical Engineering and Computer Science The
AUSTRALASIAN JOURNAL OF COMBINATORICS Volume 69(3) (2017), Pages 306 314 The maximum forcing number of a polyomino Yuqing Lin Mujiangshan Wang School of Electrical Engineering and Computer Science The
(1) Recall the different isomers mentioned in this tutorial.
 DAT Organic Chemistry - Problem Drill 08: Conformational Analysis Question No. 1 of 10 Question 1. Isomers that differ by rotation about a single bond are called: Question #01 (A) Stereoisomers (B) Constitutional
DAT Organic Chemistry - Problem Drill 08: Conformational Analysis Question No. 1 of 10 Question 1. Isomers that differ by rotation about a single bond are called: Question #01 (A) Stereoisomers (B) Constitutional
Straight. C C bonds are sp 3 hybridized. Butane, C 4 H 10 H 3 C
 Hydrocarbons Straight Chain Alkanes aren t Straight C C bonds are sp 3 hybridized Butane, C 4 H 10 Structural Shorthand Explicit hydrogens (those required to complete carbon s valence) are usually left
Hydrocarbons Straight Chain Alkanes aren t Straight C C bonds are sp 3 hybridized Butane, C 4 H 10 Structural Shorthand Explicit hydrogens (those required to complete carbon s valence) are usually left
4. Stereochemistry of Alkanes and Cycloalkanes
 4. Stereochemistry of Alkanes and Cycloalkanes Based on McMurry s Organic Chemistry, 6 th edition, Chapter 4 2003 Ronald Kluger Department of Chemistry University of Toronto The Shapes of Molecules! The
4. Stereochemistry of Alkanes and Cycloalkanes Based on McMurry s Organic Chemistry, 6 th edition, Chapter 4 2003 Ronald Kluger Department of Chemistry University of Toronto The Shapes of Molecules! The
Organic Chemistry is the chemistry of compounds containing.
 Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION DOI: 10.1038/NNANO.2012.162 Selective Molecular Sieving Through Porous Graphene Steven P. Koenig, Luda Wang, John Pellegrino, and J. Scott Bunch* *email: jbunch@colorado.edu Supplementary
SUPPLEMENTARY INFORMATION DOI: 10.1038/NNANO.2012.162 Selective Molecular Sieving Through Porous Graphene Steven P. Koenig, Luda Wang, John Pellegrino, and J. Scott Bunch* *email: jbunch@colorado.edu Supplementary
1. How do you account for the formation of ethane during chlorination of methane?
 1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
EFFECT OF BENZOCYCLOBUTADIENO-ANNELATION ON CYCLIC CONJUGATION IN PERYLENE. Ivan Gutman and Beba Stojanovska
 Macedonian Journal of Chemistry and Chemical Engineering, Vol. 30, No. 2, pp. 235 240 (2011) MJCCA9 585 ISSN 1857-5552 Received: March 4, 2011 UDC: 547.6 Accepted: May 5, 2011 Original scientific paper
Macedonian Journal of Chemistry and Chemical Engineering, Vol. 30, No. 2, pp. 235 240 (2011) MJCCA9 585 ISSN 1857-5552 Received: March 4, 2011 UDC: 547.6 Accepted: May 5, 2011 Original scientific paper
Chapters 1, 2, & 3. CHAPTER 3 *** 3-D Molecular Model Set Needed*** Saturated Hydrocarbons (AKA: Alkanes) (AKA:Paraffins)
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 1 Lecture Notes Chapters 1, 2, & 3 CAPTER 3 *** 3-D Molecular Model Set Needed*** Saturated ydrocarbons (AKA: Alkanes) (AKA:Paraffins)
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 1 Lecture Notes Chapters 1, 2, & 3 CAPTER 3 *** 3-D Molecular Model Set Needed*** Saturated ydrocarbons (AKA: Alkanes) (AKA:Paraffins)
2. A triple Diels-Alder reaction followed by a triple retro Diels Alder reaction give this interesting product:
 Lecture 8 October 6, 2011 Last time we talked a lot about highly reactive dienes, especially those developed by the group of Sam Danishefsky. One more example of an interesting diene is isobenzofuran:
Lecture 8 October 6, 2011 Last time we talked a lot about highly reactive dienes, especially those developed by the group of Sam Danishefsky. One more example of an interesting diene is isobenzofuran:
Topic 10 Organic Chemistry. Ms. Kiely IB Chemistry (SL) Coral Gables Senior High School
 Topic 10 Organic Chemistry Ms. Kiely IB Chemistry (SL) Coral Gables Senior High School -Alkanes: have low reactivity and undergo free radical substitution. -Alkenes: are more reactive than alkanes, since
Topic 10 Organic Chemistry Ms. Kiely IB Chemistry (SL) Coral Gables Senior High School -Alkanes: have low reactivity and undergo free radical substitution. -Alkenes: are more reactive than alkanes, since
Benzene and Aromaticity
 Benzene and Aromaticity Why this Chapter? Reactivity of substituted aromatic compounds is tied to their structure Aromatic compounds provide a sensitive probe for studying relationship between structure
Benzene and Aromaticity Why this Chapter? Reactivity of substituted aromatic compounds is tied to their structure Aromatic compounds provide a sensitive probe for studying relationship between structure
Alkanes and Cycloalkanes
 Chapter 3 Alkanes and Cycloalkanes Two types Saturated hydrocarbons Unsaturated hydrocarbons 3.1 Alkanes Also referred as aliphatic hydrocarbons General formula: CnH2n+2 (straight chain) and CnH2n (cyclic)
Chapter 3 Alkanes and Cycloalkanes Two types Saturated hydrocarbons Unsaturated hydrocarbons 3.1 Alkanes Also referred as aliphatic hydrocarbons General formula: CnH2n+2 (straight chain) and CnH2n (cyclic)
ORGANIC - CLUTCH CH AROMATICITY.
 !! www.clutchprep.com CONCEPT: AROMATICTY INTRODUCTION Aromatic compounds display an unusual stability for their high level of electron density. Their high level of unsaturation should make them extremely
!! www.clutchprep.com CONCEPT: AROMATICTY INTRODUCTION Aromatic compounds display an unusual stability for their high level of electron density. Their high level of unsaturation should make them extremely
Electric and Magnetic Properties Computed for Valence Bond Structures: Is There a Link between Pauling Resonance Energy and Ring Current?
 Electric and Magnetic Properties Computed for Valence Bond Structures: Is There a Link between Pauling Resonance Energy and Ring Current? Remco W. A. Havenith Debye Institute, Theoretical Chemistry Group,
Electric and Magnetic Properties Computed for Valence Bond Structures: Is There a Link between Pauling Resonance Energy and Ring Current? Remco W. A. Havenith Debye Institute, Theoretical Chemistry Group,
safety goggles 1 ball- and~stick molecular model ldt/4 students
 Text reference: Section 24.1 The physical, chemical, and biological properties of molecules are determined, to a large extent, by their three-dimensional shapes. Molecular substances made up of molecules
Text reference: Section 24.1 The physical, chemical, and biological properties of molecules are determined, to a large extent, by their three-dimensional shapes. Molecular substances made up of molecules
Elucidating the structure of light absorbing styrene. carbocation species formed within zeolites SUPPORTING INFORMATION
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 Elucidating the structure of light absorbing styrene carbocation species formed
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 Elucidating the structure of light absorbing styrene carbocation species formed
Q.1 Draw out suitable structures which fit the molecular formula C 6 H 6
 Aromatic compounds 2814 1 BENZENE Structure Primary analysis revealed benzene had an... empirical formula of and a molecular formula of 6 6 Q.1 Draw out suitable structures which fit the molecular formula
Aromatic compounds 2814 1 BENZENE Structure Primary analysis revealed benzene had an... empirical formula of and a molecular formula of 6 6 Q.1 Draw out suitable structures which fit the molecular formula
Molecular Physics. On the aromatic stabilization energy of the 4N π electron pyrene
 Molecular Physics On the aromatic stabilization energy of the N π electron pyrene Journal: Molecular Physics Manuscript ID: TMPH-00-0.R Manuscript Type: Special Issue Paper - Fritz Schaefer Date Submitted
Molecular Physics On the aromatic stabilization energy of the N π electron pyrene Journal: Molecular Physics Manuscript ID: TMPH-00-0.R Manuscript Type: Special Issue Paper - Fritz Schaefer Date Submitted
Organic Chemistry. A brief introduction
 Organic Chemistry A brief introduction Organic Chemistry the study of carbon-containing compounds and their properties excluding: CO, CO 2, CS 2, carbonates and cyanides eight million known organic compounds
Organic Chemistry A brief introduction Organic Chemistry the study of carbon-containing compounds and their properties excluding: CO, CO 2, CS 2, carbonates and cyanides eight million known organic compounds
Exam (6 pts) Show which starting materials are used to produce the following Diels-Alder products:
 Exam 1 Name CHEM 212 1. (18 pts) Complete the following chemical reactions showing all major organic products; illustrate proper stereochemistry where appropriate. If no reaction occurs, indicate NR :
Exam 1 Name CHEM 212 1. (18 pts) Complete the following chemical reactions showing all major organic products; illustrate proper stereochemistry where appropriate. If no reaction occurs, indicate NR :
Magnetic Nuclei other than 1 H
 Magnetic Nuclei other than 1 H 2 H (Deuterium): I = 1 H,D-Exchange might be used to simplify 1 H-NMR spectra since H-D couplings are generally small; - - - -O- - - -D 2 -O- triplet of triplets slightly
Magnetic Nuclei other than 1 H 2 H (Deuterium): I = 1 H,D-Exchange might be used to simplify 1 H-NMR spectra since H-D couplings are generally small; - - - -O- - - -D 2 -O- triplet of triplets slightly
Benzene and Aromatic Compounds. Chapter 15 Organic Chemistry, 8 th Edition John McMurry
 Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 4: Aromatic Compounds. Bitter almonds are the source of the aromatic compound benzaldehyde
 Chapter 4: Aromatic Compounds Bitter almonds are the source of the aromatic compound benzaldehyde Sources of Benzene Benzene, C 6 H 6, is the parent hydrocarbon of the especially stable compounds known
Chapter 4: Aromatic Compounds Bitter almonds are the source of the aromatic compound benzaldehyde Sources of Benzene Benzene, C 6 H 6, is the parent hydrocarbon of the especially stable compounds known
CHEM 112 Name: (Last) (First). Section No.: VISUALIZING ORGANIC REACTIONS THROUGH USE OF MOLECULAR MODELS
 CHEM 112 Name: (Last) (First). Section No.: VISUALIZING ORGANIC REACTIONS THROUGH USE OF MOLECULAR MODELS 1) HYDROCARBONS: a. Saturated Hydrocarbons: Construct a model for propane, C 3 H 8, using black
CHEM 112 Name: (Last) (First). Section No.: VISUALIZING ORGANIC REACTIONS THROUGH USE OF MOLECULAR MODELS 1) HYDROCARBONS: a. Saturated Hydrocarbons: Construct a model for propane, C 3 H 8, using black
Experiment 10 Organic Molecules: Description, Nomenclature and Modeling
 Experiment 10 Organic Molecules: Description, Nomenclature and Modeling Objectives The objectives for this lab are: Part I: To learn the structures of and construct models for simple organic molecules,
Experiment 10 Organic Molecules: Description, Nomenclature and Modeling Objectives The objectives for this lab are: Part I: To learn the structures of and construct models for simple organic molecules,
Alcohols, protons α to ketones. Aromatics, Amides. Acids, Aldehydes. Aliphatic. Olefins. ppm TMS
 Interpretation of 1 spectra So far we have talked about different NMR techniques and pulse sequences, but we haven t focused seriously on how to analyze the data that we obtain from these experiments.
Interpretation of 1 spectra So far we have talked about different NMR techniques and pulse sequences, but we haven t focused seriously on how to analyze the data that we obtain from these experiments.
3. What number would be used to indicate the double bond position in the IUPAC name for CH 3 CH 2 CH=CH CH 3 a. 1 b. 2 c. 3 d.
 Chapter 2 Unsaturated Hydrocarbons MULTIPLE CHOICE 1. Name a difference between a saturated and an unsaturated hydrocarbon. a. Saturated hydrocarbons are composed of only carbon and hydrogen, and unsaturated
Chapter 2 Unsaturated Hydrocarbons MULTIPLE CHOICE 1. Name a difference between a saturated and an unsaturated hydrocarbon. a. Saturated hydrocarbons are composed of only carbon and hydrogen, and unsaturated
Chapter 16. Aromatic Compounds
 Chapter 16 Aromatic Compounds Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to
Chapter 16 Aromatic Compounds Discovery of Benzene Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to
MOLECULAR STRUCTURE; LEWIS STRUCTURES AND 3-D MODELS
 Chemistry Name(s) MOLECULAR STRUCTURE; LEWIS STRUCTURES AND 3-D MODELS INTRODUCTION: Chemists are often concerned with molecular structure. It is helpful and useful to have a good understanding of the
Chemistry Name(s) MOLECULAR STRUCTURE; LEWIS STRUCTURES AND 3-D MODELS INTRODUCTION: Chemists are often concerned with molecular structure. It is helpful and useful to have a good understanding of the
Organic Chemistry - Introduction
 It s All About Carbon! Unit 15: Organic Chemistry Lesson 15.1: Hydrocarbons Organic Chemistry - Introduction Organic chemistry is the study of compounds containing carbon. Animals, plants, and other forms
It s All About Carbon! Unit 15: Organic Chemistry Lesson 15.1: Hydrocarbons Organic Chemistry - Introduction Organic chemistry is the study of compounds containing carbon. Animals, plants, and other forms
CHEM 263 Notes Oct 1, Beta-carotene (depicted below) is responsible for the orange-red colour in carrots.
 EM 263 otes ct 1, 2013 onjugated Dienes and olour ontinued Beta-carotene (depicted below) is responsible for the orange-red colour in carrots. In the below example, astaxanthin, a blue-green pigment in
EM 263 otes ct 1, 2013 onjugated Dienes and olour ontinued Beta-carotene (depicted below) is responsible for the orange-red colour in carrots. In the below example, astaxanthin, a blue-green pigment in
Organic Chemistry, Second Edition. Janice Gorzynski Smith University of Hawai i. Chapter 4 Alkanes
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 4 Alkanes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies, Inc.
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 4 Alkanes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies, Inc.
M.J. Buehler, Strength in numbers, Nature Nanotechnology, Vol. 5, pp ,
 1 M.J. Buehler, Strength in numbers, Nature Nanotechnology, Vol. 5, pp. 172-174, 2010 NANOMATERIALS Strength in numbers Self-assembly of proteins commonly associated with neurodegenerative diseases can
1 M.J. Buehler, Strength in numbers, Nature Nanotechnology, Vol. 5, pp. 172-174, 2010 NANOMATERIALS Strength in numbers Self-assembly of proteins commonly associated with neurodegenerative diseases can
Organic Chemistry 1 Lecture 5
 CEM 232 Organic Chemistry I Illinois at Chicago Organic Chemistry 1 Lecture 5 Instructor: Prof. Duncan Wardrop Time/Day: T & R, 12:30-1:45 p.m. January 26, 2010 1 Self Test Question Which of the following
CEM 232 Organic Chemistry I Illinois at Chicago Organic Chemistry 1 Lecture 5 Instructor: Prof. Duncan Wardrop Time/Day: T & R, 12:30-1:45 p.m. January 26, 2010 1 Self Test Question Which of the following
Chemistry 123: Physical and Organic Chemistry Topic 1: Organic Chemistry
 Topic 1: Mechanisms and Curved Arrows etc Reactions of Alkenes:.Similar functional groups react the same way. Why? Winter 2009 Page 73 Topic 1: Mechanisms and Curved Arrows etc Reactivity:.Electrostatic
Topic 1: Mechanisms and Curved Arrows etc Reactions of Alkenes:.Similar functional groups react the same way. Why? Winter 2009 Page 73 Topic 1: Mechanisms and Curved Arrows etc Reactivity:.Electrostatic
Vision. Cis-trans isomerism is key to vision. How rods work H 3 C CH 3. Protein opsin. 11-cis-retinal. Opsin. Rhodopsin.
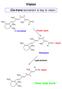 Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
Constitutional Isomers and Conformations of Alkanes & Cycloalkanes
 Discovering Molecular Models #1: Constitutional Isomers Conformations of Alkanes & Cycloalkanes There are no additional tutorial or laboratory notes. Read bring your course notes, as they provide all of
Discovering Molecular Models #1: Constitutional Isomers Conformations of Alkanes & Cycloalkanes There are no additional tutorial or laboratory notes. Read bring your course notes, as they provide all of
HISTORY OF ORGANIC CHEMISTRY
 ISTORY OF ORGANI EMISTRY In the early days of chemistry, scientists classified chemical substances into 2 groups: 1. Inorganic: those that were composed of minerals, such as rocks and nonliving matter.
ISTORY OF ORGANI EMISTRY In the early days of chemistry, scientists classified chemical substances into 2 groups: 1. Inorganic: those that were composed of minerals, such as rocks and nonliving matter.
Chapter 9 - Covalent Bonding: Orbitals
 Chapter 9 - Covalent Bonding: Orbitals 9.1 Hybridization and the Localized Electron Model A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new
Chapter 9 - Covalent Bonding: Orbitals 9.1 Hybridization and the Localized Electron Model A. Hybridization 1. The mixing of two or more atomic orbitals of similar energies on the same atom to produce new
Lecture 24 Organic Chemistry 1
 CEM 232 Organic Chemistry I at Chicago Lecture 24 Organic Chemistry 1 Professor Duncan Wardrop April 6, 2010 1 Which shorthand orbital diagram best represents the LUMO of a dienophile in a Diels-Alder
CEM 232 Organic Chemistry I at Chicago Lecture 24 Organic Chemistry 1 Professor Duncan Wardrop April 6, 2010 1 Which shorthand orbital diagram best represents the LUMO of a dienophile in a Diels-Alder
AP Chemistry Chapter 22 - Organic and Biological Molecules
 AP Chemistry Chapter - Organic and Biological Molecules.1 Alkanes: Saturated Hydrocarbons A. Straight-chain Hydrocarbons 1. Straight-chain alkanes have the formula C n H n+. Carbons are sp hybridized The
AP Chemistry Chapter - Organic and Biological Molecules.1 Alkanes: Saturated Hydrocarbons A. Straight-chain Hydrocarbons 1. Straight-chain alkanes have the formula C n H n+. Carbons are sp hybridized The
On the sextet polynomial of fullerenes
 On the sextet polynomial of fullerenes Jean-Sébastien Sereni Matěj Stehlík Abstract We show that the sextet pattern count of every fullerene is strictly smaller than the Kekulé structure count. This proves
On the sextet polynomial of fullerenes Jean-Sébastien Sereni Matěj Stehlík Abstract We show that the sextet pattern count of every fullerene is strictly smaller than the Kekulé structure count. This proves
Graphenes Aromatic Giants
 Ivan Gutman and Boris Furtula Graphenes Aromatic Giants Ivan Gutman and Boris Furtula Ivan Gutman is Professor of Physical Chemistry at the Faculty of Science, Kragujevac, Serbia. Among his interests are
Ivan Gutman and Boris Furtula Graphenes Aromatic Giants Ivan Gutman and Boris Furtula Ivan Gutman is Professor of Physical Chemistry at the Faculty of Science, Kragujevac, Serbia. Among his interests are
Excited States Calculations for Protonated PAHs
 52 Chapter 3 Excited States Calculations for Protonated PAHs 3.1 Introduction Protonated PAHs are closed shell ions. Their electronic structure should therefore be similar to that of neutral PAHs, but
52 Chapter 3 Excited States Calculations for Protonated PAHs 3.1 Introduction Protonated PAHs are closed shell ions. Their electronic structure should therefore be similar to that of neutral PAHs, but
Constitutional Isomers and Conformations of Alkanes & Cycloalkanes
 Discovering Molecular Models #1: Constitutional Isomers Conformations of Alkanes & Cycloalkanes There are no additional tutorial or laboratory notes. Read bring your course notes, as they provide all of
Discovering Molecular Models #1: Constitutional Isomers Conformations of Alkanes & Cycloalkanes There are no additional tutorial or laboratory notes. Read bring your course notes, as they provide all of
CHEMISTRY Matter and Change
 CHEMISTRY Matter and Change CHAPTER 21 Table Of Contents Section Section Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes Section Section 21.5 Aromatic Hydrocarbons Explainthe terms organic compound
CHEMISTRY Matter and Change CHAPTER 21 Table Of Contents Section Section Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes Section Section 21.5 Aromatic Hydrocarbons Explainthe terms organic compound
Molecular Shape and Molecular Polarity. Molecular Shape and Molecular Polarity. Molecular Shape and Molecular Polarity
 Molecular Shape and Molecular Polarity When there is a difference in electronegativity between two atoms, then the bond between them is polar. It is possible for a molecule to contain polar bonds, but
Molecular Shape and Molecular Polarity When there is a difference in electronegativity between two atoms, then the bond between them is polar. It is possible for a molecule to contain polar bonds, but
4. AROMATIC COMPOUNDS
 BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
BOOKS 1) Organic Chemistry Structure and Function, K. Peter C. Vollhardt, Neil Schore, 6th Edition 2) Organic Chemistry, T. W. Graham Solomons, Craig B. Fryhle 3) Organic Chemistry: A Short Course, H.
Role of Kekulé and Non-Kekulé Structures in the Radical Character of Alternant Polycyclic Aromatic Hydrocarbons: A TAO-DFT Study
 www.nature.com/scientificreports OPEN recei e : 22 pri 2016 ccepte : 06 u 2016 Pu is e : 26 u 2016 Role of Kekulé and Non-Kekulé Structures in the Radical Character of Alternant Polycyclic Aromatic Hydrocarbons:
www.nature.com/scientificreports OPEN recei e : 22 pri 2016 ccepte : 06 u 2016 Pu is e : 26 u 2016 Role of Kekulé and Non-Kekulé Structures in the Radical Character of Alternant Polycyclic Aromatic Hydrocarbons:
Chapter 3. Stabilizing Effects in Hydrocarbon Chemistry. The goal of this chapter: Iden=fy the presence of strained stabilized systems
 Stabilizing Effects in ydrocarbon Chemistry The goal of this chapter: Iden=fy the presence of strained stabilized systems Predict quan=ta=ve values of strain/stabiliza=on based on chemical equa=ons or
Stabilizing Effects in ydrocarbon Chemistry The goal of this chapter: Iden=fy the presence of strained stabilized systems Predict quan=ta=ve values of strain/stabiliza=on based on chemical equa=ons or
240 Chem. Aromatic Compounds. Chapter 6
 240 Chem Aromatic Compounds Chapter 6 1 The expressing aromatic compounds came to mean benzene and derivatives of benzene. Structure of Benzene: Resonance Description C 6 H 6 1.It contains a six-membered
240 Chem Aromatic Compounds Chapter 6 1 The expressing aromatic compounds came to mean benzene and derivatives of benzene. Structure of Benzene: Resonance Description C 6 H 6 1.It contains a six-membered
Chemistry 217 Problem Set 1
 hemistry 217 Problem Set 1 Recommended Problems from the Book (1 st ed. in parentheses): 1.1, 1.3-1.9, 1.36-1.42, 1.57-1.62 (1.1-1.8, 1.34-1.40, 1.54-1.58) Supplemental Problems: Lewis structures: Weeks,
hemistry 217 Problem Set 1 Recommended Problems from the Book (1 st ed. in parentheses): 1.1, 1.3-1.9, 1.36-1.42, 1.57-1.62 (1.1-1.8, 1.34-1.40, 1.54-1.58) Supplemental Problems: Lewis structures: Weeks,
Page 2. Q1.Consider the five cyclic compounds, A, B, C, D and E. The infrared spectra of compounds A, B, C and D are shown below.
 Q1.Consider the five cyclic compounds, A, B, C, D and E. (a) The infrared spectra of compounds A, B, C and D are shown below. Write the correct letter, A, B, C or D, in the box next to each spectrum. You
Q1.Consider the five cyclic compounds, A, B, C, D and E. (a) The infrared spectra of compounds A, B, C and D are shown below. Write the correct letter, A, B, C or D, in the box next to each spectrum. You
Structures of the Molecular Components in DNA and RNA with Bond Lengths Interpreted as Sums of Atomic Covalent Radii
 The Open Structural Biology Journal, 2008, 2, 1-7 1 Structures of the Molecular Components in DNA and RNA with Bond Lengths Interpreted as Sums of Atomic Covalent Radii Raji Heyrovska * Institute of Biophysics
The Open Structural Biology Journal, 2008, 2, 1-7 1 Structures of the Molecular Components in DNA and RNA with Bond Lengths Interpreted as Sums of Atomic Covalent Radii Raji Heyrovska * Institute of Biophysics
2. Hydrocarbons. 2.1 Composition of Petroleum
 2. Hydrocarbons 2.1 Composition of Petroleum Naturally occurring petroleum is composed of organic chemicals: approximately 11 to 13% hydrogen and 84 to 87% carbon. Traces of oxygen, sulfur, nitrogen and
2. Hydrocarbons 2.1 Composition of Petroleum Naturally occurring petroleum is composed of organic chemicals: approximately 11 to 13% hydrogen and 84 to 87% carbon. Traces of oxygen, sulfur, nitrogen and
18.1 Intro to Aromatic Compounds
 18.1 Intro to Aromatic Compounds AROMATIC compounds or ARENES include benzene and benzene derivatives. Aromatic compounds are quite common. Many aromatic compounds were originally isolated from fragrant
18.1 Intro to Aromatic Compounds AROMATIC compounds or ARENES include benzene and benzene derivatives. Aromatic compounds are quite common. Many aromatic compounds were originally isolated from fragrant
