Discovery and Optimization of Small-Molecule Ligands for the CBP/ p300 Bromodomains
|
|
|
- Bryan Chapman
- 5 years ago
- Views:
Transcription
1 pubs.acs.org/jacs Terms of Use CC-BY Downloaded via on January 27, 2019 at 03:28:13 (UTC). See for options on how to legitimately share published articles. Discovery and Optimization of Small-Molecule Ligands for the CBP/ p300 Bromodomains Duncan A. Hay,, Oleg Fedorov,, Sarah Martin,, Dean C. Singleton,, Cynthia Tallant,, Christopher Wells,, Sarah Picaud, Martin Philpott,, Octovia P. Monteiro,, Catherine M. Rogers,, Stuart J. Conway, Timothy P. C. Rooney, Anthony Tumber,, Clarence Yapp,, Panagis Filippakopoulos, Mark E. Bunnage, Susanne Mu ller,, Stefan Knapp,, Christopher J. Schofield, and Paul E. Brennan*,, Department of Chemistry, University of Oxford, South Parks Road, Oxford OX1 3TA, U.K. Structural Genomics Consortium, University of Oxford, Old Road Campus Research Building, Roosevelt Drive, Oxford, OX3 7DQ, U.K. Target Discovery Institute, University of Oxford, NDM Research Building, Roosevelt Drive, Oxford, OX3 7LD, U.K. Worldwide Medicinal Chemistry, Pfizer, Cambridge, Massachusetts 02139, United States *S Supporting Information ABSTRACT: Small-molecule inhibitors that target bromodomains outside of the bromodomain and extra-terminal (BET) sub-family are lacking. Here, we describe highly potent and selective ligands for the bromodomain module of the human lysine acetyl transferase CBP/p300, developed from a series of 5-isoxazolyl-benzimidazoles. Our starting point was a fragment hit, which was optimized into a more potent and selective lead using parallel synthesis employing Suzuki couplings, benzimidazole-forming reactions, and reductive aminations. The selectivity of the lead compound against other bromodomain family members was investigated using a thermal stability assay, which revealed some inhibition of the structurally related BET family members. To address the BET selectivity issue, X-ray crystal structures of the lead compound bound to the CREB binding protein (CBP) and the first bromodomain of BRD4 (BRD4(1)) were used to guide the design of more selective compounds. The crystal structures obtained revealed two distinct binding modes. By varying the aryl substitution pattern and developing conformationally constrained analogues, selectivity for CBP over BRD4(1) was increased. The optimized compound is highly potent (K d = 21 nm) and selective, displaying 40-fold selectivity over BRD4(1). Cellular activity was demonstrated using fluorescence recovery after photo-bleaching (FRAP) and a p53 reporter assay. The optimized compounds are cell-active and have nanomolar affinity for CBP/p300; therefore, they should be useful in studies investigating the biological roles of CBP and p300 and to validate the CBP and p300 bromodomains as therapeutic targets. INTRODUCTION CBP and p300. The CREB (cyclic-amp response element binding protein) binding protein (CBP) and E1A binding protein (p300) are ubiquitously expressed pleiotropic lysine acetyl transferases that play a key role as transcriptional coactivators in human cells. 1 5 CBP and p300 possess nine conserved functional domains which bind to general and genespecific transcription factors such as the hypoxia-inducible transcription factor (HIF) and the human tumor suppressor protein p53 (Figure 1A) Both CBP and p300 possess a single bromodomain (BRD) and a lysine acetyltransferase (KAT) domain, which are involved in the post-translational modification (PTM) and recruitment of histones and nonhistone proteins The BRD is an acetyl-lysine (Kac)- selective recognition module, whereas the KAT domain transfers an acetyl group from acetyl co-enzyme A to unmodified lysine side chains. These processes enable CBP/ p300 to exert context-dependent regulation of transcriptional control. The sequence similarity between CBP and p300 is high in the conserved functional domains, with the BRD having 96% similarity (Figure 1A). CBP and p53. Mutations of the p53 gene are common, with around 50% of human cancers encoding such mutations In response to cellular stress, p53 undergoes PTMs of the C- and N-terminal regions, including acetylation at the C-terminal region, which results in changes in the p53-dependent activation of target genes leading to cell cycle arrest, senescence, or apoptosis Lysine acetylation at lysine 382 (K382) of p53 is responsible for recruitment of CBP via its Received: December 12, 2013 Published: June 19, American Chemical Society 9308
2 Figure 1. (A) Percent conservation and domain organization of human CBP (accession no. Q92793) and p300 (accession no. Q09472). Abbreviations: ZF TAZ 1 and 2, zinc finger transcription adaptor putative zinc finger-type; KIX, kinase-inducible domain interacting domain; BRD, bromodomain; RING, really interesting new gene; PHD, plant homeodomain; KAT, lysine acetyltransferase domain; ZF ZZ, zinc finger, ZZ-type; NRC, nuclear receptor co-activator interlocking domain; CH1 3, cysteine histidine-rich regions 1 3. (B) CBP BRD-fold depicting the four α- helices (αz, αa, αb, αc) and the ZA and BC loops that form the Kac binding pocket (derived from PDB 3P1C). 27 (C) Structure of an acetyl lysine (yellow, only partial structure shown) and CBP BRD complex. Key interactions are shown, including the hydrogen bond (dotted lines) from the Kac carbonyl to N1168 and a water-mediated hydrogen bond from the Kac carbonyl to Y1125 (PDB 3P1C). 27 (D) CBP BRD ligands with reported affinities BRD, as shown by NMR titration of the CBP BRD with acetylated p53 peptides, and transfection of p53 / cells with mutated p Additionally, in a p21 luciferase assay, the CBP- Kac interaction was shown to be crucial for p53-induced p21- mediated G 1 cell cycle arrest. Additionally, chemo- and radio-therapy can cause p53- mediated tissue damage of non-cancerous tissue, implying that p53 inhibition could be used to protect healthy tissue during these therapies. 26 Thus, inhibition of the CBP BRD, and therefore p53-mediated p21 activation, has potential clinical applications. In addition to its important tumor suppressor role, hyperactive p53 is implicated in Alzheimer s disease, Parkinson s disease, spinal cord diseases, multiple sclerosis, ischemic brain injury, infectious and auto-immune diseases, and myocardial ischemia Thus, inhibitors of p53 activity represent potential points of intervention in multiple diseases. The CBP/p300 Bromodomain. Bromodomains are made up of 110 residues arranged in a characteristic structure made up of four α-helices (αz, αa, αb, αc) connected by interhelical loops, termed the BRD fold (Figure 1B). Two loop regions (ZA and BC) form the largely hydrophobic Kac side-chain binding pocket, which in most BRDs binds the Kac N-acetyl group via a hydrogen bond between the acetyl carbonyl and the NH 2 of a well-conserved asparagine residue (N1168 in CBP) and a water-mediated hydrogen bond from the acetyl carbonyl to the phenolic hydroxyl group of a conserved tyrosine (Y1125 in CBP) (Figure 1C). 27,38 42 The 61 known BRDs encoded by the human genome can be clustered into sub-families on the basis of sequence and structural similarity. 27 Selective inhibitors of these sub-families 9309
3 could serve as tools to elucidate the function of these proteins, and these have started to emerge in recent years. In particular, potent and selective inhibitors of the BRD and extra-terminal (BET) sub-family are now available from several different structural classes Pioneering work on the inhibition of the CBP BRD emerged from the Zhou group, which reported several compounds with micromolar affinities (Figure 1D) The N-acetylindole MS7972 (compound 1, K d = 19.6 μm) was shown to block the p53 CBP interaction at 50 μm in a competition assay. 28 Ischemin (compound 2, K d =19μM) inhibited p53-induced p21 activation in a luciferase reporter-gene assay (IC 50 =5μM) and down-regulated p53 target gene expression under oxidative stress conditions. 29 The cyclic peptide 3 has also been shown to bind the CBP BRD (K d =8μM) and to inhibit p53 in the reporter assay. 30 These compounds demonstrated the potential of CBP BRD inhibitors to modulate p53-mediated expression; however, they are not very potent or well characterized in terms of selectivity against other BRD-containing proteins. Recent reports also describe potent but non-selective or moderately selective CBP BRD inhibitors, including the sub-micromolar dihydroquinoxalinone inhibitor 4. 31,50 We now report on the discovery of a series of potent and selective CBP/p300 BRD inhibitors, and we demonstrate their inhibition of the CBP p53 interaction in cells. In addition, we demonstrate how compounds from the series bind in the Kac binding pocket using X-ray crystallography. Our starting point for developing selective CBP/p300 inhibitors was the reported non-selective 3,5-dimethylisoxazole BRD inhibitor, 5 (Figure 2). 43 Compound 5 was considered to RESULTS AND DISCUSSION An X-ray crystal structure of the reported dimethylisoxazole compound 7 in complex with the CBP BRD illustrated two potential regions which substituted analogues of 5 could interact with, potentially leading to improvements in potency and selectivity (Figure 3). 43 Figure 3B shows how the dimethylisoxazole of compound 7 mimics the key Kac binding interactions of the CBP BRD with an H-bond to N1168 and a water-mediated H-bond to Y1125. Figure 3C highlights the two regions targeted for analogues of 5. Region 1 is comprised of part of the ZA-channel and is largely hydrophobic, except for Figure 2. Starting point for this project. Compound 5 is a nonselective CBP and BRD4(1) inhibitor. 43 be an attractive fragment to develop CBP BRD-selective inhibitors because it has a low molecular weight (213 Da) and reasonable LipE 51 (3.5) and ligand efficiency (0.45) 52,53 for CBP, and because it has various points useful for the introduction of diversity to the core scaffold. Our aim was to develop the scaffold of compound 5 with the goal of achieving potent compounds (K d 0.1 μm) with selectivity over other BRD sub-families ( 30-fold) in vitro and displaying targetbased cellular activity (IC 50 1 μm) to enable functional studies in cellular systems. 47,54 Several reports describe 1,3-dimethylisoxazoles as potent inhibitors of the BET BRDs, including the benzimidazole compound 6. 43,45,46,55 Therefore, it was recognized that obtaining selectivity for CBP/p300 over the BET BRDs could represent a substantial challenge. Compounds would therefore initially be screened against both CBP and BRD4(1) BRDs as representative examples of their respective subfamilies. All in vitro screening and X-ray crystallography was carried out using recombinant BRDs as surrogates of full-length protein. Figure 3. (A) Compound (B) View from an X-ray crystal structure of compound 7 (carbon = yellow) bound to CBP BRD showing key H-bond interactions (PDB 4NR4). Kac (carbon = magenta) from PDB 3P1C is overlaid for reference. The dimethylisoxazole group is positioned to form an H-bond (gray dotted lines) to N1168 and a water-mediated H-bond to Y1125. The N-3 of the benzimidazole is positioned to form a water-mediated H-bond to the carbonyl of P1114. (C) Surface representation of CBP complexed with compound 7 with two shaded regions marked as potential areas to introduce functionality onto the benzimidazole scaffold in order to enhance potency and selectivity. Region 1 (blue) comprises the ZA channel which has points for interaction with polar functionality, such as the carbonyl on P1114, or the carboxamide of Q1113. Region 2 (green) is analogous to the WPF shelf in BRD4(1). R1173 was identified as a potential residue to interact with in an otherwise hydrophobic region. 9310
4 backbone carbonyls and the carboxamide of Q1113. Region 2 is analogous to the WPF shelf of BRD4(1). 56 The surface is mostly hydrophobic but also has the side-chain of R1173 as a potential site for ligand interactions that could give selectivity for CBP over BRD4(1). On the basis of this analysis, it was anticipated that analogues of compound 5 which possessed substitution on the N-1 and C-2 may be able to interact with regions 1 and 2, with the aim of improving potency and selectivity. However, in initially library chemistry, other substitution patterns were also included in anticipation that unexpected interactions may occur. Library Chemistry. In order to identify more potent and selective leads for the CBP/p300 BRD, a set of Suzuki couplings was carried out in parallel with a commercially acquired 3,5-dimethylisoxazole-4-boronic acid pinacol ester and a set of heteroaryl bromides (Scheme 1). The heteroaryl using parallel synthesis for the coupled reduction/cyclization step (Scheme 2). Dithionite-mediated reduction of the aryl Scheme 2. Synthesis of Trisubstituted Benzimidazoles a Scheme 1. Parallel Suzuki Couplings a a Reagents and conditions: 3,5-dimethylisoxazole-4-boronic acid pinacol ester, Pd(dppf)Cl 2 [dppf = 1,1 -bis(diphenylphosphino)- ferrocene], NaHCO 3, 1,2-dimethoxyethane (DME)/H 2 O, 100 C, 5 93%. See Supporting Information (SI) for specific structures of products s1 s82. bromides were chosen from the Pfizer compound collection and were selected so that products obtained would have Lipinski rule of 5 compliant properties (e.g., clog P < 5 and MW < 500), with the majority of the compounds targeted at around clog P =2 4 and MW = The set chosen consisted of 101 heteroaryl bromides, which had a variety of substituents on the heterocyclic core in order to maximize diversity. Reactions and workups were carried out in parallel, and products were purified by automated preparative HPLC. Product purity was assessed by UV, evaporative light scattering detection, and MS. The 101 reactions delivered 83 target compounds in sufficient yield and purity for biochemical testing using differential scanning fluorimetry (DSF, ΔT m ), a highthroughput assay used as a surrogate for displacement assays. 47 From this initial library, compound 8 emerged as a promising hit, with ΔT m = 4.5 C against CBP and 3.2 C against BRD4(1) at a compound concentration of 10 μm (Figure 4). To improve potency and selectivity for CBP, analogues of compound 8 varied in the C-2 position were then prepared Figure 4. CBP-selective hits from initial parallel chemistry. Mean values ± SEM (number of measurements). a Reagents and conditions: (a) 3,5-dimethylisoxazole-4-boronic acid pinacol ester, Pd(dppf)Cl 2, NaHCO 3, DME/H 2 O, 73%; (b) N,Ndimethylethylenediamine, N,N-diisopropylethylamine (DIPEA), tetrahydrofuran (THF), 86%; (c) RCH 2 CH 2 CHO, Na 2 S 2 O 4,H 2 O, EtOH, dimethyl sulfoxide (DMSO), 80 C, 8 54% (see SI for specific structures of products s83 s100); (d) NH 2 CH 2 CH(OMe) 2, DMSO; (e) PhCH 2 CH 2 CHO, Na 2 S 2 O 4,H 2 O, MeOH, 65% (two steps); (f) CF 3 CO 2 H, H 2 O, CH 2 Cl 2, microwave 150 C, 99%; (g) amine (RH), NaBH(OAc) 3, THF, 35 86%; (h) Amine (RH), NaCNBH 3, AcOH, MeOH, 42%; (i) 4-(2-aminoethyl)morpholine, DIPEA, THF, 94%; (j) Na 2 S 2 O 4, H 2 O, EtOH, 80 C, 87%; (k) RCH 2 CH 2 CO 2 H, T3P, DIEPA, EtOAc, microwave, 150 C, 31 62% (l) RCH 2 CH 2 CO 2 H, T3P, DIPEA, EtOAc, reflux, 15 63%; (m) RCH 2 CH 2 CO 2 H, 6 M aq. HCl, microwave 210 C, 16 55% (see SI for specific structures of s101 s116). nitro group of compound 11 in the presence of R-aldehydes efficiently afforded the desired benzimidazole targets which were purified by automated HPLC. The most promising compound from this set was compound 12, with an increased ΔT m = 6.3 C (4.5 C for compound 8) against CBP while maintaining BRD4(1) potency at ΔT m = 2.9 C (3.2 C for compound 8) (Figure 4). A pic 50 = 6.3 was measured for compounds 8 and 12, using a peptide displacement AlphaScreen (amplified luminescent proximity homogeneous assay screen). 58 The CBP potency represents a significant improvement on compound 5 (pic 50 = 5.4). These results implied that differential 1,2,-disusbtitution of benzimidazoles could lead to compounds with improved potency and selectivity. It was therefore decided to optimize the substituents on this template via focused synthesis to further improve potency and selectivity. N-1 Amine Optimization. Late-stage variation of the amino moiety was achieved by reductive amination of an aldehyde, obtained from the dimethyl acetal 13 (Scheme 2). The compounds obtained were screened by DSF and AlphaScreen (Table 1). Comparison of the screening 9311
5 Table 1. Structure Activity Relationships for CBP and BRD4(1) Binding As Determined by DSF and AlphaScreen for N-1 Analogues a a Values are given as mean ± SEM (number of measurements). techniques confirmed that DSF was an effective tool for ranking the potency of compounds. 59 The correlation was high enough that the general and operationally simple DSF assay was felt to be a useful surrogate for the more complex AlphaScreen in further efforts to increase the potency of the compounds. The results suggest that simple cyclic amino analogues (CBP ΔT m = C, pic 50 = ) are not as potent as the dimethylamino variant 12 (CBP ΔT m = 6.3 C, pic 50 = 6.3). However, the morpholine-containing analogue 17 gave a slight improvement with ΔT m values of 6.5 and 2.7 C for CBP and BRD4(1) respectively and was potent in CBP AlphaScreen (pic 50 = 6.8). Piperazines 18 and 19, thiomorpholine 20, and substituted morpholine derivative 21 were less potent and/or less selective for CBP over BRD4(1). With the best balance of CBP potency and BRD4(1) selectivity in the primary assays, the binding constants (K d ) of compound 17 were then determined using isothermal titration calorimetry (ITC) (Table 2). The K d value of 17 was measured at 0.32 μm against CBP, 0.35 μm against p300, and 0.94 μm against BRD4(1). These results indicate that the ITC measured selectivities of compound 17 with respect to BRD4(1) were only 3.0-fold and 2.7-fold for CBP and p300, respectively. This Table 2. ITC Results for Compound 17 CBP p300 BRD4(1) K d (μm) 0.32 ± ± ± showed that further work needed to be done in order to obtain sufficient selectivity for CBP/p300. Selectivity Optimization. In order to identify potential avenues for improving the selectivity of the series, highresolution crystal structures were determined for compound 17 in complex with CBP and BRD4(1) (Figure 5). The results suggested that differences in the observed binding modes could be exploited to improve selectivity. In the CBP complex, the morpholine moiety of compound 17 occupies an area in the ZA channel, between the targeted regions 1 and 2. The phenethyl group of compound 17 appears to be in a hydrophobic region on the edge of the pocket. In BRD4(1), compound 17 adopts a flipped binding mode with respect to side-chain orientation; notably, a water-mediated hydrogen bond from the NH 2 of the Q85 carboxamide to the benzimidazole N-3 nitrogen is apparent. The morpholine moiety points out toward solvent, whereas the phenethyl group fits into a hydrophobic region comprising the WPF shelf and ZA channel of BRD4(1). C-2 Aryl Optimization. As the phenylethyl moiety of compound 17 was observed to occupy different regions in the CBP and BRD4(1) structures, it was decided to explore the synthesis of a diverse set of compounds with substituted aryl rings to test effects on selectivity. Additionally, it was considered that the C-2 linked aryl group would be amenable to late-stage variation, allowing for an efficient synthesis of analogues. The targeted compounds were synthesized according to Scheme 2. Thus, the phenylenediamine compound 22 was formed by an S N Ar reaction of compound 10 followed by dithionite-mediated nitro reduction. Precursor 22 was then reacted with substituted carboxylic acids under dehydrating conditions (propylphosphonic anhydride (T3P) or 6 M aqueous HCl) to yield the target compounds (Table 3) and s101 s116 (see SI). The DSF results for compounds (Table 3) suggest that an electron-donating methyl group on the aryl ring is tolerated, with the para-methyl analogue 25 showing an increased ΔT m for CBP of 7.4 C. The more strongly electron-donating para-methoxy group in compound 26 gave a further increase with ΔT m = 8.1 C, and showed a larger window over BRD4(1). Conversely, the strongly electronwithdrawing nitro groups in compounds 27 and 28 were detrimental to CBP binding. The results for compounds indicated that halogens are tolerated on the phenyl ring, with the meta-substituted fluorine analogue 30 appearing optimal, with ΔT m = 7.1 C against CBP. Compounds 32 and 33 combined the para-methoxy and meta-halogen moieties, resulting in a further increase in CBP ΔT m to 9.0 and 9.6 C, respectively. Analogues demonstrated that the phenyl group can be substituted for a heteroaryl group, with the electron-rich indole-containing compound 36 representing the optimal compound from this set with ΔT m = 8.9 C against CBP. It was encouraging that increases in CBP potency for the best compounds in Table 3 were not accompanied by increases in BRD4(1) potency. Since the four aryl analogues 26, 32, 33, and 36 all indicated improved potency in the ΔT m assay, the pic 50 against CBP was determined using AlphaScreen (Table 4). The results were in the range of , indicating that these were all promising analogues warranting more detailed biophysical investigations on their selectivity for CBP over BRD4(1). Thermodynamic parameters for binding to CBP and BRD4(1) were therefore determined by ITC for this set of compounds. The results are summarized in Table
6 Journal of the American Chemical Society Table 3. Structure Activity Relationships for CBP and BRD4(1) Binding As Determined by DSF Assay for a Selection of the C-2 Analoguesa Figure 5. Views from X-ray crystal structures of compound 17 complexed to CBP (PDB 4NR5) and BRD4(1) (PDB 4NR8) BRDs. For CBP: (A) view showing the H-bond interactions between the oxygen of the isoxazole of 17 and N1168 (3.02 Å), and between the nitrogen of the isoxazole and a water (2.75 Å) (water = red spheres), and (B) surface view with shaded regions indicating regions targeted by N-1 and C-2 benzimidazole substitution according to Figure 3. For BRD4(1): (C) view showing the H-bond interactions between the oxygen of the isoxazole of 17 and N140 (3.08 Å), and between the nitrogen of the isoxazole and a water (2.91 Å); the H-bond between the benzimidazole N-3 of 17 and a water molecule is also shown (2.71 Å), and W81 from other ligand-bound structures (carbon = magenta) is overlaid to illustrate the shift in W81 side-chain position (PDB 3MXF, 4E96, 4C67),47,49,60 and (D) surface view. a Values are given as mean ΔTm ± SEM (number of measurements). Gratifyingly, the substituted aryl analogues are more potent against CBP than compound 17, with Kd in the range of μm. Additionally, the selectivity for CBP over BRD4(1), as determined by ITC Kd values, had improved to fold for these analogues, with the best results being those of compound 36, which had a CBP Kd = 30 nm and was 22-fold selective over BRD4(1). Although the selectivity had improved, efforts continued in order to achieve a greater window over BRD4(1). To achieve this objective, the obtained crystal 9313
7 Table 4. Determination of pic 50 and K d of Selected C-2 Analogues by AlphaScreen and ITC ITC K d (μm) Cmpd a CBP AlphaScreen pic 50 CBP BRD4(1) Selectivity ± 0.17 (4) ± ± fold ± (2) ± ± fold ± 0.69 (2) ± ± fold ± 0.18 (2) ± ± fold a Values are given as mean pic 50 ± SEM (number of measurements). structures (Figure 5) were employed in order to further guide design. Indole Analogue. The structure of compound 17 complexed to BRD4(1) reveals a water-mediated hydrogen bond from the benzimidazole N-3 to the protein backbone (P82) and to the carboxamide side chain of Q85; analogous interactions are absent in the equivalent CBP structure (Figure 5A,C). These observations imply that, in the absence of other effects, replacing the benzimidazole ring with an indole that does not contain the nitrogen in compound 17 should negatively impact BRD4(1) binding, but not CBP. The synthesis of the targeted indole analogue 40 is shown in Scheme 3. Aminophosphonium salt 38 was synthesized Table 5. Structure Activity Relationships for CBP and BRD4(1) Binding As Determined by ΔT m Assay for the Indole Target and Conformationally Constrained/Polar C-2 Analogues Scheme 3 a a Reagents and conditions: (a) HNO 3 conc., H 2 SO 4 conc., 0 C, 39%; (b) PPh 3, CHCl 3, quant.; (c) (i) Zn/AcOH; (ii) HCl, quant. (2 steps); (d) 3-(3-chloro-4-methoxyphenyl)propanoyl chloride, N,Ndimethylformamide (DMF)/pyridine, 56%; (e) KOt-Bu, toluene, microwave 130 C, 56%; (f) 4-(2-chloroethyl)morpholine hydrochloride, NaH, KI, DMF, 80 C, 24%, (g) 3,5-dimethylisoxazole-4- boronic acid pinacol ester, Pd(dppf)Cl 2, NaHCO 3, DME/H 2 O, 43%. according to known procedures 61 and then acylated. Basepromoted cyclization yielded the indole intermediate 39, which was alkylated and cross-coupled to give the target molecule 40. As hoped, indole 40 was completely inactive against BRD4(1) with a ΔT m <1 C (Table 5). Disappointingly, it also gave a significantly lower ΔT m for CBP (2.0 C) than the equivalent benzimidazole analogue 32. This implies that although the crystal structure of compound 17 bound to CBP shows no H-bond to the protein backbone, interaction of the electron-poor benzimidazole with the CBP protein is favored over that of the electron-rich indole. Conformationally Constrained Analogues. The ethylene moiety of compound 17, which links the benzimidazole C-2 and phenyl groups, sits in a hydrophobic region in BRD4(1) and partly occupies the space termed the WPF shelf, which contains a tryptophan residue protruding out of the pocket (Figure 5C,D). The orientation of W81 in BRD4(1) in the complex with compound 17 is unusual when compared to other BRD4(1) ligand-bound structures (Figure 5C) and shows a Values are given as mean ΔT m ± SEM (number of measurements). how BRD4(1) can accommodate hydrophobic groups in orientations other than those that occupy the typical WPF shelf. 47,48,55 It was envisaged that polar and/or constrained linkers could reduce the affinity for BRD4(1). An increase in polarity may disfavor binding in the hydrophobic WPF region, while BRD4(1) may be less able to accommodate conforma- 9314
8 tionally constrained linkers with reduced degrees of freedom, as they would be less able to avoid a steric clash with the WPF shelf by bond rotation. Synthesis of the oxygen-linked targets is shown in Scheme 4. 1,1 -Carbonyldiimidazole (CDI)-mediated cyclization of Scheme 4. Synthesis of O-Linked Targets a a Reagents and conditions: (a) CDI, THF, reflux, 78%; (b) BnBr, Ag 2 CO 3, toluene, 80 C (18%); (c) 2-hydroxyacetic acid, 6 M aq. HCl, microwave 180 C, 62%; (d) 3-fluoro-4-methoxyphenol, 1,1 - (azodicarbonyl)dipiperidine, P(n-Bu) 3,CH 2 Cl 2, 67%. modification of the N-1 ethylene linker between the morpholine moiety and the N-1 position of the benzimidazole ring. It was again proposed that by constraining the conformation of the linker it would force unfavorable interactions in BRD4(1) due to steric interactions with the WPF shelf or by changing the orientation of the phenethyl group (Figure 5). Racemic analogues containing methyl groups on the N-1 ethylene linker (compounds 52 55) were prepared according to the methodology in Scheme 2. Screening results for these compounds are shown in Table 6. While methyl groups were not well tolerated next to the benzimidazole ring (compounds 52 and 54), they were tolerated next to the morpholine ring (compounds 53 and 55). The ΔT m and AlphaScreen values for the racemic compounds were encouraging enough to prompt synthesis of the single enantiomers, according to the route shown in Scheme 5. Commercially acquired chiral (R)- and Scheme 5. Route to Single Enantiomers a phenyleneiamine 22 gave a 2-oxo precursor. Alkylation tended to favor N-substitution, but use of Ag 2 CO 3 as base gave a mixture of N- and O-alkylated isomers, which could be separated to yield the desired target 41. Compound 22 was also used to prepare a hydroxymethyl precursor, which was reacted with a phenol using Tsunodu s Mitsunobu conditions to yield target compound Analogues 43 51, which possess additional substitution or conformational constraints on the ethylene moiety linking the benzimidazole and aryl groups, were synthesized by methodology analogous to that described in Scheme 2 (see SI). Disappointingly, screening using DSF showed no improvement in potency and selectivity for the O-linked analogues (41, 42) or conformational constrained compounds (43 51) over the analogous ethylene linked compounds (Table 5). With no indication of an improvement in selectivity, attention shifted to a Reagents and conditions: (a) (R)- or (S)-1,2-diaminopropane dihydrochloride, K 2 CO 3, DMF, 80 C, 38 41%; (b) 2-bromoethyl ether, K 2 CO 3, DMF, 70 C, 27 37%; (c) Na 2 S 2 O 4,H 2 O, EtOH, 80 C; (d) R 2 CH 2 CH 2 CO 2 H, T3P, EtOAc, reflux, 38 52%. (S)-1,2-diaminopropane reacted via S N Ar with 10, predominantly at the less sterically hindered 1-amino group. This reaction gave an inseparable 4:1 mixture of isomers in favor of the desired compound. The morpholine ring was formed by Table 6. Structure Activity Relationships for CBP and BRD4(1) Binding As Determined by DSF and AlphaScreen for Conformationally Constrained N-1 Linkers R ΔT m ( C) a Cmpd CBP BRD4(1) CBP AlphaScreen pic 50 a 52 A CH 3 H 5.8 ± (3) 1.9 ± 0.27 (2) ND 53 A H CH ± 0.58 (5) 2.0 ± 0.33 (3) 7.6 ± (2) 54 B CH 3 H 5.6 ± 0.29 (3) 1.2 ± (2) ND 55 B H CH ± 0.44 (5) 2.4 ± 0.25 (3) 7.0 ± 0.15 (2) 58 A H (R)-CH ± 0.10 (3) 2.0 ± (2) 6.3 ± 0.10 (2) 59 A H (S)-CH ± 0.31 (4) 1.8 ± 0.71 (4) 7.1 ± (2) 60 B H (R)-CH ± 0.21 (3) 2.3 ± 0.25 (2) 6.3 ± (2) 61 B H (S)-CH 3 11 ± 0.17 (3) 3.3 ± 0.57 (2) 7.3 ± (2) 62 C H (R)-CH ± (3) 3.4 ± 0.54 (2) 6.7 ± (2) 63 C H (S)-CH 3 10 ± 0.11 (3) 2.3 ± 0.25 (2) 7.2 ± (2) a Compounds are racemic except where indicated. Values are given as mean ± SEM (number of measurements). 9315
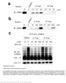
9 alkylation with 2-bromoethyl ether. At this stage, the isomeric compounds could be separated by chromatography. Nitroreduction and benzimidazole formation yielded the target compounds in >99% ee, as determined by chiral HPLC. Gratifyingly, the (R)- and (S)-enantiomers displayed clearly different affinities for CBP, with the (S)-form giving a large ΔT m of around 10 C and high potency (pic 50 > 7.0) in AlphaScreen for the three aryl variants tested (compounds 59, 61, and 63). The (S)-enantiomers were therefore analyzed by ITC, and the results are shown in Table 7. The K d for these Table 7. ITC Determination of K d for the Binding of (S)- Methyl Analogues to CBP and BRD4(1) ITC K d (μm) Cmpd CBP BRD4 CBP selectivity ± ± a 40-fold 5.2 ± 0.14 b 250-fold ± ± a 20-fold ± ± a 16-fold a BRD4(1). b BRD4(2). analogues indicated high potency ( μm). The most selective compound, 59, was shown to be 40-fold selective for CBP over BRD4(1) and 250-fold over BRD4(2). Compound 59 was also potent against p300, with K d = μm (see SI for details). Introducing a chiral methyl onto the morpholino-ethylene moiety had the desired effect of improving selectivity over BRD4(1) while maintaining CBP/ p300 potency, and validated the design strategy of introducing conformational restraints into the ethylene linker of the target compounds. Compound 59 was crystallized with CBP (Figures 6). Although the binding mode is similar to that observed for compound 17 (Figure 5A,B), the orientation of the ethylenelinked aryl group is different. In the complex with CBP and 59, there is an apparent cation π interaction between the guanidino group of R1173 and the aryl ring. This is made possible because the R1173 side chain moves with respect to the conformation observed in the complex with compound 17, forming an induced binding pocket for the aryl ring (Figure 6B). This is consistent with the observation that electrondonating groups on the aryl ring were preferred for CBP binding, as these should have a stronger interaction with the positively charged R1173. The chlorine atom on the aryl ring of 59 sits in a hydrophobic section of the induced pocket between V1174 and F1177. The chiral methyl group sits below the aryl ring, possibly helping to lock the ring in position. The induced pocket has been reported for another series of CBP inhibitors which also form cation π interactions between the inhibitors and the R1173 side chain. The combined findings perhaps suggest that the interaction of aromatic groups with R1173 represents an important feature of potent CBP inhibitors. 31 In order to investigate the wider selectivity of inhibitors in the series, compounds 17 and 59, were screened against representative members of the other BRD sub-families using DSF (Figure 7). The values obtained correlated well with available AlphaScreen IC 50 values (Spearman rank correlation, ρ 0.94, see SI). In the DSF panel, both compounds were selective for CBP/p300 BRDs. Compound 59 was particularly selective, with no significant ΔT m (>2 C) against any other BRDs apart from the BETs: BRD2(1), BRD3(1), and BRD4(1) with ΔT m between 1 and 2 C Figure 6. (A) View from X-ray crystal structure of compound 59 (carbon = yellow) complexed to CBP, showing the H-bond interaction between the oxygen of the isoxazole of 59 and N1168 (3.08 Å), and the nitrogen of the isoxazole and a water molecule (2.83 Å) (water molecules are red spheres) (PDB 4NR7). (B) Surface view from same with shaded regions indicating regions which were targeted by N-1 and C-2 benzimidazole substitution according to the strategy described in Figure 3. Overlaid with crystal structure of compound 17 (carbon = magenta) complexed to CBP. The aryl group of compound 59 and R1173 form an apparent cation π interaction. Cellular Assays. On-target cellular efficacy for the CBP BRD was investigated using a fluorescence recovery after photo-bleaching (FRAP) assay (Figure 8A). 63 HeLa cells transfected with a construct encoding a green fluorescent protein (GFP)-tagged multimerized (3 ) CBP BRD showed a rapid recovery time (t 1/2 = 0.59 s) upon photobleaching of a small area of the nucleus. The broad-spectrum histone deacetylase inhibitor, SAHA, was used to increase the extent of global lysine acetylation, so increasing recovery time (t 1/2 = 0.79 s) and expanding the assay window. An equivalent increase in the recovery time was not observed in cells transfected with a CBP BRD construct carrying the N1168F mutation, (see SI, Figure S1), consistent with the critical role of N1168 in Kac binding, and supporting the proposal that the increase in assay window due to SAHA addition is due to BRD binding. Treatment of SAHA-treated cells with compound 59 at 0.1 μm was sufficient to reduce FRAP recovery times back to unstimulated levels (t 1/2 = 0.60 s), equivalent to the N1168F mutant. The effect is indicative of displacement of the CBP BRD from acetylated chromatin. The weaker and less selective CBP inhibitor, compound 17 and the BRD4(1)-selective inhibitor 6 were unable to significantly alter the FRAP at this concentration (t 1/2 = 0.66 and 0.74 s, respectively). Conversely, in an equivalent assay using GFP-tagged BRD4, only the BRD4-selective inhibitor 6 significantly affected FRAP recovery times, with CBP inhibitors 17 and 59 showing no significant effect at 0.1 μm (Figure 8B) demonstrating the specificity of the compounds on their respective targets.
10 Figure 7. Selectivity assessment of compounds 17 and 59 against human BRD families using DSF (ΔT m ) binding assays. The 10 screened targets are labeled in black on the phylogenetic tree of the human BRD family; BRDs that were not part of the screening panel are in gray. Figure 8. Time dependence of fluorescent recovery in the bleached area in Fluorescence Recovery After Photobleaching (FRAP) assays with GFPtagged 3 CBP BRD construct (A) and full-length BRD4 (B). Half-times of fluorescence recovery (t 1/2 ) are shown as bars, which are color-coded: blue, DMSO control; green, DMSO + SAHA; yellow, 0.1 μm BRD4(1)-selective inhibitor 6; orange, 0.1 μm compound 17; and red, 0.1 μm compound 59. N1168F mutant (red hashed). Bars represent the mean ± SEM t 1/2 calculated from two or three independent experiments. Significance of groups compared with the control was determined by t-tests: *p < 0.05, **p < 0.01, ****p < (C) Inhibition of p53-driven luciferase activity by compound 59. RKO cells were transfected with p53 reporter plasmid. Cells were treated with compound 59 at the indicated concentrations for 24 h and subsequently with doxorubicin at 0.3 μm for 16 h. Each value is the mean ± SEM of a representative experiment done in eight replicates. To investigate the effect of compound 59 on the CBP p53 association in a cellular context, a luciferase reporter assay for p53 induction was used. Doxorubicin induced p53 activity was effectively inhibited by compound 59 in a dose-dependent manner (IC 50 = 1.5 μm) (Figure 8C). These results suggest that the CBP BRD inhibitor 59 inhibits the CBP co-activation of p53 target genes in cells and demonstrate the utility of 59 in a cellular context. The effect on p53 regulation by compound 59 is most likely due to its CBP inhibition, not its weaker BRD4 inhibition, as the much more potent BRD4 inhibitor, JQ1, shows p53-mediated effects at similar concentrations. 64,65 However, it was not possible to analyze the effects of less selective CBP inhibitors in the p53 reporter gene assay due to the confounding effects of BRD4 inhibition on p53. 64,66 To test if CBP BRD inhibition was cytotoxic at the concentrations where on-target efficacy was observed, U2OS osteosarcoma cells were treated with compound 59 for 24 h, and cell viability was determined using a standard 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) turnover assay (see SI, Figure S2). This showed that compound 59 had modest cytotoxicity (CC 50 =80μM), well above the levels where on-target efficacy was observed in the FRAP and p53 reporter gene assays. After 72 h of treatment with compound 59, cytotoxicity in U2OS cells increased (CC 50 =
11 μm), consistent with the effect of BRD4 inhibition at higher concentration as previously reported. 67 In order to investigate if compound 59 could also serve as a probe in animals, it was tested in in vitro ADME (absorption, distribution, metabolism, and excretion) assays (see SI, Table S6). In a human liver microsome (HLM) stability assay, no compound was detected after 60 min, implying that the metabolism of compound 59 may be too rapid for it to be useful as an oral in vivo probe. The selectivity of compound 59 against other target classes was assessed using wide ligand profiling (see SI, Table S7). 68 When tested against 136 GPCR, ion channel, enzyme, and kinase targets, compound 59 showed IC 50 <1μMonly for the adrenergic receptors α2c (0.11 μm) and α2a (0.57 μm), phosphodiesterase-5 (PDE5) (0.15 μm), and platelet-activating factor (PAF) (0.54 μm). CONCLUSIONS In summary, potent, selective, and cell-active inhibitors of the CBP/p300 BRD have been described. There is a lack of potent and selective inhibitors that target bromodomains outside the BET sub-family. The optimal compound, 59, is a highly potent inhibitor of the CBP and p300 BRDs (K d = and μm, respectively) and is 40-fold selective for CBP over BRD4(1), and highly selective over the other BRD sub-family members screened. In cells, 59 inhibits CBP-mediated p53 activity in a luciferase-based reporter assay and has low cytotoxicity. Compound 59 is expected to be useful in furthering the understanding of the role of the CBP/p300 BRD in transcriptional regulation. In the context of p53, compound 59 could serve to validate the potential of CBP BRD inhibitors as a clinical approach in the treatment of disorders related to hyperactive p53 transcription and could serve as a starting point for developing bioavailable in vivo probes and clinical candidates. ASSOCIATED CONTENT *S Supporting Information Experimental procedures and characterization data. This material is available free of charge via the Internet at pubs.acs.org. AUTHOR INFORMATION Corresponding Author paul.brennan@sgc.ox.ac.uk Notes The authors declare no competing financial interest. ACKNOWLEDGMENTS The SGC is a registered charity (number ) that receives funds from AbbVie, Bayer, Boehringer Ingelheim, the Canada Foundation for Innovation, the Canadian Institutes for Health Research, Genome Canada, GlaxoSmithKline, Janssen, Lilly Canada, the Novartis Research Foundation, the Ontario Ministry of Economic Development and Innovation, Pfizer, Takeda, and the Wellcome Trust [092809/Z/10/Z]. P.F. and S.P. are supported by a Wellcome Trust Career-Development Fellowship (095751/Z/11/Z). The authors thank the European Union, the Biotechnology and Biological Sciences Research Council (BBSRC), and the British Heart Foundation for funding. We also thank Diamond Light Source for beamtime (proposals mx8421 and mx6391), and the staff of 9318 beamlines I02 and I24 for assistance with crystal testing and data collection; Cerep for ligand profiling and ADME assays; Prof. Darren Dixon and Peter Clark for help with ee determinations; and Yue Zhu at Changchun Discovery Sciences Ltd for synthesis of selected monomers. REFERENCES (1) Goodman, R. H.; Smolik, S. Genes Dev. 2000, 14, (2) Vo, N.; Goodman, R. H. J. Biol. Chem. 2001, 276, (3) Kalkhoven, E. Biochem. Pharmacol. 2004, 68, (4) Chan, H. M.; La, T. N. B. J. Cell Sci. 2001, 114, (5) Bedford, D. C.; Kasper, L. H.; Fukuyama, T.; Brindle, P. K. Epigenetics 2010, 5, (6) Arany, Z.; Huang, L. E.; Eckner, R.; Bhattacharya, S.; Jiang, C.; Goldberg, M. A.; Bunn, H. F.; Livingston, D. M. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, (7) Ebert, B. L.; Bunn, H. F. Mol. Cell. Biol. 1998, 18, (8) Kallio, P. J.; Okamoto, K.; O Brien, S.; Carrero, P.; Makino, Y.; Tanaka, H.; Poellinger, L. EMBO J. 1998, 17, (9) Ema, M.; Hirota, K.; Mimura, J.; Abe, H.; Yodoi, J.; Sogawa, K.; Poellinger, L.; Fujii-Kuriyama, Y. EMBO J. 1999, 18, (10) Carrero, P.; Okamoto, K.; Coumailleau, P.; O Brien, S.; Tanaka, H.; Poellinger, L. Mol. Cell. Biol. 2000, 20, (11) Kung, A. L.; Wang, S.; Klco, J. M.; Kaelin, W. G.; Livingston, D. M. Nat. Med. 2000, 6, (12) Mujtaba, S.; He, Y.; Zeng, L.; Yan, S.; Plotnikova, O.; Sachchidanand; Sanchez, R.; Zeleznik-Le, N. J.; Ronai, Z.; Zhou, M. M. Mol. Cell 2004, 13, (13) Grossman, S. R. Eur. J. Biochem. 2001, 268, (14) Avantaggiati, M. L.; Ogryzko, V.; Gardner, K.; Giordano, A.; Levine, A. S.; Kelly, K. Cell 1997, 89, (15) Delvecchio, M.; Gaucher, J.; Aguilar-Gurrieri, C.; Ortega, E.; Panne, D. Nat. Struct. Mol. Biol. 2013, 20, (16) Bannister, A. J.; Kouzarides, T. Nature 1996, 384, (17) Ogryzko, V. V.; Schiltz, R. L.; Russanova, V.; Howard, B. H.; Nakatani, Y. Cell 1996, 87, (18) Gu, W.; Roeder, R. G. Cell 1997, 90, (19) Liu, L.; Scolnick, D. M.; Trievel, R. C.; Zhang, H. B.; Marmorstein, R.; Halazonetis, T. D.; Berger, S. L. Mol. Cell. Biol. 1999, 19, (20) Sakaguchi, K.; Herrera, J. E.; Saito, S.; Miki, T.; Bustin, M.; Vassilev, A.; Anderson, C. W.; Appella, E. Genes Dev. 1998, 12, (21) Vogelstein, B.; Lane, D.; Levine, A. J. Nature 2000, 408, (22) Levine, A. J. Cell 1997, 88, (23) Prives, C.; Hall, P. A. J. Pathol 1999, 187, (24) Coutts, A. S.; La Thangue, N. B. Biochem. Biophys. Res. Commun. 2005, 331, (25) Alarcon-Vargas, D.; Ronai, Z. Carcinogenesis 2002, 23, (26) Gudkov, A. V.; Komarova, E. A. Biochem. Biophys. Res. Commun. 2005, 331, (27) Filippakopoulos, P.; Picaud, S.; Mangos, M.; Keates, T.; Lambert, J.-P.; Barsyte-Lovejoy, D.; Felletar, I.; Volkmer, R.; Mu ller, S.; Pawson, T.; Gingras, A.-C.; Arrowsmith, C. H.; Knapp, S. Cell 2012, 149, (28) Sachchidanand; Resnick-Silverman, L.; Yan, S.; Mutjaba, S.; Liu, W.-j.; Zeng, L.; Manfredi, J. J.; Zhou, M.-M. Chem. Biol. 2006, 13, (29) Borah, J. C.; Mujtaba, S.; Karakikes, I.; Zeng, L.; Muller, M.; Patel, J.; Moshkina, N.; Morohashi, K.; Zhang, W.; Gerona-Navarro, G.; Hajjar, R. J.; Zhou, M.-M. Chem. Biol. 2011, 18, (30) Gerona-Navarro, G.; Yoel-Rodriguez; Mujtaba, S.; Frasca, A.; Patel, J.; Zeng, L.; Plotnikov, A. N.; Osman, R.; Zhou, M. M. J. Am. Chem. Soc. 2011, 133, (31) Rooney, T. P. C.; Filippakopoulos, P.; Fedorov, O.; Picaud, S.; Cortopassi, W. A.; Hay, D. A.; Martin, S.; Tumber, A.; Rogers, C. M.; Philpott, M.; Wang, M.; Thompson, A. L.; Heightman, T. D.; Pryde,
12 D. C.; Cook, A.; Paton, R. S.; Mu ller, S.; Knapp, S.; Brennan, P. E.; Conway, S. J. Angew. Chem., Int. Ed. 2014, 53, (32) Nayak, S. K.; Panesar, P. S.; Kumar, H. Curr. Med. Chem. 2009, 16, (33) Li, J. D.; Ghiani, C. A.; Kim, J. Y.; Liu, A. X.; Sandoval, J.; DeVellis, J.; Casaccia-Bonnefil, P. J. Neurosci. 2008, 28, (34) Duan, W.; Zhu, X.; Ladenheim, B.; Yu, Q.-S.; Guo, Z.; Oyler, J.; Cutler, R. G.; Cadet, J. L.; Greig, N. H.; Mattson, M. P. Ann. Neurol. 2002, 52, (35) Hong, L.-Z.; Zhao, X.-Y.; Zhang, H.-L. Neurosci. Bull. 2010, 26, (36) Tashiro, J.; Kikuchi, S.; Shinpo, K.; Kishimoto, R.; Tsuji, S.; Sasaki, H. J. Neurosci. Res. 2007, 85, (37) Pietrancosta, N.; Garino, C.; Laras, Y.; Queĺe ver, G.; Pierre, P.; Clavarino, G.; Kraus, J.-L. Drug Dev. Res. 2005, 65, (38) Filippakopoulos, P.; Knapp, S. FEBS Lett. 2012, 586, (39) Vidler, L. R.; Brown, N.; Knapp, S.; Hoelder, S. J. Med. Chem. 2012, 55, (40) Sanchez, R.; Zhou, M.-M. Curr. Opin. Drug Discovery Dev. 2009, 12, (41) Zeng, L.; Zhang, Q.; Gerona-Navarro, G.; Moshkina, N.; Zhou, M.-M. Structure 2008, 16, (42) Zeng, L.; Zhou, M. M. FEBS Lett. 2002, 513, (43) Hay, D.; Fedorov, O.; Filippakopoulos, P.; Martin, S.; Philpott, M.; Picaud, S.; Hewings, D. S.; Uttakar, S.; Heightman, T. D.; Conway, S. J.; Knapp, S.; Brennan, P. E. MedChemComm 2013, 4, (44) Prinjha, R. K.; Witherington, J.; Lee, K. Trends Pharmacol. Sci. 2012, 33, (45) Bamborough, P.; Diallo, H.; Goodacre, J. D.; Gordon, L.; Lewis, A.; Seal, J. T.; Wilson, D. M.; Woodrow, M. D.; Chung, C. W. J. Med. Chem. 2012, 55, (46) Hewings, D. S.; Wang, M. H.; Philpott, M.; Fedorov, O.; Uttarkar, S.; Filippakopoulos, P.; Picaud, S.; Vuppusetty, C.; Marsden, B.; Knapp, S.; Conway, S. J.; Heightman, T. D. J. Med. Chem. 2011, 54, (47) Filippakopoulos, P.; Qi, J.; Picaud, S.; Shen, Y.; Smith, W. B.; Fedorov, O.; Morse, E. M.; Keates, T.; Hickman, T. T.; Felletar, I.; Philpott, M.; Munro, S.; McKeown, M. R.; Wang, Y. C.; Christie, A. L.; West, N.; Cameron, M. J.; Schwartz, B.; Heightman, T. D.; La Thangue, N.; French, C. A.; Wiest, O.; Kung, A. L.; Knapp, S.; Bradner, J. E. Nature 2010, 468, (48) Hewings, D. S.; Fedorov, O.; Filippakopoulos, P.; Martin, S.; Picaud, S.; Tumber, A.; Wells, C.; Olcina, M. M.; Freeman, K.; Gill, A.; Ritchie, A. J.; Sheppard, D. W.; Russell, A. J.; Hammond, E. M.; Knapp, S.; Brennan, P. E.; Conway, S. J. J. Med. Chem. 2013, 56, (49) Fish, P. V.; Filippakopoulos, P.; Bish, G.; Brennan, P. E.; Bunnage, M. E.; Cook, A. S.; Federov, O.; Gerstenberger, B. S.; Jones, H.; Knapp, S.; Marsden, B.; Nocka, K.; Owen, D. R.; Philpott, M.; Picaud, S.; Primiano, M. J.; Ralph, M. J.; Sciammetta, N.; Trzupek, J. D. J. Med. Chem. 2012, 55, (50) Fedorov, O.; Lingard, H.; Wells, C.; Monteiro, O. P.; Picaud, S.; Keates, T.; Yapp, C.; Philpott, M.; Martin, S. J.; Felletar, I.; Marsden, B. D.; Filippakopoulos, P.; Muller, S.; Knapp, S.; Brennan, P. E. J. Med. Chem. 2014, 57, (51) Ryckmans, T.; Edwards, M. P.; Horne, V. A.; Correia, A. M.; Owen, D. R.; Thompson, L. R.; Tran, I.; Tutt, M. F.; Young, T. Bioorg. Med. Chem. Lett. 2009, 19, (52) Shultz, M. D. Bioorg. Med. Chem. Lett. 2013, 23, (53) Hopkins, A. L.; Groom, C. R.; Alex, A. Drug Discovery Today 2004, 9, (54) Edwards, A. M.; Bountra, C.; Kerr, D. J.; Willson, T. M. Nat. Chem. Biol. 2009, 5, (55) Dawson, M. A.; Prinjha, R. K.; Dittmann, A.; Giotopoulos, G.; Bantscheff, M.; Chan, W. I.; Robson, S. C.; Chung, C. W.; Hopf, C.; Savitski, M. M.; Huthmacher, C.; Gudgin, E.; Lugo, D.; Beinke, S.; Chapman, T. D.; Roberts, E. J.; Soden, P. E.; Auger, K. R.; Mirguet, O.; Doehner, K.; Delwel, R.; Burnett, A. K.; Jeffrey, P.; Drewes, G.; Lee, K.; Huntly, B. J. P.; Kouzarides, T. Nature 2011, 478, (56) Nicodeme, E.; Jeffrey, K. L.; Schaefer, U.; Beinke, S.; Dewell, S.; Chung, C. W.; Chandwani, R.; Marazzi, I.; Wilson, P.; Coste, H.; White, J.; Kirilovsky, J.; Rice, C. M.; Lora, J. M.; Prinjha, R. K.; Lee, K.; Tarakhovsky, A. Nature 2010, 468, (57) Lipinski, C. A.; Lombardo, F.; Dominy, B. W.; Feeney, P. J. Adv. Drug Delivery Rev. 2001, 46, (58) Philpott, M.; Yang, J.; Tumber, T.; Fedorov, O.; Uttarkar, S.; Filippakopoulos, P.; Picaud, S.; Keates, T.; Felletar, I.; Ciulli, A.; Knapp, S.; Heightman, T. D. Mol. BioSyst. 2011, 7, (59) Compounds in Table 1 were screened by AlphaScreen and DSF against a number of BRD targets. Spearman analysis shows a good rank correlation between the two assay formats (ρ = 0.91). See Supporting Information, Table S1, for assay details (60) Mirguet, O.; Gosmini, R.; Toum, J.; Clement, C. A.; Barnathan, M.; Brusq, J.-M.; Mordaunt, J. E.; Grimes, R. M.; Crowe, M.; Pineau, O.; Ajakane, M.; Daugan, A.; Jeffrey, P.; Cutler, L.; Haynes, A. C.; Smithers, N. N.; Chung, C.-w.; Bamborough, P.; Uings, I. J.; Lewis, A.; Witherington, J.; Parr, N.; Prinjha, R. K.; Nicodeme, E. J. Med. Chem. 2013, 56, (61) Prasitpan, N.; Patel, J. N.; Decroos, P. Z.; Stockwell, B. L.; Manavalan, P.; Kar, L.; Johnson, M. E.; Currie, B. L. J. Heterocycl. Chem. 1992, 29, (62) Tsunoda, T.; Nagino, C.; Oguri, M.; Ito, S. Tetrahedron Lett. 1996, 37, (63) French, C. A.; Ramirez, C. L.; Kolmakova, J.; Hickman, T. T.; Cameron, M. J.; Thyne, M. E.; Kutok, J. L.; Toretsky, J. A.; Tadavarthy, A. K.; Kees, U. R.; Fletcher, J. A.; Aster, J. C. Oncogene 2008, 27, (64) Stewart, H. J. S.; Horne, G. A.; Bastow, S.; Chevassut, T. J. T. Cancer Med. 2013, 2, (65) Delmore, J. E.; Issa, G. C.; Lemieux, M. E.; Rahl, P. B.; Shi, J.; Jacobs, H. M.; Kastritis, E.; Gilpatrick, T.; Paranal, R. M.; Qi, J.; Chesi, M.; Schinzel, A. C.; McKeown, M. R.; Heffernan, T. P.; Vakoc, C. R.; Bergsagel, P. L.; Ghobrial, I. M.; Richardson, P. G.; Young, R. A.; Hahn, W. C.; Anderson, K. C.; Kung, A. L.; Bradner, J. E.; Mitsiades, C. S. Cell 2011, 146, (66) In order to minimize the possibility of interference from BRD4 inhibition, it is recommended that compound 58 is used at relatively low concentrations in cellular studies, i.e., 2 μm, which are sufficient to inhibit p53 effects. (67) Lamoureux, F.; Baud huin, M.; Rodriguez Calleja, L.; Jacques, C.; Berreur, M.; Re dini, F.; Lecanda, F.; Bradner, J. E.; Heymann, D.; Ory, B. Nat. Commun. 2014, 5, No (68) Broader selectivity profiling was done in the CEREP BioPrint Full Panel ( 9319
Targeting Low-Druggability Bromodomains: Fragment Based Screening and Inhibitor Design against the BAZ2B Bromodomain
 pubs.acs.org/jmc Terms of Use CC-BY Targeting Low-Druggability Bromodomains: Fragment Based Screening and Inhibitor Design against the BAZ2B Bromodomain Fleur M. Ferguson, Oleg Fedorov, Apirat Chaikuad,
pubs.acs.org/jmc Terms of Use CC-BY Targeting Low-Druggability Bromodomains: Fragment Based Screening and Inhibitor Design against the BAZ2B Bromodomain Fleur M. Ferguson, Oleg Fedorov, Apirat Chaikuad,
Supporting Information
 Supporting Information Discovery and optimization of a selective ligand for the Switch/Sucrose Non-Fermenting-related bromodomains of Polybromo protein-1 by the use of virtual screening and hydration analysis.
Supporting Information Discovery and optimization of a selective ligand for the Switch/Sucrose Non-Fermenting-related bromodomains of Polybromo protein-1 by the use of virtual screening and hydration analysis.
Department of Biochemistry, University of Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland
 Supporting information Twenty crystal structures of bromodomain and PHD finger containing protein 1 (BRPF1)/ligand complexes reveal conserved binding motifs and rare interactions Jian Zhu and Amedeo Caflisch*
Supporting information Twenty crystal structures of bromodomain and PHD finger containing protein 1 (BRPF1)/ligand complexes reveal conserved binding motifs and rare interactions Jian Zhu and Amedeo Caflisch*
Table of contents 1. Thermal denaturation assay with BRD4(BD2) incubated with the covalent inhibitors vs. their. non-covalent controls.
 Supporting Information DESIGN AND CHARACTERIZATION OF NOVEL COVALENT BROMODOMAIN AND EXTRA-TERMINAL DOMAIN (BET) INHIBITORS TARGETING A METHIONINE Olesya A. Kharenko a *, Reena G. Patel a, S. David Brown
Supporting Information DESIGN AND CHARACTERIZATION OF NOVEL COVALENT BROMODOMAIN AND EXTRA-TERMINAL DOMAIN (BET) INHIBITORS TARGETING A METHIONINE Olesya A. Kharenko a *, Reena G. Patel a, S. David Brown
Receptor Based Drug Design (1)
 Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
FRAGMENT SCREENING IN LEAD DISCOVERY BY WEAK AFFINITY CHROMATOGRAPHY (WAC )
 FRAGMENT SCREENING IN LEAD DISCOVERY BY WEAK AFFINITY CHROMATOGRAPHY (WAC ) SARomics Biostructures AB & Red Glead Discovery AB Medicon Village, Lund, Sweden Fragment-based lead discovery The basic idea:
FRAGMENT SCREENING IN LEAD DISCOVERY BY WEAK AFFINITY CHROMATOGRAPHY (WAC ) SARomics Biostructures AB & Red Glead Discovery AB Medicon Village, Lund, Sweden Fragment-based lead discovery The basic idea:
Docking with Water in the Binding Site using GOLD
 Docking with Water in the Binding Site using GOLD Version 2.0 November 2017 GOLD v5.6 Table of Contents Docking with Water in the Binding Site... 2 Case Study... 3 Introduction... 3 Provided Input Files...
Docking with Water in the Binding Site using GOLD Version 2.0 November 2017 GOLD v5.6 Table of Contents Docking with Water in the Binding Site... 2 Case Study... 3 Introduction... 3 Provided Input Files...
Identifying Interaction Hot Spots with SuperStar
 Identifying Interaction Hot Spots with SuperStar Version 1.0 November 2017 Table of Contents Identifying Interaction Hot Spots with SuperStar... 2 Case Study... 3 Introduction... 3 Generate SuperStar Maps
Identifying Interaction Hot Spots with SuperStar Version 1.0 November 2017 Table of Contents Identifying Interaction Hot Spots with SuperStar... 2 Case Study... 3 Introduction... 3 Generate SuperStar Maps
Bio-elements. Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components.
 Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
Bio-elements Living organisms requires only 27 of the 90 common chemical elements found in the crust of the earth, to be as its essential components. Most of the chemical components of living organisms
Suggested solutions for Chapter 29
 s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
INVESTIGATION OF A BICYCLO [1.1.1]PENTANE AS A PHENYL REPLACEMENT WITHIN AN LpPLA 2 INHIBITOR
![INVESTIGATION OF A BICYCLO [1.1.1]PENTANE AS A PHENYL REPLACEMENT WITHIN AN LpPLA 2 INHIBITOR INVESTIGATION OF A BICYCLO [1.1.1]PENTANE AS A PHENYL REPLACEMENT WITHIN AN LpPLA 2 INHIBITOR](/thumbs/71/65562448.jpg) INVESTIGATION OF A BICYCLO [1.1.1]PENTANE AS A PHENYL REPLACEMENT WITHIN AN LpPLA 2 INHIBITOR Measom, N. D.; Down, K. D.; Hirst, D. J.; Jamieson, C.; Manas, E. S.; Patel, V. K.; and Somers, D. O. Celeste
INVESTIGATION OF A BICYCLO [1.1.1]PENTANE AS A PHENYL REPLACEMENT WITHIN AN LpPLA 2 INHIBITOR Measom, N. D.; Down, K. D.; Hirst, D. J.; Jamieson, C.; Manas, E. S.; Patel, V. K.; and Somers, D. O. Celeste
11/30/ Substituent Effects in Electrophilic Substitutions. Substituent Effects in Electrophilic Substitutions
 Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility
 (P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
(P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
Using AutoDock for Virtual Screening
 Using AutoDock for Virtual Screening CUHK Croucher ASI Workshop 2011 Stefano Forli, PhD Prof. Arthur J. Olson, Ph.D Molecular Graphics Lab Screening and Virtual Screening The ultimate tool for identifying
Using AutoDock for Virtual Screening CUHK Croucher ASI Workshop 2011 Stefano Forli, PhD Prof. Arthur J. Olson, Ph.D Molecular Graphics Lab Screening and Virtual Screening The ultimate tool for identifying
Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Calculate a rate given a species concentration change.
 Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
doi:10.1038/nature11085 Supplementary Tables: Supplementary Table 1. Summary of crystallographic and structure refinement data Structure BRIL-NOP receptor Data collection Number of crystals 23 Space group
Identification of a Chemical Probe for Bromo and Extra C Terminal Bromodomain Inhibition through Optimization of a Fragment- Derived Hit
 pubs.acs.org/jmc Downloaded via 148.251.232.83 on March 30, 2019 at 13:02:03 (UTC). See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles. Identification
pubs.acs.org/jmc Downloaded via 148.251.232.83 on March 30, 2019 at 13:02:03 (UTC). See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles. Identification
Current Literature. Development of Highly Potent and Selective Steroidal Inhibitors and Degraders of CDK8
 Current Literature Development of ighly Potent and Selective Steroidal Inhibitors and Degraders of CDK8 ACS Med. Chem. Lett. 2018, ASAP Rational Drug Development simplification Cortistatin A 16-30 steps;
Current Literature Development of ighly Potent and Selective Steroidal Inhibitors and Degraders of CDK8 ACS Med. Chem. Lett. 2018, ASAP Rational Drug Development simplification Cortistatin A 16-30 steps;
Structure-Based Drug Discovery An Overview
 Structure-Based Drug Discovery An Overview Edited by Roderick E. Hubbard University of York, Heslington, York, UK and Vernalis (R&D) Ltd, Abington, Cambridge, UK RSC Publishing Contents Chapter 1 3D Structure
Structure-Based Drug Discovery An Overview Edited by Roderick E. Hubbard University of York, Heslington, York, UK and Vernalis (R&D) Ltd, Abington, Cambridge, UK RSC Publishing Contents Chapter 1 3D Structure
Nature Structural & Molecular Biology doi: /nsmb Supplementary Figure 1. CRBN binding assay with thalidomide enantiomers.
 Supplementary Figure 1 CRBN binding assay with thalidomide enantiomers. (a) Competitive elution assay using thalidomide-immobilized beads coupled with racemic thalidomide. Beads were washed three times
Supplementary Figure 1 CRBN binding assay with thalidomide enantiomers. (a) Competitive elution assay using thalidomide-immobilized beads coupled with racemic thalidomide. Beads were washed three times
Quantum Mechanical Models of P450 Metabolism to Guide Optimization of Metabolic Stability
 Quantum Mechanical Models of P450 Metabolism to Guide Optimization of Metabolic Stability Optibrium Webinar 2015, June 17 2015 Jonathan Tyzack, Matthew Segall, Peter Hunt Optibrium, StarDrop, Auto-Modeller
Quantum Mechanical Models of P450 Metabolism to Guide Optimization of Metabolic Stability Optibrium Webinar 2015, June 17 2015 Jonathan Tyzack, Matthew Segall, Peter Hunt Optibrium, StarDrop, Auto-Modeller
3-Bromoimidazo [1,2-a]pyridines: Bromination via in situ oxidation of HBr by nitric acid
![3-Bromoimidazo [1,2-a]pyridines: Bromination via in situ oxidation of HBr by nitric acid 3-Bromoimidazo [1,2-a]pyridines: Bromination via in situ oxidation of HBr by nitric acid](/thumbs/88/116181191.jpg) Chapter 2 3-Bromoimidazo [1,2-a]pyridines: Bromination via in situ oxidation of HBr by nitric acid 2.1 INTRODUCTION Halogenated compounds play a very important role in getting higher value added pharmaceutical
Chapter 2 3-Bromoimidazo [1,2-a]pyridines: Bromination via in situ oxidation of HBr by nitric acid 2.1 INTRODUCTION Halogenated compounds play a very important role in getting higher value added pharmaceutical
Structure-based maximal affinity model predicts small-molecule druggability
 Structure-based maximal affinity model predicts small-molecule druggability Alan Cheng alan.cheng@amgen.com IMA Workshop (Jan 17, 2008) Druggability prediction Introduction Affinity model Some results
Structure-based maximal affinity model predicts small-molecule druggability Alan Cheng alan.cheng@amgen.com IMA Workshop (Jan 17, 2008) Druggability prediction Introduction Affinity model Some results
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
Design and Synthesis of Novel Benzimidazoles and Analogues of Potential Anthelmintic Activity
 Mansoura University Pharm. Organic. Chem. Dept. Design and Synthesis of ovel Benzimidazoles and Analogues of Potential Anthelmintic Activity Thesis Presented By Basem Awad Mansour Mohamed B. Pharm. Sci.,
Mansoura University Pharm. Organic. Chem. Dept. Design and Synthesis of ovel Benzimidazoles and Analogues of Potential Anthelmintic Activity Thesis Presented By Basem Awad Mansour Mohamed B. Pharm. Sci.,
Vitamin E-Labeled Polyethylenimine for in vitro and in vivo Gene Delivery
 Supporting Information Vitamin E-Labeled Polyethylenimine for in vitro and in vivo Gene Delivery Jinxing Liu, Mengke Feng, Duanwei Liang, Jiali Yang, Xinjing Tang* State Key Laboratory of Natural and Biomimetic
Supporting Information Vitamin E-Labeled Polyethylenimine for in vitro and in vivo Gene Delivery Jinxing Liu, Mengke Feng, Duanwei Liang, Jiali Yang, Xinjing Tang* State Key Laboratory of Natural and Biomimetic
NMR study of complexes between low molecular mass inhibitors and the West Nile virus NS2B-NS3 protease
 University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 NMR study of complexes between low molecular mass inhibitors and the West Nile
University of Wollongong Research Online Faculty of Science - Papers (Archive) Faculty of Science, Medicine and Health 2009 NMR study of complexes between low molecular mass inhibitors and the West Nile
Structural biology and drug design: An overview
 Structural biology and drug design: An overview livier Taboureau Assitant professor Chemoinformatics group-cbs-dtu otab@cbs.dtu.dk Drug discovery Drug and drug design A drug is a key molecule involved
Structural biology and drug design: An overview livier Taboureau Assitant professor Chemoinformatics group-cbs-dtu otab@cbs.dtu.dk Drug discovery Drug and drug design A drug is a key molecule involved
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Targeting protein-protein interactions: A hot topic in drug discovery
 Michal Kamenicky; Maria Bräuer; Katrin Volk; Kamil Ödner; Christian Klein; Norbert Müller Targeting protein-protein interactions: A hot topic in drug discovery 104 Biomedizin Innovativ patientinnenfokussierte,
Michal Kamenicky; Maria Bräuer; Katrin Volk; Kamil Ödner; Christian Klein; Norbert Müller Targeting protein-protein interactions: A hot topic in drug discovery 104 Biomedizin Innovativ patientinnenfokussierte,
sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito
 1 sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito 2016. 1. 30 1. Introduction 2 About Carbene 3 Brief history of carbene (~2000) Carbene Neutral compounds featuring a divalent carbon atom with only
1 sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito 2016. 1. 30 1. Introduction 2 About Carbene 3 Brief history of carbene (~2000) Carbene Neutral compounds featuring a divalent carbon atom with only
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Microwave Energy in Accelerating Reaction Rate of Solid-Assisted Solution Phase Synthesis
 Microwave Energy in Accelerating Reaction Rate of Solid-Assisted Solution Phase Synthesis Shahnaz Ghassemi, Discovery Chemistry Group1725 Discovery Drive Charlottesville, VA 22911 Introduction Solid-Assisted
Microwave Energy in Accelerating Reaction Rate of Solid-Assisted Solution Phase Synthesis Shahnaz Ghassemi, Discovery Chemistry Group1725 Discovery Drive Charlottesville, VA 22911 Introduction Solid-Assisted
SUPPLEMENTARY INFORMATION
 UPPLEMETARY IFRMATI In format provided by Filippakopoulos et al. (MAY 2014 upplemental Table 2: Bromodomain ligand complexes Bromodomain/ligand complexes deposited on the Protein Data Bank (PDB are given,
UPPLEMETARY IFRMATI In format provided by Filippakopoulos et al. (MAY 2014 upplemental Table 2: Bromodomain ligand complexes Bromodomain/ligand complexes deposited on the Protein Data Bank (PDB are given,
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
Introduction. Gene expression is the combined process of :
 1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
Organic Chemistry II KEY March 25, a) I only b) II only c) II & III d) III & IV e) I, II, III & IV
 rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
rganic Chemistry II KEY March 25, 2015 Exam 2: VERSIN A 1. Which of the following compounds will give rise to an aromatic conjugate base? E a) I only b) II only c) II & III d) III & IV e) I, II, III &
FRAUNHOFER IME SCREENINGPORT
 FRAUNHOFER IME SCREENINGPORT Design of screening projects General remarks Introduction Screening is done to identify new chemical substances against molecular mechanisms of a disease It is a question of
FRAUNHOFER IME SCREENINGPORT Design of screening projects General remarks Introduction Screening is done to identify new chemical substances against molecular mechanisms of a disease It is a question of
György M. Keserű H2020 FRAGNET Network Hungarian Academy of Sciences
 Fragment based lead discovery - introduction György M. Keserű H2020 FRAGET etwork Hungarian Academy of Sciences www.fragnet.eu Hit discovery from screening Druglike library Fragment library Large molecules
Fragment based lead discovery - introduction György M. Keserű H2020 FRAGET etwork Hungarian Academy of Sciences www.fragnet.eu Hit discovery from screening Druglike library Fragment library Large molecules
I5 ELECTROPHILIC SUBSTITUTIONS OF
 Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Site-Specific Prodrug Release Using Visible Light
 Site-Specific Prodrug Release Using Visible Light Michael Y. Jiang and David Dolphin* J. Am. Chem. Soc., 2008, 130, 4236-4237 Li Zhang Current literature presentation 04-26-2008 Li Zhang @ Wipf Group Page
Site-Specific Prodrug Release Using Visible Light Michael Y. Jiang and David Dolphin* J. Am. Chem. Soc., 2008, 130, 4236-4237 Li Zhang Current literature presentation 04-26-2008 Li Zhang @ Wipf Group Page
Chemistry of Benzene: Electrophilic Aromatic Substitution
 Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
Patrick, An Introduction to Medicinal Chemistry 5e Chapter 7 Enzymes as drug targets. 1) The structures of isoleucine and valine are as follows.
 Answers to end-of-chapter questions 1) The structures of isoleucine and valine are as follows. 2 C 2 2 C 2 3 C C 3 3 C C 3 Isoleucine Valine Isoleucine has a larger side chain than valine, and so there
Answers to end-of-chapter questions 1) The structures of isoleucine and valine are as follows. 2 C 2 2 C 2 3 C C 3 3 C C 3 Isoleucine Valine Isoleucine has a larger side chain than valine, and so there
Class XII: Chemistry Chapter 13: Amines Top concepts
 Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
Discovery of CREBBP Bromodomain Inhibitors by High-Throughput Docking and Hit Optimization Guided by Molecular Dynamics
 pubs.acs.org/jmc Discovery of CREBBP Bromodomain Inhibitors by High-Throughput Docking and Hit Optimization Guided by Molecular Dynamics Min Xu, Andrea Unzue, Jing Dong, Dimitrios Spiliotopoulos, Cristina
pubs.acs.org/jmc Discovery of CREBBP Bromodomain Inhibitors by High-Throughput Docking and Hit Optimization Guided by Molecular Dynamics Min Xu, Andrea Unzue, Jing Dong, Dimitrios Spiliotopoulos, Cristina
Supplemental Information
 Supplemental Information Combinatorial Readout of Unmodified H3R2 and Acetylated H3K14 by the Tandem PHD Finger of MOZ Reveals a Regulatory Mechanism for HOXA9 Transcription Yu Qiu 1, Lei Liu 1, Chen Zhao
Supplemental Information Combinatorial Readout of Unmodified H3R2 and Acetylated H3K14 by the Tandem PHD Finger of MOZ Reveals a Regulatory Mechanism for HOXA9 Transcription Yu Qiu 1, Lei Liu 1, Chen Zhao
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Design of Enzyme Literature Seminar Yun-wei Xue
 Design of Enzyme 20180714 Literature Seminar Yun-wei Xue 1 Contents 1. Introduction 2. De novo design 3. Incorporation of unnatural amino acid (main paper) 2 1.1 Advantages and limitations of natural enzyme
Design of Enzyme 20180714 Literature Seminar Yun-wei Xue 1 Contents 1. Introduction 2. De novo design 3. Incorporation of unnatural amino acid (main paper) 2 1.1 Advantages and limitations of natural enzyme
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
SUPPLEMENTARY INFORMATION
 Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
Figure S1. Secondary structure of CAP (in the camp 2 -bound state) 10. α-helices are shown as cylinders and β- strands as arrows. Labeling of secondary structure is indicated. CDB, DBD and the hinge are
CHAPTER 29 HW: AMINO ACIDS + PROTEINS
 CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
Computational chemical biology to address non-traditional drug targets. John Karanicolas
 Computational chemical biology to address non-traditional drug targets John Karanicolas Our computational toolbox Structure-based approaches Ligand-based approaches Detailed MD simulations 2D fingerprints
Computational chemical biology to address non-traditional drug targets John Karanicolas Our computational toolbox Structure-based approaches Ligand-based approaches Detailed MD simulations 2D fingerprints
Structural basis of PROTAC cooperative recognition for selective protein degradation
 SUPPLEMENTARY INFORMATION Structural basis of PROTAC cooperative recognition for selective protein degradation Morgan S. Gadd 1, Andrea Testa 1, Xavier Lucas 1, Kwok-Ho Chan, Wenzhang Chen, Douglas J.
SUPPLEMENTARY INFORMATION Structural basis of PROTAC cooperative recognition for selective protein degradation Morgan S. Gadd 1, Andrea Testa 1, Xavier Lucas 1, Kwok-Ho Chan, Wenzhang Chen, Douglas J.
Supporting Information
 S-1 Supporting Information Flaviviral protease inhibitors identied by fragment-based library docking into a structure generated by molecular dynamics Dariusz Ekonomiuk a, Xun-Cheng Su b, Kiyoshi Ozawa
S-1 Supporting Information Flaviviral protease inhibitors identied by fragment-based library docking into a structure generated by molecular dynamics Dariusz Ekonomiuk a, Xun-Cheng Su b, Kiyoshi Ozawa
Introduction to Chemoinformatics and Drug Discovery
 Introduction to Chemoinformatics and Drug Discovery Irene Kouskoumvekaki Associate Professor February 15 th, 2013 The Chemical Space There are atoms and space. Everything else is opinion. Democritus (ca.
Introduction to Chemoinformatics and Drug Discovery Irene Kouskoumvekaki Associate Professor February 15 th, 2013 The Chemical Space There are atoms and space. Everything else is opinion. Democritus (ca.
Chapter 15. Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution on Arenes. The first step is the slow, rate-determining step
 Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
Problem Set 5 Question 1
 2.32 Problem Set 5 Question As discussed in class, drug discovery often involves screening large libraries of small molecules to identify those that have favorable interactions with a certain druggable
2.32 Problem Set 5 Question As discussed in class, drug discovery often involves screening large libraries of small molecules to identify those that have favorable interactions with a certain druggable
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
AMINES. 3. From your knowledge of the effects involved, predict or explain experimental results. Important areas include:
 AMINES A STUDENT SHOULD BE ABLE TO: 1. Name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole and pyridine). Also, give the classification of
AMINES A STUDENT SHOULD BE ABLE TO: 1. Name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole and pyridine). Also, give the classification of
1. LiAlH4 :.. :.. 2. H3O +
 Ch 24 Amines Description of Amines - An amine is a compound with a nitrogen atom that has single bonds to carbon and hydrogen atoms. - An uncharged nitrogen atom normally has three bonds and a lone pair.
Ch 24 Amines Description of Amines - An amine is a compound with a nitrogen atom that has single bonds to carbon and hydrogen atoms. - An uncharged nitrogen atom normally has three bonds and a lone pair.
Benzene and Aromatic Compounds. Chapter 15 Organic Chemistry, 8 th Edition John McMurry
 Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor
 LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
Plan. Day 2: Exercise on MHC molecules.
 Plan Day 1: What is Chemoinformatics and Drug Design? Methods and Algorithms used in Chemoinformatics including SVM. Cross validation and sequence encoding Example and exercise with herg potassium channel:
Plan Day 1: What is Chemoinformatics and Drug Design? Methods and Algorithms used in Chemoinformatics including SVM. Cross validation and sequence encoding Example and exercise with herg potassium channel:
Self-stable Electrophilic Reagents for Trifluoromethylthiolation. Reporter: Linrui Zhang Supervisor: Prof. Yong Huang Date:
 Self-stable Electrophilic Reagents for Trifluoromethylthiolation Reporter: Linrui Zhang Supervisor: Prof. Yong Huang Date: 2017-12-25 Content Introduction Trifluoromethanesulfenates: Preparation and reactivity
Self-stable Electrophilic Reagents for Trifluoromethylthiolation Reporter: Linrui Zhang Supervisor: Prof. Yong Huang Date: 2017-12-25 Content Introduction Trifluoromethanesulfenates: Preparation and reactivity
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Advanced Analytical Chemistry Lecture 19. Chem 4631
 Advanced Analytical Chemistry Lecture 19 Chem 4631 Organic Electrochemistry is a multidisciplinary science overlapping the fields of organic chemistry, biochemistry, physical chemistry and electrochemistry.
Advanced Analytical Chemistry Lecture 19 Chem 4631 Organic Electrochemistry is a multidisciplinary science overlapping the fields of organic chemistry, biochemistry, physical chemistry and electrochemistry.
Targeted Covalent Inhibitors: A Risk-Benefit Perspective
 Targeted Covalent Inhibitors: A Risk-Benefit Perspective 2014 AAPS Annual Meeting and Exposition San Diego, CA, November 4, 2014 Thomas A. Baillie School of Pharmacy University of Washington Seattle, WA
Targeted Covalent Inhibitors: A Risk-Benefit Perspective 2014 AAPS Annual Meeting and Exposition San Diego, CA, November 4, 2014 Thomas A. Baillie School of Pharmacy University of Washington Seattle, WA
MULTIPLE CHOICE 2 points each
 Name: Date: Score: / 110 Chapter 1/ TEST 1 OPEN BOOK KEY Organic Chemistry MULTIPLE CHOICE 2 points each 1. An atom of which element would have an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 1? a.
Name: Date: Score: / 110 Chapter 1/ TEST 1 OPEN BOOK KEY Organic Chemistry MULTIPLE CHOICE 2 points each 1. An atom of which element would have an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 1? a.
SAR. Structure - Activity Relationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny, uhľovodíky) 2/28/2016
 Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny,
Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny,
Design Synthesis and SAR of Non-peptidic Urotensin II Agonists
 MEDICIAL CEMISTY Design Synthesis and SA of on-peptidic Urotensin II Agonists C Val Cys Tyr Lys Trp 2 Glu Thr Pro Asp Cys Phe uman urotensin II (S) pec 50 7.49 () pec 50 5.84 FL-104 AC-7954 Fredrik Lehmann
MEDICIAL CEMISTY Design Synthesis and SA of on-peptidic Urotensin II Agonists C Val Cys Tyr Lys Trp 2 Glu Thr Pro Asp Cys Phe uman urotensin II (S) pec 50 7.49 () pec 50 5.84 FL-104 AC-7954 Fredrik Lehmann
The now-banned diet drug fen-phen is a mixture of two synthetic substituted benzene: fenfluramine and phentermine.
 The now-banned diet drug fen-phen is a mixture of two synthetic substituted benzene: fenfluramine and phentermine. Chemists have synthesized compounds with structures similar to adrenaline, producing amphetamine.
The now-banned diet drug fen-phen is a mixture of two synthetic substituted benzene: fenfluramine and phentermine. Chemists have synthesized compounds with structures similar to adrenaline, producing amphetamine.
Properties of Amines
 Properties of Amines 1. Boiling Point and Water Solubility It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. The dominant factor
Properties of Amines 1. Boiling Point and Water Solubility It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. The dominant factor
Synthesis of Tethered Chromium Carbene Complexes
 SYNTHESIS OF TETHERED CHROMIUM CARBENE COMPLEXES 375 Synthesis of Tethered Chromium Carbene Complexes Nicole S. Lueck Faculty Sponsor: Curtis Czerwinski, Department of Chemistry ABSTRACT Hydroxycarbene
SYNTHESIS OF TETHERED CHROMIUM CARBENE COMPLEXES 375 Synthesis of Tethered Chromium Carbene Complexes Nicole S. Lueck Faculty Sponsor: Curtis Czerwinski, Department of Chemistry ABSTRACT Hydroxycarbene
Rapid Synthesis and Purification of Sulfahydantion Library Using Microwave Assisted Organic Synthesis and Automated Flash Chromatography
 Rapid ynthesis and Purification of ulfahydantion Library Using Microwave Assisted rganic ynthesis and Automated Flash Chromatography hahnaz Ghassemi, Ph.D. March 17, 2005 Discovery Chemistry Group 1725
Rapid ynthesis and Purification of ulfahydantion Library Using Microwave Assisted rganic ynthesis and Automated Flash Chromatography hahnaz Ghassemi, Ph.D. March 17, 2005 Discovery Chemistry Group 1725
Unit 13-NITROGEN CONTAINING ORGANIC COMPOUNDS
 Unit 13-NITROGEN CONTAINING ORGANIC COMPOUNDS Two marks: 1. Name the product obtained when a nitrile is reduced by H 2 /Ni,. Give the equation. H 2 /Ni, Primary amine: RCN RCH 2 NH 2. 2. How is nitrobenzene
Unit 13-NITROGEN CONTAINING ORGANIC COMPOUNDS Two marks: 1. Name the product obtained when a nitrile is reduced by H 2 /Ni,. Give the equation. H 2 /Ni, Primary amine: RCN RCH 2 NH 2. 2. How is nitrobenzene
Fluorine in Peptide and Protein Engineering
 Fluorine in Peptide and Protein Engineering Rita Fernandes Porto, February 11 th 2016 Supervisor: Prof. Dr. Beate Koksch 1 Fluorine a unique element for molecule design The most abundant halogen in earth
Fluorine in Peptide and Protein Engineering Rita Fernandes Porto, February 11 th 2016 Supervisor: Prof. Dr. Beate Koksch 1 Fluorine a unique element for molecule design The most abundant halogen in earth
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
Protein Structure. W. M. Grogan, Ph.D. OBJECTIVES
 Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Protein Structure W. M. Grogan, Ph.D. OBJECTIVES 1. Describe the structure and characteristic properties of typical proteins. 2. List and describe the four levels of structure found in proteins. 3. Relate
Advanced Medicinal Chemistry SLIDES B
 Advanced Medicinal Chemistry Filippo Minutolo CFU 3 (21 hours) SLIDES B Drug likeness - ADME two contradictory physico-chemical parameters to balance: 1) aqueous solubility 2) lipid membrane permeability
Advanced Medicinal Chemistry Filippo Minutolo CFU 3 (21 hours) SLIDES B Drug likeness - ADME two contradictory physico-chemical parameters to balance: 1) aqueous solubility 2) lipid membrane permeability
CHAPTER 3. Vibrational Characteristics of PTP-1B Inhibitors
 CHAPTER 3 Vibrational Characteristics of PTP-1B Inhibitors 3.1 Preface Theoretical Frequency calculations were performed on the molecules chosen in this study. Initially all the geometries were optimized
CHAPTER 3 Vibrational Characteristics of PTP-1B Inhibitors 3.1 Preface Theoretical Frequency calculations were performed on the molecules chosen in this study. Initially all the geometries were optimized
Chapter 12. Reactions of Arenes: Electrophilic Aromatic Substitution. Class Notes. A. The method by which substituted benzenes are synthesized
 Chapter 12 Reactions of Arenes: Electrophilic Aromatic Substitution Chapter 12 suggested problems: 22, 23, 26, 27, 32, 33 Class Notes I. Electrophilic aromatic substitution reactions A. The method by which
Chapter 12 Reactions of Arenes: Electrophilic Aromatic Substitution Chapter 12 suggested problems: 22, 23, 26, 27, 32, 33 Class Notes I. Electrophilic aromatic substitution reactions A. The method by which
Chem 263 Oct. 6, Single bonds, σ. e - donating Activate Activate ortho and para directing ortho and para directing
 Chem 263 ct. 6, 2009 lectrophilic Substitution of Substituted Benzenes Resonance ffect Inductive ffect C=C, π system Single bonds, σ Strong Weak e - donating Activate Activate ortho and para directing
Chem 263 ct. 6, 2009 lectrophilic Substitution of Substituted Benzenes Resonance ffect Inductive ffect C=C, π system Single bonds, σ Strong Weak e - donating Activate Activate ortho and para directing
Introduction to FBDD Fragment screening methods and library design
 Introduction to FBDD Fragment screening methods and library design Samantha Hughes, PhD Fragments 2013 RSC BMCS Workshop 3 rd March 2013 Copyright 2013 Galapagos NV Why fragment screening methods? Guess
Introduction to FBDD Fragment screening methods and library design Samantha Hughes, PhD Fragments 2013 RSC BMCS Workshop 3 rd March 2013 Copyright 2013 Galapagos NV Why fragment screening methods? Guess
Chapter 1: Atomic and Molecular Structure
 Chapter 1: Atomic and Molecular Structure LEARNING OBJECTIVES Determine the number of valence and/or core electrons for an atom or ion. Multiple Choice: 1, 6, 11 Interpret the electron configuration and
Chapter 1: Atomic and Molecular Structure LEARNING OBJECTIVES Determine the number of valence and/or core electrons for an atom or ion. Multiple Choice: 1, 6, 11 Interpret the electron configuration and
Ping-Chiang Lyu. Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University.
 Pharmacophore-based Drug design Ping-Chiang Lyu Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University 96/08/07 Outline Part I: Analysis The analytical
Pharmacophore-based Drug design Ping-Chiang Lyu Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University 96/08/07 Outline Part I: Analysis The analytical
Project I. Heterocyclic and medicinal chemistry
 Thesis projecten 2018-2019 onderzoeksgroep LOSA professor Wim Dehaen Project I. Heterocyclic and medicinal chemistry - Novel products with interesting biological properties In this line of research, novel
Thesis projecten 2018-2019 onderzoeksgroep LOSA professor Wim Dehaen Project I. Heterocyclic and medicinal chemistry - Novel products with interesting biological properties In this line of research, novel
Chapter 19: Amines. Introduction
 Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Olefin Metathesis ROMP. L n Ru= ROMP n RCM. dilute
 lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
Microcalorimetry for the Life Sciences
 Microcalorimetry for the Life Sciences Why Microcalorimetry? Microcalorimetry is universal detector Heat is generated or absorbed in every chemical process In-solution No molecular weight limitations Label-free
Microcalorimetry for the Life Sciences Why Microcalorimetry? Microcalorimetry is universal detector Heat is generated or absorbed in every chemical process In-solution No molecular weight limitations Label-free
Chapter 13 Reactions of Arenes Electrophilic Aromatic Substitution
 . 13 hapter 13 eactions of Arenes lectrophilic Aromatic ubstitution lectrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. ecall: 3 4 Y 1 4 2 1 δ
. 13 hapter 13 eactions of Arenes lectrophilic Aromatic ubstitution lectrophiles add to aromatic rings in a fashion somewhat similar to the addition of electrophiles to alkenes. ecall: 3 4 Y 1 4 2 1 δ
AMINES. 4. From your knowledge of the effects involved, predict or explain experimental results. Important areas include:
 AMINES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC or common name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole, pyridine, purine, pyrimidine,
AMINES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC or common name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole, pyridine, purine, pyrimidine,
