Apoplastic barriers to radial oxygen loss and solute
|
|
|
- Clara Casey
- 5 years ago
- Views:
Transcription
1 Research Apoplastic barriers to radial oxygen loss and solute Blackwell Publishing Ltd penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima AleS Soukup 1, William Armstrong 2, Lukas Schreiber 3, Rochus Franke 3 and Olga Votrubová 1 1 Department of Plant Physiology, Charles University, Vinièná 5, Prague, CZ , Czech Republic; 2 Department of Biological Sciences, University of Hull, Hull HU6 7RX, UK; 3 Institut für Zelluläre und Molekulare Botanik, Universität Bonn, Kirschallee 1, D Bonn, Germany Summary Author for correspondence: AleÍ Soukup Tel: Fax: asoukup@natur.cuni.cz Received: 13 July 2005 Accepted: 31 August 2006 Few studies have examined exodermal development in relation to the formation of barriers to both radial oxygen loss (ROL) and solute penetration along growing roots. Here, we report on the structural development, chemical composition and functional properties of the exodermis in two diverse wetland grasses, Glyceria maxima and Phragmites australis. Anatomical features, development, the biochemical composition of exodermal suberin and the penetration of apoplastic tracers and oxygen were examined. Striking interspecific differences in exodermal structure, suberin composition and quantity per unit surface area, and developmental changes along the roots were recorded. Towards the root base, ROL and periodic acid (H 5 IO 6 ) penetration were virtually stopped in P. australis; in G. maxima, a tight ROL barrier restricted but did not stop H 5 IO 6 penetration and the exodermis failed to stain with lipidic dyes. Cultivation in stagnant deep hypoxia conditions or oxygenated circulating solution affected the longitudinal pattern of ROL profiles in G. maxima but statistically significant changes in exodermal suberin composition or content were not detected. Interspecific differences in barrier performance were found to be related to hypodermal structure and probably to qualitative as well as quantitative variations in suberin composition and distribution within exodermal cell walls. Implications for root system function are discussed, and it is emphasized that sufficient spatial resolution to identify the effects of developmental changes along roots is crucial for realistic evaluation of exodermal barrier properties. Key words: exodermis, Glyceria maxima, permeability, Phragmites australis, radial oxygen loss, suberin. New Phytologist (2007) 173: The Authors (2006). Journal compilation New Phytologist (2006) doi: /j x Introduction Flooded soils are often characterized by oxygen shortage and accumulations of potentially toxic reduced inorganic ions and compounds derived from anoxic microbial organic matter decomposition (Patrick & Turner, 1968; Ponnamperuma, 1984; Cizkova et al., 1999). Wetland plants, however, are adapted both metabolically and structurally in numerous ways to overcome these threats. In the majority of wetland plants, aerenchyma development raises cortical porosities to much higher values than those found in nonwetland plants ( Justin & Armstrong, 1987; Seago et al., 2005), reducing respiratory demand and at the same time facilitating internal gas-phase oxygen transport to roots and rhizomes and CO 2 removal to the shoot (Armstrong, 1979; Greenway et al., 2006). The consequences of this include functional dependence of rooting 264
2 Research 265 depths on porosity ( Justin & Armstrong, 1987) in flooded soil and improved potential for radial oxygen loss (ROL) from root to sediment. The latter leads the oxidation and/or immobilization of some potential phytotoxins external to the root and some important aerobic microbial activity in the rhizosphere (Frenzel et al., 1992; Begg et al., 1994; Kirk & Kronzucker, 2005). In wetland species, ROL is usually greatest near the root apex, declining markedly in subapical parts (Armstrong, 1979; Colmer, 2003b). Because oxygen concentrations within roots increase towards the base, any subapical decline in ROL clearly indicates the development of a barrier to oxygen release. Such a barrier was first identified in bog species and in rice (Oryza sativa) (Armstrong, 1964, 1971) and tentatively attributed to lignin and suberin deposition in or on the root surface. Subsequently the barrier has been shown to vary in strength from species to species (Laan et al., 1989; McDonald et al., 2002; Garthwaite et al., 2003) and, in rice, to be inducible by transferring roots from aerated hydroponic conditions to stagnant anaerobic agar medium (Colmer et al., 1998a) and to differ among rice varieties (Colmer, 2003a). Such a barrier on the cortex periphery might function also to restrict the intake of flooded-soil-born phytotoxins (Soukup et al., 2002; Armstrong & Armstrong, 2005). Anatomical preparations, apoplastic tracer and ion movements and oxygen microelectrode studies suggest that the main barrier to ROL in Phragmites and rice appears to be the suberized part of the hypodermal cell cylinder (the exodermis) (Armstrong & Armstrong, 2001, 2005; Soukup et al., 2002). However, the barrier to ROL in the immediately subapical regions clearly also has a respiratory component in so far as oxygen consumption within the living hypodermal cells acts synergistically with physical resistance in these regions to impede the radial efflux of oxygen from the root (Armstrong & Armstrong, 2001). For this reason, it is conceivable that the reduction of ROL might occur stronger and nearer to the root tip than would be the case for compounds not consumed during their passage. A separate line of study has concentrated on the identification and role of the suberized cell layers that form apoplastic barriers in the control of nutrients and water uptake (Ferguson & Clarkson, 1976; Clarkson et al., 1987; Gierth et al., 1998; Zimmermann & Steudle, 1998). Recently, detailed chemical analyses of suberin and lignin cell wall deposits in the endodermis as well as the exodermis have been carried out for several species (Schreiber et al., 1999, 2005b). The only study dealing in any detail with chemical modification of hypodermal cell walls and their resistance to ROL is that of De Simone et al. (2003), where the composition and content of suberin and its aliphatic lipidic domain in particular were related to the efficiency of the restriction of radial losses of oxygen from roots and to flooding survival of four Amazonian tree species. However, only the apical 30 mm of the root was examined, and it was clear from the oxygen microelectrode profiles that oxygen consumption in the hypodermal and epidermal layers was also contributing to the expression of the barrier. Examples of increasing subapical resistance to nutrient and water intake have been reported frequently (e.g. Ferguson & Clarkson, 1976; Zimmerman & Steudle, 1998), but results have been variable and barriers as strong as those previously noted for ROL have rarely been recorded. As Hose et al. (2001) pointed out, however, the extent and rate at which roots suberize may depend upon stress conditions such as drought, aeration, and heavy metal or nutrient stress. In maize (Zea mays), for example, hypodermal permeability to 86 Rb (rubidium) and water can be reduced by an order of magnitude (Clarkson et al., 1987) by growing roots in damp air; stagnant agar also stimulates the onset of suberization (Soukup et al., 2004; Enstone & Peterson, 2005). The reason that barriers appeared leaky or incomplete, in some cases, may have been that studies did not include the more basal parts of the roots, or lacked the resolution to discriminate sufficiently between different zones along the root. For example, in Phragmites australis roots, in mainly oxygen-impermeable parts, numerous relatively unsuberized/weakly suberized cell areas ( windows ) can be found (Justin & Armstrong, 1987; Soukup et al., 2002) that retain some permeability to oxygen (Armstrong et al., 2000) and other tracers (Soukup et al., 2002). Lateral roots eventually emerge at these points. These have also been noted in maize (Peterson & Enstone, 1996) and rice (Armstrong & Armstrong, 2001, 2005) and it is as yet unclear to what extent they could mask the expression of barrier function in whole-root studies on nutrient and water uptake. The current study was undertaken to examine further the relationships between the pattern and composition of suberin deposits within exodermal cell walls and the ROL and solute permeability properties in adventitious roots. For this purpose, two contrasting wetland grasses, Phragmites australis and Glyceria maxima, were chosen. Both species are rhizomatous but they differ in ecophysiological preferences (Buttery & Lambert, 1965; Buttery et al., 1965) and growth strategy. G. maxima produces a superficial root system in shallow flooded zones while P. australis colonizes habitats from shallow to very deep water (Buttery & Lambert, 1965; Hejny & Husak, 1978). Convective gas flows through the rhizome system from the shoots occur in P. australis (Armstrong et al., 1992) but have not been found in G. maxima (Vretare Strand, 2002), while P. australis rhizome tissues are more tolerant to strict anoxia (Braendle & Crawford, 1987). Vlková et al. (2004) showed, in solution culture experiments, that the two species differ significantly in accumulation of toxic metals from cultivation solution, an observation that suggested some uncontrolled penetration of the solutes into root tissues, and perhaps differences in exodermal function influencing the ecophysiological tolerance of these species. Materials and Methods Plant cultivation Plants of Phragmites australis (Trin.) ex. Steud. and Glyceria maxima (Hartm.) Holmb were propagated from rhizome The Authors (2006). Journal compilation New Phytologist (2006) New Phytologist (2007) 173:
3 266 Research cuttings collected from stock plants obtained from the Institute of Botany in Trebon (South Bohemia). Cuttings bearing up to three stalks had their existing roots trimmed back before being placed in a quarter-strength Hoagland III (Hoagland & Amon, 1950) solution containing the following micronutrients: H 3 BO 3, 0.23 µm; MnCl 2, 0.71 µm; ZnSO 4, 5 nm; (NH 4 ) 6 Mo 7 O 24, 0.6 nm; CuSO 4, 1.6 nm. The nutrient solution was either slowly circulating between the cultivation vessel (15-l container) and a reservoir (40 l) or was kept stagnant (in the 15-l container). There were six plants to each of two containers per treatment. The circulating solution was well oxygenated (6 7 mg O 2 l 1 ) during mixing of the solution in the reservoir. Before use, the stagnant solution (max mg O 2 l 1 ) was enriched with 0.05% agar and deoxygenated at least overnight by flushing with nitrogen. Both solutions were changed regularly every 7th day. Plants were grown for 7 or 14 wk in a cultivation room (day:night 16 : 8 h; 20 : 16 C; 40 : 60% relative humidity) before sampling. Anatomy Segments from subapical and basal parts (within 2 cm from the root shoot junction) of new roots were sampled, sectioned with the aid of a hand microtome and stained with sudan red 7B or fluorol yellow (Brundrett et al., 1988b) to visualize lipidic compounds, HCl-phloroglucinol ( Johansen, 1940) to visualize lignin compounds and berberine-toludine blue O (Brundrett et al., 1988a) to visualize the Casparian bands and suberin lamellae in either ultraviolet (UV) or blue light excitation. Berberine was used as a 0.1% solution for 1 h and counterstained with 0.5% toluidine blue for 10 min. The sections were afterwards mounted in 50% glycerol. The same procedure was used after extraction with a CHCl 3 /CH 3 OH mixture, which was expected to unmask lipids (Gahan, 1984). The plasmalemma was stained with the FM4-64 probe (Molecular Probes, Eugene, OR, USA) to observe plasmolysis induced with 2 M sucrose. The permeability of hypodermal layers was assessed using periodic acid (0.1% aqueous solution) as described in Soukup et al. (2002). The roots were subsequently washed in water and the remaining H 5 IO 6 was removed in the following reducing solution: 1 g of KI and 1 g of Na 2 S 2 O 3 5H 2 O dissolved in 50 ml of H 2 O and acidified with 0.5 ml of 2 M HCl. Aldehydes produced in cell walls by H 5 IO 6 were detected in sections with Schiff s solution (for details, see Pearse, 1968). The possible toxic effect of H 5 IO 6 on protoplasts during long-term exposure was taken into consideration when interpreting the results. Hypodermis suberin analysis Root segments for suberin analyses were harvested from two independent cultivations. In the first cultivation, after 14 wk, basal segments 65 mm long were taken from well-established roots mm long. In the second cultivation, after 7 wk, segments 0 30, and mm behind the root tips were sampled from growing roots (length mm). Roots of similar total length and with apical regions of comparable length without laterals were grouped into single samples (usually three to five roots per plant) in order to obtain enough material for biochemical analyses. At least five replicates were included for each position and treatment, with the exception of segments at mm, where only three replicates were evaluated because of small amounts of material. Sampled segments were blotted, weighed and scanned at 600 dots per inch (dpi) and their surface areas were determined using the IMAGEJ analyser ( The dry weight was determined after drying at 65 C for 3 d. Hypodermal and attached epidermal cell layers were enzymatically isolated in a mixture of cellulase (0.5% weight/ volume (w/v); Fluka, Buchs, Switzerland) and pectinase (0.5% w/v; Fluka) in citrate buffer (ph = 3, 100 mm) as described by Schreiber et al. (1994). Isolated hypodermal material (0.5 mg) was trans-esterified with BF 3 and the released material processed as in Zeier & Schreiber (1998). For quantification and identification of the released monomers, we used an HP 5890 Series II gas chromatograph (Hewlett Packard, Palo Alto, CA, USA), equipped with a flame ionization detector, and an Agilent 6890 N gas chromatograph (Agilent Technologies, Böblingen, Germany) combined with a quadropole mass selective detector, Agilent 5973 N. The content of suberin monomers was expressed per unit of root surface area instead of per unit dry weight of hypodermal material, as this proved the most reliable and stable measure of quantity within the root wall and also the most physiologically relevant. Radial oxygen loss from root Radial oxygen loss (ROL) was measured along adventitious roots using sleeving cylindrical platinum cathodes in conjunction with Ag-AgCl anodes, after the method of Armstrong & Wright (1975), in freshly deoxygenated 0.05% w/v aqueous agar containing quarter-strength Hoagland s solution. ROL profiles were taken along the root axis from 3 mm behind the root tip up to emerging laterals and slightly beyond, as far as the laterals were not too long or too numerous to be injured by dragging the electrode along the root. Radial profiles of oxygen concentration were measured with microelectrodes as described in Armstrong et al. (2000). Statistics Results of biochemical analyses were statistically evaluated by analysis of variance (ANOVA), t-test, paired t-test to reveal differences in one or more discrete variables, and the Armitage test for trends in proportions (NCSS, 2000; Jerry Hintze, Kaysville, UT, USA). SIGMAPLOT 9 (Systat Software, Inc., Erkrath, Germany) was used for drawing graphs and regression analysis. New Phytologist (2007) 173: The Authors (2006). Journal compilation New Phytologist (2006)
4 Research 267 Fig. 1 Comparison of the structure of the hypodermis and the endodermis at the bases of well-established roots of Phragmites australis (a c) and Glyceria maxima (d e). Bars, 50 µm. (a) HCl-phloroglucinol detection of lignin in the hypodermis (hyp) of P. australis and thickened cell walls of its inner sclerenchymatous ring (scr); (b) suberized exodermis (exo) stained with sudan red 7B; (c) sudan-stained suberin lamellae of the endodermis; (d) lignin or lignin-like compounds were detected with HCl-phloroglucinol (red colouring) in hypodermal layers (hyp) of G. maxima with typically thickened middle tangential cell walls (arrow); epi, epidermis; (e) a section comparable to (d) was stained with sudan red; (g) suberin lamellae (arrow) of the endodermis stained with sudan red from the same section as (e). Results Anatomy The anatomical part of the study focused on the root cortex. Cells of the middle cortex of the apical parts of both species are radially aligned, arranged in a cubic pattern ( Justin & Armstrong, 1987), and subsequently develop similar forms of lysigenous aerenchymatous channels. The difference between the species is most pronounced in the outer cortex, with each having a distinctive form of hypodermis (Fig. 1). This develops in both species in a constitutive way and contains living protoplasts (data not shown) even in the basal parts of the roots; it is also present in well-aerated solution or soil. The hypodermis of P. australis is composed of a uniform multilayered exodermis and underlying nonexodermal sclerenchymatous ring (Fig. 1a,b). Discernible Casparian bands appeared in the exodermis but were only visible for a short period during differentiation (for a detailed description of exodermis development, see Soukup et al., 2002), being later obscured by complete suberin lamellae. Complete suberin lamellae of the exodermis were, similarly to the endodermis (Fig. 1c), strongly The Authors (2006). Journal compilation New Phytologist (2006) New Phytologist (2007) 173:
5 268 Research Fig. 2 (a) Casparian bands (arrows) in Glyceria maxima exodermis stained with berberine-toluidine blue in basal parts of a root 150 mm long bearing laterals emerging at 105 mm behind the root tip; epi, epidermis; bar, 50 µm. (b) Band plasmolysis (arrows) of the protoplast with plasmalemma attached to Casparian bands of the exodermis. Membranes were stained with FM4-64 and plasmolysed in sucrose solution. The root base of a 70-mm-long root with no laterals is shown; bar, 50 µm. (c, d) Cross-sections from the basal part of a well-established 450-mm-long root. (c) Autofluorescence under ultraviolet (UV) excitation. The cells of the epidermis (epi) were dead and sloughing. (d) Detail of striation of tangential thickened exodermal cell wall from (c). (e) Berberine-toluidine blue staining under blue excitation; arrows point to thickened tangential walls of hypodermal layers. (f) A toluidine blue-stained section of the base of a 230-mm-long root, showing autofluorescence under blue excitation. (g) The exodermis (arrows) digested with concentrated sulphuric acid; bars, 50 µm. stained with sudan red 7B (Fig. 1b) and fluorol yellow. Suberization was not histochemically detected in the sclerenchymatous ring of the hypodermis (Fig. 1b). Hypodermal cell walls were positive for lignin using HCl-phloroglucinol (Fig. 1a). G. maxima also possesses a distinct uniform hypodermis, but its character differs from that of P. australis. Its exodermis consists of two cell layers with markedly thickened adjacent tangential cell walls (Fig. 1d,e). Sometimes one subhypodermal layer may additionally develop thickened sclerenchymatous cell walls and appear to belong to the hypodermis, but fine intercellular spaces are present in the corners of cell walls and therefore we considered it to be part of the middle cortex. Further thickening of hypodermal and underlying layers may occur in some roots. Early modifications of the exodermal cell walls were recognized in G. maxima as a deposition of autofluorescent material. This significant increase in autofluorescence of exodermal cell walls was discernible very close to the root tip (up to 10 mm) and was even closer than similar cell wall modifications of the endodermis. Suberization of G. maxima hypodermis was never detected with sudan red (Fig. 1e) or fluorol yellow staining. This failure to detect suberization in the hypodermis was not caused by a deficient staining procedure as intense staining of the endodermis within the same section provided a reliable positive control (Fig. 1f). The negative sudan staining did not improve after CHCl 3 /CH 3 OH extraction, which might unmask some lipids, as suggested by (Gahan, 1984). In spite of the lack of staining for suberin, Casparian bands were discernible for a considerable distance behind the root tip, being detected with berberine-toluidine blue staining (e.g. at 7 cm in Fig. 2a) and New Phytologist (2007) 173: The Authors (2006). Journal compilation New Phytologist (2006)
6 Research 269 by band plasmolysis of the exodermis where plasmalemmae were closely adhering to cell walls in regions of Casparian bands (Fig. 2b). Casparian band staining and band plasmolysis could not be detected in older root parts after deposition of additional cell wall material in the hypodermis (Figs 2c f). The location of suberin impregnation of hypodermal cell walls can also be estimated from a comparison of the autofluorescence of unstained sections (Fig. 2c) with that of similar sections counterstained with toluidine blue (Fig. 2f) from basal parts of well-established roots. Toluidine blue quenches cell wall autofluorescence if it is bound. The presence of suberization might restrict toluidine blue penetration and binding, and thus prevent possibly quenching of cell wall autofluorescence. The suberization is thus indicated as a complete lamella in the inner cell layer and incomplete lamellae on inner periclinal and anticlinal cell walls of the outer exodermal layer. The most obvious evidence of suberization comes from the thickened adjacent tangential cell walls. Particularly in this location, periclinal striation of the fluorescence was observed in welldeveloped parts of the root (Fig. 2d), which seems to indicate that several suberin lamellae are present in this region of the cell wall. The location of suberization within the hypodermis is supported by results of sulphuric acid digestion (Fig. 2g), as only suberin is considered to resist digestion ( Johansen, 1940). Chemical analyses of suberin of hypodermal layers Suberin monomers were detected in the hypodermal layers of both species. Similarly to previous work, epidermal cell walls and lignified nonexodermal sclerenchymatous cell walls were included in the detection process as they could not be enzymatically separated from exodermal layers (De Simone et al., 2003; Schreiber et al., 2005b). Epidermal cells formed only a minor fraction of any isolated sleeve of cell walls or were even sloughed off in the basal parts of roots. As with the partially included sclerenchymatous ring of P. australis, they also did not respond to histochemical lipid tests and dissolved in sulphuric acid. Therefore, we conclude that they introduced only minor or no distortion of the results of biochemical analyses of the suberin content of the hypodermis. Considerable variation in the thickening of the hypodermal cell walls was recorded, which was inconsistent between cultivations as well as between treatments for both species. It seems that other environmental factors (movement of the nutrient solution, the mechanical properties of the rooting medium, etc.) might be more important for development of this feature than oxygen concentration alone. Thickening of the hypodermal cell walls affected values of dry weight per unit root surface area of total enzymatically extracted hypodermal material, which included other compounds in addition to suberin, but did not have a pronounced effect on suberin content per unit root surface area. The total content of aliphatics per unit surface area was several times higher in corresponding regions of P. australis roots than in G. maxima (paired t-test, P = 0.001). The overall higher variation in the content of aliphatic compounds in P. australis might be ascribed to variability in the number of hypodermal layers; in G. maxima there were regularly only two. A lower proportion of aliphatic to aromatic compounds (mean = 4.4, standard deviation (SD) = 0.96) in G. maxima compared to P. australis was recorded at each position along the root (paired t-test, P < , n = 8; Fig. 3). The major increase of phenolics in the basal part of G. maxima (Fig. 3) was connected with thickening of hypodermal cell walls, which are rich in simple phenolics in grass species. As mentioned in the previous paragraph, variation in the thickening of hypodermal cell walls and their lignification in G. maxima seems to be the reason for the higher variability in phenolic content in comparison to aliphatic compounds. This also suggests that not all the phenolics extracted after trans-esterification are necessarily related to the suberin aromatic domain. Towards the bases of roots of both species, the aliphatic content per unit root surface area increased with differentiation of hypodermal tissues (Fig. 3). Also, for both species, significant increases were recorded in the rapidly developing distal parts of the root in the segments at 0 30 and mm. Within 70 mm behind the root tip aliphatics had reached > 50% of the maximal content of the basal parts of the roots. However, the species differed significantly in relation to the composition of the aliphatic domain of hypodermal suberin (Fig. 3; for the detailed monomeric composition, see supplementary Table S1, available online). A very low content of unsubstituted monocarboxylic fatty acids (t-test, P < 0.001) was recorded in G. maxima together with higher proportions of long-chain alcohols (t-test, P < 0.001) compared with P. australis (Fig. 3). Dicarboxylic acids were detected in P. australis but not in G. maxima. Significant changes also appeared in the proportions of individual monomers along the developing root (Fig. 3). There was an increase in the chain length of aliphatic compounds deposited into the exodermis in the more differentiated part of roots further from the root tip (Armitage test, P < 0.05; Fig. 4). This shift towards longer chain aliphatic production towards the base was more pronounced in G. maxima (Fig. 4). No statistically significant difference in either composition or content of analysed compounds was found to be connected with cultivation in stagnant and circulating solutions. Permeability of the exodermis The exodermis acted as a peripheral apoplastic barrier on the root periphery in both species. Penetration of apoplastic tracers as well as ROL from the roots were restricted as differentiation of hypodermal cell walls proceeded. The profiles of ROL along G. maxima roots from stagnant and nonstagnant solutions are shown in Fig. 5 (for individual profiles, see Fig. S1 in the supplementary material). In roots from both conditions, ROL decreased within the first 30 mm The Authors (2006). Journal compilation New Phytologist (2006) New Phytologist (2007) 173:
7 270 Research Fig. 3 Contents of major suberin monomers of exodermal cell walls and their changes along the axis of roots grown in stagnant nutrient solution. The samples of basal parts correspond to those analysed in Table S1 (Supplementary Material). Error bars represent standard deviations. At least five replicates were included. The position along the root (in mm from the root tip) is indicated in the key. from the root tip. Increased oxygen leakage proximal to this zone was spatially connected to emergence of lateral roots. However, this increase, which was significant in nonstagnant cultivation, was only temporary in roots after 3 d in stagnant solution and measurable ROL declined almost to zero (Fig. 5a,b), even in the region where laterals had already emerged and the electrode was carefully pulled over them. In both stagnant and nonstagnant solutions, Afreen-Zobayed (1996) recorded profiles along roots of P. australis similar in pattern to those of the G. maxima roots from the stagnant solution, although the minimum values were reached further from the tip than in G. maxima (at c mm rather than 30 mm). In stagnant deoxygenated medium, the minimum values were close to zero; in the aerated medium, they were c. 8% of the apical value but measurements were only possible to 85 mm, where total root length was 105 mm. In G. maxima roots grown in nonstagnant solution, ROL declined with distance from the root tip but then increased towards the base and did not decrease again within the measurable part of the root (Fig. 5). Linear regression of data from 25 mm towards the base revealed an increase (slope = 1.4; r = 0.779) for roots grown in nonstagnant solution but no significant trend (slope = 0.07; r = 0.204) after 3 d in stagnant solution. The location of the ROL barrier within G. maxima roots was studied using microelectrodes that penetrated the root wall. Measurements were taken 5 and 29 mm behind the root tip. Unfortunately, the thickening of hypodermal layers made penetration by microelectrodes difficult, and further behind the root tip penetration was impossible. Therefore, only two successful replicates were recorded at 29 mm. At 5 mm from the tip, the root wall was still quite permeable to oxygen and its leakage increased the concentration of oxygen in the surrounding anoxic medium (Fig. 6a). Enhanced barrier properties were recorded 29 mm from the root tip, where the second track was taken. In this profile, the oxygen concentration approached zero at the root surface. The inflection point of a logistic curve fitted to the recorded data showed that in both replicates the highest increase was located at c. 46 µm depth from the root surface and the major differences were thus recorded across the inner hypodermal layer (Fig. 6b). The location of the ROL barrier in P. australis roots was, similar to G. maxima, in an exodermal position (Armstrong et al., 2000). The permeability of hypodermal layers was also assessed by penetration of a solution of periodic acid. In both species, the penetration from the medium bathing the roots was greatly New Phytologist (2007) 173: The Authors (2006). Journal compilation New Phytologist (2006)
8 Research 271 Fig. 4 Relative proportions of particular chain lengths of fatty acids of exodermal suberin and their changes along developing roots grown in stagnant nutrient solution. At least five replicates were included per position. The position along the root (in mm from the root tip) is indicated in the key. reduced with exodermis development (Fig. 7a,b). For G. maxima treated for 1 h, the solution pervaded the epidermis and outer tangential and partially outer radial cell walls of the outermost hypodermal layer in well-developed root parts (Fig. 7a). With more prolonged treatment (4 h), penetration was deeper (Fig. 7d) and both exodermal cell layers and adjacent cortical layers were stained. The tracer passed through the exodermis. In P. australis, only minor differences were found between the 1-h and 4-h treatments (Fig. 7c). Some additional outer exodermal cells were stained, but the deeper penetration was not regular and tracer never crossed the exodermis. The location of the cell layers that restrict penetration of periodic acid through the hypodermis, and differences in the arrangement of these cell layers, were obvious if the tracer was vacuuminfiltrated into root segments and the aerenchymatous channels of the middle cortex and then allowed to diffuse into neighbouring tissues, thus penetrating the hypodermis from both sides (Fig. 7e,f). In P. australis, the penetration stopped on the inner and outer borders of the suberized exodermis (Fig. 7e). Thickened and lignified cell walls of underlying sclerenchyma were easily penetrated (Fig. 7e). In G. maxima, the faces penetrating from the inner and outer sides met within the hypodermis and both layers were stained (Fig. 7f). Thus, the hypodermis was fully penetrated. The weaker reaction, or even lack of reaction, in the middle part of the tangential cell walls of two meeting hypodermal layers is interesting (Fig. 7f). Polysaccharides in these parts of the cell walls were obviously not oxidized with periodic acid, in spite of the fact that the acid penetrated the exodermal cell walls. The failure of the reaction might be attributable either to the absence of oxidizable material (polysaccharides) or, more likely, to the polysaccharidic material being masked by other compounds (e.g. suberin). Discussion Anatomical features Both the structure and the chemical composition of the hypodermal cell layers of roots were examined in G. maxima and P. australis, monocotyledonous wetland species of Poaceae. Both species possess an exodermal hypodermis with suberized The Authors (2006). Journal compilation New Phytologist (2006) New Phytologist (2007) 173:
9 272 Research Fig. 5 Longitudinal profiles of radial oxygen loss (ROL) measured along roots of Glyceria maxima. (a) Roots grown in nonstagnant (more oxygenated) solution (n = 4). (b) Roots grown for 3 d in stagnant hypoxic solution; high variability in the apical part reflects variation in the length of this region among individual roots (n = 5). Profiles of ROL measured for individual roots are presented in Fig. S1 (Supplementary material). Error bars represent standard deviation. cell walls as defined by Haberlandt (1918) and von Guttenberg (1968). The P. australis hypodermis is composed of a sclerenchymatous ring and multilayered exodermis, while in G. maxima a two-layered exodermis is present (Fig. 1). The exodermal layers of P. australis develop suberin lamellae very early, and Casparian bands are obscured close to the root tip (Soukup et al., 2002). Instant or almost instant deposition of suberin lamellae over Casparian bands seems to be a general feature of the exodermis, having also been found in other species tested to date (Peterson & Perumalla, 1984; Seago et al., 1999; Ma & Peterson, 2000). In roots of G. maxima, however, in the inner hypodermal layer, the Casparian bands and band plasmolysis remained discernible for a considerable distance behind the root tip (e.g. Fig. 2b). Later formation of complete suberin lamellae and additional cell wall material deposition released the attached plasmalemma from the cell wall of the inner exodermal layer. The outer exodermal layer seems to be impregnated mostly in its inner tangential and partially radial cell walls. The heaviest deposition of suberin was in the adjacent tangential cell walls of the hypodermal layers, and several parallel lamellae seem to be formed in this part of the cell wall. The pattern thus differed from the regular multilayered lamellae of P. australis. In most work published to date, the localization of suberin in tissue was confirmed by staining with sudan red or fluorol yellow lipophilic dyes. However, both stains failed to detect G. maxima exodermal lipidic cell wall material, the presence of which was confirmed biochemically. The staining of the endodermis can be seen as a positive control, and the striking difference in staining demonstrates the different properties of suberized cell walls not only between species but also between the endodermal and exodermal layers of G. maxima roots (Fig. 1e,f). The limitation of histochemical detection has been noted also by De Simone et al. (2003), and was interpreted as being attributable to low amounts of suberization. However, in the current case the quality and not the quantity of suberin New Phytologist (2007) 173: The Authors (2006). Journal compilation New Phytologist (2006)
10 Research 273 Fig. 6 Radial profiles of oxygen concentration measured with microelectrodes 5 and 29 mm behind the tip of Glyceria maxima roots grown in stagnant solution. The profiles of oxygen concentrations correspond to the root cross-sections shown in the figure. The brown track induced by wounding indicates the path of microelectrode penetration (red arrow). Both sections were stained with HClphloroglucinol for lignin. A logistic curve was fitted to discrete data. is the main difference between the species, as even in the very apical tissues of P. australis suberin lamellae are stainable, but they are not stainable in the basal parts of G. maxima. We can only hypothesize that differences in the composition and/or molecular and spatial arrangements of suberin within the cell wall or differences in its molecular context in the cell wall may be factors affecting lipid staining. Obviously, negative results for sudan staining should not always be regarded as decisive. Suberin of hypodermal layers The presence of a lipophilic (aliphatic) domain of suberin is generally connected with barrier properties (Vogt et al., 1983; Schreiber et al., 1999) described for the periderm (Groh et al., 2002), and the endodermis and exodermis (Zimmermann & Steudle, 1998; Barrowclough et al., 2000; De Simone et al., 2003). The monomeric composition of suberin significantly differed between the two species (Fig. 3, Supplementary Table S1). The ω-oh alkanoic and 2-OH alkanoic acids were the most abundant aliphatic monomers in our species. Interestingly, 2-OH alcanoic acids have been reported to be a significant monomer of suberin in maize (Zeier et al., 1999b) and, in the endodermal Casparian bands, in Clivia minimata but not in other species tested to date (Schreiber et al., 1999; Zeier et al., 1999a; Bernards, 2002). No dioic acids were detected in the hypodermal cell walls of G. maxima but were present in P. australis. α,ω-dioic acid is considered to be an aliphatic The Authors (2006). Journal compilation New Phytologist (2006) New Phytologist (2007) 173:
11 274 Research Fig. 7 Cross-sections of the hypodermis of Glyceria maxima (a, b, d, f) and Phragmites australis (c, e) root segments with hypodermis tested for permeability to periodic acid. (a) Periodic acid (as well as reducing solution) was applied to the surface of the basal part of a well-developed G. maxima root for 1 h. Red staining indicates the penetration of periodic acid, photographed as fluorescence under blue excitation. (b) Fully penetrated apical part (5 mm from the tip) of a G. maxima root after 1 h of external treatment. The purple coloration indicates tissues penetrated with periodic acid. (c, d) Tracer solution was applied externally to the root surface for 4 h. (e, f) Segments of length 1 cm were vacuuminfiltrated with tracing solution and left for 4 h to allow penetration. Segments (a, c, d) were sampled from the basal parts of wellestablished roots with length > 270 mm. epi, epidermis. Arrows indicate the middle part of adjacent cell walls with a weaker reaction. Bars, 50 µm. compound typical of, and unique to, suberin and was even suggested as a marker to differentiate between suberized and cutinized tissue (Matzke & Riederer, 1991). The only other known exception, in which dioic acids are absent, is the suberin of endodermal Casparian strips in C. minimata (Zeier & Schreiber, 1997), which remain in the first developmental stage, i.e. without complete suberin lamellae (Clarkson & Robards, 1975). Changes in suberin monomer composition were also found to accompany differentiation of the exodermis along the growing root. Lengthening of the aliphatic chains of major monomers was recorded for both genera. Suberin deposits with higher proportions of longer chain aliphatic monomers in older parts of the root (Fig. 4), reported also by Zeier et al. (1999b) for different developmental stages of the endodermis, thus seem to be a more general feature of suberized tissues. Another developmentally related trend, recorded only for P. australis, was an increase in the relative proportions of unsaturated fatty acids towards the base. The above-mentioned developmental changes in the composition as well as in the content of suberin were not restricted to particular developmental stages of the exodermis or to the short subapical region ( differentiation zone ) of the root, but took place along considerable lengths of the root behind the root tip in both species. The content of aliphatic suberin in hypodermal layers of both P. australis and G. maxima reached values comparable in order of magnitude to values measured for other plant species (for comparison, see De Simone et al., 2003; Schreiber et al., 2005a). However, considerable variation exists among tested species. In the case of Amazonian trees (De Simone et al., 2003), suberin contents measured in the first 30 mm were comparable to, or even higher than, those measured in the basal parts of the roots of our species. The more than three times higher content of aliphatics in the P. australis exodermis compared with G. maxima seems to be connected not only to a higher concentration within the cell wall material but also to a different pattern of suberin deposition and a different number of suberized exodermal cylinders. We did not find any significant difference in overall suberin content and composition between roots grown in stagnant and circulating nutrient solutions. This conclusion might be related to constitutive development of the exodermis in both species. Barrier properties of the exodermis The barrier properties of the exodermis have been described in the literature dealing with water transport (Ranathunge et al., 2003), ion uptake and penetration (Gierth et al., 1998), New Phytologist (2007) 173: The Authors (2006). Journal compilation New Phytologist (2006)
12 Research 275 apoplastic tracers (Peterson et al., 1982; Soukup et al., 2002) and movement of plant growth regulators (Seago et al., 1999; Hose et al., 2000). The properties and development of hypodermal layers are strongly affected by environmental conditions in some species (Enstone & Peterson, 1998; Degenhardt & Gimmler, 2000; Hose et al., 2001; Soukup et al., 2004) and affect the communication of root tissues with the near rhizosphere. Barriers formed subapically in hypodermal layers of wetland plants or plants from flooded soil might, by restricting ROL, be beneficial for longitudinal oxygen transport in roots (Armstrong, 1979). They can also regulate the passive transport into and out of the root of other compounds from solution in close contact with the roots. In most wetland plants tested to date, the proportion of tissues that are not shielded from the often harsh rhizosphere conditions within flooded soil seems to be minimized by the very early development of the exodermis (Seago et al., 1999, 2000a,b). Modifications of exodermal layers even precede modifications of the endodermis and appear closer to the root tip in P. australis (Soukup et al., 2002) and G. maxima. The inverse developmental sequence has been recorded for nonwetland plants, where the exodermis develops some considerable distance behind the root tip (Ferguson & Clarkson, 1976; Perumalla & Peterson, 1986). However, even in nonwetland plants the very early differentiation of the exodermis can be induced in some plants as a consequence of cultivation in rooting medium low in oxygen (Soukup et al., 2004; Enstone & Peterson, 2005) and reversed when plants are transferred into well-oxygenated soil (Soukup et al., 2004). This seems not to be the case for G. maxima and P. australis roots, where the exodermis developed close to the tip as a constitutive feature regardless of oxygen availability and also in well-aerated soil (Soukup et al., 2002). In the literature, the restriction of ROL and barrier formation have been linked to various features of tissues external to the aerenchyma. The dense arrangement of the hypodermal cylinder (Connell et al., 1999), thickening of hypodermal cell walls (Colmer et al., 1998b; Armstrong et al., 2000) and suberization and/or lignification (Armstrong et al., 1994a) were suggested to have these functions. In both species tested in our work, the barriers were shown, using microelectrodes (for P. australis, see Armstrong et al., 2000), to be in the suberized exodermis. As no other observed anatomical features other than suberin impregnation of the hypodermal cell wall were recorded in the short region (at c. 30 mm) of sharp ROL decrease along the root, the key role of this marked increase in setting up barrier properties seems obvious. The tracing of the location of the barrier to the suberized exodermis and not the thickened and lignified cell walls of sclerenchymatous hypodermis was further supported by periodic acid penetration tests. The thickened and lignified hypodermal cell walls of the sclerenchymatous ring of P. australis did not provide a significant barrier to diffusion of periodic acid and is also unlikely to be a barrier to diffusion of smaller molecules such as oxygen. Recently, De Simone et al. (2003) reported a correlation between suberin content and improved survival of tropical trees in flooded soil, thus confirming experimentally for the first time the connection between suberization and the barrier to ROL in roots. Our data provide further, more direct evidence supporting this conclusion. Major differences between the ROL profile patterns along roots grown in stagnant and nonstagnant solutions were found in G. maxima, but not in P. australis (Afreen-Zobayed, 1996). The absence of statistically significant differences in suberin content and the measured initial reduction in ROL of the subapical region of roots from both treatments show that even in nonstagnant cultivation some barrier is formed in the apical region. Reasons for the basipetal increase in ROL might include some of the following. (a) The emerging laterals puncture the exodermis and open up a radial pathway across the epidermis and hypodermal layers. We can only speculate as to whether, for example, the difference in the effectiveness of resealing after the emergence of laterals might result in a difference in the spatial pattern of ROL among treatments. (b) The ROL from laterals developed in nonstagnant medium might be higher than that from laterals developed in stagnant medium. (c) The creation of the ROL barrier in the near subapical region, which is the most metabolically active part of the root, was so quick in G. maxima that it took place during measurement (within several hours) in stagnant medium, but the barrier did not form in the more differentiated distal parts of the root. The evaluation of the functional properties of the root exodermis might be affected by cultivation treatments and/or by disregarding the presence of gaps in otherwise impermeable parts of the root, which are associated with the pre-emergent stage of lateral root development, as seen in this and other studies (Armstrong et al., 2000; Soukup et al., 2002). Such interruptions are below the spatial resolution of most measurement techniques of root radial hydraulic conductivity, solute penetration and ROL, which are almost exclusively performed on those parts of the roots still bearing no laterals. These factors therefore should be taken into account. Although suberization sufficient to create a ROL barrier appeared in the immediately subapical regions of the roots of both species, the efficiency of the exodermis in oxygen retention might be attributable in part to synergism between respiratory demand and radial diffusive resistance (Armstrong et al., 2000), an effect magnified in apical parts by high respiration rates. However, suberin content further significantly increased in the basipetal direction. A correlation between the total amount of suberin in hypodermal layers and the ability of the plant to withstand flooding (De Simone et al., 2003) thus might not always necessarily reflect only the oxygen barrier properties of the root, as the suberin content necessary to form ROL barrier is several times exceeded at some distance from the root tip, at least in the species tested. The correlation might be also related to other environmental factors of flooded soil. One of these factors might be the presence of phytotoxic compounds, which are characteristic of flooded soils The Authors (2006). Journal compilation New Phytologist (2006) New Phytologist (2007) 173:
13 276 Research (Ponnamperuma, 1984). The periodic acid penetration into root tissues across the exodermis illustrates this function of the exodermis and interspecific differences. In comparison with the tight barrier in P. australis, the penetration of the exodermis of G. maxima by periodic acid may indicate a lower selectivity of passive uptake via roots from the rhizosphere in G. maxima. Such a difference could explain the approximately three times higher accumulation of aluminium from the rooting medium in root tissues and the greater degree of middle cortex injury in G. maxima compared with P. australis (Vlková et al., 2003). However, the causal connection requires further evidence. In addition to interspecific differences in exodermis structure, composition and function, these comparisons demonstrate the variation in barrier permeability to different substances. Studies on other species have indicated that water intake is not prevented by exodermal suberization, for example in rice (Ranathunge et al., 2005; Schreiber et al., 2005b), and appears to contrast with tight ROL barrier properties (Colmer et al., 1998, 2003a). There may be several reasons for this. One possible reason is that the aerated hydroponics used in most studies (e.g. Ranathunge et al., 2005; Schreiber et al., 2005b) did not, in the species used, induce ROL barriers as strong as those developed under wetland conditions or in deeply hypoxic stagnant media. Another may relate to the positions along the root that were measured; the work on hydraulic conductivity has been neglectful of more basal regions. Recently, Garthwaite et al. (2006) observed that the induction of a ROL barrier with a stagnant nutrient solution did not significantly affect the hydraulic conductivity of Hordeum marinum roots, but the barrier to ROL was not as complete as those found here or previously in rice in stagnant anaerobic media. In contrast, Groh et al. (2002) demonstrated that the diffusional permeance of oxygen in suberized phellem of several woody species was at least an order of magnitude lower than that for water. The mechanism underlying the difference in permeability is not clear, but molecular size may play a role in transport. The water molecule, with a mean van der Waal diameter of c. 280 pm (Finney, 2001), might permeate the apoplast more easily than the oxygen molecule, with a van der Waal diameter estimated at 428 pm. Schreiber et al. (2005b) suggested that gross values of suberin content in hypodermal layers do not necessarily reflect the barrier properties of impregnated cell walls, and this suggestion is supported by the current data. The spatial arrangement of suberin deposition within the cell wall and cylinder of the exodermis should be further studied at sufficient resolution to provide the data required to elucidate the transport and pathways of various solutes across such apoplastic barriers and to explain the reported interspecific differences. Acknowledgements The presented work was supported by grant GAUK 274/ 2004/B-Bio/PrF. The authors are very grateful to the referees for their valuable comments. References Afreen-Zobayed F Factors affecting the aeration and survival of Phragmites australis. PhD thesis, University of Hull, Hull, UK. Armstrong W Oxygen diffusion from the roots on some British bog plants. Nature 204: Armstrong W Radial oxygen losses from intact rice roots as affected by distance from the apex, respiration and waterlogging. Physiologia Plantarum 25: Armstrong W Aeration in higher plants. Advances in Botanical Research 7: Armstrong J, Armstrong W Rice and Phragmites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. American Journal of Botany 88: Armstrong J, Armstrong W Rice: sulfide-induced barriers to root radial oxygen loss, Fe 2+ and water uptake, and lateral root emergence. Annals of Botany 96: Armstrong J, Armstrong W, Beckett PM Phragmites australis: venturi- and humidity-induced pressure flows enhance rhizome aeration and rhizoshpere oxidation. New Phytologist 120: Armstrong W, Braendle R, Jackson MB. 1994a. Mechanisms of flood tolerance in plants. Acta Botanica Neerlandica 43: Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modeling study with Phragmites australis. Annals of Botany 86: Armstrong W, Wright EJ Radial oxygen loss from roots: The theoretical basis for the manipulation of flux data obtained by the cylindrical platinum electrode technique. Physiologia Plantarum. 35: Barrowclough DE, Peterson CA, Steudle E Radial hydraulic conductivity along developing onion roots. Journal of Experimental Botany 51: Begg CBM, Kirk GJD, Mackenzie AF, Neue HU Root-induced iron oxidation and ph changes in the lowland rice rhizosphere. New Phytologist 128: Bernards MA Demystifying suberin. Canadian Journal of Botany 80: Braendle R, Crawford RMM Rhizome anoxia tolerance and habitat specialization in wetland plants. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats. Oxford, UK: Blackwell, Brundrett MC, Enstone DE, Peterson CA. 1988a. A berberine aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma 1446: Brundrett MC, Kendrick B, Peterson CA. 1988b. Efficient lipid staining in plant material with sudan red 7B or fluorol yellow 088 in polyethylene glycol. Biotechnic & Histochemistry 66: Buttery BR, Lambert JM Competition between Glyceria maxima and Phragmites communis in the region of Surlingham Broad. I. The competition mechanism. Journal of Ecology 53: Buttery BR, Williams WT, Lambert JM Competition between Glyceria maxima and Phragmites communis in the region of Surlingham Broad. II. The fen gradient. Journal of Ecology 53: Cizkova H, Brix H, Kopecky J, Lukavska J Organic acids in the sediment of wetland dominated by Phragmites australis: evidence of phytotoxic concentrations. Aquatic Botany 64: Clarkson DT, Robards K The endodermis, its structural development and physiological role. In: Torrey JG, Clarkson DT, eds. Structure and function of roots. London, UK: Academic Press, Clarkson DT, Robards AW, Stephens JE, Stark M Suberin lamellae in the hypodermis of maize (Zea mays) roots, development and factors affecting the permeability of the hypodermal layers. Plant, Cell & Environment 15: Colmer TD. 2003a. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Annals of Botany 91: New Phytologist (2007) 173: The Authors (2006). Journal compilation New Phytologist (2006)
Sci30264 Plant Anatomy and Physiology 1/2012 Endodermis and passage cell Bualuang FAIYUE, PhD. Endodermis and passage cell
 1. Endodermis and its development Endodermis is the innermost layer of the root cortex (Esau, 1965; Brundrett et al., 1990; Fahn, 1990; Peterson and Enstone, 1996). It is characterised by Casparian strip
1. Endodermis and its development Endodermis is the innermost layer of the root cortex (Esau, 1965; Brundrett et al., 1990; Fahn, 1990; Peterson and Enstone, 1996). It is characterised by Casparian strip
New Phytologist. Research
 Research Apoplastic barriers effectively block oxygen permeability across outer cell layers of rice roots under deoxygenated conditions: roles of apoplastic pores and of respiration Lukasz Kotula 1, Kosala
Research Apoplastic barriers effectively block oxygen permeability across outer cell layers of rice roots under deoxygenated conditions: roles of apoplastic pores and of respiration Lukasz Kotula 1, Kosala
Development of anatomical structure of roots of
 Research Development of anatomical structure of roots of Blackwell Science Ltd Phragmites australis AleS Soukup 1, Olga Votrubová 1 and Hana Čížková 2 1 Department of Plant Physiology, Charles University,
Research Development of anatomical structure of roots of Blackwell Science Ltd Phragmites australis AleS Soukup 1, Olga Votrubová 1 and Hana Čížková 2 1 Department of Plant Physiology, Charles University,
* ' ION UPTAKE IN RELATION TO THE '''-'"u.n v DEVELOPMENT OF A ROOT HYPODERMIS
 New Phytol. (igj6) JJ, 11-14. '-^ ^^ ' '''-: ^i'i^ J i.. * ' ION UPTAKE IN RELATION TO THE '''-'"u.n v DEVELOPMENT OF A ROOT HYPODERMIS :?m::,^^ BY I. B. FERGUSON* AND D. T. CLARKSON '" ' 'I ARC Letcombe
New Phytol. (igj6) JJ, 11-14. '-^ ^^ ' '''-: ^i'i^ J i.. * ' ION UPTAKE IN RELATION TO THE '''-'"u.n v DEVELOPMENT OF A ROOT HYPODERMIS :?m::,^^ BY I. B. FERGUSON* AND D. T. CLARKSON '" ' 'I ARC Letcombe
Casparian bands occur in the periderm of Pelargonium hortorum stem and root
 Annals of Botany 107: 591 598, 2011 doi:10.1093/aob/mcq267, available online at www.aob.oxfordjournals.org Casparian bands occur in the periderm of Pelargonium hortorum stem and root Chris J. Meyer and
Annals of Botany 107: 591 598, 2011 doi:10.1093/aob/mcq267, available online at www.aob.oxfordjournals.org Casparian bands occur in the periderm of Pelargonium hortorum stem and root Chris J. Meyer and
ENDODERMIS & POLARITY
 https://en.wikipedia.org/wiki/casparian_strip ENDODERMIS & POLARITY Niloufar Pirayesh 13.01.2016 PCDU SEMINAR 2 What is Endodermis? It helps with Regulate the movement of water ions and hormones. (in and
https://en.wikipedia.org/wiki/casparian_strip ENDODERMIS & POLARITY Niloufar Pirayesh 13.01.2016 PCDU SEMINAR 2 What is Endodermis? It helps with Regulate the movement of water ions and hormones. (in and
Abstract RESEARCH PAPER. Lukas Schreiber 1, *, Rochus Franke 1, Klaus-Dieter Hartmann 1, Kosala Ranathunge 2 and Ernst Steudle 2
 Journal of Experimental Botany, Vol. 56, No. 415, pp. 1427 1436, May 25 doi:1.193/jxb/eri144 Advance Access publication 4 April, 25 RESEARCH PAPER The chemical composition of suberin in apoplastic barriers
Journal of Experimental Botany, Vol. 56, No. 415, pp. 1427 1436, May 25 doi:1.193/jxb/eri144 Advance Access publication 4 April, 25 RESEARCH PAPER The chemical composition of suberin in apoplastic barriers
Received: 20 April 2000 Returned for revision: 5 June 2000 Accepted: 19 June 2000
 Annals of Botany : 7±73, doi:1.1/anbo..13, available online at http://www.idealibrary.com on Oxygen Distribution in Wetland Plant Roots and Permeability Barriers to Gas-exchange with the Rhizosphere: a
Annals of Botany : 7±73, doi:1.1/anbo..13, available online at http://www.idealibrary.com on Oxygen Distribution in Wetland Plant Roots and Permeability Barriers to Gas-exchange with the Rhizosphere: a
Journal of Plant Physiology
 Journal of Plant Physiology 168 (2011) 1249 1255 Contents lists available at ScienceDirect Journal of Plant Physiology journal homepage: www.elsevier.de/jplph Net sodium fluxes change significantly at
Journal of Plant Physiology 168 (2011) 1249 1255 Contents lists available at ScienceDirect Journal of Plant Physiology journal homepage: www.elsevier.de/jplph Net sodium fluxes change significantly at
Topic 14. The Root System. II. Anatomy of an Actively Growing Root Tip
 Topic 14. The Root System Introduction. This is the first of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Topic 14. The Root System Introduction. This is the first of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
 Question 1: What are the factors affecting the rate of diffusion? Diffusion is the passive movement of substances from a region of higher concentration to a region of lower concentration. Diffusion of
Question 1: What are the factors affecting the rate of diffusion? Diffusion is the passive movement of substances from a region of higher concentration to a region of lower concentration. Diffusion of
OCR (A) Biology A-level
 OCR (A) Biology A-level Topic 3.3: Transport in plants Notes Plants require a transport system to ensure that all the cells of a plant receive a sufficient amount of nutrients. This is achieved through
OCR (A) Biology A-level Topic 3.3: Transport in plants Notes Plants require a transport system to ensure that all the cells of a plant receive a sufficient amount of nutrients. This is achieved through
CHAPTER TRANSPORT
 CHAPTER 2 2.4 TRANSPORT Uptake of CO2 FOCUS: Uptake and transport of water and mineral salts Transport of organic substances Physical forces drive the transport of materials in plants over a range of distances
CHAPTER 2 2.4 TRANSPORT Uptake of CO2 FOCUS: Uptake and transport of water and mineral salts Transport of organic substances Physical forces drive the transport of materials in plants over a range of distances
Transport of substances in plants
 Transport of substances in plants We have already looked at why many organisms need transport systems with special reference to surface area and volume. The larger the volume : surface area ratio, the
Transport of substances in plants We have already looked at why many organisms need transport systems with special reference to surface area and volume. The larger the volume : surface area ratio, the
ABSTRACT INTRODUCTION
 bs_bs_banner Plant, Cell and Environment (2012) 35, 1618 1630 doi: 10.1111/j.1365-3040.2012.02513.x Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots
bs_bs_banner Plant, Cell and Environment (2012) 35, 1618 1630 doi: 10.1111/j.1365-3040.2012.02513.x Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots
THE ROOTS OF WILD RICE. ZIZANIA AQUATICA L.
 THE ROOTS OF WILD RICE. ZIZANIA AQUATICA L. E. L. STOVER, Eastern Illinois State Teachers College. This grass grows from Maine to Minnesota in aquatic habitats (2 and 5). It is common in marsh lands all
THE ROOTS OF WILD RICE. ZIZANIA AQUATICA L. E. L. STOVER, Eastern Illinois State Teachers College. This grass grows from Maine to Minnesota in aquatic habitats (2 and 5). It is common in marsh lands all
Exercise 12. Procedure. Aim: To study anatomy of stem and root of monocots and dicots.
 Aim: To study anatomy of stem and root of monocots and dicots. Principle: The study of internal morphology, i.e., cells of various tissues in an organ of a living body is called Anatomy. Tissue, which
Aim: To study anatomy of stem and root of monocots and dicots. Principle: The study of internal morphology, i.e., cells of various tissues in an organ of a living body is called Anatomy. Tissue, which
Root Endodermis and Exodermis: Structure, Function, and Responses to the Environment
 J Plant Growth Regul (2003) 21:335 351 DOI: 10.1007/s00344-003-0002-2 Root Endodermis and Exodermis: Structure, Function, and Responses to the Environment Daryl E. Enstone, Carol A. Peterson,* and Fengshan
J Plant Growth Regul (2003) 21:335 351 DOI: 10.1007/s00344-003-0002-2 Root Endodermis and Exodermis: Structure, Function, and Responses to the Environment Daryl E. Enstone, Carol A. Peterson,* and Fengshan
Bio Factsheet. Transport in Plants. Number 342
 Number 342 Transport in Plants This Factsheet: Explains why plants need a transport system Describes what plants transport Describes the tissues which carry out transport Outlines the position of the xylem
Number 342 Transport in Plants This Factsheet: Explains why plants need a transport system Describes what plants transport Describes the tissues which carry out transport Outlines the position of the xylem
Plant Structure. Lab Exercise 24. Objectives. Introduction
 Lab Exercise Plant Structure Objectives - Be able to identify plant organs and give their functions. - Learn distinguishing characteristics between monocot and dicot plants. - Understand the anatomy of
Lab Exercise Plant Structure Objectives - Be able to identify plant organs and give their functions. - Learn distinguishing characteristics between monocot and dicot plants. - Understand the anatomy of
ABSORPTION OF WATER MODE OF WATER ABSORPTION ACTIVE AND PASSIVE ABSORPTION AND FACTORS AFFECTING ABSORPTION.
 ABSORPTION OF WATER MODE OF WATER ABSORPTION ACTIVE AND PASSIVE ABSORPTION AND FACTORS AFFECTING ABSORPTION. PRELUDE OF WATER POTENTIAL Most organisms are comprised of at least 70% or more water. Some
ABSORPTION OF WATER MODE OF WATER ABSORPTION ACTIVE AND PASSIVE ABSORPTION AND FACTORS AFFECTING ABSORPTION. PRELUDE OF WATER POTENTIAL Most organisms are comprised of at least 70% or more water. Some
Transport in Vascular Plants
 Chapter 36 Transport in Vascular Plants PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Vascular tissue Transports nutrients throughout a plant; such
Chapter 36 Transport in Vascular Plants PowerPoint Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Vascular tissue Transports nutrients throughout a plant; such
Topic 2: Plant Structure & Growth Ch. 35 Angiosperms are the most complex plants. They are composed of cells, tissues, organs and organ systems.
 Topic 2: Plant Structure & Growth Ch. 35 Angiosperms are the most complex plants. They are composed of cells, tissues, organs and organ systems. Fig. 35.8 Plant Cells pp.798-802 Types of plant cells Include:
Topic 2: Plant Structure & Growth Ch. 35 Angiosperms are the most complex plants. They are composed of cells, tissues, organs and organ systems. Fig. 35.8 Plant Cells pp.798-802 Types of plant cells Include:
ARE YOU familiar with the sayings Get to
 Root Anatomy ARE YOU familiar with the sayings Get to the root of the problem or the root of all evil? Both these sayings suggest that the root is an essential part of something. With plants, the essential
Root Anatomy ARE YOU familiar with the sayings Get to the root of the problem or the root of all evil? Both these sayings suggest that the root is an essential part of something. With plants, the essential
RICE AND PHRAGMITES: EFFECTS OF ORGANIC ACIDS ON
 American Journal of Botany 88(8): 1359 1370. 2001. RICE AND PHRAGMITES: EFFECTS OF ORGANIC ACIDS ON GROWTH, ROOT PERMEABILITY, AND RADIAL OXYGEN LOSS TO THE RHIZOSPHERE 1 JEAN ARMSTRONG 2 AND WILLIAM ARMSTRONG
American Journal of Botany 88(8): 1359 1370. 2001. RICE AND PHRAGMITES: EFFECTS OF ORGANIC ACIDS ON GROWTH, ROOT PERMEABILITY, AND RADIAL OXYGEN LOSS TO THE RHIZOSPHERE 1 JEAN ARMSTRONG 2 AND WILLIAM ARMSTRONG
Water Acquisition and Transport - Whole Plants. 3 possible pathways for water movement across the soil-plant-atmosphere continuum
 Water transport across the entire soil-plant-atmosphere continuum Water Acquisition and Transport - Whole Plants 3 possible pathways for water movement across the soil-plant-atmosphere continuum Apoplast
Water transport across the entire soil-plant-atmosphere continuum Water Acquisition and Transport - Whole Plants 3 possible pathways for water movement across the soil-plant-atmosphere continuum Apoplast
Differences in anatomical structure and lignin content of roots of pedunculate oak and wild cherry-tree plantlets during acclimation
 BIOLOGIA PLANTARUM 48 (4): 481-489, 2004 Differences in anatomical structure and lignin content of roots of pedunculate oak and wild cherry-tree plantlets during acclimation A. SOUKUP* 1, J. MALÁ**, M.
BIOLOGIA PLANTARUM 48 (4): 481-489, 2004 Differences in anatomical structure and lignin content of roots of pedunculate oak and wild cherry-tree plantlets during acclimation A. SOUKUP* 1, J. MALÁ**, M.
Absorption of Water by Plants
 Absorption of Water by Plants Absorption of water by cells and roots Availability of Water in the Soil Soil is the major source of water for plants. The plants absorb water through root hairs from the
Absorption of Water by Plants Absorption of water by cells and roots Availability of Water in the Soil Soil is the major source of water for plants. The plants absorb water through root hairs from the
 Question 1: State the location and function of different types of meristem. Meristems are specialised regions of plant growth. The meristems mark the regions where active cell division and rapid division
Question 1: State the location and function of different types of meristem. Meristems are specialised regions of plant growth. The meristems mark the regions where active cell division and rapid division
Alaina Jane Garthwaite
 Physiological traits associated with tolerance to salinity and waterlogging in the genus Hordeum Alaina Jane Garthwaite BSc in Natural Resource Management (Hons) This thesis is presented for the degree
Physiological traits associated with tolerance to salinity and waterlogging in the genus Hordeum Alaina Jane Garthwaite BSc in Natural Resource Management (Hons) This thesis is presented for the degree
CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-11 TRANSPORT IN PLANTS
 CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-11 TRANSPORT IN PLANTS Plant transport various substance like gases, minerals, water, hormones, photosynthetes and organic solutes to short distance
CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-11 TRANSPORT IN PLANTS Plant transport various substance like gases, minerals, water, hormones, photosynthetes and organic solutes to short distance
Organs and leaf structure
 Organs and leaf structure Different types of tissues are arranged together to form organs. Structure: 2 parts (Petiole and Leaf Blade) Thin flat blade, large surface area Leaves contain all 3 types of
Organs and leaf structure Different types of tissues are arranged together to form organs. Structure: 2 parts (Petiole and Leaf Blade) Thin flat blade, large surface area Leaves contain all 3 types of
Dynamic Plant. Functions of Primary Systems. History of Plants. Plants invaded the land around 400 mya.
 Dynamic Plant Roots & Water Acquisition Roots 1) Anchor the plant 2) Absorb water 3) Absorb minerals 4) Store surplus sugars 5) Transport water, minerals and sugars and hormones 6) Produce some hormones
Dynamic Plant Roots & Water Acquisition Roots 1) Anchor the plant 2) Absorb water 3) Absorb minerals 4) Store surplus sugars 5) Transport water, minerals and sugars and hormones 6) Produce some hormones
Plant Structure and Function
 Plant Structure and Function A Meridian Biology AP Study Guide by John Ho and Tim Qi Plant Terms Growth: Growth Types Type Location Description Primary Primary Vertical growth (up-down), dominant direction
Plant Structure and Function A Meridian Biology AP Study Guide by John Ho and Tim Qi Plant Terms Growth: Growth Types Type Location Description Primary Primary Vertical growth (up-down), dominant direction
Roots and Soil Chapter 5
 Roots and Soil Chapter 5 Plant Organs Plant organs are groups of several types of tissues that together perform a particular function. Vegetative organs roots, stems, leaves make and use food, absorb water
Roots and Soil Chapter 5 Plant Organs Plant organs are groups of several types of tissues that together perform a particular function. Vegetative organs roots, stems, leaves make and use food, absorb water
Class XI Chapter 6 Anatomy of Flowering Plants Biology
 Class XI Chapter 6 Anatomy of Flowering Plants Biology Question 1: State the location and function of different types of meristem. Meristems are specialised regions of plant growth. The meristems mark
Class XI Chapter 6 Anatomy of Flowering Plants Biology Question 1: State the location and function of different types of meristem. Meristems are specialised regions of plant growth. The meristems mark
Department of Plant Biology, University of Hull, Hull, HU6 7RX, UK. (Accepted 21 February 1983)
 Netv Phytol. (1983) 94, 549-559 e. g OXYGEN DIFFUSION IN PE II. OXYGEN CONCENTRTIONS IN THE PRIMRY PE ROOT PEX S FFECTED BY GROWTH, THE PRODUCTION OF LTERLS ND RDIL OXYGEN LOSS BY W. RMSTRONG, M. T. HELY*
Netv Phytol. (1983) 94, 549-559 e. g OXYGEN DIFFUSION IN PE II. OXYGEN CONCENTRTIONS IN THE PRIMRY PE ROOT PEX S FFECTED BY GROWTH, THE PRODUCTION OF LTERLS ND RDIL OXYGEN LOSS BY W. RMSTRONG, M. T. HELY*
II. SIMPLE TISSUES Bot 404--Fall A. Introduction to Tissues (DIAGRAM allow a full page)
 II. SIMPLE TISSUES Bot 404--Fall 2004 A. Introduction to Tissues (DIAGRAM allow a full page) B. Definitions Adaxial = facing the axil; upper surface of leaf Abaxial = facing away from the axil; lower surface
II. SIMPLE TISSUES Bot 404--Fall 2004 A. Introduction to Tissues (DIAGRAM allow a full page) B. Definitions Adaxial = facing the axil; upper surface of leaf Abaxial = facing away from the axil; lower surface
Visit For All NCERT solutions, CBSE sample papers, Question papers, Notes for Class 6 to 12. Chapter-6 ANATOMY OF FLOWERING PLANTS
 Chapter-6 ANATOMY OF FLOWERING PLANTS POINTS TO REMEMBER Anatomy : Anatomy is the study of internal structure of organisms. Plant anatomy includes organisation and structure of tissues. Tissue : A group
Chapter-6 ANATOMY OF FLOWERING PLANTS POINTS TO REMEMBER Anatomy : Anatomy is the study of internal structure of organisms. Plant anatomy includes organisation and structure of tissues. Tissue : A group
Preview from Notesale.co.uk Page 20 of 34
 Page 20 of 34 (i) The role of haemoglobin in transporting oxygen and carbon dioxide To include the reversible binding of oxygen molecules, carbonic anhydrase, haemoglobinic acid, HCO3- and the chloride
Page 20 of 34 (i) The role of haemoglobin in transporting oxygen and carbon dioxide To include the reversible binding of oxygen molecules, carbonic anhydrase, haemoglobinic acid, HCO3- and the chloride
Transport of oxygen in roots of rice (Oryza sativa L.) and of water in developing grape berries (Vitis vinifera L.)
 Transport of oxygen in roots of rice (Oryza sativa L.) and of water in developing grape berries (Vitis vinifera L.) Inaugural-Dissertation zur Erlangung des Doktorgrades der Fakultät Biologie, Chemie und
Transport of oxygen in roots of rice (Oryza sativa L.) and of water in developing grape berries (Vitis vinifera L.) Inaugural-Dissertation zur Erlangung des Doktorgrades der Fakultät Biologie, Chemie und
The three principal organs of seed plants are roots, stems, and leaves.
 23 1 Specialized Tissues in Plants Seed Plant Structure The three principal organs of seed plants are roots, stems, and leaves. 1 of 34 23 1 Specialized Tissues in Plants Seed Plant Structure Roots: absorb
23 1 Specialized Tissues in Plants Seed Plant Structure The three principal organs of seed plants are roots, stems, and leaves. 1 of 34 23 1 Specialized Tissues in Plants Seed Plant Structure Roots: absorb
NOTES: CH 36 - Transport in Plants
 NOTES: CH 36 - Transport in Plants Recall that transport across the cell membrane of plant cells occurs by: -diffusion -facilitated diffusion -osmosis (diffusion of water) -active transport (done by transport
NOTES: CH 36 - Transport in Plants Recall that transport across the cell membrane of plant cells occurs by: -diffusion -facilitated diffusion -osmosis (diffusion of water) -active transport (done by transport
(Photo Atlas: Figures 9.147, 9.148, 9.150, 9.1, 9.2, )
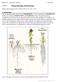 BIOL 221 Concepts of Botany Fall 2007 Topic 07: Primary Plant Body: The Root System (Photo Atlas: Figures 9.147, 9.148, 9.150, 9.1, 9.2, 9.5 9.23) A. Introduction The root has the primary functions of
BIOL 221 Concepts of Botany Fall 2007 Topic 07: Primary Plant Body: The Root System (Photo Atlas: Figures 9.147, 9.148, 9.150, 9.1, 9.2, 9.5 9.23) A. Introduction The root has the primary functions of
Biology 1030 Winter 2009
 Meeting Tissue Needs II Chapter 36 (738-755) Chapter 37 (756-770) Cellular Currency Plants harvest solar energy Photosynthesis Produces sugars Proteins, nucleic acids, lipids? H 2 O CO 2 Plants cells still
Meeting Tissue Needs II Chapter 36 (738-755) Chapter 37 (756-770) Cellular Currency Plants harvest solar energy Photosynthesis Produces sugars Proteins, nucleic acids, lipids? H 2 O CO 2 Plants cells still
TARGET STUDY MATERIAL
 TARGET STUDY MATERIAL Plus-1 Botany VOL I TARGET EDUCATIONAL INSTITUTION Target Educational institution is the one and only Entrance coaching and CBSE 10 th coaching centre at Mukkam with advanced technologies
TARGET STUDY MATERIAL Plus-1 Botany VOL I TARGET EDUCATIONAL INSTITUTION Target Educational institution is the one and only Entrance coaching and CBSE 10 th coaching centre at Mukkam with advanced technologies
Bio 10 Lecture Notes 7: Plant Diversity, Structure and Function SRJC
 Physiology study of the adaptations by which organisms function in their environ. 1.) Plants, Tissues and Function Plant types and their evolution Terrestrial plants evolved from aquatic green algae There
Physiology study of the adaptations by which organisms function in their environ. 1.) Plants, Tissues and Function Plant types and their evolution Terrestrial plants evolved from aquatic green algae There
Today: Plant Structure Exam II is on F March 31
 Next few lectures are on plant form and function Today: Plant Structure Exam II is on F March 31 Outline Plant structure I. Plant Cells structure & different types II. Types of meristems Apical meristems:
Next few lectures are on plant form and function Today: Plant Structure Exam II is on F March 31 Outline Plant structure I. Plant Cells structure & different types II. Types of meristems Apical meristems:
Chapter 6. Biology of Flowering Plants. Anatomy Seedlings, Meristems, Stems, and Roots
 BOT 3015L (Outlaw/Sherdan/Aghoram); Page 1 of 6 Chapter 6 Biology of Flowering Plants Anatomy Seedlings, Meristems, Stems, and Roots Objectives Seedling germination and anatomy. Understand meristem structure
BOT 3015L (Outlaw/Sherdan/Aghoram); Page 1 of 6 Chapter 6 Biology of Flowering Plants Anatomy Seedlings, Meristems, Stems, and Roots Objectives Seedling germination and anatomy. Understand meristem structure
PLANT STRUCTURE: PARTS (ORGANS) Roots Leaves Stems
 PLANT STRUCTURE: PARTS (ORGANS) Roots Leaves Stems ROOTS El Hiquieron. Strangulating Plant Ficusjimenezii The trees you see growing on the wall are the Higueron. The Higueronsare plants that can grow in
PLANT STRUCTURE: PARTS (ORGANS) Roots Leaves Stems ROOTS El Hiquieron. Strangulating Plant Ficusjimenezii The trees you see growing on the wall are the Higueron. The Higueronsare plants that can grow in
- Cation exchange capacity
 What is Necessary for Plant Growth? - ph (~5 to 8); - water - structural support - minerals and nutrients Macronutrients (C HOPKNS CaFe Mg) (Nutrients must be in a chemical form that plants can use.) -
What is Necessary for Plant Growth? - ph (~5 to 8); - water - structural support - minerals and nutrients Macronutrients (C HOPKNS CaFe Mg) (Nutrients must be in a chemical form that plants can use.) -
Lecture 4 Root Put line under your answer! There is only one correct answer in the multiple choice questions
 Lecture 4 Root Put line under your answer! There is only one correct answer in the multiple choice questions 1. The perception of gravity by a root is thought to take place in a) root hairs b) the region
Lecture 4 Root Put line under your answer! There is only one correct answer in the multiple choice questions 1. The perception of gravity by a root is thought to take place in a) root hairs b) the region
Chapter 30: Plant Nutrition & Transport
 Chapter 30: Plant Nutrition & Transport Carnivorous Plants Capture animals to supplement their nutrient intake Venus flytrap lures insects with sugary bait; closes on victim Cobra lily lures insects down
Chapter 30: Plant Nutrition & Transport Carnivorous Plants Capture animals to supplement their nutrient intake Venus flytrap lures insects with sugary bait; closes on victim Cobra lily lures insects down
Plant Tissues and Organs. Topic 13 Plant Science Subtopics , ,
 Plant Tissues and Organs Topic 13 Plant Science Subtopics 13.1.2, 13.1.3, 13.1.4 Objectives: List and describe the major plant organs their structure and function List and describe the major types of plant
Plant Tissues and Organs Topic 13 Plant Science Subtopics 13.1.2, 13.1.3, 13.1.4 Objectives: List and describe the major plant organs their structure and function List and describe the major types of plant
Abiotic Stress in Crop Plants
 1 Abiotic Stress in Crop Plants Mirza Hasanuzzaman, PhD Professor Department of Agronomy Sher-e-Bangla Agricultural University E-mail: mhzsauag@yahoo.com Stress Stress is usually defined as an external
1 Abiotic Stress in Crop Plants Mirza Hasanuzzaman, PhD Professor Department of Agronomy Sher-e-Bangla Agricultural University E-mail: mhzsauag@yahoo.com Stress Stress is usually defined as an external
13.4 Roots Figure 2 primary root: primary root secondary root: secondary root taproots fibrous taproots: roots. fibrous roots: adventitious roots
 10. Why is it not surprising that many hydrophytes have little or no tissue? 11. The leaves of many underwater plants are finely divided, dramatically increasing the surface area that is in contact with
10. Why is it not surprising that many hydrophytes have little or no tissue? 11. The leaves of many underwater plants are finely divided, dramatically increasing the surface area that is in contact with
MICROSEEPAGE RELATED REDOX MODELS
 INTRODUCTION The study of oxidation-reduction processes in soils began in the 1900 s (Gillespie, 1920) and has since been applied to biological, limnological, and geochemical systems (Bass Becking, 1960).
INTRODUCTION The study of oxidation-reduction processes in soils began in the 1900 s (Gillespie, 1920) and has since been applied to biological, limnological, and geochemical systems (Bass Becking, 1960).
Aeration of the root system in Alnus glutinosa L. Gaertn.
 Aeration of the root system in Alnus glutinosa L. Gaertn. P. Schröder* Bot. Inst Univ. Cologne, Gyrhofstr. 15, D-5000 Kdln 41, F.F1.G. Introduction and theoretical considerations When soils are wet or
Aeration of the root system in Alnus glutinosa L. Gaertn. P. Schröder* Bot. Inst Univ. Cologne, Gyrhofstr. 15, D-5000 Kdln 41, F.F1.G. Introduction and theoretical considerations When soils are wet or
CHAPTER 6 ANATOMY OF FLOWERING PLANTS MULTIPLE CHOICE QUESTIONS
 ANATOMY OF FLOWERING PLANTS 27 27 CHAPTER 6 ANATOMY OF FLOWERING PLANTS MULTIPLE CHOICE QUESTIONS 1. A transverse section of stem is stained first with safranin and then with fast green following the usual
ANATOMY OF FLOWERING PLANTS 27 27 CHAPTER 6 ANATOMY OF FLOWERING PLANTS MULTIPLE CHOICE QUESTIONS 1. A transverse section of stem is stained first with safranin and then with fast green following the usual
Oxygen deficiency and salinity affect cell-specific ion concentrations in adventitious roots of barley (Hordeum vulgare)
 Research Oxygen deficiency and salinity affect cell-specific ion concentrations in adventitious roots of barley (Hordeum vulgare) Lukasz Kotula 1, Peta L. Clode 2, Gustavo G. Striker 1,3, Ole Pedersen
Research Oxygen deficiency and salinity affect cell-specific ion concentrations in adventitious roots of barley (Hordeum vulgare) Lukasz Kotula 1, Peta L. Clode 2, Gustavo G. Striker 1,3, Ole Pedersen
Biology Slide 1 of 36
 Biology 1 of 36 2 of 36 Types of Roots Types of Roots What are the two main types of roots? 3 of 36 Types of Roots The two main types of roots are: taproots, which are found mainly in dicots, and fibrous
Biology 1 of 36 2 of 36 Types of Roots Types of Roots What are the two main types of roots? 3 of 36 Types of Roots The two main types of roots are: taproots, which are found mainly in dicots, and fibrous
Plant Anatomy: roots, stems and leaves
 Plant Anatomy: roots, stems and leaves The plant body has a hierarchy of organs, tissues and cells Plants, like animals, have organs composed of different tissues, which are composed of cells. Tissue is
Plant Anatomy: roots, stems and leaves The plant body has a hierarchy of organs, tissues and cells Plants, like animals, have organs composed of different tissues, which are composed of cells. Tissue is
Transport in Plants. Transport in plants. Transport across Membranes. Water potential 10/9/2016
 Transport in Plants Transport in plants How is a plant able to move water and nutrients from roots to the rest of the plant body? Especially tall trees? Sequoia can be over 300 feet tall! Transport across
Transport in Plants Transport in plants How is a plant able to move water and nutrients from roots to the rest of the plant body? Especially tall trees? Sequoia can be over 300 feet tall! Transport across
Homework for Monday: Correct potometer questions Complete transport in plants worksheet
 Transport in plants Homework for Monday: Correct potometer questions Complete transport in plants worksheet Transpiration the loss of water from a plant through evaporation Did you know? A 15m maple tree
Transport in plants Homework for Monday: Correct potometer questions Complete transport in plants worksheet Transpiration the loss of water from a plant through evaporation Did you know? A 15m maple tree
Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots
 Blackwell Science, LtdOxford, UK PCEPlant, Cell and Environment0016-8025Blackwell Science Ltd 2002 26 846 Gas transport in plants T. D. Colmer 10.1046/j.0016-8025.2002.00846.x Original ArticleBEES SGML
Blackwell Science, LtdOxford, UK PCEPlant, Cell and Environment0016-8025Blackwell Science Ltd 2002 26 846 Gas transport in plants T. D. Colmer 10.1046/j.0016-8025.2002.00846.x Original ArticleBEES SGML
Overview of Plant Tissues
 Plant Tissue Growth Key Concepts Overview of Plant Tissues Seed-bearing vascular plants have a shoot system with stems, leaves, and reproductive parts Most also have a root system These systems consist
Plant Tissue Growth Key Concepts Overview of Plant Tissues Seed-bearing vascular plants have a shoot system with stems, leaves, and reproductive parts Most also have a root system These systems consist
Structure of Hair Roots in Lysinema ciliatum R. Br. and its Implications for their Water Relations
 Annals of Botany 77: 383 388, 1996 Structure of Hair Roots in Lysinema ciliatum R. Br. and its Implications for their Water Relations W. G. ALLAWAY* and A. E. ASHFORD * School of Biological Sciences, The
Annals of Botany 77: 383 388, 1996 Structure of Hair Roots in Lysinema ciliatum R. Br. and its Implications for their Water Relations W. G. ALLAWAY* and A. E. ASHFORD * School of Biological Sciences, The
Chapter 36: Transport in Vascular Plants - Pathways for Survival
 Chapter 36: Transport in Vascular Plants - Pathways for Survival For vascular plants, the evolutionary journey onto land involved differentiation into roots and shoots Vascular tissue transports nutrients
Chapter 36: Transport in Vascular Plants - Pathways for Survival For vascular plants, the evolutionary journey onto land involved differentiation into roots and shoots Vascular tissue transports nutrients
23 2 Roots Slide 2 of 36
 2 of 36 Types of Roots Types of Roots What are the two main types of roots? 3 of 36 Types of Roots The two main types of roots are: taproots, which are found mainly in dicots, and fibrous roots, which
2 of 36 Types of Roots Types of Roots What are the two main types of roots? 3 of 36 Types of Roots The two main types of roots are: taproots, which are found mainly in dicots, and fibrous roots, which
Chapter 23 Notes Roots Stems Leaves
 Chapter 23 Notes Roots Stems Leaves I. Specialized tissue in plants - effective way to ensure the plant s survival A. Seed plant structure 1. Roots - a. Absorbs water and dissolves nutrients b. anchors
Chapter 23 Notes Roots Stems Leaves I. Specialized tissue in plants - effective way to ensure the plant s survival A. Seed plant structure 1. Roots - a. Absorbs water and dissolves nutrients b. anchors
Title: Nutrient Movement Towards and Into Plant Roots Speaker: Bill Pan. online.wsu.edu
 Title: Nutrient Movement Towards and Into Plant Roots Speaker: Bill Pan online.wsu.edu Unit 1, Lesson 4 Nutrient Movement Towards and Into Plant Roots http://soils.usda.gov/education/resources/k_12/lessons/profile/
Title: Nutrient Movement Towards and Into Plant Roots Speaker: Bill Pan online.wsu.edu Unit 1, Lesson 4 Nutrient Movement Towards and Into Plant Roots http://soils.usda.gov/education/resources/k_12/lessons/profile/
Anatomy of Flowering Plants. K C Meena PGT Biology
 Anatomy of Flowering Plants K C Meena PGT Biology Tissues A group of similar cells performing same function. Types of plant tissues - Meristematic tissues and permanent tissues. Meristematic tissues Have
Anatomy of Flowering Plants K C Meena PGT Biology Tissues A group of similar cells performing same function. Types of plant tissues - Meristematic tissues and permanent tissues. Meristematic tissues Have
! Xylem - Chief conducting tissue for water and minerals absorbed by the roots.
 + Complex Tissues! Complex tissues are made up of two or more cell types.! Xylem - Chief conducting tissue for water and minerals absorbed by the roots.! Vessels - Made of vessel elements.! Long tubes
+ Complex Tissues! Complex tissues are made up of two or more cell types.! Xylem - Chief conducting tissue for water and minerals absorbed by the roots.! Vessels - Made of vessel elements.! Long tubes
NOTES: CH 35 - Plant Structure & Growth
 NOTES: CH 35 - Plant Structure & Growth In their evolutionary journey, plants adapted to the problems of a terrestrial existence as they moved from water to land ANGIOSPERMS (flowering plants) -most diverse
NOTES: CH 35 - Plant Structure & Growth In their evolutionary journey, plants adapted to the problems of a terrestrial existence as they moved from water to land ANGIOSPERMS (flowering plants) -most diverse
2.1 PLANT TISSUE HALIMAHTUN SAEDIAH BT ABU BAKAR KOLEJ TEKNOLOGI TIMUR
 2.1 PLANT TISSUE HALIMAHTUN SAEDIAH BT ABU BAKAR KOLEJ TEKNOLOGI TIMUR GENERAL Plant cell are differentiated possessing structural adaptations that make specific functions possible. Modifications of cell
2.1 PLANT TISSUE HALIMAHTUN SAEDIAH BT ABU BAKAR KOLEJ TEKNOLOGI TIMUR GENERAL Plant cell are differentiated possessing structural adaptations that make specific functions possible. Modifications of cell
Trees are: woody complex, large, long-lived self-feeding shedding generating systems compartmented, self optimizing
 BASIC TREE BIOLOGY Trees are: woody complex, large, long-lived self-feeding shedding generating systems compartmented, self optimizing Roots: absorb water and minerals store energy support and anchor
BASIC TREE BIOLOGY Trees are: woody complex, large, long-lived self-feeding shedding generating systems compartmented, self optimizing Roots: absorb water and minerals store energy support and anchor
Groningen) (.Botanical Laboratory, day a. 1958), many investigators are inclined to assume that the ions. freely by of diffusion
 Acta Botanica Neerlandica 8 (1959) 68-76 Diffusible and Exchangeable Rubidium Ions in Pea Roots R. Brouwer*) (.Botanical Laboratory, Groningen) {received January 28th, 1959) Introduction Since the concept
Acta Botanica Neerlandica 8 (1959) 68-76 Diffusible and Exchangeable Rubidium Ions in Pea Roots R. Brouwer*) (.Botanical Laboratory, Groningen) {received January 28th, 1959) Introduction Since the concept
Tissues: - A group of cells similar in structure and performing a particular function forms a tissue.
 Plant Tissues Class- IX Tissues: - A group of cells similar in structure and performing a particular function forms a tissue. PLANT TISSUES ANIMAL TISSUES 1. Most of the plant tissues are Most of the tissues
Plant Tissues Class- IX Tissues: - A group of cells similar in structure and performing a particular function forms a tissue. PLANT TISSUES ANIMAL TISSUES 1. Most of the plant tissues are Most of the tissues
Supplementary Methods
 Supplementary Methods Modeling of magnetic field In this study, the magnetic field was generated with N52 grade nickel-plated neodymium block magnets (K&J Magnetics). The residual flux density of the magnets
Supplementary Methods Modeling of magnetic field In this study, the magnetic field was generated with N52 grade nickel-plated neodymium block magnets (K&J Magnetics). The residual flux density of the magnets
AP Biology Chapter 36
 Chapter 36 Chapter 36 Transport in Plants 2006-2007 Transport in plants - Overview H2O & minerals transport in xylem transpiration evaporation, adhesion & cohesion negative pressure Sugars transport in
Chapter 36 Chapter 36 Transport in Plants 2006-2007 Transport in plants - Overview H2O & minerals transport in xylem transpiration evaporation, adhesion & cohesion negative pressure Sugars transport in
Why Calcium is So Important
 Why Calcium is So Important Calcium - A Transportation Problem By Dr. Lynette Morgan As hydroponic growers we like to think that by supplying our plants with all the nutrients they need in the right ratios,
Why Calcium is So Important Calcium - A Transportation Problem By Dr. Lynette Morgan As hydroponic growers we like to think that by supplying our plants with all the nutrients they need in the right ratios,
BRIDGE COURSE STANDARD XI, NCERT, BIOLOGY UNIT 4- PLANT PHYSIOLOGY
 BRIDGE COURSE STANDARD XI, NCERT, BIOLOGY UNIT 4- PLANT PHYSIOLOGY Plant physiology:- It is a branch of botany which deals with the study of functions and processes curring in plants. The physiological
BRIDGE COURSE STANDARD XI, NCERT, BIOLOGY UNIT 4- PLANT PHYSIOLOGY Plant physiology:- It is a branch of botany which deals with the study of functions and processes curring in plants. The physiological
Effects of oil on internal gas transport, radial oxygen loss, gas films and bud growth in Phragmites australis
 Annals of Botany 103: 333 340, 2009 doi:10.1093/aob/mcn213, available online at www.aob.oxfordjournals.org Effects of oil on internal gas transport, radial oxygen loss, gas films and bud growth in Phragmites
Annals of Botany 103: 333 340, 2009 doi:10.1093/aob/mcn213, available online at www.aob.oxfordjournals.org Effects of oil on internal gas transport, radial oxygen loss, gas films and bud growth in Phragmites
CASE STUDY WATER ABSORPTION AND TRANSPORT IN PLANTS
 CASE STUDY WATER ABSORPTION AND TRANSPORT IN PLANTS Presentation of the problem: We need a pump to uplift water to a tank. The requirement of a pump is to pull water against the gravity. Look at the human
CASE STUDY WATER ABSORPTION AND TRANSPORT IN PLANTS Presentation of the problem: We need a pump to uplift water to a tank. The requirement of a pump is to pull water against the gravity. Look at the human
The secondary meristem result in growth in a lateral direction, such as the increase in girth of a tree.
 Chapter 9b-Stems and Material Transport Woody plants produce wood tissue and bark through the activity of secondary meristems: The secondary meristem result in growth in a lateral direction, such as the
Chapter 9b-Stems and Material Transport Woody plants produce wood tissue and bark through the activity of secondary meristems: The secondary meristem result in growth in a lateral direction, such as the
Water Transport in Onion (Allium cepa 1.) Roots'
 Plant Physiol. (1993) 101: 1305-1315 Water Transport in Onion (Allium cepa 1.) Roots' Changes of Axial and Radial Hydraulic Conductivities during Root Development Walter Melchior and Ernst Steudle* Lehrstuhl
Plant Physiol. (1993) 101: 1305-1315 Water Transport in Onion (Allium cepa 1.) Roots' Changes of Axial and Radial Hydraulic Conductivities during Root Development Walter Melchior and Ernst Steudle* Lehrstuhl
Apoplastic barriers in roots: chemical composition of
 Journal of Experimental Botany, Vol. 50, No. 337, pp. 1267 1280, August 1999 REVIEW ARTICLE Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls Lukas Schreiber1,
Journal of Experimental Botany, Vol. 50, No. 337, pp. 1267 1280, August 1999 REVIEW ARTICLE Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls Lukas Schreiber1,
Compartments and Transport. Three Major Pathways of Transport. Absorp+on of Water and Minerals by Root Cells. Bulk flow
 Plasmodesmata Channels connec+ng neighboring cells Cell membrane and cytosol are con+nuous from cell to cell Symplast Cytoplasmic con+nuum Apoplast Compartments and Transport Through plasmodesmata con+nuum
Plasmodesmata Channels connec+ng neighboring cells Cell membrane and cytosol are con+nuous from cell to cell Symplast Cytoplasmic con+nuum Apoplast Compartments and Transport Through plasmodesmata con+nuum
Plants. Tissues, Organs, and Systems
 Plants Tissues, Organs, and Systems Meristematic cells Specialized cells that are responsible for producing specialized cells, they produce three types of tissue in the body of a plant. Meristematic Cells
Plants Tissues, Organs, and Systems Meristematic cells Specialized cells that are responsible for producing specialized cells, they produce three types of tissue in the body of a plant. Meristematic Cells
GFP GAL bp 3964 bp
 Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Non Permanent Tissues - Meristematic Tissue
 PLANT TISSUES Non Permanent Tissues - Meristematic Tissue Undifferentiated plant cells that are continually dividing by mitosis Large thin walled cells No vacuole Dense cytoplasm Large nucleus Found at
PLANT TISSUES Non Permanent Tissues - Meristematic Tissue Undifferentiated plant cells that are continually dividing by mitosis Large thin walled cells No vacuole Dense cytoplasm Large nucleus Found at
Plant Anatomy: roots, stems and leaves
 Plant Anatomy: roots, stems and leaves The plant body has a hierarchy of organs, tissues and cells Plants, like animals, have organs composed of different tissues, which are composed of cells. Tissue is
Plant Anatomy: roots, stems and leaves The plant body has a hierarchy of organs, tissues and cells Plants, like animals, have organs composed of different tissues, which are composed of cells. Tissue is
Plant Structure and Function (Ch. 23)
 Plant Structure and Function (Ch. 23) Basic plant anatomy 1 root root tip root hairs Roots Roots anchor plant in soil, absorb minerals & water, & store food fibrous roots (1) mat of thin roots that spread
Plant Structure and Function (Ch. 23) Basic plant anatomy 1 root root tip root hairs Roots Roots anchor plant in soil, absorb minerals & water, & store food fibrous roots (1) mat of thin roots that spread
The Vascular Plant Body
 The Vascular Plant Body Like animals, plants are made up of specialized cells that are organized into tissues, which are themselves organized into systems of organs. The various parts of plants are adapted
The Vascular Plant Body Like animals, plants are made up of specialized cells that are organized into tissues, which are themselves organized into systems of organs. The various parts of plants are adapted
Ch. 36 Transport in Vascular Plants
 Ch. 36 Transport in Vascular Plants Feb 4 1:32 PM 1 Essential Question: How does a tall tree get the water from its roots to the top of the tree? Feb 4 1:38 PM 2 Shoot architecture and Light Capture: Phyllotaxy
Ch. 36 Transport in Vascular Plants Feb 4 1:32 PM 1 Essential Question: How does a tall tree get the water from its roots to the top of the tree? Feb 4 1:38 PM 2 Shoot architecture and Light Capture: Phyllotaxy
Plant Organs. Roots & Stems
 Plant Organs Roots & Stems I. Roots A. F(x)s = grow underground 1. Absorb water & nutrients from soil 2. Anchor plant in the soil 3. Make hormones important for growth & development I. Roots B. Structure
Plant Organs Roots & Stems I. Roots A. F(x)s = grow underground 1. Absorb water & nutrients from soil 2. Anchor plant in the soil 3. Make hormones important for growth & development I. Roots B. Structure
Two major categories. BIOLOGY 189 Fundamentals of Life Sciences. Spring 2004 Plant Structure and Function. Plant Structure and Function
 BIOLOGY 189 Fundamentals of Life Sciences Spring 2004 Plant Structure and Function 18 16 14 12 10 8 6 Examination #1 Class Average: 33/60 for 55% 4 Chapters 31-32 32 2 0 6 10 15 20 25 30 35 40 45 50 55
BIOLOGY 189 Fundamentals of Life Sciences Spring 2004 Plant Structure and Function 18 16 14 12 10 8 6 Examination #1 Class Average: 33/60 for 55% 4 Chapters 31-32 32 2 0 6 10 15 20 25 30 35 40 45 50 55
Anatomy of dicotyledonous plants
 Anatomy of dicotyledonous plants Differences between Monocotyledons and Dicotyledons All plants are classified as producing seeds or not producing seeds. Those that produce seeds are divided into flowering
Anatomy of dicotyledonous plants Differences between Monocotyledons and Dicotyledons All plants are classified as producing seeds or not producing seeds. Those that produce seeds are divided into flowering
Waterlogging tolerance of trees
 Waterlogging tolerance of trees Tapani Repo, Metla Silviculture in Changing Environment, Nov. 24-25, 2014 Contents Motivation Background concerning waterlogging tolerance An example of dormancy waterlogging
Waterlogging tolerance of trees Tapani Repo, Metla Silviculture in Changing Environment, Nov. 24-25, 2014 Contents Motivation Background concerning waterlogging tolerance An example of dormancy waterlogging
Chemistry Review. Structure of an Atom. The six most abundant elements of life. Types of chemical bonds. U n i t 2 - B i o c h e m i s t r y
 Chemistry Review Structure of an Atom are organized into shells or levels around the nucleus. Atoms are most stable when their outer or valence shell is. The six most abundant elements of life Types of
Chemistry Review Structure of an Atom are organized into shells or levels around the nucleus. Atoms are most stable when their outer or valence shell is. The six most abundant elements of life Types of
