The Biology of Methanogenic Bacteria
|
|
|
- Randolf Evans
- 6 years ago
- Views:
Transcription
1 BACTrziOLOGICAL Rzvizws, June 1977, p Vol. 41, No. 2 Copyright American Society for Microbiology Prin tted in U.S.A. The Biology of Methanogenic Bacteria J. G. ZEIKUS Department of Bacteriology, University of Wisconsin, Madison, Wisconsin INTRODUCTION... METHODS FOR STUDY... Isolation and Cultivation Techniques... Gas Chromatographic Analysis... GENERAL PROPERTIES... Species Characteristics... Selective Enrichment of Genera... Morphological Variation... Fine Structure... Coccus-type cells... Sarcina-type cells... Rod-type cells... Spirillum-type cells... Taxonomy... PHYSIOLOGICAL ASPECTS... Intermediary Metabolism... Unique biochemical components... Methane synthesis...; Nature of Autotrophic Growth in Methanobacterium thermoautotrophicum... ECOLOGICAL ASPECTS... Activities in Nature... Microbial Interactions... ACKNOWLEDGEMENTS... LITERATURE CITED... INTRODUCTION My purpose here is both to describe certain features of methanogens (i.e., methane-producing bacteria) and to view the biology of this microbial group. I first heard the term methanogen used by M. P. Bryant; this term is descriptive and eliminates confusion with another microbial group, the methane-oxidizing bacteria. The biological formation of methane (CH4) is the result of a specific type of bacterial energyyielding metabolism. Eucaryotic organisms and blue-green algae have not been reported to produce methane. Previous reviews on microbial methanogenesis by Barker (4), Stadtman (95), and Wolfe (112) summarized the state of knowledge on methanogenic bacteria and the biochemistry of methane synthesis through 1970; much of that material will not be presented here, except where importance dictates. Bacterial methanogenesis is a ubiquitous process in most anaerobic environments. The association of this event with anaerobic decomposition of organic matter in microbial habitats such as sewage sludge digesters, the rumen and intestinal tract of animals, and in sediments and muds of various aquatic habitats has been recognized and documented for more than a century. Thus, gas production commonly observed in nature can often be ascribed to growth of methanogens on specific energy sources that are formed as a result of microbial decomposition of organic matter (Fig. 1A) or in association with geochemical activity (Fig. 1B). However, detailed information on the biological properties of methanogenic bacteria has only recently materialized. Paramount to an integrated understanding of these unique microorganisms was the vivid documentation of their primary chemolithotrophic metabolism by Bryant and co-workers (10, 14). Subsequent literature that will be reviewed here has provided new scientific vistas and enables a better understanding of older studies on methanogenesis Ḟew natural groupings of microorganisms, such as those designated as parts in Bergey's Manual ofdeterminative Bacteriology (17), are as morphologically diverse as the methanogenic bacteria. Nevertheless, all methanogenic species share certain unique and unifying physiological properties. Methanogens should no longer be regarded as a mysterious group of poorly studied microbes. Indeed, the present world "energy crisis" has generated a new stimulus and scientific interest to better understand bacteria that produce natural gas.
2 VOL. 41, 1977 THE BIOLOGY OF METHANOGENIC BACTERIA 515 Downloaded from FIG. 1. (A) Ignited methane gas eminating from a hollow increment-borer bit drilled 20 cm into a cottonwood tree located on the shore oflake Wingra, Wis. Photograph is from a time exposure taken at night (from reference 117). (B) Ebullition ofgas bubbles that contained mainly CH4 and CO2 from a thermal spring in Yellowstone National Park. Methanogens were isolated from this spring, which contained geothermal hydrogen (unpublished observation). on April 21, 2018 by guest METHODS FOR STUDY Isolation and Cultivation Techniques Methanogens are perhaps the most- strictly anaerobic bacteria known, and detailed studies require the use of stringent procedures that ensure culture in the absence of oxygen. Procedures developed by Hungate have proved most successful for cultivation of fastidious anaerobes. One should refer to a recent article by Hungate (46) for a complete description of this method. Modifications of the Hungate technique described by Bryant (12) have been most widely used for the study of methanogens. This method utilizes glass test tubes (Bellco Glass, Inc., anaerobic culture tubes) that are tightly
3 516 ZEIKUS sealed with rubber stoppers during concomitant removal of gassing cannulae. Neoprene, butyl, or synthetic, but not gum, rubber are suitable as culture tube enclosures. Gases that enter the cannula (a presterilized, cotton-filled 5-ml glass syringe, fitted with a bent 2-inch [ca cm], 18-gauge needle) are scrubbed free of oxygen traces by passage through heated copper filings. A gas mixture of 80% H2 and 20% CO2 can be routinely used for culture of methanogens. However, a 50:50 mixture of H2-CO2 is preferred by some investigators (12), because this gas mixture is more dense and not as easily displaced by air when culture containers are opened. Media or substrate addition and transfer or inoculation of tubes are each accomplished by pipetting while the tubes are being gassed. Alternatively, "Hungate type" tubes (Bellco) that are screw-capped and sealed with flanged rubber stoppers can be used. Addition and transfer to these tubes utilizes the syringe methods described by Macy et al. (59). Recently, Miller and Wolin (65) have described procedures that employ serum bottles (Wheaton, Industrial Glass Division) or various glass containers fitted with serum bottle necks. Culture containers are closed with butyl rubber serum-stoppers and secured with a crimped metal seal. All inoculations are performed with a hypodermic syringe and needle. These procedures require less manipulation. The use of metal-secured containers for growth of some methanogens is advantageous because nonsecured stoppers are often blown out of culture tubes as the result of active methanol fermentation. Serum bottles are also more easily handled and less prone to breakage than test tubes and are ideal for ecological studies in the field. A new approach for cultivation of H2-oxidizing methanogens has been described by Balch and Wolfe (1). These procedures allow for good growth of methanogens under high gas pressures (2 to 4 atmospheres of H2-CO2) without the need for repeated culture gassing. The authors described procedures for preparation and use of a specialized gassing manifold, glass culture tubes for liquid cultures, and anaerobic incubators for agar plate cultures. The Hungate culture technique, with its modifications, has proven to be an excellent method for isolating fastidious anaerobes and maintaining small quantities of cells. The methods described by Bryant et al. (14) for culturing larger quantities of cells have proven acceptable for most methanogenic species (for a modification of these procedures, see Daniels and Zeikus [28]). Edwards and McBride (32) have described BACTZIUOL. Rzv. procedures for isolation and growth of methanogens that utilize a Freter-type anaerobic glove box equipped with an inner ultralow oxyen chamber. Cultures are plated in the outer anaerobic glove box and immediately placed into the inner ultralow oxygen chamber, which is periodically flushed with H2-CO2 (80:20). The inner chamber allows for growth on hydrogen and maintains the redox potential necessary for growth of methanogens. This method is considerably more expensive than Hungate procedures; however, it offers unique advantages. For example, it requires less skill and manual dexterity, and it allows for routine genetic procedures such as replica plating. In lieu of an inner chamber, plates can be incubated in specially modified pressure-cooker containers for growth under higher atmospheric pressures of H2-C02 (1). These authors (32) also described a novel screening procedure for identification of methanogenic colonies. Presumptive identification is based on the detection of fluorescent colonies when streak plates are exposed to long-wave ultraviolet light. Small colonies (less than 0.5 mm) usually do not fluoresce. All methanogens that have been examined contain a unique pigment, Factor420 (F420), which fluoresces when the oxidized form is excited by long-wave ultraviolet light. During active growth, F420 exists in a partially oxidized state (32). In addition, methanogenic bacteria will brightly fluoresce when observed by ultraviolet microscopy, although this fluorescence is short-lived (65a). Additional procedures used for identification of methanogens are based on demonstration of active methane production by isolated cultures. Gas Chromatographic Analysis During the course of physiological and ecological investigations of methanogens, it is often necessary to monitor utilized or produced metabolic gases. The energy-yielding metabolism of methane-producing bacteria often involves the oxidation of hydrogen with the concomitant reduction of carbon dioxide. Nelson and Zeikus (68) have described a gas chromatographic procedure for analysis of "4C-labeled and unlabeled metabolic gases from microbial methanogenic systems that is more rapid, sensitive, and convenient than gas chromatography-liquid scintillation techniques (61). This method identifies and quantifies gases by thermal conductivity detection and directly channels the gas chromatograph detector effluent into a gas proportional counter for radioactivity measurement. These procedures have been em-
4 VOL. 41, 1977 THE BIOLOGY OF METHANOGENIC BACTERIA 517 ployed for ecological (110, 111, 123) and physiological (122) studies of methanogens. Thermal conductivity detection is often more useful than flame ionization detection because H2, CH4, and CO2 can be accurately quantified on the same column. Although flame ionization detection is more sensitive than thermal conductivity detection, and is the preferred method for methane analysis, it is limited to CH-containing molecules and is, therefore, unsuitable for H2 and CO2. GENERAL PROPERTIES Species Characteristics Table 1 describes features of the taxonomically identified species of methanogens that are maintained in pure culture in several research laboratories. Other taxonomically described methanogenic species (13) that are presently not in culture include: Methanococcus mazei, Methanobacterium soehngenii, and Methanosarcina methanica. These species have been lost and/or were never obtained in well-documented pure culture. Numerous strains of methanogens that have been isolated by various investigators (14, 22, 71, 90, 115a, 123, 124) remain to be described in more detail before taxonomic assignment is established. Most notably these strains include Methanobacterium strain MOH (60), isolated from the Methanobacillus omelianskii symbiosis, and a recently obtained Methanobacterium strain (22) that metabolizes acetate in a complex medium. All methanogenic bacteria can use hydrogen as a sole source of reducing power for methanogenesis (i.e., as an energy source) and for cell carbon synthesis; several species utilize formate, and one species, Methanosarcina barkeri, can use methanol. Another metabolic feature shared by several species is the ability to synthesize all cellular carbon from CO2 while growing at the expense of hydrogen oxidation. However, autotrophy has been difficult to document in some species because of very slow growth in the absence of certain organic compounds. It has been well established that acetate is the major methanogenic precursor in several anaerobic ecosystems (see below, Ecological Aspects). However, acetate has not been demonstrated to serve as the sole electron donor for growth and methanogenesis in pure cultures. Previous isotopic studies of Stadtman and Barker (92, 93) that demonstrated very slow (15 weeks) acetate fermentation were performed with "highly purified" cultures of M. barkeri and Methanococcus species. These cultures contained more than one distinct morphological type. Methanosarcina species are difficult to isolate and maintain in pure culture. The work of Pine and Barker (73) and Pine and Vishniac (74) used crude enrichment cultures to demonstrate that the intact methyl group of acetate was fermented to methane. Conservation of the protons in the methyl moiety strongly suggests that CHR production from acetate was attained via a single reductive step by a single organinm. Isotopic tracer studies of Zeikus et al. (122) showed that hydrogen was required for the metabolism of acetate to methane by several pure TABLE 1. Properties of taxonomically described methanogenic species in pure culture (1977) Substrates that serve as sole electron Species name donor for both meth- Autotrophc Taxonomic description anogenesis and growth growth Methanobacteriuma arbophilicum Hydrogen Yes Zeikus and Henning (119) Methanobacterium formicium Hydrogen or formate Yes Schnellen (88) Methanobacterium ruminantium Hydrogen or formate No Smith and Hungate (91) Methanobacterium mobile Hydrogen or formate No Paynter and Hungate (72) Methanobacteriuma thermo- Hydrogen Yes Zeikus and Wolfe (116) autotrophicum Methanococcus vannieli Hydrogen or formate Not deter- Stadtman and Barker minedb (94) Methanosarcina barkeri Hydrogen or methanol Yes Schnellen (88) Methanospirilluma hungatii Hydrogen or formate Not deter- Ferry et al. (33) minedb a Type strain deposited in American Type Culture Collection. b Growth occurred in mineral salts medium that contained H2 or formate and an organic reducing agent (cysteine or sodium thioglycolate). These species may be capable of autotrophy.
5 518 ZEIKUS cultures. One strain of M. barkeri and one of Methanobacterium thermoautotrophicum produced methane in a mineral salts medium that contained acetate (1% final concentration) as the sole organic addition and an H2-N2 gas phase. Under these conditions, M. thermoautotrophicum converted approximately 5% of the acetate to methane in 150 h, and both the methyl and carboxyl moieties of acetate appeared to be reduced. Acetate conversion to CH4 by these strains was dependent on H2, C02, and acetate concentrations. Methane was not formed after prolonged incubation times (6 weeks) of either species in an acetate-mineral salts medium with an N2 or CO2 gas phase. Growth of methanogens on acetate-mineral salts medium was only observed in the presence of an H2-CO2 gas phase. Under these conditions, most of the methane formed by M. thermoautotrophicum originated from CO2 reduction. Thus, the amount of acetate converted to methane greatly depends upon the cultural conditions. The minor conversion of acetate to methane demonstrated by these pure culture studies does not account for how acetate is converted to CH4 either in nature or in mixed cultures. Cappenberg (22) has shown that an undescribed Methanobacterium species converted acetate to methane in a complex medium, although tracer studies were not used. Growth under these conditions in continuous culture was slow (65-h doubling time). When this methanogenic species was grown in continuousmixed culture with a sulfate-reducing species, acetate utilization was greatly enhanced. This Methanobacterium species can also use H2-CO2 as methanogenic substrates. Preliminary studies of Mah (Abstr. Annu. Meet. Am. Soc. Microbiol. 1976, Q27-29, p. 195) indicate that acetate dissimilation by a strain of M. barkeri in a complex medium was not dependent on H2, although acetate was utilized more rapidly in the presence of H2. This strain also uses H2-CO2 as methanogenic substrates. Factors affecting the rate of acetate conversion in a methanogenic enrichment culture were reported by Van den Berg et al. (106). The mixed culture did not use H2 or HCOOH, and methanogenesis from acetate was greatly influenced by the redox and ionic strength of the synthetic medium. Thus, acetate conversion to methane has been demonstrated in both pure and mixed cultures, although more quantitative and detailed studies are needed both to document that pure cultures of methane-producing bacteria oxidize acetate in a facile manner and to establish the significance of acetate as an energy source for growth. BACTERIOL. REV. Table 2 compares the free energy available from the metabolism of various methanogenic substrates. It is clear from these calculations that H2 (equation 1) and formate (equation 2) are nearly equivalent energy sources for methanogenesis. Less energy is available from methanol fermentation (equation 3), and limited energy is obtained by acetate fermentation (equation 4). An analysis of energy formed per mole of methane produced by these reactions demonstrates that approximately four times as much energy is available from respiration of hydrogen than from acetate fermentation. These thermodynamic calculations do not rule out the possibility of acetate-fermenting methanogens because equation 4 is exergonic. However, an acetate-fermenting methanogen would be inherently slow growing, and it would be questionable if fermentation of acetate alone would sustain the energy requirements for growth. Decker et al. (30) suggest that degradation of a substrate beyond acetate is feasible for energy production only if the oxidation of reduced coenzymes can be linked to electron transport phosphorylation. Values reported for the free energy of adenosine 5'-triphosphate (ATP) hydrolysis have been estimated to range from -8.2 kcal/mol (ca kj/mol) to kcal/mol (ca kj/mol) at physiological conditions. Normal efficiencies of energy transfer in bacteria are 30 to 50% (30, 64). Thus, reactions (e.g., equation 4) that yield less than kcal/mol (ca kj/mol) (at standard conditions) make the formation of an energy-rich compound via substrate level phosphorylation unlikely and are insufficient for cell growth (30). It should be noted that equation 4 describes an intramolecular redox proc- TABLE 2. Energy metabolism of methanogenic bacteria AGO' (kcallmol of reaction)a 1. 4H2 + HCO3- + H+ -3 CH4 + 3H HC H+ -b CH4 + 3CO2 + 2H CH30H -- 3CH4 + CO2 + 2H CH3COO- + H+ -- CH4 + CO a Calculated from published values (107) for the free energy of formation of equation reactants and products at standard conditions (250C, 1 atmosphere, and equal molar concentrations of reactants and products). Values for the free energy of formation of H2, CH4, and CO2 represented as gases; HCO3-, HC02-, and CH3CO2- represented as aqueous ions; and H+, H20, and CH20H represented as aqueous.
6 VOL. 41, 1977 ess, and, thus, energy should not be obtained by this reaction from electron transport phosphorylation (102a). Mechanistically, it is not known how energy can be obtained from acetate metabolism by methanogens. Previous isotopic tracer and deuterium incorporation studies (73, 74, 92, 93) proposed that acetate was metabolized by the following reaction: CH3*COOH' CH4*' + CO. It is important to note that this mechanism would not allow for microbial growth on acetate alone. Microbial growth on acetate as the sole reducing source would only occur if the C2 of acetate were oxidized to generate reducing equivalents. Electrons generated in this manner could then be used to convert acetate to cell material. If this occurred, significant amounts of 14CO2 would be formed from the C2 of acetate. Alternatively, acetate fermentation could be the major source of energy, but other substrates (H2 or organic compounds) would be needed to provide reducing equivalents or precursors for cell carbon synthesis. However, any alternative electron donor present in an acetate medium may also serve as an energy source for growth. Also, the repeated inability to isolate and grow acetate-fermenting methanogens on acetate alone as the sole electron donor for growth and methanogenesis (34, 66, 75, 123) may be the result of improper cultivation conditions. Carbon monoxide can also be used as a substrate by methanogens. Kluyver and Schnellen (52) demonstrated that cell suspensions of M. barkeri and Methanobacterium formicicum converted CO to C02 and CH4. Thus, while methane can be produced from carbon monoxide by some methanogens, its use as an energy source for growth is not documented. Three methanogenic bacteria have been described since 1970 and include M. thermoautotrophicum, Methanobacterium arbophilicum, and Methanospirillum hungatii. M. thermoautotrophicum type strain AH was first isolated from a sewage sludge digestor in Urbana, Ill., and has an optimum temperature for growth between 65 and 700C (116). Other thermophilic isolates that appear similar to this species have been obtained from thermophilic manure digestors, thermal springs, and decomposing algal mats associated with thermal spring effluents (Yellowstone National Park, U.S.A.). M. thermoautotrophicum strain AO, isolated from a sewage sludge digestor in Madison, Wis., appears identical to the type strain in morphology, nutrition, and guanine plus cytosine (G+C) content. M. thermoautotrophicum is an obligately chemolithotrophic autotroph, and growth of THE BIOLOGY OF METHANOGENIC BACTERIA 519 this species is not stimulated by organic additions (116). This methanogen apparently lacks the methanol dehydrogenase and formate dehydrogenase found in some other species. M. thermoautotrophicum can proliferate in 12-liter fermentor culture, with a doubling time of less than 3 h in a totally inorganic mineral salts medium, and has the shortest doubling time of the methanogens described to date. It appears that this species is the "organism of choice" for mass culturing of methane bacteria. Also, the appearance of contaminants in large fermentors is not a problem when inorganic media and high temperatures are used. M. arbophilicum type strain DH1 was isolated from wetwood of living trees (119). Other strains with nutritional and morphological properties identical to M. arbophilicum have been obtained from freshwater sediments and soil. Like M. thermoautotrophicum, this species is an obligate chemolithotroph, although growth of M. arbophilicum is greatly stimulated by organic additions. This species will grow with a doubling time of 10 h in 12-liter fermentor culture on a mineral salts medium that contains vitamins and H2-CO2. The general features of M. hungatii type strain JF1, isolated from sewage sludge, were recently described by Ferry et al. (22). M. hungatii can utilize hydrogen or formate as electron donors for methanogenesis. Growth of this methanogen was stimulated by organic supplements, and a mean doubling time of 17 h was obtained on complex medium in a 12-liter fermentor (J. G. Ferry, personal communication). A different strain of M. hungatii has recently been characterized (71). This strain differs somewhat from the type strain in temperature requirements, colony morphology, and deoxyribonucleic acid (DNA) base composition. Acetate metabolism was demonstrated, but its specific contributions to cell carbon and to methane formation were not established. Detailed nutritional studies of methanogens have been limited to relatively few species. Table 3 summarizes certain nutritional features of the best characterized strains, all of which utilize H2 and C02 for energy and cell carbon syntheses. -These methanogens do not use amino acids or N2 as nitrogen sources; and no other nitrogenous compounds have been shown to replace the obligate NH4+ requirement for growth. Methanobacterium ruminantium strain PS, M. thermoautotrophicum strain AH, and M. arbophilicum strain DH1 require sulfide as a sulfur source. Methanobacterium strain MOH can use H2S or cysteine (11). The specific vitamin requirements for growth of
7 520 ZEIKUS BACTERIOL. REV. TABLE 3. Nutrient requirements for various methanogenic bacteria Strain designation Nutritional fea- Methanobacte- Methanoture Merumnan- Merumnan- Methanobacte- rium thermoau- bacterium Methanospirilrium rumiethanobacte- rmumrhamoat- arbophili- lum hungatii tium strain Ml" tium strain PS' rium strain MOHaI totrophicum cum strain strain strain JFld AHb D Nitrogen source, NH4+ NH4 NH4+ NH4+ NH4+ NH4' Vitamins Mixture required Mixture required Mixture required Mixture not re- Mixture re- Mixture not reor stimulatory, or stimulatory or stimulatory quired and quired quired and and 2-mercap- not stimula- stimulatory toethanesul- tory fonic acid Carbon additions Acetate,'2-meth- AcetateNone None None Nonee required for ylbutyrate, growth amino acids Sulfur source Not determined H2S H2S or cysteine H2S H2S Not determined Growth stimula- Yes Yes Yes No Slight No tion by acetate Growth stimula- Yes Yes Yes No Not deter- Yes tion by amino mined acids a Data of Bryant et al. (11). b Data of Zeikus and Wolfe (116). c Data of Zeikus and Henning (119). d J. G. Ferry, personal communication. e High cell densities only achieved in complex medium. most strains are not known, except that one or more of a complex vitamin mixture may be required or highly stimulatory. M. thermoautotrophicum strain AH (116) and M. hungatii strain JF1 (J. G. Ferry, personal communication) do not require vitamins for culture, although vitamin addition stimulates growth of M. hungatii but not M. thermoautotrophicum. M. arbophilicum has an obligate requirement of one or more vitamins for growth (119). Bryant et al. (11) demonstrated that M. ruminantium strain Ml (rumen isolate) would not grow in a complex medium unless rumen fluid were added. A cofactor present in rumen fluid essential for growth of this strain has been identified as coenzyme M (2-mercaptoethanesulfonic acid) by Taylor et al. (101). Methanogens vary considerably with regard to specific carbon requirements and growth response to organic additions. M. ruminantium strain Ml (rumen isolate) requires acetate, 2- methylbutyrate, and amino acids. Isotopic incorporation studies (11) demonstrated that M. ruminantium strain Ml preferentially synthesized about 60% of cell carbon from acetate during growth on complex medium with H2- CO2. Acetate carbon was incorporated into protein, nucleic acid, and lipid fractions. 2-Methylbutyric acid is required for isoleucine biosynthesis (85). Amino acids are essential for growth of this strain, but they have not been reported as effective carbon sources (11). Methanogenic species other than M. ruminantium and Methanobacterium mobile (72) do not have specific organic requirements and can grow in the absence of volatile fatty acid and amino acid mixtures. However, M. thermoautotrophicum is the only species whose growth is not greatly enhanced by organic supplements. High cell densities in cultures of species examined, other than M. thermoautotrophicum, are best achieved in complex media (14, 121) that contain materials such as mineral salts, vitamins, yeast extract, acetate, Trypticase, and H2-CO2. Selective Enrichment and Isolation of Methanogens Many excellent procedures for enumeration and isolation of methanogenic genera and species have been published (33, 72, 90, 91). The protocol described below has been used in my laboratory for successful enrichment and isolation of methanogenic species from diverse environments. The intent here is only to provide some insight into how methanogens, differing in generic designation and sources of metabolic energy, can be obtained from nature. The addition of either 0.5 to 1% (final concentration) sodium formate or methanol, or an 80% H2-20% CO2 gas phase to the basal medium (LPBM) described in Table 4 provides a useful culture medium for enrichment and growth of methanogens that possess minimal nutrient requirements. Pure cultures are obtained from repeatedly transferred active enrichments. Purification is achieved by performing an agar roll-tube
8 VOL. 41, 1977 TABLE 4. Composition of basal medium (LPBM) used for selective enrichment and growth of some methanogenic species a Component Amount KH2PO g K2HPO4-3H2O g NH4Cl g MgCl2 H g Na2S.9H2Ob g Trace mineral solutionc... 9 ml Vitamin solutiond... 5 ml Resazurin solution (0.2%)e... 1 ml Distilled H ,000 ml a Prepared anaerobically under a 95% N2-5% CO2 gas atmosphere. Medium adjusted to ph 7.4 prior to autoclaving. Basal medium requires the addition of an electron donor (H2, formate, or methanol) for enrichment or growth of methanogens. b Sulfide solution added after sterilization. e Contains, in grams per liter of distilled water (ph to 7.0 with KOH): nitrilotriacetic acid, 4.5; FeCl2 * 4H20, 0.4; MnCl2 * 4H20, 0.1; CoCl2 * 6H2O, 0.17; ZnCl2, 0.1; CaC12, 0.02; H3BO3, 0.019; and sodium molybdate, d Optional, some methanogens require a vitamin mixture (11, 113). e Optional, an oxidation-reduction indicator. THE BIOLOGY OF METHANOGENIC BACTERIA 521 dilution series in anaerobic culture tubes that contain LPBM, 2% agar (Difco, purified), and an appropriate energy source. Well-isolated colonies are picked and used to inoculate liquid cultures. The omission of sulfate lessens the growth of sulfate-reducing bacteria, whereas omission of organic components (yeast extract, Trypticase) suppresses heterotrophic contaminants. These procedures are of limited use for the determination of the total number of methanogens in natural materials because of the specific organic growth requirements of certain species. Methanobacterium species are most numerous in lake sediments (123) and sewage sludge digestors (90). Sediment or sludge is added to anaerobic culture tubes that contain LPBM and an H2-CO2 gas phase. Incubation of enrichments at 250C and repeated weekly transfer in the same medium result in a mesophilic population of methanogenic rods. An active H2-oxidizing enrichment develops a strong negative pressure. Incubation of sludge enrichments at 650C and repeated transfer in the same medium result in selection of thermophilic species. After six to nine successive transfers, thermophilic enrichments usually contain only methanogenic rods. Formate-degrading rods can be enriched by adding sludge or sediment to anaerobic culture tubes that contain LPBM and 0.5 to 1% sodium formate. This enrichment and successive transfers should be incubated at 44 C. An active formate fermentation will increase the culture ph. Inoculation of anaerobic culture tubes that contain LPBM and 0.5 to 1% methanol with sludge or sediment will select for Methanosarcina species. The enrichments should be maintained at 30 to 40 C, and the culture tubes should be wired to hold the bungs in place, or the culture procedures of Miller and Wolin (65) should be used. One must exercise caution during culture transfer, since methanol utilization results in the development of high gas pressure. At present, other methanogenic genera have not been isolated from methanol enrichments with these conditions. Selective enrichments of Methanococcus and Methanospirillum species are not as easily obtained as those for Methanobacterium and Methanosarcina species. Methanococcus species can be enriched by inoculation of anaerobic culture tubes that contain LPBM and 0.5 to 1% sodium formate with mud or lake sediment. Enrichment cultures should be incubated at 20 C, and a 15% inoculum should be used for successive culture transfers. Methanospirillum is often associated in high numbers with Thiopedia "blooms" in the surface muds of shallow eutrophic ponds (personal observation). Inoculation of culture tubes that contain LPBM and 0.5 to 1% sodium formate with this mud (or sewage sludge), followed by incubation at 32 C and repeated transfer, will often result in enrichment of Methanospirillum. Morphological Variation Four distinct morphological types of methanogenic bacteria (121) are illustrated in Fig. 2A through D. These different cell morphologies include: sarcina, rods, spheres, and spirals. Considerable variations in cell dimension and organization as well as regularity of cell shape have been observed for individual species that are included within three of these four major cell types (Fig. 2E through H). Sarcina-type cells (Fig. 2A and E) proliferate as irregular-sized cells that tend to clump and form sandlike aggregates. Methanosarcina species often appear more like coccoid cells of Geodermatophilus species (49) than true Sarcina species (42) and may vary considerably in size. Methanosarcina strain UBS (Fig. 2E) forms larger and more irregular cell packets than M. barkeri (Fig. 2A). This strain also displays a distinctive cell life cycle that involves the formation of large, irregularly sized packets of cells from initially symmetrical cocci.
9 . 522 ZEIKUS kill 4,~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~. t^.hu~~~~~~~~~~~~~~~~~~~~~~~~~~~~ Ip C D BACTERIOL. REV. il- tm / I FIG. 2. Phase-contrast photomicrographs ofm. barkeri strain PS (A), M. thermoautotrophicum strain AO (B), M. ruminantium strain Ml (C), M. hungatii strain 3PS (D), Methanosarcina strain UBS (E), M. arbophilicum strain DH1 (F), M. thermoautotrophicum strain AH (G), and a species of Methanococcus (H). Bar indicates 5 Am (A-D) and 8,um (E-H). Figure 2A-D from reference 120.
10 VOL. 41, 1977 Rod-shaped cells are most often crooked (Fig. 2B) and can appear in chains or long filaments (Fig. 2G). M. arbophilicum (Fig. 2F) is an example of a short, straight rod that does not display filamentous growth in liquid culture. Coccal-shaped cells vary in morphology from regular to elipsoidal spheres arranged in pairs or chains. M. ruminantium, illustrated in Fig. 2C, is morphologically similar to "streptococci" in shape (91) and cytology of cell division (55). Methanococcus vanneili (94) varies in morphology from roughly spherical cells of differing diameters to pear-shaped cells. Figure 2H illustrates the appearance of an undescribed Methanococcus species cultured in my laboratory. This organism appears to divide by budding and is morphologically similar to M. vanneili (T. C. Stadtman, personal communication) but nonmotile. The cell wall of this organism and of M. vanneili is very sensitive to osmotic shock, and the cells readily lyse. Cell walls of most other methanogenic species are very resistant to osmotic, ultrasonic, mechanical, and enzymatic procedures commonly used to disrupt cells (94). Spirillum-type cells grow as regularly curved rods that form continuous helical filaments (33). The morphological features of M. hungatii (Fig. 2D) appear unique (120). Single cells have blunt ends and are motile, but they are not spiral shaped. Cell growth results in long, nonmotile helical filaments (Fig. 2D). The construction of the cell wall and its behavior in cell division of this species (120) differ from Spirillum species and other helically curved microorganisms described in Bergey's Manual ofdeterminative Bacteriology (17). Fine Structure The methanogenic bacteria are structurally diverse and display no unique features by which all species can be characterized. The wall architecture of each cell type differs significantly, although all species examined (55, 120, 124) have a gram-positive-type cell envelope structure. This is interesting since many species have been reported (13) as gram negative or gram variable. However, only Methanobacterium species have a typical gram-positive cell wall. Coccus-type cells. The fine structure of M. ruminantium is very similar to that reported for streptococci (39, 63). The cell envelope of M. ruminantium has a distinctive triple-layered appearance (Fig. 3). The wall consists of an inner electron-dense layer, closely adjoined to the plasma membrane, followed by a thicker, more electron-transparent middle layer, and a THE BIOLOGY OF METHANOGENIC BACTERIA 523 rough, irregular outer layer. Cells contain the membranous cytoplasmic bodies often associated with cell division. Cells of M. ruminantium appear to be undergoing constant cell division; before one cross wall is completed, a second division is initiated. The ultrastructure of other coccal methanogens has not been published. Preliminary studies of the cell structure of M. vannieli have been reported (J. J. Jones et al., Abstr. Annu. Meet. Am. Soc. Microbiol. 1976, I55, p. 120). This species was not sensitive to several antibiotics (penicillin, vancomycin, cycloserine) that inhibit cell wall synthesis. Freeze-fracture replicas indicated a typical cell wall that appeared as one thin uniform layer in thin sections. However, the appearance of the cell wall was not similar to that of other methanogens described (12). Sarcina-type cells. The general appearance of M. barkeri in thin section is shown in Fig. 4 through 6. The amorphorous outer layer of the wall often appears as laminations (Fig. 4), which diffusely attach to a more electron-dense layer that is closely apposed to the plasma membrane (Fig. 6). The very thick outer wall of M. barkeri bears some resemblance to that observed for Sarcina ventricula, which is composed of cellulose (43). Cells examined from exponential-phase cultures (Fig. 4) contain numerous electron-dense granules of unknown composition. Cytoplasmic regions of low electron density, with circular to oval profiles, are often present in older cells (Fig. 5). These inclusion bodies do not appear to have limiting membranes (Fig. 6) and may contain storage material. One outstanding feature of sarcina-type cells is the unusual character of cell division. Cells divide in different planes with the formation of unequal daughter cells that share a common outer wall. Thus, mature aggregates of Methanosarcina appear as unevenly segmented cocci (Fig. 5). The fine structure of M. barkeri differs from that reported for a Methanosarcina species by Zhilina (124). The cell wall of this organism was triple-layered, and the cytoplasm contained numerous gas vesicles arranged in packets. Different growth conditions may influence the ultrastructural features observed. Rod-type cells. The fine structure of bacillustype cells is illustrated in Fig. 7 through 10. All Methanobacterium species examined (117, 121) have a sharply defined, smooth, gram-positive wall and contain intracytoplasmic membranes. Internal membrane inclusions are often observed in negatively stained preparations (Fig. 10) and were first described by Langen-
11 524 ZEIKUS- BACTERIOL. REV 'I.., %a A, " AM t. "". I I' Downloaded from on April 21, 2018 by guest FIG. 3. Longitudinal section illustrating the general appearance ofm. ruminantium. The cell is undergoing cell division, and the arrow shows membrane association with the second division site. Bar indicates 0.32 gum (from reference 120). FIG. 4. Thin section of M. barkeri illustrating a multilayered outer wall (black arrows) and numerous dense cytoplasmic granules (white arrow). Bar indicates 0.45 /Im (from reference 120). berg et al. (55). Internal membranes are most cum (Fig. 9). Intracytoplasmic membranes of numerous in fast-growing species such as M. methanogens have been shown to consist of formicicum (Fig. 8) and M. thermoautotrophi- closely apposed unit membranes formed from
12 VOL. 41, 1977 THE BIOLOGY OF METHANOGENIC BACTERIA A' n Downloaded from FIG. 5. Low-power survey micrograph showing a mature M. barkeri aggregate. Bar indicates 1.26 pim (from reference 120). FIG. 6. High-power micrograph of M. barkeri illustrating an electron-dense cell wall layer (CW) attached to the cytoplasmic membrane (arrow) and inclusions (I) that may contain reserve materials. Bar indicates 0.20 pum (from reference 120). FIG. 7. Grazing section revealing the general ultrastructural appearance of Methanobacterium strain MOH. Bar indicates 0.19,um (from reference 120). on April 21, 2018 by guest invagination of the plasma membrane (117). The function of these internal membranes has not been demonstrated; they do not appear associated with cell division (Fig. 8 and 10). The energy-yielding activity of methanogenic bacteria may require coupling of enzymatic systems that are membrane bound. Perhaps more membrane surface area is required to maintain the rapid growth rates of certain Methanobacterium species grown on hydrogen and CO2.
13 526 ZEIKUS BACTERIOL. REV. > s - j He he.0 A- <.; w. s A. 8 _r ;.: A.. 1',, Of,; *.S DDL #._ r. IM 4- FIG. 8. Thin section of M. formicicum illustrating the mechanism of cell division (arrow) and numerous intracytoplasmic bodies (IM). Bar indicates 0.36 gtm (from reference 120). FIG. 9. High-power micrograph showing the cell wall (CW), cytoplasmic membrane (M), and intracytoplasmic membranes (IM) of M. thermoautotrophicum. Bar indicates 0.15 gm (from reference 120). Spirillum-type cells. The ultrastructural features of M. hungatii are shown in Fig. 11 through 16. The fine structure of this species differs dramatically from other methanogenic cell types (121) and other spiral-shaped bacteria (27, 56, 67, 83). In many respects, Methanospirillum has an ultrastructure that appears to be unique in the microbial world (120). The unusual nature of the cell ends and the outer cell envelope of M. hungatii are illustrated in phosphotungstic acid preparations (Fig. 11 through 13). Cell ends are squared-off and contain an unusually structured end component. The end component does not appear to contain cytoplasm and is bounded by structural elements that form the outer wall envelope, separating the end component from the inner wall, membrane, and cytoplasm of the cell. The outer cell envelope is composed of subunits arranged in stacked bands. The outer envelope may be a 0il brittle structure because breaks are often observed between subunit bands. Individual cells appear as long, slender, curved rods (Fig. 14). Cells divide to form spiral filaments, which are connected by structures (cell spacers) that separate individual cells within the filaments (Fig. 16). The cell spacer is bounded by the outer wall and by widely separated septa (structural elements) continuous with the inner wall. These structural elements appear to have a subunit composition similar to that of the outer wall and probably function in support. Cell spacers appear fragile, and filamentous cells tend to break apart in this area of the filament (Fig. 14). The wall ofm. hungatii consists of an outer layer (average thickness of 95 nm) composed of subunits and a more electron-dense layer (average thickness of 13.6 nm), which presumably contains peptidoglycan. The inner wall layer completely encompasses individual cells within
14 VOL. 41, 1977 THE BIOLOGY OF METHANOGENIC BACTERIA 527 FIG. 10. Negatively stained (2% phosphotungstate) cell of M. thermoautotrophicum showing cell wall topography and numerous internal structures (arrow). Bar represents 0.14 gm. the filament. Numerous granular inclusions of low electron density that probably contain reserve material are visible in thin sections. The mechanism of cell division in M. hungatii (Fig. 15) is not typical of gram-positive bacteria. Division involves the invagination of the inner wall and plasma membrane with the formation of daughter cells that are connected by a cell spacer. The outer wall does not invaginate but remains continuous and appears to maintain the integrity of the growing filament. An interpretation of the structural features observed in Methanospirillum is illustrated in Fig. 17. Taxonomy A recent taxonomic description of methaneproducing bacteria has been provided by Bryant (13). Cell shape is used as a primary property for taxonomic assignment of methanogenic genera. Physiological and nutritional properties are the basis for species designation. Schnellen's (88) cultures of M. formicicum and M. barkeri were lost; hence, the complete identity of these species in culture, at present, cannot be established. Bryant's strain of M. formicicum (55, 121) and M. barkeri strain PS (121) have been suggested as neotype strains (M. P. Bryant, personal communication). Type or neotype strains of other methanogenic species (Table 1) are presently in culture. Strains of several methanogenic species have been submitted to the American Type Culture Collection and the Deutsche Sammlung von Mikroorganismem. Hopefully, all taxonomically described species will be made available through these agencies in the future. Table 5 provides comparative data on the DNA base composition analysis of various methanogenic bacteria. These results indicate that the moles percent of G+C of methanogen DNA varies greatly, ranging from 52% for M. thermoautotrophicum to 27.5% for M. arbophilicum. The specific epithet of Methanobacterium strain MOH has not been described, although this strain is considered similar to M. formicicum (13). By observing the range in DNA G+C contents of Methanobacterium species, it is obvious that morphology and energy sources for growth alone cannot be used as a basis for species designation. Furthermore, the similarity in DNA G+C contents of Methanosarcina species, Methanobacterium species, and Methanospirillum species indicates that DNA G+C contents cannot be used as a criterion for generic designation. The differences in morphology, the dissimi-
15 528 ZEIKUS BACTERIOL. REV. 11- w., - F I i I11 Downloaded from r _ 13 _ ~~ ~ ~ ~~I*9h--I FIG. 11. Low-power micrograph ofm. hungatii negatively stained with phosphotungstic acid revealing the squared-off appearance of cell ends. A break in the outer wall envelope is indicated by an arrow. Bar represents 0.75,um (from reference 119). FIG. 12. Micrograph of Methanospirillum stained with phosphotungstic acid illustrating the unique cell end component. Bar indicates 0.14,um (from ref. 119). FIG. 13. High-power micrograph of Methanospirillum stained with phosphotungstic acid showing the arrangement of subunits in the outer wall envelope. Bar indicates 0.07,im (from reference 119). on April 21, 2018 by guest larity of ultrastructural organization, the variation in nutritional properties, and the wide range of DNA G+C values observed in this microbial group may suggest that methanogens are of diverse origin and have merely exploited a common mode of energy-yielding metabolism. Thus, the diversity observed among methaneproducing bacteria parallels that described for
16 0 I - w - 4 -, - o 15 OI _.~~~~ 1W - IP,.1 I a It, z Sp, W I I * k Downloaded from *f 0o on April 21, 2018 by guest 16 FIG. 14. Grazing section of Methanospirillum showing spiral cell and broken filaments (arrows). Bar indicates 0.71,m. FIG. 15. Thin section revealing the cell division process in Methanospirillum. Only the inner wall (IW) and cytoplasmic membranes (arrows) invaginate during cell fission. Note the continuity of the outer wall (OW) and the presence ofgranular inclusions (G) in the cytoplasm. Bar indicates 0.10 gm (from reference 119). FIG. 16. Thin section showing the separation of two Methanospirillum cells in a filament by a spacer (CS). Arrows point to structural elements within the spacer. Bar indicates 0.14 pgm (from reference 119). 529
17 530 ZEIKUS BACTERIOL. REV. FIG. 17. Schematic representative of a longitudinal section through a segment of a spiral filament of Methanospirillum to summarize and clarify the structures observed in electron micrographs. Notations: EC, end component; G, granular inclusion; N, nucleoplasm; SE, structural element; S, cell spacer; M, membranous body; OW, outer wall; IW, inner wall; CM, cytoplasmic membrane (from reference 119). TABLE 5. G+C content of the DNA of methanogenic bacteria Organism G+C (mol%) Reference Methanobacterium strain MOH 38.0 Zeikus and Wolfe (115) Methanobacterium formicicum 42.0a Methanobacterium arbophilicum strain DH Zeikus and Henning (118) Methanobacterium ruminantium strain PS 32.oa Methanobacterium thermoautotrophicum 52.0 Zeikus and Wolfe (115) strain AH Methanosarcina species 51.0 Zhilina and Alekandrushkina (125) Methanospirillum hungatii 45.0 Ferry et al. (33) Strain JF1 Strain GPI 46.5 Patel et al. (71) a Unpublished data; methods used to determine G+C content as previously reported by Zeikus and Henning (118). Cultures of M. formicicum and M. ruminantium obtained from M. P. Bryant. other bacterial groups that are distinguished on the basis of energy-yielding metabolism, such as the phototrophic bacteria (17). PHYSIOLOGICAL ASPECTS Intermediary Metabolism The metabolic feature that unites the rather diverse species of methanogenic bacteria is the capacity to couple hydrogen oxidation with the concomitant reduction of carbon dioxide. Furthermore, the ability of many species to grow autotrophically indicates the enormous biosynthetic capabilities of these microbes. Methanogens differ from other autotrophs (organisms that proliferate with CO2 as the sole carbon source) in that their CO2 metabolism involves both fixation to cell carbon and reduction to methane. At present, little is known about the initial reactions involved in CO2 reduction to methane and CO2 fixation into cellular intermediate. It has not been determined whether the initial reductive steps in CO2 fixation to cell carbon and methane involve a similar or dissimilar pathway. The mechanism coupling s methane production and ATP synthesis also remains a mystery. Unique biochemical components. Recent discoveries (23, 61), in the laboratory of R. S. Wolfe, of two biochemical components that seem unique to methanogens have provided excitement and new investigative direction toward understanding the mechanism of hydrogen oxidation and carbon dioxide reduction. Coenzyme M (CoM), a new methyl transfer coenzyme, was discovered by McBride and Wolfe (61). CoM was first isolated as a dialyzable, heat-stable cofactor in Methanobacterium strain MOH and has been found in other methanogens thus far examined (101). This cofactor was neither detected in Clostridium thermoaceticum, Clostridium sticklandii, Desulfovibrio vulgarus, nor in heart, liver, spinach, or yeast extract (61). Microbiological assays with M. ruminantium (rumen strain) showed that CoM was also not present in Escherichia coli or in a number of species of anaerobic rumen bacteria (11). CoM (HSCH2CH2SO3) was structurally identified and chemically synthesized by Taylor and Wolfe (100). This cofactor was
18 VOL. 41, 1977 initially isolated as 2,2'-dithiodiethanesulfonic acid, and the active form was described as 2- mercaptoethanesulfonic acid. These investigators also described a simplified assay for CoM (102) and related the enzymatic conditions necessary for the methylation and demethylation of this cofactor. A low-molecular-weight, fluorescent compound, F420, appears to be present in all methanogenic bacteria examined. Cheeseman et al. (23) first described the properties of this pigment and its involvement in hydrogen metabolism of Methanobacterium strain MOH. The structure of F420 has not yet been identified. It displays a conspicuous absorption peak at 420 nm when oxidized, and loses both its absorption at 420 nm and its fluorescence when reduced. Cheeseman et al. (23) suggest that the extreme oxygen sensitivity of methanogens may be associated with F420 oxidation. They propose that enzyme denaturation occurs in air, because, when reduced, enzymes are associated with F420 and stable; whereas, when oxidized, enzymes are disassociated from F420 and labile. Pyrimidine nucleotide reduction (i.e., nicotinamide adenine dinucleotide phosphate [NADP]) in methanogens has been shown to be F420 linked by Tzeng et al. (104, 105). Oxidation of formate and hydrogen in M. ruminantium are mediated via F420 and coupled to reduction of NADP. NAD cannot substitute for NADP in the following reaction scheme proposed by Tzeng et al. (104): HCOOH F420 (Oxidized C02 < }\ F420, Formate (Reduced) Dehydrogenase THE BIOLOGY OF METHANOGENIC BACTERIA 531 NADPH NADP Hydrogen oxidation by Methanobacterium strain MOH was also shown by these workers (105) to be linked to F420 as the first low-molecular-weight or anionic compound. It is interesting that F420 and CoM appear to be unique to methanogenic bacteria. The functions demonstrated for these cofactors are as a primary electron carrier (F420) and as the active methyl carrier for methanogenesis (CoM). More detailed studies may substantiate other functions for these unique biochemical components. One should now recognize that primary metabolism in methanogens may not necessarily involve mechanisms similar to those found in other C,-utilizing organisms (77). For example, an F420-associated electron carrier protein could be utilized instead of ferredoxin. Ferredoxin has not been isolated and identified from pure cultures of methanogens. Tzeng et al. (105) have reported that Clostridium pasteurianum ferredoxin does not replace F420 in F420- mediated reactions, and that F420 or crude extracts of Methanobacterium strain MOH or M. ruminantium will not replace ferredoxin in the ferredoxin-free C. pasteurianum pyruvate-ferredoxin oxidoreductase reaction. Likewise, no evidence has been presented that substantiates an active involvement of methylcobalamin during methane formation by hydrogen-grown methanogens. It would also be of interest to document the function of CoM with moieties other than methyl. It is worth noting that cytochromes of the b- or c-type have not yet been observed in any methanogenic bacteria, and M. thermoautotrophicum does not appear to have menaquinone (A. Kroger, personal communication). This is of importance for understanding energy metabolism in methanogens, as all organisms so far documented that have electron transport phosphorylation contain quinones and/or cytochromes. However, the mechanism or occurrence of oxidative phosphorylation or substrate level phosphorylation has not been established in methanogenic bacteria. Methane synthesis. Methanogenic bacteria have been shown (117, 122) to utilize H2-CO2, formate, methanol, and acetate as substrates for methanogenesis. The exact mechanism by which any of these substrates are converted to methane remains to be elucidated. Barker (4) proposed a unifying mechanism to account for methanogenesis from these substrates (Fig. 18). This scheme suggests that methanogenic substrates are bound to one or more unidentified carriers and are eventually reduced to methane with the regeneration of carriers. The discovery of CoM (61, 100) helps to verify this scheme. The work of Wolfe and co-workers (23, 61, , 104, 105) on CO2 reduction in Methanobacterium species provides a basis for understanding the terminal reaction (i.e., reduction of "active methyl" to methane). The initial reductive steps from CO2 to CH3 would at first seem to be mediated via folate enzymes as suggested by Barker (5, 6) and Stadtman (95). However, the preliminary studies of Ferry et al. (35) suggest that several species of methanogens contain varying levels of formyltetrahydrofolate synthetase and methylenetetrahydrofolate dehydrogenase, but that the levels of these enzymes detected were not reproducible
19 532 ZEIKUS 0C>, CO2 + XH -> XCOOH +2H -H2 XCHO C/+±2H allw sxch20h -H2O ±2H 4,-H20 CH3COOH + XH -2H XCH3 +2H XH + CH4 FIG. 18. Possible pathway of methane formation proposed by Barker (2). or were too low to postulate their involvement in a major metabolic pathway. A modification of Barker's scheme (4) for the reduction of CO2 (Fig. 19) was presented by Gunsalus et al. (36). This speculative scheme was based on preliminary findings and indicates the possible role of C,-S-CoM compounds in the activation and reduction of CO2 to CH3-S- CoM. Evidence was presented by these investi- OH I gators that H- C -S-CoM was biologically active H and reduced to methane. It is of interest to note that CH3-S-CoM was reported (61) as the major nonvolatile labeled product of whole cells of Methanobacterium strain MOH that were pulsed with 14CO2. The terminal reductive step of methane formation in Methanobacterium strain MOH has been demonstrated (61, 100) to occur as follows: CH-S-COM HH2, Mg2+ATP CH, + H-S-CoM Methyl reductase The role of ATP was shown to be that of an activator (112), and CoM has been shown to be one of the unknown carriers postulated by Barker. Detailed biochemical studies on methanogenesis from methanol or acetate have not been reported since the last review (117). Metabolism of formate appears to be equivalent to that of H2 and C02, after an initial oxidation via formate dehydrogenase (104). Methylcobalamin has been postulated by Blaylock and Stadtman (7, 8) to be a methyl carrier in methanol-grown cells of M. barkerii. It has not yet been documented that methyl B12 is the natural methyl BACTERIOL. REV. donor for CoM in species that grow on H2-CO2 or methanol as the methanogenic substrate. Nature of Autotrophic Growth in M. thermoautotrophicum Studies (117, 122) on the effects of organic and inorganic substrates on growth and methanogenesis demonstrate that M. thermoautotrophicum is a chemolithotrophic, autotrophic organism. This species uses molecular hydrogen to generate reducing equivalents and has an obligate CO2 requirement for growth. Unlike other methanogenic species examined, growth of M. thermoautotrophicum is not stimulated by organic additions. However, acetate can be slowly metabolized in the presence of H2 and CO2 by this species, and this feature argues against calling it an "obligate autotroph" (84). The biochemical mechanisms that account for cell carbon synthesis in methane-producing bacteria have not been determined. It is very possible that autotrophic methanogens, like methane-oxidizing bacteria (77), can employ more than one pathway for cell carbon synthesis. Recently, attempts have been made to elucidate the biochemical basis for autotrophy in M. thermoautotrophicum. Preliminary studies of Daniels and Zeikus (Abstr. Annu. Meet. Am. Soc. Microbiol. 1974, P136, p. 197) demonstrated that ribulose 1,5-bisphosphate carboxylase activity was not present in cell-free extracts of M. thermoautotrophicum, although an active phosphoenolpyruvate carboxylase was present. Analysis of the short-term 14CO2 fixation products of whole cells pulsed with labeled carbonate in the presence of H2-CO2 was reported by Daniels and Zeikus (Abstr. Annu. Meet. Am. Soc. Microbiol. 1976, I58, p. 121). These preliminary studies showed that amino acids (mainly OH CO, + HSCHCHSO3 (HS-CoL)t --OC-S-CoNM H20 2e 0 11 \ H-C- OH H-C-,-CoM H 3-CoM /Ce H20zl---(2e CH3-S-CoM j2e HS-CoNI + CH, FIG. 19. Possible role of CoM (HS-CoM) in the reduction of CO,. Proposed by R. Gunsalus et al. (36).
20 VOL. 41;, 1977 aspartate, glutamate, and alanine) and nonphosphorylated compounds accounted for the majority of non-methanogenic precursors labeled at early times. Label was not detected in 3- phosphoglyceric acid at 2 or 60 s. These findings do not suggest that the Calvin cycle is not operative in M. thermoautotrophicum. The labeling patterns observed cannot rule out the operation of the reductive carboxylic acid cycle (16), a total synthesis of acetate (57), or an unknown cell carbon synthesis mechanism. Preliminary studies on the intermediary metabolism of M. thermoautotrophicum have also been presented by Taylor et al. (99), who used enzymatic assays and analyzed the distribution of label in cell fractions of M. thermoautotrophicum after long-term incorporation of various 14C-labeled compounds. On the basis of labeling patterns observed in amino acids and nucleic acids, and the absence of detectable levels of ribulose 1,5-bisphosphate carboxylase, hexulose phosphate synthetase, and hydroxypyruvate reductase, they concluded that it was doubtful whether the Calvin cycle, the serine or hexulose pathway (77), or the total acetate synthesis path (57) exists in Methanobacterium. Autotrophy is often defined on the basis of ribulose 1,5-bisphosphate carboxylase (28, 84). If certain methanogenic species use a non-calvin CO2 fixation path, this may represent substantial proof that other mechanisms of autotrophy exist in the microbial world. However, documentation of the autotrophic path of M. thermoautotrophicum is inherently a difficult task, since the majority of the CO2 fixed (>90%) goes into CH4 intermediates. ECOLOGICAL ASPECTS Activities in Nature Methanogens have been detected in numerous primarily organotrophic ecosystems that include: the rumen and gastrointestinal tract of animals; mud, sediment, and flooded soil of marine and freshwater environments; and various waste-processing digestors. Hydrogen-oxidizing methanogens have been shown to predominate in these ecosystems (90, 123). The activity of methanogens in the rumen and sewage sludge digestors has been examined in detail, and this literature has been reviewed (40, 41, 46). Methane occasionally has been either reported or-inferred to be part of the composition of flammable gases that are sometimes trapped within the trunks of living trees. Bushong (18) reported the first analysis of gases drawn from a tree and demonstrated the presence of flammable gases in a large cottonwood THE BIOLOGY OF METHANOGENIC BACTERIA 533 tree. Visibly sound hardwood trees, including elms, poplars, willows, oaks, and maples, can contain high pressures of methane (Fig. 1A). Methane formation in these trees was demonstrated to have a microbial origin and was associated with an abnormal condition of heartwood (dead xylem tissue) known as wetwood (118). Wetwood differs from normal tree tissues because it is infested with anaerobic bacteria, is alkaline in ph, is devoid of 02, and has a high moisture content with characteristic fetid odor. One methanogenic species, M. arbophilicum, was isolated from wetwood of several cottonwood trees (119). Only one strain of M. arbophilicum (type strain DH1) was described (119) from trees that contained wetwood. Other methanogenic genera were not observed by microscopic observation of wetwood enrichment cultures. Inside living trees, anaerobic decomposition of nonligninaceous dead xylem tissue or tree nutrients results in the formation of H2 required for proliferation of methanogens. The establishment of a bacterial population in trees probably results from root injury, which provides a path of entry for indigenous soil microorganisms. The evolution of methane bubbles from the bottoms of shallow ponds or the edges of lakes is a commonplace event. However, relatively little is known about either the environmental factors that influence methanogenesis, or the in situ microbial activities responsible for this process in aquatic sediments. Most investigations of methanogenesis in both marine (26, 70, 82) and freshwater sediments (24, 32, 43) have been concerned with detection and quantification of methane evolution from sediments. Methane formed in aquatic sediments escapes into the overlying waters, where it is metabolized by other microorganisms to CO2 (86) or diffuses into the atmosphere. Analysis (123) of the activity of methanogens in sediments of Lake Mendota, Wis., revealed that methanogenesis was severely temperature limited and that the rate of methanogenesis varied seasonally. The maximum in situ temperature (23 C) attained during seasonal change was far below the temperature optimum (35 to 42 C) observed for in vitro methanogenesis. The increased rate of methanogenesis that was associated with seasonal change correlated with increased numbers of methanogens and increased rates of metabolic activity, when sediment temperature more closely approximated the optimum temperature for methanogenesis. The predominant methanogenic population was metabolically active between 4 and 45 C. All known methanogenic genera were found in Lake Mendota sedi-
21 534 ZEIKUS ments, and Methanobacterium species predominated. Thus, methanogen diversity in aquatic sediments approximates that found in sewage sludge digestors. Methanogenic isolates obtained from sediments grew when either H2, formate, or methanol was added as the sole electron donor to a mineral salts medium. Methanogens were not obtained in pure or mixed cultures that could be maintained on acetate-mineral salts medium. It is possible that the medium used inhibited (e.g., high sulfide content) acetate-fermenting methanogens, or that other supplements (e.g., high levels of yeast extract) were required to support their growth. One species isolated metabolizes H2- CO2 and acetate (127). Cappenberg (19-22) has examined the associated activities of methanogens and sulfate-reducing bacteria in Lake Vechten and in mixed culture experiments. These studies reported that lactate metabolism of sulfate reducers in the upper sulfate-containing sediment layers provides the main energy source for acetatefermenting methanogens located lower in the sediment and that sulfide production was toxic to methanogens. The turnover rate of lactate in sediments was 28.9,ug of lactate per g of mud per h. The rate of acetate disappearance was 1.99,ug of acetate per g of mud per h. The rate of acetate disappearance was approximately 70% of the observed rate of methanogenesis. Mixed-culture chemostat studies (27), using a sulfate reducer and a methanogen isolated from Lake Vechten, demonstrated that acetate metabolism by the sulfate reducer provided acetate for the methanogens, but it also produced toxic H2S. Thus, a commensal relationship between these two microbial groups was proposed. However, the ecological significance of sulfate reducer-methanogen interrelationships is probably more complex and not yet fully understood. The addition of sulfate and other compounds (e.g., nitrate, nitrite, acetylene) to sediments has been shown to inhibit methanogenesis (9, 20, 24, 58, 78). These inhibition phenomena may be either a result of channeling normal electron flow from reduction of methane carbon precursors to reduction of these alternative electron acceptors by nonmethanogens, or a direct inhibition of methanogens by the compound or a metabolite of it. Balderston and Payne (3) have shown that addition of nitrate, nitrite, nitric oxide, nitrous oxide, or sulfite inhibited H2-dependent evolution of methane from salt marsh sediments and whole-cell suspensions of methanogens. Their results suggest that inhibition of methane formation by these additions was not due to a BACTERIOL. REV. change in the redox potential of the system or to substrate competition by nonmethanogens. Some compounds shown to inhibit methanogenesis in natural environments may not inhibit methanogenic bacteria. For example, methanogens in pure culture grow well even in the presence of high sulfate concentrations. Martens and Berner (60) have demonstrated that methanogenesis in marine sediments is not initiated until sulfate is depleted from interstitial water. Cappenberg (19) reported that sulfate was depleted in actively methanogenic freshwater sediments. He proposed (22) that inhibition of methanogenesis by added sulfate (0.1%) was from the production of toxic H2S. Winfrey and Zeikus (119) have shown that sulfate inhibits methanogenesis in freshwater sediments by altering normal carbon and electron flow during anaerobic mineralization. Sulfate inhibition of methanogenesis in Lake Mendota sediments was reversed by prolonged incubation or by the addition of H2 or acetate. Radioactive tracer studies demonstrated that 14Clabeled acetate was converted to 14CH4 and 14CO2 in the absence of added sulfate, whereas only 14CO2 was found in sediments with sulfate. The addition of sulfate to sediments did not result in significant accumulation of H2S in interstitial water. It was proposed that sulfatereducing organisms assume the role of methanogens in sulfate-containing sediments by utilization of methanogenic precursors. Documentation of acetate-respiring, sulfate-reducing bacteria in pure culture will be of great interest. Recently, a Desulfotomaculum species has been described that respires acetate (109a). It would appear that sulfate-reducing bacteriamethanogen interrelationships in nature would be highly dependent on the sulfate concentration. At high sulfate concentrations, as exist in marine environments (26, 60), a competitive relationship might occur. In sulfate-depleted freshwater sediments (111), sulfate-reducing bacteria may not be active, or synergistic relationships might occur. For example, Bryant (Abstr. Annu. Meet. Am. Chem. Soc. 1969, MICE 18) presented evidence for a symbiotic association between sulfate-reducing and methane-producing bacteria. He was able to grow Desulfovibrio on lactate in the absence of sulfate in a defined-mixed culture with Methanobacterium. The methanogen functions as an alternative electron sink (in lieu of sulfate) and enables the catabolism of lactate by the sulfate reducers. However, knowledge of interspecies hydrogen transfer interactions (to be discussed more fully below) would suggest that lactate might not be an important metabolite in methanogenic environments.
22 VOL. 41, 1977 Acetate and H2-CO2 are the major in situ substrates for methanogenesis. Methanol has not been demonstrated to be a significant product of anaerobic decomposition. Formate, a product of many fermentations, is not considered to be a major substrate because it is readily cleaved into H2 and CO2. In the rumen ecosystem, Hungate et al. (47) concluded that formate was largely utilized by non-methanogenic microbes, although the H2 and CO2 derived from formate metabolism accounted for 18% of the total methane produced. The relative importance of acetate and H2-CO2 as methane precursors in organotrophic ecosystems depends largely on environmental conditions. Hungate (45) demonstrated that H2-CO2 accounts for most of the methane produced in the rumen. Oppermann et al. (69) showed that only 2 to 5.5% of the rumen methane was derived from acetate in vitro. The rumen ecosystem functions essentially as a chemostat, where volatile fatty acid concentrations are kept low because they are absorbed through the rumen wall. Also, the detention time for organic matter (1 to 3 days) may be too slow to allow for effective acetate conversion to methane (44). Jeris and McCarty (50) reported that 70% of the methane from sewage sludge was derived from acetate. Similarly, Smith and Mah (89) determined that 73% of the methane came from acetate in sludge. Cappenberg and Prins (21) calculated that approximately 70% of the methane was derived from acetate in freshwater sediments. Russian investigators (2) have studied the relative importance of acetate and H2- CO2 as methane precursors in various lakes and reported that 32 to 98% of the methane in sediments was formed through microbiological reduction of carbon dioxide by hydrogen. Thus, although acetate is the major methane precursor during anaerobic decomposition of organic matter in sediments and sludge digestors, the reported percentages derived from it may be subject to some discussion because of environmental differences. Noticeable environmental differences exist between the sludge digestor and sediment ecosystems. Sludge digestors are less nutrient limited, and the detention time for organic matter is usually 10 to 30 days. Thus, these long detention times appear sufficient for conversion of substantial amounts of volatile fatty acids, including acetate, to CH4 and CO2 (51, 97). The turnover rates for acetate in lake sediments are relatively rapid (21), and methanogenesis in this ecosystem may be substrate limited. Winfrey and Zeikus (109; Abstr. Annu. Meet. Am. Soc. Microbiol. 1976, I103, p. 128) reported that methanogenesis in Lake Mendota was limited THE BIOLOGY OF METHANOGENIC BACTERIA 535 by the small amounts of available H2 and acetate in sediments. These investigators also demonstrated that addition of H2 or acetate greatly stimulated methanogenesis and that the concentrations of these substrates greatly influenced their conversion. As the partial pressure of hydrogen was increased in sediments, carbonate was the preferred substrate for methanogenesis, whereas acetate was the preferred substrate at low partial pressures of hydrogen. Microbial methanogenesis can also be detected in association with primarily chemolithotrophic ecosystems (i.e., habitats where the environmental hydrogen present was formed as a result of geochemical processes). Work in Lake Kivu, where hydrogen of volcanic origin exists in the environment, suggests that microbial reduction of CO2 by volcanic hydrogen accounts for the major portion of methane formed in this African rift lake (31). Geochemists (37) have measured high concentrations of methane (>20%) and hydrogen (>6%) in the dissolved gases of certain thermal features in Yellowstone National Park, U.S.A. Methanogens have been isolated from several thermal springs (Fig. 1B) where geothermal hydrogen was present. Russian investigators (53) suggest that microbial reduction of geochemical hydrogen is associated with methanogenesis in petroleum, natural gas, and coal deposits. Microbial Interactions The methanogenic bacteria as a group offer the unique opportunity to study trophic interrelationships in anaerobic ecosystems. In anaerobic habitats where decomposition of organic matter is occurring, methanogens are the terminal organisms in the microbial food chain. The outstanding feature of this decomposition process is that its successful operation depends on the interaction of metabolically different bacteria. Detailed studies on interactions between methanogens and organotrophic anaerobes were initiated as a result of understanding the M. omelianskii symbiosis. Bryant et al. (10) established that ethanol fermentation by this mixed culture required a symbiotic metabolic interaction between a methanogen and an "S" organism. Analysis (79) of the metabolic coupling between the S organism and M. ruminantium grown on pyruvate revealed that methanogens alter fermentative metabolism in addition to displacing unfavorable reaction equilibria caused by high partial pressures of H2. Further detailed studies of Wolin, Bryant, and coworkers (11, 48, 65, 80, 81, 87, 115) have resulted in formation of a unified concept that
23 536 ZEIKUS describes primary metabolic interactions of methanogens and nonmethanogens known as interspecies hydrogen transfer. Disposal of electrons from reduced NAD (NADH) generated in glycolysis in order to regenerate NAD is accomplished in all fermentative bacteria, mainly via production of various reduced products such as H2, ethanol, lactate, formate, or propionate. In the rumen ecosystem, analysis of end products of carbohydratedegrading anaerobes grown in the presence and absence of methanogens (114, 115) demonstrates how methanogens alter electron flow during fermentation. Growth of various carbohydrate fermenters (in pure culture) in the absence of methanogens results in formation of, mainly, H2, CO2, formate, acetate, succinate, lactate, and ethanol, whereas growth in the presence of methanogens results mainly in production of methane, acetate, and CO2. In the presence of methanogens, products such as lactate and ethanol are produced in very small amounts and acetate increases. Weimer and Zeikus have reported (108) similar results when Clostridium thermocellum and M. thermoautotrophicum were grown on cellulose. Fermentation end products of C. thermocellum are mainly H2, CO2, ethanol, and acetate. The mixed culture formed CH4, CO2, and increased amounts of acetate as the main fermentation products. However, when grown on cellobiose, the methanogen caused only slight changes in the fermentation balance of the Clostridium, and free H2 was produced. That significant metabolic interactions between the Clostridium and the methanogen were not observed during growth on cellobiose (a soluble substrate) may be of general ecological importance. The decomposition of organic matter in nature may be limited by the rate at which insoluble biopolymers are decomposed (109). Methanogens may function as "electron sinks" (15, 44) during organic decomposition in organotrophic ecosystems by altering electron flow in the direction of hydrogen production. In theory (114, 115), the altered electron flow or interspecies hydrogen transfer that occurs during coupled growth of methanogens and nonmethanogens results in: (i) increased substrate utilization, (ii) different proportions of reduced end products, (iii) more ATP synthesized by the nonmethogens, (iv) increased growth of both organisms, and (v) displacement of unfavorable reaction equilibria İt seems obvious that metabolic interactions in the form of interspecies hydrogen transfer are operative in anaerobic environments and hold ecological significance. In the rumen and sediment ecosystem, where interspecies H2 BACTERIOL. REV. transfer appears to occur (44, 110, 115), the hydrogen partial pressure is extremely low (10-6 M). In addition, failure to detect significant concentrations of H2 in various organotrophic, methanogenic niches (44, 51, 110, 118) is probably the consequence of rapid hydrogen oxidation by methanogens that results, in part, from interspecies-hydrogen-transfer reactions. Electron-sink products of "normal" fermentations (ethanol, lactate) may be of little consequence in the metabolism of carbon compounds in anaerobic ecosystems. In this regard, Hungate (44) suggests that ethanol and lactate are not important decomposition intermediates in the rumen because electrons are diverted to methane production. Two general categories of interspecies-hydrogen-transfer interactions have been demonstrated. One category involves interactions between methanogens and carbohydrate and similar fermentative bacteria in which H2 utilized is beneficial but not essential (25, 48, 87). The second category describes interactions between methanogens and nonmethanogens in which H2 utilization is essential (8). This category of interspecies-hydrogen-transfer interactions may be involved in mixed methanogenic cultures that ferment various volatile fatty acids (4) or benzoate (34). However, the free energy of formation for fermentation of propionate (+17.8 kcal/mol [ca kj/mol]) to H2 and acetate is even more unfavorable than that of the ethanol fermentation (+1.5 kcal/mol [ca kj/mol]) by the S organism (30). It is not known that removal of hydrogen alone would render the propionate fermentation a favorable energyyielding reaction for the decomposing organism. Therefore, additional factors may be involved in certain fatty acid degradations by mixed-methanogenic-cultures. Ferry and Wolfe (34) demonstrated that a stable microbial consortium could degrade benzoate to methane. Acetate, formate, H2, and CO2 were identified as intermediates in the conversion of benzoate to methane. The organism responsible for cleavage of the benzoate ring was not isolated. The species responsible for fermenting acetate to methane was not isolated, but it was reported to be similar to the rod observed by Pretorius (75) and by Mylroie and Hungate (66). It is of interest to note that Pretorius (75) was unable to maintain a sludge enrichment that contained this rod with acetate alone. However, the addition of formate and acetate maintained this methanogenic-mixed culture. Relatively little is known about the chemical and mechanistic limitations of anaerobic decomposition of organic matter in nature. Although certain aromatic compounds (9) are me-
24 VOL. 41, 1977 tabolized to methane by mixed cultures (98), some naturally occurring compounds may not be degradable. Hackett et al. (38) reported that specifically labeled synthetic 04C-lignins were not decomposed to gaseous products during anaerobic incubation in rumen contents or lake sediments. The absence of lignin biodegradation in anaerobic ecosystems may have profound environmental implications. The gradual accumulation of lignin and lignin-derived materials over extended periods of time might form the basis for peat and coal deposits (96). ACKNOWLEDGMENTS I am indebted to my colleagues J. Ward, P. Weimer, W. Hackett, L. Daniels, D. Nelson, V. Bowen, M. Winfrey, and B. Kenealy for their significant contributions in the research program, to D. Henning and S. Klevickis for technical assistance, and to G. Fuchs and R. Thauer for discussions concerning this manuscript. It is a pleasure to thank two former mentors, T. D. Brock for stimulating my interest in microorganisms, and R. S. Wolfe for directing my attention to methanogens. For data presented here, the research was supported by the College of Agricultural and Life Sciences, University of Wisconsin, Madison, and by grant DEB from the National Science Foundation. LITERATURE CITED 1. Balch, W. E., and R. S. Wolfe New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl. Environ. Microbiol. 32: Baliaer, S. S., Z. I. Finkelstein, and M. V. Ivanov The rate of methane production by bacteria in ooze deposits of some lakes. Microbiol. Esp. 44: Balderson, W. L., and W. J. Payne Inhibition of methanogenesis in salt marsh sediments and whole-cell suspensions of methanogenic bacteria by nitrogen oxides. Appl. Environ. Microbiol. 32: Barker, H. A Biological formation of methane, p. 1. In Bacterial fermentations. John Wiley and Sons, Inc., New York. 5. Barker, H. A Biochemical functions of corrinoid compounds. Biochem. J. 105: Barker, H. A Corrinoid-dependent enzymic reactions. Annu. Rev. Biochem. 41: Blaylock, B. A Cobamide-dependent methanol-cyanocobalamin methyltransferase of Methanosarcina barkeri. Arch. Biochem. Biophys. 124: Blaylock, B. A., and T. C. Stadtman Methane biosynthesis by Methanosarcina barkeri. Properties of soluble enzyme system. Arch. Biochem. Biophys. 116: Bollag, J. M., and S. T. Czlonkowski THE BIOLOGY OF METHANOGENIC BACTERIA 537 Inhibition of methane formation in soil by various nitrogen containing compounds. Soil Biol. Biochem. 5: Bryant, M. P., E. A. Wolin, M. J. Wolin, and R. S. Wolfe Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch. Mikrobiol. 59: Bryant, M. P., S. F. Tzeng, I. M. Robinson, and A. E. Joyner Nutrient requirements of methanogenic bacteria, p In F. G. Pohland (ed.), Anaerobic biological treatment processes advances in chemistry, series 105. American Chemistry Society, Washington, D.C. 12. Bryant, M. P Commentary on the Hungate technique for culture of anaerobic bacteria. Am. J. Clin. Nutr. 25: Bryant, M. P Methane producing bacteria. In R. E. Buchanan and N. E. Gibbons (ed.), Bergey's manual of determinative bacteriology. The Williams and Wilkins Co., Baltimore. 14. Bryant, M. P., B. C. McBride, and R. S. Wolfe Hydrogen-oxidizing methane bacteria. I. Cultivation and methanogenesis. J. Bacteriol. 95: Bryant, M. P Interaction among intestinal microorganisms. Am. J. Clin. Nutr. 27: Buchanan, B. B Ferredoxin and carbon assimilation, p In W. Lovenberg (ed.), Iron-sulfur proteins, vol. 1. Academic Press Inc., New York and London. 17. Buchanan, R. E., and N. E. Gibbons Bergey's manual of determinative bacteriology. The Williams and Wilkins Co., Baltimore. 18. Bushong, F. W On gases within trees. Kans. Acad. Sci. Pt. 2 21: Cappenberg, T. E Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh water lake. I. Field observations. Antonie van Leeuwenhoek J. Microbiol. Serol. 40: Cappenberg, T. E Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh water lake. II. Inhibition experiments. Antonie van Leeuwenhoek J. Microbiol. Serol. 40: Cappenberg, T., and H. Prins Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh water lake. III. Experiments with 14C-labeled substrates. Antonie van Leeuwenhoek J. Microbiol. Serol. 40: Cappenberg, T. E A study of mixed continuous culture of sulfate-reducing and methane-producing bacteria. Microbial Ecology 2: Cheeseman, P., A. Toms-Wood, and R. S. Wolfe Isolation and properties of a fluorescent compound, factor420, from Methanobacterium strain M.O.H. J. Bacteriol. 112:
25 538 ZEIKUS 24. Chen, R. L., D. R. Keeney, J. G. Konrad, A. J. Holding, and D. A. Graetz Gas metabolism in sediments of Lake Mendota. J. Environ. Qual. 1: Chung, King Thom Inhibitory effects of H2 on growth of Clostridium cellobioparum. Appl. Environ. Microbiol. 31: Claypool, G., and I. Kaplan The origin and distribution of methane in marine sediments, p In I. R. Kaplan (ed.), Natural gases in marine sediments. Plenum Press, New York. 27. Cohen-Bazire, G., and R. Kunisawa The fine structure of Rhodospirillum rubrum. J. Cell Biol. 16: Cox, R. B., and J. R. Quayle The autotrophic growth of Micrococcus denitrificans on methanol. Biochem. J. 150: Daniels, L., and J. G. Zeikus Improved culture flask for obligate anaerobes. Appl. Microbiol. 29: Decker, K., K. Jungermann, and R. K. Thauer Energy production in anaerobic organisms. Angew. Chem. Int. Ed. Engl. 9: Deuser, W. G., E. T. Degens, and G. R. Harvey Methane in Lake Kivu: new data bearing on its origin. Science 181: Edwards, T., and B. C. McBride New method for the isolation and identification of methanogenic bacteria. Appl. Microbiol. 29: Ferry, J. G., P. H. Smith, and R. S. Wolfe Methanospirillum, a new genus of methanogenic bacteria and characterization of Methanospirillum hungatii sp. nov. Int. J. Syst. Bacteriol. 24: Ferry, J. G., and R. S. Wolfe Anaerobic degradation of benzoate to methane by a microbial consortium. Arch. Microbiol. 107: Ferry, J. G., R. D. Sherod, H. D. Peck, and L. G. Ljungdahl Autotrophic fixation of CO2 via tetrahydrofolate intermediates by Methanobacterium thermoautotrophicum. In Proceedings of Symposium on Microbial Production and Utilization of Gases (H2, CH4, CO). E. Goltze, Gottingen, in press. 36. Gunsalus, R., D. Eirich, J. Romesser, W. Balch, S. Shapiro, and R. S. Wolfe Methyltransfer and methane formation. In Proceedings of Symposium on Microbiol Formation and Utilization of Gases (H2, C02, CO). E. Goltze, Gottingen, in press. 37. Gunter, B. P., and B. C. Musgrave Gas chromatographic measurements of hydrothermal emanations for Yellowstone National Park. Geochim. Cosmochim. Acta 30: Hackett, W. F., W. J. Connors, J. K. Kirk, and J. G. Zeikus Microbial decomposition of synthetic "4C-labeled lignins in nature: lignin biodegradation in a variety of natural materials. Appl. Environ. Microbiol. 33: Higgens, M., and G. Shockman Model BACTERIOL. REV. for cell wall growth of Streptococcus faecalis. J. Bacteriol. 101: Hobson, P. N Rumen microorganisms. Prog. Ind. Microbiol. 9: Hobson, P. N., S. Bousfield, and R. Summers Anaerobic digestion of organic matter. Crit. Rev. Environ. Control 4: Holt, S. C., and E. Canale-Parola Fine structure of Sarcina maxima and Sarcina ventriculi. J. Bacteriol. 93: Howard, D. L., J. I. Frea, and R. M. Pfister The potential for methane carbon cycling in Lake Erie, p Proceedings of the Fourteenth Conference on the Great Lakes Research. 44. Hungate, R. E The rumen and its microbes. Academic Press Inc., New York. 45. Hungate, R. E Hydrogen as an intermediate in the rumen fermentation. Arch. Mikrobiol. 59: Hungate, R. E A roll tube method for cultivation of strict anaerobes, p In J. R. Norris and D. W. Ribbons (ed.), Methods in microbiology, vol Academic Press Inc., New York. 47. Hungate, R. E., W. Smith, T. Bauchop, I. Yu, and J. C. Rabinowitz Formate as an intermediate in the bovine rumen fermentation. J. Bacteriol. 102: Iannotti, E. T., D. Kafkewitz, M. J. Wolin, and M. P. Bryant Glucose fermentation products of Ruminococcus albus grown in continuous culture with Vibrio succinogenes: changes caused by interspecies transfer of H2. J. Bacteriol. 114: Ishiguro, E. E., and R. S. Wolfe Control of morphogenesis in Geodermatophilus: ultrastructural studies. J. Bacteriol. 104: Jeris, J. S., and P. L. McCarty The biochemistry of methane fermentation using "4C-tracers. J. Water Pollut. Control Fed. 37: Kirsch, E. J., and R. M. Sykes Anaerobic digestion in biological waste treatment. Prog. Ind. Microbiol. 9: Kluyver, A. J., and G. T. P. Schnellen Fermentation of carbon monoxide by pure cultures of methane bacteria. Arch. Biochem. 14: Kzerteu, V. A Production of methane by microorganisms in petroleum deposits. Geochemistry (U.S.S.R.) 4: Lawrence, A. W., and P. L. McCarty Kinetics of methane fermentation in anaerobic treatment. J. Water Pollut. Control Fed. 41:R1-S Langenberg, K. F., M. P. Bryant, and R. S. Wolfe Hydrogen-oxidizing methane bacteria. II. Electron microscopy. J. Bacteriol. 91: Lewin, R. A Saprospira grandis Gross, and suggestions for re-classifying helical, apochlorotic gliding organisms. Can. J. Microbiol. 8: Ljungdahl, L. G., and H. G. Wood Total
26 VOL. 41, 1977 synthesis of acetate from CO2 by heterotrophic bacteria. Annu. Rev. Microbiol. 23: MacGregor, A. N., and D. R. Keeney Methane formation by lake sediments during in vitro incubation. Water Resour. Bull. 9: Macy, J. M., T. E. Snellen, and R. E. Hungate Use of syringe methods for anaerobiosis. J. Clin. Nutr. 25: Martens, C. S., and R. A. Berner Methane production in the interstitial waters of sulfate-depleted marine sediments. Science 185: McBride, B. C., and R. S. Wolfe A new coenzyme of methyl transfer, coenzyme M. Biochemistry 10: McBride, B. C., and R. S. Wolfe Biochemistry of methane formation. Adv. Chem. Ser. 105: McCandless, R., M. Cohen, G. Kalmonson, and L. Guze Cores, microbial organelles possibly specific to group D streptococci. J. Bacteriol. 96: McCarty, P. L Thermodynamics of biological synthesis and growth. Adv. Water Pollut. Res. 14: Miller, T. L., and M. J. Wolin Formation of hydrogen and formate by Ruminococcus albus. J. Bacteriol. 116: a.Mink, R., and P. R. Dugan Tentative identification of methanogenic bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33: Mylroie, R. L., and R. E. Hungate Experiments on the methane bacteria in sludge. Can. J. Microbiol. 1: Murray, R. G. E On the cell wall structure of Spirillum serpens. Can. J. Microbiol. 9: Nelson, D. R., and J. G. Zeikus Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl. Microbiol. 28: Opperman, R. A., W. D. Nelson, and R. E. Bowman In vivo studies of methanogenesis in the bovine rumen; dissimilation of acetate. J. Gen. Microbiol. 25: Oremland, R. S Methane production in shallow water, tropical marine sediments. J. Appl. Microbiol. 30: Patel, G. P., L. A. Roth, L. Van den Berg, and D. S. Clark Characterization of a strain of Methanospirillum hungatii. Can J. Microbiol. 22: Paynter, M. J. B., and R. E. Hungate Characterization ofmethanobacterium mobilis, sp. n., isolated from the bovine rumen. J. Bacteriol. 95: Pine, M. J., and H. A. Barker Studies on the methane fermentation. XII. The pathway of hydrogen in acetate fermentation. J. Bacteriol. 71: Pine, M. J., and W. Vishniac The methane fermentation of acetate and methanol. J. Bacteriol. 73: THE BIOLOGY OF METHANOGENIC BACTERIA Pretorius, W. A The effect of formate on the growth of acetate utilizing methanogenic bacteria. Water Resour. 6: Prins, R. A., C. J. Van Nevel, and D. I. Demeyer Pure culture studies of inhibitors for methanogenic bacteria. Antonie van Leeuwenhoek J. Microbiol. Serol. 38: Quayle, J. R The metabolism of one-carbon compounds in microorganisms. Adv. Microb. Physiol. 7: Raimbault, M Etude de l'influence inhibitrice de l'acetylene sur la formation biologique du methane dans un sol de riziere. Ann. Microbiol. (Paris) 126A: Reddy, C. A., M. P. Bryant, and M. J. Wolin Characteristics of S organism isolated from Methanobacillus omelianskii. J. Bacteriol. 109: Reddy, C. A., M. P. Bryant, and M. J. Wolin Ferredoxin- and nicotinamide adenine dinucleotide-dependent H2 production from ethanol and formate in extracts of S organism isolated from "Methanobacillus omelianskii." J. Bacteriol. 110: Reddy, C. A., M. P. Bryant, and M. J. Wolin Ferredoxin-dependent conversion of acetaldehyde to acetate and H2 in extracts of S organism. J. Bacteriol. 110: Reeburgh, W. S Observation of gases in Chesapeake Bay sediments. Limnol. Oceanogr. 14: Remsen, C. C., S. W. Watson, T. B. Waterbury, and H. G. Truper Fine structure of Ectothiorhodospira mobilis Pelsh. J. Bacteriol. 95: Rittenberg, S. C The obligate autotroph-the demise of a concept. Antonie van Leeuwenhoek J. Microbiol. Serol. 38: Robinson, I. M., and M. J. Allison Isoleucine biosynthesis from 2-methyl butyric acid by anaerobic bacteria from the rumen. J. Bacteriol. 97: Rudd, J. W., R. D. Hamilton, and N. E. R. Campbell Measurement of microbial oxidation of methane in lake water. Limnol. Oceanogr. 19: Scherfinger, C. C., B. Linehan, and M. J. Wolin H2 production of Selenomonas ruminantium in the absence and presence of methanogenic bacteria. Appl. Microbiol. 29: Schnellen, C. G. T. P Onderzoekingen over De Methaangistring, Dissertation. Technical University of Delft. De Maasstad, Rotterdam. 89. Smith, P. H., and R. H. Mah Kinetics of acetate metabolism during sludge metabolism. Appl. Microbiol. 14: Smith, P. H The microbial ecology of sludge methanogenesis. Dev. Ind. Microbiol. 7: Smith, P. S., and R. E. Hungate Isolation and characterization of Methanobacterium ruminantium sp. n. J. Bacteriol.
27 540 ZEIKUS 75: Stadtman, T. C., and H. A. Barker Studies on the methane fermentation. VII. Tracer studies. Arch. Biochem. 21: Stadtman, T. C., and H. A. Barker Studies on the methane fermentation. IX. The origin of methane in the acetate and methanol fermentations by Methanosarcina. J. Bacteriol. 61: Stadtman, T. C., and H. A. Barker Studies on methane fermentation. X. A new formate-decomposing bacterium, Methanococcus vannieli. J. Bacteriol. 62: Stadtman, T. C Methane fermentation. Annu. Rev. Microbiol. 21: Swain, F. M Non-marine organic geochemistry. Cambridge Earth Science Series. Cambridge University Press, Cambridge. 97. Sykes, R. M., and E. J. Kirsch Fermentation patterns in the initial stages of sewage sludge digestion. Dev. Ind. Microbiol. 11: Tarvin, D., and A. M. Buswell The fermentation of organic acids and carbohydrates. J. Am. Chem. Soc. 56: Taylor, G. T., D. P. Kelly, and S. J. Prirt Intermediate metabolism in methanogenic bacteria. Proceedings of the Symposium on Microbiological Production and Utilization of Gases (H2, CH4, CO). E. Goltze, Gottingen, in press Taylor, C. D., and R. S. Wolfe Structure and methylation of coenzyme M (HSCH2CH2SO3). J. Biol. Chem. 249: Taylor, C. D., B. C. McBride, R. S. Wolfe, and M. P. Bryant Coenzyme M, essential for growth of a rumen strain of Methanobacterium ruminantium. J. Bacteriol. 120: Taylor, C. D., and R. S. Wolfe A simplified assay for coenzyme M (HSCH3CH2SO3). Resolution of methylcobalamin-coenzyme M methyltransferase and use of sodium borohydride. J. Biol. Chem. 249: a.Thauer, R. K., K. Jungermann, and K. Decker Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41: Torien, D. F., P. G. Thiel, and W. A. Pretorius Substrate flow in anaerobic digestion. In S. H. Jenkens (ed.), Advances in water pollution research, vol. 2. Pengamon Press, Ltd., Oxford Tzeng, F. S., M. P. Bryant, and R. S. Wolfe Factor 420-dependent pyridine nucleotide-linked formate metabolism of Methanobacterium ruminantium. J. Bacteriol. 121: Tzeng, S. F., R. S. Wolfe, and M. P. Bryant Factor 420-dependent pyridine nucleotide-linked hydrogenase system of Methanobacterium ruminantium. J. Bacteriol. 121: Van den Berg, L., G. B. Patel, P. S. Clark, and C. P. Lentz Factors affecting rate of BACTERIOL. REV. methane fermentation from acetic acid by enriched methanogenic cultures. Can. J. Microbiol. 22: Wagman, D. D., W. H. Evans, V. B. Parker, I. Halou, S. M. Bailey, and R. H. Schumm Selected values of chemical thermodynamic properties. Institute for Basic Standards, Technical Note U.S. Department of Commerce, National Bureau of Standards, Washington, D.C Weimer, P. J., and J. G. Zeikus Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence and presence of Methanobacterium thermoautotrophicum. Appl. Environ. Microbiol. 33: Wetzel, R. G Limnology, p The W. B. Saunders Co., Philadelphia. 109a.Widdel, F., and N. Pfennig A new anaerobic, sporing bacterium, Desulfotomaculum (emerd.) acetoxidans. Arch. Microbiol. 112: Winfrey, M. R., D. R. Nelson, S. C. Klevickis, and J. G. Zeikus Association of hydrogen metabolism with methanogenesis in Lake Mendota sediments. Appl. Environ. Microbiol. 33: Winfrey, M. R., and J. G. Zeikus Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl. Environ. Microbiol. 33: Wolfe, R. S Microbial formation of methane, p In A. H. Rose and J. F. Wilkinson (ed.), Advances in microbiological physiology, vol. 6. Academic Press, Inc., New York and London Wolin, E. A., M. J. Wolin, and R. S. Wolfe Formation of methane by bacterial extracts. J. Biol. Chem. 238: Wolin, M. J Metabolic interactions among intestinal microorganisms. Am. J. Clin. Nutr. 27: Wolin, M. J Interactions between the bacterial species of the rumen. Fourth International Symposium of Ruminant Physiology, in press. 115a.Zehnder, A. J. B., and K. Wuhrmann Physiology of a Methanobacterium strain A2. Arch. Microbiol. 111: Zeikus, J. G., and R. S. Wolfe Methanobacterium thermoautotrophicum sp. n., an anaerobic, autotrophic, extreme thermophile. J. Bacteriol. 109: Zeikus, J. G., and R. S. Wolfe Fine structure of Methanobacterium thermoautotrophicum: effect of growth temperature on morphology and ultrastructure. J. Bacteriol. 113: Zeikus, J. G., and J. G. Ward Methane formation in living trees: a microbial origin. Science 184: Zeikus, J. G., and D. L. Henning Methanobacterium arbophilicum sp. n., an obligate anaerobe isolated from wetwood in trees. Antonie van Leeuwenhoek J. Microbiol. Serol. 41:
Tentative Identification of Methanogenic Bacteria by Fluorescence Microscopy
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Mar. 1977, p. 713-717 Copyright (C 1977 American Society for Microbiology Vol. 33, No. 3 Printed in U.S.A. Tentative Identification of Methanogenic Bacteria by Fluorescence
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Mar. 1977, p. 713-717 Copyright (C 1977 American Society for Microbiology Vol. 33, No. 3 Printed in U.S.A. Tentative Identification of Methanogenic Bacteria by Fluorescence
Using the shake culture technique, it was possible to separate a Methanosarcina
 STUDIES ON THE METHANE FERMENTATION IX. THE ORIGIN OF METHANE IN THE ACETATE AND METHANOL FERMENTATIONS BY METHANOSARCINA1 THRESSA C. STADTMAN2 AND H. A. BARKER Diviaion of Plant Biochemistry, University
STUDIES ON THE METHANE FERMENTATION IX. THE ORIGIN OF METHANE IN THE ACETATE AND METHANOL FERMENTATIONS BY METHANOSARCINA1 THRESSA C. STADTMAN2 AND H. A. BARKER Diviaion of Plant Biochemistry, University
Chapter 6 Microbial Growth With a focus on Bacteria
 Chapter 6 Microbial Growth With a focus on Bacteria Temperature Minimum growth temperature Optimum growth temperature Maximum growth temperature Usually within a 30-40 degree range Microbial growth = increase
Chapter 6 Microbial Growth With a focus on Bacteria Temperature Minimum growth temperature Optimum growth temperature Maximum growth temperature Usually within a 30-40 degree range Microbial growth = increase
Classifying Prokaryotes: Eubacteria Plasma Membrane. Ribosomes. Plasmid (DNA) Capsule. Cytoplasm. Outer Membrane DNA. Flagellum.
 Bacteria The yellow band surrounding this hot spring is sulfur, a waste product of extremophilic prokaryotes, probably of the Domain Archaea, Kingdom Archaebacteria. Bacteria are prokaryotic cells (no
Bacteria The yellow band surrounding this hot spring is sulfur, a waste product of extremophilic prokaryotes, probably of the Domain Archaea, Kingdom Archaebacteria. Bacteria are prokaryotic cells (no
The Prokaryotic World
 The Prokaryotic World A. An overview of prokaryotic life There is no doubt that prokaryotes are everywhere. By everywhere, I mean living in every geographic region, in extremes of environmental conditions,
The Prokaryotic World A. An overview of prokaryotic life There is no doubt that prokaryotes are everywhere. By everywhere, I mean living in every geographic region, in extremes of environmental conditions,
Kingdom Bacteria Kingdom Archaea
 Section 5.1 Kingdom Bacteria Kingdom Archaea p. 132-139 Kingdom Bacteria General Characteristics: Cell Type: all are prokaryotic. Body Form: most are unicellular, some are colonial. Three main shapes are:
Section 5.1 Kingdom Bacteria Kingdom Archaea p. 132-139 Kingdom Bacteria General Characteristics: Cell Type: all are prokaryotic. Body Form: most are unicellular, some are colonial. Three main shapes are:
Chapter 1. Basics of Microbiology
 Chapter 1 Basics of Microbiology Objectives How microorganisms are classified (taxonomy) What they look like (morphology) The major divisions among microorganisms based upon their function in the environment
Chapter 1 Basics of Microbiology Objectives How microorganisms are classified (taxonomy) What they look like (morphology) The major divisions among microorganisms based upon their function in the environment
Chapter 19 Notes Kingdoms Archaebacteria andeubacteria
 Chapter 19 Notes Kingdoms Archaebacteria andeubacteria All bacteria are Prokaryotic. This means that they are organisms that are one-celled and do not contain a nucleus or other membrane bound organelles.
Chapter 19 Notes Kingdoms Archaebacteria andeubacteria All bacteria are Prokaryotic. This means that they are organisms that are one-celled and do not contain a nucleus or other membrane bound organelles.
Metabolic diversity is based on the Electron donors, acceptors, and carbon sources available - thermodynamics
 To date you have covered microbial community sampling using molecular techniques to identify who is present in the environment. You have also looked at various genetic mechanisms to understand how organisms
To date you have covered microbial community sampling using molecular techniques to identify who is present in the environment. You have also looked at various genetic mechanisms to understand how organisms
MICROBIOLOGIA GENERALE. The Archaea
 MICROBIOLOGIA GENERALE The Archaea The Archaea s traits 1. The cell wall of Archaea: pseudopeptidoglycan, polysaccharide, glycoprotein 2. The cytoplasmic membrane of Archaea: ether linkage, glycerol
MICROBIOLOGIA GENERALE The Archaea The Archaea s traits 1. The cell wall of Archaea: pseudopeptidoglycan, polysaccharide, glycoprotein 2. The cytoplasmic membrane of Archaea: ether linkage, glycerol
(DMB 01) M.Sc. (Previous) DEGREE EXAMINATION, DECEMBER First Year. Microbiology. Paper I INTRODUCTION TO MICROORGANISMS
 wk 7 (DMB 01) Paper I INTRODUCTION TO MICROORGANISMS PART A (5 8 = 40 marks) 1. Explain the growth of microbiology in the twentieth century. 2. Describe the structure of eukaryotic cell with a neat-labeled
wk 7 (DMB 01) Paper I INTRODUCTION TO MICROORGANISMS PART A (5 8 = 40 marks) 1. Explain the growth of microbiology in the twentieth century. 2. Describe the structure of eukaryotic cell with a neat-labeled
Red Layer Microbial Observatory Biology In-Lab Workshop Photosynthetic Microbes from Local Rivers & Beyond
 Red Layer Microbial Observatory Biology 507 - In-Lab Workshop Photosynthetic Microbes from Local Rivers & Beyond Schedule of Activities Session One 1. Microbial Diversity & the RLMO Program 2. Photosynthetic
Red Layer Microbial Observatory Biology 507 - In-Lab Workshop Photosynthetic Microbes from Local Rivers & Beyond Schedule of Activities Session One 1. Microbial Diversity & the RLMO Program 2. Photosynthetic
Kingdom Monera(Archaebacteria & Eubacteria)
 Kingdom Monera(Archaebacteria & All bacteria are prokaryotes Characteristics: 1. No nucleus Eubacteria) 2. No membrane bound organelles 3. Smaller & less ribosomes 4. Most are smaller than eukaryotes 5.
Kingdom Monera(Archaebacteria & All bacteria are prokaryotes Characteristics: 1. No nucleus Eubacteria) 2. No membrane bound organelles 3. Smaller & less ribosomes 4. Most are smaller than eukaryotes 5.
Outline. Viruses, Bacteria, and Archaea. Viruses Structure Classification Reproduction Prokaryotes Structure Reproduction Nutrition Bacteria Archaea
 Viruses, Bacteria, and Archaea Chapter 21 Viruses Structure Classification Reproduction Prokaryotes Structure Reproduction Nutrition Bacteria Archaea Outline The Viruses The Viruses Viruses are noncellular
Viruses, Bacteria, and Archaea Chapter 21 Viruses Structure Classification Reproduction Prokaryotes Structure Reproduction Nutrition Bacteria Archaea Outline The Viruses The Viruses Viruses are noncellular
Principles of Cellular Biology
 Principles of Cellular Biology آشنایی با مبانی اولیه سلول Biologists are interested in objects ranging in size from small molecules to the tallest trees: Cell Basic building blocks of life Understanding
Principles of Cellular Biology آشنایی با مبانی اولیه سلول Biologists are interested in objects ranging in size from small molecules to the tallest trees: Cell Basic building blocks of life Understanding
Kingdom Monera Bacteria
 Kingdom Monera Bacteria Common bacteria Prokaryotes Strep throat Anthrax Chlamydia E. coli Meningitis Salmonella Micrococcus(intestinal) Streptococcus mutans Haemophilusinfluenzae Cellphonious bacterious
Kingdom Monera Bacteria Common bacteria Prokaryotes Strep throat Anthrax Chlamydia E. coli Meningitis Salmonella Micrococcus(intestinal) Streptococcus mutans Haemophilusinfluenzae Cellphonious bacterious
Page 1. Name: UNIT: PHOTOSYNTHESIS AND RESPIRATION TOPIC: PHOTOSYNTHESIS
 Name: 4667-1 - Page 1 UNIT: PHOTOSYNTHESIS AND RESPIRATION TOPIC: PHOTOSYNTHESIS 1) The diagram below illustrates the movement of materials involved in a process that is vital for the energy needs of organisms.
Name: 4667-1 - Page 1 UNIT: PHOTOSYNTHESIS AND RESPIRATION TOPIC: PHOTOSYNTHESIS 1) The diagram below illustrates the movement of materials involved in a process that is vital for the energy needs of organisms.
BACTERIA AND ARCHAEA 10/15/2012
 BACTERIA AND ARCHAEA Chapter 27 KEY CONCEPTS: Structural and functional adaptations contribute to prokaryotic success Rapid reproduction, mutation, and genetic recombination promote genetic diversity in
BACTERIA AND ARCHAEA Chapter 27 KEY CONCEPTS: Structural and functional adaptations contribute to prokaryotic success Rapid reproduction, mutation, and genetic recombination promote genetic diversity in
A word of caution about a little knowing Lab organisms limit the view of the world of microbiology
 Diversity The world of living things (Figure from Madigan et al. 2002) Microbes in all three domains Two of the domains are exclusively prokaryotic and microbial The third contains both unicellular and
Diversity The world of living things (Figure from Madigan et al. 2002) Microbes in all three domains Two of the domains are exclusively prokaryotic and microbial The third contains both unicellular and
Nutritional and Biochemical Characterization of Methanospirillum hungatii
 APPLID AND NVIRONMNTAL MicaoIowLOy, Oct. 1977, p. 371-376 Copyright 1977 American Society for Microbiology Vol. 34, No. 4 Printed in U.S.A. Nutritional and Biochemical Characteriation of Methanospirillum
APPLID AND NVIRONMNTAL MicaoIowLOy, Oct. 1977, p. 371-376 Copyright 1977 American Society for Microbiology Vol. 34, No. 4 Printed in U.S.A. Nutritional and Biochemical Characteriation of Methanospirillum
BIOLOGY 10/11/2014. An Introduction to Metabolism. Outline. Overview: The Energy of Life
 8 An Introduction to Metabolism CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes
8 An Introduction to Metabolism CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes
Cells & Bacteria Notes
 Cells & Bacteria Notes 4 Major Macromolecules Macromolecules are large molecules. The four groups of macromolecules are essential to the structure and function of a cell. Group Building Block Large Molecule
Cells & Bacteria Notes 4 Major Macromolecules Macromolecules are large molecules. The four groups of macromolecules are essential to the structure and function of a cell. Group Building Block Large Molecule
BBS2710 Microbial Physiology. Module 5 - Energy and Metabolism
 BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
BBS2710 Microbial Physiology Module 5 - Energy and Metabolism Topics Energy production - an overview Fermentation Aerobic respiration Alternative approaches to respiration Photosynthesis Summary Introduction
Microbiology: An Introduction, 12e (Tortora) Chapter 2 Chemical Principles. 2.1 Multiple Choice Questions
 Microbiology An Introduction 12th Edition Tortora TEST BANK Full download at: https://testbankreal.com/download/microbiology-an-introduction-12thedition-tortora-test-bank/ Microbiology An Introduction
Microbiology An Introduction 12th Edition Tortora TEST BANK Full download at: https://testbankreal.com/download/microbiology-an-introduction-12thedition-tortora-test-bank/ Microbiology An Introduction
Announcements KEY CONCEPTS
 What do these things have in common? Announcements Lab this week: bring textbook and photo atlas. Relevant reading BEFORE lab: Ch. 30 http://i.cnn.net/cnn/specials/2001/trade.center/images/anthrax.jpg
What do these things have in common? Announcements Lab this week: bring textbook and photo atlas. Relevant reading BEFORE lab: Ch. 30 http://i.cnn.net/cnn/specials/2001/trade.center/images/anthrax.jpg
9/8/2017. Bacteria and Archaea. Three domain system: The present tree of life. Structural and functional adaptations contribute to prokaryotic success
 5 m 2 m 9/8/2017 Three domain system: The present tree of life Bacteria and Archaea Chapter 27 Structural and functional adaptations contribute to prokaryotic success Unicellular Small Variety of shapes
5 m 2 m 9/8/2017 Three domain system: The present tree of life Bacteria and Archaea Chapter 27 Structural and functional adaptations contribute to prokaryotic success Unicellular Small Variety of shapes
Vocabulary- Bacteria (34 words)
 Biology II BACTERIA Vocabulary- Bacteria (34 words) 1. Prokaryote 21. phototroph 2. Peptidoglycan 22. chemotroph 3. Methanogen 23. obligate anaerobe 4. Halophile 24. facultative anaerobe 5. Thermoacidophile
Biology II BACTERIA Vocabulary- Bacteria (34 words) 1. Prokaryote 21. phototroph 2. Peptidoglycan 22. chemotroph 3. Methanogen 23. obligate anaerobe 4. Halophile 24. facultative anaerobe 5. Thermoacidophile
Part 2. The Basics of Biology:
 Part 2 The Basics of Biology: An Engineer s Perspective Chapter 2 An Overview of Biological Basics 21 2.1 Cells 2.2 Cell Construction 2.3 Cell Nutrient 2.1 Are all cells the same? Cells Basic unit of living
Part 2 The Basics of Biology: An Engineer s Perspective Chapter 2 An Overview of Biological Basics 21 2.1 Cells 2.2 Cell Construction 2.3 Cell Nutrient 2.1 Are all cells the same? Cells Basic unit of living
Kingdom Monera. These notes are to help you check your answers in your Bacteria unit handout package that you received in class.
 Kingdom Monera These notes are to help you check your answers in your Bacteria unit handout package that you received in class. Textbook reference pages Textbook Section 17-2 & 17-3 pages 360-375 Basic
Kingdom Monera These notes are to help you check your answers in your Bacteria unit handout package that you received in class. Textbook reference pages Textbook Section 17-2 & 17-3 pages 360-375 Basic
Bacterial Morphology and Structure م.م رنا مشعل
 Bacterial Morphology and Structure م.م رنا مشعل SIZE OF BACTERIA Unit for measurement : Micron or micrometer, μm: 1μm=10-3 mm Size: Varies with kinds of bacteria, and also related to their age and external
Bacterial Morphology and Structure م.م رنا مشعل SIZE OF BACTERIA Unit for measurement : Micron or micrometer, μm: 1μm=10-3 mm Size: Varies with kinds of bacteria, and also related to their age and external
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1)
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
Introduction to Microbiology. CLS 212: Medical Microbiology Miss Zeina Alkudmani
 Introduction to Microbiology CLS 212: Medical Microbiology Miss Zeina Alkudmani Microbiology Micro- means very small (that needs a microscope to see). Microbiology is the study of very small living organisms.
Introduction to Microbiology CLS 212: Medical Microbiology Miss Zeina Alkudmani Microbiology Micro- means very small (that needs a microscope to see). Microbiology is the study of very small living organisms.
KINGDOM MONERA. Bacterial Cell Shape 8/22/2010. The Prokaryotes: Archaebacteria and Eubacteria
 KINGDOM MONERA The Prokaryotes: Archaebacteria and Eubacteria Bacteria are the most organisms living on the Earth. (i.e. 10mL of soil contains 1 x 10 10 bacteria. They are found in nearly every habitat
KINGDOM MONERA The Prokaryotes: Archaebacteria and Eubacteria Bacteria are the most organisms living on the Earth. (i.e. 10mL of soil contains 1 x 10 10 bacteria. They are found in nearly every habitat
An Introduction to Metabolism
 Chapter 8 1 An Introduction to Metabolism PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Chapter 8 1 An Introduction to Metabolism PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Microbiology. Definition of a Microorganism. Microorganisms in the Lab. The Study of Microorganisms
 Microbiology The Study of Microorganisms Definition of a Microorganism Derived from the Greek: Mikros, «small» and Organismos, organism Microscopic organism which is single celled (unicellular) or a mass
Microbiology The Study of Microorganisms Definition of a Microorganism Derived from the Greek: Mikros, «small» and Organismos, organism Microscopic organism which is single celled (unicellular) or a mass
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1 1) Which compound in Figure 2.1 is an ester? 1) A) a b c d e Answer: D 2) A scientist
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1 1) Which compound in Figure 2.1 is an ester? 1) A) a b c d e Answer: D 2) A scientist
AP Biology Review Chapters 6-8 Review Questions Chapter 6: Metabolism: Energy and Enzymes Chapter 7: Photosynthesis Chapter 8: Cellular Respiration
 AP Biology Review Chapters 6-8 Review Questions Chapter 6: Metabolism: Energy and Enzymes 1. Understand and know the first and second laws of thermodynamics. What is entropy? What happens when entropy
AP Biology Review Chapters 6-8 Review Questions Chapter 6: Metabolism: Energy and Enzymes 1. Understand and know the first and second laws of thermodynamics. What is entropy? What happens when entropy
9/25/2011. Outline. Overview: The Energy of Life. I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V.
 Chapter 8 Introduction to Metabolism Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes Overview: The Energy of Life Figure 8.1 The living cell is a miniature
Chapter 8 Introduction to Metabolism Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes Overview: The Energy of Life Figure 8.1 The living cell is a miniature
13. The diagram below shows two different kinds of substances, A and B, entering a cell.
 Name 1. In the binomial system of nomenclature, which two classification groups provide the scientific name of an organism? A) kingdom and phylum B) phylum and species C) kingdom and genus D) genus and
Name 1. In the binomial system of nomenclature, which two classification groups provide the scientific name of an organism? A) kingdom and phylum B) phylum and species C) kingdom and genus D) genus and
Name # Class Date Regents Review: Cells & Cell Transport
 Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
Prokaryotes Vs. Eukaryotes
 The Microbial World Prokaryotes Vs. Eukaryotes Mircrobes of the Ocean Primary Producers Are the organisms that produce bio-mass from inorganic compounds (autotrophs). -Photosynthetic autotrophs Phytoplankton
The Microbial World Prokaryotes Vs. Eukaryotes Mircrobes of the Ocean Primary Producers Are the organisms that produce bio-mass from inorganic compounds (autotrophs). -Photosynthetic autotrophs Phytoplankton
(Methanogenesis; xylan; formate; defined mixed culture; inhibition) J.P. Guyot
 FEMS Microbiology Letters 34 (1986) 149-153 Published by Elsevier 149 FEM 02386 Role of formate in methanogenesis from xylan by Cellulomonas sp. associated with methanogens and Desulfovibrio vulgaris:
FEMS Microbiology Letters 34 (1986) 149-153 Published by Elsevier 149 FEM 02386 Role of formate in methanogenesis from xylan by Cellulomonas sp. associated with methanogens and Desulfovibrio vulgaris:
Microbial Diversity. Yuzhen Ye I609 Bioinformatics Seminar I (Spring 2010) School of Informatics and Computing Indiana University
 Microbial Diversity Yuzhen Ye (yye@indiana.edu) I609 Bioinformatics Seminar I (Spring 2010) School of Informatics and Computing Indiana University Contents Microbial diversity Morphological, structural,
Microbial Diversity Yuzhen Ye (yye@indiana.edu) I609 Bioinformatics Seminar I (Spring 2010) School of Informatics and Computing Indiana University Contents Microbial diversity Morphological, structural,
Biology. Slide 1 of 40. End Show. Copyright Pearson Prentice Hall
 Biology 1 of 40 (p471-477) 2 of 40 Microorganisms = Microbes Microbiology is the study of living creatures too small to see with the unaided eye including : bacteria protozoa fungi algae viruses other
Biology 1 of 40 (p471-477) 2 of 40 Microorganisms = Microbes Microbiology is the study of living creatures too small to see with the unaided eye including : bacteria protozoa fungi algae viruses other
B. Correct! Bacillus anthraces produces spores that can cause anthrax. D. Incorrect! Diphtheria is caused by Corynebacterium diphtheriae.
 Microbiology - Problem Drill 09 - The Prokaryotes No. 1 of 10 1. Bacillus anthraces is most closely associated with which of the following? (A) Botulism poisoning (B) Anthrax (C) Gangrene (D) Diphtheria
Microbiology - Problem Drill 09 - The Prokaryotes No. 1 of 10 1. Bacillus anthraces is most closely associated with which of the following? (A) Botulism poisoning (B) Anthrax (C) Gangrene (D) Diphtheria
Section A: The Principles of Energy Harvest
 CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section A: The Principles of Energy Harvest 1. Cellular respiration and fermentation are catabolic, energy-yielding pathways 2. Cells recycle
CHAPTER 9 CELLULAR RESPIRATION: HARVESTING CHEMICAL ENERGY Section A: The Principles of Energy Harvest 1. Cellular respiration and fermentation are catabolic, energy-yielding pathways 2. Cells recycle
Chapter 02 The Chemical Basis of Life I: Atoms, Molecules, and Water
 Chapter 02 The Chemical Basis of Life I: Atoms, Molecules, and Water Multiple Choice Questions 1. The atomic number of an atom is A. the number of protons in the atom. B. the number of neutrons in the
Chapter 02 The Chemical Basis of Life I: Atoms, Molecules, and Water Multiple Choice Questions 1. The atomic number of an atom is A. the number of protons in the atom. B. the number of neutrons in the
Cellular Respiration: Harvesting Chemical Energy. 9.1 Catabolic pathways yield energy by oxidizing organic fuels
 Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Physiological diversity
 Physiological diversity Principles Energetic considerations Biochemical pathways Organisms Ecological relevance Physiological diversity Sulfate- and nitrate reducers (5. Nov.) Methanogens and homoacetogens
Physiological diversity Principles Energetic considerations Biochemical pathways Organisms Ecological relevance Physiological diversity Sulfate- and nitrate reducers (5. Nov.) Methanogens and homoacetogens
HYDROGEN. technique. uptake/co2 uptake, which according to equation (1) should equal 4, has
 184 BA CTERIOLOG Y: H. A. BARKER PROC. N. A. S. STUDIES ON THE METHANE FERMENTATION. VI. THE IN- FLUENCE OF CARBON DIOXIDE CONCENTRATION ON THE RATE OF CARBON DIOXIDE REDUCTION BY MOLECULAR HYDROGEN By
184 BA CTERIOLOG Y: H. A. BARKER PROC. N. A. S. STUDIES ON THE METHANE FERMENTATION. VI. THE IN- FLUENCE OF CARBON DIOXIDE CONCENTRATION ON THE RATE OF CARBON DIOXIDE REDUCTION BY MOLECULAR HYDROGEN By
Eukaryotic Cells. Figure 1: A mitochondrion
 Eukaryotic Cells Figure 1: A mitochondrion How do cells accomplish all their functions in such a tiny, crowded package? Eukaryotic cells those that make up cattails and apple trees, mushrooms and dust
Eukaryotic Cells Figure 1: A mitochondrion How do cells accomplish all their functions in such a tiny, crowded package? Eukaryotic cells those that make up cattails and apple trees, mushrooms and dust
Contains ribosomes attached to the endoplasmic reticulum. Genetic material consists of linear chromosomes. Diameter of the cell is 1 m
 1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
1. (a) Complete each box in the table, which compares a prokaryotic and a eukaryotic cell, with a tick if the statement is correct or a cross if it is incorrect. Prokaryotic cell Eukaryotic cell Contains
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1)
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
Classification. Old 5 Kingdom system. New 3 Domain system. reflects a greater understanding of evolution & molecular evidence
 Classification Old 5 Kingdom system Monera, Protists, Plants, Fungi, Animals New 3 Domain system reflects a greater understanding of evolution & molecular evidence Prokaryote: Bacteria Prokaryote: Archaebacteria
Classification Old 5 Kingdom system Monera, Protists, Plants, Fungi, Animals New 3 Domain system reflects a greater understanding of evolution & molecular evidence Prokaryote: Bacteria Prokaryote: Archaebacteria
Chapter 002 The Chemistry of Biology
 Chapter 002 The Chemistry of Biology Multiple Choice Questions 1. Anything that occupies space and has mass is called A. Atomic B. Living C. Matter D. Energy E. Space 2. The electrons of an atom are A.
Chapter 002 The Chemistry of Biology Multiple Choice Questions 1. Anything that occupies space and has mass is called A. Atomic B. Living C. Matter D. Energy E. Space 2. The electrons of an atom are A.
The invention of the microscope has opened to us a world of extraordinary numbers. A singular drop of pond water reveals countless life forms
 Biology Chapter 19 Notes - Bacteria and Viruses The invention of the microscope has opened to us a world of extraordinary numbers. A singular drop of pond water reveals countless life forms I. Classifying
Biology Chapter 19 Notes - Bacteria and Viruses The invention of the microscope has opened to us a world of extraordinary numbers. A singular drop of pond water reveals countless life forms I. Classifying
Introduction to Microbiology BIOL 220 Summer Session I, 1996 Exam # 1
 Name I. Multiple Choice (1 point each) Introduction to Microbiology BIOL 220 Summer Session I, 1996 Exam # 1 B 1. Which is possessed by eukaryotes but not by prokaryotes? A. Cell wall B. Distinct nucleus
Name I. Multiple Choice (1 point each) Introduction to Microbiology BIOL 220 Summer Session I, 1996 Exam # 1 B 1. Which is possessed by eukaryotes but not by prokaryotes? A. Cell wall B. Distinct nucleus
10/4/2016. Matter, Energy, and Life
 DISCLAIMER: Principles and concepts on atomic structure, the Periodic Table, atoms, ions, ionic and covalent compounds, metals, and nonmetals will not be covered in this course. You are expected to know
DISCLAIMER: Principles and concepts on atomic structure, the Periodic Table, atoms, ions, ionic and covalent compounds, metals, and nonmetals will not be covered in this course. You are expected to know
Bacteria. Received for publication 4 January via election sinks other than H2 (6, 18, 19). The
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, May 1977, p. 1162-1169 Copyright 1977 American Society for Microbiology Vol. 33, No. 5 Printed in U.S.A. Growth of Desulfovibrio in Lactate or Ethanol Media Low
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, May 1977, p. 1162-1169 Copyright 1977 American Society for Microbiology Vol. 33, No. 5 Printed in U.S.A. Growth of Desulfovibrio in Lactate or Ethanol Media Low
Origins - Three Domain Classification PROKARYOTES
 Bacteria Origins - Three Domain Classification EU PROKARYOTES I. Origins of Bacteria Prokaryotes Eubacteria Archaebacteria A. Prokaryotes = 1. Kingdom Eubacteria 2. Kingdom Archaebacteria 3. Prokaryote
Bacteria Origins - Three Domain Classification EU PROKARYOTES I. Origins of Bacteria Prokaryotes Eubacteria Archaebacteria A. Prokaryotes = 1. Kingdom Eubacteria 2. Kingdom Archaebacteria 3. Prokaryote
MORPHOLOGY: the study of form and structure
 MICROBIOLOGY CHAPTER 3 Bacteria Morphology 3:1 Bacteria Structure and Function MORPHOLOGY: the study of form and structure Structure of Bacteria 1. PROKARYOTIC no membrane bound nucleus nor other organelles
MICROBIOLOGY CHAPTER 3 Bacteria Morphology 3:1 Bacteria Structure and Function MORPHOLOGY: the study of form and structure Structure of Bacteria 1. PROKARYOTIC no membrane bound nucleus nor other organelles
Chapter 6- An Introduction to Metabolism*
 Chapter 6- An Introduction to Metabolism* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. The Energy of Life
Chapter 6- An Introduction to Metabolism* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. The Energy of Life
Microbial Biogeochemistry
 Microbial Biogeochemistry Chemical reactions occurring in the environment mediated by microbial communities Outline Metabolic Classifications. Winogradsky columns, Microenvironments. Redox Reactions. Microbes
Microbial Biogeochemistry Chemical reactions occurring in the environment mediated by microbial communities Outline Metabolic Classifications. Winogradsky columns, Microenvironments. Redox Reactions. Microbes
Biology Reading Assignment: Chapter 9 in textbook
 Biology 205 5.10.06 Reading Assignment: Chapter 9 in textbook HTTP://WUNMR.WUSTL.EDU/EDUDEV/LABTUTORIALS/CYTOCHROMES/CYTOCHROMES.HTML What does a cell need to do? propagate itself (and its genetic program)
Biology 205 5.10.06 Reading Assignment: Chapter 9 in textbook HTTP://WUNMR.WUSTL.EDU/EDUDEV/LABTUTORIALS/CYTOCHROMES/CYTOCHROMES.HTML What does a cell need to do? propagate itself (and its genetic program)
EFFECT OF ph AND AMMONIUM IONS ON THE PERMEABILITY
 EFFECT OF ph AND AMMONIUM IONS ON THE PERMEABILITY OF BACILLUS PASTEURII W. R. WILEY AND J. L. STOKES Department of Bacteriology and Public Health, Washington State University, Pullman, Washington ABSTRACT
EFFECT OF ph AND AMMONIUM IONS ON THE PERMEABILITY OF BACILLUS PASTEURII W. R. WILEY AND J. L. STOKES Department of Bacteriology and Public Health, Washington State University, Pullman, Washington ABSTRACT
Shape, Arrangement, and Size. Cocci (s., coccus) bacillus (pl., bacilli) 9/21/2013
 Shape, Arrangement, and Size Cocci (s., coccus) are roughly spherical cells. The other common shape is that of a rod, sometimes called a bacillus (pl., bacilli). Spiral-shaped procaryotes can be either
Shape, Arrangement, and Size Cocci (s., coccus) are roughly spherical cells. The other common shape is that of a rod, sometimes called a bacillus (pl., bacilli). Spiral-shaped procaryotes can be either
Ch. 2 BASIC CHEMISTRY. Copyright 2010 Pearson Education, Inc.
 Ch. 2 BASIC CHEMISTRY Matter and Composition of Matter Definition: Anything that has mass and occupies space Matter is made up of elements An element cannot be broken down by ordinary chemical means Atoms
Ch. 2 BASIC CHEMISTRY Matter and Composition of Matter Definition: Anything that has mass and occupies space Matter is made up of elements An element cannot be broken down by ordinary chemical means Atoms
Oceans: the cradle of life? Chapter 5. Cells: a sense of scale. Head of a needle
 Oceans: the cradle of life? Highest diversity of life, particularly archae, bacteria, and animals Will start discussion of life in the ocean with prokaryote microorganisms Prokaryotes are also believed
Oceans: the cradle of life? Highest diversity of life, particularly archae, bacteria, and animals Will start discussion of life in the ocean with prokaryote microorganisms Prokaryotes are also believed
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. C is FALSE?
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about the atom 12 6 C is FALSE? 1) A) It has 12 neutrons
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about the atom 12 6 C is FALSE? 1) A) It has 12 neutrons
METHANE FORMATION; FERMENTATION OF ETHANOL IN THE ABSENCE OF CARBON DIOXIDE BY METHANOBACILLUS OMELIANSKII'
 METHANE FORMATION; FERMENTATION OF ETHANOL IN THE ABSENCE OF CARBON DIOXIDE BY METHANOBACILLUS OMELIANSKII' A. T. JOHNS2 AND H. A. BARKER Department of Biochemistry, University of California, Berkeley,
METHANE FORMATION; FERMENTATION OF ETHANOL IN THE ABSENCE OF CARBON DIOXIDE BY METHANOBACILLUS OMELIANSKII' A. T. JOHNS2 AND H. A. BARKER Department of Biochemistry, University of California, Berkeley,
Cellular Respiration. Anaerobic vs Aerobic
 Cellular Respiration Anaerobic vs Aerobic What is Cellular Respiration? Process where organisms use GLUCOSE (sugar) to create ENERGY! The energy that is released from chemical bonds during Cellular Respiration
Cellular Respiration Anaerobic vs Aerobic What is Cellular Respiration? Process where organisms use GLUCOSE (sugar) to create ENERGY! The energy that is released from chemical bonds during Cellular Respiration
Unit 5 Cellular Energy
 Unit 5 Cellular Energy I. Enzymes (159) 1.Are CATALYSTS: Speed up chemical reactions that would otherwise happen too slowly to support life. Catalysts DO NOT make reactions happen that couldn t happen
Unit 5 Cellular Energy I. Enzymes (159) 1.Are CATALYSTS: Speed up chemical reactions that would otherwise happen too slowly to support life. Catalysts DO NOT make reactions happen that couldn t happen
Chapter Two: The Chemistry of Biology. The molecules of life make up the structure of cells Chemistry of biological molecule
 Chapter Two: The Chemistry of Biology The molecules of life make up the structure of cells Chemistry of biological molecule Atoms and Elements: Atoms: The basic units of all matter, containing three major
Chapter Two: The Chemistry of Biology The molecules of life make up the structure of cells Chemistry of biological molecule Atoms and Elements: Atoms: The basic units of all matter, containing three major
Temperature Limitation of Methanogenesis
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Jan. 1976, p. 99-107 Vol. 31, No. 1 Copyright 1976 American Society for Microbiology Printed in U.S.A. Temperature Limitation of Methanogenesis in Aquatic Sediments
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Jan. 1976, p. 99-107 Vol. 31, No. 1 Copyright 1976 American Society for Microbiology Printed in U.S.A. Temperature Limitation of Methanogenesis in Aquatic Sediments
Archaeal Cell Structure. Copyright McGraw-Hill Global Education Holdings, LLC. Permission required for reproduction or display.
 4 Archaeal Cell Structure Copyright McGraw-Hill Global Education Holdings, LLC. Permission required for reproduction or display. 1 4.1 A typical Archaeal Cell 2 Archaea Highly diverse with respect to morphology,
4 Archaeal Cell Structure Copyright McGraw-Hill Global Education Holdings, LLC. Permission required for reproduction or display. 1 4.1 A typical Archaeal Cell 2 Archaea Highly diverse with respect to morphology,
Influence of Corrinoid Antagonists on Methanogen
 JOURNAL OF BACTERIOLOGY, Apr. 1981, p. 133-140 0021-9193/81/040133-08$02.00/0 Vol. 146, No. 1 Influence of Corrinoid Antagonists on Methanogen Metabolism WILLIAM KENEALY AND J. G. ZEIKUS* Department of
JOURNAL OF BACTERIOLOGY, Apr. 1981, p. 133-140 0021-9193/81/040133-08$02.00/0 Vol. 146, No. 1 Influence of Corrinoid Antagonists on Methanogen Metabolism WILLIAM KENEALY AND J. G. ZEIKUS* Department of
Oxidation of Hydrogen and Reduction of Methanol to Methane is the Sole Energy Source for a Methanogen Isolated from Human Feces
 JOURNAL OF BACTROLOGY, Feb. 1983, p. 151-155 21-9193/83/2151-5$2./ Copyright 1983, American Society for Microbiology Vol. 153, No. 2 Oxidation of Hydrogen and Reduction of Methanol to Methane is the Sole
JOURNAL OF BACTROLOGY, Feb. 1983, p. 151-155 21-9193/83/2151-5$2./ Copyright 1983, American Society for Microbiology Vol. 153, No. 2 Oxidation of Hydrogen and Reduction of Methanol to Methane is the Sole
An Introduction to Metabolism
 CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 6 An Introduction to Metabolism Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: The Energy of Life The
CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 6 An Introduction to Metabolism Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: The Energy of Life The
Notes - Microbiology Monera
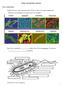 Notes - Microbiology Monera Part 1 Classification - Kingdom moneran is more commonly known as bacteria. This is the largest kingdom with inhabitants covering almost every square metre of the planet! -
Notes - Microbiology Monera Part 1 Classification - Kingdom moneran is more commonly known as bacteria. This is the largest kingdom with inhabitants covering almost every square metre of the planet! -
An Introduction to Metabolism. Chapter 8
 An Introduction to Metabolism Chapter 8 METABOLISM I. Introduction All of an organism s chemical reactions Thousands of reactions in a cell Example: digest starch use sugar for energy and to build new
An Introduction to Metabolism Chapter 8 METABOLISM I. Introduction All of an organism s chemical reactions Thousands of reactions in a cell Example: digest starch use sugar for energy and to build new
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
CELLS STRUCTURE AND FUNCTION
 CELLS STRUCTURE AND FUNCTION Jhia Anjela D. Rivera Department of Biological Sciences School of Science and Technology Centro Escolar University DISCOVERY OF CELLS Robert Hooke (1665): Observed a thin slice
CELLS STRUCTURE AND FUNCTION Jhia Anjela D. Rivera Department of Biological Sciences School of Science and Technology Centro Escolar University DISCOVERY OF CELLS Robert Hooke (1665): Observed a thin slice
Which row in the chart below identifies the lettered substances in this process?
 1. A biological process that occurs in both plants and animals is shown below. Which row in the chart below identifies the lettered substances in this process? A) 1 B) 2 C) 3 D) 4 2. All life depends on
1. A biological process that occurs in both plants and animals is shown below. Which row in the chart below identifies the lettered substances in this process? A) 1 B) 2 C) 3 D) 4 2. All life depends on
Chapter 2: Fundamentals of Chemistry. Question Type: Multiple Choice. 1) Which of the following pairs is mismatched?
 Microbiology Principles and Explorations 9th Edition Black TEST BANK Full clear download at: https://testbankreal.com/download/microbiology-principles-explorations- 9th-edition-black-test-bank/ Microbiology
Microbiology Principles and Explorations 9th Edition Black TEST BANK Full clear download at: https://testbankreal.com/download/microbiology-principles-explorations- 9th-edition-black-test-bank/ Microbiology
Outline. Cell Structure and Function. Cell Theory Cell Size Prokaryotic Cells Eukaryotic Cells Organelles. Chapter 4
 Cell Structure and Function Chapter 4 Cell Theory Cell Size Prokaryotic Cells Eukaryotic Cells Organelles! Nucleus Outline! Endomembrane System! Cytoskeleton! Centrioles, Cilia, and Flagella 1 2 Cell Theory
Cell Structure and Function Chapter 4 Cell Theory Cell Size Prokaryotic Cells Eukaryotic Cells Organelles! Nucleus Outline! Endomembrane System! Cytoskeleton! Centrioles, Cilia, and Flagella 1 2 Cell Theory
Biology 30 The Chemistry of Living Things
 Biology 30 The Chemistry of Living Things Hierarchy of organization: Chemistry: MATTER: Periodic Table: ELEMENT: Ex. oxygen, gold, copper, carbon COMPOUND: Ex. salt (NaCl), H 2 O ELEMENTS ESSENTIAL TO
Biology 30 The Chemistry of Living Things Hierarchy of organization: Chemistry: MATTER: Periodic Table: ELEMENT: Ex. oxygen, gold, copper, carbon COMPOUND: Ex. salt (NaCl), H 2 O ELEMENTS ESSENTIAL TO
Chapter 6 # METABOLISM PowerPoint Image Slideshow
 COLLEGE BIOLOGY PHYSICS Chapter 6 # METABOLISM Chapter Title PowerPoint Image Slideshow Figure 8.1 Metabolism Figure 6.2 Energy from the sun. Plants photosynthesis Herbivores eat those plants Carnivores
COLLEGE BIOLOGY PHYSICS Chapter 6 # METABOLISM Chapter Title PowerPoint Image Slideshow Figure 8.1 Metabolism Figure 6.2 Energy from the sun. Plants photosynthesis Herbivores eat those plants Carnivores
Aquatic Chemistry (10 hrs)
 Aquatic Chemistry (10 hrs) Water -The quality and quantity of water available to human have been vital factors in determining their well-being. -More then 70% of the earth is covered by water. Living cells
Aquatic Chemistry (10 hrs) Water -The quality and quantity of water available to human have been vital factors in determining their well-being. -More then 70% of the earth is covered by water. Living cells
Outline 10: Origin of Life. Better Living Through Chemistry
 Outline 10: Origin of Life Better Living Through Chemistry What is Life? Internal chemical activity providing growth, repair, and generation of energy. The ability to reproduce. The capacity to respond
Outline 10: Origin of Life Better Living Through Chemistry What is Life? Internal chemical activity providing growth, repair, and generation of energy. The ability to reproduce. The capacity to respond
9/8/2010. Chapter 4. Structures Internal to the Cell Wall. The Plasma Membrane. Functional Anatomy of Prokaryotic and Eukaryotic Cells
 Chapter 4 Functional Anatomy of Prokaryotic and Eukaryotic Cells Johana Meléndez Part II slides 39-87 Lectures prepared by Christine L. Case Structures Internal to the Cell Wall Learning Objectives 4-8
Chapter 4 Functional Anatomy of Prokaryotic and Eukaryotic Cells Johana Meléndez Part II slides 39-87 Lectures prepared by Christine L. Case Structures Internal to the Cell Wall Learning Objectives 4-8
Basic Chemistry. Chemistry Review. Bio 250: Anatomy & Physiology
 Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Metabolism Review. A. Top 10
 A. Top 10 Metabolism Review 1. Energy production through chemiosmosis a. pumping of H+ ions onto one side of a membrane through protein pumps in an Electron Transport Chain (ETC) b. flow of H+ ions across
A. Top 10 Metabolism Review 1. Energy production through chemiosmosis a. pumping of H+ ions onto one side of a membrane through protein pumps in an Electron Transport Chain (ETC) b. flow of H+ ions across
Bacteria. Prepared by. Doua a Hamadi Gellan Ibrahim Rahma Younis Doua a Abdul-Hadi Doua a Amjad Hanin Laith Khamael Dawood
 Bacteria Prepared by Doua a Hamadi Gellan Ibrahim Rahma Younis Doua a Abdul-Hadi Doua a Amjad Hanin Laith Khamael Dawood History of Bacteriology Doua a Hamadi Bacteria were first observed by Antonie van
Bacteria Prepared by Doua a Hamadi Gellan Ibrahim Rahma Younis Doua a Abdul-Hadi Doua a Amjad Hanin Laith Khamael Dawood History of Bacteriology Doua a Hamadi Bacteria were first observed by Antonie van
Metabolism: Energy and Enzymes. February 24 th, 2012
 Metabolism: Energy and Enzymes February 24 th, 2012 1 Outline Forms of Energy Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration
Metabolism: Energy and Enzymes February 24 th, 2012 1 Outline Forms of Energy Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration
Section 19 1 Bacteria (pages )
 Chapter 19 Bacteria and Viruses Section 19 1 Bacteria (pages 471 477) How do the two groups of prokaryotes differ? What factors are used to identify prokaryotes? What is the importance of bacteria? 13.
Chapter 19 Bacteria and Viruses Section 19 1 Bacteria (pages 471 477) How do the two groups of prokaryotes differ? What factors are used to identify prokaryotes? What is the importance of bacteria? 13.
MICROBIAL GROUPS CE 421/521
 MICROBIAL GROUPS CE 421/521 Chapter 10 in Vaccari et.al. www.ibuf.coartuja.csic.es www.environmentaleverage.com www.astrosurf.com www.lbl.gov www.library.thinkquest.org www.ecosys.uni-erlangen.de erlangen.de
MICROBIAL GROUPS CE 421/521 Chapter 10 in Vaccari et.al. www.ibuf.coartuja.csic.es www.environmentaleverage.com www.astrosurf.com www.lbl.gov www.library.thinkquest.org www.ecosys.uni-erlangen.de erlangen.de
Research Science Biology The study of living organisms (Study of life)
 Scientific method Why is there a hypothesis and prediction? If only prediction: then there is no way to finish the prediction and conclude whether the results support the hypothesis If surfaces are sampled
Scientific method Why is there a hypothesis and prediction? If only prediction: then there is no way to finish the prediction and conclude whether the results support the hypothesis If surfaces are sampled
4/17/2014. Prokaryotes have inhabited the Earth for billions of years
 Prokaryotes have inhabited the Earth for billions of years Fossil evidence shows that prokaryotes were abundant 3.5 bya, and they evolved alone for the following 2 billion years. Prokaryotes are ubiquitous,
Prokaryotes have inhabited the Earth for billions of years Fossil evidence shows that prokaryotes were abundant 3.5 bya, and they evolved alone for the following 2 billion years. Prokaryotes are ubiquitous,
1- Which of the following molecules stores hereditary information? A. ATP B. DNA C. protein D. carbohydrates
 Question 1: Multiple Choice (20 Marks) 1- Which of the following molecules stores hereditary information? A. ATP B. DNA C. protein D. carbohydrates 2- What is the name of the molecule in plants that stores
Question 1: Multiple Choice (20 Marks) 1- Which of the following molecules stores hereditary information? A. ATP B. DNA C. protein D. carbohydrates 2- What is the name of the molecule in plants that stores
Principles of Biotechnology Lectures of week 4 MICROBIOLOGY AND BIOTECHNOLOGY
 Principles of Biotechnology Lectures of week 4 MICROBIOLOGY AND BIOTECHNOLOGY INTRODUCTION TO MICROBIOLOGY What are microbes? Germs, microbe s s microorganisms are minute living things that individually
Principles of Biotechnology Lectures of week 4 MICROBIOLOGY AND BIOTECHNOLOGY INTRODUCTION TO MICROBIOLOGY What are microbes? Germs, microbe s s microorganisms are minute living things that individually
