For more information about how to cite these materials visit
|
|
|
- Abner Boyd
- 6 years ago
- Views:
Transcription
1 Author: Robert Lyons, Ph.D., 008 License: Unless otherwise noted, this material is made available under the terms of the reative ommons Attribution Share Alike.0 License: We have reviewed this material in accordance with U.S. opyright Law and have tried to maximize your ability to use, share, and adapt it. The citation key on the following slide provides information about how you may share and adapt this material. opyright holders of content included in this material should contact with any questions, corrections, or clarification regarding the use of content. For more information about how to cite these materials visit Any medical information in this material is intended to inform and educate and is not a tool for self-diagnosis or a replacement for medical evaluation, advice, diagnosis or treatment by a healthcare professional. Please speak to your physician if you have questions about your medical condition. Viewer discretion is advised: Some medical content is graphic and may not be suitable for all viewers.
2 itation Key for more information see: Use + Share + Adapt { ontent the copyright holder, author, or law permits you to use, share and adapt. } Public Domain Government: Works that are produced by the U.S. Government. (17 US 105) Public Domain Expired: Works that are no longer protected due to an expired copyright term. Public Domain Self Dedicated: Works that a copyright holder has dedicated to the public domain. reative ommons Zero Waiver reative ommons Attribution License reative ommons Attribution Share Alike License reative ommons Attribution Noncommercial License reative ommons Attribution Noncommercial Share Alike License GNU Free Documentation License Make Your wn Assessment { ontent pen.michigan believes can be used, shared, and adapted because it is ineligible for copyright. } Public Domain Ineligible: Works that are ineligible for copyright protection in the U.S. (17 US 10(b)) *laws in your jurisdiction may differ { ontent pen.michigan has used under a Fair Use determination. } Fair Use: Use of works that is determined to be Fair consistent with the U.S. opyright Act. (17 US 107) *laws in your jurisdiction may differ ur determination DES NT mean that all uses of this rd-party content are Fair Uses and we D NT guarantee that your use of the content is Fair. To use this content you should do your own independent analysis to determine whether or not your use will be Fair.
3 M1 Renal: Nitrogen Metabolism (and Related Topics) Amino Acid Metabolism (Nitrogen metabolism) Folate Metabolism ( ne-arbon pathways ) Nucleotide Metabolism Dr. Robert Lyons Assistant Professor, Biological hemistry Director, DNA Sequencing ore Web: Fall 008
4 Supplementary study material on the Web:
5 Amino Acid metabolism Amino acids Folate metabolism Glu, Gln, Asp, N Methylene TF Met ycle Urea oxaloacetate P u rin e s DNA RNA P y rim id in e s TA ycle fumarate Uric Acid (energy) Nucleic Acid metabolism
6 Protein Degradation: Endogenous proteins degrade continuously - Damaged - Mis-folded - Un-needed Dietary protein intake - mostly degraded Nitrogen Balance - expresses the patient s current status - are they gaining or losing net Nitrogen?
7 Transaminases ollect Amines General reaction overview: R 1 N amino acid + R R + R 1!-keto acid (typically alpha-ketoglutarate)!-keto acid N amino acid (typically glutamate) Details of reaction mechanism: R P amino acid N + + N pyridoxal phosphate R P N + N R P + N N R P N + N R P!-keto acid + N + N pyridoxamine phosphate
8 Transfer the amine back to an acceptor α-keto acid P N + N pyridoxamine phosphate + R!-keto acid P + N pyridoxal phosphate + R N amino acid
9 In peripheral tissues, transaminases tend to form Glutamate when they catabolize amino acids In other words, alpha-ketoglutarate is the preferred acceptor, and Glutamate is the resulting amino acid: Some amino acid + α-ketoglutarate à some alpha keto acid + Glutamate
10 Glutamate can donate its amines to form other amino acids as needed A specific example - production of Aspartate in liver (described a few slides from now): Glutamate + oxaloacetate à α-ketoglutarate + aspartate
11 Ge tt in g A m in es In to the L iv e r Glutamate Dehydrogenase: NAD(P) N (mito) glutamate NAD(P)!-ketoglutarate + N ammonia Glutamine Synthetase: N glutamate ATP N ADP P i + + N glutamine N
12 . In th e L iv er: Pre cursers for Urea ycle Glutamine is hydrolyzed to glutamate and ammonia: N glutamine N N N glutamate Ammonia can also be formed by the glutamate dehydrogenase reaction and several other reactions as well. Glutamate donates its amino group to form aspartate: Glutamate-aspartate aminotransferase: (- ) N Glutamate + + ( -) ( -) oxaloacetate (- )!-keto glutarate (- ) N aspartate.
13 ATP ADP + P i N arbamoyl phosphate N P N P i N N N rnithine Liver mitochondrion Liver cytoplasm N itrulline N N N N N Urea N 5 rnithine N N N N Arginine ATP AMP + PP i N itrulline N N N N N aspartate Argininosuccinate 4 Fumarate
14 a rb a m o y l p h o s p h a t e s y n th e ta s e I (- ) A T P P N N A T P P N b ic ar b on at e A D P c ar bo ny l ph os p ha te P i ca rb a ma te A D P ca rb a mo yl ph os p ha te
15 rn it h in e T ra n s c a rb a m o y la s e a rb a m o y l p ho s p ha t e N P ( -) P i ( -) (+ ) N (- ) N N N (+ ) N (+ ) rn ith in e it rullin e
16 A rg in in o s u c c in a te s y n t h e t a s e N N itrulline N aspartate N N N N N Argininosuccinate ATP AMP + PP i
17 A r g in in o s u c c in a t e ly a s e N N N N N N Argininosuccinate Fumarate N Arginine N
18 A rg in a s e N N N N Arginine N N Urea N N rnithine
19 ATP ADP + P i N arbamoyl phosphate N P N P i N N N rnithine Liver mitochondrion Liver cytoplasm N itrulline N N N N N Urea N 5 rnithine N N N N Arginine ATP AMP + PP i N itrulline N N N N N aspartate Argininosuccinate 4 Fumarate
20 Urea ycle onnects to TA ycle Urea rnithine Urea ycle itrulline N Aspartate Arginine Argininosuccinate Malate xaloacetate Fumarate TA ycle itrate!-ketoglutarate
21 Ge tt in g A m in es In to the L iv e r Glutamate Dehydrogenase: NAD(P) N (mito) glutamate NAD(P)!-ketoglutarate + N ammonia Glutamine Synthetase: N glutamate ATP N ADP P i + + N glutamine N
22 ATP ADP + P i N arbamoyl phosphate N P N P i N N N rnithine Liver mitochondrion Liver cytoplasm N itrulline N N N N N Urea N 5 rnithine N N N N Arginine ATP AMP + PP i N itrulline N N N N N aspartate Argininosuccinate 4 Fumarate
23 P S I is S t im u la t e d b y N A G ( - ) N ( +) + o A - N -a ce ty l g lu ta m a te s y n th e tas e ( - ) N g lu t a m a t e a c et y l o A N -a c et y l g lu t a m a te (N A G ) ( re p e a t in g t h e fig u re fro m p a g e o f y o u r h an d o u t ) ( -) A T P P N N A T P P N bi ca rb o na te A D P c a rb on y l p ho s ph at e P i c ar ba m at e A D P c ar ba m oy l p ho sp h at e
24 -- Muscle (Amines) Glucose Pyruvate Glutamate Amino acids Alanine!-ketoglutarate blood transport Alanine!-ketoglutarate Urea Glucose Pyruvate Glutamate N Liver
25 omplicating the picture: ther tissues may be involved Muscle: Amino acids: Transamination Deamination purine deamination: Alanine Glutamate N 4 Glutamine Intestine: Glutamine Alanine N 4 itrulline Glutamine Kidney: itrulline N N 4 Arginine Alanine Liver: Glutamine N 4 Arginine Urea Glu Aspartate
26 Why is Ammonia Toxic?
27 Why is Ammonia Toxic? Possible neurotoxic effects on glutamate levels (and also GABA) (due to shifting equilibria of reactions involving these compounds)
28 Why is Ammonia Toxic? Possible neurotoxic effects on glutamate levels (and also GABA) (due to shifting equilibria of reactions involving these compounds) Possible metabolic/energetics effects: - alpha-ketoglutarate levels - glutamate levels - glutamine
29 ATP ADP + P i N arbamoyl phosphate N P N P i N N N rnithine Liver mitochondrion Liver cytoplasm N itrulline N N N N N Urea N 5 rnithine N N N N Arginine ATP AMP + PP i N itrulline N N N N N aspartate Argininosuccinate 4 Fumarate
30 Inherited Defects of Urea ycle Enzymes: Diagnosis Defects are diagnosed based on the metabolites seen in the blood and/or urine. PSD TD ASD ALD AD No elevation except ammonia; diagnosed by elimination. Elevated P causes synthesis of rotate Elevated citrulline Elevated argininosuccinate Elevated arginine
31 ATP ADP + P i N arbamoyl phosphate N P N P i N N N rnithine Liver mitochondrion Liver cytoplasm N itrulline N N N N N Urea N 5 rnithine N N N N Arginine ATP AMP + PP i N itrulline N N N N N aspartate Argininosuccinate 4 Fumarate
32 P S I is S t im u la t e d b y N A G ( - ) N ( +) + o A - N -a ce ty l g lu ta m a te s y n th e tas e ( - ) N g lu t a m a t e a c et y l o A N -a c et y l g lu t a m a te (N A G ) ( re p e a t in g t h e fig u re fro m p a g e o f y o u r h an d o u t ) ( -) A T P P N N A T P P N bi ca rb o na te A D P c a rb on y l p ho s ph at e P i c ar ba m at e A D P c ar ba m oy l p ho sp h at e
33 ATP ADP + P i N arbamoyl phosphate N P N P i N N N rnithine Liver mitochondrion Liver cytoplasm N itrulline N N N N N Urea N 5 rnithine N N N N Arginine ATP AMP + PP i N itrulline N N N N N aspartate Argininosuccinate 4 Fumarate
34 linical Management of Urea ycle Defects Dialysis to remove ammonia Provide the patient with alternative ways to excrete nitrogenous compounds: * Intravenous sodium benzoate or phenylacetate * Supplemental arginine carbamoyl phosphate Excreted by kidney N Urea N rnithine Arginine ATP AMP+PP i itrulline Aspartate X ASD Argininosuccinate Dietary Arginine Levulose - acidifies the gut Low protein diet X ALD Excreted by kidney
35 Degrading the Amino Acid arbon Backbone
36 Easily-degraded products after transamination: N aspartate transamination oxaloacetate N glutamate N alanine transamination transamination!-ketoglutarate pyruvate We also already know how to degrade Glutamine: glutaminase Glutamine > glutamate + ammonia and by analogy, how to degrade Asparagine: asparaginase Asparagine > aspartate + ammonia
37 Amino Acids are categorized as Glucogenic or ketogenic or both. Many amino acids are purely glucogenic: Glutamate, aspartate, alanine, glutamine, asparagine, Some amino acids are both gluco- and ketogenic: Threonine, isoleucine, phenylalanine, tyrosine, tryptophan The only PURELY ketogenic Amino Acids: leucine, lysine
38 N N N Arginine N Amino acids with 5-carbon backbones tend to form α ketoglutarate Urea (via the urea cycle) N N N rnithine!-ketoglutarate glutamate glutamate semialdehyde NAD(P) N Proline NAD(P) N glutamate N N glutamine N! - ketoglutarate
39 Degradation and Biosynthesis of Serine and Glycine NAD NAD Glycine Synthase: N + N 4 Glycine TF 5 10 N -N - methylene TF Serine ydroxymethyltransferase: N N Serine TF 5 10 N -N - methylene TF Glycine N 4 Serine Dehydratase: N Serine N N
40 S N Methionine ATP + PPi + Pi S Adenine N S-Adenosyl Methionine Methionine ycle And Biological Methyl Groups tetrahydrofolate N5 methyl tetrahydrofolate S N omocysteine Bios ynthetic Methylation reaction S Adenine Methyl acceptor * see examples Methylated acceptor N S-Adenosyl omocysteine N Serine S N ysteine (remainder of homocysteine degraded for energy)
41 Phenylalanine and Tyrosine (Normal path shown in black, pathological reaction shown in red) N Phenylalanine Phenylketonuria (no phenylalanine hydroxylase) Tetrahydrobiopterin + Dihydrobiopterin + Enzyme: Phenylalanine hydroxylase N Tyrosine omogentisate Deficiency: Alkaptonuria chronosis Enzyme: homogentisate dioxygenase Phenylpyruvate (you don t need to know the rest)
42 Branched hain Amino Acids Isoleucine Leucine Valine N N N (+ )!"KG!"KG!"KG Transamination Glu Glu Glu + NAD, oas + + NAD, oas NAD, oas --- Branched-chain!-keto acid dehydrogenase --- NAD + S-oA NAD + NAD + S-oA S-oA (continues on to degradation path similar to #-oxidation of fatty acids)
43 Synthesis of Bioactive Amines N Tyrosine Tyrosine hydroxylase N Dihydroxyphenylalanine (L-DPA) N Dopamine N Norepinephrine N Epinephrine
44 Synthesis of Bioactive Amines N Tryptophan N Tryptophan hydroxylase N 5-hydroxytryptophan N PLP-dependent decvarboxylation NAD+ N N Serotonin
45 Synthesis of Bioactive Amines Glutamate N Glutamate decarboxylase (PLP-dependent ) N!-aminobutyric acid (GABA) N N istidine N (- ) istidine decarboxylase (PLP-dependent ) N N N istamine
46 NN-Essential Amino Acids: Glutamate, aspartate, alanine, glutamine, asparagine, (proline), glycine, serine (cysteine, tyrosine) Essential Amino Acids: Arginine (!), phenylalanine, methionine, histidine, Isoleucine, leucine, valine, threonine, tryptophan, lysine
47 Additional Source Information for more information see: Slide 4: Robert Lyons Slide 5: Robert Lyons Slide 7: Robert Lyons Slide 8: Robert Lyons Slide 11: Robert Lyons Slide 1: Robert Lyons Slide 1: Robert Lyons Slide 14: Robert Lyons Slide 15: Robert Lyons Slide 16: Robert Lyons Slide 17: Robert Lyons Slide 18: Robert Lyons Slide 19: Robert Lyons Slide 0: Robert Lyons Slide 1: Robert Lyons Slide : Robert Lyons Slide : Robert Lyons Slide 4: Robert Lyons Slide 5: Robert Lyons Slide 9: Robert Lyons Slide 1: Robert Lyons Slide : Robert Lyons Slide : Robert Lyons Slide 4: Robert Lyons Slide 6: Robert Lyons Slide 8: Robert Lyons Slide 9: Robert Lyons Slide 40: Robert Lyons Slide 41: Robert Lyons Slide 44: Robert Lyons Slide 45: Robert Lyons
Proteins: Characteristics and Properties of Amino Acids
 SBI4U:Biochemistry Macromolecules Eachaminoacidhasatleastoneamineandoneacidfunctionalgroupasthe nameimplies.thedifferentpropertiesresultfromvariationsinthestructuresof differentrgroups.thergroupisoftenreferredtoastheaminoacidsidechain.
SBI4U:Biochemistry Macromolecules Eachaminoacidhasatleastoneamineandoneacidfunctionalgroupasthe nameimplies.thedifferentpropertiesresultfromvariationsinthestructuresof differentrgroups.thergroupisoftenreferredtoastheaminoacidsidechain.
Systematic approaches to study cancer cell metabolism
 Systematic approaches to study cancer cell metabolism Kivanc Birsoy Laboratory of Metabolic Regulation and Genetics The Rockefeller University, NY Cellular metabolism is complex ~3,000 metabolic genes
Systematic approaches to study cancer cell metabolism Kivanc Birsoy Laboratory of Metabolic Regulation and Genetics The Rockefeller University, NY Cellular metabolism is complex ~3,000 metabolic genes
BIS Office Hours
 BIS103-001 001 ffice ours TUE (2-3 pm) Rebecca Shipman WED (9:30-10:30 am) TUE (12-1 pm) Stephen Abreu TUR (12-1 pm) FRI (9-11 am) Steffen Abel Lecture 2 Topics Finish discussion of thermodynamics (ΔG,
BIS103-001 001 ffice ours TUE (2-3 pm) Rebecca Shipman WED (9:30-10:30 am) TUE (12-1 pm) Stephen Abreu TUR (12-1 pm) FRI (9-11 am) Steffen Abel Lecture 2 Topics Finish discussion of thermodynamics (ΔG,
Hypergraphs, Metabolic Networks, Bioreaction Systems. G. Bastin
 Hypergraphs, Metabolic Networks, Bioreaction Systems. G. Bastin PART 1 : Metabolic flux analysis and minimal bioreaction modelling PART 2 : Dynamic metabolic flux analysis of underdetermined networks 2
Hypergraphs, Metabolic Networks, Bioreaction Systems. G. Bastin PART 1 : Metabolic flux analysis and minimal bioreaction modelling PART 2 : Dynamic metabolic flux analysis of underdetermined networks 2
Chemistry Chapter 22
 hemistry 2100 hapter 22 Proteins Proteins serve many functions, including the following. 1. Structure: ollagen and keratin are the chief constituents of skin, bone, hair, and nails. 2. atalysts: Virtually
hemistry 2100 hapter 22 Proteins Proteins serve many functions, including the following. 1. Structure: ollagen and keratin are the chief constituents of skin, bone, hair, and nails. 2. atalysts: Virtually
For more information about how to cite these materials visit
 Author(s): MELO 3D Project Team, 2012 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share-Alike 3.0 License: http://creativecommons.org/licenses/by-sa/2.0/
Author(s): MELO 3D Project Team, 2012 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share-Alike 3.0 License: http://creativecommons.org/licenses/by-sa/2.0/
Enzyme Catalysis & Biotechnology
 L28-1 Enzyme Catalysis & Biotechnology Bovine Pancreatic RNase A Biochemistry, Life, and all that L28-2 A brief word about biochemistry traditionally, chemical engineers used organic and inorganic chemistry
L28-1 Enzyme Catalysis & Biotechnology Bovine Pancreatic RNase A Biochemistry, Life, and all that L28-2 A brief word about biochemistry traditionally, chemical engineers used organic and inorganic chemistry
Discussion Section (Day, Time): TF:
 ame: Chemistry 27 Professor Gavin MacBeath arvard University Spring 2004 Final Exam Thursday, May 28, 2004 2:15 PM - 5:15 PM Discussion Section (Day, Time): Directions: TF: 1. Do not write in red ink.
ame: Chemistry 27 Professor Gavin MacBeath arvard University Spring 2004 Final Exam Thursday, May 28, 2004 2:15 PM - 5:15 PM Discussion Section (Day, Time): Directions: TF: 1. Do not write in red ink.
Amino Acids and Peptides
 Amino Acids Amino Acids and Peptides Amino acid a compound that contains both an amino group and a carboxyl group α-amino acid an amino acid in which the amino group is on the carbon adjacent to the carboxyl
Amino Acids Amino Acids and Peptides Amino acid a compound that contains both an amino group and a carboxyl group α-amino acid an amino acid in which the amino group is on the carbon adjacent to the carboxyl
Range of Certified Values in Reference Materials. Range of Expanded Uncertainties as Disseminated. NMI Service
 Calibration and Capabilities Amount of substance,, Russian Federation (Ural Scientific and Research Institiute Metrology, Rosstandart) (D.I. Mendeleyev Institute Metrology, Rosstandart) The uncertainty
Calibration and Capabilities Amount of substance,, Russian Federation (Ural Scientific and Research Institiute Metrology, Rosstandart) (D.I. Mendeleyev Institute Metrology, Rosstandart) The uncertainty
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but not the only one!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids repeated
Properties of amino acids in proteins one of the primary roles of DNA (but not the only one!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids repeated
Lecture 15: Realities of Genome Assembly Protein Sequencing
 Lecture 15: Realities of Genome Assembly Protein Sequencing Study Chapter 8.10-8.15 1 Euler s Theorems A graph is balanced if for every vertex the number of incoming edges equals to the number of outgoing
Lecture 15: Realities of Genome Assembly Protein Sequencing Study Chapter 8.10-8.15 1 Euler s Theorems A graph is balanced if for every vertex the number of incoming edges equals to the number of outgoing
Discussion Section (Day, Time):
 Chemistry 27 Spring 2005 Exam 3 Chemistry 27 Professor Gavin MacBeath arvard University Spring 2005 our Exam 3 Friday April 29 th, 2005 11:07 AM 12:00 PM Discussion Section (Day, Time): TF: Directions:
Chemistry 27 Spring 2005 Exam 3 Chemistry 27 Professor Gavin MacBeath arvard University Spring 2005 our Exam 3 Friday April 29 th, 2005 11:07 AM 12:00 PM Discussion Section (Day, Time): TF: Directions:
Chem Lecture 10 Lipid, Amino Acid, and Nucleotide Metabolism Part III: Nucleotide Metabolism
 Chem 352 - Lecture 10 Lipid, Amino Acid, and Nucleotide Metabolism Part III: Nucleotide Metabolism Lipid Metabolism Chem 352, Lecture 10, Part I: Lipid Metabolism 2 Lipid Metabolism Question: Draw a general
Chem 352 - Lecture 10 Lipid, Amino Acid, and Nucleotide Metabolism Part III: Nucleotide Metabolism Lipid Metabolism Chem 352, Lecture 10, Part I: Lipid Metabolism 2 Lipid Metabolism Question: Draw a general
Lecture 14 - Cells. Astronomy Winter Lecture 14 Cells: The Building Blocks of Life
 Lecture 14 Cells: The Building Blocks of Life Astronomy 141 Winter 2012 This lecture describes Cells, the basic structural units of all life on Earth. Basic components of cells: carbohydrates, lipids,
Lecture 14 Cells: The Building Blocks of Life Astronomy 141 Winter 2012 This lecture describes Cells, the basic structural units of all life on Earth. Basic components of cells: carbohydrates, lipids,
Basic Principles of Protein Structures
 Basic Principles of Protein Structures Proteins Proteins: The Molecule of Life Proteins: Building Blocks Proteins: Secondary Structures Proteins: Tertiary and Quartenary Structure Proteins: Geometry Proteins
Basic Principles of Protein Structures Proteins Proteins: The Molecule of Life Proteins: Building Blocks Proteins: Secondary Structures Proteins: Tertiary and Quartenary Structure Proteins: Geometry Proteins
Chemical Properties of Amino Acids
 hemical Properties of Amino Acids Protein Function Make up about 15% of the cell and have many functions in the cell 1. atalysis: enzymes 2. Structure: muscle proteins 3. Movement: myosin, actin 4. Defense:
hemical Properties of Amino Acids Protein Function Make up about 15% of the cell and have many functions in the cell 1. atalysis: enzymes 2. Structure: muscle proteins 3. Movement: myosin, actin 4. Defense:
Using Higher Calculus to Study Biologically Important Molecules Julie C. Mitchell
 Using Higher Calculus to Study Biologically Important Molecules Julie C. Mitchell Mathematics and Biochemistry University of Wisconsin - Madison 0 There Are Many Kinds Of Proteins The word protein comes
Using Higher Calculus to Study Biologically Important Molecules Julie C. Mitchell Mathematics and Biochemistry University of Wisconsin - Madison 0 There Are Many Kinds Of Proteins The word protein comes
Discussion Section (Day, Time):
 Chemistry 27 pring 2005 Exam 3 Chemistry 27 Professor Gavin MacBeath arvard University pring 2005 our Exam 3 Friday April 29 th, 2005 11:07 AM 12:00 PM Discussion ection (Day, Time): TF: Directions: 1.
Chemistry 27 pring 2005 Exam 3 Chemistry 27 Professor Gavin MacBeath arvard University pring 2005 our Exam 3 Friday April 29 th, 2005 11:07 AM 12:00 PM Discussion ection (Day, Time): TF: Directions: 1.
Exam III. Please read through each question carefully, and make sure you provide all of the requested information.
 09-107 onors Chemistry ame Exam III Please read through each question carefully, and make sure you provide all of the requested information. 1. A series of octahedral metal compounds are made from 1 mol
09-107 onors Chemistry ame Exam III Please read through each question carefully, and make sure you provide all of the requested information. 1. A series of octahedral metal compounds are made from 1 mol
Periodic Table. 8/3/2006 MEDC 501 Fall
 Periodic Table 8/3/2006 MEDC 501 Fall 2006 1 rbitals Shapes of rbitals s - orbital p -orbital 8/3/2006 MEDC 501 Fall 2006 2 Ionic Bond - acl Electronic Structure 11 a :: 1s 2 2s 2 2p x2 2p y2 2p z2 3s
Periodic Table 8/3/2006 MEDC 501 Fall 2006 1 rbitals Shapes of rbitals s - orbital p -orbital 8/3/2006 MEDC 501 Fall 2006 2 Ionic Bond - acl Electronic Structure 11 a :: 1s 2 2s 2 2p x2 2p y2 2p z2 3s
PROTEIN STRUCTURE AMINO ACIDS H R. Zwitterion (dipolar ion) CO 2 H. PEPTIDES Formal reactions showing formation of peptide bond by dehydration:
 PTEI STUTUE ydrolysis of proteins with aqueous acid or base yields a mixture of free amino acids. Each type of protein yields a characteristic mixture of the ~ 20 amino acids. AMI AIDS Zwitterion (dipolar
PTEI STUTUE ydrolysis of proteins with aqueous acid or base yields a mixture of free amino acids. Each type of protein yields a characteristic mixture of the ~ 20 amino acids. AMI AIDS Zwitterion (dipolar
Lecture'18:'April'2,'2013
 CM'224' 'rganic'chemistry'ii Spring'2013,'Des'Plaines' 'Prof.'Chad'Landrie 2 3 N cysteine (Cys) S oxidation S S 3 N cystine N 3 Lecture'18:'April'2,'2013 Disaccharides+&+Polysaccharides Amino+acids++(26.1926.3)
CM'224' 'rganic'chemistry'ii Spring'2013,'Des'Plaines' 'Prof.'Chad'Landrie 2 3 N cysteine (Cys) S oxidation S S 3 N cystine N 3 Lecture'18:'April'2,'2013 Disaccharides+&+Polysaccharides Amino+acids++(26.1926.3)
For more information about how to cite these materials visit
 Author(s): Kerby Shedden, Ph.D., 2010 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
Author(s): Kerby Shedden, Ph.D., 2010 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
Student Handout 2. Human Sepiapterin Reductase mrna Gene Map A 3DMD BioInformatics Activity. Genome Sequencing. Sepiapterin Reductase
 Project-Based Learning ctivity Human Sepiapterin Reductase mrn ene Map 3DMD BioInformatics ctivity 498 ---+---------+--------- ---------+---------+---------+---------+---------+---------+---------+---------+---------+---------
Project-Based Learning ctivity Human Sepiapterin Reductase mrn ene Map 3DMD BioInformatics ctivity 498 ---+---------+--------- ---------+---------+---------+---------+---------+---------+---------+---------+---------+---------
Practice Midterm Exam 200 points total 75 minutes Multiple Choice (3 pts each 30 pts total) Mark your answers in the space to the left:
 MITES ame Practice Midterm Exam 200 points total 75 minutes Multiple hoice (3 pts each 30 pts total) Mark your answers in the space to the left: 1. Amphipathic molecules have regions that are: a) polar
MITES ame Practice Midterm Exam 200 points total 75 minutes Multiple hoice (3 pts each 30 pts total) Mark your answers in the space to the left: 1. Amphipathic molecules have regions that are: a) polar
MITOCW watch?v=0xajihttcns
 MITOCW watch?v=0xajihttcns The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=0xajihttcns The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
For more information about how to cite these materials visit
 Author(s): Kerby Shedden, Ph.D., 2010 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
Author(s): Kerby Shedden, Ph.D., 2010 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
CHEM Organic Chemistry with Biological Applications EXAM 1A (250 points)
 UCSC, Binder Name Student ID # Section Day/Time CEM 109 rganic Chemistry with Biological Applications EXAM 1A (250 points) D NT BEGIN TE EXAM R TURN TE PAGE UNTIL INSTRUCTED T D S. In the meantime, please
UCSC, Binder Name Student ID # Section Day/Time CEM 109 rganic Chemistry with Biological Applications EXAM 1A (250 points) D NT BEGIN TE EXAM R TURN TE PAGE UNTIL INSTRUCTED T D S. In the meantime, please
Protein Secondary Structure Prediction
 part of Bioinformatik von RNA- und Proteinstrukturen Computational EvoDevo University Leipzig Leipzig, SS 2011 the goal is the prediction of the secondary structure conformation which is local each amino
part of Bioinformatik von RNA- und Proteinstrukturen Computational EvoDevo University Leipzig Leipzig, SS 2011 the goal is the prediction of the secondary structure conformation which is local each amino
The Select Command and Boolean Operators
 The Select Command and Boolean Operators Part of the Jmol Training Guide from the MSOE Center for BioMolecular Modeling Interactive version available at http://cbm.msoe.edu/teachingresources/jmol/jmoltraining/boolean.html
The Select Command and Boolean Operators Part of the Jmol Training Guide from the MSOE Center for BioMolecular Modeling Interactive version available at http://cbm.msoe.edu/teachingresources/jmol/jmoltraining/boolean.html
Part 4 The Select Command and Boolean Operators
 Part 4 The Select Command and Boolean Operators http://cbm.msoe.edu/newwebsite/learntomodel Introduction By default, every command you enter into the Console affects the entire molecular structure. However,
Part 4 The Select Command and Boolean Operators http://cbm.msoe.edu/newwebsite/learntomodel Introduction By default, every command you enter into the Console affects the entire molecular structure. However,
INTRODUCTION. Amino acids occurring in nature have the general structure shown below:
 Biochemistry I Laboratory Amino Acid Thin Layer Chromatography INTRODUCTION The primary importance of amino acids in cell structure and metabolism lies in the fact that they serve as building blocks for
Biochemistry I Laboratory Amino Acid Thin Layer Chromatography INTRODUCTION The primary importance of amino acids in cell structure and metabolism lies in the fact that they serve as building blocks for
For more information about how to cite these materials visit
 Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Peter Hitchcock, PH.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Non-commercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
7.05 Spring 2004 February 27, Recitation #2
 Recitation #2 Contact Information TA: Victor Sai Recitation: Friday, 3-4pm, 2-132 E-mail: sai@mit.edu ffice ours: Friday, 4-5pm, 2-132 Unit 1 Schedule Recitation/Exam Date Lectures covered Recitation #2
Recitation #2 Contact Information TA: Victor Sai Recitation: Friday, 3-4pm, 2-132 E-mail: sai@mit.edu ffice ours: Friday, 4-5pm, 2-132 Unit 1 Schedule Recitation/Exam Date Lectures covered Recitation #2
For more information about how to cite these materials visit
 Author(s): Kerby Shedden, Ph.D., 2010 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
Author(s): Kerby Shedden, Ph.D., 2010 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Share Alike 3.0 License: http://creativecommons.org/licenses/by-sa/3.0/
Principles of Biochemistry
 Principles of Biochemistry Fourth Edition Donald Voet Judith G. Voet Charlotte W. Pratt Chapter 4 Amino Acids: The Building Blocks of proteins (Page 76-90) Chapter Contents 1- Amino acids Structure: 2-
Principles of Biochemistry Fourth Edition Donald Voet Judith G. Voet Charlotte W. Pratt Chapter 4 Amino Acids: The Building Blocks of proteins (Page 76-90) Chapter Contents 1- Amino acids Structure: 2-
National Nutrient Database for Standard Reference Release 28 slightly revised May, 2016
 National base for Standard Reference Release 28 slightly revised May, 206 Full Report (All s) 005, Cheese, cottage, lowfat, 2% milkfat Report Date: February 23, 208 02:7 EST values and weights are for
National base for Standard Reference Release 28 slightly revised May, 206 Full Report (All s) 005, Cheese, cottage, lowfat, 2% milkfat Report Date: February 23, 208 02:7 EST values and weights are for
METABOLISM CHAPTER 04 BIO 211: ANATOMY & PHYSIOLOGY I. Dr. Lawrence G. Altman Some illustrations are courtesy of McGraw-Hill.
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. CELLULAR METABOLISM Dr. Lawrence G. Altman
CELL METABOLISM OVERVIEW Keep the big picture in mind as we discuss the particulars!
 BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
BIO 211: ANATOMY & PHYSIOLOGY I CHAPTER 04 CELLULAR METABOLISM 1 Please wait 20 seconds before starting slide show. Mouse click or Arrow keys to navigate. Hit ESCAPE Key to exit. Dr. Lawrence G. Altman
Translation. A ribosome, mrna, and trna.
 Translation The basic processes of translation are conserved among prokaryotes and eukaryotes. Prokaryotic Translation A ribosome, mrna, and trna. In the initiation of translation in prokaryotes, the Shine-Dalgarno
Translation The basic processes of translation are conserved among prokaryotes and eukaryotes. Prokaryotic Translation A ribosome, mrna, and trna. In the initiation of translation in prokaryotes, the Shine-Dalgarno
Studies Leading to the Development of a Highly Selective. Colorimetric and Fluorescent Chemosensor for Lysine
 Supporting Information for Studies Leading to the Development of a Highly Selective Colorimetric and Fluorescent Chemosensor for Lysine Ying Zhou, a Jiyeon Won, c Jin Yong Lee, c * and Juyoung Yoon a,
Supporting Information for Studies Leading to the Development of a Highly Selective Colorimetric and Fluorescent Chemosensor for Lysine Ying Zhou, a Jiyeon Won, c Jin Yong Lee, c * and Juyoung Yoon a,
Solutions In each case, the chirality center has the R configuration
 CAPTER 25 669 Solutions 25.1. In each case, the chirality center has the R configuration. C C 2 2 C 3 C(C 3 ) 2 D-Alanine D-Valine 25.2. 2 2 S 2 d) 2 25.3. Pro,, Trp, Tyr, and is, Trp, Tyr, and is Arg,
CAPTER 25 669 Solutions 25.1. In each case, the chirality center has the R configuration. C C 2 2 C 3 C(C 3 ) 2 D-Alanine D-Valine 25.2. 2 2 S 2 d) 2 25.3. Pro,, Trp, Tyr, and is, Trp, Tyr, and is Arg,
5) An amino acid that doesn't exist in proteins: - Tyrosine - Tryptophan - Cysteine - ornithine* 6) How many tripeptides can be formed from using one
 Biochemistry First 1) An amino acid that does not have L & D: - proline - serine - Glycine* - valine 2) An amino acid that exists in beta-bends and turns: - Gly* - Pro - Arg - Asp 3) An amino acid that
Biochemistry First 1) An amino acid that does not have L & D: - proline - serine - Glycine* - valine 2) An amino acid that exists in beta-bends and turns: - Gly* - Pro - Arg - Asp 3) An amino acid that
Bioinformatics: Network Analysis
 Bioinformatics: Network Analysis Flux Balance Analysis and Metabolic Control Analysis COMP 572 (BIOS 572 / BIOE 564) - Fall 2013 Luay Nakhleh, Rice University 1 Flux Balance Analysis (FBA) Flux balance
Bioinformatics: Network Analysis Flux Balance Analysis and Metabolic Control Analysis COMP 572 (BIOS 572 / BIOE 564) - Fall 2013 Luay Nakhleh, Rice University 1 Flux Balance Analysis (FBA) Flux balance
Prediction of protein function from sequence analysis
 Prediction of protein function from sequence analysis Rita Casadio BIOCOMPUTING GROUP University of Bologna, Italy The omic era Genome Sequencing Projects: Archaea: 74 species In Progress:52 Bacteria:
Prediction of protein function from sequence analysis Rita Casadio BIOCOMPUTING GROUP University of Bologna, Italy The omic era Genome Sequencing Projects: Archaea: 74 species In Progress:52 Bacteria:
12/6/12. Dr. Sanjeeva Srivastava IIT Bombay. Primary Structure. Secondary Structure. Tertiary Structure. Quaternary Structure.
 Dr. anjeeva rivastava Primary tructure econdary tructure Tertiary tructure Quaternary tructure Amino acid residues α Helix Polypeptide chain Assembled subunits 2 1 Amino acid sequence determines 3-D structure
Dr. anjeeva rivastava Primary tructure econdary tructure Tertiary tructure Quaternary tructure Amino acid residues α Helix Polypeptide chain Assembled subunits 2 1 Amino acid sequence determines 3-D structure
UNIT TWELVE. a, I _,o "' I I I. I I.P. l'o. H-c-c. I ~o I ~ I / H HI oh H...- I II I II 'oh. HO\HO~ I "-oh
 UNT TWELVE PROTENS : PEPTDE BONDNG AND POLYPEPTDES 12 CONCEPTS Many proteins are important in biological structure-for example, the keratin of hair, collagen of skin and leather, and fibroin of silk. Other
UNT TWELVE PROTENS : PEPTDE BONDNG AND POLYPEPTDES 12 CONCEPTS Many proteins are important in biological structure-for example, the keratin of hair, collagen of skin and leather, and fibroin of silk. Other
BCH 4053 Exam I Review Spring 2017
 BCH 4053 SI - Spring 2017 Reed BCH 4053 Exam I Review Spring 2017 Chapter 1 1. Calculate G for the reaction A + A P + Q. Assume the following equilibrium concentrations: [A] = 20mM, [Q] = [P] = 40fM. Assume
BCH 4053 SI - Spring 2017 Reed BCH 4053 Exam I Review Spring 2017 Chapter 1 1. Calculate G for the reaction A + A P + Q. Assume the following equilibrium concentrations: [A] = 20mM, [Q] = [P] = 40fM. Assume
Read more about Pauling and more scientists at: Profiles in Science, The National Library of Medicine, profiles.nlm.nih.gov
 2018 Biochemistry 110 California Institute of Technology Lecture 2: Principles of Protein Structure Linus Pauling (1901-1994) began his studies at Caltech in 1922 and was directed by Arthur Amos oyes to
2018 Biochemistry 110 California Institute of Technology Lecture 2: Principles of Protein Structure Linus Pauling (1901-1994) began his studies at Caltech in 1922 and was directed by Arthur Amos oyes to
Structures in equilibrium at point A: Structures in equilibrium at point B: (ii) Structure at the isoelectric point:
 ame 21 F10-Final Exam Page 2 I. (42 points) (1) (16 points) The titration curve for L-lysine is shown below. Provide (i) the main structures in equilibrium at each of points A and B indicated below and
ame 21 F10-Final Exam Page 2 I. (42 points) (1) (16 points) The titration curve for L-lysine is shown below. Provide (i) the main structures in equilibrium at each of points A and B indicated below and
Dental Biochemistry Exam The total number of unique tripeptides that can be produced using all of the common 20 amino acids is
 Exam Questions for Dental Biochemistry Monday August 27, 2007 E.J. Miller 1. The compound shown below is CH 3 -CH 2 OH A. acetoacetate B. acetic acid C. acetaldehyde D. produced by reduction of acetaldehyde
Exam Questions for Dental Biochemistry Monday August 27, 2007 E.J. Miller 1. The compound shown below is CH 3 -CH 2 OH A. acetoacetate B. acetic acid C. acetaldehyde D. produced by reduction of acetaldehyde
Supporting Information
 Supporting Information Proteomic Analyses of Cysteine Redox in High-fat-fed and Fasted Mouse Livers: Implications for Liver Metabolic Homeostasis Yixing Li 1#, Zupeng Luo 1#, Xilong Wu 2, Jun Zhu 2, Kai
Supporting Information Proteomic Analyses of Cysteine Redox in High-fat-fed and Fasted Mouse Livers: Implications for Liver Metabolic Homeostasis Yixing Li 1#, Zupeng Luo 1#, Xilong Wu 2, Jun Zhu 2, Kai
ChemWiki BioWiki GeoWiki StatWiki PhysWiki MathWiki SolarWiki
 Ashley Robison My Preferences Site Tools Popular pages MindTouch User Guide FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it
Ashley Robison My Preferences Site Tools Popular pages MindTouch User Guide FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it
CHEMISTRY ATAR COURSE DATA BOOKLET
 CHEMISTRY ATAR COURSE DATA BOOKLET 2018 2018/2457 Chemistry ATAR Course Data Booklet 2018 Table of contents Periodic table of the elements...3 Formulae...4 Units...4 Constants...4 Solubility rules for
CHEMISTRY ATAR COURSE DATA BOOKLET 2018 2018/2457 Chemistry ATAR Course Data Booklet 2018 Table of contents Periodic table of the elements...3 Formulae...4 Units...4 Constants...4 Solubility rules for
Method of Enzyme Assay
 Method of Enzyme Assay Objective To study the different methods for determining enzyme activity. Enzyme assays: Are laboratory methods for measuring enzymatic activity. All enzyme assays measure either
Method of Enzyme Assay Objective To study the different methods for determining enzyme activity. Enzyme assays: Are laboratory methods for measuring enzymatic activity. All enzyme assays measure either
CHAPTER 29 HW: AMINO ACIDS + PROTEINS
 CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
Viewing and Analyzing Proteins, Ligands and their Complexes 2
 2 Viewing and Analyzing Proteins, Ligands and their Complexes 2 Overview Viewing the accessible surface Analyzing the properties of proteins containing thousands of atoms is best accomplished by representing
2 Viewing and Analyzing Proteins, Ligands and their Complexes 2 Overview Viewing the accessible surface Analyzing the properties of proteins containing thousands of atoms is best accomplished by representing
Biochemistry by Mary K. Campbell & Shawn O. Farrell 8th. Ed. 2016
 3 Biochemistry by Mary K. Campbell & Shawn. Farrell 8th. Ed. 2016 3-1 3 Amino Acids & Peptides 3-2 3 Learning bjectives 1. What are amino acids, and what is their threedimensional structure? 2. What are
3 Biochemistry by Mary K. Campbell & Shawn. Farrell 8th. Ed. 2016 3-1 3 Amino Acids & Peptides 3-2 3 Learning bjectives 1. What are amino acids, and what is their threedimensional structure? 2. What are
A Plausible Model Correlates Prebiotic Peptide Synthesis with. Primordial Genetic Code
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 A Plausible Model Correlates Prebiotic Peptide Synthesis with Primordial Genetic Code Jianxi Ying,
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 A Plausible Model Correlates Prebiotic Peptide Synthesis with Primordial Genetic Code Jianxi Ying,
Sequence comparison: Score matrices. Genome 559: Introduction to Statistical and Computational Genomics Prof. James H. Thomas
 Sequence comparison: Score matrices Genome 559: Introduction to Statistical and omputational Genomics Prof James H Thomas Informal inductive proof of best alignment path onsider the last step in the best
Sequence comparison: Score matrices Genome 559: Introduction to Statistical and omputational Genomics Prof James H Thomas Informal inductive proof of best alignment path onsider the last step in the best
Discussion Section (Day, Time):
 Chemistry 27 Spring 2005 Exam 1 Chemistry 27 Professor Gavin MacBeath arvard University Spring 2005 our Exam 1 Friday, February 25, 2005 11:07 AM 12:00 PM Discussion Section (Day, Time): TF: Directions:
Chemistry 27 Spring 2005 Exam 1 Chemistry 27 Professor Gavin MacBeath arvard University Spring 2005 our Exam 1 Friday, February 25, 2005 11:07 AM 12:00 PM Discussion Section (Day, Time): TF: Directions:
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Protein structure. Protein structure. Amino acid residue. Cell communication channel. Bioinformatics Methods
 Cell communication channel Bioinformatics Methods Iosif Vaisman Email: ivaisman@gmu.edu SEQUENCE STRUCTURE DNA Sequence Protein Sequence Protein Structure Protein structure ATGAAATTTGGAAACTTCCTTCTCACTTATCAGCCACCT...
Cell communication channel Bioinformatics Methods Iosif Vaisman Email: ivaisman@gmu.edu SEQUENCE STRUCTURE DNA Sequence Protein Sequence Protein Structure Protein structure ATGAAATTTGGAAACTTCCTTCTCACTTATCAGCCACCT...
Course syllabus for Chemistry 109C Organic Chemistry
 Course syllabus for Chemistry 109C Organic Chemistry Class meets: Tue, Thu 5:00 6:15 PM Chem 1179 Spring 2005 Instructor: Prof. Kalju Kahn, Office: PSB-N 1511, E-mail: kalju@chem.ucsb.edu Phone: 893-6157
Course syllabus for Chemistry 109C Organic Chemistry Class meets: Tue, Thu 5:00 6:15 PM Chem 1179 Spring 2005 Instructor: Prof. Kalju Kahn, Office: PSB-N 1511, E-mail: kalju@chem.ucsb.edu Phone: 893-6157
Systems II. Metabolic Cycles
 Systems II Metabolic Cycles Overview Metabolism is central to cellular functioning Maintenance of mass and energy requirements for the cell Breakdown of toxic compounds Consists of a number of major pathways
Systems II Metabolic Cycles Overview Metabolism is central to cellular functioning Maintenance of mass and energy requirements for the cell Breakdown of toxic compounds Consists of a number of major pathways
Solutions. Solutions. Water Basics 10/24/ Water Properties
 0/24/206 O Water Basics Polar: part of a molecule is slightly positive, while another part is slightly negative Oxygen hogs electrons from hydrogen; results in negative charge on oxygen and positive charge
0/24/206 O Water Basics Polar: part of a molecule is slightly positive, while another part is slightly negative Oxygen hogs electrons from hydrogen; results in negative charge on oxygen and positive charge
Chemistry review for Bio 260 answer key
 Chemistry review for Bio 260 answer key Atomic structure Q1. What are the atomic numbers for Nitrogen and Oxygen? A. Nitrogen is atomic number 7. Oxygen is atomic number 8. Q2. If an atom has 11 protons,
Chemistry review for Bio 260 answer key Atomic structure Q1. What are the atomic numbers for Nitrogen and Oxygen? A. Nitrogen is atomic number 7. Oxygen is atomic number 8. Q2. If an atom has 11 protons,
Sequence comparison: Score matrices
 Sequence comparison: Score matrices http://facultywashingtonedu/jht/gs559_2013/ Genome 559: Introduction to Statistical and omputational Genomics Prof James H Thomas FYI - informal inductive proof of best
Sequence comparison: Score matrices http://facultywashingtonedu/jht/gs559_2013/ Genome 559: Introduction to Statistical and omputational Genomics Prof James H Thomas FYI - informal inductive proof of best
Patrick: An Introduction to Medicinal Chemistry 5e Chapter 03
 01) Which of the following statements is not true regarding the active site of an enzyme? a. An active site is normally on the surface of an enzyme. b. An active site is normally hydrophobic in nature.
01) Which of the following statements is not true regarding the active site of an enzyme? a. An active site is normally on the surface of an enzyme. b. An active site is normally hydrophobic in nature.
Chapter 3 - Amino Acids
 hapter 3 Amino Acids αamino Acids are the main constituents of proteins. But they also can function as neurotransmitters (glutamate, γaminobutyric acid), hormones (thyroxine; see right), as bacterial cell
hapter 3 Amino Acids αamino Acids are the main constituents of proteins. But they also can function as neurotransmitters (glutamate, γaminobutyric acid), hormones (thyroxine; see right), as bacterial cell
BENG 183 Trey Ideker. Protein Sequencing
 BENG 183 Trey Ideker Protein Sequencing The following slides borrowed from Hong Li s Biochemistry Course: www.sb.fsu.edu/~hongli/4053notes Introduction to Proteins Proteins are of vital importance to biological
BENG 183 Trey Ideker Protein Sequencing The following slides borrowed from Hong Li s Biochemistry Course: www.sb.fsu.edu/~hongli/4053notes Introduction to Proteins Proteins are of vital importance to biological
XCMS-MRM and METLIN-MRM: a cloud library and public resource for targeted analysis of small molecules
 SUPPLEMENTARY Brief Communication INFORMATION https://doi.org/10.1038/s41592-018-0110-3 In the format provided by the authors and unedited. XCMS-MRM and METLIN-MRM: a cloud library and public resource
SUPPLEMENTARY Brief Communication INFORMATION https://doi.org/10.1038/s41592-018-0110-3 In the format provided by the authors and unedited. XCMS-MRM and METLIN-MRM: a cloud library and public resource
Protein Structure Bioinformatics Introduction
 1 Swiss Institute of Bioinformatics Protein Structure Bioinformatics Introduction Basel, 27. September 2004 Torsten Schwede Biozentrum - Universität Basel Swiss Institute of Bioinformatics Klingelbergstr
1 Swiss Institute of Bioinformatics Protein Structure Bioinformatics Introduction Basel, 27. September 2004 Torsten Schwede Biozentrum - Universität Basel Swiss Institute of Bioinformatics Klingelbergstr
The Structure of Enzymes!
 The Structure of Enzymes Levels of Protein Structure 0 order amino acid composition Primary Secondary Motifs Tertiary Domains Quaternary ther sequence repeating structural patterns defined by torsion angles
The Structure of Enzymes Levels of Protein Structure 0 order amino acid composition Primary Secondary Motifs Tertiary Domains Quaternary ther sequence repeating structural patterns defined by torsion angles
The Structure of Enzymes!
 The Structure of Enzymes Levels of Protein Structure 0 order amino acid composition Primary Secondary Motifs Tertiary Domains Quaternary ther sequence repeating structural patterns defined by torsion angles
The Structure of Enzymes Levels of Protein Structure 0 order amino acid composition Primary Secondary Motifs Tertiary Domains Quaternary ther sequence repeating structural patterns defined by torsion angles
EXAM 1 Fall 2009 BCHS3304, SECTION # 21734, GENERAL BIOCHEMISTRY I Dr. Glen B Legge
 EXAM 1 Fall 2009 BCHS3304, SECTION # 21734, GENERAL BIOCHEMISTRY I 2009 Dr. Glen B Legge This is a Scantron exam. All answers should be transferred to the Scantron sheet using a #2 pencil. Write and bubble
EXAM 1 Fall 2009 BCHS3304, SECTION # 21734, GENERAL BIOCHEMISTRY I 2009 Dr. Glen B Legge This is a Scantron exam. All answers should be transferred to the Scantron sheet using a #2 pencil. Write and bubble
BI/CH421 Biochemistry I Exam 1 09/29/2014
 Part I: Multiple choice for each question, circle the choice that best answers the question. Do not write the letter in the margin to indicate your answer, circle it. 3 points each. 1. For a reaction with
Part I: Multiple choice for each question, circle the choice that best answers the question. Do not write the letter in the margin to indicate your answer, circle it. 3 points each. 1. For a reaction with
Protein Struktur (optional, flexible)
 Protein Struktur (optional, flexible) 22/10/2009 [ 1 ] Andrew Torda, Wintersemester 2009 / 2010, AST nur für Informatiker, Mathematiker,.. 26 kt, 3 ov 2009 Proteins - who cares? 22/10/2009 [ 2 ] Most important
Protein Struktur (optional, flexible) 22/10/2009 [ 1 ] Andrew Torda, Wintersemester 2009 / 2010, AST nur für Informatiker, Mathematiker,.. 26 kt, 3 ov 2009 Proteins - who cares? 22/10/2009 [ 2 ] Most important
1 large 50g. 1 extra large 56g
 National base for Standard Reference Release 28 slightly revised May, 206 Full Report (All s) 023, Egg, whole, raw, fresh Report Date: February 08, 208 06:8 EST values and weights are for edible portion.
National base for Standard Reference Release 28 slightly revised May, 206 Full Report (All s) 023, Egg, whole, raw, fresh Report Date: February 08, 208 06:8 EST values and weights are for edible portion.
Module No. 31: Peptide Synthesis: Definition, Methodology & applications
 PAPER 9: TECHNIQUES USED IN MOLECULAR BIOPHYSICS I Module No. 31: Peptide Synthesis: Definition, Methodology & applications Objectives: 1. Introduction 2. Synthesis of peptide 2.1. N-terminal protected
PAPER 9: TECHNIQUES USED IN MOLECULAR BIOPHYSICS I Module No. 31: Peptide Synthesis: Definition, Methodology & applications Objectives: 1. Introduction 2. Synthesis of peptide 2.1. N-terminal protected
National Stable Isotope Resource at Los Alamos
 ational table Isotope Resource at Los Alamos I ational Institute for Biological Imaging and Bioengineering lifford J. Unkefer 2,, 15, 17,18 1 utline ynthetic apabilities Example ynthesis of chiral methyl
ational table Isotope Resource at Los Alamos I ational Institute for Biological Imaging and Bioengineering lifford J. Unkefer 2,, 15, 17,18 1 utline ynthetic apabilities Example ynthesis of chiral methyl
Ch. 2 BASIC CHEMISTRY. Copyright 2010 Pearson Education, Inc.
 Ch. 2 BASIC CHEMISTRY Matter and Composition of Matter Definition: Anything that has mass and occupies space Matter is made up of elements An element cannot be broken down by ordinary chemical means Atoms
Ch. 2 BASIC CHEMISTRY Matter and Composition of Matter Definition: Anything that has mass and occupies space Matter is made up of elements An element cannot be broken down by ordinary chemical means Atoms
Sequence comparison: Score matrices. Genome 559: Introduction to Statistical and Computational Genomics Prof. James H. Thomas
 Sequence comparison: Score matrices Genome 559: Introduction to Statistical and omputational Genomics Prof James H Thomas FYI - informal inductive proof of best alignment path onsider the last step in
Sequence comparison: Score matrices Genome 559: Introduction to Statistical and omputational Genomics Prof James H Thomas FYI - informal inductive proof of best alignment path onsider the last step in
Basic Concepts of Metabolism. Stages of Catabolism. Key intermediates 10/12/2015. Chapter 15, Stryer Short Course
 Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
Basic Concepts of Metabolism Chapter 15, Stryer Short Course Digestion Formation of key intermediate small molecules Formation of ATP Stages of Catabolism Key intermediates 1 Fundamental Needs for Energy
1. Amino Acids and Peptides Structures and Properties
 1. Amino Acids and Peptides Structures and Properties Chemical nature of amino acids The!-amino acids in peptides and proteins (excluding proline) consist of a carboxylic acid ( COOH) and an amino ( NH
1. Amino Acids and Peptides Structures and Properties Chemical nature of amino acids The!-amino acids in peptides and proteins (excluding proline) consist of a carboxylic acid ( COOH) and an amino ( NH
C. Incorrect! Catalysts themselves are not altered or consumed during the reaction.
 Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Human Physiology - Problem Drill 04: Enzymes and Energy Question No. 1 of 10 Instructions: (1) Read the problem and answer choices carefully, (2) Work the problems on paper as needed, (3) Pick the answer,
Beaming in your answers
 Bio 111 andout for Biochemistry 1 This handout contains: 1. Today s iclicker Questions 2. The handout for today s lecture. iclicker Question #13A - before lecture Your lunch consists primarily of polymers.
Bio 111 andout for Biochemistry 1 This handout contains: 1. Today s iclicker Questions 2. The handout for today s lecture. iclicker Question #13A - before lecture Your lunch consists primarily of polymers.
The Evolution of the Ribosome and the Genetic Code
 Life 2014, 4, 227-249; doi:10.3390/life4020227 Article OPEN ACCESS life ISSN 2075-1729 www.mdpi.com/journal/life The Evolution of the Ribosome and the Genetic Code Hyman Hartman 1, * and Temple F. Smith
Life 2014, 4, 227-249; doi:10.3390/life4020227 Article OPEN ACCESS life ISSN 2075-1729 www.mdpi.com/journal/life The Evolution of the Ribosome and the Genetic Code Hyman Hartman 1, * and Temple F. Smith
Dental Biochemistry EXAM I
 Dental Biochemistry EXAM I August 29, 2005 In the reaction below: CH 3 -CH 2 OH -~ ethanol CH 3 -CHO acetaldehyde A. acetoacetate is being produced B. ethanol is being oxidized to acetaldehyde C. acetaldehyde
Dental Biochemistry EXAM I August 29, 2005 In the reaction below: CH 3 -CH 2 OH -~ ethanol CH 3 -CHO acetaldehyde A. acetoacetate is being produced B. ethanol is being oxidized to acetaldehyde C. acetaldehyde
Chapter 15 part 2. Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry. ATP 4- + H 2 O ADP 3- + P i + H +
 Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Biochemistry I Introduction to Metabolism Bioenergetics: Thermodynamics in Biochemistry ATP 4- + 2 ADP 3- + P i 2- + + Chapter 15 part 2 Dr. Ray 1 Energy flow in biological systems: Energy Transformations
Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Outer Glycolysis mitochondrial membrane Glucose ATP
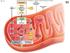 Fig. 7.5 uter Glycolysis mitochondrial membrane Glucose Intermembrane space xidation Mitochondrial matrix Acetyl-oA Krebs FAD e NAD + FAD Inner mitochondrial membrane e Electron e Transport hain hemiosmosis
Fig. 7.5 uter Glycolysis mitochondrial membrane Glucose Intermembrane space xidation Mitochondrial matrix Acetyl-oA Krebs FAD e NAD + FAD Inner mitochondrial membrane e Electron e Transport hain hemiosmosis
SEQUENCE ALIGNMENT BACKGROUND: BIOINFORMATICS. Prokaryotes and Eukaryotes. DNA and RNA
 SEQUENCE ALIGNMENT BACKGROUND: BIOINFORMATICS 1 Prokaryotes and Eukaryotes 2 DNA and RNA 3 4 Double helix structure Codons Codons are triplets of bases from the RNA sequence. Each triplet defines an amino-acid.
SEQUENCE ALIGNMENT BACKGROUND: BIOINFORMATICS 1 Prokaryotes and Eukaryotes 2 DNA and RNA 3 4 Double helix structure Codons Codons are triplets of bases from the RNA sequence. Each triplet defines an amino-acid.
ANSWERS TO CASE STUDIES Chapter 2: Drug Design and Relationship of Functional Groups to Pharmacologic Activity
 ANSWERS TO CASE STUDIES Chapter 2: Drug Design and Relationship of Functional Groups to Pharmacologic Activity Absorption/Acid-Base Case (p. 42) Question #1: Drug Cetirizine Clemastin e Functional groups
ANSWERS TO CASE STUDIES Chapter 2: Drug Design and Relationship of Functional Groups to Pharmacologic Activity Absorption/Acid-Base Case (p. 42) Question #1: Drug Cetirizine Clemastin e Functional groups
A rapid and highly selective colorimetric method for direct detection of tryptophan in proteins via DMSO acceleration
 A rapid and highly selective colorimetric method for direct detection of tryptophan in proteins via DMSO acceleration Yanyan Huang, Shaoxiang Xiong, Guoquan Liu, Rui Zhao Beijing National Laboratory for
A rapid and highly selective colorimetric method for direct detection of tryptophan in proteins via DMSO acceleration Yanyan Huang, Shaoxiang Xiong, Guoquan Liu, Rui Zhao Beijing National Laboratory for
Unit Two Chemistry of the Human Body
 I. Introduction to atoms Unit Two Chemistry of the Human Body A. Chemistry is the branch of science that concerns itself with the structure of matter, including the interaction between atoms. 1. Atoms-
I. Introduction to atoms Unit Two Chemistry of the Human Body A. Chemistry is the branch of science that concerns itself with the structure of matter, including the interaction between atoms. 1. Atoms-
Method of Enzyme Assay
 Method of Enzyme Assay Objective To study the different methods for determining enzyme activity. Use these method in diagnosis of certain diseases How to follow a reaction? Enzyme assays: Are laboratory
Method of Enzyme Assay Objective To study the different methods for determining enzyme activity. Use these method in diagnosis of certain diseases How to follow a reaction? Enzyme assays: Are laboratory
Sheet #3 Dr. Mamoun Ahram 06/07/2014. Peptide Bonds
 Peptide Bonds Features of peptide bond: Zigzag structure : when you look at it you notice that it s going up,down,up Notice the figure below A structure that consumes the least amount of energy to form
Peptide Bonds Features of peptide bond: Zigzag structure : when you look at it you notice that it s going up,down,up Notice the figure below A structure that consumes the least amount of energy to form
Cellular Respiration Chapter 5 Notes
 Cellular Respiration Chapter 5 Notes Some Terms to Know Aerobic = WITH oxygen Anaerobic = without oxygen NAD electron carrier = NADH FAD electron carrier = FADH 2 Cellular Respiration a way for cells to
Cellular Respiration Chapter 5 Notes Some Terms to Know Aerobic = WITH oxygen Anaerobic = without oxygen NAD electron carrier = NADH FAD electron carrier = FADH 2 Cellular Respiration a way for cells to
Potentiometric Titration of an Amino Acid. Introduction
 NAME: Course: DATE Sign-Off: Performed: Potentiometric Titration of an Amino Acid Introduction In previous course-work, you explored the potentiometric titration of a weak acid (HOAc). In this experiment,
NAME: Course: DATE Sign-Off: Performed: Potentiometric Titration of an Amino Acid Introduction In previous course-work, you explored the potentiometric titration of a weak acid (HOAc). In this experiment,
