Dynamic regulation of the expression of neurotrophin receptors by Runx3
|
|
|
- Wilfrid Hunt
- 5 years ago
- Views:
Transcription
1 RESEARCH ARTICLE 1703 Development 135, (2008) doi: /dev Dynamic regulation of the expression of neurotrophin receptors by Runx3 Souichiro Nakamura 1, *, Kouji Senzaki 1, *, Masaaki Yoshikawa 1, Mika Nishimura 1, Ken-ichi Inoue 2, Yoshiaki Ito 2, Shigeru Ozaki 1 and Takashi Shiga 1, Sensory neurons in the dorsal root ganglion (DRG) specifically project axons to central and peripheral targets according to their sensory modality. However, the molecular mechanisms that govern sensory neuron differentiation and the axonal projections remain unclear. The Runt-related transcription factors, Runx1 and Runx3, are expressed in DRG neuronal subpopulations, suggesting that they might regulate the cell specification and the trajectories of specific axons. Here, we show that parvalbuminpositive DRG neurons fail to differentiate from the onset in Runx3 / mice. By contrast, TrkC-positive DRG neurons differentiate normally at embryonic day (E) 11.5, but disappear by E13.5 in Runx3 / mice. Subsequently, TrkC-positive DRG neurons reappear but in smaller numbers than in the wild type. In Runx3 / mice, central axons of the TrkC-positive DRG neurons project to the dorsal spinal cord but not to the ventral and intermediate spinal cord, whereas the peripheral axons project to skin but not to muscle. These results suggest that Runx3 controls the acquisition of distinct proprioceptive DRG neuron identities, and that TrkC-positive DRG neurons consist of two subpopulations: Runx3-dependent early-appearing proprioceptive neurons that project to the ventral and intermediate spinal cord and muscle; and Runx3-independent late-appearing cutaneous neurons that project to the dorsal spinal cord and skin. Moreover, we show that the number of TrkA-positive DRG neurons is reduced in Runx3 / mice, as compared with the wild type. These results suggest that Runx3 positively regulates the expression of TrkC and TrkA in DRG neurons. KEY WORDS: Runx, Transcription factor, Dorsal root ganglion, Proprioceptive neuron, TrkC (Ntrk3), TrkA (Ntrk1), Parvalbumin, Cell fate specification, Axonal projection INTRODUCTION Somatosensory information is conveyed from the periphery to the spinal cord by primary sensory neurons located in the dorsal root ganglion (DRG). In the DRG, two major subpopulations of sensory neurons are defined: TrkA (Ntrk1)-expressing (TrkA + ) cutaneous neurons, which convey information from thermoreceptors and nociceptors in the skin; and TrkC (Ntrk3)-expressing (TrkC + ) proprioceptive neurons, which convey information regarding muscle length and tension (for a review, see Snider, 1994). These distinct sensory neuron subpopulations have different molecular characteristics, and terminate in different laminar targets within the spinal cord and peripheral targets. Trk receptors play crucial roles in neuronal survival, differentiation and axon projection of DRG neurons (Bibel and Barde, 2000; Huang and Reichardt, 2001; Markus et al., 2002; Patel et al., 2003; Snider, 1994). A recent study suggested that the selective expression and signaling of Trk receptors in DRG neurons are involved in axonal target selection in the spinal cord (Moqrich et al., 2004). However, the molecular mechanisms that govern sensory neuron differentiation and the axonal projections remain unclear. In mammals, the Runt-related (Runx) transcription factor family consists of three members: Runx1, 2 and 3. These Runx transcription factors interact with a common co-factor, polyomavirus enhancerbinding protein 2 [Pebp2; also known as core binding factor β (Cbfβ)] with highly conserved Runt DNA-binding domain that belongs to the 1 Graduate School of Comprehensive Human Sciences, University of Tsukuba, Tennodai, Tsukuba, Ibaraki , Japan. 2 Institute of Molecular and Cell Biology, 61 Biopolis Drive, Proteos, Singapore , Singapore. *These authors contributed equally to this work Author for correspondence ( tshiga@md.tsukuba.ac.jp) Accepted 3 March 2008 Runx transcription factors, and play important roles in developmental processes of various cell types including hematopoietic cells, osteoblasts and gastric epithelial cells (for reviews, see Coffman, 2003; Ito, 2004). Runx1 and Runx3 are also expressed in subtypes of neurons in the peripheral and central nervous systems (Simeone et al., 1995; Theriault et al., 2004; Theriault et al., 2005). In the DRG, reciprocal expression during early stages of development has been reported: Runx1 is initially expressed in TrkA + presumptive cutaneous neurons, while Runx3 is expressed in TrkC + presumptive proprioceptive neurons (Chen et al., 2006a; Chen et al., 2006b; Kramer et al., 2006; Levanon et al., 2001; Marmigère et al., 2006). These Runx transcription factors play crucial roles in the cell fate specification and axonal projections of DRG neurons (Chen et al., 2006a; Chen et al., 2006b; Inoue et al., 2002; Kramer et al., 2006; Levanon et al., 2001; Levanon et al., 2002; Marmigère et al., 2006; Yoshikawa et al., 2007) (for a review, see Marmigère and Ernfors, 2007). However, there seem to be some discrepancies concerning the role of Runx3. Inoue et al. showed that TrkC + DRG neurons are differentiated and maintained, but the projection of proprioceptive axons to both central and peripheral targets is severely impaired in Runx3 / embryos and newborns (Inoue et al., 2002). By contrast, initial appearance and subsequent loss of proprioceptive phenotypes including TrkC expression was reported in DRG neurons of Runx3 / embryos, suggesting the possibility of cell death of proprioceptive DRG neurons (Levanon et al., 2002). More recently, it has been shown that TrkC + DRG neuron numbers are decreased, with a concomitant increase in TrkB (Ntrk2)-expressing (TrkB + ) DRG neurons, in Runx3 / embryos by embryonic day (E) 12.5 (Kramer et al., 2006; Inoue et al., 2007). Runx3 promotes DRG neuron differentiation to a solitary TrkC + phenotype by repressing TrkB expression in hybrid TrkB + /TrkC + DRG neurons, and by maintaining the expression of TrkC within prospective proprioceptive DRG neurons (Kramer et al., 2006; Inoue et al.,
2 1704 RESEARCH ARTICLE Development 135 (9) 2007). These findings raise the question as to the role of Runx3 in the control of cell fate specification of proprioceptive DRG neurons, including its role in the expression of TrkC. In this study, to gain further insight into the function of Runx3 in cell type specification and axonal projections of DRG neurons, we performed a cell fate analysis of Runx3 / dorsal root ganglia (DRGs) during embryonic and neonatal stages. We show that Runx3 is required for the cell fate specification and the axonal projection of proprioceptive DRG neurons. However, a subset of TrkC + DRG neurons appears independently of Runx3 that may subserve cutaneous sensation. Therefore, TrkC + DRG neurons are divided into two subpopulations on the basis of Runx3 dependency: Runx3- dependent early-appearing proprioceptive neurons; and Runx3- independent late-appearing cutaneous neurons. Moreover, we show that Runx3 positively regulates the expression of TrkA and calcitonin gene-related peptide (CGRP; Calca) in addition to TrkC and parvalbumin (PV; Pvalb), suggesting that Runx3 might be involved in the development of cutaneous, as well as of proprioceptive, DRG neurons. Considering our previous study reporting that Runx1 negatively regulates the expression of TrkA, CGRP and TrkC (Yoshikawa et al., 2007), it can be suggested that Runx1 and Runx3 have antagonistic roles in the development of DRG neuron subpopulations. MATERIALS AND METHODS Genotyping and maintenance of animals The strategy used to inactivate Runx3 in the mouse germline was described previously (Inoue et al., 2002; Li et al., 2002). Bax-deficient mice (Knudson et al., 1995) were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were bred in a clean room in the Laboratory Animal Resource Center at the University of Tsukuba. Runx3 +/ and Runx3 +/+ mice were obtained from mating of Runx3 +/ mice. Runx3 +/ ; Bax +/ double-knockout mice and Runx3 +/+ ; Bax +/ control mice were obtained from the mating of Runx3 +/ ; Bax +/ double heterozygotes. Timed embryos were obtained by overnight mating, and the morning when the vaginal plug was observed was considered E0.5. For genotyping of Runx3 deficiency, PCRs were performed using primer pairs for wild-type (NA, 5 -GACTGTG CATGCACCTTT - CAC CAA-3 and CB, 5 -TAGGGCTCAGTAGC ACTTACGTCG-3 ) or mutant (NA and C2, 5 -ATGAAACGCCGAGTTAACGCCATCA-3 ) allele detection. For genotyping of Bax deficiency, PCRs were performed using a set of three primers: Bax exon 5 forward primer (5 -TGATCAGA AC - CATCATG-3 ), Bax intron 5 reverse primer (5 -GTTGACCAGAGTG - GCGTAGG-3 ) and Neo reverse primer (5 -CCGCTTCCATTGCTCAG - CGG-3 ). All experiments followed the Guide for the Care and Use of Laboratory Animals described by the National Institutes of Health (USA), and were approved by the Animal Experimentation Committee of the University of Tsukuba. Immunohistochemistry For cryostat sections, E11.5 and E13.5 whole mouse embryos were immersed overnight at 4 C in a fixative containing 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) (ph 7.4). Embryos at E14.5 and older were perfused transcardially with the same fixative and immersed overnight at 4 C. The trunk at thoracic segments (Th) and lower limbs including the gastrocnemius muscle were dissected and immersed in 30% sucrose in 0.1 M PB and frozen in Tissue-Tek OCT compound (Sakura Finetek, Japan). Ten or 12 μm transverse sections of Th or axial sections of legs were cut and collected onto MAS-coated glass slides (Matsunami Glass, Japan) and air-dried for 1 hour. If needed, sections were subjected to heat-induced epitope retrieval by heating to 105 C for 5 minutes in REAL Target Retrieval Solution (Dako). After treatment for 30 minutes at room temperature (RT) with 0.3% H 2 O 2 in methanol, the sections were incubated for 1 hour at RT in a blocking solution containing 1% BSA and % Triton X-100 in PBS. For immunohistochemical analysis, the following antibodies were used: rabbit anti-runx1 (Sigma; 1:1000 dilution), mouse anti-runx3 (Abnova; 1:2000), rabbit anti-pv (Swant; 1:2000), goat anti-trkc (R&D Systems; 1:2500), rabbit anti-calretinin (CR) (Swant; 1:2000), rabbit anti-vesicular glutamate transporter 1 (Vglut1) (a gift from Dr M. Watanabe, Hokkaido University, Sapporo, Japan; 1:1000), mouse anti-islet1 (Developmental Studies Hybridoma Bank; 1:200), rabbit anti-cgrp (Chemicon; 1:4000), rabbit anti-trka (a gift from Dr F. Reichardt, University of California, San Francisco, CA; 1:4000), rabbit anti-trkb (Upstate; 1:2000), rabbit antisomatostatin (SOM) (Protos Biotech; 1:2000), rabbit anti-c-ret (IBL; 1:100) and rabbit anti-calbindin-28k (CB) (Swant; 1:1000). The specificity of antibodies against Runx1 and Runx3 was verified by the absence of immunoreactivity in DRGs of Runx1 / and Runx3 / mice, respectively (Yoshikawa et al., 2007) (data not shown). The sections were incubated for hours at 4 C with each of the primary antibodies in the blocking solution. For single staining, the sections were incubated with a biotinylated secondary antibody for 1 hour at RT, followed with the peroxidaseconjugated avidin-biotin complex (Vector Laboratories; 1:100) for 30 minutes at RT. The positive reactions were visualized with 3,3 diaminobenzidine (DAB) using the ImmunoPure Metal Enhanced DAB Substrate Kit (Pierce). For caspase 3 immunostaining, cryostat sections were incubated with anti-active caspase 3 antibodies (Promega; 1:1000), followed by incubation with Alexa Fluor 488-labeled donkey anti-rabbit IgG (Invitrogen; 1:1000). For double or triple staining, cryostat sections were incubated with anti-runx3 antibodies, followed by incubation with biotinylated horse anti-mouse IgG (Vector Laboratories; 1:500) and Pacific Blue-conjugated streptavidin (Invitrogen; 1:500). The sections were then incubated with antibody against PV, CR, TrkC, TrkA, TrkB, CGRP or Runx1, followed by Alexa Fluor 488-labeled donkey anti-rabbit or anti-goat IgG, or Alexa Fluor 594-labeled donkey anti-rabbit or anti-goat IgG (Invitrogen; 1:1000). Samples from Runx3 / and Runx3 +/+ littermates were processed simultaneously for immunohistochemistry. DiI labeling Newborn mice were deeply anesthetized with diethylether and perfused transcardially with 4% PFA in 0.1 M PB. In order to retrogradely label DRG axons, crystals of a lipophilic fluorescent dye (DiI) were placed in several segments of the thoracic nerves after cutting off the ventral roots. The dyes were allowed to transport for 3 days at 37 C and the preparations were then cut in 50 μm sections for microscopy. Images of DiI-labeled spinal cord were collected on an Axioimager microscope (Carl Zeiss). Cell counting For counts of Islet1 + DRG neurons, DRGs in the tenth thoracic segment (Th10) at E13.5, E16.5 and postnatal day (P) 0, and for counts of caspase 3 + DRG neurons, Th4 DRGs at E17.5 and P0, were serially sectioned at 10 μm. For counts of TrkC +, CR + and PV + DRG neurons, Th10, Th11 and Th12 DRGs from E13.5 to P0, and for counts of Vglut1 +, TrkA +, TrkB +, c-ret +, CB +, SOM + and CGRP + DRG neurons, Th8, Th12, Th6, Th7, Th11, Th12 and Th9 DRGs at P0, were serially sectioned at 10 μm. The number of immunoreactive neurons was determined by counting neurons that contained a nucleus and showed a signal intensity in the cytoplasm >2.5-fold above the noise level in the same sections. The total number of immunoreactive neurons was counted from the level-matched DRGs of each genotype (5-6 DRGs from three animals at each stage were examined). Measurement of DRG volume Using serial sections (10 μm) containing whole DRGs (Th10) at E13.5, E16.5 and P0, the areas of the DRG in each section were measured and the DRG volume calculated using AxioVision imaging software (Carl Zeiss). Measurement of signal intensity of the immunoreactivity of axonal projections in the spinal cord For measurements of TrkC, PV and Vglut1 immunoreactivity within the spinal cord, Th10, Th11 and Th8 spinal cord, respectively, at P0 were sectioned by cryostat at 10 μm. After immunostaining, images of TrkC, PV and Vglut1 immunoreactive axons in the spinal cord were collected on a LSM510META confocal microscope (Carl Zeiss) or an Axioplan2 imaging microscope (Carl Zeiss). The spinal cord was divided into ten equal parts along the dorsoventral axis and the signal intensity of each part measured using ImageJ (version 1.34s, NIH). The signal intensity of each part in Runx3 / and wild-type spinal cord was quantified by comparison to the total
3 Runx3 regulates Trk expression RESEARCH ARTICLE 1705 signal intensity of the whole spinal cord of the wild type. Spinal cord sections from wild-type and Runx3 / littermates were processed simultaneously during the immunohistochemical process, and six spinal cord sections from two to three mice of each genotype were analyzed quantitatively. Statistical analysis Quantitative analyses were performed on three pairs of embryos or newborns from three independent pregnant mice. Statistical analyses were performed by ANOVA followed by post-hoc analysis (Fisher s protected least significant difference test). Differences were considered significant if the probability of error was less than 5%. All results were expressed as the mean ±s.e.m. RESULTS Decreased number of DRG neurons in Runx3 / mice First, we examined whether the loss of the Runx3 gene affects the number of DRG neurons, using Islet1 as a pan-neuronal marker (Fig. 1A-C). The number of Islet1 + DRG neurons did not differ in wildtype and Runx3 / mice at E13.5 and E16.5. By contrast, Islet1 + DRG neurons were decreased to about 80% of wild-type levels in Runx3 / mice at P0 (Fig. 1C, Table 1). Similarly, the DRG volume did not differ at E13.5 and E16.5, but decreased to about 85% in Runx3 / mice at P0 (Fig. 1D, Table 1). Next, we examined the effects of Runx3 deficiency on the apoptosis of DRG neurons (Fig. 1E-G). The number of active caspase 3-immunoreactive apoptotic DRG cells did not differ in wild-type and Runx3 / mice at E17.5 (Runx3 +/+, 42.83±3.40 versus Runx3 /, 41.33±4.15; n=6) and P0 (4.50±1.98 versus 3.50±1.15; n=6), suggesting that Runx3 inactivation does not induce caspase 3-dependent apoptosis. Cell fate specification of proprioceptive DRG neurons in Runx3 / mice To clarify whether the decrease in the number of Runx3 / DRG neurons was due to the loss of specific neurons, we examined the number of proprioceptive DRG neurons using antibodies against selective markers PV, TrkC and calretinin [CR; calbindin 2 (Calb2)] (Copray et al., 1994; Honda, 1995; Kucera et al., 2002; Mu et al., 1993). In the wild-type mice, PV was expressed at a low level in the DRG (Th12) at E13.5 (data not shown), and subsequently PV expression increased. The number of PV + DRG neurons increased by about 3-fold from E14.5 to E15.5, and thereafter decreased slightly until P0 (Fig. 2A-C,G). By contrast, PV + DRG neurons were virtually absent from E13.5 to P0 in the Runx3 / mice (Fig. 2D- F,G). Table 1. Number of DRG neurons and volume of DRGs in Runx3 +/+ and Runx3 / mice Stage Runx3 +/+ Runx3 / Number of Islet1 + DRG neurons (Th10) E ±351.0 (6) ±412.9 (5) E ±224.7 (6) ±502.6 (4) P ±292.5 (5) ±118.2 (5)* Volume of DRGs ( 10 5 μm 3 ) (Th10) E ±19.0 (5) 91.2±18.9 (5) E ±3.7 (6) 157.7±3.2 (6) P ±7.4 (6) 214.9±5.2 (6)* Statistical analyses were performed by ANOVA followed by post-hoc analysis (Fisher s protected least significant difference test). Data are presented as mean ±s.e.m. The number of DRGs is shown in parentheses. *P<0.01. Fig. 1. Number of DRG neurons and DRG volume are decreased in P0 Runx3 / mice with no differences in the number of caspase 3 + DRG cells between wild-type and Runx3 / mice. (A,B) Islet1 + neurons in DRGs of wild-type (A) and Runx3 / (B) mice at P0. (C,D) Quantitative analysis of the total number of Islet1 + DRG neurons (C) and the DRG volume (D) at the level of Th10 in wild-type (white bars) and Runx3 / (black bars) mice at E13.5, E16.5 and P0. (E,F) Immunoreactivity (green) of caspase 3 in DRGs of wild-type (E) and Runx3 / (F) mice at E17.5. The dotted line outlines the DRG. (G) Quantitative analysis of the number of caspase 3 + DRG cells in wildtype (white bars) and Runx3 / (black bars) mice at E17.5 and P0. Data are shown as mean ±s.e.m.; *P<0.01. Scale bars: 100 μm. TrkC was expressed in DRGs (Th10) of both the wild-type and Runx3 / mice at E11.5 (Fig. 3A,F). In the wild-type mice, TrkC expression was maintained at E13.5 and the number of TrkC + neurons increased by about 3-fold from E13.5 to E15.5 (Fig. 3B,C,K). By contrast, TrkC + neurons were hardly detected in Runx3 / mice at E13.5 (Fig. 3G,K). TrkC expression reappeared by E14.5 in Runx3 / mice, but the number of TrkC + neurons was significantly smaller than in the wild type (Fig. 3C-E,H-K). At P0, TrkC + neurons were decreased to about 65% in Runx3 / mice (213.2±8.1, n=6, P<0.01) as compared with the wild-type mice (330.0±12.7, n=6) (Fig. 3K). Next, to evaluate the contribution of apoptosis to the disappearance of TrkC + DRG neurons in Runx3 / mice at E13.5, we examined the expression of TrkC in DRGs of Runx3 / ; Bax / double-knockout and Runx3 +/+ ; Bax / control mice. TrkC expression was maintained in Runx3 +/+ ; Bax / but not in Runx3 / ; Bax / DRGs at E13.5 (Fig. 1L-O), suggesting that the disappearance of TrkC + DRG neurons is not due to apoptosis. CR was expressed in a limited number of DRG neurons (see Fig. S1 in the supplementary material). In the wild-type mice, CR was expressed at a low level in DRGs (Th11) at E13.5 and the expression
4 1706 RESEARCH ARTICLE Development 135 (9) Fig. 2. Loss of expression of parvalbumin in Runx3 / DRGs during development. (A-F) Photomicrographs indicate parvalbumin (PV) immunoreactivity (brown) at E14.5 (A,D), E18.5 (B,E) and P0 (C,F) in wild-type (A-C) and Runx3 / (D-F) DRGs at the level of Th12. (G) Quantification of PV + DRG neurons from E14.5 to P0. The number of PV + neurons in wild-type (white squares) and Runx3 / (black squares) mice are shown, and virtually no neurons express PV in Runx3 / DRGs through these developmental stages. SC, spinal cord. Data are shown as mean±s.e.m.; *P< Scale bar: 100 μm. increased subsequently (see Fig. S1A-C in the supplementary material). In Runx3 / mice, CR was also expressed, but the number of CR + neurons was reduced to 51.4%, 63.1%, 54.3% and 77.7% at E14.5, E15.5, E18.5 and P0, respectively, as compared with that in wild type (see Fig. S1D-G in the supplementary material). In summary, these results suggest that Runx3 is required for both the appearance of PV expression and the maintenance of TrkC expression until E13.5, but not for the initial appearance of TrkC. Projection of proprioceptive DRG afferents in Runx3 / mice Because the expression of PV and TrkC was lost or decreased in Runx3 / mice, we next analyzed the axonal projection of these neurons. We also analyzed Vglut1 (Slc17a7) expression, as a marker for a subpopulation of proprioceptive DRG neurons (Alvarez et al., 2004; Landry et al., 2004). TrkC + afferents projected to the dorsal horn, the intermediate zone and the ventral horn of the spinal cord in P0 wild-type mice. In Runx3 / mice, however, the projection to the dorsal horn was observed, but not to the intermediate zone and the ventral horn (Fig. 4A). PV + afferents projected to the ventral horn through the dorsal horn in the wild-type mice at P0. In Runx3 / mice, no projection to the spinal cord was observed throughout developmental stages from E14.5 to P0 (Fig. 4B, data not shown). Vglut1 + afferents projected to the deep layer of the dorsal horn, the intermediate zone and the ventral horn in the wild-type mice, whereas the projection to the ventral horn was not observed in Runx3 / mice (Fig. 4C). To investigate whether the loss of projections to the ventral horn in Runx3 / mice results from the apoptosis of proprioceptive DRG neurons, we examined the projections of DRG afferents using DiI labeling in Runx3 / ; Bax / mice and Runx3 +/+ ; Bax / mice at P0. DiI-labeled afferents projected to the ventral horn of the spinal cord in Runx3 +/+ ; Bax / mice, but not in Runx3 / ; Bax / mice (see Fig. S2 in the supplementary material), suggesting that the disappearance of proprioceptive DRG afferents is not due to apoptosis of the DRG neurons. Next, we identified the TrkC + neurons by examining the co expression of cutaneous markers in the wild-type and Runx3 / mice at P0. In the skin of the lower limb, the co expression of TrkC and TrkA (Fig. 4D,E), and of TrkC and CGRP (Fig. 4F,G), were observed in some fibbers of both the wild-type and Runx3 / mice. In the DRG, some TrkC + neurons coexpressed CGRP or TrkB, both in the wildtype and Runx3 / mice (see Fig. S3 in the supplementary material). In the muscle, however, TrkC + and PV + afferents were observed in wild-type but not in Runx3 / mice (Fig. 4H-J, data not shown). In summary, these results suggest that Runx3 is required for the proper projection of proprioceptive afferents. In addition, TrkC + neurons observed in Runx3 / mice may subserve cutaneous sensation. Expression of various markers of DRG neuron subtypes As mentioned above, the number of Islet1 + neurons was reduced by as much as 1300 in the Runx3 / mice at P0, but only a limited decrease of TrkC +, PV + and CR + neurons was detected. To clarify the participation of other types of neurons in this decrease, we examined the number of Vglut1 + neurons as the subpopulation of proprioceptive DRG neurons, and TrkA +, TrkB +, c-ret +, calbindin- 28K (CB) +, somatostatin (SOM) + and CGRP + neurons as cutaneous DRG neurons (Fig. 5). The number of Vglut1 + neurons was decreased to 69% in Runx3 / mice as compared with the wild-type mice (788.5±39.5 versus 546.2±18.7, n=6 for both Runx3 +/+ and Runx3 /, P<0.001) (Fig. 5A). Moreover, the number of TrkA + neurons was decreased to 69% in Runx3 / mice (4767.2±59.7 versus ±148.8, n=6, P<0.001) (Fig. 5B), SOM + neurons to 57% (471.8±39.4 versus 268.0±13.9, n=6, P<0.01) (Fig. 5F) and CGRP + neurons to 82% (804.2±31.6 versus 659.0±29.7, n=6, P<0.01) (Fig. 5G). TrkB + neurons were decreased, but not significantly (1484.2±133.0 versus ±134.3, n=6, P=0.067) (Fig. 5C). By contrast, the number of CB + neurons increased to 128% (510.8±17.5 versus 651.3±22.0, n=6, P<0.01) (Fig. 5E), whereas the number of c-ret + neurons was unchanged in Runx3 / mice (1186.0±129.0 versus ±53.4, n=6, P=0.35) (Fig. 5D). These results indicate that Runx3 deficiency affected the expression of cutaneous marker molecules differentially in DRG neurons at P0. Co expression of Runx3 with DRG neuron subtype markers and Runx1 To clarify whether the changes in the expression of TrkC, PV, CR, TrkA and TrkB in Runx3 / mice were regulated cell-autonomously by Runx3, the co expression of Runx3 with these marker molecules was examined in wild-type mice. The majority of TrkC + DRG neurons (83.0±1.3%, n=69) were Runx3-positive at E13.5 (Fig. 6A,C), but only one-third of TrkC + neurons coexpressed Runx3 at P0 (30.1±3.0%, n=17, P<0.001)
5 Runx3 regulates Trk expression RESEARCH ARTICLE 1707 Fig. 3. Transient loss of TrkC expression in Runx3 / DRGs during development. (A-J) Photomicrographs showing TrkC immunoreactivity (brown) at E11.5 (A,F), E13.5 (B,G), E14.5 (C,H), E18.5 (D,I) and P0 (E,J) in wildtype (A-E) and Runx3 / (F-J) DRGs at the level of Th10. (K) Quantification of TrkC + DRG neurons from E13.5 to P0. The number of TrkC + DRG neurons was consistently smaller in Runx3 / (black circles) than in wild type (white circles) mice between E13.5 and P0. (L-O) Immunoreactivity of TrkC in Runx3 +/+ ; Bax / (L,M) and in Runx3 / ; Bax / (N,O) DRGs at the level of Th10. The DRGs of L and N are shown at higher magnification in M and O, respectively. TrkC was expressed in Runx3 +/+ ; Bax / DRG neurons but not in Runx3 / ; Bax / DRG neurons. Data are shown as mean ±s.e.m.; *P<0.05, **P<0.01, ***P< Scale bars: 100 μm in J and in N for L,N; 50 μm in O for M,O. (Fig. 6B,C). By contrast, almost all PV + neurons coexpressed Runx3 and TrkC (Fig. 6D), whereas CR + neurons coexpressed TrkC but not Runx3 at P0 (see Fig. S4 in the supplementary material). Moreover, the subpopulation of TrkA + and TrkB + neurons coexpressed Runx3 at P0 (Fig. 6E,F). These findings suggest that Runx3 might regulate the expression of TrkC, PV, TrkA and TrkB cell-autonomously. We also investigated the co expression of Runx3 and Runx1 in wild-type mice. Few Runx3 + DRG neurons coexpressed Runx1 at E16.5, but almost all Runx3 + neurons coexpressed Runx1 at E18.5 and P0 (Fig. 6G,H; data not shown). These findings revealed that Runx3 expression changes dynamically during development, suggesting that Runx3 might function in concert with Runx1 in DRG neuron subsets at late developmental stages. DISCUSSION In the present study, to clarify the roles of Runx3 in the development of DRG neurons, we analyzed neuronal profiles and axonal projections in Runx3 / mice using immunohistochemistry for specific neuronal subtype markers. We found that TrkC + DRG neurons consist of two subpopulations: an early-developing subpopulation that maintains TrkC expression until E13.5 depending on Runx3; and a late-developing subpopulation that starts TrkC expression from E14.5 independently of Runx3. From the analysis of the axonal projections to central and peripheral targets, it could be suggested that early-developing Runx3-dependent TrkC + DRG neurons may subserve proprioception, whereas latedeveloping Runx3-independent neurons may subserve cutaneous sensation. We also provide evidence that Runx3 positively regulates the expression of TrkA, CGRP and SOM, as well as TrkC, PV, CR and Vglut1, suggesting that Runx3 might be involved in the development of cutaneous, as well as proprioceptive, DRG neurons. Considering our previous study reporting the negative regulation of TrkA, CGRP, SOM and TrkC by Runx1 (Yoshikawa et al., 2007), it can be suggested that Runx1 and Runx3 have antagonistic roles in the development of DRG neuron subsets. Proprioceptive DRG neurons are lost from the onset of development under Runx3 deficiency Several lines of evidence have suggested that Runx3 plays a crucial role in the cell fate specification of proprioceptive DRG neurons (Inoue et al., 2002; Levanon et al., 2002; Kramer et al., 2006). The expression of PV, a specific marker for proprioceptive DRG neurons (Copray et al., 1994; Honda, 1995), was not detected in DRGs of Runx3 / newborn mice (Inoue et al., 2002; Levanon et al., 2002). The present study confirmed the loss of PV + DRG neurons in Runx3 / mice at P0 and further showed that PV + DRG neurons were virtually absent from Runx3 / mice from E13.5 to P0, whereas PV + DRG neurons appeared by E13.5 in the wild-type mice (Fig. 2). Therefore, the present study suggests that Runx3 is required for the induction of PV expression, and it is likely that proprioceptive DRG neurons fail to develop from the onset under Runx3 deficiency. The loss of proprioceptive DRG neurons in Runx3 / mice was also supported by the absence of axonal projections to the central and peripheral targets (Inoue et al., 2002; Levanon et al., 2002) (the
6 1708 RESEARCH ARTICLE Development 135 (9) Fig. 4. DRG axon projections to the spinal cord, skin and muscle in wild-type and Runx3 / mice at P0. (A-C) Representative photomicrographs of the immunoreactivity of TrkC (A,A ; green), PV (B,B ; brown) and Vglut1 (C,C ; brown) in the spinal cord of wild-type (A - C ) and Runx3 / (A -C ) mice. The spinal cords shown in A and B were from the same littermates, those in C from different littermates. The relative signal intensity of the immunoreactivity of TrkC (A ), PV (B ) and Vglut1 (C ) in the spinal cords along the dorsoventral axis in wild-type (white squares) and Runx3 / (black squares) mice. In total, six spinal cord sections from two to three animals of each genotype were analyzed. TrkC + afferents project to the dorsal horn (DH), the intermediate zone (IZ) and the ventral horn (VH) in the wild-type mice (A,A ), but not to the intermediate zone and the ventral horn in Runx3 / mice (A,A ). PV + afferents project to the ventral horn in the wild-type (B,B ), but not in Runx3 / mice (B,B ). Vglut1 + afferents project to the dorsal horn, the intermediate zone and the ventral horn in the wild type (C,C ), but not to the ventral horn in Runx3 / mice (C,C ). (D-G) Double staining of TrkC + (D,E ; green) and TrkA + (D,E ; red), and of TrkC + (F,G ; green) and CGRP + (F,G ; red) axons in the skin of wild-type (D,F) and Runx3 / (E,G) mice from the same littermates. Arrowheads indicate TrkC + /TrkA + or TrkC + /CGRP + axons (yellow in D -G ). (H-J) Immunoreactivity of TrkC (H,I ; green) and PV (I ; red) in the muscle of wild-type mice (merge in I ), and of TrkC (J; green) in Runx3 / mice. TrkC + axons project to the muscle in wild-type but not in Runx3 / mice. PV immunoreactivity was not observed in the muscle of Runx3 / mice (data not shown). Data are shown as mean ±s.e.m.; *P<0.05, **P<0.01, ***P< Scale bars: 100 μm in A,H,J; 50 μm in E,G ; 20 μm in I. present study). In the present study, PV +, TrkC + and Vglut1 + afferents did not extend to the ventral horn in Runx3 / mice, whereas these afferents projected to the ventral horn in the wild-type mice (Fig. 4A-C). In accordance with the central projection, no TrkC + afferents projected to the muscle in Runx3 / mice (Fig. 4H- J). The loss of proprioceptive DRG neurons and their axonal projections in Runx3 / mice may not be caused by apoptosis, as no proprioceptive neuron-specific markers and axonal projections were observed in Runx3 / ; Bax / double-knockout mice, in which DRG neurons were rescued from apoptosis during embryonic development (White et al., 1998). A recent study has shown that Runx3 regulates the axon guidance of proprioceptive DRG neurons (Chen et al., 2006a). Gain- and loss-of-function studies in chick embryos revealed that Runx3 activity determines the axonal projections to the ventral horn, the intermediate zone and the dorsal horn in the spinal cord. Dynamic regulation of TrkC expression by Runx3 There seems to be some inconsistency regarding TrkC expression in Runx3 / DRG neurons. Levanon et al. showed that Runx3 / DRG neurons begin to express TrkC initially, and then lose the TrkC expression completely by E13.5 (Levanon et al., 2002). By contrast, Inoue et al. demonstrated that Runx3 / DRG neurons maintained both TrkC immunoreactivity at E14.0 and TrkC mrna expression at P0 (Inoue et al., 2002). The present study examined in detail TrkC expression in DRGs from E11.5 to P0. TrkC was expressed in DRGs of both wild-type and Runx3 / mice at E11.5, as shown in the previous study (Levanon et al., 2002). In contrast to the continuous TrkC expression in the wild-type DRG, we found that TrkC + DRG neurons disappeared completely by E13.5 in Runx3 / mice, and then TrkC expression resumed by E14.5 to be maintained through P0. Therefore, the present study revealed that Levanon et al. and Inoue et al. observed different aspects of the dynamic TrkC
7 Runx3 regulates Trk expression RESEARCH ARTICLE 1709 Fig. 5. Changes in the number of DRG neuron subtypes in Runx3 / mice. (A-G) Immunoreactivity (brown) of Vglut1 (A,A ), TrkA (B,B ), TrkB (C,C ), c-ret (D,D ), calbindin-28k (CB; E,E ), somatostatin (SOM; F,F ) and CGRP (G,G ) in wild-type (A -G ) and Runx3 / (A -G ) mice. Boxed areas of immunoreactive neurons are shown at higher magnification in the insets at the left bottom of each panel. (A -G ) Quantification of the number of DRG neuron subtypes in wild-type (white bars) and Runx3 / (black bars) mice. Data are shown as mean ±s.e.m.; *P<0.05, **P< Scale bar: 100 μm. expression in Runx3 / DRGs: the initial appearance of TrkC expression, its transient loss and recovery. We also showed that the disappearance of TrkC + DRG neurons in E13.5 Runx3 / mice is not due to apoptosis, as TrkC expression was maintained in Runx3 +/+ ; Bax / but not in Runx3 / ; Bax / DRGs at E13.5 (Fig. 3L-O). Therefore, the present study showed that Runx3 is required for the maintenance of TrkC expression until E13.5. Although TrkC expression recovered by E14.5, our quantitative analysis revealed a constant decrease in the number of TrkC + DRG neurons in Runx3 / from E13.5 to P0, as compared with wild type (Fig. 3). The reduction in the number of TrkC + neurons (by ) in Runx3 / mice was comparable to the number of PV + DRG neurons in the wild-type mice (Fig. 2G, Fig. 3K). Considering that almost all PV + DRG neurons coexpressed TrkC (Fig. 6De), the TrkC + DRG neurons lost in Runx3 / mice may correspond to TrkC + /PV + proprioceptive neurons. We also investigated the coexpression of Runx3 and TrkC in wild-type mice, and showed that most TrkC + DRG neurons coexpressed Runx3 at E13.5, whereas about 30% of TrkC + neurons coexpressed Runx3 at P0 (Fig. 6A-C). This level of coexpression in the wild-type mice at P0 was almost comparable to the reduction (by ~35%) in the number of TrkC + DRG neurons in Runx3 / mice at P0. Taken together, it is likely that Runx3 is required for the maintenance of the initial TrkC expression and the proper development of proprioceptive neurons. In Runx3 / mice, a subset of DRG neurons recovers TrkC expression independently of Runx3 from E14.5 (Fig. 3). Because these later-appearing TrkC + DRG neurons projected to the dorsal spinal cord and the skin and coexpressed markers of cutaneous DRG neurons, such as TrkA and CGRP, these neurons seem to be cutaneous in nature. Indeed, previous studies have shown that a subset of TrkC + DRG neurons send axons to the skin (Bronzetti et al., 1995; McMahon et al., 1994; Oakley and Karpinski, 2002; Oakley et al., 2000). The expression of TrkC in non-proprioceptive DRG neurons has also been reported (Genç et al., 2004). Erzurumlu s group examined neurotrophin 3 (Ntf3) knockout mice and found that no DRG neurons express TrkC at E15, but TrkC + DRG neurons appear at P0, although these do not express PV (Genç et al., 2004). Considering that Ntf3 knockout mice lack proprioceptive DRG neurons (Ernfors et al., 1994; Fariñas et al., 1994), these TrkC + /PV DRG neurons should be other than proprioceptive neurons. Taken together, it seems likely that TrkC expression around E13.5 is regulated by Runx3, and that during the critical period TrkC plays pivotal roles in the proper cell fate specification of proprioceptive neurons. Runx3 regulates the expression of cutaneous DRG neuron-specific markers We demonstrated that the total number of DRG neurons was greatly reduced (by as much as 1300) in Runx3 / mice at P0 (Fig. 1C). The reduction of DRG neurons at P0 was not affected by caspase 3- dependent apoptosis (Fig. 1E-G). Because the magnitude of this reduction cannot be explained by the loss of PV + (nearly 150), TrkC + (nearly 150) and/or Vglut1 + (nearly 250) proprioceptive DRG neurons, we examined the number of TrkA +, TrkB +, CB +, CGRP +, SOM +, c-ret + cutaneous DRG neurons. We found that DRG neurons that express TrkA, CGRP and SOM were decreased by nearly 1500, 150 and 200 in Runx3 / mice, respectively (Fig. 5). TrkB + DRG
8 1710 RESEARCH ARTICLE Development 135 (9) Fig. 6. Coexpression of Runx3 and neuron subtype markers in the wild-type DRG. (A,B) Double staining of Runx3 (A,B ; green) and TrkC (A,B ; red) at E13.5 (A) and P0 (B) in Th9 DRGs; (A,B ) merge. (C) The ratio of Runx3 + /TrkC + DRG neurons at E13.5 and P0. (D) Triple staining of PV (a), Runx3 (b) and TrkC (c) in Th9 DRGs at P0; (d-f) merges. Some images are shown with pseudo coloring (A,B,D). (E,F) Double staining of Runx3 (red) in combination with TrkA (green) (E) and TrkB (green) (F) in Th10 DRGs at P0. (G,H) Double staining of Runx3 (green) and Runx1 (red) at E16.5 (G) and E18.5 (H) in Th10 DRGs. Data are shown as mean ± s.e.m.; *P< Scale bars: 100 μm. neurons seemed to be decreased, but no significant difference was detected. By contrast, the number of CB + DRG neurons was increased, whereas that of c-ret + neurons was unchanged in Runx3 / mice. The expression of c-ret may be regulated by Runx1, because the number of c-ret + DRG neurons was increased in Runx1 / mice (Chen et al., 2006b; Yoshikawa et al., 2007). The present studies suggest that Runx3 might be involved in the development of subsets of cutaneous DRG neurons as well as proprioceptive neurons, by regulating the expression of TrkA, CB, SOM and CGRP. Unfortunately, functions of cutaneous sensation cannot be analyzed in Runx3 / mice because they die shortly after birth. In addition, the regulatory mechanisms underlying the expression of these molecules by Runx3 are not well known. There seem to be two possibilities: cell-autonomous regulation and indirect regulation. In vivo analysis of the coexpression of Runx3 with these molecules during development and loss- and gain-of-function studies are needed to reveal the regulatory mechanisms of Runx3. It is possible that the regulation of TrkA and TrkB by Runx3 is cell-autonomous, because a subpopulation of TrkA + and TrkB + DRG neurons coexpressed Runx3 (Fig. 6E,F). It has been reported that Runx3 transcriptionally represses TrkB expression and that TrkB + DRG neurons increased from E11 to E13 but not at later embryonic stages in Runx3 / mice (Kramer et al., 2006; Inoue et al., 2007). In contrast to this increase of TrkB + DRG neurons in Runx3 / mice during early embryonic stages, the present study showed that these neurons tended to decrease in Runx3 / mice at birth. The tendency at P0 might result from cell death due to the mismatch of neurotrophic factors between overproduced TrkB + DRG neurons and their axonal targets (Oppenheim, 1991).
9 Runx3 regulates Trk expression RESEARCH ARTICLE 1711 Runx3 and Runx1 have antagonistic effects on the development of subsets of DRG neurons The present study showed that the total number of DRG neurons was decreased in P0 Runx3 / mice. It is interesting to see that the total number of DRG neurons increases in Runx1 / mice (Chen et al., 2006b; Yoshikawa et al., 2007). Furthermore, the present study showed that the number of TrkA +, CGRP +, SOM + and TrkC + neurons was decreased in Runx3 / mice, suggesting that Runx3 positively regulates the expression of TrkA, CGRP, SOM and TrkC. In striking contrast, we have previously shown that DRG neurons that express TrkA, CGRP, SOM and TrkC were increased in Runx1 / mice (Yoshikawa et al., 2007). These results suggest that Runx1 and Runx3 might be involved antagonistically in the development of subsets of DRG neurons. Further studies that include their downstream targets will be needed to understand the roles of Runx1 and Runx3. We thank Drs M. Watanabe and F. Reichardt for providing antibodies against Vglut1 and TrkA, respectively. This study was supported by Grant-in-Aid for Scientific Research from the 21st Century COE Program from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan to M.Y., S.O. and T.S., and Grant-in-Aid for Young Scientists (B) to K.S. from MEXT. Supplementary material Supplementary material for this article is available at References Alvarez, F. J., Villalba, R. M., Zerda, R. and Schneider, S. P. (2004). Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J. Comp. Neurol. 472, Bibel, M. and Barde, Y. A. (2000). Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 14, Bronzetti, E., Ciriaco, E., Germana, G. and Vega, J. A. (1995). Immunohistochemical localization of neurotrophin receptor proteins in human skin. Ital. J. Anat. Embryol. 100, Chen, A. I., de Nooij, J. C. and Jessell, T. M. (2006a). Graded activity of transcription factor Runx3 specifies the laminar termination pattern of sensory axons in the developing spinal cord. Neuron 49, Chen, C. L., Broom, D. C., Liu, Y., de Nooij, J. C., Li, Z., Cen, C., Samad, O. A., Jessell, T. M., Woolf, C. J. and Ma, Q. (2006b). Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron 49, Coffman, J. A. (2003). Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell Biol. Int. 27, Copray, J. C., Mantingh-Otter, I. J. and Brouwer, N. (1994). Expression of calcium-binding proteins in the neurotrophin-3-dependent subpopulation of rat embryonic dorsal root ganglion cells in culture. Brain Res. Dev. Brain Res. 81, Ernfors, P., Lee, K.-E., Kucera, J. and Jaenish, R. (1994). Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell 77, Fariñas, I., Jones, K. R., Backus, C., Wang, X. Y. and Reichardt, L. F. (1994). Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature 369, Genç, B., Özdinler, P. H., Mendoza, A. E. and Erzurumlu, R. S. (2004). A chemoattractant role for NT-3 in proprioceptive axon guidance. PLoS Biol. 2, Honda, C. N. (1995). Differential distribution of calbindin-d28k and parvalbumin in somatic and visceral sensory neurons. Neuroscience 68, Huang, E. J. and Reichardt, L. F. (2001). Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, Inoue, K., Ozaki, S., Shiga, T., Ito, K., Masuda, T., Okado, N., Iseda, T., Kawaguchi, S., Ogawa, M., Bae, S. C. et al. (2002). Runx3 controls the axonal projection of proprioceptive dorsal root ganglion neurons. Nat. Neurosci. 5, Inoue, K., Ito, K., Osato, M., Lee, B., Bae, S. C. and Ito, Y. (2007). The transcription factor Runx3 represses the neurotrophin receptor TrkB during lineage commitment of dorsal root ganglion neurons. J. Biol. Chem. 282, Ito, Y. (2004). Oncogenic potential of the RUNX gene family: overview. Oncogene 23, Knudson, C. M., Tung, K. S., Tourtellotte, W. G., Brown, G. A. and Korsmeyer, S. J. (1995). Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science 270, Kramer, I., Sigrist, M., de Nooij, J. C., Taniuchi, I., Jessell, T. M. and Arber, S. (2006). A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron 49, Kucera, J., Cooney, W., Que, A., Szeder, V., Stancz-Szeder, H. and Walro, J. (2002). Formation of supernumerary muscle spindles at the expense of Golgi tendon organs in ER81-deficient mice. Dev. Dyn. 223, Landry, M., Bouali-Benazzouz, R., El Mestikawy, S., Ravassard, P. and Nagy, F. (2004). Expression of vesicular glutamate transporters in rat lumbar spinal cord, with a note on dorsal root ganglia. J. Comp. Neurol. 468, Levanon, D., Brenner, O., Negreanu, V., Bettoun, D., Woolf, E., Eilam, R., Lotem, J., Gat, U., Otto, F., Speck, N. et al. (2001). Spatial and temporal expression pattern of Runx3 (Aml2) and Runx1 (Aml1) indicates non-redundant functions during mice embryogenesis. Mech. Dev. 109, Levanon, D., Bettoun, D., Harris-Cerruti, C., Woolf, E., Negreanu, V., Eilam, R., Bernstein, Y., Goldenberg, D., Xiao, C., Fliegauf, M. et al. (2002). The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J. 21, Li, Q. L., Ito, K., Sakakura, C., Fukamachi, H., Inoue, K., Chi, X. Z., Lee, K. Y., Nomura, S., Lee, C. W., Han, S. B. et al. (2002). Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 109, Markus, A., Patel, T. D. and Snider, W. D. (2002). Neurotrophic factors and axonal growth, Curr. Opin. Neurobiol. 12, Marmigère, F. and Ernfors, P. (2007). Specification and connectivity of neuronal subtypes in the sensory lineage. Nat. Rev. Neurosci. 8, Marmigère, F., Montelius, A., Wegner, M., Groner, Y., Reichardt, L. F. and Ernfors, P. (2006). The Runx1/AML1 transcription factor selectively regulates development and survival of TrkA nociceptive sensory neurons. Nat. Neurosci. 9, McMahon, S. B., Armanini, M. P., Ling, L. H. and Phillips, H. S. (1994). Expression and co expression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron 12, Moqrich, A., Earley, T. J., Watson, J., Andahazy, M., Backus, C., Martin- Zanca, D., Wright, D. E., Reichardt, L. F. and Patapoutian, A. (2004). Expressing TrkC from the TrkA locus causes a subset of dorsal root ganglia neurons to switch fate. Nat. Neurosci. 7, Mu, X., Silos-Santiago, I., Carroll, S. L. and Snider, W. D. (1993). Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J. Neurosci. 13, Oakley, R. A. and Karpinski, B. A. (2002). Target-independent specification of proprioceptive sensory neurons. Dev. Biol. 249, Oakley, R. A., Lefcort, F. B., Plouffe, P., Ritter, A. and Frank, E. (2000). Neurotrophin-3 promotes the survival of a limited subpopulation of cutaneous sensory neurons. Dev. Biol. 224, Oppenheim, R. W. (1991). Cell death during development of the nervous system. Annu. Rev. Neurosci. 14, Patel, T. D., Kramer, I., Kucera, J., Niederkofler, V., Jessell, T. M., Arber, S. and Snider, W. D. (2003). Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron 38, Simeone, A., Daga, A. and Calabi, F. (1995). Expression of runt in the mice embryo. Dev. Dyn. 203, Snider, W. D. (1994). Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell 77, Theriault, F. M., Roy, P. and Stifani, S. (2004). AML1/Runx1 is important for the development of hindbrain cholinergic branchiovisceral motor neurons and selected cranial sensory neurons. Proc. Natl. Acad. Sci. USA 101, Theriault, F. M., Nuthall, H. N., Dong, Z., Lo, R., Barnabe-Heider, F., Miller, F. D. and Stifani, S. (2005). Role for Runx1 in the proliferation and neuronal differentiation of selected progenitor cells in the mammalian nervous system. J. Neurosci. 25, White, F. A., Keller-Peck, C. R., Knudson, C. M., Korsmeyer, S. J. and Snider, W. D. (1998). Widespread elimination of naturally occurring neuronal death in Bax deficient mice. J. Neurosci. 18, Yoshikawa, M., Senzaki, K., Yokomizo, T., Takahashi, S., Ozaki, S. and Shiga, T. (2007). Runx1 selectively regulates cell fate specification and axonal projections of dorsal root ganglion neurons. Dev. Biol. 303,
Runx1 selectively regulates cell fate specification and axonal projections of dorsal root ganglion neurons
 Developmental Biology 303 (2007) 663 674 www.elsevier.com/locate/ydbio Runx1 selectively regulates cell fate specification and axonal projections of dorsal root ganglion neurons Masaaki Yoshikawa a, Kouji
Developmental Biology 303 (2007) 663 674 www.elsevier.com/locate/ydbio Runx1 selectively regulates cell fate specification and axonal projections of dorsal root ganglion neurons Masaaki Yoshikawa a, Kouji
A Role for Runx Transcription Factor Signaling in Dorsal Root Ganglion Sensory Neuron Diversification
 Neuron 49, 379 393, February 2, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.neuron.2006.01.008 A Role for Runx Transcription Factor Signaling in Dorsal Root Ganglion Sensory Neuron Diversification Ina Kramer,
Neuron 49, 379 393, February 2, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.neuron.2006.01.008 A Role for Runx Transcription Factor Signaling in Dorsal Root Ganglion Sensory Neuron Diversification Ina Kramer,
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
Supplementary Figure 1
 Supplementary Figure 1 Single-cell RNA sequencing reveals unique sets of neuropeptides, transcription factors and receptors in specific types of sympathetic neurons (a) Dissection of paravertebral SGs
Supplementary Figure 1 Single-cell RNA sequencing reveals unique sets of neuropeptides, transcription factors and receptors in specific types of sympathetic neurons (a) Dissection of paravertebral SGs
Supplementary Figure 1
 Supplementary Figure 1 Number of genes detected. Distribution of the number of detected genes, including only the UCSC mrna gene models (i.e. excluding many non-coding RNAs, ribosomal and trnas and expressed
Supplementary Figure 1 Number of genes detected. Distribution of the number of detected genes, including only the UCSC mrna gene models (i.e. excluding many non-coding RNAs, ribosomal and trnas and expressed
Cell Death & Trophic Factors II. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Type III neuregulin 1 regulates pathfinding of sensory axons in the developing spinal cord and periphery
 RESEARCH ARTICLE 4887 Development 138, 4887-4898 (2011) doi:10.1242/dev.072306 2011. Published by The Company of Biologists Ltd Type III neuregulin 1 regulates pathfinding of sensory axons in the developing
RESEARCH ARTICLE 4887 Development 138, 4887-4898 (2011) doi:10.1242/dev.072306 2011. Published by The Company of Biologists Ltd Type III neuregulin 1 regulates pathfinding of sensory axons in the developing
Formation of the Cortex
 Formation of the Cortex Neuronal Birthdating with 3 H-thymidine 3H-thymidine is incorporated into the DNA during the S-phase (replication of DNA). It marks all mitotic cells Quantitative technique. (you
Formation of the Cortex Neuronal Birthdating with 3 H-thymidine 3H-thymidine is incorporated into the DNA during the S-phase (replication of DNA). It marks all mitotic cells Quantitative technique. (you
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Overexpression of YFP::GPR-1 in the germline.
 Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Neurotrophin-3 Promotes the Differentiation of Muscle Spindle Afferents in the Absence of Peripheral Targets
 The Journal of Neuroscience, June 1, 1997, 17(11):4262 4274 Neurotrophin-3 Promotes the Differentiation of Muscle Spindle Afferents in the Absence of Peripheral Targets Robert A. Oakley, 1 Frances B. Lefcort,
The Journal of Neuroscience, June 1, 1997, 17(11):4262 4274 Neurotrophin-3 Promotes the Differentiation of Muscle Spindle Afferents in the Absence of Peripheral Targets Robert A. Oakley, 1 Frances B. Lefcort,
NIH Public Access Author Manuscript Nat Neurosci. Author manuscript; available in PMC 2009 June 30.
 NIH Public Access Author Manuscript Published in final edited form as: Nat Neurosci. 2006 February ; 9(2): 180 187. doi:10.1038/nn1631. The Runx1/AML1 transcription factor selectively regulates development
NIH Public Access Author Manuscript Published in final edited form as: Nat Neurosci. 2006 February ; 9(2): 180 187. doi:10.1038/nn1631. The Runx1/AML1 transcription factor selectively regulates development
Pacinian Corpuscle Development Involves Multiple Trk Signaling Pathways
 DEVELOPMENTAL DYNAMICS 231:551 563, 2004 RESEARCH ARTICLE Pacinian Corpuscle Development Involves Multiple Trk Signaling Pathways J. Šedý, 1,5 V. Szeder, 1,2 J.M. Walro, 3 Z.G. Ren, 2 O. Naňka, 1 L. Tessarollo,
DEVELOPMENTAL DYNAMICS 231:551 563, 2004 RESEARCH ARTICLE Pacinian Corpuscle Development Involves Multiple Trk Signaling Pathways J. Šedý, 1,5 V. Szeder, 1,2 J.M. Walro, 3 Z.G. Ren, 2 O. Naňka, 1 L. Tessarollo,
A Developmental Switch in the Response of DRG Neurons to ETS Transcription Factor Signaling
 Wright State University CORE Scholar Neuroscience, Cell Biology & Physiology Faculty Publications Neuroscience, Cell Biology & Physiology 5-2005 A Developmental Switch in the Response of DRG Neurons to
Wright State University CORE Scholar Neuroscience, Cell Biology & Physiology Faculty Publications Neuroscience, Cell Biology & Physiology 5-2005 A Developmental Switch in the Response of DRG Neurons to
Nature Neuroscience: doi: /nn.2662
 Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Neural development its all connected
 Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Original Article Delayed but not loss of gliogenesis in Rbpj-deficient trigeminal ganglion
 Int J Clin Exp Pathol 2013;6(7):1261-1271 www.ijcep.com /ISSN:1936-2625/IJCEP1303056 Original Article Delayed but not loss of gliogenesis in Rbpj-deficient trigeminal ganglion Ze-Lan Hu 1, Xin Zhang 1,
Int J Clin Exp Pathol 2013;6(7):1261-1271 www.ijcep.com /ISSN:1936-2625/IJCEP1303056 Original Article Delayed but not loss of gliogenesis in Rbpj-deficient trigeminal ganglion Ze-Lan Hu 1, Xin Zhang 1,
Selective Regulation of trkc Expression by NT3 in the Developing Peripheral Nervous System
 The Journal of Neuroscience, August 1, 1999, 19(15):6559 6570 Selective Regulation of trkc Expression by NT3 in the Developing Peripheral Nervous System Sean Wyatt, Gayle Middleton, Epaminondas Doxakis,
The Journal of Neuroscience, August 1, 1999, 19(15):6559 6570 Selective Regulation of trkc Expression by NT3 in the Developing Peripheral Nervous System Sean Wyatt, Gayle Middleton, Epaminondas Doxakis,
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8
 Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
Cellular Neurobiology BIPN 140 Fall 2016 Problem Set #8 1. Inductive signaling is a hallmark of vertebrate and mammalian development. In early neural development, there are multiple signaling pathways
The growth rate of sensory nerve fibres in the mammalian embryo
 Development 00, 307-3 (987) Printed in Great Britain The Company of Biologists Limited 987 307 The growth rate of sensory nerve fibres in the mammalian embryo ALUN M. DAVIES Department of Anatomy, Si George's
Development 00, 307-3 (987) Printed in Great Britain The Company of Biologists Limited 987 307 The growth rate of sensory nerve fibres in the mammalian embryo ALUN M. DAVIES Department of Anatomy, Si George's
Conclusions. The experimental studies presented in this thesis provide the first molecular insights
 C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
Populations of NGF-dependent neurones differ in their requirement for BAX to undergo apoptosis in the absence of NGF/TrkA signalling in vivo
 Development 128, 4715-4728 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV1729 4715 Populations of NGF-dependent neurones differ in their requirement for BAX to undergo apoptosis
Development 128, 4715-4728 (2001) Printed in Great Britain The Company of Biologists Limited 2001 DEV1729 4715 Populations of NGF-dependent neurones differ in their requirement for BAX to undergo apoptosis
Muscle sensory neurons require neurotrophin-3 from peripheral tissues
 Development 121, 1341-1350 (1995) Printed in Great Britain The Company of Biologists Limited 1995 1341 Muscle sensory neurons require neurotrophin-3 from peripheral tissues during the period of normal
Development 121, 1341-1350 (1995) Printed in Great Britain The Company of Biologists Limited 1995 1341 Muscle sensory neurons require neurotrophin-3 from peripheral tissues during the period of normal
Trophic Factors. Trophic Factors. History 2. History Growth Factors. Giles Plant
 217 - Growth Factors Giles Plant Role in: Growth and Trophic Factors Soluble/diffusible factors - polypeptides Proliferation Differentiation (ie Cancer) Survival (degenerative diseases) Innervation Maintenance
217 - Growth Factors Giles Plant Role in: Growth and Trophic Factors Soluble/diffusible factors - polypeptides Proliferation Differentiation (ie Cancer) Survival (degenerative diseases) Innervation Maintenance
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice Supplementary Figure 2.
 Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
THE PROBLEMS OF DEVELOPMENT. Cell differentiation. Cell determination
 We emphasize these points from Kandel in Bi/CNS 150 Bi/CNS/NB 150: Neuroscience Read Lecture Lecture Friday, October 2, 2015 Development 1: pp 5-10 Introduction Brains evolved All higher animals have brains
We emphasize these points from Kandel in Bi/CNS 150 Bi/CNS/NB 150: Neuroscience Read Lecture Lecture Friday, October 2, 2015 Development 1: pp 5-10 Introduction Brains evolved All higher animals have brains
Bcl-2 Accelerates the Maturation of Early Sensory Neurons
 The Journal of Neuroscience, May 1, 1998, 18(9):3344 3350 Bcl-2 Accelerates the Maturation of Early Sensory Neurons Gayle Middleton, Luzia G. P. Piñón, Sean Wyatt, and Alun M. Davies School of Biological
The Journal of Neuroscience, May 1, 1998, 18(9):3344 3350 Bcl-2 Accelerates the Maturation of Early Sensory Neurons Gayle Middleton, Luzia G. P. Piñón, Sean Wyatt, and Alun M. Davies School of Biological
Bio 3411, Fall 2006, Lecture 19-Cell Death.
 Types of Cell Death Questions : Apoptosis (Programmed Cell Death) : Cell-Autonomous Stereotypic Rapid Clean (dead cells eaten) Necrosis : Not Self-Initiated Not Stereotypic Can Be Slow Messy (injury can
Types of Cell Death Questions : Apoptosis (Programmed Cell Death) : Cell-Autonomous Stereotypic Rapid Clean (dead cells eaten) Necrosis : Not Self-Initiated Not Stereotypic Can Be Slow Messy (injury can
The neural crest presents a model system for studying neural
 Transient expression of the bhlh factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate Mariela Zirlinger*, Liching Lo*, Jill McMahon, Andrew P. McMahon,
Transient expression of the bhlh factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate Mariela Zirlinger*, Liching Lo*, Jill McMahon, Andrew P. McMahon,
C. elegans L1 cell adhesion molecule functions in axon guidance
 C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
Characterization of Neurotrophin and Trk Receptor Functions in Developing Sensory Ganglia: Direct NT-3 Activation of TrkB Neurons In Vivo
 Neuron, Vol. 21, 325 334, August, 1998, Copyright 1998 by Cell Press Characterization of Neurotrophin and Trk Receptor Functions in Developing Sensory Ganglia: Direct NT-3 Activation of TrkB Neurons In
Neuron, Vol. 21, 325 334, August, 1998, Copyright 1998 by Cell Press Characterization of Neurotrophin and Trk Receptor Functions in Developing Sensory Ganglia: Direct NT-3 Activation of TrkB Neurons In
SUPPLEMENTARY INFORMATION
 med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its
 Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its transcriptional activity in wild-type embryo. A gradient of canonical
Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its transcriptional activity in wild-type embryo. A gradient of canonical
PROPRIOCEPTIVE AFFERENTS SURVIVE IN THE MASSETER MUSCLE OF trkc KNOCKOUT MICE
 Proprioceptive afferents survive in the masseter muscle of trkc knockout mice Neuroscience Vol. 95, No. 1, pp. 209 216, 2000 209 Copyright 1999 IBRO. Published by Elsevier Science Ltd Pergamon Printed
Proprioceptive afferents survive in the masseter muscle of trkc knockout mice Neuroscience Vol. 95, No. 1, pp. 209 216, 2000 209 Copyright 1999 IBRO. Published by Elsevier Science Ltd Pergamon Printed
MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning
 MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning Learning Goals for Lecture 29 4.1 Describe the contributions of early developmental events in the embryo to the formation
MCDB 4777/5777 Molecular Neurobiology Lecture 29 Neural Development- In the beginning Learning Goals for Lecture 29 4.1 Describe the contributions of early developmental events in the embryo to the formation
Question Set # 4 Answer Key 7.22 Nov. 2002
 Question Set # 4 Answer Key 7.22 Nov. 2002 1) A variety of reagents and approaches are frequently used by developmental biologists to understand the tissue interactions and molecular signaling pathways
Question Set # 4 Answer Key 7.22 Nov. 2002 1) A variety of reagents and approaches are frequently used by developmental biologists to understand the tissue interactions and molecular signaling pathways
10/2/2015. Chapter 4. Determination and Differentiation. Neuroanatomical Diversity
 Chapter 4 Determination and Differentiation Neuroanatomical Diversity 1 Neurochemical diversity: another important aspect of neuronal fate Neurotransmitters and their receptors Excitatory Glutamate Acetylcholine
Chapter 4 Determination and Differentiation Neuroanatomical Diversity 1 Neurochemical diversity: another important aspect of neuronal fate Neurotransmitters and their receptors Excitatory Glutamate Acetylcholine
9/4/2015 INDUCTION CHAPTER 1. Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology. Fig 1.
 INDUCTION CHAPTER 1 Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology Fig 1.1 1 EVOLUTION OF METAZOAN BRAINS GASTRULATION MAKING THE 3 RD GERM LAYER
INDUCTION CHAPTER 1 Neurons are similar across phyla Thus, many different model systems are used in developmental neurobiology Fig 1.1 1 EVOLUTION OF METAZOAN BRAINS GASTRULATION MAKING THE 3 RD GERM LAYER
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
Comparison of the expression patterns of several sox genes between Oryzias latipes and Danio rerio
 Urun 1 Comparison of the expression patterns of several sox genes between Oryzias latipes and Danio rerio Fatma Rabia URUN ilkent University, nkara - TURKEY High mobility group domain containing transcription
Urun 1 Comparison of the expression patterns of several sox genes between Oryzias latipes and Danio rerio Fatma Rabia URUN ilkent University, nkara - TURKEY High mobility group domain containing transcription
Developmental Biology
 Developmental Biology 360 (2011) 77 86 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology The transcription factor Cux2 marks
Developmental Biology 360 (2011) 77 86 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology The transcription factor Cux2 marks
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
3/8/ Complex adaptations. 2. often a novel trait
 Chapter 10 Adaptation: from genes to traits p. 302 10.1 Cascades of Genes (p. 304) 1. Complex adaptations A. Coexpressed traits selected for a common function, 2. often a novel trait A. not inherited from
Chapter 10 Adaptation: from genes to traits p. 302 10.1 Cascades of Genes (p. 304) 1. Complex adaptations A. Coexpressed traits selected for a common function, 2. often a novel trait A. not inherited from
1. What are the three general areas of the developing vertebrate limb? 2. What embryonic regions contribute to the developing limb bud?
 Study Questions - Lecture 17 & 18 1. What are the three general areas of the developing vertebrate limb? The three general areas of the developing vertebrate limb are the proximal stylopod, zeugopod, and
Study Questions - Lecture 17 & 18 1. What are the three general areas of the developing vertebrate limb? The three general areas of the developing vertebrate limb are the proximal stylopod, zeugopod, and
Supporting Information
 Supporting Information Cao et al. 10.1073/pnas.1306220110 Gram - bacteria Hemolymph Cytoplasm PGRP-LC TAK1 signalosome Imd dfadd Dredd Dnr1 Ikk signalosome P Relish Nucleus AMP and effector genes Fig.
Supporting Information Cao et al. 10.1073/pnas.1306220110 Gram - bacteria Hemolymph Cytoplasm PGRP-LC TAK1 signalosome Imd dfadd Dredd Dnr1 Ikk signalosome P Relish Nucleus AMP and effector genes Fig.
Fig. S1. Proliferation and cell cycle exit are affected by the med mutation. (A,B) M-phase nuclei are visualized by a-ph3 labeling in wild-type (A)
 Fig. S1. Proliferation and cell cycle exit are affected by the med mutation. (A,B) M-phase nuclei are visualized by a-ph3 labeling in wild-type (A) and mutant (B) 4 dpf retinae. The central retina of the
Fig. S1. Proliferation and cell cycle exit are affected by the med mutation. (A,B) M-phase nuclei are visualized by a-ph3 labeling in wild-type (A) and mutant (B) 4 dpf retinae. The central retina of the
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
Nature Methods: doi: /nmeth Supplementary Figure 1. In vitro screening of recombinant R-CaMP2 variants.
 Supplementary Figure 1 In vitro screening of recombinant R-CaMP2 variants. Baseline fluorescence compared to R-CaMP1.07 at nominally zero calcium plotted versus dynamic range ( F/F) for 150 recombinant
Supplementary Figure 1 In vitro screening of recombinant R-CaMP2 variants. Baseline fluorescence compared to R-CaMP1.07 at nominally zero calcium plotted versus dynamic range ( F/F) for 150 recombinant
Cells. Steven McLoon Department of Neuroscience University of Minnesota
 Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Massive loss of neurons in embryos occurs during normal development (!)
 Types of Cell Death Apoptosis (Programmed Cell Death) : Cell-Autonomous Stereotypic Rapid Clean (dead cells eaten) Necrosis : Not Self-Initiated Not Stereotypic Can Be Slow Messy (injury can spread) Apoptosis
Types of Cell Death Apoptosis (Programmed Cell Death) : Cell-Autonomous Stereotypic Rapid Clean (dead cells eaten) Necrosis : Not Self-Initiated Not Stereotypic Can Be Slow Messy (injury can spread) Apoptosis
Geert Geeven. April 14, 2010
 iction of Gene Regulatory Interactions NDNS+ Workshop April 14, 2010 Today s talk - Outline Outline Biological Background Construction of Predictors The main aim of my project is to better understand the
iction of Gene Regulatory Interactions NDNS+ Workshop April 14, 2010 Today s talk - Outline Outline Biological Background Construction of Predictors The main aim of my project is to better understand the
Role of Organizer Chages in Late Frog Embryos
 Ectoderm Germ Layer Frog Fate Map Frog Fate Map Role of Organizer Chages in Late Frog Embryos Organizer forms three distinct regions Notochord formation in chick Beta-catenin localization How does beta-catenin
Ectoderm Germ Layer Frog Fate Map Frog Fate Map Role of Organizer Chages in Late Frog Embryos Organizer forms three distinct regions Notochord formation in chick Beta-catenin localization How does beta-catenin
Specification of Motor Axon Trajectory by Ephrin-B:EphB Signaling: Symmetrical Control of Axonal Patterning in the Developing Limb
 Article Specification of Motor Axon Trajectory by Ephrin-B:EphB Signaling: Symmetrical Control of Axonal Patterning in the Developing Limb Victor Luria, 1,6 Dayana Krawchuk, 9 Thomas M. Jessell, 2,3,4,6
Article Specification of Motor Axon Trajectory by Ephrin-B:EphB Signaling: Symmetrical Control of Axonal Patterning in the Developing Limb Victor Luria, 1,6 Dayana Krawchuk, 9 Thomas M. Jessell, 2,3,4,6
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila
 Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Biology 218, practise Exam 2, 2011
 Figure 3 The long-range effect of Sqt does not depend on the induction of the endogenous cyc or sqt genes. a, Design and predictions for the experiments shown in b-e. b-e, Single-cell injection of 4 pg
Figure 3 The long-range effect of Sqt does not depend on the induction of the endogenous cyc or sqt genes. a, Design and predictions for the experiments shown in b-e. b-e, Single-cell injection of 4 pg
AP Biology Gene Regulation and Development Review
 AP Biology Gene Regulation and Development Review 1. What does the regulatory gene code for? 2. Is the repressor by default active/inactive? 3. What changes the repressor activity? 4. What does repressor
AP Biology Gene Regulation and Development Review 1. What does the regulatory gene code for? 2. Is the repressor by default active/inactive? 3. What changes the repressor activity? 4. What does repressor
The majority of cells in the nervous system arise during the embryonic and early post
 Introduction Introduction The majority of cells in the nervous system arise during the embryonic and early post natal period. These cells are derived from population of neural stem cells first shown by
Introduction Introduction The majority of cells in the nervous system arise during the embryonic and early post natal period. These cells are derived from population of neural stem cells first shown by
Midterm 1. Average score: 74.4 Median score: 77
 Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Physiology 2 nd year. Neuroscience Optional Lecture
 Academic year 2018/2019 Physiology 2 nd year Semester 1 Curricula Nervous system physiology Blood physiology Acid-base equilibrium Bibliography: Boron & Boulpaep Medical Physiology, 3 rd edition Physiology
Academic year 2018/2019 Physiology 2 nd year Semester 1 Curricula Nervous system physiology Blood physiology Acid-base equilibrium Bibliography: Boron & Boulpaep Medical Physiology, 3 rd edition Physiology
Cooperation between GDNF/Ret and ephrina/epha4 Signals for Motor-Axon Pathway Selection in the Limb
 Neuron 50, 35 47, April 6, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.neuron.2006.02.020 Cooperation between GDNF/Ret and ephrina/epha4 Signals for Motor-Axon Pathway Selection in the Limb Edgar R. Kramer,
Neuron 50, 35 47, April 6, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.neuron.2006.02.020 Cooperation between GDNF/Ret and ephrina/epha4 Signals for Motor-Axon Pathway Selection in the Limb Edgar R. Kramer,
SUPPLEMENTARY INFORMATION
 GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11419 Supplementary Figure 1 Schematic representation of innate immune signaling pathways induced by intracellular Salmonella in cultured macrophages. a, During the infection Salmonella
doi:10.1038/nature11419 Supplementary Figure 1 Schematic representation of innate immune signaling pathways induced by intracellular Salmonella in cultured macrophages. a, During the infection Salmonella
Independent Development of Sensory and Motor Innervation Patterns in Embryonic Chick Hindlimbs
 Developmental Biology 208, 324 336 (1999) Article ID dbio.1999.9212, available online at http://www.idealibrary.com on Independent Development of Sensory and Motor Innervation Patterns in Embryonic Chick
Developmental Biology 208, 324 336 (1999) Article ID dbio.1999.9212, available online at http://www.idealibrary.com on Independent Development of Sensory and Motor Innervation Patterns in Embryonic Chick
Developmental Biology Lecture Outlines
 Developmental Biology Lecture Outlines Lecture 01: Introduction Course content Developmental Biology Obsolete hypotheses Current theory Lecture 02: Gametogenesis Spermatozoa Spermatozoon function Spermatozoon
Developmental Biology Lecture Outlines Lecture 01: Introduction Course content Developmental Biology Obsolete hypotheses Current theory Lecture 02: Gametogenesis Spermatozoa Spermatozoon function Spermatozoon
Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant
 9 10 11 Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant Makoto Kazama 1 *, Sanae Ogiwara, Takashi Makiuchi 1, Kazuhiro Yoshida 1, Kumiko Nakada-Tsukui, Tomoyoshi
9 10 11 Behavior of DNA-lacking mitochondria in Entamoeba histolytica revealed by organelle transplant Makoto Kazama 1 *, Sanae Ogiwara, Takashi Makiuchi 1, Kazuhiro Yoshida 1, Kumiko Nakada-Tsukui, Tomoyoshi
Lbx1 Specifies Somatosensory Association Interneurons in the Dorsal Spinal Cord
 Neuron, Vol. 34, 535 549, May 16, 2002, Copyright 2002 by Cell Press Lbx1 Specifies Somatosensory Association Interneurons in the Dorsal Spinal Cord Michael K. Gross, Mirella Dottori, and Martyn Goulding
Neuron, Vol. 34, 535 549, May 16, 2002, Copyright 2002 by Cell Press Lbx1 Specifies Somatosensory Association Interneurons in the Dorsal Spinal Cord Michael K. Gross, Mirella Dottori, and Martyn Goulding
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Supplemental material
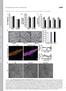 Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
JCB Article. Lineage-specific requirements of -catenin in neural crest development
 JCB Article Lineage-specific requirements of -catenin in neural crest development Lisette Hari, 1 Véronique Brault, 2 Maurice Kléber, 1 Hye-Youn Lee, 1 Fabian Ille, 1 Rainer Leimeroth, 1 Christian Paratore,
JCB Article Lineage-specific requirements of -catenin in neural crest development Lisette Hari, 1 Véronique Brault, 2 Maurice Kléber, 1 Hye-Youn Lee, 1 Fabian Ille, 1 Rainer Leimeroth, 1 Christian Paratore,
Lmx1b controls the differentiation and migration of the superficial
 Research article 3693 Lmx1b controls the differentiation and migration of the superficial dorsal horn neurons of the spinal cord Yu-Qiang Ding 1, *, Jun Yin 1, Artur Kania 2, Zhong-Qiu Zhao 1, Randy L.
Research article 3693 Lmx1b controls the differentiation and migration of the superficial dorsal horn neurons of the spinal cord Yu-Qiang Ding 1, *, Jun Yin 1, Artur Kania 2, Zhong-Qiu Zhao 1, Randy L.
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03
 Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
SAD Kinases Sculpt Axonal Arbors of Sensory Neurons through Long- and Short-Term Responses to Neurotrophin Signals
 Neurotrophin Signals, (2013), http://dx.doi.org/10.1016/j.neuron.2013.05.017 Article SAD Kinases Sculpt Axonal Arbors of Sensory s through Long- and Short-Term Responses to Neurotrophin Signals Brendan
Neurotrophin Signals, (2013), http://dx.doi.org/10.1016/j.neuron.2013.05.017 Article SAD Kinases Sculpt Axonal Arbors of Sensory s through Long- and Short-Term Responses to Neurotrophin Signals Brendan
Sonic hedgehog (Shh) signalling in the rabbit embryo
 Sonic hedgehog (Shh) signalling in the rabbit embryo In the first part of this thesis work the physical properties of cilia-driven leftward flow were characterised in the rabbit embryo. Since its discovery
Sonic hedgehog (Shh) signalling in the rabbit embryo In the first part of this thesis work the physical properties of cilia-driven leftward flow were characterised in the rabbit embryo. Since its discovery
Retrograde BMP Signaling Regulates Trigeminal Sensory Neuron Identities and the Formation of Precise Face Maps
 Article Retrograde BMP Signaling Regulates Trigeminal Sensory Neuron Identities and the Formation of Precise Face Maps Liberty K. Hodge, 1,2 Matthew P. Klassen, 3 Bao-Xia Han, 1 Glenn Yiu, 4 Joanna Hurrell,
Article Retrograde BMP Signaling Regulates Trigeminal Sensory Neuron Identities and the Formation of Precise Face Maps Liberty K. Hodge, 1,2 Matthew P. Klassen, 3 Bao-Xia Han, 1 Glenn Yiu, 4 Joanna Hurrell,
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1178343/dc1 Supporting Online Material for Starvation Protects Germline Stem Cells and Extends Reproductive Longevity in C. elegans This PDF file includes: Giana Angelo
www.sciencemag.org/cgi/content/full/1178343/dc1 Supporting Online Material for Starvation Protects Germline Stem Cells and Extends Reproductive Longevity in C. elegans This PDF file includes: Giana Angelo
DOWNLOAD OR READ : THE NEURONAL CYTOSKELETON MOTOR PROTEINS AND ORGANELLE TRAFFICKING IN THE AXON PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : THE NEURONAL CYTOSKELETON MOTOR PROTEINS AND ORGANELLE TRAFFICKING IN THE AXON PDF EBOOK EPUB MOBI Page 1 Page 2 the neuronal cytoskeleton motor proteins and organelle trafficking in
DOWNLOAD OR READ : THE NEURONAL CYTOSKELETON MOTOR PROTEINS AND ORGANELLE TRAFFICKING IN THE AXON PDF EBOOK EPUB MOBI Page 1 Page 2 the neuronal cytoskeleton motor proteins and organelle trafficking in
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Nature Neuroscience: doi: /nn.2717
 Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Developmental Biology 3230 Midterm Exam 1 March 2006
 Name Developmental Biology 3230 Midterm Exam 1 March 2006 1. (20pts) Regeneration occurs to some degree to most metazoans. When you remove the head of a hydra a new one regenerates. Graph the inhibitor
Name Developmental Biology 3230 Midterm Exam 1 March 2006 1. (20pts) Regeneration occurs to some degree to most metazoans. When you remove the head of a hydra a new one regenerates. Graph the inhibitor
Sig2GRN: A Software Tool Linking Signaling Pathway with Gene Regulatory Network for Dynamic Simulation
 Sig2GRN: A Software Tool Linking Signaling Pathway with Gene Regulatory Network for Dynamic Simulation Authors: Fan Zhang, Runsheng Liu and Jie Zheng Presented by: Fan Wu School of Computer Science and
Sig2GRN: A Software Tool Linking Signaling Pathway with Gene Regulatory Network for Dynamic Simulation Authors: Fan Zhang, Runsheng Liu and Jie Zheng Presented by: Fan Wu School of Computer Science and
Identification of dividing, determined sensory neuron precursors in the mammalian neural crest
 Development 126, 3545-3559 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV9668 3545 Identification of dividing, determined sensory neuron precursors in the mammalian neural crest
Development 126, 3545-3559 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV9668 3545 Identification of dividing, determined sensory neuron precursors in the mammalian neural crest
CELL REPLICATION. Fluorescent light microscopy showing mitosis, especially immunolabelled cytoskeleton and tubulin
 CELL REPLICATION Fluorescent light microscopy showing mitosis, especially immunolabelled cytoskeleton and tubulin Cell REPLICATION PROLIFERATION MUTIPLICATION DIVISION CELL REPLICATION Fluorescent light
CELL REPLICATION Fluorescent light microscopy showing mitosis, especially immunolabelled cytoskeleton and tubulin Cell REPLICATION PROLIFERATION MUTIPLICATION DIVISION CELL REPLICATION Fluorescent light
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Figure S1. Programmed cell death in the AB lineage occurs in temporally distinct
 SUPPLEMENTAL FIGURE LEGENDS Figure S1. Programmed cell death in the AB lineage occurs in temporally distinct waves. (A) A representative sub-lineage (ABala) of the C. elegans lineage tree (adapted from
SUPPLEMENTAL FIGURE LEGENDS Figure S1. Programmed cell death in the AB lineage occurs in temporally distinct waves. (A) A representative sub-lineage (ABala) of the C. elegans lineage tree (adapted from
7.06 Cell Biology EXAM #3 April 21, 2005
 7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 April 21, 2005 This is an open book exam, and you are allowed access to books, a calculator, and notes but not computers or any other types of electronic devices. Please write
MBios 401/501: Lecture 14.2 Cell Differentiation I. Slide #1. Cell Differentiation
 MBios 401/501: Lecture 14.2 Cell Differentiation I Slide #1 Cell Differentiation Cell Differentiation I -Basic principles of differentiation (p1305-1320) -C-elegans (p1321-1327) Cell Differentiation II
MBios 401/501: Lecture 14.2 Cell Differentiation I Slide #1 Cell Differentiation Cell Differentiation I -Basic principles of differentiation (p1305-1320) -C-elegans (p1321-1327) Cell Differentiation II
Embryonic Schwann cell development: the biology of Schwann cell precursors and early Schwann cells
 J. Anat. (1997) 191, pp. 501 505, with 2 figures Printed in the United Kingdom 501 Review Embryonic Schwann cell development: the biology of Schwann cell precursors and early Schwann cells K. R. JESSEN
J. Anat. (1997) 191, pp. 501 505, with 2 figures Printed in the United Kingdom 501 Review Embryonic Schwann cell development: the biology of Schwann cell precursors and early Schwann cells K. R. JESSEN
Developmental Zoology. Ectodermal derivatives (ZOO ) Developmental Stages. Developmental Stages
 Developmental Zoology (ZOO 228.1.0) Ectodermal derivatives 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis
Developmental Zoology (ZOO 228.1.0) Ectodermal derivatives 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis
Neurons and Nervous Systems
 34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
178 Part 3.2 SUMMARY INTRODUCTION
 178 Part 3.2 Chapter # DYNAMIC FILTRATION OF VARIABILITY WITHIN EXPRESSION PATTERNS OF ZYGOTIC SEGMENTATION GENES IN DROSOPHILA Surkova S.Yu. *, Samsonova M.G. St. Petersburg State Polytechnical University,
178 Part 3.2 Chapter # DYNAMIC FILTRATION OF VARIABILITY WITHIN EXPRESSION PATTERNS OF ZYGOTIC SEGMENTATION GENES IN DROSOPHILA Surkova S.Yu. *, Samsonova M.G. St. Petersburg State Polytechnical University,
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Nervous System Organization
 The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
Organization of Vertebrate Body. Organization of Vertebrate Body
 The Animal Body and Principles of Regulation Chapter 43 There are four levels of organization: 1. Cells 2. Tissues 3. Organs 4. Organ systems Bodies of vertebrates are composed of different cell types
The Animal Body and Principles of Regulation Chapter 43 There are four levels of organization: 1. Cells 2. Tissues 3. Organs 4. Organ systems Bodies of vertebrates are composed of different cell types
ANATOMY AND PHYSIOLOGY Revised 11/2010
 ANATOMY AND PHYSIOLOGY Revised 11/2010 DESCRIPTION OF COURSE: Covers the basics of human anatomy and physiology including anatomical terminology, basic biochemistry, cells and tissues, and the integumentary,
ANATOMY AND PHYSIOLOGY Revised 11/2010 DESCRIPTION OF COURSE: Covers the basics of human anatomy and physiology including anatomical terminology, basic biochemistry, cells and tissues, and the integumentary,
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo
 Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Pathfinding by zebrafish motoneurons in the absence of normal pioneer axons
 Development 114, 825-831 (1992) Printed in Great Britain The Company of Biologists Limited 1992 825 Pathfinding by zebrafish motoneurons in the absence of normal pioneer axons SUSAN H. PIKE, ELOINE F.
Development 114, 825-831 (1992) Printed in Great Britain The Company of Biologists Limited 1992 825 Pathfinding by zebrafish motoneurons in the absence of normal pioneer axons SUSAN H. PIKE, ELOINE F.
Bio Section III Organogenesis. The Neural Crest and Axonal Specification. Student Learning Objectives. Student Learning Objectives
 Bio 127 - Section III Organogenesis The Neural Crest and Axonal Specification Gilbert 9e Chapter 10 Student Learning Objectives 1. You should understand that the neural crest is an evolutionary advancement
Bio 127 - Section III Organogenesis The Neural Crest and Axonal Specification Gilbert 9e Chapter 10 Student Learning Objectives 1. You should understand that the neural crest is an evolutionary advancement
Waithe et al Supplementary Figures
 Waithe et al Supplementary Figures Supplementary Figure 1 Expression and properties of WT and W391A mutant YFP- Ca V 2.2. A Immunoblot using Ca V 2.2 Ab for untransfected cells (UT, lane 1), YFP-Ca V 2.2
Waithe et al Supplementary Figures Supplementary Figure 1 Expression and properties of WT and W391A mutant YFP- Ca V 2.2. A Immunoblot using Ca V 2.2 Ab for untransfected cells (UT, lane 1), YFP-Ca V 2.2
