Magnetic Resonance Imaging in Medicine
|
|
|
- Jonas Rose
- 5 years ago
- Views:
Transcription
1 Institute for Biomedical Engineering University and ETH Zurich Gloriastrasse 35 CH Zurich Switzerland Magnetic Resonance Imaging in Medicine D. Meier, P. Boesiger, S. Kozerke 2012
2 All rights reserved. No part of this manuscript may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of the authors.
3 `lkqbkq N fåíêççìåíáçåkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkn O kìåäé~ê=j~öåéíáå=oéëçå~ååé KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKP OKN j~öåéíáå=ãçãéåíkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkp OKO qüé=_äçåü=éèì~íáçåë KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKR OKP qüé=ãçíáçå=çñ=ëéáåë=áå=ëí~íáå=ã~öåéíáå=ñáéäçëi=êéä~ñ~íáçå=éüéåçãéå~kkkkkkkkkkkkkkkkkkkkkkkkkt OKQ mìäëéç=kjox=íüé=ñêéé=fåçìåíáçå=aéå~óx=cçìêáéê=pééåíêçëåçéókkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkknm OKR péáå=éåüç KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKNQ P fã~öé=cçêã~íáçåkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkknt PKN péäéåíáîé=éñåáí~íáçå=~åç=áåçìåíáçå=çñ=ëéáå=éåüçéë KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKNU PKO mêçàéåíáçå=êéåçåëíêìåíáçå=íéåüåáèìékkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkknv PKP cçìêáéê=áã~öáåö KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKON PKQ péáå=éåüç=~åç=öê~çáéåí=éåüç=áã~öáåökkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkor PKR c~ëí=áã~öáåö=íéåüåáèìéë KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKOT Q bñåáí~íáçå=péèìéååéë=~åç=fã~öé=`çåíê~ëí KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKPP QKN qüé=é~êíá~ä=ë~íìê~íáçå=ãççéw=fã~öé=`çåíê~ëí KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKPQ QKO qüé=ëéáåjéåüç=ãççéw=fã~öé=`çåíê~ëí KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKPS QKP qüé=áåîéêëáçå=êéåçîéêó=ãéíüçç KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKPU R fåëíêìãéåí~íáçå KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKQN RKN j~öåéíëkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkqn RKO dê~çáéåí=póëíéã KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKQP RKP pééåíêçãéíéê=~åç=oc=åçáä=ëóëíéãkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkqq RKQ qüé=åçãéìíéê=ëóëíéã KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKQR S cìååíáçå~ä=fåñçêã~íáçå=ïáíü=jof KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKQT SKN _äççç=ñäçï=ãé~ëìêéãéåíi=ãçíáçå=~å~äóëáë=~åç=jo=~åöáçöê~éüókkkkkkkkkkkkkkkkkkkkkkkkkkkkkqt SKO m~ê~ã~öåéíáå=åçåíê~ëí=~öéåíëkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkru SKP aáññìëáçå=fã~öáåö KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKSM SKQ cìååíáçå~ä=fã~öáåö=eñjoffkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkksp T içå~äáòéç=jo=pééåíêçëåçéó KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKTN TKN `ÜÉãáÅ~ä=ëÜáÑí KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKTN TKO ^Åèìáëáíáçå=~åÇ=péÉÅíê~ä=oÉëçäìíáçå KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKTO TKP içå~äáò~íáçå KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKTP TKQ t~íéê=~åç=c~í=pìééêéëëáçå KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKUQ TKR gj`çìéäáåö=~åç=pééåíê~ä=bçáíáåö KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKUV ^ ^éééåçáñw=cçìêáéêjqê~åëñçêã~íáçå KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKVT ^KN cçìêáéêjpéêáéëkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkvt ^KO cçìêáéê=fåíéöê~äëkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkvt ^KP mêçééêíáéë=çñ=cçìêáéê=qê~åëñçêã~íáçåëkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkvu ^KQ bñ~ãéäéëw KKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKKVV ^KR p~ãéäáåö=qüéçêéã=çñ=pü~ååçåkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkknmo _ ^éééåçáñw=mêçíçå=jéí~äçäáíéëkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkknmp iáíéê~íìêé=kkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkknmr fåçéñ=kkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkknmt Magnetic Resonance Imaging and Spectroscopy i
4 ii Institute for Biomedical Engineering, UZH / ETHZ
5 Introduction 1 INTRODUCTION Imaging techniques, based on magnetic resonance, are widely available in medical diagnostics. The phenomenon of Nuclear Magnetic Resonance (NMR) was discovered in 1946 by FELIX BLOCH at Stanford University and by EDWARD PURCELL at Harvard University. Since then, the NMR technique has been applied in physics and chemistry to a gradually increasing extent, e.g. for following a chemical reaction, to analyze solutions and to determine the structures of molecules and crystals. Since 1972, techniques are available, to correlate signal contributions to a spatial distribution within a sample. After whole-body magnets could be built, this became of interest for medical diagnostics: non-invasive examinations of anatomical structures and of biochemical processes inside the human body become possible. Magnetic Resonance Imaging (MRI) is a technique, which is nowadays widely used in radiological diagnostics. Based on the magnetic properties of hydrogen nuclei, it creates images of slices of the human body in any selected direction or of any three-dimensional volume of the body. Contrary to X-ray, in MRI multi-dimensional information is obtained. By choice of the excitation and detection technique, either the density distribution of the hydrogen nuclei or the relaxation time T 1 or T 2 can be displayed. Routine diagnostics are generally combinations of these three quantities, T 1, T 2 and different weights are obtained. Special techniques allow to determine blood flow in larger vessels or motion of the heart wall. MR images allow substantially more differentiated examination of anatomical structures inside the body, than was possible ever before, due to better soft tissue contrast. Especially for spine and heart imaging the possibility of image acquisition in any slice direction without moving the patient is of great advantage. Besides, from the images information may be extracted about the biochemical environment of the hydrogen nuclei and therefore, about certain functional aspects, of important for early detection of diseases. Another technique is local Magnetic Resonance Spectroscopy (MRS), which allows to follow biochemical processes inside the body. It produces spatially resolved NMR-spectra of elements like 1 H, 13 C, 23 Na and 31 P. Different resonance lines represent different chemical substances of these elements. Signal amplitudes or line intensities are a measure for the concentration of the corresponding substances. The spectra allow the investigation of biochemical processes like the phosphorus metabolism in the brain or other organs, or the non-invasive study of effects of pharmaceutics in a living body. The combination of these techniques with MR imaging may lead to powerful procedures for localized investigations of the central nervous system, the heart, the kidneys, the liver, or other organs. Magnetic Resonance Imaging and Spectroscopy 1
6 1 2 Institute for Biomedical Engineering UZH / ETHZ
7 Nuclear Magnetic Resonance 2 NUCLEAR MAGNETIC RESONANCE 2.1 MAGNETIC MOMENT NMR is based on the fact that several atomic nuclei, such as 1 H, 13 C, 14 N, 19 F, 23 Na and 31 P possess an inherent angular momentum or spin. This spin is a result of the rotation of the nucleus about a fixed axis through that nucleus, so that the spin describes a rotation about his axis with angular momentum J. Since the nucleus consists of charged particles, a magnetic moment results = J (Eq. 2.1) similar to a macroscopic magnetic moment generated by a circular electrical current. The gyromagnetic ratio is unique for each type of nuclei. The quantum mechanical laws state that this angular momentum is quantized. Along the z-direction of a coordinate system (x, y, z) the possible values for the angular momentum are J z = hm (Eq. 2.2) h is Planck's constant; m is the magnetic quantum number, which describes the quantum mechanical eigenvalue of the system. m may have values m = I, I-1,..., -I where spin quantum number I is a nucleus specific half or whole number. The total number of possible eigenvalues (stationary states) of a nucleus is therefore 2I +1. Nuclei of isotopes 1 H, 13 C and 31 P, to which we will restrict in the following, show a spin quantum number I = ½. The two possible eigenvalues of the angular momentum are therefore characterized by magnetic quantum numbers m ½. The z-components of their magnetic moment amount 1 z = hm = -- h 2 (Eq. 2.3) The magnetic moment of the nucleus may have two directions in the coordinate system in such way that the z-component is given by (Eq. 2.3). The two energy states or eigenvalues of nuclei with m +½ and m -½ are degenerate in a magnetic field-free space, i.e. both situations are energetically identical. In a static magnetic field of flux B o in the z-direction of the coordinate system the degeneracy is lost by interaction of the nuclear moments with the field. The situation where the z-components of magnetic moment is parallel to magnetic field B o is energetically more favorable, as also expected Magnetic Resonance Imaging and Spectroscopy 3
8 2 Magnetic moment from classical physics. The potential energy on both situations amounts after normalizing to E m = 0 in a space free of any magnetic field is given by: E m = z B o = hb o m (Eq. 2.4) It increases linearly with the field strength for m +½ and decreases for m -½ (Figure 2-1). In thermal equilibrium at spin temperature T s, the populations of both energy levels are slightly different. The difference between the populations of the two levels, n is governed by Boltzmann statistics: nh B o n = n 12 n k B T s (Eq. 2.5) where n = number of magnetic moments per unit volume, and n +1/2, n -1/2 the populations of levels with m +½ and m -½ respectively. The level with the lower energy shows the higher population. For the magnetization, i.e. the macroscopic magnetic dipolar moment per unit volume of a sample, defined as the sum of dipole moments per unit volume, M o i results from the difference in the populations = n hb o n M o n z h 2 = = 2k B T z = B s 4k B T o = o T s B o s i (Eq. 2.6) (Eq. 2.7) where k B is the Boltzmann constant and nuclei. o the magnetic susceptibility of the E m m = -1/2 E m m = -1/2 E m = +1/2 m = +1/2 B o n n Figure 2-1 Energy levels for nuclei with I =½ in external magnetic field Bo (left). Populations n1/ 2, n-1/2 of the energy levels with m=+½ and m= -½ respectively, for a particular Bo. 4 Institute for Biomedical Engineering UZH / ETHZ
9 Nuclear Magnetic Resonance TABLE 2-1 Spin, gyromagnetic ratio, natural abundance and relative sensitivity of nuclei of biological interest. Isotope Spin Quantum number Gyromagnetic Ratio [MHz/Tesla] Natural Abundance [%] Relative Sensitivity for equal number of Spins a and Constant Field Relative Sensitivity corrected for Natural Abundance 1 H 1/ C 1/ N / F 1/ Na 3/ P 1/ K 3/ Ca 7/ a. For calculation of the measurable relative signal strength these values should be multiplied by the relative concentrations of these atoms (for spectroscopy of the corresponding metabolites) in the tissue of interest and eventually be corrected for spin. By irradiation with quanta of corresponding energy, transitions between the two levels are induced by flipping the magnetic moments. This energy is attained by alternating an electromagnetic field with a frequency that fulfils the BOHR condition: E = h o (Eq. 2.8) From this and (Eq. 2.4) the so-called Larmor frequency o = B o (Eq. 2.9) can be calculated. For 1 H nuclei (protons) in magnetic fields with fluxes of 0.5 and 1.5 Tesla respectively, as they are often applied for MR imaging, the resonance frequencies o = o B o 2 are about 21 MHz and 63 MHz (see Table 2-1). 2.2 THE BLOCH EQUATIONS The time dependent behavior of an ensemble of nuclear moments under the influence of external static and time dependent magnetic fields can be approximated by laws from classical physics. The temporal change of the angular Magnetic Resonance Imaging and Spectroscopy 5
10 2 The Bloch equations momentum J and with it the temporal change of the magnetic moments = J are equal to the torque that works on. The torque that acts on a magnetic moment in a magnetic field B is given by the vector products B. This results in the motion equation d = B dt (Eq. 2.10) B contains all magnetic field components acting on the magnetic moment, i.e. both time dependent and time independent components. The magnetization M is defined as the sum of the magnetic moment vectors of the analyzed nuclei per unit volume of the macroscopic sample (Eq. 2.6). Thus, from (Eq. 2.10) movement of this magnetization may be described by: dm = M B dt (Eq. 2.11) or in Cartesian coordinates: dm x = M dt y B z M z B y dm y = M dt z B x M x B z (Eq. 2.12) dm z = M dt x B y M y B x In general the magnetic field B in a magnetic resonance experiment consists of a static magnetic component B o (0, 0, B z B o ) in the z-direction of the accordingly chosen coordinate system and of a time dependent component B(t) (B x (t), B y (t), 0) in the xy-plane orthogonal to B o. However, the resulting equations do not fully describe the system of spins, since effects of relaxation due to interaction between spins (spin-spin relaxation) and between spins and the environment (spin-lattice relaxation) are neglected. Bloch assumed, that these phenomena could be treated as first order processes and therefore, in relations (Eq. 2.12) could be represented by linear terms with time constants T 1 and T 2. This results in the Bloch equations 6 Institute for Biomedical Engineering UZH / ETHZ
11 Nuclear Magnetic Resonance dm x = M dt y B z M z B y M T x 2 dm y = M dt z B x M x B z M T y 2 (Eq. 2.13) dm z = M dt x B y M y B x M T z M o 1 These equations describe the motion of the macroscopic magnetization under influence of a stationary magnetic field B o in z-direction, a time dependent field B(t), and relaxation mechanisms. The relaxation effects act upon disturbance of the equilibrium state, which may be caused e.g. by switching on the time dependent field during a short time interval. Thereby the system restores thermodynamic equilibrium, i.e. the x- and y-components of the magnetization disappear and the z-component returns to the equilibrium value. M o is determined by the population difference (Eq. 2.5). The time constants T 1 and T 2 governing these relaxation processes are denoted as longitudinal and transversal relaxation times respectively, since they describe the change in magnetization along the axis of the static field B o and within the plane transversal to B o, respectively. 2.3 THE MOTION OF SPINS IN STATIC MAGNETIC FIELDS, RELAXATION PHENOMENA To investigate the motion of the magnetization under influence of a magnetic field, first consider the simple case where the magnetization only experiences the static magnetic field B o. To solve the BLOCH equations, a coordinate system is chosen with its z-axis along the direction of B o. By neglecting the relaxation terms the equations are reduced to or in components dm = M B dt o with B o = (0, 0, B o ) (Eq. 2.14) dm x = B dt o M y dm y = B dt o M x (Eq. 2.15) dm z dt = 0 Magnetic Resonance Imaging and Spectroscopy 7
12 2 The motion of spins in static magnetic fields, relaxation phenomena B o m o M M z z y x Figure 2-2 Precession of magnetization on a conical surface about the direction of the stationary field B o. Then, as solution it is found M x = m o cos o t + M y = m o sin o t + M z = const (Eq. 2.16) with o = B o or o = B o if the direction of rotation is taken into consideration. The magnetization precesses on a conical surface about the field direction B o (Figure 2-2) with precession frequency or Larmor frequency o =B o. The values of m o and M z are determined by the initial condition. The Larmor frequencies are located in the radio-frequency range. For nuclei of biological interest for given field strength they may be calculated applying the gyromagnetic ratios listed in Table 2-1. To investigate the influence of the relaxation terms we transform the equation (Eq. 2.11) according to the formula for the transformation dm dt lab = dm dt + M rot (Eq. 2.17) into a coordinate system (x', y', z' = z) that rotates about the field direction with the Larmor frequency o = B o. The time dependence of the magnetization with respect to (x', y', z') is thus defined by dm dt = M B o M rot (Eq. 2.18) 8 Institute for Biomedical Engineering UZH / ETHZ
13 Nuclear Magnetic Resonance and with o o dm dt = rot Including the relaxation terms results in 0 (Eq. 2.19) dm x' dt dm y' dt dm z' dt = = = M T x' M T y' M T z' M o 1 (Eq. 2.20) for the rotating system, with solutions M x' M x'o e t T 2 = M y' M y'o e t T 2 = M z' = M z'o M o e t T 1 + M o (Eq. 2.21) M x'o, M y'o and M z'o = M zo represent the values in the initial situation. This result shows that magnetization components orthogonal to the field B o decrease with time constant T 2. This decrease results from slightly fluctuating magnetic fields originating from collisions between molecules and from interaction between nuclei (spin-spin interaction). As a consequence, the individual spins acquire slightly fluctuating Larmor frequencies, so that their precessions run out of phase and the transversal vector components of the macroscopic magnetization disappear. This dephasing of the spins is accelerated by inhomogeneities of the field B o, so that the components M x and M y in general decrease with time constants T 2 T T 2 = T T inhom (Eq. 2.22) Magnetic Resonance Imaging and Spectroscopy 9
14 2 Pulsed NMR; the free Induction Decay; Fourier Spectroscopy TABLE 2-2 Relaxation times of 1 H nuclei in several tissues, measured in vivo at B o = 0.5 and 1.5Tesla. The variance of the presented numbers is about ±20%, depending on the authors. Region T 1 for 1.5 T [ms] T 1 for 0.5 T [ms] T 2 [ms] White Matter Grey Matter CSF >2500 >2500 >2000 Skeletal Muscle Kidney Liver Fat At the same time, the magnetization component M z in field direction recovers with time constant T 1 towards its thermal equilibrium value M o, which is determined by the population difference of the energy levels. With the same time constant,m o will build up when a sample is transferred from a field-free space into a magnetic field. T 1 is larger than T 2 (Table 2-2), since spin flips are induced by the environment (spin-lattice interaction) and the flip energy exchange has to be performed with the environment. 2.4 PULSED NMR; THE FREE INDUCTION DECAY; FOURIER SPECTROSCOPY A coil around the sample with its axis perpendicular to B o, e.g. in x-direction of the laboratory system, can be applied to induce an alternating magnetic field B x t = 2B 1 cost. To solve the Bloch equations for the magnetization under influence of this alternating field, it is split into two rotating components of amplitude B 1. One rotates clockwise, the other one counter clockwise (Figure 2-3). B R = B 1 icost + jsint B L = B 1 icost jsint (Eq. 2.23) i, j = are unit vectors in x-, y-direction. 10 Institute for Biomedical Engineering UZH / ETHZ
15 Nuclear Magnetic Resonance y B R x B X B L Figure 2-3 A linear oscillating field can be split into two rotating components. To avoid having to consider both components separately, is replaced by z with z > 0 or z <0, so that both rotation directions are covered. Thus it can be restricted to one oscillating field B 1 = B 1 icos z t + jsin z t (Eq. 2.24) Neglecting the relaxation terms the motion equation (Eq. 2.9) for magnetization M becomes dm = M B B dt o + 1 t (Eq. 2.25) The time dependence of B 1 can be removed by transformation to a coordinate frame (x',y',z') with unit vectors (i', j', k') that rotates around the z-axis with z. dm dt = rot M z + B o k' + B1 i' (Eq. 2.26) The first term with z results from differentiation of the unit vectors. Magnetic resonance only occurs if z + B o 0. Since this can only be the case for z < 0, we will restrict to this rotation direction of the oscillating field and replace z = with > 0: this results in dm dt = M B o --- k' + B 1 i' rot (Eq. 2.27) Magnetic Resonance Imaging and Spectroscopy 11
16 2 Pulsed NMR; the free Induction Decay; Fourier Spectroscopy dm dt rot = M B eff (Eq. 2.28) with B eff = B o --- k' + B1 i' (Eq. 2.29) This motion equation in the rotating coordinate system (x', y', z') is formally identical to (Eq. 2.11) describing the free precession under influence of a static magnetic field. The solution represents precession of the magnetization in the rotating frame around the effective field direction B eff with precession frequency eff = B eff (Figure 2-4). The z'-component of the effective magnetic field becomes smaller. Therefore the angle of the precession can inreaese as the frequency of the magnetic field approaches the Larmor frequency of the spins o = B o. At resonance = o = B o, the z-component of B eff disappears, so that B eff = B 1 = B 1 i'. The angle becomes 90 and the rotation of the magnetization with frequency eff = 1 = B 1 takes place in the y'z'-plane orthogonal to B 1. This motion is often called RF precession or nutation. As B o is usually about 10 6 times larger than B 1, the resonance is really sharp. As soon as differs only slightly from o, B eff will be almost equal to B o and the radio frequency field B 1 has no marked influence on the magnetization. Only if the influence of B 1 will become relevant 10 o 6 o and induce rotation of the magnetization away from the field direction. z` B o B eff z` B eff M y` y` B 1 x` x` Figure 2-4 Left: Effective field; Right: Motion of magnetic moments in a rotating coordinate system. 12 Institute for Biomedical Engineering UZH / ETHZ
17 Nuclear Magnetic Resonance Rotating Frame z Laboratory Frame z x RF =B 1 y x RF =B 1 y Figure 2-5 Precession of magnetization under influence of a stationary magnetic field B o and an oscillating field B 1 during a 90 pulse starting at equilibrium. The trajectory in the laboratory system is plotted in the right and the motion in the rotating frame in the left graph. Assuming magnetization M is the z'-direction, a sinus-like alternating field B x (t) 2 B 1 cos(t) of frequency o an duration T p is switched on, will rotate magnetization M over an angle in the y'z'-plane with: = 1 T p = B 1 T p (Eq. 2.30) With a carefully selected amplitude B 1 and time T P of this radio-frequency pulse, it becomes possible to induce nutation in the rotating frame by a well-defined angle, e.g. over = 90 or =180. The excitation pulse is therefore, called a 90 pulse or a 180 pulse. In the laboratory frame this motion is superimposed onto the precession with frequency o >> 1, so that the tip of the magnetization M describes a spiral-shaped trajectory about the field direction on a spherical surface (Figure 2-5). After switching off a 90 pulse, the magnetization will precess according to solution (Eq. 2.16) in the xy-plane of the laboratory system. This induces a sinus-shaped signal in the coil, which can be used as a receiver coil after the excitation pulse. Due to relaxation effects this signal will decay with time con- * stant T since usually T, inducing in the coil a so-called Free Induction 2 2 «T2 Decay (FID) signal. This signal is experimentally mixed with excitation frequency (chapter 5). With an exact match of the excitation frequency with the Larmor frequency o, the result is a signal which decays with time constant * T 2 (Figure 2-6a). If the excitation frequency is close enough for resonance effects but does not exactly coincide with the precession frequency o of the spins, an interference pattern will be observed. This pattern is modulated by the frequency difference o and with the amplitude, decreasing with T 2 * (Figure 2-6b). Magnetic Resonance Imaging and Spectroscopy 13
18 2 Spin echo a b c Signal t Signal t FT-Signal Figure 2-6 Time signal for = (a) and for (b) and the corresponding frequency spectrum (c). Fourier transformation of this interference pattern leads to the frequency spectrum (Figure 2-6c), from which resonance lines can be identified and by which the spatial distribution of signal parts can be identified. With constant sample temperature and constant magnetic field, the spectral intensity represents a measure for the magnetization M o of the sample, from which nuclear spin densities may be derived. However, to determine relaxation times T 1 and T 2, more sophisticated methods of nuclear magnetic resonance have to be applied, in which signals are analyzed after several consecutive excitation pulses (see Section 2.5 and chapter 4). 2.5 SPIN ECHO In most imaging procedures, instead of measuring FID signals, so-called spin echoes are evoked, using extra excitation pulses. Furthermore, series of these echoes allow determination of relaxation time T 2 of a sample. In the 1950s, HAHN developed the following method: First the magnetization is rotated from its equilibrium position (z-direction) by a 90 pulse into the y-direction of the rotating frame (Figure 2-7 and Figure 2-8). Due to transversal relaxation and field inhomogeneities the magnetization that precesses in the x'y'-plane will dephase as the individual spins rotate with slightly different Larmor frequencies (T 2 relaxation). As a consequence, the amount M decreases. After a time interval T i an 180 pulse, the so-called spin echo pulse, rotates the spins about the x'-axis from positive y'- direction to negative y'-direction. The spins that were processing faster before the pulse (due to field inhomogeneities), still precess faster, so that the spins will approach each other again. At time T E = 2T i all spins are in phase again in the negative y'-direction. T E is called the echo time. The rephasing of spins generates the reappearance of an FID signal, the so-called spin echo, in the detection coil with its maximum amplitude at time T E. After reaching this maximum again a normal free induction decay follows, because the spins will dephase again 14 Institute for Biomedical Engineering UZH / ETHZ
19 Nuclear Magnetic Resonance. 90 o 180 o RF t Signal e t * T 2 e t T 2 t T E /2 T E /2 Figure 2-7 Excitation pulse sequence and signals in a spin-echo experiment. This spin echo signal is phase-shifted by 180 with respect to the original FID signal, since the rephasing of spins takes place in the negative y'-direction; echo signals without phase shift may be generated if the phase of the 180 pulse is shifted by 90, so that the magnetization rotates around the y'-axis. The amplitude of the spin echo signal is in both cases equal to that of the first FID signal if no spin-spin relaxation (T 2 relaxation) occurs. 90 o 180 o z' y' x' Figure 2-8 Effect of a spin echo pulse: dephasing after the excitation pulse (upper row), followed by an echo pulse (middle) and the rephasing (lower row). Magnetic Resonance Imaging and Spectroscopy 15
20 2 Spin echo However, the transversal magnetization undergoes during the time interval T E a stochastic dephasing of spins (T 2 relaxation), which is not reversed by the 180 pulse. Consequently the signal amplitude decays with T 2 (Figure 2-7). More 180 pulses may be applied to generate further spin echoes. A least squares fit procedure allows an estimation of T 2 from the amplitudes of these signals (chapter 4). 16 Institute for Biomedical Engineering UZH / ETHZ
21 Image Formation 3 IMAGE FORMATION Each MR technique considered up to now, detects a signal originating from all examined nuclei in the total sample volume. A spatial assignment of signal parts within the sample was not performed. If in addition to the static magnetic field B o a magnetic field, whose field strength linearly depends on the spatial coordinates, is produced by gradient coils, the resonance frequency within the sample also becomes dependent on the spatial coordinates of the volume element under investigation. Based on this principle many different techniques for producing two- and three-dimensional images have been developed. The first proposition was made by DAMADIAN in The first technique actually was realized by LAUTERBUR in 1973 and was able to measure a number of onedimensional projections in different orientations within a plane of an object. From these projections the image was reconstructed by procedures used in computer tomography. First two techniques for the acquisition of MR slice images are presented; then the widely used method based on Fouriertransformation technique is discussed. To facilitate discussion the following conventions are used: qüé=åççêçáå~íé=ëóëíéã=áë=åüçëéå=áå=íüé=ï~ó=íü~í=íüé=ëí~íáå=ã~öåéíáå=ñáéäç= _ ç =éçáåíë=áå=íüé=òjçáêéåíáçåw=_ ç =Z=EMI=MI=_ ç FK qüé=~ççáíáçå~ä=ñáéäçë=åêé~íéç=äó=íüé=öê~çáéåí=åçáäë=éçáåí=áå=òjçáêéåíáçåi=ákék= G = G x G y G z = B z x B z y B z z cçê=êé~ëçåë=çñ=ëáãéäáåáíó=éçëáíáçå=çéééåçéåí=~ççáíáçå~ä=ñáéäçë=çñ=íüé=âáåç B z B === xg x x z B = I== yg ~åç= x z y z = zg y z = z z ~êé=çéåçíéç=d ñ I=d ó =~åç=d ò K päáåé=áã~öéë=~êé=áã~öéë=çñ=éä~åéë=ééêééåçáåìä~ê=íç=íüé=òj~ñáëk=líüéê=ëäáåé= çêáéåí~íáçåë=å~å=äé=åêé~íéç=äó=ééêãìí~íáçåë=çñ=íüé=íáãé=ëéèìéååé=çñ= Öê~ÇáÉåí=ÑáÉäÇë=d ñ I=d ó =~åç=d ò K qüé=ê~çáçjñêéèìéååó=åçáäë=~êé=~ééäáéç=áå=~=ï~ó=íü~í=íüé=éêççìåéç= ~äíéêå~íáåö=ã~öåéíáå=ñáéäçë=~êé=ééêééåçáåìä~ê=íç=íüé=ëí~íáå=ã~öåéíáå=ñáéäçi= ákék=íü~í=_ N EíFZE_ ñ EíFI=_ ó EíFI=MFK qüé=íéêã=éä~åé=áë=ìëéç=ñçê=ëäáåéë=çñ=íüêééjçáãéåëáçå~ä=îçäìãé=éäéãéåíëx= ëáãáä~êäó=îçäìãé=éäéãéåíë=~äçåö=~=äáåé=~êé=ëüçêíäó=çéåçíéç=~ë=äáåéëk Magnetic Resonance Imaging and Spectroscopy 17
22 3 Selective excitation and induction of spin echoes 3.1 SELECTIVE EXCITATION AND INDUCTION OF SPIN ECHOES The first step of this technique is the so-called excitation phase of time duration (Figure 3-1). A small-band 90 o radio frequency excitation pulse in combination with a gradient G z is applied to the volume. The resonance condition (Eq. 2.9) is only fulfilled for a thin slice z of the sample, orthogonal to the z-axis at position z o (Figure 3-2), so that only the magnetic moments of this slice are excited. This sequence part is called slice-selective excitation. G z G y G x G z t RF t Signal t T I T I T s T W Figure 3-1 Timing diagram for slice selective excitation of a slice, orthogonal to the z-axis and production of spin echoes of a line in this slice. B o ; G z G y z z y x y Figure 3-2 Selective excitation of a slice, orthogonal to the z-axis and selective production of spin echoes within this slice under G y. 18 Institute for Biomedical Engineering UZH / ETHZ
23 Image Formation After an immediately following second phase, an FID appears under influence of a gradient G y, that is interrupted at T P +T I by a small band spin echo pulse (180 o pulse) that only acts on the magnetization of a line of width y at position y o. The spins within this line are refocused to precession in phase; a spin echo signal is built up (Figure 3-1). The decay of this echo signal is finally registered spatially selective by influence of G x. After a Fourier transform the signal contributions can be identified according to their frequency and thus be attributed to the volume elements along the line. By modification of the gradient strengths G y and G z or the frequency spectrum of the excitation pulses, signals from each line of any slice of the object may be acquired. Imaging a slice in n n pixels requires n imaging cycles, by which the signal of each volume element is acquired once. (Example: n = 256, T R =1second, imaging time = 4.3 minutes). 3.2 PROJECTION RECONSTRUCTION TECHNIQUE The first step of the most basic projection reconstruction technique for producing two-dimensional slice images of three-dimensional objects is a slice selective excitation, as explained in the previous chapter. After switching off G z a resulting gradient G xy is applied by addition of vector components G x and G y, with azimuth angle, where is determined by the relative strengths of G x and G y by tan = G y G x. G x G x1 G x2 G x3 t G y G y1 G y2 G y3 t G xy = G x +G y G xy1 G xy2 G xy3 t RF G z G t z G z t t Signal t T R T R T R Figure 3-3 Projection on frequency axis of signal information from lines within the excited slice and orthogonal to G xy. Magnetic Resonance Imaging and Spectroscopy 19
24 3 Projection reconstruction technique B o, G z Signal z G xy x y Figure 3-4 Time diagram for creating an image of a slice with the simple projection reconstruction technique. Therefore, spins along each line in the slice, perpendicular to G xy, show the same resonance frequencies. From the frequency spectrum of the resulting FID signal, which is caused by superposition of signals from all lines in the slice, the contributions from the different lines can be extracted; they represent projections under the angle ( - 90 ) of the densities of the spins, inducing signals along each line, orthogonal to G xy. By changing the relative field strength of G x and G y with G x +G y has to be constant (Figure 3-4, 3-5), n projections under different angles i -90 (i=1...n) that are needed for reconstruction of an image can be obtained. The reconstruction results from algorithms that closely resemble X-ray (computer-) tomography algorithms. The imaging time, is similar to the time needed for selective excitation and induction of spin echoes, since the n projections are acquired in n imaging cycles. However, since in every cycle the signals from all volume elements of the slice are acquired, an averaging over n cycles occurs. This results in an improvement of the signal-to-noise ratio by the square root of n or a reduction of image noise by this factor respectively. Figure 3-5 Projections of line signals on the frequency axis under different angles. 20 Institute for Biomedical Engineering UZH / ETHZ
25 Signal RF Image Formation For three-dimensional imaging the signals from n parallel cross sections are projected on to the frequency axis in each cycle. In order to obtain this, the entire object is excited non-selectively. Under influence of a field gradient G=(G x,g y,g z ) in a direction characterized by azimuth and polar angles and the signal of the planes orthogonal to G are recorded. The signal contributions in every frequency interval are induced by the spins in one of these planes (slices). From n n of such projections of slices under n different angles i and j with i,j =1...n the three-dimensional image matrix can be reconstructed. 3.3 FOURIER IMAGING The first Fourier imaging technique was proposed by KUMAR, WELTI and ERNST in The combined advantage of high sensitivity due to repeated excitation and signal detection of the slice or the total volume of interest is combined with an easy image reconstruction by a multi-dimensional Fourier transformation. G z G y G x G z t t t t T p T y T x T w T R Figure 3-6 Time diagram for simple Fourier imaging of a slice. The gradient G y is increased successively from cycle to cycle. Magnetic Resonance Imaging and Spectroscopy 21
26 3 Fourier imaging For the acquisition of a two-dimensional image of a particular slice a selective slice excitation under control of a field gradient G z is performed (Figure 3-6). Before the FID signal in the interval T x is recorded under gradient G x, a socalled phase encoding gradient G y is applied during a time interval T y accomplishes that the spins precess with different frequencies, y ( B0 yg y ) according to their y-coordinate (Figure 3-7). After switching off G y at the end of the time interval T y the spins show different phases accord to their y-coordinate. Thus, the y-coordinates are encoded into the phases. During the consecutive registration of the signals under a gradient G x during time interval T x the x-coordinate is encoded in the Larmor frequency B xg ). ( 0 x To get a deeper understanding of the technique, we calculate the signal ij (t) of the magnetization of a volume element (i,j) at position (x i, y j ) within the plane (x, y, z o ). It is proportional to the density *(x i, y j, z o ) of excited spins at this location. The frequency of the signal ij (t) is determined by the local field strength at the location with coordinate x i, during the registration of the signal, while the phase at time T y is determined by the field strength at the location with coordinate y j during the phase encoding interval. Thus x ij t x i y j z o e i x it + y j (Eq. 3.1) Demodulation with carrier frequency x i = G x x i y j = y j T y = G y y j T y Inserting this in (Eq. 3.1) yields o = B o yields: ij t x i y j z o e i G xx i t + G y y j T y (Eq. 3.2) Digital sampling of the signal in k =1... N channels at time t =T xk for an experiment with certain phase encoding gradient G yl results in sampled values of ij kl = ij x i y j T xk G yl x i y j z o e i G xx i T xk + G y y j T y (Eq. 3.3) After definition of the wave vector k with its components k xk = G x T xk and k yl = G yl T y, the FID signal of the entire slice can be calculated by summation of signals over the slice: s kl = sk xk k yl = ij kl i j j i x i y j z o e ik xkx i + k yl y j (Eq. 3.4) 22 Institute for Biomedical Engineering UZH / ETHZ
27 Image Formation G y Signal T y G x T x ~ x B=B o + y*g y B=B o + x*g x Figure 3-7 Phase encoding (left) and frequency encoding (right) of the Fourier imaging sequence. +/y k y = G y T y 0 k x = G x T x -/y -/x 0 +/x Figure 3-8 Schematic representation of the k-space. For the reconstruction of a complete image, N such experiments with l =1...N, different gradient strengths G yl are required. In each experiment the FID or Magnetic Resonance Imaging and Spectroscopy 23
28 3 Fourier imaging preferably, an echo signal is sampled as a function of T x in N channels T xk, so that finally, a matrix with N N data points is obtained. The density * (x i,y j,z o ) of the spins that induce signals can be derived from a two-dimensional Fourier transform of this data matrix: x i y j z o s kl e ik xkx i + k yl y j k l s kl e ik xkx i ik yl y j = e l k (Eq. 3.5) As it is evident from the second part of (Eq. 3.5) this two-dimensional Fourier transform can be reduced to two series each of N one-dimensional transforms, that can be performed successively. The space spanned by k x and k y is called k-space (Figure 3-8). The imaging time and the signal-to-noise ratio, which can be achieved in this time, are almost identical to those of the projection reconstruction technique. The advantages of the Fourier imaging technique are a considerably lower sensitivity to inhomogeneities of the static magnetic field, to non-linearities of gradient fields and to the eddy currents induced in the metal parts by switching of the gradient fields, as well as in the straight forward reconstruction. For creating three-dimensional images, the entire sample is excited nonselectively. To allow spatial attribution of the signals also in the third dimension, a second period of duration T z, is fitted in after the first phase encoding period T y with G yl, during which the gradients G zm perform the phase encoding in the third dimension. The image reconstruction from the data, recorded under gradient G x is achieved by three-dimensional Fourier transform. Slice Profile S(z) z ( z) ( Bo z Gz ) Puls-Profile S() Figure 3-9 Slice profile in function of the pulse profile and the selection gradient. 24 Institute for Biomedical Engineering UZH / ETHZ
29 Image Formation The slice selection is achieved by an excitation pulse with low bandwidth in combination with a gradient. The resulting slice profile is directly related to the frequency spectrum of the excitation pulse, the width of the resonance line and the gradient field strength (Figure 3-9). In practice most often pulses shaped by sinc or Gaussian functions are applied. Application of strong gradients may reduce the influence of the width of the resonance line by a great extent, so that for most applications a sufficiently well shaped slice results. 3.4 SPIN ECHO AND GRADIENT ECHO IMAGING If we carefully consider the pulse sequence depicted in Figure 3-6 we realize that by that sequence only the right half of k-space with k x > 0 (Figure 3-8) is sampled, since G x > 0 and T x >0. For imaging of non-moving structures this usually is no serious problem since the k-space matrix is Hermitian and missing data may be completed. However for living subjects this is no longer true, because motion (beating heart, blood flow, moving intestine etc.) induces phase shifts for the MRI signal (chapter 6.1). Thus severe image artifacts may occur. To improve image quality modified pulse sequences are applied which allow the assessment of the entire k-space. These sequences are based on spin echo or gradient echo techniques. Figure 3-10 shows the timing diagram and the corresponding motion in k-space of a sequence for spin echo imaging. In the first step slice selective excitation is performed. The slight dephasing of the spin during the excitation which is induced by the slice selection gradient is compensated for by the short inversion period of the gradient immediately after the excitation. The first step is followed immediately by a phase encoding period with a gradient G y. Simultaneous with G y a gradient G x is applied. The two gradients induce a motion from position A to B in k-space (as described in Figure 3-10). G z t k G T y y y G y t B G x t k x G x RF A t Signal t A T E /2 T E /2 t B t C t D t C D Figure 3-10 Time diagram of a spin echo imaging sequence (left) and corresponding path in the k-space (right). Magnetic Resonance Imaging and Spectroscopy 25
30 3 Spin echo and gradient echo imaging The subsequent slice selective echo pulse gives rise to motion from B to C. Then the subsequent echo signal is sampled along C to D, while another gradient G x is on. Further rows in k-space are sampled with identical experiments, but with different phase encoding gradients G y. However, this refocusing is often generated by a proper inversion of the read-out gradient G x, as shown in Figure The excitation sequence of the spin echo mode consists of at least two sequential RF pulses (Figure 2-7). The first 90 pulse forces the spins to precess in the xy-plane. The spins then dephase with time constant T 2, so (Figure 2-6) that the FID signal disappears. In clinical applications, so-called gradient echo sequences are much more often used, instead of spin echo sequences. The corresponding pulse sequence together with k-space motion is depicted in Figure After slice selective excitation the phase encoding gradient G y together with a negative gradient G x cause motion from A to B. During that period the signal decays very fast ( T 2 relaxation) because of the defocussing effect of G x. By inversion of G x a refocussing of the magnetization is achieved, so that a so-called gradient echo signal occurs. This gradient echo signal is sampled moving along B to C in k-space by application of the positive gradient G x. Gradient echo techniques hold the advantage of not using energy intensive 180 pulses. Therefore, the specific absorption rate (SAR) of radio wave energy and thus tissue heating may be considerably reduced. They also open up the possibility to apply excitation pulses causing excitation flip angles 90. Indeed, for < 90 the precessing component of the magnetization is smaller inducing a lower signal and therefore, a lower signal-to-noise ratio in the image. However, a certain part of the magnetization remains in direction of the main magnetic field and can immediately be used for subsequent experiments without any waiting period for regrowth of magnetization by T 1 relaxation between the experiments. k G T y y y G z t G y t B C G x t A k G T x x x RF t Signal t t A t B t C Figure 3-11 Time diagram of a gradient echo imaging sequence (left) and corresponding path in the k-space (right). 26 Institute for Biomedical Engineering UZH / ETHZ
31 Image Formation 3.5 FAST IMAGING TECHNIQUES In all imaging techniques discussed so far, the acquisition (or imaging) time, is determined by the selected spatial resolution, which directly determines the number of required experiments with different phase encoding gradients to be performed. Furthermore it is related to the repetition time T R, by which experiments are repeated. This repetition time influences the tissue contrast in the image (see Section 4.2), but also the signal-to-noise ratio, if during the acquisition due to incomplete T l relaxation only a fraction of the magnetization is available for a next excitation. These considerations lead to image acquisition times of typically 2-4 minutes per slice. The total imaging time can be reduced in several ways. The waiting time between two consecutive excitations of the same slice can be used for imaging other slices. In the multi-slice Fourier imaging technique, the rows k yk in k-space for different images are measured one after another, before returning to the next line k y(k+1) of the first slice. This way an imaging time of 2-4 minutes of typically 32 or 64 slice images can be acquired GRADIENT ECHO Further possibilities for reducing imaging time are based on reduction of the repetition time T R. In order to minimize saturation effects, excitation pulses with flip angles smaller than 90 may be applied (Section 3.4). With such pulses a part of the magnetization will remain in the field direction after each excitation, already present for the next flip. After a few experiments a pseudostationary situation is reached, so that an equal initial magnetization is available for each experiment. T1 rel sin T R TR T1 1 e 1 cose T R T 1 Figure 3-12 Relative signal intensity as a function of T R /T 1 for various excitation angles. Magnetic Resonance Imaging and Spectroscopy 27
32 3 Fast imaging techniques After 8 excitation cycles, the steady state is built up and allows high quality images. The SNR is much lower as just a fraction of the total magnetization is used. Figure 3-12 shows the signal intensity, which is a measure of the achievable signal-to-noise ratio in the image as a function of T R /T 1 for various excitation angles. For known T 1 and fixed repetition time T R the flip angle for the best signal intensity is calculated from the Ernst relation T R T 1 cos opt = e (Eq. 3.6) For estimation of the optimized flip angle an average value for T 1 of the examined tissue has to be filled in. The technique with reduced flip angles can be combined with the multislice technique. It also may be expanded to very fast imaging techniques, where additional problems remain to be solved: If T R is reduced to the order of T 2 (T R < 100ms), the precession of the previous excitation cycle has still not fully decayed at the moment of the next excitation. During signal recording interference with signals of previous imaging cycles occurs, which appears as extensive image artifacts. To suppress such interference, additional gradients are applied between consecutive experiments to destroy magnetization components from previous experiments. Several possibilities are proposed and discussed for this purpose; dependent on the chosen techniques these appear under different names like FFE, TFE, (FLASH, FISP, GRASS, RARE). Such techniques basically allow registration of images in less than a second (sub-second imaging) ECHO PLANAR IMAGING Other ultra-fast imaging techniques are Echo Planar Imaging (EPI) and spiral imaging. EPI is based on an idea of MANSFIELD and co-workers and has been published many years ago. In the extreme case the technique of Echo Planar Imaging (EPI), also based on two-dimensional Fourier imaging, allows acquisition of images with only one excitation. After excitation and optimal 180 spin echo pulse (Figure 3-13) a large number of gradient echoes (see Section 4.2) is induced by repeated inversion of the read-out gradient. The echo signals are phase-encoded by applying shorttime phase encoding gradients, so that the entire k-space is traversed (y-direction in Figure 3-14). After recording of a sufficient number of echo signals, the total k-space is covered and the image can be reconstructed by two-dimensional Fourier transformation. The k-space sampling procedure is visualized in Figure A similar sequence without an RF refocusing pulse allows fast acquisition of gradient recalled FID signals. 28 Institute for Biomedical Engineering UZH / ETHZ
33 Image Formation Phase Encod. G y t Frequency Encod. G x t 180 o 90 o RF t Signal t t A t B t C t D t E t F Figure 3-13 Spinecho EPI sequence with sampling of the spin echo signal. k G T y y y B A k G T x x x E C F D Figure 3-14 k-space trajectory for SE EPI sequence SPIRAL IMAGING Spiral Imaging is based on sampling of the k-space along a spiral starting in the center of the k-space. (Figure 3-15 right). This is achieved by a sinusoidal modulation of the two gradients G x and G y with slowly growing amplitude starting from zero (Figure 3-15 left). The signal sampling is performed with constant frequency, so that sampling points occur with constant spacing on the spiral. For the reconstruction, first interpolation of the data to a rectangular grid is Magnetic Resonance Imaging and Spectroscopy 29
34 3 Fast imaging techniques performed, and finally, the image can be reconstructed through a two-dimensional Fourier transformation. 90 o t G x t G y t Signal t Figure 3-15 Acquisition scheme (left) and k-space trajectory (right) for spiral imaging PARALLEL IMAGING In all imaging techniques, discussed so far the k-space is sampled sequentially. Imaging can become faster by using gradient systems with higher performance allowing faster motion in k-space. However gradients are today so powerful that the patients own electrophysiology becomes a natural limit. Incident neuronal stimulation can occur by use of strong gradients as they are available on powerful commercial scanners. SMASH (SiMultaneous Acquisition of Spatial Harmonics) and SENSE (SEN- Sitivity Encoding) are two novel techniques developed for the purpose of speeding up imaging on conventional MRI scanners. The basic idea of the two techniques is to use receiver coils with distinct spatial sensitivities for achieving signal localization partly in parallel. Surface coils, in particular, have strongly inhomogeneous sensitivities. Therefore, data acquired in parallel with distinctly positioned surface coils contains distinct information about the spatial distribution of signal sources in the sample. By taking this information into account, gradient encoding can partly be made redundant and encoding steps may be skipped. Thereby scan time is reduced, the maximum reduction factor is determined by the number of used coils. While for SMASH receiver coil arrays are needed with a well defined overall sinusoidal spatial sensitivity, with SENSE considerable savings in scan time can be achieved by use of simple coil arrays. This gives much more flexibility in daily applications of the procedure. The two techniques are called parallel imaging techniques, because data of several lines in k-space is sampled parallel (simultaneously) by the dif- 30 Institute for Biomedical Engineering UZH / ETHZ
35 Image Formation ferent coils. To understand the basic idea of SENSE we discuss the most simple case of a scan time reduction by a factor of two by application of a surface coil array with two coils. Leaving the field-of-view unchanged reduction of the image acquisition time is achieved by sampling only each n th line in k-space. Therefore, for conventional imaging, folding or aliasing artifacts occur in the image in the phase encoding direction (Figure 3-16). For n equal 2, the pixel value P(x,y) of the image (right image in the figure) is composed of two contributions S(x,y), S(x,y+y) from the positions (x,y) and (x,y+y) of the object (left image in Figure 3-16), y representing the foldover distance. Expressing the acquired signal in a formula we obtain: Pxy = Sxy Cxy + Sxy + ycxy + y (Eq. 3.7) where S(x,y) and S(x,y+y) stand for the signal, generated by the volume elements of the object at the two respective positions, P(x,y) for the signal value of pixel (x,y). in the image, and C(x,y) and C(x,y+y) for the coil sensitivity at the respective positions. If now two receiver coils are applied with different sensitivities C 1 and C 2 for the same image position (x,y) they record different signal values P 1 (x,y) and P 2 (x,y): P 1 xy = Sxy C 1 xy + Sxy + yc 1 xy + y P 2 xy = Sxy C 2 xy + Sxy + yc 2 xy + y (Eq. 3.8) If the coil sensitivities C 1 (x,y) and C 2 (x,y) are known from a previous calibration procedure, the unaliased image can be reconstructed by solving the two equations for S(x,y) and S(x,y+y). For a further reduction of the examination time, more coils can be applied; with arrays of 5-6 coils reduction factors of 3-4 may typically be achieved. However, the reconstruction procedure becomes more demanding and new reconstruction algorithms have to be applied. Magnetic Resonance Imaging and Spectroscopy 31
36 3 Fast imaging techniques o (x,y) o (x,y) o (x,y+y) Figure 3-16 Phantom (left) and its image (right) acquired with reduced line sampling in k-space. By sampling of every second line in the phase encoding direction only aliasing occurs. One of the strengths of SENSE is that it may be combined with virtually all standard MR imaging modalities. In combination with echo planar or spiral imaging real time image acquisition, a temporal resolution down to ms becomes possible. However more efforts are needed, mainly for the initial determination of coil sensitivities and for some extra calculation in generating images from raw data. Image reconstruction has particularly been optimized for maximum signal-to-noise ratio (SNR) of the resulting image. For sensitivity, encoding as for all fast imaging techniques SNR is an important issue. Theoretical analysis yields that in SENSE the loss in SNR caused by reducing scan time is at least equivalent to that faced when scan time is reduced by using stronger gradients. However, additional noise enhancement may occur when the geometrical relations of the coils used are not ideal. Sensitivity encoding is mainly called for when acquisition cannot be speed up by conventional means and some loss in SNR is acceptable. In this respect, the so-called 3D techniques form a highly interesting class of applications. 3D techniques are for instance used to image the blood vessels of the abdomen, neck, brain and all-important coronary arteries supplying the heart. The basic SNR of 3D techniques is relatively high, while the need for threedimensional gradient encoding makes conventional 3D scans rather long. Therefor,e these methods are excellent fields of application for sensitivity encoding. The scan time reduction is also significant for neuroscience where the so-called event-related functional brain imaging is receiving increasing attention. In experiments of this kind, the dynamics of brain activation in response to a single stimulus is investigated, requiring highest temporal resolution. Another promising 3D application is Spectroscopic Imaging (SI) (see chapter 7) where in a brain slice the spectral composition of the resonance signal is resolved in addition to the two spatial dimensions. This method enables highly intriguing studies of the distribution of brain metabolites. 32 Institute for Biomedical Engineering UZH / ETHZ
37 Excitation Sequences and Image Contrast 4 EXCITATION SEQUENCES AND IMAGE CONTRAST The analysis of FID signals or spin echoes following an excitation pulse offers the possibility to determine relative spin densities in a measured sample provided that the spins were in thermodynamic equilibrium before the excitation. The time interval between the excitation pulse and 99% recovery of the longitudinal component is about 5T 1. If a second pulse is given at time T R <5T 1, only the recovered part of magnetization flips to the xy-plane. Consequently a reduced signal amplitude occurs. Since different types of tissue are characterized by different relaxation times T 1 (Figure 4-1), the amount of recovered magnetization and thus the brightness of the tissue and the image contrast become dependent on T 1 and on the repetition time T R. Further contrast relations may be accomplished by the use of other excitation sequences with several excitation pulses, i.e. on the excitation mode. Signal amplitudes, and therefore, image contrast on one hand depend on the experimental parameters such as selected excitation mode for repetitive excitation of the same spins and from time intervals between several pulses. On the other hand, signal amplitudes and image contrast are determined by physical or physiological characteristics of the tissue, such as spin density and relaxation times T 1 and T 2 ; T 1 and T 2 in turn depend on magnetic field strength Water Fat Muscle Grey M. White M. Liver Blood Bone A Density T1 [ms] 10*T2 [ms] Figure 4-1 Relative spin density and relaxation time T 1 and T 2 (scaled up by a factor of 10) for several tissues at 1.5 Tesla compared to X-ray absorption coefficients A. Magnetic Resonance Imaging and Spectroscopy 33
38 4 The partial saturation mode: Image Contrast Experience has shown that soft tissue contrast in MR images is much higher than in X-ray computer tomograms or ultrasound echograms. This is because the quantities measured in MR, such as 1 H spin density or especially relaxation times T 1 and T 2, differ much more in different tissues than X-ray absorption coefficients or Ultrasound scattering coefficients (Figure 4-1). 4.1 THE PARTIAL SATURATION MODE: IMAGE CONTRAST Partial saturation occurs, if a sample is repeatedly excited by 90 o pulses with repetition time T R (Figure 4-2). The signal amplitude PS and thus also the pixel brightness or pixel intensity I of a volume element are according to simple argumentation (solution of the differential equation for M z in (Eq. 2.21) with initial condition M z (t =0) = 0 ) proportional to T R T 1 PS M o 1 e (Eq. 4.1) Since the magnetization M o = M o (object)/n x n y n z of a volume element is proportional to spin density, (Eq. 4.1) may be written as T R T 1 PS T 1 T R 1 e (Eq. 4.2) 90 o 90 o RF M 1 T1 e t t Signal t T R Figure 4-2 Reduced signal intensity because of partial saturation as a consequence of repeated excitation by 90 pulses.this effect is independent of the non presented gradients. 34 Institute for Biomedical Engineering UZH / ETHZ
39 Excitation Sequences and Image Contrast The contrast C between two kinds of tissue A and B is defined as the difference in image intensity between these tissues A and B and equals C = PS A T 1A T R PS B T 1B T R T R T R T 1A T A 1 e 1B B 1 e (Eq. 4.3) If the repetition time T R >>T 1, then the signal intensity will mainly depend on ; the resulting images are therefore, 1 H spin density weighted images. The shorter T 1 is chosen, the stronger signal intensity is influenced by spin-lattice relaxation. For short T R <T 1, so-called T 1 -weighted images are obtained; tissues with short T 1 values become bright, while tissues with long T 1 become dark, since strong saturation occurs. How can the experimental parameter T R be chosen so that image contrast between the two tissues A and B becomes maximum? To answer this question the relation above (Eq. 4.3) is differentiated to T R and set equal to zero, which results in T R C = T 1A T 1B A T 1B ln T 1B T 1A B T 1A (Eq. 4.4) As described in Section 3.5, the flip angle is often reduced which leads to considerably changed image contrast. With decrease of flip angle both signal amplitude and image contrast become dependent on the choice of flip angle, which has to be included in concerning formulas. 0.8 Partial Saturation GM 0.4 GM/CSF Signal Intensities CSF WM Contrast GM/CSF WM/GM TR [ms] TR [ms] Figure 4-3 Signal intensity and image contrast for white (WM) and grey matter (GM) and cerebrospinal fluid (CSF) at 1.5 Tesla as a function of T R in the partial saturation mode. Relative spin densities are taken from Figure 4-1. Magnetic Resonance Imaging and Spectroscopy 35
40 4 The spin-echo mode: Image Contrast 4.2 THE SPIN-ECHO MODE: IMAGE CONTRAST In practice mostly spin echo and gradient echo excitation sequences are used. In spin echo sequences a refocusing of the precessing spins is induced by one or more 180 pulses, as described in Section 3.4 (Figure 3-10 on page 25). The following 180 pulse at time T E /2 inverts the y-components of the magnetization (Figure 2-8 on page 15), so that the spins rephase and generate a spin echo, of which the amplitude is reduced with respect to the preceding FID by a factor of exp T E T 2. If the pulse sequences are repeatedly applied in time intervals T R, then the amplitude SE of the induced spin echo signal becomes for T E <T R SE T 1 T 2 T E T R 1 e T R T 1 e T E T 2 (Eq. 4.5) The amplitude is dependent on the quantities, T 1, and T 2 of the spin system and on measurement parameters T R and T E. The signal amplitude increases with increase of, T 2 and T R and with decrease of T 1 and T E. If T R >>T 1 then [l - exp(-t R /T 1 )] 1 and the signal will be determined by T E and T 2. For the case T E <<T 2 follows exp(-t E /T 2 )1, so that signal dependence on T R and T 1 predominates. If the selected repetition time is short compared to the T 1 values of the tissues, a partial saturation will occur. If echo times are also short, the resulting images will be T 1 -weighted, so that tissues with short T 1 appear bright and structures with long T 1 values appear dark. Sequences with long T R and T E result in T 2 -weighted images in which structures with short T 2 values appear dark and with long T 2 values appear bright. Proton density weighted images are obtained with long T R and short T E. Typical values for T R and T E are 2000ms / 10ms for proton density weighted, 500ms /10ms for T 1 -weighted, and 2000ms /100ms for T 2 -weighted images, which leads to acquisition times of 2 to 8 minutes for a conventional 256 x 256 pixel image. Figure 4-4 shows signal amplitudes and contrast C of two tissues A and B calculated as T R T R T 1A T 1B C A 1 e B 1 e (Eq. 4.6) for white and grey matter (T 2 =80ms and 100ms) and CSF (T 2 =145ms). At T R = 2s and T E = 60 ms contrast inversion occurs between grey matter and CSF. An extension of the spin echo technique is the pulse sequence of CARR and PURCELL. After a 90 excitation pulse several 180 pulses at distances of T E apart are given and several spin echo signals are generated. This offers the 36 Institute for Biomedical Engineering UZH / ETHZ
41 Excitation Sequences and Image Contrast possibility to obtain a sequence of spin echo images at times T E,2T E,3T E,.. without increase in acquisition time per slice. Figure 4-5 shows images of a spin-echo sequence with echo times of 10, 50, 100, 200ms and with T R = 500ms. The inversion of the contrast (of such T 2 -weighted images) between CSF and surrounding brain tissue is clearly visible: In the first image the fluids in the ventricles, the sulci and eyes appear dark, while in the last image these are bright due to the long relaxation times. The intensity of the grey matter reduces from image to image in accordance with Figure The generation of gradient echoes by gradient inversion is explained in Figure 4-4, where the echo is the result of inversion of the read-out gradient G x. By this gradient inversion, however, only magnetization components that were dephased under influence of G x can be refocused, so that these experiments cannot be used for T 2 determinations. The advantage of gradient echo image generation is that very short echo times become possible. In combination with short repetition times and reduced flip angles for excitation this allows very short imaging times. Besides the 180 pulses are not needed, so that the risk of tissue heating by high RF load with short repetition times is avoided. The image intensities for different tissues can be calculated in a similar way as for the spin echo mode. Spin Echo GM 0.15 GM/CSF Signal Intensities WM Contrast WM/CSF WM/GM 0.05 CSF Echo Time [ms] Echo Time [ms] Figure 4-4 Signal intensity and image contrast for white (WM) and grey matter (GM) and CSF at 1.5 Tesla, as a function of echo time T E for T R = 0.5 s in a spin echo experiment. Figure 4-5 Spin echo images acquired at B o = 1.5 Tesla with T R = 0.5 s and T E =10, 50, 100 and 200 ms. Magnetic Resonance Imaging and Spectroscopy 37
42 4 The inversion recovery method 4.3 THE INVERSION RECOVERY METHOD In the used inversion recovery mode, the excitation sequence consists of two pulses, so that the measurable signal intensity becomes dependent on repetition time T R and time interval T I between the two pulses. The 180 pulse inverses the magnetization, after which it relaxes back to the original value in the direction of the static field due to spin lattice relaxation. The subsequent 90 o pulse flips the magnetization, which is available at time T I, to the xy-plane, so that in the coil an FID signal is induced. The signal is dependent on spin density, relaxation time T 1 and time constants T I and T R of the pulse sequence. The amplitude is dependent on IR T 1 T 2 T I T R 1 2e T I T e T R T 1 (Eq. 4.7) For small values of T I compared to T 1 the magnetization is still in negative z-direction at the moment of the 90 pulse. It is then flipped into the xy-plane and an FID signal is observed with its phase shifted over 180 compared to the signal that is found when the magnetization is in positive z-direction before the pulse. Since in imaging techniques in general only the amplitude of the signal and not the phase is detected, the two situations cannot be distinguished. The pixel intensity is therefore, determined only by the modulus of (Eq. 4.7) and thus is IR T 1 T 2 T I T R 1 2e T I T e T R T 1 (Eq. 4.8) Inversion Recovery WM/CSF Signal Intensities GM WM Contrast WM/GM 0.1 CSF -0.1 GM/CSF Inversion Time [ms] Inversion Time [ms] Figure 4-6 Signal intensity and image contrast for white and grey brain matter and the cerebrospinal fluid as a function of T I for T R = 0.5 with inversion recovery excitation. 38 Institute for Biomedical Engineering UZH / ETHZ
43 Excitation Sequences and Image Contrast The image contrast C being the difference in image intensity between two tissues A and B becomes T I T R T C 1A T 1A T A 1 2e + e 1B T 1B B 1 2e + e T I T R (Eq. 4.9) If a repetition time is chosen T R >5T 1, then the magnetization will recover to its equilibrium value M o, before each excitation. The signal amplitude becomes dependent only on the spin density and the ratio T I /T 1. If in addition the two pulses of a sequence are selected to follow close after each other (T I 0), an almost pure spin density image will result, which shows poor tissue contrast. The contrast will increase with increasing T I. The signal amplitudes and image contrast for the example of grey and white matter and CSF are given in Figure 4-7 as function of T I. Because of the phase-insensitive processing of the signals (modulus images) tissues with different relaxation times may appear in the same shade of grey in images. If the 90 o pulse is given exactly at the moment where the magnetization passes through zero, which is according to (Eq. 4.7) at time T R T T I = T 1 1 ln2 ln e + 1 (Eq. 4.10) then no FID signal will occur. The corresponding tissue appears dark in the image. Furthermore, contrast inversion may occur for similar reasons, if increase of T 1 makes tissues dark that appeared bright compared to neighboring structures and vice versa. Drastic changes of contrast can be seen between a relative small range of the inversion time -for the choosen parameters between 100 and 200 ms. Minimal contrast (Grey matter/csf) is clearly demonstrated in the third image of Figure 4-7. Minimal contrast of Grey and White matter is demonstrated in the left image of Figure 4-7 in accordance to the contrast curve of Figure 4-6 at T I equal 50ms. Figure 4-7 Images acquired with the inversion recovery method. T R =0.5s and T I =100,140,160 and 180ms (1.5 Tesla). Magnetic Resonance Imaging and Spectroscopy 39
44 4 The inversion recovery method 40 Institute for Biomedical Engineering UZH / ETHZ
45 Instrumentation 5 INSTRUMENTATION An MRI scanner consists of three subsystems: the magnet with gradient coils and radio-frequency coils and the computer controlled patient support, the MR spectrometer with RF units and waveform generator, and finally, the computer system with operator's console, which also contains the image reconstruction and display units. Figure 5-1 shows the block diagram of a typical scanner. 5.1 MAGNETS The central part of the MRI scanner is the magnet system, which generates the static magnetic field B o. In the first prototypes of MRI scanners magnets with resistive Helmholtz coils were used, that needed electrical feeds of up to 80kW and could produce fluxes of 0.08 to 0.2Tesla. With special iron walling, which at the same time reduced the stray field, these could be raised to 0.25Tesla. Figure 5-1 Block diagram of a MRI scanner Magnetic Resonance Imaging and Spectroscopy 41
46 5 Magnets TABLE 5-1 Typical specifications for today's actively shielded superconductive whole-body MR magnets. Magnetic field strength 1.5 ( - 3.0) Tesla 5 mtesla line axial 3.9 m 5 mtesla line radial 2.5 m Homogeneity (peak-peak) [10cm] 0.1 ppm [25 cm] 1.5 ppm [50 cm] 5.0 ppm Weight 3500 kg Stability 0.1 ppm/h Gradient Strength 30 mt/m - 80mT/m Slew Rate 150 mt/m/ms Rise Time 0.2 ms Radiofrequency Power kw Patient Bore 60 cm Capacity He 1400 l Boil-off Liquid He 0.04 l/h Today, however, mostly superconductive systems with fluxes of 0.5 to 3.0Tesla are used, with high field stability and homogeneity and low energy consumption. After start-up only cooling liquid and eventually a minimum current for normally conductive shimming coils to compensate for field inhomogeneities are used. The supply of a magnet with liquid helium for cooling hardly causes any problems for the new generation of scanners with cryo-generators Higher field strengths imply an increasing population difference between energy levels, which leads to better signal-to-noise ratios or alternatively to shorter imaging acquisition times ratios with equal signal-to-noise ratio. While with resistive magnets, for imaging 16 slices, acquisition times of up to 1 hour were needed, similar measurements are possible in less than 1 minutes with superconductive magnets. For this reason, almost exclusively superconductive magnets are used for clinical applications nowadays. The higher price is compensated with a higher image quality and of higher number of examinations attainable. On the other hand, site planning needs more attention, since the magnets have large stray fields. However, with the new compact magnet systems with active magnetic field shielding these problems may easily be controlled. For magnetic resonance imaging with 1 H nuclei systems with magnetic fluxes of 0.5 to 3Tesla are typically available. Image contrast is slightly reduced in conventional proton images of high-field systems, since relaxation time differences between different tissues become smaller with increasing magnetic field strength. However, since signalto-noise ratio increases the contrast-to-noise ratio improves for higher field strength and for fixed acquisition time. The spatial image resolution is consid- 42 Institute for Biomedical Engineering UZH / ETHZ
47 Instrumentation erably determined by homogeneity of the static magnetic field and the strength, linearity and reproducibility of gradient fields. The homogeneity of the existing magnets should be sufficient for imaging. However, for spectroscopic investigations and for chemical shift imaging the region of high homogeneity 0.1ppm) should be extended. 5.2 GRADIENT SYSTEM The gradient-system is equipped with three independent coil-units, which induce linear magnetic gradient fields in the three spatial directions of the scanner bore. The gradient coil producing the gradient parallel to the bore -the z- gradient, is primarily a pair of Helmholtz coils with current flowing in opposite directions. Each coil consists of several windings. For garantuing a linear gradient field for a big FOV those windings are seperated in two parts. The gradients for the directions orthogonal to the bore (x- and y-gradients)consist of two coils, which are radially divided in two sectors, as depicted in (--> insert Figure 5.2 german). The currents again flow in opposite directions. Z-Gradient z x z Input Current y Input Current X-Gradient Input Current Figure 5-2 Schematics of the z- and x-gradientcoils. The gradient systems should be switched rapidly and reproducible, so that spatial decoding of signal information of an imaging cycle in all three spatial directions is very precise. This requires a switching of currents of up to 500Ampere in the gradient coils have to be switched on and off within fractions of milliseconds, which imposes strong demands on the gradient power amplifier. The resolution and imaging speed might be limited by the eddy currents induced in the tissues by switching on and off the gradient fields. The shielding effect of the eddy currents may limit penetration of additional fields into the body for a certain time after switching. Magnetic Resonance Imaging and Spectroscopy 43
48 5 Spectrometer and RF coil system 5.3 SPECTROMETER AND RF COIL SYSTEM The purpose of the spectrometer unit and the RF coil system is to excite spins and to detect signals from the spins. Excitation requires pulses of high RF field strengths. For given excitation angles amplitude and duration of the pulses may be estimated with the aid of (Eq. 2.30). In order to prevent considerable relaxation during the excitation pulses, the pulse duration should not exceed the order of 1-2ms. Consequently, an RF power of 5 to 25kW is required for whole body cases. The frequency spectrum of the pulses should be selectable with great precision, so that during excitation a well defined slice can be obtained. AD Converter Filter Main Receiver Linear Attenuator Low-Noise Preamplifier Reference 0 o 90 o Frequency Switch Box Coil + Probe Frequency Synthesizer Pulse Modulator linear Modulator Power Amplifier Waveform Generator Gradient Amplifier X-Coil Y-Coil Z-Coil Figure 5-3 RF Signal flow in the spectrometer For excitation of the spins the transmitter coil produces an alternating RF field B 1, which should be homogeneous over the total volume of interest and orthogonal to the static magnetic field B o. For imaging techniques with 1 H nuclei saddle coils are often used for the low- and mid-field range, which corresponds to resonance frequencies of below 20 to a maximum of 40MHz. For higher field strengths with resonance frequencies in the range of 60 to 130MHz resonance circles can not be produced any more, as the high capacity of the windings prodces a resonance frequency of the coil which is below the nuclear resonance frequency and thus birdcage coils are preferred. Most coils are quadrature coils, thus transmitting only one component of the RF- field (circular polarisation). For whole-body imaging the coil, which is mounted on the inner wall of the magnet bore, is switched to function as the receiving antenna after the excitation pulse. However, for images with high spatial resolution of particular body areas separate coils may be used. For image formation of the head, these are coils resembling the total body coil in a smaller dimension. They realize a 44 Institute for Biomedical Engineering UZH / ETHZ
49 Instrumentation much better filling factor and therefore show for volume elements of similar size a better S/N ratio, or reach a better spatial resolution within similar measurement time and with similar noise level. For high-resolution imaging of other organs close to the surface, like eyes, spine, heart, female breast, etc., surface coils are used for receiving the magnetic resonance signals that are specially adapted for each application. These surface coils are applied directly to the body surface at the place of interest. Surface coils expose a spatially limited sensitivity area. Since noise is mainly generated by the biological system, this limitation of the sensitivity area results in a reduction of image noise. With the existing surface coils also in routine clinical diagnostics excellent image quality is obtained. Signals received in the coil are pre-amplified and, demodulated by mixing with the excitation frequency, then digitized and transferred to the computer system for reconstruction. Usually complex signals which correspond to the x and y component in the rotating coordinate frame are sampled from which after reconstruction amplitude or phase images are computed. 5.4 THE COMPUTER SYSTEM On one hand, the computer system controls and manages the total measurement procedure including the gradient fields and the high frequency excitation pulses. On the other hand, it is used for data acquisition. In order to be able to manage all and guarantee an efficient data evaluation, nowadays computer systems of medium performance (e.g. PC with up to 4 CPU) are used. The performance of these computers is improved by supplementing them with array processors, which enable image reconstruction times of seconds or less for real time imaging. Registration of image data usually is performed in matrices of 256 x 256 or 512 x512 pixels of at least 16 bits; the desired spatial resolution for selectable slice thickness of 1 to 10 mm typically amounts for whole-body images 2x 2 mm 2 and for head images 1x1mm 2. For special investigations data can also be acquired in matrices of 1024x1024 pixels, which may result in spatial resolutions down to 0.2 x0.2 mm 2. For first survey acquisitions with fast imaging techniques often matrices of 128 x128 or 64 x l28 pixels are sufficient. Visualization of images by means of display units connected to the computer is usually done with image matrices interpolated to 512 x512 or 1024 x1024 pixels. Magnetic Resonance Imaging and Spectroscopy 45
50 5 The computer system 46 Institute for Biomedical Engineering UZH / ETHZ
51 Functional Information with MRI 6 FUNCTIONAL INFORMATION WITH MRI 6.1 BLOOD FLOW MEASUREMENT, MOTION ANALYSIS AND MR ANGIOGRAPHY Motion of nuclear spins influences the MRI signals by two different effects: On one hand, motion modifies the signal amplitudes and thus the brightness e.g. of vessels with flowing blood. On the other hand, motion along magnetic field gradients modulates the phase of the MRI signal. Often the two effects cause unwanted image artifacts; however, they also may be used for the assessment of motion of structures or organs within the human body. The two effects can be applied for quantification of heart motion and of blood flow within vessels, or for imaging of vessels (MR Angiography: MRA). Because the two effects modify either the amplitude of the signal or its phase, procedures making use of these effects are either called amplitude contrast procedures or phase contrast procedures. Amplitude contrast effects are often called inflow effects or time-of-flight effects AMPLITUDE CONTRAST EFFECTS We consider a slice of thickness z orthogonal to the z-axis and a vessel with flowing blood passing it in z-direction. The nuclei of the slice are excited repeatedly by 90 pulses with a repetition time T R. Thus the magnetization of the tissue as well as of the blood become partially saturated and recover with the corresponding relaxation time T 1 of the tissue and blood. If the blood within the vessel moves with uniform velocity v through the imaging plane between repetitive excitations, the nuclei within the slice will partially be replaced by fresh nuclei which have not been previously excited by RF pulses, because they come from outside the excited slice or volume (Figure 6-1). Vessel t=0 t=t R Saturation z Flow Flow Figure 6-1 Dependence of the signal amplitude on the flow velocity for partial saturation of blood in a vessel, passing orthogonal through the slice. Magnetic Resonance Imaging and Spectroscopy 47
52 6 Blood flow measurement, motion analysis and MR angiography Figure 6-2 Dependence of the signal amplitude on the flow velocity for partial saturation of blood in a vessel, passing orthogonal through the slice T 1 =1sec, z=1cm). The replaced spins are unsaturated and produce full signal which may be considerably enhanced compared to that of the surrounding tissue. This effect is called flow related enhancement. The amplitude of the enhanced signal flow PS depends on many factors and can be calculated by straightforward geometrical considerations according to Figure 6-1: flow PS v z TR T 1 z vt R 1 e T R T 1 + vt R (Eq. 6.1) Signal amplitudes as function of the flow velocity are plotted in Figure 6-2. Maximum signal amplitude occurs when the flow velocity v is sufficiently high so that during the interval T R all spins of the slice within the vessel are replaced by unsaturated spins, i.e. when v z/t R. Higher flow velocities do not further increase the signal amplitude. Since blood flow in most vessels is pulsatile, image acquisition often is synchronized to the heart beat, i.e. to the ECG signal which is measured during the examination (ECG triggering). If for image acquisition spin echo sequences are used, the dependence of the signal amplitude on the flow velocity becomes more complicated. In addition to the signal enhancement by the inflow of unsaturated spins, an outflow of excited spins occur between the slice selective excitation and the refocusing echo pulses. The outflowing spins are replaced by unexcited spins, which do not form an echo signal after the 180 pulse and therefore do not 48 Institute for Biomedical Engineering UZH / ETHZ
53 Functional Information with MRI contribute to the echo signal. A signal reduction occurs by a factor (1-T E v/ 2z). Considering both effects, i.e. the inflow of unsaturated spins between the repetitive excitation and the outflow between the excitation and the echo formation pulse the amplitude of the echo signal becomes flow v z TR T SE E T 1 T flow 2 = 1 TE v 2z e T E T 2 PS (Eq. 6.2) This formula is valid for v z/t R and 0 v 2z/T E. Figure 6-3 shows corresponding signal amplitudes. Figure 6-3 shows that the signal amplitude is a rather complex and non unique function of flow velocity v, of relaxation times T 1 and T 2 of the blood, and of the experimental parameters T R and T E. A quantitative analysis requires knowledge of all these parameters and of the excitation angle. Since in addition, often because of inhomogeneous velocity distribution within the voxels, a reduction or even a cancellation of the signal (signal voids, see Section 6.1.2) occurs, amplitude contrast effects are rather used for visualization of vessels (inflow angiography) or for qualitative investigation of blood flow than its quantification. Figure 6-3 Dependence of the signal amplitude on the flow velocity for spin echo excitation. Magnetic Resonance Imaging and Spectroscopy 49
54 6 Blood flow measurement, motion analysis and MR angiography Saturation effects can be applied for the assessment of motion as well. For these so-called tagging techniques, the motion of saturated spins which produce reduced signal amplitude or even no signal at all is directly followed by acquisition of time series of images. By specially designed pulse sequences highly sophisticated saturation patterns may be produced. For motion analysis of the human heart, binomial pulses are often applied, together with corresponding field gradients, in between the pulses in the imaging plane. Due to the periodic frequency spectrum a periodic pattern of stripes of saturated spins occurs. This procedure may be repeated with a field gradient orthogonal to the first one within the same imaging plane to produce a rectangular grid pattern. This pattern moves with the myocardium during the heart beat cycle. From the translation, the rotation and the deformation of the grid pattern on subsequently acquired images the motion can be derived. Figure 6-4 shows from top-left to bottom right the left ventricle at four different instants during the heart beat cycle. The bottom-left image shows maximum contraction at end diastole. Figure 6-4 Four tagged images of the left ventricle of a patient with hypertrophic cardiomyopathy at four different instants during the heart beat cycle; top-left a few milliseconds after the application of the grid, bottom-left at maximum contraction at end diastole. 50 Institute for Biomedical Engineering UZH / ETHZ
55 Functional Information with MRI PHASE CONTRAST EFFECTS If spins move between excitation and signal detection along a magnetic field gradient, then they experience a magnetic field, which changes according to their spatial coordinate. As a consequence, the magnetization will progressively precess faster if moving along a gradient or slower if moving into the opposite direction. The acquired signal shows a modified phase angle compared to stationary spins which remain at the same location and experience constant field strength. Assuming a constant flow velocity v z along a gradient G z the phase angle difference =in comparison to that of stationary magnetization can be calculated = v z G z t t dt (Eq. 6.3) The integration has to be performed over the entire time between the spin excitation and signal acquisition during which the gradient is switched on. If the time course and the amplitude of the gradient is known, from the flow velocity distribution over the vessel lumen can be determined pixelwise from phase images. t = t o + t M Blood Vessel G z t = t o M Flow v z Figure 6-5 Induction of a phase shift by motion of the magnetization along a gradient G z. Magnetic Resonance Imaging and Spectroscopy 51
56 6 Blood flow measurement, motion analysis and MR angiography Blood Vessel z y V x (z) x (V x ) y x M xy Figure 6-6 Signal voids induced by a dispersion of the phase values within the volume element. For complex flow conditions, as they may occur in human vessels with pulsatile flow or for complicated vessel structures such as bifurcations or vessel valves, accelerations and higher order motion terms may occur. Furthermore, in practical cases, vessels usually do not only run along one field gradient, but in all three space direction interacting with all three gradient components. All these effects produce additional phase shifts. Because in imaging usually spatial localization is performed on basis of phase values (see Section 3.3 to Section 3.5), misalignment of the voxel information occurs and thus severe image artifacts are introduced. Often signal voids (Figure 6-6) appear because flow velocity inhomogeneities within a volume element cause a phase dispersion and corresponding destructive interference of the signal contributions. All these artifacts may prevent quantitative analysis. 52 Institute for Biomedical Engineering UZH / ETHZ
57 Functional Information with MRI Figure 6-7 Axial flow velocity components downstream the bifurcation of the abdominal aorta into the iliacae communes of a healthy volunteer at 16 different time instants during the heart beat cycle. The plot at bottom left shows the complexity of the profile at 445ms behind the R wave of ECG in detail. For the development of sequences with high flow artifact suppression and for flow measurement we consider these flow induced phase shifts for more general situations. The phase (r,t E ) of the measured signal is given by rt E = rt T E = Brt dt 0 = T E 0 T E 0 B o r + rt Gt dt (Eq. 6.4) In this equation r(t) represents the space vector of the moving magnetization and G(t) = (G x (t), G y (t), G z (t)) encompasses all gradients acting between excitation of the magnetization and signal detection. For moving magnetization r(t) will be developed into a Taylor series leading to Magnetic Resonance Imaging and Spectroscopy 53
58 6 Blood flow measurement, motion analysis and MR angiography T E rt = B o r o T E + r o Gt dt 0 T E + v o 0 Gt tdt T E a 2 o Gt t 2 dt 0 (Eq. 6.5) T E b Gt t 3 6 o dt with r o = rt = 0 v o = dr dt 0 = a o = d 2 a dt 2 0 = b o = d 3 b dt 3 0 = vt = 0 at = 0 bt = 0 (Eq. 6.6) The first term of (Eq. 6.5) is constant and is induced by the static magnetic field. The second term comprises the phase values induced by the gradient field components which are used for spatial localization. The third term is called velocity term: Its value is determined by the velocity components of the blood or moving tissue. The next terms contain phase contributions induced by the accelerations and higher order motion terms. All terms contain scalar products of space or motion vectors with corresponding gradient moments. Since the three gradient components act during different time intervals within the image formation procedure complicated relations between motion and the induced phase values arise. From (Eq. 6.5) it can be recognized that for conventional imaging of anatomical structures flow induced phase shifts may cause severe flow related image artefacts, as the phase information is used for spatial encoding. These effects are especially unwanted in accurate vessel depiction of flow measurements. Additionally so called 'flow voids', i.e. signal annihilation due to the phase dispersion appear if there are different velocities in one single voxel appearent, causing intravoxel dephasing. These artifacts may be reduced by shortening the echo time T E. Therefore, mainly sequences using only the second part of the echo signal, are used. However, by such procedure also the integrals used for spatial localization and, therefore, spatial resolution of the image are reduced, to a lower extent, because T E is only contained linearly in 54 Institute for Biomedical Engineering UZH / ETHZ
59 Functional Information with MRI these integrals. Furthermore so-called flow compensating gradients may be added which cause the time integrals to disappear. This has to be done in such way that the constant terms inducing spatial encoding do not disappear (Figure 6-7). Thereby the velocity induced phase shifts disappear and with them all artifacts caused by that term. Basically, contributions of higher order terms may be suppressed by such procedure; however because more time is needed for the additional gradients, longer T E occur thus destroying the benefits of short echo times. Therefore practical considerations make it generally impractical to compensate for others than velocity terms. For further improvement of image quality image acquisition is often synchronized to cardiac motion, i.e. to ECG, and to breathing motion. Breathing motion is recorded by additional sensors or by so-called navigator signals. For conventional imaging saturation of spins flowing into the image slice may be applied in addition for suppression of image artifacts. G z t G y t G x t RF t T E Figure 6-8 Gradient echo sequence with additional gradients for compensation of velocity induced phase shifts. Magnetic Resonance Imaging and Spectroscopy 55
60 6 Blood flow measurement, motion analysis and MR angiography MAGNETIC RESONANCE ANGIOGRAPHY Most common MRA methods share two fundamental steps, including ^Åèìáëáíáçå=çÑ=~=ÑäçïJëÉåëáíáîÉ=áã~ÖÉ=ïáíÜ=ëìééêÉëëáçå=çÑ=ëáÖå~ä=çÑ= ëí~íáçå~êó=ä~åâöêçìåç=íáëëìé=ñçê=éãéü~ëáòáåö=î~ëåìä~ê=~å~íçãó déåéê~íáçå=çñ=~=éêçàéåíáçå=áã~öé=ñçê=îáëì~äáò~íáçå=çñ=íüé=î~ëåìä~íìêék The requirement for a projection results from the tortuosity of the vessel inherent in many of the regions of interest, such as e.g. the neurovascular anatomy. Use of 3D image acquisition permits the most flexible data set for projection, since the projection direction can be selected freely for viewing of any alternative projection. Image acquisition relies on use of either inflow or phase effects to generate images with high signal intensity in the vascular anatomy and a low background signal from surrounding tissue. Accordingly, the methods are called inflow MRA or Phase Contrast Angiography (PCA). The projection is often done with the so-called Maximum Intensity Projection (MIP). For inflow angiography, stacks of images of thin continuous or slightly overlapping slices are measured using fast gradient echo sequences to produce the 3D data set For fast flowing blood often full 3D acquisition procedures are used. The shorter T R the better is the suppression of stationary tissue. The smaller the slice thickness the higher are the inflow effects and the smaller the intravoxel dephasing. The inflow effect is highest if the slices are lying orthogonal by the vessel. To avoid inflow of already saturated blood into the slice, the slice sequencing should be in opposite direction to the main blood flow. To avoid flow effects in one direction, i.e. for imaging of arteries or veins only in extremities, the blood flowing into the slice from one direction can be presaturated by REST pulses (Regional Saturation Technique). For phase contrast angiography, images are used in which the phase values of the signals are gray scale coded instead of the amplitude on conventional images. For suppression of the phase values occurring for each acquisition in order to measure only flow induced phase values usually three images are acquired with phase encoding of motion in one spatial direction only, while for the other two directions flow compensation is used. Furthermore, an image with flow compensation (Figure 6-8) in all three directions is measured for reference. By a subtraction procedure (Figure 6-9) then images with phase values of each velocity component separately are computed, from which the final angiogram is calculated. Further processing is performed by MIP. Figure 6-10 shows an inflow angiogram and a phase contrast angiogram respectively of vessels in the head and neck). 56 Institute for Biomedical Engineering UZH / ETHZ
61 Functional Information with MRI Flow sensitive Image X Difference Image X Flow sensitive Image Y Flow compensated Image Difference Image Y PCA Image Flow sensitive Image Z Subtraction Difference Image Z Combination Calculated Measured Figure 6-9 The construction of a phase contrast angiogram. Example: Figure 6-10 Transversal inflow angiogram of the human brain (left) and coronal phase contrast angiogram of brain and neck (right) of a healthy volunteer. Magnetic Resonance Imaging and Spectroscopy 57
62 6 Paramagnetic contrast agents QUANTITATIVE ASSESSMENT OF BLOOD FLOW For quantitative assessment only methods, based on phase contrast effects, can be applied. Basically, the same images as for phase contrast angiography are acquired, however, time resolved at different time instants during the heart beat cycle. From exact knowledge of the gradient moments from special calibration procedures from the phase values, the velocity components are calculated pixelwise for each measured time instant according to (Eq. 6.5). Figure 6-7 shows the axial flow velocity components as they have been measured downstream the bifurcation of the abdominal aorta into the iliacae communes of a healthy volunteer. For synchronization of data acquisition with the heart beat ECG triggering was performed. By integration of the velocity values over the vessel lumen, instant flow rates can be computed. By integration of these instantaneous flow rates over the heart beat interval, mean flow rates may be determined. Highly dedicated techniques have been designed for assessment of detailed flow structure of complex flow patterns such as in vessel with bifurcations or downstream of heart valves. Most of them are either based on gradient echo imaging techniques or on echo planar or spiral acquisition procedures. Spiral imaging proved to be very powerful because the center of k-space is sampled with extremely short echo time and thus with high suppression of flow induced phase artifacts. With very new techniques it is even possible to move the imaging slice with the motion of vessels, as it often occurs with vessels, located very close to or on the myocardium. Such procedures reduces (or remove) through plane artifacts. Furthermore, phase shifts which are induced by the motion of the vessel itself may easily be corrected for. 6.2 PARAMAGNETIC CONTRAST AGENTS In most images, intrinsic parameters such as spin density, T 1 and T 2 are the primary sources of image contrast. In certain cases, however, MRI fails to provide adequate discrimination between tissue structures. Ions or molecules with one or more unpaired electrons, when placed in a magnetic field, generate a magnetic moment which tends to align with the applied field and is 3-8 orders of magnitude stronger than the moment generated by the nuclei. These ions or molecules are referred to as paramagnetic, superparamagnetic, or ferromagnetic ions, depending on their specific electronic configuration. Of great interest in MRI is a class of paramagnetic compounds such as Gadolinium (Gd 3+ ), Iron (Fe 2+, Fe 3+ ), and Manganese (Mn 2+ ). Basically, all substances accelerate T 1 relaxation, but Gd 3+ has gained the most widespread acceptance. The acute toxicity of Gd 3+ at clinically relevant dosages restricts its use as free ion. Chelation to molecules such as diethylene triamine pentaacetic acid (DTPA) reduces this toxicity and permits intravenous injection in clinical applications. 58 Institute for Biomedical Engineering UZH / ETHZ
63 Functional Information with MRI Figure 6-11 Native and contrast enhanced image of the human kidneys after administration of Gd-DTPA. When administered, the predominant effect of Gd-DTPA is to decrease the T 1 values of the tissues where it is accumulated. A T 1 weighted sequence easily demonstrates such effects (Figure 6-11). The use of a dynamic T 1 weighted gradient echo scan in combination with subtraction of a pre-contrast reference image allows for time-resolved monitoring of contrast wash-in and wash-out from which perfusion may be estimated. Contrast agents are also used for sensitivity enhancement in MRA. A bolus of contrast agent is administered into the veins in a very short time. This bolus then passes through the heart and some instants later the arteries. If with fast imaging techniques time series of images are acquired the first passage of the bolus through the arteries can be followed. Images sampled at the time where maximum intensity occurs clearly demonstrate the pattern of vessels with considerably enhanced brightness (Figure 6-12). Subsequently, the contrast agent flows in the tissue and then in the veins (3rd image and following panels of Figure 6-12). After a certain delay the agent is disseminate in the whole blood and tissues. If the concentration is small, the effect will be negligeable. At the end, the kidneys will remove the contrast agent from the body. For angiography of the coronary arteries new contrast agents are in development, where the gadolinium ions are built into larger molecules. Therefore, they stay longer in the vessel and more time is available for data acquisition. In addition, the inflow of the contrast agent into the tissue is reduced, leading to a higher contrast between the vessels and the surrounding tissue. Magnetic Resonance Imaging and Spectroscopy 59
64 6 Diffusion Imaging Figure 6-12 First passage of a contrast agent bolus (Gd-DTPA) through the abdominal aorta and the kidney. The images are acquired with the SENSE technique with temporal resolution of 4s. 6.3 DIFFUSION IMAGING Diffusion weighted imaging (DWI) can characterize water diffusion properties across tissue non-invasively and in vivo at each voxel of an image. The clinical relevance of DWI turned out to be very prominent as it can e.g. detect stroke in its acute phase. The technique is based on the measurement of Brownian motion of molecules. These motions obey Fick s second law of diffusion: c = j = D 2 c t (Eq. 6.7) with c, the molecule concentration, j the molecular flux density and D the diffusion coefficient. The displacements resulting from self-diffusion are given by the EINSTEIN SMOLUCHOWSKI relation: R = 2D (Eq. 6.8) whereas is the diffusion time. The diffusion coefficient in mm2/s can be described as: D = (Eq. 6.9) with, the free path length and the molecular velocity. Typical values are 0.8 µm 2 /ms for gray matter and µm 2 /ms for white matter. In MR the diffusion time is typically relatively long, and therefore the diffusion is not completely free, but restricted by the geometry of tissue, so the actual diffusion coefficient measured with MR is the apparent diffusion coefficient. 60 Institute for Biomedical Engineering UZH / ETHZ
65 Functional Information with MRI 90 o 180 o G G G ABBILDUNG 6-1 mêáååáéäéë=çñ=~=çáññìëáçå=ïéáöüíéç=éìäëé=ëéèìéååé Figure 6-1 shows a typical DWI sequence with the application of a linear gradient in a single direction, the so called dephasing gradient. The thus introduced differences in the precession rate lead to dispersion of the phase and signal loss analog to the T 2 relaxation process. However, if another gradient pulse is subsequently applied with the same direction and time period but of opposite magnitude (rephrasing gradient), such dispersion can be refocused or rephased. If the protons moved in between a pair of the gradient applications cannot be perfect refocused as shown in Figure 6-1. The resulting image characterizes the diffusion process along the applied gradient direction, whereas the signal S depends on the time between the two lobes of the bipolar gradient, the gradient strength and G, the time the gradient is applied and the diffusion coefficient D (Figure 6-13). Magnetic Resonance Imaging and Spectroscopy 61
66 6 Diffusion Imaging b=0 b=200 b=400 b=600 b=800 b=1000 M b [s/mm Figure 6-13 Exponential decay of the magnetization M in function of the b-value. The attenuation is given by S 2 G e 3 -- D = = e bd S 0 (Eq. 6.1) with b = 2 G (Eq. 6.2) the so called b value, describing the relationship between the signal attenuation and the diffusion coefficient. S 0 is the unweighted image i.e. b = 0. A further improvement of DWI is diffusion tensor imaging (DTI) which allows to measure and to characterize the diffusion process in 3D. Highly ordered organs, such as the brain white matter, show anisotropic diffusion and the diffusion can reveal the actual organization tissue. In DTI, the diffusion coefficient D no longer is a scalar but a symmetric, 3x3 Tensor which can be calculated by applying the above described gradient lobes in at least 6 different directions. The three orthogonal eigenvectors of the tensor, weighted by the corresponding eigenvalues, span a so called diffusion ellipsoid, which can be regarded as a 3D visualization of the diffusion distribution within a single voxel. Thereby, the tensor s principle eigenvector corresponds to the direction of the main diffusivity and thus indicates the course of the underlying fiber bundles. Based on this concept, DTI allows the characterization of the axonal architecture in white matter networks. Figure 6-14 shows a colorcoded DTI image. 62 Institute for Biomedical Engineering UZH / ETHZ
67 Functional Information with MRI Figure 6-14 Example of a colorcoded DTI image. 6.4 FUNCTIONAL IMAGING (FMRI) BASIC PRINCIPLES OF FMRI Over the last decade functional magnetic resonance imaging (fmri) has emerged to the most often used technique for mapping human brain function. Almost all sensory and cognitive systems have been studied with this technique in health and disease, in order to explore the functional topography of our brains and changes in functional organization during development and learning, but also to learn about the neural correlates of functional abnormalities and functional reorganization after brain damage. FMRI allows to non-invasively detect subtle changes in blood oxygenation that are driven by brain activation, and the functional contrast is hence termed blood oxygenation level dependent (BOLD) contrast. The BOLD signal is measured by the effects of local magnetic susceptibility field gradients that are induced by local alterations in deoxyhemoglobin content (see chapter 6.4.2). Magnetic Resonance Imaging and Spectroscopy 63
68 6 Functional Imaging (fmri) log(size[m]) Brain Map Column Layer MEG & ERP Optical dyes fmri PET Micro lesions Lesions 2-Deoxyglucose Neuron Dendrite -5-6 Single unit Patch clamp Light microscopy Synapse Non-Invasive Invasive log(time[s] Figure 6-15 Functional mapping methods. Figure 6-15 illustrates how fmri ranks among other functional mapping methods in terms of spatial and temporal resolution and invasiveness of the technique. Single cell recordings allow to record synaptic transmission of action potentials at their very source with high spatial and temporal precision. In contrast, fmri provides a tool to study networks of neuronal populations that interact with each other over large scale distances across the whole brain. Although fmri can not compete with the temporal resolution provided by EEG (electro-encephalography) and MEG (magneto-encephalography) or single cell recordings, it is not invasive like the latter and spatially more precise than the former. In contrast, PET (positron emission tomography) permits to selectively study distinct brain metabolites, however at a longer timescale and with less spatial specificity. Hence, fmri turned a method of choice to study human brain function, enabling comparatively high spatial and temporal specificity while being a non-invasive, widespread available, versatile and harmless procedure THE INFORMATION CONTENT OF A FMRI IMAGE. It is important to note that the functional signal measured with fmri only indirectly measures brain activation and originates from the combined effect of distinct vascular events and metabolic changes (Figure 6-16). Neuronal activation is accompanied by a release of one or more mediators in the central neuronal layers, for example NO, K, and/or adenosine, which induce a smoothing of the muscle cells on the arterial side and subsequently cause the blood vessels to dilate, which in turn triggers an increase in blood flow and volume. However, the precise mechanism that mediates between electrical activity and the 64 Institute for Biomedical Engineering UZH / ETHZ
69 Functional Information with MRI hemodynamic response as well as the sequence and interplay between metabolic, physiological and hemodynamic events are not yet fully understood. Various models have been brought up to describe activity-induced coupling between cerebral blood flow and oxygen consumption: spanning uncoupling, transient uncoupling and tight coupling. Even at a biochemical level the exact mechanism that drives synaptic glucose metabolism is rather controversial. The Astrocyte-Neuron Lactate Shuttle Hypothesis proposed by PELLERIN & MAG- ISTRETTI, which states that astrocytes provide lactate as an energy substrate for neurons, has recently been critically reviewed by CHIH & ROBERTS, supporting the conventional hypothesis, which contends that activation-induced energy demand is met predominantly by metabolizing glucose oxidatively. Moreover, the very coupling mechanisms as well as the vascular reactivity supposedly differ between brain regions and also in health and disease. Fortunately, it has recently been proven that the BOLD signal indeed tightly correlates with change in local field potentials in postsynaptic neurons that occurs as a result of changes in excitatory (glutamatergic) neurotransmission. Figure 6-16 Neurovascular coupling cascade. Magnetic Resonance Imaging and Spectroscopy 65
70 6 Functional Imaging (fmri) Figure 6-17 Results of an fmri experiment An increase in neuronal activity will consume more oxygen and therefore cause a decrease of the oxyhemoglobine concentration in downstream blood. The vascular system will respond to this decrease with a bigger blood flow and cerebral volume. In fact the vascular system will overreact and the oxygen consumption will rapidly (order of one second) be compensate by the increase of blood flow, so that the net deoxyhemoglobin concentration will decrease, implying a bigger magnetic field homogeneity and a bigger T 2*. This increase in T 2* will manifest in a bigger MRI signal. An fmri experiment essentially includes the acquisition of a time series of susceptibility (T 2* )-weighted images during which the subject is presented with a specific task that alternates with a baseline state. Further, a statistical map is generated by analyzing the functional time series with respect to correlation between the induced signal changes and the course of the applied functional task. Finally, an anatomical scan is acquired for structural reference, as outlined in Figure For the interpretation of the resulting data it should precisely be indicated what is actually meant by 'activation', since it might be quantified by either the size or significance of the observed clusters of activation, or the amplitude of the BOLD signal change GRADIENT- VERSUS SPIN-ECHO BASED FMRI STATIC AND DYNAMIC DEPHASING EFFECTS The BOLD signal arises from the paramagnetism of venous blood deoxyhemoglobin and functionally driven alterations in blood oxygenation (i.e. change in deoxyhemoglobin content). The paramagnetic deoxyhemoglobin gives rise to two magnetic susceptibility field gradients: one is intravascular and the sec- 66 Institute for Biomedical Engineering UZH / ETHZ
71 Functional Information with MRI ond between the venous vessel and the surrounding diamagnetic tissue. This field implies a dephasing of the water spins. Two different spin-dephasing mechanisms contribute to the BOLD signal, 2 dynamic (diffusion) effects (~ B o ) and static dephasing effects (~ B o ). The static dephasing effect arises from loss of coherence of tissue water spins in the susceptibility gradient field between the intra- and extravascular compartments. The extravascular dynamic effect is based upon diffusion of tissue water spins in the susceptibility gradient field around blood vessels. The intravascular BOLD effect is a consequence of the diffusion of blood water spins in the susceptibility gradients around deoxygenated red blood cells within the blood vessels. The so-called transverse relaxation times T 2 and T 2* are the times that describe the decay of the signal due to these spin-dephasing effects. T 2* is defined by the following equation: = T * T (Eq. 6.3) where represents the relaxation effect evoked by static field inhomogeneities EXTRAVASCULAR AND INTRAVASCULAR BOLD-EFFECT The extra-vascular BOLD effect arises from both static dephasing and diffusion effect experienced by tissue water spins in the susceptibility gradient field around the blood vessels. If the range of the susceptibility gradient field around the vessel is larger than the diffusion length, static dephasing effects contribute more to the signal loss than diffusion effects (Figure 6-18). Typically, small vessels show mostly diffusion losses. The intra-vascular BOLD effect arises from the diffusion of blood water spins in the susceptibility gradient field around deoxygenated red blood cells within the blood vessels and is characterized by rapid exchange of water molecules between red blood cells and blood plasma. Not only does the susceptibility difference between the intra- and extravascular space change with blood oxygenation and hence activation, but also the transversal relaxation time T 2 of blood itself is dependent on oxygenation ("LUTZ-MEIBOOM MODEL"). The latter can give rise to a BOLD signal in, and the former around large veins, remote from the actual site of activation, when changes in blood oxygenation propagate downstream in the venous vascular bed. Hence the intra- as well as the extravascular BOLD signal arising from larger veins needs to be eliminated in order to map functional activation with high spatial specificity. Magnetic Resonance Imaging and Spectroscopy 67
72 6 Functional Imaging (fmri) SPIN-ECHO AND GRADIENT-ECHO BASED FMRI The functional contrast obtained with spin-echo (SE) based fmri differs from the one underlying gradient-echo (GE) based fmri. While dynamic effects are measured with spin-echo (T 2 -weighted) weighted sequences, both dynamic and static dephasing effects contribute to signal changes measured with gradientecho (T 2* -weighted) sequences. In an SE experiment, static dephasing effects that contribute to the BOLD signal are refocused, which results in reduced functional signal by a factor of 2 4, depending on the underlying vascular architecture. Furthermore, the same static dephasing effects give rise to signal dropout in regions like the orbitofrontal cortex, where the MR signal is dephased in the magnetic field inhomogeneity generated between the air-filled cavity of the frontal sinus and the surrounding brain tissue. Figure 6-18 Outlined are the effects of vessel radius on the variation of the transverse relaxation times T 2 and T 2*. Each curve corresponds to a different susceptibility difference between vascular and extravascular compartments (e.g. different contrast agents). SE-based fmri shows a greater micro-vascular selectivity in the extra-vascular BOLD signal, because non-specific extravascular signal changes in the vicinity of large vessels are rephased. with: IV = intravascular, EV = extravascular, = magnetic susceptibility difference, (1-Y) = deoxygenation of the blood, bl, bs = blood-volume fractions of small and large vessels, constants ( = 1 free diffusion, = 0.5 restricted diffusion). Susceptibility plots and equations were taken from literature. 68 Institute for Biomedical Engineering UZH / ETHZ
73 Functional Information with MRI The 'brain-vein'-problem constitutes one of the most pertinent methodological fmri issues. Several properties of the BOLD signal, however, might help to surmount this problem. First, the extra- and intravascular contributions to the BOLD signal change with field strength. While the extravascular signal contribution from small vessels increases with field strength, the intravascular contribution generally decreases as T 2 (blood) decreases with the field strength. Hence, SE-based functional contrast mechanism along with a long echo-time 1 and high field strength provides for a high intrinsic spatial specificity of the functional signal. Second, unspecific signal contributions from downstream activation in large venous vessels can be separated by using bipolar gradients that dephase the intravascular signal or, third, venograms can be used in order to elucidate where large vessels are located. Furthermore, magnetization transfer selectively suppresses the signal that arises from water molecules in the vicinity of macromolecules. It therefore attenuates signal from tissue protons, because brain tissue contains macromolecules in high concentration or protons within capillaries due to their rapid exchange with tissue, but leaves protons in large vessels unaffected and can hence be used complimentary to diffusion-weighting. 1. in order to ensure that the signal from blood water is completely dephased by the time the image is acquired Magnetic Resonance Imaging and Spectroscopy 69
74 6 Functional Imaging (fmri) 70 Institute for Biomedical Engineering UZH / ETHZ
75 Localized MR Spectroscopy 7 LOCALIZED MR SPECTROSCOPY 7.1 CHEMICAL SHIFT Until now we assumed for the MR imaging technique that nuclei of the same isotope show identical Larmor frequencies, apart from stochastic dephasing effects. The same gyromagnetic ratio implies the same precession frequencies, as long as the individual spins experience the same local magnetic field. However, electrons circling around the nucleus, slightly shield the nucleus from the external magnetic field so that the nuclear spin feels a slightly weaker magnetic field (see Figure 7-1). Consequently, the Larmor frequency becomes dependent on the electronic configuration and thus on the molecular structure around the atom. Therefore, nuclei of identical atoms in different molecules or at different positions in the same molecule emit slightly distinguishable frequencies after excitation. These frequency differences are called chemical shifts and are measured with respect to the frequency of a reference substance. This chemical shift is the basis for NMR spectroscopy, as applied in chemistry and biochemistry. Its origin lies in the phenomenon that the external magnetic field induces circular currents in the electron cloud around a nucleus. These create supplemental magnetic fields at the location of the nucleus, which - according to LENZ's rule - are in a direction such that the inducing field is compensated, which explains why the original field strength is reduced. The configuration of the electron clouds that induce the shielding effect is determined by the chemical bonds of the atom of interest. B e B 3 4 R + e - Figure 7-1 Induced local magnetic field B reduced by the motion of the electrons, which shields the nucleus due to the opposite direction with respect to the external static field B o. The quantity is the measurable frequency shift that results from shielding effects. It represents the difference between the measured frequency and the Larmor frequency stand of the nuclei of a standard substance, normalized with the resonance frequency of unbound atoms o. It is defined as Magnetic Resonance Imaging and Spectroscopy 71
76 7 Acquisition and Spectral Resolution = stand o (Eq. 7.1) and expressed in units of 10-6 or ppm (parts per million). The quantity is independent of the magnetic field strength and always refers to a standard substance. Comparison of measured values with values, listed in tables allows the identification of the chemical substances under investigation. Concentrations of substances may be estimated from line intensities after calibration of the measurement system. 7.2 ACQUISITION AND SPECTRAL RESOLUTION The spectral resolution, meaning the separation of two contigeous adjacent neighboring metabolites is mainly determined by few parameters. Most important is the experimental setup of the hardware and the probe, in a sense that the spatial homogeneity within the selected volume. This is done by a separate shimming, where small magnetic fields (in all directions) are superimposed and dynamically optimized over the main static field for getting a much better local field homogenity. With such conditions, the best spectral resolution can be achieved, given by the volume and the tissue characteristics. It is obvious that a smaller and mostly homogeneous volume will bring the best result. The signal acquisition determines also the spectral resolution. The mathematical background, (given from the Fourier transformation), between the length of acquired time signal T acq and the spectral resolution, the frequency difference between two data points is given by T 1 acq = (Eq. 7.2) From this it becomes obvious, that a shortening of the acquisition time (or the repetition time) will result in a reduced spectral resolution. This effect is illustrated in Figure 7-2, where a 31 P spectrum of the calf muscle is acquired with two different acquisition times. It is clearly visible, that with a shortening by a factor of 4 (from 336msec to 84msec) the structure of the three ATP metabolites (see Figure 7-8) is vanishing (left graphs). On the other hand, an increase of the sampling time will also increase the noise, which can also clearly seen in Figure 7-2 (lower right). 72 Institute for Biomedical Engineering UZH / ETHZ
77 Localized MR Spectroscopy Figure 7-2 Effect of length of signal acquisition and the resulting spectral resolution: (left: FID acquisition window 84msec, right: 336msec). 7.3 LOCALIZATION In NMR spectroscopy of living organisms, it is an important task to match the measured spectra in a defined region of the body. For example, it is useless, to determine the 31 P spectrum of the human head. It is more likely of interest to record a spectrum of a particular part of the brain, liver, kidneys, a muscle or even the heart muscle. Different kinds of localization methods can be distinguished: äçå~äáò~íáçå=ïáíü=ëìêñ~åé=åçáäë ëáåöäé=îçñéä=ãéíüççë= - ëéäéåíáîé=éñåáí~íáçå=eékök=mobppi=pqb^jf - ëéäéåíáîé=çéjéñåáí~íáçå=eçìíéê=îçäìãé=ëìééêéëëáçåf - ÉåÅçÇáåÖ=ëÅÜÉãÉë=EÉKÖK=fpfpF ãìäíájîçñéä=íéåüåáèìéë - ÇÉêáîÉÇ=Ñêçã=ëáåÖäÉ=îçñÉä=íÉÅÜåáèìÉë - ÅÜÉãáÅ~ä=ëÜáÑí=áã~ÖáåÖ=Eáå=çåÉI=íïç=çê=íÜêÉÉ=ÇáãÉåëáçåëF Magnetic Resonance Imaging and Spectroscopy 73
78 7 Localization In the following sections the surface coil and the single voxel methods ISIS, PRESS, and STEAM will be discussed SURFACE COILS The easiest way for a volume selection is using a so-called surface coil. It is applied to the body surface directly at the region of interest and characterized by a non-sharply defined half-spherical sensitivity area. By appropriate choice of pulse sequences this area can be further confined. The advantage of surface coils is the high sensitivity, which results in a signal-to-noise ratio superior to that of the body coil. The decisive disadvantages are the limitation to localize only structures in the body that lie close to the surface and the inaccuracy of the localization, since the area that was identified in a magnetic resonance image is not directly transferable to the patient under examination. Besides, only elementary resonance experiments can be realized. A surface coil is not able to produce a homogeneous B 1 field in the tissue and has therefore a non-uniform sensitivity. z P x a a x B o y 0.8 B 1 (x) b B 1 (x) sin() 0.8 c 0.6 x=a/2 0.6 x=a/2 0.4 x=a x=3a/2 0.2 x=a x=3a/2 0 a/2 a 3a/2 2a 0 a/2 a 3a/2 2a Figure 7-3 a) Diagram illustrating a circular surface coil (with a radius a). A point P in space is described by the radial and axial coordinates (, x). b) Spatial dependence of B 1 () c) B 1 () sin, plotted as a function of and x, which are expressed in units of the coil radius a. B 1 () is normalized to 1.0 at (,x)=(0,0), i.e. at the coil center. 74 Institute for Biomedical Engineering UZH / ETHZ
79 Localized MR Spectroscopy a y [mm] y [mm] 10 b o 60 o 90 o o 60 o 90 o z [mm] o 270 o 270 o z [mm] Figure 7-4 Inhomogeneous B 1 distribution. The pulse angle is a function of the distance (x,y) to the coil isocenter and of the pulse power. a) 90 excitation pulse, b) 180 excitation pulse. According to the flip angle calculation = B 1 T p, also the flip angle distribution is inhomogeneous in the tissue. Therefore the flip angles are not everywhere optimal (Figure 7-4). As alternative, adiabatic pulses can be used, which create a more uniform flip angle distribution over a wide range of B 1 field strength. They have a lower power and, therefore, a longer duration in order to obtain the same flip angles. The term adiabatic describes the fact that the direction of the effective field changes slowly with respect to its strength. In this case the magnetization follows the direction of the effective field in the rotating frame. Having a rotating field B 1 of frequency, perpendicular to the static field B o, we can make use of this principle in the following way: Starting far below resonance, the magnetization is nearly parallel to the effective field in the rotating frame, as << B o. Approaching resonance, both magnitude and direction of the effective field will change. When resonance is approached the magnetization M remains parallel to B eff in the rotating frame. Thus, exactly at resonance, the magnetization will lie along B 1, making a 90 angle with B o - (as shown in Figure 7-5). Magnetic Resonance Imaging and Spectroscopy 75
80 7 Localization Amplitude z` t B o B eff o Frequency M x` t y` B 1 Figure 7-5 B eff as the vector in the rotating frame. Continuing on through the resonance, the magnetization would end up by pointing in the negative z-direction. This technique is called adiabatic inversion. The advantage of adiabatic pulses is the fact, that the magnetization can be turned completely independent of the strength of B 1 and thus independent of inhomogeneities of the B 1 field LOCALIZATION BY GRADIENTS The disadvantages encountered when using surface coils can be eliminated by volume selective measurement techniques in which selection of a particular volume is achieved by a combination of carefully designed excitation pulse sequences and corresponding gradient sequences. Several techniques were proposed: The first Volume Selective Excitation (VSE) technique was realized in 1984 by AUE and SEELIG on a small system for animal experiments. In the years more techniques followed like SPARS (SPatially Resolved Spectroscopy) by den HOLLANDER and LUYTEN, ISIS (image selective in vivo spectroscopy) by ORDIDGE, STEVE (Stimulated Echo Volume Excitation) by FRAHM et al., VSR (Volume Selective Refocusing) by MCKINNON and BOESIGER, and many other techniques. With several of these techniques successful studies of living organisms were completed. Since then many new sequences have been developed. Three of the single voxel techniques used today will be further explained here: ISIS, which nowadays has proved its validity in 31 P spectroscopy, PRESS, which distinguishes itself for 1 H spectroscopy by high suppression of signals from outside the selected volume, and STEAM, which is also widely used for 1 H spectroscopy. 76 Institute for Biomedical Engineering UZH / ETHZ
81 Localized MR Spectroscopy z y x Figure 7-6 The intersection of three orthogonal slices defines a volume. All methods presented in this chapter use three slice selections in three orthogonal directions. In practice, the pulses are usually combinations of sinc- and Gausspulses, resulting in relatively well defined slices. Because of different s nuclei with different bonding patterns have different Larmor frequencies. This effect also leads to slightly shifted slices for different metabolites: x = G x (Eq. 7.3) Some numerical examples: 1 H at 1.5 Tesla = 3ppm G x = 10mT/m x = 0.5mm 31 P at 1.5 Tesla =15ppm G x = 10mT/m x = 2.5mm ISIS The excitation pulse and gradient sequence for ISIS is outlined in Figure 7-7. We concentrate on the first part of the preparation and the acquisition period: A small band 180 pulse together with a gradient G x inverts the magnetization of a slice orthogonal to the x-axis of the sample, because only in this slice the resonance condition is fulfilled (slice selective inversion). It is assumed - here and in the following - that the gradients are sufficiently strong so that the line splitting in the spectra due to chemical shifts and other interactions may be neglected. Since the resonance condition is not fulfilled outside of that slice the magnetization is not affected. After excitation of the whole sample by a nonselective 90 excitation pulse. The signals are recorded signals originating from the inverted slice show a phase shift of 180 or an inverted sign in comparison to the signals from outside the slice. If we now perform the experiment twice, once with and a second time without the selective inversion pulse and then Magnetic Resonance Imaging and Spectroscopy 77
82 7 Localization subtract the two resulting signals from each other, we obtain a signal only from the magnetization of the inverted slice. In two or three dimensions, four or eight experiments, respectively, are necessary with all possible combinations of inversions switched on and off. For confining a volume element in all three spatial directions (Figure 7-7), the sequence is extended by two further small-banded 180 inversion pulses with the corresponding gradients, which are slice selective to the remaining two spatial directions. The experiment then is carried out eight times according to a phase cycling scheme with all possible combinations of 180 pulses on or off. The resulting eight signals are then added with appropriate signs in such a way that all signals interfere destructively, except the signal from the selected volume element. Figure 7-7 shows the corresponding scheme for localization in two dimensions. 1. Cycle 90 o + 2. Cycle 3. Cycle 4. Cycle 5. Cycle 180 o 180 o 180 o 180 o 180 o Cycle 7. Cycle 8. Cycle 180 o 180 o 180 o 180 o 180 o 180 o 180 o G z G y G x Time Figure 7-7 Cycling scheme of the selective inversion pulses of ISIS in three dimensions with the add-subtract of the FID-signals. 78 Institute for Biomedical Engineering UZH / ETHZ
83 Localized MR Spectroscopy Figure 7-8 Localized 31 P spectrum 1 of a human calf muscle. ISIS is nowadays mainly used for 31 P spectroscopy, since signal acquisition can start immediately after the 90 excitation pulse. Signal losses due to the relatively short T 2 relaxation time of many 31 P metabolites remain small. The increase in measurement time due to the eight measurement cycles required to obtain a spectrum of the selected volume element is hardly a disadvantage, since the low 31 P metabolite concentrations in tissue (typically in the order of a few millimole per dm 3 of tissue) always require multiple signal acquisitions for the improvement of the signal-to-noise ratio. Figure 7-8 shows a spectrum of the calf muscle, acquired using the total body MR machine Philips Gyroscan ACS-NT (1.5Tesla) in a measurement time of a few minutes. From left to right the resonance lines of inorganic bound phosphorous (P i ), of Phosphocreatine (PCr) and three lines of the energy-rich Adenosine Triphosphate (ATP) can be identified. ATP produces three resonance lines corresponding to the three chemically different positions of the 31 P atoms in the molecule. Due to interactions between these spins, each of the three lines shows a fine structure visible as a splitting into two, respectively three lines. The resonance signal of ADP, which is present in about ten times lower concentration than ATP, coincides with the and lines of ATP. Furthermore, the exact position of the inorganically bound 31 P indicates the acidity (ph) in the tissue PRESS PRESS is widely used for localization of 1 H spectra. The double spin echo method allows a good localization in one measurement and has a strong suppression of signals outside the selected volume. The three slice selective pulses are a 90 excitation pulse and two 180 refocusing pulses. While the first echo 1. The resonance lines originate from 31 P nuclei of (from left to right): the anorganic bounded Phosphor (Pi), the phosphocreatine (PCr) and the three different positions in the ATP molecule (-,- and -ATP). Magnetic Resonance Imaging and Spectroscopy 79
84 7 Localization between the two 180 pulses is not acquired, the second echo is measured either completely, starting the acquisition immediately after the second refocusing pulse, or only after the echo top (starting after the total echo time). G z G y G x t 180 o 180 o 90 o RF t Signal t Figure 7-9 Pulse and gradient sequence for PRESS. -CH 2 a CH=CH -CH x -CH 3 H 2O b H 2O c Figure H spectra localized with PRESS in the calf areas that are marked in the images. Spectra of a) bony marrow, b) muscle tissue, c) muscle tissue together with subcutaneous fat. 80 Institute for Biomedical Engineering UZH / ETHZ
85 Localized MR Spectroscopy The excitation pulse and the small band echo pulses that follow the 90 pulse become slice selective by field gradients in the three spatial directions. The final echo signal, therefore, originates only from the volume element in the intersection point of the three slice planes. Figure 7-10 shows a transversal slice of the lower leg. Three volumes are indicated, which were selected by PRESS for 1 H spectroscopic examination. Spectrum (a) originates from the bony marrow and shows resonance lines of fatty substances. Spectrum (b) from the muscle tissue is dominated by the water line, while in spectrum (c) from muscle tissue and subcutaneous fat both lines appear STEAM STEAM (Stimulated Echo Acquisition Mode) uses three slice selective 90 pulses forming a stimulated echo after the total of the echo time T E and the mixing time T M. It allows shorter echo times and better water suppression than PRESS, but the signal intensities are only half of those obtained with PRESS. G z t G y t 90 o G x t RF t T E /2 T M T E /2 Figure 7-11 Pulse and gradient sequence for STEAM. The Press part determines the measurement volume Magnetic Resonance Imaging and Spectroscopy 81
86 7 Localization CHEMICAL SHIFT IMAGING The spins within one (or more) selected slice(s) can also be spatially encoded by applying gradients within the slice(s). The basic mechanism of chemical shift imaging which is also often called Spectroscopic Imaging, (SI) is almost the same as in the imaging mode, except, that the signal acquisition ( readout ) has to be performed without any gradient. To fulfill this restriction, phase encoding steps in two spatial directions are applied. To get a complete image the experiment has to be performed n n times, which makes the acquisition time rather long. Also for signal to noise considerations, the voxel sizes has to be significantly larger than in imaging sequences, typical minimal values are 1cm 3 for proton signals and 25cm 3 for phosphorus. Together with a rather long repetition time (>1s) and a spatial resolution up to 32 32, acquisition times longer than 15 minutes have to be considered. After acquisition of all profiles and applying a 2-D Fourier transformation we end up with n n localized signals. After applying an additional Fourier transformation to this dataset we get n n spectra. Using the SI procedure for protons, it is important to supress signals from water and fatty tissues. Therefore in basic application within the human brain, the SI method is combined with water suppression (Section 7.4) and PRESS, where the press volume is a large area within brain tissue only. The principle of this sequence is represented in Figure phase encod- Typical frequency signals of an experiment with ing step is figured out in Figure G z G y G x RF 90 o 180 o 180o TIME Water suppression PRESS Localization Phase Encoding Figure 7-12 Pulse and gradient sequences for chemical shift imaging sequences. 82 Institute for Biomedical Engineering UZH / ETHZ
87 Localized MR Spectroscopy Figure H spectra of a spectroscopic imaging sequence within the human brain with a spatial resolution of 16x16 pixels. Figure 7-14 Metabolite map of NAA in a transversal slice in the human brain. Magnetic Resonance Imaging and Spectroscopy 83
MRI Physics I: Spins, Excitation, Relaxation
 MRI Physics I: Spins, Excitation, Relaxation Douglas C. Noll Biomedical Engineering University of Michigan Michigan Functional MRI Laboratory Outline Introduction to Nuclear Magnetic Resonance Imaging
MRI Physics I: Spins, Excitation, Relaxation Douglas C. Noll Biomedical Engineering University of Michigan Michigan Functional MRI Laboratory Outline Introduction to Nuclear Magnetic Resonance Imaging
Introduction to Biomedical Imaging
 Alejandro Frangi, PhD Computational Imaging Lab Department of Information & Communication Technology Pompeu Fabra University www.cilab.upf.edu MRI advantages Superior soft-tissue contrast Depends on among
Alejandro Frangi, PhD Computational Imaging Lab Department of Information & Communication Technology Pompeu Fabra University www.cilab.upf.edu MRI advantages Superior soft-tissue contrast Depends on among
Magnetic Resonance Imaging. Pål Erik Goa Associate Professor in Medical Imaging Dept. of Physics
 Magnetic Resonance Imaging Pål Erik Goa Associate Professor in Medical Imaging Dept. of Physics pal.e.goa@ntnu.no 1 Why MRI? X-ray/CT: Great for bone structures and high spatial resolution Not so great
Magnetic Resonance Imaging Pål Erik Goa Associate Professor in Medical Imaging Dept. of Physics pal.e.goa@ntnu.no 1 Why MRI? X-ray/CT: Great for bone structures and high spatial resolution Not so great
Nuclear Magnetic Resonance Imaging
 Nuclear Magnetic Resonance Imaging Jeffrey A. Fessler EECS Department The University of Michigan NSS-MIC: Fundamentals of Medical Imaging Oct. 20, 2003 NMR-0 Background Basic physics 4 magnetic fields
Nuclear Magnetic Resonance Imaging Jeffrey A. Fessler EECS Department The University of Michigan NSS-MIC: Fundamentals of Medical Imaging Oct. 20, 2003 NMR-0 Background Basic physics 4 magnetic fields
The NMR Inverse Imaging Problem
 The NMR Inverse Imaging Problem Nuclear Magnetic Resonance Protons and Neutrons have intrinsic angular momentum Atoms with an odd number of proton and/or odd number of neutrons have a net magnetic moment=>
The NMR Inverse Imaging Problem Nuclear Magnetic Resonance Protons and Neutrons have intrinsic angular momentum Atoms with an odd number of proton and/or odd number of neutrons have a net magnetic moment=>
Nuclear Magnetic Resonance Imaging
 Nuclear Magnetic Resonance Imaging Simon Lacoste-Julien Electromagnetic Theory Project 198-562B Department of Physics McGill University April 21 2003 Abstract This paper gives an elementary introduction
Nuclear Magnetic Resonance Imaging Simon Lacoste-Julien Electromagnetic Theory Project 198-562B Department of Physics McGill University April 21 2003 Abstract This paper gives an elementary introduction
Principles of Magnetic Resonance Imaging
 Principles of Magnetic Resonance Imaging Hi Klaus Scheffler, PhD Radiological Physics University of 1 Biomedical Magnetic Resonance: 1 Introduction Magnetic Resonance Imaging Contents: Hi 1 Introduction
Principles of Magnetic Resonance Imaging Hi Klaus Scheffler, PhD Radiological Physics University of 1 Biomedical Magnetic Resonance: 1 Introduction Magnetic Resonance Imaging Contents: Hi 1 Introduction
The Basics of Magnetic Resonance Imaging
 The Basics of Magnetic Resonance Imaging Nathalie JUST, PhD nathalie.just@epfl.ch CIBM-AIT, EPFL Course 2013-2014-Chemistry 1 Course 2013-2014-Chemistry 2 MRI: Many different contrasts Proton density T1
The Basics of Magnetic Resonance Imaging Nathalie JUST, PhD nathalie.just@epfl.ch CIBM-AIT, EPFL Course 2013-2014-Chemistry 1 Course 2013-2014-Chemistry 2 MRI: Many different contrasts Proton density T1
Introduction to MRI. Spin & Magnetic Moments. Relaxation (T1, T2) Spin Echoes. 2DFT Imaging. K-space & Spatial Resolution.
 Introduction to MRI Spin & Magnetic Moments Relaxation (T1, T2) Spin Echoes 2DFT Imaging Selective excitation, phase & frequency encoding K-space & Spatial Resolution Contrast (T1, T2) Acknowledgement:
Introduction to MRI Spin & Magnetic Moments Relaxation (T1, T2) Spin Echoes 2DFT Imaging Selective excitation, phase & frequency encoding K-space & Spatial Resolution Contrast (T1, T2) Acknowledgement:
Topics. The concept of spin Precession of magnetic spin Relaxation Bloch Equation. Bioengineering 280A Principles of Biomedical Imaging
 Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 2006 MRI Lecture 1 Topics The concept of spin Precession of magnetic spin Relaxation Bloch Equation 1 Spin Intrinsic angular momentum of
Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 2006 MRI Lecture 1 Topics The concept of spin Precession of magnetic spin Relaxation Bloch Equation 1 Spin Intrinsic angular momentum of
NMR and MRI : an introduction
 Intensive Programme 2011 Design, Synthesis and Validation of Imaging Probes NMR and MRI : an introduction Walter Dastrù Università di Torino walter.dastru@unito.it \ Introduction Magnetic Resonance Imaging
Intensive Programme 2011 Design, Synthesis and Validation of Imaging Probes NMR and MRI : an introduction Walter Dastrù Università di Torino walter.dastru@unito.it \ Introduction Magnetic Resonance Imaging
Physical fundamentals of magnetic resonance imaging
 Physical fundamentals of magnetic resonance imaging Stepan Sereda University of Bonn 1 / 26 Why? Figure 1 : Full body MRI scan (Source: [4]) 2 / 26 Overview Spin angular momentum Rotating frame and interaction
Physical fundamentals of magnetic resonance imaging Stepan Sereda University of Bonn 1 / 26 Why? Figure 1 : Full body MRI scan (Source: [4]) 2 / 26 Overview Spin angular momentum Rotating frame and interaction
V27: RF Spectroscopy
 Martin-Luther-Universität Halle-Wittenberg FB Physik Advanced Lab Course V27: RF Spectroscopy ) Electron spin resonance (ESR) Investigate the resonance behaviour of two coupled LC circuits (an active rf
Martin-Luther-Universität Halle-Wittenberg FB Physik Advanced Lab Course V27: RF Spectroscopy ) Electron spin resonance (ESR) Investigate the resonance behaviour of two coupled LC circuits (an active rf
Basic MRI physics and Functional MRI
 Basic MRI physics and Functional MRI Gregory R. Lee, Ph.D Assistant Professor, Department of Radiology June 24, 2013 Pediatric Neuroimaging Research Consortium Objectives Neuroimaging Overview MR Physics
Basic MRI physics and Functional MRI Gregory R. Lee, Ph.D Assistant Professor, Department of Radiology June 24, 2013 Pediatric Neuroimaging Research Consortium Objectives Neuroimaging Overview MR Physics
M R I Physics Course. Jerry Allison Ph.D., Chris Wright B.S., Tom Lavin B.S., Nathan Yanasak Ph.D. Department of Radiology Medical College of Georgia
 M R I Physics Course Jerry Allison Ph.D., Chris Wright B.S., Tom Lavin B.S., Nathan Yanasak Ph.D. Department of Radiology Medical College of Georgia M R I Physics Course Spin Echo Imaging Hahn Spin Echo
M R I Physics Course Jerry Allison Ph.D., Chris Wright B.S., Tom Lavin B.S., Nathan Yanasak Ph.D. Department of Radiology Medical College of Georgia M R I Physics Course Spin Echo Imaging Hahn Spin Echo
Fundamental MRI Principles Module 2 N. Nuclear Magnetic Resonance. X-ray. MRI Hydrogen Protons. Page 1. Electrons
 Fundamental MRI Principles Module 2 N S 1 Nuclear Magnetic Resonance There are three main subatomic particles: protons positively charged neutrons no significant charge electrons negatively charged Protons
Fundamental MRI Principles Module 2 N S 1 Nuclear Magnetic Resonance There are three main subatomic particles: protons positively charged neutrons no significant charge electrons negatively charged Protons
G Medical Imaging. Outline 4/13/2012. Physics of Magnetic Resonance Imaging
 G16.4426 Medical Imaging Physics of Magnetic Resonance Imaging Riccardo Lattanzi, Ph.D. Assistant Professor Department of Radiology, NYU School of Medicine Department of Electrical and Computer Engineering,
G16.4426 Medical Imaging Physics of Magnetic Resonance Imaging Riccardo Lattanzi, Ph.D. Assistant Professor Department of Radiology, NYU School of Medicine Department of Electrical and Computer Engineering,
Contrast Mechanisms in MRI. Michael Jay Schillaci
 Contrast Mechanisms in MRI Michael Jay Schillaci Overview Image Acquisition Basic Pulse Sequences Unwrapping K-Space Image Optimization Contrast Mechanisms Static and Motion Contrasts T1 & T2 Weighting,
Contrast Mechanisms in MRI Michael Jay Schillaci Overview Image Acquisition Basic Pulse Sequences Unwrapping K-Space Image Optimization Contrast Mechanisms Static and Motion Contrasts T1 & T2 Weighting,
Magnetic Resonance Imaging in a Nutshell
 Magnetic Resonance Imaging in a Nutshell Oliver Bieri, PhD Department of Radiology, Division of Radiological Physics, University Hospital Basel Department of Biomedical Engineering, University of Basel,
Magnetic Resonance Imaging in a Nutshell Oliver Bieri, PhD Department of Radiology, Division of Radiological Physics, University Hospital Basel Department of Biomedical Engineering, University of Basel,
BMB 601 MRI. Ari Borthakur, PhD. Assistant Professor, Department of Radiology Associate Director, Center for Magnetic Resonance & Optical Imaging
 BMB 601 MRI Ari Borthakur, PhD Assistant Professor, Department of Radiology Associate Director, Center for Magnetic Resonance & Optical Imaging University of Pennsylvania School of Medicine A brief history
BMB 601 MRI Ari Borthakur, PhD Assistant Professor, Department of Radiology Associate Director, Center for Magnetic Resonance & Optical Imaging University of Pennsylvania School of Medicine A brief history
MR Fundamentals. 26 October Mitglied der Helmholtz-Gemeinschaft
 MR Fundamentals 26 October 2010 Mitglied der Helmholtz-Gemeinschaft Mitglied der Helmholtz-Gemeinschaft Nuclear Spin Nuclear Spin Nuclear magnetic resonance is observed in atoms with odd number of protons
MR Fundamentals 26 October 2010 Mitglied der Helmholtz-Gemeinschaft Mitglied der Helmholtz-Gemeinschaft Nuclear Spin Nuclear Spin Nuclear magnetic resonance is observed in atoms with odd number of protons
Fundamental MRI Principles Module Two
 Fundamental MRI Principles Module Two 1 Nuclear Magnetic Resonance There are three main subatomic particles: protons neutrons electrons positively charged no significant charge negatively charged Protons
Fundamental MRI Principles Module Two 1 Nuclear Magnetic Resonance There are three main subatomic particles: protons neutrons electrons positively charged no significant charge negatively charged Protons
FREQUENCY SELECTIVE EXCITATION
 PULSE SEQUENCES FREQUENCY SELECTIVE EXCITATION RF Grad 0 Sir Peter Mansfield A 1D IMAGE Field Strength / Frequency Position FOURIER PROJECTIONS MR Image Raw Data FFT of Raw Data BACK PROJECTION Image Domain
PULSE SEQUENCES FREQUENCY SELECTIVE EXCITATION RF Grad 0 Sir Peter Mansfield A 1D IMAGE Field Strength / Frequency Position FOURIER PROJECTIONS MR Image Raw Data FFT of Raw Data BACK PROJECTION Image Domain
Lecture 12 February 11, 2016
 MATH 262/CME 372: Applied Fourier Analysis and Winter 2016 Elements of Modern Signal Processing Lecture 12 February 11, 2016 Prof. Emmanuel Candes Scribe: Carlos A. Sing-Long, Edited by E. Bates 1 Outline
MATH 262/CME 372: Applied Fourier Analysis and Winter 2016 Elements of Modern Signal Processing Lecture 12 February 11, 2016 Prof. Emmanuel Candes Scribe: Carlos A. Sing-Long, Edited by E. Bates 1 Outline
EL-GY 6813/BE-GY 6203 Medical Imaging, Fall 2016 Final Exam
 EL-GY 6813/BE-GY 6203 Medical Imaging, Fall 2016 Final Exam (closed book, 1 sheets of notes double sided allowed, no calculator or other electronic devices allowed) 1. Ultrasound Physics (15 pt) A) (9
EL-GY 6813/BE-GY 6203 Medical Imaging, Fall 2016 Final Exam (closed book, 1 sheets of notes double sided allowed, no calculator or other electronic devices allowed) 1. Ultrasound Physics (15 pt) A) (9
Part II: Magnetic Resonance Imaging (MRI)
 Part II: Magnetic Resonance Imaging (MRI) Contents Magnetic Field Gradients Selective Excitation Spatially Resolved Reception k-space Gradient Echo Sequence Spin Echo Sequence Magnetic Resonance Imaging
Part II: Magnetic Resonance Imaging (MRI) Contents Magnetic Field Gradients Selective Excitation Spatially Resolved Reception k-space Gradient Echo Sequence Spin Echo Sequence Magnetic Resonance Imaging
Relaxation times in nuclear magnetic resonance
 Relaxation times in TEP Related topics Nuclear spins, atomic nuclei with a magnetic moment, precession movement of the nuclear spins, Landau-Lifshitz equation, Bloch equation, magnetisation, resonance
Relaxation times in TEP Related topics Nuclear spins, atomic nuclei with a magnetic moment, precession movement of the nuclear spins, Landau-Lifshitz equation, Bloch equation, magnetisation, resonance
Chemistry 431. Lecture 23
 Chemistry 431 Lecture 23 Introduction The Larmor Frequency The Bloch Equations Measuring T 1 : Inversion Recovery Measuring T 2 : the Spin Echo NC State University NMR spectroscopy The Nuclear Magnetic
Chemistry 431 Lecture 23 Introduction The Larmor Frequency The Bloch Equations Measuring T 1 : Inversion Recovery Measuring T 2 : the Spin Echo NC State University NMR spectroscopy The Nuclear Magnetic
Sketch of the MRI Device
 Outline for Today 1. 2. 3. Introduction to MRI Quantum NMR and MRI in 0D Magnetization, m(x,t), in a Voxel Proton T1 Spin Relaxation in a Voxel Proton Density MRI in 1D MRI Case Study, and Caveat Sketch
Outline for Today 1. 2. 3. Introduction to MRI Quantum NMR and MRI in 0D Magnetization, m(x,t), in a Voxel Proton T1 Spin Relaxation in a Voxel Proton Density MRI in 1D MRI Case Study, and Caveat Sketch
K-space. Spin-Warp Pulse Sequence. At each point in time, the received signal is the Fourier transform of the object s(t) = M( k x
 Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 2015 MRI Lecture 4 k (t) = γ 2π k y (t) = γ 2π K-space At each point in time, the received signal is the Fourier transform of the object
Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 2015 MRI Lecture 4 k (t) = γ 2π k y (t) = γ 2π K-space At each point in time, the received signal is the Fourier transform of the object
Basis of MRI Contrast
 Basis of MRI Contrast MARK A. HORSFIELD Department of Cardiovascular Sciences University of Leicester Leicester LE1 5WW UK Tel: +44-116-2585080 Fax: +44-870-7053111 e-mail: mah5@le.ac.uk 1 1.1 The Magnetic
Basis of MRI Contrast MARK A. HORSFIELD Department of Cardiovascular Sciences University of Leicester Leicester LE1 5WW UK Tel: +44-116-2585080 Fax: +44-870-7053111 e-mail: mah5@le.ac.uk 1 1.1 The Magnetic
NMR Spectroscopy of Polymers
 UNESCO/IUPAC Course 2005/2006 Jiri Brus NMR Spectroscopy of Polymers Brus J 1. part At the very beginning the phenomenon of nuclear spin resonance was studied predominantly by physicists and the application
UNESCO/IUPAC Course 2005/2006 Jiri Brus NMR Spectroscopy of Polymers Brus J 1. part At the very beginning the phenomenon of nuclear spin resonance was studied predominantly by physicists and the application
The Physical Basis of Nuclear Magnetic Resonance Part I ESMRMB. Jürgen R. Reichenbach
 The Physical Basis of Nuclear agnetic Resonance Part I Jürgen R. Reichenbach odule 1 October 17, 216 Outline of odule Introduction Spin and magnetic moment Spin precession, Larmor frequency agnetic properties
The Physical Basis of Nuclear agnetic Resonance Part I Jürgen R. Reichenbach odule 1 October 17, 216 Outline of odule Introduction Spin and magnetic moment Spin precession, Larmor frequency agnetic properties
NUCLEAR MAGNETIC RESONANCE. The phenomenon of nuclear magnetic resonance will be used to study magnetic moments of nuclei.
 14 Sep 11 NMR.1 NUCLEAR MAGNETIC RESONANCE The phenomenon of nuclear magnetic resonance will be used to study magnetic moments of nuclei. Theory: In addition to its well-known properties of mass, charge,
14 Sep 11 NMR.1 NUCLEAR MAGNETIC RESONANCE The phenomenon of nuclear magnetic resonance will be used to study magnetic moments of nuclei. Theory: In addition to its well-known properties of mass, charge,
Part III: Sequences and Contrast
 Part III: Sequences and Contrast Contents T1 and T2/T2* Relaxation Contrast of Imaging Sequences T1 weighting T2/T2* weighting Contrast Agents Saturation Inversion Recovery JUST WATER? (i.e., proton density
Part III: Sequences and Contrast Contents T1 and T2/T2* Relaxation Contrast of Imaging Sequences T1 weighting T2/T2* weighting Contrast Agents Saturation Inversion Recovery JUST WATER? (i.e., proton density
Biomedical Imaging Magnetic Resonance Imaging
 Biomedical Imaging Magnetic Resonance Imaging Charles A. DiMarzio & Eric Kercher EECE 4649 Northeastern University May 2018 Background and History Measurement of Nuclear Spins Widely used in physics/chemistry
Biomedical Imaging Magnetic Resonance Imaging Charles A. DiMarzio & Eric Kercher EECE 4649 Northeastern University May 2018 Background and History Measurement of Nuclear Spins Widely used in physics/chemistry
The Theory of Nuclear Magnetic Resonance Behind Magnetic Resonance Imaging. Catherine Wasko Physics 304 Physics of the Human Body May 3, 2005
 The Theory of Nuclear Magnetic Resonance Behind Magnetic Resonance Imaging Catherine Wasko Physics 304 Physics of the Human Body May 3, 2005 Magnetic resonance imaging (MRI) is a tool utilized in the medical
The Theory of Nuclear Magnetic Resonance Behind Magnetic Resonance Imaging Catherine Wasko Physics 304 Physics of the Human Body May 3, 2005 Magnetic resonance imaging (MRI) is a tool utilized in the medical
Introduction to Magnetic Resonance Imaging (MRI) Pietro Gori
 Introduction to Magnetic Resonance Imaging (MRI) Pietro Gori Enseignant-chercheur Equipe IMAGES - Télécom ParisTech pietro.gori@telecom-paristech.fr September 20, 2017 P. Gori BIOMED 20/09/2017 1 / 76
Introduction to Magnetic Resonance Imaging (MRI) Pietro Gori Enseignant-chercheur Equipe IMAGES - Télécom ParisTech pietro.gori@telecom-paristech.fr September 20, 2017 P. Gori BIOMED 20/09/2017 1 / 76
Apodization. Gibbs Artifact. Bioengineering 280A Principles of Biomedical Imaging. Fall Quarter 2013 MRI Lecture 5. rect(k x )
 Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 2013 MRI Lecture 5 GE Medical Systems 2003 Gibbs Artifact Apodization rect(k ) Hanning Window h(k )=1/2(1+cos(2πk ) 256256 image 256128
Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 2013 MRI Lecture 5 GE Medical Systems 2003 Gibbs Artifact Apodization rect(k ) Hanning Window h(k )=1/2(1+cos(2πk ) 256256 image 256128
Chapter 14:Physics of Magnetic Resonance
 Chapter 14:Physics of Magnetic Resonance Slide set of 141 slides based on the chapter authored by Hee Kwon Song of the publication (ISBN 978-92-0-131010-1): Diagnostic Radiology Physics: A Handbook for
Chapter 14:Physics of Magnetic Resonance Slide set of 141 slides based on the chapter authored by Hee Kwon Song of the publication (ISBN 978-92-0-131010-1): Diagnostic Radiology Physics: A Handbook for
Spin Dynamics Basics of Nuclear Magnetic Resonance. Malcolm H. Levitt
 Spin Dynamics Basics of Nuclear Magnetic Resonance Second edition Malcolm H. Levitt The University of Southampton, UK John Wiley &. Sons, Ltd Preface xxi Preface to the First Edition xxiii Introduction
Spin Dynamics Basics of Nuclear Magnetic Resonance Second edition Malcolm H. Levitt The University of Southampton, UK John Wiley &. Sons, Ltd Preface xxi Preface to the First Edition xxiii Introduction
MRI in Review: Simple Steps to Cutting Edge Part I
 MRI in Review: Simple Steps to Cutting Edge Part I DWI is now 2 years old... Mike Moseley Radiology Stanford DWI, b = 1413 T2wt, 28/16 ASN 21 San Francisco + Disclosures: Funding NINDS, NCRR, NCI 45 minutes
MRI in Review: Simple Steps to Cutting Edge Part I DWI is now 2 years old... Mike Moseley Radiology Stanford DWI, b = 1413 T2wt, 28/16 ASN 21 San Francisco + Disclosures: Funding NINDS, NCRR, NCI 45 minutes
Bloch Equations & Relaxation UCLA. Radiology
 Bloch Equations & Relaxation MRI Systems II B1 I 1 I ~B 1 (t) I 6 ~M I I 5 I 4 Lecture # Learning Objectives Distinguish spin, precession, and nutation. Appreciate that any B-field acts on the the spin
Bloch Equations & Relaxation MRI Systems II B1 I 1 I ~B 1 (t) I 6 ~M I I 5 I 4 Lecture # Learning Objectives Distinguish spin, precession, and nutation. Appreciate that any B-field acts on the the spin
Topics. Spin. The concept of spin Precession of magnetic spin Relaxation Bloch Equation
 Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 2005 MRI Lecture 1 Topics The concept of spin Precession of magnetic spin Relaation Bloch Equation Spin Intrinsic angular momentum of elementary
Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 2005 MRI Lecture 1 Topics The concept of spin Precession of magnetic spin Relaation Bloch Equation Spin Intrinsic angular momentum of elementary
NMR course at the FMP: NMR of organic compounds and small biomolecules - II -
 NMR course at the FMP: NMR of organic compounds and small biomolecules - II - 16.03.2009 The program 2/76 CW vs. FT NMR What is a pulse? Vectormodel Water-flip-back 3/76 CW vs. FT CW vs. FT 4/76 Two methods
NMR course at the FMP: NMR of organic compounds and small biomolecules - II - 16.03.2009 The program 2/76 CW vs. FT NMR What is a pulse? Vectormodel Water-flip-back 3/76 CW vs. FT CW vs. FT 4/76 Two methods
BME I5000: Biomedical Imaging
 BME I5000: Biomedical Imaging Lecture 9 Magnetic Resonance Imaging (imaging) Lucas C. Parra, parra@ccny.cuny.edu Blackboard: http://cityonline.ccny.cuny.edu/ 1 Schedule 1. Introduction, Spatial Resolution,
BME I5000: Biomedical Imaging Lecture 9 Magnetic Resonance Imaging (imaging) Lucas C. Parra, parra@ccny.cuny.edu Blackboard: http://cityonline.ccny.cuny.edu/ 1 Schedule 1. Introduction, Spatial Resolution,
Index. p, lip, 78 8 function, 107 v, 7-8 w, 7-8 i,7-8 sine, 43 Bo,94-96
 p, lip, 78 8 function, 107 v, 7-8 w, 7-8 i,7-8 sine, 43 Bo,94-96 B 1,94-96 M,94-96 B oro!' 94-96 BIro!' 94-96 I/r, 79 2D linear system, 56 2D FFT, 119 2D Fourier transform, 1, 12, 18,91 2D sinc, 107, 112
p, lip, 78 8 function, 107 v, 7-8 w, 7-8 i,7-8 sine, 43 Bo,94-96 B 1,94-96 M,94-96 B oro!' 94-96 BIro!' 94-96 I/r, 79 2D linear system, 56 2D FFT, 119 2D Fourier transform, 1, 12, 18,91 2D sinc, 107, 112
10.4 Continuous Wave NMR Instrumentation
 10.4 Continuous Wave NMR Instrumentation coherent detection bulk magnetization the rotating frame, and effective magnetic field generating a rotating frame, and precession in the laboratory frame spin-lattice
10.4 Continuous Wave NMR Instrumentation coherent detection bulk magnetization the rotating frame, and effective magnetic field generating a rotating frame, and precession in the laboratory frame spin-lattice
NMR, the vector model and the relaxation
 NMR, the vector model and the relaxation Reading/Books: One and two dimensional NMR spectroscopy, VCH, Friebolin Spin Dynamics, Basics of NMR, Wiley, Levitt Molecular Quantum Mechanics, Oxford Univ. Press,
NMR, the vector model and the relaxation Reading/Books: One and two dimensional NMR spectroscopy, VCH, Friebolin Spin Dynamics, Basics of NMR, Wiley, Levitt Molecular Quantum Mechanics, Oxford Univ. Press,
Spectral Broadening Mechanisms
 Spectral Broadening Mechanisms Lorentzian broadening (Homogeneous) Gaussian broadening (Inhomogeneous, Inertial) Doppler broadening (special case for gas phase) The Fourier Transform NC State University
Spectral Broadening Mechanisms Lorentzian broadening (Homogeneous) Gaussian broadening (Inhomogeneous, Inertial) Doppler broadening (special case for gas phase) The Fourier Transform NC State University
Magnetic Resonance Imaging (MRI)
 Magnetic Resonance Imaging Introduction The Components The Technology (MRI) Physics behind MR Most slides taken from http:// www.slideworld.org/ viewslides.aspx/magnetic- Resonance-Imaging- %28MRI%29-MR-Imaging-
Magnetic Resonance Imaging Introduction The Components The Technology (MRI) Physics behind MR Most slides taken from http:// www.slideworld.org/ viewslides.aspx/magnetic- Resonance-Imaging- %28MRI%29-MR-Imaging-
Chapter 7. Nuclear Magnetic Resonance Spectroscopy
 Chapter 7 Nuclear Magnetic Resonance Spectroscopy I. Introduction 1924, W. Pauli proposed that certain atomic nuclei have spin and magnetic moment and exposure to magnetic field would lead to energy level
Chapter 7 Nuclear Magnetic Resonance Spectroscopy I. Introduction 1924, W. Pauli proposed that certain atomic nuclei have spin and magnetic moment and exposure to magnetic field would lead to energy level
Classical Description of NMR Parameters: The Bloch Equations
 Classical Description of NMR Parameters: The Bloch Equations Pascale Legault Département de Biochimie Université de Montréal 1 Outline 1) Classical Behavior of Magnetic Nuclei: The Bloch Equation 2) Precession
Classical Description of NMR Parameters: The Bloch Equations Pascale Legault Département de Biochimie Université de Montréal 1 Outline 1) Classical Behavior of Magnetic Nuclei: The Bloch Equation 2) Precession
2.1.1 A Brief History of NMR The conception of NMR sprouted after the Pauli s prediction of nuclear spin in
 CHAPTER--2 BASICS OF NMR IMAGING AND SPECTROSCOPY 2.1 Introduction 2.1.1 A Brief History of NMR The conception of NMR sprouted after the Pauli s prediction of nuclear spin in 1924. Later Gorter (1936)
CHAPTER--2 BASICS OF NMR IMAGING AND SPECTROSCOPY 2.1 Introduction 2.1.1 A Brief History of NMR The conception of NMR sprouted after the Pauli s prediction of nuclear spin in 1924. Later Gorter (1936)
A Hands on Introduction to NMR Lecture #1 Nuclear Spin and Magnetic Resonance
 A Hands on Introduction to NMR 22.920 Lecture #1 Nuclear Spin and Magnetic Resonance Introduction - The aim of this short course is to present a physical picture of the basic principles of Nuclear Magnetic
A Hands on Introduction to NMR 22.920 Lecture #1 Nuclear Spin and Magnetic Resonance Introduction - The aim of this short course is to present a physical picture of the basic principles of Nuclear Magnetic
NMR/MRI examination (8N080 / 3F240)
 NMR/MRI examination (8N080 / 3F240) Remarks: 1. This test consists of 3 problems with at total of 26 sub-questions. 2. Questions are in English. You are allowed to answer them in English or Dutch. 3. Please
NMR/MRI examination (8N080 / 3F240) Remarks: 1. This test consists of 3 problems with at total of 26 sub-questions. 2. Questions are in English. You are allowed to answer them in English or Dutch. 3. Please
Principles of Nuclear Magnetic Resonance Microscopy
 Principles of Nuclear Magnetic Resonance Microscopy Paul T. Callaghan Department of Physics and Biophysics Massey University New Zealand CLARENDON PRESS OXFORD CONTENTS 1 PRINCIPLES OF IMAGING 1 1.1 Introduction
Principles of Nuclear Magnetic Resonance Microscopy Paul T. Callaghan Department of Physics and Biophysics Massey University New Zealand CLARENDON PRESS OXFORD CONTENTS 1 PRINCIPLES OF IMAGING 1 1.1 Introduction
Classical Description of NMR Parameters: The Bloch Equations
 Classical Description of NMR Parameters: The Bloch Equations Pascale Legault Département de Biochimie Université de Montréal 1 Outline 1) Classical Behavior of Magnetic Nuclei: The Bloch Equation 2) Precession
Classical Description of NMR Parameters: The Bloch Equations Pascale Legault Département de Biochimie Université de Montréal 1 Outline 1) Classical Behavior of Magnetic Nuclei: The Bloch Equation 2) Precession
Magnetic resonance imaging MRI
 Magnetic resonance imaging MRI Introduction What is MRI MRI is an imaging technique used primarily in medical settings that uses a strong magnetic field and radio waves to produce very clear and detailed
Magnetic resonance imaging MRI Introduction What is MRI MRI is an imaging technique used primarily in medical settings that uses a strong magnetic field and radio waves to produce very clear and detailed
COPYRIGHTED MATERIAL. Production of Net Magnetization. Chapter 1
 Chapter 1 Production of Net Magnetization Magnetic resonance (MR) is a measurement technique used to examine atoms and molecules. It is based on the interaction between an applied magnetic field and a
Chapter 1 Production of Net Magnetization Magnetic resonance (MR) is a measurement technique used to examine atoms and molecules. It is based on the interaction between an applied magnetic field and a
Basic p rinciples COPYRIGHTED MATERIAL. Introduction. Atomic s tructure
 1 Basic p rinciples Introduction 1 Atomic structure 1 Motion in the atom 2 MR active nuclei 2 The hydrogen nucleus 4 Alignment 4 Precession 8 The Larmor equation 9 Introduction The basic principles of
1 Basic p rinciples Introduction 1 Atomic structure 1 Motion in the atom 2 MR active nuclei 2 The hydrogen nucleus 4 Alignment 4 Precession 8 The Larmor equation 9 Introduction The basic principles of
Physics of MR Image Acquisition
 Physics of MR Image Acquisition HST-583, Fall 2002 Review: -MRI: Overview - MRI: Spatial Encoding MRI Contrast: Basic sequences - Gradient Echo - Spin Echo - Inversion Recovery : Functional Magnetic Resonance
Physics of MR Image Acquisition HST-583, Fall 2002 Review: -MRI: Overview - MRI: Spatial Encoding MRI Contrast: Basic sequences - Gradient Echo - Spin Echo - Inversion Recovery : Functional Magnetic Resonance
Field trip: Tuesday, Feb 5th
 Pulse Sequences Field trip: Tuesday, Feb 5th Hardware tour of VUIIIS Philips 3T Meet here at regular class time (11.15) Complete MRI screening form! Chuck Nockowski Philips Service Engineer Reminder: Project/Presentation
Pulse Sequences Field trip: Tuesday, Feb 5th Hardware tour of VUIIIS Philips 3T Meet here at regular class time (11.15) Complete MRI screening form! Chuck Nockowski Philips Service Engineer Reminder: Project/Presentation
Chem 325 NMR Intro. The Electromagnetic Spectrum. Physical properties, chemical properties, formulas Shedding real light on molecular structure:
 Physical properties, chemical properties, formulas Shedding real light on molecular structure: Wavelength Frequency ν Wavelength λ Frequency ν Velocity c = 2.998 10 8 m s -1 The Electromagnetic Spectrum
Physical properties, chemical properties, formulas Shedding real light on molecular structure: Wavelength Frequency ν Wavelength λ Frequency ν Velocity c = 2.998 10 8 m s -1 The Electromagnetic Spectrum
CONTENTS. 2 CLASSICAL DESCRIPTION 2.1 The resonance phenomenon 2.2 The vector picture for pulse EPR experiments 2.3 Relaxation and the Bloch equations
 CONTENTS Preface Acknowledgements Symbols Abbreviations 1 INTRODUCTION 1.1 Scope of pulse EPR 1.2 A short history of pulse EPR 1.3 Examples of Applications 2 CLASSICAL DESCRIPTION 2.1 The resonance phenomenon
CONTENTS Preface Acknowledgements Symbols Abbreviations 1 INTRODUCTION 1.1 Scope of pulse EPR 1.2 A short history of pulse EPR 1.3 Examples of Applications 2 CLASSICAL DESCRIPTION 2.1 The resonance phenomenon
Tissue Characteristics Module Three
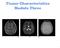 Tissue Characteristics Module Three 1 Equilibrium State Equilibrium State At equilibrium, the hydrogen vector is oriented in a direction parallel to the main magnetic field. Hydrogen atoms within the vector
Tissue Characteristics Module Three 1 Equilibrium State Equilibrium State At equilibrium, the hydrogen vector is oriented in a direction parallel to the main magnetic field. Hydrogen atoms within the vector
MRS: IN VIVO SPECTROSCOPIC IMAGING MAIN POINTS
 MRS: IN VIVO SPECTROSCOPIC IMAGING MAIN POINTS 1. A MR spectrum can identify many metabolites other than water by: Locating the peak(s) determined by a characteristic chemical shift (ppm) resulting from
MRS: IN VIVO SPECTROSCOPIC IMAGING MAIN POINTS 1. A MR spectrum can identify many metabolites other than water by: Locating the peak(s) determined by a characteristic chemical shift (ppm) resulting from
1 Magnetism, Curie s Law and the Bloch Equations
 1 Magnetism, Curie s Law and the Bloch Equations In NMR, the observable which is measured is magnetization and its evolution over time. In order to understand what this means, let us first begin with some
1 Magnetism, Curie s Law and the Bloch Equations In NMR, the observable which is measured is magnetization and its evolution over time. In order to understand what this means, let us first begin with some
Low Field MRI of Laser Polarized Noble Gases. Yuan Zheng, 4 th year seminar, Feb, 2013
 Low Field MRI of Laser Polarized Noble Gases Yuan Zheng, 4 th year seminar, Feb, 2013 Outline Introduction to conventional MRI Low field MRI of Laser Polarized (LP) noble gases Spin Exchange Optical Pumping
Low Field MRI of Laser Polarized Noble Gases Yuan Zheng, 4 th year seminar, Feb, 2013 Outline Introduction to conventional MRI Low field MRI of Laser Polarized (LP) noble gases Spin Exchange Optical Pumping
RADIOLOGIV TECHNOLOGY 4912 COMPREHENSEIVE REVIEW/MRI WORSHEET #1- PATIENT CARE AND SAFETY/PHYSICAL PRINCIPLES
 RADIOLOGIV TECHNOLOGY 4912 COMPREHENSEIVE REVIEW/MRI WORSHEET #1- PATIENT CARE AND SAFETY/PHYSICAL PRINCIPLES 1. What are potential consequences to patients and personnel should there be a release of gaseous
RADIOLOGIV TECHNOLOGY 4912 COMPREHENSEIVE REVIEW/MRI WORSHEET #1- PATIENT CARE AND SAFETY/PHYSICAL PRINCIPLES 1. What are potential consequences to patients and personnel should there be a release of gaseous
Doppler echocardiography & Magnetic Resonance Imaging. Doppler echocardiography. History: - Langevin developed sonar.
 1 Doppler echocardiography & Magnetic Resonance Imaging History: - Langevin developed sonar. - 1940s development of pulse-echo. - 1950s development of mode A and B. - 1957 development of continuous wave
1 Doppler echocardiography & Magnetic Resonance Imaging History: - Langevin developed sonar. - 1940s development of pulse-echo. - 1950s development of mode A and B. - 1957 development of continuous wave
Biochemistry 530 NMR Theory and Practice
 Biochemistry 530 NMR Theory and Practice Gabriele Varani Department of Biochemistry and Department of Chemistry University of Washington Lecturer: Gabriele Varani Biochemistry and Chemistry Room J479 and
Biochemistry 530 NMR Theory and Practice Gabriele Varani Department of Biochemistry and Department of Chemistry University of Washington Lecturer: Gabriele Varani Biochemistry and Chemistry Room J479 and
Outlines: (June 11, 1996) Instructor:
 Magnetic Resonance Imaging (June 11, 1996) Instructor: Tai-huang Huang Institute of Biomedical Sciences Academia Sinica Tel. (02) 2652-3036; Fax. (02) 2788-7641 E. mail: bmthh@ibms.sinica.edu.tw Reference:
Magnetic Resonance Imaging (June 11, 1996) Instructor: Tai-huang Huang Institute of Biomedical Sciences Academia Sinica Tel. (02) 2652-3036; Fax. (02) 2788-7641 E. mail: bmthh@ibms.sinica.edu.tw Reference:
Bioengineering 278" Magnetic Resonance Imaging" Winter 2010" Lecture 1! Topics:! Review of NMR basics! Hardware Overview! Quadrature Detection!
 Bioengineering 278" Magnetic Resonance Imaging" Winter 2010" Lecture 1 Topics: Review of NMR basics Hardware Overview Quadrature Detection Boltzmann Distribution B 0 " = µ z $ 0 % " = #h$ 0 % " = µ z $
Bioengineering 278" Magnetic Resonance Imaging" Winter 2010" Lecture 1 Topics: Review of NMR basics Hardware Overview Quadrature Detection Boltzmann Distribution B 0 " = µ z $ 0 % " = #h$ 0 % " = µ z $
Spin. Nuclear Spin Rules
 Spin Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 2012 MRI Lecture 1 Intrinsic angular momentum of elementary particles -- electrons, protons, neutrons. Spin is quantized. Key concept
Spin Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 2012 MRI Lecture 1 Intrinsic angular momentum of elementary particles -- electrons, protons, neutrons. Spin is quantized. Key concept
The physics US and MRI. Prof. Peter Bogner
 The physics US and MRI Prof. Peter Bogner Sound waves mechanical disturbance, a pressure wave moves along longitudinal wave compression rarefaction zones c = nl, (c: velocity, n: frequency, l: wavelength
The physics US and MRI Prof. Peter Bogner Sound waves mechanical disturbance, a pressure wave moves along longitudinal wave compression rarefaction zones c = nl, (c: velocity, n: frequency, l: wavelength
Principles of MRI. Vinyl Record. Last time: Today: Homework Due tonight! EE225E / BIO265. Transforms a temporal signal to a spatial signal
 What is this? ` Principles of MRI Lecture 05 EE225E / BIO265 Instructor: Miki Lustig UC Berkeley, EECS The first NMR spectrum of ethanol 1951. 1 2 Today Last time: Linear systems, Fourier Transforms, Sampling
What is this? ` Principles of MRI Lecture 05 EE225E / BIO265 Instructor: Miki Lustig UC Berkeley, EECS The first NMR spectrum of ethanol 1951. 1 2 Today Last time: Linear systems, Fourier Transforms, Sampling
Exam 8N080 - Introduction to MRI
 Exam 8N080 - Introduction to MRI Friday April 10 2015, 18.00-21.00 h For this exam you may use an ordinary calculator (not a graphical one). In total there are 5 assignments and a total of 50 points can
Exam 8N080 - Introduction to MRI Friday April 10 2015, 18.00-21.00 h For this exam you may use an ordinary calculator (not a graphical one). In total there are 5 assignments and a total of 50 points can
Introductory MRI Physics
 C HAPR 18 Introductory MRI Physics Aaron Sodickson EXRNAL MAGNETIC FIELD, PROTONS AND EQUILIBRIUM MAGNETIZATION Much of the bulk of the magnetic resonance imaging (MRI) scanner apparatus is dedicated to
C HAPR 18 Introductory MRI Physics Aaron Sodickson EXRNAL MAGNETIC FIELD, PROTONS AND EQUILIBRIUM MAGNETIZATION Much of the bulk of the magnetic resonance imaging (MRI) scanner apparatus is dedicated to
NMR Spectroscopy: A Quantum Phenomena
 NMR Spectroscopy: A Quantum Phenomena Pascale Legault Département de Biochimie Université de Montréal Outline 1) Energy Diagrams and Vector Diagrams 2) Simple 1D Spectra 3) Beyond Simple 1D Spectra 4)
NMR Spectroscopy: A Quantum Phenomena Pascale Legault Département de Biochimie Université de Montréal Outline 1) Energy Diagrams and Vector Diagrams 2) Simple 1D Spectra 3) Beyond Simple 1D Spectra 4)
Spectral Broadening Mechanisms. Broadening mechanisms. Lineshape functions. Spectral lifetime broadening
 Spectral Broadening echanisms Lorentzian broadening (Homogeneous) Gaussian broadening (Inhomogeneous, Inertial) Doppler broadening (special case for gas phase) The Fourier Transform NC State University
Spectral Broadening echanisms Lorentzian broadening (Homogeneous) Gaussian broadening (Inhomogeneous, Inertial) Doppler broadening (special case for gas phase) The Fourier Transform NC State University
Measuring Spin-Lattice Relaxation Time
 WJP, PHY381 (2009) Wabash Journal of Physics v4.0, p.1 Measuring Spin-Lattice Relaxation Time L.W. Lupinski, R. Paudel, and M.J. Madsen Department of Physics, Wabash College, Crawfordsville, IN 47933 (Dated:
WJP, PHY381 (2009) Wabash Journal of Physics v4.0, p.1 Measuring Spin-Lattice Relaxation Time L.W. Lupinski, R. Paudel, and M.J. Madsen Department of Physics, Wabash College, Crawfordsville, IN 47933 (Dated:
Ferdowsi University of Mashhad
 Spectroscopy in Inorganic Chemistry Nuclear Magnetic Resonance Spectroscopy spin deuterium 2 helium 3 The neutron has 2 quarks with a -e/3 charge and one quark with a +2e/3 charge resulting in a total
Spectroscopy in Inorganic Chemistry Nuclear Magnetic Resonance Spectroscopy spin deuterium 2 helium 3 The neutron has 2 quarks with a -e/3 charge and one quark with a +2e/3 charge resulting in a total
Lecture 02 Nuclear Magnetic Resonance Spectroscopy Principle and Application in Structure Elucidation
 Application of Spectroscopic Methods in Molecular Structure Determination Prof. S. Sankararaman Department of Chemistry Indian Institution of Technology Madras Lecture 02 Nuclear Magnetic Resonance Spectroscopy
Application of Spectroscopic Methods in Molecular Structure Determination Prof. S. Sankararaman Department of Chemistry Indian Institution of Technology Madras Lecture 02 Nuclear Magnetic Resonance Spectroscopy
Technical University of Denmark
 Technical University of Denmark Page 1 of 11 pages Written test, 9 December 2010 Course name: Introduction to medical imaging Course no. 31540 Aids allowed: none. "Weighting": All problems weight equally.
Technical University of Denmark Page 1 of 11 pages Written test, 9 December 2010 Course name: Introduction to medical imaging Course no. 31540 Aids allowed: none. "Weighting": All problems weight equally.
Lecture 21. Nuclear magnetic resonance
 Lecture 21 Nuclear magnetic resonance A very brief history Stern and Gerlach atomic beam experiments Isidor Rabi molecular beam exp.; nuclear magnetic moments (angular momentum) Felix Bloch & Edward Purcell
Lecture 21 Nuclear magnetic resonance A very brief history Stern and Gerlach atomic beam experiments Isidor Rabi molecular beam exp.; nuclear magnetic moments (angular momentum) Felix Bloch & Edward Purcell
BASIC MRI PHYSICS SPIN GYMNASTICS Don Plewes PhD, Walter Kucharczyk MD
 BASIC MRI PHYSICS SPIN GYMNASTICS Don Plewes PhD, Walter Kucharczyk MD Introduction To understand MRI, it is first necessary to understand the physics of proton Nuclear Magnetic Resonance (NMR). The most
BASIC MRI PHYSICS SPIN GYMNASTICS Don Plewes PhD, Walter Kucharczyk MD Introduction To understand MRI, it is first necessary to understand the physics of proton Nuclear Magnetic Resonance (NMR). The most
Tissue Parametric Mapping:
 Tissue Parametric Mapping: Contrast Mechanisms Using SSFP Sequences Jongho Lee Department of Radiology University of Pennsylvania Tissue Parametric Mapping: Contrast Mechanisms Using bssfp Sequences Jongho
Tissue Parametric Mapping: Contrast Mechanisms Using SSFP Sequences Jongho Lee Department of Radiology University of Pennsylvania Tissue Parametric Mapping: Contrast Mechanisms Using bssfp Sequences Jongho
MRI Physics II: Gradients, Imaging. Douglas C. Noll, Ph.D. Dept. of Biomedical Engineering University of Michigan, Ann Arbor
 MRI Physics II: Gradients, Imaging Douglas C., Ph.D. Dept. of Biomedical Engineering University of Michigan, Ann Arbor Magnetic Fields in MRI B 0 The main magnetic field. Always on (0.5-7 T) Magnetizes
MRI Physics II: Gradients, Imaging Douglas C., Ph.D. Dept. of Biomedical Engineering University of Michigan, Ann Arbor Magnetic Fields in MRI B 0 The main magnetic field. Always on (0.5-7 T) Magnetizes
Topics. The History of Spin. Spin. The concept of spin Precession of magnetic spin Relaxation
 Topics Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 2008 MRI Lecture 1 The concept of spin Precession of magnetic spin Relaation Spin The History of Spin Intrinsic angular momentum
Topics Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 2008 MRI Lecture 1 The concept of spin Precession of magnetic spin Relaation Spin The History of Spin Intrinsic angular momentum
Chapter 1 Introduction
 Chapter 1 Introduction A journey of a thousand miles must begin with a single step. LaoZi Tomography is an important area in the ever-growing field of imaging science. The term tomos (rofio
Chapter 1 Introduction A journey of a thousand miles must begin with a single step. LaoZi Tomography is an important area in the ever-growing field of imaging science. The term tomos (rofio
Technical University of Denmark
 Technical University of Denmark Page 1 of 10 pages Written test, 12 December 2012 Course name: Introduction to medical imaging Course no. 31540 Aids allowed: None. Pocket calculator not allowed "Weighting":
Technical University of Denmark Page 1 of 10 pages Written test, 12 December 2012 Course name: Introduction to medical imaging Course no. 31540 Aids allowed: None. Pocket calculator not allowed "Weighting":
Functional Magnetic Resonance Imaging (FMRI) is an imaging technique for
 Chapter 2 Principles of FMRI Functional Magnetic Resonance Imaging (FMRI) is an imaging technique for examining brain function. Since its first appearance in 1991 (Belliveau et al.[8]) the use of FMRI
Chapter 2 Principles of FMRI Functional Magnetic Resonance Imaging (FMRI) is an imaging technique for examining brain function. Since its first appearance in 1991 (Belliveau et al.[8]) the use of FMRI
Biophysical Chemistry: NMR Spectroscopy
 Relaxation & Multidimensional Spectrocopy Vrije Universiteit Brussel 9th December 2011 Outline 1 Relaxation 2 Principles 3 Outline 1 Relaxation 2 Principles 3 Establishment of Thermal Equilibrium As previously
Relaxation & Multidimensional Spectrocopy Vrije Universiteit Brussel 9th December 2011 Outline 1 Relaxation 2 Principles 3 Outline 1 Relaxation 2 Principles 3 Establishment of Thermal Equilibrium As previously
Spin. Nuclear Spin Rules
 Spin Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 203 MRI Lecture Intrinsic angular momentum of elementary particles -- electrons, protons, neutrons. Spin is quantized. Key concept
Spin Bioengineering 280A Principles of Biomedical Imaging Fall Quarter 203 MRI Lecture Intrinsic angular momentum of elementary particles -- electrons, protons, neutrons. Spin is quantized. Key concept
The Nuclear Emphasis
 The Nuclear Emphasis Atoms are composed of electrons and nuclei we ll focus almost exclusively on the physical properties of the nucleus and the chemicoelectronic attributes of its environment. The nucleus
The Nuclear Emphasis Atoms are composed of electrons and nuclei we ll focus almost exclusively on the physical properties of the nucleus and the chemicoelectronic attributes of its environment. The nucleus
Background II. Signal-to-Noise Ratio (SNR) Pulse Sequences Sampling and Trajectories Parallel Imaging. B.Hargreaves - RAD 229.
 Background II Signal-to-Noise Ratio (SNR) Pulse Sequences Sampling and Trajectories Parallel Imaging 1 SNR: Signal-to-Noise Ratio Signal: Desired voltage in coil Noise: Thermal, electronic Noise Thermal
Background II Signal-to-Noise Ratio (SNR) Pulse Sequences Sampling and Trajectories Parallel Imaging 1 SNR: Signal-to-Noise Ratio Signal: Desired voltage in coil Noise: Thermal, electronic Noise Thermal
Physics of Imaging Systems Basic Principles of Magnetic Resonance Imaging II
 1 3/14/2019 Page 1 Master s Program in Medical Physics Physics of Imaging Systems Basic Principles of Magnetic Resonance Imaging II Chair in Faculty of Medicine Mannheim University of Heidelberg Theodor-Kutzer-Ufer
1 3/14/2019 Page 1 Master s Program in Medical Physics Physics of Imaging Systems Basic Principles of Magnetic Resonance Imaging II Chair in Faculty of Medicine Mannheim University of Heidelberg Theodor-Kutzer-Ufer
Magnetic Resonance Spectroscopy EPR and NMR
 Magnetic Resonance Spectroscopy EPR and NMR A brief review of the relevant bits of quantum mechanics 1. Electrons have spin, - rotation of the charge about its axis generates a magnetic field at each electron.
Magnetic Resonance Spectroscopy EPR and NMR A brief review of the relevant bits of quantum mechanics 1. Electrons have spin, - rotation of the charge about its axis generates a magnetic field at each electron.
MRI in Practice. Catherine Westbrook MSc, DCRR, CTC Senior Lecturer Anglia Polytechnic University Cambridge UK. John Talbot MSc, DCRR
 MRI in Practice Third edition Catherine Westbrook MSc, DCRR, CTC Senior Lecturer Anglia Polytechnic University Cambridge UK and Carolyn Kaut RothRT(R) (MR) (CT) (M) (CV) Fellow SMRT (Section for Magnetic
MRI in Practice Third edition Catherine Westbrook MSc, DCRR, CTC Senior Lecturer Anglia Polytechnic University Cambridge UK and Carolyn Kaut RothRT(R) (MR) (CT) (M) (CV) Fellow SMRT (Section for Magnetic
