Thermodynamics Heat & Internal Energy Heat Capacity
|
|
|
- Abraham Tate
- 5 years ago
- Views:
Transcription
1 Thermodynamics Heat & Internal Energy Heat Capacity Lana Sheridan De Anza College April 23, 2018
2 Last time the ideal gas equation moles and molecules
3 Overview finish applying the ideal gas equation thermal energy introduced heat capacity
4 Ideal Gas Equation The equation of state for an ideal gas: PV = nrt where P is pressure V is volume n is the number of moles (amount of gas) R = J mol 1 K 1 is the universal gas constant T is temperature The LHS and RHS of this equation both have units of Joules (energy).
5 73. Review. A steel guitar string with a diameter of 1.00 mm is stretched between supports 80.0 cm apart. The temperature is 0.08C. (a) Find the mass per unit length of Problem #74 is ure the rce nci of surater the ub- ne- A cylinder this string. is (Use closed the value by7.86 a piston kg/m connected 3 for the density.) (b) to a spring of constant The fundamental frequency of transverse N/m. With the spring relaxed, the cylinder is filled with oscillations of the string is 200 Hz. What is the tension 5.00 in the L of string? gasnext, at athe pressure temperature of 1.00 is raised atm to 30.08C. and a temperature of Find 20.0 the resulting values of (c) the tension and (d) the C. fundamental frequency. Assume both the Young s modulus If the of 20.0 piston has N/m 2 aand cross-sectional the average coefficient area of m 2 and (a) negligible expansion mass, a how 3 10high 26 (8C) will 21 have it rise constant when values the temperature is between 0.08C raised to 250 and 30.08C. C? 74. A cylinder is closed by W a piston connected to a spring of constant N/m (see Fig. P19.74). With the spring relaxed, the cylinder is filled with 5.00 L of gas at a pressure of 1.00 atm and a temperature of 20.08C. (a) If the piston has a cross- sectional area of m 2 and negligible mass, how high will it rise when the temperature is raised to 1 Serway 2508C? & Figure P19.74 (b) Jewett, What page is 588. k T 20.0 C h T 250 C
6 Problem #74 (a) Find h. PV = nrt We have enough info to know the number of moles, n, or we can work around that because the amount of gas does not change as it is heated. Also, kh + P 0 A = P f A P f V f T f and P i = P 0 and V f = V i + Ah. = P iv i T i
7 Problem #74 (a) Find h. PV = nrt We have enough info to know the number of moles, n, or we can work around that because the amount of gas does not change as it is heated. Also, kh + P 0 A = P f A P f V f T f and P i = P 0 and V f = V i + Ah. = P iv i T i T f P f V f = P i V i T ( ) i kh A + P T f 0 (V i + Ah) = P 0 V i T i ( ) ( kh 2 kvi + A + AP 0 h + P 0 V i 1 T ) f = 0 T i
8 Problem #74 (a) Find h. Solving quadratic: ( ) ( kh 2 kvi + A + AP 0 h + P 0 V i 1 T ) f = 0 T i Remember: T f = K, T i = K
9 Problem #74 (a) Find h. Solving quadratic: ( ) ( kh 2 kvi + A + AP 0 h + P 0 V i 1 T ) f = 0 T i Remember: T f = K, T i = K positive solution: h = m
10 is stretched between supports 80.0 cm apart. The temperature is 0.08C. (a) Find the mass per unit length of this string. (Use the value kg/m 3 for the density.) (b) The fundamental frequency of transverse Problem #74 is ure the rce nci of surater the ubne- per A cylinder oscillations is closed by a piston connected to a spring of constant of the string is 200 Hz. What is the tension in the string? N/m. Next, the With temperature the spring is raised relaxed, to 30.08C. the cylinder is filled with Find the resulting values of (c) the tension and (d) the 5.00 L of gas at a pressure of 1.00 atm and a temperature of fundamental 20.0 frequency. Assume both the Young s modulus C. of N/m 2 and the average coefficient of expansion a (b) What is the pressure 26 (8C) of 21 have constant values the gas at 250 C? between 0.08C and 30.08C. 74. A cylinder is closed by W a piston connected to a spring of constant N/m (see k Fig. P19.74). With the spring relaxed, the cylinder is filled with 5.00 L of gas at a pressure of 1.00 atm and a h temperature of 20.08C. (a) If the piston has a cross- sectional area of m 2 and negligible mass, how high will it rise when the T 20.0 C T 250 C temperature is raised to 2508C? (b) What is Figure P19.74 the Serway pressure & Jewett, of the gas page at 2508C? 588.
11 Problem #74 (b) Find P f. We already have the expression we need: P f = kh/a + P 0
12 Problem #74 (b) Find P f. We already have the expression we need: P f = kh/a + P 0 P f = Pa
13 Heat and Energy In the late 1700s, the theory was that heat was a kind of fluid that would pass from one object to another. Burning an object would release this trapped fluid, which would explain why fire is hot.
14 Heat and Energy In the late 1700s, the theory was that heat was a kind of fluid that would pass from one object to another. Burning an object would release this trapped fluid, which would explain why fire is hot. Benjamin Thompson (Count Rumford, or simply Rumford ) was an American Loyalist soldier during the American revolution and moved to Europe after the war. While studying canon manufacture in Munich, he noticed that the process of boring canons (drilling out the barrel) produced an incredible amount of heat, especially if the drill bit was dull.
15 Heat and Energy This fluid model could not explain why friction of the drill bit on the canon would produce enough heat to keep water boiling, basically for as long as the drilling continued. Wearing away the canon metal was not producing the heating (a dull bit wears away the metal more slowly), the friction was.
16 Heat and Energy James Prescott Joule realized that this meant there was an equivalence between work and heat: both were kinds of energy transfers. He did many experiments to quantify this relationship. About 4,180 Joules of mechanical energy are needed to increase the temperature of 1 kg of water by 1 C. (More on this later.)
17 Heat and Energy James Prescott Joule realized that this meant there was an equivalence between work and heat: both were kinds of energy transfers. He did many experiments to quantify this relationship. About 4,180 Joules of mechanical energy are needed to increase the temperature of 1 kg of water by 1 C. (More on this later.) We conclude that there is another type of energy: a hot object has more energy than a similar cold object.
18 Thermal Energy and Internal Energy thermal energy 1 The energy that an object has as a result of its temperature. Internal energy, E int or U The energy that a system has as a result of its temperature and all other molecular motions, effects, and configurations, when viewed from a reference frame at rest with respect to the center of mass of the system. Internal energy can be thought of as a combination of kinetic and potential energies of microscopic particles, but it is different from mechanical energy, because it cannot be directly converted into work. 1 This definition is not universal!
19 Internal energy vs. Heat Internal energy: U is the symbol most commonly used for internal energy, but it should not be confused with potential energy! They are different: potential energy can be directly converted to work, internal energy cannot. The textbook uses E int for internal energy.
20 Internal energy vs. Heat Internal energy: U is the symbol most commonly used for internal energy, but it should not be confused with potential energy! They are different: potential energy can be directly converted to work, internal energy cannot. The textbook uses E int for internal energy. Heat, Q Energy that is transferred into or out of a system in thermal contact with its environment because of a temperature difference between the system and environment. Heat and internal energy are not the same thing. Heat changes the internal energy of the system.
21 Bond Energy bond energy The energy that an object has as a result of the configuration of its constituent particles at a microscopic level. internal energy = thermal energy + bond energy Intuitively, bond energy is an intermolecular potential energy of all of the atoms or molecules due to how they are bonded, and thermal energy is the the kinetic energy of the random motion of the atoms or molecules.
22 Units of Internal Energy and Heat: Calories The units of both internal energy and heat are Joules, J. However, heat was not always understood to be an amount of energy, so other units have been defined for it, and are still sometimes used. 1 calorie is the heat required to raise the temperature of 1 gram of water by 1 degree Celsius.
23 Units of Internal Energy and Heat: Calories The units of both internal energy and heat are Joules, J. However, heat was not always understood to be an amount of energy, so other units have been defined for it, and are still sometimes used. 1 calorie is the heat required to raise the temperature of 1 gram of water by 1 degree Celsius. The calories listed on food labels are sometimes called Calories (capital C) because they are in fact kilocalories. 1 Calorie = 1 kilocalorie = the heat required to raise the temperature of 1 kilogram of water by 1 degree Celsius. 1 calorie = 4.18 Joules.
24 First Law of Thermodynamics Where this is headed: 1st Law The change in the internal energy of a system is equal to the sum of the heat added to the system and the work done on the system. E int = W + Q This is just the conservation of energy written in a different way! It takes into account that heat is energy.
25 Heat Capacity Before we look more closely at the first law, let s look at the effect of adding heat to a substance that is not near a phase change. It requires less energy to raise the temperature of some objects compared to others.
26 Heat Capacity Before we look more closely at the first law, let s look at the effect of adding heat to a substance that is not near a phase change. It requires less energy to raise the temperature of some objects compared to others. Obviously, a small amount of water requires less heat to raise its temperature by 1 degree than a large amount of water. But even two objects of the same mass may require different amounts of heat to change their temperature by 1 degree if they are made of different materials. Different materials have different heat capacities.
27 Heat Capacity Heat Capacity, C of a sample of substance is the quantity of heat required to change the temperature of that sample by 1 degree C (or K). Q = C T where T is the change in temperature and Q is the heat. Q T and C is the constant of proportionality.
28 Specific Heat Capacity However, it is usually more useful to compare one kind of substance to another for a given mass (eg. 1 kg). Specific Heat Capacity 2, c of a substance is the quantity of heat required to change the temperature of a unit mass of that substance by 1 degree C (or K). Q = cm T m is the mass of the object. For example, water has a specific heat capacity c = 4186 J kg 1 K 1. C = cm 2 Here, this refers to a process where temperature changes at constant pressure. In solids and liquids this distinction is not too significant.
29 Specific Heat Capacity Different materials have different heat capacities. for Lead, c = 129 J kg 1 K 1 for Hydrogen, c = J kg 1 K 1 Hydrogen gas s heat capacity is phenomenally high. (Its molar mass is small.) Most substances are in the range J kg 1 K 1. This means that water also has quite a high heat capacity (4186 J kg 1 K 1 ). This has an effect on Earth s weather and climate, since oceans make most of Earth s surface.
30 Summary applying the ideal gas equation thermal energy introduced heat capacity Homework Serway & Jewett: Look at examples new: Ch 20, onward from page 615. (OQs: 3, 5, 7; CQs: 3, 11); Probs: 1, (3, 9, 13, 63, 69)
Thermodynamics Thermal Expansion The Ideal Gas Equation
 Thermodynamics Thermal Expansion The Ideal Gas Equation Lana Sheridan De Anza College April 19, 2017 Last time applications of Bernoulli s equation heat, thermal equilibrium, and the 0th law temperature
Thermodynamics Thermal Expansion The Ideal Gas Equation Lana Sheridan De Anza College April 19, 2017 Last time applications of Bernoulli s equation heat, thermal equilibrium, and the 0th law temperature
Thermodynamics Heat Capacity Phase Changes
 Thermodynamics Heat Capacity Phase Changes Lana Sheridan De Anza College April 24, 2018 Last time finish applying the ideal gas equation thermal energy introduced heat capacity Overview heat capacity phase
Thermodynamics Heat Capacity Phase Changes Lana Sheridan De Anza College April 24, 2018 Last time finish applying the ideal gas equation thermal energy introduced heat capacity Overview heat capacity phase
Dual Program Level 1 Physics Course
 Dual Program Level 1 Physics Course Assignment 15 Due: 11/Feb/2012 14:00 Assume that water has a constant specific heat capacity of 4190 J/kg K at all temperatures between its melting point and boiling
Dual Program Level 1 Physics Course Assignment 15 Due: 11/Feb/2012 14:00 Assume that water has a constant specific heat capacity of 4190 J/kg K at all temperatures between its melting point and boiling
Thermal Energy. solid. liquid. gas
 Heat (Chapter 10) What is heat? What is the relationship between quantity of heat and temperature? What happens to a body (solid, liquid, gas) when thermal energy is added or removed? Thermal Energy Solid:
Heat (Chapter 10) What is heat? What is the relationship between quantity of heat and temperature? What happens to a body (solid, liquid, gas) when thermal energy is added or removed? Thermal Energy Solid:
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Application of the First Law To The Construction of an Engine
 CHEM 331 Physical Chemistry Fall 2014 Application of the First Law To The Construction of an Engine So, now we are in the thick of it. We have established a method for measuring the Internal Energy change
CHEM 331 Physical Chemistry Fall 2014 Application of the First Law To The Construction of an Engine So, now we are in the thick of it. We have established a method for measuring the Internal Energy change
HEAT AND THERMODYNAMICS
 HEAT AND THERMODYNAMICS 1. THE ABSOLUTE TEMPERATURE SCALE In the textbook you have been introduced to the concept of temperature, and to the fact that there is a natural zero of temperature, the temperature
HEAT AND THERMODYNAMICS 1. THE ABSOLUTE TEMPERATURE SCALE In the textbook you have been introduced to the concept of temperature, and to the fact that there is a natural zero of temperature, the temperature
Thermodynamics Second Law Heat Engines
 Thermodynamics Second Law Heat Engines Lana Sheridan De Anza College May 10, 2018 Last time entropy (microscopic perspective) Overview heat engines heat pumps Carnot engines Heat Engines Steam engines
Thermodynamics Second Law Heat Engines Lana Sheridan De Anza College May 10, 2018 Last time entropy (microscopic perspective) Overview heat engines heat pumps Carnot engines Heat Engines Steam engines
CHAPTER 16 A MACROSCOPIC DESCRIPTION OF MATTER
 CHAPTER 16 A MACROSCOPIC DESCRIPTION OF MATTER This brief chapter provides an introduction to thermodynamics. The goal is to use phenomenological descriptions of the microscopic details of matter in order
CHAPTER 16 A MACROSCOPIC DESCRIPTION OF MATTER This brief chapter provides an introduction to thermodynamics. The goal is to use phenomenological descriptions of the microscopic details of matter in order
- The empirical gas laws (including the ideal gas equation) do not always apply.
 145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
CHEM What is Energy? Terminology: E = KE + PE. Thermodynamics. Thermodynamics
 Thermodynamics 2 Thermodynamics The study of energy changes accompanying physical and chemical processes. From the laws of thermodynamics, one can: 1. Predict the results of chemical reactions 2. Ascertain
Thermodynamics 2 Thermodynamics The study of energy changes accompanying physical and chemical processes. From the laws of thermodynamics, one can: 1. Predict the results of chemical reactions 2. Ascertain
Waves Standing Waves Sound Waves
 Waves Standing Waves Sound Waves Lana Sheridan De Anza College May 23, 2018 Last time finish up reflection and transmission standing waves Warm Up Question: Standing Waves and Resonance In the following
Waves Standing Waves Sound Waves Lana Sheridan De Anza College May 23, 2018 Last time finish up reflection and transmission standing waves Warm Up Question: Standing Waves and Resonance In the following
Thermal energy. Thermal energy is the internal energy of a substance. I.e. Thermal energy is the kinetic energy of atoms and molecules.
 Thermal energy Thermal energy is the internal energy of a substance. I.e. Thermal energy is the kinetic energy of atoms and molecules. Heat is the transfer of thermal energy between substances. Until the
Thermal energy Thermal energy is the internal energy of a substance. I.e. Thermal energy is the kinetic energy of atoms and molecules. Heat is the transfer of thermal energy between substances. Until the
16-1. Sections Covered in the Text: Chapter 17. Example Problem 16-1 Estimating the Thermal Energy of a gas. Energy Revisited
 Heat and Work Sections Covered in the Text: Chapter 17 In this note we continue our study of matter in bulk. Here we investigate the connection between work and heat in bulk matter. Work and heat are both
Heat and Work Sections Covered in the Text: Chapter 17 In this note we continue our study of matter in bulk. Here we investigate the connection between work and heat in bulk matter. Work and heat are both
Fluids, Thermodynamics, Waves, and Optics Fluids
 Fluids, Thermodynamics, Waves, and Optics Fluids Lana Sheridan De Anza College April 10, 2018 Overview static fluids pressure liquid pressure Pascal s law Elastic Properties of Solids We are considering
Fluids, Thermodynamics, Waves, and Optics Fluids Lana Sheridan De Anza College April 10, 2018 Overview static fluids pressure liquid pressure Pascal s law Elastic Properties of Solids We are considering
(Heat capacity c is also called specific heat) this means that the heat capacity number c for water is 1 calorie/gram-k.
 Lecture 23: Ideal Gas Law and The First Law of Thermodynamics 1 (REVIEW) Chapter 17: Heat Transfer Origin of the calorie unit A few hundred years ago when people were investigating heat and temperature
Lecture 23: Ideal Gas Law and The First Law of Thermodynamics 1 (REVIEW) Chapter 17: Heat Transfer Origin of the calorie unit A few hundred years ago when people were investigating heat and temperature
Thermodynamics Molecular Model of a Gas Molar Heat Capacities
 Thermodynamics Molecular Model of a Gas Molar Heat Capacities Lana Sheridan De Anza College May 3, 2018 Last time modeling an ideal gas at the microscopic level rms speed of molecules equipartition of
Thermodynamics Molecular Model of a Gas Molar Heat Capacities Lana Sheridan De Anza College May 3, 2018 Last time modeling an ideal gas at the microscopic level rms speed of molecules equipartition of
Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law
 Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law Units of Chapter 17 & 19 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work
Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law Units of Chapter 17 & 19 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work
Lecture 21. Temperature. Thermal Expansion. Heat and Internal Energy. Cutnell+Johnson: , Temperature
 Lecture 21 Temperature Thermal Expansion Heat and Internal Energy Cutnell+Johnson: 12.1-12.7, 14.3 Temperature So far in this class we ve usually talked about large objects, and we ve treated the object
Lecture 21 Temperature Thermal Expansion Heat and Internal Energy Cutnell+Johnson: 12.1-12.7, 14.3 Temperature So far in this class we ve usually talked about large objects, and we ve treated the object
the energy of motion!
 What are the molecules of matter doing all the time?! Heat and Temperature! Notes! All matter is composed of continually jiggling atoms or molecules! The jiggling is! If something is vibrating, what kind
What are the molecules of matter doing all the time?! Heat and Temperature! Notes! All matter is composed of continually jiggling atoms or molecules! The jiggling is! If something is vibrating, what kind
CHAPTER 17 WORK, HEAT, & FIRST LAW OF THERMODYNAMICS
 CHAPTER 17 WORK, HEAT, and the FIRST LAW OF THERMODYNAMICS In this chapter, we will examine various thermal properties of matter, as well as several mechanisms by which energy can be transferred to and
CHAPTER 17 WORK, HEAT, and the FIRST LAW OF THERMODYNAMICS In this chapter, we will examine various thermal properties of matter, as well as several mechanisms by which energy can be transferred to and
The Kinetic Theory of Matter. Temperature. Temperature. Temperature. Temperature. Chapter 6 HEAT
 The Kinetic Theory of Matter Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in
The Kinetic Theory of Matter Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in
1985B4. A kilogram sample of a material is initially a solid at a temperature of 20 C. Heat is added to the sample at a constant rate of 100
 1985B4. A 0.020-kilogram sample of a material is initially a solid at a temperature of 20 C. Heat is added to the sample at a constant rate of 100 joules per second until the temperature increases to 60
1985B4. A 0.020-kilogram sample of a material is initially a solid at a temperature of 20 C. Heat is added to the sample at a constant rate of 100 joules per second until the temperature increases to 60
Chapter 10 Temperature and Heat
 Chapter 10 Temperature and Heat Thermodynamics deals with 1. Temperature. 2. The transfer and transformation of energy. 3. The relationship between macroscopic properties and microscopic dynamics. Temperature
Chapter 10 Temperature and Heat Thermodynamics deals with 1. Temperature. 2. The transfer and transformation of energy. 3. The relationship between macroscopic properties and microscopic dynamics. Temperature
Fluids Bernoulli s equation conclusion
 Chapter 11 Fluids Bernoulli s equation conclusion 11.9 Bernoulli s Equation W NC = ( P 2! P 1 )V W NC = E 1! E 2 = 1 mv 2 + mgy 2 1 1 ( )! ( 1 "v 2 + "gy 2 2 2 ) ( P 2! P 1 ) = 1 "v 2 + "gy 2 1 1 NC Work
Chapter 11 Fluids Bernoulli s equation conclusion 11.9 Bernoulli s Equation W NC = ( P 2! P 1 )V W NC = E 1! E 2 = 1 mv 2 + mgy 2 1 1 ( )! ( 1 "v 2 + "gy 2 2 2 ) ( P 2! P 1 ) = 1 "v 2 + "gy 2 1 1 NC Work
A thermodynamic system is taken from an initial state X along the path XYZX as shown in the PV-diagram.
 AP Physics Multiple Choice Practice Thermodynamics 1. The maximum efficiency of a heat engine that operates between temperatures of 1500 K in the firing chamber and 600 K in the exhaust chamber is most
AP Physics Multiple Choice Practice Thermodynamics 1. The maximum efficiency of a heat engine that operates between temperatures of 1500 K in the firing chamber and 600 K in the exhaust chamber is most
Fluids Bernoulli s equation conclusion
 Chapter 11 Fluids Bernoulli s equation conclusion 11.9 Bernoulli s Equation W NC = ( P 2! P 1 )V W NC = E 1! E 2 = 1 mv 2 + mgy 2 1 1 ( )! ( 1 "v 2 + "gy 2 2 2 ) ( P 2! P 1 ) = 1 "v 2 + "gy 2 1 1 NC Work
Chapter 11 Fluids Bernoulli s equation conclusion 11.9 Bernoulli s Equation W NC = ( P 2! P 1 )V W NC = E 1! E 2 = 1 mv 2 + mgy 2 1 1 ( )! ( 1 "v 2 + "gy 2 2 2 ) ( P 2! P 1 ) = 1 "v 2 + "gy 2 1 1 NC Work
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chemical Thermodynamics
 Quiz A 42.8 ml solution of ammonia (NH 3 ) is titrated with a solution of 0.9713 M hydrochloric acid. The initial reading on the buret containing the HCl was 47.13 ml and the final reading when the endpoint
Quiz A 42.8 ml solution of ammonia (NH 3 ) is titrated with a solution of 0.9713 M hydrochloric acid. The initial reading on the buret containing the HCl was 47.13 ml and the final reading when the endpoint
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
- Joule (J): SI unit for energy. It's defined based on the equation for kinetic energy. from. mass. velocity
 153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
Temperature, Thermal Expansion and the Gas Laws
 Temperature, Thermal Expansion and the Gas Laws z x Physics 053 Lecture Notes Temperature,Thermal Expansion and the Gas Laws Temperature and Thermometers Thermal Equilibrium Thermal Expansion The Ideal
Temperature, Thermal Expansion and the Gas Laws z x Physics 053 Lecture Notes Temperature,Thermal Expansion and the Gas Laws Temperature and Thermometers Thermal Equilibrium Thermal Expansion The Ideal
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
Thermodynamics Thermal Equilibrium Temperature
 Thermodynamics Thermal Equilibrium Temperature Lana Sheridan De Anza College April 18, 2017 Last time Torricelli s Law applications of Bernoulli s equation Overview heat, thermal equilibrium, and the 0th
Thermodynamics Thermal Equilibrium Temperature Lana Sheridan De Anza College April 18, 2017 Last time Torricelli s Law applications of Bernoulli s equation Overview heat, thermal equilibrium, and the 0th
Thermodynamics Test Wednesday 12/20
 Thermodynamics Test Wednesday 12/20 HEAT AND TEMPERATURE 1 Temperature Temperature: A measure of how hot (or cold) something is Specifically, a measure of the average kinetic energy of the particles in
Thermodynamics Test Wednesday 12/20 HEAT AND TEMPERATURE 1 Temperature Temperature: A measure of how hot (or cold) something is Specifically, a measure of the average kinetic energy of the particles in
Mechanics Units, Dimensional Analysis, and Unit Conversion
 Mechanics Units, Dimensional Analysis, and Unit Conversion Lana Sheridan De Anza College Sept 25, 2018 Last time introduced the course basic ideas about science and physics Overview introduce SI units
Mechanics Units, Dimensional Analysis, and Unit Conversion Lana Sheridan De Anza College Sept 25, 2018 Last time introduced the course basic ideas about science and physics Overview introduce SI units
October 5 A. Bell Work B. Have 4.2 Notes I Out for a stamp C. Today we will work on 4.2 Notes II D. Homework: 4.2 Notes II (due tomorrow end of
 October 5 A. Bell Work B. Have 4.2 Notes I Out for a stamp C. Today we will work on 4.2 Notes II D. Homework: 4.2 Notes II (due tomorrow end of class) Read 4.3 and take notes (due Monday) 4.2 Key Concept:
October 5 A. Bell Work B. Have 4.2 Notes I Out for a stamp C. Today we will work on 4.2 Notes II D. Homework: 4.2 Notes II (due tomorrow end of class) Read 4.3 and take notes (due Monday) 4.2 Key Concept:
Chapter 8 Thermochemistry
 William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy
 Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Temp vs. Heat. Absolute Temperature Scales. Common Temperature Scales. Thermal Energy. Heat and Temperature are not the same!!
 Thermal Energy Heat and Temperature are not the same!! Cold is the absence of heat, not an energy Same concept as light/dark Cold can t come in, heat flows out Heat flows from High Temp Low Temp Temp vs.
Thermal Energy Heat and Temperature are not the same!! Cold is the absence of heat, not an energy Same concept as light/dark Cold can t come in, heat flows out Heat flows from High Temp Low Temp Temp vs.
Waves Solutions to the Wave Equation Sine Waves Transverse Speed and Acceleration
 Waves Solutions to the Wave Equation Sine Waves Transverse Speed and Acceleration Lana Sheridan De Anza College May 17, 2018 Last time pulse propagation the wave equation Overview solutions to the wave
Waves Solutions to the Wave Equation Sine Waves Transverse Speed and Acceleration Lana Sheridan De Anza College May 17, 2018 Last time pulse propagation the wave equation Overview solutions to the wave
NATIONAL 5 PHYSICS THERMODYNAMICS
 NATIONAL 5 PHYSICS THERMODYNAMICS HEAT AND TEMPERATURE Heat and temperature are not the same thing! Heat Heat is a type of energy. Like all types of energy it is measured in joules (J). The heat energy
NATIONAL 5 PHYSICS THERMODYNAMICS HEAT AND TEMPERATURE Heat and temperature are not the same thing! Heat Heat is a type of energy. Like all types of energy it is measured in joules (J). The heat energy
Thermochemistry is study of changes in energy (heat) associated with physical or chemical changes.
 Thermochemistry Thermochemistry and Energy and Temperature Thermochemistry is study of changes in energy (heat) associated with physical or chemical changes. Force = push F= m a (mass x acceleration) force
Thermochemistry Thermochemistry and Energy and Temperature Thermochemistry is study of changes in energy (heat) associated with physical or chemical changes. Force = push F= m a (mass x acceleration) force
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
2,000-gram mass of water compared to a 1,000-gram mass.
 11.2 Heat To change the temperature, you usually need to add or subtract energy. For example, when it s cold outside, you turn up the heat in your house or apartment and the temperature goes up. You know
11.2 Heat To change the temperature, you usually need to add or subtract energy. For example, when it s cold outside, you turn up the heat in your house or apartment and the temperature goes up. You know
Waves Pulse Propagation The Wave Equation
 Waves Pulse Propagation The Wave Equation Lana Sheridan De Anza College May 16, 2018 Last time oscillations simple harmonic motion (SHM) spring systems energy in SHM introducing waves kinds of waves wave
Waves Pulse Propagation The Wave Equation Lana Sheridan De Anza College May 16, 2018 Last time oscillations simple harmonic motion (SHM) spring systems energy in SHM introducing waves kinds of waves wave
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 17 Thermochemistry
 Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Waves Wave Speed on a String Pulse Propagation The Wave Equation
 Waves Wave Speed on a String Pulse Propagation The Wave Equation Lana Sheridan De Anza College May 16, 2018 Last time oscillations simple harmonic motion (SHM) spring systems energy in SHM introducing
Waves Wave Speed on a String Pulse Propagation The Wave Equation Lana Sheridan De Anza College May 16, 2018 Last time oscillations simple harmonic motion (SHM) spring systems energy in SHM introducing
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Physics 111. Lecture 35 (Walker: ) Thermal Physics I: Temperature Thermal Expansion. April 29, Temperature (T)
 Physics 111 Lecture 35 (Walker: 16.1-3) Thermal Physics I: Temperature Thermal Expansion April 29, 2009 Lecture 35 1/26 Temperature (T) Temperature (T) is a measure of how hot or cold something is Temperature
Physics 111 Lecture 35 (Walker: 16.1-3) Thermal Physics I: Temperature Thermal Expansion April 29, 2009 Lecture 35 1/26 Temperature (T) Temperature (T) is a measure of how hot or cold something is Temperature
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
Chapter 11 Ideal gases
 OCR (A) specifications: 5.4.10c,d,e,i,j,k Chapter 11 Ideal gases Worksheet Worked examples Practical: Determining absolute zero of temperature from the pressure law End-of-chapter test Marking scheme:
OCR (A) specifications: 5.4.10c,d,e,i,j,k Chapter 11 Ideal gases Worksheet Worked examples Practical: Determining absolute zero of temperature from the pressure law End-of-chapter test Marking scheme:
Chapter 2 Heat, Temperature and the First Law of Thermodynamics
 Chapter 2 Heat, Temperature and the First Law of Thermodynamics 2.1. Temperature and the Zeroth Law of Thermodynamics 2.2. Thermal Expansion 2.3. Heat and the Absorption of Heat by Solids and Liquids 2.4.
Chapter 2 Heat, Temperature and the First Law of Thermodynamics 2.1. Temperature and the Zeroth Law of Thermodynamics 2.2. Thermal Expansion 2.3. Heat and the Absorption of Heat by Solids and Liquids 2.4.
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
Chapter 19 The First Law of Thermodynamics
 Chapter 19 The First Law of Thermodynamics The first law of thermodynamics is an extension of the principle of conservation of energy. It includes the transfer of both mechanical and thermal energy. First
Chapter 19 The First Law of Thermodynamics The first law of thermodynamics is an extension of the principle of conservation of energy. It includes the transfer of both mechanical and thermal energy. First
Physics 1501 Lecture 35
 Physics 1501: Lecture 35 Todays Agenda Announcements Homework #11 (Dec. 2) and #12 (Dec. 9): 2 lowest dropped Honors students: see me after the class! Todays topics Chap.16: Temperature and Heat» Latent
Physics 1501: Lecture 35 Todays Agenda Announcements Homework #11 (Dec. 2) and #12 (Dec. 9): 2 lowest dropped Honors students: see me after the class! Todays topics Chap.16: Temperature and Heat» Latent
Lesson 6 Matter. Introduction: Connecting Your Learning
 Lesson 6 Matter Introduction: Connecting Your Learning The previous lessons discussed mechanics and many forms of motion. Lesson 6 introduces the second major topic in physics, which is matter. This lesson
Lesson 6 Matter Introduction: Connecting Your Learning The previous lessons discussed mechanics and many forms of motion. Lesson 6 introduces the second major topic in physics, which is matter. This lesson
Ch06. Energy. Thermochemistry, understanding energy, heat & work. version 1.5
 Ch06 Energy Thermochemistry, understanding energy, heat & work. version 1.5 Nick DeMello, PhD. 2007-2016 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal
Ch06 Energy Thermochemistry, understanding energy, heat & work. version 1.5 Nick DeMello, PhD. 2007-2016 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal
Keep the Heat Test School Name. Team Number
 Keep the Heat Test 1-28-2012 School Name Team Number Circle the all of the correct answer to the below questions. One or more of the answers can be correct, if more than on one answer is correct, circle
Keep the Heat Test 1-28-2012 School Name Team Number Circle the all of the correct answer to the below questions. One or more of the answers can be correct, if more than on one answer is correct, circle
Thermodynamics Heat Transfer
 Thermodynamics Heat Transfer Lana Sheridan De Anza College April 30, 2018 Last time heat transfer conduction Newton s law of cooling Overview continue heat transfer mechanisms conduction over a distance
Thermodynamics Heat Transfer Lana Sheridan De Anza College April 30, 2018 Last time heat transfer conduction Newton s law of cooling Overview continue heat transfer mechanisms conduction over a distance
Chapter 17. Work, Heat, and the First Law of Thermodynamics Topics: Chapter Goal: Conservation of Energy Work in Ideal-Gas Processes
 Chapter 17. Work, Heat, and the First Law of Thermodynamics This false-color thermal image (an infrared photo) shows where heat energy is escaping from a house. In this chapter we investigate the connection
Chapter 17. Work, Heat, and the First Law of Thermodynamics This false-color thermal image (an infrared photo) shows where heat energy is escaping from a house. In this chapter we investigate the connection
Distinguish between an isothermal process and an adiabatic process as applied to an ideal gas (2)
 1. This question is about thermodynamic processes. (a) Distinguish between an isothermal process and an adiabatic process as applied to an ideal gas.......... An ideal gas is held in a container by a moveable
1. This question is about thermodynamic processes. (a) Distinguish between an isothermal process and an adiabatic process as applied to an ideal gas.......... An ideal gas is held in a container by a moveable
Thermodynamics. Internal Energy. Study of energy and its transformations Thermochemistry
 Internal Energy 5.1- Thermodynamics Study of energy and its transformations Thermochemistry Study of energy changes that accompany chemical and physical changes. Energy the capacity to do work or transfer
Internal Energy 5.1- Thermodynamics Study of energy and its transformations Thermochemistry Study of energy changes that accompany chemical and physical changes. Energy the capacity to do work or transfer
T (K or C) Q (quant.) Phase change. (quant.) C T. Temperature change 14-0
 T (K or C) G L S Q Q (quant.) Phase change Q (quant.) C T Temperature change 14-0 Temperature How do we keep track of energy when it is distributed over many, many [~10 23 ] objects that are constantly
T (K or C) G L S Q Q (quant.) Phase change Q (quant.) C T Temperature change 14-0 Temperature How do we keep track of energy when it is distributed over many, many [~10 23 ] objects that are constantly
1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy.
 Worksheet 1 1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy. The first law of thermodynamics is the conservation
Worksheet 1 1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy. The first law of thermodynamics is the conservation
Thermodynamics part II.
 Thermodynamics part II. a.) Fenomenological thermodynamics macroscopic description b.) Molecular thermodynamics microscopic description b1.) kinetical gas theory b2.) statistical thermodynamics Measuring
Thermodynamics part II. a.) Fenomenological thermodynamics macroscopic description b.) Molecular thermodynamics microscopic description b1.) kinetical gas theory b2.) statistical thermodynamics Measuring
CHEM Thermodynamics. Work. There are two ways to change the internal energy of a system:
 There are two ways to change the internal energy of a system: Thermodynamics Work 1. By flow of heat, q Heat is the transfer of thermal energy between and the surroundings 2. By doing work, w Work can
There are two ways to change the internal energy of a system: Thermodynamics Work 1. By flow of heat, q Heat is the transfer of thermal energy between and the surroundings 2. By doing work, w Work can
Lecture 10. What is energy? Professor Hicks Inorganic Chemistry (CHE151) Ability to do work. Work means moving something against a force
 Lecture 10 Professor Hicks Inorganic Chemistry (CHE151) Ability to do work What is energy? Work means moving something against a force Energy thought of as an imaginary liquid that gets moved from one
Lecture 10 Professor Hicks Inorganic Chemistry (CHE151) Ability to do work What is energy? Work means moving something against a force Energy thought of as an imaginary liquid that gets moved from one
SPH3U1 Lesson 03 Energy
 THERMAL ENERGY AND LATENT HEAT LEARNING GOALS Students will learn: Heat changes the amount of thermal energy in an object Temperature is a measure of the average thermal energy in an object Heat capacity
THERMAL ENERGY AND LATENT HEAT LEARNING GOALS Students will learn: Heat changes the amount of thermal energy in an object Temperature is a measure of the average thermal energy in an object Heat capacity
Week 1 Temperature, Heat and the First Law of Thermodynamics. (Ch. 19 of Serway&J.)
 Week 1 Temperature, Heat and the First Law of Thermodynamics. (Ch. 19 of Serway&J.) (Syllabus) Temperature Thermal Expansion Temperature and Heat Heat and Work The first Law Heat Transfer Temperature Thermodynamics:
Week 1 Temperature, Heat and the First Law of Thermodynamics. (Ch. 19 of Serway&J.) (Syllabus) Temperature Thermal Expansion Temperature and Heat Heat and Work The first Law Heat Transfer Temperature Thermodynamics:
Kinetic Theory continued
 Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Gases and Kinetic Molecular Theory
 1 Gases and Kinetic Molecular Theory 1 CHAPTER GOALS 1. Comparison of Solids, Liquids, and Gases. Composition of the Atmosphere and Some Common Properties of Gases 3. Pressure 4. Boyle s Law: The Volume-Pressure
1 Gases and Kinetic Molecular Theory 1 CHAPTER GOALS 1. Comparison of Solids, Liquids, and Gases. Composition of the Atmosphere and Some Common Properties of Gases 3. Pressure 4. Boyle s Law: The Volume-Pressure
- Apply closed system energy balances, observe sign convention for work and heat transfer.
 CHAPTER : ENERGY AND THE FIRST LAW OF THERMODYNAMICS Objectives: - In this chapter we discuss energy and develop equations for applying the principle of conservation of energy. Learning Outcomes: - Demonstrate
CHAPTER : ENERGY AND THE FIRST LAW OF THERMODYNAMICS Objectives: - In this chapter we discuss energy and develop equations for applying the principle of conservation of energy. Learning Outcomes: - Demonstrate
Thermodynamic Processes and Thermochemistry
 General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
Thermodynamics Boltzmann (Gibbs) Distribution Maxwell-Boltzmann Distribution Second Law Entropy
 Thermodynamics Boltzmann (Gibbs) Distribution Maxwell-Boltzmann Distribution Second Law Entropy Lana Sheridan De Anza College May 8, 2017 Last time modeling an ideal gas at the microscopic level pressure,
Thermodynamics Boltzmann (Gibbs) Distribution Maxwell-Boltzmann Distribution Second Law Entropy Lana Sheridan De Anza College May 8, 2017 Last time modeling an ideal gas at the microscopic level pressure,
Energy Transformations
 Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Chapter 10. Thermal Physics. Thermodynamic Quantities: Volume V and Mass Density ρ Pressure P Temperature T: Zeroth Law of Thermodynamics
 Chapter 10 Thermal Physics Thermodynamic Quantities: Volume V and Mass Density ρ Pressure P Temperature T: Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion of Solids and Liquids Ideal
Chapter 10 Thermal Physics Thermodynamic Quantities: Volume V and Mass Density ρ Pressure P Temperature T: Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion of Solids and Liquids Ideal
Temperature Thermal Expansion Ideal Gas Law Kinetic Theory Heat Heat Transfer Phase Changes Specific Heat Calorimetry Heat Engines
 Temperature Thermal Expansion Ideal Gas Law Kinetic Theory Heat Heat Transfer Phase Changes Specific Heat Calorimetry Heat Engines Zeroeth Law Two systems individually in thermal equilibrium with a third
Temperature Thermal Expansion Ideal Gas Law Kinetic Theory Heat Heat Transfer Phase Changes Specific Heat Calorimetry Heat Engines Zeroeth Law Two systems individually in thermal equilibrium with a third
Archimedes Principle
 Archimedes Principle applies in air the more air an object displaces, the greater the buoyant force on it if an object displaces its weight, it hovers at a constant altitude if an object displaces less
Archimedes Principle applies in air the more air an object displaces, the greater the buoyant force on it if an object displaces its weight, it hovers at a constant altitude if an object displaces less
States of Matter. We can explain the properties that we observe in the various states of matter with these postulates.
 States of Matter Kinetic Molecular Theory When discussing the properties of matter, it is not enough just to classify them. We must also create a model that helps to explain the properties that we see.
States of Matter Kinetic Molecular Theory When discussing the properties of matter, it is not enough just to classify them. We must also create a model that helps to explain the properties that we see.
Chapter 6 Thermochemistry 許富銀
 Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
- Kinetic energy: energy of matter in motion. gravity
 148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
Handout 11: Ideal gas, internal energy, work and heat. Ideal gas law
 Handout : Ideal gas, internal energy, work and heat Ideal gas law For a gas at pressure p, volume V and absolute temperature T, ideal gas law states that pv = nrt, where n is the number of moles and R
Handout : Ideal gas, internal energy, work and heat Ideal gas law For a gas at pressure p, volume V and absolute temperature T, ideal gas law states that pv = nrt, where n is the number of moles and R
Questions Chapter 18 Temperature, Heat, and the First Law of Thermodynamics
 Questions Chapter 18 Temperature, Heat, and the First Law of Thermodynamics 18-1 What is Physics? 18-2 Temperature 18-3 The Zeroth Law of Thermodynamics 18-4 Measuring Temperature 18-5 The Celsius and
Questions Chapter 18 Temperature, Heat, and the First Law of Thermodynamics 18-1 What is Physics? 18-2 Temperature 18-3 The Zeroth Law of Thermodynamics 18-4 Measuring Temperature 18-5 The Celsius and
Ready for some more SCIENCE Homer? ( Homer gives his brain a pep talk )
 Ready for some more SCIENCE Homer? ( Homer gives his brain a pep talk ) REVIEW: THE TWO LAWS OF THERMODYNAMICS #1 First Law (2 simple ways of understanding it) Energy can be transformed (changed from one
Ready for some more SCIENCE Homer? ( Homer gives his brain a pep talk ) REVIEW: THE TWO LAWS OF THERMODYNAMICS #1 First Law (2 simple ways of understanding it) Energy can be transformed (changed from one
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics. Thermodynamics and Statistical Physics
 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Thermodynamics and Statistical Physics Key contents: Temperature scales Thermal expansion Temperature and heat, specific heat Heat and
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Thermodynamics and Statistical Physics Key contents: Temperature scales Thermal expansion Temperature and heat, specific heat Heat and
A). Yes. B). No. Q15 Is it possible for a solid metal ball to float in mercury?
 Q15 Is it possible for a solid metal ball to float in mercury? A). Yes. B). No. The upward force is the weight of liquid displaced and the downward force is the weight of the ball. If the density of the
Q15 Is it possible for a solid metal ball to float in mercury? A). Yes. B). No. The upward force is the weight of liquid displaced and the downward force is the weight of the ball. If the density of the
Thermodynamics. Atoms are in constant motion, which increases with temperature.
 Thermodynamics SOME DEFINITIONS: THERMO related to heat DYNAMICS the study of motion SYSTEM an object or set of objects ENVIRONMENT the rest of the universe MICROSCOPIC at an atomic or molecular level
Thermodynamics SOME DEFINITIONS: THERMO related to heat DYNAMICS the study of motion SYSTEM an object or set of objects ENVIRONMENT the rest of the universe MICROSCOPIC at an atomic or molecular level
Kinetic Theory continued
 Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Physics 1010: The Physics of Everyday Life. TODAY Heat and Thermodynamics Thermometers, temperature scales; conduction, convection, radiation.
 Physics 1010: The Physics of Everyday Life TODAY Heat and Thermodynamics Thermometers, temperature scales; conduction, convection, radiation. 1 Today s topics Heat and thermometers Burning - conversion
Physics 1010: The Physics of Everyday Life TODAY Heat and Thermodynamics Thermometers, temperature scales; conduction, convection, radiation. 1 Today s topics Heat and thermometers Burning - conversion
The following gas laws describes an ideal gas, where
 Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as
Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as
In the name of Allah
 In the name of Allah Physical chemistry- 2 nd state semester 1 Petroleum and petrochemical engineering. Lecture No. 5,6 Thermodynamics 6-11-2016 27-11-2016 Assistance prof. Dr. Luma Majeed Ahmed lumamajeed2013@gmail.com,
In the name of Allah Physical chemistry- 2 nd state semester 1 Petroleum and petrochemical engineering. Lecture No. 5,6 Thermodynamics 6-11-2016 27-11-2016 Assistance prof. Dr. Luma Majeed Ahmed lumamajeed2013@gmail.com,
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
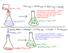 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
Handout 11: Ideal gas, internal energy, work and heat. Ideal gas law
 Handout : Ideal gas, internal energy, work and heat Ideal gas law For a gas at pressure p, volume V and absolute temperature T, ideal gas law states that pv = nrt, where n is the number of moles and R
Handout : Ideal gas, internal energy, work and heat Ideal gas law For a gas at pressure p, volume V and absolute temperature T, ideal gas law states that pv = nrt, where n is the number of moles and R
MATTER AND HEAT. Chapter 4 OUTLINE GOALS
 Chapter 4 MATTER AND HEAT OUTLINE Temperature and Heat 4.1 Temperature 4.2 Heat 4.3 Metabolic Energy Fluids 4.4 Density 4.5 Pressure 4.6 Buoyancy 4.7 The Gas Laws Kinetic Theory of Matter 4.8 Kinetic Theory
Chapter 4 MATTER AND HEAT OUTLINE Temperature and Heat 4.1 Temperature 4.2 Heat 4.3 Metabolic Energy Fluids 4.4 Density 4.5 Pressure 4.6 Buoyancy 4.7 The Gas Laws Kinetic Theory of Matter 4.8 Kinetic Theory
Chemistry 104 Chapter Two PowerPoint Notes
 Measurements in Chemistry Chapter 2 Physical Quantities Measurable physical properties such as height, volume, and temperature are called Physical quantity. A number and a unit of defined size is required
Measurements in Chemistry Chapter 2 Physical Quantities Measurable physical properties such as height, volume, and temperature are called Physical quantity. A number and a unit of defined size is required
Sparks CH301. THERMODYNAMICS Quantifying Heat Flow Physical Change. UNIT 4 Day 2
 Sparks CH301 THERMODYNAMICS Quantifying Heat Flow Physical Change UNIT 4 Day 2 You can burn 55,000 calories by drinking 6 glasses of ice water. What are we going to learn today? State Functions Review
Sparks CH301 THERMODYNAMICS Quantifying Heat Flow Physical Change UNIT 4 Day 2 You can burn 55,000 calories by drinking 6 glasses of ice water. What are we going to learn today? State Functions Review
THE SECOND LAW OF THERMODYNAMICS. Professor Benjamin G. Levine CEM 182H Lecture 5
 THE SECOND LAW OF THERMODYNAMICS Professor Benjamin G. Levine CEM 182H Lecture 5 Chemical Equilibrium N 2 + 3 H 2 2 NH 3 Chemical reactions go in both directions Systems started from any initial state
THE SECOND LAW OF THERMODYNAMICS Professor Benjamin G. Levine CEM 182H Lecture 5 Chemical Equilibrium N 2 + 3 H 2 2 NH 3 Chemical reactions go in both directions Systems started from any initial state
