Thermochemistry is study of changes in energy (heat) associated with physical or chemical changes.
|
|
|
- Aubrey Dixon
- 6 years ago
- Views:
Transcription
1 Thermochemistry Thermochemistry and Energy and Temperature Thermochemistry is study of changes in energy (heat) associated with physical or chemical changes. Force = push F= m a (mass x acceleration) force units: N (newton) = kg m s -2 Work = force x distance W = F d energy units: J (joule) = kg m 2 s -2 Energy is the capacity to do work Forms of energy are electrical, mechanical, chemical, nuclear, etc. Conservation of energy means energy is never created or destroyed but always changed from one form to another Units and conversion factors joule (J) J = kgm 2 s -2 J and kj are SI (metric) units calorie (cal) 1 cal = J kilocalorie (kcal) kcal = 4184 J kilojoule (kj) kj = 1000 J diet calorie (Cal) Cal= 1000 cal = 4184J Values for water fp and bp below: Temperature Freezing Point Boiling Point o F Fahrenheit o C Celsius K Kelvin and scales to convert temperature are o F = 1.8(C) + 32 o C = ( o F 32)/1.8 K = o C For T in equation always use K T=273K but only 0 o C For T in equation can use K or o C (will be the same T change) Example: if T=273K then T= 0 o C however if T=10K then T=10 o C
2 Note on R and R Gas constant R can be written as R = L atm/mol K which is used to calc amounts such as P,V,or n from PV=nRT and R = 8.31 J/mol K which is used for to calc energy (J) from RT or nrt Make sure units work correctly in any calculation you do. Temperature and Heat Temperature is the degree of hotness Heat is the amount of energy associated with thermal motion Small metal foil at 100 o C has less heat energy than large block of metal at 100 o C even though temperatures are the same. Energy Potential Energy = position energy (chemical bonds) Kinetic Energy = motion energy (heat) First Law of Thermodynamics First Law is conservation of energy. or Energy of universe is constant. Heat flow exothermic (heat out of system) (-) and endothermic (heat into system) (+) System is whatever we are studying or measuring. So universe is divided into system and surroundings. First Law of Thermodynamics says that total energy change is sum of changes of heat and work going into or out of system. ΔU = q + w or E = q + w different books use U or E for internal energy ΔU or E = change in internal energy q = heat (random motion) w = work (organized motion)
3 Heat changes First Law of Thermodynamics means (conservation of energy) or (energy of universe is constant) so notice below if heat leaves system then it goes to surroundings and therefore energy of universe is constant same true if heat goes into system from surroundings Exothermic Endothermic ( hermoho_f/thermoho.htm\) Signs: + into system à system energy increases endothermic (energy in) out of system à system energy decreases exothermic (energy out) Work gas expansion (generally we are more interested in heat) Imagine a piston moving in a cylinder then to calculate work (w) of surroundings recall w = (force) (distance) = F(Δd) and can multiple by (A/A) where A= area of top of cylinder w = F(Δd) (A/A) and regroup terms w = (F/A)(A Δd) and recall that pressure is defined as force/area so P=F/A and the change in A d is same as change in volume V so therefore the work of gas expansion is given by w = P(ΔV) and work of system (gas in cylinder is opposite sign) w = P( V)
4 ( Units: atm = force/area, Pascal = N/m 2, Pa = 1 atm Work of expanding gas useful conversion 101 J = 1 L atm Pressure conversion: 1 atm = 1.01 x 10 5 Pa = 760 torr Example What is the work of the gas that expands by 10.0L against an external pressure of exactly 5.5 atmosphere? convert atm to N/m 2 w = P V P = 5.5atm ( 1.01 x 10 5 N/m 2 /atm) because pascal Pa = N/m 2 P = 5.56x10 5 N/m 2 V = 10.0L ( 1 m 3 /1000L) V = 1.0x10-2 m 3 because there are 1000 liters in 1 cubic meter
5 so w = (5.56x10 5 N/m 2 ) (1.0x10-2 m 3 ) w = 5.56x10 3 Nm = 5.56x10 3 J or 5.56 kj since a (newton)(meter) = joule and 1000J = 1 kj or using conversion that 101 J = 1 atm L then w = P V w = (5.5 atm)(+10 L) ( 101 J/atmL) = 5.56x10 3 J (same result) Calorimetry (measuring heat changes) q = C ΔΤ q = heat (J) C = heat capacity (J/ o C) ΔT = temp change ( o C) Heat capacity = heat required to raise temp by 1 o C Specific heat = heat capacity of 1g of substance (heat capacity) (mass) (specific heat, can be per gram or per mol) Symbol (C) (m) (s) Units (J/ o C) (g) (J/g o C) (J/K) (g) (J/ g K) or if per mole (J/K) mol (J/mol K) C = m s Water (liquid) specific heat is 1.00 cal/g o C or 4.18 J/g o C Or per mol specific heat = (18g/mol)(4.18 J/g o C) = 75.2 J/mol o C Combining equations q = C T and C = m s we get q = m s T heat = (mass)(specific heat) (change in temperature) or if done in moles then q = (mol) (s mol ) T heat = (moles) (heat per mol) (change in temperature) Change is always the difference of (final value initial value) Δ = final initial
6 Example How much heat is put into 100. g of water to raise temperature from 25 to 75 degrees Celsius? The specific heat of water is J/ g o C 100. g of Water with temp change 25à 75 o C q = m s (ΔT) = C (T 2 T 1 ) where C = m s q = (100g)(4.184 J/g o C)(75.0 o C 25.0 o C) q = J = 20.9 kj endothermic Heat goes into water (the system) Suppose same water cools 75à 25 o C then q = kj exothermic Heat leaves water (the system) to go to surroundings) Example using per mole How much heat is put into 100. g of water to raise temperature from 25 to 75 degrees Celsius? The molar heat capacity of water is J/mol o C moles = 100. g (mol/18.0 g) = mol q = m s (ΔT) = C (T 2 T 1 ) where C = m s q = (5.556 mol) (75.31 J/g o C) (75.0 o C 25.0 o C) = or 20.9kJ notice final answer in problems above should be 3 sig fig 2.09x10 4 J or 20.9kJ Calorimeter device to measure changes in heat Bomb Calorimeter shown below (
7 Calorimeter is used to measure C and T and thus find q for a reaction A bomb calorimeter is one that uses a metal chamber to rapidly burn a sample in an oxygen atmosphere Example: 3.00g of glucose was burned in an excess of oxygen in bomb calorimeter with metal holder ( bomb ) heat capacity of 2.21 kj/ o C. and 1.2kg of water where water has a specific heat capacity of kj/kg o C. The temp change upon combustion of glucose and oxygen was 19.0à 25.5 o C Question what is the heat evolved from the combustion of 1.00 mol of glucose? Total heat capacity C total = C cal + C H2O = (2.21 kj/ o C + 1.2kg(4.18kJ/kg o C)) = (7.23 kj/ o C) Heat absorbed by calorimeter and water q = C total (ΔT) q = 7.23 kj/ o C ( o C) q = kj absorbed by water calorimeter so therefore q= kj for heat released from combustion of glucose and per mole Heat evolved = (-47.0 kj/ 3.00g glucose)(180g/1mol) ΔU = x 10 3 kj/mol of glucose Reaction C 6 H 12 O 6 (s) + 6O 2 (g) à 6CO 2 (g) + 6H 2 O (l) ΔU = x 10 3 kj/mol Next section will show that if the same amount of gas is on reactant and product side then U = H so for above H = x 10 3 kj/mol Remember ΔU or ΔE for is used for internal energy. Some books use U and some use E for change in internal energy
8 Connection between Internal Energy and Enthalpy Internal Energy (ΔE or ΔU) = q V constant volume heat change Enthalpy ΔH = q P constant pressure heat change Often ignore the difference because it is small However, if need to calculate exactly U = q + w and heat is q = H and work is w = - (PV) so U = H - (PV) ΔH = (ΔU) + Δ (PV) and it is the gas produced or used up that causes change in PV so Δ (PV) = Δ (nrt) = RT(Δn gas ) and therefore ΔH = ΔU + RT(Δn gas ) where Δn gas = Σn gas (prod) Σn gas (react) where n gas is moles of gas on product or reactant side of chemical equation For Example: If in a reaction among other things happening 4 mol gas à 5 mol gas then Δn gas = Σn gas (prod) Σn gas (react) Δn gas = 5-4 Δn gas = + 1 mol and RT(Δn gas ) = (8.31 J/molK)(298K)(+ 1 mol) = 2476 J = 2.5 kj so then H = U + 2.5kJ this could be a small difference if H is hundreds of kj Usually the difference between ΔH and ΔU is small ΔH = ΔU if only liquid and solid that is no gas among reactants or products or if same moles of gas on product and reactant sides so n gas = 0 Bomb Calorimetry ( in closed container) gives us U but Usually we are interested in enthalpy (ΔH)
9 Reaction in a beaker open to room is at constant pressure but volume may change if gas produced or used up so in a beaker heat change is H so to repeat Internal Energy Enthalpy ΔU= q v (constant volume) heat change ΔH = q p (constant pressure) heat change ΔH = (ΔU) + Δ (PV) but if no increase or decrease in gas then (PV) = 0 and H = U Thermochemical Equations Enthalpy is the heat content (H) Enthalpy change is ΔH = H products - H reactants So H is the heat change for chemical reactions H = (-) is exothermic heat given off (feels hot) H = (+) is endothermic heat taken in (feels cool) Examples: For H 2 (g) + (1/2)O 2 (g) à H 2 O (g) kj we say for reaction above ΔH = -286 kj since heat given off for the reverse or opposite reaction H 2 O (g) kj à H 2 (g) + (1/2)O 2 (g) we say ΔH= +286 kj since heat was put into reaction Remember heat in (reactant side) H = + heat out (product side) H = Applications: Our society runs on exothermic reactions we burn coal, oil, natural gas (methane, CH 4 ), and gasoline (a mixture of different hydrocarbons) to heat our homes, run our cars, and produce electricity. Our society would collapse without exothermic reactions!
10 We eat food that our body metabolizes to produce energy and our brain needs a constant regular supply of glucose sugar molecules that are used for energy production. We would die without exothermic reactions! H tells us what the energy change will be but not how fast it will happen. We will see later that: Thermodynamics tell us what is likely to happen (where is equilibrium) Kinetics tells us how fast it will happen (speed of reaction) Enthalpies depend on amounts given for reaction as written and can depend on pressure and temperature. If P=1.00 atm and T=25 o C then at standard state and can indicate by H o We will often just write H instead of H o even if at standard state. How do you calculate ΔH for reaction? 1. Law of Hess 2. Enthalpies of Formation 3. Bond Energies 1. Law of Hess Change in enthalpy for reaction may be broken down into several steps so the way you get from reactants to products will give same overall H A à B à C will have same H as A à C Example: If C (graphite) + (1/2) O 2 à CO H= kJ CO + (1/2) O 2 à CO 2 H = kJ added together gives C(graphite) + O 2 (g) à CO 2 (g) and also H = Rules: ΔH is for reaction as written a) If reverse reaction, change sign of ΔH ( or can multiple value by 1) b) If multiply or divide reaction by constant, do the same to ΔH Example: If C + O 2 (g) à CO 2 (g) and H = kJ then reverse and get CO 2 (g) à C + O 2 (g) and H = kj multiple this by 3 and get 3CO 2 (g) à 3C + 3O 2 (g) and H = kj
11 or another example If A à B ΔH = +5 kj then B à A ΔH = -5 kj 3A à 3B ΔH = +15 kj 2B à 2A ΔH = -10 kj Formal Procedure to get H: In Hess s Law approach we use reactions with known ΔH values to find unknown ΔH for a reaction that is combination of known ones Example: Given ΔH (a) 2CO à 2C + O kj (b) CO + (1/2) O 2 à CO kj Find C + O 2 à CO 2 (a) 2C + O 2 à 2CO kj (a)/2 C + (1/2) O 2 à CO kj (b) CO + (1/2) O 2 à CO kj (a)/2 + (b) C + O 2 à CO 2 so then H = H a /2 + H b = (+221.0)/2 + (-283.0) = kj Example combining reactions Given: (a) 4NH 3 + 3O 2 à 2N 2 + 6H 2 O (b) N 2 O + H 2 à N 2 + H 2 O (c) H 2 + (1/2) O 2 à H 2 O ΔH H a = kj H b = -367 kj H c = -286 kj Then Find H for the reaction:
12 2NH 3 + 3N 2 O à 4N 2 + 3H 2 O ΔH =? Look for components found only in one reaction NH 3 only in (a) N 2 O only in (b) Try (a)/2 + 3(b) and combine to give: 2NH 3 + 3/2O 2 + 3N 2 O + 3H 2 à N 2 + 3H 2 O + 3N 2 + 3H 2 O but need to get rid of 3H 2 + (3/2) O 2 à 3H 2 O so -3(c) Therefore (a)/2 + 3(b) 3(c) gives the desired reaction 2NH 3 + 3N 2 O à 4N 2 + 3H 2 O and ΔH = (-1531)/2 + 3(-367) 3(-286) = 1009 kj whatever you do to reactions do the same thing to the H values 2. Enthalpies of Formation Standard state of substance is 25 o C and 1.00 atm Standard enthalpy of formation ΔH f o is change in heat when 1 mol of substance is made from elements in their standard states at 25 o C at 1.00 atm ΔH f o for example to make water need to combine hydrogen and oxygen H 2 (g) + (1/2) O 2 (g) à H 2 O (l) and find from experiment ΔH = kj or to make chloroform CHCl 3 C (s) + (1/2) H 2 (g) + 3/2Cl 2 à CHCl 3 (l) and measure that ΔH = -132 kj ΔH f o for pure element always equals zero Can use ΔH f o values to calculate ΔH for any reaction using ΔH = ΣΔH f o (products) ΣΔH f o (reactants) where Σ means sum or add together all values ΔH f o values are found in thermodynamic table(s) in your textbook look up values for homework and values will be given for exam questions
13 Example: 2NH 3 (g) + 3Cl 2 (g) à N 2 (g) + 6HCl (g) From table: kj/mol ΔH = [ 1 (0) + 6(-92.3)] [2( ) + 3(0)] kj = [-553.8] [ ] = kj Example calculate H (enthalpy change) and also E (internal energy change): for OF 2 (g) + H 2 O (g) à O 2 (g) + 2HF (g) where o ΔH f kj/mol Find ΔH ΔH = [ 1(0) + 2(-269) ] [ 1(23) + 1 (-242) ] H = [-538] [ - 219] H = kj and to find ΔU recall that ΔH = ΔU + RTΔn gas Use this equation: ΔH = ΔU + RTΔn gas (-319 kj) = ΔU + (3 2 mol)(8.31 J/molK)(298 K)(kJ/ 1000J) (-319 kj) = ΔU kj kj = ΔU So H and U are less than 1% difference often we can ignore this small difference if H is a large value and not much gas is produced or used up. 3. Bond Energies (not as accurate as above but used to see what is happening in a reaction and get approximate answer) Bond dissociation energy is the energy required to break single bond or double bond or triple bond that holds two atoms together BE = Bond energy (energy to break bond) and
14 ΔH = Σ BE (bond broken) + Σ BE(bonds formed) Energy In (+) Energy Out (-) Example given reaction H 2 + (1/2) O 2 à H 2 O and BE values given H H 432 kj/mol O=O 495 kj/mol O H 467 kj/mol then for reaction H = 1(H H ) + (1/2) ( O=O ) + 2(O H ) ΔH = 1(+432) + (1/2) (+495) + 2 ( - 467) units are 2 mol( -467kJ/mol) H = (680) + (-934) kj H = -254 kj for reaction as written Chemical reactions break bonds and form bonds so Put energy in to break bond Get energy out when form bond and BE different for single,double, or triple because triple bond hardest to break Diatomic Nitrogen: Single bond ΒΕ= 159 kj/mol Double bond ΒΕ= 418 kj/mol Triple bond ΒΕ= 941 kj/mol
15 Tables shown below are available to find bond energy values Example: Find H for reaction given 2NH 3 + Cl 2 à N 2 + 6HCl and BE values N-H Cl-Cl N = N H-Cl and remember ΔH = Σ BE (bond broken) + Σ BE(bonds formed)
16 Energy In (+) Energy Out (-) reactant reactant product product break 6 break 3 form 1 form 6 6(389) 3(243) 1(-941) 6(-431) Sum all values! Result is ΔH= -464 kj value is close but a little different than using heats of formation Note that the BE Tables are average bond energy values Consider H O H Bond energy to break the first bond is 501 kj/mol Bond energy to break the second is 425 kj/mol Average = 463 kj/mol Easier to break the second bond and we use just average values Example of H application U.S. Military uses Meals Ready to Eat (MRE) Uses reaction of magnesium with water to heat food in meal. Calculate enthalpy change for reaction below with Hf (kj/mol) values given Mg (s) + 2H 2 O (l) à Mg(OH) 2 (s) + H 2 (g) + heat ΔH f so ΔH = [ 1(-924.5) + 1(0)] [1(0) + 2(-285.8)] = kj ΔH = kj for reaction as written. Another Example Given above how much magnesium is needed to heat 1.00L of water from 20.0 to 90.0 o C? If specific heat of water is 4.184J/g K and water density is d=1.0g/ml then 1.00L of water is same as 1000mL or 1000g and then the heat q required to raise temp is q = m s T q= (1000g ) ( 4.184J/g K ) ( o C)
17 q= 2.93x10 5 J or 293kJ remember for o C or K the T is same [90-20] = 70 o C for K T would be [ ( ) - ( )] or [ ] = 70 K so 293kJ required as input but 353kJ given off by 1 mol of Mg so (293kJ) /(353kJ/mol)= mol or 0.830mol (24.3g/mol) = 20.2g of Mg to raise temp of water by 70 o C For more information on MRE see below from Marshall Brain. "How MREs Work". April 15, (January 21, 2007) Flameless Heaters Most human beings much prefer a warm meal to a cold one, especially if they're in cold or wet conditions. Eating cold spaghetti or cold beef stew is definitely no fun. A hot meal, on the other hand, can lift a soldier's spirits. Because of the importance of a hot meal, all military MREs come packaged with a flameless heater. The flameless heater uses a simple chemical reaction to provide sufficient heat to warm the food. Chemical heating is actually a pretty widespread natural phenomenon. Everyone has seen iron rust. Rust is a natural process in which iron atoms combine with oxygen atoms to create reddish, crumbly iron oxide. The process is normally very slow, but we all know that wet iron rusts faster. Iron exposed to salty ocean water rusts the fastest. When iron turns to rust, the oxidation process generates heat. But rust forms so slowly that the heat generated is unnoticeable. We are all familiar with much faster oxidation reactions as well. For example, when you "oxidize" the carbon atoms in a charcoal briquette, they get quite hot. We use the word burning to describe this high-speed sort of oxidation. The idea behind a flameless heater is to use the oxidation of a metal to generate heat. Magnesium metal works better than iron because it rusts much more quickly. To make a flameless heater, magnesium dust is mixed with salt and a little iron dust in a thin, flexible pad about the size of a playing card. To activate the heater, a soldier adds a little water. Within seconds the flameless heater reaches the boiling point (of water) and is bubbling and steaming. To heat the meal, the soldier simply inserts the heater and the MRE pouch back in the box that the pouch came in. Ten minutes later, dinner is served!
Chapter 6 Energy and Chemical Change. Brady and Senese 5th Edition
 Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Ch 6. Energy and Chemical Change. Brady & Senese, 5th Ed.
 Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat.
 CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
CHAPTER 17 Thermochemistry
 CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Chapter 8 Thermochemistry
 William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
Thermochemistry. Using Heats of Reaction - Hess s Law - Standard Enthalpies of Formation - Fuels Foods, Commercial Fuels, and Rocket Fuels
 Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)
![= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj) = (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)](/thumbs/89/101004910.jpg) CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
Chapter 8 Thermochemistry: Chemical Energy
 Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy
 Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A
 ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A PART 1: KEY TERMS AND SYMBOLS IN THERMOCHEMISTRY System and surroundings When we talk about any kind of change, such as a chemical
ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A PART 1: KEY TERMS AND SYMBOLS IN THERMOCHEMISTRY System and surroundings When we talk about any kind of change, such as a chemical
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Introduction to Thermochemistry. Thermochemistry Unit. Definition. Terminology. Terminology. Terminology 07/04/2016. Chemistry 30
 Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 8 Thermochemistry: Chemical Energy. Chemical Thermodynamics
 Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
I. The Nature of Energy A. Energy
 I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
Thermochemistry. Section The flow of energy
 Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Class work on Calorimetry. January 11 and 12, 2011
 Class work on Calorimetry January 11 and 12, 2011 Name 1. The number of calories needed to raise the temperature of 100 grams of water 10 degrees Celsius is the same as the number of calories needed to
Class work on Calorimetry January 11 and 12, 2011 Name 1. The number of calories needed to raise the temperature of 100 grams of water 10 degrees Celsius is the same as the number of calories needed to
Chapter 6 Thermochemistry
 Chapter 6 Thermochemistry Thermochemistry Thermochemistry is a part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? - Will a reaction proceed
Chapter 6 Thermochemistry Thermochemistry Thermochemistry is a part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? - Will a reaction proceed
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
THERMOCHEMISTRY & DEFINITIONS
 THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
Thermochemistry: Energy Flow and Chemical Reactions
 Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
The following gas laws describes an ideal gas, where
 Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as
Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as
Thermochemistry-Part 1
 Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
Lecture Outline. 5.1 The Nature of Energy. Kinetic Energy and Potential Energy. 1 mv
 Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Section 9: Thermodynamics and Energy
 Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Name Class Date. As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings.
 Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Ch. 17 Thermochemistry
 Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
CHEMISTRY. Chapter 5 Thermochemistry
 CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
Thermochemistry: Heat and Chemical Change
 Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Chapter 5 Thermochemistry. 許富銀 ( Hsu Fu-Yin)
 Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 3. Thermochemistry: Energy Flow and Chemical Change. 5.1 Forms of Energy and Their Interconversion
 Chapter 3 Thermochemistry: Energy Flow and Chemical Change 5.1 Forms of Energy and Their Interconversion 5.2 Enthalpy: Chemical Change at Constant Pressure 5.3 Calorimetry: Measuring the Heat of a Chemical
Chapter 3 Thermochemistry: Energy Flow and Chemical Change 5.1 Forms of Energy and Their Interconversion 5.2 Enthalpy: Chemical Change at Constant Pressure 5.3 Calorimetry: Measuring the Heat of a Chemical
Energy, Heat and Chemical Change
 Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Reaction Energy. Thermochemistry
 Reaction Energy Thermochemistry Thermochemistry The study of the transfers of energy as heat that accompany chemical reactions & physical changes Thermochemistry -In studying heat changes, think of defining
Reaction Energy Thermochemistry Thermochemistry The study of the transfers of energy as heat that accompany chemical reactions & physical changes Thermochemistry -In studying heat changes, think of defining
Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes
 Thermochemistry Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy
Thermochemistry Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy
Thermochemistry Chapter 4
 Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
CP Chapter 17 Thermochemistry
 CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
Thermochemistry Chapter 8
 Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School
 Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93
 Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Thermodynamics Test Clio Invitational January 26, 2013
 Thermodynamics Test Clio Invitational January 26, 2013 School Name: Team Number: Variables specified: s = specific heat C = heat capacity H f = heat of fusion H v = heat of vaporization Given information:
Thermodynamics Test Clio Invitational January 26, 2013 School Name: Team Number: Variables specified: s = specific heat C = heat capacity H f = heat of fusion H v = heat of vaporization Given information:
2. If the volume of a container holding a gas is reduced, what will happen to the presure within the container?
 1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Thermochemistry 14.notebook. November 24, Thermochemistry. Energy the capacity to do work or produce heat. translational.
 Thermochemistry Energy the capacity to do work or produce heat POTENTIAL ENERGY KINETIC ENERGY (energy of motion) "stored" bond energy TEMPERATURE and HEAT vibrational rotational translational a measure
Thermochemistry Energy the capacity to do work or produce heat POTENTIAL ENERGY KINETIC ENERGY (energy of motion) "stored" bond energy TEMPERATURE and HEAT vibrational rotational translational a measure
Gravity is a force which keeps us stuck to the earth. The Electrostatic force attracts electrons to protons in an atom.
 Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Ch. 6 Enthalpy Changes
 Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions
 Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Chapter 6 Thermochemistry 許富銀
 Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Thermochemistry: Part of Thermodynamics
 Thermochemistry: Part of Thermodynamics Dr. Vickie M. Williamson @vmwilliamson Student Version 1 Chemical Thermodynamics! Thermodynamics: study of the energy changes associated with physical and chemical
Thermochemistry: Part of Thermodynamics Dr. Vickie M. Williamson @vmwilliamson Student Version 1 Chemical Thermodynamics! Thermodynamics: study of the energy changes associated with physical and chemical
_ + Units of Energy. Energy in Thermochemistry. Thermochemistry. Energy flow between system and surroundings. 100º C heat 50º C
 Units of Energy Like we saw with pressure, many different units are used throughout the world for energy. SI unit for energy 1kg m 1J = 2 s 2 Joule (J) calorie (cal) erg (erg) electron volts (ev) British
Units of Energy Like we saw with pressure, many different units are used throughout the world for energy. SI unit for energy 1kg m 1J = 2 s 2 Joule (J) calorie (cal) erg (erg) electron volts (ev) British
Chapter 5 - Thermochemistry
 Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energetics. Topic
 Energetics Topic 5.1 5.2 Topic 5.1 Exothermic and Endothermic Reactions?? total energy of the universe is a constant if a system loses energy, it must be gained by the surroundings, and vice versa Enthalpy
Energetics Topic 5.1 5.2 Topic 5.1 Exothermic and Endothermic Reactions?? total energy of the universe is a constant if a system loses energy, it must be gained by the surroundings, and vice versa Enthalpy
Thermodynamics. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
Unit 7 Thermochemistry Chemistry 020, R. R. Martin
 Unit 7 Thermochemistry Chemistry 020, R. R. Martin 1. Thermochemistry Heat is a form of energy - which may take many forms: - Kinetic energy due to motion, ½ mv 2 - Potential energy due to position - Electrical
Unit 7 Thermochemistry Chemistry 020, R. R. Martin 1. Thermochemistry Heat is a form of energy - which may take many forms: - Kinetic energy due to motion, ½ mv 2 - Potential energy due to position - Electrical
Exam 4, Enthalpy and Gases
 CHEM 1100 Dr. Stone November 8, 2017 Name_ G Exam 4, Enthalpy and Gases Equations and constants you may need: ΔE system = q + w PV = nrt R = 0.0821 (L*atm)/(mole*K) w = -PΔV K.E. = 1 2 m *µ 2 rms µ rms=
CHEM 1100 Dr. Stone November 8, 2017 Name_ G Exam 4, Enthalpy and Gases Equations and constants you may need: ΔE system = q + w PV = nrt R = 0.0821 (L*atm)/(mole*K) w = -PΔV K.E. = 1 2 m *µ 2 rms µ rms=
Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions
 Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions Terms, definitions, topics Joule, calorie (Re-read p 57-58) Thermochemistry Exothermic reaction Endothermic reaction
Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions Terms, definitions, topics Joule, calorie (Re-read p 57-58) Thermochemistry Exothermic reaction Endothermic reaction
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
5.1 Exothermic and endothermic reactions
 Topic 5: Energetics 5.1 Exothermic and endothermic reactions Chemical reactions involve the breaking and making of bonds. Breaking bonds requires energy,whereas energy is given out when new bonds are formed.
Topic 5: Energetics 5.1 Exothermic and endothermic reactions Chemical reactions involve the breaking and making of bonds. Breaking bonds requires energy,whereas energy is given out when new bonds are formed.
Chapter 6. Thermochemistry. Chapter 6. Chapter 6 Thermochemistry. Chapter 6 Thermochemistry Matter vs Energy 2/16/2016
 Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry 6.1 Chemical Hand Warmers 6.2 The Nature of Energy: Key Definitions 6.3 The First Law of Thermodynamics: There is no Free Lunch 6.4 6.5 Measuring
Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry 6.1 Chemical Hand Warmers 6.2 The Nature of Energy: Key Definitions 6.3 The First Law of Thermodynamics: There is no Free Lunch 6.4 6.5 Measuring
Name Date Class THERMOCHEMISTRY
 Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Energy Heat Work Heat Capacity Enthalpy
 Energy Heat Work Heat Capacity Enthalpy 1 Prof. Zvi C. Koren 20.07.2010 Thermodynamics vs. Kinetics Thermodynamics Thermo = Thermo + Dynamics E (Note: Absolute E can never be determined by humans!) Can
Energy Heat Work Heat Capacity Enthalpy 1 Prof. Zvi C. Koren 20.07.2010 Thermodynamics vs. Kinetics Thermodynamics Thermo = Thermo + Dynamics E (Note: Absolute E can never be determined by humans!) Can
CHAPTER 17: THERMOCHEMISTRY. Mrs. Brayfield
 CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
AP* Chemistry THERMOCHEMISTRY
 AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
s Traditionally, we use the calorie as a unit of energy. The nutritional Calorie, Cal = 1000 cal. Kinetic Energy and Potential Energy
 AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
Unit 14. States of Matter & Thermochemistry
 Unit 14 Flashback: States of Matter & Thermochemistry Characteristic Solids Liquids Gases Shape Volume Density Fluidity Compressibility Picture Phase Diagram Shows the relationship between solid, liquid,
Unit 14 Flashback: States of Matter & Thermochemistry Characteristic Solids Liquids Gases Shape Volume Density Fluidity Compressibility Picture Phase Diagram Shows the relationship between solid, liquid,
Energy Ability to produce change or do work. First Law of Thermodynamics. Heat (q) Quantity of thermal energy
 THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
Heat. Heat Terminology 04/12/2017. System Definitions. System Definitions
 System Definitions Heat Physical Science 20 Ms. Hayduk Heat Terminology System: the part of the universe being studied (big Earth, or small one atom) Surroundings: the part of the universe outside the
System Definitions Heat Physical Science 20 Ms. Hayduk Heat Terminology System: the part of the universe being studied (big Earth, or small one atom) Surroundings: the part of the universe outside the
10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics
 Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 Chapter 10 Thermochemistry Heat
Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 Chapter 10 Thermochemistry Heat
The Nature of Energy Energy is the ability to do work or produce Heat, q or Q, is ; flows due to temperature differences (always to )
 CP Chapter 17 Thermochemistry 2014-2015 Thermochemistry Thermochemistry is the study of energy that occur during chemical and physical changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry 2014-2015 Thermochemistry Thermochemistry is the study of energy that occur during chemical and physical changes (changes of state) The Nature of Energy Energy is the ability
Energy & Chemistry. Internal Energy (E) Energy and Chemistry. Potential Energy. Kinetic Energy. Energy and Chemical Reactions: Thermochemistry or
 Page III-5-1 / Chapter Five Lecture Notes Energy & Chemistry Energy and Chemical Reactions: Thermochemistry or Thermodynamics Chapter Five Burning peanuts supplies sufficient energy to boil a cup of water
Page III-5-1 / Chapter Five Lecture Notes Energy & Chemistry Energy and Chemical Reactions: Thermochemistry or Thermodynamics Chapter Five Burning peanuts supplies sufficient energy to boil a cup of water
3.2 Calorimetry and Enthalpy
 3.2 Calorimetry and Enthalpy Heat Capacity Specific heat capacity (c) is the quantity of thermal energy required to raise the temperature of 1 g of a substance by 1 C. The SI units for specific heat capacity
3.2 Calorimetry and Enthalpy Heat Capacity Specific heat capacity (c) is the quantity of thermal energy required to raise the temperature of 1 g of a substance by 1 C. The SI units for specific heat capacity
Chapter 6: Thermochemistry
 Chem 1045 General Chemistry by Ebbing and Gammon, 8th Edition George W.J. Kenney, Jr Last Update: 24-Oct-2008 Chapter 6: Thermochemistry These Notes are to SUPPLIMENT the Text, They do NOT Replace reading
Chem 1045 General Chemistry by Ebbing and Gammon, 8th Edition George W.J. Kenney, Jr Last Update: 24-Oct-2008 Chapter 6: Thermochemistry These Notes are to SUPPLIMENT the Text, They do NOT Replace reading
2013, 2011, 2009, 2008 AP
 Lecture 15 Thermodynamics I Heat vs. Temperature Enthalpy and Work Endothermic and Exothermic Reactions Average Bond Enthalpy Thermodynamics The relationship between chemical reactions and heat. What causes
Lecture 15 Thermodynamics I Heat vs. Temperature Enthalpy and Work Endothermic and Exothermic Reactions Average Bond Enthalpy Thermodynamics The relationship between chemical reactions and heat. What causes
Unit 15 Energy and Thermochemistry Notes
 Name KEY Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Name KEY Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
CHEMISTRY: Chapter 10 Prep-Test
 CHEMISTRY: Chapter 10 Prep-Test Matching Match each item with the correct statement below. a. calorimeter d. temperature b. calorie e. specific heat c. joule f. heat 1. quantity of heat needed to raise
CHEMISTRY: Chapter 10 Prep-Test Matching Match each item with the correct statement below. a. calorimeter d. temperature b. calorie e. specific heat c. joule f. heat 1. quantity of heat needed to raise
Lecture Presentation. Chapter 6. Thermochemistry. Sherril Soman Grand Valley State University Pearson Education, Inc.
 Lecture Presentation Chapter 6 Thermochemistry Sherril Soman Grand Valley State University Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron 4 Fe(s)
Lecture Presentation Chapter 6 Thermochemistry Sherril Soman Grand Valley State University Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron 4 Fe(s)
Thermochemistry AP Chemistry Lecture Outline
 Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Chemistry. Friday, March 30 th Monday, April 9 th, 2018
 Chemistry Friday, March 30 th Monday, April 9 th, 2018 Do-Now: BrainPOP: Heat 1. Write down today s FLT 2. Distinguish between exothermic and endothermic processes. 3. What is the specific heat of water?
Chemistry Friday, March 30 th Monday, April 9 th, 2018 Do-Now: BrainPOP: Heat 1. Write down today s FLT 2. Distinguish between exothermic and endothermic processes. 3. What is the specific heat of water?
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
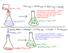 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
Chapter Objectives. Chapter 9 Energy and Chemistry. Chapter Objectives. Energy Use and the World Economy. Energy Use and the World Economy
 Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Energy Changes in Reactions p
 Energy Changes in Reactions p.126 210 Heat vs. temperature: Heat is a form of energy, it is transferred from one system to another Temperature is an indication of the intensity of heat, it measures the
Energy Changes in Reactions p.126 210 Heat vs. temperature: Heat is a form of energy, it is transferred from one system to another Temperature is an indication of the intensity of heat, it measures the
Name: Class: Date: ID: A
 Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
Chapter 11. Thermochemistry. 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages
 Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Energy Ability to produce change or do work. First Law of Thermodynamics. Heat (q) Quantity of thermal energy
 THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
Enthalpy and Internal Energy
 Enthalpy and Internal Energy H or ΔH is used to symbolize enthalpy. The mathematical expression of the First Law of Thermodynamics is: ΔE = q + w, where ΔE is the change in internal energy, q is heat and
Enthalpy and Internal Energy H or ΔH is used to symbolize enthalpy. The mathematical expression of the First Law of Thermodynamics is: ΔE = q + w, where ΔE is the change in internal energy, q is heat and
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Topic 05 Energetics : Heat Change. IB Chemistry T05D01
 Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
Thermochemistry. Chapter 6. Dec 19 8:52 AM. Thermochemistry. Energy: The capacity to do work or to produce heat
 Chapter 6 Dec 19 8:52 AM Intro vocabulary Energy: The capacity to do work or to produce heat Potential Energy: Energy due to position or composition (distance and strength of bonds) Kinetic Energy: Energy
Chapter 6 Dec 19 8:52 AM Intro vocabulary Energy: The capacity to do work or to produce heat Potential Energy: Energy due to position or composition (distance and strength of bonds) Kinetic Energy: Energy
