10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics
|
|
|
- Ferdinand Marshall
- 5 years ago
- Views:
Transcription
1 Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1
2 Chapter 10 Thermochemistry Heat Capacity, Cp, is the amount of heat required to raise the temperature of a substance by one degree at constant pressure. Cp is always a positive number Q and T must be both negative or positive If q is negative, then heat is evolved or given off and the temperature decreases If q is positive, then heat is absorbed and the temperature increases OFB Chap. 10 2
3 Heat Capacity (continued) C p units are energy per temp. change C p units are Joule/ o K or JK -1 Molar heat capacity Units are J/K/mole or JK -1 mol -1 OFB Chap. 10 3
4 Specific Heat Capacity The word Specific before the name of a physical quantity very often means divided by the mass Specific Heat Capacity is the amount of heat required to raise the temperature of one gram of material by one degree Kelvin (at constant pressure) Cs Hg(l) SiO O H 2 O 4.18 OFB Chap. 10 4
5 OFB Chap. 10 5
6 Chapter 10 Thermochemistry Example (not in book) a.) Calculate the molar heat capacity of quartz (SiO 2 ) b) Calculate the amount of heat required to raise 45.0 Kg of rock (quartz) by 15 C. Strategy a) Use relationship c p =Mc s b) Use q=mc s T OFB Chap. 10 6
7 Example (not in book) a.) Calculate the molar heat capacity of quartz (SiO 2 ), if c s SiO2=0.739 J/K/g b) Calculate the amount of heat required to raise 45.0 Kg of rock (quartz) by 15 C. Solution a) Find the molar mass of quartz and Use relationship c p =Mc s b) Use q= mc s T OFB Chap. 10 7
8 Exercise 10-1 Exactly kj of heat is absorbed at a constant pressure by a sample of gaseous helium. The temperature increases by 15.0K. a.) Compute the heat capacity of the sample b.) The mass of the helium sample is 6.42 kg. Compute the specific heat capacity and molar heat capacity of helium. Strategy a.) Use the relationship C p =q/ T B.) Use c s =C p /m and use c p =C p /n OFB Chap. 10 8
9 Exercise 10-1 Exactly kj of heat is absorbed at a constant pressure by a sample of gaseous helium. The temperature increases by 15.0K. a.) Compute the heat capacity of the sample b.) The mass of the helium sample is 6.42 kg. Compute the specific heat capacity and molar heat capacity of helium. Solution a.) Use the relationship C p =q/ T b.) Use c s =C p /m and use c p =C p /n OFB Chap. 10 9
10 Equilibration Temperature When 2 bodies are placed in contact, heat is exchanged until they reach a common final temperature Heat gained by the cooler body equals the heat lost by the warmer body If q is positive, heat is gained If q is negative, heat is lost Can also write similar equations for c s OFB Chap
11 Chapter 10 Thermochemistry Example (not in book) A 43.9g piece of Copper (c s =0.385 J g -1 K -1 ) at 135 C is plunged into 254g of water (c s =4.18 J g -1 K -1 ) at 39 C. Assuming no heat is lost to the surroundings, what will be the final temperature? a) C b) 87.0 C c) 53.1 C d) 40.5 C e) None of these OFB Chap
12 Also Try 10-2 Example and Exercise OFB Chap
13 Chapter 10 Thermochemistry For processes carried out at constant pressure, the heat absorbed equals a change in Enthalpy, H. q p = H (note p denotes constant pressure) Enthalpy is a state property, which means it depends on its initial and final state, not the path to get there. OFB Chap
14 Enthalpy of Reaction If a chemical reaction occurs at constant pressure then (Heat absorbed) = q p = H H may be either positive or negative If H positive = H > 0 = q > 0 Means heat is absorbed And is called Endothermic If H negative = H < 0 = q < 0 Means heat is given off And is called Exothermic OFB Chap
15 CO (g) + ½ O 2 (g) CO 2 (g) H= -283kJ An exothermic reaction If doubled (x2), double H 2CO (g) + 1O 2 (g) 2CO 2 (g) H= -566kJ If the original reaction is reversed CO 2 (g) CO (g) + ½ O 2 (g) H= +283kJ An endothermic reaction You can Try Example 10-5 OFB Chap
16 Phase Changes Phase changes are not chemical reactions but involve enthalpy change Note that there are no T units, these occur at constant temperatures for freezing, fusion, vaporization or condensation OFB Chap
17 Typical Exam question How much heat energy is needed to decompose 9.74g of HBr (g) (M =80.9 g/mol) into its elements? H 2 + Br 2 2HBr (g) H = kjmol -1 Strategy: Decomposition is actually the reverse reaction H to burn 2 moles of HBr is 72.8kJ/mol OFB Chap
18 Typical Exam question How much heat energy is needed to decompose 9.74g of HBr (g) (M =80.9 g/mol) into its elements? H 2 + Br 2 2HBr (g) H = kjmol -1 Solution: 2HBr (g) H 2 + Br 2 H = kjmol -1 OFB Chap
19 Hess s Law Sometimes its difficult or even impossible to conduct some experiments in the lab. Because Enthalpy (H) is a State Property, it doesn t matter what path is taken as long as the initial reactants lead to the final product. Hess s Law is about adding or subtracting equations and enthalpies. Hess s Law: If two or more chemical equations are added to give a new equation, then adding the enthalpies of the reactions that they represent gives the enthalpy of the new reaction. OFB Chap
20 Hess s Law Unknown C (graphite) + 1/2 O2 (g) CO (g) H =? This is Hess s Law. It always works no matter how complicated the path. OFB Chap
21 Standard State Enthalpies Designated using a superscript (pronounced naught) This is needed because Enthalpy varies with temperature Is written as H H ο or H ο or H ο o o 25 C K If no temperature is indicated in the subscript, assume 25 C. OFB text appendix D lists standard enthalpies of formation for a variety of chemical species at 1 atm and 25 C. OFB Chap
22 Standard State Enthalpies H is the sum of products minus the sum of the reactants For a general reaction a A + b B c C + d D OFB Chap
23 Chapter 10 Thermochemistry Exercise 10-7 Suppose hydrazine and oxygen react to give dinitrogen pentaoxide and water vapor: 2N 2 H 4 (l) + 7O 2 (g) 2N 2 O 5 (s) + 4H 2 O (g) Calculate the H of this reaction, given that the reaction: N 2 (g) + 5/2O 2 (g) N 2 O 5 (s) has a H of -43 kj. OFB Chap
24 10-7 Exercise 2N 2 H 4 (l) + 7O 2 (g) 2N 2 O 5 (s) + 4H 2 O (g) What values to use? H f N 2 O 5 (s) given H =-43.1 kjmol -1 H f H 2 O (g) look up in App. D H f N 2 H 4 (l) look up in App. D H f O 2 (g) look up in App. D H f = kjmol -1 OFB Chap
25 Typical Exam question Given the following thermo chemical equations, calculate the standard enthalpy of formation for propane, C 3 H 8 (g) 1. C(s) + H 2. H 3. H = (-2452) kjmol Answers o H 2(g) o C3H 8(g) o = = O 2(g) (-393) kjmol + 1/2O 2(g) (-286) kjmol + 5O 2(g) A. (3131) kjmol -1 B. 129 C D. (1773) E. None of these CO 2(g) H2O(l) 3CO 2(g) 4H2O(l) OFB Chap
26 General aa + bb cc + dd H o = o c H C + d H o D a H o A b H o B OFB Chap
27 Bond Enthalpies The breaking of chemical bonds in stable substances often generates highly reactive products (or intermediates) CH 4 CH3 + H H = kjmol -1 Called Bond Enthalpy OFB Table 10-3 p 462 gives Average Bond Enthalpies OFB Chap
28 Average Bond Enthalpies C 2 H 6 C 2 H 5 + H H = kjmol -1 CHF 3 CF 3 + H H = kjmol -1 CHCl 3 CCl 3 + H H = kjmol -1 CHBr 3 CBr 3 + H H = kjmol -1 average H C-H = kjmol -1 Applications of Bond Enthalpy Given a reaction 1 st Step is break all bonds to give free atoms in the gas phase (Endothermic) 2 nd Step is form new bonds for the products. (Exothermic) OFB Chap
29 Applications of Bond Enthalpy Given a reaction 1 st Step is break all bonds to give free atoms in the gas phase (Endothermic) 2 nd Step is form new bonds for the products. (Exothermic) OFB Chap
30 Exercise Estimate the Standard Enthalpy of reaction for the gas-phase reaction that forms methanol from methane and water and compare it with the H r obtained from the data in Appendix D. CH 4 (g) + H 2 O(g) CH 3 OH(g) + H 2 (g) OFB Chap
31 CH 4 (g) + H 2 O(g) CH 3 OH(g) + H 2 (g) H H C H H + H H O H H C O H H + H H Broken 4 C-H 2 O-H kjmol -1 Endothermic Formed 3 C-H 1 O-H 1 C-O 1 H-H kjmol -1 Exothermic H= H bonds broken + H bonds formed =+2578+(-2489)= + 89 kjmol -1 (estimated) Using Appendix D H = 116 kjmol -1 OFB Chap
32 Chapter 10 Thermochemistry First Law of Thermodynamics The change in the internal energy of a system is equal to the work done on it plus the heat transferred to it. E = q + w Second Law of Thermodynamics In a real spontaneous process the Entropy of the universe (meaning the system plus its surroundings) must increase. S universe > 0 Third Law of Thermodynamics In any thermodynamic process involving only pure phases at equilibrium, the entropy change, S, approaches zero at absolute zero temperature; also the entropy of a crystalline substance approaches zero. S = 0 at 0 K OFB Chap
33 First Law of Thermodynamics E = q + w q was previously defined as the Heat Absorbed by a system If q > 0, heat is absorbed If q < 0, heat is given off w is the work done on the body Recall w = F x d w = E Kinetic = (1/2mv 2 ) w = E potential = mg h In chemistry this kind of mechanical work is Pressure- Volume work (P-V) OFB Chap
34 Force exerted by heating = P 1 A Where P 1 is the pressure inside the vessel Where A is the Area of the piston P ext = P 1 if balanced V = A h W = -P ext V Units are atm L or L atm Where 1 L atm = Joules OFB Chap
35 W = -P ext V Expansion V > 0 therefore w < 0 The system does work on the surroundings Compression V < 0 therefore w > 0 The surroundings have done wok on the system Try Example and Exercise Calculate the work done on a gas and express it in Joules OFB Chap
36 Typical exam questions (1-4) A gas is compressed from 39.92L to 12.97L at a constant pressure of 5.00 atm. In the course of this compression 9.82 kj of energy is released Q1 The heat q for this process is a 135 kj b -135 kj c kj d 9.82 kj e can not be determined OFB Chap
37 A gas is compressed from 39.92L to 12.97L at a constant pressure of 5.00 atm. In the course of this compression 9.82 kj of energy is released Q2 The work w for this process is a 135 L atm b -135 L atm c L atm d 9.82 L atm e can not be determined OFB Chap
38 A gas is compressed from 39.92L to 12.97L at a constant pressure of 5.00 atm. In the course of this compression 9.82 kj of energy is released Q3 E for the process is a kj b 3.86 kj c kj d 125 kj e none of these OFB Chap
39 A gas is compressed from 39.92L to 12.97L at a constant pressure of 5.00 atm. In the course of this compression 9.82 kj of energy is released Q4 H for the process is a kj b kj c 3.83 kj d 9.82 KJ e none of these OFB Chap
40 Enthalpy and Energy 1 st Law of Thermodynamics E = q + w At constant volume V =0 Thus w = -P ext V = 0 E = q v (constant volume) Recall as previously stated H = q p (constant pressure) Enthalpy is defined as H = E + PV or Expressed as a change in Enthalpy H = E + (PV) Rearrange E = H + (PV) If PV = nrt is the ideal gas law (PV) = (nrt)= RT n g OFB Chap
41 Chapter 10 Thermochemistry (PV) = RT n g n g is the change in the total chemical amount of gases in a reaction n g = Total moles of product gases minus the Total moles of reactant gases OFB Chap
42 Example Calculate the internal 25 C for the following reaction 1 C(graphite) + O 2(g) 2 H = kj 1CO(g) OFB Chap
43 Example Calculate the internal 25 C for the following reaction 1 C(graphite) + O 2(g) 2 H = kj 1CO(g) OFB Chap
44 Chapter 10 Thermochemistry Summary c p Heat Capacity where Cp p units are JK = C p denotes Heat Capacity at constant pressure T Molar Heat Capacity C = n -1 = mol = T 1 q T f - T = i heat absorbed T Specific Heat Capacity Cp -1 cs = units are JK g m -1 n 1 Equilibration Temperature c p1 n 1 c p1 1 1 p2 ( f i ) 1 2 p2 ( f i ) 2 T T q T = q = n = n 2 2 c c T T 2 T OFB Chap
45 Enthalpy, H. If H positive = H > 0 = q > 0 Means heat is absorbed And is called Endothermic If H negative = H < 0 = q < 0 Means heat is given off And is called Exothermic Hess s Law: If two or more chemical equations are added to give a new equation, then adding the enthalpies of the reactions that they represent gives the enthalpy of the new reaction. q p = H (note p denotes constant pressure) Enthalpy is a state property, which means it depends on its initial and final state, not the path to get there. OFB Chap
46 Standard State Enthalpies H is the sum of products minus the sum of the reactants For a general reaction a A + b B c C + d D H o = c H o f (C) + d H o f (D) a H o f (A) b H o f (B) Bond Enthalpies H= H bonds broken + H bonds formed First Law of Thermodynamics The change in the internal energy of a system is equal to the work done on it plus the heat transferred to it. E = q + w OFB Chap
47 Enthalpy and Energy Enthalpy is defined as H = E + PV or Expressed as a change in Enthalpy H = E + (PV) For gases, If PV = nrt is the ideal gas law (PV) = (nrt)= RT ng OFB Chap
48 Chapter 10 Thermochemistry Examples / exercises 10-1, 10-2, 10-5, 10-6, 10-7, 10-8, 10-9, 10-10, 10-11, HW Problems 11, 13, 19, 23, 33, 37, 40, 43, 49, 53, 59, 63 OFB Chap
10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics
 Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 OFB Chap. 10 2 Thermite Reaction
Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 OFB Chap. 10 2 Thermite Reaction
Major Concepts Calorimetry (from last time)
 Major Concepts Calorimetry (from last time) Heat capacity Molar heat capacity (per mole) Specific heat capacity (per mass) Standard state enthalpies: Hº Physical Changes Chemical Changes Hess's Law Balancing
Major Concepts Calorimetry (from last time) Heat capacity Molar heat capacity (per mole) Specific heat capacity (per mass) Standard state enthalpies: Hº Physical Changes Chemical Changes Hess's Law Balancing
Chapter 8. Thermochemistry 강의개요. 8.1 Principles of Heat Flow. 2) Magnitude of Heat Flow. 1) State Properties. Basic concepts : study of heat flow
 강의개요 Basic concepts : study of heat flow Chapter 8 Thermochemistry Calorimetry : experimental measurement of the magnitude and direction of heat flow Thermochemical Equations Copyright 2005 연세대학교이학계열일반화학및실험
강의개요 Basic concepts : study of heat flow Chapter 8 Thermochemistry Calorimetry : experimental measurement of the magnitude and direction of heat flow Thermochemical Equations Copyright 2005 연세대학교이학계열일반화학및실험
Chapter 8 Thermochemistry: Chemical Energy. Chemical Thermodynamics
 Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
I PUC CHEMISTRY CHAPTER - 06 Thermodynamics
 I PUC CHEMISTRY CHAPTER - 06 Thermodynamics One mark questions 1. Define System. 2. Define surroundings. 3. What is an open system? Give one example. 4. What is closed system? Give one example. 5. What
I PUC CHEMISTRY CHAPTER - 06 Thermodynamics One mark questions 1. Define System. 2. Define surroundings. 3. What is an open system? Give one example. 4. What is closed system? Give one example. 5. What
Energy, Heat and Chemical Change
 Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
I. The Nature of Energy A. Energy
 I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
CHAPTER 10: THERMOCHEMISTRY
 CHAPTER 10: THERMOCHEMISTRY Definition of heat Calorimetry and relationship between heat and temperature changes Enthalpy Hess s Law Bond enthalpies First Law of Thermodynamics The Zeroth Law Zeroth Law
CHAPTER 10: THERMOCHEMISTRY Definition of heat Calorimetry and relationship between heat and temperature changes Enthalpy Hess s Law Bond enthalpies First Law of Thermodynamics The Zeroth Law Zeroth Law
CHEMISTRY. Chapter 5 Thermochemistry
 CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chemical Thermodynamics
 Quiz A 42.8 ml solution of ammonia (NH 3 ) is titrated with a solution of 0.9713 M hydrochloric acid. The initial reading on the buret containing the HCl was 47.13 ml and the final reading when the endpoint
Quiz A 42.8 ml solution of ammonia (NH 3 ) is titrated with a solution of 0.9713 M hydrochloric acid. The initial reading on the buret containing the HCl was 47.13 ml and the final reading when the endpoint
Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy
 Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Chapter 8. Thermochemistry
 Chapter 8 Thermochemistry Copyright 2001 by Harcourt, Inc. All rights reserved. Requests for permission to make copies of any part of the work should be mailed to the following address: Permissions Department,
Chapter 8 Thermochemistry Copyright 2001 by Harcourt, Inc. All rights reserved. Requests for permission to make copies of any part of the work should be mailed to the following address: Permissions Department,
Lecture 2. Review of Basic Concepts
 Lecture 2 Review of Basic Concepts Thermochemistry Enthalpy H heat content H Changes with all physical and chemical changes H Standard enthalpy (25 C, 1 atm) (H=O for all elements in their standard forms
Lecture 2 Review of Basic Concepts Thermochemistry Enthalpy H heat content H Changes with all physical and chemical changes H Standard enthalpy (25 C, 1 atm) (H=O for all elements in their standard forms
Chapter 8 Thermochemistry: Chemical Energy
 Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Chapter 17: Spontaneity, Entropy, and Free Energy
 Chapter 17: Spontaneity, Entropy, and Free Energy Review of Chemical Thermodynamics System: the matter of interest Surroundings: everything in the universe which is not part of the system Closed System:
Chapter 17: Spontaneity, Entropy, and Free Energy Review of Chemical Thermodynamics System: the matter of interest Surroundings: everything in the universe which is not part of the system Closed System:
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Module Tag CHE_P10_M6
 Subject Chemistry Paper No and Title 10: Physical Chemistry- III (Classical Thermodynamics, Non-Equilibrium Thermodynamics, Module No and 6, Thermochemistry and Hess s law Title Module Tag CHE_P10_M6 TABLE
Subject Chemistry Paper No and Title 10: Physical Chemistry- III (Classical Thermodynamics, Non-Equilibrium Thermodynamics, Module No and 6, Thermochemistry and Hess s law Title Module Tag CHE_P10_M6 TABLE
Section 1 - Thermochemistry
 Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
ENERGY (THERMOCHEMISTRY) Ch 1.5, 6, 9.10, , 13.3
 ENERGY (THERMOCHEMISTRY) Ch 1.5, 6, 9.10, 11.5-11.7, 13.3 Thermochemistry Prediction and measurement of energy transfer, in the form of heat, that accompanies chemical and physical processes. Chemical
ENERGY (THERMOCHEMISTRY) Ch 1.5, 6, 9.10, 11.5-11.7, 13.3 Thermochemistry Prediction and measurement of energy transfer, in the form of heat, that accompanies chemical and physical processes. Chemical
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
Thermodynamics- Chapter 19 Schedule and Notes
 Thermodynamics- Chapter 19 Schedule and Notes Date Topics Video cast DUE Assignment during class time One Review of thermodynamics ONE and TWO Review of thermo Wksheet Two 19.1-4; state function THREE
Thermodynamics- Chapter 19 Schedule and Notes Date Topics Video cast DUE Assignment during class time One Review of thermodynamics ONE and TWO Review of thermo Wksheet Two 19.1-4; state function THREE
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Thermochemistry-Part 1
 Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Unit 7 Kinetics and Thermodynamics
 17.1 The Flow of Energy Heat and Work Unit 7 Kinetics and Thermodynamics I. Energy Transformations A. Temperature 1. A measure of the average kinetic energy of the particles in a sample of matter B. Heat
17.1 The Flow of Energy Heat and Work Unit 7 Kinetics and Thermodynamics I. Energy Transformations A. Temperature 1. A measure of the average kinetic energy of the particles in a sample of matter B. Heat
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
AP CHEMISTRY NOTES 4-1 THERMOCHEMISTRY: ENTHALPY AND ENTROPY
 AP CHEMISTRY NOTES 4-1 THERMOCHEMISTRY: ENTHALPY AND ENTROPY Reaction Rate how fast a chemical reaction occurs Collision Theory In order for a chemical reaction to occur, the following conditions must
AP CHEMISTRY NOTES 4-1 THERMOCHEMISTRY: ENTHALPY AND ENTROPY Reaction Rate how fast a chemical reaction occurs Collision Theory In order for a chemical reaction to occur, the following conditions must
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
0. Graphite is thermodynamically less stable that diamond under standard conditions. 1. True 2. False
 0. Graphite is thermodynamically less stable that diamond under standard conditions. 1. True 2. False 1. Which statement would be the best interpretation of the First Law of Thermodynamics? 1. The total
0. Graphite is thermodynamically less stable that diamond under standard conditions. 1. True 2. False 1. Which statement would be the best interpretation of the First Law of Thermodynamics? 1. The total
CHAPTER 3 THE FIRST LAW OF THERMODYNAM- ICS
 CHAPTER 3 THE FIRST LAW OF THERMODYNAM- ICS Introduction In this chapter, we discuss the First Law of Thermodynamics (energy cannot be created or destroyed). In our discussion, we will define some important
CHAPTER 3 THE FIRST LAW OF THERMODYNAM- ICS Introduction In this chapter, we discuss the First Law of Thermodynamics (energy cannot be created or destroyed). In our discussion, we will define some important
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
I. Multiple Choice Questions (Type-I)
 I. Multiple Choice Questions (Type-I) 1. Thermodynamics is not concerned about. (i) energy changes involved in a chemical reaction. the extent to which a chemical reaction proceeds. the rate at which a
I. Multiple Choice Questions (Type-I) 1. Thermodynamics is not concerned about. (i) energy changes involved in a chemical reaction. the extent to which a chemical reaction proceeds. the rate at which a
Thermodynamics. Thermodynamics of Chemical Reactions. Enthalpy change
 Thermodynamics 1 st law (Cons of Energy) Deals with changes in energy Energy in chemical systems Total energy of an isolated system is constant Total energy = Potential energy + kinetic energy E p mgh
Thermodynamics 1 st law (Cons of Energy) Deals with changes in energy Energy in chemical systems Total energy of an isolated system is constant Total energy = Potential energy + kinetic energy E p mgh
Thermodynamics. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
Chemical Thermodynamics
 Chemical Thermodynamics 1 Thermodynamics Thermodynamics is a Greek term which means, heat power. Thermodynamics is the study of energy and its transformations. 2 Thermodynamics Thermochemistry how we observe,
Chemical Thermodynamics 1 Thermodynamics Thermodynamics is a Greek term which means, heat power. Thermodynamics is the study of energy and its transformations. 2 Thermodynamics Thermochemistry how we observe,
Energy and Chemical Change
 Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy & Chemistry. Internal Energy (E) Energy and Chemistry. Potential Energy. Kinetic Energy. Energy and Chemical Reactions: Thermochemistry or
 Page III-5-1 / Chapter Five Lecture Notes Energy & Chemistry Energy and Chemical Reactions: Thermochemistry or Thermodynamics Chapter Five Burning peanuts supplies sufficient energy to boil a cup of water
Page III-5-1 / Chapter Five Lecture Notes Energy & Chemistry Energy and Chemical Reactions: Thermochemistry or Thermodynamics Chapter Five Burning peanuts supplies sufficient energy to boil a cup of water
Thermochemistry Chapter 4
 Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Review of Terms. Additional Review. Energy, Enthalpy, & Thermochemistry
 Energy, Enthalpy, & Thermochemistry 9.1 The Nature of Energy 9. Enthalpy 9. Thermodynamics of Ideal Gases 9.4 alorimetry 9.5 Hess s Law 9.6 Standard Enthalpies of Formation 9.7 Present Sources of Energy
Energy, Enthalpy, & Thermochemistry 9.1 The Nature of Energy 9. Enthalpy 9. Thermodynamics of Ideal Gases 9.4 alorimetry 9.5 Hess s Law 9.6 Standard Enthalpies of Formation 9.7 Present Sources of Energy
Thermochemistry. Mr.V
 Thermochemistry Mr.V Introduction to Energy changes System Surroundings Exothermic Endothermic Internal energy Enthalpy Definitions System A specified part of the universe which is under investigation
Thermochemistry Mr.V Introduction to Energy changes System Surroundings Exothermic Endothermic Internal energy Enthalpy Definitions System A specified part of the universe which is under investigation
Chemical Thermodynamics. Chemical Thermodynamics. Changes of State. Chemical Thermodynamics. State Functions. State Functions 11/25/13
 Chemical Thermodynamics n Thermodynamics is the study of the energetics and order of a system. n A system is the thing we want to study it can be a chemical reaction, a solution, an automobile, or the
Chemical Thermodynamics n Thermodynamics is the study of the energetics and order of a system. n A system is the thing we want to study it can be a chemical reaction, a solution, an automobile, or the
Chapter 6 Thermochemistry
 Chapter 6 Thermochemistry Contents and Concepts Understanding Heats of Reaction The first part of the chapter lays the groundwork for understanding what we mean by heats of reaction. 1. Energy and Its
Chapter 6 Thermochemistry Contents and Concepts Understanding Heats of Reaction The first part of the chapter lays the groundwork for understanding what we mean by heats of reaction. 1. Energy and Its
Thermochemistry: Part of Thermodynamics
 Thermochemistry: Part of Thermodynamics Dr. Vickie M. Williamson @vmwilliamson Student Version 1 Chemical Thermodynamics! Thermodynamics: study of the energy changes associated with physical and chemical
Thermochemistry: Part of Thermodynamics Dr. Vickie M. Williamson @vmwilliamson Student Version 1 Chemical Thermodynamics! Thermodynamics: study of the energy changes associated with physical and chemical
Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat.
 CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Energy the capacity to do work or produce heat A. two basic types of
Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Energy the capacity to do work or produce heat A. two basic types of
Chapter 5: Thermochemistry. Molecular Kinetic Energy -Translational energy E k, translational = 1/2mv 2 -Rotational energy 5.
 Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Unit 7 Thermochemistry Chemistry 020, R. R. Martin
 Unit 7 Thermochemistry Chemistry 020, R. R. Martin 1. Thermochemistry Heat is a form of energy - which may take many forms: - Kinetic energy due to motion, ½ mv 2 - Potential energy due to position - Electrical
Unit 7 Thermochemistry Chemistry 020, R. R. Martin 1. Thermochemistry Heat is a form of energy - which may take many forms: - Kinetic energy due to motion, ½ mv 2 - Potential energy due to position - Electrical
Chapter 17: Energy and Kinetics
 Pages 510-547 S K K Chapter 17: Energy and Kinetics Thermochemistry: Causes of change in systems Kinetics: Rate of reaction progress (speed) Heat, Energy, and Temperature changes S J J Heat vs Temperature
Pages 510-547 S K K Chapter 17: Energy and Kinetics Thermochemistry: Causes of change in systems Kinetics: Rate of reaction progress (speed) Heat, Energy, and Temperature changes S J J Heat vs Temperature
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
TODAY 0. Why H = q (if p ext =p=constant and no useful work) 1. Constant Pressure Heat Capacity (what we usually use)
 361 Lec 7 Fri 9sep15 TODAY 0. Why H = q (if p ext =p=constant and no useful work) 1. Constant Pressure Heat Capacity (what we usually use) 2. Heats of Chemical Reactions: r H (mechanics of obtaining from
361 Lec 7 Fri 9sep15 TODAY 0. Why H = q (if p ext =p=constant and no useful work) 1. Constant Pressure Heat Capacity (what we usually use) 2. Heats of Chemical Reactions: r H (mechanics of obtaining from
CHEMISTRY 109 #25 - REVIEW
 CHEMISTRY 109 Help Sheet #25 - REVIEW Chapter 4 (Part I); Sections 4.1-4.6; Ch. 9, Section 9.4a-9.4c (pg 387) ** Review the appropriate topics for your lecture section ** Prepared by Dr. Tony Jacob http://www.chem.wisc.edu/areas/clc
CHEMISTRY 109 Help Sheet #25 - REVIEW Chapter 4 (Part I); Sections 4.1-4.6; Ch. 9, Section 9.4a-9.4c (pg 387) ** Review the appropriate topics for your lecture section ** Prepared by Dr. Tony Jacob http://www.chem.wisc.edu/areas/clc
Thermodynamic Processes and Thermochemistry
 General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
2013, 2011, 2009, 2008 AP
 Lecture 15 Thermodynamics I Heat vs. Temperature Enthalpy and Work Endothermic and Exothermic Reactions Average Bond Enthalpy Thermodynamics The relationship between chemical reactions and heat. What causes
Lecture 15 Thermodynamics I Heat vs. Temperature Enthalpy and Work Endothermic and Exothermic Reactions Average Bond Enthalpy Thermodynamics The relationship between chemical reactions and heat. What causes
THERMOCHEMISTRY -1. Dr. Sapna Gupta
 THERMOCHEMISTRY -1 Dr. Sapna Gupta THERMODYNAMICS Thermodynamics: Relationship between heat and other forms of energy Thermochemistry: Study of heat absorbed or evolved by chemical reactions. Energy: Capacity
THERMOCHEMISTRY -1 Dr. Sapna Gupta THERMODYNAMICS Thermodynamics: Relationship between heat and other forms of energy Thermochemistry: Study of heat absorbed or evolved by chemical reactions. Energy: Capacity
Contents and Concepts
 Contents and Concepts 1. First Law of Thermodynamics Spontaneous Processes and Entropy A spontaneous process is one that occurs by itself. As we will see, the entropy of the system increases in a spontaneous
Contents and Concepts 1. First Law of Thermodynamics Spontaneous Processes and Entropy A spontaneous process is one that occurs by itself. As we will see, the entropy of the system increases in a spontaneous
Contents and Concepts
 Contents and Concepts 1. First Law of Thermodynamics Spontaneous Processes and Entropy A spontaneous process is one that occurs by itself. As we will see, the entropy of the system increases in a spontaneous
Contents and Concepts 1. First Law of Thermodynamics Spontaneous Processes and Entropy A spontaneous process is one that occurs by itself. As we will see, the entropy of the system increases in a spontaneous
Section 3.0. The 1 st Law of Thermodynamics. (CHANG text Chapter 4) 3.1. Revisiting Heat Capacities Definitions and Concepts
 Section 3.0. The 1 st Law of Thermodynamics (CHANG text Chapter 4) 3.1. Revisiting Heat Capacities 3.2. Definitions and Concepts 3.3. The First Law of THERMODYNAMICS 3.4. Enthalpy 3.5. Adiabatic Expansion
Section 3.0. The 1 st Law of Thermodynamics (CHANG text Chapter 4) 3.1. Revisiting Heat Capacities 3.2. Definitions and Concepts 3.3. The First Law of THERMODYNAMICS 3.4. Enthalpy 3.5. Adiabatic Expansion
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
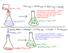 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Thermochemistry Lecture
 Thermochemistry Lecture Jennifer Fang 1. Enthalpy 2. Entropy 3. Gibbs Free Energy 4. q 5. Hess Law 6. Laws of Thermodynamics ENTHALPY total energy in all its forms; made up of the kinetic energy of the
Thermochemistry Lecture Jennifer Fang 1. Enthalpy 2. Entropy 3. Gibbs Free Energy 4. q 5. Hess Law 6. Laws of Thermodynamics ENTHALPY total energy in all its forms; made up of the kinetic energy of the
= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)
![= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj) = (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)](/thumbs/89/101004910.jpg) CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
Chapter Objectives. Chapter 9 Energy and Chemistry. Chapter Objectives. Energy Use and the World Economy. Energy Use and the World Economy
 Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Exam 4, Enthalpy and Gases
 CHEM 1100 Dr. Stone November 8, 2017 Name_ G Exam 4, Enthalpy and Gases Equations and constants you may need: ΔE system = q + w PV = nrt R = 0.0821 (L*atm)/(mole*K) w = -PΔV K.E. = 1 2 m *µ 2 rms µ rms=
CHEM 1100 Dr. Stone November 8, 2017 Name_ G Exam 4, Enthalpy and Gases Equations and constants you may need: ΔE system = q + w PV = nrt R = 0.0821 (L*atm)/(mole*K) w = -PΔV K.E. = 1 2 m *µ 2 rms µ rms=
Energy Relationships in Chemical Reactions
 Energy Relationships in Chemical Reactions What is heat? What is a state function? What is enthalpy? Is enthalpy a state function? What does this mean? How can we calculate this? How are the methods the
Energy Relationships in Chemical Reactions What is heat? What is a state function? What is enthalpy? Is enthalpy a state function? What does this mean? How can we calculate this? How are the methods the
THERMOCHEMISTRY & DEFINITIONS
 THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
Warm up. 1) What is the conjugate acid of NH 3? 2) What is the conjugate base of HNO 2? 3) If the ph is 9.2, what is the [H 3 O + ], poh, and [OH - ]?
![Warm up. 1) What is the conjugate acid of NH 3? 2) What is the conjugate base of HNO 2? 3) If the ph is 9.2, what is the [H 3 O + ], poh, and [OH - ]? Warm up. 1) What is the conjugate acid of NH 3? 2) What is the conjugate base of HNO 2? 3) If the ph is 9.2, what is the [H 3 O + ], poh, and [OH - ]?](/thumbs/78/77877522.jpg) Warm up 1) What is the conjugate acid of NH 3? 2) What is the conjugate base of HNO 2? 3) If the ph is 9.2, what is the [H 3 O + ], poh, and [OH - ]? 4) What is the concentration of H 2 SO 4 if 30.1 ml
Warm up 1) What is the conjugate acid of NH 3? 2) What is the conjugate base of HNO 2? 3) If the ph is 9.2, what is the [H 3 O + ], poh, and [OH - ]? 4) What is the concentration of H 2 SO 4 if 30.1 ml
THERMODYNAMICS I. TERMS AND DEFINITIONS A. Review of Definitions 1. Thermodynamics = Study of the exchange of heat, energy and work between a system
 THERMODYNAMICS I. TERMS AND DEFINITIONS A. Review of Definitions 1. Thermodynamics = Study of the exchange of heat, energy and work between a system and its surroundings. a. System = That part of universe
THERMODYNAMICS I. TERMS AND DEFINITIONS A. Review of Definitions 1. Thermodynamics = Study of the exchange of heat, energy and work between a system and its surroundings. a. System = That part of universe
For more info visit
 Basic Terminology: Terms System Open System Closed System Isolated system Surroundings Boundary State variables State Functions Intensive properties Extensive properties Process Isothermal process Isobaric
Basic Terminology: Terms System Open System Closed System Isolated system Surroundings Boundary State variables State Functions Intensive properties Extensive properties Process Isothermal process Isobaric
McCord CH301 Exam 5 Dec 5, 2017
 425 version last name first name signature McCord CH301 Exam 5 Dec 5, 2017 50070 BUR 106 Tuesday TTh 9:30 am - 11 pm Remember to refer to the Periodic Table handout that is separate from this exam copy.
425 version last name first name signature McCord CH301 Exam 5 Dec 5, 2017 50070 BUR 106 Tuesday TTh 9:30 am - 11 pm Remember to refer to the Periodic Table handout that is separate from this exam copy.
General Chemistry I. Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University. Module 4: Chemical Thermodynamics
 General Chemistry I Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University Module 4: Chemical Thermodynamics Zeroth Law of Thermodynamics. First Law of Thermodynamics (state quantities:
General Chemistry I Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University Module 4: Chemical Thermodynamics Zeroth Law of Thermodynamics. First Law of Thermodynamics (state quantities:
Gilbert Kirss Foster. Chapter 9. Thermochemistry. Energy Changes in Chemical Reactions
 Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Chapter 5 THERMO. THERMO chemistry. 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation
 Chapter 5 THERMO THERMO chemistry 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation Chemical Equations 1 st WRITE the Chemical Equation 2 nd BALANCE the Chemical Equation
Chapter 5 THERMO THERMO chemistry 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation Chemical Equations 1 st WRITE the Chemical Equation 2 nd BALANCE the Chemical Equation
Chapter 19. Chemical Thermodynamics. Chemical Thermodynamics
 Chapter 19 Enthalpy A thermodynamic quantity that equal to the internal energy of a system plus the product of its volume and pressure exerted on it by its surroundings; Enthalpy is the amount of energy
Chapter 19 Enthalpy A thermodynamic quantity that equal to the internal energy of a system plus the product of its volume and pressure exerted on it by its surroundings; Enthalpy is the amount of energy
Ch. 6 Enthalpy Changes
 Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
CHAPTER 17 Thermochemistry
 CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
Contents and Concepts
 Contents and Concepts 1. First Law of Thermodynamics Spontaneous Processes and Entropy A spontaneous process is one that occurs by itself. As we will see, the entropy of the system increases in a spontaneous
Contents and Concepts 1. First Law of Thermodynamics Spontaneous Processes and Entropy A spontaneous process is one that occurs by itself. As we will see, the entropy of the system increases in a spontaneous
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Thermochemistry HW. PSI Chemistry
 Thermochemistry HW PSI Chemistry Name Energy 1) Objects can possess energy as: (a) endothermic energy (b) potential energy A) a only B) b only C) c only D) a and c E) b and c (c) kinetic energy 2) The
Thermochemistry HW PSI Chemistry Name Energy 1) Objects can possess energy as: (a) endothermic energy (b) potential energy A) a only B) b only C) c only D) a and c E) b and c (c) kinetic energy 2) The
Exothermic process is any process that gives off heat transfers thermal energy from the system to the surroundings. H 2 O (l) + energy
 Exothermic process is any process that gives off heat transfers thermal energy from the system to the surroundings. H 2 O (g) H 2 O (l) + energy Endothermic process is any process in which heat has to
Exothermic process is any process that gives off heat transfers thermal energy from the system to the surroundings. H 2 O (g) H 2 O (l) + energy Endothermic process is any process in which heat has to
Lecture Outline. 5.1 The Nature of Energy. Kinetic Energy and Potential Energy. 1 mv
 Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 16. Thermodynamics. Thermochemistry Review. Calculating H o rxn. Predicting sign for H o rxn. Creative Commons License
 Chapter 16 Thermodynamics GCC CHM152 Creative Commons License Images and tables in this file have been used from the following sources: OpenStax: Creative Commons Attribution License 4.0. ChemWiki (CC
Chapter 16 Thermodynamics GCC CHM152 Creative Commons License Images and tables in this file have been used from the following sources: OpenStax: Creative Commons Attribution License 4.0. ChemWiki (CC
The following gas laws describes an ideal gas, where
 Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as
Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as
Saturday Study Session 1 3 rd Class Student Handout Thermochemistry
 Saturday Study Session 1 3 rd Class Student Handout Thermochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1. C 2 H 4 (g) + 3 O 2 (g) 2 CO 2 (g)
Saturday Study Session 1 3 rd Class Student Handout Thermochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1. C 2 H 4 (g) + 3 O 2 (g) 2 CO 2 (g)
Energy. Different types of energy exist (heat, potential, kinetic, chemical, nuclear etc.)
 Change in Energy Energy Different types of energy exist (heat, potential, kinetic, chemical, nuclear etc.) Heat - the energy transferred between objects that are at different temperatures. Unit of heat
Change in Energy Energy Different types of energy exist (heat, potential, kinetic, chemical, nuclear etc.) Heat - the energy transferred between objects that are at different temperatures. Unit of heat
10 Enthalpy changes Answers to Activity and Practice questions
 Page 150 151 Activity: Measuring the enthalpy change for the reaction of zinc with copper sulfate solution 1 The graph should have: axes with scales and labels points plotted accurately a clean, smooth
Page 150 151 Activity: Measuring the enthalpy change for the reaction of zinc with copper sulfate solution 1 The graph should have: axes with scales and labels points plotted accurately a clean, smooth
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes
 Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16
 Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
Thermochemistry: Heat and Chemical Change
 Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Energy Heat Work Heat Capacity Enthalpy
 Energy Heat Work Heat Capacity Enthalpy 1 Prof. Zvi C. Koren 20.07.2010 Thermodynamics vs. Kinetics Thermodynamics Thermo = Thermo + Dynamics E (Note: Absolute E can never be determined by humans!) Can
Energy Heat Work Heat Capacity Enthalpy 1 Prof. Zvi C. Koren 20.07.2010 Thermodynamics vs. Kinetics Thermodynamics Thermo = Thermo + Dynamics E (Note: Absolute E can never be determined by humans!) Can
Chapter 8 Thermochemistry
 William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
Chapter 5: Thermochemistry
 Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
- The empirical gas laws (including the ideal gas equation) do not always apply.
 145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A
 ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A PART 1: KEY TERMS AND SYMBOLS IN THERMOCHEMISTRY System and surroundings When we talk about any kind of change, such as a chemical
ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A PART 1: KEY TERMS AND SYMBOLS IN THERMOCHEMISTRY System and surroundings When we talk about any kind of change, such as a chemical
_ + Units of Energy. Energy in Thermochemistry. Thermochemistry. Energy flow between system and surroundings. 100º C heat 50º C
 Units of Energy Like we saw with pressure, many different units are used throughout the world for energy. SI unit for energy 1kg m 1J = 2 s 2 Joule (J) calorie (cal) erg (erg) electron volts (ev) British
Units of Energy Like we saw with pressure, many different units are used throughout the world for energy. SI unit for energy 1kg m 1J = 2 s 2 Joule (J) calorie (cal) erg (erg) electron volts (ev) British
THE SECOND LAW OF THERMODYNAMICS. Professor Benjamin G. Levine CEM 182H Lecture 5
 THE SECOND LAW OF THERMODYNAMICS Professor Benjamin G. Levine CEM 182H Lecture 5 Chemical Equilibrium N 2 + 3 H 2 2 NH 3 Chemical reactions go in both directions Systems started from any initial state
THE SECOND LAW OF THERMODYNAMICS Professor Benjamin G. Levine CEM 182H Lecture 5 Chemical Equilibrium N 2 + 3 H 2 2 NH 3 Chemical reactions go in both directions Systems started from any initial state
Chapter 2 First Law Formalism
 Chapter 2 First Law Formalism 2.1 The Special Character of State Variables A gas can be characterized by a set of state variables. Some, such as temperature, pressure, and volume, are measured directly
Chapter 2 First Law Formalism 2.1 The Special Character of State Variables A gas can be characterized by a set of state variables. Some, such as temperature, pressure, and volume, are measured directly
