Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
|
|
|
- Martha Stone
- 5 years ago
- Views:
Transcription
1 Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Energy the capacity to do work or produce heat A. two basic types of energy 1) potential energy at rest; energy of position 2) kinetic energy of motion (1/2 mv 2 ) B. other subcategories of energy radiant light thermal heat mechanical gravitational electrical sound atomic nuclear chemical energy (chemical potential energy) stored in chemical bonds C. Law of Conservation of Energy in a physical or chemical change, energy cannot be created nor destroyed, it merely changes form D. heat (q) 1) energy flowing from warmer to cooler objects or areas 2) units: Joule (J), calorie (cal); diet Calorie (Cal) II. Heat Capacity A. heat capacity amount of heat required to change a substance s temperature by exactly 1 C; common unit: J / C B. calorie (calorie with a lower-case c) amount of heat required to raise the temp of 1 g of pure water by 1 C C. Calorie (Calorie, with an upper-case c) diet Calorie = 1000 calories D. Joule SI unit of heat and energy; amount of heat required to raise the temp of 1 g of pure water by C. UNIT CONVERSIONS: 1 Cal (diet Calorie) = 1000 cal = 1 kcal = J 1 J = cal J = 1 cal E. heat changes can be measured with a calorimeter, which is a covered container III. Specific Heat Capacity A. Specific heat (c or c p) amount of heat required to raise the temperature of 1 g of a substance by exactly 1 C. B. c deals with heat requirements and heat retention : 1) low c = low requirements and retention = heats up quickly and cools down quickly 2) high c = high requirements and retention = heats up slowly and cools down slowly C. common unit: J / g C D. heat changes can be measured with a calorimeter E. metals have low specific heats F. water has the highest specific heat of common substances = J/g C 1
2 G. equations Δ = delta = change in ΔT = (Tfinal Tinitial) H. examples q = m c ΔT EXAMPLE 1) The temperature of an 89.1 g piece of metal rises from 22.0 C to 51.1 C when the metal absorbs 794 J of energy. What is the specific heat of the metal? c = m = 89.1 g q = 794 J T = = 29.1 C c = q = 794 J = J m ΔT (89.1 g )(29.1 C) g C q m ΔT EXAMPLE 2) How much heat energy is needed to increase the temperature of 44.7 g of water from 20.0 to 36.3 C? m = 44.7 g ΔT = 16.3 C C = J/g C ΔT = 36.3 C 20.0 C = 16.3 C c = q q = m ΔT c q = 44.7 g (16.3 C) J = 3050 J or 3.05 kj m ΔT g C IV. calorimetry the accurate and precise measurement of the heat change for chemical reactions and physical changes A. equation q = ΔH = m c ΔT (rearrangement of the specific heat equation) q = ΔH = (mass of water)(specific heat of water)(change in temp) CALORIMETER Source: prenhall B. typical lab steps for determination of specific heat of an unknown metal 1) water is measured and poured into the calorimeter 2) initial temp of water is recorded 2
3 3) metal is heated and temperature of metal is recorded 4) metal is added to the calorimeter and lid is closed immediately 5) mixture is stirred 6) final temp is recorded heat lost by metal = heat gained by water -ΔHm = +ΔHw q lost by metal = q gained by H 2O qm = qw - [ (mm) (cm) (ΔTm) ] = (mw) (c w) (ΔTw) - [(mm) (cm) (Tfinal, m Tinitial, m)] = (mw) (c w) (Tfinal, w Tinitial, w) EXAMPLE 3) A g piece of Bauckium metal is heated thoroughly in a boiling water bath. The calorimeter is filled with ml of water. The room temperature is 26.5 C. The final calorimeter temperature at the end of the experiment is 28.4 C. What is the specific heat of Bauckium? - [(mm) (cm) (Tfinal, m Tinitial, m)] = (mw) (c w) (Tfinal, w Tinitial, w) (cm) = (mw) (c w) (Tfinal, w Tinitial, w) (mm) (Tfinal, m Tinitial, m) [(20.00 g) (cm) ( C) ] = ( g)(4.184 J/g C)( C) cm = cm = 1.53 J/g C EXAMPLE 4) A g piece of Bauckium metal is heated thoroughly in a boiling water bath. The calorimeter is filled with ml of water. The room temperature is 26.5 C. The specific heat of Bauckium metal is 1.53 J/g C. What is the final temperature of the system at the end of the experiment? - [(mm) (cm) (Tfinal, m Tinitial, m)] = (mw) (c w) (Tfinal, w Tinitial, w) - [(20.00 g) (1.53 J/g C) (x C) ] = ( g)(4.184 J/g C)(x-26.5 C) - (30.6)(x-100) = (x-26.5) -30.6x = x = x 28.4 = C V. Chemical Energy and the Universe A. thermochemistry 1) the study of heat changes in chemical reactions and physical changes 2) the study of heat flow between a system and its surroundings 3
4 a. system specific part being analyzed b. surroundings everything outside the system (usually the immediate area) c. universe = system + surroundings 3) thermochemical equations equations that show heat changes B. enthalpy 1) (H) heat content of a system at constant pressure 2) ΔH is used interchangeably with q 3) change in enthalpy = ΔH; heat change for a process at constant pressure; usually measured in kj (kilojoules) 4) enthalpy (heat) of reaction heat absorbed or released in a chemical rxn.: ΔH rxn = ΔH products - ΔH reactants ENDOTHERMIC: +ΔH heat absorbed into system; surroundings cool down (A + B + ENERGY C + D) +ΔH EXOTHERMIC: -ΔH heat released from system; surroundings heat up (A + B C + D + ENERGY) -ΔH VI. Thermochemical Equations A. thermochemical equation a balanced chemical equation including physical states of all reactants and products and the energy change (usually ΔH) B. ΔH relates to the coefficients of the balanced equation C. examples EXAMPLE 5) 2H 2 (g) + O 2 (g) 2H 2O (l) ΔH = kj How much energy is produced if 100. grams of H 2 are converted to water? 100. g H 2 x 1 mol H 2 x kj = x 10 4 kj (-14,300) 2.00 g H 2 2 mol H 2 EXAMPLE 6) 2H 2 (g) + O 2 (g) 2H 2O (l) ΔH = kj How many grams of H 2 would be required to produce 5.00 x 10 3 kj? 5.00 x 10 3 kj x 2 mol H 2 x 2.00 g H 2 = 35.0 g H kj 1 mol H 2 Chemical change heat transfers: ΔHr or ΔHrxn = generic ENTHALPY (HEAT) OF REACTION = heat absorbed or released in a chemical rxn. ΔHcomb = MOLAR ENTHALPY (HEAT) OF COMBUSTION = heat released in combustion of 1 mol of substance ΔHf = ENTHALPY (HEAT) OF FORMATION = heat absorbed or released to make 1 mol of a cmpd from its elements ΔH f = STANDARD ENTHALPY (HEAT) OF FORMATION = heat absorbed or released to make 1 mol of a cmpd from its elements in their standard states at 298 K (25 C) 4
5 VII. Heat and Changes of State A. math problems use ΔH related to coefficients B. problems discuss melting, freezing, boiling, condensing, dissolving Physical change heat transfers: ΔH vap = MOLAR ENTHALPY (HEAT) OF VAPORIZATION = heat required to vaporize 1 mol of a liquid q = (mass / molar mass) ( Hvap) ΔH fus = MOLAR ENTHALPY (HEAT) OF FUSION= heat required to melt 1 mol of a solid q = (mass / molar mass) ( Hfus) ΔH solid = MOLAR ENTHALPY (HEAT) OF SOLIDIFICATION = heat energy released freezing 1 mol of substance ΔH fus H 2O = 6.01 kj/mol ΔHfus = - ΔHsolid ΔHcond = MOLAR ENTHALPY (HEAT) OF CONDENSATION = heat energy released condensing 1 mol of substance ΔH vap H 2O = 40.7 kj/mol ΔHvap = - ΔHcond ΔHsoln = MOLAR ENTHALPY (HEAT) OF SOLUTION = heat energy change due to the dissolving of 1 mol of substance C. examples EXAMPLE 7) 53.1 g of H 2O exists as a liquid at 0 C, but it is about to freeze. What is the heat change, in kj, to convert the water to a solid at 0 C? ΔH fus H 2O = 6.01 kj/mol This problem deals with freezing. ΔH solid ΔH fus = - ΔH solid q = 53.1 g H 2O x 1 mol H 2O x kj = kj g H 2O mol EXAMPLE 8) 49.5 g of H 2O is being boiled at its boiling point of 100 C. How many kj are required? ΔH vap H 2O = 40.7 kj/mol q = 49.5 g H 2O x 1 mol H 2O x 40.7 kj = +112 kj g H 2O mol VIII. Hess Law (Germain Hess ) A. Hess Law of Heat Summation if two or more thermochemical equations can be added to produce a final equation, the overall ΔH for a rxn. equals the sum of all ΔH s for the individual steps involved ΔH rxn = Σ [(cf)(δh f products )] - Σ [(cf)(δh f reactants)] where cf = coefficient from the balanced equation 5
6 B. If a chemical equation is written in reverse order, the sign of ΔH must be changed (+ to - OR - to +) example: C (graphite) O 2 CO ΔH = -110 kj/mol CO C O 2 ΔH = +110 kj/mol C. If coefficients are multiplied by 2, 3, 4, etc. the same number must be multiplied by the original ΔH 2 C + O 2 2 CO ΔH = -220 kj/mol (multiplied by 2) 6 C + 3 O 2 6 CO ΔH = -660 kj/mol (multiplied by 6) D. example EXAMPLE 9) From the following data, calculate the enthalpy of the third reaction. CH 4 + 2O 2 CO 2 + 2H 2O ΔH = -890 kj/mol H 2O (l) H 2O (g) ΔH = 44 kj/mol at 298 K CH O 2(g) CO 2(g) + 2 H 2O(g) ΔH rxn =? ΔH rxn = Σ [(cf)(δh f products )] - Σ [(cf)(δh f reactants)] where cf = coefficient from the balanced equation ** ** The 2 H 2O s in the first equation must be accounted for in the second equation, so the coefficients are multiplied by 2 (and so is the ΔH, since it is listed as kj per mole). If coefficients are 1, this is not necessary. Add the two equations to give the third one: CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2O (l) ΔH = -890 kj/mol 2H 2O (l) 2H 2O (g) ΔH = 88 kj/mol ** add the equations add the enthalpies CH 4 (g) + 2 O 2(g) CO 2(g) + 2 H 2O(g) ΔH = -802 kj/mol IX. Standard Heats of Formation A. standard state of an element 1) pure, at 1 atm pressure, in the normal phase at 298 K (25 C) 2) enthalpy of an element in its standard state at 298K (25 C) = 0 examples: Ar (g), O 2 (g), C (solid graphite), Br 2 (l); all diatomics = 0 B. values can be looked up in reference tables C. example EXAMPLE 10) Calculate ΔHcomb, in kj, from the burning of CO to form CO 2. from tables: ΔH f CO2 = kj/mol & ΔH f CO = kj/mol 2CO + O2 2CO2 ΔH f O2 = 0 ΔH rxn = Σ [(cf)(δh f products )] - Σ [(cf)(δh f reactants)] ΔH rxn = [(2 mol CO2)( kj)] - [(2 mol CO)( kj)] + (0 kj/mol) = mol mol = -566 kj 6
7 X. Reaction Spontaneity A. spontaneous process a physical or chemical change that, once begun, occurs without outside manipulation (may need help getting started) B. entropy (S) 1) disorder in a system 2) typical unit: J/K (Joules per Kelvin) 3) Second Law of Thermodynamics spontaneous process show an increase in entropy 4) ΔS rxn = ΔS products - ΔS reactants 5) ΔS = + with increased entropy (favorable) 6) ΔS = - with decreased entropy (unfavorable) C. ΔH (enthalpy) conditions 1) - ΔH is favorable (exothermic) 2) + ΔH is unfavorable (endothermic) D. Gibbs free energy (G) energy available to do work; useful energy Gibbs free energy equation (Gibbs function, free enthalpy equation): ΔG = ΔH - TΔS or ΔGsystem = ΔHsystem - TΔSsystem E. example EXAMPLE 11) Determine ΔG and the overall spontaneity of a system under the following conditions: ΔH system = 452 kj ΔS system = 55.7 J/K T = 165 K ΔS system = 55.7 J x 1 kj = kj K 1000 J K ΔG system = ΔH system - TΔS system ΔG system = 452 kj - (165 K)( kj) = = 442 kj; nonspontaneous K EXAMPLE 12) Determine ΔG and the overall spontaneity of a system under the following conditions: ΔH system = kj ΔS system = 138 J/K T = 273 K ΔS system = 138 J x 1 kj = kj K 1000 J K ΔG system = ΔH system - TΔS system ΔG system = kj - (273 K)(0.138 kj) = = kj; spontaneous K 7
8 - ΔH = exothermic (favorable) + ΔH = endothermic (unfavorable) +ΔS = increased entropy (favorable) +ΔS = decreased entropy (unfavorable) - ΔG = spontaneous (favorable) +ΔG = nonspontaneous (unfavorable) 8
Thermochemistry. Energy and Chemical Change
 Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
CP Chapter 17 Thermochemistry
 CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
Ch. 17 Thermochemistry
 Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy
 Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Thermochemistry. Energy and Chemical Change
 Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
CHAPTER 17 Thermochemistry
 CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
The Nature of Energy Energy is the ability to do work or produce Heat, q or Q, is ; flows due to temperature differences (always to )
 CP Chapter 17 Thermochemistry 2014-2015 Thermochemistry Thermochemistry is the study of energy that occur during chemical and physical changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry 2014-2015 Thermochemistry Thermochemistry is the study of energy that occur during chemical and physical changes (changes of state) The Nature of Energy Energy is the ability
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Thermochemistry-Part 1
 Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
I. The Nature of Energy A. Energy
 I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Chapter 17 Thermochemistry
 Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chemical Thermodynamics
 Chemical Thermodynamics 1 Thermodynamics Thermodynamics is a Greek term which means, heat power. Thermodynamics is the study of energy and its transformations. 2 Thermodynamics Thermochemistry how we observe,
Chemical Thermodynamics 1 Thermodynamics Thermodynamics is a Greek term which means, heat power. Thermodynamics is the study of energy and its transformations. 2 Thermodynamics Thermochemistry how we observe,
Chapter 11. Thermochemistry: Heat & Chemical Change
 Chapter 11 Thermochemistry: Heat & Chemical Change The Flow of Energy Thermochemistry: Study of heat changes that occur during physical processes and chemical reactions Energy Energy is the capacity to
Chapter 11 Thermochemistry: Heat & Chemical Change The Flow of Energy Thermochemistry: Study of heat changes that occur during physical processes and chemical reactions Energy Energy is the capacity to
Energy. Different types of energy exist (heat, potential, kinetic, chemical, nuclear etc.)
 Change in Energy Energy Different types of energy exist (heat, potential, kinetic, chemical, nuclear etc.) Heat - the energy transferred between objects that are at different temperatures. Unit of heat
Change in Energy Energy Different types of energy exist (heat, potential, kinetic, chemical, nuclear etc.) Heat - the energy transferred between objects that are at different temperatures. Unit of heat
Energy Ability to produce change or do work. First Law of Thermodynamics. Heat (q) Quantity of thermal energy
 THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
Energy and Chemical Change
 Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy Transformations
 Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes
 Thermochemistry Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy
Thermochemistry Thermochemistry: the study of energy (in the from of heat) changes that accompany physical & chemical changes heat flows from high to low (hot cool) endothermic reactions: absorb energy
Energy Ability to produce change or do work. First Law of Thermodynamics. Heat (q) Quantity of thermal energy
 THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
Name Class Date. As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings.
 Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Section 9: Thermodynamics and Energy
 Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
17.4 Calculating Heats Essential Understanding Heats of reaction can be calculated when it is difficult or
 17.4 Calculating Heats of Reaction Essential Understanding Heats of reaction can be calculated when it is difficult or impossible to measure them directly. Lesson Summary Hess s Law Hess s law provides
17.4 Calculating Heats of Reaction Essential Understanding Heats of reaction can be calculated when it is difficult or impossible to measure them directly. Lesson Summary Hess s Law Hess s law provides
Thermochemistry. Section The flow of energy
 Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
CHAPTER 17: THERMOCHEMISTRY. Mrs. Brayfield
 CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Unit 7 Kinetics and Thermodynamics
 17.1 The Flow of Energy Heat and Work Unit 7 Kinetics and Thermodynamics I. Energy Transformations A. Temperature 1. A measure of the average kinetic energy of the particles in a sample of matter B. Heat
17.1 The Flow of Energy Heat and Work Unit 7 Kinetics and Thermodynamics I. Energy Transformations A. Temperature 1. A measure of the average kinetic energy of the particles in a sample of matter B. Heat
Chapter 8 Thermochemistry: Chemical Energy. Chemical Thermodynamics
 Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Topic 05 Energetics : Heat Change. IB Chemistry T05D01
 Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
Name Date Class SECTION 16.1 PROPERTIES OF SOLUTIONS
 SOLUTIONS Practice Problems In your notebook, solve the following problems. SECTION 16.1 PROPERTIES OF SOLUTIONS 1. The solubility of CO 2 in water at 1.22 atm is 0.54 g/l. What is the solubility of carbon
SOLUTIONS Practice Problems In your notebook, solve the following problems. SECTION 16.1 PROPERTIES OF SOLUTIONS 1. The solubility of CO 2 in water at 1.22 atm is 0.54 g/l. What is the solubility of carbon
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School
 Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
Name Date Class THERMOCHEMISTRY
 Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
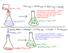 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
AP* Chemistry THERMOCHEMISTRY
 AP* Chemistry THERMOCHEMISTRY Let s begin with terms for you to master: Heat (q) Two systems with different temperatures that are in thermal contact will exchange thermal energy, the quantity of which
AP* Chemistry THERMOCHEMISTRY Let s begin with terms for you to master: Heat (q) Two systems with different temperatures that are in thermal contact will exchange thermal energy, the quantity of which
Name: Class: Date: ID: A
 Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
All chemical reactions involve changes in energy. Typically this energy comes in the form of heat.
 Topic: Thermochemistry Essential Question: How does energy flow in chemical reactions? Name: Class: Date: / / Period: All chemical reactions involve changes in energy. Typically this energy comes in the
Topic: Thermochemistry Essential Question: How does energy flow in chemical reactions? Name: Class: Date: / / Period: All chemical reactions involve changes in energy. Typically this energy comes in the
Ch. 6 Enthalpy Changes
 Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Chapter 5 - Thermochemistry
 Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Reaction Energy. Thermochemistry
 Reaction Energy Thermochemistry Thermochemistry The study of the transfers of energy as heat that accompany chemical reactions & physical changes Thermochemistry -In studying heat changes, think of defining
Reaction Energy Thermochemistry Thermochemistry The study of the transfers of energy as heat that accompany chemical reactions & physical changes Thermochemistry -In studying heat changes, think of defining
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
Thermodynamics Cont. Subtitle
 Thermodynamics Cont. Subtitle System vs. Surroundings The system- the reactants and products of a reaction The surroundings- everything that surrounds a reaction Thermochemistry is concerned with the flow
Thermodynamics Cont. Subtitle System vs. Surroundings The system- the reactants and products of a reaction The surroundings- everything that surrounds a reaction Thermochemistry is concerned with the flow
Chapter 17: Energy and Kinetics
 Pages 510-547 S K K Chapter 17: Energy and Kinetics Thermochemistry: Causes of change in systems Kinetics: Rate of reaction progress (speed) Heat, Energy, and Temperature changes S J J Heat vs Temperature
Pages 510-547 S K K Chapter 17: Energy and Kinetics Thermochemistry: Causes of change in systems Kinetics: Rate of reaction progress (speed) Heat, Energy, and Temperature changes S J J Heat vs Temperature
Chapter 8 Thermochemistry: Chemical Energy
 Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Saturday Study Session 1 3 rd Class Student Handout Thermochemistry
 Saturday Study Session 1 3 rd Class Student Handout Thermochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1. C 2 H 4 (g) + 3 O 2 (g) 2 CO 2 (g)
Saturday Study Session 1 3 rd Class Student Handout Thermochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1. C 2 H 4 (g) + 3 O 2 (g) 2 CO 2 (g)
Thermochemistry Lecture
 Thermochemistry Lecture Jennifer Fang 1. Enthalpy 2. Entropy 3. Gibbs Free Energy 4. q 5. Hess Law 6. Laws of Thermodynamics ENTHALPY total energy in all its forms; made up of the kinetic energy of the
Thermochemistry Lecture Jennifer Fang 1. Enthalpy 2. Entropy 3. Gibbs Free Energy 4. q 5. Hess Law 6. Laws of Thermodynamics ENTHALPY total energy in all its forms; made up of the kinetic energy of the
Thermochemistry Chapter 8
 Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Thermochemistry: Energy Flow and Chemical Reactions
 Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Chapter 10 Lecture Notes: Thermodynamics
 Chapter 10 Lecture Notes: Thermodynamics During this unit of study, we will cover three main areas. A lot of this information is NOT included in your text book, which is a shame. Therefore, the notes you
Chapter 10 Lecture Notes: Thermodynamics During this unit of study, we will cover three main areas. A lot of this information is NOT included in your text book, which is a shame. Therefore, the notes you
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chapter 11. Thermochemistry. 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages
 Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
CHEMISTRY. Chapter 5 Thermochemistry
 CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
- Joule (J): SI unit for energy. It's defined based on the equation for kinetic energy. from. mass. velocity
 153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
Thermodynamics Test Clio Invitational January 26, 2013
 Thermodynamics Test Clio Invitational January 26, 2013 School Name: Team Number: Variables specified: s = specific heat C = heat capacity H f = heat of fusion H v = heat of vaporization Given information:
Thermodynamics Test Clio Invitational January 26, 2013 School Name: Team Number: Variables specified: s = specific heat C = heat capacity H f = heat of fusion H v = heat of vaporization Given information:
Energy, Heat and Chemical Change
 Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Name: Date: Period: #: UNIT 4 NOTES & EXAMPLE PROBLEMS. W = kg m s 2 m= kg m2. Pressure =
 BACKGROUND ON UNITS Acceleration = velocity time Force (F) = mass acceleration = m a F = kg m s 2 = kg m s 2 UNIT 4 NOTES & EXAMPLE PROBLEMS = m s s Work (W)= Force distance = F d = m s 2 = m s-2 W = kg
BACKGROUND ON UNITS Acceleration = velocity time Force (F) = mass acceleration = m a F = kg m s 2 = kg m s 2 UNIT 4 NOTES & EXAMPLE PROBLEMS = m s s Work (W)= Force distance = F d = m s 2 = m s-2 W = kg
AP* Chemistry THERMOCHEMISTRY
 AP* Chemistry THERMOCHEMISTRY Let s begin with terms for you to master: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system is known as the internal
AP* Chemistry THERMOCHEMISTRY Let s begin with terms for you to master: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system is known as the internal
ENTHALPY CHANGE CHAPTER 4
 ENTHALPY CHANGE CHAPTER 4 ENTHALPY Is the total energy of a system. E k = Kinetic energy. Vibrational Rotational Translational E due to motion H = E k + E p E P = Potential energy Attractive force b/w
ENTHALPY CHANGE CHAPTER 4 ENTHALPY Is the total energy of a system. E k = Kinetic energy. Vibrational Rotational Translational E due to motion H = E k + E p E P = Potential energy Attractive force b/w
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry. Chapter 6. Dec 19 8:52 AM. Thermochemistry. Energy: The capacity to do work or to produce heat
 Chapter 6 Dec 19 8:52 AM Intro vocabulary Energy: The capacity to do work or to produce heat Potential Energy: Energy due to position or composition (distance and strength of bonds) Kinetic Energy: Energy
Chapter 6 Dec 19 8:52 AM Intro vocabulary Energy: The capacity to do work or to produce heat Potential Energy: Energy due to position or composition (distance and strength of bonds) Kinetic Energy: Energy
Chapter 5 Thermochemistry. 許富銀 ( Hsu Fu-Yin)
 Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Unit 4: Thermochemistry
 Unit 4: Thermochemistry The making and breaking of bonds only happen as a result of energy being exchanged. Some reactions give off energy and some take in energy. This unit is all about the energy of
Unit 4: Thermochemistry The making and breaking of bonds only happen as a result of energy being exchanged. Some reactions give off energy and some take in energy. This unit is all about the energy of
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Energy and Chemical Change
 Energy and Chemical Change Reviewing Vocabulary Match the definition in Column A with the term in Column B. h e d p c f a r m t j i s l u k n q g o Column A 1. The ability to do work or produce heat 2.
Energy and Chemical Change Reviewing Vocabulary Match the definition in Column A with the term in Column B. h e d p c f a r m t j i s l u k n q g o Column A 1. The ability to do work or produce heat 2.
Chemical Thermodynamics
 Page III-16-1 / Chapter Sixteen Lecture Notes Chemical Thermodynamics Thermodynamics and Kinetics Chapter 16 Chemistry 223 Professor Michael Russell How to predict if a reaction can occur, given enough
Page III-16-1 / Chapter Sixteen Lecture Notes Chemical Thermodynamics Thermodynamics and Kinetics Chapter 16 Chemistry 223 Professor Michael Russell How to predict if a reaction can occur, given enough
Enthalpies of Reaction
 Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Thermodynamics C Test
 Northern Regional: January 19 th, 2019 Thermodynamics C Test Name(s): Team Name: School Name: Team Number: Rank: Score: Science Olympiad North Florida Regional at the University of Florida Thermodynamics
Northern Regional: January 19 th, 2019 Thermodynamics C Test Name(s): Team Name: School Name: Team Number: Rank: Score: Science Olympiad North Florida Regional at the University of Florida Thermodynamics
Thermodynamics- Chapter 19 Schedule and Notes
 Thermodynamics- Chapter 19 Schedule and Notes Date Topics Video cast DUE Assignment during class time One Review of thermodynamics ONE and TWO Review of thermo Wksheet Two 19.1-4; state function THREE
Thermodynamics- Chapter 19 Schedule and Notes Date Topics Video cast DUE Assignment during class time One Review of thermodynamics ONE and TWO Review of thermo Wksheet Two 19.1-4; state function THREE
Chapter 19 Chemical Thermodynamics
 Chapter 19 Chemical Thermodynamics Kinetics How fast a rxn. proceeds Equilibrium How far a rxn proceeds towards completion Thermodynamics Study of energy relationships & changes which occur during chemical
Chapter 19 Chemical Thermodynamics Kinetics How fast a rxn. proceeds Equilibrium How far a rxn proceeds towards completion Thermodynamics Study of energy relationships & changes which occur during chemical
Gilbert Kirss Foster. Chapter 9. Thermochemistry. Energy Changes in Chemical Reactions
 Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Unit 15 Energy and Thermochemistry Notes
 Name KEY Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Name KEY Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes
 Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
CHM 1046 FINAL REVIEW
 CHM 1046 FINAL REVIEW Prepared & Presented By: Marian Ayoub PART I Chapter Description 6 Thermochemistry 11 States of Matter; Liquids and Solids 12 Solutions 13 Rates of Reactions 18 Thermodynamics and
CHM 1046 FINAL REVIEW Prepared & Presented By: Marian Ayoub PART I Chapter Description 6 Thermochemistry 11 States of Matter; Liquids and Solids 12 Solutions 13 Rates of Reactions 18 Thermodynamics and
Thermochemistry. The study of energy changes that occur during chemical reactions and changes in state.
 Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Chapter 8. Thermochemistry 강의개요. 8.1 Principles of Heat Flow. 2) Magnitude of Heat Flow. 1) State Properties. Basic concepts : study of heat flow
 강의개요 Basic concepts : study of heat flow Chapter 8 Thermochemistry Calorimetry : experimental measurement of the magnitude and direction of heat flow Thermochemical Equations Copyright 2005 연세대학교이학계열일반화학및실험
강의개요 Basic concepts : study of heat flow Chapter 8 Thermochemistry Calorimetry : experimental measurement of the magnitude and direction of heat flow Thermochemical Equations Copyright 2005 연세대학교이학계열일반화학및실험
CHM 111 Dr. Kevin Moore
 CHM 111 Dr. Kevin Moore Kinetic Energy Energy of motion E k 1 2 mv 2 Potential Energy Energy of position (stored) Law of Conservation of Energy Energy cannot be created or destroyed; it can only be converted
CHM 111 Dr. Kevin Moore Kinetic Energy Energy of motion E k 1 2 mv 2 Potential Energy Energy of position (stored) Law of Conservation of Energy Energy cannot be created or destroyed; it can only be converted
THERMOCHEMISTRY -1. Dr. Sapna Gupta
 THERMOCHEMISTRY -1 Dr. Sapna Gupta THERMODYNAMICS Thermodynamics: Relationship between heat and other forms of energy Thermochemistry: Study of heat absorbed or evolved by chemical reactions. Energy: Capacity
THERMOCHEMISTRY -1 Dr. Sapna Gupta THERMODYNAMICS Thermodynamics: Relationship between heat and other forms of energy Thermochemistry: Study of heat absorbed or evolved by chemical reactions. Energy: Capacity
Chapter 5: Thermochemistry. Molecular Kinetic Energy -Translational energy E k, translational = 1/2mv 2 -Rotational energy 5.
 Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Thermodynamics. Thermodynamics of Chemical Reactions. Enthalpy change
 Thermodynamics 1 st law (Cons of Energy) Deals with changes in energy Energy in chemical systems Total energy of an isolated system is constant Total energy = Potential energy + kinetic energy E p mgh
Thermodynamics 1 st law (Cons of Energy) Deals with changes in energy Energy in chemical systems Total energy of an isolated system is constant Total energy = Potential energy + kinetic energy E p mgh
Thermochemistry: Heat and Chemical Change
 Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Chapter 5: Thermochemistry
 Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Thermodynamics. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
MCAT General Chemistry Discrete Question Set 19: Thermochemistry & Thermodynamics
 MCAT General Chemistry Discrete Question Set 19: Thermochemistry & Thermodynamics Question No. 1 of 10 1: A metal with a high heat capacity is put on a hot plate. What will happen? Question #01 A. The
MCAT General Chemistry Discrete Question Set 19: Thermochemistry & Thermodynamics Question No. 1 of 10 1: A metal with a high heat capacity is put on a hot plate. What will happen? Question #01 A. The
Topic 5: Energetics. Heat & Calorimetry. Thursday, March 22, 2012
 Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93
 Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Heating Curve Worksheet If this curve is read from right to left, it is a Cooling Curve.
 Heating Curve Worksheet If this curve is read from right to left, it is a Cooling Curve. The diagram below illustrates the steps involved to convert 10g of solid ice at -20 C to 10g of gaseous steam at
Heating Curve Worksheet If this curve is read from right to left, it is a Cooling Curve. The diagram below illustrates the steps involved to convert 10g of solid ice at -20 C to 10g of gaseous steam at
Section 1 - Thermochemistry
 Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
THERMOCHEMISTRY CHAPTER 11
 THERMOCHEMISTRY CHAPTER 11 ENERGY AND HEAT nthermochemistry: The study of the energy changes that accompany chemical reactions and changes in the physical states of matter. ENERGY AND HEAT nwork: Energy
THERMOCHEMISTRY CHAPTER 11 ENERGY AND HEAT nthermochemistry: The study of the energy changes that accompany chemical reactions and changes in the physical states of matter. ENERGY AND HEAT nwork: Energy
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions
 Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Unit 15 Energy and Thermochemistry Notes
 Name Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Name Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
THERMOCHEMISTRY. This section explains the relationship between energy and heat, and distinguishes between heat capacity and specific heat.
 I Name _ Date _ Class _ THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY-HEAT AND WORK (pages 505-510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
I Name _ Date _ Class _ THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY-HEAT AND WORK (pages 505-510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Name: General Chemistry Chapter 11 Thermochemistry- Heat and Chemical Change
 Name: General Chemistry Chapter 11 Thermochemistry- Heat and Chemical Change Notepack 1 Section 11.1: The Flow of Energy Heat (Pages 293 299) 1. Define the following terms: a. Thermochemistry b. Energy
Name: General Chemistry Chapter 11 Thermochemistry- Heat and Chemical Change Notepack 1 Section 11.1: The Flow of Energy Heat (Pages 293 299) 1. Define the following terms: a. Thermochemistry b. Energy
