Thermochemistry. Using Heats of Reaction - Hess s Law - Standard Enthalpies of Formation - Fuels Foods, Commercial Fuels, and Rocket Fuels
|
|
|
- Derrick Green
- 5 years ago
- Views:
Transcription
1 Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring Heats of Reaction Using Heats of Reaction - Hess s Law - Standard Enthalpies of Formation - Fuels Foods, Commercial Fuels, and Rocket Fuels
2 Thermochemistry After Reaction
3 Understanding Heats of Reaction Thermodynamics is the science of the relationships between heat and other forms of energy. Thermochemistry is one area of thermodynamics. It concerns the study of the quantity of heat absorbed or evolved (given off) by chemical reactions.
4 Energy and Its Units We can define energy briefly as the potential or capacity to move matter. According to this definition, energy is not a material thing but rather a property of matter. Energy exists in different forms that can be interconverted. Kinetic Energy: Kinetic energy is the energy associated with an object by virtue of its motion. An object of mass m and speed or velocity v has kinetic energy Ek equal to This formula shows that the kinetic energy of an object depends on both its mass and its speed. A heavy object can move more slowly than a light object and still have the same kinetic energy.
5 Energy and Its Units Consider the kinetic energy of a person whose mass is 59.0 kg and whose speed is 26.8 m/s. (This is equivalent to a person with a mass of 130 lb traveling in an automobile going 60 miles per hour.) You substitute the mass and speed into the formula. The calorie (cal) is a non-si unit of energy commonly used by chemists, originally defined as the amount of energy required to raise the temperature of one gram of water by one degree Celsius. A person weighing 130 lb and traveling 60 miles per hour has a kinetic energy of
6 Potential Energy Potential energy is the energy an object has by virtue of its position in a field of force. The potential energy of the water is Ep = mgh Here Ep is the potential energy of a quantity of water at the top of the dam, m is the mass of the water, g is the constant acceleration of gravity, and h is the height of the water measured from some standard level.
7 Ep = mgh
8 Internal Energy The sum of the kinetic and potential energies of the particles making up a substance is referred to as the internal energy, U, of the substance. Therefore, the total energy, Etot, of a quantity of water equals the sum of its kinetic and potential energies as a whole (Ek + Ep) plus its internal energy. Etot = Ek + Ep + U Normally when you study a substance in the laboratory, the substance is at rest in a vessel. Its kinetic energy as a whole is zero. Moreover, its potential energy as a whole is constant, and you can take it to be zero. In this case, the total energy of the substance equals its internal energy, U.
9 One type of chemical reactions evolve, or release, heat. Other type of reactions absorb heat. Both types of reaction involve a heat of reaction. Suppose that you are interested in studying the change of a thermodynamic property (such as internal energy) during a physical or chemical change. The substance or mixture of substances under study in which a change occurs is called the thermodynamic system (or simply system). The surroundings are everything in the vicinity of the thermodynamic system.
10 Heat & Thermodynamic System Heat is defined as the energy that flows into or out of a system because of a difference in temperature between the thermodynamic system and its surroundings. As long as a system and surroundings are in thermal contact, energy (heat) flows between them to establish temperature equality, or thermal equilibrium. Heat flows from a region of higher temperature to one of lower temperature; once the temperatures become equal, heat flow stops.
11 Heat of Reaction The heat of reaction (at a given temperature) is the value of q required to return a system to the given temperature at the completion of the reaction. Chemical reactions or physical changes are classified as exothermic or endothermic. An exothermic process is a chemical reaction or a physical change in which heat is evolved (q is negative). An endothermic process is a chemical reaction or a physical change in which heat is absorbed (q is positive). Experimentally, in the exothermic reaction, the reaction flask initially warms; in the endothermic reaction, the reaction flask initially cools.
12 Heat of Reaction
13 Heat of Reaction
14 Enthalpy There is a property of substances called enthalpy that is related to the heat of reaction qp. Enthalpy (denoted by H) is an extensive property of a substance that can be used to obtain the heat absorbed or evolved in a chemical reaction. Enthalpy is an extensive property. An extensive property is a property that depends on the amount of substance. Other examples of extensive properties are mass and volume. Enthalpy is a state function. A state function is a property of a system that depends only on its present state, which is determined by variables such as temperature and pressure, and is independent of any previous history of the system. This means that a change in enthalpy does not depend on how the change was made, but only on the initial state and final state of the system.
15 Enthalpy of Reaction The change in enthalpy, ΔH, for a reaction at a given temperature and pressure (called the enthalpy of reaction) is obtained by subtracting the enthalpy of the reactants from the enthalpy of the products. ΔH = Hfinal - Hinitial. Since you start from reactants and end with products, the enthalpy of reaction is ΔH = H(products) - H(reactants) Because H is a state function, the value of ΔH is independent of the details of the reaction. It depends only on the initial state (the reactants) and the final state (the products).
16 Enthalpy of Reaction The enthalpy of reaction equals the heat of reaction at constant pressure. ΔH = qp To illustrate the concepts we have introduced, consider the reaction at 25 C of sodium metal and water, carried out in a beaker open to the atmosphere at 1.00 atm pressure. 2Na(s) + 2H2O(l ) = 2NaOH(aq) + H2(g) 2 mol of sodium metal reacts with 2 mol of water to evolve kj of heat. Because heat evolves, the reaction is exothermic, and you write qp = kj. So, the enthalpy of reaction, or change of enthalpy for the reaction, is ΔH = kj.
17 Enthalpy & Internal Energy The enthalpy, H, is defined precisely as the internal energy, U, plus pressure, P, times volume, V. H = U + PV Consider a reaction system at constant pressure P. We will label the initial quantities (those for reactants) with a subscript i and the final quantities (those for products) with a subscript f. Then ΔH = Hf Hi = (Uf + PVf) (Ui + PVi) Or, ΔH = (Uf Ui) + P(Vf Vi) = ΔU + PΔV Or, ΔU = ΔH PΔV The last term in this equation ( PΔV) is the energy required by the system to change volume against the constant pressure of the atmosphere. This required energy is called the pressure volume work. The internal energy of the system changes because energy leaves or enters the system as heat (ΔH), and because the system increases or decreases in volume against the constant pressure of the atmosphere ( PΔV).
18 Enthalpy & Internal Energy Consider a specific reaction. When 2 mol of sodium metal and 2 mol of water react in a beaker, 1 mol of hydrogen gas forms and heat evolves. If you measured this heat, you would find it to be kj, indicating that kj of energy has left the system in the form of heat. Because hydrogen gas forms during the reaction, the volume of the system increases. To expand, the system must push back the atmosphere, and this requires energy equal to the pressure volume work. It may be easier to see this pressure volume work if you replace the constant pressure of the atmosphere by an equivalent pressure from a piston-and-weight assembly. When hydrogen gas is released during the reaction, it pushes upward on the piston and raises the weight. It requires energy to lift a weight upward in a gravitational field. Calculate the pressure volume work. Find out the internal energy of the system.
19 At 25 C and 1.00 atm pressure, you find that it is PΔV = ΔnRT = 2.5 kj. In the sodium water reaction, the internal energy changes by kj because heat evolves and changes by 2.5 kj because pressure volume work is done. The total change of internal energy is ΔU = ΔH PΔV = kj kj = kj ΔU does not differ a great deal from ΔH. This is the case in most reactions, so the heat of reaction at constant pressure is approximately equal to the change of internal energy.
20 Thermochemical Equations A thermochemical equation is the chemical equation for a reaction (including phase labels) in which the equation is given a molar interpretation, and the enthalpy of reaction for these molar amounts is written directly after the equation. For the reaction of sodium and water, you would write 2Na(s) + 2H2O(l ) 2NaOH(aq) + H2(g); ΔH = kj This equation says that 2 mol of sodium reacts with 2 mol of water to produce 2 mol of sodium hydroxide and 1 mol of hydrogen gas, and kj of heat evolves.
21 Thermochemical Equations Thermochemical equation includes phase labels. This is because the enthalpy change, ΔH, depends on the phase of the substances. Consider the reaction of hydrogen and oxygen to produce water. If the product is water vapor, 2 mol of H2 burn to release kj of heat. 2H2(g) + O2(g) 2H2O(g); ΔH = kj On the other hand, if the product is liquid water, the heat released is kj. 2H2(g) + O2(g) 2H2O(l ); ΔH = kj In this case, additional heat is released when water vapor condenses to liquid
22 Thermochemical Equations 1. When a thermochemical equation is multiplied by any factor, the value of ΔH for the new equation is obtained by multiplying the value of ΔH in the original equation by that same factor. 1. When a chemical equation is reversed, the value of ΔH is reversed in sign.
23
24 Applying Stoichiometry to Heats of Reaction
25 Measuring Heats of Reaction The heat capacity (C) of a sample of substance is the quantity of heat needed to raise the temperature of the sample of substance one degree Celsius (or one kelvin). Changing the temperature of the sample from an initial temperature ti to a final temperature tf requires heat equal to Where Δt is the change of temperature and equals tf ti. Suppose a piece of iron requires 6.70 J of heat to raise the temperature by one degree Celsius. Its heat capacity is therefore 6.70 J/ C. The quantity of heat required to raise the temperature of the piece of iron from 25.0 C to 35.0 C is q = CΔt = (6.70 J/ C) (35.0 C C) = 67.0 J. C is directly proportional to the amount of substance. Often heat capacities are listed for molar amounts of substances. The molar heat capacity of a substance is its heat capacity for one mole of substance.
26 Measuring Heats of Reaction The specific heat capacity (or simply specific heat) is the quantity of heat required to raise the temperature of one gram of a substance by one degree Celsius (or one kelvin) at constant pressure. To find the heat q required to raise the temperature of a sample, you multiply the specific heat of the substance, s, by the mass in grams, m, and the temperature change, Δt. The specific heats and molar heat capacities of substances depend somewhat on temperature. Water has a specific heat of 4.18 J/(g C) that is, 4.18 joules per gram per degree Celsius. In terms of calories, the specific heat of water is 1.00 cal/(g C).
27 Measuring Heats of Reaction
28 Calorimeter
29 Calorimeter A bomb calorimeter This type of calorimeter can be used to determine the heat of combustion of a substance. The figure shows a sample of graphite being burned in oxygen within a vessel called the bomb. An electric current passing through the ignition coil starts the graphite burning. The temperature of the water surrounding the bomb rises as heat flows from the exothermic reaction of graphite and oxygen.
30
31 Hess s Law Hess s law of heat summation states that for a chemical equation that can be written as the sum of two or more steps, the enthalpy change for the overall equation equals the sum of the enthalpy changes for the individual steps. ΔH2 =?
32 Applying Hess s Law
33
34
35 Standard Enthalpies of Formation The term standard state refers to the standard thermodynamic conditions chosen for substances when listing or comparing thermodynamic data: 1 atm pressure and the specified temperature (usually 25 C). The enthalpy change for a reaction in which reactants in their standard states yield products in their standard states is denoted ΔH. The quantity ΔH is called the standard enthalpy of reaction. The standard enthalpy of formation (also called the standard heat of formation) of a substance, denoted!h"f, is the enthalpy change for the formation of one mole of the substance in its standard state from its elements in their reference form and in their standard states. The reference form of an element for the purpose of specifying the formation reaction is usually the stablest form (physical state and allotrope) of the element under standard thermodynamic conditions. The reference form of oxygen at 25 C is O2(g); the reference form of carbon at 25 C is graphite.
36 Equilibrium Constant for the Sum of Reactions You write the formation reaction for 1 mol of liquid water as follows: The standard enthalpy change for this reaction is kj per mole of H2O. Therefore, the thermochemical equation is
37
38
39
40 Foods as Fuels Foods fill three needs of the body: - supply substances for the growth and repair of tissue - supply substances for the synthesis of compounds used in the regulation of body processes - supply energy (about 80% of the energy is for heat, rest is used for muscular action, chemical processes, and other body processes) Combustion of glucose: One gram of glucose yields 15.6 kj (3.73 kcal) of heat when burned. Combustion of a representative fat, glyceryl trimyristate, C45H86O6: One gram of this fat yields 38.5 kj (9.20 kcal) of heat when burned. The average values quoted for carbohydrates and fats are 4.0 kcal/g and 9.0 kcal/g, respectively.
41 Fossil Fuels Fuel values of coals are rated in Btu s (British thermal units, 1 Btu = 1054 J) per pound, which are essentially heats of combustion per pound of coal. A typical value is 13,200 Btu/lb (1 Btu/lb = 2.32 J/g). So, the combustion of coal in oxygen yields about 30.6 kj/g. Compare this value with the heat of combustion of pure carbon (graphite). Combustion of Methane: This value of ΔH is equivalent to 50.1 kj per gram of fuel. Combustion of Octane: This value of ΔH is equivalent to 44.4 kj/g.
42 Sources of Energy Consumed
43 Rocket Fuels This value of ΔH is equivalent to 120 kj/g of fuel (H2) compared with 50 kj/g of methane. The second and third stages of the Saturn V launch vehicle that sent a three man Apollo crew to the moon used a hydrogen/oxygen system. The launch vehicle contained liquid H2 (boiling at 253 C) and liquid oxygen, or LOX (boiling at 183 C).
44 Rocket Fuels The first stage of liftoff (Russian space shuttle) used kerosene and oxygen, and an unbelievable 550 metric tons ( kg) of kerosene were burned in 2.5 minutes! Kerosene is approximately C12H26. The thermochemical equation is This value of ΔH is equivalent to 44.1 kj/g. Thus, g of fuel generated kj in 150 s (2.5 min) The landing module for the Apollo mission used a fuel made of hydrazine, N2H4, and a derivative of hydrazine. The oxidizer was dinitrogen tetroxide, N2O4. These substances are normally liquids and are therefore easier to store than liquid hydrogen and oxygen.
Chapter 6 Thermochemistry
 Chapter 6 Thermochemistry Contents and Concepts Understanding Heats of Reaction The first part of the chapter lays the groundwork for understanding what we mean by heats of reaction. 1. Energy and Its
Chapter 6 Thermochemistry Contents and Concepts Understanding Heats of Reaction The first part of the chapter lays the groundwork for understanding what we mean by heats of reaction. 1. Energy and Its
Chapter 6: Thermochemistry
 Chem 1045 General Chemistry by Ebbing and Gammon, 8th Edition George W.J. Kenney, Jr Last Update: 24-Oct-2008 Chapter 6: Thermochemistry These Notes are to SUPPLIMENT the Text, They do NOT Replace reading
Chem 1045 General Chemistry by Ebbing and Gammon, 8th Edition George W.J. Kenney, Jr Last Update: 24-Oct-2008 Chapter 6: Thermochemistry These Notes are to SUPPLIMENT the Text, They do NOT Replace reading
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY -1. Dr. Sapna Gupta
 THERMOCHEMISTRY -1 Dr. Sapna Gupta THERMODYNAMICS Thermodynamics: Relationship between heat and other forms of energy Thermochemistry: Study of heat absorbed or evolved by chemical reactions. Energy: Capacity
THERMOCHEMISTRY -1 Dr. Sapna Gupta THERMODYNAMICS Thermodynamics: Relationship between heat and other forms of energy Thermochemistry: Study of heat absorbed or evolved by chemical reactions. Energy: Capacity
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
s Traditionally, we use the calorie as a unit of energy. The nutritional Calorie, Cal = 1000 cal. Kinetic Energy and Potential Energy
 AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Topic 05 Energetics : Heat Change. IB Chemistry T05D01
 Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Gravity is a force which keeps us stuck to the earth. The Electrostatic force attracts electrons to protons in an atom.
 Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Lecture Outline. 5.1 The Nature of Energy. Kinetic Energy and Potential Energy. 1 mv
 Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
CHEMISTRY. Chapter 5 Thermochemistry
 CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
Thermochemistry-Part 1
 Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Reaction Energy. Thermochemistry
 Reaction Energy Thermochemistry Thermochemistry The study of the transfers of energy as heat that accompany chemical reactions & physical changes Thermochemistry -In studying heat changes, think of defining
Reaction Energy Thermochemistry Thermochemistry The study of the transfers of energy as heat that accompany chemical reactions & physical changes Thermochemistry -In studying heat changes, think of defining
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Thermochemistry: energy considerations associated with chemical and physical change Energy: the capacity of a system to do work or produce heat potential energy energy of position;
Chapter 6: Thermochemistry Thermochemistry: energy considerations associated with chemical and physical change Energy: the capacity of a system to do work or produce heat potential energy energy of position;
Chapter 6. Thermochemistry. Chapter 6. Chapter 6 Thermochemistry. Chapter 6 Thermochemistry Matter vs Energy 2/16/2016
 Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry 6.1 Chemical Hand Warmers 6.2 The Nature of Energy: Key Definitions 6.3 The First Law of Thermodynamics: There is no Free Lunch 6.4 6.5 Measuring
Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry 6.1 Chemical Hand Warmers 6.2 The Nature of Energy: Key Definitions 6.3 The First Law of Thermodynamics: There is no Free Lunch 6.4 6.5 Measuring
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy
 Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
If neither volume nor pressure of the system changes, w = 0 and ΔE = q = ΔH. The change in internal energy is equal to the change in enthalpy.
 5 Thermochemistry Visualizing Concepts 5.1 The book s potential energy is due to the opposition of gravity by an object of mass m at a distance d above the surface of the earth. Kinetic energy is due to
5 Thermochemistry Visualizing Concepts 5.1 The book s potential energy is due to the opposition of gravity by an object of mass m at a distance d above the surface of the earth. Kinetic energy is due to
Gilbert Kirss Foster. Chapter 9. Thermochemistry. Energy Changes in Chemical Reactions
 Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Thermochemistry: Energy Flow and Chemical Reactions
 Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
All chemical reactions involve changes in energy. Typically this energy comes in the form of heat.
 Topic: Thermochemistry Essential Question: How does energy flow in chemical reactions? Name: Class: Date: / / Period: All chemical reactions involve changes in energy. Typically this energy comes in the
Topic: Thermochemistry Essential Question: How does energy flow in chemical reactions? Name: Class: Date: / / Period: All chemical reactions involve changes in energy. Typically this energy comes in the
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Name Class Date. As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings.
 Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Energy & Chemistry. Internal Energy (E) Energy and Chemistry. Potential Energy. Kinetic Energy. Energy and Chemical Reactions: Thermochemistry or
 Page III-5-1 / Chapter Five Lecture Notes Energy & Chemistry Energy and Chemical Reactions: Thermochemistry or Thermodynamics Chapter Five Burning peanuts supplies sufficient energy to boil a cup of water
Page III-5-1 / Chapter Five Lecture Notes Energy & Chemistry Energy and Chemical Reactions: Thermochemistry or Thermodynamics Chapter Five Burning peanuts supplies sufficient energy to boil a cup of water
Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat.
 CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
Chapter 8 Thermochemistry
 William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
Chapter 6. Thermochemistry
 Chapter 6 Thermochemistry Section 5.6 The Kinetic Molecular Theory of Gases http://www.scuc.txed.net/webpages/dmackey/files /chap06notes.pdf ..\..\..\..\..\..\Videos\AP Videos\Thermochemistry\AP
Chapter 6 Thermochemistry Section 5.6 The Kinetic Molecular Theory of Gases http://www.scuc.txed.net/webpages/dmackey/files /chap06notes.pdf ..\..\..\..\..\..\Videos\AP Videos\Thermochemistry\AP
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Chapter 5 Thermochemistry. 許富銀 ( Hsu Fu-Yin)
 Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions
 Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Thermodynamics. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
Energy and Chemical Change
 Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 16.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Selected Questions on Chapter 5 Thermochemistry
 Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
Chapter Objectives. Chapter 9 Energy and Chemistry. Chapter Objectives. Energy Use and the World Economy. Energy Use and the World Economy
 Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Class work on Calorimetry. January 11 and 12, 2011
 Class work on Calorimetry January 11 and 12, 2011 Name 1. The number of calories needed to raise the temperature of 100 grams of water 10 degrees Celsius is the same as the number of calories needed to
Class work on Calorimetry January 11 and 12, 2011 Name 1. The number of calories needed to raise the temperature of 100 grams of water 10 degrees Celsius is the same as the number of calories needed to
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Name Date Class SECTION 16.1 PROPERTIES OF SOLUTIONS
 SOLUTIONS Practice Problems In your notebook, solve the following problems. SECTION 16.1 PROPERTIES OF SOLUTIONS 1. The solubility of CO 2 in water at 1.22 atm is 0.54 g/l. What is the solubility of carbon
SOLUTIONS Practice Problems In your notebook, solve the following problems. SECTION 16.1 PROPERTIES OF SOLUTIONS 1. The solubility of CO 2 in water at 1.22 atm is 0.54 g/l. What is the solubility of carbon
Chapter 5 - Thermochemistry
 Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Ch. 17 Thermochemistry
 Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
THERMOCHEMISTRY & DEFINITIONS
 THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
Thermochemistry. Section The flow of energy
 Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Chapter 6 Thermochemistry 許富銀
 Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93
 Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Name Date Class THERMOCHEMISTRY
 Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Chapter 8 Thermochemistry: Chemical Energy. Chemical Thermodynamics
 Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
The Nature of Energy Energy is the ability to do work or produce Heat, q or Q, is ; flows due to temperature differences (always to )
 CP Chapter 17 Thermochemistry 2014-2015 Thermochemistry Thermochemistry is the study of energy that occur during chemical and physical changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry 2014-2015 Thermochemistry Thermochemistry is the study of energy that occur during chemical and physical changes (changes of state) The Nature of Energy Energy is the ability
17.4 Calculating Heats Essential Understanding Heats of reaction can be calculated when it is difficult or
 17.4 Calculating Heats of Reaction Essential Understanding Heats of reaction can be calculated when it is difficult or impossible to measure them directly. Lesson Summary Hess s Law Hess s law provides
17.4 Calculating Heats of Reaction Essential Understanding Heats of reaction can be calculated when it is difficult or impossible to measure them directly. Lesson Summary Hess s Law Hess s law provides
1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy.
 Worksheet 1 1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy. The first law of thermodynamics is the conservation
Worksheet 1 1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy. The first law of thermodynamics is the conservation
Chemical Thermodynamics. Chemical Thermodynamics. Changes of State. Chemical Thermodynamics. State Functions. State Functions 11/25/13
 Chemical Thermodynamics n Thermodynamics is the study of the energetics and order of a system. n A system is the thing we want to study it can be a chemical reaction, a solution, an automobile, or the
Chemical Thermodynamics n Thermodynamics is the study of the energetics and order of a system. n A system is the thing we want to study it can be a chemical reaction, a solution, an automobile, or the
Thermochemistry 14.notebook. November 24, Thermochemistry. Energy the capacity to do work or produce heat. translational.
 Thermochemistry Energy the capacity to do work or produce heat POTENTIAL ENERGY KINETIC ENERGY (energy of motion) "stored" bond energy TEMPERATURE and HEAT vibrational rotational translational a measure
Thermochemistry Energy the capacity to do work or produce heat POTENTIAL ENERGY KINETIC ENERGY (energy of motion) "stored" bond energy TEMPERATURE and HEAT vibrational rotational translational a measure
Chapter 6 Energy and Chemical Change. Brady and Senese 5th Edition
 Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Enthalpy and Internal Energy
 Enthalpy and Internal Energy H or ΔH is used to symbolize enthalpy. The mathematical expression of the First Law of Thermodynamics is: ΔE = q + w, where ΔE is the change in internal energy, q is heat and
Enthalpy and Internal Energy H or ΔH is used to symbolize enthalpy. The mathematical expression of the First Law of Thermodynamics is: ΔE = q + w, where ΔE is the change in internal energy, q is heat and
I. The Nature of Energy A. Energy
 I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
Chapter 5: Thermochemistry. Problems: , , 5.100, 5.106, 5.108, , 5.121, 5.126
 Chapter 5: Thermochemistry Problems: 5.1-5.95, 5.97-98, 5.100, 5.106, 5.108, 5.118-5.119, 5.121, 5.126 Energy: Basic Concepts and Definitions energy: capacity to do work or to produce heat thermodynamics:
Chapter 5: Thermochemistry Problems: 5.1-5.95, 5.97-98, 5.100, 5.106, 5.108, 5.118-5.119, 5.121, 5.126 Energy: Basic Concepts and Definitions energy: capacity to do work or to produce heat thermodynamics:
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
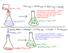 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
Chemical Thermodynamics
 Chemical Thermodynamics 1 Thermodynamics Thermodynamics is a Greek term which means, heat power. Thermodynamics is the study of energy and its transformations. 2 Thermodynamics Thermochemistry how we observe,
Chemical Thermodynamics 1 Thermodynamics Thermodynamics is a Greek term which means, heat power. Thermodynamics is the study of energy and its transformations. 2 Thermodynamics Thermochemistry how we observe,
Lecture Presentation. Chapter 5. Thermochemistry Pearson Education, Inc.
 Lecture Presentation Chapter 5 Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work. Energy used to cause the temperature of an
Lecture Presentation Chapter 5 Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work. Energy used to cause the temperature of an
Chapter 3. Thermochemistry: Energy Flow and Chemical Change. 5.1 Forms of Energy and Their Interconversion
 Chapter 3 Thermochemistry: Energy Flow and Chemical Change 5.1 Forms of Energy and Their Interconversion 5.2 Enthalpy: Chemical Change at Constant Pressure 5.3 Calorimetry: Measuring the Heat of a Chemical
Chapter 3 Thermochemistry: Energy Flow and Chemical Change 5.1 Forms of Energy and Their Interconversion 5.2 Enthalpy: Chemical Change at Constant Pressure 5.3 Calorimetry: Measuring the Heat of a Chemical
Thermochemistry AP Chemistry Lecture Outline
 Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Name: Class: Date: ID: A
 Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
AP* Chapter 6. Thermochemistry
 AP* Chapter 6 Thermochemistry Section 6.1 The Nature of Energy Energy Capacity to do work or to produce heat. Law of conservation of energy energy can be converted from one form to another but can be neither
AP* Chapter 6 Thermochemistry Section 6.1 The Nature of Energy Energy Capacity to do work or to produce heat. Law of conservation of energy energy can be converted from one form to another but can be neither
AP* Chemistry THERMOCHEMISTRY
 AP* Chemistry THERMOCHEMISTRY Let s begin with terms for you to master: Heat (q) Two systems with different temperatures that are in thermal contact will exchange thermal energy, the quantity of which
AP* Chemistry THERMOCHEMISTRY Let s begin with terms for you to master: Heat (q) Two systems with different temperatures that are in thermal contact will exchange thermal energy, the quantity of which
CP Chapter 17 Thermochemistry
 CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
CHM 111 Dr. Kevin Moore
 CHM 111 Dr. Kevin Moore Kinetic Energy Energy of motion E k 1 2 mv 2 Potential Energy Energy of position (stored) Law of Conservation of Energy Energy cannot be created or destroyed; it can only be converted
CHM 111 Dr. Kevin Moore Kinetic Energy Energy of motion E k 1 2 mv 2 Potential Energy Energy of position (stored) Law of Conservation of Energy Energy cannot be created or destroyed; it can only be converted
Chapter 11. Thermochemistry. 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages
 Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Lecture outline: Chapter 5
 Lecture outline: Chapter 5 1. The nature of energy Thermochemistryh 2. First law of thermodynamics 3. Enthalpies of reaction 4. Hess law 5. Enthalpies of formation 1 Chemical Reactivity (1) Does a chemical
Lecture outline: Chapter 5 1. The nature of energy Thermochemistryh 2. First law of thermodynamics 3. Enthalpies of reaction 4. Hess law 5. Enthalpies of formation 1 Chemical Reactivity (1) Does a chemical
Chapter 8 Thermochemistry: Chemical Energy
 Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Section 9: Thermodynamics and Energy
 Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
Section 9: Thermodynamics and Energy The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 9.01 Law of Conservation of Energy Chemistry (11)(A)
ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A
 ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A PART 1: KEY TERMS AND SYMBOLS IN THERMOCHEMISTRY System and surroundings When we talk about any kind of change, such as a chemical
ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A PART 1: KEY TERMS AND SYMBOLS IN THERMOCHEMISTRY System and surroundings When we talk about any kind of change, such as a chemical
AP CHEMISTRY. Unit 5 Thermochemistry. Jeff Venables Northwestern High School
 AP CHEMISTRY Unit 5 Thermochemistry Jeff Venables Northwestern High School Kinetic Energy and Potential Energy Kinetic energy - the energy of motion: Ek = 1 mv 2 Potential energy - the energy an object
AP CHEMISTRY Unit 5 Thermochemistry Jeff Venables Northwestern High School Kinetic Energy and Potential Energy Kinetic energy - the energy of motion: Ek = 1 mv 2 Potential energy - the energy an object
= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)
![= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj) = (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)](/thumbs/89/101004910.jpg) CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
Chapter 17 Thermochemistry
 Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes
 Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Ch 6. Energy and Chemical Change. Brady & Senese, 5th Ed.
 Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
- Kinetic energy: energy of matter in motion. gravity
 148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
5.1 Exothermic and endothermic reactions
 Topic 5: Energetics 5.1 Exothermic and endothermic reactions Chemical reactions involve the breaking and making of bonds. Breaking bonds requires energy,whereas energy is given out when new bonds are formed.
Topic 5: Energetics 5.1 Exothermic and endothermic reactions Chemical reactions involve the breaking and making of bonds. Breaking bonds requires energy,whereas energy is given out when new bonds are formed.
Thermochemistry. Energy and Chemical Change
 Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
47 people in recitation yesterday. Expect quizzes there and in class.
 Announcements 47 people in recitation yesterday. Expect quizzes there and in class. Chapter 6 Problems: 6.9, 6.11, 6.13(except c), 6.19, 6.23, 6.34, 6.38, 6.42, 6.51, 6.53, 6.54, 6.57, 6.64, 6.66, 6.69,
Announcements 47 people in recitation yesterday. Expect quizzes there and in class. Chapter 6 Problems: 6.9, 6.11, 6.13(except c), 6.19, 6.23, 6.34, 6.38, 6.42, 6.51, 6.53, 6.54, 6.57, 6.64, 6.66, 6.69,
Chemistry 123: Physical and Organic Chemistry Topic 2: Thermochemistry
 Topic 2: Introduction, Topic 2: Thermochemistry Text: Chapter 7 and 19 (~ 3 weeks) 2.0 Introduction, terminology and scope 2.1 Enthalapy and Energy Change in a chemical process; 1st law of Thermodynamics
Topic 2: Introduction, Topic 2: Thermochemistry Text: Chapter 7 and 19 (~ 3 weeks) 2.0 Introduction, terminology and scope 2.1 Enthalapy and Energy Change in a chemical process; 1st law of Thermodynamics
Chapter 6 Thermochemistry
 Chapter 6 Thermochemistry Thermochemistry Thermochemistry is a part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? - Will a reaction proceed
Chapter 6 Thermochemistry Thermochemistry Thermochemistry is a part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? - Will a reaction proceed
Thermochemistry. Chapter 6. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
Thermochemistry is study of changes in energy (heat) associated with physical or chemical changes.
 Thermochemistry Thermochemistry and Energy and Temperature Thermochemistry is study of changes in energy (heat) associated with physical or chemical changes. Force = push F= m a (mass x acceleration) force
Thermochemistry Thermochemistry and Energy and Temperature Thermochemistry is study of changes in energy (heat) associated with physical or chemical changes. Force = push F= m a (mass x acceleration) force
Chapter 6 Review. Part 1: Change in Internal Energy
 Chapter 6 Review This is my own personal review, this should not be the only thing used to study. You should also study using notes, PowerPoint, homework, ect. I have not seen the exam, so I cannot say
Chapter 6 Review This is my own personal review, this should not be the only thing used to study. You should also study using notes, PowerPoint, homework, ect. I have not seen the exam, so I cannot say
Ch. 6 Enthalpy Changes
 Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
