Ch06. Energy. Thermochemistry, understanding energy, heat & work. version 1.5
|
|
|
- Millicent Carr
- 5 years ago
- Views:
Transcription
1 Ch06 Energy Thermochemistry, understanding energy, heat & work. version 1.5 Nick DeMello, PhD
2 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal & Chemical Systems keeping perspective consistent is essential! the system & the surroundings types of systems open, closed, isolated Changes, Values & Size What is change? (over time) Measuring changes State Functions vs Path Functions Changes in Energy Internal Energy Heat & Work Energy Transfer/Change by Heat Heat (Temperature Change) Heat Capacity Specific Heat Capacity Molar Heat Capacity Heat Transfer by Work Pressure-Volume Work Measuring Changes in Internal Energy Bomb Calorimetry 2
3 Energy: Kinetic & Potential Energy (E) is the capacity to do work or transmit heat. All energy is kinetic or potential. Kinetic Energy is energy of motion swinging a bat, moving a piston, rolling a ball The movement of particles is thermal energy. Thermal energy is a kind of kinetic energy. Potential Energy is energy of position the position of the edge of a cliff, top of a hill, of a bow string, rubber band, of ions in a battery. The position of atoms in a molecule is chemical energy. Chemical energy is a kind of potential energy. 36 4
4 Energy: Kinetic & Potential Energy (E) is the capacity to do work or transmit heat. All energy is kinetic or potential. Kinetic Energy is energy of motion swinging a bat, moving a piston, rolling a ball The movement of particles is thermal energy. Thermal energy is a kind of kinetic energy. Potential Energy is energy of position the position of the edge of a cliff, top of a hill, of a bow string, rubber band, of ions in a battery. The position of atoms in a molecule is chemical energy. Chemical energy is a kind of potential energy. 4 4
5 Units of Energy The SI (Système international) unit of energy is the joule (J), a derived unit named after the British scientist James Joule. It s common to find energy reported in kilojoules (kj). A joule is defined as: 1 J = 1 kg m 2 / s 2 A joule is a unit derived from kilograms, meters, and seconds. This is a definition, therefore an exact conversion factor - sig figs. The calorie (cal) preceded the joule as a standard of energy and is still widely used. The calorie was originally experimentally determined. We later redefined calories so we would have an exact conversion factor. You must be able to convert between joules and calories. 1 cal = J (memorize this exact conversion factor - sig figs) Calories (cal) are sometimes called small calories. A large calorie (Cal) is 1000 small calories. The calories you see on supermarket packages are large calories, also called food calories or kilocalories. Liters-atmospheres (L atm) is also a unit of energy. You won t have to do this conversion but I ll use it in some derivations. 1 L atm = J (you do not need to memorize this measured conversion factor, it will be provided if needed - 6 sig figs) 5
6 Why Calories to Joules is an Exact Number The original calorie was defined as the energy it takes to raise 1 grams of water 1 degree. The temperature at which that experiment is said to occur differs from source to source, resulting in different conversion factors. 1 calorie (at 4 C) = J (measured) 1 calorie (at 15 C) = J (measured) 1 calorie (at 20 C) = J (measured) In 1929, the calorie used to report internationally accepted boiling point data (the international Steam Tables) was standardized by defining it to be equal to exactly 180/43 Joules, in an attempt to remove that uncertainty. 1 calorie = 180/43 J (exact - a definition) 1 = J (measured, the fraction rounded to 5 s.f.) By 1956, we found it more convenient to do calculations with decimals than fractions. The calorie was again redefined to be exactly J to make it an exact number in decimal form. 1 calorie = J (exact - a definition) The calorie was later redefined to be equal to exactly J and this calorie is now used for all thermochemical tables. 1 calorie = J (exact - a definition) This final calorie is called the Thermochemical calorie, this is the calorie we will use in this class. 6
7 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal & Chemical Systems keeping perspective consistent is essential! the system & the surroundings types of systems open, closed, isolated Changes, Values & Size What is change? (over time) Measuring changes State Functions vs Path Functions Changes in Energy Internal Energy Heat & Work Energy Transfer/Change by Heat Heat (Temperature Change) Heat Capacity Specific Heat Capacity Molar Heat Capacity Heat Transfer by Work Pressure-Volume Work Measuring Changes in Internal Energy Bomb Calorimetry 7
8 Internal Energy A system is any portion of the universe we single out for study. Internal Energy is the energy contained within that system. Chemists care about: the thermal energy (kinetic energy) the chemical energy (potential energy) Not all systems have the same energy. A cold brick may have less energy than a hot brick. A hot brick may have less energy than a block of wood. A block of wood may have less energy than a lump of coal. A lump of coal might have less energy than a vial of nitroglycerine. We want to understand how to predict and effect the energy of a system. We want to know what substances may be richer in internal energy. We want to discover how to unlock energy and put it to use. We want to understand how to trap energy in systems to store it for later. We want to understand how to measure and control changes to a systems energy. 8
9 System and Surroundings A system is any portion of the universe we single out for study. The surroundings are the rest of the universe (anything not in the system we have selected). Anything that leaves the system, must enter the surroundings. A open system is one where matter and energy may both exchange between the system and it s surroundings. In a closed system matter cannot exchange, but energy can exchange. An isolated system is one where neither may exchange between the system and it s surroundings. Isolated systems are effectively a universe of their own (at least it s our goal to approximate that). When we do thermochemical experiments we will do our experiments in isolated apparatus. Always understand the boundaries of the system you re looking at. If the system is not isolated, you have to account for all energy gained or lost by that system to it s surroundings all energy that crosses those boundaries. 9
10 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal & Chemical Systems keeping perspective consistent is essential! the system & the surroundings types of systems open, closed, isolated Changes, Values & Size What is change? (over time) Measuring changes State Functions vs Path Functions Changes in Energy Internal Energy Heat & Work Energy Transfer/Change by Heat Heat (Temperature Change) Heat Capacity Specific Heat Capacity Molar Heat Capacity Heat Transfer by Work Pressure-Volume Work Measuring Changes in Internal Energy Bomb Calorimetry 10
11 Measurements of Size & Position Properties can be intensive or extensive. Measurements of extensive properties answer the question how much? These are measurements of size (extent). They say how many units are contained in the sample. Example: Mass, contains 50.0 grams Volume, contains 32 ml Measurements of intensive properties answer the question how far? These are measurements of position. They say how many units are between that value and a reference point. Measurements of position can be positive or negative. They require a reference point. Example: Speed, 55 mph faster (than another object) Brightness, 0.23 lumen brighter (than another source) Hardness, 7.0 Moh (70% the hardness of diamond) Density, 19.3 g/ml (19.3 times as dense as water) A B C D 11
12 Change (Δ) Change is the difference between two measurements, over time. We always assume time moves forward. So change is always final minus initial. The change of a value is represented with the delta symbol (Δ). ΔX = Xfinal - Xinitial XFINAL is -2. XINITIAL is +3. Change can be represented by: A single value (which can be positive or negative) ΔX = -5 A size and a direction (example: decreased) X decreased by +5 (the size of the change is +5) XFINAL The change in X is (-2) - (+3) = -5 XINITIAL 12 ΔX = -5
13 Measuring Changes A path is a group of steps you took to accomplish a change. Different paths can be used to change from the same initial state to the same final state. You can go from +3 to -2 by: subtracting 8 and adding 3 subtracting 2 and subtracting 3 or many other paths Some properties associated with change depend on the path you took to produce the change, others don t. If I drive from Madison WI to Helena MT. how much gasoline I use will be a function of my path. my change in time zones will not. If I climb a hill the miles I walk will be a function of my path my change in elevation will not. XFINAL XINITIAL Path A Path B 13
14 Measuring Changes A path is a group of steps you took to accomplish a change. Different paths can be used to change from the same initial state to the same final state. You can go from +3 to -2 by: subtracting 8 and adding 3 subtracting 2 and subtracting 3 or many other paths Some properties associated with change depend on the path you took to produce the change, others don t. If I drive from Madison WI to Helena MT. how much gasoline I use will be a function of my path. my change in time zones will not. If I climb a hill the miles I walk will be a function of my path my change in elevation will not. XFINAL XINITIAL 14
15 Measuring Changes A state property is a property that can be measured considering only a systems condition it s state. A state function is the equation that calculates the property. A path property is a property that requires you to measure it s path to determine the property. A path function is the equation that calculates the property. Example 1: The fuel level in a gas tank is a state property. How much fuel went into the tank is a path property.. Example 2: Altitude can be calculated with a state function. Miles traveled must be calculated with a path function XFINAL XINITIAL 15
16 Internal Energy A system is any portion of the universe we single out for study. Internal Energy is the energy contained within that system. Chemists care about: the thermal energy (kinetic energy) the chemical energy (potential energy) Work (w) is the energy used to cause an object to move against a force. Lifting a box, pulling a bow, throwing a stone. Heat (q or Q) is energy that flows between two samples due to their difference in temperature. Thermal energy moving from a hot material to a cold one. ΔEsys = q + w Internal Energy is a state function. (this will be very important) Work and Heat are path functions. 16 We can t easily measure internal energy. But we can measure work and heat. If we know the work and heat that is done to a system, we can know how internal energy changes.
17 Why it matters that ΔEsys is a state function. Internal energy is all chemical energy and thermal energy in a system. How much energy is in a burning coal, an ice cube, or 4,000 L of petroleum is useful information. We have no idea how to measure internal energy. (practically) However it is possible to measure work and heat. And if we add all the work and heat related to a process we can calculate the change in internal energy of that process. We can do an experiment to find out how the internal energy changes when a coal burns, ice cube melts, or 4,000 L of petroleum are consumed. And because ΔEsys is a state function once we know it's value for that coal, ice cube or 4,000 L of petroleum we know it for every identical lump of coal, cube of ice, or equivalent volume of petroleum. ΔE = Efinal - Einitial ΔE = Eproducts - Ereactants ΔEsys = q + w 17
18 Finding ΔEsys ΔEsys = q + w When we use q and w it's extremely important we keep our perspective consistent so use the correct sign with each number! Example: A system undergoes the following processes, what is the net change in internal energy? The system releases 15 J heat while doing 65 J of work. The system does 34 J of work while absorbing 67 J of heat. The system absorbs 35 J of heat, releases 15 J of heat, and has 23 J of work done on it. 18 So how do we find q?
19 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal & Chemical Systems keeping perspective consistent is essential! the system & the surroundings types of systems open, closed, isolated Changes, Values & Size What is change? (over time) Measuring changes State Functions vs Path Functions Changes in Energy Internal Energy Heat & Work Energy Transfer/Change by Heat Heat (Temperature Change) Heat Capacity Specific Heat Capacity Molar Heat Capacity Heat Transfer by Work Pressure-Volume Work Measuring Changes in Internal Energy Bomb Calorimetry 19
20 A word about ΔT ΔT in Kelvin or Celsius is the Same Number A temperature T (value) is not the same in Celsius, Kelvin or Fahrenheit. The size of each degree is the same in Celsius and Kelvin. A change in temperature ΔT (change) is the same in Celsius and Kelvin. We generally measure temperature in Celsius, it must be in Kelvin for most calculations. For equations involving ΔT this conversion is trivial and often skipped. 20 Note: Temperatures in C and K are not the same. But a difference in C and K are identical! Note: Fahrenheit is evil. Never use it. For anything.
21 Introducing Heat Capacity Heat (q or Q) is energy that flows between two systems due to their difference in temperature. Observation: The more energy an object gains, the hotter it becomes (it s temperature goes up). Observation: Different objects have different capacities to hold heat, before their temperature increases. E T ΔE α ΔT q α ΔT 21
22 Introducing Heat Capacity Heat (q or Q) is energy that flows between two systems due to their difference in temperature. Observation: The more energy an object gains, the hotter it becomes (it s temperature goes up). Observation: Different objects have different capacities to hold heat, before their temperature increases. Heat capacity (C) is the heat an object can hold before it rises 1. Think of this as resistance to heat or capacity to hold heat at each degree. Heat Capacity varies between objects. Size matters, a bigger frying pan can hold more heat than a smaller one. Substance matters, an oven mitt takes more heat to raise it s temperature than the frying pan. C = 2,835 J / C E T ΔE α ΔT q α ΔT q = C ΔT C = J / C If we want to explore heat capacity as a function of the substance we need to factor out size (amount of substance). 22 C = 62.0 J / C
23 Specific Heat Capacity & Molar Heat Capacity Heat capacity (C) is the heat an object can hold before it rises 1. Think of it as resistance to heat or capacity to hold heat at each degree. We can factor out size (amount of substance), by dividing heat capacity by the moles or grams of a substance. This gives us two new kinds of heat capacity: Molar Heat Capacity (Cm) is the heat capacity per mole (n) of a substance. Cm = C / n or C = Cm * n Specific Heat Capacity (Cs) is the heat capacity per gram (m) of a substance. (Often abbreviated as Specific Heat) Cs = C / m or C = Cs * m There are three forms of the heat capacity equation! q = C ΔT q = Cm n ΔT q = Cs m ΔT Specific Heat and Molar Heat Capacity are related by molar mass. C = 2,835 J / C Cm n = Cs m Cm / Cs = m/n = grams/moles = molar mass Cs = 0.45 J / g C Cm = 25.1 J / mol C 23 These two new heat capacities are a property of the substance! They don t change! You might need to use any of the three heat capacity equations depending on the problem. C = 62.0 J / C
24 Heat Capacity Note: Temperatures in C and K are not the same. But a difference in C and K are identical! A 12.5 gram block of metal cools from 53.2 C to 28.7 C. It s specific heat is 19 J/g-K. How much energy was released? q = Cs m ΔT 24
25 Problem: How much heat does it take to warm 143 grams of water from 25.0 C to 78.0 C. The specific heat of water is J/g K. Solution
26 Problem: A 62.0 C iron cube weighing 149 grams looses 2.34 kj of energy, what is it s final temperature? The specific heat of iron is J/g K. Solution
27 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal & Chemical Systems keeping perspective consistent is essential! the system & the surroundings types of systems open, closed, isolated Changes, Values & Size What is change? (over time) Measuring changes State Functions vs Path Functions Changes in Energy Internal Energy Heat & Work Energy Transfer/Change by Heat Heat (Temperature Change) Heat Capacity Specific Heat Capacity Molar Heat Capacity Heat Transfer by Work Pressure-Volume Work Measuring Changes in Internal Energy Bomb Calorimetry 27
28 Energy Transfer The law of conservation of matter: Matter can never be created nor destroyed. The first law of thermodynamics: Energy can never be created nor destroyed. At the atomic scale and above, there are no exceptions to these laws. So energy that leaves one system must go into another. If one system has a negative ΔE another must have a positive ΔE If we consider only heat, that means if one system has a positive q another must have a negative q. If A Looses 25 J then then B gains 25 J The size of both changes will be 25 J The direction of the changes will be opposite. qa = -1 x qb A ΔEA = -25 J qa = -25 J A looses +25 J B ΔEB = +25 J qb = +25 J B gains +25 J Note words like gain or loose provide that direction so be careful to not use a double negative: Never say qb lost -25 J! A B 28
29 Heat Capacity / Heat Transfer Note: Temperatures in C and K are not the same. But a difference in C and K are identical! A 12.5 gram block of metal cools from 53.2 C to 28.7 C. It s specific heat is 19 J/g-K. How much energy was released? q = Cs m ΔT It cooled in water. How much energy was gained by the water? 29
30 Heat Capacity A 12.5 gram block at 53.2 C is dropped into 255 grams of liquid at 25.8 C. The specific heat of the metal is 19 J/g-K. The liquid and metal come to a final temperature of 32.7 C. What is the heat capacity of the liquid?
31 Problem: Metal Cooling in Water A g sample of an unknown metal is heated to 99.0 C and then was dropped into 50.0 grams of 25.0 C water. The temperature of the water rose to 28.1 C, water has specific heat of J/g-K. (a) How many joules of energy did the water absorb? (b) How many joules of energy did the metal lose? (c) What is the specific heat capacity of the metal? 31
32 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal & Chemical Systems keeping perspective consistent is essential! the system & the surroundings types of systems open, closed, isolated Changes, Values & Size What is change? (over time) Measuring changes State Functions vs Path Functions Changes in Energy Internal Energy Heat & Work Energy Transfer/Change by Heat Heat (Temperature Change) Heat Capacity Specific Heat Capacity Molar Heat Capacity Heat Transfer by Work Pressure-Volume Work Measuring Changes in Internal Energy Bomb Calorimetry 32
33 Pressure-Volume Work Heat (q) is one way to change internal energy. Another way is work (w). Work is the energy used to move an object. Heat and work are the primary ways the internal energy of chemical systems change. ΔE = q + w Chemical systems do work by changing volume under pressure. P x ΔV For example, burning gasoline converts it to gases which pushes a piston. By convention, w and ΔV must be opposite in sign, so w and ΔE can have the same sign w = P x ΔV ΔE = q - P x ΔV 33
34 Pressure-Volume Work Chemical reactions most commonly work on their surroundings ( push things ) by changing volume and thereby exerting pressure. w = -P ΔV (reminder: positive work is work done on the system, so work done by the system is negative) 34
35 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal & Chemical Systems keeping perspective consistent is essential! the system & the surroundings types of systems open, closed, isolated Changes, Values & Size What is change? (over time) Measuring changes State Functions vs Path Functions Changes in Energy Internal Energy Heat & Work Energy Transfer/Change by Heat Heat (Temperature Change) Heat Capacity Specific Heat Capacity Molar Heat Capacity Heat Transfer by Work Pressure-Volume Work Measuring Changes in Internal Energy Bomb Calorimetry 35
36 Calorimetry We re interested in chemical reactions. We want to know how the energies change when one substance turns into another. We want to know if we re putting energy into a substance by chemical reaction, or if we re releasing it. We know that energy is the result of all work and heat that occurs in the reaction. Heat can be determined by measuring temperature and knowing heat capacity. But work is a lot harder to track. So how do we find that change? A C3H8 + 8 O2 (g) 3 CO2 (g) + 4 H2O (g) B Measuring energy changes is called calorimetry. ΔX = Xfinal - Xinitial 36 ΔE = EProducts - EReactants ΔE = q + w EProducts - EReactants = q + w A B
37 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal & Chemical Systems keeping perspective consistent is essential! the system & the surroundings types of systems open, closed, isolated Changes, Values & Size What is change? (over time) Measuring changes State Functions vs Path Functions Changes in Energy Internal Energy Heat & Work Energy Transfer/Change by Heat Heat (Temperature Change) Heat Capacity Specific Heat Capacity Molar Heat Capacity Heat Transfer by Work Pressure-Volume Work Measuring Changes in Internal Energy Bomb Calorimetry 37
38 Bomb Calorimetry ΔE = q + w w = -P ΔV ΔE = q + (-P ΔV) if constant volume: then ΔV = 0 ΔE = qv + (-P)(0) A bomb is a rigid sealed container for chemical reactions. ΔE = qv This means, if volume is constant we can measure changes in internal energy just by measuring heat flow qv 38 q = C ΔT q = Cm n ΔT q = Cs m ΔT
39 Finding ΔE w/ Bomb Calorimetry A bomb calorimeter has a heat capacity of 5.42 kj/ C. If you burn moles in the calorimeter and see the temperature rise 3.60 C, what is the reactions ΔE in kj/mole? q = C ΔT q = Cm n ΔT q = Cs m ΔT Disadvantages: - the equipment is expensive - the experiment is difficult - it s not exactly the reaction we re interested in, in the real world volume changes. 39
40 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal & Chemical Systems keeping perspective consistent is essential! the system & the surroundings types of systems open, closed, isolated Changes, Values & Size What is change? (over time) Measuring changes State Functions vs Path Functions Changes in Energy Internal Energy Heat & Work Energy Transfer/Change by Heat Heat (Temperature Change) Heat Capacity Specific Heat Capacity Molar Heat Capacity Heat Transfer by Work Pressure-Volume Work Measuring Changes in Internal Energy Bomb Calorimetry 40
41 Questions?
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
THERMOCHEMISTRY & DEFINITIONS
 THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
Chapter 5: Thermochemistry. Problems: , , 5.100, 5.106, 5.108, , 5.121, 5.126
 Chapter 5: Thermochemistry Problems: 5.1-5.95, 5.97-98, 5.100, 5.106, 5.108, 5.118-5.119, 5.121, 5.126 Energy: Basic Concepts and Definitions energy: capacity to do work or to produce heat thermodynamics:
Chapter 5: Thermochemistry Problems: 5.1-5.95, 5.97-98, 5.100, 5.106, 5.108, 5.118-5.119, 5.121, 5.126 Energy: Basic Concepts and Definitions energy: capacity to do work or to produce heat thermodynamics:
Lecture Outline. 5.1 The Nature of Energy. Kinetic Energy and Potential Energy. 1 mv
 Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
s Traditionally, we use the calorie as a unit of energy. The nutritional Calorie, Cal = 1000 cal. Kinetic Energy and Potential Energy
 AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93
 Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Thermochemistry-Part 1
 Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
What is energy??? The ability to do work or produce heat. Potential Energy (PE) energy due to position or composition
 Chapter 10: Energy What is energy??? The ability to do work or produce heat. Potential Energy (PE) energy due to position or composition Kinetic Energy (KE) energy due to motion Law of Conservation of
Chapter 10: Energy What is energy??? The ability to do work or produce heat. Potential Energy (PE) energy due to position or composition Kinetic Energy (KE) energy due to motion Law of Conservation of
Chapter 5 Thermochemistry. 許富銀 ( Hsu Fu-Yin)
 Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Name Class Date. As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings.
 Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A
 ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A PART 1: KEY TERMS AND SYMBOLS IN THERMOCHEMISTRY System and surroundings When we talk about any kind of change, such as a chemical
ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A PART 1: KEY TERMS AND SYMBOLS IN THERMOCHEMISTRY System and surroundings When we talk about any kind of change, such as a chemical
Chapter 6 Energy and Chemical Change. Brady and Senese 5th Edition
 Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions
 Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions Terms, definitions, topics Joule, calorie (Re-read p 57-58) Thermochemistry Exothermic reaction Endothermic reaction
Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions Terms, definitions, topics Joule, calorie (Re-read p 57-58) Thermochemistry Exothermic reaction Endothermic reaction
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy
 Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Thermochemistry 14.notebook. November 24, Thermochemistry. Energy the capacity to do work or produce heat. translational.
 Thermochemistry Energy the capacity to do work or produce heat POTENTIAL ENERGY KINETIC ENERGY (energy of motion) "stored" bond energy TEMPERATURE and HEAT vibrational rotational translational a measure
Thermochemistry Energy the capacity to do work or produce heat POTENTIAL ENERGY KINETIC ENERGY (energy of motion) "stored" bond energy TEMPERATURE and HEAT vibrational rotational translational a measure
CHAPTER 17 Thermochemistry
 CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
Gravity is a force which keeps us stuck to the earth. The Electrostatic force attracts electrons to protons in an atom.
 Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Ch 6. Energy and Chemical Change. Brady & Senese, 5th Ed.
 Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Lecture Presentation. Chapter 5. Thermochemistry. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 5 Thermochemistry John D. Bookstaver St. Charles Community College Cottleville, MO Thermochemistry Thermodynamics is the study of energy and its transformations. Thermochemistry
Lecture Presentation Chapter 5 Thermochemistry John D. Bookstaver St. Charles Community College Cottleville, MO Thermochemistry Thermodynamics is the study of energy and its transformations. Thermochemistry
Chapter 11. Thermochemistry: Heat & Chemical Change
 Chapter 11 Thermochemistry: Heat & Chemical Change The Flow of Energy Thermochemistry: Study of heat changes that occur during physical processes and chemical reactions Energy Energy is the capacity to
Chapter 11 Thermochemistry: Heat & Chemical Change The Flow of Energy Thermochemistry: Study of heat changes that occur during physical processes and chemical reactions Energy Energy is the capacity to
Energy Conversions. Energy. the ability to do work or produce heat. energy energy due to composition or position of an object
 Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
Topic 5: Energetics. Heat & Calorimetry. Thursday, March 22, 2012
 Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Thermochemistry AP Chemistry Lecture Outline
 Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Thermochemistry: Energy Flow and Chemical Reactions
 Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Chapter 17 Thermochemistry
 Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Thermochemistry. Section The flow of energy
 Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
If neither volume nor pressure of the system changes, w = 0 and ΔE = q = ΔH. The change in internal energy is equal to the change in enthalpy.
 5 Thermochemistry Visualizing Concepts 5.1 The book s potential energy is due to the opposition of gravity by an object of mass m at a distance d above the surface of the earth. Kinetic energy is due to
5 Thermochemistry Visualizing Concepts 5.1 The book s potential energy is due to the opposition of gravity by an object of mass m at a distance d above the surface of the earth. Kinetic energy is due to
Topic 05 Energetics : Heat Change. IB Chemistry T05D01
 Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
Chapter 11. Thermochemistry. 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages
 Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Energy Transformations
 Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
Chemistry 104 Chapter Two PowerPoint Notes
 Measurements in Chemistry Chapter 2 Physical Quantities Measurable physical properties such as height, volume, and temperature are called Physical quantity. A number and a unit of defined size is required
Measurements in Chemistry Chapter 2 Physical Quantities Measurable physical properties such as height, volume, and temperature are called Physical quantity. A number and a unit of defined size is required
Chapter 6. Thermochemistry. Chapter 6. Chapter 6 Thermochemistry. Chapter 6 Thermochemistry Matter vs Energy 2/16/2016
 Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry 6.1 Chemical Hand Warmers 6.2 The Nature of Energy: Key Definitions 6.3 The First Law of Thermodynamics: There is no Free Lunch 6.4 6.5 Measuring
Chapter 6 Thermochemistry Chapter 6 Chapter 6 Thermochemistry 6.1 Chemical Hand Warmers 6.2 The Nature of Energy: Key Definitions 6.3 The First Law of Thermodynamics: There is no Free Lunch 6.4 6.5 Measuring
Chem 115 Class Notes 3/13/08 KF. THERMOCHEMISTRY (Ch.5)
 Chem 115 Class Notes 3/13/08 KF THERMOCHEMISTRY (Ch.5) - thermochemistry is the portion of thermodynamics that deals with the relationship between chemical reactions and the exchange of energy in the form
Chem 115 Class Notes 3/13/08 KF THERMOCHEMISTRY (Ch.5) - thermochemistry is the portion of thermodynamics that deals with the relationship between chemical reactions and the exchange of energy in the form
Chapter 6 Thermochemistry 許富銀
 Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Name Date Class THERMOCHEMISTRY
 Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
CHEMISTRY: Chapter 10 Prep-Test
 CHEMISTRY: Chapter 10 Prep-Test Matching Match each item with the correct statement below. a. calorimeter d. temperature b. calorie e. specific heat c. joule f. heat 1. quantity of heat needed to raise
CHEMISTRY: Chapter 10 Prep-Test Matching Match each item with the correct statement below. a. calorimeter d. temperature b. calorie e. specific heat c. joule f. heat 1. quantity of heat needed to raise
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6. Thermochemistry
 Chapter 6 Thermochemistry Section 5.6 The Kinetic Molecular Theory of Gases http://www.scuc.txed.net/webpages/dmackey/files /chap06notes.pdf ..\..\..\..\..\..\Videos\AP Videos\Thermochemistry\AP
Chapter 6 Thermochemistry Section 5.6 The Kinetic Molecular Theory of Gases http://www.scuc.txed.net/webpages/dmackey/files /chap06notes.pdf ..\..\..\..\..\..\Videos\AP Videos\Thermochemistry\AP
Chapter 8 Thermochemistry
 William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
Lecture Presentation. Chapter 6. Thermochemistry. Sherril Soman Grand Valley State University Pearson Education, Inc.
 Lecture Presentation Chapter 6 Thermochemistry Sherril Soman Grand Valley State University Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron 4 Fe(s)
Lecture Presentation Chapter 6 Thermochemistry Sherril Soman Grand Valley State University Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron 4 Fe(s)
Thermodynamics. Internal Energy. Study of energy and its transformations Thermochemistry
 Internal Energy 5.1- Thermodynamics Study of energy and its transformations Thermochemistry Study of energy changes that accompany chemical and physical changes. Energy the capacity to do work or transfer
Internal Energy 5.1- Thermodynamics Study of energy and its transformations Thermochemistry Study of energy changes that accompany chemical and physical changes. Energy the capacity to do work or transfer
Chapter 6. The First Law of Thermodynamics and Heat Exchange Processes
 Chapter 6 The First Law of Thermodynamics and Heat Exchange Processes Nature of Energy Chemistry is the study of matter, its changes and the energy associated with these changes. Energy is anything that
Chapter 6 The First Law of Thermodynamics and Heat Exchange Processes Nature of Energy Chemistry is the study of matter, its changes and the energy associated with these changes. Energy is anything that
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Archimedes Principle
 Archimedes Principle applies in air the more air an object displaces, the greater the buoyant force on it if an object displaces its weight, it hovers at a constant altitude if an object displaces less
Archimedes Principle applies in air the more air an object displaces, the greater the buoyant force on it if an object displaces its weight, it hovers at a constant altitude if an object displaces less
= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)
![= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj) = (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)](/thumbs/89/101004910.jpg) CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 8 Thermochemistry: Chemical Energy. Chemical Thermodynamics
 Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
THERMODYNAMICS 11/14/2018 THERMOCHEMISTRY OBJECT OF THE THERMODYNAMICS FUNDAMMENTAL ASPECT OF THERMODYNAMICS
 THERMODYNAMICS Thermochemistry In thermodynamics we study the energy changes that accompany physical and chemical processes. Usually these energy changes involve heat hence the thermo- part of the term.
THERMODYNAMICS Thermochemistry In thermodynamics we study the energy changes that accompany physical and chemical processes. Usually these energy changes involve heat hence the thermo- part of the term.
Page 1 SPH3U. Heat. What is Heat? Thermal Physics. Waterloo Collegiate Institute. Some Definitions. Still More Heat
 SPH3U Thermal Physics electrons and holes in semiconductors An Introductory ourse in Thermodynamics converting energy into work magnetism thin films and surface chemistry thermal radiation (global warming)
SPH3U Thermal Physics electrons and holes in semiconductors An Introductory ourse in Thermodynamics converting energy into work magnetism thin films and surface chemistry thermal radiation (global warming)
The following gas laws describes an ideal gas, where
 Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as
Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as
BRCC CHM 101 Class Notes Chapter 1 Page 1 of 7
 BRCC CHM 101 Class Notes Chapter 1 Page 1 of 7 Chemistry - the study of matter, its behavior and interactions. matter - anything that takes up space and has mass mass - the substance which makes up the
BRCC CHM 101 Class Notes Chapter 1 Page 1 of 7 Chemistry - the study of matter, its behavior and interactions. matter - anything that takes up space and has mass mass - the substance which makes up the
Thermochemistry Chapter 8
 Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School
 Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy.
 Worksheet 1 1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy. The first law of thermodynamics is the conservation
Worksheet 1 1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy. The first law of thermodynamics is the conservation
Lecture 10. What is energy? Professor Hicks Inorganic Chemistry (CHE151) Ability to do work. Work means moving something against a force
 Lecture 10 Professor Hicks Inorganic Chemistry (CHE151) Ability to do work What is energy? Work means moving something against a force Energy thought of as an imaginary liquid that gets moved from one
Lecture 10 Professor Hicks Inorganic Chemistry (CHE151) Ability to do work What is energy? Work means moving something against a force Energy thought of as an imaginary liquid that gets moved from one
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions
 Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Chemistry 123: Physical and Organic Chemistry Topic 2: Thermochemistry
 Topic 2: Introduction, Topic 2: Thermochemistry Text: Chapter 7 and 19 (~ 3 weeks) 2.0 Introduction, terminology and scope 2.1 Enthalapy and Energy Change in a chemical process; 1st law of Thermodynamics
Topic 2: Introduction, Topic 2: Thermochemistry Text: Chapter 7 and 19 (~ 3 weeks) 2.0 Introduction, terminology and scope 2.1 Enthalapy and Energy Change in a chemical process; 1st law of Thermodynamics
Most hand warmers work by using the heat released from the slow oxidation of iron: The amount your hand temperature rises depends on several factors:
 Lecture Presentation Chapter 6 Thermochemistry Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron: Exothermic reaction 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O
Lecture Presentation Chapter 6 Thermochemistry Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron: Exothermic reaction 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O
- Joule (J): SI unit for energy. It's defined based on the equation for kinetic energy. from. mass. velocity
 153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
Chapter 11. Energy in Thermal Processes
 Chapter 11 Energy in Thermal Processes Energy Transfer When two objects of different temperatures are placed in thermal contact, the temperature of the warmer decreases and the temperature of the cooler
Chapter 11 Energy in Thermal Processes Energy Transfer When two objects of different temperatures are placed in thermal contact, the temperature of the warmer decreases and the temperature of the cooler
Thermochemistry. Using Heats of Reaction - Hess s Law - Standard Enthalpies of Formation - Fuels Foods, Commercial Fuels, and Rocket Fuels
 Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
Chapter 6 Thermochemistry
 Chapter 6 Thermochemistry Thermochemistry Thermochemistry is a part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? - Will a reaction proceed
Chapter 6 Thermochemistry Thermochemistry Thermochemistry is a part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? - Will a reaction proceed
Chapter 2 Energy and Matter 2.1 Energy
 Chapter 2 Energy and Matter 2.1 Energy 1 Energy Energy makes objects move. makes things stop. is needed to do work. 2 Work Work is done when you climb. you lift a bag of groceries. you ride a bicycle.
Chapter 2 Energy and Matter 2.1 Energy 1 Energy Energy makes objects move. makes things stop. is needed to do work. 2 Work Work is done when you climb. you lift a bag of groceries. you ride a bicycle.
CP Chapter 17 Thermochemistry
 CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Energy and Chemical Change Section 15.1 Energy Section 15.2 Heat Section 15.3 Thermochemical Equations Section 15.4 Calculating Enthalpy Change Section 15.5 Reaction Spontaneity Click a hyperlink or folder
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Energetics. Topic
 Energetics Topic 5.1 5.2 Topic 5.1 Exothermic and Endothermic Reactions?? total energy of the universe is a constant if a system loses energy, it must be gained by the surroundings, and vice versa Enthalpy
Energetics Topic 5.1 5.2 Topic 5.1 Exothermic and Endothermic Reactions?? total energy of the universe is a constant if a system loses energy, it must be gained by the surroundings, and vice versa Enthalpy
Ch. 7: Thermochemistry
 Thermodynamics and Thermochemistry Thermodynamics concerns itself with energy and its relationship to the large scale bulk properties of a system that are measurable: Volume, Temperature, Pressure, Heat
Thermodynamics and Thermochemistry Thermodynamics concerns itself with energy and its relationship to the large scale bulk properties of a system that are measurable: Volume, Temperature, Pressure, Heat
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Lecture Presentation. Chapter 5. Thermochemistry Pearson Education, Inc.
 Lecture Presentation Chapter 5 Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work. Energy used to cause the temperature of an
Lecture Presentation Chapter 5 Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work. Energy used to cause the temperature of an
Introduction to Thermochemistry. Thermochemistry Unit. Definition. Terminology. Terminology. Terminology 07/04/2016. Chemistry 30
 Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
SPECIFIC HEAT CAPACITY AND HEAT OF FUSION
 SPECIFIC HEAT CAPACITY AND HEAT OF FUSION Apparatus on each table: Thermometer, metal cube, complete calorimeter, outer calorimeter can (aluminum only), balance, 4 styrofoam cups, graduated container,
SPECIFIC HEAT CAPACITY AND HEAT OF FUSION Apparatus on each table: Thermometer, metal cube, complete calorimeter, outer calorimeter can (aluminum only), balance, 4 styrofoam cups, graduated container,
Thermodynamics Test Clio Invitational January 26, 2013
 Thermodynamics Test Clio Invitational January 26, 2013 School Name: Team Number: Variables specified: s = specific heat C = heat capacity H f = heat of fusion H v = heat of vaporization Given information:
Thermodynamics Test Clio Invitational January 26, 2013 School Name: Team Number: Variables specified: s = specific heat C = heat capacity H f = heat of fusion H v = heat of vaporization Given information:
- Kinetic energy: energy of matter in motion. gravity
 148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
Ch. 17 Thermochemistry
 Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Thermochemistry. The study of energy changes that occur during chemical reactions and changes in state.
 Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Chapter 4. Properties of Matter
 Chapter 4 Properties of Matter A burning log undergoes chemical change resulting in the release of energy in the form of heat and light. The physical properties of the log change during the Introduction
Chapter 4 Properties of Matter A burning log undergoes chemical change resulting in the release of energy in the form of heat and light. The physical properties of the log change during the Introduction
Thermodynamic Processes and Thermochemistry
 General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
Chapter Objectives. Chapter 9 Energy and Chemistry. Chapter Objectives. Energy Use and the World Economy. Energy Use and the World Economy
 Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
- The empirical gas laws (including the ideal gas equation) do not always apply.
 145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
Energy, Heat and Temperature. Introduction
 Energy, Heat and Temperature Introduction 3 basic types of energy: Potential (possibility of doing work because of composition or position) Kinetic (moving objects doing work) Radiant (energy transferred
Energy, Heat and Temperature Introduction 3 basic types of energy: Potential (possibility of doing work because of composition or position) Kinetic (moving objects doing work) Radiant (energy transferred
Thermodynamics. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law
 Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law Units of Chapter 17 & 19 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work
Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law Units of Chapter 17 & 19 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work
Thermochemistry. Chapter 6. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
SUMMARY OF PROPERTIES OF MATTER State Shape Volume Particles Compressibility Solid Definite Definite Densely packed Very slight
 MATTER & ITS FORMS Matter is defined as anything that has mass and occupies space. Matter can be classified by its states: solid, liquid, and gas. Solid: Densely packed matter with definite shape and volume.
MATTER & ITS FORMS Matter is defined as anything that has mass and occupies space. Matter can be classified by its states: solid, liquid, and gas. Solid: Densely packed matter with definite shape and volume.
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
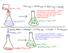 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
CHEM 103 CHEMISTRY I
 CHEM 103 CHEMISTRY I CHAPTER 5 THERMOCHEMISTRY Inst. Dr. Dilek IŞIK TAŞGIN Inter-Curricular Courses Department Çankaya University, Inc. Energy Energy is the ability to do work or transfer heat. Energy
CHEM 103 CHEMISTRY I CHAPTER 5 THERMOCHEMISTRY Inst. Dr. Dilek IŞIK TAŞGIN Inter-Curricular Courses Department Çankaya University, Inc. Energy Energy is the ability to do work or transfer heat. Energy
Enthalpies of Reaction
 Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Thermochemistry. The study of energy transfers and chemical reactions
 Thermochemistry The study of energy transfers and chemical reactions Energy Energy is the ability to do work Work = Force x distance SI unit is the Joule (J) 1000 J = 1 kj other unit: calorie (cal) 1000
Thermochemistry The study of energy transfers and chemical reactions Energy Energy is the ability to do work Work = Force x distance SI unit is the Joule (J) 1000 J = 1 kj other unit: calorie (cal) 1000
The Kinetic Theory of Matter. Temperature. Temperature. Temperature. Temperature. Chapter 6 HEAT
 The Kinetic Theory of Matter Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in
The Kinetic Theory of Matter Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in
