Glauconite and celadonite" two separate mineral species
|
|
|
- Roy Rogers
- 6 years ago
- Views:
Transcription
1 MINERALOGICAL MAGAZINE, SEPTEMBER I978, VOL. 42, PP Glauconite and celadonite" two separate mineral species H. A. BUCKLEY, J. C. BEVAN, K. M. BROWN, AND L. R. JOHNSON Department of Mineralogy, British Museum (Natural History), Cromwell Road, London SW7 58D AND V. C. FARMER The Macaulay Institute for Soil Research, Craigiebuckler, Aberdeen AB 9 2QJ S U M M A RY. Analyses o f glauconites and celadonites from continental sedimentary rocks and sea-floor basalts using X-ray diffraction, electron probe microanalysis, infra-red spectroscopy, and X-ray fluorescence spectroscopy are reported. The minerals are shown to be distinct species; each an isomorphous replacement series, glauconite having an average half unit cell of Ko.85(Fe 3+, A13+)x.34 (Mg 2+, Fe2+)o.66(Si3.76Alo.z4)Olo(OH)2 whereas celadonite approaches the ideal half unit cell of K(Fe 3+, A13+)(Mg 2+, Fe2+)Si4Oxo(OH)2. Considerable Fe 3+- AI vl interchangeability occurs in the octahedral layer in both minerals and considerable substitution of aluminium in the tetrahedral layer of glauconites results in the more disordered IMd type of structure compared with the more highly ordered IM structure of celadonites. Some mixed layer glauconite-smectites and celadonites were also examined and could be distinguished from true glauconites and celadonites by chemical analysis, XRD, and IR techniques. It is proposed that the terms 'glauconite' and 'celadonite' should be used only for those minerals containing less than 5 ~ layering. inter- CONTROVERSY over the structure, composition, and terminology of glauconite and celadonite has existed for decades. It is well established that both are iron-rich, hydrous silicates with a dioctahedral structure, within which considerable chemical variations can occur. Although the two minerals are found in widely differing environments, glauconite occurring in recent and fossil sediments and celadonite in altered volcanic rocks, they are considered by some authors (Foster, I969) to form an isomorphous replacement series. This paper deals with the identification and characterization of the pure mineral species. A major problem in clay analysis is sample purity; for classical wet chemical techniques, the size of the sample invariably precludes removal of trace amounts of other minerals. This paper Copyright the Macaulay Institute records the analyses of both minerals using X-ray diffraction (XRD), infra-red spectrometry (IR), electron probe microanalysis (EPMA), and X-ray fluorescence (XRF). Modern XRD and IR techniques analyse a relatiyely large sample compared with EPMA; they do, however, distinguish and identify most of the minerals present. Since the areas of individual analysis by EPMA approach I pm in diameter, most impurities can be avoided by using this technique. The external morphology of selected samples was determined using a Cambridge Instruments' 6oo Stereoscan. Sample selection and preparation. Fifty-seven glauconite and twenty-five celadonite samples were examined initially by XRD and those containing greater than 5 ~ interstratification or other impurities were rejected. Twenty-six glauconites and twenty celadonites were then selected for further investigation by IR while eighteen of the glauconites and fifteen of the celadonites were analysed by EPMA. The glauconites came from a wide variety of localities: land outcrops, boreholes, and submarine continental shelf deposits (Table I). The celadonites came from ocean-floor basalts and continental volcanic rocks (Table II). The glauconite Was separated from the host rock by gentle crushing followed by cleaning in an ultrasonic bath. There have been many descriptions of the various types of glauconitic pellets found in rocks (e.g. McRae, I972): in the present work only two distinctions are made. Most of the glauconite is in the form of dark green pellets IOO-IOOO #m in size, which bear the suffix 'D' in Tables I and III. A much rarer form occurs as light green lumps (suffix 'L' in Table I), which lack the typical pelletal form and from their external morphology appear to have formed subsequent to the 'D' pellets, since they bear the impressions of adjoining mineral grains.
2 TABLE I. Location, description, and analysis of glauconite samples Geological horizon and locality Description No. XRD IR analysis analysis Lower Greensand Probably Gault/Lower Greensand junction beds, Glyndebourne Bore BDK 4128 *, Sussex Folkestone Beds, Oxney Bore K544", 835 ft, Kent Probably Gault/Lower Greensand junction beds, Oxney Bore Bw I72O*, 805 ft, Kent Gault/Lower Greensand junction beds, Dover Bore, BM. I9O5, 2o2, Dover, Kent Folkestone Stone Beds, Copt Point, Folkestone, Kent Folkestone Beds, 2o ft below base of Gault, Copt Point, Folkestone, Kent Folkestone Beds, I ft below base of Gault, Copt Point, Folkestone, Kent Folkestone Beds, 189 in. below base of GaulL Copt Point, Folkestone, Kent Folkestone Beds, Sandling, Kent (sample FGt of Padgham, I97O) Upper Greensand Gault, Glauconite bed of Bed XII, Abbots Cliff, Folkestone (19i 5 slip), base of bed Gault, Glauconite bed of Bed XII, Abbots Cliff, Folkestone (19I 5 slip), just above base Gault, Glauconite bed of Bed XII, Abbots Cliff, Folkestone (i9i 5 slip), near base of bed Gault, Glauconite bed of Bed XII, Abbots Cliff, Folkestone (19I 5 slip), near top of bed Lower Chalk, Glauconitic Marl Abbots Cliff, Folkestone (1975 exposure), near base Lower Chalk, Glauconitic Marl Abbots Cliff, Folkestone (1975 exposure), top of seam Cambridge Greensand, Barrington, near Cambridge Cambridge Greensand, Hauxton Road, Cambridge Lower Chalkl Glauconitic Marl, Glyndebourne Bore BDK 2792", 48.3 m Eocene Upper Bracklesham Beds, Shepherds Gutter, Hampshire* Upper Bracklesham Beds, Kings Garn Gutter, Brook, Hampshire'~ Sea Floor Samples Mudstone, from 3 l~ 33' N. Io ~ 04. 5' W., 15o m (sample 873 of Summerhayes, 197I ) Phosphorite, from 31~ 22' N. lo ~ I6' W., 400 m (sample 154 of Summerhayes, I971) Glauconitic phosphatic conglomerate, 31 ~ 21 "5' N. i o ~ 19"5' W., 500 m (sample 155 of Summerhayes, I971) Calcareous mudstone, from 31~ 27' N. IO ~ 24' W. 75 ~ m (sample 882 of Summerhayes, I97I) Glauconitic phosphatic conglomerate, 3 I~ I I'3' N. IO ~ o4' W., I5O m (sample 833 of Summerhayes, 197I) Silty limestone, from 31~ I I-2' N. IO ~ o6' W., I4O m (sample 834 of Summerhayes, I97I) DSDP I-2, 24-5 cm from 33 ~ 37' S. 45 ~ o 9' E., IO3O m, on Madagascar Ridge olive green pellets IL g, cl, q g, i, q dark green pellets 5 D g, m, c, q, go diffuse, c rare light green lumps na light green massive 4 L g, cl g large light green lumps 7 L g g, c, q, i small dark green pellets 7 D g, c g, c large light green lumps -- g stained pebbles botryoidal dark green lumps -- apatite with g dark green pellets 8D g, cl g, q light green lumps 8L g, cl g, q, i large light green lamps 9 L g, cl, q, p g, i, q large dark green lumps 9 D g, cl g, q dark green pellets 1 I D g g rare light green lumps na dark green pellets iod g, q, c, f, cl g large olive green lumps 24L g, cl g, q dark olive pellets 24D g, cl g small dark green pellets t7d g, mi g small light green lumps I7L g or m g, c, q small dark green pellets 16D g g rare light green lumps 16L el g, c, q small dark green pellets I5D g, q g rare light green lumps I5L cl, q, f g, c, q small dark green pellets I4D g, cl g small light green lumps 14L g, q, c g, q, c small dark green pellets I3D g, mi, q, c, a g, c small light green lumps x3l cl, q, c g, c, q small dark green pellets 12D g, cl, q, f, a g, c, q small light green lumps 12L cl g, c, q mm pellets i8a g, mi g o-i6-o.2 mm pellets 18b g g, c dark green pellets I9D g g, c rare light green lumps I9L g, mi na dark green pellets 2D g na rare light green lumps 2L cl na olive green pellets 21 g, mi g; q, c dark green grains -- q na light and dark green pellets, 23L g g some with iron stains shiny, dark green grains 25D mi, q na light olive green grains 25L g, cl g black pellets 27L g, q g large green pellets 27 g na large dark green botryoidal pellets 29D g, mi, a g light green pellets 34L g, mi, c, f na dark green pellets 34 D g, mi, a g light olive pellets 35L g, mi g, c light olive pellets 36 mi, c g, c green casts of fora- -- m, c, mi na minifera g = glauconite, m = montmorillonite, mi = mixed layer clays, cl = other clays, i = illite, q = quart~ c = calcite, f = feldspar, go = goethite staining, p = plagioclase, a = apatite, D = dark green pellets, L = light green lumps, (rare), na = not analysed, nd = not determined. * Samples provided by permission of The Director, Institute of Geological Sciences. t Samples provided by A. J. Fleet. Potassium-argon dates: for 27L 49 Ma, 29D I4-4 Ma, 34 L and D I3"I Ma, 35 L IO.6 Ma, from Summerhayes (197o). DSDP samples provided by the Deep Sea Drilling Project.
3 GLAUCONITE AND CELADONITE 375 The continental celadonites were separated from the host rock by crushing, after which the mineral could be readily hand picked from the debris. The ocean-floor basalts contain thin veins of celadonite from which the mineral can be picked using a sharp needle. Most of the celadonites appear to be homogenous when viewed through a binocular microscope (x IOO), although small, glassy, white crystals of a zeolite (probably thomsonite) could be seen in part of BM I9O7, 662, and sample DSDP I7- I7o-I6-L 24-6 cm, contains both light (A', Table IV) and dark (A) green celadonite in a vein. After the samples had been polished for EPMA, however, considerable surface variation due to inhomogeneity could be seen. It was noted that after gentle grinding in an agate mortar and pestle in preparation for XRD analysis, the two minerals displayed marked colour differences: the celadonites exhibited a distinctly blue- green colour, whilst the glauconites were grassygreen and notably softer. Microstructure. Several continental glauconite grains and celadonites from the ocean-floor basalts were examined by a scanning electron microscope (SEM) to see if any differences in the crystalline form of the minerals could be observed. Celadonite 'B', found growing in free space in a vesicle, forms radiating clusters of needles (fig. IA); smectite from the same vesicle forms shorter blade-like radiating crystals (fig. IB). No mixing of the two types was observed and the radial nature of both minerals persisted in material that did not grow into free space but formed veins. Some glauconite pellets appear to be composed of numerous platy crystals, which often show a swirling foliation (fig. IC), while other crystals exhibit a more or less ordered appearance (fig. ID), and are similar to features described by Odin TABLE II. Location, description and analysis of celadonite samples Sample locality Description No. XRD 1R analysis analysis DSDP* I7-I7Oq6-L 24-6 cm green vein in basalt A cl, m, q cl I I ~ 48' N. I77 ~ 37' E., 5792 m DSDP* cc green vesicles in basalt B el, m, a, q d, s 29 ~ 56' S. 36 ~ o4' E., 2088 m DSDP*I7-I64-27-I, Io7 Io cm mineral in crack in C m, cl, c c, cl i3 ~ I2.I 4' N. I6I ~ 30-98' W., 5499 m basalt DSDP* I5-I51-I3-2, 67-8 cm green mud -- m, c, q, f, el, cy cl sili- I5 ~ 0I' N. 73 ~ 24' W., 2029 m cates DSDP* ~7-I , 89-9o cm dark green mineral in -- m, i nocl 03 ~ 45' N. I75 ~ 04' W., 4962 m basalt DSDP* I7q , 95-6 cm dark green mineral in -- m, f, a, q c, cl, silio7 ~ o4' N. I76~ 49' W, 3176 m breccia cates DSDP* I7-i69-II-I, 87-9 cm mineral in cracks in -- m, cl, f, c el 5%, e, Io ~ 40' N. I73 ~ 33' E., 54o7 m basalt silicates DSDP* I7-I71-27-I, I28-3t cm green mineral in inter- -- tridymite na I9 ~ 07' N. I69 ~ 27' W., 2290 m pillow voids DSDP* 26-25oAq, I35 cm green mineral in basalt -- el, m na 33 ~ 27' S. 39 ~ 22' E., 5II9 m BM 8982o, unknown locality massive D ci cl BM 19o7, 662 Thorshaven, Stromo, Faeroes nodules E cl cl BM 192I, 223 Brentonico, Verona, Italy massive F ci cl BM 1922, 973 Omaru District, New Zealand massive G cl cl BM 1913, 333 Hall, Iceland earthy, massive H cl, m cl BM I948, 18 Truckee River, Washoe Co., Nevada massive, in basalt I ck m el, s BM Monte Baldo, Verona, Italy crystals in tuff -- cl, q, m, cy cl, q, ch BM Marcano, Tyrol, Italy crystals in matrix -- el, q, p, cy cl, p BM 1399 Vat di Fassa, Italy pseudomorphs after -- ci, cy, m el BM 14oo Val di Fassa, Italy BM Most, Bohemia BM 1937, I95 Buffaure, Val di Fassa, Italy BM x937, I388 Guarapuara, Parana, Brazil a'ugite crystals in porphyry el, c, q cl, c, q massive g, mi, q cl pseudomorphs after -- cl, mi, ch el, eh, c, q augite cavities in basalt cl, ch, c, q, f cl, ch, q cl = celadonite, g = glauconite, i = illite, cy = other clays, s = saponite, ch = chlorite, m = montmorillonite, c = calcite, q = quartz, f = feldspar, a = amphibole, na = not analysed. * Samples provided by the Deep Sea Drilling Project.
4 376 H.A. BUCKLEY, J. C. BEVAN, K. M. BROWN, AND L. R. JOHNSON (I975). These are not always visible on the surface of a pellet since a covering, presumably also of glauconitic material, is sometimes present. This may be similar to the corona described by Zumpe (1971) and Odom (I976), although major chemical differences between 'corona' and core were not revealed by EPMA. Results of individual analytical methods XRD analysis. All the samples were examined by XRD as a preliminary to further work. The initial results are shown in Tables I and II where the impurities are also listed. Discrepancies between these results and those from IR analyses are caused by the larger sample used for XRD. All the celadonites have sharp basal and hkl reflections (fig. 2) indicating a IM type structure, while the glauconites gave broader basal reflections and reduced hkl reflections, indicating a IMd type structure (fig. 2), and enabling differentiation between the two minerals to be made. These findings are in agreement with those of Carroll (197o). The measurement of basal spacings for clay mineral differentiation is unreliable when mixed-layering is present; FIG. I. Scanning electron microscope photographs of: (A) celadonite; (B) smectite, both from the vesicle in specimen 'B'; (c) part of the surface of a glauconite grain showing the crystal orientation; (D) a different surface texture on the same grain (from sample IoD). (130) (112) ( ) (1~) (020) (11T) (021) (ooi) nite \ ) I I Dec lees 20 Cu-Ka I I I I I! I ! 20 5 I lo /~ Glauconite!! 8 6 FIG. 2. XRD traces showing the characteristic peaks of glauconite 27L and celadonite E.
5 GLAUCONITE AND CELADONITE 377 the d 060 spacings can, however, be used to distinguish between celadonites and glauconites, particularly when used in conjunction with the Fe 3 + content of the unit cell (fig. 3). IR analysis. Samples were pre-ground under alcohol and then incorporated in KBr discs as described by Russell (I974). Spectra were recorded over the range 4ooo-25o cm- 1 in a Perkin Elmer 577 spectrometer. For all but one sample, celadonites were clearly distinguishable from glauconites by their spectra, and samples of the infrared spectra of these two minerals are given in fig. 4. Celadonites exhibit extremely sharp absorption bands throughout their spectra, in which two to four narrow OH stretching bands are clearly resolved in the 36Io-353o cm-1 region, whereas glauconites give much broader absorption bands in which no more than three maxima can be poorly resolved in the region of OH stretching. A further point of distinction is that the band associated with an OH bending vibration, lying at 8oo cm- 1 in celadonites, is either entirely shifted to 815 cm- 1 or partially displaced to give a doublet in glauconites. The sole exception, BM 327o9, has a spectrum with the sharp absorption bands of celadonites, but exhibits a doublet at 8to-8t5 cm-1. The greater sharpness of the celadonite spectra reflects their more highly ordered structure within their individual layers, associated with the very low extent of substitution of A1 for Si in the tetrahedral layer (Table IV) and the regular distribution of divalent and trivalent ions in the octahedral layer. The 8I 5 cm- ~ band of glauconites arises from Fe23 OH groups, which are normally absent from celadonites. The exception BM 327o9 is a mixed-layer celadonite-nontronite. 1" d celadonites 9 glauconites 1.51~-~ [] mixed-layered celadonite / o mixed-layered glaucqnites "510" 1'508" Cl BA IF: me Q ~=1 md =B 1290 OIL o5d a2~ 9 elld 9 9 o27l 16L 2D,15D 14D9 ~lel~0 9D~160 o32709 BG Octahedral Fe 3+,!, 1.'8 FIG. 3. Relationship of d 060 spacing to Fea ions. E ~L i1 I- "%. rldo 9~ -,,6o ~ 3~ CM -z FIG. 4. Infra-red spectra of celadonites G and E, and glauconites 27L and I ID, illustrating the extremes of spectral sharpness and diffuseness found within each species of mica. EP M A electron probe micro-analysis. The grains to be examined were set in resin in perspex mounts, ground by hand using wet and dry silicon carbide paper laps with paraffin lubricant, and polished by hand on paper laps with oil-based diamond pastes and lubricants. After polishing, the mounts were cleansed in diethyl ether, transferred in groups of nine to a brass specimen holder, and coated using a carbon beam evaporator. The specimens were analysed using a Cambridge Instruments' 'Geoscan' microprobe with an accelerating voltage of t 5 kv and a specimen current of approximately o.6 x to-7 A. The standards used were independently analysed minerals, and the results were corrected using the method outlined by Sweatman and Long (t969), using the BM-IC-NPL computer program (Mason et al., I969). With each nine-specimen, eight-element analysis, an independent mineral standard, Kakanui augite (Mason, ~966; Mason and Allen, I973) was run. Each specimen was analysed separately by two operators, each examining different grains. A minimum of six points on each grain were analysed, taking two ten-second counts on each spot. Inhomogeneous specimens were analysed after first determining the areas of celadonite or glauconite, using potassium content
6 378 H.A. BUCKLEY, J. C. BEVAN, K. M. BROWN, AND L. R. JOHNSON AI "4 0 \ \ \ \ \ \ \ \ \ \E \ A~\ E ~ \ 9 ~11 111~ 12D,15D N H "iir SO//18b 9 celadonites \c\ 9~!~ 9 glauconites A D\F e~34d~ I L~' ~r',_ 2 9DN" ~e--,, "~'~ 9 z/.~ 2~L I! I I I I I- "4 "8 1.2 Fe3+ 3SL 11 ~ -u FIG. 5- Relationship between the number of octahedral A1 ions and trivalent Fe ions per half unit cell from the EPMA analyses. Dashed lines indicate the position of the average R 3 content of I.O 4 for celadonites and 1.34 for glauconites. Sample 327o 9 is a mixed-layered celadonite, 23L and 25L are mixed-layered glauconites. as a guide. Since the 'Geoscan' has two spectrometers enabling two elements to be determined simultaneously iron and potassium were first analysed together followed by analysis for magnesium and again, iron, which ensured that the correct area was being examined. Since none of the ferruginous impurities present were known to contain major potassium, this method is considered valid for the samples studied. It is assumed that all the H20- was driven off by the electron beam; the low totals of some analyses (Tables III and IV) may be due to the effects of imperfect polish on the specimens, in particular those with an 'L' suffix. The extent of compositional variation within a sample of celadonite is shown by the analyses of E and E' and A and A'. In the latter pair there is considerable octahedral Fe 3 +-A13 + reciprocal variation although the total trivalent octahedral occupancy remains constant (fig. 5). Element distribution photographs taken of this specimen by EPMA show the potassium to have an even distribution over a wide area while aluminium and iron show a reciprocal relationship. There is a sharp boundary between the two celadonites in some areas (fig. 6), while in others the one grades into the other. Areas of the A' celadonite can be seen to have slightly higher Mg contents than the rest, which is in agreement with chemical analyses obtained for other samples of A and A'. Celadonite A' also has a much smaller d o6o spacing (~-5o65/~) compared with other members of this species. XRF and wet chemistry. Five samples analysed by EPMA were re-analysed by XRF (Tables III and IV) in order to confirm the results. The samples were prepared by fusing with lithium metaborate (t:4) and analysed as pressed glass discs using international standards prepared in the same way for comparison. The analyses compare closely with FIG. 6. EPMA photographs of samples A and A 1 showing (A) backscattered electrons, (B) potassium distribution, (c) distribution of dark green celadonite type A (stippled) and light blue-green celadonite type A x (plain), (D) distribution of iron and (E) distribution of aluminium.
7 GLAUCONITE AND CELADONITE TABLE III. Electron probe microanalyses and structural formulae of glauconites 379 I 5 9 D lid I2D I3D I4D I4D* I5D I6D I6L SiO2 39"77 49"47 51'53 51"45 51"36 51"12 52'87 49"90 52"39 51' TiOz 0"55 o'o4 o'o o'oo o'oo 0"o4 o'o6 o"o 4 o'oo o'o9 A12Oa 6"12 9" "9o 6'71 7"87 7"80 7"32 6"25 7"68 Fe203 15"16 17"37 17"27 14"81 17"73 17"91 I7"14 15'6o 18"14 17"68 17"98 FeO '47 3"43 4"37 2"7o 3'99 4" MuO nd nd nd nd nd nd nd o.oi nd nd nd MgO "88 2"72 4"18 4"o9 4'3o 4"4 ~ 4 '2I 4 "o8 3'69 CaO ' o '32 0"23 0"43 Na20 o'64 o-oo o.o2 o-oo 0.07 o'o3 o-o3 0.o8 o.oo 0.02 o'05 K o i I H20+ nd nd nd nd nd nd nd 5'35 nd nd nd P205 nd nd nd nd nd nd nd I.I8 nd nd nd CO2 nd nd nd nd nd nd nd 0-54 nd nd nd Total 78"5 o 94 " TM 92"40 92"32 95" "25 92"4 o 94"24 Numbers of ions on the basis of 22 (O,OH,F) Si 3"57 3"62 3"77 3"81 3"77 3'77 3'76 3"77 3"75 3"79 3"77 A o o.25 o A1 o-21 o'42 0"31 0"44 0"36 o'35 0"42 o'46 o'36 o'33 0"32 Fe o " "98 0"99 0" Fe 2+ 0"64 o'43 0'37 o"51 o.2i o o'i 7 0" Mg o "42 o'3 ~ o'46 0'45 0"45 o'49 0"45 o'45 o'39 Ti 0"04 o.oo o-oo o.oi o.oo o-oo o-oo o.oo o.oo o.oo o.oi Ca 0"05 o-i I "04 0'05 0" "06 0"05 0"04 0.o6 Na o. I I o-oo o.oo o'oo o.o t o.oo o-oo o'o I o.oo o.oo o'oo K o.62 o'71 o'76 o'76 o'79 0"79 o'76 o'78 o-81 o.8o o'7o ]~R 3 + R '09 2" " ZR I' I-32 i-29 ZA 0.78 o o-8o o-85 o o' I8b I8b* L 27L 29D 34D 34 D* 35 L 35 L* SiO2 51"95 49"70 52'00 49" "43 5o.12 50"64 49"50 48"05 45"3 ~ TiO2 o.o 7 o-io o.o2 o'o4 o'o5 o'o5 o.o2 o'o4 o-o8 o'o5 o'15 A1203 8"87 9"3o 8"55 9'4I 5'81 4"25 2'4I 3'45 3" "3o Fe203 19"65 16"5o 19"62 21"oo 21-1o 21"6o "37 21'2o 31"92 24"40 FeO 2"98 2-2o I "o7 2"49 2"5o 1"59 I'IO MnO nd o'o2 nd nd nd nd nd nd o.oi nd o.oi MgO 3"71 4"0o 3"84 yo '32 4"9o 3"87 3"9 o CaO o-68 1"32 o'65 0"35 o.57 o.45 o.16 o" ~ 4"06 Na20 o.oi 2.19 o.oo 0.03 o.oo 0.25 o.o2 0"03 o"13 o"19 0"3 ~ K20 8'23 7"85 8" "55 8"31 8"19 8"15-8" "82[ HzO + nd 5.32 nd nd nd nd nd nd 5-69 nd 8"35 PzO5 nd 0"43 nd nd nd nd nd nd o.61 nd 0.25 CO2 nd o'4o nd nd nd nd nd nd o.59 nd 3.83 Total 96"15 99"33 95"49 94'39 8o o-66 98"45 95"15 loo'79 Numbers of ions on the basis of 22 (O,OH,F) Si 3"67 3"65 3"69 3"57 3"34 3"69 3'83 3"82 3'81 3"57 3"69 A1 o'33 o'35 o'31 o'43 o.61 o.31 o'17 o-18 o-19 o-19 o-31 Al o'4i o'45 o'4o o'37 o-oo o-o8 o'o4 o'13 o"13 o.oo o.io Fe 3+ 1"o4 o'9i 1"o5 1"15 1" Fe 2+ o'17 o"13 o"15 o o.i2 o-13 o.16 o Mg o'39 o'44 o'41 0"33 o'29 0"55 o'72 o.6o 0'55 o'43 o'38 Ti o.oo o-oo o.oo o'oo o-oo o.oo o.oo o'oo o.oo o.oo o.oo Ca o. I o "05 o. I I 0"07 0"03 o'o3 o.oo 0-06 o.oo Na o.oo o-31 o.oo o.oo o'oo 0"04 o-oo o'oo o.o I 0'03 0"05 K o'74 0'73 0" o-82 o'79 o'78 0"84 o'65 o'5o ]ER3+R 2+ 2" 'Ol 'oi 2"06 2"05 2"07 2"30 2"04 ER s+ 1" "36 1"78 1"59 EA 0"84 I'IO 0'83 0'85 0"74 0"93 0"82 o'81 0"85 0"74 0"55 nd = not determined; in structural formula, P205 deducted as Cas(PO,)aOH; CaO and CO2 as CaCOa. * Additional analysis by XRF (analyst G. C. Jones), with CO2 and H20 determined by CHN analyser (analyst G. C. Jones) and Fe3+/Fe 2+ determined by V. K. Din and A. J. Easton. Analysts J. C. Bevan and K. M. Brown.
8 380 H. A. BUCKLEY, J. C. BEVAN, K. M. BROWN, AND L. R. JOHNSON TABLE I V. Electron probe microanalyses and structural formulae of celadonites A A' B C D D* E E' F G H I Q BM BM 327o9 * 327o9 SiO2 54"84 53"64 52"27 52"58 53"40 53"00 56"05 54"14 50' '43 TiO2 0.o0 nd o-oo o.2o o.oo 0.00 o.oo 0"04 A1203 1"69 5'85 4"17 5"28 2"51 2" o I Fe203 14" o 14.6I o lo.3o o I1-75 FeO 5"88 3"28 5"39 3"37 3"53 3"80 3"98 4"47 3" "68 MnO nd nd nd nd nd o'o2 nd nd nd nd nd MgO 6"98 7"94 6-o "95 5'71 5"45 6' CaO 0-07 o.12 0"07 0"68 0" o"1o 0.I Na20 o-15 nd o-o2 o"31 o'o 5 o'38 0"05 0.oo o-02 0.oo o'o3 K20 8"93 8"97 8.2o '38 lo'29 9"89 8'75 9" H20+ nd nd nd nd nd 4"59 nd nd nd nd nd P2Os nd nd nd nd nd 0.1o nd nd nd nd nd CO2 nd nd nd nd nd o.75 lad nd nd nd nd Total 93"35 88"94 84'98 91"o '12 92'76 92"20 86"07 91"o3 79"99 Numbers of ions on the basis of 22 (O,OH,F) Si 4 "ol 3'98 4"09 3'83 3"96 3"94 4 "01 3"94 4 "O1 3"95 3"99 A1 0.oo o'o2 0.oo o.17 o.04 o'o6 o'oo o'o6 0.0o o'o5 o'oi A1 o'15 O'49 o'38 o'28 o'18 o'19 0"58 o' "02 o'41 Fe 3+ o'81 o,5i "78 0"87 0'92 0"56 0'63 0'95 I.O8 0"75 Fe 2+ o o o.37 o o.19 o'25 Mg o'75 o-88 o-71 o o'63 0" o'64 o'71 0"45 Ti o-oo nd 0.oo 0.oi o-oo 0.oi 0.oo 0.oo o-oo 0.0o o-oo Ca o.oi O.Ol 0.00 O.lO O.Ol o-oo 0.Ol '05 Na 0.o2 o-oo o-oo o-o4 o-oo 0-o 5 o.oo 0.o0 0.0o 0.o0 0.oo K o-82 0"73 0"97 0"89 o'94 0" o' XR3+R "08 I' "98 1"91 2"oo I'98 2'00 1'86 ZR a+ 0"96 1'o0 0"87 1"o6 1"o 5 I'II I'14 1"I1 1'o8 I'I0 1'16 A "87 0"97 0"89 0"97 o' '94 0"93 47"23 55"80 49'70 52" ' "87 5"76 1"9o 2'15 i o "81 3"39 2"30 2"07 nd nd 0-02 nd 9"oo 6"78 3"7o 3"34 o'39 0"36 1'95 1"o4 0"00 0"8l 0" '57 9"28 6"65 6"72 nd nd 5'78 nd nd nd 0"o9 nd nd nd 4"00 nd 83'54 94"75 98"39 89"71 3"83 3"91 3'92 3"95 o"i7 0' O'IO 0"39 0"I0 0"15 0"77 O'66 I'30 1'24 0.I 9 O'20 0"15 O'I 3 1"08 O' "37 0"00 0"01 0" "O6 O'O6 0"00 O' O' I I 0"OO O'OO 0"88 O'85 0"67 0" O'87 1"O5 1'40 1"39 O' O'81 nd = not determined; in structural formula, P2Os deducted as Cas(POa)3OH; CaO and CO2 as CaCO3. * Additional analysis by XRF (analyst G. C. Jones), with CO2 and H20 determined by CHN analyser (analyst G. C. Jones) and Fe3+/Fe 2+ determined by V. K. Din and A. J. Easton. Analysts J. C. Bevan and K. M. Brown. Sample A contains light blue-green mineral A' grading into green mineral A. Sample E is divided into E (matrix) and E' (smooth areas in the matrix). BM 327o9 is a mixed layer celadonite nontronite. Sample Q is a re-analysis of Kempe (1974). the EPMA results with the exception of 35L, which is a mixed-layer species. The Fe 2 +/Fe 3 ratio was determined using a modification (Easton, t972) of the ferrous 2,2'-dipyridyl iron complex (Riley and Williams, I959). This ratio was then applied to the total iron determined by microprobe. Discussion The conventional distinction between glauconite and celadonite, based on mode of origin, is to a large extent reflected in certain features of chemical composition, X-ray diffraction pattern, infra-red spectra, and crystal morphology of the samples examined here; taken together, these features distinguish unambiguously celadonites from glauconites. The essential differences are revealed by the chemical analysis, and in particular by the half unit cell contents (Tables III and IV, fig. 5). The total number of octahedral trivalent atoms per half unit cell (R 3+) is constant for each species, being on average I.o 4 for celadonites and t'34 for glauconites, but within these limits considerable variation in the amounts of A1 vi and Fe 3 + exists (fig. 5), although Fe 3+ remains predominant. Further, celadonites show less A1 tetrahedral substitution; thirteen of the fifteen analysed celadonites have fewer than o-i A13 ions per four tetrahedral sites, while the remaining two samples (C and I) fall just on the lower limit (o-i7) of the glauconite field (0. I7-0"6I A13 +). The average R 3 + determined for glauconites and celadonites from the current analyses is lower than that found by previous workers. The average R 3 + of I-48 for glauconites is calculated from the results of Odom (i976), Foster (i969), Thompson and Hower (I975), Bentnor and Kastner (I965), Hendricks and Ross (I940, and Weaver and Pollard (i973). The average R 3 + for celadonites calculated
9 from the results of Foster (I969), Wise and Eugster 0964), Hendricks and Ross (I94I), and Kohyama et al. (I97I) is i.i 5. These samples plot along lines parallel to the current results (fig. 5) and show a similar degree of AI vi-fe 3+ substitution. The reason for the difference is uncertain: the fact that the earlier work was done by bulk wet-chemical analysis may be a contributory factor. EPMA work by Kastner (i976) on a celadonite from DSDP basalt sample , 9o- 2 Cll~ gave a R 3+ value of I-o3 when the average celadonite Fe 3 +/Fe 2+ ratio of 2.6 from the current work was applied and is in line with the present results rather than those of previous workers. A strong correlation was found for both glauconite and celadonite between the Fe 3 content and the d o6o spacing (fig. 3), the former having a d o6o greater than r5i A, the latter less than 1.5I A. Exceptions to the above generalizations fall into three groups: 23L and 35L; BM 327o9; and IL, 5 D, and IID. The first group consists of mixed layer glauconite-smectites (about Io and 25~ mixedlayering respectively) and were included in order to illustrate the abnormal trends that mixed-layering induces. Figs. 3 and 5 show their octahedral Fe 3 + content and d o6o spacing to be much greater than in the pure minerals. Of the second type, BM 327o 9 was at first thought to be a mixed-layer glauconitesmectite from the XRD trace, while the IR data indicated that it was a mixed-layer celadonite. This is supported by its position on figs. 3 and 5 where it shows trends away from the pure celadonites similar to those shown by z3l and 35L relative to pure glauconites. In addition, all the mixed-layer species show considerably higher total octahedral occupancies than the norm for glauconites and celadonites. The third group differ from the others in an altogether different respect. These samples have the morphology and IR and XRD patterns of glauconites, but have higher Fe 2 + and lower Mg contents than normal. The environment from which i ID was extracted was very much reduced, containing pyrite and free sulphur; LL and 5D are from the same bed but from inland boreholes. It is suspected that these samples were initially 'normal' glauconite but have since undergone considerable reduction. From the data available it appears that glauconites from different outcrops in the same area and geological horizon have similar compositions. The glauconites from the Lower and Upper Greensand plot separately, as do the Moroccan continental shelf deposits. There also seems to be a loss of Fe 3 and Mg and a corresponding gain of A! and Fe: + with increasing age: oceanic glauconites also show higher oxidation states. GLAUCONITE AND CELADONITE Conclusions 381 L Celadonites and glauconites form separate mineral species: within each group considerable chemical variation can be found. A rough distinction between them can be made from their overall bulk chemical analysis, celadonites usually having higher MgO and K20 but lower total Fe203 than glauconites. Further, each of the two minerals is an individual isomorphous replacement series, celadonites approaching the ideal half unit cell of the type: K(Fe 3 +, AI 3 (Mg 2 +, Fe 2 +) Si4010(OH)2, whilst the glauconites have an average half unit cell of the type: K0.as(Fe 3 A13+)1.34 (Mg 1+, Fe2 +)0.66 (5i3-76, A10.24) O10(OH)2. In the latter the divergence of the octahedral layer from the optimum R3+R 2+ ratio of I: I and the considerable substitution of aluminium in the tetrahedral layer presumably leads to the relative disorder within the layers of this structure and a lower energy of formation. 2. Celadonites and glauconites can be differentiated readily by both IR and XRD: in the case of the latter, particularly so when the d o6o spacings are plotted against the ferric iron content of the half unit cell (fig. 3)- 3- An apparent systematic discrepancy between chemical data as determined by EPMA and bulk 'wet' chemical methods was noted, the cause of which is not known. This results in lower A120 3 and R 3+ occupancy in the present data than in that previously reported. 4. Glauconites from a given geological horizon generally have a similar composition, whereas celadonites may show a wide range of composition within the same bulk sample. 5. The term 'glauconite' or 'glauconitic' has been used in several contexts: to describe glauconitebearing sediments; for individual grains or pellets containing glauconite; or to denote a specific mineral species (McRae, I972 ). Some authors (e.g. Thompson and Hower, I975) refer to the mixedlayer minerals, which consist of interstratified layers of glauconite and smectite, as 'glauconite' even though it may be the minor constituent. On the basis of the results reported here it is suggested that the term 'glauconite' be reserved for use in the specific mineralogical sense; that both constituents be specified in naming mixed-layer clays; and that the adjective 'glauconitic' be used in all other contexts. Acknowledgements. Thanks are due to the Deep-Sea Drilling Project and National Science Foundation, the Director of the Institute of Geological Sciences, London, and Dr. A. J. Fleet of the Open University for providing some of the samples used in this study. The authors would
10 382 H.A. BUCKLEY, J. C. BEVAN, K. M. BROWN, AND L. R. JOHNSON like to thank Dr. D. R. C. Kempe of the British Museum (Natural History) for critically reading this manuscript and his editorial comments. REFERENCES Bentnor (Y. K.) and Kastner (M.), I965. Jour. Geol. Pert. 35, Carroll (D.), 197o. Geol. Soc. Am. Spec. Pap. 126, 1-8o. Easton (A. J.), Chemical analysis of silicate rocks, 1-258, Elsevier, London. Foster (M. D.), U.S. Geol. Surv. Prof. Pap. 614-F, I- I7. Hendricks (S. B.) and Ross (C. S.), Am. Mineral. 26, 683-7o8. Kastner (M.), In Schlanger (S. O.) and Johnson (E. D.), Initial Reports Deep-Sea Drilling Project, 35, 518 I9. Kempe (D. R. C.), In Davis (T. A.) and Luyendyk (B. P.), Ibid. 26, 465-5o3. Kohyama (N.), Shimode (S.), and Sudo (T.), I97 I. Mineral. J. Japan, 6, Mason (B.), N.Z.J. Geophys. 9, 474-8o. --and Allen (R. O.), N.Z.J. Geophys. 16, Mason (P.), Frost (M. T.), and Reed (S. J. B.), National Physical Lab. I.M.S. Report I. McRae (S. G.), Earth-Sci. Rev. 8, Odin (G. S.), Ph.D. Thesis, Univ. P. et M. Curie de Paris. Odom (I. E.), Clays Clay Miner, 24, Padgham (R. C.), 197o. Unpublished Ph.D. Thesis, Univ. of London. Riley (J. P.) and Williams (M. P.), I959. Mikrochim. Acta, 4, Russell (J. D.), In Farmer (V. C.), The infra-red spectra of minerals, Min. Soc. Lond, II-25. Summerhayes (C. P.), 197o. Unpublished Ph.D. Thesis, Univ. of London. Sweatman (T. R.) and Long (J. V. P.), J. Petrol. 10, Thompson (G. R.) and Hower (J.), Clays Clay Miner. 23, o. Weaver (C. E.) and Pollard (L. D.), The chemistry of clay minerals, Elsevier, Wise (S. E.) and Eugster (H. P.), Am. Mineral. 49, IO Zumpe (H. H.), I97 I. Mineral. Mag. 38, [Manuscript received 23 June I977, revised z 9 December "977]
Metcalf and Buck. GSA Data Repository
 GSA Data Repository 2015035 Metcalf and Buck Figure DR1. Secondary ionization mass-spectrometry U-Pb zircon geochronology plots for data collected on two samples of Wilson Ridge plutonic rocks. Data presented
GSA Data Repository 2015035 Metcalf and Buck Figure DR1. Secondary ionization mass-spectrometry U-Pb zircon geochronology plots for data collected on two samples of Wilson Ridge plutonic rocks. Data presented
 26. MIXED-LAYER ILLITE/MONTMORILLONITE CLAYS FROM SITES 146 AND 149 Herman E. Roberson, State University of New York, Binghamton, New York INTRODUCTION The purpose of this report is to describe the clay
26. MIXED-LAYER ILLITE/MONTMORILLONITE CLAYS FROM SITES 146 AND 149 Herman E. Roberson, State University of New York, Binghamton, New York INTRODUCTION The purpose of this report is to describe the clay
Chlorites from granitic rocks of the central Sierra Nevada batholith, California
 MNERALOGCAL MAGAZNE, MARCH 973, VOL. 39, PP- 58-64 Chlorites from granitic rocks of the central Sierra Nevada batholith, California F. C. W. DODGE U.S. Geological Survey, Menlo Park, California 9425, U.S.A.
MNERALOGCAL MAGAZNE, MARCH 973, VOL. 39, PP- 58-64 Chlorites from granitic rocks of the central Sierra Nevada batholith, California F. C. W. DODGE U.S. Geological Survey, Menlo Park, California 9425, U.S.A.
Report on samples from the Great Basin Science Sample and Records Library
 Jonathan G. Price, Ph.D. State Geologist and Director Nevada Bureau of Mines and Geology Office telephone: 775-784-6691 extension 5 1664 North Virginia Street Home telephone: 775-329-8011 University of
Jonathan G. Price, Ph.D. State Geologist and Director Nevada Bureau of Mines and Geology Office telephone: 775-784-6691 extension 5 1664 North Virginia Street Home telephone: 775-329-8011 University of
NOTE TOSUDITE CRYSTALLIZATION IN THE KAOLINIZED GRANITIC CUPOLA OF MONTEBRAS, CREUSE, FRANCE
 Clay Minerals (1986) 21, 225-230 225 NOTE TOSUDITE CRYSTALLIZATION IN THE KAOLINIZED GRANITIC CUPOLA OF MONTEBRAS, CREUSE, FRANCE Albite, muscovite granite and greisens of the Montebras cupola, Creuse,
Clay Minerals (1986) 21, 225-230 225 NOTE TOSUDITE CRYSTALLIZATION IN THE KAOLINIZED GRANITIC CUPOLA OF MONTEBRAS, CREUSE, FRANCE Albite, muscovite granite and greisens of the Montebras cupola, Creuse,
Arsenic and Other Trace Elements in Groundwater in the Southern San Joaquin Valley of California
 Arsenic and Other Trace Elements in Groundwater in the Southern San Joaquin Valley of California Dirk Baron Geological Sciences California State University, Bakersfield Trace Element Maximum Contaminant
Arsenic and Other Trace Elements in Groundwater in the Southern San Joaquin Valley of California Dirk Baron Geological Sciences California State University, Bakersfield Trace Element Maximum Contaminant
ROCK CLASSIFICATION AND IDENTIFICATION
 Name: Miramar College Grade: GEOL 101 - Physical Geology Laboratory SEDIMENTARY ROCK CLASSIFICATION AND IDENTIFICATION PRELAB SECTION To be completed before labs starts: I. Introduction & Purpose: The
Name: Miramar College Grade: GEOL 101 - Physical Geology Laboratory SEDIMENTARY ROCK CLASSIFICATION AND IDENTIFICATION PRELAB SECTION To be completed before labs starts: I. Introduction & Purpose: The
Igneous Rocks. Sedimentary Rocks. Metamorphic Rocks
 Name: Date: Igneous Rocks Igneous rocks form from the solidification of magma either below (intrusive igneous rocks) or above (extrusive igneous rocks) the Earth s surface. For example, the igneous rock
Name: Date: Igneous Rocks Igneous rocks form from the solidification of magma either below (intrusive igneous rocks) or above (extrusive igneous rocks) the Earth s surface. For example, the igneous rock
PETROGRAPHIC MINERALOGICAL ANALYSIS OF AGGREGATES FROM DEVOLL HYDROPOWER PROJECT
 PETROGRAPHIC MINERALOGICAL ANALYSIS OF AGGREGATES FROM DEVOLL HYDROPOWER PROJECT Rezarta QEMALLAJ 1,dr. ing. Alma GOLGOTA 1 Authors Affiliations: Prof.Dr. Marie Koçi 1 ( KIBE1 Laborator, Durres, Albania)
PETROGRAPHIC MINERALOGICAL ANALYSIS OF AGGREGATES FROM DEVOLL HYDROPOWER PROJECT Rezarta QEMALLAJ 1,dr. ing. Alma GOLGOTA 1 Authors Affiliations: Prof.Dr. Marie Koçi 1 ( KIBE1 Laborator, Durres, Albania)
Speciation of Individual Mineral Particles of Micrometer Size by the Combined Use of ATR-FT-IR Imaging and Quantitative ED-EPMA Techniques
 Speciation of Individual Mineral Particles of Micrometer Size by the ombined Use of ATR-FT-IR Imaging and Quantitative ED-EPMA Techniques Md Abdul Malek, Hae-Jin Jung, JiYeon Ryu, BoHwa Kim, Young-hul
Speciation of Individual Mineral Particles of Micrometer Size by the ombined Use of ATR-FT-IR Imaging and Quantitative ED-EPMA Techniques Md Abdul Malek, Hae-Jin Jung, JiYeon Ryu, BoHwa Kim, Young-hul
A Closer Look At Hydrothermal Alteration and Fluid-Rock Interaction Using Scanning Electron Microscopy
 Proceedings World Geothermal Congress 2015 Melbourne, Australia, 19-25 April 2015 A Closer Look At Hydrothermal Alteration and Fluid-Rock Interaction Using Scanning Electron Microscopy Bridget Y. Lynne
Proceedings World Geothermal Congress 2015 Melbourne, Australia, 19-25 April 2015 A Closer Look At Hydrothermal Alteration and Fluid-Rock Interaction Using Scanning Electron Microscopy Bridget Y. Lynne
Mineral Characterization and Crystalline Nature of Quartz in Ponnaiyar River Sediments, Tamilnadu, India
 American-Eurasian Journal of Scientific Research 4 (2): 03-07 2009 ISSN 88-6785 IDOSI Publications 2009 Mineral Characterization and Crystalline Nature of Quartz in Ponnaiyar River Sediments Tamilnadu
American-Eurasian Journal of Scientific Research 4 (2): 03-07 2009 ISSN 88-6785 IDOSI Publications 2009 Mineral Characterization and Crystalline Nature of Quartz in Ponnaiyar River Sediments Tamilnadu
Preliminary Progress Report. Mineralogy and Geochemistry of Well Cuttings. Prepared for: Gastem USA. Colgate University Department of Geology
 Preliminary Progress Report Mineralogy and Geochemistry of Well Cuttings Prepared for: Gastem USA By: Jaclyn Baughman Alison MacNamee Bruce Selleck Colgate University Department of Geology July 30, 2010
Preliminary Progress Report Mineralogy and Geochemistry of Well Cuttings Prepared for: Gastem USA By: Jaclyn Baughman Alison MacNamee Bruce Selleck Colgate University Department of Geology July 30, 2010
A. IGNEOUS Rocks formed by cooling and hardening of hot molten rock called magma (within crust or at its surface).
 EARTH SCIENCE 11 CHAPTER 5 NOTES KEY How Earth's Rocks Were Formed Early geologists believed that the physical features of the Earth were formed by sudden spectacular events called CATASTROPHES. Modern
EARTH SCIENCE 11 CHAPTER 5 NOTES KEY How Earth's Rocks Were Formed Early geologists believed that the physical features of the Earth were formed by sudden spectacular events called CATASTROPHES. Modern
I.S : What s in it and the role of the Geologist
 Institute of Geologists of Ireland Pyrite Course I.S. 398-1: What s in it and the role of the Geologist Michael L.J. Maher 4 December, 2013 Responsibilities of Geologist You re only the messenger! Classification
Institute of Geologists of Ireland Pyrite Course I.S. 398-1: What s in it and the role of the Geologist Michael L.J. Maher 4 December, 2013 Responsibilities of Geologist You re only the messenger! Classification
EPMA IMAGES. Figure 9. Energy-dispersive spectra of spot mineral analyses in sample 89GGR-33A for locations 1-5 in Figure 8.
 EPMA IMAGES The attached images and mineral data can be used to supplement an instrument-based lab, or serve as the basis for lab that can be completed without an instrument. Please provide credit for
EPMA IMAGES The attached images and mineral data can be used to supplement an instrument-based lab, or serve as the basis for lab that can be completed without an instrument. Please provide credit for
Mineralogical & Chemical Studies of Gel-e-sarshooy (shampoo clay) in Manian-Iran
 Mineralogical & Chemical Studies of Gel-e-sarshooy (shampoo clay) in Manian-Iran Zohre Moosavinasab Dep.Of geology, Islamic azad university- Estahban Branch-Iran Phone No.:0973496 E mail: z_moosavinasab@yahoo.com.
Mineralogical & Chemical Studies of Gel-e-sarshooy (shampoo clay) in Manian-Iran Zohre Moosavinasab Dep.Of geology, Islamic azad university- Estahban Branch-Iran Phone No.:0973496 E mail: z_moosavinasab@yahoo.com.
Sediment and sedimentary rocks Sediment
 Sediment and sedimentary rocks Sediment From sediments to sedimentary rocks (transportation, deposition, preservation and lithification) Types of sedimentary rocks (clastic, chemical and organic) Sedimentary
Sediment and sedimentary rocks Sediment From sediments to sedimentary rocks (transportation, deposition, preservation and lithification) Types of sedimentary rocks (clastic, chemical and organic) Sedimentary
ENVI.2030L Rock Identification
 ENVI.2030L Rock Identification Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give an endless variety of rocks - aggregates
ENVI.2030L Rock Identification Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give an endless variety of rocks - aggregates
Rocks Rock- A group of minerals, glass, mineroid bound together in some way.
 Rocks Rock- A group of minerals, glass, mineroid bound together in some way. All rocks fit into one of three categories: Igneous- formed by the cooling and hardening of hot molten rock Sedimentary- formed
Rocks Rock- A group of minerals, glass, mineroid bound together in some way. All rocks fit into one of three categories: Igneous- formed by the cooling and hardening of hot molten rock Sedimentary- formed
Breeding et al., Data Repository Material Figure DR1. Athens. Study Area
 Breeding, Ague, and Brocker 1 Figure DR1 21 o 24 Greece o A 38 o Athens Tinos 37 o Syros Attic-Cycladic Blueschist Belt Syros Kampos B Study Area Ermoupoli N Vari Unit Cycladic HP-LT Unit Marble horizons
Breeding, Ague, and Brocker 1 Figure DR1 21 o 24 Greece o A 38 o Athens Tinos 37 o Syros Attic-Cycladic Blueschist Belt Syros Kampos B Study Area Ermoupoli N Vari Unit Cycladic HP-LT Unit Marble horizons
LAB 2: SILICATE MINERALS
 GEOLOGY 640: Geology through Global Arts and Artifacts LAB 2: SILICATE MINERALS FRAMEWORK SILICATES The framework silicates quartz and feldspar are the most common minerals in Earth s crust. Quartz (SiO
GEOLOGY 640: Geology through Global Arts and Artifacts LAB 2: SILICATE MINERALS FRAMEWORK SILICATES The framework silicates quartz and feldspar are the most common minerals in Earth s crust. Quartz (SiO
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 GLS100-01 Quiz#7 chapters 5 and 6 Fall 2009 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Clay minerals formed from gabbro or diorite bedrock
GLS100-01 Quiz#7 chapters 5 and 6 Fall 2009 Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Clay minerals formed from gabbro or diorite bedrock
Wednesday 22 May 2013 Morning
 Wednesday 22 May 2013 Morning AS GCE GEOLOGY F792/01 Rocks Processes and Products *F713000613* Candidates answer on the Question Paper. OCR supplied materials: None Other materials required: Ruler (cm/mm)
Wednesday 22 May 2013 Morning AS GCE GEOLOGY F792/01 Rocks Processes and Products *F713000613* Candidates answer on the Question Paper. OCR supplied materials: None Other materials required: Ruler (cm/mm)
GEOL.3250 Geology for Engineers Sedimentary & Metamorphic Rocks
 GEOL.3250 Geology for Engineers Sedimentary & Metamorphic Rocks Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give
GEOL.3250 Geology for Engineers Sedimentary & Metamorphic Rocks Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give
CHEMICAL, PHYSICAL, AND MINERALOGICAL PROPERTIES OF CERTAIN SOIL PROFILES IN THE LOWER MISSISSIPPI DELTA B. N. DRISKELL ABSTRACT
 CHEMICAL, PHYSICAL, AND MINERALOGICAL PROPERTIES OF CERTAIN SOIL PROFILES IN THE LOWER MISSISSIPPI DELTA B. N. DRISKELL Louisiana State University ABSTRACT The soils of the lower Mississippi Delta are
CHEMICAL, PHYSICAL, AND MINERALOGICAL PROPERTIES OF CERTAIN SOIL PROFILES IN THE LOWER MISSISSIPPI DELTA B. N. DRISKELL Louisiana State University ABSTRACT The soils of the lower Mississippi Delta are
Sedimentary Rocks. Weathering. Mechanical & Chemical Weathering. Sediments. Lithification. Deposition. Transport. Erosion.
 Lithification Sedimentary Rocks Sediments Deposition Transport Erosion Weathering Weathering The sediments that make up sedimentary rocks are produced by: Mechanical & Chemical Weathering Mechanical Weathering
Lithification Sedimentary Rocks Sediments Deposition Transport Erosion Weathering Weathering The sediments that make up sedimentary rocks are produced by: Mechanical & Chemical Weathering Mechanical Weathering
Geology for Engineers Rocks
 89.325 Geology for Engineers Rocks Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give an endless variety of rocks -
89.325 Geology for Engineers Rocks Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give an endless variety of rocks -
Treatment of Data. Methods of determining analytical error -Counting statistics -Reproducibility of reference materials -Homogeneity of sample
 Treatment of Data Methods of determining analytical error -Counting statistics -Reproducibility of reference materials -Homogeneity of sample Detection Limits Assessment of analytical quality -Analytical
Treatment of Data Methods of determining analytical error -Counting statistics -Reproducibility of reference materials -Homogeneity of sample Detection Limits Assessment of analytical quality -Analytical
muscovite PART 4 SHEET SILICATES
 muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
EPS 50 Lab 4: Sedimentary Rocks
 Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
The effect of isomorphous substitutions on the intensities of (OO1) reflections of mica- and chlorite-type structures.
 657 The effect of isomorphous substitutions on the intensities of (OO1) reflections of mica- and chlorite-type structures. By GEORGE BROWN', B.Sc. Pedology Department, Rothamsted Experimental Station,
657 The effect of isomorphous substitutions on the intensities of (OO1) reflections of mica- and chlorite-type structures. By GEORGE BROWN', B.Sc. Pedology Department, Rothamsted Experimental Station,
Possible chemical controls of illite/smectite composition during diagenesis
 MINERALOGICAL MAGAZINE, JUNE 1985, VOL. 49, PP. 387 391 Possible chemical controls of illite/smectite composition during diagenesis B. VELDE Laboratoire de Grologie, ER 224 CNRS, Ecole Normal Suprrieure,
MINERALOGICAL MAGAZINE, JUNE 1985, VOL. 49, PP. 387 391 Possible chemical controls of illite/smectite composition during diagenesis B. VELDE Laboratoire de Grologie, ER 224 CNRS, Ecole Normal Suprrieure,
Log Interpretation Parameters Determined by Analysis of Green River Oil Shale Samples: Initial Steps
 Log Interpretation Parameters Determined by Analysis of Green River Oil Shale Samples: Initial Steps Michael M. Herron Susan L. Herron Malka Machlus Schlumberger-Doll Research Log Interpretation in Green
Log Interpretation Parameters Determined by Analysis of Green River Oil Shale Samples: Initial Steps Michael M. Herron Susan L. Herron Malka Machlus Schlumberger-Doll Research Log Interpretation in Green
THIS IS A NEW SPECIFICATION
 THIS IS A NEW SPECIFICATION ADVANCED SUBSIDIARY GCE GEOLOGY Rocks Processes and Products F792 * OCE / 11038 * Candidates answer on the question paper OCR Supplied Materials: None Other Materials Required:
THIS IS A NEW SPECIFICATION ADVANCED SUBSIDIARY GCE GEOLOGY Rocks Processes and Products F792 * OCE / 11038 * Candidates answer on the question paper OCR Supplied Materials: None Other Materials Required:
THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION
 THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION By J. M. OADES* and W. N. TOWNSEND Department of Agriculture, The University of Leeds. [Received 30th August, 1962] ABSTRACT It is suggested
THE USE OF PIPERIDINE AS AN AID TO CLAY-MINERAL IDENTIFICATION By J. M. OADES* and W. N. TOWNSEND Department of Agriculture, The University of Leeds. [Received 30th August, 1962] ABSTRACT It is suggested
1/31/2013. Weathering Includes Physical, Chemical, Biological processes. Weathering Mechanisms. Wind abrasion forming Ventifacts
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Slake Durability of a Deep Red Stratum Sandstone under Different Environments
 An Interdisciplinary Response to Mine Water Challenges - Sui, Sun & Wang (eds) 2014 China University of Mining and Technology Press, Xuzhou, ISBN 978-7-5646-2437-8 Slake Durability of a Deep Red Stratum
An Interdisciplinary Response to Mine Water Challenges - Sui, Sun & Wang (eds) 2014 China University of Mining and Technology Press, Xuzhou, ISBN 978-7-5646-2437-8 Slake Durability of a Deep Red Stratum
23/9/2013 ENGINEERING GEOLOGY. Chapter 2: Rock classification:
 ENGINEERING GEOLOGY Chapter 2: Rock classification: ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks
ENGINEERING GEOLOGY Chapter 2: Rock classification: ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks
Native nickel from the Jerry River, South Westland, New Zealand: an example of natural refining
 MINERALOGICAL MAGAZINE, SEPTEMBER I975, VOL. 4 0, PP. 247-5I Native nickel from the Jerry River, South Westland, New Zealand: an example of natural refining G. A. CHALLIS New Zealand Geological Survey,
MINERALOGICAL MAGAZINE, SEPTEMBER I975, VOL. 4 0, PP. 247-5I Native nickel from the Jerry River, South Westland, New Zealand: an example of natural refining G. A. CHALLIS New Zealand Geological Survey,
APPENDIX F PHOTOGRAPHS OF CORE SAMPLES
 EKATI DIAMOND MINE LONG LAKE CONTAINMENT FACILITY INVESTIGATION 2013 EBA FILE: E14103013-01.002 MAY 2014 ISSUED FOR USE APPENDIX F PHOTOGRAPHS OF CORE SAMPLES LLCF Geotechnical Site Investigation Report_IFU.docx
EKATI DIAMOND MINE LONG LAKE CONTAINMENT FACILITY INVESTIGATION 2013 EBA FILE: E14103013-01.002 MAY 2014 ISSUED FOR USE APPENDIX F PHOTOGRAPHS OF CORE SAMPLES LLCF Geotechnical Site Investigation Report_IFU.docx
Weathering Cycle Teacher Notes
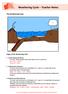 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
Igneous Rock Classification, Processes and Identification Physical Geology GEOL 100
 Igneous Rock Classification, Processes and Identification Physical Geology GEOL 100 Ray Rector - Instructor Major Concepts 1) Igneous rocks form directly from the crystallization of a magma or lava 2)
Igneous Rock Classification, Processes and Identification Physical Geology GEOL 100 Ray Rector - Instructor Major Concepts 1) Igneous rocks form directly from the crystallization of a magma or lava 2)
Surname. Number OXFORD CAMBRIDGE AND RSA EXAMINATIONS ADVANCED SUBSIDIARY GCE F792 GEOLOGY. Rocks Processes and Products
 Candidate Forename Centre Number Candidate Surname Candidate Number OXFORD CAMBRIDGE AND RSA EXAMINATIONS ADVANCED SUBSIDIARY GCE F792 GEOLOGY Rocks Processes and Products WEDNESDAY 20 MAY 2009: Afternoon
Candidate Forename Centre Number Candidate Surname Candidate Number OXFORD CAMBRIDGE AND RSA EXAMINATIONS ADVANCED SUBSIDIARY GCE F792 GEOLOGY Rocks Processes and Products WEDNESDAY 20 MAY 2009: Afternoon
LAB 2 IDENTIFYING MATERIALS FOR MAKING SOILS: ROCK AND PARENT MATERIALS
 LAB 2 IDENTIFYING MATERIALS FOR MAKING SOILS: ROCK AND PARENT MATERIALS Learning outcomes The student is able to: 1. understand and identify rocks 2. understand and identify parent materials 3. recognize
LAB 2 IDENTIFYING MATERIALS FOR MAKING SOILS: ROCK AND PARENT MATERIALS Learning outcomes The student is able to: 1. understand and identify rocks 2. understand and identify parent materials 3. recognize
9/4/2015. Feldspars White, pink, variable Clays White perfect Quartz Colourless, white, red, None
 ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks Chapter 3.0: Weathering & soils Chapter 4.0: Geological
ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks Chapter 3.0: Weathering & soils Chapter 4.0: Geological
Sample collection was restricted to pumice from plinian fallout, from thin distal ignimbrite, and from
 DATA REPOSITORY APPENDIX 1. ANALYTICAL TECHNIQUES Sample collection was restricted to pumice from plinian fallout, from thin distal ignimbrite, and from proximal lithic breccias deposits (Fig. DR1 and
DATA REPOSITORY APPENDIX 1. ANALYTICAL TECHNIQUES Sample collection was restricted to pumice from plinian fallout, from thin distal ignimbrite, and from proximal lithic breccias deposits (Fig. DR1 and
Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn?
 Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn? EPMA - what is it? Precise and accurate quantitative chemical analyses of micron-size
Electron probe microanalysis - Electron microprobe analysis EPMA (EMPA) What s EPMA all about? What can you learn? EPMA - what is it? Precise and accurate quantitative chemical analyses of micron-size
Rocks. Rocks are composed of 1 or more minerals. Rocks are classified based on how they formed (origin). 3 classes of rocks:
 ROCKS Rocks If a mineral is a naturally occurring homogeneous solid, inorganically formed, with a definite chemical composi:on and a crystalline structure then what is a rock? Rocks Rocks are composed
ROCKS Rocks If a mineral is a naturally occurring homogeneous solid, inorganically formed, with a definite chemical composi:on and a crystalline structure then what is a rock? Rocks Rocks are composed
The most common elements that make up minerals are oxygen, silicon, aluminum, iron, calcium, potassium, and magnesium
 Mineralogy: The Study of Minerals and their Properties A Mineral! Occurs! Is a! Is a substance (element or compound)! Has atoms arrange in an orderly pattern ( )! Is (not formed by any process involving
Mineralogy: The Study of Minerals and their Properties A Mineral! Occurs! Is a! Is a substance (element or compound)! Has atoms arrange in an orderly pattern ( )! Is (not formed by any process involving
CERAMIC MATERIALS I. Asst. Prof. Dr. Ayşe KALEMTAŞ
 CERAMIC MATERIALS I akalemtas@mu.edu.tr, akalemtas@gmail.com, Phone: 211 19 17 Metallurgical and Materials Engineering Department Traditional Ceramics Clay products Main Components Clay Feldspar Silica
CERAMIC MATERIALS I akalemtas@mu.edu.tr, akalemtas@gmail.com, Phone: 211 19 17 Metallurgical and Materials Engineering Department Traditional Ceramics Clay products Main Components Clay Feldspar Silica
As compaction and cementation of these sediments eventually occur, which area will become siltstone? A) A B) B C) C D) D
 1. A student obtains a cup of quartz sand from a beach. A saltwater solution is poured into the sand and allowed to evaporate. The mineral residue from the saltwater solution cements the sand grains together,
1. A student obtains a cup of quartz sand from a beach. A saltwater solution is poured into the sand and allowed to evaporate. The mineral residue from the saltwater solution cements the sand grains together,
About Earth Materials
 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Drill Cuttings Analysis: How to Determine the Geology of a Formation and Reservoir
 Drill Cuttings Analysis: How to Determine the Geology of a Formation and Reservoir Chuck Stringer ASA Manager Southern Region 2015 TECH MKT_2014-BD-REG-1673 1 The one item that has lacked serious consideration
Drill Cuttings Analysis: How to Determine the Geology of a Formation and Reservoir Chuck Stringer ASA Manager Southern Region 2015 TECH MKT_2014-BD-REG-1673 1 The one item that has lacked serious consideration
Review - Unit 2 - Rocks and Minerals
 Review - Unit 2 - Rocks and Minerals Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral.
Review - Unit 2 - Rocks and Minerals Base your answers to questions 1 and 2 on the diagram below, which shows the results of three different physical tests, A, B, and C, that were performed on a mineral.
Instrumental Characterization of Montmorillonite Clay by FT-IR and XRD from J.K.U.A.T Farm, in the Republic of Kenya
 Instrumental Characterization of Montmorillonite Clay by FT-IR and XRD from J.K.U.A.T Farm, in the Republic of Kenya Maina,E.W. Wanyika, H.J. Gacanja, A.N. Department of Chemistry, Faculty of Science,
Instrumental Characterization of Montmorillonite Clay by FT-IR and XRD from J.K.U.A.T Farm, in the Republic of Kenya Maina,E.W. Wanyika, H.J. Gacanja, A.N. Department of Chemistry, Faculty of Science,
Copyright SOIL STRUCTURE and CLAY MINERALS
 SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
Lesson Seven: Metamorphic Rocks
 Name: Date: GEOL1 Physical Geology Laboratory Manual College of the Redwoods Lesson Seven: Metamorphic Rocks Background Reading: Metamorphic Rocks Metamorphic Rocks These are rocks that have been changed
Name: Date: GEOL1 Physical Geology Laboratory Manual College of the Redwoods Lesson Seven: Metamorphic Rocks Background Reading: Metamorphic Rocks Metamorphic Rocks These are rocks that have been changed
Highlights and Breakthroughs article for Thomas Bristow David Bish et al.,:the origin and
 1 Revision 1 2 3 4 Highlights and Breakthroughs article for Thomas Bristow David Bish et al.,:the origin and implications of clay minerals from Yellowknife Bay, Gale crater, Mars. 5 6 7 8 9 10 11 12 13
1 Revision 1 2 3 4 Highlights and Breakthroughs article for Thomas Bristow David Bish et al.,:the origin and implications of clay minerals from Yellowknife Bay, Gale crater, Mars. 5 6 7 8 9 10 11 12 13
EFFECT OF CALIBRATION SPECIMEN PREPARATION TECHNIQUES ON NARROW RANGE X-RAY FLUORESCENCE CALIBRATION ACCURACY
 Copyright(c)JCPDS-International Centre for Diffraction Data 2000,Advances in X-ray Analysis,Vol.43 424 EFFECT OF CALIBRATION SPECIMEN PREPARATION TECHNIQUES ON NARROW RANGE X-RAY FLUORESCENCE CALIBRATION
Copyright(c)JCPDS-International Centre for Diffraction Data 2000,Advances in X-ray Analysis,Vol.43 424 EFFECT OF CALIBRATION SPECIMEN PREPARATION TECHNIQUES ON NARROW RANGE X-RAY FLUORESCENCE CALIBRATION
Student Name: College: Grade:
 Student Name: College: Grade: Physical Geology Laboratory IGNEOUS MINERALS AND ROCKS IDENTIFICATION - INTRODUCTION & PURPOSE: In this lab you will learn to identify igneous rocks in hand samples from their
Student Name: College: Grade: Physical Geology Laboratory IGNEOUS MINERALS AND ROCKS IDENTIFICATION - INTRODUCTION & PURPOSE: In this lab you will learn to identify igneous rocks in hand samples from their
Thickness, Compositional and Textural Variability, and Genesis of El-Lajjun Oil Shale, Central Jordan
 Thickness, Compositional and Textural Variability, and Genesis of El-Lajjun Oil Shale, Central Jordan H Alnawafleh 1, D Large 2 & B Spiro 3 1 Department of Mining Engineering, Al-Hussein Bin Talal University,
Thickness, Compositional and Textural Variability, and Genesis of El-Lajjun Oil Shale, Central Jordan H Alnawafleh 1, D Large 2 & B Spiro 3 1 Department of Mining Engineering, Al-Hussein Bin Talal University,
THE ROCK CYCLE & ROCKS. Subtitle
 THE ROCK CYCLE & ROCKS Subtitle 3. Three rocks that do not have minerals or are composed of nonmineral matter. Coal Pumuce Obsidian THE ROCK CYCLE Why do scientists study rocks? Rocks contain clues about
THE ROCK CYCLE & ROCKS Subtitle 3. Three rocks that do not have minerals or are composed of nonmineral matter. Coal Pumuce Obsidian THE ROCK CYCLE Why do scientists study rocks? Rocks contain clues about
Sedimentology & Stratigraphy. Thanks to Rob Viens for slides
 Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Which sample best shows the physical properties normally associated with regional metamorphism? (1) A (3) C (2) B (4) D
 1 Compared to felsic igneous rocks, mafic igneous rocks contain greater amounts of (1) white quartz (3) pink feldspar (2) aluminum (4) iron 2 The diagram below shows how a sample of the mineral mica breaks
1 Compared to felsic igneous rocks, mafic igneous rocks contain greater amounts of (1) white quartz (3) pink feldspar (2) aluminum (4) iron 2 The diagram below shows how a sample of the mineral mica breaks
Cation Exchange Capacity, CEC
 Cation Exchange Capacity, CEC The basic building blocks of clay minerals are: silicon atoms surrounded by four oxygen atoms (tetrahedra), and aluminium atoms surrounded by six hydroxide groups (dioctahedra),
Cation Exchange Capacity, CEC The basic building blocks of clay minerals are: silicon atoms surrounded by four oxygen atoms (tetrahedra), and aluminium atoms surrounded by six hydroxide groups (dioctahedra),
Lab 6: Metamorphic Rocks
 Introduction The Earth s crust is in a constant state of change. For example, plutonic igneous rocks are exposed at the surface through uplift and erosion. Many minerals within igneous rocks are unstable
Introduction The Earth s crust is in a constant state of change. For example, plutonic igneous rocks are exposed at the surface through uplift and erosion. Many minerals within igneous rocks are unstable
Geos 306, Mineralogy Final Exam, Dec 12, pts
 Name: Geos 306, Mineralogy Final Exam, Dec 12, 2014 200 pts 1. (9 pts) What are the 4 most abundant elements found in the Earth and what are their atomic abundances? Create a reasonable hypothetical charge-balanced
Name: Geos 306, Mineralogy Final Exam, Dec 12, 2014 200 pts 1. (9 pts) What are the 4 most abundant elements found in the Earth and what are their atomic abundances? Create a reasonable hypothetical charge-balanced
Quartz. ! Naturally occurring - formed by nature. ! Solid - not liquid or gas. Liquid water is not a mineral
 GEOL 110 - Minerals, Igneous Rocks Minerals Diamond Azurite Quartz Why Study Minerals?! Rocks = aggregates of minerals! Importance to Society?! Importance to Geology? 5 part definition, must satisfy all
GEOL 110 - Minerals, Igneous Rocks Minerals Diamond Azurite Quartz Why Study Minerals?! Rocks = aggregates of minerals! Importance to Society?! Importance to Geology? 5 part definition, must satisfy all
High-purity limestone assessment : from mine to market
 High-purity limestone assessment : from mine to market Clive Mitchell Industrial Minerals Specialist, British Geological Survey Email: cjmi@bgs.ac.uk Website: www.mineralsuk.com Outline Introduction Limestone
High-purity limestone assessment : from mine to market Clive Mitchell Industrial Minerals Specialist, British Geological Survey Email: cjmi@bgs.ac.uk Website: www.mineralsuk.com Outline Introduction Limestone
Lab 4: Mineral Identification April 14, 2009
 Name: Lab 4: Mineral Identification April 14, 2009 While about 3000 minerals have been recognized as valid species, very few of these are commonly seen. Comprehensive mineralogy texts typically deal with
Name: Lab 4: Mineral Identification April 14, 2009 While about 3000 minerals have been recognized as valid species, very few of these are commonly seen. Comprehensive mineralogy texts typically deal with
APPENDICES. Appendix 1
 Corthouts, T.L., Lageson, D.R., and Shaw, C.A., 2016, Polyphase deformation, dynamic metamorphism and metasomatism of Mount Everest s summit limestone, east central Himalaya, Nepal/Tibet: Lithosphere,
Corthouts, T.L., Lageson, D.R., and Shaw, C.A., 2016, Polyphase deformation, dynamic metamorphism and metasomatism of Mount Everest s summit limestone, east central Himalaya, Nepal/Tibet: Lithosphere,
Geology 252, Historical Geology, California State University, Los Angeles - professor: Dr. Alessandro Grippo
 LAB # 1 - CLASTIC ROCKS Background: - Mechanical and Chemical Weathering - Production of Clastic Sediment - Classification of Sediment according to size: Gravel, Sand, Silt, Clay - Erosion, Transportation
LAB # 1 - CLASTIC ROCKS Background: - Mechanical and Chemical Weathering - Production of Clastic Sediment - Classification of Sediment according to size: Gravel, Sand, Silt, Clay - Erosion, Transportation
Soil Mechanics Prof. B.V.S. Viswanadham Department of Civil Engineering Indian Institute of Technology, Bombay Lecture 3
 Soil Mechanics Prof. B.V.S. Viswanadham Department of Civil Engineering Indian Institute of Technology, Bombay Lecture 3 In the previous lecture we have studied about definitions of volumetric ratios and
Soil Mechanics Prof. B.V.S. Viswanadham Department of Civil Engineering Indian Institute of Technology, Bombay Lecture 3 In the previous lecture we have studied about definitions of volumetric ratios and
1. Gravel-size 2. Sand-size 3. Silt-size 4. Clay-size 5. Microcrystalline 6. Macrocrystalline
 Name: GEOL 101 - Physical Geology Lab Grade: SEDIMENTARY & METAMORPHIC ROCK CLASSIFICATION and IDENTIFICATION SEDIMENTARY PRE-ID SECTION To be completed before observing hand samples: I. Introduction &
Name: GEOL 101 - Physical Geology Lab Grade: SEDIMENTARY & METAMORPHIC ROCK CLASSIFICATION and IDENTIFICATION SEDIMENTARY PRE-ID SECTION To be completed before observing hand samples: I. Introduction &
Sedimentary Rocks. Origin, Properties and Identification. Geology Laboratory GEOL 101 Lab Ray Rector - Instructor
 Sedimentary Rocks Origin, Properties and Identification Geology Laboratory GEOL 101 Lab Ray Rector - Instructor Sedimentary Rock Origin and Identification Lab Pre-Lab Internet Link Resources 1) http://www.rockhounds.com/rockshop/rockkey/index.html
Sedimentary Rocks Origin, Properties and Identification Geology Laboratory GEOL 101 Lab Ray Rector - Instructor Sedimentary Rock Origin and Identification Lab Pre-Lab Internet Link Resources 1) http://www.rockhounds.com/rockshop/rockkey/index.html
COMPO- SITION. Euhedral skeletal. Twinned, zoned. Euhedral. Calcic. Anhedral. Mafic. brown clay.
 SITE 9-9A-24X-CC (Piece,-2 cm) ROCK NAME: Basaltic vitrophyre. GRAIN : y to 2.2 mm. TEXTURE: Spherulitic; microporphyritic; subophitic. WHERE SAMPLED: At top of contact with volcaniclastic. Green clay
SITE 9-9A-24X-CC (Piece,-2 cm) ROCK NAME: Basaltic vitrophyre. GRAIN : y to 2.2 mm. TEXTURE: Spherulitic; microporphyritic; subophitic. WHERE SAMPLED: At top of contact with volcaniclastic. Green clay
Plasma driven ammonia decomposition on Fe-catalyst: eliminating surface nitrogen poisoning
 Supporting Information for Plasma driven ammonia decomposition on Fe-catalyst: eliminating surface nitrogen poisoning Contents: 1. Scheme of the DBD plasma-driven catalysis reactor, Scheme S1. 2. XRF analysis
Supporting Information for Plasma driven ammonia decomposition on Fe-catalyst: eliminating surface nitrogen poisoning Contents: 1. Scheme of the DBD plasma-driven catalysis reactor, Scheme S1. 2. XRF analysis
Chapter 8 Lecture. Earth: An Introduction to Physical Geology. Twelfth Edition. Metamorphism. Rocks. Tarbuck and Lutgens Pearson Education, Inc.
 Chapter 8 Lecture Earth: An Introduction to Physical Geology Twelfth Edition Metamorphism and dmetamorphic Rocks Tarbuck and Lutgens Chapter 8 Metamorphic Rocks What Is Metamorphism? Metamorphism means
Chapter 8 Lecture Earth: An Introduction to Physical Geology Twelfth Edition Metamorphism and dmetamorphic Rocks Tarbuck and Lutgens Chapter 8 Metamorphic Rocks What Is Metamorphism? Metamorphism means
Supplementary information
 Supplementary information Sample details Samples used were from the Natural History Museum, London, UK: collections BM1968 P37 and BM1957 1056, and are listed in Supplementary Table1 and Table 2. Supplementary
Supplementary information Sample details Samples used were from the Natural History Museum, London, UK: collections BM1968 P37 and BM1957 1056, and are listed in Supplementary Table1 and Table 2. Supplementary
Data Repository item
 Mineralogical and Geochemical Evolution of a Basalt-Hosted Fossil Soil (Late Triassic, Ischigualasto Formation, Northwest Argentina): Potential for Paleoenvironmental Reconstruction * Neil J. Tabor 1,
Mineralogical and Geochemical Evolution of a Basalt-Hosted Fossil Soil (Late Triassic, Ischigualasto Formation, Northwest Argentina): Potential for Paleoenvironmental Reconstruction * Neil J. Tabor 1,
Sediments and Sedimentary Rocks
 Sediments and Sedimentary Rocks (Shaping Earth s Surface, Part 2) Science 330 Summer 2005 What is a sedimentary rock? Products of mechanical and chemical weathering Account for about 5 percent of Earth
Sediments and Sedimentary Rocks (Shaping Earth s Surface, Part 2) Science 330 Summer 2005 What is a sedimentary rock? Products of mechanical and chemical weathering Account for about 5 percent of Earth
WEDNESDAY, 21 MAY 1.00 PM 3.30 PM. Date of birth Day Month Year Scottish candidate number
 FOR OFFICIAL USE Total X043/2/0 NATIONAL QUALIFICATIONS 204 WEDNESDAY, 2 MAY.00 PM 3.30 PM GEOLOGY HIGHER Fill in these boxes and read what is printed below. Full name of centre Town Forename(s) Surname
FOR OFFICIAL USE Total X043/2/0 NATIONAL QUALIFICATIONS 204 WEDNESDAY, 2 MAY.00 PM 3.30 PM GEOLOGY HIGHER Fill in these boxes and read what is printed below. Full name of centre Town Forename(s) Surname
Perryite in the Kota-Kota and South Oman enstatite chondrites
 850 Perryite in the Kota-Kota and South Oman enstatite chondrites By S. J. B. REED Department of Mineralogy, British Museum (Natural History), Cromwell Road, London, S.W. 7 [Taken as read 6 June 1968]
850 Perryite in the Kota-Kota and South Oman enstatite chondrites By S. J. B. REED Department of Mineralogy, British Museum (Natural History), Cromwell Road, London, S.W. 7 [Taken as read 6 June 1968]
GSA Data Repository
 GSA Data Repository 2019057 1 METHODS Grain Boundary Imaging and Orientation Analysis Backscatter electron (BSE) maps of thin sections were acquired using the FEI Verios XHR scanning electron microscope
GSA Data Repository 2019057 1 METHODS Grain Boundary Imaging and Orientation Analysis Backscatter electron (BSE) maps of thin sections were acquired using the FEI Verios XHR scanning electron microscope
Sedimentary Rocks. Origin, Properties and Identification. Physical Geology GEOL 101 Lab Ray Rector - Instructor
 Sedimentary Rocks Origin, Properties and Identification Physical Geology GEOL 101 Lab Ray Rector - Instructor Sedimentary Rock Origin and Identification Lab Pre-Lab Internet Link Resources 1) http://www.rockhounds.com/rockshop/rockkey/index.html
Sedimentary Rocks Origin, Properties and Identification Physical Geology GEOL 101 Lab Ray Rector - Instructor Sedimentary Rock Origin and Identification Lab Pre-Lab Internet Link Resources 1) http://www.rockhounds.com/rockshop/rockkey/index.html
Name. GEOL.3250 Geology for Engineers Igneous Rocks
 Name GEOL.3250 Geology for Engineers Igneous Rocks I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give an endless variety
Name GEOL.3250 Geology for Engineers Igneous Rocks I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give an endless variety
Engineering Geology ECIV 2204
 Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (3) Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth
Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (3) Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth
Which rock is shown? A) slate B) dunite C) gneiss D) quartzite
 1. Which metamorphic rock will have visible mica crystals and a foliated texture? A) marble B) quartzite C) schist D) slate 2. The recrystallization of unmelted material under high temperature and pressure
1. Which metamorphic rock will have visible mica crystals and a foliated texture? A) marble B) quartzite C) schist D) slate 2. The recrystallization of unmelted material under high temperature and pressure
Paleo Lab #4 - Sedimentary Environments
 Paleo Lab #4 - Sedimentary Environments page - 1. CHARACTERISTICS OF SEDIMENT Grain size and grain shape: The sizes and shapes of sedimentary particles (grains) are modified considerably during their transportation
Paleo Lab #4 - Sedimentary Environments page - 1. CHARACTERISTICS OF SEDIMENT Grain size and grain shape: The sizes and shapes of sedimentary particles (grains) are modified considerably during their transportation
Name Class Date. In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements.
 CHAPTER 5 Igneous Rocks SECTION 5.1 What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. basaltic
CHAPTER 5 Igneous Rocks SECTION 5.1 What are igneous rocks? In your textbook, read about the nature of igneous rocks. Use each of the terms below just once to complete the following statements. basaltic
WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY
 WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY Brandon E. Schwab Department of Geology Humboldt State University
WORKING WITH ELECTRON MICROPROBE DATA FROM A HIGH PRESSURE EXPERIMENT CALCULATING MINERAL FORMULAS, UNIT CELL CONTENT, AND GEOTHERMOMETRY Brandon E. Schwab Department of Geology Humboldt State University
Lecture 6. Physical Properties. Solid Phase. Particle Composition
 Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Examining Minerals and Rocks
 Examining Minerals and Rocks What is a mineral? A mineral is homogenous, naturally occurring substance formed through geological processes that has a characteristic chemical composition, a highly ordered
Examining Minerals and Rocks What is a mineral? A mineral is homogenous, naturally occurring substance formed through geological processes that has a characteristic chemical composition, a highly ordered
Geology 229 Engineering Geology. Lecture 6. Basic Rock Classification and Engineering Considerations (West, Chs. 2, 3, 4, 5)
 Geology 229 Engineering Geology Lecture 6 Basic Rock Classification and Engineering Considerations (West, Chs. 2, 3, 4, 5) Outline of this Lecture 1. Rock types and rock cycle 2. Geological and engineering
Geology 229 Engineering Geology Lecture 6 Basic Rock Classification and Engineering Considerations (West, Chs. 2, 3, 4, 5) Outline of this Lecture 1. Rock types and rock cycle 2. Geological and engineering
Quiz 1. 3) Which of the following planetary bodies has the least number of impact craters on its surface? A) Mercury B) Mars C) the Moon D) Earth
 Quiz 1 1) Earth's atmosphere is unique among the moons and planets in that A) it has a nitrogen (N2) rich atmosphere. B) it is rich in oxygen (O2) and nitrogen (N2). C) it is rich in carbon dioxide because
Quiz 1 1) Earth's atmosphere is unique among the moons and planets in that A) it has a nitrogen (N2) rich atmosphere. B) it is rich in oxygen (O2) and nitrogen (N2). C) it is rich in carbon dioxide because
The structural formula of a hydrous amphibole.
 717 The structural formula of a hydrous amphibole. By G. D. NIC~OLLS, B.A., Ph.D., F.G.S. and J. ZVSSMAN, M.A., Ph.D. Department of Geology, University of Manchester. T [Read January 27, 1955.] HE majority
717 The structural formula of a hydrous amphibole. By G. D. NIC~OLLS, B.A., Ph.D., F.G.S. and J. ZVSSMAN, M.A., Ph.D. Department of Geology, University of Manchester. T [Read January 27, 1955.] HE majority
Sediment. Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface
 Sediment Some basic terminology Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface Erosion: removal of weathered rock and minerals from one place to
Sediment Some basic terminology Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface Erosion: removal of weathered rock and minerals from one place to
Crust Elements. Elements of Earth. Minerals. Crystals. Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air
 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
