Loudon Chapter 9 Review: Reactions of Alkyl Halides Jacquie Richardson, CU Boulder Last updated 5/1/2016
|
|
|
- Erin Griffin
- 5 years ago
- Views:
Transcription
1 Chapter 9 covers reactions you can do with alkyl halides. For the most part, these break down into two categories: substitution and elimination. Substitution results in replacing the halogen with some other group. Elimination results in removing the halogen, along with a hydrogen on a neighboring atom (the β ), to create a new double bond. There are several terms that are useful to know here: The substrate is the molecule having this reaction performed on it. The leaving group or LG is the group that dissociates from the substrate. In this chapter, all the leaving groups are halogens. The base/nucleophile is the molecule that attacks the substrate. If it performs substitution, then it s acting as a nucleophile (because it s attacking a C atom); if it performs elimination then it s acting as a base (because it s attacking an atom). Often it can do a mixture of both jobs: Reaction Kinetics The reactions we ll see can be subdivided based on kinetics (how fast the reaction occurs, or the reaction rate). Reaction rate is defined as: The rate is often set by the concentrations of one or more reactants; concentrations are usually given in square brackets. For a reaction like A + B C, the rate can be measured at various concentrations of each reactant. For example, if double the concentration of A or the concentration of B doubles the rate, then the rate law would be rate = k [A][B]. For a different reaction, perhaps doubling the concentration of A quadruples the rate but doubling the concentration of B has no effect. In this case the rate law would be rate = k [A] 2. Overall kinetic order is the sum of the powers in the rate law for a reaction. For rate = k [A][B], it s second-order overall (first-order in A, and first-order in B). For rate = k [A] 2, it would also be second-order overall (second-order in A, and zeroth-order in B). The order is determined by how many molecules are involved in the rate-determining step. First-order reactions involve only one molecule and are unimolecular, second-order reactions involve two and are bimolecular, and third-order reactions involve three and are termolecular. k, the rate constant, varies from reaction to reaction. A bigger k gives a faster reaction. It s controlled by the activation energy, G - a smaller activation energy gives bigger k. It s also controlled by temperature higher temperature gives a bigger k. The substitution and elimination reactions in this chapter each have one unimolecular and one bimolecular option. They are: S N1: First-order substitution E1: First-order elimination S N2: Second-order substitution E2: Second-order elimination Looking at the mechanism for each one will explain why it behaves the way it does. 1
2 S N2 The attacking nucleophile forms a bond to the substrate at the same time the leaving group is dissociating. This is a concerted, one-step reaction. Br S N 2 Nu Br Nu Br Nu Transition state- don't draw as part of the mech Since both the substrate and the nucleophile are involved in the RDS, the reaction is secondorder. This reaction has several interesting characteristics, as a result of its mechanism being what it is. Since the carbon has bonds or partial bonds to five atoms at once during the transition state, space is at a premium. The fewer R groups and the more s there are on the central carbon (in other words, the less sterically hindered the carbon is), the faster this reaction will go. The trend is Me > 1 > 2 > 3. Some examples of relative S N2 rates are given below these are just examples under a very specific set of conditions, but the same general trends hold for all S N2 reactions. Since the nucleophile attacks from the side opposite to the leaving group, inversion of stereochemistry occurs. We ve seen this before in the opening of bromonium rings it s similar to an umbrella turning inside-out. If the leaving group had a bold bond before, then the new group will have a dashed bond or vice-versa. This also means that as long as the leaving group and the incoming group have the same priority ranking in CIP rules, the molecule will convert from R to S or vice-versa at the attacked carbon. Why does S N2 happen this way? It has to do with the shapes of the frontier molecular orbitals involved. The OMO is the lone pair on the nucleophilic atom (the O in EtO - 2
3 in this example) and the LUMO is the bond that is about to break between the carbon and the leaving group (the C-Br σ*). For the reaction to occur, these orbitals must overlap each other. The shapes of the C-Br σ and σ* orbitals are shown here: The largest lobe of the σ* orbital is on C, pointing in the opposite direction from the Br. For the lone pair on the nucleophile to overlap with this, it must perform backside attack from about 180 away from the leaving group. This is why backside attack on carbon is required it s the only way for the orbitals to overlap properly. The S N2 mechanism looks very similar to an acid-base reaction: The only difference is that an alkyl group is getting transferred, instead of a proton. The rates for these reactions, though, are very different. Acid-base occurs almost instantaneously, while S N2 takes minutes to hours. This means that if both reactions are a possibility (as determined by relative pka values, like we saw in Ch. 3), acid-base will happen faster. owever, the product of the acid-base reaction can often go on to do its own S N2 reaction on the substrate, giving an unexpected side product. Nucleophilicity vs. Basicity: Basicity is how good something is at forming new bonds to, and nucleophilicity is how good something is at forming new bonds to something other than (in this case, C). We can use pka values to compare basicity. There s not a single number that describes nucleophilicity, but we can get an estimate of it by comparing S N2 rates under the same conditions. It turns out that nucleophilicity follows different trends in protic vs. aprotic solvents. In protic solvents: o If the charge-bearing atoms are within the same row of the periodic table, more basic = more nucleophilic. 3
4 o If the charge-bearing atoms are within the same column of the periodic table, more basic = less nucleophilic. ere are some examples within the same row. The S N2 rate constants are all for a reaction with C 3I in C 3O. Base/Nu pka S N2 rate C 3O x 10-4 PhO x 10-5 AcO x 10-6 F x 10-8 Note that even though three of these have the same charge-bearing atom, they all have different pka values and therefore different nucleophilicities. ere are some examples within the same column. The S N2 rates are all for the same reaction again. Base/Nu pka S N2 rate I x 10-3 Br x 10-5 Cl x 10-6 F x 10-8 So iodide (I - ) is a weak base but a really good nucleophile in protic solvents. Why is this? It has to do with hydrogen-bonding to protic solvents. Small atoms are the best at hydrogen bonds, especially when they re stronger bases. Unfortunately, this means they re so wrapped up in -bonds to the solvent molecules that they re not able to attack the substrate as effectively. owever, switching to an aprotic solvent reverses this trend! In aprotic solvents: o More basic = more nucleophilic, for both directions in periodic table ere are some rates again, for S N2 in two different solvents: C 3O (protic) and DMF (aprotic). Base/Nu pka S N2 rate in C 3O S N2 rate in DMF I x Br x Cl x F x 10-8 >3 By taking away the option of -bonding to solvent, not only are we speeding up the rates of all S N2 reactions, but we re also reversing the trend within a column of the periodic table. In general, aprotic solvents are the best choice for fast S N2 reactions, but for practical reasons (solubility, boiling point, etc). protic solvents are sometime used. Leaving group ability: since the C-LG bond breaks during the rate-determining step, it can really slow down the reaction if it s a difficult bond to break. This is based on bond strength for the C-LG bond, which is largely set by how stable the LG is with a negative charge after it leaves. Based on pka values for X acids, F - is the strongest base, so it s least stable with a negative charge, so it forms the strongest bond to C, so it s the worst leaving group. So for S N2 rates on alkyl halides, 4
5 R-F << R-Cl < R-Br < R-I E2 This is another second-order reaction, but an elimination instead of a substitution. The base removes the at the same time the LG dissociates. Like S N2, this is a concerted reaction everything happens at once. Since everything is happening at once, geometry is important here just like it is for S N2. You need antiplanar (also called antiperiplanar) geometry: the four atoms (, C, C, and the leaving group) are all in the same plane, with the and the leaving group pointing opposite directions. If you can t get antiplanar geometry, E2 can t happen. This comes up most often on rings, which are rotationally constrained more than linear chains. For E2 to happen on a cyclohexane, the leaving group and the must be up axial or down axial on adjacent carbons. Regiochemistry: often, there are multiple different protons that could be pulled off during an elimination, to form different alkenes. Zaitsev s Rule says that the more stable, substituted alkene will be formed as the major product. Almost all bases obey Zaitsev s Rule, with one major exception: bases which are large and sterically hindered, like t-butoxide. This is because, being large and hindered, it takes too much energy to squeeze them up against the substrate to pick off the proton that would give the best product. Instead, they go for the short-term of a lower activation energy and take the easiest proton to access the one that gives the less substituted alkene. For both these cases (Zaitsev and anti-zaitsev bases) there s usually not an overwhelming majority in favor of one product or another. Product ratios are usually 2 or 3 to 1. owever, this is still enough that we can more-or-less target the desired product by choosing the right base. 5
6 Leaving-groups effects: the same as for S N2. RF is the slowest, RI is the fastest. Kinetic isotope effect (KIE): For reasons based on lowest-state vibrational energy, C- bonds are just slightly weaker than C-D bonds. This means that if a C- bond gets broken during the RDS of a reaction, the reaction will happen slightly slower if we replace the with a D. This is called the primary deuterium isotope effect, and can be used to prove that the E2 mechanism involves losing an /D in the RDS. S N1/E1 These two reactions are covered together since they have so much in common. In both reactions, the leaving group dissociates first and leaves a carbocation behind, then the Base/Nu comes in to attack either C or. Since the RDS involves only the alkyl halide, it s first-order. For both of these reactions, the overall arrows are exactly the same as for S N2 or E2, but they re broken into multiple steps instead of all simultaneous. This means it s a stepwise reaction, rather than concerted. Most of the behavior of these reactions is determined by the fact that they go through carbocations. More substituted carbocations are more stable, so the reaction rates are the opposite of S N2: Me < 1 < 2 < 3. Leaving-groups effects: the same as for S N2. RF is the slowest, RI is the fastest. E1 & S N1 have the same rate-determining step, but different product-determining steps. Anything that speeds up E1 will also speed up S N1, so these two reactions usually occur together and give a mixture of products. E1 & S N1 are fastest in polar protic solvents. This is because the carbocation benefits from being stabilized by the solvent. If there s nothing more nucleophilic around, the solvent itself can react with the substrate this is solvolysis. E1 obeys Zaitsev s rule. owever, like we ve seen for carbocations previously, rearrangements are possible! (And for S N1 as well). 6
7 S N1: The substrate goes through a carbocation intermediate, so you d expect completely scrambled stereochemistry like for the carbocation reactions in Ch. 7. owever, this is not quite accurate! The inverted product is favored by a slight margin. This is because the leaving group dissociates, but it is still nearby, partially blocking access to one face of the carbocation. If the carbocation existed for a long time this wouldn t happen, because the leaving group would have time to drift away. The fact that it still has an effect on the outcome means that the carbocation is only in existence for a short time (~10-8 seconds). Predicting Reaction Outcomes ow can we predict which one of these four reactions will actually happen? We need to look at the three molecules involved: substrate, solvent, and Base/Nu. 1. Classify substrate as Me, 1 o, 2 o, or 3 o. Check for neopentyl effect (3 o β C). 2. Classify solvent as protic or aprotic. Protic solvents are generally things like RO, RCO 2, or 2O. Aprotic solvents are generally the ones that go by acronyms: DMSO, DMF, TF, DCM, chloroform, and acetone. In general, polar protic solvents favor E1 and S N1 reactions, while polar aprotic solvents favor E2 and S N2 reactions. Usually this preference is not strong enough to control the choice of reaction, but check for acidbase reactions with the Base/Nu. 3. Classify base/nucleophile as strong or weak base, good or poor nucleophile. Strong Bases (usually Weak Bases (usually in in aprotic solvents) protic solvents) Good Nucleophiles O -, RO - (if R isn t I -, Br -, S -, RS -, CN - -, N 3 bulky), RC C - Poor Nucleophiles Cl -, F -, RCO 2-, 2O, RO, RCO 2 Strong bases are technically anything with a pka greater than about 15. In general, anything with a negative charge on C, N, or O is strong, unless there s additional stabilization coming from somewhere (resonance, etc.). If it s a strong base then it s assumed to be a good nucleophile, unless it s very bulky like tbuo -. Weak bases are anything with a pka below 15. Weak base/good nucleophiles fall into two categories: those at the stronger end of the weak base categories like N 3 - (pka of 9.4) and CN - (pka of 4.7), and those with a minus charge on large atoms (I, Br or S). Weak base/poor nucleophiles are anything outside of this category, including molecules with no negative charge at all. 4. Use this information to select which mechanism(s) will occur. If you have a strong base: You must do S N2 or E2 only! Carbocations (from E1 or S N1) can t exist in the presence of strong bases. E2 is the default, unless both the 7
8 substrate and the base/nucleophile are unhindered. owever, if S N2 turns out to be the predicted mechanism, it can still be blocked by the neopentyl effect, giving NR. Strong base/good nucleophile: o S N2 on Me and 1 o S N2/E2 on 2 o E2 on 3 Strong base/poor nucleophile: o S N2 on Me o E2 on 1, 2, 3 If you have a weak base: E1/S N1 mix is the default, but S N2 can happen under the right circumstances. Weak base/good nucleophile: o S N2 on Me, 1, 2 o S N1/E1 on 3 Weak base/poor nucleophile: o S N2 on Me, 1 o S N1/E1 on 2, 3 5. Apply this mechanism to the substrate. In example below, the substrate is 2 o, the solvent is protic, and the Base/Nu is a strong base/good nucleophile. This combination gives you a mixture of E2 and S N2 as the favored outcome. Using S N2 gives you the first product shown, and E2 gives you the second product. ere, the solvent is also working as the base/nucleophile. This is an example of solvolysis. You have a 1 o substrate, protic solvent, and weak base/poor nucleophile, giving you an S N2 outcome. WB/PN, protic S N 2 1 o Br 2 O ere, you have a 2 o substrate, protic solvent, and weak base/poor nucleophile, giving you an S N1/E1 outcome. O Sometimes a predicted outcome will be blocked by other reasons. Even though an S N1/E1 mix is predicted here, the E1 product isn t formed because it violates Bredt s rule. 8
9 Organometallics Anything with a bond between a carbon atom and a metal atom is an organometallic compound. Two useful types are Grignard and organolithiums, which are both made from alkyl halides. The mechanism for this goes by a complicated single-electron-transfer mechanism that we won t cover. For Grignards, you must have some kind of ether solvent (usually TF or diethyl ether). For organolithiums, ethers or alkane solvents like hexanes will work. The organometallic that s formed has a big delta negative charge on carbon, which makes it act like a very, very strong base in fact, it can be drawn as an ionic compound or a covalent compound, since it s midway in between. They react strongly with anything protic: This can be used as a way to turn alkyl halides into alkanes, in two steps. This is the first example we ll see of a multistep synthesis: using the product of one reaction as a starting material for the next. We can also use this to label molecules with deuterium in selected places, by using D 2O instead of 2O. Carbenes and Carbenoids Both the eliminations we looked at were β eliminations- you lose an and an LG from adjacent carbons. It s also possible to do an α elimination, where you lose an and an LG from the same carbon. These only happen under specific circumstances. To make this happen, you need a substrate that s good at stabilizing a minus charge, and a strong, bulky base. This creates a carbene, which has an unfilled octet. It behaves like a carbon with a negative charge and a positive charge at the same time, although you shouldn t draw it this way. It s a neutral carbon with both an empty orbital (so it s a great electrophile) and a lone pair (so it s a great nucleophile). The one reaction that we ll see it doing involves both behaviors at the same time. This is the same mechanism as that simultaneous attack/back-attack thing 9
10 we used to make bromonium ions, only the three-membered ring it makes is permanent. Since we re making an all-carbon cyclopropane ring, this is called a cyclopropanation. This particular reaction creates a ring with two halogens attached to one corner, but what if we don t want those halogens? In that case, we can use Simmonds-Smith cyclopropanation instead. Combining diiodomethane with zinc-copper couple creates something that s very similar to a Grignard, but with Zn instead of Mg. It s not a true carbene - it doesn t have a full positive and negative charge, but it does have sizeable delta positive (due to the bond to iodine) and delta negative (due to the bond to zinc) charges. For this reason, it behaves like a milder carbene, and is called a carbenoid. Again, it does the same attack and back-attack to make a three-membered ring. Radical halogenations of alkanes This is a way to create alkyl halides from alkanes (not from alkenes, which we saw in Ch. 4 & 5). ere, instead of adding an and a Br to a double bond, we re pulling an off the molecule and replacing it with a halogen. No radical initiator is needed the dihalogen will split into radicals on its own, as long as you use UV light (written as hν), or heat (written as ). 1. Initiation Br Br 2. Propagation Br 2Br C 2 I 2 Zn-Cu TF I Br Br 3. Termination: any step that involves 2 radicals getting together, for example: ZnI Br + Br This works best for chlorine and bromine. For iodine it s too endothermic and reacts too slowly, and for fluorine it s too exothermic and goes out-of-control until most s are replaced. Bromine is significantly endothermic, so it s slow and energy-poor. For this reason, bromine will carefully select which to pull off so that only the most stable radicals are formed. The net result is that radical halogenations with Br 2 only adds a Br at the most 10
11 substituted carbons. Chlorine, on the other hand, is much less endothermic and can afford to create radicals anywhere. It s a lot less predictable and basically useless unless every on the molecule is equivalent. Multistep Synthesis Synthesis involves stringing together multiple reactions to get from a starting material to a target molecule or final product, via one or more synthetic intermediates. We only care about showing overall reactions here, no arrow-pushing mechanisms. Now that we ve covered reactions that create alkyl halides (in Ch. 4 & 5) and reactions that use them, we can start putting them together for synthesis. A common case is where we have a functional group on one carbon, and we want to end up with a functional group on a neighboring carbon instead. The only way we know of (at present) to gain some kind of functional group handle on the rightmost carbon is by forming an alkene between the two carbons by doing an elimination, and then adding things back to the alkene: Now we can choose between Zaitsev/anti-Zaitsev eliminations, and Markovnikov/anti- Markovnikov additions, to control the regiochemistry of both reactions. The elimination in this case needs to be anti-zaitsev, and the addition needs to be anti-markovnikov. What about this synthesis? The elimination is neither Zaitsev nor anti-zaitsev, since there s only one choice. owever, we need to be careful this is a 1 alkyl halide and will undergo S N2 if we use a strong base that s not hindered. For this reason, we still use tbuona. ow about this one? 11
12 We need to add a group anti-markovnikov as the last step, but we haven t covered any ways to add an OAc group. Instead, we can add a bromine in the correct location first, and then replace it later by an S N2 reaction. Bromine is a very convenient placeholder atom, since we have control over where it adds to the alkene (it s the only halogen where we can choose Markovnikov or anti-markovnikov), and it s also easy to replace afterwards (since it s such a good LG). We can use some protic solvent to promote S N2. One more synthesis: We only know one reaction that can do anything with alkanes: radical halogenation. Our first step will be to add a bromine atom to the most substituted carbon. From there, we can eliminate to make an alkene, and then perform a Simmonds-Smith cyclopropanation. Sometimes it s easiest to work backwards from the target this is called retrosynthetic analysis. Often this is shown with a two-line retrosynthetic arrow, which basically means is synthesized from and can also be shown by a regular arrow pointing in the opposite direction. Something that s very helpful for synthesis is keeping track of all the functional group interconversions. Appendix V in Loudon has a good listing of which reactions we can use to get to and from a given functional group this is a good place to review the methods we ve covered so far. Finally, one more convention: the cut here or disconnect line shown as either a slightly wavy line or a straight line with a knob at each end. This can be used in either the forward or the backwards direction. 12
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
7. Haloalkanes (text )
 2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
Loudon Chapter 14 Review: Reactions of Alkynes Jacquie Richardson, CU Boulder Last updated 1/16/2018
 An alkyne is any molecule with a triple bond between two carbon atoms. This triple bond consists of one σ bond and two π bonds: the σ bond exists on a straight line between carbon atoms, while one π bond
An alkyne is any molecule with a triple bond between two carbon atoms. This triple bond consists of one σ bond and two π bonds: the σ bond exists on a straight line between carbon atoms, while one π bond
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
Nucleophilic Substitution and Elimination
 Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Some Arrow-Pushing Guidelines (Section 1.14) 1. Arrows follow electron movement.
 Chem 350 Jasperse Ch. 1 Notes 1 Note: The headers and associated chapters don t actually jive with the textbook we are using this summer. But otherwise this highlights a lot of the chemistry from Organic
Chem 350 Jasperse Ch. 1 Notes 1 Note: The headers and associated chapters don t actually jive with the textbook we are using this summer. But otherwise this highlights a lot of the chemistry from Organic
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Chemistry CHM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl Halides: Substitution Reactions - Chapter 6 (Wade)
 rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
rganic Chemistry CM 314 Dr. Laurie S. Starkey, Cal Poly Pomona Alkyl alides: Substitution Reactions - Chapter 6 (Wade) Chapter utline I. Intro to RX (6-1 - 6-7) II. Substitution Reactions A) S N 2 (6-8,
Loudon Chapter 19 Review: Aldehydes and Ketones CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Loudon Chapter 10 Review: Alcohols & Thiols Jacquie Richardson, CU Boulder Last updated 4/26/2016
 Alcohols (R) and thiols (RS) have many reactions in common with alkyl halides, but they don t do everything exactly the same. The main difference between this and alkyl halide chemistry is that unlike
Alcohols (R) and thiols (RS) have many reactions in common with alkyl halides, but they don t do everything exactly the same. The main difference between this and alkyl halide chemistry is that unlike
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 11, Part 1: Polar substitution reactions involving alkyl halides
 hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Chapter 9. Nucleophilic Substitution and ß-Elimination
 Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Essential Organic Chemistry. Chapter 9
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Walden discovered a series of reactions that could interconvert (-)-malic acid and (+)-malic acid.
 Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Alkyl halides are polarized in the C-X bond, making carbon δ+ (electrophilic). A nucleophilecan attack this carbon, displacing
Chapter 11: Reactions of alkyl halides: nucleophilic substitutions and eliminations Alkyl halides are polarized in the C-X bond, making carbon δ+ (electrophilic). A nucleophilecan attack this carbon, displacing
Loudon Chapter 17 Review: Allylic/Benzylic Reactivity
 Chapter 17 is all about reactions that happen at the position one away from an aromatic ring, or one away from a double bond. These are called the benzylic and allylic positions respectively. Benzyl and
Chapter 17 is all about reactions that happen at the position one away from an aromatic ring, or one away from a double bond. These are called the benzylic and allylic positions respectively. Benzyl and
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2018
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
CONCERTED sp 2 H. HO Et
 Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
BSc. II 3 rd Semester. Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1
 BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
C h a p t e r S e v e n : Substitution Reactions S N 2 O H H H O H H. Br -
 C h a p t e r S e v e n : Substitution Reactions Br Br S N 2 CM 321: Summary of Important Concepts YConcepts for Chapter 7: Substitution Reactions I. Nomenclature of alkyl halides, R X A. Common name:
C h a p t e r S e v e n : Substitution Reactions Br Br S N 2 CM 321: Summary of Important Concepts YConcepts for Chapter 7: Substitution Reactions I. Nomenclature of alkyl halides, R X A. Common name:
Substitution and Elimination reactions
 PART 3 Substitution and Elimination reactions Chapter 8. Substitution reactions of RX 9. Elimination reactions of RX 10. Substit n/elimin n of other comp ds 11. Organometallic comp ds 12. Radical reactions
PART 3 Substitution and Elimination reactions Chapter 8. Substitution reactions of RX 9. Elimination reactions of RX 10. Substit n/elimin n of other comp ds 11. Organometallic comp ds 12. Radical reactions
Elimination reactions
 Chapter 9 Elimination reactions E2 and E1 reactions Competition between S N and E Elimination reactions Ch 9 #2 elimination and/or substitution 2 mechanisms ~ E2 and E1 E2: bimolecular elimination rxn
Chapter 9 Elimination reactions E2 and E1 reactions Competition between S N and E Elimination reactions Ch 9 #2 elimination and/or substitution 2 mechanisms ~ E2 and E1 E2: bimolecular elimination rxn
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Homework problems Chapters 6 and Give the curved-arrow formalism for the following reaction: CH 3 OH + H 2 C CH +
 omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
Chapter 7 Substitution Reactions 7.1 Introduction to Substitution Reactions Substitution Reactions: two reactants exchange parts to give new products
 hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2015
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
Loudon Chapter 15 Review: Dienes and Aromaticity Jacquie Richardson, CU Boulder Last updated 1/28/2019
 This chapter looks at the behavior of carbon-carbon double bonds when several of them are in the same molecule. There are several possible ways they can be grouped. Conjugated dienes have a continuous
This chapter looks at the behavior of carbon-carbon double bonds when several of them are in the same molecule. There are several possible ways they can be grouped. Conjugated dienes have a continuous
Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
+ + CH 11: Substitution and Elimination Substitution reactions
 C 11: Substitution and Elimination Substitution reactions Things to sort out: Nucleophile Electrophile -- > substrate Leaving Group S N 2 S N 1 E 1 E 2 Analysis Scheme Kinetics Reaction profile Substrates
C 11: Substitution and Elimination Substitution reactions Things to sort out: Nucleophile Electrophile -- > substrate Leaving Group S N 2 S N 1 E 1 E 2 Analysis Scheme Kinetics Reaction profile Substrates
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
Reactions of Alkyl Halides with Nucleophiles and Bases a substitution reaction
 Reactions of Alkyl Halides with Nucleophiles and Bases a substitution reaction Nucleophilic substitution and base induced elimination are among most widely occurring and versatile reaction types in organic
Reactions of Alkyl Halides with Nucleophiles and Bases a substitution reaction Nucleophilic substitution and base induced elimination are among most widely occurring and versatile reaction types in organic
Learning Guide for Chapter 7 - Organic Reactions I
 Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. cleophiles and Electrophiles V. Acids and Bases What a chemical
Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. cleophiles and Electrophiles V. Acids and Bases What a chemical
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Elimination Reactions Heating an alkyl halide with a strong base causes elimination of a. molecule of HX
 Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Loudon Chapter 7 Review: Cyclic Compounds Jacquie Richardson, CU Boulder Last updated 8/24/2017
 Compounds with a single ring are monocyclic. For example: Assuming they have no double or triple bonds, they each have one degree of unsaturation. This means that their formulas follow the pattern C nh
Compounds with a single ring are monocyclic. For example: Assuming they have no double or triple bonds, they each have one degree of unsaturation. This means that their formulas follow the pattern C nh
Chapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution
 hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
Elimination Reactions. Chapter 6 1
 Elimination Reactions Chapter 6 1 E1 Mechanism Step 1: halide ion leaves, forming a carbocation. Step 2: Base abstracts H + from adjacent carbon forming the double bond. Chapter 6 2 E1 Energy Diagram E1:
Elimination Reactions Chapter 6 1 E1 Mechanism Step 1: halide ion leaves, forming a carbocation. Step 2: Base abstracts H + from adjacent carbon forming the double bond. Chapter 6 2 E1 Energy Diagram E1:
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
Alkyl Halides. Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group.
 Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat #
 Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
The Electrophile. S N 2 and E2 least stable most stable least hindered most hindered. S N 1 and E1. > x > >
 The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
10. Organohalides. Based on McMurry s Organic Chemistry, 7 th edition
 10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
Lab 11 Guide: Nucleophilic Substitution (Nov 10 16)
 Lab 11 Guide: Nucleophilic Substitution (Nov 10 16) Nucleophilic Substitution of Alkyl Halides, Exp. 17A, B, and C, pages 187-192 in Taber This week you will be doing examining real life S N 1 and S N
Lab 11 Guide: Nucleophilic Substitution (Nov 10 16) Nucleophilic Substitution of Alkyl Halides, Exp. 17A, B, and C, pages 187-192 in Taber This week you will be doing examining real life S N 1 and S N
Dr. Anand Gupta Mr Mahesh Kapil
 Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
Lecture 21 Organic Chemistry 1
 CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
ORGANIC - CLUTCH CH. 8 - ELIMINATION REACTIONS.
 !! www.clutchprep.com CONCEPT: THE E2 MECHANISM A strong nucleophile reacts with an inaccessible leaving group to produce beta-elimination in one-step. E2 Properties (Circle One) Nucleophile = Strong /
!! www.clutchprep.com CONCEPT: THE E2 MECHANISM A strong nucleophile reacts with an inaccessible leaving group to produce beta-elimination in one-step. E2 Properties (Circle One) Nucleophile = Strong /
Chapter 8 Reactions of Alkenes
 Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Classes of Halides. Chapter 6 Alkyl Halides: Nucleophilic Substitution and Elimination. Polarity and Reactivity. Classes of Alkyl Halides
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
The Study of Chemical Reactions. Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order.
 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
CHEM Lecture 7
 CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
Reaction mechanism 1/17/19. HCl. HCl. 3 radical can form here. Br 2. hint!
 If the reagents or reaction conditions favor radicals, then it is likely to be a radical reaction. Look for hints like peroxide, AIBN, NBS, hn (UV light). 3 radical can form here Reaction mechanism How
If the reagents or reaction conditions favor radicals, then it is likely to be a radical reaction. Look for hints like peroxide, AIBN, NBS, hn (UV light). 3 radical can form here Reaction mechanism How
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 9:Nucleophiles & Substitution Reactions
 1. a. Place the following nucleophiles in order of strength (1= strongest; 3 = weakest). i. ii. b. Place the following in order of leaving group ability (1= best; 7 = worst). (A pka table may help you!)
1. a. Place the following nucleophiles in order of strength (1= strongest; 3 = weakest). i. ii. b. Place the following in order of leaving group ability (1= best; 7 = worst). (A pka table may help you!)
Chapter 8 Alkyl Halides and Elimination Reactions
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 8 Alkyl Halides and Elimination Reactions Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 8 Alkyl Halides and Elimination Reactions Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Chapter 6 Chemical Reactivity and Mechanisms
 Chapter 6 Chemical Reactivity and Mechanisms 6.1 Enthalpy Enthalpy (ΔH or q) is the heat energy exchange between the reaction and its surroundings at constant pressure Breaking a bond requires the system
Chapter 6 Chemical Reactivity and Mechanisms 6.1 Enthalpy Enthalpy (ΔH or q) is the heat energy exchange between the reaction and its surroundings at constant pressure Breaking a bond requires the system
1-What is substitution reaction? 2-What are can Nucleophilic Substitution Reaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2
 1-What is substitution reaction? 2-What are can Nucleophilic Substitution eaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2 1- SUBSTITUTION EACTIONS 1-Substitution eaction In this type
1-What is substitution reaction? 2-What are can Nucleophilic Substitution eaction? 3- SN1 reaction. 4-SN2 reaction 5- mechanisms of SN1&SN2 1- SUBSTITUTION EACTIONS 1-Substitution eaction In this type
Classes of Alkenes. Alkenes and Alkynes. Saturated compounds (alkanes): Have the maximum number of hydrogen atoms attached to each carbon atom.
 Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Loudon Chapter 18 Review: Vinyl/Aryl Reactivity Jacquie Richardson, CU Boulder Last updated 2/21/2016
 Chapter 18 covers leaving groups that are directly attached to double-bonded sp 2 carbons. These molecules don t do most of the regular alkyl halide chemistry from Ch. 9 (S N1/ S N2/E1), but they can do
Chapter 18 covers leaving groups that are directly attached to double-bonded sp 2 carbons. These molecules don t do most of the regular alkyl halide chemistry from Ch. 9 (S N1/ S N2/E1), but they can do
Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid
![Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid](/thumbs/95/123929992.jpg) Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
PAPER No. 5: REACTION MECHANISM MODULE No. 2: Types of Organic Reaction Mechanisms
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
CHEMISTRY Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay
 EMISTRY 2600 Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay rganic Reactions (EM 2500 Review) Most reactions in organic chemistry fall into one (or more)
EMISTRY 2600 Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay rganic Reactions (EM 2500 Review) Most reactions in organic chemistry fall into one (or more)
Writing a Correct Mechanism
 Chapter 2 1) Balancing Equations Writing a Correct Mechanism 2) Using Arrows to show Electron Movement 3) Mechanisms in Acidic and Basic Media 4) Electron rich Species: Nucleophile or Base? 5) Trimolecular
Chapter 2 1) Balancing Equations Writing a Correct Mechanism 2) Using Arrows to show Electron Movement 3) Mechanisms in Acidic and Basic Media 4) Electron rich Species: Nucleophile or Base? 5) Trimolecular
Dehydrohalogenation of Alkyl Halides E2 and E1 Reactions in Detail
 Dehydrohalogenation of Alkyl Halides E2 and E1 Reactions in Detail b-elimination Reactions Overview dehydration of alcohols: X = H; Y = OH dehydrohalogenation of alkyl halides: X = H; Y = Br, etc. X C
Dehydrohalogenation of Alkyl Halides E2 and E1 Reactions in Detail b-elimination Reactions Overview dehydration of alcohols: X = H; Y = OH dehydrohalogenation of alkyl halides: X = H; Y = Br, etc. X C
ORGANIC - EGE 5E CH. 7 - NUCLEOPHILIC SUBSTITUTION AND ELIMINATION REACTIONS
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO SUBSTITUTION Previously, we discussed the various ways that acids could react with bases: Recall that in these mechanisms, electrons always travel from density
!! www.clutchprep.com CONCEPT: INTRODUCTION TO SUBSTITUTION Previously, we discussed the various ways that acids could react with bases: Recall that in these mechanisms, electrons always travel from density
Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms
 Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms Dr. Solomon Derese 1 A chemical reaction is the transformation of one chemical or collection of chemicals into another
Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms Dr. Solomon Derese 1 A chemical reaction is the transformation of one chemical or collection of chemicals into another
Química Orgânica I. Organic Reactions
 Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
1.13 Acid-Base Reactions: Lone-Pair Donors & Acceptors
 1.13 Acid-Base Reactions: Lone-Pair Donors & Acceptors I, Cl, N 3, 3 P 4 pka 10 to 5 Super strong acids 3 + pka 1.7 RC 2 pka ~ 5 acids Ph pka ~ 10 get 2, R pka ~ 16 weaker RCC (alkynes) pka ~ 26 RN 2 pka
1.13 Acid-Base Reactions: Lone-Pair Donors & Acceptors I, Cl, N 3, 3 P 4 pka 10 to 5 Super strong acids 3 + pka 1.7 RC 2 pka ~ 5 acids Ph pka ~ 10 get 2, R pka ~ 16 weaker RCC (alkynes) pka ~ 26 RN 2 pka
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2
 Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Study of Chemical Reactions
 Study of Chemical Reactions Introduction to Mechanisms There are four different types of organic reactions: Additions Eliminations Substitutions Rearrangements 149 Addition Reactions Occur when 2 reactants
Study of Chemical Reactions Introduction to Mechanisms There are four different types of organic reactions: Additions Eliminations Substitutions Rearrangements 149 Addition Reactions Occur when 2 reactants
Physical Properties: Structure:
 Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
What are radicals? H. Cl. Chapter 10 Radical Reactions. Production of radicals. Reactions of radicals. Electronic structure of methyl radical
 What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations
 11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
Reactions SN2 and SN1
 Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
Reactions SN2 and SN1 Reactivity: Functional groups can be interconverted using a great variety of reagents. Millions of organic molecules have been synthesized via a series of functional-group interconversions.
(CH 3 ) 3 COH. CH 3 ONa
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Chem 341 Jasperse Ch Handouts 1
 Chem 341 Jasperse Ch. 5 + 10 Handouts 1 Ch. 5 The Study of Chemical Reactions 5.1 Four general types of chemical reactions 1. Addition reactions 2. Elimination Reactions 3. Substitution Reactions 4. Rearrangement
Chem 341 Jasperse Ch. 5 + 10 Handouts 1 Ch. 5 The Study of Chemical Reactions 5.1 Four general types of chemical reactions 1. Addition reactions 2. Elimination Reactions 3. Substitution Reactions 4. Rearrangement
Chapter 11: Nucleophilic Substitution and Elimination Walden Inversion
 hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
hapter 11: Nucleophilic Substitution and Elimination Walden Inversion (S)-(-) Malic acid [a] D = -2.3 Ag 2, 2 Pl 5 l Ag 2, 2 ()-2-hlorosuccinic acid l (-)-2-hlorosuccinic acid Pl 5 ()-() Malic acid [a]
Only five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those five.
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
LECTURE #14 Thurs., Oct.20, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
Learning Guide for Chapter 11 - Alkenes I
 Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Chapter 8. Alkenes and Alkynes II: Addition Reactions. Ch. 8-1
 hapter 8 Alkenes and Alkynes II: Addition Reactions h. 8-1 1. Addition Reactions of Alkenes E + E Nu Nu h. 8-2 1A. ow To Understand Additions to Alkenes This is an addition reaction: E Nu added across
hapter 8 Alkenes and Alkynes II: Addition Reactions h. 8-1 1. Addition Reactions of Alkenes E + E Nu Nu h. 8-2 1A. ow To Understand Additions to Alkenes This is an addition reaction: E Nu added across
PRACTICE PROBLEMS UNIT 8
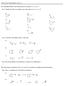 PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
PRACTICE PROBLEMS UNIT 8 8A. Identify halides and carbocations as being 1 o, 2 o, or 3 o 8A.1 assify the following halides and carbocations as 1 o, 2 o, or 3 o. 8A.2 Circle the most stable cation in each
UCF - ORGANIC CHEMISTRY 1 - PROF. DAOUDI UCF PROF. DAOUDI EXAM 3 REVIEW.
 UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
Chapter 3 An Introduction to Organic Reactions: Acids and Bases
 There are 4 types of Organic Reactions Chapter 3 An Introduction to Organic Reactions: SUBSTITUTION: ADDITION: X Y + A X A + Y Example Example A B + X Y A B X Y ELIMINATION There are 4 Types of Organic
There are 4 types of Organic Reactions Chapter 3 An Introduction to Organic Reactions: SUBSTITUTION: ADDITION: X Y + A X A + Y Example Example A B + X Y A B X Y ELIMINATION There are 4 Types of Organic
The carbon-carbon double bond is the distinguishing feature of alkenes.
 Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Chapter 15. Free Radical Reactions
 Grossman, CE 230 Chapter 15. Free Radical Reactions A free radical is a species containing one or more unpaired electrons. Free radicals are electrondeficient species, but they are usually uncharged, so
Grossman, CE 230 Chapter 15. Free Radical Reactions A free radical is a species containing one or more unpaired electrons. Free radicals are electrondeficient species, but they are usually uncharged, so
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams.
 2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
2311A and B Practice Problems to help Prepare for Final from Previous Marder Exams. Disclaimer.: Use only to help learn what you need to know and don t expect the final to be in the same form. 1 1. Short
Learning Guide for Chapter 7 - Organic Reactions I
 Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
