CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
|
|
|
- Britney Wilkerson
- 6 years ago
- Views:
Transcription
1 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1.
2 FIRST SEMESTER: KEY TO ASSIGNMENT 1 DUE DATE: 1 March 2016 UNIQUE NUMBER: Correct Answer: (2) It contains a σ molecular orbital formed by the overlap of one carbon sp 2 hybrid orbitals and one hydrogen sp 3 hybrid orbital. Based on the structure of the following compound, the above statement is INCORRECT because a hydrogen atom only has a 1s orbital available for overlap with another orbital. All the other statements are correct. 2. Correct Answer: (1) The above DOES NOT represent the appropriate hybridization state of the carbon atom in the structure shown. The C is part of a C=O bond and therefore it is sp 2 hybridized. All the other options represent the correct hybridization state of the carbon atom in the respective structure shown. 2
3 CE1502/201/1 3. Correct Answer: (2) C 2 C 2 N 2 and N The pair of compounds above is constitutional isomers because they have the same molecular formula (C 3 9 N). The other options: (1) ClC 2 C 2 and C 2 C 2 Cl The SAME COMPOUND (3) C 2 C 2 and ( ) 2 CC 2 Compound on the left has a molecular formula of C 4 10 and the one on the right has a molecular formula of C The compounds have no relationship. (4) C 2 OC 2 and C 2 C 2 O Compound on the left has a molecular formula of C 4 10 O and the one on the right has a molecular formula of C 3 8 O the compounds have no relationship. 4. Correct Answer: (4) O N The structure above is another resonance form of the following organic molecule: O N 3
4 The molecule undergoes resonance delocalization as follows: O N O N 5. Correct Answer: (3) 3 <4< 1<2 The above arrangement represents the compounds shown below in order of increasing Bronsted basicity (weakest first). 1. N O 3. Cl 4. 2 O A Bronsted base is a species that can accept a proton. The proton has no electrons therefore a Bronsted base must contain an electron pair which is available for donation to the proton. The more readily a compound is able to accept a proton, the stronger the base. If we compare the molecules N 3 and 2 O, the atoms N and O are both in the same row in the periodic table. Oxygen is more electronegative than N and will therefore attract its lone pair of electrons stronger than nitrogen. The nitrogen will donate its lone pair of electrons more readily to a proton than the oxygen atom, therefore the N 3 molecule is a stronger Bronsted base than 2 O. The Cl atom has 7 electrons in its outer shell. The Cl atom will not accept a proton, it will rather accept an electron in order to obtain an octet structure. The O ion is the strongest Bronsted base because when it accepts a proton 2 O is formed as the conjugate acid. The water molecule ( 2 O) is a weak acid. 4
5 CE1502/201/1 6. Correct Answer: (3) BCl 3 A Lewis base is a species that have a pair of electrons that can be donated to form a new bond. The B atom in BCl 3 does not have any lone pairs of electrons- it has an empty orbital which can accept an electron pair. BCl 3 is therefore a Lewis acid. The other options are explained below: (1) The oxygen atom has two lone pairs of electrons and therefore can act as a Lewis base. O (2) The nitrogen atom has one lone pair of electrons and therefore can act as a Lewis base. N (4) The oxygen atom has two lone pairs of electrons and therefore can act as a Lewis base. O 7. Correct Answer: (4) C C + The above is a CORRECT process of bond breaking. All the other options are incorrect. The corrected versions are shown below: (1) 5
6 (2) (3) The process should be the same as in (1) above. 8. Correct Answer: (4) 3,5-dimethylheptane The above is the correct IUPAC name of the following compound: C 2 CC 2 C C2 According to the IUPAC convention, the longest continuous carbon chain has 7 carbon atoms; 7 C s heptane. Begin numbering at a branch to give substituents the lowest possible number - in this case, from either side 3-methyl and 5-methyl. Two methyl groups 3,5-dimethyl; name the substituents in alphabetical order. 9. Correct Answer: (2) and The above pair of drawings represents the identical conformations of the same compound. The first structure is the Sawhorse representation of the conformation and the second structure is the Newman projection of the same compound. The two structures represent the same conformation because the groups and atoms attached to the C-2 and C-3 are in the same 6
7 CE1502/201/1 relationship with respect to each other in each structure. The other options: (1) Both conformations are staggered but they are different. and Anti conformation Gauche conformation (3) The two structures represent different conformations - in the structure of the left, the group (in top C) is between a group and a atom of the other C atom. In the structure of the right, the group of the one C is between a group and another group of the other C atom. 3 C and (4) The conformations below do not have the same molecular formula each conformation represents a different compound. The first Newman projection is a conformation of 2- methylbutane and the second Newman projection represents a conformation of n-butane. 3 C and 7
8 10. Correct Answer: (1) The above molecule has the MOST 1 o hydrogens. A discussion of all the options where the hydrogens, attached to primary carbons, are shown below: (1) A primary C (1 o C) is attached to one other C atom the hydrogens attached to a primary C are called 1 o hydrogens. There are four methyl groups and there are therefore twelve, (4x3 = 12), 1 o hydrogens. (2) There are three methyl groups and there are therefore nine, (3x3 = 9), 1 o hydrogens. (3) There are no primary C s all C s are secondary, 2 o, carbons and the compound has zero 1 o hydrogens. (4) There are two methyl groups and there are therefore six, (2x3 = 6), 1 o hydrogens. N.B. You should be able to identify the secondary and tertiary carbons/ hydrogens. 8
9 CE1502/201/1 11. Correct Answer: (2) secondary free radicals are more stable than primary free radicals The reaction of chlorine with butane in the presence of light gives primarily 2-chlorobutane and not 1- chlorobutane as the reaction product because secondary free radicals are more stable than primary free radicals. Butane can form primary and secondary carbon radical intermediates in the reaction. ; 1 o C radical 2 o C radical The more stable carbon radical intermediate forms the major product. 12. Correct Answer: (2) Br The above compound is the major monosubstituted organic product formed in the following reaction: ( ) 3 CC 2 C( ) 2 + Br 2 light Explanation: The above chemical equation can be rewritten as: 9
10 The halogenation reaction of alkanes proceeds via a radical intermediate. Possible radicals that can form in the reaction are: ; ; ; 1 o C radical 3 o C radical 2 o C radical 1 o C radical The tertiary carbon radical is the most stable radical and will produce the major product. 13. Correct Answer: (1) Cl 2 catalyst Cl + Cl The above reaction is a substitution reaction. The chemical equation is redrawn below: In the above reaction a Cl replaces/substitutes a atom. The other options: (2) The reaction below is an addition reaction: Br 2 Br (3) In this reaction isomerization takes place. Br acid catalyst 10
11 CE1502/201/1 (4) The reaction below is an elimination reaction: O acid catalyst + 2 O 14. Correct Answer: (1) 1-bromo-2-ethyl-4,4-dimethylcyclohexane Of the options given, above is the correct IUPAC name of the following compound: According to the IUPAC convention, the longest continuous carbon chain is a ring with 6 C s cyclohexane. Begin numbering at a position on the ring to give substituents the lowest possible number. There are two possibilities: 1) Begin numbering at the C attached to the methyl groups- then there are substituents on C-1, C-1, C-3 and C-4 2) Begin numbering at the C attached to the Br atom - then there are substituents on C-1, C-2, C-4 and C-4 On C-1 there is a bromine atom 1-bromo; on C-2 there is an ethyl group 2-ethyl; on C-4 there are two substituents 4,4-dimethyl; name substituents in alphabetical order. 15. Correct Answer: (3) The above nucleophilic substitution reaction takes place TE FASTEST. 11
12 The nucleophile, - O, is a strong nucleophile which promotes nucleophilic substitution reaction via the S N 2 reaction mechanism. The alkyl halide is a methyl alkyl bromide which can easily react in such a reaction. The other options: (1) Br O O + Br 2 O This alkyl halide is a secondary alkyl bromide which can undergo S N 2 reaction. owever, the reaction of primary alkyl halides is always faster than the reaction of primary alkyl halides under similar reaction conditions (2) Br O O 2 O + Br The alkyl halide is a tertiary alkyl halide which, due to crowding at the tertiary C, cannot undergo S N 2 reaction to give the product as shown. (4) C 2 Br C 2 O O + Br 2 O The alkyl halide is a primary alkyl halide which, compared to the alkyl halide in (3), has a larger group attached to the C where the nucleophile, - O, must attack. This S N 2 reaction will therefore take place at a slower rate than the reaction in (3). 12
13 CE1502/201/1 16. Correct Answer: (3) Under the given reaction conditions, the nucleophilic substitution reaction will proceed according to the S N 1 reaction mechanism. Thus each reaction will proceed via a carbocation intermediate. In the compound shown above, the Br group is the leaving group and will produce a primary carbocation which is the least stable carbocation. In the other examples: (1) S N 1 reaction takes place readily with this substrate although this is a secondary alkyl halide. This compound will form the following carboacation which is stabilized by resonance. (2) A resonance stabilized carbocation is formed in the reaction. (Draw resonance structures and redraw the Ph group as a ring structure) (4) A tertiary carbocation is formed which is also stabilized by resonance. 17. Correct Answer: (1) I O + O + I In the above reaction, the alkyl halide is a tertiary alkyl iodide which, due to steric hindrance (crowding), cannot undergo S N 2 reactions easily. Also, O is a poor nucleophile which promotes S N 1 reactions. 13
14 The other options: (2) The substrate is a primary alkyl halide and - S is a strong nucleophile both reagents promote S N 2 reactions. (3) The substrate is a secondary alkyl halide and - CN is a strong nucleophile both reagents promote S N 2 reactions. (4) The S N 2 reaction can take place. 18. Correct Answer: (4) PhS - The above anion has an excess of electrons which can easily be donates when it reacts as a nucleophile in an S N 2-type reaction. The PhS - ion will react the fastest with for example, C 2 -I in a substitution reaction because it is the strongest nucleophile. (1) 2 O is a weak nucleophile it has lone pairs of electrons on the electronegative O atom and the electrons are not readily donated. (2) The compound, Cl, has a polar C-Cl bond and tends to be electrophilic. It will thus not act as a nucleophile. (3) The structure of the anion, COO -, does not make sense. 19. Correct Answer: (2) 4,5,5-trimethyl-2-hexanol The above is the correct IUPAC name for the following compound: O 14
15 CE1502/201/1 According to the IUPAC convention, the longest continuous carbon chain that contains the O group has 6 C s hexane. Begin numbering at the end that will give the O substituent the lowest possible number; i.e. O is on C-2, change the ending from e to -ol 2-ol; three methyl groups on C-4, C-5, C-5 4,5,5-trimethyl. Name the substituents in alphabetical order. 20. Correct Answer: (1) C 2 C O In a tertiary alcohol the O group is attached to the tertiary carbon. The other options (2) A primary alcohol: C C 2 O (1) An aromatic alcohol phenol: O (2) A secondary alcohol: C O 15
16 21. Correct Answer: (1) O Alcohols undergo dehydration in the presence of a strong acid. In the presence of acid the O atom of becomes protonated to form the oxonium ion. The O group which is a poor leaving group is converted to a good leaving group. Loss of water gives a carbocation and the reaction which produces the most stable carbocation which will react the fastest. Intermediates formed in the options: (1) This compound leads to the formation of a tertiary carbocation which is very stable. (2) This compound leads to the formation of a primary carbocation which is very unstable. (3) This compound leads to the formation of a secondary carbocation which is less stable than a tertiary carbocation. (4) This compound forms of a secondary carbocation which is less stable than a tertiary carbocation. 22. Correct Answer: (3) O + Cl O Cl O + Cl The above represents the CORRECT mechanism for a nucleophilic substitution reaction specifically for a S N 2 reaction. All the other options are incorrect. 16
17 CE1502/201/1 23. Correct Answer: (4) + 2 O The above step is not part of the mechanism of the dehydration reaction. The reaction mechanism is: O O O + 2 O O + 2 O carbocation rearrangement + 2 O 17
18 24. Correct Answer: (4) The above compound has the highest boiling point because its molecules can undergo hydrogen bonding due to the presence of the O- bond. 25. Correct Answer: (2) 2-butanol According to the structure of each compound, below, the compound shown in (3) has a chiral (asymmetric) carbon atom because there are 4 different groups or atoms attached to C-2. (1) 3-Bromopentane Br (2) 2-butanol O (3) cyclopentanol O (4) 2-Methylpropane 26. Correct Answer: (2) (S)-hexan-3-ol The above name is the correct one according to the Cahn-Ingold-Prelog rules of the following compound: 18
19 CE1502/201/1 C 2 C 2 C 2 O According to the Cahn-Ingold-Prelog rules, assigning priorities to the groups/atoms around the chiral center: Group Priority O 1 C 2 C 2 2 C C 2 C O C 2 counter clockwise -S 27. Correct Answer: (3) Sodium ethoxide is a strong base and in the reaction with the tertiary alkyl bromide, E2 elimination occurs: 19
20 Br C C OEt 3 C + Br According to Zaitsev s rule, the product with the most substituted double bond will predominate. Therefore, the product formed above is the major product. The product formed (1) is a minor elimination product. The product shown in (2) is as a result of a nucleophilic substitution reaction. The product shown in (3) will not form in this reaction. 28. Correct Answer: (3) C Br + C 2 O C OC 2 ; S N 1 C 2 C 2 The alkyl halide is a tertiary alkyl bromide which will produce a stable carbocation intermediate. Therefore, the S N 1 reaction will occur. Explanation of the options: (1) This reaction is not an elimination reaction but a substitution reaction. (2) The reaction is an elimination reaction not a substitution reaction proceeds via the E2 mechanism. (3) This is the reaction of a tertiary alkyl halide and a poor nucleophile, C 2 O. These reagents undergo nucleophilic substitution reaction via the S N 1 reaction mechanism. (4) This is a substitution reaction and not an elimination reaction (it does not proceed via an E2 reaction mechanism). A primary alkyl halide forms an unstable carbocation and the reaction of a primary alkyl halide with a strong nucleophile like - O goes via the 20
21 CE1502/201/1 29. Correct Answer: (1) 3-methoxybutan-1-ol The above name corresponds to the following compound according to the IUPAC nomenclature: This compound has an O group and an O group this is an alcohol and the O group is a substituent. According to the IUPAC convention, the longest continuous carbon chain that contains the O group has 4 C s butane. Begin numbering at the end that will give the O substituent the lowest possible number; the O is on C-1, change ending -e to ol 1-ol; the O group is on C-2 2-methoxy. Write the name in alphabetical order. 30. Correct Answer: (4) The above reaction is the most appropriate to prepare the following compound: The reaction of an alkoxide with an alkyl halide proceed is a S N 2 reaction. Evaluation of the S N 2 reaction with each option: (1) This is a tertiary alkyl halide which undergoes S N 1 reaction instead of S N 2 reaction 21
22 (2) Under the reaction conditions, both reagents contain a poor leaving group which makes substitution to form the product very difficult. (3) The nucleophile cannot attack the carbon attached to the O group because the O group is a poor leaving group. The nucleophile is also a strong base and will abstract the from the O group. (4) This is a reaction between a strong nucleophile and a primary alkyl halide which contains a good leaving group, Br, therefore, the desired product will form. 22
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Homework problems Chapters 6 and Give the curved-arrow formalism for the following reaction: CH 3 OH + H 2 C CH +
 omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
Exam Analysis: Organic Chemistry, Midterm 1
 Exam Analysis: Organic Chemistry, Midterm 1 1) TEST BREAK DOWN: There are three independent topics covered in the first midterm, which are hybridization, structure and isomerism, and resonance. The test
Exam Analysis: Organic Chemistry, Midterm 1 1) TEST BREAK DOWN: There are three independent topics covered in the first midterm, which are hybridization, structure and isomerism, and resonance. The test
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7
 Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
Sevada Chamras, Ph.D. Glendale Community College Chemistry 105 Exam. 3 Lecture Notes Chapters 6 & 7 Description: Examples: 3 Major Types of Organic Halides: 1. Alkyl Halides: Chapter 6 (Part 1/2) : Alkyl
(CH 3 ) 3 COH. CH 3 ONa
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
The C-X bond gets longerand weakergoing down the periodic table.
 Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Physical Properties: Structure:
 Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Chapter 2: Alkanes MULTIPLE CHOICE
 Chapter 2: Alkanes MULTIPLE CHOICE 1. Which of the following orbitals is properly described as an antibonding orbital? a. sp + 1s d. sp 2 1s b. sp 2 + 1s e. sp 2 + sp 2 sp 3 + 1s D DIF: Easy REF: 2.2 2.
Chapter 2: Alkanes MULTIPLE CHOICE 1. Which of the following orbitals is properly described as an antibonding orbital? a. sp + 1s d. sp 2 1s b. sp 2 + 1s e. sp 2 + sp 2 sp 3 + 1s D DIF: Easy REF: 2.2 2.
1. What is the letter designation given to dumbbell shaped orbitals like the one depicted below?
 1. What is the letter designation given to dumbbell shaped orbitals like the one depicted below? 2. Which of the following does not have an octet of electrons surrounding the central atom? A. B 3 B. C
1. What is the letter designation given to dumbbell shaped orbitals like the one depicted below? 2. Which of the following does not have an octet of electrons surrounding the central atom? A. B 3 B. C
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
Introduction to Alkenes and Alkynes
 Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Class XI Chapter 13 Hydrocarbons Chemistry
 Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Chemistry 110. Bettelheim, Brown, Campbell & Farrell. Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes.
 Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes
 Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
The Final Learning Experience
 Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #5 Reactions of Alcohols and Related Reactions The Final Learning Experience Wednesday, December 20, 2000, 1:00-3:00 Name:
Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #5 Reactions of Alcohols and Related Reactions The Final Learning Experience Wednesday, December 20, 2000, 1:00-3:00 Name:
Chapter 17. Reactions of Aromatic Compounds
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides"
 Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Organic Chemistry Worksheets
 Highlight the single longest, continuous carbon-carbon chain. Note the alkyl branches that are connected to the root chain. Count the carbons in the root chain, starting from the end closest to the alkyl
Highlight the single longest, continuous carbon-carbon chain. Note the alkyl branches that are connected to the root chain. Count the carbons in the root chain, starting from the end closest to the alkyl
8. What is the slow, rate-determining step, in the acidcatalyzed dehydration of 2-methyl-2-propanol?
 CHEMISTRY 313-03 MIDTERM # 2 answer key October 25, 2011 Statistics: Average: 68 pts (68%); Highest: 100 pts (100%); Lowest: 30 pts (30%) Number of students performing at or above average: 56 (54%) Number
CHEMISTRY 313-03 MIDTERM # 2 answer key October 25, 2011 Statistics: Average: 68 pts (68%); Highest: 100 pts (100%); Lowest: 30 pts (30%) Number of students performing at or above average: 56 (54%) Number
CHAPTER 2. Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules
 CHAPTER 2 Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules 2-1 Kinetics and Thermodynamics of Simple Chemical Processes Chemical thermodynamics: Is concerned with the extent that
CHAPTER 2 Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules 2-1 Kinetics and Thermodynamics of Simple Chemical Processes Chemical thermodynamics: Is concerned with the extent that
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
The Electrophile. S N 2 and E2 least stable most stable least hindered most hindered. S N 1 and E1. > x > >
 The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
The Electrophile 1 Recall that electrophile means electron- loving. When considering substitution and elimination reactions we must consider the carbon attached to the leaving group. Is it a primary, secondary,
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Chapter 2: An Introduction to Organic Compounds
 Chapter : An Introduction to Organic Compounds I. FUNCTIONAL GROUPS: Functional groups with similar structure/reactivity may be "grouped" together. A. Functional Groups With Carbon-Carbon Multiple Bonds.
Chapter : An Introduction to Organic Compounds I. FUNCTIONAL GROUPS: Functional groups with similar structure/reactivity may be "grouped" together. A. Functional Groups With Carbon-Carbon Multiple Bonds.
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Chapter 7 Alkenes; Elimination Reactions
 hapter 7 Alkenes; Elimination Reactions Alkenes Alkenes contain a carbon-carbon double bond. They are named as derivatives of alkanes with the suffix -ane changed to -ene. The parent alkane is the longest
hapter 7 Alkenes; Elimination Reactions Alkenes Alkenes contain a carbon-carbon double bond. They are named as derivatives of alkanes with the suffix -ane changed to -ene. The parent alkane is the longest
Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid
![Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid](/thumbs/95/123929992.jpg) Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Chapter 7 Substitution Reactions
 Chapter 7 Substitution Reactions Review of Concepts Fill in the blanks below. To verify that your answers are correct, look in your textbook at the end of Chapter 7. Each of the sentences below appears
Chapter 7 Substitution Reactions Review of Concepts Fill in the blanks below. To verify that your answers are correct, look in your textbook at the end of Chapter 7. Each of the sentences below appears
CHEM120 - ORGANIC CHEMISTRY WORKSHEET 1
 EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
C C. sp 2. π M.O. atomic. orbitals. carbon 1. σ M.O. molecular. orbitals. H C C rotate D. D H zero overlap of p orbitals: π bond broken!
 Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BDE ~ 80 kcal/mol π = BDE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in
Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BDE ~ 80 kcal/mol π = BDE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in
Alkanes and Cycloalkanes
 Chapter 3 Alkanes and Cycloalkanes Two types Saturated hydrocarbons Unsaturated hydrocarbons 3.1 Alkanes Also referred as aliphatic hydrocarbons General formula: CnH2n+2 (straight chain) and CnH2n (cyclic)
Chapter 3 Alkanes and Cycloalkanes Two types Saturated hydrocarbons Unsaturated hydrocarbons 3.1 Alkanes Also referred as aliphatic hydrocarbons General formula: CnH2n+2 (straight chain) and CnH2n (cyclic)
10. Organohalides. Based on McMurry s Organic Chemistry, 7 th edition
 10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
10. Organohalides Based on McMurry s Organic Chemistry, 7 th edition What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain
CHEM Lecture 7
 CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Classes of Alkenes. Alkenes and Alkynes. Saturated compounds (alkanes): Have the maximum number of hydrogen atoms attached to each carbon atom.
 Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Chapter 3. Alkenes And Alkynes
 Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Alkenes. Dr. Munther A. M-Ali For 1 st Stage Setudents
 Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2014 Dr. Rainer Glaser
 Chemistry 2030 Survey of Organic Chemistry Fall Semester 2014 Dr. Rainer Glaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Thursday, September 18, 2014, 8:00 8:50 am Question 1. Atomic Structure
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2014 Dr. Rainer Glaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Thursday, September 18, 2014, 8:00 8:50 am Question 1. Atomic Structure
Chemistry 123: Physical and Organic Chemistry Topic 1: Organic Chemistry
 Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
CHEM 241 ALCOHOLS AND ALKYL HALIDES CHAP 5 ASSIGN
 CEM 241 ALCOOLS AND ALKYL ALIDES CAP 5 ASSIGN 1. What is the IUPAC name of the compound below? A. 3-isobutyl-2-hexanol B. 2-methyl-5-propyl-6-heptanol C. 2-methyl-5-(1-hydroxyethyl)octane D. 6-methyl-3-propyl-2-heptanol
CEM 241 ALCOOLS AND ALKYL ALIDES CAP 5 ASSIGN 1. What is the IUPAC name of the compound below? A. 3-isobutyl-2-hexanol B. 2-methyl-5-propyl-6-heptanol C. 2-methyl-5-(1-hydroxyethyl)octane D. 6-methyl-3-propyl-2-heptanol
What is the major product of the following reaction?
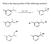 What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI - 87
 HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
Chapter 3. Organic Compounds: Alkanes and Their Stereochemistry
 Chapter 3. Organic Compounds: Alkanes and Their Stereochemistry Functional Group: Be able to identify and name any of the functional groups listed on Table 3.1, pages 76-77. Summary of important functional
Chapter 3. Organic Compounds: Alkanes and Their Stereochemistry Functional Group: Be able to identify and name any of the functional groups listed on Table 3.1, pages 76-77. Summary of important functional
(c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
 KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chem 3719 Klein Chapter Practice Problems
 Chem 379 Klein Chapter Practice Problems Dr. Peter Norris, 208 Klein Chapter Problems : Review of General Chemistry. Draw viable structures for molecules with the following molecular formulae. Remember
Chem 379 Klein Chapter Practice Problems Dr. Peter Norris, 208 Klein Chapter Problems : Review of General Chemistry. Draw viable structures for molecules with the following molecular formulae. Remember
Chapter 7: Alcohols, Phenols and Thiols
 Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
CHAPTER 16 - CHEMISTRY OF BENZENE: ELECTROPHILIC AROMATIC SUBSTITUTION
 CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
An alkane homolog differs only in the number of CH 2 groups. Example: butane: CH 3 CH 2 CH 2 CH 3 and pentane CH 3 CH 2 CH 2 CH 2 CH 3 are homolgs.
 Structure and Stereochemistry of Alkanes Reading: Wade chapter 3, sections 3-1- 3-9 Study Problems: 3-33, 3-37, 3-39, 3-40, 3-42 Key Concepts and Skills: Explain and predict trends in the physical properties
Structure and Stereochemistry of Alkanes Reading: Wade chapter 3, sections 3-1- 3-9 Study Problems: 3-33, 3-37, 3-39, 3-40, 3-42 Key Concepts and Skills: Explain and predict trends in the physical properties
Chapter 4 Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis"
 Chapter 4 Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis" Alkanes = saturated hydrocarbons" Simplest alkane = methane C 4" " We can build additional alkanes by adding
Chapter 4 Alkanes: Nomenclature, Conformational Analysis, and an Introduction to Synthesis" Alkanes = saturated hydrocarbons" Simplest alkane = methane C 4" " We can build additional alkanes by adding
Facebook: UCI ORganic Chemistry Peer Tutoring King 51 Fall 2017
 1 Organic Chemistry 51A Professor King Final Exam Review Session December 7 th, 2017 Will Cabanela (rcabanel@uci.edu) Amanda Pinski (apinski@uci.edu) Zachary Valley (zvalley@uci.edu) http://sites.uci.edu/ochemtutors
1 Organic Chemistry 51A Professor King Final Exam Review Session December 7 th, 2017 Will Cabanela (rcabanel@uci.edu) Amanda Pinski (apinski@uci.edu) Zachary Valley (zvalley@uci.edu) http://sites.uci.edu/ochemtutors
First Midterm Exam Monday, October 16, You may use model kits but no other material with chemical information without instructor approval.
 CH 334 Form A First Midterm Exam Monday, October 16, 2017 Name KEY You may use model kits but no other material with chemical information without instructor approval. Please do not use any electronic devices
CH 334 Form A First Midterm Exam Monday, October 16, 2017 Name KEY You may use model kits but no other material with chemical information without instructor approval. Please do not use any electronic devices
CHEM1902/ N-8 November Consider the following reaction sequences beginning with the carboxylic acid, E.
 CEM1902/4 2014--8 ovember 2014 Consider the following reaction sequences beginning with the carboxylic acid, E. 6 ame compounds E and G. E: propionic acid G: methyl propionate Propose structures for compounds
CEM1902/4 2014--8 ovember 2014 Consider the following reaction sequences beginning with the carboxylic acid, E. 6 ame compounds E and G. E: propionic acid G: methyl propionate Propose structures for compounds
CHE 275 NUCLEOPHILIC SUBSTITUTUION CHAP 8 ASSIGN. 1. Which best depicts the partial charges on methyl bromide and sodium methoxide?
 CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
16.1 Draw Newman projections for these staggered and three eclipsed conformations of 1, 2 - dichlorobutane as viewed downt he central C-C bond.
 CAPTER 16 Practice Exercises 16.1 Draw Newman projections for these staggered and three eclipsed conformations of 1, 2 - dichlorobutane as viewed downt he central C-C bond. 16.3 (a) The more stable conformation
CAPTER 16 Practice Exercises 16.1 Draw Newman projections for these staggered and three eclipsed conformations of 1, 2 - dichlorobutane as viewed downt he central C-C bond. 16.3 (a) The more stable conformation
HALOALKANES. Structure Contain the functional group C-X where X is a halogen (F,Cl,Br or I)
 aloalkanes AS3 1 ALOALKANES Structure ontain the functional group X where X is a halogen (F,l, or I) Types aloalkanes halogen is attached to an aliphatic skeleton alkyl group aloarenes halogen is attached
aloalkanes AS3 1 ALOALKANES Structure ontain the functional group X where X is a halogen (F,l, or I) Types aloalkanes halogen is attached to an aliphatic skeleton alkyl group aloarenes halogen is attached
Chapter Organic Chemistry Basics
 29 Chapter Organic Chemistry Basics 2. A similarity between optical and geometrical isomerism is that [2002] (a) each forms equal number of isomers for a given compound (b) if in a compound one is present
29 Chapter Organic Chemistry Basics 2. A similarity between optical and geometrical isomerism is that [2002] (a) each forms equal number of isomers for a given compound (b) if in a compound one is present
Chapter 3 AN INTRODUCTION TO ORGANIC COMPOUNDS NOMENCLATURE, PHYSICAL PROPERTIES, REPRESENTATION OF STRUCTURE AND
 ORGANIC CHEMISTRY, 2 ND EDITION PAULA YURKANIS BRUICE Chapter 3 AN INTRODUCTION TO ORGANIC COMPOUNDS NOMENCLATURE, PHYSICAL PROPERTIES, AND REPRESENTATION OF STRUCTURE RAED M. AL-ZOUBI, ASSISTANT PROFESSOR
ORGANIC CHEMISTRY, 2 ND EDITION PAULA YURKANIS BRUICE Chapter 3 AN INTRODUCTION TO ORGANIC COMPOUNDS NOMENCLATURE, PHYSICAL PROPERTIES, AND REPRESENTATION OF STRUCTURE RAED M. AL-ZOUBI, ASSISTANT PROFESSOR
Chapter 9. Nucleophilic Substitution and ß-Elimination
 Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Chapter 9 Nucleophilic Substitution and ß-Elimination Nucleophilic Substitution Nucleophile: From the Greek meaning nucleus loving. A molecule or ion that donates a pair of electrons to another atom or
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 19: Amines. Introduction
 Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Ethers & Epoxides. Chapter 5. Dr. Seham ALTERARY. Chem 340-2nd semester
 Ethers & Epoxides Chapter 5 Dr. Seham ALTERARY Chapter s out line Ethers Definition; General formula; Classification and Types Nomenclature - Common Names. - IUPAC Naming. Physical Properties General methods
Ethers & Epoxides Chapter 5 Dr. Seham ALTERARY Chapter s out line Ethers Definition; General formula; Classification and Types Nomenclature - Common Names. - IUPAC Naming. Physical Properties General methods
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
Organic Halogen Compounds
 8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
Organic Chemistry, 7 L. G. Wade, Jr. Chapter , Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Lesmahagow High School CfE Advanced Higher Chemistry
 Lesmahagow High School AHChemistry Organic Chemistry& Instrumental Analysis Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Alkanes, Alkenes and
Lesmahagow High School AHChemistry Organic Chemistry& Instrumental Analysis Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Alkanes, Alkenes and
trans-cyclooctene 4-fluoro-1-butanol 2-cyclohexenol 4. (5 pts) Provide structural formula for each of the following molecules: Br OH Cl OH
 CEMISTRY 313-01 MIDTERM # 2 answer key March 12, 2009 Statistics: Average: 72 pts (72%); ighest: 98 pts (98%); Lowest: 21 pts (21%) umber of students performing at or above average: 22 (50%) umber of students
CEMISTRY 313-01 MIDTERM # 2 answer key March 12, 2009 Statistics: Average: 72 pts (72%); ighest: 98 pts (98%); Lowest: 21 pts (21%) umber of students performing at or above average: 22 (50%) umber of students
Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008
 Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008 Lesson Date Assignment Lesson Objective Description Lesson Problems 4 14-Jan Chapter 1 Quiz Describe how bond polarity
Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008 Lesson Date Assignment Lesson Objective Description Lesson Problems 4 14-Jan Chapter 1 Quiz Describe how bond polarity
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Text Sections (N0 1.9, 9-11) Homework: Chapter 1:
 CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
3.1 Introduction to Organic Chemistry
 3.1 Introduction to Organic hemistry Organic hemistry is the study of carbon chemistry as carbon has the ability to join together in chains, rings, balls etc. arbon also joins with other elements easily
3.1 Introduction to Organic hemistry Organic hemistry is the study of carbon chemistry as carbon has the ability to join together in chains, rings, balls etc. arbon also joins with other elements easily
OChem1 Old Exams. Chemistry 3719 Practice Exams
 OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
20 Halogenoalkanes:substitution and elimination reactions
 20 alogenoalkanes:substitution and elimination reactions s to worked examples WE 20.1 Allylic halogenations (on p. 922 in Chemistry 3 ) 1-(Chloromethyl)benzene (benzyl chloride, PhC 2 ) is made on a large
20 alogenoalkanes:substitution and elimination reactions s to worked examples WE 20.1 Allylic halogenations (on p. 922 in Chemistry 3 ) 1-(Chloromethyl)benzene (benzyl chloride, PhC 2 ) is made on a large
CHEMISTRY 241 Section 004 EXAMINATION I TUESDAY, October 11, :30-11:50 AM Professor William P. Dailey NAME: QUESTIONS POINTS SCORE
 CEMISTRY 241 Section 004 EXAMIATI I TUESDAY, ctober 11, 2005 10:30-11:50 AM Professor William P. Dailey AME: Student ID number : QUESTIS PITS SCRE 1. 16 2. 10 3. 12 4. 12 5. 12 6. 8 7. 9 8. 9 9. 15 10.
CEMISTRY 241 Section 004 EXAMIATI I TUESDAY, ctober 11, 2005 10:30-11:50 AM Professor William P. Dailey AME: Student ID number : QUESTIS PITS SCRE 1. 16 2. 10 3. 12 4. 12 5. 12 6. 8 7. 9 8. 9 9. 15 10.
1) (100 pts) 5) (20 pts) 3) (35 pts) 4) (25pts. Total (200 pts) More Tutorial at
 Name: Perm number: Question 1) (100 pts) 2) (20 pts) 3) (35 pts) 4) (25pts 5) (20 pts) Total (200 pts) Your score 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
Name: Perm number: Question 1) (100 pts) 2) (20 pts) 3) (35 pts) 4) (25pts 5) (20 pts) Total (200 pts) Your score 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
Electrophilic Aromatic Substitution
 Chem 263 ct. 8, 2013 lectrophilic Aromatic Substitution Benzene appears to be a remarkably stable and unreactive compared to alkenes, such as cyclohexene or ethylene, or even alkanes, such as cyclohexane
Chem 263 ct. 8, 2013 lectrophilic Aromatic Substitution Benzene appears to be a remarkably stable and unreactive compared to alkenes, such as cyclohexene or ethylene, or even alkanes, such as cyclohexane
FIRST HOUR EXAMINATION
 Name: ANSWERS CEM 331 FIRST UR EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided. If I have to guess as to what or where
Name: ANSWERS CEM 331 FIRST UR EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided. If I have to guess as to what or where
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations
 11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
Alkanes. Introduction
 Introduction Alkanes Recall that alkanes are aliphatic hydrocarbons having C C and C H bonds. They can be categorized as acyclic or cyclic. Acyclic alkanes have the molecular formula C n H 2n+2 (where
Introduction Alkanes Recall that alkanes are aliphatic hydrocarbons having C C and C H bonds. They can be categorized as acyclic or cyclic. Acyclic alkanes have the molecular formula C n H 2n+2 (where
2. Which functional groups and structural features are present in the following molecule (strychnine)?
 Chapter 1-2: 1. Which of the following species has a negative charge but NO lone pair of valance shell nonbonding electrons? [all atoms have complete valance shell of electrons, but lone pairs are not
Chapter 1-2: 1. Which of the following species has a negative charge but NO lone pair of valance shell nonbonding electrons? [all atoms have complete valance shell of electrons, but lone pairs are not
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
Organic Chemistry. Unit 10
 Organic Chemistry Unit 10 Halides Primary Carbons Secondary Carbons Tertiary Carbons IMPORTANCE?? REACTIONS!! Benzene C6H6 Aromatic functional group - C6H5 (IUPAC name - phenyl) Substitution Reactions
Organic Chemistry Unit 10 Halides Primary Carbons Secondary Carbons Tertiary Carbons IMPORTANCE?? REACTIONS!! Benzene C6H6 Aromatic functional group - C6H5 (IUPAC name - phenyl) Substitution Reactions
Organic Chemistry Practice Problems: Solutions
 rganic Chemistry Practice Problems: Solutions 1. 2. a. B, A b. D, B c. A, D d. D, A a. Resonance b. Electronegativity of fluorine atoms F F c. Neither is very acidic, but the oxygen will help stabilise
rganic Chemistry Practice Problems: Solutions 1. 2. a. B, A b. D, B c. A, D d. D, A a. Resonance b. Electronegativity of fluorine atoms F F c. Neither is very acidic, but the oxygen will help stabilise
I. Multiple Choice Questions (Type-I)
 Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
CHAPTER 15. Practice exercises 15.1
 CAPTER 15 Practice exercises 15.1 15.3 (a) C 9 18 : isobutylcyclopentane C 11 22 : sec-butylcycloheptane C 6 2 : 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene 2,3-dimethyl-2-butene 3,3-dimethyl-1-butyne
CAPTER 15 Practice exercises 15.1 15.3 (a) C 9 18 : isobutylcyclopentane C 11 22 : sec-butylcycloheptane C 6 2 : 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene 2,3-dimethyl-2-butene 3,3-dimethyl-1-butyne
6-2 This exercise is worked out on page 220 as "Working with Concepts".
 Copyright 2009 James K Whitesell 6-1 Although we can approach this exercise from a chemical perspective, one can also teach a non-chemist how to derive the answer once the name of the starting material
Copyright 2009 James K Whitesell 6-1 Although we can approach this exercise from a chemical perspective, one can also teach a non-chemist how to derive the answer once the name of the starting material
