less stable intermediate
|
|
|
- Theodora Neal
- 6 years ago
- Views:
Transcription
1 166 CAPTER 4 INTRODUCTION TO ALKENES. STRUCTURE AND REACTIVITY STANDARD FREE ENERGY higher-energy transition state less stable intermediate (C 3 ) 2 CC 2 Br _ (C 3 ) 2 C A C 2 + Br (C 3 ) 2 CC 2 L Br slower reaction lower-energy transition state DG DG (C 3 ) 2 C A C 2 + Br faster reaction more stable intermediate (C 3 ) 3 C Br _ (C 3 ) 3 C L Br reaction coordinate reaction coordinate Figure 4.15 A reaction free-energy diagram for the two possible modes of Br addition to 2-methylpropene. ammond s postulate states that the energy of each transition state is approximated by the energy of the corresponding carbocation. The formation of tert-butyl bromide (right panel) is faster than the formation of isobutyl bromide (left panel) because it involves the more stable carbocation intermediate and therefore the transition state of lower energy. state of lower energy than the transition state for protonation to give a primary carbocation. The stabilities of the carbocations themselves do not determine which reaction is faster; the relative free energies of the transition states for carbocation formation determine the relative rates of the two processes. Only the validity of ammond s postulate allows us to make the connection between carbocation energy and transition-state energy. We need ammond s postulate because the structures of transition states are uncertain, whereas the structures of reactants, products, and reactive intermediates are known. Therefore, knowing that a transition state resembles a particular species (for example, a carbocation) helps us to make a good guess about the transition-state structure. In this text, we ll frequently analyze or predict reaction rates by considering the structures and stabilities of reactive intermediates such as carbocations. When we do this, we are assuming that the transition states and the corresponding reactive intermediates have similar structures and energies; in other words, we are invoking ammond s postulate. PROBLEM 4.31 Apply ammond s postulate to decide which reaction is faster: addition of Br to 2-methylpropene or addition of Br to trans-2-butene. Assume that the energy difference between the starting alkenes can be ignored. Why is this assumption necessary? 4.9 CATALYSIS Some reactions take place much more rapidly in the presence of certain substances that are themselves left unchanged by the reaction. A substance that increases the rate of a reaction without being consumed is called a catalyst. A practical example of a catalyst is platinum in the catalytic converter on the modern automobile. The platinum catalyst in the converter
2 4.9 CATALYSIS 167 no catalyst STANDARD FREE ENERGY reactants a catalyst does not change G + catalyst G a catalyst increases the rate (decreases G ) G G products reaction coordinate Figure 4.16 A reaction free-energy diagram comparing a hypothetical catalyzed reaction (red curve) to the uncatalyzed reaction (blue curve). brings about the rapid oxidation (combustion) of hydrocarbon exhaust emissions. This reaction would not occur were it not for the catalyst; yet the catalyst is left unchanged by the combustion reaction. The catalyst increases the rate of the combustion reaction by many orders of magnitude. ere are some important points about catalysts. 1. A catalyst increases the reaction rate. This means that it lowers the standard free energy of activation for a reaction (Fig. 4.16). 2. A catalyst is not consumed. It may be consumed in one step of a catalyzed reaction, but if so, it is regenerated in a subsequent step. An implication of points 1 and 2 is that a catalyst that strongly accelerates a reaction can be used in very small amounts. Many expensive catalysts are practical for this reason. 3. A catalyst does not affect the energies of reactants and products. In other words, a catalyst does not affect the DG of a reaction and consequently also does not affect the equilibrium constant (Fig. 4.16). 4. A catalyst accelerates both the forward and reverse of a reaction by the same factor. The last point follows from the fact that, at equilibrium, the rates of a reaction and its reverse are equal. If a catalyst does not affect the equilibrium constant (point 3) but increases the reaction rate in one direction, equality of rates at equilibrium requires that the rate of the reverse reaction must be increased by the same factor. When a catalyst and the reactants exist in separate phases, the catalyst is called a heterogeneous catalyst. The catalyst in the catalytic converter of an automobile is a heterogeneous catalyst because it is a solid and the reactants are gases. In other cases, a reaction in solution may be catalyzed by a soluble catalyst. A catalyst that is soluble in a reaction solution is called a homogeneous catalyst.
3 168 CAPTER 4 INTRODUCTION TO ALKENES. STRUCTURE AND REACTIVITY A large number of organic reactions are catalyzed. In this section, we ll introduce the idea of catalysis by considering three examples of catalyzed alkene reactions. The first example, catalytic hydrogenation, is a very important example of heterogeneous catalysis. The second example, hydration, is an example of homogeneous catalysis. The last example involves catalysis of a biological reaction by an enzyme. Catalyst Poisons Although in theory catalysts should function indefinitely, in practice many catalysts, particularly heterogeneous catalysts, slowly become less effective. It is as if they wear out. One reason for this behavior is that they slowly absorb impurities, called catalyst poisons, from the surroundings; these impurities impede the functioning of the catalyst. An example of this phenomenon also occurs with the catalytic converter. Lead is a potent poison of the catalyst in a catalytic converter.this fact, as well as the desire to eliminate atmospheric lead pollution, are the major reasons why leaded gasoline is no longer used in automotive engines in the United States. A. Catalytic ydrogenation of Alkenes When a solution of an alkene is stirred under an atmosphere of hydrogen, nothing happens. But if the same solution is stirred under hydrogen in the presence of a metal catalyst, the hydrogen is rapidly absorbed by the solution. The hydrogen is consumed because it undergoes an addition to the alkene double bond. M y % cyclohexene C 3 (C 2 ) 5 C A C octene + 2 Pt/C Pt/C ` C 3 (C 2 ) 5 C 2 L C 3 octane (4.38) (4.39) These reactions are examples of catalytic hydrogenation, an addition of hydrogen to an alkene in the presence of a catalyst. Catalytic hydrogenation is one of the best ways to convert alkenes into alkanes. Catalytic hydrogenation is an important reaction in both industry and the laboratory. The inconvenience of using a special apparatus for the handling of a flammable gas (dihydrogen) is more than offset by the great utility of the reaction. In the preceding reactions, the catalyst is written over the reaction arrows. Pt/C is read as Platinum supported on carbon or simply Platinum on carbon. This catalyst is a finely divided platinum metal that has been precipitated, or supported, on activated charcoal. A number of noble metals, such as platinum, palladium, and nickel, are useful as hydrogenation catalysts, and they are often used in conjunction with solid support materials such as alumina (Al 2 O 3 ), barium sulfate (BaSO 4 ), or, as in the previous examples, activated carbon. ydrogenation can be carried out at room temperature and pressure or, for especially difficult cases, at higher temperature and pressure in a bomb (a closed vessel designed to withstand high pressures). Because hydrogenation catalysts are insoluble in the reaction solution, they are examples of heterogeneous catalysts. (Soluble hydrogenation catalysts are also known and, although important, are not so widely used; Sec. 18.6D.) Even though they involve relatively expensive noble metals, heterogeneous hydrogenation catalysts are very practical because they can ) % M cyclohexane
4 4.9 CATALYSIS 169 be filtered off and reused. Furthermore, because they are exceedingly effective, they can be used in very small amounts. For example, typical catalytic hydrogenation reactions can be run with reactant-to-catalyst ratios of 100 or more. ow do hydrogenation catalysts work? Research has shown that both the hydrogen and the alkene must be adsorbed on the surface of the catalyst for a reaction to occur. The catalyst is believed to form reactive metal carbon and metal hydrogen bonds that ultimately are broken to form the products and to regenerate the catalyst sites. Beyond this, the chemical details of catalytic hydrogenation are poorly understood. This is not a reaction for which a simple curved-arrow mechanism can be written. The mechanism of noble-metal catalysis is an active area of research in many branches of chemistry. The benzene ring is inert to conditions under which normal double bonds react readily: cl C A C Pt/C cl C 2 L C 3 (4.40) styrene ethylbenzene (Benzene rings can be hydrogenated, however, with certain catalysts under conditions of high temperature and pressure.) You will learn that many other alkene reactions do not affect the double bonds of a benzene ring. The relative inertness of benzene rings toward the conditions of alkene reactions was one of the great puzzles of organic chemistry that was ultimately explained by the theory of aromaticity, which is introduced in Chapter 15. PROBLEMS 4.32 Give the product formed when each of the following alkenes reacts with a large excess of hydrogen in the presence of Pd/C. (a) 1-pentene (b) (E)-1,3-hexadiene 4.33 (a) Give the structures of five alkenes, each with the formula C 6 12, that would give hexane as the product of catalytic hydrogenation. (b) ow many alkenes containing one double bond can react with 2 over a Pt/C catalyst to give methylcyclopentane? Give their structures. (int: See Study Problem 4.9, p. 153.) B. ydration of Alkenes The alkene double bond undergoes reversible addition of water in the presence of moderately concentrated strong acids such as 2 SO 4, ClO 4, and NO 3. 3 C C A C 2 + L O 3 C 2-methylpropene (in excess; solvent) 1 M NO 3 C 3 3 CLCLC 3 O 2-methyl-2-propanol (tert-butyl alcohol) (4.41) The addition of the elements of water is in general called hydration. ence, the addition of water to the alkene double bond is called alkene hydration. ydration does not occur at a measurable rate in the absence of an acid, and the acid is not consumed in the reaction. ence, alkene hydration is an acid-catalyzed reaction. Because the catalyzing acid is soluble in the reaction solution, it is a homogeneous catalyst.
5 170 CAPTER 4 INTRODUCTION TO ALKENES. STRUCTURE AND REACTIVITY Notice that this reaction, like the addition of Br, is regioselective. As in the addition of Br, the hydrogen adds to the carbon of the double bond with the smaller number of alkyl substituents. The more electronegative partner of the LO bond, the O group, like the Br in Br addition, adds to the carbon of the double bond with the greater number of alkyl substituents. In this reaction, the manner in which the catalyst functions can be understood by considering the mechanism of the reaction, which is very similar to that of Br addition. In the first step of the reaction, which is the rate-limiting step, the double bond is protonated so as to give the more stable carbocation. Because water is present, the actual acid is the hydrated proton ( 3 O ). 3 C C A C 2 3 C L O C 1 C L C O 1 3 C (4.42a) This is a Brønsted acid base reaction. Because this is the rate-limiting step, the rate of the hydration reaction increases when the rate of this step increases. The strong acid 3 O is more effective than the considerably weaker acid water in protonating a weak base (the alkene). If a strong acid is not present, the reaction does not occur because water alone is too weak an acid to protonate the alkene. In the next step of the hydration reaction, the Lewis base water combines with the carbocation in a Lewis acid base association reaction: (C 3 ) 3 C 3O 2 2 (C 3 ) 3 C L O 2 2 (4.42b) Finally, a proton is lost to solvent in another Brønsted acid base reaction to give the alcohol product and regenerate the catalyzing acid 3 O : 1 (C 3 ) 3 C L O (C 3 ) 3 C L O + 3 O (4.42c) 1 2 O Notice three things about this mechanism. First, it consists entirely of Lewis acid base and Brønsted acid base reactions. Second, although the proton consumed in Eq. 4.42a is not the same as the one produced in Eq. 4.42c, there is no net consumption of protons. Finally, the base is Eq. 4.42c is water. Some students are tempted to use hydroxide ion in a situation like this because it is a stronger base. owever, there is essentially no hydroxide in a 1 M nitric acid or sulfuric acid solution. Nor is hydroxide needed, because the acid on the left of Eq. 4.42c is a strong acid. Whenever 3 O + acts as an acid, its conjugate base 2 O acts as the base. (Read again about amphoteric compounds on p. 97 if this isn t clear.) More generally, acids and their conjugate bases always act in tandem in acid base catalysis. Because the hydration reaction involves carbocation intermediates, some alkenes give rearranged hydration products. O 3 C LC LC A C O 3 O 3 C LC LC 2 LC 3 C 3 C 3 (4.43)
6 4.9 CATALYSIS 171 PROBLEMS 4.34 Give the mechanism for the reaction in Eq Show each step of the mechanism separately with careful use of the curved-arrow notation. Explain why the rearrangement takes place The alkene 3,3-dimethyl-1-butene undergoes acid-catalyzed hydration with rearrangement. Use the mechanism of hydration and rearrangement to predict the structure of the hydration product of this alkene (a) Unlike the alcohol product Eq. 4.41, the product in Eq does not come to equilibrium with the starting alkene. owever, it does come to equilibrium with two other alkenes. What are their structures? (b) Why isn t the alkene starting material in Eq part of the equilibrium mixture? The equilibrium constants for many alkene hydrations are close enough to unity that the hydration reaction can be run in reverse. The reverse of alkene hydration is called alcohol dehydration. The direction in which the reaction is run depends on the application of Le Châtelier s principle, which states that if an equilibrium is disturbed, it will react so as to offset the disturbance. For example, if the alkene is a gas (as in Eq. 4.41), the reaction vessel can be pressurized with the alkene. The equilibrium reacts to the excess of alkene by forming more alcohol. Neutralization of the acid catalyst stops the reaction and permits isolation of the alcohol. This strategy is used particularly in industrial applications. One such application of alkene hydration is the commercial preparation of ethyl alcohol (ethanol) from ethylene: 2 C A C O 3 PO 4 (absorbed on solid support) 300 C 3 C L C 2 L O (4.44) ethylene ethanol A high temperature is required because the hydration of ethylene is very slow at ordinary temperatures (see Problem 4.37). Recall (Sec. 4.8B) that increasing the temperature accelerates a reaction. This reaction was at one time a major source of industrial ethanol. Although it is still used, its importance has decreased as the fermentation of sugars from biomass (for example, corn) has become more prevalent. To run the hydration reaction in the reverse (dehydration) direction, the alkene is removed as it is formed, typically by distillation. (Alkenes have significantly lower boiling points than alcohols, as we ll further discuss in Sec. 8.3B.) The equilibrium responds by forming more alkene. Alcohol dehydration is more widely used than alkene hydration in the laboratory. We ll consider this reaction in Sec Alkene hydration and alcohol dehydration illustrate two important points. First is one of the key points about catalysis: a catalyst accelerates the forward and reverse reactions of an equilibrium by the same factor. For example, because alkene hydration is acid-catalyzed, alcohol dehydration is acid-catalyzed as well. A second point is that alkene hydration and alcohol dehydration occur by the forward and reverse of the same mechanism. Generally, if a reaction occurs by a certain mechanism, the reverse reaction under the same conditions occurs by the exact reverse of that mechanism. This statement is called the principle of microscopic reversibility. Microscopic reversibility requires, for example, that if you know the mechanism of alkene hydration, then you know the mechanism of alcohol dehydration as well. A consequence of microscopic reversibility is that the rate-limiting transition states of a reaction and its reverse are the same. For example, if the rate-limiting step of alkene hydration is protonation of the double bond to form the carbocation intermediate (Eq. 4.42a), then the rate-limiting step of alcohol dehydration is the reverse of the same equation deprotonation of the carbocation to give the alkene.
7 172 CAPTER 4 INTRODUCTION TO ALKENES. STRUCTURE AND REACTIVITY PROBLEMS 4.37 Explain why the hydration of ethylene is a very slow reaction. (int: Think about the structure of the reactive intermediate and apply ammond s postulate.) 4.38 Isopropyl alcohol is produced commercially by the hydration of propene. Show the mechanistic steps of this process. If you do not know the structure of isopropyl alcohol, try to deduce it by analogy from the structure of propene and the mechanism of alkene hydration. C. Enzyme Catalysis Catalysis is not limited to the laboratory or chemical industry. The biological processes of nature involve thousands of chemical reactions, most of which have their own unique naturally occurring catalysts. These biological catalysts are called enzymes. (The structures of enzymes are discussed in Sec ) Under physiological conditions, most important biological reactions would be too slow to be useful in the absence of their enzyme catalysts. Enzyme catalysts are important not only in nature; they are finding increasing use both in industry and in the laboratory. Many of the best characterized enzymes are soluble in aqueous solution and hence are homogeneous catalysts. owever, other enzymes are immobilized within biological substructures such as membranes and can be viewed as heterogeneous catalysts. An example of an important enzyme-catalyzed addition to an alkene is the hydration of fumarate ion to malate ion. _ O O S C L C A C L C S O fumarate O _ + 2 O fumarase (an enzyme) O O O S S L C L C L C 2 LC L malate (4.45) This reaction is catalyzed by the enzyme fumarase. It is one reaction in the Krebs cycle, or citric acid cycle, a series of reactions that plays a central role in the generation of energy in biological systems. Fumarase catalyzes only this reaction. The effectiveness of fumarase catalysis can be appreciated by the following comparison: At physiological p and temperature (p = 7, 37 C), the enzyme-catalyzed reaction is about times faster than the same reaction in the absence of enzyme. _ O O _ KEY IDEAS IN CAPTER 4 Alkenes are compounds containing carbon carbon double bonds. Alkene carbon atoms, as well as other trigonal planar atoms, are sp 2 -hybridized. The carbon carbon double bond consists of a s bond and a p bond. The p electrons are more reactive than the s electrons and can be donated to Brønsted or Lewis acids. In the IUPAC substitutive nomenclature of alkenes, the principal chain, which is the carbon chain containing the greatest number of double bonds, is
CHEM Lecture 7
 CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds.
 This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
Lecture 11 Organic Chemistry 1
 EM 232 rganic hemistry I at hicago Lecture 11 rganic hemistry 1 Professor Duncan Wardrop February 16, 2010 1 Self Test Question What is the product(s) of the following reaction? 3 K( 3 ) 3 A 3 ( 3 ) 3
EM 232 rganic hemistry I at hicago Lecture 11 rganic hemistry 1 Professor Duncan Wardrop February 16, 2010 1 Self Test Question What is the product(s) of the following reaction? 3 K( 3 ) 3 A 3 ( 3 ) 3
Chemistry 110. Bettelheim, Brown, Campbell & Farrell. Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes.
 Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes
 Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, Organic and Biochemistry Chapter 12 Alkenes & Alkynes Chapter 12 Alkenes are hydrocarbons which have one or more
Vision. Cis-trans isomerism is key to vision. How rods work H 3 C CH 3. Protein opsin. 11-cis-retinal. Opsin. Rhodopsin.
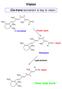 Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
Vision Cis-trans isomerism is key to vision. 3 C 11 12 3 C C 3 3 C O C 3 11-cis-retinal Protein opsin 3 C 11 12 3 C C 3 3 C N Opsin C 3 Rhodopsin Light photons 3 C N Opsin 3 C 11 12 3 C C 3 C 3 ow rods
ADDITION OF HYDROGEN HALIDES TO CONJUGATED DIENES A. 1,2- and 1,4-Additions 700 CHAPTER 15 DIENES, RESONANCE, AND AROMATICITY
 700 CAPTER 15 DIENES, RESONANCE, AND AROMATICITY 15.18 Give the structures of the starting materials that would yield each of the compounds below in Diels Alder reactions. Pay careful attention to stereochemistry,
700 CAPTER 15 DIENES, RESONANCE, AND AROMATICITY 15.18 Give the structures of the starting materials that would yield each of the compounds below in Diels Alder reactions. Pay careful attention to stereochemistry,
As time allows, additional practice questions for Exam 2 follow. This is an incomplete collection. Content & emphasis will vary.
 Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Alkenes. Dr. Munther A. M-Ali For 1 st Stage Setudents
 Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
The Chemistry of Ethers, Epoxides, Glycols, and Sulfides
 The Chemistry of Ethers, Epoxides, Glycols, and Sulfides The chemistry of ethers is closely intertwined with the chemistry of alkyl halides, alcohols, and alkenes. Ethers, however, are considerably less
The Chemistry of Ethers, Epoxides, Glycols, and Sulfides The chemistry of ethers is closely intertwined with the chemistry of alkyl halides, alcohols, and alkenes. Ethers, however, are considerably less
Allylic and Benzylic Reactivity
 17 17 Allylic and Benzylic Reactivity An allylic group is a group on a carbon adjacent to a double bond. A benzylic group is a group on a carbon adjacent to a benzene ring or substituted benzene ring.
17 17 Allylic and Benzylic Reactivity An allylic group is a group on a carbon adjacent to a double bond. A benzylic group is a group on a carbon adjacent to a benzene ring or substituted benzene ring.
Electrophilic Aromatic Substitution
 Chem 263 ct. 8, 2013 lectrophilic Aromatic Substitution Benzene appears to be a remarkably stable and unreactive compared to alkenes, such as cyclohexene or ethylene, or even alkanes, such as cyclohexane
Chem 263 ct. 8, 2013 lectrophilic Aromatic Substitution Benzene appears to be a remarkably stable and unreactive compared to alkenes, such as cyclohexene or ethylene, or even alkanes, such as cyclohexane
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Organic Chemistry. REACTIONS Grade 12 Physical Science Mrs KL Faling
 Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
Learning Guide for Chapter 11 - Alkenes I
 Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Chem101 General Chemistry. Lecture 11 Unsaturated Hydrocarbons
 hem101 General hemistry Lecture 11 Unsaturated ydrocarbons Unsaturated ydrocarbons ontain one or more double or triple carbon-carbon bond. University of Wisconsin-Eau laire hem101 - Lecture 11 2 Unsaturated
hem101 General hemistry Lecture 11 Unsaturated ydrocarbons Unsaturated ydrocarbons ontain one or more double or triple carbon-carbon bond. University of Wisconsin-Eau laire hem101 - Lecture 11 2 Unsaturated
Organic Chemistry Worksheets
 Highlight the single longest, continuous carbon-carbon chain. Note the alkyl branches that are connected to the root chain. Count the carbons in the root chain, starting from the end closest to the alkyl
Highlight the single longest, continuous carbon-carbon chain. Note the alkyl branches that are connected to the root chain. Count the carbons in the root chain, starting from the end closest to the alkyl
10/26/2010. An Example of a Polar Reaction: Addition of H 2 O to Ethylene. to Ethylene
 6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Electrophilic Aromatic Substitution
 Chem 263 Sept 29, 2016 lectrophilic Aromatic Substitution Benzene appears to be a remarkably stable (36 kcal/mole more) and unreactive compared to alkenes, such as cyclohexene or ethylene, or even alkanes,
Chem 263 Sept 29, 2016 lectrophilic Aromatic Substitution Benzene appears to be a remarkably stable (36 kcal/mole more) and unreactive compared to alkenes, such as cyclohexene or ethylene, or even alkanes,
The following quiz contains 30 questions valued at 1 point/question unless otherwise noted. Name:
 Chemistry 261 Quiz 2 Practice Fall 2017 The following quiz contains 30 questions valued at 1 point/question unless otherwise noted ame: ELECTR DEFICIET CMPUDS AD LEWIS ACID-BASE DEFIITIS 1. Which of the
Chemistry 261 Quiz 2 Practice Fall 2017 The following quiz contains 30 questions valued at 1 point/question unless otherwise noted ame: ELECTR DEFICIET CMPUDS AD LEWIS ACID-BASE DEFIITIS 1. Which of the
Organic Chemistry. Alkynes
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkynes by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkynes by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
LECTURE #13 Tues., Oct.18, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CEM 221 section 01 LECTURE #13 Tues., Oct.18, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSGNED READNGS: TODAY S CLASS: Sections 4.1 4.6 NEXT CLASS: rest of Ch.4 http://artsandscience.concordia.ca/facstaff/p-r/rogers
CEM 221 section 01 LECTURE #13 Tues., Oct.18, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSGNED READNGS: TODAY S CLASS: Sections 4.1 4.6 NEXT CLASS: rest of Ch.4 http://artsandscience.concordia.ca/facstaff/p-r/rogers
Chapter 3. Alkenes And Alkynes
 Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Elimination Reactions Heating an alkyl halide with a strong base causes elimination of a. molecule of HX
 Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Chapter 10 Lecture Outline
 Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction
Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction
Chapter 7: Alcohols, Phenols and Thiols
 Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Ethers & Epoxides. Chapter 5. Dr. Seham ALTERARY. Chem 340-2nd semester
 Ethers & Epoxides Chapter 5 Dr. Seham ALTERARY Chapter s out line Ethers Definition; General formula; Classification and Types Nomenclature - Common Names. - IUPAC Naming. Physical Properties General methods
Ethers & Epoxides Chapter 5 Dr. Seham ALTERARY Chapter s out line Ethers Definition; General formula; Classification and Types Nomenclature - Common Names. - IUPAC Naming. Physical Properties General methods
C C. sp 2. π M.O. atomic. orbitals. carbon 1. σ M.O. molecular. orbitals. H C C rotate D. D H zero overlap of p orbitals: π bond broken!
 Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BDE ~ 80 kcal/mol π = BDE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in
Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BDE ~ 80 kcal/mol π = BDE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in
Chapter 3 An Introduction to Organic Reactions: Acids and Bases
 There are 4 types of Organic Reactions Chapter 3 An Introduction to Organic Reactions: SUBSTITUTION: ADDITION: X Y + A X A + Y Example Example A B + X Y A B X Y ELIMINATION There are 4 Types of Organic
There are 4 types of Organic Reactions Chapter 3 An Introduction to Organic Reactions: SUBSTITUTION: ADDITION: X Y + A X A + Y Example Example A B + X Y A B X Y ELIMINATION There are 4 Types of Organic
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
8. What is the slow, rate-determining step, in the acidcatalyzed dehydration of 2-methyl-2-propanol?
 CHEMISTRY 313-03 MIDTERM # 2 answer key October 25, 2011 Statistics: Average: 68 pts (68%); Highest: 100 pts (100%); Lowest: 30 pts (30%) Number of students performing at or above average: 56 (54%) Number
CHEMISTRY 313-03 MIDTERM # 2 answer key October 25, 2011 Statistics: Average: 68 pts (68%); Highest: 100 pts (100%); Lowest: 30 pts (30%) Number of students performing at or above average: 56 (54%) Number
5. Reactions of Alkenes (text )
 2009, Department of hemistry, The University of Western Ontario 5.1 5. Reactions of Alkenes (text 5.1 5.5) A. Addition Reactions In hapter 4, we saw that π bonds have electron density on two sides of the
2009, Department of hemistry, The University of Western Ontario 5.1 5. Reactions of Alkenes (text 5.1 5.5) A. Addition Reactions In hapter 4, we saw that π bonds have electron density on two sides of the
Introduction to Alkenes and Alkynes
 Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 19: Alkenes and Alkynes
 Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Organic Chemistry Review: Topic 10 & Topic 20
 Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Chapter 7 - Alkenes and Alkynes I
 Andrew Rosen Chapter 7 - Alkenes and Alkynes I 7.1 - Introduction - The simplest member of the alkenes has the common name of ethylene while the simplest member of the alkyne family has the common name
Andrew Rosen Chapter 7 - Alkenes and Alkynes I 7.1 - Introduction - The simplest member of the alkenes has the common name of ethylene while the simplest member of the alkyne family has the common name
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Chapter 3 Alkenes and Alkynes. Excluded sections 3.15&3.16
 Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Learning Guide for Chapter 13 - Alkynes
 Learning Guide for Chapter 13 - Alkynes I. Introduction to Alkynes - p 1 II. Natural ccurrence and Uses of Alkynes - p 5 III. Physical Properties of Alkynes - p 7 IV. Spectroscopy of Alkynes - p 7 V. Nomenclature
Learning Guide for Chapter 13 - Alkynes I. Introduction to Alkynes - p 1 II. Natural ccurrence and Uses of Alkynes - p 5 III. Physical Properties of Alkynes - p 7 IV. Spectroscopy of Alkynes - p 7 V. Nomenclature
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides"
 Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Chapter 2 Alkanes and Cycloalkanes: Introduction to Hydrocarbons
 Chapter 2 Alkanes and Cycloalkanes: Introduction to Hydrocarbons 2.1 Classes of Hydrocarbons Classes of Hydrocarbons Hydrocarbons only contain carbon and hydrogen atoms. Hydrocarbons are either classed
Chapter 2 Alkanes and Cycloalkanes: Introduction to Hydrocarbons 2.1 Classes of Hydrocarbons Classes of Hydrocarbons Hydrocarbons only contain carbon and hydrogen atoms. Hydrocarbons are either classed
Reaction Rates and Equilibrium
 CHAPTER 7 14 SECTION Chemical Reactions Reaction Rates and Equilibrium KEY IDEAS As you read this section, keep these questions in mind: How can you increase the rate of a reaction? What does a catalyst
CHAPTER 7 14 SECTION Chemical Reactions Reaction Rates and Equilibrium KEY IDEAS As you read this section, keep these questions in mind: How can you increase the rate of a reaction? What does a catalyst
Learning Guide for Chapter 14 - Alcohols (I)
 Learning Guide for Chapter 14 - Alcohols (I) I. Introduction to Alcohols and Thiols II. Acid/base Behavior of Alcohols, Phenols, and Thiols III. Nomenclature of Alcohols IV. Synthesis of Alcohols Previous
Learning Guide for Chapter 14 - Alcohols (I) I. Introduction to Alcohols and Thiols II. Acid/base Behavior of Alcohols, Phenols, and Thiols III. Nomenclature of Alcohols IV. Synthesis of Alcohols Previous
16.1 Draw Newman projections for these staggered and three eclipsed conformations of 1, 2 - dichlorobutane as viewed downt he central C-C bond.
 CAPTER 16 Practice Exercises 16.1 Draw Newman projections for these staggered and three eclipsed conformations of 1, 2 - dichlorobutane as viewed downt he central C-C bond. 16.3 (a) The more stable conformation
CAPTER 16 Practice Exercises 16.1 Draw Newman projections for these staggered and three eclipsed conformations of 1, 2 - dichlorobutane as viewed downt he central C-C bond. 16.3 (a) The more stable conformation
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
The carbon-carbon double bond is the distinguishing feature of alkenes.
 Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Alcohols: Contain a hydroxy group( OH) bonded to an sp 2 or sp 3 hybridized
 Lecture Notes hem 51B S. King hapter 9 Alcohols, Ethers, and Epoxides I. Introduction Alcohols, ether, and epoxides are 3 functional groups that contain σ-bonds. Alcohols: ontain a hydroxy group( ) bonded
Lecture Notes hem 51B S. King hapter 9 Alcohols, Ethers, and Epoxides I. Introduction Alcohols, ether, and epoxides are 3 functional groups that contain σ-bonds. Alcohols: ontain a hydroxy group( ) bonded
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
More Nomenclature: Common Names for Selected Aromatic Groups. Aryl = Ar = aromatic group. It is a broad term, and includes any aromatic rings.
 More Nomenclature: Common Names for Selected Aromatic Groups Phenyl group = or Ph = C 6 H 5 = Aryl = Ar = aromatic group. It is a broad term, and includes any aromatic rings. Benzyl = Bn = It has a -CH
More Nomenclature: Common Names for Selected Aromatic Groups Phenyl group = or Ph = C 6 H 5 = Aryl = Ar = aromatic group. It is a broad term, and includes any aromatic rings. Benzyl = Bn = It has a -CH
Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 INTRODUCTION TO ORGANIC AND BIOCHEMISTRY QUIZ 5 Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the IUPAC name
INTRODUCTION TO ORGANIC AND BIOCHEMISTRY QUIZ 5 Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the IUPAC name
Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
H H C C. Alkenes C n H 2n unsaturated hydrocarbons. C 2 H 4 ethylene. Functional group = carbon-carbon double bond
 Alkenes C n H 2n unsaturated hydrocarbons C 2 H 4 ethylene H H C C H H Functional group = carbon-carbon double bond sp 2 hybridization => flat, 120 o bond angles σ bond & π bond => H 2 C=CH 2 No rotation
Alkenes C n H 2n unsaturated hydrocarbons C 2 H 4 ethylene H H C C H H Functional group = carbon-carbon double bond sp 2 hybridization => flat, 120 o bond angles σ bond & π bond => H 2 C=CH 2 No rotation
17.3 REACTIONS INVOLVING ALLYLIC AND BENZYLIC ANIONS
 798 HAPTER 17 ALLYLI AND BENZYLI REATIVITY Because the unpaired electron is shared by two different carbons, this radical can react in the final propagation step to give two different products. Reaction
798 HAPTER 17 ALLYLI AND BENZYLI REATIVITY Because the unpaired electron is shared by two different carbons, this radical can react in the final propagation step to give two different products. Reaction
CHAPTER 3 ALKENES, ALKYNES & CONJUGATE DIENES
 CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
Chapter 13 Alkenes and Alkynes & Aromatic Compounds
 Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
EXPERIMENT 8 Reactions of Hydrocarbons
 EXPERIMENT 8 Reactions of Hydrocarbons Properties and Identification of Hydrocarbons Purpose: a) To identify saturated and unsaturated hydrocarbons using properties and reactions. b) Study substitution
EXPERIMENT 8 Reactions of Hydrocarbons Properties and Identification of Hydrocarbons Purpose: a) To identify saturated and unsaturated hydrocarbons using properties and reactions. b) Study substitution
Electronegativity Scale F > O > Cl, N > Br > C, H
 Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
Organic Chem Chapter 12 Alkanes Organic chemistry is the study of carbon compounds. Carbon has several properties that are worth discussing: Tetravalent Always forms 4 bonds Can form multiple bonds (double
Organic Chemistry, Second Edition. Janice Gorzynski Smith University of Hawai i. Chapter 10 Alkenes
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry
 Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry 1 Structure and Bonding 2 Structure and Bonding Rotation around the C=C bond is restricted 90 rotation The p orbitals are orthogonal
Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry 1 Structure and Bonding 2 Structure and Bonding Rotation around the C=C bond is restricted 90 rotation The p orbitals are orthogonal
Chapter 8 Reactions of Alkenes
 Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Loudon Chapter 14 Review: Reactions of Alkynes Jacquie Richardson, CU Boulder Last updated 1/16/2018
 An alkyne is any molecule with a triple bond between two carbon atoms. This triple bond consists of one σ bond and two π bonds: the σ bond exists on a straight line between carbon atoms, while one π bond
An alkyne is any molecule with a triple bond between two carbon atoms. This triple bond consists of one σ bond and two π bonds: the σ bond exists on a straight line between carbon atoms, while one π bond
1. Root of name depends on longest chain of C containing the double bond; ends in "ene"
 Alkenes (β-carotene, an antioxidant pigment) n 2n (acyclic) n 2n-2 (cyclic) R R R R Key features sp 2 -hybridized carbons, 120 o bond angles σ + π bonding between = planar geometry around = "unsaturated"
Alkenes (β-carotene, an antioxidant pigment) n 2n (acyclic) n 2n-2 (cyclic) R R R R Key features sp 2 -hybridized carbons, 120 o bond angles σ + π bonding between = planar geometry around = "unsaturated"
Lecture Notes Chem 51B S. King I. Conjugation
 Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
Chapter 8: Ethers and Epoxides. Diethyl ether in starting fluid
 Chapter 8: Ethers and Epoxides Diethyl ether in starting fluid 8.1 Nomenclature of Ethers Ethers are usually named by giving the name of each alkyl or aryl group, in alphabetical order, followed by the
Chapter 8: Ethers and Epoxides Diethyl ether in starting fluid 8.1 Nomenclature of Ethers Ethers are usually named by giving the name of each alkyl or aryl group, in alphabetical order, followed by the
CHAPTER 15. Practice exercises 15.1
 CAPTER 15 Practice exercises 15.1 15.3 (a) C 9 18 : isobutylcyclopentane C 11 22 : sec-butylcycloheptane C 6 2 : 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene 2,3-dimethyl-2-butene 3,3-dimethyl-1-butyne
CAPTER 15 Practice exercises 15.1 15.3 (a) C 9 18 : isobutylcyclopentane C 11 22 : sec-butylcycloheptane C 6 2 : 1-ethyl-1-methylcyclopropane 15.5 (a) 3,3-dimethyl-1-pentene 2,3-dimethyl-2-butene 3,3-dimethyl-1-butyne
Chapter 4. Reactions of alkenes. Addition reactions Carbocations Selectivity of reactions
 Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
BRCC CHM 102 Class Notes Chapter 11 Page 1 of 9
 BRCC CHM 102 Class Notes Chapter 11 Page 1 of 9 Chapter 11 Alkanes and Cycloalkanes hydrocarbons compounds that contain only carbon and hydrogen * 4 families: 1) alkanes only single bonds (includes cycloalkanes)
BRCC CHM 102 Class Notes Chapter 11 Page 1 of 9 Chapter 11 Alkanes and Cycloalkanes hydrocarbons compounds that contain only carbon and hydrogen * 4 families: 1) alkanes only single bonds (includes cycloalkanes)
Organic Chemistry. Organic chemistry is the chemistry of compounds containing carbon.
 Organic Chemistry Organic Chemistry Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a
Organic Chemistry Organic Chemistry Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Introduction to Chemical Reactions. Chapter 6
 Introduction to Chemical Reactions Chapter 6 Instructional Goals 1. Given the reactants and product in a chemical reaction, the student will be able to write and balance chemical equations. 2. Identify
Introduction to Chemical Reactions Chapter 6 Instructional Goals 1. Given the reactants and product in a chemical reaction, the student will be able to write and balance chemical equations. 2. Identify
Experiment 5 Reactions of Hydrocarbons
 Experiment 5 Reactions of ydrocarbons ydrocarbons are compounds that only contain carbon and hydrogen. ydrocarbons can be classified further by the type of bonds they contain. If a hydrocarbon contains
Experiment 5 Reactions of ydrocarbons ydrocarbons are compounds that only contain carbon and hydrogen. ydrocarbons can be classified further by the type of bonds they contain. If a hydrocarbon contains
Chapter 17 Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
BIOB111 - Tutorial activities for session 8
 BIOB111 - Tutorial activities for session 8 General topics for week 4 Session 8 Physical and chemical properties and examples of these functional groups (methyl, ethyl in the alkyl family, alkenes and
BIOB111 - Tutorial activities for session 8 General topics for week 4 Session 8 Physical and chemical properties and examples of these functional groups (methyl, ethyl in the alkyl family, alkenes and
Chemistry 261 Exam 2 Practice Fall 2017
 Chemistry 261 Exam 2 Practice Fall 2017 The following practice examination contains 30 questions valued at 3 point/question unless otherwise noted. Wednesday s exam will also contain 30 questions, with
Chemistry 261 Exam 2 Practice Fall 2017 The following practice examination contains 30 questions valued at 3 point/question unless otherwise noted. Wednesday s exam will also contain 30 questions, with
BAE 820 Physical Principles of Environmental Systems
 BAE 820 Physical Principles of Environmental Systems Catalysis of environmental reactions Dr. Zifei Liu Catalysis and catalysts Catalysis is the increase in the rate of a chemical reaction due to the participation
BAE 820 Physical Principles of Environmental Systems Catalysis of environmental reactions Dr. Zifei Liu Catalysis and catalysts Catalysis is the increase in the rate of a chemical reaction due to the participation
Chapter 6 H 2 H 3 C C H CH 3 C H H 2 C C CH 3. (b) =2 H 2 C C C H H C H CH 2 C CH 3 H 3 C C C CH 3. (c) =2
 hapter 6 6.1 alculate the degree of the unsaturation in the following hydrocarbons: 8 14 ; 5 6 (c) 12 20 (d) 20 32 (e) 40 56 =2 =3 (c) =3 (d) =5 (e) =13 6.2 alculate the degree of the unsaturation in the
hapter 6 6.1 alculate the degree of the unsaturation in the following hydrocarbons: 8 14 ; 5 6 (c) 12 20 (d) 20 32 (e) 40 56 =2 =3 (c) =3 (d) =5 (e) =13 6.2 alculate the degree of the unsaturation in the
Classes of Alkenes. Alkenes and Alkynes. Saturated compounds (alkanes): Have the maximum number of hydrogen atoms attached to each carbon atom.
 Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem reaction what is added to the C=C what kind of molecule results addition of HX HX only
 I. Addition Reactions of Alkenes Introduction Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem 2310 An addition reaction always involves changing a double bond to a single bond and adding a new bond
I. Addition Reactions of Alkenes Introduction Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem 2310 An addition reaction always involves changing a double bond to a single bond and adding a new bond
7. Haloalkanes (text )
 2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
Learning Guide for Chapter 12 - Alkenes (II)
 Learning Guide for Chapter 12 - Alkenes (II) I. Addition reactions of alkenes Introduction to addition reactions Catalytic hydrogenation of alkenes Hydroxylation of alkenes Epoxidation of alkenes Cyclopropanation
Learning Guide for Chapter 12 - Alkenes (II) I. Addition reactions of alkenes Introduction to addition reactions Catalytic hydrogenation of alkenes Hydroxylation of alkenes Epoxidation of alkenes Cyclopropanation
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
CO 2 and CO activation
 2 and activation Most organic chemicals are currently made commercially from ethylene, a product of oil refining. It is possible that in the next several decades we may have to shift toward other carbon
2 and activation Most organic chemicals are currently made commercially from ethylene, a product of oil refining. It is possible that in the next several decades we may have to shift toward other carbon
Alcohols. Contents. Structure. structure
 Page 1 of 9 Alcohols Contents structure Physical Properties Classification of Alcohols Nomenclature of Alcohols Preparation of Alcohols Oxidation of Alcohols oxidation of aldehydes Structure Alcohols can
Page 1 of 9 Alcohols Contents structure Physical Properties Classification of Alcohols Nomenclature of Alcohols Preparation of Alcohols Oxidation of Alcohols oxidation of aldehydes Structure Alcohols can
Unit 2. Answers to examination-style questions. Answers Marks Examiner s tips. 1 (a) heat energy change at constant pressure
 (a) heat energy change at constant pressure This is in the spec but not so well known. Learn it. (b) N 2 (g) + ½O 2 (g) N 2 O(g) (c) (i) D = (bonds broken) (bonds made) = ½(945) + (3/2)(59) 3(278) = 23
(a) heat energy change at constant pressure This is in the spec but not so well known. Learn it. (b) N 2 (g) + ½O 2 (g) N 2 O(g) (c) (i) D = (bonds broken) (bonds made) = ½(945) + (3/2)(59) 3(278) = 23
Unsaturated hydrocarbons. Chapter 13
 Unsaturated hydrocarbons Chapter 13 Unsaturated hydrocarbons Hydrocarbons which contain at least one C-C multiple (double or triple) bond. The multiple bond is a site for chemical reactions in these molecules.
Unsaturated hydrocarbons Chapter 13 Unsaturated hydrocarbons Hydrocarbons which contain at least one C-C multiple (double or triple) bond. The multiple bond is a site for chemical reactions in these molecules.
Hydrogen iodide is a strong acid and will drive the reverse reaction, meaning the forward reaction will not occur.
 EM 261 Oct 18, 2018 Photosynthesis and Related Reactions O 2 2 O 6 12 O 6 2 O N 3, S, Fe, u, o, other Natural Products D-Glucose R O R OR Ionic substitution S N 1 & R X X 2 hv Petroleum/ Alkanes R N 2
EM 261 Oct 18, 2018 Photosynthesis and Related Reactions O 2 2 O 6 12 O 6 2 O N 3, S, Fe, u, o, other Natural Products D-Glucose R O R OR Ionic substitution S N 1 & R X X 2 hv Petroleum/ Alkanes R N 2
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
What are radicals? H. Cl. Chapter 10 Radical Reactions. Production of radicals. Reactions of radicals. Electronic structure of methyl radical
 What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
Organic Chemistry The Functional Group Approach. Organic Chemistry The Functional Group Approach
 Organic Chemistry The Functional Group Approach OH Br alkane (no F.G.) alcohol halide alkene non-polar (grease, fats) O NH alkyne aromatic aldehyde/ketone imine linear flat Organic Chemistry The Functional
Organic Chemistry The Functional Group Approach OH Br alkane (no F.G.) alcohol halide alkene non-polar (grease, fats) O NH alkyne aromatic aldehyde/ketone imine linear flat Organic Chemistry The Functional
Alkenes. Bonding and Structure: Carbons in the double bond of butene are sp 2 hybridized. Side on p-p orbital overlap creates a π-bond.
 Alkenes Bonding and Structure: Carbons in the double bond of butene are sp 2 hybridized. Side on p-p orbital overlap creates a π-bond. Angles around the carbons in the double bond are ~ 120º. Thus, all
Alkenes Bonding and Structure: Carbons in the double bond of butene are sp 2 hybridized. Side on p-p orbital overlap creates a π-bond. Angles around the carbons in the double bond are ~ 120º. Thus, all
Kinetics. Chapter 14. Chemical Kinetics
 Lecture Presentation Chapter 14 Yonsei University In kinetics we study the rate at which a chemical process occurs. Besides information about the speed at which reactions occur, kinetics also sheds light
Lecture Presentation Chapter 14 Yonsei University In kinetics we study the rate at which a chemical process occurs. Besides information about the speed at which reactions occur, kinetics also sheds light
Cracking. 191 minutes. 186 marks. Page 1 of 27
 3.1.6.2 Cracking 191 minutes 186 marks Page 1 of 27 Q1. (a) Gas oil (diesel), kerosine (paraffin), mineral oil (lubricating oil) and petrol (gasoline) are four of the five fractions obtained by the fractional
3.1.6.2 Cracking 191 minutes 186 marks Page 1 of 27 Q1. (a) Gas oil (diesel), kerosine (paraffin), mineral oil (lubricating oil) and petrol (gasoline) are four of the five fractions obtained by the fractional
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 4-3: Continue Alkynes: An Introduction to Organic Synthesis Based on: McMurry s Organic Chemistry,
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 4-3: Continue Alkynes: An Introduction to Organic Synthesis Based on: McMurry s Organic Chemistry,
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
Theoretical Models for Chemical Kinetics
 Theoretical Models for Chemical Kinetics Thus far we have calculated rate laws, rate constants, reaction orders, etc. based on observations of macroscopic properties, but what is happening at the molecular
Theoretical Models for Chemical Kinetics Thus far we have calculated rate laws, rate constants, reaction orders, etc. based on observations of macroscopic properties, but what is happening at the molecular
