Process Analytical Technology. How much oxygen is inside?
|
|
|
- Peregrine Moore
- 5 years ago
- Views:
Transcription
1 Process Analytical Technology How much oxygen is inside? Jacqueline Waldvogel, Yves Wittwer
2 Process Analytics monitoring of process parameters to ensure: cost-effective production product quality efficient planning of maintenances safe plant operation two important concepts related to Process Analytics: feed-forward: proactive, avoid errors before they occur feed-back: reactive, correct errors after they already occurred W. Kessler. Prozessanalytik: Strategien und Fallbeispiele aus der industriellen Praxis Jacqueline Waldvogel, Yves Wittwer
3 Process Analytics for both correction mechanism, online monitoring of certain process parameters is necessary Jacqueline Waldvogel, Yves Wittwer
4 Offline Analysis Pro: analysis is performed by experts flexible cheap appropriate surrounding sample is transported to a laboratory with highly-skilled staff W. Kessler. Prozessanalytik: Strategien und Fallbeispiele aus der industriellen Praxis Contra: slow «ownership of data» not guaranteed direct process control is not possible Jacqueline Waldvogel, Yves Wittwer
5 Atline Analysis (exline) Pro: relatively fast Contra: usually without qualified personal instruments have to be more solid manually or (half-) automatized sampling and analysis near by the process W. Kessler. Prozessanalytik: Strategien und Fallbeispiele aus der industriellen Praxis Jacqueline Waldvogel, Yves Wittwer
6 Online Analysis Pro: fast highly specific analyser feedforward and feedback control possible usually analysis in a bypass Contra: expensive calibration more difficult sampling is accident-sensitive condition: t R (investigated property) > t R (sensor) The time needed for a investigated property to change must be smaller than the time required for a complete measurement (including data evaluation). W. Kessler. Prozessanalytik: Strategien und Fallbeispiele aus der industriellen Praxis Jacqueline Waldvogel, Yves Wittwer
7 Inline Analysis Pro: direct information obtained no sampling needed (therefore less accident-sensitive) similarities to online analysis, but without sample-taking. Pro expense calibration no sample-pre-treatement possible high requirements for instruments W. Kessler. Prozessanalytik: Strategien und Fallbeispiele aus der industriellen Praxis Jacqueline Waldvogel, Yves Wittwer
8 Why monitoring oxygen? large interest in measuring oxygen quantitatively variety of different methods available oxygen is important in many fields, e.g: medicine biology industry Jacqueline Waldvogel, Yves Wittwer
9 Industrial usage of O 2 : A few examples production of: sulfuric acid from sulfur S + O 2 SO 2 2SO 2 + O 2 SO 3 H 2 SO 4 nitric acid (Ostwald process) ethylene oxide hydrogen peroxide (Anthraquinone process) Goor, G.; Glenneberg, J.; Jacobi, S. Ullmann s Encyclopedia of Industrial Chemistry, 18, Jacqueline Waldvogel, Yves Wittwer
10 Considered reaction continuous process direct oxidation silver-based catalyst prevent further oxidation to CO 2 and H 2 O oxygen concentration as crucial parameter -> monitoring of oxygen Jacqueline Waldvogel, Yves Wittwer
11 Needs in this case: typical concentration of oxygen: 6 8 % temperature range: C pressure range: bar high reliability of the analytical method/ instrument gas is consisting different components (organic compounds) fluctuations of gas pressure might be possible installation of the instrument in explosive protected area (ATEX zone 2) Jacqueline Waldvogel, Yves Wittwer
12 ATEX Atmosphères Explosibles ATEX /34/EU: equipment directive ATEX /92/EG: workplace directive explosion protection high-risk areas are divided in zones by ATEX frequency duration of dangerous explosive atmosphere Jacqueline Waldvogel, Yves Wittwer
13 ATEX Jacqueline Waldvogel, Yves Wittwer
14 ATEX avoid ignition sources self ignition, static electricity, ultrasonic, hot surfaces, sparks, -> electrical parts are especially critical ignition protection flush critical components with inert gas (electrical) separate electrical parts in case of emergency protect electrical components from explosion by stable covers W. Kessler. Prozessanalytik: Strategien und Fallbeispiele aus der industriellen Praxis Jacqueline Waldvogel, Yves Wittwer
15 Major methods for oxygen determination Chemical Reactions Winkler method Chromogenic reactions Electrochemical Lambda electrode Clark electrode Gas chromatography Optical Absorption spectroscopy Quenching based Paramagnetic sensors Jacqueline Waldvogel, Yves Wittwer
16 Chemical methods: Winkler method developed 1888 by Ludwig W. Winkler only for dissolved oxygen easy to use due to commercially available kits hod_50_tests_kit/ew , Jacqueline Waldvogel, Yves Wittwer
17 Chemical methods: Winkler Method Step 1: fixation of dissolved oxygen 2 Mn 2+ + O OH - 2 MnO(OH) 2 Step 2: Use Mn(IV) to generate I 2 in an amount, which is proportional to the original concentration of O 2 MnO(OH) 2 + 2I - + 4H + Mn 2+ + I 2 + 3H 2 O Step 3: determine amount of I 2 by titration (eventually with the help of an indicator) 2S 2 O I 2 2S 4 O I - respectively with I 3 - Winkler, L.W. Die Bestimmung des im Wasser gelösten Sauerstoffes. (1888). Jacqueline Waldvogel, Yves Wittwer
18 Chemical methods: Chromogenic Method colour change of dye due to reaction with oxygen example: Ageless Eye reduction usually done chemically (e.g. by ascorbic acid) Jacqueline Waldvogel, Yves Wittwer
19 Chemical methods: Ageless Eye application in food packaging other examples for chromogenic reactions: haemoglobin based based on oxide formation of nickel Wang X., Wolfbeis O.S., Chem. Soc. Rev., 2014, 43, 3666ff. Jacqueline Waldvogel, Yves Wittwer
20 Chemical methods: Pros and Cons Pros Cons - easy to use - no continuous online measurements - often cheap (Winkler Kit: 41.50$ for 50 tests) - time intensive methods - readout often directly by eye, but - subjective readout - very low lifetimes conclusions concerning our case: no online measurements possible no real-time correction of process parameters possible difficult implementation into the plant not suitable for our case Jacqueline Waldvogel, Yves Wittwer
21 Electrochemical methods example: Lambda-type electrode ZrO 2 behaves like a solid electrolyte for oxygen (needs high T >650 C) ZrO 2 pumping disc pumps oxygen from p1 p2 generation of different oxygen concentrations in p2 and p1 according to the Nernst equation a voltage is generated at the ZrO 2 sensing disc Jacqueline Waldvogel, Yves Wittwer
22 Electrochemical methods Nernst equation measured voltage is compared to reference voltages V 1 and V 5 as V 1 is reached, chamber (p2) is evacuated, as V 5 is reached it is pressurized time needed for one cycle is proportional to sample oxygen concentration Jacqueline Waldvogel, Yves Wittwer
23 Electrochemical methods example: Clark electrode O 2 enters the cell through a barrier (e.g. a membrane) cathode: O 2 + 2e - + 2H 2 O H 2 O 2 + 2OH - H 2 O 2 + 2e - 2 OH - anode: 4Ag 4Ag + + 4e - measure current, which is proportional to the amount of O 2 entering the cell many other cell types available as well picture: Jacqueline Waldvogel, Yves Wittwer
24 Electrochemical methods: Pros and Cons Pros Cons - fast - lifetime: 1-2 years - easy to use - aging requires regular calibration - inline analysis possible - temperature dependence - pressure dependence - interferences possible conclusions concerning our case: inline/ online measurements possible real-time measurements possible possible problems because of process conditions and ATEX guidelines in principle suitable for our case Jacqueline Waldvogel, Yves Wittwer
25 Paramagnetic sensors based on paramagnetic properties of triplet oxygen. working principle: sample gas and auxiliary gas (N 2 ) are injected and divided into two streams sample gas and auxiliary meet in ring-shape path at B side, a magnetic field is created, which draws the oxygen of the sample gas into. the flow rate at B decreases in comparison with the one at A. thermistors at point A and B determine the flowrate, which is converted into an electrical signal. its difference is proportional to the amount of oxygen in the sample gas Jacqueline Waldvogel, Yves Wittwer
26 Paramagnetic sensors: Pros and Cons Pros Cons - long-term stable measurements - interferences with other paramagnetic compounds - high resistance to vibrations - only works at low temperatures - high sensitivity (0-1 vol-%) - no moisture allowed - fast response ( 3s) - only works at low pressure - easy calibration conclusions concerning our case: Suitable for online analysis Process temperature too high Process pressure too high not suitable for our case Jacqueline Waldvogel, Yves Wittwer
27 Gas Chromatography An example separation of gases with GC possible example: Analysis of power plant flue gases carrier Gas: H2 pressure: 2.2 bar two columns Porapak Q (apolar polymer) molecular sieve 5A detector: TCD %20Technical%20Notes/Chromatography/Gas%20Chromatography/AN GC-Flue%20Gases- TRACE% AN10351-EN.pdf, Jacqueline Waldvogel, Yves Wittwer
28 Gas Chromatography Detection? GC-MS: may have some problems with low mass of oxygen GC-IR: does not work since O 2 is IR-inactive TCD (thermal conductivity detector) Jacqueline Waldvogel, Yves Wittwer
29 Thermal Conductivity Detector, TCD measuring thermal conductivity of the sample and a reference electrically heated filament surrounded by gas (sample or reference) heat is conducted by gas to detector block conductivity is dependant of the gas (composition) reference and gas cell form a wheatstone bridge record a voltage difference svg, Jacqueline Waldvogel, Yves Wittwer
30 Gas Chromatography: Pros and Cons Pros Cons - high precision - slow - case specific - potential use of explosive gases conclusions concerning our case: no online measurements possible no real-time correction of process parameters possible process pressure is too high problems with ATEX guidelines? Not suitable for our case Jacqueline Waldvogel, Yves Wittwer
31 Absorption spectroscopy absorption bands of O 2 : 760 nm, one deep in the UV 760 nm: b 1 Σ + g Χ 3 Σ - g (triplet singlet) emission peak: 1270 nm UV/Vis absorption continuous measurement Wang X., Wolfbeis O.S., Chem. Soc. Rev., 2014, 43, 3666ff Jacqueline Waldvogel, Yves Wittwer
32 Absorption spectroscopy TDLAS: Tuneable diode laser absorption spectroscopy excitation by tuneable laser measure absorption to determine concentration, temperature or pressure Pro: high sensitivity wide temperature range (up to 1000 C) ser-absorption-photonik pdf, Jacqueline Waldvogel, Yves Wittwer
33 Absorption spectroscopy: Pros and Cons Pros Cons - easy to handle - expensive instrument (TDLAS) - fast (TDLAS) - bulky instrumentation - interferences (H 2 O, CO 2 ) conclusions concerning our case: online measurement possible real-time measurement possible problems with interferences? in principle suitable for our case Wang X., Wolfbeis O.S., Chem. Soc. Rev., 2014, 43, 3666ff. ser-absorption-photonik pdf, Jacqueline Waldvogel, Yves Wittwer
34 Quenching based methods Jablonski diagram dynamic quenching: collision induced energy transfer Jacqueline Waldvogel, Yves Wittwer
35 Quenching based methods Stern-Volmer equation describes the relation between the luminescence intensity and the oxygen concentration Stern-Volmer equation: F 0 / F: fluorescence without/ with oxygen, K SV : Stern-Volmer constant (function of probe lifetime and polymeric solvent) Wang X., Wolfbeis O.S., Chem. Soc. Rev., 2014, 43, 3666ff. Jacqueline Waldvogel, Yves Wittwer
36 Quenching based methods in reality luminescence intensity is often not linearly dependent on oxygen concentration this is caused by different surroundings of the fluorophore by the polymer (see next slide) this can be corrected by using e.g. multiple Stern-Volmer constants representing the different environments f: fraction of total emission corresponding to each component Wang X., Wolfbeis O.S., Chem. Soc. Rev., 2014, 43, 3666ff. Jacqueline Waldvogel, Yves Wittwer
37 Quenching based methods optical isolation non-transparent layer avoid interferences e.g. by ambient light oxygen sensing layer luminophore solid support polymer matrix/ layer Wang X., Wolfbeis O.S., Chem. Soc. Rev., 2014, 43, 3666ff. Jacqueline Waldvogel, Yves Wittwer
38 Oxygen sensing layer in principle, every luminophore interacting with oxygen can be used properties of the luminophore (e.g. stability, lifetime of excited states, ) mainly (but not only) determine properties of sensor suitable luminophores are for example: fullerenes diverse metal-ligand complexes porphyrins Wang X., Wolfbeis O.S., Chem. Soc. Rev., 2014, 43, 3666ff. Jacqueline Waldvogel, Yves Wittwer
39 Solid support either protection layer or host matrix for luminophore requirements optically transparent oxygen permeability long-term stability if used as host matrix, solid support and luminophore must be compatible diverse functions protection of luminophore modify luminophore properties, e.g. lifetime of excited state selective diffusion of oxygen for example: silicones Wang X., Wolfbeis O.S., Chem. Soc. Rev., 2014, 43, 3666ff. Jacqueline Waldvogel, Yves Wittwer
40 Quenching based methods: Pros and Cons Pros Cons - highly adjustable - possible interferences with other quenchers, e.g. SO 2, NO x, many olefins, halogenated organic species, humidity,... - high sensitivity possible - not many sensors working at high temperatures - fast - extremely case specific conclusions concerning our case: online/ inline measurements possible real-time measurements possible difficult to find proper sensor, e.g. Mo 6 Cl 12 sensor can be used at high temperature (> 600 C) In principle suitable for our case Wang X., Wolfbeis O.S., Chem. Soc. Rev., 2014, 43, 3666ff. Jacqueline Waldvogel, Yves Wittwer
41 Conclusion suitable electrochemical methods absorption spectroscopy quenching based methods not suitable chemical methods paramagnetic sensors gas chromatography Jacqueline Waldvogel, Yves Wittwer
42 Abbreviations LMB: leucomethylene blue MB: methylene blue Jacqueline Waldvogel, Yves Wittwer
Thermo Scientific Electrochemistry Products
 Comparing Performance of DO Methods The world leader in serving science in Water and Seawaters Thermo Scientific Electrochemistry Products Dissolved Oxygen Measurement Oxygen is essential for most of the
Comparing Performance of DO Methods The world leader in serving science in Water and Seawaters Thermo Scientific Electrochemistry Products Dissolved Oxygen Measurement Oxygen is essential for most of the
Process Analyzer and Sampling Systems
 Process Analyzer and Sampling Systems Course Price 3050 Course Description Analytical instruments used for online chemical analysis of process streams or plant environments are generally called Process
Process Analyzer and Sampling Systems Course Price 3050 Course Description Analytical instruments used for online chemical analysis of process streams or plant environments are generally called Process
 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS)
 New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS) Jean-Nicolas Adami, PhD Head of Strategic Product Group Gas Analytics Mettler-Toledo GmbH,
New advances in folded pathlength technology for Process Tunable Diode Laser Absorption Spectrometers (TDLAS) Jean-Nicolas Adami, PhD Head of Strategic Product Group Gas Analytics Mettler-Toledo GmbH,
Surface Mount UV LED. NUVA33 Series PART NUMBERING SYSTEM. WAVELENGTH CODES Code Nominal Wavelength
 FEATURES SURFACE MOUNT 3.4mm x 3.4mm x 2.37mm WAVELENGTH 365 ~ 45nm FOR UV CURING, PHOTO CATALYST & SENSOR LIGHTING RoHS COMPLIANT COMPATIBLE WITH REFLOW SOLDERING TAPE AND REEL PACKAGING SPECIFICATIONS
FEATURES SURFACE MOUNT 3.4mm x 3.4mm x 2.37mm WAVELENGTH 365 ~ 45nm FOR UV CURING, PHOTO CATALYST & SENSOR LIGHTING RoHS COMPLIANT COMPATIBLE WITH REFLOW SOLDERING TAPE AND REEL PACKAGING SPECIFICATIONS
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Batteries (Electrochemical Power Sources)
 Batteries (Electrochemical Power Sources) 1. Primary (single-discharge) batteries. => finite quantity of the reactants 2. Secondary or rechargeable batteries => regeneration of the original reactants by
Batteries (Electrochemical Power Sources) 1. Primary (single-discharge) batteries. => finite quantity of the reactants 2. Secondary or rechargeable batteries => regeneration of the original reactants by
White Paper. Overview: NDIR Definition:
 Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
LAB007 Industrial Analytical Chemistry & Process Analyzer System
 LAB007 Industrial Analytical Chemistry & Process Analyzer System H.H. Sheikh Sultan Tower (0) Floor Corniche Street Abu Dhabi U.A.E www.ictd.ae ictd@ictd.ae Course Introduction: Analytical instruments
LAB007 Industrial Analytical Chemistry & Process Analyzer System H.H. Sheikh Sultan Tower (0) Floor Corniche Street Abu Dhabi U.A.E www.ictd.ae ictd@ictd.ae Course Introduction: Analytical instruments
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Hydrogen production by electrolysis. Ann Cornell, Department of Chemical Engineering, KTH
 Hydrogen production by electrolysis Ann Cornell, Department of Chemical Engineering, KTH amco@kth.se Sources for hydrogen International Energy Agency. Technology Roadmap Hydrogen and Fuel Cells, 2015
Hydrogen production by electrolysis Ann Cornell, Department of Chemical Engineering, KTH amco@kth.se Sources for hydrogen International Energy Agency. Technology Roadmap Hydrogen and Fuel Cells, 2015
Gaseous CEMS technology
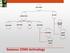 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
Basic Digestion Principles
 Basic Digestion Principles 1 From Samples to Solutions Direct Analytical Method Solid Sample Problems: Mech. Sample Preparation (Grinding, Sieving, Weighing, Pressing, Polishing,...) Solid Sample Autosampler
Basic Digestion Principles 1 From Samples to Solutions Direct Analytical Method Solid Sample Problems: Mech. Sample Preparation (Grinding, Sieving, Weighing, Pressing, Polishing,...) Solid Sample Autosampler
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
Hydrogen & Chlorine monitoring for the Chlor-Alkali Industry
 technical datasheet Application Note - AN9041 Hydrogen & Chlorine monitoring for the Chlor-Alkali Industry Measurement Technology Limited (MTL) part of Eaton's Crouse-Hinds business, design and manufacture
technical datasheet Application Note - AN9041 Hydrogen & Chlorine monitoring for the Chlor-Alkali Industry Measurement Technology Limited (MTL) part of Eaton's Crouse-Hinds business, design and manufacture
Unit 4: Chemical Changes (Higher Content)
 Metals react with oxygen to produce metal oxides. E.g. Copper + Oxygen > Copper Oxide The reactions are oxidation reactions because the metals gain oxygen. Reactivity of Metals Metal Extraction Metals
Metals react with oxygen to produce metal oxides. E.g. Copper + Oxygen > Copper Oxide The reactions are oxidation reactions because the metals gain oxygen. Reactivity of Metals Metal Extraction Metals
ICP-3000 Inductively Coupled Plasma Optical Emission Spectrometer
 Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer is powerful simultaneous full
Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer is powerful simultaneous full
Covalent (sharing of electron pairs) Ionic ( electrostatic attraction between oppositely charged ions)
 Covalent (sharing of electron pairs) Ionic ( electrostatic attraction between oppositely charged ions) Metallic (electrostatic attraction between + metal ions and delocalised electrons) Group 1 ions 1+
Covalent (sharing of electron pairs) Ionic ( electrostatic attraction between oppositely charged ions) Metallic (electrostatic attraction between + metal ions and delocalised electrons) Group 1 ions 1+
Questions on Instrumental Methods of Analysis
 Questions on Instrumental Methods of Analysis 1. Which one of the following techniques can be used for the detection in a liquid chromatograph? a. Ultraviolet absorbance or refractive index measurement.
Questions on Instrumental Methods of Analysis 1. Which one of the following techniques can be used for the detection in a liquid chromatograph? a. Ultraviolet absorbance or refractive index measurement.
a. An emission line as close as possible to the analyte resonance line
 Practice Problem Set 5 Atomic Emission Spectroscopy 10-1 What is an internal standard and why is it used? An internal standard is a substance added to samples, blank, and standards. The ratio of the signal
Practice Problem Set 5 Atomic Emission Spectroscopy 10-1 What is an internal standard and why is it used? An internal standard is a substance added to samples, blank, and standards. The ratio of the signal
Department of Chemistry Physical Chemistry Göteborg University
 Department of Chemistry Physical Chemistry Göteborg University &RQVWUXFWLRQRIDSXOVHGG\HODVHU 3OHDVHREVHUYHWKDWWKHVDIHW\SUHFDXWLRQVRQSDJHPXVW EHIROORZHGRWKHUZLVHWKHUHLVDULVNRIH\HGDPDJH Renée Andersson -97,
Department of Chemistry Physical Chemistry Göteborg University &RQVWUXFWLRQRIDSXOVHGG\HODVHU 3OHDVHREVHUYHWKDWWKHVDIHW\SUHFDXWLRQVRQSDJHPXVW EHIROORZHGRWKHUZLVHWKHUHLVDULVNRIH\HGDPDJH Renée Andersson -97,
AL108 Process Analyzers & Sampling Systems
 AL108 Process Analyzers & Sampling Systems AL108 Rev.002 CMCT COURSE OUTLINE Page 1 of 8 Training Description: Analytical instruments used for online chemical analysis of process streams or plant environments
AL108 Process Analyzers & Sampling Systems AL108 Rev.002 CMCT COURSE OUTLINE Page 1 of 8 Training Description: Analytical instruments used for online chemical analysis of process streams or plant environments
Sampling for Gases and Vapors
 Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Overview Brochure Comprehensive range of instrumentation for the measurement of moisture and oxygen
 Overview Brochure 2016 Comprehensive range of instrumentation for the measurement of moisture and oxygen Overview Brochure Relative Humidity Probes and Transmitters Repeatable RH and temperature measurement
Overview Brochure 2016 Comprehensive range of instrumentation for the measurement of moisture and oxygen Overview Brochure Relative Humidity Probes and Transmitters Repeatable RH and temperature measurement
Pros and Cons of Water Analysis Methods
 Water Lens, LLC 4265 San Felipe, Suite 1100 Houston, Texas 77027 Office: (844) 987-5367 www.waterlensusa.com Pros and Cons of Water Analysis Methods Prepared by: Adam Garland, CTO Water Lens, LLC ICP-MS/OES
Water Lens, LLC 4265 San Felipe, Suite 1100 Houston, Texas 77027 Office: (844) 987-5367 www.waterlensusa.com Pros and Cons of Water Analysis Methods Prepared by: Adam Garland, CTO Water Lens, LLC ICP-MS/OES
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
GC Instruments. GC Instruments - Sample Introduction
 GC Instruments 1 Fairly simple instrumentation Maintaining constant average pressure is important! Pressure controls flow rate T influences retention (k ) Flow rate monitoring Changing flow rate changes
GC Instruments 1 Fairly simple instrumentation Maintaining constant average pressure is important! Pressure controls flow rate T influences retention (k ) Flow rate monitoring Changing flow rate changes
Supplementary material
 Supplementary material Title: Photocatalytic Hydrogen Production from a noble metal free system based on a water soluble porphyrin derivative and a cobaloxime catalyst Authors: Theodore Lazarides, Milan
Supplementary material Title: Photocatalytic Hydrogen Production from a noble metal free system based on a water soluble porphyrin derivative and a cobaloxime catalyst Authors: Theodore Lazarides, Milan
Application Note: Ocean Optics in the Teaching and Research Laboratories Susquehanna University Department of Chemistry July 2014
 Application Note: Ocean Optics in the Teaching and Research Laboratories Susquehanna University Department of Chemistry July 2014 Authors: Dr. Swarna Basu (Associate Professor of Chemistry), Dr. Wade Johnson
Application Note: Ocean Optics in the Teaching and Research Laboratories Susquehanna University Department of Chemistry July 2014 Authors: Dr. Swarna Basu (Associate Professor of Chemistry), Dr. Wade Johnson
Analytical Strategy: HS 2014 Rafael Hodel, Stefanie Jucker. Quality Control: Quantitative Nuclear Magnetic Resonance
 Analytical Strategy: HS 2014 Rafael Hodel, Stefanie Jucker Quality Control: Quantitative Nuclear Magnetic Resonance Quality Control (QC) - Why? 1) Purchasing raw materials and educts Did we get what we
Analytical Strategy: HS 2014 Rafael Hodel, Stefanie Jucker Quality Control: Quantitative Nuclear Magnetic Resonance Quality Control (QC) - Why? 1) Purchasing raw materials and educts Did we get what we
Spectroscopy in Transmission
 Spectroscopy in Transmission + Reflectance UV/VIS - NIR Absorption spectra of solids and liquids can be measured with the desktop spectrograph Lambda 9. Extinctions up to in a wavelength range from UV
Spectroscopy in Transmission + Reflectance UV/VIS - NIR Absorption spectra of solids and liquids can be measured with the desktop spectrograph Lambda 9. Extinctions up to in a wavelength range from UV
AASS, Örebro University
 Mobile Robotics Achim J. and Lilienthal Olfaction Lab, AASS, Örebro University 1 Contents 1. "Classical" Electronic Nose 2. Further Gas Sensing Technologies for Mobile Robots 3. (Signal Processing in Electronic
Mobile Robotics Achim J. and Lilienthal Olfaction Lab, AASS, Örebro University 1 Contents 1. "Classical" Electronic Nose 2. Further Gas Sensing Technologies for Mobile Robots 3. (Signal Processing in Electronic
Chapter 7. Oxidation-Reduction Reactions
 Chapter 7 Oxidation-Reduction Reactions Chapter Map Oxidation Historically oxidation meant reacting with oxygen. 2Zn(s) + O 2 (g) 2ZnO(s) Zn Zn 2+ + 2e or 2Zn 2Zn 2+ + 4e O + 2e O 2 or O 2 + 4e 2O 2 Oxidation
Chapter 7 Oxidation-Reduction Reactions Chapter Map Oxidation Historically oxidation meant reacting with oxygen. 2Zn(s) + O 2 (g) 2ZnO(s) Zn Zn 2+ + 2e or 2Zn 2Zn 2+ + 4e O + 2e O 2 or O 2 + 4e 2O 2 Oxidation
EE 5344 Introduction to MEMS CHAPTER 7 Biochemical Sensors. Biochemical Microsensors
 I. Basic Considerations & Definitions 1. Definitions: EE 5344 Introduction to MEMS CHAPTER 7 Biochemical Sensors Chemical/ Biological quantity Biochemical Microsensors Electrical Signal Ex: Chemical species
I. Basic Considerations & Definitions 1. Definitions: EE 5344 Introduction to MEMS CHAPTER 7 Biochemical Sensors Chemical/ Biological quantity Biochemical Microsensors Electrical Signal Ex: Chemical species
novaa 800 D Atomic Absorption Spectrometer
 Technical Data Atomic Absorption Spectrometer Cpt : +27 (0) 21 905 0476 Jhb : +27 (0) 11 794 Dbn : +27 (0) 31 266 2454 1/7 General The is a compact atomic absorption spectrometer with deuterium background
Technical Data Atomic Absorption Spectrometer Cpt : +27 (0) 21 905 0476 Jhb : +27 (0) 11 794 Dbn : +27 (0) 31 266 2454 1/7 General The is a compact atomic absorption spectrometer with deuterium background
Chapter 20. Electrochemistry. Chapter 20 Problems. Electrochemistry 7/3/2012. Problems 15, 17, 19, 23, 27, 29, 33, 39, 59
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College Cottleville, MO Chapter 20 Problems
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College Cottleville, MO Chapter 20 Problems
for sodium ion (Na + )
 3.4 Unit 2 Chemistry 2 Throughout this unit candidates will be expected to write word equations for reactions specified. Higher tier candidates will also be expected to write and balance symbol equations
3.4 Unit 2 Chemistry 2 Throughout this unit candidates will be expected to write word equations for reactions specified. Higher tier candidates will also be expected to write and balance symbol equations
Atomic Absorption Spectrometer ZEEnit P series
 Atomic Absorption Spectrometer ZEEnit P series Technical Data ZEEnit series Update 07/2014 OBue 1/ 5 ZEEnit P series Variable high-end AA Spectrometer with Deuterium and Zeeman Background Correction with
Atomic Absorption Spectrometer ZEEnit P series Technical Data ZEEnit series Update 07/2014 OBue 1/ 5 ZEEnit P series Variable high-end AA Spectrometer with Deuterium and Zeeman Background Correction with
PHYSICS. ElectroChemical Effects. Rishi Gopie
 ElectroChemical Effects Rishi Gopie ELECTRO CHEMICAL EFFECTS Cells A cell is a device that converts chemical energy to electrical energy by producing electrons during chemical reactions. It gives a steady
ElectroChemical Effects Rishi Gopie ELECTRO CHEMICAL EFFECTS Cells A cell is a device that converts chemical energy to electrical energy by producing electrons during chemical reactions. It gives a steady
Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases and vapours Date: January 15-17, 2016 Language: English
 Course n : Course 3 Title: RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases
Course n : Course 3 Title: RESPIRATORY PHYSIOLOGY, PHYSICS AND PATHOLOGY IN RELATION TO ANAESTHESIA AND INTENSIVE CARE Sub-category: Physics and Principles of Measurement Topic: Monitoring anesthetic gases
Introduction to electrochemistry
 Introduction to electrochemistry Oxidation reduction reactions involve energy changes. Because these reactions involve electronic transfer, the net release or net absorption of energy can occur in the
Introduction to electrochemistry Oxidation reduction reactions involve energy changes. Because these reactions involve electronic transfer, the net release or net absorption of energy can occur in the
Fluorescence Spectrophotometry
 Chemistry 422L Manual Page 27 I. Introduction Fluorescence Spectrophotometry Ru(bpy) 3 2+, where bpy = 2, 2' bipyridine, has been one of the most widely studied metal complexes in recent years. Interest
Chemistry 422L Manual Page 27 I. Introduction Fluorescence Spectrophotometry Ru(bpy) 3 2+, where bpy = 2, 2' bipyridine, has been one of the most widely studied metal complexes in recent years. Interest
Applications of Voltaic Cells
 Applications of Voltaic Cells Lesson 4 chapter 13 Objective You will be able to explain how the development of the voltaic cell had affected society. Dry Cells Since voltaic cells are not portable, dry
Applications of Voltaic Cells Lesson 4 chapter 13 Objective You will be able to explain how the development of the voltaic cell had affected society. Dry Cells Since voltaic cells are not portable, dry
Direct, Reliable, and Correct Oxygen Measurement
 Direct, Reliable, and Correct Oxygen Measurement February, 2015 Oxygen-dependent quenching of phosphorescence has been used extensively for the last 25 years as a reliable method for measuring oxygen.
Direct, Reliable, and Correct Oxygen Measurement February, 2015 Oxygen-dependent quenching of phosphorescence has been used extensively for the last 25 years as a reliable method for measuring oxygen.
ELECTROCHEMICAL COMPRESSION OF PRODUCT HYDROGEN FROM PEM ELECTROLYZER STACK
 ELECTROCHEMICAL COMPRESSION OF PRODUCT HYDROGEN FROM PEM ELECTROLYZER STACK N.V. Dale 1,*, C. Y. Biaku 1, M. D. Mann 1, H. Salehfar 2, A. J. Peters 2 Abstract The low volumetric energy density of hydrogen
ELECTROCHEMICAL COMPRESSION OF PRODUCT HYDROGEN FROM PEM ELECTROLYZER STACK N.V. Dale 1,*, C. Y. Biaku 1, M. D. Mann 1, H. Salehfar 2, A. J. Peters 2 Abstract The low volumetric energy density of hydrogen
RoHS. Specification CUD8AF1C. 서식 Rev: 00
 Specification RoHS CUD8AF1C 1 [ Contents ] 1. Description 2. Outline dimensions 3. Characteristics of CUD8AF1C 4. Characteristic diagrams 5. Binning & Labeling 6. Reel packing 7. Recommended solder pad
Specification RoHS CUD8AF1C 1 [ Contents ] 1. Description 2. Outline dimensions 3. Characteristics of CUD8AF1C 4. Characteristic diagrams 5. Binning & Labeling 6. Reel packing 7. Recommended solder pad
Reference literature. (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters )
 September 17, 2018 Reference literature (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters 13-14 ) Reference.: https://slideplayer.com/slide/8354408/ Spectroscopy Usual Wavelength Type of Quantum
September 17, 2018 Reference literature (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters 13-14 ) Reference.: https://slideplayer.com/slide/8354408/ Spectroscopy Usual Wavelength Type of Quantum
Experiment. Quantification of Ascorbic acid by Fluorescence Spectroscopy1
 Experiment. Quantification of Ascorbic acid by Fluorescence Spectroscopy Modified 10/2017 Experiment. Quantification of Ascorbic acid by Fluorescence Spectroscopy1 Objective: The goal of this experiment
Experiment. Quantification of Ascorbic acid by Fluorescence Spectroscopy Modified 10/2017 Experiment. Quantification of Ascorbic acid by Fluorescence Spectroscopy1 Objective: The goal of this experiment
Ultra-High Vacuum Technology. Sputter Ion Pumps l/s
 Ultra-High Vacuum Technology 30-400 l/s 181.06.01 Excerpt from the Product Chapter C15 Edition November 2007 Contents General General..........................................................................
Ultra-High Vacuum Technology 30-400 l/s 181.06.01 Excerpt from the Product Chapter C15 Edition November 2007 Contents General General..........................................................................
Achim J. Lilienthal Erik Schaffernicht Achim J. Lilienthal. AASS, Örebro University
 Achim J. Lilienthal Erik Schaffernicht Mobile Achim J. Achim J. Lilienthal Room T1222 Robotics and Lilienthal Olfaction Room T1211 Lab, Erik Schaffernicht achim.lilienthal@oru.se AASS, Örebro University
Achim J. Lilienthal Erik Schaffernicht Mobile Achim J. Achim J. Lilienthal Room T1222 Robotics and Lilienthal Olfaction Room T1211 Lab, Erik Schaffernicht achim.lilienthal@oru.se AASS, Örebro University
GCSE CHEMISTRY REVISION LIST
 GCSE CHEMISTRY REVISION LIST OCR Gateway Chemistry (J248) from 2016 Topic C1: Particles C1.1 Describe the main features of the particle model in terms of states of matter and change of state Explain, in
GCSE CHEMISTRY REVISION LIST OCR Gateway Chemistry (J248) from 2016 Topic C1: Particles C1.1 Describe the main features of the particle model in terms of states of matter and change of state Explain, in
ELECTROCHEMICAL TECHNIQUES, OSMOMETRY AND THE PRINCIPLES OF RADIOACTIVITY
 ELECTROCHEMICAL TECHNIQUES, OSMOMETRY AND THE PRINCIPLES OF RADIOACTIVITY ELECTROCHEMISTY ELECTROCHEMISTRY IS THE STUDY OF CHEMICAL REACTIONS THAT RESULT IN THE FLOW OF ELECTRONS (CURRENT) OR THE DEVELOPMENT
ELECTROCHEMICAL TECHNIQUES, OSMOMETRY AND THE PRINCIPLES OF RADIOACTIVITY ELECTROCHEMISTY ELECTROCHEMISTRY IS THE STUDY OF CHEMICAL REACTIONS THAT RESULT IN THE FLOW OF ELECTRONS (CURRENT) OR THE DEVELOPMENT
Vernier Probe Inventory List
 Physics Equipment Current Probe Used to measure currents in low-voltage AC and DC circuits, or it can be used in electrochemistry experiments. Differential Voltage Used to measure voltages in low voltage
Physics Equipment Current Probe Used to measure currents in low-voltage AC and DC circuits, or it can be used in electrochemistry experiments. Differential Voltage Used to measure voltages in low voltage
ISEmax CAM40/CAS40. Technical Information
 Technical Information Online measurement of nutrient parameters Ion-selective electrode system for the continuous measurement of ammonium and nitrate Application The ion-selective electrode system works
Technical Information Online measurement of nutrient parameters Ion-selective electrode system for the continuous measurement of ammonium and nitrate Application The ion-selective electrode system works
Analytical techniques: Environmental samples. Lecture 2 Universidade do Algarve
 Analytical techniques: Environmental samples Lecture 2 Universidade do Algarve Terms, definitions & applications Difference between technique and method: Analytical technique: Fundamental scientific application
Analytical techniques: Environmental samples Lecture 2 Universidade do Algarve Terms, definitions & applications Difference between technique and method: Analytical technique: Fundamental scientific application
In terms of production, nitric acid is the third most widely produced acid across the world.
 In terms of production, nitric acid is the third most widely produced acid across the world. It has a wide range of uses in agriculture, industry and medicine where it is used as a fertiliser and in the
In terms of production, nitric acid is the third most widely produced acid across the world. It has a wide range of uses in agriculture, industry and medicine where it is used as a fertiliser and in the
e - Galvanic Cell 1. Voltage Sources 1.1 Polymer Electrolyte Membrane (PEM) Fuel Cell
 Galvanic cells convert different forms of energy (chemical fuel, sunlight, mechanical pressure, etc.) into electrical energy and heat. In this lecture, we are interested in some examples of galvanic cells.
Galvanic cells convert different forms of energy (chemical fuel, sunlight, mechanical pressure, etc.) into electrical energy and heat. In this lecture, we are interested in some examples of galvanic cells.
LAB #3: FLUROESCENCE SPECTROSCOPY AND ELECTRON TRANSFER (This lab is adapted from the U of MN Phsyical Chemistry lab manual)
 Chemistry 372 Gustavus Adolphus College A. Purpose LAB #3: FLUROESCENCE SPECTROSCOPY AND ELECTRON TRANSFER (This lab is adapted from the U of MN Phsyical Chemistry lab manual) In this experiment, you will
Chemistry 372 Gustavus Adolphus College A. Purpose LAB #3: FLUROESCENCE SPECTROSCOPY AND ELECTRON TRANSFER (This lab is adapted from the U of MN Phsyical Chemistry lab manual) In this experiment, you will
ISA QATAR : 90 th Seminar
 ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
ANALYTICAL INSTRUMENTATION & MAINTENANCE SYSTEMS (AIMS) ISA QATAR : 90 th Seminar UTILIZATION OF LASERS IN THE FIELD OF GAS ANALYZERS Presenter: ZAHEER JUDDY Electromagnetic Spectrum Electromagnetic Spectrum
Introduction Oxidation/reduction reactions involve the exchange of an electron between chemical species.
 Introduction Oxidation/reduction reactions involve the exchange of an electron between chemical species. The species that loses the electron is oxidized. The species that gains the electron is reduced.
Introduction Oxidation/reduction reactions involve the exchange of an electron between chemical species. The species that loses the electron is oxidized. The species that gains the electron is reduced.
Supporting Information
 Supporting Information Photocatalytic H 2 production in aqueous solution with host-guest inclusions formed by insertion of an FeFe-hydrogenase mimic and organic dye into cyclodextrins respectively Xueqiang
Supporting Information Photocatalytic H 2 production in aqueous solution with host-guest inclusions formed by insertion of an FeFe-hydrogenase mimic and organic dye into cyclodextrins respectively Xueqiang
C2 Revision Pack (Please keep this pack with you)
 Name: C2 Revision Pack (Please keep this pack with you) Follow all the steps below... 1) Practice all the maths and working scientifically questions PRACTICE ALL THESE QUESTIONS! Maths and Science Skills
Name: C2 Revision Pack (Please keep this pack with you) Follow all the steps below... 1) Practice all the maths and working scientifically questions PRACTICE ALL THESE QUESTIONS! Maths and Science Skills
Monitoring Nitrogen Trichloride in Chlorine Manufacture. solvent. O NaOH + 1 /2H 2
 Application Summary Analytes: (nitrogen trichloride) Detector: OMA-300 Process Analyzer Process Stream: headspace gas above saturated CCl 4 solvent Typical Measurement Range: 0-20,000 ppm Introduction
Application Summary Analytes: (nitrogen trichloride) Detector: OMA-300 Process Analyzer Process Stream: headspace gas above saturated CCl 4 solvent Typical Measurement Range: 0-20,000 ppm Introduction
Chapter 9 Oxidation-Reduction Reactions. An Introduction to Chemistry by Mark Bishop
 Chapter 9 Oxidation-Reduction Reactions An Introduction to Chemistry by Mark Bishop Chapter Map Oxidation Historically, oxidation meant reacting with oxygen. 2Zn(s) + O 2 (g) 2ZnO(s) Zn Zn 2+ + 2e or 2Zn
Chapter 9 Oxidation-Reduction Reactions An Introduction to Chemistry by Mark Bishop Chapter Map Oxidation Historically, oxidation meant reacting with oxygen. 2Zn(s) + O 2 (g) 2ZnO(s) Zn Zn 2+ + 2e or 2Zn
N Goalby chemrevise.org
 Redox Equilibria Electrochemical cells This type of cell can be called a Voltaic cell or Galvanic cell. Voltaic cells convert energy from spontaneous, exothermic chemical processes to electrical energy.
Redox Equilibria Electrochemical cells This type of cell can be called a Voltaic cell or Galvanic cell. Voltaic cells convert energy from spontaneous, exothermic chemical processes to electrical energy.
RoHS. Specification CUD8DF1A. Drawn Approval Approval. 서식 Rev: 00
 Specification RoHS CUD8DF1A SVC Customer Drawn Approval Approval 1 [ Contents ] 1. Description 2. Outline dimensions 3. Characteristics of CUD8DF1A 4. Characteristic diagrams 5. Binning & Labeling 6. Reel
Specification RoHS CUD8DF1A SVC Customer Drawn Approval Approval 1 [ Contents ] 1. Description 2. Outline dimensions 3. Characteristics of CUD8DF1A 4. Characteristic diagrams 5. Binning & Labeling 6. Reel
Understanding ph Troubleshooting and Diagnostic information
 Understanding ph Troubleshooting and Diagnostic information The desire is to achieve an accurate, reliable measurement with a reasonable electrode life expectancy while minimizing frequency or complexity
Understanding ph Troubleshooting and Diagnostic information The desire is to achieve an accurate, reliable measurement with a reasonable electrode life expectancy while minimizing frequency or complexity
Electrolytic processes Notes
 Edexcel GCSE Chemistry Topic 3: Chemical changes Electrolytic processes Notes 3.22 Recall that electrolytes are ionic compounds in the molten state or dissolved in water When an ionic substance is melted
Edexcel GCSE Chemistry Topic 3: Chemical changes Electrolytic processes Notes 3.22 Recall that electrolytes are ionic compounds in the molten state or dissolved in water When an ionic substance is melted
ELECTROCHEMISTRY Chapter 19, 4.9
 ELECTROCHEMISTRY Chapter 19, 4.9 Overview of an Electrochemical Process at Constant T and P ΔG = ΔG o + RT ln Q = welec (maximum) Note: I below stands for current measured in amperes = qecell = ItEcell
ELECTROCHEMISTRY Chapter 19, 4.9 Overview of an Electrochemical Process at Constant T and P ΔG = ΔG o + RT ln Q = welec (maximum) Note: I below stands for current measured in amperes = qecell = ItEcell
The ph-responsive behaviour of aqueous solutions of poly(acrylic acid) is dependent on molar mass
 Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2016 The ph-responsive behaviour of aqueous solutions of poly(acrylic acid) is dependent on molar
Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2016 The ph-responsive behaviour of aqueous solutions of poly(acrylic acid) is dependent on molar
2401 Gas (liquid) Chromatography
 2401 Gas (liquid) Chromatography Chromatography Scheme Gas chromatography - specifically gas-liquid chromatography - involves a sample being vaporized and injected onto the head of the chromatographic
2401 Gas (liquid) Chromatography Chromatography Scheme Gas chromatography - specifically gas-liquid chromatography - involves a sample being vaporized and injected onto the head of the chromatographic
Earlier Lecture. In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide.
 41 1 Earlier Lecture In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide. Silicon diodes have negligible i 2 R losses. Cernox RTDs offer high response
41 1 Earlier Lecture In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide. Silicon diodes have negligible i 2 R losses. Cernox RTDs offer high response
LABORATORY OF ELEMENTARY BIOPHYSICS
 LABORATORY OF ELEMENTARY BIOPHYSICS Experimental exercises for III year of the First cycle studies Field: Applications of physics in biology and medicine Specialization: Molecular Biophysics Fluorescence
LABORATORY OF ELEMENTARY BIOPHYSICS Experimental exercises for III year of the First cycle studies Field: Applications of physics in biology and medicine Specialization: Molecular Biophysics Fluorescence
Far UV Absorbance Detector
 Far UV Absorbance Detector Theory Most organic and inorganic species absorb strongly in the far UV. Notable exceptions are the inert gases, helium and nitrogen which absorb very weakly in this region.
Far UV Absorbance Detector Theory Most organic and inorganic species absorb strongly in the far UV. Notable exceptions are the inert gases, helium and nitrogen which absorb very weakly in this region.
Chapter 15 Molecular Luminescence Spectrometry
 Chapter 15 Molecular Luminescence Spectrometry Two types of Luminescence methods are: 1) Photoluminescence, Light is directed onto a sample, where it is absorbed and imparts excess energy into the material
Chapter 15 Molecular Luminescence Spectrometry Two types of Luminescence methods are: 1) Photoluminescence, Light is directed onto a sample, where it is absorbed and imparts excess energy into the material
(18) WMP/Jun10/CHEM5
 Electrochemistry 18 7 The electrons transferred in redox reactions can be used by electrochemical cells to provide energy. Some electrode half-equations and their standard electrode potentials are shown
Electrochemistry 18 7 The electrons transferred in redox reactions can be used by electrochemical cells to provide energy. Some electrode half-equations and their standard electrode potentials are shown
Electrode half-equation. H 2O(l)
 Q1.This table shows some standard electrode potential data. Electrode half-equation E ϴ / V Au + (aq) + e Au(s) +1.68 O 2(g) + 2H + (aq) + 2e H 2O(l) +1.23 Ag + (aq) + e Ag(s) +0.80 Fe 3+ (aq) + e Fe 2+
Q1.This table shows some standard electrode potential data. Electrode half-equation E ϴ / V Au + (aq) + e Au(s) +1.68 O 2(g) + 2H + (aq) + 2e H 2O(l) +1.23 Ag + (aq) + e Ag(s) +0.80 Fe 3+ (aq) + e Fe 2+
Combustion Ion Chromatography. Fast and reliable determination of halogens and sulfur
 Combustion Ion Chromatography Fast and reliable determination of halogens and sulfur Combustion digestion and ion chromatography combined in one system 02 Combustion Ion Chromatography (CIC) extends the
Combustion Ion Chromatography Fast and reliable determination of halogens and sulfur Combustion digestion and ion chromatography combined in one system 02 Combustion Ion Chromatography (CIC) extends the
The electrolysis of sodium chloride solution produces useful substances. covalent ionic non-metallic
 1 The electrolysis of sodium chloride solution produces useful substances. (a) (i) Choose a word from the box to complete the sentence. covalent ionic non-metallic Electrolysis takes place when electricity
1 The electrolysis of sodium chloride solution produces useful substances. (a) (i) Choose a word from the box to complete the sentence. covalent ionic non-metallic Electrolysis takes place when electricity
Permeation Measurement Testing Techniques. Michelle Stevens MOCON, Inc.
 Permeation Measurement Testing Techniques Michelle Stevens MOCON, Inc. Permeation Permeation Permeation is simply the flux of molecules through a material normalized to the pressure drop across the film.
Permeation Measurement Testing Techniques Michelle Stevens MOCON, Inc. Permeation Permeation Permeation is simply the flux of molecules through a material normalized to the pressure drop across the film.
Nanotechnology and Solar Energy. Solar Electricity Photovoltaics. Fuel from the Sun Photosynthesis Biofuels Split Water Fuel Cells
 Nanotechnology and Solar Energy Solar Electricity Photovoltaics Fuel from the Sun Photosynthesis Biofuels Split Water Fuel Cells Solar cell A photon from the Sun generates an electron-hole pair in a semiconductor.
Nanotechnology and Solar Energy Solar Electricity Photovoltaics Fuel from the Sun Photosynthesis Biofuels Split Water Fuel Cells Solar cell A photon from the Sun generates an electron-hole pair in a semiconductor.
M.Sc. (Final) DEGREE EXAMINATION, DECEMBER Second Year Chemistry Paper V ANALYTICAL CHEMISTRY. PART A (4 10 = 40 marks)
 (DCHE 21) M.Sc. (Final) DEGREE EXAMINATION, DECEMBER 2009. Second Year Chemistry Paper V ANALYTICAL CHEMISTRY Time : Three hours Maximum : 100 marks PART A (4 10 = 40 marks) Answer any FOUR questions.
(DCHE 21) M.Sc. (Final) DEGREE EXAMINATION, DECEMBER 2009. Second Year Chemistry Paper V ANALYTICAL CHEMISTRY Time : Three hours Maximum : 100 marks PART A (4 10 = 40 marks) Answer any FOUR questions.
Supplementary Figure S1. Verifying the CH 3 NH 3 PbI 3-x Cl x sensitized TiO 2 coating UV-vis spectrum of the solution obtained by dissolving the
 Supplementary Figure S1. Verifying the CH 3 NH 3 PbI 3-x Cl x sensitized TiO 2 coating UV-vis spectrum of the solution obtained by dissolving the spiro-ometad from a perovskite-filled mesoporous TiO 2
Supplementary Figure S1. Verifying the CH 3 NH 3 PbI 3-x Cl x sensitized TiO 2 coating UV-vis spectrum of the solution obtained by dissolving the spiro-ometad from a perovskite-filled mesoporous TiO 2
ii) Describe the various types of detectors used in IR spectroscopy.
 (DCHE 21) ASSIGNMENT - 1, DEC - 2014. PAPER - V : ANALYTICAL 1) Describe a method for the determination of manganese in complex matrices. 2) Describe the instrumentation and working principles of IR. 3)
(DCHE 21) ASSIGNMENT - 1, DEC - 2014. PAPER - V : ANALYTICAL 1) Describe a method for the determination of manganese in complex matrices. 2) Describe the instrumentation and working principles of IR. 3)
Chromatography. Gas Chromatography
 Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Fluorescence 2009 update
 XV 74 Fluorescence 2009 update Jablonski diagram Where does the energy go? Can be viewed like multistep kinetic pathway 1) Excite system through A Absorbance S 0 S n Excite from ground excited singlet
XV 74 Fluorescence 2009 update Jablonski diagram Where does the energy go? Can be viewed like multistep kinetic pathway 1) Excite system through A Absorbance S 0 S n Excite from ground excited singlet
ION Pumps for UHV Systems, Synchrotrons & Particle Accelerators. Mauro Audi, Academic, Government & Research Marketing Manager
 ION Pumps for UHV Systems, Synchrotrons & Particle Accelerators Mauro Audi, Academic, Government & Research Marketing Manager ION Pumps Agilent Technologies 1957-59 Varian Associates invents the first
ION Pumps for UHV Systems, Synchrotrons & Particle Accelerators Mauro Audi, Academic, Government & Research Marketing Manager ION Pumps Agilent Technologies 1957-59 Varian Associates invents the first
Excited State Processes
 Excited State Processes Photophysics Fluorescence (singlet state emission) Phosphorescence (triplet state emission) Internal conversion (transition to singlet gr. state) Intersystem crossing (transition
Excited State Processes Photophysics Fluorescence (singlet state emission) Phosphorescence (triplet state emission) Internal conversion (transition to singlet gr. state) Intersystem crossing (transition
Amperometric biosensors
 Electrochemical biosensors II: Amperometric biosensors Lecture 2 Amperometric Sensors: Problem formulation amperometric techniques have some selectivity as every RedOx reaction has it s own characteristic
Electrochemical biosensors II: Amperometric biosensors Lecture 2 Amperometric Sensors: Problem formulation amperometric techniques have some selectivity as every RedOx reaction has it s own characteristic
COURSE MATERIAL: Unit 3 (Part 1) Polymer Science LT8501 (Click the link Detail to download)
 COURSE MATERIAL: Unit 3 (Part 1) Polymer Science LT8501 (Click the link Detail to download) Dr. Debasis Samanta Senior Scientist & AcSIR Assistant Professor Polymer Science & Technology Department., CSIR-CLRI,
COURSE MATERIAL: Unit 3 (Part 1) Polymer Science LT8501 (Click the link Detail to download) Dr. Debasis Samanta Senior Scientist & AcSIR Assistant Professor Polymer Science & Technology Department., CSIR-CLRI,
ELECTROCHEMISTRY. these are systems involving oxidation or reduction there are several types METALS IN CONTACT WITH SOLUTIONS OF THEIR IONS
 Electrochemistry 1 ELECTROCHEMISTRY REDOX Reduction gain of electrons Cu 2+ (aq) + 2e > Cu(s) Oxidation removal of electrons Zn(s) > Zn 2+ (aq) + 2e HALF CELLS these are systems involving oxidation or
Electrochemistry 1 ELECTROCHEMISTRY REDOX Reduction gain of electrons Cu 2+ (aq) + 2e > Cu(s) Oxidation removal of electrons Zn(s) > Zn 2+ (aq) + 2e HALF CELLS these are systems involving oxidation or
Chemical Storage Guide
 1 P a g e Chemical Storage Guide It is the responsibility of every occupant, owner, tenant, contractor, employee & visitor and ALL users of this facility to ensure they take all reasonably practical steps
1 P a g e Chemical Storage Guide It is the responsibility of every occupant, owner, tenant, contractor, employee & visitor and ALL users of this facility to ensure they take all reasonably practical steps
GCSE OCR Revision Chemistry. GCSE OCR Revision Chemistry. GCSE OCR Revision Chemistry. Bonding. GCSE OCR Revision Chemistry
 Particle Model and Atomic Structure The following symbols describe two different substances. Deduce all the information you can from these symbols. 13 C 12 6 6 C 1 Particle Model and Atomic Structure The
Particle Model and Atomic Structure The following symbols describe two different substances. Deduce all the information you can from these symbols. 13 C 12 6 6 C 1 Particle Model and Atomic Structure The
Co-Ni/Al 2 O 3 catalysts for CO 2 methanation at atmospheric pressure
 Co-Ni/Al 2 O 3 catalysts for CO 2 methanation at atmospheric pressure K. Nifantiev, O. Byeda, B. Mischanchuk, E. Ischenko a Taras Shevchenko National university of Kyiv, Kyiv, Ukraine knifantiev@gmail.com
Co-Ni/Al 2 O 3 catalysts for CO 2 methanation at atmospheric pressure K. Nifantiev, O. Byeda, B. Mischanchuk, E. Ischenko a Taras Shevchenko National university of Kyiv, Kyiv, Ukraine knifantiev@gmail.com
Chapter 17: Fundamentals of Spectrophotometry
 Chapter 17: Fundamentals of Spectrophotometry Spectroscopy: the science that deals with interactions of matter with electromagnetic radiation or other forms energy acoustic waves, beams of particles such
Chapter 17: Fundamentals of Spectrophotometry Spectroscopy: the science that deals with interactions of matter with electromagnetic radiation or other forms energy acoustic waves, beams of particles such
6.1. Expressing and Measuring Reaction Rates. Expressing Reaction Rates
 Expressing and Measuring Reaction Rates 6.1 As you learned in the Unit 3 opener, nitroglycerin is an explosive that was used to clear the way for railroads across North America. It decomposes instantly.
Expressing and Measuring Reaction Rates 6.1 As you learned in the Unit 3 opener, nitroglycerin is an explosive that was used to clear the way for railroads across North America. It decomposes instantly.
0.5W White SPMWHT346EA3
 Product Family Data Sheet Rev. 09 2016.11.01 1 Middle Power LED PLCC Series 0.5W White SPMWHT346EA3 Features & Benefits Package : Au Plated 4 pad design package with silicone resin Dimension : 3.2 mm x
Product Family Data Sheet Rev. 09 2016.11.01 1 Middle Power LED PLCC Series 0.5W White SPMWHT346EA3 Features & Benefits Package : Au Plated 4 pad design package with silicone resin Dimension : 3.2 mm x
2101 Atomic Spectroscopy
 2101 Atomic Spectroscopy Atomic identification Atomic spectroscopy refers to the absorption and emission of ultraviolet to visible light by atoms and monoatomic ions. It is best used to analyze metals.
2101 Atomic Spectroscopy Atomic identification Atomic spectroscopy refers to the absorption and emission of ultraviolet to visible light by atoms and monoatomic ions. It is best used to analyze metals.
