An experimental investigation of the effects of n- decane on the supercritical pyrolysis of toluene
|
|
|
- Octavia Wilson
- 5 years ago
- Views:
Transcription
1 Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 214 An experimental investigation of the effects of n- decane on the supercritical pyrolysis of toluene Catherine A. Grubb Louisiana State University and Agricultural and Mechanical College, Follow this and additional works at: Part of the Chemical Engineering Commons Recommended Citation Grubb, Catherine A., "An experimental investigation of the effects of n-decane on the supercritical pyrolysis of toluene" (214). LSU Master's Theses This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact
2 AN EXPERIMENTAL INVESTIGATION OF THE EFFECTS OF N-DECANE ON THE SUPERCRITICAL PYROLYSIS OF TOLUENE A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Science in The Department of Chemical Engineering by Catherine A. Grubb B.S., Louisiana State University, 211 May 214
3 For my grandmothers ii
4 Acknowledgements I would firstly like to thank my graduate advisor, Professor Mary J. Wornat. She has always believed in my abilities and encouraged me in my every endeavor. This work would never have come to fruition without her help and guidance. I will never forget the lessons I have learned from her. I will always strive to embody the exceptional qualities she instills in all her students: honesty, integrity, and an intense dedication to do my very best. Secondly, I wish to thank the members of my committee, Dr. Michael Benton and Dr. John Flake, for their time and the many words of wisdom they ve bestowed upon me over the years. I have learned so much from them. I would also like to thank the Air Force Office of Scientific Research for providing the funds for this research. This journey would not have been complete without my colleagues, Dr. Nimesh Poddar; Mr. Venkateswaran Subramanian Kalpathy; Ms. Eva Caspary; and Dr. Sean Bagley, and my academic brother, Dr. Franz Ehrenhauser. Their knowledge and support throughout my tenure here have been invaluable. Finally, this list would be incomplete if I did not acknowledge my gracious family. I cannot forget my parents, Robert and Wendy Grubb, who have supported and cared for me throughout my years, or my brother, who I could always count on for a unique anecdote to make me laugh. Recently, I have gained a father-in-law and mother-in-law who never cease to amaze me with their caring hearts. Without their wonderful advice (and delicious care packages), I don t know how we would ve survived these past few years. Lastly, to my incredible husband, Dr. Nimesh Poddar, who has loved me through everything and never given up on me. His support throughout this time has never wavered and I can never thank him enough for it. I could not have done this without him, both professionally and personally. iii
5 Table of Contents Acknowledgements... iii List of Tables... vii List of Figures... viii Abstract... xii Chapter I. Introduction Motivation Background Previous toluene pyrolysis studies Previous alkane pyrolysis studies Exploration of fuel component interactions through model fuel mixtures Structure of this thesis Chapter II. Experimental Methods and Analysis Introduction Supercritical fuel pyrolysis reactor system Product analysis Gas-phase product analysis Condensed-phase product analysis Summary... 2 Chapter III. Effects of n-decane Addition and Temperature on Supercritical Toluene Pyrolysis: Fuel Conversion and Yields of Aliphatic and One-ring Aromatic Products Introduction Fuel conversion The conversion of toluene The conversion of n-decane Aliphatic and one-ring aromatic products of supercritical n-decane-doped toluene pyrolysis n-alkanes and 1-alkenes Effects of n-decane addition on the yields of n-alkanes and 1-alkenes Effects of temperature on the yields of n-alkanes and 1-alkenes One-ring aromatics: Benzene, ethylbenzene, styrene, and xylenes Effects of n-decane addition on the yields of benzene, ethylbenzene, styrene, and xylenes Effects of temperature on the yields of benzene, ethylbenzene, styrene, and xylenes One-ring aromatics: n-alkylbenzenes (n C 2 ), allylbenzene, and 4-phenyl-1- butene Effects of n-decane on the yields of n-alkylbenzenes (n C 2 ), allylbenzene, and 4-phenyl-1-butene iv
6 Effects of temperature on the yields of n-alkylbenzenes (n C 2 ), allylbenzene, and 4-phenyl-1-butene Summary Chapter IV. Effects of n-decane Addition and Temperature on Supercritical Toluene Pyrolysis: Yields of Two- and Three-ring Aromatic Products Introduction Two- and three-ring aromatic products of supercritical n-decane-doped toluene pyrolysis Two-ring aromatics: Bi-tolyl products Effects of n-decane on the yields of bi-tolyl products Effects of temperature on yields of bi-tolyl products Two-ring aromatics: Indene and methylindenes Effects of n-decane on the yields of indene and methylindenes Effects of temperature on the yields of indene and methylindenes Two-ring aromatics: Naphthalene and methylnaphthalenes Effects of n-decane on the yields of naphthalene and methylnaphthalenes Effects of temperature on the yields of naphthalene and methylnaphthalenes Three-ring aromatics: Fluorene and methylfluorenes Effects of n-decane on the yields of fluorene and methylfluorenes Effects of temperature on the yields of fluorene and methylfluorenes Three-ring aromatics: 9,1-Dihydrophenanthrene and phenanthrene Effects of n-decane on the yields of 9,1-dihydrophenanthrene and phenanthrene Effects of temperature on the yields of 9,1-dihydrophenanthrene and phenanthrene Three-ring aromatics: 9,1-Dihydroanthracene and anthracene Effects of n-decane on the yields of 9,1-dihydroanthracene and anthracene Effects of temperature on the yields of 9,1-dihydroanthracene and anthracene Summary Chapter V. Conclusions and Recommendations Summary Recommendations for future work References Appendix A. Product Analysis: GC Calibrations Appendix B. Conversion of Toluene and n-decane from Supercritical n-decane-doped Toluene Pyrolysis Appendix C. Products of Supercritical n-decane-doped Toluene Pyrolysis v
7 Appendix D. Yields of Products of Supercritical n-decane-doped Toluene Pyrolysis Vita vi
8 List of Tables 3.1. Average toluene conversion for each condition investigated Average n-decane conversion for each condition investigated A1. Response factors and GC retention times for C 1 -C 6 hydrocarbons A2. Response factors and GC retention times for one-to three-ring aromatics A3. Response factors used in the quantification of other condensed-phase products not listed in Table A B1. n-decane added (vol %) and conversion of n-decane and toluene (%) in supercritical n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, 133 sec B2. n-decane added (vol %) and conversion of n-decane and toluene (%) in supercritical n-decane-doped toluene pyrolysis at 6 C, 94.6 atm, 133 sec C1. Gas-phase products of supercritical n-decane-doped toluene pyrolysis C2. Condensed-phase products of supercritical n-decane-doped toluene pyrolysis D1(a). Average yields of gas-phase products of supercritical n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, and 133 sec D1(b). Average yields of gas-phase products of supercritical n-decane-doped toluene pyrolysis at 6 C, 94.6 atm, and 133 sec D2(a). Average yields of condensed-phase products of supercritical n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, and 133 sec D2(b). Average yields of condensed-phase products of supercritical n-decane-doped toluene pyrolysis at 6 C, 94.6 atm, and 133 sec vii
9 List of Figures 2.1. Supercritical fuel pyrolysis reactor system Bond-dissociation energies of toluene Bond-dissociation energies of n-decane Yields of (a) n-alkanes and (b) 1-alkenes (C 2 -C 9 ), excluding C 5 species, from supercritical n-decane and n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, and 133 sec Yields of (a) methane, (b) ethane, (c) propane, and (d) n-butane from supercritical n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, 133 sec Yields of (a) n-heptane, (b) n-octane, and (c) n-nonane from supercritical n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, 133 sec Yields of (a) ethylene, (b) propene, and (c) 1-butene from supercritical n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, 133 sec Yields of (a) 1-hexene, (b) 1-heptene, (c) 1-octene, and (d) 1-nonene from supercritical n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, 133 sec Yields, as functions n-decane fed, of (a) methane; (b) ethane; (c) propane; (d) n-butane; (e) n-heptane; (f) n-octane; (g) n-nonane at 57 C ( ) and 6 C ( ) from supercritical n-decane-doped toluene pyrolysis at a constant pressure and residence time of 94.6 atm and 133 sec, respectively Yields, as functions n-decane fed, of (a) ethylene; (b) propene; (c) 1-butene; (d) 1-hexene; (e) 1-heptene; (f) 1-octene; (g) 1-nonene at 57 C ( ) and 6 C ( ) from supercritical n-decane doped toluene pyrolysis at a constant pressure and residence time of 94.6 atm and 133 sec, respectively Yields of n-alkanes from supercritical n-decane-doped toluene experiments at (a) 57 C and (b) 6 C, 94.6 atm, and 133 sec Yields of 1-alkenes from supercritical n-decane-doped toluene experiments at (a) 57 C and (b) 6 C, 94.6 atm, and 133 sec Yields of (a) benzene, (b) ethylbenzene, (c) styrene, (d) m-& p-xylene, and (e) o-xylene from supercritical n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, 133 sec Yields of one-ring aromatics from supercritical n-decane-doped toluene pyrolysis at (a) 57 C and (b) 6 C, 94.6 atm, and 133 sec viii
10 3.14. Yields, as functions n-decane fed, of (a) benzene, (b) ethylbenzene, (c) styrene, (d) m-and p-xylene, and (e) o-xylene at 57 C ( ) and 6 C ( ) from supercritical n-decane doped toluene pyrolysis at a constant pressure and residence time of 94.6 atm and 133 sec, respectively Yields, as functions n-decane fed, of (a) to (i) n-alkylbenzenes (n C 2 ), (j) allylbenzene, and (k) 4-phenyl-1-butene at 57 C ( ) and 6 C ( ) from supercritical n-decane-doped toluene pyrolysis at a constant pressure and residence time of 94.6 atm and 133 sec, respectively Yields of (a) ethylbenzene, (b) n-propylbenzene, and (c) n-butylbenzene from supercritical n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, and 133 sec Yields of n-alkylbenzenes (n C 2 ) from supercritical n-decane-doped toluene pyrolysis at (a) 57 C and (b) 6 C, 94.6 atm, and 133 sec Yields of allylbenzene and 4-phenyl-1-butene from supercritical n-decane-doped toluene pyrolysis at (a) 57 C and (b) 6 C, 94.6 atm, and 133 sec Yields, as functions of n-decane fed, of: (a) biphenyl; (b) 2-methylbiphenyl; (c) 3- methylbiphenyl; (d) the summed yields of 4-methylbiphenyl and 2,3 -dimethylbiphenyl; (e) 2,2 -dimethylbiphenyl; (f) 2,4 -dimethylbiphenyl; (g) 3,3 -dimethylbiphenyl; (h) 3,4 - dimethylbiphenyl; and (i) 4,4 -dimethylbiphenyl at 57 C ( ) and 6 C ( ) from supercritical n-decane-doped toluene pyrolysis at a constant pressure and residence time of 94.6 atm and 133 sec, respectively Yields, as functions of n-decane fed, of: (a) bibenzyl; (b) trans-stilbene; (c) diphenylmethane; (d) 2-methyldiphenylmethane; (e) 3-methyliphenylmethane; (f) 4-methyldiphenylmethane at 57 C ( ) and 6 C ( ) from supercritical n-decane-doped toluene pyrolysis at a constant pressure and residence time of 94.6 atm and 133 sec, respectively Yields of: 3,3 -dimethylbiphenyl; 3,4 -dimethylbiphenyl; the summed yields of 4- methylbiphenyl and 2,3 -dimethyl-biphenyl; 2,4 -dimethylbiphenyl; 4,4 -dimethylbiphenyl; 2,2 -dimethylbiphenyl; 3-methylbiphenyl; 2-methylbiphenyl; and biphenyl from supercritical n-decane-doped toluene pyrolysis at (a) 57 C and (b) 6 C, 94.6 atm, and 133 sec Yields of bibenzyl, diphenylmethane, 3-methyldiphenylmethane, 4-methyldiphenylmethane, 2-methyldiphenylmethane, and trans-stilbene from supercritical n-decanedoped toluene pyrolysis at (a) 57 C and (b) 6 C, 94.6 atm, and 133 sec Yields of (a) indene; (b) 2-methylindene; (c) 3-methylindene from supercritical n-decanedoped toluene pyrolysis at 57 C, 94.6 atm, and 133 sec Bond-dissociation energies of indene, 3-methylindene, and naphthalene ix
11 4.7. Yields, as functions of n-decane fed, of: (a) indene; (b) 2-methylindene; (c) 3- methylindene at 57 C ( ) and 6 C ( ) from supercritical n-decane-doped toluene pyrolysis at a constant pressure and residence time of 94.6 atm and 133 sec, respectively Yields of indene, 2-methylindene, and 3-methylindene from supercritical n-decane-doped toluene pyrolysis at (a) 57 C and (b) 6 C, 94.6 atm, and 133 sec Yields of (a) naphthalene; (b) 1-methylnaphthalene; (c) 2-methylnaphthalene from supercritical n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, and 133 sec Yields, as functions of n-decane fed, of: (a) naphthalene; (b) 1-methylnaphthalene; (c) 2-methylnaphthalene at 57 C ( ) and 6 C ( ) from supercritical n-decane-doped toluene pyrolysis at a constant pressure and residence time of 94.6 atm and 133 sec, respectively Yields of naphthalene, 2-methylnaphthalene, and 1-methylnaphthalene from supercritical n-decane-doped toluene pyrolysis at (a) 57 C and (b) 6 C, 94.6 atm, and 133 sec Yields of (a) fluorene; (b) 1-methylfluorene; (c) 2-methylfluorene; (d) the summed yields of all other methylfluorenes from supercritical n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, and 133 sec Yields of 1-methylfluorene, 2-methylfluorene, and fluorene from supercritical n-decane and n-decane-doped toluene pyrolysis at 57 C, 94.6 atm, and 133 sec Yields, as functions of n-decane fed, of: (a) fluorene; (b) 1-methylfluorene; (c) 2-methylfluorene (d) an unknown methylfluorene; (e) a second unknown methylfluorene at 57 C ( ) and 6 C ( ) from supercritical n-decane-doped toluene pyrolysis at a constant pressure and residence time of 94.6 atm and 133 sec, respectively Yields of 1-methylfluorene, two unidentified methylfluorene, 2-methylfluorene, and fluorene from supercritical n-decane-doped toluene pyrolysis at (a) 57 C and (b) 6 C, 94.6 atm, and 133 sec Yields, as functions of n-decane fed, of (a) 9,1-dihydrophenanthrene and (b) phenanthrene at 57 C ( ) and 6 C ( ) from supercritical n-decane-doped toluene pyrolysis at a constant pressure and residence time of 94.6 atm and 133 sec, respectively Yields of phenanthrene from supercritical n-decane-doped toluene at 57 C, 94.6 atm, and 133 sec Bond-dissociation energies of 9,1-dihydrophenanthrene and phenanthrene Yields of 9,1-dihydrophenanthrene and phenanthrene from supercritical n-decane-doped toluene pyrolysis at (a) 57 C and (b) 6 C, 94.6 atm, and 133 sec x
12 4.2. Yields, as functions of n-decane fed, of (a) 9,1-dihydroanthracene and (b) anthracene at 57 C ( ) and 6 C ( ) from supercritical n-decane-doped toluene pyrolysis at a constant pressure and residence time of 94.6 atm and 133 sec, respectively Yields of anthracene from supercritical n-decane-doped toluene at 57 C, 94.6 atm, and 133 sec Bond-dissociation energies of 9,1-dihydroanthracene and anthracene Yields of 9,1-dihydroanthracene and anthracene from supercritical n-decane-doped toluene pyrolysis at (a) 57 C and (b) 6 C, 94.6 atm, and 133 sec A1. GC calibration curve of naphthalene xi
13 Abstract Future and current high-speed jet aircraft will require their fuels to act as the primary coolants as well as propellants. Fuels will be exposed to severe temperatures and pressures in hypersonic aircraft, up to 7 C and 13 atm, respectively, conditions that are supercritical for most pure hydrocarbons. Under supercritical conditions, hydrocarbon fuels undergo pyrolytic reactions, which may lead to the formation of polycyclic aromatic hydrocarbons (PAH), known precursors to carbonaceous solid deposits. Such deposits may clog fuel lines and injection nozzles, hindering safe engine performance. Hence, it is important to understand the reactions that lead to the formation of PAH. While jet fuels are composed primarily of alkanes, a significant portion of their composition is comprised of aromatics. In our effort to understand PAH formation, we must first understand the interactions of aliphatic and aromatic fuel components. Therefore, the aromatic model fuel toluene (critical temperature, 319 C; critical pressure, 41 atm) has been pyrolyzed both alone and in the presence of the aliphatic model fuel n-decane (critical temperature, 345 C; critical pressure, 2.8 atm) in an isothermal flow reactor at 57 C and 6 C, 94.6 atm, and 133 sec. Analyses of 12 gas-phase and 53 condensed-phase hydrocarbon products were performed with gas chromatography (GC) with flame-ionization detection (FID) and GC/FID coupled with mass spectrometry (MS), respectively. Results indicate that n-decane addition increases toluene conversion and product yields. In n-decane-doped toluene pyrolysis at 57 C, n-decane conversion is inhibited by the smaller radical pool relative to n-decane-only pyrolysis at the same temperature. 1-Alkene formation is generally enhanced compared to n-alkanes at 57 C, a result that contrasts with results obtained from n-decane-only pyrolysis at the same temperature. Additionally, results suggest that interactions of alkenes and benzylic-type radicals are important to the xii
14 formation of high-ring number aromatics. Increasing the temperature to 6 C increases the conversions of both toluene and n-decane and causes a general rise in product yields. Yields of aliphatics and n-alkylbenzenes with long-carbon chains decrease at 6 C due to decomposition of the alkyl chain. Product yields as functions of n-decane concentration at 57 C and 6 C are presented, and possible product formation pathways are discussed. xiii
15 Chapter I. Introduction 1.1 Motivation At the high-speeds envisioned for current and future jet aircraft, fuels will be required to act not only as a propellant, but also as the primary coolant. 1-6 In hypersonic aircraft, temperatures may reach upwards of 7 C for long durations; 1,7 this is significantly above the critical temperatures for most jet fuels and pure hydrocarbons. 8 Additionally, in order to ensure a liquid-like fuel density throughout the engine, pressures are high in aircraft fuel systems (up to 13 atm), often above the critical pressures of current jet fuels. 8 Surrounding air temperatures in hypersonic flight are high; therefore, as it would require the use of comparatively large heat exchangers, it would be inefficient to use air as the sole means of cooling in high-speed flight. 2,7 Fuel, therefore, must be able to absorb high heat loads in order to effectively cool the engine; such fuels are known as endothermic fuels. Endothermic hydrocarbon fuels absorb heat two ways: via physical heating (increasing the fuel temperature) and via endothermic reactions. 1,2,7 These endothermic reactions produce smaller, high-energy products; 1,9,1 however, other, less desirable products are also created in the process. Additionally, reactions forming larger, high molecular weight products can occur. These products may agglomerate in fuel lines and injection nozzles, significantly hindering engine performance. 1,1-12 As oxidation reactions promote solid deposition, limiting the amount of dissolved oxygen present by deoxygenating the fuel prior to use or adding additives such as oxygen scavengers 4,1-14 vastly improves the thermal oxidative stability of a fuel. It should be noted that under supercritical conditions, the chemical and physical properties of a fluid are very different from those observed at lower pressures. 15,16 At the high pressures imposed on the fuel, pyrolytic reactions can begin to occur at temperatures above 1
16 48 C. 1,1 These reactions can lead to the formation of polycyclic aromatic hydrocarbons (PAH), 9,17-19 known precursors to carbonaceous solid deposits. As mentioned earlier, these solid deposits can lead to a decrease in engine performance and, in the worst case, engine failure. Current hydrocarbon jet fuels are primarily composed of n-and i-alkanes and naphthenes, but a significant portion of the fuel is composed of aromatic molecules. 3,9 The presence of both alkanes and aromatics in jet fuels necessitates knowledge of the interactions of the two fuel components. 9,18,19 Previous studies 2-23 have shown these interactions to be important to the overall understanding of pyrolytic reactions. The purpose of this study is to investigate the interactions of the model fuels toluene and n-decane under supercritical conditions. Because both toluene and n-decane are actual components of jet fuels 39,76 and their individual fuel pyrolysis has been well-studied, they are ideally suited for this endeavor. 1.2 Background Previous toluene pyrolysis studies Aromatic and alicyclic compounds can make up a significant portion of jet fuels. 3,9 Several model fuel studies have endeavored to understand the behavior of aromatics in pyrolysis and oxidation environments. Hein and Mesée 24 were amongst the first to investigate the decomposition of toluene. The pyrolysis of toluene at 6 C 14 C in the presence of mercury vapor yielded dibenzylmercury, indicating the presence of benzyl radicals in the reaction environment. Subsequent toluene pyrolysis experiments by Szwarc 25 substantiated this hypothesis. From these results, Szwarc 25 developed a possible toluene decomposition mechanism. The free-radical mechanism devised included the unimolecular decomposition of toluene (R1), two possible 2
17 mechanisms of propagation reactions one involving H abstraction from the aromatic ring of toluene by free hydrogen (R2a) and ipso addition of H to toluene (R2b); the other involving the breakage of the aryl-methyl C-C bond of toluene (R3a) and H abstraction from toluene by phenyl (R3b) and termination reactions (R4). The author determined that the bond dissociation energy of the methyl C-H bond in toluene to be 77.5 kcal/mol. Due to the low activation energy of that bond, Szwarc 25 determined that R3a (production of phenyl radicals) is unlikely to occur. R1 R2a R2b R3a R3b R4 In the same work, 25 all three xylene isomers were also pyrolyzed. It was found that m-and p-xylene produced similar ratios of hydrogen to methane (though p-xylene produced slightly more hydrogen), while o-xylene pyrolysis yielded higher amounts of methane, providing evidence that o-xylene may have weaker aryl-methyl C-C bonds. Blades et al 26 also pyrolyzed toluene, in an effort to verify the results of Szwarc. 25 In addition to the termination product bibenzyl, dimethylbiphenyls and styrene were found. The dimerization of toluene to dimethylbiphenyls was similar to the findings of Brooks, 27 who studied the pyrolysis of toluene in a static reactor at 684 C. Brooks 27 discovered the reactions of 3
18 toluene are autocatalytic, leading to an increase in rates as the residence time increased, a point which was noted by Blades et al 26 but not explained. Blades and Steacie 28 verified the Szwarc mechanism and also determined that the benzyl radical could abstract hydrogen from toluene (or any molecule RH) to form methylphenyl radicals and toluene (R5). Takahasi 29 and Wilen and Eliel 3 later suggested the hydrogen abstraction from the toluene ring could also be performed by free hydrogen or methyl radicals (R6 and R7). Subsequent work by Colket and Seery 31 in a single-pulse shock tube at C supports these authors assertions. Colket and Seery 31 also determined that phenyl production is minimal compared to benzyl production, under the conditions employed there. R5 R6 R7 Pamidimukkala et al 32 performed toluene pyrolysis experiments at high temperatures (T > 1 C) and found that at high temperatures, phenyl production (R3a) would drive the decomposition mechanism. Earlier results obtained from the high-temperature pyrolysis of toluene in quartz and tungsten Knudsen cells by Smith 33 showed that at temperatures above 1 C, the aromatic ring of the benzyl radical ruptures, producing cyclopentadienyl radicals, 4
19 acetylene, vinylacetylene, and propargyl radicals. None of the previous authors noted this behavior; as such, ring-rupturing reactions were not incorporated into their mechanisms. Several works have explored the high pressure pyrolysis of toluene behind reflected shock waves 34,35 and in flow reactors 36,37 and gold cells, 38 although temperature conditions differed. Sivaramakrishnan performed their experiments 34 and simulations 35 in a temperature and pressure range of C and bars, respectively. They noted the formation of acetylene and diacetylene, along with one-ring aromatic species. These results are consistent with ring-opening reactions reported by Smith 33 at high temperatures. Ledesma et al 36 explored the supercritical pyrolysis of toluene in a plug-flow reactor at 535 C and 1 atm. Those authors found that product yields increased substantially with increasing pressure. McClaine and Wornat 37 analyzed the products formed from supercritical toluene pyrolysis at 535 C and 1 atm and the possible reaction mechanisms occurring in the system were explored. Nguyen 39 investigated the effects of pressure and temperature on the supercritical pyrolysis of toluene in the same reactor system as Ledesma et al 36 and McClaine et al. 37 Pressures varied from 5-1 atm, while temperatures varied from C. Nguyen found that, while product yields rose substantially when either temperature or pressure increased, temperature has a greater effect on product yields than pressure. No acetylenic species were found as products in any of the three preceding works, 36,37,39 likely due to the much lower temperatures explored there. It should also be noted that no oxygen was present in the fuel of the preceding three works. 36,37,39 It was previously mentioned that oxidation reactions in the fuel may lead to the formation of solid deposits. 1 In aromatic oxidation studies 4-43 ring-opening reactions were enhanced in the presence of oxygen. However, as Ledesma et al, 36 McClaine et al, 37 and Nguyen 39 did not add oxygen to the fuel and because no products of ring-opening reactions were observed, it can be 5
20 said that oxidation reactions are not playing a role in the decomposition of toluene in the systems investigated. Lannuzel et al 38 performed toluene pyrolysis experiments at temperatures of 35 4 C and a pressure of 7 bar in confined gold cells. The products observed are consistent with previous studies and additionally include the identities of specific isomers of C 14 H 14 bi-aryls, like 2,3 -dimethylbiphenyl and 4-methyldiphenylmethane. Lannuzel et al 38 expanded on the established toluene pyrolysis mechanism by including bimolecular initiation reactions of toluene, which may be important in geological conditions (i.e. low temperature, high pressure). Savage and Klein devised a generalized model for the pyrolysis of n-alkylbenzenes (n > C 4 ) using lumped reactions. 23,46-48 The overall reaction is shown below (R9), where n is the number of carbons in the alkyl chain. They agreed with the findings of Mushrush and Hazlett 22 in that long-chain alkyl aromatic species that are present in asphaltenes and jet fuels could be responsible for the formation of n-alkanes during the thermolysis of those mixtures. R9 The pyrolysis of n-butylbenzene under near critical and supercritical conditions was studied by Yu and Eser 49 in a 45-5 μl Pyrex glass tube reactor and a 316 stainless steel tubing bomb. The major products of n-butylbenzene pyrolysis, especially at low conversions of the reactant, are toluene and styrene, although the yields of these vary with pressure: At low pressures, styrene formation is favored, while at high pressures, toluene formation is. Other products are also present, but in much smaller yields. Yu and Eser used the decomposition reactions developed by Savage and Klein 23,48 and Leigh and Szwarc 5,51 in their decomposition mechanism. 6
21 1.2.2 Previous alkane pyrolysis studies Rice 52 was amongst the first to investigate the decomposition of alkanes from thermolytic reactions. Rice recognized that alkyl radicals decomposed into alkenes and other, smaller radicals and hydrogen. The new radicals could then react with other molecules in the system, propagating a chain reaction. Rice studied the thermal decompositions of saturated hydrocarbons. They found that unimolecular decomposition (R1) was the initial reaction in alkane decomposition, followed by hydrogen abstraction (R11), and dehydrogenation to alkenes and hydrogen (R12a and R12b). The radical products of these reactions could continue the chain reaction. R1 R11 R12a R12b Rice et al 53 expanded on the previous work by Rice 52 by extending this free-radical mechanism to other organic compounds, such as aldehydes and ketones. They used metal bromides to identify the different radicals produced. Rice and Herzfeld 54 derived reactions mechanisms for the decompositions of ethane, acetone, dimethylether, and acetylaldehyde. Rice and Herzlfeld also investigated the hydrogenation of ethylene. Later, Kossiakoff et al 55 theorized on the resonance structures of radicals, noting that primary, secondary, and tertiary hydrocarbon radicals display different behavior. The authors theorized that there may be differences in the associated C-H bond energies in hydrocarbons. For instance, the decomposition of isopentyl radicals can occur two ways: to 1-butene and methyl 7
22 and to propene and ethyl. Because the radical can be shared over two carbons, the ethyl radical is more stable than the methyl radical. Additionally, in the decomposition of C 6 C 9 n-alkanes, Kossiakoff et al 55 determined that radical isomerizations (R13) must occur to account for the high yields of certain products. R13 Gas-phase thermolysis of C 9 C 22 n-alkanes at 1 atm was undertaken by Zhou et al. 56 Pyrolysis of the individual compounds as well as mixtures was performed in a stainless steel reactor at temperatures ranging from C. Primary alkenes (C n-2 to C n ) were noted as major products from the experiments, with ethylene yields being the highest; ethane and methane were the two highest yield alkane products. The authors showed that the major products of gasphase alkane pyrolysis at low pressures are 1-alkenes; however, as pressures increase, the selectivity to 1-alkenes decreases. Dominé 57 pyrolyzed n-hexane under supercritical conditions in a constant-pressure goldtube reactor. Experiments were performed at several pressures (21, 18, 38, and 156 bar) to observe any possible effects; temperatures ranged from C. The author found that as pressure increases, the yields of smaller (C 2 C 4 ) alkenes and alkanes decrease significantly. The author also noted that at high temperatures, the rate constants of the reactions therein would be essentially independent of pressure, limiting the observed effects. A free-radical mechanism based on the work of Rice et al was used to investigate the possible reactions occurring, but also included bimolecular reactions to account for the high-density, high-viscosity state of n- hexane under supercritical conditions. 8
23 Song et al, 58 Khorasheh and Gray, 59 and Yu and Eser 6 investigated the pyrolysis of longchain n-alkanes at high pressures. Song et al 58 performed n-tetradecane pyrolysis experiments at 45 C and pressures of 2 9 MPa for 6 48 minutes in tubing bombs. The authors found that at low residence times, n-alkanes are preferentially formed over 1-alkenes (unlike the work of Zhou et al 56 ). The authors proposed a new scheme for pyrolysis of long-chain n-alkanes at high pressures to account for the increase in hydrogen abstraction over β scission (1-alkene production) and the increase of bimolecular reactions, incorporating elements from Kossiakoff and Rice. 55 Khorasheh and Gray 59 thermally cracked n-hexadecane in a tubular flow reactor at 13.9 MPa, with temperatures and residence times ranging from C and.6 2. hours, respectively. The authors reported similar results to Song et al, 58 in that n-alkanes were also preferentially formed. Yu and Eser 6 found that, in the pyrolysis of n-alkanes (n = C 1, C 12, and C 14 ) at and near supercritical conditions, as conversion and pressure increased, the yields of 1- alkenes decreased, while alkane yields increased. They also noted that C 2 and C 3 aliphatic yields were the highest of the aliphatic products quantified. Yu and Eser 61 also investigated the decomposition of decalin at and near supercritical conditions ( C and atm). Ring-cracking reactions, similar to those observed by Stewart et al 62 in supercritical decalin pyrolysis, were common in this environment. Those authors 62 explored the reactions of decalin, tetralin, and n-decane under supercritical conditions at C and.2 1 MPa. Decalin pyrolysis led to the formation of methylhexahydroindanes, alkenes, and methylenecyclohexene, among others. Tetralin pyrolysis favored methylindenes, n-butylbenzene, and naphthalene formation. From these products, the authors concluded that ring-contraction reactions represented a major portion of the decomposition pathways of decalin and tetralin, but dehydrogenation reactions are also 9
24 significant. Pyrolysis of n-decane led to higher n-alkane yields than conventional gas-phase pyrolysis. The presence of decalin and tetralin stabilized n-decane slightly, decreasing its conversion. Stewart 63 also examined the reactions of supercritical methylcyclohexane pyrolysis, in addition to decalin and tetralin. Major products of supercritical methylcyclohexane pyrolysis include dimethylcylcopentanes and methylcyclopentane, as well as other aliphatic products, indicating that ring-contraction reactions take place in this system as well Exploration of fuel component interactions through model fuel mixtures This work is not the first to explore the interactions of aromatic and aliphatic fuel components. 17,64-71 Scott 64 investigated the interactions of toluene and alkanes at high temperatures. Scott pyrolyzed propane with toluene at temperatures of 8 C to 12 C in a steel flow reactor at low pressures. Other alkanes were also pyrolyzed with toluene at 11 C and the results were compared. It was found that in propane/toluene co-pyrolysis at 11 C and a residence time of 1.4 ms, benzyl and methyl radicals react preferentially over benzyl-benzyl reactions, enhancing the formation of ethylbenzene and styrene over benzene and bibenzyl, while increasing the conversion of toluene. In pure toluene pyrolysis at 12 C and 1.3 ms, the opposite occurs: benzene and bibenzyl yields are higher, while ethylbenzene and styrene yields decrease. In addition, toluene s conversion is lower. Savage 65 developed a reaction model to investigate the reactions between n- pentadecylbenzene and n-tridecylcyclohexane at low concentrations. They found that, upon the addition of the other respective component, both n-pentadecylbenzene and n-tridecylcyclohexane s conversion increased. Their results indicate that the radical pool size increases with the addition of n-pentadecylbenzene, while n-tridecylcyclohexane contributes reactive alkyl radicals, 1
25 both of which increase the conversion of the opposing fuel component. However, these findings are only valid at low concentrations of the alkylbenzene and the alkylcyclohexane at higher concentrations, the interactions become too complex. In a stainless steel plug-flow reactor at 35 bar and moderate temperatures (5 727 C), Maurice and Edwards 17 performed both experiments (up to 6 C) and computations (up to 727 C) with n-decane/toluene mixtures (9/1 and 8/2 n-decane/toluene by volume) to investigate their interactions. Their results show that n-decane significantly affects the conversion of toluene at 6 C (n-decane itself is fully converted at this temperature). The products of these reactions include xylenes; n-alkylbenzenes; alkenylbenzenes; cycloalkanes and alkenes, along with alkylated versions; several alkanes, alkenes, and a few dienes; and some low yield bitolyl products with the C x H y formula C 14 H 14. Naphthalene formation is observed in the presence of toluene, but concentrations are low; benzene is formed in significant amounts at all conditions. n-dodecane was pyrolyzed by Yu and Eser 66,67 in dual component mixtures with n- decane, n-tetradecane, n-butylbenzene, and n-butylcylcohexane at 425 C at both sub-and supercritical pressures in Pyrex glass tubes for 15-6 minutes. Yu and Eser 66 found that, in the mixtures of n-alkanes, the reaction products formed did not vary significantly from those formed in pure n-alkane pyrolysis. Under supercritical conditions, n-dodecane/n-butylbenzene mixtures preferentially form n-alkanes, toluene, and heavier aromatics. The n-dodecane/n-butylcyclohexane mixture produced alkane and alkenes, cyclohexanes and hexenes, and alkylcyclohexanes and hexenes. n-butylbenzene/n-butylcyclohexane produced mainly cyclohexane and hexane, cyclohexylbenzenes, and phenylalkanes. Yu and Eser found that, in general, the conversions of each fuel component were affected by the other in this way: The lighter fuel component s 11
26 conversion increased, while the heavier component s conversion appears to be inhibited. According to the authors, this effect is entirely due to the size of the radical pool relative to each fuel component s pure environment; in the light fuel s system, the pool is smaller, so its conversion increases, where the opposite is true for the heavy component. Sakai et al 68 investigated the oxidation kinetics of a ternary mixture of n-heptane, i- octane, and toluene. They adjusted a previously established model from Ogura et al 72 to include alkane-toluene reactions and to include a toluene sub-mechanism developed by Pitz et al 73 for an adiabatic homogeneous reactor to depict the behavior observed in reflected shock waves. Different pressures (2 5 atm), temperatures ( C), and equivalency ratios (.25 1.) were investigated. The authors found that at temperatures below 827 C, toluene reacts with hydroxyl preferentially, and the subsequently formed benzyl radical reacts with O 2. Crossreactions between toluene and alkane components include reactions of allene and alkenes with benzyl radicals, although they acknowledge the dearth of information on the reactions of alkenes and aromatic radical, such as benzyl (R14). R14 Free-radical reactions in the cracking of a bitumen residue were investigated by Blanchard and Gray. 69 They diluted the residue with 1-methylnaphthalene to probe the interactions of different components of complex mixtures. The validity of the radical capping mechanism, where hydrogen donors (such as solvents or molecular hydrogen) help to stabilize radicals, was also discussed. The residue/1-methylnaphthalene mixture was reacted in a batch reactor at 4 C and 13.8 MPa. Several binaphthyls were formed when pure 1- methylnaphthalene was reacted alone; the yield and number of these dimers only increased when 12
27 the residue was added. The presence of the solvent 1-methylnaphthalene decreased the conversion and coke production of the residue. Bounaceur et al 7 used simulations to explore the effects of tetralin on the pyrolysis reactions of n-hexadecane and compared the results with previous toluene/n-hexadecane experiments. At temperatures, pressures, and experimental durations of 38 C, 5 bar, and up to 4 days, respectively, the addition of either tetralin or toluene reduced the conversion of n- hexadecane, although by varying amounts. In tetralin doping experiments, the pyrolysis products of tetralin decreased the inhibition of n-hexadecane when tetralin was present in concentrations greater than 2 mol %. In contrast, toluene s major radical product, the benzyl radical, was shown to stabilize n-hexadecane and significantly reduced its conversion. However, as temperature increased, the authors found tetralin s inhibiting power increased while toluene s decreased. Lannuzel et al 71 expanded upon the work of Bounaceur et al. 7 The authors pyrolyzed n- octane with 1 mol% toluene diluted in argon in gold cells at temperatures and pressures of 35 C-45 C and up to 7 bar, respectively, for anywhere from 24 hours to one month. They used a lumped reaction model to simulate the n-octane/toluene reaction environment and found a good agreement with experimental data. Within their model, several mechanisms were taken into account. n-octane pyrolysis mechanisms followed a general free-radical mechanism. The toluene pyrolysis mechanism includes bimolecular interactions, metathesis, ipso addition of hydrogen or methyl to toluene, benzyl addition to toluene, and termination reactions, adapted from Szwarc. 25 The n-octane/toluene pyrolysis mechanisms include mainly hydrogen-transfer between toluene and alkyl radicals and n-octane and those radicals produced by toluene, benzyl/alkene reactions, hydrogen-transfer reactions between toluene and n-octane with phenylalkyl radicals (formed 13
28 from benzyl/alkene reactions see R14), and termination reactions. Their results indicate that at high pressures and low temperatures (T 45 C), toluene does not inhibit the radical addition reactions present in n-octane pyrolysis, except at very low conversions of the alkane. At high conversions, alkene production increases, and so do their reactions with benzyl, effectively limiting the inhibition power of toluene. At low pressures, toluene inhibits the conversion of n- octane. 1.3 Structure of this thesis This work will endeavor to shed some light on the interactions between aliphatic and aromatic components of jet fuel. To accomplish this task, some background information on the reactor system and analytical techniques used is needed and will therefore be provided in Chapter II. Next, the effects of both n-decane addition and increasing temperature on both toluene and n-decane s conversion and the products of supercritical toluene pyrolysis will be discussed, along with the most plausible reaction mechanisms responsible for the products formed. The observed effects on the conversion and on aliphatic and one-ring aromatic product yields will be discussed in Chapter III and the effects on the yields of two-and three-ring aromatic products will be discussed in Chapter IV. Lastly, the major findings of this work will be given and possible avenues of future research will be discussed in Chapter V. 14
29 Chapter II. Experimental Methods and Analysis 2.1 Introduction Prior to investigating the results of any experimental endeavor, we must first understand the materials and conditions employed so that those results may be properly interpreted. To that end, this chapter discusses first, the reactor system used in the supercritical pyrolysis of neat toluene and toluene doped with n-decane and second, the methods used to analyze the data. Toluene is relatively unreactive at these temperatures; therefore, analytical methods used here are limited. 2.2 Supercritical fuel pyrolysis reactor system Both the supercritical pyrolysis experiments of pure toluene (critical temperature, 319 C; critical pressure, 41 atm) and n-decane (critical temperature, 345 C; critical pressure, 2.8 atm) doped toluene experiments are performed in an isothermal, isobaric reactor system designed by Davis, 74 and previously used by Stewart, 62,63 Ledesma et al, 36 McClaine et al, 37 Somers et al, 75 Bagley et al, and Nguyen 39 for the supercritical pyrolysis of other model fuels. This reactor has been modified 76 to attain higher temperatures. A schematic of the reactor system is presented in Figure 2.1. Upon the outset of any experiment, the liquid fuel is first sparged with nitrogen (UHP, % pure) for approximately three hours to ensure that no dissolved oxygen, which can introduce auto-oxidative effects, 1 is present in the fuel. After sparging, the fuel is loaded into a high-pressure nonreciprocating pump for delivery to the reactor. The reactor tubing is composed of silica-lined stainless steel (i.d., 1 mm; o.d., 1.59 mm). The silica lining prevents any wallcatalyzed deposit formation which could occur from contact with unlined stainless steel. The 15
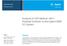
30 Ultra-high purity N 2 Cooling water out Pressure gauge Cooling water in Filter Backpressure regulator Heat exchanger Waste Gas phase products Sparge vessel Fuel Syringe pump Fluidized alumina bath Reactor tubing Condensed phase product collector Gas sampling bag Bubble flowmeter Figure 2.1. Supercritical reactor fuel pyrolysis reactor system. 16
31 tubing is immersed in a temperature-controlled fluidized-alumina bath, establishing isothermality over the entire reactor length. Prior to entering and immediately upon leaving the fluidized-alumina bath, the tubing passes through a water-cooled (25 C) shell-and-tube heat-exchanger to assure a precise thermal history for the fuel and a constant residence time for each experiment. Once the now-quenched products and any unreacted fuel exit the heat exchanger, they pass through a stainless-steel frit (hole size: 1 µm) to prevent any solids which may have been formed from exiting the reactor and enter a back pressure regulator which maintains the constant system pressure throughout the experiment. Upon exiting the back-pressure regulator, the products and fuel continue on to the product-collection apparatus, where they are separated by phase for subsequent analysis. The condensed-phase-product-collection apparatus is immersed in an ice-water bath; gas-phase products are diverted to a Teflon gas-sampling bag for analysis. We chose the experimental conditions to: 1) simulate actual reaction conditions expected in high-speed aircraft and 2) determine the effects of n-decane and temperature on the yields of supercritical pyrolysis of toluene to better understand product formation in mixtures of aliphatic and aromatic compounds in fuels. This particular system is able to attain and function at temperatures up to 7 C (± 1 C), pressures up to 11 atm (±.2 atm), and residence times in the minutes. However, as Bagley et al and Kalpathy et al 8 noted, at 57 C and 94.6 atm, n- decane pyrolysis begins to produce solid deposits. Nguyen 39 performed supercritical toluene experiments up to temperatures and pressures of 685 C and 1 atm without solid formation occurring. In this work, experiments are performed at temperatures of 57 C and 6 C and a constant pressure of 94.6 atm while varying the concentration of the n-decane dopant in pure toluene. Levels of n-decane corresponded to approximately 1, 5, 7.5, and 1 vol % of the fed fuel. The 17
32 residence time is held constant at 133 sec by setting both the flowrate of the high-pressure pump and the reactor tubing length to constant values of 53 ml/hr and 53 cm, respectively. 2.3 Product analysis From Figure 2.1, we see that the unreacted fuel and pyrolysis products are separated by phase in the collection apparatus. Gas-phase pyrolysis products are collected in a Teflon gassampling bag and analyzed by gas chromatography (GC) with flame-ionization detection (FID), while condensed-phase products are collected in a glass vial and transferred to a 6 ml amber vial for storage after analysis. In this work, GC/FID coupled with mass spectrometry (MS) is used to analyze all condensed-phase products Gas-phase product analysis Before analysis, gas-phase products are first diluted in nitrogen (UHP, % pure), then injected onto an Agilent Model 698 GC/FID. Separation is achieved using a GS-GasPro fused silica capillary column (length, 3 m; i.d.,.32 mm; manufactured by J&W Scientific). The carrier gas used is helium ( % pure) with a flowrate of 5 ml/min. The injection volume used is 1 ml and the split flow ratio is 5:1. The initial oven temperature is held at 35 C for two minutes, and then ramped up to 24 C at a rate of 1 C/min. After reaching 24 C, the temperature is held constant for 1 minutes. Prior to identifying any products, reference standards are shot to establish their retention times and to calibrate the instrument. To calibrate the instrument, the reference standards are injected at several concentrations and the FID areas of the peaks of each compound was measured. From the areas and the concentrations of the compounds, individual response factors (RFs) are calculated. These calibrations help to establish the linear range for each compound and provide a range of concentrations which correspond to specific peak areas. 18
Defense Technical Information Center Compilation Part Notice
 UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP012132 TITLE: Fuels Combustion Research: Supercritical Fuel Pyrolysis DISTRIBUTION: Approved for public release, distribution
UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP012132 TITLE: Fuels Combustion Research: Supercritical Fuel Pyrolysis DISTRIBUTION: Approved for public release, distribution
Alkanes and Cycloalkanes
 Alkanes and Cycloalkanes Families of Organic Compounds Organic compounds can be grouped into families by their common structural features We shall survey the nature of the compounds in a tour of the families
Alkanes and Cycloalkanes Families of Organic Compounds Organic compounds can be grouped into families by their common structural features We shall survey the nature of the compounds in a tour of the families
3. Organic Compounds: Alkanes and Cycloalkanes
 3. Organic Compounds: Alkanes and Cycloalkanes Based on McMurry s Organic Chemistry, 6 th edition, Chapter 3 2003 Ronald Kluger Department of Chemistry University of Toronto 1 Families of Organic Compounds!
3. Organic Compounds: Alkanes and Cycloalkanes Based on McMurry s Organic Chemistry, 6 th edition, Chapter 3 2003 Ronald Kluger Department of Chemistry University of Toronto 1 Families of Organic Compounds!
Unit 7 ~ Learning Guide Name:
 Unit 7 ~ Learning Guide : Instructions: Using a pencil, complete the following notes as you work through the related lessons. Show ALL work as is explained in the lessons. You are required to have this
Unit 7 ~ Learning Guide : Instructions: Using a pencil, complete the following notes as you work through the related lessons. Show ALL work as is explained in the lessons. You are required to have this
EARLY CARBONIZATION CHEMISTRY OF FCC DECANT OILS HEATED IN A FLOW REACTOR
 EARLY CARBONIZATION CHEMISTRY OF FCC DECANT OILS HEATED IN A FLOW REACTOR Richard P. Dutta, Kevin Kelleher, and Semih Eser The Energy Institute, Penn State University 29 Academic Projects Bldg, University
EARLY CARBONIZATION CHEMISTRY OF FCC DECANT OILS HEATED IN A FLOW REACTOR Richard P. Dutta, Kevin Kelleher, and Semih Eser The Energy Institute, Penn State University 29 Academic Projects Bldg, University
2. Hydrocarbons. 2.1 Composition of Petroleum
 2. Hydrocarbons 2.1 Composition of Petroleum Naturally occurring petroleum is composed of organic chemicals: approximately 11 to 13% hydrogen and 84 to 87% carbon. Traces of oxygen, sulfur, nitrogen and
2. Hydrocarbons 2.1 Composition of Petroleum Naturally occurring petroleum is composed of organic chemicals: approximately 11 to 13% hydrogen and 84 to 87% carbon. Traces of oxygen, sulfur, nitrogen and
Simplified Chemical Kinetic Models for High-Temperature Oxidation of C 1 to C 12 n-alkanes
 Simplified Chemical Kinetic Models for High-Temperature Oxidation of C 1 to C 1 n-alkanes B. Sirjean, E. Dames, D. A. Sheen, H. Wang * Department of Aerospace and Mechanical Engineering, University of
Simplified Chemical Kinetic Models for High-Temperature Oxidation of C 1 to C 1 n-alkanes B. Sirjean, E. Dames, D. A. Sheen, H. Wang * Department of Aerospace and Mechanical Engineering, University of
Ultra-Inert chemistry for Trace Level Analysis
 Ultra-Inert chemistry for Trace Level Analysis Cikui Liang, Ph.D. Challenges and Needs of Today s Laboratories Challenges Qualification/quantification of trace samples Keep instrument up and running Needs
Ultra-Inert chemistry for Trace Level Analysis Cikui Liang, Ph.D. Challenges and Needs of Today s Laboratories Challenges Qualification/quantification of trace samples Keep instrument up and running Needs
Chapter 22. Organic and Biological Molecules
 Chapter 22 Organic and Biological Molecules The Bonding of Carbon Organic chemistry is the chemistry of compounds containing carbon. Because carbon can form single, double, and triple bonds, the following
Chapter 22 Organic and Biological Molecules The Bonding of Carbon Organic chemistry is the chemistry of compounds containing carbon. Because carbon can form single, double, and triple bonds, the following
C. Saggese, N. E. Sanchez, A. Callejas, A. Millera, R. Bilbao, M. U. Alzueta, A. Frassoldati, A. Cuoci, T. Faravelli, E. Ranzi
 Dipartimento di Chimica, Materiali e Ingegneria Chimica G. Natta Politecnico di Milano in collaboration with: A Kinetic Modeling Study of Polycyclic Aromatic Hydrocarbons (PAHs) and Soot Formation in Acetylene
Dipartimento di Chimica, Materiali e Ingegneria Chimica G. Natta Politecnico di Milano in collaboration with: A Kinetic Modeling Study of Polycyclic Aromatic Hydrocarbons (PAHs) and Soot Formation in Acetylene
An Investigation of Precursors of Combustion Generated Soot Particles in Premixed Ethylene Flames Based on Laser-Induced Fluorescence
 7 th Annual CE-CERT-SJTU Student Symposium An Investigation of Precursors of Combustion Generated Soot Particles in Premixed Ethylene Flames Based on Laser-Induced Fluorescence Chen Gu Problems of Fossil
7 th Annual CE-CERT-SJTU Student Symposium An Investigation of Precursors of Combustion Generated Soot Particles in Premixed Ethylene Flames Based on Laser-Induced Fluorescence Chen Gu Problems of Fossil
Practice Packet Unit 11: Organic Chemistry
 Regents Chemistry: Mr. Palermo Practice Packet Unit 11: Organic Chemistry www.mrpalermo.com 1 LESSON 1: Introduction to Organic Chemistry 1. How many times does carbon bond and why? 2. A student investigated
Regents Chemistry: Mr. Palermo Practice Packet Unit 11: Organic Chemistry www.mrpalermo.com 1 LESSON 1: Introduction to Organic Chemistry 1. How many times does carbon bond and why? 2. A student investigated
Combustion and Flame. The oxidation of a gasoline surrogate in the negative temperature coefficient region
 Combustion and Flame 156 (2009) 549 564 Contents lists available at ScienceDirect Combustion and Flame www.elsevier.com/locate/combustflame The oxidation of a gasoline surrogate in the negative temperature
Combustion and Flame 156 (2009) 549 564 Contents lists available at ScienceDirect Combustion and Flame www.elsevier.com/locate/combustflame The oxidation of a gasoline surrogate in the negative temperature
Geol Supplementary Notes 463-RWR-1,2 GEOL RWR-1 GENERAL INTRODUCTION TO PETROLEUM GEOLOGY: OUTLINE OF MATERIAL TO BE COVERED
 GEOL 463.3 RWR-1 GENERAL INTRODUCTION TO PETROLEUM GEOLOGY: OUTLINE OF MATERIAL TO BE COVERED Recommended sections to read in the textbook: Chapters 1 and 2 (p. 2-22): Background to development of petroleum
GEOL 463.3 RWR-1 GENERAL INTRODUCTION TO PETROLEUM GEOLOGY: OUTLINE OF MATERIAL TO BE COVERED Recommended sections to read in the textbook: Chapters 1 and 2 (p. 2-22): Background to development of petroleum
Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 INTRODUCTION TO ORGANIC AND BIOCHEMISTRY QUIZ 5 Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the IUPAC name
INTRODUCTION TO ORGANIC AND BIOCHEMISTRY QUIZ 5 Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the IUPAC name
Experiment 5 Reactions of Hydrocarbons
 Experiment 5 Reactions of ydrocarbons ydrocarbons are compounds that only contain carbon and hydrogen. ydrocarbons can be classified further by the type of bonds they contain. If a hydrocarbon contains
Experiment 5 Reactions of ydrocarbons ydrocarbons are compounds that only contain carbon and hydrogen. ydrocarbons can be classified further by the type of bonds they contain. If a hydrocarbon contains
Alkanes and Cycloalkanes
 Chapter 3 Alkanes and Cycloalkanes Two types Saturated hydrocarbons Unsaturated hydrocarbons 3.1 Alkanes Also referred as aliphatic hydrocarbons General formula: CnH2n+2 (straight chain) and CnH2n (cyclic)
Chapter 3 Alkanes and Cycloalkanes Two types Saturated hydrocarbons Unsaturated hydrocarbons 3.1 Alkanes Also referred as aliphatic hydrocarbons General formula: CnH2n+2 (straight chain) and CnH2n (cyclic)
Analysis of USP Method <467> Residual Solvents on the Agilent 8890 GC System
 Application Note Residual Solvent Analysis of USP Method Residual Solvents on the Agilent 889 GC System Author Lukas Wieder, Jie Pan, and Rebecca Veeneman Agilent Technologies, Inc. 8 Centerville Road
Application Note Residual Solvent Analysis of USP Method Residual Solvents on the Agilent 889 GC System Author Lukas Wieder, Jie Pan, and Rebecca Veeneman Agilent Technologies, Inc. 8 Centerville Road
QUESTION 1 The boiling temperature of hydrocarbons making up crude oil depends on the strength of intermolecular forces known as:
 QUESTION 1 The boiling temperature of hydrocarbons making up crude oil depends on the strength of intermolecular forces known as: B C D Hydrogen bonding. Dipole-dipole interactions. Dispersion forces.
QUESTION 1 The boiling temperature of hydrocarbons making up crude oil depends on the strength of intermolecular forces known as: B C D Hydrogen bonding. Dipole-dipole interactions. Dispersion forces.
Organic Chemistry Worksheets
 Highlight the single longest, continuous carbon-carbon chain. Note the alkyl branches that are connected to the root chain. Count the carbons in the root chain, starting from the end closest to the alkyl
Highlight the single longest, continuous carbon-carbon chain. Note the alkyl branches that are connected to the root chain. Count the carbons in the root chain, starting from the end closest to the alkyl
Supported molybdenum oxides as effective catalysts for the catalytic fast pyrolysis of lignocellulosic biomass
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 ELECTRONIC SUPPLEMENTARY INFORMATION Supported molybdenum oxides as effective catalysts
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 ELECTRONIC SUPPLEMENTARY INFORMATION Supported molybdenum oxides as effective catalysts
Refereed Publications of M. J. Wornat
 Refereed Publications of M. J. Wornat Kalpathy, S. V., Bagley, S. P., and Wornat, M. J., The Effects of Temperature on the Yields of the Major Aliphatic and Aromatic Products of Supercritical n-decane
Refereed Publications of M. J. Wornat Kalpathy, S. V., Bagley, S. P., and Wornat, M. J., The Effects of Temperature on the Yields of the Major Aliphatic and Aromatic Products of Supercritical n-decane
Some Reactions of Hydrocarbons Experiment #2
 Some Reactions of Hydrocarbons Experiment #2 Objective: To distinguish alkanes, alkenes and aromatic hydrocarbons by their chemical reactions and reactivity. Introduction Hydrocarbons are organic compounds
Some Reactions of Hydrocarbons Experiment #2 Objective: To distinguish alkanes, alkenes and aromatic hydrocarbons by their chemical reactions and reactivity. Introduction Hydrocarbons are organic compounds
Organic Chemistry. REACTIONS Grade 12 Physical Science Mrs KL Faling
 Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
ORGANIC CHEMISTRY: SATURATED HYDROCARBONS
 19 09/16/2013 13:54:37 Page 283 APTER 19 ORGANI EMISTRY: SATURATED YDROARBONS SOLUTIONS TO REVIEW QUESTIONS 1. Two of the major reasons for the large number of organic compounds is the ability of carbon
19 09/16/2013 13:54:37 Page 283 APTER 19 ORGANI EMISTRY: SATURATED YDROARBONS SOLUTIONS TO REVIEW QUESTIONS 1. Two of the major reasons for the large number of organic compounds is the ability of carbon
Organic Chemistry. A. Introduction
 Organic Chemistry A. Introduction 1. Organic chemistry is defined as the chemistry of CARBON compounds. There are a huge number of organic compounds. This results from the fact that carbon forms chains
Organic Chemistry A. Introduction 1. Organic chemistry is defined as the chemistry of CARBON compounds. There are a huge number of organic compounds. This results from the fact that carbon forms chains
Chemical Kinetics of HC Combustion
 Spark Ignition Engine Combustion MAK65E Chemical Kinetics of HC Combustion Prof.Dr. Cem Soruşbay Istanbul Technical University Chemical Kinetics of HC Combustion Introduction Elementary reactions Multi-step
Spark Ignition Engine Combustion MAK65E Chemical Kinetics of HC Combustion Prof.Dr. Cem Soruşbay Istanbul Technical University Chemical Kinetics of HC Combustion Introduction Elementary reactions Multi-step
PETE 203: Properties of oil
 PETE 203: Properties of oil Prepared by: Mr. Brosk Frya Ali Koya University, Faculty of Engineering, Petroleum Engineering Department 2013 2014 Lecture no. (2): Crude oil chemistry and composition 5. Crude
PETE 203: Properties of oil Prepared by: Mr. Brosk Frya Ali Koya University, Faculty of Engineering, Petroleum Engineering Department 2013 2014 Lecture no. (2): Crude oil chemistry and composition 5. Crude
Organic Chemistry. Organic chemistry is the chemistry of compounds containing carbon.
 Organic Chemistry Organic Chemistry Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a
Organic Chemistry Organic Chemistry Organic chemistry is the chemistry of compounds containing carbon. In this chapter we will discuss the structural features of organic molecules, nomenclature, and a
Experimental Determination of Pyrolysis Products from Carbon/Resin Ablative Materials
 Experimental Determination of Pyrolysis Products from Carbon/Resin Ablative Materials Hsi-Wu Wong, Jay Peck, and Robin Edwards Aerodyne Research, Inc., Billerica, MA Guillaume Reinisch University of Texas
Experimental Determination of Pyrolysis Products from Carbon/Resin Ablative Materials Hsi-Wu Wong, Jay Peck, and Robin Edwards Aerodyne Research, Inc., Billerica, MA Guillaume Reinisch University of Texas
Chromatography. Gas Chromatography
 Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Organic Chemistry. Pre-lab Assignment. Purpose. Background. Experiment 14. Before coming to lab: Hydrocarbons. Read the lab thoroughly.
 Experiment 14 Pre-lab Assignment Before coming to lab: Read the lab thoroughly. rganic hemistry Answer the pre-lab questions that appear at the end of this lab exercise. The questions should be answered
Experiment 14 Pre-lab Assignment Before coming to lab: Read the lab thoroughly. rganic hemistry Answer the pre-lab questions that appear at the end of this lab exercise. The questions should be answered
Site Specific Conditional Sampler Garfield County, Colorado. VOC Data Summaries. Prepared for
 Site Specific Conditional Sampler Garfield County, Colorado VOC Data Summaries Prepared for Garfield County Public Health 195 West 14 th Street Rifle, Colorado 81650 Prepared by 1901 Sharp Point Dr., Suite
Site Specific Conditional Sampler Garfield County, Colorado VOC Data Summaries Prepared for Garfield County Public Health 195 West 14 th Street Rifle, Colorado 81650 Prepared by 1901 Sharp Point Dr., Suite
Chapter 12 Alkanes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 12 Alkanes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Introduction
Chapter 12 Alkanes Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Introduction
Organic Chemistry. A brief introduction
 Organic Chemistry A brief introduction Organic Chemistry the study of carbon-containing compounds and their properties excluding: CO, CO 2, CS 2, carbonates and cyanides eight million known organic compounds
Organic Chemistry A brief introduction Organic Chemistry the study of carbon-containing compounds and their properties excluding: CO, CO 2, CS 2, carbonates and cyanides eight million known organic compounds
Hydrocarbons. Chapter 22-23
 Chapter 22-23 Hydrocarbons Organic Compounds All Carbon containing compounds Except carbon oxides, carbides, and carbonates which are inorganic. CO & CO2 Na4C CaCO3 +8 oxidation change CH 4 + O 2 CO 2
Chapter 22-23 Hydrocarbons Organic Compounds All Carbon containing compounds Except carbon oxides, carbides, and carbonates which are inorganic. CO & CO2 Na4C CaCO3 +8 oxidation change CH 4 + O 2 CO 2
Experiment 8: Chlorination of 1-Chlorobutane
 1 Experiment 8: Chlorination of 1-Chlorobutane Alkanes contain only nonpolar carbon-hydrogen and carbon-carbon single bonds, which makes them unreactive toward most acidic and basic reagents. They can,
1 Experiment 8: Chlorination of 1-Chlorobutane Alkanes contain only nonpolar carbon-hydrogen and carbon-carbon single bonds, which makes them unreactive toward most acidic and basic reagents. They can,
General Chemistry Unit 7A ( )
 Organic Chemistry Allotropes Isomers Hydrocarbons o Alkanes o Alkenes o Alkynes o Aromatics Alkyl Halides General Chemistry Unit 7A (2017-2018) 1 2 3 4 Parent Chain: Methane Ethane CH4 C2H6 Propane C3H8
Organic Chemistry Allotropes Isomers Hydrocarbons o Alkanes o Alkenes o Alkynes o Aromatics Alkyl Halides General Chemistry Unit 7A (2017-2018) 1 2 3 4 Parent Chain: Methane Ethane CH4 C2H6 Propane C3H8
1. What is the letter of the alphabet in parentheses that follows EXAM I in the title above? a. a b. b c. c d. d e. e
 HEM 102, EXAM I ( a ) 1. What is the letter of the alphabet in parentheses that follows EXAM I in the title above? a. a b. b c. c d. d e. e 2. Which compound has the most constitutional isomers? a. 2 H
HEM 102, EXAM I ( a ) 1. What is the letter of the alphabet in parentheses that follows EXAM I in the title above? a. a b. b c. c d. d e. e 2. Which compound has the most constitutional isomers? a. 2 H
4) Interpret in words the equation: P4O10 (s) + 6 H2O (l) 4 H3PO4 (aq)
 CHEM102 Chemistry II Spring 09-10 Mid-term Exam/Faculty of Agricultural Sciences and Technologies Student Registration No: Instructor: Prof.Dr.Hüseyin Oğuz Student Name-Surname: Dept. of Computer Information
CHEM102 Chemistry II Spring 09-10 Mid-term Exam/Faculty of Agricultural Sciences and Technologies Student Registration No: Instructor: Prof.Dr.Hüseyin Oğuz Student Name-Surname: Dept. of Computer Information
Chemistry 1110 Exam 4 Study Guide
 Chapter 10 Chemistry 1110 Exam 4 Study Guide 10.1 Know that unstable nuclei can undergo radioactive decay. Identify alpha particles, beta particles, and/or gamma rays based on physical properties such
Chapter 10 Chemistry 1110 Exam 4 Study Guide 10.1 Know that unstable nuclei can undergo radioactive decay. Identify alpha particles, beta particles, and/or gamma rays based on physical properties such
Selection of a Capillary
 Selection of a Capillary GC Column - Series 3 Mark Sinnott Application Engineer March 19, 2009 Page 1 Typical Gas Chromatographic System Mol-Sieve Traps Fixed Restrictors Regulators Injection Port Detector
Selection of a Capillary GC Column - Series 3 Mark Sinnott Application Engineer March 19, 2009 Page 1 Typical Gas Chromatographic System Mol-Sieve Traps Fixed Restrictors Regulators Injection Port Detector
10/27/10. Chapter 27. Injector typically 50 C hotter than oven
 Sample and solvent are vaporized onto the head of a column Vaporized solvent and solute are carried through the column by an inert gas (mobile phase) The mobile phase does not interact with compounds of
Sample and solvent are vaporized onto the head of a column Vaporized solvent and solute are carried through the column by an inert gas (mobile phase) The mobile phase does not interact with compounds of
AP Chemistry Chapter 22 - Organic and Biological Molecules
 AP Chemistry Chapter - Organic and Biological Molecules.1 Alkanes: Saturated Hydrocarbons A. Straight-chain Hydrocarbons 1. Straight-chain alkanes have the formula C n H n+. Carbons are sp hybridized The
AP Chemistry Chapter - Organic and Biological Molecules.1 Alkanes: Saturated Hydrocarbons A. Straight-chain Hydrocarbons 1. Straight-chain alkanes have the formula C n H n+. Carbons are sp hybridized The
AN INTEGRATED SYSTEM USING TEMPERATURE BASED SAMPLING FOR POLYMER CHARACTERIZATION
 AN INTEGRATED SYSTEM USING TEMPERATURE BASED SAMPLING FOR POLYMER CHARACTERIZATION Paper # 164-8P Pittsburgh Conference 24 T. Wampler, C. Zawodny, L. Mancini CDS Analytical, Inc 465 Limestone Road, Oxford,
AN INTEGRATED SYSTEM USING TEMPERATURE BASED SAMPLING FOR POLYMER CHARACTERIZATION Paper # 164-8P Pittsburgh Conference 24 T. Wampler, C. Zawodny, L. Mancini CDS Analytical, Inc 465 Limestone Road, Oxford,
Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
 Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
Section 21.1 Introduction to Hydrocarbons Section 1 Objectives: Explain the terms organic compound and organic chemistry. Section 21.2 Alkanes Chapter 21: Hydrocarbons Section 21.3 Alkenes and Alkynes
All organic compounds contain carbon, however, not all carbon containing compounds are classified as organic. Organic compounds covalently bonded
 Chapter 20 All organic compounds contain carbon, however, not all carbon containing compounds are classified as organic. Organic compounds covalently bonded compounds containing carbon, excluding carbonates
Chapter 20 All organic compounds contain carbon, however, not all carbon containing compounds are classified as organic. Organic compounds covalently bonded compounds containing carbon, excluding carbonates
I. Multiple Choice Questions (Type-I)
 Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Q1. Which one of the following is least likely to occur in the reaction between methane and chlorine?
 Q1. Which one of the following is least likely to occur in the reaction between methane and chlorine? A B C D C 4 + Cl C 3 + Cl C 3 + Cl C 3 Cl + C 3 + Cl 2 C 3 Cl + Cl C 3 Cl + Cl C 2 Cl + Cl (Total 1
Q1. Which one of the following is least likely to occur in the reaction between methane and chlorine? A B C D C 4 + Cl C 3 + Cl C 3 + Cl C 3 Cl + C 3 + Cl 2 C 3 Cl + Cl C 3 Cl + Cl C 2 Cl + Cl (Total 1
Polymer plants continue to seek ways to increase production and efficiency without compromising safety.
 Polyethylene Polypropylene APPLICATION NOTE NOTE Polymer plants continue to seek ways to increase production and efficiency without compromising safety. Process gas analysis is integral to the control
Polyethylene Polypropylene APPLICATION NOTE NOTE Polymer plants continue to seek ways to increase production and efficiency without compromising safety. Process gas analysis is integral to the control
Chemistry 11. Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons
 Chemistry 11 Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons 2 1. Unsaturated hydrocarbons So far, we have studied the hydrocarbons in which atoms are connected exclusively by
Chemistry 11 Unit 10 Organic Chemistry Part III Unsaturated and aromatic hydrocarbons 2 1. Unsaturated hydrocarbons So far, we have studied the hydrocarbons in which atoms are connected exclusively by
National 5 Chemistry. Unit 2: Nature s Chemistry. Topic 1 Hydrocarbons
 St Ninian s High School Chemistry Department National 5 Chemistry Unit 2: Nature s Chemistry Topic 1 Hydrocarbons Summary Notes Name Learning Outcomes After completing this topic you should be able to
St Ninian s High School Chemistry Department National 5 Chemistry Unit 2: Nature s Chemistry Topic 1 Hydrocarbons Summary Notes Name Learning Outcomes After completing this topic you should be able to
High Performance Liquid Chromatography
 Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
High Performance Liquid Chromatography
 Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 1 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Updated: 3 November 2014 Print version High Performance Liquid Chromatography David Reckhow CEE 772 #18 1 HPLC System David Reckhow CEE 772 #18 2 1 Instrument Basics PUMP INJECTION POINT DETECTOR COLUMN
Thermal decomposition of norbornane. (bicyclo[2.2.1]heptane) dissolved in benzene.
![Thermal decomposition of norbornane. (bicyclo[2.2.1]heptane) dissolved in benzene. Thermal decomposition of norbornane. (bicyclo[2.2.1]heptane) dissolved in benzene.](/thumbs/78/76964261.jpg) Thermal decomposition of norbornane (bicyclo[2.2.1]heptane) dissolved in benzene. Experimental study and mechanism investigation. Olivier HERBINET,* Baptiste SIRJEAN, Frédérique BATIN-LECLERC, René FOURNET
Thermal decomposition of norbornane (bicyclo[2.2.1]heptane) dissolved in benzene. Experimental study and mechanism investigation. Olivier HERBINET,* Baptiste SIRJEAN, Frédérique BATIN-LECLERC, René FOURNET
Organic Chemistry. February 18, 2014
 Organic Chemistry February 18, 2014 What does organic mean? Organic Describes products Grown through natural biological process Without synthetic materials In the 18 th century Produced by a living system
Organic Chemistry February 18, 2014 What does organic mean? Organic Describes products Grown through natural biological process Without synthetic materials In the 18 th century Produced by a living system
CH 2252 Instrumental Methods of Analysis Unit V Gas Chromatography. M. Subramanian
 CH 2252 Instrumental Methods of Analysis Unit V Gas Chromatography M. Subramanian Assistant Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam 603
CH 2252 Instrumental Methods of Analysis Unit V Gas Chromatography M. Subramanian Assistant Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam 603
Chapter 13 Alkenes and Alkynes & Aromatic Compounds
 Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
CHEMISTRY - TRO 4E CH.21 - ORGANIC CHEMISTRY.
 !! www.clutchprep.com TOPI: ORGANI EMISTRY Organic hemistry is the study of carbon and the other common nonmetals it is connected to:,, &. Some organic molecules are made of just carbons and hydrogens
!! www.clutchprep.com TOPI: ORGANI EMISTRY Organic hemistry is the study of carbon and the other common nonmetals it is connected to:,, &. Some organic molecules are made of just carbons and hydrogens
THE NEW QUANTITATIVE ANALYTICAL METHOD FOR ULTRATRACE SULFUR COMPOUNDS IN NATURAL GAS
 International Gas Union Research Conference 14 THE NEW QUANTITATIVE ANALYTICAL METHOD FOR ULTRATRACE SULFUR COMPOUNDS IN NATURAL GAS Main author Hironori IMANISHI Tokyo Gas Co., Ltd. JAPAN himanishi@tokyo-.co.jp
International Gas Union Research Conference 14 THE NEW QUANTITATIVE ANALYTICAL METHOD FOR ULTRATRACE SULFUR COMPOUNDS IN NATURAL GAS Main author Hironori IMANISHI Tokyo Gas Co., Ltd. JAPAN himanishi@tokyo-.co.jp
Unit 2 Nature s Chemistry Question Booklet
 Farr igh School NATIONAL 5 EMISTRY Unit 2 Nature s hemistry Question Booklet 1 omologous Series 1. What is meant by a homologous series? 2. What is the general formula for the alkanes? 3. opy and complete
Farr igh School NATIONAL 5 EMISTRY Unit 2 Nature s hemistry Question Booklet 1 omologous Series 1. What is meant by a homologous series? 2. What is the general formula for the alkanes? 3. opy and complete
Unit 5: Organic Chemistry
 Unit 5: Organic Chemistry Organic chemistry: discipline in chemistry focussing strictly on the study of hydrocarbons compounds made up of carbon & hydrogen Organic compounds can contain other elements
Unit 5: Organic Chemistry Organic chemistry: discipline in chemistry focussing strictly on the study of hydrocarbons compounds made up of carbon & hydrogen Organic compounds can contain other elements
Ashwani Gupta. Mb: Class IX-X: X: Math & Science Class XI-XII: XII: Accts., Eco. & B. Stds. Carbon and its compounds.
 Carbon and its compounds MCQ s How many unshared pairs of electrons are present on a nitrogen atom in a molecule of ammonia? 1. 1 2. 2 3. 0 4. 3 What is the estimated number of carbon compounds whose formulae
Carbon and its compounds MCQ s How many unshared pairs of electrons are present on a nitrogen atom in a molecule of ammonia? 1. 1 2. 2 3. 0 4. 3 What is the estimated number of carbon compounds whose formulae
A. They all have a benzene ring structure in the molecule. B. They all have the same molecular formula. C. They all have carbon and hydrogen only
 Ch 21 G12 CoreI- Choose the best answer, then transfer your answers to page (1) [32 marks; 2 each] 1. What characteristic do all aromatic hydrocarbons share? A. They all have a benzene ring structure in
Ch 21 G12 CoreI- Choose the best answer, then transfer your answers to page (1) [32 marks; 2 each] 1. What characteristic do all aromatic hydrocarbons share? A. They all have a benzene ring structure in
3.2 Alkanes. Refining crude oil. N Goalby chemrevise.org 40 C 110 C 180 C. 250 C fuel oil 300 C 340 C. Fractional Distillation: Industrially
 3.2 Alkanes Refining crude oil Fractional Distillation: Industrially Petroleum is a mixture consisting mainly of alkane hydrocarbons Petroleum fraction: mixture of hydrocarbons with a similar chain length
3.2 Alkanes Refining crude oil Fractional Distillation: Industrially Petroleum is a mixture consisting mainly of alkane hydrocarbons Petroleum fraction: mixture of hydrocarbons with a similar chain length
A wide range kinetic modelling study of laminar flame speeds of reference fuels and their mixtures
 A wide range kinetic modelling study of laminar flame speeds of reference fuels and their mixtures A. Frassoldati, R. Grana, A. Cuoci, T. Faravelli, E. Ranzi Dipartimento di Chimica, Materiali e Ingegneria
A wide range kinetic modelling study of laminar flame speeds of reference fuels and their mixtures A. Frassoldati, R. Grana, A. Cuoci, T. Faravelli, E. Ranzi Dipartimento di Chimica, Materiali e Ingegneria
Hydrocarbons. Table Alkanes
 Hydrocarbons Hydrocarbons may be divided into two groups, aliphatic and aromatic. The aliphatics can be subdivided into i) alkanes, ii) alkenes, and iii) alkynes. The alkanes contain single covalent bonds
Hydrocarbons Hydrocarbons may be divided into two groups, aliphatic and aromatic. The aliphatics can be subdivided into i) alkanes, ii) alkenes, and iii) alkynes. The alkanes contain single covalent bonds
II. 1. TRANSFORMATIONS UNDER THE ACTION OF HEAT OR IRRADIATION
 CHAPTER II HYDROCARBON TRANSFORMATIONS THAT DO NOT INVOLVE METALS OR THEIR COMPOUNDS n this chapter we will briefly survey the main types of hydrocarbon transformations that occur without the participation
CHAPTER II HYDROCARBON TRANSFORMATIONS THAT DO NOT INVOLVE METALS OR THEIR COMPOUNDS n this chapter we will briefly survey the main types of hydrocarbon transformations that occur without the participation
CHAPTER 2. Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules
 CHAPTER 2 Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules 2-1 Kinetics and Thermodynamics of Simple Chemical Processes Chemical thermodynamics: Is concerned with the extent that
CHAPTER 2 Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules 2-1 Kinetics and Thermodynamics of Simple Chemical Processes Chemical thermodynamics: Is concerned with the extent that
Name Date Class HYDROCARBONS
 22.1 HYDROCARBONS Section Review Objectives Describe the relationship between number of valence electrons and bonding in carbon Define and describe alkanes Relate the polarity of hydrocarbons to their
22.1 HYDROCARBONS Section Review Objectives Describe the relationship between number of valence electrons and bonding in carbon Define and describe alkanes Relate the polarity of hydrocarbons to their
How To Select the Correct GC Column. Simon Jones Application Engineer
 How To Select the Correct GC Column Simon Jones Application Engineer Things to Consider Is it Volatile enough to chromatograph by GC? Is it a Gas or a Liquid? How are we getting the Sample Injected? What
How To Select the Correct GC Column Simon Jones Application Engineer Things to Consider Is it Volatile enough to chromatograph by GC? Is it a Gas or a Liquid? How are we getting the Sample Injected? What
3. What number would be used to indicate the double bond position in the IUPAC name for CH 3 CH 2 CH=CH CH 3 a. 1 b. 2 c. 3 d.
 Chapter 2 Unsaturated Hydrocarbons MULTIPLE CHOICE 1. Name a difference between a saturated and an unsaturated hydrocarbon. a. Saturated hydrocarbons are composed of only carbon and hydrogen, and unsaturated
Chapter 2 Unsaturated Hydrocarbons MULTIPLE CHOICE 1. Name a difference between a saturated and an unsaturated hydrocarbon. a. Saturated hydrocarbons are composed of only carbon and hydrogen, and unsaturated
Methane contains atoms of two elements, combined chemically. Methane is a mixture of two different elements.
 Q1.Methane (CH 4) is used as a fuel. (a) The displayed structure of methane is: Draw a ring around a part of the displayed structure that represents a covalent bond. (b) Why is methane a compound? Tick
Q1.Methane (CH 4) is used as a fuel. (a) The displayed structure of methane is: Draw a ring around a part of the displayed structure that represents a covalent bond. (b) Why is methane a compound? Tick
Zn/H-ZSM-5 zeolite as catalyst for benzene alkylation with isobutane
 Prog. Catal, Vol. 13, No. 1-2, pp. 35-41 (24) Prog. Catal. Zn/H-ZSM-5 zeolite as catalyst for benzene alkylation with isobutane Adriana Urdă *, Ioan Săndulescu, Ioan-Cezar Marcu University of Bucharest,
Prog. Catal, Vol. 13, No. 1-2, pp. 35-41 (24) Prog. Catal. Zn/H-ZSM-5 zeolite as catalyst for benzene alkylation with isobutane Adriana Urdă *, Ioan Săndulescu, Ioan-Cezar Marcu University of Bucharest,
Organic Chemistry 17.1
 Organic Chemistry 17.1 Introduction to Organic Compounds Naming Alkanes Isomers of Alkanes Naming Cycloalkanes What are Organic Compounds? (1807) The term organic compound originated Meant compounds derived
Organic Chemistry 17.1 Introduction to Organic Compounds Naming Alkanes Isomers of Alkanes Naming Cycloalkanes What are Organic Compounds? (1807) The term organic compound originated Meant compounds derived
Selection of a Capillary GC Column
 Selection of a Capillary GC Column Mark Sinnott Application Engineer March 13, 2008 Page 1 Typical Gas Chromatographic System Mol-Sieve Traps Fixed Restrictors Regulators Injection Port Detector Electrometer
Selection of a Capillary GC Column Mark Sinnott Application Engineer March 13, 2008 Page 1 Typical Gas Chromatographic System Mol-Sieve Traps Fixed Restrictors Regulators Injection Port Detector Electrometer
The School For Excellence 2018 Unit 3 & 4 Chemistry Topic Notes Page 1
 The term fractional distillation refers to a physical method used to separate various components of crude oil. Fractional distillation uses the different boiling temperatures of each component, or fraction,
The term fractional distillation refers to a physical method used to separate various components of crude oil. Fractional distillation uses the different boiling temperatures of each component, or fraction,
SAMPLE FINAL 3150:110
 SAMPLE FINAL 3150:110 Print out a copy. Answer the questions on your own. Check the answers in GOBC Ans.pdf. Good luck! 1. Given the following: Element Electronegativity 2.1 Li 1.0 C 2.5 F 4.0 Al 1.5 2.8
SAMPLE FINAL 3150:110 Print out a copy. Answer the questions on your own. Check the answers in GOBC Ans.pdf. Good luck! 1. Given the following: Element Electronegativity 2.1 Li 1.0 C 2.5 F 4.0 Al 1.5 2.8
AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES
 JPACSM 127 AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES Trace Analytical Inc. Menlo Park, CA ABSTRACT GC based gas analyzers with Reduction Gas Detector (RGD) and Flame Ionization
JPACSM 127 AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES Trace Analytical Inc. Menlo Park, CA ABSTRACT GC based gas analyzers with Reduction Gas Detector (RGD) and Flame Ionization
TDTS 16 Round-the-clock, on-line and cryogen-free monitoring of hydrocarbons from acetylene to trimethylbenzene in ambient air
 Application TDTS 16 Round-the-clock, on-line and cryogen-free monitoring of hydrocarbons from acetylene to trimethylbenzene in ambient air Summary This Application Note describes validation of a thermal
Application TDTS 16 Round-the-clock, on-line and cryogen-free monitoring of hydrocarbons from acetylene to trimethylbenzene in ambient air Summary This Application Note describes validation of a thermal
Organic Chemistry is the chemistry of compounds containing.
 Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Automated Characterization of Compounds in Fire Debris Samples
 125 Sandy Drive, Newark, Delaware 19713 USA tel: 302-737-4297 fax: 302-737-7781 www.midi-inc.com Automated Characterization of Compounds in Fire Debris Samples Application Note Forensics Fire Debris Analysis
125 Sandy Drive, Newark, Delaware 19713 USA tel: 302-737-4297 fax: 302-737-7781 www.midi-inc.com Automated Characterization of Compounds in Fire Debris Samples Application Note Forensics Fire Debris Analysis
The names and formulae of three hydrocarbons in the same homologous series are:... (1) Which homologous series contains ethane, propane and butane?
 Q1. This question is about hydrocarbons. (a) The names and formulae of three hydrocarbons in the same homologous series are: Ethane C 2 H 6 Propane C 3 H 8 Butane C 4 H 10 The next member in the series
Q1. This question is about hydrocarbons. (a) The names and formulae of three hydrocarbons in the same homologous series are: Ethane C 2 H 6 Propane C 3 H 8 Butane C 4 H 10 The next member in the series
1. How do you account for the formation of ethane during chlorination of methane?
 1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
Mass Spectrometry Instrumentation
 Mass Spectrometry Instrumentation A mass spectrometer is composed of an inlet system (which introduces the sample to the instrument and vaporizes the sample) A molecular leak (which produces a steady stream
Mass Spectrometry Instrumentation A mass spectrometer is composed of an inlet system (which introduces the sample to the instrument and vaporizes the sample) A molecular leak (which produces a steady stream
Detailed kinetic study of anisole pyrolysis and oxidation to understand tar formation during biomass combustion and gasification
 Detailed kinetic study of anisole pyrolysis and oxidation to understand tar formation during biomass combustion and gasification Milena Nowakowska, Olivier Herbinet, Anthony Dufour, Pierre-Alexandre Glaude
Detailed kinetic study of anisole pyrolysis and oxidation to understand tar formation during biomass combustion and gasification Milena Nowakowska, Olivier Herbinet, Anthony Dufour, Pierre-Alexandre Glaude
Validation of New VPH GC/MS Method using Multi-Matrix Purge and Trap Sample Prep System
 Validation of New VPH GC/MS Method using Multi-Matrix Purge and Trap Sample Prep System Application Note Abstract The Massachusetts Department of Environmental Protection (MassDEP) developed the Method
Validation of New VPH GC/MS Method using Multi-Matrix Purge and Trap Sample Prep System Application Note Abstract The Massachusetts Department of Environmental Protection (MassDEP) developed the Method
Chapter 27: Gas Chromatography
 Chapter 27: Gas Chromatography Gas Chromatography Mobile phase (carrier gas): gas (He, N 2, H 2 ) - do not interact with analytes - only transport the analyte through the column Analyte: volatile liquid
Chapter 27: Gas Chromatography Gas Chromatography Mobile phase (carrier gas): gas (He, N 2, H 2 ) - do not interact with analytes - only transport the analyte through the column Analyte: volatile liquid
e.g. propan-2-ol ethane-1,1-diol propane-1,2,3-triol H H
 Alcohols General The functional group is - The homologous series has general formula n 2n+1 The names end in ol, with a number if needed to indicate where on the carbon skeleton the group is located. When
Alcohols General The functional group is - The homologous series has general formula n 2n+1 The names end in ol, with a number if needed to indicate where on the carbon skeleton the group is located. When
Combustible Gas Catalytic Bead (0-100 %LEL) Part No FM Performance Certified 1,4 FM Performance Certified 1
 Sensor Data Sheet Document No. 365-2211-31 (Rev D) Combustible Gas Catalytic Bead (0-100 %LEL) Part No. 823-0211-31 FM Performance Certified 1,4 FM Performance Certified 1 Minimum Indicated Concentration...
Sensor Data Sheet Document No. 365-2211-31 (Rev D) Combustible Gas Catalytic Bead (0-100 %LEL) Part No. 823-0211-31 FM Performance Certified 1,4 FM Performance Certified 1 Minimum Indicated Concentration...
Chapter 3: Structure and Nomenclature of Organic Compounds Focus on Alkanes
 hapter 3: Structure and Nomenclature of rganic ompounds Focus on Alkanes rganic molecules are composed of one or more functional groups attached to one or more hydrocarbon groups (alkyl or groups) I. Functional
hapter 3: Structure and Nomenclature of rganic ompounds Focus on Alkanes rganic molecules are composed of one or more functional groups attached to one or more hydrocarbon groups (alkyl or groups) I. Functional
Understanding Gas Chromatography
 Understanding Gas Chromatography What is Really Going on Inside the Box? Simon Jones GC Applications Engineer Page 1 Group/Presentation Title Month ##, 200X ?? K? Page 2 Typical GC System Gas supply Injector
Understanding Gas Chromatography What is Really Going on Inside the Box? Simon Jones GC Applications Engineer Page 1 Group/Presentation Title Month ##, 200X ?? K? Page 2 Typical GC System Gas supply Injector
Fast Analysis of Aromatic Solvent with 0.18 mm ID GC column. Application. Authors. Introduction. Abstract. Gas Chromatography
 Fast Analysis of Aromatic Solvent with.8 mm ID GC column Application Gas Chromatography Authors Yun Zou Agilent Technologies (Shanghai) Co. Ltd. Ying Lun Road Waigaoqiao Free Trade Zone Shanghai 3 P.R.
Fast Analysis of Aromatic Solvent with.8 mm ID GC column Application Gas Chromatography Authors Yun Zou Agilent Technologies (Shanghai) Co. Ltd. Ying Lun Road Waigaoqiao Free Trade Zone Shanghai 3 P.R.
EXPERIMENT 8 Reactions of Hydrocarbons
 EXPERIMENT 8 Reactions of Hydrocarbons Properties and Identification of Hydrocarbons Purpose: a) To identify saturated and unsaturated hydrocarbons using properties and reactions. b) Study substitution
EXPERIMENT 8 Reactions of Hydrocarbons Properties and Identification of Hydrocarbons Purpose: a) To identify saturated and unsaturated hydrocarbons using properties and reactions. b) Study substitution
DEVELOPMENT AND EVALUATION OF THE SAPRC-99 CHEMICAL MECHANISM
 DEVELOPMENT AND EVALUATION OF THE SAPRC-99 CHEMICAL MECHANISM W. P. L. Carter Air Pollution Research Center University of California, Riverside, CA 92521 ALL MECHANISMS DESIGN OBJECTIVES CAN BE USED IN
DEVELOPMENT AND EVALUATION OF THE SAPRC-99 CHEMICAL MECHANISM W. P. L. Carter Air Pollution Research Center University of California, Riverside, CA 92521 ALL MECHANISMS DESIGN OBJECTIVES CAN BE USED IN
CHEM 203 Exam 1. Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 CHEM 203 Exam 1 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following elements is a large percentage of both the earth's
CHEM 203 Exam 1 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following elements is a large percentage of both the earth's
Chapter 11. Introduction to Organic Chemistry
 hapter 11 Introduction to rganic hemistry Properties of arbon and its compounds 2 Properties of arbon and its compounds 3 Properties of arbon and its compounds 4 Properties of arbon and its compounds 5
hapter 11 Introduction to rganic hemistry Properties of arbon and its compounds 2 Properties of arbon and its compounds 3 Properties of arbon and its compounds 4 Properties of arbon and its compounds 5
MOLECULER MODELS/ISOMERS ORGANIC STRUCTURES AND NAMING
 REVISED 10/14 EMISTRY 1101L MOLEULER MODELS/ISOMERS ORGANI STRUTURES AND NAMING NOTE: This lab does not require safety glasses or lab coats. INTRODUTION Electron Dot Structures: Electron dot structures,
REVISED 10/14 EMISTRY 1101L MOLEULER MODELS/ISOMERS ORGANI STRUTURES AND NAMING NOTE: This lab does not require safety glasses or lab coats. INTRODUTION Electron Dot Structures: Electron dot structures,
HISTORY OF ORGANIC CHEMISTRY
 hemistry 52 hapter 12 ISTORY OF ORGANI EMISTRY In the early days of chemistry, scientists classified chemical substances into 2 groups: 1. Inorganic: those that were composed of minerals, such as rocks
hemistry 52 hapter 12 ISTORY OF ORGANI EMISTRY In the early days of chemistry, scientists classified chemical substances into 2 groups: 1. Inorganic: those that were composed of minerals, such as rocks
Chromatographic Methods of Analysis Section: 5 Gas Chromatography (GC) Prof. Tarek A. Fayed
 Chromatographic Methods of Analysis Section: 5 Gas Chromatography (GC) Prof. Tarek A. Fayed Gas Chromatography (GC) In gas chromatography, the sample is vaporized and injected onto the head of a chromatographic
Chromatographic Methods of Analysis Section: 5 Gas Chromatography (GC) Prof. Tarek A. Fayed Gas Chromatography (GC) In gas chromatography, the sample is vaporized and injected onto the head of a chromatographic
