Thermodynamics Lecture Series
|
|
|
- Hugh Houston
- 6 years ago
- Views:
Transcription
1 Termodynamics Lecture Series Ideal Ranke Cycle Te Practical Cycle Applied Sciences Education Researc Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA ttp://www5.uitm.edu.my/faculties/fsg/drjj1.tml
2 Steam Power Plant Example: A steam power cycle. Combustion Products Fuel Steam Turbe Mecanical Energy to Generator Air Pump Heat Excanger Coolg Water System System Boundary for for Termodynamic Analysis
3 Workg fluid: Water Second Law Hig T Res., T H Furnace q q H Purpose: Produce work, W out, ω out Steam Power Plant ω net,out q out q L Low T Res., T L Water from river An Energy-Flow diagram for a SPP
4 Second Law Dream Enge Wat is te maximum performance of real enges if it can never acieve 100%?? Carnot Cycle P - ν diagram for a Carnot (ideal) power plant P, kpa 1 q 4 desired output η required put 3 η rev q q q ω out net q,out rev q out ν, m 3 /kg
5 Second Law Will a Process Happen Carnot Prciples For eat enges contact wit te same ot and cold reservoir P1: η 1 η η 3 (Equality) P: η real < η rev (Inequality) Consequence q L TL q H TH rev (K) (K) ; η rev 1 q q L H rev ηreal η rev 1 Processes satisfyg Carnot Prciples obeys te Second Law of Termodynamics T T L H (K) (K)
6 Second Law Will a Process Happen Clausius Inequality : Sum of Q/T a cyclic process must be zero for reversible processes and negative for real processes δq kj δq kj 0, 0, T K T kg K δq 0, reversible T δq < 0, real T δq > 0, impossible T
7 Copyrigt Te McGraw-Hill Companies, Inc. Permission required for reproduction or display. Isolated systems FIGURE 6-6 Te entropy cange of an isolated system is te sum of te entropy canges of its components, and is never less tan zero. 6-3
8 S Entropy Quantifyg Disorder Increase of Entropy Prciple closed system isolated Te entropy of an isolated (closed and adiabatic) system undergog any process, will always crease. S eat For pure substance: and Ten S gen + S gen S sys + S S sys m(s s1 ) S surr ( Q Q ) T out surr m( s s1 ) + surr surr 0 ( ) Q T surr net, Surroundg System
9 Entropy Quantifyg Disorder Entropy Balance for any general system For any system undergog any process, Energy must be conserved (E E out E sys ) Mass must be conserved (m m out m sys ) Entropy will always be generated except for reversible processes Entropy balance is (S S out + S gen S sys )
10 Copyrigt Te McGraw-Hill Companies, Inc. Permission required for reproduction or display. Entropy Transfer FIGURE 6-61 Mecanisms of entropy transfer for a general system. 6-18
11 Entropy Quantifyg Disorder Entropy Balance Steady-flow device Q S Q out + W W out S out + S gen Ssys m ϑ 0 out So, m ϑ S gen, kw S out S Ten: S gen S eat + S mass S eat + S mass out Q out Q gen + m s m s Tout exit T S let
12 Turbe: Entropy Quantifyg Disorder Entropy Balance Steady-flow device Q out + W W out m ( ϑexit ϑlet ), kw Q Assume adiabatic, ke mass 0, pe mass 0 Entropy Balance S gen S gen were m m W m( ) out 4 3, kw Q out kw + m 4 s4 m 3 s3, out T K Q T ( s s ) m 4 3, m let kw K exit Out In,3
13 Q Entropy Quantifyg Disorder Entropy Balance Steady-flow device Mixg Camber: Q out + W W out m ϑ exit m ϑ Q Q out + W W out m3 3 m m1 1 Q out Q kw S gen + m 3 s3 m s m1 s1, Tout T K were m let m exit 1 let,, kw kw 3
14 Steam Power Plant Vapor Cycle External combustion Fuel (q H ) from nuclear reactors, natural gas, carcoal Workg fluid is H O Ceap, easily available & ig entalpy of vaporization fg Cycle is closed termodynamic cycle Alternates between liquid and gas pase Can Can Carnot cycle be used for representg real SPP?? Aim: To decrease ratio of T L /T H
15 Vapor Cycle Carnot Cycle Efficiency of a Carnot Cycle SPP TL ηrev 1 1 T H 0.55 TL ηrev 1 1 T H 0.67
16 Vapor Cycle Carnot Cycle Impracticalities of Carnot Cycle T, C T crit T H T L q q H qout ql Isotermal expansion: T H limited to only T crit for H O. Hig moisture at turbe exit Not economical to design pump to work -pase (end of Isotermal compression) No assurance can get same x for every cycle (end of Isotermal compression) s 1 s s 3 s 4 s, kj/kg K
17 Vapor Cycle Alternate Carnot Cycle T H Impracticalities of Alternate Carnot Cycle T, C T crit T L q q H Still Problematic Isotermal expansion but at variable pressure Pump to very ig pressure Can te boiler susta te ig P? s 1 s q out q L s 3 s 4 s, kj/kg K
18 Vapor Cycle Ideal Ranke Cycle Overcomg Impracticalities of Carnot Cycle Supereat Supereat te H O at a constant pressure (isobaric expansion) Can easily acieve desired T H iger tan T crit. reduces moisture content at turbe exit Remove all excess eat at condenser Pase is sat. liquid at condenser exit, ence need only a pump to crease pressure Quality is zero for every cycle at condenser exit (pump let)
19 Vapor Cycle Ideal Ranke Cycle Workg fluid: Water Hig T Res., T H Furnace q q H Boiler ω Pump Turbe ω out q -q out ω out - ω Condenser q out q L Low T Res., T L Water from river q -q out ω net,out A Scematic diagram for a Steam Power Plant
20 Vapor Cycle Ideal Ranke Cycle T- s diagram for an Ideal Ranke Cycle T H T crit T, C q q H boiler P H 3 turbe P L T sat@p ω out T L T sat@p4 ω pump 1 s 1 s q out q L condenser 4 s 3 s 4 s, kj/kg K
21 Copyrigt Te McGraw-Hill Companies, Inc. Permission required for reproduction or display. FIGURE 9- Te simple ideal Ranke cycle. 9-
22 Vapor Cycle Ideal Ranke Cycle Energy Analysis In, q q H Out,3 Boiler Assume ke 0, pe 0 for te movg mass, kj/kg q q out + ω ω out θ out θ, kj/kg q exit let, kj/kg q 3, kj/kg Q m( 3 ), kj Q m ( ), kw 3
23 Vapor Cycle Ideal Ranke Cycle Energy Analysis Assume ke 0, pe 0 for te movg mass, kj/kg q q out + ω ω out θ out θ, kj/kg 0 q out exit let -q out 1 4, Out,1 Condenser In,4 So, q out 4 1, kj/kg Q out m( 4 1), kj q out q L ( ) kw Q out m 4 1,
24 Vapor Cycle Ideal Ranke Cycle Energy Analysis In,3 q q out + ω ω out θ out θ, kj/kg Turbe ω out ω out exit let, kj/kg - ω out 4 3, kj/kg So, ω out 3 4, kj/kg Out,4 W out m( 3 4), kj W out m ( ), kw 3 4 Assume ke 0, pe 0 for te movg mass, kj/kg
25 Vapor Cycle Ideal Ranke Cycle Energy Analysis Out, q q out + ω ω out θ out θ, kj/kg ω 0 exit let, kj/kg ω 1, kj/kg For reversible pumps were ω ω pump pump,, ν ω Pdν + νdp ν ν 1 ν f So, W m( 1), kj 1 ( P ) P1 P 1 W m ν 1 Pump dp In,1 ( ), kw 1
26 Vapor Cycle Ideal Ranke Cycle Energy Analysis Efficiency η η ω ω net q net q,out,out q ω η q out 3 q q out ω ( ) 4 1 ( ) 1
27 Vapor Cycle Ideal Ranke Cycle T- s diagram for an Ideal Ranke Cycle T H T crit T, C q q H Note tat P 1 P 4 boiler P H 3 turbe P L s 1 s f@p1 1 f@p1 s 3 T sat@p L T sat@p4 pump ω 1 s 1 s q out q L condenser 4 s 3 s 4 ω out s 4 [s f +xs fg s 3 x s 3 s 4 [ f +x fg s, kj/kg K s f P P 4 1 +ν (P P 1 ); were ν ν 1 ν P 1
28 Vapor Cycle Ideal Ranke Cycle Energy Analysis Increasg Efficiency Must crease ω net,out q q out Increase area under process cycle Decrease condenser pressure; P 1 P 4 P m > P sat@tcoolg+10 deg C Supereat T 3 limited to metullargical strengt of boiler Increase boiler pressure; P P 3 Will decrease quality (an crease moisture). Mimum x is 89.6%.
29 Copyrigt Te McGraw-Hill Companies, Inc. Permission required for reproduction or display. FIGURE 9-6 Te effect of lowerg te condenser pressure on te ideal Ranke cycle. Lowerg Condenser Pressure 9-4
30 Copyrigt Te McGraw-Hill Companies, Inc. Permission required for reproduction or display. FIGURE 9-7 Te effect of supereatg te steam to iger temperatures on te ideal Ranke cycle. Supereatg Steam 9-5
31 Copyrigt Te McGraw-Hill Companies, Inc. Permission required for reproduction or display. Increasg Boiler Pressure FIGURE 9-8 Te effect of creasg te boiler pressure on te ideal Ranke cycle. 9-6
32 FIGURE 9-10 T-s diagrams of te tree cycles discussed Example 9 3. Copyrigt Te McGraw-Hill Companies, Inc. Permission required for reproduction or display. 9-8
33 Vapor Cycle Reeat Ranke Cycle Hig T Reservoir, T H ω Pump 1 q q H Boiler q reeat Hig P turbi ne Low P turbi ne ω out,1 ω out, Condenser 6 q out q L Low T Reservoir, T L
34 FIGURE 9-11 Te ideal reeat Ranke cycle. Copyrigt Te McGraw-Hill Companies, Inc. Permission required for reproduction or display. 9-9
35 Vapor Cycle Reeat Ranke Cycle Reeatg creases η and reduces moisture turbe T, C q reeat 5-4 T 3 5 H T q primary 3 - crit P 3 P 4 P ω 5 out T sat@p3 T ω sat@p4 out, II P 6 P 1 4 T L T sat@p1 1 ω q out s, kj/kg K s 1 s s 3 s 4 s 5 s 6
36 Vapor Cycle Reeat Ranke Cycle Energy Analysis q q primary +q reeat q out 6-1 ω net,out ω out,1 + ω out, - ω η ω net,out q q q q out ( ) η ω net, out q ω out1 + ω q out ω
37 Vapor Cycle Reeat Ranke Cycle Energy Analysis were s 6 [s f +xs fg Use x and s 5 s 6 6 [ f +x fg Knowg s 5 and T 5, P 5 needs to be estimated (usually approximately a quarter of P 3 to ensure x is around 89%. On te property table, coose P 5 so tat te entropy is lower tan s 5 above. Ten can fd
38 Vapor Cycle Reeat Ranke Cycle Energy Analysis were s 1 s f@p1 1 f@p1 s 3 s ν (P P 1 ); were ν ν 1 ν P 1 P 5 P 4. From P 4 and s 4, lookup for 4 te table. If not found, ten do terpolation.
39 Copyrigt Te McGraw-Hill Companies, Inc. Permission required for reproduction or display. Supercritical Ranke Cycle FIGURE 9-9 A supercritical Ranke cycle. 9-7
Thermodynamics Lecture Series
 Thermodynamics Lecture Series Second Law uality of Energy Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@hotmail.com http://www.uitm.edu.my/faculties/fsg/drjj.html
Thermodynamics Lecture Series Second Law uality of Energy Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti Teknologi MARA email: drjjlanita@hotmail.com http://www.uitm.edu.my/faculties/fsg/drjj.html
Quotes. Review - First Law. Review - First Law. Review - First Law. Review - First Law. Thermodynamics Lecture Series
 8//005 herodynaics ecture Series Entropy uantifyg Energy Degradation Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti eknologi MARA eail: drjjlanita@hotail.co http://www3.uit.edu.y/staff/drjj/
8//005 herodynaics ecture Series Entropy uantifyg Energy Degradation Applied Sciences Education Research Group (ASERG) Faculty of Applied Sciences Universiti eknologi MARA eail: drjjlanita@hotail.co http://www3.uit.edu.y/staff/drjj/
Lecture 38: Vapor-compression refrigeration systems
 ME 200 Termodynamics I Lecture 38: Vapor-compression refrigeration systems Yong Li Sangai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Cuan Road Sangai, 200240, P. R. Cina Email
ME 200 Termodynamics I Lecture 38: Vapor-compression refrigeration systems Yong Li Sangai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Cuan Road Sangai, 200240, P. R. Cina Email
Thermodynamics Lecture Series
 Thermodynamics ecture Series Reference: Chap 0 Halliday & Resnick Fundamental of Physics 6 th edition Kinetic Theory of Gases Microscopic Thermodynamics Applied Sciences Education Research Group (ASERG)
Thermodynamics ecture Series Reference: Chap 0 Halliday & Resnick Fundamental of Physics 6 th edition Kinetic Theory of Gases Microscopic Thermodynamics Applied Sciences Education Research Group (ASERG)
Existing Resources: Supplemental/reference for students with thermodynamics background and interests:
 Existing Resources: Masters, G. (1991) Introduction to Environmental Engineering and Science (Prentice Hall: NJ), pages 15 29. [ Masters_1991_Energy.pdf] Supplemental/reference for students with thermodynamics
Existing Resources: Masters, G. (1991) Introduction to Environmental Engineering and Science (Prentice Hall: NJ), pages 15 29. [ Masters_1991_Energy.pdf] Supplemental/reference for students with thermodynamics
Chapters 19 & 20 Heat and the First Law of Thermodynamics
 Capters 19 & 20 Heat and te First Law of Termodynamics Te Zerot Law of Termodynamics Te First Law of Termodynamics Termal Processes Te Second Law of Termodynamics Heat Engines and te Carnot Cycle Refrigerators,
Capters 19 & 20 Heat and te First Law of Termodynamics Te Zerot Law of Termodynamics Te First Law of Termodynamics Termal Processes Te Second Law of Termodynamics Heat Engines and te Carnot Cycle Refrigerators,
Chapter 7. Entropy. by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn
 Chapter 7 Entropy by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics: An Engineering Approach, 5th ed.,
Chapter 7 Entropy by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics: An Engineering Approach, 5th ed.,
= T. (kj/k) (kj/k) 0 (kj/k) int rev. Chapter 6 SUMMARY
 Capter 6 SUMMARY e second la of termodynamics leads to te definition of a ne property called entropy ic is a quantitative measure of microscopic disorder for a system. e definition of entropy is based
Capter 6 SUMMARY e second la of termodynamics leads to te definition of a ne property called entropy ic is a quantitative measure of microscopic disorder for a system. e definition of entropy is based
Applied Thermodynamics. Gas Power Cycles
 Applied Thermodynamics Gas Power Cycles By: Mohd Yusof Taib Faculty of Mechanical Engineering myusof@ump.edu.my Chapter Description Aims To identify and recognized ideal thermodynamics cycle. To analyze
Applied Thermodynamics Gas Power Cycles By: Mohd Yusof Taib Faculty of Mechanical Engineering myusof@ump.edu.my Chapter Description Aims To identify and recognized ideal thermodynamics cycle. To analyze
Entropy and the Second Law of Thermodynamics
 Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
CHAPTER 7 ENTROPY. Copyright Hany A. Al-Ansary and S. I. Abdel-Khalik (2014) 1
 CHAPTER 7 ENTROPY S. I. Abdel-Khalik (2014) 1 ENTROPY The Clausius Inequality The Clausius inequality states that for for all cycles, reversible or irreversible, engines or refrigerators: For internally-reversible
CHAPTER 7 ENTROPY S. I. Abdel-Khalik (2014) 1 ENTROPY The Clausius Inequality The Clausius inequality states that for for all cycles, reversible or irreversible, engines or refrigerators: For internally-reversible
Chapter 7. Entropy: A Measure of Disorder
 Chapter 7 Entropy: A Measure of Disorder Entropy and the Clausius Inequality The second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of microscopic
Chapter 7 Entropy: A Measure of Disorder Entropy and the Clausius Inequality The second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of microscopic
King Fahd University of Petroleum & Minerals
 King Fahd University of Petroleum & Minerals Mechanical Engineering Thermodynamics ME 04 BY Dr. Haitham Bahaidarah My Office Office Hours: :00 0:00 am SMW 03:00 04:00 pm UT Location: Building Room # 5.4
King Fahd University of Petroleum & Minerals Mechanical Engineering Thermodynamics ME 04 BY Dr. Haitham Bahaidarah My Office Office Hours: :00 0:00 am SMW 03:00 04:00 pm UT Location: Building Room # 5.4
Thermodynamics: An Engineering Approach Seventh Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, Chapter 7 ENTROPY
 Thermodynamics: An Engineering Approach Seventh Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 7 ENTROPY Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Thermodynamics: An Engineering Approach Seventh Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 7 ENTROPY Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
ENTROPY. Chapter 7. Mehmet Kanoglu. Thermodynamics: An Engineering Approach, 6 th Edition. Yunus A. Cengel, Michael A. Boles.
 Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Chapter 7 ENTROPY Mehmet Kanoglu Copyright The McGraw-Hill Companies, Inc. Permission required
Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Chapter 7 ENTROPY Mehmet Kanoglu Copyright The McGraw-Hill Companies, Inc. Permission required
Carnot Factor of a Vapour Power Cycle with Regenerative Extraction
 Journal of Modern Pysics, 2017, 8, 1795-1808 ttp://www.scirp.org/journal/jmp ISSN Online: 2153-120X ISSN Print: 2153-1196 arnot Factor of a Vapour Power ycle wit Regenerative Extraction Duparquet Alain
Journal of Modern Pysics, 2017, 8, 1795-1808 ttp://www.scirp.org/journal/jmp ISSN Online: 2153-120X ISSN Print: 2153-1196 arnot Factor of a Vapour Power ycle wit Regenerative Extraction Duparquet Alain
Lecture 35: Vapor power systems, Rankine cycle
 ME 00 Thermodynamics I Spring 015 Lecture 35: Vapor power systems, Rankine cycle Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai, 0040, P. R.
ME 00 Thermodynamics I Spring 015 Lecture 35: Vapor power systems, Rankine cycle Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai, 0040, P. R.
(1)5. Which of the following equations is always valid for a fixed mass system undergoing an irreversible or reversible process:
 Last Name First Name ME 300 Engineering Thermodynamics Exam #2 Spring 2008 March 28, 2008 Form A Note : (i) (ii) (iii) (iv) Closed book, closed notes; one 8.5 x 11 sheet allowed. 60 points total; 60 minutes;
Last Name First Name ME 300 Engineering Thermodynamics Exam #2 Spring 2008 March 28, 2008 Form A Note : (i) (ii) (iii) (iv) Closed book, closed notes; one 8.5 x 11 sheet allowed. 60 points total; 60 minutes;
In the next lecture...
 16 1 In the next lecture... Solve problems from Entropy Carnot cycle Exergy Second law efficiency 2 Problem 1 A heat engine receives reversibly 420 kj/cycle of heat from a source at 327 o C and rejects
16 1 In the next lecture... Solve problems from Entropy Carnot cycle Exergy Second law efficiency 2 Problem 1 A heat engine receives reversibly 420 kj/cycle of heat from a source at 327 o C and rejects
( )( ) 7 MPa q in = = 10 kpa q out. 1 h. = s. Thus, and = 38.9% (b) (c) The rate of heat rejection to the cooling water and its temperature rise are
 . A team poer plant operate on a imple ideal Ranke cycle beteen te peciied preure limit. e termal eiciency o te cycle, te ma lo rate o te team, and te temperature rie o te coolg ater are to be determed.
. A team poer plant operate on a imple ideal Ranke cycle beteen te peciied preure limit. e termal eiciency o te cycle, te ma lo rate o te team, and te temperature rie o te coolg ater are to be determed.
Special Topic: Binary Vapor Cycles
 0- Special opic: Bary Vapor ycle 0- Bary poer cycle i a cycle ic i actually a combation o to cycle; one te ig temperature region, and te oter te lo temperature region. It purpoe i to creae termal eiciency.
0- Special opic: Bary Vapor ycle 0- Bary poer cycle i a cycle ic i actually a combation o to cycle; one te ig temperature region, and te oter te lo temperature region. It purpoe i to creae termal eiciency.
SOLUTION: Consider the system to be the refrigerator (shown in the following schematic), which operates over a cycle in normal operation.
 Soln_21 An ordinary household refrigerator operating in steady state receives electrical work while discharging net energy by heat transfer to its surroundings (e.g., the kitchen). a. Is this a violation
Soln_21 An ordinary household refrigerator operating in steady state receives electrical work while discharging net energy by heat transfer to its surroundings (e.g., the kitchen). a. Is this a violation
Thermodynamics: An Engineering Approach Seventh Edition in SI Units Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011.
 Thermodynamics: An Engineering Approach Seventh Edition in SI Units Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 7 ENTROPY Mehmet Kanoglu University of Gaziantep Copyright The McGraw-Hill
Thermodynamics: An Engineering Approach Seventh Edition in SI Units Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 7 ENTROPY Mehmet Kanoglu University of Gaziantep Copyright The McGraw-Hill
8-4 P 2. = 12 kw. AIR T = const. Therefore, Q &
 8-4 8-4 Air i compreed teadily by a compreor. e air temperature i mataed contant by eat rejection to te urroundg. e rate o entropy cange o air i to be determed. Aumption i i a teady-low proce ce tere i
8-4 8-4 Air i compreed teadily by a compreor. e air temperature i mataed contant by eat rejection to te urroundg. e rate o entropy cange o air i to be determed. Aumption i i a teady-low proce ce tere i
I. (20%) Answer the following True (T) or False (F). If false, explain why for full credit.
 I. (20%) Answer the following True (T) or False (F). If false, explain why for full credit. Both the Kelvin and Fahrenheit scales are absolute temperature scales. Specific volume, v, is an intensive property,
I. (20%) Answer the following True (T) or False (F). If false, explain why for full credit. Both the Kelvin and Fahrenheit scales are absolute temperature scales. Specific volume, v, is an intensive property,
= for reversible < for irreversible
 CHAPER 6 Entropy Copyright he McGraw-Hill Companies, Inc. Permission required for reproduction or display. he Clausius Inequality: δ 0 Cyclic integral his inequality is valid for all cycles, reversible
CHAPER 6 Entropy Copyright he McGraw-Hill Companies, Inc. Permission required for reproduction or display. he Clausius Inequality: δ 0 Cyclic integral his inequality is valid for all cycles, reversible
5/6/ :41 PM. Chapter 6. Using Entropy. Dr. Mohammad Abuhaiba, PE
 Chapter 6 Using Entropy 1 2 Chapter Objective Means are introduced for analyzing systems from the 2 nd law perspective as they undergo processes that are not necessarily cycles. Objective: introduce entropy
Chapter 6 Using Entropy 1 2 Chapter Objective Means are introduced for analyzing systems from the 2 nd law perspective as they undergo processes that are not necessarily cycles. Objective: introduce entropy
Lecture 44: Review Thermodynamics I
 ME 00 Thermodynamics I Lecture 44: Review Thermodynamics I Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai, 0040, P. R. China Email : liyo@sjtu.edu.cn
ME 00 Thermodynamics I Lecture 44: Review Thermodynamics I Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai, 0040, P. R. China Email : liyo@sjtu.edu.cn
Delft University of Technology DEPARTMENT OF AEROSPACE ENGINEERING
 Delft University of Technology DEPRTMENT OF EROSPCE ENGINEERING Course: Physics I (E-04) Course year: Date: 7-0-0 Time: 4:00-7:00 Student name and itials (capital letters): Student number:. You have attended
Delft University of Technology DEPRTMENT OF EROSPCE ENGINEERING Course: Physics I (E-04) Course year: Date: 7-0-0 Time: 4:00-7:00 Student name and itials (capital letters): Student number:. You have attended
Kelvin Planck Statement of the Second Law. Clausius Statement of the Second Law
 Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent
Kelv Planck Statement of te Second aw It is imossible to construct an enge wic, oeratg a cycle, will roduce no oter effect tan te extraction of eat from a sgle reservoir and te erformance of an equivalent
Spring_#7. Thermodynamics. Youngsuk Nam.
 Spring_#7 Thermodynamics Youngsuk Nam ysnam1@khu.ac.kr You can t connect the dots looking forward; you can only connect them looking backwards. So you have to trust that the dots will somehow connect in
Spring_#7 Thermodynamics Youngsuk Nam ysnam1@khu.ac.kr You can t connect the dots looking forward; you can only connect them looking backwards. So you have to trust that the dots will somehow connect in
Consequences of Second Law of Thermodynamics. Entropy. Clausius Inequity
 onsequences of Second Law of hermodynamics Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & echnology BUE Dhaka-000, Bangladesh zahurul@me.buet.ac.bd
onsequences of Second Law of hermodynamics Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & echnology BUE Dhaka-000, Bangladesh zahurul@me.buet.ac.bd
Exergy and the Dead State
 EXERGY The energy content of the universe is constant, just as its mass content is. Yet at times of crisis we are bombarded with speeches and articles on how to conserve energy. As engineers, we know that
EXERGY The energy content of the universe is constant, just as its mass content is. Yet at times of crisis we are bombarded with speeches and articles on how to conserve energy. As engineers, we know that
The Second Law of Thermodynamics
 he Second Law of hermodynamics So far We have studied the second law by looking at its results We don t have a thermodynamic property that can describe it In this chapter we will develop a mathematical
he Second Law of hermodynamics So far We have studied the second law by looking at its results We don t have a thermodynamic property that can describe it In this chapter we will develop a mathematical
General Physics I. New Lecture 27: Carnot Cycle, The 2nd Law, Entropy and Information. Prof. WAN, Xin
 General Pysics I New Lecture 27: Carnot Cycle, e 2nd Law, Entropy and Information Prof. AN, Xin xinwan@zju.edu.cn ttp://zimp.zju.edu.cn/~xinwan/ Carnot s Engine Efficiency of a Carnot Engine isotermal
General Pysics I New Lecture 27: Carnot Cycle, e 2nd Law, Entropy and Information Prof. AN, Xin xinwan@zju.edu.cn ttp://zimp.zju.edu.cn/~xinwan/ Carnot s Engine Efficiency of a Carnot Engine isotermal
ME 2322 Thermodynamics I PRE-LECTURE Lesson 23 Complete the items below Name:
 Lesson 23 1. (10 pt) Write the equation for the thermal efficiency of a Carnot heat engine below: T η = T 1 L H 2. (10 pt) Can the thermal efficiency of an actual engine ever exceed that of an equivalent
Lesson 23 1. (10 pt) Write the equation for the thermal efficiency of a Carnot heat engine below: T η = T 1 L H 2. (10 pt) Can the thermal efficiency of an actual engine ever exceed that of an equivalent
c Dr. Md. Zahurul Haq (BUET) Entropy ME 203 (2017) 2 / 27 T037
 onsequences of Second Law of hermodynamics Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & echnology BUE Dhaka-000, Bangladesh zahurul@me.buet.ac.bd
onsequences of Second Law of hermodynamics Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & echnology BUE Dhaka-000, Bangladesh zahurul@me.buet.ac.bd
MAE 11. Homework 8: Solutions 11/30/2018
 MAE 11 Homework 8: Solutions 11/30/2018 MAE 11 Fall 2018 HW #8 Due: Friday, November 30 (beginning of class at 12:00p) Requirements:: Include T s diagram for all cycles. Also include p v diagrams for Ch
MAE 11 Homework 8: Solutions 11/30/2018 MAE 11 Fall 2018 HW #8 Due: Friday, November 30 (beginning of class at 12:00p) Requirements:: Include T s diagram for all cycles. Also include p v diagrams for Ch
Lecture 10: Carnot theorem
 ecture 0: Carnot teorem Feb 7, 005 Equivalence of Kelvin and Clausius formulations ast time we learned tat te Second aw can be formulated in two ways. e Kelvin formulation: No process is possible wose
ecture 0: Carnot teorem Feb 7, 005 Equivalence of Kelvin and Clausius formulations ast time we learned tat te Second aw can be formulated in two ways. e Kelvin formulation: No process is possible wose
Section A 01. (12 M) (s 2 s 3 ) = 313 s 2 = s 1, h 3 = h 4 (s 1 s 3 ) = kj/kgk. = kj/kgk. 313 (s 3 s 4f ) = ln
 0. (a) Sol: Section A A refrigerator macine uses R- as te working fluid. Te temperature of R- in te evaporator coil is 5C, and te gas leaves te compressor as dry saturated at a temperature of 40C. Te mean
0. (a) Sol: Section A A refrigerator macine uses R- as te working fluid. Te temperature of R- in te evaporator coil is 5C, and te gas leaves te compressor as dry saturated at a temperature of 40C. Te mean
Consequences of Second Law of Thermodynamics. Entropy. Clausius Inequity
 onsequences of Second Law of hermodynamics Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & echnology BUE Dhaka-000, Bangladesh zahurul@me.buet.ac.bd
onsequences of Second Law of hermodynamics Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & echnology BUE Dhaka-000, Bangladesh zahurul@me.buet.ac.bd
ENERGY TRANSFER BY WORK: Electrical Work: When N Coulombs of electrical charge move through a potential difference V
 Weight, W = mg Where m=mass, g=gravitational acceleration ENERGY TRANSFER BY WOR: Sign convention: Work done on a system = (+) Work done by a system = (-) Density, ρ = m V kg m 3 Where m=mass, V =Volume
Weight, W = mg Where m=mass, g=gravitational acceleration ENERGY TRANSFER BY WOR: Sign convention: Work done on a system = (+) Work done by a system = (-) Density, ρ = m V kg m 3 Where m=mass, V =Volume
ME Thermodynamics I
 Homework - Week 01 HW-01 (25 points) Given: 5 Schematic of the solar cell/solar panel Find: 5 Identify the system and the heat/work interactions associated with it. Show the direction of the interactions.
Homework - Week 01 HW-01 (25 points) Given: 5 Schematic of the solar cell/solar panel Find: 5 Identify the system and the heat/work interactions associated with it. Show the direction of the interactions.
Entropy and the Second Law of Thermodynamics
 Entropy and the Second Law of hermodynamics Reading Problems 6-, 6-2, 6-7, 6-8, 6-6-8, 6-87, 7-7-0, 7-2, 7-3 7-39, 7-46, 7-6, 7-89, 7-, 7-22, 7-24, 7-30, 7-55, 7-58 Why do we need another law in thermodynamics?
Entropy and the Second Law of hermodynamics Reading Problems 6-, 6-2, 6-7, 6-8, 6-6-8, 6-87, 7-7-0, 7-2, 7-3 7-39, 7-46, 7-6, 7-89, 7-, 7-22, 7-24, 7-30, 7-55, 7-58 Why do we need another law in thermodynamics?
VI. Entropy. VI. Entropy
 A. Introduction. he first law: energy cannot be created or destroyed. he second law: certain processes do occur and certain processes don t 3. he magic vortex tube. Will it work or won t it? Cold air kg
A. Introduction. he first law: energy cannot be created or destroyed. he second law: certain processes do occur and certain processes don t 3. he magic vortex tube. Will it work or won t it? Cold air kg
Physics 207 Lecture 23
 ysics 07 Lecture ysics 07, Lecture 8, Dec. Agenda:. Finis, Start. Ideal gas at te molecular level, Internal Energy Molar Specific Heat ( = m c = n ) Ideal Molar Heat apacity (and U int = + W) onstant :
ysics 07 Lecture ysics 07, Lecture 8, Dec. Agenda:. Finis, Start. Ideal gas at te molecular level, Internal Energy Molar Specific Heat ( = m c = n ) Ideal Molar Heat apacity (and U int = + W) onstant :
= for reversible < for irreversible
 CAPER 6 Entropy Copyright he McGraw-ill Companies, Inc. Permission required for reproduction or display. he Clausius Inequality: 0 his inequality is valid for all cycles, reversible or irreversible Cycle
CAPER 6 Entropy Copyright he McGraw-ill Companies, Inc. Permission required for reproduction or display. he Clausius Inequality: 0 his inequality is valid for all cycles, reversible or irreversible Cycle
Engineering Thermodynamics. Chapter 6. Entropy: a measure of Disorder 6.1 Introduction
 Engineering hermodynamics AAi Chapter 6 Entropy: a measure of Disorder 6. Introduction he second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of
Engineering hermodynamics AAi Chapter 6 Entropy: a measure of Disorder 6. Introduction he second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of
CLAUSIUS INEQUALITY. PROOF: In Classroom
 Chapter 7 ENTROPY CLAUSIUS INEQUALITY PROOF: In Classroom 2 RESULTS OF CLAUSIUS INEQUALITY For internally reversible cycles δq = 0 T int rev For irreversible cycles δq < 0 T irr A quantity whose cyclic
Chapter 7 ENTROPY CLAUSIUS INEQUALITY PROOF: In Classroom 2 RESULTS OF CLAUSIUS INEQUALITY For internally reversible cycles δq = 0 T int rev For irreversible cycles δq < 0 T irr A quantity whose cyclic
Solutions to Homework #10
 Solution to Homeork #0 0-6 A teady-lo Carnot enge it ater a te orkg luid operate at peciied condition. e termal eiciency, te preure at te turbe let, and te ork put are to be determed. Aumption Steady operatg
Solution to Homeork #0 0-6 A teady-lo Carnot enge it ater a te orkg luid operate at peciied condition. e termal eiciency, te preure at te turbe let, and te ork put are to be determed. Aumption Steady operatg
CHAPTER 8 ENTROPY. Blank
 CHAPER 8 ENROPY Blank SONNAG/BORGNAKKE SUDY PROBLEM 8-8. A heat engine efficiency from the inequality of Clausius Consider an actual heat engine with efficiency of η working between reservoirs at and L.
CHAPER 8 ENROPY Blank SONNAG/BORGNAKKE SUDY PROBLEM 8-8. A heat engine efficiency from the inequality of Clausius Consider an actual heat engine with efficiency of η working between reservoirs at and L.
Spring_#8. Thermodynamics. Youngsuk Nam
 Spring_#8 Thermodynamics Youngsuk Nam ysnam1@khu.ac.krac kr Ch.7: Entropy Apply the second law of thermodynamics to processes. Define a new property called entropy to quantify the secondlaw effects. Establish
Spring_#8 Thermodynamics Youngsuk Nam ysnam1@khu.ac.krac kr Ch.7: Entropy Apply the second law of thermodynamics to processes. Define a new property called entropy to quantify the secondlaw effects. Establish
SIMPLE RANKINE CYCLE. 3 expander. boiler. pump. condenser 1 W Q. cycle cycle. net. shaft
 SIMPLE RANKINE CYCLE um boiler exander condener Steady Flow, Oen Sytem - region ace Steady Flow Energy Equation for Procee m (u Pum Proce,, Boiler Proce,, V ρg) 0, 0, Exanion Proce,, 0, Condener Proce,,
SIMPLE RANKINE CYCLE um boiler exander condener Steady Flow, Oen Sytem - region ace Steady Flow Energy Equation for Procee m (u Pum Proce,, Boiler Proce,, V ρg) 0, 0, Exanion Proce,, 0, Condener Proce,,
CHAPTER 6 THE SECOND LAW OF THERMODYNAMICS
 CHAPTER 6 THE SECOND LAW OF THERMODYNAMICS S. I. Abdel-Khalik (2014) 1 CHAPTER 6 -- The Second Law of Thermodynamics OUTCOME: Identify Valid (possible) Processes as those that satisfy both the first and
CHAPTER 6 THE SECOND LAW OF THERMODYNAMICS S. I. Abdel-Khalik (2014) 1 CHAPTER 6 -- The Second Law of Thermodynamics OUTCOME: Identify Valid (possible) Processes as those that satisfy both the first and
ME 2322 Thermodynamics I PRE-LECTURE Lesson 23 Complete the items below Name:
 Lesson 23 1. (10 pt) Write the equation for the thermal efficiency of a Carnot heat engine below: 1 L H 2. (10 pt) Can the thermal efficiency of an actual engine ever exceed that of an equivalent Carnot
Lesson 23 1. (10 pt) Write the equation for the thermal efficiency of a Carnot heat engine below: 1 L H 2. (10 pt) Can the thermal efficiency of an actual engine ever exceed that of an equivalent Carnot
CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES
 Thermodynamics: An Engineering Approach 8th Edition in SI Units Yunus A. Çengel, Michael A. Boles McGraw-Hill, 2015 CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES Lecture slides by Dr. Fawzi Elfghi
Thermodynamics: An Engineering Approach 8th Edition in SI Units Yunus A. Çengel, Michael A. Boles McGraw-Hill, 2015 CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES Lecture slides by Dr. Fawzi Elfghi
The first law of thermodynamics. U = internal energy. Q = amount of heat energy transfer
 Thermodynamics Investigation of the energy transfer by heat and work and how natural systems behave (Q) Heat transfer of energy due to temp differences. (W) Work transfer of energy through mechanical means.
Thermodynamics Investigation of the energy transfer by heat and work and how natural systems behave (Q) Heat transfer of energy due to temp differences. (W) Work transfer of energy through mechanical means.
Physics 202 Homework 5
 Physics 202 Homework 5 Apr 29, 2013 1. A nuclear-fueled electric power plant utilizes a so-called boiling water reac- 5.8 C tor. In this type of reactor, nuclear energy causes water under pressure to boil
Physics 202 Homework 5 Apr 29, 2013 1. A nuclear-fueled electric power plant utilizes a so-called boiling water reac- 5.8 C tor. In this type of reactor, nuclear energy causes water under pressure to boil
ME6301- ENGINEERING THERMODYNAMICS UNIT I BASIC CONCEPT AND FIRST LAW PART-A
 ME6301- ENGINEERING THERMODYNAMICS UNIT I BASIC CONCEPT AND FIRST LAW PART-A 1. What is meant by thermodynamics system? (A/M 2006) Thermodynamics system is defined as any space or matter or group of matter
ME6301- ENGINEERING THERMODYNAMICS UNIT I BASIC CONCEPT AND FIRST LAW PART-A 1. What is meant by thermodynamics system? (A/M 2006) Thermodynamics system is defined as any space or matter or group of matter
Chapter 7: The Second Law of Thermodynamics
 Chapter 7: he Second Law of hermodynamics he second law of thermodynamics asserts that processes occur in a certain direction and that the energy has quality as well as quantity he first law places no
Chapter 7: he Second Law of hermodynamics he second law of thermodynamics asserts that processes occur in a certain direction and that the energy has quality as well as quantity he first law places no
Unit Workbook 2 - Level 5 ENG U64 Thermofluids 2018 UniCourse Ltd. All Rights Reserved. Sample
 Pearson BTEC Level 5 Higher Nationals in Engineering (RQF) Unit 64: Thermofluids Unit Workbook 2 in a series of 4 for this unit Learning Outcome 2 Vapour Power Cycles Page 1 of 26 2.1 Power Cycles Unit
Pearson BTEC Level 5 Higher Nationals in Engineering (RQF) Unit 64: Thermofluids Unit Workbook 2 in a series of 4 for this unit Learning Outcome 2 Vapour Power Cycles Page 1 of 26 2.1 Power Cycles Unit
Number of extra papers used if any
 Last Name: First Name: Thermo no. ME 200 Thermodynamics 1 Fall 2018 Exam Circle your structor s last name Division 1 (7:0): Naik Division (1:0): Wassgren Division 6 (11:0): Sojka Division 2 (9:0): Choi
Last Name: First Name: Thermo no. ME 200 Thermodynamics 1 Fall 2018 Exam Circle your structor s last name Division 1 (7:0): Naik Division (1:0): Wassgren Division 6 (11:0): Sojka Division 2 (9:0): Choi
ME 200 Final Exam December 14, :00 a.m. to 10:00 a.m.
 CIRCLE YOUR LECTURE BELOW: First Name Last Name 7:30 a.m. 8:30 a.m. 10:30 a.m. 11:30 a.m. Boregowda Boregowda Braun Bae 2:30 p.m. 3:30 p.m. 4:30 p.m. Meyer Naik Hess ME 200 Final Exam December 14, 2015
CIRCLE YOUR LECTURE BELOW: First Name Last Name 7:30 a.m. 8:30 a.m. 10:30 a.m. 11:30 a.m. Boregowda Boregowda Braun Bae 2:30 p.m. 3:30 p.m. 4:30 p.m. Meyer Naik Hess ME 200 Final Exam December 14, 2015
Content. Entropy and principle of increasing entropy. Change of entropy in an ideal gas.
 Entropy Content Entropy and principle of increasing entropy. Change of entropy in an ideal gas. Entropy Entropy can be viewed as a measure of molecular disorder, or molecular randomness. As a system becomes
Entropy Content Entropy and principle of increasing entropy. Change of entropy in an ideal gas. Entropy Entropy can be viewed as a measure of molecular disorder, or molecular randomness. As a system becomes
Basic thermodynamics. heat to the high temperature reservoir.
 Consider a heat engine that is operating in a cyclic process takes heat (QH) from a high temperature reservoir & converts completely into work (W), violating the Kelvin Planck statement. Let the work W,
Consider a heat engine that is operating in a cyclic process takes heat (QH) from a high temperature reservoir & converts completely into work (W), violating the Kelvin Planck statement. Let the work W,
ECE309 THERMODYNAMICS & HEAT TRANSFER MIDTERM EXAMINATION. Instructor: R. Culham. Name: Student ID Number:
 ECE309 THERMODYNAMICS & HEAT TRANSFER MIDTERM EXAMINATION June 19, 2015 2:30 pm - 4:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Permitted
ECE309 THERMODYNAMICS & HEAT TRANSFER MIDTERM EXAMINATION June 19, 2015 2:30 pm - 4:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Permitted
Availability and Irreversibility
 Availability and Irreversibility 1.0 Overview A critical application of thermodynamics is finding the maximum amount of work that can be extracted from a given energy resource. This calculation forms the
Availability and Irreversibility 1.0 Overview A critical application of thermodynamics is finding the maximum amount of work that can be extracted from a given energy resource. This calculation forms the
CHAPTER. The First Law of Thermodynamics: Closed Systems
 CHAPTER 3 The First Law of Thermodynamics: Closed Systems Closed system Energy can cross the boundary of a closed system in two forms: Heat and work FIGURE 3-1 Specifying the directions of heat and work.
CHAPTER 3 The First Law of Thermodynamics: Closed Systems Closed system Energy can cross the boundary of a closed system in two forms: Heat and work FIGURE 3-1 Specifying the directions of heat and work.
ME Thermodynamics I. Lecture Notes and Example Problems
 ME 227.3 Thermodynamics I Lecture Notes and Example Problems James D. Bugg September 2018 Department of Mechanical Engineering Introduction Part I: Lecture Notes This part contains handout versions of
ME 227.3 Thermodynamics I Lecture Notes and Example Problems James D. Bugg September 2018 Department of Mechanical Engineering Introduction Part I: Lecture Notes This part contains handout versions of
Readings for this homework assignment and upcoming lectures
 Homework #3 (group) Tuesday, February 13 by 4:00 pm 5290 exercises (individual) Thursday, February 15 by 4:00 pm extra credit (individual) Thursday, February 15 by 4:00 pm Readings for this homework assignment
Homework #3 (group) Tuesday, February 13 by 4:00 pm 5290 exercises (individual) Thursday, February 15 by 4:00 pm extra credit (individual) Thursday, February 15 by 4:00 pm Readings for this homework assignment
Lecture 29-30: Closed system entropy balance
 ME 200 Thermodynamics I Spring 2016 Lecture 29-30: Closed system entropy balance Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai, 200240, P.
ME 200 Thermodynamics I Spring 2016 Lecture 29-30: Closed system entropy balance Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai, 200240, P.
This follows from the Clausius inequality as a consequence of the second law of thermodynamics. Therefore. (for reversible process only) (22.
 Entropy Clausius inequality can be used to analyze the cyclic process in a quantitative manner. The second law became a law of wider applicability when Clausius introduced the property called entropy.
Entropy Clausius inequality can be used to analyze the cyclic process in a quantitative manner. The second law became a law of wider applicability when Clausius introduced the property called entropy.
6-5. H 2 O 200 kpa 200 C Q. Entropy Changes of Pure Substances
 Canges f ure Substances 6-0C Yes, because an ternally reversible, adiabatic prcess vlves n irreversibilities r eat transfer. 6- e radiatr f a steam eatg system is itially filled wit supereated steam. e
Canges f ure Substances 6-0C Yes, because an ternally reversible, adiabatic prcess vlves n irreversibilities r eat transfer. 6- e radiatr f a steam eatg system is itially filled wit supereated steam. e
Chapter 5. Mass and Energy Analysis of Control Volumes
 Chapter 5 Mass and Energy Analysis of Control Volumes Conservation Principles for Control volumes The conservation of mass and the conservation of energy principles for open systems (or control volumes)
Chapter 5 Mass and Energy Analysis of Control Volumes Conservation Principles for Control volumes The conservation of mass and the conservation of energy principles for open systems (or control volumes)
10. Heat devices: heat engines and refrigerators (Hiroshi Matsuoka)
 10 Heat devices: heat engines and refrigerators (Hiroshi Matsuoka) 1 In this chapter we will discuss how heat devices work Heat devices convert heat into work or work into heat and include heat engines
10 Heat devices: heat engines and refrigerators (Hiroshi Matsuoka) 1 In this chapter we will discuss how heat devices work Heat devices convert heat into work or work into heat and include heat engines
c Dr. Md. Zahurul Haq (BUET) Thermodynamic Processes & Efficiency ME 6101 (2017) 2 / 25 T145 = Q + W cv + i h 2 = h (V2 1 V 2 2)
 Thermodynamic Processes & Isentropic Efficiency Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & Technology (BUET Dhaka-1000, Bangladesh zahurul@me.buet.ac.bd
Thermodynamic Processes & Isentropic Efficiency Dr. Md. Zahurul Haq Professor Department of Mechanical Engineering Bangladesh University of Engineering & Technology (BUET Dhaka-1000, Bangladesh zahurul@me.buet.ac.bd
Teaching schedule *15 18
 Teaching schedule Session *15 18 19 21 22 24 Topics 5. Gas power cycles Basic considerations in the analysis of power cycle; Carnot cycle; Air standard cycle; Reciprocating engines; Otto cycle; Diesel
Teaching schedule Session *15 18 19 21 22 24 Topics 5. Gas power cycles Basic considerations in the analysis of power cycle; Carnot cycle; Air standard cycle; Reciprocating engines; Otto cycle; Diesel
Chapter 5. Mass and Energy Analysis of Control Volumes. by Asst. Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn
 Chapter 5 Mass and Energy Analysis of Control Volumes by Asst. Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics:
Chapter 5 Mass and Energy Analysis of Control Volumes by Asst. Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics:
ME 200 Thermodynamics 1 Fall 2017 Exam 3
 ME 200 hermodynamics 1 Fall 2017 Exam Circle your structor s last name Division 1: Naik Division : Wassgren Division 6: Braun Division 2: Sojka Division 4: Goldenste Division 7: Buckius Division 8: Meyer
ME 200 hermodynamics 1 Fall 2017 Exam Circle your structor s last name Division 1: Naik Division : Wassgren Division 6: Braun Division 2: Sojka Division 4: Goldenste Division 7: Buckius Division 8: Meyer
Compressor 1. Evaporator. Condenser. Expansion valve. CHE 323, October 8, Chemical Engineering Thermodynamics. Tutorial problem 5.
 CHE 33, October 8, 014. Cemical Engineering Termodynamics. Tutorial problem 5. In a simple compression refrigeration process, an adiabatic reversible compressor is used to compress propane, used as a refrigerant.
CHE 33, October 8, 014. Cemical Engineering Termodynamics. Tutorial problem 5. In a simple compression refrigeration process, an adiabatic reversible compressor is used to compress propane, used as a refrigerant.
 Two mark questions and answers UNIT II SECOND LAW 1. Define Clausius statement. It is impossible for a self-acting machine working in a cyclic process, to transfer heat from a body at lower temperature
Two mark questions and answers UNIT II SECOND LAW 1. Define Clausius statement. It is impossible for a self-acting machine working in a cyclic process, to transfer heat from a body at lower temperature
Thermodynamics [ENGR 251] [Lyes KADEM 2007]
![Thermodynamics [ENGR 251] [Lyes KADEM 2007] Thermodynamics [ENGR 251] [Lyes KADEM 2007]](/thumbs/79/79128760.jpg) CHAPTER V The first law of thermodynamics is a representation of the conservation of energy. It is a necessary, but not a sufficient, condition for a process to occur. Indeed, no restriction is imposed
CHAPTER V The first law of thermodynamics is a representation of the conservation of energy. It is a necessary, but not a sufficient, condition for a process to occur. Indeed, no restriction is imposed
ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER. 20 June 2005
 ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER 20 June 2005 Midterm Examination R. Culham & M. Bahrami This is a 90 minute, closed-book examination. You are permitted to use one 8.5 in. 11 in. crib
ECE309 INTRODUCTION TO THERMODYNAMICS & HEAT TRANSFER 20 June 2005 Midterm Examination R. Culham & M. Bahrami This is a 90 minute, closed-book examination. You are permitted to use one 8.5 in. 11 in. crib
Chapter 4. Entropy and the Second Law. Soong Ho Um Sungkyunkwan University Chemical Engineering
 Chapter 4. Entropy and the Second Law Soong Ho Um Sungkyunkwan University Chemical Engineering 1 st Law to 2 nd Law The first law, discussed in the previous chapter, expresses a fundamental principle,
Chapter 4. Entropy and the Second Law Soong Ho Um Sungkyunkwan University Chemical Engineering 1 st Law to 2 nd Law The first law, discussed in the previous chapter, expresses a fundamental principle,
Second Law of Thermodynamics -
 Second Law of Thermodynamics - REVIEW ENTROPY EXAMPLE Dr. Garrick 1/19/09 First Law of Thermodynamics you can t win! First Law of Thermodynamics: Energy cannot be Created or Destroyed the total energy
Second Law of Thermodynamics - REVIEW ENTROPY EXAMPLE Dr. Garrick 1/19/09 First Law of Thermodynamics you can t win! First Law of Thermodynamics: Energy cannot be Created or Destroyed the total energy
Previous lecture. Today lecture
 Previous lecture ds relations (derive from steady energy balance) Gibb s equations Entropy change in liquid and solid Equations of & v, & P, and P & for steady isentropic process of ideal gas Isentropic
Previous lecture ds relations (derive from steady energy balance) Gibb s equations Entropy change in liquid and solid Equations of & v, & P, and P & for steady isentropic process of ideal gas Isentropic
Name: I have observed the honor code and have neither given nor received aid on this exam.
 ME 235 FINAL EXAM, ecember 16, 2011 K. Kurabayashi and. Siegel, ME ept. Exam Rules: Open Book and one page of notes allowed. There are 4 problems. Solve each problem on a separate page. Name: I have observed
ME 235 FINAL EXAM, ecember 16, 2011 K. Kurabayashi and. Siegel, ME ept. Exam Rules: Open Book and one page of notes allowed. There are 4 problems. Solve each problem on a separate page. Name: I have observed
III. Evaluating Properties. III. Evaluating Properties
 F. Property Tables 1. What s in the tables and why specific volumes, v (m /kg) (as v, v i, v f, v g ) pressure, P (kpa) temperature, T (C) internal energy, u (kj/kg) (as u, u i, u f, u g, u ig, u fg )
F. Property Tables 1. What s in the tables and why specific volumes, v (m /kg) (as v, v i, v f, v g ) pressure, P (kpa) temperature, T (C) internal energy, u (kj/kg) (as u, u i, u f, u g, u ig, u fg )
v v = = Mixing chamber: = 30 or, = s6 Then, and = 52.4% Turbine Boiler process heater Condenser 7 MPa Q in 0.6 MPa Q proces 10 kpa Q out
 0-0- A cogeneration plant i to generate poer and proce eat. art o te team extracted rom te turbe at a relatively ig preure i ued or proce eatg. e poer produced and te utilization actor o te plant are to
0-0- A cogeneration plant i to generate poer and proce eat. art o te team extracted rom te turbe at a relatively ig preure i ued or proce eatg. e poer produced and te utilization actor o te plant are to
FINAL EXAM. ME 200 Thermodynamics I, Spring 2013 CIRCLE YOUR LECTURE BELOW:
 ME 200 Thermodynamics I, Spring 2013 CIRCLE YOUR LECTURE BELOW: Div. 5 7:30 am Div. 2 10:30 am Div. 4 12:30 am Prof. Naik Prof. Braun Prof. Bae Div. 3 2:30 pm Div. 1 4:30 pm Div. 6 4:30 pm Prof. Chen Prof.
ME 200 Thermodynamics I, Spring 2013 CIRCLE YOUR LECTURE BELOW: Div. 5 7:30 am Div. 2 10:30 am Div. 4 12:30 am Prof. Naik Prof. Braun Prof. Bae Div. 3 2:30 pm Div. 1 4:30 pm Div. 6 4:30 pm Prof. Chen Prof.
UBMCC11 - THERMODYNAMICS. B.E (Marine Engineering) B 16 BASIC CONCEPTS AND FIRST LAW PART- A
 UBMCC11 - THERMODYNAMICS B.E (Marine Engineering) B 16 UNIT I BASIC CONCEPTS AND FIRST LAW PART- A 1. What do you understand by pure substance? 2. Define thermodynamic system. 3. Name the different types
UBMCC11 - THERMODYNAMICS B.E (Marine Engineering) B 16 UNIT I BASIC CONCEPTS AND FIRST LAW PART- A 1. What do you understand by pure substance? 2. Define thermodynamic system. 3. Name the different types
Chapter 19. Heat Engines
 Chapter 19 Heat Engines QuickCheck 19.11 The efficiency of this Carnot heat engine is A. Less than 0.5. B. 0.5. C. Between 0.5 and 1.0. D. 2.0. E. Can t say without knowing Q H. 2013 Pearson Education,
Chapter 19 Heat Engines QuickCheck 19.11 The efficiency of this Carnot heat engine is A. Less than 0.5. B. 0.5. C. Between 0.5 and 1.0. D. 2.0. E. Can t say without knowing Q H. 2013 Pearson Education,
Lecture 9. Heat engines. Pre-reading: 20.2
 Lecture 9 Heat engines Pre-reading: 20.2 Review Second law when all systems taking part in a process are included, the entropy remains constant or increases. No process is possible in which the total entropy
Lecture 9 Heat engines Pre-reading: 20.2 Review Second law when all systems taking part in a process are included, the entropy remains constant or increases. No process is possible in which the total entropy
ME Thermodynamics I
 HW-6 (5 points) Given: Carbon dioxide goes through an adiabatic process in a piston-cylinder assembly. provided. Find: Calculate the entropy change for each case: State data is a) Constant specific heats
HW-6 (5 points) Given: Carbon dioxide goes through an adiabatic process in a piston-cylinder assembly. provided. Find: Calculate the entropy change for each case: State data is a) Constant specific heats
First Law of Thermodynamics
 First Law of Thermodynamics During an interaction between a system and its surroundings, the amount of energy gained by the system must be exactly equal to the amount of energy lost by the surroundings.
First Law of Thermodynamics During an interaction between a system and its surroundings, the amount of energy gained by the system must be exactly equal to the amount of energy lost by the surroundings.
NEWCOMEN ATMOSPHERIC ENGINE
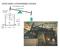 NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m F 7 stroke 5 sia steam 8 50 F water NECMEN AMSPERIC ENGINE atmoseric ressure Ford Museum, 760 strokes/m 5 sia steam 8 F 50 F water 7
FUNDAMENTALS OF THERMODYNAMICS
 FUNDAMENTALS OF THERMODYNAMICS SEVENTH EDITION CLAUS BORGNAKKE RICHARD E. SONNTAG University of Michigan John Wiley & Sons, Inc. PUBLISHER ASSOCIATE PUBLISHER ACQUISITIONS EDITOR SENIOR PRODUCTION EDITOR
FUNDAMENTALS OF THERMODYNAMICS SEVENTH EDITION CLAUS BORGNAKKE RICHARD E. SONNTAG University of Michigan John Wiley & Sons, Inc. PUBLISHER ASSOCIATE PUBLISHER ACQUISITIONS EDITOR SENIOR PRODUCTION EDITOR
first law of ThermodyNamics
 first law of ThermodyNamics First law of thermodynamics - Principle of conservation of energy - Energy can be neither created nor destroyed Basic statement When any closed system is taken through a cycle,
first law of ThermodyNamics First law of thermodynamics - Principle of conservation of energy - Energy can be neither created nor destroyed Basic statement When any closed system is taken through a cycle,
+ m B1 = 1. u A1. u B1. - m B1 = V A. /v A = , u B1 + V B. = 5.5 kg => = V tot. Table B.1.
 5.6 A rigid tank is divided into two rooms by a membrane, both containing water, shown in Fig. P5.6. Room A is at 200 kpa, v = 0.5 m3/kg, VA = m3, and room B contains 3.5 kg at 0.5 MPa, 400 C. The membrane
5.6 A rigid tank is divided into two rooms by a membrane, both containing water, shown in Fig. P5.6. Room A is at 200 kpa, v = 0.5 m3/kg, VA = m3, and room B contains 3.5 kg at 0.5 MPa, 400 C. The membrane
ME 354 THERMODYNAMICS 2 MIDTERM EXAMINATION. Instructor: R. Culham. Name: Student ID Number: Instructions
 ME 354 THERMODYNAMICS 2 MIDTERM EXAMINATION February 14, 2011 5:30 pm - 7:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Answer all questions
ME 354 THERMODYNAMICS 2 MIDTERM EXAMINATION February 14, 2011 5:30 pm - 7:30 pm Instructor: R. Culham Name: Student ID Number: Instructions 1. This is a 2 hour, closed-book examination. 2. Answer all questions
