Capillarity. ESS5855 Lecture Fall 2010
|
|
|
- Frank Robertson
- 5 years ago
- Views:
Transcription
1 Capillarity ESS5855 Lecture Fall 2010 Capillarity: the tendency of a liquid in a narrow tube or pore to rise or fall as a result of surface tension (The concise Oxford Dictionary) Surface tension: the tension of the surface film of a liquid, which tends to minimize surface area (The concise Oxford Dictionary) Surface tension (γ) results from inter-molecular forces: a molecule in the interior of a liquid experiences interactions with other molecules equally from all sides, while a molecule at the surface is only affected by molecules below it in the liquid
2
3 Surface Tension As a result, the interfacial molecules are pulled into the bulk (to the extent allowed by their finite size and repulsive interactions) and the net density of molecules in the surface region is decreased There is more space between surface molecules and the springs acting between them are therefore stretched beyond their equilibrium length, creating a tension pulling along the surface working to keep the molecules together The force of the springs pulling along the surface, then, is the surface tension or surface free energy Interfaces In order for two phases to exist in contact, there must be a region through which the intensive properties of the system change from those of one phase to those of the other In order for such a boundary to be stable, it must possess an interfacial free energy such that work must be done to extend or enlarge the boundary or interface If not and if no other external forces act to separate the phases, then no energy will be required to increase the interfacial area and random forces will distort, fold, and convolute the interface until the phases become mixed
4 Surface Free Energy Free energy: the total amount of energy in a physical system that can be converted to do work Quantity derived from the relationships studied in thermodynamics and used as a measure of the relative stability of a physical system, i.e., the tendency of the system to change Atoms and molecules at interfaces, because of their special environment, often possess energies significantly different from those of the same species in a bulk situation Interfaces Nature will always act so as to attain a situation of minimum total free energy In the case of a two-phase system, if the presence of the interface results in a higher (positive) free energy, the interface will spontaneously be reduced to a minimum - the two phases will tend to separate to the greatest extent possible within the imposed constraints
5 Capillarity The macroscopic motion of a fluid system under the influence of its own surface and interfacial forces Such flow is similar to other types of hydraulic flow in that it results from the presence of a pressure differential between two hydraulically connected regions of the liquid mass The direction of flow is such as to decrease the pressure difference When the difference vanishes, or when there is no longer a mechanism to reduce the difference, flow ceases
6 Capillarity The topic of capillarity concerns interfaces that are sufficiently mobile to assume an equilibrium shape The common examples are meniscuses, thin films, and drops formed by liquids in air or in another liquid Capillarity deals with macroscopic and statistical behavior of interfaces rather than the details of their molecular structure Surface Tension/Free Energy Although referred to as a force per unit length, surface tension may equally well be thought of as a free energy per unit area l F dx A soap film stretched across a wire frame with one movable side
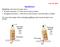
7 Capillary rise
8 R θ r
9 P b P b Important functions of capillary forces in practical situations. (a) As two emulsion drops approach, the pressure at the nearest surfaces increases, deforming the drops and enlarging the radius of curvature in the immediate area. That deformation causes the capillary pressure in the regions outside that area to decrease in a relative sense, suctioning continuous phase from between the drops and increasing the likelihood of contact and film rupture or coalescence. (b) In capillary displacement, the liquid that preferentially wets the solid will displace the less wetting liquid.
10 Contact angle: 1. surface tension and contact angle are two different but closely related things 2. surface tension is a property of the interface between two phases, whereas the contact angle describes the edge of the two-phase boundary where it ends at a third phase 3. two phases must be specified to describe surface tension; three are needed to describe contact angle γ γ γ
11 Wenzel s Model Metastable states
12 Cassie-Baxter Model
13 = c c c c
14
15 A B ΔG = ( σ A σ B + σ AB ) da A (σ=γ: surface tension)
16 ΔG = ( σ σ B A - S B/A + σ AB ) da B ( = σa + σb σab 2σBB)
17 P3
18 Bashforth-Adams Equation
19 ( ) ( )
20 Drop in Motion
21 dp 3Vη = 2 dx ς
22
Surface and Interfacial Tensions. Lecture 1
 Surface and Interfacial Tensions Lecture 1 Surface tension is a pull Surfaces and Interfaces 1 Thermodynamics for Interfacial Systems Work must be done to increase surface area just as work must be done
Surface and Interfacial Tensions Lecture 1 Surface tension is a pull Surfaces and Interfaces 1 Thermodynamics for Interfacial Systems Work must be done to increase surface area just as work must be done
emulsions, and foams March 21 22, 2009
 Wetting and adhesion Dispersions in liquids: suspensions, emulsions, and foams ACS National Meeting March 21 22, 2009 Salt Lake City Ian Morrison 2009 Ian Morrison 2009 Lecure 2 - Wetting and adhesion
Wetting and adhesion Dispersions in liquids: suspensions, emulsions, and foams ACS National Meeting March 21 22, 2009 Salt Lake City Ian Morrison 2009 Ian Morrison 2009 Lecure 2 - Wetting and adhesion
DLVO interaction between the spheres
 DLVO interaction between the spheres DL-interaction energy for two spheres: D w ( x) 64c π ktrϕ e λ DL 2 x λ 2 0 0 D DLVO interaction w ( x) 64πkTRϕ e λ DLVO AR /12x 2 x λd 2 0 D Lecture 11 Contact angle
DLVO interaction between the spheres DL-interaction energy for two spheres: D w ( x) 64c π ktrϕ e λ DL 2 x λ 2 0 0 D DLVO interaction w ( x) 64πkTRϕ e λ DLVO AR /12x 2 x λd 2 0 D Lecture 11 Contact angle
The Origins of Surface and Interfacial Tension
 The Origins of Surface and Interfacial Tension Imbalance of intermolecular forces exists at the liquid-air interface γ la= the surface tension that exists at the liquid-air interface Suppose we have a
The Origins of Surface and Interfacial Tension Imbalance of intermolecular forces exists at the liquid-air interface γ la= the surface tension that exists at the liquid-air interface Suppose we have a
Lecture 7 Contact angle phenomena and wetting
 Lecture 7 Contact angle phenomena and Contact angle phenomena and wetting Young s equation Drop on the surface complete spreading Establishing finite contact angle γ cosθ = γ γ L S SL γ S γ > 0 partial
Lecture 7 Contact angle phenomena and Contact angle phenomena and wetting Young s equation Drop on the surface complete spreading Establishing finite contact angle γ cosθ = γ γ L S SL γ S γ > 0 partial
Capillarity and Wetting Phenomena
 ? Pierre-Gilles de Gennes Frangoise Brochard-Wyart David Quere Capillarity and Wetting Phenomena Drops, Bubbles, Pearls, Waves Translated by Axel Reisinger With 177 Figures Springer Springer New York Berlin
? Pierre-Gilles de Gennes Frangoise Brochard-Wyart David Quere Capillarity and Wetting Phenomena Drops, Bubbles, Pearls, Waves Translated by Axel Reisinger With 177 Figures Springer Springer New York Berlin
Chapter -6(Section-1) Surface Tension
 Chapter -6(Section-1) Surface Tension Free surface of the liquid tends to minimize the surface area. e.g.(1)if the small quantity of mercury is allowed to fall on the floor, it converted in to small spherical
Chapter -6(Section-1) Surface Tension Free surface of the liquid tends to minimize the surface area. e.g.(1)if the small quantity of mercury is allowed to fall on the floor, it converted in to small spherical
Chemical Potential. Combining the First and Second Laws for a closed system, Considering (extensive properties)
 Chemical Potential Combining the First and Second Laws for a closed system, Considering (extensive properties) du = TdS pdv Hence For an open system, that is, one that can gain or lose mass, U will also
Chemical Potential Combining the First and Second Laws for a closed system, Considering (extensive properties) du = TdS pdv Hence For an open system, that is, one that can gain or lose mass, U will also
Colloidal Particles at Liquid Interfaces: An Introduction
 1 Colloidal Particles at Liquid Interfaces: An Introduction Bernard P. Binks and Tommy S. Horozov Surfactant and Colloid Group, Department of Chemistry, University of Hull, Hull, HU6 7RX, UK 1.1 Some Basic
1 Colloidal Particles at Liquid Interfaces: An Introduction Bernard P. Binks and Tommy S. Horozov Surfactant and Colloid Group, Department of Chemistry, University of Hull, Hull, HU6 7RX, UK 1.1 Some Basic
3.091 Introduction to Solid State Chemistry. Lecture Notes No. 6a BONDING AND SURFACES
 3.091 Introduction to Solid State Chemistry Lecture Notes No. 6a BONDING AND SURFACES 1. INTRODUCTION Surfaces have increasing importance in technology today. Surfaces become more important as the size
3.091 Introduction to Solid State Chemistry Lecture Notes No. 6a BONDING AND SURFACES 1. INTRODUCTION Surfaces have increasing importance in technology today. Surfaces become more important as the size
MECHANICAL PROPERTIES OF FLUIDS
 CHAPTER-10 MECHANICAL PROPERTIES OF FLUIDS QUESTIONS 1 marks questions 1. What are fluids? 2. How are fluids different from solids? 3. Define thrust of a liquid. 4. Define liquid pressure. 5. Is pressure
CHAPTER-10 MECHANICAL PROPERTIES OF FLUIDS QUESTIONS 1 marks questions 1. What are fluids? 2. How are fluids different from solids? 3. Define thrust of a liquid. 4. Define liquid pressure. 5. Is pressure
Surface chemistry. Liquid-gas, solid-gas and solid-liquid surfaces.
 Surface chemistry. Liquid-gas, solid-gas and solid-liquid surfaces. Levente Novák & István Bányai, University of Debrecen Dept of Colloid and Environmental Chemistry http://kolloid.unideb.hu/~kolloid/
Surface chemistry. Liquid-gas, solid-gas and solid-liquid surfaces. Levente Novák & István Bányai, University of Debrecen Dept of Colloid and Environmental Chemistry http://kolloid.unideb.hu/~kolloid/
Jacco Snoeijer PHYSICS OF FLUIDS
 Jacco Snoeijer PHYSICS OF FLUIDS dynamics dynamics freezing dynamics freezing microscopics of capillarity Menu 1. surface tension: thermodynamics & microscopics 2. wetting (statics): thermodynamics & microscopics
Jacco Snoeijer PHYSICS OF FLUIDS dynamics dynamics freezing dynamics freezing microscopics of capillarity Menu 1. surface tension: thermodynamics & microscopics 2. wetting (statics): thermodynamics & microscopics
Chapter II: Reversible process and work
 Chapter II: Reversible process and work 1- Process Defined by change in a system, a thermodynamic process is a passage of a thermodynamic system from an initial to a final state of thermodynamic equilibrium.
Chapter II: Reversible process and work 1- Process Defined by change in a system, a thermodynamic process is a passage of a thermodynamic system from an initial to a final state of thermodynamic equilibrium.
4/14/11. Chapter 12 Static equilibrium and Elasticity Lecture 2. Condition for static equilibrium. Stability An object is in equilibrium:
 About Midterm Exam 3 When and where Thurs April 21 th, 5:45-7:00 pm Rooms: Same as Exam I and II, See course webpage. Your TA will give a brief review during the discussion session. Coverage: Chapts 9
About Midterm Exam 3 When and where Thurs April 21 th, 5:45-7:00 pm Rooms: Same as Exam I and II, See course webpage. Your TA will give a brief review during the discussion session. Coverage: Chapts 9
Boundary Conditions in Fluid Mechanics
 Boundary Conditions in Fluid Mechanics R. Shankar Subramanian Department of Chemical and Biomolecular Engineering Clarkson University The governing equations for the velocity and pressure fields are partial
Boundary Conditions in Fluid Mechanics R. Shankar Subramanian Department of Chemical and Biomolecular Engineering Clarkson University The governing equations for the velocity and pressure fields are partial
CHAPTER 1 Fluids and their Properties
 FLUID MECHANICS Gaza CHAPTER 1 Fluids and their Properties Dr. Khalil Mahmoud ALASTAL Objectives of this Chapter: Define the nature of a fluid. Show where fluid mechanics concepts are common with those
FLUID MECHANICS Gaza CHAPTER 1 Fluids and their Properties Dr. Khalil Mahmoud ALASTAL Objectives of this Chapter: Define the nature of a fluid. Show where fluid mechanics concepts are common with those
AMME2261: Fluid Mechanics 1 Course Notes
 Module 1 Introduction and Fluid Properties Introduction Matter can be one of two states: solid or fluid. A fluid is a substance that deforms continuously under the application of a shear stress, no matter
Module 1 Introduction and Fluid Properties Introduction Matter can be one of two states: solid or fluid. A fluid is a substance that deforms continuously under the application of a shear stress, no matter
Course Level Author Mentor
 Interfacial energy Course: Phase transformations Level: UG Author: Satish S Kamath, Department of Chemical Engineering, NITK-Surathkal Mentor: M P Gururajan Interfacial energy In materials, the formation
Interfacial energy Course: Phase transformations Level: UG Author: Satish S Kamath, Department of Chemical Engineering, NITK-Surathkal Mentor: M P Gururajan Interfacial energy In materials, the formation
We may have a general idea that a solid is hard and a fluid is soft. This is not satisfactory from
 Chapter 1. Introduction 1.1 Some Characteristics of Fluids We may have a general idea that a solid is hard and a fluid is soft. This is not satisfactory from scientific or engineering point of view. In
Chapter 1. Introduction 1.1 Some Characteristics of Fluids We may have a general idea that a solid is hard and a fluid is soft. This is not satisfactory from scientific or engineering point of view. In
Chapter 8 Surface phenomena and dispersion system 8.1 Surface tension
 Chapter 8 Surface phenomena and dispersion system Levine p. 384-387 13.1 he interphase region 1.1 Some important concepts and phenomena (1) Surface and Interface Surface: he boundary between non-gaseous
Chapter 8 Surface phenomena and dispersion system Levine p. 384-387 13.1 he interphase region 1.1 Some important concepts and phenomena (1) Surface and Interface Surface: he boundary between non-gaseous
Phase Transitions. Ehrenfest Classification of Phase Transitions. Curved Surfaces Bubbles, cavities and droplets Nucleation
 Phase Transitions Chapter 6 of Atkins Sections 6.7-6.10 Ehrenfest Classification of Phase Transitions Physical Liquid Surface Surface Tension Curved Surfaces Bubbles, cavities and droplets Nucleation Capillary
Phase Transitions Chapter 6 of Atkins Sections 6.7-6.10 Ehrenfest Classification of Phase Transitions Physical Liquid Surface Surface Tension Curved Surfaces Bubbles, cavities and droplets Nucleation Capillary
Fluid Mechanics-61341
 An-Najah National University College of Engineering Fluid Mechanics-61341 Chapter [1] Fundamentals 1 The Book (Elementary Fluid Mechanics by Street, Watters and Vennard) Each chapter includes: Concepts
An-Najah National University College of Engineering Fluid Mechanics-61341 Chapter [1] Fundamentals 1 The Book (Elementary Fluid Mechanics by Street, Watters and Vennard) Each chapter includes: Concepts
Multiphase Flow and Heat Transfer
 Multiphase Flow and Heat Transfer ME546 -Sudheer Siddapureddy sudheer@iitp.ac.in Surface Tension The free surface between air and water at a molecular scale Molecules sitting at a free liquid surface against
Multiphase Flow and Heat Transfer ME546 -Sudheer Siddapureddy sudheer@iitp.ac.in Surface Tension The free surface between air and water at a molecular scale Molecules sitting at a free liquid surface against
Praktikum zur. Materialanalytik
 Praktikum zur Materialanalytik Functionalized Surfaces B510 Stand: 20.10.2017 Table of contents Introduction 2 Basics 2 Surface tension 2 From wettability to the contact angle 4 The Young equation 5 Wetting
Praktikum zur Materialanalytik Functionalized Surfaces B510 Stand: 20.10.2017 Table of contents Introduction 2 Basics 2 Surface tension 2 From wettability to the contact angle 4 The Young equation 5 Wetting
Fluid Mechanics Introduction
 Fluid Mechanics Introduction Fluid mechanics study the fluid under all conditions of rest and motion. Its approach is analytical, mathematical, and empirical (experimental and observation). Fluid can be
Fluid Mechanics Introduction Fluid mechanics study the fluid under all conditions of rest and motion. Its approach is analytical, mathematical, and empirical (experimental and observation). Fluid can be
CHAPTER 3: SURFACE AND INTERFACIAL TENSION. To measure surface tension using both, the DuNouy ring and the capillary tube methods.
 CHAPTER 3: SURFACE AND INTERFACIAL TENSION Objective To measure surface tension using both, the DuNouy ring and the capillary tube methods. Introduction In a fluid system, the presence of two or more phases
CHAPTER 3: SURFACE AND INTERFACIAL TENSION Objective To measure surface tension using both, the DuNouy ring and the capillary tube methods. Introduction In a fluid system, the presence of two or more phases
A drop forms when liquid is forced out of a small tube. The shape of the drop is determined by a balance of pressure, gravity, and surface tension
 A drop forms when liquid is forced out of a small tube. The shape of the drop is determined by a balance of pressure, gravity, and surface tension forces. 2 Objectives 3 i i 2 1 INTRODUCTION Property:
A drop forms when liquid is forced out of a small tube. The shape of the drop is determined by a balance of pressure, gravity, and surface tension forces. 2 Objectives 3 i i 2 1 INTRODUCTION Property:
*blood and bones contain colloids. *milk is a good example of a colloidal dispersion.
 Chap. 3. Colloids 3.1. Introduction - Simple definition of a colloid: a macroscopically heterogeneous system where one component has dimensions in between molecules and macroscopic particles like sand
Chap. 3. Colloids 3.1. Introduction - Simple definition of a colloid: a macroscopically heterogeneous system where one component has dimensions in between molecules and macroscopic particles like sand
2. Determine the surface tension of water with the capillary-rise method.
 Fakultät für Physik und Geowissenschaften Physikalisches Grundpraktikum M19e Surface Tension Tasks 1. Determine the surface tension σ of an organic liquid using the anchor-ring method. Use three different
Fakultät für Physik und Geowissenschaften Physikalisches Grundpraktikum M19e Surface Tension Tasks 1. Determine the surface tension σ of an organic liquid using the anchor-ring method. Use three different
LECTURE 4: Marangoni flows
 LECTURE 4: Marangoni flows Marangoni flows are those driven by surface tension gradients. In general, surface tension σ depends on both the temperature and chemical composition at the interface; consequently,
LECTURE 4: Marangoni flows Marangoni flows are those driven by surface tension gradients. In general, surface tension σ depends on both the temperature and chemical composition at the interface; consequently,
Lecture Outline Chapter 6. Physics, 4 th Edition James S. Walker. Copyright 2010 Pearson Education, Inc.
 Lecture Outline Chapter 6 Physics, 4 th Edition James S. Walker Chapter 6 Applications of Newton s Laws Units of Chapter 6 Frictional Forces Strings and Springs Translational Equilibrium Connected Objects
Lecture Outline Chapter 6 Physics, 4 th Edition James S. Walker Chapter 6 Applications of Newton s Laws Units of Chapter 6 Frictional Forces Strings and Springs Translational Equilibrium Connected Objects
LECTURE 4 FLUID FLOW & SURFACE TENSION. Lecture Instructor: Kazumi Tolich
 LECTURE 4 FLUID FLOW & SURFACE TENSION Lecture Instructor: Kazumi Tolich Lecture 4 2 Reading chapter 15.6 to 15.9 Continuity equation Bernoulli s equation n Torricelli s law Viscosity Surface tension Equation
LECTURE 4 FLUID FLOW & SURFACE TENSION Lecture Instructor: Kazumi Tolich Lecture 4 2 Reading chapter 15.6 to 15.9 Continuity equation Bernoulli s equation n Torricelli s law Viscosity Surface tension Equation
BFC FLUID MECHANICS BFC NOOR ALIZA AHMAD
 BFC 10403 FLUID MECHANICS CHAPTER 1.0: Principles of Fluid 1.1 Introduction to Fluid Mechanics 1.2 Thermodynamic Properties of a Fluid: Density, specific weight, specific gravity, viscocity (kelikatan)berat
BFC 10403 FLUID MECHANICS CHAPTER 1.0: Principles of Fluid 1.1 Introduction to Fluid Mechanics 1.2 Thermodynamic Properties of a Fluid: Density, specific weight, specific gravity, viscocity (kelikatan)berat
Fluid flow Pressure Bernoulli Principle Surface Tension
 Lecture 9. Fluid flow Pressure Bernoulli Principle Surface Tension A v L A is the area Fluid flow Speed of a fluid in a pipe is not the same as the flow rate Relating: Fluid flow rate to Average speed
Lecture 9. Fluid flow Pressure Bernoulli Principle Surface Tension A v L A is the area Fluid flow Speed of a fluid in a pipe is not the same as the flow rate Relating: Fluid flow rate to Average speed
+ S/y. The wetted portion of the surface is then delimited by a certain contact line L (here a
 EUROPHYSICS LETTERS Europhys. Lett., 21 (4), pp. 483-488 (1993) 1 February 1993 Contact Line Elasticity of a Completely Wetting Liquid Rising on a Wall. E. RAPHAEL(*)( ) and J. F. JoA"Y(**) (*) Institute
EUROPHYSICS LETTERS Europhys. Lett., 21 (4), pp. 483-488 (1993) 1 February 1993 Contact Line Elasticity of a Completely Wetting Liquid Rising on a Wall. E. RAPHAEL(*)( ) and J. F. JoA"Y(**) (*) Institute
10 - FLUID MECHANICS Page 1
 0 - FLUID MECHANICS Page Introduction Fluid is a matter in a state which can flow. Liquids, gases, molten metal and tar are examples of fluids. Fluid mechanics is studied in two parts: ( i ) Fluid statics
0 - FLUID MECHANICS Page Introduction Fluid is a matter in a state which can flow. Liquids, gases, molten metal and tar are examples of fluids. Fluid mechanics is studied in two parts: ( i ) Fluid statics
Wetting Transitions at Fluid Interfaces and Related Topics
 Wetting Transitions at Fluid Interfaces and Related Topics Kenichiro Koga Department of Chemistry, Faculty of Science, Okayama University Tsushima-Naka 3-1-1, Okayama 7-853, Japan Received April 3, 21
Wetting Transitions at Fluid Interfaces and Related Topics Kenichiro Koga Department of Chemistry, Faculty of Science, Okayama University Tsushima-Naka 3-1-1, Okayama 7-853, Japan Received April 3, 21
8.2 Surface phenomenon of liquid. Out-class reading: Levine p Curved interfaces
 Out-class reading: Levine p. 387-390 13.2 Curved interfaces https://news.cnblogs.com/n/559867/ 8.2.1 Some interesting phenomena 8.2.1 Some interesting phenomena Provided by Prof. Yu-Peng GUO of Jilin
Out-class reading: Levine p. 387-390 13.2 Curved interfaces https://news.cnblogs.com/n/559867/ 8.2.1 Some interesting phenomena 8.2.1 Some interesting phenomena Provided by Prof. Yu-Peng GUO of Jilin
INTERFACIAL PHENOMENA GRADING SCHEME
 18.357 INTERFACIAL PHENOMENA Professor John W. M. Bush Fall 2010 Office 2-346 MW 2-3:30 Phone: 253-4387 (office) Room 2-135 email: bush@math.mit.edu Office hours: after class, available upon request GRADING
18.357 INTERFACIAL PHENOMENA Professor John W. M. Bush Fall 2010 Office 2-346 MW 2-3:30 Phone: 253-4387 (office) Room 2-135 email: bush@math.mit.edu Office hours: after class, available upon request GRADING
Liquids and solids are essentially incompressible substances and the variation of their density with pressure is usually negligible.
 Properties of Fluids Intensive properties are those that are independent of the mass of a system i.e. temperature, pressure and density. Extensive properties are those whose values depend on the size of
Properties of Fluids Intensive properties are those that are independent of the mass of a system i.e. temperature, pressure and density. Extensive properties are those whose values depend on the size of
Module 3: "Thin Film Hydrodynamics" Lecture 11: "" The Lecture Contains: Micro and Nano Scale Hydrodynamics with and without Free Surfaces
 The Lecture Contains: Micro and Nano Scale Hydrodynamics with and without Free Surfaces Order of Magnitude Analysis file:///e /courses/colloid_interface_science/lecture11/11_1.htm[6/16/2012 1:39:56 PM]
The Lecture Contains: Micro and Nano Scale Hydrodynamics with and without Free Surfaces Order of Magnitude Analysis file:///e /courses/colloid_interface_science/lecture11/11_1.htm[6/16/2012 1:39:56 PM]
Most substances can be in three states: solid, liquid, and gas.
 States of Matter Most substances can be in three states: solid, liquid, and gas. Solid Particles Have Fixed Positions The particles in a solid are very close together and have an orderly, fixed arrangement.
States of Matter Most substances can be in three states: solid, liquid, and gas. Solid Particles Have Fixed Positions The particles in a solid are very close together and have an orderly, fixed arrangement.
Hemodynamics II. Aslı AYKAÇ, PhD. NEU Faculty of Medicine Department of Biophysics
 Hemodynamics II Aslı AYKAÇ, PhD. NEU Faculty of Medicine Department of Biophysics Laplace s Law Relates the pressure difference across a closed elastic membrane on liquid film to the tension in the membrane
Hemodynamics II Aslı AYKAÇ, PhD. NEU Faculty of Medicine Department of Biophysics Laplace s Law Relates the pressure difference across a closed elastic membrane on liquid film to the tension in the membrane
Fluid flow Pressure Bernoulli Principle Surface Tension
 Lecture 9. Fluid flow Pressure Bernoulli Principle Surface Tension Fluid flow Speed of a fluid in a pipe is not the same as the flow rate Depends on the radius of the pipe. example: Low speed Large flow
Lecture 9. Fluid flow Pressure Bernoulli Principle Surface Tension Fluid flow Speed of a fluid in a pipe is not the same as the flow rate Depends on the radius of the pipe. example: Low speed Large flow
Chemistry of Life: Water and Solutions
 Chemistry of Life: Water and Solutions Unit Objective I can describe the role of organic and inorganic chemicals important to living things. During this unit, we will answer the following very important
Chemistry of Life: Water and Solutions Unit Objective I can describe the role of organic and inorganic chemicals important to living things. During this unit, we will answer the following very important
THE PHYSICS OF FOAM. Boulder School for Condensed Matter and Materials Physics. July 1-26, 2002: Physics of Soft Condensed Matter. 1.
 THE PHYSICS OF FOAM Boulder School for Condensed Matter and Materials Physics July 1-26, 2002: Physics of Soft Condensed Matter 1. Introduction Formation Microscopics 2. Structure Experiment Simulation
THE PHYSICS OF FOAM Boulder School for Condensed Matter and Materials Physics July 1-26, 2002: Physics of Soft Condensed Matter 1. Introduction Formation Microscopics 2. Structure Experiment Simulation
6.Surface Tension (Marks: 04/05)
 Q. Q.1 Define the following terms. 1) Intermolecular force of attraction. 2) Cohesive force 3) Adhesive force 4) Molecular range. 5) Sphere of influence. Ans:- 1)Intermolecular force of attraction:- The
Q. Q.1 Define the following terms. 1) Intermolecular force of attraction. 2) Cohesive force 3) Adhesive force 4) Molecular range. 5) Sphere of influence. Ans:- 1)Intermolecular force of attraction:- The
LESSON No. 9 WORK TRANSFER: In thermodynamics the work can be defined as follows:
 LESSON No. 9 WORK TRANSFER: In thermodynamics the work can be defined as follows: Work shall be done by the system if the total effect outside the system is equivalent to the raising of weight and this
LESSON No. 9 WORK TRANSFER: In thermodynamics the work can be defined as follows: Work shall be done by the system if the total effect outside the system is equivalent to the raising of weight and this
Unsaturated Flow (brief lecture)
 Physical Hydrogeology Unsaturated Flow (brief lecture) Why study the unsaturated zone? Evapotranspiration Infiltration Toxic Waste Leak Irrigation UNSATURATAED ZONE Aquifer Important to: Agriculture (most
Physical Hydrogeology Unsaturated Flow (brief lecture) Why study the unsaturated zone? Evapotranspiration Infiltration Toxic Waste Leak Irrigation UNSATURATAED ZONE Aquifer Important to: Agriculture (most
9 MECHANICAL PROPERTIES OF SOLIDS
 9 MECHANICAL PROPERTIES OF SOLIDS Deforming force Deforming force is the force which changes the shape or size of a body. Restoring force Restoring force is the internal force developed inside the body
9 MECHANICAL PROPERTIES OF SOLIDS Deforming force Deforming force is the force which changes the shape or size of a body. Restoring force Restoring force is the internal force developed inside the body
Chapter 10. Solids and Fluids
 Chapter 10 Solids and Fluids Surface Tension Net force on molecule A is zero Pulled equally in all directions Net force on B is not zero No molecules above to act on it Pulled toward the center of the
Chapter 10 Solids and Fluids Surface Tension Net force on molecule A is zero Pulled equally in all directions Net force on B is not zero No molecules above to act on it Pulled toward the center of the
Chapter 9: Solids and Fluids
 Chapter 9: Solids and Fluids State of matters: Solid, Liquid, Gas and Plasma. Solids Has definite volume and shape Can be crystalline or amorphous Molecules are held in specific locations by electrical
Chapter 9: Solids and Fluids State of matters: Solid, Liquid, Gas and Plasma. Solids Has definite volume and shape Can be crystalline or amorphous Molecules are held in specific locations by electrical
Peter Buhler. NanothermodynamicS
 Peter Buhler NanothermodynamicS Introduction 7 Symbols and definitions 9 1. On the basic concepts of classical thermodynamics 1 5 1.1. Internal energy, heat, and work 1 7 1.1.1. Internal energy is a property
Peter Buhler NanothermodynamicS Introduction 7 Symbols and definitions 9 1. On the basic concepts of classical thermodynamics 1 5 1.1. Internal energy, heat, and work 1 7 1.1.1. Internal energy is a property
Module17: Intermolecular Force between Surfaces and Particles. Lecture 23: Intermolecular Force between Surfaces and Particles
 Module17: Intermolecular Force between Surfaces and Particles Lecture 23: Intermolecular Force between Surfaces and Particles 1 We now try to understand the nature of spontaneous instability in a confined
Module17: Intermolecular Force between Surfaces and Particles Lecture 23: Intermolecular Force between Surfaces and Particles 1 We now try to understand the nature of spontaneous instability in a confined
Frieder Mugele. Physics of Complex Fluids. University of Twente. Jacco Snoeier Physics of Fluids / UT
 coorganizers: Frieder Mugele Physics of Comple Fluids Jacco Snoeier Physics of Fluids / UT University of Twente Anton Darhuber Mesoscopic Transport Phenomena / Tu/e speakers: José Bico (ESPCI Paris) Daniel
coorganizers: Frieder Mugele Physics of Comple Fluids Jacco Snoeier Physics of Fluids / UT University of Twente Anton Darhuber Mesoscopic Transport Phenomena / Tu/e speakers: José Bico (ESPCI Paris) Daniel
Property of liquid and Phase Diagram for EN 2017
 Property of Liquid and Phase Diagram by Assist.Prof.Dr.Choosak Poonsawat choosak@kku.ac.th FB: เคม อ ช ศ กด You should know - Intermolecular forces - Properties of Liquids - Phase Diagram Properties of
Property of Liquid and Phase Diagram by Assist.Prof.Dr.Choosak Poonsawat choosak@kku.ac.th FB: เคม อ ช ศ กด You should know - Intermolecular forces - Properties of Liquids - Phase Diagram Properties of
Energy Considerations
 Physics 42200 Waves & Oscillations Lecture 4 French, Chapter 3 Spring 2016 Semester Matthew Jones Energy Considerations The force in Hooke s law is = Potential energy can be used to describe conservative
Physics 42200 Waves & Oscillations Lecture 4 French, Chapter 3 Spring 2016 Semester Matthew Jones Energy Considerations The force in Hooke s law is = Potential energy can be used to describe conservative
Shape of the Interfaces
 NPTEL Chemical Engineering Interfacial Engineering Module : Lecture 3 Shape of the Interfaces Dr. Pallab Ghosh Associate Professor Department of Chemical Engineering IIT Guwahati, Guwahati 781039 India
NPTEL Chemical Engineering Interfacial Engineering Module : Lecture 3 Shape of the Interfaces Dr. Pallab Ghosh Associate Professor Department of Chemical Engineering IIT Guwahati, Guwahati 781039 India
Interfaces and interfacial energy
 Interfaces and interfacial energy 1/14 kinds: l/g }{{ l/l } mobile s/g s/l s/s Example. Estimate the percetage of water molecules on the surface of a fog droplet of diameter (i) 0.1 mm (naked eye visibility
Interfaces and interfacial energy 1/14 kinds: l/g }{{ l/l } mobile s/g s/l s/s Example. Estimate the percetage of water molecules on the surface of a fog droplet of diameter (i) 0.1 mm (naked eye visibility
An Analytical Approach for Determination of Riverbank Erosion under Action of Capillary Cohesion, Viscous Force and Force due to Pore Pressure
 An Analytical Approach for Determination of Riverbank Erosion under Action of Capillary Cohesion, Viscous Force and Force due to Pore Pressure Sanchayan Mukherjee 1, Bimalendu Pal 2, Debasish Mandi 2,
An Analytical Approach for Determination of Riverbank Erosion under Action of Capillary Cohesion, Viscous Force and Force due to Pore Pressure Sanchayan Mukherjee 1, Bimalendu Pal 2, Debasish Mandi 2,
Sta$s$cal mechanics of hystere$c capillary phenomena: predic$ons of contact angle on rough surfaces and liquid reten$on in unsaturated porous media
 Sta$s$cal mechanics of hystere$c capillary phenomena: predic$ons of contact angle on rough surfaces and liquid reten$on in unsaturated porous media Michel Louge h@p://grainflowresearch.mae.cornell.edu/
Sta$s$cal mechanics of hystere$c capillary phenomena: predic$ons of contact angle on rough surfaces and liquid reten$on in unsaturated porous media Michel Louge h@p://grainflowresearch.mae.cornell.edu/
the expansion for the Helmholtz energy derived in Appendix A, part 2, the expression for the surface tension becomes: σ = ( a + ½ k(ρ) ρ 2 x ) dx
 Motivation The surface tension plays a major role in interfacial phenomena. It is the fundamental quantity that determines the pressure change across a surface due to curvature. This in turn is the basis
Motivation The surface tension plays a major role in interfacial phenomena. It is the fundamental quantity that determines the pressure change across a surface due to curvature. This in turn is the basis
VISCOSITY MEASUREMENT MEAN MOLECULAR MASS DETERMINATION
 VISCOSITY MEASUREMENT MEAN MOLECULAR MASS DETERMINATION Author: dr Marek Studziński Editor: dr hab. Agnieszka Ewa Wiącek Task 11 VISCOSITY MEASUREMENT MEAN MOLECULAR MASS DETERMINATION I. Aim of the task
VISCOSITY MEASUREMENT MEAN MOLECULAR MASS DETERMINATION Author: dr Marek Studziński Editor: dr hab. Agnieszka Ewa Wiącek Task 11 VISCOSITY MEASUREMENT MEAN MOLECULAR MASS DETERMINATION I. Aim of the task
Instability & Patterning of Thin Polymer Films Prof. R. Mukherjee Department of Chemical Engineering Indian Institute Of Technology, Kharagpur
 Instability & Patterning of Thin Polymer Films Prof. R. Mukherjee Department of Chemical Engineering Indian Institute Of Technology, Kharagpur Lecture No. # 33 Spontaneous Instability & Dewetting of Thin
Instability & Patterning of Thin Polymer Films Prof. R. Mukherjee Department of Chemical Engineering Indian Institute Of Technology, Kharagpur Lecture No. # 33 Spontaneous Instability & Dewetting of Thin
5.2 Surface Tension Capillary Pressure: The Young-Laplace Equation. Figure 5.1 Origin of surface tension at liquid-vapor interface.
 5.2.1 Capillary Pressure: The Young-Laplace Equation Vapor Fo Fs Fs Fi Figure 5.1 Origin of surface tension at liquid-vapor interface. Liquid 1 5.2.1 Capillary Pressure: The Young-Laplace Equation Figure
5.2.1 Capillary Pressure: The Young-Laplace Equation Vapor Fo Fs Fs Fi Figure 5.1 Origin of surface tension at liquid-vapor interface. Liquid 1 5.2.1 Capillary Pressure: The Young-Laplace Equation Figure
Reaction at the Interfaces
 Reaction at the Interfaces Lecture 1 On the course Physics and Chemistry of Interfaces by HansJürgen Butt, Karlheinz Graf, and Michael Kappl Wiley VCH; 2nd edition (2006) http://homes.nano.aau.dk/lg/surface2009.htm
Reaction at the Interfaces Lecture 1 On the course Physics and Chemistry of Interfaces by HansJürgen Butt, Karlheinz Graf, and Michael Kappl Wiley VCH; 2nd edition (2006) http://homes.nano.aau.dk/lg/surface2009.htm
CE MECHANICS OF FLUIDS UNIT I
 CE 6303- MECHANICS OF FLUIDS UNIT I 1. Define specific volume of a fluid and write its unit [N/D-14][M/J-11] Volume per unit mass of a fluid is called specific volume. Unit: m3 / kg. 2. Name the devices
CE 6303- MECHANICS OF FLUIDS UNIT I 1. Define specific volume of a fluid and write its unit [N/D-14][M/J-11] Volume per unit mass of a fluid is called specific volume. Unit: m3 / kg. 2. Name the devices
Class XI Physics Syllabus One Paper Three Hours Max Marks: 70
 Class XI Physics Syllabus 2013 One Paper Three Hours Max Marks: 70 Class XI Weightage Unit I Physical World & Measurement 03 Unit II Kinematics 10 Unit III Laws of Motion 10 Unit IV Work, Energy & Power
Class XI Physics Syllabus 2013 One Paper Three Hours Max Marks: 70 Class XI Weightage Unit I Physical World & Measurement 03 Unit II Kinematics 10 Unit III Laws of Motion 10 Unit IV Work, Energy & Power
FOAM DRAINAGE part 3
 FOAM DRAINAGE part 3 gas effect : coupling coarsening and drainage () C 2 F 6 :almost «insoluble», so no coarsening N 2 : much more soluble, significantly faster coarsening Free-drainage, liquid fraction
FOAM DRAINAGE part 3 gas effect : coupling coarsening and drainage () C 2 F 6 :almost «insoluble», so no coarsening N 2 : much more soluble, significantly faster coarsening Free-drainage, liquid fraction
Solid-liquid interface
 Lecture Note #9 (Spring, 2017) Solid-liquid interface Reading: Shaw, ch. 6 Contact angles and wetting Wetting: the displacement from a surface of one fluid by another. A gas is displaced by a liquid at
Lecture Note #9 (Spring, 2017) Solid-liquid interface Reading: Shaw, ch. 6 Contact angles and wetting Wetting: the displacement from a surface of one fluid by another. A gas is displaced by a liquid at
P = 1 3 (σ xx + σ yy + σ zz ) = F A. It is created by the bombardment of the surface by molecules of fluid.
 CEE 3310 Thermodynamic Properties, Aug. 27, 2010 11 1.4 Review A fluid is a substance that can not support a shear stress. Liquids differ from gasses in that liquids that do not completely fill a container
CEE 3310 Thermodynamic Properties, Aug. 27, 2010 11 1.4 Review A fluid is a substance that can not support a shear stress. Liquids differ from gasses in that liquids that do not completely fill a container
Line Tension Effect upon Static Wetting
 Line Tension Effect upon Static Wetting Pierre SEPPECHER Université de Toulon et du Var, BP 132 La Garde Cedex seppecher@univ tln.fr Abstract. Adding simply, in the classical capillary model, a constant
Line Tension Effect upon Static Wetting Pierre SEPPECHER Université de Toulon et du Var, BP 132 La Garde Cedex seppecher@univ tln.fr Abstract. Adding simply, in the classical capillary model, a constant
Chem 728 Introduction to Solid Surfaces
 Chem 728 Introduction to Solid Surfaces Solids: hard; fracture; not compressible; molecules close to each other Liquids: molecules mobile, but quite close to each other Gases: molecules very mobile; compressible
Chem 728 Introduction to Solid Surfaces Solids: hard; fracture; not compressible; molecules close to each other Liquids: molecules mobile, but quite close to each other Gases: molecules very mobile; compressible
of Nebraska - Lincoln
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Xiao Cheng Zeng Publications Published Research - Department of Chemistry 10-1-2006 Homogeneous nucleation at high supersaturation
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Xiao Cheng Zeng Publications Published Research - Department of Chemistry 10-1-2006 Homogeneous nucleation at high supersaturation
Physics 141 Rotational Motion 2 Page 1. Rotational Motion 2
 Physics 141 Rotational Motion 2 Page 1 Rotational Motion 2 Right handers, go over there, left handers over here. The rest of you, come with me.! Yogi Berra Torque Motion of a rigid body, like motion of
Physics 141 Rotational Motion 2 Page 1 Rotational Motion 2 Right handers, go over there, left handers over here. The rest of you, come with me.! Yogi Berra Torque Motion of a rigid body, like motion of
Complexity of Two-Phase Flow in Porous Media
 1 Complexity of Two-Phase Flow in Porous Media Rennes September 16, 2009 Eyvind Aker Morten Grøva Henning Arendt Knudsen Thomas Ramstad Bo-Sture Skagerstam Glenn Tørå Alex Hansen 2 Declining oil production,
1 Complexity of Two-Phase Flow in Porous Media Rennes September 16, 2009 Eyvind Aker Morten Grøva Henning Arendt Knudsen Thomas Ramstad Bo-Sture Skagerstam Glenn Tørå Alex Hansen 2 Declining oil production,
Introduction. Statistical physics: microscopic foundation of thermodynamics degrees of freedom 2 3 state variables!
 Introduction Thermodynamics: phenomenological description of equilibrium bulk properties of matter in terms of only a few state variables and thermodynamical laws. Statistical physics: microscopic foundation
Introduction Thermodynamics: phenomenological description of equilibrium bulk properties of matter in terms of only a few state variables and thermodynamical laws. Statistical physics: microscopic foundation
PORE-SCALE PHASE FIELD MODEL OF TWO-PHASE FLOW IN POROUS MEDIUM
 Excerpt from the Proceedings of the COMSOL Conference 2010 Paris PORE-SCALE PHASE FIELD MODEL OF TWO-PHASE FLOW IN POROUS MEDIUM Igor Bogdanov 1*, Sylvain Jardel 1, Anis Turki 1, Arjan Kamp 1 1 Open &
Excerpt from the Proceedings of the COMSOL Conference 2010 Paris PORE-SCALE PHASE FIELD MODEL OF TWO-PHASE FLOW IN POROUS MEDIUM Igor Bogdanov 1*, Sylvain Jardel 1, Anis Turki 1, Arjan Kamp 1 1 Open &
8.2 Surface phenomena of liquid. Out-class reading: Levine p Curved interfaces
 Out-class reading: Levine p. 387-390 13.2 Curved interfaces 8.2.1 Some interesting phenomena Evolution of bubbles on porous surface. 8.2.1 Some interesting phenomena Addition of a seed in Supersaturated
Out-class reading: Levine p. 387-390 13.2 Curved interfaces 8.2.1 Some interesting phenomena Evolution of bubbles on porous surface. 8.2.1 Some interesting phenomena Addition of a seed in Supersaturated
MECHANICAL PROPERTIES OF SOLIDS
 Chapter Nine MECHANICAL PROPERTIES OF SOLIDS MCQ I 9.1 Modulus of rigidity of ideal liquids is (a) infinity. (b) zero. (c) unity. (d) some finite small non-zero constant value. 9. The maximum load a wire
Chapter Nine MECHANICAL PROPERTIES OF SOLIDS MCQ I 9.1 Modulus of rigidity of ideal liquids is (a) infinity. (b) zero. (c) unity. (d) some finite small non-zero constant value. 9. The maximum load a wire
I. Borsi. EMS SCHOOL ON INDUSTRIAL MATHEMATICS Bedlewo, October 11 18, 2010
 : an : an (Joint work with A. Fasano) Dipartimento di Matematica U. Dini, Università di Firenze (Italy) borsi@math.unifi.it http://web.math.unifi.it/users/borsi porous EMS SCHOOL ON INDUSTRIAL MATHEMATICS
: an : an (Joint work with A. Fasano) Dipartimento di Matematica U. Dini, Università di Firenze (Italy) borsi@math.unifi.it http://web.math.unifi.it/users/borsi porous EMS SCHOOL ON INDUSTRIAL MATHEMATICS
Phase Transitions & Interfaces
 Phase Transitions & Interfaces Chapter 6 of Atkins, 7th Ed. Chapter 4 and 18, 8th Ed. Sections 6.7-6.10, 7th Ed.; 4.7, 18.7, 18.8 in 8th Ed. Ehrenfest Classification of Phase Transitions Physical Liquid
Phase Transitions & Interfaces Chapter 6 of Atkins, 7th Ed. Chapter 4 and 18, 8th Ed. Sections 6.7-6.10, 7th Ed.; 4.7, 18.7, 18.8 in 8th Ed. Ehrenfest Classification of Phase Transitions Physical Liquid
Spri ringer. INTERFACIAL TRANSPORT PHENOMENA 2 nd Edition. John C. Slattery Department ofaerospace Engineering Texas A&M University
 INTERFACIAL TRANSPORT PHENOMENA 2 nd Edition John C. Slattery Department ofaerospace Engineering Texas A&M University Leonard Sagis Department of Agrotechnology & Food Science Wageningen University Eun-Suok
INTERFACIAL TRANSPORT PHENOMENA 2 nd Edition John C. Slattery Department ofaerospace Engineering Texas A&M University Leonard Sagis Department of Agrotechnology & Food Science Wageningen University Eun-Suok
Part II Fundamentals of Fluid Mechanics By Munson, Young, and Okiishi
 Part II Fundamentals of Fluid Mechanics By Munson, Young, and Okiishi WHAT we will learn I. Characterization of Fluids - What is the fluid? (Physical properties of Fluid) II. Behavior of fluids - Fluid
Part II Fundamentals of Fluid Mechanics By Munson, Young, and Okiishi WHAT we will learn I. Characterization of Fluids - What is the fluid? (Physical properties of Fluid) II. Behavior of fluids - Fluid
Equilibrium. the linear momentum,, of the center of mass is constant
 Equilibrium is the state of an object where: Equilibrium the linear momentum,, of the center of mass is constant Feb. 19, 2018 the angular momentum,, about the its center of mass, or any other point, is
Equilibrium is the state of an object where: Equilibrium the linear momentum,, of the center of mass is constant Feb. 19, 2018 the angular momentum,, about the its center of mass, or any other point, is
Computer Animation: Animation of Soap Bubble Dynamics, Cluster Formation and Collision
 Journal of the Applied Mathematics, Statistics and Informatics (JAMSI), 1 (2005), No. 2 Computer Animation: Animation of Soap Bubble Dynamics, Cluster Formation and Collision Roman Ďurikovič1 Abstract
Journal of the Applied Mathematics, Statistics and Informatics (JAMSI), 1 (2005), No. 2 Computer Animation: Animation of Soap Bubble Dynamics, Cluster Formation and Collision Roman Ďurikovič1 Abstract
Interfacial dynamics
 Interfacial dynamics Interfacial dynamics = dynamic processes at fluid interfaces upon their deformation Interfacial rheological properties: elasticity, viscosity, yield stress, Relation between macroscopic
Interfacial dynamics Interfacial dynamics = dynamic processes at fluid interfaces upon their deformation Interfacial rheological properties: elasticity, viscosity, yield stress, Relation between macroscopic
Intermolecular forces are classified into four major types.
 Intermolecular forces are classified into four major types. 1. Ion-dipole: IMF s that occur between neighboring an ion solution and a polar molecule (dipole) also in solution. Na+ 2. Dipole-dipole: IMF
Intermolecular forces are classified into four major types. 1. Ion-dipole: IMF s that occur between neighboring an ion solution and a polar molecule (dipole) also in solution. Na+ 2. Dipole-dipole: IMF
DIVIDED SYLLABUS ( ) - CLASS XI PHYSICS (CODE 042) COURSE STRUCTURE APRIL
 DIVIDED SYLLABUS (2015-16 ) - CLASS XI PHYSICS (CODE 042) COURSE STRUCTURE APRIL Unit I: Physical World and Measurement Physics Need for measurement: Units of measurement; systems of units; SI units, fundamental
DIVIDED SYLLABUS (2015-16 ) - CLASS XI PHYSICS (CODE 042) COURSE STRUCTURE APRIL Unit I: Physical World and Measurement Physics Need for measurement: Units of measurement; systems of units; SI units, fundamental
MECHANICAL PROPERTIES OF FLUIDS:
 Important Definitions: MECHANICAL PROPERTIES OF FLUIDS: Fluid: A substance that can flow is called Fluid Both liquids and gases are fluids Pressure: The normal force acting per unit area of a surface is
Important Definitions: MECHANICAL PROPERTIES OF FLUIDS: Fluid: A substance that can flow is called Fluid Both liquids and gases are fluids Pressure: The normal force acting per unit area of a surface is
CHAPTER 2. SOIL-WATER POTENTIAL: CONCEPTS AND MEASUREMENT
 SSC107 Fall 2000 Chapter 2, Page - 1 - CHAPTER 2. SOIL-WATER POTENTIAL: CONCEPTS AND MEASUREMENT Contents: Transport mechanisms Water properties Definition of soil-water potential Measurement of soil-water
SSC107 Fall 2000 Chapter 2, Page - 1 - CHAPTER 2. SOIL-WATER POTENTIAL: CONCEPTS AND MEASUREMENT Contents: Transport mechanisms Water properties Definition of soil-water potential Measurement of soil-water
Mohamed Daoud Claudine E.Williams Editors. Soft Matter Physics. With 177 Figures, 16 of them in colour
 Mohamed Daoud Claudine E.Williams Editors Soft Matter Physics With 177 Figures, 16 of them in colour Contents 1. Droplets: CapiUarity and Wetting 1 By F. Brochard-Wyart (With 35 figures) 1.1 Introduction
Mohamed Daoud Claudine E.Williams Editors Soft Matter Physics With 177 Figures, 16 of them in colour Contents 1. Droplets: CapiUarity and Wetting 1 By F. Brochard-Wyart (With 35 figures) 1.1 Introduction
Chapter One Reviews of Thermodynamics Update on 2013/9/13
 Chapter One Reviews of Thermodynamics Update on 2013/9/13 (1.1). Thermodynamic system An isolated system is a system that exchanges neither mass nor energy with its environment. An insulated rigid tank
Chapter One Reviews of Thermodynamics Update on 2013/9/13 (1.1). Thermodynamic system An isolated system is a system that exchanges neither mass nor energy with its environment. An insulated rigid tank
Interfacial Instabilities in a Microfluidic Hele-Shaw Cell: Supplemental
 Supplementary Material (ESI) for Soft Matter This journal is The Royal Society of Chemistry 2008 Interfacial Instabilities in a Microfluidic Hele-Shaw Cell: Supplemental Michinao Hashimoto 1, Piotr Garstecki
Supplementary Material (ESI) for Soft Matter This journal is The Royal Society of Chemistry 2008 Interfacial Instabilities in a Microfluidic Hele-Shaw Cell: Supplemental Michinao Hashimoto 1, Piotr Garstecki
Fluid Mechanics. du dy
 FLUID MECHANICS Technical English - I 1 th week Fluid Mechanics FLUID STATICS FLUID DYNAMICS Fluid Statics or Hydrostatics is the study of fluids at rest. The main equation required for this is Newton's
FLUID MECHANICS Technical English - I 1 th week Fluid Mechanics FLUID STATICS FLUID DYNAMICS Fluid Statics or Hydrostatics is the study of fluids at rest. The main equation required for this is Newton's
Lecture 15 Strain and stress in beams
 Spring, 2019 ME 323 Mechanics of Materials Lecture 15 Strain and stress in beams Reading assignment: 6.1 6.2 News: Instructor: Prof. Marcial Gonzalez Last modified: 1/6/19 9:42:38 PM Beam theory (@ ME
Spring, 2019 ME 323 Mechanics of Materials Lecture 15 Strain and stress in beams Reading assignment: 6.1 6.2 News: Instructor: Prof. Marcial Gonzalez Last modified: 1/6/19 9:42:38 PM Beam theory (@ ME
Transduction Based on Changes in the Energy Stored in an Electrical Field
 Lecture 6-1 Transduction Based on Changes in the Energy Stored in an Electrical Field Electric Field and Forces Suppose a charged fixed q 1 in a space, an exploring charge q is moving toward the fixed
Lecture 6-1 Transduction Based on Changes in the Energy Stored in an Electrical Field Electric Field and Forces Suppose a charged fixed q 1 in a space, an exploring charge q is moving toward the fixed
TOPICS. Density. Pressure. Variation of Pressure with Depth. Pressure Measurements. Buoyant Forces-Archimedes Principle
 Lecture 6 Fluids TOPICS Density Pressure Variation of Pressure with Depth Pressure Measurements Buoyant Forces-Archimedes Principle Surface Tension ( External source ) Viscosity ( External source ) Equation
Lecture 6 Fluids TOPICS Density Pressure Variation of Pressure with Depth Pressure Measurements Buoyant Forces-Archimedes Principle Surface Tension ( External source ) Viscosity ( External source ) Equation
Microfluidics 2 Surface tension, contact angle, capillary flow
 MT-0.6081 Microfluidics and BioMEMS Microfluidics 2 Surface tension, contact angle, capillary flow 28.1.2017 Ville Jokinen Surface tension & Surface energy Work required to create new surface = surface
MT-0.6081 Microfluidics and BioMEMS Microfluidics 2 Surface tension, contact angle, capillary flow 28.1.2017 Ville Jokinen Surface tension & Surface energy Work required to create new surface = surface
