NANOSCALE AND SURFACE PHYSICS LAB MANUAL
|
|
|
- Penelope Gilmore
- 5 years ago
- Views:
Transcription
1 NANOSCALE AND SURFACE PHYSICS LAB MANUAL KATIE MITCHELL DEPARTMENT OF PHYSICS AND ENGINEERING PHYSICS JANUARY 2010 Version 1.1
2 1. Ultrahigh Vacuum 1.1 What is ultrahigh vacuum? Ultrahigh vacuum (UHV) is defined as the pressure range between 10-7 and Pa (7.5 x and 7.5 x Torr) (American Vacuum Society definition [1]). For comparison, atmospheric pressure is approximately 100 kpa or 760 Torr. An overview of different pressure ranges is shown in Fig Fig Overview of pressure ranges below atmospheric pressure. Ultrahigh vacuum (UHV) is typically used in surface science research and in semiconductor fabrication, as well as in particle and electron storage rings, and can easily be obtained using a range of commercial components. Extreme high vacuum (xhv) (pressures below Pa) can only be obtained using very specialized and careful procedures [1]. Approximate pressures inside an incandescent light bulb and at the surface of the moon are shown for comparison. UHV pressures are usually measured in Torr, mbar or Pa (the SI unit). In surface science research in North America, Torr is most commonly used, and it is the unit that we use in our lab. Table 1.1 lists the conversions between these units. Note that Torr and mbar are of the same order of magnitude. Table 1.1. Pressure unit conversions [2]. Pa (pascal) bar mbar (10-3 bar) Torr 1 Pa 1 N/m x
3 1.2 Why do we need ultrahigh vacuum? We need ultrahigh vacuum for surface science studies for two principal reasons: (1) In order to study a well-defined surface that is free of contaminants, we need to be able to clean the surface, and to maintain the surface in a clean state for the duration of the experiment. (2) Many experimental probes, such as low energy electrons and vacuum ultraviolet light are scattered or absorbed at atmospheric pressures, so these techniques can only be performed in vacuum. We can estimate what kind of vacuum is needed to satisfy these two conditions by using the kinetic theory of gases [3,4,5]. Assuming that we have an ideal gas at pressure p (Pa) and temperature T (K), with n molecules per unit volume, then the ideal gas law tells us that where k is Boltzmann s constant. p n (1.1) kt The mean free path is defined as the mean distance travelled by a molecule between collisions with other molecules. Assuming a single species of molecule with molecular diameter d and n molecules per unit volume, the mean free path can be estimated to be [5] 1 (1.2) 2 n 2 d Another quantity of interest is the monolayer (ML) coverage time. This is defined as the time taken for a surface to be completely covered by a single layer of gas molecules. The ML coverage time will depend on the structure of the surface in question and on the sticking probability of the impinging gas molecules. However, we can get an order of magnitude estimate by using parameters appropriate for a typical molecule, and assuming a sticking probability of one. For gas molecules of mass m (kg), at pressure p (Pa) and temperature T (K), and for a ML density of N 0 molecules/m 2, we have [3,4] N0 2 mkt (1.3) p We can use these expressions to get a feel for the numbers involved. In high and ultrahigh vacuum, the dominant gases in a chamber are typically H 2, H 2 O and CO. We will follow Venables [3], and choose CO as a representative gas. CO has a molecular diameter d of nm. Given d, a close-packed monolayer of CO has a density of N (see Fig. 1.2) Using T 295 K, m m kg (given that the molecular weight of CO is 28) and k J/K, we find the values given in Table
4 Fig Schematic diagram of a close-packed monolayer of molecules, with each molecule represented as a sphere. A unit cell is outlined in black. For a close-packed layer, the internal angles of the unit cell are 60 and 120. For CO, the molecular diameter and thus the length of the unit cell side, is nm [3]. Since there is one molecule per unit cell, the density N0 of molecules on the surface is 1 area of unit cell, which in this case is N m Table 1.2. Variation of molecular density, mean free path and monolayer coverage time with pressure. For these calculations, we have used the molecular weight and diameter of CO, and a condensed close-packed monolayer of CO for the definition of monolayer coverage. Pressure Molecular density Mean Free Path Monolayer coverage time p (Torr) n=n/v (cm -3 ) λ (m) (s) 760 (atmosphere) 2.49 x x x x x x 10 5 (690 km) 3.0 x 10 4 (8.3 hrs) From Table 1.2, we can see the tremendous difference between conditions at atmospheric pressure and at ultrahigh vacuum. We can also see that even at UHV, there are still a large number of gas molecules per cubic centimetre. At 10-6 torr, mean free paths are already much longer than the dimensions of a typical UHV chamber, so pressures better than this are sufficient for the use of experimental techniques involving probes such as low energy electrons. However, the requirement for a clean surface places more stringent requirements on the pressure. Pressures in the UHV range are needed in order to keep most surfaces clean for the duration of a typical experiment. 1.3 Ultrahigh vacuum components and materials A typical ultrahigh vacuum system consists of a stainless steel chamber with a variety of ports to accommodate pumps, gauges, instrumentation, sample manipulation, windows, and interconnection with other chambers. Connections to the ports are made via CF or conflat flanges, which are bolted together. Conflat flanges are sealed by means of a copper gasket which is sandwiched between knife edges on the mating stainless steel faces (see Fig.1.3). 4
5 Fig Schematic diagram of a conflat flange before assembly, showing the copper gasket between the two mating surfaces (diagram from Trinos Vacuum Systems Inc. Catalog). If ultrahigh vacuum pressures are needed, a new gasket should be used each time the flange is bolted on, and the bolts should be tightened evenly around the perimeter of the flange. It is more important to tighten the bolts evenly than to do them up extremely tightly. An important precaution: if the knife edge on either flange is damaged (for example by scratching or hitting), it may no longer be possible to make a UHV seal. It is therefore of great importance to avoid damaging the knife edges. Always protect them with an old copper gasket when they are exposed. The pressure in an ultrahigh vacuum system is the result of a competition between the rate at which gas molecules enter the chamber, and the rate at which they are pumped out. Since there are always gas molecules entering even the best UHV chamber, continuous pumping is needed in order to achieve UHV. Sources of gas molecules entering the chamber include leaks through any connections (flanges etc.), leaks from trapped gases (such as inside screwholes), diffusion through the chamber walls, and desorption of water and other gases from all inside surfaces. When a UHV chamber is first pumped down after being vented, the dominant gas inside is water, desorbing from the walls. In order to get rid of this water, UHV chambers are baked at 100 C for hrs. This speeds up the desorption of water, and after cooling down, UHV can be achieved. After bakeout, the dominant gases in the chamber are usually hydrogen and carbon monoxide [1,3]. Because the vacuum is very sensitive to the desorption rate of materials inside the chamber, many common materials are unsuitable for use in UHV because of their high vapour pressure. In particular, most organic materials including lubricants must not be used in a UHV chamber. Table 1.3 shows a selection of suitable and unsuitable materials for UHV. Not only do we have to be careful about what materials go into an ultrahigh vacuum chamber, we also need to be careful about how we clean and handle components that will go into UHV. In particular, all components need to be degreased before going into the vacuum chamber. To degrease components, we clean them in the ultrasonic bath for about ten minutes, in a beaker of isopropanol (isopropyl alcohol, or IPA). Very dirty components may also need to be scrubbed with IPA (for example, machined components that still have grease in screw threads, etc.) When handling UHV components and when mounting samples, clean powder-free vinyl or latex gloves should be worn at all times. This prevents grease from your hands getting onto the components. Dust can be removed from components and samples by blowing a stream of dry nitrogen gas across them. All tools used for assembling 5
6 components and mounting samples should first be cleaned in IPA. We usually keep a box of clean tools on the bench, ready for use. Table 1.3. A selection of suitable and unsuitable materials for use in UHV, taken from the HASYLAB Vacuum Guidelines for Beamlines and Experiments, HASYLAB Overview of the Ultrahigh Vacuum System Our ultrahigh vacuum system is designed for the preparation and characterization of clean single crystal surfaces and ultrathin films, and for studying these surfaces using scanning tunnelling and atomic force microscopy. The UHV system consists of two chambers, pumps and accessories mounted on a steel frame. The steel frame is supported by a Newport vibration isolation system consisting of three pneumatic stabilizers. The stabilizers are required to reduce vibrations when doing scanning tunnelling microscopy (STM) or atomic force microscopy (AFM) experiments. Figure 2.1 shows a schematic overview of the system, seen from above. Some ports have been omitted for clarity. In Figure 2.2, a photograph of the system is shown. The shaded area represents the chamber and components supplied by RHK Technology [6] as part of the UHV 350 AFM/STM system. RHK also supplied the steel frame and Newport isolation system. The STM/AFM chamber is pumped by a Physical Electronics ion getter pump (mounted directly under the chamber, below the table), and also by a Leybold 300 l/s turbomolecular pump through the load-lock. The load-lock is used to introduce samples and STM/AFM probes into the chamber without having to break vacuum. Samples and probes are transferred into the chamber using the magnetic transfer arm shown. 6
7 Figure 2.1 Overview of the UHV system. The UHV system is mounted on a table which covers a steel frame. The frame is supported by three pneumatic stabilizers, which reduce vibration. Figure 2.2 Photograph of the UHV system. 7
8 The RHK STM/AFM chamber is connected by a gate valve to the sample preparation/analysis chamber. This chamber is a 12 diameter stainless steel sphere with 26 ports, and was designed by us and manufactured by Torrovap [7]. It is aligned with the RHK chamber such that samples may be transferred from one chamber to the other using the magnetically coupled transfer arm mounted on the preparation chamber. The preparation chamber is pumped by a Gamma Vacuum ion getter pump (mounted directly underneath the chamber and below the table), and by a Varian 550 l/s turbomolecular pump. The turbo pump is connected to the chamber by large diameter tubing and gate valve in order to maximize the conductance between chamber and pump. This provides optimum pump down speed for removing gases from the chamber. The centrepiece of the preparation/analysis chamber is the sample manipulator which accommodates samples mounted in RHK sample holders, allows them to be transferred on and off in vacuum, positions the samples for the various prep/analysis tools, and provides heating, cooling and electrical connections to the samples. The manipulator is a Vacuum Generators (VG) HPT-WX5 manipulator which provides 5-axes of motion, electrical and liquid nitrogen feedthroughs. It is mounted on the top 6 flange of the chamber and allows the sample to be moved in the x, y and z directions (see Fig. 2.3), and rotated about the vertical axis of the chamber. An additional rotary feedthrough can be used to control another degree of freedom. We use it to move the sample heater to different positions for sample transfer and heating. The manipulator head was designed as part of an EP495 capstone project, by Greg Florizone, Lindsey Hinther, David Muir, and Benjamin Wilson (2004), and won the 2004 Engineering Innovative Design Competition at the University of Saskatchewan. It has been further improved by Jeff Svajlenko, who worked with us as an NSERC USRA student in the summer of (a) (b) Figure 2.3 (a) Vacuum Generators (VG) HPT-WX5 manipulator. (b) The manipulator head inside the chamber. The sample is mounted in an RHK sample holder (gold-coloured disk) which has been transferred onto the manipulator. 8
9 For analysis, the prep/analysis chamber features 1) Low Energy Electron Diffraction (LEED) and Auger Electron Spectroscopy (AES) We have an OCI BDL-800 reverse view LEED/Auger instrument (see Fig. 2.4) [8]. This instrument can be used to provide information on crystalline order at the surface and surface structure (LEED), and on surface chemical composition (Auger). The diffraction images can be captured by a CCD camera connected to the data acquisition computer. (a) (b) (c) Figure 2.4 (a) Two views of the LEED/Auger spectrometer mounted on the chamber. The CCD camera can be seen on the right, in the second view. (b) LEED pattern from a clean Cu(110) single crystal. The LEED pattern is a Fourier transform of the surface periodicity, and the dimensions of the unit cell in the pattern can be used to calculate the dimensions of the surface unit cell. (c) Auger spectrum obtained from the same copper crystal the presence of Cu, as well as of S contamination, can be seen in the spectrum. (Images (b) and (c) courtesy of Trevor Coulman, Univ. of Saskatchewan). 2) Mass Spectrometry We have a Hiden HAL IV RC PIC-RGA 301 quadrupole mass spectrometer. This spectrometer has a mass range of 300 amu and can detect partial pressures down to 5 x mbar [9]. It can be used to monitor residual gases in the chamber, and also to perform temperature programmed desorption (TPD) measurements. TPD is a technique that is used to study molecular adsorption at a surface. The mass spectrometer is mounted directly opposite the LEED/Auger spectrometer. For sample preparation, the prep/analysis chamber features 1) On-sample temperature measurement using either a K-type thermocouple, or an infrared pyrometer (Land Cyclops 100B) 9
10 2) A tungsten wire heater incorporated into the manipulator head for radiative and electron beam heating (300 to >900 K) 3) Liquid nitrogen cooling incorporated into the manipulator head (temp range to be determined) 4) An OCI IG70 sputter ion gun (0-300 ev energy range) for noble gas sputter cleaning and low energy ion implantation [8] 5) A Mantis Quad-EV 4-pocket e-beam metal evaporator for deposition of ultrathin metal films [10] 6) A home-built Knudsen cell for low temperature deposition ( K) of organic molecules such as amino acids [11] 7) A Sigma quartz crystal microbalance for calibrating deposition rates for our evaporators [12] 8) A gas line to supply the ion sputter gun, and for dosing of other gases (eg. oxygen, nitrogen). The gas line accommodates up to four gas bottles, and may be baked for improved cleanliness. 3. Overview of the pumps and pressure gauges It is vital to understand the function and limitations of each of the pumps and pressure gauges used in the UHV system. Figure 3.1 shows a schematic diagram of our pumping and pressure measurement system, and Figure 3.2 shows a photograph for comparison. The types of pumps and pressure gauges that we use are described on the following pages. Please use the schematic diagram and photograph in Figs. 3.1 and 3.2 to familiarize yourself with the location of the pumps, pressure gauges and valves on the UHV system, and to find them on the actual system. It may also help to use the photographs of the pumps and pressure gauges in the following sections of chapter 3. Make sure you understand what each component does and how the schematic diagram relates to the actual vacuum system. When opening and closing valves, and turning pumps on and off, it is very important to take your time, to double check the status of all valves and pumps, and to monitor the pressure continuously. If something unexpected happens, stop or reverse what you were doing until you can figure out what has happened. 10
11 Figure 3.1 Schematic diagram of the main pumping and pressure measurement system for our UHV chambers (pumping and pressure measurement for the gas line are shown separately). This diagram shows the connectivity of the pumps and gauges, and the location of the valves. Figure 3.2 Photograph of the UHV system, showing the gate valves in red, for comparison to the schematic diagram. 11
12 3.1 Pumps Scroll Pump Varian TriScroll 300 dry vacuum pumps are used as backing pumps for both of our turbo pumps. A scroll pump is a mechanical pump in which two metal scrolls are interleaved. One scroll orbits with respect to the other, trapping gas and forcing it through the pump (see Figure 3.3a) [13]. The Varian TriScroll 300 pump uses this mechanism, but with a patented scroll design and several stages of pumping for high pumping speed and low ultimate pressure. The maximum pumping speed is 250 l/min (4.2 l/s), with an ultimate pressure of 1.3x10-2 mbar. An important feature of the Varian TriScroll 300 is that it is a dry vacuum pump it does not contain any oil for lubrication, and thus there is no risk of hydrocarbons contaminating the vacuum system. (a) (b) Figure 3.3 (a) The interleaved scrolls of a scroll pump are shown in grey and blue. Here, the grey scroll orbits eccentrically (its centre moves but the scroll does not rotate) with respect to the blue, in order to trap gas and compress it. Gas enters at the side (yellow arrow) and exits at the centre (red arrow). ( (b) One of our Varian Triscroll 300 pumps. These pumps sit on the floor beside the UHV system Turbomolecular (Turbo) Pump The turbomolecular pump (usually referred to as a turbo pump) is a type of molecular drag pump. Gas is pumped by a molecular drag pump through momentum transfer from a rapidly rotating solid surface to the gas molecules. A turbo pump consists of a bladed molecular turbine, with closely spaced rotor and stator blades (see Fig.3.4) Typical rotation frequencies are in the range of 50,000 rpm. If the mean free path of the gas molecules is longer than the distance between rotor and stator blades, then the gas molecules will collide primarily with the blades rather than with each other, and pumping will be efficient. This is called the molecular flow range. If this condition isn t met, collisions between the gas molecules will interfere with the pumping. For a typical rotor-stator spacing on the order of mm, the molecular flow 12
13 range occurs for pressures Pa (~ 10 mbar). Because of this, a turbo can t pump against atmospheric pressure, and needs a backing pump, such as the scroll pump described above [14]. Fig. 3.4 A cut through a representative turbomolecular pump (image Wikimedia Commons), showing the rotor and stator blades, angled in opposite directions. The turbo pump is very widely used in high vacuum and ultrahigh vacuum applications, and is the only mechanical pump that, in combination with a backing pump, can reach pressures below 8 10 Pa (in the ultrahigh vacuum range) [14]. It is also very reliable. Care must be taken not to expose a turbo running at full speed to atmospheric pressure the sudden deceleration caused by the inrush of air may damage the pump. We have two turbo pumps: Figure 3.5 Leybold 300 l/s turbo pump and controller. This pump is used for the load-lock, and can also pump the whole system through the load-lock. When I took this picture, the turbo was off, hence the rotational frequency of 0 Hz. 13
14 Figure 3.6 Varian 550 l/s turbo pump and controller. This pump is used for the preparation chamber, and can also be used to pump the whole system. Because it is a bigger pump and is connected to the chamber through large diameter tubing, it can pump the system down much faster than the Leybold turbo. When I took the picture, this pump was running at its normal frequency of 42 krpm (what is that in Hz?) Note the fan mounted on the side of the turbo. Turbo pumps need to be actively cooled during operation Sputter Ion Pump The sputter ion pump works by ionizing and capturing gas molecules. The gas is trapped within the pump. This pump is very important for its ability to reach ultrahigh vacuum pressures, and because it has no moving parts and hence creates no vibration. This makes it ideal to use with scanning probe microscopes. Figure 3.7 shows a schematic diagram illustrating the basic principles of a sputter ion pump. (a) (b) Figure 3.7 (a) Schematic diagram illustrating the physics of a sputter ion pump. The shaded area represents the region inside the vacuum envelope. The cylindrical anodes are held at a positive high voltage, and the Ti cathode plates are grounded. See text for a description of ion pump operation. (b) Perspective drawing corresponding to the diagram in (a) (image, Thermionics Vacuum Products, Ion Pump Guide). Inside the vacuum envelope, an array of cylindrical anodes is held at a positive high voltage (7 kv for our pump), and these anodes are sandwiched between grounded titanium plates. Permanent magnets 14
15 outside the pump generate a magnetic field on the order of 1000 Gauss parallel to the axes of the cylindrical anodes. When the high voltage is applied to the pump, a plasma discharge is created. Electrons are trapped in helical paths in the cylindrical anodes, creating ions when they collide with gas molecules. The positive ions are accelerated towards the Ti cathodes where they may be buried or reflected and then buried on another pump surface. The bombardment of the cathodes also causes Ti to be sputtered onto all of the pump surfaces. Pumping occurs through burial in the pump surfaces and also through Ti gettering : Ti surfaces are very reactive and will chemically bind many gas molecules which land on them [15]. Ion pumps are usually started when the vacuum pressure is in the range of 10-6 Torr. If an ion pump is started at higher pressures, above 10-3 Torr, a glow discharge will result, no pumping action occurs, and previously pumped gases will be desorbed from pump surfaces. We have two sputter ion pumps, one for each chamber. Fig. 3.8 shows a photograph of one of our pumps and controllers. Figure 3.8 Physical Electronics 300 l/s ion pump and controller. This pump is mounted underneath the STM/AFM chamber. A titanium sublimation pump (TSP, see below) is incorporated into our ion pump. The connections for the TSP are the four black/orange cables. The status of the ion pump power supply is shown in the top readout on the controller. The exact operating voltage of the pump varies with the chamber pressure, but at UHV, the operating voltage is approximately 7 kv. At the time I took this picture, it was 7430 V Titanium Sublimation Pump The titanium sublimation pump (TSP) is a type of getter pump. Getter pumps rely on the capture of molecules by chemical reaction with a solid surface. A TSP generally consists of a feedthrough onto which Ti rods are mounted, and from which Ti is evaporated onto the inside surfaces of the pump. A TSP can be located within its own housing, or incorporated into an ion pump. Figure 3.9 shows a typical TSP 15
16 feedthrough. A high current (~ 50 A) is passed through one of the titanium rods, heating the rod and causing Ti to evaporate onto the walls of the pump. Gas molecules which subsequently collide with the pump walls may react with the Ti and be adsorbed onto the surface. As the Ti film is used up the pumping speed decreases. A Ti sublimation pump is therefore operated by periodically passing a current through the Ti rod in order to replenish the getter film on the pump walls. Pumping speed may be increased, particularly for non-reactive gases, by cooling the pump walls using either water or liquid nitrogen. In this case, the pump acts as a combination getter/cryopump. Figure 3.9. Titanium sublimation pump feedthrough, showing the Ti elements (wavy Ti rods). A high current is passed through these rods in order to heat them and cause Ti to be evaporated onto the pump surfaces (image Our titanium sublimation pumps are mounted on our ion pumps. The TSP current and cooling feedthroughs can be seen in Fig. 3.8, and the TSP is controlled by the second power supply in the ion pump controller. 3.2 Pressure Gauges Convectron Gauge Convectron is a trademark of Helix Corp., and refers to their convection-enhanced pirani gauge. It measures pressures in the range atmosphere to 10-4 Torr. The pirani gauge was invented by Marcello Pirani in 1906, and consists of a wire, often Platinum, open to the gas whose pressure is being measured. A current is passes through the wire, heating it, and the wire also loses heat to the surrounding gas. The rate at which it loses heat to the gas depends on the rate at which gas molecules hit the wire, and thus on the pressure. The equilibrium temperature of the wire thus depends on the gas pressure. Since the resistance of a wire varies with its temperature, the resistance can be used to measure the wire temperature and thus the gas pressure. At temperatures above about 10 Torr, heat transfer is dominated by convection in the gas surrounding the wire, and convection enhanced gauges are designed with a geometry that allows this convection heat loss to be quantified in order to provide accurate pressure measurements above 10 Torr (SRS brochure). The lower limit of pressure measurement of these gauges occurs when heat losses due to radiation, and conduction through the wire, dominate the heat losses to the surrounding gas. This occurs at Torr [2]. 16
17 Figure 3.10 This photo shows the load-lock, gate valve and Leybold turbo. One of the two convectron gauges is circled in white, while the other one is hidden behind the transfer arm. The convectron gauge that you can see is used to monitor the pressure in the backing line, between the turbo and scroll pump. The gauge controller is used to control the two convectron gauges as well as the Bayard Alpert gauge on the load-lock. When I took this picture, the Leybold turbo and backing pump were off, and the convectron gauge on the backing line is reading 5.7x10 2 Torr atmospheric pressure within the accuracy of the gauge. The convectron gauge on the load-lock is reading 3.2 Torr, indicating that the load-lock is still under a partial vacuum Cold Cathode Gauge A cold cathode gauge is an ionization gauge in which there is no hot filament. The cold cathode gauge uses crossed electric and magnetic fields (usually 2-6 kv and 1-2 kg) to trap electrons. These trapped electrons ionize gas molecules, which are then trapped by the cathode. The current of ions to the cathode is used as a measure of the gas pressure. Cold cathode gauges rely on a random event such as a cosmic ray, field emission, or a photon to release an electron at the cathode and start the discharge. The starting time is quick (seconds) at pressures in the range of 10-6 Torr, but at lower pressures can be very long (hours at Torr). The operating range of cold cathode gauges is typically Torr [2] Bayard Alpert Ionization Gauge The Bayard Alpert ionization gauge is the standard gauge used to measure ultrahigh vacuum pressures, and is a hot cathode ionization gauge. It can measure pressures in the range of Torr. The Bayard Alpert gauge consists of a cathode filament which is heated by a current, and produces electrons by thermionic emission. Typical emission currents are 10 ma. The emitted electrons are accelerated through a grid, where they may ionize gas molecules. Ions produced in this way are accelerated toward the collector filament, producing a current. The ion current is linearly proportional to the gas density and pressure, allowing a pressure measurement to be made [2]. A typical Bayard Alpert gauge is shown in Fig
18 Figure Photo of a Bayard Alpert ionization gauge (Varian Inc.) The lower pressure limit of Bayard Alpert gauges is determined by two processes which cause a current at the collector unrelated to the gas pressure [2]: 1) Photoelectric effect: electrons hitting the grid can produce X-rays which may hit the collector and cause a current due to photoemission. This effect is called the X-ray limit, and may be reduced by using very small diameter grid and collector wires. 2) Electron stimulated desorption: electrons hitting the collector may cause the desorption of positive ions from gases adsorbed on the collector. This effect can be reduced by bakeout and degassing of the gauge. If Bayard Alpert gauges are operated at high pressures near the limit of operation, the filament lifetime will be greatly reduced, and the filament may be damaged. Even under normal operation, the hot filament needs to be replaced periodically. Our two Bayard Alpert gauges (usually referred to simply as ion gauges ) are shown in Figs and Figure 3.12 Granville-Phillips Bayard-Alpert gauge on the STM/AFM chamber. This gauge is located under the table, just above the ion pump. The controller shows a pressure of 6.4 x10-10 Torr. 18
19 Figure 3.13 Varian Bayard-Alpert gauge on the prep chamber. This gauge is located just above the table at the bottom of the chamber. Looking in a chamber window, you can see the glow from the gauge s hot filament. The controller shows a pressure of 1.0x10-9 Torr. 19
20 7. Useful Texts and Websites 7.1 Vacuum Practice The Physical Basis of Ultrahigh Vacuum, P.A. Redhead, J.P. Hobson, and E.V. Kornelsen, American Vacuum Society Classics, American Institute of Physics, Surface Science Physics at Surfaces, Zangwill Surface Science, Kolasinski Surfaces, Attard and Barnes Semiconductor Surfaces and Interfaces, Monch The Surface Science of Metal Oxides, Henrich and Cox 7.3 Scanning Probe Microscopy Introduction to Scanning Tunneling Microscopy, Chen Scanning Probe Microscopy and Spectroscopy, Wiesendanger Scanning Tunneling Microscopy III, Wiesendanger & Guntherodt 8. References 1. P.A. Redhead, in Foundations of Vacuum Science and Technology, (J.M. Lafferty, ed.), p.625. John Wiley & Sons, Inc., New York, R.N. Peacock, in Foundations of Vacuum Science and Technology, (J.M. Lafferty, ed.), p.375. John Wiley & Sons, Inc., New York, John A. Venables, Lecture Notes on Surfaces and Thin Films, Sect. 2.1, Arizona State University, October 2009 ( 4. Roger M. Nix, An Introduction to Surface Chemistry, School of Biological and Chemical Sciences, Queen Mary, University of London, June 2003 ( 5. W.R. Salzman, Kinetic Molecular Theory of Gases, Physical Chemistry Dynamic Text, Dept. of Chemistry, University of Arizona, October 2006 ( 6. RHK Technology, Inc., Troy, Michigan, USA ( 7. Torrovap Industries, Inc., Markham, ON ( 20
21 8. OCI Vacuum Microengineering, Inc., London, ON ( 9. Hiden Analytical, Warrington, England ( 10. Mantis Deposition Ltd., Thame, Oxon, UK ( 11. E.A. Dubiel, A Knudsen Cell for Controlled Deposition of L-Cysteine and L-Methionine on Au(111), MSc Thesis, University of Saskatchewan, Sigma Instruments, ( 13. Nigel T.M. Dennis, in Foundations of Vacuum Science and Technology, (J.M. Lafferty, ed.), p.167. John Wiley & Sons, Inc., New York, Jörgen Henning, in Foundations of Vacuum Science and Technology, (J.M. Lafferty, ed.), p.233. John Wiley & Sons, Inc., New York, Hinrich Henning, in Foundations of Vacuum Science and Technology, (J.M. Lafferty, ed.), p.317. John Wiley & Sons, Inc., New York,
Vacuum Pumps. Two general classes exist: Gas transfer physical removal of matter. Mechanical, diffusion, turbomolecular
 Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Lecture 3 Vacuum Science and Technology
 Lecture 3 Vacuum Science and Technology Chapter 3 - Wolf and Tauber 1/56 Announcements Homework will be online from noon today. This is homework 1 of 4. 25 available marks (distributed as shown). This
Lecture 3 Vacuum Science and Technology Chapter 3 - Wolf and Tauber 1/56 Announcements Homework will be online from noon today. This is homework 1 of 4. 25 available marks (distributed as shown). This
Vacuum. Kai Schwarzwälder, Institut für Physik Universität Basel October 6 th 2006
 Physics,, Technology and Techniques of the Vacuum Kai Schwarzwälder, Institut für Physik Universität Basel October 6 th 2006 Outline Introduction and basics Defintion of Vacuum Vacuum A vacuum is a volume
Physics,, Technology and Techniques of the Vacuum Kai Schwarzwälder, Institut für Physik Universität Basel October 6 th 2006 Outline Introduction and basics Defintion of Vacuum Vacuum A vacuum is a volume
Sputter Ion Pump (Ion Pump) By Biswajit
 Sputter Ion Pump (Ion Pump) By Biswajit 08-07-17 Sputter Ion Pump (Ion Pump) An ion pump is a type of vacuum pump capable of reaching pressures as low as 10 11 mbar under ideal conditions. An ion pump
Sputter Ion Pump (Ion Pump) By Biswajit 08-07-17 Sputter Ion Pump (Ion Pump) An ion pump is a type of vacuum pump capable of reaching pressures as low as 10 11 mbar under ideal conditions. An ion pump
DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD
 Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
( KS A ) (1) , vapour, vapor (USA) , saturation vapour pressure. , standard reference conditions for gases. , degree of saturation
 ( KS A 3014-91 ) (1), standard reference conditions for gases 0, 101325 Pa (1 =760mmHg ), vacuum, low ( rough ) vacuum 100Pa, medium vacuum 100 01 Pa, high vacuum 01 10 5 Pa, ultra high vacuum ( UHV )
( KS A 3014-91 ) (1), standard reference conditions for gases 0, 101325 Pa (1 =760mmHg ), vacuum, low ( rough ) vacuum 100Pa, medium vacuum 100 01 Pa, high vacuum 01 10 5 Pa, ultra high vacuum ( UHV )
ION Pumps for UHV Systems, Synchrotrons & Particle Accelerators. Mauro Audi, Academic, Government & Research Marketing Manager
 ION Pumps for UHV Systems, Synchrotrons & Particle Accelerators Mauro Audi, Academic, Government & Research Marketing Manager ION Pumps Agilent Technologies 1957-59 Varian Associates invents the first
ION Pumps for UHV Systems, Synchrotrons & Particle Accelerators Mauro Audi, Academic, Government & Research Marketing Manager ION Pumps Agilent Technologies 1957-59 Varian Associates invents the first
Ultra-High Vacuum Technology. Sputter Ion Pumps l/s
 Ultra-High Vacuum Technology 30-400 l/s 181.06.01 Excerpt from the Product Chapter C15 Edition November 2007 Contents General General..........................................................................
Ultra-High Vacuum Technology 30-400 l/s 181.06.01 Excerpt from the Product Chapter C15 Edition November 2007 Contents General General..........................................................................
Vacuum. Residual pressure can thwart the best cryogenic design. Each gas molecule collision carries ~kt from the hot exterior to the cold interior.
 Vacuum Residual pressure can thwart the best cryogenic design Each gas molecule collision carries ~kt from the hot exterior to the cold interior. 1 millitorr = 3.5x10¹³/cm³ Gas atoms leaving the hot surfaces
Vacuum Residual pressure can thwart the best cryogenic design Each gas molecule collision carries ~kt from the hot exterior to the cold interior. 1 millitorr = 3.5x10¹³/cm³ Gas atoms leaving the hot surfaces
Lecture 4. Ultrahigh Vacuum Science and Technology
 Lecture 4 Ultrahigh Vacuum Science and Technology Why do we need UHV? 1 Atmosphere = 760 torr; 1 torr = 133 Pa; N ~ 2.5 10 19 molecules/cm 3 Hertz-Knudsen equation p ZW 1/ 2 ( 2mk T) At p = 10-6 Torr it
Lecture 4 Ultrahigh Vacuum Science and Technology Why do we need UHV? 1 Atmosphere = 760 torr; 1 torr = 133 Pa; N ~ 2.5 10 19 molecules/cm 3 Hertz-Knudsen equation p ZW 1/ 2 ( 2mk T) At p = 10-6 Torr it
Earlier Lecture. In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide.
 41 1 Earlier Lecture In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide. Silicon diodes have negligible i 2 R losses. Cernox RTDs offer high response
41 1 Earlier Lecture In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide. Silicon diodes have negligible i 2 R losses. Cernox RTDs offer high response
Surface Analysis - The Principal Techniques
 Surface Analysis - The Principal Techniques Edited by John C. Vickerman Surface Analysis Research Centre, Department of Chemistry UMIST, Manchester, UK JOHN WILEY & SONS Chichester New York Weinheim Brisbane
Surface Analysis - The Principal Techniques Edited by John C. Vickerman Surface Analysis Research Centre, Department of Chemistry UMIST, Manchester, UK JOHN WILEY & SONS Chichester New York Weinheim Brisbane
Rough (low) vacuum : Medium vacuum : High vacuum (HV) : Ultrahigh vacuum (UHV) :
 4. UHV & Effects of Gas Pressure 4.1 What is Ultra-high Vacuum (UHV)? 4.2 Why is UHV required for Surface Studies? 4.3 Exercises - Effects of Gas Pressure Vacuum technology has advanced considerably over
4. UHV & Effects of Gas Pressure 4.1 What is Ultra-high Vacuum (UHV)? 4.2 Why is UHV required for Surface Studies? 4.3 Exercises - Effects of Gas Pressure Vacuum technology has advanced considerably over
VACUUM PUMPING METHODS
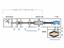 VACUUM PUMPING METHODS VACUUM PUMPS (METHODS) Positive Displacement Vacuum Gas Transfer Vacuum Kinetic Vacuum Entrapment Vacuum Adsorption Reciprocating Displacement Rotary Drag Fluid Entrainment Ion Transfer
VACUUM PUMPING METHODS VACUUM PUMPS (METHODS) Positive Displacement Vacuum Gas Transfer Vacuum Kinetic Vacuum Entrapment Vacuum Adsorption Reciprocating Displacement Rotary Drag Fluid Entrainment Ion Transfer
Vacuum for Accelerators
 Vacuum for Accelerators Oswald Gröbner Baden, 22 September 2004 1) Introduction and some basics 2) Pumps used in accelerators 3) Desorption phenomena 4) Practical examples Oswald Gröbner, Retired from
Vacuum for Accelerators Oswald Gröbner Baden, 22 September 2004 1) Introduction and some basics 2) Pumps used in accelerators 3) Desorption phenomena 4) Practical examples Oswald Gröbner, Retired from
Photoemission Spectroscopy
 FY13 Experimental Physics - Auger Electron Spectroscopy Photoemission Spectroscopy Supervisor: Per Morgen SDU, Institute of Physics Campusvej 55 DK - 5250 Odense S Ulrik Robenhagen,
FY13 Experimental Physics - Auger Electron Spectroscopy Photoemission Spectroscopy Supervisor: Per Morgen SDU, Institute of Physics Campusvej 55 DK - 5250 Odense S Ulrik Robenhagen,
To move a particle in a (straight) line over a large distance
 2.1 Introduction to Vacuum Technology 2.1.1 Importance of Vacuum Technology for Processing and Characterization Under partial vacuum conditions (pressures orders of magnitude below ambient atmospheric
2.1 Introduction to Vacuum Technology 2.1.1 Importance of Vacuum Technology for Processing and Characterization Under partial vacuum conditions (pressures orders of magnitude below ambient atmospheric
UHV - Technology. Oswald Gröbner
 School on Synchrotron Radiation UHV - Technology Trieste, 20-21 April 2004 1) Introduction and some basics 2) Building blocks of a vacuum system 3) How to get clean ultra high vacuum 4) Desorption phenomena
School on Synchrotron Radiation UHV - Technology Trieste, 20-21 April 2004 1) Introduction and some basics 2) Building blocks of a vacuum system 3) How to get clean ultra high vacuum 4) Desorption phenomena
Study of Distributed Ion-Pumps in CESR 1
 Study of Distributed Ion-Pumps in CESR 1 Yulin Li, Roberto Kersevan, Nariman Mistry Laboratory of Nuclear Studies, Cornell University Ithaca, NY 153-001 Abstract It is desirable to reduce anode voltage
Study of Distributed Ion-Pumps in CESR 1 Yulin Li, Roberto Kersevan, Nariman Mistry Laboratory of Nuclear Studies, Cornell University Ithaca, NY 153-001 Abstract It is desirable to reduce anode voltage
Previous Lecture. Electron beam lithoghraphy e - Electrons are generated in vacuum. Electron beams propagate in vacuum
 Previous Lecture Electron beam lithoghraphy e - Electrons are generated in vacuum Electron beams propagate in vacuum Lecture 6: Vacuum & plasmas Objectives From this vacuum lecture you will learn: What
Previous Lecture Electron beam lithoghraphy e - Electrons are generated in vacuum Electron beams propagate in vacuum Lecture 6: Vacuum & plasmas Objectives From this vacuum lecture you will learn: What
Ion Pump Applications
 Gamma Vacuum Gamma Vacuum designs, manufactures, and services ion pumps, titanium sublimation pumps, and their controls. We have grown to be an industry leading ion pump supplier through superior quality,
Gamma Vacuum Gamma Vacuum designs, manufactures, and services ion pumps, titanium sublimation pumps, and their controls. We have grown to be an industry leading ion pump supplier through superior quality,
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/44295 holds various files of this Leiden University dissertation. Author: Badan, C. Title: Surface-structure dependence of water-related adsorbates on platinum
Cover Page The handle http://hdl.handle.net/1887/44295 holds various files of this Leiden University dissertation. Author: Badan, C. Title: Surface-structure dependence of water-related adsorbates on platinum
CapaciTorr HV Pumps. making innovation happen,together
 CapaciTorr HV Pumps making innovation happen,together CapaciTorr HV HIGHLIGHTS General Features High pumping speed for all active gases High sorption capacity and increased lifetime Constant pumping speed
CapaciTorr HV Pumps making innovation happen,together CapaciTorr HV HIGHLIGHTS General Features High pumping speed for all active gases High sorption capacity and increased lifetime Constant pumping speed
Secondary Ion Mass Spectrometry (SIMS)
 CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
CHEM53200: Lecture 10 Secondary Ion Mass Spectrometry (SIMS) Major reference: Surface Analysis Edited by J. C. Vickerman (1997). 1 Primary particles may be: Secondary particles can be e s, neutral species
Technical description of photoelectron spectrometer Escalab 250Xi
 Technical description of photoelectron spectrometer Escalab 250Xi Resource center Physical Methods of Surface Investigations 2014 Table of contents Common description 3 Analytical chamber 8 Preparation
Technical description of photoelectron spectrometer Escalab 250Xi Resource center Physical Methods of Surface Investigations 2014 Table of contents Common description 3 Analytical chamber 8 Preparation
Ma5: Auger- and Electron Energy Loss Spectroscopy
 Ma5: Auger- and Electron Energy Loss Spectroscopy 1 Introduction Electron spectroscopies, namely Auger electron- and electron energy loss spectroscopy are utilized to determine the KLL spectrum and the
Ma5: Auger- and Electron Energy Loss Spectroscopy 1 Introduction Electron spectroscopies, namely Auger electron- and electron energy loss spectroscopy are utilized to determine the KLL spectrum and the
Vacuum II. G. Franchetti CAS - Bilbao. 30/5/2011 G. Franchetti 1
 Vacuum II G. Franchetti CAS Bilbao 30/5/2011 G. Franchetti 1 Index Creating Vacuum (continuation) Measuring Vacuum Partial Pressure Measurements 30/5/2011 G. Franchetti 2 Laminar flow Cold surface Diffusion
Vacuum II G. Franchetti CAS Bilbao 30/5/2011 G. Franchetti 1 Index Creating Vacuum (continuation) Measuring Vacuum Partial Pressure Measurements 30/5/2011 G. Franchetti 2 Laminar flow Cold surface Diffusion
NEXTorr HV 100 HIGHLIGHTS
 NEXTorr HV 100 HIGHLIGHTS General Features High pumping speed for all active gases Pumping speed for noble gases and methane High sorption capacity and increased lifetime Constant pumping speed in HV and
NEXTorr HV 100 HIGHLIGHTS General Features High pumping speed for all active gases Pumping speed for noble gases and methane High sorption capacity and increased lifetime Constant pumping speed in HV and
Surface Analysis - The Principal Techniques
 Surface Analysis - The Principal Techniques 2nd Edition Editors johnc.vickerman Manchester Interdisciplinary Biocentre, University of Manchester, UK IAN S. GILMORE National Physical Laboratory, Teddington,
Surface Analysis - The Principal Techniques 2nd Edition Editors johnc.vickerman Manchester Interdisciplinary Biocentre, University of Manchester, UK IAN S. GILMORE National Physical Laboratory, Teddington,
Experimental techniques of Surface Science and how a theorist understands them
 Experimental techniques of Surface Science and how a theorist understands them Karsten Reuter Fritz-Haber-Institut der Max-Planck-Gesellschaft Faradayweg 4-6, D-14195 Berlin reuter@fhi-berlin.mpg.de 0.
Experimental techniques of Surface Science and how a theorist understands them Karsten Reuter Fritz-Haber-Institut der Max-Planck-Gesellschaft Faradayweg 4-6, D-14195 Berlin reuter@fhi-berlin.mpg.de 0.
Vacuum Technology for Particle Accelerators
 Vacuum Technology for Particle Accelerators (Max IV laboratory) CERN Accelerator School: Introduction to Accelerator Physics 8 th October 2016, Budapest, Hungary 1 Contents What is vacuum and why do we
Vacuum Technology for Particle Accelerators (Max IV laboratory) CERN Accelerator School: Introduction to Accelerator Physics 8 th October 2016, Budapest, Hungary 1 Contents What is vacuum and why do we
Chemistry Instrumental Analysis Lecture 34. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Generation of vacuum (pumps) and measurements
 Generation of vacuum (pumps) and measurements Generation of vacuum (pumps): ᴥ pumping systems general considerations on their use and match with physical quantities introduced for the dimensioning of vacuum
Generation of vacuum (pumps) and measurements Generation of vacuum (pumps): ᴥ pumping systems general considerations on their use and match with physical quantities introduced for the dimensioning of vacuum
Scanning Tunneling Microscopy Studies of the Ge(111) Surface
 VC Scanning Tunneling Microscopy Studies of the Ge(111) Surface Anna Rosen University of California, Berkeley Advisor: Dr. Shirley Chiang University of California, Davis August 24, 2007 Abstract: This
VC Scanning Tunneling Microscopy Studies of the Ge(111) Surface Anna Rosen University of California, Berkeley Advisor: Dr. Shirley Chiang University of California, Davis August 24, 2007 Abstract: This
PHYSICAL VAPOR DEPOSITION OF THIN FILMS
 PHYSICAL VAPOR DEPOSITION OF THIN FILMS JOHN E. MAHAN Colorado State University A Wiley-Interscience Publication JOHN WILEY & SONS, INC. New York Chichester Weinheim Brisbane Singapore Toronto CONTENTS
PHYSICAL VAPOR DEPOSITION OF THIN FILMS JOHN E. MAHAN Colorado State University A Wiley-Interscience Publication JOHN WILEY & SONS, INC. New York Chichester Weinheim Brisbane Singapore Toronto CONTENTS
Jumbo Cross Beam Ionizer
 Jumbo Cross Beam Ionizer Version 1.0 September 30, 2008 Page 1 of 19 Table of Contents Cross Beam Ionizer... 1 Table of Contents... 2 1.0 Packing List... 3 1.1 Packing List for Cross Beam Ionizer... 3
Jumbo Cross Beam Ionizer Version 1.0 September 30, 2008 Page 1 of 19 Table of Contents Cross Beam Ionizer... 1 Table of Contents... 2 1.0 Packing List... 3 1.1 Packing List for Cross Beam Ionizer... 3
Repetition: Physical Deposition Processes
 Repetition: Physical Deposition Processes PVD (Physical Vapour Deposition) Evaporation Sputtering Diode-system Triode-system Magnetron-system ("balanced/unbalanced") Ion beam-system Ionplating DC-glow-discharge
Repetition: Physical Deposition Processes PVD (Physical Vapour Deposition) Evaporation Sputtering Diode-system Triode-system Magnetron-system ("balanced/unbalanced") Ion beam-system Ionplating DC-glow-discharge
CapaciTorr HV 200 HIGHLIGHTS
 CapaciTorr HV 200 General Features High pumping speed for all active gases High sorption capacity and increased lifetime Costant pumping speed in HV, UHV and XHV Reversible pumping of hydrogen and its
CapaciTorr HV 200 General Features High pumping speed for all active gases High sorption capacity and increased lifetime Costant pumping speed in HV, UHV and XHV Reversible pumping of hydrogen and its
Generation of vacuum (pumps): Vacuum (pressure) measurements:
 Generation of vacuum (pumps) p and measurements Generation of vacuum (pumps): ᴥ pumping p systems general considerations on their use and match with physical quantities introduced for the dimensioning
Generation of vacuum (pumps) p and measurements Generation of vacuum (pumps): ᴥ pumping p systems general considerations on their use and match with physical quantities introduced for the dimensioning
Advanced Lab Course. X-Ray Photoelectron Spectroscopy 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT Qualitative analysis Chemical Shifts 7
 Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Advanced Lab Course X-Ray Photoelectron Spectroscopy M210 As of: 2015-04-01 Aim: Chemical analysis of surfaces. Content 1 INTRODUCTION 1 2 BASICS 1 3 EXPERIMENT 3 3.1 Qualitative analysis 6 3.2 Chemical
Experimental 2.1 Introduction. Ultra high vacuum.
 2 Experimental 2.1 Introduction. Ultra high vacuum. The interest in solid-gas (or solid-vacuum) interfaces has been increasing rapidly during the last three decades. On one hand this is due to the practical
2 Experimental 2.1 Introduction. Ultra high vacuum. The interest in solid-gas (or solid-vacuum) interfaces has been increasing rapidly during the last three decades. On one hand this is due to the practical
k T m 8 B P m k T M T
 I. INTRODUCTION AND OBJECTIVE OF THE EXPERIENT The techniques for evaporation of chemicals in a vacuum are widely used for thin film deposition on rigid substrates, leading to multiple applications: production
I. INTRODUCTION AND OBJECTIVE OF THE EXPERIENT The techniques for evaporation of chemicals in a vacuum are widely used for thin film deposition on rigid substrates, leading to multiple applications: production
Extrel Application Note
 Extrel Application Note Real-Time Plasma Monitoring and Detection of Trace H 2 O and HF Species in an Argon Based Plasma Jian Wei, 575 Epsilon Drive, Pittsburgh, PA 15238. (Presented at the 191st Electrochemical
Extrel Application Note Real-Time Plasma Monitoring and Detection of Trace H 2 O and HF Species in an Argon Based Plasma Jian Wei, 575 Epsilon Drive, Pittsburgh, PA 15238. (Presented at the 191st Electrochemical
Deuterium Gas Analysis by Residual Gas Analyzer
 Journal of Physics: Conference Series Deuterium Gas Analysis by Residual Gas Analyzer To cite this article: B K Das et al 2012 J. Phys.: Conf. Ser. 390 012009 View the article online for updates and enhancements.
Journal of Physics: Conference Series Deuterium Gas Analysis by Residual Gas Analyzer To cite this article: B K Das et al 2012 J. Phys.: Conf. Ser. 390 012009 View the article online for updates and enhancements.
Lecture 10. Vacuum Technology and Plasmas Reading: Chapter 10. ECE Dr. Alan Doolittle
 Lecture 10 Vacuum Technology and Plasmas Reading: Chapter 10 Vacuum Science and Plasmas In order to understand deposition techniques such as evaporation, sputtering,, plasma processing, chemical vapor
Lecture 10 Vacuum Technology and Plasmas Reading: Chapter 10 Vacuum Science and Plasmas In order to understand deposition techniques such as evaporation, sputtering,, plasma processing, chemical vapor
LECTURE 5 SUMMARY OF KEY IDEAS
 LECTURE 5 SUMMARY OF KEY IDEAS Etching is a processing step following lithography: it transfers a circuit image from the photoresist to materials form which devices are made or to hard masking or sacrificial
LECTURE 5 SUMMARY OF KEY IDEAS Etching is a processing step following lithography: it transfers a circuit image from the photoresist to materials form which devices are made or to hard masking or sacrificial
Continued Work toward XHV for the Jefferson Lab Polarized Electron Source
 Continued Work toward XHV for the Jefferson Lab Polarized Electron Source Marcy Stutzman, Philip Adderley, Matt Poelker Thomas Jefferson National Accelerator Facility Newport News, VA 23601 Thomas Jefferson
Continued Work toward XHV for the Jefferson Lab Polarized Electron Source Marcy Stutzman, Philip Adderley, Matt Poelker Thomas Jefferson National Accelerator Facility Newport News, VA 23601 Thomas Jefferson
Vacuum I. G. Franchetti CAS - Bilbao. 30/5/2011 G. Franchetti 1
 Vacuum I G. Franchetti CAS - Bilbao 30/5/2011 G. Franchetti 1 Index Introduction to Vacuum Vacuum and the Beam Flow Regimes Creating Vacuum 30/5/2011 G. Franchetti 2 Vacuum in accelerators All beam dynamics
Vacuum I G. Franchetti CAS - Bilbao 30/5/2011 G. Franchetti 1 Index Introduction to Vacuum Vacuum and the Beam Flow Regimes Creating Vacuum 30/5/2011 G. Franchetti 2 Vacuum in accelerators All beam dynamics
Angular Correlation Experiments
 Angular Correlation Experiments John M. LoSecco April 2, 2007 Angular Correlation Experiments J. LoSecco Notre Dame du Lac Nuclear Spin In atoms one can use the Zeeman Effect to determine the spin state.
Angular Correlation Experiments John M. LoSecco April 2, 2007 Angular Correlation Experiments J. LoSecco Notre Dame du Lac Nuclear Spin In atoms one can use the Zeeman Effect to determine the spin state.
Vacuum System of Synchrotron radiation sources
 3 rd ILSF Advanced School on Synchrotron Radiation and Its Applications September 14-16, 2013 Vacuum System of Synchrotron radiation sources Prepared by: Omid Seify, Vacuum group, ILSF project Institute
3 rd ILSF Advanced School on Synchrotron Radiation and Its Applications September 14-16, 2013 Vacuum System of Synchrotron radiation sources Prepared by: Omid Seify, Vacuum group, ILSF project Institute
Table 1: Residence time (τ) in seconds for adsorbed molecules
 1 Surfaces We got our first hint of the importance of surface processes in the mass spectrum of a high vacuum environment. The spectrum was dominated by water and carbon monoxide, species that represent
1 Surfaces We got our first hint of the importance of surface processes in the mass spectrum of a high vacuum environment. The spectrum was dominated by water and carbon monoxide, species that represent
PHOTOELECTRON SPECTROSCOPY IN AIR (PESA)
 PHOTOELECTRON SPECTROSCOPY IN AIR (PESA) LEADERS IN GAS DETECTION Since 1977 Model AC-3 Features: Atmospheric pressure operation (unique in the world) Estimate work function, ionization potential, density
PHOTOELECTRON SPECTROSCOPY IN AIR (PESA) LEADERS IN GAS DETECTION Since 1977 Model AC-3 Features: Atmospheric pressure operation (unique in the world) Estimate work function, ionization potential, density
INTRODUCTION Strained Silicon Monochromator Magnesium Housing Windows for Monochromator Shutter and Collimator Fission Detector HOPG Monochromator
 Design for a Four-Blade Neutron Interferometer INTRODUCTION Strained Silicon Monochromator The neutron beam used for this interferometer is separated from the NIST reactor's main beam using a strained
Design for a Four-Blade Neutron Interferometer INTRODUCTION Strained Silicon Monochromator The neutron beam used for this interferometer is separated from the NIST reactor's main beam using a strained
BALKAN PHYSICS LETTERS Bogazici University Press 15 November 2016 BPL, 24, , pp , (2016)
 139 BALKAN PHYSICS LETTERS Bogazici University Press 15 November 2016 BPL, 24, 241016, pp. 139 145, (2016) VACUUM STUDIES ON ELECTRON GUN AND INJECTOR LINE OF TARLA FACILITY E. COŞGUN, E.P. DEMİRCİ, Ö.
139 BALKAN PHYSICS LETTERS Bogazici University Press 15 November 2016 BPL, 24, 241016, pp. 139 145, (2016) VACUUM STUDIES ON ELECTRON GUN AND INJECTOR LINE OF TARLA FACILITY E. COŞGUN, E.P. DEMİRCİ, Ö.
Electrical Discharges Characterization of Planar Sputtering System
 International Journal of Recent Research and Review, Vol. V, March 213 ISSN 2277 8322 Electrical Discharges Characterization of Planar Sputtering System Bahaa T. Chaid 1, Nathera Abass Ali Al-Tememee 2,
International Journal of Recent Research and Review, Vol. V, March 213 ISSN 2277 8322 Electrical Discharges Characterization of Planar Sputtering System Bahaa T. Chaid 1, Nathera Abass Ali Al-Tememee 2,
What Is Air Temperature?
 2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
2.2 Read What Is Air Temperature? In Learning Set 1, you used a thermometer to measure air temperature. But what exactly was the thermometer measuring? What is different about cold air and warm air that
CHAPTER 6: Etching. Chapter 6 1
 Chapter 6 1 CHAPTER 6: Etching Different etching processes are selected depending upon the particular material to be removed. As shown in Figure 6.1, wet chemical processes result in isotropic etching
Chapter 6 1 CHAPTER 6: Etching Different etching processes are selected depending upon the particular material to be removed. As shown in Figure 6.1, wet chemical processes result in isotropic etching
TMT4320 Nanomaterials November 10 th, Thin films by physical/chemical methods (From chapter 24 and 25)
 1 TMT4320 Nanomaterials November 10 th, 2015 Thin films by physical/chemical methods (From chapter 24 and 25) 2 Thin films by physical/chemical methods Vapor-phase growth (compared to liquid-phase growth)
1 TMT4320 Nanomaterials November 10 th, 2015 Thin films by physical/chemical methods (From chapter 24 and 25) 2 Thin films by physical/chemical methods Vapor-phase growth (compared to liquid-phase growth)
Physical Vapor Deposition
 Physical Vapor Deposition EVAPORATION SPUTTERING Typically used for metallization of semiconductors. Both Evaporation & Sputtering are done in vacuum environments. Typically: y Evaporation Pressures are
Physical Vapor Deposition EVAPORATION SPUTTERING Typically used for metallization of semiconductors. Both Evaporation & Sputtering are done in vacuum environments. Typically: y Evaporation Pressures are
The Franck-Hertz Experiment
 The Franck-Hertz Experiment 1/30/06 The Franck-Hertz experiment, first undertaken shortly after Bohr s theory of the atom was presented, provided one of the early indications that atoms had discrete energy
The Franck-Hertz Experiment 1/30/06 The Franck-Hertz experiment, first undertaken shortly after Bohr s theory of the atom was presented, provided one of the early indications that atoms had discrete energy
The Vacuum Case for KATRIN
 The Vacuum Case for KATRIN Institute of Nuclear Physics, Forschungszentrum Karlsruhe,Germany, for the KATRIN Collaboration Lutz.Bornschein@ik.fzk.de The vacuum requirements of the KATRIN experiment have
The Vacuum Case for KATRIN Institute of Nuclear Physics, Forschungszentrum Karlsruhe,Germany, for the KATRIN Collaboration Lutz.Bornschein@ik.fzk.de The vacuum requirements of the KATRIN experiment have
Gaetano L Episcopo. Scanning Electron Microscopy Focus Ion Beam and. Pulsed Plasma Deposition
 Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
Gaetano L Episcopo Scanning Electron Microscopy Focus Ion Beam and Pulsed Plasma Deposition Hystorical background Scientific discoveries 1897: J. Thomson discovers the electron. 1924: L. de Broglie propose
6.5 Optical-Coating-Deposition Technologies
 92 Chapter 6 6.5 Optical-Coating-Deposition Technologies The coating process takes place in an evaporation chamber with a fully controlled system for the specified requirements. Typical systems are depicted
92 Chapter 6 6.5 Optical-Coating-Deposition Technologies The coating process takes place in an evaporation chamber with a fully controlled system for the specified requirements. Typical systems are depicted
Hiden SIMS Secondary Ion Mass Spectrometers. Analysers for surface, elemental and molecular analysis
 Hiden SIMS Secondary Ion Mass Spectrometers Analysers for surface, elemental and molecular analysis vacuum analysis surface science plasma diagnostics gas analysis SIMS Versatility SIMS is a high sensitivity
Hiden SIMS Secondary Ion Mass Spectrometers Analysers for surface, elemental and molecular analysis vacuum analysis surface science plasma diagnostics gas analysis SIMS Versatility SIMS is a high sensitivity
MOLECULAR SURFACE PUMPING: CRYOPUMPING
 51 MOLECULAR SURFACE PUMPING: CRYOPUMPING C. Benvenuti CERN, Geneva, Switzerland Abstract Weak van der Waals attractive forces may provide molecular gas pumping on surfaces cooled at sufficiently low temperatures.
51 MOLECULAR SURFACE PUMPING: CRYOPUMPING C. Benvenuti CERN, Geneva, Switzerland Abstract Weak van der Waals attractive forces may provide molecular gas pumping on surfaces cooled at sufficiently low temperatures.
3. Experimental Methods and Instrumentation.
 3. Experimental Methods and Instrumentation. 3.1 UHV Setup An ultrahigh vacuum chamber pumped by a turbo molecular pump with a base pressure in the low 10-10 mbar range was used (figure 3.1). This chamber
3. Experimental Methods and Instrumentation. 3.1 UHV Setup An ultrahigh vacuum chamber pumped by a turbo molecular pump with a base pressure in the low 10-10 mbar range was used (figure 3.1). This chamber
Metal Deposition. Filament Evaporation E-beam Evaporation Sputter Deposition
 Metal Deposition Filament Evaporation E-beam Evaporation Sputter Deposition 1 Filament evaporation metals are raised to their melting point by resistive heating under vacuum metal pellets are placed on
Metal Deposition Filament Evaporation E-beam Evaporation Sputter Deposition 1 Filament evaporation metals are raised to their melting point by resistive heating under vacuum metal pellets are placed on
VACUUM SYSTEM FOR STANFORD STORAGE R7NG, SPEAR* U. Curmnings, N. Dean, F. Johnson, J. Jurow, J. Voss ERRATA
 SLAC-m-797 VACUUM SYSTEM FOR STANFORD STORAGE R7NG, SPEAR* U. Curmnings, N. Dean, F. Johnson, J. Jurow, J. Voss ERRATA 1. On page 4, the electron bombardment dose should be 3.6 x lc6 instead of 3.6 x 103.
SLAC-m-797 VACUUM SYSTEM FOR STANFORD STORAGE R7NG, SPEAR* U. Curmnings, N. Dean, F. Johnson, J. Jurow, J. Voss ERRATA 1. On page 4, the electron bombardment dose should be 3.6 x lc6 instead of 3.6 x 103.
Lab 5 - ELECTRON CHARGE-TO-MASS RATIO
 81 Name Date Partners Lab 5 - ELECTRON CHARGE-TO-MASS RATIO OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass ratio
81 Name Date Partners Lab 5 - ELECTRON CHARGE-TO-MASS RATIO OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass ratio
(1) E. & F. N. SPON London VACUUM MANUAL. Edited by. W. Steckelmacher. J. Yarwood. L. Holland
 Vacuum Manual VACUUM MANUAL Edited by L. Holland Formerly Director of Research Edwards High Vacuum; Associate Reader in Physics, Brunei University W. Steckelmacher Formerly Deputy Director of Research
Vacuum Manual VACUUM MANUAL Edited by L. Holland Formerly Director of Research Edwards High Vacuum; Associate Reader in Physics, Brunei University W. Steckelmacher Formerly Deputy Director of Research
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons
 Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
JARA FIT Ferienprakticum Nanoelektronik Experiment: Resonant tunneling in quantum structures
 JARA FIT Ferienprakticum Nanoelektronik 2013 Experiment: Resonant tunneling in quantum structures Dr. Mihail Ion Lepsa, Peter Grünberg Institut (PGI 9), Forschungszentrum Jülich GmbH 1. Introduction The
JARA FIT Ferienprakticum Nanoelektronik 2013 Experiment: Resonant tunneling in quantum structures Dr. Mihail Ion Lepsa, Peter Grünberg Institut (PGI 9), Forschungszentrum Jülich GmbH 1. Introduction The
Electron beam scanning
 Electron beam scanning The Electron beam scanning operates through an electro-optical system which has the task of deflecting the beam Synchronously with cathode ray tube which create the image, beam moves
Electron beam scanning The Electron beam scanning operates through an electro-optical system which has the task of deflecting the beam Synchronously with cathode ray tube which create the image, beam moves
Vacuum Technology and film growth. Diffusion Resistor
 Vacuum Technology and film growth Poly Gate pmos Polycrystaline Silicon Source Gate p-channel Metal-Oxide-Semiconductor (MOSFET) Drain polysilicon n-si ion-implanted Diffusion Resistor Poly Si Resistor
Vacuum Technology and film growth Poly Gate pmos Polycrystaline Silicon Source Gate p-channel Metal-Oxide-Semiconductor (MOSFET) Drain polysilicon n-si ion-implanted Diffusion Resistor Poly Si Resistor
Lab 5 - ELECTRON CHARGE-TO-MASS RATIO
 79 Name Date Partners OBJECTIVES OVERVIEW Lab 5 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
79 Name Date Partners OBJECTIVES OVERVIEW Lab 5 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
Lab 6 - ELECTRON CHARGE-TO-MASS RATIO
 101 Name Date Partners OBJECTIVES OVERVIEW Lab 6 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
101 Name Date Partners OBJECTIVES OVERVIEW Lab 6 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
Nanoscale Surface Physics PHY 5XXX
 SYLLABUS Nanoscale Surface Physics PHY 5XXX Spring Semester, 2006 Instructor: Dr. Beatriz Roldán-Cuenya Time: Tuesday and Thursday 4:00 to 5:45 pm Location: Theory: MAP 306, Laboratory: MAP 148 Office
SYLLABUS Nanoscale Surface Physics PHY 5XXX Spring Semester, 2006 Instructor: Dr. Beatriz Roldán-Cuenya Time: Tuesday and Thursday 4:00 to 5:45 pm Location: Theory: MAP 306, Laboratory: MAP 148 Office
Surface Defects on Natural MoS 2
 Supporting Information: Surface Defects on Natural MoS 2 Rafik Addou 1*, Luigi Colombo 2, and Robert M. Wallace 1* 1 Department of Materials Science and Engineering, The University of Texas at Dallas,
Supporting Information: Surface Defects on Natural MoS 2 Rafik Addou 1*, Luigi Colombo 2, and Robert M. Wallace 1* 1 Department of Materials Science and Engineering, The University of Texas at Dallas,
Lab 6 - Electron Charge-To-Mass Ratio
 Lab 6 Electron Charge-To-Mass Ratio L6-1 Name Date Partners Lab 6 - Electron Charge-To-Mass Ratio OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine
Lab 6 Electron Charge-To-Mass Ratio L6-1 Name Date Partners Lab 6 - Electron Charge-To-Mass Ratio OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine
MODERN TECHNIQUES OF SURFACE SCIENCE
 MODERN TECHNIQUES OF SURFACE SCIENCE Second edition D. P. WOODRUFF & T. A. DELCHAR Department ofphysics, University of Warwick CAMBRIDGE UNIVERSITY PRESS Contents Preface to first edition Preface to second
MODERN TECHNIQUES OF SURFACE SCIENCE Second edition D. P. WOODRUFF & T. A. DELCHAR Department ofphysics, University of Warwick CAMBRIDGE UNIVERSITY PRESS Contents Preface to first edition Preface to second
Thermal Coatings for In-vacuum Radiation Cooling LIGO-T C R. Abbott, S. Waldman, Caltech 12 March, 2007
 Thermal Coatings for In-vacuum Radiation Cooling LIGO-T070054-00-C R. Abbott, S. Waldman, Caltech 12 March, 2007 1. Overview and Background 1.1. There are instances in LIGO where the use of electronics
Thermal Coatings for In-vacuum Radiation Cooling LIGO-T070054-00-C R. Abbott, S. Waldman, Caltech 12 March, 2007 1. Overview and Background 1.1. There are instances in LIGO where the use of electronics
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Nova 600 NanoLab Dual beam Focused Ion Beam IITKanpur
 Nova 600 NanoLab Dual beam Focused Ion Beam system @ IITKanpur Dual Beam Nova 600 Nano Lab From FEI company (Dual Beam = SEM + FIB) SEM: The Electron Beam for SEM Field Emission Electron Gun Energy : 500
Nova 600 NanoLab Dual beam Focused Ion Beam system @ IITKanpur Dual Beam Nova 600 Nano Lab From FEI company (Dual Beam = SEM + FIB) SEM: The Electron Beam for SEM Field Emission Electron Gun Energy : 500
Studying Metal to Insulator Transitions in Solids using Synchrotron Radiation-based Spectroscopies.
 PY482 Lecture. February 28 th, 2013 Studying Metal to Insulator Transitions in Solids using Synchrotron Radiation-based Spectroscopies. Kevin E. Smith Department of Physics Department of Chemistry Division
PY482 Lecture. February 28 th, 2013 Studying Metal to Insulator Transitions in Solids using Synchrotron Radiation-based Spectroscopies. Kevin E. Smith Department of Physics Department of Chemistry Division
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
ENVG FALL ICP-MS (Inductively Coupled Plasma Mass Spectrometry) Analytical Techniques
 ENVG 60500 FALL 2013 ICP-MS (Inductively Coupled Plasma Mass Spectrometry) Analytical Techniques HISTORY In the 1940s, arc and high-voltage spark spectrometry became widely utilized for metal analysis
ENVG 60500 FALL 2013 ICP-MS (Inductively Coupled Plasma Mass Spectrometry) Analytical Techniques HISTORY In the 1940s, arc and high-voltage spark spectrometry became widely utilized for metal analysis
STRONG DOUBLE LAYER STRUCTURE IN THERMIONIC VACUUM ARC PLASMA *
 STRONG DOUBLE LAYER STRUCTURE IN THERMIONIC VACUUM ARC PLASMA * V. TIRON 1, L. MIHAESCU 1, C.P. LUNGU 2 and G. POPA 1 1 Faculty of Physics, Al. I. Cuza University, 700506, Iasi, Romania 2 National Institute
STRONG DOUBLE LAYER STRUCTURE IN THERMIONIC VACUUM ARC PLASMA * V. TIRON 1, L. MIHAESCU 1, C.P. LUNGU 2 and G. POPA 1 1 Faculty of Physics, Al. I. Cuza University, 700506, Iasi, Romania 2 National Institute
= 6 (1/ nm) So what is probability of finding electron tunneled into a barrier 3 ev high?
 STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
.Fritjaf Capra, The Tao of Physics
 Probing inside the atom and investigating its structure, science transcended the limits of our sensory imagination. From this point on, it could no longer rely with absolute certainty on logic and common
Probing inside the atom and investigating its structure, science transcended the limits of our sensory imagination. From this point on, it could no longer rely with absolute certainty on logic and common
Effect of Spiral Microwave Antenna Configuration on the Production of Nano-crystalline Film by Chemical Sputtering in ECR Plasma
 THE HARRIS SCIENCE REVIEW OF DOSHISHA UNIVERSITY, VOL. 56, No. 1 April 2015 Effect of Spiral Microwave Antenna Configuration on the Production of Nano-crystalline Film by Chemical Sputtering in ECR Plasma
THE HARRIS SCIENCE REVIEW OF DOSHISHA UNIVERSITY, VOL. 56, No. 1 April 2015 Effect of Spiral Microwave Antenna Configuration on the Production of Nano-crystalline Film by Chemical Sputtering in ECR Plasma
Mass Spectrometry in MCAL
 Mass Spectrometry in MCAL Two systems: GC-MS, LC-MS GC seperates small, volatile, non-polar material MS is detection devise (Agilent 320-MS TQ Mass Spectrometer) Full scan monitoring SIM single ion monitoring
Mass Spectrometry in MCAL Two systems: GC-MS, LC-MS GC seperates small, volatile, non-polar material MS is detection devise (Agilent 320-MS TQ Mass Spectrometer) Full scan monitoring SIM single ion monitoring
Peltier Application Note
 Peltier Application Note Early 19th century scientists, Thomas Seebeck and Jean Peltier, first discovered the phenomena that are the basis for today s thermoelectric industry. Seebeck found that if you
Peltier Application Note Early 19th century scientists, Thomas Seebeck and Jean Peltier, first discovered the phenomena that are the basis for today s thermoelectric industry. Seebeck found that if you
Revision Guide. Chapter 7 Quantum Behaviour
 Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
Auger Electron Spectroscopy (AES)
 1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
EXPERIMENT 5. The Franck-Hertz Experiment (Critical Potentials) Introduction
 EXPERIMENT 5 The Franck-Hertz Experiment (Critical Potentials) Introduction In the early part of the twentieth century the structure of the atom was studied in depth. In the process of developing and refining
EXPERIMENT 5 The Franck-Hertz Experiment (Critical Potentials) Introduction In the early part of the twentieth century the structure of the atom was studied in depth. In the process of developing and refining
Investigation #9 OBSERVATION OF THE PHOTOELECTRIC EFFECT
 Name: Investigation #9 Partner(s): OBSERVATION OF THE PHOTOELECTRIC EFFECT As mentioned in the previous investigation, one well-known phenomenon that defied explanation based on the well-established theories
Name: Investigation #9 Partner(s): OBSERVATION OF THE PHOTOELECTRIC EFFECT As mentioned in the previous investigation, one well-known phenomenon that defied explanation based on the well-established theories
Cryogenic Engineering Prof. M. D. Atrey Department of Mechanical Engineering Indian Institute of Technology, Bombay
 Cryogenic Engineering Prof. M. D. Atrey Department of Mechanical Engineering Indian Institute of Technology, Bombay Lecture No. # 41 Instrumentation in Cryogenics (Refer Slide Time: 00:31) So, welcome
Cryogenic Engineering Prof. M. D. Atrey Department of Mechanical Engineering Indian Institute of Technology, Bombay Lecture No. # 41 Instrumentation in Cryogenics (Refer Slide Time: 00:31) So, welcome
20.2 Ion Sources. ions electrospray uses evaporation of a charged liquid stream to transfer high molecular mass compounds into the gas phase as MH n
 20.2 Ion Sources electron ionization produces an M + ion and extensive fragmentation chemical ionization produces an M +, MH +, M +, or M - ion with minimal fragmentation MALDI uses laser ablation to transfer
20.2 Ion Sources electron ionization produces an M + ion and extensive fragmentation chemical ionization produces an M +, MH +, M +, or M - ion with minimal fragmentation MALDI uses laser ablation to transfer
Chapter Six: X-Rays. 6.1 Discovery of X-rays
 Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
X- ray Photoelectron Spectroscopy and its application in phase- switching device study
 X- ray Photoelectron Spectroscopy and its application in phase- switching device study Xinyuan Wang A53073806 I. Background X- ray photoelectron spectroscopy is of great importance in modern chemical and
X- ray Photoelectron Spectroscopy and its application in phase- switching device study Xinyuan Wang A53073806 I. Background X- ray photoelectron spectroscopy is of great importance in modern chemical and
