Calcium Coding and Adaptive Temporal Computation in Cortical Pyramidal Neurons
|
|
|
- Beatrix Preston
- 6 years ago
- Views:
Transcription
1 Calcium Coding and Adaptive Temporal Computation in Cortical Pyramidal Neurons XIAO-JING WANG Center for Complex Systems and Department of Physics, Brandeis University, Waltham, Massachusetts Wang, Xiao-Jing. Calcium coding and adaptive temporal com- while the Poisson input is totally uncorrelated in time. Possible putation in cortical pyramidal neurons. J. Neurophysiol. 79: functional implications of these results are discussed , In this work, we present a quantitative theory of temporal spike-frequency adaptation in cortical pyramidal cells. Our model pyramidal neuron has two-compartments (a INTRODUCTION soma and a dendrite ) with a voltage-gated Ca 2/ conduc- Cortical neurons display a large repertoire of voltage- and tance (g Ca )andaca 2/ -dependent K / conductance (g AHP ) located calcium-gated potassium ion channels with kinetic time conat the dendrite or at both compartments. Its frequency-current relations are comparable with data from cortical pyramidal cells, stants ranging from milliseconds to seconds ( Llinás 1988; and the properties of spike-evoked intracellular [Ca 2/ ] transients Rudy 1988; Storm 1990). The diversity and richness of K / are matched with recent dendritic [Ca 2/ ] imaging measurements. conductances indicate that they likely contribute to neuronal Spike-frequency adaptation in response to a current pulse is charturing the waveform of action potentials or regulating the input-output computation in ways more complex than sculpacterized by an adaptation time constant t adap and percentage adaptation of spike frequency F adap [% (peak 0 steady state)/ overall membrane excitability. For example, slow K / curpeak]. We show how t adap and F adap can be derived in terms of rents, in interplay with Ca 2/ and/orna / currents, can generthe biophysical parameters of the neural membrane and [Ca 2/ ] ate rhythmic firing patterns intrinsic to single neurons (Llidynamics. Two simple, experimentally testable, relations be- nás 1988; Wang and Rinzel 1995). Or a slowly inactivating tween t adap and F adap are predicted. The dependence of t adap and K / current can integrate synaptic inputs in a temporal-his- F adap on current pulse intensity, electrotonic coupling between tory dependent manner ( Storm 1988; Turrigiano et al. 1996; the two compartments, g AHP as well the [Ca 2/ ] decay time constant t Wang 1993). Moreover, K / channels at dendritic sites are Ca, is assessed quantitatively. In addition, we demonstrate that the intracellular [Ca 2/ ] signal can encode the instantaneous capable of modifying cable properties and may regulate syn- neuronal firing rate and that the conductance-based model can aptic transmission ( Hoffman et al. 1997) and prevent input be reduced to a simple calcium-model of neuronal activity that saturation ( Bernander et al. 1994; Wilson 1995). faithfully predicts the neuronal firing output even when the input Spike-frequency adaptation that depends on a Ca 2/ -gated varies relatively rapidly in time (tens to hundreds of millisec- K / conductance is a conspicuous neuronal firing characterisonds). Extensive simulations have been carried out for the model tic exhibited by a majority of ( regular spiking ) pyramidal neuron with random excitatory synaptic inputs mimicked by a neurons in neocortex and hippocampus ( Avoli et al. 1994; Poisson process. Our findings include 1) the instantaneous firing Connors et al. 1982; Foehring et al. 1991; Gustafsson and frequency (averaged over trials) shows strong adaptation similar Wigström 1981; Lanthorn et al. 1984; Lorenzon and Foehrto the case with current pulses; 2) whentheg AHP is blocked, the ing 1992; Mason and Larkman 1990; McCormick et al. dendritic g Ca could produce a hysteresis phenomenon where the neuron is driven to switch randomly between a quiescent state 1985). In response to a constant current pulse, the firing and a repetitive firing state. The firing pattern is very irregular frequency of an adapting neuron is initially high then de- with a large coefficient of variation of the interspike intervals creases to a lower steady-state plateau level within hundreds (ISI CV ú 1). The ISI distribution shows a long tail but is not of milliseconds. This phenomenon has been studied intenbimodal. 3) By contrast, in an intrinsically bursting regime (with sively in in vitro slice experiments (as is the case for all different parameter values), the model neuron displays a random afore-cited references). Recently, Ahmed et al. ( 1993; B. temporal mixture of single action potentials and brief bursts of Ahmed, C. Anderson, R. J. Douglas; K.A.C. Martin, unpubspikes. Its ISI distribution is often bimodal and its power spec- lished results) observed and quantified spike-frequency adtrum has a peak. 4) The spike-adapting current I AHP, as delayed aptation of in vivo cortical neurons with intracellular reinhibition through intracellular Ca 2/ accumulation, generates a cordings from the primary visual cortex of the anesthetized forward masking effect, where a masking input dramatically cat. They found that when subjected to a injected current reduces or completely suppresses the neuronal response to a pulse, the adaptation time course of cortical cells can be subsequent test input. When two inputs are presented repetitively fitted empirically by an exponential time course ( Ahmed et in time, this mechanism greatly enhances the ratio of the real. 1993; unpublished results), i.e., the instantaneous firing sponses to the stronger and weaker inputs, fulfilling a cellular form of lateral inhibition in time. 5) The[Ca 2/ ]-dependent I AHP rate f (t) Å f ss / ( f 0 0 f ss ) exp(0t/t adap ), where f 0 is the provides a mechanism by which the neuron unceasingly adapts initial firing rate, f ss is the steady-state firing rate, and t adap to the stochastic synaptic inputs, even in the stationary state is an adaptation time constant. Thus this time course is following the input onset. This creates strong negative correlations between output ISIs in a frequency-dependent manner, adaptation of firing frequency F adap Å ( f 0 0 f ss )/f 0. characterized by two quantities: t adap and the percentage Ahmed /98 $5.00 Copyright 1998 The American Physiological Society 1549
2 1550 X.-J. WANG et al. (1993; unpublished results) found that t adap Å suppressed. Furthermore, if the input consists of temporally ms and F adap Å 50 70% with a significant difference between uncorrelated excitatory postsynaptic potentials ( EPSPs) ( a superficial and deep layer neurons. They also per- Poisson process), spike-frequency adaptation leads to strong formed computer simulations that reproduced many of their anticorrelation between the consecutive interspike intervals observations. of the output spike train. These results indicate that the adaptation In this modeling work, we present a quantitative study mechanism is operative even in the stationary state of spike-frequency adaptation temporal dynamics, which, in after the input onset, and suggest a direct means to assess particular, yields analytical expressions for t adap and F adap in its efficacy from extracellularly recorded spike trains. terms of the cellular biophysical parameters. We also explore possible implications of this phenomenon in the real-time METHODS input-output computation of cortical neurons. Compared with an early quantitative work modeling spike-frequency Model adaptation in motoneurons by Baldsissera and Gustafsson The neuron model has two compartments, representing the den- ( 1974), the present study benefitted from a number of recent drite and the soma plus axonal initial segment, respectively ( Pinsky experimental findings and quantitative data about the cellular and Rinzel 1994). Many of our results can be obtained with a mechanisms underlying the spike-frequency adaptation phe- single compartment. However, we used a two-compartment model nomenon. First, it is well known that the spike-frequency for three reasons. 1) Ca 2/ imaging measurements from pyramidal adaptation is produced mainly by a voltage-independent, cells show spike-evoked [Ca 2/ ] transient that is much larger at Ca 2/ -dependent K / current, although other K / currents the dendrite than at the soma (Jaffe et al. 1994; Schiller et al. (such as the M current) also are involved to a lesser degree 1995; Svoboda et al. 1997; Yuste et al. 1994), and the [Ca 2/ ] influx is produced primarily by Ca 2/ entry through voltage-gated ( Madison and Nicoll 1984; Madison et al. 1987; McCormick channels (Miyakawa et al. 1992). In the present model, we focus and Williamson 1989). This current is associated with the mainly on spike-frequency adaptation that is caused by a dendritic slow after hyperpolarization (AHP) after a burst of spikes, [Ca 2/ ]-dependent I AHP. 2) We wanted to see whether our theoretihence is called the AHP current (I AHP ) (Hotson and Prince cal analysis can be carried out even with two (or more) compart- 1980; Lancaster and Adams 1986; Schwindt et al. 1988). ments. When I AHP is present both at soma and dendrite, we show Second, it has been demonstrated by photolytic manipulation that there are two calcium modes and the spike-frequency adaptation of Ca 2/ that the intrinsic gating of I AHP is rapid; its slow time course should be described as a sum of two exponenof activation is thus attributable to the kinetics of the cyto- tials. And 3) with an appropriate choice of parameters, the neuron plasmic calcium concentration [Ca 2/ ] (Lancaster and model displays burst firing patterns that require weak electrotonic Zucker 1994). Third, spike-evoked [Ca 2/ ] transients now interactions between the two compartments. The dendritic compartment has a high-threshold calcium current can be measured by fluorescence imaging techniques ( see (L type), I Ca, and a calcium-dependent potassium current I AHP. Yuste and Tank 1996 for a review). Recently, to overcome The somatic compartment contains spike generating currents (I Na the problem that a [Ca 2/ ] indicator dye like Fura-2 is also and I K ) and possibly also I Ca and I AHP. The somatic and dendritic a[ca 2/ ] buffer, Helmchen et al. (1996) used increasingly membrane potentials V s and V d obey the following current-balance low concentrations of Fura-2 and, by extrapolation to zero equations dye concentration, obtained measurements of putatively indv s C m Å0I L 0I Na 0 I K 0 I Ca 0 I AHP 0 g c dt p (V s 0 V d ) / I (1) trinsic [Ca 2/ ] transient signals. Their estimated spikeevoked [Ca 2/ ] transient from dendrites of cortical pyramidal neurons are larger and faster than previously reported. In g c (1 0 p) (V d 0 V s ) 0 I syn (2) dv d C m Å0I L 0I Ca 0 I AHP 0 the model pyramidal neuron of the present paper, the calcium dt dynamics (spike-evoked influx and decay) is constrained by where C m Å 1 mf/cm 2 and I L Å g L (V 0 V L ) is the leak current. accurate measurements of Helmchen et al. ( 1996). Following Pinsky and Rinzel ( 1994), we express the current flows Can calcium signaling perform interesting sensory compu- between the soma and dendrite [proportional to (V s 0 V d )] in tation in cortical neurons? In previous computational models, microamperes per square centimeter, with the coupling conducspike-frequency adaptation has been incorporated as a gain tance g c Å 2 ms/cm 2, and the parameter p Å somatic area/total control mechanism for neuronal excitability ( Barkai and area Å 0.5. Other, voltage-gated currents are described below. The Hasselmo 1994; Douglas et al. 1995). However, the adaptasoma or by a random synaptic input I syn of the a-amino-3-hydroxy- cell is either excited by an injected current I (in ma/cm 2 ) to the tion temporal dynamics, i.e., its role in moment-to-moment neural computation in response to time-varying inputs, has 5-methyl-4-isoxazolepropionic acid type to the dendrite. I syn Å not been emphasized. Through spike-frequency adaptation, g syn s(v 0 E syn ); the gating variable s obeys the equation ds/dt Å h(t) 0 s/t s, where h(t) is a Poisson point-process with a rate l, [Ca 2/ ] dynamics produces a forward masking phenome- E syn Å 0mV,t s Å0.5 ms, and g syn Å 0.08 ms/cm 2 (if present). non: the neuronal response to a stimulus may be masked The voltage-dependent currents are described by the Hodgkindue to another stimulus that precedes it in time as was dem- Huxley formalism (Hodgkin and Huxley 1952). Thus a gating onstrated experimentally in the cricket auditory neurons ( So- variable x satisfies a first-order kinetics bel and Tank 1994). Results reported here suggest that such dx an effect also exists in cortical pyramidal cells and may be dt Å f x[a x (V )(1 0 x) 0 b x (V)x] Å f x [x (V) 0 x]/t x (V) (3) used to selectively respond to temporal input patterns. In particular, when two or several competing inputs are pre- The sodium current I Na Å g Na m 3 (V )h(v 0 V Na ), where the fast sented, the neuronal output is sharply tuned to the strong- activation variable is replaced by its steady state, m Å a m /(a m / est input, and the responses to weaker inputs are greatly b m ), a m Å00.1(V / 33)/ {exp[00.1(v / 33)] 0 1}, b m Å
3 4 exp[0(v / 58)/12], a h Å 0.07 exp[0(v / 50)/10], and b h Å 1/{exp[00.1(V / 20)] / 1}. The delayed rectifier I K Å g K n 4 (V 0 V K ), where a n Å 00.01(V / 34)/ {exp[00.1(v / 34)] 0 1}, and b n Å exp[0(v / 44)/25]. The temperature factor f h Å f n Å 4. The high-threshold calcium current I Ca Å g Ca m (V 0 V Ca ), where m is replaced by its steady-state m (V ) Å 1/{1 / exp[0(v / 20)]/ 9} ( Kay and Wong 1987). The voltage-independent, calcium-activated potassium current I AHP Å g AHP [[Ca 2/ ]/([Ca 2/ ] / K D )](V 0 V K ), with K D Å 30 mm. The intracellular calcium concentration [Ca 2/ ] is assumed to be governed by a leaky-integrator (Helmchen et al. 1996; Tank et al. 1995; Traub 1982) d[ca 2/ ] Å0aI Ca 0 [Ca 2/ ]/t Ca (4) dt where a is proportional to S/V with S being the membrane area and V the volume immediately beneath the membrane ( Yamada et al. 1989). We used a Å [in mm (msma) 01 cm 2 ] so that the [Ca 2/ ] influx per spike is Ç200 nm (Helmchen et al. 1996). The various extrusion and buffering mechanisms are described collectively by a first-order decay process with a time constant t Ca Å 80 ms (Helmchen et al. 1996; Markram et al. 1995; Svoboda et al. 1997). The parameter values for the spike-generating currents and the I AHP were chosen so that the model displayed the initial as well as the adapted f-i curves similar to the data of McCormick et al. (1985) and Mason and Larkman (1990): g L Å 0.1, g Na Å 45, g K Å 18, dendritic g Ca Å 1, and g AHP Å 5 (in ms/cm 2 ); V L Å065, V Na Å/55, V K Å080, V Ca Å/120 (in mv). The somatic g Ca and g AHP are zero except for Fig. 7. The resting state (with I Å 0 and g syn Å 0) is at V s Å064.8 and V d Å064 mv. The model was simulated on a Silicon Graphics Workstation, using a fourth-order Runge-Kutta method, with time step dt Å ms. CALCIUM COMPUTATION OF PYRAMIDAL NEURONS 1551 Statistical analysis With Poisson synaptic inputs, each random output train of spike times is converted into a sequence of interspike intervals {t 1, t 2, t 3,...,t N }. The interspike interval (ISI) histogram is tabulated, with a mean m and a standard deviation s given by m Å 1 N N 1 N t n ; s Å N (t n 0 m) 2 FIG. 1. Spike-frequency adaptation characteristics. A: an example of nå1 nå1 spike-frequency adaptation in response to a current pulse. Adaptation is accompanied by a gradual increase of the fast spike afterhyperpolarization The ISI variability is quantified by the coefficient of variation (AHP; top, inset). Each action potential generates a [Ca 2/ ] influx of Ç200 nm (bottom, inset), and the adaptation time course follows that of [Ca 2/ ] (CV) (Softky and Koch 1993; Tuckwell 1988) (hence I AHP ) accumulation. Slow AHP after the spike firing mirrors the CV Å s/m (6) [Ca 2/ ] decay process. B: 1st, 3rd, and 5th instantaneous firing rates and the steady-state firing rate vs. the applied current intensity (left). Initial f- The statistical interdependence between consecutive ISIs is mea- I curves are nonlinear, but the steady-state f-i relation is essentially linear. sured by the coefficient of correlation (CC) (Perkel et al. 1967; Plateau [Ca 2/ ] level is a linear function of the steady-state firing rate, with Tuckwell 1988) a slope of Ç13 nm/hz (right). N01 1 subroutine Spctrm.c from Numerical Recipes ( Press et al. 1989), CC Å»(t n/1 0 m)(t n 0 m) N 0 1 (t n/1 0 m)(t n 0 m) nå1 Å (7) modified by Yinghui Liu. s 2 s 2 which is between 01 and /1. The CC can be interpreted as follows. RESULTS Suppose that we have an ISI return map, where t n/1 is plotted against t n. To assess how ISIs (t n/1 ) depend on their preceding Time course of spike-frequency adaptation values (t n ), we calculate the conditional average of t n/1 for each given t In response to a depolarizing current pulse, the model n,»t n/1 Ét n. If this function of t n is linear, then its slope is the same as the CC (see APPENDIX A for a derivation of this neuron initially fires at a high frequency, then adapts to statement). a lower steady-state frequency ( Fig. 1A). Spike-frequency The temporal correlations are further assessed by the power adaptation is accompanied by a gradual increase of the fast spectrum of the spike train (with a time bin of 1 ms), using the spike AHP (from 053 to 057 mv, see Fig. 1A, inset). This
4 1552 X.-J. WANG firing pattern is in parallel with the time course of Ca 2/ accumulation, at a rate of á200 nm/spike [comparable with the [Ca 2/ ] imaging measurements from proximal apical dendrites of cortical layer V pyramidal cells ( Helmchen et al. 1996)]. The I AHP increases with [Ca 2/ ], hence the cell is gradually hyperpolarized and the firing frequency is decreased in time. In the steady state, an equilibrium is reached in the [Ca 2/ ] dynamics, when the spike-evoked [Ca 2/ ] influx rate is balanced with the [Ca 2/ ] decay rate. After the current pulse, there is a long-lasting AHP that mirrors the Ca 2/ (hence the I AHP ) decay (Fig. 1A). Frequency-current f-i curves are shown in Fig. 1B ( left), for the initial first, third, and fifth interspike intervals, as well as the steady state. At the onset of repetitive firing ( rheobase I á 0.5), the firing frequency starts at zero, through a homoclinic bifurcation of the saddle-node type (see also Crook et al. 1997). It is noticeable that the initial f-i curves are quite nonlinear, but the steady-state f-i relation is very close to linear, similar to regular spiking pyramidal neurons (cf. Figs. 8 9 in Mason and Larkman 1990). Intuitively, the adapting AHP-current provides a delayed negative feedback to the cell. It is larger at higher firing frequencies, thus the difference between the initial f 0 and the final f ss increases with the current intensity. As a result, the steadystate input-output relation is linearized by the I AHP. We also computed the mean dendritic [Ca 2/ ]asiwas varied. Its steady-state plateau level depends linearly on f ss (Fig. 1B, right), with a slope á 17 nm/hz (compared with the measured 16 nm/hz from dendrites of layer V pyramidal cells) (Helmchen et al. 1996). In this sense, the dendritic Ca 2/ level encodes the neuronal firing activity ( Helmchen et al. 1996; Johnston 1996). For the spike train in Fig. 1, the instantaneous firing rate f (t) is defined as the reciprocal of ISIs (Fig. 2A, top). Its time course can be well fitted by a mono-exponential curve, f (t) Å A / B exp(0t/t adap ), where A Å f ss, and B Å f 0 0 FIG. 2. Theoretical derivation of the spike-frequency adaptation time f ss. The empirical best fit is given by f (t) Å 116 / 156 course. A: instantaneous firing rate [ f Å 1/interspike interval (ISI)] and exp(0t/33). Thus t [Ca 2/ adap Å 33 ms, and the percentage adaptacurve: empirical best fit. Blue curve: computed using a square-root fit for ] as function of time. Red curves: linear theory predictions. Green tion F adap Å ( f 0 0 f ss )/f 0 Å B/(A / B) Å 57%. The [Ca 2/ ] the f-vs.-[ca 2/ ] relation (see B). B: neuronal firing rate as function of also follows an exponential time course with the same time [Ca 2/ ]., data obtained when [Ca 2/ ] was varied as a parameter., data constant t adap, the steady-state plateau is [Ca 2/ ] ss Å 1.74 from A with f plotted against [Ca 2/ ] averaged over each individual ISI. mm. Note that t adap is much shorter than the decay time Two data sets yield a same curve, demonstrating that the functional depen- constant t dence of f on [Ca 2/ ] can be obtained with [Ca 2/ Ca Å 80 ms. We now show how this adaptation ] varied as a parameter. Red curve: linear regression fit; blue curve: square-root fit. C: average I time course, given a constant current pulse, can be predicted Ca as function of [Ca 2/ ], fitted by a straight line (red). In B and C, the dashed quantitatively from the biophysics of the membrane dynam- vertical lines indicate the plateau [Ca 2/ ] level in A. ics Eqs We shall see further that this description leads to a calcium-coding model of neuronal output, even when mine the functional [Ca 2/ ] dependence of the firing frethe input varies temporally. quency f ([Ca 2/ ]) as well as other voltage-dependent quanti- The fast-slow variable dissection method (Baer et al. ties like»i Ca, where»x denotes an average of x over a 1995; Ermentrout 1994; Guckenheimer et al. 1997; Rinzel typical ISI. The functional [Ca 2/ ] dependence of f ([Ca 2/ ]) 1987; Wang and Rinzel 1995) is based on the observation and of»i Ca was found to be approximately linear (Figs. 2, that Ca 2/ evolves much more slowly than the membrane B and C) potential and the other channel gating variables of the model f á f 0 0 G f [Ca 2/ ];»I CA á»i Ca 0 / G cc [Ca 2/ ] (8) and that this slow [Ca 2/ ](t) determines the adaptation time course. Thus we can first analyze the fast subsystem and where f 0 Å 271 Hz is the initial firing frequency, and G f Å the slow [Ca 2/ ] dynamics separately then put them back 0df /d[ca 2/ ] Å 84 (in Hz/mM) is a negative-feedback together. This is done in three steps (see APPENDIX B for gain parameter for the firing frequency.»i Ca 0 Å028.8 details). In step 1, the fast electrical subsystem is analyzed while considering the slow [Ca 2/ ] as if it was a static parameter rather than a dynamical variable. This allows us to deter- ma/cm 2 is the initial»i Ca, G cc Å d»i Ca /d[ca 2/ ] Å 10 (in ma/cm 2 mm) is a negative-feedback gain parameter for the [Ca 2/ ]-dynamics (Ahmed et al., unpublished results), and
5 ag cc (in 1/ms) is the rate at which the [Ca 2/ ] influx is reduced by [Ca 2/ ] itself. In step 2, we turn to the [Ca 2/ ] dynamics Eq. 4. Because it does not vary a lot within a sufficiently short ISI, I Ca is substituted by»i Ca, which is a function of [Ca 2/ ]. The resulting equation now only depends on [Ca 2/ ] with d[ca 2/ ] dt Solving Eq. 9, we obtain CALCIUM COMPUTATION OF PYRAMIDAL NEURONS 1553 Å (0a»I Ca 0 ) 0 [Ca 2/ ]/t adap (9) 1 Å ag cc / 1 (10) t adap t Ca [Ca 2/ ](t) Å [Ca 2/ ] ss [1 0 exp(0t/t adap )] (11) with [Ca 2/ ] ss Å0a»I Ca 0 t adap. Note that the adaptation time constant t adap (Eq. 10) is always smaller than t Ca due to the presence of the negative feedback term ag cc. For instance, with a Å and G cc Å 10, ag cc Å 0.02 is larger than 1/t Ca Å , hence contributes more to t adap.we have t adap Å 30.8 ms, whereas t Ca Å 80 ms; and [Ca 2/ ] ss Å 1.77 mm. Finally, in step 3, we insert the time course for [Ca 2/ ] into the expression f Å f 0 0 G f [Ca 2/ ], which yields the adaptation process for the firing frequency as function of time f (t) Å f ss / ( f 0 0 f ss )exp(0t/t adap ) Å 122 / 149 exp(0t/30.8) (12) with f ss Å f 0 0 G f [Ca 2/ ] ss. The theoretically predicted time course for spike-frequency adaptation is shown in Fig. 2A (red curve), which compares well with the empirical fit FIG. 3. Dependence on applied current intensity I. A: f 0, G f,»i Ca 0, and (green curve). However, there is a small discrepancy betion time constant t adap increases, and the percentage adaptation F adap de- G cc as functions of I., empirical fitting functions of Eq. 15. B: adaptatween the numerical and predicted f ss. This is mainly due to creases, with I. C: examples with 4 current intensities (indicated on the the fact that f ([Ca 2/ ]) is not exactly linear. Indeed, f goes right), the smooth curves are theoretical predictions. to zero at a critical value of [Ca 2/ ] á 2.2 mm (when the I AHP becomes too strong) via a homoclinic bifurcation of d[ca 2/ ]/dt Å0a(»I Ca 0 (I) / G cc (I)[Ca 2/ ]) 0 [Ca 2/ ]/t Ca (14) the saddle-node type. The mathematical theory of such a bifurcation predicts that, near the bifurcation, f ([Ca 2/ ]) berectifying function [x] / Å x if x 0, and 0 otherwise. The where the firing rate is always positive by using the half- haves as a square-root function of [Ca 2/ ] (see Guckendependence on the current intensity I of the four quantities heimer et al. 1997; Rinzel and Ermentrout 1987). We found that a square-root function fits well even the global f 0, G f,»i Ca 0, and G cc are shown in Fig. 3A. We observe f ([Ca 2/ ]): f([ca 2/ ]) Å [Ca 2/ ], except that t adap is larger and F adap is smaller with larger current near the bifurcation threshold ( Fig. 2B, blue curve). Using intensities ( see also Ahmed et al. unpublished results). This this nonlinear expression for f ([Ca 2/ ]) and the same is because at higher I, spike width becomes slightly nar- [Ca 2/ ](t) as before, f (t) can now be very accurately precurves in Fig. 3A can be fitted reasonably well by logarith- rower, hence Ca 2/ influx per spike is reduced. All four dicted (Fig. 2A, blue curve). In the following, we shall limit ourselves to the linear approximation. mic functions Note that the percentage adaptation f 0 (I) Å 9[ln (I/0.3)] / G f (I) Å [ln (I/0.3)] / F adap Å ( f 0 0 f ss )/f 0 Å (0a»I Ca 0 / f 0 )G f t adap (13)»I Ca 0 (I) Å084[ln (I/0.3)] / G cc (I) Å [ln (I/0.3)] / (15) where I 0 Å 0.3 is the estimated rheobase (The actual rheo- base is somewhat larger, I 0 á 0.5). Note that such a curvefitting is purely empirical and is not based on theoretical grounds (see also Agin 1964; Koch et al. 1995). Equations 14 and 15 completely describe the calcium model of neuronal activity. For this model to be useful, it should be able to predict the output firing rate f (t), even when the input current I(t) varies temporally in an arbitrary fashion. In Helmchen et al. (1996), the dendritic [Ca 2/ ] which depends on the [Ca 2/ ] dynamics and the I AHP only through the factor G f t adap. Calcium model of neuronal activity We have described above how to reduce the biophysical membrane model of the neuron to a calcium-model, for a given applied current I f (t) Å [ f 0 (I) 0 G f ( I)[Ca 2/ ]] /
6 1554 X.-J. WANG signal was shown to encode firing frequency of a cortical layer V pyramidal neuron, when the input changed at the time scale of seconds. Intuitively, one expects that the calcium-model of neuronal discharges to be valid only when the temporal change of I(t)is slower than both the individual ISIs and the [Ca 2/ ] dynamics. Because of the adapting I AHP, the effective [Ca 2/ ] time constant is given by t adap, typically much shorter than t Ca. Hence, one can expect that the [Ca 2/ ] code could remain effective even for input changes much more rapidly than in seconds. An example is shown in Fig. 4. Driven by a chaotic input current I(t) (Fig. 4, bottom), the membrane potential fires spikes irregularly (Fig. 4, top). The instantaneous firing rate ( the reciprocal of the ISI) and [Ca 2/ ] as function of time are shown in Fig. 4, middle (in blue), superimposed with the predictions from the calciummodel ( in red). This example suggests that the calcium model can indeed predict the instantaneous firing rate, even for relatively rapid time changes ( within tens to hundreds of milliseconds) of the input current. Dependence on the electrotonic coupling g c We next consider how the spike-frequency adaptation properties depend on the various biophysical parameters of the model. First, the spike-frequency adaptation is influenced strongly by the electrotonic coupling g c, which controls the two-way current flow between the somatic and dendritic compartments. An example is illustrated in Fig. 5A, with two different values of g c and a same current pulse to the soma. With a larger g c, there is greater current loss to the dendrite, hence the initial firing frequency is lower (Fig. 5A, left). On the other hand, the dendritic membrane potential repolarizes more rapidly after the somatic spike, the dendritic spike width is narrower, and the [Ca 2/ ] influx per spike is reduced (Fig. 5A, right). This leads to a slower [Ca 2/ ] accumulation, larger t adap, and smaller percentage adaptation F adap (Fig. 5B). As in the case of varying the applied current I (Fig. 3B), the two quantities t adap and F adap change in an antagonistic manner ( faster adaptation time course is correlated with a greater degree of adaptation). On the other hand, burst firing of spikes can be observed when the electrotonic coupling g c between the two compartments is sufficiently small, and the dendritic membrane area is significantly larger than the somatic one so that the coupling is asymmetric and the soma-to-dendrite influence is weak ( Mainen and Sejnowski 1996; Pinsky and Rinzel 1994; Rhodes and Gray 1994; Traub 1982; Traub et al. 1994). Similar to the intrinsically bursting pyramidal cells in layer V neocortex ( Kim and Connors 1993; Mason and Larkman FIG. 4. Calcium coding of neuronal electrical activity. In response to a 1990; McCormick et al. 1985; Nishimura et al. 1996), with temporally varying input I(t) (bottom), the cell s firing (blue dots, middle moderate current intensity the model neuron fires doubles top) and [Ca 2/ ] time course (blue curve, middle bottom) are well predicted by the reduced calcium model Eqs. 14 and 15 (red curves). of spikes repetitively at low frequencies (Ç4 Hz); and (more typically) current injection of higher intensities elicits an initial burst of spikes followed by a train of single spikes Relations between t adap and F adap (Fig. 5C). This firing pattern can be viewed as an extremely In addition to the neuronal electrotonic structure, spikestrong form of spike-frequency adaptation, produced by the frequency adaptation depends also on the channel conducsame set of ion channels as the adaptation phenomenon (if these ion channels are located at dendritic sites sufficiently isolated from the soma). tances g Ca and g AHP, as well as the [Ca 2/ ] kinetic parameters a and t Ca. These dependences were explored within the framework of our calcium-model. First, the initial firing rate
7 CALCIUM COMPUTATION OF PYRAMIDAL NEURONS 1555 f 0, GV f, and»i Ca 0 (computed with [Ca 2/ ] as a static parameter) are all independent of t Ca, hence F adap is simply proportional to t adap. The slope of the linear curve, however, depends on the input current intensity I. By contrast, if t Ca is held constant and ag Ca g AHP is increased (Fig. 6C), adaptation is faster (t adap is smaller) and the percentage adaptation F adap is larger. The plot of F adap versus t adap shows a linear relation with a negative slope (Fig. 6D). In Fig. 6D, we also plotted the F adap versus t adap simulation data, obtained when the input current intensity I is varied (Fig. 3B) and when the electrotonic coupling g c is varied (Fig. 5B). Quite surprisingly, in all cases, the F adap -t adap curve is linear with approximately the same slope á 1/65, which is close to, but differs from, the reciprocal of the [Ca 2/ ] decay time constant 1/t Ca Å 1/80. To gain some insights about this general relation between F adap and t adap, let us suppose that the ratio between the initial value and the gain parameter is roughly the same for f and»i Ca, i.e., f 0 /G f á (0»I Ca 0 )/G cc. With this assumption, we then can write 0»I Ca 0 G f / f 0 á G cc. Inserting this relation into Eq. 13, we have FIG. 5. Dependence on the electrotonic coupling g c between the 2 compartments. A: with an increased g c, the initial firing frequency is lower but the steady-state frequency remains the same (left). Reduced percentage adaptation is due to a narrower dendritic spike (because V d repolarizes more rapidly at larger g c ), hence a smaller [Ca 2/ ] influx per spike (right). B: t adap increases, and F adap decreases, with g c. C: burst firing patterns with modified electrotonic properties (g c Å 1.4, P Å 0.3) and g Ca Å 0.5, g AHP Å 18. f 0 should not depend on any of these parameters. Second, because»i Ca Å»I Ca 0 / G cc [Ca 2/ ],»I Ca 0 and G cc should be proportional to g Ca. Third, both gain parameters G f and G cc must be proportional to g AHP. In summary, we can write G f Å GV fg AHP,»I Ca 0 Å»IV Ca 0 g Ca, G cc Å GV ccg Ca g AHP (16) Inserting these scaling relations into Eqs. 10 and 13 we have 1/t adap Å (ag Ca g AHP )GV cc / 1/t Ca FIG. 6. Dependence of t adap and F adap on t Ca (A and B) and the combina- tion ag Ca g AHP (C and D) (cf. Eq. 17). Three curves in each panel correspond to different applied current intensities. B: F adap vs. t adap from data in A is linear with a positive slope (which depends on I). D: F adap vs. t adap from data in C: is linear with a negative slope á01/t Ca. This is also true for data obtained with I (Fig. 3B) org c (Fig. 5B) being varied as parameter. F adap Å (ag Ca g AHP )(0GV f»ivca 0 /f 0 )t adap (17) From Eq. 17 we can conclude that t adap and F adap depend only on t Ca (a neuron s [Ca 2/ ] extrusion and buffering properties) and on the combination ag Ca g AHP (the product of the spike-evoked [Ca 2/ ]-influx size ag Ca and the adaptation conductance g AHP ). Given fixed ag Ca g AHP,ast Ca is increased (Fig. 6A), the adaptation is slower (t adap is larger), but the plateau [Ca 2/ ] level is higher (cf. Eqs. 4 and 11), hence F adap is larger. A plot of F adap versus t adap is linear with a positive slope (Fig. 6B). This is evident in Eq. 17, where
8 1556 X.-J. WANG F adap á ag cc t adap (18) or because ag cc Å 1/t adap 0 1/t Ca (Eq. 10), we have F adap á 1 0 t adap /t Ca (19) which is the desired relation between F adap and t adap. This theoretical prediction is directly testable by experimental measurements from cortical pyramidal neurons. Two calcium modes We have developed our calcium model of neuronal activity Eqs. 14 and 15 with the ion currents I Ca and I AHP localized at the dendrite. In real cortical pyramidal neurons, of course, these channels are distributed widely on the dendritic trees as well as the soma (Jaffe et al. 1994; Johnston et al. 1996; Yuste et al. 1994). It is of interest to investigate whether our approach can be generalized to such cases where the [Ca 2/ ] signaling and [Ca 2/ ]-dependent spike-frequency adaptation are distributed across multiple compartments. For our two-compartment model, suppose that the distributions of g Ca and g AHP are uniform at the soma and dendrite, g Ca Å 1 and g AHP Å 5 (in ms/cm 2 ) for both compartments. We then have two equations like Eq. 4 for somatic and dendritic [Ca 2/ ], respectively d[ca 2/ ] s dt d[ca 2/ ] d dt Å0a s I Ca,s 0 [Ca 2/ ] s /t Ca,s Å0a d I Ca,d 0 [Ca 2/ ] d /t Ca,d (20) Because the parameter a is proportional to the surface:volume ratio, it should be much smaller for the soma than for the dendrite. Similarly, t Ca is expected to be longer at the soma than at the dendrite. For instance, the spike-evoked FIG. 7. Two Ca 2/ modes. With g Ca and g AHP on both the somatic and [Ca 2/ ] transient peak amplitude and decay rate (1/t Ca ) can dendritic compartments, the time course of spike-frequency adaptation is a be three times as large in some dendritic sites as in the soma sum of 2 exponentials, with t adap,1 Å 29.4 ms and t adap,2 Å 191 ms. Faster ( Schiller et al. 1995). With the simple two-compartment component (largely due to the dendritic g AHP ) dominates (top). Dendritic [Ca 2/ ](t) is not monotonic and displays a maximum at around t max Å 106 model, let us assume that for the dendritic compartment, ms. Red curves: empirical fits. a d Å and t Ca,d Å 80 ms, whereas for the somatic compartment we multiple the right-hand side of Eq. 4 by a factor of 1 / 3, so that a s Å and t Ca,s Å 240 ms. As shown in Fig. 7, with two calcium modes, [Ca 2/ ] is smaller than would be expected without a somatic I AHP s (t),. [Ca 2/ ] Hence, the nonmonotonic behavior of [Ca 2/ ] d d (t), and f (t) can be empirically well fitted by a sum (t). of two exponentials, with the first rapid phase followed by The theoretical analysis for the adaptation time course can a second much slower phase (Fig. 7, red curves) be generalized in this case, but only approximately. As is detailed in APPENDIX C, our liner approach yields good esti- [Ca 2/ ] s (t) Å exp(0t/t adap,1 ) exp(0t/t adap,2 ) mates for the two adaptation time constants t adap,1 and t adap,2. [Ca 2/ ] d (t) Å exp(0t/t adap,1 ) / 0.5 exp(0t/t adap,2 ) However, the steady state plateaus and the actual time courses cannot be predicted accurately unless nonlinear in- teractions between the two [Ca 2/ ] modes are taken into account. f (t) Å 73 / 225 exp(0t/t adap,1 ) / 16 exp(0t/t adap,2 ) (21) where t adap,1 Å 29.4 ms and t adap,2 Å 191 ms. None that the faster [Ca 2/ ] mode (which is primarily of the dendritic origin) dominates the spike-frequency adaptation, while the slower mode is only 16/ 225 Å 7% of the faster mode. Moreover, the time course of [Ca 2/ ] d can be nonmonotonic (Fig. 7). In the first rapid phase of adaptation, [Ca 2/ ] s is small, both [Ca 2/ ] d and the firing rate converge to their plateau levels as if the somatic I AHP did not exist. But as [Ca 2/ ] s (hence the somatic I AHP ) slowly accumulates, the firing rate further decreases in the second phase of adaptation, which leads to a decay of [Ca 2/ ] d to its actual final plateau, which Adaptation to stochastic Poisson input So far, spike-frequency adaptation of cortical pyramidal neurons has been investigated with current pulse stimulation. However, in in vivo conditions, pyramidal cells are driven by synaptic inputs that are stochastic and vary unceasingly in time. As a first step toward addressing the question of spike-frequency adaptation to natural stimuli, we stimulated the response of our model neuron to random synaptic inputs
9 CALCIUM COMPUTATION OF PYRAMIDAL NEURONS 1557 FIG. 8. Adaptation to a Poisson synaptic input. A: an example of the membrane potential and [Ca 2/ ] time course (top and middle top). Cell initially fires rapidly that appears as a burst of spikes, followed by a firing pattern that is uneven in time. Instantaneous firing rate (averaged over 100 trials) has a single exponential time course (middle bottom). ISI variability increases with decreasing firing rate (bottom). Red curves: theoretical predictions. B: linear dependence of f and»i Ca on [Ca 2/ ]. C: ISI return map (t n/1 vs. t n ). Blue curve: conditional average of t n/1 for each fixed t n, which is approximately linear with a negative slope k Å (Dendritic g AHP Å 8 ms/cm 2.) generated by a Poisson process with rate l. As illustrated in f 0 Å 213 (in Hz). (Note, again, t adap is much shorter than Fig. 8A, when the Poisson input is turned on, the cell initially t Ca Å 80 ms.) These curves fit accurately with the simulation fires rapidly (at 180 Hz), then the firing rate is reduced in data (red curves in Fig. 8A). parallel with the accumulation of [Ca 2/ ]. The instantaneous Driven by random EPSPs, the neuronal firing activity disfiring frequency calculated by averaging over trials shows a plays fluctuations. We calculated the coefficient of variation time course similar to that with a constant current pulse. of the ISIs (cf. METHODS). The CV displays a time course This time course can be predicted using the same analysis that mirrors the mean instantaneous firing rate ( Fig. 8A): as before, except that now we use trial-averaged firing rate its initial value is low but not zero (Ç0.1), increases as the f and calcium current I Ca. As shown in Fig. 8B, both f and firing rate decreases, and plateaus at a value close to 0.5.»I Ca are approximately linear with [Ca 2/ ] when the latter The ISI variability is expected to be larger with lower firing is considered as a parameter frequencies when the cell acts more like a coincidence detector than a temporal integrator of excitatory inputs ( Perkel et ];»I Ca Å»I Ca 0 / G cc [Ca 2/ ] (22) f Å f 0 0 G f [Ca al. 1967; Softky and Koch 1993; Stern 1965). where f 0 Å 213 Hz, G f Å 252 Hz/mM,»I Ca 0 Å022.6 ma/ Because the firing is now random, the membrane potential cm 2, and G cc Å 27.5 ma/cm 2 mm. Solving Eq. 4 with I Ca shows two noteworthy features. First the initial response substituted by»i Ca, we obtain appears as a burst, even though the neuron shows typical [Ca 2/ ](t) Å [Ca 2/ ] ss (1 0 exp(0t/t adap )) adaptation time course and no burst with a current pulse f (t) Å f ss / ( f 0 0 f ss ) exp(0t/t adap ) (23) (Fig. 1). Second, in the steady state after the transient burst, fast doublets can be observed typically after a relatively long where t adap Å 14.8 ms, [Ca 2/ ] ss Å 0.67 mm, f ss Å 44.2 and silent time. This phenomenon can be understood in terms of
10 1558 X.-J. WANG temporal correlations caused by the spike-adapting current I AHP. Statistically, if by chance the cell happens to fire rapidly in a short time window, significant [Ca 2/ ] influx will be produced, the enhanced I AHP will hyperpolarize the cell, and the firing rate subsequently will be reduced. Conversely, during a time period of relative low firing, I AHP decreases as a result of the [Ca 2/ ] decay, and the probability of firing increases. In short, the adaptation generates negative statistical temporal correlations between ISIs. This is shown in Fig. 8C by the ISI return map (computed in the steady state) where the (n / 1)th ISI is plotted against the nth ISI. Given ISI n, the average ISI n/1 is calculated (blue curve) and yields a linear relation with a slope k Å00.3. This slope is the same as the coefficient of correlation between successive ISIs (the CC, see METHODS). It is about a third of the maximum negative correlation ( k Å01) possible. The steady-state input-output relation, i.e., the mean firing rate f versus the Poisson input rate l, is linear (Fig. 9A, red curve). We also computed the ISI CV and CC as function of l; they are plotted against the mean output frequency f (Fig. 9, B and C). One observes that the CV changes slightly with f,ranging (Fig. 9B, red curve). The CC shows a much stronger frequency dependence with a large negative peak at f á 20 Hz (Fig. 9C, red curve). This strong frequency dependence can be explained in terms of two time constants: t Ca Å 80 ms and t adap á 15 ms (for the sake of argument, we assume that t adap remains about the same as l is varied). The negative ISI correlation is produced by the spike-adapting current I AHP, and the effect is large only in a neuronal firing rate range such that the mean ISI (1/ f )is between t adap and t Ca. This is because when the firing rate f is very low ( f õ 1/t Ca Ç 10 Hz), [Ca 2/ ] decays back to baseline between spikes so that I AHP cannot accumulate and k á 0. On the other hand, at very high firing rates ( f ú 1/ t adap Ç 70 Hz), no significant adaptation dynamics takes place during two consecutive ISIs, and their correlation should again be small. This result demonstrates that, when the input is stochastic, the [Ca 2/ ]-dependent current I AHP strongly sculptures the firing patterns by producing negative correlations between ISIs in a frequency-dependent manner, even in the stationary state where the trial-averaged firing rate is constant. Stochastic burst firing cited by a constant input current ( within an appropriate range), there is a bistability where both the resting state and a state of repetitive spiking are possible (data not shown). Because Poisson synaptic inputs are not constant in time, the model neuron is driven to switch randomly between the two states (Fig. 10A). There is no significant ISI correlation in the absence of I AHP (Fig. 10B). The switching is not periodic but stochastic, and the ISI distribution is not bi- modal (Fig. 10C). The large CV is related to the presence of a long tail in the ISI distribution, with large ISIs corre- sponding to the time epochs when the cells is in the silent state (Fig. 10C). Note that hysteresis between two states has been suggested previously to be a cause of large ISI variability, although in these cases the ISI distribution is bimodal (Stern et al. 1997; Wilbur and Rinzel 1983). Such We confirmed that the ISI correlation ( CC) is essentially zero when the spike-frequency adaptation is absent by blocking either g AHP or g Ca (Fig. 9C, blue and green curves). Interestingly, with g AHP Å 0, the ISI variability (CV) is ú1 at low frequencies, whereas it is always õ1 ifg Ca Å 0 (Fig. 9B). Note that the behavior with g AHP Å 0 should be the same as the initial unadapted firing state when g AHP is not blocked (but I AHP Å 0). Therefore, the f-l curves with or without g AHP blockade in Fig. 9A can be considered as the initial and the plateau input-output relations, respectively, under control conditions. The firing is highly irregular (with CV ú 1) when the g AHP is blocked because of a hysteresis phenomenon that is produced by a dendritic calcium plateau potential ( cf. Marder et al. 1996). Indeed, when the neuron model is ex- FIG. 9. Dependence on the Poisson input rate l. A: average steady-state firing rate f as function of l. ISI variability (CV) (B) and ISI correlation (CC) (C) are plotted as function of f. Red: control (with dendritic g Ca Å 1 and g AHP Å 8 ms/cm 2 ), blue: g AHP Å 0, green: g Ca Å 0. Note that when g AHP Å 0 but g Ca is present, the CV is larger than 1 at low firing frequencies (B). On the other hand, the CC is virtually 0, hence the ISIs are essentially uncorrelated in time when g AHP Å 0 (C). In control condition, CC depends strongly on firing frequency and displays a negative peak at á 20 Hz.
11 CALCIUM COMPUTATION OF PYRAMIDAL NEURONS 1559 FIG. 10. A: an example with g AHP Å 0 and l Å 0.3 khz, which shows high ISI variability (CV ú 1). Membrane potential randomly switches in time between a resting (inactive) phase and a firing (active) phase. B: consecutive ISIs are not correlated in time. C: ISI distribution shows a long tail that spans several mean ISI (blue curve), in contrast with the case of Fig. 8 with adaptation (red curve). ISI distributions are plotted against ISI/»ISI. D: power spectrum of the spike train has a large amplitude at low frequencies (blue curve), whereas the adaptation current I AHP filters out response power at low-frequencies (red curve). dynamics with many long time scales also is reflected in state and a firing state is characterized by a long tail in the the power spectrum of the spike train, which displays large ISI distribution and a high power at low frequencies. By amplitudes at low frequencies ( Fig. 10D, blue curve). By contrast, an intrinsically bursting neuron is characterized by contrast, in an adapting state with g AHP x 0, powers at lower a random temporal mixture of bursts and single spikes, a frequencies are strongly suppressed ( Fig. 10D, red curve). strong negative ISI correlation, a bimodal ISI distribution Such high-pass filtering behavior is a common and important and a peak in the power spectrum that depends on the level signature of temporally adapting dynamics. of the excitatory drive. Although random switching between two states gives rise to an apparently bursty pattern of neuronal firing (Fig. 10), we emphasize that it is very different from an intrinsically Forward masking bursting behavior that does not require random switching by We investigated possible computational implications of the fluctuating inputs. We simulated the bursting state of Fig. spike-frequency adaptation, in particular a forward masking 5C with Poisson inputs ( Fig. 11). In this case, the membrane effect suggested by the experiments of Sobel and Tank (1994) potential displayed a temporal mixture of single spikes and on the cricket Omega auditory neurons. Namely, when two bursts of spikes ( Fig. 11A). Unlike the bistable dynamics, or several inputs are presented sequentially in time, neuronal bursting state showed a large negative correlation between response to the first input would activate an I AHP with a delay, ISIs due to the g AHP (Fig. 11B). Furthermore, the ISI distri- thereby inhibiting responses to subsequent inputs. To see if bution can be bimodal: a broad peak at typical ISI values such an effect also occurred in our pyramidal neuron model, between single spikes, whereas a second sharp peak at we first presented to the cell a masking input pulse, then ISI á 3 5 ms corresponds to the short ISIs within a burst a test input pulse after a time interval of t ms (Fig. 12). (Fig. 11C). The bimodality is most evident at low mean Indeed, the response to the test input was reduced dramatically firing rates when the two maxima are well separated (Fig. by the masking input (Fig. 12, A and B). The peak response 11C, blue and red curves). The bimodality also is reflected to the test input was decreased in proportion with the amplitude by the presence of a distinct peak in the power spectrum of of the masking input, i.e., there was a linear relationship bespike trains ( Fig. 11D). At higher mean firing rates, the tween the two with a negative slope (Fig. 12C). The masking broad peak moves closer to, and eventually overlaps with, effect was produced by the residual [Ca 2/ ], which was accuthe sharp peak of the ISI distribution ( Fig. 11C, black mulated by the neuronal firing during the masking input and curve), and the hump in the power spectrum disappears which did not have enough time to return to its baseline be- (Fig. 11D). tween the two pulses as long as t was not too large. Therefore, To summarize, stochastic switching between a resting a significant amount of residual I AHP inhibited the neuronal
Spike-Frequency Adaptation of a Generalized Leaky Integrate-and-Fire Model Neuron
 Journal of Computational Neuroscience 10, 25 45, 2001 c 2001 Kluwer Academic Publishers. Manufactured in The Netherlands. Spike-Frequency Adaptation of a Generalized Leaky Integrate-and-Fire Model Neuron
Journal of Computational Neuroscience 10, 25 45, 2001 c 2001 Kluwer Academic Publishers. Manufactured in The Netherlands. Spike-Frequency Adaptation of a Generalized Leaky Integrate-and-Fire Model Neuron
Linearization of F-I Curves by Adaptation
 LETTER Communicated by Laurence Abbott Linearization of F-I Curves by Adaptation Bard Ermentrout Department of Mathematics, University of Pittsburgh, Pittsburgh, PA 15260, U.S.A. We show that negative
LETTER Communicated by Laurence Abbott Linearization of F-I Curves by Adaptation Bard Ermentrout Department of Mathematics, University of Pittsburgh, Pittsburgh, PA 15260, U.S.A. We show that negative
Consider the following spike trains from two different neurons N1 and N2:
 About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
A Universal Model for Spike-Frequency Adaptation
 A Universal Model for Spike-Frequency Adaptation Jan Benda 1 & Andreas V. M. Herz 2 1 Department of Physics, University of Ottawa, Ottawa, Ontario, K1N 6N5, Canada j.benda@biologie.hu-berlin.de 2 Institute
A Universal Model for Spike-Frequency Adaptation Jan Benda 1 & Andreas V. M. Herz 2 1 Department of Physics, University of Ottawa, Ottawa, Ontario, K1N 6N5, Canada j.benda@biologie.hu-berlin.de 2 Institute
Topics in Neurophysics
 Topics in Neurophysics Alex Loebel, Martin Stemmler and Anderas Herz Exercise 2 Solution (1) The Hodgkin Huxley Model The goal of this exercise is to simulate the action potential according to the model
Topics in Neurophysics Alex Loebel, Martin Stemmler and Anderas Herz Exercise 2 Solution (1) The Hodgkin Huxley Model The goal of this exercise is to simulate the action potential according to the model
The Spike Response Model: A Framework to Predict Neuronal Spike Trains
 The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
arxiv: v1 [q-bio.nc] 13 Feb 2018
![arxiv: v1 [q-bio.nc] 13 Feb 2018 arxiv: v1 [q-bio.nc] 13 Feb 2018](/thumbs/76/73604465.jpg) Gain control with A-type potassium current: I A as a switch between divisive and subtractive inhibition Joshua H Goldwyn 1*, Bradley R Slabe 2, Joseph B Travers 3, David Terman 2 arxiv:182.4794v1 [q-bio.nc]
Gain control with A-type potassium current: I A as a switch between divisive and subtractive inhibition Joshua H Goldwyn 1*, Bradley R Slabe 2, Joseph B Travers 3, David Terman 2 arxiv:182.4794v1 [q-bio.nc]
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests
 Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Biological Modeling of Neural Networks
 Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
Voltage-clamp and Hodgkin-Huxley models
 Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best) Koch, Chapters 6, 8, 9 See also Clay, J. Neurophysiol. 80:903-913 (1998) (for a recent version of the HH squid axon model) Rothman
Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best) Koch, Chapters 6, 8, 9 See also Clay, J. Neurophysiol. 80:903-913 (1998) (for a recent version of the HH squid axon model) Rothman
Synaptic dynamics. John D. Murray. Synaptic currents. Simple model of the synaptic gating variable. First-order kinetics
 Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
An Introductory Course in Computational Neuroscience
 An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
Model Neurons II: Conductances and Morphology
 Chapter 6 Model Neurons II: Conductances and Morphology 6.1 Levels of Neuron Modeling In modeling neurons, we must deal with two types of complexity; the intricate interplay of active conductances that
Chapter 6 Model Neurons II: Conductances and Morphology 6.1 Levels of Neuron Modeling In modeling neurons, we must deal with two types of complexity; the intricate interplay of active conductances that
Basic elements of neuroelectronics -- membranes -- ion channels -- wiring
 Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wires -- signal propagation -- processing
Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wires -- signal propagation -- processing
Overview Organization: Central Nervous System (CNS) Peripheral Nervous System (PNS) innervate Divisions: a. Afferent
 Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Integration of synaptic inputs in dendritic trees
 Integration of synaptic inputs in dendritic trees Theoretical Neuroscience Fabrizio Gabbiani Division of Neuroscience Baylor College of Medicine One Baylor Plaza Houston, TX 77030 e-mail:gabbiani@bcm.tmc.edu
Integration of synaptic inputs in dendritic trees Theoretical Neuroscience Fabrizio Gabbiani Division of Neuroscience Baylor College of Medicine One Baylor Plaza Houston, TX 77030 e-mail:gabbiani@bcm.tmc.edu
Voltage-clamp and Hodgkin-Huxley models
 Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best Koch, Chapters 6, 8, 9 See also Hodgkin and Huxley, J. Physiol. 117:500-544 (1952. (the source Clay, J. Neurophysiol. 80:903-913
Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best Koch, Chapters 6, 8, 9 See also Hodgkin and Huxley, J. Physiol. 117:500-544 (1952. (the source Clay, J. Neurophysiol. 80:903-913
Action Potentials and Synaptic Transmission Physics 171/271
 Action Potentials and Synaptic Transmission Physics 171/271 Flavio Fröhlich (flavio@salk.edu) September 27, 2006 In this section, we consider two important aspects concerning the communication between
Action Potentials and Synaptic Transmission Physics 171/271 Flavio Fröhlich (flavio@salk.edu) September 27, 2006 In this section, we consider two important aspects concerning the communication between
Frequency Adaptation and Bursting
 BioE332A Lab 3, 2010 1 Lab 3 January 5, 2010 Frequency Adaptation and Bursting In the last lab, we explored spiking due to sodium channels. In this lab, we explore adaptation and bursting due to potassium
BioE332A Lab 3, 2010 1 Lab 3 January 5, 2010 Frequency Adaptation and Bursting In the last lab, we explored spiking due to sodium channels. In this lab, we explore adaptation and bursting due to potassium
How to read a burst duration code
 Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Conductance-Based Integrate-and-Fire Models
 NOTE Communicated by Michael Hines Conductance-Based Integrate-and-Fire Models Alain Destexhe Department of Physiology, Laval University School of Medicine, Québec, G1K 7P4, Canada A conductance-based
NOTE Communicated by Michael Hines Conductance-Based Integrate-and-Fire Models Alain Destexhe Department of Physiology, Laval University School of Medicine, Québec, G1K 7P4, Canada A conductance-based
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten. Lecture 2a. The Neuron - overview of structure. From Anderson (1995)
 Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
/639 Final Solutions, Part a) Equating the electrochemical potentials of H + and X on outside and inside: = RT ln H in
 580.439/639 Final Solutions, 2014 Question 1 Part a) Equating the electrochemical potentials of H + and X on outside and inside: RT ln H out + zf 0 + RT ln X out = RT ln H in F 60 + RT ln X in 60 mv =
580.439/639 Final Solutions, 2014 Question 1 Part a) Equating the electrochemical potentials of H + and X on outside and inside: RT ln H out + zf 0 + RT ln X out = RT ln H in F 60 + RT ln X in 60 mv =
Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity
 Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity Jorge F. Mejias 1,2 and Joaquín J. Torres 2 1 Department of Physics and Center for
Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity Jorge F. Mejias 1,2 and Joaquín J. Torres 2 1 Department of Physics and Center for
Exercises. Chapter 1. of τ approx that produces the most accurate estimate for this firing pattern.
 1 Exercises Chapter 1 1. Generate spike sequences with a constant firing rate r 0 using a Poisson spike generator. Then, add a refractory period to the model by allowing the firing rate r(t) to depend
1 Exercises Chapter 1 1. Generate spike sequences with a constant firing rate r 0 using a Poisson spike generator. Then, add a refractory period to the model by allowing the firing rate r(t) to depend
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
Electrophysiology of the neuron
 School of Mathematical Sciences G4TNS Theoretical Neuroscience Electrophysiology of the neuron Electrophysiology is the study of ionic currents and electrical activity in cells and tissues. The work of
School of Mathematical Sciences G4TNS Theoretical Neuroscience Electrophysiology of the neuron Electrophysiology is the study of ionic currents and electrical activity in cells and tissues. The work of
Neuronal Firing Sensitivity to Morphologic and Active Membrane Parameters
 Neuronal Firing Sensitivity to Morphologic and Active Membrane Parameters Christina M. Weaver 1,2,3*, Susan L. Wearne 1,2,3* 1 Laboratory of Biomathematics, Mount Sinai School of Medicine, New York, New
Neuronal Firing Sensitivity to Morphologic and Active Membrane Parameters Christina M. Weaver 1,2,3*, Susan L. Wearne 1,2,3* 1 Laboratory of Biomathematics, Mount Sinai School of Medicine, New York, New
Dendritic computation
 Dendritic computation Dendrites as computational elements: Passive contributions to computation Active contributions to computation Examples Geometry matters: the isopotential cell Injecting current I
Dendritic computation Dendrites as computational elements: Passive contributions to computation Active contributions to computation Examples Geometry matters: the isopotential cell Injecting current I
BME 5742 Biosystems Modeling and Control
 BME 5742 Biosystems Modeling and Control Hodgkin-Huxley Model for Nerve Cell Action Potential Part 1 Dr. Zvi Roth (FAU) 1 References Hoppensteadt-Peskin Ch. 3 for all the mathematics. Cooper s The Cell
BME 5742 Biosystems Modeling and Control Hodgkin-Huxley Model for Nerve Cell Action Potential Part 1 Dr. Zvi Roth (FAU) 1 References Hoppensteadt-Peskin Ch. 3 for all the mathematics. Cooper s The Cell
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 2, W.W. Lytton, Hodgkin-Huxley Model) 9. Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 2, W.W. Lytton, Hodgkin-Huxley Model) 9. Passive and active membrane models In the previous lecture we have considered a passive
Lecture 11 : Simple Neuron Models. Dr Eileen Nugent
 Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Physiology Unit 2. MEMBRANE POTENTIALS and SYNAPSES
 Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Characterizing the firing properties of an adaptive
 Characterizing the firing properties of an adaptive analog VLSI neuron Daniel Ben Dayan Rubin 1,2, Elisabetta Chicca 2, and Giacomo Indiveri 2 1 Department of Bioengineering, Politecnico di Milano, P.zza
Characterizing the firing properties of an adaptive analog VLSI neuron Daniel Ben Dayan Rubin 1,2, Elisabetta Chicca 2, and Giacomo Indiveri 2 1 Department of Bioengineering, Politecnico di Milano, P.zza
Activity Driven Adaptive Stochastic. Resonance. Gregor Wenning and Klaus Obermayer. Technical University of Berlin.
 Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
Reduction of Conductance Based Models with Slow Synapses to Neural Nets
 Reduction of Conductance Based Models with Slow Synapses to Neural Nets Bard Ermentrout October 1, 28 Abstract The method of averaging and a detailed bifurcation calculation are used to reduce a system
Reduction of Conductance Based Models with Slow Synapses to Neural Nets Bard Ermentrout October 1, 28 Abstract The method of averaging and a detailed bifurcation calculation are used to reduce a system
A Mathematical Study of Electrical Bursting in Pituitary Cells
 A Mathematical Study of Electrical Bursting in Pituitary Cells Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Collaborators on
A Mathematical Study of Electrical Bursting in Pituitary Cells Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Collaborators on
Channels can be activated by ligand-binding (chemical), voltage change, or mechanical changes such as stretch.
 1. Describe the basic structure of an ion channel. Name 3 ways a channel can be "activated," and describe what occurs upon activation. What are some ways a channel can decide what is allowed to pass through?
1. Describe the basic structure of an ion channel. Name 3 ways a channel can be "activated," and describe what occurs upon activation. What are some ways a channel can decide what is allowed to pass through?
Neocortical Pyramidal Cells Can Control Signals to Post-Synaptic Cells Without Firing:
 Neocortical Pyramidal Cells Can Control Signals to Post-Synaptic Cells Without Firing: a model of the axonal plexus Erin Munro Department of Mathematics Boston University 4/14/2011 Gap junctions on pyramidal
Neocortical Pyramidal Cells Can Control Signals to Post-Synaptic Cells Without Firing: a model of the axonal plexus Erin Munro Department of Mathematics Boston University 4/14/2011 Gap junctions on pyramidal
Action Potential Initiation in the Hodgkin-Huxley Model
 Action Potential Initiation in the Hodgkin-Huxley Model The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published Version
Action Potential Initiation in the Hodgkin-Huxley Model The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published Version
Neurophysiology. Danil Hammoudi.MD
 Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Membrane equation. VCl. dv dt + V = V Na G Na + V K G K + V Cl G Cl. G total. C m. G total = G Na + G K + G Cl
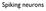 Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
How Spike Generation Mechanisms Determine the Neuronal Response to Fluctuating Inputs
 628 The Journal of Neuroscience, December 7, 2003 23(37):628 640 Behavioral/Systems/Cognitive How Spike Generation Mechanisms Determine the Neuronal Response to Fluctuating Inputs Nicolas Fourcaud-Trocmé,
628 The Journal of Neuroscience, December 7, 2003 23(37):628 640 Behavioral/Systems/Cognitive How Spike Generation Mechanisms Determine the Neuronal Response to Fluctuating Inputs Nicolas Fourcaud-Trocmé,
Simulation of Cardiac Action Potentials Background Information
 Simulation of Cardiac Action Potentials Background Information Rob MacLeod and Quan Ni February 7, 2 Introduction The goal of assignments related to this document is to experiment with a numerical simulation
Simulation of Cardiac Action Potentials Background Information Rob MacLeod and Quan Ni February 7, 2 Introduction The goal of assignments related to this document is to experiment with a numerical simulation
Effects of synaptic conductance on the voltage distribution and firing rate of spiking neurons
 PHYSICAL REVIEW E 69, 051918 (2004) Effects of synaptic conductance on the voltage distribution and firing rate of spiking neurons Magnus J. E. Richardson* Laboratory of Computational Neuroscience, Brain
PHYSICAL REVIEW E 69, 051918 (2004) Effects of synaptic conductance on the voltage distribution and firing rate of spiking neurons Magnus J. E. Richardson* Laboratory of Computational Neuroscience, Brain
Spike Frequency Adaptation Affects the Synchronization Properties of Networks of Cortical Oscillators
 LETTER Communicated by John Rinzel Spike Frequency Adaptation Affects the Synchronization Properties of Networks of Cortical Oscillators Sharon M. Crook Center for Computational Biology, Montana State
LETTER Communicated by John Rinzel Spike Frequency Adaptation Affects the Synchronization Properties of Networks of Cortical Oscillators Sharon M. Crook Center for Computational Biology, Montana State
Neural Modeling and Computational Neuroscience. Claudio Gallicchio
 Neural Modeling and Computational Neuroscience Claudio Gallicchio 1 Neuroscience modeling 2 Introduction to basic aspects of brain computation Introduction to neurophysiology Neural modeling: Elements
Neural Modeling and Computational Neuroscience Claudio Gallicchio 1 Neuroscience modeling 2 Introduction to basic aspects of brain computation Introduction to neurophysiology Neural modeling: Elements
Communicated by John Rinzel
 ARTICLE Communicated by John Rinzel Dynamics of Membrane Excitability Determine Interspike Interval Variability: A Link Between Spike Generation Mechanisms and Cortical Spike Train Statistics Boris S.
ARTICLE Communicated by John Rinzel Dynamics of Membrane Excitability Determine Interspike Interval Variability: A Link Between Spike Generation Mechanisms and Cortical Spike Train Statistics Boris S.
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell
 Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
Peripheral Nerve II. Amelyn Ramos Rafael, MD. Anatomical considerations
 Peripheral Nerve II Amelyn Ramos Rafael, MD Anatomical considerations 1 Physiologic properties of the nerve Irritability of the nerve A stimulus applied on the nerve causes the production of a nerve impulse,
Peripheral Nerve II Amelyn Ramos Rafael, MD Anatomical considerations 1 Physiologic properties of the nerve Irritability of the nerve A stimulus applied on the nerve causes the production of a nerve impulse,
Neural Conduction. biologyaspoetry.com
 Neural Conduction biologyaspoetry.com Resting Membrane Potential -70mV A cell s membrane potential is the difference in the electrical potential ( charge) between the inside and outside of the cell. The
Neural Conduction biologyaspoetry.com Resting Membrane Potential -70mV A cell s membrane potential is the difference in the electrical potential ( charge) between the inside and outside of the cell. The
Introduction and the Hodgkin-Huxley Model
 1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
An analytical model for the large, fluctuating synaptic conductance state typical of neocortical neurons in vivo
 An analytical model for the large, fluctuating synaptic conductance state typical of neocortical neurons in vivo Hamish Meffin Anthony N. Burkitt David B. Grayden The Bionic Ear Institute, 384-388 Albert
An analytical model for the large, fluctuating synaptic conductance state typical of neocortical neurons in vivo Hamish Meffin Anthony N. Burkitt David B. Grayden The Bionic Ear Institute, 384-388 Albert
Physiology Unit 2. MEMBRANE POTENTIALS and SYNAPSES
 Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES In Physiology Today Ohm s Law I = V/R Ohm s law: the current through a conductor between two points is directly proportional to the voltage across the
Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES In Physiology Today Ohm s Law I = V/R Ohm s law: the current through a conductor between two points is directly proportional to the voltage across the
Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued
 Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued Filip Piękniewski Faculty of Mathematics and Computer Science, Nicolaus Copernicus University, Toruń, Poland
Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued Filip Piękniewski Faculty of Mathematics and Computer Science, Nicolaus Copernicus University, Toruń, Poland
A realistic neocortical axonal plexus model has implications for neocortical processing and temporal lobe epilepsy
 A realistic neocortical axonal plexus model has implications for neocortical processing and temporal lobe epilepsy Neocortical Pyramidal Cells Can Send Signals to Post-Synaptic Cells Without Firing Erin
A realistic neocortical axonal plexus model has implications for neocortical processing and temporal lobe epilepsy Neocortical Pyramidal Cells Can Send Signals to Post-Synaptic Cells Without Firing Erin
CORRELATION TRANSFER FROM BASAL GANGLIA TO THALAMUS IN PARKINSON S DISEASE. by Pamela Reitsma. B.S., University of Maine, 2007
 CORRELATION TRANSFER FROM BASAL GANGLIA TO THALAMUS IN PARKINSON S DISEASE by Pamela Reitsma B.S., University of Maine, 27 Submitted to the Graduate Faculty of the Department of Mathematics in partial
CORRELATION TRANSFER FROM BASAL GANGLIA TO THALAMUS IN PARKINSON S DISEASE by Pamela Reitsma B.S., University of Maine, 27 Submitted to the Graduate Faculty of the Department of Mathematics in partial
3.3 Simulating action potentials
 6 THE HODGKIN HUXLEY MODEL OF THE ACTION POTENTIAL Fig. 3.1 Voltage dependence of rate coefficients and limiting values and time constants for the Hodgkin Huxley gating variables. (a) Graphs of forward
6 THE HODGKIN HUXLEY MODEL OF THE ACTION POTENTIAL Fig. 3.1 Voltage dependence of rate coefficients and limiting values and time constants for the Hodgkin Huxley gating variables. (a) Graphs of forward
Ch. 5. Membrane Potentials and Action Potentials
 Ch. 5. Membrane Potentials and Action Potentials Basic Physics of Membrane Potentials Nerve and muscle cells: Excitable Capable of generating rapidly changing electrochemical impulses at their membranes
Ch. 5. Membrane Potentials and Action Potentials Basic Physics of Membrane Potentials Nerve and muscle cells: Excitable Capable of generating rapidly changing electrochemical impulses at their membranes
Deconstructing Actual Neurons
 1 Deconstructing Actual Neurons Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference: The many ionic
1 Deconstructing Actual Neurons Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference: The many ionic
6.3.4 Action potential
 I ion C m C m dφ dt Figure 6.8: Electrical circuit model of the cell membrane. Normally, cells are net negative inside the cell which results in a non-zero resting membrane potential. The membrane potential
I ion C m C m dφ dt Figure 6.8: Electrical circuit model of the cell membrane. Normally, cells are net negative inside the cell which results in a non-zero resting membrane potential. The membrane potential
Fast neural network simulations with population density methods
 Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
3 Action Potentials - Brutal Approximations
 Physics 172/278 - David Kleinfeld - Fall 2004; Revised Winter 2015 3 Action Potentials - Brutal Approximations The Hodgkin-Huxley equations for the behavior of the action potential in squid, and similar
Physics 172/278 - David Kleinfeld - Fall 2004; Revised Winter 2015 3 Action Potentials - Brutal Approximations The Hodgkin-Huxley equations for the behavior of the action potential in squid, and similar
AT2 Neuromodeling: Problem set #3 SPIKE TRAINS
 AT2 Neuromodeling: Problem set #3 SPIKE TRAINS Younesse Kaddar PROBLEM 1: Poisson spike trains Link of the ipython notebook for the code Brain neuron emit spikes seemingly randomly: we will aim to model
AT2 Neuromodeling: Problem set #3 SPIKE TRAINS Younesse Kaddar PROBLEM 1: Poisson spike trains Link of the ipython notebook for the code Brain neuron emit spikes seemingly randomly: we will aim to model
80% of all excitatory synapses - at the dendritic spines.
 Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Channel Noise in Excitable Neuronal Membranes
 Channel Noise in Excitable Neuronal Membranes Amit Manwani, Peter N. Steinmetz and Christof Koch Computation and Neural Systems Program, M-S 9-74 California Institute of Technology Pasadena, CA 95 fquixote,peter,kochg@klab.caltech.edu
Channel Noise in Excitable Neuronal Membranes Amit Manwani, Peter N. Steinmetz and Christof Koch Computation and Neural Systems Program, M-S 9-74 California Institute of Technology Pasadena, CA 95 fquixote,peter,kochg@klab.caltech.edu
Subthreshold cross-correlations between cortical neurons: Areference model with static synapses
 Neurocomputing 65 66 (25) 685 69 www.elsevier.com/locate/neucom Subthreshold cross-correlations between cortical neurons: Areference model with static synapses Ofer Melamed a,b, Gilad Silberberg b, Henry
Neurocomputing 65 66 (25) 685 69 www.elsevier.com/locate/neucom Subthreshold cross-correlations between cortical neurons: Areference model with static synapses Ofer Melamed a,b, Gilad Silberberg b, Henry
Dynamical Systems in Neuroscience: Elementary Bifurcations
 Dynamical Systems in Neuroscience: Elementary Bifurcations Foris Kuang May 2017 1 Contents 1 Introduction 3 2 Definitions 3 3 Hodgkin-Huxley Model 3 4 Morris-Lecar Model 4 5 Stability 5 5.1 Linear ODE..............................................
Dynamical Systems in Neuroscience: Elementary Bifurcations Foris Kuang May 2017 1 Contents 1 Introduction 3 2 Definitions 3 3 Hodgkin-Huxley Model 3 4 Morris-Lecar Model 4 5 Stability 5 5.1 Linear ODE..............................................
5.4 Modelling ensembles of voltage-gated ion channels
 5.4 MODELLING ENSEMBLES 05 to as I g (Hille, 200). Gating currents tend to be much smaller than the ionic currents flowing through the membrane. In order to measure gating current, the ionic current is
5.4 MODELLING ENSEMBLES 05 to as I g (Hille, 200). Gating currents tend to be much smaller than the ionic currents flowing through the membrane. In order to measure gating current, the ionic current is
COGNITIVE SCIENCE 107A
 COGNITIVE SCIENCE 107A Electrophysiology: Electrotonic Properties 2 Jaime A. Pineda, Ph.D. The Model Neuron Lab Your PC/CSB115 http://cogsci.ucsd.edu/~pineda/cogs107a/index.html Labs - Electrophysiology
COGNITIVE SCIENCE 107A Electrophysiology: Electrotonic Properties 2 Jaime A. Pineda, Ph.D. The Model Neuron Lab Your PC/CSB115 http://cogsci.ucsd.edu/~pineda/cogs107a/index.html Labs - Electrophysiology
Synaptic Input. Linear Model of Synaptic Transmission. Professor David Heeger. September 5, 2000
 Synaptic Input Professor David Heeger September 5, 2000 The purpose of this handout is to go a bit beyond the discussion in Ch. 6 of The Book of Genesis on synaptic input, and give some examples of how
Synaptic Input Professor David Heeger September 5, 2000 The purpose of this handout is to go a bit beyond the discussion in Ch. 6 of The Book of Genesis on synaptic input, and give some examples of how
Stochastic Oscillator Death in Globally Coupled Neural Systems
 Journal of the Korean Physical Society, Vol. 52, No. 6, June 2008, pp. 19131917 Stochastic Oscillator Death in Globally Coupled Neural Systems Woochang Lim and Sang-Yoon Kim y Department of Physics, Kangwon
Journal of the Korean Physical Society, Vol. 52, No. 6, June 2008, pp. 19131917 Stochastic Oscillator Death in Globally Coupled Neural Systems Woochang Lim and Sang-Yoon Kim y Department of Physics, Kangwon
How do synapses transform inputs?
 Neurons to networks How do synapses transform inputs? Excitatory synapse Input spike! Neurotransmitter release binds to/opens Na channels Change in synaptic conductance! Na+ influx E.g. AMA synapse! Depolarization
Neurons to networks How do synapses transform inputs? Excitatory synapse Input spike! Neurotransmitter release binds to/opens Na channels Change in synaptic conductance! Na+ influx E.g. AMA synapse! Depolarization
Dynamical Constraints on Computing with Spike Timing in the Cortex
 Appears in Advances in Neural Information Processing Systems, 15 (NIPS 00) Dynamical Constraints on Computing with Spike Timing in the Cortex Arunava Banerjee and Alexandre Pouget Department of Brain and
Appears in Advances in Neural Information Processing Systems, 15 (NIPS 00) Dynamical Constraints on Computing with Spike Timing in the Cortex Arunava Banerjee and Alexandre Pouget Department of Brain and
Chapter 24 BIFURCATIONS
 Chapter 24 BIFURCATIONS Abstract Keywords: Phase Portrait Fixed Point Saddle-Node Bifurcation Diagram Codimension-1 Hysteresis Hopf Bifurcation SNIC Page 1 24.1 Introduction In linear systems, responses
Chapter 24 BIFURCATIONS Abstract Keywords: Phase Portrait Fixed Point Saddle-Node Bifurcation Diagram Codimension-1 Hysteresis Hopf Bifurcation SNIC Page 1 24.1 Introduction In linear systems, responses
MATH 3104: THE HODGKIN-HUXLEY EQUATIONS
 MATH 3104: THE HODGKIN-HUXLEY EQUATIONS Parallel conductance model A/Prof Geoffrey Goodhill, Semester 1, 2009 So far we have modelled neuronal membranes by just one resistance (conductance) variable. We
MATH 3104: THE HODGKIN-HUXLEY EQUATIONS Parallel conductance model A/Prof Geoffrey Goodhill, Semester 1, 2009 So far we have modelled neuronal membranes by just one resistance (conductance) variable. We
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34
 NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
In#uence of dendritic topologyon "ring patterns in model neurons
 Neurocomputing 38}40 (2001) 183}189 In#uence of dendritic topologyon "ring patterns in model neurons Jacob Duijnhouwer, Michiel W.H. Remme, Arjen van Ooyen*, Jaap van Pelt Netherlands Institute for Brain
Neurocomputing 38}40 (2001) 183}189 In#uence of dendritic topologyon "ring patterns in model neurons Jacob Duijnhouwer, Michiel W.H. Remme, Arjen van Ooyen*, Jaap van Pelt Netherlands Institute for Brain
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons
 Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
Propagation& Integration: Passive electrical properties
 Fundamentals of Neuroscience (NSCS 730, Spring 2010) Instructor: Art Riegel; email: Riegel@musc.edu; Room EL 113; time: 9 11 am Office: 416C BSB (792.5444) Propagation& Integration: Passive electrical
Fundamentals of Neuroscience (NSCS 730, Spring 2010) Instructor: Art Riegel; email: Riegel@musc.edu; Room EL 113; time: 9 11 am Office: 416C BSB (792.5444) Propagation& Integration: Passive electrical
Single-Neuron Modeling of LSO Unit Responses
 Single-Neuron Modeling of LSO Unit Responses MIRIAM ZACKSENHOUSE, 1 DON H. JOHNSON, 1 JEROME WILLIAMS, 1 AND CHIYEKO TSUCHITANI 2 1 Department of Electrical and Computer Engineering, Rice University, Houston
Single-Neuron Modeling of LSO Unit Responses MIRIAM ZACKSENHOUSE, 1 DON H. JOHNSON, 1 JEROME WILLIAMS, 1 AND CHIYEKO TSUCHITANI 2 1 Department of Electrical and Computer Engineering, Rice University, Houston
Numerical Simulation of Bistability between Regular Bursting and Chaotic Spiking in a Mathematical Model of Snail Neurons
 International Journal of Theoretical and Mathematical Physics 2015, 5(5): 145-150 DOI: 10.5923/j.ijtmp.20150505.08 Numerical Simulation of Bistability between Regular Bursting and Chaotic Spiking in a
International Journal of Theoretical and Mathematical Physics 2015, 5(5): 145-150 DOI: 10.5923/j.ijtmp.20150505.08 Numerical Simulation of Bistability between Regular Bursting and Chaotic Spiking in a
Information Maximization in Single Neurons
 nformation Maximization in Single Neurons Martin Stemmler and Christof Koch Computation and Neural Systems Program Caltech 139-74 Pasadena CA 91 125 Email: stemmler@klab.caltech.edu.koch@klab.caltech.edu
nformation Maximization in Single Neurons Martin Stemmler and Christof Koch Computation and Neural Systems Program Caltech 139-74 Pasadena CA 91 125 Email: stemmler@klab.caltech.edu.koch@klab.caltech.edu
Exercise 15 : Cable Equation
 Biophysics of Neural Computation : Introduction to Neuroinformatics WS 2008-2009 Prof. Rodney Douglas, Kevan Martin, Hans Scherberger, Matthew Cook Ass. Frederic Zubler fred@ini.phys.ethz.ch http://www.ini.uzh.ch/
Biophysics of Neural Computation : Introduction to Neuroinformatics WS 2008-2009 Prof. Rodney Douglas, Kevan Martin, Hans Scherberger, Matthew Cook Ass. Frederic Zubler fred@ini.phys.ethz.ch http://www.ini.uzh.ch/
An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding
 NOTE Communicated by Michael Hines An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding A. Destexhe Z. F. Mainen T. J. Sejnowski The Howard Hughes Medical
NOTE Communicated by Michael Hines An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding A. Destexhe Z. F. Mainen T. J. Sejnowski The Howard Hughes Medical
Roles of Fluctuations. in Pulsed Neural Networks
 Ph.D. Thesis Roles of Fluctuations in Pulsed Neural Networks Supervisor Professor Yoichi Okabe Takashi Kanamaru Department of Advanced Interdisciplinary Studies, Faculty of Engineering, The University
Ph.D. Thesis Roles of Fluctuations in Pulsed Neural Networks Supervisor Professor Yoichi Okabe Takashi Kanamaru Department of Advanced Interdisciplinary Studies, Faculty of Engineering, The University
Lecture 2. Excitability and ionic transport
 Lecture 2 Excitability and ionic transport Selective membrane permeability: The lipid barrier of the cell membrane and cell membrane transport proteins Chemical compositions of extracellular and intracellular
Lecture 2 Excitability and ionic transport Selective membrane permeability: The lipid barrier of the cell membrane and cell membrane transport proteins Chemical compositions of extracellular and intracellular
When is an Integrate-and-fire Neuron like a Poisson Neuron?
 When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
Introduction to Neural Networks. Daniel Kersten. Lecture 2. Getting started with Mathematica. Review this section in Lecture 1
 Introduction to Neural Networks Daniel Kersten Lecture 2 Getting started with Mathematica Review this section in Lecture 1 2 Lect_2_TheNeuron.nb The Neuron - overview of structure From Anderson (1995)
Introduction to Neural Networks Daniel Kersten Lecture 2 Getting started with Mathematica Review this section in Lecture 1 2 Lect_2_TheNeuron.nb The Neuron - overview of structure From Anderson (1995)
1 Hodgkin-Huxley Theory of Nerve Membranes: The FitzHugh-Nagumo model
 1 Hodgkin-Huxley Theory of Nerve Membranes: The FitzHugh-Nagumo model Alan Hodgkin and Andrew Huxley developed the first quantitative model of the propagation of an electrical signal (the action potential)
1 Hodgkin-Huxley Theory of Nerve Membranes: The FitzHugh-Nagumo model Alan Hodgkin and Andrew Huxley developed the first quantitative model of the propagation of an electrical signal (the action potential)
Patterns of Synchrony in Neural Networks with Spike Adaptation
 Patterns of Synchrony in Neural Networks with Spike Adaptation C. van Vreeswijky and D. Hanselz y yracah Institute of Physics and Center for Neural Computation, Hebrew University, Jerusalem, 9194 Israel
Patterns of Synchrony in Neural Networks with Spike Adaptation C. van Vreeswijky and D. Hanselz y yracah Institute of Physics and Center for Neural Computation, Hebrew University, Jerusalem, 9194 Israel
All-or-None Principle and Weakness of Hodgkin-Huxley Mathematical Model
 All-or-None Principle and Weakness of Hodgkin-Huxley Mathematical Model S. A. Sadegh Zadeh, C. Kambhampati International Science Index, Mathematical and Computational Sciences waset.org/publication/10008281
All-or-None Principle and Weakness of Hodgkin-Huxley Mathematical Model S. A. Sadegh Zadeh, C. Kambhampati International Science Index, Mathematical and Computational Sciences waset.org/publication/10008281
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Sample HHSim Exercises
 I. Equilibrium Potential II. Membrane Potential III. The Action Potential IV. The Fast Sodium Channel V. The Delayed Rectifier VI. Voltage-Gated Channel Parameters Part I: Equilibrium Potential Sample
I. Equilibrium Potential II. Membrane Potential III. The Action Potential IV. The Fast Sodium Channel V. The Delayed Rectifier VI. Voltage-Gated Channel Parameters Part I: Equilibrium Potential Sample
Signal processing in nervous system - Hodgkin-Huxley model
 Signal processing in nervous system - Hodgkin-Huxley model Ulrike Haase 19.06.2007 Seminar "Gute Ideen in der theoretischen Biologie / Systembiologie" Signal processing in nervous system Nerve cell and
Signal processing in nervous system - Hodgkin-Huxley model Ulrike Haase 19.06.2007 Seminar "Gute Ideen in der theoretischen Biologie / Systembiologie" Signal processing in nervous system Nerve cell and
Dynamical phase transitions in periodically driven model neurons
 Dynamical phase transitions in periodically driven model neurons Jan R. Engelbrecht 1 and Renato Mirollo 2 1 Department of Physics, Boston College, Chestnut Hill, Massachusetts 02467, USA 2 Department
Dynamical phase transitions in periodically driven model neurons Jan R. Engelbrecht 1 and Renato Mirollo 2 1 Department of Physics, Boston College, Chestnut Hill, Massachusetts 02467, USA 2 Department
The Bayesian Brain. Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester. May 11, 2017
 The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
Computational Explorations in Cognitive Neuroscience Chapter 2
 Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
