Synaptic Input. Linear Model of Synaptic Transmission. Professor David Heeger. September 5, 2000
|
|
|
- Ezra Tate
- 6 years ago
- Views:
Transcription
1 Synaptic Input Professor David Heeger September 5, 2000 The purpose of this handout is to go a bit beyond the discussion in Ch. 6 of The Book of Genesis on synaptic input, and give some examples of how neurons compute things. Read that chapter first. In this handout, I consider membrane potential to be the key quantity. I take the point of view that action potentials are just a way of encoding (for transmission) the continuous membrane potential signals. In fact, the spike encoding/decoding process may perform important additional processing functions in some neural systems, but I ignore this possibility here. Instead I think of the neural membrane as the brain s primary functional computing element, loosely analogous to a transistor in computer. By wiring transistors together in different ways, you can compute many different functions. Similarly, by using different combinations and spatial arrangements of synapses, a dendritic tree can compute many different functions. The inputs to the neural membrane are the synaptic conductances g j evoked by the presynaptic spiking at a number of synapses, and the output is the membrane potential V m. The neural membrane performs two basic functions. First, it acts as a reconstruction filter that transforms the presynaptic spike encoded signal back into a continuous/analog signal. Second, it computes an output membrane potential signal by combining its inputs. Linear Model of Synaptic Transmission An approximate model of synaptic transmission is to assume that each spike evokes a change in the conductance of the postsynaptic membrane with a characteristic time-course. For the simulation results in this handout, I used the alpha function: ff =u g peak t t peak exp 1 t t peak ; (1) where ff is the conductance of the postsynaptic membrane, g peak is the peak conductance change evoked by a single spike, and t peak is the time (after the spike) of the peak conductance change. The u function is the unit step function that makes ff causal, i.e., ff =0for all time before t = 0. The alpha function is a parametric model that has been fit to approximate experimentally observed synaptic conductance changes. It is not derived from considerations of how ion channels 1
2 actually work. For some synapses, the alpha function is a poor approximation, so people sometimes use more complicated formulae, but the basic idea is the same. For a train of spikes, the postsynaptic conductance change is given by a sum of time-shifted alpha functions: X g syn = ff(t t j ) (2) where t j are the spike times. The spike train itself f is considered to be a sum of impulses: f = j X j ffi(t t j ); where the ffi(t t j ) function is the unit impulse signal that is nonzero only when there is a spike. Putting this together with Eq. 2 we see that the postsynaptic conductance is given by a convolving the spike train with an alpha function: g syn = f Λ ff = = = = Z f (s)ff(t s)ds Z 2 4 X j X»Z ffi(s t j ) j X ff(t t j ): j 3 5 ff(t s) ds ffi(s t j ) ff(t s) ds The last line follows from the one above it because the integral is zero except when s = t j for one of the spike times. This linear model of synaptic transmission assumes that each and every spike evokes the same characteristic conductance change. This not correct in detail for two reasons. First, the effect of each spike is not really independent of the others; sometimes a pair or series of spikes spaced closely in time will result in conductance changes that are either bigger or smaller than expected by the linear model, phenomena known as synaptic facilitation and depression. Second, synapses are unreliable; only a small fraction of spikes (sometimes less than one-tenth) evoke postsynaptic conductance changes. In fact, recent evidence strongly suggests that changes in synaptic reliability may be the cause of synaptic facilitation and depression (Allen and Stevens, 1994; Dobrunz and Stevens, 1997; Tsodyks and Markram, 1997; Abbott et al., 1997). We will stick with the simple linear model for the time being even though it is not completely accurate, but we will cover synaptic reliability, facilitation, and depression later in the course. Post-Synaptic Potential The membrane equation, for a passive neural membrane, with a number of synapses is: C dv m dt +g l[v m E l ]+g 1 [V m E 1 ]+g 2 [V m E 2 ]+:::++g N [V m E N ]=0; (3) 2
3 0 Membrane potential (mv) Synaptic Conductance (us) Figure 1: Steady-state postsynaptic potential as a function of postsynaptic conductance. curve: g l =0:01 us. Dashed curve: g l =0:04 us. Other paramaters: E syn =0mV, E l = 70 mv. where V m is the membrane potential, C is membrane capacitance, g j is the postsynaptic conductance evoked by each synapse, E j is the reversal potential of the ion channels in that syanpse, g l is the constant leak conductance, and E l is the reversal potential of the leak channel. In general, the output membrane potential depends nonlinearly on the input conductances. For a simple example, let s assume that there is one synapse with a reversal potential of 0 mv, and that we can hold the conductance of that synapse at a fixed level for some relatively long period of time (long relative to the membrane time constant). Then the membrane potential reaches the following steady state value: V m = g syne syn E l : (4) g syn This function comes from the membrane equation (Eq. 3) by setting dv m =dt =0, and it is plotted for two different values of g l in Fig. 1. When g syn is large, the membrane potential saturates: V m ß E syn : This, of course, must be the case. There is no way that a synapse could drive the membrane beyond the reversal potential of that synapse. When g syn = g l, the membrane potential is at half the saturating level. When g syn fi g l, the membrane potential fluctuation is very nearly proportional to g syn : V m ß g syne syn g l + E l : In general, for time-varying synaptic conductances, there is no closed form solution to the membrane equation, Eq. 3. There are closed-form solutions for a handful of interesting special 3 Solid
4 cases (see below), but otherwise we have no recourse but to resort to numerical methods and computer simulations. Once you know the postsynaptic membrane potential, it is a simple matter to compute the synaptic current: I syn =g syn [V m E syn ] Temporal Integration and Reconstruction The inputs to most neurons (other than those in the retina) are in the form of trains of action potentials. But for the purposes of this handout, I do not want to get too hung up on exact timing of individual spikes in spike trains. So I will consider the inputs to be instantaneous firing rate signals, denoted r, and expressed in units of impulses per second. For example, let s say that a particular input neuron is driven to spike very regularly, at 10 msec intervals, over an extended period of time. Then its instantaneous firing rate is constant over time, r = 100 imp/sec. In most cases, however, we will want to work with time-varying signals. There are (at least) two ways that we could construct an instantaneous firing rate signal from spike trains. The first way to construct an instantaneous firing rate signal is called a post-stimulus time histogram (PSTH), and it involves recording from a neuron for many repeated presentations of the same stimulus (e.g., injected current, visual stimulus, whatever). Break the stimulus presentation period into short time bins, and count the number of spikes that occur in each time bin, collapsed across many repeats of the stimulus. The second way to construct an instantaneous firing rate signal is to record the time of each spike along with its interspike interval (the time interval until the next spike occurs) and plot one over the interspike interval as a function of spike time. A third method for constructing an instantaneous firing rate signal, available only to the theoretical/computational neuroscientist, is to make it up. That is what we ll do in what follows. Regardless of how we construct the instantaneous firing rate signals for each of the input neurons, the point I want to make here is that the instantaneous firing rate signals are really all we need because the postsynaptic neural membrane acts as a lowpass, reconstruction filter. Figure 2A shows an example of a theoretical (made-up) instantaneous firing rate signal, and Fig 2B shows a spike train computed from Fig 2A assuming an ideal Poisson spike generator (see the Poisson Spike Model handout). Figure 2C shows the postsynaptic conductance computed using the linear (alpha function) model of synaptic transmission discussed above. The thin, noisy curve shows the postsynaptic conductance computed by convolving an alpha function with the spike train (from Fig. 2B). And the bold line shows the postsynaptic conductance computed by convolving an alpha function with the instantaneous firing rate (from Fig. 2A). Finally, Fig. 2D shows the postsynaptic membrane potential computed from both conductances in Fig. 2C. The membrane potential evoked by the spike train is noisy, but otherwise very similar to that computed from the continuous/analog instantaneous firing rate signal. The time constant of the membrane is long relative to the spike and alpha function durations. This results in a temporal summation of the series of inputs. This lowpass filtering property of the membrane acts to reconstruct a continuous, analog signal from the discrete spike events. 4
5 Firing Rate (imp/sec) A Spikes B Synaptic Conductance (us) C Membrane potential (mv) D Time (msec) Time (msec) Figure 2: A: Continuous/analog instantaneous firing rate signal. B: Spike train computed from A assuming an ideal Poisson spike generator. C: Postsynaptic conductance. Bold line computed by convolving the instantaneous firing rate signal from A with an alpha function. Thin, noisy curve computed by convolving the spike train from B with the alpha function. D: Postsynaptic membrane potentials computed from C using the membrane equation. Due to the low-pass filtering properties of the membrane, the two curves are very similar. Parameters: E l = 70 mv, g l = 0:01 us, C =0:5 nf, E syn =0mV, g peak =0:0184 us, t peak =1msec. 5
6 20 Membrane potential (mv) A Membrane potential (mv) B Time (msec) Time (msec) Figure 3: A: Postsynaptic membrane potential, computed as in Fig. 2, except with C = 0:1 nf, hence reducing the membrane time constant by a factor of 5. Other parameters are the same as in Fig. 2, e.g., g peak = 0:0184 us. With a short membrane time constant, the evoked membrane potential is much noisier. B: Same as A except with 20 inputs, each an independent Poisson spike process, and each with g peak = 0:9 ns, 1/20th as big as in A. With 20 inputs instead of one, the noise is greatly attenuated. If the time constant is reduced, as demonstrated in Fig. 3A, the evoked membrane potential is very noisy. The noise can be attenuated again by having muliple copies of the input. In Fig. 3B there are 20 inputs, each with the same average firing, but generated as independent Poisson processes. Since there are now 20 inputs, each of the synapses has a g peak that is 1/20th as big. This new value, g peak =0:9 ns is close to the actual conductance change at individual synapses. Computing With Synapses In this section I give some simple examples of how a neural membrane can do computations. In particular, I show how to compute a linear combination (via addition and subtraction) of a pair of input signals and I show how to multiply/divide a pair of input signals. These examples are not meant to be physiologically realistic. Rather, they should be taken as simplified exercises of working with the equations. I will use the convention that V m denotes voltage with respect to the extracellular potential, whereas V denotes voltage with respect to the resting potential. The resting potential in what follows depends both on a leak conductance and on a tonic level of background activity. Adding and subtracting. input signals, We want to use a neural membrane to compute a linear sum of two V =fi 1 +fi 2 ; 6
7 where fi 2 and fi 2 are constants. Equivalently, we can write the desired output membrane potential with respect to the extracellular potential, V m =fi 1 +fi 2 +V rest : (5) The output, V or V m, is the membrane potential with units of mv. The inputs, and, might be instantaneous firing rate signals with units of spikes/sec, or they might be the membrane potentials of a pair of presynaptic neurons (e.g., in the retina where there are no spikes). We ll assume the former (instantaneous firing rates) in what follows. Equation 5 seems like a relatively simple goal. The inputs give rise to postsynaptic conductance changes which in turn combine to produce the postsynaptic membrane potential. The problem is that the membrane potential is not a linear function of the synaptic conductance (see Eq. 4 above). Synaptic currents are summed, but then divided by the total membrane conductance. The trick is to use a complementary (push-pull) arrangement of inputs to hold the total conductance at a fixed constant level even though the synaptic currents are varying over time. Such a push-pull arrangement of inputs has been used to model the linearity of retinal ganglion cell responses (e.g., Gaudiano, 1994), and the receptive field properties of V1 simple cells (e.g., Carandini and Heeger, 1994). In an idealized push-pull model we replace our two inputs with a set of four inputs, two excitatory and two inhibitory: r e1 =s + r e2 =s + r i1 =s r i2 =s where s is the spontaneous firing rate of all four input neurons. Choosing s> and s> guarantees that the four input signals are positive, as firing rates must be. Each of the four inputs gives rise to a postsynaptic conductance: g e1 =g 1 + fi 0 1 g e2 =g 2 + fi 0 2 g i1 =g 1 fi 0 1 g i2 =g 2 fi 0 2 where fi 0, and fi0 are new constants, and where g = fi 0 s and g 1 2 = fi 0 s are the conductances that 2 result from the spontaneous firing. The membrane equation is: C dv m dt [V m E l ]+g e1 [V m E e ]+g i1 [V m E i ]+g e2 [V m E e ]+g i2 [V m E i ]=0 Here E e and E i are, respectively, the reversal potentials of the excitatory and inhibitory synaptic ion channels. In addition, g l and E l are, respectively, the leak conductance and leak reversal potential. The goal now is to show that V m is a linear summation of the inputs (Eq. 5). To simplify matters, let s begin by assuming that the inputs and the postsynaptic conductances are constant over time. We will return to the general (time-varying) case below. When the postsynaptic conductances are 7
8 constant over time, then the membrane potential is also constant over time, dvm dt where and where Or, as desired. V m = g e1e e + g i1 E i + g e2 E e + g i2 E i E l g e1 + g i1 + g e2 + g i2 = fi0 1 (E e E i )+fi 0 2 (E e E i )+(g 1 + g 2 )(E e + E i )+g l E l 2g 1 +2g 2 = fi 1 + fi 2 + V rest fi 1 = fi 0 E e E i fi 1 2 = fi 0 E e E i ; 2 2g 1 +2g 2 2g 1 +2g 2 V rest The solution for time-vary input signals is: = (g 1 + g 2 )(E e + E i )+g l E l 2g 1 +2g 2 : V = fi 1 + fi 2 ; V m =[u 1 C e (t=fi ) ] Λ [fi 1 +fi 2 ] + V rest ; =0, hence, where fi = C=g as usual, and g =2g 1 +2g 2 is the total conductance. The membrane potential is simply a lowpass filtered version of the linear sum of the two inputs. It would be a good exercise for you to try to derive this result from the above equations. In deriving this result, it is critical that the total conductance is constant over time even though the conductances of the 4 individual synapses change over time. The additional lowpass filter is unavoidable because of the capacitance of the membrane. It is, however, a feature, not a bug. In the brain (excluding in the retina), the input signals are spike trains, not the continuous/analog instantaneous firing rate signals that I have used here. The lowpass filtering property of the membrane acts to reconstruct a continuous, analog signal from the discrete spike events (see above). Figure 4 shows an example. Panel A shows two of the four continuous (instantaneous firing rate) input signals. Panel B shows the spike trains computed from A assuming a Poisson spike generator. Panel C shows the synaptic conductances computed in two ways: (1) using the instantaneous firing rates as inputs, and (2) using the spike trains as the inputs. Panel D shows the postsynaptic membrane potentials computed from C. Notice in comparing C and D how the membrane s lowpass filter greatly attenuates the noise in the postsynaptic conductance. Multiplying/Dividing. signals, We want to use a neural membrane to compute a ratio of two input V = fi 1 fi 2 +fl ; 8
9 200 A B Firing Rate (imp/sec) Spikes Synaptic Conductance (us) Time (msec) C Membrane potential (mv) Time (msec) Figure 4: A: Instantaneous firing rates of two of the input signals. B: Spike trains computed from A using a Poisson spike generator. C: Synaptic conductances computed by convolving A and B and an alpha function. D: Postsynaptic membrane potentials computed from A and B. Parameters: E l = 70 mv, g l = 0:01 us, C = 0:5 nf, E e = 0 mv, E i = 70 mv, g peak = 0:0184 us, t peak =1msec. D 9
10 for some constants, fi 1, fi 2, and fl. Equivalently, we can write the desired output membrane potential with respect to the extracellular potential, V m = fi 1 fi 2 +fl + V rest: (6) As before we replace with a pair of complementary inputs: g e =g 1 + fi 0 1 g i =g 1 fi 0 1 ; for a new constant fi 0, so that the sum of these two conductances g 1 e +g i = 2g 1 is constant over time. For the second input, we choose the conductance to be proportional to the presynaptic firing rate: g sh =fi 2 ; where the subscript in g sh stands for shunting. The membrane equation is: C dv m dt [V m E l ]+g e [V m E e ]+g i [V m E i ]+g sh [V m E sh ]=0 As before, we begin by assuming that the inputs are constant over time, and solve for the steady state membrane potential, V m = g ee e + g i E i + g sh E sh E l 2g 1 + g sh where the resting potential (when = =0) is: = fi0 1 (E e E i )+fi 2 E sh fi 2 +2g 1 + V rest ; V rest = g 1(E e + E i )+g l E l 2g 1 : Now we assume that the reversal potential of the shunting synapse is equal to the resting potential, E sh = V rest, and write the membrane potential with respect to V rest : V = fi0 1 (E e E i ) fi 2 +2g 1 = fi 1 fi 2 + fl ; where fi 1 = fi 0 1 (E e E i ) fl =2g 1 : The trick here is that I chose E sh = V rest = 0. Shunting inhibition refers to this situation in which the reversal potential of the synaptic ion channel equals the resting potential, and it is a widely cited proposal for how neurons might perform division (Coombs et al., 1955; Koch and Poggio, 1987). Because the equilibrium potential for chloride, E cl, is close to a cell s resting potential, the GABA-A receptor has been proposed as a potential shunting channel. Chloride 10
11 shunting, however, only approximates division because E cl is not exactly equal to V rest. Exact division can be implemented with two synaptic conductances, one excitatory and one inhibitory, that increase (and decrease) in proportion. For example, let s assume that shunting is subserved by a combination of chloride and sodium. The synaptic current combining both ion species is the sum of the two (chloride, sodium) components: i.e., and With E syn = V rest =0, i.e., g syn (V E syn )=g K (V E k )+g Na (V E Na ); g syn = g K + g Na ; g syn E syn = g K E k + g Na E Na g K E k + g Na E Na =0; g Na g K = E K E Na : In words, we can get a perfect shunting channel by choosing the two conductances so that their ratio equals minus the ratio of their reversal potentials (noting that E K and E Na are specified with respect to V rest, and hence have opposite sign). Unfortunately, there is no exact solution to the above equations when varies arbitrarily over time. However, we can get an solution by assuming that is piecewise constant, i.e., that it is constant for some period of time (long relative to the membrane time constant), and then it swtiches instantaneously to a new constant value. Then the time-varying solution is: V =[u 1 C e (t=fi ) ] Λ " fi 1 fi 2 +fl where fi = C=g as usual, and g = g sh +2g 1 is the total conductance. Having vary over time is not problematic because the complementary inputs g e and g i guarantee that has no effect on the total membrane conductance. The membrane potential is simply a lowpass filtered version of the quotient: =( + fl). The input controls the total conductance of membrane. This has two effects: (1) it changes the gain (sensitivity to the input) since is scaled by + fl, and (2) it changes the dynamics since the cell s time constant (fi = C=g) is also scaled by conductance. This link between gain and dynamics is one of the signatures of shunting inhibition. Changes in gain and dynamics are linked in a variety of neural systems including: vestibulo-ocular reflex (Lisberger and Sejnowski, 1992), turtle photoreceptors (Baylor et al., 1974), and in V1 neurons (Carandini and Heeger, 1994). # ; 11
12 References L F Abbott, J A Varela, K Sen, and S B Nelson. Synaptic depression and cortical gain control. Science, 275: , C Allen and C F Stevens. An evaluation of causes for unreliability of synaptic transmission. Proc. Natl. Acad. Sci., 91: , D A Baylor and A L Hodgkin. Changes in time scale and sensitivity in turtle photoreceptors. Journal of Physiology (London), 242: , M Carandini and D J Heeger. Summation and division by neurons in primate visual cortex. Science, 264: , J S Coombs, J C Eccles, and P Fatt. The inhibitory suppression of reflex discharges from motoneurones. J. Physiol. (Lond.), 130: , L E Dobrunz and C F Stevens. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron, 18: , P Gaudiano. Simulations of x and y retinal ganglion cell behavior with a nonlinear push-pull model of spatiotemporal processing. Vision Research, 34: , C Koch and T Poggio. Biophysics of computation: neurons, synapses and membranes. In G M Edelman, W E Gall, and W M Cowan, editors, Synaptic function. Wiley, NY, S G Lisberger and T J Sejnowski. Motor learning in a recurrent network model based on the vestibulo-ocular reflex. Nature, 360: , M V Tsodyks and H Markram. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc. Natl. Acad. Sci., 94: ,
Synaptic dynamics. John D. Murray. Synaptic currents. Simple model of the synaptic gating variable. First-order kinetics
 Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Consider the following spike trains from two different neurons N1 and N2:
 About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
Biological Modeling of Neural Networks
 Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
How do synapses transform inputs?
 Neurons to networks How do synapses transform inputs? Excitatory synapse Input spike! Neurotransmitter release binds to/opens Na channels Change in synaptic conductance! Na+ influx E.g. AMA synapse! Depolarization
Neurons to networks How do synapses transform inputs? Excitatory synapse Input spike! Neurotransmitter release binds to/opens Na channels Change in synaptic conductance! Na+ influx E.g. AMA synapse! Depolarization
Integration of synaptic inputs in dendritic trees
 Integration of synaptic inputs in dendritic trees Theoretical Neuroscience Fabrizio Gabbiani Division of Neuroscience Baylor College of Medicine One Baylor Plaza Houston, TX 77030 e-mail:gabbiani@bcm.tmc.edu
Integration of synaptic inputs in dendritic trees Theoretical Neuroscience Fabrizio Gabbiani Division of Neuroscience Baylor College of Medicine One Baylor Plaza Houston, TX 77030 e-mail:gabbiani@bcm.tmc.edu
How to read a burst duration code
 Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten. Lecture 2a. The Neuron - overview of structure. From Anderson (1995)
 Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Neuron. Detector Model. Understanding Neural Components in Detector Model. Detector vs. Computer. Detector. Neuron. output. axon
 Neuron Detector Model 1 The detector model. 2 Biological properties of the neuron. 3 The computational unit. Each neuron is detecting some set of conditions (e.g., smoke detector). Representation is what
Neuron Detector Model 1 The detector model. 2 Biological properties of the neuron. 3 The computational unit. Each neuron is detecting some set of conditions (e.g., smoke detector). Representation is what
3 Detector vs. Computer
 1 Neurons 1. The detector model. Also keep in mind this material gets elaborated w/the simulations, and the earliest material is often hardest for those w/primarily psych background. 2. Biological properties
1 Neurons 1. The detector model. Also keep in mind this material gets elaborated w/the simulations, and the earliest material is often hardest for those w/primarily psych background. 2. Biological properties
Fast neural network simulations with population density methods
 Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
This script will produce a series of pulses of amplitude 40 na, duration 1ms, recurring every 50 ms.
 9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
Electrodiffusion Model of Electrical Conduction in Neuronal Processes
 i. ISMS OF CONDlTlONlff PLASTICITY ditecl by Charks B. Woody, Daniel L. Alk~n, and James b. McGauyh (Plenum Publishing Corporation, 19 23 Electrodiffusion Model of Electrical Conduction in Neuronal Processes
i. ISMS OF CONDlTlONlff PLASTICITY ditecl by Charks B. Woody, Daniel L. Alk~n, and James b. McGauyh (Plenum Publishing Corporation, 19 23 Electrodiffusion Model of Electrical Conduction in Neuronal Processes
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding
 NOTE Communicated by Michael Hines An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding A. Destexhe Z. F. Mainen T. J. Sejnowski The Howard Hughes Medical
NOTE Communicated by Michael Hines An Efficient Method for Computing Synaptic Conductances Based on a Kinetic Model of Receptor Binding A. Destexhe Z. F. Mainen T. J. Sejnowski The Howard Hughes Medical
Dynamic Stochastic Synapses as Computational Units
 LETTER Communicated by Laurence Abbott Dynamic Stochastic Synapses as Computational Units Wolfgang Maass Institute for Theoretical Computer Science, Technische Universität Graz, A 8010 Graz, Austria Anthony
LETTER Communicated by Laurence Abbott Dynamic Stochastic Synapses as Computational Units Wolfgang Maass Institute for Theoretical Computer Science, Technische Universität Graz, A 8010 Graz, Austria Anthony
Exercises. Chapter 1. of τ approx that produces the most accurate estimate for this firing pattern.
 1 Exercises Chapter 1 1. Generate spike sequences with a constant firing rate r 0 using a Poisson spike generator. Then, add a refractory period to the model by allowing the firing rate r(t) to depend
1 Exercises Chapter 1 1. Generate spike sequences with a constant firing rate r 0 using a Poisson spike generator. Then, add a refractory period to the model by allowing the firing rate r(t) to depend
Biosciences in the 21st century
 Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests
 Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Action Potentials and Synaptic Transmission Physics 171/271
 Action Potentials and Synaptic Transmission Physics 171/271 Flavio Fröhlich (flavio@salk.edu) September 27, 2006 In this section, we consider two important aspects concerning the communication between
Action Potentials and Synaptic Transmission Physics 171/271 Flavio Fröhlich (flavio@salk.edu) September 27, 2006 In this section, we consider two important aspects concerning the communication between
Membrane equation. VCl. dv dt + V = V Na G Na + V K G K + V Cl G Cl. G total. C m. G total = G Na + G K + G Cl
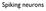 Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
An Introductory Course in Computational Neuroscience
 An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
CSE/NB 528 Final Lecture: All Good Things Must. CSE/NB 528: Final Lecture
 CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
Effects of synaptic conductance on the voltage distribution and firing rate of spiking neurons
 PHYSICAL REVIEW E 69, 051918 (2004) Effects of synaptic conductance on the voltage distribution and firing rate of spiking neurons Magnus J. E. Richardson* Laboratory of Computational Neuroscience, Brain
PHYSICAL REVIEW E 69, 051918 (2004) Effects of synaptic conductance on the voltage distribution and firing rate of spiking neurons Magnus J. E. Richardson* Laboratory of Computational Neuroscience, Brain
Broadband coding with dynamic synapses
 Broadband coding with dynamic synapses Benjamin Lindner Max-Planck-Institute for the Physics of Complex Systems, Nöthnitzer Str. 38 1187 Dresden, Germany André Longtin Department of Physics and Center
Broadband coding with dynamic synapses Benjamin Lindner Max-Planck-Institute for the Physics of Complex Systems, Nöthnitzer Str. 38 1187 Dresden, Germany André Longtin Department of Physics and Center
Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits
 Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits Wolfgang Maass, Robert Legenstein, Nils Bertschinger Institute for Theoretical Computer Science Technische
Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits Wolfgang Maass, Robert Legenstein, Nils Bertschinger Institute for Theoretical Computer Science Technische
Model neurons!!poisson neurons!
 Model neurons!!poisson neurons! Suggested reading:! Chapter 1.4 in Dayan, P. & Abbott, L., heoretical Neuroscience, MI Press, 2001.! Model neurons: Poisson neurons! Contents: Probability of a spike sequence
Model neurons!!poisson neurons! Suggested reading:! Chapter 1.4 in Dayan, P. & Abbott, L., heoretical Neuroscience, MI Press, 2001.! Model neurons: Poisson neurons! Contents: Probability of a spike sequence
Quantitative Electrophysiology
 ECE 795: Quantitative Electrophysiology Notes for Lecture #4 Wednesday, October 4, 2006 7. CHEMICAL SYNAPSES AND GAP JUNCTIONS We will look at: Chemical synapses in the nervous system Gap junctions in
ECE 795: Quantitative Electrophysiology Notes for Lecture #4 Wednesday, October 4, 2006 7. CHEMICAL SYNAPSES AND GAP JUNCTIONS We will look at: Chemical synapses in the nervous system Gap junctions in
Balance of Electric and Diffusion Forces
 Balance of Electric and Diffusion Forces Ions flow into and out of the neuron under the forces of electricity and concentration gradients (diffusion). The net result is a electric potential difference
Balance of Electric and Diffusion Forces Ions flow into and out of the neuron under the forces of electricity and concentration gradients (diffusion). The net result is a electric potential difference
Introduction to Neural Networks. Daniel Kersten. Lecture 2. Getting started with Mathematica. Review this section in Lecture 1
 Introduction to Neural Networks Daniel Kersten Lecture 2 Getting started with Mathematica Review this section in Lecture 1 2 Lect_2_TheNeuron.nb The Neuron - overview of structure From Anderson (1995)
Introduction to Neural Networks Daniel Kersten Lecture 2 Getting started with Mathematica Review this section in Lecture 1 2 Lect_2_TheNeuron.nb The Neuron - overview of structure From Anderson (1995)
Basic elements of neuroelectronics -- membranes -- ion channels -- wiring. Elementary neuron models -- conductance based -- modelers alternatives
 Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wiring neurons together -- synapses
Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wiring neurons together -- synapses
Exercise 15 : Cable Equation
 Biophysics of Neural Computation : Introduction to Neuroinformatics WS 2008-2009 Prof. Rodney Douglas, Kevan Martin, Hans Scherberger, Matthew Cook Ass. Frederic Zubler fred@ini.phys.ethz.ch http://www.ini.uzh.ch/
Biophysics of Neural Computation : Introduction to Neuroinformatics WS 2008-2009 Prof. Rodney Douglas, Kevan Martin, Hans Scherberger, Matthew Cook Ass. Frederic Zubler fred@ini.phys.ethz.ch http://www.ini.uzh.ch/
Activity Driven Adaptive Stochastic. Resonance. Gregor Wenning and Klaus Obermayer. Technical University of Berlin.
 Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
Dendritic computation
 Dendritic computation Dendrites as computational elements: Passive contributions to computation Active contributions to computation Examples Geometry matters: the isopotential cell Injecting current I
Dendritic computation Dendrites as computational elements: Passive contributions to computation Active contributions to computation Examples Geometry matters: the isopotential cell Injecting current I
Neurophysiology. Danil Hammoudi.MD
 Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Subthreshold cross-correlations between cortical neurons: Areference model with static synapses
 Neurocomputing 65 66 (25) 685 69 www.elsevier.com/locate/neucom Subthreshold cross-correlations between cortical neurons: Areference model with static synapses Ofer Melamed a,b, Gilad Silberberg b, Henry
Neurocomputing 65 66 (25) 685 69 www.elsevier.com/locate/neucom Subthreshold cross-correlations between cortical neurons: Areference model with static synapses Ofer Melamed a,b, Gilad Silberberg b, Henry
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell
 Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Effects of Betaxolol on Hodgkin-Huxley Model of Tiger Salamander Retinal Ganglion Cell 1. Abstract Matthew Dunlevie Clement Lee Indrani Mikkilineni mdunlevi@ucsd.edu cll008@ucsd.edu imikkili@ucsd.edu Isolated
Processing of Time Series by Neural Circuits with Biologically Realistic Synaptic Dynamics
 Processing of Time Series by Neural Circuits with iologically Realistic Synaptic Dynamics Thomas Natschläger & Wolfgang Maass Institute for Theoretical Computer Science Technische Universität Graz, ustria
Processing of Time Series by Neural Circuits with iologically Realistic Synaptic Dynamics Thomas Natschläger & Wolfgang Maass Institute for Theoretical Computer Science Technische Universität Graz, ustria
Basic elements of neuroelectronics -- membranes -- ion channels -- wiring
 Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wires -- signal propagation -- processing
Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wires -- signal propagation -- processing
Introduction and the Hodgkin-Huxley Model
 1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
Membrane Potentials, Action Potentials, and Synaptic Transmission. Membrane Potential
 Cl Cl - - + K + K+ K + K Cl - 2/2/15 Membrane Potentials, Action Potentials, and Synaptic Transmission Core Curriculum II Spring 2015 Membrane Potential Example 1: K +, Cl - equally permeant no charge
Cl Cl - - + K + K+ K + K Cl - 2/2/15 Membrane Potentials, Action Potentials, and Synaptic Transmission Core Curriculum II Spring 2015 Membrane Potential Example 1: K +, Cl - equally permeant no charge
The Bayesian Brain. Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester. May 11, 2017
 The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
Math in systems neuroscience. Quan Wen
 Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Nervous Tissue. Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation
 Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Lecture 11 : Simple Neuron Models. Dr Eileen Nugent
 Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
SPIKE TRIGGERED APPROACHES. Odelia Schwartz Computational Neuroscience Course 2017
 SPIKE TRIGGERED APPROACHES Odelia Schwartz Computational Neuroscience Course 2017 LINEAR NONLINEAR MODELS Linear Nonlinear o Often constrain to some form of Linear, Nonlinear computations, e.g. visual
SPIKE TRIGGERED APPROACHES Odelia Schwartz Computational Neuroscience Course 2017 LINEAR NONLINEAR MODELS Linear Nonlinear o Often constrain to some form of Linear, Nonlinear computations, e.g. visual
Chapter 9. Nerve Signals and Homeostasis
 Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
Propagation& Integration: Passive electrical properties
 Fundamentals of Neuroscience (NSCS 730, Spring 2010) Instructor: Art Riegel; email: Riegel@musc.edu; Room EL 113; time: 9 11 am Office: 416C BSB (792.5444) Propagation& Integration: Passive electrical
Fundamentals of Neuroscience (NSCS 730, Spring 2010) Instructor: Art Riegel; email: Riegel@musc.edu; Room EL 113; time: 9 11 am Office: 416C BSB (792.5444) Propagation& Integration: Passive electrical
AT2 Neuromodeling: Problem set #3 SPIKE TRAINS
 AT2 Neuromodeling: Problem set #3 SPIKE TRAINS Younesse Kaddar PROBLEM 1: Poisson spike trains Link of the ipython notebook for the code Brain neuron emit spikes seemingly randomly: we will aim to model
AT2 Neuromodeling: Problem set #3 SPIKE TRAINS Younesse Kaddar PROBLEM 1: Poisson spike trains Link of the ipython notebook for the code Brain neuron emit spikes seemingly randomly: we will aim to model
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 2, W.W. Lytton, Hodgkin-Huxley Model) 9. Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 2, W.W. Lytton, Hodgkin-Huxley Model) 9. Passive and active membrane models In the previous lecture we have considered a passive
Adaptation in the Neural Code of the Retina
 Adaptation in the Neural Code of the Retina Lens Retina Fovea Optic Nerve Optic Nerve Bottleneck Neurons Information Receptors: 108 95% Optic Nerve 106 5% After Polyak 1941 Visual Cortex ~1010 Mean Intensity
Adaptation in the Neural Code of the Retina Lens Retina Fovea Optic Nerve Optic Nerve Bottleneck Neurons Information Receptors: 108 95% Optic Nerve 106 5% After Polyak 1941 Visual Cortex ~1010 Mean Intensity
Roles of Fluctuations. in Pulsed Neural Networks
 Ph.D. Thesis Roles of Fluctuations in Pulsed Neural Networks Supervisor Professor Yoichi Okabe Takashi Kanamaru Department of Advanced Interdisciplinary Studies, Faculty of Engineering, The University
Ph.D. Thesis Roles of Fluctuations in Pulsed Neural Networks Supervisor Professor Yoichi Okabe Takashi Kanamaru Department of Advanced Interdisciplinary Studies, Faculty of Engineering, The University
A Three-dimensional Physiologically Realistic Model of the Retina
 A Three-dimensional Physiologically Realistic Model of the Retina Michael Tadross, Cameron Whitehouse, Melissa Hornstein, Vicky Eng and Evangelia Micheli-Tzanakou Department of Biomedical Engineering 617
A Three-dimensional Physiologically Realistic Model of the Retina Michael Tadross, Cameron Whitehouse, Melissa Hornstein, Vicky Eng and Evangelia Micheli-Tzanakou Department of Biomedical Engineering 617
Tuning tuning curves. So far: Receptive fields Representation of stimuli Population vectors. Today: Contrast enhancment, cortical processing
 Tuning tuning curves So far: Receptive fields Representation of stimuli Population vectors Today: Contrast enhancment, cortical processing Firing frequency N 3 s max (N 1 ) = 40 o N4 N 1 N N 5 2 s max
Tuning tuning curves So far: Receptive fields Representation of stimuli Population vectors Today: Contrast enhancment, cortical processing Firing frequency N 3 s max (N 1 ) = 40 o N4 N 1 N N 5 2 s max
Decoding. How well can we learn what the stimulus is by looking at the neural responses?
 Decoding How well can we learn what the stimulus is by looking at the neural responses? Two approaches: devise explicit algorithms for extracting a stimulus estimate directly quantify the relationship
Decoding How well can we learn what the stimulus is by looking at the neural responses? Two approaches: devise explicit algorithms for extracting a stimulus estimate directly quantify the relationship
لجنة الطب البشري رؤية تنير دروب تميزكم
 1) Hyperpolarization phase of the action potential: a. is due to the opening of voltage-gated Cl channels. b. is due to prolonged opening of voltage-gated K + channels. c. is due to closure of the Na +
1) Hyperpolarization phase of the action potential: a. is due to the opening of voltage-gated Cl channels. b. is due to prolonged opening of voltage-gated K + channels. c. is due to closure of the Na +
Marr's Theory of the Hippocampus: Part I
 Marr's Theory of the Hippocampus: Part I Computational Models of Neural Systems Lecture 3.3 David S. Touretzky October, 2015 David Marr: 1945-1980 10/05/15 Computational Models of Neural Systems 2 Marr
Marr's Theory of the Hippocampus: Part I Computational Models of Neural Systems Lecture 3.3 David S. Touretzky October, 2015 David Marr: 1945-1980 10/05/15 Computational Models of Neural Systems 2 Marr
Neurons and Nervous Systems
 34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity
 Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity Jorge F. Mejias 1,2 and Joaquín J. Torres 2 1 Department of Physics and Center for
Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity Jorge F. Mejias 1,2 and Joaquín J. Torres 2 1 Department of Physics and Center for
Computational Explorations in Cognitive Neuroscience Chapter 2
 Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
Probabilistic Models in Theoretical Neuroscience
 Probabilistic Models in Theoretical Neuroscience visible unit Boltzmann machine semi-restricted Boltzmann machine restricted Boltzmann machine hidden unit Neural models of probabilistic sampling: introduction
Probabilistic Models in Theoretical Neuroscience visible unit Boltzmann machine semi-restricted Boltzmann machine restricted Boltzmann machine hidden unit Neural models of probabilistic sampling: introduction
Outline. Neural dynamics with log-domain integrator circuits. Where it began Biophysics of membrane channels
 Outline Neural dynamics with log-domain integrator circuits Giacomo Indiveri Neuromorphic Cognitive Systems group Institute of Neuroinformatics niversity of Zurich and ETH Zurich Dynamics of Multi-function
Outline Neural dynamics with log-domain integrator circuits Giacomo Indiveri Neuromorphic Cognitive Systems group Institute of Neuroinformatics niversity of Zurich and ETH Zurich Dynamics of Multi-function
Nervous System Organization
 The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
Dendrites - receives information from other neuron cells - input receivers.
 The Nerve Tissue Neuron - the nerve cell Dendrites - receives information from other neuron cells - input receivers. Cell body - includes usual parts of the organelles of a cell (nucleus, mitochondria)
The Nerve Tissue Neuron - the nerve cell Dendrites - receives information from other neuron cells - input receivers. Cell body - includes usual parts of the organelles of a cell (nucleus, mitochondria)
Neural Encoding I: Firing Rates and Spike Statistics
 Chapter 1 Neural Encoding I: Firing Rates and Spike Statistics 1.1 Introduction Neurons are remarkable among the cells of the body in their ability to propagate signals rapidly over large distances. They
Chapter 1 Neural Encoding I: Firing Rates and Spike Statistics 1.1 Introduction Neurons are remarkable among the cells of the body in their ability to propagate signals rapidly over large distances. They
A Model for Real-Time Computation in Generic Neural Microcircuits
 A Model for Real-Time Computation in Generic Neural Microcircuits Wolfgang Maass, Thomas Natschläger Institute for Theoretical Computer Science Technische Universitaet Graz A-81 Graz, Austria maass, tnatschl
A Model for Real-Time Computation in Generic Neural Microcircuits Wolfgang Maass, Thomas Natschläger Institute for Theoretical Computer Science Technische Universitaet Graz A-81 Graz, Austria maass, tnatschl
Synapse Model. Neurotransmitter is released into cleft between axonal button and dendritic spine
 Synapse Model Neurotransmitter is released into cleft between axonal button and dendritic spine Binding and unbinding are modeled by first-order kinetics Concentration must exceed receptor affinity 2 MorphSynapse.nb
Synapse Model Neurotransmitter is released into cleft between axonal button and dendritic spine Binding and unbinding are modeled by first-order kinetics Concentration must exceed receptor affinity 2 MorphSynapse.nb
Hopfield Neural Network and Associative Memory. Typical Myelinated Vertebrate Motoneuron (Wikipedia) Topic 3 Polymers and Neurons Lecture 5
 Hopfield Neural Network and Associative Memory Typical Myelinated Vertebrate Motoneuron (Wikipedia) PHY 411-506 Computational Physics 2 1 Wednesday, March 5 1906 Nobel Prize in Physiology or Medicine.
Hopfield Neural Network and Associative Memory Typical Myelinated Vertebrate Motoneuron (Wikipedia) PHY 411-506 Computational Physics 2 1 Wednesday, March 5 1906 Nobel Prize in Physiology or Medicine.
Annales UMCS Informatica AI 1 (2003) UMCS. Liquid state machine built of Hodgkin-Huxley neurons pattern recognition and informational entropy
 Annales UMC Informatica AI 1 (2003) 107-113 Annales UMC Informatica Lublin-Polonia ectio AI http://www.annales.umcs.lublin.pl/ Liquid state machine built of Hodgkin-Huxley neurons pattern recognition and
Annales UMC Informatica AI 1 (2003) 107-113 Annales UMC Informatica Lublin-Polonia ectio AI http://www.annales.umcs.lublin.pl/ Liquid state machine built of Hodgkin-Huxley neurons pattern recognition and
Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued
 Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued Filip Piękniewski Faculty of Mathematics and Computer Science, Nicolaus Copernicus University, Toruń, Poland
Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued Filip Piękniewski Faculty of Mathematics and Computer Science, Nicolaus Copernicus University, Toruń, Poland
Nervous Tissue. Neurons Neural communication Nervous Systems
 Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Compartmental Modelling
 Modelling Neurons Computing and the Brain Compartmental Modelling Spring 2010 2 1 Equivalent Electrical Circuit A patch of membrane is equivalent to an electrical circuit This circuit can be described
Modelling Neurons Computing and the Brain Compartmental Modelling Spring 2010 2 1 Equivalent Electrical Circuit A patch of membrane is equivalent to an electrical circuit This circuit can be described
Divisive Inhibition in Recurrent Networks
 Divisive Inhibition in Recurrent Networks Frances S. Chance and L. F. Abbott Volen Center for Complex Systems and Department of Biology Brandeis University Waltham MA 2454-911 Abstract Models of visual
Divisive Inhibition in Recurrent Networks Frances S. Chance and L. F. Abbott Volen Center for Complex Systems and Department of Biology Brandeis University Waltham MA 2454-911 Abstract Models of visual
The Spike Response Model: A Framework to Predict Neuronal Spike Trains
 The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
Chapter 48 Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Neural variability and Poisson statistics
 Neural variability and Poisson statistics January 15, 2014 1 Introduction We are in the process of deriving the Hodgkin-Huxley model. That model describes how an action potential is generated by ion specic
Neural variability and Poisson statistics January 15, 2014 1 Introduction We are in the process of deriving the Hodgkin-Huxley model. That model describes how an action potential is generated by ion specic
Channel Noise in Excitable Neuronal Membranes
 Channel Noise in Excitable Neuronal Membranes Amit Manwani, Peter N. Steinmetz and Christof Koch Computation and Neural Systems Program, M-S 9-74 California Institute of Technology Pasadena, CA 95 fquixote,peter,kochg@klab.caltech.edu
Channel Noise in Excitable Neuronal Membranes Amit Manwani, Peter N. Steinmetz and Christof Koch Computation and Neural Systems Program, M-S 9-74 California Institute of Technology Pasadena, CA 95 fquixote,peter,kochg@klab.caltech.edu
MEMBRANE POTENTIALS AND ACTION POTENTIALS:
 University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
Organization of the nervous system. Tortora & Grabowski Principles of Anatomy & Physiology; Page 388, Figure 12.2
 Nervous system Organization of the nervous system Tortora & Grabowski Principles of Anatomy & Physiology; Page 388, Figure 12.2 Autonomic and somatic efferent pathways Reflex arc - a neural pathway that
Nervous system Organization of the nervous system Tortora & Grabowski Principles of Anatomy & Physiology; Page 388, Figure 12.2 Autonomic and somatic efferent pathways Reflex arc - a neural pathway that
Conductance-Based Integrate-and-Fire Models
 NOTE Communicated by Michael Hines Conductance-Based Integrate-and-Fire Models Alain Destexhe Department of Physiology, Laval University School of Medicine, Québec, G1K 7P4, Canada A conductance-based
NOTE Communicated by Michael Hines Conductance-Based Integrate-and-Fire Models Alain Destexhe Department of Physiology, Laval University School of Medicine, Québec, G1K 7P4, Canada A conductance-based
Mid Year Project Report: Statistical models of visual neurons
 Mid Year Project Report: Statistical models of visual neurons Anna Sotnikova asotniko@math.umd.edu Project Advisor: Prof. Daniel A. Butts dab@umd.edu Department of Biology Abstract Studying visual neurons
Mid Year Project Report: Statistical models of visual neurons Anna Sotnikova asotniko@math.umd.edu Project Advisor: Prof. Daniel A. Butts dab@umd.edu Department of Biology Abstract Studying visual neurons
Overview Organization: Central Nervous System (CNS) Peripheral Nervous System (PNS) innervate Divisions: a. Afferent
 Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Control and Integration. Nervous System Organization: Bilateral Symmetric Animals. Nervous System Organization: Radial Symmetric Animals
 Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Decoding Input Signals in Time Domain A Model Approach
 Journal of Computational Neuroscience 6, 237 249, 24 c 24 Kluwer Academic Publishers. Manufactured in The Netherlands. Decoding Input Signals in Time Domain A Model Approach JIANFENG FENG AND DAVID BROWN
Journal of Computational Neuroscience 6, 237 249, 24 c 24 Kluwer Academic Publishers. Manufactured in The Netherlands. Decoding Input Signals in Time Domain A Model Approach JIANFENG FENG AND DAVID BROWN
PROPERTY OF ELSEVIER SAMPLE CONTENT - NOT FINAL. The Nervous System and Muscle
 The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
Computational Neuroscience. Lubica Benuskova Lecture 1
 Computational Neuroscience Lubica Benuskova Lecture 1 1 Outline Brief history of Computational Neuroscience, i.e. computational modelling of biological neurons Blue Brain project Basic concepts of computational
Computational Neuroscience Lubica Benuskova Lecture 1 1 Outline Brief history of Computational Neuroscience, i.e. computational modelling of biological neurons Blue Brain project Basic concepts of computational
80% of all excitatory synapses - at the dendritic spines.
 Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Nerve Signal Conduction. Resting Potential Action Potential Conduction of Action Potentials
 Nerve Signal Conduction Resting Potential Action Potential Conduction of Action Potentials Resting Potential Resting neurons are always prepared to send a nerve signal. Neuron possesses potential energy
Nerve Signal Conduction Resting Potential Action Potential Conduction of Action Potentials Resting Potential Resting neurons are always prepared to send a nerve signal. Neuron possesses potential energy
Voltage-clamp and Hodgkin-Huxley models
 Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best Koch, Chapters 6, 8, 9 See also Hodgkin and Huxley, J. Physiol. 117:500-544 (1952. (the source Clay, J. Neurophysiol. 80:903-913
Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best Koch, Chapters 6, 8, 9 See also Hodgkin and Huxley, J. Physiol. 117:500-544 (1952. (the source Clay, J. Neurophysiol. 80:903-913
Neurons and conductance-based models
 Neurons and conductance-based models Jaeseung Jeong, Ph.D Department of Bio and Brain Engineering, KAIST Bilateralization ( 양측편재화 ): HAROLD:Hemispheric Asymmetry Reduction in Older Adults Prototypical
Neurons and conductance-based models Jaeseung Jeong, Ph.D Department of Bio and Brain Engineering, KAIST Bilateralization ( 양측편재화 ): HAROLD:Hemispheric Asymmetry Reduction in Older Adults Prototypical
Spike-Frequency Adaptation of a Generalized Leaky Integrate-and-Fire Model Neuron
 Journal of Computational Neuroscience 10, 25 45, 2001 c 2001 Kluwer Academic Publishers. Manufactured in The Netherlands. Spike-Frequency Adaptation of a Generalized Leaky Integrate-and-Fire Model Neuron
Journal of Computational Neuroscience 10, 25 45, 2001 c 2001 Kluwer Academic Publishers. Manufactured in The Netherlands. Spike-Frequency Adaptation of a Generalized Leaky Integrate-and-Fire Model Neuron
The Neuron - F. Fig. 45.3
 excite.org(anism): Electrical Signaling The Neuron - F. Fig. 45.3 Today s lecture we ll use clickers Review today 11:30-1:00 in 2242 HJ Patterson Electrical signals Dendrites: graded post-synaptic potentials
excite.org(anism): Electrical Signaling The Neuron - F. Fig. 45.3 Today s lecture we ll use clickers Review today 11:30-1:00 in 2242 HJ Patterson Electrical signals Dendrites: graded post-synaptic potentials
Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling
 Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Efficient temporal processing with biologically realistic dynamic synapses
 INSTITUTE OF PHYSICS PUBLISHING NETWORK: COMPUTATION IN NEURAL SYSTEMS Network: Comput. Neural Syst. 12 (21) 75 87 www.iop.org/journals/ne PII: S954-898X(1)2361-3 Efficient temporal processing with biologically
INSTITUTE OF PHYSICS PUBLISHING NETWORK: COMPUTATION IN NEURAL SYSTEMS Network: Comput. Neural Syst. 12 (21) 75 87 www.iop.org/journals/ne PII: S954-898X(1)2361-3 Efficient temporal processing with biologically
Structure and Measurement of the brain lecture notes
 Structure and Measurement of the brain lecture notes Marty Sereno 2009/2010!"#$%&'(&#)*%$#&+,'-&.)"/*"&.*)*-'(0&1223 Neurons and Models Lecture 1 Topics Membrane (Nernst) Potential Action potential/voltage-gated
Structure and Measurement of the brain lecture notes Marty Sereno 2009/2010!"#$%&'(&#)*%$#&+,'-&.)"/*"&.*)*-'(0&1223 Neurons and Models Lecture 1 Topics Membrane (Nernst) Potential Action potential/voltage-gated
Resting Distribution of Ions in Mammalian Neurons. Outside Inside (mm) E ion Permab. K Na Cl
 Resting Distribution of Ions in Mammalian Neurons Outside Inside (mm) E ion Permab. K + 5 100-81 1.0 150 15 +62 0.04 Cl - 100 10-62 0.045 V m = -60 mv V m approaches the Equilibrium Potential of the most
Resting Distribution of Ions in Mammalian Neurons Outside Inside (mm) E ion Permab. K + 5 100-81 1.0 150 15 +62 0.04 Cl - 100 10-62 0.045 V m = -60 mv V m approaches the Equilibrium Potential of the most
Spatiotemporal Response Properties of Optic-Flow Processing Neurons
 Article Spatiotemporal Response Properties of Optic-Flow Processing Neurons Franz Weber, 1,3, * Christian K. Machens, 2 and Alexander Borst 1 1 Department of Systems and Computational Neurobiology, Max-Planck-Institute
Article Spatiotemporal Response Properties of Optic-Flow Processing Neurons Franz Weber, 1,3, * Christian K. Machens, 2 and Alexander Borst 1 1 Department of Systems and Computational Neurobiology, Max-Planck-Institute
Neurons. The Molecular Basis of their Electrical Excitability
 Neurons The Molecular Basis of their Electrical Excitability Viva La Complexity! Consider, The human brain contains >10 11 neurons! Each neuron makes 10 3 (average) synaptic contacts on up to 10 3 other
Neurons The Molecular Basis of their Electrical Excitability Viva La Complexity! Consider, The human brain contains >10 11 neurons! Each neuron makes 10 3 (average) synaptic contacts on up to 10 3 other
