The Crystal Structure
|
|
|
- Juliana Garrison
- 6 years ago
- Views:
Transcription
1 The Crystl Structure INTRODUCTION Intermoleculr ttrction is minimum in the gseous stte nd this disppers completely when the gs is idel. The interction is stronger in liquids nd is strongest in solids. Therml motion of the molecules increses or decreses by rising or lowering of temperture. The ttrctive interction between the molecules tries to keep them together nd the therml motion is opposed to tht. Hence, it is possible to chnge substnce from one stte to nother by chnging its temperture. If liquid is llowed to cool slowly, the molecules will rrnge themselves in n orderly mnner nd this will finlly result in crystlline solid. If, on the other hnd, cooling is rpid, the molecules will not be ble to rrnge themselves in order. Rpid densifiction will give glss or n morphous solid. It is not true tht the molecules nd toms in solid hve rigidly fixed coordintes. But they move only smll distnce bout their equilibrium positions. In this book, we re concerned with crystlline solids nd the word solid nd crystlline solid will be used synonymously. Wht is the stble stte of given mteril will depend on its free energy. The stble stte will be the one tht hs the lowest free energy under the given conditions. Free energy A is relted to internl energy U nd entropy S of the system s A = U ST...(1.1) Internl energy is lowered by n orderly rrngement of the toms, molecules or ions s tht will led to mximum energy of interction. But this will minimise the entropy. Since internl energy nd entropy mke opposite contribution to free energy, the stte of mtter will be determined by the reltive contributions of U nd ST to A. If interction is strong, U is highly negtive nd ST cn overcome the contribution of the former only t high tempertures. Such substnce will remin s solid even t reltively high temperture. The bsic feture of crystlline solid is the regulr rrngement of the toms nd molecules. At the mcro level, this trnsltes into crystls hving shrp boundries with cler cut shpes. It is these beutiful shpes of nturl crystls tht ttrcted humn ttention for ges. This beutiful shpe nd colour dded to their vlue s gems. Erly study of crystls begn with the observtion of their shpes nd this is known s Geometric Crystllogrphy. The description of crystl symmetry in terms of point lttice begn in the mid-nineteenth century. This ws followed by X-ry crystl structure determintion following the work of Lue nd Brgg on X-ry diffrction by crystls. In the second qurter of the twentieth century, the presence of lttice defects nd their role in determining the properties of crystls were recognised. We shll not try to follow the development of the subject in chronologicl order s the development of knowledge in n re of science does not tke plce in the sme
2 2 Solid Stte Chemistry logicl wy s one would like to see it. But only fter enough knowledge gets ccumulted tht subject is put in logicl perspective. Here we shll follow the rtionl rther thn the chronologicl course of development of the subject. Crystl lttice It is esy to imgine crystl s periodic rrngement of points s shown in figure 1.1. A point my represent n tom or group toms rrnged round it in rel crystl. Let us begin with single point. Repeted trnsltion of this point through fixed distnce ( periodic trnsltion) will generte liner rry of points. This movement is denoted by trnsltion vector. If we dd second t^1 trnsltion, it will generte periodiclly repeting t^2 points on plne nd this is known s plne lttice. If third trnsltion is dded, we get three t^3 dimensionl rrngement of the points tht is clled Figure 1.1: A two dimensionl plne lttice spce lttice. The lttice points re imginry. In rel crystl, they re occupied by toms or groups of toms tht re rrnged in regulr fshion bout the lttice points. This tom or the group of toms is the bsis nd the rrngement of the imginry points is the lttice. The rel crystl is then: bsis lttice = crystl. A two dimensionl pttern s is usully found on curtin cloth or wll pper is nlogous to two dimensionl crystl lttice. We cn hve n rry of squrely rrnged points (Figure 1.2) or the points my be rrnged long inclined lines (Figure 1.2b). We cn select single motif nd plce this motif in the sme wy bout ech lttice points. This will give two different ptterns (Figure. 1.2c nd 1.2d). By selecting different motif, we my get still different pttern nd lrge number of ptterns cn be generted from limited number of motifs nd lttice rrngements. Unit cell We hve seen tht two noncolliner trnsltions give rise to plne lttice nd introducing third trnsltion (not on the sme plne) genertes spce lttice. Since ny line joining two lttice points is trnsltion nd there cn be wide choice of trnsltion, the question rises s to which two trnsltion should one select to describe plne lttice. A few such combintions re shown in figure 1.3. It is seen tht they generte two dimensionl units clled unit cell. Combintion of t^, t^ or t^, t^ leds to cells hving only one lttice point per cell. These re known s primitive unit cell. The combintion t^, t^ genertes double cell. There cn be mny more multiple cells. The 5 6 unit cell of lttice cn be primitive or multiple. A repetition of the two dimensionl unit cell by trnsltion in two directions genertes the plne lttice. This my be extended to three dimensionl lttice tht my be generted by trnsltion of three dimensionl unit cell.
3 The Crystl Structure 3 ( ) () c ( b) ( d) Figure 1.2: Two different plin lttices with identicl motif leding to two different ptterns t 3 t 4 t 6 t 5 t 2 t 1 Figure 1.3: Different combintions of trnsltion nd the unit cell The three trnsltions re selected long the three edges of the chosen unit cell. The choice of unit cell for rel crystl is done bsed on the convenience nd conventions. It cn be primitive cell or multiple cell. The three selected xes long the edges of the unit cell re clled the crystllogrphic xes, b nd c nd the ngles between them α, β nd γ s shown in figure 1.4. As hs lredy been sid, the erly yers of study of solids were concerned mostly with crystl geometry. This ws followed by the study of crystl symmetry. Brvis in 1848 hd shown tht ll structures cn be generted by using only 14 types of spce lttice (14 types of unit cell). These 14 types of unit cells will give rise to 230 types of lttice structures (spce group) by performing such symmetry opertions s b g Figure 1.4: A unit cell showing the edges nd the ngles c b
4 4 Solid Stte Chemistry (i) trnsltion, (ii) rottion, (iii) trnsltion rottion nd (iv) reflection. It ws shown by Federov nd independently by Brlow tht it is not possible to hve rrngement of lttice points other thn thn these 230 types tht cn repet itself infinitely in three dimensions. The 14 types of Brvis lttices re shown in figure 1.5. sc bcc fcc hexgonl Simple bcc tetrgonl orthorhombic rhombohedrl monoclinic triclinic Figure 1.5: Fourteen types of Brvis lttice All rel crystls belong to nyone of the 230 spce groups. The imginry lttice points re occupied by toms or groups of toms. Tble 1.1 shows the clssifiction nd the geometric properties of the vrious Brvis lttices. Tble 1.1: Clssifiction of the fourteen Brvis lttices System Spce Lttice Condition Cubic simple cubic = b = c body centered cubic α = β = γ = 90 fce centered cubic Hexgonl hexgonl prism = b c α = β = 90 ; γ = 120 Tetrgonl tetrgonl prism = b c tetrgonl bcc prism α = β = γ = 90 (Contd.)
5 The Crystl Structure 5 Orthorhombic rectngulr prism b c bc rectngulr prism α = β = γ = 90 fc rectngulr prism bse centered prism Rhombohedrl rhombohedron = b = c α = β = γ 90 Monoclinic prllelopiped b c bc prllelopiped α = β = 90 ; γ 90 Triclinic triclinic prllelopiped b c α β γ 1.2 CRYSTAL PLANES AND MILLER INDICES In rel crystl, the lttice points re occupied by toms. One cn think of very lrge number of lttice plnes nd one set of prllel plnes cn be distinguished from nother set by their orienttion. Miller indices re the lbels used to distinguish one set of prllel plnes from nother. It is set of three numbers h k l tht defines set of prllel plnes in crystl. The following procedure is generlly followed to determine the Miller indices. 1. Choose n origin; 2. Find out the intercept tht the first such plne mkes with the three crystllogrphic xes; 3. Obtin their reciprocls; 4. Eliminte frctions. The set of numbers thus generted in reltion with the xes, b nd c re h, k nd l respectively. A set of prllel plnes re lbelled by set of hkl numbers. Different set of prllel plnes hve different hkl or Miller indices. This is illustrted in figure 1.6. Here, the plne nerest to the origin nd cutting the b xis t 1 1, the xis t 2b 3 nd the c xis t 1 is shown. This 2c mkes 1 3 intercept long xis, 1 2 intercept long b xis nd 1 long c xis. 2 c c b b Figure 1.6: (322) set of plnes
6 6 Solid Stte Chemistry We cn write b c intercept 1/3 1/2 1/2 reciprocl Hence this plne nd set of prllel plnes seprted by distnce d hve the Miller indices (322). It should be noted tht point on pper ctully represents line of points when one considers the three dimensionl lttice. Hence on the plne of pper line of points ctully represents plne. The mjor dvntge of the Miller indices is tht it permits to express interplnr distnce d hkl of set of hkl plnes in terms of lttice prmeters, b, c, α, β nd γ. For cubic crystl d hkl = h k l 1.3 DIFFRACTION OF X-RAYS e In 1912, von Lue first suggested tht since the lttice points in rel crystl re occupied by toms, the crystl lttice should ct s three-dimensionl diffrction grting for X-rys. This should hppen becuse X-rys hve wvelength of the dimension of interplnr distnces in rel crystl. Shortly fter this, W.L. Brgg showed tht wvelength of the X-ry undergoing diffrction by crystl is relted to the interplnr distnces by the fmous Brgg s eqution Brgg s Lw of Diffrction Let there be set of lttice plnes consisting of n rry of toms s shown in figure 1.7. The X-ry bem incident on plne t ngle θ will be reflected from the plne such tht the ngle of j c c c 2 (100) c 3 23 b b (010) c b b (001) c b b 3 (111) 3 (101) (011) Figure 1.7: Different sets of crystl plnes in cubic crystl
7 The Crystl Structure 7 reflection is lso θ nd prt of the intensity will pss though the crystl undevited from its pth. Reflection is cused by the interction of the electromgnetic rdition with the electrons of the toms in the lttice. In order tht the intensity of the reflection is sufficiently strong, reflected wves from the successive plnes seprted by d hkl should be in phse. From figure 1.8, it is seen tht the pth difference of the wves from successive plnes is 2d sin θ. In order tht the wves trvelling from successive plnes re in phse the condition nλ = 2d hkl sin θ (1.2) should be stisfied. This is Brgg s condition of reflection nd is known s Brgg s lw. q A Pth diff. = BC BD n l = 2AB sin q = 2d sin q q q C B D Figure 1.8: Diffrction of X-ry from set of plnes 1.4 RECIPROCAL LATTICE The concept of reciprocl lttice is very useful in X-ry crystllogrphy. It ws Ewld who developed the reltion between the diffrcted X-ry bems. The crystl, insted of being seen s different sets of prllel plnes, my be represented by norml drwn perpendiculr to ech set of prllel plnes from common point s origin. The length of the norml is proportionl to 1/d hkl. This length nd direction of the norml is used to represent set of prllel plnes. If point is plced t the end of ech such norml, n rry of points is generted. Ech point then represents set of prllel equidistnt lttice plnes nd hence, ech point is represented by set of Miller indices (hkl) of the crystl. This rry of points is known s the reciprocl lttice. The reciprocl lttice vector d hkl hs direction sme s the norml to the d hk l plnes nd its mgnitude is 1/d hkl. We see tht the rrngements of the points in the reciprocl lttice hs the sme symmetry s the lttice points of the rel crystl. The concept of reciprocl lttice is prticulrly helpful in understnding diffrction of X- rys by crystl plnes. Let us rewrite the Brgg s eqution in reciprocl lttice s sin θ hkl = λ 2 = 1 d hkl d hkl 2 λ (1.3) Here, we hve tried to relte the mgnitude of the reciprocl lttice vector to diffrction ngle nd the wvelength of the X-ry. In order to see the geometric consequence of this eqution, let us imgine sphere of rdius 1/λ = AO s shown in figure 1.9.
8 8 Solid Stte Chemistry P s hkl A q q C q 2q O Figure 1.9: Reltion between reciprocl lttice point nd X-ry diffrction Let AO lso be the direction of the X-ry bem incident on the crystl plne t C, the centre of the sphere. If θ is the Brgg s ngle, the reflected bem will strike the sphere (shown s circle here) t the point P mking n ngle 2θ with the pssing bem. It should be noted tht the ngle between the incident bem nd AP is θ. Since 2 sin θ hk l /λ = 1/d hk l, we cn see tht 1/d hk l λ = OP. Thus, the point P is the reciprocl lttice point for the set of plnes from which the X-ry bem is reflected. Also, since the Brgg s diffrction conditions re stisfied, the diffrcted bem will touch the sphere (circle in the picture) t point P. The sme will be the cse with other set of prllel plnes except tht they will strike the sphere t some other point which re the reciprocl lttice points for the respective set of plnes. The three-dimensionl sphere is clled the sphere of reflection or Ewld sphere. 1.5 POWDER METHOD The most common method of determining the structure of crystlline solid is X-ry diffrction. There re mny experimentl vritions. Complete determintion of the crystl structure by locting the coordintes of ll the toms requires good single crystl of, t lest, bout 1 mm size. But most substnces crystllize s polycrystlline solids which mens tht ech prticle is mde of number of rndomly oriented tiny crystls. Growing single crystl needs some Diffrcted bem X-ry O 2q 2q Diffrcted bem Figure 1.10: Diffrction of X-ry by rndomly oriented crystls
9 The Crystl Structure 9 specil techniques nd is not lwys esy. However, it is possible to get importnt structurl informtion by recording the X-ry diffrction pttern of the powdered polycrystlline smples. This is commonly known s the powder method. Synthetic chemists prepre mny solid mterils in the lbortory. Before proceeding further, one would like to know whether the desired structure hs been formed. This is done by recording the X-ry powder diffrction pttern of the mteril nd compring this with tht of the known pttern. Extensive powder diffrction dt hve been compiled in the ASTM X-Ry Dt Files tht mkes such comprison possible. Moreover, the powder diffrction ptterns cn provide importnt structurl informtion such s the type of Brvis lttice, size of the unit cell nd the spce group. In the cse of simple crystl, it is even possible to determine the coordintes of ll the toms by nlyzing these ptterns. No surprise tht this method hs become useful tool of the solid stte chemists. If set of Miller plnes stisfy the Brgg s condition, the reflected bem will emerge mking n ngle 2θ with the undeflected bem. Since the crystls re rndomly oriented, the sme Miller plnes in nother crystl my stisfy Brgg s condition, but the deflected bem t ngle 2θ my hve different direction s shown in the figure Since the number of crystls is very lrge, ll kind of orienttions re possible nd the diffrcted X-ry will form cone with ngle 4θ Debye-Scherrer Method Debye nd Scherrer devised specilly designed cmer tht used photogrphic film for detecting the diffrcted X-ry bems. The finely powdered smple in the shpe of thin rod is mounted verticlly inside the cmer which is surrounded by strip of photogrphic film. The rod-like shpe of the powdered smple is chieved by filling the powder in short cpillry mde of glss or some polymer. The crystls inside the tube re rndomly oriented. Hence plnes with different Miller indices will stisfy the Brgg s condition of reflection of X-ry. So s not to miss ny set of prllel plnes fulfilling the reflection condition, the rod is slowly rotted by motor. In rndomly oriented crystls, there re innumerble orienttions of the sme set of plnes mking the ngle θ hkl with the incident X-ry bem. Hence the reflected X-ry will pper s cone tht mkes n ngle 2θ hkl with the direction of the bem. This cone will strike the wll of the cmer in the form of circle tht will be registered in the thin strip of the film s two lines equidistnt from the centre of the circle (see figure 1.11). If S is the mesured distnce between the centre of the two lines (prt of the circle), then 4θ hkl = S R rdins This gives S 180 θ hkl = degree 4R π Thus, by mesuring S, the corresponding vlue of θ cn be found out. The interplnr distnce d hkl cn be found out using Brgg s eqution. Since 180 = 57.3, clcultions cn be simplified if the rdius of the cmer is mde 57.3 mm π or its integrl multiple. Then S cn be mesured in mm nd cn be esily converted to θ in degree. This is the reson why cmers re mde either of 57.3 mm or mm rdius. The min disdvntges of the Debye-Scherrer method re: (i) the precision is limited by the mesurement of S, (ii) the time of exposure is lrge, often severl hours, (iii) mesurement of the
10 10 Solid Stte Chemistry intensity of the lines is not very stisfctory nd it is time consuming. For these resons, this method is hrdly used in recent times nd hs been replced by the utomtic X-ry powder diffrctometer. We shll not discuss here the different sources of error while recording the X-ry diffrction pttern using the Debye-Scherrer cmer s it is rrely used nowdys. Diffrction cones X-ry in X-ry out Film ( ) (b) Figure 1.11: Diffrction from powdered smple using Debye-Scherrer cmer: () diffrcted cones nd (b) the prt of the cones s pirs of lines in the uncoiled film The Powder Diffrctometer In this instrument, the powder smple in the form of thin circulr disc of bout 15 mm dimeter 1-2 mm thickness is plced on holder stnding verticlly. The holder cn be rotted bout n xis perpendiculr to the tble. The X-ry bem is llowed to fll on the smple mking n ngle with the surfce. An X-ry photon counter rottes in circle round the smple detecting ll the reflected bems in turn. For better focussing of the reflected bem on the detector, the smple is rotted t speed hlf of tht of the detector. The detector converts the intensity into current nd the diffrction ngles is plotted s 2θ ginst the current on strip-chrt recorder. As n exmple, the diffrction pttern of potssium bromide crystls is shown in figure This method is fst nd is much more precise both in terms of θ nd intensity mesurement q Figure 1.12: Powder diffrction pttern of potssium bromide
11 The Crystl Structure Indexing the Powder Ptterns Identifying every d vlue in the powder pttern of pure solid substnce with s set of Miller indices hkl is known s indexing of powder pttern. This is done using the equtions tht relte the lttice prmeters for the cyrstl, b, c nd α, β, γ with d hkl. Indexing is simple for cubic crystls nd not difficult for tetrgonl nd hexgonl crystls, but my be quite difficult for crystls of low symmetry like those with monoclinic or triclinic unit cells. For cubic crystls, 2 = (h 2 k 2 l 2 )d 2 hkl (1.4) Since is constnt for given substnce, it is possible to choose the hkl vlues for ll d vlues in such wy so tht the right hnd side of the bove eqution gives constnt vlue. Thus the lttice prmeter of the cubic crystl cn be found out. Rerrnging the bove eqution, we get 2 = h 2 k 2 l 2 (1.5) 2 d The vlue of is chosen such tht 2 /d 2 is n integer for ll the observed vlues of d. This mkes indexing esier. An lterntive to this numericl method of indexing is grphicl technique. According to this method, some d vlues re clculted with ssumed vlues of using eqution (1.5) for different hkl. Vlues of between 0 to 20 Å re sufficient. is then plotted ginst d using cm chrt pper giving number of stright lines ech corresponding to set of hkl. A strip of chrt pper is cut nd the experimentl d vlues re mrked using the sme scle s used in the chrt pper. The strip is then slid over the chrt pper (see figure 1.13) so tht ech nd every mrk on it coincides with one of the stright lines of some hkl. The hkl vlues for ech d is thus identified. For indexing tetrgonl crystl, one cn use the eqution When l = 0, 1 2 = h 2 k 2 l 2 d hkl 2 d hk0 = h 2 2 (1.6) c k (1.7) This eqution mkes it possible to find out for those plnes with l = 0. After getting, vlue of c cn be found out using eqution (1.6) for the rest of the lines. A grphicl method for indexing tetrgonl crystls ws devised by Hull nd Dvey. For hexgonl system, the eqution used is When l = 0, it becomes 1 2 d hkl = (h 2 hk K 2 ) l2 c 2 (1.8) 2 d hk0 = 3 2 (1.9) 4 h 2 hk k 2 which is used for finding. Knowing, c cn be found out using eqution (1.8). Powder ptterns of crystls of low symmetry re difficult to index nd will not be considered here.
12 12 Solid Stte Chemistry d = , Å d, Å Figure 1.13: Indexing cubic crystl using grphicl method Wht is mesured in the diffrction pttern re the ngles 2θ nd the intensity of the peks. Lrger the ngle, more ccurtely it cn be mesured. Lrge ngles correspond to higher hkl plnes. Hence, the lttice prmeter clculted for the high hkl plnes is more ccurte. For exmple, let us sy tht we hve clculted the lttice prmeter for cubic crystl using eqution (1.4) for ech hkl. Insted of verging these vlues of, it will mke more sense if we verge only those vlues tht hve been clculted for the lrge hkl plnes. If it is suspected tht the instrument itself is source of inccurcy, the smple my be mixed with some crystlline solid for which the d vlues re ccurtely known. These lines cn then be used for clibrtion. Accurte mesurement of density of the crystl is very useful s it helps in finding out the number of formul units per unit cell. For this, the number of formul units per unit cell is ssumed nd the mss of n unit cell is clculted from the tomic weight of the elements. The volume of the unit cell is clculted from the lttice constnts obtined from X-ry diffrction.
13 The Crystl Structure 13 Density (ρ x-ry ) is clculted s nm N ρ xry = volume g/cm3 where n is the number of formul units, m is the mss (in mu) of the formul unit, N is Avogdro number. This density known s X-ry density is then compred with the density mesured by ny stndrd experimentl method. A comprison of the two densities will give the correct number of formul units per unit cell. Intensity of the Lines Intensity of the diffrcted lines depend on the scttering power of the toms tht occupy Miller plne. Higher the number of electrons in the tom, higher is its scttering power. This is so becuse the scttering of the electromgnetic rdition tkes plce by interction with electrons. Plnes tht hve hevy toms will give more intense diffrction lines, wheres it is difficult to detect very light toms by X-ry diffrction becuse of their poor scttering power. It is esy to predict the powder pttern of solid with cubic unit cell. Let us tke the exmple of solid hving one kind of tom such s metl. A primitive cubic structure is expected to show the diffrction lines corresponding to ll possible hkl vlues. But two cubic metls, tungsten nd copper show diffrction ptterns tht re quite different. Copper tht hs fcc unit cell gives reflection corresponding to (111), (200), (220), (311), (222), (400), (331) etc. wheres the powder pttern of bcc tungsten gives X-ry diffrction lines for plnes (110), (200), (211), (220), (301), (222), (321)... This mrked difference between the two diffrction ptterns cn be understood by considering the fcc nd bcc lttice s consisting of two interpenetrting primitive cubic sublttices A nd B in two diffeent wys. The distnce trvelled by prllel X-ry bems from the sme hkl plnes of the two sublttices is not equl nd there will be pth difference between the two wves. If the pth difference is n integrl multiple of the X-ry wvelength λ, the two wves will superimpose giving high intensity to the diffrcted bem. We sy the two wves re in phse. If the two wves re out of phse, they will interfere destructively nd no diffrction line will be observed. The phse difference is phse difference = 2π(hx ky lz) where the tom of B sublttice is locted t the point (x by cz) ssuming the tom of A sublttice s the origin. Note tht the plne under considertion in both the sublttice is the sme hkl plne. When the phse difference is π rdins, the mplitudes of the two wves interfere destructively nd if the toms t the two sublttices re the sme (with identicl scttering power), the intensity will completely vnish. If the unit cell is bcc, x = y = z = 1 nd the phse 2 difference = π (h k l). Hence ll reflections for plnes for which h k l is odd will vnish. The systemtic bsence of the lines for plnes for which (h k l) is odd indictes the cubic crystl s bcc. It will only show those lines for which (h k l) is n even number. No such restriction exists for primitive cubic unit cell. It cn be similrly shown tht for fcc unit cell, (h, k, l) should either be ll even or ll odd in order tht the wves from both sublttices re in phse. So, fcc metl will show only those lines for which (h, k, l) re ll even or ll odd. In the light of this discussion, the difference between the X-ry diffrction ptterns of copper nd tungsten cn be pprecited. Let us next consider the X-ry powder diffrction ptterns of two similr fcc binry crystls KCl nd NCl. Their diffrction ptterns re shown in figure 1.14.
14 14 Solid Stte Chemistry () (b) Figure 1.14: X-ry powder ptterns () KCl nd (b) NCl The first thing tht strikes us is tht the ngles θ (given by the position of the lines) due to the sme set of Miller plnes re slightly lrger in NCl. This is becuse the NCl unit cell is slightly smller thn tht of KCl. The more striking difference is the bsence of certin lines like (111), (311), (511) etc. in KCl lthough these lines re present in powder pttern of NCl. From systemtic bsence rules for fcc crystls, we know tht the lines with mixed indices should be bsent for both NCl nd KCl. This is indeed so. Further, for NCl we see tht the successive orders of (111) plnes (these re 111, 222, 333, 444 etc.) re lterntely wek nd strong. For exmple, the reflection from (111) is wek nd tht from (222) is strong nd so on. We know tht in the binry fcc compounds like NCl or KCl, the (111) plnes re lterntely occupied by N (or K ) nd Cl ions. If the scttered wves from two or more such plnes contining only sodium re in phse nd intensify the reflection, the plnes contining sodium will be out of phse with plnes contining chlorine nd will interfere destructively thus diminishing the intensity. This is the reson why (111) reflection in NCl is wek nd (222) reflection is strong. For KCl, the lternte (111) plnes hve potssium nd chlorine. These two ions hve the sme number of electrons nd identicl scttering power. So fr s X-ry is concerned, K nd Cl re identicl. Since the rdition scttered by these plnes re out of phse, the reflected wves will get completely nnihilted nd the reflections from (111), (333) etc. will not be seen. X-ry scttering power of n tom f is proportionl to the number of electrons in the tom. One need not consider the scttering power of ll the infinite number of toms in crystl. It is enough to consider the toms of one unit cell. We must know the loction of the toms in unit cell. This will enble us to determine wht is clled the structure fctor (F hkl ) for reflection hkl. i hx ky lz ihx ky lz 1 2 π ( ) n 2 π ( ) n n n F hkl = fe... fe (1.10) where N is the number of toms in the unit cell nd x n y n z n defines the coordinte of n tom. Tking the position of ction s the origin, NCl crystl will hve in its unit cell N t 000, , nd nd Cl ions t , , nd Putting these coordintes 2 nd simplifying, we get πih ( k l) 0 πih ( k) πih ( l) πik ( l) F hkl = [ f f e ][ e e e e ] N Cl (1.11)
15 The Crystl Structure 15 Since e πin is 1 when n is even nd 1 when n is odd, the Miller indices hkl with ll even or ll odd will mke the terms within the second squre brckets in eqution (1.11) equl to 4. With mixed hkl, this prt of the eqution becomes zero. So the structure fctor for NCl is ( ) F hkl = 4[ f f π ] e ih k l which cn be further simplified s F hkl = 4(f N f Cl ) when h, k, l re ll even, nd F hkl = 4(f N f Cl ) when h, k, l re ll odd. N Cl (1.12) 2 Intensity of the reflected X-ry bem is proportionl to F hkl. This mens tht if the ction nd the nion hd the sme scttering power, ll reflections with h, k, l ll odd will vnish s it hppens in the cse of KCl. These lines pper s wek reflections for NCl becuse of the unequl scttering power of sodium nd chloride ions. The powder method is indequte for locting the coordintes of every single tom in complex solid tht needs single crystl X-ry structure determintion. This is beyond the scope of this book Use of X-ry Powder Ptterns Despite ll its limittions, X-ry powder method hs become the most importnt tool for chemists who study solid mterils, metls nd inorgnic solids in prticulr. It is most commonly used to find out if the desired phse is present in synthesized or nturl mteril by compring its X-ry pttern with those of the known mterils of similr composition. The presence of ny impurity phse cn be detected by this method. In solid stte rection to synthesize some cermic mteril, it cn be used to check the completion of the rection. For simple structures, it llows to determine the unit cell type nd size, the number of formul units per unit cell nd in some simple cses, even intensity nlysis is possible. Its one limittion is tht the lines become brod nd finlly dispper s the prticle size decreses. The most pproprite size of the prticles should be between 100 nm to 10 3 nm. Another shortcoming is its inbility to identify hydrogen toms thus severely restricting its ppliction to orgnic compounds. 1.6 CRYSTAL DEFECTS So fr in this chpter we hve considered the crystl lttice in which ll the sites ment for some toms re occupied ppropritely. In the NCl structure, for exmple, every site ment for sodium re occupied by sodium nd the sme is true for chlorine toms. It did not hppen tht chlorine tom occupied site tht is ment for sodium tom. Such crystl is clled perfect or n idel crystl. A perfect rrngement gives the crystl the highest possible negtive vlue of lttice energy nd hence pprently such structure should be most stble. Stbility, however, is determined by free energy G which for solid my be tken s A, the Helmholtz free energy expressed s A = U TS Further, we cn write lttice energy U L in plce of internl energy U. This eqution tells us tht A = U L will be minimum only when T = 0 K, i.e., when the contribution of entropy towrds free energy is zero. Entropy of n disordered (imperfect) crystl is higher thn tht of n ordered crystl. Thus, t ny temperture other thn 0 K, two fctors will contribute to free energy.
16 16 Solid Stte Chemistry Perfection in the crystl will minimize the free energy by mking the internl energy U L more negtive, nd the contribution from entropy ST will try to minimize the sme by mking the crystl more imperfect thus incresing S. The equilibrium structure t ny temperture other thn 0 K will hve some disorder or imperfection. We see tht the presence of defects in crystl is thermodynmic requirement for stbility. The min defects in crystl re of three types: point defects, disloctions nd grin boundries Point Defects The simplest type of point defect in crystl is vcncy. When lttice site tht normlly is to be occupied by n tom or n ion is left vcnt, we hve point defect known s vcncy nd it hs the symbol V. If the vcnt site ws supposed to be occupied by n tom X in the crystl, the symbol is V X mening the vcncy occurs t site tht is ment for n X tom. If n tom or ion is removed from its norml position in the lttice nd is plced t n interstitil site, the point defect is known s n interstitil. If M is the tom tht is t n interstitil site, it is given the symobl M i. Similrly, there my be n interstitil X i which mens n X tom insted of being present t its norml site, occupies n interstitil site. If n tom A in crystl of composition AB insted of being present t site ment for it goes to occupy site which is normlly occupied by n tom B, the point defect is clled misplced tom. The symbol for this is B A. Here the letter B is the site for tom B nd the subscript shows the tom tht is occupying the site. It goes without sying tht AA^ nd BB men norml lttice sites but A B nd B A men misplced toms. A crystl my hve in it vriety of point defects but which type will predominte depends on the energetics of defect formtion. Mny crystls show combintion of defects i.e., one type of defect is ssocited with n equivlent number of defects of nother type. Such combintion defects re prticulrly importnt in ionic crystls for mintining overll chrge neutrlity of the crystl. Two such defects of prticulr importnce re the Frenkel nd Schottky defects. When n tom or ion is removed from its norml site nd plced t n interstitil site, the crystl hs simultneously vcncy nd one interstitil. This combintion is known s Frenkel defect. In crystl, when ction vcncy exists with n equivlent number of nion vcncies necessry for its chrge neutrlity, the combintion is known s Schottky defect. Frenkel nd Schottky defects re shown in figure ( ) ( b) Figure 1.15: () Frenkel defect nd (b) Schottky defect in n ionic crystl
17 The Crystl Structure 17 The formtion of Schottky defect is ccompnied by the cretion of n equivlent number of new lttice sites or removl of the ions to the gs phse from the lttice. The presence of foreign toms constitutes nother type of point defect. These toms or ions cn be present either in interstitil or in substitutionl positions. In n ionic crystl, the introduction of foreign tom is dependent on its ionic rdius nd electronic structure. Sometimes, it is possible for the sme ion to enter the lttice both interstitilly nd substitutionlly. Substitution of lttice ion by n impurity ion of different chrge will disturb the chrge blnce. In order to mintin the chrge neutrlity of the lttice, such substitution my be ccompnied either by the cretion of lttice vcncy or by the chnge of the oxidtion stte of n ion in the lttice. Substitution of Ag by Cd 2 in AgCl leds to the former, wheres substitution of Ni 2 by Li in NiO lttice gives rise to the ltter. These two cses re illustrted below, one creting ction vcncy, nd the other vlence defect. Here Ni 3 is known s vlence defect. Ag Cl Ag Cl Ni 2 O 2 Ni 2 O 2 Cl Cd 2 Cl Ag O 2 Ni 3 O 2 Ni 2 Ag Cl Cl Ni 2 O 2 Li O 2 Cl Ag Cl Ag O 2 Ni 2 O 2 Ni 2 Vcncy produced by Vlence defect produced Cd 2 substitution by Li substitution Unlike the inherent thermodynmic defects, the impurity defects cn be delibertely dded in controlled wy tht is known s doping. This helps in modifying the properties of the solid. There is nother type of defect tht very much influences the electronic properties of the solid. These re the electronic defects. Let us ssume tht solid hs n nion vcncy. The nion vcncy is the site tht normlly should hve been occupied by n nion. Hence this site (surrounded by ctions) hs strong positive potentil nd cn trp electrons. It will need some energy to free the trpped electron. When sodium chloride is heted in sodium vpour, we see tht is cquires yellow colour. The heting in excess sodium cretes new lttice sites for sodium. To mintin the structure, n equl number of chloride sites re lso creted, but the ltter re vcnt. The electron of the sodium tom tht is ionized in the NCl mtrix is trpped by the chloride ion vcncy. This trpped electron cn be freed into the crystl by bsorbing visible light nd hence the colour. A trpped electron t n nion vcncy is n electronic defect nd it is known s F centre (from Germn Frbe mening colour). In fct, mny of the point defects my hve chrge different from the norml chrge of the originl site due to trpped electrons or holes. These electrons nd holes cn be relesed to the conduction bnd or to the vlence bnd of the solid nd this will modify its electronic properties. The symbols of the point defects then should lso include the informtion of the chrge tht they crry. It is more convenient to tlk of n effective chrge. The effective chrge of point defect is tken s the difference of its chrge from the chrge of the ion tht normlly occupies tht site. Let us illustrte this with some exmples. Let us sy tht there is ction vcncy in the MgO lttice. This vcnt site ws originlly occupied by Mg 2 ion tht hd chrge 2. Now it hs zero chrge nd hence its effective chrge is 2. We write the symbol V Mg, where the superscript stnds for two negtive effective chrges. If the ction vcncy cptures hole, its effective chrge becomes uninegtive nd the symbol used is V Mg. If it cptures nother hole, it will hve rel chrge 2, but zero effective chrge nd will be denoted s V X Mg. An oxygen vcncy with zero chrge is hving two effective
18 18 Solid Stte Chemistry positive chrges nd will be represented s V O. If it cptures one electron, its effective chrge will be unipositive nd the symbol for it is V O. If it cptures second electron, its effective chrge will be zero nd the symbol is VO X, lthough its true chrge is 2. Similrly, the chrge of other types of point defects should be included in the symbol. Thus, if bivlent metl ion goes to normlly unoccupied interstitil site, the effective chrge of the interstitil site becomes 2 becuse originlly this site did not hve ny chrge. In this cse, however, the rel chrge too is 2. The inclusion of the effective chrge in the nottion of the point defects is importnt s complete blnce of effecticve chrge will hve to be mintined in writing blnced equtions for defect rections Defect Equtions It should be remembered tht the totl effective positive nd negtive chrges should be equl in crystl. In writing n eqution involving point defects, the following rules should be followed. 1. The rtio of the number of regulr ctionic nd nionic sites in solid is constnt. If the crystl hs NCl structure, this rtio is unity. If the solid crystllizes with the fluorite structure, this rtio is 1: 2. This will be so even if the solid is non-stoichiometric. This mens tht if we crete N lttice site, we re obliged lso to crete Cl lttice site even if tht site is to be kept vcnt. 2. Defect eqution my include cretion of new lttice sites s well s elimintion of the existing lttice sites i.e., the totl number of lttice sites my chnge. 3. The totl mss blnce should be mintined which mens the totl number of ech type of toms on both sides of the eqution should be the sme. 4. The totl effective chrge should be sme on both sides, i.e., chrge blnce should be mintined. Next, let us try to write some defect equtions following the rules stted bove. Here only stoichiometric compounds will be considered. Non-stoichiometeric oxides will be considered lter. Let us first consider Schottky defects in stoichiometric oxide MO. The eqution for the formtion of Schottky defect cn be written s M M O O l V M V O M M O O The regulr lttice sites on the right hnd side mens tht the formtion of Schottky defect requires tht new lttice sites re creted. Cncelling common terms, we write O l V M V O where O stnds for perfect lttice. Here we hve ssumed tht the doubly chrged vcncies predominte. It is obvious tht the doubly chrged vcncies cn cpture or relese electrons nd cn either become singly chrged or neutrl. If these species re to be included in the eqution, the corresponding electrons or holes re lso to be dded. A Frenkel defect is creted by trnsferring regulr ion to n interstitil site. Suppose the interstitil ion is ction s is generlly the cse, we cn write M M l V M M i ssuming tht the doubly chrged interstitils nd vcncies predominte.
19 The Crystl Structure 19 We hve considered the importnt types of point defect. Next we shll look into the concentrtion of the thermodynmic defects under specific conditions. The equilibrium concentrtion of the inherent thermodynmic defects is dependent on temperture. It is possible to clculte this concentrtion t ny given temperture provided the energy required for their formtion is known. Here we shll derive the equtions tht relte the defect concentrtion to temperture Vcncy Concentrtion in Metl The chnge of n idel crystl t constnt volume nd temperture to defect crystl with vcnt lttice sites will be ccompnied by chnge in Helmholtz free energy such tht A = U T S (1.13) where A, U nd S re the respective chnges in free energy, internl energy nd entropy. At equilibrium, the chnge in free energy with the chnge in the number of vcncies n will be zero. We cn write F AfI = 0 (1.14) HG n KJ TV, If ε is the energy needed to crete vcncy, U = nε. (1.15) Neglecting the contribution to entropy by the vibrtion of the toms which in ny cse is smll, the entropy of the defect structure will be essentilly its configurtionl entropy ( S conf. = S). Hence, S = k ln w (1.16) where k is the Boltzmnn constnt nd w the thermodynmic probbility which is equl to the number of wys n vcnt sites nd (N n) occupied sites cn be rrnged mong the N vilble lttice sites. This will give N! w = (1.17) N nf! n! Substituting this in eqution (1.16) nd pplying Stirling s pproximtion, we get It is now possible to write f f (1.18) S =k N ln N N n ln N n nln n A = nε kt N ln N N n ln N n nln n f f (1.19) Differentiting A with respect to n nd equting to zero (condition of equilibrium concentrtion of defects), we get ε = kt N nflnn nf nln n n This will give ε = kt ln N n n nd n N n F = exp HG ε kt I K J (1.20)
20 20 Solid Stte Chemistry Since N >> n, n F N = exp HG ε kt If E V is the energy necessry to crete one mole of vcncy, then = ε Avogdro s number nd E V n N = exp F H G EV I K J RT I K J (1.21) Thus, knowing the energy of fromtion of vcncies, it is possible to clculte the number of vcncies per mole of metl toms t given temperture Schottky Defect Concentrtion in n Ionic Crystl Let us next clculte the equilibrium concentrtion of Schottky defects in n ionic crystl of stoichiometry MX. Let ε s be the energy necessry to crete Schottky defect. This mens tht this is the energy needed to produce one ction nd one nion vcncy in MX. If the totl ctionic site is N nd the number of ction vcncy is n s, the number of occupied ctionic sites will be (N n s ). The number of wys in which n s ction vcncies nd (N n s ) ctionic sites cn be rrnged in N vilble ctionic sites is w = n! N n! n! (1.22) b sg s There will be n s nion vcncy since stoichiometry is MX. The number of wys in which n s nion vcncies nd (N n s ) nions cn be distributed mong N nionic sites is lso equl to w given by eqution (1.22). The totl probbility W = w.w. The configurtionl entropy is N! S = 2k ln (1.23) bn nsg! ns! which mens We cn now write b sg b sg s s (1.24) S =2k Nln N N n ln N n n ln n b sg b sg s s (1.25) A =n s ε 2kT Nln N N n ln N n n ln n In order to minimize A, we differentite the bove eqution with respect to n s nd equte tht to zero. After rerrnging the result, we get ns ε = exp s (1.26) N 2kT If E s is the energy of formtion of one mole of Schottky defect in the crystl MX, then ns Es = exp (1.27) N 2RT The expression for Schottky defect concentrtion for crystl of composition MX 2 cn be similrly deduced. F HG F HG I K J I K J
21 The Crystl Structure Frenkel Defect Concentrtion A crystl with Frenkel defects will hve equl number of vcncies nd interstitils. Let the number of Frenkel defects be nf. If N is the totl number of lttice sites, there will (N n f ) occupied sites. The number of wys in which n f vcncies (N n f ) occupied sites cn be rrnged in N vilble lttice sites gives the probbility N! w = (1.28) N n! n! Let the totl number of interstitil sites be N*. The number of wys in which n f interstitil toms nd (N* n f ) unoccupied interstitil sites cn rrnge gives the probbility N *! w* = (1.29) en* nfj! nf! Totl configurtionl probbility W = w.w* In this cse, we get N! N *! S = k[ln ln en n! n! N* n! n! ] fj f e fj f The chnge in free energy in forming n f number of Frenkel defects is Solving A = n f kt[ln F HG A n f I KJ VT, n f ( N n )( N* n ) f 2 f e N! ln N n! n! f j f N *! N* n! n! ] e j e j f f f f (1.30) (1.31) = 0 (1.32) F = exp HG Since n f is very much smller thn both N nd N*, we get n F f ε f I = exp = exp NN * HG ktkj F Ef I (1.33) HG ktkj where E f is the energy needed to form mole of Frenkel defects. The concentrtion of the vrious types of point defects present t temperture T cn be clculted if the energy of defect formtion is known Disloctions Disloctions lso known s line defects nd re of two kinds. They re edge disloction nd screw disloction. An edge disloction is wht would hppen if hlf plne is inserted into crystl. The disloction extends long line perpendiculr to the plne of the pper. The structure is distorted ner the disloction nd severl lttice constnts re to be covered before this distortion disppers. Edge disloction cn move long the crystl under sher force. Suppose force is pplied in ε f kt I KJ
22 22 Solid Stte Chemistry opposite directions t the two ends of crystl. Insted of seprting it into two prts tht would need lrge number of bonds to be broken, toms on one side cn just move short distnce. This will mke the disloction move long the crystl in the direction of the sher force. Movement of the edge disloctions in the crystl re responsible for their plstic property. Edge disloction my form ccidentlly during crystl growth or my be produced by bending crystl. Disloction density in common crystl is of the order 10 6 /cm 2. Figure 1.16: Edge disloction Edge disloction re given the symbol s shown in figure 1.16 where the disloction line is perpendiculr to the plne of the pper. Disloctions cn be identified by tking electron microscope pictures t mgnifiction 10 5 or higher where the lttice plnes pper s stright lines. Disloctions show up s disruption in these stright lines. The other type of line defect is known s screw disloction. Screw disloction results from movement of one prt of crystl reltive to nother. The distnce of movement is less thn lttice constnt nd hence the coordintion number of the toms ner the screw disloction does not chnge. The screw disloction only leds to distortion of the bonds in its vicinity. If one moves from lttice point to lttice point round the disloction, one will move up s if in spirl stircse. The nme screw disloction is derived from this (see figure 1.17). Disloctions severely ffect the mechnicl properties of solids. Figure 1.17: Screw disloction
23 The Crystl Structure Plne Defects or Grin Boundries So fr we discussed defects tht re present within crystl. Mny solids however does not exist s single crystl but s ggregte of lrge number of smll crystls or grins. These grins hve different orienttions. As result, the environment of the toms ner the boundry of two such grins is bound to be different from wht it is within crystllite of grin. The periodicity of the toms will be destroyed t nd ner the grin boundry region nd this region will be noncrystlline. If this non-crystlline region is firly thick, ech crystl will try to force the toms of this region to orient themselves in ccordnce with its own structure. This will be so becuse the irregulrity in the non-crystlline region will increse the energy of the solid. Grin boundries my be wide-ngle (figure 1.18) or smll-ngle depending upon the tilt of the ngle of the boundry. Smll-ngle grin boundries cn be treted s series of disloctions. When the grin boundry becomes reflection plne, the two crystls on either side of the reflection plne constitutes twin. Figure 1.18: Wide-ngle grin boundry Grin boundries seriously ffect the mechnicl properties of solids. They cn be removed by het tretment. At high temperture when the mobility of the toms increses, some grins grow t the expense of others nd this process is known s secondry recrystlliztion. SUGGESTED READING 1. Azroff, L.V. Introduction to Solids, TMH edition, Tt McGrw-Hill, New Delhi. 2. Azroff, L.V. nd Buerger, M.J. The Powder Method in X-ry Crystllogrphy, McGrw-Hill, Epifnov, G.I. Solid Stte Physics, MIR, Moscow, Hnny, N.B. Solid Stte Chemistry, Prentice-Hll, New Jersey, Keer, H.V. Principles of the Solid Stte, Wiley Estern, New Delhi, Kittel, C. Introduction to Solid Stte, 3rd edition, John Wiley, Megw, H.D. Crystl Structure: A Working Approch, Sunders College Publ., 1973.
QUB XRD Course. The crystalline state. The Crystalline State
 QUB XRD Course Introduction to Crystllogrphy 1 The crystlline stte Mtter Gseous Stte Solid stte Liquid Stte Amorphous (disordered) Crystlline (ordered) 2 The Crystlline Stte A crystl is constructed by
QUB XRD Course Introduction to Crystllogrphy 1 The crystlline stte Mtter Gseous Stte Solid stte Liquid Stte Amorphous (disordered) Crystlline (ordered) 2 The Crystlline Stte A crystl is constructed by
fiziks Institute for NET/JRF, GATE, IIT JAM, M.Sc. Entrance, JEST, TIFR and GRE in Physics
 Solid Stte Physics JEST-0 Q. bem of X-rys is incident on BCC crystl. If the difference between the incident nd scttered wvevectors is K nxˆkyˆlzˆ where xˆ, yˆ, zˆ re the unit vectors of the ssocited cubic
Solid Stte Physics JEST-0 Q. bem of X-rys is incident on BCC crystl. If the difference between the incident nd scttered wvevectors is K nxˆkyˆlzˆ where xˆ, yˆ, zˆ re the unit vectors of the ssocited cubic
PHY 140A: Solid State Physics. Solution to Midterm #1
 PHY 140A: Solid Stte Physics Solution to Midterm #1 TA: Xun Ji 1 October 24, 2006 1 Emil: jixun@physics.ucl.edu Problem #1 (20pt)Clculte the pcking frction of the body-centered cubic lttice. Solution:
PHY 140A: Solid Stte Physics Solution to Midterm #1 TA: Xun Ji 1 October 24, 2006 1 Emil: jixun@physics.ucl.edu Problem #1 (20pt)Clculte the pcking frction of the body-centered cubic lttice. Solution:
Analytical Methods for Materials
 Anlyticl Methods for Mterils Lesson 7 Crystl Geometry nd Crystllogrphy, Prt 1 Suggested Reding Chpters 2 nd 6 in Wsed et l. 169 Slt crystls N Cl http://helthfreedoms.org/2009/05/24/tble-slt-vs-unrefined-se-slt--primer/
Anlyticl Methods for Mterils Lesson 7 Crystl Geometry nd Crystllogrphy, Prt 1 Suggested Reding Chpters 2 nd 6 in Wsed et l. 169 Slt crystls N Cl http://helthfreedoms.org/2009/05/24/tble-slt-vs-unrefined-se-slt--primer/
Crystalline Structures The Basics
 Crystlline Structures The sics Crystl structure of mteril is wy in which toms, ions, molecules re sptilly rrnged in 3-D spce. Crystl structure = lttice (unit cell geometry) + bsis (tom, ion, or molecule
Crystlline Structures The sics Crystl structure of mteril is wy in which toms, ions, molecules re sptilly rrnged in 3-D spce. Crystl structure = lttice (unit cell geometry) + bsis (tom, ion, or molecule
Chapter 4 Contravariance, Covariance, and Spacetime Diagrams
 Chpter 4 Contrvrince, Covrince, nd Spcetime Digrms 4. The Components of Vector in Skewed Coordintes We hve seen in Chpter 3; figure 3.9, tht in order to show inertil motion tht is consistent with the Lorentz
Chpter 4 Contrvrince, Covrince, nd Spcetime Digrms 4. The Components of Vector in Skewed Coordintes We hve seen in Chpter 3; figure 3.9, tht in order to show inertil motion tht is consistent with the Lorentz
Kai Sun. University of Michigan, Ann Arbor
 Ki Sun University of Michign, Ann Arbor How to see toms in solid? For conductors, we cn utilize scnning tunneling microscope (STM) to see toms (Nobel Prize in Physics in 1986) Limittions: (1) conductors
Ki Sun University of Michign, Ann Arbor How to see toms in solid? For conductors, we cn utilize scnning tunneling microscope (STM) to see toms (Nobel Prize in Physics in 1986) Limittions: (1) conductors
a * a (2,1) 1,1 0,1 1,1 2,1 hkl 1,0 1,0 2,0 O 2,1 0,1 1,1 0,2 1,2 2,2
 18 34.3 The Reciprocl Lttice The inverse of the intersections of plne with the unit cell xes is used to find the Miller indices of the plne. The inverse of the d-spcing etween plnes ppers in expressions
18 34.3 The Reciprocl Lttice The inverse of the intersections of plne with the unit cell xes is used to find the Miller indices of the plne. The inverse of the d-spcing etween plnes ppers in expressions
What is solid state physics?
 Wht is solid stte physics? Explins the properties of solid mterils. Explins the properties of collection of tomic nuclei nd electrons intercting with electrosttic forces. Formultes fundmentl lws tht govern
Wht is solid stte physics? Explins the properties of solid mterils. Explins the properties of collection of tomic nuclei nd electrons intercting with electrosttic forces. Formultes fundmentl lws tht govern
1 Which of the following summarises the change in wave characteristics on going from infra-red to ultraviolet in the electromagnetic spectrum?
 Which of the following summrises the chnge in wve chrcteristics on going from infr-red to ultrviolet in the electromgnetic spectrum? frequency speed (in vcuum) decreses decreses decreses remins constnt
Which of the following summrises the chnge in wve chrcteristics on going from infr-red to ultrviolet in the electromgnetic spectrum? frequency speed (in vcuum) decreses decreses decreses remins constnt
Miller indices and Family of the Planes
 SOLID4 Miller Indices ltest Fmily of Plnes nd Miller indices; Miller indices nd Fmily of the Plnes The geometricl fetures of the crystls represented by lttice points re clled Rtionl. Thus lttice point
SOLID4 Miller Indices ltest Fmily of Plnes nd Miller indices; Miller indices nd Fmily of the Plnes The geometricl fetures of the crystls represented by lttice points re clled Rtionl. Thus lttice point
UNIVERSITY OF OSLO. Faculty of Mathematics and Natural Sciences
 UNIVERSITY OF OSLO Fculty of Mthemtics nd Nturl Sciences Midterm exm in MENA3100 Dy of exm: 19 th Mrch 2018 Exm hours: 14:30 17:30 This exmintion pper consists of 4 pges including 1 ppendix pge. Permitted
UNIVERSITY OF OSLO Fculty of Mthemtics nd Nturl Sciences Midterm exm in MENA3100 Dy of exm: 19 th Mrch 2018 Exm hours: 14:30 17:30 This exmintion pper consists of 4 pges including 1 ppendix pge. Permitted
The Properties of Stars
 10/11/010 The Properties of Strs sses Using Newton s Lw of Grvity to Determine the ss of Celestil ody ny two prticles in the universe ttrct ech other with force tht is directly proportionl to the product
10/11/010 The Properties of Strs sses Using Newton s Lw of Grvity to Determine the ss of Celestil ody ny two prticles in the universe ttrct ech other with force tht is directly proportionl to the product
IV. CONDENSED MATTER PHYSICS
 IV. CONDENSED MATTER PHYSICS UNIT I CRYSTAL PHYSICS Lecture - II Dr. T. J. Shinde Deprtment of Physics Smt. K. R. P. Kny Mhvidyly, Islmpur Simple Crystl Structures Simple cubic (SC) Fce centered cubic
IV. CONDENSED MATTER PHYSICS UNIT I CRYSTAL PHYSICS Lecture - II Dr. T. J. Shinde Deprtment of Physics Smt. K. R. P. Kny Mhvidyly, Islmpur Simple Crystl Structures Simple cubic (SC) Fce centered cubic
Measuring Electron Work Function in Metal
 n experiment of the Electron topic Mesuring Electron Work Function in Metl Instructor: 梁生 Office: 7-318 Emil: shling@bjtu.edu.cn Purposes 1. To understnd the concept of electron work function in metl nd
n experiment of the Electron topic Mesuring Electron Work Function in Metl Instructor: 梁生 Office: 7-318 Emil: shling@bjtu.edu.cn Purposes 1. To understnd the concept of electron work function in metl nd
SUMMER KNOWHOW STUDY AND LEARNING CENTRE
 SUMMER KNOWHOW STUDY AND LEARNING CENTRE Indices & Logrithms 2 Contents Indices.2 Frctionl Indices.4 Logrithms 6 Exponentil equtions. Simplifying Surds 13 Opertions on Surds..16 Scientific Nottion..18
SUMMER KNOWHOW STUDY AND LEARNING CENTRE Indices & Logrithms 2 Contents Indices.2 Frctionl Indices.4 Logrithms 6 Exponentil equtions. Simplifying Surds 13 Opertions on Surds..16 Scientific Nottion..18
State space systems analysis (continued) Stability. A. Definitions A system is said to be Asymptotically Stable (AS) when it satisfies
 Stte spce systems nlysis (continued) Stbility A. Definitions A system is sid to be Asymptoticlly Stble (AS) when it stisfies ut () = 0, t > 0 lim xt () 0. t A system is AS if nd only if the impulse response
Stte spce systems nlysis (continued) Stbility A. Definitions A system is sid to be Asymptoticlly Stble (AS) when it stisfies ut () = 0, t > 0 lim xt () 0. t A system is AS if nd only if the impulse response
The Predom module. Predom calculates and plots isothermal 1-, 2- and 3-metal predominance area diagrams. Predom accesses only compound databases.
 Section 1 Section 2 The module clcultes nd plots isotherml 1-, 2- nd 3-metl predominnce re digrms. ccesses only compound dtbses. Tble of Contents Tble of Contents Opening the module Section 3 Stoichiometric
Section 1 Section 2 The module clcultes nd plots isotherml 1-, 2- nd 3-metl predominnce re digrms. ccesses only compound dtbses. Tble of Contents Tble of Contents Opening the module Section 3 Stoichiometric
STRUCTURAL ISSUES IN SEMICONDUCTORS
 Chpter 1 STRUCTURAL ISSUES IN SEMICONDUCTORS Most semiconductor devices re mde from crystlline mterils. The following gures provide n overview of importnt crystlline properties of semiconductors, like
Chpter 1 STRUCTURAL ISSUES IN SEMICONDUCTORS Most semiconductor devices re mde from crystlline mterils. The following gures provide n overview of importnt crystlline properties of semiconductors, like
Vyacheslav Telnin. Search for New Numbers.
 Vycheslv Telnin Serch for New Numbers. 1 CHAPTER I 2 I.1 Introduction. In 1984, in the first issue for tht yer of the Science nd Life mgzine, I red the rticle "Non-Stndrd Anlysis" by V. Uspensky, in which
Vycheslv Telnin Serch for New Numbers. 1 CHAPTER I 2 I.1 Introduction. In 1984, in the first issue for tht yer of the Science nd Life mgzine, I red the rticle "Non-Stndrd Anlysis" by V. Uspensky, in which
Scientific notation is a way of expressing really big numbers or really small numbers.
 Scientific Nottion (Stndrd form) Scientific nottion is wy of expressing relly big numbers or relly smll numbers. It is most often used in scientific clcultions where the nlysis must be very precise. Scientific
Scientific Nottion (Stndrd form) Scientific nottion is wy of expressing relly big numbers or relly smll numbers. It is most often used in scientific clcultions where the nlysis must be very precise. Scientific
200 points 5 Problems on 4 Pages and 20 Multiple Choice/Short Answer Questions on 5 pages 1 hour, 48 minutes
 PHYSICS 132 Smple Finl 200 points 5 Problems on 4 Pges nd 20 Multiple Choice/Short Answer Questions on 5 pges 1 hour, 48 minutes Student Nme: Recittion Instructor (circle one): nme1 nme2 nme3 nme4 Write
PHYSICS 132 Smple Finl 200 points 5 Problems on 4 Pges nd 20 Multiple Choice/Short Answer Questions on 5 pges 1 hour, 48 minutes Student Nme: Recittion Instructor (circle one): nme1 nme2 nme3 nme4 Write
Name Solutions to Test 3 November 8, 2017
 Nme Solutions to Test 3 November 8, 07 This test consists of three prts. Plese note tht in prts II nd III, you cn skip one question of those offered. Some possibly useful formuls cn be found below. Brrier
Nme Solutions to Test 3 November 8, 07 This test consists of three prts. Plese note tht in prts II nd III, you cn skip one question of those offered. Some possibly useful formuls cn be found below. Brrier
Lecture V. Introduction to Space Groups Charles H. Lake
 Lecture V. Introduction to Spce Groups 2003. Chrles H. Lke Outline:. Introduction B. Trnsltionl symmetry C. Nomenclture nd symols used with spce groups D. The spce groups E. Derivtion nd discussion of
Lecture V. Introduction to Spce Groups 2003. Chrles H. Lke Outline:. Introduction B. Trnsltionl symmetry C. Nomenclture nd symols used with spce groups D. The spce groups E. Derivtion nd discussion of
13: Diffusion in 2 Energy Groups
 3: Diffusion in Energy Groups B. Rouben McMster University Course EP 4D3/6D3 Nucler Rector Anlysis (Rector Physics) 5 Sept.-Dec. 5 September Contents We study the diffusion eqution in two energy groups
3: Diffusion in Energy Groups B. Rouben McMster University Course EP 4D3/6D3 Nucler Rector Anlysis (Rector Physics) 5 Sept.-Dec. 5 September Contents We study the diffusion eqution in two energy groups
Intro to Nuclear and Particle Physics (5110)
 Intro to Nucler nd Prticle Physics (5110) Feb, 009 The Nucler Mss Spectrum The Liquid Drop Model //009 1 E(MeV) n n(n-1)/ E/[ n(n-1)/] (MeV/pir) 1 C 16 O 0 Ne 4 Mg 7.7 14.44 19.17 8.48 4 5 6 6 10 15.4.41
Intro to Nucler nd Prticle Physics (5110) Feb, 009 The Nucler Mss Spectrum The Liquid Drop Model //009 1 E(MeV) n n(n-1)/ E/[ n(n-1)/] (MeV/pir) 1 C 16 O 0 Ne 4 Mg 7.7 14.44 19.17 8.48 4 5 6 6 10 15.4.41
CHM Physical Chemistry I Chapter 1 - Supplementary Material
 CHM 3410 - Physicl Chemistry I Chpter 1 - Supplementry Mteril For review of some bsic concepts in mth, see Atkins "Mthemticl Bckground 1 (pp 59-6), nd "Mthemticl Bckground " (pp 109-111). 1. Derivtion
CHM 3410 - Physicl Chemistry I Chpter 1 - Supplementry Mteril For review of some bsic concepts in mth, see Atkins "Mthemticl Bckground 1 (pp 59-6), nd "Mthemticl Bckground " (pp 109-111). 1. Derivtion
Jackson 2.26 Homework Problem Solution Dr. Christopher S. Baird University of Massachusetts Lowell
 Jckson 2.26 Homework Problem Solution Dr. Christopher S. Bird University of Msschusetts Lowell PROBLEM: The two-dimensionl region, ρ, φ β, is bounded by conducting surfces t φ =, ρ =, nd φ = β held t zero
Jckson 2.26 Homework Problem Solution Dr. Christopher S. Bird University of Msschusetts Lowell PROBLEM: The two-dimensionl region, ρ, φ β, is bounded by conducting surfces t φ =, ρ =, nd φ = β held t zero
interatomic distance
 Dissocition energy of Iodine molecule using constnt devition spectrometer Tbish Qureshi September 2003 Aim: To verify the Hrtmnn Dispersion Formul nd to determine the dissocition energy of I 2 molecule
Dissocition energy of Iodine molecule using constnt devition spectrometer Tbish Qureshi September 2003 Aim: To verify the Hrtmnn Dispersion Formul nd to determine the dissocition energy of I 2 molecule
Materials Analysis MATSCI 162/172 Laboratory Exercise No. 1 Crystal Structure Determination Pattern Indexing
 Mterils Anlysis MATSCI 16/17 Lbortory Exercise No. 1 Crystl Structure Determintion Pttern Inexing Objectives: To inex the x-ry iffrction pttern, ientify the Brvis lttice, n clculte the precise lttice prmeters.
Mterils Anlysis MATSCI 16/17 Lbortory Exercise No. 1 Crystl Structure Determintion Pttern Inexing Objectives: To inex the x-ry iffrction pttern, ientify the Brvis lttice, n clculte the precise lttice prmeters.
- 5 - TEST 2. This test is on the final sections of this session's syllabus and. should be attempted by all students.
 - 5 - TEST 2 This test is on the finl sections of this session's syllbus nd should be ttempted by ll students. Anything written here will not be mrked. - 6 - QUESTION 1 [Mrks 22] A thin non-conducting
- 5 - TEST 2 This test is on the finl sections of this session's syllbus nd should be ttempted by ll students. Anything written here will not be mrked. - 6 - QUESTION 1 [Mrks 22] A thin non-conducting
LECTURE 14. Dr. Teresa D. Golden University of North Texas Department of Chemistry
 LECTURE 14 Dr. Teres D. Golden University of North Texs Deprtment of Chemistry Quntittive Methods A. Quntittive Phse Anlysis Qulittive D phses by comprison with stndrd ptterns. Estimte of proportions of
LECTURE 14 Dr. Teres D. Golden University of North Texs Deprtment of Chemistry Quntittive Methods A. Quntittive Phse Anlysis Qulittive D phses by comprison with stndrd ptterns. Estimte of proportions of
1.Bravais Lattices The Bravais lattices Bravais Lattice detail
 1.Brvis Lttices 12.1. The Brvis lttices 2.2.4 Brvis Lttice detil The Brvis lttice re the distinct lttice types which when repeted cn fill the whole spce. The lttice cn therefore be generted by three unit
1.Brvis Lttices 12.1. The Brvis lttices 2.2.4 Brvis Lttice detil The Brvis lttice re the distinct lttice types which when repeted cn fill the whole spce. The lttice cn therefore be generted by three unit
Lecture 13 - Linking E, ϕ, and ρ
 Lecture 13 - Linking E, ϕ, nd ρ A Puzzle... Inner-Surfce Chrge Density A positive point chrge q is locted off-center inside neutrl conducting sphericl shell. We know from Guss s lw tht the totl chrge on
Lecture 13 - Linking E, ϕ, nd ρ A Puzzle... Inner-Surfce Chrge Density A positive point chrge q is locted off-center inside neutrl conducting sphericl shell. We know from Guss s lw tht the totl chrge on
13.3 CLASSICAL STRAIGHTEDGE AND COMPASS CONSTRUCTIONS
 33 CLASSICAL STRAIGHTEDGE AND COMPASS CONSTRUCTIONS As simple ppliction of the results we hve obtined on lgebric extensions, nd in prticulr on the multiplictivity of extension degrees, we cn nswer (in
33 CLASSICAL STRAIGHTEDGE AND COMPASS CONSTRUCTIONS As simple ppliction of the results we hve obtined on lgebric extensions, nd in prticulr on the multiplictivity of extension degrees, we cn nswer (in
Energy Bands Energy Bands and Band Gap. Phys463.nb Phenomenon
 Phys463.nb 49 7 Energy Bnds Ref: textbook, Chpter 7 Q: Why re there insultors nd conductors? Q: Wht will hppen when n electron moves in crystl? In the previous chpter, we discussed free electron gses,
Phys463.nb 49 7 Energy Bnds Ref: textbook, Chpter 7 Q: Why re there insultors nd conductors? Q: Wht will hppen when n electron moves in crystl? In the previous chpter, we discussed free electron gses,
CALCULATED POWDER X-RAY DIFFRACTION LINE PROFILES VIA ABSORPTION
 16 17 CALCULATED POWDER X-RAY DFFRACTON LNE PROFLES VA ABSORPTON Keji Liu nd Heifen Chen School of Mteril Science nd Engineering, Shnghi nstitute of Technology, Shnghi, Chin 2233 ABSTRACT We hve clculted
16 17 CALCULATED POWDER X-RAY DFFRACTON LNE PROFLES VA ABSORPTON Keji Liu nd Heifen Chen School of Mteril Science nd Engineering, Shnghi nstitute of Technology, Shnghi, Chin 2233 ABSTRACT We hve clculted
7.2 The Definite Integral
 7.2 The Definite Integrl the definite integrl In the previous section, it ws found tht if function f is continuous nd nonnegtive, then the re under the grph of f on [, b] is given by F (b) F (), where
7.2 The Definite Integrl the definite integrl In the previous section, it ws found tht if function f is continuous nd nonnegtive, then the re under the grph of f on [, b] is given by F (b) F (), where
KINEMATICS OF RIGID BODIES
 KINEMTICS OF RIGID ODIES Introduction In rigid body kinemtics, e use the reltionships governing the displcement, velocity nd ccelertion, but must lso ccount for the rottionl motion of the body. Description
KINEMTICS OF RIGID ODIES Introduction In rigid body kinemtics, e use the reltionships governing the displcement, velocity nd ccelertion, but must lso ccount for the rottionl motion of the body. Description
What is thin film/layer?
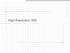 High-esolution XD Wht is thin film/lyer? Mteril so thin tht its chrcteristics re dominted primrily by two dimensionl effects nd re mostly different thn its bulk properties Source: semiconductorglossry.com
High-esolution XD Wht is thin film/lyer? Mteril so thin tht its chrcteristics re dominted primrily by two dimensionl effects nd re mostly different thn its bulk properties Source: semiconductorglossry.com
We partition C into n small arcs by forming a partition of [a, b] by picking s i as follows: a = s 0 < s 1 < < s n = b.
![We partition C into n small arcs by forming a partition of [a, b] by picking s i as follows: a = s 0 < s 1 < < s n = b. We partition C into n small arcs by forming a partition of [a, b] by picking s i as follows: a = s 0 < s 1 < < s n = b.](/thumbs/93/114277851.jpg) Mth 255 - Vector lculus II Notes 4.2 Pth nd Line Integrls We begin with discussion of pth integrls (the book clls them sclr line integrls). We will do this for function of two vribles, but these ides cn
Mth 255 - Vector lculus II Notes 4.2 Pth nd Line Integrls We begin with discussion of pth integrls (the book clls them sclr line integrls). We will do this for function of two vribles, but these ides cn
#6A&B Magnetic Field Mapping
 #6A& Mgnetic Field Mpping Gol y performing this lb experiment, you will: 1. use mgnetic field mesurement technique bsed on Frdy s Lw (see the previous experiment),. study the mgnetic fields generted by
#6A& Mgnetic Field Mpping Gol y performing this lb experiment, you will: 1. use mgnetic field mesurement technique bsed on Frdy s Lw (see the previous experiment),. study the mgnetic fields generted by
Math 8 Winter 2015 Applications of Integration
 Mth 8 Winter 205 Applictions of Integrtion Here re few importnt pplictions of integrtion. The pplictions you my see on n exm in this course include only the Net Chnge Theorem (which is relly just the Fundmentl
Mth 8 Winter 205 Applictions of Integrtion Here re few importnt pplictions of integrtion. The pplictions you my see on n exm in this course include only the Net Chnge Theorem (which is relly just the Fundmentl
Exam 1 Solutions (1) C, D, A, B (2) C, A, D, B (3) C, B, D, A (4) A, C, D, B (5) D, C, A, B
 PHY 249, Fll 216 Exm 1 Solutions nswer 1 is correct for ll problems. 1. Two uniformly chrged spheres, nd B, re plced t lrge distnce from ech other, with their centers on the x xis. The chrge on sphere
PHY 249, Fll 216 Exm 1 Solutions nswer 1 is correct for ll problems. 1. Two uniformly chrged spheres, nd B, re plced t lrge distnce from ech other, with their centers on the x xis. The chrge on sphere
1 1. Crystallography 1.1 Introduction 1.2 Crystalline and Non-crystalline materials crystalline materials single crystals polycrystalline material
 P g e. Crystllogrphy. Introduction Crystllogrphy is the brnch of science tht dels bout the crystl structures of elements. The crystl structures of elements re studied by mens of X-ry diffrction or electron
P g e. Crystllogrphy. Introduction Crystllogrphy is the brnch of science tht dels bout the crystl structures of elements. The crystl structures of elements re studied by mens of X-ry diffrction or electron
Properties of Integrals, Indefinite Integrals. Goals: Definition of the Definite Integral Integral Calculations using Antiderivatives
 Block #6: Properties of Integrls, Indefinite Integrls Gols: Definition of the Definite Integrl Integrl Clcultions using Antiderivtives Properties of Integrls The Indefinite Integrl 1 Riemnn Sums - 1 Riemnn
Block #6: Properties of Integrls, Indefinite Integrls Gols: Definition of the Definite Integrl Integrl Clcultions using Antiderivtives Properties of Integrls The Indefinite Integrl 1 Riemnn Sums - 1 Riemnn
7.1 Integral as Net Change and 7.2 Areas in the Plane Calculus
 7.1 Integrl s Net Chnge nd 7. Ares in the Plne Clculus 7.1 INTEGRAL AS NET CHANGE Notecrds from 7.1: Displcement vs Totl Distnce, Integrl s Net Chnge We hve lredy seen how the position of n oject cn e
7.1 Integrl s Net Chnge nd 7. Ares in the Plne Clculus 7.1 INTEGRAL AS NET CHANGE Notecrds from 7.1: Displcement vs Totl Distnce, Integrl s Net Chnge We hve lredy seen how the position of n oject cn e
DIRECT CURRENT CIRCUITS
 DRECT CURRENT CUTS ELECTRC POWER Consider the circuit shown in the Figure where bttery is connected to resistor R. A positive chrge dq will gin potentil energy s it moves from point to point b through
DRECT CURRENT CUTS ELECTRC POWER Consider the circuit shown in the Figure where bttery is connected to resistor R. A positive chrge dq will gin potentil energy s it moves from point to point b through
Physics 9 Fall 2011 Homework 2 - Solutions Friday September 2, 2011
 Physics 9 Fll 0 Homework - s Fridy September, 0 Mke sure your nme is on your homework, nd plese box your finl nswer. Becuse we will be giving prtil credit, be sure to ttempt ll the problems, even if you
Physics 9 Fll 0 Homework - s Fridy September, 0 Mke sure your nme is on your homework, nd plese box your finl nswer. Becuse we will be giving prtil credit, be sure to ttempt ll the problems, even if you
Week 10: Line Integrals
 Week 10: Line Integrls Introduction In this finl week we return to prmetrised curves nd consider integrtion long such curves. We lredy sw this in Week 2 when we integrted long curve to find its length.
Week 10: Line Integrls Introduction In this finl week we return to prmetrised curves nd consider integrtion long such curves. We lredy sw this in Week 2 when we integrted long curve to find its length.
How do we solve these things, especially when they get complicated? How do we know when a system has a solution, and when is it unique?
 XII. LINEAR ALGEBRA: SOLVING SYSTEMS OF EQUATIONS Tody we re going to tlk bout solving systems of liner equtions. These re problems tht give couple of equtions with couple of unknowns, like: 6 2 3 7 4
XII. LINEAR ALGEBRA: SOLVING SYSTEMS OF EQUATIONS Tody we re going to tlk bout solving systems of liner equtions. These re problems tht give couple of equtions with couple of unknowns, like: 6 2 3 7 4
Improper Integrals, and Differential Equations
 Improper Integrls, nd Differentil Equtions October 22, 204 5.3 Improper Integrls Previously, we discussed how integrls correspond to res. More specificlly, we sid tht for function f(x), the region creted
Improper Integrls, nd Differentil Equtions October 22, 204 5.3 Improper Integrls Previously, we discussed how integrls correspond to res. More specificlly, we sid tht for function f(x), the region creted
Period #2 Notes: Electronic Structure of Atoms
 Period # Notes: Electronic Structure of Atoms The logicl plce (for civil engineers) to begin in describing mterils is t the tomic scle. The bsic elements of the tom re the proton, the neutron, nd the electron:
Period # Notes: Electronic Structure of Atoms The logicl plce (for civil engineers) to begin in describing mterils is t the tomic scle. The bsic elements of the tom re the proton, the neutron, nd the electron:
The Thermodynamics of Aqueous Electrolyte Solutions
 18 The Thermodynmics of Aqueous Electrolyte Solutions As discussed in Chpter 10, when slt is dissolved in wter or in other pproprite solvent, the molecules dissocite into ions. In queous solutions, strong
18 The Thermodynmics of Aqueous Electrolyte Solutions As discussed in Chpter 10, when slt is dissolved in wter or in other pproprite solvent, the molecules dissocite into ions. In queous solutions, strong
Strategy: Use the Gibbs phase rule (Equation 5.3). How many components are present?
 University Chemistry Quiz 4 2014/12/11 1. (5%) Wht is the dimensionlity of the three-phse coexistence region in mixture of Al, Ni, nd Cu? Wht type of geometricl region dose this define? Strtegy: Use the
University Chemistry Quiz 4 2014/12/11 1. (5%) Wht is the dimensionlity of the three-phse coexistence region in mixture of Al, Ni, nd Cu? Wht type of geometricl region dose this define? Strtegy: Use the
LUMS School of Science and Engineering
 LUMS School of Science nd Engineering PH- Solution of ssignment Mrch, 0, 0 Brvis Lttice Answer: We hve given tht c.5(î + ĵ + ˆk) 5 (î + ĵ + ˆk) 0 (î + ĵ + ˆk) c (î + ĵ + ˆk) î + ĵ + ˆk + b + c î, b ĵ nd
LUMS School of Science nd Engineering PH- Solution of ssignment Mrch, 0, 0 Brvis Lttice Answer: We hve given tht c.5(î + ĵ + ˆk) 5 (î + ĵ + ˆk) 0 (î + ĵ + ˆk) c (î + ĵ + ˆk) î + ĵ + ˆk + b + c î, b ĵ nd
Conducting Ellipsoid and Circular Disk
 1 Problem Conducting Ellipsoid nd Circulr Disk Kirk T. McDonld Joseph Henry Lbortories, Princeton University, Princeton, NJ 08544 (September 1, 00) Show tht the surfce chrge density σ on conducting ellipsoid,
1 Problem Conducting Ellipsoid nd Circulr Disk Kirk T. McDonld Joseph Henry Lbortories, Princeton University, Princeton, NJ 08544 (September 1, 00) Show tht the surfce chrge density σ on conducting ellipsoid,
R. I. Badran Solid State Physics
 I Bdrn Solid Stte Physics Crystl vibrtions nd the clssicl theory: The ssmption will be mde to consider tht the men eqilibrim position of ech ion is t Brvis lttice site The ions oscillte bot this men position
I Bdrn Solid Stte Physics Crystl vibrtions nd the clssicl theory: The ssmption will be mde to consider tht the men eqilibrim position of ech ion is t Brvis lttice site The ions oscillte bot this men position
Higher Checklist (Unit 3) Higher Checklist (Unit 3) Vectors
 Vectors Skill Achieved? Know tht sclr is quntity tht hs only size (no direction) Identify rel-life exmples of sclrs such s, temperture, mss, distnce, time, speed, energy nd electric chrge Know tht vector
Vectors Skill Achieved? Know tht sclr is quntity tht hs only size (no direction) Identify rel-life exmples of sclrs such s, temperture, mss, distnce, time, speed, energy nd electric chrge Know tht vector
Recitation 3: More Applications of the Derivative
 Mth 1c TA: Pdric Brtlett Recittion 3: More Applictions of the Derivtive Week 3 Cltech 2012 1 Rndom Question Question 1 A grph consists of the following: A set V of vertices. A set E of edges where ech
Mth 1c TA: Pdric Brtlett Recittion 3: More Applictions of the Derivtive Week 3 Cltech 2012 1 Rndom Question Question 1 A grph consists of the following: A set V of vertices. A set E of edges where ech
Summary of equations chapters 7. To make current flow you have to push on the charges. For most materials:
 Summry of equtions chpters 7. To mke current flow you hve to push on the chrges. For most mterils: J E E [] The resistivity is prmeter tht vries more thn 4 orders of mgnitude between silver (.6E-8 Ohm.m)
Summry of equtions chpters 7. To mke current flow you hve to push on the chrges. For most mterils: J E E [] The resistivity is prmeter tht vries more thn 4 orders of mgnitude between silver (.6E-8 Ohm.m)
 Rel Gses 1. Gses (N, CO ) which don t obey gs lws or gs eqution P=RT t ll pressure nd tempertures re clled rel gses.. Rel gses obey gs lws t extremely low pressure nd high temperture. Rel gses devited
Rel Gses 1. Gses (N, CO ) which don t obey gs lws or gs eqution P=RT t ll pressure nd tempertures re clled rel gses.. Rel gses obey gs lws t extremely low pressure nd high temperture. Rel gses devited
Section 14.3 Arc Length and Curvature
 Section 4.3 Arc Length nd Curvture Clculus on Curves in Spce In this section, we ly the foundtions for describing the movement of n object in spce.. Vector Function Bsics In Clc, formul for rc length in
Section 4.3 Arc Length nd Curvture Clculus on Curves in Spce In this section, we ly the foundtions for describing the movement of n object in spce.. Vector Function Bsics In Clc, formul for rc length in
References and Resources:
 Surfce nd Interfce Science Physics 627; Chemistry 542 Lectures 4 Feb 3, 2013 Determining Surfce Structure Diffrction methods: LEED; RHEED Rel Spce: STEM References nd Resources: Woodruff nd Delchr (2 nd
Surfce nd Interfce Science Physics 627; Chemistry 542 Lectures 4 Feb 3, 2013 Determining Surfce Structure Diffrction methods: LEED; RHEED Rel Spce: STEM References nd Resources: Woodruff nd Delchr (2 nd
University of Alabama Department of Physics and Astronomy. PH126: Exam 1
 University of Albm Deprtment of Physics nd Astronomy PH 16 LeClir Fll 011 Instructions: PH16: Exm 1 1. Answer four of the five questions below. All problems hve equl weight.. You must show your work for
University of Albm Deprtment of Physics nd Astronomy PH 16 LeClir Fll 011 Instructions: PH16: Exm 1 1. Answer four of the five questions below. All problems hve equl weight.. You must show your work for
Goals: Determine how to calculate the area described by a function. Define the definite integral. Explore the relationship between the definite
 Unit #8 : The Integrl Gols: Determine how to clculte the re described by function. Define the definite integrl. Eplore the reltionship between the definite integrl nd re. Eplore wys to estimte the definite
Unit #8 : The Integrl Gols: Determine how to clculte the re described by function. Define the definite integrl. Eplore the reltionship between the definite integrl nd re. Eplore wys to estimte the definite
Point Lattices: Bravais Lattices
 Physics for Solid Stte Applictions Februry 18, 2004 Lecture 7: Periodic Structures (cont.) Outline Review 2D & 3D Periodic Crystl Structures: Mthemtics X-Ry Diffrction: Observing Reciprocl Spce Point Lttices:
Physics for Solid Stte Applictions Februry 18, 2004 Lecture 7: Periodic Structures (cont.) Outline Review 2D & 3D Periodic Crystl Structures: Mthemtics X-Ry Diffrction: Observing Reciprocl Spce Point Lttices:
2. VECTORS AND MATRICES IN 3 DIMENSIONS
 2 VECTORS AND MATRICES IN 3 DIMENSIONS 21 Extending the Theory of 2-dimensionl Vectors x A point in 3-dimensionl spce cn e represented y column vector of the form y z z-xis y-xis z x y x-xis Most of the
2 VECTORS AND MATRICES IN 3 DIMENSIONS 21 Extending the Theory of 2-dimensionl Vectors x A point in 3-dimensionl spce cn e represented y column vector of the form y z z-xis y-xis z x y x-xis Most of the
13.4 Work done by Constant Forces
 13.4 Work done by Constnt Forces We will begin our discussion of the concept of work by nlyzing the motion of n object in one dimension cted on by constnt forces. Let s consider the following exmple: push
13.4 Work done by Constnt Forces We will begin our discussion of the concept of work by nlyzing the motion of n object in one dimension cted on by constnt forces. Let s consider the following exmple: push
Consequently, the temperature must be the same at each point in the cross section at x. Let:
 HW 2 Comments: L1-3. Derive the het eqution for n inhomogeneous rod where the therml coefficients used in the derivtion of the het eqution for homogeneous rod now become functions of position x in the
HW 2 Comments: L1-3. Derive the het eqution for n inhomogeneous rod where the therml coefficients used in the derivtion of the het eqution for homogeneous rod now become functions of position x in the
Chapter One Crystal Structure
 Chpter One Crystl Structure Drusy Qurtz in Geode Tbulr Orthoclse Feldspr Encrusting Smithsonite Peruvin Pyrite http://www.rockhounds.com/rockshop/xtl 1 Snow crystls the Beltsville Agriculturl Reserch Center
Chpter One Crystl Structure Drusy Qurtz in Geode Tbulr Orthoclse Feldspr Encrusting Smithsonite Peruvin Pyrite http://www.rockhounds.com/rockshop/xtl 1 Snow crystls the Beltsville Agriculturl Reserch Center
Method of Localisation and Controlled Ejection of Swarms of Likely Charged Particles
 Method of Loclistion nd Controlled Ejection of Swrms of Likely Chrged Prticles I. N. Tukev July 3, 17 Astrct This work considers Coulom forces cting on chrged point prticle locted etween the two coxil,
Method of Loclistion nd Controlled Ejection of Swrms of Likely Chrged Prticles I. N. Tukev July 3, 17 Astrct This work considers Coulom forces cting on chrged point prticle locted etween the two coxil,
20 MATHEMATICS POLYNOMIALS
 0 MATHEMATICS POLYNOMIALS.1 Introduction In Clss IX, you hve studied polynomils in one vrible nd their degrees. Recll tht if p(x) is polynomil in x, the highest power of x in p(x) is clled the degree of
0 MATHEMATICS POLYNOMIALS.1 Introduction In Clss IX, you hve studied polynomils in one vrible nd their degrees. Recll tht if p(x) is polynomil in x, the highest power of x in p(x) is clled the degree of
Crystals. Fig From Principles of Electronic Materials and Devices, Third Edition, S.O. Kasap ( McGraw-Hill, 2005)
 Crystls Mterils will often orgnize themselves by minimizing energy to hve long rnge order. This order results in periodicity tht determines mny properties of the mteril. We represent this periodicity by
Crystls Mterils will often orgnize themselves by minimizing energy to hve long rnge order. This order results in periodicity tht determines mny properties of the mteril. We represent this periodicity by
( dg. ) 2 dt. + dt. dt j + dh. + dt. r(t) dt. Comparing this equation with the one listed above for the length of see that
 Arc Length of Curves in Three Dimensionl Spce If the vector function r(t) f(t) i + g(t) j + h(t) k trces out the curve C s t vries, we cn mesure distnces long C using formul nerly identicl to one tht we
Arc Length of Curves in Three Dimensionl Spce If the vector function r(t) f(t) i + g(t) j + h(t) k trces out the curve C s t vries, we cn mesure distnces long C using formul nerly identicl to one tht we
25 Which of the following summarises the change in wave characteristics on going from infra-red to ultraviolet in the electromagnetic spectrum?
 PhysicsndMthsTutor.com 25 Which of the following summrises the chnge in wve chrcteristics on going from infr-red to ultrviolet in the electromgnetic spectrum? 972//M/J/2 frequency speed (in vcuum) decreses
PhysicsndMthsTutor.com 25 Which of the following summrises the chnge in wve chrcteristics on going from infr-red to ultrviolet in the electromgnetic spectrum? 972//M/J/2 frequency speed (in vcuum) decreses
( ) ( ) Chapter 5 Diffraction condition. ρ j
 Grdute School of Engineering Ngo Institute of Technolog Crstl Structure Anlsis Tkshi Id (Advnced Cermics Reserch Center) Updted Nov. 3 3 Chpter 5 Diffrction condition In Chp. 4 it hs been shown tht the
Grdute School of Engineering Ngo Institute of Technolog Crstl Structure Anlsis Tkshi Id (Advnced Cermics Reserch Center) Updted Nov. 3 3 Chpter 5 Diffrction condition In Chp. 4 it hs been shown tht the
SOLUTIONS FOR ADMISSIONS TEST IN MATHEMATICS, COMPUTER SCIENCE AND JOINT SCHOOLS WEDNESDAY 5 NOVEMBER 2014
 SOLUTIONS FOR ADMISSIONS TEST IN MATHEMATICS, COMPUTER SCIENCE AND JOINT SCHOOLS WEDNESDAY 5 NOVEMBER 014 Mrk Scheme: Ech prt of Question 1 is worth four mrks which re wrded solely for the correct nswer.
SOLUTIONS FOR ADMISSIONS TEST IN MATHEMATICS, COMPUTER SCIENCE AND JOINT SCHOOLS WEDNESDAY 5 NOVEMBER 014 Mrk Scheme: Ech prt of Question 1 is worth four mrks which re wrded solely for the correct nswer.
DETERMINATION OF MECHANICAL PROPERTIES OF NANOSTRUCTURES WITH COMPLEX CRYSTAL LATTICE USING MOMENT INTERACTION AT MICROSCALE
 Determintion RevAdvMterSci of mechnicl 0(009) -7 properties of nnostructures with complex crystl lttice using DETERMINATION OF MECHANICAL PROPERTIES OF NANOSTRUCTURES WITH COMPLEX CRYSTAL LATTICE USING
Determintion RevAdvMterSci of mechnicl 0(009) -7 properties of nnostructures with complex crystl lttice using DETERMINATION OF MECHANICAL PROPERTIES OF NANOSTRUCTURES WITH COMPLEX CRYSTAL LATTICE USING
THREE-DIMENSIONAL KINEMATICS OF RIGID BODIES
 THREE-DIMENSIONAL KINEMATICS OF RIGID BODIES 1. TRANSLATION Figure shows rigid body trnslting in three-dimensionl spce. Any two points in the body, such s A nd B, will move long prllel stright lines if
THREE-DIMENSIONAL KINEMATICS OF RIGID BODIES 1. TRANSLATION Figure shows rigid body trnslting in three-dimensionl spce. Any two points in the body, such s A nd B, will move long prllel stright lines if
ragsdale (zdr82) HW2 ditmire (58335) 1
 rgsdle (zdr82) HW2 ditmire (58335) This print-out should hve 22 questions. Multiple-choice questions my continue on the next column or pge find ll choices before nswering. 00 0.0 points A chrge of 8. µc
rgsdle (zdr82) HW2 ditmire (58335) This print-out should hve 22 questions. Multiple-choice questions my continue on the next column or pge find ll choices before nswering. 00 0.0 points A chrge of 8. µc
The solutions of the single electron Hamiltonian were shown to be Bloch wave of the form: ( ) ( ) ikr
 Lecture #1 Progrm 1. Bloch solutions. Reciprocl spce 3. Alternte derivtion of Bloch s theorem 4. Trnsforming the serch for egenfunctions nd eigenvlues from solving PDE to finding the e-vectors nd e-vlues
Lecture #1 Progrm 1. Bloch solutions. Reciprocl spce 3. Alternte derivtion of Bloch s theorem 4. Trnsforming the serch for egenfunctions nd eigenvlues from solving PDE to finding the e-vectors nd e-vlues
Wave Phenomena Physics 15c
 Wve Phenomen Physics 15c Lecture Diffrction (H&L Chpter 11) Wht We Did Lst Time! Studied interference! or more wves overlp " Amplitudes dd up " Intensity = (mplitude) does not dd up! Thin-film interference!
Wve Phenomen Physics 15c Lecture Diffrction (H&L Chpter 11) Wht We Did Lst Time! Studied interference! or more wves overlp " Amplitudes dd up " Intensity = (mplitude) does not dd up! Thin-film interference!
Problem Set 4: Mostly Magnetic
 University of Albm Deprtment of Physics nd Astronomy PH 102 / LeClir Summer 2012 nstructions: Problem Set 4: Mostly Mgnetic 1. Answer ll questions below. Show your work for full credit. 2. All problems
University of Albm Deprtment of Physics nd Astronomy PH 102 / LeClir Summer 2012 nstructions: Problem Set 4: Mostly Mgnetic 1. Answer ll questions below. Show your work for full credit. 2. All problems
Prof. Anchordoqui. Problems set # 4 Physics 169 March 3, 2015
 Prof. Anchordoui Problems set # 4 Physics 169 Mrch 3, 15 1. (i) Eight eul chrges re locted t corners of cube of side s, s shown in Fig. 1. Find electric potentil t one corner, tking zero potentil to be
Prof. Anchordoui Problems set # 4 Physics 169 Mrch 3, 15 1. (i) Eight eul chrges re locted t corners of cube of side s, s shown in Fig. 1. Find electric potentil t one corner, tking zero potentil to be
ADVANCEMENT OF THE CLOSELY COUPLED PROBES POTENTIAL DROP TECHNIQUE FOR NDE OF SURFACE CRACKS
 ADVANCEMENT OF THE CLOSELY COUPLED PROBES POTENTIAL DROP TECHNIQUE FOR NDE OF SURFACE CRACKS F. Tkeo 1 nd M. Sk 1 Hchinohe Ntionl College of Technology, Hchinohe, Jpn; Tohoku University, Sendi, Jpn Abstrct:
ADVANCEMENT OF THE CLOSELY COUPLED PROBES POTENTIAL DROP TECHNIQUE FOR NDE OF SURFACE CRACKS F. Tkeo 1 nd M. Sk 1 Hchinohe Ntionl College of Technology, Hchinohe, Jpn; Tohoku University, Sendi, Jpn Abstrct:
Fundamentals of Analytical Chemistry
 Homework Fundmentls of nlyticl hemistry hpter 9 0, 1, 5, 7, 9 cids, Bses, nd hpter 9(b) Definitions cid Releses H ions in wter (rrhenius) Proton donor (Bronsted( Lowry) Electron-pir cceptor (Lewis) hrcteristic
Homework Fundmentls of nlyticl hemistry hpter 9 0, 1, 5, 7, 9 cids, Bses, nd hpter 9(b) Definitions cid Releses H ions in wter (rrhenius) Proton donor (Bronsted( Lowry) Electron-pir cceptor (Lewis) hrcteristic
2.57/2.570 Midterm Exam No. 1 March 31, :00 am -12:30 pm
 2.57/2.570 Midterm Exm No. 1 Mrch 31, 2010 11:00 m -12:30 pm Instructions: (1) 2.57 students: try ll problems (2) 2.570 students: Problem 1 plus one of two long problems. You cn lso do both long problems,
2.57/2.570 Midterm Exm No. 1 Mrch 31, 2010 11:00 m -12:30 pm Instructions: (1) 2.57 students: try ll problems (2) 2.570 students: Problem 1 plus one of two long problems. You cn lso do both long problems,
Classical Mechanics. From Molecular to Con/nuum Physics I WS 11/12 Emiliano Ippoli/ October, 2011
 Clssicl Mechnics From Moleculr to Con/nuum Physics I WS 11/12 Emilino Ippoli/ October, 2011 Wednesdy, October 12, 2011 Review Mthemtics... Physics Bsic thermodynmics Temperture, idel gs, kinetic gs theory,
Clssicl Mechnics From Moleculr to Con/nuum Physics I WS 11/12 Emilino Ippoli/ October, 2011 Wednesdy, October 12, 2011 Review Mthemtics... Physics Bsic thermodynmics Temperture, idel gs, kinetic gs theory,
CAPACITORS AND DIELECTRICS
 Importnt Definitions nd Units Cpcitnce: CAPACITORS AND DIELECTRICS The property of system of electricl conductors nd insultors which enbles it to store electric chrge when potentil difference exists between
Importnt Definitions nd Units Cpcitnce: CAPACITORS AND DIELECTRICS The property of system of electricl conductors nd insultors which enbles it to store electric chrge when potentil difference exists between
Applications of Bernoulli s theorem. Lecture - 7
 Applictions of Bernoulli s theorem Lecture - 7 Prcticl Applictions of Bernoulli s Theorem The Bernoulli eqution cn be pplied to gret mny situtions not just the pipe flow we hve been considering up to now.
Applictions of Bernoulli s theorem Lecture - 7 Prcticl Applictions of Bernoulli s Theorem The Bernoulli eqution cn be pplied to gret mny situtions not just the pipe flow we hve been considering up to now.
Acceptance Sampling by Attributes
 Introduction Acceptnce Smpling by Attributes Acceptnce smpling is concerned with inspection nd decision mking regrding products. Three spects of smpling re importnt: o Involves rndom smpling of n entire
Introduction Acceptnce Smpling by Attributes Acceptnce smpling is concerned with inspection nd decision mking regrding products. Three spects of smpling re importnt: o Involves rndom smpling of n entire
Chapter 14. Matrix Representations of Linear Transformations
 Chpter 4 Mtrix Representtions of Liner Trnsformtions When considering the Het Stte Evolution, we found tht we could describe this process using multipliction by mtrix. This ws nice becuse computers cn
Chpter 4 Mtrix Representtions of Liner Trnsformtions When considering the Het Stte Evolution, we found tht we could describe this process using multipliction by mtrix. This ws nice becuse computers cn
W. We shall do so one by one, starting with I 1, and we shall do it greedily, trying
 Vitli covers 1 Definition. A Vitli cover of set E R is set V of closed intervls with positive length so tht, for every δ > 0 nd every x E, there is some I V with λ(i ) < δ nd x I. 2 Lemm (Vitli covering)
Vitli covers 1 Definition. A Vitli cover of set E R is set V of closed intervls with positive length so tht, for every δ > 0 nd every x E, there is some I V with λ(i ) < δ nd x I. 2 Lemm (Vitli covering)
Riemann Sums and Riemann Integrals
 Riemnn Sums nd Riemnn Integrls Jmes K. Peterson Deprtment of Biologicl Sciences nd Deprtment of Mthemticl Sciences Clemson University August 26, 203 Outline Riemnn Sums Riemnn Integrls Properties Abstrct
Riemnn Sums nd Riemnn Integrls Jmes K. Peterson Deprtment of Biologicl Sciences nd Deprtment of Mthemticl Sciences Clemson University August 26, 203 Outline Riemnn Sums Riemnn Integrls Properties Abstrct
Riemann Sums and Riemann Integrals
 Riemnn Sums nd Riemnn Integrls Jmes K. Peterson Deprtment of Biologicl Sciences nd Deprtment of Mthemticl Sciences Clemson University August 26, 2013 Outline 1 Riemnn Sums 2 Riemnn Integrls 3 Properties
Riemnn Sums nd Riemnn Integrls Jmes K. Peterson Deprtment of Biologicl Sciences nd Deprtment of Mthemticl Sciences Clemson University August 26, 2013 Outline 1 Riemnn Sums 2 Riemnn Integrls 3 Properties
Physics 3323, Fall 2016 Problem Set 7 due Oct 14, 2016
 Physics 333, Fll 16 Problem Set 7 due Oct 14, 16 Reding: Griffiths 4.1 through 4.4.1 1. Electric dipole An electric dipole with p = p ẑ is locted t the origin nd is sitting in n otherwise uniform electric
Physics 333, Fll 16 Problem Set 7 due Oct 14, 16 Reding: Griffiths 4.1 through 4.4.1 1. Electric dipole An electric dipole with p = p ẑ is locted t the origin nd is sitting in n otherwise uniform electric
Partial Derivatives. Limits. For a single variable function f (x), the limit lim
 Limits Prtil Derivtives For single vrible function f (x), the limit lim x f (x) exists only if the right-hnd side limit equls to the left-hnd side limit, i.e., lim f (x) = lim f (x). x x + For two vribles
Limits Prtil Derivtives For single vrible function f (x), the limit lim x f (x) exists only if the right-hnd side limit equls to the left-hnd side limit, i.e., lim f (x) = lim f (x). x x + For two vribles
NOT TO SCALE. We can make use of the small angle approximations: if θ á 1 (and is expressed in RADIANS), then
 3. Stellr Prllx y terrestril stndrds, the strs re extremely distnt: the nerest, Proxim Centuri, is 4.24 light yers (~ 10 13 km) wy. This mens tht their prllx is extremely smll. Prllx is the pprent shifting
3. Stellr Prllx y terrestril stndrds, the strs re extremely distnt: the nerest, Proxim Centuri, is 4.24 light yers (~ 10 13 km) wy. This mens tht their prllx is extremely smll. Prllx is the pprent shifting
5.2 Volumes: Disks and Washers
 4 pplictions of definite integrls 5. Volumes: Disks nd Wshers In the previous section, we computed volumes of solids for which we could determine the re of cross-section or slice. In this section, we restrict
4 pplictions of definite integrls 5. Volumes: Disks nd Wshers In the previous section, we computed volumes of solids for which we could determine the re of cross-section or slice. In this section, we restrict
