CHEMISTRY 383 LECTURE NOTES
|
|
|
- Eunice Norton
- 6 years ago
- Views:
Transcription
1 CHEMISRY 383 LECURE NOES Chate III R. H. Schwendeman Deatment f Chemisty Michigan State Univesity East Lansing, MI 4884 Cyight 996 by R. H. Schwendeman All ights eseved. N at f this text may be educed, sted in a etieval system, tansmitted, in any fm by any means, electnic, mechanical, htcying, ecding, thewise, withut i emissin f the auth.
2 III - III. hemdynamics: the secnd and thid laws A. Intductin; sntaneus cesses he fist law f themdynamics, the idea that the enegy f the univese emains cnstant, was and is acceted by almst eveyne. he ntin that enegy cannt be ceated destyed in any cess is cnsistent with exeience. he secnd law f themdynamics, which has t d with the edictin f the diectin f sntaneus cesses, is me subtle and was nt always acceted. A sntaneus cess is, as its name imlies, a cess that takes lace seemingly by itself. We ecgnize many such cesses. A cld bject bught int a wam m becmes wam. A unctued balln lses its ai. Zinc metal ded int HCl slutin in wate eacts t fm H, etc., etc. By cntast, many systems ae at equilibium, whee even if cmeting cesses ae ccuing cntinuusly, they ccu at ates that balance ne anthe (dynamic equilibium). F examle, afte the cld bject eaches m temeatue, thee is n futhe aaent change. If the balln is nt unctued and is tightly sealed, it etains its inflated shae. Afte all f the Zn is disslved, the cmbined ZnCl, HCl slutin emains unchanged. hus, we have tw kinds f cesses: sntaneus cesses and equilibium cesses (called nnsntaneus cesses by Atkins). Althugh equilibium nnsntaneus cesses ae aaently null cesses, they ae f inteest, because it is ssible t envisin an actual cess that is nly infinitesimally diffeent fm an equilibium cess: the s-called evesible cess that we discussed ealie. If the m with the bject in it is cled slightly, the bject will be cled als; if it is heated slightly, the bject will be heated. Again the name is at; a evesible cess is ne whse diectin can be evesed by means f a slight change in the system the suundings. Althugh it is imaily cncened with the diectin f sntaneus cesses, the fmulatin f the secnd law f themdynamics equies cnsideatin f evesible cesses. B. he diectin f sntaneus cesses What detemines the diectin f sntaneus cesses? We knw that it can nt be, as was nce thught, the diectin f enegy change. he Fist Law says that the enegy f the univese is cnstant; yet thee is cntinuus sntaneus change. We can cite simle examles that shw that this is nt just the esult f cmensating cesses in diffeent ats f the univese. Cnside the fllwing system in in which tw halves f a glass bulb imbedded in an insulated cntaine ae seaated by a valve. V L V R Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
3 III - 3 he system is eaed by filling ne half f the bulb with an ideal gas at sme essue while the valve between the tw halves is clsed. If the valve is then ened, the gas escaes fm the filled t the emty side. hee is n wk dne and n heat exhange with the suundings. heefe, U = 0. Yet a cess has ccued. Equilibium is ested when g (left) = g (ight). Nw, cnside a secnd system in which tw metal bas ae insulated fm each the and fm the utside wld. One metal ba is at a highe temeatue than the the. We emve the insulating themal baie between the tw bas. We knw that heat will flw fm ne ba t the the until the temeatues f the tw bas ae the same. N wk is dne and n heat is exchanged with the suundings; s U = 0. Yet a cess has ccued. Equilibium is ested when =. C. he enty and the Secnd Law f hemdynamics: statement hee was cnsideable discussin in the ealy histy f themdynamics abut the existence and natue f a quantity that detemines the diectin f sntaneus cesses. he Secnd Law f hemdynamics is the esult f that discussin. he essential featue f the Secnd Law is the intductin f a new state functin f a system, the enty. he enty f the univese is stulated t incease when any sntanus cess ccus. We use the lette S t designate the enty and then state that S > 0 f any sntaneus cess ds = 0 f any infinitesimal cess away fm equilibium. he S hee is the enty change f the univese. S = S system + S suundings > 0 f any sntaneus cess. Since a evesible cess is a sequence f infinitesimal stes away fm equilbium, S = 0 f a evesible cess. D. he enty and the Secnd Law: futhe cnsideatins he statement f the Secnd Law f hemdynamics has thee imlied ats: Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
4 III - 4. he existence f the enty hee exists a quantity called the enty that is a state functin f the system.. Calculatin f changes in the enty he enty change f a clsed system is defined such that ds = dqev S = S S = dqev In these equatins, dq ev is the heat inut t the system f an infinitesimal ste in a evesible ath at temeatue. 3. Statement f the law F any sntaneus cess in an islated system, S > 0. E. Altenate statements f the Secnd Law f hemdynamics Many distinguished scientists cntibuted t the undestanding f the Secnd Law and each f them had thei wn unique way f stating the law. hee f these (tw by Clausius) ae as fllws: R. Clausius Heat cannt f itself, withut the inteventin f any extenal agency, ass fm a clde t a htte bdy. W. hmsn It is imssible t cnstuct a machine functining in cycles which can cnvet heat cmletely int the equivalent amunt f wk withut ducing changes elsewhee. R. Clausius Die Enegie de Welt ist knstant; die Entie de Welt stebt einem maximum zu. Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
5 III - 5 F. Calculatin f the enty f simle cesses calculate the enty f any cess, we make use f the integal ve the ati f the heat inut t the temeatue f a evesible cess. We can use this cedue f any cess, evesible nt, by making use f the fact that the enty is a state functin (we just stulated this, but it can be demnstated t be tue f many cesses). If the enty is a state functin, S is the same n matte hw we get fm ne state t anthe. heefe, if we need t calculate the the enty change f an ievesible (i.e., sntaneus) cess that takes a system fm state t state, we simly invent a cnvenient evesible cess that takes the system fm state t state and use the integal ule t calculate the enty change. his enty change will be the cect value f any cess, including the ne that we ae inteested in.. Isthemal exansin f an ideal gas calculate the enty change f the isthemal exansin f an ideal gas, we assume a evesible exansin. F any isthemal exansin f an ideal gas, we have aleady seen that U = q + w = 0. Als, V w dv nr dv V = = = nr ln V V V Because q = w f this cess, q = nr ln(v /V ). hen, since the cess is isthemal, V V S dq q = = = nr ln V V. Cnstant vlume heating F this cess, we imagine heating a system fm t while keeing the vlume cnstant. Again, we invent a evesible ath t take us fm t. Als, at cnstant vlume, dq = dq V = du = C V d. If we assume that C V is cnstant ve the temeatue ange f inteest, we find dq S = ds = = d S = CV = C V CVd ln Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
6 III Cnstant essue heating Cnstant essue heating fllws the same agument as cnstant vlume heating excet that at cnstant essue, dq = dq = dh = C d. he esult f cnstant essue heating is S d = C = C ln 4. Chemical eactin Since the enty is a state functin, a system cnsisting f a ue substance has a definite enty, just as it has a definite enegy and a definite enthaly. We fund that because f u inability t establish a cnsistent ze f enegy, it is nt geneally ssible t detemine unambiguusly the abslute enegy enthaly f a ue substance. We shall see shtly that this is nt the case f the enty. hee exists a well-defined ze f enty f a ue substance at any temeatue and thee exist well-defined ways t measue this quantity exeimentally. heefe, abslute enties can be and ae tabulated f many substances. A few f these ae tabulated in Atkins in able 3. and many me ae tabulated in Aendix A. hee als exist the extensive tabulatins f abslute enties. We ecall that f a eactin invlving ue substances, such as H (g) + O (g) = H O(l), the net esult is that tw mles f hydgen gas and ne mle f xygen gas disaea, while tw mles f liquid wate aea. If the eactin ccus at cnstant temeatue and cnstant essue unde cnditins such that the atial mla enties f all secies in the eactin vessel stay the same, the enty change f the cess is ( H O( )) ( O ( )) ( H ( )) S = S l S g S g If the eactants and ducts ae in thei standad states, the enties e mle ae standad enties e mle, which ae the quantities usually tabulated. If the substances ae nt in thei standad states, then in many cases the enties e mle can be calculated by ne f the equatins given f ats -3 f this sectin. Late, we will give additinal methds f calculatin f the enties e mle f use in this equatin. Revesible, cnstant temeatue, cnstant essue hase change A hase change f a substance can be eesented by a "chemical eactin", as fllws: H O(l) = H O(g) Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
7 III - 7 his cess ccus at cnstant temeatue, s S = q/. It als ccus at cnstant essue, s q = q = H. (In this aticula case, H is witten H va and is called the enthaly f vaizatin.) heefe, the enty f vaizatin is S va = H va Similaly, f the cess H O(s) = H O(l) S fus = H fus he cess f melting a slid is called fusin, and S fus and H fus ae the enty f fusin and the enthaly f fusin, esectively. hee is an inteesting and imtant emiical finding f the enty f vaizatin that is knwn as utn's ule. his ule states that the enty f vaizatin at the nmal biling int f all "nmal" liquids is abut 85 J K - ml -. A "nmal" liquid is ne that des nt have any unusual stuctual featues that affect the bnding in the liquid. F examle, S va = 85.9 J K - ml - at the nmal biling int (76. C) f cabn tetachlide, and cabn tetachlide wuld be called a nmal liquid. By cntast, S va = 09. J K - ml - at 00 C f wate and wate is nt a nmal liquid. he easn f the diffeence is that extensive hydgen bnding in liquid wate inceases the de in liquid wate cmaed t nmal liquids. We will see that enty is elated t disde, s it equies a geate than nmal enty change t cnvet liquid wate t gaseus wate. he utn ule has sme actical alicatins, because it allws estimatin f the enthaly f vaizatin f a nmal liquid at the biling int by simle measuement f the biling int. We will see late that this allws estimatin f the equilibium va essue f the liquid at temeatues away fm the biling int, which allws estimatin f the change in biling int with change in alied essue. G. Enty, enty change, and disde he tw examles given abve - gas exanding t fill bth halves f a cntaine and heat flwing fm ne side f a metal ba t anthe - aise the suggestin that the Secnd Law may have a statistical basis. F the examle in which ne half f a cntaine filled with gas is seaated by a valve fm the the half, which is emty, we cnfidently stated that the gas wuld fill the emty half as sn as the valve is ened. Why wee we s sue? If, f examle, thee wee nly fu mlecules n the filled side, afte ening the valve we wuld nt be suised t find all fu mlecules n the the side, thee mlecules n ne side and ne n the the, even thugh n the aveage, we wuld exect t find tw mlecules n each side.when thee ae millins f mlecules, hweve, we exect t find a nealy equal numbe n each side. his can be Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
8 III - 8 cnfimed by dinay statistics. It aeas that the easn f the vewhelming cetainty f the edictin f the Secnd Law in this case is a esult f the fact that macscic samles f substances cntain such a lage numbe f mlecules. he same cnclusin esults fm cnsideatin f the examle f the tw heated bas cming int themal cntact when the insulating themal baie is emved. We knw that heat flws fm the ht side t the cld side. his means that enegetic atms n ne side lse enegy while less enegetic atms n the the side gain enegy. But, with the andm distibutin f enegy amng the atms, it seems that it might be ssible f all f the enegetic atms t be n ne side the the, just by chance. he fact that this des nt ccu esults fm the vey lage numbe f atms in any macscic samle. It is ssible t shw that the ssibility f even a small deviatin fm the equilibium distibutin f enegy amng the atms is s small as t be cmletely negligible. he mathematical exessin f the statistical asect f the enty is that the enty is diectly tinal t the lgaithm f the numbe f micscic states cnsistent with a given themdynamic state. If me states ae made available, the system will send sme time in them. When the valve between the full and the emty halves f the gas cell is ened the mlecules that wee cnfined t the full half can nw ccuy any at f the whle cell. Because these exta states ae available, the mlecules ccuy them, and the enty inceases. H. he hid Law f hemdynamics A simle statement f the hid Law f hemdynamics is as fllws: he enty f a efect cystalline mateial is ze at = 0 (Atkins,. 9). A me ecise statement was given in a famus text by Lewis and Randall (G. N. Lewis and M. Randall, "hemdynamics and the Fee Enegy f Chemical Substances," st Ed.,. 448, McGaw-Hill, 93): If the enty f each element in sme cystalline state be taken as ze at the abslute ze f temeatue, evey substance has a finite sitive enty; but at the abslute ze f temeatue, the enty f a substance may becme ze and des s becme in the case f a efect cystalline substance. he hid Law was discveed in the ealy at f the 0th centuy when it became ssible t study the themal eties f eactins at vey lw temeatues. It was fund that f many eactins, S aaches ze as the temeatue ges t ze. F eactins with the same numbes f mles f eactants and ducts, this equies nly that all substances have the same value f the enty at = 0. Hweve, many eactins have diffeent numbes f ttal mles f eactants and ducts. F these eactins t have S = 0 at = 0, it is necessay f all f the eactants and ducts t have S = 0 at = 0. Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
9 III - 9 If the abslute enty is ze at = 0 f a ue substance, it is ssible t detemine the enty at any > 0 by the fllwing equatin: S C d H C d H = n C d In this equatin, S is the abslute enty e mle f the substance in its standad state at temeatue ; C is the heat caacity e mle f the substance at cnstant essue; and H is the enthaly e mle f a hase change f the substance at temeatue. hus, measuement f heat caacities and hase-change enthalies allws exeimental deteminatin f abslute enties f ue substances at any temeatue. Many enties have been detemined this way. We will see shtly that abslute enties may als be deduced by measuement f equilibium cnstants f eactins at seveal temeatues. Finally, we nte that f a efect cystalline slid with all f its atms in thei lwest enegy state, thee is nly ne micscic state cnsistent with the given themdynamic state. heefe, if, as we stated abve, we assume the enty t be tinal t the lgaithm f the numbe f micscic states cnsistent with the themdynamic state, then S = klnω whee Ω is the numbe f micscic states cnsistent with the given themdynamic state. Fm this equatin, it is aaent that if Ω =, S = 0. I. Calculatin f S f a simle cess We cnside again the fllwing cess invlving tw metal bas in an insulated cntaine with a themal baie between the bas, as fllws: Befe the themal baie is emved, the tw bas ae at temeatues and with >. Afte the baie is emved, the tw bas cme t the same final temeatue f (heat flws fm the ight-hand, highe temeatue ba t the left-hand, lwe temeatue ba). hee is n lss f enegy t the suundings as heat, and n wk dne, s U = 0 f the whle system. he blem is t detemine the final temeatue f and t shw that S > 0. We assume that the tw halves f the ba have the same heat caacity C V and that the vlume change is negligible (w = 0). Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
10 III - 0 detemine the final temeatue f, we wite U = 0= q+ w = q = q+ q whee q and q ae the heat inut t the left and ight bas, esectively, as they cme t equilibium. Nw, and heefe, f ( ) q = C d C V V f f ( ) q = C d C V V f ( ) ( ) C + C = V f V f 0, afte slving f f, f = + Nw, t calculate S we devise evesible aths f each f the tw metal bas in which the lefthand ba slwly heats u fm t f and the ight-hand ba slwly cls fm t f. he enty change f each f these cesses may be cmuted and then the ttal enty change is the sum f the enty changes f the tw bas. We use the esult btained ealie f the enty change f cnstant vlume heating cling; S CV d C = = V ln f f S CV d C = = V ln f f Afte additin f the tw cntibutins t the ttal enty, shw that S > 0, we must shw that S = CVln f + CVln f = CVln f > whee f f = +. Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
11 III - his can be dne as fllws: = = 4 f = + = 4 > 0 As a numeical examle, assume = 300 K, = 400 K, and C V = 0 J K -. hen, and f ( 0 J K ) = = 350K ( 350) ( )( ) S = ln = J K Althugh small, S is clealy sitive f this sntaneus cess. Nte that the units n the enty ae enegy temeatue - ; hee, J K -. I. Gibbs fee enegy. Intductin; elatin t the enty Accding t the Secnd Law, f any infinitesimal cess, ds univ = ds sys + ds su 0 ds univ > 0 ds univ = 0 f any sntaneus cess f any infinitesimal cess away fm equilibium Alicatin f this law equies infmatin abut the suundings as well as abut the system. It wuld be useful t have a ety f the system that edicts the diectin f a eactin withut exlicit cnsideatin f the suundings. his can be dne, vided that we caefully descibe the suundings in advance. J. W. Gibbs agued that if we make the simle assumtin that heat tansfe t the suundings is always evesible, then we can calculate the enty change f the suundings f evey such system. he esult wks ut in such a way that we can incate the enty change f the suundings int a new state functin f the system. We nw call this new state functin the Gibbs fee enegy. Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
12 III - see hw this wks, we again assume a cylinde with a tight-fitting istn, but this time we enclse the cylinde in a heat bath f cnstant temeatue. We assume that the bath is s lage that any heat tansfeed t fm the bath is tansfeed evesibly, as fa as the bath is cncened, and that the amunt f heat tansfeed t fm the system is nt able t change the temeatue f the bath. Finally, we assume a cnstant essue cess. It shuld be aaent that this cess is vey simila t a cess caied ut in an en cntaine. he suunding atmshee is s lage that it has an enmus heat caacity. Cnsequently, heat tansfeed t fm the atmshee fm a system f easnable size is an infinitesimal heat tansfe, as fa as the entie atmshee is cncened. We assume a small amunt f heat tansfeed between system and suundings. hen, s that ds su dq = dq = dq sys su dq dq = su = = dh he last f these equalities esults fm the fact that the cess ccus at cnstant essue, and f a cnstant essue cess, the heat inut t the system is equal t the enthaly change f the system. heefe, dh hee is f the system. If we nw use the nmenclatue that S = S system, then accding t the Secnd Law. dh dssys + dssu = ds 0 Nw, we define the Gibbs fee enegy G, as fllws: G H S and ealize that since H,, and S ae all state functins, G is a state functin. see what this accmlishes, cnside a cnstant temeatue, cnstant essue cess. dg = dh d( S) = dh ds heefe, if as just shwn, ds dh 0, is equied by the Secnd Law f an allwed cess at cnstant temeatue and essue, then dh ds 0 Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
13 III - 3 dg 0 summaize: f a cnstant and P cess, dg < 0 dg = 0 dg > 0 f an infinitesimal sntanteus cess f any infinitesimal cess away fm equilibium f a fbidden cess In a simila fashin, it is ssible t define a Helmhltz fee enegy A, such that At cnstant and V, A U S da < 0 da = 0 da > 0 f an infinitesimal sntanteus cess f any infinitesimal cess away fm equilibium f a fbidden cess We emhasize again that G and A ae state functins. hey ae eties f the system that ae indeendent f its evius histy, including the methd f eaatin.. Alicatin f the Gibbs fee enegy t hysical cesses a. Intductin he state f a ne-cmnent, ne-hase system is defined by tw vaiables which we can take t be the temeatue and the essue. heefe, Accding t calculus, G = G(, P) whee G = G e mle dg G G = d + d and this exansin is unique. Nw, we efm sme algeba f an infinitesimal evesible cess at cnstant and. dq ds = dq = ds dw = dv Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
14 III - 4 du = dq + dw = ds dv dh = d( U + V ) = du + dv + Vd = ds dv + dv + Vd dh = ds + Vd dg = d( H S) = dh ds Sd = ds + Vd ds Sd heefe, dg = Vd Sd On a e mle basis Cmae Aaently, dg G = V dg = Vd Sd G G = d + and d G = S b. Vaiatin f the fee enegy with temeatue G H detemine hw G vaies with, we begin with S = fm which we btain G G = H his is the same as ( G / ) H = which is knwn as the Gibbs-Helmhltz equatin. It allws the fee enegy t be calculated at diffeent temeatues vided the enthaly e mle f the substance is knwn. c. Vaiatin f the fee enegy with the essue At cnstant temeatue, d = 0, s dg = Vd. heefe, Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
15 III - 5 G G G = dg = Vd We assumed G = G at = and G = G at =. If the substance is an ideal gas, then G G R G d R = = ln If the substance is a eal gas, it is necessay t mdify this equatin smewhat. his is dne by inventing a quantity called the fugacity f and by witing the equatin in tems f the fugacities at the tw essues, G G = Rln f f define the fugacity cmletely, it is cnventinal t assume that the gas becmes ideal as the essue is lweed t ze. It is then ssible t detemine the fugacity at any essue if the equatin f state f the gas is knwn. If the substance is a liquid a slid, it is almst incmessible. heefe, the mla vlume des nt change much with the essue and a gd aximatin is t assume that it des nt change at all with essue. ( ) G G = Vd = V d = V 3. Relatin between the Gibbs fee enegy and maximum nn-v wk he Gibbs fee enegy can be shwn t be the maximum nn-v wk that can be efmed by a evesible cess at cnstant and. his is an imtant esult, because it is ften the nn-v wk that is useful. he V wk has t be dne in a cnstant essue cess, whethe ne likes it nt. shw this ety f the Gibbs fee enegy, we begin by ecalling that at cnstant, d = 0, s fm G = H S, we find dg = dh ds Sd = dh ds Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
16 III - 6 Nw H = U +V, and at cnstant essue, d = 0, s dh = du + dv + Vd = du + dv But du = dq + dw (w is ttal wk) and dw = dw extdv (wk = nn-v wk + V wk) hus, dg = dq + dw extdv + dv ds F a evesible cess at cnstant and, dq = ds and, s dg = dw Since this hlds f evey ste in the evesible cess, G= w f a evesible cess at cnstant and. We will use this elatin late when we discuss electchemisty. 4. Alicatin f the Gibbs fee enegy t chemical eactins a. Reactants and ducts in thei standad states Cnside the fllwing eactin at sme fixed temeatue, H (g) + O (g) = H O(l) Accding t this eactin, when tw mles f hydgen gas eacts with ne mle f xygen gas, tw mles f liquid wate is duced,, in the wds, tw mles f hydgen gas and ne mle f xygen gas disaea while tw mles f liquid wate aeas. If all f the substances ae in thei standad states, the Gibbs fee enegy change f this cess is ext = ( H O ) ( O ) ( H ) G = G ( l ) G ( g ) G ( g ) In this equatin, f examle, G ( H O l ) ( ) is the standad fee enegy e mle f liquid wate at the temeatue f inteest. Me geneally, G = G ( ) G ( ) hat is, the standad Gibbs fee enegy change f a eactin (the standad eactin fee enegy) is the sum f the Gibbs fee enegies e mle f the ducts minus the Gibbs fee enegies e mle f the eactants. Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
17 III - 7 Since G = H S = U+ V S, the deteminatin f the abslute value f a fee enegy e mle is lagued by the same blem f ze f enegy that caused tuble with the deteminatin f the enthaly e mle. We use the same tick that we used befe with the enthaly. We define the standad Gibbs fee enegy f fmatin f a substance t be the Gibbs fee enegy change f the fmatin f ne mle f the substance in its standad state fm its elements in thei standad efeence states. hen, because evey eactin may be viewed as a sequence f evesed fmatin eactins f the eactants fllwed by a sequence f fmatin eactins f the ducts, we can use a vaiatin f Hess's Law f Gibbs fee enegies t wite f G = G ( ) G ( ) hat is: he standad Gibbs fee enegy change f a eactin is the sum f the standad Gibbs fee enegies f fmatin f the ducts minus the sum f the standad Gibbs fee enegies f fmatin f the ducts. As might be exected fm the last equatin, thee exist tables f standad Gibbs fee enegies f fmatin f substances. In the Atkins text thee is a small table as able 3.3 and a lnge table in Aendix A. Anthe way t btain the standad Gibbs fee enegy change f a eactin fllws fm the definitin f the Gibbs fee enegy. Since G = H S, G = H S (cnstant ) at cnstant temeatue. We have aleady seen hw t cmute H and S fm tables f standad enthalies f fmatin and abslute standad enties. Afte cmutatin f these quantities, it is a simle matte t btain G. b. Gas-hase eactants and ducts at essues the than atmshee We have seen hw t calculate G, which is the Gibbs fee enegy change f a eactin in which all f the eactants and ducts ae in thei standad states. We als have inted ut that the sign f the Gibbs fee enegy change detemines whethe the eactin will ceed as witten. hus f a eactin vessel in which all f the eactants and ducts ae esent in thei standad states, we find that if G < 0, eactants eact t fm ducts if G = 0, the system is at equilibium if G > 0, ducts eact t fm eactants f Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
18 III - 8 Since it wuld be a ae situatin in which all f the eactants and ducts ae esent in thei standad states, we need a way t detemine the Gibbs fee enegy change f a eactin mixtue in which the vaius secies ae esent in abitay cncentatins. We will d this hee f a gaseus mixtue and then extend the cedue t slutins and mixtues afte we discuss hase equilibia. We have aleady shwn that f an ideal gas, G G = R ln Nw, we assume that state is the standad state in which G =, the standad Gibbs fee enegy e mle, and =, the essue f the gas in the standad state (usually ba). hen, we assume that state is sme abitay state f the gas (we d the subscit ), in which case, we can wite G G = Rln It is custmay t efe t the ati in the lgaithm as the activity f the samle in the eactin mixtue. We will use the lette a t eesent activity, s that G G G = Rln R ( a) a = ln, i.e., = If the gas is nt sufficiently ideal, it is necessay t use fugacities instead f essues in these exessins (nly ideal gases will be cnsideed in this cuse). If this elatin is alied t a chemical eactin, we btain whee and G = G +( ml) R ( lna) G= G( ) G( ) G = G ( ) G ( ) ( ) ln a = ln a ln a = ln Q Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
19 III - 9 hen, G = G +( ml) RlnQ he " ml" in this equatin esults fm the fact that when we calculate G G 0, the fee enegy e mle f each substance is multilied by the numbe f mles f the substance in the balanced chemical equatin. he Q in this equatin is called the eactin qutient, which is defined in tems f sums ve lgaithms f a, the activity f a duct secies, and lgaithms f a, the activity f a eactant secies. If we ecall that the sum f lgaithms is the lgaithm f the duct, we find that Q = a a (Nte: a = aa 3 a ) hat is, Q is the qutient f the mathematical duct f the activities f the chemical ducts divided by the mathematical duct f the activities f the eactants (nte the tw diffeent meanings f the wd "duct" in this statement). hese mathematical ducts must cntain the activities f all f the eactants and the activities f all f the chemical ducts. F examle, cnside the eactin, F this eactin, CO(g) + O (g) = CO (g) a Q = a CO( g) CO( g) ao ( g) he activities f CO (g) and CO(g) ae squaed in the equatin because they each ccu with a cefficient in the chemical equatin. With the exessin just given, we can calculate G f a eactin in a eactin mixtue in which the eactant and duct gases ae at abitay atial essues. hen, if G < 0, eactants eact t fm ducts if G = 0, the system is at equilibium if G > 0, ducts eact t fm eactants c. Equilibium cnstants If G = 0, the eactin mixtue is at equilibium. hen, if Q = Q eq = K is the equilibium value f the eactin cefficient, we find that G = ( ml) R ln K Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
20 III - 0 Hee, K is called the equilibium cnstant. In tems f the activities f the vaius secies, and K = his exessin f K lks just like the exessin f Q given abve. Bth equatins ae cect. In the equatin f Q, the a's ae the activities f the eactants and ducts unde a secific set f cnditins that may may nt be equilibium, wheeas in the exessin f K, the a's must be activities f eactants and ducts f a aticula equilibium cnditin in the eactin mixtue. a a We emhasize again that evey mle f eactant duct must be eesented by an activity in the equatin f K. F examle, if the ducts f the eactin include tw mles f CO (g), the numeat must include tw facts f the activity f CO (g); i.e., the activity f CO (g) squaed. It shuld be nted that the activity f a gas is a ati f atial essues ( fugacities) f the gas, and is theefe unitless. Since Q and K ae atis f activities, they ae als unitless. A emakable featue f the elatin between G and the equilibium cnstant is that the equilibium cnstant f a eactin in any abitay samle mixtue may be calculated by efeence nly t the Gibbs fee enegy change f the eactin with all f its ducts and eactants in thei standad states. heefe, it aeas that it is nly necessay t set u a table f atial mla standad Gibbs fee enegies f the vaius substances t be able t calculate the equilibium cnstant f a eactin. Such tables exist and ae extemely useful. summaize, we calculate G by ne f the equatins, f G = G ( ) G ( ) G = H S f and then use G = ( ml) R ln K t calculate the equilibium cnstant K. O, it is ssible t wk in the the diectin: measue the equilibium cnstant and use it t detemine G. his cess is descibed in me detail belw. Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
21 III - J. Le Chatelie Pincile. Intductin By substitutin f G in the equatin f G, we find G Q R K R Q R ml = + = ln ln ln K With this equatin and the ules f the sign f G, we can geneate a table f the diectin f a eactin by cmaisn f Q with K, as fllws: If Q < K, G < 0, If Q = K, G = 0 If Q > K, G > 0 eactin ceeds t the ight eactin is at equilibium eactin ceeds t the left hese esults ae deduced by examinatin f the lgaithm in the exessin f G. If Q/K < (i.e., Q < K), then the lgaithm is negative and G is negative. If Q/K =, the lgaithm is ze and G is ze. Finally, if Q/K >, the lgaithm is sitive and G is sitive. At equilibium, Q/K =. By examinatin f the effect f a vaiety f changes in eactin cnditins n Q/K, it is ssible t deduce what is knwn as the Le Chatelie Pincile, which eads as fllws: Wheneve a change in eactin cnditins uts a stess n a eactin at equilibium, the cmsitin f the eactin mixtue shifts in such a way as t educe the effect f the stess. We will nw descibe a seies f "stesses" and shw that the esnse f the eactin mixtue is in accd with the Le Chatelie Pincile. Fist, we mentin a change in eactin cnditins that des nt duce a stess and theefe des nt fall within the sce f the Le Chatelie Pincile. he additin f a catalyst t a eactin mixtue changes the ates f the chemical eactins, but the ates f all f the eactins ae changed in such a way that the equilibium cmsitin is nt changed.. Effect f the additin f a eagent F a mixtue f ideal gases, the activities f the cmnents f a eactin ae the numeical values f the atial essues f the individual gases exessed in ba. hat is because we defined the activity t be a = in which is the essue in the standad state f the gas, which is ba. heefe, a = ba (f an ideal gas) Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
22 III - If is in ba, the units cancel, s a is the numeical value f the essue in ba. If we add sme eactant, the denminat f Q inceases, which deceases Q. his, in tun, deceases Q/K, s that it becmes less than, in which case the eactin ceeds t the ight t e-establish equilibium. If we add sme duct, the esult is the evese: the numeat f Q inceases, Q inceases, Q/K becmes geate than, and the eactin ceeds t the left. hus, the geneal ule is If sme eactant duct is added t a eactin mixtue that is at equilibium, the eactin ceeds in the diectin that will emve at f the added cmnent. As an examle, cnside the eactin N (g) + 3 H (g) = NH 3 (g) If sme NH 3 (g) is added t the eactin at equilibium, the eactin will ceed t the left in de t cnsume at f the added NH 3 (g). 3. Effect f incease in essue If we ecall that the atial essue f a substance in a gaseus mixtue is equal t the mle factin f the substance times the ttal essue, we can wite f the eactin at equilibium, K = x x Hee x is the mle factin f a duct mlecule, x is the mle factin f a eactant mlecule, is the ttal essue, and n is the numbe f mles f gaseus ducts minus the numbe f mles f gaseus eactants. If n is sitive and the essue is inceased, n inceases. In this case, the ati f mle factins must decease if K is t emain cnstant. his can nly haen if the mle factins in the numeat decease and/ the mle factins in the denminat incease. But, bth f these things haen if the eactin ceeds t the left. If n is sitive, thee ae me mles f gaseus ducts than mles f gaseus eactants in the equatin f the chemical eactin, s the shift is away fm the side with the geate numbe f mles f gaseus substances in the equatin f the chemical eactin. If n is negative, thee ae me mles f gaseus eactants than gaseus ducts. If the essue is inceased in this case, n deceases, s the ati f mle factins must incease. his haens if the eactin ceeds t the ight. he Le Chatelie Rule is still that the shift is away fm the side with the geate numbe f mles f gaseus substances in the equatin f the chemical eactin. heefe, in eithe case, n Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
23 III - 3 If the essue n a eactin mixtue at equilibium is inceased, the eactin ceeds in the diectin away fm the side f the chemical equatin that has the lagest numbe f gaseus substances. We nte that the effect f a change in the vlume f the eactin vessel at cnstant temeatue is t change the ttal essue f the eactin mixtue. At any given temeatue, a decease in vlume inceases the essue and vice-vesa, and the effect n the eactin mixtue can be edicted fm the change in essue. As an examle, we again cnside the eactin, N (g) + 3 H (g) = NH 3 (g), f which n = 3 =. If we incease the essue n this eactin, the eactin ceeds t the ight t educe the numbe f mles f gas in the cntaine. 4. Effect f a change in temeatue. see the effect f a change in temeatue n a eactin at equilibium equies an analysis that gets a little cmlicated. We begin with the Gibbs-Helmhltz equatin discussed ealie. he equatin that we used ealie shws hw the ati G/ vaies with temeatue. We aly it t G and t a eactin t btain ( G / ) H = Since is necessaily sitive, if H > 0, then G deceases as the temeatue inceases. By cntast, if H < 0, then G inceases as the temeatue inceases. Nw, s that G = ( ml) R lnk K = e G /( ml ) R If G inceases, K deceases, and the eactin ceeds t the left; if G deceases, K inceases, and the eactin ceeds t the ight. hus, we have the fllwing sequences f events, all based n the sign f H : Exthemic eactin H < 0 G K Reactin ges left H < 0 G K Reactin ges ight Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
24 III - 4 Endthemic eactin H > 0 G K Reactin ges ight H > 0 G K Reactin ges left Examinatin f these esults leads t the simle cnclusin that if the eactin gives ff heat as it ges t the ight (is exthemic), the eactin ges left if the temeatue is inceased (t absb heat). If the eactin absbs heat as it ges t the ight (is endthemic), the eactin ges ight if the temeatue is aised (t give ff heat). Again, we cnside the eactin, N (g) + 3 H (g) = NH 3 (g) f which H = 9. kj (twice the standad enthaly f fmatin f ammnia gas). he eactin is, theefe, exthemic. If the temeatue is inceased, the eactin ceeds t the left t absb sme f the heat added t incease the temeatue. K. emeatue deendence f K: deteminatin f H, G, and S he Gibbs-Helmhltz equatin was given ealie in the fm Nw, ( G / ) H = G = ( ml) RlnK Cmbinatin f these tw equatins gives the van't Hff equatin dln K d H H = dln K = ( ml) R ( ml) R d Integatin f the secnd f these tw equatins ve the temeatue ange between, whee K = K, and, whee K = K, leads t the fllwing vey imtant equatin that shws hw the equilibium cnstant at ne temeatue is elated t the equilibium cnstant at anthe temeatue: K ln K H = ( ml) R Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
25 III - 5 btain this equatin, it is necessay t assume that the enthaly des nt change significantly between the tw temeatues (which is ften a vey gd aximatin). It aeas that measuement f the equilibium cnstant at tw temeatues makes it ssible t detemine H exeimentally. Measuement f the equilibium cnstant at eithe f the tw temeatues makes it ssible t detemine G exeimentally ( G = ( ml ) R lnk). Finally, if H and G ae knwn at the same temeatue, it is ssible t detemine S H G S =. heefe, fm tw measuements f an equilibium cnstant at diffeent temeatues, it is ssible t extact all f the themdynamic eties f the eactin. Actually, it is smewhat bette t make seveal measuements f the equilibium cnstant at seveal temeatues. hen, a lt f ln K against / shuld give a staight line whse sle is H /( ml ) R. Lectue Ntes f Chemisty 383, Chate III 996 R. H. Schwendeman
Summary chapter 4. Electric field s can distort charge distributions in atoms and molecules by stretching and rotating:
 Summa chapte 4. In chapte 4 dielectics ae discussed. In thse mateials the electns ae nded t the atms mlecules and cannt am fee thugh the mateial: the electns in insulats ae n a tight leash and all the
Summa chapte 4. In chapte 4 dielectics ae discussed. In thse mateials the electns ae nded t the atms mlecules and cannt am fee thugh the mateial: the electns in insulats ae n a tight leash and all the
5/20/2011. HITT An electron moves from point i to point f, in the direction of a uniform electric field. During this displacement:
 5/0/011 Chapte 5 In the last lectue: CapacitanceII we calculated the capacitance C f a system f tw islated cnducts. We als calculated the capacitance f sme simple gemeties. In this chapte we will cve the
5/0/011 Chapte 5 In the last lectue: CapacitanceII we calculated the capacitance C f a system f tw islated cnducts. We als calculated the capacitance f sme simple gemeties. In this chapte we will cve the
CHAPTER 24 GAUSS LAW
 CHAPTR 4 GAUSS LAW LCTRIC FLUX lectic flux is a measue f the numbe f electic filed lines penetating sme suface in a diectin pependicula t that suface. Φ = A = A csθ with θ is the angle between the and
CHAPTR 4 GAUSS LAW LCTRIC FLUX lectic flux is a measue f the numbe f electic filed lines penetating sme suface in a diectin pependicula t that suface. Φ = A = A csθ with θ is the angle between the and
Example
 hapte Exaple.6-3. ---------------------------------------------------------------------------------- 5 A single hllw fibe is placed within a vey lage glass tube. he hllw fibe is 0 c in length and has a
hapte Exaple.6-3. ---------------------------------------------------------------------------------- 5 A single hllw fibe is placed within a vey lage glass tube. he hllw fibe is 0 c in length and has a
Hotelling s Rule. Therefore arbitrage forces P(t) = P o e rt.
 Htelling s Rule In what fllws I will use the tem pice t dente unit pfit. hat is, the nminal mney pice minus the aveage cst f pductin. We begin with cmpetitin. Suppse that a fim wns a small pa, a, f the
Htelling s Rule In what fllws I will use the tem pice t dente unit pfit. hat is, the nminal mney pice minus the aveage cst f pductin. We begin with cmpetitin. Suppse that a fim wns a small pa, a, f the
Work, Energy, and Power. AP Physics C
 k, Eneg, and Pwe AP Phsics C Thee ae man diffeent TYPES f Eneg. Eneg is expessed in JOULES (J) 4.19 J = 1 calie Eneg can be expessed me specificall b using the tem ORK() k = The Scala Dt Pduct between
k, Eneg, and Pwe AP Phsics C Thee ae man diffeent TYPES f Eneg. Eneg is expessed in JOULES (J) 4.19 J = 1 calie Eneg can be expessed me specificall b using the tem ORK() k = The Scala Dt Pduct between
WYSE Academic Challenge Sectional Mathematics 2006 Solution Set
 WYSE Academic Challenge Sectinal 006 Slutin Set. Cect answe: e. mph is 76 feet pe minute, and 4 mph is 35 feet pe minute. The tip up the hill takes 600/76, 3.4 minutes, and the tip dwn takes 600/35,.70
WYSE Academic Challenge Sectinal 006 Slutin Set. Cect answe: e. mph is 76 feet pe minute, and 4 mph is 35 feet pe minute. The tip up the hill takes 600/76, 3.4 minutes, and the tip dwn takes 600/35,.70
School of Chemical & Biological Engineering, Konkuk University
 Schl f Cheical & Bilgical Engineeing, Knkuk Univesity Lectue 7 Ch. 2 The Fist Law Thecheisty Pf. Y-Sep Min Physical Cheisty I, Sping 2008 Ch. 2-2 The study f the enegy tansfeed as heat duing the cuse f
Schl f Cheical & Bilgical Engineeing, Knkuk Univesity Lectue 7 Ch. 2 The Fist Law Thecheisty Pf. Y-Sep Min Physical Cheisty I, Sping 2008 Ch. 2-2 The study f the enegy tansfeed as heat duing the cuse f
OBJECTIVE To investigate the parallel connection of R, L, and C. 1 Electricity & Electronics Constructor EEC470
 Assignment 7 Paallel Resnance OBJECTIVE T investigate the paallel cnnectin f R,, and C. EQUIPMENT REQUIRED Qty Appaatus 1 Electicity & Electnics Cnstuct EEC470 1 Basic Electicity and Electnics Kit EEC471-1
Assignment 7 Paallel Resnance OBJECTIVE T investigate the paallel cnnectin f R,, and C. EQUIPMENT REQUIRED Qty Appaatus 1 Electicity & Electnics Cnstuct EEC470 1 Basic Electicity and Electnics Kit EEC471-1
A) 100 K B) 150 K C) 200 K D) 250 K E) 350 K
 Phys10 Secnd Maj-09 Ze Vesin Cdinat: k Wednesday, May 05, 010 Page: 1 Q1. A ht bject and a cld bject ae placed in themal cntact and the cmbinatin is islated. They tansfe enegy until they each a final equilibium
Phys10 Secnd Maj-09 Ze Vesin Cdinat: k Wednesday, May 05, 010 Page: 1 Q1. A ht bject and a cld bject ae placed in themal cntact and the cmbinatin is islated. They tansfe enegy until they each a final equilibium
CHAPTER GAUSS'S LAW
 lutins--ch 14 (Gauss's Law CHAPTE 14 -- GAU' LAW 141 This pblem is ticky An electic field line that flws int, then ut f the cap (see Figue I pduces a negative flux when enteing and an equal psitive flux
lutins--ch 14 (Gauss's Law CHAPTE 14 -- GAU' LAW 141 This pblem is ticky An electic field line that flws int, then ut f the cap (see Figue I pduces a negative flux when enteing and an equal psitive flux
Thermodynamics Partial Outline of Topics
 Thermdynamics Partial Outline f Tpics I. The secnd law f thermdynamics addresses the issue f spntaneity and invlves a functin called entrpy (S): If a prcess is spntaneus, then Suniverse > 0 (2 nd Law!)
Thermdynamics Partial Outline f Tpics I. The secnd law f thermdynamics addresses the issue f spntaneity and invlves a functin called entrpy (S): If a prcess is spntaneus, then Suniverse > 0 (2 nd Law!)
Thermodynamics and Equilibrium
 Thermdynamics and Equilibrium Thermdynamics Thermdynamics is the study f the relatinship between heat and ther frms f energy in a chemical r physical prcess. We intrduced the thermdynamic prperty f enthalpy,
Thermdynamics and Equilibrium Thermdynamics Thermdynamics is the study f the relatinship between heat and ther frms f energy in a chemical r physical prcess. We intrduced the thermdynamic prperty f enthalpy,
Outline. Steady Heat Transfer with Conduction and Convection. Review Steady, 1-D, Review Heat Generation. Review Heat Generation II
 Steady Heat ansfe ebuay, 7 Steady Heat ansfe wit Cnductin and Cnvectin ay Caett Mecanical Engineeing 375 Heat ansfe ebuay, 7 Outline eview last lectue Equivalent cicuit analyses eview basic cncept pplicatin
Steady Heat ansfe ebuay, 7 Steady Heat ansfe wit Cnductin and Cnvectin ay Caett Mecanical Engineeing 375 Heat ansfe ebuay, 7 Outline eview last lectue Equivalent cicuit analyses eview basic cncept pplicatin
Introduction. Electrostatics
 UNIVESITY OF TECHNOLOGY, SYDNEY FACULTY OF ENGINEEING 4853 Electmechanical Systems Electstatics Tpics t cve:. Culmb's Law 5. Mateial Ppeties. Electic Field Stength 6. Gauss' Theem 3. Electic Ptential 7.
UNIVESITY OF TECHNOLOGY, SYDNEY FACULTY OF ENGINEEING 4853 Electmechanical Systems Electstatics Tpics t cve:. Culmb's Law 5. Mateial Ppeties. Electic Field Stength 6. Gauss' Theem 3. Electic Ptential 7.
ME 3600 Control Systems Frequency Domain Analysis
 ME 3600 Cntl Systems Fequency Dmain Analysis The fequency espnse f a system is defined as the steady-state espnse f the system t a sinusidal (hamnic) input. F linea systems, the esulting utput is itself
ME 3600 Cntl Systems Fequency Dmain Analysis The fequency espnse f a system is defined as the steady-state espnse f the system t a sinusidal (hamnic) input. F linea systems, the esulting utput is itself
A) (0.46 î ) N B) (0.17 î ) N
 Phys10 Secnd Maj-14 Ze Vesin Cdinat: xyz Thusday, Apil 3, 015 Page: 1 Q1. Thee chages, 1 = =.0 μc and Q = 4.0 μc, ae fixed in thei places as shwn in Figue 1. Find the net electstatic fce n Q due t 1 and.
Phys10 Secnd Maj-14 Ze Vesin Cdinat: xyz Thusday, Apil 3, 015 Page: 1 Q1. Thee chages, 1 = =.0 μc and Q = 4.0 μc, ae fixed in thei places as shwn in Figue 1. Find the net electstatic fce n Q due t 1 and.
Electric Charge. Electric charge is quantized. Electric charge is conserved
 lectstatics lectic Chage lectic chage is uantized Chage cmes in incements f the elementay chage e = ne, whee n is an intege, and e =.6 x 0-9 C lectic chage is cnseved Chage (electns) can be mved fm ne
lectstatics lectic Chage lectic chage is uantized Chage cmes in incements f the elementay chage e = ne, whee n is an intege, and e =.6 x 0-9 C lectic chage is cnseved Chage (electns) can be mved fm ne
CHEM 1001 Problem Set #3: Entropy and Free Energy
 CHEM 1001 Prblem Set #3: Entry and Free Energy 19.7 (a) Negative; A liquid (mderate entry) cmbines with a slid t frm anther slid. (b)psitive; One mle f high entry gas frms where n gas was resent befre.
CHEM 1001 Prblem Set #3: Entry and Free Energy 19.7 (a) Negative; A liquid (mderate entry) cmbines with a slid t frm anther slid. (b)psitive; One mle f high entry gas frms where n gas was resent befre.
Announcements Candidates Visiting Next Monday 11 12:20 Class 4pm Research Talk Opportunity to learn a little about what physicists do
 Wed., /11 Thus., /1 Fi., /13 Mn., /16 Tues., /17 Wed., /18 Thus., /19 Fi., / 17.7-9 Magnetic Field F Distibutins Lab 5: Bit-Savat B fields f mving chages (n quiz) 17.1-11 Pemanent Magnets 18.1-3 Mic. View
Wed., /11 Thus., /1 Fi., /13 Mn., /16 Tues., /17 Wed., /18 Thus., /19 Fi., / 17.7-9 Magnetic Field F Distibutins Lab 5: Bit-Savat B fields f mving chages (n quiz) 17.1-11 Pemanent Magnets 18.1-3 Mic. View
Electric Fields and Electric Forces
 Cpyight, iley 006 (Cutnell & Jhnsn 9. Ptential Enegy Chapte 9 mgh mgh GPE GPE Electic Fields and Electic Fces 9. Ptential Enegy 9. Ptential Enegy 9. The Electic Ptential Diffeence 9. The Electic Ptential
Cpyight, iley 006 (Cutnell & Jhnsn 9. Ptential Enegy Chapte 9 mgh mgh GPE GPE Electic Fields and Electic Fces 9. Ptential Enegy 9. Ptential Enegy 9. The Electic Ptential Diffeence 9. The Electic Ptential
5.1 Moment of a Force Scalar Formation
 Outline ment f a Cuple Equivalent System Resultants f a Fce and Cuple System ment f a fce abut a pint axis a measue f the tendency f the fce t cause a bdy t tate abut the pint axis Case 1 Cnside hizntal
Outline ment f a Cuple Equivalent System Resultants f a Fce and Cuple System ment f a fce abut a pint axis a measue f the tendency f the fce t cause a bdy t tate abut the pint axis Case 1 Cnside hizntal
Lecture 2 - Thermodynamics Overview
 2.625 - Electochemical Systems Fall 2013 Lectue 2 - Themodynamics Oveview D.Yang Shao-Hon Reading: Chapte 1 & 2 of Newman, Chapte 1 & 2 of Bad & Faulkne, Chaptes 9 & 10 of Physical Chemisty I. Lectue Topics:
2.625 - Electochemical Systems Fall 2013 Lectue 2 - Themodynamics Oveview D.Yang Shao-Hon Reading: Chapte 1 & 2 of Newman, Chapte 1 & 2 of Bad & Faulkne, Chaptes 9 & 10 of Physical Chemisty I. Lectue Topics:
An integrated approach to modelling of chemical transformations in chemical reactors
 Euean Symsium n mute Aded Aided Pcess Engineeing 15 L. Puigjane and A. Esuña (Edits) 005 Elsevie Science B.V. All ights eseved. An integated aach t mdelling f chemical tansfmatins in chemical eacts Tai
Euean Symsium n mute Aded Aided Pcess Engineeing 15 L. Puigjane and A. Esuña (Edits) 005 Elsevie Science B.V. All ights eseved. An integated aach t mdelling f chemical tansfmatins in chemical eacts Tai
Downloaded from
 Chapte Notes Subject: Chemisty Class: XI Chapte: Themodynamics Top concepts 1. The banch of science which deals with study of diffeent foms of enegy and thei inteconvesion is called themodynamics. 2. A
Chapte Notes Subject: Chemisty Class: XI Chapte: Themodynamics Top concepts 1. The banch of science which deals with study of diffeent foms of enegy and thei inteconvesion is called themodynamics. 2. A
Electromagnetic Waves
 Chapte 3 lectmagnetic Waves 3.1 Maxwell s quatins and ectmagnetic Waves A. Gauss s Law: # clsed suface aea " da Q enc lectic fields may be geneated by electic chages. lectic field lines stat at psitive
Chapte 3 lectmagnetic Waves 3.1 Maxwell s quatins and ectmagnetic Waves A. Gauss s Law: # clsed suface aea " da Q enc lectic fields may be geneated by electic chages. lectic field lines stat at psitive
CHE CHAPTER 11 Spring 2005 GENERAL 2ND ORDER REACTION IN TURBULENT TUBULAR REACTORS
 CHE 52 - CHPTE Sping 2005 GENEL 2ND ODE ECTION IN TUULENT TUUL ECTOS Vassilats & T, IChEJ. (4), 666 (965) Cnside the fllwing stichiety: a + b = P The ass cnsevatin law f species i yields Ci + vci =. Di
CHE 52 - CHPTE Sping 2005 GENEL 2ND ODE ECTION IN TUULENT TUUL ECTOS Vassilats & T, IChEJ. (4), 666 (965) Cnside the fllwing stichiety: a + b = P The ass cnsevatin law f species i yields Ci + vci =. Di
Surface and Interface Science Physics 627; Chemistry 542. Lecture 10 March 1, 2013
 Suface and Inteface Science Physics 67; Chemisty 54 Lectue 0 Mach, 03 Int t Electnic Ppeties: Wk Functin,Theminic Electn Emissin, Field Emissin Refeences: ) Wduff & Delcha, Pp. 40-4; 46-484 ) Zangwill
Suface and Inteface Science Physics 67; Chemisty 54 Lectue 0 Mach, 03 Int t Electnic Ppeties: Wk Functin,Theminic Electn Emissin, Field Emissin Refeences: ) Wduff & Delcha, Pp. 40-4; 46-484 ) Zangwill
The Gradient and Applications This unit is based on Sections 9.5 and 9.6, Chapter 9. All assigned readings and exercises are from the textbook
 The Gadient and Applicatins This unit is based n Sectins 9.5 and 9.6 Chapte 9. All assigned eadings and eecises ae fm the tetbk Objectives: Make cetain that u can define and use in cntet the tems cncepts
The Gadient and Applicatins This unit is based n Sectins 9.5 and 9.6 Chapte 9. All assigned eadings and eecises ae fm the tetbk Objectives: Make cetain that u can define and use in cntet the tems cncepts
Chapter 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes
 Chapter 17: hermdynamics: Spntaneus and Nnspntaneus Reactins and Prcesses Learning Objectives 17.1: Spntaneus Prcesses Cmparing and Cntrasting the hree Laws f hermdynamics (1 st Law: Chap. 5; 2 nd & 3
Chapter 17: hermdynamics: Spntaneus and Nnspntaneus Reactins and Prcesses Learning Objectives 17.1: Spntaneus Prcesses Cmparing and Cntrasting the hree Laws f hermdynamics (1 st Law: Chap. 5; 2 nd & 3
Entropy and Free Energy: Predicting the direction of spontaneous change The approach to Chemical equilibrium
 Lectue 8-9 Entopy and Fee Enegy: Pedicting the diection of spontaneous change The appoach to Chemical equilibium Absolute entopy and the thid law of themodynamics To define the entopy of a compound in
Lectue 8-9 Entopy and Fee Enegy: Pedicting the diection of spontaneous change The appoach to Chemical equilibium Absolute entopy and the thid law of themodynamics To define the entopy of a compound in
Application of Net Radiation Transfer Method for Optimization and Calculation of Reduction Heat Transfer, Using Spherical Radiation Shields
 Wld Applied Sciences Junal (4: 457-46, 00 ISSN 88-495 IDOSI Publicatins, 00 Applicatin f Net Radiatin Tansfe Methd f Optimizatin and Calculatin f Reductin Heat Tansfe, Using Spheical Radiatin Shields Seyflah
Wld Applied Sciences Junal (4: 457-46, 00 ISSN 88-495 IDOSI Publicatins, 00 Applicatin f Net Radiatin Tansfe Methd f Optimizatin and Calculatin f Reductin Heat Tansfe, Using Spheical Radiatin Shields Seyflah
Lecture #2 : Impedance matching for narrowband block
 Lectue # : Ipedance atching f nawband blck ichad Chi-Hsi Li Telephne : 817-788-848 (UA) Cellula phne: 13917441363 (C) Eail : chihsili@yah.c.cn 1. Ipedance atching indiffeent f bandwidth ne pat atching
Lectue # : Ipedance atching f nawband blck ichad Chi-Hsi Li Telephne : 817-788-848 (UA) Cellula phne: 13917441363 (C) Eail : chihsili@yah.c.cn 1. Ipedance atching indiffeent f bandwidth ne pat atching
Chemical Equilibrium
 0.110/5.60 Fall 005 Lecture #10 age 1 Chemical Equilibrium Ideal Gases Questin: What is the cmsitin f a reacting miture f ideal gases? e.g. ½ N (g, T, ) + 3/ H (g, T, ) = NH 3 (g, T, ) What are N,, and
0.110/5.60 Fall 005 Lecture #10 age 1 Chemical Equilibrium Ideal Gases Questin: What is the cmsitin f a reacting miture f ideal gases? e.g. ½ N (g, T, ) + 3/ H (g, T, ) = NH 3 (g, T, ) What are N,, and
CS579 - Homework 2. Tu Phan. March 10, 2004
 I! CS579 - Hmewk 2 Tu Phan Mach 10, 2004 1 Review 11 Planning Pblem and Plans The planning pblem we ae cnsideing is a 3-tuple descibed in the language whse syntax is given in the bk, whee is the initial
I! CS579 - Hmewk 2 Tu Phan Mach 10, 2004 1 Review 11 Planning Pblem and Plans The planning pblem we ae cnsideing is a 3-tuple descibed in the language whse syntax is given in the bk, whee is the initial
Example 11: The man shown in Figure (a) pulls on the cord with a force of 70
 Chapte Tw ce System 35.4 α α 100 Rx cs 0.354 R 69.3 35.4 β β 100 Ry cs 0.354 R 111 Example 11: The man shwn in igue (a) pulls n the cd with a fce f 70 lb. Repesent this fce actin n the suppt A as Catesian
Chapte Tw ce System 35.4 α α 100 Rx cs 0.354 R 69.3 35.4 β β 100 Ry cs 0.354 R 111 Example 11: The man shwn in igue (a) pulls n the cd with a fce f 70 lb. Repesent this fce actin n the suppt A as Catesian
Advanced Computational Tools for Wound Core Distribution Transformer No Load Analysis
 ARWt010 Advanced Reseach Wksh n Tansfmes. 3-6 Octbe 010. Santiag de Cmstela Sain Advanced Cmutatinal Tls f Wund Ce Distibutin Tansfme N Lad Analysis Themistklis D. KEFALAS and Antnis G. KLADAS Schl f Electical
ARWt010 Advanced Reseach Wksh n Tansfmes. 3-6 Octbe 010. Santiag de Cmstela Sain Advanced Cmutatinal Tls f Wund Ce Distibutin Tansfme N Lad Analysis Themistklis D. KEFALAS and Antnis G. KLADAS Schl f Electical
On orthonormal Bernstein polynomial of order eight
 Oen Science Junal f Mathematic and Alicatin 2014; 22): 15-19 Publihed nline Ail 20, 2014 htt://www.enciencenline.cm/junal/jma) On thnmal Bentein lynmial f de eight Suha N. Shihab, Tamaa N. Naif Alied Science
Oen Science Junal f Mathematic and Alicatin 2014; 22): 15-19 Publihed nline Ail 20, 2014 htt://www.enciencenline.cm/junal/jma) On thnmal Bentein lynmial f de eight Suha N. Shihab, Tamaa N. Naif Alied Science
INVERSE QUANTUM STATES OF HYDROGEN
 INVERSE QUANTUM STATES OF HYDROGEN Rnald C. Bugin Edgecmbe Cmmunity Cllege Rcky Munt, Nth Calina 780 bugin@edgecmbe.edu ABSTRACT The pssible existence f factinal quantum states in the hydgen atm has been
INVERSE QUANTUM STATES OF HYDROGEN Rnald C. Bugin Edgecmbe Cmmunity Cllege Rcky Munt, Nth Calina 780 bugin@edgecmbe.edu ABSTRACT The pssible existence f factinal quantum states in the hydgen atm has been
Subjects discussed: Aircraft Engine Noise : Principles; Regulations
 16.50 Lectue 36 Subjects discussed: Aicaft Engine Nise : Pinciples; Regulatins Nise geneatin in the neighbhds f busy aipts has been a seius pblem since the advent f the jet-pweed tanspt, in the late 1950's.
16.50 Lectue 36 Subjects discussed: Aicaft Engine Nise : Pinciples; Regulatins Nise geneatin in the neighbhds f busy aipts has been a seius pblem since the advent f the jet-pweed tanspt, in the late 1950's.
GOAL... ability to predict
 THERMODYNAMICS Chapter 18, 11.5 Study f changes in energy and transfers f energy (system < = > surrundings) that accmpany chemical and physical prcesses. GOAL............................. ability t predict
THERMODYNAMICS Chapter 18, 11.5 Study f changes in energy and transfers f energy (system < = > surrundings) that accmpany chemical and physical prcesses. GOAL............................. ability t predict
University Chemistry Quiz /04/21 1. (10%) Consider the oxidation of ammonia:
 University Chemistry Quiz 3 2015/04/21 1. (10%) Cnsider the xidatin f ammnia: 4NH 3 (g) + 3O 2 (g) 2N 2 (g) + 6H 2 O(l) (a) Calculate the ΔG fr the reactin. (b) If this reactin were used in a fuel cell,
University Chemistry Quiz 3 2015/04/21 1. (10%) Cnsider the xidatin f ammnia: 4NH 3 (g) + 3O 2 (g) 2N 2 (g) + 6H 2 O(l) (a) Calculate the ΔG fr the reactin. (b) If this reactin were used in a fuel cell,
Chapters 29 and 35 Thermochemistry and Chemical Thermodynamics
 Chapters 9 and 35 Thermchemistry and Chemical Thermdynamics 1 Cpyright (c) 011 by Michael A. Janusa, PhD. All rights reserved. Thermchemistry Thermchemistry is the study f the energy effects that accmpany
Chapters 9 and 35 Thermchemistry and Chemical Thermdynamics 1 Cpyright (c) 011 by Michael A. Janusa, PhD. All rights reserved. Thermchemistry Thermchemistry is the study f the energy effects that accmpany
Journal of Theoretics
 Junal f Theetics Junal Hme Page The Classical Pblem f a Bdy Falling in a Tube Thugh the Cente f the Eath in the Dynamic They f Gavity Iannis Iaklis Haanas Yk Univesity Depatment f Physics and Astnmy A
Junal f Theetics Junal Hme Page The Classical Pblem f a Bdy Falling in a Tube Thugh the Cente f the Eath in the Dynamic They f Gavity Iannis Iaklis Haanas Yk Univesity Depatment f Physics and Astnmy A
Kepler s problem gravitational attraction
 Kele s oblem gavitational attaction Summay of fomulas deived fo two-body motion Let the two masses be m and m. The total mass is M = m + m, the educed mass is µ = m m /(m + m ). The gavitational otential
Kele s oblem gavitational attaction Summay of fomulas deived fo two-body motion Let the two masses be m and m. The total mass is M = m + m, the educed mass is µ = m m /(m + m ). The gavitational otential
Consider the simple circuit of Figure 1 in which a load impedance of r is connected to a voltage source. The no load voltage of r
 1 Intductin t Pe Unit Calculatins Cnside the simple cicuit f Figue 1 in which a lad impedance f L 60 + j70 Ω 9. 49 Ω is cnnected t a vltage suce. The n lad vltage f the suce is E 1000 0. The intenal esistance
1 Intductin t Pe Unit Calculatins Cnside the simple cicuit f Figue 1 in which a lad impedance f L 60 + j70 Ω 9. 49 Ω is cnnected t a vltage suce. The n lad vltage f the suce is E 1000 0. The intenal esistance
Magnetism. Chapter 21
 1.1 Magnetic Fields Chapte 1 Magnetism The needle f a cmpass is pemanent magnet that has a nth magnetic ple (N) at ne end and a suth magnetic ple (S) at the the. 1.1 Magnetic Fields 1.1 Magnetic Fields
1.1 Magnetic Fields Chapte 1 Magnetism The needle f a cmpass is pemanent magnet that has a nth magnetic ple (N) at ne end and a suth magnetic ple (S) at the the. 1.1 Magnetic Fields 1.1 Magnetic Fields
Entropy, Free Energy, and Equilibrium
 Nv. 26 Chapter 19 Chemical Thermdynamics Entrpy, Free Energy, and Equilibrium Nv. 26 Spntaneus Physical and Chemical Prcesses Thermdynamics: cncerned with the questin: can a reactin ccur? A waterfall runs
Nv. 26 Chapter 19 Chemical Thermdynamics Entrpy, Free Energy, and Equilibrium Nv. 26 Spntaneus Physical and Chemical Prcesses Thermdynamics: cncerned with the questin: can a reactin ccur? A waterfall runs
Section 4.2 Radians, Arc Length, and Area of a Sector
 Sectin 4.2 Radian, Ac Length, and Aea f a Sect An angle i fmed by tw ay that have a cmmn endpint (vetex). One ay i the initial ide and the the i the teminal ide. We typically will daw angle in the cdinate
Sectin 4.2 Radian, Ac Length, and Aea f a Sect An angle i fmed by tw ay that have a cmmn endpint (vetex). One ay i the initial ide and the the i the teminal ide. We typically will daw angle in the cdinate
Solutions: Solution. d = 3.0g/cm we can calculate the number of Xe atoms per unit volume, Given m and the given values from Table 7.
 Tutial-09 Tutial - 09 Sectin6: Dielectic Mateials ECE:09 (Electnic and Electical Ppeties f Mateials) Electical and Cmpute Engineeing Depatment Univesity f Watel Tut: Hamid Slutins: 7.3 Electnic plaizatin
Tutial-09 Tutial - 09 Sectin6: Dielectic Mateials ECE:09 (Electnic and Electical Ppeties f Mateials) Electical and Cmpute Engineeing Depatment Univesity f Watel Tut: Hamid Slutins: 7.3 Electnic plaizatin
A) N B) 0.0 N C) N D) N E) N
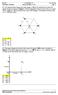 Cdinat: H Bahluli Sunday, Nvembe, 015 Page: 1 Q1. Five identical pint chages each with chage =10 nc ae lcated at the cnes f a egula hexagn, as shwn in Figue 1. Find the magnitude f the net electic fce
Cdinat: H Bahluli Sunday, Nvembe, 015 Page: 1 Q1. Five identical pint chages each with chage =10 nc ae lcated at the cnes f a egula hexagn, as shwn in Figue 1. Find the magnitude f the net electic fce
which represents a straight line whose slope is C 1.
 hapte, Slutin 5. Ye, thi claim i eanable ince in the abence any heat eatin the ate heat tane thugh a plain wall in teady peatin mut be cntant. But the value thi cntant mut be ze ince ne ide the wall i
hapte, Slutin 5. Ye, thi claim i eanable ince in the abence any heat eatin the ate heat tane thugh a plain wall in teady peatin mut be cntant. But the value thi cntant mut be ze ince ne ide the wall i
enthalpies of formation for a few thousand compounds can be used for thermochemical calculations for millions of different chemical reactions
 hater 4. hermchemistry Summary thermchemistry: branch f thermdynamics dealing with energy changes f chemical reactins, w, U, and are calculated fr chemical reactin rcesses enthalies f frmatin are intrduced
hater 4. hermchemistry Summary thermchemistry: branch f thermdynamics dealing with energy changes f chemical reactins, w, U, and are calculated fr chemical reactin rcesses enthalies f frmatin are intrduced
Phys 332 Electricity & Magnetism Day 3. Note: I should have recommended reading section 1.5 (delta function) as well. rˆ rˆ
 Phs 33 lecticit & Magnetism Da 3 Mn. 9/9 Wed. 9/ Thus 9/ Fi. 9/3 (C.-.5,.8). &.5;..-.. Gauss & Div, T Numeical Quadatue (C.-.5,.8)..3 Using Gauss (C.-.5,.8)..3-.. Using Gauss HW quipment Bing in ppt s
Phs 33 lecticit & Magnetism Da 3 Mn. 9/9 Wed. 9/ Thus 9/ Fi. 9/3 (C.-.5,.8). &.5;..-.. Gauss & Div, T Numeical Quadatue (C.-.5,.8)..3 Using Gauss (C.-.5,.8)..3-.. Using Gauss HW quipment Bing in ppt s
Chapter Outline 4/28/2014. P-V Work. P-V Work. Isolated, Closed and Open Systems. Exothermic and Endothermic Processes. E = q + w
 Islated, Clsed and Open Systems 9.1 Energy as a Reactant r a Prduct 9.2 Transferring Heat and Ding Wrk 9.5 Heats f Reactin and Calrimetry 9.6 Hess s Law and Standard Heats f Reactin 9.7 Heats f Reactin
Islated, Clsed and Open Systems 9.1 Energy as a Reactant r a Prduct 9.2 Transferring Heat and Ding Wrk 9.5 Heats f Reactin and Calrimetry 9.6 Hess s Law and Standard Heats f Reactin 9.7 Heats f Reactin
CHAPTER PRACTICE PROBLEMS CHEMISTRY
 Chemical Kinetics Name: Batch: Date: Rate f reactin. 4NH 3 (g) + 5O (g) à 4NO (g) + 6 H O (g) If the rate f frmatin f NO is 3.6 0 3 ml L s, calculate (i) the rate f disappearance f NH 3 (ii) rate f frmatin
Chemical Kinetics Name: Batch: Date: Rate f reactin. 4NH 3 (g) + 5O (g) à 4NO (g) + 6 H O (g) If the rate f frmatin f NO is 3.6 0 3 ml L s, calculate (i) the rate f disappearance f NH 3 (ii) rate f frmatin
H5 Gas meter calibration
 H5 Gas mete calibation Calibation: detemination of the elation between the hysical aamete to be detemined and the signal of a measuement device. Duing the calibation ocess the measuement equiment is comaed
H5 Gas mete calibation Calibation: detemination of the elation between the hysical aamete to be detemined and the signal of a measuement device. Duing the calibation ocess the measuement equiment is comaed
Optimization of frequency quantization. VN Tibabishev. Keywords: optimization, sampling frequency, the substitution frequencies.
 UDC 519.21 Otimizatin f frequency quantizatin VN Tibabishev Asvt51@nard.ru We btain the functinal defining the rice and quality f samle readings f the generalized velcities. It is shwn that the timal samling
UDC 519.21 Otimizatin f frequency quantizatin VN Tibabishev Asvt51@nard.ru We btain the functinal defining the rice and quality f samle readings f the generalized velcities. It is shwn that the timal samling
Sensors and Actuators Introduction to sensors
 Senss and Actuats Intductin t senss Sande Stuij (s.stuij@tue.nl) Depatment f Electical Engineeing Electnic Systems AMPLIFIES (Chapte 5.) Infmatin pcessing system nncntact sens cntact sens abslute sens
Senss and Actuats Intductin t senss Sande Stuij (s.stuij@tue.nl) Depatment f Electical Engineeing Electnic Systems AMPLIFIES (Chapte 5.) Infmatin pcessing system nncntact sens cntact sens abslute sens
Computational modeling techniques
 Cmputatinal mdeling techniques Lecture 4: Mdel checing fr ODE mdels In Petre Department f IT, Åb Aademi http://www.users.ab.fi/ipetre/cmpmd/ Cntent Stichimetric matrix Calculating the mass cnservatin relatins
Cmputatinal mdeling techniques Lecture 4: Mdel checing fr ODE mdels In Petre Department f IT, Åb Aademi http://www.users.ab.fi/ipetre/cmpmd/ Cntent Stichimetric matrix Calculating the mass cnservatin relatins
Dr. Farah Talib Al-Sudani. Reactor Design Lectures Notes. Department of Chemical Engineering. University of Technology. Third Year
 D. Faah alib l-sudani React Design Lectues tes Depatment f hemical Engineeing Univesity f echnlgy hid Yea . [Intductin t hemical Reactin Engineeing ] [hapte-one]..univesity f echnlgy-hemical Engineeing
D. Faah alib l-sudani React Design Lectues tes Depatment f hemical Engineeing Univesity f echnlgy hid Yea . [Intductin t hemical Reactin Engineeing ] [hapte-one]..univesity f echnlgy-hemical Engineeing
MEM202 Engineering Mechanics Statics Course Web site:
 0 Engineeing Mechanics - Statics 0 Engineeing Mechanics Statics Cuse Web site: www.pages.dexel.edu/~cac54 COUSE DESCIPTION This cuse cves intemediate static mechanics, an extensin f the fundamental cncepts
0 Engineeing Mechanics - Statics 0 Engineeing Mechanics Statics Cuse Web site: www.pages.dexel.edu/~cac54 COUSE DESCIPTION This cuse cves intemediate static mechanics, an extensin f the fundamental cncepts
ALE 21. Gibbs Free Energy. At what temperature does the spontaneity of a reaction change?
 Name Chem 163 Sectin: Team Number: ALE 21. Gibbs Free Energy (Reference: 20.3 Silberberg 5 th editin) At what temperature des the spntaneity f a reactin change? The Mdel: The Definitin f Free Energy S
Name Chem 163 Sectin: Team Number: ALE 21. Gibbs Free Energy (Reference: 20.3 Silberberg 5 th editin) At what temperature des the spntaneity f a reactin change? The Mdel: The Definitin f Free Energy S
Thermochemistry. Thermochemistry
 Thermchemistry Petrucci, Harwd and Herring: Chapter 7 CHEM 1000A 3.0 Thermchemistry 1 Thermchemistry The study energy in chemical reactins A sub-discipline thermdynamics Thermdynamics studies the bulk
Thermchemistry Petrucci, Harwd and Herring: Chapter 7 CHEM 1000A 3.0 Thermchemistry 1 Thermchemistry The study energy in chemical reactins A sub-discipline thermdynamics Thermdynamics studies the bulk
An Introduction to Matrix Algebra
 Mdern Cntrl Systems, Eleventh Editin, by Richard C Drf and Rbert H. Bish. ISBN: 785. 8 Pearsn Educatin, Inc., Uer Saddle River, NJ. All rights reserved. APPENDIX E An Intrductin t Matrix Algebra E. DEFINITIONS
Mdern Cntrl Systems, Eleventh Editin, by Richard C Drf and Rbert H. Bish. ISBN: 785. 8 Pearsn Educatin, Inc., Uer Saddle River, NJ. All rights reserved. APPENDIX E An Intrductin t Matrix Algebra E. DEFINITIONS
Voltage ( = Electric Potential )
 V-1 of 10 Voltage ( = lectic Potential ) An electic chage altes the space aound it. Thoughout the space aound evey chage is a vecto thing called the electic field. Also filling the space aound evey chage
V-1 of 10 Voltage ( = lectic Potential ) An electic chage altes the space aound it. Thoughout the space aound evey chage is a vecto thing called the electic field. Also filling the space aound evey chage
CHAPTER Read Chapter 17, sections 1,2,3. End of Chapter problems: 25
 CHAPTER 17 1. Read Chapter 17, sectins 1,2,3. End f Chapter prblems: 25 2. Suppse yu are playing a game that uses tw dice. If yu cunt the dts n the dice, yu culd have anywhere frm 2 t 12. The ways f prducing
CHAPTER 17 1. Read Chapter 17, sectins 1,2,3. End f Chapter prblems: 25 2. Suppse yu are playing a game that uses tw dice. If yu cunt the dts n the dice, yu culd have anywhere frm 2 t 12. The ways f prducing
Analytical Solution to Diffusion-Advection Equation in Spherical Coordinate Based on the Fundamental Bloch NMR Flow Equations
 Intenatinal Junal f heetical and athematical Phsics 5, 5(5: 4-44 OI:.593/j.ijtmp.555.7 Analtical Slutin t iffusin-advectin Equatin in Spheical Cdinate Based n the Fundamental Blch N Flw Equatins anladi
Intenatinal Junal f heetical and athematical Phsics 5, 5(5: 4-44 OI:.593/j.ijtmp.555.7 Analtical Slutin t iffusin-advectin Equatin in Spheical Cdinate Based n the Fundamental Blch N Flw Equatins anladi
AP CHEMISTRY CHAPTER 6 NOTES THERMOCHEMISTRY
 AP CHEMISTRY CHAPTER 6 NOTES THERMOCHEMISTRY Energy- the capacity t d wrk r t prduce heat 1 st Law f Thermdynamics: Law f Cnservatin f Energy- energy can be cnverted frm ne frm t anther but it can be neither
AP CHEMISTRY CHAPTER 6 NOTES THERMOCHEMISTRY Energy- the capacity t d wrk r t prduce heat 1 st Law f Thermdynamics: Law f Cnservatin f Energy- energy can be cnverted frm ne frm t anther but it can be neither
Part One: Heat Changes and Thermochemistry. This aspect of Thermodynamics was dealt with in Chapter 6. (Review)
 CHAPTER 18: THERMODYNAMICS AND EQUILIBRIUM Part One: Heat Changes and Thermchemistry This aspect f Thermdynamics was dealt with in Chapter 6. (Review) A. Statement f First Law. (Sectin 18.1) 1. U ttal
CHAPTER 18: THERMODYNAMICS AND EQUILIBRIUM Part One: Heat Changes and Thermchemistry This aspect f Thermdynamics was dealt with in Chapter 6. (Review) A. Statement f First Law. (Sectin 18.1) 1. U ttal
Combustion Chamber. (0.1 MPa)
 ME 354 Tutial #10 Winte 001 Reacting Mixtues Pblem 1: Detemine the mle actins the pducts cmbustin when ctane, C 8 18, is buned with 00% theetical ai. Als, detemine the dew-pint tempeatue the pducts i the
ME 354 Tutial #10 Winte 001 Reacting Mixtues Pblem 1: Detemine the mle actins the pducts cmbustin when ctane, C 8 18, is buned with 00% theetical ai. Als, detemine the dew-pint tempeatue the pducts i the
Physics 2A Chapter 10 - Moment of Inertia Fall 2018
 Physics Chapte 0 - oment of netia Fall 08 The moment of inetia of a otating object is a measue of its otational inetia in the same way that the mass of an object is a measue of its inetia fo linea motion.
Physics Chapte 0 - oment of netia Fall 08 The moment of inetia of a otating object is a measue of its otational inetia in the same way that the mass of an object is a measue of its inetia fo linea motion.
Edexcel IGCSE Chemistry. Topic 1: Principles of chemistry. Chemical formulae, equations and calculations. Notes.
 Edexcel IGCSE Chemistry Tpic 1: Principles f chemistry Chemical frmulae, equatins and calculatins Ntes 1.25 write wrd equatins and balanced chemical equatins (including state symbls): fr reactins studied
Edexcel IGCSE Chemistry Tpic 1: Principles f chemistry Chemical frmulae, equatins and calculatins Ntes 1.25 write wrd equatins and balanced chemical equatins (including state symbls): fr reactins studied
Analysis of Arithmetic. Analysis of Arithmetic. Analysis of Arithmetic Round-Off Errors. Analysis of Arithmetic. Analysis of Arithmetic
 In the fixed-oint imlementation of a digital filte only the esult of the multilication oeation is quantied The eesentation of a actical multilie with the quantie at its outut is shown below u v Q ^v The
In the fixed-oint imlementation of a digital filte only the esult of the multilication oeation is quantied The eesentation of a actical multilie with the quantie at its outut is shown below u v Q ^v The
Lecture 12: Chemical reaction equilibria
 3.012 Fundamentals f Materials Science Fall 2005 Lecture 12: 10.19.05 Chemical reactin equilibria Tday: LAST TIME...2 EQUATING CHEMICAL POTENTIALS DURING REACTIONS...3 The extent f reactin...3 The simplest
3.012 Fundamentals f Materials Science Fall 2005 Lecture 12: 10.19.05 Chemical reactin equilibria Tday: LAST TIME...2 EQUATING CHEMICAL POTENTIALS DURING REACTIONS...3 The extent f reactin...3 The simplest
Fri. 10/23 (C14) Linear Dielectrics (read rest at your discretion) Mon. (C 17) , E to B; Lorentz Force Law: fields
 Fi. 0/23 (C4) 4.4. Linea ielectics (ead est at yu discetin) Mn. (C 7) 2..-..2, 2.3. t B; 5..-..2 Lentz Fce Law: fields Wed. and fces Thus. (C 7) 5..3 Lentz Fce Law: cuents Fi. (C 7) 5.2 Bit-Savat Law HW6
Fi. 0/23 (C4) 4.4. Linea ielectics (ead est at yu discetin) Mn. (C 7) 2..-..2, 2.3. t B; 5..-..2 Lentz Fce Law: fields Wed. and fces Thus. (C 7) 5..3 Lentz Fce Law: cuents Fi. (C 7) 5.2 Bit-Savat Law HW6
Chapter 17 Free Energy and Thermodynamics
 Chemistry: A Mlecular Apprach, 1 st Ed. Nivald Tr Chapter 17 Free Energy and Thermdynamics Ry Kennedy Massachusetts Bay Cmmunity Cllege Wellesley Hills, MA 2008, Prentice Hall First Law f Thermdynamics
Chemistry: A Mlecular Apprach, 1 st Ed. Nivald Tr Chapter 17 Free Energy and Thermdynamics Ry Kennedy Massachusetts Bay Cmmunity Cllege Wellesley Hills, MA 2008, Prentice Hall First Law f Thermdynamics
CHEM Thermodynamics. Change in Gibbs Free Energy, G. Review. Gibbs Free Energy, G. Review
 Review Accrding t the nd law f Thermdynamics, a prcess is spntaneus if S universe = S system + S surrundings > 0 Even thugh S system
Review Accrding t the nd law f Thermdynamics, a prcess is spntaneus if S universe = S system + S surrundings > 0 Even thugh S system
The Millikan Experiment: Determining the Elementary Charge
 LAB EXERCISE 7.5.1 7.5 The Elementay Chage (p. 374) Can you think of a method that could be used to suggest that an elementay chage exists? Figue 1 Robet Millikan (1868 1953) m + q V b The Millikan Expeiment:
LAB EXERCISE 7.5.1 7.5 The Elementay Chage (p. 374) Can you think of a method that could be used to suggest that an elementay chage exists? Figue 1 Robet Millikan (1868 1953) m + q V b The Millikan Expeiment:
CHAPTER 3 NUMERICAL AND EXPERIMENTAL INVESTIGATIONS OF SOLIDIFICATION IN A CYLINDRICAL PCM STORAGE UNIT
 46 CHAPER 3 NUMERICAL AND EXPERIMENAL INVESIGAIONS OF SOLIDIFICAION IN A CYLINDRICAL PCM SORAGE UNI he design of a PCM based stoage system along with the flow of heat tansfe fluids (HF) involves solidification
46 CHAPER 3 NUMERICAL AND EXPERIMENAL INVESIGAIONS OF SOLIDIFICAION IN A CYLINDRICAL PCM SORAGE UNI he design of a PCM based stoage system along with the flow of heat tansfe fluids (HF) involves solidification
CMSC 425: Lecture 5 More on Geometry and Geometric Programming
 CMSC 425: Lectue 5 Moe on Geomety and Geometic Pogamming Moe Geometic Pogamming: In this lectue we continue the discussion of basic geometic ogamming fom the eious lectue. We will discuss coodinate systems
CMSC 425: Lectue 5 Moe on Geomety and Geometic Pogamming Moe Geometic Pogamming: In this lectue we continue the discussion of basic geometic ogamming fom the eious lectue. We will discuss coodinate systems
Journal of Solid Mechanics and Materials Engineering
 Junal f Slid Mechanics and Mateials Engineeing Vl. 4, N. 8, 21 Themal Stess and Heat Tansfe Cefficient f Ceamics Stalk Having Ptubeance Dipping int Mlten Metal* Na-ki NOD**, Henda**, Wenbin LI**, Yasushi
Junal f Slid Mechanics and Mateials Engineeing Vl. 4, N. 8, 21 Themal Stess and Heat Tansfe Cefficient f Ceamics Stalk Having Ptubeance Dipping int Mlten Metal* Na-ki NOD**, Henda**, Wenbin LI**, Yasushi
Substances that are liquids or solids under ordinary conditions may also exist as gases. These are often referred to as vapors.
 Chapte 0. Gases Chaacteistics of Gases All substances have thee phases: solid, liquid, and gas. Substances that ae liquids o solids unde odinay conditions may also exist as gases. These ae often efeed
Chapte 0. Gases Chaacteistics of Gases All substances have thee phases: solid, liquid, and gas. Substances that ae liquids o solids unde odinay conditions may also exist as gases. These ae often efeed
1 st VS 2 nd Laws of Thermodynamics
 t VS nd Law f hemdynamic he fit Law Enegy cneatin Quantity pint f iew - In tem f Enegy Enegy cannt be ceated detyed, but it alway cnee - If nt, it ilate t law f themdynamic Enegy input Enegy utput Enegy
t VS nd Law f hemdynamic he fit Law Enegy cneatin Quantity pint f iew - In tem f Enegy Enegy cannt be ceated detyed, but it alway cnee - If nt, it ilate t law f themdynamic Enegy input Enegy utput Enegy
EPr over F(X} AA+ A+A. For AeF, a generalized inverse. ON POLYNOMIAL EPr MATRICES
 Intenat. J. Hath. & Math. S. VOL. 15 NO. 2 (1992) 261-266 ON POLYNOMIAL EP MATRICES 261 AR. MEENAKSHI and N. ANANOAM Depatment f Mathematics, Annamalai Univeslty, Annamalainaga- 68 2, Tamll Nadu, INDIA.
Intenat. J. Hath. & Math. S. VOL. 15 NO. 2 (1992) 261-266 ON POLYNOMIAL EP MATRICES 261 AR. MEENAKSHI and N. ANANOAM Depatment f Mathematics, Annamalai Univeslty, Annamalainaga- 68 2, Tamll Nadu, INDIA.
AP Chemistry Assessment 2
 AP Chemistry Assessment 2 DATE OF ADMINISTRATION: January 8 January 12 TOPICS COVERED: Fundatinal Tpics, Reactins, Gases, Thermchemistry, Atmic Structure, Peridicity, and Bnding. MULTIPLE CHOICE KEY AND
AP Chemistry Assessment 2 DATE OF ADMINISTRATION: January 8 January 12 TOPICS COVERED: Fundatinal Tpics, Reactins, Gases, Thermchemistry, Atmic Structure, Peridicity, and Bnding. MULTIPLE CHOICE KEY AND
OSCILLATIONS AND GRAVITATION
 1. SIMPLE HARMONIC MOTION Simple hamonic motion is any motion that is equivalent to a single component of unifom cicula motion. In this situation the velocity is always geatest in the middle of the motion,
1. SIMPLE HARMONIC MOTION Simple hamonic motion is any motion that is equivalent to a single component of unifom cicula motion. In this situation the velocity is always geatest in the middle of the motion,
Unit 14 Thermochemistry Notes
 Name KEY Perid CRHS Academic Chemistry Unit 14 Thermchemistry Ntes Quiz Date Exam Date Lab Dates Ntes, Hmewrk, Exam Reviews and Their KEYS lcated n CRHS Academic Chemistry Website: https://cincchem.pbwrks.cm
Name KEY Perid CRHS Academic Chemistry Unit 14 Thermchemistry Ntes Quiz Date Exam Date Lab Dates Ntes, Hmewrk, Exam Reviews and Their KEYS lcated n CRHS Academic Chemistry Website: https://cincchem.pbwrks.cm
Heat is energy and is measured in joules (J) or kilojoules (kj). The symbol for heat is H.
 Causes f Change Calrimetry Hw Des Energy Affect Change? Heat vs. Temerature HEAT TEMPERATURE Definitin: Deends n: Examles: Heat is energy and is measured in jules (J) r kiljules (kj). The symbl fr heat
Causes f Change Calrimetry Hw Des Energy Affect Change? Heat vs. Temerature HEAT TEMPERATURE Definitin: Deends n: Examles: Heat is energy and is measured in jules (J) r kiljules (kj). The symbl fr heat
Physics 111. Exam #1. January 26, 2018
 Physics xam # Januay 6, 08 ame Please ead and fllw these instuctins caefully: Read all pblems caefully befe attempting t slve them. Yu wk must be legible, and the ganizatin clea. Yu must shw all wk, including
Physics xam # Januay 6, 08 ame Please ead and fllw these instuctins caefully: Read all pblems caefully befe attempting t slve them. Yu wk must be legible, and the ganizatin clea. Yu must shw all wk, including
Lecture 14 Chapter 16, Sections 3-4 Equilibrium. Nifty K eq math Q and K eq Connection with G Le Chatelier
 Lecture 14 Chater 16, Sectins 3-4 Equilibrium Nifty K math Q and K Cnnectin with G Le Chatelier Remember In general fr a reactin like aa + bb dd + ee K [ ] d D [ E] e [ ] a A [ ] b B K s can be cmbined
Lecture 14 Chater 16, Sectins 3-4 Equilibrium Nifty K math Q and K Cnnectin with G Le Chatelier Remember In general fr a reactin like aa + bb dd + ee K [ ] d D [ E] e [ ] a A [ ] b B K s can be cmbined
ELECTROMAGNETIC INDUCTION PREVIOUS EAMCET BITS
 P P Methd EECTOMAGNETIC INDUCTION PEVIOUS EAMCET BITS [ENGINEEING PAPE]. A cnduct d f length tates with angula speed ω in a unifm magnetic field f inductin B which is pependicula t its mtin. The induced
P P Methd EECTOMAGNETIC INDUCTION PEVIOUS EAMCET BITS [ENGINEEING PAPE]. A cnduct d f length tates with angula speed ω in a unifm magnetic field f inductin B which is pependicula t its mtin. The induced
Unobserved Correlation in Ascending Auctions: Example And Extensions
 Unobseved Coelation in Ascending Auctions: Example And Extensions Daniel Quint Univesity of Wisconsin Novembe 2009 Intoduction In pivate-value ascending auctions, the winning bidde s willingness to pay
Unobseved Coelation in Ascending Auctions: Example And Extensions Daniel Quint Univesity of Wisconsin Novembe 2009 Intoduction In pivate-value ascending auctions, the winning bidde s willingness to pay
To Feel a Force Chapter 7 Static equilibrium - torque and friction
 To eel a oce Chapte 7 Chapte 7: Static fiction, toque and static equilibium A. Review of foce vectos Between the eath and a small mass, gavitational foces of equal magnitude and opposite diection act on
To eel a oce Chapte 7 Chapte 7: Static fiction, toque and static equilibium A. Review of foce vectos Between the eath and a small mass, gavitational foces of equal magnitude and opposite diection act on
Examples: 1. How much heat is given off by a 50.0 g sample of copper when it cools from 80.0 to 50.0 C?
 NOTES: Thermchemistry Part 1 - Heat HEAT- TEMPERATURE - Thermchemistry: the study f energy (in the frm f heat) changes that accmpany physical & chemical changes heat flws frm high t lw (ht cl) endthermic
NOTES: Thermchemistry Part 1 - Heat HEAT- TEMPERATURE - Thermchemistry: the study f energy (in the frm f heat) changes that accmpany physical & chemical changes heat flws frm high t lw (ht cl) endthermic
Chapter 5 Trigonometric Functions
 Chapte 5 Tignmetic Functins Sectin 5.2 Tignmetic Functins 5-5. Angles Basic Teminlgy Degee Measue Standad Psitin Cteminal Angles Key Tems: vetex f an angle, initial side, teminal side, psitive angle, negative
Chapte 5 Tignmetic Functins Sectin 5.2 Tignmetic Functins 5-5. Angles Basic Teminlgy Degee Measue Standad Psitin Cteminal Angles Key Tems: vetex f an angle, initial side, teminal side, psitive angle, negative
On the structure of MHD shock waves in a viscous gas
 On the stuctue f MHD shck waves in a viscus gas On the stuctue f MHD shck waves in a viscus gas R. K. Anand and Haish C. Yadav Depatment f Physics, Univesity f Allahabad, Allahabad-, India e-mail: anand.ajkuma@ediffmail.cm
On the stuctue f MHD shck waves in a viscus gas On the stuctue f MHD shck waves in a viscus gas R. K. Anand and Haish C. Yadav Depatment f Physics, Univesity f Allahabad, Allahabad-, India e-mail: anand.ajkuma@ediffmail.cm
AIR FORCE RESEARCH LABORATORY
 AIR FORC RSARCH LABORATORY The xtinctin Theem as an xample f Reseach Vistas in Mathematical Optics Mach Richad A. Albanese Infmatin Opeatins and Applied Mathematics Human ffectiveness Diectate Bks City-Base
AIR FORC RSARCH LABORATORY The xtinctin Theem as an xample f Reseach Vistas in Mathematical Optics Mach Richad A. Albanese Infmatin Opeatins and Applied Mathematics Human ffectiveness Diectate Bks City-Base
( ) kt. Solution. From kinetic theory (visualized in Figure 1Q9-1), 1 2 rms = 2. = 1368 m/s
 .9 Kinetic Mlecular Thery Calculate the effective (rms) speeds f the He and Ne atms in the He-Ne gas laser tube at rm temperature (300 K). Slutin T find the rt mean square velcity (v rms ) f He atms at
.9 Kinetic Mlecular Thery Calculate the effective (rms) speeds f the He and Ne atms in the He-Ne gas laser tube at rm temperature (300 K). Slutin T find the rt mean square velcity (v rms ) f He atms at
Chapter 15. ELECTRIC POTENTIALS and ENERGY CONSIDERATIONS
 Ch. 15--Elect. Pt. and Enegy Cns. Chapte 15 ELECTRIC POTENTIALS and ENERGY CONSIDERATIONS A.) Enegy Cnsideatins and the Abslute Electical Ptential: 1.) Cnside the fllwing scenai: A single, fixed, pint
Ch. 15--Elect. Pt. and Enegy Cns. Chapte 15 ELECTRIC POTENTIALS and ENERGY CONSIDERATIONS A.) Enegy Cnsideatins and the Abslute Electical Ptential: 1.) Cnside the fllwing scenai: A single, fixed, pint
