Volterra kernels and effective connectivity
|
|
|
- Elmer McLaughlin
- 6 years ago
- Views:
Transcription
1 Volterra kernels and effective connectivity Karl J Friston The Wellcome Dept. of Cognitive Neurology, University College London Queen Square, London, UK WC1N 3BG Tel (44) Fax (44) k.friston@fil.ion.ucl.ac.uk Contents I. Introduction II. Neuronal Transients III. Neuronal Codes IV. Evidence for nonlinear coupling V. The neural basis of nonlinear coupling VI Conclusion References Box 1: Dynamical systems and Volterra kernels I INTRODUCTION The purpose of this brief chapter is to establish the Volterra formulation of effective connectivity from a conceptual and neurobiological point of view. Recall from the previous chapter that the generalised convolution representation of dynamic systems, afforded by Volterra expansions, is just anther way of describing the input-output behaviour of systems that have an equivalent state-space representation. In the next chapter we will deal with 1
2 state-space representations in more detail. Before proceeding to explicit input-state-output models we will look at why the Volterra formulation can be so useful. A The brain and dynamic systems The brain can be regarded as an ensemble of connected dynamical systems and as such conforms to some simple principles relating the inputs and outputs of its constituent parts. The ensuing implications, for the way we think about, and measure, neuronal interactions can be quite profound. These range from implications for which aspects of neuronal activity are important to measure and how to characterise coupling among neuronal populations, to implications pertaining to dynamic instability and complexity, that is necessary for adaptive self-organisation. This chapter focuses on the first issue by looking at neuronal interactions, coupling and implicit neuronal codes from a dynamical perspective. By considering the brain in this light one can show that a sufficient description of neuronal activity must comprise activity at the current time and its recent history. This history constitutes a neuronal transient. Such transients represent an essential metric of neuronal interactions and, implicitly, a code employed in the functional integration of brain systems. The nature of transients, expressed conjointly in different neuronal populations, reflects the underlying coupling among brain systems. A complete description of this coupling, or effective connectivity, can be expressed in terms of generalised convolution kernels [Volterra kernels] that embody high-order or nonlinear interactions. This coupling may be synchronous, and possibly oscillatory, or asynchronous. A critical distinction between synchronous and asynchronous coupling is that the former is essentially linear and the latter is nonlinear. The nonlinear nature of asynchronous coupling enables context-sensitive interactions that characterise real brain dynamics, suggesting that it plays an important role in functional integration. Brain states are inherently labile, with a complexity and transience that renders their invariant characteristics elusive. The position adopted in this chapter is that the best approach is to embrace these dynamical aspects. Its aim is to introduce the notion of neuronal transients and the underlying framework (Friston 2000). The central tenet is that the dynamics of neuronal systems can be viewed as a succession of transient spatiotemporal patterns of activity. These transients are shaped by the brain's anatomical infrastructure, principally connections, which have been selected to ensure the adaptive nature of the resulting dynamics. Although rather obvious, this formulation embodies one fundamental point; namely that any description of brain state should have an explicit temporal dimension. 2
3 In other words, measures of brain activity are only meaningful when specified over periods of time. This is particularly important in relation to fast dynamic interactions among neuronal populations that are characterised by synchrony. Synchronisation has become popular in the past years (e.g. Gray and Singer 1991, Eckhorn et al. 1992, Engel et al 1991) and yet represents only one possible sort of interaction. This chapter is divided into four sections. In the first we review the conceptual basis of neuronal transients. This section uses an equivalence, between two mathematical formulations of nonlinear systems, to show that descriptions of brain dynamics, in terms of (i) neuronal transients and (ii) the coupling among interacting brain systems, is complete and sufficient. The second section uses this equivalence to motivate a taxonomy of neuronal codes and establish the relationship among neuronal transients, asynchronous coupling, dynamic correlations and nonlinear interactions. In the third section we illustrate nonlinear coupling using magnetoencephalography (MEG) data. The final section discusses some neurobiological mechanisms that might mediate nonlinear coupling. II. NEURONAL TRANSIENTS The assertion that meaningful measures of brain dynamics have a temporal domain is neither new nor contentious (e.g. von der Malsburg 1983, Optican and Richmond 1987, Engel et al 1991, Aertsen et al 1994, Freeman and Barrie 1994, Abeles et al 1995, decharms and Merzenich 1996). A straightforward analysis demonstrates its veracity: Suppose that one wanted to posit some variables x that represented a complete and self-consistent description of brain activity. In short, everything needed to determine the evolution of the brain's state, at a particular place and time, was embodied in these measurements. Consider a component of the brain (e.g. a neuron or neuronal population). If such a set of variables existed for this component system they would satisfy some immensely complicated nonlinear state equation x( t) t = f ( x( t), u( t)) 1 where x is a huge vector of state variables which range from depolarisation at every point in the dendritic tree to the phosphorylation status of every relevant enzyme, from the biochemical status of every glial cell compartment to every aspect of gene expression.. u(t) 3
4 represents a set of inputs conveyed by afferent from other regions. Eq(1) simply says that the changes in the state variables are nonlinear functions of the variables themselves and some inputs. The vast majority of these variables are hidden and not measurable directly. However, there are a small number of derived measurements y that can be made, y( t) = λ( x( t)) 2 such as activities of whole cells or populations. These activities could be measured in many ways, for example firing at the initial segment of an axon or local field potentials. The problem is that a complete and sufficient description appears unattainable, given that the underlying state variables cannot be observed directly. This is not the case. The resolution of this apparent impasse rests upon two things. (i) Firstly, a fundamental mathematical equivalence relating the inputs and outputs of a dynamical system and (ii) the fact that these measurable outputs constitute the inputs to other cells or populations. Box 1 about here A Convolution and state-space representations Assume that every neuron in the brain is modelled by a nonlinear dynamical system of the sort described by Eq(1). Under this assumption it can be shown that the output is a function of the recent history of its inputs. y ( t) = h( u( t σ )) 3 where u( t τ ) represents the inputs in the recent past. Furthermore, this relationship can be expressed as a Volterra series of the inputs (see Box 1). The critical thing here is that we never need to know the underlying and 'hidden' variables that describe the details of each cell's electrochemical and biochemical status. We only need to know the history of its inputs, which, of course, are the outputs of other cells. Eq(3) is, in principle, a sufficient description of brain dynamics and involves the variables u ( t σ ) = y ( t σ ) that represent activity at all times σ preceding the moment in question. These are simply neuronal transients. The degree of transience depends on how far back in time it is necessary to go to fully capture the brain's dynamics. The sensible nature of Eq(3) can be readily seen. For example, if we wanted to determine the behaviour of a cell in V1 (primary visual cortex) then 4
5 we would need to know the activity of all connected cells in the immediate vicinity over the last millisecond or so to account for propagation delays down afferent axons. We would also need to know the activity in distant sources, like the lateral geniculate nucleus and higher cortical areas, some ten or more milliseconds ago. In short we need the recent history of all inputs. Transients can be expressed in terms of firing rates (e.g. chaotic oscillations, Freeman and Barrie 1994) or individual spikes (e.g. syn-fire chains, Abeles et al. 1994; 1995). Transients are not just a mathematical abstraction, they have real implications at a number of levels: For example, the emergence of fast oscillatory interactions among simulated neuronal populations depends upon the time delays implicit in axonal transmission and the timeconstants of postsynaptic responses. Another slightly more subtle aspect of this formulation is that changes in synaptic efficacy, such as short-term potentiation or depression, take some time to be mediated by intracellular mechanisms. This means that the interaction between inputs at different times, that models these activity-dependent effects, again depends on the relevant history of activity 1 levels of description The above arguments lead to a conceptual model of the brain that comprises a collection of dynamical systems (e.g. cells or populations of cells), each representing as an input-stateoutput model, where the state remains forever hidden. However the inputs and outputs are accessible and are causally related where the output of one system constitutes the input to another. A complete description therefore comprises the nature of these relationships (the Volterra series corresponding to the function h) and the neuronal transients. This constitutes a mesoscopic level of description that permits a degree of black-boxness but with no loss of information. The equivalence, in terms of specifying the behaviour of a neuronal system, between microscopic and mesoscopic levels of description is critical. In short, the equivalence means that all the information inherent in unobservable microscopic variables that determine the response of a neuronal system is embodied in the history of its observable inputs and outputs. Although the microscopic level of description may be more mechanistically informative, from the point of view of response prediction, neuronal transients are an entirely equivalent representation 1. 1 We have focussed on the distinction between microscopic and mesoscopic levels of description. The macroscopic level is reserved for approaches, exemplified by synergistics (Haken 1983), that characterise the 5
6 B Effective connectivity and Volterra kernels The first conclusion so far is that neuronal transients are necessary to specify brain dynamics. The second conclusion is that a complete model of the influence one neuronal population exerts over another should take the form of a Volterra series 2. This implies that a complete characterisation of these influences (i.e. effective connectivity) comprises the Volterra kernels that are applied to the inputs to yield the outputs. Effective connectivity refers explicitly to "the influence that one neural system exerts over another, either at a synaptic (i.e. synaptic efficacy) or population level" (Friston 1995a). It has been proposed (Aertsen and Preißl 1991) that "the notion of effective connectivity should be understood as the experiment- and time-dependent, simplest possible circuit diagram that would replicate the observed timing relationships between the recorded neurons". See the previous chapter. If effective connectivity is the influence that one neural system exerts over another it should be possible, given the effective connectivity and the afferent activity, to predict the response of a recipient population. This is precisely what Volterra kernels do. Any model of effective connectivity can be expressed as a Volterra series and any measure of effective connectivity can be reduced to a set of Volterra kernels (see Box 1). An important aspect of effective connectivity is its context-sensitivity. Effective connectivity is simply the 'effect' that input has on the output of a target system. This effect will be sensitive to other inputs, its own history and, of course, the microscopic state and causal architecture intrinsic to the target population. This intrinsic dynamical structure is embodied in the Volterra kernels. In short, Volterra kernels are synonymous with effective connectivity because they characterise the measurable effect that an input has on its target. An example of using Volterra kernels, to characterise context-sensitive changes in effective connectivity, was provided in the previous chapter (see Figure 16). This example used hemodynamic responses to changes in neuronal activity as measured with functional magnetic resonance imaging (fmri). spatiotemporal evolution of brain dynamics in terms of a small number of macroscopic order parameters [see Kelso (1995) for an engaging exposition]. Order parameters are created and determined by the co-operation of microscopic quantities and yet, at the same time, govern the behaviour of the whole system. See Jirsa et al (1995) for a nice example. 2 An important qualification here is that each system is 'controllable'. Systems which are not 'controlled' have quasi-periodic or chaotic behaviours that are maintained by interactions among the states of the system. Although an important issue at the microscopic level, it is fairly easy to show that the mean field approximation to any ensemble of subsystems is controllable. 6
7 III. NEURONAL CODES Functional integration refers to the concerted interactions among neuronal populations that mediate perceptual binding, sensorimotor integration and cognition. It pertains to the mechanisms of, and constraints under which, the state of one population influences that of another. It has been suggested by many that functional integration, among neuronal populations, uses transient dynamics that represent a temporal 'code'. A compelling proposal is that population responses, encoding a percept, become organised in time, through reciprocal interactions, to discharge in synchrony (von der Malsburg 1985, Singer 1994). The use of the term 'encoding' here speaks directly to the notion of codes. Here a neuronal code is taken to be a metric that reveals interactions among neuronal systems by enabling some prediction of the response in one population given the same sort of measure in another 3. Clearly, from the previous section, neuronal transients represent the most generic form of code because, given the Volterra kernels, the output can, in principle, be predicted exactly. Neuronal transients have a number of attributes (e.g. inter-spike interval, duration, mean level of firing, predominant frequency etc.) and any of these could be contenders for a more parsimonious code. The problem of identifying possible codes can be reduced to identifying the form the Volterra kernels in Box 1 can take. If we know their form then we can say which aspects of the input will cause a response. Conversely, it follows that the different forms of kernels should specify the various codes that might be encountered. This is quite an important point and leads to a clear formulation of what can and cannot constitute a code. We will review different codes in terms of the different sorts of kernels that could mediate them. A Instantaneous vs. temporal codes The first kernel characteristic that engenders a coding taxonomy is kernel depth. The limiting case here is when the kernels support shrinks to a point in time. This means that the only relevant history is the immediate activity of inputs (all earlier activities are 'ignored' by the kernel). In this case the activity in any unit is simply a nonlinear function of current activities elsewhere. An example of this is instantaneous rate coding. 3 Although the term code is not being used to denote anything that 'codes' for something in the environment, it could be used to define some aspect of an evoked transient that expresses a high mutual information with a stimulus parameter (e.g. Optican and Richmond 1987, Tovee et al 1993). 7
8 Rate coding considers spike-trains as stochastic processes whose first order moments (i.e. mean activity) describe neuronal interactions. These moments may be in terms of spikes themselves or other compound events (e.g. the average rate of bursting, Bair et al 1994). Interactions based on rate coding are usually assessed in terms of cross-correlations. From the dynamical perspective instantaneous rate codes are considered insufficient. This is because they predict nothing about a cell, or population, response unless one knows the microscopic state of that cell or population. The distinction between rate and temporal coding (see Shadlen & Newsome 1995, de Ruyter van Steveninck 1997) centres on whether the precise timing of individual spikes is sufficient to facilitate meaningful neuronal interactions. In temporal coding the exact time at which an individual spike occurs is the important measure and the spike-train is considered as a point process. There are clear examples of temporal codes that have predictive validity; for example, the primary cortical representation of sounds by the co-ordination of action potential timing (decharms and Merzenich 1996). These codes depend on the relative timing of action potentials and implicitly, an extended temporal frame of reference. They therefore fall into the class of transient codes, where selective responses to particular inter-spike intervals are modelled by temporally extended second order kernels. A nice example is provided by de Ruyter van Steveninck et al (1997) who show that the temporal patterning of spike trains, elicited in fly motion-sensitive neurons by natural stimuli, can carry twice the amount of information than an equivalent [Poisson] rate code. B Transient codes: Synchronous vs. asynchronous The second distinction, assuming the kernels have a non-trivial depth, is whether they comprise high order terms or not. Expansions that encompass just first-order terms are only capable of meditating linear or synchronous interactions. Higher-order kernels confer nonlinearity on the influence of an input that leads to asynchronous interactions. Mathematically, if there are only first-order terms then the Fourier transform of the Volterra kernel completely specifies the relationship (the transfer function) between the spectral density of input and output in a way that precludes interactions among frequencies, or indeed inputs. In other words, the expression of any frequency in a recipient cell is predicted exactly by the expression of the same frequency in the source (after some scaling by the transfer function). 1 Synchronous codes 8
9 The proposal most pertinent to these forms of code is that population responses, participating in the encoding of a percept, become organised in time through reciprocal interactions so that they come to discharge in synchrony (von der Malsburg 1985, Singer 1994) with regular periodic bursting. It should be noted that synchronisation does not necessarily imply oscillations. However, synchronised activity is usually inferred operationally by oscillations implied by the periodic modulation of cross-correlograms of separable spike trains (e.g. Gray and Singer 1991, Eckhorn et al. 1992) or measures of coherence in multichannel electrical and neuromagnetic time-series (e.g. Llinas et al 1994). The underlying mechanism of these frequency-specific interactions is usually attributed to phase-locking among neuronal populations (e.g. Sporns et al 1992; Aertsen and Preißl 1991). The key aspect of these measures is that they refer to the extended temporal structure of synchronised firing patterns, either in terms of spiking (e.g. syn-fire chains, Abeles et al 1994, Lumer et al 1997) or oscillations in the ensuing population dynamics (e.g. Singer 1994). Many aspects of functional integration and feature linking in the brain are thought to be mediated by synchronised dynamics among neuronal populations (Singer 1994). Synchronisation reflects the direct, reciprocal exchange of signals between two populations, whereby the activity in one population influences the second, such that the dynamics become entrained and mutually reinforcing. In this way the binding of different features of an object may be accomplished, in the temporal domain, through the transient synchronisation of oscillatory responses. This 'dynamical linking' defines their short-lived functional association. Physiological evidence is compatible with this theory (e.g. Engel et al 1991, Fries et al 1997). Synchronisation of oscillatory responses occurs within as well as among visual areas, for example between homologous areas of the left and right hemispheres and between areas at different levels of the visuomotor pathway (Engel et al 1991, Roelfsema et al 1997). Synchronisation in the visual cortex appears to depend on stimulus properties, such as continuity, orientation and motion coherence. The problem with synchronisation is that there is nothing essentially dynamic about synchronous interactions per se. As argued by Erb and Aertsen (1992) "the question might not be so much how the brain functions by virtue of oscillations, as most researchers working on cortical oscillations seem to assume, but rather how it manages to do so in spite of them". In order to establish dynamic cell assemblies it is necessary to create and destroy synchronised couplings. It is precisely these dynamic aspects that speak to changes in synchrony (e.g. Desmedt and Tomberg 1994) and the asynchronous transitions between synchronous states as the more pertinent phenomenon. In other words, it is the successive 9
10 reformulation of dynamic cell assemblies, through nonlinear or asynchronous interactions, that is at the heart of 'dynamical linking' (Singer 1994). 2 Asynchronous codes An alternative perspective on neuronal codes is provided by dynamic correlations (Aertsen et al 1994) as exemplified in Vaadia et al (1995). A fundamental phenomenon, observed by Vaadia et al (1995), is that, following behaviourally salient events, the degree of coherent firing between two neurons can change profoundly and systematically over the ensuing second or so. One implication is that a complete model of neuronal interactions has to accommodate dynamic changes in correlations, modulated on time-scales of ms. Neuronal transients provide a simple explanation for this temporally modulated coherence or dynamic correlation. Imagine that two neurons respond to an event with a similar transient. For example, if two neurons respond to an event with decreased firing for 400ms, and this decrease was correlated over epochs, then positive correlations between the two firing rates would be seen for the first 400 of the epoch, and then fade away, exhibiting a dynamic modulation of coherence. In other words, a transient modulation of covariance can be equivalently formulated as covariance in the expression of transients. The generality of this equivalence can be established using singular value decomposition (SVD) of the jointperistimulus time histogram (J-PSTH) as described in Friston (1995b). This is simply a mathematical device to show that dynamic changes in coherence are equivalent to the coherent expression of neural transients. In itself it is not important, in the sense that dynamic correlations are just as valid a characterisation as neuronal transients and indeed may provide more intuitive insights into how this phenomenon is mediated (e.g. Riehle et al 1997). A more important observation is that J-PSTHs can be asymmetric about the leading diagonal. This suggests that coupled transients in two units can have a different patterning of activity. This can only be explained by asynchronous or nonlinear coupling. C Summary In summary, the critical distinction between synchronous and asynchronous coupling is the difference between linear and nonlinear interactions among units or populations 4. This difference is reduces to the existence of high-order Volterra kernels in the mediating the 4 The term 'generalised synchrony' has been introduced to include nonlinear inter-dependencies (see Schiff et al 1996). Generalised synchrony subsumes synchronous and asynchronous coupling. An elegant method for making inferences about generalised synchrony is described in Schiff et al (1996). This approach is 10
11 input-output behaviour of coupled cortical regions. There is a close connection between asynchronous/nonlinear coupling and the expression of distinct transients in two brain regions: Both would be expressed as dynamic correlations, or, in the EEG, as event-related changes in synchronisation (e.g.. induced oscillations). If the full transient model is correct then important transactions among cortical areas will be overlooked by techniques that are predicated on rate coding (e.g. correlations, covariance patterns, spatial modes etc.) or synchronisation models (e.g. coherence analysis and cross-correlograms). Clearly the critical issue here is whether there is direct evidence for nonlinear or asynchronous coupling that would render high-order Volterra kernels necessary. IV. EVIDENCE FOR NONLINEAR COUPLING Why is asynchronous coupling so important? The reason is that asynchronous interactions embody all the nonlinear interactions implicit in functional integration and it is these that mediate the context-sensitive nature of neuronal interactions. Nonlinear interactions among cortical areas render the effective connectivity among them inherently dynamic and contextual. Compelling examples of context-sensitive interactions include the attentional modulation of evoked responses in functionally specialised sensory areas (e.g. Treue and Maunsell 1996) and other contextually dependent dynamics [see Phillips and Singer (1997)]. Whole classes of empirical phenomena such as extra-classical receptive field effects rely on nonlinear or asynchronous interactions. A Nonlinear coupling and asynchronous interactions If the temporal structures of recurring transients in two parts of the brain are distinct, then the prevalence of certain frequencies in one cortical area should predict the expression of different frequencies in another. In contrast, synchronisation posits the expression of the same frequencies. Correlations among different frequencies therefore provide a basis for discriminating between synchronous and asynchronous coupling. Consider time-series from two neuronal populations or cortical areas. Synchrony requires that the expression of a particular frequency (e.g. 40 Hz) in one time series will be coupled with the expression of the same frequency in the other. In other words, the modulation of particularly interesting from our point of view because it calls upon the recent history of the dynamics through the use of temporal embedding to reconstruct the attractors analysed. 11
12 this frequency in one area can be explained or predicted by its modulation in the second. Conversely, asynchronous coupling suggests that the power at a reference frequency, say 40 Hz, can be predicted by the spectral density in the second time-series at frequencies other than 40 Hz. These predictions can be tested empirically using standard time-frequency and regression analyses as described in Friston (2000). Figure 1 shows an example of this sort of analysis, revealing the dynamic changes in spectral density between 8 and 64 Hz over 16 seconds. The cross-correlation matrix of the time-dependent expression of different frequencies in the parietal and prefrontal regions is shown in the lower left panel. There is anecdotal evidence here for both synchronous and asynchronous coupling. Synchronous coupling, based upon the co-modulation of the same frequencies, is manifest as hot-spots along, or near, the leading diagonal of the cross-correlation matrix (e.g. around 20Hz). More interesting are correlations between high frequencies in one time-series and low frequencies in another. In particular note that the frequency modulation at about 34Hz in the parietal region (second time-series) could be explained by several frequencies in the prefrontal region. The most profound correlations are with lower frequencies in the first time-series (26Hz). Using a simple regression framework, statistical inferences can be made about the coupling within and between different frequencies (see Friston 2000 for details). A regression analysis shows that coupling at 34Hz has significant synchronous and asynchronous components, where as the coupling at 48Hz is purely asynchronous (middle and right peaks in the graphs) i.e. a coupling between beta dynamics in the premotor region and gamma dynamics in the parietal region. Figure 1 about here V. THE NEURAL BASIS OF NONLINEAR COUPLING In Friston (1997) it was suggested that, from a neurobiological perspective, the distinction between nonlinear [asynchronous] and linear [synchronous] interactions could be viewed in the following way. Synchronisation emerges from the reciprocal exchange of signals between two populations, where each drives the other, such that the dynamics become entrained and mutually reinforcing. In asynchronous coding the afferents from one population exert a modulatory influence, not on the activity of the second, but on the interactions within it (e.g. a modulation of effective connectivity or synaptic efficacies within 12
13 the target population) leading to changes in the dynamics intrinsic to the second population. In this model there is no necessary synchrony between the intrinsic dynamics that ensue and the temporal pattern of modulatory input. To test this hypothesis one would need to demonstrate that asynchronous coupling emerges when extrinsic connections are changed from driving connections to modulatory connections. Clearly this cannot be done in the real brain. However, we can use computational techniques to create a biologically realistic model of interacting populations and test this hypothesis directly. A. Interactions between simulated populations Two populations were simulated using the model described in Friston (2000). This model simulates entire neuronal populations in a deterministic fashion based on known neurophysiological mechanisms. In particular we modelled three sorts of synapse, fast inhibitory (GABA), fast excitatory (AMPA) and slower voltage-dependent synapses (NMDA). Connections intrinsic to each population used only GABA and AMPA-like synapses. Simulated glutaminergic extrinsic connections between the two populations used either driving AMPA-like synapses or modulatory NMDA-like synapses. Transmission delays for extrinsic connections were fixed at 8ms. By using realistic time constants the characteristic oscillatory dynamics of each population were expressed in the gamma range. Figure 2 about here The results of coupling two populations with unidirectional AMPA-like connections are shown in the top of Figure 2 in terms of the simulated local field potentials (LFP). Occasional transients in the driving population were evoked by injecting a depolarising current, of the same magnitude, at random intervals (dotted lines). The tight synchronised coupling that ensues is evident. This example highlights the point that near-linear coupling can arise even in the context of loosely coupled, highly nonlinear neuronal oscillators of the sort modelled here. Contrast these entrained dynamics under driving connections with those that emerge when the connection is modulatory or NMDA-like (lower panel in Figure 2). Here there is no synchrony and, as predicted, fast transients of an oscillatory nature are facilitated by the low frequency input from the first population that has a lower frequency (c.f. the MEG analyses above). This is a nice example of asynchronous coupling that is underpinned by nonlinear modulatory interactions between neuronal populations. The nature of the coupling can be characterised using the time-frequency analysis (identical in every 13
14 detail) applied to the neuromagnetic data of the previous section. The results for the NMDA simulation are presented in Figure 3. The cross-correlation matrix resembles that obtained with the MEG data in Figure 1. Both in terms of the variance, and inference, asynchronous coupling supervenes at most frequencies but, as in the real data, mixed coupling is also evident. These results can be taken as a heuristic conformation of the notion that modulatory, in this case voltage-dependent, interactions are sufficiently nonlinear to account for the emergence of asynchronous coupling. Figure 3 about here B. Modulatory interactions and nonlinear coupling In summary, asynchronous coupling is synonymous with nonlinear coupling. Nonlinear coupling can be framed in terms of the modulation of intrinsic interactions, within a cortical area or neuronal population, by extrinsic input offered by afferents from other parts of the brain. This mechanism predicts that the modulation of fast (e.g. gamma) activity in one cortical area can be predicted by much slower changes in other areas. This form of coupling is very different from coherence or other measures of synchronous coupling and concerns the relationship between the first-order dynamics in one area and the second order dynamics (spectral density) expressed in another. In terms of the above NMDA simulation, transient depolarisation in the modulating population causes a short-lived increased input to the second. These afferents impinge on voltage-sensitive NMDA-like synapses with time constants (in the model) of about 100ms. These synapses open and slowly close again, remaining open long after an afferent volley. Because of their voltage-sensitive nature this input will have no effect on the dynamics intrinsic to the second population unless there is already a substantial degree of depolarisation. If there is then, through self-excitation and inhibition, the concomitant opening of fast excitatory and inhibitory channels will generally increase membrane conductance, decrease the effective membrane time constants and lead to fast oscillatory transients. This is what we observe in the lower panel of Figure 2. In relation to the MEG analyses, the implied modulatory mechanisms, that may underpin this effect, are entirely consistent with the anatomy, laminar specificity and functional role attributed to prefrontal efferents (Rockland and Pandya 1979, Selemon and Goldman-Rakic 1988). VI CONCLUSION 14
15 In this chapter we have dealt with some interesting and interrelated aspects of effective connectivity, neuronal codes, nonlinear coupling, neuronal transients and dynamic correlations (e.g. induced oscillations). The key points can be summarised as follows Starting with the premise that the brain can be represented as an ensemble of connected input-state-output systems (e.g. cellular compartments, cells or populations of cells) there exists an equivalent input-output formulation in terms of a Volterra series. This is simply a functional expansion of each system's inputs that produces its outputs (where the outputs to one system constitute the inputs to another). The existence of this expansion suggests that the history of inputs, or neuronal transients, and the Volterra kernels are a complete and sufficient specification of brain dynamics (to the extent they are controllable). This is the primary motivation for framing dynamics in terms of neuronal transients and using Volterra kernels to model effective connectivity. The Volterra formulation provides constraints on the form that neuronal interactions and implicit codes must conform to. There are two limiting cases; (i) when the kernel decays very quickly and (ii) when high order kernels disappear. The first case corresponds to instantaneous codes (e.g. rate codes) and the second to synchronous interactions (e.g. synchrony codes). High order kernels in the Volterra formulation of effective connectivity speak to nonlinear interactions and implicitly to asynchronous coupling. Asynchronous coupling implies coupling among the expression of different frequencies. Coupling among different frequencies is easy to demonstrate using neuromagnetic measurements of real brain dynamics. This implies that nonlinear, asynchronous coupling is a prevalent component of functional integration. High order kernels correspond to modulatory interactions that can be construed as a nonlinear effect of inputs that interact bilinearly with the intrinsic states of the recipient system. This implies that driving connections may be linear and engender 15
16 synchronous interactions. Conversely, modulatory connections, being nonlinear, may be revealed by asynchronous coupling and be expressed in high order kernels. 16
17 Box 1: Dynamical systems and Volterra kernels Input-state-output systems and Volterra series Neuronal systems are inherently nonlinear and lend themselves to modelling with nonlinear dynamical systems. However due to the complexity of biological systems it is difficult to find analytic equations that describe them adequately. Even if these equations were known the state variables are often not observable. An alternative approach to identification is to adopt a very general model (Wray and Green 1994) and focus on the inputs and outputs. Consider the single input-single output (SISO) system x& ( t) = f ( x( t), u( t)) y( t) = λ( x( t)) B.1 The Fliess fundamental formula (Fliess et al 1983) describes the causal relationship between the outputs and the recent history of the inputs. This relationship can be expressed as a Volterra series which expresses the output y(t) as a nonlinear convolution of the inputs u(t), critically without reference to the state variables x(t). This series is simply a functional Taylor expansion of y(t) in Eq(3) (main text). t y( t) = h( u( t σ )) = K κ i ( σ 1, Kσ i ) u( t σ 1) Ku( t σ i ) dσ 1 Kdσ i i= 1 0 t 0 i y( t) κ i ( σ 1, Kσ i ) = u( t σ ) K u( t σ ) 1 i B.2 were κ σ, K σ ) is the ith order kernel. Volterra series have been described as a 'power i ( 1 i series with memory' and are generally thought of as a high-order or 'nonlinear convolution' of the inputs to provide an output. See Bendat (1990) for a fuller discussion. Volterra kernels and effective connectivity Volterra kernels are essential in characterising the effective connectivity or influences that one neuronal system exerts over another because they represent the causal input-output 17
18 18 characteristics of the system in question. Neurobiologically they have a simple and compelling interpretation they are synonymous with effective connectivity: From B.2 K, ) ( ) ( ) ( ), (, ) ( ) ( ) ( σ σ σ σ κ σ σ κ = = t u t u t y t u t y It is evident that the first-order kernel embodies the response evoked by a change in input at σ 1 t. In other words, it is a time-dependant measure of driving efficacy. Similarly the second order kernel reflects the modulatory influence of the input at 1 σ t on the response evoked by input at 2 σ t. And so on for higher orders.
19 Figure Legends Figure 1 Time-frequency and regression analysis of MEG time-series designed to characterise the relative contribution of synchronous and asynchronous coupling. Neuromagnetic data were acquired from a normal subject using a KENIKRON 37 channel MEG system at onemillisecond intervals for periods of up to two minutes. During this time the subject made volitional joystick movements to the left, every two seconds or so. Paired epochs were taken from a left prefrontal and left parietal region. Top panels: The two times series (plots) and their corresponding time-frequency profiles (images). The first time-series comes from the left prefrontal region. The second comes from the left superior parietal region. Lower left panel: This is a simple characterisation of the coupling among frequencies in the two regions and represents the [squared] cross-correlations of the time-varying expression of different frequencies from the upper panels. Lower right panels: These are the results of a linear regression analysis that partitions the variance in the second (parietal) time-series into components that can be attributed to synchronous (broken lines) and asynchronous (solid lines) contributions from the first (prefrontal) time series. The upper graph shows the relative contribution in terms of the proportion of variance explained and in terms of the significance using a semi-log plot of the corresponding p-values (lower graph). The dotted line in the latter corresponds to p = This example was chosen because it illustrates three sorts of coupling (synchronous, asynchronous and mixed). From inspection of the cross correlation matrix it is evident that power in the beta range (20Hz) in the second time-series is correlated with similar frequency modulation in the first, albeit at a slightly lower frequency. The resulting correlations appear just off the leading diagonal (broken line) on the upper left. The proportion of variance explained by synchronous and asynchronous coupling is roughly the same and, in terms of significance, synchrony supervenes (see upper graph). In contrast, the high correlations, between 48Hz in the second time-series and 26Hz in the first, are well away from the leading diagonal, with little evidence of correlations within either of these frequencies. The regression analysis confirms that, at this frequency, asynchronous coupling prevails. The variation at about 34Hz in the parietal region could be explained by several frequencies in the prefrontal region. A formal analysis shows that both synchronous and asynchronous coupling coexist at this frequency (i.e. the middle peak in the graphs). 19
20 Figure 2 Simulated local field potentials (LFP) of two coupled populations using two different sorts of postsynaptic responses (AMPA and NMDA-like) to connections from the first to the target population. The dotted line shows the depolarisation effected by sporadic injections of current into the first population. The key thing to note is that under AMPA-like or driving connections the second population is synchronously entrained by the first. When the connections are modulatory or voltage-dependent (NMDA), the effects are much more subtle and resemble a frequency modulation. These data were simulated using a biologically plausible model of excitatory and inhibitory subpopulations. The model was deterministic with variables pertaining to the collective, probabilistic, behaviour of the subpopulations (c.f. a mean field treatment). See Friston (2000) for details. Figure 3 As for Figure 1, but here using the simulated data employing voltage-dependent NMDA-like connections. The coupling here includes some profoundly asynchronous [nonlinear] components involving frequencies in the gamma range implicated in the analyses of real (MEG) data shown in Figure 1. In particular note the asymmetrical cross-correlation matrix and the presence of asynchronous and mixed coupling implicit in the p-value plots on the lower right. 20
21 References Abeles M, Bergman H, Gat I, Meilijson I, Seidmann E, Tishby N and Vaadia E (1995) Cortical activity flips among quasi-stationary states. Proc. Natl. Acad. Sci. 92: Aertsen A, Erb M and Palm G (1994) Dynamics of functional coupling in the cerebral cortex: an attempt at a model-based interpretation, Physica D 75: Aertsen A & Preißl H (1991) Dynamics of activity and connectivity in physiological neuronal Networks in Non Linear Dynamics and Neuronal Networks Ed Schuster HG VCH publishers Inc., New York NY USA p Bair W, Koch C, Newsome W and Britten K (1994) Relating temporal properties of spike trains from area MT neurons to the behaviour of the monkey. In Temporal Coding in the Brain Eds. Buzsaki, Llinas R, Singer W, Berthoz A and Christen T. Springer Verlag, Berlin. p Bendat JS (1990) Nonlinear System Analysis and Identification from Random Data. John Wiley and Sons, New York USA Büchel C and Friston KJ (1997) Modulation of connectivity in visual pathways by attention: Cortical interactions evaluated with structural equation modelling and fmri. Cerebral Cortex 7: decharms RC and Merzenich MM (1996) Primary cortical representation of sounds by the co-ordination of action potential timing. Nature 381: de Ruyter van Steveninck RR, Lewen GD, Strong SP, Koberie R and Bialek W (1997) Reproducibility and variability in neural spike trains. Science 275: Desmedt JE and Tomberg C (1994) Transient phase-locking of 40Hz electrical oscillations in prefrontal and parietal human cortex reflects the process of conscious somatic perception. Neurosci. Lett. 168: Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ (1988) Coherent oscillations: a mechanism of feature linking in the visual cortex?. Multiple electrode and correlation analysis in the cat. Biol. Cybern 60: Erb M and Aertsen A (1992) Dynamics of activity in biology-oriented neural network models: Stability analysis at low firing rates. In Information processing in the cortex. Experiments and Theory. Eds. Aertsen A and Braitenberg V. Springer-Verlag, Berlin p Engel A K, Konig P and Singer W (1991) Direct physiological evidence for scene segmentation by temporal coding. Proc. Natl. Acad Sci USA vol. 88:
22 Fliess M, Lamnabhi M and Lamnabhi-Lagarrigue F (1983) An algebraic approach to nonlinear functional expansions. IEEE Transactions on Circuits and Systems 30: Freeman W and Barrie J (1994) Chaotic oscillations and the genesis of meaning in cerebral cortex. In Temporal Coding in the Brain Eds. Buzsaki, Llinas R, Singer W, Berthoz A and Christen T. Springer Verlag, Berlin. p13-38 Fries P, Roelfsema PR, Engel A, Konig P and Singer W, 1997, Synchronisation of oscillatory responses in visual cortex correlates with perception in inter-ocular rivalry. Pro Natl Acad Sci USA vol. 94: Friston KJ. (1995a) Functional and effective connectivity in neuroimaging: A synthesis. Human Brain Mapp. 2:56-78 Friston KJ. (1995b) Neuronal transients. Proc. Roy. Soc. Series B. 261: Friston KJ (1997) Transients metastability and neuronal dynamics. NeuroImage 5: Friston KJ (2000) The Labile Brain I: Neuronal Transients and nonlinear coupling. Phil. Trans. R. Soc. (Lond.) 355: Friston KJ and Büchel C (2000) Attentional modulation of effective connectivity from V2 to V5 in humans Pro Natl Acad Sci USA vol. 97: Gerstein GL & Perkel DH (1969) Simultaneously recorded trains of action potentials: Analysis and functional interpretation Science 164: Gray CM & Singer W (1989) Stimulus specific neuronal oscillations in orientation columns of cat visual cortex Proc. Natl. Acad. Sci USA 86: Haken H (1983) Synergistics: An introduction. Springer Berlin. 3rd Edition Jirsa VK, Friedrich R and Haken H (1995) Reconstruction of the spatio-temporal dynamics of a human magnetoencephalogram. Physica D 89: Kelso JAS (1995) Dynamic Patterns: The self-organisation of brain and behaviour. MIT Press, USA Llinas R Ribary U Joliot M and Wang X-J (1994) Content and context in temporal thalamocortical binding. In Temporal Coding in the Brain Eds. Buzsaki, Llinas R, Singer W, Berthoz A and Christen T. Springer Verlag, Berlin. p Lumer ED, Edelman GM, Tononi G, (1997) Neural Dynamics in a model of the thalamocortical System II. The role of neural synchrony tested through perturbations of spike timing. Cerebral Cortex 7: Phillips WA and Singer W (1997) In search of common foundations for cortical computation. Behavioural and Brain Sciences. 20:
23 Riehle A, Grun S, Diesmann M and Aertsen A (1997) Spike synchronisation and rate modulation differentially involved in motor cortical function. Science 278: Rockland K. S., Pandya D. N. (1979) Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain-Res 179: 3-20 Schiff SJ, So P, Chang T, Burke RE and Sauer T (1996) Detecting dynamical interdependence and generalised synchrony though mutual prediction in a neuronal ensemble. Physical Review E 54: Selemon L. D., Goldman Rakic P. S. (1988) Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 8: Shadlen MN and Newsome WT (1995) Noise, neural codes and cortical organisation. Current Opinion in Neurobiology. 4: Singer W (1994) Time as coding space in neocortical processing: A hypothesis. In Temporal Coding in the Brain Eds. Buzsaki, Llinas R, Singer W, Berthoz A and Christen T. Springer Verlag, Berlin. p51-80 Sporns O, Gally, JA, Reeke GN & Edelman GM (1989) Reentrant Signalling among simulated neuronal groups leads to coherence in their oscillatory activity Proc. Natl. Acad. Sci USA 86: Tovee MJ, Rolls ET, Treves A and Bellis RP (1993) Information encoding and the response of single neurons in the primate temporal visual cortex. J. Neurophysiology 70: Treue S, Maunsell HR (1996) Attentional modulation of visual motion processing in cortical areas MT and MST. Nature 382: Vaadia E, Haalman I, Abeles M, Bergman H, Prut Y, Slovin H and Aertsen A (1995) Dynamics of neuronal interactions in monkey cortex in relation to behavioural events. Nature 373: von der Malsburg C (1985) Nervous structures with dynamical links. Ber Bunsenges. Phys. Chem. 89: Wray J and Green GGR (1994) Calculation of the Volterra kernels of non-linear dynamic systems using an artificial neuronal network. Biol. Cybern. 71:
Consider the following spike trains from two different neurons N1 and N2:
 About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
Self-organized Criticality and Synchronization in a Pulse-coupled Integrate-and-Fire Neuron Model Based on Small World Networks
 Commun. Theor. Phys. (Beijing, China) 43 (2005) pp. 466 470 c International Academic Publishers Vol. 43, No. 3, March 15, 2005 Self-organized Criticality and Synchronization in a Pulse-coupled Integrate-and-Fire
Commun. Theor. Phys. (Beijing, China) 43 (2005) pp. 466 470 c International Academic Publishers Vol. 43, No. 3, March 15, 2005 Self-organized Criticality and Synchronization in a Pulse-coupled Integrate-and-Fire
Neural Networks 1 Synchronization in Spiking Neural Networks
 CS 790R Seminar Modeling & Simulation Neural Networks 1 Synchronization in Spiking Neural Networks René Doursat Department of Computer Science & Engineering University of Nevada, Reno Spring 2006 Synchronization
CS 790R Seminar Modeling & Simulation Neural Networks 1 Synchronization in Spiking Neural Networks René Doursat Department of Computer Science & Engineering University of Nevada, Reno Spring 2006 Synchronization
Will Penny. 21st April The Macroscopic Brain. Will Penny. Cortical Unit. Spectral Responses. Macroscopic Models. Steady-State Responses
 The The 21st April 2011 Jansen and Rit (1995), building on the work of Lopes Da Sliva and others, developed a biologically inspired model of EEG activity. It was originally developed to explain alpha activity
The The 21st April 2011 Jansen and Rit (1995), building on the work of Lopes Da Sliva and others, developed a biologically inspired model of EEG activity. It was originally developed to explain alpha activity
Dynamic Causal Modelling for EEG/MEG: principles J. Daunizeau
 Dynamic Causal Modelling for EEG/MEG: principles J. Daunizeau Motivation, Brain and Behaviour group, ICM, Paris, France Overview 1 DCM: introduction 2 Dynamical systems theory 3 Neural states dynamics
Dynamic Causal Modelling for EEG/MEG: principles J. Daunizeau Motivation, Brain and Behaviour group, ICM, Paris, France Overview 1 DCM: introduction 2 Dynamical systems theory 3 Neural states dynamics
ATTRACTORS IN SONG. KARL FRISTON and STEFAN KIEBEL
 Attractors in song: 1 New Mathematics and Natural Computation Vol. 5, No. 1 (9) 1-3 World Scientific Publishing Company ATTRACTORS IN SONG KARL FRISTON and STEFAN KIEBEL The Wellcome Trust Centre of Neuroimaging
Attractors in song: 1 New Mathematics and Natural Computation Vol. 5, No. 1 (9) 1-3 World Scientific Publishing Company ATTRACTORS IN SONG KARL FRISTON and STEFAN KIEBEL The Wellcome Trust Centre of Neuroimaging
The homogeneous Poisson process
 The homogeneous Poisson process during very short time interval Δt there is a fixed probability of an event (spike) occurring independent of what happened previously if r is the rate of the Poisson process,
The homogeneous Poisson process during very short time interval Δt there is a fixed probability of an event (spike) occurring independent of what happened previously if r is the rate of the Poisson process,
Dynamic Causal Modelling for fmri
 Dynamic Causal Modelling for fmri André Marreiros Friday 22 nd Oct. 2 SPM fmri course Wellcome Trust Centre for Neuroimaging London Overview Brain connectivity: types & definitions Anatomical connectivity
Dynamic Causal Modelling for fmri André Marreiros Friday 22 nd Oct. 2 SPM fmri course Wellcome Trust Centre for Neuroimaging London Overview Brain connectivity: types & definitions Anatomical connectivity
Dynamic Modeling of Brain Activity
 0a Dynamic Modeling of Brain Activity EIN IIN PC Thomas R. Knösche, Leipzig Generative Models for M/EEG 4a Generative Models for M/EEG states x (e.g. dipole strengths) parameters parameters (source positions,
0a Dynamic Modeling of Brain Activity EIN IIN PC Thomas R. Knösche, Leipzig Generative Models for M/EEG 4a Generative Models for M/EEG states x (e.g. dipole strengths) parameters parameters (source positions,
Processing of Time Series by Neural Circuits with Biologically Realistic Synaptic Dynamics
 Processing of Time Series by Neural Circuits with iologically Realistic Synaptic Dynamics Thomas Natschläger & Wolfgang Maass Institute for Theoretical Computer Science Technische Universität Graz, ustria
Processing of Time Series by Neural Circuits with iologically Realistic Synaptic Dynamics Thomas Natschläger & Wolfgang Maass Institute for Theoretical Computer Science Technische Universität Graz, ustria
Dynamical Constraints on Computing with Spike Timing in the Cortex
 Appears in Advances in Neural Information Processing Systems, 15 (NIPS 00) Dynamical Constraints on Computing with Spike Timing in the Cortex Arunava Banerjee and Alexandre Pouget Department of Brain and
Appears in Advances in Neural Information Processing Systems, 15 (NIPS 00) Dynamical Constraints on Computing with Spike Timing in the Cortex Arunava Banerjee and Alexandre Pouget Department of Brain and
Effective Connectivity & Dynamic Causal Modelling
 Effective Connectivity & Dynamic Causal Modelling Hanneke den Ouden Donders Centre for Cognitive Neuroimaging Radboud University Nijmegen Advanced SPM course Zurich, Februari 13-14, 2014 Functional Specialisation
Effective Connectivity & Dynamic Causal Modelling Hanneke den Ouden Donders Centre for Cognitive Neuroimaging Radboud University Nijmegen Advanced SPM course Zurich, Februari 13-14, 2014 Functional Specialisation
SPATIOTEMPORAL ANALYSIS OF SYNCHRONIZATION OF NEURAL ENSEMBLES FOR SPATIAL DISCRIMINATIONS IN CAT STRIATE CORTEX
 SPATIOTEMPORAL ANALYSIS OF SYNCHRONIZATION OF NEURAL ENSEMBLES FOR SPATIAL DISCRIMINATIONS IN CAT STRIATE CORTEX Jason M Samonds Department of Biomedical Engineering Vanderbilt University The auto-content
SPATIOTEMPORAL ANALYSIS OF SYNCHRONIZATION OF NEURAL ENSEMBLES FOR SPATIAL DISCRIMINATIONS IN CAT STRIATE CORTEX Jason M Samonds Department of Biomedical Engineering Vanderbilt University The auto-content
The Bayesian Brain. Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester. May 11, 2017
 The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
Causality and communities in neural networks
 Causality and communities in neural networks Leonardo Angelini, Daniele Marinazzo, Mario Pellicoro, Sebastiano Stramaglia TIRES-Center for Signal Detection and Processing - Università di Bari, Bari, Italy
Causality and communities in neural networks Leonardo Angelini, Daniele Marinazzo, Mario Pellicoro, Sebastiano Stramaglia TIRES-Center for Signal Detection and Processing - Università di Bari, Bari, Italy
An Introductory Course in Computational Neuroscience
 An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
Spike Count Correlation Increases with Length of Time Interval in the Presence of Trial-to-Trial Variation
 NOTE Communicated by Jonathan Victor Spike Count Correlation Increases with Length of Time Interval in the Presence of Trial-to-Trial Variation Robert E. Kass kass@stat.cmu.edu Valérie Ventura vventura@stat.cmu.edu
NOTE Communicated by Jonathan Victor Spike Count Correlation Increases with Length of Time Interval in the Presence of Trial-to-Trial Variation Robert E. Kass kass@stat.cmu.edu Valérie Ventura vventura@stat.cmu.edu
Disambiguating Different Covariation Types
 NOTE Communicated by George Gerstein Disambiguating Different Covariation Types Carlos D. Brody Computation and Neural Systems Program, California Institute of Technology, Pasadena, CA 925, U.S.A. Covariations
NOTE Communicated by George Gerstein Disambiguating Different Covariation Types Carlos D. Brody Computation and Neural Systems Program, California Institute of Technology, Pasadena, CA 925, U.S.A. Covariations
Synfire Waves in Small Balanced Networks
 Synfire Waves in Small Balanced Networks Yuval Aviel 1,3, David Horn 2 and Moshe Abeles 1 1 Interdisciplinary Center for Neural Computation, Hebrew University, Jerusalem, Israel. 2 School of Physics and
Synfire Waves in Small Balanced Networks Yuval Aviel 1,3, David Horn 2 and Moshe Abeles 1 1 Interdisciplinary Center for Neural Computation, Hebrew University, Jerusalem, Israel. 2 School of Physics and
Decision-making and Weber s law: a neurophysiological model
 European Journal of Neuroscience, Vol. 24, pp. 901 916, 2006 doi:10.1111/j.14-9568.2006.04940.x Decision-making and Weber s law: a neurophysiological model Gustavo Deco 1 and Edmund T. Rolls 2 1 Institucio
European Journal of Neuroscience, Vol. 24, pp. 901 916, 2006 doi:10.1111/j.14-9568.2006.04940.x Decision-making and Weber s law: a neurophysiological model Gustavo Deco 1 and Edmund T. Rolls 2 1 Institucio
EEG- Signal Processing
 Fatemeh Hadaeghi EEG- Signal Processing Lecture Notes for BSP, Chapter 5 Master Program Data Engineering 1 5 Introduction The complex patterns of neural activity, both in presence and absence of external
Fatemeh Hadaeghi EEG- Signal Processing Lecture Notes for BSP, Chapter 5 Master Program Data Engineering 1 5 Introduction The complex patterns of neural activity, both in presence and absence of external
A Three-dimensional Physiologically Realistic Model of the Retina
 A Three-dimensional Physiologically Realistic Model of the Retina Michael Tadross, Cameron Whitehouse, Melissa Hornstein, Vicky Eng and Evangelia Micheli-Tzanakou Department of Biomedical Engineering 617
A Three-dimensional Physiologically Realistic Model of the Retina Michael Tadross, Cameron Whitehouse, Melissa Hornstein, Vicky Eng and Evangelia Micheli-Tzanakou Department of Biomedical Engineering 617
A Novel Chaotic Neural Network Architecture
 ESANN' proceedings - European Symposium on Artificial Neural Networks Bruges (Belgium), - April, D-Facto public., ISBN ---, pp. - A Novel Neural Network Architecture Nigel Crook and Tjeerd olde Scheper
ESANN' proceedings - European Symposium on Artificial Neural Networks Bruges (Belgium), - April, D-Facto public., ISBN ---, pp. - A Novel Neural Network Architecture Nigel Crook and Tjeerd olde Scheper
How to read a burst duration code
 Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Probabilistic Models in Theoretical Neuroscience
 Probabilistic Models in Theoretical Neuroscience visible unit Boltzmann machine semi-restricted Boltzmann machine restricted Boltzmann machine hidden unit Neural models of probabilistic sampling: introduction
Probabilistic Models in Theoretical Neuroscience visible unit Boltzmann machine semi-restricted Boltzmann machine restricted Boltzmann machine hidden unit Neural models of probabilistic sampling: introduction
A Multivariate Time-Frequency Based Phase Synchrony Measure for Quantifying Functional Connectivity in the Brain
 A Multivariate Time-Frequency Based Phase Synchrony Measure for Quantifying Functional Connectivity in the Brain Dr. Ali Yener Mutlu Department of Electrical and Electronics Engineering, Izmir Katip Celebi
A Multivariate Time-Frequency Based Phase Synchrony Measure for Quantifying Functional Connectivity in the Brain Dr. Ali Yener Mutlu Department of Electrical and Electronics Engineering, Izmir Katip Celebi
Bursting and Chaotic Activities in the Nonlinear Dynamics of FitzHugh-Rinzel Neuron Model
 Bursting and Chaotic Activities in the Nonlinear Dynamics of FitzHugh-Rinzel Neuron Model Abhishek Yadav *#, Anurag Kumar Swami *, Ajay Srivastava * * Department of Electrical Engineering, College of Technology,
Bursting and Chaotic Activities in the Nonlinear Dynamics of FitzHugh-Rinzel Neuron Model Abhishek Yadav *#, Anurag Kumar Swami *, Ajay Srivastava * * Department of Electrical Engineering, College of Technology,
Activity Driven Adaptive Stochastic. Resonance. Gregor Wenning and Klaus Obermayer. Technical University of Berlin.
 Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
Hierarchy. Will Penny. 24th March Hierarchy. Will Penny. Linear Models. Convergence. Nonlinear Models. References
 24th March 2011 Update Hierarchical Model Rao and Ballard (1999) presented a hierarchical model of visual cortex to show how classical and extra-classical Receptive Field (RF) effects could be explained
24th March 2011 Update Hierarchical Model Rao and Ballard (1999) presented a hierarchical model of visual cortex to show how classical and extra-classical Receptive Field (RF) effects could be explained
Fast neural network simulations with population density methods
 Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity
 Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity Jorge F. Mejias 1,2 and Joaquín J. Torres 2 1 Department of Physics and Center for
Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity Jorge F. Mejias 1,2 and Joaquín J. Torres 2 1 Department of Physics and Center for
Synaptic Input. Linear Model of Synaptic Transmission. Professor David Heeger. September 5, 2000
 Synaptic Input Professor David Heeger September 5, 2000 The purpose of this handout is to go a bit beyond the discussion in Ch. 6 of The Book of Genesis on synaptic input, and give some examples of how
Synaptic Input Professor David Heeger September 5, 2000 The purpose of this handout is to go a bit beyond the discussion in Ch. 6 of The Book of Genesis on synaptic input, and give some examples of how
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons
 Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
September 16, 2004 The NEURON Book: Chapter 2
 Chapter 2 The ing perspective This and the following chapter deal with concepts that are not NEURON-specific but instead pertain equally well to any tools used for neural ing. Why? In order to achieve
Chapter 2 The ing perspective This and the following chapter deal with concepts that are not NEURON-specific but instead pertain equally well to any tools used for neural ing. Why? In order to achieve
Phase-coupling in Two-Dimensional Networks of Interacting Oscillators
 Phase-coupling in Two-Dimensional Networks of Interacting Oscillators Ernst Niebur, Daniel M. Kammen, Christof Koch, Daniel Ruderman! & Heinz G. Schuster2 Computation and Neural Systems Caltech 216-76
Phase-coupling in Two-Dimensional Networks of Interacting Oscillators Ernst Niebur, Daniel M. Kammen, Christof Koch, Daniel Ruderman! & Heinz G. Schuster2 Computation and Neural Systems Caltech 216-76
Information Theory and Neuroscience II
 John Z. Sun and Da Wang Massachusetts Institute of Technology October 14, 2009 Outline System Model & Problem Formulation Information Rate Analysis Recap 2 / 23 Neurons Neuron (denoted by j) I/O: via synapses
John Z. Sun and Da Wang Massachusetts Institute of Technology October 14, 2009 Outline System Model & Problem Formulation Information Rate Analysis Recap 2 / 23 Neurons Neuron (denoted by j) I/O: via synapses
Computational Explorations in Cognitive Neuroscience Chapter 2
 Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
PERFORMANCE STUDY OF CAUSALITY MEASURES
 PERFORMANCE STUDY OF CAUSALITY MEASURES T. Bořil, P. Sovka Department of Circuit Theory, Faculty of Electrical Engineering, Czech Technical University in Prague Abstract Analysis of dynamic relations in
PERFORMANCE STUDY OF CAUSALITY MEASURES T. Bořil, P. Sovka Department of Circuit Theory, Faculty of Electrical Engineering, Czech Technical University in Prague Abstract Analysis of dynamic relations in
Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits
 Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits Wolfgang Maass, Robert Legenstein, Nils Bertschinger Institute for Theoretical Computer Science Technische
Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits Wolfgang Maass, Robert Legenstein, Nils Bertschinger Institute for Theoretical Computer Science Technische
A Model for Real-Time Computation in Generic Neural Microcircuits
 A Model for Real-Time Computation in Generic Neural Microcircuits Wolfgang Maass, Thomas Natschläger Institute for Theoretical Computer Science Technische Universitaet Graz A-81 Graz, Austria maass, tnatschl
A Model for Real-Time Computation in Generic Neural Microcircuits Wolfgang Maass, Thomas Natschläger Institute for Theoretical Computer Science Technische Universitaet Graz A-81 Graz, Austria maass, tnatschl
Nonlinear reverse-correlation with synthesized naturalistic noise
 Cognitive Science Online, Vol1, pp1 7, 2003 http://cogsci-onlineucsdedu Nonlinear reverse-correlation with synthesized naturalistic noise Hsin-Hao Yu Department of Cognitive Science University of California
Cognitive Science Online, Vol1, pp1 7, 2003 http://cogsci-onlineucsdedu Nonlinear reverse-correlation with synthesized naturalistic noise Hsin-Hao Yu Department of Cognitive Science University of California
arxiv: v3 [q-bio.nc] 17 Oct 2018
![arxiv: v3 [q-bio.nc] 17 Oct 2018 arxiv: v3 [q-bio.nc] 17 Oct 2018](/thumbs/96/126993392.jpg) Evaluating performance of neural codes in model neural communication networks Chris G. Antonopoulos 1, Ezequiel Bianco-Martinez 2 and Murilo S. Baptista 3 October 18, 2018 arxiv:1709.08591v3 [q-bio.nc]
Evaluating performance of neural codes in model neural communication networks Chris G. Antonopoulos 1, Ezequiel Bianco-Martinez 2 and Murilo S. Baptista 3 October 18, 2018 arxiv:1709.08591v3 [q-bio.nc]
Influence of Criticality on 1/f α Spectral Characteristics of Cortical Neuron Populations
 Influence of Criticality on 1/f α Spectral Characteristics of Cortical Neuron Populations Robert Kozma rkozma@memphis.edu Computational Neurodynamics Laboratory, Department of Computer Science 373 Dunn
Influence of Criticality on 1/f α Spectral Characteristics of Cortical Neuron Populations Robert Kozma rkozma@memphis.edu Computational Neurodynamics Laboratory, Department of Computer Science 373 Dunn
A Mass Model of Interconnected Thalamic Populations including both Tonic and Burst Firing Mechanisms
 International Journal of Bioelectromagnetism Vol. 12, No. 1, pp. 26-31, 2010 www.ijbem.org A Mass Model of Interconnected Thalamic Populations including both Tonic and Burst Firing Mechanisms Pirini M.
International Journal of Bioelectromagnetism Vol. 12, No. 1, pp. 26-31, 2010 www.ijbem.org A Mass Model of Interconnected Thalamic Populations including both Tonic and Burst Firing Mechanisms Pirini M.
Statistical models for neural encoding
 Statistical models for neural encoding Part 1: discrete-time models Liam Paninski Gatsby Computational Neuroscience Unit University College London http://www.gatsby.ucl.ac.uk/ liam liam@gatsby.ucl.ac.uk
Statistical models for neural encoding Part 1: discrete-time models Liam Paninski Gatsby Computational Neuroscience Unit University College London http://www.gatsby.ucl.ac.uk/ liam liam@gatsby.ucl.ac.uk
CSE/NB 528 Final Lecture: All Good Things Must. CSE/NB 528: Final Lecture
 CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
Spectral Interdependency Methods
 Spectral Interdependency Methods Mukesh Dhamala* Department of Physics and Astronomy, Neuroscience Institute, Georgia State University, Atlanta, Georgia, USA Synonyms Coherence and Granger causality spectral
Spectral Interdependency Methods Mukesh Dhamala* Department of Physics and Astronomy, Neuroscience Institute, Georgia State University, Atlanta, Georgia, USA Synonyms Coherence and Granger causality spectral
Rhythms in the gamma range (30 80 Hz) and the beta range
 Gamma rhythms and beta rhythms have different synchronization properties N. Kopell, G. B. Ermentrout, M. A. Whittington, and R. D. Traub Department of Mathematics and Center for BioDynamics, Boston University,
Gamma rhythms and beta rhythms have different synchronization properties N. Kopell, G. B. Ermentrout, M. A. Whittington, and R. D. Traub Department of Mathematics and Center for BioDynamics, Boston University,
A neural mass model for MEG/EEG: coupling and neuronal dynamics
 NeuroImage 20 (2003) 1743 1755 www.elsevier.com/locate/ynimg A neural mass model for MEG/EEG: coupling and neuronal dynamics Olivier David* and Karl J. Friston Wellcome Department of Imaging Neuroscience,
NeuroImage 20 (2003) 1743 1755 www.elsevier.com/locate/ynimg A neural mass model for MEG/EEG: coupling and neuronal dynamics Olivier David* and Karl J. Friston Wellcome Department of Imaging Neuroscience,
Visual motion processing and perceptual decision making
 Visual motion processing and perceptual decision making Aziz Hurzook (ahurzook@uwaterloo.ca) Oliver Trujillo (otrujill@uwaterloo.ca) Chris Eliasmith (celiasmith@uwaterloo.ca) Centre for Theoretical Neuroscience,
Visual motion processing and perceptual decision making Aziz Hurzook (ahurzook@uwaterloo.ca) Oliver Trujillo (otrujill@uwaterloo.ca) Chris Eliasmith (celiasmith@uwaterloo.ca) Centre for Theoretical Neuroscience,
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
Kantian metaphysics to mind-brain. The approach follows Bacon s investigative method
 82 Basic Tools and Techniques As discussed, the project is based on mental physics which in turn is the application of Kantian metaphysics to mind-brain. The approach follows Bacon s investigative method
82 Basic Tools and Techniques As discussed, the project is based on mental physics which in turn is the application of Kantian metaphysics to mind-brain. The approach follows Bacon s investigative method
Effects of Interactive Function Forms in a Self-Organized Critical Model Based on Neural Networks
 Commun. Theor. Phys. (Beijing, China) 40 (2003) pp. 607 613 c International Academic Publishers Vol. 40, No. 5, November 15, 2003 Effects of Interactive Function Forms in a Self-Organized Critical Model
Commun. Theor. Phys. (Beijing, China) 40 (2003) pp. 607 613 c International Academic Publishers Vol. 40, No. 5, November 15, 2003 Effects of Interactive Function Forms in a Self-Organized Critical Model
Membrane equation. VCl. dv dt + V = V Na G Na + V K G K + V Cl G Cl. G total. C m. G total = G Na + G K + G Cl
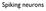 Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Synchronization in Spiking Neural Networks
 CS 790R Seminar Modeling & Simulation Synchronization in Spiking Neural Networks ~ Lecture 8 ~ René Doursat Department of Computer Science & Engineering University of Nevada, Reno Spring 2005 Synchronization
CS 790R Seminar Modeling & Simulation Synchronization in Spiking Neural Networks ~ Lecture 8 ~ René Doursat Department of Computer Science & Engineering University of Nevada, Reno Spring 2005 Synchronization
Lecture 4: Importance of Noise and Fluctuations
 Lecture 4: Importance of Noise and Fluctuations Jordi Soriano Fradera Dept. Física de la Matèria Condensada, Universitat de Barcelona UB Institute of Complex Systems September 2016 1. Noise in biological
Lecture 4: Importance of Noise and Fluctuations Jordi Soriano Fradera Dept. Física de la Matèria Condensada, Universitat de Barcelona UB Institute of Complex Systems September 2016 1. Noise in biological
When is an Integrate-and-fire Neuron like a Poisson Neuron?
 When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
The Spike Response Model: A Framework to Predict Neuronal Spike Trains
 The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
Neurophysiology. Danil Hammoudi.MD
 Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
!) + log(t) # n i. The last two terms on the right hand side (RHS) are clearly independent of θ and can be
 Supplementary Materials General case: computing log likelihood We first describe the general case of computing the log likelihood of a sensory parameter θ that is encoded by the activity of neurons. Each
Supplementary Materials General case: computing log likelihood We first describe the general case of computing the log likelihood of a sensory parameter θ that is encoded by the activity of neurons. Each
The Variance of Covariance Rules for Associative Matrix Memories and Reinforcement Learning
 NOTE Communicated by David Willshaw The Variance of Covariance Rules for Associative Matrix Memories and Reinforcement Learning Peter Dayan Terrence J. Sejnowski Computational Neurobiology Laboratory,
NOTE Communicated by David Willshaw The Variance of Covariance Rules for Associative Matrix Memories and Reinforcement Learning Peter Dayan Terrence J. Sejnowski Computational Neurobiology Laboratory,
of the dynamics. There is a competition between the capacity of the network and the stability of the
 Special Issue on the Role and Control of Random Events in Biological Systems c World Scientic Publishing Company LEARNING SYNFIRE CHAINS: TURNING NOISE INTO SIGNAL JOHN HERTZ and ADAM PRUGEL-BENNETT y
Special Issue on the Role and Control of Random Events in Biological Systems c World Scientic Publishing Company LEARNING SYNFIRE CHAINS: TURNING NOISE INTO SIGNAL JOHN HERTZ and ADAM PRUGEL-BENNETT y
Layer 3 patchy recurrent excitatory connections may determine the spatial organization of sustained activity in the primate prefrontal cortex
 Neurocomputing 32}33 (2000) 391}400 Layer 3 patchy recurrent excitatory connections may determine the spatial organization of sustained activity in the primate prefrontal cortex Boris S. Gutkin *, G. Bard
Neurocomputing 32}33 (2000) 391}400 Layer 3 patchy recurrent excitatory connections may determine the spatial organization of sustained activity in the primate prefrontal cortex Boris S. Gutkin *, G. Bard
Phase Response Properties and Phase-Locking in Neural Systems with Delayed Negative-Feedback. Carter L. Johnson
 Phase Response Properties and Phase-Locking in Neural Systems with Delayed Negative-Feedback Carter L. Johnson Faculty Mentor: Professor Timothy J. Lewis University of California, Davis Abstract Oscillatory
Phase Response Properties and Phase-Locking in Neural Systems with Delayed Negative-Feedback Carter L. Johnson Faculty Mentor: Professor Timothy J. Lewis University of California, Davis Abstract Oscillatory
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests
 Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Biological Modeling of Neural Networks
 Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
The Mixed States of Associative Memories Realize Unimodal Distribution of Dominance Durations in Multistable Perception
 The Mixed States of Associative Memories Realize Unimodal Distribution of Dominance Durations in Multistable Perception Takashi Kanamaru Department of Mechanical Science and ngineering, School of Advanced
The Mixed States of Associative Memories Realize Unimodal Distribution of Dominance Durations in Multistable Perception Takashi Kanamaru Department of Mechanical Science and ngineering, School of Advanced
Subthreshold cross-correlations between cortical neurons: Areference model with static synapses
 Neurocomputing 65 66 (25) 685 69 www.elsevier.com/locate/neucom Subthreshold cross-correlations between cortical neurons: Areference model with static synapses Ofer Melamed a,b, Gilad Silberberg b, Henry
Neurocomputing 65 66 (25) 685 69 www.elsevier.com/locate/neucom Subthreshold cross-correlations between cortical neurons: Areference model with static synapses Ofer Melamed a,b, Gilad Silberberg b, Henry
Patterns, Memory and Periodicity in Two-Neuron Delayed Recurrent Inhibitory Loops
 Math. Model. Nat. Phenom. Vol. 5, No. 2, 2010, pp. 67-99 DOI: 10.1051/mmnp/20105203 Patterns, Memory and Periodicity in Two-Neuron Delayed Recurrent Inhibitory Loops J. Ma 1 and J. Wu 2 1 Department of
Math. Model. Nat. Phenom. Vol. 5, No. 2, 2010, pp. 67-99 DOI: 10.1051/mmnp/20105203 Patterns, Memory and Periodicity in Two-Neuron Delayed Recurrent Inhibitory Loops J. Ma 1 and J. Wu 2 1 Department of
Integration of synaptic inputs in dendritic trees
 Integration of synaptic inputs in dendritic trees Theoretical Neuroscience Fabrizio Gabbiani Division of Neuroscience Baylor College of Medicine One Baylor Plaza Houston, TX 77030 e-mail:gabbiani@bcm.tmc.edu
Integration of synaptic inputs in dendritic trees Theoretical Neuroscience Fabrizio Gabbiani Division of Neuroscience Baylor College of Medicine One Baylor Plaza Houston, TX 77030 e-mail:gabbiani@bcm.tmc.edu
Dynamical Embodiments of Computation in Cognitive Processes James P. Crutcheld Physics Department, University of California, Berkeley, CA a
 Dynamical Embodiments of Computation in Cognitive Processes James P. Crutcheld Physics Department, University of California, Berkeley, CA 94720-7300 and Santa Fe Institute, 1399 Hyde Park Road, Santa Fe,
Dynamical Embodiments of Computation in Cognitive Processes James P. Crutcheld Physics Department, University of California, Berkeley, CA 94720-7300 and Santa Fe Institute, 1399 Hyde Park Road, Santa Fe,
Discriminative Direction for Kernel Classifiers
 Discriminative Direction for Kernel Classifiers Polina Golland Artificial Intelligence Lab Massachusetts Institute of Technology Cambridge, MA 02139 polina@ai.mit.edu Abstract In many scientific and engineering
Discriminative Direction for Kernel Classifiers Polina Golland Artificial Intelligence Lab Massachusetts Institute of Technology Cambridge, MA 02139 polina@ai.mit.edu Abstract In many scientific and engineering
Decoding Input Signals in Time Domain A Model Approach
 Journal of Computational Neuroscience 6, 237 249, 24 c 24 Kluwer Academic Publishers. Manufactured in The Netherlands. Decoding Input Signals in Time Domain A Model Approach JIANFENG FENG AND DAVID BROWN
Journal of Computational Neuroscience 6, 237 249, 24 c 24 Kluwer Academic Publishers. Manufactured in The Netherlands. Decoding Input Signals in Time Domain A Model Approach JIANFENG FENG AND DAVID BROWN
THE LOCUST OLFACTORY SYSTEM AS A CASE STUDY FOR MODELING DYNAMICS OF NEUROBIOLOGICAL NETWORKS: FROM DISCRETE TIME NEURONS TO CONTINUOUS TIME NEURONS
 1 THE LOCUST OLFACTORY SYSTEM AS A CASE STUDY FOR MODELING DYNAMICS OF NEUROBIOLOGICAL NETWORKS: FROM DISCRETE TIME NEURONS TO CONTINUOUS TIME NEURONS B. QUENET 1 AND G. HORCHOLLE-BOSSAVIT 2 1 Equipe de
1 THE LOCUST OLFACTORY SYSTEM AS A CASE STUDY FOR MODELING DYNAMICS OF NEUROBIOLOGICAL NETWORKS: FROM DISCRETE TIME NEURONS TO CONTINUOUS TIME NEURONS B. QUENET 1 AND G. HORCHOLLE-BOSSAVIT 2 1 Equipe de
arxiv:quant-ph/ v1 17 Oct 1995
 PHYSICS AND CONSCIOUSNESS Patricio Pérez arxiv:quant-ph/9510017v1 17 Oct 1995 Departamento de Física, Universidad de Santiago de Chile Casilla 307, Correo 2, Santiago, Chile ABSTRACT Some contributions
PHYSICS AND CONSCIOUSNESS Patricio Pérez arxiv:quant-ph/9510017v1 17 Oct 1995 Departamento de Física, Universidad de Santiago de Chile Casilla 307, Correo 2, Santiago, Chile ABSTRACT Some contributions
Estimation of information-theoretic quantities
 Estimation of information-theoretic quantities Liam Paninski Gatsby Computational Neuroscience Unit University College London http://www.gatsby.ucl.ac.uk/ liam liam@gatsby.ucl.ac.uk November 16, 2004 Some
Estimation of information-theoretic quantities Liam Paninski Gatsby Computational Neuroscience Unit University College London http://www.gatsby.ucl.ac.uk/ liam liam@gatsby.ucl.ac.uk November 16, 2004 Some
+ + ( + ) = Linear recurrent networks. Simpler, much more amenable to analytic treatment E.g. by choosing
 Linear recurrent networks Simpler, much more amenable to analytic treatment E.g. by choosing + ( + ) = Firing rates can be negative Approximates dynamics around fixed point Approximation often reasonable
Linear recurrent networks Simpler, much more amenable to analytic treatment E.g. by choosing + ( + ) = Firing rates can be negative Approximates dynamics around fixed point Approximation often reasonable
An algorithm for detecting oscillatory behavior in discretized data: the damped-oscillator oscillator detector
 An algorithm for detecting oscillatory behavior in discretized data: the damped-oscillator oscillator detector David Hsu, Murielle Hsu, He Huang and Erwin B. Montgomery, Jr Department of Neurology University
An algorithm for detecting oscillatory behavior in discretized data: the damped-oscillator oscillator detector David Hsu, Murielle Hsu, He Huang and Erwin B. Montgomery, Jr Department of Neurology University
Neuron. Detector Model. Understanding Neural Components in Detector Model. Detector vs. Computer. Detector. Neuron. output. axon
 Neuron Detector Model 1 The detector model. 2 Biological properties of the neuron. 3 The computational unit. Each neuron is detecting some set of conditions (e.g., smoke detector). Representation is what
Neuron Detector Model 1 The detector model. 2 Biological properties of the neuron. 3 The computational unit. Each neuron is detecting some set of conditions (e.g., smoke detector). Representation is what
High-dimensional geometry of cortical population activity. Marius Pachitariu University College London
 High-dimensional geometry of cortical population activity Marius Pachitariu University College London Part I: introduction to the brave new world of large-scale neuroscience Part II: large-scale data preprocessing
High-dimensional geometry of cortical population activity Marius Pachitariu University College London Part I: introduction to the brave new world of large-scale neuroscience Part II: large-scale data preprocessing
EEG/MEG Inverse Solution Driven by fmri
 EEG/MEG Inverse Solution Driven by fmri Yaroslav Halchenko CS @ NJIT 1 Functional Brain Imaging EEG ElectroEncephaloGram MEG MagnetoEncephaloGram fmri Functional Magnetic Resonance Imaging others 2 Functional
EEG/MEG Inverse Solution Driven by fmri Yaroslav Halchenko CS @ NJIT 1 Functional Brain Imaging EEG ElectroEncephaloGram MEG MagnetoEncephaloGram fmri Functional Magnetic Resonance Imaging others 2 Functional
 Max-Planck-Institut fur Mathematik in den Naturwissenschaften Leipzig Synchronized chaos and other coherent states for two coupled neurons by Frank Pasemann Preprint-Nr.: 44 1998 Synchronized Chaos and
Max-Planck-Institut fur Mathematik in den Naturwissenschaften Leipzig Synchronized chaos and other coherent states for two coupled neurons by Frank Pasemann Preprint-Nr.: 44 1998 Synchronized Chaos and
Principles of DCM. Will Penny. 26th May Principles of DCM. Will Penny. Introduction. Differential Equations. Bayesian Estimation.
 26th May 2011 Dynamic Causal Modelling Dynamic Causal Modelling is a framework studying large scale brain connectivity by fitting differential equation models to brain imaging data. DCMs differ in their
26th May 2011 Dynamic Causal Modelling Dynamic Causal Modelling is a framework studying large scale brain connectivity by fitting differential equation models to brain imaging data. DCMs differ in their
Artificial Neural Network and Fuzzy Logic
 Artificial Neural Network and Fuzzy Logic 1 Syllabus 2 Syllabus 3 Books 1. Artificial Neural Networks by B. Yagnanarayan, PHI - (Cover Topologies part of unit 1 and All part of Unit 2) 2. Neural Networks
Artificial Neural Network and Fuzzy Logic 1 Syllabus 2 Syllabus 3 Books 1. Artificial Neural Networks by B. Yagnanarayan, PHI - (Cover Topologies part of unit 1 and All part of Unit 2) 2. Neural Networks
3 Detector vs. Computer
 1 Neurons 1. The detector model. Also keep in mind this material gets elaborated w/the simulations, and the earliest material is often hardest for those w/primarily psych background. 2. Biological properties
1 Neurons 1. The detector model. Also keep in mind this material gets elaborated w/the simulations, and the earliest material is often hardest for those w/primarily psych background. 2. Biological properties
Mid Year Project Report: Statistical models of visual neurons
 Mid Year Project Report: Statistical models of visual neurons Anna Sotnikova asotniko@math.umd.edu Project Advisor: Prof. Daniel A. Butts dab@umd.edu Department of Biology Abstract Studying visual neurons
Mid Year Project Report: Statistical models of visual neurons Anna Sotnikova asotniko@math.umd.edu Project Advisor: Prof. Daniel A. Butts dab@umd.edu Department of Biology Abstract Studying visual neurons
SUPPLEMENTARY INFORMATION
 Supplementary discussion 1: Most excitatory and suppressive stimuli for model neurons The model allows us to determine, for each model neuron, the set of most excitatory and suppresive features. First,
Supplementary discussion 1: Most excitatory and suppressive stimuli for model neurons The model allows us to determine, for each model neuron, the set of most excitatory and suppresive features. First,
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Modulation ofexcitatory synaptic coupling facilitates synchronization and complex dynamics in a nonlinear model ofneuronal dynamics
 Neurocomputing 52 54 (23) 151 158 www.elsevier.com/locate/neucom Modulation ofexcitatory synaptic coupling facilitates synchronization and complex dynamics in a nonlinear model ofneuronal dynamics Michael
Neurocomputing 52 54 (23) 151 158 www.elsevier.com/locate/neucom Modulation ofexcitatory synaptic coupling facilitates synchronization and complex dynamics in a nonlinear model ofneuronal dynamics Michael
Model of a Biological Neuron as a Temporal Neural Network
 Model of a Biological Neuron as a Temporal Neural Network Sean D. Murphy and Edward W. Kairiss Interdepartmental Neuroscience Program, Department of Psychology, and The Center for Theoretical and Applied
Model of a Biological Neuron as a Temporal Neural Network Sean D. Murphy and Edward W. Kairiss Interdepartmental Neuroscience Program, Department of Psychology, and The Center for Theoretical and Applied
Synchrony in Neural Systems: a very brief, biased, basic view
 Synchrony in Neural Systems: a very brief, biased, basic view Tim Lewis UC Davis NIMBIOS Workshop on Synchrony April 11, 2011 components of neuronal networks neurons synapses connectivity cell type - intrinsic
Synchrony in Neural Systems: a very brief, biased, basic view Tim Lewis UC Davis NIMBIOS Workshop on Synchrony April 11, 2011 components of neuronal networks neurons synapses connectivity cell type - intrinsic
DESYNCHRONIZATION TRANSITIONS IN RINGS OF COUPLED CHAOTIC OSCILLATORS
 Letters International Journal of Bifurcation and Chaos, Vol. 8, No. 8 (1998) 1733 1738 c World Scientific Publishing Company DESYNCHRONIZATION TRANSITIONS IN RINGS OF COUPLED CHAOTIC OSCILLATORS I. P.
Letters International Journal of Bifurcation and Chaos, Vol. 8, No. 8 (1998) 1733 1738 c World Scientific Publishing Company DESYNCHRONIZATION TRANSITIONS IN RINGS OF COUPLED CHAOTIC OSCILLATORS I. P.
To cognize is to categorize revisited: Category Theory is where Mathematics meets Biology
 To cognize is to categorize revisited: Category Theory is where Mathematics meets Biology Autonomous Systems Laboratory Technical University of Madrid Index 1. Category Theory 2. The structure-function
To cognize is to categorize revisited: Category Theory is where Mathematics meets Biology Autonomous Systems Laboratory Technical University of Madrid Index 1. Category Theory 2. The structure-function
Observed Brain Dynamics
 Observed Brain Dynamics Partha P. Mitra Hemant Bokil OXTORD UNIVERSITY PRESS 2008 \ PART I Conceptual Background 1 1 Why Study Brain Dynamics? 3 1.1 Why Dynamics? An Active Perspective 3 Vi Qimnü^iQ^Dv.aamics'v
Observed Brain Dynamics Partha P. Mitra Hemant Bokil OXTORD UNIVERSITY PRESS 2008 \ PART I Conceptual Background 1 1 Why Study Brain Dynamics? 3 1.1 Why Dynamics? An Active Perspective 3 Vi Qimnü^iQ^Dv.aamics'v
A Rate and History-Preserving Resampling Algorithm for Neural Spike Trains
 A Rate and History-Preserving Resampling Algorithm for Neural Spike Trains Matthew T. Harrison mtharris@cmu.edu Department of Statistics Carnegie Mellon University Pittsburgh, PA 15213 Stuart Geman stuart
A Rate and History-Preserving Resampling Algorithm for Neural Spike Trains Matthew T. Harrison mtharris@cmu.edu Department of Statistics Carnegie Mellon University Pittsburgh, PA 15213 Stuart Geman stuart
Analysis of Neural Networks with Chaotic Dynamics
 Chaos, Solitonr & Fructals Vol. 3, No. 2, pp. 133-139, 1993 Printed in Great Britain @60-0779/93$6.00 + 40 0 1993 Pergamon Press Ltd Analysis of Neural Networks with Chaotic Dynamics FRANCOIS CHAPEAU-BLONDEAU
Chaos, Solitonr & Fructals Vol. 3, No. 2, pp. 133-139, 1993 Printed in Great Britain @60-0779/93$6.00 + 40 0 1993 Pergamon Press Ltd Analysis of Neural Networks with Chaotic Dynamics FRANCOIS CHAPEAU-BLONDEAU
Nervous Tissue. Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation
 Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Hopfield Neural Network and Associative Memory. Typical Myelinated Vertebrate Motoneuron (Wikipedia) Topic 3 Polymers and Neurons Lecture 5
 Hopfield Neural Network and Associative Memory Typical Myelinated Vertebrate Motoneuron (Wikipedia) PHY 411-506 Computational Physics 2 1 Wednesday, March 5 1906 Nobel Prize in Physiology or Medicine.
Hopfield Neural Network and Associative Memory Typical Myelinated Vertebrate Motoneuron (Wikipedia) PHY 411-506 Computational Physics 2 1 Wednesday, March 5 1906 Nobel Prize in Physiology or Medicine.
arxiv:physics/ v1 [physics.bio-ph] 19 Feb 1999
![arxiv:physics/ v1 [physics.bio-ph] 19 Feb 1999 arxiv:physics/ v1 [physics.bio-ph] 19 Feb 1999](/thumbs/83/88796750.jpg) Odor recognition and segmentation by coupled olfactory bulb and cortical networks arxiv:physics/9902052v1 [physics.bioph] 19 Feb 1999 Abstract Zhaoping Li a,1 John Hertz b a CBCL, MIT, Cambridge MA 02139
Odor recognition and segmentation by coupled olfactory bulb and cortical networks arxiv:physics/9902052v1 [physics.bioph] 19 Feb 1999 Abstract Zhaoping Li a,1 John Hertz b a CBCL, MIT, Cambridge MA 02139
Decoding Synchronized Oscillations within the Brain: Phase-Delayed Inhibition Provides a Robust Mechanism for Creating a Sharp Synchrony Filter
 Decoding Synchronized Oscillations within the Brain: Phase-Delayed Inhibition Provides a Robust Mechanism for Creating a Sharp Synchrony Filter Mainak Patel 1 and Badal Joshi 2 1 Mainak Patel, Mathematics
Decoding Synchronized Oscillations within the Brain: Phase-Delayed Inhibition Provides a Robust Mechanism for Creating a Sharp Synchrony Filter Mainak Patel 1 and Badal Joshi 2 1 Mainak Patel, Mathematics
