Decoding Input Signals in Time Domain A Model Approach
|
|
|
- Olivia Lee
- 5 years ago
- Views:
Transcription
1 Journal of Computational Neuroscience 6, , 24 c 24 Kluwer Academic Publishers. Manufactured in The Netherlands. Decoding Input Signals in Time Domain A Model Approach JIANFENG FENG AND DAVID BROWN COGS, University of Sussex, Brighton, BN 9QH, UK jianfeng@cogs.susx.ac.uk ( Received July 3, 22; Revised December 6, 23; Accepted January 3, 24 Action Editor: Barry J. Richmond Abstract. From an observation of efferent interspike intervals of a neuron, we consider how to decode the input temporal information. It is found that the integrate-and-fire model is blind in the temporal domain due to the fact that its efferent firing rate is independent of the input temporal frequency. The conclusion is then confirmed for the integrate-and-fire model with correlated inputs, with reversal potentials, with a nonlinear leakage and with a subthreshold oscillation. For the Hodgkin-Huxley model, however, in terms of efferent firing rates alone, it is possible to read out the input temporal information. Keywords: the integrate-and-fire model, decoding, the Hodgkin-Huxley model, input frequency, tuning curves. Introduction To understand how the nervous system encodes and then decodes the external input information is one of the fundamental issues in neuroscience. Due to nonlinear relationships between the input and output of a neuron, together with the intrinsic randomness in the nervous system, the problem largely remains elusive. Suppose that a (model) neuron receives inputs from N s synapses, each sending Poisson EPSPs with a rate a( + cos(2π Ft))/2 (.) where a (magnitude) and F (temporal frequency) are both constants, t is time. Assume that we have recorded N interspike intervals {T i, i =,...,N}, can the mean firing rate of the neuron tell us about the drive frequency F? The biological meaning of the question is clear. For example, suppose that a sinusoidal grating is moving across the receptive field of a neuron in V. The input signal of this neuron might be described by Eq. (.). We seek to determine the input temporal information (input frequency) using observations of the neuron activity. As another example, consider a neuron in the primary somatosensory cortex, as reported early in experiments in Lamotte and Mountcastle (975), it receives vibrating stimuli as described by Eq. (.). It is reported (Lamotte and Mountcastle, 975) that neurons fire with different firing rates when the temporal input frequency F varies. The integrate-and-fire model is first introduced in Section 2. Tuning curves of the integrate-and-fire model are estimated, in terms of both temporal-average and spatio-average. It is found that for the integrateand-fire model, the tuning curve with respect to the input temporal frequency F is almost flat, implying that the model is not sensitive to the input temporal frequency at all. In other words, the output firing rate contains no information on the input temporal frequency. Our result, that the integrate-and-fire model is insensitive to long time scale input fluctuations, is then verified for various situations: with correlated inputs, with large EPSP sizes, with reversal potentials, with nonlinear leakage and with a subthreshold oscillation. The observed phenomenon poses a fundamental problem for the encoding and decoding capability
2 238 Feng and Brown of the integrate-and-fire model in the time domain, or more generally, of the widely believed integrate and fire mechanisms of a neuron. We also briefly consider the impact of the results on simple discrimination tasks. Compared with the integrate-and-fire model, the tuning curve of the Hodgkin-Huxley model exhibits totally different behaviour and is dealt with in Section 3. The tuning curve is not flat, not monotone, but has two maximum points and one minimum point. The nonmonotone behaviour reflects the subthreshold oscillation of the Hodgkin-Huxley model. We arrive at the conclusion that it is not possible to uniquely read out the input temporal frequency, at least not above about 5 Hz. Taken together, the answer to the previous question is: in terms of efferent firing rates alone, we can not read out the temporal information in the integrate-andfire model, or a model based upon the integrate and fire mechanism, but can successfully decode the temporal information in the Hodgkin-Huxley model. 2. The Integrate-and-Fire Model 2.. The Model Fortwo given quantities V thre (threshold) > V rest (resting potential) and when v t < V thre, the membrane potential v t satisfies the following dynamics: dv t = v t V rest + di syn (t) γ (2.) v = V rest where γ is the decay time, I syn (t)isthe synaptic input given by di syn (t) = µ(t) + σ (t)db t (2.2) with µ(t),σ(t) and the standard Brownian motion B t. Once v t is greater than V thre,itisreset to V rest, i.e. v = V rest. More specifically we confine ourselves to Poisson process inputs defined by µ(t) = wλ(t)( r), σ 2 (t) = w 2 λ(t)(+r) (2.3) where w is the magnitude of EPSPs (excitatory postsynaptic potentials) and IPSPs (inhibitory postsynaptic potentials), λ(t) = λ E (t)n s is the input rate with N s as the number of active synapses and λ E (t) asthe firing rate of each synapse, r is the ratio between inhibitory inputs and excitatory inputs. Of course, Eq. (2.3) is an approximation, the so-called usual approximation (Tuckwell, 988), to point process inputs such as Poisson process inputs. However, the approximation accuracy is quite good in most parameter regions of the integrate-and-fire model and has been checked by many authors (Tuckwell, 988; Gerstner and Kistler, 22). In particular, when r = the neuron exclusively receives excitatory inputs; when r = the inhibitory and excitatory input is exactly balanced. Here for the simplicity of notation, we assume that the EPSP and IPSP size are equal. We refer the reader to Koch (999) for a more complete and biologically oriented formulation of synaptic inputs. The model defined by Eq. (2.) is called the integrate-and-fire model (Feng, 997; Koch, 999). When the integrate-and-fire model receives correlated inputs, µ(t) remains identical as in Eq. (2.3), but σ (t) takes the following form σ 2 (t) = w 2 λ E (t)n s ( + (N s )c)( + r) (2.4) where c is the correlation coefficient of synaptic inputs. For a most recent review on the functional role of correlated inputs, we refer the reader to Salinas and Sejnowski (2). There are two driving forces deterministic, γµ and stochastic, σγ that depolarize the cell to fire. When V thre <γµ, the deterministic force alone is large enough to ensure that the cell fires and the stochastic force is only a perturbation of the system. In this case, as we have discussed in Feng (2), the interspike intervals are usually very regular. The other case is V thre >γµ.now the stochastic force plays a major role in pushing the cell to cross its firing threshold. As a consequence, the cell fires with very irregular spike trains. In what follows, we define T (F) = inf{t : v t V thre } (2.5) as the firing time (interspike intervals) Temporal-Average We consider the IF model with synaptic inputs with the rate λ E (t) = a[ + cos(2π Ft)]/2 (2.6) where a is the magnitude and F is the input temporal frequency ranging from to a few hundred Hz. The
3 Decoding Input Signals in Time Domain 239 parameters are V thre = 2 mv, V rest = mv,γ = 2 msec, N s =, a = Hz, and w =, otherwise specified elsewhere. Note that our choice of parameters is in agreement with data from experiments (see for example, Shadlen and Newsome, 994; Feng, 2), which is equivalent to the assumption that a neuron receives three hundred active synaptic inputs, each fires with a rate of 3 Hz. Mathematically, the maximum effective input to the neuron, as defined below, is γ N s λ E ()( r) = 2. ( r) = 2 ( r) when λ E () = Hz. In our following simulations, we consider the case of r [, ]. Hence the maximum effective input is the same order of magnitude of the threshold when r >. Note that when λ E () is smaller (see for example, Fig. ), the maximum effective input becomes smaller. When r =, the maximum effective input is zero. When we calculate the mean of interspike intervals, a refractory period of 5 msec is added. spikes are generated to calculate the histogram, mean and, i.e. = T 2 (F) ( T (F) ) 2 /[ T (F) +5]. As readily checked from all following figures, a larger number of interspike intervals will not alter all conclusions below. In this subsection we consider the case that the output frequency is obtained via averaging over the time domain. By this (temporal-average) we mean that after each spike the time t in Eq. (2.6) is not reset to zero and so the the initial state of the input signal is possibly different for each spike. The case that the mean interspike intervals are obtained by averaging over an ensemble of neurons is discussed in the following subsection. The temporal-average might be a realistic situation for spatio-average as well if we assume that the initial states of the ensemble of neurons are all different, i.e. when the input signal is applied, all neurons are in different states Tuning Curves. We first consider tuning curves of the integrate-and-fire model with respect to a (magnitude of the input) and F (temporal frequency of the input). Figure (upper panel) depicts the membrane potential of the integrate-and-fire model vs. time with different input temporal frequencies F (F = and Hz). It is easily seen that with a fast temporal frequency input, the neuron fires more regularly, but with a slow temporal frequency input, its spike trains become more clustered. The histograms of interspike intervals are different. Nevertheless, it is surprisingly seen that the efferent firing rates are independent of the temporal frequency. For example, in Fig., middle panel (left), we see that the efferent firing rate is about 58 Hz, when r =.6, and is insensitive to the temporal frequency. Figure, middle panel (right), tells us that the of efferent spike trains are very irregular, with a above.5. The tuning curves of the integrate-and-fire model with respect to a, F are shown in the bottom panel of Fig.. As we have mentioned above, the tuning curve reveals an interesting phenomenon: it is independent of F, and, as we all known, is a sigmoidal function of a (see bottom panel, right). Denote the efferent frequency of the model f (a, F). We then see there are two functions f and f 2 satisfying f (a, F) = f (F) f 2 (a) (2.7) The tuning curve of the model with respect to a, F is separable. In fact, we could further assert that f (a, F) = f 2 (a) (2.8) since f (a, F) is independent of F. In Fig., in order to show the model behaviour in whole parameter regions of r, the neuron fires slowly when r =. One might argue that if the neuron fires at a reasonable firing rate, the behaviour might be different. To answer the question, we simulate two different cases: increasing w to its upper limit w = 2 and increasing correlations between input synapses. As shown in Fig. 2, we see that both when w = 2 and c =. (Zohary et al., 994), our conclusions remain true. In fact Fig. 2 indicates that both the mean and are quite flat. The possible function of r =, exactly balanced inputs, and c >, correlated inputs, have been intensively discussed in the past few years (see for example Feng, 2; Salinas and Sejnowski, 2). It is interesting to see that when r =, both the first (mean) and second () order statistics almost contain no information about F. When c =, r =.6 the of efferent interval does depend on the input temporal frequency Decoding. Now we are in the position to answer the question raised in Introduction. As a matter of fact, the answer now is trivial since the efferent firing rate does not contain any information about the temporal input frequency. Hence we conclude that it is impossible to read out F based upon the mean efferent
4 2 Feng and Brown 8 8 Membrane potential (mv) 2 Membrane potential (mv) Time (msec) r = x r =.6 * r = 8 2 Time (msec) r = x r =.6 * r = a = Hz a = 3 Hz a = 2 Hz 5 a = Hz a = 3 Hz a = 25 Hz a = 2 Hz a = Hz a = 5 Hz a = Hz a = 3 Hz Figure. Behaviour of the integrate-and-fire model. Upper panel: membrane potential vs. time with r =.6, F = Hz (left) and F = Hz (right). Regularly oscillating curves are µ and σ. Middle panel: output firing rate λ (left) and (right) vs. input frequencies F with r =,.6,. Bottom panel: output frequency (Hz) vs. input temporal frequency F with different magnitude a, r = (left) and r =.6 (right).
5 Decoding Input Signals in Time Domain X: w =, c =., r = O: w = 2, c =., r = X: w =, c =., r = O: w = 2, c =., r = Figure 2. Output firing rate (Hz) and for the integrate-and-fire model vs. input frequency (Hz) with w = 2., c =., r =. and w =., c =., r =, all other parameters are the same as in Fig., comparing with Fig.. 2 O r = 3 8 O r =.6 2 r = O r = 2 r =.6 O O O r = Figure 3. Output firing rate vs. input temporal frequency F =, 2,..., with r =,,.6 for the integrate-and-fire model. The efferent firing rate (marked by o ) at F = isobtained with the input defined by Eq. (2.9) rather than F =. firing rate. For fixed F, the decoding of input amplitude a is easy since the tuning curve f 2 (a) ismonotone and the maximum likelihood estimate has a unique solution Underpinning Mechanisms. We naturally ask why the neuron is so insensitive to the input temporal frequency. A straightforward hypothesis would be that the integrate-and-fire model averages out the information in time domain. If this idea is true, then the firing rate of the neuron model considered in the previous subsections should be equal to the firing rate of the neuronal model with inputs [ T ]/ λ(t) = a lim + T cos(2π Ft)/T 2 = a/2 (2.9) We next check whether this is the case. To this end, we extend Fig. to the extreme case: the input frequency is as low as one Hz. Figure 3 shows the output firing rate vs. the input rates from to Hz. It is easily seen that the output firing rate remains a constant. When the temporal input frequency is zero, the output firing rate is calculated when the input takes the form of Eq. (2.9) rather than F =. It is readily seen that
6 242 Feng and Brown our hypothesis the mean firing rate of the integrateand-fire model averages out temporal information is well supported by Fig. 3. If the neuron receives input with F =, the output firing rate is.933 Hz with a of.947 when r =, Hz with a of.488 when r =.6, and.5333 Hz with a being.223 when r =. Therefore there is a sudden jump on the value of efferent firing rates from F = tof >, an interesting phenomenon. The integrate-and-fire model can easily detect whether there is an oscillating signal present or not, but can not tell how fast the period of the signal is IF Model with a Subthreshold Oscillation. To further check the validity of our hypothesis above, we consider the model with a subthreshold oscillation. In fact, there are many discussions in the literature about subthreshold oscillations and their possible functions in information processing in nervous systems. But according to the hypothesis in the previous subsections we would conclude that for the integrate-andfire model, a subthreshold oscillation would have no impact on the efferent firing rate. To confirm it, we simulate the model defined by Eq. (2.). A subthreshold oscillation k(cos(2πωt)+) is introduced in the integrate-and-fire model, where k and ω are constants. dv t = v t V rest + di syn (t) γ + k(cos(2πωt) + ) (2.) v = V rest Again Fig. 4 supports our hypothesis: the subthreshold oscillation is totally averaged out when the mean Membrane potential (mv) 2 Membrane potential (mv) Time (msec) k = 4 k = 3 k = 2 3 k = Time (msec) x k = 4 o k = 3 + k = 2 * k = Figure 4. The behaviour of the integrate-and-fire model with a subthreshold oscillation, ω =.5, r =. Upper panel: membrane potential vs. time, F = Hz (left) and F = Hz (right). The subthreshold oscillation, regularly oscillating curve, is added with k = 4. Bottom panel: output frequency (Hz), left, and, right, vs. input frequency with k =, 2, 3, 4.
7 Decoding Input Signals in Time Domain 243 of the efferent interspike intervals is considered. The stronger the subthreshold oscillation is, the higher the efferent firing rate. It is interesting to note that although the mean firing rate is independent of the subthreshold oscillation, is not. When the subthreshold oscillation is strong enough k = 2, 3, 4, there is a peak in at the frequency of the subthreshold oscillation ω = 5 Hz Models with Reversal Potentials. A slightly more biologically realistic model than the integrateand-fire model defined above is the integrate-and-fire model with reversal potentials defined by where dz t = Z t V rest γ + d Ī syn (Z t, t) (2.) N s rn s Ī syn (Z t, t) = ā(v E Z t ) E i (t)+ b(v I Z t ) I j (t) i= j= V E and V I are the reversal potentials V I < V rest < V E, ā(v E V rest ), b(v I V rest ) are the magnitude of single EPSP and IPSP when Z t = V rest, and E i (t) (I j (t)) are the incoming EPSP (IPSP) trains (Poisson processes). We could rewrite Eq. (2.) in the following form dz t = (Z t V rest ) ( ) Ns γ + ā rn s de i (t) + b di i (t) i= i= N s + ā(v E V rest ) de i (t) i= rn s + b(v I V rest ) di j (t) j= [ Ns = (Z t V rest ) γ + ā de i (t) i= ] rn s N s + b di i (t) + w de i (t) i= i= rn s + w di j (t) (2.2) j= Therefore the difference between the model with and without reversal potentials is that the latter has a decay rate depending on incoming signals. From the conclusions of the previous subsections we would expect that the model is still insensitive to the temporal input frequency since essentially the model is a leaky integrate and fire device. Figure 5 fully confirms our conclusions. The model is independent of the input temporal frequency. The parameters used in the model with reversal potentials are ā =., b =., V E = mv, V I = mv, with all other parameters the same as the model without reversal potentials. Again, this choice of parameters is in agreement with the published literature, see for example Feng (2) and references therein r =, c = r =, c =.5 r =.6, c= r =, c = x r =.6, c =. o r =, c = Figure 5. Output firing rate and vs. input frequency for the integrate and fire model with reversal potentials. To keep output firing rates in the range between to Hz, we have used different c values in simulations, as indicated in the figure.
8 244 Feng and Brown 9 8 r =.6, c =. + r =.6, c =. 7 5 r =, c = 2 O r =, c = x r =, c =. r =, c = Figure 6. Output frequency and vs. input temporal frequency for the IF-FHN model Models with Nonlinear Leakage. The integrate-and-fire model is the simplest neuron model which mimics certain properties of a biological neuron and is linear before resetting. Another slightly more complex model is the IF-FHN model, an integrate-and-fire model but with a nonlinear leakage coefficient, as in a biophysical model. In terms of the output signal-to-noise ratio, we know that the IF and IF-FHN model behave in totally opposite ways when they receive correlated inputs (see Feng, 2) for a review). We then naturally ask that whether the phenomenon observed in the previous subsections with partially correlated inputs is true only for the IF model or not. To this end we simulate the IF-FHN model defined by dv(t) = (/β + γα)v(t) + γ ( + α) v(t)2 γv(t)3 3 + di syn(t) (2.3) when v(t) <v thre = 5. We can rewrite the IF-FHN model in the following form [ dv(t) = (/β + γα) γ ( + α) v(t) ] + γv(t)2 v(t) + di syn(t) (2.4) 3 an integrate-and-fire model with a nonlinear leakage term (Feng, 2) + γα γ ( + α)v(t) β + γv(t)2 3 The parameters are v rest =, γ = 5,α =.2,β = 2.5, w = 2., N s = 3. Note that to ensure output firing rates in similar regions for different models, the value of N s and w used in the IF-FHN model is higher than that in the IF model, but still in the physiologically plausible region. As shown in Fig. 6, the output frequency is flat for different model parameters. This confirms our main conclusion: a model with an integrate and fire mechanism is insensitive to the fluctuations in time domain, no matter whether the leakage is linear, nonlinear (as in the IF-FHN model), or depending on incoming signals (as in the integrate-and-fire model with reversal potentials) Application to Discrimination Tasks. As an application of the results in the previous subsections, we investigate the impacts of our results on discrimination tasks. The problem is stated as follows. Suppose that a neuron receives a vibrating stimulus with a frequency F, and another stimulus with a frequency F 2. In terms of the histograms of the interspike intervals, can we tell one stimulus from the other? In experiments, it has been confirmed that single neuron activity contains enough information to account for psychophysical behaviours and the issue is currently a hot topic in neuroscience (see for example Kast, 2; Parker and Newsome, 998; Romo and Salinas, 2). Before carrying out a simulation, we could have a guess on whether the information contained in the interspike intervals is enough to discriminate between different inputs (see Feng and Liu, 23 for a theoretical treatment). From the previous results, we know
9 Decoding Input Signals in Time Domain 245 Figure 7. Histograms of interspike intervals with different input temporal frequencies with r =.6 (left) and r = (right). When r =.6, a slowly vibrating stimulus results in a bimodal histogram (F = 25 Hz); while a fast stimulus gives rise to a uni-modal distribution. When r =, exactly balanced inputs, the efferent interspike intervals histograms are uni-modal and are similar to Poisson process. that the histogram of efferent interspike intervals will be centred around its mean value which is identical for different input temporal frequencies. Therefore we could imagine that histograms with different temporal frequencies will mix with each other. This is exactly the case as shown in Fig. 7. Figure 7 tells us that there is very little information about input temporal frequencies, even in the histograms. From the classical discrimination theory (Lehmann and Casella, 999) we know that to tell one stimulus from the other, we require that their histograms should be almost separable. It is interesting to see that for different model parameters, the histogram is very different. When r =.6, a slow oscillating signal (F = 25 Hz) results in a bi-modal histogram, a fast oscillating signal (F = 75 Hz) ensures a uni-modal histogram. When r =, exactly balanced inputs, the histograms are both uni-modal and therefore can be approximated by a renewal process of Gamma distributed interspike intervals. As we pointed out before, the integrate-and-fire model is blind on discriminating between signals with different oscillating periods Spatio-Average In this subsection we consider alternative ways of averaging interspike intervals. Suppose that the membrane potentials of all neurons are at identically initial levels, i.e. synchronize their membrane potentials. Note that each neuron still receives independent inputs. The mean interspike intervals are obtained by averaging over all N neurons and only the first spike of each neuron is taken into account. Here in our simulations we take N =. Under the spatio-average of single spike assumption above, we could expect that the mean of interspike intervals of the integrate-and-fire model will depend on the input temporal frequency. In fact it is easy to see that when the mean is small enough, neurons do not have a chance to sample one single period of the input signal. Therefore it makes no sense to talk about averaging out the temporal information and the spatioaverage of single spike assumption is incorrect. Results in Fig. 8 are in agreement with our expectations. When r =.6 and the mean output firing rate is above 5 Hz, the efferent firing rate is mildly sensitive to the input temporal frequency. Now we turn to another scenario: averaging over a group of neurons within a short time window. This seems a more reasonable assumption and is widely used in dealing with experimental data. In Fig. 9, output firing rates are obtained by averaging over N = neurons and each neuron with a time window of 2 msec. The initial value of each neurons is random, uniformly distributed between the resting potential and the threshold. Figure 9 indicates that the model is again insensitive to the input temporal frequency. 3. A Comparison with the HH Model Finally we turn out attentions to the Hodgkin-Huxley model, the simplest biophysical model.
10 246 Feng and Brown r = r = r =.6 r =.6 r =.4 r = Figure 8. Output firing rate and vs. input frequency F with r =,,.6 for the integrate-and-fire model. Spatio-averaging with a single spike of each neuron is performed r =, c = r =.6, c = r =, c = r =.6, c = Figure 9. Output firing rate and vs. input frequency F with r =,.6 for the integrate-and-fire model. Output firing rates are obtained by averaging over neurons and 2 msec. Compare with Figs. and Tuning Curves In Fig. we show the tuning curve of the Hodgkin- Huxley model with respect to F. The Hodgkin-Huxley model is the standard one (see Appendix) (Brown et al., 999) with N independent Poisson process inputs with a rate a( + cos(2π Ft))/2 (3.) Synaptic input in the HH model is I = N i= wα(t t i, j )(V (t) E i ) where N =,w =.2, E i = mv, V (t) is the membrane potential at time t, α(t) = t exp( t/τ)/τ with τ = 2 msec, and t i, j, j =, 2,..., are Poisson process with rates defined by Eq. (3.) and a = 8 Hz. The efferent frequency vs. afferent frequency F is plotted in Fig.. After subtracting a refractory period of around 2 msec (Brown et al., 999), the efferent spike trains can be roughly approximated by a Poisson process. In Fig., it is clearly shown that when afferent frequency is around 63 Hz, the efferent frequency arrives at its maximum, around 63 Hz as well. The reason we observe the maximum firing at 63 Hz lies in the fact that the Hodgkin-Huxley model oscillates at a frequency of 63 Hz when it receives a subthreshold stimulus, the so-called subthreshold oscillation. Hence the maximum firing depicted in Fig. reveals the
11 Decoding Input Signals in Time Domain Figure. Tuning curve for the Hodgkin-Huxley model with a = 8 Hz. When F = 63 Hz, the coherence between the external input and the subthreshold oscillation drives the neuron to fire at its largest firing rate. coherence between the input and subthreshold oscillation frequencies. When afferent frequency is around 2 Hz, doubling the internal frequency, the efferent frequency rises again and arrives its local maximum. Between and 2 Hz, there is a local minimum of frequency Decoding From Fig., we see that there are two regions of the output frequency vs. the input frequency F. When the output frequency is below around 5 Hz, from the output firing rate, we can uniquely tell the input frequency. Above around 5 Hz and apart from the firing rates corresponding to the minimum and maximum values (i.e. output frequencies corresponding to 63, 95 and 26 Hz), for a given output firing rate, we can have either multi solutions or no solution (above the global maximum value). In comparison with the case of the integrate-and-fire model, we have at most four solutions here. In this region, how to pick up one solution out of the multi solutions is problematic. Of course, we can make a convention to select one out of the multi solutions, for example, the smallest one. Hence we conclude that for the Hodgkin-Huxley model, in terms of the output firing rates alone, it is possible to decode the input frequency F.Ofcourse, as we all know from the theory of population coding that with many HH neurons, each with different tuning curves, it is possible to uniquely read out the input frequency. We will pursue the topic in further publications. The results also provide us with a natural solution to the general issue of how to decode the input frequency F. The biophysical mechanisms behind the Hodgkin- Huxley model are the key (see next Section for more discussions). 4. Discussion Based upon neuronal models, we have presented a simple study on how to read out the input information. It is somewhat surprising to see that, using the mean interspike interval, one of the most widely used neuron models, the integrate-and-fire model, is incapable of reading out the input temporal information. To the best of our knowledge, the phenomenon has not been reported in the literature. The implication of the result is two folds. For neuroscience, the result indicates that the integrate and fire mechanism is not enough to account for experimental data, which clearly demonstrate that many neurons are good at decoding the temporal information (Romo and Salinas, 2). What is the key mechanism in a biological neuron to enable it to read out the temporal information is still unclear and is one of our future topics. For engineering applications, our result clearly shows that the integrate and fire model, despite its computational advantage over biophysical models, is not sufficient if we intend to encode and then decode the input temporal information. One might argue that in a nervous system, encoding and decoding is accomplished at a system level rather than at a single neuron level. This is definitely true. However, firstly a study at a single neuron level is theoretically more tractable than a group of neurons; secondly whether the nervous system employs the lower envelope principle (decoding based upon a single neuron) or pooling models (therefore decoding in terms of a group of neurons) is still unknown. Thirdly there are ample examples demonstrated that single neuron activity contains enough information to account for psychophysical experiment data. Hence we hope our approach is informative and opens up many intriguing issues for further study. The problem as posed is actually a special case of input temporal information, where the input is harmonic with a single frequency F. If the neuron was linear, this would provide (when studied over all frequencies)
12 248 Feng and Brown a complete description of the system. However, this is not so for any of the models, and there is the possibility of nonlinear interactions between frequencies, resulting in the results not being valid for more complex temporal inputs. This is one of our future topics. The interspike interval distribution is not a complete representation of the output of the neuron this would be the case if successive interspike intervals were independent, but they are not in practice. In present paper, we have only considered the case that the efferent interspike intervals are independent. In the biological case, the situation could be different. For example, when a cell exhibits bursting activity (Lisman, 997), its interspike intervals are correlated. There is a continuous endeavour to find a canonical model for neurons. We have shown here that neuron models with a subthreshold oscillation, with a nonlinear leakage and with a leakage depending on incoming signals do not work for decoding the input temporal information. However we have not tested a few other important mechanisms. For example, synaptic depression and facilitation might be helpful in reading out the input temporal information. Including synaptic depression and facilitation in the integrate-and-fire model would be equivalent to having a mechanism of incoming signal dependent oscillations. Furthermore we have only considered the simplest case of encoding and decoding one dimensional information. To generalize our approach to high dimensional cases is illuminating, for example, to decode (a, F)interms of mean and of efferent spike trains. Many aspects related to the encoding and decoding have been addressed in the past decades, but the majority of them deal with experimental data. Here we present a simple approach to methods of reading out the encoded information, using neuronal models. It is usually not easy to directly investigate the issue for an in vivo biological system since the input information often intermingles with many other uncontrollable factors. The advantage of a modelling approach lies in the fact that we can manage to thoroughly understand the underpinning mechanisms and hopefully such an approach could provide an excellent template for biological systems. Appendix Here is a detailed list of the equations in the Hodgkin- Huxley model. The Hodgkin-Huxley model is written by CdV = g Na m 3 h(v V Na ) g K n 4 (V V K ) g L (V V L ) + I. (4.) The term m 3 h describes the sodium channel activity and n 4 reflects the potassium channel activity. The remaining equations in the Hodgkin-Huxley model are as following. dn and = n n τ n, dm = m m τ m, dh = h h τ h n = α n α m, m =, h = α h α n + β n α m + β m τ n = with, τ m =, τ h = α n + β n α m + β m.(v + 55) α n = exp ( ) V +55 ( β n =.25 exp V + 65 ) 8.(V + ) α m = exp ( ) V + ( β m = 4exp V + 65 ) 8 ( α h =.7 exp V + 65 ) 2 β h = exp ( ) V α h + β h α h + β h The parameters used in Eq. (4.) are C =, g Na = 2, g K = 36, g L =.3, V k = 77, V Na = 5, V L = Acknowledgment We are grateful to the referees for their valuable comments on the paper. Figure was generated by Dr. F. Liu. Partially supported by grants from UK EPSRC (R54569), (S2574), (S3443), and (S6383).
13 Decoding Input Signals in Time Domain 249 References Abbott LF, Dayan P (999) The effect of correlated variability on the accuracy of a population code. Neural Computation. : 9. Brown D, Feng J, Feerick S (999) Variability of firing of Hodgkin- Huxley and FitzHugh-Nagumo neurons with stochastic synaptic input. Phys. Rev. Letts. 82: Dayan P, Abbott LF (2) Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. MIT Press. Feng J (997) Behaviours of spike output jitter in the integrate-andfire model. Phys. Rev. Lett. 79: Feng J (2) Is the integrate-and-fire model good enough? A review. Neural Networks 2: Feng J, Liu F (23) Discriminating between input signals via single neuron activity (submitted). Available at cogs.susx.ac.uk/users/jianfeng/publication.html. Georgopoulos AP, Kalaska JK, Caminiti R, Massey JT (982) On the relations between the directions of two-dimensional arm movements and cell discharge in primate motor cortex. J. of Neurosci. 2: Gerstner W, Kistler W (22). Spiking Neuron Models Single Neurons, Populations, Plasticity. Cambridge University Press, Cambridge. Goldman MS, Maldonado P, Abbott LF (22) Redundancy reduction and sustained firing with stochastic depressing synapses. J. Neurosci. 22: Kast B (2) Decisions, decisions. Nature 4: Koch C (999) Biophysics of Computation. Oxford University Press, New York, pp Lamotte RH, Mountcastle VB (975) Capacities of humans and monkeys to discriminate between vibratory stimuli of different frequency and amplitude: A correlation between neural events and psychophysical measurements. J. Neurophysiol. 38: Lehmann E, Casella G (999) Theory of Point Estimation. Springer- Verlag, New York, Berlin etc. Lisman JE (997) Bursts as a unit of neural informaiton: Making unreliable synapses reliable. Trends in Neuroacience 2: Parker AJ, Newsome WT (998) Sense and the single neuron: Probing the physiology of perception. Annu. Rev. Neurosci. 2: Poggio GF, Talbot WH (98) Mechanisms of static and dynamic stereopsis in foveal cortex of the rhesus monkey. J. Physiology 35: Romo R, Salinas E (2) Touch and go: Decision-making mechanisms in somatosensation. Annu. Rev. Neurosci. 24: Salinas E and Sejnowski T (2) Correlated neuronal activity and the flow of neural information. Nature Rev. Neurosci. 2: Shadlen MN, Newsome WT (994) Noise, neural codes and cortical organization. Curr. Opin. Neurobiol. 4: Tuckwell HC (988) Introduction to Theoretical Neurobiology, vol. 2. Cambridge University Press. Zohary E, Shadlen MN, Newsome WT (994) Correlated neuronal discharge rate and its implications for psychophysical performance Nature 37: 43. Wandell BA (995) Foundations of Vision. Sunderland, MA, Sinauer Associates.
Increasing Inhibitory Input Increases Neuronal Firing Rate: Why And When? Diffusion Process Cases
 Increasing Inhibitory Input Increases Neuronal Firing Rate: Why And When? Diffusion Process Cases Jianfeng Feng Gang Wei Abstract Increasing inhibitory input to single neuronal models, such as the FitzHugh-
Increasing Inhibitory Input Increases Neuronal Firing Rate: Why And When? Diffusion Process Cases Jianfeng Feng Gang Wei Abstract Increasing inhibitory input to single neuronal models, such as the FitzHugh-
Exercises. Chapter 1. of τ approx that produces the most accurate estimate for this firing pattern.
 1 Exercises Chapter 1 1. Generate spike sequences with a constant firing rate r 0 using a Poisson spike generator. Then, add a refractory period to the model by allowing the firing rate r(t) to depend
1 Exercises Chapter 1 1. Generate spike sequences with a constant firing rate r 0 using a Poisson spike generator. Then, add a refractory period to the model by allowing the firing rate r(t) to depend
Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity
 Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity Jorge F. Mejias 1,2 and Joaquín J. Torres 2 1 Department of Physics and Center for
Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity Jorge F. Mejias 1,2 and Joaquín J. Torres 2 1 Department of Physics and Center for
Consider the following spike trains from two different neurons N1 and N2:
 About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
Activity Driven Adaptive Stochastic. Resonance. Gregor Wenning and Klaus Obermayer. Technical University of Berlin.
 Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
Neural Modeling and Computational Neuroscience. Claudio Gallicchio
 Neural Modeling and Computational Neuroscience Claudio Gallicchio 1 Neuroscience modeling 2 Introduction to basic aspects of brain computation Introduction to neurophysiology Neural modeling: Elements
Neural Modeling and Computational Neuroscience Claudio Gallicchio 1 Neuroscience modeling 2 Introduction to basic aspects of brain computation Introduction to neurophysiology Neural modeling: Elements
The Spike Response Model: A Framework to Predict Neuronal Spike Trains
 The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
When is an Integrate-and-fire Neuron like a Poisson Neuron?
 When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
Model neurons!!poisson neurons!
 Model neurons!!poisson neurons! Suggested reading:! Chapter 1.4 in Dayan, P. & Abbott, L., heoretical Neuroscience, MI Press, 2001.! Model neurons: Poisson neurons! Contents: Probability of a spike sequence
Model neurons!!poisson neurons! Suggested reading:! Chapter 1.4 in Dayan, P. & Abbott, L., heoretical Neuroscience, MI Press, 2001.! Model neurons: Poisson neurons! Contents: Probability of a spike sequence
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons
 Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
An Introductory Course in Computational Neuroscience
 An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
Biological Modeling of Neural Networks
 Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
Synaptic Input. Linear Model of Synaptic Transmission. Professor David Heeger. September 5, 2000
 Synaptic Input Professor David Heeger September 5, 2000 The purpose of this handout is to go a bit beyond the discussion in Ch. 6 of The Book of Genesis on synaptic input, and give some examples of how
Synaptic Input Professor David Heeger September 5, 2000 The purpose of this handout is to go a bit beyond the discussion in Ch. 6 of The Book of Genesis on synaptic input, and give some examples of how
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten. Lecture 2a. The Neuron - overview of structure. From Anderson (1995)
 Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Single-Compartment Neural Models
 Single-Compartment Neural Models BENG/BGGN 260 Neurodynamics University of California, San Diego Week 2 BENG/BGGN 260 Neurodynamics (UCSD) Single-Compartment Neural Models Week 2 1 / 18 Reading Materials
Single-Compartment Neural Models BENG/BGGN 260 Neurodynamics University of California, San Diego Week 2 BENG/BGGN 260 Neurodynamics (UCSD) Single-Compartment Neural Models Week 2 1 / 18 Reading Materials
Effects of Interactive Function Forms in a Self-Organized Critical Model Based on Neural Networks
 Commun. Theor. Phys. (Beijing, China) 40 (2003) pp. 607 613 c International Academic Publishers Vol. 40, No. 5, November 15, 2003 Effects of Interactive Function Forms in a Self-Organized Critical Model
Commun. Theor. Phys. (Beijing, China) 40 (2003) pp. 607 613 c International Academic Publishers Vol. 40, No. 5, November 15, 2003 Effects of Interactive Function Forms in a Self-Organized Critical Model
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
Basic elements of neuroelectronics -- membranes -- ion channels -- wiring
 Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wires -- signal propagation -- processing
Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wires -- signal propagation -- processing
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 2, W.W. Lytton, Hodgkin-Huxley Model) 9. Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 2, W.W. Lytton, Hodgkin-Huxley Model) 9. Passive and active membrane models In the previous lecture we have considered a passive
Effects of synaptic conductance on the voltage distribution and firing rate of spiking neurons
 PHYSICAL REVIEW E 69, 051918 (2004) Effects of synaptic conductance on the voltage distribution and firing rate of spiking neurons Magnus J. E. Richardson* Laboratory of Computational Neuroscience, Brain
PHYSICAL REVIEW E 69, 051918 (2004) Effects of synaptic conductance on the voltage distribution and firing rate of spiking neurons Magnus J. E. Richardson* Laboratory of Computational Neuroscience, Brain
Decoding. How well can we learn what the stimulus is by looking at the neural responses?
 Decoding How well can we learn what the stimulus is by looking at the neural responses? Two approaches: devise explicit algorithms for extracting a stimulus estimate directly quantify the relationship
Decoding How well can we learn what the stimulus is by looking at the neural responses? Two approaches: devise explicit algorithms for extracting a stimulus estimate directly quantify the relationship
Spike Count Correlation Increases with Length of Time Interval in the Presence of Trial-to-Trial Variation
 NOTE Communicated by Jonathan Victor Spike Count Correlation Increases with Length of Time Interval in the Presence of Trial-to-Trial Variation Robert E. Kass kass@stat.cmu.edu Valérie Ventura vventura@stat.cmu.edu
NOTE Communicated by Jonathan Victor Spike Count Correlation Increases with Length of Time Interval in the Presence of Trial-to-Trial Variation Robert E. Kass kass@stat.cmu.edu Valérie Ventura vventura@stat.cmu.edu
Decision-making and Weber s law: a neurophysiological model
 European Journal of Neuroscience, Vol. 24, pp. 901 916, 2006 doi:10.1111/j.14-9568.2006.04940.x Decision-making and Weber s law: a neurophysiological model Gustavo Deco 1 and Edmund T. Rolls 2 1 Institucio
European Journal of Neuroscience, Vol. 24, pp. 901 916, 2006 doi:10.1111/j.14-9568.2006.04940.x Decision-making and Weber s law: a neurophysiological model Gustavo Deco 1 and Edmund T. Rolls 2 1 Institucio
Membrane equation. VCl. dv dt + V = V Na G Na + V K G K + V Cl G Cl. G total. C m. G total = G Na + G K + G Cl
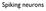 Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
How to read a burst duration code
 Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
!) + log(t) # n i. The last two terms on the right hand side (RHS) are clearly independent of θ and can be
 Supplementary Materials General case: computing log likelihood We first describe the general case of computing the log likelihood of a sensory parameter θ that is encoded by the activity of neurons. Each
Supplementary Materials General case: computing log likelihood We first describe the general case of computing the log likelihood of a sensory parameter θ that is encoded by the activity of neurons. Each
Neuronal Dynamics: Computational Neuroscience of Single Neurons
 Week 4 part 5: Nonlinear Integrate-and-Fire Model 4.1 From Hodgkin-Huxley to 2D Neuronal Dynamics: Computational Neuroscience of Single Neurons Week 4 Recing detail: Two-dimensional neuron models Wulfram
Week 4 part 5: Nonlinear Integrate-and-Fire Model 4.1 From Hodgkin-Huxley to 2D Neuronal Dynamics: Computational Neuroscience of Single Neurons Week 4 Recing detail: Two-dimensional neuron models Wulfram
Neural variability and Poisson statistics
 Neural variability and Poisson statistics January 15, 2014 1 Introduction We are in the process of deriving the Hodgkin-Huxley model. That model describes how an action potential is generated by ion specic
Neural variability and Poisson statistics January 15, 2014 1 Introduction We are in the process of deriving the Hodgkin-Huxley model. That model describes how an action potential is generated by ion specic
AT2 Neuromodeling: Problem set #3 SPIKE TRAINS
 AT2 Neuromodeling: Problem set #3 SPIKE TRAINS Younesse Kaddar PROBLEM 1: Poisson spike trains Link of the ipython notebook for the code Brain neuron emit spikes seemingly randomly: we will aim to model
AT2 Neuromodeling: Problem set #3 SPIKE TRAINS Younesse Kaddar PROBLEM 1: Poisson spike trains Link of the ipython notebook for the code Brain neuron emit spikes seemingly randomly: we will aim to model
The homogeneous Poisson process
 The homogeneous Poisson process during very short time interval Δt there is a fixed probability of an event (spike) occurring independent of what happened previously if r is the rate of the Poisson process,
The homogeneous Poisson process during very short time interval Δt there is a fixed probability of an event (spike) occurring independent of what happened previously if r is the rate of the Poisson process,
Dynamical systems in neuroscience. Pacific Northwest Computational Neuroscience Connection October 1-2, 2010
 Dynamical systems in neuroscience Pacific Northwest Computational Neuroscience Connection October 1-2, 2010 What do I mean by a dynamical system? Set of state variables Law that governs evolution of state
Dynamical systems in neuroscience Pacific Northwest Computational Neuroscience Connection October 1-2, 2010 What do I mean by a dynamical system? Set of state variables Law that governs evolution of state
Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits
 Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits Wolfgang Maass, Robert Legenstein, Nils Bertschinger Institute for Theoretical Computer Science Technische
Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits Wolfgang Maass, Robert Legenstein, Nils Bertschinger Institute for Theoretical Computer Science Technische
Lecture 11 : Simple Neuron Models. Dr Eileen Nugent
 Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Neurophysiology of a VLSI spiking neural network: LANN21
 Neurophysiology of a VLSI spiking neural network: LANN21 Stefano Fusi INFN, Sezione Roma I Università di Roma La Sapienza Pza Aldo Moro 2, I-185, Roma fusi@jupiter.roma1.infn.it Paolo Del Giudice Physics
Neurophysiology of a VLSI spiking neural network: LANN21 Stefano Fusi INFN, Sezione Roma I Università di Roma La Sapienza Pza Aldo Moro 2, I-185, Roma fusi@jupiter.roma1.infn.it Paolo Del Giudice Physics
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Fast neural network simulations with population density methods
 Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Linearization of F-I Curves by Adaptation
 LETTER Communicated by Laurence Abbott Linearization of F-I Curves by Adaptation Bard Ermentrout Department of Mathematics, University of Pittsburgh, Pittsburgh, PA 15260, U.S.A. We show that negative
LETTER Communicated by Laurence Abbott Linearization of F-I Curves by Adaptation Bard Ermentrout Department of Mathematics, University of Pittsburgh, Pittsburgh, PA 15260, U.S.A. We show that negative
CSE/NB 528 Final Lecture: All Good Things Must. CSE/NB 528: Final Lecture
 CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
Visual motion processing and perceptual decision making
 Visual motion processing and perceptual decision making Aziz Hurzook (ahurzook@uwaterloo.ca) Oliver Trujillo (otrujill@uwaterloo.ca) Chris Eliasmith (celiasmith@uwaterloo.ca) Centre for Theoretical Neuroscience,
Visual motion processing and perceptual decision making Aziz Hurzook (ahurzook@uwaterloo.ca) Oliver Trujillo (otrujill@uwaterloo.ca) Chris Eliasmith (celiasmith@uwaterloo.ca) Centre for Theoretical Neuroscience,
Computing with inter-spike interval codes in networks ofintegrate and fire neurons
 Neurocomputing 65 66 (2005) 415 420 www.elsevier.com/locate/neucom Computing with inter-spike interval codes in networks ofintegrate and fire neurons Dileep George a,b,, Friedrich T. Sommer b a Department
Neurocomputing 65 66 (2005) 415 420 www.elsevier.com/locate/neucom Computing with inter-spike interval codes in networks ofintegrate and fire neurons Dileep George a,b,, Friedrich T. Sommer b a Department
Voltage-clamp and Hodgkin-Huxley models
 Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best Koch, Chapters 6, 8, 9 See also Hodgkin and Huxley, J. Physiol. 117:500-544 (1952. (the source Clay, J. Neurophysiol. 80:903-913
Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best Koch, Chapters 6, 8, 9 See also Hodgkin and Huxley, J. Physiol. 117:500-544 (1952. (the source Clay, J. Neurophysiol. 80:903-913
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests
 Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Roles of Fluctuations. in Pulsed Neural Networks
 Ph.D. Thesis Roles of Fluctuations in Pulsed Neural Networks Supervisor Professor Yoichi Okabe Takashi Kanamaru Department of Advanced Interdisciplinary Studies, Faculty of Engineering, The University
Ph.D. Thesis Roles of Fluctuations in Pulsed Neural Networks Supervisor Professor Yoichi Okabe Takashi Kanamaru Department of Advanced Interdisciplinary Studies, Faculty of Engineering, The University
Coarse-grained event tree analysis for quantifying Hodgkin-Huxley neuronal network dynamics
 J Comput Neurosci (212) 32:55 72 DOI 1.17/s1827-11-339-7 Coarse-grained event tree analysis for quantifying Hodgkin-Huxley neuronal network dynamics Yi Sun Aaditya V. Rangan Douglas Zhou David Cai Received:
J Comput Neurosci (212) 32:55 72 DOI 1.17/s1827-11-339-7 Coarse-grained event tree analysis for quantifying Hodgkin-Huxley neuronal network dynamics Yi Sun Aaditya V. Rangan Douglas Zhou David Cai Received:
Divisive Inhibition in Recurrent Networks
 Divisive Inhibition in Recurrent Networks Frances S. Chance and L. F. Abbott Volen Center for Complex Systems and Department of Biology Brandeis University Waltham MA 2454-911 Abstract Models of visual
Divisive Inhibition in Recurrent Networks Frances S. Chance and L. F. Abbott Volen Center for Complex Systems and Department of Biology Brandeis University Waltham MA 2454-911 Abstract Models of visual
Neuronal Dynamics: Computational Neuroscience of Single Neurons
 Week 5 part 3a :Three definitions of rate code Neuronal Dynamics: Computational Neuroscience of Single Neurons Week 5 Variability and Noise: The question of the neural code Wulfram Gerstner EPFL, Lausanne,
Week 5 part 3a :Three definitions of rate code Neuronal Dynamics: Computational Neuroscience of Single Neurons Week 5 Variability and Noise: The question of the neural code Wulfram Gerstner EPFL, Lausanne,
Self-organized Criticality and Synchronization in a Pulse-coupled Integrate-and-Fire Neuron Model Based on Small World Networks
 Commun. Theor. Phys. (Beijing, China) 43 (2005) pp. 466 470 c International Academic Publishers Vol. 43, No. 3, March 15, 2005 Self-organized Criticality and Synchronization in a Pulse-coupled Integrate-and-Fire
Commun. Theor. Phys. (Beijing, China) 43 (2005) pp. 466 470 c International Academic Publishers Vol. 43, No. 3, March 15, 2005 Self-organized Criticality and Synchronization in a Pulse-coupled Integrate-and-Fire
The Bayesian Brain. Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester. May 11, 2017
 The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
Interspike interval distributions of spiking neurons driven by fluctuating inputs
 J Neurophysiol 106: 361 373, 2011. First published April 27, 2011; doi:10.1152/jn.00830.2010. Interspike interval distributions of spiking neurons driven by fluctuating inputs Srdjan Ostojic Center for
J Neurophysiol 106: 361 373, 2011. First published April 27, 2011; doi:10.1152/jn.00830.2010. Interspike interval distributions of spiking neurons driven by fluctuating inputs Srdjan Ostojic Center for
Chapter 9. Nerve Signals and Homeostasis
 Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
This document is downloaded from DR-NTU, Nanyang Technological University Library, Singapore.
 This document is downloaded from DR-NTU, Nanyang Technological University Library, Singapore. Title Small-signal neural models and their applications Author(s) Basu, Arindam Citation Basu, A. (01). Small-signal
This document is downloaded from DR-NTU, Nanyang Technological University Library, Singapore. Title Small-signal neural models and their applications Author(s) Basu, Arindam Citation Basu, A. (01). Small-signal
Voltage-clamp and Hodgkin-Huxley models
 Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best) Koch, Chapters 6, 8, 9 See also Clay, J. Neurophysiol. 80:903-913 (1998) (for a recent version of the HH squid axon model) Rothman
Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best) Koch, Chapters 6, 8, 9 See also Clay, J. Neurophysiol. 80:903-913 (1998) (for a recent version of the HH squid axon model) Rothman
A General Framework for Neurobiological Modeling: An Application to the Vestibular System 1
 A General Framework for Neurobiological Modeling: An Application to the Vestibular System 1 Chris Eliasmith, a M. B. Westover, b and C. H. Anderson c a Department of Philosophy, University of Waterloo,
A General Framework for Neurobiological Modeling: An Application to the Vestibular System 1 Chris Eliasmith, a M. B. Westover, b and C. H. Anderson c a Department of Philosophy, University of Waterloo,
(Feed-Forward) Neural Networks Dr. Hajira Jabeen, Prof. Jens Lehmann
 (Feed-Forward) Neural Networks 2016-12-06 Dr. Hajira Jabeen, Prof. Jens Lehmann Outline In the previous lectures we have learned about tensors and factorization methods. RESCAL is a bilinear model for
(Feed-Forward) Neural Networks 2016-12-06 Dr. Hajira Jabeen, Prof. Jens Lehmann Outline In the previous lectures we have learned about tensors and factorization methods. RESCAL is a bilinear model for
Topics in Neurophysics
 Topics in Neurophysics Alex Loebel, Martin Stemmler and Anderas Herz Exercise 2 Solution (1) The Hodgkin Huxley Model The goal of this exercise is to simulate the action potential according to the model
Topics in Neurophysics Alex Loebel, Martin Stemmler and Anderas Herz Exercise 2 Solution (1) The Hodgkin Huxley Model The goal of this exercise is to simulate the action potential according to the model
How do synapses transform inputs?
 Neurons to networks How do synapses transform inputs? Excitatory synapse Input spike! Neurotransmitter release binds to/opens Na channels Change in synaptic conductance! Na+ influx E.g. AMA synapse! Depolarization
Neurons to networks How do synapses transform inputs? Excitatory synapse Input spike! Neurotransmitter release binds to/opens Na channels Change in synaptic conductance! Na+ influx E.g. AMA synapse! Depolarization
Dynamical Constraints on Computing with Spike Timing in the Cortex
 Appears in Advances in Neural Information Processing Systems, 15 (NIPS 00) Dynamical Constraints on Computing with Spike Timing in the Cortex Arunava Banerjee and Alexandre Pouget Department of Brain and
Appears in Advances in Neural Information Processing Systems, 15 (NIPS 00) Dynamical Constraints on Computing with Spike Timing in the Cortex Arunava Banerjee and Alexandre Pouget Department of Brain and
High-conductance states in a mean-eld cortical network model
 Neurocomputing 58 60 (2004) 935 940 www.elsevier.com/locate/neucom High-conductance states in a mean-eld cortical network model Alexander Lerchner a;, Mandana Ahmadi b, John Hertz b a Oersted-DTU, Technical
Neurocomputing 58 60 (2004) 935 940 www.elsevier.com/locate/neucom High-conductance states in a mean-eld cortical network model Alexander Lerchner a;, Mandana Ahmadi b, John Hertz b a Oersted-DTU, Technical
Conductance-Based Integrate-and-Fire Models
 NOTE Communicated by Michael Hines Conductance-Based Integrate-and-Fire Models Alain Destexhe Department of Physiology, Laval University School of Medicine, Québec, G1K 7P4, Canada A conductance-based
NOTE Communicated by Michael Hines Conductance-Based Integrate-and-Fire Models Alain Destexhe Department of Physiology, Laval University School of Medicine, Québec, G1K 7P4, Canada A conductance-based
Introduction and the Hodgkin-Huxley Model
 1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
1 Introduction and the Hodgkin-Huxley Model Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 Reference:
Communicated by John Rinzel
 ARTICLE Communicated by John Rinzel Dynamics of Membrane Excitability Determine Interspike Interval Variability: A Link Between Spike Generation Mechanisms and Cortical Spike Train Statistics Boris S.
ARTICLE Communicated by John Rinzel Dynamics of Membrane Excitability Determine Interspike Interval Variability: A Link Between Spike Generation Mechanisms and Cortical Spike Train Statistics Boris S.
Neural Decoding. Chapter Encoding and Decoding
 Chapter 3 Neural Decoding 3.1 Encoding and Decoding In chapters 1 and 2, we considered the problem of predicting neural responses to known stimuli. The nervous system faces the reverse problem, determining
Chapter 3 Neural Decoding 3.1 Encoding and Decoding In chapters 1 and 2, we considered the problem of predicting neural responses to known stimuli. The nervous system faces the reverse problem, determining
Liquid Computing in a Simplified Model of Cortical Layer IV: Learning to Balance a Ball
 Liquid Computing in a Simplified Model of Cortical Layer IV: Learning to Balance a Ball Dimitri Probst 1,3, Wolfgang Maass 2, Henry Markram 1, and Marc-Oliver Gewaltig 1 1 Blue Brain Project, École Polytechnique
Liquid Computing in a Simplified Model of Cortical Layer IV: Learning to Balance a Ball Dimitri Probst 1,3, Wolfgang Maass 2, Henry Markram 1, and Marc-Oliver Gewaltig 1 1 Blue Brain Project, École Polytechnique
Basic elements of neuroelectronics -- membranes -- ion channels -- wiring. Elementary neuron models -- conductance based -- modelers alternatives
 Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wiring neurons together -- synapses
Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wiring neurons together -- synapses
Comparing integrate-and-fire models estimated using intracellular and extracellular data 1
 Comparing integrate-and-fire models estimated using intracellular and extracellular data 1 Liam Paninski a,b,2 Jonathan Pillow b Eero Simoncelli b a Gatsby Computational Neuroscience Unit, University College
Comparing integrate-and-fire models estimated using intracellular and extracellular data 1 Liam Paninski a,b,2 Jonathan Pillow b Eero Simoncelli b a Gatsby Computational Neuroscience Unit, University College
Bursting and Chaotic Activities in the Nonlinear Dynamics of FitzHugh-Rinzel Neuron Model
 Bursting and Chaotic Activities in the Nonlinear Dynamics of FitzHugh-Rinzel Neuron Model Abhishek Yadav *#, Anurag Kumar Swami *, Ajay Srivastava * * Department of Electrical Engineering, College of Technology,
Bursting and Chaotic Activities in the Nonlinear Dynamics of FitzHugh-Rinzel Neuron Model Abhishek Yadav *#, Anurag Kumar Swami *, Ajay Srivastava * * Department of Electrical Engineering, College of Technology,
MATH 3104: THE HODGKIN-HUXLEY EQUATIONS
 MATH 3104: THE HODGKIN-HUXLEY EQUATIONS Parallel conductance model A/Prof Geoffrey Goodhill, Semester 1, 2009 So far we have modelled neuronal membranes by just one resistance (conductance) variable. We
MATH 3104: THE HODGKIN-HUXLEY EQUATIONS Parallel conductance model A/Prof Geoffrey Goodhill, Semester 1, 2009 So far we have modelled neuronal membranes by just one resistance (conductance) variable. We
How Spike Generation Mechanisms Determine the Neuronal Response to Fluctuating Inputs
 628 The Journal of Neuroscience, December 7, 2003 23(37):628 640 Behavioral/Systems/Cognitive How Spike Generation Mechanisms Determine the Neuronal Response to Fluctuating Inputs Nicolas Fourcaud-Trocmé,
628 The Journal of Neuroscience, December 7, 2003 23(37):628 640 Behavioral/Systems/Cognitive How Spike Generation Mechanisms Determine the Neuronal Response to Fluctuating Inputs Nicolas Fourcaud-Trocmé,
Probabilistic Models in Theoretical Neuroscience
 Probabilistic Models in Theoretical Neuroscience visible unit Boltzmann machine semi-restricted Boltzmann machine restricted Boltzmann machine hidden unit Neural models of probabilistic sampling: introduction
Probabilistic Models in Theoretical Neuroscience visible unit Boltzmann machine semi-restricted Boltzmann machine restricted Boltzmann machine hidden unit Neural models of probabilistic sampling: introduction
What is the neural code? Sekuler lab, Brandeis
 What is the neural code? Sekuler lab, Brandeis What is the neural code? What is the neural code? Alan Litke, UCSD What is the neural code? What is the neural code? What is the neural code? Encoding: how
What is the neural code? Sekuler lab, Brandeis What is the neural code? What is the neural code? Alan Litke, UCSD What is the neural code? What is the neural code? What is the neural code? Encoding: how
Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued
 Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued Filip Piękniewski Faculty of Mathematics and Computer Science, Nicolaus Copernicus University, Toruń, Poland
Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued Filip Piękniewski Faculty of Mathematics and Computer Science, Nicolaus Copernicus University, Toruń, Poland
Neural Coding: Integrate-and-Fire Models of Single and Multi-Neuron Responses
 Neural Coding: Integrate-and-Fire Models of Single and Multi-Neuron Responses Jonathan Pillow HHMI and NYU http://www.cns.nyu.edu/~pillow Oct 5, Course lecture: Computational Modeling of Neuronal Systems
Neural Coding: Integrate-and-Fire Models of Single and Multi-Neuron Responses Jonathan Pillow HHMI and NYU http://www.cns.nyu.edu/~pillow Oct 5, Course lecture: Computational Modeling of Neuronal Systems
The Resonate-and-fire Neuron: Time Dependent and Frequency Selective Neurons in Neural Networks
 Bucknell University Bucknell Digital Commons Master s Theses Student Theses 2010 The Resonate-and-fire Neuron: Time Dependent and Frequency Selective Neurons in Neural Networks Himadri Mukhopadhyay Bucknell
Bucknell University Bucknell Digital Commons Master s Theses Student Theses 2010 The Resonate-and-fire Neuron: Time Dependent and Frequency Selective Neurons in Neural Networks Himadri Mukhopadhyay Bucknell
Dendritic cable with active spines: a modelling study in the spike-diffuse-spike framework
 Dendritic cable with active spines: a modelling study in the spike-diffuse-spike framework Yulia Timofeeva a, Gabriel Lord a and Stephen Coombes b a Department of Mathematics, Heriot-Watt University, Edinburgh,
Dendritic cable with active spines: a modelling study in the spike-diffuse-spike framework Yulia Timofeeva a, Gabriel Lord a and Stephen Coombes b a Department of Mathematics, Heriot-Watt University, Edinburgh,
Synfire Waves in Small Balanced Networks
 Synfire Waves in Small Balanced Networks Yuval Aviel 1,3, David Horn 2 and Moshe Abeles 1 1 Interdisciplinary Center for Neural Computation, Hebrew University, Jerusalem, Israel. 2 School of Physics and
Synfire Waves in Small Balanced Networks Yuval Aviel 1,3, David Horn 2 and Moshe Abeles 1 1 Interdisciplinary Center for Neural Computation, Hebrew University, Jerusalem, Israel. 2 School of Physics and
DEVS Simulation of Spiking Neural Networks
 DEVS Simulation of Spiking Neural Networks Rene Mayrhofer, Michael Affenzeller, Herbert Prähofer, Gerhard Höfer, Alexander Fried Institute of Systems Science Systems Theory and Information Technology Johannes
DEVS Simulation of Spiking Neural Networks Rene Mayrhofer, Michael Affenzeller, Herbert Prähofer, Gerhard Höfer, Alexander Fried Institute of Systems Science Systems Theory and Information Technology Johannes
Broadband coding with dynamic synapses
 Broadband coding with dynamic synapses Benjamin Lindner Max-Planck-Institute for the Physics of Complex Systems, Nöthnitzer Str. 38 1187 Dresden, Germany André Longtin Department of Physics and Center
Broadband coding with dynamic synapses Benjamin Lindner Max-Planck-Institute for the Physics of Complex Systems, Nöthnitzer Str. 38 1187 Dresden, Germany André Longtin Department of Physics and Center
Fast and exact simulation methods applied on a broad range of neuron models
 Fast and exact simulation methods applied on a broad range of neuron models Michiel D Haene michiel.dhaene@ugent.be Benjamin Schrauwen benjamin.schrauwen@ugent.be Ghent University, Electronics and Information
Fast and exact simulation methods applied on a broad range of neuron models Michiel D Haene michiel.dhaene@ugent.be Benjamin Schrauwen benjamin.schrauwen@ugent.be Ghent University, Electronics and Information
Synaptic dynamics. John D. Murray. Synaptic currents. Simple model of the synaptic gating variable. First-order kinetics
 Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Neural Encoding I: Firing Rates and Spike Statistics
 Chapter 1 Neural Encoding I: Firing Rates and Spike Statistics 1.1 Introduction Neurons are remarkable among the cells of the body in their ability to propagate signals rapidly over large distances. They
Chapter 1 Neural Encoding I: Firing Rates and Spike Statistics 1.1 Introduction Neurons are remarkable among the cells of the body in their ability to propagate signals rapidly over large distances. They
3 Neural Decoding. 3.1 Encoding and Decoding. (r 1, r 2,..., r N ) for N neurons is a list of spike-count firing rates, although,
 3 Neural Decoding 3.1 Encoding and Decoding In chapters 1 and 2, we considered the problem of predicting neural responses to known stimuli. The nervous system faces the reverse problem, determining what
3 Neural Decoding 3.1 Encoding and Decoding In chapters 1 and 2, we considered the problem of predicting neural responses to known stimuli. The nervous system faces the reverse problem, determining what
Overview Organization: Central Nervous System (CNS) Peripheral Nervous System (PNS) innervate Divisions: a. Afferent
 Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Single-Cell and Mean Field Neural Models
 1 Single-Cell and Mean Field Neural Models Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 The neuron
1 Single-Cell and Mean Field Neural Models Richard Bertram Department of Mathematics and Programs in Neuroscience and Molecular Biophysics Florida State University Tallahassee, Florida 32306 The neuron
Empirical Bayes interpretations of random point events
 INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS A: MATHEMATICAL AND GENERAL J. Phys. A: Math. Gen. 38 (25) L531 L537 doi:1.188/35-447/38/29/l4 LETTER TO THE EDITOR Empirical Bayes interpretations of
INSTITUTE OF PHYSICS PUBLISHING JOURNAL OF PHYSICS A: MATHEMATICAL AND GENERAL J. Phys. A: Math. Gen. 38 (25) L531 L537 doi:1.188/35-447/38/29/l4 LETTER TO THE EDITOR Empirical Bayes interpretations of
Analytical Integrate-and-Fire Neuron Models with Conductance-Based Dynamics for Event-Driven Simulation Strategies
 LETTER Communicated by Arnaud Delorme Analytical Integrate-and-Fire Neuron Models with Conductance-Based Dynamics for Event-Driven Simulation Strategies Michelle Rudolph Rudolph@iaf.cnrs-gif.fr Alain Destexhe
LETTER Communicated by Arnaud Delorme Analytical Integrate-and-Fire Neuron Models with Conductance-Based Dynamics for Event-Driven Simulation Strategies Michelle Rudolph Rudolph@iaf.cnrs-gif.fr Alain Destexhe
Neurons and conductance-based models
 Neurons and conductance-based models Jaeseung Jeong, Ph.D Department of Bio and Brain Engineering, KAIST Bilateralization ( 양측편재화 ): HAROLD:Hemispheric Asymmetry Reduction in Older Adults Prototypical
Neurons and conductance-based models Jaeseung Jeong, Ph.D Department of Bio and Brain Engineering, KAIST Bilateralization ( 양측편재화 ): HAROLD:Hemispheric Asymmetry Reduction in Older Adults Prototypical
Processing of Time Series by Neural Circuits with Biologically Realistic Synaptic Dynamics
 Processing of Time Series by Neural Circuits with iologically Realistic Synaptic Dynamics Thomas Natschläger & Wolfgang Maass Institute for Theoretical Computer Science Technische Universität Graz, ustria
Processing of Time Series by Neural Circuits with iologically Realistic Synaptic Dynamics Thomas Natschläger & Wolfgang Maass Institute for Theoretical Computer Science Technische Universität Graz, ustria
Computational Explorations in Cognitive Neuroscience Chapter 2
 Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
Simple models of neurons!! Lecture 4!
 Simple models of neurons!! Lecture 4! 1! Recap: Phase Plane Analysis! 2! FitzHugh-Nagumo Model! Membrane potential K activation variable Notes from Zillmer, INFN 3! FitzHugh Nagumo Model Demo! 4! Phase
Simple models of neurons!! Lecture 4! 1! Recap: Phase Plane Analysis! 2! FitzHugh-Nagumo Model! Membrane potential K activation variable Notes from Zillmer, INFN 3! FitzHugh Nagumo Model Demo! 4! Phase
arxiv: v1 [q-bio.nc] 20 Oct 2017
![arxiv: v1 [q-bio.nc] 20 Oct 2017 arxiv: v1 [q-bio.nc] 20 Oct 2017](/thumbs/88/114888549.jpg) Point Neurons with ConductanceBased Synapses in the Neural Engineering Framework Andreas Stöckel, Aaron R. Voelker, and Chris Eliasmith arxiv:1710.07659v1 [qbio.nc] 20 Oct 2017 Centre for Theoretical Neuroscience,
Point Neurons with ConductanceBased Synapses in the Neural Engineering Framework Andreas Stöckel, Aaron R. Voelker, and Chris Eliasmith arxiv:1710.07659v1 [qbio.nc] 20 Oct 2017 Centre for Theoretical Neuroscience,
Dynamic Stochastic Synapses as Computational Units
 LETTER Communicated by Laurence Abbott Dynamic Stochastic Synapses as Computational Units Wolfgang Maass Institute for Theoretical Computer Science, Technische Universität Graz, A 8010 Graz, Austria Anthony
LETTER Communicated by Laurence Abbott Dynamic Stochastic Synapses as Computational Units Wolfgang Maass Institute for Theoretical Computer Science, Technische Universität Graz, A 8010 Graz, Austria Anthony
Factors affecting phase synchronization in integrate-and-fire oscillators
 J Comput Neurosci (26) 2:9 2 DOI.7/s827-6-674-6 Factors affecting phase synchronization in integrate-and-fire oscillators Todd W. Troyer Received: 24 May 25 / Revised: 9 November 25 / Accepted: November
J Comput Neurosci (26) 2:9 2 DOI.7/s827-6-674-6 Factors affecting phase synchronization in integrate-and-fire oscillators Todd W. Troyer Received: 24 May 25 / Revised: 9 November 25 / Accepted: November
Action Potential Initiation in the Hodgkin-Huxley Model
 Action Potential Initiation in the Hodgkin-Huxley Model The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published Version
Action Potential Initiation in the Hodgkin-Huxley Model The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published Version
This script will produce a series of pulses of amplitude 40 na, duration 1ms, recurring every 50 ms.
 9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
Dynamical Systems in Neuroscience: Elementary Bifurcations
 Dynamical Systems in Neuroscience: Elementary Bifurcations Foris Kuang May 2017 1 Contents 1 Introduction 3 2 Definitions 3 3 Hodgkin-Huxley Model 3 4 Morris-Lecar Model 4 5 Stability 5 5.1 Linear ODE..............................................
Dynamical Systems in Neuroscience: Elementary Bifurcations Foris Kuang May 2017 1 Contents 1 Introduction 3 2 Definitions 3 3 Hodgkin-Huxley Model 3 4 Morris-Lecar Model 4 5 Stability 5 5.1 Linear ODE..............................................
Neuronal Tuning: To Sharpen or Broaden?
 NOTE Communicated by Laurence Abbott Neuronal Tuning: To Sharpen or Broaden? Kechen Zhang Howard Hughes Medical Institute, Computational Neurobiology Laboratory, Salk Institute for Biological Studies,
NOTE Communicated by Laurence Abbott Neuronal Tuning: To Sharpen or Broaden? Kechen Zhang Howard Hughes Medical Institute, Computational Neurobiology Laboratory, Salk Institute for Biological Studies,
Entrainment and Chaos in the Hodgkin-Huxley Oscillator
 Entrainment and Chaos in the Hodgkin-Huxley Oscillator Kevin K. Lin http://www.cims.nyu.edu/ klin Courant Institute, New York University Mostly Biomath - 2005.4.5 p.1/42 Overview (1) Goal: Show that the
Entrainment and Chaos in the Hodgkin-Huxley Oscillator Kevin K. Lin http://www.cims.nyu.edu/ klin Courant Institute, New York University Mostly Biomath - 2005.4.5 p.1/42 Overview (1) Goal: Show that the
Maximum Likelihood Estimation of a Stochastic Integrate-and-Fire Neural Model
 Maximum Likelihood Estimation of a Stochastic Integrate-and-Fire Neural Model Jonathan W. Pillow, Liam Paninski, and Eero P. Simoncelli Howard Hughes Medical Institute Center for Neural Science New York
Maximum Likelihood Estimation of a Stochastic Integrate-and-Fire Neural Model Jonathan W. Pillow, Liam Paninski, and Eero P. Simoncelli Howard Hughes Medical Institute Center for Neural Science New York
Characterizing the firing properties of an adaptive
 Characterizing the firing properties of an adaptive analog VLSI neuron Daniel Ben Dayan Rubin 1,2, Elisabetta Chicca 2, and Giacomo Indiveri 2 1 Department of Bioengineering, Politecnico di Milano, P.zza
Characterizing the firing properties of an adaptive analog VLSI neuron Daniel Ben Dayan Rubin 1,2, Elisabetta Chicca 2, and Giacomo Indiveri 2 1 Department of Bioengineering, Politecnico di Milano, P.zza
Channel Noise in Excitable Neuronal Membranes
 Channel Noise in Excitable Neuronal Membranes Amit Manwani, Peter N. Steinmetz and Christof Koch Computation and Neural Systems Program, M-S 9-74 California Institute of Technology Pasadena, CA 95 fquixote,peter,kochg@klab.caltech.edu
Channel Noise in Excitable Neuronal Membranes Amit Manwani, Peter N. Steinmetz and Christof Koch Computation and Neural Systems Program, M-S 9-74 California Institute of Technology Pasadena, CA 95 fquixote,peter,kochg@klab.caltech.edu
