Influence of surface conductivity on the apparent zeta potential of TiO2 nanoparticles
|
|
|
- Charity Welch
- 5 years ago
- Views:
Transcription
1 Influence of urface conductvty on the apparent zeta potental of TO2 nanopartcle Phlppe Leroy, Chrtophe Tournaat, Mohamed Bz To cte th veron: Phlppe Leroy, Chrtophe Tournaat, Mohamed Bz. Influence of urface conductvty on the apparent zeta potental of TO2 nanopartcle. Journal of Collod and Interface Scence, Elever, 2011, 356 (2), pp < /j.jc >. <hal > HAL Id: hal Submtted on 14 Jun 2011 HAL a mult-dcplnary open acce archve for the depot and demnaton of centfc reearch document, whether they are publhed or not. The document may come from teachng and reearch nttuton n France or abroad, or from publc or prvate reearch center. L archve ouverte plurdcplnare HAL, et detnée au dépôt et à la dffuon de document centfque de nveau recherche, publé ou non, émanant de établement d enegnement et de recherche frança ou étranger, de laboratore publc ou prvé.
2 The nfluence of urface conductvty on the apparent zeta potental of TO 2 nanopartcle Phlppe Leroy 1*, Chrtophe Tournaat 1, Mohamed Bz 1 1 BRGM, French Geologcal Survey, 3 Avenue C. Gullemn, Orléan, France * Correpondng author and malng addre: Phlppe Leroy BRGM Water Dvon (EAU/VAP) 3 Avenue Claude Gullemn Orléan Cedex 2, France E-mal: p.leroy@brgm.fr Tel: +33 (0) Fax: +33 (0) Intended for publcaton n Journal of Collod and Interface Scence 1
3 Abtract Zeta potental a phyco-chemcal parameter of partcular mportance to decrbe on adorpton and electrotatc nteracton between charged partcle. Neverthele, th fundamental parameter ll-contraned, becaue t expermental nterpretaton complex, partcularly for very mall and charged TO 2 nanopartcle. The exce of electrcal charge at the nterface reponble for urface conductance, whch can gnfcantly lower the electrophoretc meaurement, and hence the apparent zeta potental. Conequently, the ntrnc zeta potental can have a larger ampltude, even n the cae of mple 1:1 electrolyte lke NaCl and KCl. Surface conductance of TO 2 nanopartcle mmered n a NaCl oluton etmated ung a urface complexaton model, and th parameter and partcle ze are ncorporated nto Henry' model n order to determne a contraned value of the zeta potental from electrophore. Interor conductvty of the agglomerate calculated ung a dfferental elf-content model. The ampltude of etmated zeta potental greater than that derved from the von Smoluchowk equaton and correpond to the electrc potental at the Outer Helmholtz Plane calculated by our urface complexaton model. Conequently, the hear plane may be located cloe to the OHP, contradctng the aumpton of the preence of a tagnant dffue layer at the TO 2 /water nterface. Keyword: zeta potental, electrophoretc moblty, TO 2, nanopartcle, urface conductvty, extended Stern model. 2
4 1. Introducton Ttanum doxde wdely ued a TO 2 nanopartcle and ha a large varety of potental applcaton n, for example, the bomedcal, optcal, and electronc feld [1, 2]. Due to ther mall ze, nanopartcle have a very hgh urface area to volume rato and are thu of great centfc nteret a they are a brdge between bulk materal and atomc or molecular tructure. The properte of materal change a ther ze decreae to nanocale and the proporton of urface atom become gnfcant. One of thee properte, the urface onzaton of ttanum doxde nanopartcle n contact wth an electrolytc oluton, ha been tuded extenvely [1, 3-10]. It well known that the complexaton reacton at the urface of an oxde mneral are trongly nfluenced by the development of the urface charge. The prmary urface charge determned by the reacton of proton wth the urface. Surface complexaton reacton between the urface te and the on from the bulk electrolyte at the Stern and n the dffue layer neutralze the urface charge [11]. Surface charge properte are prmarly determned ung proton ttraton data. Thee data can be modeled ung varou electrotatc model uch a the dffue double layer, bac Stern, trple layer or CD-MUSIC model [1, 3-10]. Mot ophtcated model are able to reproduce data for a wde range of expermental condton but rely on the fttng of a large number of parameter whoe phycal gnfcance not alway eay to jutfy. Moreover, the unquene of a et of parameter not alway obvou. On the other hand, le ophtcated model rely on the fttng of fewer parameter but often fal to reproduce the data under all of the expermental condton tuded. At preent, there no conenu on whch the bet model to repreent charged urface at oxde-water nterface. Of all the phyco-chemcal parameter characterzng the old/water nterface, the zeta potental partcularly mportant. It the potental at the uppoed lppng plane that 3
5 eparate the tatonary and moble phae n tangental flow of the lqud wth repect to the urface. For example, n the cae of a partcle undergong electrophore, becaue of the electrotatc nteracton between the appled electrc feld and the hydrated counter-on n the dffue layer, the nterface develop a urface of hear [12]. The electrc potental at the lppng plane of partcular nteret f we wh to etmate the crtcal coagulaton concentraton when tudyng nanopartcle agglomeraton, for example [5, 12, 13]. The zeta potental alo a key parameter for the tudy of the tranport properte of electrcally charged materal lke oxde and clay mneral [14-16]. It uually make t poble to optmze the parameter of the electrotatc urface complexaton model whle amlatng the poton of the hear-plane to the poton of the head end of the dffue layer [10, 17]. Th nterpretaton ha, however, been challenged by recent tude on ttanum doxde nanopartcle that how that ttraton data cannot be reproduced together wth zeta potental value wthout havng a hearplane poton that change a a functon of the onc trength. Bourka et al. [9] and Panagotou et al. [1] have hown that the hear plane poton (n log cale) lnearly dependant on the log value of the onc trength. Th phycal model lead, however, to a urface repreentaton n whch the hear-plane can be a far a 210 Å from the urface at an onc trength of 10-4 M (Fgure 32 of [1]), the entre volume between the oxde urface and th hear-plane beng condered to be tagnant. Moreover, th phycal repreentaton of the oxde urface rule out any ue of the zeta potental a a contrant for urface charge model. The nature of the phycal property that caue the eparaton between the tagnant dffue layer and the moble dffue layer, however, not gven. Molecular dynamc tude and X-ray meaurement on oxde and alumnolcate urface tend to how that dffue layer water properte (denty, moblty, molecule orentaton) are very mlar to thoe of bulk water [18-20]. 4
6 Electrophoretc moblty meaurement are uually ued to determne the zeta potental. However, zeta potental etmaton can be erroneou due to the uncertanty concernng the value of the converon factor ued [12]. Th could explan the oberved hft of the hear-plane poton a a functon of the onc trength n urface complexaton model. Numerou author [18-26] emphaze that the anomalou urface conductvty of partcle mght explan the low zeta potental value (n ampltude) determned from electrophore compared to value etmated by urface complexaton model at the OHP and electrcal conductvty meaurement. They alo tate that the lateral moton of adorbed counteron at the Stern layer mut not be dregarded for ome materal havng a large exce of electrcal charge at ther urface (lke clay mneral or latex upenon). Lttle work ha been done to characterze the urface conductance due to the Stern layer for ttanum doxde nanopartcle [27]. In addton, the urface conductvty of TO 2 nanopartcle may be trong due to the mall ze of the elementary partcle. The am of th work to tudy the poble nfluence of TO 2 nanopartcle urface conductvty on t electrophoretc moblty n the hope that th wll lead to zeta potental value n agreement wth thoe predcted by electrotatc urface complexaton model (wthout conderng large varaton of the dtance between the outer boundary of the compact layer and the nner boundary of the dffue layer). Surface pecaton model are needed to calculate the urface conductance of TO 2. For th reaon, the model developed recently by Panagotou et al. [1] and alternatve model are crtcally evaluated n the econd chapter of th tudy. In the thrd chapter, we dcu the theore ued to convert electrophoretc moblty to zeta potental and calculate urface conductance due to the double layer. In the fourth chapter, we decrbe our modelng 5
7 trategy and ue the propoed methodology to etmate the ntrnc zeta potental of ttanum doxde P25 n a NaCl electrolyte from electrophore. 2. Electrotatc model for ttanum oxde 2.1. Panagotou et al. model (2008) Panagotou et al. [1] propoed a Trple Plane Model (TPM, [6]) for the ttanum oxde (P25) old-oluton nterface n NaNO 3 and KNO 3 electrolyte oluton. Th model baed on a tate-of-the-art decrpton of the TO 2 urface properte wth regard to protonaton-deprotonaton procee ung the recent ab-nto calculaton and DFT development for th materal. Two man urface functonal group were found to be reponble for the urface reactvty: T 2 O H + T 2 OH log K 1, (1) TO H + TOH log K 2. (2) Total urface te denty wa fxed at a value of 5.6 te nm -2 obtaned from crytallographc conderaton. Total urface area wa 50 m 2 g -1 accordng to BET meaurement. The urface onzaton model wa then combned wth a trple plane model, and the predcton were ued together wth potentometrc ttraton, mcroelectrophore and treamng potental experment to decrbe the electrochemcal properte of the TO 2 urface. Both urface te were condered to behave mlarly wth a gven caton (Na + and K + ) or anon (NO - 3 ). Th mplfcaton enabled the author to reduce the number of adjutable parameter for ther model: T 2 O Na + T 2 O Na + log K Na, (3) 6
8 TO Na + TO Na + log K Na, (4) T 2 OH NO 3- T 2 OH NO 3 - log K NO3, (5) TOH NO 3- TOH NO 3 - log K NO3. (6) In the trple plane model, the charge of orbng caton and anon not attrbuted to only one electrotatc plane but dtrbuted over the three plane (0, 1, 2), thu addng two addtonal fttng parameter (Δz 0 and Δz 1 or Δz 1 and Δz 2 ) for each orbed pece at each urface te (Fg. 1). Agan, both urface te were condered to behave mlarly wth a gven caton or anon. Fg. 1. Schematc drawng of the bac Stern model of Bourka et al. [9], our bac Stern model, the trple plane model of Panagotou et al. [1], and our extended Stern model for a negatvely charged urface of ttanum doxde. At a gven pcture, from left to rght: metal on, urface hydroxyl, prmary and econdary water layer, compact layer, dffue layer. 7
9 The parameter of th model are gven for a NaNO 3 electrolyte background n Table 1. We reproduced ther model ung PHREEQC v2.17 [28]. We were not able to reproduce ther ttraton curve ung the parameter n the reference publcaton. The tabulated protonaton/deprotonaton contant (log K 1 and log K 2 ) had to be changed lghtly n order to obtan reult n full agreement wth the data. Hereafter, we refer to th modfed model a Reference model (Table 1). Surface charge predcton are gven n Fg. 2 together wth urface potental at the 2-plane, whch condered to be the head end of the dffue layer. Th parameter can be compared to the zeta potental. Fg. 2. TO 2 urface charge and potental at the head end of the dffue layer predcted by dfferent urface complexaton model at three onc trength I n NaNO 3. Lne depct the reference model (TPM) reult whle ymbol depct the reult of alternatve model (BSM, ESM, Bdentate, Table 1). 8
10 Panagotou et al. [1] reported zeta potental value far below the potental value of the 2-plane (n abolute value). They nterpreted th to be a conequence of a hft of the hear plane (where the zeta potental located) from th 2-plane. Whle calculatng the dtance d from the 2-plane to the hear plane, they found a log-log lnear relatonhp between the onc trength I (n M) and d (n nm): log d = log I (7) 2.2. Alternatve model Whle the data of Panagotou et al. [1] could be very well reproduced by ther TPM model, t could alo be reproduced wth the mpler Bac Stern Model (BSM). The BSM parameter (Table 1) gve urface charge reult nearly dentcal to thoe of the TPM reference model n NaNO 3 electrolyte (Fg. 2) although the BSM requre only fve fttng parameter ntead of ten for TPM. Th rae the queton of the unquene of the TPM parameter et. In prncple, th problem can be overcome by fttng a large range of data obtaned under the ame expermental condton but tetng dfferent electrolyte type. Panagotou et al. [1] provded ttraton data n a KNO 3 electrolyte background and were able to ft them adequately only by attrbutng an aocaton contant (log K K = -1.1) and a charge dtrbuton (Δz 1 K = 0, Δz 2 K = 1) for K + at the urface, whle keepng the other parameter contant. A good agreement can alo be obtaned wth the BSM model (Fg. 3) addng only an aocaton contant (log K K = - 1.7) for K +. There a dcrepancy between the ttraton predcton of the two model at M, but the dfference ncreae wth alnty, and are therefore greater and gnfcant for a alnty of 0.3 M and ph > 9.5. Conequently, the addton of fve fttng parameter for the trple plane model rele on a very retrcted ubet of expermental data pont. 9
11 Fg. 3. TO 2 urface properte predcted by dfferent urface complexaton model at three onc trength n KNO 3. Lne depct the TPM reference model reult whle ymbol depct the reult of alternatve model (Table 1). The rght de of Fg. 2 and Fg. 3 how that the choce of a gven model nfluence the predcton of the electrcal potental at the head end of the dffue layer. For thee two model, however, predcted potental are much hgher n ampltude than reported zeta potental from electrophore [3], corroboratng the hypothe of a hear plane located n the dffue layer. A an alternatve model, an Extended Stern Model (ESM) can reproduce perfectly the reference TPM for urface charge but dmnh the potental at the head end of the dffue layer. One et of th model parameter, amongt thoe teted uccefully, gven n Table 1. Correpondng ttraton and potental curve are hown n Fg. 2 and Fg.3 for NaNO 3 and KNO 3 electrolyte background, repectvely. Accordng to Panagotou et al. [1], the hgh capactance value of the reference TPM are n agreement wth theoretcal and expermental tude concernng the locaton of the 10
12 frt two water overlayer and the electrolyte counteron at the rutle urface, taken here a a good analogue of the anatae urface [29-34]. Predota and Vlcek and Predota et al. [31, 33] howed that the frt hydraton layer at the rutle urface ~1.8 Å from the urface termnal oxygen atom and that Na + caton are located at ~1 1.8 Å. Thee dtance are n agreement wth the capactance value C 1 = 3.2 F m -2 that locate 1-plane at d 1 = 1.7 Å from the 0-plane, whle conderng the followng equaton wth the mean relatve delectrc contant, ε r,1, equal to 60 (ε 0 the delectrc contant of the vacuum, C V -1 m -1 ) : C 1(2) ε 0ε r,1(2) =, d 1(2) (8) where C 2, d 2 and ε r,2 are the capactance, dtance and the mean relatve delectrc contant between the 1- and 2-plane, repectvely. The propoed ESM model ha a lower capactance C 1 = 2.5 F m -2. It could therefore be condered to not be n agreement wth above reult. However, parameter ε r,1 ll-defned. Takng a value of ε r,1 = 47 enable u to arrve at the ame dtance d 1 wth the ESM model. Conequently, avalable data at the molecular level combned wth ttraton data cannot be ued to determne whch the bet repreentatve model nce both ε r,1 value are reaonable [35]. We were, however, not able to acheve a good ft of ttraton data wth model havng a C 1 value lower than 2.5 F m -2. Th eem to confrm the preence of a layer wth a hgh capactance and Na orpton cloe to the urface. On the contrary, C 2 could be et at a value a low a 1 F m -2 n the ESM, n marked contrat wth the 4.2 F m -2 value of the reference TPM. The poton of the econd plane alo ubject to dcuon. Panagotou et al. [1] condered that the econd plane end the compact layer at a dtance of 3.4 Å from the urface. However, molecular dynamc calculaton how gnfcant water denty ocllaton up to 12 Å from the urface aocated wth 11
13 contnuou vcoty change [33]. A a conequence, the choce of a prece locaton for the econd plane cannot be ealy jutfed. Moreover, the calculaton of the capactance value for the regon between the 1- and 2-plane rele heavly on the modeler choce for ε r,2. Theoretcal calculaton ndcate that the greatet porton of orbed Na + form bdentate complexe. We tred to roughly ncorporate th nformaton by conderng the followng equlbra n another alternatve model (the Bdentate model n Table 1): 2 T 2 O Na + (T 2 O ) 2 Na + log K Na, (9) 2TO Na + (TO ) 2 Na + log K Na. (10) The charge of counter Na + wa attrbuted partly to the 0-plane and partly to the 1-plane, nce Na + wa alo hown to be partly dehydrated at the urface and engaged n nner phere complexe [34]. Fg. 2 agan how that th model can match almot perfectly the reference TPM ttraton predcton. Note alo that, dependng on the choen urface complexaton model,.e. TPM, BSM, ESM, or Bdentate, there may be dfferent locaton and then defnton of the tagnant layer (whch not necearly a monolayer of orbed counteron). For example, n the cae of a NaNO 3 electrolyte, the tagnant layer located between 1 and 2-plane for TPM, at the 1-plane for BSM, at the 1-plane for ESM and between the 0 and 1-plane for Bdentate Implcaton for mappng the "ttanum doxde/electrolyte oluton" nterface Th analy how that ttraton data combned wth urface complexaton model uch a TPM or ESM cannot yeld a unque and unambguou et of nterfacal parameter. Theoretcal calculaton and modelng at the molecular cale can help u valdate the model lkelhood but uncertante reman. 12
14 Th ugget that the relatonhp oberved between the poton of the hear plane and the onc trength (Eq. (7)) model dependant. Fg. 2 and Fg. 3 how that the reference TPM and the BSM predct nearly dentcal potental at the head end of the dffue layer, n agreement wth the fact that the reference TPM an mprovement on the BSM publhed earler by Bourka et al. [9]. However, the propoed ESM and Bdentate model predct lower potental (n abolute value) than the reference model. Predcted potental reman, however, hgher n abolute value than commonly reported zeta potental from electrophore. Conequently, the queton of the poton of the hear plane n relaton to the head end of the dffue layer reman. Zeta potental calculaton from electrophore are alo model-dependant. In the followng chapter, everal model to convert electrophoretc meaurement to zeta potental are brefly revewed. 3. From the electrophoretc moblty to the zeta potental 3.1. Electroknetc theore The mot well-known and wdely ued theory of electrophore wa developed by von Smoluchowk [36, 37]. He tuded the movement of the lqud adjacent to a flat, electrcally charged urface under the nfluence of an electrc feld appled parallel to the nterface. Von Smoluchowk [36] ued the Stoke equaton and calculated the electrcal (ung Poon equaton) and vcou force on an element of volume of the lqud to expre the electrophoretc moblty a a functon of the zeta potental. The von Smoluchowk equaton lnearly relate the electrophoretc moblty μ e (n m 2-1 V - 1 ) to the electrcal potental at the hear plane (ζ, n V): ε μ = ζ η e, (11) 13
15 where η the dynamc vcoty (n Pa ; η = Pa at T = 298 K) and ε the delectrc permttvty of water (ε = ε 0 ε r = F m -1 at T = 298 K). The von Smoluchowk equaton vald only f the thckne of the dffue layer ngnfcant compared to the ze of the partcle,.e. for thn double layer, κa >> 1, where κ the nvere of the Debye length (n m -1 ) and a the partcle radu (n m). The nvere of the Debye length gven by: κ = F 2 z εrt 2 c b, (12) κ = 2 F 2 I εrt, (13) where F the Faraday contant (96485 C mol -1 ), R the ga contant (8.314 J mol -1 K -1 ), c the on concentraton (n mol m -3 ), z ther valency, and I the onc trength. The ymbol b refer to the bulk on. For mall phercal partcle havng a thck double layer, κa << 1, the appled electrc feld not nfluenced by the preence of the partcle and the effect of the retardaton force due to the double layer on the mgraton of the partcle neglgble. Hückel [38] condered that the man retardaton force the frctonal retance of the medum. He uppoed that the electrcal conductvty of the partcle the ame a that of the urroundng medum. Therefore, the electrc feld not dtorted by the partcle. He wrote the followng equaton, whch vald for κa << 1: 2ε μ = ζ 3η e. (14) Henry [39, 40] revted Hückel theory by conderng that the conductvty of the partcle dfferent from that of the urroundng medum. In th cae, the appled electrc feld wll be dtorted o that the opotental value can be around the partcle 14
16 urface. Accordng to Henry [40], the partcle conductvty alter the hape of the potental dtrbuton of the appled feld n the lqud, modfe the flud moton wthn the electrcal double layer, and therefore change the tree of the flud exerted on the partcle. Conequently, th conductvty lead to the mutual dtorton of the appled feld and the feld of the double layer, and hence low the electrophoretc moton. For phercal partcle wth arbtrary double-layer thckne, Henry [40] wrote: 2ε μe = F ( κa, K p, K )ζ, 3η (15) [ f ( κ ) 1] F( κ a, K, K ) = 1 + 2λ a, (16) p 1 K = 2 + K p λ, p 2K + 2K (17) K p σ p =, σ b (18) K σ σ = =, b Σ aσ b (19) ( κa) 5( κa) ( κa) ( κa) f ( κ a) = a t κa κ exp( ) exp( ) dt, for κa < 1, 96 t f ( κa) = +, for κa > 1, κa 2κ a κ a (20) (21) where σ the electrcal conductvty (n S m -1 ), Σ the urface conductance of the electrcal double layer, the ubcrpt p,, b correpondng, repectvely, to the partcle nteror, the partcle urface and the urroundng medum (the bulk electrolyte). The urface conductance expree the exce of electrcal conductvty at the old urface compared to that of the bulk electrolyte. K correpond to the well- 15
17 known Dukhn number, Du (ee [18, 41] and [42] for more detal). Accordng to Eq. (15) to (17) and by replacng K wth Du, we obtan: 2ε 1 K p 2Du μ = η 2 + K p + 2Du [ f ( κa) ] ζ e. (22) In the abence of urface conductance and n the cae of an nulatng partcle, Henry theory lead to von Smoluchowk equaton for large κa value (f(κa) = 1.5) and Hückel equaton for κa << 1 (thck double layer, f(κa) = 1). For κa >> 1, f(κa) = 1.5, and Eq. (22) reduce to: 2ε 1 K μ e = 1 + 3η 2 + K p p 2Du ζ. + 2 Du (23) In the cae of nulatng partcle, K p = 0, and Eq. (23) correpond to O Bren formula [43] wthn the lmt of a DC appled electrc feld and dregardng the nertal term n h theory. O Bren [43] developed a complete pcture of the frequency dependent delectrc repone of a dlute upenon of phere wth thn double layer. Note that all of the equaton preented here, except Smoluchowk equaton, conder a Debye- Hückel onc atmophere,.e. that electrc potental n the dffue layer follow a Debye- Hückel dtrbuton. Conequently, the analytcal equaton that we ue to etmate the zeta potental from the electrophoretc moblty are vald for low zeta potental ( ζ 25.7 mv n the cae of 1:1 electrolyte at T = 298 K), but they can tll be appled for zeta potental of greater ampltude [12]. In addton, Eq. (11) to (23) do not conder pherodal partcle, partcle volume fracton, polydperty of the ample,.e. agglomerate of dfferent ze, and dffue layer overlappng. Several electroknetc model make t poble to etmate urface conductance for pherodal partcle (for example, [44]), conder polydpervty of the ample and nanopartcle agglomeraton [45], and dffue layer overlappng [46]. 16
18 Moreover, Mangeldorf and Whte numercal model, whch take nto account partcle ze effect and the adorpton of on and ther moblty n the nner part of the EDL [21], can be more accurate than the analytcal oluton we ue, epecally at low onc trength and at ph value far from the PZC where the ampltude of the urface electrc potental hgh. However, the electrcal conductvty of the partcle nteror σ p can be determned ung the o-called dfferental elf-content model [47, 48]. Th model conder mall contguty between the partcle and allow the determnaton of the electrcal conductvty of the agglomerate correpondng to the fnal concentraton of ncluon (here the elementary nanopartcle of conductvty σ e ) by addton of nfntemal porton of ncluon: σ σ b p σ + σ 1 e 2 σ = ( ) φ dω d, σ σ σ 0 e 1 Ω 3 (24) where φ the ntra-aggregate poroty, Ω = v/(v+v) the volume fracton of the elementary nanopartcle n the agglomerate wth v and V the total volume (n m -3 ) of elementary partcle and of water, repectvely. By ntegratng Eq. (24), we obtan: σ σ p b σ σ e e σ σ b p D = φ, (25) where D = 1/3 n the cae of phercal elementary partcle. Eq. (25) ha been generalzed to non-phercal partcle, but t not very practcal becaue t an equaton of the form σ = f D, φ, σ, σ, σ ). Revl [48] found an analytcal oluton of p ( b e p Eq. (25) by conderng D = 1/2 for dk-haped partcle: σ b σ = FΘ + 1 ( Θ) ( ) Θ + Θ + p FΘ F 2, (26) 17
19 σ e Θ = σ b 2Σ = a σ e b, (27) 2 F = φ. (28) Accordng to Revl [48], D = 1/2 correpond to a partcle hape uually found n mot porou meda. In addton, followng the approach of [45], the electrophoretc moblty due to the polydperty of the ample, ze, can be calculated by: μ e,.e. to agglomerate poeng dfferent N μe f ( a ) a Δa = 1 μ e = N, 3 f ( a ) a Δa = 1 3 (29) where N the number of dfferent rad, f(a ) repreent the dcretzed veron of the Partcle Sze Dtrbuton (PSD), Δa the radu nterval, and μ e the electrophoretc moblty of agglomerate wth radu a ± Δa /2. Accordng to Eq. (29), the electrophoretc moblty of the polydpere ytem the volume average of the moblte of partcle wth dfferent ze n the dtrbuton. Henry [40] and O Bren [43] condered that only the counteron n the dffue layer are reponble for the urface conductvty. They dd not conder the nfluence of the Stern layer on partcle urface conductvty [18-23]. Accordng to thee author, the zeta potental of (electrcally) charged upenon lke polytyrene lattce, determned from electrophore, are lower n ampltude than the zeta potental value etmated from electrcal conductvty meaurement. In the cae of electrophore, thee author aume that the lateral movement of on at the Stern layer n repone to the appled electrc feld can explan uch dcrepancy. The preence of moble counteron at the Stern layer and n the dffue layer lower the ampltude of zeta potental nferred from 18
20 moblty meaurement and rae thoe from conductvty meaurement, compared to the zeta potental that correpond to the ntrnc partcle charge. The anomalou urface conductance lnked to the Stern layer,.e. to on below the hear plane, can be determned for oxde mneral ung electrcal conductvty meaurement [25, 27, 42, 49] or a urface complexaton model lke a Trple Layer Model (TLM, [15, 50]) where the aocated equlbrum orpton contant are calbrated by ttraton experment. Surface complexaton model calculate the exce of counteron at the nterface and thereby make t poble to etmate the (pecfc) urface conductance of the partcle [50, 51] Determnaton of the urface conductance The dffue layer urface conductance The urface conductance of the Electrcal Double Layer (EDL) the enhanced conductvty due to the preence of a double layer at the partcle urface ([40, 49-51]; ee Fg. 4). We conder here the urface conductance due only to the preence of the dffue layer at the partcle urface. In the followng chapter, we wll conder the nfluence of the Stern layer on the urface conductance. 19
21 Fg. 4. Schematc repreentaton of the electro-chemcal properte of a upenon of phercal oxde nanopartcle. The partcle ha a local exce of electrcal conductvty at t nterface σ(χ). The urface conductance Σ etmated by ntegratng σ(χ) - σ b over the thckne of the Stern and dffue layer. Accordng to Revl and Glover [51], the urface conductance Σ (n S) can be decrbed by the ndvdual pecfc urface conductance of each on n the dffue layer, Σ = ez Σ. (30) Σ, The total urface conductance Σ made up of an electro-mgraton, Σ e, and an electro-omotc urface conductance, e o o Σ, Σ = Σ + Σ. (31) The electro-mgraton urface conductance due to the exce of Ohmc conductvty n the EDL [15], 20
22 χ [ σ ( χ σ ] dχ Σ = 0 ), e D b (32) where χ repreent the urface/oluton dtance n m and χ D the total thckne of the dffue layer (uually, χ D = 2κ -1 ). The parameter σ(χ) and σ b are the electrcal conductvty at the old/oluton nterface and n the bulk pore water defned, repectvely, by: σ ( χ) = Fz β c ( χ), b b σ b = Fzβ c, (33) (34) where β the on moblty (n m 2-1 V -1 ) at the partcle urface and n the bulk pore, b water. The electrcal conductvty of the oluton due to electro-mgraton gven by an equvalent Ohm law determned ung the generalzed Nernt-Planck equaton and the electrochemcal potental equaton for the on pece [14, 52] (electro-omo neglected). The local electrcal conductvty σ(χ) gven by analogy wth the free electrolyte conductvty ([51]). By ncorporatng Eq. (33) and (34) n Eq. (32), we obtan: b b [ β c ( χ) β c ] e D Σ = Fz dχ. χ 0 (35) Accordng to Eq. (35), by conderng the ame on moblty at the nterface (dffue b layer here) and n the bulk pore water,.e. β = β = β, we can determne e Σ [15, 53]: b [ c ( χ) c ] D zβ χ e Σ = F dχ, 0 χ e b D ± zfψ ( χ) Σ = F zβ c exp 1 dχ, 0 RT (36) (37) ( ) ψ ( χ) = ψ d exp κχ, (38) 21
23 where ψ d the electrc potental at the head end of the dffue layer. The term ± correpond to the gn of the electrcal charge aocated wth the pece ( + for caton and for anon). It relatvely eay to fnd an analytcal oluton for the on contrbuton to the urface conductance ung Eq. (30), (37)-(38) and lnearzng the exponental functon of the Boltzmann dtrbuton ([51] and Eq. (191) to (194) of [54]): Σ Σ e e 1 b ± zfψ d 2κ F zβ c exp 1, 2RT 1 b ± zfψ d 2κ β Na c exp 1, 2RT (39) (40) where Na the Avogadro Number ( te mol -1 ) and by conderng a dffue layer havng a thckne of χ = 2κ 1 D. At the urface of the ttanum doxde partcle (for condton other than the IEP and for low onc trength, typcally 0.01 M for oxde mneral, [10]), counter-on are predomnant n the dffue layer. When an electrc feld appled, t reult n a olvent convecton and, conequently, a urplu conductvty called electro-omotc conductvty. The electro-omotc contrbuton to the urface conductance decrbed by [51]: o D Σ = ρ( χ) β ( χ) dχ, χ 0 o (41) o o Σ = ez Σ, (42) ( χ ) = ± ρ F ( 1) z c ( χ), (43) ε βo( χ) = [ ψ ( χ) ψ d ], η (44) 22
24 where β o the electro-omotc moblty (n m 2-1 V -1 ) and ρ the volume charge denty n the dffue layer (n C m -3 ). Accordng to Bkerman [55]: Σ o 4εRT 1 κ Na η b ± zfψ d c exp 2RT 1. (45) The on contrbuton of the electro-omotc urface conductance determned ung Eq. (42) and (45): Σ o 4εRT κ ηez 1 Na c b ± zfψ d exp 2RT 1. (46) By combnng Eq. (40) and (46), the total on contrbuton to the urface conductance gven by: Σ B 1 b ± zfψ d = 2κ B Na c exp 1, 2RT 2εRT = β +. ηez (47) (48) In Eq. (47) and (48), we have aumed that the on moblte are the ame n the bulk electrolyte and n the dffue layer. Thee equaton correpond thoe of Bkerman [55] for characterzng the on contrbuton to the urface conductance of the partcle (due to the appled electrc feld). In h approach, the moble counteron and coon n the dffue layer are only reponble for the urface conductance. Moreover, accordng to Eq. (48), the onc electro-omotc contrbuton to the urface conductance mut not be neglected. For example, for a NaCl oluton and at T = 298 K, the Na + and Cl - on moblty value n the bulk electrolyte are and m 2-1 V -1, repectvely [15]. The econd term n Eq. (48), for z = 1, ε = F m -1, η = Pa, F = C mol -1, R = J mol -1 K -1, and T = 298 K, m 2-1 V -1, whch of the ame order of magntude a the frt term. 23
25 For a bnary ymmetrc electrolyte, z + = z - = z, and the urface conductance determned ung Eq. (30) and (47): Σ 1 b Fzψ d = 4Fzκ c B coh 1, 2RT (49) aumng that B ( + ) = B ( + ) = B, (50) and c = b b b ( + ) = c ( ) c. (51) Eq. (49) aume that both anon and caton have the ame moblty. By ung Eq. (19), (34), (49), and the Nernt-Enten relatonhp for on dffuvty, D RTβ =, Fz (52) we obtan Bkerman equaton [25, 49, 55]: 2 Fzψ d Du = coh κa 2RT 2ε ηd RT Fz 2. (53) In Eq. (53), the nfluence of ph on urface conductvty not taken nto account. Takng nto conderaton the preence of H + and OH - on, we obtan the followng equaton for urface conductance, accordng to Eq. (30), (47), and for a bnary ymmetrc background electrolyte lke NaCl or KCl [50]: Σ 2κ Fz b ph ( c B B ) 1 = ( + ) ( + ) H + Fzψ d exp 1 2RT ph p K ( c B + 10 B ) Fz exp 2RT, b ψ f d + ( ) ( ) 1000 OH 1 (54) where pk f the negatve log of the docaton contant of water (14 at T = 298K). 24
26 The Stern layer and total urface conductance For ome hghly-charged mneral lke clay, the contrbuton of the compact Stern layer to urface conductvty mut not be neglected [53]. However, t ha not been extenvely tuded for ttanum doxde nanopartcle. Accordng to everal author (e.g. [18-23]), both the dffue and Stern layer contrbute to the pecfc urface conductance: Σ = Σ Σ. (55) dffue + Stern The Stern layer contrbuton can be decrbed by [50, 53]: Stern St St Σ = zeβ Γ, (56) where the upercrpt St correpond to the Stern layer, and Γ St the urface te denty of adorbed counteron at the Stern layer (n te m -2 ). Eq. (56) decrbe the lateral movement of adorbed caton and anon [56, 57], and conequently aume that the on pece are not mmoble n the Stern layer [16]. The on moblty value at the Stern layer are tll relatvely unknown. For clay mneral and quartz, Revl and Glover [50] and Revl et al. [58] condered that the on moblte of everal caton lke Na +, K +, Ca 2+ are at leat one order of magntude maller than thoe n the bulk electrolyte. For lca mneral, Leroy et al. [15] have hypothezed that the urface on moblte are mlar to thoe of the bulk. Molecular dynamc mulaton at the old/water nterface mut be of partcular mportance to characterze the on and water moblte n the compact layer. For example, n ther work concernng the characterzaton of the urface properte of rutle, Predota et al. [32] condered a urface water moblty that 10% of the bulk on moblty at 3.7 Å from the urface. Accordng to Eq. (54) to (56), the total urface conductance of a partcle can be decrbed by: 25
27 Σ 2κ Fz b ph ( c B B ) 1 = ( + ) ( + ) + H Fzψ d exp 1 2RT b ph p K Fzψ f d ( c ) B( ) + 10 B ) exp 1 + OH 1000 zeβ 2RT St St + ( Γ. (57) A een by Eq. (57), t eay to etmate urface conductance f the electrcal potental at the head end of the dffue layer ψ d and the urface te S t Γ are known. Thee parameter can be etmated by a complete urface complexaton model of the S t TO 2 /water nterface. The on moblte of the counteron n the compact layer β are the only unknown. Below, we ue th approach to determne the zeta potental of TO 2 nanopartcle ung the partcle ze and the electrophoretc moblty data of Foy [3]. 4. Comparon wth expermental data 4.1. Modelng trategy Frtly, the functon f(κa) determned accordng to Eq. (12), (20) and (21). Secondly, ttanum doxde urface complexaton model etmate the urface conductance of the double layer Σ (Eq. (57)). The ubequent calculaton of the electrcal conductvty of the bulk electrolyte baed on t on compoton, σ b, (Eq. (34)) and the radu of an agglomerate made up of elementary partcle, a, make t poble to determne the Dukhn number, Du (Eq. 19). Thrdly, the parameter correpondng to the electrcal conductvty of the agglomerate nteror, K p, calculated thank to Eq. (26) to (28) for a gven value of the ntra-aggregate poroty φ and elementary partcle radu a e. Fnally, the zeta potental etmated from the electrophoretc meaurement ung the calculated value of f(κa), Du, K p, and Eq. (22) for the cae of Henry theory (ee Fg. 5). By comparon, the von Smoluchowk equaton (Eq. (11)) alo ued to convert 26
28 electrophoretc moblte to zeta potental value. The urface complexaton calculaton were done wth PHREEQC v2.17 [28] and the converon calculaton were done ung a MATLAB routne. Fg. 5. Modelng trategy for determnng the zeta potental from electrophore. Raw electrophoretc meaurement are neceary for thee calculaton. Unfortunately, only pre-proceed zeta potental are avalable n mot publcaton. Foy [3] provde electrophoretc meaurement for ttanum doxde P25 for a wde range of electrolyte concentraton but n a NaCl electrolyte background. Accordng to the comparatve modelng of Bourka et al. [9], NO - 3 and Cl - behave mlarly at the TO 2 urface. Th could be verfed by atfactorly reproducng the urface charge data that Rdley et al. [2] obtaned on the anatae ST-01 ung the ame model a the ESM lted n Table 1 and the ame parameter for Cl - a for NO - 3 (not hown) Input data of the electroknetc model Foy [3] dd ttraton and electrophoretc meaurement on TO 2 P25 (Degua, Germany). The crytallne form anatae (95 %). The denty meaured by helum 27
29 pycnometry after dryng 3.76 g cm -3. The etmated pecfc urface area (ung N 2 adorpton and mmeron calormetry) 54 ± 3 m 2 g -1. The ze of an elementary partcle approxmately 30 nm, but P25 nanopartcle can form aggregate meaurng everal mcrometer [1]. Foy [3] meaured electrophoretc moblte for ph value compred between 4 and 11, and alnte (NaCl) of 10-4, 10-3, and 10-2 M. Furthermore, he ued X-ray meaurement to obtan the partcle ze dtrbuton a a functon of ph at 10-3 M. At 10-3 M, we ue a normal dtrbuton n agreement wth the partcle ze dtrbuton meaured by Foy [3] and calculate the Debye length to etmate the functon f(κa). For other alnte, we alo ue a normal dtrbuton for the partcle ze. Eq. (12), (48) and (57) make t poble to calculate the pecfc urface conductance ung the value of ψ 2 (ψ 2 = ψ d here), Γ St Na, Γ St Cl determned by the ESM urface complexaton model (Fg. 6). The Na +, Cl -, H + and OH - on moblty value n the bulk and dffue layer (β ) are 5.17, 7.89, 36.20, and m 2-1 V -1, repectvely (from PHREEQC phreeqd.dat databae). The on moblte at the Stern layer reman unknown. We ued β = β, accordng to the urface conductvty model of Leroy et St al. [15] for lca mneral, to etmate urface conductance. Surface conductance ncreae gnfcantly wth alnty and wth the dfference between ph and IEP (6.5 here) (Fg. 6). Note that we retrct the nvetgated ph range at 10-4 and 10-3 M to have a contant onc trength. 28
30 Fg. 6. The electrcal potental at the 2-plane, the urface te dente of adorbed odum and chlorde at the Stern layer (1-plane), and the urface conductance veru ph for three alnte (NaCl). Accordng to Eq. (19), the Dukhn number (Du) determned ung (1) a normal dtrbuton for the partcle ze a (n agreement at 10-3 M wth the partcle ze dtrbuton meaured by Foy [3]), (2) the etmated urface conductance Σ, and (3) the electrcal conductvty of the bulk electrolyte σ b. The fttng parameter are the ntraaggregate poroty φ and the PSD at 10-4 and 10-2 M Zeta potental from electrophoretc data Accordng to the partcle ze meaurement of Foy at 10-3 M [3] (Fg. 7), ttanum doxde nanopartcle are hghly agglomerated at ph value cloe to the IEP, leadng to 20-µm agglomerate. At extreme ph value (ph = 4, ph =10) agglomerate reach ze 29
31 compred between one and three mcrometer. Thu, κa >>1 and ncreae gnfcantly at ph value cloe to the IEP. A a reult, the Dukhn number low n ampltude except for bac ph where t ncreae trongly (Fg. 7). Zeta potental nferred from electrophore ung our model are n very good agreement wth the ESM predcton aumng ψ 2 = ψ d (Fg. 7). We ue φ = 0.4, whch n accordance wth the ntraaggregate poroty value meaured recently by Xu et al. [59] for P25 (φ = 0.38) from the N 2 deorpton otherm ung the cylndrcal pore model (BJH method). The zeta potental calculaton ung the Smoluchowk equaton are alo preented by comparon. Ther ampltude are not n agreement wth the ESM predcton becaue the Smoluchowk equaton neglect urface conductvty. PSD found at other alnte eem to be phycally realtc (Fg. 8). Sze of agglomerated nanopartcle ph-and alnty-dependent, and ncreae gnfcantly cloe to the IEP and at hgh onc trength. The Dukhn number alo dmnh gnfcantly n thee phyco-chemcal condton. In addton, for 10-4 and 10-2 M, zeta potental calculated from electrophore ung our modelng approach are alo n very good agreement wth the ESM predcton (Fg. 9), except for ph value cloe to the IEP. We ue φ = 0.4. Our modelng reult ugget that the hear plane may be located cloe to the OHP, n contradcton wth the hypothe of a tagnant dffue layer havng a alnty-dependant thckne at the TO 2 water nterface [1, 9]. 30
32 Fg. 7. Input data of the model of Henry [40] and zeta potental veru ph at 10-3 M from electrophore (full trangle, our model; empty trangle, Smoluchowk equaton predcton). The lne on zeta potental fgure the ESM predcton aumng ψ 2 = ψ d. Fg. 8. Input data of the model of Henry [40] veru ph at 10-4, 10-3, and 10-2 M. 31
33 Fg. 9. Zeta potental veru ph at 10-4, 10-3, and 10-2 M from electrophore. The lne are the ESM predcton aumng ψ 2 = ψ d. 5. Concluon We have developed an extended Stern layer model to characterze the electrochemcal properte of ttanum doxde n a 1:1 electrolyte (NaCl). Th model gnfcantly lower the ampltude of electrcal potental at the OHP compared to that of other recent urface complexaton model [1, 9] wthout alterng the qualty of the ttraton data predcton. Henry model [40] ued to convert electrophoretc moblty meaurement to zeta potental value takng nto account agglomerate ze and urface conductance. Electrcal conductvty nde the agglomerate calculated ung the dfferental elfcontent model of Sen et al. [47]. The theory of partcle urface conductance due to the dffue and Stern layer decrbed n depth. By combnng the exce of electrcal charge calculated at the compact Stern layer and n the dffue layer wth Henry equaton [40], we how that the hear plane may be located cloe to the OHP, contradctng the aumpton of the preence of a tagnant dffue layer at the TO 2 /water nterface a mentoned by Bourka et al. [9] and Panagotou et al. [1]. 32
34 In the future, we wll ue a numercal model to convert electrophoretc and electrcal conductvty meaurement to zeta potental value under arbtrary condton ncludng hgh zeta potental ampltude, partcle volume fracton, polydperty of the ample, dffue layer overlappng, and urface conductance. In addton, we wll compare our modelng reult to a much more refned et of experment (phercal and monodpere/polydpere partcle) ncludng electrcal conductvty meaurement. Acknowledgment Th tudy wa done wthn the framework of the NANOSEP Project (ANR-08-ECOT- 009). The author are grateful to French Natonal Reearch Agency for fnancal upport. 33
35 Reference 1. G. D. Panagotou; T. Pet; K. Bourka; C. S. Garoufal; A. Tev; N. Spano; C. Kordul; A. Lycourghot, Advance n Collod and Interface Scence 2008, 142, (1-2), M. K. Rdley; V. A. Hackley; M. L. Macheky, Langmur 2006, 22, (26), A. Foy. Analy of adorpton of on and polyacrylc acd n aqueou dperon of ttane doxde. PhD the, Faculté de Scence et de Technque de l'unverté de Franche-Comté-Beançon, A. Foy; A. Mpandou; J. M. Lamarche; N. Jaffrezcrenault, Collod and Surface 1982, 5, (4), N. Kallay; M. Colc; D. W. Fuertenau; H. M. Jang; E. Matjevc, Collod and Polymer Scence 1994, 272, (5), T. Hemtra; W. H. Van Remdjk, Journal of Collod and Interface Scence 1996, 179, T. Hemtra; P. Venema; W. H. Van Remdjk, Journal of Collod and Interface Scence 1996, 184, N. Saha; D. A. Sverjenky, Geochmca Et Comochmca Acta 1997, 61, (14), K. Bourka; T. Hemtra; W. H. Van Remdjk, Langmur 2001, 17, (3), D. A. Sverjenky, Geochmca et Comochmca Acta 2005, 69, (2), J. Lyklema, Fundamental of nterface and collod cence: Lqud-flud nterface. Academc Pre: London, R. J. Hunter, Zeta Potental n Collod Scence: Prncple and Applcaton. Academc Pre: New York, B. V. Derjagun; L. Landau, Acta Phyco-Chmca (URSS) 1941, 14, A. Revl; P. Leroy, Journal of Geophycal Reearch-Sold Earth 2004, 109, B03208, /2003JB P. Leroy; A. Revl; A. Kemna; P. Coenza; A. Ghorban, Journal of Collod and Interface Scence 2008, 321, (1), C. Tournaat; Y. Chapron; P. Leroy; M. Bz; F. Boulahya, Journal of Collod and Interface Scence 2009, 339, P. Leroy; A. Revl; S. Altmann; C. Tournaat, Geochmca et Comochmca Acta 2007, 71, (5), J. Lyklema; S. S. Dukhn; V. N. Shlov, Journal of Electroanalytcal Chemtry 1983, 143, (1-2), C. F. Zukok; D. A. Savlle, Journal of Collod and Interface Scence 1986, 114, (1), C. F. Zukok; D. A. Savlle, Journal of Collod and Interface Scence 1986, 114, (1), C. S. Mangeldorf; L. R. Whte, Journal of the Chemcal Socety-Faraday Tranacton 1990, 86, (16), C. S. Mangeldorf; L. R. Whte, Journal of the Chemcal Socety-Faraday Tranacton 1998, 94, (17), C. S. Mangeldorf; L. R. Whte, Journal of the Chemcal Socety-Faraday Tranacton 1998, 94, (16), V. E. Shubn; R. J. Hunter; R. W. Obren, Journal of Collod and Interface Scence 1993, 159, (1),
36 25. J. Sonnefeld; M. Lobbu; W. Vogelberger, Collod and Surface a- Phycochemcal and Engneerng Apect 2001, 195, (1-3), M. L. Jmenez; F. J. Arroyo; F. Carrque; A. V. Delgado, Journal of Collod and Interface Scence 2007, 316, (2), N. Spano; A. Tev; P. G. Koutouko; M. Mnor; A. van der Lnde; J. Lyklema, Collod and Surface a-phycochemcal and Engneerng Apect 1998, 141, (1), D. L. Parkhurt; C. A. J. Appelo 1999, Uer' gude to PHREEQC (Veron 2) - A computer program for pecaton, batch-reacton, one-dmenonal tranport, and nvere geochemcal calculaton. 29. A. Tlocca; A. Sellon, Langmur 2004, 20, (19), A. Tlocca; A. Sellon, Journal of Phycal Chemtry B 2004, 108, (15), M. Predota; A. V. Bandura; P. T. Cummng; J. D. Kubck; D. J. Weolowk; A. A. Chalvo; M. L. Macheky, Journal of Phycal Chemtry B 2004, 108, (32), M. Predota; P. T. Cummng; D. J. Weolowk, Journal of Phycal Chemtry C 2007, 111, (7), M. Predota; L. Vlcek, Journal of Phycal Chemtry B 2007, 111, (5), M. Predota; Z. Zhang; P. Fenter; D. J. Weolowk; P. T. Cummng, Journal of Phycal Chemtry B 2004, 108, (32), T. Hemtra; W. H. Van Remdjk, Journal of Collod and Interface Scence 2006, 301, (1), M. Von Smoluchowk, n: Handbuch der Elektrztat und de Magnetmu, L. Graetz, (Ed.) Barth, Lepzg, 1921; Vol. II, pp M. Von Smoluchowk, Bulletn Internatonal de l Academe de Scence de Cracove 1903, 8, E. Hückel, Phykalche Zetchrft 1924, 25, D. C. Henry, Proc. R. Soc. Lond. A 1931, 133, D. C. Henry, Tranacton of the Faraday Socety 1948, 44, S. S. Dukhn; V. N. Shlov, Delectrc phenomena and the double layer n dpere ytem and polyelectrolyte. John Wley and Son: New York, A. Crepy; A. Boleve; A. Revl, Journal of Collod and Interface Scence 2007, 305, (1), R. W. O'Bren, Journal of Collod and Interface Scence 1986, 113, (1), R. W. Obren; D. N. Ward, Journal of Collod and Interface Scence 1988, 121, (2), S. Ahuall; F. J. Arroyo; A. V. Delgado, Journal of Collod and Interface Scence 2010, 343, (1), F. Carrque; F. J. Arroyo; A. V. Delgado, Journal of Collod and Interface Scence 2002, 252, (1), P. N. Sen; C. Scala; M. H. Cohen, Geophyc 1981, 46, (5), A. Revl, Journal of Geophycal Reearch-Sold Earth 2000, 105, (B7), R. W. O'Bren; D. W. Cannon; W. N. Rowland, Journal of Collod and Interface Scence 1995, 173, (2), A. Revl; P. W. J. Glover, Geophycal Reearch Letter 1998, 25, (5), A. Revl; P. W. J. Glover, Phycal Revew B 1997, 55, (3), S. R. De Groot; P. Mazur, Non-equlbrum thermodynamc. North-Holland Pub. Co.: Amterdam,
37 53. P. Leroy; A. Revl, Journal of Collod and Interface Scence 2004, 270, (2), S. Prde, Phycal Revew B 1994, 50, (21), J. J. Bkerman, Tranacton of the Faraday Socety 1940, 36, C. T. O Konk, Journal of Phycal Chemtry 1960, 64, J. M. Schurr, Journal of Phycal Chemtry 1964, 68, A. Revl; L. M. Cathle; S. Loh; J. A. Nunn, Journal of Geophycal Reearch- Sold Earth 1998, 103, (B10), J. H. Xu; W. L. Da; J. L; Y. Cao; H. L; K. Fan, Journal of Photochemtry and Photobology a-chemtry 2008, 195,
38 Table 1. Surface and nterface parameter for the Panagotou et al. model [1] and alternatve model. Parameter Orgnal Reference BSM ESM Bdentate Panagotou et model (TPM) al. model log K log K log K Na (K) -1.7 (-1.1) -1.7 (-1.1) -1.7 (-1.7) -1.4 (-1.1) -1 log K NO * Δz 0 Na Δz 1 Na (K) 0.7 (0) 0.7 (0) n.a. 1 (0.7) 0.45 (0.7) Δz 2 Na (K) 0.3 (1) 0.3 (1) n.a. 0 (0.3) 0 (0.3) Δz 1 NO n.a Δz 2 NO n.a. 0 0 C 1 (F m -2 ) C 2 (F m -2 ) * In the BSM, NO - 3 anon act a ndfferent anon 37
39 Fgure capton Fg. 1. Schematc drawng of the bac Stern model of Bourka et al. [9], our bac Stern model, the trple plane model of Panagotou et al. [1], and our extended Stern model for a negatvely charged urface of ttanum doxde. At a gven pcture, from left to rght: metal on, urface hydroxyl, prmary and econdary water layer, compact layer, dffue layer. Fg. 2. TO 2 urface charge and potental at the head end of the dffue layer predcted by dfferent urface complexaton model at three onc trength n NaNO 3. Lne depct the reference model (TPM) reult whle ymbol depct the reult of alternatve model (BSM, ESM, Bdentate, Table 1). Fg. 3. TO 2 urface properte predcted by dfferent urface complexaton model at three onc trength n KNO 3. Lne depct the TPM reference model reult whle ymbol depct the reult of alternatve model (Table 1). Fg. 4. Schematc repreentaton of the electro-chemcal properte of a upenon of phercal oxde nanopartcle. The partcle ha a local exce of electrcal conductvty at t nterface σ(χ). The urface conductance Σ etmated by ntegratng σ(χ) - σ b over the thckne of the Stern and dffue layer. Fg. 5. Modelng trategy for determnng the zeta potental from electrophore. Fg. 6. The electrcal potental at the 2-plane, the urface te dente of adorbed chlorde and odum at the Stern layer (1-plane), and the urface conductance veru ph for three alnte (NaCl). Fg. 7. Input data of the model of Henry [40] and zeta potental veru ph at 10-3 M from electrophore (full trangle, our model; empty trangle, Smoluchowk equaton predcton). The lne on zeta potental fgure the ESM predcton aumng ψ 2 = ψ d. 38
Introduction to Interfacial Segregation. Xiaozhe Zhang 10/02/2015
 Introducton to Interfacal Segregaton Xaozhe Zhang 10/02/2015 Interfacal egregaton Segregaton n materal refer to the enrchment of a materal conttuent at a free urface or an nternal nterface of a materal.
Introducton to Interfacal Segregaton Xaozhe Zhang 10/02/2015 Interfacal egregaton Segregaton n materal refer to the enrchment of a materal conttuent at a free urface or an nternal nterface of a materal.
Additional File 1 - Detailed explanation of the expression level CPD
 Addtonal Fle - Detaled explanaton of the expreon level CPD A mentoned n the man text, the man CPD for the uterng model cont of two ndvdual factor: P( level gen P( level gen P ( level gen 2 (.).. CPD factor
Addtonal Fle - Detaled explanaton of the expreon level CPD A mentoned n the man text, the man CPD for the uterng model cont of two ndvdual factor: P( level gen P( level gen P ( level gen 2 (.).. CPD factor
The influence of Stern layer conductance on the. dielectrophoretic behaviour of latex nanospheres
 The nfluence of Stern layer conductance on the delectrophoretc behavour of latex nanophere Mchael Pycraft Hughe* Bomedcal Engneerng Group, Unverty of Surrey, Guldford, GU2 7XH, UK Ncola Gavn Green Boelectronc
The nfluence of Stern layer conductance on the delectrophoretc behavour of latex nanophere Mchael Pycraft Hughe* Bomedcal Engneerng Group, Unverty of Surrey, Guldford, GU2 7XH, UK Ncola Gavn Green Boelectronc
Electrical double layer: revisit based on boundary conditions
 Electrcal double layer: revst based on boundary condtons Jong U. Km Department of Electrcal and Computer Engneerng, Texas A&M Unversty College Staton, TX 77843-318, USA Abstract The electrcal double layer
Electrcal double layer: revst based on boundary condtons Jong U. Km Department of Electrcal and Computer Engneerng, Texas A&M Unversty College Staton, TX 77843-318, USA Abstract The electrcal double layer
Chapter 11. Supplemental Text Material. The method of steepest ascent can be derived as follows. Suppose that we have fit a firstorder
 S-. The Method of Steepet cent Chapter. Supplemental Text Materal The method of teepet acent can be derved a follow. Suppoe that we have ft a frtorder model y = β + β x and we wh to ue th model to determne
S-. The Method of Steepet cent Chapter. Supplemental Text Materal The method of teepet acent can be derved a follow. Suppoe that we have ft a frtorder model y = β + β x and we wh to ue th model to determne
Specification -- Assumptions of the Simple Classical Linear Regression Model (CLRM) 1. Introduction
 ECONOMICS 35* -- NOTE ECON 35* -- NOTE Specfcaton -- Aumpton of the Smple Clacal Lnear Regreon Model (CLRM). Introducton CLRM tand for the Clacal Lnear Regreon Model. The CLRM alo known a the tandard lnear
ECONOMICS 35* -- NOTE ECON 35* -- NOTE Specfcaton -- Aumpton of the Smple Clacal Lnear Regreon Model (CLRM). Introducton CLRM tand for the Clacal Lnear Regreon Model. The CLRM alo known a the tandard lnear
Harmonic oscillator approximation
 armonc ocllator approxmaton armonc ocllator approxmaton Euaton to be olved We are fndng a mnmum of the functon under the retrcton where W P, P,..., P, Q, Q,..., Q P, P,..., P, Q, Q,..., Q lnwgner functon
armonc ocllator approxmaton armonc ocllator approxmaton Euaton to be olved We are fndng a mnmum of the functon under the retrcton where W P, P,..., P, Q, Q,..., Q P, P,..., P, Q, Q,..., Q lnwgner functon
Chapter 6 The Effect of the GPS Systematic Errors on Deformation Parameters
 Chapter 6 The Effect of the GPS Sytematc Error on Deformaton Parameter 6.. General Beutler et al., (988) dd the frt comprehenve tudy on the GPS ytematc error. Baed on a geometrc approach and aumng a unform
Chapter 6 The Effect of the GPS Sytematc Error on Deformaton Parameter 6.. General Beutler et al., (988) dd the frt comprehenve tudy on the GPS ytematc error. Baed on a geometrc approach and aumng a unform
Two-Layered Model of Blood Flow through Composite Stenosed Artery
 Avalable at http://pvamu.edu/aam Appl. Appl. Math. ISSN: 93-9466 Vol. 4, Iue (December 9), pp. 343 354 (Prevouly, Vol. 4, No.) Applcaton Appled Mathematc: An Internatonal Journal (AAM) Two-ayered Model
Avalable at http://pvamu.edu/aam Appl. Appl. Math. ISSN: 93-9466 Vol. 4, Iue (December 9), pp. 343 354 (Prevouly, Vol. 4, No.) Applcaton Appled Mathematc: An Internatonal Journal (AAM) Two-ayered Model
On the SO 2 Problem in Thermal Power Plants. 2.Two-steps chemical absorption modeling
 Internatonal Journal of Engneerng Reearch ISSN:39-689)(onlne),347-53(prnt) Volume No4, Iue No, pp : 557-56 Oct 5 On the SO Problem n Thermal Power Plant Two-tep chemcal aborpton modelng hr Boyadjev, P
Internatonal Journal of Engneerng Reearch ISSN:39-689)(onlne),347-53(prnt) Volume No4, Iue No, pp : 557-56 Oct 5 On the SO Problem n Thermal Power Plant Two-tep chemcal aborpton modelng hr Boyadjev, P
You must not circulate this book in any other binding or cover and you must impose this same condition on any acquirer.
 6 Interfacal thermodynamc: Gbb equaton Luuk K. Koopal Chapter 6, Interfacal thermodynamc: Gbb equaton n Interface Scence, Second edton, 008, Wagenngen Unverty, Wagenngen, The Netherland. Avalable va: http://www.reearchgate.net/profle/luuk_koopal
6 Interfacal thermodynamc: Gbb equaton Luuk K. Koopal Chapter 6, Interfacal thermodynamc: Gbb equaton n Interface Scence, Second edton, 008, Wagenngen Unverty, Wagenngen, The Netherland. Avalable va: http://www.reearchgate.net/profle/luuk_koopal
APPROXIMATE FUZZY REASONING BASED ON INTERPOLATION IN THE VAGUE ENVIRONMENT OF THE FUZZY RULEBASE AS A PRACTICAL ALTERNATIVE OF THE CLASSICAL CRI
 Kovác, Sz., Kóczy, L.T.: Approxmate Fuzzy Reaonng Baed on Interpolaton n the Vague Envronment of the Fuzzy Rulebae a a Practcal Alternatve of the Clacal CRI, Proceedng of the 7 th Internatonal Fuzzy Sytem
Kovác, Sz., Kóczy, L.T.: Approxmate Fuzzy Reaonng Baed on Interpolaton n the Vague Envronment of the Fuzzy Rulebae a a Practcal Alternatve of the Clacal CRI, Proceedng of the 7 th Internatonal Fuzzy Sytem
Gouy-Chapman model (1910) The double layer is not as compact as in Helmholtz rigid layer.
 CHE465/865, 6-3, Lecture 1, 7 nd Sep., 6 Gouy-Chapman model (191) The double layer s not as compact as n Helmholtz rgd layer. Consder thermal motons of ons: Tendency to ncrease the entropy and make the
CHE465/865, 6-3, Lecture 1, 7 nd Sep., 6 Gouy-Chapman model (191) The double layer s not as compact as n Helmholtz rgd layer. Consder thermal motons of ons: Tendency to ncrease the entropy and make the
Two Approaches to Proving. Goldbach s Conjecture
 Two Approache to Provng Goldbach Conecture By Bernard Farley Adved By Charle Parry May 3 rd 5 A Bref Introducton to Goldbach Conecture In 74 Goldbach made h mot famou contrbuton n mathematc wth the conecture
Two Approache to Provng Goldbach Conecture By Bernard Farley Adved By Charle Parry May 3 rd 5 A Bref Introducton to Goldbach Conecture In 74 Goldbach made h mot famou contrbuton n mathematc wth the conecture
A NUMERICAL MODELING OF MAGNETIC FIELD PERTURBATED BY THE PRESENCE OF SCHIP S HULL
 A NUMERCAL MODELNG OF MAGNETC FELD PERTURBATED BY THE PRESENCE OF SCHP S HULL M. Dennah* Z. Abd** * Laboratory Electromagnetc Sytem EMP BP b Ben-Aknoun 606 Alger Algera ** Electronc nttute USTHB Alger
A NUMERCAL MODELNG OF MAGNETC FELD PERTURBATED BY THE PRESENCE OF SCHP S HULL M. Dennah* Z. Abd** * Laboratory Electromagnetc Sytem EMP BP b Ben-Aknoun 606 Alger Algera ** Electronc nttute USTHB Alger
Not at Steady State! Yes! Only if reactions occur! Yes! Ideal Gas, change in temperature or pressure. Yes! Class 15. Is the following possible?
 Chapter 5-6 (where we are gong) Ideal gae and lqud (today) Dente Partal preure Non-deal gae (next tme) Eqn. of tate Reduced preure and temperature Compreblty chart (z) Vapor-lqud ytem (Ch. 6) Vapor preure
Chapter 5-6 (where we are gong) Ideal gae and lqud (today) Dente Partal preure Non-deal gae (next tme) Eqn. of tate Reduced preure and temperature Compreblty chart (z) Vapor-lqud ytem (Ch. 6) Vapor preure
No! Yes! Only if reactions occur! Yes! Ideal Gas, change in temperature or pressure. Survey Results. Class 15. Is the following possible?
 Survey Reult Chapter 5-6 (where we are gong) % of Student 45% 40% 35% 30% 25% 20% 15% 10% 5% 0% Hour Spent on ChE 273 1-2 3-4 5-6 7-8 9-10 11+ Hour/Week 2008 2009 2010 2011 2012 2013 2014 2015 2017 F17
Survey Reult Chapter 5-6 (where we are gong) % of Student 45% 40% 35% 30% 25% 20% 15% 10% 5% 0% Hour Spent on ChE 273 1-2 3-4 5-6 7-8 9-10 11+ Hour/Week 2008 2009 2010 2011 2012 2013 2014 2015 2017 F17
Method Of Fundamental Solutions For Modeling Electromagnetic Wave Scattering Problems
 Internatonal Workhop on MehFree Method 003 1 Method Of Fundamental Soluton For Modelng lectromagnetc Wave Scatterng Problem Der-Lang Young (1) and Jhh-We Ruan (1) Abtract: In th paper we attempt to contruct
Internatonal Workhop on MehFree Method 003 1 Method Of Fundamental Soluton For Modelng lectromagnetc Wave Scatterng Problem Der-Lang Young (1) and Jhh-We Ruan (1) Abtract: In th paper we attempt to contruct
AP Statistics Ch 3 Examining Relationships
 Introducton To tud relatonhp between varable, we mut meaure the varable on the ame group of ndvdual. If we thnk a varable ma eplan or even caue change n another varable, then the eplanator varable and
Introducton To tud relatonhp between varable, we mut meaure the varable on the ame group of ndvdual. If we thnk a varable ma eplan or even caue change n another varable, then the eplanator varable and
Supporting Information. Hydroxyl Radical Production by H 2 O 2 -Mediated. Conditions
 Supportng Informaton Hydroxyl Radcal Producton by H 2 O 2 -Medated Oxdaton of Fe(II) Complexed by Suwannee Rver Fulvc Acd Under Crcumneutral Frehwater Condton Chrtopher J. Mller, Andrew L. Roe, T. Davd
Supportng Informaton Hydroxyl Radcal Producton by H 2 O 2 -Medated Oxdaton of Fe(II) Complexed by Suwannee Rver Fulvc Acd Under Crcumneutral Frehwater Condton Chrtopher J. Mller, Andrew L. Roe, T. Davd
728. Mechanical and electrical elements in reduction of vibrations
 78. Mechancal and electrcal element n reducton of vbraton Katarzyna BIAŁAS The Slean Unverty of Technology, Faculty of Mechancal Engneerng Inttute of Engneerng Procee Automaton and Integrated Manufacturng
78. Mechancal and electrcal element n reducton of vbraton Katarzyna BIAŁAS The Slean Unverty of Technology, Faculty of Mechancal Engneerng Inttute of Engneerng Procee Automaton and Integrated Manufacturng
Small signal analysis
 Small gnal analy. ntroducton Let u conder the crcut hown n Fg., where the nonlnear retor decrbed by the equaton g v havng graphcal repreentaton hown n Fg.. ( G (t G v(t v Fg. Fg. a D current ource wherea
Small gnal analy. ntroducton Let u conder the crcut hown n Fg., where the nonlnear retor decrbed by the equaton g v havng graphcal repreentaton hown n Fg.. ( G (t G v(t v Fg. Fg. a D current ource wherea
8 Waves in Uniform Magnetized Media
 8 Wave n Unform Magnetzed Meda 81 Suceptblte The frt order current can be wrtten j = j = q d 3 p v f 1 ( r, p, t) = ɛ 0 χ E For Maxwellan dtrbuton Y n (λ) = f 0 (v, v ) = 1 πvth exp (v V ) v th 1 πv th
8 Wave n Unform Magnetzed Meda 81 Suceptblte The frt order current can be wrtten j = j = q d 3 p v f 1 ( r, p, t) = ɛ 0 χ E For Maxwellan dtrbuton Y n (λ) = f 0 (v, v ) = 1 πvth exp (v V ) v th 1 πv th
Scattering of two identical particles in the center-of. of-mass frame. (b)
 Lecture # November 5 Scatterng of two dentcal partcle Relatvtc Quantum Mechanc: The Klen-Gordon equaton Interpretaton of the Klen-Gordon equaton The Drac equaton Drac repreentaton for the matrce α and
Lecture # November 5 Scatterng of two dentcal partcle Relatvtc Quantum Mechanc: The Klen-Gordon equaton Interpretaton of the Klen-Gordon equaton The Drac equaton Drac repreentaton for the matrce α and
Verification of Selected Precision Parameters of the Trimble S8 DR Plus Robotic Total Station
 81 Verfcaton of Selected Precon Parameter of the Trmble S8 DR Plu Robotc Total Staton Sokol, Š., Bajtala, M. and Ježko, J. Slovak Unverty of Technology, Faculty of Cvl Engneerng, Radlnkého 11, 81368 Bratlava,
81 Verfcaton of Selected Precon Parameter of the Trmble S8 DR Plu Robotc Total Staton Sokol, Š., Bajtala, M. and Ježko, J. Slovak Unverty of Technology, Faculty of Cvl Engneerng, Radlnkého 11, 81368 Bratlava,
MULTIPLE REGRESSION ANALYSIS For the Case of Two Regressors
 MULTIPLE REGRESSION ANALYSIS For the Cae of Two Regreor In the followng note, leat-quare etmaton developed for multple regreon problem wth two eplanator varable, here called regreor (uch a n the Fat Food
MULTIPLE REGRESSION ANALYSIS For the Cae of Two Regreor In the followng note, leat-quare etmaton developed for multple regreon problem wth two eplanator varable, here called regreor (uch a n the Fat Food
2.3 Least-Square regressions
 .3 Leat-Square regreon Eample.10 How do chldren grow? The pattern of growth vare from chld to chld, o we can bet undertandng the general pattern b followng the average heght of a number of chldren. Here
.3 Leat-Square regreon Eample.10 How do chldren grow? The pattern of growth vare from chld to chld, o we can bet undertandng the general pattern b followng the average heght of a number of chldren. Here
Supplementary information: Efficient mass transport by optical advection
 Supplementary nformaton: Effcent ma tranport by optcal advecton Veerachart Kaorndenukul, Sergey Sukhov, and Artde Dogaru CREOL, The College of Optc and Photonc Unverty of Central lorda, 4 Central lorda
Supplementary nformaton: Effcent ma tranport by optcal advecton Veerachart Kaorndenukul, Sergey Sukhov, and Artde Dogaru CREOL, The College of Optc and Photonc Unverty of Central lorda, 4 Central lorda
CONDITIONAL MOMENT CLOSURE MODELLING OF SOOT FORMATION IN TURBULENT NON-PREMIXED, ETHYLENE-AIR FLAMES
 CONDITIONAL MOMENT CLOSURE MODELLING OF SOOT FORMATION IN TURBULENT NON-PREMIXED, ETHYLENE-AIR FLAMES Yunard, Robert M. Woolley and Mchael Farweather Energy and Reource Reearch Inttute, School of Proce,
CONDITIONAL MOMENT CLOSURE MODELLING OF SOOT FORMATION IN TURBULENT NON-PREMIXED, ETHYLENE-AIR FLAMES Yunard, Robert M. Woolley and Mchael Farweather Energy and Reource Reearch Inttute, School of Proce,
Electromagnetic scattering. Graduate Course Electrical Engineering (Communications) 1 st Semester, Sharif University of Technology
 Electromagnetc catterng Graduate Coure Electrcal Engneerng (Communcaton) 1 t Semeter, 1390-1391 Sharf Unverty of Technology Content of lecture Lecture : Bac catterng parameter Formulaton of the problem
Electromagnetc catterng Graduate Coure Electrcal Engneerng (Communcaton) 1 t Semeter, 1390-1391 Sharf Unverty of Technology Content of lecture Lecture : Bac catterng parameter Formulaton of the problem
 Ice crytal nucleaton, growth and breakage modellng n a craped urface heat exchanger H. Benkhelfa (a, M. Arellano (a,b G. Alvarez (b, D. Flck (a (a UMR n 45 AgroParTech/INRA, Ingénere-Procédé-Alment, 6
Ice crytal nucleaton, growth and breakage modellng n a craped urface heat exchanger H. Benkhelfa (a, M. Arellano (a,b G. Alvarez (b, D. Flck (a (a UMR n 45 AgroParTech/INRA, Ingénere-Procédé-Alment, 6
Estimation of Finite Population Total under PPS Sampling in Presence of Extra Auxiliary Information
 Internatonal Journal of Stattc and Analy. ISSN 2248-9959 Volume 6, Number 1 (2016), pp. 9-16 Reearch Inda Publcaton http://www.rpublcaton.com Etmaton of Fnte Populaton Total under PPS Samplng n Preence
Internatonal Journal of Stattc and Analy. ISSN 2248-9959 Volume 6, Number 1 (2016), pp. 9-16 Reearch Inda Publcaton http://www.rpublcaton.com Etmaton of Fnte Populaton Total under PPS Samplng n Preence
KEY POINTS FOR NUMERICAL SIMULATION OF INCLINATION OF BUILDINGS ON LIQUEFIABLE SOIL LAYERS
 KY POINTS FOR NUMRICAL SIMULATION OF INCLINATION OF BUILDINGS ON LIQUFIABL SOIL LAYRS Jn Xu 1, Xaomng Yuan, Jany Zhang 3,Fanchao Meng 1 1 Student, Dept. of Geotechncal ngneerng, Inttute of ngneerng Mechanc,
KY POINTS FOR NUMRICAL SIMULATION OF INCLINATION OF BUILDINGS ON LIQUFIABL SOIL LAYRS Jn Xu 1, Xaomng Yuan, Jany Zhang 3,Fanchao Meng 1 1 Student, Dept. of Geotechncal ngneerng, Inttute of ngneerng Mechanc,
Pythagorean triples. Leen Noordzij.
 Pythagorean trple. Leen Noordz Dr.l.noordz@leennoordz.nl www.leennoordz.me Content A Roadmap for generatng Pythagorean Trple.... Pythagorean Trple.... 3 Dcuon Concluon.... 5 A Roadmap for generatng Pythagorean
Pythagorean trple. Leen Noordz Dr.l.noordz@leennoordz.nl www.leennoordz.me Content A Roadmap for generatng Pythagorean Trple.... Pythagorean Trple.... 3 Dcuon Concluon.... 5 A Roadmap for generatng Pythagorean
FREE VIBRATION ANALYSIS OF CLAMPED-FREE COMPOSITE CYLINDRICAL SHELLS WITH AN INTERIOR RECTANGULAR PLATE
 FREE VIBRATION ANALYSIS OF CLAPED-FREE COPOSITE CYLINDRICAL SHELLS WITH AN INTERIOR RECTANGULAR PLATE Young-Shn Lee and young-hwan Cho Department of echancal Degn Engneerng, Chungnam Natonal Unverty, 0
FREE VIBRATION ANALYSIS OF CLAPED-FREE COPOSITE CYLINDRICAL SHELLS WITH AN INTERIOR RECTANGULAR PLATE Young-Shn Lee and young-hwan Cho Department of echancal Degn Engneerng, Chungnam Natonal Unverty, 0
Lecture 2 Thermodynamics and stability October 30, 2009
 The tablty of thn (oft) flm Lecture 2 Thermodynamc and tablty October 30, 2009 Ian Morron 2009 Revew of Lecture 1 The djonng preure a jump n preure at the boundary. It doe not vary between the plate. 1
The tablty of thn (oft) flm Lecture 2 Thermodynamc and tablty October 30, 2009 Ian Morron 2009 Revew of Lecture 1 The djonng preure a jump n preure at the boundary. It doe not vary between the plate. 1
MODELLING OF TRANSIENT HEAT TRANSPORT IN TWO-LAYERED CRYSTALLINE SOLID FILMS USING THE INTERVAL LATTICE BOLTZMANN METHOD
 Journal o Appled Mathematc and Computatonal Mechanc 7, 6(4), 57-65 www.amcm.pcz.pl p-issn 99-9965 DOI:.75/jamcm.7.4.6 e-issn 353-588 MODELLING OF TRANSIENT HEAT TRANSPORT IN TWO-LAYERED CRYSTALLINE SOLID
Journal o Appled Mathematc and Computatonal Mechanc 7, 6(4), 57-65 www.amcm.pcz.pl p-issn 99-9965 DOI:.75/jamcm.7.4.6 e-issn 353-588 MODELLING OF TRANSIENT HEAT TRANSPORT IN TWO-LAYERED CRYSTALLINE SOLID
V. Electrostatics. Lecture 25: Diffuse double layer structure
 V. Electrostatcs Lecture 5: Dffuse double layer structure MIT Student Last tme we showed that whenever λ D L the electrolyte has a quas-neutral bulk (or outer ) regon at the geometrcal scale L, where there
V. Electrostatcs Lecture 5: Dffuse double layer structure MIT Student Last tme we showed that whenever λ D L the electrolyte has a quas-neutral bulk (or outer ) regon at the geometrcal scale L, where there
Extended Prigogine Theorem: Method for Universal Characterization of Complex System Evolution
 Extended Prgogne Theorem: Method for Unveral Characterzaton of Complex Sytem Evoluton Sergey amenhchkov* Mocow State Unverty of M.V. Lomonoov, Phycal department, Rua, Mocow, Lennke Gory, 1/, 119991 Publhed
Extended Prgogne Theorem: Method for Unveral Characterzaton of Complex Sytem Evoluton Sergey amenhchkov* Mocow State Unverty of M.V. Lomonoov, Phycal department, Rua, Mocow, Lennke Gory, 1/, 119991 Publhed
Team. Outline. Statistics and Art: Sampling, Response Error, Mixed Models, Missing Data, and Inference
 Team Stattc and Art: Samplng, Repone Error, Mxed Model, Mng Data, and nference Ed Stanek Unverty of Maachuett- Amhert, USA 9/5/8 9/5/8 Outlne. Example: Doe-repone Model n Toxcology. ow to Predct Realzed
Team Stattc and Art: Samplng, Repone Error, Mxed Model, Mng Data, and nference Ed Stanek Unverty of Maachuett- Amhert, USA 9/5/8 9/5/8 Outlne. Example: Doe-repone Model n Toxcology. ow to Predct Realzed
Improvements on Waring s Problem
 Improvement on Warng Problem L An-Png Bejng, PR Chna apl@nacom Abtract By a new recurve algorthm for the auxlary equaton, n th paper, we wll gve ome mprovement for Warng problem Keyword: Warng Problem,
Improvement on Warng Problem L An-Png Bejng, PR Chna apl@nacom Abtract By a new recurve algorthm for the auxlary equaton, n th paper, we wll gve ome mprovement for Warng problem Keyword: Warng Problem,
Root Locus Techniques
 Root Locu Technque ELEC 32 Cloed-Loop Control The control nput u t ynthezed baed on the a pror knowledge of the ytem plant, the reference nput r t, and the error gnal, e t The control ytem meaure the output,
Root Locu Technque ELEC 32 Cloed-Loop Control The control nput u t ynthezed baed on the a pror knowledge of the ytem plant, the reference nput r t, and the error gnal, e t The control ytem meaure the output,
Problem #1. Known: All required parameters. Schematic: Find: Depth of freezing as function of time. Strategy:
 BEE 3500 013 Prelm Soluton Problem #1 Known: All requred parameter. Schematc: Fnd: Depth of freezng a functon of tme. Strategy: In thee mplfed analy for freezng tme, a wa done n cla for a lab geometry,
BEE 3500 013 Prelm Soluton Problem #1 Known: All requred parameter. Schematc: Fnd: Depth of freezng a functon of tme. Strategy: In thee mplfed analy for freezng tme, a wa done n cla for a lab geometry,
Electrostatic Potential from Transmembrane Currents
 Electrostatc Potental from Transmembrane Currents Let s assume that the current densty j(r, t) s ohmc;.e., lnearly proportonal to the electrc feld E(r, t): j = σ c (r)e (1) wth conductvty σ c = σ c (r).
Electrostatc Potental from Transmembrane Currents Let s assume that the current densty j(r, t) s ohmc;.e., lnearly proportonal to the electrc feld E(r, t): j = σ c (r)e (1) wth conductvty σ c = σ c (r).
Confidence intervals for the difference and the ratio of Lognormal means with bounded parameters
 Songklanakarn J. Sc. Technol. 37 () 3-40 Mar.-Apr. 05 http://www.jt.pu.ac.th Orgnal Artcle Confdence nterval for the dfference and the rato of Lognormal mean wth bounded parameter Sa-aat Nwtpong* Department
Songklanakarn J. Sc. Technol. 37 () 3-40 Mar.-Apr. 05 http://www.jt.pu.ac.th Orgnal Artcle Confdence nterval for the dfference and the rato of Lognormal mean wth bounded parameter Sa-aat Nwtpong* Department
PROBABILITY-CONSISTENT SCENARIO EARTHQUAKE AND ITS APPLICATION IN ESTIMATION OF GROUND MOTIONS
 PROBABILITY-COSISTET SCEARIO EARTHQUAKE AD ITS APPLICATIO I ESTIATIO OF GROUD OTIOS Q-feng LUO SUARY Th paper preent a new defnton of probablty-content cenaro earthquae PCSE and an evaluaton method of
PROBABILITY-COSISTET SCEARIO EARTHQUAKE AD ITS APPLICATIO I ESTIATIO OF GROUD OTIOS Q-feng LUO SUARY Th paper preent a new defnton of probablty-content cenaro earthquae PCSE and an evaluaton method of
Wind - Induced Vibration Control of Long - Span Bridges by Multiple Tuned Mass Dampers
 Tamkang Journal of Scence and Engneerng, Vol. 3, o., pp. -3 (000) Wnd - Induced Vbraton Control of Long - Span Brdge by Multple Tuned Ma Damper Yuh-Y Ln, Ch-Mng Cheng and Davd Sun Department of Cvl Engneerng
Tamkang Journal of Scence and Engneerng, Vol. 3, o., pp. -3 (000) Wnd - Induced Vbraton Control of Long - Span Brdge by Multple Tuned Ma Damper Yuh-Y Ln, Ch-Mng Cheng and Davd Sun Department of Cvl Engneerng
Modeling of Wave Behavior of Substrate Noise Coupling for Mixed-Signal IC Design
 Modelng of Wave Behavor of Subtrate Noe Couplng for Mxed-Sgnal IC Degn Georgo Veron, Y-Chang Lu, and Robert W. Dutton Center for Integrated Sytem, Stanford Unverty, Stanford, CA 9435 yorgo@gloworm.tanford.edu
Modelng of Wave Behavor of Subtrate Noe Couplng for Mxed-Sgnal IC Degn Georgo Veron, Y-Chang Lu, and Robert W. Dutton Center for Integrated Sytem, Stanford Unverty, Stanford, CA 9435 yorgo@gloworm.tanford.edu
Calculating Jacobian coefficients of primitive constraints with respect to Euler parameters
 Calculatng Jacoban coeffcent of prmtve contrant wth repect to Euler parameter Yong Lu, Ha-Chuan Song, Jun-Ha Yong To cte th veron: Yong Lu, Ha-Chuan Song, Jun-Ha Yong. Calculatng Jacoban coeffcent of prmtve
Calculatng Jacoban coeffcent of prmtve contrant wth repect to Euler parameter Yong Lu, Ha-Chuan Song, Jun-Ha Yong To cte th veron: Yong Lu, Ha-Chuan Song, Jun-Ha Yong. Calculatng Jacoban coeffcent of prmtve
Image Registration for a Series of Chest Radiograph Images
 Proceedng of the 5th WE Internatonal Conference on gnal Proceng, Itanbul, Turkey, May 7-9, 006 (pp179-184) Image Regtraton for a ere of Chet Radograph Image Omar Mohd. Rjal*, Norlza Mohd. Noor, hee Lee
Proceedng of the 5th WE Internatonal Conference on gnal Proceng, Itanbul, Turkey, May 7-9, 006 (pp179-184) Image Regtraton for a ere of Chet Radograph Image Omar Mohd. Rjal*, Norlza Mohd. Noor, hee Lee
Start Point and Trajectory Analysis for the Minimal Time System Design Algorithm
 Start Pont and Trajectory Analy for the Mnmal Tme Sytem Degn Algorthm ALEXANDER ZEMLIAK, PEDRO MIRANDA Department of Phyc and Mathematc Puebla Autonomou Unverty Av San Claudo /n, Puebla, 757 MEXICO Abtract:
Start Pont and Trajectory Analy for the Mnmal Tme Sytem Degn Algorthm ALEXANDER ZEMLIAK, PEDRO MIRANDA Department of Phyc and Mathematc Puebla Autonomou Unverty Av San Claudo /n, Puebla, 757 MEXICO Abtract:
Physics 111. CQ1: springs. con t. Aristocrat at a fixed angle. Wednesday, 8-9 pm in NSC 118/119 Sunday, 6:30-8 pm in CCLIR 468.
 c Announcement day, ober 8, 004 Ch 8: Ch 10: Work done by orce at an angle Power Rotatonal Knematc angular dplacement angular velocty angular acceleraton Wedneday, 8-9 pm n NSC 118/119 Sunday, 6:30-8 pm
c Announcement day, ober 8, 004 Ch 8: Ch 10: Work done by orce at an angle Power Rotatonal Knematc angular dplacement angular velocty angular acceleraton Wedneday, 8-9 pm n NSC 118/119 Sunday, 6:30-8 pm
CHAPTER 9 LINEAR MOMENTUM, IMPULSE AND COLLISIONS
 CHAPTER 9 LINEAR MOMENTUM, IMPULSE AND COLLISIONS 103 Phy 1 9.1 Lnear Momentum The prncple o energy conervaton can be ued to olve problem that are harder to olve jut ung Newton law. It ued to decrbe moton
CHAPTER 9 LINEAR MOMENTUM, IMPULSE AND COLLISIONS 103 Phy 1 9.1 Lnear Momentum The prncple o energy conervaton can be ued to olve problem that are harder to olve jut ung Newton law. It ued to decrbe moton
Kinetic-Energy Density-Functional Theory on a Lattice
 h an open acce artcle publhed under an ACS AuthorChoce Lcene, whch permt copyng and redtrbuton of the artcle or any adaptaton for non-commercal purpoe. Artcle Cte h: J. Chem. heory Comput. 08, 4, 407 4087
h an open acce artcle publhed under an ACS AuthorChoce Lcene, whch permt copyng and redtrbuton of the artcle or any adaptaton for non-commercal purpoe. Artcle Cte h: J. Chem. heory Comput. 08, 4, 407 4087
Phys 402: Raman Scattering. Spring Introduction: Brillouin and Raman spectroscopy. Raman scattering: how does it look like?
 Phy 402: Raman Scatterng Sprng 2008 1 Introducton: Brlloun and Raman pectrocopy Inelatc lght catterng medated by the electronc polarzablty of the medum a materal or a molecule catter rradant lght from
Phy 402: Raman Scatterng Sprng 2008 1 Introducton: Brlloun and Raman pectrocopy Inelatc lght catterng medated by the electronc polarzablty of the medum a materal or a molecule catter rradant lght from
MODEL OF CEMENTING ANNULUS GAS CHANNELING BASED ON THE LATTICE BOLTZMANN THEORY
 Journal of Theoretcal and Appled Informaton Technology 20 th February 203. Vol. 48 No.2 2005-203 JATIT & LLS. All rght reerved. ISSN: 2-8645 www.jatt.org E-ISSN: 87-35 MODEL OF CEMENTING ANNULUS GAS CHANNELING
Journal of Theoretcal and Appled Informaton Technology 20 th February 203. Vol. 48 No.2 2005-203 JATIT & LLS. All rght reerved. ISSN: 2-8645 www.jatt.org E-ISSN: 87-35 MODEL OF CEMENTING ANNULUS GAS CHANNELING
Chapter 7 Four-Wave Mixing phenomena
 Chapter 7 Four-Wave Mx phenomena We wll dcu n th chapter the general nonlnear optcal procee wth four nteract electromagnetc wave n a NLO medum. Frt note that FWM procee are allowed n all meda (nveron or
Chapter 7 Four-Wave Mx phenomena We wll dcu n th chapter the general nonlnear optcal procee wth four nteract electromagnetc wave n a NLO medum. Frt note that FWM procee are allowed n all meda (nveron or
Physics 120. Exam #1. April 15, 2011
 Phyc 120 Exam #1 Aprl 15, 2011 Name Multple Choce /16 Problem #1 /28 Problem #2 /28 Problem #3 /28 Total /100 PartI:Multple Choce:Crclethebetanwertoeachqueton.Anyothermark wllnotbegvencredt.eachmultple
Phyc 120 Exam #1 Aprl 15, 2011 Name Multple Choce /16 Problem #1 /28 Problem #2 /28 Problem #3 /28 Total /100 PartI:Multple Choce:Crclethebetanwertoeachqueton.Anyothermark wllnotbegvencredt.eachmultple
Quantitative Discrimination of Effective Porosity Using Digital Image Analysis - Implications for Porosity-Permeability Transforms
 2004, 66th EAGE Conference, Pars Quanttatve Dscrmnaton of Effectve Porosty Usng Dgtal Image Analyss - Implcatons for Porosty-Permeablty Transforms Gregor P. Eberl 1, Gregor T. Baechle 1, Ralf Weger 1,
2004, 66th EAGE Conference, Pars Quanttatve Dscrmnaton of Effectve Porosty Usng Dgtal Image Analyss - Implcatons for Porosty-Permeablty Transforms Gregor P. Eberl 1, Gregor T. Baechle 1, Ralf Weger 1,
Chapter 13: Multiple Regression
 Chapter 13: Multple Regresson 13.1 Developng the multple-regresson Model The general model can be descrbed as: It smplfes for two ndependent varables: The sample ft parameter b 0, b 1, and b are used to
Chapter 13: Multple Regresson 13.1 Developng the multple-regresson Model The general model can be descrbed as: It smplfes for two ndependent varables: The sample ft parameter b 0, b 1, and b are used to
Module 5. Cables and Arches. Version 2 CE IIT, Kharagpur
 odule 5 Cable and Arche Veron CE IIT, Kharagpur Leon 33 Two-nged Arch Veron CE IIT, Kharagpur Intructonal Objectve: After readng th chapter the tudent wll be able to 1. Compute horzontal reacton n two-hnged
odule 5 Cable and Arche Veron CE IIT, Kharagpur Leon 33 Two-nged Arch Veron CE IIT, Kharagpur Intructonal Objectve: After readng th chapter the tudent wll be able to 1. Compute horzontal reacton n two-hnged
An O(N) algorithm for Stokes and Laplace interactions of particles
 Syracue Unverty From the SelectedWork of Ahok S. Sangan 1996 An O(N) algorthm for Stoke and Laplace nteracton of partcle Ahok S. Sangan, Syracue Unverty Guobao Mo, Syracue Unverty Avalable at: http://work.bepre.com/ahok_angan/16/
Syracue Unverty From the SelectedWork of Ahok S. Sangan 1996 An O(N) algorthm for Stoke and Laplace nteracton of partcle Ahok S. Sangan, Syracue Unverty Guobao Mo, Syracue Unverty Avalable at: http://work.bepre.com/ahok_angan/16/
Uncertainty in measurements of power and energy on power networks
 Uncertanty n measurements of power and energy on power networks E. Manov, N. Kolev Department of Measurement and Instrumentaton, Techncal Unversty Sofa, bul. Klment Ohrdsk No8, bl., 000 Sofa, Bulgara Tel./fax:
Uncertanty n measurements of power and energy on power networks E. Manov, N. Kolev Department of Measurement and Instrumentaton, Techncal Unversty Sofa, bul. Klment Ohrdsk No8, bl., 000 Sofa, Bulgara Tel./fax:
1) Silicon oxide has a typical surface potential in an aqueous medium of ϕ,0
 1) Slcon oxde has a typcal surface potental n an aqueous medum of ϕ, = 7 mv n 5 mm l at ph 9. Whch concentraton of catons do you roughly expect close to the surface? What s the average dstance between
1) Slcon oxde has a typcal surface potental n an aqueous medum of ϕ, = 7 mv n 5 mm l at ph 9. Whch concentraton of catons do you roughly expect close to the surface? What s the average dstance between
1. Mean-Field Theory. 2. Bjerrum length
 1. Mean-Feld Theory Contnuum models lke the Posson-Nernst-Planck equatons are mean-feld approxmatons whch descrbe how dscrete ons are affected by the mean concentratons c and potental φ. Each on mgrates
1. Mean-Feld Theory Contnuum models lke the Posson-Nernst-Planck equatons are mean-feld approxmatons whch descrbe how dscrete ons are affected by the mean concentratons c and potental φ. Each on mgrates
3 Implementation and validation of analysis methods
 3 Implementaton and valdaton of anal method 3. Preface When mplementng new method bacall three cae can be dfferentated: - Implementaton of offcal method (nternatonall approved, valdated method, e.g. method
3 Implementaton and valdaton of anal method 3. Preface When mplementng new method bacall three cae can be dfferentated: - Implementaton of offcal method (nternatonall approved, valdated method, e.g. method
Improvements on Waring s Problem
 Imrovement on Warng Problem L An-Png Bejng 85, PR Chna al@nacom Abtract By a new recurve algorthm for the auxlary equaton, n th aer, we wll gve ome mrovement for Warng roblem Keyword: Warng Problem, Hardy-Lttlewood
Imrovement on Warng Problem L An-Png Bejng 85, PR Chna al@nacom Abtract By a new recurve algorthm for the auxlary equaton, n th aer, we wll gve ome mrovement for Warng roblem Keyword: Warng Problem, Hardy-Lttlewood
Information Acquisition in Global Games of Regime Change (Online Appendix)
 Informaton Acquton n Global Game of Regme Change (Onlne Appendx) Mchal Szkup and Iabel Trevno Augut 4, 05 Introducton Th appendx contan the proof of all the ntermedate reult that have been omtted from
Informaton Acquton n Global Game of Regme Change (Onlne Appendx) Mchal Szkup and Iabel Trevno Augut 4, 05 Introducton Th appendx contan the proof of all the ntermedate reult that have been omtted from
FREE DIFFUSION INTRODUCTION
 2 FREE DIFFUSION INTRODUCTION In th chapter, we conder the mplet of tranport procee: the pave dffuon of a olute that occur when t electrochemcal potental on the two de of a permeable barrer are dfferent.
2 FREE DIFFUSION INTRODUCTION In th chapter, we conder the mplet of tranport procee: the pave dffuon of a olute that occur when t electrochemcal potental on the two de of a permeable barrer are dfferent.
Frequency dependence of the permittivity
 Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
Supplementary Notes for Chapter 9 Mixture Thermodynamics
 Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Alpha Risk of Taguchi Method with L 18 Array for NTB Type QCH by Simulation
 Proceedng of the World Congre on Engneerng 00 Vol II WCE 00, July -, 00, London, U.K. Alpha Rk of Taguch Method wth L Array for NTB Type QCH by Smulaton A. Al-Refae and M.H. L Abtract Taguch method a wdely
Proceedng of the World Congre on Engneerng 00 Vol II WCE 00, July -, 00, London, U.K. Alpha Rk of Taguch Method wth L Array for NTB Type QCH by Smulaton A. Al-Refae and M.H. L Abtract Taguch method a wdely
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM
 ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
Quick Visit to Bernoulli Land
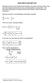 Although we have een the Bernoull equaton and een t derved before, th next note how t dervaton for an uncopreble & nvcd flow. The dervaton follow that of Kuethe &Chow ot cloely (I lke t better than Anderon).
Although we have een the Bernoull equaton and een t derved before, th next note how t dervaton for an uncopreble & nvcd flow. The dervaton follow that of Kuethe &Chow ot cloely (I lke t better than Anderon).
Rise and Dissolution Modeling of CO 2 Droplet in the Ocean
 Kyuhu Unverty Global COE Program Journal of Novel Carbon Reource Scence Vol. 7 pp. 1-17 Feb. 013 Re and Doluton Modelng of CO Droplet n the Ocean Changhong Hu *1* Makoto Sueyoh *1* Fe Jang *3 Kmnor Shtahma
Kyuhu Unverty Global COE Program Journal of Novel Carbon Reource Scence Vol. 7 pp. 1-17 Feb. 013 Re and Doluton Modelng of CO Droplet n the Ocean Changhong Hu *1* Makoto Sueyoh *1* Fe Jang *3 Kmnor Shtahma
Coupling t- formulation with surface impedance boundary condition for eddy current crack detection
 Couplng t- formulaton wth urface mpedance boundary condton for eddy current crack detecton C. Guérn, G. Meuner, F. Foucher To cte th veron: C. Guérn, G. Meuner, F. Foucher. Couplng t- formulaton wth urface
Couplng t- formulaton wth urface mpedance boundary condton for eddy current crack detecton C. Guérn, G. Meuner, F. Foucher To cte th veron: C. Guérn, G. Meuner, F. Foucher. Couplng t- formulaton wth urface
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Appendix II Summary of Important Equations
 W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
ENTROPY BOUNDS USING ARITHMETIC- GEOMETRIC-HARMONIC MEAN INEQUALITY. Guru Nanak Dev University Amritsar, , INDIA
 Internatonal Journal of Pure and Appled Mathematc Volume 89 No. 5 2013, 719-730 ISSN: 1311-8080 prnted veron; ISSN: 1314-3395 on-lne veron url: http://.jpam.eu do: http://dx.do.org/10.12732/jpam.v895.8
Internatonal Journal of Pure and Appled Mathematc Volume 89 No. 5 2013, 719-730 ISSN: 1311-8080 prnted veron; ISSN: 1314-3395 on-lne veron url: http://.jpam.eu do: http://dx.do.org/10.12732/jpam.v895.8
Research of Dependence of Heat Conductivity of The Clap-Raw And Its Components From Temperature And Humidity
 ISSN: 35-38 Internatonal Journal of AdvancedReearch n Scence, Engneerng and Technology Vol. 5, Iue, December 8 Reearch of Dependence of Heat Conductvty of The Clap-Raw And It Component From Temperature
ISSN: 35-38 Internatonal Journal of AdvancedReearch n Scence, Engneerng and Technology Vol. 5, Iue, December 8 Reearch of Dependence of Heat Conductvty of The Clap-Raw And It Component From Temperature
2010 Black Engineering Building, Department of Mechanical Engineering. Iowa State University, Ames, IA, 50011
 Interface Energy Couplng between -tungsten Nanoflm and Few-layered Graphene Meng Han a, Pengyu Yuan a, Jng Lu a, Shuyao S b, Xaolong Zhao b, Yanan Yue c, Xnwe Wang a,*, Xangheng Xao b,* a 2010 Black Engneerng
Interface Energy Couplng between -tungsten Nanoflm and Few-layered Graphene Meng Han a, Pengyu Yuan a, Jng Lu a, Shuyao S b, Xaolong Zhao b, Yanan Yue c, Xnwe Wang a,*, Xangheng Xao b,* a 2010 Black Engneerng
NUMERICAL DIFFERENTIATION
 NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
FEEDDBACK CONTROL OF PIEZO-LAMINATE COMPOSITE PLATE. Hafez Ave, Tehran 15914, Iran
 ICSV14 Carn Autrala 9-12 July, 2007 FEEDDBACK CONTROL OF PIEZO-LAMINATE COMPOSITE PLATE A. Yelagh Tamjan 1, M. Abouhamze 1, R. Mrzaefar 1, A.R. Ohad 1, M.R. Elam 1 1 Department of Mechancal Engneerng,
ICSV14 Carn Autrala 9-12 July, 2007 FEEDDBACK CONTROL OF PIEZO-LAMINATE COMPOSITE PLATE A. Yelagh Tamjan 1, M. Abouhamze 1, R. Mrzaefar 1, A.R. Ohad 1, M.R. Elam 1 1 Department of Mechancal Engneerng,
Resonant FCS Predictive Control of Power Converter in Stationary Reference Frame
 Preprnt of the 9th World Congre The Internatonal Federaton of Automatc Control Cape Town, South Afrca. Augut -9, Reonant FCS Predctve Control of Power Converter n Statonary Reference Frame Lupng Wang K
Preprnt of the 9th World Congre The Internatonal Federaton of Automatc Control Cape Town, South Afrca. Augut -9, Reonant FCS Predctve Control of Power Converter n Statonary Reference Frame Lupng Wang K
Weierstraß-Institut. für Angewandte Analysis und Stochastik. Leibniz-Institut im Forschungsverbund Berlin e. V. Preprint ISSN
 Weertraß-Inttut für Angewandte Analy und Stochatk Lebnz-Inttut m Forchungverbund Berln e. V. Preprnt ISSN 2198-5855 A new perpectve on the electron tranfer: Recoverng the Butler-Volmer equaton n non-equlbrum
Weertraß-Inttut für Angewandte Analy und Stochatk Lebnz-Inttut m Forchungverbund Berln e. V. Preprnt ISSN 2198-5855 A new perpectve on the electron tranfer: Recoverng the Butler-Volmer equaton n non-equlbrum
Assignment 4. Adsorption Isotherms
 Insttute of Process Engneerng Assgnment 4. Adsorpton Isotherms Part A: Compettve adsorpton of methane and ethane In large scale adsorpton processes, more than one compound from a mxture of gases get adsorbed,
Insttute of Process Engneerng Assgnment 4. Adsorpton Isotherms Part A: Compettve adsorpton of methane and ethane In large scale adsorpton processes, more than one compound from a mxture of gases get adsorbed,
Distributed Control for the Parallel DC Linked Modular Shunt Active Power Filters under Distorted Utility Voltage Condition
 Dtrbted Control for the Parallel DC Lnked Modlar Shnt Actve Power Flter nder Dtorted Utlty Voltage Condton Reearch Stdent: Adl Salman Spervor: Dr. Malabka Ba School of Electrcal and Electronc Engneerng
Dtrbted Control for the Parallel DC Lnked Modlar Shnt Actve Power Flter nder Dtorted Utlty Voltage Condton Reearch Stdent: Adl Salman Spervor: Dr. Malabka Ba School of Electrcal and Electronc Engneerng
Chapter 2. Electrode/electrolyte interface: ----Structure and properties
 Chapter 2 Electrode/electrolyte nterface: ----Structure and propertes Electrochemcal reactons are nterfacal reactons, the structure and propertes of electrode / electrolytc soluton nterface greatly nfluences
Chapter 2 Electrode/electrolyte nterface: ----Structure and propertes Electrochemcal reactons are nterfacal reactons, the structure and propertes of electrode / electrolytc soluton nterface greatly nfluences
Electrochemistry Thermodynamics
 CHEM 51 Analytcal Electrochemstry Chapter Oct 5, 016 Electrochemstry Thermodynamcs Bo Zhang Department of Chemstry Unversty of Washngton Seattle, WA 98195 Former SEAC presdent Andy Ewng sellng T-shrts
CHEM 51 Analytcal Electrochemstry Chapter Oct 5, 016 Electrochemstry Thermodynamcs Bo Zhang Department of Chemstry Unversty of Washngton Seattle, WA 98195 Former SEAC presdent Andy Ewng sellng T-shrts
Joint Source Coding and Higher-Dimension Modulation
 Jont Codng and Hgher-Dmenon Modulaton Tze C. Wong and Huck M. Kwon Electrcal Engneerng and Computer Scence Wchta State Unvert, Wchta, Kana 676, USA {tcwong; huck.kwon}@wchta.edu Abtract Th paper propoe
Jont Codng and Hgher-Dmenon Modulaton Tze C. Wong and Huck M. Kwon Electrcal Engneerng and Computer Scence Wchta State Unvert, Wchta, Kana 676, USA {tcwong; huck.kwon}@wchta.edu Abtract Th paper propoe
Electric and magnetic field sensor and integrator equations
 Techncal Note - TN12 Electrc and magnetc feld enor and ntegrator uaton Bertrand Da, montena technology, 1728 oen, Swtzerland Table of content 1. Equaton of the derate electrc feld enor... 1 2. Integraton
Techncal Note - TN12 Electrc and magnetc feld enor and ntegrator uaton Bertrand Da, montena technology, 1728 oen, Swtzerland Table of content 1. Equaton of the derate electrc feld enor... 1 2. Integraton
LECTURER: PM DR MAZLAN ABDUL WAHID PM Dr Mazlan Abdul Wahid
 H E A R A N S F E R HEA RANSFER SME 4463 LECURER: PM DR MAZLAN ABDUL WAHID http://www.fkm.utm.my/~mazlan C H A P E R 3 Dr Mazlan - SME 4463 H E A R A N S F E R Chapter 5 ranent Conducton PM Dr Mazlan Abdul
H E A R A N S F E R HEA RANSFER SME 4463 LECURER: PM DR MAZLAN ABDUL WAHID http://www.fkm.utm.my/~mazlan C H A P E R 3 Dr Mazlan - SME 4463 H E A R A N S F E R Chapter 5 ranent Conducton PM Dr Mazlan Abdul
Statistical Properties of the OLS Coefficient Estimators. 1. Introduction
 ECOOMICS 35* -- OTE 4 ECO 35* -- OTE 4 Stattcal Properte of the OLS Coeffcent Etmator Introducton We derved n ote the OLS (Ordnary Leat Square etmator ˆβ j (j, of the regreon coeffcent βj (j, n the mple
ECOOMICS 35* -- OTE 4 ECO 35* -- OTE 4 Stattcal Properte of the OLS Coeffcent Etmator Introducton We derved n ote the OLS (Ordnary Leat Square etmator ˆβ j (j, of the regreon coeffcent βj (j, n the mple
A NEW APPROACH IN THE RAYLEIGH - SCHRÖDINGER PERTURBATION THEORY FOR THE ROVIBRATIONAL PROBLEM
 Lebanee Scence Journal, Vol., No., A NEW APPROACH IN THE RAYLEIGH - SCHRÖDINGER PERTURBATION THEORY FOR THE ROVIBRATIONAL PROBLEM M. Korek Faculty of Scence, Berut Arab Unerty, P.O.Box - Rad El Solh, Berut
Lebanee Scence Journal, Vol., No., A NEW APPROACH IN THE RAYLEIGH - SCHRÖDINGER PERTURBATION THEORY FOR THE ROVIBRATIONAL PROBLEM M. Korek Faculty of Scence, Berut Arab Unerty, P.O.Box - Rad El Solh, Berut
The discrete dipole approximation: an overview and recent developments
 The dcrete dpole approxmaton: an overvew and recent development M.A. Yurkn a,b, and A.G. Hoektra a a Secton Computatonal Scence, Faculty of Scence, Unverty of Amterdam, Krulaan 40, 1098 SJ, Amterdam, The
The dcrete dpole approxmaton: an overvew and recent development M.A. Yurkn a,b, and A.G. Hoektra a a Secton Computatonal Scence, Faculty of Scence, Unverty of Amterdam, Krulaan 40, 1098 SJ, Amterdam, The
2. SINGLE VS. MULTI POLARIZATION SAR DATA
 . SINGLE VS. MULTI POLARIZATION SAR DATA.1 Scatterng Coeffcent v. Scatterng Matrx In the prevou chapter of th document, we dealt wth the decrpton and the characterzaton of electromagnetc wave. A t wa hown,
. SINGLE VS. MULTI POLARIZATION SAR DATA.1 Scatterng Coeffcent v. Scatterng Matrx In the prevou chapter of th document, we dealt wth the decrpton and the characterzaton of electromagnetc wave. A t wa hown,
Analytical and Empirical Study of Particle Swarm Optimization with a Sigmoid Decreasing Inertia W eight
 Electrcal and Electronc 47 Analytcal and Emprcal Study of Partcle Sarm Optmzaton th a Sgmod Decreang Inerta W eght And Adranyah, Shamudn H. M. Amn Departmentof Electrcal, Faculty ofindutral Engneerng,
Electrcal and Electronc 47 Analytcal and Emprcal Study of Partcle Sarm Optmzaton th a Sgmod Decreang Inerta W eght And Adranyah, Shamudn H. M. Amn Departmentof Electrcal, Faculty ofindutral Engneerng,
Buckling analysis of piezoelectric composite plates using NURBSbased isogeometric finite elements and higher-order shear deformation theory
 Proceedng of the 3 rd nternatonal Conference on Fracture Fatgue and Wear, pp. 134-140, 014 Bucklng analy of pezoelectrc compote plate ung NURBSbaed ogeometrc fnte element and hgher-order hear deformaton
Proceedng of the 3 rd nternatonal Conference on Fracture Fatgue and Wear, pp. 134-140, 014 Bucklng analy of pezoelectrc compote plate ung NURBSbaed ogeometrc fnte element and hgher-order hear deformaton
PORE STRUCTURE AND THERMAL CONDUCTIVITY OF BURNT CLAY BRICKS INTRODUCTION
 PORE STRUCTURE AND THERMAL CONDUCTIVITY OF BURNT CLAY BRICKS Olga Koronthalyova, Peter Matasovsky Insttute of Constructon and Archtecture, Slovak Academy of Scences, Dubravska 9, 845 43 Bratslava, Slovaka.
PORE STRUCTURE AND THERMAL CONDUCTIVITY OF BURNT CLAY BRICKS Olga Koronthalyova, Peter Matasovsky Insttute of Constructon and Archtecture, Slovak Academy of Scences, Dubravska 9, 845 43 Bratslava, Slovaka.
