Weierstraß-Institut. für Angewandte Analysis und Stochastik. Leibniz-Institut im Forschungsverbund Berlin e. V. Preprint ISSN
|
|
|
- Gladys McGee
- 5 years ago
- Views:
Transcription
1 Weertraß-Inttut für Angewandte Analy und Stochatk Lebnz-Inttut m Forchungverbund Berln e. V. Preprnt ISSN A new perpectve on the electron tranfer: Recoverng the Butler-Volmer equaton n non-equlbrum thermodynamc Wolfgang Dreyer, Clemen Guhlke, Rüdger Müller ubmtted: December 22, 215 Weertra-Inttute Mohrentr Berln Germany E-Mal: Wolfgang.Dreyer@wa-berln.de Clemen.Guhlke@wa-berln.de Ruedger.Mueller@wa-berln.de No. 224 Berln Mathematc Subject Clafcaton. 35Q6, 35Q79, 8A17. Key word and phrae. electrolyte, double-layer, Butler-Volmer, thermodynamc.
2 Edted by Weertraß-Inttut für Angewandte Analy und Stochatk WIAS Lebnz-Inttut m Forchungverbund Berln e. V. Mohrentraße Berln Germany Fax: E-Mal: preprnt@wa-berln.de World Wde Web:
3 Abtract Undertandng and correct mathematcal decrpton of electron tranfer reacton a central queton n electrochemtry. Typcally the electron tranfer reacton are decrbed by the Butler-Volmer equaton whch ha t orgn n knetc theore. The Butler-Volmer equaton relate nterfacal reacton rate to bulk quantte lke the electrotatc potental and electrolyte concentraton. Snce n the clacal form, the valdty of the Butler-Volmer equaton lmted to ome mple electrochemcal ytem, many attempt have been made to generalze the Butler-Volmer equaton. Baed on non-equlbrum thermodynamc we have recently derved a reduced model for the electrode-electrolyte nterface. Th reduced model nclude urface reacton and adorpton but doe not reolve the charge layer at the nterface. Intead t locally electroneutral and contently ncorporate all feature of the double layer nto a et of nterface condton. In the context of th reduced model we are able to derve a general Butler-Volmer equaton. We dcu the applcaton of the new Butler-Volmer equaton to dfferent cenaro lke electron tranfer reacton at metal electrode, the ntercalaton proce n lthum-ron-phophate electrode and adorpton procee. We llutrate the theory by an example of electroplatng. 1 Introducton Energy converon n battere, fuel-cell or redox-flow-cell requre electrochemcal urface reacton to take place at the contact between electrode and electrolyte. In electroly or electroplatng, on the other hand, electrcal energy ued to drve a chemcal reacton. In all thee applcaton, t oberved that the urface reacton rate R, or equvalently the electrc current denty j e, related to a potental dfference at the nterface, the urface overpotental η S. The mot mple relaton of th knd the emprcal Tafel-equaton [Taf5] η S = a + b logj e, 1 where the coeffcent b called the Tafel-lope and a a further phenomenologcal coeffcent. A more general relaton that account for multaneou anodc oxdaton reacton and cathodc reducton reacton on the ame electrode urface the Butler-Volmer equaton [BF1, BRGA2, NTA4], j e = A exp αa e η S C exp α C e η S. 2 Heren A/C are called anodc and cathodc exchange current, repectvely, whch can be general functon of the temperature T and the concentraton of the dfferent chemcal pece. The tranfer coeffcent α A and α C are condered a phenomenologcal coeffcent. The Boltzmann 1
4 contant denoted by k and e the elementary charge. If the exchange current are contant, the Butler-Volmer equaton predct contant Tafel-lope at larger overpotental. Such a behavor can n fact be oberved, mot pronounced n the cae of the hydrogen reducton reacton whch how contant Tafel lope over a range of everal decade of the current j e [Vet61]. In other reacton a tronger dependence of the Tafel lope on potental and temperature can be oberved. Thee devaton from the lnear Tafel behavor are explaned wthn the Marcu-Huh theore [Mar56, Mar65, Mar93, Hu58, Hu99]. We do not conder thee theore here n detal but only refer to the comment n the concludng dcuon of our reult n Sect. 7. The Butler-Volmer equaton condered to be the central equaton n phenomenologcal electrode knetc [BRGA2, p. 153]. All the more t urprng to fnd n the lterature dfference n the way the potental are defned, cf. [NTA4] v. [BvSB9] or [LZ11], and how the exchange current depend on the concentraton, cf. [NTA4] v. [LZ13]. Orgnally, the dervaton of the Butler-Volmer equaton baed on knetc theory [But24, EGV3]. Recently ome approache have been made to gve the Butler-Volmer equaton a thermodynamc jutfcaton. A thermodynamc defnton of an overpotental a a urface exce quantty wa ntroduced n [KRB96] and n [KR3] an attempt wa taken to gve a jutfcaton of the Butler-Volmer equaton wthn a meocopc thermodynamc theory. Recently a Butler-Volmer equaton for oxdaton reacton n fuel cell wa derved wthn the GENERIC framework [BKÖ14], and n the context of phae eparatng electrode materal, a Butler-Volmer equaton n non-equlbrum thermodynamc wa derved [Baz13]. In th paper we derve a general Butler-Volmer of the form 2 whch not retrcted to a pecfc applcaton cenaro. The dervaton baed on a thermodynamc content model for electrochemcal ytem n non-equlbrum and follow from ratonal argument. By th procedure we want to clarfy characterzaton of the dfferent quantte n the Butler-Volmer equaton and the tructure of functonal dependence between them. For the dervaton of 2 t mportant to bear n mnd: 1 The defnton of η S not evdent from modelng baed on non-equlbrum thermodynamc where the et of thermodynamc varable contan the electrc feld E = ϕ but not a potental ϕ telf or ome potental dfference. 2 The nterfacal Maxwell equaton requre that the electrc potental ϕ contnuou at an nterface. Therefore no natural potental dfference ext, whch can be ued to defne an overpotental. 3 The generalty of A/C retrcted by the 2nd law of thermodynamc. In conequence, the forward and backward reacton can not be modeled ndependently. 4 In order to have a general form of the Butler-Volmer equaton that doe not depend on a pecfc expermental etup, the functon have to be expreed n term of the chemcal potental ntead of concentraton or partcle dente. Uually Butler-Volmer equaton eem to mplctly aume that materal are modeled a deal mxture of gae. The underlyng model for electrochemcal ytem n thermodynamc non-equlbrum wa formulated [DGM15]. We call th the complete model nce the dffue charge layer are patally 2
5 reolved. The conttutve law for the nterfacal reacton rate are not of Butler-Volmer type. Under the aumpton of approprate calng relaton, the double layer can aymptotcally be condered n equlbrum. Th allow the dervaton of a locally electroneutral reduced bulk model uch that all feature of the double layer are contently ncorporated nto a et of jump condton at the nterface, ee [DGM15]. Wthn th reduced model, we are able to defne an overpotental and to recover relaton of Butler-Volmer type for the nterfacal reacton rate. Our approach to generalze the Butler-Volmer equaton contrary to thoe uggeted prevouly. Intead of tartng from tandard Butler-Volmer equaton and then ung a-pror aumpton about a pecfc electrochemcal ytem wth t partcular double-layer tructure to derve new Butler-Volmer equaton, we tart from a general thermodynamcally content model and can derve a general Butler-Volmer equaton whch under approprate aumpton reduce to the clacal varant. Outlne. After the ntroducton of the notaton n the followng ecton, we recaptulate n Secton 3 the reduced bulk model of [DGM15]. In Secton 4, we derve a general Butler-Volmer equaton and then dcu n Secton 5 t applcaton to dfferent relevant cenaro. For llutraton of the Butler-Volmer equaton and the nteracton wth the bulk tranport, we conder the example of copper depoton and doluton n Secton 6. Fnally, n Secton 7, we dcu the range of valdty of the underlyng aumpton, compare our reult wth the lterature and dcu the role of the double layer tructure. 2 Decrpton of reactng mxture Here we ntroduce the notaton for the decrpton of reactng mxture n ubdoman eparated by planar electrochemcal nterface. For mplcty we conder a planar tuaton where two onedmenonal regon Ω ± R are eparated by an nterface I = Ω + Ω. For quantte defned n the bulk doman there wll often be correpondng quantte on the nterface I, ndcated by a ubcrpt. 2.1 Conttuent and chemcal reacton In each of the two doman Ω ± and on the nterface I we conder a mxture of everal conttuent. The total number of conttuent n the ubdoman Ω ± denoted by N + 1 and the et of conttuent M = {A, A 1,, A N }, uually ndexed by α {, 1,, N}. In general we have dfferent conttuent n Ω + and Ω, but for the mplcty of notaton th fact wll only be ndcated f neceary. We aume that each conttuent of Ω ± alo preent on I, but n addton there may be conttuent that are excluvely preent on I. Accordngly, the number of conttuent on I N S N and the et of conttuent M S = {A, A 1,, A NS }. A conttuent A α ha the atomc ma m α and may be carrer of charge z α e, where z α the charge number and e the elementary charge. We may have chemcal reacton among the conttuent. There are M bulk reacton and n addton there may be M S urface reacton of 3
6 the general form a A + + a N A N a A + + a NS A NS R f ba + + b N A N for {1,, M}, 3a R b R f R b b A + + b NS A NS for {1,, M S }. 3b The contant a α, b α are potve nteger and γ α = b α a α denote the tochometrc coeffcent of the reacton. The reacton from left to rght called forward reacton wth reacton rate Rf >. The reacton n the revere drecton wth rate R b > the backward reacton. The net reacton rate defned a R = Rf R b. Snce charge and ma have to be conerved by every ngle reacton n the bulk and on the nterface, we have N z α γα = α= N m α γα = α= and and N S α= N S α= z α γα =, m α γα =. 4a 4b 2.2 Bac quantte In each pont x Ω ± and at any tme t >, the tate of the mxture characterzed by the number dente n α α=,1,,n, the barycentrc velocty v, the temperature T and the electrc potental ϕ. On the nterface I the mxture characterzed at any t by the number dente of the nterfacal conttuent, n α α=,1,,ns, the velocty w of the nterface, the nterfacal temperature T and electrc potental ϕ. Multplcaton of the number dente n α by the ma m α gve the partal ma dente: ρ α = m α n α and ρ α = m α n α. 5 For the mxture, the ma denty defned by ρ = N α= The free charge dente are defned a n F = ρ α and ρ = N z α e n α and n F = α= N α= ρ α. 6 N z α e nα. 7 α= 4
7 .8.6 complete model reducded model ϕ [V].4.2 λ L metal electrolyte x [nm] Fgure 1: Electrc potental ϕ at an nterface n equlbrum. The complete model reolve the boundary layer and the electrc potental contnuou dahed lne. The lmt λ yeld a reduced model, where the electrc potental can be dcontnuou at the nterface old lne. 2.3 Jump at nterface We ntroduce the boundary value and the jump of a generc functon ut, x n Ω ± at the nterface I a u ± I = lm u and [u ] = u + x Ω ± I u I. 8 I In cae that the functon u not defned n ether Ω + or n Ω, we et the correpondng value n 8 to zero. The normal ν to the nterface I alway pont from Ω to Ω +. In th one-dmenonal ettng, we have ν = ±1. 3 Reduced bulk model for the thn double layer lmt In a prevou work [DGM15] we derved a thermodynamc content model, whch decrbe the electrochemcal nterface between two arbtrary mxture. Th model we call the complete model, becaue t patally reolve the charge layer n the vcnty of the nterface, the double layer. The charactertc length cale for the charge layer λl ref = ε e 2 n ref, 9 where k the Boltzmann contant, n ref denote a charactertc value for the number denty and L ref a charactertc length of the ytem. For example, L ref can be the dtance between two electrode and n ref can be related to the anon and caton denty n an electrolyte. Then, the length λl ref repreent the well known Debye length. For a oluton of.1mol per lter, λl ref m. Snce often λ 1, we appled n [DGM15] the method of formal aymptotc analy to derve from the complete model a reduced bulk model for the lmt λ. Th reduced model characterzed by mplfed bulk equaton and new urface equaton for the thn double layer nterface. The Fgure 1 how the the electrotatc potental at a metal-electrolyte nterface gven by the complete model and t approxmaton by the reduced model. 5
8 Model aumpton. The reduced model derved from the complete model under the followng aumpton, cf. [DGM15]: 1 The parameter λ atfe the condton λ 1. 2 The ytem under conderaton can be treated a a one dmenonal problem. 3 The electrc feld qua-tatc, the magnetc feld can be gnored. 4 Qua-tatc momentum balance, vcoty n the bulk doman neglgble. 5 Iothermal cae, the bulk temperature T and urface temperature T are contant and atfy T = T. 3.1 Bulk and nterface equaton The reduced model rele on unveral balance equaton whch are ndependent of the pecfc materal. In addton we need conttutve equaton for the materal at hand. In the othermal cae the unveral equaton are the balance equaton of ma and momentum. In addton there a local electroneutralty condton that a conequence of Maxwell equaton n the aymptotc lmt λ. Bulk equaton. In the bulk Ω ±, the electrc potental ϕ, the velocty v and the number dente n α for α =, 1,, N are determned by t m α n α + x m α n α v + J α = M γαm α R for α =,..., N, 1a =1 x σ =, N z α e n α =. α= 1b 1c The quantte J α and σ are the ma flux of the conttuent A α and the Cauchy tre tenor, repectvely. Note that there are only N ndependent dffuon fluxe and the flux J determned by the de condton N α= J α =. Interface equaton. dente n α for α =, 1,, N S we have For the nterfacal peed w, the electrc potental ϕ and the number M S [m α n α v wν + J α ν ] = γ α m α R, α =,, N S, 11a =1 q + + n F + q =. [σ ] =, 11b 11c 6
9 The lat equaton the electroneutralty condton of the double layer I, whch a conequence of the Maxwell equaton n the aymptotc lmt λ, cf. [DGM15]. In general the determnaton of q ± requre the oluton of an addtonal ytem of equaton that patally reolve the layer tructure. Therefore the nterface equaton 11a-11c are not a cloed equaton ytem contanng excluvely the varable of the reduced ytem that were ntroduced above. But n many relevant cae, e.g. for the nterface at a metal electrode, an explct oluton of the nterface electroneutralty condton 11c not neceary. Snce t can be decoupled from the ret of the equaton ytem, and then erve only to determne one remanng urface number denty wthn a pot proceng tep. 3.2 Conttutve equaton The unveral equaton 1a 11b need to be upplemented by conttutve equaton for the ma fluxe J α, the reacton rate R, R and the Cauchy tre tenor σ. The conttutve equaton are retrcted by the prncple of materal objectvty and the 2 nd law of thermodynamc. Free energy and chemcal potental. In order to cover a wde cla of dfferent materal we ue free energy functon of the general form ρψ = ρ ˆψT, ρ,..., ρ N, ρψ = ρ ˆψ T, ρ,..., ρ N. 12 S Note that due to the aymptotc lmt, the free energy functon are ndependent of the electrc feld, cf. [DGM15]. The chemcal potental of the bulk and urface materal are defned by µ α = ρψ ρ α and µ α = ρψ. 13 ρ α Conttutve equaton n bulk. For the dffuon fluxe, the preure and the reacton rate we chooe the followng relaton that guarantee the contency wth the 2 nd law of thermodynamc partcularly the non negatvene of the entropy producton N µβ µ J α = M αβ x + e zβ z x ϕ, α = 1,, N, 14a T T m β m β=1 σ = p wth p = ρ ˆψ R = R [ exp β A α= N m α n α µ α, α= N γαm α µ α exp 1 β A N ] γαm α µ α. α= 14b 14c Here R and A denote potve phenomenologcal coeffcent and M αβ defne the moblty matrx that mut be potve defnte. Although not requred by the 2 nd law of thermodynamc, we aume < β < 1. The quantty p the preure whch gven by the Gbb-Duhem equaton 14b 2. All conttutve equaton are related to each other nce they hare the dependency on the chemcal potental and thu on the free energy ρψ. 7
10 Conttutve equaton for the nterface. A n the volume, the conttutve relaton are all related to the urface free energy ρψ and can not be modeled ndependently of each other. We chooe the followng thermodynamc content conttutve equaton for the reacton rate and the ma fluxe on I [ A R = R exp β N S γ αm α µ α α= A exp 1 β N S ] γ αm α µ α, 15a α= ρv w ± ± = L µ + z e I m ϕ ± µ I + z e m ϕ, 15b ρα v wν + J α ν ± I = M ± α exp β α ± mα B ± α D α ± exp β ± α 1 mα B ± α D α ±. 15c where R, A, L ±, M α and B ± α denote potve phenomenologcal coeffcent. The coeffcent β and β α ± are uually called ymmetry factor. Although not requred by the 2nd law of thermodynamc, we aume < β, β α ± < 1. By D α ± we denote the drvng force for adorpton, D ± α = µα + zαe m α ϕ ± I µ + z e m ϕ ± I µ α + zαe m α ϕ µ + z βe m ϕ. 16 We hghlght, that n [DGM15] lnear relaton were choen for all conttutve law except for the reacton n the volume and on the urface, where an Arrhenu-type exponental form wa choen. Here we alo chooe the exponental relaton for the adorpton n 15c. We note that near to equlbrum,.e. D ± α 1, 15c can be lnearzed reultng n the conttutve equaton NS ρα v w + J α ± = I β=1 M ± αβ [ µβ + z βe m β ϕ ± µ I + z e m ϕ ± I µ β + z βe m β ϕ µ + z βe m ϕ ], 17 ± whch concde wth the repectve equaton n [DGM15, eq. 3], f the matrx M αβ choen dagonal. 3.3 Remark Electrotatc potental. When dervng a trctly thermodynamcally content model, the proper thermodynamc varable the electrc feld E = ϕ, not the electrotatc potental ϕ telf [MR59, dm84, Mül85]. The explct dependence of the conttutve relaton 15b and 15c on ϕ only jutfed a a conequence of the formal aymptotc approach n [DGM15]. Moreover, n the reduced model above, ϕ not requred to be contnuou acro the nterface and hence we have to ntroduce on I the varable ϕ that n general not an one-ded lmt of ϕ n the bulk. 8
11 Electrochemcal potental. It remarkable that the conttutve relaton 14a, 15b and 15c depend explctly on the electrochemcal potental µ e α = µ α + zαe m α ϕ and µ α = µ e α + zαe m α ϕ. 18 If we ue the electroneutralty condton 1c and the charge conervaton for chemcal reacton 4b we can alo expre the conttutve equaton of the Cauchy tre tenor σ and the reacton rate R and R n term of electrochemcal potental. Such that all conttutve equaton 14 and 15 can be expreed n term of electrochemcal potental ntead of chemcal potental. Electrc current. In the reduced model, the electrc current gven by the mple relaton j e = N α= z α e m α J α. 19 Moreover, we can derve from 1 and 11 the followng tatonary balance equaton for the electrc current j e n the bulk and on the nterface x j e = and [j e ν ] =. 2 The electrc current contant n each ubvolume Ω ± and contnuou at the nterface I. Hence j e even a global contant n the whole electrochemcal ytem. Electrc current reacton rate. In the reduced model there a mple relaton between the electrc current j e and the reacton rate R. We ntroduce the ubet J + M of all pece that are preent n Ω +. Then multplyng n Ω + the urface balance 11a for the pece A α J + by z α e /m α and ubequent ummaton together wth 1c yeld M S γα z α e R = =1 α J + α J + zα e n α v wν + zαe m α J α ν + I 1c,19,2 = j e ν I. 21 If there only one urface reacton,.e. M S = 1, we get the drect proportonalty j e ν I = γ α J + α z α e R n Ω ±, 22 that allow the alternatve formulaton of the Butler-Volmer equaton 2 a a logarthmc relaton between electrc current and the overpotental. 4 Dervaton of the Butler-Volmer equaton The above reduced model form the thermodynamc content ba for a dervaton of the Butler-Volmer equaton. The dervaton not retrcted to a ngle urface reacton. Therefore 9
12 we derve for each urface reacton a correpondng Butler-Volmer equaton. The am of the, dervaton to dentfy exchange rate R f/b, coeffcent α f/b and the overpotental η S n term of bulk quantte: number dente n α and electrc potental ϕ. In contrat, the conttutve relaton 15a for the reacton rate depend only on the nterfacal quantte: nterfacal number dente nα and urface electrc potental ϕ. The neceary relaton between bulk and nterfacal quantte etablhed by the conttutve relaton 15b and 15c for the ma fluxe. Decompoton of the et of bulk conttuent. For the dervaton of the Butler-Volmer equaton, t neceary to decompoe the et of all bulk conttuent nto two djont et M = M + M, M + M =, uch that all conttuent A α M + are preent n Ω + and all conttuent A α M are preent n Ω. In general, t poble that a conttuent preent n both bulk doman Ω + and Ω. Then, the decompoton of the et M nto the ubet M ± not unquely defned. Aumpton of fat adorpton. The conttutve equaton 15b and 15c for the ma fluxe are the boundary condton that decrbe the adorbton of the conttuent at the nterface I. Here we are only ntereted n the lmt cae of fat adorpton,.e. L ± and M ± α. Then, we obtan the contnuty of the electrochemcal potental at the nterface µα + zαe m α ϕ ± I = µ α + zαe m α ϕ for α M. 23 Equlbrum, Nernt equaton. At frt we conder the equlbrum,.e. R =, to how the bac tep for the dervaton of the Butler-Volmer equaton wthn the reduced model. We denote all quantte that depend on the equlbrum tate by an overbar. For every urface reacton, we deduce from R = and 15a the law of ma acton, vz. = αm α µ α. 24 γ α M S Accordng to 4a, each reacton conerve electrc charge. Therefore = αm α µ α + zαe m α ϕ. 25 α M S γ Next we ue the fat adorpton lmt 23 to replace the nterfacal quantte by bulk quantte, = γα mα µ α + z α e ϕ ± + γ I α mα µ α + z α e ϕ, 26 α M α M S \M where n the frt um the bulk value taken from Ω + f α M + and ele taken from Ω. A an abbrevaton we defne the coeffcent Γ + = αz α, Γ = αz α and Γ = γ αz α 27 γ α M + γ α M α M S \M 1
13 The conervaton of charge 4a mple Γ + + Γ + Γ =. Now we obtan for the th reacton the alternatve repreentaton of the ma acton law = γαm α µ α ± + γ I αm α µ α + Γ +e [ ϕ ] + Γ e ϕ ϕ I. 28 α M α M S \M We oberve that by ung the conttutve equaton 23, we are able to expre the urface law of ma acton 24 n term of bulk quantte. By th we automatcally ntroduce an electrc potental dfference [ ϕ ] acro the nterface. In the cae M S \ M =, and hence Γ = and Γ +, 28 reduce to the well known Nernt-equaton Γ +e [ ϕ ] = α M γ α m α µ α ± I. 29 Generalzed Butler-Volmer equaton. Next we conder an arbtrary tme dependent tate. In order to replace the urface chemcal potental n 15a by the bulk chemcal potental, we apply the ame tep lke n the dervaton of the Nernt equaton n the above veron 28. Therefore we frt rewrte the drvng force of the chemcal reacton 15a a αm α µ α = γαm α µ α ± + γ I αm α µ α + Γ +e [ϕ ] + Γ e ϕ ϕ I α M γ α M S α M S \M Becaue we want to ntroduce the overpotental η S a the devaton from an equlbrum potental, we ubtract the law of ma acton 28 from 3 to get γαm α µ α = γαm α µα µ α ± + γ I αm α µ α µ α 31 α M S α M + Γ +e [ϕ ϕ ] + Γ e ϕ α M S \M ϕ I ϕ ϕ For each urface reacton we defne t repectve overpotental by [ϕ ϕ ] + Γ ηs Γ + ϕ ϕ I := ϕ ϕ I, f Γ + ϕ ϕ I ϕ ϕ I, f Γ+ = Inertng the dentty 31 nto 15a yeld net reacton rate R n the form of a Butler-Volmer equaton, vz. R = R, f wth the tranfer coeffcent a β A Γ αf +, f Γ +, = β A Γ, f Γ + =, exp α f e η S R, b I 3 32 exp + α b e η S β A Γ and αb +, f Γ +, = 1 β A Γ, f Γ + =, 34 11
14 and the exchange rate R, f R, b = R exp = R exp β 1 β [ A γ αm α µα µ α ± + I A α M α M S \M [ γ αm α µα µ α ± + I α M α M S \M γαm α µ α µ γαm α µ α µ ] α α, 35a ]. 35b Contency of conttutve equaton. Due to the knetc approach, Butler-Volmer equaton n the tandard lterature uually contan an explct dependency on the number dente n α, cf. [But24, EGV3, NTA4, BRGA2, BF1]. In contrat, the general Butler-Volmer equaton above ha only an ndrect dependency on n α becaue of the chemcal potental. Such a formulaton n term of the chemcal potental can alo been found n Butler-Volmer equaton whch have been propoed recently for pecfc applcaton lke L-on battere or fuel cell, cf. [LZ13, Baz13, BKÖ14]. We want to emphaze the mportance of the chemcal potental for a content modelng: In the formulaton of the conttutve equaton 14 and 15, we followed the approach of non-equlbrum thermodynamc that mpoe compatblty contrant. Th ha the advantage, that the modelng conderably mplfed, becaue once the free energy denty pecfed, all conttutve equaton are determned unquely and n a compatble way, up to the choce of phenomenologcal coeffcent. Compared to 15a, urface chemcal potental µ α have been replaced by bulk chemcal potental µ α n By th, we ntroduced a dependency of the conttutve law for the urface reacton on the bulk free energy ρψ. Hence the urface reacton rate are alo related to the conttutve equaton n the bulk 14 and can not be modeled ndependently of each other. Wthn our framework, a Butler-Volmer equaton formulated n term of n α would mply a pecfc form of the free energy dente and thu mpoe compatblty contrant for the other conttutve equaton n the bulk and the urface. Smplfcaton n the abence of excluve urface pece. Although mot of the urface quantte have been replaced by volume quantte, the general Butler-Volmer equaton tll contan urface quantte. However, n many electrochemcal ytem the conttuent on the urface are alo preent n at leat one of the bulk phae,.e. M S \ M =. Th lead to a repreentaton of the Butler-Volmer equaton that ndependent from the urface quantte and we get a ngle overpotental for all urface reacton a well a mplfed reacton coeffcent whch excluvely depend on bulk quantte, η S = [ϕ ϕ ], 36 A = R exp β γ αm α µα µ α ±, 37 I α M A = R exp 1 β γ αm α µα µ α ±. 38 I R, f R, b α M 12
15 The potental dfference η S then decrbe the devaton of the actual potental dfference ϕ + I ϕ I from the equlbrum voltage ϕ + I ϕ I of the bulk phae that n accordance wth uual defnton n electrochemtry [BRGA2, NTA4]. 5 Adapton to dfferent electrochemcal ytem In th ecton, we tudy two cenaro for the applcaton of Butler-Volmer equaton: Frt, we conder a prototypcal metal-electrolyte nterface lke t can be found n many electrochemcal applcaton. Next we turn to the electron tranfer reacton n modern lthum-on-battere. Here we are ntereted n the decrpton of the ntercalaton materal FePO 4 that undergoe a phae tranton durng the ntercalaton proce. Moreover, we dcu the orgn of Butler-Volmer type relaton n the cae of lthum depoton from an electrolyte to a metallc lthum. In partcular we demontrate that Butler-Volmer type equaton do not alway orgnate from an electron tranfer reacton, but t can reult from an ntercalaton proce or a low adorpton proce ntead. 5.1 General metal electrode We conder a metal-electrolyte nterface wth an electron tranfer reacton of the form R f O + n e R. 39 Rb Here O and R denote the oxdzed and the reduced pece, repectvely, and n the number of tranfered electron. The backward reacton R b n 39 the oxdaton and the forward reacton R f the reducton. The tochometrc coeffcent related to 39 are γ O = 1, γ e = n and γ R = We decrbe the metal a a mxture of free electron e and metal on M. The electrolyte a mxture contng of a olvent S a well a anon and caton. The oxdzed pece O alway a potvely charged caton of the electrolyte. If the reduced pece doe not concde wth M, then we aume R to be a further caton pece of the electrolyte. Becaue there are no excluve urface pece nvolved n the reacton 39, we can aume that all pece whch are preent on the metal-electrolyte-nterface, are alo preent n Ω + or Ω,.e. M S \ M =, and hence Γ =. Followng the conventon [NTA4, pp. 7] we want the anodc oxdaton reacton to be domnant for potve overpotental η S. From 33 and 34 we ee that Γ + ha to be potve and thu we chooe Ω + to repreent the metal electrode. We denote the electrc potental n the metal by ϕ M = ϕ + I and the electrc potental on the electrolyte de by ϕ E = ϕ I. The overpotental then gven by η S = ϕ M ϕ E ϕ M ϕ E
16 Specfc bulk materal model for metal and electrolyte. To get a fully explct Butler-Volmer equaton we have to pecfy free energy dente for the metal and the electrolyte. Here we wll only ntroduce the chemcal potental and refer for the correpondng free energy dente to [DGL14a, DGL14b, DGM13]. The elatc model for the metal appled n [DGL14b] mple that the number denty of the metallc on n M contant. Then, the electro-neutralty condton 1c requre that the electron denty n e alo contant. Thu n an othermal proce, alo the chemcal potental of the metal on and electron n the bulk are contant n pace and tme, and we et µ M = µ M and µ e = µ e. 42 The electrolyte aumed to be an deal mxture. Then the chemcal potental of the electrolyte pece are gven by [DGM15, DGL14a] µ α = g α T, p + m α ln y α for α = A, C, S. 43 Here y α = n α / β n β denote the mole fracton of conttuent A α and g α the Gbb free energy of the correpondng pure ubtance. In general the preure dependence of g α of outmot mportance n the complete model, ee [DGM13, DGL14a], whch contan more nformaton on the thermodynamc modelng of electrolyte. Here, n the reduced model, the preure contant due to the momentum balance equaton 1b and the mple conttutve equaton 14b. Cae I: The metal on are not nvolved n reacton,.e. M R. We obtan the coeffcent Γ + = n and α f = βan and α b = 1 βan. 44 In equlbrum,.e. R =, the Nernt equaton 28 take the form ϕ M ϕ E = ne ln ȳo ȳ R + 1 ne m O g O m R g R + n m e µ e. 45 Snce the chemcal potental of the electron are contant, the equlbrum potental ϕ M ϕ E vare logarthmcally wth mole fracton ȳ O and ȳ R of the on when preure p and temperature T are kept fxed. In Butler-Volmer equaton 33 for the reacton 46 the contrbuton of the electron vanh due to 42. Wth γ O = 1, γ R = 1 and µ O and µ R accordng to 43, the Butler-Volmer equaton read β βan e ȳr y 1β 1 βan e O A R = R exp ȳo ȳ O y R η y R A S R exp η S ȳ R y O 46 wth the overpotental η S gven by 41. When applyng 22, we get a Butler-Volmer equaton a a relaton between overpotental and electrc current,.e. j e ν I = jc exp β A n e η S 1 βan e ja exp η S, 47 14
17 wth the repectve anodc and cathodc exchange current j C = ne R ȳr ȳ O y O y R β A ȳo and ja y R = ne R ȳ R y O 1β A. 48 When R >, then the domnant reacton the reducton, whch depend on the avalablty of uffcently many partcle of pece O. Keepng R > fxed, then we conclude from 46 that y O requre η S. Analogouly, aumng R < a precrbed contant rate, then the oxdaton reacton domnant and y R mple η S +. Further, for y O 1 we ee that already a mall overpotental, η S /e, uffcent to reach a rate R <. Therefore the Butler-Volmer equaton 46 mple the expected behavor that the oxdaton favorable for a low concentraton of the oxdzed pece. Cae II: The meal on are the reduced pece,.e. M = R. We obtan the coeffcent Γ + = z R + n = z O and α f = βaz O and α b = 1 βaz O. 49 In equlbrum,.e. R =, the Nernt equaton 28 take the form ϕ R ϕ E = z O e ln ȳ O + 1 z O e m O g O m R µ R + n m e µ e. 5 Snce the chemcal potental of the metal on and electron are contant, there reman only a logarthmc dependence of the equlbrum potental ϕ M ϕ E on ȳ O. The Butler-Volmer equaton 33 for the reacton 46 mplfe conderably becaue the contrbuton of the chemcal potental of e and R vanh due to 42. Wth γ O = 1 and µ O accordng to 43, the Butler-Volmer equaton read R = R yo ȳ O βa exp β Az O e ȳo η S R wth the overpotental η S gven by 41. From 22, we get j e ν I = jc exp β y O Az O e η S ja exp 1βA exp 1 β 1 β wth the repectve anodc and cathodc exchange current 1βA ȳo ja = z O e R and jc = z O e R y O Az O e η S 51 Az O e η S, 52 yo ȳ O βa. 53 We hghlght two dfference to the prevou cae and 46. Frt, n 51 there no mechanm for η S to blow up f an oxdaton at a fxed rate R < precrbed. Second, the tranfer coeffcent n front of η S dffer between 46 and 51 f z R and hence z O n. 15
18 5.2 Lthum ron phophate electrode The electrode materal L y FePO 4 LFP a cheap and afe electrode materal for lthumon battere [PNG97]. A LFP electrode cont of a metallc carrer fol, carbon-coated LFP nano-partcle, bnder and further addtve mprovng the electrc and onc conductvty and the mechancal properte of the battery. We decrbe the LFP nano partcle a a bnary mxture of crytallne FePO 4 - and L-atom. The lthum atom can move freely through the FePO 4 crytal lattce [HVdVMC4, Baz13, DJG + 1]. Snce FePO 4 ha a low electrc conductvty, we can neglect the electron tranport nde the nano partcle. To etablh electrc conductvty of the LFP, the LFP partcle are coated wth a thn carbon layer [MDCK + 7]. Therefore we have free electron e on the urface, n addton to the electrolyte and electrode pece, a well a the non-reactng carbon. The electrolyte cont of an organc olvent S, lthum on L + and the aocated anon A. At the ron phophate-electrolyte nterface, we conder the electron tranfer reacton The tochometrc coeffcent are L + + e L. 54 γ L + = 1, γ e = 1 and γ L = We let Ω + repreent the doman of the ron phophate electrode. Accordngly the electrolyte occupe the doman Ω. Then we get the coeffcent Γ + =, Γ = 1, and Γ = For the phenomenologcal coeffcent α f,b we obtan α f = βa and α b = 1 βa. 57 Snce we model the LFP-electrode a mxture of electrcally neutral pece, there no pace charge layer nde the electrode. In conequence the electrc potental patally contant and atfe ϕ + I = ϕ. Denotng the electrc potental n the electrolyte by ϕ E = ϕ I and n the ron phophate by ϕ LFP = ϕ + I, the overpotental n 32 can be expreed a η S = ϕ LFP ϕ E ϕ LFP ϕ E. 58 Specfc materal model. A before, the electrolyte modeled a a mple mxture wth chemcal potental gven by the conttutve equaton 43. The chemcal potental of the urface electron aumed to be contant,.e. µ e = µ e. Lthum-on battere wth LFP-electrode are characterzed by a phae tranton between lthum-poor and lthum-rch phae nde the LFP-electrode [PNG97, WSvA + 12, LML + 15]. There are everal approache to model for LFP electrode takng the phae tranton nto account [ZB14, BCB11, HVdVMC4, DGH11, DJG + 1]. A common feature of all thee model a non-monotone chemcal potental of lthum 16
19 n LFP. The mplet conttutve equaton for the chemcal potental µ L, that account for heat of oluton and the entropy of mxng, gven by µ L = L m L 1 2y L + m L lnyl ln1 y L. 59 Here y L = n L /n FePO4 the mole fracton of lthum and L the heat of oluton. Mechancal contrbuton due to the volume change of LFP durng the lthum ntercalaton are not condered here. Butler-Volmer equaton for LFP. The Butler-Volmer equaton for the reacton 54 at the ron phophate-electrolyte nterface gven by βae 1 βa e R = Rf exp η S Rb exp η S 6 wth the coeffcent R f = R R b = R yl + ȳ L + ȳl + y L + 1 y L ȳ L βa exp + 2L yl ȳ L, 61a 1 ȳ L y L 1 ȳ L y L exp 1 y L ȳ L 2L yl ȳ L 1βA. 61b Compared to the Butler-Volmer equaton 46 for the metal-electrolyte nterface, the tructure of the tranfer coeffcent more complex becaue of the dependency on the lthum mole fracton y L. For ntercalaton electrode, the overpotental hould blow up f the electrode completely flled or empty, cf. [LZ13]. Th requrement atfed by the Butler-Volmer equaton 6: Durng the ntercalaton of lthum n the electrode, the reducton the domnatng reacton and thu the reacton rate n 6 ha to be potve. Aume an ntercalaton proce wth a fxed potve rate R >. For y L 1, the tranfer coeffcent yeld and Rf and R b +. Then, the Butler-Volmer equaton 6 mple η S. Analogouly, for dentercalaton proce wth a contant negatve reacton rate R <, the overpotental ha to ncreae η S + for y L. 5.3 Lthum electrode The metallc lthum electrode n contact wth an lthum conducton electrolyte erve a an example where the Butler-Volmer equaton doe not orgnate from an electron tranfer reacton. Intead, the Butler-Volmer equaton here can reult from two dfferent procee: urface reacton wthout charge tranfer and adorpton. Let the doman Ω + repreent the metallc lthum electrode and Ω repreent the electrolyte doman. The electrolyte cont of lthum on L +, the aocated anon A and a olvent S. A already decrbed n Secton 5.1, the metal electrode cont of potve metal on L + M and free electron e. Snce L + and L + M have the ame charge number, an electrc current can not orgnate from an electron tranfer reacton 17
20 between thee two pece. However meaurement how, that alo th proce generate an exponental relatonhp of Butler-Volmer type between the overpotental of the lthum-electrolyte nterface and electrc current [MBS87]. Therefore th behavor ha to orgnate form one of the three mechanm: 1 Adorpton of the lthum on from the electrolytc oluton to the metal-electrolyte nterface. 2 Intercalaton of lthum from the electrolyte phae to the metal phae,.e. L + M L + at I Adorpton of lthum on and electron from the metal to the metal-electrolyte nterface. In general the thrd mechanm aumed fat compared to the other, and therefore can not the orgn of the non-lnear relatonhp between overpotental and current. The fat adorpton modeled by L + + n 15b and M e n 15c. We obtan on the lthum metal de the equaton µ L + M + e m L + M ϕ + I = µ L+ M + e m L + M ϕ and µ e e m e ϕ + I = µ e e m e ϕ. 63 We denote the potental n the lthum metal wth ϕ M = ϕ + I and n the electrolyte wth ϕ E = ϕ I. Accordngly, we defne the overpotental a η S = ϕ M ϕ E ϕ M ϕ M. Now we how that both cae, a low reacton and a low adorpton, reult n a Butler-Volmer equaton of the ame type. We ue the ame conttutve relaton for the metal and the electrolyte a n Secton 5.1. Cae 1: Slow reacton and fat adorpton. Let the reacton 62 be the lmtng proce. We can aume that the fat adorpton aumpton 23 atfed,.e. µ α + zαe m α ϕ + I = µ α + zαe m α ϕ α = L +, A, S, 64 and the reult of Secton 4 are applcable. In partcular we can ue the reult of Secton 5.1, nce the dervaton hold alo n the cae where no electron are nvolved n the reacton 39. We have j e ν I = jc exp wth anodc and cathodc exchange current β Az L + e η S ja exp 1 β Az L + e η S, 65 j A = z L +e R ȳl + y L + 1βA and j C = z L +e R yl + ȳ L + βa
21 Cae 2: Fat reacton and low adorpton. The reacton 62 fat compared to the adorpton. We can aume that the knetc parameter for the reacton rate meet R n 15a. Then the law of ma acton read µ = µ. 67 L+ L+ M To mplfy the argumentaton n the followng, we aume that the adorpton of the olvent S= A to the metal-electrolyte nterface fat,.e. L + n 15b, and we have µ S I = µ S. 68 Further, we aume that the anon cannot adorb at the metal urface,.e. M A = n 15c, and we obtan from the conttutve relaton 15c ρa v wν + J A ν =. I 69 Thee aumpton yeld that the electrc current j e at the nterface gven by the lthum flux j e ν I = e m L + ρl +v wν + J L +ν I. 7 Th relaton follow from the mple relaton 19 for the electrc current, the local electroneutralty condton 1c and the equaton 69. The lthum flux n 7 gven by the conttutve relaton for the adorpton, equaton 15c, ρl +v wν + J L +ν I =M L + exp β m L + L B + L D + L exp β + Here, the drvng force defned a µl D L = z L + e ϕ m L + I L + 1 m L + B L + D L µ L+ + z L + e m L + ϕ. 72 Ung the relaton 63 1 we can wrte the drvng force a µl D L = z L + e ϕ µl m L + I + + z L + e ϕ M m L + I A n the dervaton of the Butler-Volmer µ equaton, we ntroduce an equlbrum tate n whch the drvng force vanhe, leadng to L + z + L + e ϕ = µ m L + I L + + z L + e ϕ +. The drvng M m L + I force 73 can be wrtten a D L = µ + L + µ L + µ I L + µ L + + z L + e η I m S, 74 L + where we have replaced the electrc potental by the overpotental η S = ϕ ϕ + I ϕ ϕ I. Wth the chemcal potental 43 for the lthum on of the electrolyte and the contant chemcal potental for lthum metal accordng to 42, we get for the current β j e ν I = jc L B exp + L z + L +e 1 β η S ja L B exp + L z + L +e η S 19 75
22 wth the exchange coeffcent j A = M L + z L +e m L + 1β ȳl + L + B y L + L + and j C = M L + z L +e m L + β yl + L + B L Thee relaton are dentcal to the Butler-Volmer equaton 65 reultng from the urface reacton. Th remarkable, becaue of the thermodynamc orgn of the non-lnear relaton, whch are derved n one cae from an adorpton proce and n the other cae from an urface reacton. Therefore we can not conclude from a Tafel-plot whether the adorpton or the urface reacton the lmtng urface proce. ȳ L + 6 Example Electroplatng The electroplatng of metal erve a a mple example of an electrochemcal proce wth a urface reacton that can be decrbed by a teady tate. We conder an aqueou copper ulfate oluton between two parallel copper plate, a hown n Fgure 2. The doman of the electrolyte Ω and we let Ω + denote both electrode. We model copper electrode a a bnary mxture of free electron e and cuprou on Cu +. The electrolyte cont of water H 2 O a the olvent S, the anon SO 2 4 and the caton Cu 2+, n the followng alo denoted by A and C, repectvely. j e Ω + e A ν + Cu Cu 2+ I A Ω SO 2 4 H 2O Fgure 2: Expermental etup for electroplatng: aqueou copper ulfate oluton bounded by two copper electrode. The urface normal ν alway pont to the electrode. The role of the electrode a anode or cathode depend on the drecton of the mpoed current j e ν. When a current j e appled to the electrode, copper oxdzed at the anode A and cuprc on are dolved from the the anode nterface I A nto the electrolyte. On the other de, at the nterface I C, the cuprc on are reduced and ncorporated nto cathode C. If the plate are uffcently large the proce one dmenonal. The overall doluton/depoton proce can be plt nto everal tep: I C Cu + adorpton/deorpton of Cu + between electrode bulk and urface, ν e C + Ω j e electron tranfer reacton Cu 2+ + e Cu adorpton/deorpton of Cu 2+ between urface and caton of the electrolyte. The adorpton n the frt and thrd tep condered nherently fat compared to 77 uch that aumpton 23 vald. 2
23 Bulk tranport. The momentum balance 1b mple that the preure n Ω + and Ω contant p = p. Snce we do not conder olvated on here, we may aume that all partcle are of the ame ze. Then the conttutve model for an ncompreble mple mxture [DGM15, DGL14a] together wth global electroneutralty accordng to 1c lead to the relaton n A + n C + n S = n ref, 78a z A n A + z C n C =, 78b where n ref contant total number denty of partcle. The pece A, C and S of the electrolyte atfy the tatonary veron of the ma balance equaton 1a. In the abence of bulk reacton we have to olve n Ω x manav + JA =, 79a x m C n C v + J C =, x ρv =, 79b 79c where we replaced the partal balance of S by the total ma balance. In addton there are two more contrant. Frt, we have to pecfy the total amount of copper ulphate dolved nto the water by precrbng the average number denty n C of caton n the electrolyte. Second, an abolute reference value for ϕ ha to be defned, e.g. ϕ = at I C. The boundary condton 11a for the pece A, C and S at the nterface are m α γ α R A/C = m α n α v w A/C ν + J α ν I A /I C, 8 where R A/C and w A/C denote the reacton rate and the nterfacal peed at I A and I C, repectvely. For the reacton 77 we have γ C = 1 and γ A = γ S =. We multply 8 wth z α e /m α for each α. Then ummaton and the electroneutralty 78b yeld z C e RA/C = zαe m α J α ν I A/C, 81 α {A,C,S} Wth 21 we conclude that z C e R A/C = j e ν and get the boundary condton m α γ α j e = z C e m α n α v w A/C + J α I A/C for α {A, C, S}. 82 Summng the boundary condton 82 for α = A, C, S how m C γ C j e = z C e ρ v w A/C I A/C. 83 From 79c we conclude w A = w C. Thu we can chooe a coordnate ytem uch that w A = w C =. The explct form of the boundary condton 82 m A n A v + J A =, z C e m C n C v + J C = m C γ C j e, z C e ρ v = m C γ C j e. 84a 84b 84c 21
24 n C x [ mol /l] j e [ A/m 2 ] x [ cm ] ϕx [ V ] j e [ A/m 2 ] x [ cm ] Fgure 3: Soluton of 84 over pace for dfferent appled current and a alt concentraton of.5 mol/l. Left: We oberve almot lnear concentraton profle n C wth a lope proportonal to j e. Rght: electrotatc potental ϕ n the electrolyte doman Ω. The boundary condton can be extended to hold n the whole electrolyte doman Ω by applyng 79. We emphaze the drect proportonalty between the electrc current j e and the barycentrc velocty v n the electrolyte n 84c. For th reaon a long a electrc current flow, the barycentrc velocty can not be neglected, a t uually done n the lterature. To olve the ytem 84 numercally, we chooe a dagonal moblty matrx wth M αα = B α T m 2 αn α leadng to the dffuve fluxe J α = B α m α x n α mα n α e m n x n + n α z α xϕ, α = A, C. 85 Note that there no preure dependence n 85 becaue x p =. Smulaton for Ω of length L = 1cm and ung materal parameter accordng to Table 1 yeld oluton n C and ϕ a plotted n Fgure 3. We oberve a nearly lnear patal dtrbuton of the caton wth a lope that proportonal to the mpoed current j e. Wth ncreang current, we oberve a nonlnear behavor of the electrotatc potental ϕ near the cathode, where the caton concentraton get low. A: SO 2 4 C: Cu 2+ aq S: H 2 O m A = u m C = u m S = u B A = /kg B C = /kg Table 1: Materal parameter ued for the calculaton, cf. [Ld5]. Butler-Volmer equaton. A Butler-Volmer equaton for the reacton 77 can be derved n the context of cae II of Sect The choce of Ω + above and 77 mply Γ + = z C = 2. Expermental meaurement n [BM59] how that α A 1.5 and α C.5. We thu chooe A = 1 and β = 1/4 to get from 51 at I A and I C 1/4 nc R = R exp 1 n C 2 e η S 3/4 nc 3 e R exp n C 2 η S
25 5 η [ mv ] anode catode logj e j e [ A / m 2 ] Fgure 4: Tafel plot from the reacton 77 and the computed electrolyte concentraton at I A and I C accordng to 84 wth n C =.5mol/l. Dahed lne for fxed caton concentraton n C = n C at I A and I C how the lnear Tafel lope n the large overpotental regme. Ung the electrolyte concentraton at the nterface from the prevou computaton, we can determne from 86 the overpotental at the anode and cathode, ee Fgure 4. In the Tafel plot we oberve a nearly lnear lope over one decade of the mpoed current dente. When j e get larger, the Tafel plot devate from the lnear behavor. Th effect due to the dependency of the exchange current on the concentraton of the pece. The oberved devaton much more pronounced at the cathode, where n C for larger mpoed current dente. Polarographc curve. From the computed overpotental we can not drectly nfer the potental dfference [ϕ ] over the double layer, nce by defnton η S alo depend on the choen equlbrum value. But, nce n the expermental etup condered here the electrode cont of the ame materal, the equlbrum potental [ ϕ ] the ame at I A and I C. We denote the electrotatc potental n the bulk of the electrode A and C by ϕ A and ϕ C, repectvely. Then the voltage over the complete electrochemcal cell ϕ A ϕ C = η A S + ϕ I A ϕ I C η C S, 87 where η A/C S denote the overpotental at I A/C, repectvely. At a certan mpoed current where n C approache at I C, the overpotental at the cathode, and thu alo ϕ A ϕ C, ha to blow up, a dcued n Sect Motvated by the dffuon lmted current n [BBC5], we defne j e d := 4z Ce B C n C L. 88 For a dluted electrolyte, we oberve that the blow up of the cell voltage occur cloe to j e = jd e, ee Fgure 5. Increang the alt concentraton,.e. ncreang n C, the characterzaton of the lmtng current by jd e get le and le harp, a the blow up of the cell voltage occur at hgher mpoed current. Th dcrepancy ha to attrbuted to the mpler bulk model n [BBC5], where v = aumed n Ω and the on olvent nteracton neglected. 23
26 n C [ mol /l] ϕ A - ϕ C [V] j e [A / m 2 ] n C [ mol /l] ϕ A - ϕ C [V] j e / j e d Fgure 5: Lmtng current caung a blow up of the cell voltage. Left: polarographc curve for dfferent alt concentraton. we oberve a blow up of the cell voltage for dfferent appled current j e. Rght: polarographc curve where the current recaled by jd e defned n Dcuon 7.1 Valdty of the Butler-Volmer equaton The Butler-Volmer equaton not a unveral natural law but t valdty lmted to certan applcaton cenaro. Our general Butler-Volmer equaton 33 can only be vald a long a the aumpton of the underlyng reduced bulk model hold. Frt of all, the reduced bulk model requre that the Debye length mall. That mean, we chooe a charactertc length cale L ref for the electrochemcal ytem under conderaton, uch that the overall ze of the ytem comparable to L ref and the curvature of urface le than 1/L ref. Then the Debye length, whch control the wdth of boundary layer, ha to be maller than L ref by ome order of magntude. In conequence, the Butler-Volmer equaton 33 can not be appled n the context of nano-ytem. Second, the dervaton of the reduced bulk model baed on qua-equlbrum of the boundary layer. For th, t neceary that relaxaton tme of the layer are mall compared to the macrocopc expermental tmecale. In [DGM15], a macrocopc tme cale of t ref = 1 wa ued. Th certanly rule out the applcaton of 33 n procee where exctaton by hort pule or medum to hgh frequence are appled. Moreover n the reduced model, we aumed othermal ytem. If the urface reacton are trongly endothermc or exothermc, the energy balance can not be neglected and the procedure of the aymptotc analy of [DGM15] ha to be appled to the larger ytem of equaton and mght pobly lead to dfferent relaton. Fnally, we remark that n ome cae t mght be more approprate to aume that the number dente of the urface pece are comparable to thoe n the volume. Th would necetate the ncluon of a materal model for the urface pece and lead to dfferent couplng condton of the urface pece to the bulk equaton. The Marcu-Huh theory often ued n tuaton where the clacal Butler-Volmer equaton fal. In partcular, the Marcu-Huh theory able to decrbe curved Tafel lope whch are nterpreted a a dependency of the tranfer coeffcent on the overpotental. Curved Tafel lope have been reported e.g. n [ST75], but uually th requre non-teady tate technque. 24
27 Expermental condton to oberve curved Tafel lope under teady-tate condton were decrbed n [Fel1] and expermental reult have been reported for a redox couple that ha a pathologcally mall rate contant [FV11]. We note that becaue the Butler-Volmer equaton 33 formulated n term of chemcal potental ntead of number dente, t eem poble that applyng a materal model dfferent from the mple mxture would allow for curved Tafel behavor. The ame alo hold true for temperature dependence of Tafel lope. In a ere of comparatve tude [LWH + 11, HWLP + 11, HLRC12], expermental data of quare wave voltammetry and cyclc voltammetry wa ftted to Marcu-Huh and Butler-Volmer theory. The reult ndcate ome quanttatve weakne of the Marcu-Huh theory. We remark that cyclc voltammetry or quare-wave voltammetry requre the ncluon of a tme cale nto the model equaton that at leat one order of magntude lower than the t ref = 1 condered here. Thu, n our pont of vew, a mulaton of thee procee hould be baed on the the complete model of [DGM15]. 7.2 Comparon wth the lterature General metal electrode. The textbook lterature provde Nernt- and the Butler-Volmer equaton for the electron tranfer reacton 39 at a metal electrode whch we can compare wth the repectve equaton n Sect For the Nernt equaton, we fnd [NTA4, 8.2]: ϕ M ϕ E = kc ȳ O ln. 89 ne k A ȳ R Here k A/C are the rate contant related to the anodc and cathodc reacton, repectvely. The ame tructure of the Nernt equaton can be found e.g. [BRGA2, 7.4] and [BF1, 3.2.2], only the rate contant are replaced by a reference potental. All thee equaton how the ame logarthmc dependency on the equlbrum concentraton ȳ O and ȳ R a 45. For the Butler-Volmer equaton, the overpotental η S defned n [NTA4, 8.21] dentcal to 41 and n our notaton the author tate the Butler-Volmer equaton: [NTA4, 8.24]: j e ν I = j exp βn e 1 βn η S j e exp η S. 9 The ame tructure of the equaton can be found n [BRGA2, 7.23]. We oberve that the exchange current are the ame for the anodc and the cathodc current,.e. j A = j C = j, what not compatble wth our approach to guarantee the contency wth the 2 nd law of thermodynamc. Moreover j e then alway mple η S. In contrat, the overpotental defned n Secton 4 depend on the choen equlbrum tate wth t repectve electrolyte concentraton at the nterface. A devaton from the concentraton of the reference tate then mple a non-vanhng overpotental even for j e =. The dependency on the concentraton pecfed a [NTA4, 8.23]: j = ne k β A k1β C y β R y1β O. 91 A a conequence, for a low amount of the oxdzed pece y O 1, the Butler-Volmer equaton 9 mple that a hgh overpotental η S requred n order to mantan an oxdaton 25
Introduction to Interfacial Segregation. Xiaozhe Zhang 10/02/2015
 Introducton to Interfacal Segregaton Xaozhe Zhang 10/02/2015 Interfacal egregaton Segregaton n materal refer to the enrchment of a materal conttuent at a free urface or an nternal nterface of a materal.
Introducton to Interfacal Segregaton Xaozhe Zhang 10/02/2015 Interfacal egregaton Segregaton n materal refer to the enrchment of a materal conttuent at a free urface or an nternal nterface of a materal.
Additional File 1 - Detailed explanation of the expression level CPD
 Addtonal Fle - Detaled explanaton of the expreon level CPD A mentoned n the man text, the man CPD for the uterng model cont of two ndvdual factor: P( level gen P( level gen P ( level gen 2 (.).. CPD factor
Addtonal Fle - Detaled explanaton of the expreon level CPD A mentoned n the man text, the man CPD for the uterng model cont of two ndvdual factor: P( level gen P( level gen P ( level gen 2 (.).. CPD factor
Specification -- Assumptions of the Simple Classical Linear Regression Model (CLRM) 1. Introduction
 ECONOMICS 35* -- NOTE ECON 35* -- NOTE Specfcaton -- Aumpton of the Smple Clacal Lnear Regreon Model (CLRM). Introducton CLRM tand for the Clacal Lnear Regreon Model. The CLRM alo known a the tandard lnear
ECONOMICS 35* -- NOTE ECON 35* -- NOTE Specfcaton -- Aumpton of the Smple Clacal Lnear Regreon Model (CLRM). Introducton CLRM tand for the Clacal Lnear Regreon Model. The CLRM alo known a the tandard lnear
Harmonic oscillator approximation
 armonc ocllator approxmaton armonc ocllator approxmaton Euaton to be olved We are fndng a mnmum of the functon under the retrcton where W P, P,..., P, Q, Q,..., Q P, P,..., P, Q, Q,..., Q lnwgner functon
armonc ocllator approxmaton armonc ocllator approxmaton Euaton to be olved We are fndng a mnmum of the functon under the retrcton where W P, P,..., P, Q, Q,..., Q P, P,..., P, Q, Q,..., Q lnwgner functon
Scattering of two identical particles in the center-of. of-mass frame. (b)
 Lecture # November 5 Scatterng of two dentcal partcle Relatvtc Quantum Mechanc: The Klen-Gordon equaton Interpretaton of the Klen-Gordon equaton The Drac equaton Drac repreentaton for the matrce α and
Lecture # November 5 Scatterng of two dentcal partcle Relatvtc Quantum Mechanc: The Klen-Gordon equaton Interpretaton of the Klen-Gordon equaton The Drac equaton Drac repreentaton for the matrce α and
Chapter 11. Supplemental Text Material. The method of steepest ascent can be derived as follows. Suppose that we have fit a firstorder
 S-. The Method of Steepet cent Chapter. Supplemental Text Materal The method of teepet acent can be derved a follow. Suppoe that we have ft a frtorder model y = β + β x and we wh to ue th model to determne
S-. The Method of Steepet cent Chapter. Supplemental Text Materal The method of teepet acent can be derved a follow. Suppoe that we have ft a frtorder model y = β + β x and we wh to ue th model to determne
No! Yes! Only if reactions occur! Yes! Ideal Gas, change in temperature or pressure. Survey Results. Class 15. Is the following possible?
 Survey Reult Chapter 5-6 (where we are gong) % of Student 45% 40% 35% 30% 25% 20% 15% 10% 5% 0% Hour Spent on ChE 273 1-2 3-4 5-6 7-8 9-10 11+ Hour/Week 2008 2009 2010 2011 2012 2013 2014 2015 2017 F17
Survey Reult Chapter 5-6 (where we are gong) % of Student 45% 40% 35% 30% 25% 20% 15% 10% 5% 0% Hour Spent on ChE 273 1-2 3-4 5-6 7-8 9-10 11+ Hour/Week 2008 2009 2010 2011 2012 2013 2014 2015 2017 F17
Not at Steady State! Yes! Only if reactions occur! Yes! Ideal Gas, change in temperature or pressure. Yes! Class 15. Is the following possible?
 Chapter 5-6 (where we are gong) Ideal gae and lqud (today) Dente Partal preure Non-deal gae (next tme) Eqn. of tate Reduced preure and temperature Compreblty chart (z) Vapor-lqud ytem (Ch. 6) Vapor preure
Chapter 5-6 (where we are gong) Ideal gae and lqud (today) Dente Partal preure Non-deal gae (next tme) Eqn. of tate Reduced preure and temperature Compreblty chart (z) Vapor-lqud ytem (Ch. 6) Vapor preure
Small signal analysis
 Small gnal analy. ntroducton Let u conder the crcut hown n Fg., where the nonlnear retor decrbed by the equaton g v havng graphcal repreentaton hown n Fg.. ( G (t G v(t v Fg. Fg. a D current ource wherea
Small gnal analy. ntroducton Let u conder the crcut hown n Fg., where the nonlnear retor decrbed by the equaton g v havng graphcal repreentaton hown n Fg.. ( G (t G v(t v Fg. Fg. a D current ource wherea
HYDRODYNAMIC LIMIT FOR A GAS WITH CHEMICAL REACTIONS
 October 3, 003 8:48 WSPC/Trm Sze: 9n x 6n for Proceedng bp HYDRODYNAMIC LIMIT FOR A GAS WITH CHEMICAL REACTIONS M. BISI Dpartmento d Matematca, Unvertà d Mlano, Va Saldn 50, 0133 Mlano, Italy, E-mal: b@mat.unm.t
October 3, 003 8:48 WSPC/Trm Sze: 9n x 6n for Proceedng bp HYDRODYNAMIC LIMIT FOR A GAS WITH CHEMICAL REACTIONS M. BISI Dpartmento d Matematca, Unvertà d Mlano, Va Saldn 50, 0133 Mlano, Italy, E-mal: b@mat.unm.t
Electrochemical Equilibrium Electromotive Force
 CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
Extended Prigogine Theorem: Method for Universal Characterization of Complex System Evolution
 Extended Prgogne Theorem: Method for Unveral Characterzaton of Complex Sytem Evoluton Sergey amenhchkov* Mocow State Unverty of M.V. Lomonoov, Phycal department, Rua, Mocow, Lennke Gory, 1/, 119991 Publhed
Extended Prgogne Theorem: Method for Unveral Characterzaton of Complex Sytem Evoluton Sergey amenhchkov* Mocow State Unverty of M.V. Lomonoov, Phycal department, Rua, Mocow, Lennke Gory, 1/, 119991 Publhed
On the SO 2 Problem in Thermal Power Plants. 2.Two-steps chemical absorption modeling
 Internatonal Journal of Engneerng Reearch ISSN:39-689)(onlne),347-53(prnt) Volume No4, Iue No, pp : 557-56 Oct 5 On the SO Problem n Thermal Power Plant Two-tep chemcal aborpton modelng hr Boyadjev, P
Internatonal Journal of Engneerng Reearch ISSN:39-689)(onlne),347-53(prnt) Volume No4, Iue No, pp : 557-56 Oct 5 On the SO Problem n Thermal Power Plant Two-tep chemcal aborpton modelng hr Boyadjev, P
You must not circulate this book in any other binding or cover and you must impose this same condition on any acquirer.
 6 Interfacal thermodynamc: Gbb equaton Luuk K. Koopal Chapter 6, Interfacal thermodynamc: Gbb equaton n Interface Scence, Second edton, 008, Wagenngen Unverty, Wagenngen, The Netherland. Avalable va: http://www.reearchgate.net/profle/luuk_koopal
6 Interfacal thermodynamc: Gbb equaton Luuk K. Koopal Chapter 6, Interfacal thermodynamc: Gbb equaton n Interface Scence, Second edton, 008, Wagenngen Unverty, Wagenngen, The Netherland. Avalable va: http://www.reearchgate.net/profle/luuk_koopal
CHAPTER X PHASE-CHANGE PROBLEMS
 Chapter X Phae-Change Problem December 3, 18 917 CHAPER X PHASE-CHANGE PROBLEMS X.1 Introducton Clacal Stefan Problem Geometry of Phae Change Problem Interface Condton X. Analytcal Soluton for Soldfcaton
Chapter X Phae-Change Problem December 3, 18 917 CHAPER X PHASE-CHANGE PROBLEMS X.1 Introducton Clacal Stefan Problem Geometry of Phae Change Problem Interface Condton X. Analytcal Soluton for Soldfcaton
A NUMERICAL MODELING OF MAGNETIC FIELD PERTURBATED BY THE PRESENCE OF SCHIP S HULL
 A NUMERCAL MODELNG OF MAGNETC FELD PERTURBATED BY THE PRESENCE OF SCHP S HULL M. Dennah* Z. Abd** * Laboratory Electromagnetc Sytem EMP BP b Ben-Aknoun 606 Alger Algera ** Electronc nttute USTHB Alger
A NUMERCAL MODELNG OF MAGNETC FELD PERTURBATED BY THE PRESENCE OF SCHP S HULL M. Dennah* Z. Abd** * Laboratory Electromagnetc Sytem EMP BP b Ben-Aknoun 606 Alger Algera ** Electronc nttute USTHB Alger
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Chapter 6 The Effect of the GPS Systematic Errors on Deformation Parameters
 Chapter 6 The Effect of the GPS Sytematc Error on Deformaton Parameter 6.. General Beutler et al., (988) dd the frt comprehenve tudy on the GPS ytematc error. Baed on a geometrc approach and aumng a unform
Chapter 6 The Effect of the GPS Sytematc Error on Deformaton Parameter 6.. General Beutler et al., (988) dd the frt comprehenve tudy on the GPS ytematc error. Baed on a geometrc approach and aumng a unform
AP Statistics Ch 3 Examining Relationships
 Introducton To tud relatonhp between varable, we mut meaure the varable on the ame group of ndvdual. If we thnk a varable ma eplan or even caue change n another varable, then the eplanator varable and
Introducton To tud relatonhp between varable, we mut meaure the varable on the ame group of ndvdual. If we thnk a varable ma eplan or even caue change n another varable, then the eplanator varable and
Supplementary Notes for Chapter 9 Mixture Thermodynamics
 Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
MULTIPLE REGRESSION ANALYSIS For the Case of Two Regressors
 MULTIPLE REGRESSION ANALYSIS For the Cae of Two Regreor In the followng note, leat-quare etmaton developed for multple regreon problem wth two eplanator varable, here called regreor (uch a n the Fat Food
MULTIPLE REGRESSION ANALYSIS For the Cae of Two Regreor In the followng note, leat-quare etmaton developed for multple regreon problem wth two eplanator varable, here called regreor (uch a n the Fat Food
Kinetic-Energy Density-Functional Theory on a Lattice
 h an open acce artcle publhed under an ACS AuthorChoce Lcene, whch permt copyng and redtrbuton of the artcle or any adaptaton for non-commercal purpoe. Artcle Cte h: J. Chem. heory Comput. 08, 4, 407 4087
h an open acce artcle publhed under an ACS AuthorChoce Lcene, whch permt copyng and redtrbuton of the artcle or any adaptaton for non-commercal purpoe. Artcle Cte h: J. Chem. heory Comput. 08, 4, 407 4087
Root Locus Techniques
 Root Locu Technque ELEC 32 Cloed-Loop Control The control nput u t ynthezed baed on the a pror knowledge of the ytem plant, the reference nput r t, and the error gnal, e t The control ytem meaure the output,
Root Locu Technque ELEC 32 Cloed-Loop Control The control nput u t ynthezed baed on the a pror knowledge of the ytem plant, the reference nput r t, and the error gnal, e t The control ytem meaure the output,
1) Silicon oxide has a typical surface potential in an aqueous medium of ϕ,0
 1) Slcon oxde has a typcal surface potental n an aqueous medum of ϕ, = 7 mv n 5 mm l at ph 9. Whch concentraton of catons do you roughly expect close to the surface? What s the average dstance between
1) Slcon oxde has a typcal surface potental n an aqueous medum of ϕ, = 7 mv n 5 mm l at ph 9. Whch concentraton of catons do you roughly expect close to the surface? What s the average dstance between
MODELLING OF TRANSIENT HEAT TRANSPORT IN TWO-LAYERED CRYSTALLINE SOLID FILMS USING THE INTERVAL LATTICE BOLTZMANN METHOD
 Journal o Appled Mathematc and Computatonal Mechanc 7, 6(4), 57-65 www.amcm.pcz.pl p-issn 99-9965 DOI:.75/jamcm.7.4.6 e-issn 353-588 MODELLING OF TRANSIENT HEAT TRANSPORT IN TWO-LAYERED CRYSTALLINE SOLID
Journal o Appled Mathematc and Computatonal Mechanc 7, 6(4), 57-65 www.amcm.pcz.pl p-issn 99-9965 DOI:.75/jamcm.7.4.6 e-issn 353-588 MODELLING OF TRANSIENT HEAT TRANSPORT IN TWO-LAYERED CRYSTALLINE SOLID
Team. Outline. Statistics and Art: Sampling, Response Error, Mixed Models, Missing Data, and Inference
 Team Stattc and Art: Samplng, Repone Error, Mxed Model, Mng Data, and nference Ed Stanek Unverty of Maachuett- Amhert, USA 9/5/8 9/5/8 Outlne. Example: Doe-repone Model n Toxcology. ow to Predct Realzed
Team Stattc and Art: Samplng, Repone Error, Mxed Model, Mng Data, and nference Ed Stanek Unverty of Maachuett- Amhert, USA 9/5/8 9/5/8 Outlne. Example: Doe-repone Model n Toxcology. ow to Predct Realzed
Pythagorean triples. Leen Noordzij.
 Pythagorean trple. Leen Noordz Dr.l.noordz@leennoordz.nl www.leennoordz.me Content A Roadmap for generatng Pythagorean Trple.... Pythagorean Trple.... 3 Dcuon Concluon.... 5 A Roadmap for generatng Pythagorean
Pythagorean trple. Leen Noordz Dr.l.noordz@leennoordz.nl www.leennoordz.me Content A Roadmap for generatng Pythagorean Trple.... Pythagorean Trple.... 3 Dcuon Concluon.... 5 A Roadmap for generatng Pythagorean
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
728. Mechanical and electrical elements in reduction of vibrations
 78. Mechancal and electrcal element n reducton of vbraton Katarzyna BIAŁAS The Slean Unverty of Technology, Faculty of Mechancal Engneerng Inttute of Engneerng Procee Automaton and Integrated Manufacturng
78. Mechancal and electrcal element n reducton of vbraton Katarzyna BIAŁAS The Slean Unverty of Technology, Faculty of Mechancal Engneerng Inttute of Engneerng Procee Automaton and Integrated Manufacturng
The influence of Stern layer conductance on the. dielectrophoretic behaviour of latex nanospheres
 The nfluence of Stern layer conductance on the delectrophoretc behavour of latex nanophere Mchael Pycraft Hughe* Bomedcal Engneerng Group, Unverty of Surrey, Guldford, GU2 7XH, UK Ncola Gavn Green Boelectronc
The nfluence of Stern layer conductance on the delectrophoretc behavour of latex nanophere Mchael Pycraft Hughe* Bomedcal Engneerng Group, Unverty of Surrey, Guldford, GU2 7XH, UK Ncola Gavn Green Boelectronc
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
The multivariate Gaussian probability density function for random vector X (X 1,,X ) T. diagonal term of, denoted
 Appendx Proof of heorem he multvarate Gauan probablty denty functon for random vector X (X,,X ) px exp / / x x mean and varance equal to the th dagonal term of, denoted he margnal dtrbuton of X Gauan wth
Appendx Proof of heorem he multvarate Gauan probablty denty functon for random vector X (X,,X ) px exp / / x x mean and varance equal to the th dagonal term of, denoted he margnal dtrbuton of X Gauan wth
Modeling of Wave Behavior of Substrate Noise Coupling for Mixed-Signal IC Design
 Modelng of Wave Behavor of Subtrate Noe Couplng for Mxed-Sgnal IC Degn Georgo Veron, Y-Chang Lu, and Robert W. Dutton Center for Integrated Sytem, Stanford Unverty, Stanford, CA 9435 yorgo@gloworm.tanford.edu
Modelng of Wave Behavor of Subtrate Noe Couplng for Mxed-Sgnal IC Degn Georgo Veron, Y-Chang Lu, and Robert W. Dutton Center for Integrated Sytem, Stanford Unverty, Stanford, CA 9435 yorgo@gloworm.tanford.edu
CHEMICAL REACTIONS AND DIFFUSION
 CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
Electrochemistry Thermodynamics
 CHEM 51 Analytcal Electrochemstry Chapter Oct 5, 016 Electrochemstry Thermodynamcs Bo Zhang Department of Chemstry Unversty of Washngton Seattle, WA 98195 Former SEAC presdent Andy Ewng sellng T-shrts
CHEM 51 Analytcal Electrochemstry Chapter Oct 5, 016 Electrochemstry Thermodynamcs Bo Zhang Department of Chemstry Unversty of Washngton Seattle, WA 98195 Former SEAC presdent Andy Ewng sellng T-shrts
V. Electrostatics. Lecture 25: Diffuse double layer structure
 V. Electrostatcs Lecture 5: Dffuse double layer structure MIT Student Last tme we showed that whenever λ D L the electrolyte has a quas-neutral bulk (or outer ) regon at the geometrcal scale L, where there
V. Electrostatcs Lecture 5: Dffuse double layer structure MIT Student Last tme we showed that whenever λ D L the electrolyte has a quas-neutral bulk (or outer ) regon at the geometrcal scale L, where there
Improvements on Waring s Problem
 Improvement on Warng Problem L An-Png Bejng, PR Chna apl@nacom Abtract By a new recurve algorthm for the auxlary equaton, n th paper, we wll gve ome mprovement for Warng problem Keyword: Warng Problem,
Improvement on Warng Problem L An-Png Bejng, PR Chna apl@nacom Abtract By a new recurve algorthm for the auxlary equaton, n th paper, we wll gve ome mprovement for Warng problem Keyword: Warng Problem,
Problem #1. Known: All required parameters. Schematic: Find: Depth of freezing as function of time. Strategy:
 BEE 3500 013 Prelm Soluton Problem #1 Known: All requred parameter. Schematc: Fnd: Depth of freezng a functon of tme. Strategy: In thee mplfed analy for freezng tme, a wa done n cla for a lab geometry,
BEE 3500 013 Prelm Soluton Problem #1 Known: All requred parameter. Schematc: Fnd: Depth of freezng a functon of tme. Strategy: In thee mplfed analy for freezng tme, a wa done n cla for a lab geometry,
8 Waves in Uniform Magnetized Media
 8 Wave n Unform Magnetzed Meda 81 Suceptblte The frt order current can be wrtten j = j = q d 3 p v f 1 ( r, p, t) = ɛ 0 χ E For Maxwellan dtrbuton Y n (λ) = f 0 (v, v ) = 1 πvth exp (v V ) v th 1 πv th
8 Wave n Unform Magnetzed Meda 81 Suceptblte The frt order current can be wrtten j = j = q d 3 p v f 1 ( r, p, t) = ɛ 0 χ E For Maxwellan dtrbuton Y n (λ) = f 0 (v, v ) = 1 πvth exp (v V ) v th 1 πv th
Statistical Properties of the OLS Coefficient Estimators. 1. Introduction
 ECOOMICS 35* -- OTE 4 ECO 35* -- OTE 4 Stattcal Properte of the OLS Coeffcent Etmator Introducton We derved n ote the OLS (Ordnary Leat Square etmator ˆβ j (j, of the regreon coeffcent βj (j, n the mple
ECOOMICS 35* -- OTE 4 ECO 35* -- OTE 4 Stattcal Properte of the OLS Coeffcent Etmator Introducton We derved n ote the OLS (Ordnary Leat Square etmator ˆβ j (j, of the regreon coeffcent βj (j, n the mple
Variable Structure Control ~ Basics
 Varable Structure Control ~ Bac Harry G. Kwatny Department of Mechancal Engneerng & Mechanc Drexel Unverty Outlne A prelmnary example VS ytem, ldng mode, reachng Bac of dcontnuou ytem Example: underea
Varable Structure Control ~ Bac Harry G. Kwatny Department of Mechancal Engneerng & Mechanc Drexel Unverty Outlne A prelmnary example VS ytem, ldng mode, reachng Bac of dcontnuou ytem Example: underea
CHAPTER 9 LINEAR MOMENTUM, IMPULSE AND COLLISIONS
 CHAPTER 9 LINEAR MOMENTUM, IMPULSE AND COLLISIONS 103 Phy 1 9.1 Lnear Momentum The prncple o energy conervaton can be ued to olve problem that are harder to olve jut ung Newton law. It ued to decrbe moton
CHAPTER 9 LINEAR MOMENTUM, IMPULSE AND COLLISIONS 103 Phy 1 9.1 Lnear Momentum The prncple o energy conervaton can be ued to olve problem that are harder to olve jut ung Newton law. It ued to decrbe moton
APPLICATIONS: CHEMICAL AND PHASE EQUILIBRIA
 5.60 Sprn 2007 Lecture #28 pae PPLICTIOS: CHMICL D PHS QUILIBRI pply tattcal mechanc to develop mcrocopc model for problem you ve treated o far wth macrocopc thermodynamc 0 Product Reactant Separated atom
5.60 Sprn 2007 Lecture #28 pae PPLICTIOS: CHMICL D PHS QUILIBRI pply tattcal mechanc to develop mcrocopc model for problem you ve treated o far wth macrocopc thermodynamc 0 Product Reactant Separated atom
Method Of Fundamental Solutions For Modeling Electromagnetic Wave Scattering Problems
 Internatonal Workhop on MehFree Method 003 1 Method Of Fundamental Soluton For Modelng lectromagnetc Wave Scatterng Problem Der-Lang Young (1) and Jhh-We Ruan (1) Abtract: In th paper we attempt to contruct
Internatonal Workhop on MehFree Method 003 1 Method Of Fundamental Soluton For Modelng lectromagnetc Wave Scatterng Problem Der-Lang Young (1) and Jhh-We Ruan (1) Abtract: In th paper we attempt to contruct
Physics 111. CQ1: springs. con t. Aristocrat at a fixed angle. Wednesday, 8-9 pm in NSC 118/119 Sunday, 6:30-8 pm in CCLIR 468.
 c Announcement day, ober 8, 004 Ch 8: Ch 10: Work done by orce at an angle Power Rotatonal Knematc angular dplacement angular velocty angular acceleraton Wedneday, 8-9 pm n NSC 118/119 Sunday, 6:30-8 pm
c Announcement day, ober 8, 004 Ch 8: Ch 10: Work done by orce at an angle Power Rotatonal Knematc angular dplacement angular velocty angular acceleraton Wedneday, 8-9 pm n NSC 118/119 Sunday, 6:30-8 pm
Electromagnetic scattering. Graduate Course Electrical Engineering (Communications) 1 st Semester, Sharif University of Technology
 Electromagnetc catterng Graduate Coure Electrcal Engneerng (Communcaton) 1 t Semeter, 1390-1391 Sharf Unverty of Technology Content of lecture Lecture : Bac catterng parameter Formulaton of the problem
Electromagnetc catterng Graduate Coure Electrcal Engneerng (Communcaton) 1 t Semeter, 1390-1391 Sharf Unverty of Technology Content of lecture Lecture : Bac catterng parameter Formulaton of the problem
II. Equilibrium Thermodynamics. Lecture 8: The Nernst Equation
 II. Equlbrum Thermodynamcs Lecture 8: The Nernst Equaton Notes by MIT Student, re-wrtten by MZB 2014 February 27, 2014 1 Chemcal Actvty The fundamental thermodynamc quantty controllng transport and reactons
II. Equlbrum Thermodynamcs Lecture 8: The Nernst Equaton Notes by MIT Student, re-wrtten by MZB 2014 February 27, 2014 1 Chemcal Actvty The fundamental thermodynamc quantty controllng transport and reactons
Two-Layered Model of Blood Flow through Composite Stenosed Artery
 Avalable at http://pvamu.edu/aam Appl. Appl. Math. ISSN: 93-9466 Vol. 4, Iue (December 9), pp. 343 354 (Prevouly, Vol. 4, No.) Applcaton Appled Mathematc: An Internatonal Journal (AAM) Two-ayered Model
Avalable at http://pvamu.edu/aam Appl. Appl. Math. ISSN: 93-9466 Vol. 4, Iue (December 9), pp. 343 354 (Prevouly, Vol. 4, No.) Applcaton Appled Mathematc: An Internatonal Journal (AAM) Two-ayered Model
Appendix II Summary of Important Equations
 W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
W. M. Whte Geochemstry Equatons of State: Ideal GasLaw: Coeffcent of Thermal Expanson: Compressblty: Van der Waals Equaton: The Laws of Thermdynamcs: Frst Law: Appendx II Summary of Important Equatons
Circuit Theorems. Introduction
 //5 Crcut eorem ntroducton nearty Property uperpoton ource Tranformaton eenn eorem orton eorem Maxmum Power Tranfer ummary ntroducton To deelop analy technque applcable to lnear crcut. To mplfy crcut analy
//5 Crcut eorem ntroducton nearty Property uperpoton ource Tranformaton eenn eorem orton eorem Maxmum Power Tranfer ummary ntroducton To deelop analy technque applcable to lnear crcut. To mplfy crcut analy
Supporting Information. Hydroxyl Radical Production by H 2 O 2 -Mediated. Conditions
 Supportng Informaton Hydroxyl Radcal Producton by H 2 O 2 -Medated Oxdaton of Fe(II) Complexed by Suwannee Rver Fulvc Acd Under Crcumneutral Frehwater Condton Chrtopher J. Mller, Andrew L. Roe, T. Davd
Supportng Informaton Hydroxyl Radcal Producton by H 2 O 2 -Medated Oxdaton of Fe(II) Complexed by Suwannee Rver Fulvc Acd Under Crcumneutral Frehwater Condton Chrtopher J. Mller, Andrew L. Roe, T. Davd
2. SINGLE VS. MULTI POLARIZATION SAR DATA
 . SINGLE VS. MULTI POLARIZATION SAR DATA.1 Scatterng Coeffcent v. Scatterng Matrx In the prevou chapter of th document, we dealt wth the decrpton and the characterzaton of electromagnetc wave. A t wa hown,
. SINGLE VS. MULTI POLARIZATION SAR DATA.1 Scatterng Coeffcent v. Scatterng Matrx In the prevou chapter of th document, we dealt wth the decrpton and the characterzaton of electromagnetc wave. A t wa hown,
This appendix presents the derivations and proofs omitted from the main text.
 Onlne Appendx A Appendx: Omtted Dervaton and Proof Th appendx preent the dervaton and proof omtted from the man text A Omtted dervaton n Secton Mot of the analy provded n the man text Here, we formally
Onlne Appendx A Appendx: Omtted Dervaton and Proof Th appendx preent the dervaton and proof omtted from the man text A Omtted dervaton n Secton Mot of the analy provded n the man text Here, we formally
Module 5. Cables and Arches. Version 2 CE IIT, Kharagpur
 odule 5 Cable and Arche Veron CE IIT, Kharagpur Leon 33 Two-nged Arch Veron CE IIT, Kharagpur Intructonal Objectve: After readng th chapter the tudent wll be able to 1. Compute horzontal reacton n two-hnged
odule 5 Cable and Arche Veron CE IIT, Kharagpur Leon 33 Two-nged Arch Veron CE IIT, Kharagpur Intructonal Objectve: After readng th chapter the tudent wll be able to 1. Compute horzontal reacton n two-hnged
 Ice crytal nucleaton, growth and breakage modellng n a craped urface heat exchanger H. Benkhelfa (a, M. Arellano (a,b G. Alvarez (b, D. Flck (a (a UMR n 45 AgroParTech/INRA, Ingénere-Procédé-Alment, 6
Ice crytal nucleaton, growth and breakage modellng n a craped urface heat exchanger H. Benkhelfa (a, M. Arellano (a,b G. Alvarez (b, D. Flck (a (a UMR n 45 AgroParTech/INRA, Ingénere-Procédé-Alment, 6
FREE DIFFUSION INTRODUCTION
 2 FREE DIFFUSION INTRODUCTION In th chapter, we conder the mplet of tranport procee: the pave dffuon of a olute that occur when t electrochemcal potental on the two de of a permeable barrer are dfferent.
2 FREE DIFFUSION INTRODUCTION In th chapter, we conder the mplet of tranport procee: the pave dffuon of a olute that occur when t electrochemcal potental on the two de of a permeable barrer are dfferent.
Entropy generation in a chemical reaction
 Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Entropy generaton n a chemcal reacton E Mranda Área de Cencas Exactas COICET CCT Mendoza 5500 Mendoza, rgentna and Departamento de Físca Unversdad aconal de San Lus 5700 San Lus, rgentna bstract: Entropy
Energy, Entropy, and Availability Balances Phase Equilibria. Nonideal Thermodynamic Property Models. Selecting an Appropriate Model
 Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
Lecture 4. Thermodynamcs [Ch. 2] Energy, Entropy, and Avalablty Balances Phase Equlbra - Fugactes and actvty coeffcents -K-values Nondeal Thermodynamc Property Models - P-v-T equaton-of-state models -
4.2 Chemical Driving Force
 4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
Weak McCoy Ore Extensions
 Internatonal Mathematcal Forum, Vol. 6, 2, no. 2, 75-86 Weak McCoy Ore Extenon R. Mohammad, A. Mouav and M. Zahr Department of Pure Mathematc, Faculty of Mathematcal Scence Tarbat Modare Unverty, P.O.
Internatonal Mathematcal Forum, Vol. 6, 2, no. 2, 75-86 Weak McCoy Ore Extenon R. Mohammad, A. Mouav and M. Zahr Department of Pure Mathematc, Faculty of Mathematcal Scence Tarbat Modare Unverty, P.O.
More metrics on cartesian products
 More metrcs on cartesan products If (X, d ) are metrc spaces for 1 n, then n Secton II4 of the lecture notes we defned three metrcs on X whose underlyng topologes are the product topology The purpose of
More metrcs on cartesan products If (X, d ) are metrc spaces for 1 n, then n Secton II4 of the lecture notes we defned three metrcs on X whose underlyng topologes are the product topology The purpose of
2.3 Least-Square regressions
 .3 Leat-Square regreon Eample.10 How do chldren grow? The pattern of growth vare from chld to chld, o we can bet undertandng the general pattern b followng the average heght of a number of chldren. Here
.3 Leat-Square regreon Eample.10 How do chldren grow? The pattern of growth vare from chld to chld, o we can bet undertandng the general pattern b followng the average heght of a number of chldren. Here
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Lecture 12: Discrete Laplacian
 Lecture 12: Dscrete Laplacan Scrbe: Tanye Lu Our goal s to come up wth a dscrete verson of Laplacan operator for trangulated surfaces, so that we can use t n practce to solve related problems We are mostly
Lecture 12: Dscrete Laplacan Scrbe: Tanye Lu Our goal s to come up wth a dscrete verson of Laplacan operator for trangulated surfaces, so that we can use t n practce to solve related problems We are mostly
NUMERICAL DIFFERENTIATION
 NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
APPROXIMATE FUZZY REASONING BASED ON INTERPOLATION IN THE VAGUE ENVIRONMENT OF THE FUZZY RULEBASE AS A PRACTICAL ALTERNATIVE OF THE CLASSICAL CRI
 Kovác, Sz., Kóczy, L.T.: Approxmate Fuzzy Reaonng Baed on Interpolaton n the Vague Envronment of the Fuzzy Rulebae a a Practcal Alternatve of the Clacal CRI, Proceedng of the 7 th Internatonal Fuzzy Sytem
Kovác, Sz., Kóczy, L.T.: Approxmate Fuzzy Reaonng Baed on Interpolaton n the Vague Envronment of the Fuzzy Rulebae a a Practcal Alternatve of the Clacal CRI, Proceedng of the 7 th Internatonal Fuzzy Sytem
Quick Visit to Bernoulli Land
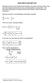 Although we have een the Bernoull equaton and een t derved before, th next note how t dervaton for an uncopreble & nvcd flow. The dervaton follow that of Kuethe &Chow ot cloely (I lke t better than Anderon).
Although we have een the Bernoull equaton and een t derved before, th next note how t dervaton for an uncopreble & nvcd flow. The dervaton follow that of Kuethe &Chow ot cloely (I lke t better than Anderon).
Osmotic pressure and protein binding
 Osmotc pressure and proten bndng Igor R. Kuznetsov, KochLab Symposum talk 5/15/09 Today we take a closer look at one of the soluton thermodynamcs key ponts from Steve s presentaton. Here t s: d[ln(k off
Osmotc pressure and proten bndng Igor R. Kuznetsov, KochLab Symposum talk 5/15/09 Today we take a closer look at one of the soluton thermodynamcs key ponts from Steve s presentaton. Here t s: d[ln(k off
COMPUTATIONAL HEAT PRADIP DUTTA INDIAN INSTITUTE OF SCIENCE BANGALORE
 COMPUTATIONAL HEAT TRANSFER AND FLUID FLOW PRADIP DUTTA DEPARTMENT OF MECHANICAL ENGINEERING INDIAN INSTITUTE OF SCIENCE BANGALORE Otlne Bac of Heat ttranfer Mathematcalh l decrpton of fld flow and heat
COMPUTATIONAL HEAT TRANSFER AND FLUID FLOW PRADIP DUTTA DEPARTMENT OF MECHANICAL ENGINEERING INDIAN INSTITUTE OF SCIENCE BANGALORE Otlne Bac of Heat ttranfer Mathematcalh l decrpton of fld flow and heat
Phys 402: Raman Scattering. Spring Introduction: Brillouin and Raman spectroscopy. Raman scattering: how does it look like?
 Phy 402: Raman Scatterng Sprng 2008 1 Introducton: Brlloun and Raman pectrocopy Inelatc lght catterng medated by the electronc polarzablty of the medum a materal or a molecule catter rradant lght from
Phy 402: Raman Scatterng Sprng 2008 1 Introducton: Brlloun and Raman pectrocopy Inelatc lght catterng medated by the electronc polarzablty of the medum a materal or a molecule catter rradant lght from
Start Point and Trajectory Analysis for the Minimal Time System Design Algorithm
 Start Pont and Trajectory Analy for the Mnmal Tme Sytem Degn Algorthm ALEXANDER ZEMLIAK, PEDRO MIRANDA Department of Phyc and Mathematc Puebla Autonomou Unverty Av San Claudo /n, Puebla, 757 MEXICO Abtract:
Start Pont and Trajectory Analy for the Mnmal Tme Sytem Degn Algorthm ALEXANDER ZEMLIAK, PEDRO MIRANDA Department of Phyc and Mathematc Puebla Autonomou Unverty Av San Claudo /n, Puebla, 757 MEXICO Abtract:
( ) 1/ 2. ( P SO2 )( P O2 ) 1/ 2.
 Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Estimation of Finite Population Total under PPS Sampling in Presence of Extra Auxiliary Information
 Internatonal Journal of Stattc and Analy. ISSN 2248-9959 Volume 6, Number 1 (2016), pp. 9-16 Reearch Inda Publcaton http://www.rpublcaton.com Etmaton of Fnte Populaton Total under PPS Samplng n Preence
Internatonal Journal of Stattc and Analy. ISSN 2248-9959 Volume 6, Number 1 (2016), pp. 9-16 Reearch Inda Publcaton http://www.rpublcaton.com Etmaton of Fnte Populaton Total under PPS Samplng n Preence
PHYS 705: Classical Mechanics. Calculus of Variations II
 1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
1 PHYS 705: Classcal Mechancs Calculus of Varatons II 2 Calculus of Varatons: Generalzaton (no constrant yet) Suppose now that F depends on several dependent varables : We need to fnd such that has a statonary
Towards the modeling of microgalvanic coupling in aluminum alloys : the choice of boundary conditions
 Presented at the COMSOL Conference 2008 Boston COMSOL Conference BOSTON November 9-11 2008 Towards the modelng of mcrogalvanc couplng n alumnum alloys : the choce of boundary condtons Ncolas Murer *1,
Presented at the COMSOL Conference 2008 Boston COMSOL Conference BOSTON November 9-11 2008 Towards the modelng of mcrogalvanc couplng n alumnum alloys : the choce of boundary condtons Ncolas Murer *1,
Two Approaches to Proving. Goldbach s Conjecture
 Two Approache to Provng Goldbach Conecture By Bernard Farley Adved By Charle Parry May 3 rd 5 A Bref Introducton to Goldbach Conecture In 74 Goldbach made h mot famou contrbuton n mathematc wth the conecture
Two Approache to Provng Goldbach Conecture By Bernard Farley Adved By Charle Parry May 3 rd 5 A Bref Introducton to Goldbach Conecture In 74 Goldbach made h mot famou contrbuton n mathematc wth the conecture
Linear Approximation with Regularization and Moving Least Squares
 Lnear Approxmaton wth Regularzaton and Movng Least Squares Igor Grešovn May 007 Revson 4.6 (Revson : March 004). 5 4 3 0.5 3 3.5 4 Contents: Lnear Fttng...4. Weghted Least Squares n Functon Approxmaton...
Lnear Approxmaton wth Regularzaton and Movng Least Squares Igor Grešovn May 007 Revson 4.6 (Revson : March 004). 5 4 3 0.5 3 3.5 4 Contents: Lnear Fttng...4. Weghted Least Squares n Functon Approxmaton...
Computer Control Systems
 Computer Control ytem In th chapter we preent the element and the bac concept of computercontrolled ytem. The dcretaton and choce of amplng frequency wll be frt examned, followed by a tudy of dcrete-tme
Computer Control ytem In th chapter we preent the element and the bac concept of computercontrolled ytem. The dcretaton and choce of amplng frequency wll be frt examned, followed by a tudy of dcrete-tme
Advanced Quantum Mechanics
 Advanced Quantum Mechancs Rajdeep Sensarma! sensarma@theory.tfr.res.n ecture #9 QM of Relatvstc Partcles Recap of ast Class Scalar Felds and orentz nvarant actons Complex Scalar Feld and Charge conjugaton
Advanced Quantum Mechancs Rajdeep Sensarma! sensarma@theory.tfr.res.n ecture #9 QM of Relatvstc Partcles Recap of ast Class Scalar Felds and orentz nvarant actons Complex Scalar Feld and Charge conjugaton
Frequency dependence of the permittivity
 Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE
 CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
CHAPTER 5 NUMERICAL EVALUATION OF DYNAMIC RESPONSE Analytcal soluton s usually not possble when exctaton vares arbtrarly wth tme or f the system s nonlnear. Such problems can be solved by numercal tmesteppng
Solution Methods for Time-indexed MIP Models for Chemical Production Scheduling
 Ian Davd Lockhart Bogle and Mchael Farweather (Edtor), Proceedng of the 22nd European Sympoum on Computer Aded Proce Engneerng, 17-2 June 212, London. 212 Elever B.V. All rght reerved. Soluton Method for
Ian Davd Lockhart Bogle and Mchael Farweather (Edtor), Proceedng of the 22nd European Sympoum on Computer Aded Proce Engneerng, 17-2 June 212, London. 212 Elever B.V. All rght reerved. Soluton Method for
Chapter 11: Simple Linear Regression and Correlation
 Chapter 11: Smple Lnear Regresson and Correlaton 11-1 Emprcal Models 11-2 Smple Lnear Regresson 11-3 Propertes of the Least Squares Estmators 11-4 Hypothess Test n Smple Lnear Regresson 11-4.1 Use of t-tests
Chapter 11: Smple Lnear Regresson and Correlaton 11-1 Emprcal Models 11-2 Smple Lnear Regresson 11-3 Propertes of the Least Squares Estmators 11-4 Hypothess Test n Smple Lnear Regresson 11-4.1 Use of t-tests
CONDITIONAL MOMENT CLOSURE MODELLING OF SOOT FORMATION IN TURBULENT NON-PREMIXED, ETHYLENE-AIR FLAMES
 CONDITIONAL MOMENT CLOSURE MODELLING OF SOOT FORMATION IN TURBULENT NON-PREMIXED, ETHYLENE-AIR FLAMES Yunard, Robert M. Woolley and Mchael Farweather Energy and Reource Reearch Inttute, School of Proce,
CONDITIONAL MOMENT CLOSURE MODELLING OF SOOT FORMATION IN TURBULENT NON-PREMIXED, ETHYLENE-AIR FLAMES Yunard, Robert M. Woolley and Mchael Farweather Energy and Reource Reearch Inttute, School of Proce,
Verification of Selected Precision Parameters of the Trimble S8 DR Plus Robotic Total Station
 81 Verfcaton of Selected Precon Parameter of the Trmble S8 DR Plu Robotc Total Staton Sokol, Š., Bajtala, M. and Ježko, J. Slovak Unverty of Technology, Faculty of Cvl Engneerng, Radlnkého 11, 81368 Bratlava,
81 Verfcaton of Selected Precon Parameter of the Trmble S8 DR Plu Robotc Total Staton Sokol, Š., Bajtala, M. and Ježko, J. Slovak Unverty of Technology, Faculty of Cvl Engneerng, Radlnkého 11, 81368 Bratlava,
Lectures - Week 4 Matrix norms, Conditioning, Vector Spaces, Linear Independence, Spanning sets and Basis, Null space and Range of a Matrix
 Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
If two volatile and miscible liquids are combined to form a solution, Raoult s law is not obeyed. Use the experimental data in Table 9.
 9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
9.9 Real Solutons Exhbt Devatons from Raoult s Law If two volatle and mscble lquds are combned to form a soluton, Raoult s law s not obeyed. Use the expermental data n Table 9.3: Physcal Chemstry 00 Pearson
Electrical double layer: revisit based on boundary conditions
 Electrcal double layer: revst based on boundary condtons Jong U. Km Department of Electrcal and Computer Engneerng, Texas A&M Unversty College Staton, TX 77843-318, USA Abstract The electrcal double layer
Electrcal double layer: revst based on boundary condtons Jong U. Km Department of Electrcal and Computer Engneerng, Texas A&M Unversty College Staton, TX 77843-318, USA Abstract The electrcal double layer
Numerical Heat and Mass Transfer
 Master degree n Mechancal Engneerng Numercal Heat and Mass Transfer 06-Fnte-Dfference Method (One-dmensonal, steady state heat conducton) Fausto Arpno f.arpno@uncas.t Introducton Why we use models and
Master degree n Mechancal Engneerng Numercal Heat and Mass Transfer 06-Fnte-Dfference Method (One-dmensonal, steady state heat conducton) Fausto Arpno f.arpno@uncas.t Introducton Why we use models and
χ x B E (c) Figure 2.1.1: (a) a material particle in a body, (b) a place in space, (c) a configuration of the body
 Secton.. Moton.. The Materal Body and Moton hyscal materals n the real world are modeled usng an abstract mathematcal entty called a body. Ths body conssts of an nfnte number of materal partcles. Shown
Secton.. Moton.. The Materal Body and Moton hyscal materals n the real world are modeled usng an abstract mathematcal entty called a body. Ths body conssts of an nfnte number of materal partcles. Shown
Robert Eisberg Second edition CH 09 Multielectron atoms ground states and x-ray excitations
 Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
A particle in a state of uniform motion remain in that state of motion unless acted upon by external force.
 The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
Lecture. Polymer Thermodynamics 0331 L Chemical Potential
 Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
Prof. Dr. rer. nat. habl. S. Enders Faculty III for Process Scence Insttute of Chemcal Engneerng Department of Thermodynamcs Lecture Polymer Thermodynamcs 033 L 337 3. Chemcal Potental Polymer Thermodynamcs
Electrostatic Potential from Transmembrane Currents
 Electrostatc Potental from Transmembrane Currents Let s assume that the current densty j(r, t) s ohmc;.e., lnearly proportonal to the electrc feld E(r, t): j = σ c (r)e (1) wth conductvty σ c = σ c (r).
Electrostatc Potental from Transmembrane Currents Let s assume that the current densty j(r, t) s ohmc;.e., lnearly proportonal to the electrc feld E(r, t): j = σ c (r)e (1) wth conductvty σ c = σ c (r).
Module 1 : The equation of continuity. Lecture 1: Equation of Continuity
 1 Module 1 : The equaton of contnuty Lecture 1: Equaton of Contnuty 2 Advanced Heat and Mass Transfer: Modules 1. THE EQUATION OF CONTINUITY : Lectures 1-6 () () () (v) (v) Overall Mass Balance Momentum
1 Module 1 : The equaton of contnuty Lecture 1: Equaton of Contnuty 2 Advanced Heat and Mass Transfer: Modules 1. THE EQUATION OF CONTINUITY : Lectures 1-6 () () () (v) (v) Overall Mass Balance Momentum
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 16 8/4/14 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 214. Real Vapors and Fugacty Henry s Law accounts or the propertes o extremely dlute soluton. s shown n Fgure
Lecture 16 8/4/14 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 214. Real Vapors and Fugacty Henry s Law accounts or the propertes o extremely dlute soluton. s shown n Fgure
and decompose in cycles of length two
 Permutaton of Proceedng of the Natona Conference On Undergraduate Reearch (NCUR) 006 Domncan Unverty of Caforna San Rafae, Caforna Apr - 4, 007 that are gven by bnoma and decompoe n cyce of ength two Yeena
Permutaton of Proceedng of the Natona Conference On Undergraduate Reearch (NCUR) 006 Domncan Unverty of Caforna San Rafae, Caforna Apr - 4, 007 that are gven by bnoma and decompoe n cyce of ength two Yeena
Physics 5153 Classical Mechanics. D Alembert s Principle and The Lagrangian-1
 P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
NAME and Section No. it is found that 0.6 mol of O
 NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
