FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BSC PROGRAMME NAME.. ST. #:. QUALIFICATION(S): CENTRE:.
|
|
|
- Anna Charles
- 5 years ago
- Views:
Transcription
1 FACULTY OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BSC PROGRAMME NAME.. ST. #:. QUALIFICATION(S): CENTRE:. MODE OF STUDY: FM PM DM CLASS VENUE: (FHAS Aud., or Mining Aud.) COURSE NAME: BASIC SCIENCE COURSE CODE: BSC410S DATE: 12 MAY 2017 DURATION: 2 HOURS MARKS: 90 BASIC SCIENCE TEST 2, SEMESTER 1, MEMO EXAMINER(S): MR. V. INDONGO DR. E. HESS DR. L. MWAPAGHA INSTRUCTIONS: 1. Answer all the questions in this questions paper. 2. For Multiple Choice questions, CYCLE the correct answer with a blue/black INK pen. 3. No books, notes and other additional aids are allowed. 4. A Periodic Table is attached at the back of this question paper. PERMISSIBLE MATERIALS Only a scientific calculator is allowed This paper consists of 10 pages including this cover page and the Periodic Table 1
2 SECTION A: BIOLOGY [30 MARKS] 1. Amino acids that are not synthesized in the body and must be obtained from the diet are called. [2] Essential amino acids 2. A process that destroys pathogens through simple heat is. [2] Pasteurization 3. What gives yoghurt a sour taste? [2] Lactic acid 4. What is the best-known function of vitamin K? [2] Blood clotting 5. How is sweet wine produced? [2] Sweet wine is produced when the fermentation process stops before all of the sugar has been converted into alcohol. 6. Name two commonly used species of yeast in beer making. [2] I. Saccharomyces cerevisiae II. Saccharomyces uvarum / Saccharomyces carlsbergensis 7. The main function of rennet in the manufacture of hard cheese is to. [2] lower the ph of milk and form of curds 8. A starter culture in the food industry is. [2] bacteria that are used in the fermentation of food products 9. What determines whether a mineral is a macromineral or a micro- (trace) mineral? [2] Macrominerals are found in and used by the body in the largest amounts. Microminerals are found in and used by the body in smaller amounts. 10. Define undernutrition and overnutrition. [3] Undernutrition is poor health resulting from the depletion of nutrients due to inadequate nutrient intake over time. Overnutrition is due to the regular consumption of excess calories, fats, saturated fats, and cholesterol. 11. State the three (3) stages of wastewater treatment: [3] I. Primary II. Secondary III. Tertiary 2
3 12. What are the differences between a monosaccharide, disaccharide and polysaccharide? [3] A monosaccharide is a single sugar unit (e.g., glucose, fructose, and galactose). A disaccharide (e.g., maltose, sucrose, and lactose) is a molecule of two single sugar units. A polysaccharide (e.g., starch and fiber) is a long chain of sugar units. 13. State three (3) reasons why sulphites are added during the wine making process? [3] I. Arrest fermentation II. As preservatives to prevent spoilage and oxidation at several stages of the winemaking. III. Protects wine from bacteria. SECTION B: CHEMISTRY 30 MARKS 1. In each of the following lists, identify the two terms which are related and provide an explanation as to either why the two terms you have chosen are related or why the third is not related. [3] a. Kelvin, Decimetre and Kilometre Decimetre and kilometre are related as they measure length/distance. Kelvin is the units for temperature b. Kilogram, Cubic centimetre and litre Cubic centrimetre and litre are related as they measure volume. Kilogram is the units for mass c. Time, Distance and seconds Seconds is the units for time. Distance is not related. 2. Differentiate between the terms accuracy and precision. [2] Accuracy how a series of measurements related to the true value Precision how a series of measurements related to each other 3. Using a Kelvin thermometer, the temperature in a freezer was recorded to be 169 K. Convert the temperature to Degree Celsius ( o C) and Fahrenheit ( o F) using the appropriate formulae. [3] o C = K 273 = = (1 mark) 3
4 o F = (1.8 x o C) + 32 (1 mark) = (1.8 x -104) + 32 = ( 1 mark) 4. Do the following calculations and record the answer to the correct number of significant figures or where applicable, to the correct number of decimal places. [2] a. (0.250 / 25.00) x = b = Apply the rules of rounding off numbers and round off the numbers below to the number of significant figures stated. [2] a. Round off nm to two significant figures = nm b. Round off g to three significant figures = How many significant figures does each of the following measurements have? [2] a m FOUR b Kg FOUR 7. Determine whether each of the following statements is true or false. If false, correct or state why the statement is false. [10] (a) Bases change litmus paper to blue. True (b) Ammonia is an acid. False it s a base (c) Properties of acids include being corrosive and having a sour taste. True (d) The chemical formula for sulfuric acid is H 2 SO 4. 4
5 True (e) Neutral solutions have a ph of 0. False has ph = 7 8. Complete the following reactions: [4] (a) Zn (s) + 2 HCl (aq) H 2 (g) + ZnCl 2 (2) (b) NaOH (aq) + HCl (aq) NaCl + H 2 O (2) 9. Complete the following sentences: [2] (a) An acid is a proton Donors (1) (b) Water soluble bases are called Alkalis (1) SECTION C: PHYSICS 30 MARKS hold quarks together. (1) A. Gravity B. Electromagnetic force C. Strong nuclear force D. Weak nuclear force 1.2 A unit of force called Newton is equivalent to. (1) A. g.m/s 2 B. kg.ms -1 C. c.ms -2 D. kg.ms Suppose an object of mass 8.9 kg falls from a height of 16 cm to the ground. This object has a weight of. (1) A. 8.9 kg B. 9.8 kg.n C N D J 1.4 The weight of an object is known as. (1) a. The mass of the object in space b. The force of an object due to gravity. c. The gravitational acceleration of the object. 5
6 d. Newton s laws of motion. 1.5 For a force to be observed, two bodies. (1) A. Must be in contact. B. Do not need to be contact. C. Need to have equal forces. D. Must have a uniform velocity. 1.6 The gravitational acceleration for the moon and the earth is. (1) A. The same B. Different C m/s 2 D. 9.8 m/s Suppose a force of 10 N is exerted on a brick and an acceleration of 2.5 m/s/s is observed. What is the mass of the brick? (1) A. 25 kg B. 4 kg C. 10 kg D. 2.5 kg 1.8 An electron and a proton are two particles. (1) A. Nucleons B. Positively charged C. Negatively charged D. Unlike charged 1.9 When an atom undergoes fission process, it is a result of. (1) A. Weak nuclear force B. Strong nuclear force C. Gravitational force D. Electromagnetic force 1.10 According to one Newton s law of motion, mass is. (1) A. A constant of proportionality. B. Directly proportional to acceleration. C. Directly proportional to force applied on a body. D. Changing as a constant force is applied on a body If a force applied on a body doubles, then acceleration. (1) A. Doubles 6
7 B. Triples C. Decreases by a half D. Remains the same 1.12 A contact force acting in the opposite direction when an ink pen is slid on the surface of the book is known as. (1) A. Friction B. Magnetic force C. Applied force D. Tension 1.13 The SI unit for frequency is. (1) A. Kg/s B. m C. m/s D. Hz 1.14 A is a disturbance that transfers energy through a medium from one location to another. (1) A. Force B. Wave C. Wavelength D. Friction 1.15 Type of wave with vibrations perpendicular to the direction of the motion of the wave is known as; (1) A. Longitudinal wave B. Transverse wave C. Both longitudinal and transverse waves D. None of the above 1.16 Which one is a renewable source of energy? (1) A. nuclear energy B. geothermal gas C. natural gas D. coal 1.17 Briefly discuss how electricity is generated from nuclear. (4) Fission of uranium heat water to make steam steam turns turbines electrical power sent around the country. [one mark for each step] A student with a mass of 8200 g stands at the top of a hill of height 0.4 Km (Position A), 7
8 as shown in the diagram below. Note: Answers should be in SI units a. What is the student s gravitational potential energy at the top of the hill? (2) Note: Gravitational acceleration (g) = 9.8 m.s -2 Gravitational potential energy (PE) = mgh (1 mark) 1000) m = (8200 /1000) Kg x 9.8m.s -2 x (0.4 x = Kg.m 2.s -2 = Joules (1 mark) b. Assuming that energy is conserved and there is no friction, what will the student s velocity be at the bottom of the hill (position B)? (3) PE at position A = KE at position B Therefore, KE at position B = J (1 mark) Thus, KE = ½ mv 2 (1 mark) J = ½ (82 Kg)v 2 v = m.s -1 (1 mark) 1.19 How do you understand the weight of an object? (1) 8
9 The weight of an object is force due to gravity State the difference between transverse and longitudinal waves (4) Transverse wave the vibration of particles is perpendicular (form 90 o ) to the direction of a wave. Longitudinal wave the vibrations of particles is parallel to the direction of a wave. 9
10 10
POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BASIC SCIENCE FULLTIME TEST 2
 POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BASIC SCIENCE FULLTIME TEST 2 INITIALS: SURNAME: STUDENT NUMBER: MODE OF STUDY: QUALIFICATION: LECTURE
POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BASIC SCIENCE FULLTIME TEST 2 INITIALS: SURNAME: STUDENT NUMBER: MODE OF STUDY: QUALIFICATION: LECTURE
POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES
 POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES NQF LEVEL: LEVEL 4 COURSE NAME: COURSE CODE: BASIC SCIENCE BSC410S DATE: NOVEMBER 2013 DURATION:
POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES NQF LEVEL: LEVEL 4 COURSE NAME: COURSE CODE: BASIC SCIENCE BSC410S DATE: NOVEMBER 2013 DURATION:
Energy, Work, and Power
 Matthew W. Milligan, Work, and Power Conservation Laws an Alternative to Newton s Laws Matthew W. Milligan, Work, and Power I. - kinetic and potential - conservation II. Work - dot product - work-energy
Matthew W. Milligan, Work, and Power Conservation Laws an Alternative to Newton s Laws Matthew W. Milligan, Work, and Power I. - kinetic and potential - conservation II. Work - dot product - work-energy
POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES
 POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES NQF LEVEL: LEVEL 4 COURSE NAME: COURSE CODE: BASIC SCIENCE BSC410S DATE: JANUARY 2016 DURATION: 3
POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES NQF LEVEL: LEVEL 4 COURSE NAME: COURSE CODE: BASIC SCIENCE BSC410S DATE: JANUARY 2016 DURATION: 3
Study Guide: Basic Chemistry, Water, Life Compounds and Enzymes
 Study Guide: Basic Chemistry, Water, Life Compounds and Enzymes 1. Lipids are good energy-storage molecules because a) the can absorb a large amount of energy while maintaining a constant temperature b)
Study Guide: Basic Chemistry, Water, Life Compounds and Enzymes 1. Lipids are good energy-storage molecules because a) the can absorb a large amount of energy while maintaining a constant temperature b)
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1)
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
Physical Science Study Guide
 Name: Class: Date: Physical Science Study Guide Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The electrons in a water molecule are gathered nearest
Name: Class: Date: Physical Science Study Guide Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The electrons in a water molecule are gathered nearest
Chapter 3: Force, Work and Energy
 Chapter 3: Force and Force Equilibrium Chapter 3: Force, Work and Energy Chapter 3: Force, Work and Energy 3.1 Mass and Weight 3.2 Newton's Law of Gravitation 3.3 Force and Newton's 3 Laws of Motion 3.4
Chapter 3: Force and Force Equilibrium Chapter 3: Force, Work and Energy Chapter 3: Force, Work and Energy 3.1 Mass and Weight 3.2 Newton's Law of Gravitation 3.3 Force and Newton's 3 Laws of Motion 3.4
Figure ) Letter E represents a nucleic acid building block known as a. Answer: nucleotide Diff: 3 Page Ref: 54
 Essentials of Human Anatomy and Physiology, 10e (Marieb) Chapter 2 Basic Chemistry 2.1 Short Answer Figure 2.1 Using Figure 2.1, identify the following: 1) Which letter represents a carbohydrate polymer?
Essentials of Human Anatomy and Physiology, 10e (Marieb) Chapter 2 Basic Chemistry 2.1 Short Answer Figure 2.1 Using Figure 2.1, identify the following: 1) Which letter represents a carbohydrate polymer?
POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BASIC SCIENCE PARTTIME TEST 2
 POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BASIC SCIENCE PARTTIME TEST 2 INITIALS: SURNAME: STUDENT NUMBER: MODE OF STUDY: QUALIFICATION: LECTURE
POLYTECHNIC OF NAMIBIA SCHOOL OF HEALTH AND APPLIED SCIENCES DEPARTMENT OF NATURAL AND APPLIED SCIENCES BASIC SCIENCE PARTTIME TEST 2 INITIALS: SURNAME: STUDENT NUMBER: MODE OF STUDY: QUALIFICATION: LECTURE
KINETIC AND POTENTIAL ENERGY. Chapter 6 (cont.)
 KINETIC AND POTENTIAL ENERGY Chapter 6 (cont.) The Two Types of Mechanical Energy Energy- the ability to do work- measured in joules Potential Energy- energy that arises because of an object s position
KINETIC AND POTENTIAL ENERGY Chapter 6 (cont.) The Two Types of Mechanical Energy Energy- the ability to do work- measured in joules Potential Energy- energy that arises because of an object s position
9/8/17. K h D Base d c m m = 5 km 2 km = 2000 m
 9/6/17 Scientific Method Process to test hypothesis to answer a question Parts of the Scientific Method: Observation Question Research Hypothesis Experiment/ Procedure Analysis Results Control Group no
9/6/17 Scientific Method Process to test hypothesis to answer a question Parts of the Scientific Method: Observation Question Research Hypothesis Experiment/ Procedure Analysis Results Control Group no
Chapter 02. Lecture and Animation Outline
 Chapter 02 Lecture and Animation Outline To run the animations you must be in Slideshow View. Use the buttons on the animation to play, pause, and turn audio/text on or off. Please Note: Once you have
Chapter 02 Lecture and Animation Outline To run the animations you must be in Slideshow View. Use the buttons on the animation to play, pause, and turn audio/text on or off. Please Note: Once you have
Chapter 2 Chemical Aspects of Life
 Chapter 2 Chemical Aspects of Life Multiple Choice Questions 1. Anything that has weight and occupies space can be described as A. an atom. B. matter. C. a compound. D. a molecule. #1 Learning Outcome:
Chapter 2 Chemical Aspects of Life Multiple Choice Questions 1. Anything that has weight and occupies space can be described as A. an atom. B. matter. C. a compound. D. a molecule. #1 Learning Outcome:
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1)
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
Chapter 2 The Chemistry of Life
 Chapter 2 The Chemistry of Life I. Water Liquid Naturally occurring It expands liquid to solid Covers more than 75% of our surface Most abundant in living organisms most important inorganic compound for
Chapter 2 The Chemistry of Life I. Water Liquid Naturally occurring It expands liquid to solid Covers more than 75% of our surface Most abundant in living organisms most important inorganic compound for
Ch. 2 BASIC CHEMISTRY. Copyright 2010 Pearson Education, Inc.
 Ch. 2 BASIC CHEMISTRY Matter and Composition of Matter Definition: Anything that has mass and occupies space Matter is made up of elements An element cannot be broken down by ordinary chemical means Atoms
Ch. 2 BASIC CHEMISTRY Matter and Composition of Matter Definition: Anything that has mass and occupies space Matter is made up of elements An element cannot be broken down by ordinary chemical means Atoms
Chapter 2: Fundamentals of Chemistry. Question Type: Multiple Choice. 1) Which of the following pairs is mismatched?
 Microbiology Principles and Explorations 9th Edition Black TEST BANK Full clear download at: https://testbankreal.com/download/microbiology-principles-explorations- 9th-edition-black-test-bank/ Microbiology
Microbiology Principles and Explorations 9th Edition Black TEST BANK Full clear download at: https://testbankreal.com/download/microbiology-principles-explorations- 9th-edition-black-test-bank/ Microbiology
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1 1) Which compound in Figure 2.1 is an ester? 1) A) a b c d e Answer: D 2) A scientist
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1 1) Which compound in Figure 2.1 is an ester? 1) A) a b c d e Answer: D 2) A scientist
1/23/2012. Atoms. Atoms Atoms - Electron Shells. Chapter 2 Outline. Planetary Models of Elements Chemical Bonds
 Chapter 2 Outline Atoms Chemical Bonds Acids, Bases and the p Scale Organic Molecules Carbohydrates Lipids Proteins Nucleic Acids Are smallest units of the chemical elements Composed of protons, neutrons
Chapter 2 Outline Atoms Chemical Bonds Acids, Bases and the p Scale Organic Molecules Carbohydrates Lipids Proteins Nucleic Acids Are smallest units of the chemical elements Composed of protons, neutrons
GLOSSARY OF PHYSICS TERMS. v-u t. a =
 GLOSSARY OF PHYSICS TERMS Scalar: A quantity that has magnitude only. Vector: A quantity that has magnitude and direction. Speed is the distance travelled per unit time. OR the rate of change of distance.
GLOSSARY OF PHYSICS TERMS Scalar: A quantity that has magnitude only. Vector: A quantity that has magnitude and direction. Speed is the distance travelled per unit time. OR the rate of change of distance.
Physical Science midterm study guide. Chapter 1 and 2
 Physical Science midterm study guide Chapter 1 and 2 1. Explain the difference between a scientific law and a scientific theory a. Laws generalize observations b. Theories explain observations 2. Select
Physical Science midterm study guide Chapter 1 and 2 1. Explain the difference between a scientific law and a scientific theory a. Laws generalize observations b. Theories explain observations 2. Select
Chemistry of Life 10/1/2010. What makes up the chemistry of life?
 A. Students will be able to identify and define the parts of an atom. Chemistry of Life At the Completion of this Unit, Students will be able to: A. Identify and define the parts of an atom. B. Demonstrate
A. Students will be able to identify and define the parts of an atom. Chemistry of Life At the Completion of this Unit, Students will be able to: A. Identify and define the parts of an atom. B. Demonstrate
Energy: Forms and Changes
 Energy: Forms and Changes Nature of Energy Energy is all around you! You can hear energy as sound. You can see energy as light. And you can feel it as wind. Nature of Energy You use energy when you: hit
Energy: Forms and Changes Nature of Energy Energy is all around you! You can hear energy as sound. You can see energy as light. And you can feel it as wind. Nature of Energy You use energy when you: hit
GCSE (9 1) Physics A (Gateway Science) J249/04 Paper 4, P5 P8 and P9 (Higher Tier)
 Oxford Cambridge and RSA GCSE (9 1) Physics A (Gateway Science) J249/04 Paper 4, P5 P8 and P9 (Higher Tier) H Year 11 Test Time allowed: 1 hour 45 minutes You must have: a ruler (cm/mm) the Data Sheet
Oxford Cambridge and RSA GCSE (9 1) Physics A (Gateway Science) J249/04 Paper 4, P5 P8 and P9 (Higher Tier) H Year 11 Test Time allowed: 1 hour 45 minutes You must have: a ruler (cm/mm) the Data Sheet
What is Energy? In science, energy is the ability to do work. Work is done when a force causes an object to move in the direction of the force.
 What is Energy? In science, energy is the ability to do work. Work is done when a force causes an object to move in the direction of the force. Energy Energy is the ability to do work. (reminder=what is
What is Energy? In science, energy is the ability to do work. Work is done when a force causes an object to move in the direction of the force. Energy Energy is the ability to do work. (reminder=what is
Chapter 02 Chemical Composition of the Body
 Chapter 02 Chemical Composition of the Body 1. In an atom, the number of Student: A. Protons always equals the number of neutrons B. Of protons always equals the number of electrons C. Of neutrons always
Chapter 02 Chemical Composition of the Body 1. In an atom, the number of Student: A. Protons always equals the number of neutrons B. Of protons always equals the number of electrons C. Of neutrons always
Pre Comp Review Questions 7 th Grade
 Pre Comp Review Questions 7 th Grade Section 1 Units 1. Fill in the missing SI and English Units Measurement SI Unit SI Symbol English Unit English Symbol Time second s second s. Temperature Kelvin K Fahrenheit
Pre Comp Review Questions 7 th Grade Section 1 Units 1. Fill in the missing SI and English Units Measurement SI Unit SI Symbol English Unit English Symbol Time second s second s. Temperature Kelvin K Fahrenheit
P.M. THURSDAY, 15 January hour
 Surname Centre Number Candidate Number Other Names 0 GCSE 4473/02 W15-4473-02 ADDITIONAL SCIENCE/PHYSICS PHYSICS 2 HIGHER TIER P.M. THURSDAY, 15 January 2015 1 hour For s use Question Maximum Mark 1. 15
Surname Centre Number Candidate Number Other Names 0 GCSE 4473/02 W15-4473-02 ADDITIONAL SCIENCE/PHYSICS PHYSICS 2 HIGHER TIER P.M. THURSDAY, 15 January 2015 1 hour For s use Question Maximum Mark 1. 15
Chapter 4. Energy. Work Power Kinetic Energy Potential Energy Conservation of Energy. W = Fs Work = (force)(distance)
 Chapter 4 Energy In This Chapter: Work Kinetic Energy Potential Energy Conservation of Energy Work Work is a measure of the amount of change (in a general sense) that a force produces when it acts on a
Chapter 4 Energy In This Chapter: Work Kinetic Energy Potential Energy Conservation of Energy Work Work is a measure of the amount of change (in a general sense) that a force produces when it acts on a
Chapter 4. Dynamics: Newton s Laws of Motion
 Chapter 4 Dynamics: Newton s Laws of Motion Types of Forces: An Overview In nature there are two general types of forces, fundamental and nonfundamental. Fundamental Forces -- three have been identified,
Chapter 4 Dynamics: Newton s Laws of Motion Types of Forces: An Overview In nature there are two general types of forces, fundamental and nonfundamental. Fundamental Forces -- three have been identified,
LIFE OF CELL. Jhia Anjela D. Rivera 1,2 1. BS Biology Graduate, Department of Biology, College of Science, Polytechnic University of the Philippines 2
 LIFE OF CELL Jhia Anjela D. Rivera 1,2 1 BS Biology Graduate, Department of Biology, College of Science, Polytechnic University of the Philippines 2 MS Biology Student, Graduate School, Centro Escolar
LIFE OF CELL Jhia Anjela D. Rivera 1,2 1 BS Biology Graduate, Department of Biology, College of Science, Polytechnic University of the Philippines 2 MS Biology Student, Graduate School, Centro Escolar
SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question.
 ch 2 chemical basis of life Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Fill in the blank or provide a short answer: 1) When a change in matter
ch 2 chemical basis of life Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. Fill in the blank or provide a short answer: 1) When a change in matter
1 Some Basic Concepts of Chemistry
 1 Some Basic Concepts of Chemistry Multiple Choice Questions (MCQs) Q. 1 Two students performed the same experiment separately and each one of them recorded two readings of mass which are given below.
1 Some Basic Concepts of Chemistry Multiple Choice Questions (MCQs) Q. 1 Two students performed the same experiment separately and each one of them recorded two readings of mass which are given below.
Energy present in a variety of forms. Energy can be transformed form one form to another Energy is conserved (isolated system) ENERGY
 ENERGY Energy present in a variety of forms Mechanical energy Chemical energy Nuclear energy Electromagnetic energy Energy can be transformed form one form to another Energy is conserved (isolated system)
ENERGY Energy present in a variety of forms Mechanical energy Chemical energy Nuclear energy Electromagnetic energy Energy can be transformed form one form to another Energy is conserved (isolated system)
Guided Notes Unit 1: Biochemistry
 Name: Date: Block: Chapter 2: The Chemistry of Life I. Concept 2.1: Atoms, Ions, and Molecules a. Atoms Guided Notes Unit 1: Biochemistry i. Atom: _ ii. (They are SUPER small! It would take 3 million carbon
Name: Date: Block: Chapter 2: The Chemistry of Life I. Concept 2.1: Atoms, Ions, and Molecules a. Atoms Guided Notes Unit 1: Biochemistry i. Atom: _ ii. (They are SUPER small! It would take 3 million carbon
Chapter 02 Chemical Basis of Life. Multiple Choice Questions
 Seeleys Essentials of Anatomy and Physiology 8th Edition VanPutte Test Bank Full Download: http://testbanklive.com/download/seeleys-essentials-of-anatomy-and-physiology-8th-edition-vanputte-test-bank/
Seeleys Essentials of Anatomy and Physiology 8th Edition VanPutte Test Bank Full Download: http://testbanklive.com/download/seeleys-essentials-of-anatomy-and-physiology-8th-edition-vanputte-test-bank/
BIOCHEMISTRY 10/9/17 CHEMISTRY OF LIFE. Elements: simplest form of a substance - cannot be broken down any further without changing what it is
 BIOCHEMISTRY CHEMISTRY OF LIFE Elements: simplest form of a substance - cannot be broken down any further without changing what it is THE ATOM Just like cells are the basic unit of life, the ATOM is the
BIOCHEMISTRY CHEMISTRY OF LIFE Elements: simplest form of a substance - cannot be broken down any further without changing what it is THE ATOM Just like cells are the basic unit of life, the ATOM is the
Name: Block: Date: Microbiology Chapters 1 and 2 Review
 Name: Block: Date: Microbiology Chapters 1 and 2 Review Complete the following short answer questions. 1. Define microbiology. The study of microorganisms. 2. What are the six major groups of organisms
Name: Block: Date: Microbiology Chapters 1 and 2 Review Complete the following short answer questions. 1. Define microbiology. The study of microorganisms. 2. What are the six major groups of organisms
Chemistry: The Study of Change
 Chemistry: The Study of Change Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chemistry: A Science for the 21 st Century Health and Medicine Sanitation
Chemistry: The Study of Change Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chemistry: A Science for the 21 st Century Health and Medicine Sanitation
Full file at https://fratstock.eu
 VanMeter: Microbiology for the Healthcare Professional Chapter 02: Chemistry of Life Test Bank MULTIPLE CHOICE 1. The atomic number equals the number of a. Protons b. Neutrons c. Electrons d. Protons and
VanMeter: Microbiology for the Healthcare Professional Chapter 02: Chemistry of Life Test Bank MULTIPLE CHOICE 1. The atomic number equals the number of a. Protons b. Neutrons c. Electrons d. Protons and
Chapter 6 The Chemistry of Life
 Chapter 6 The Chemistry of Life Atoms: The Building Blocks of Life Both living and non-living things have atoms Everything, living and non, is made of Atoms. An elements is something you can break down
Chapter 6 The Chemistry of Life Atoms: The Building Blocks of Life Both living and non-living things have atoms Everything, living and non, is made of Atoms. An elements is something you can break down
Chapter 02 Testbank. 1. Anything that occupies space and has mass is called. A. an electron. B. living. C. matter. D. energy. E. space.
 Chapter 02 Testbank Student: 1. Anything that occupies space and has mass is called A. an electron. B. living. C. matter. D. energy. E. space. 2. The electrons of an atom are A. always equal to the number
Chapter 02 Testbank Student: 1. Anything that occupies space and has mass is called A. an electron. B. living. C. matter. D. energy. E. space. 2. The electrons of an atom are A. always equal to the number
chapter A solution is a mixture composed of two or more substances that are physically blended but not chemically combined.
 chapter 02 True / False Questions 1. Minerals are organic elements extracted from the soil by plants. True False 2. Molecules composed of two or more atoms are called compounds. True False 3. Hydrogen,
chapter 02 True / False Questions 1. Minerals are organic elements extracted from the soil by plants. True False 2. Molecules composed of two or more atoms are called compounds. True False 3. Hydrogen,
Energy and the Environment
 Energy and the Environment Energy physics definition the capacity to do work and conjunction used to connect grammatically coordinate words, phrases, or clauses the Environment the aggregate of surrounding
Energy and the Environment Energy physics definition the capacity to do work and conjunction used to connect grammatically coordinate words, phrases, or clauses the Environment the aggregate of surrounding
Introduction. Chapter 1. The Study of Chemistry. The scientific method is a systematic approach to research
 1 Introduction Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 2 Macroscopic The Study of Chemistry Microscopic 2 3 The scientific method is a systematic
1 Introduction Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 2 Macroscopic The Study of Chemistry Microscopic 2 3 The scientific method is a systematic
Chapter 02 Testbank. 1. Anything that occupies space and has mass is called. A. an electron. B. living. C. matter. D. energy. E. space.
 Chapter 02 Testbank Student: 1. Anything that occupies space and has mass is called A. an electron. B. living. C. matter. D. energy. E. space. 2. The electrons of an atom are A. always equal to the number
Chapter 02 Testbank Student: 1. Anything that occupies space and has mass is called A. an electron. B. living. C. matter. D. energy. E. space. 2. The electrons of an atom are A. always equal to the number
Chapter 9. Preview. Objectives Defining Temperature. Thermal Equilibrium. Thermal Expansion Measuring Temperature. Section 1 Temperature and
 Section 1 Temperature and Thermal Equilibrium Preview Objectives Defining Temperature Thermal Equilibrium Thermal Expansion Measuring Temperature Section 1 Temperature and Thermal Equilibrium Objectives
Section 1 Temperature and Thermal Equilibrium Preview Objectives Defining Temperature Thermal Equilibrium Thermal Expansion Measuring Temperature Section 1 Temperature and Thermal Equilibrium Objectives
1- Proteins are made up of many. a. Sugars b. Fats c. Amino acids
 The first question: Choose the right answer: 1- Proteins are made up of many. a. Sugars b. Fats c. Amino acids 2- Feces are stored and expelled by the. a. Small intestine b. Large intestine c. Appendix
The first question: Choose the right answer: 1- Proteins are made up of many. a. Sugars b. Fats c. Amino acids 2- Feces are stored and expelled by the. a. Small intestine b. Large intestine c. Appendix
100 Physics Facts. 1. The standard international unit (SI unit) for mass (m) is. kg (kilograms) s (seconds)
 100 Physics Facts 1. The standard international unit (SI unit) for mass (m) is. kg (kilograms) 2. The standard international unit (SI unit) for time (t) is. s (seconds) 3. The standard international unit
100 Physics Facts 1. The standard international unit (SI unit) for mass (m) is. kg (kilograms) 2. The standard international unit (SI unit) for time (t) is. s (seconds) 3. The standard international unit
Science Year 10 Unit 1 Biology
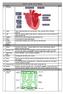 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
2/18/2013 CHEMISTRY OF CELLS. Carbon Structural Formations. 4 Classes of Organic Compounds (biomolecules)
 CHEMISTRY OF CELLS 11 elements make up all organisms C, O, N, H: 96% weight of human body ORGANIC CHEMISTRY Organic compounds: contain C Inorganic compounds: no C Bonding and Structural Formulas H and
CHEMISTRY OF CELLS 11 elements make up all organisms C, O, N, H: 96% weight of human body ORGANIC CHEMISTRY Organic compounds: contain C Inorganic compounds: no C Bonding and Structural Formulas H and
Knox Academy. S4 Chemistry
 Unit 2: Nature s Chemistry Summary Book Name: Summary 2.1: Plants and their Products 1. Animals eat as food, humans grow particular plants as food crops. 2. Plants produce carbohydrates, and oils which
Unit 2: Nature s Chemistry Summary Book Name: Summary 2.1: Plants and their Products 1. Animals eat as food, humans grow particular plants as food crops. 2. Plants produce carbohydrates, and oils which
Atomic weight = Number of protons + neutrons
 1 BIOLOGY Elements and Compounds Element is a substance that cannot be broken down to other substances by chemical reactions. Essential elements are chemical elements required for an organism to survive,
1 BIOLOGY Elements and Compounds Element is a substance that cannot be broken down to other substances by chemical reactions. Essential elements are chemical elements required for an organism to survive,
Copy into Note Packet and Return to Teacher
 Copy into Note Packet and Return to Teacher Section 1: Nature of Matter Objectives: Differentiate between atoms and elements. Analyze how compounds are formed. Distinguish between covalent bonds, hydrogen
Copy into Note Packet and Return to Teacher Section 1: Nature of Matter Objectives: Differentiate between atoms and elements. Analyze how compounds are formed. Distinguish between covalent bonds, hydrogen
St Olave s Physics Department. Year 11 Mock Revision Checklist
 St Olave s Physics Department Year 11 Mock Revision Checklist The following checklists include all the topics that will be included in the Year 11 Mock exam. Students should use the tickboxes to check
St Olave s Physics Department Year 11 Mock Revision Checklist The following checklists include all the topics that will be included in the Year 11 Mock exam. Students should use the tickboxes to check
Chemical Basis of Life
 Chemical Basis of Life Jan 30 11:42 AM In order to understand digestion and nutrition, we need some basic biochemistry Chemistry studies the composition of matter and its changes as well as the change
Chemical Basis of Life Jan 30 11:42 AM In order to understand digestion and nutrition, we need some basic biochemistry Chemistry studies the composition of matter and its changes as well as the change
NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE. Honors Biology I
 NOTE/STUDY GUIDE: Unit 1-2, Biochemistry Honors Biology I, Mr. Doc Miller, M.Ed. North Central High School Name: Period: Seat #: Date: NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE Honors Biology I Unit
NOTE/STUDY GUIDE: Unit 1-2, Biochemistry Honors Biology I, Mr. Doc Miller, M.Ed. North Central High School Name: Period: Seat #: Date: NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE Honors Biology I Unit
BIOCHEMISTRY BIOCHEMISTRY INTRODUCTION ORGANIZATION? MATTER. elements into the order and appearance we now
 BIOCHEMISTRY MR. HULSE BVHS BIOLOGY MATTER Matter - anything that occupies space and has mass Lacked clarity and flow BIOCHEMISTRY INTRODUCTION Biochemistry study of chemical and physiological process
BIOCHEMISTRY MR. HULSE BVHS BIOLOGY MATTER Matter - anything that occupies space and has mass Lacked clarity and flow BIOCHEMISTRY INTRODUCTION Biochemistry study of chemical and physiological process
1.4 recall and use the relationship between acceleration, velocity and time: 1.6 determine acceleration from the gradient of a velocity-time graph
 Physics Section 1: Forces and motion b) Movement and position c) Forces, movement and shape d) Astronomy 1.1 use the following units: kilogram (kg), metre (m), metre/second (m/s), metre/second 2 (m/s 2
Physics Section 1: Forces and motion b) Movement and position c) Forces, movement and shape d) Astronomy 1.1 use the following units: kilogram (kg), metre (m), metre/second (m/s), metre/second 2 (m/s 2
ATom,ion, molwcul in the dily live
 ATom,ion, molwcul in the dily live Chemistry of Life All matter is composed of tiny particles called atoms. There are 109 types of atoms. A substance made up of one kind of atom is called an element. An
ATom,ion, molwcul in the dily live Chemistry of Life All matter is composed of tiny particles called atoms. There are 109 types of atoms. A substance made up of one kind of atom is called an element. An
Lab: Using indicator dyes to examine macromolecules in food.
 Lab: Using indicator dyes to examine macromolecules in food. Chemistry deals with the study of matter. Matter: Anything that takes up space and has mass (rock, bug, human). Atoms are the fundamental units
Lab: Using indicator dyes to examine macromolecules in food. Chemistry deals with the study of matter. Matter: Anything that takes up space and has mass (rock, bug, human). Atoms are the fundamental units
CORE CONCEPTS & TERMINOLOGY FALL 2010
 CORE CONCEPTS & TERMINOLOGY FALL 2010 The following concepts and terms will be covered by all BIO 120 lecture instructors. Presentation of additional concepts is left to the discretion of the individual
CORE CONCEPTS & TERMINOLOGY FALL 2010 The following concepts and terms will be covered by all BIO 120 lecture instructors. Presentation of additional concepts is left to the discretion of the individual
UNIT 2 CHEMISTRY. Atomic Structure: Ionic Bond: Covalent Bond: Hydrogen Bond:
 UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
Basic Chemistry. Chapter 2 BIOL1000 Dr. Mohamad H. Termos
 Basic Chemistry Chapter 2 BIOL1000 Dr. Mohamad H. Termos Chapter 2 Objectives Following this chapter, you should be able to describe: - Atoms, molecules, and ions - Composition and properties - Types of
Basic Chemistry Chapter 2 BIOL1000 Dr. Mohamad H. Termos Chapter 2 Objectives Following this chapter, you should be able to describe: - Atoms, molecules, and ions - Composition and properties - Types of
Energy Review Guide Name: Date: Period:
 Name: Date: Period: You are to use this review guide in addition to the study cards you should have already created using the guide previously given to you. I can state and explain the Law of Conservation
Name: Date: Period: You are to use this review guide in addition to the study cards you should have already created using the guide previously given to you. I can state and explain the Law of Conservation
Conceptual Understanding
 Name Period Conceptual Understanding 1. Define work in scientific terms, and give the formula. What is it measured in? Work is a force applied over a distance to move and object. Force applied and object
Name Period Conceptual Understanding 1. Define work in scientific terms, and give the formula. What is it measured in? Work is a force applied over a distance to move and object. Force applied and object
APES CHAPTER 2 NOTES (MRS. BAUCK): ENVIRONMENTAL SYSTEMS
 APES CHAPTER 2 NOTES (MRS. BAUCK): ENVIRONMENTAL SYSTEMS MODULE 4: Systems and Matter I. Matter A. general chemistry terms for review 1) matter anything that takes up space and has mass 2) element a) a
APES CHAPTER 2 NOTES (MRS. BAUCK): ENVIRONMENTAL SYSTEMS MODULE 4: Systems and Matter I. Matter A. general chemistry terms for review 1) matter anything that takes up space and has mass 2) element a) a
Today. Work, Energy, Power loose ends Temperature Second Law of Thermodynamics
 Today Announcements: HW#5 is due by 8:00 am Wed. Feb. 5th. Extra Credit Exam due by Tomorrow 8am. Work, Energy, Power loose ends Temperature Second Law of Thermodynamics ISP09s9 Lecture 11-1- Energy and
Today Announcements: HW#5 is due by 8:00 am Wed. Feb. 5th. Extra Credit Exam due by Tomorrow 8am. Work, Energy, Power loose ends Temperature Second Law of Thermodynamics ISP09s9 Lecture 11-1- Energy and
Today. Exam 1. The Electric Force Work, Energy and Power. Comments on exam extra credit. What do these pictures have in common?
 Today Exam 1 Announcements: The average on the first exam was 31/40 Exam extra credit is due by :00 pm Thursday February 18th. (It opens on LONCAPA today) The Electric Force Work, Energy and Power Number
Today Exam 1 Announcements: The average on the first exam was 31/40 Exam extra credit is due by :00 pm Thursday February 18th. (It opens on LONCAPA today) The Electric Force Work, Energy and Power Number
Angel International School -Manipay 2 nd Term Examination April, 2015 Science
 c Grade 10 Angel International School -Manipay 2 nd Term Examination April, 2015 Science Duration: 2.5 Hours PART I Index No:- 1) To which group of organisms does Yeast belong? a) Bactria c) Algae b) Fungi
c Grade 10 Angel International School -Manipay 2 nd Term Examination April, 2015 Science Duration: 2.5 Hours PART I Index No:- 1) To which group of organisms does Yeast belong? a) Bactria c) Algae b) Fungi
ENERGY. Unit 12: IPC
 ENERGY Unit 12: IPC WHAT IS ENERGY? Energy- is the ability to do work. Energy is the ability to cause a change. Energy can change an object s: motion shape temperature color THERMAL internal motion of
ENERGY Unit 12: IPC WHAT IS ENERGY? Energy- is the ability to do work. Energy is the ability to cause a change. Energy can change an object s: motion shape temperature color THERMAL internal motion of
Table of Contents. Performance Standards
 Table of Contents Letter to the Student............................................ 5 Letter to the Family............................................. 6 Georgia Performance Standards Correlation Chart..................
Table of Contents Letter to the Student............................................ 5 Letter to the Family............................................. 6 Georgia Performance Standards Correlation Chart..................
Chapter 02 Chemical Composition of the Body
 Chapter 02 Chemical Composition of the Body Multiple Choice Questions 1. Water makes up of the total body weight of an average adult. A. 50-60% B. 55-65% C. 60-70% D. 65-75% Learning Outcome: 02.01 2.
Chapter 02 Chemical Composition of the Body Multiple Choice Questions 1. Water makes up of the total body weight of an average adult. A. 50-60% B. 55-65% C. 60-70% D. 65-75% Learning Outcome: 02.01 2.
Pre Comp Review Questions 8 th Grade Answers
 Pre Comp Review Questions 8 th Grade Answers Section 1 Units 1. Fill in the missing SI and English Units Measurement SI Unit SI Symbol English Unit English Symbol Time second s second s. Temperature Kelvin
Pre Comp Review Questions 8 th Grade Answers Section 1 Units 1. Fill in the missing SI and English Units Measurement SI Unit SI Symbol English Unit English Symbol Time second s second s. Temperature Kelvin
GCSE PHYSICS. Materials For this paper you must have: a ruler a scientific calculator the Physics Equations Sheet (enclosed).
 Please write clearly in block capitals. Centre number Candidate number Surname Forename(s) Candidate signature GCSE PHYSICS Foundation Tier Paper 2F F Specimen 2018 (set 2) Time allowed: 1 hour 45 minutes
Please write clearly in block capitals. Centre number Candidate number Surname Forename(s) Candidate signature GCSE PHYSICS Foundation Tier Paper 2F F Specimen 2018 (set 2) Time allowed: 1 hour 45 minutes
Hole s Human Anatomy and Physiology Eleventh Edition. Chapter 2
 Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. C is FALSE?
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about the atom 12 6 C is FALSE? 1) A) It has 12 neutrons
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about the atom 12 6 C is FALSE? 1) A) It has 12 neutrons
The Story of Energy. Forms and Functions
 The Story of Energy Forms and Functions What are 5 things E helps us do? Batteries store energy! This car uses a lot of energy Even this sleeping puppy is using stored energy. We get our energy from FOOD!
The Story of Energy Forms and Functions What are 5 things E helps us do? Batteries store energy! This car uses a lot of energy Even this sleeping puppy is using stored energy. We get our energy from FOOD!
CHE Thermodynamics of Chemical Processes
 CHE 3010 - Thermodynamics of Chemical Processes Venkat Padmanabhan, PhD Department of Chemical Engineering Tennessee Tech University Lecture 2 - Basic Concepts 8/29/2018 CHE 3010 - Thermodynamics Tennessee
CHE 3010 - Thermodynamics of Chemical Processes Venkat Padmanabhan, PhD Department of Chemical Engineering Tennessee Tech University Lecture 2 - Basic Concepts 8/29/2018 CHE 3010 - Thermodynamics Tennessee
FORCE. Definition: Combining Forces (Resultant Force)
 1 FORCE Definition: A force is either push or pull. A Force is a vector quantity that means it has magnitude and direction. Force is measured in a unit called Newtons (N). Some examples of forces are:
1 FORCE Definition: A force is either push or pull. A Force is a vector quantity that means it has magnitude and direction. Force is measured in a unit called Newtons (N). Some examples of forces are:
 Unit 1 SOME BASIC CONCEPTS OF CHEMISTRY I. Multiple Choice Questions (Type-I) 1. Two students performed the same experiment separately and each one of them recorded two readings of mass which are given
Unit 1 SOME BASIC CONCEPTS OF CHEMISTRY I. Multiple Choice Questions (Type-I) 1. Two students performed the same experiment separately and each one of them recorded two readings of mass which are given
Chapter 02 - Life, Matter, and Energy. Multiple Choice Questions
 Essentials of Anatomy and Physiology 1st Edition Saladin TEST BANK Full clear download (no formatting errors) at: https://testbankreal.com/download/essentials-anatomy-physiology-1stedition-saladin-test-bank/
Essentials of Anatomy and Physiology 1st Edition Saladin TEST BANK Full clear download (no formatting errors) at: https://testbankreal.com/download/essentials-anatomy-physiology-1stedition-saladin-test-bank/
W = Fd cos θ. W = (75.0 N)(25.0 m) cos (35.0º) = 1536 J = J. W 2400 kcal =
 8 CHAPTER 7 WORK, ENERGY, AND ENERGY RESOURCES generator does negative work on the briefcase, thus removing energy from it. The drawing shows the latter, with the force from the generator upward on the
8 CHAPTER 7 WORK, ENERGY, AND ENERGY RESOURCES generator does negative work on the briefcase, thus removing energy from it. The drawing shows the latter, with the force from the generator upward on the
Biology 30 The Chemistry of Living Things
 Biology 30 The Chemistry of Living Things Hierarchy of organization: Chemistry: MATTER: Periodic Table: ELEMENT: Ex. oxygen, gold, copper, carbon COMPOUND: Ex. salt (NaCl), H 2 O ELEMENTS ESSENTIAL TO
Biology 30 The Chemistry of Living Things Hierarchy of organization: Chemistry: MATTER: Periodic Table: ELEMENT: Ex. oxygen, gold, copper, carbon COMPOUND: Ex. salt (NaCl), H 2 O ELEMENTS ESSENTIAL TO
SIC CONCEPTS TS OF CHEMISTRY. Unit. I. Multiple Choice Questions (Type-I)
 Unit 1 SOME BASIC B SIC CONCEPTS CONCEP TS OF CHEMISTRY CHEMIS I. Multiple Choice Questions (Type-I) 1. Two students performed the same experiment separately and each one of them recorded two readings
Unit 1 SOME BASIC B SIC CONCEPTS CONCEP TS OF CHEMISTRY CHEMIS I. Multiple Choice Questions (Type-I) 1. Two students performed the same experiment separately and each one of them recorded two readings
N5 Physics. Key Definitions. Name:
 N5 Physics Key s Name: Contents Page 3 Key s Electricity Equations Electricity Page 4 Key s Dynamics and Space Equations Dynamics and Space Page 5 Key s Properties of Matter Equations Properties of Matter
N5 Physics Key s Name: Contents Page 3 Key s Electricity Equations Electricity Page 4 Key s Dynamics and Space Equations Dynamics and Space Page 5 Key s Properties of Matter Equations Properties of Matter
Biochemistry. The Chemistry of Life
 Biochemistry The Chemistry of Life Biochemistry The life processes (Chapter 1) are chemical in nature. Chemical reactions occur in life. Living things are made of chemical compounds. The Atom- The Basic
Biochemistry The Chemistry of Life Biochemistry The life processes (Chapter 1) are chemical in nature. Chemical reactions occur in life. Living things are made of chemical compounds. The Atom- The Basic
Energy: The ability to cause changes. thermodynamics stems from therme (heat) and dynamis (power).
 Energy: The ability to cause changes. thermodynamics stems from therme (heat) and dynamis (power). Thermodynamics: The science of energy. Conservation of energy principle: During an interaction, energy
Energy: The ability to cause changes. thermodynamics stems from therme (heat) and dynamis (power). Thermodynamics: The science of energy. Conservation of energy principle: During an interaction, energy
Biology: The Core (Simon) Chapter 2 The Chemistry of Life. Multiple-Choice Questions
 Biology: The Core (Simon) Chapter 2 The Chemistry of Life Multiple-Choice Questions 1) The chemical name for table salt is sodium chloride, or simply NaCl. What type of chemical is NaCl? A) Compound B)
Biology: The Core (Simon) Chapter 2 The Chemistry of Life Multiple-Choice Questions 1) The chemical name for table salt is sodium chloride, or simply NaCl. What type of chemical is NaCl? A) Compound B)
Motion. Argument: (i) Forces are needed to keep things moving, because they stop when the forces are taken away (evidence horse pulling a carriage).
 1 Motion Aristotle s Study Aristotle s Law of Motion This law of motion was based on false assumptions. He believed that an object moved only if something was pushing it. His arguments were based on everyday
1 Motion Aristotle s Study Aristotle s Law of Motion This law of motion was based on false assumptions. He believed that an object moved only if something was pushing it. His arguments were based on everyday
Chapter 2. Chemical Principles
 Chapter 2 Chemical Principles Insert Fig CO 2 The Structure of Atoms Chemistry is the study of interactions between atoms and molecules The atom is the smallest unit of matter that enters into chemical
Chapter 2 Chemical Principles Insert Fig CO 2 The Structure of Atoms Chemistry is the study of interactions between atoms and molecules The atom is the smallest unit of matter that enters into chemical
Hole s Human Anatomy and Physiology Tenth Edition. Chapter 2
 PowerPoint Lecture Outlines to accompany Hole s Human Anatomy and Physiology Tenth Edition Shier w Butler w Lewis Chapter 2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
PowerPoint Lecture Outlines to accompany Hole s Human Anatomy and Physiology Tenth Edition Shier w Butler w Lewis Chapter 2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
An atom is the smallest unit of an element. It has: A general understanding of chemistry is necessary for understanding human physiology.
 8/29/11 Chapter 2 I. Atoms, Ions, and Chemical Bonds Chemical Composition of the Body Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Body
8/29/11 Chapter 2 I. Atoms, Ions, and Chemical Bonds Chemical Composition of the Body Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Body
UNIT 2 CHEMISTRY. Atomic Structure: Ionic Bond: Covalent Bond: Hydrogen Bond:
 UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
UNIT 2 CHEMISTRY Atomic Structure: Ionic Bond: Hydrogen Bond: Covalent Bond: 1 Carbohydrates: >energy yield- >elements- >monomers- >functions- >examples- >misc- Lipids: Proteins: Nucleic Acids: I. Energy
Full file at Essentials of Anatomy & Physiology (Martini/ Bartholomew) Chapter 2 The Chemical Level of Organization
 Essentials of Anatomy & Physiology (Martini/ Bartholomew) Chapter 2 The Chemical Level of Organization Multiple Choice 1) An unstable isotope that emits subatomic particles spontaneously is called A) a
Essentials of Anatomy & Physiology (Martini/ Bartholomew) Chapter 2 The Chemical Level of Organization Multiple Choice 1) An unstable isotope that emits subatomic particles spontaneously is called A) a
(D) The subtraction of from 4.8 giving the correct number of significant
 HCCS CHEM 1405 PRACTICE EXAM I: 5 th & 6 th & 7 th editions of Corwin s Introductory Chemistry: Concepts and Critical Thinking. Multiple Choices: Choose the best (one) answer. Show in bold. Questions break-down:
HCCS CHEM 1405 PRACTICE EXAM I: 5 th & 6 th & 7 th editions of Corwin s Introductory Chemistry: Concepts and Critical Thinking. Multiple Choices: Choose the best (one) answer. Show in bold. Questions break-down:
Wiley Plus Reminder! Assignment 1
 Wiley Plus Reminder! Assignment 1 6 problems from chapters and 3 Kinematics Due Monday October 5 Before 11 pm! Chapter 4: Forces and Newton s Laws Force, mass and Newton s three laws of motion Newton s
Wiley Plus Reminder! Assignment 1 6 problems from chapters and 3 Kinematics Due Monday October 5 Before 11 pm! Chapter 4: Forces and Newton s Laws Force, mass and Newton s three laws of motion Newton s
the universal solvent
 Chapter 7: Acids, Bases, and Solutions Solution a homogeneous mixture Solutions have the same properties throughout, containing solute particles (molecules or ions) that are too small to see Solvent the
Chapter 7: Acids, Bases, and Solutions Solution a homogeneous mixture Solutions have the same properties throughout, containing solute particles (molecules or ions) that are too small to see Solvent the
New GCSE 4463/02 SCIENCE A HIGHER TIER PHYSICS 1
 Surname Other Names Centre Number 0 Candidate Number New GCSE 4463/02 SCIENCE A HIGHER TIER PHYSICS 1 P.M. FRIDAY, 15 June 2012 1 hour ADDITIONAL MATERIALS In addition to this paper you may require a calculator.
Surname Other Names Centre Number 0 Candidate Number New GCSE 4463/02 SCIENCE A HIGHER TIER PHYSICS 1 P.M. FRIDAY, 15 June 2012 1 hour ADDITIONAL MATERIALS In addition to this paper you may require a calculator.
