Electricity and magnetism Solid state physics
|
|
|
- Ferdinand Barnett
- 5 years ago
- Views:
Transcription
1 Physics: Physics: Electricity and magnetism Solid state physics Thermoelectricity he direct conversion of heat into electrical energy, or the reverse, in solid or liquid conductors by means of three interrelated phenomena the Seebeck effect, the Peltier effect, and the Thomson effect including the influence of magnetic fields upon each. The Seebeck effect concerns the electromotive force (emf) generated in a circuit composed of two different conductors whose junctions are maintained at different temperatures. The Peltier effect refers to the reversible heat generated at the junction between two different conductors when a current passes through the junction. The Thomson effect involves the reversible generation of heat in a single current-carrying conductor along which a temperature gradient is maintained. Specifically excluded from the definition of thermoelectricity are the phenomena of Joule heating and thermionic emission. See also: Electromotive force (emf); Joule's law; Thermionic emission The three thermoelectric effects are described in terms of three coefficients: the absolute thermoelectric power (or thermopower) S, the Peltier coefficient, and the Thomson coefficient, each of which is defined for a homogeneous conductor at a given temperature. These coefficients are connected by the Kelvin relations, which convert complete knowledge of one into complete knowledge of all three. It is therefore necessary to measure only one of the three coefficients; usually the thermopower S is chosen. The combination of electrical resistivity, thermal conductivity, and thermopower (more generally, these quantities as tensors) is sufficient to provide a complete description of the electronic transport properties of conductors for which the electric current and heat current are linear functions of both the applied electric field and the temperature gradient. See also: Electrical conductivity of metals Thermoelectric effects have significant applications in both science and technology and show promise of more importance in the future. Studies of thermoelectricity in metals and semiconductors yield information about electronic structure and about the interactions between electrons and both lattice vibrations and impurities. Practical applications include the measurement of temperature, generation of power, cooling, and heating. Thermocouples are widely used for temperature measurement, providing both accuracy and sensitivity. Research has been undertaken concerning the direct thermoelectric generation of electricity using the heat produced by nuclear reactions or generated at automobile exhausts. Cooling units using the Peltier effect have been constructed in sizes up to those of home refrigerators. Development of thermoelectric heating has also been undertaken. Seebeck Effect In 1821, T. J. Seebeck discovered that when two different conductors are joined into a loop and a temperature difference is maintained between the two junctions, an emf is generated. Such a loop is called a thermocouple, and the emf generated is called a thermoelectric (or Seebeck) emf. Measurements The magnitude of the emf generated by a thermocouple is standardly measured using the system shown in Fig. 1. Here the contact points between conductors A and B are called junctions. Each junction is maintained at a well-controlled temperature (either T 1 or T 0 ) by immersion in a bath or connection to a heat reservoir. These baths or reservoirs are indicated by the white squares. From each junction, conductor A is brought to a measuring device M, usually a potentiometer. When the potentiometer is balanced, no current flows, thereby allowing direct measurement of the opencircuit emf, undiminished by resistive losses and unperturbed by spurious effects arising from Joule heating or from Peltier heating and cooling at the junctions. This open-circuit emf is the thermoelectric emf. See also: Potentiometer Fig. 1 Diagram of apparatus usually used for measuring thermoelectric (Seebeck) emf E AB (T 0,T 1 ). M is an instrument for measuring potential.
2 Equations According to the experimentally established law of Magnus, for homogeneous conductors A and B the thermoelectric emf depends only upon the temperatures of the two junctions and not upon either the shapes of the samples or the detailed forms of the temperature distributions along them. This emf can thus be symbolized E AB (T 0,T 1 ). According to both theory and experiment, if one of the conductors, say B, is a superconductor in its superconducting state, it makes no contribution to E AB (except for very small effects near the superconducting transition temperature T c, discussed below). That is, when B is superconducting, E AB (T 0,T 1 ) is determined solely by conductor A, and can be written as E A (T 0,T 1 ). See also: Superconductivity It is convenient to express this emf in terms of a property that depends only upon a single temperature. Such a property is the absolute thermoelectric power (or, simply, thermopower) S A(T), defined so that Eq. (1) is valid. If E A(T, T + T) is known for example, from measurements involving a superconductor S A(T) can be determined from Eq. (2). If Eq. (1) is valid for any homogeneous conductor, then it ought to apply to both sides of the thermocouple shown in Fig. 1. Indeed, it has been verified experimentally that the emf E AB (T 0,T 1 ) produced by a thermocouple is just the difference between the emfs, calculated using Eq. (1), produced by its two arms. This result can be derived as follows. Employing the usual sign convention, to calculate E AB (T 0,T 1 ), begin at the cooler bath, T 0, integrate S A (T) dt along conductor A up to the warmer bath, T 1, and then return to T 0 along conductor B by integrating S B (T) dt. This circular excursion produces E AB(T 0,T 1), given by Eq. (3). Inverting the last integral in Eq. (3) gives Eq. (4), which from Eq. (1) can be rewritten as Eq. (5). Alternatively, combining the two integrals in Eq. (4) gives Eq. (6). Defining S AB according to Eq. (7) then yields Eq. (8). (1) (2) (3) (4) (5)
3 Equation (6) shows that E AB (T 0,T 1 ) can be calculated for a given thermocouple whenever the thermopowers S A (T) and S B (T) are known for its two constitutents over the temperature range T 0 to T 1. By convention, the signs of S A(T) and S B(T) are chosen so that, if the temperature difference T 1 - T 0 is taken small enough so that S A(T) and S B(T) can be presumed constant, then S A(T) S B(T) when the emf E AB (T 0,T 1 ) has the polarity indicated in Fig. 1. Results of equations These equations lead directly to the following experimentally and theoretically verified results. Uniform temperature In a circuit kept at a uniform temperature throughout, E = 0, even though the circuit may consist of a number of different conductors. This result follows directly from Eq. (8), since dt = 0 everywhere throughout the circuit. It follows also from thermodynamic reasoning. If E did not equal 0, the circuit could drive an electrical motor and make it do work. But the only source of energy would be heat from the surroundings which, by assumption, are at the same uniform temperature as the circuit. Thus, a contradiction with the second law of thermodynamics would result. See also: Chemical thermodynamics Homogeneous conductor A circuit composed of a single, homogeneous conductor cannot produce a thermoelectric emf. This follows from Eq. (6) when S B (T) is set equal to S A (T). It is important to emphasize that in this context "homogeneous" means perfectly uniform throughout. A sample made of an isotropic material can be inhomogeneous either because of small variations in chemical composition or because of strain. Figure 2 shows the thermoelectric emf generated by a thermocouple in which one arm is a cold-rolled copper (Cu) sample, and the other arm is the same material after annealing at an elevated temperature to remove the effects of the strain introduced by the cold-rolling. Figure 3 shows how the addition of impurities can change the thermopower of a pure metal. An additional effect can occur in a noncubic material. As illustrated in Fig. 4, a thermocouple formed from two samples cut in different orientations from a noncubic single crystal can generate a thermoelectric emf even if each sample is highly homogeneous. See also: Crystal structure Fig. 2 Thermoelectric emf of a thermocouple formed from pure annealed and pure cold-worked copper. The cold junction reference temperature is 4.2 K (-452 F). F = (K 1.8) (After R. H. Kropschot and F. J. Blatt, Thermoelectric power of cold-rolled pure copper, Phys. Rev., 116: , 1959) Fig. 3 Thermopower S from 0 to 300 K for pure silver (Ag) and a series of dilute silver-gold (Ag-Au) alloys, F = (K 1.8) (After R. S. Crisp and J. Rungis, Thermoelectric power and thermal conductivity in the silver-gold alloy system from K, Phil. Mag., 22: , 1970) Fig. 4 Thermopower S of zinc parallel (A) and perpendicular (B) to the hexagonal axis. F = (K 1.8) (After V. A. Rowe and P. A. Schroeder, Thermopower of Mg, Cd and Zn between 1.2 and 300 K, J. Phys. Chem. Sol., 31:1-8, 1970) If material B is superconducting, so that S B = 0, Eq. (5) reduces to E AB(T 0,T 1) = E A(T 0,T 1), as assumed above. Source of emf Finally, Eq. (6) makes clear that the source of the thermoelectric emf in a thermocouple lies in the bodies of the two materials of which it is composed, rather than at the junctions. This serves to emphasize that thermoelectric emfs are not related to the contact potential or Volta effect, which is a potential difference across the junction between two different metals arising from the difference between their Fermi energies. The contact potential is present even in the absence of temperature gradients or electric currents. See also: Contact potential difference Peltier Effect In 1834, C. A. Peltier discovered that when an electric current passes through two different conductors connected in a loop, one of the two junctions between the conductors cools and the other warms. If the direction of the current is reversed, the effect also reverses: the first junction warms and the second cools. In 1853, Quintus Icilius showed that the rate of heat output or intake at each junction is directly proportional to the current i. The Peltier coefficient AB is defined as the (6) (7) (8)
4 heat generated per second per unit current flow through the junction between materials A and B. By convention, AB is taken to be positive when cooling occurs at the junction through which current flows from conductor A to conductor B. Quintus Icilius's result guarantees that the Peltier coefficient is independent of the magnitude of the current i. Additional experiments have shown that it is also independent of the shapes of the conductors. It therefore depends only upon the two materials and the temperature of the junction, and can be written as AB (T) or, alternatively, as A (T) - B(T), where A(T) and A(T) are the Peltier coefficients for materials A and B, respectively. The second form emphasizes that the Peltier coefficient is a bulk property which can be defined for a single conductor. Because of the difficulty of measuring heat input or output from a junction, as well as complications resulting from the simultaneous presence of Joule heating and the Thomson effect, AB(T) has rarely been quantitatively measured. Rather its value is usually determined from the Kelvin relations, using experimental values for S AB. The Peltier effect does, however, form the basis for thermoelectric heating and cooling. Thomson Effect and Kelvin Relations When an electric current passes through a conductor that is maintained at a constant temperature, heat is generated at a rate proportional to the square of the current. This phenomenon is called Joule heat, and its magnitude for any given material is determined by the electrical resistivity of the material. In 1854, William Thomson (Lord Kelvin), in an attempt to explain discrepancies between experimental results and a relationship between AB and S AB that he had derived from thermodynamic analysis of a thermocouple, postulated the existence of an additional reversible generation of heat when a temperature gradient is applied to a current-carrying conductor. This heat, called Thomson heat, is proportional to the product of the current and the temperature gradient. It is reversible in the sense that the conductor changes from a generator of Thomson heat to an absorber of Thomson heat when the direction of either the current or the temperature gradient (but not both at once) is reversed. By contrast, Joule heating is irreversible in that heat is generated for both directions of current flow. The magnitude of Thomson heat generated (or absorbed) is determined by the Thomson coefficient. Using reasoning based upon equilibrium thermodynamics, Thomson derived results equivalent to Eqs. (9) and (10), called the Kelvin (or Kelvin-Onsager) relations. Here A is the Thomson coefficient, defined as the heat generated per second per unit current flow per unit temperature gradient when current flows through conductor A in the presence of a temperature gradient. Equation (10) can be integrated to give Eq. (11), in which the third law of thermodynamics has been evoked to set S A(0) = 0. Using Eq. (11), S A(T) can be determined from measurements on a single conductor. In practice, however, accurate measurements of A are very difficult to make; therefore, they have been carried out for only a few metals notably lead (Pb), platinum (Pt), and tungsten (W) which then serve as standards for determining S B(T) by using measurements of S AB(T) in conjunction with Eq. (7). Long after the Thomson heat was observed and the Kelvin relations were verified experimentally, debate raged over the validity of the derivation employed by Thomson. However, the theory of irreversible processes, developed by L. Onsager in 1931, and by others, yields the same equations and thus provides them with a firm foundation. Thermopowers of Metals and Semiconductors Since the Kelvin relations provide recipes for calculating any two of the thermoelectric coefficients, S,, and, from the third, only one of the three coefficients need be measured to determine the thermoelectric properties of any given material. Although there are circumstances under which one of the other two coefficients may be preferred, because of ease and accuracy of measurement, it is usually the thermopower S that is measured. Reference materials Because S must be measured using a thermocouple, the quantity measured experimentally is S A - S B, the difference between the thermopowers of the two conductors constituting the thermocouple. Only when one of the arms of the thermocouple is superconducting and therefore has zero thermopower can the absolute thermopower of the other be directly measured. At temperatures up to about 18 K (-427 F) superconducting niobium-tin (Nb 3Sn) wire can be used for conductor B, (9) (10) (11)
5 thereby permitting direct determination of S A. Direct measurements have been extended to just above 90 K (-298 F) using the YBa 2 Cu 3 O 7 high-temperature superconductors, and should be extendable to above 120 K (-244 F) using still higher-temperature superconductors. For even higher temperatures, a standard thermoelectric material is needed. For historical reasons, the reference material up to about room temperature has been chosen to be Pb. Until the mid-1970s, the thermopower of Pb was calculated from Thomson coefficient measurements made in the early 1930s, and all published values of S were ultimately traceable to those measurements. In 1977, new measurements of the Thomson coefficient of Pb were made up to 350 K (170 F). The revised values of S Pb are listed in Table 1. Above 50 K (-370 F) they differ from the old values by microvolt/k; older values of S must be corrected for these differences if accuracy is important. Measurements in 1985 of the Thomson coefficients of Pt and W allow these two metals to be used as references up to 1600 K (2900 F) or 1800 K (3250 F), respectively. Table 1. Absolute thermoelectric power of S of pure lead berween 0 and 350 K* T, K S Pb, V/K
6 *After R. B. Roberts, the absolute scale of thermoelectricity, Phil. Mag., 36:91-107, C = K - 273, 15; F = (K 1.8) Temperature variation Figure 5 shows the variation with temperature of the thermopowers of four different pure metals. The data for gold (Au), aluminum (Al), and Pt are typical of those for most simple metals and for some transition metals as well. The thermopower S consists of a slowly varying portion that increases approximately linearly with absolute temperature, upon which is superimposed a "hump" at lower temperatures. In analyzing these results, S is written as the sum of two terms, as in Eq. (12), (12) where S d, called the electron-diffusion component, is the slowly varying portion, and S g, called the phonon-drag component, is the hump. For some transition metals, on the other hand, the behavior of S is more complex as illustrated by the data for rhodium (Rh) in Fig. 5. Figure 6 shows comparative data for a sample p-type semiconductor. The separation of S into S d and S g is still valid, but at high temperatures S d now varies more weakly than linearly with temperature. Note also the different ordinate scales in Fig. 5 ( V/K) and Fig. 6 (mv/k) the thermopower of a semiconductor can be a thousand times larger than that of a metal. Fig. 5 Thermopower S of the metals gold (Au), aluminum (Al), platinum (Pt), and rhodium (Rh) as a function of temperature. The differences between the solid curves for Pt, Al, and Au and the broken lines indicate the magnitude of the phonon-drag component S. F = (K 1.8)
7 Fig. 6 Thermopower S of p-type germanium ( acceptors per cubic centimeter) and calculated value for the electron-diffusion thermopower S d. (After C. Harring, The role of low-frequency phonons in thermoelectricity and thermal conductivity, Proc. Int. Coll. 1956, Garmisch-Partenkirchen, Vieweg. Braunschweig, 1958) Theory When a small temperature difference T is established across a conductor, heat is carried from its hot end to its cold end by the flow of both electrons and phonons (quantized lattice vibrations). If the electron current is constrained to be zero for example by the insertion of a high-resistance measuring device in series with the conductor the electrons will redistribute themselves in space so as to produce an emf along the conductor. This is the thermoelectric emf. If the phonon flow could somehow be turned off, this emf would be just S d T. However, the phonon flow cannot be turned off, and as the phonons move down the sample, they interact with the electrons and "drag" them along. This process produces an additional contribution to the emf, S g T. See also: Conduction (heat); Lattice vibrations; Phonon; Thermal conduction in solids Source of S d The conduction electrons in a metal are those having energies near the Fermi energy. Only these electrons are important for thermoelectricity. As illustrated in Fig. 7, in a metal, the energy distribution of these electrons varies with the temperature. At high temperatures, the metal has more high-energy electrons and fewer low-energy ones than when it is at low temperatures. This means that if a temperature gradient is established along a metal sample, the total number of electrons will remain constant, but the hot end will have more high-energy electrons than the cold end, and the cold end will have more low-energy electrons than the hot end. The high-energy electrons will diffuse toward the cold end, and the low-energy electrons will diffuse toward the hot end. However, in general, the diffusion rate is a function of electron energy, and thus a net electron current will result. This current will cause electrons to pile up at one end of the metal (usually the cold end) and thereby produce an emf that opposes the further flow of electrons. When the emf becomes large enough, the current will be reduced to zero. This equilibrium emf is the thermoelectric emf arising from electron diffusion. Essentially the same argument applies to semiconductors, except that in that case the electrons (or holes) are those just above (or just below) the band gap. See also: Free-electron theory of metals; Hole states in solids; Semiconductor Fig. 7 Variation with energy of the number of conduction electrons n( ) in a metal in the vicinity of the Fermi energy for two different temperatures. A small variation of with temperature has been neglected. S d for a metal For a completely free-electron metal, S d should be given by Eq. (13), where k is Boltzmann's constant, e is the charge on an electron, T is the absolute (Kelvin) temperature, and is the Fermi energy of the metal. According to Eq. (13), S d should be negative since e is a negative quantity and should increase linearly with T. In Table 2 the predictions of Eq. (13) are compared with experiment for a number of the most free-electron-like metals. Equation (13) correctly predicts the general size of S d, but often misses the actual value by a factor of 2 or more and in several cases predicts the wrong sign. To understand the thermopowers of real metals, it is necessary to use a more sophisticated model that takes into account interactions between the electrons in the metal and the crystal lattice, as well as scattering of the electrons by impurities and phonons. The proper generalization of Eq. (13) is Eq. (14), where ( ) is a generalized energy-dependent conductivity defined so that ( ) is the experimental electrical conductivity of the metal, and the logarithmic derivative with respect to the energy is to be evaluated at =. For free electrons, Eq. (14) reduces to Eq. (13). But more generally, Eq. (14) is able to account for all of the deviations of experiment from Eq. (13). If the logarithmic derivative is negative, S d will be positive; S d will differ in size from Eq. (13) if the logarithmic derivative does not have the free-electron value (3/2) -1 ; and S d(t) will deviate from a linear dependence on T if the logarithmic derivative is temperature-dependent. Table 2. Comparision between theoretical values of S and experimental data Thermopower (S), V/K (13) (14)
8 Metal Theoretical values at 0 C according to Eq. (13) Experimental data at approximately 0 C Lithium (Li) Sodium (Na) -3-6 Potassium (K) Copper (Cu) Gold (Au) Aluminum (Al) In metals, research on S d has attacked such diverse topics as understanding changes due to alloying with both magnetic and nonmagnetic impurities, strain, application of pressure or magnetic fields, and changes near phase transitions. In some cases the changes can be dramatic. Figure 8 shows that the addition of very small amounts of the magnetic impurity iron (Fe) can produce enormous changes in S d for copper (Cu) at low temperatures. Sample 1 (in which the deviation of the thermopower from zero is too small to be seen with the chosen scale) is most representative of pure Cu because the Fe is present as an oxide and is thus not in "magnetic form." Figure 9 shows that at low temperatures application of a magnetic field H to Al can cause S d to change sign. [To obtain a temperature-independent quantity, S d has been divided by the absolute temperature T. To remove the effects of varying impurity concentrations, H has been divided by (4.2 K)nec, where (4.2 K) is the sample resistivity at 4.2 K, n is the number of electrons per unit volume in the metal, and c is the speed of light.] Figure 10 illustrates the significant changes that can occur in S when a metal melts. Fig. 8 Low-temperature thermopowers of various samples of copper containing vary small concentrations of iron (Fe). Specific compositions of samples 1-8 are unknown. F = (K 1.8) (After A. V. Gold et al., The Thermoelectric power of pure copper, Phil. Mag., 5: , 1960) Fig. 9 Variation with magnetic field H of the low-temperature electron-diffusion thermopower S d of aluminum (Al) and various dilute aluminum-based alloys. Sample labeled Al-Cu is a second sample of Al- Cu. (After R. S. Averback, C. H. Stephan, and J. Bass, Magnetic field dependence of the thermopower of dilute aluminum alloys, J. Low Temp. Phys., 12: , 1973) Fig. 10 Changes in the thermopowers of gold (Au) and silver (Ag) upon melting. F = ( C 1.8) (After R. A. Howe and J. E. Enderby, The thermoelectric power of liquid Ag-Au, Phil. Mag., 16: , 1967) Substantial effort has been devoted to the study of thermoelectricity in liquid metals and liquid metal alloys. There has also been considerably interest in the thermopower of quasi-onedimensional conductors (Fig. 11), in amorphous metals (also called metallic glasses or metglasses), in many-body effects in thermoelectricity, and in thermoelectric effects in inhomogeneous superconductors, such as loops consisting of two different superconducting materials. Thermoelectric effects in superconductors are much smaller than those in normal metals and are generally visible only very near T c. Their study gives insight into nonequilibrium processes in superconductors. See also: Metallic glasses Fig. 11 Thermopower of highly conducting salts of the form (Donor) + (TCNQ) - 2. By 300 K (80 F) all of the thermopowers are very close to the "entropy per carrier" of (k/e) In 2 = -60 V/K, where k is Boltzmann's constant and e is the electron charge. F = ( K 1.8) (After F. J. Blatt and P. A. Schroeder, eds., Thermoelectricity in Metallic Conductors, Plenum Press, 1978) S d for a semiconductor Equation (13) is appropriate for a free-electron gas that obeys Fermi-Dirac statistics. The conduction electrons in a metal obey these statistics. However, there are so few conduction electrons in a semiconductor that, to a good approximation, they can be treated as though they obey different statistics Maxwell-Boltzmann statistics. For free electrons obeying these statistics, S d is given by Eq. (15), (15)
9 which predicts that S d should be temperature-independent and have the value S d = -130 times; 10-6 V/K. For a p-type extrinsic semiconductor, in which the carriers are approximated as free holes, the sign of S d will be reversed to positive. Examination of the data of Fig. 6 shows that S d is nearly independent of temperature but is considerably larger than predicted by Eq. (15). Again, a complete understanding of the thermopowers of semiconductors requires the generalization of Eq. (15). The appropriate generalizations are different for single-band and multiband semiconductors, the latter being considerably more complicated. For a single-band (extrinsic) semiconductor, the generalization is relatively straightforward and yields predictions for S d which, in agreement with experiment, vary slowly with temperature and are several times larger than the prediction of Eq. (15). [The white curve for S d in Fig. 6 is calculated from this generalization.] Experimental interest in the thermopower of semiconductors concerns topics similar to those for metals. In addition, the large magnitudes of the thermopowers of semiconductors continue to spur efforts to develop materials better suited for electric power generation and thermoelectric cooling. See also: Band theory of solids; Boltzmann statistics; Fermi-Dirac statistics Source of S g Unlike the behavior of S d, which is determined in both metals and semiconductors primarily by the properties of the charge carriers, the behavior of S g is determined in both cases primarily by the properties of the phonons. At low temperatures, phonons scatter mainly from electrons or impurities rather than from other phonons. The initial increase in S g with increasing temperature at the very lowest temperatures in Figs. 5 and 6 results from an increasing number of phonons becoming available to drag the electrons along. However, as the temperature increases, the phonons begin to scatter more frequently from each other. Eventually, phonon-phonon scattering becomes dominant, the electrons are no longer dragged along, and S g falls off in magnitude with increasing temperature. Interest in phonon drag is associated with such questions as whether it is the sole source of the humps in Figs. 5 and 6, how it changes as impurities are added, and how it is affected by a magnetic field. Applications The most important practical application of thermoelectric phenomena to date is in the accurate measurement of temperature. The phenomenon involved is the Seebeck effect. Of less importance so far are the direct generation of electrical power by application of heat (also involving the Seebeck effect) and thermoelectric cooling and heating (involving the Peltier effect). A basic system suitable for all four applications is illustrated schematically in Fig. 12. Several thermocouples are connected together in series to form a thermopile, a device with increased output (for power generation or cooling and heating) or sensitivity (for temperature measurement) relative to a single thermocouple. The junctions forming one end of the thermopile are all at the same low temperature T L, and the junctions forming the other end are at the high temperature T H. The thermopile is connected to a device D that differs for each application. For temperature measurement, the temperature T L is fixed, for example, by means of a bath; the temperature T H becomes the running temperature T that is to be measured; and the device is a potentiometer for measuring the thermoelectric emf generated by the thermopile. For power generation, the temperature T L is fixed by connection to a heat sink; the temperature T H is fixed at a value determined by the output of the heat source and the thermal conductance of the thermopile; and the device is whatever is to be run by the electricity that is generated. For heating or cooling, the device is a current generator that passes current through the thermopile. If the current flows in the proper direction, the junctions at T H will heat up and those at T L will cool down. If T H is fixed by connection to a heat sink, thermoelectric cooling will be provided at T L. Alternatively, if T L is fixed, thermoelectric heating will be provided at T H. If the heat sink is room-temperature, such a system has the advantage that at any given location it can be converted from a cooler to a heater merely by reversing the direction of the current. Fig. 12 Thermopile, a battery of thermocouples connected in series; D is a device appropriate to the particular application.
10 Temperature measurement In principle, any material property that varies with temperature can serve as the basis for a thermometer. In practice, the two properties most often used for precision thermometry are electrical resistance and thermoelectric emf. Thermocouples are widely employed to measure temperature in both scientific research and industrial processes. In the United States alone, several hundred tons of thermocouple materials are produced annually. See also: Temperature measurement; Thermocouple Construction of instruments In spite of their smaller thermopowers, metals are usually preferred to semiconductors for precision temperature measurements because they are cheaper, are easier to fabricate into convenient forms such as thin wires, and have more reproducible thermoelectric properties. With modern potentometric systems, standard metallic thermocouples provide temperature sensitivity adequate for most needs small fractions of a degree Celsius are routinely obtained. If greater sensitivity is required, several thermocouples can be connected in series to form a thermopile (Fig. 12). A 10- element thermopile provides a temperature sensitivity 10 times as great as that of each of its constituent thermocouples. However, the effects of any inhomogeneities are also enhanced 10 times. The thermocouple system standardly used to measure temperature is shown in Fig. 13. It consists of wires of three metals, A, B, and C, where C is usually the metal Cu. The junction between the wires of metals A and B is located at the temperature to be measured T. Each of these two wires is joined to a wire of metal C at the reference temperature T 0. The other ends of the two wires of metal C are connected to the potentiometer at room temperature T r. Integrating the appropriate thermopowers around the circuit of Fig. 13 yields the total thermoelectric emf E in terms of the separate emf's generated by each of the four pieces of wire, as given in Eq. (16). Fig. 13 Thermocouple system standardly used to measure temperature; M is a measuring device, usually a potentiometer, which is at room temperature. (16)
11 If the two wires C1 and C2 have identical thermoelectric characteristics, the last two terms in Eq. (16) cancel, and, with the use of Eq. (5), Eq. (17) results. That is, two matched pieces of metal C produce no contribution to the thermelectric emf of the circuit shown in Fig. 13, provided their ends are maintained at exactly the same two temperatures. This means that it is not necessary to use either of the sometimes expensive metals making up the thermocouple to go from the reference-temperature bath to the potentiometer. That part of the circuit can be constructed of any uniform, homogeneous metal. Copper is often used because it is inexpensive, is available in adequate purity to ensure uniform, homogeneous samples when handled with care, can be obtained in a wide variety of wire diameters, and can be either spotwelded or soldered to the ends of the thermocouple wires, Special low-thermal emf alloys are available for making solder connections to Cu in thermocouple circuits. Choice of materials Characteristics that make a thermocouple suitable as a general-purpose thermometer include adequate sensitivity over a wide temperature range, stability against physical and chemical change under various conditions of use and over extended periods of time, availability in a selection of wire diameters, and moderate cost. No single pair of thermocouple materials satisfies all needs. Platinum versus platinum-10% rhodium can be used up to 1700 C (3100 F). A thermocouple combining the two alloys chromel and alumel gives greater sensitivity and an emf that is closely linear with temperature, but cannot be used to as high a temperature. A combination of Cu versus the alloy constantan also has high sensitivity above room temperature and adequate sensitivity down to as low as 15 K (-433 F). For temperatures of 4 K (-452 F) or lower, special gold-cobalt alloys versus Cu or gold-iron alloys versus chromel are used. Thermocouple tables To use a thermocouple composed of metals A and B as a thermometer, it is necessary to know how E AB (T 0,T) varies with temperature T for some reference temperature T 0. According to Eq. (6), E AB(T 0,T 1) can be determined for any two temperatures T 0 and T 1 if both S A(T) and S B(T) are known for all temperatures between T 0 and T 1. Knowledge of S A(T) and S B(T) allows construction of a table of values for E AB (T 0,T) using any arbitrary reference temperature T 0. Such tables are available for the thermocouples mentioned above, and for some others as well, usually with a reference temperature of 0 C (32 F). A table of E AB(T 0,T) for one reference temperature T 0 can be converted into a table for any other reference temperature T 2 merely by subtracting a constant value E AB (T 0,T 2 ) from each entry in the table to give Eq. (18). (17)
12 Here E AB (T 0,T 2 ) is a positive quantity when T 2 is greater than T 0 and when S AB (T) is positive between T 0 and T 2. Other uses Thermoelectric power generators, heaters, or coolers made from even the best presently available materials have the disadvantages of relatively low efficiencies and concomitant high cost per unit of output. Their use has therefore been largely restricted to situations in which these disadvantages are outweighed by such advantages as small size, low maintenance due to lack of moving parts, quiet and vibration-free performance, light weight, and long life. Figure of merit A measure of the utility of a given thermoelectric material for power generation, cooling, or heating at an absolute temperature T is provided by the dimensionless figure of merit ZT given by Eq. (19). (18) Here S is the thermopower of the material, is its electrical conductivity, and is its thermal conductivity. The largest values for ZT are obtained in semimetals and highly doped semiconductors, which are the materials normally used in practical thermoelectric devices. As illustrated in Fig. 14, for most materials ZT varies with temperature, going through maxima at different temperatures. Thus, combining available materials into thermocouples often results in values of ZT too small to be competitive over a wide enough temperature range to be useful. The best available values of ZT 1 yield conversion efficiencies of only a few percent. Values of ZT 2 over a wide enough temperature range could make thermoelectrics competitive for some uses described below, and values of ZT 4 over wide temperature ranges in materials with high-temperature stability and affordable cost might revolutionalize heating, cooling, and power generation. See also: Thermoelectric power generator Fig. 14 Representative values of the dimensionless figure of merit ZT as a function of temperature for p- type -Zn 4Sb 3 (diamonds). These values are to be compared with those for state-of-the-art p-type thermoelectric materials: PbTe- and Bi 2Te 3-based alloys and TAGS (Te-Ag-Ge-Sb) alloys. F = ( C 1.8) (From T. Caillat, J.-P. Fleurial and A. Borschevsky, A low thermal conductivity compound for thermoelectric applications: -Zn 4Sb 3, 15th IEEE International Conference on Thermoelectrics p. 151, 1996) For a long time, little progress was made in increasing ZT beyond the values for the established BiTe- and PbTe-based thermoelectric materials shown in Fig. 14, although no rigorous theoretical limit on the value of ZT is known. However, research on thermoelectric materials has accelerated due to the discovery of values of ZT 1 in -Zn 4 Sb 3 (Fig. 14) and ternary filled skutterudites (Fig. 15) of the form LnT 4Pn 12 (Ln = rare earth or Th; T = Fe, Ru, Os, Co, Rh, Ir; Pn = P, As, Sb), as well as new ideas that might lead to larger ZT. Fig. 15 Dimensionless figure of merit ZT as a function of temperature for cerium (Ce)-filled skutterudite samples with different compositions. F = ( C 1.8) (From J.-P. Fleurial et al., High figure of merit in Ce-filled skutterudites, 15th IEEE International Conference on Thermoelectrics, p. 91, 1996) The quantity of importance in a thermoelectric device is the figure of merit of the thermocouple rather than the separate figures of merit of its constituents. Although at least one constituent should have a high figure of merit, two constituents with high figures of merit do not guarantee that the figure of merit of the thermocouple will be high. For example, if the thermopowers of the two constituents are identical, the figure of merit of the couple will be zero. Just as the figures of merit for single materials vary with temperature, so do the figures of merit for thermocouples formed from two such materials. This means that one thermocouple can be better than another in one temperature range but less efficient in another. To take maximum advantage of the different properties of different couples, thermocouples are often cascaded (Fig. 16). Cascading produces power generation in stages, the higher temperature of each stage being determined by the heat rejected from the stage above. Thus, the highest and lowest temperatures T 4 and T 1 are fixed by connection to external reservoirs, whereas the middle temperatures T 3 and T 2 are determined by the properties of the materials. By cascading, a series of thermocouples can be used simultaneously in the temperature ranges where their figures of merit are highest. Cascaded thermocouple systems have achieved conversion efficiencies as high as 10-15%. Fig. 16 Three-level cascade consisting of three different thermocouples (A versus B, C versus D, and E versus F) at four temperatures (T). (19)
13 Thermoelectric generators A thermoelectric generator requires a heat source and a thermocouple. Kerosine lamps and firewood have been used as heat sources to produce a few watts of electricity in remote locations, and systems using motor fuel burners have produced 100 W. Test systems using sunlight have also been constructed. Radioactive sources, especially strontium-90 ( 90 Sr) and plutonium-238 ( 238 Pu), have provided the heat to activate small rugged thermoelectric batteries for use in lighthouses, in navigation buoys, at isolated weather stations or oil platforms, and in spaceships. Such small nuclear batteries operate pacemakers implanted in humans and data transmission systems for deep-space planetary probes. Thermopiles based on silicon-germanium (SiGe) alloys and powered by plutonium-238 supplied more than 100 W of power at an efficiency of over 6% to the Voyager 1 and Voyager 2 spacecraft for the 12 years ( ) of their missions to the outer planets, and are expected to remain electrically active until at least the year Nuclear-powered batteries for medical use must be designed to remain intact following the maximum credible accident. Capabilities such as retention of integrity after crushing by 1 ton, or impact at 50 m/s (112 mi/h), or salt-water corrosion for centuries, or cremation at temperatures up to 1300 C (2400 F) for half an hour are required. Investigations have been made of the feasibility of thermoelectric generation using the copious heat generated by nuclear reactors, or the heat generated in the exhaust system of automobiles. Such uses would require the development of more efficient thermoelectric materials able to operate for a long time at the high temperatures that are encountered. See also: Nuclear battery; Nuclear power; Radioactivity; Space power systems Peltier cooling With available values of ZT, thermoelectric refrigerators suitable for use in homes or automobiles are more expensive and less efficient than standard vapor-compression-cycle refrigerators. Their use is thus largely restricted to situations in which lower maintenance, increased life, absence of vibration, or quiet performance are essential, or to situations (as in space vehicles or artificial satellites) in which the compressor type of refrigerator is impractical. A number are in use in hotels and other large facilities. A typical unit of about 50-liter (13-gallon) capacity requires a dc power input of 40 W, has a refrigerative capacity of 20 kcal/h (23 W), and a time to cool of 4-5 h. Larger ZT would also make thermoelectricity competitive for cooling of high-power electronic devices. See also: Refrigeration For lower temperatures, the proper choice of thermoelectric materials and the use of cascading can result in a reduction in temperature at the coldest junctions of as much as 150 C (270 F). Temperature drops of 100 C (180 F) have been obtained in single crystals of the semimetal bismuth through use of the thermomagnetic Ettingshausen effect. A large enough ZT down to temperatures of -200 C (-328 F) could allow widespread use of new high-temperature superconductors in electronic devices. See also: Thermomagnetic effects Small cooling units with capacities of 10 W or less have been developed for miscellaneous applications such as cold traps for vacuum systems, cooling controls for thermocouple reference junctions, cooling devices for scientific equipment such as infrared detectors, cold stages on microscopes or on microtomes used for sectioning cooled tissues, and electronic equipment coolers. However, major commercial success of thermoelectric refrigeration requires thermocouple materials with higher values of ZT. Thermoelectric heating A thermoelectric heater referenced to room temperature is nothing more than a thermoelectric refrigerator with the current reversed. No large heaters have been marketed. However, various small household convenience devices have been developed, such as a baby-bottle cooler-warmer that cools the bottle until just before feeding time and then automatically switches to a heating cycle to warm it, and a thermoelectric hostess cart. See also: Electricity Jack Bass How to cite this article Suggested citation for this article: Jack Bass, "Thermoelectricity", in AccessScience@McGraw-Hill, DOI / , last modified: August 8, For Further Study Topic Page: Physics: Electricity and magnetism Topic Page: Physics: Solid state physics Bibliography F. J. Blatt et al., Thermoelectric Power of Metals, Plenum Press, New York, 1976 F. J. Blatt and P. A. Schroeder (eds.), Thermoelectricity in Metallic Conductors, Plenum Press, New York, 1978 F. J. DiSalvo, Thermoelectric Cooling and Power Generation, Science, 285: , 1999 D. M. Rowe (ed.), CRC Handbook of Thermoelectrics, CRC Press, Boca Raton, FL, 1995
14 Proceedings of the 15th IEEE International Conference on Thermoelectrics, IEEE Catalog no. 96TH8169, 1996 T. M. Tritt et al. (eds.), Thermoelectric Materials 1998 The Next Generation Materials for Small-Scale Refrigeration and Power Generation Applications, MRS Soc. Proc., vol. 545, 1998 Customer Privacy Notice Copyright The McGraw-Hill Companies. All rights reserved. Any use is subject to the Terms of Use and Notice. Additional credits and copyright information. For further information about this site contact Last modified: Sep 30, 2003.
Thermoelectric effect
 Thermoelectric effect See Mizutani the temperature gradient can also induce an electrical current. linearized Boltzmann transport equation in combination with the relaxation time approximation. Relaxation
Thermoelectric effect See Mizutani the temperature gradient can also induce an electrical current. linearized Boltzmann transport equation in combination with the relaxation time approximation. Relaxation
Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect
 Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect Objectives In this lecture you will learn the following Seebeck effect and thermo-emf. Thermoelectric series of metals which can be used to form
Module 4 : THERMOELECTRICITY Lecture 21 : Seebeck Effect Objectives In this lecture you will learn the following Seebeck effect and thermo-emf. Thermoelectric series of metals which can be used to form
Sensing, Computing, Actuating
 Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Sensing, Computing, ctuating Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC EFFECT (Chapter 5.11) 3 Thermocouple cylinder head temperature (thermocouple)
Energy Conversion in the Peltier Device
 Laboratory exercise 4 Energy Conversion in the Peltier Device Preface The purpose of this exercise is to become familiar with the Peltier effect. Students will observe Peltier device working as a heat
Laboratory exercise 4 Energy Conversion in the Peltier Device Preface The purpose of this exercise is to become familiar with the Peltier effect. Students will observe Peltier device working as a heat
Thermoelectric effect
 Hiroyuki KOIZUMI 1. Principle Thermoelectric effect Seebeck effect Temperature difference ΔT Voltage difference ΔV Peltier effect I Q Thomson effect I Current Q Heat transfer Thermoelectric effect Seebeck
Hiroyuki KOIZUMI 1. Principle Thermoelectric effect Seebeck effect Temperature difference ΔT Voltage difference ΔV Peltier effect I Q Thomson effect I Current Q Heat transfer Thermoelectric effect Seebeck
Sensors and Actuators Sensors Physics
 Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
Sensors and ctuators Sensors Physics Sander Stuijk (s.stuijk@tue.nl) Department of Electrical Engineering Electronic Systems 2 THERMOELECTRIC SENSORS (Chapter 3.9, 16.4) 3 Thermoelectric effect thermoelectric
Using a Mercury itc with thermocouples
 Technical Note Mercury Support Using a Mercury itc with thermocouples Abstract and content description This technical note describes how to make accurate and reliable temperature measurements using an
Technical Note Mercury Support Using a Mercury itc with thermocouples Abstract and content description This technical note describes how to make accurate and reliable temperature measurements using an
Section 7. Temperature Measurement
 Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Section 7 Temperature Measurement 7/25/2017 Engineering Measurements 7 1 Working Definition Temperature is a measure of the average kinetic energy of the molecules that make of a substance. After time,
Temperature Measurement
 MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
MECE 3320 Measurements & Instrumentation Temperature Measurement Dr. Isaac Choutapalli Department of Mechanical Engineering University of Texas Pan American Introduction Temperature is one of the most
Chapter 1 Overview of Semiconductor Materials and Physics
 Chapter 1 Overview of Semiconductor Materials and Physics Professor Paul K. Chu Conductivity / Resistivity of Insulators, Semiconductors, and Conductors Semiconductor Elements Period II III IV V VI 2 B
Chapter 1 Overview of Semiconductor Materials and Physics Professor Paul K. Chu Conductivity / Resistivity of Insulators, Semiconductors, and Conductors Semiconductor Elements Period II III IV V VI 2 B
SENSORS and TRANSDUCERS
 SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
SENSORS and TRANSDUCERS Tadeusz Stepinski, Signaler och system The Thermal Energy Domain Physics» Seebeck effect» Peltier effect» Thomson effect Thermal effects in semiconductors Thermoelectric sensors
Lecture 9: Metal-semiconductor junctions
 Lecture 9: Metal-semiconductor junctions Contents 1 Introduction 1 2 Metal-metal junction 1 2.1 Thermocouples.......................... 2 3 Schottky junctions 4 3.1 Forward bias............................
Lecture 9: Metal-semiconductor junctions Contents 1 Introduction 1 2 Metal-metal junction 1 2.1 Thermocouples.......................... 2 3 Schottky junctions 4 3.1 Forward bias............................
Introduction to Thermoelectric Materials and Devices
 Introduction to Thermoelectric Materials and Devices 4th Semester of 2012 2012.03.29, Thursday Department of Energy Science Sungkyunkwan University Radioisotope Thermoelectric Generator (PbTe) Space probe
Introduction to Thermoelectric Materials and Devices 4th Semester of 2012 2012.03.29, Thursday Department of Energy Science Sungkyunkwan University Radioisotope Thermoelectric Generator (PbTe) Space probe
Thermoelectric materials. Hyo-Jeong Moon
 Thermoelectric materials Hyo-Jeong Moon Electrical conductivity Thermoelectric materials Ratio of current density to electric field, when no temperature gradient is present. Thermal conductivity Ratio
Thermoelectric materials Hyo-Jeong Moon Electrical conductivity Thermoelectric materials Ratio of current density to electric field, when no temperature gradient is present. Thermal conductivity Ratio
Experimental Setup for the Measurement of Low Temperature Seebeck Coefficient of Single Crystal and Bulk Materials
 Journal of Control & Instrumentation IN: 9-697 (online), IN: 347-737 (print) Volume 5, Issue www.stmjournals.com Experimental etup for the Measurement of Low Temperature eebeck Coefficient of ingle Crystal
Journal of Control & Instrumentation IN: 9-697 (online), IN: 347-737 (print) Volume 5, Issue www.stmjournals.com Experimental etup for the Measurement of Low Temperature eebeck Coefficient of ingle Crystal
EXPERIMENT VARIATION OF THERMO-EMF WITH TEMPERATURE. Structure. 7.1 Introduction Objectives
 EXPERIMENT 7 VARIATION OF THERMO-EMF WITH TEMPERATURE Thermo-EMF Structure 7.1 Introduction Objectives 7.2 Working Principle of a Potentiometer 7.3 Measurement of Thermo-EMF and its Variation with Temperature
EXPERIMENT 7 VARIATION OF THERMO-EMF WITH TEMPERATURE Thermo-EMF Structure 7.1 Introduction Objectives 7.2 Working Principle of a Potentiometer 7.3 Measurement of Thermo-EMF and its Variation with Temperature
I m. R s. Digital. R x. OhmmetersxSeries Shunt Digital. R m
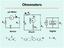 µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
µa Meter I I s I m s E Digital x I Voltmeter x x E µa Meter m Is OhmmetersxSeries Shunt Digital EIx= = ()E sm x mxvi= x Shunt Ohmmeter Shunt s x E µa Meter I m I m V m E ) ( v I E ) ( E v E v E I When
Experiment The Hall Effect Physics 2150 Experiment No. 12 University of Colorado
 Experiment 12 1 Introduction The Hall Effect Physics 2150 Experiment No. 12 University of Colorado The Hall Effect can be used to illustrate the effect of a magnetic field on a moving charge to investigate
Experiment 12 1 Introduction The Hall Effect Physics 2150 Experiment No. 12 University of Colorado The Hall Effect can be used to illustrate the effect of a magnetic field on a moving charge to investigate
From Last Time Important new Quantum Mechanical Concepts. Atoms and Molecules. Today. Symmetry. Simple molecules.
 Today From Last Time Important new Quantum Mechanical Concepts Indistinguishability: Symmetries of the wavefunction: Symmetric and Antisymmetric Pauli exclusion principle: only one fermion per state Spin
Today From Last Time Important new Quantum Mechanical Concepts Indistinguishability: Symmetries of the wavefunction: Symmetric and Antisymmetric Pauli exclusion principle: only one fermion per state Spin
HARVESTING HEAT TO CREATE ELECTRICITY: A NEW WORLD RECORD
 HARVESTING HEAT TO CREATE ELECTRICITY: A NEW WORLD RECORD Approximately 90% of world s electricity is generated in turbines moved by hot steam, which, unfortunately, operate only at 30 to 40 percent efficiency.
HARVESTING HEAT TO CREATE ELECTRICITY: A NEW WORLD RECORD Approximately 90% of world s electricity is generated in turbines moved by hot steam, which, unfortunately, operate only at 30 to 40 percent efficiency.
Chemistry: The Study of Change Chang & Goldsby 12 th edition
 Chemistry: The Study of Change Chang & Goldsby 12 th edition modified by Dr. Hahn Chapter 1 Chemistry: A Science for the 21 st Century (2) Materials and Technology Polymers, liquid crystals photovoltaic
Chemistry: The Study of Change Chang & Goldsby 12 th edition modified by Dr. Hahn Chapter 1 Chemistry: A Science for the 21 st Century (2) Materials and Technology Polymers, liquid crystals photovoltaic
Temperature Scales. Temperature, and Temperature Dependent on Physical Properties. Temperature. Temperature Scale
 Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Temperature Scales The Celsius, Fahrenheit, and Kelvin Temperature Scales: Temperature, and Temperature Dependent on Physical Properties Physics Enhancement Programme Dr. M.H. CHAN, HKBU 9 T F T 5 T T
Superconductor. Superconductor Materials Materials Eng. Dep. Kufa Univ. Dr. Sabah M. Thahab
 Superconductor Materials What's a superconductor? Superconductors have two outstanding features: 1). Zero electrical resistivity. This means that an electrical current in a superconducting ring continues
Superconductor Materials What's a superconductor? Superconductors have two outstanding features: 1). Zero electrical resistivity. This means that an electrical current in a superconducting ring continues
Clean Energy: Thermoelectrics and Photovoltaics. Akram Boukai Ph.D.
 Clean Energy: Thermoelectrics and Photovoltaics Akram Boukai Ph.D. Solar Energy Use Hydrocarbons vs. Photons Arabian Oil: 600 years Sun: 1.5 billion years The Sun can Power both Solar Cells and Thermoelectrics
Clean Energy: Thermoelectrics and Photovoltaics Akram Boukai Ph.D. Solar Energy Use Hydrocarbons vs. Photons Arabian Oil: 600 years Sun: 1.5 billion years The Sun can Power both Solar Cells and Thermoelectrics
Peltier Application Note
 Peltier Application Note Early 19th century scientists, Thomas Seebeck and Jean Peltier, first discovered the phenomena that are the basis for today s thermoelectric industry. Seebeck found that if you
Peltier Application Note Early 19th century scientists, Thomas Seebeck and Jean Peltier, first discovered the phenomena that are the basis for today s thermoelectric industry. Seebeck found that if you
15. Compare the result with the value you have taken above Compare the calculated pressure value with the actual pressure value that you have
 105) to convert it to. 15. Compare the result with the value you have taken above. 17. 16. Compare the calculated pressure value with the actual pressure value that you have taken from the, is it the same?
105) to convert it to. 15. Compare the result with the value you have taken above. 17. 16. Compare the calculated pressure value with the actual pressure value that you have taken from the, is it the same?
Review of Semiconductor Physics. Lecture 3 4 Dr. Tayab Din Memon
 Review of Semiconductor Physics Lecture 3 4 Dr. Tayab Din Memon 1 Electronic Materials The goal of electronic materials is to generate and control the flow of an electrical current. Electronic materials
Review of Semiconductor Physics Lecture 3 4 Dr. Tayab Din Memon 1 Electronic Materials The goal of electronic materials is to generate and control the flow of an electrical current. Electronic materials
efficiency can be to Carnot primarily through the thermoelectric figure of merit, z, defined by
 USING THE COMPATIBILITY FACTOR TO DESIGN HIGH EFFICIENCY SEGMENTED THERMOELECTRIC GENERATORS G. Jeffrey Snyder*, and T. Caillat Jet Propulsion Laboratory/California Institute of Technology 4800, Oak Grove
USING THE COMPATIBILITY FACTOR TO DESIGN HIGH EFFICIENCY SEGMENTED THERMOELECTRIC GENERATORS G. Jeffrey Snyder*, and T. Caillat Jet Propulsion Laboratory/California Institute of Technology 4800, Oak Grove
A MODEL BASED APPROACH TO EXHAUST THERMOELECTRICS. Quazi Hussain, David Brigham, and Clay Maranville Research & Advanced Engineering
 A MODEL BASED APPROACH TO EXHAUST HEAT RECOVERY USING THERMOELECTRICS Quazi Hussain, David Brigham, and Clay Maranville Research & Advanced Engineering Ford Motor Company Objective Investigate potential
A MODEL BASED APPROACH TO EXHAUST HEAT RECOVERY USING THERMOELECTRICS Quazi Hussain, David Brigham, and Clay Maranville Research & Advanced Engineering Ford Motor Company Objective Investigate potential
V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). Makariy A.
 V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). 590B Maariy A. Tanatar September 28, 2009 Thermo- galvano-magnetic effects
V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). 590B Maariy A. Tanatar September 28, 2009 Thermo- galvano-magnetic effects
Thermionic power generation at high temperatures using SiGe/ Si superlattices
 JOURNAL OF APPLIED PHYSICS 101, 053719 2007 Thermionic power generation at high temperatures using SiGe/ Si superlattices Daryoosh Vashaee a and Ali Shakouri Jack Baskin School of Engineering, University
JOURNAL OF APPLIED PHYSICS 101, 053719 2007 Thermionic power generation at high temperatures using SiGe/ Si superlattices Daryoosh Vashaee a and Ali Shakouri Jack Baskin School of Engineering, University
Electro - Principles I
 Electro - Principles I Page 10-1 Atomic Theory It is necessary to know what goes on at the atomic level of a semiconductor so the characteristics of the semiconductor can be understood. In many cases a
Electro - Principles I Page 10-1 Atomic Theory It is necessary to know what goes on at the atomic level of a semiconductor so the characteristics of the semiconductor can be understood. In many cases a
materials and their properties
 materials and their properties macroscopic properties phase state strength / stiffness electrical conductivity chemical properties color / transparence spectroscopical properties surface properties density
materials and their properties macroscopic properties phase state strength / stiffness electrical conductivity chemical properties color / transparence spectroscopical properties surface properties density
Fundamentals of Heat Transfer (Basic Concepts)
 Fundamentals of Heat Transfer (Basic Concepts) 1 Topics to be covered History Thermodynamics Heat transfer Thermodynamics versus Heat Transfer Areas and Applications of Heat Transfer Heat Transfer problems
Fundamentals of Heat Transfer (Basic Concepts) 1 Topics to be covered History Thermodynamics Heat transfer Thermodynamics versus Heat Transfer Areas and Applications of Heat Transfer Heat Transfer problems
Energy Levels Zero energy. From Last Time Molecules. Today. n- and p-type semiconductors. Energy Levels in a Metal. Junctions
 Today From Last Time Molecules Symmetric and anti-symmetric wave functions Lightly higher and lower energy levels More atoms more energy levels Conductors, insulators and semiconductors Conductors and
Today From Last Time Molecules Symmetric and anti-symmetric wave functions Lightly higher and lower energy levels More atoms more energy levels Conductors, insulators and semiconductors Conductors and
Research to Improve Photovoltaic (PV) Cell Efficiency by Hybrid Combination of PV and Thermoelectric Cell Elements.
 UNIVERSITY OF CENTRAL FLORIDA Research to Improve Photovoltaic (PV) Cell Efficiency by Hybrid Combination of PV and Thermoelectric Cell Elements. Page 129 PI: Nicoleta Sorloaica-Hickman, Robert Reedy Students:
UNIVERSITY OF CENTRAL FLORIDA Research to Improve Photovoltaic (PV) Cell Efficiency by Hybrid Combination of PV and Thermoelectric Cell Elements. Page 129 PI: Nicoleta Sorloaica-Hickman, Robert Reedy Students:
Chapter 1 INTRODUCTION AND BASIC CONCEPTS
 Heat and Mass Transfer: Fundamentals & Applications 5th Edition in SI Units Yunus A. Çengel, Afshin J. Ghajar McGraw-Hill, 2015 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu University of Gaziantep
Heat and Mass Transfer: Fundamentals & Applications 5th Edition in SI Units Yunus A. Çengel, Afshin J. Ghajar McGraw-Hill, 2015 Chapter 1 INTRODUCTION AND BASIC CONCEPTS Mehmet Kanoglu University of Gaziantep
A thesis submitted to Cardiff University in the candidature for the degree of Doctor of Philosophy By. Nadhrah Md Yatim, BSc. (Hons), MSc.
 Development of Open-Short Circuit Dimensionless Figure-of-Merit (ZT) Measurement Technique for Investigation of Thermoelements and Segmented Thermoelectric Structures A thesis submitted to Cardiff University
Development of Open-Short Circuit Dimensionless Figure-of-Merit (ZT) Measurement Technique for Investigation of Thermoelements and Segmented Thermoelectric Structures A thesis submitted to Cardiff University
4. I-V characteristics of electric
 KL 4. - characteristics of electric conductors 4.1 ntroduction f an electric conductor is connected to a voltage source with voltage a current is produced. We define resistance being the ratio of the voltage
KL 4. - characteristics of electric conductors 4.1 ntroduction f an electric conductor is connected to a voltage source with voltage a current is produced. We define resistance being the ratio of the voltage
Resistivity and Temperature Coefficients (at 20 C)
 Homework # 4 Resistivity and Temperature Coefficients (at 0 C) Substance Resistivity, Temperature ( m) Coefficient, (C ) - Conductors Silver.59 x 0-0.006 Copper.6 x 0-0.006 Aluminum.65 x 0-0.0049 Tungsten
Homework # 4 Resistivity and Temperature Coefficients (at 0 C) Substance Resistivity, Temperature ( m) Coefficient, (C ) - Conductors Silver.59 x 0-0.006 Copper.6 x 0-0.006 Aluminum.65 x 0-0.0049 Tungsten
Temperature Measurement
 Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Temperature Measurement Temperature is one of the most common measurements What is Temperature? Intuitively understood as sensation of hot/cold Early Researchers: Galileo (1564-1642) Newton (1642-1727)
Laboratory 12: Three Thermodynamics Experiments
 Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
UMEÅ UNIVERSITY Department of Physics Agnieszka Iwasiewicz Leif Hassmyr Ludvig Edman SOLID STATE PHYSICS HALL EFFECT
 UMEÅ UNIVERSITY Department of Physics 2004-04-06 Agnieszka Iwasiewicz Leif Hassmyr Ludvig Edman SOLID STATE PHYSICS HALL EFFECT 1. THE TASK To measure the electrical conductivity and the Hall voltage for
UMEÅ UNIVERSITY Department of Physics 2004-04-06 Agnieszka Iwasiewicz Leif Hassmyr Ludvig Edman SOLID STATE PHYSICS HALL EFFECT 1. THE TASK To measure the electrical conductivity and the Hall voltage for
Lecture 36: Temperatue Measurements
 Lecture 36: Temperatue Measurements Contents Principle of thermocouples Materials for themocouples Cold junction compensation Compensating wires Selection of thermocouples Illustration of gas temperature
Lecture 36: Temperatue Measurements Contents Principle of thermocouples Materials for themocouples Cold junction compensation Compensating wires Selection of thermocouples Illustration of gas temperature
Temperature. Sensors. Measuring technique. Eugene V. Colla. 10/25/2017 Physics 403 1
 Temperature. Sensors. Measuring technique. Eugene V. Colla 10/25/2017 Physics 403 1 Outline Temperature Sensors Measuring equipment and ideas Sensor calibration Temperature scales 10/25/2017 Physics 403
Temperature. Sensors. Measuring technique. Eugene V. Colla 10/25/2017 Physics 403 1 Outline Temperature Sensors Measuring equipment and ideas Sensor calibration Temperature scales 10/25/2017 Physics 403
Advantages / Disadvantages of semiconductor detectors
 Advantages / Disadvantages of semiconductor detectors Semiconductor detectors have a high density (compared to gas detector) large energy loss in a short distance diffusion effect is smaller than in gas
Advantages / Disadvantages of semiconductor detectors Semiconductor detectors have a high density (compared to gas detector) large energy loss in a short distance diffusion effect is smaller than in gas
Lecture 11 Temperature Sensing. ECE 5900/6900 Fundamentals of Sensor Design
 EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
EE 4900: Fundamentals of Sensor Design Lecture 11 Temperature Sensing 1 Temperature Sensing Q: What are we measuring? A: Temperature 2 SI Units: Celcius ( C), Kelvin (K) British Units: Fahrenheit ( F)
The Underground Experimental Investigation of Thermocouples
 The Underground Experimental Investigation of Thermocouples April 14, 21 Abstract This experiment investigates a K-type thermocouple in order to demonstrate the Seebeck and Peltier coefficients. Temperature
The Underground Experimental Investigation of Thermocouples April 14, 21 Abstract This experiment investigates a K-type thermocouple in order to demonstrate the Seebeck and Peltier coefficients. Temperature
Electronic Circuits for Mechatronics ELCT 609 Lecture 2: PN Junctions (1)
 Electronic Circuits for Mechatronics ELCT 609 Lecture 2: PN Junctions (1) Assistant Professor Office: C3.315 E-mail: eman.azab@guc.edu.eg 1 Electronic (Semiconductor) Devices P-N Junctions (Diodes): Physical
Electronic Circuits for Mechatronics ELCT 609 Lecture 2: PN Junctions (1) Assistant Professor Office: C3.315 E-mail: eman.azab@guc.edu.eg 1 Electronic (Semiconductor) Devices P-N Junctions (Diodes): Physical
Semiconductor physics I. The Crystal Structure of Solids
 Lecture 3 Semiconductor physics I The Crystal Structure of Solids 1 Semiconductor materials Types of solids Space lattices Atomic Bonding Imperfection and doping in SOLIDS 2 Semiconductor Semiconductors
Lecture 3 Semiconductor physics I The Crystal Structure of Solids 1 Semiconductor materials Types of solids Space lattices Atomic Bonding Imperfection and doping in SOLIDS 2 Semiconductor Semiconductors
V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). Makariy A.
 V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). 590B Maariy A. Tanatar November 14, 2008 Thermo- galvano-magnetic effects Seebec
V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). 590B Maariy A. Tanatar November 14, 2008 Thermo- galvano-magnetic effects Seebec
Superconductivity. The Discovery of Superconductivity. Basic Properties
 Superconductivity Basic Properties The Discovery of Superconductivity Using liquid helium, (b.p. 4.2 K), H. Kamerlingh Onnes found that the resistivity of mercury suddenly dropped to zero at 4.2 K. H.
Superconductivity Basic Properties The Discovery of Superconductivity Using liquid helium, (b.p. 4.2 K), H. Kamerlingh Onnes found that the resistivity of mercury suddenly dropped to zero at 4.2 K. H.
Thermal conductivity: An example of structure-property relations in crystals Ram Seshadri
 Thermal conductivity: An example of structure-property relations in crystals Ram Seshadri Materials Department, and Department of Chemistry and Biochemistry Materials Research Laboratory University of
Thermal conductivity: An example of structure-property relations in crystals Ram Seshadri Materials Department, and Department of Chemistry and Biochemistry Materials Research Laboratory University of
CENTER FOR NONLINEAR AND COMPLEX SYSTEMS. Como - Italy
 CENTER FOR NONLINEAR AND COMPLEX SYSTEMS Como - Italy Providing a sustainable supply of energy to the world s population will become a major societal problem for the 21 st century as fossil fuel supplies
CENTER FOR NONLINEAR AND COMPLEX SYSTEMS Como - Italy Providing a sustainable supply of energy to the world s population will become a major societal problem for the 21 st century as fossil fuel supplies
Carrier Mobility and Hall Effect. Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India
 Carrier Mobility and Hall Effect 1 Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India http://folk.uio.no/ravi/semi2013 calculation Calculate the hole and electron densities
Carrier Mobility and Hall Effect 1 Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India http://folk.uio.no/ravi/semi2013 calculation Calculate the hole and electron densities
Introduction of Nano Science and Tech. Thermal and Electric Conduction in Nanostructures. Nick Fang
 Introduction of Nano Science and Tech Thermal and Electric Conduction in Nanostructures Nick Fang Course Website: nanohub.org Compass.illinois.edu ME 498 2006-09 Nick Fang, University of Illinois. All
Introduction of Nano Science and Tech Thermal and Electric Conduction in Nanostructures Nick Fang Course Website: nanohub.org Compass.illinois.edu ME 498 2006-09 Nick Fang, University of Illinois. All
Introduction to Semiconductor Physics. Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India
 Introduction to Semiconductor Physics 1 Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India http://folk.uio.no/ravi/cmp2013 Review of Semiconductor Physics Semiconductor fundamentals
Introduction to Semiconductor Physics 1 Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India http://folk.uio.no/ravi/cmp2013 Review of Semiconductor Physics Semiconductor fundamentals
Thermodynamics. 1.1 Introduction. Thermodynamics is a phenomenological description of properties of macroscopic systems in thermal equilibrium.
 1 hermodynamics 1.1 Introduction hermodynamics is a phenomenological description of properties of macroscopic systems in thermal equilibrium. Imagine yourself as a post-newtonian physicist intent on understanding
1 hermodynamics 1.1 Introduction hermodynamics is a phenomenological description of properties of macroscopic systems in thermal equilibrium. Imagine yourself as a post-newtonian physicist intent on understanding
Keep the Heat Test School Name. Team Number
 Keep the Heat Test 1-28-2012 School Name Team Number Circle the all of the correct answer to the below questions. One or more of the answers can be correct, if more than on one answer is correct, circle
Keep the Heat Test 1-28-2012 School Name Team Number Circle the all of the correct answer to the below questions. One or more of the answers can be correct, if more than on one answer is correct, circle
Lecture 6: Irreversible Processes
 Materials Science & Metallurgy Master of Philosophy, Materials Modelling, Course MP4, Thermodynamics and Phase Diagrams, H. K. D. H. Bhadeshia Lecture 6: Irreversible Processes Thermodynamics generally
Materials Science & Metallurgy Master of Philosophy, Materials Modelling, Course MP4, Thermodynamics and Phase Diagrams, H. K. D. H. Bhadeshia Lecture 6: Irreversible Processes Thermodynamics generally
Chapter 3: Electric Current And Direct-Current Circuits
 Chapter 3: Electric Current And Direct-Current Circuits 3.1 Electric Conduction 3.1.1 Describe the microscopic model of current Mechanism of Electric Conduction in Metals Before applying electric field
Chapter 3: Electric Current And Direct-Current Circuits 3.1 Electric Conduction 3.1.1 Describe the microscopic model of current Mechanism of Electric Conduction in Metals Before applying electric field
Semiconductors. Semiconductors also can collect and generate photons, so they are important in optoelectronic or photonic applications.
 Semiconductors Semiconducting materials have electrical properties that fall between true conductors, (like metals) which are always highly conducting and insulators (like glass or plastic or common ceramics)
Semiconductors Semiconducting materials have electrical properties that fall between true conductors, (like metals) which are always highly conducting and insulators (like glass or plastic or common ceramics)
Analysis of Thermoelectric Generator Performance by Use of Simulations and Experiments
 Journal of ELECTRONIC MATERIALS, Vol. 43, No. 6, 2014 DOI: 10.1007/s11664-014-3020-x Ó 2014 The Author(s). This article is published with open access at Springerlink.com Analysis of Thermoelectric Generator
Journal of ELECTRONIC MATERIALS, Vol. 43, No. 6, 2014 DOI: 10.1007/s11664-014-3020-x Ó 2014 The Author(s). This article is published with open access at Springerlink.com Analysis of Thermoelectric Generator
The Electromagnetic Properties of Materials
 The lectromagnetic Properties of Materials lectrical conduction Metals Semiconductors Insulators (dielectrics) Superconductors Magnetic materials Ferromagnetic materials Others Photonic Materials (optical)
The lectromagnetic Properties of Materials lectrical conduction Metals Semiconductors Insulators (dielectrics) Superconductors Magnetic materials Ferromagnetic materials Others Photonic Materials (optical)
ELECTRICITY & CIRCUITS
 ELECTRICITY & CIRCUITS Reason and justice tell me there s more love for humanity in electricity and steam than in chastity and vegetarianism. Anton Chekhov LIGHTNING, PART 2 Electricity is really just
ELECTRICITY & CIRCUITS Reason and justice tell me there s more love for humanity in electricity and steam than in chastity and vegetarianism. Anton Chekhov LIGHTNING, PART 2 Electricity is really just
CLASS 12th. Semiconductors
 CLASS 12th Semiconductors 01. Distinction Between Metals, Insulators and Semi-Conductors Metals are good conductors of electricity, insulators do not conduct electricity, while the semiconductors have
CLASS 12th Semiconductors 01. Distinction Between Metals, Insulators and Semi-Conductors Metals are good conductors of electricity, insulators do not conduct electricity, while the semiconductors have
EECS130 Integrated Circuit Devices
 EECS130 Integrated Circuit Devices Professor Ali Javey 8/30/2007 Semiconductor Fundamentals Lecture 2 Read: Chapters 1 and 2 Last Lecture: Energy Band Diagram Conduction band E c E g Band gap E v Valence
EECS130 Integrated Circuit Devices Professor Ali Javey 8/30/2007 Semiconductor Fundamentals Lecture 2 Read: Chapters 1 and 2 Last Lecture: Energy Band Diagram Conduction band E c E g Band gap E v Valence
V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). Makariy A.
 V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). 590B Makariy A. Tanatar November 12, 2008 Resistivity Typical resistivity temperature
V, I, R measurements: how to generate and measure quantities and then how to get data (resistivity, magnetoresistance, Hall). 590B Makariy A. Tanatar November 12, 2008 Resistivity Typical resistivity temperature
R measurements (resistivity, magnetoresistance, Hall). Makariy A. Tanatar
 R measurements (resistivity, magnetoresistance, Hall). 590B Makariy A. Tanatar April 18, 2014 Resistivity Typical resistivity temperature dependence: metals, semiconductors Magnetic scattering Resistivities
R measurements (resistivity, magnetoresistance, Hall). 590B Makariy A. Tanatar April 18, 2014 Resistivity Typical resistivity temperature dependence: metals, semiconductors Magnetic scattering Resistivities
EE301 Electronics I , Fall
 EE301 Electronics I 2018-2019, Fall 1. Introduction to Microelectronics (1 Week/3 Hrs.) Introduction, Historical Background, Basic Consepts 2. Rewiev of Semiconductors (1 Week/3 Hrs.) Semiconductor materials
EE301 Electronics I 2018-2019, Fall 1. Introduction to Microelectronics (1 Week/3 Hrs.) Introduction, Historical Background, Basic Consepts 2. Rewiev of Semiconductors (1 Week/3 Hrs.) Semiconductor materials
Semiconductor Physics
 Semiconductor Physics Motivation Is it possible that there might be current flowing in a conductor (or a semiconductor) even when there is no potential difference supplied across its ends? Look at the
Semiconductor Physics Motivation Is it possible that there might be current flowing in a conductor (or a semiconductor) even when there is no potential difference supplied across its ends? Look at the
Properties of Materials
 Tao Deng, dengtao@sjtu.edu.cn 1 1896 1920 1987 2006 Properties of Materials Chapter 3 Electrical Properties of Materials Tao Deng 3.1.4.4 The superconducting tunneling effect (Josephson effect) Tao Deng,
Tao Deng, dengtao@sjtu.edu.cn 1 1896 1920 1987 2006 Properties of Materials Chapter 3 Electrical Properties of Materials Tao Deng 3.1.4.4 The superconducting tunneling effect (Josephson effect) Tao Deng,
Slide 1. Temperatures Light (Optoelectronics) Magnetic Fields Strain Pressure Displacement and Rotation Acceleration Electronic Sensors
 Slide 1 Electronic Sensors Electronic sensors can be designed to detect a variety of quantitative aspects of a given physical system. Such quantities include: Temperatures Light (Optoelectronics) Magnetic
Slide 1 Electronic Sensors Electronic sensors can be designed to detect a variety of quantitative aspects of a given physical system. Such quantities include: Temperatures Light (Optoelectronics) Magnetic
Unit 6 Current Electricity and Circuits
 Unit 6 Current Electricity and Circuits 2 Types of Electricity Electricity that in motion. Electricity that in motion. Occurs whenever an moves through a. 2 Types of Current Electricity Electricity that
Unit 6 Current Electricity and Circuits 2 Types of Electricity Electricity that in motion. Electricity that in motion. Occurs whenever an moves through a. 2 Types of Current Electricity Electricity that
Lecture 2 Electrons and Holes in Semiconductors
 EE 471: Transport Phenomena in Solid State Devices Spring 2018 Lecture 2 Electrons and Holes in Semiconductors Bryan Ackland Department of Electrical and Computer Engineering Stevens Institute of Technology
EE 471: Transport Phenomena in Solid State Devices Spring 2018 Lecture 2 Electrons and Holes in Semiconductors Bryan Ackland Department of Electrical and Computer Engineering Stevens Institute of Technology
Chapter 4: Bonding in Solids and Electronic Properties. Free electron theory
 Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal an electron gas. regards a metal as a box in which electrons are free to move. assumes nuclei
Chapter 4: Bonding in Solids and Electronic Properties Free electron theory Consider free electrons in a metal an electron gas. regards a metal as a box in which electrons are free to move. assumes nuclei
THERMOELECTRIC PROPERTIES OF V-VI SEMICONDUCTOR ALLOYS AND NANOCOMPOSITES
 THERMOELECTRIC PROPERTIES OF V-VI SEMICONDUCTOR ALLOYS AND NANOCOMPOSITES Submitted by OVGU CEYDA YELGEL to the University of Exeter as a thesis for the degree of Doctor of Philosophy in Physics. August
THERMOELECTRIC PROPERTIES OF V-VI SEMICONDUCTOR ALLOYS AND NANOCOMPOSITES Submitted by OVGU CEYDA YELGEL to the University of Exeter as a thesis for the degree of Doctor of Philosophy in Physics. August
Applications: Thermoelectrics
 Page 1 of 5 Applications: Thermoelectrics Quantum dots breathes life back into Thermoelectrics Shortcomings of Traditional Thermoelectric devices Thermoelectrics is the science and technology associated
Page 1 of 5 Applications: Thermoelectrics Quantum dots breathes life back into Thermoelectrics Shortcomings of Traditional Thermoelectric devices Thermoelectrics is the science and technology associated
eterostrueture Integrated Thermionic Refrigeration
 eterostrueture Integrated Thermionic Refrigeration Ali Shakouri, and John E. Bowers Department of Electrical and Computer Engineering University of California, Santa Barbara, CA USA 936 ABSTRACT Thermionic
eterostrueture Integrated Thermionic Refrigeration Ali Shakouri, and John E. Bowers Department of Electrical and Computer Engineering University of California, Santa Barbara, CA USA 936 ABSTRACT Thermionic
IC Temperature Sensor Provides Thermocouple Cold-Junction Compensation
 IC Temperature Sensor Provides Thermocouple Cold-Junction Compensation Introduction Due to their low cost and ease of use, thermocouples are still a popular means for making temperature measurements up
IC Temperature Sensor Provides Thermocouple Cold-Junction Compensation Introduction Due to their low cost and ease of use, thermocouples are still a popular means for making temperature measurements up
Physics Nov Cooling by Expansion
 Physics 301 19-Nov-2004 25-1 Cooling by Expansion Now we re going to change the subject and consider the techniques used to get really cold temperatures. Of course, the best way to learn about these techniques
Physics 301 19-Nov-2004 25-1 Cooling by Expansion Now we re going to change the subject and consider the techniques used to get really cold temperatures. Of course, the best way to learn about these techniques
Influence of electric field dynamics on the generation of thermo emf by some advanced thermocouples in the high temperature range
 Volume 11 Issue 11 ISSN : 0974-7486 Influence of electric field dynamics on the generation of thermo emf by some advanced thermocouples in the high temperature range Jaspal Singh, S.S.Verma* Department
Volume 11 Issue 11 ISSN : 0974-7486 Influence of electric field dynamics on the generation of thermo emf by some advanced thermocouples in the high temperature range Jaspal Singh, S.S.Verma* Department
Emissivity: Understanding the difference between apparent and actual infrared temperatures
 Emissivity: Understanding the difference between apparent and actual infrared temperatures By L. Terry Clausing, P.E. ASNT Certified NDT Level III T/IR, for Fluke Corporation Application Note Taking infrared
Emissivity: Understanding the difference between apparent and actual infrared temperatures By L. Terry Clausing, P.E. ASNT Certified NDT Level III T/IR, for Fluke Corporation Application Note Taking infrared
Class 22 - Second Law of Thermodynamics and Entropy
 Class 22 - Second Law of Thermodynamics and Entropy The second law of thermodynamics The first law relates heat energy, work and the internal thermal energy of a system, and is essentially a statement
Class 22 - Second Law of Thermodynamics and Entropy The second law of thermodynamics The first law relates heat energy, work and the internal thermal energy of a system, and is essentially a statement
For their 1948 discovery of the transistor, John Bardeen, Walter Brattain, and William Shockley were awarded the 1956 Nobel prize in physics.
 Modern Physics (PHY 3305) Lecture Notes Modern Physics (PHY 3305) Lecture Notes Solid-State Physics: Superconductivity (Ch. 10.9) SteveSekula, 1 April 2010 (created 1 April 2010) Review no tags We applied
Modern Physics (PHY 3305) Lecture Notes Modern Physics (PHY 3305) Lecture Notes Solid-State Physics: Superconductivity (Ch. 10.9) SteveSekula, 1 April 2010 (created 1 April 2010) Review no tags We applied
A Comparison of Figureof Merit for Some Common ThermocouplesintheHighTemperatureRange
 Global Journal of Researches in Engineering Electrical and Electronics Engineering Volume 13 Issue 11 Version 1.0 Year 2013 Type: Double Blind Peer Reviewed International Research Journal Publisher: Global
Global Journal of Researches in Engineering Electrical and Electronics Engineering Volume 13 Issue 11 Version 1.0 Year 2013 Type: Double Blind Peer Reviewed International Research Journal Publisher: Global
Demonstration Some simple theoretical models Materials How to make superconductors Some applications
 Superconductivity Demonstration Some simple theoretical models Materials How to make superconductors Some applications How do we show superconductivity? Superconductors 1. have an electrical resistivity
Superconductivity Demonstration Some simple theoretical models Materials How to make superconductors Some applications How do we show superconductivity? Superconductors 1. have an electrical resistivity
Chemistry: The Central Science. Chapter 20: Electrochemistry
 Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
1 Arranging the Elements
 CHAPTER 12 1 Arranging the Elements SECTION The Periodic Table BEFORE YOU READ After you read this section, you should be able to answer these questions: How are elements arranged on the periodic table?
CHAPTER 12 1 Arranging the Elements SECTION The Periodic Table BEFORE YOU READ After you read this section, you should be able to answer these questions: How are elements arranged on the periodic table?
Core Concepts. PowerPoint Lectures to accompany Physical Science, 8e. Chapter 4 Heat and Temperature. New Symbols for this Chapter 2/14/2011
 PowerPoint Lectures to accompany Physical Science, 8e Chapter 4 Heat and Temperature Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. New Symbols for this Chapter
PowerPoint Lectures to accompany Physical Science, 8e Chapter 4 Heat and Temperature Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. New Symbols for this Chapter
LAB I WHAT IS IN IT AND WHY?
 LAB I WHAT IS IN IT AND WHY? Study Questions: 1. Make a list of 3 examples for each of the following material properties: 1. Mechanical properties 2. Electrical properties 3. Optical properties 4. Magnetic
LAB I WHAT IS IN IT AND WHY? Study Questions: 1. Make a list of 3 examples for each of the following material properties: 1. Mechanical properties 2. Electrical properties 3. Optical properties 4. Magnetic
Chapter 11. Energy in Thermal Processes
 Chapter 11 Energy in Thermal Processes Energy Transfer When two objects of different temperatures are placed in thermal contact, the temperature of the warmer decreases and the temperature of the cooler
Chapter 11 Energy in Thermal Processes Energy Transfer When two objects of different temperatures are placed in thermal contact, the temperature of the warmer decreases and the temperature of the cooler
Chem 481 Lecture Material 3/20/09
 Chem 481 Lecture Material 3/20/09 Radiation Detection and Measurement Semiconductor Detectors The electrons in a sample of silicon are each bound to specific silicon atoms (occupy the valence band). If
Chem 481 Lecture Material 3/20/09 Radiation Detection and Measurement Semiconductor Detectors The electrons in a sample of silicon are each bound to specific silicon atoms (occupy the valence band). If
Lecture 11: Coupled Current Equations: and thermoelectric devices
 ECE-656: Fall 011 Lecture 11: Coupled Current Euations: and thermoelectric devices Professor Mark Lundstrom Electrical and Computer Engineering Purdue University, West Lafayette, IN USA 9/15/11 1 basic
ECE-656: Fall 011 Lecture 11: Coupled Current Euations: and thermoelectric devices Professor Mark Lundstrom Electrical and Computer Engineering Purdue University, West Lafayette, IN USA 9/15/11 1 basic
Lecture 18: Semiconductors - continued (Kittel Ch. 8)
 Lecture 18: Semiconductors - continued (Kittel Ch. 8) + a - Donors and acceptors J U,e e J q,e Transport of charge and energy h E J q,e J U,h Physics 460 F 2006 Lect 18 1 Outline More on concentrations
Lecture 18: Semiconductors - continued (Kittel Ch. 8) + a - Donors and acceptors J U,e e J q,e Transport of charge and energy h E J q,e J U,h Physics 460 F 2006 Lect 18 1 Outline More on concentrations
Device Testing and Characterization of Thermoelectric Nanocomposites
 Device Testing and Characterization of Thermoelectric Nanocomposites By Andrew Muto B.S., Mechanical Engineering (2005) Northeastern University Submitted to the Department of Mechanical Engineering in
Device Testing and Characterization of Thermoelectric Nanocomposites By Andrew Muto B.S., Mechanical Engineering (2005) Northeastern University Submitted to the Department of Mechanical Engineering in
Exact Solution of a Constrained. Optimization Problem in Thermoelectric Cooling
 Applied Mathematical Sciences, Vol., 8, no. 4, 77-86 Exact Solution of a Constrained Optimization Problem in Thermoelectric Cooling Hongyun Wang Department of Applied Mathematics and Statistics University
Applied Mathematical Sciences, Vol., 8, no. 4, 77-86 Exact Solution of a Constrained Optimization Problem in Thermoelectric Cooling Hongyun Wang Department of Applied Mathematics and Statistics University
Supplementary Information for On-chip cooling by superlattice based thin-film thermoelectrics
 Supplementary Information for On-chip cooling by superlattice based thin-film thermoelectrics Table S1 Comparison of cooling performance of various thermoelectric (TE) materials and device architectures
Supplementary Information for On-chip cooling by superlattice based thin-film thermoelectrics Table S1 Comparison of cooling performance of various thermoelectric (TE) materials and device architectures
ISSUES TO ADDRESS...
 Chapter 12: Electrical Properties School of Mechanical Engineering Choi, Hae-Jin Materials Science - Prof. Choi, Hae-Jin Chapter 12-1 ISSUES TO ADDRESS... How are electrical conductance and resistance
Chapter 12: Electrical Properties School of Mechanical Engineering Choi, Hae-Jin Materials Science - Prof. Choi, Hae-Jin Chapter 12-1 ISSUES TO ADDRESS... How are electrical conductance and resistance
High Temperature Measurement System Design for Thermoelectric Materials In Power Generation Application
 Mat. Res. Soc. Symp. Proc. Vol. 793 24 Materials Research Society S9.4.1 High Temperature Measurement System Design for Thermoelectric Materials In Power Generation Application Sim Loo, Jarrod Short, Kuei
Mat. Res. Soc. Symp. Proc. Vol. 793 24 Materials Research Society S9.4.1 High Temperature Measurement System Design for Thermoelectric Materials In Power Generation Application Sim Loo, Jarrod Short, Kuei
